Attached files
| file | filename |
|---|---|
| EX-32.2 - KIWA BIO-TECH PRODUCTS GROUP CORP | ex32-2.htm |
| EX-32.1 - KIWA BIO-TECH PRODUCTS GROUP CORP | ex32-1.htm |
| EX-31.2 - KIWA BIO-TECH PRODUCTS GROUP CORP | ex31-2.htm |
| EX-31.1 - KIWA BIO-TECH PRODUCTS GROUP CORP | ex31-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| [X] | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2016
OR
| [ ] | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Transition Period from ______ to ______
Commission File Number: 000-33167
KIWA BIO-TECH PRODUCTS GROUP CORPORATION
(Exact name of registrant as specified in its charter)
| Nevada | 77-0632186 | |
| (State
or other jurisdiction of incorporation or organization) |
(I.R.S.
Employer Identification No.) |
| 310 N. Indian Hill Blvd., #702 |
| Claremont, California 91711 |
| (Address of principal executive offices) |
| (626) 715-5855 |
| (Registrant’s telephone number, including area code) |
| Securities
registered pursuant to Section 12(b) of the Act: |
Securities registered pursuant to Section 12(g) of the Act: (Title of Each Class) | |
| None | Common Stock, $0.001 par value |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. [ ] Yes [X] No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. [ ] Yes [X] No
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. [X] Yes [ ] No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). [X] Yes [ ] No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [ ] Yes [X] No
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer [ ] | Accelerated filer [ ] |
| Non-accelerated filer [ ] | Smaller reporting company [X] |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). [ ] Yes [X] No
The aggregate market value of voting and non-voting common stock held by non-affiliates of the registrant, based upon the closing bid quotation for the registrant’s common stock, as reported on the OTC Markets Group, Inc., as of June 30, 2016, the last business day of the registrant’s most recently completed second fiscal quarter, was approximately $3,557,238 (based on the closing sale price of the common stock as reported by the OTC QB) on June 30, 2016.
The number of shares of registrant’s common stock outstanding as of April 17, 2017 was 9,798,981.
Annual Report on Form 10-K
For the Fiscal Year Ended December 31, 2016
INDEX
TABLE OF CONTENTS
| 2 |
Special Note Regarding Forward-Looking Statements
On one or more occasions, we may make forward-looking statements in this Annual Report on Form 10-K regarding our assumptions, projections, expectations, targets, intentions or beliefs about future events. Words or phrases such as “anticipates,” “may,” “will,” “should,” “believes,” “estimates,” “expects,” “intends,” “plans,” “predicts,” “projects,” “targets,” “will likely result,” “will continue” or similar expressions identify forward-looking statements. These forward-looking statements are only our predictions and involve numerous assumptions, risks and uncertainties, including, but not limited to those listed below and those business risks and factors described elsewhere in this report and our other Securities and Exchange Commission filings.
We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. However, your attention is directed to any further disclosures made on related subjects in our subsequent annual and periodic reports filed with the Securities and Exchange Commission on Forms 10-K, 10-Q and 8-K and Proxy Statements on Schedule 14A.
References herein to “we,” “us,” “our” or “the Company” refer to Kiwa Bio-Tech Products Group Corporation and its wholly-owned and majority-owned subsidiaries unless the context specifically states or implies otherwise.
The Company
1. Organizational History
We are the result of a share exchange transaction completed in March 2004 between the shareholders of Tintic Gold Mining Company (“Tintic”), a corporation originally incorporated in the state of Utah on June 14, 1933 to perform mining operations in Utah, and the shareholders of Kiwa Bio-Tech Products Group Ltd. (“Kiwa BVI”), a company originally organized under the laws of the British Virgin Islands on June 5, 2002. The share exchange resulted in a change of control of Tintic, with former Kiwa BVI stockholders owning approximately 89% of Tintic on a fully diluted basis and Kiwa BVI surviving as a wholly-owned subsidiary of Tintic. Subsequent to the share exchange transaction, Tintic changed its name to Kiwa Bio-Tech Products Group Corporation. On July 21, 2004, we completed our reincorporation in the State of Delaware. On March 8, 2017, we completed our reincorporation in the State of Nevada.
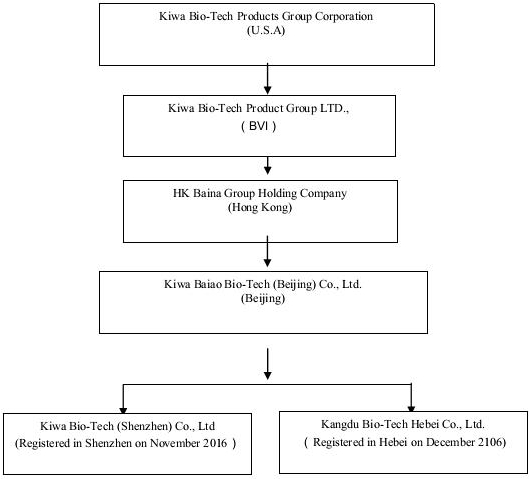
The Company operates through a series of subsidiaries in the Peoples Republic of China as detailed in the following Organizational Chart. The Company had previously operated its business through its subsidiaries Kiwa Bio-Tech Products (Shandong) Co., Ltd. (“Kiwa Shandong”) and Tianjin Kiwa Feed Co., Ltd. (“Kiwa Tianjin”). Kiwa Tianjin has been dissolved since July 11, 2012. On February 11, 2017, the Company entered an Equity Transfer Agreement with Dian Shi Cheng Jing (Beijing) Technology Co. (“Transferee”) to transfer all of shareholders’ right, title and interest in Kiwa Shandong to the Transferee for USD $1.00. Currently, the completion of transfer is under the government processing.
| 3 |
2. Overview of Business
We develop, manufacture, distribute and market innovative, cost-effective and environmentally safe bio-technological products for agriculture. Our products are designed to enhance the quality of human life by increasing the value, quality and productivity of crops and decreasing the negative environmental impact of chemicals and other wastes.
| 4 |
Bio-fertilizers
We have developed six bio-fertilizer products with bacillus species (“bacillus spp”) and/or photosynthetic bacteria as core ingredients. Some of our products contain ingredients of both photosynthesis and bacillus bacteria. Bacillus spp is a species of bacteria that interacts with plants and promotes biological processes. It is highly effective for promoting plant growth, enhancing yield, improving quality and elevating resistances. Photosynthetic bacteria are a group of green and purple bacteria. Bacterial photosynthesis differs from green plant photosynthesis in that bacterial photosynthesis occurs in an anaerobic environment and does not produce oxygen. Photosynthetic bacteria can enhance the photosynthetic capacity of green plants by increasing the utilization of sunlight, which helps keep the photosynthetic process at a vigorous level, enhances the capacity of plants to transform inorganic materials to organic products, and boosts overall plant health and productivity.
Our bacillus bacteria based fertilizers are protected by patents. In 2004, we acquired patent no. ZL 93101635.5 entitled “Highly Effective Composite Bacteria for Enhancing Yield and the Related Methodology for Manufacturing” from China Agricultural University (“CAU”) for the aggregate purchase of $480,411, consisting of $60,411 in cash and 5,000 shares of our common stock, valued at $84.00 per share (aggregate value of $420,000). Our photosynthetic bacteria based fertilizers are also protected by trade secret laws.
The patent acquired from CAU covers six different species of bacillus which have been tested as bio-fertilizers to enhance yield and plant health. The production methods of the six species are also patented. The patent has expired on February 19, 2013.There are no limitations under this agreement on our exclusive use of the patent. Pursuant to our agreement with CAU, the University agreed to provide research and technology support services at no additional cost to us in the event we decide to use the patent to produce commercial products. These research and technology support services include: (1) furnishing faculty or graduate-level researchers to help bacteria culturing, sampling, testing, trial production and production formula adjustment; (2) providing production technology and procedures to turn the products into powder form while keeping live required bacteria in the products; (3) establishing quality standards and quality control systems; (4) providing testing and research support for us to obtain necessary sale permits from the Chinese government; and (5) cooperation in developing derivative products.
On January 5, 2011, the State Intellectual Property Office of the PRC (“Intellectual Property Office”) granted Kiwa two Certificates of Patent of Invention for (1) “A cucumber dedicated composite anti-continuous cropping effect probiotics and their specific strains with related application” with patent number of “ZL 2008 1 0144492.6”; and (2) “Cotton dedicated composite anti-continuous cropping effect probiotics and their special strains with related application” with patent number of “ZL 2008 1 0144491.1” These two patents have been developed by Kiwa-CAU R&D Center. These two patents will expire on August 5, 2028. These two patents can be used to develop specific environment-friendly bio-fertilizer.
We have obtained five fertilizer registration certificates from the Chinese government - four covering our bacillus bacteria fertilizer and one covering our photosynthetic bacteria fertilizer. The five registration certificates are: (1) Microorganism Microbial Inoculum Fertilizer Registration Certificate issued by the PRC Ministry of Agriculture; (2) Photosynthetic Bacteria Fertilizer Registration Certificate issued by the PRC Ministry of Agriculture; (3) Amino Acid Foliar Fomular Fertilizer Registration Certificate issued by the PRC Ministry of Agriculture; (4) Organic Fertilizer Registration Certificate issued by Agriculture Department of Shandong Province; and (5) Organic Matter-Decomposing Inoculants Registration Certificate issued by the PRC Ministry of Agriculture on February 16, 2008. Protected by these five fertilizer registration certificates and five trademarks under the names of “KANGTAN” (Chinese translation name for Kiwa), “ZHIGUANGYOU,” “PUGUANGFU,” “JINWA” and “KANGGUAN,” we have developed six series of bio-fertilizer products with bacillus spp and/or photosynthetic bacteria as core ingredients. Valid period of fertilizer registration certificates is five years and may be extended for another five years upon application from the owner of fertilizer registration certificates. The Company has determined to re-apply the Fertilizer Registration Certificate issued by the PRC Ministry of Agriculture.
| 5 |
Kiwa-CAU Research and Development Center
In July 2006, we established a new research center with CAU which is known as Kiwa-CAU Bio-Tech Research & Development Center (the “Kiwa-CAU R&D Center”). Pursuant to an agreement between CAU and Kiwa Shandong dated November 14, 2006, Kiwa agreed to contribute RMB 1 million (approximately $160,000) each year to fund research at Kiwa-CAU R&D Center. Under the above agreement, the Kiwa-CAU R&D Center is responsible for fulfilling the overall research-and-development functions of Kiwa Shandong, including: (1) development of new technologies and new products (which will be shared by Kiwa and CAU); (2) subsequent perfection of existing product-related technologies; and (3) training quality-control personnel and technicians and technical support for marketing activities. The Company has spent $224,704 and $178,388 for its research and development activities during the years ended December 31, 2016 and 2015, respectively. The costs incurred by Company’s research and development activities are not borne directly by customers.
During fiscal 2014, Kiwa-CAU R&D Center had successfully isolated several strains of endophytic bacillus from plants. A number of strains had been observed to have the capability of boosting crop yield and dispelling chemical pesticide residual from soil. These strains could be used for developing not only new biological preparation but also environmental protection preparation.
Pursuant to the agreement on joint incorporation of the research and development center between CAU and Kiwa dated November 14, 2006, Kiwa agreed to invest RMB 1 million (approximately $160,000) each year to fund research at the R&D Center. The term of this agreement was ten years starting from July 1, 2006. Prof. Qi Wang, who became one of our directors in July 2007, has acted as the Director of Kiwa-CAU R&D Center since July 2006. The Company has negotiated a follow-up Cooperation Agreement for the technologies with China Agricultural University.
On November 5, 2015, the Company signed a strategic cooperation agreement (the “Agreement”) with China Academy of Agricultural Science (“CAAS”)’s Institute of Agricultural Resources & Regional Planning (“IARRP”) and Institute of Agricultural Economy & Development (“IAED”). Pursuant to the Agreement, the Company will form a strategic partnership with the two institutes and establish an “International Cooperation Platform for Internet and Safe Agricultural Products”. To fund the cooperation platform’s R&D activities, the Company will provide RMB 1 million (approximately $160,000) per year to the Spatial Agriculture Planning Method & Applications Innovation Team that belongs to the Institutes. The term of the Agreement is for three years beginning November 20, 2015. Prof. Yong Chang Wu, the authorized representative of IARRP, CAAS, is also one of the Company’s directors effective since November 20, 2015 until March 13, 2017.
On February 23, 2017, the Company agreed to a strategic relationship with ETS (Tianjin) Biological Science and Technology Development Co., Ltd. (“ETS”). The partnership will include the deployment and strategic use of ETS biotechnology to produce of bio-fertilizers for use in both China and internationally. Kiwa and ETS, together with the certain Chinese government departments, will work together to enhance China’s microbial fertilizer industry standards and China’s food safety industry chain standards. The parties will work together on the development of microbial technology and products in agriculture, environmental protection, soil management and other fields. Relying on the Chinese Academy of Sciences, ETS Environmental and Agricultural Microbial Technology Research Center and biotechnology project research results, Kiwa has introduced the ETS core technology to complete bio-fertilizer upgrading, transformation and to develop new product lines. In order to meet the growing global consumer demand to increase food supply and develop sustainable farming we are applying sustainable use of biotechnology and the use of biotechnology products to replace chemical products, which will strengthen environmental protection and promote international cooperation. As a result of strict management of many agricultural chemicals, such chemicals will continue to be abandoned, resulting is a growing demand for bio-fertilizers. It has been widely accepted that the application of ETS biotechnology facilitates agricultural sustainability and helps to protect the soil and improve grain output. The technology focuses on keeping soil healthy by restoring healthy microbes that are naturally present in healthy soils. As the technology gains worldwide recognition, it is imperative to popularize bio-fertilizer in developing countries to fulfill the needs of growing populations and promote environmentally friendly agriculture. Through the cooperation of Kiwa and ETS, the parties aim to enhance the usage of the bio-fertilizers in China. The cooperation will bring technological transformation and support for Kiwa to improve its existing manufacturing techniques. Kiwa and ETS will also collaborate to establish a comprehensive platform for producing, supplying, and marketing in China. Ultimately, Kiwa would look to introduce these products to the international market, including the United States.
| 6 |
Other
On November 30, 2015, we entered into an acquisition agreement (the “Agreement”) with the shareholders of Caber Holdings LTD, whose Chinese name is Hong Kong Baina Group Co., Ltd, located in Hong Kong (“Baina Hong Kong”), and Oriental Baina Co. Ltd. (hereinafter referred to as “Baina Beijing”), Baina Hong Kong’s wholly-owned subsidiary in Beijing, China. As a result of this Agreement, Kiwa renamed Baina Beijing to Kiwa Baiao Bio-Tech (Beijing) Co., Ltd., which replaced Kiwa Bio-Tech (Shandong) Co., Ltd (“Kiwa Shandong”) to operate Kiwa’s bio-fertilizer market expansion and become Kiwa’s platform for future acquisitions of new agricultural-related projects in China. In accordance with the terms of the Agreement, Kiwa agreed to pay US$30,000 to the Baina Hong Kong Shareholders for the acquisition of 100% of the equity of Baina Hong Kong. The Company paid RMB 220,000 (approximately $34,000) for the acquisition. The acquisition was completed on January 7, 2016. Both Baina Hong Kong and Baina Beijing had no activities before the acquisition date and had no assets and liabilities.
Thereafter, Baina Beijing formed two new subsidiaries—Kiwa Bio-Tech (Shenzhen) Co., Ltd (Registered in Shenzhen on November 2016); Kangdu Bio-Tech Hebei Co., Ltd. (Registered in Hebei on December 2106) to operate in specific markets in China.
On December 17, 2015, we entered into a distribution agreement (the “Agreement”) with Kangtan Gerui (Beijing) Bio-Tech Co., Ltd. (“Gerui”) and formally awarded Gerui a right to sell and distribute the Company’s fertilizer products in 3 major agricultural regions of China— Hainan Province, Hunan Province and Xinjiang Autonomous Region. The Company’s Research and Development department has been conducting application experiments in Hainan and Hunan Provinces since August 2015, in accordance with the market requirements. The experiment data indicates that the Company’s fertilizer products have fulfilled the requirements of reduction of content of heavy metals in soil and improved crop yield. Gerui was founded in Beijing in April 2015 and relies on the sales network of China’s Supply and Marketing Cooperatives system. Currently, the Company and Gerui do not hold any interest in each other; however, a collaboration and integration may take place in the future. The term of the Agreement is for a period of three years commencing December 17, 2015. In September 2016, Kiwa Baiao Bio-Tech (Beijing) Co., Ltd obtained a fertilizer sales permit from the Chinese government and began to sale the products directly to customers in those 3 major agricultural regions. In September 2016, Kiwa Baiao Bio-Tech (Beijing) Co., Ltd obtained a fertilizer sales permit from the Chinese government and began to sale the products directly to customers in those 3 major agricultural regions.
| 7 |
On February 27, 2017, the Company signed a strategic cooperation agreement with the Beijing Zhongpin Agricultural Science and Technology Development Center (“Zhongpin Center”). Zhongpin Center is the Chinese Agricultural Science and Technology Innovation and Development Committee’s executive implementation agency (referred to as the Agricultural Science and Technology Commission). The Agricultural Science and Technology Commission is set up by the Chinese Central Government for the construction of the National Ecological Security Agriculture Industrial Chain standardization system. This includes the establishment of National Ecology Safe Agricultural Industrial Parks to build China’s Ecological Security and Agricultural Industrial in an orderly business environment, including completion of the National Soil Remediation Program and governance of the various government functions of the institutions. Through the guidance and support by the Zhongpin Center, Kiwa will participate and be involved in China’s National Soil Remediation Program and construction of the National Ecological Security Agriculture Industrial Chain Standardization System’s operation and process.
Smaller reporting companies are not required to provide the information required by this item.
| 8 |
ITEM 1B. Unresolved Staff Comments
None.
In June 2002, Kiwa Shandong entered into an agreement with Zoucheng Municipal Government granting us the use of at least 15.7 acres in Shandong Province, China at no cost for 10 years to construct a manufacturing facility. Under the agreement, we have the option to pay a fee of approximately RMB 480,000 (approximately $78,155) per acre for the land use right at the expiration of the 10-year period. We may not transfer or pledge the temporary land use right. In the same agreement, we have also committed to invest approximately $18 million to $24 million for developing the manufacturing and research facilities in Zoucheng, Shandong Province. On February 11, 2017 Kiwa Bio-Tech Products (Shandong) entered an Equity Transfer Agreement with Dian Shi Cheng Jing (Beijing) Technology Co. (“Transferee”) to transfer all of shareholders’ right, title and interest in Kiwa Shandong to the Transferee for USD $1.00. Currently, the completion of transfer is under the government processing.
None.
ITEM 4. Mine Safety Disclosures
Not applicable.
ITEM 5. Market for Registrants’ Common Equity, Related Stockholder Matters and Issuer Purchasers of Equity Securities
Market Information
The Company’s common stock has been quoted on the OTCQB under the symbol “KWBT” since March 30, 2004.
The following table sets forth the high and low bid quotations per share of our common stock as reported on the OTCQB for the periods indicated. The high and low bid quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions. All prices are adjusted to reflect the Company’s one for 200 reverse split which went effective January 28, 2016.
| Fiscal Year 2016 | High | Low | ||||||
| First Quarter | $ | 2.24 | $ | 0.20 | ||||
| Second Quarter | $ | 1.98 | $ | 1.30 | ||||
| Third Quarter | $ | 1.65 | $ | 0.62 | ||||
| Fourth Quarter | $ | 1.43 | $ | 0.80 | ||||
| Fiscal Year 2015 | High | Low | ||||||
| First Quarter | $ | 1.47 | $ | 0.12 | ||||
| Second Quarter | $ | 0.44 | $ | 0.02 | ||||
| Third Quarter | $ | 0.32 | $ | 0.12 | ||||
| Fourth Quarter | $ | 0.70 | $ | 0.12 | ||||
Holders
As of December 31, 2016, there were approximately 461 shareholders of record of our common shares.
Dividend Policy
We have not paid any dividends on our common shares since our inception and do not anticipate that dividends will be paid at any time in the immediate future.
Equity Compensation Plan Information
The information required by Item 5 regarding securities authorized for issuance under equity compensation plans is included in Item 12 of this report.
| 9 |
Recent Sales of Unregistered Securities
The following is a list of securities issued for cash or converted with debentures or as stock compensation to consultants during the period from January 1, 2016 through April 14, 2017, which were not registered under the Securities Act:
| Name of Purchaser | Issue Date | Security | Shares | Consideration | ||||||||
| YVONNE WANG | 3/24/16 | Common | 240,000 | Debenture Conversion | ||||||||
| WEI LI | 3/24/16 | Common | 2,900,000 | Debenture Conversion | ||||||||
| MARK E. CRONE | 3/25/16 | Common | 1,000 | Legal Fees | ||||||||
| ZENG MIN QING | 5/24/16 | Common | 125,000 | Stock Purchase | ||||||||
| CHENG TZU-YUN | 5/24/16 | Common | 50,000 | Stock Purchase | ||||||||
| ZHIMING ZHU | 5/24/16 | Common | 45,000 | Stock Purchase | ||||||||
| HANG ZHAO | 7/20/16 | Common | 20,000 | Stock Purchase | ||||||||
| SHIWEI XIE | 7/20/16 | Common | 10,000 | Stock Purchase | ||||||||
| XIAOQIANG YU | 7/20/16 | Common | 30,000 | Stock Purchase | ||||||||
| HANG ZHAO | 8/10/16 | Common | 20,000 | Stock Purchase | ||||||||
| XIAOQIANG YU | 8/10/16 | Common | 10,000 | Stock Purchase | ||||||||
| XIANGRON CHEN | 8/10/16 | Common | 40,000 | Stock Purchase | ||||||||
| JIMMY ZHOU | 8/24/16 | Common | 101,947 | Consultant Fees | ||||||||
| WEIHONG SHAN | 10/6/16 | Common | 150,000 | Stock Purchase | ||||||||
| LIFENG LIU | 10/6/16 | Common | 100,000 | Stock Purchase | ||||||||
| ZHENPING LI | 10/6/16 | Common | 100,000 | Stock Purchase | ||||||||
| QI JIANG | 10/6/16 | Common | 550,000 | Stock Purchase | ||||||||
| LEI HOU | 10/6/16 | Common | 100,000 | Stock Purchase | ||||||||
| BING ZHANG | 10/6/16 | Common | 150,000 | Stock Purchase | ||||||||
| DEMEI YANG | 10/6/16 | Common | 150,000 | Stock Purchase | ||||||||
| WSMG ADVISORS, INC. | 10/6/16 | Common | 100,000 | Consultant Fees | ||||||||
| EQUITIES.COM, INC | 10/6/16 | Common | 30,808 | Consultant Fees | ||||||||
| GENG LIU | 10/19/16 | Common | 500,000 | Consultant Fees | ||||||||
| LIXIN TIAN | 10/19/16 | Common | 500,000 | Consultant Fees | ||||||||
| XU LIU | 10/19/16 | Common | 500,000 | Consultant Fees | ||||||||
| QINGKE XING | 10/19/16 | Common | 60,000 | Consultant Fees | ||||||||
| GROWTH CIRCLE INC. | 10/19/16 | Common | 20,000 | Consultant Fees | ||||||||
| WILLIAM FARRANCE | 11/29/16 | Common | 125,000 | Stock Purchase | ||||||||
| JUNWEI ZHENG | 3/3/17 | Common | 920,000 | Stock Purchase | ||||||||
| YUAN WANG | 3/3/17 | Common | 80,000 | Stock Purchase | ||||||||
| YUAN WANG | 3/3/17 | Common | 70,000 | Consultant Fees | ||||||||
ITEM 6. Selected Financial Data
Not required.
ITEM 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
This Annual Report on Form 10-K for the fiscal year ended December 31, 2016 contains “forward-looking” statements within the meaning of Section 21E of the Securities and Exchange Act of 1934, as amended, including statements that include the words “believes,” “expects,” “anticipates,” or similar expressions. These forward-looking statements include, among others, statements concerning our expectations regarding our working capital requirements, financing requirements, business, growth prospects, competition and results of operations, and other statements of expectations, beliefs, future plans and strategies, anticipated events or trends, and similar expressions concerning matters that are not historical facts. The forward-looking statements in this Annual Report on Form 10-K for the fiscal year ended December 31, 2016 involve known and unknown risks, uncertainties and other factors (described in “Business-Risk Factors” under Item 1) that could cause our actual results, performance or achievements to differ materially from those expressed in or implied by the forward-looking statements contained herein.
Overview
The Company took its present corporate form in March 2004 when the shareholders of Tintic Gold Mining Company, a Utah public corporation (“Tintic”), entered into a share exchange transaction with the shareholders of Kiwa BVI, a privately-held British Virgin Islands corporation that left the shareholders of Kiwa BVI owning a majority of Tintic and Kiwa BVI a wholly-owned subsidiary of Tintic, see “Business - The Company” under Item 1. For accounting purposes this transaction was treated as an acquisition of Tintic Gold Mining Company by Kiwa BVI in the form of a reverse triangular merger and a recapitalization of Kiwa BVI and its wholly owned subsidiary, Kiwa Shandong. On July 21, 2004, we completed our reincorporation in the State of Delaware. On March 8, 2017, we completed our reincorporation in the State of Nevada.
The Company operates through a series of subsidiaries in the Peoples Republic of China as detailed in the Organizational Chart on page 4, above. The Company had previously operated its business through its subsidiaries Kiwa Bio-Tech Products (Shandong) Co., Ltd. (“Kiwa Shandong”) and Tianjin Kiwa Feed Co., Ltd. (“Kiwa Tianjin”). Kiwa Tianjin has been dissolved since July 11, 2012. On February 11, 2017, the Company entered an Equity Transfer Agreement with Dian Shi Cheng Jing (Beijing) Technology Co. (“Transferee”) to transfer all of shareholders’ right, title and interest in Kiwa Shandong to the Transferee for USD $1.00. Currently, the completion of transfer is under the government processing.
| 10 |
We generated revenue in fiscal year 2016. We incurred a net income of $963,296 and a net loss $677,358 during fiscal 2016 and 2015, respectively.
Due to our limited revenues, we have relied on the proceeds from loans from both unrelated and related parties to provide the resources necessary to fund the development of our business plan and operations. Our financing activities generated $1,053,358 and $148,009 during fiscal 2016 and 2015, respectively. These funds are insufficient to execute our business plan as currently contemplated, which may result in the risks described in “Risk Factors” under Item 1 - Business.
Going Concern
The consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business.
As of December 31, 2016, the Company’s current liabilities substantially exceeded its current assets by $5,729,622. Although the company’s operations for the year ended December 31, 2016 resulted net income of $963,296, it had an accumulated deficit of $19,489,400 and stockholders’ deficiency of $5,601,213 at December 31, 2016. These circumstances, among others, raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. The financial statements also do not include any adjustments relating to the recoverability and classification of recorded asset amounts, or amounts and classifications of liabilities that might be necessary should the Company be unable to continue as a going concern.
The Company already raised additional fund through equity financing and plans to raise additional funds from domestic and foreign banks and/or financial institutions to increase working capital in order to meet capital demands. Please refer to Note 15 (Subsequent Evens) for additional information.
Trends and Uncertainties in Regulation and Government Policy in China
Foreign Exchange Policy Changes
China is considering allowing its currency to be freely exchangeable for other major currencies. This change will result in greater liquidity for revenues generated in Renminbi (“RMB”). We would benefit by having easier access to and greater flexibility with capital generated in and held in the form of RMB. The majority of our assets are located in China and most of our earnings are currently generated in China, and are therefore denominated in RMB. Changes in the RMB-U.S. Dollar exchange rate will impact our reported results of operations and financial condition. In the event that RMB appreciates over the next year as compared to the U.S. Dollar, our earnings will benefit from the appreciation of the RMB. However, if we have to use U.S. Dollars to invest in our Chinese operations, we will suffer from the depreciation of U.S. Dollars against the RMB. On the other hand, if the value of the RMB were to depreciate compared to the U.S. Dollar, then our reported earnings and financial condition would be adversely affected when converted to U.S. Dollars.
On July 21, 2005, the People’s Bank of China announced it would appreciate the RMB, increasing the RMB-U.S. Dollar exchange rate from approximately US$1.00 = RMB 8.28 to approximately US$1.00 = RMB 8.11. So far the trend of such appreciation continues. The exchange rate of U.S. Dollar against RMB on December 31, 2016 was US$1.00 = RMB 6.945.
Critical Accounting Policies and Estimates
We prepared our consolidated financial statements in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires the use of estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the reporting period. Management periodically evaluates the estimates and judgments made. Management bases its estimates and judgments on historical experience and on various factors that are believed to be reasonable under current circumstances. Actual results may differ from these estimates as a result of different assumptions or conditions.
The following critical accounting policies affect the more significant judgments and estimates used in the preparation of our consolidated financial statements. In addition, you should refer to our accompanying audited balance sheets as of December 31, 2016 and 2015, and the audited statements of comprehensive loss, equity movement and cash flows for the fiscal years ended December 31, 2016 and 2015, and the related notes thereto, for further discussion of our accounting policies.
Fair value of warrants and options
We have adopted ASC Topic 815, “Accounting for Derivative Instruments and Hedging Activities” to recognize warrants relating to loans and warrants issued to consultants as compensation as derivative instruments in our consolidated financial statements.
| 11 |
We also adopted ASC Topic 718, “Share Based Payment” to recognize options granted to employees as derivative instruments in our consolidated financial statements.
We calculate the fair value of the warrants and options using the Black-Scholes Model.
Income Taxes
The Company accounts for income taxes under the provisions of FASB ASC Topic 740, “Income Tax,” which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the consolidated financial statements or tax returns. Deferred tax assets and liabilities are recognized for the future tax consequence attributable to the difference between the tax bases of assets and liabilities and their reported amounts in the financial statements. Deferred tax assets and liabilities are measured using the enacted tax rate expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. The Company establishes a valuation when it is more likely than not that the assets will not be recovered.
ASC Topic 740.10.30 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740.10.40 provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. We have no material uncertain tax positions for any of the reporting periods presented.
Major Customers and Suppliers.
Bio-fertilizer products
During 2016, the following were Kiwa’s major customers:
1. Qingzhou City Agricultural Production Materials Co., Ltd. second wholesale department (11.3% of sales)
2. Huarong County Yinfeng fertilizer industry LTD. (81% of sales)
During 2016, the following were Kiwa’s major suppliers:
1. Weifang Druek Fertilizer Co., Ltd (92% of Subcontractor Production)
2 Lianyungang Chuangyi Logistics Co., Ltd (Logistics provider)
3. Linshu Shangrun Color Printing Co., Ltd (Packing provider)
Results of Operations
Net Sales
Net sales were $9,617,845 and $0 for the years ended December 31, 2016 and 2015, respectively.
Cost of Sales
Cost of sales was $7,672,451 and $0 for the years ended December 31, 2016 and 2015, respectively.
Gross Profit/Loss
Gross profit for fiscal 2016 was $1,945,394 compared to $0 for fiscal 2015.
Licensing Revenue
Licensing revenue totaled $786,329 for the year ended December 31, 2016.
General and Administrative
General and administrative expenses were $874,097 and $313,589 for the years ended December 31, 2016 and 2015, respectively, an increase of $560,508 or 178%. General and administrative expenses include professional fees, officers’ compensation, depreciation and amortization, salaries, travel and entertainment, rent, office expense and telephone expense etc.
| 12 |
Research and Development
Research and development expenses increased significantly by $45,445 or 25% to $224,433 for the year ended December 31, 2016, compared to $178,988 for the prior comparable period. Research and development expense mainly consists of the expenses of maintaining Kiwa-CAU R&D Center, which began operation in July 2006 (see “Business-Intellectual Property and Product Lines- Kiwa-CAU R&D Center” under Item 1 in Part I). On November 5, 2015, the Company signed a strategic cooperation agreement (the “Agreement”) with China Academy of Agricultural Science (“CAAS”)’s Institute of Agricultural Resources & Regional Planning (“IARRP”) and Institute of Agricultural Economy & Development (“IAED”). Pursuant to the Agreement, the Company will form a strategic partnership with the two institutes and establish an “International Cooperation Platform for Internet and Safe Agricultural Products”. To fund the cooperation platform’s R&D activities, the Company will provide RMB 1 million (approximately $160,000) per year to the Spatial Agriculture Planning Method & Applications Innovation Team that belongs to the Institutes. During the years ended December 31, 2016 and 2015, the Company accrued $224,433 and $178,988 for research and development respectively.
Penalty Expense
We charged $77,575 and $72,512 of liquidated damages in connection with the 6% Notes to penalty expenses during the years ended December 31, 2016 and 2015, respectively. The increase of penalty expense was mainly due to accrued and unpaid interest on the 6% Notes.
Interest Expenses
Net interest expense was $112,977 and $112,629 in the fiscal years of 2016 and 2015, respectively, representing a increase of $348 or 0.3%.
Net Income & (Loss)
During the fiscal year 2016, net income was $963,296, comparing with net loss $677,358 for the same period of 2015, representing an increase of $1,640,654.
Comprehensive Gain & (Loss)
Comprehensive income increased by $1,446,652 from $(253,293) for fiscal 2015, as compared to $1,193,359 for the comparable year of 2016.
Liquidity and Capital Resources
Since inception of our ag-biotech business in 2002, we have relied on the proceeds from the sale of our equity securities and loans from both unrelated and related parties to provide the resources necessary to fund our operations and the execution of our business plan. During fiscal 2016 and 2015, we raised $1,053,358 and $148,009 in total from stock purchase agreements and related party loans. To some extent, these advances improved our short-term liquidity. However, as of December 31, 2016, our current liabilities substantially exceeded current assets by $5,729,622, reflecting a current ratio of 0.4451, whereas current liabilities exceeded current assets by $11,103.261, reflecting a current ratio of 0.0043 as of December 31, 2015. During the years ended December 31, 2016 and 2015, we did not issue any shares resulting from the conversion of principal of the 6% Notes into our common stock.
| 13 |
As of December 31, 2016 and 2015, we had cash of $13,469 and $721, respectively. The change is outlined as follows:
During the fiscal year of 2016, net cash used in our operating activities was $1,142,9741, compared to $150,208 for the comparable period of 2015. Such cash was mainly used for maintaining operations of a public company and working capital for our bio-fertilizer business.
During the fiscal year of 2016, we incurred approximately $79,083 in investment activity, including $34,112 in acquiring a subsidiary.
During the year ended December 31, 2016, we generated cash inflow for $1,053,358 from financing activities, compared to $148,009 of cash inflow during the year ended December 31, 2015, from financing activities.
As of December 31, 2016, we had an accumulated deficit of $19,489,400, down from $20,324,812 deficit at December 31, 2015, as a result of a net income of $967,296 including $127,884 for statutory reserve for the year ended December 31, 2016 and compared to a net loss of $677,358 for 2015.
We still require additional working capital to accomplish our business objectives and to sustain our operations. Continuously, we intend to raise additional capital through the issuance of debt or equity securities to fund the development of our planned business operations, although there can be no assurance that we will be successful in obtaining this financing. The following factors, among others, may significantly harm our ability to obtain required additional financing.
Given the facts that:
| (1) | Outstanding 6% Notes. As of December 31, 2016, the amount of outstanding 6% Notes was $150,250. The 6% Notes have been in default since June 2009. |
| (2) | Outstanding note payable of $360,000 as of December 31, 2016. This note has been in default since July 2007. |
Contractual Obligations
(1) Operation of Kiwa-CAU R&D Center
Pursuant to the agreement on joint incorporation of the research and development center between CAU and Kiwa Shandong dated November 14, 2006, Kiwa Shandong agrees to invest RMB 1 million (approximately $160,000) each year to fund research at the R&D Center. The term of this agreement is ten years starting from July 1, 2006. Prof. Qi Wang, who became one of our directors in July 2007, has acted as the Director of Kiwa-CAU R&D Center since July 2006.
(2) Investment in manufacturing and research facilities in Zoucheng, Shandong Province in China
According to the Project Agreement with Zoucheng Municipal Government in 2002, we have committed to investing approximately $18 million to $24 million for developing the manufacturing and research facilities in Zoucheng, Shandong Province. As of December 31, 2016, we had invested approximately $1.91 million for the project. On February 11, 2017, the Company entered an Equity Transfer Agreement with Dian Shi Cheng Jing (Beijing) Technology Co. (“Transferee”) to transfer all of shareholders’ right, title and interest in Kiwa Shandong to the Transferee for USD $1.00. Currently, the completion of transfer is under the government processing.
| 14 |
(3) Strategic cooperation with two institutes in China
On November 5, 2015, the Company signed a strategic cooperation agreement (the “Agreement”) with China Academy of Agricultural Science (“CAAS”)’s Institute of Agricultural Resources & Regional Planning (“IARRP”) and Institute of Agricultural Economy & Development (“IAED”). Pursuant to the Agreement, the Company will form a strategic partnership with the two institutes and establish an “International Cooperation Platform for Internet and Safe Agricultural Products”. To fund the cooperation platform’s R&D activities, the Company will provide RMB 1 million (approximately $160,000) per year to the Spatial Agriculture Planning Method & Applications Innovation Team that belongs to the Institutes. The term of the Agreement is for three years beginning November 20, 2015.
(4) Acquisition of Caber Holdings LTD (Hong Kong Baina Group Co. LTD) in China
On November 30, 2015, we entered into an acquisition agreement (the “Agreement”) with the shareholders of Caber Holdings LTD, whose Chinese name is Hong Kong Baina Group Co., Ltd, located in Hong Kong (“Baina Hong Kong”), and Oriental Baina Co. Ltd. (hereinafter referred to as “Baina Beijing”), Baina Hong Kong’s wholly-owned subsidiary in Beijing, China. When this acquisition is completed, Kiwa will rename Baina Beijing to Kiwa Baiao Bio-Tech (Beijing) Co., Ltd. Kiwa Baiao Co. Ltd will replace Kiwa’s current subsidiary in China - Kiwa Bio-Tech (Shandong) Co., Ltd (“Kiwa Shandong”) - to operate Kiwa’s bio-fertilizer market expansion and become Kiwa’s platform for future acquisitions of new agricultural-related projects in China. In accordance with the terms of the Agreement, Kiwa agreed to pay US$30,000 to the Baina Hong Kong Shareholders for the acquisition of 100% of the equity of Baina Hong Kong. As of December 31, 2015, the Company has paid RMB 220,000 (approximately $34,000) for the acquisition. The acquisition was completed on January 7, 2016. Both Baina Hong Kong and Baina Beijing had no activities before the acquisition date and had no assets and liabilities. Thereafter, Baina Beijing formed two new subsidiaries—Kiwa Bio-Tech (Shenzhen) Co., Ltd (Registered in Shenzhen on November 2016); Kangdu Bio-Tech Hebei Co., Ltd. (Registered in Hebei on December 2106) to operate in specific markets in China.
(5) Distribution agreement with Kangtan Gerui Bio-Tech in China
On December 17, 2015, Kiwa Bio-Tech Products Group Corporation (the “Company”) entered into a distribution agreement (the “Agreement”) with Kangtan Gerui (Beijing) Bio-Tech Co., Ltd. (“Gerui”) and formally awarded Gerui a right to sell and distribute the Company’s fertilizer products in 3 major agricultural regions of China— Hainan Province, Hunan Province and Xinjiang Autonomous Region. The Company’s Research and Development department has been conducting application experiments in Hainan and Hunan Provinces since August 2015, in accordance with the market requirements. The experiment data indicates that the Company’s fertilizer products have fulfill the requirements of reduction of content of heavy metals in soil and improve crop yield. Gerui was founded in Beijing in April 2015 and relies on the sales network of China’s Supply and Marketing Cooperatives system. Currently, the Company and Gerui do not hold any interest in each other; however, a collaboration and integration may take place in the future. The term of the Agreement is for a period of three years commencing December 17, 2015. In September 2016, Kiwa Baiao Bio-Tech (Beijing) Co., Ltd obtained a fertilizer sales permit from the Chinese government and began to sale the products directly to customers in those 3 major agricultural regions.
(6) Lease payments
(1) On April 29, 2016, Kiwa Baiao Bio-Tech (Beijing) Co., Ltd. entered an office lease agreement with two-year team. Monthly lease payment and building management fee totaled RMB 77,867 or approximately USD $11,622.
(2) On November 11, 2017, Kiwa Baiao Bio-Tech (Beijing) Co., Ltd. entered an apartment lease agreement for its employees. The lease term is one year with monthly lease payment of RMB 6,000 or approximately USD $896.
(3) In March 1, 2017, Kiwa Bio-Tech (Shenzhen) Co., Ltd, a newly established subsidiary entered an office lease agreement with one-year term. Monthly lease payment is RMB 29,000 or approximately of USD $4,320.
Off-Balance Sheet Arrangements
At December 31, 2016, we did not have any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities, established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. As such, we are not exposed to any financing, liquidity, market or credit risk that could arise had we engaged in such relationships.
Recent Accounting Pronouncements
See Note 2 to the Consolidated Financial Statements under Item 8, Part II.
| 15 |
ITEM 7A. Quantitative and Qualitative Disclosures about Market Risk
Not required.
ITEM 8. Financial Statements and Supplementary Data
The full text of our audited consolidated financial statements as of December 31, 2016 and 2015 begins on page F-1 of this annual report.
ITEM 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
On February 15, 2017, the Board of Directors of Kiwa Bio-Tech Products Group Corporation (“Kiwa” or “Company”) decided to engage DYH & Co. as independent principal accountant and auditor to report on the Company’s financial statements for the fiscal year ended December 31, 2016, including performing the required quarterly reviews.
In conjunction with the new engagement, the Company has dismissed its former accountant, Paritz & Co., P.A., Hackensack, NJ (“Paritz”), as the Company’s principal accountant effective February 22, 2016. Paritz has served the Company well since 2013. Under Item 304 of Regulation S-K, the reason for the auditor change is dismissal, not resignation nor declining to stand for re-election.
During the two most recent fiscal years and the interim period through the date of the dismissal, there were no disagreements with Paritz on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedure, which disagreements, if not resolved to Paritz’s satisfaction, would have caused Paritz to make reference to the subject matter of the disagreements in connection with its reports.
ITEM 9A. Controls and Procedures
Disclosure Controls and Procedures
Our management, under the supervision and with the participation of our Chief Executive Officer (“CEO”) and Chief Financial Officer (“CFO”), has evaluated the effectiveness of our disclosure controls and procedures as defined in SEC Rules 13a-15(e) and 15d-15(e) as of the end of the period covered by this Annual Report. Our disclosure controls and procedures are designed to ensure that information required to be disclosed in the reports we file or submit under the Securities Exchange Act of 1934 (“Exchange Act”) is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management including our CEO and CFO, to allow timely decisions regarding required disclosures. Based on their evaluation, our CEO and CFO have concluded that, as of December 31, 2016, our disclosure controls and procedures were ineffective.
Management Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Our internal control over financial reporting was designed to provide reasonable assurance to the Company’s management and board of directors regarding the preparation and fair presentation of published consolidated financial statements. Internal control over financial reporting is promulgated under the Exchange Act as a process designed by, or under the supervision of, the Company’s principal executive and principal financial officers and effected by the Company’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Internal control over financial reporting, no matter how well designed, has inherent limitations and may not prevent or detect misstatements. Therefore, even effective internal control over financial reporting can only provide reasonable assurance with respect to the financial statement preparation and presentation.
Our management has conducted, with the participation of our CEO and CFO, an assessment, including testing of the effectiveness, of our internal control over financial reporting as of December 31, 2016. Management’s assessment of internal control over financial reporting was conducted using the criteria in Internal Control over Financial Reporting - Guidance for Smaller Public Companies issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Based on such evaluation, management identified deficiencies that were determined to be a material weakness.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. Because of the material weakness described below, management concluded that our internal control over financial reporting was ineffective as of December 31, 2016.
| 16 |
The specific material weakness identified by the Company’s management as of December 31, 2016 is described as follows:
| ● | The Company did not have sufficient and skilled accounting personnel with an appropriate level of technical accounting knowledge and experience in the application of accounting principles generally accepted in the United States of America commensurate with the Company’s financial reporting requirements, which resulted in a number of internal control deficiencies that were identified as being significant. The Company’s management determined that the number and nature of these significant deficiencies, when aggregated, constituted a material weakness. | |
| ● | The Company lacks qualified resources to perform the internal audit functions properly. In addition, the scope and effectiveness of the Company’s internal audit function are yet to be developed. | |
| ● | We currently do not have an audit committee. |
Remediation Initiative
| ● | We are committed to establishing the internal audit functions but due to limited qualified resources in the region, we were not able to hire sufficient internal audit resources before the end of 2016. However, internally we established a central management center to recruit more senior qualified people in order to improve our internal control procedures. Externally, we are looking forward to engaging an accounting firm to assist the Company in improving the Company’s internal control system based on COSO Framework. | |
| ● | We intend to establish an audit committee of the board of directors as soon as practicable. We envision that the audit committee will be primarily responsible for reviewing the services performed by our independent auditors, evaluating our accounting policies and our system of internal controls. |
Conclusion
Despite the material weakness and deficiencies reported above, the Company’s management believes that its consolidated financial statements included in this report fairly present in all material respects the Company’s financial condition, results of operations and cash flows for the periods presented and that this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report.
This Annual Report does not include an attestation report of the Company’s registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to temporary rules of the SEC that permit the Company to provide only management’s report in this Annual Report.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting during the fiscal year ended December 31, 2016 that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
| 17 |
ITEM 10. Directors, Executive Officers and Corporate Governance
Directors and Executive Officers
Set forth below are the names of our directors and executive officers, their ages, their offices with us, if any, their principal occupations or employment for the past five years. The directors listed below will serve until the Company’s next annual meeting of the stockholders:
| Name | Age | Position | Director Since | |||
| Yvonne Wang | 39 | Acting President, CEO and CFO and Director | 2015 | |||
| Feng Li | 28 | Secretary and Director | 2015 | |||
| Qi Wang | 49 | Vice President of Technology, Director | 2007 | |||
Yong Lin Song Xiao Qiang Yu |
50 39 |
CTO, Director of Technology and Director Sales and Marketing Director |
2017 2016 |
Yvonne Wang Ms. Wang became our Chairman in November 2015. She served as corporate Secretary from 2005 to 2015. Prior to 2005, she served as an executive assistant and a manager of the Company’s U.S. office between April 2003 and September 2005. She obtained her B.S. degree of Business Administration from University of Phoenix.
On August 11, 2106 became Company’s acting president, CEO and CFO.
Feng Li became our Secretary and a Director in 2015. From 2011- 2012, Ms. Li has served as an assistant project manager for SCHSAsia, a boutique business consulting firm specializing in events and project management for overseas company wishing to expand into the Asia Pacific arena. From 2012 until 2014, Ms. Li served as a campaigner for WildAid China Office, a non-profit organization with focus on raising public awareness on wildlife and climate change related issues.
Qi Wang became our Vice President - Technical on July 19, 2005 and was elected as one of our directors of the Company on July 18, 2007. Prof. Wang has also acted as Director of Kiwa-CAU R&D Center since July 2006. Prof. Wang served as a Professor and Advisor for Ph.D. students in the Department of Plant Pathology, China Agricultural University (“CAU”) since January 2005. Prior to that, he served as an assistant professor and lecturer of CAU since June 1997. He obtained his master degree and Ph.D. in agricultural science from CAU in July 1994 and July 1997, respectively. Prof. Wang received his bachelor’s degree of science from Inner Mongolia Agricultural University in July 1989. He is a committee member of various scientific institutes in China, including the National Research and Application Center for Increasing-Yield Bacteria, Chinese Society of Plant Pathology, Chinese Association of Animal Science and Veterinary Medicine. Prof. Wang’s unique expertise in the field of agriculture offers significant knowledge and experience to the Board of Directors when making critical operational decisions.
Yong Lin Song became our CTO and Director of Technology responsible for the Company’s R&D operations on March 2016 and as one of our directors of the Company on March 2017. Mr. Song is a senior agronomist at the Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences. He has 29 years of experience in microbial R&D and technology promotion and has led many national agricultural projects. In 2001, he was responsible for technological achievement transformation and technology promotion of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences. In 2009, he served as deputy secretary general of the Chinese Society of Plant Nutrition and Fertilizer Science.
Xiao Qiang Yu became our Sales and Marketing Director on June 2016 and is responsible for managing the overall marketing strategy of Kiwa, which includes brand expansion, sale targets, strategic planning and corporate communications. Mr. Yu participated in Chinese fertilizer market since 1999. Mr. Yu has over 15 years of marketing, management and strategy experience from two major fertilizer companies in China.
| 18 |
Family Relationships
There are no family relationships among our directors or executive officers.
Involvement in Certain Legal Proceedings
None of our directors or executive officers has, during the past ten years:
| (a) | Had any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
| (b) | Been convicted in a criminal proceeding or subject to a pending criminal proceeding; |
| (c) | Been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or any federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities, futures, commodities or banking activities; and |
| (d) | Been found by a court of competent jurisdiction (in a civil action), the Securities and Exchange Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated. |
Section 16(a) Beneficial Ownership Compliance
Section 16(a) of the Securities Exchange Act of 1934 requires our officers, directors and certain persons holding more than 10 percent of a registered class of our common stock to file with the SEC initial reports of ownership and reports of changes in ownership of our common stock. Officers, directors and certain other shareholders are required by the SEC to furnish the Company with copies of all Section 16(a) forms they file. To the best of the Company’s knowledge, based solely upon a review of the copies of such reports, the Company believes that all Section 16(a) filing requirements applicable to its officers, directors and certain other shareholders were complied with during the fiscal year ended December 31, 2016.
Code of Ethics
We have adopted a Code of Business Conduct and Ethics (the “Code”) that is applicable to all employees, consultants and members of the Board of Directors, including the Chief Executive Officer, Chief Financial Officer and Secretary. This Code embodies our commitment to conduct business in accordance with the highest ethical standards and applicable laws, rules and regulations. We will provide any person a copy of the Code, without charge, upon written request to the Company’s Secretary. Requests should be addressed in writing to Ms. Yvonne Wang; 310 N. Indian Hill Blvd., #702 Claremont, California 91711.
Board Composition; Audit Committee and Financial Expert
Our Board of Directors is currently composed of four members: Yvonne Wang, Feng Li, Qi Wang and Yong Lin Song. All board actions require the approval of a majority of the directors in attendance at a meeting at which a quorum is present.
| 19 |
We currently do not have an audit committee. We intend, however, to establish an audit committee of the board of directors as soon as practicable. We envision that the audit committee will be primarily responsible for reviewing the services performed by our independent auditors, evaluating our accounting policies and our system of internal controls. Currently such functions are performed by our Board of Directors.
The Board does not have a “financial expert” as defined by SEC rules implementing Section 407 of the Sarbanes-Oxley Act.
Board meetings and committees; annual meeting attendance.
During fiscal year 2016, the Board of Directors did not have any meetings.
ITEM 11. Executive Compensation
We currently have no Compensation Committee. The Board of Directors is currently performing the duties and responsibilities of Compensation Committee. In addition, we have no formal compensation policy. We decide on our executives’ compensation based on average compensation levels of similar companies in the U.S. or China, depending on consideration of many factors such as where the executive works. Our Chief Executive Officer’s compensation is approved by the Board of Directors. Other named executive officers’ compensation are proposed by our Chief Executive Officer and approved by the Board of Directors.
Our Stock Incentive Plan is administered by the Board of Directors. Any amendment to our Stock Incentive Plan requires majority approval of the stockholders of the Company.
The Company had no officers or directors whose total compensation during either 2016 or 2015 exceeded $100,000.
Currently, the main forms of compensation provided to each of our executive officers are: (1) annual salary; (2) non-equity Incentive Plan; and (3) the granting of incentive stock options subject to approval by our Board of Directors.
Summary Compensation Table
Summary Compensation Table
| Name and principal position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Nonqualified Deferred Compensation Earnings ($) | All
Other Compensation ($) | Total ($) | |||||||||||||||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | |||||||||||||||||
| Yvonne Wang, | 2016 | 84,000 | Nil | Nil | Nil | Nil | Nil | Nil | 84,000 | |||||||||||||||||
| Acting President, CEO and CFO | 2015 | 51,000 | Nil | Nil | Nil | Nil | Nil | Nil | 51,000 | |||||||||||||||||
| Yong Lin Song, | 2016 | 29,828 | Nil | Nil | Nil | Nil | Nil | Nil | 29,828 | |||||||||||||||||
| CTO, Director of Technology | 2015 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | |||||||||||||||||
| Xiao Qiang Yu, | 2016 | 30,259 | Nil | Nil | Nil | Nil | Nil | Nil | 30,259 | |||||||||||||||||
| Sales and Marketing Director | 2015 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | |||||||||||||||||
Employment Contracts and Termination of Employment and Change of Control Arrangements
There are no compensatory plans or arrangements with respect to a named executive officer that would result in payments or installments in excess of $100,000 upon the resignation, retirement or other termination of such executive officer’s employment with us or from a change-in-control.
| 20 |
Stock Incentive Plan and Option Grants
2016 Stock Incentive Plan
On March 8, 2017, pursuant to the consent of the holders of a majority of the votes entitled to be cast on the matter, were approved of the Kiwa Bio-Tech Products Group Corporation 2016 Employee, Director and Consultant Stock Plan. The Plan is a key aspect of our compensation program, designed to attract, retain, and motivate the highly qualified individuals required for our long-term success.
No options were granted under the Plan during 2016.
Option Exercises and Stock Vested
No stock options were exercised by any officers or directors during 2016 and 2015. We did not adjust or amend the exercise price of any stock options previously awarded to any named executive officers during 2016 and 2015.
| 21 |
Director Compensation for 2016
We currently have no policy in effect for providing compensation to our directors for their services on our Board of Directors, and did not compensate our directors in 2016 for services performed as directors.
ITEM 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following table sets forth as of April 7, 2017 certain information with respect to the beneficial ownership of our common stock by (i) each of our executive officers, (ii) each person who is known by us to beneficially own more than 5% of our outstanding common stock, and (iii) all of our directors and executive officers as a group. Percentage ownership is calculated based on 9,798,981 shares of our common stock and 500,000 shares of our Series A Preferred Stock outstanding as of April 7, 2017. None of the shares listed below are issuable pursuant to stock options or warrants of the Company.
| Title of class | Name and Address of Beneficial Ownership(1) | Amount
and Nature of Beneficial Owner (2) |
Percentage of class | |||||||
| Common Stock | Yvonne Wang | 240,000 | 2.45 | % | ||||||
| Common Stock | Feng Li (3) | 1,965,326 | 20.06 | % | ||||||
| Common Stock | Qi Wang | - | - | |||||||
| Common Stock | Yong Lin Song | - | - | |||||||
| Common Stock | All officers and directors as a group | 2,205,326 | 22.51 | % | ||||||
| Ser. A Pref. Stock | Yvonne Wang | 250,000 | 50.00 | % | ||||||
| Ser. A Pref. Stock | Feng Li | 250,000 | 50.00 | % | ||||||
| Ser. A Pref. Stock | All officers and directors as a group | 500,000 | 100.00 | % | ||||||
| 5% Holders: | ||||||||||
| Common Stock | Troniya Industrial Incubator, Inc. | 1,000,000 | 10.21 | % | ||||||
| Common Stock | Liu Geng | 500,000 | 5.10 | % | ||||||
| Common Stock | Liu Xu | 500,000 | 5.10 | % | ||||||
| Common Stock | Tian Lixin | 500,000 | 5.10 | % | ||||||
| Common Stock | Zheng JunWei | 920,000 | 9.39 | % | ||||||
| Common Stock | Wei Li (3) | 1,965,326 | 20.06 | % | ||||||
| (1) | The address for all holders is 310 N. Indian Hill Blvd, #702, Claremont, CA 91711. |
| (2) | In determining beneficial ownership of our Common Stock and Series A Preferred Stock, the number of shares shown includes shares which the beneficial owner may acquire upon exercise of debentures, warrants and options which may be acquired within 60 days. Unless otherwise stated, each beneficial owner has sole power to vote and dispose of its shares. |
| (3) | Includes 61,784 shares of common stock held by All Star Technology, Inc., a British Virgin Islands international business company. Feng Li’s father, Wei Li, exercises voting and investment control over the shares held by All Star Technology, Inc. Wei Li is a principal stockholder of All Star Technology, Inc. and may be deemed to beneficially own such shares, but disclaims beneficial ownership in such shares held by All Star Technology, Inc. except to the extent of his pecuniary interest therein. Mr. Li has pledged all of his common stock of the Company as collateral security for the Company’s obligations under certain 6% Convertible Notes owed by the Company. |
| 22 |
Change in Control
None.
ITEM 13. Certain Relationships and Related Transactions, and Director Independence
For description of transactions with related parties, see Note 6 to Consolidated Financial Statements under Item 8 in Part II.
Under the independence standard set forth in Rule 4200(a) (15) of the Market Place Rules of the Nasdaq Stock Market, which is the independence standard that we have chosen to report under.
ITEM 14. Principal Accountant Fees and Services
Fees Paid to Independent Public Accountants for 2016 and 2015.
Audit Fees
All of the services described below were approved by our board of directors prior to performance of such services. The board of directors has determined that the payments made to its independent accountants for these services are compatible with maintaining such auditor’s independence.
The aggregate audit fees for 2016 and 2015 paid and payable to DYH & Company and Paritz & Company, P.A. were approximately $38,000 and $30,500, respectively. The amounts include: (1) fees for professional services rendered by DYH & Company and Paritz & Company, P.A. in connection with the audit of our consolidated financial statements; (2) reviews of our quarterly reports on the Form 10-Q.
| 23 |
Audit-Related Fees
Audit-related fees for 2016 and 2015 were $nil.
Tax Fees
Tax service fees billed by Paritz & Co., P.A. as tax consultant for 2016 and 2015 were $3,400 and $2,750, respectively.
All Other Fees
None.
Policy on Audit Committee Pre-Approval of Audit and Permissible Non-Audit Services of Independent Auditors
Since we did not have a formal audit committee, our board of directors served as our audit committee. We have not adopted pre-approval policies and procedures with respect to our accountants in 2016. All of the services provided and fees charged by our independent registered accounting firms in 2016 were approved by the board of directors.
| 24 |
ITEM 15. Exhibits and Financial Statement Schedules
Exhibit No. |
Description | |
| 31.1 | Certification of Chief Executive Officer pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934 | |
| 31.2 | Certification of Chief Financial Officer pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934 | |
| 32.1 | Certification of Chief Executive Officer, pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 32.2 | Certification of Chief Financial Officer, pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 101.INS | XBRL Instance Document | |
| 101.SCH | XBRL Taxonomy Extension Schema Document | |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document |
| 25 |
Kiwa Bio-Tech Products Group Corporation
Pursuant to the requirements of Section 13 or 15(d) of the Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Date: April 17, 2017
| KIWA BIO-TECH PRODUCTS GROUP CORPORATION. | ||
| By: | /s/ Yvonne Wang | |
Yvonne Wang Chief Executive Officer (Principal Executive Officer) | ||
| By: | /s/ Yvonne Wang | |
Yvonne Wang Chief Financial Officer (Principal Financial and Accounting Officer) | ||
Pursuant to the requirements of Section 13 or 15(d) of the Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf of the registrant and in the capacities and on the dates indicated.
| /s/ Yvonne Wang | Chief Executive Officer and Director | April 17, 2017 | ||
| Yvonne Wang | (Principal Executive Officer) | |||
| /s/ Yvonne Wang | Chief Financial Officer | April 17, 2017 | ||
| Yvonne Wang | (Principal Financial and Accounting Officer) | |||
| /s/ Feng Li | Secretary and Director | April 17, 2017 | ||
| Feng Li | ||||
| /s/ Qi Wang | Vice President of Technology and Director | April 17, 2017 | ||
| Qi Wang | ||||
| /s/ Yong Lin Song | CTO, Director of Technology and Director | April 17, 2017 | ||
| Yong Lin Song |
| 26 |
Kiwa Bio-Tech Products Group Corporation
Index to Consolidated Financial Statements
| 27 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To Board of Directors and the Stockholders of
Kiwa Bio-Tech Products Group Corporation
We have audited the accompanying consolidated balance sheet of Kiwa Bio-Tech Products Group Corporations as of December 31, 2015 and the related consolidated statement of comprehensive loss, changes in stockholders’ deficiency and cash flows for the year then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audit in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Kiwa Bio-Tech Products Group Corporation as of December 31, 2015, and the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
/s/ Paritz & Company, P.A.
Hackensack, New Jersey
April 14, 2016
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To
the Board of Directors and
Stockholders of Kiwa Bio-Tech Products Group Corporation
We have audited the accompanying consolidated balance sheet of Kiwa Bio-Tech Products Group Corporation as of December 31, 2016, and the related statements of income, comprehensive income, stockholders’ equity, and cash flows for the year ended December 31, 2016. Kiwa Bio-Tech Products Group Corporation’s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Kiwa Bio-Tech Products Group Corporation as of December 31, 2016, and the results of its operations and its cash flows for the year ended December 31, 2016, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the consolidated financial statements, the Company’s current liabilities substantially exceeded its current assets by $5,729,622 at December 31, 2016. Although the Company reported net income approximately $963,296 for its fiscal year ended December 31, 2016, it had an accumulated deficit of $19,489,400 as of December 31, 2016. These circumstances, among others, raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regarding to these matters are described in Note 3, which include raising additional equity financing. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ DYH & Company
Irvine, California
April 17, 2017
| F-2 |
KIWA BIO-TECH PRODUCTS GROUP CORPORATION
| December 31, 2016 | December 31, 2015 | |||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | 13,469 | $ | 721 | ||||
| Accounts receivable | 1,122,754 | - | ||||||
| Prepaid expenses | 92,504 | - | ||||||
| Other receivable | 1,561,331 | 47,453 | ||||||
| Advance to suppliers | 1,805,044 | - | ||||||
| Total current assets | 4,595,102 | 48,174 | ||||||
| Property, plant and equipment - net | 59,778 | 2,807 | ||||||
| Deposit | 34,519 | - | ||||||
| Goodwill | 34,112 | - | ||||||
| Total non-current assets | 128,409 | 2,807 | ||||||
| Total assets | $ | 4,723,511 | $ | 50,981 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIENCY | ||||||||
| Current liabilities | ||||||||
| Accounts payable | $ | 1,318,802 | $ | 269,360 | ||||
| Accrued expenses | 35,715 | 30,887 | ||||||
| Advances from customers | 12,883 | 13,800 | ||||||
| Construction costs payable | 255,539 | 273,722 | ||||||
| Due to related parties - trade | 1,283,215 | 1,143,978 | ||||||
| Due to related parties - non-trade | 100,798 | 3,166,198 | ||||||
| Convertible notes payable | 150,250 | 150,250 | ||||||
| Notes payable | 360,000 | 360,000 | ||||||
| Unsecured loans payable | 1,655,343 | 1,773,131 | ||||||
| Salary payable | 1,688,353 | 1,632,881 | ||||||
| Taxes payable | 919,255 | 478,209 | ||||||
| Penalty payable | 482,327 | 404,752 | ||||||
| Interest payable | 1,042,661 | 930,062 | ||||||
| Other payables | 1,019,583 | 524,205 | ||||||
| Total current liabilities | 10,324,724 | 11,151,435 | ||||||
| SHAREHOLDER’S EQUITY | ||||||||
Preferred stock - $0.001 par value, Authorized 20,000,000 shares. Issued and outstanding 500,000 and 500,000 shares at December 31, 2016 and 2015, respectively. |
500 | 500 | ||||||
Common stock - $0.001 per value. Authorized 100,000,000 shares. Issued and outstanding $8,728,981 and 2,000,000 shares at December 31, 2016 and 2015 |
8,729 | 2,000 | ||||||
| Additional paid-in capital | 13,789,990 | 9,490,837 | ||||||
| Statutory Reserve | 127,884 | - | ||||||
| Accumulated deficit | (19,489,400 | ) | (20,324,812 | ) | ||||
| Accumulated other comprehensive loss | (38,916 | ) | (268,979 | ) | ||||
| Total stockholders’ deficiency | (5,601,213 | ) | (11,100,454 | ) | ||||
| Total liabilities and shareholder’s equity | $ | 4,723,511 | $ | 50,981 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-3 |
KIWA BIO-TECH PRODUCTS GROUP CORPORATION
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
| Years Ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Revenue | $ | 9,617,845 | $ | - | ||||
| Cost of goods sold | 7,672,451 | - | ||||||
| Gross profit | 1,945,394 | - | ||||||
| Operating expenses | ||||||||
| Research and development | 224,433 | 178,988 | ||||||
| Selling expenses | 49,295 | - | ||||||
| General and administrative | 874,097 | 313,589 | ||||||
| Total operating expenses | 1,147,825 | 492,577 | ||||||
| Operating income (loss) | 797,569 | (492,577 | ) | |||||
| Other income | ||||||||
| License revenue | 786,329 | - | ||||||
| Other expense | ||||||||
| Penalty expense | (77,575 | ) | (72,152 | ) | ||||
| Interest expense | (112,977 | ) | (112,629 | ) | ||||
| Other expense | (3,770 | ) | - | |||||
| Total other expense | (194,322 | ) | (184,781 | ) | ||||
| Net income (loss) before income tax | 1,389,576 | (677,358 | ) | |||||
| Income tax provision | (426,280 | ) | - | |||||
| Net income (loss) | 963,296 | (677,358 | ) | |||||
| Other comprehensive income | ||||||||
| Foreign currency translation adjustment | 230,063 | 424,065 | ||||||
| Total comprehensive income (loss) | $ | 1,193,359 | $ | (253,293 | ) | |||
| Net income (loss) per common share - basic | $ | 0.17 | $ | (0.34 | ) | |||
| Net income (loss) per common share - diluted | $ | 0.11 | $ | (0.34 | ) | |||
| Weighted average number of common shares outstanding - basic | 5,645,263 | 2,000,000 | ||||||
| Weighted average number of common shares outstanding - diluted | 8,872,655 | 2,000,000 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 |
KIWA BIO-TECH PRODUCTS GROUP CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ DEFICIENCY
| Common Stock | Preferred Stock | Additional Paid-in | Statutory | Accumulated | Accumulated Other Comprehensive | Total Stockholders’ | ||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Reserve | Deficit | Loss | Deficiency | ||||||||||||||||||||||||||||
| Balance, December 31, 2014 (Restated) | 2,000,000 | $ | 2,000 | - | $ | - | $ | 8,491,337 | $ | - | $ | (19,647,454 | ) | $ | (693,044 | ) | $ | (11,847,161 | ) | |||||||||||||||||
| Issuance of 500,000 shares of preferred stock as debt cancellation on December 14, 2015 | - | - | 500,000 | 500 | 999,500 | - | - | 1,000,000 | ||||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | (677,358 | ) | - | (677,358 | ) | ||||||||||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | - | - | - | 424,065 | 424,065 | |||||||||||||||||||||||||||
| Balance, December 31, 2015 | 2,000,000 | 2,000 | 500,000 | 500 | 9,490,837 | - | (20,324,812 | ) | (268,979 | ) | (11,100,454 | ) | ||||||||||||||||||||||||
| Issuance of common shares for cash | 1,775,000 | 1,775 | - | - | 857,884 | - | 859,659 | |||||||||||||||||||||||||||||
| Issuance of common shares for Liabilities settlement | 3,243,173 | 3,243 | - | - | 3,188,731 | - | 3,191,974 | |||||||||||||||||||||||||||||
| Issuance of common shares for consulting service | 1,710,808 | 1,711 | - | - | 252,539 | - | 254,250 | |||||||||||||||||||||||||||||
| Net income | - | - | - | - | - | 127,884 | 835,412 | 963,296 | ||||||||||||||||||||||||||||
| Foreign currency translation adjustments | 230,063 | 230,063 | ||||||||||||||||||||||||||||||||||
| Balance, December 31, 2016 | 8,728,981 | $ | 8,729 | 500,000 | $ | 500 | $ | 13,789,991 | $ | 127,884 | $ | (19,489,400 | ) | $ | (38,916 | ) | $ | (5,601,212 | ) | |||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
KIWA BIO-TECH PRODUCTS GROUP CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Years Ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Cash flows from operating activities: | ||||||||
| Net income (loss) | $ | 963,296 | $ | (677,358 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | 23,980 | 4,352 | ||||||
| Bad debt | 55,240 | - | ||||||
| Provision for penalty payable | 77,575 | 72,152 | ||||||
| Accrued interest on convertible notes and note payable | 112,599 | 112,538 | ||||||
| Changes in operating assets and liabilities: | - | - | ||||||
| Accounts receivable | (1,229,249 | ) | - | |||||
| Other receivable | (1,619,623 | ) | - | |||||
| Advance to supplier | (1,887,446 | ) | - | |||||
| Prepaid expense | (59,154 | ) | - | |||||
| Deposit | (36,095 | ) | (33,921 |
) | ||||
| Account payable | 1,116,061 | - | ||||||
| Accrued expense | 7,117 | - | ||||||
| Salary payable | 93,860 | 125,924 | ||||||
| Taxes payable | 494,397 | 62,915 | ||||||
| Due to related parties - trade | 217,293 | 178,988 | ||||||
| Other payables | 527,175 | 4,202 | ||||||
| Net cash used in operating activities | (1,142,974 | ) | (150,208 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Purchase of property, plant and equipment | (79,083 | ) | - | |||||
| Net cash used in investing activities | (79,083 | ) | - | |||||
| Cash flows from financing activities: | ||||||||
| Proceeds from related parties, net of payments to related parties | 139,085 | 148,009 | ||||||
| Proceeds from sale of common stock | 914,273 | - | ||||||
| Net cash provided by financing activities | 1,053,358 | 148,009 | ||||||
| Effect of exchange rate change | 181,447 | (43 | ) | |||||
| Cash and cash equivalents: | ||||||||
| Net increase (decrease) | 12,748 | (2,242 | ) | |||||
| Balance at beginning of year | 721 | 2,963 | ||||||
| Balance at end of year | $ | 13,469 | $ | 721 | ||||
| Non-cash financing activities: | ||||||||
| Issuance of common stock for debts settlement | $ | 3,137,359 | $ | 1,000,000 | ||||
| Issuance of common stock for consulting service | 254,250 | |||||||
| Supplemental Disclosures of Cash flow Information: | ||||||||
| Cash paid for interest | $ | $ | - | |||||
| Cash paid for income taxes | $ | $ | - | |||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-6 |
KIWA BIO-TECH PRODUCTS GROUP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2016
1. Description of Business and Organization
Organization – Kiwa Bio-Tech Products Group Corporation (“the Company”) is the result of a share exchange transaction accomplished on March 12, 2004 between the shareholders of Kiwa Bio-Tech Products Group Ltd. (“Kiwa BVI”), a company originally organized under the laws of the British Virgin Islands on June 5, 2002 and Tintic Gold Mining Company (“Tintic”), a corporation originally incorporated in the state of Utah on June 14, 1933 to perform mining operations in Utah. The share exchange resulted in a change of control of Tintic, with former Kiwa BVI stockholders owning approximately 89% of Tintic on a fully diluted basis and Kiwa BVI surviving as a wholly-owned subsidiary of Tintic. Subsequent to the share exchange transaction, Tintic changed its name to Kiwa Bio-Tech Products Group Corporation. On July 21, 2004, the Company completed its reincorporation in the State of Delaware. On March 8, 2017, we completed our reincorporation in the State of Nevada.
The Company operates through a series of subsidiaries in the Peoples Republic of China as detailed in the following Organizational Chart. The Company had previously operated its business through its subsidiaries Kiwa Bio-Tech Products (Shandong) Co., Ltd. (“Kiwa Shandong”) and Tianjin Kiwa Feed Co., Ltd. (“Kiwa Tianjin ”). Kiwa Tianjin has been dissolved since July, 11, 2012. On February 11, 2017, the Company entered an Equity Transfer Agreement with Dian Shi Cheng Jing (Beijing) Technology Co. (“Transferee”) to transfer all of shareholders’ right, title and interest in Kiwa Shandong to the Transferee for USD $1.00. Currently, the completion of transfer is under the government processing.
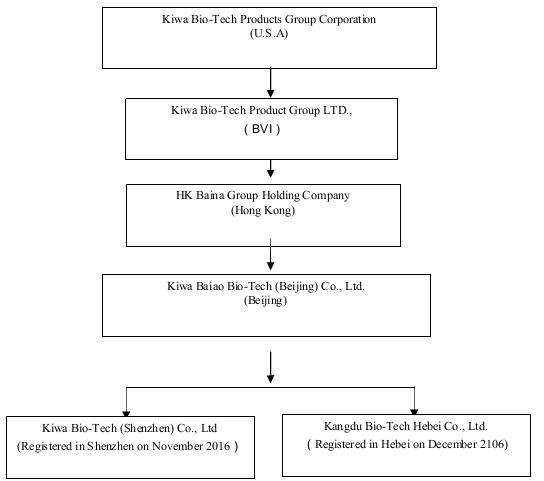
Business – The Company’s business plan is to develop and market innovative, manufacture, distribute cost-effective and environmentally safe bio-technological products for agriculture markets primarily in China. The Company has acquired technologies to produce and market bio-fertilizer.
2. Summary of Significant Accounting Policies
Principle of Consolidation - These consolidated financial statements include the financial statements of the Company and its wholly-owned subsidiaries, Kiwa BVI, Hong Kong Baina Group Holding Company, Kiwa Baiao Bio-Tech (Beijing) Co., Ltd, Kiwa Bio-Tech Products (Shandong) Co., Ltd. (“Kiwa Shandong”). All significant inter-company balances or transactions are eliminated on consolidation.
Reverse Split - On January 14, 2016, the Company filed a Certificate of Amendment of its Certificate of Incorporation with the State of Delaware with reference to a 1-for-200 reverse stock split with respect to its Common Stock with effective date of January 28, 2016. In connection with the reverse split, the Company’s authorized capital was amended to be 120,000,000 shares, comprising 100,000,000 shares of Common Stock par value $0.001 and 20,000,000 shares of Preferred Stock par value $0.001. All relevant information relating to numbers of shares, options and per share information have been retrospectively adjusted to reflect the reverse stock split for all periods presented.
Use of Estimates - The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant accounting estimates include the valuation of securities issued, deferred tax assets and related valuation allowance.
| F-7 |
Certain of our estimates, including evaluating the collectability of accounts receivable and the fair market value of long-lived assets, could be affected by external conditions, including those unique to our industry, and general economic conditions. It is possible that these external factors could have an effect on our estimates that could cause actual results to differ from our estimates. We re-evaluate all of our accounting estimates annually based on these conditions and record adjustments when necessary.
Cash and Cash Equivalents
The Company considers all highly liquid investments with a maturity of three months or less to be cash and cash equivalents. At times, such investments may be in excess of Federal Deposit Insurance Corporation (FDIC) insurance limit.
Accounts Receivables - Accounts receivables represent customer accounts receivables. The allowance for doubtful accounts is based on a combination of current sales, historical charge offs and specific accounts identified as high risk. Uncollectible accounts receivable are charged against the allowance for doubtful accounts when all reasonable efforts to collect the amounts due have been exhausted. Such allowances, if any, would be recorded in the period the impairment is identified.
Allowance for doubtful accounts
The Company provides an allowance for doubtful accounts equal to the estimated uncollectible amounts. The Company’s estimate is based on historical collection experience and a review of the current status of trade accounts receivable. It is reasonably possible that the Company’s estimate of the allowance for doubtful accounts will change. There was no allowance for doubtful accounts at December 31, 2016 and December 31, 2015.
Inventories - Inventories are stated at the lower of cost, determined on the weighted average method, and net realizable value. Work in progress and finished goods are composed of direct material, direct labor and a portion of manufacturing overhead. Net realizable value is the estimated selling price in the ordinary course of business, less estimated costs to complete and dispose.
Property, plant and equipment - Property, plant and equipment are stated at cost less accumulated depreciation and accumulated impairment losses, if any. Gains or losses on disposals are reflected as gain or loss in the year of disposal. The cost of improvements that extend the life of property, plant and equipment are capitalized. These capitalized costs may include structural improvements, equipment and fixtures. All ordinary repair and maintenance costs are expensed as incurred. Depreciation for financial reporting purposes is provided using the straight-line method over the estimated useful lives of the assets as follows:
| Useful Life | ||
| (In years) | ||
| Buildings | 30 - 35 | |
| Machinery and equipment | 5 - 10 | |
| Automobiles | 8 | |
| Office equipment | 2 - 5 | |
| Computer software | 3 |
Impairment of Long-Lived Assets - The Company’s long-lived assets consist of property, equipment and intangible assets. The Company evaluates its investment in long-lived assets, including property and equipment, for recoverability whenever events or changes in circumstances indicate the net carrying amount may not be recoverable. Judgments regarding potential impairment are based on legal factors, market conditions and operational performance indicators, among others. In assessing the impairment of property and equipment, the Company makes assumptions regarding the estimated future cash flows and other factors to determine the fair value of the respective assets.
Fair value of warrants and options - The Company adopted ASC Topic 815, “Accounting for Derivative Instruments and Hedging Activities” to recognize warrants relating to loans and warrants issued to consultants as compensation as derivative instruments in our consolidated financial statements. The Company also adopted ASC Topic 718, “Share Based Payment” to recognize options granted to employees as derivative instruments in our consolidated financial statements. The Company calculates the fair value of the warrants and options using the Black-Scholes Model.
| F-8 |
Revenue Recognition – The Company applies paragraph 605-10-S99-1 of the FASB Accounting Standards Codification for revenue recognition. The Company recognizes revenue when it is realized or realizable and earned. The Company considers revenue realized or realizable and earned when all of the following criteria are met: (i) persuasive evidence of an arrangement exists, (ii) the product has been shipped or the services have been rendered to the customer, (iii) the sales price is fixed or determinable, and (iv) collectability is reasonably assured.
The Company derives its revenues from sales contracts with its customer with revenues being generated upon delivery of products. Persuasive evidence of an arrangement is demonstrated via invoice; and the sales price to the customer is fixed upon acceptance of the purchase order and there is no separate sales rebate, discount, or volume incentive.
Shipping and Handling Costs - Substantially all costs of shipping and handling of products to customers are included in selling. Shipping and handling costs for the years ended December 31, 2016 and 2015 were $480,892 and $ nil, respectively.
Income Taxes - The Company accounts for income taxes under the provisions of FASB ASC Topic 740, “Income Tax,” which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the consolidated financial statements or tax returns. Deferred tax assets and liabilities are recognized for the future tax consequence attributable to the difference between the tax bases of assets and liabilities and their reported amounts in the financial statements. Deferred tax assets and liabilities are measured using the enacted tax rate expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. The Company establishes a valuation when it is more likely than not that the assets will not be recovered.
ASC Topic 740.10.30 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740.10.40 provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. We have no material uncertain tax positions for any of the reporting periods presented.
Foreign Currency Translation and Other Comprehensive Income - The Company uses United States dollars (“US Dollar” or “US$” or “$”) for financial reporting purposes. However, the Company maintains the books and records in its functional currency, Chinese Renminbi (“RMB”), being the primary currency of the economic environment in which its operations are conducted. In general, the Company translates its assets and liabilities into U.S. dollars using the applicable exchange rates prevailing at the balance sheet date, and the statement of comprehensive loss and the statement of cash flow are translated at average exchange rates during the reporting period. Equity accounts are translated at historical rates. Adjustments resulting from the translation of the Company’s financial statements are recorded as accumulated other comprehensive income.
Other comprehensive income for the years ended December 31, 2016 and 2015 represented foreign currency translation adjustments and were included in the consolidated statements of comprehensive loss.
The exchange rates used to translate amounts in RMB into U.S. Dollars for the purposes of preparing the consolidated financial statements were as follows:
| As of December 31, | ||||||||
| 2016 | 2015 | |||||||
| Balance sheet items, except for equity accounts | 6.94 | 6.4857 | ||||||
| Years ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Items in the statements of comprehensive loss | 6.62 | 6.2281 | ||||||
Advertising Costs - The Company charges all advertising costs to expense as incurred. The total amounts of advertising costs charged to selling, general and administrative expense were $nil for the years ended December 31, 2016 and 2015, respectively.
Research and Development Costs - Research and development costs are charged to expense as incurred. During the years ended December 31, 2016 and 2015, research and development costs were $224,433 and $178,988, respectively.
| F-9 |
Net Loss Per Common Share - Basic loss per common share is calculated by dividing net loss attributable to Kiwa stockholders by the weighted-average number of shares of common stock outstanding during the period. Diluted loss per common share includes dilutive effect of dilutive securities (stock options, warrants, convertible debt, stock subscription and other stock commitments issuable). These potentially dilutive securities were not included in the calculation of loss per share for the periods presented because the Company incurred a loss during such periods and thus the effect would have been anti-dilutive. Accordingly, basic and diluted loss per common share is the same for all periods presented. As of December 31, 2016 and 2015, potentially dilutive securities aggregated 8,872,655 (632,204 post-reverse split shares) and 126,440,833 (632,204 post-reverse split shares) shares of common stock, respectively.
Fair Value of Financial Instruments
The Company follows paragraph 825-10-50-10 of the FASB Accounting Standards Codification for disclosures about fair value of its financial instruments and paragraph 820- 10-35-37 of the FASB Accounting Standards Codification (“Paragraph 820-10-35-37”) to measure the fair value of its financial instruments. Paragraph 820-10-35-37 establishes a framework for measuring fair value with U.S. GAAP, and expands disclosures about fair value measurements.
To increase consistency and comparability in fair value measurements and related disclosures, Paragraph 820-10-35-37 establishes a fair value hierarchy which prioritizes the inputs to valuation techniques used to measure fair value into three (3) broad levels. The fair value hierarchy gives the highest priority to quoted prices (unadjusted) in active markets for identical assets or liabilities and the lowest priority to unobservable inputs. The three (3) levels of fair value hierarchy defined by Paragraph 820-10-35-37 are described below:
| ● | Level 1: quoted market prices available in active markets for identical assets or liabilities as of the reporting date. | |
| ● | Level 2: pricing inputs other than quoted prices in active markets included in Level 1, which are either directly or indirectly observable as of the reporting date. | |
| ● | Level 3: Pricing inputs that are generally observable inputs and not corroborated by market data. |
Financial assets are considered Level 3 when their fair values are determined using pricing models, discounted cash flow methodologies or similar techniques and at least one significant model assumption or input is unobservable.
The fair value hierarchy gives the highest priority to quoted prices (unadjusted) in active markets for identical assets or liabilities and the lowest priority to unobservable inputs. If the inputs used to measure the financial assets and liabilities fall within more than one level described above, the categorization is based on the lowest level input that is significant to the fair value measurement of the instrument.
The fair value hierarchy gives the highest priority to quoted prices (unadjusted) in active markets for identical assets or liabilities and the lowest priority to unobservable inputs. If the inputs used to measure the financial assets and liabilities fall within more than one level described above, the categorization is based on the lowest level input that is significant to the fair value measurement of the instrument.
The carrying amount of the Company’s financial assets and liabilities, such as cash and cash equivalent, prepaid expenses, accounts payable and accrued expenses, approximate their fair value because of the short maturity of those instruments.
Transactions involving related parties cannot be presumed to be carried out on an arm’s-length basis, as the requisite conditions of competitive, free-market dealings may not exist. Representations about transactions with related parties, if made, shall not imply that the related party transactions were consummated on terms equivalent to those that prevail in arm’s-length transactions unless such representations can be substantiated.
It is not however practical to determine the fair value of advances from stockholders, if any, due to their related party nature.
| F-10 |
Related Parties
The Company follows subtopic 850-10 of the FASB Accounting Standards Codification for the identification of related parties and disclosure of related party transactions. Pursuant to Section 850-10-20 the related parties include: a) affiliates of the Company; b) entities for which investments in their equity securities would be required, absent the election of the fair value option under the Fair Value Option Subsection of Section 825–10–15, to be accounted for by the equity method by the investing entity; c) trusts for the benefit of employees, such as pension and profit-sharing trusts that are managed by or under the trusteeship of management; d) principal owners of the Company; e) management of the Company; f) other parties with which the Company may deal if one party controls or can significantly influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully pursuing its own separate interests; and g) other parties that can significantly influence the management or operating policies of the transacting parties or that have an ownership interest in one of the transacting parties and can significantly Influence the other to an extent that one or more of the transacting parties might be prevented from fully pursuing its own separate interests.
The consolidated financial statements shall include disclosures of material related party transactions, other than compensation arrangements, expense allowances, and other similar items in the ordinary course of business. However, disclosure of transactions that are eliminated in the preparation of consolidated financial statements is not required in those statements. The disclosures shall include: a. the nature of the relationship(s) involved; b. a description of the transactions, including transactions to which no amounts or nominal amounts were ascribed, for each of the periods for which income statements are presented, and such other information deemed necessary to an understanding of the effects of the transactions on the consolidated financial statements; c. the dollar amounts of transactions for each of the periods for which income statements are presented and the effects of any change in the method of establishing the terms from that used in the preceding period; and d. amounts due from or to related parties as of the date of each balance sheet presented and, if not otherwise apparent, the terms and manner of settlement.
Commitments and Contingencies
The Company follows subtopic 450-20 of the FASB Accounting Standards Codification to report accounting for contingencies. Certain conditions may exist as of the date the consolidated financial statements are issued, which may result in a loss to the Company but which will only be resolved when one or more future events occur or fail to occur. The Company assesses such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company evaluates the perceived merits of any legal proceedings or unasserted claims as well as the perceived merits of the amount of relief sought or expected to be sought therein.
| F-11 |
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s consolidated financial statements. If the assessment indicates that a potential material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, and an estimate of the range of possible losses, if determinable and material, would be disclosed.
Loss contingencies considered remote are generally not disclosed unless they involve guarantees, in which case the guarantees would be disclosed. Management does not believe, based upon information available at this time that these matters will have a material adverse effect on the Company’s financial position, results of operations or cash flows. However, there is no assurance that such matters will not materially and adversely
Stock Based Compensation
The Company accounts for employee and non-employee stock awards under ASC 718, whereby equity instruments issued to employees for services are recorded based on the fair value of the instrument issued and those issued to non-employees are recorded based on the fair value of the consideration received or the fair value of the equity instrument, whichever is more reliably measurable.
No stock based compensation was issued or outstanding as of December 31, 2016 and 2015.
Income Tax Provision
Income taxes are accounted for using the asset and liability method. Deferred income taxes are provided for temporary differences in recognizing certain income, expense and credit items for financial reporting purposes and tax reporting purposes. Such deferred income taxes primarily relate to the difference between the tax basis of assets and liabilities and their financial reporting amounts. Deferred tax assets and liabilities are measured by applying enacted statutory tax rates applicable to the future years in which deferred tax assets or liabilities are expected to be settled or realized. There were no material deferred tax assets or liabilities as of December 31, 2016 and December 31, 2015.
As of December 31, 2016, and 2015, the Company did not identify any material uncertain tax positions. As of December 31, 2016, the Company’s returns are subject to examination by federal and state taxing authorities, generally for three years and four years, respectively, after they are filed.
Net Income (Loss) Per Common Share
Net income (loss) per common share is computed pursuant to section 260-10-45 of the FASB Accounting Standards Codification. Basic net income (loss) per common share is computed by dividing net income (loss) by the weighted average number of common shares outstanding during the period.
| F-12 |
Diluted net income (loss) per common share is computed by dividing net income (loss) by the weighted average number of shares of common stock and potentially outstanding shares of common stock during the period to reflect the potential dilution that could occur from common shares issuable through contingent shares issuance arrangement, stock options or warrants.
There were no potentially dilutive debt or equity instruments issued and outstanding at any time during the twelve-month periods ended December 31, 2016 and 2015.
Cash Flows Reporting
The Company adopted paragraph 230-10-45-24 of the FASB Accounting Standards Codification for cash flows reporting, classifies cash receipts and payments according to whether they stem from operating, investing, or financing activities and provides definitions of each category, and uses the indirect or reconciliation method (“Indirect method”) as defined by paragraph 230-10-45-25 of the FASB Accounting Standards Codification to report net cash flow from operating activities by adjusting net income to reconcile it to net cash flow from operating activities by removing the effects of (a) all deferrals of past operating cash receipts and payments and all accruals of expected future operating cash receipts and payments and (b) all items that are included in net income that do not affect operating cash receipts and payments. The Company reports the reporting currency equivalent of foreign currency cash flows, using the current exchange rate at the time of the cash flows and the effect of exchange rate changes on cash held in foreign currencies is reported as a separate item in the reconciliation of beginning and ending balances of cash and cash equivalents and separately provides information about investing and financing activities not resulting in cash receipts or payments in the period pursuant to paragraph 830-230-45-1 of the FASB Accounting Standards Codification.
Subsequent Events
The Company follows the guidance in Section 855-10-50 of the FASB Accounting Standards Codification for the disclosure of subsequent events. The Company will evaluate subsequent events through the date when the financial statements were issued. Pursuant to ASU 2010-09 of the FASB Accounting Standards Codification, the Company as an SEC filer considers its financial statements issued when they are widely distributed to users, such as through filing them on EDGAR.
Recent accounting pronouncements
In November 2015, FASB issued ASU 2015-17, “Balance Sheet Classification of Deferred Taxes.” ASU 2015-17 requires that deferred tax liabilities and assets be classified as noncurrent in a classified statement of financial position. ASU 2015-17 is effective for financial statements issued for fiscal years beginning after December 15, 2016, and interim periods within those fiscal years. The Company does not expect these changes to have a material impact on the Company’s consolidated financial statements.
In January 2016, Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2016-01, “Recognition and Measurement of Financial Assets and Financial Liabilities.” ASU 2016-01 requires equity investments to be measured at fair value with changes in fair value recognized in net income; simplifies the impairment assessment of equity investments without readily determinable fair values by requiring a qualitative assessment to identify impairment; Eliminates the requirement for public business entities to disclose the method(s) and significant assumptions used to estimate the fair value that is required to be disclosed for financial instruments measured at amortized cost on the balance sheet; requires public business entities to use the exit price notion when measuring the fair value of financial instruments for disclosure purposes; requires an entity to present separately in other comprehensive income the portion of the total change in the fair value of a liability resulting from a change in the instrument-specific credit risk when the entity has elected to measure the liability at fair value in accordance with the fair value option for financial instruments; requires separate presentation of financial assets and financial liabilities by measurement category and form of financial assets on the balance sheet or the accompanying notes to the financial statements and clarifies that an entity should evaluate the need for a valuation allowance on a deferred tax asset related to available-for-sale securities in combination with the entity’s other deferred tax assets. ASU 2016-01 is effective for financial statements issued for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. The Company does not expect these changes to have a material impact on the Company’s consolidated financial statements.
| F-13 |
In March 2016, the FASB issued ASU 2016-09—Compensation—Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting. The amendments in this guidance are relating to employee share-based compensation. Under the new guidance, we are required to recognize the tax effects of stock compensation as income tax expense or benefit in the income statement and treat the tax effects of exercised or vested awards as discrete items in the reporting period in which they occur. Excess tax benefits are required to be classified as operating activities, and shares we withhold on behalf of employees for tax purposes are required to be classified as financing activities. We may make an accounting policy election to continue to estimate the number of awards that are expected to vest or account for forfeitures when they occur. The threshold to qualify for equity classification permits withholding up to the maximum statutory tax rates. This guidance is required to be adopted in the first quarter of 2017. We are currently evaluating the impact this guidance will have on our consolidated financial statements.
In August 2016, the FASB issued ASU 2016-15—Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments (a consensus of the Emerging Issues Task Force). The amendments in this guidance on eight specific cash flow issues with regard to how cash receipts and cash payments are presented and classified in the statement of cash flows in order to clarify existing guidance and reduce diversity in practice. The guidance is required to be adopted in the first quarter of 2018 on a retrospective basis, unless it is impracticable to apply, in which case it should be applied prospectively as of the earliest date practicable. Early adoption is permitted. We are currently evaluating the impact this guidance will have on our consolidated statement of cash flows.
In January 2017, the FASB issued ASU 2017-01, “Business Combinations (Topic 805): Clarifying the Definition of a Business.” The amendments in this guidance are clarifying the definition of a business to assist entities when determining whether an integrated set of assets and activities meets the definition of a business. The update provides that when substantially all the fair value of the assets acquired is concentrated in a single identifiable asset or a group of similar identifiable assets, the set is not a business. The guidance is effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years. The adoption of this new guidance is not expected to have a material impact on our consolidated financial statements.
In January 2017, the FASB issued ASU 2017-04—Intangibles—Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment. The amendments in this guidance to eliminate the requirement to calculate the implied fair value of goodwill to measure goodwill impairment charge (Step 2). As a result, an impairment charge will equal the amount by which a reporting unit’s carrying amount exceeds its fair value, not to exceed the amount of goodwill allocated to the reporting unit. An entity still has the option to perform the qualitative assessment for a reporting unit to determine if the quantitative impairment test is necessary. The amendment should be applied on a prospective basis. The guidance is effective for goodwill impairment tests in fiscal years beginning after December 15, 2019. Early adoption is permitted for goodwill impairment tests performed after January 1, 2017. The impact of this guidance for the Company will depend on the outcomes of future goodwill impairment tests.
There were other updates recently issued. The Company does not believe that other than disclosed above, the recently issued, but not yet adopted, accounting pronouncements will have a material impact on its financial position, results of operations or cash flows.
Goodwill and Other Intangibles
In accordance with Accounting Standards Update (ASU) No. 2014-02, management evaluates goodwill on an annual basis in the fourth quarter of more frequently if management believes indicators of impairment exist. Such indicators could, but are not limited to (1) a significant adverse change in legal factors or in business climate, (2) unanticipated competition, or (3) an adverse action or assessment by a regulator. The Company first assesses qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount, including goodwill. If management concludes that it is more likely than not that the fair value of a reporting unit is less than its carrying amount, management conducts a two-step quantitative goodwill impairment test. The first step of the impairment test involves comparing the fair value of the applicable reporting unit with its carrying value. The Company estimates the fair value of its reporting units using a combination of the income, or discounted cash flows, approach and the market approach, with utilizes comparable companies’ data. If the carrying amount of a reporting unit exceeds the reporting unit’s fair value, management performs the second step of the goodwill impairment test. The second step of the goodwill impairment test involves comparing the implied fair value of the affected reporting unit’s goodwill with the carrying value of that goodwill. The amount, by which the carrying value of the goodwill exceeds its implied fair value, if any, is recognized as an impairment loss. The Company’s evaluation of goodwill completed during the year resulted in no impairment losses.
3. Going Concern
The consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business.
As of December 31, 2016, the Company’s current liabilities substantially exceeded its current assets by $5,729,622, had an accumulated deficit of $19,489,400, and stockholders’ deficiency of $5,601,213. These circumstances, among others, raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. The financial statements also do not include any adjustments relating to the recoverability and classification of recorded asset amounts, or amounts and classifications of liabilities that might be necessary should the Company be unable to continue as a going concern.
The management of the Company already raised additional equity during the first quarter of 2017 for approximately $1,000,000. (Please refer to Note 14 for additional information) The Company is generating additional revenue while seeking additional equity financing. Management is very optimistic about the Company’s continue profitability for the coming years.
4. Property, Plant and Equipment
Property, plant and equipment, net consisted of the following:
| December 31, 2016 | December 31, 2015 | |||||||
| Property, Plant and Equipment | ||||||||
| Buildings | $ | - | $ | 1,308,785 | ||||
| Machinery and equipment | - | 595,623 | ||||||
| Automobiles | - | 85,769 | ||||||
| Office equipment | 942 | 104,843 | ||||||
| Furniture | 8,276 | - | ||||||
| Leasehold improvement | 70,871 | - | ||||||
| Computer software | - | 22,304 | ||||||
| Property, plant and equipment - total | $ | 80,089 | $ | 2,117,324 | ||||
| Less: accumulated depreciation | (20,311 | ) | (762,791 | ) | ||||
| Less: impairment on long-lived assets | - | (1,351,726 | ) | |||||
| Property, plant and equipment - net | $ | 59,778 | $ | 2,807 | ||||
The building is on a piece of land the use right of which was granted to Kiwa Bio-Tech Products (Shandong) Co., Ltd. by local government free for 10 years and then for another 20 years on a fee calculated according to Kiwa Shandong’s net profit. Since Kiwa Shandong did not generate any net profit, no fee is payable.
Depreciation expense was $22,340 and $4,352 for the years ended December 31, 2016 and 2015, respectively.
Impairment on long-lived assets was $nil for the years ended December 31, 2016 and 2015, respectively.
All of our property, plant and equipment have been held as collateral to secure the 6% Notes (see Note 8).
5. Goodwill
On November 30, 2015, Kiwa Bio-tech Products Group Ltd in BVI ("Kiwa BVI") entered an acquisition agreement with shareholders of Caber Holdings Ltd. (“Acquiree”) in Hong Kong to acquire 100 percent entity interest of the acquiree, including a wholly owned subsidiary, Oriental Baina Co., Ltd. in Beijing for US$30,000. The acquisition was completed in January, 2016. On the acquisition date, there was no any asset or liability acquired, and thus no fair value was allocated to asset and liability. Including legal fee and government fees, the total payment of approximately $34,112 ($30,000 plus legal fee and government fees totaled $4,112) was recorded as goodwill. The fair value of the goodwill is tested prior to the year-end 2016, and management determined there is no impairment to the goodwill as of December 31, 2016.
6. Construction Costs Payable
Construction costs payable represents remaining amounts to be paid for the first phase of construction of bio-fertilizer facility in Shandong. The balance of construction costs payable as of December 31, 2016 and 2015 was $255,539 and $273,722, respectively.
| F-14 |
7. Related Party Transactions
Amounts due to related parties consisted of the following as of December 31, 2016 and 2015:
| Item | Nature | Notes | December 31, 2016 | December 31, 2015 | ||||||||||||
| Mr. Wei Li (“Mr. Li”) | Non-trade | (1 | ) | $ | - | $ | 2,879,307 | |||||||||
| Kangtai Xinnong Agriculture Tech (Beijing) Co., Ltd. (“Kangtai”) | Non-trade | (2 | ) | - | (12,173 | ) | ||||||||||
| Ms. Yvonne Wang (“Ms. Wang”) | Non-trade | (3 | ) | 100,798 | 299,064 | |||||||||||
| Subtotal | 100,798 | 3,166,198 | ||||||||||||||
| Kiwa-CAU R&D Center | Trade | (4 | ) | 1,122,754 | 1,125,553 | |||||||||||
| CAAS IARRP and IAED Institutes | Trade | (5 | ) | 160,461 | 18,425 | |||||||||||
| Subtotal | 1,283,215 | 1,143,978 | ||||||||||||||
| Total | $ | 1,384,013 | $ | 4,310,176 | ||||||||||||
(1) Mr. Li
Mr. Li was the Chairman of the Board until November 20, 2015 and was the Chief Executive Officer of the Company until July 1, 2015.
Advances and Loans
On December 14, 2015, Mr. Li assigned $500,000 of obligation owed by the Company to his daughter, Feng Li. On the same day, Feng Li subscribed for the purchase of 250,000 shares of preferred stock for the aggregate amount of $500,000, and agreed to the concurrent cancellation of debt owed by the Company.
On March 24, 2016, the Company issued 2,900,000 shares of common stock to Mr. Li to settle down entire outstanding balance of $2,879,307. Subsequently in June 2016, Mr. Li transferred 1,000,000 shares to Troniya Industria Incubator Co., Ltd as a personal collateral for RMB 3.2 million received in Kiwa Baiao Bio-Tech (Beijing) Co., Ltd.’s account.
Guarantees for the Company
Mr. Li has pledged without any compensation from the Company all of his common stock of the Company as collateral for the Company’s obligations under the 6% Notes (see Note 9).
(2) Kangtai
Kangtai is a private company and is 64% owned by Mr. Li. Mr. Li is the Chairman of Kangtai.
(3) Ms. Wang
Ms. Wang is the Secretary of the Company until November 20, 2015. Effective as of November 20, 2015, the Company appointed Ms. Wang as the Chairman of the Board. Effective August 11, 2016, the Company’s Board of Directors has assigned Ms. Wang the additional titles of Acting President, Acting Chief Executive Officer and Acting Chief Financial Officer.
On December 14, 2015, Ms. Wang subscribed for the purchase of 250,000 shares of preferred stock for the aggregate amount of $500,000, and agrees to the concurrent cancellation of debt owed by the Company.
On March 24, 2016, the Company issued 240,000 shares of common stock to Ms. Wang to pay off the loan balance of $240,000. During the year ended December 31, 2016, Ms. Wang paid various expenses on behalf of the Company. As of December 31, 2016, the amount due to Ms. Wang was $100,798.
| F-15 |
(4) Kiwa-CAU R&D Center
In November 2006, Kiwa and China Agricultural University (the “CAU”) agreed to jointly establish a new research and development center, named Kiwa-CAU R&D Center. The term of the agreement was ten years commencing July 1, 2006.
| ● | Pursuant to the agreement, Kiwa agree to invest RMB 1 million (approximately $160,000) each year to fund research at Kiwa-CAU R&D Center. Prof. Qi Wang, a director of the Company, is also the director of Kiwa-CAU R&D Center. The Company recorded $75,528 and $160,563 research and development expenses related to this R&D Center for the years ended December 31, 2016 and 2015, respectively. |
(5) CAAS IARRP and IAED Institutes
On November 5, 2015, the Company signed a strategic cooperation agreement (the “Agreement”) with China Academy of Agricultural Science (“CAAS”)’s Institute of Agricultural Resources & Regional Planning (“IARRP”) and Institute of Agricultural Economy & Development (“IAED”). The term of the Agreement was three years commencing November 20, 2015.
| ● | Pursuant to the agreement, Kiwa agree to invest RMB 1 million (approximately $160,000) each year to the Spatial Agriculture Planning Method & Applications Innovation Team that belongs to the Institutes. Prof. Yong Chang Wu, the authorized representative of IARRP, CAAS, is also one of the Company's directors effective since November 20, 2015 until March 13, 2017. The Company recorded $149,176 and $18,425 research and development expenses related to the institutes, for the years ended December 31, 2016 and 2015, respectively, |
Research and Development expenses for the years ended December 31, 2016 and 2015, totaled $224,704 and $178,988, respectively.
8. Unsecured Loans Payable
Unsecured loans payable consisted of the following:
| Item | December 31, 2016 | December 31, 2015 | ||||||
| Unsecured loan payable to Zoucheng Municipal Government, non-interest bearing, becoming due within three years from Kiwa Shandong’s first profitable year on a formula basis, interest has not been imputed due to the undeterminable repayment date | $ | 1,269,880 | $ | 1,387,668 | ||||
| Unsecured loan payable to Zoucheng Science & Technology Bureau, non-interest bearing, it is due in Kiwa Shandong’s first profitable year, interest has not been imputed due to the undeterminable repayment date | 385,463 | 385,463 | ||||||
| Total | $ | 1,655,343 | $ | 1,773,131 | ||||
The Company qualifies for non-interest bearing loans under a Chinese government sponsored program to encourage economic development in certain industries and locations in China. To qualify for the favorable loan terms, a company must meet the following criteria: (1) be a technology company with innovative technology or product (as determined by the Science Bureau of the central Chinese government); (2) operate in specific industries that the Chinese government has determined are important to encourage development, such as agriculture, environmental, education, and others; and (3) be located in an undeveloped area such as Zoucheng, Shandong Province, where the manufacturing facility of the Company is located.
According to the Company’s project agreement, Zoucheng Municipal Government granted the Company use of at least 15.7 acres in Shandong Province, China at no cost for 10 years to construct a manufacturing facility. Under the agreement, the Company has the option to pay a fee of RMB 480,000 ($77,100) per acre for the land use right after the 10-year period until May 2012. The Company may not transfer or pledge the temporary land use right. The Company also committed to invest approximately $18 million to $24 million for developing the manufacturing and research facilities in Zoucheng, Shandong Province. As of December 31, 2016, the Company invested approximately $2 million for the property, plant and equipment of the project and these assets were impaired as of December 31, 2016.
| F-16 |
9. Convertible Notes Payable
Convertible notes payable consists of 6% secured convertible notes issued to FirsTrust Group Inc. on June 29, 2006. The notes beard interest at 6% and were due on June 29, 2009. Once the note is pass due, the interest rate increased to 15% per annum. The Company accrued $22,977 and $22,538 interest expense on convertible notes for the years ended December 31, 2016 and 2015, respectively. Interest payable to FirstTrust Group Inc. totaled $183,361 and $160,762 at December 31, 2016 and 2015, respectively.
The conversion price of the 6% Notes is based on a 40% discount to the average of the trading price of the Company’s common stock on the OTC Bulletin Board over a 20-day trading period. The conversion price is also adjusted for certain subsequent issuances of equity securities of the Company at prices below the conversion price then in effect. The 6% Notes contain a volume limitation that prohibits the holder from further converting the 6% Notes if doing so would cause the holder and its affiliates to hold more than 4.99% of the Company’s outstanding common stock. In addition, the holder of the 6% Notes agrees that they may not convert more than their pro-rata share (based on original principal amount) of the greater of $120,000 principal amount of the 6% Notes per calendar month or the average daily dollar volume calculated during the 10 business days prior to a conversion, per conversion. This conversion limit has since been eliminated pursuant to an agreement by the Company and the Purchasers.
The Company incurs a financial penalty in cash or shares at the option of the Company (equal to 2% of the outstanding amount of the Notes per month plus accrued and unpaid interest on the Notes, prorated for partial months) if it breaches this or other affirmative covenants in the Purchase Agreement, including a covenant to maintain a sufficient number of authorized shares under its Certificate of Incorporation to cover at least 110% of the stock issuable upon full conversion of the Notes. Pursuant to the relevant provisions for liquidated damages in the Purchase Agreement, the Company has accrued the penalty of $77,575 and $72,152 for the years ended December 31, 2016 and 2015, respectively.
The 6% Notes require the Company to procure the Purchaser’s consent prior to taking certain actions including the payment of dividends, repurchasing stock, incurring debt, guaranteeing obligations, merging or restructuring the Company, or selling significant assets.
The Company’s obligations under the 6% Notes are secured by a first priority security interest in the Company’s intellectual property pursuant to an Intellectual Property Security Agreement with the Purchasers, and by a first priority security interest in all of the Company’s other assets pursuant to a Security Agreement with the Purchasers. In addition, Mr. Li, the Company’s former Chief Executive Officer until July 1, 2015, has pledged all of his common stock of the Company as collateral for the Company’s obligations under the 6% Notes. The intellectual property pledged had a cost of $592,901 which carrying value of $179,897 was fully impaired during the year ended December 31, 2009.
| F-17 |
10. Note payable
On May 29, 2007, the Company issued a $360,000 promissory note to an unrelated individual. This note bears interest at 18% per annum and due on July 27, 2007. This note is currently in default and bears interest of 25% per annum (the “Default rate”) until paid in full. This note is secured by a pledge of 6,178,336 (post-reverse split 30,892) shares of the Company’s common stock owned by Investlink (China) Limited, a British Virgin Island corporation. The Company accrued $90,000 and $90,000 interest expense on note payable for the years ended December 31, 2016 and 2015, respectively.
11.Stockholders’ Deficiency
On December 14, 2015, the Company issued 500,000 shares of preferred stock for the aggregate amount of $1,000,000 as debt cancellation owed to two related party individuals (see Note 7).
In March, 2016, the Company issued 3,140,000 shares of common stock to Mr. Li and Ms. Wang for debt and salary payable settlement for an aggregate amount of $3,141,000 (See Note 7). In addition, the Company issued 101,947 common shares to Jimmy Zhou, former CEO in August 2016, to settle payable to him for $50,974. All of issuances of common shares for settlement of debts were based the stock price on the transaction dates.
During the year, the Company issued 1,775,000 common shares for cash at $0.8 per share for an aggregate subscribe price equivalent to $1,420,000, of which $859,659 has received while approximately $560,341 remaining subscribe receivable at December 31, 2016.
During the year ended December 31, 2016, the Company issued 1,711,808 common shares to four consulting companies as compensation for their consulting service received, totaled $254,250 approximately for the year ended December 31, 2016.
12. Stock-based Compensation
On December 12, 2006, the Company granted options for 2,000,000 shares (10,000 post-reverse split shares) of its common stock under its 2004 Stock Incentive Plan. Summary of options issued and outstanding at December 31, 2016 and 2015 and the movements during the years then ended are as follows:
| F-18 |
| Number
of underlying shares | Weighted- Average Exercise Price Per Share | Aggregate Intrinsic Value | Weighted-
Average Contractual Life Remaining in Years | |||||||||||||
| Outstanding at December 31, 2014 | 6,163 | $ | 35 | $ | - | 2 | ||||||||||
| Exercised | - | - | - | |||||||||||||
| Expired | - | - | - | |||||||||||||
| Forfeited | - | - | - | |||||||||||||
| Outstanding at December 31, 2015 | 6,163 | $ | 35 | $ | - | 1 | ||||||||||
| Exercised | - | - | - | |||||||||||||
| Expired | 6,163 | $ | 35 | - | ||||||||||||
| Forfeited | - | - | - | |||||||||||||
| Outstanding at December 31, 2016 | - | - | $ | - | - | |||||||||||
| Exercisable at December 31, 2016 | - | - | $ | - | - | |||||||||||
As of December 31, 2016, no stock options or other stock-based compensation was outstanding and all prior grants of stock options had expired as of that date.
13. Income Tax
In accordance with the current tax laws in China, Kiwa Shandong is subject to a corporate income tax rate of 25% on its taxable income. However, Kiwa Shandong has not provided for any corporate income taxes since it had no taxable income for the years ended December 31, 2016 and 2015.
No provision for taxes is made for U.S. income tax as the Company has no taxable income in the U.S. In accordance with the relevant tax laws in the British Virgin Islands, Kiwa BVI, as an International Business Company, is exempt from income taxes.
A reconciliation of the provision for income taxes determined at the local income tax rate to the Company’s effective income tax rate is as follows:
| Years ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Pre-tax income (loss) | $ | 1,389,576 | $ | (677,358 | ) | |||
| U.S. federal corporate income tax rate | 34 | % | 34 | % | ||||
| Income tax computed at U.S. federal corporate income tax rate | 472,456 | (230,302 | ) | |||||
| Reconciling items: | ||||||||
| Rate differential for PRC earnings | (139,923 | ) | 22,439 | |||||
| Change of valuation allowance | 301,813 | 171,993 | ||||||
| Non-deductible expenses | (208,066 | ) | 35,870 | |||||
| Effective tax expense | $ | 426,280 | $ | - | ||||
| F-19 |
The Company had deferred tax assets as follows:
| December 31, 2016 | December 31, 2015 | |||||||
| Net operating losses carried forward | $ | 3,475,563 | $ | 3,398,402 | ||||
| Less: Valuation allowance | (3,475,563 | ) | (3,398,402 | ) | ||||
| Net deferred tax assets | $ | - | $ | - | ||||
As of December 31, 2016 and 2015, the Company had approximately $3.5 million and $8 million net operating loss carryforwards available to reduce future taxable income. Net operating loss of the Company could be carried forward and taken against any taxable income for a period of not more than twenty years from the year of the initial loss pursuant to Section 172 of the Internal Revenue Code of 1986, as amended. The net operating loss of Kiwa Shandong could be carried forward for a period of not more than five years from the year of the initial loss pursuant to relevant PRC tax laws and regulations. It is more likely than not that the deferred tax assets cannot be utilized in the future because there will not be significant future earnings from the entity which generated the net operating loss. Therefore, the Company recorded a full valuation allowance on its deferred tax assets.
As of December 31, 2016 and 2015, the Company has no material unrecognized tax benefits which would favorably affect the effective income tax rate in future periods and does not believe that there will be any significant increases or decreases of unrecognized tax benefits within the next twelve months. No interest or penalties relating to income tax matters have been imposed on the Company during the two years ended December 31, 2016 and 2015, and no provision for interest and penalties is deemed necessary as of December 31, 2016 and 2015.
According to the PRC Tax Administration and Collection Law, the statute of limitations is three years if the underpayment of taxes is due to computational errors made by the taxpayer or its withholding agent. The statute of limitations extends to five years under special circumstances, which are not clearly defined. In the case of a related party transaction, the statute of limitation is ten years. There is no statute of limitation in the case of tax evasion.
14. Commitments and Contingencies
The Company has the following material contractual obligations:
(1) Investment in manufacturing and research facilities in Zoucheng, Shandong Province in China
According to the Project Agreement with Zoucheng Municipal Government in 2002, we have committed to investing approximately $18 million to $24 million for developing the manufacturing and research facilities in Zoucheng, Shandong Province. As of December 31, 2016, we had invested approximately $1.91 million for the project. On February 11, 2017, the Company entered an Equity Transfer Agreement with Dian Shi Cheng Jing (Beijing) Technology Co. (“Transferee”) to transfer all of shareholders’ right, title and interest in Kiwa Shandong to the Transferee for USD $1.00. Currently, the completion of transfer is under the government processing.
(2) Strategic cooperation with two institutes in China
On November 5, 2015, the Company signed a strategic cooperation agreement (the “Agreement”) with China Academy of Agricultural Science (“CAAS”)’s Institute of Agricultural Resources & Regional Planning (“IARRP”) and Institute of Agricultural Economy & Development (“IAED”). Pursuant to the Agreement, the Company will form a strategic partnership with the two institutes and establish an “International Cooperation Platform for Internet and Safe Agricultural Products”. To fund the cooperation platform’s R&D activities, the Company will provide RMB 1 million (approximately $160,000) per year to the Spatial Agriculture Planning Method & Applications Innovation Team that belongs to the Institutes. The term of the Agreement is for three years beginning November 20, 2015. Prof. Yong Chang Wu, the authorized representative of IARRP, CAAS, is also one of the Company’s directors effective since November 20, 2015 until March 13, 2017.
| F-20 |
(3) Distribution agreement with Kangtan Gerui Bio-Tech in China
On December 17, 2015, Kiwa Bio-Tech Products Group Corporation (the “Company”) entered into a distribution agreement (the “Agreement”) with Kangtan Gerui (Beijing) Bio-Tech Co., Ltd. (“Gerui”) and formally awarded Gerui a right to sell and distribute the Company’s fertilizer products in 3 major agricultural regions of China— Hainan Province, Hunan Province and Xinjiang Autonomous Region. The Company’s Research and Development department has been conducting application experiments in Hainan and Hunan Provinces since August 2015, in accordance with the market requirements. The experiment data indicates that the Company’s fertilizer products have fulfill the requirements of reduction of content of heavy metals in soil and improve crop yield. Gerui was founded in Beijing in April 2015 and relies on the sales network of China’s Supply and Marketing Cooperatives system. Currently, the Company and Gerui do not hold any interest in each other; however, a collaboration and integration may take place in the future. The term of the Agreement is for a period of three years commencing December 17, 2015. In September 2016, Kiwa Baiao Bio-Tech (Beijing) Co., Ltd obtained a fertilizer sales permit from the Chinese government and began to sale the products directly to customers in those 3 major agricultural regions.
(4) Lease payments
(1) On April 29, 2016, Kiwa Baiao Bio-Tech (Beijing) Co., Ltd. entered an office lease agreement with two-year team. Monthly lease payment and building management fee totaled RMB 77,867 or approximately USD $11,622.
(2) On November 11, 2017, Kiwa Baiao Bio-Tech (Beijing) Co., Ltd. entered an apartment lease agreement for its employees. The lease term is one year with monthly lease payment of RMB 6,000 or approximately USD $896.
(3) In March 1, 2017, Kiwa Bio-Tech (Shenzhen) Co., Ltd, a newly established subsidiary entered an office lease agreement with one-year term. Monthly lease payment is RMB 29,000 or approximately of USD $4,320.
The future lease payments at December 31, 2016 are summarized below.
| Beijing Office | Beijing Apartment | Shenzhen Office | Total | |||||||||||||
| 2017 | $ | 139,462 | $ | 3,632 | $ | 4,328 | $ | 147,422 | ||||||||
| 2018 | $ | 44,555 | - | - | $ | 44,555 | ||||||||||
| Thereafter | - | - | - | - | ||||||||||||
15. Subsequent Events
The Company has evaluated the existence of significant events subsequent to the balance sheet date through the date these financial statements were issued and has determined that there were no subsequent events or transactions which would require recognition or disclosure in the financial statements, other than noted herein.
On February 11, 2017, the Company executed an Equity Transfer Agreement with Dian Shi Cheng Jing (Beijing) Technology Co. (“Transferee”) whereby the Company transferred all of its right, title and interest in Kiwa Bio-Tech Products (Shandong) Co., Ltd. (“Shandong”) to the Transferee for the RMB equivalent of US$1.00. In connection with the transaction, the Transferee received all assets of Shandong which are estimated to be approximately RMB 14,057,713 at the effective date and assumed all liabilities of Shandong which are estimated to be approximately RMB 59,446,513 at the effective date. In connection with this transaction, Transferee agreed to indemnify the Company for any liability or claims of any third party(ies) against Shandong or the Company for five (5) years. The transaction is subject to obtaining Chinese government approval for the transaction, which the parties agrees to use their best efforts to obtain prior to December 31, 2017. The completion of transfer is under government processing.
| F-21 |
On February 15, 2017, the Company completed the sale of 1,000,000 shares of Kiwa Common Stock (each a “Share”) at a price of $1.00 per share (total sale proceeds were $1,000,000) to Junwei Zheng in a private transaction which was exempt from registration under Section 4(a)(2) of the Securities Act of 1933, as amended (the “Act”) and Regulation S promulgated under the Act since, among other things, the transaction did not involve a public offering and the securities were acquired for investment purposes only and not with a view to or for sale in connection with any distribution thereof and were purchased by an investor who is not a resident of the United States. The net proceeds will be used for the further development of Kiwa products and distribution, as well as for general working capital.
On February 23, 2017, the Company has agreed to a strategic relationship with ETS (Tianjin) Biological Science and Technology Development Co., Ltd. (“ETS”). The partnership will include the deployment and strategic use of ETS biotechnology to produce of bio-fertilizers for use in both China and internationally. Kiwa and ETS, together with the certain Chinese government departments, will work together to enhance China’s microbial fertilizer industry standards and China’s food safety industry chain standards. The parties will work together on the development of microbial technology and products in agriculture, environmental protection, soil management and other fields. Relying on the Chinese Academy of Sciences, ETS Environmental and Agricultural Microbial Technology Research Center and biotechnology project research results, Kiwa has introduced the ETS core technology to complete bio-fertilizer upgrading, transformation and to develop new product lines. In order to meet the growing global consumer demand to increase food supply and develop sustainable farming we are applying sustainable use of biotechnology and the use of biotechnology products to replace chemical products, which will strengthen environmental protection and promote international cooperation. As a result of strict management of many agricultural chemicals, such chemicals will continue to be abandoned, resulting is a growing demand for bio-fertilizers. It has been widely accepted that the application of ETS biotechnology facilitates agricultural sustainability and helps to protect the soil and improve grain output. The technology focuses on keeping soil healthy by restoring healthy microbes that are naturally present in healthy soils. As the technology gains worldwide recognition, it is imperative to popularize bio-fertilizer in developing countries to fulfill the needs of growing populations and promote environmentally friendly agriculture. Through the cooperation of Kiwa and ETS, the parties aim to enhance the usage of the bio-fertilizers in China. The cooperation will bring technological transformation and support for Kiwa to improve its existing manufacturing techniques. Kiwa and ETS will also collaborate to establish a comprehensive platform for producing, supplying, and marketing in China. Ultimately, Kiwa would look to introduce these products to the international market, including the United States.
On February 27, 2017, the Company has signed a strategic cooperation agreement with the Beijing Zhongpin Agricultural Science and Technology Development Center (“Zhongpin Center”). Zhongpin Center is the Chinese Agricultural Science and Technology Innovation and Development Committee’s executive implementation agency (referred to as the Agricultural Science and Technology Commission). The Agricultural Science and Technology Commission is set up by the Chinese Central Government for the construction of the National Ecological Security Agriculture Industrial Chain standardization system. This includes the establishment of National Ecology Safe Agricultural Industrial Parks to build China’s Ecological Security and Agricultural Industrial in an orderly business environment, including completion of the National Soil Remediation Program and governance of the various government functions of the institutions. Through the guidance and support by the Zhongpin Center, Kiwa will participate and be involved in China’s National Soil Remediation Program and construction of the National Ecological Security Agriculture Industrial Chain Standardization System’s operation and process.
On March 8, 2017, pursuant to the consent of the holders of a majority of the votes entitled to be cast on the matter and the approval of the majority of the directors of the Company, the Company was converted from a Delaware corporation to a Nevada corporation by filing of Articles of Conversion and Articles of Incorporation in the State of Nevada and filing a Certificate of Dissolution in the State of Delaware.
On March 8, 2017, pursuant to the consent of the holders of a majority of the votes entitled to be cast on the matter and the approval of the Kiwa Bio-Tech Products Group Corporation 2016 Employee, Director and Consultant Stock Plan.
On March 13, 2017, Yong Change Wu was removed as a director of the Company by the consent of the holders of a majority of the votes entitled to be cast on the matter and the approval of the majority of the directors of the Company. Immediately thereafter, the Board appointed Yong Lin Song as a director of the Company to be effective immediately.
| F-22 |
