Attached files
| file | filename |
|---|---|
| EX-32.1 - KIWA BIO-TECH PRODUCTS GROUP CORP | v179093_ex32-1.htm |
| EX-31.2 - KIWA BIO-TECH PRODUCTS GROUP CORP | v179093_ex31-2.htm |
| EX-31.1 - KIWA BIO-TECH PRODUCTS GROUP CORP | v179093_ex31-1.htm |
|
|
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
10-K
|
x
|
ANNUAL
REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
|
For the
fiscal year ended December 31, 2009
|
¨
|
TRANSITION
REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
|
For the
Transition Period from ______ to ______
Commission
file number: 000-33167
KIWA
BIO-TECH PRODUCTS GROUP CORPORATION
(Exact
name of registrant as specified in its charter)
|
Delaware
|
77-0632186
|
|
|
(State
or other jurisdiction of
incorporation
or organization)
|
(I.R.S.
Employer Identification
No.)
|
|
310
N. Indian Hill Blvd., #702 Claremont, California 91711
|
||
|
(Address
of principal executive
offices)
|
||
|
(626)
715-5855
|
||
|
(Registrant’s
telephone number,
including
area code)
|
|
Securities
registered pursuant to
Section
12(b) of the Act:
|
Name
of each exchange on which
registered
|
|
|
None
|
OTC
Bulletin Board
|
|
Securities
registered pursuant to
Section
12(g) of the Act:
(Title
of Each Class)
|
||
|
Common
Stock, $0.001 par value
|
Indicate
by check mark if the registrant is a well-know seasoned issuer, as defined in
Rule 405 of the Securities Act. ¨ Yes x No
Indicate
by check mark if the registrant is not required to file reports pursuant to
Section 13 or Section 15(d) of the Act. ¨ Yes x No
Indicate
by check mark whether the Registrant (1) has filed all reports required to be
filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the
preceding 12 months (or for such shorter period that the registrant was required
to file such reports), and (2) has been subject to such filing requirements for
the past 90 days. x Yes ¨ No
Indicate
by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K is not contained herein, and will not be contained, to the best
of the Registrant’s knowledge, in definitive proxy or information statements
incorporated by reference in Part III of this Form 10-K or any amendment to this
Form 10-K. ¨
Indicate
by check mark whether the Registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer or a smaller reporting company. See
definitions of “large accelerated filer,” “accelerated filer” and “smaller
reporting company” in Rule 12b-2 of the Exchange Act.
|
Large
accelerated filer ¨
|
Accelerated
filer ¨
|
|
Non-accelerated
filer ¨
|
Smaller
reporting company x
|
Indicate
by check mark whether the Registrant is a shell company (as defined in Rule
12b-2 of the Exchange Act). ¨ Yes x No
The
aggregate market value of voting and non-voting common stock held by
non-affiliates of the registrant, based upon the closing bid quotation for the
registrant’s common stock, as reported on the OTC Bulletin Board quotation
service, as of June 30, 2009 was approximately $560,000.
|
The number of shares of
registrant’s common stock outstanding as of March 29, 2010 was
400,000,000.
|
Annual
Report on Form 10-K
For the
Fiscal Year Ended December 31, 2009
INDEX
TABLE
OF CONTENTS
|
PART
I
|
1
|
|
SPECIAL
NOTE REGARDING FORWARD-LOOKING STATEMENTS
|
1
|
|
ITEM
1. BUSINESS
|
1
|
|
ITEM
1A. RISK FACTORS
|
13
|
|
ITEM
2. PROPERTY
|
27
|
|
ITEM
3. LEGAL PROCEEDINGS
|
28
|
|
ITEM
4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
|
28
|
|
PART
II
|
30
|
|
ITEM
5. MARKET OF REGISTRANTS’ COMMON EQUITY, RELATED STOCKHOLDER MATTER AND
ISSUER PURCHASES OF EQUITY SECURITY
|
30
|
|
ITEM
6. SELECTED FINANCIAL DATE
|
31
|
|
ITEM
7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS
OF OPERATIONS
|
31
|
|
ITEM
8. FINANCIAL STATEMENTS
|
41
|
|
ITEM
9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND
FINANCIAL DISCLOSURE
|
41
|
|
ITEM
9A(T). CONTROLS AND PROCEDURES
|
42
|
|
ITEM
9B. OTHER INFORMATION
|
44
|
|
PART
III
|
44
|
|
ITEM
10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE
GOVERNANCE.
|
44
|
|
ITEM
11. EXECUTIVE COMPENSATION
|
47
|
|
ITEM
12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND
RELATED STOCKHOLDER MATTERS
|
50
|
|
ITEM
13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR
INDEPENDENCE
|
51
|
|
ITEM
14. PRINCIPAL ACCOUNTING FEES AND SERVICES
|
52
|
|
PART
IV
|
53
|
|
ITEM
15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
|
53
|
|
SIGNATURES
|
55
|
Part
I
On one or
more occasions, we may make forward-looking statements in this Annual Report on
Form 10-K regarding our assumptions, projections, expectations, targets,
intentions or beliefs about future events. Words or phrases such as
“anticipates,” “may,” “will,” “should,” “believes,” “estimates,” “expects,”
“intends,” “plans,” “predicts,” “projects,” “targets,” “will likely result,”
“will continue” or similar expressions identify forward-looking
statements. These forward-looking statements are only our predictions
and involve numerous assumptions, risks and uncertainties, including, but not
limited to those listed below and those business risks and factors described
elsewhere in this report and our other Securities and Exchange Commission
filings.
We
undertake no obligation to publicly update or revise any forward-looking
statements, whether as a result of new information, future events or
otherwise. However, your attention is directed to any further
disclosures made on related subjects in our subsequent annual and periodic
reports filed with the Securities and Exchange Commission on Forms 10-K, 10-Q
and 8-K and Proxy Statements on Schedule 14A.
References
herein to “we,” “us,” “our” or “the Company” refer to Kiwa Bio-Tech Products
Group Corporation and its wholly-owned and majority-owned subsidiaries unless
the context specifically states or implies otherwise.
|
Item
1.
|
Business
|
The
Company
We are
the result of a share exchange transaction completed in March 2004 between the
shareholders of Tintic Gold Mining Company (“Tintic”), a corporation originally
incorporated in the state of Utah on June 14, 1933 to perform mining operations
in Utah, and the shareholders of Kiwa Bio-Tech Products Group Ltd. (“Kiwa BVI”),
a company originally organized under the laws of the British Virgin Islands on
June 5, 2002. The share exchange resulted in a change of control of
Tintic, with former Kiwa BVI stockholders owning approximately 89% of Tintic on
a fully diluted basis and Kiwa BVI surviving as a wholly-owned subsidiary of
Tintic. Subsequent to the share exchange transaction, Tintic changed
its name to Kiwa Bio-Tech Products Group Corporation. On July 21,
2004, we completed our reincorporation in the State of Delaware.
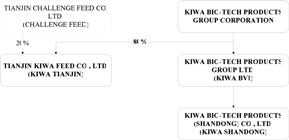
We have
established two subsidiaries in China: (1) Kiwa Bio-Tech Products (Shandong)
Co., Ltd. (“Kiwa Shandong”) in 2002 and (2) Tianjin Kiwa Feed Co., Ltd. (“Kiwa
Tianjin”) in July 2006. The following chart summarizes our
organizational and ownership structure.
1
Bio-fertilizer
We have
developed a number of bio-fertilizer and other products for plants and are
developing more. In 2002, Kiwa BVI chartered Kiwa Shandong, a
wholly-owned subsidiary organized under the laws of PRC, as its offshore
fertilizer manufacturing base to capitalize on low cost, high quality
manufacturing advantages available in China. In October 2003, Kiwa
Shandong completed the first phase of construction of its manufacturing facility
in Shandong Province, China. In November 2003, Kiwa Shandong began
shipping its first bio-fertilizer product to the agricultural market in
China. Since then, we have been devoting ourselves to expand our
market share and further upgrade our facility.
In June
2008, Kiwa Shandong received approval from the Ministry of Commerce of the PRC
to sell fertilizer products of other manufacturers on a wholesale basis,
including chemical fertilizers, complex fertilizers and compound
fertilizers. Based on applicable tax laws in China, Kiwa Shandong’s
new business items will be exempt from value-added tax.
Bio-enhanced
Feed
On July
11, 2006, we entered into a joint venture with Tianjin Challenge Feed Co., Ltd.
(“Challenge Feed”) to engage in the developing, manufacturing and marketing of
biologically enhanced feed for livestock. The joint venture is
through Kiwa Tianjin, our 80% subsidiary formed under the laws of
PRC. Pursuant to the joint venture agreement between the Company and
Challenge Feed, we invested $480,000 in cash for our 80% equity share of Kiwa
Tianjin and Challenge Feed invested machinery and equipment used in one of Kiwa
Tianjin’s two bio-enhanced feed production lines with an agreed value of
$120,000 for the remaining 20% equity. We also lease another
production line from Challenge Feed.
On
December 22, 2009, Kiwa Tianjin filed a lawsuit against Challenge Feed in the
local court of Wuqing District, Tianjin, where Kiwa Tianjin is
domiciled. In the lawsuit, Kiwa Tianjin asserted that Challenge Feed
unlawfully disposed of the assets held by Kiwa Tianjin. The local
court is currently reviewing the complaint and related documents filed with
it. As a result, Kiwa Tianjin could no longer use its assets
including machinery and inventory in normal course of operation. As
of December 31, 2009, the Company has classified its bio-enhanced feed business
through Kiwa Tianjin as discontinued operations.
AF-01
Anti-viral Aerosol
On May 8,
2006 we entered into a Technology Transfer Agreement with Jinan Kelongboao
Bio-Tech Co., Ltd. (“JKB”), which will become fully effective when we have
finished paying the first installment of consideration of RMB 3,000,000
according to the payment schedule in the contract. As of December 31,
2009, the Company has paid RMB 1,000,000 for the first installment of
consideration of the contract. Pursuant to the agreement, JKB agreed
to transfer its AF-01 anti-viral aerosol technology for veterinary medicine
applications to the Company. The AF-01 anti-viral aerosol technology
is a broad-spectrum antiviral agent with potent inhibitory and/or viricidal
effects on a variety of RNA viruses found in animals and fowls such as bird
flu. We acquired the exclusive production right and other related
rights to produce an anti-viral aerosol veterinary drug. Our hope is
to develop a commercialized product in the form of a spray for applying in fowl
houses and other animal holding facilities to prevent and cure virus-caused
diseases.
2
In addition, pursuant to the Technology Transfer Agreement, JKB will
exclusively supply to us the raw material medicine for AF-01 anti-viral aerosol,
which must have an index of 200,000 zymolysis units per
milliliter. There is no alternative supplier if JKB fails to perform
its supply obligations in the contract.
We are
now in the process of applying for statutory licenses for the AF-01
technology. Before marketing this product, we will need to: (1)
successfully complete a safety evaluation, pre-clinical study, pharmacological
and toxicological test, clinical trial report, stability test report,
environmental impact report, residue depletion test and other obligatory
experiments by statutory authorities; (2) pass an evaluation by the veterinary
drug evaluation institution established by Administrative Department for
Veterinary Medicine of State Council (the “Administrative Department”) and pass
a sample quality retrial by a test institution established by the Administrative
Department after the application is accepted; (3) acquire a Registration
Certificate of New Veterinary Drug from the Administrative Department compliant
with its drug qualification standards; (4) acquire a company or factory with GMP
qualification and submit the application for Approval Number of Veterinary Drug
Products in the name of the acquired company to the Administrative Department;
and (5) pass an evaluation of manufacturing requirements by the Administrative
Department and procure a Veterinary Drug Manufacturing License. There
can be no assurance that we can acquire such prerequisite approvals and
licenses, or how much time it will take.
Such
procedures are subject to Regulations on Administration of Veterinary Drugs
promulgated by Decree No. 404 of the State Council of China on April 9, 2004,
Measures for Registering of Veterinary Drugs and Measures for Administration of
Approval Number of Veterinary Drug Products promulgated by Decrees No. 44 and
No. 45 respectively of the PRC Ministry of Agriculture on November 24, 2004, and
other applicable rules and regulations of China.
The
Company is currently looking for GMP-qualified veterinary drug manufacturers to
set up a new joint venture.
Strategies
With the
world’s largest population to feed, China’s demand for agricultural products is
immense. Problems with pollution and soil contamination have
increased pressure on the Chinese government to conserve land and enhance
environmental protection. Serious diseases such as H5N1 avian flu are spreading
around the world and have threatened animal husbandry. More
critically, such diseases have threatened the health and safety of humans
through possible bird to human and human to human transmission. China
thus faces an urgent need to improve unit land yield, prevent and treat such
diseases, and reduce pollution. We plan to address this need through
the development of our ag-biotech inputs which may resolve many of these
problems in environmentally friendly ways. To exploit this
opportunity, our core strategies are as follows:
General Operational
Strategy
|
l
|
Build
a platform for world-class biotechnological research and development
results to be commercialized into products for applications in
agriculture;
|
|
l
|
Invest
in mature technologies that will not require large amounts of research
expense to develop into commercial
products;
|
|
l
|
Utilize
proprietary technology to supply ag-biotech inputs to the market at lower
cost than our competitors;
|
|
l
|
Constructing
or acquiring new production facilities, improving established facilities;
to improve our manufacturing capability in
China;
|
|
l
|
Building
and strengthening our “KIWA” brand so as to become one of the leading
companies in China’s “biological, safe and environment-friendly”
agricultural inputs industry;
|
|
l
|
Establish
strategic alliances for research and development, sales and distribution
and customer acquisition with complimentary entities in the
biological-agriculture industry;
and
|
3
|
l
|
Enhance
overall management systems, operational structure and corporate
governance.
|
Sales
Strategy
|
l
|
Our
sales strategy involves utilizing both a direct sales force and
distribution networks. Our distribution efforts are expected to include
the following:
|
|
|
o
|
Choosing
green food/organic food planting bases or other demonstrative agricultural
products producers, carrying out regional field tests, fanning out from a
point to an area, cultivating market
network;
|
|
|
o
|
Leveraging
government, industrial organizations (such as “China Green Food
Association”) to strengthen existing sales network in rural areas, thereby
reach end-users in a more cost-effective manner; cut off selling expenses
occurred during middle tiers of supply chain to boost end-user’s
value;
|
|
|
o
|
Cooperating
with special agricultural production materials distributors who also help
farmers resell their products; focusing on large-to-medium size
wholesalers of agricultural production materials at provincial and
municipal levels;
|
|
|
o
|
Establishing
a three-level distribution network consisting of a company-centralized
sales office, prefectural representative offices and direct distributors
in villages and towns; and
|
|
|
o
|
Leveraging
existing sales channels and network of affiliates’ products to save costs
of building the network from
scratch.
|
Strategy Regarding
Customers
|
l
|
Our
targeted customers include major agricultural companies and growers that
can realize significant financial benefits from using our products
including:
|
|
|
o
|
Provide
high value-added agricultural products (such as fruits, vegetables, meat,
eggs that meet the requirements of green food/organic food) to Chinese
agricultural products producers;
|
|
|
o
|
Agricultural
products producers located in China who are exporting to Japan, Korea,
Europe, US and other regional markets of the
world;
|
|
|
o
|
Chinese
agricultural products producers who have generated internal needs of
ag-biotech inputs to solve the problems of soil-caused diseases,
anti-biotic drug tolerance, leftover and others;
and
|
|
|
o
|
“Green”
or organic growers throughout the
world.
|
Given the
global trend of customers favoring environmentally safe green food and
organically grown food, producers’ needs for higher yields and better quality
and increasing pressure of treating and preventing such diseases as H5N1 avian
flu, we also foresee strong market needs in other international markets
including East Asia and Southeast Asia. We plan to explore these
markets when the time is right.
Intellectual Property and
Product Lines
Our goal
is to build a platform to commercialize bio-technological research and
development results for applications in agriculture and environmental
protection. In this respect, we are working on developing cooperative
research relationships with several universities and institutions in
China. When our liquidity position improves, we also intend to
continue to acquire technologies to reduce research and development costs and
shorten commercialization cycles.
Bio-fertilizer
We have
developed six series of bio-fertilizer products with bacillus spp and/or
photosynthetic bacteria as core ingredients. Bacillus spp is one
species of bacteria that interacts with plants and promotes biological
processes. It is highly effective for promoting plant growth,
enhancing yield, improving quality and elevating
resistances. Photosynthetic bacteria are a group of green and purple
bacteria. Bacterial photosynthesis differs from green plant
photosynthesis in that bacterial photosynthesis occurs in an anaerobic
environment and does not produce oxygen. Photosynthetic bacteria can
help enhance the photosynthetic capacity of green plants by increasing the
utilization of sunlight. This helps keep the photosynthetic process
at a vigorous level, enhance the capacity of plants to transform inorganic
materials to organic products. It greatly boosts overall plant health
and the productivity of agricultural products.
4
Our bacillus bacteria based fertilizers are protected by
patents. On April 12, 2004, we entered into an agreement with China
Agricultural University (“CAU”) to acquire from the university Chinese patent
no. ZL 93101635.5 entitled “Highly Effective Composite Bacteria for Enhancing
Yield and the Related Methodology for Manufacturing.” The aggregate
purchase consideration under the agreement was $480,411, of which $60,411 was
paid in cash in 2004. For the balance of the consideration, we issued
1,000,000 shares of our common stock to CAU in September 2004, valued at $0.42
per share (aggregate value of $420,000) based on the market value on July 20,
2004, the date when the transfer of the patent was approved. Our
photosynthetic bacteria based fertilizers are protected by trade secret.
The
patent acquired from CAU covers six different species of bacillus which have
been tested as bio-fertilizers to enhance yield and plant health. The
production methods of the six species are also patented. The patent
will expire on February 9, 2013. There are no limitations under this
agreement on our exclusive use of the patent. Pursuant to our
agreement with CAU, the university agreed to provide research and technology
support services at no additional cost to us in the event we decide to use the
patent to produce commercial products. These research and technology
support services include: (1) furnishing faculty or graduate-level researchers
to help bacteria culturing, sampling, testing, trial production and production
formula adjustment; (2) providing production technology and procedures to turn
the products into powder form while keeping live required bacteria in the
products; (3) establishing quality standards and quality control systems; (4)
providing testing and research support for us to obtain necessary sale permits
from the Chinese government; and (5) cooperation in developing derivative
products.
We have
obtained five fertilizer registration certificates from the Chinese government -
four covering our bacillus bacteria fertilizer and one covering our
photosynthetic bacteria fertilizer. Some of our products contain
ingredients of both photosynthesis and bacillus bacteria. The five
registration certificates are: (1) Microorganism Microbial Inoculum Fertilizer
Registration Certificate issued by the PRC Ministry of Agriculture; (2)
Photosynthetic Bacteria Fertilizer Registration Certificate issued by the PRC
Ministry of Agriculture; (3) Amino Acid Foliar Fomular Fertilizer Registration
Certificate issued by the PRC Ministry of Agriculture; (4) Organic Fertilizer
Registration Certificate issued by Agriculture Department of Shandong Province;
and (5) Organic Matter-Decomposing Inoculants Registration Certificate issued by
the PRC Ministry of Agriculture on February 16, 2008. Protected by
these five fertilizer registration certificates and five trademarks under the
names of “KANGTAN” (Chinese translation name for Kiwa), “ZHIGUANGYOU,”
“PUGUANGFU,” “JINWA” and “KANGGUAN,” we have developed six series of
bio-fertilizer products with bacillus spp and/or photosynthetic bacteria as core
ingredients.
We also
obtained two fertilizer product licenses from the Vietnamese government in
November 2006, one is used for leaf fertilizer and the other for organic
fertilizer.
Bio-enhanced
Feed
We have
developed our own special concentrated and supportive feeds prescriptions,
mainly for fowl, fish and pigs. We add distilled materials from
animal blood, bacillus spp or other ingredients to standard livestock feed to
improve quality and function. Our feed products can enhance digestion
and inhibit disease in animals, in some circumstances functioning as a
substitute for antibiotic additives. Currently we have different feed
prescriptions for fowl, fish and swine at different growth stages.
5
The Company has engaged in bio-enhanced feed business through its
majority-owned subsidiary, Kiwa Tianjin. On December 22, 2009, Kiwa
Tianjin filed a lawsuit against Challenge Feed. In the lawsuit, Kiwa
Tianjin asserted that Challenge Feed unlawfully disposed of the assets held by
Kiwa Tianjin, such assets include (1) machinery and equipment and (2)
inventories.
As a
result, Kiwa Tianjin could no longer use its assets including machinery and
inventory in normal course of operation. As of December 31, 2009, the
Company has classified its bio-enhanced feed business through Kiwa Tianjin as
discontinued operations.
AF-01
Anti-viral Aerosol
AF-01
anti-viral aerosol is a broad-spectrum antiviral agent with potent inhibitory
and/or viricidal effects on a variety of RNA viruses found in fowl and other
animals, initially discovered and developed by the Institute of Medicinal
Biotechnology, Chinese Academy of Medical Sciences (“IMB”). Pursuant
to a related technical appraisal report certified by the PRC Ministry of Health,
the current owners of technology rights are IMB and its medium test center,
Jinan Kelongboao Bio-Tech Co., Ltd. (“JKB”). IMB designated JKB as
its custodian to apply and dispose all rights of the AF-01 technology on IMB’s
behalf. Pursuant to a technical appraisal report certified by the PRC
Ministry of Health, no adverse effects have been found of this agent, and it is
not irritating or erosive to the skin, mucous membrane or the eyes of the
recipient animal after swallowing or inhalation.
On May 8,
2006, we entered into a Technology Transfer Agreement with
JKB. Pursuant to the agreement, JKB agreed to transfer to us its
AF-01 anti-viral aerosol technology for veterinary medicine
applications. The AF-01 technology, which can be used to prevent and
cure virus-caused diseases by aerosol spray, is recognized by a technological
achievement appraisal certificate issued by relevant administration of the
Chinese government. Under the agreement JKB will facilitate
transferring of the technology by providing consulting services to us and to
cooperate with us in the development of an animal drug product for the
market.
We plan
to develop a commercialized product in the form of spray for applying in hen
houses and other animal holding facilities to prevent and cure virus-caused
diseases. Before marketing this product, we must acquire statutory
licenses in accordance with the rules and regulations of the PRC government. See
subsection entitled “The Company” under Business in Part I.
Kiwa-CAU
Research and Development Center
In July
2006, we established a new research center with CAU through our subsidiary, Kiwa
Shandong, which goes under the name, Kiwa-CAU Bio-Tech Research &
Development Center (the “Kiwa-CAU R&D Center”). Pursuant to an
agreement reached between CAU and Kiwa Shandong on November 14, 2006, Kiwa
Shandong agreed to contribute RMB 1 million (approximately $146,300) each year
to fund research at Kiwa-CAU R&D Center. Under the above
agreement, the Kiwa-CAU R&D Center is responsible for fulfilling the overall
research-and-development functions of Kiwa Shandong, including: (1) development
of new technologies and new products (which will be shared by Kiwa and CAU); (2)
subsequent perfection of existing product-related technologies; and (3) training
quality-control personnel and technicians and technical support for marketing
activities.
During
fiscal 2009, Kiwa-CAU R&D Center had concentrated on the following filed of
works:
|
1.
|
Screening
of growth-promoting bacteria;
|
|
2.
|
Screening
of bio-control bacteria;
|
|
3.
|
Screening
of environmental microbiology;
|
|
4.
|
Studies
on fermentation technology and related production
process;
|
|
5.
|
Analysis
of soil and fertilizer nutrients and fertilization program
development;
|
|
6.
|
Organic
Fertilizer Application Techniques;
and
|
|
7.
|
Technical
training and services.
|
6
During fiscal 2009, Kiwa-CAU R&D Center had successfully isolated
forty-one strains of endophytic bacillus from plants. A number of strains had
been observed to have the capability of boosting crop yield, dispelling chemical
pesticide residual from soil. These strains could be used for not
only developing new biological preparation but also environmental protection
preparation.
Market
Overview
Modern
agricultural practices largely rely on heavy use of chemical fertilizers,
pesticides and veterinary drugs that can cause tremendous harm to the
environment, soils and human health. Such practices have been under
increasing public scrutiny across the world, leading to increased consumer
demand for agricultural practices that are more environmentally
friendly. China has only 9.26% of the world’s arable land but needs
to feed over 1.3 billion people, or approximately 22.9% of the world’s
population. If the situation continues unchanged, the largest
population in the world could potentially face severe food and water shortages
and an increasingly polluted living environment. One solution to the
environmental problem is to develop environmentally friendly fertilizer,
veterinary drugs and animal feed.
China’s
agricultural production has steadily increased for more than 20 years due to
agricultural policy reform, improved agricultural technology and recent
government support programs, including price supports, export incentives, direct
payment and tax incentives. The following table shows the increase in
output of major agriculture products between 1970 and 2006:
|
Data item
|
2006
|
2005
|
2004
|
2003
|
2002
|
2001
|
2000
|
1999
|
1990
|
1980
|
1970
|
|||||||||||||||||||||||||||||||||
|
Corn
|
145,482 | 139,365 | 130,287 | 115,830 | 121,310 | 114,094 | 106,001 | 128,084 | 96,821 | 62,600 | 33,030 | |||||||||||||||||||||||||||||||||
|
Cotton
|
6,746 | 5,714 | 6,324 | 4,860 | 4,916 | 5,324 | 4,417 | 3,829 | 4,508 | 2,707 | 2,277 | |||||||||||||||||||||||||||||||||
|
Early
rice
|
31,868 | 31,873 | 32,217 | 29,484 | 30,288 | 34,002 | 37,623 | 40,973 | 51,649 | 49,140 | 37,410 | |||||||||||||||||||||||||||||||||
|
Late
rice
|
34,669 | 34,614 | 32,959 | 31,903 | 35,244 | 41,754 | 41,423 | 48,120 | 50,438 | 36,710 | 26,320 | |||||||||||||||||||||||||||||||||
|
Middle
rice
|
116,034 | 114,104 | 113,914 | 99,268 | 109,007 | 101,382 | 108,861 | 109,394 | 89,661 | 44,410 | 39,320 | |||||||||||||||||||||||||||||||||
|
Wheat
|
104,467 | 97,445 | 91,952 | 86,488 | 90,290 | 93,874 | 99,637 | 113,879 | 98,220 | 55,210 | 29,185 | |||||||||||||||||||||||||||||||||
(All in
thousand tons)
Source:
ERS-United States Department of Agriculture
According
to Organic Products Market in China 2006, a publication issued by USDA in June
2006, China has the potential to become a world power in the organic foods
industry. Home to one-fifth of the world’s population, a growing number of its
Chinese consumers are making more health-conscious purchases. The country
continues to attempt to increase organic export production as well as boost
domestic demand. With the growth of the international market for organic
products, some products in China are now being grown to international organic
standards for export with the help of third-party global certification groups.
Other products continue to target the domestic market with certification by
local or provincial bodies. In 2003, the total turnover for the “Green Foods”
market reached approximately $11.9 billion with $8.7 billion wholesale for the
domestic market. An initiative by the government to promote pure foods led to
development of an organic food market that continues to show growth potential.
Organic farms in China are beginning to resemble Western counterparts in farming
practices, certification and retail promotion.
In
response to the increasingly severe deterioration in food safety, environment
pollution, rural area stability and other challenges, the Chinese government
attaches high importance to the problems of farmers, rural areas and
agriculture. From January 1, 2006, the agricultural tax had been abolished. From
2004, the Central People’s Government of the PRC continuously issued “Number One
Document” regarding rural areas of China. The latest “Number One Document”
issued on January 30, 2008, contains wider-range of policies promoting
sustainable development of agriculture, for example, promoting income level of
billions of farmers, strengthening supervision of farm inputs and actively
developing green-food and organic food. In April 2007, the State Council of the
PRC promulgated the “‘Eleven-Five’ National Program on Boosting Food and Drug
Safety”, calling for strengthening agricultural input quality and safety
control; setting up demonstrational bases for agricultural products and food
based on “recycling economy” model; speeding up building bases for
Uncontaminated Food/Agricultural Products), GAP (Good Agricultural Practices),
Green Food and Organic Food. In July 2007, the State Council of the PRC
promulgated “Special Rules of the State Council on Strengthening the Supervision
and Management of the Safety of Food and Other Products” requiring compliance of
laws, administrative regulations and national compulsory standards for producers
when using agricultural input; producers and dealers of export products must
guarantee the compliance of importing country’s relevant standards or
contractual standards; any violators will face severe punishment. These policies
are in favor of our Company in the following three ways:
7
|
l
|
The
trend in government policy development is propitious to expedite more
agricultural products’ producers to accept ag-biotech input in a quicker
fashion;
|
|
l
|
Preferential
policies in rural areas increase farmers’ income level, therefore they can
afford to buy more of our products, thus increase our sales
volume.
|
|
l
|
Resulting
from certain changes in new policies’ procedures, we expect to receive
stronger support from relevant industrial associations and government
departments when promoting our
products.
|
Bio-fertilizer
Market
To
increase the overall crop yield, farmers in China use vast amounts of chemical
fertilizers. According to U.S. Department of Agriculture, the use of fertilizer
in China rocketed from 10,863,000 tons in 1979 to 47,662,000 tons in 2005,
underpinning a compound annual growth rate of 5.85%. Together with
the continuous growth of total fertilizer consumption, the way that Chinese
farmers apply fertilizer is also changing. From 1979 to 2005, the
percentage of Nitrogenous fertilizer application to total amount of fertilizer
consumption decreased gradually from 76% to 47%. In the meanwhile, the
percentage of Phosphate fertilizer and Potash fertilizer increased steadily.
Most importantly, in 1976, 100% of China’s fertilizer consumption was chemical
fertilizer (including Nitrogenous fertilizer, Phosphate fertilizer and Potash
fertilizer); while in 2005, the rate decreased to 73%. Other fertilizer,
including bio-fertilizer has been gradually accepted by Chinese farmers. (Source: ERS-United States
Department of Agriculture)
The
excessive use of chemical fertilizer in China is also reflected by the
China-to-U.S. rate of chemical fertilizer application. According to
data quoted from U.S. Department of Agriculture, Chinese farmers applied 2.05
times the nitrogen fertilizer and 1.8 times the phosphate fertilizer compared to
their U.S. counterparts in 2005.
Use of
chemical fertilizer in China is now higher than it has ever
been. This increase in use of chemical fertilizer has led to a series
of severe problems including degradation of the soil structure, natural
biodiversity and ecological system stability. Promoting the use of
bio-fertilizer together with chemical fertilizer is one of the solutions to
solve these problems.
In the
U.S. and European countries, the amount of bio-fertilizer consumption in the
agricultural production accounts for over 20% of the total amount of fertilizer
consumption. The output increased at the speed of 10% to 20% each year.
According to the statistics, total amount of bio-fertilizer production was 1.5
million tons, total sales volume about RMB4 billion Yuan in 2001, which accounts
for about 1% of total commercial fertilizer consumption. Analysis indicates that
bio-fertilizer will possess about 10% of market share in 2010, which is
forecasted to be about 14 million tons of market demands. Therefore, the market
potential of bio-fertilizer is immense.
Our
serial commercialized products, with bacillus and/or Photosynthesis Biological
Catalyst as core ingredients, capitalize on this market trend and we hope to
become one of the leaders in developing green technologies for productive, more
sustainable agriculture in China.
Our main
markets have so far been in China, mostly in Shandong (sown area 10,736,100
hectares, accounted for 6.9% of China; value of crop output RMB203,400 million
Yuan, 10.4% of China in 2005), Jiangsu (sown area 7,641,200 hectares, accounted
for 4.9% of China; value of crop output RMB129,110 million Yuan, 6.6% of China
in 2005), Zhejiang (sown area 2,837,900 hectares, accounted for 1.8% of China;
value of crop output RMB65,480 million Yuan, 3.3% of China in 2005), Hebei
Provinces (sown area 8,785,500 hectares, accounted for 5.7% of China; value of
crop output RMB125,800 million Yuan, 6.4% of China in 2005), Xinjiang Uygur
Autonomous Region (sown area 3,731,200 hectares, accounted for 2.4%; value of
crop output RMB59,580 million Yuan, 3.0% of China in 2005), and Northeast area
of China all these are the primary large agricultural provinces in China. (Source: ERS-United States
Department of Agriculture)
8
Virus-caused
diseases remain the most deadly category of animal disease in
clinic. They are not only spreading quickly but also claiming a high
fatality rate. The unique nature of virus reproduction makes it
difficult to find a cure for it in the laboratory. Virus-caused
diseases account for a large proportion of all animal infectious diseases; the
death rate is 36.8%, the second highest among all causes, second only to
bacteria. However, in China only a very small proportion of
veterinary drugs are anti-viral. The income breakdown of the Chinese
veterinary drug industry is as follows: antimicrobial agent 37%, medication
additive 21%, biological products 14%, insecticide-agent 14%, health-care
products 6%, environmental hygiene products 4%, and others 4%.
Virus can
be divided into two categories, one group is DNA virus and other group is RNA
virus. Our planned anti-viral aerosol product is based on AF-01
technology. It will be in the form of spray and be capable of
preventing/curing various RNA virus-caused diseases in various breading
farms. Bird-flu is caused by typical RNA virus, it is the first virus
caused animal disease that AF-01 will target.
In recent
years, it was reported that human being could be infected by H5N1 and sometimes
it could be fatal. According to the World Health Organization (the
“WHO”) to date, human cases have been reported in six countries, most of which
are in Asia: Cambodia, China, Indonesia, Thailand, Turkey, and
Vietnam. The first patients in the current outbreak, which were
reported from Vietnam, developed symptoms in December 2003 but were not
confirmed as H5N1 infection until January 11, 2004. Thailand reported
its first cases on January 23, 2004. The first case in Cambodia was
reported on February 2, 2005. The next country to report cases was
Indonesia, which confirmed its first infection on July 21,
2005. China’s first two cases were reported on November 16,
2005. Confirmation of the first cases in Turkey came on January 5,
2006, followed by the first reported case in Iraq on January 30,
2006. All human cases have coincided with outbreaks of highly
pathogenic H5N1 avian influenza in poultry. To date, Vietnam has been
the most severely affected country, with more than 90 cases.
The use
of appropriate antiseptics is an effective prevention method against avian
influenza. As indicated by our Technical Appraisal Report (No.
GuoWeiKeChengJianZi (2004) A0101) certified by the Ministry of Health of China,
our planned product with bio-active glycopeptides produced by actinomycetes as
the functioning element has been demonstrated to be an effective antiseptic to
prevent the spread of H5N1. Furthermore, we believe this product has competitive
differential compared with other existing chemical disinfectors. If we are able
to complete approval procedures to develop our intended anti-viral aerosol agent
product, we believe that it will have the potential to attract a significant
share of the Chinese market upon launching and benefit from large government
orders.
Competition
We have
two different product lines: (1) bio-fertilizer and (2) veterinary disinfectants
and drugs. The market condition and competition confronting us are
different and vary with respect to each of the three product lines.
9
Bio-fertilizer
According
to statistics, so far there are about 400 microbial fertilizer producers in
China, most of which are small-scale, workshop producing enterprises with
backward equipment and production processes and poor quality. Some of
the producers over-exaggerate product effectiveness, employ improper artifice
and even produce fake and shoddy products, all of which has caused losses to
farmers and lowered the reputation of bio-fertilizer.
Due to
the unique products that we offer and the very early stage of the bio-fertilizer
market in China, we believe there is limited direct competition for our products
in the Chinese marketplace. We believe that we have product
differentiation and cost advantages (cost to customer) that will enable us to be
more profitable than our competitors, in terms of profitability, for the
following reasons, among others:
|
l
|
Highly
effective in boosting crop yield and quality while being environmentally
friendly;
|
|
l
|
Lower
price point and higher return on investment to end users;
and
|
|
l
|
Complimentary
to existing use of chemical fertilizer which will help minimize switching
costs for end users.
|
In
addition, we face competition from large chemical fertilizer manufacturers in
China. These chemical fertilizer manufacturers have provided chemical
fertilizers to farmers in China for more than ten years and customers are more
accustomed to using their established products as compared to our
products.
AF-01
Anti-viral Aerosol
Our
planed AF-01 anti-viral aerosol belongs to the scope of bio-veterinary
drugs. According to the Ministry of Agriculture of PRC, approximately
1,700 veterinary drug manufacturers were awarded GMP qualifications as of
December 31, 2007. Few of these manufacturers have annual net sales
of over RMB100 million; some generate more than RMB50 million each year; while
most of these companies have annual net sales about RMB10 million. In
the meantime, some of the manufacturers remain small scale workshop-production
level.
AF-01
anti-viral aerosol is very much different from other ordinary veterinary
drugs. First of all, it is fundamentally a biological product; and
secondly it is an anti-viral biological product. The combination of
these two features has equipped AF-01 with distinct product differentiation and
competitive advantage. The income structure of Chinese veterinary
drug industry is as follows: antimicrobial agent 37%, medication additive 21%,
biological product 14%, insecticide-agent 14%, health-care product 6%,
environmental hygiene product 4%, and others 4%. From the perspective of income
structure, it can be seen that anti-viral veterinary drug holds a very small
proportion and that of biological product is as low as 14% (most of them are
vaccines); therefore our planned product will face limited competition once the
commercialized product has been developed.
One of
the most severe diseases that AF-01 anti-viral aerosol is against is avian
flu. According to the Ministry of Agriculture of PRC, there are nine
Chinese companies that are developing/distributing anti-avian flu vaccine, which
can be regarded as substitutes of our product. However, as one of the two ways
to prevent/cure avian flu, anti-viral aerosol is different from vaccine in first
these is no residues; and second there will not be any drug failure in case
virus variation. Thus we believe once our commercialized product has been
successfully developed, it will have a greater market potential and social value
than vaccines.
Other
potential competitors of our veterinary drugs product line also include some
veterinary disinfector manufacturers.
10
Raw Materials and
Suppliers
The key
raw materials used in manufacturing of our products are available from a wide
variety of supply sources. Historically, we have not experienced any
difficulties in procuring adequate quantities of raw materials for use in our
bio-fertilizer and biologically enhanced livestock feed production. We do not
have long-term agreements with our suppliers due to the availability of other
numerous suppliers that have the ability to supply our required raw materials to
us on fairly short notice. We typically place purchase orders when we need raw
material supplies.
Bio-fertilizer
The major
raw materials for our bio-fertilizer production can be divided into two
categories: (1) growth media such as sodium acetate, glucose and turf for
culturing bacillus spp. and (2) photosynthetic and bacillus bacteria, which are
the core ingredients for our finished products. Some other main ingredients
include urea, aminophenol, humus, diammonium phosphate, and dipotassium hydrogen
phosphate. Prior to the completion of our bacillus manufacturing facility
upgrade in Shandong, we had purchased semi-manufactured bacillus
goods.
Our top
two suppliers accounted for 85.7% and 6.4% of our net purchases for the fiscal
year ended December 31, 2009, respectively. No other single supplier accounted
for more than 10%.
Customers
Bio-fertilizer
With
respect to bio-fertilizer, we have a total of 34 customers as of December 31,
2009, of which two customers accounted for 15.9% and 7.4% of our net sales for
the fiscal year ended December 31, 2009, respectively. No other single customer
accounted for more than 7% of our revenues in this product line.
Seasonality
Bio-fertilizer
Our
operating results have been and are expected to continue to be subject to
seasonal trends. This trend is dependent on numerous factors, including the
markets in which we are operating in, growing seasons, climate, economic
conditions and numerous other factors beyond our control. Generally, we expect
the second and third quarters will be stronger than the first and fourth
quarters, primarily because the second and third quarters correspond with the
growing seasons in our primary markets in China. It is during those growing
seasons when application of our products by our customers would be most
beneficial and we therefore expect greater demand for our products during those
periods. There can be no assurance that these operating patterns will
occur.
11
Bio-enhanced Feed
Our
operating results for livestock feed products are also expected to continue to
be subject to seasonal factors. The main seasonal factors that would
influence our livestock feed product line operating results include farming
seasons, climate, traditional holidays, animal diseases as well as other factors
that the management cannot control. Generally speaking, our operating
results in this product line in the second and third quarters are expected be
better than those from the first and fourth quarters since fishponds in the
first and fourth quarters are frozen and pisciculturists stop fishing by
then. Consequently they do not purchase our fish feed products during
the first and fourth quarters of each year. Our livestock feed
factory does not produce fish feed during most of the first and fourth
quarters. There is no guarantee for those operating result circles
will repeat themselves and management would adjust our plan in accordance with
changes occurred.
AF-01
Anti-viral Aerosol
We have
not identified any patterns from our AF-01 anti-viral aerosol product business,
as it is still in the development stage.
Employees
We
currently employ 30 full-time employees in China and 1 in the United
States. We also have 43 seasonal employees in China. We
have full-time workers of 20 and management staff of 10.
Regulatory
Concerns
Our
production must comply with bio-fertilizer, livestock feed production and
testing procedure standards promulgated by the PRC Ministry of Agriculture or
local administrative authorities. We have complied with the
applicable PRC government standard production and testing
procedures. As for AF-01 anti-viral aerosol, we are now in the
process of applying for statutory licenses for the AF-01 technology in
accordance with relevant regulations (See subsection entitled “The Company—
AF-01 Anti-viral Aerosol” in this Item 1).
Environmental
Matters
Our two
manufacturing facilities, Kiwa Shandong and Kiwa Tianjin, have passed
environmental impact assessment by local environmental
authorities. Photosynthesis bacteria, bacillus ssp, and actinomycetes
are environmentally friendly and are not known to cause any environmental
problems.
Legal
Proceedings
On
December 22, 2009, Tianjin Kiwa filed a lawsuit against Challenge Feed in the
local court of Wuqing District, Tianjin, where Kiwa Tianjin is
domiciled.
In the
lawsuit, Kiwa Tianjin asserted that Challenge Feed unlawfully disposed of the
assets held by Kiwa Tianjin, such assets include:
(1)
Machinery and equipment. Challenge Feed entered into a settlement
agreement with one of its creditors, in accordance with which Challenge Feed
agreed to transfer title of the machinery and equipment, which had been assigned
to Kiwa Tianjin in 2006 in connection with the establishment of Kiwa Tianjin as
a joint venture between the Company and Challenge Feed, to repay Challenge
Feed’s debt. Challenge Feed did not obtain Kiwa Tianjin’s consent nor
inform Kiwa Tianjin of such transfer.
12
(2) Inventories. Kiwa Tianjin had a long standing
agreement to lease Challenge Feed’s factory facilities and warehouse for storage
of its inventory. Challenge Feed has disposed Kiwa Tianjin’s
inventories including raw materials, packages and finished goods stored in the
factory to repay Challenge Feed’s debt without any permission from Kiwa
Tianjin.
Kiwa
Tianjin is seeking damages against Challenge Feed in the amount of approximately
RMB 2.2 million in total. The local court is currently reviewing the
complaint and related documents filed with it.
|
Item
1A.
|
Risk
Factors
|
We
operate in a market environment that is difficult to predict and that involves
significant risks and uncertainties, many of which will be beyond our
control. The following risk factors and other information included in
this annual report should be carefully considered. The risks and
uncertainties described below are not the only ones we
face. Additional risks and uncertainties not presently known to us or
that we currently deem immaterial also may impair our business
operations. If any of the following risks occur, our business,
financial condition, operating results, and cash flows could be materially
adversely affected.
(1)
Risks Related to Our Business
We
have not yet generated any profits and if we do not become profitable or obtain
additional funding to implement our business plan our ability to continue as a
going concern is in doubt.
Overview of the Company’s
Financial Condition as of December 31, 2009
As of
December 31, 2009, the Company had accumulated deficit of $16,394,930, among
which, $3,714,529 and $3,632,188 were incurred during twelve months ended
December 31, 2009 and 2008, respectively.
As of
December 31, 2009, we had cash and cash equivalents of $28,765 and total current
assets of $169,982; at the same time, we had current liabilities of $6,553,102,
denoting current ratio of 0.03 and quick ratio of 0.005. At the end
of fiscal 2009, we also had long-term liabilities of $1,804,780.
On June
29, 2006, the Company entered into a securities purchase agreement with six
institutional investors (the “Purchasers”) for the issuance and sale of (1) 6%
secured convertible notes, due three years from the date of issuance, in the
aggregate principal amount of $2,450,000, convertible into shares of the
Company’s common stock (the “6% Notes”), and (2) warrants to purchase 12,250,000
shares of the Company’s common stock (the “Warrants”). As of December
31, 2009, the outstanding principal of 6% Notes was $1,518,171. On
June 29, 2009, the 6% Notes were due. The Company has informed the
Purchasers of its inability to repay the outstanding balance on the due
date. Therefore, the 6% Notes are in default.
To the
extent that we are unable to successfully raise the capital necessary to fund
our future cash requirements on a timely basis and under acceptable terms and
conditions, we will not have sufficient cash resources to maintain operations
and repay our liabilities, and may have to curtail or cease operations and
consider a formal or informal restructuring or reorganization.
Overview of the Company’s
Operating Results for the Twelve Months Ended December 31, 2009 and
2008
During
twelve months ended December 31, 2009 and 2008, our sales revenue from
continuing operations was $38,292 and $226,869, respectively. The
Company gross profit was $6,091 and $60,035, denoting a gross profit margin of
15.9% and 26.5%, respectively. During fiscal year of 2009 and 2008,
our loss from continuing operations was $3,388,109 and $3,425,657,
respectively. Net loss attributable to Kiwa Shareholders for both
periods was $3,714,529 and $3,632,188, respectively.
13
Overview of the Company’s
Cash flow Status for the Twelve Months Ended December 31, 2009 and
2008
During
fiscal year ended December 31, 2009 and 2008, our operating activities for
continuing operations used net cash of $566,232 and $622,611,
respectively. We also invested $7,320 and $48,111 in purchasing
property and equipment during both periods. Although our financing
activities for continuing operations provided net cash of $918,217 and $901,615
in the fiscal year of 2009 and 2008, we had cash of only $28,765 and $18,609 on
December 31, 2009 and 2008, respectively.
The Company’s Ability of
Raising New Finance
Continuous
losses and low share price has deteriorate the Company’s ability of raising new
finance. As of December 31, 2009, the closing price of our common
stock reported by on the OTC Bulletin Board was $0.003. The market
value of the Company was $1,200,000, which makes it very hard to arrange new
financing on equity financing basis. The Company’s obligations under
the 6% Notes and the Warrants are secured by a first priority security interest
in the Company’s intellectual property pursuant to an Intellectual Property
Security Agreement with the Purchasers, and by a first priority security
interest in all of the Company’s other assets pursuant to a Security Agreement
with the Purchasers. In addition, the Company’s Chief Executive
Officer has pledged all of his common stock of the Company as collateral
security for the Company’s obligations under the 6% Notes and the
Warrants. As a result, the Company does not have assets to secure the
obligations of new debt.
The 6%
Notes require the Company to procure the Purchaser’s consent to take certain
actions including paying dividends, repurchasing stock, incur debt, guaranty
obligations, merge or restructuring the Company, or selling significant
assets. Our ability of raising new finance is limited.
Kiwa Shandong’s Ability to
Continue as a Going Concern is in Doubt
Kiwa
Shandong is our wholly-owned subsidiary of engaging in researching, developing,
producing and marketing bio-fertilizer. However, since its inception
in 2002, Kiwa Shandong has not generated material revenue. Moreover,
Kiwa Shandong has never been profitable. As of December 31, 2009,
Kiwa Shandong has accumulated deficit of $3,263,469.
In June
2002, we entered into an agreement with Zoucheng Municipal Government granting
us the use of at least 15.7 acres in Shandong Province, China at no cost for 10
years to construct a manufacturing facility. Pursuant to relevant
China laws and regulations, we had paid tenure tax on quarterly basis at the
rate of approximately $1,660 per acre. However, from January 1, 2007,
China central government adopted a series of policies to strengthen land
management, including doubled tenure tax to $3,320 per acre. In
February 2008, the Ministry of Land and Resources of China issued “Controlling
Indexes of Construction Land Use for Industrial Projects,” which requires the
building coverage should not be less than 30%. Up to now, the current
situation in Kiwa Shandong does not meet this requirement. There is
no assurance that local authority would not reduce the acreage of land granted
to us to use at no cost.
On
December 31, 2009, we launched a complete test on the recoverability of our
long-lived assets in Kiwa Shandong. Based on our analysis, Kiwa
Shandong’s long-lived assets were impaired. Management is assessing
the usage of our long-lived assets in Kiwa Shandong; it is possible that we
would dispose some of our long-lived assets in the future.
Given the
tight cash flow status of the Company, we may have to curtail or cease
operations and consider a formal or informal restructuring or reorganization in
Kiwa Shandong. For example, we may consider reduce the acreage of
land we use in Kiwa Shandong to lower tax expenditure.
14
On
December 22, 2009, Kiwa Tianjin filed a lawsuit against Challenge Feed in the
local court of Wuqing District, Tianjin, where Kiwa Tianjin is
domiciled. In the lawsuit, Kiwa Tianjin asserted that Challenge Feed
unlawfully disposed of the assets held by Kiwa Tianjin, such assets
include:
(1)
Machinery and equipment. Challenge Feed entered into a settlement
agreement with one of its creditors, in accordance with which Challenge Feed
agreed to transfer title of the machinery and equipment, which had been assigned
to Kiwa Tianjin in 2006 in connection with the establishment of Kiwa Tianjin as
a joint venture between the Company and Challenge Feed, to repay Challenge
Feed’s debt. Challenge Feed did not obtain Kiwa Tianjin’s consent nor
inform Kiwa Tianjin of such transfer.
(2)
Inventories. Kiwa Tianjin had a long standing agreement to lease
Challenge Feed’s factory facilities and warehouse for storage of its
inventory. Challenge Feed has disposed of Kiwa Tianjin’s
inventories including raw materials, packages and finished goods stored in the
factory to repay Challenge Feed’s debt without any permission from Kiwa
Tianjin.
The local
court is currently reviewing the complaint and related documents filed with
it.
As a
result, Kiwa Tianjin could no longer use its assets including machinery and
inventory in normal course of operation. As of December 31, 2009, the
Company has classified its bio-enhanced feed business through Kiwa Tianjin as
discontinued operations.
In
Conclusion
The
Company’s ability to continue as a going concern is in doubt. We
expect to continue to have operating losses for the foreseeable future as we are
still in the process of exploring market, further research and product
tests. We will require additional capital to implement our business
plan and continue operating. To the extent that we are unable to
successfully raise the capital necessary to fund our future cash requirements on
a timely basis and under acceptable terms and conditions, we will not have
sufficient cash resources to maintain operations, and may have to curtail or
cease operations and consider a formal or informal restructuring or
reorganization.
Our
independent auditors have added an explanatory paragraph to their audit opinion
issued in connection with our financial statements for the latest seven fiscal
years, which states that the financial statements raise substantial doubt as to
our ability to continue as a going concern. Our ability to make
operations profitable or obtain additional funding will determine our ability to
continue as a going concern.
We
depend on a few customers for a significant portion of our revenue and are still
in the initial stage of market development.
We do not
have long-term contracts with any of our customers. Generally we sign
an annual distribution agreement with each customer and purchases in most cases
occur on an order-by-order basis. Relationships exist as long as
there is a perceived benefit to both parties. A decision by a major
customer, whether motivated by competitive considerations, financial
difficulties and economic conditions or otherwise, to decrease its purchases
from us or to change its manner of doing business with us, could adversely
affect our business and financial condition.
During
fiscal 2009, three customers accounted for 59.2% of our net sales in
bio-fertilizer product line. During fiscal 2009, three customers
accounted for 29.0% of our net sales in bio-enhanced feed product
line. The customer concentration in this production line has been
increasing. The loss of any of our significant customers would result
in a material reduction in our sales and results of operations.
15
We are
still in the initial stage of market development and need more time to construct
a robust customer base. There can be no assurances that we will be
able to retain these customers. Our inability to generate new
customers and retain old customers could negatively impact our business and our
ability to continue as a going concern.
Our
business is subject to seasonal fluctuations.
Our
operating results have been and are expected to continue to be subject to
seasonal trends. This trend is dependent on numerous factors,
including the markets in which we operate, growing seasons, climate, economic
conditions and numerous other factors beyond our control.
Our
operating results may fluctuate significantly, which may result in volatility or
have an adverse effect on the market price of our common stock.
We have
experienced, and expect to continue to experience, substantial variation in our
net sales and operating results from quarter to quarter. Our business
is subject to seasonal fluctuations due to growing seasons in different
markets. We believe the factors that influence this variability of
quarterly results include:
|
l
|
the
timing and size of orders from major
customers;
|
|
l
|
budgeting
and purchasing cycles of customers;
|
|
l
|
the
timing of enhancements to products or new products introduced by us or our
competitors;
|
|
l
|
changes
in pricing policies made by us, our competitors or suppliers, including
possible decreases in average selling prices of products in response to
competitive pressures;
|
|
l
|
fluctuations
in general economic conditions;
|
|
l
|
the
status of operating cash flow; and
|
|
l
|
natural
disasters and contagious animal
diseases.
|
We may
also choose to reduce prices or to increase spending in response to competition
or to pursue new market opportunities. Due to fluctuations in our
revenue and operating expenses, we believe that period-to-period comparisons of
our results of operations are not a good indication of our future
performance. It is possible that in some future quarter or quarters
our operating results will be below the expectations of securities analysts or
investors. In that case, our stock price could fluctuate
significantly or decline.
From
January 1, 2009 to December 31, 2009, the market close price for our common
stock as quoted on the OTC Bulletin Board has ranged from a low of $0.0003 to a
high of $0.008 per share. High volatility in the market price of our
common stock may result in lower prices for our common stock, making it more
difficult for us to obtain equity financing on terms and conditions which are
favorable to us, if at all. We expect to continue to incur losses in
the future as we develop and market our initial products. As a
result, we will be dependent on additional debt or equity financing to fund our
operations. If such financing is not available on terms which are
acceptable to us, we may have to delay development of new products and/or reduce
sales and marketing efforts for our existing products. Such actions
may have an adverse effect on our results of operations. In addition,
uncertainties with respect to our ability to raise additional capital would make
operational planning more difficult for management.
16
Revocation of our right to use
patents or other intellectual property rights could adversely impact the growth
of our business.
We
acquired a patent in April 2004 from CAU, entitled “Highly Effective Composite
Bacteria for Enhancing Yield and the Related Methodology for Manufacturing,”
issued by the China Intellectual Property Bureau. On May 8, 2006, we
entered into a technology transfer agreement with JKB with respect to the
technology transfer and related technical service for the AF-01 anti-viral
aerosol, which will become fully effective when we have finished paying the
first installment of consideration according to the payment schedule in the
contract. So far we have not yet fully paid the first
installment. If our rights under this patent and technology transfer
agreement are challenged or if we default on our obligations under applicable
Chinese regulatory requirements, our right to use these forms of intellectual
property could be revoked and we would no longer be permitted to use them in our
research, development, manufacturing and sales activities. Such a
revocation or default could have an adverse impact on the growth of our business
by reducing the introduction of new products, and consequently,
sales.
Our
success depends in part on our successful development and sale of products
currently in the research and development stage.
Some of
our product candidates are still in the research and development
stage. The successful development of new products is uncertain and
subject to a number of significant risks. Potential products that
appear to be promising at early stages of development may not reach the market
for a number of reasons, including but not limited to, the cost and time of
development. Potential products may be found to be ineffective or
cause harmful side effects, fail to receive necessary regulatory approvals, be
difficult to manufacture on a large scale or be uneconomical or fail to win
market acceptance. For example, before marketing of the planned
veterinary drug based on AF-01 technology, there are several tests, trial,
evaluation, government approval and other procedures that are
required. Our failure to successfully develop and sell new products
may delay or eliminate future acquisition plans and would most likely slow our
development. Our plans to introduce additional proprietary products
may not be realized as expected, if at all.
As above
mentioned, the China bio-fertilizer market is still in a very early stage and is
very fragmented with many potential customers, but with no single producer or
small group of producers dominating the market. To some extent,
however, we also face competition from large chemical fertilizer manufacturers
in China. These chemical fertilizer manufacturers have provided
chemical fertilizers to farmers in China for over twenty years and customers are
more accustomed to using their established products as compared with new
products. The livestock feed industry is fully developed in
China. We are new entrants to the livestock feed industry, and our
production capacity is small relative to that of the whole
industry.
We plan
to develop a commercialized product using AF-01 anti-viral aerosol
technology. We are now in the process of applying for prerequisite
statutory licenses. There can be no assurance that we can acquire
such prerequisite approvals and licenses, or how much time it will
take.
There can
be no assurance that any of our intended products will be successfully developed
or that we will achieve significant revenues from such products even if they are
successfully developed. Our success is dependent upon our ability to
develop and market our products on a timely basis. There can be no
assurance that we will be successful in developing or marketing such products or
taking advantage of the perceived demand for such products. In
addition, there can be no assurance that products or technologies developed by
others will not render our products or technologies non-competitive or
obsolete.
17
Failure to adequately expand to
address expanding market opportunities could have a material adverse effect on
our business and results of operations.
We
anticipate that a significant expansion of operations will be required to
address potential market opportunities. There can be no assurances
that we will expand our operations in a timely or sufficiently large manner to
capitalize on these market opportunities. The anticipated substantial
growth is expected to place a significant strain on our managerial, operational
and financial resources and systems. While management believes it
must implement, improve and effectively use our operational, management,
research and development, marketing, financial and employee training systems to
manage anticipated substantial growth, there can be no assurances that these
practices will be successful.
The
products we hope to develop based on AF-01 technology will depend on an
exclusive supply relationship for raw materials.
Pursuant
to our Technology Transfer Agreement with JKB, they will have the exclusive
right to supply us the raw material medicine for AF-01 anti-viral
aerosol. Although the exclusive supply relationship may help to
prevent new entrants from producing similar products, our ability to produce our
products in a timely manner will depend on JKB fulfilling its supply obligation
for the raw material. If we desired to produce raw material medicine
by ourselves, we would have to acquire additional technology and negotiate with
JKB and IMB. There can be no assurance that we can acquire the required
technology with an acceptable price. Consequently without JKB’s
cooperation and performance of its obligations, we may not be able to execute
our business plan on this project, even if we successfully acquire all
prerequisite certificates for producing and marketing this veterinary drug
product.
Our
success depends in part upon our ability to retain and recruit key
personnel.
Our
success is highly dependent upon the continued services of our executive
officers, key product development personnel and key scientific
personnel. Given the intense competition for qualified management and
product development personnel in our industry, the loss of the services of any
key management or product development personnel may significantly and
detrimentally affect our business and prospects. We maintain
employment agreements with all members of management or key
personnel. Pursuant to our joint agreement with CAU, it must make
available at least six R&D staff to join the Kiwa-CAU R&D Center, at
least three of whom must have professor or doctorate degrees, and at least two
who must have master degrees. There can be no assurance that we will
be able to retain these personnel, and it may be time-consuming and costly to
recruit qualified replacement personnel.
We
currently do not have sufficient revenues to support our business activities,
expect operating losses continue, and will require additional financing which we
may not be able to secure.
We
require substantial working capital to fund our business. In the
short term, we still need to continue building out our bio-fertilizer
manufacturing facility, adjust our product formula to improve product stability
and optimize our product offerings, expand our sales and marketing efforts in
China, expand our distribution base in China, maintain operation of Kiwa-CAU
R&D Center, introduce new veterinary drug products and acquire a small or
medium sized biotechnology company or a factory with GMP qualification for this
new product. In the long term, we plan to become a commercialization
platform for world-class biotechnological research and development results for
applications in agriculture, natural resources conservation and environment
protection, launch our products in the Southeast Asia, United States and other
markets, continue our introduction of new products, create formal strategic
alliances with selected United States companies to co-develop and/or co-market
products in the United States and China, and form an international biotechnology
research center in China for the research and development of agricultural,
environmental and medical applications.
18
During
fiscal year of 2009, our sales revenue decreased significantly as compared to
that of 2008, at the same time we are continuing to experience
losses. We currently do not have sufficient revenues to support our
business activities and we expect operating losses to continue. We
will require additional capital to fund our operations and finance our research
and development activities. Funding, whether from a public or private
offering of debt or equity, a bank loan or a collaborative agreement, may not be
available when needed or on favorable terms. Further, any significant
equity or debt financing will require us to give priority to holders of the 6%
secured convertible notes (“6% Notes”) under the terms of a securities purchase
agreement dated June 29, 2006, which may raise the difficulty level of
completing a financing. (For more details regarding the 6% Notes see Note 12 to
consolidated financial statements under Item 8, Part II.) If we are unable to
obtain necessary financing in the amounts and on terms deemed acceptable, we
will have to limit, delay, scale back or eliminate our research and development
activities or future operations. Any of the foregoing may adversely
affect our business and cause us to discontinue as a going concern.
The
risks associated with raising capital through collaborations and licensing
agreements could adversely affect our business.
We will
be required to raise additional capital to fund our operations and finance our
research and development activities through collaborative and/or licensing
agreements. Under these agreements, we may be subject to various
restrictive covenants which could significantly limit our operating and
financial flexibility and may limit our ability to respond to changes in our
business or competitive environment. If we are unable to obtain
necessary financing in the amounts and on terms deemed acceptable, we may have
to limit, delay, scale back or eliminate our research and development activities
or future operations. Any of the foregoing may adversely affect our
business.
Restrictions
on currency exchange may limit our ability to effectively receive and use our
revenue.
Since
most of our future revenues may be in the form of China Renminbi, any future
restrictions on currency exchanges may limit our ability to use revenue
generated in Renminbi to fund our business activities outside China or to make
dividend or other payments in U.S. Dollars. Although the Chinese
government introduced regulations since 1996 to allow greater convertibility of
Renminbi, for current account transactions significant restrictions still
remain, including primarily the restriction that foreign invested enterprises
may only buy, sell and/or remit foreign currencies at those banks authorized to
conduct foreign exchange business after providing valid commercial
documents. In addition, conversion of Renminbi for capital account
items, including direct investment and loans, is subject to governmental
approval in China, and companies are required to open and maintain separate
foreign exchange accounts for capital account items. We cannot be
certain that the Chinese regulatory authorities will not impose more stringent
restrictions on the convertibility of Renminbi, especially with respect to
foreign exchange transactions.
We may
also be subject to foreign exchange risk and foreign ownership
restrictions. The Chinese government is loosening its control on
foreign exchange transactions, and has steadily appreciated Renminbi relative to
the U.S. dollar since July 2005. However, there can be no assurance
that this policy will continue. More liberal foreign exchange
policies will reduce our foreign exchange risk by increasing the liquidity of
revenues generated in Renminbi. Fluctuations in the exchange rate of
Renminbi against the U.S. Dollar could adversely affect our results of
operations by affecting our reported earnings for any given
period. In addition, foreign ownership restrictions could also impact
our ability to expand our business through investment and acquisition
opportunities. If we are unable to pursue such strategic
opportunities due to foreign ownership regulations, the growth of our business
could be limited.
19
Changes in China’s political, social,
economic or legal systems could materially harm our business.
All of
our manufacturing and production as well as the majority of our sales occur in
China. Consequently, an investment in our common stock may be
adversely affected by the political, social and economic environment in
China. Under its current leadership, China has been pursuing economic
reform policies, including the encouragement of private economic activities and
greater economic decentralization. There can be no assurance,
however, that the Chinese government will continue to pursue such policies, that
such policies will be successful if pursued, or that such policies will not be
significantly altered from time to time.
Our
business and prospects are dependent upon agreements and regulatory approval
with various entities controlled by Chinese governmental
instrumentalities. Historically, our operations in China have
received relatively favorable treatment from these instrumentalities as a result
of the Chinese government’s policies of encouraging economic development and
innovation, especially in underdeveloped regions. However, our
operations and prospects would be materially and adversely affected by a change
in China’s economic policies, which could make it more difficult for us to
obtain necessary approvals from governmental authorities and to obtain economic
incentives from governmental authorities. In addition, if the Chinese
government elects not to honor certain contracts as a result of political
change, it might be difficult to enforce these contracts against such
governmental entities in China. In addition, the legal system of
China relating to foreign investments is both new and continually evolving, and
currently there can be no certainty as to the application of its laws and
regulations in particular instances.
For
example, in June 2002, we entered into an agreement with Zoucheng Municipal
Government granting us the use of at least 15.7 acres in Shandong Province,
China at no cost for 10 years to construct a manufacturing
facility. Pursuant to relevant China laws and regulations, we had
paid tenure tax on quarterly basis at the rate of approximately $1,660 per
acre. However, from January 1, 2007, China central government adopted
a series of policies to strengthen land management, including doubled tenure tax
to $3,320 per acre. In February 2008, the Ministry of Land and
Resources of China issued “Controlling Indexes of Construction Land Use for
Industrial Projects,” which requires the building coverage should not be less
than 30%. Up to now, the current situation in Kiwa Shandong does not
meet this requirement. The Company is also considering reduce the
acreage that we leased and return part of the land to local authority to lower
down taxes.
A
slow-down in the Chinese economy may adversely affect our growth and
profitability.
The
growth of the Chinese economy has been uneven across geographic regions and
economic sectors. There can be no assurance that growth of the
Chinese economy will be steady or that any recessionary conditions will not have
a negative effect on our business. To the extent that there is a
slow-down in the Chinese economy, the agricultural industry may be adversely
affected. Consequently, the growth and profitability of our
bio-fertilizer business and bio-enhanced feed business may drop
down. The financial tsunami has significantly slow down the growth of
world economy. There can be no assurance that Chinese economy and our
growth and profitability will not be affected.
Any
recurrence of SARS, avian influenza or another widespread public health problem,
could adversely affect our business and results of operations.
A renewed
outbreak of SARS, avian influenza, highly pathogenic blue-ear disease or another
widespread public health problem in China, where most of our revenue is derived,
could have a negative effect on our operations. Our operations may be
impacted by a number of health-related factors, including the following: (1)
quarantines or closures of some of our offices and factories which would
severely disrupt our operations, (2) the sickness or death of our key officers
and employees, (3) a general slowdown in the Chinese economy, especially rapid
decrease of stockbreeding
20
Any of the foregoing events or other unforeseen consequences of
public health problems could adversely affect our business and results of
operations.
Our
ability to generate revenues could suffer if the Chinese ag-biotechnology market
does not develop as anticipated.
The
agriculture-biotechnology market in China, the primary market in which we do
business, is in the early stages of development. While we believe the
market opportunity looks promising, we expect that the market will take several
years to develop. While it is difficult to project exactly how long
it will take to develop the ag-biotechnology industry in China, we anticipate
that it will take at least ten years to reach a level of development that is
similar to the current state of the industry in the United
States. Successful development of the ag-biotechnology market in
China depends on the following: (1) continuation of governmental and consumer
trends favoring the use of products and technologies designed to create
sustainable agriculture; (2) educating the Chinese agricultural community and
consumers about the uses of ag-biotechnology products; and (3) certain
institutional developments such as governmental agricultural subsidies designed
to promote the use of environmentally friendly ag-biotechnological
products.
There are
no assurances that these trends will continue, governmental subsidies will be
offered, or that the Chinese agricultural community and consumers will be
successfully educated about the uses of ag-biotechnology
products. The conduct of business in the ag-biotechnology market
involves high risks. There can be no assurances that the
ag-biotechnology market in China will develop sufficiently to facilitate our
profitable operation. While we believe that we will benefit from our
first-mover advantage in a growing market, existing competitors and new entrants
in the ag-biotechnology market are expected to create fierce competition in the
future as the market evolves. Competitors and new entrants may
introduce new products into the market that may detrimentally affect sales of
our existing products, and consequently our revenues. We intend to
fund operations through sales, debt and equity financings until such time as the
ag-biotechnology market in China is sufficiently developed to support our
profitable operation.
We
may not be able to adequately protect our intellectual property rights, and may
be exposed to infringement claims from third parties.
Our
success will depend in part on our ability to obtain patent protection for our
technology, to preserve our trade secrets and to operate without infringing on
the proprietary rights of third parties. We have several trademarks
registered in China, which will be protected by the trademark laws in China for
ten years and are renewable at the expiration of the initial ten-year
term. In addition, we acquired a China patent in 2004 from CAU
entitled “Highly Effective Composite Bacteria for Enhancing Yield and the
Related Methodology for Manufacturing,” issued by the China Intellectual
Property Bureau, which has a remaining term of five years, and entered into a
Technology Transfer Agreement with JKB on the technology transfer and related
technical service for the AF-01 technology.
We may
also file patents with the PRC Intellectual Property Bureau and/or the U.S.
Patent and Trademark Office as we deem appropriate, or buy other patents such as
above said anti-viral aerosol technologies. There can be no assurance
that the patents applied for will be reviewed in a timely manner, that any
additional patents will be issued or that any patents issued will afford
meaningful protection against competitors with similar technology or that any
patents issued will not be challenged by third parties. There also
can be no assurance that others will not independently develop similar
technologies, duplicate our technologies or design around our technologies
whether or not patented. There also can be no assurance that we will
have sufficient resources to maintain a patent infringement lawsuit should
anyone be found or believed to be infringing our patents. There also
can be no assurance that the technology ultimately used by us will be covered in
any additional patent applications that we may file. We do not
believe that our technology infringes on the patent rights of third
parties. However, there can be no assurance that certain aspects of
our technology will not be challenged by the holders of other patents or that we
will not be required to license or otherwise acquire from third parties the
right to use additional technology. The failure to overcome such
challenges or obtain such licenses or rights on acceptable terms could have a
material adverse affect on our results of operations and financial
condition.
21
The processes and know-how of importance to our technology are
dependent upon the skills, knowledge and experience of our technical personnel,
consultants and advisors and such skills, knowledge and experience are not
patentable. To help protect our rights, we require employees,
significant consultants and advisors with access to proprietary information to
enter into confidentiality and proprietary rights agreements. There
can be no assurance, however, that these agreements will provide adequate
protection for our trade secrets, know-how or proprietary information in the
event of any unauthorized use or disclosure. There can be no
assurance that we will be able to obtain a license for any technology that we
may require to conduct our business or that, if obtainable, such technology can
be licensed at a reasonable cost. The cost of obtaining and enforcing
patent protection and of protecting proprietary technology may involve a
substantial commitment of our resources. Any such commitment may
divert resources from other areas of our operations. We may be
required to license or sublicense certain technology or patents in order to
commence operations. There can be no assurance that we will be able
to obtain any necessary licenses or to do so on satisfactory
terms. In addition, we could incur substantial costs in defending
ourselves against suits brought by other parties for infringement of
intellectual property rights and there are no assurances that we will have the
resources to do so.
We
may become involved in intellectual property litigation, the defense of which
could adversely impact our business operations.
Currently
we have one patent in China (Patent Number ZL93 101635.5 and International
patent classification Number A01N 63/00), which covers six different species of
bacillus which have been tested as bio-fertilizers to enhance yield and plant
health as well as the production methods of the six species. The
patent will expire on February 19, 2013. Pursuant to our Technology
Transfer Agreement with JKB, we will acquire the AF-01 anti-viral aerosol
technology when we have fully paid the first installment of the purchase price
and other conditions to the contract have been fulfilled, such as issuance by
the PRC Ministry of Agriculture of a new medicine certificate in respect of the
technology.
While we
have not received any allegations, complaints or threats of litigation relating
to any intellectual property rights, we may, from time to time, become involved
in litigation regarding patent and other intellectual property
rights. From time to time, we may receive notices from third parties
of potential infringement and claims of potential
infringement. Defending these claims could be costly and time
consuming and would divert the attention of management and key personnel from
other business issues. The complexity of the technology involved and
the uncertainty of intellectual property litigation increase these
risks. Claims of intellectual property infringement also might
require us to enter into costly royalty or license
agreements. However, we may be unable to obtain royalty or license
agreements on terms acceptable to us, or at all. In addition, third parties may
attempt to appropriate the confidential information and proprietary technologies
and processes used in our business, which we may be unable to prevent and which
would harm the businesses and our prospects.
We
face technical risks associated with commercializing our technology which could
have a material adverse impact on our business results and
operations.
A key to
our future success is the ability to produce our planed animal flu disinfector,
livestock feed and bacillus series of products at lower costs than our
competitors. Although we are currently utilizing our proprietary
technology to produce such products at lower costs, our method for producing
such products on a commercial basis has only recently begun. Further,
although results from recent independent tests and our early production results
have been encouraging, the ability of our technology to commercially produce
such products at consistent levels is still being evaluated. There
can be no assurance that we will continue to be able to produce such products at
lower costs than our competitors, nor that our technology will be able to
commercially produce such products at consistent levels.
22
We
have limited business insurance coverage.
We do not
have any business liability insurance coverage for our operations. Any business
disruption, litigation or natural disaster might result in substantial costs and
diversion of resources.
If
we fail to maintain an effective system of internal controls, we may not be able
to accurately report our financial results. As a result, current and potential
stockholders could lose confidence in our financial reporting, which would harm
our business and the trading price of our stock.
As
a public company, we are subject to report our internal control structure and
procedures for financial reporting in our annual reports on Form 10-K, as a
requirement of Section 404 of the U.S. Sarbanes-Oxley Act of 2002 by the U.S.
Securities and Exchange Commission (the “SEC”). The report must
contain an assessment by management about the effectiveness of our internal
controls over financial reporting.
Our
management has concluded that our internal controls over our financial reporting
are ineffective, as required by Section 404 of the U.S. Sarbanes-Oxley Act, for
the fiscal year ending December 31, 2009. Any failure to implement
and maintain improvements in the controls over our financial reporting, or
difficulties encountered in the implementation of any improvements in our
controls, could cause us to fail to meet our reporting
obligations. Any failure to improve our internal controls to address
these identified weaknesses could also cause investors to lose confidence in our
reported financial information, which could have a negative impact on the
trading price of our stock.
(2)
Risk Related to Our Common Stock
If
an active trading market for our securities does not remain in existence, the
market price of our securities may decline and stockholders’ liquidity may be
reduced.
Our
common stock is quoted on the OTC Bulletin Board; however, trading volume is
very limited. We cannot guarantee that trading volumes to sustain a
regular trading market will ever develop. The OTC Bulletin Board is
an inter-dealer, over-the-counter market that provides significantly less
liquidity than the NASDAQ’s automated quotation system. Market prices
for our common stock will be influenced by a number of factors, including but
not limited to: (1) the issuance of new equity securities; (2) changes in
interest rates; (3) competitive developments, including announcements by
competitors of new products or services or significant contracts, acquisitions,
strategic partnerships, joint ventures or capital commitments; (4) variations in
quarterly operating results; (5) change in financial estimates by securities
analysts; (6) the depth and liquidity of the market for our common stock; (7)
investor perceptions of our company and the ag-biotechnology industry generally;
and (8) general economic and other conditions.
23
The
designation of our common stock as “penny stock” could impact the trading market
for our common stock due to broker-dealer requirements imposed by the
designation of our common stock as “penny stock.”
Our
common stock is a “penny stock” as defined in Rules 15g-2 through 15g-6
promulgated under Section 15(g) of the Securities Exchange Act of 1934, as
amended, as it meets the following definitions: (i) the stock trades at a price
less than $5.00 per share; (ii) it is not traded on a “recognized” national
exchange, or even if so, has a price less than $5.00 per share; and (iii) is
issued by a company with net tangible assets less than $2.0 million, if in
business more than a continuous three years, or with average revenues of less
than $6.0 million for the past three years. The principal result or
effect of being designated as a “penny stock” is that securities broker-dealers
cannot recommend the stock but must trade in it on an unsolicited
basis.
Section
15(g) of the Securities Exchange Act of 1934, as amended, and Rule 15g-2
promulgated thereunder by the Securities and Exchange Commission require
broker-dealers dealing in penny stocks to provide potential investors with a
document disclosing the risks of penny stocks and to obtain a manually signed
and dated written receipt of the document before effecting any transaction in a
penny stock for the investor’s account.
Potential
investors in our common stock are urged to obtain and read such disclosure
carefully before purchasing any shares that are deemed to be “penny
stock.” Moreover, Rule 15g-9 requires broker-dealers in penny stocks
to approve the account of any investor for transactions in such stocks before
selling any penny stock to that investor. This procedure requires the
broker-dealer to (i) obtain from the investor information concerning his or her
financial situation, investment experience and investment objectives; (ii)
reasonably determine, based on that information, that transactions in penny
stocks are suitable for the investor and that the investor has sufficient
knowledge and experience as to be reasonably capable of evaluating the risks of
penny stock transactions; (iii) provide the investor with a written statement
setting forth the basis on which the broker-dealer made the determination in
(ii) above; and (iv) receive a signed and dated copy of such statement from the
investor, confirming that it accurately reflects the investor’s financial
situation, investment experience and investment
objectives. Compliance with these requirements may make it more
difficult for holders of our common stock to resell their shares to third
parties or to otherwise dispose of them in the market or otherwise.
Provisions
in our charter and the corporate law of our state of incorporation could deter
or prevent an acquisition or change of control.
Provisions
of our certificate of incorporation may deter or prevent a change in control of
management. Specifically, our certificate of incorporation allows our
Board of Directors to issue 20,000,000 shares of preferred stock, in one or more
series and with such rights and preferences including voting rights, without
further stockholder approval. In the event that the Board of
Directors designates additional series of preferred stock with rights and
preferences, including super-majority voting rights, and issues such preferred
stock, the preferred stock could make our acquisition by means of a tender
offer, a proxy contest or otherwise, more difficult, and could also make the
removal of incumbent officers and directors more difficult. As a
result, these provisions may have an anti-takeover effect. The
preferred stock authorized in our certificate of incorporation may inhibit
changes of control.
In
addition, we are subject to the provisions of Section 203 of the Delaware
General Corporation Law. That section provides, with some exceptions,
that a Delaware corporation may not engage in any of a broad range of business
combinations with a person or affiliate, or associate of the person, who is an
“interested stockholder” for a period of three years from the date that the
person became an interested stockholder unless: (i) the transaction resulting in
a person becoming an interested stockholder, or the business combination, is
approved by the Board of Directors of the corporation before the person becomes
an interested stockholder; (ii) the interested stockholder acquires 85% or more
of the outstanding voting stock of the corporation in the same transaction that
makes it an interested stockholder, excluding shares owned by persons who are
both officers and directors of the corporation, and shares held by some employee
stock ownership plans; or (iii) on or after the date the person becomes an
interested stockholder, the business combination is approved by the
corporation’s Board of Directors and by the holders of at least 66 2/3% of the
corporation’s outstanding voting stock at an annual or special meeting,
excluding shares owned by the interested stockholder. An “interested
stockholder” is defined as any person that is (a) the owner of 15% or more of
the outstanding voting stock of the corporation or (b) an affiliate or associate
of the corporation and was the owner of 15% or more of the outstanding voting
stock of the corporation at any time within the three-year period immediately
prior to the date on which it is sought to be determined whether the person is
an interested stockholder.
24
These provisions could also limit the price that future investors
might be willing to pay in the future for our common stock. This
could have the effect of delaying, deferring or preventing a change in control
of our Company and/or a change in the members our Board of
Directors. The issuance of preferred stock could also effectively
limit or dilute the voting power of our stockholders. Accordingly,
such provisions of our certificate of incorporation, as amended, may discourage
or prevent an acquisition or disposition of our business that could otherwise be
in the best interest of our stockholders.
Investors
should not rely on an investment in our common stock for dividend income as we
do not intend to pay dividends in the foreseeable future.
We do not
anticipate paying any cash dividends on our common stock in the foreseeable
future. We intend to retain any earnings to finance the growth of our
business. We cannot assure you that we will ever pay cash
dividends. Therefore, investors should not rely on an investment in
our common stock if they require dividend income. The only income in
the foreseeable future such investors will receive from an investment in our
common stock will come from increases in the market price of our common
stock. There can be no assurances that the market price of our common
stock will increase or continue to increase, and such increases will most likely
be uncertain and unpredictable. Whether we pay any cash dividends in
the future will depend on the financial condition, results of operations and
other factors that the Board of Directors will consider.
It
may be difficult for investors to enforce a service of process or enforce
liabilities against us.
We are
incorporated in the State of Delaware, and our principal executive offices are
located in the State of California. However, substantially all our
fixed assets and operations are located in the PRC. In addition, some
of our directors and officers are Chinese citizens and residents. As
a result, it may be more difficult for investors or other third parties to
attach our assets in enforcement of a judgment against us or to enforce
liabilities and obligations against us in certain circumstances. It
may also be difficult to enforce service of process against directors and
officers in China.
Entering
into equity or debt financings could result in dilution to existing
stockholders.
We will
be required to raise additional capital to fund our operations and finance our
research and development activities through a public or private offering of debt
or equity securities. Any equity financing could result in dilution
to the existing stockholders as a direct result of our issuance of additional
shares of our capital stock. Debt financings will result in interest
expense and likely subject us to negative covenants that would limit our
operational flexibility, and if convertible into equity, could also dilute
then-existing stockholders.
25
For example, we issued $2,450,000 of convertible notes (6% Notes) in
2006, of which $931,829 of principal have been converted into 318,398,409 shares
and the balance of $1,518,171 may be converted into an estimated 1,167,823,796
shares of our common stock based on the average price of three lowest prices
within 20 trading days before December 31, 2009. We also have
outstanding 6% Note Warrants to purchase 12,250,000 shares of common
stock. The conversion ratio of the 6% Notes is based on the market
price of our stock at any given point in time. Consequently, the
number of shares of common stock issuable upon conversion of the outstanding 6%
Notes and certain of our other outstanding convertible notes will increase if
the market price of our stock declines. Such debt financings may
cause immediate and substantial dilution to our existing stockholders.
The
shares of common stock allocated for conversion of the 6% Notes are not adequate
and we are required to amend our certificate of incorporation to increase our
authorized shares of common stock. We may incur substantial costs in
connection therewith. Since the annual meeting of stockholders did
not approve an proposed amendment to our certificate of incorporation, we cannot
repay 6% Notes by issuing shares of common stocks.
Pursuant
to the securities purchase agreement in connection with the 6% Notes, we must
reserve for purposes of issuance a number of shares of common stock that is no
less than 110% of the number of shares of common stock issuable upon full
conversion of the 6% Notes based on the average conversion price of the 6% Notes
and full exercise of the 6% Note Warrants based on the average exercise price of
the 6% Note Warrants. Based on our current market price and the
potential decrease in our market price as a result of the issuance of shares
upon conversion of the 6% Notes, we have made a good faith estimate as to the
amount of shares of common stock that we are required to allocate for conversion
of the 6% Notes. The annual meeting of stockholders of the Company
for 2009 did not approve a proposed amendment of certificate of incorporation
increasing the authorized shares from 400,000,000 to
800,000,000. Given the fact that as of December 31, 2009, the Company
had issued 400,000,000 shares of common stock, the Company could not repay
outstanding 6% Notes by further issuing shares of common stock. As of
December 31, 2009, amount of authorized shares does not meet the requirements as
set forth by the security purchase agreement in connection with the 6%
Notes.
In the
future, we may have to further amend our certificate of incorporation to
increase the number of authorized common stock, which could incur substantial
costs.
Future
sales by our stockholders may negatively affect our stock price and our ability
to raise funds in new stock offerings.
Sales of
our common stock in the public market could lower the market price of our common
stock. Sales may also make it more difficult for us to sell equity
securities or equity-related securities in the future at a time and price that
our management deems acceptable or at all. As of December 31, 2009,
we had 400,000,000 shares of common stock outstanding, most of which we estimate
have been held more than two years and are freely tradable under Rule
144. In the Form SB-2 declared effective on October 30, 2006, we
registered up to 27,685,365 shares of common stock for resale, which may be sold
without restriction under securities laws. In November of 2007, the
SEC adopted significant amendments to Rule 144, pursuant to which holding period
of non-affiliates before resale of restricted shares of a reporting company has
been shortened to six months. The sale of these shares may adversely
affect the market price of our common stock.
The
sale of our stock under the Securities Purchase Agreement could encourage short
sales by third parties, which could contribute to the future decline of our
stock price.
In many
circumstances the provision of financing based on the distribution of
equity/convertible notes for companies that are quoted on the OTC Bulletin Board
has the potential to cause a significant downward pressure on the price of
common stock. Since the registration statement for this offering is
effective, the number of freely tradable shares will significantly increase,
thus there is a possibility that the balance of sell side pressure would
overwhelmingly exceed that of the buying side. As a consequence, the
price of shares will drop considerably. This is especially the case
if the shares being placed into the market exceed the market’s ability to take
up the increased stock or if we have not performed in such a manner to show that
the equity funds raised will be used to grow our business. Such an
event could place further downward pressure on the price of our common
stock.
26
During
fiscal 2009, the lowest trading price of our common stocks was
$0.0003. There is no assurance that the share price will not further
drop down.
If there
are significant short sales of stock, the price decline that would result from
this activity will cause the share price to decline more so which in turn may
cause long holders of the stock to sell their shares thereby contributing to
sales of stock in the market. If there is an imbalance on the sell side of the
market for the stock, the price will decline significantly and
quickly. It is not possible to predict if the circumstances exist
under which short sales could materialize or to what level our stock price could
decline. In some companies that have been subjected to short sales
the stock price has dropped to near zero.
|
Item
2.
|
Property
|
In June
2002, Kiwa Shandong entered into an agreement with Zoucheng Municipal Government
granting us the use of at least 15.7 acres in Shandong Province, China at no
cost for 10 years to construct a manufacturing facility. Under the
agreement, we have the option to pay a fee of approximately RMB 480,000
($70,297) per acre for the land use right at the expiration of the 10-year
period. We may not transfer or pledge the temporary land use
right. In the same agreement, we have also committed to invest
approximately $18 million to $24 million for developing the manufacturing and
research facilities in Zoucheng, Shandong Province. As of December
31, 2009, we had invested approximately $1.91 million in plant and equipment for
the project.
From
January 1, 2007, China central government adopted a series of policies to
strengthen land management, including doubled tenure tax to $3,320 per
acre. In February 2008, the Ministry of Land and Resources of China
issued “Controlling Indexes of Construction Land Use for Industrial Projects,”
which requires the building coverage should not be less than 30%. Up
to now, the current situation in Kiwa Shandong does not meet this requirement.
As a company operating ag-biotech business, the building coverage may differ
from that of typical manufacturers in other industries. However,
there is no assurance that local authorities would not take back part of the
land.
The core
ingredient of our bio-fertilizer products is bacillus
spp. Photosynthetic bacteria are one of the ingredients used in some
of our products. However, the upgrade was not fully completed due to
shortage of capital. The Company plans to finish upgrading bacillus
spp manufacturing facilities in 2010 if the required financing could be
successfully raised.
With the
formation of Kiwa Tianjin in July 2006, Challenge Feed, the minority
shareholder, invested machinery and equipment used in one of its two
bio-enhanced feed production lines at an agreed value of
$120,000. The Company has also entered into a lease agreement with
Challenge Feed to lease another concentrated feed product line for three
years. Under the lease agreement, we also lease Challenge
Feeds’s other facilities for three years commencing on August 1, 2006: (1) an
office building with floor area of approximately 800 square meters; (2)
storehouses with floor area approximately 2,500 square meters; and (3) two
workshops with floor area of approximately 1,200 square meters. The
total monthly rental is RMB 50,000 ($7,300). As of December 31, 2009,
the lease agreement has expired.
On
December 22, 2009, Kiwa Tianjin filed a lawsuit against Challenge Feed in the
local court of Wuqing District, Tianjin, where Kiwa Tianjin is
domiciled.
27
In the
lawsuit, Kiwa Tianjin asserted that Challenge Feed unlawfully disposed of the
assets held by Kiwa Tianjin, such assets include:
(1)
Machinery and equipment. Challenge Feed entered into a settlement
agreement with one of its creditors, in accordance with which Challenge Feed
agreed to transfer title of the machinery and equipment, which had been assigned
to Kiwa Tianjin in 2006 in connection with the establishment of Kiwa Tianjin as
a joint venture between the Company and Challenge Feed, to repay Challenge
Feed’s debt. Challenge Feed did not obtain Kiwa Tianjin’s consent nor
inform Kiwa Tianjin of such transfer.
(2)
Inventories. Kiwa Tianjin had a long standing agreement to lease
Challenge Feed’s factory facilities and warehouse for storage of its
inventory. Challenge Feed has disposed Kiwa Tianjin’s inventories
including raw materials, packages and finished goods stored in the factory to
repay Challenge Feed’s debt without any permission from Kiwa
Tianjin.
The local
court is currently reviewing the complaint and related documents filed with
it.
|
Item
3.
|
Legal
Proceedings
|
On
December 22, 2009, Kiwa Tianjin filed a lawsuit against Challenge Feed, the
Company’s 20% joint venture partner in Kiwa Tianjin, in the local court of
Wuqing District, Tianjin, where Kiwa Tianjin is domiciled.
In the
lawsuit, Kiwa Tianjin asserted that Challenge Feed unlawfully disposed of the
assets held by Kiwa Tianjin, such assets include:
(1)
Machinery and equipment. Challenge Feed entered into a settlement
agreement with one of its creditors, in accordance with which Challenge Feed
agreed to transfer title of the machinery and equipment, which had been assigned
to Kiwa Tianjin in 2006 in connection with the establishment of Kiwa Tianjin as
a joint venture between the Company and Challenge Feed, to repay Challenge
Feed’s debt. Challenge Feed did not obtain Kiwa Tianjin’s consent nor
inform Kiwa Tianjin of such transfer.
(2)
Inventories. Kiwa Tianjin had a long standing agreement to lease
Challenge Feed’s factory facilities and warehouse for storage of its
inventory. Challenge Feed has disposed Kiwa Tianjin’s inventories
including raw materials, packages and finished goods stored in the factory to
repay Challenge Feed’s debt without any permission from Kiwa
Tianjin.
The local
court is currently reviewing the complaint and related documents filed with
it.
|
Item
4.
|
Submission
of Matters to a Vote of Security
Holders
|
The
Company hosted annual stockholders’ meeting of 2009 on December 28,
2009.
Only
stockholders of record at the close of business on August 24, 2009 were entitled
to vote at the meeting. As of August 24, 2009, number of shares of
common stock issued and outstanding and entitled to vote was
400,000,000. On December 28, 2009, 215,483,661 shares of common
stock, representing 53.9% of total number of shares entitled to vote, voted at
the meeting in person or through proxies.
28
At our
annual meeting held on December 28, 2009, five nominees for director have been
elected to serve a one-year term on the Board of Directors set to expire at the
2010 annual meeting of stockholders and until their respective successors are
elected and qualified. Directors elected are Mr. Wei Li, Mr. Lianjun
Luo, Mr. Xucheng Hu, Mr. Yunlong Zhang and Prof. Qi Wang.
The
following table summarizes voting results of electing members of Board of
Directors.
|
FOR
|
WITHHOLD
|
FOR %
|
||||||||||
|
Wei
Li
|
214,736,363 | 747,298 | 99.7 | % | ||||||||
|
Xucheng
Hu
|
214,728,072 | 755,589 | 99.6 | % | ||||||||
|
Lianjun
Luo
|
214,696,168 | 787,493 | 99.6 | % | ||||||||
|
Yunlong
Zhang
|
214,686,420 | 797,241 | 99.6 | % | ||||||||
|
Qi
Wang
|
214,696,168 | 787,493 | 99.6 | % | ||||||||
The
annual meeting also ratified the appointment of AGCA, Inc. as the Company’s
independent auditor for fiscal 2009.
|
FOR
|
WITHHOLD
|
ABSTAINED
|
||||||||||
|
TOTAL
|
194,520,839 | 2,439,092 | 18,523,730 | |||||||||
The
annual meeting of stockholders did not approve of changing our certificate of
incorporation by increasing the number of authorized common stocks from
400,000,000 to 800,000,000.
|
|
FOR
|
WITHHOLD
|
ABSTAINED
|
|||||||||
|
TOTAL
|
91,956,551 | 74,514,400 | 49,012,710 | |||||||||
29
Part
II
|
Item
5.
|
Market
for Registrants’ Common Equity, Related Stockholder Matters and Issuer
Purchasers of Equity Securities
|
Market
Information
The
Company’s common stock has been quoted on the OTC Bulletin Board of the NASD
under the symbol “KWBT.OB” since March 30, 2004, and was quoted under the symbol
“TTGM.OB” prior to the merger in March 2004. The merger transaction
is described in “The Company” under Item 1- Business. During fiscal
2009, the market price for our common stock has ranged from $0.0003 to
$0.008.
The
following table sets forth the high and low bid quotations per share of our
common stock as reported on the OTC Bulletin Board for the periods
indicated. The high and low bid quotations reflect inter-dealer
prices, without retail mark-up, mark-down or commission and may not necessarily
represent actual transactions.
|
Fiscal
Year 2009
|
High
|
Low
|
||||||
|
First
Quarter
|
$ | 0.008 | $ | 0.0005 | ||||
|
Second
Quarter
|
$ | 0.0062 | $ | 0.0003 | ||||
|
Third
Quarter
|
$ | 0.0055 | $ | 0.0011 | ||||
|
Fourth
Quarter
|
$ | 0.0075 | $ | 0.0021 | ||||
|
Fiscal
Year 2008
|
High
|
Low
|
||||||
|
First
Quarter
|
$ | 0.21 | $ | 0.10 | ||||
|
Second
Quarter
|
$ | 0.115 | $ | 0.058 | ||||
|
Third
Quarter
|
$ | 0.11 | $ | 0.0125 | ||||
|
Fourth
Quarter
|
$ | 0.048 | $ | 0.0009 | ||||
Holders
As of
March 20, 2010, there were approximately 428 shareholders of record of our
common shares.
Dividend
Policy
We have
not paid any dividends on our common shares since our inception and do not
anticipate that dividends will be paid at any time in the immediate
future.
Equity Compensation Plan
Information
The
information required by Item 5 regarding securities authorized for issuance
under equity compensation plans is included in Item 12 of this
report.
30
|
Item
6.
|
Selected
Financial Data
|
Not
required.
|
Item
7.
|
Management’s
Discussion and Analysis of Financial Condition and Results of
Operation
|
This
Annual Report on Form 10-K for the fiscal year ended December 31, 2009 contains
“forward-looking” statements within the meaning of Section 21E of the Securities
and Exchange Act of 1934, as amended, including statements that include the
words “believes,” “expects,” “anticipates,” or similar
expressions. These forward-looking statements include, among others,
statements concerning our expectations regarding our working capital
requirements, financing requirements, business, growth prospects, competition
and results of operations, and other statements of expectations, beliefs, future
plans and strategies, anticipated events or trends, and similar expressions
concerning matters that are not historical facts. The forward-looking
statements in this Annual Report on Form 10-K for the fiscal year ended December
31, 2009 involve known and unknown risks, uncertainties and other factors
(described in “Business-Risk Factors” under Item 1) that could cause our actual
results, performance or achievements to differ materially from those expressed
in or implied by the forward-looking statements contained herein.
Overview
The
Company took its present corporate form in March 2004 when the shareholders of
Tintic Gold Mining Company, a Utah public corporation (“Tintic”), entered into a
share exchange transaction with the shareholders of Kiwa BVI, a privately-held
British Virgin Islands corporation that left the shareholders of Kiwa BVI owning
a majority of Tintic and Kiwa BVI a wholly-owned subsidiary of Tintic, See
“Business - The Company” under Item 1. For accounting purposes this
transaction was treated as an acquisition of Tintic Gold Mining Company by Kiwa
BVI in the form of a reverse triangular merger and a recapitalization of Kiwa
BVI and its wholly owned subsidiary, Kiwa Shandong. On July 21, 2004,
we completed our reincorporation in the State of Delaware.
We have
established two subsidiaries in China: (1) Kiwa Shandong in 2002, a wholly-owned
subsidiary, and (2) Kiwa Tianjin in July 2006, of which we hold 80%
equity. At the end of 2009, Kiwa Tianjin could no longer use its
assets including machinery and inventory in normal course of
operation. The Company has classified the bio-enhanced feed business
as discontinued operations. Our company chart is presented and our
businesses, including bio-fertilizer, fertilizer trade and AF-01 anti-viral
aerosol, are described in detail in “Business - The Company” under Item
I.
We
generated approximately $38,292 and $226,869 in revenue from continuing
operations in fiscal years 2009 and 2008, respectively, reflecting a decrease of
83.1%. We incurred a net loss of $3,388,109 and $3,425,657 from
continuing operations and net loss of $$392,512 and $258,164 from discontinued
operations during fisacal 2009 and 2008, respectively.
Due to
our limited revenues from sales and continuous losses, we have relied on the
proceeds from the sale of our equity securities and loans from both unrelated
and related parties to provide the resources necessary to fund the development
of our business plan and operations. Our financing activities of
continuing operations generated $918,217 and $901,615 net cash inflow in total
during the twelve months ended December 31, 2009 and 2008,
respectively. These funds are insufficient to execute our business
plan as currently contemplated, which may result in the risks described in “Risk
Factors” under Item 1-Business.
31
Going
Concern
Our
consolidated financial statements have been prepared assuming that we will
continue as a going concern, which contemplates the realization of assets and
the satisfaction of liabilities in the normal course of business. The
carrying amounts of assets and liabilities presented in the consolidated
financial statements do not purport to represent the realizable or settlement
values.
Overview of the Company’s
Financial Condition as of December 31, 2009
As of
December 31, 2009, the Company had accumulated deficit of $16,394,930, among
which, $3,714,529 and $3,632,188 were incurred during twelve months ended
December 31, 2009 and 2008, respectively.
As of
December 31, 2009, we had cash and cash equivalents of $28,765 and total current
assets of $169,982; at the same time, we had current liabilities of $6,553,102,
denoting current ratio of 0.03 and quick ratio of 0.005. At the end
of fiscal 2009, we also had long-term liabilities of $1,804,780.
On June
29, 2006, the Company entered into a securities purchase agreement with six
institutional investors for the issuance and sale of (1) 6% secured convertible
notes, due three years from the date of issuance, in the aggregate principal
amount of $2,450,000, convertible into shares of the Company’s common stock, and
(2) warrants to purchase 12,250,000 shares of the Company’s common
stock. As of December 31, 2009, the outstanding principal of 6% Notes
was $1,518,171. On June 29, 2009, the 6% Notes were
due. The Company has informed the Purchasers of its inability to
repay the outstanding balance on the due date. Therefore, the 6%
Notes are in default.
To the
extent that we are unable to successfully raise the capital necessary to fund
our future cash requirements on a timely basis and under acceptable terms and
conditions, we will not have sufficient cash resources to maintain operations
and repay our liabilities, and may have to curtail or cease operations and
consider a formal or informal restructuring or reorganization.
Overview of the Company’s
Operating Results for the Twelve Months Ended December 31, 2009 and
2008
During
twelve months ended December 31, 2009 and 2008, our sales revenue was $38,292
and $226,869, respectively. The Company gross profit was $6,091 and
$60,035, denoting a gross profit margin of 15.9% and 26.5%,
respectively. During fiscal years of 2009 and 2008, our operating
loss was $2,073,310 and $1,839,450. Net loss attributable to Kiwa
shareholders for both periods was $3,714,529 and $3,632,188,
respectively.
Overview of the Company’s
Cash flow Status for the Twelve Months Ended December 31, 2009 and
2008
During
fiscal year ended December 31, 2009 and 2008, our operating activities of
continuing operations used net cash of $566,232 and $622,611,
respectively. We also invested $7,320 and $48,111 in purchasing
property and equipment during both periods. Our financing activities
of continuing operations provided net cash of $918,217 and $901,615 in the
fiscal year of 2009 and 2008. Our operating activities of
discontinued operations used net cash of $326,433 and 212,136 for fiscal 2009
and 2008, respectively. Our investing activities of discontinued
activities generated nil and $5,863 during the same period. We had
cash of only $28,765 and $18,609 on December 31, 2009 and 2008,
respectively.
The Company’s Ability of
Raising New Finance
Continuous
losses and low share price has deteriorate the Company’s ability of raising new
finance. As of December 31, 2009, the closing price of our common
stock reported by on the OTC Bulletin Board was $0.003. The market
value of the Company was $1,200,000, which makes it very hard to arrange new
financing on equity financing basis. The Company’s obligations under
the 6% Notes and the Warrants are secured by a first priority security interest
in the Company’s intellectual property pursuant to an Intellectual Property
Security Agreement with the Purchasers, and by a first priority security
interest in all of the Company’s other assets pursuant to a Security Agreement
with the Purchasers. In addition, the Company’s Chief Executive
Officer has pledged all of his common stock of the Company as collateral
security for the Company’s obligations under the 6% Notes and the
Warrants. As a result, the Company does not have assets to secure the
obligations of new debt.
32
The 6%
Notes require the Company to procure the Purchaser’s consent to take certain
actions including to pay dividends, repurchasing stock, incur debt, guaranty
obligations, merge or restructuring the Company, or selling significant
assets. Our ability of raising new finance is limited.
Kiwa Shandong’s Ability to
Continue as a Going Concern is in Doubt
Kiwa
Shandong is our wholly-owned subsidiary of engaging in researching, developing,
producing and marketing bio-fertilizer. However, since its inception
in 2002, Kiwa Shandong has not generated material revenue. Moreover,
Kiwa Shandong has never been profitable. As of December 31, 2009,
Kiwa Shandong has accumulated deficit of $3,263,469.
In June
2002, we entered into an agreement with Zoucheng Municipal Government granting
us the use of at least 15.7 acres in Shandong Province, China at no cost for 10
years to construct a manufacturing facility. Pursuant to relevant
China laws and regulations, we had paid tenure tax on quarterly basis at the
rate of approximately $1,660 per acre. However, from January 1, 2007,
China central government adopted a series of policies to strengthen land
management, including doubled tenure tax to $3,320 per acre. In
February 2008, the Ministry of Land and Resources of China issued “Controlling
Indexes of Construction Land Use for Industrial Projects,” which requires the
building coverage should not be less than 30%. Up to now, the current
situation in Kiwa Shandong does not meet this requirement. There is
no assurance that local authority would not reduce the acreage of land granted
to us to use at no cost.
On
December 31, 2009, we launched a complete test on the recoverability of our
long-lived assets in Kiwa Shandong. Based on our analysis, Kiwa
Shandong’s long-lived assets were impaired. Management is assessing
the usage of our long-lived assets in Kiwa Shandong; it is possible that we
would dispose some of our long-lived assets in the future.
Given the
tight cash flow status of the Company, we may have to curtail or cease
operations and consider a formal or informal restructuring or reorganization in
Kiwa Shandong. For example, we may consider reduce the acreage of
land we use in Kiwa Shandong to lower tax expenditure.
Kiwa Tianjin’s Bio-enhanced
Feed Business has been Classified as Discontinued Operations
On
December 22, 2009, Kiwa Tianjin filed a lawsuit against Challenge Feed in the
local court of Wuqing District, Tianjin, where Kiwa Tianjin is
domiciled. In the lawsuit, Kiwa Tianjin asserted that Challenge Feed
unlawfully disposed of the assets held by Kiwa Tianjin, such assets
include:
(1)
Machinery and equipment. Challenge Feed entered into a settlement
agreement with one of its creditors, in accordance with which Challenge Feed
agreed to transfer title of the machinery and equipment, which had been assigned
to Kiwa Tianjin in 2006 in connection with the establishment of Kiwa Tianjin as
a joint venture between the Company and Challenge Feed, to repay Challenge
Feed’s debt. Challenge Feed did not obtain Kiwa Tianjin’s consent nor
inform Kiwa Tianjin of such transfer.
(2)
Inventories. Kiwa Tianjin had a long standing agreement to lease
Challenge Feed’s factory facilities and warehouse for storage of its
inventory. Challenge Feed has disposed Kiwa Tianjin’s inventories
including raw materials, packages and finished goods stored in the factory to
repay Challenge Feed’s debt without any permission from Kiwa
Tianjin.
Kiwa
Tianjin is seeking damages against Challenge Feed in the amount of approximately
RMB 2.2 million in total. The local court is currently reviewing the
complaint and related documents filed with it.
33
Taking
into consideration of the fact that Kiwa Tianjin will not be able to operate
normally with its own machinery and equipment in the factory leased from
Challenge Feed, the Company has classified the bio-enhanced feed business as
discontinued operations.
In
Conclusion
The
Company’s ability to continue as a going concern is in doubt. We
expect to continue to have operating losses for the foreseeable future as we are
still in the process of exploring market, further research and product
tests. We will require additional capital to implement our business
plan and continue operating. To the extent that we are unable to
successfully raise the capital necessary to fund our future cash requirements on
a timely basis and under acceptable terms and conditions, we will not have
sufficient cash resources to maintain operations, and may have to curtail or
cease operations and consider a formal or informal restructuring or
reorganization.
Our
independent auditors have added an explanatory paragraph to their audit opinion
issued in connection with our financial statements for the latest seven fiscal
years, which states that the financial statements raise substantial doubt as to
our ability to continue as a going concern. Our ability to make
operations profitable or obtain additional funding will determine our ability to
continue as a going concern.
Trends and Uncertainties in
Regulation and Government Policy in China
Foreign
Exchange Policy Changes
China is
considering allowing its currency to be freely exchangeable for other major
currencies. This change will result in greater liquidity for revenues
generated in Renminbi (“RMB”). We would benefit by having easier
access to and greater flexibility with capital generated in and held in the form
of RMB. The majority of our assets are located in China and most of
our earnings are currently generated in China, and are therefore denominated in
RMB. Changes in the RMB-U.S. Dollar exchange rate will impact our reported
results of operations and financial condition. In the event that RMB appreciates
over the next year as compared to the U.S. Dollar, our earnings will benefit
from the appreciation of the RMB. However, if we have to use U.S.
Dollars to invest in our Chinese operations, we will suffer from the
depreciation of U.S. Dollars against the RMB. On the other hand, if
the value of the RMB were to depreciate compared to the U.S. Dollar, then our
reported earnings and financial condition would be adversely affected when
converted to U.S. Dollars.
On July
21, 2005, the People’s Bank of China announced it would appreciate the RMB,
increasing the RMB-U.S. Dollar exchange rate from approximately US$1.00 =
RMB8.28 to approximately US$1.00 = RMB8.11. So far the trend of such
appreciation continues; the exchange rate of U.S. Dollar against RMB on December
31, 2009 was US$1.00 = RMB6.8282.
Critical Accounting Policies
and Estimates
We
prepared our consolidated financial statements in accordance with accounting
principles generally accepted in the United States of America. The
preparation of these financial statements requires the use of estimates and
assumptions that affect the reported amounts of assets and liabilities and the
disclosure of contingent assets and liabilities at the date of the financial
statements and the reported amount of revenues and expenses during the reporting
period. Management periodically evaluates the estimates and judgments
made. Management bases its estimates and judgments on historical
experience and on various factors that are believed to be reasonable under
current circumstances. Actual results may differ from these estimates
as a result of different assumptions or conditions.
34
The following critical accounting policies affect the more
significant judgments and estimates used in the preparation of our consolidated
financial statements. In addition, you should refer to our
accompanying audited balance sheets as of December 31, 2009 and 2008, and the
audited statements of operations, equity movement and cash flows for the fiscal
years ended December 31, 2009 and 2008, and the related notes thereto, for
further discussion of our accounting policies.
Accounts
Receivables
The
Company performs ongoing credit evaluations of its customers and establishes an
allowance for doubtful accounts when amounts are not considered fully
collectable. According to the Company’s credit policy, the Company
generally provides 100% bad debt provision for the amounts outstanding over 365
days after the deduction of the amount subsequently settled after the balance
sheet date, which management believes is consistent with industry practice in
the China region.
Terms of
our sales vary from cash on delivery to a credit term up to three to twelve
months. Ordinarily, we require our customers to pay between 20% and
60% of the purchase price of an order placed, depending on the results of our
credit investigations, prior to shipment. The remaining balance is
due within twelve months, unless other terms are approved by
management. As stated in the “Business - Risk Factors” under Item 1,
the agriculture-biotechnology market in China is in the early stages of
development and we are still in the process of exploring the new
market. We may also distribute our bio-products to special
wholesalers with favorable payment terms with a focus on the
future. We maintain a policy that all sales are final and we do not
allow returns. However, in the event of defective products, we may
allow customers to exchange the defective products for new products within the
quality guarantee period. In the event of any exchange, the customers
pay all transportation expenses.
Inventories
Inventories
are stated at the lower of cost, determined on the weighted average method, and
net realizable value. Work in progress and finished goods are
composed of direct material, direct labor and a portion of manufacturing
overhead. Net realizable value is the estimated selling price in the
ordinary course of business, less estimated costs to complete and
dispose.
Impairment of Long-Lived
Assets
Our
long-lived assets consist of property, equipment and intangible
assets. As of December 31, 2009, the net value of property and
equipment and intangible assets was $255,483 and nil, respectively, which
represented approximately 58.0% and 0% of our total assets,
respectively.
We
periodically evaluate our investment in long-lived assets, including property
and equipment, for recoverability whenever events or changes in circumstances
indicate the net carrying amount may not be recoverable. Our
judgments regarding potential impairment are based on legal factors, market
conditions and operational performance indicators, among others. In
assessing the impairment of property and equipment, we make assumptions
regarding the estimated future cash flows and other factors to determine the
fair value of the respective assets.
Based on
our analysis, we charged $804,780 and $639,492 as loss from impairment of
long-lived assets during twelve months ended December 31, 2009 and 2008,
respectively.
35
Fair value of warrants and
options
We have
adopted ASC Topic 815, “Accounting for Derivative Instruments and Hedging
Activities” to recognize warrants relating to loans and warrants issued to
consultants as compensation as derivative instruments in our consolidated
financial statements.
We also
adopt ASC Topic 718 “Share Based Payment” to recognize options granted to
employees as derivative instruments in our consolidated financial
statements.
We
calculate fair value of the warrants and options with Black-Schole
Model.
Revenue
Recognition
We
recognize revenue for our products in accordance with Securities and Exchange
Commission Staff Accounting Bulletin (“SAB”) No. 101, “Revenue Recognition in
Financial Statements,” as amended by SAB No. 104, “Revenue Recognition.” Sales
represent the invoiced value of goods, net of value added tax, supplied to
customers, and are recognized upon delivery of goods and passage of
title.
Income
Taxes
The
Company accounts for income taxes under the provisions of SFAS No. 109,
“Accounting for Income Taxes” (codified to FASB ASC Topic 740, “Income Taxes”),
which requires recognition of deferred tax assets and liabilities for the
expected future tax consequences of events that have been included in the
consolidated financial statements or tax returns. Deferred tax assets and
liabilities are recognized for the future tax consequence attributable to the
difference between the tax bases of assets and liabilities and their reported
amounts in the financial statements. Deferred tax assets and liabilities are
measured using the enacted tax rate expected to apply to taxable income in the
years in which those temporary differences are expected to be recovered or
settled. The effect on deferred tax assets and liabilities of a change in tax
rates is recognized in income in the period that includes the enactment date.
The Company establishes a valuation when it is more likely than not that the
assets will not be recovered.
Major Customers and
Suppliers
Bio-fertilizer
products
We had a
total of 34 customers as of December 31, 2009, of which two customers accounted
for 15.9%and 7.4% of our net sales for the fiscal year ended December 31, 2009,
respectively. No other single customer accounted for more than 7% of our
revenues. For the fiscal year ended December 31, 2008, we had three significant
customers accounting for 40.6%, 10.9% and 7.8% of our net sales, respectively,
and no other single customer accounted for more than 7% of our
revenues.
Three
suppliers accounted for 16.3%, 11.6% and 11.4% of our net sales of our net
purchase for the fiscal year ended December 31, 2008. Comparably, two suppliers
accounted for 85.7% and 6.4% of net purchases for fiscal 2009, respectively.
Historically our existing suppliers have met our needs. In addition, the raw
materials used in our bio-fertilizer products are widely available from a
variety of alternative sources.
36
Results of
Operations
Net
Sales
Net sales
from continuing operations were $38,292 and $226,869 for the twelve months ended
December 31, 2009, representing a $188,577 or 83.1% decrease. The tremendous
decrease was mainly due to loss of customers who are extremely price-sensitive
and are reluctant to settle payment at the place of order.
Cost
of Sales
Cost of
sales from continuing operations was $32,201 and $166,834 for the twelve months
ended December 31, 2009 and 2008, respectively. The decrease of $134,633 or
80.7% in cost of sales was primarily due to decrease of net sales from
continuing operations.
Gross
Profit
Gross
profit from our continuing operations was $6,091 and $60,035, representing a
profit margin of 15.9% and 26.5% for the twelve months ended December 31, 2009
and 2008, respectively.
This
decrease of $53,944 in gross profit is mainly due to drop down of gross profit
in both bio-fertilizer and bio-enhanced feed business.
Gross
profit margin of bio-fertilizer business reduced gradually in 2009. This was due
to the fact that we sold higher percentage (in terms of quantity of products
sold) low-end products in 2009 than did 2008, which has undermined our
profitability of this segment.
Consulting
and Professional Fees
Consulting
and professional fees of continuing operations occurred in principal operations
were $271,042 and $358,142 for the twelve months ended December 31, 2009 and
2008, respectively, representing a decrease of $87,100 or 24.3%. Fair value of
warrants issued to a financial advisor, who introduced 6% Notes purchasers to
the Company, had been charged to consulting and professional fees in three years
time from June 2006. As the 6% Notes fell due by the end of June 2009, our
consulting and professional fees decreased.
Officers’
Compensation
Officers’
compensation was $230,214 and $240,993 for the twelve months ended December 31,
2009 and 2008, denoting $10,779 or 4.5% decrease.
37
General
and Administrative
General
and administrative expenses of continuing operations were $1,164,733 and
$914,539 for the twelve months ended December 31, 2009 and 2008, an increase of
$250,194 or 27.4%. General and administrative expenses include
salaries, travel and entertainment, rent, office expense, telephone expense and
insurance costs. The increase is mainly resulted from the expansion of our
operating activities, which led to the increased amount of rental, salaries,
travel expenses and office expenses. We also charged $442,527 and
$152,750 of liquidated damages in connection with 6% Notes into general and
administrative expenses during twelve months ended December 31, 2009 and 2008,
respectively. (See Note 12 of Notes to Consolidated Financial
Statements)
Selling
Expenses
During
2009, selling expenses of continuing operations were $75,994, a $31,426 or 70.5%
increase from $44,568 of the selling expenses from continuing operations in
2008. This increase was mainly attributable to our adjustment of
marketing and sales policies in our fertilizer business.
Research
and Development
Research
and development expenses decreased by $874 or 0.5% to $192,103, for the twelve
months ended December 31, 2009, as compared to $192,977 for the twelve months
ended December 31, 2008. The research and development expense mainly
consist of the expenses of maintaining Kiwa-CAU R&D Centre, which began
operation in July 2006. (See “Business-Intellectual Property and Product Lines-
Kiwa-CAU R&D Center” under Item 1 in Part I).
Depreciation
and Amortization
Depreciation
and amortization of continuing operations, excluding depreciation included in
cost of production and deprecation of research equipment, increased $23,143 or
18.9% to $145,315, for the twelve months ended December 31, 2009, as compared to
$122,172 for the same period of 2008. For fiscal years
2009 and 2008, part of the depreciation of manufacturing facilities was booked
as operating expense, which would normally be booked as production costs when
production capacity reaches an adequate level.
Loss
From Disposal of Obsolete Inventory
During
twelve months ended December 31, 2009 and 2008, the Company incurred $55,183 and
$192,798 loss resulting from disposal of obsolete inventory of continuing
operations. Due to unique nature of bio-fertilizer products, some raw
materials of bio-fertilizer products have a shorter term of validity than
chemical substance. Since the Company kept on adjusting its product
mix, some raw materials purchased years ago cannot be used to in production of
current products and had been stored for a period of time that is longer than
its period of validity. We decided to dispose these raw materials as
obsolete inventory.
Loss
From Impairment of Long-lived Assets
At the
end of fiscal years 2009 and 2008, we launched a complete test of the
recoverability of long-lived assets. Based on evaluations, the
Company charged $804,780 and $639,492 into losses of fiscal 2009 and 2008,
respectively.
Net
Interest Expenses
Net
interest expense was $532,626 in 2009 and $753,917 in 2008, representing a
$221,291 or 29.4% decrease. The interest expenses for both periods
are consisted of amortization of fair value of warrants in connection with 6%
Notes, and (2) interest charges on our loans.
38
Other
Income
Other
income during twelve months ended December 31, 2009 was $77,790 and was nil
during comparable period of 2008. This income was generated by our
bio-fertilizer business.
Loss
from Discontinued Operations
During
twelve months ended December 31, 2009 and 2008, the Company has incurred net
loss of $392,512 and $258,164 from discontinued operations. During
both periods, discontinued operations refer to bio-enhanced feed
business.
Net
Loss Attributable to Kiwa Shareholders
Net loss
attributable to Kiwa shareholders for fiscal 2009 and 2008 was $3,714,529 and
$3,632,188, representing $82,341 or 2.3% increase. This increase
resulted from the following factors: (1) decrease in gross profit of $53,944 or
89.9%; (2) increase in operating expenses of $179,916 or 9.5%; (3) decrease in
loss from disposal of obsolete inventory from $192,798 in 2008 to $55,183 in
2009; (4) increase in loss from impairment of long-lived assets from $639,492 in
fiscal 2008 to $804,780 in 2009; (5) decrease in interest expenses of $221,291
or 29.4%; (5) other income of $77,790 in 2009 and nil in 2008; (6) loss from
discontinued operations increased from $258,164 in 2008 to $392,512 in
209 and (7) $66,092 and $51,633 in net loss attributable to
non-controlling interest in subsidiary in 2009 and 2008,
respectively.
Comprehensive
Loss
Comprehensive
loss increased by $62,183 or 1.7% to $3,718,566 for the twelve months ended
December 31, 2009, as compared to $3,656,383 for the comparable period of
2008. The increase in comprehensive loss in the current year as
compared to fiscal 2008 is due to an increase of $82,341 in net loss
attributable to Kiwa shareholders and a decrease of $20,158 in other
comprehensive loss.
Liquidity and Capital
Resources
Since
inception of our ag-biotech business in 2002, we have relied on the proceeds
from the sale of our equity securities and loans from both unrelated and related
parties to provide the resources necessary to fund our operations and the
execution of our business plan. During fiscal 2009, we raised
$1,009,844 in total from our related parties through several advance and repaid
$87,407 to related parties. To some extent, these fundraisings
improved our short-term liquidity, however as of December 31, 2009, our current
liabilities exceeded current assets by $6,383,120, reflecting a current ratio of
0.03:1, compared to current liabilities exceeded current assets by $4,000,056,
reflecting a current ratio 0.49:1, as of December 31, 2008. The sharp
downturn of our short-term liquidity was mainly due to current assets hold by
Kiwa Tianjin was disposed by Challenge Feed without the Company’s permission,
therefore, inventory and other current assets hold by Kiwa Tianjin was charged
into losses. During twelve months ended December 31, 2009, we issued
260,285,794 shares resulting from the conversion of $104,151 principal of 6%
Notes into our common stock. During comparable period of 2008,
52,739,530 shares of common stock had been issued for the conversion of $436,302
principal and $116,873 interest by 6% Notes Purchasers. Since the
Company does not have sufficient financial capability to repay or buy-back 6%
Notes by means of cash and has issued 400,000,000 shares of common stocks, it is
expected that we could not issue mores shares to repay principal and interest of
6% Notes. The Company may have to further amend its certificate of
incorporation increasing authorized number of common shares. (See
Note12 of Notes to Consolidated Financial Statements).
39
As of
December 31, 2009 and 2008, we had cash of $28,765 and $18,609,
respectively. The change is outlined as follows.
During
2009, our operating activities from continuing operations utilized cash of
$566,233, as compared with $622,611 used by operations in 2008. Such
cash was mainly used for working capital for our bio-fertilizer and bio-enhanced
feed businesses, purchase of inventory, a market development fee for
bio-fertilizer, and repayment of accounts payable to venders and service
providers.
During
2009, our investing activities utilized $7,320 in acquiring property and
equipment. Comparatively, in 2008 we spent $48,111 for the purchase
of equipment to finish the first stage to upgrade our facility in Kiwa
Shandong.
During
the twelve months ended December 31, 2009, we generated $918,217 from financing
activities, consisting of proceeds from related parties of $1,009,844, which was
offset in part by repayment of $87,407 to related parties and long-term
borrowings of $4,220. During the fiscal year ended December 31, 2008,
we generated $901,615 from financing activities, consisting of proceeds from
issuance of common stocks of $650,000 and from related parties of $802,574,
which was offset in part by repayment of $545,353 to related parties and
long-term borrowings of $5,606.
Operating
activities of discontinued operations used $326,433 in the twelve months ended
December 31, 2009 and $212,136 during the comparable period of
2008.
Investing
activities of discontinued operations has generated nil and $5,863 in fiscal
2009 and 2008, respectively.
As of
December 31, 2009, we had an accumulated deficit of $16,394,930, which was made
up in part of a net loss attributable to Kiwa shareholders of $3,714,529
(including non-cash expenses of $1,721,577) and $3,632,188 (including non-cash
expenses of $1,532,958) during 2009 and 2008, respectively. Our
operating activities incurred net loss $3,388,109 and $3,425,657 during twelve
months ended December 31, 2009 and 2008. Our discontinued operations
has incurred $392,512 and $258,164 in fiscal 2009 and 2008,
respectively. We do not anticipate generating sufficient positive
operating cash inflow to fund our planned operations.
In 2010,
we plan to keep on upgrading of Kiwa Shandong’s fermentation facilities, which
will allow us to fully utilize the patent we acquired from CAU and further
reduce production costs. In the meantime, we will focus on expanding
our bio-fertilizer sales. We will concentrate on (1) adjusting our
product mix to increase the proportion of high-margin products, (2) improving
our current equipment, and (3) setting up new production lines. With
respect to the AF-01 technology, our hope is to (1) close the acquisition of a
GMP-qualified veterinary factory, (2) close a first round of investment, and (3)
procure approval of a number of veterinary drug products for the AF-01
technology.
Currently
we have insufficient cash resources to accomplish our objectives. We
will need to seek additional sources of funding to sustain our
operations. In the next year, we intend to raise additional capital
through the issuance of debt or equity securities to fund the development of our
planned business operations, although there can be no assurance that we will be
successful in obtaining this financing.
To the
extent that we are unable to successfully raise the capital necessary to fund
our future cash requirements on a timely basis and under acceptable terms and
conditions, we will not have sufficient cash resources to maintain operations,
and may have to curtail operations and consider a formal or informal
restructuring or reorganization.
40
Off-Balance Sheet
Arrangements
At
December 31, 2009, we did not have any relationships with unconsolidated
entities or financial partnerships, such as entities often referred to as
structured finance or special purpose entities, established for the purpose of
facilitating off-balance sheet arrangements or other contractually narrow or
limited purposes. As such, we are not exposed to any financing,
liquidity, market or credit risk that could arise if we had engaged in such
relationships.
Recent Accounting
Pronouncements
See Note
2 to the Consolidated Financial Statements under Item 8, Part II.
|
Item
8.
|
Financial
Statements
|
The
Consolidated Financial Statements of Kiwa Bio-Tech Products Group Corporation
and its subsidiaries including the notes thereto, together with the reports
thereon of AGCA, Inc. for fiscal year ended December 31, 2009 and Consolidated
Financial Statements of Kiwa Bio-Tech Products Group Corporation and its
subsidiaries including the notes thereto, together with the reports from thereon
of Mao & Company, CPAs, Inc. (“Mao & Company”) are presented beginning
on page F-1.
|
Item
9.
|
Changes
in and Disagreements with Accountants on Accounting and Financial
Disclosure
|
Effective
as of June 2, 2009, the Company dismissed Mao & Company, the Company's
independent registered public accounting firm. The decision to change
accountants was approved by the Company's Board of Directors. Mao
& Company reported on the Company's consolidated financial statements for
the years ended December 31, 2008 and 2007. For these periods and up
to June 2, 2009, there were no disagreements with Mao & Company on any
matter of accounting principle or practices, financial statement disclosure, or
auditing scope or procedure, which disagreement(s), if not resolved to the
satisfaction of Mao & Company, would have caused it to make reference
thereto in its report on the financial statements for such
years. During such years, there were no reportable events within the
meaning set forth in Item 304(a)(1)(v) of Regulation S-K.
The
reports of Mao & Company on the financial statements of the Company for the
fiscal years ended December 31, 2008 and 2007 did not contain any adverse
opinion or disclaimer of opinion and were not qualified or modified as to
uncertainty, audit scope or accounting principles.
The
Company has engaged AGCA, Inc. (“AGCA”) to assume the role of its new principal
independent accountants. The decision to engage AGCA was approved by the Board
of Directors on June 2, 2009. The Company signed the AGCA engagement
letter on June 2, 2009 after AGCA completed its internal procedures related to
new attest client acceptance. During the fiscal years ended December
31, 2008 and 2007 and through June 2, 2009, the Company did not consult with
AGCA on (i) the application of accounting principles to a specified transaction,
either completed or proposed, or the type of audit opinion that may be rendered
on the Company’s financial statements, and AGCA did not provide either in a
written report or oral advice to the Company that was an
important factor considered by the Company in reaching a decision as to any
accounting, auditing, or financial reporting issue; or (ii) the subject of any
disagreement, as defined in Item 304 (a)(1)(v) of Regulation S-K and the related
instructions, or a reportable event within the meaning set forth in Item 304
(a)(1)(V) of Regulation S-K.
41
At the
annual meeting of shareholders on December 28, 2009, the proposal of the
appointment of AGCA, Inc. as the Company’s independent auditors for the fiscal
year ended December 31, 2009 was approved by the required votes of our
shareholders. Mao & Company had audited our Annual Report on Form
10-K for the fiscal year ended December 31, 2008.
Through
March 29, 2010, there was not any disagreement with our current certifying
accountants on any matter of accounting principles or practices, financial
statement disclosure, or auditing scope and procedures.
Item
9A(T). Controls and Procedures
Disclosure Controls and
Procedures
Our
management, under the supervision and with the participation of our Chief
Executive Officer (“CEO”) and Chief Financial Officer (“CFO”), has evaluated the
effectiveness of our disclosure controls and procedures as defined in SEC Rules
13a-15(e) and 15d-15(e) as of the end of the period covered by this Annual
Report. Our disclosure controls and procedures are designed to ensure
that information required to be disclosed in the reports we file or submit under
the Securities Exchange Act of 1934 (“Exchange Act”) is recorded, processed,
summarized, and reported within the time periods specified in the SEC’s forms,
and that such information is accumulated and communicated to our management
including our CEO and CFO, to allow timely decisions regarding required
disclosures. Based on their evaluation, our CEO and CFO have
concluded that, as of December 31, 2009, our disclosure controls and procedures
were ineffective.
Management Report on
Internal Control over Financial Reporting
Our
management is responsible for establishing and maintaining adequate internal
control over financial reporting, as such term is defined in Exchange Act Rules
13a-15(f) and 15d-15(f). Our internal control over financial reporting was
designed to provide reasonable assurance to the Company’s management and board
of directors regarding the preparation and fair presentation of published
consolidated financial statements. Internal control over financial reporting is
promulgated under the Exchange Act as a process designed by, or under the
supervision of, the Company’s principal executive and principal financial
officers and effected by the Company’s board of directors, management and other
personnel, to provide reasonable assurance regarding the reliability of
financial reporting and the preparation of financial statements for external
purposes in accordance with generally accepted accounting principles. Internal
control over financial reporting, no matter how well designed, has inherent
limitations and may not prevent or detect misstatements. Therefore, even
effective internal control over financial reporting can only provide reasonable
assurance with respect to the financial statement preparation and
presentation.
Our
management has conducted, with the participation of our CEO and CFO, an
assessment, including testing of the effectiveness, of our
internal control over financial reporting as of December 31, 2009. Management’s
assessment of internal control over financial reporting was conducted using the
criteria in Internal Control over Financial Reporting - Guidance for Smaller
Public Companies issued by the Committee of Sponsoring Organizations of the
Treadway Commission. Based on such evaluation, management identified
deficiencies that were determined to be a material weakness.
A
material weakness is a deficiency, or a combination of deficiencies, in internal
control over financial reporting, such that there is a reasonable possibility
that a material misstatement of the company’s annual or interim financial
statements will not be prevented or detected on a timely basis. Because of the
material weakness described below, management concluded that our internal
control over financial reporting was ineffective as of December 31,
2009.
42
The
specific material weakness identified by the Company’s management as of December
31, 2009 is described as follows:
• The
Company is lacking qualified resources to perform the internal audit functions
properly. In addition, the scope and effectiveness of the Company’s internal
audit function are yet to be developed.
• We
currently do not have an audit committee.
Remediation
Initiative
• We
are committed to establishing the internal audit functions but due to limited
qualified resources in the region, we were not able to hire sufficient internal
audit resources before the end of 2009. However, internally we established a
central management center to recruit more senior qualified people in order to
improve our internal control procedures. Externally, we are looking forward to
engage an accounting firm to assist the Company in improving the Company’s
internal control system based on COSO Framework. During fiscal 2009, the Company
engaged a consulting firm to provide training services regarding U.S. GAAP,
financial statement and SOX compliance to management staff. In the future, we
also will increase our efforts to hire the qualified resources.
•
We intend to
establish an audit committee of the board of directors as soon as
practicable. We envision that the audit committee will be primarily
responsible for reviewing the services performed by our independent auditors,
evaluating our accounting policies and our system of internal
controls.
Conclusion
The
Company did not have sufficient and skilled accounting personnel with an
appropriate level of technical accounting knowledge and experience in the
application of generally accepted accounting principles accepted in the United
States of America commensurate with the Company’s financial reporting
requirements, which resulted in a number of internal control deficiencies that
were identified as being significant. The Company’s management
believes that the number and nature of these significant deficiencies, when
aggregated, was determined to be a material weakness.
Despite
of the material weakness and deficiencies reported above, the Company’s
management believes that its consolidated financial statements included in this
report fairly present in all material respects the Company’s financial
condition, results of operations and cash flows for the periods presented and
that this report does not contain any untrue statement of a material fact or
omit to state a material fact necessary to make the statements made, in light of
the circumstances under which such statements were made, not misleading with
respect to the period covered by this report.
This
Annual Report does not include an attestation report of the Company’s registered
public accounting firm regarding internal control over financial
reporting. Management’s report was not subject to attestation by the
Company’s registered public accounting firm pursuant to temporary rules of the
SEC that permit the Company to provide only management's report in this Annual
Report.
43
Changes in Internal Control
over Financial Reporting
There
were no changes in our internal control over financial reporting during the
fiscal year ended December 31, 2009 that materially affected, or are reasonably
likely to materially affect, our internal control over financial
reporting.
Item
9B. Other Information
None.
Part
III
|
Item
10.
|
Directors,
Executive Officers and Corporate
Governance
|
Directors and Executive
Officers
Set forth
below are the names of our directors and executive officers, their ages, their
offices with us, if any, their principal occupations or employment for the past
five years. The directors listed below will serve until the Company’s
next annual meeting of the stockholders:
|
Name
|
Age
|
Position
|
||
|
Wei
Li
|
49
|
Chief
Executive Officer, Chief Financial Officer (from January 4, 2009 to
February 17, 2009) and Chairman of the Board of
Directors
|
||
|
Steven
Ning Ma
|
53
|
Chief
Financial Officer and Chief Operating Officer (from February 18,
2009)
|
||
|
Lianjun
Luo
|
40
|
Chief
Financial Officer (to December 31, 2008), Director
|
||
|
Xucheng
Hu
|
47
|
Director
(since December 30, 2008)
|
||
|
Dachang
Ju
|
69
|
Director
(to December 30, 2008)
|
||
|
Yunlong
Zhang
|
46
|
Director
(General Manager of Kiwa Shandong until February 2009)
|
||
|
Qi
Wang
|
43
|
Director
and Vice President - Technical
|
||
|
Yvonne
Wang
|
31
|
Corporate
Secretary
|
||
|
Dianyuan
Song
|
50
|
Vice
President - Marketing
|
||
|
Xin
Ma
|
33
|
Vice
President (since January 4, 2009) Associate Chief Financial Officer (from
January 2006 to January 3,
2009)
|
Wei Li
became our Chief Executive Officer and Chairman of the Board of Directors on
March 12, 2004. Mr. Li was appointed as Chief Financial Officer on
January 4, 2009 by the Board of Directors. From January 1, 2004 to
the time of the Tintic/Kiwa merger, Mr. Li was the acting Chief Executive
Officer of Kiwa Bio-Tech Products Group Ltd. Mr. Li founded Kiwa
Bio-Tech Products Group Ltd. to capitalize on the growth of the ag-biotechnology
industry in China. Prior to founding Kiwa Bio-Tech Products Group
Ltd., Mr. Li founded China Star, an entity which provides integrated financial
services and/or venture investments to growth businesses in
China. Mr. Li served as President of China Star from June 1993 to
January 2004. In 1989, Mr. Li founded Xinhua International Market Development
Co. Ltd., a company which engaged in investing in China’s high tech,
pharmaceutical, medical device, media, entertainment and real estate industries.
Mr. Li holds a B.S. in Finance from Hunan Finance and Economics
University.
Steven Ning
Ma became our Chief Financial Officer and Chief Operating Officer on
February 18, 2009. Prior to joining the Company, Mr. Ma served as
Managing Director of SAS Conserve de Provence from 2006 to
2008. Prior to that, Mr. Ma was the Senior Managing Partner of HJV
(Hejun) Consulting (Ltd.) from 2004 to 2005. Mr. Ma received his
Master degree in Economics/Finance from the Graduate School of Chinese Academy
of Sciences. He is also a Ph.D. Candidate in Financial Economics from
Wageningen University, Netherlands.
44
Lianjun
Luo became our Chief Financial Officer on March 12, 2004, and one of our
directors on March 27, 2004. On December 31, 2008, the employment
agreement between the Company and Mr. Lianjun Luo expired, Mr. Luo does not
serve as Chief Financial Officer since then. Mr. Luo served as the
Chief Executive Officer of Kiwa Bio-Tech Products Group Ltd. from October 2002
to December 2003. From January 2002 to October 2002, Mr. Luo served
as the Chief Financial Officer of China Star. From August 2000 to
December 2001, Mr. Luo served as manager of Security Department and Assistant to
President at Jilin Hengfa Group Ltd., a Chinese drug manufacturing company,
responsible for the company’s preparation for an aborted IPO and for merger and
acquisition activities. From May 1998 to July 2000, Mr. Luo worked as
manager of Investment Department and Associate General Manager for Hongli
Enterprise Ltd., a Chinese investment company on merger and acquisition
transactions. Mr. Luo obtained his law degree from China University
of Political Science and Law in 1993. Mr. Luo is a certified public accountant
and lawyer in China.
Xucheng Hu
became one of our directors since December 30, 2008. He has been the
Executive Director of New Capital International Investment Limited, a listed
company on the Hong Kong Stock Exchange, since August 2003. Prior to
that engagement, Mr. Hu acted as Executive Director of China Property
Development (Holdings) Limited and Asia Director of ING Real
Estate. Over the past 10 years, he has been working with the Beijing
International Trade Association and the Beijing International Trade Research
Institute, during which period his responsibilities included performing
financial and economic research and providing professional advice on the Beijing
municipal government’s cross-provincial investments and foreign investments,
participating in the decision-making process for granting export rights to
Beijing government-owned enterprises, evaluating investment proposal, and
supervising sino-foreign investments in Beijing. Mr. Hu graduated
with a bachelor degree in economics from Beijing Economics College in
1983.
Dachang Ju
became one of our directors on March 12, 2004. From 1987 to
1999 when he retired, Mr. Ju worked as General Manager of XinShen Company, an
investment firm in China. He was responsible for the company's daily
operations and investment decision making. He served as a board
member of Kiwa Bio-Tech Products Group Ltd. since 2003 and a board member of
China Star from 1999 to 2000. Mr. Ju holds a B.S. in mathematics from
Capital Normal University in Beijing, China.
Yunlong
Zhang became one of our directors on March 27, 2004. From May 2000 to
2007, Mr. Zhang had been the General Manager of China Star, responsible for the
group’s daily operations. From 1994 to 2000, Mr. Zhang served as the head of the
Investment Department at China National Economic and Systems Reform Research and
Services Center, an economic reform think tank for the central government. Mr.
Zhang holds a degree in statistics.
Qi Wang
became our Vice President - Technical on July 19, 2005 and was elected one of
our directors of the Company on July 18, 2007. Prof. Wang also acts as Director
of Kiwa-CAU R&D Center since July 2006. Prof. Wang served as a Professor and
Advisor for Ph.D. students in Department of Plant Pathology, China Agricultural
University since January 2005. Prior to that, he served as an assistant
professor and lecturer of CAU since June 1997. He obtained his master degree and
Ph.D. in agricultural science from CAU in July 1994 and July 1997, respectively.
Prof. Wang received his bachelor’s degree of science from Inner Mongolia
Agricultural University in July 1989. He is a committee member of various
scientific institutes in China, including the National Research and Application
Center for Increasing-Yield Bacteria, Chinese Society of Plant Pathology,
Chinese Association of Animal Science and Veterinary Medicine.
Yvonne
Wang became our Secretary in September 2005. Prior to that, she served as
an executive assistant and a manager of the Company’s US office between April
2003 and September 2005. She obtained her B.S. degree of Business Administration
in July 2001 from University of Phoenix. She is also a Realtor and committees in
California, and a certified Notary Public from California’s Secretary of
State.
45
Dianyuan
Song became our Vice President-Marketing in February 2008, prior to that
Mr. Song served as Marketing and Sales Director of Kiwa since he joined Kiwa in
October 2007. Mr. Song served as a member of senior management of HuaKen Group
of China, which is a large enterprise group specializing in marketing and
distributing of fertilizer in China. Mr. Song holds a Bachelor’s degree from
Agriculture University of Shenyang.
Xin Ma
became our Vice President on January 4, 2009, prior to that Mr. Ma was our
Associate Chief Financial Officer in January 2006. Prior to that Mr.
Ma served as financial controller of LangChao Group. He obtained his
MSc. Degrees of Management and of Finance in 2005 and 2006 respectively from the
University of Leicester.
Family
Relationships
There are
no family relationships among our directors or executive officers.
Section 16(a) Beneficial
Ownership Compliance
Section
16(a) of the Securities Exchange Act of 1934 requires our officers, directors
and certain persons holding more than 10 percent of a registered class of our
common stock to file with the Securities and Exchange Commission initial reports
of ownership and reports of changes in ownership of our common
stock. Officers, directors and certain other shareholders are
required by the Securities and Exchange Commission to furnish the Company with
copies of all Section 16(a) forms they file. During fiscal 2009,
there are a number of filings were not made on a timely basis.
|
l
|
The
Company’s Annual Report on Form 10-K for fiscal year ended December 31,
2008 was filed with the SEC on May 18,
2009.
|
|
l
|
The
Company’s Quarterly Report on Form 10-Q for three months ended March 31,
2009 was filed with the SEC on June 15,
2009.
|
Code of
Ethics
We have
adopted a Code of Business Conduct and Ethics (the “Code”) that is applicable to
all employees, consultants and members of the Board of Directors, including the
Chief Executive Officer, Chief Financial Officer and Secretary. This
Code embodies our commitment to conduct business in accordance with the highest
ethical standards and applicable laws, rules and regulations. We will
provide any person a copy of the Code, without charge, upon written request to
the Company’s Secretary. Requests should be addressed in writing to
Ms. Yvonne Wang; 310 N. Indian Hill Blvd., #702 Claremont, California
91711.
Director Nominees
Recommended by Stockholders
We have
not implemented any changes to the procedures by which stockholders may
recommend nominees to our board of directors since we last disclosed those
procedures in our most recent proxy statement.
Board Composition; Audit
Committee and Financial Expert
Our Board
of Directors is currently composed of five members: Wei Li, Lianjun Luo, Xucheng
Hu, Yunlong Zhang and Qi Wang. All board actions require the approval
of a majority of the directors in attendance at a meeting at which a quorum is
present.
46
We
currently do not have an audit committee. We intend, however, to
establish an audit committee of the board of directors as soon as
practicable. We envision that the audit committee will be primarily
responsible for reviewing the services performed by our independent auditors,
evaluating our accounting policies and our system of internal
controls. Currently such functions are performed by our Board of
Directors.
The Board
has determined that at least one person on the Board, Lianjun Luo, qualifies as
a “financial expert” as defined by SEC rules implementing Section 407 of the
Sarbanes-Oxley Act. Mr. Luo does not meet the definition of an
“independent” director set forth in Rule 4200(a)(15) of the Market Place Rules
of the Nasdaq Stock Market, which is the independence standard that we have
chosen to report under.
Board meetings and
committees; annual meeting attendance.
During
fiscal year 2009, the Board of Directors had six meetings in
total. All members of the Board of Directors attended all six
meetings. The Company requires all members of the Board of Directors
are required to attend the annual meetings of securities holders. On
December 30, 2009, all members of the Board of Directors attended the annual
meetings of securities holders for 2009.
|
Item
11.
|
Executive
Compensation
|
We
currently have no Compensation Committee. The Board of Directors is
currently performing the duties and responsibilities of Compensation
Committee. In addition, we have no formal compensation
policy. We decide on our executives’ compensation based on average
compensation levels of similar companies in U.S. or China, depending on
consideration of many factors such as where the executive works. Our
Chief Executive Officer’s compensation is approved by the Board of
Directors. Other named executive officers’ compensation are proposed
by our Chief Executive Officer and approved by the Board of
Directors.
Our Stock
Incentive Plan is administered by the Board of Directors. Any
amendment to our Stock Incentive Plan requires majority approval of the
stockholders of the Company.
The
Company had no officers or directors whose total compensation during either 2009
or 2008 exceeded $100,000.
Currently,
the main forms of compensation provided to each of our executive officers are:
(1) annual salary; (2) non-equity Incentive Plan; and (3) the granting of
incentive stock options subject to approval by our Board of
Directors.
Summary Compensation
Table
Summary
Compensation Table
|
Name and principal
position
|
Year
|
|
Salary
($)
|
Bonus
($)
|
Stock
Awards
($)
|
Option
Awards
($)(1)
|
Non-Equity
Incentive Plan
Compensation
($)
|
Nonqualified
Deferred
Compensation
Earnings ($)
|
All Other
Compensation
($)
|
Total
($)
|
|||||||||||
|
(a)
|
(b)
|
|
(c)
|
(d)
|
(e)
|
(f)
|
(g)
|
(h)
|
(i)
|
(j)
|
|||||||||||
|
Wei
Li, CEO
|
2009
|
|
72,000 |
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
72,000 | |||||||||||
|
Wei
Li, CEO
|
2008
|
|
72,000 |
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
72,000 | |||||||||||
|
Steven
Ning Ma, CFO
|
2009
|
74,400 |
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
74,400 | ||||||||||||
|
Lianjun
Luo, CFO
|
2008
|
48,000 |
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
48,000 | ||||||||||||
47
|
(1)
|
Options
granted on December 12, 2006. For material terms of the grant,
see additional information below under subheading entitled “2004 Stock
Incentive Plan” under this Item 10. The fair value of these
options at the date of grant was estimated using a Black-Scholes option
pricing model.
|
Employment Contracts and
Termination of Employment and Change of Control Arrangements
On
February 2, 2009, we entered into an employment agreement with our Chief
Executive Officer and Chief Financial Officer, Wei Li, for a three-year term,
commencing on January 1, 2009. Pursuant to this agreement, Mr. Li
receives a salary at the rate of $96,000 per annum, of which $72,000 will be
paid in equal monthly installments of $6,000 during the period of employment,
prorated for any partial employment period, and $24,000 is paid as an annual
performance bonus in three months after each employment year. Mr. Li
may receive such annual increases in salary as may be determined by our Board of
Directors at our annual meeting. Mr. Li is also entitled to an annual
grant of stock options under our employee stock option plan as determined by the
Board of Directors. Mr. Li is entitled to three-month’s severance if
his employment is terminated without cause.
On July
31, 2006, we entered into an employment agreement with our Chief Financial
Officer, Lianjun Luo, for a three-year term, commencing on January 1,
2006. Pursuant to this 2006 agreement, we paid pay Mr. Luo an annual
salary at the rate per annum of RMB480,000 (approximately $65,700), of which
RMB384,000 was paid in equal monthly installments of RMB32,000 during the period
of employment, prorated for any partial employment period, and RMB96,000 was
paid as an annual performance bonus in three months after each employment year
for the successful completion of all goals and objectives of that
year. Mr. Luo was entitled to an annual grant of stock options under
our employee stock option plan as determined by the Board of
Directors. Mr. Luo was entitled to three month’s severance if his
employment is terminated without cause. The employment agreement
between the Company and Mr. Lianjun Luo expired on December 31,
2008. Both parties agreed not to renew the contract.
On
February 18, 2009, the Company and Mr. Steven Ning Ma entered into an employment
agreement with the Company. Pursuant to the employment agreement, Mr. Ma is
entitled to annual salary of RMB636,000 (approximately US$93,000), among which
RMB42,400 (approximately US$6,200) payable monthly and RMB127,200 (approximately
$18,600) in one lump sum, as a performance bonus, three months following the
anniversary of his employment provided that Mr. Ma meets all goals and
objectives set by the Company. Mr. Ma’s employment may be terminated
at any time for cause or with thirty days’ written notice without
cause. The employment agreement is automatically terminated upon
death or permanent disability. Upon termination without cause, Mr. Ma
is entitled to severance payment equal to three months’ salary including all
non-cash benefits, if the termination is due to death or permanent disability;
Mr. Ma is entitled to six months’ salary. The employment agreement
also contains confidentiality provisions and provisions against competition with
the Company and solicitation of customers for 12 months following termination of
employment.
There are
no compensatory plans or arrangements with respect to a named executive officer
that would result in payments or installments in excess of $100,000 upon the
resignation, retirement or other termination of such executive officer's
employment with us or from a change-in-control.
48
Stock Incentive Plan and
Option Grant
2004
Stock Incentive Plan
On May
10, 2004, our Board of Directors approved equity incentive awards to certain of
our directors, officers and employees and/or consultants and adopted, subject to
stockholder approval, our 2004 Stock Incentive Plan (the “Plan”). Our
stockholders approved the Plan on June 3, 2004, and an amendment to the Plan on
September 12. 2006. There are 3,047,907 shares reserved for issuance
of options and other stock awards under the Plan. The number of
shares that may be granted to any participant in a fiscal year is
500,000. Options issued under the Plan will expire not more than ten
years from the date of grant.
The Plan
is a key aspect of our compensation program, designed to attract, retain, and
motivate the highly qualified individuals required for our long-term
success.
Stock
Option Grant
On
December 12, 2006, our Board of Directors granted 2,000,000 options under the
Plan, of which 823,700 shares were granted to the current executive officers and
directors. The exercise price was $0.175, equal to the closing price
of our common stock on December 12, 2006. Pursuant to the approval of
Board of Directors, after each of the first and second anniversaries of the
grant date, 33% percent of the options will become exercisable. After
the third anniversary of the grant date, 34% of the options will become
exercisable.
During
2009, a total number of 182,800 unexercised stock options were returned to the
Plan pool following the separation of certain company
employees. These stock options are available for future
grant.
On
December 12, 2009, 1,232,600outstanding stock options were vested, among which
691,500 stock options in total are held by our current executive
officers.
No
options were granted under the Plan during 2009.
Outstanding
Equity Awards at 2009 Fiscal Year-End
The
following table sets forth the status of all outstanding equity awards of the
Company as of December 31, 2009.
Outstanding Equity
Awards at Fiscal Year-End
|
Option
Awards
|
Stock
Awards
|
|||||||||||||||||
|
Name
|
Number of
Securities
Underlying
Unexercised
Options (#)
Exercisable
|
Number of
Securities
Underlying
Unexercised
Options (#)
Unexercisable
|
Equity
Incentive
Plan
Awards:
Number of
Securities
Underlying
Unexercised
Unearned
Options (#)
|
Option
Exercise
Price ($)
|
Option
Expiration
Date
|
Number
of
Shares
or Units
of
Stock
That
Have
Not
Vested
(#)
|
Value
of
Shares
or
Units
of
Stock
That
Have
Not
Vested
($)
|
Equity
Incentive
Plan
Awards:
Number
of
Unearned
Shares,
Units or
Other
Rights
That
Have Not
Vested
(#)
|
Equity
Incentive
Plan
Awards:
Market
or Payout
Value of
Unearned
Shares,
Units or
Other
Rights
That
Have Not
Vested
(#)
|
|||||||||
|
(a)
|
(b)
|
(c)
|
(d)
|
(e)
|
(f)
|
(g)
|
(h)
|
(i)
|
(j)
|
|||||||||
|
Wei
Li
|
182,800 |
Nil
|
182,800 | 0.175 |
12/04/16
|
Nil
|
Nil
|
Nil
|
Nil
|
|||||||||
|
Steven
Ning Ma
|
Nil
|
Nil
|
Nil
|
Nil
|
N/A |
Nil
|
Nil
|
Nil
|
Nil
|
|||||||||
|
Lianjun
Luo
|
132,200 |
Nil
|
132,200 | 0.175 |
12/04/16
|
Nil
|
Nil
|
Nil
|
Nil
|
|||||||||
49
|
(1)
|
See
information contained in subheading entitled “Stock Option Grant” under
heading “2004 Stock Incentive
Plan.”
|
Option
Exercises and Stock Vested
No stock
options were exercised by any officers or directors during 2008 and
2009. We did not adjust or amend the exercise price of any stock
options previously awarded to any named executive officers during 2008 and
2009.
|
Option Exercises and Stock Vested
|
||||||||
|
|
|
Option Awards
|
|
Stock Awards
|
||||
|
Name
|
|
Number of Shares
Acquired on
Exercise (#)
|
|
Value Realized on
Exercise ($)
|
|
Number of Shares
Acquired on
Vesting (#)
|
|
Value Realized on
Vesting ($)
|
|
(a)
|
|
(b)
|
|
(c)
|
|
(d)
|
|
(e)
|
|
Wei Li
|
|
Nil
|
|
Nil
|
|
Nil
|
|
Nil
|
|
Steven Ning Ma
|
|
Nil
|
|
Nil
|
|
Nil
|
|
Nil
|
|
Lianjun Luo
|
Nil
|
Nil
|
Nil
|
Nil
|
||||
Director Compensation for 2009
We currently have no policy in effect for
providing compensation to our directors for their services on our Board of
Directors, and did not compensate our directors in 2009 for services performed
as directors.
|
Item
12.
|
Security
Ownership of Certain Beneficial Owners and Management and Related
Stockholder Matters
|
The
following table sets forth as of December 31, 2009 certain information with
respect to the beneficial ownership of our common stock by (i) each of our
executive officers, (ii) each person who is known by us to beneficially own more
than 5% of our outstanding common stock, and (iii) all of our directors and
executive officers as a group. Percentage ownership is calculated
based on 400,000,000 shares of our common stock outstanding as of December 31,
2009. None of the shares listed below are issuable pursuant to stock
options or warrants of the Company.
|
Title of class
|
Name and Address of Beneficial Ownership
|
Amount and Nature of
Beneficial Owner
|
Percentage of class
|
|||||||||
|
Common
Stock
|
|
Wei
Li(1)
|
13,064,794 | 3.3 | % | |||||||
|
Common
Stock
|
|
Dachang
Ju(2)
|
10,062,088 | 2.5 | % | |||||||
|
Common
Stock
|
|
Lianjun
Luo
|
1,305,562 |
Ü
|
||||||||
|
Common
Stock
|
|
Qi
Wang
|
- | - | ||||||||
|
Common
Stock
|
|
Yunlong
Zhang
|
308,916 |
Ü
|
||||||||
|
Common
Stock
|
|
All
Star Technology Inc.
|
12,356,672 | 3.1 | % | |||||||
|
Common
Stock
|
|
InvestLink
(China) Limited
|
10,062,288 | 2.5 | % | |||||||
|
Common
Stock
|
|
All
officers and directors as a group (5 persons)
|
24,741,360 | 6.2 | % | |||||||
|
Ü
|
Less
than 1%
|
|
(1).
|
Consists
of shares held by All Star Technology Inc., a British Virgin Islands
international business company. Wei Li exercises voting and
investment control over the shares held by All Star Technology
Inc. Wei Li is a principal stockholder of All Star Technology
Inc. and may be deemed to beneficially own such shares, but disclaims
beneficial ownership in such shares held by All Star Technology Inc.
except to the extent of his pecuniary interest
therein.
|
50
|
(2).
|
Consists
of 7,812,088 shares of common stock held directly by InvestLink (China)
Limited (“Investlink”) and 2,250,000 shares of common stock held by
InvestLink as custodian for Guisheng Chen. InvestLink has the
sole power to vote or direct the vote and dispose or direct the
disposition of 10,062,088 shares but disclaims beneficial ownership of
such shares except to the extent of its pecuniary interest
therein. Dachang Ju exercises voting and investment control
over the shares held by InvestLink. Dachang Ju is a principal
stockholder of InvestLink and may be deemed to beneficially own such
shares, but disclaims beneficial ownership in such shares held by
InvestLink except to the extent of his pecuniary interest
therein.
|
Under the
terms of the 6% Notes and 6% Note Warrants, the notes and warrants are
exercisable by any holder only to the extent that the number of shares of common
stock issuable pursuant to such securities, together with the number of shares
of common stock owned by such holder and its affiliates (but not including
shares of common stock underlying unconverted shares of callable secured
convertible notes or unexercised portions of the warrants) would not exceed
4.99% of the then outstanding common stock as determined in accordance with
Section 13(d) of the Exchange Act. Therefore, the table above does
not include beneficial ownership information of the following holders of the 6%
Notes and 6% Note Warrants of the Company: AJW Partners, LLC, AJW Offshore,
Ltd., AJW Qualified Partners, LLC, New Millennium Capital Partners II, LLC,
Double U Master Fund LP, and Nite Capital LP.
Equity Compensation Plan
Information
The
following table sets forth certain information as of December 31, 2009 about our
equity compensation plans under which our equity securities are authorized for
issuance.
Equity
Compensation Plan Information
|
Plan category
|
Number of securities to be
issued upon exercise of
outstanding options,
warrants and rights
|
Weighted-average exercise
price of outstanding options,
warrants and rights
|
Number of securities
remaining available for future
issuance under equity
compensation plans
(excluding securities reflected
in column (a))
|
|||||||||
|
(a)
|
(b)
|
(c)
|
||||||||||
|
Equity
compensation plans approved by security holders
|
1,232,600 | $ | 0.175 | 767,400 | ||||||||
|
Equity
compensation plans not approved by security holders
|
- | - |
|
|||||||||
|
Total
|
1,232,600 | - | 767,400 | |||||||||
Change in
Control
None.
|
Item
13.
|
Certain
Relationships and Related Transactions, and Director
Independence.
|
For
description of transactions with related parties, see Note 13 to Consolidated
Financial Statements under Item 8 in Part II.
Under the
independence standard set forth in Rule 4200(a) (15) of the Market Place Rules
of the Nasdaq Stock Market, which is the independence standard that we have
chosen to report under, none of the members of the Board of Directors are
independent.
The
relationships between our directors and the Company are as follows:
51
Mr. Wei
Li is a principal stockholder of All Star Technology Inc, which holds 12,356,672
shares of our common stock. Mr. Li may be deemed to beneficially own
such shares and exercises voting and investment control over such shares. Mr. Li
is also Chief Executive Officer of the Company.
Mr.
Dachang Ju is a principal stockholder of InvestLink (China) Limited, which holds
directly 7,812,088 shares of our common stock and 2,250,000 shares of common
stock as custodian, Mr. Ju may be deemed to beneficially own such shares. Mr. Ju
exercises voting and investment control over such shares.
Mr.
Lianjun Luo was Chief Financial Officer of the Company for the fiscal year of
2008. On December 31, 2008, the employment agreement between the
Company and Mr. Lianjun Luo expired. Both parties agreed not to renew
this agreement.
Prof. Qi
Wang is the Director of Kiwa-CAU R&D centre and also Vice President of the
Company.
Mr.
Yunlong Zhang is Vice President of the Company.
|
Item
14.
|
Principal
Accounting Fees and Services
|
Fees Paid
to Independent Public Accountants for 2009 and 2008.
Audit
Fees
AGCA,
Inc. audited our financial statements for year-end 2009, and reviewed our
quarterly financial statements for 2009. Since we do not have a
formal audit committee, our entire Board of Directors serves as our audit
committee. We have not adopted pre-approval policies and procedures
with respect to the Company’s accountants, but our shareholders’ meeting and
board of directors approved the engagement of AGCA, Inc. before
engagement. All of the services described below were approved by our
board of directors prior to performance. The board of directors has
determined that the payments made to its independent accountant for these
services are compatible with maintaining such auditor's
independence.
The
aggregate audit fees for 2009 were approximately $130,000. The
amounts include fees for professional services rendered by AGCA, Inc. in
connection with the audit of our consolidated financial statements for the 2009
fiscal year and reviews of our quarterly reports on the Form 10-Q for the first,
second and third quarters of 2009 fiscal year.
Mao &
Company, CPAs, Inc. audited our financial statements for year-end 2008, and
reviewed our quarterly financial statements for 2008. Since we do not
have a formal audit committee, our entire Board of Directors serves as our audit
committee. We have not adopted pre-approval policies and procedures
with respect to the Company’s accountants, but our shareholders’ meeting and
board of directors approved the engagement of Mao & Company, CPAs, Inc.
before engagement. All of the services described below were approved
by our board of directors prior to performance. The board of
directors has determined that the payments made to its independent accountant
for these services are compatible with maintaining such auditor's
independence.
The
aggregate audit fees for 2008 were approximately $74,612. The amounts
include fees for professional services rendered by Mao & Company, CPAs, Inc.
in connection with the audit of our consolidated financial statements for the
2008 fiscal year and reviews of our quarterly reports on the Form 10-Q for the
first, second and third quarters of 2008 fiscal year.
52
Audit-Related
Fees
Audit-related
fees for 2009 and 2008 were nil.
Tax Fees
Tax
service fees billed to a tax consultant for 2009 and 2008 were $4,500 in each
year..
All Other
Fees
During
2009, AGCA, Inc. has charged the Company $1,500 for IDD call, company search,
issuance of review reports, copying, photocopying, courier,
etc. There were no additional aggregate fees billed by Mao &
Company, CPAs, Inc. for 2008 for other services rendered to the
Company.
Policy on Audit Committee
Pre-Approval of Audit and Permissible Non-Audit Services of Independent
Auditors
Since we
did not have a formal audit committee, our board of directors served as our
audit committee. We have not adopted pre-approval policies and
procedures with respect to our accountants in 2009. All of the
services provided and fees charged by our independent registered accounting
firms in 2009 were approved by the board of directors.
|
Item
15.
|
Exhibits
and Financial Statement Schedules
|
|
Exhibit
No.
|
Description
|
Incorporated by
Reference in
Document
|
Exhibit No. in
Incorporated
Document
|
|||
|
3.1
|
Certificate
of Incorporation, effective as of July 21, 2004
|
Form
8-K filed on July 23, 2004
|
3.1
|
|||
|
3.2
|
Bylaws,
effective as of July 22, 2004
|
Form
8-K filed on July 23, 2004
|
3.2
|
|||
|
3.3
|
Certificate
of Amendment to Certificate of Incorporation, effective as of January 9,
2009
|
Filed
herewith
|
3.3
|
|||
|
10.1
|
Advance
Agreement by and between Wei Li and the Company dated January 10,
2008
|
Form
8-K filed on January 11, 2008
|
10.01
|
|||
|
10.2
|
Stock
Purchase Agreement between Kiwa Bio-Tech Products Group Corporation and
Yuxin Zhou dated February 19, 2008
|
Form
8-K filed on February 22, 2008
|
10.01
|
|||
|
10.3
|
Consulting
Agreement between the Company and Robert Schechter dated January 10,
2008
|
Form
10-Q filed on August 11, 2008
|
10.1
|
|||
|
10.4
|
Contract
for Joint Venture between the Company and Hebei Huaxing Pharmaceuticals
Co., Ltd. dated May 22, 2008
|
Form
8-K filed on May 27, 2008
|
10.1
|
53
|
10.5
|
Term
Sheet for Redemption Convertible Notes dated September 25, 2008 between
the Company and AJW Offshore Ltd., AJW Qualified Partners LLC, AJW
Partners LLC, and New Millennium Capital Partners II LLC
|
Form
10-Q filed on November 12, 2008
|
10.3
|
|||
|
10.6
|
Term
Sheet for Redemption Convertible Notes dated September 25, 2008 between
the Company and FirsTrust Group, Inc. dated October 7,
2008
|
Form
10-Q filed on November 12, 2008
|
10.4
|
|||
|
10.7
|
2004
Stock Incentive Plan, amended in 2006
|
Form
Pre 14A filed on July 28, 2006
|
Appendix
A
|
|||
|
10.8
|
Employment
Agreement dated July 31, 2006, between the Company and Lianjun
Luo
|
Form
8-K filed on August 7, 2006
|
10.02
|
|||
|
10.9
|
Employment
Agreement dated February 2, 2009 by and between the Company and Wei
Li.
|
Form
8-K filed on February 2, 2009
|
10.1
|
|||
|
10.10
|
Employment
Agreement dated February 18, 2009 by and between the Company and Steven
Ning Ma.
|
Form
8-K filed on February 19, 2009
|
10.1
|
|||
|
10.11
|
Letter
from Mao & Company, CPAs, Inc. dated June 7, 2009 to the Securities
and Exchange Commission
|
Form
8-K filed on June 8, 2009
|
16.1
|
|||
|
14
|
Code
of Ethics
|
Form
10-K filed on May 18, 2009
|
14.1
|
|||
|
21
|
List
of Subsidiaries
|
Form
10-KSB filed on April 2, 2007
|
21
|
|||
|
31.1
|
Certification
of Chief Executive Officer pursuant to Rule 13a-14(a)/15d-14(a) of the
Securities Exchange Act of 1934
|
Filed
herewith.
|
||||
|
31.2
|
Certification
of Chief Financial Officer pursuant to Rule 13a-14(a)/15d-14(a) of the
Securities Exchange Act of 1934
|
Filed
herewith.
|
||||
|
32.1
|
Certification
of Chief Executive Officer and Chief Financial Officer, pursuant to 18
U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act
of 2002
|
Filed
herewith.
|
54
Kiwa
Bio-Tech Products Group Corporation
Signatures
Pursuant
to the requirements of Section 13 or 15(d) of the Exchange Act of 1934, the
registrant has duly caused this report to be signed on its behalf by the
undersigned, thereunto duly authorized.
Date:
March 29, 2010
|
KIWA
BIO-TECH PRODUCTS GROUP
CORPORATION.
|
||
|
By:
|
/s/ Wei Li
|
|
|
Wei
Li
|
||
|
Chief
Executive Officer
|
||
|
(Principal
Executive Officer)
|
||
|
By:
|
/s/ Steven Ning Ma
|
|
|
Steven
Ning Ma
|
||
|
Chief
Financial Officer
|
||
|
(Principal
Financial and Accounting
Officer)
|
||
Pursuant
to the requirements of Section 13 or 15(d) of the Exchange Act of 1934, the
registrant has duly caused this report to be signed on its behalf of the
registrant and in the capacities and on the dates indicated.
|
/s/
Wei Li
|
Chief Executive Officer
and
Chairman
of the Board of Directors (Principal
Executive
Officer)
|
March
29, 2010
|
||
|
Wei
Li
|
||||
|
/s/
Steven Ning Ma
|
Chief
Financial Officer
(Principal
Financial and Accounting Officer)
|
March
29, 2010
|
||
|
Steven
Ning Ma
|
||||
|
/s/
Xucheng Hu
|
Director
|
March
29, 2010
|
||
|
Xucheng
Hu
|
||||
|
/s/
Yunlong Zhang
|
Director
|
March
29, 2010
|
||
|
Yunlong
Zhang
|
||||
|
/s/
Lianjun Luo
|
Director
|
March
29, 2010
|
||
|
Lianjun
Luo
|
||||
|
/s/
Qi Wang
|
Director
|
March
29, 2010
|
||
|
Qi
Wang
|
55
Kiwa
Bio-Tech Products Group Corporation
Index
to Consolidated Financial Statements
|
Reports
of Independent Registered Public Accounting Firms
|
F-1
|
|
Consolidated
Balance Sheets
|
F-3
|
|
Consolidated
Statements of Operations and Comprehensive Income
|
F-4
|
|
Consolidated
Statement of Stockholders’ Equity
|
F-5
|
|
Consolidated
Statements of Cash Flows
|
F-6
|
|
Notes
to Consolidated Financial Statements
|
F-7
|
Report
of Independent Registered Public Accounting Firm
To the
Board of Directors and Stockholders of
Kiwa
Bio-Tech Products Group Corporation:
We have
audited the accompanying consolidated balance sheets of Kiwa Bio-Tech Products
Group Corporation and its subsidiaries (the “Company”) as of December 31, 2008,
and the related consolidated statements of operations and comprehensive income,
stockholders’ equity (deficiency), and cash flows for the year then ended. The
Company’s management is responsible for these financial statements. Our
responsibility is to express an opinion on these financial statements based on
our audits.
We
conducted our audits in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require that we plan
and perform the audit to obtain reasonable assurance about whether the financial
statements are free of material misstatement. The Company is not required to
have, nor were we engaged to perform, an audit of its internal control over
financial reporting. Our audit included consideration of internal control over
financial reporting as a basis for designing audit procedures that are
appropriate in the circumstances, but not for the purpose of expressing an
opinion on the effectiveness of the Company’s internal control over financial
reporting. Accordingly, we express no such opinion. An audit also includes
examining, on a test basis, evidence supporting the amounts and disclosures in
the financial statements, assessing the accounting principles used and
significant estimates made by management, as well as evaluating the overall
financial statement presentation. We believe that our audits provide a
reasonable basis for our opinion.
In our
opinion, the consolidated financial statements referred to above present fairly,
in all material respects, the financial position of the Company as of December
31, 2008, and the consolidated result of its operations and its cash flows for
the year then ended in conformity with accounting principles generally accepted
in the United States of America.
The
accompanying consolidated financial statements have been prepared assuming that
the Company will continue as a going concern. As discussed in Note 1 to the
consolidated financial statements, the Company has suffered recurring losses
from operations, has debts maturing in 2009 but a working capital deficit and a
net capital deficiency as of December 31, 2008 that raise substantial doubt
about its ability to continue as a going concern. Management’s plans in regard
to these matters are also described in Note 1. The consolidated financial
statements do not include any adjustments that might result from the outcome of
this uncertainty.
/s/ Mao
& Company CPAs, Inc.
New York,
New York
March 6,
2009 – except for discontinued operation disclosure in note 18 and the related
reclassification presentation in the financial statements, for which the date is
March 27, 2010.
F-1
Report of Independent Registered
Public Accounting Firm
To the
Board of Directors and Stockholders of
Kiwa
Bio-Tech Products Group Corporation:
We have
audited the accompanying consolidated balance sheets of Kiwa Bio-Tech Products
Group Corporation and subsidiaries (the “Company”) as of December 31, 2009 and
the related consolidated statements of operations and comprehensive loss,
stockholders’ equity, and cash flows for the year then ended. These consolidated
financial statements are the responsibility of the Company’s management. Our
responsibility is to express an opinion on these consolidated financial
statements based on our audits. The consolidated financial statements of
Kiwa Bio-Tech Products Group Corporation and subsidiaries for the year ended
December 31, 2008 were audited by other auditors whose report dated March 6,
2009 (except for discontinued operation disclosure in note 18 and the related
reclassification presentation in the financial statements, for which the date is
March 27, 2010.) expressed an unqualified opinion on those
statements.
We
conducted our audits in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require that we plan
and perform the audit to obtain reasonable assurance about whether the
consolidated financial statements are free of material misstatement. The Company
is not required to have, nor were we engaged to perform, an audit of its
internal control over financial reporting. Our audit included consideration of
internal control over financial reporting as a basis for designing audit
procedures that are appropriate in the circumstances, but not for the purpose of
expressing an opinion on the effectiveness of the Company’s internal control
over financial reporting. Accordingly, we express no such opinion. An
audit also includes examining, on a test basis, evidence supporting the amounts
and disclosures in the consolidated financial statements. An audit also includes
assessing the accounting principles used and significant estimates made by
management, as well as evaluating the overall financial statement presentation.
We believe that our audits provide a reasonable basis for our
opinion.
In our
opinion, the consolidated financial statements referred to above present fairly,
in all material respects, the consolidated financial position of Kiwa Bio-Tech
Products Group Corporation and subsidiaries as of December 31, 2009, the
consolidated results of their operations and their cash flows for the year then
ended in conformity with accounting principles generally accepted in the United
States of America.
These
consolidated financial statements have been prepared assuming that the Company
will continue as a going concern. As discussed
in Note 1 to the consolidated financial statements, the Company has operating
and liquidity concerns, has incurred an accumulated deficit of $16,394,930 as of
December 31, 2009. This condition raises substantial doubt about the Company's
ability to continue as a going concern. The
consolidated financial statements do not include any adjustments to reflect the
possible future effects on the recoverability and classification of assets or
the amounts and classification of liabilities that may result from the outcome
of these uncertainties.
/s/ AGCA,
Inc.
Arcadia,
California
March 29,
2010
F-2
KIWA
BIO-TECH PRODUCTS GROUP CORPORATION
CONSOLIDATED
BALANCE SHEETS
(AUDITED)
|
December
31,
|
||||||||
|
2009
|
2008
|
|||||||
|
ASSETS
|
||||||||
|
Current
assets
|
||||||||
|
Cash and cash equivalents
|
$ | 28,765 | $ | 18,609 | ||||
|
Accounts receivable, net
|
2,441 | 83,836 | ||||||
|
Inventories
|
6,717 | 55,447 | ||||||
|
Prepaid expenses
|
- | 6,889 | ||||||
|
Prepayment for fertilizer trade
|
- | 2,955,550 | ||||||
|
Other current assets
|
131,674 | 62,290 | ||||||
|
Current assets of discontinued operation
|
385 | 727,633 | ||||||
|
Total
current assets
|
169,982 | 3,910,254 | ||||||
|
Property,
Plant and Equipment
|
||||||||
|
Buildings
|
1,243,137 | 1,241,972 | ||||||
|
Machinery and equipment
|
565,745 | 565,218 | ||||||
|
Automobiles
|
81,467 | 81,390 | ||||||
|
Office equipment
|
99,159 | 99,071 | ||||||
|
Computer software
|
21,186 | 21,166 | ||||||
|
Property,
plant and equipment - total
|
2,010,694 | 2,008,817 | ||||||
|
Less: accumulated depreciation
|
(688,617 | ) | (568,988 | ) | ||||
|
Less: impairment on long-lived assets
|
(1,066,594 | ) | (542,285 | ) | ||||
|
Property,
plant and equipment - net
|
255,483 | 897,544 | ||||||
|
Construction
in progress
|
- | 71,887 | ||||||
|
Intangible
asset - net
|
- | 151,231 | ||||||
|
Deferred
financing costs
|
- | 47,793 | ||||||
|
Deposit
to purchase proprietary technology
|
- | 126,443 | ||||||
|
Property,
plant and equipment of discontinued operation
|
- | 118,542 | ||||||
|
Total
assets
|
$ | 425,465 | $ | 5,323,694 | ||||
|
LIABILITIES
AND SHAREHOLDERS’ DEFICIENCY
|
||||||||
|
Current
liabilities
|
||||||||
|
Accounts payable
|
$ | 279,248 | $ | 382,714 | ||||
|
Advances from customers
|
13,107 | 146,241 | ||||||
|
Construction costs payable
|
290,429 | 297,472 | ||||||
|
Due to related parties - trade
|
351,484 | 3,189,653 | ||||||
|
Due to related parties - non-trade
|
1,819,507 | 897,070 | ||||||
|
Convertible notes payable, net
|
1,518,171 | 1,273,391 | ||||||
|
Salary payable
|
493,153 | 301,892 | ||||||
|
Taxes payable
|
85,937 | 16,179 | ||||||
|
Penalty payable
|
595,277 | 152,750 | ||||||
|
Current portion of long-term liabilities
|
4,253 | 3,857 | ||||||
|
Other payable
|
997,021 | 690,194 | ||||||
|
Current liabilities of discontinued operation
|
105,515 | 558,897 | ||||||
|
Total
current liabilities
|
6,553,102 | 7,910,310 | ||||||
|
Long-term
liabilities, less current portion
|
||||||||
|
Unsecured loans payable
|
1,684,192 | 1,682,615 | ||||||
|
Bank notes payable
|
7,671 | 11,881 | ||||||
|
Long-term convertible notes payable - net
|
112,917 | 112,917 | ||||||
|
Total
long-term liabilities
|
1,804,780 | 1,807,413 | ||||||
|
Shareholders’
deficiency
|
||||||||
|
Common stock - $0.001 par value
Authorized 400,000,000 shares. Issued
and
outstanding 400,000,000 and 139,399,206 shares
at
December 31, 2009 and 2008
|
400,000 | 139,399 | ||||||
|
Preferred stock - $0.001 par value
Authorized 20,000,000 shares, none
issued
|
- | - | ||||||
|
Additional paid-in capital
|
8,093,337 | 10,269,855 | ||||||
|
Stock-based compensation reserve
|
- | (135,843 | ) | |||||
|
Deficit accumulated
|
(16,394,930 | ) | (14,706,710 | ) | ||||
|
Accumulated other comprehensive income (deficiency)
|
(30,824 | ) | (26,787 | ) | ||||
|
Total
Kiwa shareholders’ deficiency
|
(7,932,417 | ) | (4,460,086 | ) | ||||
|
Non-controlling
interest
|
- | 66,057 | ||||||
|
Total
deficiency
|
(7,932,417 | ) | (4,394,029 | ) | ||||
|
Total
liabilities and shareholders' deficiency
|
$ | 425,465 | $ | 5,323,694 | ||||
SEE
ACCOMPANYING NOTES
F-3
KIWA
BIO-TECH PRODUCTS GROUP CORPORATION
CONSOLIDATED
STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(AUDITED)
|
Year
Ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Net
sales
|
$ | 38,292 | $ | 226,869 | ||||
|
Cost of sales
|
32,201 | 166,834 | ||||||
|
Gross
profit
|
6,091 | 60,035 | ||||||
|
Operating
expenses
|
||||||||
|
Consulting and professional fees
|
271,042 | 358,142 | ||||||
|
Officers’ compensation
|
230,214 | 240,993 | ||||||
|
General and administrative
|
1,164,733 | 914,539 | ||||||
|
Selling expenses
|
75,994 | 44,568 | ||||||
|
Research and development
|
192,103 | 192,977 | ||||||
|
Depreciation and amortization
|
145,315 | 122,172 | ||||||
|
Allowance for doubtful accounts
|
- | 26,094 | ||||||
|
Total
operating expenses
|
2,079,401 | 1,899,485 | ||||||
|
Operating
loss
|
(2,073,310 | ) | (1,839,450 | ) | ||||
|
Loss
from disposal of obsolete inventory
|
(55,183 | ) | (192,798 | ) | ||||
|
Loss
from impairment of long-lived assets
|
(804,780 | ) | (639,492 | ) | ||||
|
Interest
expense
|
(532,626 | ) | (753,917 | ) | ||||
|
Other
income
|
77,790 | - | ||||||
|
Loss
from continuing operations
|
(3,388,109 | ) | (3,425,657 | ) | ||||
|
Loss
from discontinued operation
|
(392,512 | ) | (258,164 | ) | ||||
|
Net
loss
|
(3,780,621 | ) | (3,683,821 | ) | ||||
|
Net
loss attributable to non-controlling interest
|
66,092 | 51,633 | ||||||
|
Net
loss attributable to Kiwa shareholders
|
(3,714,529 | ) | (3,632,188 | ) | ||||
|
Other
comprehensive loss
|
||||||||
|
Translation adjustment
|
(4,037 | ) | (24,195 | ) | ||||
|
Comprehensive
loss
|
$ | (3,718,566 | ) | $ | (3,656,383 | ) | ||
|
Net
(loss) per common share - basic and diluted -contiuing
operations
|
$ | (0.010 | ) | $ | (0.037 | ) | ||
|
Net
(loss) per common share - basic and diluted -discontiued
operations
|
(0.001 | ) | (0.003 | ) | ||||
|
Weighted
average number of common
shares outstanding-basic and diluted
|
351,197,778 | 93,624,204 | ||||||
SEE
ACCOMPANYING NOTES
F-4
KIWA
BIO-TECH PRODUCTS GROUP CORPORATION
CONSOLIDATED
STATEMENTS OF STOCKHOLDERS' DEFICIENCY
(AUDITED)
|
Kiwa
Shareholders
|
|||||||||||||||||||||||||||||||
|
Common
Stock
|
Additional
Paid-in
Capital
|
Stock-based
Compensation
Reserve
|
Accumulated
Deficits
|
Other
Comprehensive
Deficiency
|
Non-controlling
interest
|
Total
|
|||||||||||||||||||||||||
|
Shares
|
Amount
|
||||||||||||||||||||||||||||||
|
Balance,
December 31, 2007
|
81,519,676 | 81,520 | 9,217,876 | (307,053 | ) | (11,074,522 | ) | (2,592 | ) | 110,838 | (1,973,933 | ) | |||||||||||||||||||
|
Issuance
of 140,000 shares of common stock to an Investor Relations consultant on
February 27, 2008
|
140,000 | 140 | 19,460 | - | - | - | - | 19,600 | |||||||||||||||||||||||
|
Issuance
of 5,000,000 shares of common stock to an investor for the consideration
of $650,000 on March 14, 2008
|
5,000,000 | 5,000 | 645,000 | - | - | - | - | 650,000 | |||||||||||||||||||||||
|
Issuance
of common stock for conversion of principal and interest of 6% Notes
during fiscal year ended December 31, 2008
|
52,739,530 | 52,739 | 387,519 | - | - | - | - | 440,258 | |||||||||||||||||||||||
|
Amortizaton
of fair value of warrants issued to a financing consultant during fiscal
year ended December 31, 2008
|
- | - | - | 77,181 | - | - | - | 77,181 | |||||||||||||||||||||||
|
Amortization
of fari value of employee stock options granted in 2006
|
- | - | - | 94,029 | - | - | - | 94,029 | |||||||||||||||||||||||
|
Net
loss for the fiscal year ended December 31, 2008
|
- | - | - | - | (3,632,188 | ) | - | (3,632,188 | ) | ||||||||||||||||||||||
|
Foreign
currency translation difference
|
- | - | - | - | - | (24,195 | ) | (45,113 | ) | (69,308 | ) | ||||||||||||||||||||
|
Net
loss attributible to non-controlling interest
|
- | - | - | - | - | - | 332 | 332 | |||||||||||||||||||||||
|
Balance,
December 31, 2008 (as previously reported)
|
139,399,206 | $ | 139,399 | $ | 10,269,855 | $ | (135,843 | ) | $ | (14,706,710 | ) | $ | (26,787 | ) | $ | 66,057 | $ | (4,394,029 | ) | ||||||||||||
|
Cumulative
effective of reclassification of warrants under ASC Topic
815
|
- | - | (2,026,309 | ) | - | 2,026,309 | - | - | - | ||||||||||||||||||||||
|
Balance,
January 1, 2009, as adjusted
|
139,399,206 | $ | 139,399 | $ | 8,243,546 | $ | (135,843 | ) | $ | (12,680,401 | ) | $ | (26,787 | ) | $ | 66,057 | $ | (4,394,029 | ) | ||||||||||||
|
Issuance
of 75,000 shares of common stock to a legal service provider as
compensation on January 8, 2009
|
75,000 | 75 | 5,925 | - | - | - | - | 6,000 | |||||||||||||||||||||||
|
Issuance
of 140,000 shares of common stock to an Investor Relations consultant on
February 18, 2009
|
140,000 | 140 | - | - | - | - | - | 140 | |||||||||||||||||||||||
|
Issuance
of 100,000 shares of common stock to an Investor Relations consultant on
February 23, 2009
|
100,000 | 100 | - | - | - | - | - | 100 | |||||||||||||||||||||||
|
Issuance
of common stock for conversion of principal of 6% Notes during twelve
months ended December 31, 2009
|
260,285,794 | 260,286 | (156,134 | ) | - | - | - | - | 104,152 | ||||||||||||||||||||||
|
Amortizaton
of fair value of warrants issued to a financing consultant during twelve
months ended December 31, 2009
|
- | - | - | 46,380 | - | - | - | 46,380 | |||||||||||||||||||||||
|
Amortization
of fari value of employee stock options granted in 2006
|
- | - | - | 89,463 | - | - | - | 89,463 | |||||||||||||||||||||||
|
Net
loss attributable to Kiwa shareholders for the twelve months ended
December 31, 2009
|
- | - | - | - | (3,714,529 | ) | - | - | (3,714,529 | ) | |||||||||||||||||||||
|
Foreign
currency translation difference
|
- | - | - | - | - | (4,037 | ) | 35 | (4,002 | ) | |||||||||||||||||||||
|
Net
loss attributable to non-controlling interest
|
- | - | - | - | - | - | (66,092 | ) | (66,092 | ) | |||||||||||||||||||||
|
Balance,
December 31, 2009
|
400,000,000 | $ | 400,000 | $ | 8,093,337 | - | $ | (16,394,930 | ) | $ | (30,824 | ) | - | $ | (7,932,417 | ) | |||||||||||||||
SEE
ACCOMPANYING NOTES
F-5
KIWA BIO-TECH PRODUCTS GROUP
CORPORATION
CONSOLIDATED
STATEMENTS OF CASH FLOWS
(AUDITED)
|
Year
Ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Cash
flows from operating activities:
|
||||||||
|
Net
loss attributable to Kiwa shareholders
|
$ | (3,714,529 | ) | $ | (3,632,188 | ) | ||
|
Net
loss from discontinued operations, net of income taxes
|
326,421 | 206,531 | ||||||
|
Adjustments
to reconcile net loss to net cash used in
operating activities:
|
||||||||
|
Depreciation and amortization
|
201,984 | 317,379 | ||||||
|
Impairment loss on long-lived assets
|
908,926 | 639,492 | ||||||
|
Amortization of detachable warrants, options and stocks
as
compensation
|
538,808 | 844,069 | ||||||
|
Provision for doubtful debt and inventory
impairment
|
(1,544 | ) | 275,613 | |||||
|
Provision for penalty payable
|
442,527 | 152,750 | ||||||
|
Non-controlling interest
|
(66,092 | ) | (51,633 | ) | ||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Accounts receivable
|
544,601 | (102,577 | ) | |||||
|
Inventories
|
290,076 | 273,745 | ||||||
|
Prepaid expenses
|
20,450 | (7,669 | ) | |||||
|
Prepayment to supplier - fertilizer
trade
|
- | (2,955,550 | ) | |||||
|
Other current assets
|
(57,976 | ) | (6,060 | ) | ||||
|
Accounts payable
|
(612,195 | ) | 420,938 | |||||
|
Salary payable
|
247,646 | - | ||||||
|
Taxes payable
|
69,714 | - | ||||||
|
Advances from customers
|
(146,182 | ) | (10,353 | ) | ||||
|
Due to related parties-trade
|
147,908 | 57,352 | ||||||
|
Other payable
|
293,224 | - | ||||||
|
Advances from related party - fertilizer
trade
|
- | 2,955,550 | ||||||
|
Net
cash used in operating activities
|
(566,233 | ) | (622,611 | ) | ||||
|
Cash
flows from investing activities:
|
||||||||
|
Purchase of property and equipment
|
(7,320 | ) | (48,111 | ) | ||||
|
Net
cash used in investing activities
|
(7,320 | ) | (48,111 | ) | ||||
|
Cash
flows from financing activities:
|
||||||||
|
Proceeds from issuance of common stock
|
- | 650,000 | ||||||
|
Proceeds from related parties
|
1,009,844 | 802,574 | ||||||
|
Repayment to related parties
|
(87,407 | ) | (545,353 | ) | ||||
|
Repayment of long-term borrowings
|
(4,220 | ) | (5,606 | ) | ||||
|
Net
cash provided by financing activities
|
918,217 | 901,615 | ||||||
|
Effect
of exchange rate changes on cash and cash equivalents
|
(8,075 | ) | (66,088 | ) | ||||
|
Cash
flows from discontinued operations
|
||||||||
|
Net
cash (used in) discontinued operating activities
|
(326,433 | ) | (212,136 | ) | ||||
|
Net
cash provided by discontinued investing
activites
|
- | 5,863 | ||||||
|
Net
cash (used in) discontinued financing activites
|
- | - | ||||||
|
Cash
and cash equivalents:
|
||||||||
|
Net increase (decrease)
|
10,156 | (41,468 | ) | |||||
|
Balance at beginning of year
|
18,609 | 60,077 | ||||||
|
Balance
at end of year
|
$ | 28,765 | $ | 18,609 | ||||
|
Supplemental
Disclosures of Cash flow Information:
|
||||||||
|
Cash
paid for interest
|
$ | 1,360 | $ | 1,008 | ||||
|
Cash
paid for income taxes
|
$ | - | $ | - | ||||
|
Non-cash
operating, investing and financing activities:
|
||||||||
|
Issuance of common stock for conversion of
convertible notes payable and
interest
|
104,152 | 440,258 | ||||||
|
Issuance of stock as compensation to
consultants
|
6,000 | 19,600 | ||||||
|
Conversion
of accrued interests into principal
|
- | 112,917 | ||||||
|
Setoff
of prepayment for fertilizer trade against due to
related
parties (see Note 10(2))
|
2,957,107 | - | ||||||
SEE
ACCOMPANYING NOTES
F-6
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
Notes
to Consolidated Financial Statements
|
1.
|
Description
of Business and Organization
|
Organization – The Kiwa
Bio-Tech Products Group Corporation (“the Company”) is the result of a share
exchange transaction accomplished on March 12, 2004 between the shareholders of
Kiwa Bio-Tech Products Group Ltd. (“Kiwa BVI”), a company originally organized
under the laws of the British Virgin Islands on June 5, 2002 and Tintic Gold
Mining Company (“Tintic”), a corporation originally incorporated in the state of
Utah on June 14, 1933 to perform mining operations in Utah. The share
exchange resulted in a change of control of Tintic, with former Kiwa BVI
stockholders owning approximately 89% of Tintic on a fully diluted basis and
Kiwa BVI surviving as a wholly-owned subsidiary of Tintic. Subsequent to
the share exchange transaction, Tintic changed its name to Kiwa Bio-Tech
Products Group Corporation. On July 21, 2004, the Company completed its
reincorporation in the State of Delaware.
The
Company has established two subsidiaries in China: (1) Kiwa Bio-Tech Products
(Shandong) Co., Ltd. (“Kiwa Shandong”) in 2002 and (2) Tianjin Kiwa Feed Co.,
Ltd. (“Kiwa Tianjin”) in July 2006. The following chart summarizes the
Company’s organizational and ownership structure.

Business – The Company’s
business plan is to develop, manufacture, distribute and market innovative,
cost-effective and environmentally safe bio-technological products for
agriculture markets located primarily in China. The Company has acquired
technologies to produce and market bio-fertilizer and also is developing a
veterinary drug based on AF-01 anti-viral aerosol technology.
Going Concern and Management
Plan - The consolidated financial statements have been prepared assuming
that the Company will continue as a going concern, which contemplates the
realization of assets and the satisfaction of liabilities in the normal course
of business. The carrying amounts of assets and liabilities presented in
the consolidated financial statements do not purport to represent the realizable
or settlement values.
As of
December 31, 2009, the Company had cash of $28,765, current ratio of 0.03 and
quick ratio of 0.005. The Company had an accumulated deficit of
$16,394,930 and incurred net loss attributable to Kiwa shareholders of
$3,714,529 and $3,632,188 during the years ended December 31, 2009 and 2008,
respectively. This trend is expected to continue. These factors cast
substantial doubt about the Company’s ability to continue as a going
concern.
F-7
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
In
addition, during the year ended December 31, 2009, Challenge Feed, the 20%
minority shareholder of Kiwa Tianjin, without the Company’s prior permission,
transferred titles to machinery and equipment as well as inventories of Kiwa
Tianjin to its own creditors to settle its own debts. On December 22,
2009, Kiwa Tianjin filed a lawsuit against Challenge Feed in the local court of
Wuqing District, Tianjin, where Kiwa Tianjin is domiciled. In the lawsuit,
Kiwa Tianjin asserted that Challenge Feed unlawfully disposed of the assets held
by Kiwa Tianjin. Whilst the lawsuit is in process, Kiwa Tianjin has been unable
to carry on the normal business and has classified bio-enhanced feed business
through Kiwa Tianjin as discontinued operations.
Management
is in the course of sourcing additional capital and considering ways to
restructure or adjust the Company’s operations and product mix so as to increase
profit margins in the future. However, there is no guarantee that these
actions will be successful.
These
consolidated financial statements do not include any adjustments that might
result from the outcome of this uncertainty.
|
2.
|
Summaries
of Significant Accounting Policies
|
Principle of Consolidation -
These consolidated financial statements include the financial statements of the
Company and its wholly-owned subsidiaries, Kiwa BVI and Kiwa Bio-Tech Products
(Shandong) Co., Ltd. (“Kiwa Shandong”), and also its majority-owned subsidiary,
Tianjin Kiwa Feed Co., Ltd. (“Kiwa Tianjin”). All significant
inter-company balances or transactions are eliminated on
consolidation.
Basis of Preparation - The
accompanying consolidated financial statements have been prepared in accordance
with the rules and regulations of the Securities and Exchange Commission and do
not include all information and footnotes necessary for a complete presentation
of financial statements in conformity with accounting principles generally
accepted in the United States (“US GAAP”).
Use of Estimates - The
preparation of financial statements in conformity with US GAAP requires
management to make estimates and assumptions that affect the reported amounts of
assets and liabilities, disclosure of contingent assets and liabilities at the
date of the consolidated financial statements, and the reported amounts of
revenues and expenses during the reporting period. Actual results could
differ from those estimates. Significant accounting estimates include bad
debt provision, impairment of inventory and long-lived assets, depreciation and
amortization and fair value of warrants and options.
Country Risk - As the
Company’s principal operations are conducted in China, the Company is subject to
special considerations and significant risks not typically associated with
companies operating in North America and Western Europe. These risks
include, among others, risks associated with the political, economic and legal
environments and foreign currency exchange limitations encountered in
China. The Company’s results of operations may be adversely affected by
changes in the political and social conditions in China, and by changes in
governmental policies with respect to laws and regulations, among other
things.
In
addition, all of the Company’s transactions undertaken in China are denominated
in China Renminbi (“RMB”), which must be converted into other currencies before
remittance out of China may be made. Both the conversion of RMB into
foreign currencies and the remittance of foreign currencies out of China require
the approval from the Chinese government. In recent years, the Chinese
government has gradually loosened its control over foreign exchange, especially
with respect to current foreign exchange accounts, for instance, by removing the
requirement for advance examination and approval to open a current foreign
exchange account and by increasing the quota for foreign exchange
accounts.
Credit Risk - The Company
performs ongoing credit evaluations of its customers and intends to establish an
allowance for doubtful accounts when amounts are not considered fully
collectable. According to the Company’s credit policy, the Company
generally provides 100% bad debt provision for the amounts outstanding over 365
days after the deduction of the amount subsequently settled after the balance
sheet date, which management believes is consistent with industry practice in
China region.
F-8
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
Foreign Currency Translation
-.The Company uses United States dollars (“US Dollar” or “US$” or “$”) for
financial reporting purposes. However, the Company maintains the books and
records in its functional currency, Chinese Renminbi (“RMB”), being the primary
currency of the economic environment in which its operations are
conducted. In general, the Company translates its assets and liabilities
into U.S. dollars using the applicable exchange rates prevailing at the balance
sheet date, and the statement of income is translated at average exchange rates
during the reporting period. Equity accounts are translated at historical
rates. Adjustments resulting from the translation of the Company’s
financial statements are recorded as accumulated other comprehensive
income.
The
exchange rates used to translate amounts in RMB into U.S. Dollars for the
purposes of preparing the consolidated financial statements were as
follows:
|
As
of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Balance
sheet items, except for equity accounts
|
6.8282 | 6.8346 | ||||||
|
Twelve months ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Items
in the statements of income
|
6.8310 | 6.9480 | ||||||
Advertising Costs - The
Company charges all advertising costs to expense as incurred. The total
amounts of advertising costs charged to selling, general and administrative
expense were $44,988 and $9,517 for the years ended December 31, 2009 and 2008,
respectively.
Research and Development Costs
- Research and development costs are charged to expense as incurred.
During the years ended December 31, 2009 and 2008, research and development
costs were $192,103 and $192,977, respectively.
Shipping and Handling Costs -
Substantially all costs of shipping and handling of products to customers are
included in selling, general and administrative expense. Shipping and
handling costs for the years ended December 31, 2009 and 2008 were $5,999 and
$12,758, respectively.
Net Loss Per Common Share -
Basic loss per common share is calculated by dividing net loss attributable to
Kiwa shareholders by the weighted-average number of shares of common stock
outstanding during the period. Diluted loss per common share includes
dilutive effect of dilutive securities (stock options, warrants, convertible
debt, stock subscription and other stock commitments issuable). These
potentially dilutive securities were not included in the calculation of loss per
share for the periods presented because the Company incurred a loss during such
periods and thus the effect would have been anti-dilutive. Accordingly,
basic and diluted loss per common share is the same for all periods
presented. As of December 31, 2009, potentially dilutive securities
aggregated 1,467,435,018 shares of common stock.
Reclassification from Prior Period
Financial Statements - The balance sheet as of December 31, 2008 and the
statements of operations and cash flow have been reclassified to reflect the
discontinued operations (see Note 18) and to conform to the current year
presentation.
F-9
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
Fair
value measurements
ASC Topic
820, Fair Value Measurement and Disclosures, defines fair value as the exchange
price that would be received for an asset or paid to transfer a liability (an
exit price) in the principal or most advantageous market for the asset or
liability in an orderly transaction between market participants on the
measurement date. This topic also establishes a fair value hierarchy which
requires classification based on observable and unobservable inputs when
measuring fair value. There are three levels of inputs that may be used to
measure fair value:
Level 1 -
Quoted prices in active markets for identical assets or
liabilities.
Level 2 -
Observable inputs other than Level 1 prices such as quoted prices for similar
assets or liabilities; quoted prices in markets that are not active; or other
inputs that are observable or can be corroborated by observable market data for
substantially the full term of the assets or liabilities.
Level 3 -
Unobservable inputs that are supported by little or no market activity and that
are significant to the fair value of the assets or liabilities.
The
carrying values of cash and cash equivalents, trade receivables and payables,
and short-term debts approximate their fair values due to their short
maturities.
There
were no assets and liabilities measured at fair value on a nonrecurring basis as
of December 31, 2009.
Recent
accounting pronouncement adopted
In June
2009, the FASB established the FASB Accounting Standards CodificationTM (ASC) as
the single source of authoritative U.S generally accepted accounting principles
(GAAP) recognized by the FASB to be applied to nongovernmental entities. Rules
and interpretive releases of the Securities and Exchange Commission (“SEC”)
under authority of federal securities laws are also sources of authoritative
GAAP for SEC registrants. The ASC superseded all previously existing non-SEC
accounting and reporting standards, and any prior sources of U.S. GAAP not
included in the ASC or grandfathered are not authoritative. New accounting
standards issued subsequent to June 30, 2009 are communicated by the FASB
through Accounting Standards Updates (ASUs). The ASC did no change current U.S.
GAAP but changes the approach by referencing authoritative literature by topic
(each a “Topic”) rather than by type of standard. The ASC has been effective for
the Company effective July 1, 2009. Adoption of the ASC did not have a material
impact on the Company’s Consolidated Financial Statements, but references in the
Company’s Notes to Consolidated Financial Statements to former FASB positions,
statements, interpretations, opinions, bulletins or other pronouncements are now
presented as references to the corresponding Topic in the ASC.
Effective
January 1, 2009, the Company adopted FASB ASC 350-30 and ASC 275-10-50 (formerly
FSP FAS 142-3, “Determination of the Useful Life of Intangible Assets”), which
amends the factors that should be considered in developing renewal or extension
assumptions used to determine the useful life of a recognized intangible asset
under SFAS No. 142 ("SFAS 142"), “Goodwill and Other Intangible Assets.” The
Company will apply ASC 350-30 and ASC 275-10-50 prospectively to intangible
assets acquired subsequent to the adoption date. The adoption of these
revised provisions had no impact on the Company’s Consolidated Financial
Statements.
Effective
January 1, 2009, the Company adopted FASB ASC 815-10-65 (formerly SFAS 161,
“Disclosures about Derivative Instruments and Hedging Activities”), which amends
and expands previously existing guidance on derivative instruments to require
tabular disclosure of the fair value of derivative instruments and their gains
and losses., This ASC also requires disclosure regarding the credit-risk related
contingent features in derivative agreements, counterparty credit risk, and
strategies and objectives for using derivative instruments. The adoption of this
ASC did not have a material impact on the Company’s Consolidated Financial
Statements.
F-10
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
During
2008, the Company adopted FASB ASC 820-10 (formerly FSP FAS 157-2, "Effective
Date of FASB Statement 157"), which deferred the provisions of previously issued
fair value guidance for nonfinancial assets and liabilities to the first fiscal
period beginning after November 15, 2008. Deferred nonfinancial assets and
liabilities include items such as goodwill and other nonamortizable intangibles.
Effective January 1, 2009, the Company adopted the fair value guidance for
nonfinancial assets and liabilities. The adoption of FASB ASC 820-10 did not
have a material impact on the Company’s Consolidated Financial
Statements.
Effective
January 1, 2009, the Company adopted FASB ASC 810-10-65 (formerly SFAS 160,
"Noncontrolling Interests in Consolidated Financial Statements — an amendment of
ARB No. 51"), which amends previously issued guidance to establish accounting
and reporting standards for the noncontrolling interest in a subsidiary and for
the deconsolidation of a subsidiary. It clarifies that a noncontrolling interest
in a subsidiary, which is sometimes referred to as minority interest, is an
ownership interest in the consolidated entity that should be reported as equity.
Among other requirements, this Statement requires that the consolidated net
income attributable to the parent and the noncontrolling interest be clearly
identified and presented on the face of the consolidated income statement.
The adoption of the provisions in this ASC did not have a material impact on the
Company’s Consolidated Financial Statements.
Effective
January 1, 2009, the Company adopted FASB ASC 805-10, (formerly SFAS 141R,
"Business Combinations"), which establishes principles and requirements for how
an acquirer recognizes and measures in its financial statements the identifiable
assets acquired, the liabilities assumed, any noncontrolling interest in an
acquiree and the goodwill acquired. In addition, the provisions in this
ASC require that any additional reversal of deferred tax asset valuation
allowance established in connection with our fresh start reporting on January 7,
1998 be recorded as a component of income tax expense rather than as a reduction
to the goodwill established in connection with the fresh start reporting. The
Company will apply ASC 805-10 to any business combinations subsequent to
adoption.
Effective
January 1, 2009, the Company adopted FASB ASC 805-20 (formerly FSP FAS 141R-1,
"Accounting for Assets Acquired and Liabilities Assumed in a Business
Combination That Arise from Contingencies"), which amends ASC 805-10 to require
that an acquirer recognize at fair value, at the acquisition date, an asset
acquired or a liability assumed in a business combination that arises from a
contingency if the acquisition-date fair value of that asset or liability can be
determined during the measurement period. If the acquisition-date fair value of
such an asset acquired or liability assumed cannot be determined, the acquirer
should apply the provisions of ASC Topic 450, Contingences, to determine whether
the contingency should be recognized at the acquisition date or after such date.
FSP The adoption of ASC 805-20 did not have a material impact on the Company’s
Consolidated Financial Statements.
Effective
July 1, 2009, the Company adopted FASB ASC 825-10-65 (formerly FASB Staff
Position (“FSP”) No. FAS 107-1 and Accounting Principles Board 28-1, "Interim
Disclosures about Fair Value of Financial Instruments"), which amends previous
guidance to require disclosures about fair value of financial instruments for
interim reporting periods of publicly traded companies as well as in annual
financial statements. The adoption of FASB ASC 825-10-65 did not have a material
impact on the Company’s Consolidated Financial Statements.
F-11
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
Effective
July 1, 2009, the Company adopted FASB ASC 320-10-65 (formerly FSP FAS 115-2 and
FAS 124-2, "Recognition and Presentation of Other-Than-Temporary Impairments").
Under ASC 320-10-65, an other-than-temporary impairment must be recognized
if the Company has the intent to sell the debt security or the Company is more
likely than not will be required to sell the debt security before its
anticipated recovery. In addition, ASC 320-10-65 requires impairments related to
credit loss, which is the difference between the present value of the cash flows
expected to be collected and the amortized cost basis for each security, to be
recognized in earnings while impairments related to all other factors to be
recognized in other comprehensive income. The adoption of ASC 320-10-65 did not
have a material impact on the Company’s Consolidated Financial
Statements.
Effective
July 1, 2009, the Company adopted FASB ASC 820-10-65 (formerly FSP FAS 157-4,
"Determining Fair Value When the Volume and Level of Activity for the Asset or
Liability Have Significantly Decreased and Identifying Transactions That Are Not
Orderly"), which provides guidance on how to determine the fair value of assets
and liabilities when the volume and level of activity for the asset or liability
has significantly decreased when compared with normal market activity for the
asset or liability as well as guidance on identifying circumstances that
indicate a transaction is not orderly. The adoption of ASC 820-10-65 did not
have a material impact on the Company’s Consolidated Financial
Statements.
New
accounting pronouncement to be adopted
In
December 2008, the FASB issued ASC 715, Compensation – Retirement Benefits
(formerly FASB FSP FAS 132(R)-1, “Employers’ Disclosures about Postretirement
Benefit Plan Assets”), which expands the disclosure requirements about plan
assets for defined benefit pension plans and postretirement plans. The Company
is required to adopt these disclosure requirements in the fourth quarter of
2009. It is expected the adoption of these disclosure requirements will have no
material effect on the Company’s Consolidated Financial Statements.
In June
2009, the FASB issued SFAS No. 166, “Accounting for Transfers of Financial
Assets – an amendment of FASB Statement No. 140,” (not yet reflected in FASB
ASC). SFAS No. 166 limits the circumstances in which a financial asset should be
derecognized when the transferor has not transferred the entire financial asset
by taking into consideration the transferor’s continuing involvement. The
standard requires that a transferor recognize and initially measure at fair
value all assets obtained (including a transferor’s beneficial interest) and
liabilities incurred as a result of a transfer of financial assets accounted for
as a sale. The concept of a qualifying special-purpose entity is removed from
SFAS No. 140, “Accounting for Transfers and Servicing of Financial Assets and
Extinguishments of Liabilities,” along with the exception from applying FIN
46(R), “Consolidation of Variable Interest Entities.” The standard is effective
for the first annual reporting period that begins after November 15, 2009 (i.e.
the Company’s fiscal year beginning January 1, 2010), for interim periods within
the first annual reporting period, and for interim and annual reporting periods
thereafter. Earlier application is prohibited. It is expected the adoption of
this Statement will have no material effect on the Company’s Consolidated
Financial Statements.
In June
2009, the FASB issued SFAS No. 167, “Amendments to FASB Interpretation No.
46(R),” (not yet reflected in FASB ASC). The standard amends FIN No. 46(R) to
require a company to analyze whether its interest in a variable interest entity
(“VIE”) gives it a controlling financial interest. A company must assess whether
it has an implicit financial responsibility to ensure that the VIE operates as
designed when determining whether it has the power to direct the activities of
the VIE that significantly impact its economic performance. Ongoing
reassessments of whether a company is the primary beneficiary are also required
by the standard. SFAS No. 167 amends the criteria to qualify as a primary
beneficiary as well as how to determine the existence of a VIE. The standard
also eliminates certain exceptions that were available under FIN No. 46(R). This
Statement will be effective as of the beginning of each reporting entity’s first
annual reporting period that begins after November 15, 2009 (i.e. the
Company’s fiscal year beginning January 1, 2010), for interim periods within
that first annual reporting period, and for interim and annual reporting periods
thereafter. Earlier application is prohibited. Comparative disclosures will be
required for periods after the effective date. As such, the Company will adopt
this Statement for interim and annual periods ending after January 1,
2010. It is expected the adoption of this Statement will have no material
effect on the Company’s Consolidated Financial Statements.
F-12
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
In
August, 2009, the FASB issued ASC Update No. 2009-05 (“Update 2009-05”) to
provide guidance on measuring the fair value of liabilities under FASB ASC 820
(formerly SFAS 157, "Fair Value Measurements"). The Company is required to
adopt Update 2009-05 in the fourth quarter of 2009. It is expected the
adoption of this Update will have no material effect on the Company’s
Consolidated Financial Statements.
In
October 2009, the FASB concurrently issued the following ASC
Updates:
·
ASU No. 2009-14 - Software (Topic 985): Certain Revenue Arrangements That
Include Software Elements (formerly EITF Issue No. 09-3). This standard removes
tangible products from the scope of software revenue recognition guidance and
also provides guidance on determining whether software deliverables in an
arrangement that includes a tangible product, such as embedded software, are
within the scope of the software revenue guidance.
·
ASU No. 2009-13 - Revenue Recognition (Topic 605): Multiple-Deliverable Revenue
Arrangements (formerly EITF Issue No. 08-1). This standard modifies the
revenue recognition guidance for arrangements that involve the delivery of
multiple elements, such as product, software, services or support, to a customer
at different times as part of a single revenue generating transaction.
This standard provides principles and application guidance to determine whether
multiple deliverables exist, how the individual deliverables should be separated
and how to allocate the revenue in the arrangement among those separate
deliverables. The standard also expands the disclosure requirements for multiple
deliverable revenue arrangements.
These
Accounting Standards Updates should be applied on a prospective basis for
revenue arrangements entered into or materially modified in fiscal years
beginning on or after June 15, 2010, with earlier application permitted.
Alternatively, an entity can elect to adopt these standards on a retrospective
basis, but both these standards must be adopted in the same period using the
same transition method. The Company expects to apply this standard on a
prospective basis for revenue arrangements entered into or materially modified
beginning January 1, 2011. The Company is currently evaluating the
potential impact these standards may have on its financial position and results
of operations.
In
January 2010, the FASB issued the following ASC Updates:
·
ASU No. 2010-01—Equity (Topic
505): Accounting for Distributions to Shareholders with Components of Stock and
Cash. This Update clarifies that the stock portion of a distribution to
shareholders that allows them to elect to receive cash or stock with a potential
limitation on the total amount of cash that all shareholders can elect to
receive in the aggregate is considered a share issuance that is reflected in EPS
prospectively and is not a stock dividend for purposes of applying Topics 505
and 260 (Equity and Earnings Per Share). The amendments in this Update are
effective for interim and annual periods ending on or after December 15, 2009
with retrospective application.
·
ASU No. 2010-02—Consolidation
(Topic 810): Accounting and Reporting for Decreases in Ownership of a
Subsidiary. This Update amends Subtopic 810-10 and related guidance to
clarify that the scope of the decrease in ownership provisions of the Subtopic
and related guidance applies to (i) a subsidiary or group of assets that is a
business or nonprofit activity; (ii) a subsidiary that is a business or
nonprofit activity that is transferred to an equity method investee or
joint venture; and (iii) an exchange of a group of assets that constitutes a
business or nonprofit activity for a noncontrolling interest in an entity, but
does not apply to: (i) sales of in substance real estate; and (ii) conveyances
of oil and gas mineral rights. The amendments in this Update are effective
beginning in the period that an entity adopts FAS 160 (now included in Subtopic
810-10).
·
ASU No. 2010-06—Fair Value
Measurements and Disclosures (Topic 820): Improving Disclosures about Fair
Value Measurements. This Update amends Subtopic 820-10 that require
new disclosures about transfers in and out of Levels 1 and 2 and activity in
Level 3 fair value measurements. This Update also amends Subtopic 820-10 to
clarify certain existing disclosures. The new disclosures and clarifications of
existing disclosures are effective for interim and annual reporting periods
beginning after December 15, 2009, except for the disclosures about purchases,
sales, issuances, and settlements in the roll forward of activity in Level
3 fair value measurements, which are effective for fiscal years beginning after
December 15, 2010.
The
Company expects that the adoption of the above Updates issued in January 2010
will not have any significant impact on its financial position and results of
operations.
F-13
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
|
3.
|
Accounts
Receivable
|
The
following table sets forth gross amount, bad-debt allowance and net amount of
accounts receivable as of December 31, 2009 and 2008, repectively.
|
Item
|
December 31, 2009
|
December 31, 2008
|
||||||
|
Accounts
receivables - gross
|
$ | 2,441 | $ | 380,035 | ||||
|
Write-off
for doubtful accounts
|
- | (296,199 | ) | |||||
|
Accounts
receivables - net
|
$ | 2,441 | $ | 83,836 | ||||
|
4.
|
Prepayment
for fertilizer trade
|
On
November 25, 2008, Kiwa Shandong and Oriental Chemical Industrial Corp., Ltd.
(the “Oriental Chemical”) entered into “Chemical Fertilizer Sales Agreement,”
(the “Chemical Fertilizer Purchase Agreement”) pursuant to which Oriental
Chemical will sell to Kiwa Shandong 6,700 tons of chemical fertilizer products
at the tentative price of RMB3,000 per ton. Under this agreement, Kiwa
Shandong prepaid $2,955,550 to Oriental Chemical. As of December 20, 2009,
Kiwa Shandong did not purchase any chemical fertilizer under the Chemical
Fertilizer Purchase Agreement. Please also refer to Note 10 “Related Party
Transaction – Kangtai – Fertilizer trade” section below.
On
December 12, 2008, Kiwa Shandong and Kangtai entered into “Chemical Fertilizer
Sales Agreement,” (the “Chemical Fertilizer Sales Agreement”) pursuant to which,
Kiwa Shandong will sell to Kangtai 6,700 tons of chemical fertilizer products at
the tentative price of RMB 3,130 per ton. Under this agreement, Kangtai
prepaid to Kiwa Shandong $2,957,973. As of December 20, 2009, Kiwa
Shandong did not sell any chemical fertilizer to Kangtai under the Chemical
Fertilizer Sales Agreement.
On
December 20, 2009, Kiwa Shandong, Oriental Chemical and Kangtai had entered into
“Chemical Fertilizer Sales Termination Agreement,” (the “Chemical Fertilizer
Termination Agreement”) pursuant to which Oriental Chemical and Kiwa Shandong
confirmed that Chemical Fertilizer Purchase Agreement has been terminated.
Kiwa Shandong and Kangtai also confirmed that Chemical Fertilizer Sales
Agreement was also terminated. After the Chemical Fertilizer Termination
Agreement took effect, Kiwa Shandong will no longer have any responsibilities
nor interests under Chemical Fertilizer Purchase Agreement and Chemical
Fertilizer Sales Agreement.
|
5.
|
Property,
Plant and Equipment
|
The total
gross amount of property, plant and equipment was $2,010,694 and $2,008,817 as
of December 31, 2009 and 2008, respectively.
The
building is on a piece of land the use right of which was granted to Kiwa
Bio-Tech Products (Shandong) Co., Ltd. ("Kiwa Shandong) by local government free
for 10 years. Then for another 20 years on a fee calculated according to Kiwa
Shandong's net profit. Since Kiwa Shandong did not generate any net profit, no
fee is payable.
Depreciation
expense was $119,096 and $153,019 for the years ended December 31, 2009 and
2008, respectively.
F-14
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
Impairment
on long-lived assets was $523,801 and $542,285 for the years ended December 31,
2009 and 2008, respectively.
All of
our property, plant and equipment have been held as collaterals to secure the 6%
Notes (See Note 12 below).
|
6.
|
Intangible
Assets
|
The
Company’s intangible asset as of December 31, 2009 consisted of a patent as
follows:
|
Amortization Year
|
Gross carrying
value
|
Accumulated
amount of
amortization
|
Impairment on
Intangible
Assets
|
Net Value at
December 31,
2009
|
||||||||||||||
| 8.5 | $ | 592,901 | $ | 413,004 | $ | 179,897 | $ | nil | ||||||||||
This
patent is held as collateral to secure the 6% Notes (See Note 12
below).
|
7.
|
Deferred
Financing Costs
|
The
financing costs relating to 6% Notes (See Note 15 below) were nil and $47,793 as
of December 31, 2009 and 2008, respectively. These costs consist of
financing commission paid to an investment bank, legal service fees, insurance
premium and other related costs. The costs are being amortized over the
three-year term of the 6% Notes, starting at various dates of each tranche of 6%
Notes in 2006.
|
8.
|
Deposit
to Purchase the Proprietary
Technology
|
The
balance of $126,443 as of December 31, 2008 is partial payment of the first
installment of the transfer fee for the Anti-viral Aerosol technology pursuant
to a Technology Transfer Agreement dated May 8, 2006 (See Note 17 below).
Since the Company did not make full payment for the first installment of
consideration, the deposit to purchase proprietary technology had been charged
to expenses.
|
9.
|
Construction
Costs Payable
|
Construction
costs payable represents remaining amounts to be paid for the first phase of
construction of bio-fertilizer facility in Shandong. The balance of
construction costs payment on December 31, 2009 and 2008 was $290,429 and
$297,472, respectively.
|
10.
|
Related
Party Transactions
|
Amounts
due to related parties consisted of the following as of December 31, 2009 and
December 31, 2008:
F-15
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
|
Nature
|
Notes
|
December 31, 2009
|
December 31, 2008
|
|||||||||
|
Item
|
||||||||||||
|
Mr.
Wei Li ("Mr. Li")
|
Non-trade
|
(1)
|
$ | 1,693,036 | $ | 837,347 | ||||||
|
Kangtai
International Logistics (Beijing) Co., Ltd. ("Kangtai")
|
Non-trade
|
(2)
|
(45,029 | ) | (57,277 | ) | ||||||
|
Ms.
Yvonne Wang ("Ms. Wang")
|
Non-trade
|
(3)
|
171,500 | 117,000 | ||||||||
|
Subtotal
|
$ | 1,819,507 | $ | 897,070 | ||||||||
|
Kiwa-CAU
R&D Center
|
Trade
|
(4)
|
351,484 | 234,103 | ||||||||
|
Kangtai
International Logistics (Beijing) Co., Ltd.
|
(2)
|
- | 2,955,550 | |||||||||
| Subtotal | $ | 383,513 | $ | 3,190,872 | ||||||||
| Total | $ | 2,203,020 | $ | 4,087,942 | ||||||||
(1)
Mr. Li
Mr. Li is
the Chairman of the Board, Chief Executive Officer and Chief Financial Officer
of the Company.
Advances
and Loans
As of
December 31, 2008, the balance due to Mr. Li was $837,347. During the year
ended December 31, 2009, Mr. Li advanced $943,096 to the Company and was repaid
$87,407. As of December 31, 2009, the balance due to Mr. Li was
$1,693,036. Mr. Li has agreed that the Company may repay the balance when
its cash flow circumstance allows.
Motor
Vehicle Lease
In
December 2004, the Company entered into an agreement with Mr. Li, pursuant to
which Mr. Li leases to the Company a motor vehicle. The monthly rental
payment is $2,200. The Company has extended this lease agreement with Mr.
Li to the end of fiscal year 2010.
Guarantees
for the Company
Mr. Li
has pledged without any compensation from the Company all of his common stock of
the Company as collateral security for the Company’s obligations under the 6%
Notes (See Note 12 below).
(2)
Kangtai
Non-trade
Kangtai
International Logistics (Beijing) Co., Ltd., formerly named China Star
Investment Management Co., Ltd., is a private company, 28% owned by Mr. Li. Mr.
Li is the Chairman of Kantai.
The
balance due from Kangtai on December 31, 2008 was $57,277. During twelve
months ended December 31, 2009, Kangtai has repaid $12,248 to the Company.
As of December 31, 2009, the balance due from Kangtai was
$45,029.
F-16
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
Fertilizer
trade
On
December 12, 2008, Kiwa Shandong and Kangtai entered into “Chemical Fertilizer
Sales Agreement,” (the “Chemical Fertilizer Sales Agreement”) pursuant to which,
Kiwa Shandong will sell to Kangtai 6,700 tons of chemical fertilizer products at
the tentative price of RMB3,130 per ton. Under this agreement, Kangtai
prepaid to Kiwa Shandong $2,955,550. As of December 31, 2008, Kiwa
Shandong did not sell any chemical fertilizer to Kangtai under the Chemical
Fertilizer Sales Agreement.
On
November 25, 2008, Kiwa Shandong and Oriental Chemical Industrial Corp., Ltd.
(the “Oriental Chemical”) entered into “Chemical Fertilizer Sales Agreement,”
(the “Chemical Fertilizer Purchase Agreement”) pursuant to which Oriental
Chemical will sell to Kiwa Shandong 6,700 tons of chemical fertilizer products
at the tentative price of RMB3,000 per ton. Under this agreement, Kiwa
Shandong prepaid to Oriental Chemical $2,955,550. As of December 31, 2008,
Kiwa Shandong did not purchase any chemical fertilizer under the Chemical
Fertilizer Purchase Agreement.
On
December 20, 2009, Kiwa Shandong, Oriental Chemical and Kangtai had entered into
“Chemical Fertilizer Sales Termination Agreement,” (the “Chemical Fertilizer
Termination Agreement”) pursuant to which Oriental Chemical and Kiwa Shandong
confirmed that Chemical Fertilizer Purchase Agreement has been terminated.
Kiwa Shandong and Kangtai also confirmed that Chemical Fertilizer Sales
Agreement was also terminated. After the Chemical Fertilizer Termination
Agreement took effect, Kiwa Shandong will no longer have any responsibilities
nor interests under Chemical Fertilizer Purchase Agreement and Chemical
Fertilizer Sales Agreement. Kiwa Shandong, Oriental Chemical and Kangtai
had confirmed that prepayment by Kangtai to Kiwa Shandong under the Chemical
Fertilizer Sales Agreement and the prepayment by Kiwa Shandong to Oriental
Chemical under the Chemical Fertilizer Purchase Agreement has been set
off.
(3)
Ms. Wang
Ms. Wang
is the Secretary of the Company.
As of
December 31, 2008, the amount due to Ms. Wang was $117,000. During the
year ended December 31, 2009, Ms. Wang has advanced $54,500 to the
Company. As of December 31, 2009, balance due to Ms. Wang was
$171,500. Ms. Wang has agreed that the Company may repay the balance when
its cash flow circumstance allows.
(4)
Kiwa-CAU R&D Center
In
November 2006 Kiwa and China Agricultural University (the “CAU”) agreed to
jointly set up a new research and development center, named Kiwa-CAU R&D
Center. The term of the agreement was ten years commencing from July 1,
2006.
Pursuant
to the agreement, Kiwa agree to invest RMB 1 million (approximately $146,300)
each year to fund research at Kiwa-CAU R&D Center. Prof. Qi Wang,
director of the Company, is also the director of Kiwa-CAU R&D Center.
The agreement also stipulated that the Kiwa-CAU R&D Center shall complete
the following tasks each year:
|
l
|
Three
new technological achievements get
patented;
|
|
l
|
Two
technological achievements pass the provincial level or ministerial level
scientific and technological achievements
qualification;
|
|
l
|
Develop
two new products which can be
commercialized..
|
During
the fiscal year 2009, Kiwa-CAU R&D Center had concentrated on the following
filed of works:
|
1.
|
Screening
of growth-promoting bacteria;
|
F-17
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
|
2.
|
Screening
of bio-control bacteria;
|
|
3.
|
Screening
of environmental microbiology;
|
|
4.
|
Studies
on fermentation technology and related production
process;
|
|
5.
|
Analysis
of soil and fertilizer nutrients and fertilization program
development;
|
|
6.
|
Organic
Fertilizer Application Techniques;
and
|
|
7.
|
Technical
training and services.
|
During
fiscal 2009, Kiwa-CAU R&D Center had successfully isolated forty-one strains
of endophytic bacillus from plants. A number of strains had been observed to
have the capability of boosting crop yield, dispelling chemical pesticide
residual from soil. These strains could be used for not only developing
new biological preparation but also environmental protection
preparation.
Management
has assessed Kiwa-CAU R&D center’s performance for the fiscal year ended
December 31, 2009; it is believed that Kiwa-CAU R&D center has achieved its
goals.
As of
December 31, 2008, the outstanding balance due to Kiwa-CAU R&D Center was
$234,103. During the year ended December 31, 2009, the Company paid
$29,290 to Kiwa-CAU R&D Center. As of December 31, 2009, the
outstanding balance due to Kiwa-CAU R&D Center was $351,484.
|
11.
|
Unsecured
Loans Payable
|
The
balance of unsecured loans payable was $1,684,192 and $1,682,615 as of December
31, 2009 and December 31, 2008, respectively. The movements from the
balances at December 31, 2008 to December 31, 2009 arose from the effect of
changes in the exchange rates prevailing at the balance sheet dates.
Unsecured loans payable consisted of the following at December 31, 2009 and
December 31, 2008:
|
Item
|
December 31, 2009
|
December 31, 2008
|
||||||
|
Unsecured
loan payable to Zoucheng Municipal Government, non-interest bearing,
becoming due within three years from Kiwa Shandong’s first profitable year
on a formula basis, interest has not been imputed due to the
undeterminable repayment date
|
$ | 1,318,063 | $ | 1,316,829 | ||||
|
Unsecured
loan payable to Zoucheng Science & Technology Bureau, non-interest
bearing, it is due in Kiwa Shandong’s first profitable year, interest has
not been imputed due to the undeterminable repayment date
|
366,129 | 365,786 | ||||||
|
Total
|
$ | 1,684,192 | $ | 1,682,615 | ||||
The
Company qualifies for non-interest bearing loans under a Chinese government
sponsored program to encourage economic development in certain industries and
locations in China. To qualify for the favorable loan terms, a company
must meet the following criteria: (1) be a technology company with innovative
technology or product (as determined by the Science Bureau of the central
Chinese government); (2) operate in specific industries that the Chinese
government has determined are important to encourage development, such as
agriculture, environmental, education, and others; and (3) be located in an
undeveloped area such as Zoucheng, Shandong Province, where the manufacturing
facility of the Company is located.
F-18
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
According
to the Company’s project agreement, Zoucheng Municipal Government granted the
Company use of at least 15.7 acres in Shandong Province, China at no cost for 10
years to construct a manufacturing facility. Under the agreement, the
Company has the option to pay a fee of RMB480,000 ($70,297) per acre for the
land use right after the 10-year period. The Company may not transfer or
pledge the temporary land use right. The Company also committed to invest
approximately $18 million to $24 million for developing the manufacturing and
research facilities in Zoucheng, Shandong Province. As of December 31,
2009, the Company invested approximately $1.91 million for the property, plant
and equipment of the project.
|
12.
|
Long-Term
Convertible Notes Payable
|
On June
29, 2006, the Company entered into a securities purchase agreement (the
“Purchase Agreement”) with six institutional investors (collectively, the
“Purchasers”) for the issuance and sale of (1) 6% secured convertible notes, due
three years from the date of issuance, in the aggregate principal amount of
$2,450,000 (the “6% Notes”), convertible into shares of the Company’s common
stock, and (2) warrants (the “Warrants”) to purchase 12,250,000 shares of the
Company’s common stock.
In
conjunction with the sale and issuance of the 6% Notes, the Company entered into
a Registration Rights Agreement, amended in October 2006, the requirements of
which the Company met by filing its registration statement on Form SB-2 on
August 11, 2006 and subsequently amended on October 20, 2006 and June 29,
2007.
Closings
for the sale of the 6% Notes occurred on June 29, August 15 and October 31, 2006
for $857,500, $735,000 and $857,500 principal amount, respectively. The
Company received $2,450,000 in aggregate from the three sales of the 6%
Notes.
The
conversion price of the 6% Notes is based on a 40% discount to the average of
the trading price of the Company’s common stock on the OTC Bulletin Board over a
20-day trading period. The conversion price is also adjusted for certain
subsequent issuances of equity securities of the Company at prices below the
conversion price then in effect. The 6% Notes contain a volume limitation
that prohibits the holder from converting further 6% Notes if doing so would
cause the holder and its affiliates to hold more than 4.99% of the Company’s
outstanding common stock. In addition, each holder of 6% Notes agrees that
they may not convert more than their pro-rata share (based on original principal
amount) of the greater of $120,000 principal amount of 6% Notes per calendar
month or the average daily dollar volume calculated during the 10 business days
prior to a conversion, per conversion. This conversion limit has since
been eliminated pursuant to an agreement by the Company and the Purchasers (see
discussion below).
The
exercise price of the Warrants is $0.45 per share, subject to anti-dilution
adjustments pursuant to a broad-based weighted average formula for subsequent
issues of equity securities by the Company below the trading price of the
shares. The Purchase Agreement requires the Company to maintain a reserve
of authorized common stock equal to 110% of the number of shares issuable upon
full conversion of the 6% Notes and exercise of the Warrants. The Purchase
Agreement imposes financial penalties in cash (equal to 2% of the number of
shares that the Purchaser is entitled to multiplied by the market price for each
day) if the authorized number of shares of common stock is insufficient to
satisfy the reserve requirements. The 6% Notes and the Warrants also impose
financial penalties on the Company if it fails to timely deliver common stock
upon conversion of the 6% Notes and exercise of the Warrants,
respectively.
To enable
reservation of a sufficient amount of authorized shares that may be issued
pursuant to conversion of the 6% Notes and exercise of the Warrants, the
Purchase Agreement required the Company to amend its Certificate of
Incorporation to increase the number of authorized shares of common stock.
At the annual meeting for 2006, which was held on September 12, 2006, a proposal
to amend our Certificate of Incorporation to increase the number of authorized
shares of common stock, from 100,000,000 shares to 200,000,000 shares was
approved by the required vote of our stockholders. At the annual meeting
held for 2008 on December 30, 2008 we further amended our Certificate of
Incorporation by increasing the number of authorized shares of common stock from
200,000,000 to 400,000,000. At our annual meeting for 2009, which was hold
on December 28, 2009, the proposal of further amend the Certificate of
Incorporation to increase the number of authorized shares from 400,000,000 to
800,000,000 was not approved by stockholders.
F-19
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
The
Company incurs a financial penalty in cash or shares at the option of the
Company (equal to 2% of the outstanding amount of the Notes per months plus
accrued and unpaid interest on the Notes, prorated for partial months) if it
breaches this or other affirmative covenants in the Purchase Agreement,
including a covenant to maintain a sufficient number of authorized shares under
its Certificate of Incorporation to cover at least 110% of the stock issuable
upon full conversion of the Notes and the Warrants. Pursuant to the
relevant provisions for liquidated damages in the Purchase Agreement, as of
December 31, 2009, the Company has accrued a total amount of $595,277 penalty,
which was included in general and administrative expenses. .
The 6%
Notes require the Company to procure the Purchaser’s consent to take certain
actions including to pay dividends, repurchase stock, incur debt, guaranty
obligations, merge or restructure the Company, or sell significant
assets.
The
Company’s obligations under the 6% Notes and the Warrants are secured by a first
priority security interest in the Company’s intellectual property pursuant to an
Intellectual Property Security Agreement with the Purchasers, and by a first
priority security interest in all of the Company’s other assets pursuant to a
Security Agreement with the Purchasers. In addition, the Company’s Chief
Executive Officer has pledged all of his common stock of the Company as
collateral security for the Company’s obligations under the 6% Notes and the
Warrants. The Purchasers are accredited investors as defined under the
Securities Act and the 6% Notes and the Warrants and the underlying common stock
upon conversion and exercise will be issued without registration under the
Securities Act in reliance on the exemption provided by Rule 506 under
Regulation D under the Securities Act.
The fair
value of the Warrants underlying the three sales of the 6% Notes (amounting to
4,287,500 shares, 3,675,000 shares and 4,287,500 shares respectively) at the
time of their issuance was determined to be $545,477, $416,976 and $505,503
calculated pursuant to the Black-Scholes option pricing model. The fair
value was recorded as a reduction to 6% Notes payable and was charged to
operations as interest expense in accordance with effective interest method
within the period of the 6% Notes.
The
Purchasers of the 6% Notes and Warrants were introduced to the Company by an
investment bank pursuant to an engagement letter agreement with the
Company. Pursuant to the engagement letter, the investment bank received a
cash fee equal to 8% of the aggregate proceeds raised in the financing and to
warrants in the quantity equal to 8% of the securities issued in the
financing. The Company recorded the cash fee and other direct costs
incurred for the issuance of the convertible loan in aggregate of $30,000 as
deferred debt issuance costs. Debt issuance costs were amortized on the
straight-line method over the term of the 6% Notes, with the amounts amortized
being recognized as interest expense. As of June 30, 2009 the debt
issuance costs were fully amortized.
The
warrants issued to the investment bank in connection with each tranche of 6%
Notes (amounting to 343,000 shares, 294,000 shares and 343,000 shares) are
exercisable for three years and have an exercise price equal to $0.2598.
The fair value of these warrants at the time of their issuance was determined to
be $94,005, $60,324 and $77,214 calculated pursuant to the Black-Scholes option
pricing mode. As of June 29, 2009, warrants issued to the investment bank
had expired.
On
January 31, 2008, the Company entered into three Callable Secured Convertible
Notes Agreements (“2% Notes”) with four of the Company’s 6% Notes purchasers
converting their unpaid interest of $112,917 in total, into principal with an
interest rate of 2% per annum, which will be due on January 31, 2011.
Other terms of the 2% Notes are similar to the 6% Notes. No principal of the 2%
Notes has been converted so far. As of December 31, 2009, the outstanding
principal balance on the 2% Notes was $112,917.
F-20
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
On
September 25 and October 7, 2008, the Company entered into an agreement with the
Purchasers to redeem all of the 6% Notes and 2% Notes. Under the
redemption agreement, the Purchasers agreed to waive their participation right
with respect to any new financing that closes before October 31, 2008, and
suspend conversions of principal and interest under the 6% Notes and 2% Notes
from September 25 to October 31, 2008. The Company agreed to redeem the
notes for a specified price if a new financing was completed before October 31,
2008. Under the redemption agreement, if the Company failed to redeem the
notes by October 31, 2008, the 6% Notes and 2% Notes would be automatically
amended to remove limitations on the Purchasers’ right to convert under the 6%
Notes and 2% Notes no more than (1) $120,000 per calendar month; and (2) the
average daily dollar volume calculated during the ten (10) business days prior
to a conversion, per conversion.
On
October 27, 2008, the Company had informed the Purchasers that the Company would
not be able to redeem the 6% Notes and the 2% Notes due to failure to close an
anticipated new financing. Therefore, the amendment to 6% Notes and 2%
Notes took effective and the Purchasers resumed conversion.
During
twelve months ended December 31, 2009, the Purchasers converted $104,152
principal and nil interest into 260,285,794 shares of common stocks. As of
December 31, 2009, face amount of convertible notes outstanding was
$1,518,171.
On June
3, 2009, the Company has received Notice of Default from four of 6% Notes
purchasers for reason of the Company’s failure to timely file registration or
effect registration. However, the Company believes that such Notes
Purchasers’ claim is not valid and has not made any provision for liquidated
damages in this regard.
On June
29, 2009, the 6% Notes were due. The Company has informed the Purchasers
of its inability to repay the outstanding balance on the due date.
Therefore, the 6% Notes are in default.
|
13.
|
Equity-Based
Transactions
|
During
the year ended December 31, 2009, the Company effected the following
equity-based transactions:
|
a)
|
Expenses
paid by issuance of common
stocks:
|
i.
Issuance of 75,000 shares as partial settlement of legal fees on January 8,
2009.
Shares of
the Company during the year were rarely traded at around par value and
accordingly, the agreed price of $0.08 is adopted as the fair value of this
transaction.
ii.
Issuance of 240,000 shares as partial settlement of investor relationship
consulting fees on February 18 and 23, 2009 in accordance of an agreement dated
July 1, 2008.
Shares of
the Company during the year were rarely traded at around par value and
accordingly, the par value is adopted as the fair value of these
transactions.
Fair
values of the above transactions were accounted for as expenses during the
year.
|
|
b)
|
Convertible
notes of $104,152 were converted, under difference periods of fewer than
10% of total issued capital each occasion, into 260,285,794 common
stocks.
|
After the
above transactions, the Company’s issued and outstanding common stocks increased
to 400,000,000 shares as of December 31, 2009.
F-21
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
|
14.
|
Stock-based
Compensation
|
On
December 12, 2006, the Company granted options for 2,000,000 shares of its
common stock under its 2004 Stock Incentive Plan. During fiscal 2007 and
2008, 362,100 and 222,500 stock options were returned to the Company when the
holders separated from the Company without exercising the options. As of
December 31, 2009, 1,232,600 options were outstanding. As of December 31,
2009, none of the stock options were exercised.
The
exercise price of all of our outstanding options was $0.175 per share, equal to
the closing price of our common stock on December 12, 2006. On each of the
first and second anniversaries of the grant date, 33% percent of the options
will become exercisable. On the third anniversary of the grant date, 34%
of the options will become exercisable.
The
Company has adopted ASC Topic 718 effective as of January 1, 2006. The
fair value of the options granted at the grant date was determined to be
$320,154 (approximately $0.16 per share), calculated pursuant to the
Black-Scholes option pricing model. The calculated fair value is
recognized as expense over the applicable vesting periods, using the
straight-line attribution method. Unamortized fair value of stock options
granted to those who separated from the Company has been charged to expense,
while the options returned to the Company. During the twelve months ended
December 31, 2009 and 2008, $89,463 and $94,028 was charged to expense,
respectively.
|
15.
|
Segment
Reporting
|
Our
principal business is bio-fertilizer for fiscal 2008. We used to engage in
livestock feed business and chemical fertilizer trade business. At the end
of fiscal year 2009, we made arrangements to terminate all agreements related to
chemical fertilizer trade. At the end of fiscal 2009, we classified
livestock feed business as discontinued operation. Management believes
that the following table highlights relevant information to the chief operation
decision makers for measuring business performances and financing needs and
preparing the corporate budget and other items. As most of the Company’s
customers are located in China, no geographical segment information is
presented.
F-22
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
|
Item
|
Bio-fertilizer
|
Livestock Feed
(Discontined)
|
Chemical Fertilizer
Trade (1)
|
Corporate (2)
|
Total
|
|||||||||||||||
|
Fiscal
Year Ended December 31, 2009
|
||||||||||||||||||||
|
Net
sales
|
$ | 38,292 | $ | - | $ | - | $ | - | $ | 38,292 | ||||||||||
|
Gross
profit
|
6,091 | - | - | - | 6,091 | |||||||||||||||
|
Operating
expenses
|
424,735 | - | - | 1,654,666 | 2,079,401 | |||||||||||||||
|
Operating
loss
|
(418,644 | ) | - | - | (1,654,666 | ) | (2,073,310 | ) | ||||||||||||
|
Loss
from disposal of obsolete inventory
|
(55,183 | ) | - | - | - | (55,183 | ) | |||||||||||||
|
Loss
from impairment of long-lived assets
|
(678,337 | ) | - | - | (126,443 | ) | (804,780 | ) | ||||||||||||
|
Interest
income (expense)
|
(246 | ) | - | - | (532,380 | ) | (532,626 | ) | ||||||||||||
|
Other
income
|
5,856 | - | - | 71,934 | 77,790 | |||||||||||||||
|
Non-controlling
interest
|
- | - | - | - | - | |||||||||||||||
|
Net
loss attributable to Kiwa shareholders
|
$ | (1,146,554 | ) | $ | - | $ | - | $ | (2,241,555 | ) | $ | (3,388,109 | ) | |||||||
|
Total
assets as of December 31, 2009
|
$ | 252,638 | $ | 385 | $ | - | $ | 172,442 | $ | 425,465 | ||||||||||
|
Fiscal
Year Ended December 31, 2008
|
||||||||||||||||||||
|
Net
sales
|
$ | 226,869 | $ | - | $ | - | $ | - | $ | 226,869 | ||||||||||
|
Gross
profit
|
60,035 | - | - | - | 60,035 | |||||||||||||||
|
Operating
expenses
|
360,320 | - | 2,323 | 1,536,842 | 1,899,485 | |||||||||||||||
|
Operating
loss
|
(300,285 | ) | - | (2,323 | ) | (1,536,842 | ) | (1,839,450 | ) | |||||||||||
|
Loss
from disposal of obsolete inventory
|
(192,798 | ) | - | - | - | (192,798 | ) | |||||||||||||
|
Loss
from impairment of long-term assets
|
(639,492 | ) | - | - | - | (639,492 | ) | |||||||||||||
|
Interest
income (expense)
|
(438 | ) | - | - | (753,479 | ) | (753,917 | ) | ||||||||||||
|
Non-controlling
interest
|
- | - | - | - | - | |||||||||||||||
|
Net
loss attributable to Kiwa shareholders
|
$ | (1,133,013 | ) | $ | - | $ | (2,323 | ) | $ | (2,290,321 | ) | $ | (3,425,657 | ) | ||||||
|
Total
assets as of December 31, 2008
|
$ | 1,223,645 | $ | 846,175 | $ | 2,955,550 | $ | 298,324 | $ | 5,323,694 | ||||||||||
(1)
On June 23rd, 2008, Kiwa
Bio-Tech Products (Shangdong) Co., Ltd. (Kiwa Shandong) received approval from
the Ministry of Commerce of the People's Republic of China, ratifying authority
for Kiwa Shandong to sell fertilizer products, including chemical fertilizers,
complex fertilizers and compound fertilizers to other companies.
(2)
Beijing Representative Office
of Kiwa Shandong fulfills part of corporate managerial function. Most of its
expenses relating to this function were categorized into corporate
segment.
|
16.
|
Income
Tax
|
There is
no provision (benefit) for income taxes for the years ended December 31, 2009
and 2008 since the Company and its subsidiaries have incurred operating losses
and have established a valuation allowance equal to the total deferred tax
asset.
The loss
generated in the U.S., British Virgin Islands and China (Kiwa Shandong and Kiwa
Tianjin) before income taxes in 2009 and 2008, respectively, was as
follows:
|
Years Ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Income
(Loss) in U.S. before income taxes
|
$ | (1,483,597 | ) | $ | (1,228,183 | ) | ||
|
Income
(Loss) in British Virgin Islands before income taxes
|
(120,000 | ) | (120,000 | ) | ||||
|
Income
(Loss) in Kiwa Shandong before income taxes
|
(1,784,512 | ) | (2,093,534 | ) | ||||
|
Income
(Loss) in Kiwa Tianjin before income taxes
|
(326,420 | ) | (190,471 | ) | ||||
|
Total
|
$ | (3,714,529 | ) | $ | (3,632,188 | ) | ||
F-23
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
The tax
effect of temporary differences and operating loss carryforwards is as follows
as of December 31, 2009 and 2008:
|
Year
Ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Deferred
tax assets
|
||||||||
|
Net operating loss carryforwards
|
$ | 1,311,782 | $ | 1,130,492 | ||||
|
Allowance for doubtful accounts receivable
|
|
0 | 50,529 | |||||
|
Value difference of intangible assets
|
0 | 27,987 | ||||||
|
Impairment of inventories
|
8,277 | 28,920 | ||||||
|
Impairment of long-lived asset
|
101,751 | 73,239 | ||||||
|
Accrued expenses
|
137,285 | 40,286 | ||||||
| 1,559,095 | 1,351,453 | |||||||
|
Deferred
tax liabilities
|
||||||||
|
Prepaid expenses
|
0 | (375 | ) | |||||
|
Deferred financing cost
|
0 | (7,169 | ) | |||||
| 0 | (7,544 | ) | ||||||
|
Valuation
allowance
|
(1,559,095 | ) | (1,343,909 | ) | ||||
|
Net
deferred tax assets
|
$ | - | $ | - | ||||
In
accordance with the current tax laws in China, Kiwa Shandong and Kiwa Tianjin
would normally be subject to a corporate income tax rate of 25% on its taxable
income. However, in accordance with the relevant income laws in China, Kiwa
Shandong and Kiwa Tianjin are exempt from corporate income taxes for their first
two profitable years and are entitled to a 50% tax reduction for the succeeding
three years. After the Enterprise Income Tax Law of the PRC promulgated on
March 16, 2007 took effect as of January 1, 2008, fiscal year 2009 shall be
regarded as the second profitable year for determining eligibility of these
benefits even if Kiwa Shandong or Kiwa Tianjin have not been profitable in 2009.
Kiwa Shandong and Kiwa Tianjin have not provided for any corporate income taxes
since they had no taxable income for the years ended December 31, 2009 and 2008.
The difference between the effective income tax rate and the expected statutory
rate for Kiwa Shandong and Kiwa Tianjin was as follows:
|
Year Ended December 31, 2009
|
Year Ended December 31, 2008
|
|||||||
|
Statutory
rate
|
25.0 | % | 25.0 | % | ||||
|
Income
tax holiday
|
(25.0 | )% | (25.0 | )% | ||||
|
Effective
income tax rate
|
- | - | ||||||
In
accordance with the relevant tax laws in the British Virgin Islands, Kiwa BVI,
as an International Business Company, is exempt from income taxes.
Net
operating loss of the Company could be carried forward and taken against any
taxable income for a period of not more than twenty years from the year of the
initial loss pursuant to Section 172 of the Internal Revenue Code of 1986, as
amended. The net operating loss of Kiwa Shandong and Kiwa Tianjin could be
carried forward for a period of not more than five years from the year of the
initial loss pursuant to relevant P.R.C. tax laws and regulations.
|
17.
|
Commitments
and Contingencies
|
The
Company has the following material contractual obligations:
(1)
Operating lease commitments
The
Company leased an office in Beijing on July 15, 2007. The operating lease
agreement will expire at January 14, 2010. The monthly rental payment for
the office is RMB 96,074 (approximately $14,068). Rent expense under the
operating leases for the twelve months ended December 31, 2009 and 2008 was
$153,759 and $132,000, respectively.
F-24
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
Lease
commitments under the foregoing lease agreements are as follows:
|
Fiscal
Year
|
Amount
|
|||
|
2010
|
$ | 6,027 | ||
|
2011
|
- | |||
|
2012
|
- | |||
|
2013
|
- | |||
|
2014
|
- | |||
|
Thereafter
|
- | |||
|
Total
|
$ | 6,027 | ||
(2)
Technology acquisition
On May 8,
2006 the Company entered into a Technology Transfer Agreement with Jinan
Kelongboao Bio-Tech Co. Ltd. (“JKB”). Pursuant to the agreement, JKB agreed to
transfer its AF-01 Anti-viral Aerosol technology for veterinary medicines to the
Company. Pursuant to the agreement the Company will pay JKB a transfer fee of
RMB10 million (approximately $1.369 million), of which RMB 6 million will be
paid in cash and RMB 4 million will be paid in stock. The cash portion will be
paid in installments, the first installment RMB 3 million was set for May 23,
2006 initially, of which RMB 1 million has been paid and both parties have
agreed to extend the remaining RMB 2 million to the date when the application
for new veterinary drug certificate is accepted. Three other installments
of RMB 1 million are due upon the achievement of certain milestones, the last
milestone being the issuance by the PRC Ministry of Agriculture of a new
medicine certificate in respect of the technology. The RMB 4 million stock
payment will be due 90 days after the AF-01 technology is approved by the
appropriate PRC department for use as a livestock disinfector for preventing
bird flu. The agreement will become effective when the first installment has
been fully paid.
During
the year ended December 31, 2009, no payment was made to JKB. The Company
is still pursuing to acquire AF-01 technology and develop veterinary drug
product based on this technology. There were no changes to the terms of
the Technology Transfer Agreement.
(3)
Operation of Kiwa-CAU R&D
Center
Pursuant
to the agreement on joint incorporation of the research and development center
between CAU and Kiwa Shandong dated November 14, 2006, Kiwa Shandong agrees to
invest RMB1 million (approximately $146,451) each year to fund research at the
R&D Center. The term of this Agreement is ten years starting from July
1, 2006. Qi Wang, one of our director commencing in July 2007 acts as
Director of Kiwa-CAU R&D Center since July 2006.
(4)
Investment in manufacturing and research
facilities in Zoucheng, Shandong Province in China
According
to the Project Agreement with Zoucheng Municipal Government in 2002, the Company
has committed to investing approximately $18 million to $24 million for
developing the manufacturing and research facilities in Zoucheng, Shandong
Province. As of December 31, 2009, the Company had invested approximately $1.91
million for the project.
F-25
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
(5)
PRC employee costs
According
to the prevailing laws and regulations of the PRC, the Company’s subsidiaries in
the PRC are required to cover its employees with medical, retirement and
unemployment insurance programs. Management believes that due to the
transient nature of its employees, they do not need to provide all employees
with such social insurances, and have not paid the social insurances for all
employees.
In the
event that any current or former employee files a complaint with the PRC
government, the Company's subsidiaries may be subject to making up the social
insurances as well as administrative fines. As the Company believes that
these fines would not be material, no provision has been made in this
regard.
|
18.
|
Discontinued
Operation
|
In
accordance with the provisions of ASC topic 360 (formerly SFAS No. 144),
“Accounting for the Impairment or Disposal of Long-Lived Assets,” the disposal
of our bio-enhanced feed business segment is presented as assets and liabilities
of a discontinued operation in the accompanying consolidated financial
statements.
The
consolidated balance sheets at December 31, 2009 and 2008 were updated to
reflect the assets and liabilities of the bio-enhanced feed business segment as
a discontinued operation. The following table summarizes the assets and liabilities of the discontinued operation, excluding
intercompany balances eliminated in consolidation, at December 31, 2009 and
2008, respectively:
|
December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Assets
|
||||||||
|
Cash
and cash equivalents
|
$ | 385 | $ | 377 | ||||
|
Accounts
receivable - net
|
- | 406,224 | ||||||
|
Inventories
- net
|
- | 296,339 | ||||||
|
Prepaid
expenses
|
- | 13,551 | ||||||
|
Other
current assets
|
- | 11,142 | ||||||
|
Property,
plant and equipment - net
|
- | 118,542 | ||||||
|
Total
assets
|
$ | 385 | $ | 846,175 | ||||
|
Liabilities
|
||||||||
|
Accounts
payable
|
- | $ | 514,238 | |||||
|
Advances
from customers
|
- | 12,959 | ||||||
|
Due
to related-parties-trade
|
32,029 | 1,219 | ||||||
|
Salary
payable
|
73,486 | 16,972 | ||||||
|
Other
payable
|
- | 13,509 | ||||||
|
Total
liabilities
|
$ | 105,515 | $ | 558,897 | ||||
The
income statement for the year ended December 31, 2008 was adjusted to reflect
the bio-enhanced feed business segment as a discontinued operation. The
following results of operations of bio-enhanced feed business are presented as a
loss from a discontinued operation in the consolidated statements of
operations:
F-26
Kiwa
Bio-Tech Products Group Corporation
Notes to
Consolidated Financial Statements
(Audited)
|
Fiscal year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Net
sales
|
$ | 3,615,860 | $ | 8,948,868 | ||||
|
Gross
profit
|
55,636 | 157,483 | ||||||
|
Operating
expenses
|
115,758 | 415,608 | ||||||
|
Operating
loss
|
(60,122 | ) | (258,125 | ) | ||||
|
Interest
expense
|
- | (39 | ) | |||||
|
Impairment
on long-lived assets
|
(104,146 | ) | - | |||||
|
Loss
from discontinued operation
|
(228,244 | ) | - | |||||
|
Non-controlling
interest
|
66,092 | 51,633 | ||||||
|
Net
loss from discontinued operations
|
$ | (326,420 | ) | $ | (206,531 | ) | ||
During
the year ended December 31, 2009, Challenge Feed, the 20% minority shareholder
of Kiwa Tianjin, without our prior permission, transferred titles to machinery
and equipment as well as inventories of Kiwa Tianjin to its own creditors to
settle its own debts. On December 22, 2009, Kiwa Tianjin filed a lawsuit against
Challenge Feed in the local court of Wuqing District, Tianjin, where Kiwa
Tianjin is domiciled. In the lawsuit, Kiwa Tianjin asserted that Challenge
Feed unlawfully disposed of the assets held by Kiwa Tianjin. The
local court is currently reviewing the complaint and related documents filed
with it. Assets disposed by Challenge Feed includes inventory
amounting to $221,848 and fixed assets at the amount of
$104,190. As a result, Kiwa Tianjin could no longer use its
assets including machinery and inventory in its normal course of
operation. As of December 31, 2009, the Company has classified its
bio-enhanced feed business through Kiwa Tianjin as discontinued
operations.
F-27
