Attached files
| file | filename |
|---|---|
| EX-32.2 - EX-32.2 - Kintara Therapeutics, Inc. | ktra-ex322_8.htm |
| EX-32.1 - EX-32.1 - Kintara Therapeutics, Inc. | ktra-ex321_9.htm |
| EX-31.2 - EX-31.2 - Kintara Therapeutics, Inc. | ktra-ex312_10.htm |
| EX-31.1 - EX-31.1 - Kintara Therapeutics, Inc. | ktra-ex311_11.htm |
| EX-10.1 - EX-10.1 - Kintara Therapeutics, Inc. | ktra-ex101_319.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-Q
(Mark One)
☑ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended September 30, 2020
or
☐ TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file number: 001-37823
Kintara Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
|
Nevada |
|
99-0360497 |
|
(State or other jurisdiction of |
|
(I.R.S. Employer |
|
12707 High Bluff Dr., Suite 200 |
|
92130 |
|
(Address of principal executive offices) |
|
(zip code) |
(858) 350-4364
(Registrant’s telephone number, including area code)
N/A
(Former name, former address and former fiscal year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
|
Trading Symbol(s) |
|
Name of Each Exchange on Which Registered |
|
Common Stock |
|
KTRA |
|
The Nasdaq Capital Market |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☑ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company”, and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
☐ |
|
Accelerated filer |
☐ |
|
Non-accelerated filer |
☑ |
|
Smaller reporting company |
☑ |
|
Emerging growth company |
☐ |
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act) Yes ☐ No ☑
Number of shares of common stock outstanding as of November 9, 2020 was 24,662,299.
|
|
|
|
|
Page No. |
|
|
|
|
|
|
|
Item 1. |
|
|
1 |
|
|
Item 2. |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
|
19 |
|
Item 3. |
|
|
40 |
|
|
Item 4 |
|
|
40 |
|
|
|
|
|
|
|
|
Item 1. |
|
|
41 |
|
|
Item 1A. |
|
|
41 |
|
|
Item 2. |
|
Unregistered Sales of Equity Securities and Use of Proceeds. |
|
41 |
|
Item 3. |
|
|
41 |
|
|
Item 4. |
|
|
41 |
|
|
Item 5. |
|
|
41 |
|
|
Item 6. |
|
|
42 |
i
PART 1. - FINANCIAL INFORMATION
Kintara Therapeutics, Inc.
Condensed Consolidated Interim Financial Statements
(Unaudited)
For the three months ended September 30, 2020
(expressed in US dollars unless otherwise noted)
1
Condensed Consolidated Interim Balance Sheets
(In thousands, except par value amounts)
|
|
|
|
|
|
|
September 30, 2020 |
|
|
June 30, 2020 |
|
||
|
|
|
Note |
|
|
$ |
|
|
$ |
|
|||
|
|
|
|
|
|
|
(unaudited) |
|
|
|
|
|
|
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
Current assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
|
|
|
|
|
22,602 |
|
|
|
2,392 |
|
|
Prepaid expenses and deposits |
|
|
|
|
|
|
343 |
|
|
|
356 |
|
|
Interest, taxes and other receivables |
|
|
|
|
|
|
10 |
|
|
|
9 |
|
|
Deferred loan costs |
|
|
5 |
|
|
|
— |
|
|
|
94 |
|
|
|
|
|
|
|
|
|
22,955 |
|
|
|
2,851 |
|
|
Intangible assets - net |
|
|
|
|
|
|
1 |
|
|
|
2 |
|
|
Property and equipment |
|
|
3 |
|
|
|
175 |
|
|
|
— |
|
|
Deferred financing costs |
|
|
6 |
|
|
|
— |
|
|
|
85 |
|
|
Total assets |
|
|
|
|
|
|
23,131 |
|
|
|
2,938 |
|
|
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued liabilities |
|
|
|
|
|
|
1,566 |
|
|
|
2,011 |
|
|
Loan payable, net of deferred loan costs |
|
|
5 |
|
|
|
441 |
|
|
|
— |
|
|
Related party payables |
|
|
4 |
|
|
|
382 |
|
|
|
664 |
|
|
|
|
|
|
|
|
|
2,389 |
|
|
|
2,675 |
|
|
Milestone payment liability |
|
|
3 |
|
|
|
188 |
|
|
|
— |
|
|
Total liabilities |
|
|
|
|
|
|
2,577 |
|
|
|
2,675 |
|
|
Stockholders’ equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock |
|
|
|
|
|
|
|
|
|
|
|
|
|
Authorized |
|
|
|
|
|
|
|
|
|
|
|
|
|
5,000 shares, $0.001 par value |
|
|
|
|
|
|
|
|
|
|
|
|
|
Issued and outstanding |
|
|
|
|
|
|
|
|
|
|
|
|
|
279 Series A shares at September 30, 2020 (June 30, 2020 – 279) |
|
4,6 |
|
|
|
279 |
|
|
|
279 |
|
|
|
649 Series B shares at September 30, 2020 (June 30, 2020 – 649) |
|
|
6 |
|
|
|
4,525 |
|
|
|
4,525 |
|
|
25 Series C shares at September 30, 2020 (June 30, 2020 – 0) |
|
|
6 |
|
|
|
18,355 |
|
|
|
— |
|
|
Common stock |
|
|
|
|
|
|
|
|
|
|
|
|
|
Authorized |
|
|
|
|
|
|
|
|
|
|
|
|
|
95,000 shares at September 30, 2020 and June 30, 2020, $0.001 par value |
|
|
|
|
|
|
|
|
|
|
|
|
|
24,466 issued at September 30, 2020 (June 30, 2020 – 11,458) |
|
|
6 |
|
|
|
24 |
|
|
|
11 |
|
|
Additional paid-in capital |
|
|
6 |
|
|
|
89,777 |
|
|
|
65,148 |
|
|
Accumulated deficit |
|
|
|
|
|
|
(92,427 |
) |
|
|
(69,721 |
) |
|
Accumulated other comprehensive income |
|
|
|
|
|
|
21 |
|
|
|
21 |
|
|
Total stockholders’ equity |
|
|
|
|
|
|
20,554 |
|
|
|
263 |
|
|
Total liabilities and stockholders’ equity |
|
|
|
|
|
|
23,131 |
|
|
|
2,938 |
|
|
Nature of operations, corporate history, and liquidity risk and management plans (note 1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Subsequent events (note 9) |
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
2
Condensed Consolidated Interim Statements of Operations
(Unaudited)
(In thousands, except per share amounts)
|
|
|
|
|
|
|
Three months ended September 30, |
|
|||||
|
|
|
Note |
|
|
2020 |
|
|
2019 |
|
|||
|
|
|
|
|
|
|
$ |
|
|
$ |
|
||
|
Expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
|
|
|
|
1,357 |
|
|
|
721 |
|
|
General and administrative |
|
|
|
|
|
|
1,534 |
|
|
|
914 |
|
|
Merger costs |
|
|
3 |
|
|
|
500 |
|
|
|
— |
|
|
In-process research and development |
|
|
3 |
|
|
|
16,094 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
19,485 |
|
|
|
1,635 |
|
|
Other (income) loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign exchange loss |
|
|
|
|
|
|
(1 |
) |
|
|
— |
|
|
Amortization of deferred loan costs |
|
|
5 |
|
|
|
27 |
|
|
|
— |
|
|
Interest expense |
|
|
5 |
|
|
|
8 |
|
|
|
— |
|
|
Interest income |
|
|
|
|
|
|
(1 |
) |
|
|
(29 |
) |
|
|
|
|
|
|
|
|
33 |
|
|
|
(29 |
) |
|
Net loss for the period |
|
|
|
|
|
|
19,518 |
|
|
|
1,606 |
|
|
Computation of basic loss per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss for the period |
|
|
|
|
|
|
19,518 |
|
|
|
1,606 |
|
|
Deemed dividend recognized on beneficial conversion features of Series C Preferred stock issuance |
|
|
6 |
|
|
|
3,181 |
|
|
|
— |
|
|
Series A Preferred cash dividend |
|
|
6 |
|
|
|
2 |
|
|
|
2 |
|
|
Series B Preferred stock dividend |
|
|
6 |
|
|
|
5 |
|
|
|
2 |
|
|
Net loss for the period attributable to common stockholders |
|
|
|
|
|
|
22,706 |
|
|
|
1,610 |
|
|
Basic and fully diluted loss per share |
|
|
|
|
|
|
1.33 |
|
|
|
0.21 |
|
|
Basic and fully diluted weighted average number of shares |
|
|
|
|
|
|
17,106 |
|
|
|
7,539 |
|
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
3
Condensed Consolidated Interim Statements of Stockholders’ Equity
(Unaudited)
(In thousands)
For the three months ended September 30, 2020 and 2019
|
|
|
Number of shares |
|
|
Common stock $ |
|
|
Additional paid-in capital $ |
|
|
Accumulated other comprehensive income $ |
|
|
Preferred stock $ |
|
|
Accumulated deficit $ |
|
|
Stockholders' equity $ |
|
|||||||
|
Balance - June 30, 2020 |
|
|
11,458 |
|
|
|
11 |
|
|
|
65,148 |
|
|
|
21 |
|
|
|
4,804 |
|
|
|
(69,721 |
) |
|
|
263 |
|
|
Adgero merger (note 3) |
|
|
12,011 |
|
|
|
12 |
|
|
|
16,713 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
16,725 |
|
|
Issuance of Series C Preferred stock |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
25,028 |
|
|
|
— |
|
|
|
25,028 |
|
|
Series C placement agent warrants |
|
|
— |
|
|
|
— |
|
|
|
3,287 |
|
|
|
— |
|
|
|
(3,287 |
) |
|
|
— |
|
|
|
— |
|
|
Series C Preferred stock share issuance costs |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(3,386 |
) |
|
|
— |
|
|
|
(3,386 |
) |
|
Deemed dividend recognized on beneficial conversion features of Series C Preferred stock issuance |
|
|
— |
|
|
|
— |
|
|
|
3,181 |
|
|
|
— |
|
|
|
— |
|
|
|
(3,181 |
) |
|
|
— |
|
|
Exercise of warrants for cash |
|
|
993 |
|
|
|
1 |
|
|
|
993 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
994 |
|
|
Warrants issued for services |
|
|
— |
|
|
|
— |
|
|
|
45 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
45 |
|
|
Stock option expense |
|
|
— |
|
|
|
— |
|
|
|
405 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
405 |
|
|
Series A Preferred cash dividend |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2 |
) |
|
|
(2 |
) |
|
Series B Preferred stock dividend |
|
|
4 |
|
|
|
— |
|
|
|
5 |
|
|
|
— |
|
|
|
— |
|
|
|
(5 |
) |
|
|
— |
|
|
Loss for the period |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(19,518 |
) |
|
|
(19,518 |
) |
|
Balance - September 30, 2020 |
|
|
24,466 |
|
|
|
24 |
|
|
|
89,777 |
|
|
|
21 |
|
|
|
23,159 |
|
|
|
(92,427 |
) |
|
|
20,554 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance - June 30, 2019 |
|
|
3,839 |
|
|
|
4 |
|
|
|
57,543 |
|
|
|
21 |
|
|
|
4,978 |
|
|
|
(60,578 |
) |
|
|
1,968 |
|
|
Issuance of shares and warrants - net of issue costs |
|
|
4,895 |
|
|
|
5 |
|
|
|
6,578 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
6,583 |
|
|
Exercise of pre-funded warrants for cash |
|
|
2,655 |
|
|
|
2 |
|
|
|
24 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
26 |
|
|
Conversion of Series B Preferred stock to common stock |
|
|
6 |
|
|
|
— |
|
|
|
174 |
|
|
|
— |
|
|
|
(174 |
) |
|
|
— |
|
|
|
— |
|
|
Shares issued for services |
|
|
7 |
|
|
|
— |
|
|
|
5 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
5 |
|
|
Stock option expense |
|
|
— |
|
|
|
— |
|
|
|
51 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
51 |
|
|
Series A Preferred cash dividend |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2 |
) |
|
|
(2 |
) |
|
Series B Preferred stock dividend |
|
|
4 |
|
|
|
— |
|
|
|
2 |
|
|
|
— |
|
|
|
— |
|
|
|
(2 |
) |
|
|
— |
|
|
Loss for the period |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1,606 |
) |
|
|
(1,606 |
) |
|
Balance - September 30, 2019 |
|
|
11,406 |
|
|
|
11 |
|
|
|
64,377 |
|
|
|
21 |
|
|
|
4,804 |
|
|
|
(62,188 |
) |
|
|
7,025 |
|
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
4
Condensed Consolidated Interim Statements of Cash Flows
(Unaudited)
(In thousands)
|
|
|
|
|
|
|
Three months ended September 30, |
|
|||||
|
|
|
|
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
Note |
|
|
$ |
|
|
$ |
|
|||
|
Cash flows from operating activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss for the period |
|
|
|
|
|
|
(19,518 |
) |
|
|
(1,606 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortization of intangible assets |
|
|
|
|
|
|
1 |
|
|
|
3 |
|
|
In-process research and development |
|
|
3 |
|
|
|
16,094 |
|
|
|
— |
|
|
Amortization of deferred loan costs |
|
|
5 |
|
|
|
27 |
|
|
|
— |
|
|
Interest expense |
|
|
5 |
|
|
|
8 |
|
|
|
— |
|
|
Shares issued for services |
|
|
6 |
|
|
|
— |
|
|
|
5 |
|
|
Warrants issued for services |
|
|
6 |
|
|
|
45 |
|
|
|
— |
|
|
Stock option expense |
|
|
6 |
|
|
|
405 |
|
|
|
51 |
|
|
Changes in operating assets and liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Prepaid expenses and deposits |
|
|
|
|
|
|
24 |
|
|
|
56 |
|
|
Interest, taxes and other receivables |
|
|
|
|
|
|
— |
|
|
|
(44 |
) |
|
Accounts payable and accrued liabilities |
|
|
|
|
|
|
(914 |
) |
|
|
(617 |
) |
|
Related party payables |
|
|
|
|
|
|
(282 |
) |
|
|
(114 |
) |
|
Net cash used in operating activities |
|
|
|
|
|
|
(4,110 |
) |
|
|
(2,266 |
) |
|
Cash flows from investing activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash acquired on merger with Adgero |
|
|
3 |
|
|
|
969 |
|
|
|
— |
|
|
Net cash provided by investing activities |
|
|
|
|
|
|
969 |
|
|
|
— |
|
|
Cash flows from financing activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net proceeds from the issuance of shares and warrants |
|
|
6 |
|
|
|
21,859 |
|
|
|
6,583 |
|
|
Warrants exercised for cash |
|
|
6 |
|
|
|
994 |
|
|
|
26 |
|
|
Proceeds from loan |
|
|
5 |
|
|
|
500 |
|
|
|
— |
|
|
Series A preferred cash dividend |
|
|
4 |
|
|
|
(2 |
) |
|
|
(2 |
) |
|
Net cash provided by financing activities |
|
|
|
|
|
|
23,351 |
|
|
|
6,607 |
|
|
Decrease in cash and cash equivalents |
|
|
|
|
|
|
20,210 |
|
|
|
4,341 |
|
|
Cash and cash equivalents – beginning of period |
|
|
|
|
|
|
2,392 |
|
|
|
3,719 |
|
|
Cash and cash equivalents – end of period |
|
|
|
|
|
|
22,602 |
|
|
|
8,060 |
|
|
Supplementary information (note 7) |
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
5
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
September 30, 2020
(expressed in US dollars unless otherwise noted)
|
1 |
Nature of operations, corporate history, and liquidity risk and management plans |
Nature of operations
Kintara Therapeutics, Inc. (formerly DelMar Pharmaceuticals, Inc.) (the “Company”) is a clinical stage drug development company with a focus on the development of novel cancer therapies for patients with unmet medical needs. The Company is developing two late-stage, Phase 3-ready therapeutics - VAL-083 for glioblastoma multiforme and REM-001 for cutaneous metastatic breast cancer. In order to accelerate the Company’s development timelines, it leverages existing preclinical and clinical data from a wide range of sources. The Company may seek marketing partnerships in order to potentially offset clinical costs and to generate future royalty revenue from approved indications of its product candidates.
On June 9, 2020, the Company entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”), by and among Adgero Acquisition Corp., the Company’s wholly-owned subsidiary incorporated in the State of Delaware (“Merger Sub”), and Adgero Biopharmaceuticals Holdings, Inc., a Delaware corporation (“Adgero”). On August 19, 2020, upon the terms and subject to the conditions set forth in the Merger Agreement, Merger Sub merged with and into Adgero (the “Merger”), the separate corporate existence of Merger Sub ceased and Adgero continued its existence under Delaware law as the surviving corporation in the Merger and became a direct, wholly-owned subsidiary of the Company. As a result of the Merger, each issued and outstanding share of Adgero common stock, par value $0.0001 per share (the “Adgero Common Stock”) (other than treasury shares held by Adgero), was converted automatically into the right to receive 1.5740 shares (the “Exchange Ratio”) of the Company’s common stock, and cash in lieu of any fractional shares. Also, each outstanding warrant to purchase Adgero Common Stock was converted into a warrant exercisable for that number of shares of the Company’s common stock equal to the product of (x) the aggregate number of shares of Adgero Common Stock for which such warrant was exercisable and (y) the Exchange Ratio.
Following the completion of the Merger, the Company changed its name from DelMar Pharmaceuticals, Inc. to Kintara Therapeutics, Inc. and began trading on Nasdaq under the symbol “KTRA”.
Corporate history
The Company is a Nevada corporation formed on June 24, 2009 under the name Berry Only, Inc. On January 25, 2013, the Company entered into and closed an exchange agreement (the “Exchange Agreement”), with Del Mar Pharmaceuticals (BC) Ltd. (“Del Mar (BC)”), 0959454 B.C. Ltd. (“Callco”), and 0959456 B.C. Ltd. (“Exchangeco”) and the security holders of Del Mar (BC). Upon completion of the Exchange Agreement, Del Mar (BC) became a wholly-owned subsidiary of the Company (the “Reverse Acquisition”).
Kintara Therapeutics, Inc. is the parent company of Del Mar (BC), a British Columbia, Canada corporation and Adgero, a Delaware corporation, which are clinical stage companies with a focus on the development of drugs for the treatment of cancer. The Company is also the parent company to Callco and Exchangeco which are British Columbia, Canada corporations. Callco and Exchangeco were formed to facilitate the Reverse Acquisition. In connection with the Merger, the Company also became the parent company of Adgero Biopharmaceuticals, Inc. (“Adgero Bio”), formerly a wholly-owned subsidiary of Adgero.
References to the Company refer to the Company and its wholly-owned subsidiaries.
Liquidity risk and management plans
During the three months ended September 30, 2020, the Company reported a net loss of $19.5 million. As of September 30, 2020, the Company had $22.6 million of cash and cash equivalents and used $4.1 million of cash in its operating activities during the three months ended September 30, 2020. The Company is in the clinical stage and has not generated any revenues to-date. The Company does not have the prospect of achieving revenues until such time that its product candidates are commercialized, or partnered, which may not ever occur. In the future, the Company will require additional funding to maintain its clinical trials, research and development projects, and for general operations. The Company may tailor its drug development programs based on the amount of funding the Company is able to raise in the future. During the three months ended September 30, 2020, the Company completed a private placement in three closings for aggregate net proceeds of approximately $21.6 million (note 6). The Company believes that based on its current estimates, the cash and cash equivalents at September 30, 2020 of $22.6 million, as well as cash from the proceeds from stock purchase warrants exercised subsequent to September 30, 2020, will be sufficient to fund its planned operations for at least
6
the next twelve months from the date these condensed consolidated interim financial statements are issued. However, the coronavirus (“COVID-19”) pandemic has created significant economic uncertainty and volatility in the credit and capital markets. The ultimate impact of the COVID-19 pandemic on the Company’s ability to raise additional capital in the future is unknown and will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the COVID-19 outbreak and any new information which may emerge concerning the severity of the COVID-19 pandemic.
|
2 |
Significant accounting policies |
Basis of presentation
The condensed consolidated interim financial statements of the Company have been prepared in accordance with United States Generally Accepted Accounting Principles (“U.S. GAAP”) and are presented in United States dollars. The functional currency of the Company and each of its subsidiaries is the United States dollar.
The accompanying condensed consolidated interim financial statements include the accounts of the Company and its wholly-owned subsidiaries, Adgero, Adgero Bio, Del Mar BC, Callco, and Exchangeco. All intercompany balances and transactions have been eliminated in consolidation.
The principal accounting policies applied in the preparation of these condensed consolidated interim financial statements are set out below and have been consistently applied to all periods presented.
Certain prior period balances have been reclassified to conform with the current period’s presentation.
Unaudited interim financial data
The accompanying unaudited condensed consolidated interim financial statements have been prepared in accordance with the rules and regulations of the Securities and Exchange Commission for interim financial information. Accordingly, they do not include all of the information and the notes required by U.S. GAAP for complete financial statements. These unaudited condensed consolidated interim financial statements should be read in conjunction with the audited financial statements of the Company as at June 30, 2020 included in our Form 10-K. In the opinion of management, the unaudited condensed consolidated interim financial statements reflect all adjustments, consisting of normal and recurring adjustments, necessary for a fair presentation. The results for three months ended September 30, 2020 are not necessarily indicative of the results to be expected for the fiscal year ending June 30, 2021, or for any other future annual or interim period.
Use of estimates
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions about future events that affect the reported amounts of assets, liabilities, expenses, contingent assets, and contingent liabilities as at the end of, or during, the reporting period. Actual results could significantly differ from those estimates. Significant areas requiring management to make estimates include the fair value of the milestone payment liability, the valuation of equity instruments issued for services, and clinical trial accruals. Further details of the nature of these assumptions and conditions may be found in the relevant notes to these condensed consolidated interim financial statements.
Loss per share
Income or loss per share is calculated based on the weighted average number of common shares outstanding. For the three-month periods ended September 30, 2020 and 2019 diluted loss per share does not differ from basic loss per share since the effect of the Company’s warrants, stock options, and convertible preferred shares is anti-dilutive. As of September 30, 2020, potential common shares of 11,858,152 (2019 – 9,683,596) related to outstanding common share warrants, 2,152,701 (2019 – nil) related to outstanding Series C preferred stock warrants, 6,543,569 (2019 – 780,000) related to stock options, 162,177 (2019 – 162,177) relating to outstanding Series B convertible preferred shares, and 21,516,484 (2019 – nil) relating to outstanding Series C convertible preferred shares were excluded from the calculation of net loss per common share.
Acquired in-process research and development expense
The Company acquired in-process research and development assets in connection with its Merger with Adgero. As the acquired in-process research and development assets were deemed to have no current or alternative future use, an expense of $16.1 million was recognized in the condensed consolidated interim statements of operations for the three month period ended September 30, 2020.
7
Property and equipment is stated at cost less accumulated depreciation. Depreciation is calculated on a straight-line basis over its estimated useful life of five years. Depreciation expense is recognized from the date the equipment is put into use.
Recent accounting pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (“FASB”) or other standard setting bodies that are adopted by the Company as of the specified effective date.
Not yet adopted
Accounting Standards Update (“ASU”) 2020-06 — Debt - Debt with conversion and other options (subtopic 470-20) and derivatives and hedging – contracts in entity’s own equity (subtopic 815-40): accounting for convertible instruments and contracts in an entity’s own equity
The amendments in this update are intended to simplify the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments and contracts on an entity’s own equity. The ASU is part of the FASB’s simplification initiative, which aims to reduce unnecessary complexity in U.S. GAAP. For public business entities that are not smaller reporting companies, the ASU’s amendments are effective for fiscal years beginning after December 15, 2021, and interim periods within those fiscal years. For all other entities, the effective date is for fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. The guidance may be early adopted for fiscal years beginning after December 15, 2020, and interim periods within those fiscal years. The Company has not yet evaluated the impact of adoption of this ASU on its condensed consolidated interim financial statements and related disclosures.
During the three-months ended September 30, 2020, other than ASU 2020-06 there have been no new, or existing recently issued, accounting pronouncements that are of significance, or potential significance, that impact the Company’s condensed consolidated interim financial statements.
|
3 |
Merger |
As described in Note 1, on August 19, 2020, the Company completed its Merger with Adgero in accordance with the terms of the Merger Agreement. To determine the accounting for this transaction under ASU 2017-01, an assessment must be made as to whether an integrated set of assets and activities should be accounted for as an acquisition of a business or an asset acquisition. The guidance requires an initial screen test to determine if substantially all of the fair value of the gross assets acquired is concentrated in a single asset or group of similar assets. If that screen is met, the set is not a business. In connection with the Merger, substantially all of the fair value is concentrated in in-process research and development (“IPR&D”). As such, the Merger has been treated as an acquisition of Adgero assets and an assumption of Adgero liabilities.
Under the terms of the Merger Agreement, upon closing of the Merger, the Company issued 11,439,013 shares of Company common stock and 2,313,904 stock purchase warrants to the security holders of Adgero (“Adgero Warrants”). The Adgero Warrants are exercisable at $3.18 per share (note 6). The Adgero Warrants were valued using a Black-Scholes valuation with a weighted-average risk-free interest rate of 0.21%, a term of one year, a volatility of 115.96%, and a dividend rate of 0%. The estimated volatility of the Company’s common stock at the date of measurement is based on the historical volatility of the Company. The risk-free interest rate is based on rates published by the government for bonds with a maturity similar to the expected remaining life of the instrument at the valuation date. The expected term has been estimated using the remaining life of the warrant. Also, in conjunction with the Merger, the Company issued 571,951 shares of common stock to the placement agent as a success fee. The common shares issued to the former Adgero stockholders as well as the success fee shares, have been value at $1.34 per share which was the closing price of the Company’s common stock on August 19, 2020, the date the Merger closed.
The Company incurred approximately $1.55 million of legal, consulting and other professional fees related to the Merger, of which approximately $1.1 million had been incurred in the year ended June 30, 2020. The transaction costs have been classified as merger expenses in the accompanying unaudited condensed consolidated interim statement of operations for the three months ended September 30, 2020.
8
The following summarizes total consideration transferred to the Adgero stockholders under the Merger as well as the assets acquired and liabilities assumed under the Merger:
|
|
|
$ (in thousands) |
|
|
|
Consideration: |
|
|
|
|
|
Common stock |
|
|
15,328 |
|
|
Warrants |
|
|
630 |
|
|
Success fee shares |
|
|
766 |
|
|
|
|
|
16,724 |
|
|
Net assets acquired: |
|
|
|
|
|
Cash |
|
|
(969 |
) |
|
Other current assets |
|
|
(11 |
) |
|
Property and equipment |
|
|
(175 |
) |
|
Accounts payable and accrued liabilities |
|
|
337 |
|
|
Milestone payment liability |
|
|
188 |
|
|
In-process research and development |
|
|
16,094 |
|
Property and equipment include office furniture that was subsequently sold and laboratory equipment that has not yet been put into use.
The milestone payment liability relates to an asset purchase agreement with St. Cloud Investments, LLC (“St. Cloud”) that Adgero has regarding the acquisition of REM-001. The Agreement, as amended, is dated November 26, 2012 (the “St. Cloud Agreement”). Pursuant to the terms of the St. Cloud Agreement, the Company is obligated to make certain payments under the agreement. The future contingent amounts payable under that agreement are as follows:
|
|
• |
Upon the earlier of (i) a subsequent equity financing to take place after the Company conducts a Phase 2B clinical study in which fifty patients complete the study and their clinical data can be evaluated or (ii) the commencement of a clinical study intended to be used as a definitive study for market approval in any country, the Company is obligated to pay an aggregate amount of $300,000 in cash or an equivalent amount of common stock, with $240,000 to St. Cloud and $60,000 to an employee of the Company; and |
|
|
• |
Upon receipt of regulatory approval of REM-001 Therapy, the Company is obligated to pay an aggregate amount of $700,000 in cash or an equivalent amount of common stock, with $560,000 to St. Cloud and $140,000 to an employee of the Company. |
With respect to the $300,000 and $700,000 potential milestone payments referenced above (each a “Milestone Payment”), if either such Milestone Payment becomes payable, and in the event the Company elects to pay either such Milestone Payment in shares of its common stock, the value of the common stock will equal the average of the closing price per share of the Company’s common stock over the twenty (20) trading days following the first public announcement of the applicable event described above.
The milestone payment liability has been determined using the discounted cash flow value of the two respective milestone payments. A discount rate of 79% has been used which accounts for the probability of success given the phase of clinical development of REM-001. The term is based on an estimate of the planned timing of completion of the respective milestones that would result in payment of the milestones.
The fair value of the IPR&D assets is expensed as a charge in the condensed consolidated interim statements of operations for the three months ended September 30, 2020 as there is no alternative use for these assets.
|
4 |
Related party transactions |
Valent Technologies, LLC Agreements
One of the Company’s officers is a principal of Valent Technologies, LLC (“Valent”) and as result Valent is a related party to the Company.
On September 12, 2010, the Company entered into a Patent Assignment Agreement (the “Valent Assignment Agreement”) with Valent pursuant to which Valent transferred to the Company all its right, title and interest in, and to, the patents for VAL-083 owned by Valent. The Company now owns all rights and title to VAL-083 and is responsible for the drug’s further development and commercialization. In accordance with the terms of the Valent Assignment Agreement, Valent is entitled to receive a future royalty on
9
all revenues derived from the development and commercialization of VAL-083. In the event that the Company terminates the agreement, the Company may be entitled to receive royalties from Valent’s subsequent development of VAL-083 depending on the development milestones the Company has achieved prior to the termination of the Valent Assignment Agreement.
On September 30, 2014, the Company entered into an exchange agreement (the “Valent Exchange Agreement”) with Valent and Del Mar (BC). Pursuant to the Valent Exchange Agreement, Valent exchanged its loan payable in the outstanding amount of $278,530 (including aggregate accrued interest to September 30, 2014 of $28,530), issued to Valent by Del Mar (BC), for 278,530 shares of the Company’s Series A Preferred Stock. The Series A Preferred Stock has a stated value of $1.00 per share (the “Series A Stated Value”) and is not convertible into common stock. The holder of the Series A Preferred Stock is entitled to dividends at the rate of 3% of the Series A Stated Value per year, payable quarterly in arrears. For the three months ended September 30, 2020 and 2019 respectively, the Company recorded $2,089 related to the dividend paid to Valent. The dividends have been recorded as a direct increase in accumulated deficit.
Related party payables
At September 30, 2020 there is an aggregate amount of $382,004 (June 30, 2020 - $663,865) payable to the Company’s officers and directors for fees, expenses, and accrued liabilities.
|
5 |
Loan from National Brain Tumor Society and National Foundation for Cancer Research |
|
|
|
$ (in thousands) |
|
|
|
Balance – June 30, 2020 |
|
|
— |
|
|
Funding |
|
|
500 |
|
|
Financing costs |
|
|
(94 |
) |
|
Interest expense |
|
|
8 |
|
|
Amortization of deferred financing costs |
|
|
27 |
|
|
Balance – September 30, 2020 |
|
|
441 |
|
During the period ended September 30, 2020, the Company received a loan of $500,000 from National Brain Tumor Society (“NBTS”) and the National Foundation for Cancer Research to support VAL-083's preparation for participation in the Global Coalition for Adaptive Research's (“GCAR”) sponsored trial, Glioblastoma (“GBM”) Adaptive Global Innovative Learning Environment (“GBM AGILE”) study (the “NBTS Loan”). In relation to the NBTS Loan, the Company issued 125,000 share purchase warrants which are exercisable at a price of $1.09 per common share until June 19, 2025 and had been included in deferred financing costs as at June 30, 2020 (“NBTS Warrants”). The NBTS Loan is secured by a promissory note, accrues interest at a rate of 6% per annum and matures on June 19, 2021.
The NBTS Warrants were valued at $93,701 using a Black-Scholes valuation with a risk-free interest rate of 0.37%, a term of 5 years, a volatility of 89.82%, and a dividend rate of 0%. The estimated volatility of the Company’s common stock at the date of measurement is based on the historical volatility of the Company. The risk-free interest rate is based on rates published by the government for bonds with a maturity similar to the expected remaining life of the instrument at the valuation date. The expected term has been estimated using the remaining life of the warrant.
|
6 |
Stockholders’ equity |
Preferred stock
Series C Preferred stock
|
|
|
Series C Preferred Stock |
|
|||||
|
|
|
Number of shares |
|
|
$ (in thousands) |
|
||
|
Balance – June 30, 2020 |
|
|
— |
|
|
|
— |
|
|
Issuance |
|
|
25,028 |
|
|
|
18,355 |
|
|
Balance – September 30, 2020 |
|
|
25,028 |
|
|
|
18,355 |
|
10
In connection with the Merger (note 3), the Company issued 25,028 shares of Series C Convertible Preferred Stock (the “Series C Preferred Stock”) in three separate closings of a private placement (Series C-1, C-2, and C-3) in August, 2020. Each share of Series C Preferred Stock was issued at a purchase price of $1,000 per share and is convertible into shares of common stock based on the respective conversion prices which were determined at the closing of each round of the private placement. Subject to ownership limitations, the owners of the Series C Preferred Stock are entitled to receive dividends, payable in shares of common stock at a rate of 10%, 15%, 20% and 25% of the number of shares of common stock issuable upon conversion of the Series C Preferred Stock, on the 12th, 24th, 36th and 48th month, anniversary of the initial closing of the private placement which occurred on August 19, 2020. The Series C Preferred Stock dividends do not require declaration by the Board of Directors and are accrued annually as of the date the dividend is earned in an amount equal to the applicable rate of the stated value. Any outstanding shares of Series C Preferred Stock will automatically convert to shares of common stock on August 19, 2024.
The conversion prices for the Series C-1 Preferred Stock, Series C-2 Preferred Stock and Series C-3 Preferred Stock are $1.16, $1.214 and $1.15, respectively. Based on the conversion prices of the three respective classes of the Series C Preferred Stock, the 25,028 shares of Series C Preferred Stock will be convertible into an aggregate of 21,516,484 shares of common stock. The cumulative dividends to be issued on the 12th, 24th, 36th and 48th month anniversary of the initial closing of the private placement are 15,061,952.
The conversion feature of the Series C Convertible Preferred Stock at the time of issuance was determined to be beneficial on the commitment date. Because the Series C Convertible Preferred Stock was perpetual with no stated maturity date, and the conversions could occur any time from inception, the Company immediately recorded a non-cash deemed dividend of $3.18 million related to the beneficial conversion feature arising from the issuance of Series C Convertible Preferred Stock. This non-cash deemed dividend increased the Company’s net loss attributable to common stockholders and net loss per share.
The Series C Preferred Stock shall with respect to distributions of assets and rights upon the occurrence of a liquidation, rank (i) senior to the Company’s common stock and (ii) senior to any other class or series of capital stock of the Company hereafter created which does not expressly rank pari passu with, or senior to, the Series C Preferred Stock. The Series C Preferred Stock shall be pari passu in liquidation to the Company’s Series A and Series B Preferred Stock. The liquidation value of the Series C Preferred Stock at September 30, 2020 is the stated value of $25,028,000.
Total gross proceeds from the private placement were $25 million, or $21.6 million in net proceeds after deducting financing costs of $3.4 million with respect to agent commissions and expenses, as well as legal and accounting fees. Of the total financing costs, $84,944 was deferred as of June 30, 2020. In addition, the Company issued warrants to purchase 2,504 shares of Series C Stock to the placement agent (“2020 Agent Warrants”) that are convertible into an aggregate 2,152,701 shares of common stock.
A total of 25,028 (June 30, 2020 – Nil) shares of Series C Preferred Stock are outstanding as of September 30, 2020, such that a total of 21,516,484 (June 30, 2020 – Nil) shares of common stock are issuable upon conversion of the Series C Preferred Stock as at September 30, 2020. Converted shares are rounded up to the nearest whole share.
Series B Preferred Stock
During the year ended June 30, 2016, the Company issued an aggregate of 902,238 shares of Series B Preferred Stock at a purchase price of $8.00 per share. Each share of Series B Preferred Stock is convertible into 0.25 shares of common stock equating to a conversion price of $32.00 (the “Conversion Price”) and will automatically convert to common stock at the earlier of 24 hours following regulatory approval of VAL-083 with a minimum closing bid price of $80.00, or five years from the respective final closing dates. The holders of the Series B Preferred Stock are entitled to an annual cumulative, in arrears, dividend at the rate of 9% payable quarterly. The 9% dividend accrues quarterly commencing on the date of issue and is payable quarterly on September 30, December 31, March 31, and June 30 of each year commencing on June 30, 2016. Dividends are payable solely by delivery of shares of common stock, in an amount for each holder equal to the aggregate dividend payable to such holder with respect to the shares of Series B Preferred Stock held by such holder divided by the Conversion Price. The Series B Preferred Stock does not contain any repricing features. Each share of Series B Preferred Stock entitles its holder to vote with the common stock on an as-converted basis.
The Series B Preferred Stock shall with respect to distributions of assets and rights upon the occurrence of a liquidation, rank (i) senior to the Company’s common stock and (ii) senior to any other class or series of capital stock of the Company hereafter created which does not expressly rank pari passu with, or senior to, the Series B Preferred Stock. The Series B Preferred Stock shall be pari passu in liquidation to the Company’s Series A and Series C Preferred Stock. The liquidation value of the Series B Preferred Stock at September 30, 2020 is the stated value of $5.2 million (June 30, 2020 - $5.2 million).
11
In addition, the Company and the holders entered into a royalty agreement, pursuant to which the Company will pay the holders of the Series B Preferred Stock, in aggregate, a low, single-digit royalty based on their pro rata ownership of the Series B Preferred Stock on products sold directly by the Company or sold pursuant to a licensing or partnering arrangement (the “Royalty Agreement”).
Upon conversion of a holder’s Series B Preferred Stock to common stock, such holder shall no longer receive ongoing royalty payments under the Royalty Agreement but will be entitled to receive any residual royalty payments that have vested. Rights to the royalties shall vest during the first three years following the applicable closing date, in equal thirds to holders of the Series B Preferred Stock on each of the three vesting dates, upon which vesting dates such royalty amounts shall become vested royalties.
Pursuant to the Series B Preferred Stock dividend, during the three months ended September 30, 2020, the Company issued 3,700 (2019 – 3,700) shares of common stock and recognized $5,180 (2019 – $2,046) as a direct increase in accumulated deficit. During the three months ended September 30, 2020 there were no conversions (2019 - 25,000) of Series B Preferred Stock for nil (2019 – 6,250) shares of common stock.
A total of 648,613 (June 30, 2020 – 648,613) shares of Series B Preferred Stock are outstanding as of September 30, 2020, such that a total of 162,177 (June 30, 2020 – 162,177) shares of common stock are issuable upon conversion of the Series B Preferred Stock as at September 30, 2020. Converted shares are rounded up to the nearest whole share.
Series A Preferred Stock
Effective September 30, 2014, the Company filed a Certificate of Designation of Series A Preferred Stock (the “Series A Certificate of Designation”) with the Secretary of State of Nevada. Pursuant to the Series A Certificate of Designation, the Company designated 278,530 shares of preferred stock as Series A Preferred Stock. The shares of Series A Preferred Stock have a stated value of $1.00 per share (the “Series A Stated Value”) and are not convertible into common stock. The holder of the Series A Preferred Stock is entitled to dividends at the rate of 3% of the Series A Stated Value per year, payable quarterly in arrears. Upon any liquidation of the Company, the holder of the Series A Preferred Stock will be entitled to be paid, out of any assets of the Company available for distribution to stockholders, the Series A Stated Value of the shares of Series A Preferred Stock held by such holder, plus any accrued but unpaid dividends thereon, prior to any payments being made with respect to the common stock. The Series A Preferred Stock is held by Valent (note 4).
The Series A Preferred Stock shall with respect to distributions of assets and rights upon the occurrence of a liquidation, rank (i) senior to the Company’s common stock, and (ii) senior to any other class or series of capital stock of the Company hereafter created which does not expressly rank pari passu with, or senior to, the Series A Preferred Stock. The Series A Preferred Stock shall be pari passu in liquidation to the Company’s Series B and Series C Preferred Stock. The liquidation value of the Series A Preferred stock at September 30, 2020 and June 30, 2020 was $278,530.
There was no change to the Series A Preferred stock for the three months ended September 30, 2020 or 2019.
Common stock
Stock Issuances
Three months ended September 30, 2019
Underwritten public offering
On August 16, 2019, the Company closed on the sale of (i) 4,895,000 shares of its common stock, par value $0.001 per share (the “Common Stock”), (ii) pre-funded warrants (“PFW”) to purchase an aggregate of 2,655,000 shares of Common Stock and (iii) common warrants to purchase an aggregate of 7,762,500 shares of Common Stock (“2020 Investor Warrants”), including 800,000 shares of Common Stock and 2020 Investor Warrants to purchase an aggregate of 1,012,500 shares of Common Stock sold pursuant to a partial exercise by the underwriters of the underwriters’ option to purchase additional securities, in the Company’s underwritten public offering (the “Offering”). Each share of Common Stock or PFW, as applicable, was sold together with a 2020 Investor Warrant to purchase one share of Common Stock at a combined effective price to the public of $1.00 per share of Common Stock and accompanying 2020 Investor Warrant.
The net proceeds from the Offering, including from the partial exercise of the underwriters’ option to purchase additional securities, were $6,582,966 after deducting underwriting discounts and commissions, and other offering expenses.
The 2020 Investor Warrants are exercisable at $1.00 per share until their expiry on August 16, 2024 and the PFW are exercisable at $0.01 per share at any time after August 16, 2019. The Company also issued 377,500 warrants to the underwriters of the
12
Offering. The underwriter warrants are exercisable at $1.15 per share commencing February 10, 2020 until their expiry on August 14, 2022.
During the three months ended September 30, 2019, all of the 2,655,000 PFW were exercised at $0.01 per PFW for proceeds of $26,550.
Shares issued for services
During the three months ended September 30, 2020, the Company issued Nil (2019 – 6,925) shares of common stock for services resulting in the recognition of $Nil (2019 – $4,843) in expense. All of the shares issued for services for the three months ended September 30, 2019 have been recognized as research and development expense.
2017 Omnibus Incentive Plan
The Company’s board of directors has approved adoption of the Company’s 2017 Omnibus Equity Incentive Plan (the “2017 Plan”) that has also been approved by the Company’s stockholders. The board of directors also approved a form of Performance Stock Unit Award Agreement to be used in connection with grants of performance stock units (“PSUs”) under the 2017 Plan. Under the 2017 Plan, 6,700,000 shares of Company common stock are currently reserved for issuance, less the number of shares of common stock issued under the Del Mar (BC) 2013 Amended and Restated Stock Option Plan (the “Legacy Plan”) or that are subject to grants of stock options made, or that may be made, under the Legacy Plan. As of September 30, 2020, a total of 164,235 shares of common stock are currently outstanding under the Legacy Plan and/or are subject to outstanding stock options granted under the Legacy Plan, and a total of 6,379,334 shares of common stock have been issued under the 2017 Plan and/or are subject to outstanding stock options granted under the 2017 Plan leaving 156,431 shares of common stock available at September 30, 2020 for issuance under the 2017 Plan if all such options under the Legacy Plan were exercised.
The maximum number of shares of Company common stock with respect to which any one participant may be granted awards during any calendar year is 8% of the Company’s fully diluted shares of common stock on the date of grant (excluding the number of shares of common stock issued under the 2017 Plan and/or the Legacy Plan or subject to outstanding awards granted under the 2017 Plan and/or the Legacy Plan). No award will be granted under the 2017 Plan on or after July 7, 2027, but awards granted prior to that date may extend beyond that date.
During the three months ended September 30, 2020, a total of 222,584 stock options issued to directors of the Company were amended such that the period to exercise vested stock options from the date of termination of continuous service with the Company was extended from 90 days to one year. Of the total of 222,584, 66,850 had their expiry increased from September 26, 2020 to June 26, 2021 and 155,734 had their expiry increased from November 19, 2020 to August 19, 2021. As a result of the amendments, a total of $8,569 stock-based compensation expense has been recognized. In addition, 250,000 stock options previously granted to an officer of the Company were amended such that the vesting of the stock options was changed from a completely contingent vesting to a time-based vesting such that 1/6th of the stock options vest on the six-month anniversary of the amendment date with the remaining portion vesting in equal monthly installments over a period of 30 months commencing on the seven-month anniversary of the amendment date. A total compensation expense of $319,376 will be recognized over the amended vesting period for the 250,000 stock options.
During the three months ended September 30, 2020, a total of 4,758,687 stock options were granted to executive officers and directors of the Company. Of these, 4,698,687 have an exercise price of $1.70 per share and 60,000 have an exercise price of $1.355 per share. Of the total granted, 4,278,687 stock options vest as to 1/6 on the six month anniversary of the grant date with the remaining portion vesting in equal monthly installments over a period of 30 months commencing on the seven month anniversary of the grant date. Of the total stock options granted to executive officers and directors, 480,000 vest in 12 equal monthly installments beginning on October 15, 2020. All of the stock options granted have a 10-year term and are subject to cancellation upon the grantees’ termination of service for the Company, with certain exceptions.
Stock Options
The following table sets forth changes in stock options outstanding under all plans:
|
|
|
Number of stock options outstanding (in thousands) |
|
|
Weighted average exercise price |
|
||
|
Balance – June 30, 2020 |
|
|
1,559 |
|
|
|
4.61 |
|
|
Granted |
|
|
4,999 |
|
|
|
1.68 |
|
|
Forfeited |
|
|
(14 |
) |
|
|
1.42 |
|
|
Balance – September 30, 2020 |
|
|
6,544 |
|
|
|
2.38 |
|
13
The following table summarizes stock options outstanding and exercisable under all plans at September 30, 2020:
|
Exercise price $ |
|
|
Number Outstanding at September 30, 2020 (in thousands) |
|
|
Weighted average remaining contractual life (years) |
|
|
Number exercisable at September 30, 2020 (in thousands) |
|
||||
|
|
0.61 |
|
|
|
1,010 |
|
|
|
8.93 |
|
|
|
566 |
|
|
|
0.74 |
|
|
|
250 |
|
|
|
9.12 |
|
|
|
— |
|
|
|
1.36 |
|
|
|
300 |
|
|
|
9.98 |
|
|
|
— |
|
|
|
1.70 |
|
|
|
4,699 |
|
|
|
9.96 |
|
|
|
— |
|
|
|
6.10 |
|
|
|
30 |
|
|
|
8.11 |
|
|
|
26 |
|
|
|
7.00 |
|
|
|
3 |
|
|
|
7.73 |
|
|
|
3 |
|
|
|
8.70 |
|
|
|
12 |
|
|
|
7.09 |
|
|
|
12 |
|
|
|
9.83 |
|
|
|
83 |
|
|
|
7.64 |
|
|
|
65 |
|
|
|
10.60 |
|
|
|
4 |
|
|
|
7.54 |
|
|
|
3 |
|
|
|
11.70 |
|
|
|
30 |
|
|
|
2.41 |
|
|
|
30 |
|
|
|
14.94 |
|
|
|
3 |
|
|
|
1.67 |
|
|
|
3 |
|
|
|
20.00 |
|
|
|
13 |
|
|
|
1.02 |
|
|
|
13 |
|
|
|
21.10 |
|
|
|
14 |
|
|
|
6.77 |
|
|
|
14 |
|
|
|
29.60 |
|
|
|
5 |
|
|
|
4.35 |
|
|
|
5 |
|
|
|
37.60 |
|
|
|
5 |
|
|
|
5.36 |
|
|
|
5 |
|
|
|
41.00 |
|
|
|
4 |
|
|
|
6.11 |
|
|
|
4 |
|
|
|
42.00 |
|
|
|
41 |
|
|
|
2.31 |
|
|
|
41 |
|
|
|
44.80 |
|
|
|
3 |
|
|
|
5.36 |
|
|
|
3 |
|
|
|
49.50 |
|
|
|
22 |
|
|
|
3.82 |
|
|
|
22 |
|
|
|
53.20 |
|
|
|
8 |
|
|
|
5.60 |
|
|
|
8 |
|
|
|
61.60 |
|
|
|
2 |
|
|
|
2.50 |
|
|
|
2 |
|
|
|
92.00 |
|
|
|
3 |
|
|
|
2.67 |
|
|
|
3 |
|
|
|
|
|
|
|
6,544 |
|
|
|
|
|
|
|
828 |
|
Included in the number of stock options outstanding are 2,500 stock options granted at an exercise price of CA$20.00. The exercise price of these options shown in the above table have been converted to US$14.94 using the period ending closing exchange rate. Stock options granted during the three months ended September 30, 2020 have been valued using a Black-Scholes pricing model with the following assumptions:
|
|
|
September 30, 2020 |
|
|
|
|
Dividend rate |
|
|
— |
|
% |
|
Volatility |
|
121% to 153 |
|
% |
|
|
Risk-free rate |
|
0.19% to 0.42 |
|
% |
|
|
Term – years |
|
0.4 to 5.8 |
|
|
|
The estimated volatility of the Company’s common stock at the date of issuance of the stock options is based on the historical volatility of the Company. The risk-free interest rate is based on rates published by the government for bonds with a maturity similar to the expected remaining life of the stock options at the valuation date. The expected life of the stock options has been estimated using the plain vanilla method.
The Company has recognized the following amounts as stock option expense for the periods noted (in thousands):
|
|
|
Three months ended September 30, |
|
|||||
|
|
|
2020 $ |
|
|
2019 $ |
|
||
|
Research and development |
|
|
91 |
|
|
8 |
|
|
|
General and administrative |
|
|
314 |
|
|
|
43 |
|
|
|
|
|
405 |
|
|
|
51 |
|
All of the stock option expense for the periods ended September 30, 2020 and 2019 has been recognized as additional paid in capital. The aggregate intrinsic value of stock options outstanding at September 30, 2020 was $977,465 (2019 - $0) and the aggregate
14
intrinsic value of stock options exercisable at September 30, 2020 was $446,950 (2019 - $0). As of September 30, 2020, there was $7,802,208 in unrecognized compensation expense that will be recognized over the next 3.0 years. No stock options granted under the Company’s equity plans have been exercised during the three months ended September 30, 2020. Upon the exercise of stock options new shares will be issued.
The following table sets forth changes in unvested stock options under all plans:
|
|
|
Number of Options (in thousands) |
|
|
Weighted average exercise price $ |
|
||
|
Unvested at June 30, 2020 |
|
|
858 |
|
|
|
0.98 |
|
|
Granted |
|
|
4,999 |
|
|
|
1.68 |
|
|
Vested |
|
|
(127 |
) |
|
|
1.18 |
|
|
Forfeited |
|
|
(14 |
) |
|
|
1.42 |
|
|
Unvested at September 30, 2020 |
|
|
5,716 |
|
|
|
1.59 |
|
The aggregate intrinsic value of unvested stock options at September 30, 2020 was $530,516 (2019 - $0). The unvested stock options have a remaining weighted average contractual term of 9.83 (2019 – 9.76) years.
Common Stock Warrants
The following table sets forth changes in outstanding common stock warrants:
|
|
|
Number of Warrants (in thousands) |
|
|
Weighted average exercise price $ |
|
||
|
Balance – June 30, 2020 |
|
|
10,309 |
|
|
|
2.71 |
|
|
Issuance of Adgero Warrants |
|
|
2,314 |
|
|
|
3.18 |
|
|
Exercise of warrants (i) |
|
|
(994 |
) |
|
|
1.00 |
|
|
Warrants issued for services (ii) |
|
|
330 |
|
|
|
1.49 |
|
|
Expiry of warrants (iii) |
|
|
(101 |
) |
|
|
30.00 |
|
|
Balance – September 30, 2020 |
|
|
11,858 |
|
|
|
2.67 |
|
|
i) |
A total of 994,000 2020 Investor Warrants were exercised at $1.00 per share. |
|
ii) |
Warrants issued for services are exercisable at various prices and expire at the various dates noted in the table below. |
|
iii) |
The warrant expiries include the 2015 Investor Warrants, the 2015 Agent Warrants, and certain warrants issued for services. All of the expired warrants were exercisable at $30 per share. |
15
The following table summarizes the Company’s outstanding common stock warrants as of September 30, 2020:
|
Description of warrants |
|
Number (in thousands) |
|
|
Exercise price $ |
|
|
Expiry date |
||
|
2020 Investor warrants |
|
|
6,744 |
|
|
|
1.00 |
|
|
August 16, 2024 |
|
2019 Investor warrants |
|
|
760 |
|
|
|
3.10 |
|
|
June 5, 2024 |
|
2019 Investor warrants |
|
|
280 |
|
|
|
12.50 |
|
|
September 22, 2022 |
|
2017 Investor warrants |
|
|
208 |
|
|
|
35.00 |
|
|
April 19, 2022 |
|
NBTS Warrants |
|
|
125 |
|
|
|
1.09 |
|
|
June 19, 2025 (i) |
|
Warrants issued for services |
|
|
25 |
|
|
|
30.00 |
|
|
December 1, 2020 to February 1, 2021 |
|
Warrants issued for services |
|
|
6 |
|
|
|
17.80 |
|
|
January 25, 2023 |
|
Warrants issued for services |
|
|
34 |
|
|
|
11.70 |
|
|
February 27, 2023 |
|
Warrants issued for services |
|
|
14 |
|
|
|
9.00 |
|
|
September 15, 2023 and October 11, 2023 |
|
Warrants issued for services |
|
|
280 |
|
|
|
0.75 |
|
|
November 18, 2023 |
|
Warrants issued for services |
|
|
250 |
|
|
|
0.64 |
|
|
January 20, 2024 |
|
Warrants issued for services |
|
|
330 |
|
|
|
1.49 |
|
|
September 22, 2023 |
|
2020 Underwriter Warrants |
|
|
377 |
|
|
|
1.15 |
|
|
August 14, 2022 |
|
2019 Agent warrants |
|
|
47 |
|
|
|
3.88 |
|
|
June 3, 2024 |
|
2018 Agent warrants |
|
|
40 |
|
|
|
12.50 |
|
|
September 20, 2022 |
|
2017 Agent warrants |
|
|
14 |
|
|
|
40.60 |
|
|
April 12, 2022 |
|
2016 Agent warrants |
|
|
10 |
|
|
|
40.00 |
|
|
May 12, 2021 |
|
Adgero Warrants |
|
|
1,206 |
|
|
|
3.18 |
|
|
April 8, 2021 |
|
Adgero Warrants |
|
|
353 |
|
|
|
3.18 |
|
|
August 31, 2021 |
|
Adgero Warrants |
|
|
755 |
|
|
|
3.18 |
|
|
January 17, 2022 |
|
|
|
|
11,858 |
|
|
|
|
|
|
|
|
(i) |
NBTS Warrants were issued in connection with respect to the NBTS Loan (note 5). |
Series C Preferred Stock Warrants
In connection with the Series C Preferred Stock private placement, the Company issued 2,504 Series C Stock purchase warrants (the “Series C Warrants”). The Series C Warrants have an exercise price of $1,000 per share, provide for a cashless exercise feature, and are exercisable for a period of four years from August 19, 2020. The Series C Preferred Stock issuable upon exercise of the Series C Warrants is convertible into shares of common stock in the same manner as each respective series of outstanding Series C Stock, and will be entitled to the same dividend rights as each respective series.
The 2020 Agent Warrants were valued at a total of approximately $3.3 million using a binomial pricing model with a risk-free interest rate of 0.27%, a term of 4.0 years, and a volatility of 95.2% to 95.8%. The estimated volatility of the Company’s common stock at the date of measurement is based on the historical volatility of the Company’s common stock. The risk-free interest rate is based on rates published by the government for bonds with a maturity similar to the expected remaining life of the instrument at the valuation date. The expected term has been estimated using the contractual term of the warrant.
The following table sets forth changes in outstanding Series C Warrants:
|
|
|
Number of Warrants |
|
|
Conversion price $ |
|
||
|
Balance – June 30, 2020 |
|
|
— |
|
|
|
|
|
|
Issuance of Preferred Series C-1 Warrants |
|
|
1,959 |
|
|
|
1.16 |
|
|
Issuance of Preferred Series C-2 Warrants |
|
|
219 |
|
|
|
1.21 |
|
|
Issuance of Preferred Series C-3 Warrants |
|
|
326 |
|
|
|
1.15 |
|
|
Balance – September 30, 2020 |
|
|
2,504 |
|
|
|
|
|
16
The following table summarizes the Company’s outstanding Series C Warrants as of September 30, 2020:
|
Description of warrants |
|
Number |
|
|
Conversion price $ |
|
|
Number of conversion shares (in thousands) |
|
|
Cumulative common stock dividends (in thousands) |
|
|
||||
|
Series 1 |
|
|
1,959 |
|
|
|
1.16 |
|
|
|
1,689 |
|
|
|
1,182 |
|
|
|
Series 2 |
|
|
219 |
|
|
|
1.21 |
|
|
|
180 |
|
|
|
126 |
|
|
|
Series 3 |
|
|
326 |
|
|
|
1.15 |
|
|
|
283 |
|
|
|
198 |
|
|
|
|
|
|
2,504 |
|
|
|
|
|
|
|
2,152 |
|
|
|
1,506 |
|
|
|
7 |
Supplementary statement of cash flows information |
The Company incurred the following non-cash investing and financing transactions (in thousands):
|
|
|
Three months ended September 30, 2020 |
|
|
Three months ended September 30, 2019 |
|
||
|
Series B Preferred Stock common stock dividend (note 6) |
|
|
5 |
|
|
|
2 |
|
|
Deemed dividend recognized on beneficial conversion features of Series C Preferred stock issuance (note 6) |
|
|
3,181 |
|
|
|
— |
|
|
Non-cash issue costs (note 6) |
|
|
3,287 |
|
|
|
— |
|
|
Issue costs in accounts payable (note 6) |
|
|
193 |
|
|
|
— |
|
|
Income taxes paid |
|
|
— |
|
|
|
— |
|
|
Interest paid |
|
|
— |
|
|
|
— |
|
|
8 |
Financial instruments |
The Company has financial instruments that are measured at fair value. To determine the fair value, the Company uses the fair value hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use to value an asset or liability and are developed based on market data obtained from independent sources. Unobservable inputs are inputs based on assumptions about the factors market participants would use to value an asset or liability. The three levels of inputs that may be used to measure fair value are as follows:
|
|
• |
Level one - inputs utilize quoted prices (unadjusted) in active markets for identical assets or liabilities; |
|
|
• |
Level two - inputs are inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly such as interest rates, foreign exchange rates, and yield curves that are observable at commonly quoted intervals; and |
|
|
• |
Level three - unobservable inputs developed using estimates and assumptions, which are developed by the reporting entity and reflect those assumptions that a market participant would use. |
Assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurements. Changes in the observability of valuation inputs may result in a reclassification of levels for certain securities within the fair value hierarchy.
The Company’s financial instruments consist of cash and cash equivalents, other receivables, accounts payable, related party payables and loan payable. The carrying values of cash and cash equivalents, other receivables, accounts payable and related party payables approximate their fair values due to the immediate or short-term maturity of these financial instruments. The fair value of the loan payable is equal to its principal and accrued interest of $508,466 as at September 30, 2020.
17
Warrant exercises
Subsequent to September 30, 2020, 161,084 warrants were exercised at $1.00 per share for gross proceeds of $161,084.
Stock option exercises
Subsequent to September 30, 2020, 35,000 stock options were exercised at $0.61 per share for gross proceeds of $21,350.
Stock option amendments
On November 11, 2020, the Board of Directors approved the acceleration of vesting of 279,675 stock options to purchase shares of the Company’s common stock previously granted on September 5, 2019 to an executive officer of the Company. The exercise price of the stock options is $0.61 per share
The Company has evaluated its subsequent events from September 30, 2020 through the date these condensed consolidated interim financial statements were issued and has determined that there are no subsequent events requiring disclosure in these condensed consolidated interim financial statements other than the items noted below.
18
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
This Management’s Discussion and Analysis (“MD&A”) contains “forward-looking statements”, within the meaning of the Private Securities Litigation Reform Act of 1995, which represent our projections, estimates, expectations, or beliefs concerning, among other things, financial items that relate to management’s future plans or objectives or to our future economic and financial performance. In some cases, you can identify these statements by terminology such as “may”, “should”, “plans”, “believe”, “will”, “anticipate”, “estimate”, “expect” “project”, or “intend”, including their opposites or similar phrases or expressions. You should be aware that these statements are projections or estimates as to future events and are subject to a number of factors that may tend to influence the accuracy of the statements. These forward-looking statements should not be regarded as a representation by us or any other person that our events or plans will be achieved. You should not unduly rely on these forward-looking statements, which speak only as of the date of this report. Except as may be required under applicable securities laws, we undertake no obligation to publicly revise any forward-looking statement to reflect circumstances or events after the date of this report or to reflect the occurrence of unanticipated events.
You should review the factors and risks we describe under “Risk Factors” in this report on Form 10-K for the year ended June 30, 2020 and in our other filings with the Securities and Exchange Commission, available at www.sec.gov. Actual results may differ materially from any forward-looking statement.
Impact of Coronavirus (“COVID-19”) on our Operations, Financial Condition, Liquidity and Results of Operations
In December 2019, a novel strain of coronavirus, COVID-19, was reported to have surfaced in Wuhan, China and on March 11, 2020 it was declared a pandemic by the World Health Organization. The ultimate impact of the COVID-19 pandemic on our operations is unknown and will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the COVID-19 outbreak, new information which may emerge concerning the duration and severity of the COVID-19 pandemic, and any additional preventative and protective actions that governments, or us, may determine are needed.
To date, the COVID-19 pandemic has not caused significant disruption to our clinical studies. Each of our ongoing Phase 2 clinical studies is being conducted at a single site which has reduced the risk of disruption. Patient visits are currently taking place on schedule for both the MD Anderson Cancer Center study being conducted in Houston, Texas and the Sun Yat-sen University Cancer Center study being conducted in China. In addition, thus far, any disruptions to patient treatments have been within allowances under each study protocol. Access to the sites by our clinical monitors has been limited during the COVID-19 pandemic but the recording of study data in both studies and patient treatments at both study sites are being conducted per protocol at this time.
We have cash available to fund planned operations into the fourth quarter of calendar 2021. However, the COVID-19 pandemic has created significant economic uncertainty and volatility in the credit and capital markets. The ultimate impact of the COVID-19 pandemic on our ability to raise additional capital is unknown and will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the COVID-19 outbreak and new information which may emerge concerning the severity of the COVID-19 pandemic. We may not be able to raise sufficient additional capital and may tailor our drug candidate development programs based on the amount of funding we are able to raise in the future. Nevertheless, there is no assurance that these initiatives will be successful.
Background
Kintara Therapeutics, Inc. (formerly DelMar Pharmaceuticals, Inc.) is a clinical stage, biopharmaceutical company focused on the development and commercialization of new cancer therapies.
On June 9, 2020, we entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”), by and among Adgero Acquisition Corp., our wholly-owned subsidiary incorporated in the State of Delaware (“Merger Sub”), and Adgero Biopharmaceuticals Holdings, Inc., a Delaware corporation (“Adgero”). On August 19, 2020, upon the terms and subject to the conditions set forth in the Merger Agreement, Merger Sub merged with and into Adgero (the “Merger”), the separate corporate existence of Merger Sub ceased and Adgero continued its existence under Delaware law as the surviving corporation in the Merger and became our direct, wholly-owned subsidiary. As a result of the Merger, each issued and outstanding share of Adgero common stock, par value $0.0001 per share (the “Adgero Common Stock”) (other than treasury shares held by Adgero), was converted automatically into the right to receive 1.5740 shares (the “Exchange Ratio”) of our common stock, and cash in lieu of any fractional shares. Also, each outstanding warrant to purchase Adgero Common Stock was converted into a warrant exercisable for that number of shares of our common stock equal to the product of (x) the aggregate number of shares of Adgero Common Stock for which such warrant was exercisable and (y) the Exchange Ratio.
Following the completion of the Merger, we changed our name from DelMar Pharmaceuticals, Inc. to Kintara Therapeutics, Inc. and began trading on Nasdaq under the symbol “KTRA”.
19
We are the parent company of Del Mar (BC), a British Columbia, Canada corporation, and Adgero. We are also the parent company to Callco and Exchangeco which are British Columbia, Canada corporations. Callco and Exchangeco were formed to facilitate the Reverse Acquisition.
References to “we”, “us”, and “our”, refer to Kintara and our wholly-owned subsidiaries, Del Mar (BC), Adgero, Callco and Exchangeco.
We are dedicated to the development of novel cancer therapies for patients with unmet medical needs. Our mission is to benefit patients by developing and commercializing anti-cancer therapies for patients whose solid tumors exhibit features that make them resistant to, or unlikely to respond to, currently available therapies, with particular focus on orphan cancer indications.
Our two lead candidates are VAL-083, a novel, validated, DNA-targeting agent, for the treatment of drug-resistant solid tumors such as glioblastoma multiforme (“GBM”) and potentially other solid tumors, including ovarian cancer, non-small cell lung cancer (“NSCLC”), and diffuse intrinsic pontine glioma (“DIPG”) and REM-001, a late-stage photodynamic therapy (“PDT”) for the treatment of cutaneous metastatic breast cancer (“CMBC”). PDT is a treatment that uses light sensitive compounds, or photosensitizers, that, when exposed to specific wavelengths of light, act as a catalyst to produce a form of oxygen that induces local tumor cell death.
Recent Highlights
|
|
• |
On October 21, 2020, we announced we had entered into a definitive agreement with the Global Coalition for Adaptive Research (“GCAR”) to include VAL-083 in GCAR’s Glioblastoma Adaptive Global Innovative Learning Environment (“GBM AGILE”) Study, an adaptive clinical study platform for patients with GBM. We plan to utilize the GBM AGILE study to serve as the basis for VAL-083’s new drug application submission and registration. |
|
|
• |
On August 21, 2020, we announced we had regained compliance with the minimum bid price requirement for continued listing on The Nasdaq Capital Market. As a result of our shares having had a closing bid price at, or above, $1.00 per share for a minimum of ten (10) consecutive business days, our stock had regained compliance with the minimum bid price requirement and the matter is now closed. |
|
|
• |
On August 19, 2020, we completed our merger with Adgero and through three closings of a private placement, the first of which also closed on August 19, 2020, we raised aggregate gross proceeds of approximately $25 million, or net proceeds of approximately $21.6 million. |
Private Placement of Series C Preferred Stock
In conjunction with the closing of the Merger, and through a series of three private placement closings, we issued a total of 25,028 shares of Series C Convertible Preferred Stock (the “Series C Stock”) at a purchase price of $1,000 per share for total aggregate gross proceeds of approximately $25 million, or net proceeds of approximately $21.6 million. Each closing of the private placement was priced at-the-market under the rules of the Nasdaq Stock Market.
The Series C Stock was issued in three series (C-1, C-2, and C-3) at conversion prices equal to $1.16, $1.214 and $1.15, respectively. As result, we issued a total of 25,028 shares of Series C Stock, which will be convertible into an aggregate of 21,516,484 shares of common stock. The Series C Stock will be entitled to receive dividends, payable in shares of common stock at a rate of 10%, 15%, 20% and 25% of the number of shares of common stock issuable upon conversion of the Series C Stock, on the 12th, 24th, 36th and 48th month, anniversary of the initial closing of the private placement which occurred on August 19, 2020; provided, that the holder of such shares has not converted the shares of Series C Stock prior to the applicable dividend rate.
In connection with the private placement, we entered into a Placement Agency Agreement (the “Placement Agency Agreement”), with Aegis Capital Corp., which acted as our exclusive placement agent (the “Placement Agent”) for the private placement. Pursuant to the terms of the Placement Agency Agreement, in connection with the three closings of the private placement, we paid the Placement Agent an aggregate cash fee of $2,502,800, a non-accountable expense allowance of approximately $650,840 and issued to the Placement Agent, or its designees, warrants to purchase 2,504 shares of Series C Stock (the “Placement Agent Warrants”). The Placement Agent Warrants have an exercise price of $1,000 per share, provide for a cashless exercise feature and are exercisable for a period of four years from the date of the initial closing of the private placement. The Series C Stock issuable upon exercise of the Placement Agent Warrants will be convertible into shares of common stock and will be entitled to the same dividend rights as the outstanding Series C Stock. In addition, and as compensation for advisory services rendered in connection with the Merger, we issued 571,951 shares of common stock to the Placement Agent.
20
(calendar quarters)
Below are our planned, or expected, milestones for the respective time periods noted:
Q4 2020
|
|
• |
VAL-083: First Patient Enrolled - GCAR GBM AGILE Registration Study |
|
|
• |
VAL-083: Top Line Results - Phase 2 Newly-Diagnosed GBM Study |
Q1 2021
|
|
• |
VAL-083: Top Line Results - Phase 2 Recurrent GBM Study |
Q2 2021
|
|
• |
VAL-083: Top Line Results - Phase 2 Adjuvant GBM Study |
|
|
• |
REM-001: First patient enrolled – CMBC lead-in study |
Q4 2021
|
|
• |
REM-001: Top Line Results – CMBC lead-in study |
H1 2022
|
|
• |
VAL-083: Graduation from Stage 1 to Stage 2 - GCAR GBM AGILE Registration Study |
Product Pipeline
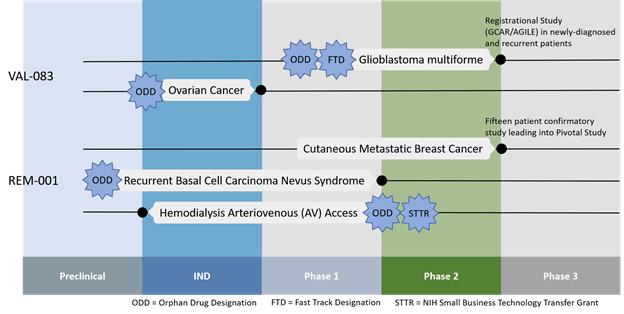
21
Background
VAL-083 is a first-in-class, small-molecule, DNA-targeting chemotherapeutic that has demonstrated activity against a range of tumor types in prior Phase 1 and Phase 2 clinical studies sponsored by the US National Cancer Institute (“NCI”). “First-in-class” means that VAL-083 embodies a unique molecular structure which is not an analogue or derivative of any approved product, or product under development, for the treatment of cancer. As part of our business strategy, we leverage and build upon these prior NCI investments and data from more than 40 NCI- Phase 1 and Phase 2 clinical studies, which includes an estimated 1,100 patient safety database.
Prior studies of VAL-083 have shown increased median overall survival benefits versus radiation alone validating the tumor affecting properties of VAL-083. Our recent research has highlighted the opportunities afforded by VAL-083’s unique mechanism of action and its potential to address unmet medical needs in a well-defined and acknowledged biomarker selected population within the larger GBM population. We are thus focusing our initial development efforts on patients whose tumors exhibit biological features that make them resistant to, or unlikely to respond to, currently available therapies as identified by the National Comprehensive Cancer Network (“NCCN”). For example, our research demonstrating VAL-083’s activity in GBM independent of the O6-methyl guanine methyltransferase (“MGMT”) methylation status allows us to focus patient selection based on this important biomarker and thus improve the probability of success in our current and future clinical studies.
We are currently conducting two open-label, biomarker driven Phase 2 studies in MGMT-unmethylated GBM. MGMT is a DNA-repair enzyme that is associated with resistance to temozolomide (“TMZ”), the current standard-of-care chemotherapy used in the treatment of GBM. Greater than 60% of GBM patients have MGMT-unmethylated tumors and exhibit a high expression of MGMT, which is correlated with TMZ treatment failure and poor patient outcomes as indicated in the NCCN guidelines for GBM treatment published in September 2017. Our research to-date demonstrates that VAL-083’s anti-tumor activity is independent of MGMT expression. In our current Phase 2 studies we are using MGMT as a biomarker to identify patients for treatment with VAL-083 in three distinct GBM patient populations:
|
|
• |
MGMT-unmethylated GBM, currently comprising two ongoing, separate Phase 2 clinical studies for: |
|
|
▪ |
GBM patients in two study arms at MD Anderson Cancer Center: |
|
|
• |
as adjuvant therapy immediately following concomitant TMZ treatment with chemoradiation; and |
|
|
• |
in Avastin®-naïve recurrent GBM patients; |
|
|
▪ |
Newly diagnosed GBM patients at Sun Yat-sen University Cancer Center (“SYSUCC”). |
On June 4, 2020, we accepted an invitation from the Global Coalition for Adaptive Research (“GCAR”) to include VAL-083 in GCAR’s Glioblastoma Adaptive Global Innovative Learning Environment (“GBM AGILE”) Study, an adaptive clinical study platform for patients with GBM. On October 21, 2020, we announced we had entered into a definitive agreement with GCAR. We plan to utilize the GBM AGILE Study to serve as the basis for VAL-083’s new drug application submission and registration.
GBM AGILE is an international effort to develop potential therapies for newly-diagnosed and recurrent GBM utilizing an FDA approved master protocol with multiple drugs from multiple sponsors tested simultaneously, and over time, against a common control arm with a primary endpoint of overall survival. We intend to use results from the VAL-083 arm of the GBM AGILE study to file for FDA approval, assuming results support such a filing. GBM AGILE is a Phase II/Phase III study which employs a cost-efficient, adaptive study design with a Stage 1 learning and adapting phase and a Stage 2 expansion and confirmation phase. GBM AGILE will consist of up to a maximum 200 patients stratified by three subtypes: newly-diagnosed methylated, newly-diagnosed unmethylated, and recurrent. The effort is led by key opinion leaders in the GBM field and has the collective support of an international group of more than 130 clinicians, researchers, biostatisticians, imagers, pathologists, leaders from government and industry, and patient advocates.
We have also undertaken research in ovarian cancer. Ovarian cancer is the fifth most common cancer in women and is the leading cause of death among women diagnosed with gynecological malignancies. We are in the process of evaluating the best path forward in ovarian cancer including the potential combination of VAL-083 with PARP inhibitors. The FDA granted orphan drug designation for the use of VAL-083 in the treatment of ovarian cancer in 2016.
In addition to our clinical development activities in the United States, pursuant to our collaboration with Guangxi Wuzhou Pharmaceutical (Group) Co. Ltd. (“Guangxi Wuzhou Pharmaceutical Company”), we have provided Guangxi Wuzhou Pharmaceutical Company certain commercial rights to VAL-083 in China where it is approved as a chemotherapy for the treatment of chronic myelogenous leukemia (“CML”) and lung cancer. Guangxi Wuzhou Pharmaceutical Company is the only manufacturer presently licensed by the China Food and Drug Administration (“CFDA”) to produce the product for the China market.
22
We have a broad patent portfolio to protect our intellectual property. Our patent applications claim composition of matter and methods of use of VAL-083 and related compounds, synthetic methods, and quality controls for the manufacturing process of VAL-083. We believe that our portfolio of intellectual property rights provides a defensible market position for the commercialization of VAL-083. In addition, VAL-083 has been designated by the FDA as an orphan drug under the Orphan Drug Act and the European Medicines Agency (“EMA”) for the treatment of gliomas, including GBM. The FDA has also granted Orphan Drug description to VAL-083 for the treatment of medulloblastoma and ovarian cancer.
Our corporate development strategy is to advance VAL-083 on an indication-by-indication basis, and then to consider out-licensing when it has matured enough to warrant proper licensing valuations. In addition to VAL-083’s applicability to multiple solid tumor indications, we are also constantly evaluating licensing, or acquiring additional product candidates, in order to establish a product pipeline and to position us for long-term sustainability and growth of shareholder value. We believe the experience of our clinical development team will position us to efficiently develop possible drug candidates that we may acquire, or license, in the future.
We intend to continue to evaluate options for our strategic direction. These options may include raising additional capital, the acquisition of another company and/or complementary assets, our sale, or another type of strategic partnership.
MGMT-unmethylated GBM
GBM is the most common and the most lethal form of glioma. According to the Central Brain Tumor Registry of the United States, GBM occurs with an incidence of 3.20 per 100,000 person-years. Approximately 13,000 new cases of GBM were diagnosed in the United States and 16,000 in Europe during 2017. Within the GBM patient population, approximately two-thirds of patients are unmethylated with respect to their MGMT status.
Measurement of MGMT (O6-methyl guanine methyltransferase) methylation status has become routine in clinical practice as a biomarker that correlates with resistance to the standard-of-care chemotherapy with TMZ (Temodar®), and patient outcomes in GBM. Approximately two-thirds of GBM patients’ tumors are characterized as “MGMT-unmethylated” and exhibit a high expression of MGMT, a naturally occurring DNA-repair enzyme, the activity of which nullifies the chemotherapeutic activity of TMZ. The lack of specific therapies for MGMT-unmethylated GBM is a significant unmet medical need. Importantly, the 2017 update to the NCCN guidelines states that the treatment benefit of TMZ is likely to be lower in GBM patients with an unmethylated MGMT promoter.
We have demonstrated that VAL-083’s anti-tumor mechanism is active independent from the MGMT status in vitro. We believe this suggests the potential of VAL-083 as a replacement for the current standard-of-care chemotherapy, temozolomide, in MGMT-unmethylated GBM. We are therefore utilizing MGMT-methylation status to identify GBM patients who are unlikely to respond to temozolomide and including only MGMT-unmethylated patients in our current clinical studies of VAL-083.
We believe that our research, in the context of the 2017 update to the NCCN guidelines, highlights this unmet need and the opportunity for VAL-083 as a potential new standard-of-care in the treatment of MGMT-unmethylated GBM.
VAL-083 Clinical Studies
Phase 2 Study in Newly-Diagnosed MGMT-unmethylated GBM
In September 2017, we initiated a single arm, biomarker driven, open-label Phase 2 study in newly-diagnosed MGMT-unmethylated GBM patients at SYSUCC in Guangzhou, China. The study is being conducted under our collaboration agreement with Guangxi Wuzhou Pharmaceutical Company.
In this Phase 2 study, VAL-083 is being combined with radiotherapy as a potential replacement for standard-of-care chemoradiation with temozolomide in patients with MGMT-unmethylated GBM. The goals of the study are to confirm the safety of the three-day VAL-083 dosing regimen in combination with radiotherapy and to investigate efficacy outcomes of the combination of VAL-083 and radiotherapy in MGMT-unmethylated GBM patients.
We have completed enrollment of this study with a total of 29 newly-diagnosed, MGMT-unmethylated GBM patients. The efficacy endpoints of the study include tumor response, as assessed by the Response Assessment in NeuroOncology (“RANO”), and progression-free survival (“PFS”), progression-free survival at six months (“PFS6”), and overall survival (“OS”), compared to historical results in the target population. The study is being conducted in two parts: (1) Dose-confirmation: VAL-083 in cohorts (20, 30 and 40 mg/m2/day IV daily x 3 every 21 days) to assess safety and activity when administered concurrently with x-ray therapy (“XRT”) to confirm the maximum tolerated dose (“MTD”), and (2) Expansion: VAL-083 will be studied in up to 20 additional patients at the target dose, as determined by the dose-confirmation part of the study, administered concurrently with XRT. Assessments of safety and tolerability will be used to support further clinical development of VAL-083 in combination with radiotherapy. Pharmacokinetic assessments of VAL-083 in plasma and cerebral spinal fluid (“CSF”) will be used to correlate drug exposure in the central nervous system with patient outcomes.
23
Dose-confirming cohorts studying 20, 30, and 40 mg/m2/day x three every 21 days have been completed. Based on the dose confirmation phase of the study, we have selected 30 mg/m2/day for combination with irradiation for the treatment of newly-diagnosed MGMT-unmethylated GBM patients. This study is fully enrolled at 29 patients.
On June 22, 2020 at the AACR’s Virtual Annual Meeting II, we provided an update on patient data as follows:
|
|
• |
For the 25 patients initially receiving the treatment dose that will be carried forward in the GBM AGILE pivotal study (30 mg/m2/day on days 1, 2 and 3 of a 21-day cycle) median progression-free survival (“PFS”) was reported to be 8.7 months (confidence interval, or CI 6.0-12.0 months) as of the May 15, 2020 cut-off date. |
|
|
• |
Overall PFS (n=29) with VAL-083 was also 8.7 months (CI 6.4-11.2 months). |
While this is not a head-to-head study, historically, temozolomide (“TMZ”) has been demonstrated to have 6.9 months PFS in unmethylated GBM patients. Other doses were also examined as part of the dose escalation aspect of the study, and all but the 20 mg/m2/day dose also demonstrated superior PFS to the historical comparator. A median of eight cycles of treatment has been received by all patients who had either completed treatment, or remain in active treatment. Nine patients have received ten or more cycles.
Phase 2 Study in MGMT-unmethylated GBM in Collaboration with University of Texas MD Anderson Cancer Center
In February 2017, we initiated a biomarker driven, open-label, single-arm Phase 2 study in collaboration with MDACC. This biomarker-driven study (testing for MGMT methylation status) has been amended to enroll up to 83 patients (35 with a starting dose of 40 mg/m2/day and 48 with a starting dose of 30 mg/m2/day) to determine the potential of VAL-083 treatment to improve overall survival in GBM patients whose tumors have recurred following treatment with temozolomide. These patients will not have been treated previously with Avastin®. In addition, this study has been amended to add a new adjuvant patient arm. This arm will include up to 36 patients previously treated with TMZ in combination with radiation who, rather than being treated with additional cycles of TMZ, will begin treatment with VAL-083.
Recurrent Study Arm
The patients in the recurrent study arm are receiving second-line therapy with VAL-083 following TMZ failure. As of May 28, 2020, 72 patients (out of a planned 83) have been enrolled in the recurrent arm of this study.
On June 22, 2020 at the AACR’s Virtual Annual Meeting II, we provided an update on patient data as follows:
|
|
• |
In recurrent GBM, for the 37 patients initially receiving the intended treatment dose that will be carried forward in the GBM AGILE pivotal study (30 mg/m2/day on days 1, 2 and 3 of a 21-day cycle), median overall survival (mOS) is currently 8.5 months (CI 5.7-14.3 months) as of the May 28, 2020 cut-off date. |
|
|
• |
Overall mOS for the 72 patients who have completed at least one cycle of treatment was 7.1 months (CI 5.8-9.9 months). |
The safety profile in this study has been well within the existing safety monitoring guidelines described in the study protocol. However, in consultation with the principal investigator at MDACC, we have amended the protocol for this clinical study to modify the starting dose of VAL-083 to 30 mg/m2/day on days 1, 2 and 3, of a 21-day cycle. This modification may improve tolerance in this patient population and thereby potentially increase overall exposure to VAL-083 by increasing the number of cycles of drug patients may be able to receive. We have modified the patient screening platelet count, from 100,000/µL to 125,000/µL, for the same reasons. Safety data from this study will become part of the overall safety dossier to support future filings with the FDA and other regulatory agencies.
It is important for this GBM patient population, which has been heavily pre-treated with temozolomide, to be able to be treated with multiple cycles of VAL-083 without significant hematological toxicities. We believe the modified dose of VAL-083, in addition to the change in patient eligibility platelet counts, should help provide for enhanced patient safety. We believe a positive outcome from this study will help support approval of VAL-083 for the treatment of MGMT-unmethylated recurrent GBM.
A detailed description of this study can be found at clinicatrials.gov, Identifier Number: NCT02717962.
Adjuvant Study Arm
On July 24, 2019, we announced the enrollment of the first patient in the adjuvant arm of the Phase 2 study being conducted at MDACC. The adjuvant arm was originally planned for 24 patients, but based on encouraging outcomes, we plan to increase the adjuvant arm enrollment from the originally planned 24 patients to include up to 12 additional patients. These patients will have had initial cycles of temozolomide concomitant with radiation but will not have yet started subsequent cycles of TMZ (i.e., maintenance stage TMZ patients). Published data from Tanguturi et al (2017 Nero-Oncology) indicates that MGMT-unmethylated patients receiving current standard of care have a median progression-free survival of 6.9 months.
24
On June 22, 2020 at the AACR’s Virtual Annual Meeting II, we provided an update on patient data as follows:
|
|
• |
As of the data cut-off date of May 28, 2020, 19 evaluable subjects have completed at least one 21-day cycle of treatment, with a total of 25 subjects enrolled. Enrollment for this arm was initiated in July 2019, and all 25 subjects enrolled to-date were alive at the data cut-off date. |
As noted above, patients in the recurrent arm of the MDACC clinical study have been heavily pre-treated with temozolomide. Based on published data from our MDACC and SYSUCC clinical studies, we believe there is a significant opportunity to treat GBM patients in the pre-temozolomide maintenance stage (i.e., adjuvant). At the AACR’s annual meeting in April 2019, we reported that myelosuppression (thrombocytopenia and neutropenia) is the most common adverse event associated with VAL-083.
Safety Across Studies
Three subjects have experienced a serious adverse event (“SAE”), possibly related to VAL-083 in the newly-diagnosed study as of May 15, 2020, while as of May 28, 2020, 10 subjects have experienced a possibly drug-related SAE in the recurrent arm of the Phase 2 Study in MGMT-unmethylated GBM, and one patient has experienced a possibly drug-related SAE in the adjuvant arm of that study.
VAL-083 Fast Track Designation
The FDA has granted us Fast Track designation for VAL-083 in recurrent GBM.
Fast Track designation is designed to expedite the review of drugs that show promise in treating life-threatening diseases and address unmet medical needs, with the goal of getting new treatments to patients earlier. Fast Track designation provides sponsors with an opportunity for increased frequency for communication with the FDA to ensure an optimal development plan and to collect appropriate data needed to support drug approval. Additional benefits of the Fast Track designation may include an Accelerated Approval, a Priority Review, and a Rolling Review. Accelerated Approval is granted to drugs that demonstrate an effect on a surrogate, or intermediate endpoints, reasonably likely to predict clinical benefit. Priority Review shortens the FDA review process for a new drug from ten months to six months and is appropriate for drugs that demonstrate significant improvements in both safety and efficacy of an existing therapy. Rolling Review provides a drug company the opportunity to submit completed sections of its New Drug Application (“NDA”) for review by the FDA. Typically, NDA reviews do not commence until the drug company has submitted the entire application to the FDA. Through the Fast Track designation, the FDA attempts to ensure that questions raised during the drug development process are resolved quickly, often leading to earlier approval and increased access for patients.
Current Treatments for Gliomas and Glioblastoma Multiforme
Gliomas are a type of Central Nervous System (“CNS”) tumor that arises from glial cells in the brain or spine. Glial cells are the cells surrounding nerves. Their primary function is to provide support and protection for neurons in the CNS.
GBM is the most common and the most lethal form of glioma. According to the Central Brain Tumor Registry of the United States, GBM occurs with an incidence of 3.20 per 100,000 person-years. Approximately 13,000 new cases of GBM were diagnosed in the United States and 16,000 in Europe during 2017.
Common symptoms of GBM include headaches, seizures, nausea, weakness, paralysis and personality or cognitive changes such as loss of speech or difficulty in thinking clearly. GBM progresses quickly and patients’ conditions deteriorate rapidly progressing to death. The outlook for GBM patients is generally poor. The overall median survival in newly diagnosed GBM patients with best available treatments is less than 15 months, and two-year and five-year survival rates are approximately 30% and 10%, respectively. Median overall survival in newly-diagnosed, unmethylated GBM patients is 12.2 months.
In September 2017, the NCCN updated treatment guidelines for GBM. The recommended treatment regimen for GBM includes surgical resection to remove as much of the tumor as possible (“debulking”) followed by radiotherapy with concomitant and adjuvant chemotherapy with temozolomide with or without tumor treating fields (“TTF”). GBM patients whose tumors exhibit an unmethylated promoter for the gene encoding the DNA repair enzyme MGMT, a biomarker correlated with resistance to temozolomide, may be treated with radiation alone following surgery.
Patients with an unmethylated MGMT promoter have high levels of MGMT, a naturally-occurring DNA repair enzyme that repairs tumor-fighting lesions induced by TMZ thus allowing a patient’s tumor to continue to grow despite treatment, which leads to poor outcomes. Measurement of MGMT methylation status has become routine in clinical practice as biomarker that correlates with response to TMZ and patient outcomes in GBM.
25
Probability of GBM Patient Survival Correlated to Expression of MGMT Enzyme (Unmethylated promoter = High MGMT Expression and Significantly Shorter Survival)
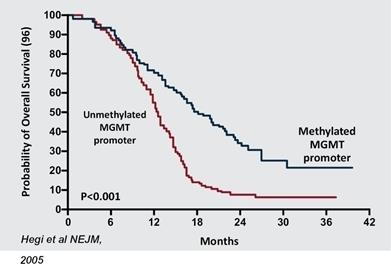
TTF (Optune®) is a non-invasive technique for adults with GBM. TTF uses alternating electrical fields to disrupt tumor cell division, or cause cell death, thereby preventing the tumor from growing or spreading as quickly. A clinical study reported that GBM patients treated with TTF combined with TMZ experienced longer survival than those treated with TMZ alone.
The majority of GBM patients’ tumors recur within 6 – 12 months of initial treatment. Experimental therapy through clinical studies is recommended under NCCN guidelines for eligible patients. NCCN guidelines also recommend treatment with systemic chemotherapy, such as lomustine (“CCNU”). For patients who are eligible for additional surgical debulking, local chemotherapy with carmustine (“BCNU”) wafers may be employed. CCNU and BCNU target the same DNA-site as TMZ and are also subject to MGMT-related resistance.
Avastin (Avastin®, an anti-VEGF antibody) recently received full approval in the US, Canada, Australia, and Japan as a single agent for patients with recurrent GBM following prior therapy. Avastin carries an FDA “black-box warning” related to severe, sometimes fatal, side effects such as gastrointestinal perforations, wound healing complications and hemorrhage. There are no data demonstrating an improvement in disease-related symptoms or increased survival for GBM patients treated with Avastin.
Recurrent GBM patients, especially those whose tumors progress following treatment with Avastin, have limited or no treatment options and a very poor prognosis. According to published literature, the median survival for GBM patients whose tumors progress following Avastin is less than five months.
VAL-083 Mechanism of Action
Chemotherapy forms the basis of treatment in nearly all cancers. We believe that VAL-083 may be effective in treating tumors exhibiting biological features that cause resistance to currently available chemotherapy, particularly for patients who have failed, or become resistant to, other treatment regimens.
Our research suggests that VAL-083 attacks cancer cells via a unique mechanism of action which is distinct from other chemotherapies used in the treatment of cancer. Our data indicate that VAL-083 forms inter-strand crosslinks at the N7 position of guanine on the DNA of cancer cells. Our data also indicate that this crosslink forms rapidly and is not easily repaired by the cancer cell, resulting in cell-cycle arrest and lethal double-strand DNA breaks in cancer cells. VAL-083 readily crosses the blood brain barrier. Published preclinical and clinical research demonstrate that VAL-083 is absorbed more readily in tumor cells than in normal cells.
In vitro, our data also demonstrate that VAL-083’s distinct mechanism may be able to overcome drug resistance against a range of cancers. For example, VAL-083 is active against MGMT-unmethylated GBM cells which are resistant to treatment with temozolomide and nitrosoureas. VAL-083 also retains a high level of activity in p53 mutated non-small cell lung cancer (“NSCLC”), ovarian cancer and medulloblastoma cell lines that are resistant to platinum-based chemotherapy.
26
Importantly, clinical activity against each of the tumors mentioned above was established in prior NCI-sponsored Phase 2 clinical studies. We believe that these historical clinical data and our own research support the development of VAL-083 as a potential new treatment for multiple types of cancer.
The main dose-limiting toxicity (“DLT”) related to the administration of VAL-083 in previous NCI-sponsored clinical studies and our own clinical studies is myelosuppression, particularly thrombocytopenia. Myelosuppression, including thrombocytopenia, is a common side effect of chemotherapy. Myelosuppression is the decrease in cells responsible for providing immunity, carrying oxygen, and causing normal blood clotting. Thrombocytopenia is a reduction in platelet counts which assist in blood clotting. Modern medicine allows for better management of myelosuppressive side effects. We believe this offers the potential opportunity to improve upon the drug’s already established efficacy profile by substantially increasing the dose of VAL-083 that can be safely administered to cancer patients.
There is no evidence of lung, liver, or kidney toxicity even with prolonged treatment by VAL-083. Data from the Chinese market where the drug has been approved for more than 15 years supports the safety findings of the NCI studies.
REM-001
Background
Through REM-001, we are developing our photodynamic therapy (“PDT”) for the treatment of rare, unmet medical needs. PDT is a treatment that uses light sensitive compounds, or photosensitizers, that, when exposed to specific wavelengths of light, act as catalysts to produce a form of oxygen that induces local tumor cell death. REM-001 consists of three parts, the laser light source, the light delivery device, and the REM-001 drug product (collectively, the “REM-001 Therapy”). REM-001 consists of an active pharmaceutical ingredient (“API”) in a lipid formulation. The REM-001 API is SnET2 (“tin ethyl etiopurpurin”) which is a second-generation PDT photosensitizer agent. We believe REM-001 possesses multiple advantages over earlier generation PDT compounds.
Our lead indication for REM-001 is CMBC which is a disease that may strike individuals with advanced breast cancer and for which effective treatment options are limited. In four Phase 2 and/or Phase 3 clinical studies in CMBC patients, primarily targeting patients who had previously received chemotherapy and failed radiation therapy, REM-001 Therapy was able to reduce or eliminate a substantial number of the treated CMBC tumors. Specifically, our analysis of the data collected from these studies indicates that in approximately 80% of evaluable tumor sites treated with REM-001 Therapy, there was a complete response; meaning that follow-up clinical assessments indicated no visible evidence of the tumor remaining. We believe clinical data indicates that REM-001 Therapy holds promise as a treatment to locally eliminate, or slow the growth of, treated cutaneous cancerous tumors in this difficult-to-treat patient population.
In 2012, Adgero acquired certain assets and regulatory filings, including REM-001 Therapy developed by Miravant Medical Technologies, and its wholly-owned subsidiaries (collectively, “Miravant”), and the associated technology, clinical data and intellectual property, from a creditor of Miravant. The primary motivation behind the acquisition of the technology was to secure the rights to the REM-001 Therapy and its associated technology, proprietary processes and regulatory filings which have already undergone substantial clinical development which we believe will help expedite the process of gaining regulatory approval to market our REM-001 Therapy.
Miravant initiated commercial development of REM-001 and its associated device components in the 1990s. This led to late-stage clinical studies in CMBC and also in an aspect of “wet” age-related macular degeneration (“AMD”). Of these two indications, AMD represented a much larger market, and in 1998, for what we believe were primarily business reasons, Miravant discontinued its CMBC program and, together with, or through its corporate partners, ultimately focused its REM-001 development efforts on AMD. In 2004, Miravant submitted a new drug application (“NDA”) to the FDA for the use of REM-001 to treat an aspect of AMD. The FDA reviewed this submission and granted Miravant an approvable letter for REM-001 in the treatment of AMD, with final approval contingent on, among other things, the successful completion of a Phase 3 study. Miravant ceased operations in 2006 prior to completing this study.
While Miravant did not pursue the CMBC indication through to approval, it did compile substantial clinical data in the four Miravant CMBC Studies. The first two of these studies were Phase 2/3 studies that treated 68 CMBC patients who, for the most part, previously failed radiation therapy, and were then treated with REM-001 Therapy. Miravant compiled both safety and efficacy data for these two studies. At the time Miravant discontinued its CMBC program, REM-001 Therapy was also being tested in two additional Phase 2 or 3 clinical studies that treated a total of 81 CMBC patients. Our review of internal Miravant records indicates that data was collected in all four studies generally in accordance with Good Clinical Practice and the data was analyzed for safety, and reports were filed with the FDA. Our review also indicates that Miravant never conducted an efficacy analysis of the 81 patients in the last two studies which were not yet complete when Miravant discontinued its CMBC program.
27
Based on our analysis of both the 81 CMBC patients, and data collected from the initial 68 patients, we believe REM-001 Therapy provided promising safety and efficacy in CMBC patients and that, taken together, these results provide strong support for REM-001 Therapy as a potential therapy for this disease. Furthermore, we believe the approvable letter previously granted to Miravant with respect to its NDA for REM-001 in an aspect of AMD may indicate that many of the elements required for approval have already been completed for REM-001.
Numerous approaches have been utilized to treat CMBC patients, including various forms of chemotherapy, radiation therapy, surgical excision, hyperthermia, cryotherapy, electro-chemotherapy, topical drugs, and intra-lesional chemotherapy injections. However, for the most part, we believe that these therapies are often inadequate given the limited efficacy, toxicities and/or side effects of each. We believe our REM-001 Therapy has several advantages for this indication: it can be highly directed to the tumor site, has minimal systemic effects or normal tissue toxicities, can be used in conjunction with other therapies, and can be periodically repeated.
As a result of our review, we submitted questions to the FDA under a Type C format to review the technology and results and determine the anticipated requirements for regulatory approval. On March 3, 2017, we received FDA’s written response to these questions. Based on that response, we believe our plans to manufacture REM-001 by revising the prior quality standards to meet the currently-recommended regulatory standards will be acceptable. The FDA also indicated our plans for utilizing light delivery devices that have been shown to be functionally equivalent to the devices used by Miravant will be acceptable.
In October 2017, we held a Type B face-to-face guidance meeting with the FDA that was primarily focused on the design of a Phase 3 study in CMBC. Then, in May 2018, we held a Type B end-of-phase 2 meeting with the FDA that focused on our plans for addressing CMC and device topics related to our CMBC effort. In these interactions, the FDA provided guidance on a number of clinical parameters it would like us to measure in the planned clinical study, and on the associated CMC and device plans. Based on the FDA’s responses, we plan to conduct a Phase 3 clinical study in CMBC to test the safety and efficacy of REM-001 Therapy for marketing approval. In June 2018, we submitted to the FDA a Phase 3 protocol and statistical analysis plan incorporating feedback received from FDA at the October 2017 meeting. We have also undertaken extensive discussions with clinical research organizations to carry out this study and have received detailed proposals from five of these organizations. Since our May 2018 meeting, we have engaged a contract manufacturer who has manufactured the starting material for our API and manufactured two API lots under GMP. We are currently planning to undertake GMP manufacturing of finished drug product for use in the planned clinical study.
We also believe REM-001 Therapy holds promise as a treatment for cutaneous metastatic cancers other than CMBC, as well as locally-advanced basal cell cancer such as often occurs in patients with Basal Cell Carcinoma Nevus Syndrome (“BCCNS”) and cutaneously recurrent basal cell cancer. On January 16, 2018, the FDA granted our request that tin ethyl etiopurpurin (the active pharmaceutical ingredient in REM-001) be designated as an orphan drug for treatment of BCCNS. Following this designation, we contacted clinical experts in BCCNS and related indications to seek their guidance on the most appropriate clinical pathway for REM-001 Therapy in these indications.
We believe REM-001 Therapy also holds promise for certain cardiovascular conditions, including de novo treatment of cardiovascular access sites in hemodialysis patients to ameliorate current high failure rates. We also hold an orphan drug designation that was initially awarded to Miravant for tin ethyl etiopurpurin for the prevention of access graft failure in hemodialysis patients. We have been working to further develop this indication, including engaging with a key opinion leader in this area and submitting an NIH grant proposal for late stage preclinical research that we believe could lead directly to an IND and clinical study. On July 17, 2020 we received notification that that grant had been awarded.
REM-001 Regulatory Filings
The initial investigational new drug (“IND”) filing for REM-001 Therapy was IND 39,940 which was filed in June 1992 with the FDA’s Division of Oncology and Pulmonary Drug Products. This IND is now under the purview of the FDA’s Division of Oncology Products. All CMBC studies were conducted under this IND. Miravant kept this IND in place but in 2005 they placed it on inactive status since they had focused their REM-001 development efforts on ophthalmology. In 2012, following St Cloud’s foreclosure action on Miravant and our subsequent purchase of the Miravant assets, St. Cloud transferred ownership of this IND to us. This transfer was formally recognized by the FDA with a Change of Sponsor letter dated December 14, 2012. Our interactions with the FDA for CMBC are under the auspices of this IND. It is our expectation, based on input from regulatory consultants, that clinical development in CMBC, non-CMBC cutaneous metastatic cancer and BCCNS basal cell nevus syndrome would be conducted under this IND. Recent FDA approvals in locally advanced basal cell cancers, which included patients with BCCNS, have been under the purview of the FDA’s Division of Oncology Products.
28
As part of our purchase agreement with St. Cloud, sponsorship of two other INDs was transferred to us. On February 25, 2013, the FDA’s Division of Dermatology and Dental Products notified us with a Change of Sponsor letter that it recognized us as the sponsor of IND 50,116. On May 8, 2013 the FDA’s Division of Transplant and Ophthalmology Products notified us with a Change of Sponsor letter that it recognized Adgero as the sponsor of IND 49,648. At this time, we do not anticipate any of our planned, or contemplated, clinical development activities would be under either of these INDs.
REM-001 Therapy
Our REM-001 Therapy product consists of three parts, the DD series laser light source (or equivalent), the ML2-0400 light delivery device (or equivalent) and the drug REM-001. Pursuant to the Miravant oncology IND, the FDA previously approved all three components to be used together in certain Miravant CMBC Studies. In use, the drug REM-001 is first administered by intravenous infusion and allowed to distribute within the body and be taken up by the tumors. Tumors are then illuminated with light using the light delivery device, which is attached to the laser light source, so that the accumulated drug REM-001 can be activated for the desired clinical effect. Our analysis of clinical data collected in the Miravant CMBC Studies shows that REM-001 Therapy provides a stronger reaction in tumor tissues than in healthy tissues, which was a goal with REM-001’s formulation.
Our plan is to use new lasers that are functionally equivalent to the Miravant DD2, the laser used in certain prior Miravant clinical studies, for CMBC. The Miravant DD2 lasers are capable of delivering two watts of optical power centered at a wavelength of 664 nanometers. Based on our interactions with the FDA, we believe that use of such new functionally equivalent lasers will be acceptable to the FDA.
The light delivery devices we plan to use in our CMBC program are the same basic design developed and used previously by Miravant in its clinical studies. In the case of cutaneous treatment, such as with CMBC, the light delivery device consists of an optical fiber which has a modified end to allow it to deliver a uniform light treatment field to the tumor. Our plan is to have clinical light delivery devices built by a contract medical device manufacturer using the basic Miravant design and tested to the same performance specifications as used previously.
The REM-001 Drug
REM-001 is a light activated photosensitizer drug used in PDT. During light activation, photosensitizer drugs act as a catalyst and absorb light energy which they transfer to surrounding oxygen-containing molecules to create reactive oxygen species (“ROS”). ROS can initiate various biological mechanisms of action:
|
|
• |
Apoptosis—Certain photosensitizer drugs associate with the cells’ mitochondria. When light activated, these drugs generate ROS that alter mitochondria membrane permeability to allow the release of activators that initiate a programmed cell death process known as apoptosis. Apoptosis is a desirable means of inducing tumor cell death as it is the body’s natural mode for eliminating damaged cells. |
|
|
• |
Necrosis—At higher doses these photosensitizer-generated ROS can overwhelm a cell and induce cellular necrosis. |
|
|
• |
Anti-angiogenesis—As they grow, tumors develop their own micro-vasculature network. ROS can be used to create permeability in these micro-vessels which reduces their effectiveness and cuts off the tumor’s blood supply. |
|
|
• |
Immune Response—PDT is known to induce an immune response including activation of CD8+ T cells to attack tumor cells. Such T cells provide one of the key mechanisms making up the body’s immune response system, which response may enhance anti-tumor immunity. Therapeutic drugs that produce such an immune response are known as immunotherapies. We believe that immunotherapies are promising areas of cancer treatment and are being developed as either monotherapies or in combination with other treatments. |
REM-001 has been shown to induce apoptosis and, in treating an aspect of AMD, to have anti-angiogenesis properties. REM-001 is a second-generation photosensitizer drug designed with the following attributes to overcome several of the shortcomings of earlier, first generation photosensitizer drugs such as Photofrin:
|
|
• |
It is activated with longer wavelength, deeper penetrating light; |
|
|
• |
It has a stronger light absorption coefficient; |
|
|
• |
It is a synthetic single molecule; and |
|
|
• |
It causes transient photosensitivity of shorter duration. |
29
PDT carries what we believe is an inherent safety advantage since it uses photosensitizer compounds that are largely inactive except when they are being illuminated by intense light at specific wavelengths. Nevertheless, drug molecules, including photosensitizer molecules, can carry safety or toxicology risks on their own. REM-001 has previously undergone preclinical and clinical studies throughout its development cycle and has undergone certain tests typically required for FDA drug approval. REM-001 has been safely administered to over 1,100 patients in prior clinical studies. Most significantly, REM-001 has been previously reviewed by the FDA as part of the NDA submitted by Miravant for the use of REM-001 to treat an aspect of AMD, a non-CMBC indication. Following that review, the FDA granted an approvable letter for REM-001 in an aspect of AMD in 2004, with final approval contingent on, among other things, the successful completion of a Phase 3 study. While not definitive, we believe this letter, along with feedback we received from FDA meetings, indicates that it is unlikely that there will be significant safety or toxicology issues associated with REM-001 that would ultimately prevent marketing approval.
Based on our review of the clinical data of the Miravant CMBC Studies, we believe pain was the most common treatment-related adverse event experienced by patients in these studies. The second most common safety issue experienced with REM-001 was a transient photosensitivity, meaning extended exposure in bright light and direct sunlight should be avoided. Transient photosensitivity occurs with all photosensitizers to some degree. We believe this issue can be addressed by minimizing one’s exposure to bright light and sunlight for two to four weeks after treatment. In general, the potentially treatment-related adverse events observed in these CMBC studies were expected in nature (pain, edema, skin photosensitivity) and severity, and mostly resolved during the course of the studies.
Current and Experimental Treatments for CMBC
As with many cancers, the current standard treatment for CMBC is surgical excision. However, this is often not feasible due to the extent of the tumor field or the condition of the skin, particularly in patients who have had radiation therapy. A number of other therapies have been used on patients with CMBC, including various forms of chemotherapy, radiation therapy, hyperthermia, cryotherapy, electro-chemotherapy, topical drugs and intra-lesional chemotherapy injections. Researchers have also attempted to combine therapies in an effort to improve efficacy. However, we believe that these therapies are often inadequate given the limited efficacy, toxicities and/or side effects of each. The side effects associated with therapies may be particularly difficult for patients who may have already experienced extensive surgery along with a full course of radiation and/or systemic chemotherapy. Also, the fact that CMBC tumors continue to develop following these therapies is a signal that the tumor cells may have developed a resistance to some of these approaches. Based on our discussions with clinicians and literature reviews, and its March 3, 2017 response from FDA, we believe that treatment of unresectable CMBC tumors is a largely unmet medical need, particularly in patients who have already received extensive radiation and chemotherapy.
Clinical Results in CMBC
While we have not conducted any clinical studies, we have undertaken an analysis of the Phase 1 and four Phase 2 and/or Phase 3 CMBC clinical studies done previously with REM-001 Therapy by Miravant (the “Miravant CMBC Studies”) and have concluded that, in these studies, REM-001 Therapy provided higher tumor response rates than are generally seen with alternative CMBC treatments but this program was discontinued in 1998. Our review of Miravant’s records further indicates that, following this decision, Miravant continued to monitor patients in the CMBC studies and collected data as required by protocol, but they conducted no further treatment of CMBC patients with REM-001 Therapy. We believe that Miravant primarily chose to discontinue this program in order to focus its REM-001 development efforts on an aspect of “wet” AMD.
Phase 1 Clinical Study
A Phase 1 dose escalation clinical study was initially conducted by Miravant to establish the REM-001 dosimetry to be used in subsequent safety and efficacy studies. The study was initiated in 1993 and enrolled 22 patients with a variety of types of cutaneous cancer lesions. Of these, 213 cutaneous cancer lesions were treated using escalating REM-001 drug and light doses. This study used earlier generation light delivery devices than those used in later studies but these devices provided equivalent light output to those units used in later studies. In these studies, REM-001 drug doses ranged from 0.1 mg/kg to 1.2 mg/kg, light doses ranged from 100 to 200 J/cm2 and treatment time-points ranged from 24 to 72 hours. This study indicated that a drug dose in excess of 0.8 mg/kg and a light dose of 200 J/cm2 administered at 24 hours provided a high overall response rate when delivered in a variety of cutaneous cancer lesions. The previously tested dose of 1.2mg/kg was then tested further in a second Phase 1 trial, where it was administered to 27 cutaneous tumor lesions and provided a 66% complete response rate and a 90% overall response rate. Based on these results, this dosimetry was used in subsequent CMBC studies, including the Miravant CMBC Studies described below.
30
Miravant conducted four Phase 2/3 studies with REM-001 Therapy for the treatment of CMBC as summarized below. These studies all used the same dosimetry as described above and most of the patients had been previously treated with radiation therapy and chemotherapy. The light delivery devices used in these studies were the ML1-0400 or the functionally equivalent ML2-0400. The laser light source used in three of the studies was the Miravant DD2 laser and one study used the KTP model laser manufactured by LaserScope. Each study was conducted under Miravant’s REM-001 cancer Investigational New Drug Application (“IND”) using Good Clinical Practices with safety and efficacy data collected accordingly. In connection with our acquisition of the Miravant assets, ownership of that IND has been transferred to us.
The table below summarizes the Miravant CMBC Studies. Studies CA008, CA009 and CA019 required that the patients enrolled had received prior radiation therapy. Study CA013 did not have this specific inclusion requirement but our review of the data indicates that at least 50 of the 56 patients in CA013 had received prior radiation therapy. A second difference across the studies is that studies CA008, CA009 and CA019 had a 24-week follow-up period while study CA013 had a 52-week follow-up period. Also, in studies CA008 and CA009 two tumor lesions on each patient were randomly selected as controls and did not receive light activation. CA013 was conducted in Europe by a corporate partner of Miravant. Beyond these differences and those device differences noted above, we believe there were no other substantive differences between the studies and that all studies enrolled similar patients.
Table of Phase 2 and/or 3 Miravant CMBC Studies
(Note: SnET2 is now called REM-001.)
|
Study Title |
|
Phase |
|
|
Location |
|
Total Patients |
|
|
Total Patients Previously Treated with Radiotherapy |
|
|
Included Randomly Selected Control Tumors |
|||
|
CA008: Open-Label Randomized No Treatment Concurrent Controlled Study of Single Dose Tin Ethyl Etiopurpurin (SnET2) Photodynamic Therapy (PDT) in Patients with Advanced Breast Cancer Who Have Failed Radiation Therapy for the Management of Cutaneous Metastatic Breast Carcinoma (24 Week Follow Up) |
|
2/3 |
|
|
U.S. |
|
|
32 |
|
|
|
32 |
|
|
Yes |
|
|
CA009: Open-Label Randomized No Treatment Concurrent Controlled Study of Single Dose Tin Ethyl Etiopurpurin (SnET2) Photodynamic Therapy (PDT) in Patients with Advanced Breast Cancer Who Have Failed Radiation Therapy for the Management of Cutaneous Metastatic Breast Carcinoma (24 Week Follow Up) |
|
2/3 |
|
|
U.S. |
|
|
36 |
|
|
|
36 |
|
|
Yes |
|
|
CA013: Multinational, Open-Label Study of Single Dose Tin Ethyl Etiopurpurin (SnET2) Photodynamic Therapy (PDT) in Patients with Advanced Breast Cancer for the Management of Cutaneous Metastases of Breast Carcinoma (52 Week Follow Up) |
|
|
2 |
|
|
Europe |
|
|
56 |
|
|
|
50 |
|
|
No |
|
CA019: Open-Label Study of Single Dose Tin Ethyl Etiopurpurin (SnET2) Photodynamic Therapy (PDT) in Patients with Advanced Breast Cancer Who Have Failed Radiation Therapy for the Management of Cutaneous Metastatic Breast Carcinoma (24 Week Follow Up) |
|
|
3 |
|
|
U.S. |
|
|
25 |
|
|
|
25 |
|
|
No |
31
The primary endpoints for studies CA008 and CA009 were objective tumor response rate, quality-of-life change, device performance and patient safety. Our review of the tumor response rate and quality-of-life endpoints indicated they were defined as follows:
|
|
• |
Tumor Response: Measured as paired response difference, as calculated by the percentage of a patient’s evaluable lesions that respond minus the percentage of the patient’s control lesions that respond with this difference averaged over all treated patients. |
|
|
• |
Quality of Life Change: Measured using the Dermatologic Life Quality Index (DLQI, A.Y. Finlay and O.K. Khan, “Dermatology Life Quality Index (DLQI—a simple practical measure for routine clinical use”. Clinical and Experimental Dermatology 1994; 19: 210-2 16) with change measured from baseline measurements. |
The following table shows the results of these two endpoints for studies CA008 and CA009 as calculated by Miravant. In some cases, patients dropped out of the study before lesion responses could be assessed or they did not complete their quality of life questionnaires. The Eligible Patients column in this and the following tables refers to the number of patients in each case for which sufficient data is available to calculate the relevant endpoint.
|
|
|
Tumor Response as Measured by Paired Response Endpoint |
|
24 Week Quality of Life Change |
|
|||||||||||||
|
Study |
|
Eligible Patients (N) |
|
|
Mean ± SD (%) |
|
P value |
|
Eligible Patients (N) |
|
|
Mean ± SD |
|
P value |
|
|||
|
CA008 |
|
|
18 |
|
|
33% ± 37% |
|
< 0.001 |
|
|
7 |
|
|
0.4 ± 4.8 |
|
|
0.813 |
|
|
CA009 |
|
|
19 |
|
|
39% ± 47% |
|
< 0.001 |
|
|
10 |
|
|
-0.3 ± 4.1 |
|
|
0.554 |
|
The FDA typically requires a p value of 0.05 or less for approval. Based on the above results, it appears that the Paired Response endpoint achieved statistical significance in both the CA008 and CA009 studies. However, it is our understanding that FDA questions the strength of this data, in part due to the small number of patients involved as well as the fact that each patient had only two control lesions.
Following discussions with the FDA, an endpoint called Clinical Success was added as an additional measure of tumor response. This was defined as follows:
|
|
• |
Clinical Success: Clinical success is determined by a two-step process. First, for each patient, clinical success occurs when the fraction of evaluable lesions that respond minus the fraction of evaluable lesions that progress is greater than 0.5. Second, for the entire study, an average rate of clinical success is determined, simply by taking the ratio of individual patients who are clinical successes to the total number of eligible patients. Note this endpoint does not involve the control lesions or any other control, so a p-value is not appropriate since p-values refer to the difference between a treated and a control group. In such uncontrolled settings, the statistical measure commonly used by regulatory agencies instead of a p-value is the confidence interval, which is provided in the charts below. |
The clinical success rates for studies CA008 and CA009 as calculated by Miravant are provided in the following table:
|
|
|
Tumor Response as Measured by Clinical Success |
||||||||
|
Study |
|
Eligible Patients (N) |
|
|
Average Rate of Clinical Success (%) |
|
|
95% Confidence Interval |
||
|
CA008 |
|
|
20 |
|
|
|
60 |
% |
|
39% - 81% |
|
CA009 |
|
|
20 |
|
|
|
50 |
% |
|
28% - 72% |
No significant device failures were observed in either study. Secondary endpoints in CA008 and CA009 were patient disease burden, duration of response and patient pain assessment. Miravant’s analysis indicated, for patients for which data was available, there was a treatment benefit in disease burden (p = 0.0017 for CA008, p = 0.0020 for CA009) and duration of response (p < 0.001 for CA008, not significant in CA009) when comparing treated and control lesions. In terms of pain, there was no significant change in pain in CA008 and a treatment related increase in pain at 4 Weeks post-treatment in CA009. Treatment related pain, particularly during the first month after treatment, was the most commonly reported adverse event and was often treated with analgesics.
32
Studies CA013 and CA019 used similar endpoints with one notable exception. Tumor Response as Measured by Paired Response was not possible in these studies since this measurement relies on control lesions and CA013 and CA019 did not include controls. Miravant did not conduct an efficacy analysis of these two studies but we have conducted an analysis of the Quality of Life and Clinical Success endpoints used in the pivotal CA008 and CA009 studies. Results from that analysis are shown in the following table:
|
|
|
Clinical Success |
|
24 Week Quality of Life Change |
|
|||||||||||||||
|
Study |
|
Eligible Patients (N) |
|
|
Average Rate of Clinical Success (%) |
|
|
95% Confidence Interval |
|
Eligible Patients (N) |
|
|
Mean ± SD |
|
P value |
|
||||
|
CA013 |
|
|
32 |
|
|
|
88 |
% |
|
71% - 97% |
|
|
16 |
|
|
1.3 ± 3.6 |
|
|
1.00 |
|
|
CA019 |
|
|
18 |
|
|
|
83 |
% |
|
45% - 86% |
|
|
11 |
|
|
2.5 ± 4.7 |
|
|
1.00 |
|
We have not attempted any further analysis of the endpoints included in these two studies.
The most common adverse events seen in these four studies (CA008, CA009, CA013, CA019) were pain and photosensitivity, both of which are expected with this therapy. In the four studies there were a total of 17 serious adverse events (SAE’s) that were judged by investigators to be possibly, probably or definitely related to treatment. None of these were classified by the investigator as life threatening and none resulted in death. Of the 17 SAE’s, eight were related to necrosis of the treated lesions, three were related to treatment field infection, 4 were treatment related pain, one was a photosensitivity skin reaction and one was an allergic reaction.
We believe that the data from these studies show that REM-001 Treatment is a promising therapy for CMBC. However, because there are no approved therapies for CMBC, we have no basis for comparing these results to existing therapies. Based on the FDA’s March 3, 2017 response, we believe the FDA will view these results as supportive data and our plan is to conduct a new pivotal Phase 3 study to support a new drug application.
The figure below shows the results of our initial preliminary analysis of Miravant clinical data and depicts the percentage of evaluable lesions in each Miravant CMBC Study for which there was a complete response; i.e. where all visible clinical evidence of the tumor is gone after treatment with REM-001 Therapy.
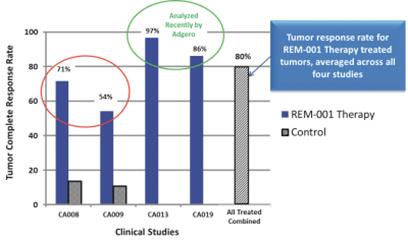
Clinical Development Plans
CMBC
Based on the FDA’s guidance, our plan is to conduct a Phase 3 clinical study in CMBC. Our plan is to include a first confirmatory element to test the safety and efficacy of REM-001 Therapy for marketing approval. In June 2018, we submitted to FDA a Phase 3 protocol and statistical analysis plan incorporating feedback received from the FDA at our October 2017 meeting.
33
At this time, we estimate the necessary study design will be a pivotal Phase 3 multi-center study that would enroll approximately 100-150 CMBC patients who have received prior radiation therapy and chemotherapy. This study has been designed with input from the FDA with the goal of gaining expedited development and review through one or more of the FDA’s expedited programs. Following our meeting with the FDA, we undertook further analysis of the original Miravant study data and concluded that the data may support use of a lower dose than Miravant used in its original study design. Use of such a lower dose may have potential benefits including faster post-treatment healing and response assessment and lower drug exposure. Based on this analysis and discussions with regulatory and clinical consultants, including prior FDA employees or consultants, and clinical research organizations, we plan to add a preliminary confirmatory element to our Phase 3 study. This confirmatory element anticipates treating up to 15 patients at a lower dose than used by Miravant. Patients treated in this confirmatory phase will not be included in the pivotal study efficacy population but their results should provide an indication that a lower dose may be as effective as the original Miravant dose and they may be used to provide a further preliminary confirmation of the potential of REM-001 Therapy in CMBC and if the results are sufficiently compelling we may use them as guidance for the use of a slightly lowered dose in the pivotal study. This confirmatory phase was included in the protocol submitted to FDA in June 2018 and we have not received comment on this from FDA although based on guidance from our regulatory consultants we believe the FDA will be supportive of this design.
If approved, the FDA grants five years of data exclusivity for a new chemical entity (“NCE”). A drug is an NCE if the FDA has not previously approved any other new drug containing the same active ingredient. We believe that REM-001 would qualify for this form of exclusivity.
Corporate History
We are a Nevada corporation formed on June 24, 2009 under the name Berry Only Inc. On January 25, 2013, we entered into and closed an exchange agreement (the “Exchange Agreement”), with Del Mar Pharmaceuticals (BC) Ltd. (“Del Mar (BC)”), 0959454 B.C. Ltd. (“Callco”), and 0959456 B.C. Ltd. (“Exchangeco”) and the security holders of Del Mar (BC). Upon completion of the Exchange Agreement, Del Mar (BC) became a wholly-owned subsidiary of ours (the “Reverse Acquisition”).
On August 19, 2020, we acquired Adgero Biopharmaceuticals Holdings Inc. (“Adgero”) and changed our name from DelMar Pharmaceuticals, Inc. to Kintara Therapeutics, Inc. We are the parent company to the following entities:
|
|
• |
Del Mar (BC), a British Columbia, Canada corporation incorporated on April 6, 2010, which is a clinical stage company with a focus on the development of drugs for the treatment of cancer; |
|
|
• |
Adgero, a Delaware corporation incorporated on October 26, 2015, which is a clinical stage company with a focus on the development of photodynamic therapy for the treatment of rare, unmet medical needs, specifically orphan cancer indications; |
|
|
• |
Adgero Biopharmaceuticals, Inc. a Delaware corporation incorporated on November 16, 2007; and |
|
|
• |
Callco and Exchangeco which are British Columbia, Canada corporations. Callco and Exchangeco were formed to facilitate the Reverse Acquisition. |
Outstanding Securities
As of November 9, 2020, we had 24,662,299 shares of common stock issued and outstanding, outstanding warrants to purchase 11,697,068 shares of common stock, warrants to purchase 2,504 Series C Preferred Stock that upon exercise are convertible into 2,152,701 shares of common stock, outstanding stock options to purchase 6,508,569 shares of common stock, 648,613 outstanding shares of Series B Preferred Stock that are convertible into 162,177 shares of common stock, 25,028 outstanding shares of Series C Preferred Stock that are convertible into 21,516,484 shares of common stock. All common stock warrants and stock options are convertible, or exercisable into, one share of common stock. Each Series B convertible preferred share is convertible into 0.25 shares of common stock. The Series C Preferred Stock (issued in three series) is convertible into shares of common stock at $1.16 per share (Series C-1), $1.214 per share (Series C-2) or $1.15 per share (Series C-3), respectively. The Series C Preferred stock purchase warrants are convertible into Series C Preferred Stock at $1,000 per share for either Series C-1, Series C-2, or Series C-3 Preferred Stock, as applicable.
Selected Quarterly Information
The financial information reported herein has been prepared in accordance with accounting principles generally accepted in the United States. Our functional currency at September 30, 2020 and June 30, 2020 is the US$. The following tables represent selected financial information for us for the periods presented.
34
|
|
|
September 30, 2020 $ |
|
|
June 30, 2020 $ |
|
||
|
|
|
(in thousands) |
|
|||||
|
Cash and cash equivalents |
|
|
22,602 |
|
|
|
2,392 |
|
|
Working capital |
|
|
20,566 |
|
|
|
176 |
|
|
Total assets |
|
|
23,131 |
|
|
|
2,938 |
|
|
Total stockholders’ equity |
|
|
20,554 |
|
|
|
263 |
|
Selected Statement of Operations Data
For the three months ended:
|
|
|
September 30, 2020 |
|
|
September 30, 2019 |
|
||
|
|
|
$ |
|
|
$ |
|
||
|
|
|
(in thousands, except per share data) |
|
|||||
|
Expenses |
|
|
|
|
|
|
|
|
|
Research and development |
|
|
1,357 |
|
|
|
721 |
|
|
General and administrative |
|
|
1,534 |
|
|
|
914 |
|
|
Merger costs |
|
|
500 |
|
|
|
— |
|
|
In-process research and development |
|
|
16,094 |
|
|
|
— |
|
|
|
|
|
19,485 |
|
|
|
1,635 |
|
|
Other (income) loss |
|
|
|
|
|
|
|
|
|
Foreign exchange loss |
|
|
(1 |
) |
|
|
— |
|
|
Amortization of deferred loan costs |
|
|
27 |
|
|
|
— |
|
|
Interest expense |
|
|
8 |
|
|
|
— |
|
|
Interest income |
|
|
(1 |
) |
|
|
(29 |
) |
|
|
|
|
33 |
|
|
|
(29 |
) |
|
Net loss for the period |
|
|
19,518 |
|
|
|
1,606 |
|
|
Deemed dividend recognized on beneficial conversion features of Series C Preferred stock issuance |
|
|
3,181 |
|
|
|
— |
|
|
Series A Preferred cash dividend |
|
|
2 |
|
|
|
2 |
|
|
Series B Preferred stock dividend |
|
|
5 |
|
|
|
2 |
|
|
Net loss for the period attributable to common stockholders |
|
|
22,706 |
|
|
|
1,610 |
|
|
Basic and fully diluted weighted average number of shares |
|
|
17,106 |
|
|
|
7,539 |
|
|
Basic and fully diluted loss per share |
|
|
1.33 |
|
|
|
0.21 |
|
Expenses net of non-cash, share-based compensation expense – non-GAAP
The following table discloses research and development, and general and administrative expenses net of non-cash, share-based compensation payment expense. The disclosure has been provided to reconcile the total operational expenses on a GAAP basis and the non-GAAP operational expenses net of non-cash stock-based compensation in order to provide an estimate of cash used in research and development, and general and administrative expense. Management uses the cash basis of expenses for forecasting and budget purposes to determine the allocation of resources and to plan for future financing opportunities.
35
|
|
|
September 30, 2020 $ |
|
|
September 30, 2019 $ |
|
||
|
|
|
(in thousands) |
|
|||||
|
Research and development - GAAP |
|
|
1,357 |
|
|
|
721 |
|
|
Less: non-cash, share-based compensation expense |
|
|
(91 |
) |
|
|
(13 |
) |
|
Research and development net of non-cash, share-based, compensation expense – Non-GAAP |
|
|
1,266 |
|
|
|
708 |
|
|
General and administrative - GAAP |
|
|
1,534 |
|
|
|
914 |
|
|
Less: non-cash, share-based compensation expense |
|
|
(359 |
) |
|
|
(43 |
) |
|
General and administrative net of non-cash, share-based, compensation expense – Non-GAAP |
|
|
1,175 |
|
|
|
871 |
|
Results of Operations
Comparison of the three months ended September 30, 2020 and September 30, 2019
|
|
|
Three months ended |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
September 30, 2020 $ |
|
|
September 30, 2019 $ |
|
|
Change $ |
|
|
Change % |
|
||||
|
|
|
(in thousands) |
|
|
|
|
|
|||||||||
|
Expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
1,357 |
|
|
|
721 |
|
|
|
636 |
|
|
|
88 |
|
|
General and administrative |
|
|
1,534 |
|
|
|
914 |
|
|
|
620 |
|
|
|
68 |
|
|
Merger costs |
|
|
500 |
|
|
|
— |
|
|
|
500 |
|
|
|
— |
|
|
In-process research and development |
|
|
16,094 |
|
|
|
— |
|
|
|
16,094 |
|
|
|
— |
|
|
|
|
|
19,485 |
|
|
|
1,635 |
|
|
|
17,850 |
|
|
|
|
|
|
Other (income) loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign exchange loss |
|
|
(1 |
) |
|
|
— |
|
|
|
(1 |
) |
|
|
— |
|
|
Amortization of deferred loan costs |
|
|
27 |
|
|
|
— |
|
|
|
27 |
|
|
|
— |
|
|
Interest expense |
|
|
8 |
|
|
|
— |
|
|
|
8 |
|
|
|
— |
|
|
Interest income |
|
|
(1 |
) |
|
|
(29 |
) |
|
|
28 |
|
|
|
(97 |
) |
|
|
|
|
33 |
|
|
|
(29 |
) |
|
|
62 |
|
|
|
|
|
|
Net loss |
|
|
19,518 |
|
|
|
1,606 |
|
|
|
17,912 |
|
|
|
|
|
Research and Development
Research and development expenses increased to $1,357 for the three months ended September 30, 2020 from $721 for the three months ended September 30, 2019. The increase was largely attributable to higher clinical development, intellectual property and non-cash, share-based compensation expenses incurred during the three months ended September 30, 2020 compared to the three months ended September 30, 2019.
Clinical development costs have increased in the current period compared to the prior period largely due to the initiation costs related to GCAR. In addition, with the acquisition of the REM-001 technology as part of the Adgero merger, costs relating to drug manufacturing activity have been incurred. We expect our research and development costs to be higher in fiscal year 2021 than fiscal year 2020 as our GCAR GBM AGILE study commences. In addition, our planned clinical activities, including drug manufacturing, for REM-001 commenced during the quarter ended September 30, 2020.
Intellectual property costs increased in the three months ended September 30, 2020 compared to the three months ended September 30, 2019 as we have incurred more foreign office actions in the current period than the prior period. Patent costs can vary considerably depending on the filing of new patents, conversion of the provisional applications to PCT applications, foreign office actions, and actual filing costs. Non-cash, share-based compensation expense increased for the three months ended September 30, 2020, due to stock options granted in September 2020.
36
General and administrative expenses were $1,534 for the three months ended September 30, 2020 compared to $914 for the three months ended September 30, 2019. A significant portion of the increase was due to higher professional fees, office and sundry expenses, and non-cash, share-based compensation expense incurred in the current three months compared to the prior three months.
Professional fees increased during the three months ended September 30, 2020 compared to the three months ended September 30, 2019 primarily due to higher legal and accounting fees in the current period. Office and sundry increased in the three months ended September 30, 2020 compared to the three months ended September 30, 2019 due primarily to costs of higher directors’ and officers’ liability insurance.
In relation to general and administrative expenses during the three months ended September 30, 2020, we incurred non-cash, share-based compensation expense relating to warrants issued for services and stock option expense while during the three months ended September 30, 2019, we incurred non-cash, share-based compensation expense relating to stock option expense only. The higher stock option expense in the current period compared to the prior period was due to stock options granted in September 2020.
Merger Costs
Merger costs of $500 relate to expenditures with respect to the Adgero transaction and have been expensed.
Acquired In-Process Research and Development Expense
We acquired in-process research and development assets in connection with our merger with Adgero. As the acquired in-process research and development assets were deemed to have no current or alternative future use, an expense of $16.1 million was recognized in the condensed consolidated interim statements of operations for the three month period ended September 30, 2020.
Preferred Share Dividends
For each of the three months ended September 30, 2020 and 2019, we recorded $2 related to the dividend payable to Valent on the Series A preferred stock. The dividend has been recorded as a direct increase in accumulated deficit for both periods.
During the three months ended September 30, 2020, we issued 3,700 (2019 – 3,700) shares of common stock as a dividend on the Series B Preferred stock and recognized $5 (2019 - $2) as a direct increase in accumulated deficit.
Liquidity and Capital Resources
Three months ended September 30, 2020 compared to the three months ended September 30, 2019
|
|
|
September 30, 2020 $ |
|
|
September 30, 2019 $ |
|
|
Change $ |
|
|
Change % |
|
||||
|
|
|
(in thousands) |
|
|
|
|
|
|||||||||
|
Cash flows from operating activities |
|
|
(4,110 |
) |
|
|
(2,266 |
) |
|
|
(1,844 |
) |
|
|
81 |
|
|
Cash flows from investing activities |
|
|
969 |
|
|
|
— |
|
|
|
969 |
|
|
|
— |
|
|
Cash flows from financing activities |
|
|
23,351 |
|
|
|
6,607 |
|
|
|
16,744 |
|
|
|
253 |
|
Operating Activities
Net cash used in operating activities increased to $4,110 for the three months ended September 30, 2020 from $2,266 for the three months ended September 30, 2019. During the three months ended September 30, 2020 and 2019, we reported net losses of $19,518 and $1,606, respectively. Partially offsetting the higher loss in the current period compared to the prior period was the recognition of $16,094 of acquired in-process research and development expense related to the Adgero merger. Additional changes in adjustments to reconcile net loss to net cash used in operating activities for the three months ended September 30, 2020 included stock option expense of $405 being recognized during the current period compared to $51 in the prior period. The most significant changes in working capital for the three months ended September 30, 2020 were from uses of cash due to a decrease in accounts payable and accrued liabilities of $914 as well as $282 related to a decrease in related party payables. The most significant change in working capital for the three months ended September 30, 2019 was cash from a reduction in accounts payable and accrued liabilities of $617.
37
As part of the Adgero merger that closed on August 19, 2020, we acquired $969 in cash. There were no investing activities during the three months ended September 30, 2019.
Financing Activities
During the three months ended September 30, 2020, we received $21.6 million in net proceeds from the completion of a private placement of Series C Preferred stock and $994 from exercise of stock purchase warrants. Also, during the three months ended September 30, 2020, we received proceeds from the NBTS Loan of $500.
During the three months ended September 30, 2019, we received $6,583 in net proceeds from the completion of an underwritten public offering by us of common stock, pre-funded warrants, and common stock purchase warrants. Additionally, we received $26 pursuant to the exercise of warrants in the current period.
Liquidity Risk and Capital Expenditure Requirements
Liquidity Risk and Management Plans
(See note 1 to the condensed consolidated interim financial statements)
During the three months ended September 30, 2020, the Company reported a net loss of $19.5 million. As of September 30, 2020, the Company had $22.6 million of cash and cash equivalents and used $4.1 million of cash in its operating activities during the three months ended September 30, 2020. The Company is in the clinical stage and has not generated any revenues to-date. The Company does not have the prospect of achieving revenues until such time that its product candidates are commercialized, or partnered, which may not ever occur. In the future, the Company will require additional funding to maintain its clinical trials, research and development projects, and for general operations. The Company may tailor its drug development programs based on the amount of funding the Company is able to raise in the future. During the three months ended September 30, 2020, the Company completed a private placement in three closings for aggregate net proceeds of approximately $21.6 million (note 6). The Company believes that based on its current estimates, the cash and cash equivalents at September 30, 2020 of $22.6 million, as well as cash from the proceeds from stock purchase warrants exercised subsequent to September 30, 2020, will be sufficient to fund its planned operations for at least the next twelve months from the date these condensed consolidated interim financial statements are issued. However, the coronavirus (“COVID-19”) pandemic has created significant economic uncertainty and volatility in the credit and capital markets. The ultimate impact of the COVID-19 pandemic on the Company’s ability to raise additional capital in the future is unknown and will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the COVID-19 outbreak and any new information which may emerge concerning the severity of the COVID-19 pandemic.
Our future funding requirements will depend on many factors, including but not limited to:
|
|
• |
the rate of progress and cost of our clinical studies, preclinical studies and other discovery and research and development activities; |
|
|
• |
the costs associated with establishing manufacturing and commercialization capabilities; |
|
|
• |
the costs of acquiring or investing in businesses, product candidates and technologies; |
|
|
• |
the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
|
|
• |
the costs and timing of seeking and obtaining FDA and other regulatory approvals; |
|
|
• |
the effect of competing technological and market developments; |
|
|
• |
the economic and other terms and timing of any collaboration, licensing or other arrangements into which we may enter; and |
|
|
• |
the impact of us being a public entity. |
38
Until we can generate a sufficient amount of product revenue to finance our cash requirements, which we may never do, we expect to finance future cash needs primarily through public or private equity offerings, or strategic collaborations. The sale of equity and convertible debt securities may result in dilution to our stockholders and certain of those securities may have rights senior to those of our shares of capital stock. If we raise additional funds through the issuance of preferred stock, convertible debt securities or other debt financing, these securities or other debt could contain covenants that would restrict our operations. Any other third-party funding arrangement could require us to relinquish valuable rights. Economic conditions may affect the availability of funds and activity in equity markets. We do not know whether additional funding will be available on acceptable terms, or at all. If we are not able to secure additional funding when needed, we may have to delay, reduce the scope of or eliminate one or more of our clinical trials or research and development programs or make changes to our operating plan. In addition, we may have to seek a partner for one or more of our product candidates at an earlier stage of development, which would lower the economic value of those programs to us.
Critical Accounting Policies
The preparation of financial statements, in conformity with generally accepted accounting principles in the United States, requires companies to establish accounting policies and to make estimates that affect both the amount and timing of the recording of assets, liabilities, revenues and expenses. Some of these estimates require judgments about matters that are inherently uncertain and therefore actual results may differ from those estimates.
A detailed presentation of all of our significant accounting policies and the estimates derived therefrom is included in Note 2 to our consolidated financial statements for the year ended June 30, 2020 contained in our Form 10-K. While all of the significant accounting policies are important to our consolidated financial statements, the following accounting policies and the estimates derived therefrom are critical:
|
|
• |
Warrants and shares issued for services |
|
|
• |
Stock options |
|
|
• |
Accruals for research and development expenses and clinical trials |
Warrants and shares issued for services
We have issued equity instruments for services provided by employees and nonemployees. The equity instruments are valued at the fair value of the instrument granted.
Stock options
We recognize compensation costs resulting from the issuance of stock-based awards to employees, non-employees and directors as an expense in the statement of operations over the service period based on a measurement of fair value for each stock-based award. Prior to our adoption of ASU 2018-07, Compensation-Stock Compensation (Topic 718), Improvements to Nonemployee Share-Based Payment Accounting (“ASU 2018-07”), stock options granted to non-employee consultants were revalued at the end of each reporting period until vested using the Black-Scholes option-pricing model and the changes in their fair value were recorded as adjustments to expense over the related vesting period. For the three-months ended September 30, 2020, the determination of grant-date fair value for stock option awards was estimated using the Black-Scholes model, which includes variables such as the expected volatility of our share price, the anticipated exercise behavior of its grantee, interest rates, and dividend yields. For the three-months ended September 30, 2020, we utilized the plain vanilla method to determine the expected life of stock options. These variables are projected based on our historical data, experience, and other factors. Changes in any of these variables could result in material adjustments to the expense recognized for share-based payments. Such value is recognized as expense over the requisite service period, net of actual forfeitures, using the accelerated attribution method. We recognize forfeitures as they occur. The estimation of stock awards that will ultimately vest requires judgment, and to the extent actual results, or updated estimates, differ from current estimates, such amounts are recorded as a cumulative adjustment in the period estimates are revised.
39
Accruals for research and development expenses and clinical trials
As part of the process of preparing our financial statements, we are required to estimate our expenses resulting from our obligations under contracts with vendors, clinical research organizations and consultants, and under clinical site agreements in connection with conducting clinical trials. The financial terms of these contracts are subject to negotiations, which vary from contract to contract and may result in payment terms that do not match the periods over which materials or services are provided under such contracts. Our objective is to reflect the appropriate expenses in our financial statements by matching those expenses with the period in which services are performed and efforts are expended. We account for these expenses according to the timing of various aspects of the expenses. We determine accrual estimates by taking into account discussion with applicable personnel and outside service providers as to the progress of clinical trials, or the services completed. During the course of a clinical trial, we adjust our clinical expense recognition if actual results differ from our estimates. We make estimates of our accrued expenses as of each balance sheet date based on the facts and circumstances known to us at that time. Our clinical trial accruals are dependent upon the timely and accurate reporting of contract research organizations and other third-party vendors. Although we do not expect our estimates to be materially different from amounts actually incurred, our understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result in us reporting amounts that are too high or too low for any particular period. For years ended June 30, 2020 and 2019, there were no material adjustments to our prior period estimates of accrued expenses for clinical trials.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
Not required for a smaller reporting company.
Item 4. Controls and Procedures.
Disclosure Controls and Procedures
Management, with the participation of the Chief Executive Officer and Chief Financial Officer, conducted an evaluation (as required by Rule 13a-15 under the Exchange Act) of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this report. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were not effective as of the end of the period covered by this report, due to the material weakness in internal control over financial reporting as discussed in the Company’s Annual Report on Form 10-K for the year ended June 30, 2020, filed with the SEC on September 18, 2020.
Changes in internal controls
There have been no changes in our internal control over financial reporting that occurred during the quarter ended September 30, 2020 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
40
There are no legal proceedings the Company is party to or any of its property is subject to.
None
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
During the three months ended September 30, 2020, we issued 3,700 shares of common stock as dividends on our outstanding shares of Series B Preferred Stock. In addition, we issued 330,000 stock purchase warrants for services. The warrants have an exercise price of $1.4905 per share.
In connection with the foregoing, we relied upon the exemption from registration provided by Section 4(a)(2) of the Securities Act of 1933, as amended, for transactions not involving a public offering.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
Not applicable.
On November 11, 2020, our Board of Directors (the “Board”) approved the terms of the First Amended Executive Employment Agreement, by and between Saiid Zarrabian and Kintara Therapeutics, Inc., including (i) that Mr. Zarrabian will be eligible to receive an annual (fiscal year) performance bonus of up to fifty percent (50%) of his base salary, subject to the achievement of performance targets or criteria established by the Board for such year in consultation with Mr. Zarrabian and (ii) that in the event of overachievement of such targets or criteria for a given fiscal year, the Board may increase the bonus for such year to up to an additional twenty-five percent (25%) of Mr. Zarrabian’s base salary. In addition, on November 11, 2020, the Board approved the acceleration of vesting of 279,675 stock options to purchase shares of our common stock previously granted to Mr. Zarrabian on September 5, 2019, and which have an exercise price of $0.61 per share.
41
|
10.1 |
|
|
|
|
|
|
|
31.1 |
|
|
|
|
|
|
|
31.2 |
|
|
|
|
|
|
|
32.1 |
|
|
|
|
|
|
|
32.2 |
|
|
|
|
|
|
|
EX-101.INS |
|
XBRL Instance Document * |
|
|
|
|
|
EX-101.SCH |
|
XBRL Taxonomy Extension Schema Document * |
|
|
|
|
|
EX-101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase * |
|
|
|
|
|
EX-101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase * |
|
|
|
|
|
EX-101.LAB |
|
XBRL Taxonomy Extension Labels Linkbase * |
|
|
|
|
|
EX-101.PRE |
|
XBRL Taxonomy Extension Presentation Linkbase * |
|
* |
Filed herewith |
|
** |
Furnished herewith |
|
† |
Exhibit is a management contract or compensatory plan or arrangement. |
42
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
Kintara Therapeutics, Inc. |
|
|
|
|
|
|
Date: November 12, 2020 |
By: |
/s/ Saiid Zarrabian |
|
|
|
Saiid Zarrabian |
|
|
|
Chief Executive Officer (Principal Executive Officer) |
|
Date: November 12, 2020 |
By: |
/s/ Scott Praill |
|
|
|
Scott Praill |
|
|
|
Chief Financial Officer (Principal Financial and Accounting Officer) |
43
