Attached files
| file | filename |
|---|---|
| EX-32.1 - CERTIFICATION - Kintara Therapeutics, Inc. | f10q1215ex32i_delmarpharma.htm |
| EX-31.1 - CERTIFICATIONS - Kintara Therapeutics, Inc. | f10q1215ex31i_delmarpharma.htm |
| EX-32.2 - CERTIFICATION - Kintara Therapeutics, Inc. | f10q1215ex32ii_delmarpharma.htm |
| EX-31.2 - CERTIFICATIONS - Kintara Therapeutics, Inc. | f10q1215ex31ii_delmarpharma.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-Q
(Mark One)
| þ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For the quarterly period ended December 31, 2015 |
or
| o | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For the transition period from __________________ to __________________________ |
| Commission file number: 000-54801 |
| DelMar Pharmaceuticals, Inc. |
| (Exact name of registrant as specified in its charter) |
| Nevada | 99-0360497 | |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
|
Suite 720-999 West Broadway Vancouver, British Columbia, Canada |
V5Z 1K5 | |
| (Address of principal executive offices) | (zip code) |
| (604) 629-5989 |
| (Registrant's telephone number, including area code) |
| N/A |
| (Former name, former address and former fiscal year, if changed since last report) |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes þ No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes þ No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer o | |
|
Non-accelerated filer o (Do not check if smaller reporting company) |
Smaller reporting company þ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act)
Yes o No þ
Indicated the number of shares outstanding of each of the issuer's classes of common stock, as of the latest practicable date, 40,253,056 shares of common stock are issued and outstanding as of February 12, 2016.
| 1 |
TABLE OF CONTENTS
| Page No. | ||||
| PART I - FINANCIAL INFORMATION | ||||
| Item 1. | Financial Statements. | 3 | ||
| Item 2. | Management's Discussion and Analysis of Financial Condition and Results of Operations. | 23 | ||
| Item 3. | Quantitative and Qualitative Disclosures About Market Risk. | 51 | ||
| Item 4 | Controls and Procedures. | 51 | ||
| PART II - OTHER INFORMATION | ||||
| Item 1. | Legal Proceedings. | 52 | ||
| Item 1A. | Risk Factors. | 52 | ||
| Item 2. | Unregistered Sales of Equity Securities and Use of Proceeds. | 52 | ||
| Item 3. | Defaults Upon Senior Securities. | 52 | ||
| Item 4. | Mine Safety Disclosures. | 52 | ||
| Item 5. | Other Information. | 52 | ||
| Item 6. | Exhibits. | 52 |
| 2 |
PART 1. - FINANCIAL INFORMATION
Item 1. Financial Statements.
DelMar Pharmaceuticals, Inc.
Consolidated Condensed Interim Financial Statements
(Unaudited)
For the six months ended December 31, 2015
(expressed in US dollars unless otherwise noted)
| 3 |
DelMar Pharmaceuticals, Inc.
Consolidated Condensed Interim Balance Sheets
(Unaudited)
(expressed in US dollars unless otherwise noted)
| Note | December 31, 2015 $ | June 30, 2015 $ | ||||||||||
| As Restated | ||||||||||||
| Assets | ||||||||||||
| Current assets | ||||||||||||
| Cash and cash equivalents | 1,957,009 | 1,754,433 | ||||||||||
| Taxes and other receivables | 16,349 | 25,831 | ||||||||||
| Prepaid expenses | 167,502 | 245,038 | ||||||||||
| Deferred costs | - | 550,119 | ||||||||||
| 2,140,860 | 2,575,421 | |||||||||||
| Website development costs - net | 43,733 | - | ||||||||||
| 2,184,593 | 2,575,421 | |||||||||||
| Liabilities | ||||||||||||
| Current liabilities | ||||||||||||
| Accounts payable and accrued liabilities | 479,101 | 762,265 | ||||||||||
| Related party payables | 5 | 56,734 | 90,820 | |||||||||
| 535,835 | 853,085 | |||||||||||
| Stock option liability | 179,445 | 179,445 | ||||||||||
| Derivative liability | 6 | 1,352,584 | 2,364,381 | |||||||||
| 2,067,864 | 3,396,911 | |||||||||||
| Stockholders’ accumulated equity (deficit) | ||||||||||||
| Preferred stock | ||||||||||||
| Authorized | ||||||||||||
| 5,000,000 shares, $0.001 par value | ||||||||||||
| Issued and outstanding | ||||||||||||
| 278,530 Series A shares at December 31, 2015 (June 30, 2015 – 278,530) | 4 | 278,530 | 278,530 | |||||||||
| 1 special voting share at December 31, 2015 (June 30, 2015 – 1) | - | - | ||||||||||
| Common stock | ||||||||||||
| Authorized | ||||||||||||
| 200,000,000 shares, $0.001 par value, 44,309,098 issued at December 31, 2015 (June 30, 2015 – 39,455,931) | 7 | 44,309 | 39,456 | |||||||||
| Additional paid-in capital | 7 | 21,686,527 | 17,363,208 | |||||||||
| Warrants | 7 | 971,735 | 89,432 | |||||||||
| Accumulated deficit | (22,885,550 | ) | (18,613,294 | ) | ||||||||
| Accumulated other comprehensive income | 21,178 | 21,178 | ||||||||||
| 116,729 | (821,490 | ) | ||||||||||
| 2,184,593 | 2,575,421 | |||||||||||
Going concern, nature of operations, and corporate history (note 1)
Restatement of previously issued financial statements (note 2)
The accompanying notes are an integral part of these consolidated condensed interim financial statements.
| 4 |
DelMar Pharmaceuticals, Inc.
Consolidated Condensed Interim Statement of Loss and Comprehensive Loss
(Unaudited)
(expressed in US dollars unless otherwise noted)
| Note | Three months ended December 31, 2015 $ | Three months ended December 31, 2014 $ | Six months ended December 31, 2015 $ | Six months ended December 31, 2014 $ | ||||||||||||||||
| (as restated) | (as restated) | |||||||||||||||||||
| Expenses | ||||||||||||||||||||
| Research and development | 789,187 | 612,169 | 1,393,032 | 1,283,796 | ||||||||||||||||
| General and administrative | 890,672 | 656,229 | 1,364,697 | 1,101,229 | ||||||||||||||||
| 1,679,859 | 1,268,398 | 2,757,729 | 2,385,025 | |||||||||||||||||
| Other (income) loss | ||||||||||||||||||||
| Change in fair value of derivative liability | 6 | 680,188 | (892,326 | ) | 1,219,634 | (329,357 | ) | |||||||||||||
| Change in fair value of derivative liability due to change in warrant terms | 6 | 242,400 | 143,532 | 263,965 | (23,658 | ) | ||||||||||||||
| Loss on exchange of warrants | 6 | - | 92,843 | - | 92,843 | |||||||||||||||
| Foreign exchange loss | 44,253 | 7,295 | 26,780 | 9,686 | ||||||||||||||||
| Interest expense | - | - | - | 2,091 | ||||||||||||||||
| Interest income | (10 | ) | (109 | ) | (30 | ) | (261 | ) | ||||||||||||
| 966,831 | (648,765 | ) | 1,510,349 | (248,656 | ) | |||||||||||||||
| Net and comprehensive loss for the period | 2,646,690 | 619,633 | 4,268,078 | 2,136,369 | ||||||||||||||||
| Basic loss per share | 0.06 | 0.02 | 0.10 | 0.06 | ||||||||||||||||
| Basic weighted average number of shares | 43,979,516 | 37,798,183 | 43,234,639 | 37,125,074 | ||||||||||||||||
The accompanying notes are an integral part of these consolidated condensed interim financial statements.
| 5 |
DelMar Pharmaceuticals, Inc.
Consolidated Condensed Interim Statement of Cash Flows
(Unaudited)
(expressed in US dollars unless otherwise noted)
| Six months ended December 31, | ||||||||
| 2015 $ | 2014 $ | |||||||
| As Restated | ||||||||
| Cash flows from operating activities | ||||||||
| Loss for the period | (4,268,078 | ) | (2,136,369 | ) | ||||
| Items not affecting cash | ||||||||
| Amortization | 2,572 | - | ||||||
| Accrued interest | - | 2,091 | ||||||
| Change in fair value of derivative liability | 1,219,634 | (329,357 | ) | |||||
| Change in fair value of derivative liability due to change in warrant terms | 263,965 | (23,658 | ) | |||||
| Loss on exchange of warrants | - | 92,843 | ||||||
| Shares issued for services | 80,400 | - | ||||||
| Warrants issued for services | 181,571 | - | ||||||
| Share-based compensation | 114,868 | 260,510 | ||||||
| (2,405,068 | ) | (2,133,940 | ) | |||||
| Changes in non-cash working capital | ||||||||
| Taxes and other receivables | 9,482 | (4,510 | ) | |||||
| Prepaid expenses | 77,536 | 78,770 | ||||||
| Accounts payable and accrued liabilities | (283,164 | ) | 11,621 | |||||
| Related party payables | (34,086 | ) | (17,301 | ) | ||||
| (230,232 | ) | 68,580 | ||||||
| (2,635,300 | ) | (2,065,360 | ) | |||||
| Cash flows from investing activities | ||||||||
| Web site development costs | (16,762 | ) | - | |||||
| (16,762 | ) | - | ||||||
| Cash flows from financing activities | ||||||||
| Net proceeds from issuance of shares and warrants | 2,453,633 | - | ||||||
| Series A preferred stock dividend | (4,178 | ) | (2,089 | ) | ||||
| Net proceeds from the exercise of warrants | 405,183 | 1,266,177 | ||||||
| 2,854,638 | 1,264,088 | |||||||
| Increase (decrease) in cash and cash equivalents | 202,576 | (801,272 | ) | |||||
| Cash and cash equivalents - beginning of period | 1,754,433 | 4,759,711 | ||||||
| Cash and cash equivalents - end of period | 1,957,009 | 3,958,439 | ||||||
| Supplementary information | ||||||||
| Issuance of preferred shares for the settlement of the loan payable to Valent (note 4) | - | 278,530 | ||||||
| Reclassification of derivative liability upon the exercise of Investor Warrants (note 6) | 247,440 | 391,422 | ||||||
| Reclassification of derivative liability upon the exchange of Investor Warrants (note 6) | - | 305,112 | ||||||
| Reclassification of derivative liability upon the amendment of warrants (note 6) | 2,277,550 | 975,278 | ||||||
| Reclassification of stock option liability upon the forfeiture of stock options | - | 38,038 | ||||||
| Deferred costs recognized as equity issue costs | 550,119 | - | ||||||
The accompanying notes are an integral part of these consolidated condensed interim financial statements.
| 6 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2015
(expressed in US dollars unless otherwise noted)
| 1 | Going concern, nature of operations, and corporate history |
Going concern
These
financial statements have been prepared on a going concern basis which assumes that DelMar Pharmaceuticals, Inc. (“the Company”)
will continue its operations for the foreseeable future and contemplates the realization of assets and the settlement of liabilities
in the normal course of business.
For the six months ended December 31, 2015 the Company reported a net
loss of $4,268,078 and for the fiscal year ended June 30, 2015, the Company reported a net loss of $4,347,767. The Company also
reported negative cash flows from operations of $2,635,300 for the six months ended December 31, 2015 and an accumulated deficit
of $22,885,550 at that date. As at December 31, 2015, the Company has cash and cash equivalents on hand of $1,957,009 and a working
capital balance of $1,605,025. The Company has not begun to generate revenues from its product candidate and the Company does
not have the prospect of achieving revenues in the near future. The Company will require additional funding to maintain its research
and development projects and for general operations. These circumstances indicate the existence of a material uncertainty that
casts substantial doubt as to the ability of the Company to meet its obligations as they come due.
Consequently, management is pursuing various financing alternatives to fund the Company’s operations so it can continue as a going concern. During the six months ended December 31, 2015 the Company received net proceeds of $1,903,514 from a public offering of common stock and common stock purchase warrants (note 7) and $405,183 from the exercise of warrants. However, the net proceeds from these sources are not enough to fund all of the Company’s planned activities. Management plans to secure the necessary additional financing through the issue of new equity and/or entering into strategic partnership arrangements. Nevertheless, there is no assurance that these initiatives will be successful.
These financial statements do not give effect to any adjustments to the amounts and classification of assets and liabilities that may be necessary should the Company be unable to continue as a going concern. Such adjustments could be material.
Nature of operations
The Company is a clinical stage drug development company with a focus on the treatment of cancer. We are conducting clinical trials in the United States with our product candidate, VAL-083, as a potential new treatment for glioblastoma multiforme (“GBM”), the most common and aggressive form of brain cancer. We have also acquired certain exclusive commercial rights to VAL-083 in China where it is approved as a chemotherapy for the treatment of chronic myelogenous leukemia (“CML”) and lung cancer. In order to accelerate our development timelines and reduce technical risk, we leverage existing clinical and commercial data from a wide range of sources. We plan to seek marketing partnerships in China in order to potentially generate future royalty revenue.
| 7 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2015
(expressed in US dollars unless otherwise noted)
The address of the Company’s administrative offices is Suite 720 - 999 West Broadway, Vancouver, British Columbia, V5Z 1K5 and the Company’s clinical operations are located at 3485 Edison Way, Suite R, Menlo Park, California, 94025.
Corporate history
The Company is a Nevada corporation formed on June 24, 2009 under the name Berry Only Inc. On January 25, 2013 (the “Closing Date”), the Company entered into and closed an exchange agreement (the “Exchange Agreement”), with Del Mar Pharmaceuticals (BC) Ltd. (“DelMar (BC)”), 0959454 B.C. Ltd. (“Callco”), and 0959456 B.C. Ltd. (“Exchangeco”) and the security holders of DelMar (BC). Upon closing of the Exchange Agreement, DelMar (BC) became a wholly-owned subsidiary of the Company (the “Reverse Acquisition”). As a result of the shareholders of DelMar (BC) having a controlling interest in the Company subsequent to the Reverse Acquisition, for accounting purposes the transaction is a capital transaction with DelMar (BC) being the accounting acquirer even though the legal acquirer is the Company.
DelMar Pharmaceuticals, Inc. is the parent company of DelMar (BC), a British Columbia, Canada corporation, and Callco and Exchangeco which are British Columbia, Canada corporations. Callco and Exchangeco were formed to facilitate the Reverse Acquisition.
References to the Company refer to the Company and its wholly-owned subsidiaries, DelMar (BC), Callco and Exchangeco.
| 2 | Restatement of previously issued financial statements |
In our 2015 Annual Report on Form 10-K/A, we restated our previously issued consolidated financial statements and the related disclosures for the fiscal years ended June 30, 2015 and June 30, 2014 and for each of the quarters ended March 31, 2013, June 30, 2013, September 30, 2013, December 31, 2013, March 31, 2014, September 30, 2014, December 31, 2014, and March 31, 2015 (the "Restated Periods").
The restatement is the result of our corrections for the effect of financial statement errors attributable to the incorrect accounting for certain warrants issued for placement agent services issued on March 6, 2013 (the “2013 Placement Agent Warrants”). The 2013 Placement Agent Warrants were improperly accounted for as equity instruments at the time they were issued. During the preparation of our financial statements for the first quarter of fiscal 2016, we discovered that the 2013 Placement Agent Warrants represented a derivative liability and should not have been recognized as equity. The exercise price of the 2013 Placement Agent Warrants is subject to adjustment in certain circumstances. The public equity financing that was completed in August 2015 resulted in the exercise price of the 2013 Placement Agent Warrants being reduced. Accordingly, we have classified the 2013 Placement Agent Warrants as a derivative liability on the Consolidated Balance Sheets at June 30, 2015 and June 30, 2014 as well as recognized the gain/loss from the revaluation of the derivative liability in the Consolidated Statement of Operations and Comprehensive Loss of the years ended June 30, 2015 and June 30, 2014. We have also reflected the cumulative impact of the fair value adjustments from March 6, 2013 to June 30, 2014 in the accumulated deficit.
| 8 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2015
(expressed in US dollars unless otherwise noted)
The aggregate impacts of correcting the errors relating to the Placement Agent Warrants, as of, and for the three and six months ended December 31, 2014 were as follows:
| Three Months Ended December 31, 2014 | |||||||||||||
| As previously reported $ | Restatement adjustment $ | As restated $ | |||||||||||
| Change in fair value of derivative liability | (435,200 | ) | (457,126 | ) | (892,326 | ) | |||||||
| Loss for the period | 1,076,759 | (457,126 | ) | 619,633 | |||||||||
| Basic and diluted loss per share | 0.03 | (0.01 | ) | 0.02 | |||||||||
| Six Months Ended December 31, 2014 | |||||||||||||
| As previously reported $ | Restatement adjustment $ | As restated $ | |||||||||||
| Change in fair value of derivative liability | (66,606 | ) | (262,751 | ) | (329,357 | ) | |||||||
| Loss for the period | 2,399,120 | (262,751 | ) | 2,136,369 | |||||||||
| Basic and diluted loss per share | 0.06 | 0.00 | 0.06 | ||||||||||
| As At December 31, 2014 | |||||||||||||
| As previously reported $ | Restatement adjustment $ | As restated $ | |||||||||||
| Derivative liability | 1,567,291 | 1,518,889 | 3,086,180 | ||||||||||
| Additional paid-in capital | 16,625,081 | (136,800 | ) | 16,488,281 | |||||||||
| Warrants | 6,187,805 | (6,048,994 | ) | 138,811 | |||||||||
| Accumulated deficit | (21,064,623 | ) | 4,666,905 | 16,397,718 | ) | ||||||||
We assessed the impact of these errors on our previously issued financial statements and concluded that the combined impact of these errors was material to our financial statements. Consequently, we have restated the prior period financial statements identified above. All amounts in our consolidated financial statements in this Quarterly Report on Form 10-Q affected by the restatement adjustments reflect such amounts as restated.
| 9 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2015
(expressed in US dollars unless otherwise noted)
| 3 | Significant accounting policies |
Basis of presentation
The consolidated condensed interim financial statements of the Company have been prepared in accordance with United States Generally Accepted Accounting Principles (“U.S. GAAP”) and are presented in United States dollars. The Company’s functional currency is the United States dollar.
The accompanying consolidated condensed interim financial statements include the accounts of the Company and its wholly-owned subsidiaries, DelMar BC, Callco, and Exchangeco. All intercompany balances and transactions have been eliminated.
The principal accounting policies applied in the preparation of these financial statements are set out below and have been consistently applied to all periods presented.
Unaudited interim financial data
The accompanying unaudited December 31, 2015 consolidated condensed interim balance sheet, the consolidated condensed interim statements of loss and comprehensive loss for the three and six months ended December 31, 2015 and 2014, and consolidated condensed cash flows for the six months ended December 31, 2015 and 2014, and the related interim information contained within the notes to the consolidated condensed interim financial statements have been prepared in accordance with the rules and regulations of the Securities and Exchange Commission for interim financial information. Accordingly, they do not include all of the information and the notes required by U.S. GAAP for complete financial statements. These consolidated condensed interim financial statements should be read in conjunction with the audited financial statements of the Company as at June 30, 2015 filed in our amended Form 10-K/A filed with the Securities and Exchange Commission on November 16, 2015. In the opinion of management, the unaudited consolidated condensed interim financial statements reflect all adjustments, consisting of normal and recurring adjustments, necessary for the fair presentation of the Company’s financial position at December 31, 2015 and results of its operations for the three and six months ended December 31, 2015 and 2014, and its cash flows for the six months ended December 31, 2015 and 2014. The results for three and six months ended December 31, 2015 are not necessarily indicative of the results to be expected for the fiscal year ending June 30, 2016 or for any other future annual or interim period.
| 10 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2015
(expressed in US dollars unless otherwise noted)
Use of estimates
The preparation of consolidated condensed interim financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions about future events that affect the reported amounts of assets, liabilities, expenses, contingent assets and contingent liabilities as at the end or during the reporting period. Actual results could significantly differ from those estimates. Significant areas requiring management to make estimates include the derivative liability and the valuation of equity instruments issued for services. There have been no changes to the methodology used in determining these estimates from the period ended June 30, 2015.
Intangible assets – website development costs
Website development costs are stated at cost less accumulated amortization. The Company capitalizes website development costs associated with graphics design and development of the website application and infrastructure. Costs related to planning, content input, and website operations are expensed as incurred. The Company amortizes website development costs on a straight-line basis over three years. The website costs consist of $16,762 in cash costs and $29,543 in non-cash consideration in the form of the issuance of warrants. The Company recognized $2,572 (2014 - $nil) in amortization during the three and six months ended December 31, 2015.
Loss per share
Loss per share is calculated based on the weighted average number of common shares outstanding. For the three and six month periods ended December 31, 2015 and 2014 diluted loss per share does not differ from basic loss per share since the effect of the Company’s warrants and stock options are anti-dilutive. At December 31, 2015, potential common shares of 17,888,945 (December 31, 2014 – 15,409,745) relating to warrants and 3,635,000 (December 31, 2014 – 3,415,000) relating to stock options were excluded from the calculation of net loss per common share because their inclusion would be anti-dilutive.
Recent accounting pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (“FASB”) or other standard setting bodies that are adopted by the Company as of the specified effective date. Unless otherwise discussed, we believe that the impact of recently issued standards that are not yet effective will not have a material impact on our financial position or results of operations upon adoption.
Accounting Standards Update (“ASU”) 2014-15 - Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern
The objective of the guidance is to require management to explicitly assess an entity's ability to continue as a going concern, and to provide related footnote disclosures in certain circumstances. In connection with each annual and interim period, management will assess if there is substantial doubt about an entity's ability to continue as a going concern within one year after the issuance date of an entity’s financial statements. The new standard defines substantial doubt and provides examples of indicators thereof. The definition of substantial doubt incorporates a likelihood threshold of "probable" similar to the current use of that term in U.S. GAAP for loss contingencies. The new standard will be effective for all entities in the first annual period ending after December 15, 2016 (December 31, 2016 for calendar year-end entities). Earlier application is permitted. The Company is currently assessing this standard for its impact on future reporting periods.
| 11 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2015
(expressed in US dollars unless otherwise noted)
| 4 | Valent Technologies, LLC |
On September 30, 2014, the Company entered into an exchange agreement (the “Valent Exchange Agreement”) with Valent Technologies, LLC (“Valent”), an entity owned by the Company’s Chief Scientific Officer and director, and DelMar (BC). Pursuant to the Valent Exchange Agreement, Valent exchanged its loan payable in the outstanding amount of $278,530 (including aggregate accrued interest to December 31, 2014 of $28,530), issued to Valent by DelMar (BC), for 278,530 shares of the Company’s Series A Preferred Stock. The shares of Series A Preferred Stock have a stated value of $1.00 per share (the “Stated Value”) and are not convertible into common stock. The holder of the Series A Preferred Stock is entitled to dividends at the rate of 3% of the Stated Value per year, payable quarterly in arrears.
For the three and six months ended December 31, 2015, the Company recorded $2,089 and $4,178 respectively related to the dividend payable to Valent. The dividends have been recorded as a direct increase in accumulated deficit. For the six months ended December 31, 2014 the Company accrued $2,091 in interest expense on the loan payable to the date of the conversion on September 30, 2014 and $2,089 related to the dividend for the period from October 1, 2014 to December 31, 2014.
| 5 | Related party transactions |
During the six months ended December 31, 2015
Pursuant to consulting agreements with the Company’s officers, the Company recognized a total of $240,000 in compensation expense for the six months ended December 31, 2015.
Included in accounts payable at December 31, 2015 is an aggregate amount of $56,734 (June 30, 2015 - $90,820) owed to the Company’s officers and directors for fees and expenses. The Company pays related party payables incurred for fees and expenses under normal commercial terms.
The Company paid $85,000 in directors’ fees during the six months ended December 31, 2015.
The Company recorded $4,178 in dividends related to the preferred stock issued to Valent (note 4).
| 12 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2015
(expressed in US dollars unless otherwise noted)
During the six months ended December 31, 2014
Effective September 30, 2014, the Company entered into and closed an agreement with Valent to exchange its loan with Valent for 278,530 shares of preferred stock of the Company (note 4).
Pursuant to consulting agreements with the Company’s officers the Company recognized a total of $265,000 in compensation expense for the six months ended December 31, 2014.
The Company recognized $48,500 in directors’ fees during the six months ended December 31, 2014.
| 6 | Derivative liability |
The Company has issued common stock purchase warrants. Based on the terms of certain of these warrants the Company determined that the warrants were a derivative liability which is recognized at fair value at the date of the transaction and re-measured at fair value each reporting period with the changes in fair value recorded in the consolidated condensed statement of loss and comprehensive loss.
Investor Warrants
In connection with the Reverse Acquisition (note 1), during the quarter ended March 31, 2013 the Company issued units consisting of one share of common stock and one five-year warrant (the “Investor Warrants”) to purchase one share of common stock at an exercise price of $0.80. The exercise price of the Investor Warrants is subject to adjustment in the event that the Company issues common stock at a price lower than the exercise price, subject to certain exceptions. As a result of the financing completed by the Company during the three months ended September 30, 2015 (note 7) the exercise price of the Investor Warrants was reduced from $0.80 to $0.786. As a result of the price being reduced, the Company has recognized a loss of $8,098 on the revaluation of the warrants.
Investor Warrant exercises
During the six months ended December 31, 2015, 515,500 Investor Warrants were exercised for 515,500 shares of common stock at an exercise price of $0.786 per share. The Company received proceeds of $405,183 from these exercises. The warrants that have been exercised were revalued at their exercise date and then the reclassification to equity was recorded resulting in $247,440 of the derivative liability being reclassified to equity.
During the six months ended December 31, 2014 the Company concluded a tender offer whereby the holders of the Investor Warrants had the opportunity to exercise their warrants at an exercise price of $0.65. Under the tender offer, a total of 762,227 warrants were exercised for net proceeds of $470,676 after payment by the Company of a 5% warrant agent fee of $24,772.
| 13 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2015
(expressed in US dollars unless otherwise noted)
In addition, during the six months ended December 31, 2014, 1,223,847 warrants were exercised for 1,223,847 shares of common stock at an exercise price of $0.65 per warrant. The Company received proceeds of $795,501 from these exercises.
As a result of all of the warrant exercises during the six months ended December 31, 2014, the Company received net proceeds of $1,266,177 from the exercise of 1,986,074 warrants. The warrants that have been exercised were revalued at their exercise date and then the reclassification to equity was recorded resulting in $391,422 of the derivative liability being reclassified to equity.
Investor Warrant exchange
On December 31, 2014, the Company issued 414,889 shares of common stock in exchange for 1,244,666 Investor Warrants. The Investor Warrants that have been exchanged were revalued at their exchange date and then a reclassification to equity was recorded. The reclassification to equity upon the exchange was $305,112. The Company recognized a loss of $92,843 at the time of the exchange.
The 3,857,363 Investor Warrants outstanding at December 31, 2015 all have an exercise price of $0.786 and have been re-valued at December 31, 2015 using the adjusted exercise price of $0.786 and using a probability valuation model using the following assumptions: dividend rate - 0%, volatility – 99%, risk free rate – 1.18% and a term of approximately 2.25 years.
2013 Placement Agent Warrants
Also in connection with the Reverse Acquisition (note 1), on March 6, 2013 the Company issued 5,250,000 warrants (the “2013 Placement Agent Warrants”) that are exercisable at $0.80 per share until March 6, 2018 but can be exercised on a cashless basis. The exercise price of the 2013 Placement Agent Warrants is subject to adjustment in the event that the Company sells common stock at a price lower than the exercise price, subject to certain exceptions. As a result of the financing completed by the Company during the quarter ended September 30, 2015 (note 7) the exercise price of the 2013 Placement Agent Warrants was reduced from $0.80 to $0.786. As a result of the price being reduced, the Company has recognized a loss of $13,467.
On December 30, 2015, the Company entered into amendments (the “2013 Placement Agent Warrant Amendments”) with the holders of the 2013 Placement Agent Warrants. Pursuant to the 2013 Placement Agent Warrant Amendments, 5,050,000 2013 Placement Agent Warrants were amended to extend the expiration date to June 30, 2019 and remove the provision requiring an adjustment of the exercise price in the event the Company sells common stock at a purchase price lower than the current warrant exercise price. As a result of the 2013 Placement Agent Warrant Amendments, the Company has recognized a loss of $242,400 and has reclassified $2,277,550 from the derivative liability to equity resulting in an increase to equity of $2,035,150. The 2013 Placement Agent Warrants were revalued to the date of the amendment and were then reclassified to equity.
| 14 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2015
(expressed in US dollars unless otherwise noted)
Dividend Warrants
In connection with the Reverse Acquisition (note 1), effective January 24, 2013, the Company effected a warrant dividend (the “Warrant Dividend”) pursuant to which the Company issued one five-year warrant to purchase one share of common stock at an exercise price of $1.25 for each outstanding share of common stock (the “Dividend Warrants”). Pursuant to the Warrant Dividend, the Company issued an aggregate of 3,250,007 Dividend Warrants.
On October 31, 2014, the Company and all of its Dividend Warrant holders entered into amendments to the Dividend Warrants such that the Company’s redemption rights and certain provisions of the Dividend Warrant agreements relating to potential cash settlement of the Dividend Warrants were removed. The Dividend Warrants were revalued to the date of the amendment on October 31, 2014 which resulted in a reclassification to equity of $975,278.
2015 Agent Warrants
As part of the Company’s financing completed during the quarter ended September 30, 2015 (note 7), the Company issued 93,908 warrants to certain placement agents (“2015 Agent Warrants”). The 2015 Agent Warrants are exercisable at a per share price equal to $0.75 during the five-year period commencing six months from the effective date of the Offering, which period shall not extend further than five years from the effective date of the Offering. Therefore, all 2015 Agent Warrants expire on July 15, 2020.
The 93,908 2015 Agent Warrants outstanding at December 31, 2015 have been re-valued at December 31, 2015 using a simulated probability valuation model using the following assumptions: dividend rate - 0%, volatility – 88%, risk free rate – 1.63% and a term of approximately 4.75 years.
Warrants issued for services
In a prior period, the Company issued 300,000 warrants for services. The warrants were issued on September 12, 2013 and are exercisable on a cashless basis at an exercise price of $1.76 for five years. The warrants have been measured at December 31, 2014 using a simulated probability valuation model using the following assumptions: dividend rate - 0%, volatility – 96%, risk free rate – 1.31% and a term of approximately 3.0 years.
| 15 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2015
(expressed in US dollars unless otherwise noted)
The Company’s derivative liability is summarized as follows:
| December 31, 2015 $ | June 30, 2015 $ | ||||||||
| (as restated) | |||||||||
| Opening balance | 2,364,381 | 5,111,007 | |||||||
| Issuance of 2015 Agent Warrants | 29,594 | - | |||||||
| Change in fair value of warrants | 1,219,634 | (627,433 | ) | ||||||
| Change in fair value due to change in warrant terms | 263,965 | (23,658 | ) | ||||||
| Reclassification to equity upon amendment of warrants | (2,277,550 | ) | (975,278 | ) | |||||
| Reclassification to equity upon exchange of warrants | - | (728,835 | ) | ||||||
| Reclassification to equity upon exercise of warrants | (247,440 | ) | (391,422 | ) | |||||
| Closing balance | 1,352,584 | 2,364,381 | |||||||
| 7 | Stockholders’ equity |
Preferred stock
Authorized
5,000,000 preferred shares, $0.001 par value
Issued and outstanding
Special voting shares – at December 31, 2015 – 1 (June 30, 2015 – 1)
Series A shares – at December 31, 2015 – 278,530 (June 30, 2015 – 278,530)
Effective September 30, 2014 pursuant to the Company’s Valent Exchange Agreement (note 4), the Company filed the Series A Certificate of Designation with the Secretary of State of Nevada. Pursuant to the Series A Certificate of Designation, the Company designated 278,530 shares of preferred stock as Series A Preferred Stock. The shares of Series A Preferred Stock have a stated value of $1.00 per share (the “Stated Value”) and are not convertible into common stock. The holder of the Series A Preferred Stock is entitled dividends at the rate of 3% of the Stated Value per year, payable quarterly in arrears. Upon any liquidation of the Company, the holder of the Series A Preferred Stock will be entitled to be paid, out of any assets of the Company available for distribution to stockholders, the Stated Value of the shares of Series A Preferred Stock held by such holder, plus any accrued but unpaid dividends thereon, prior to any payments being made with respect to the common stock.
Common stock
Authorized
200,000,000 common shares, $0.001 par value
Issued and outstanding
December 31, 2015 – 44,309,098 (June 30, 2015 – 39,455,931)
| 16 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2015
(expressed in US dollars unless otherwise noted)
The issued and outstanding common shares at December 31, 2015 include 4,056,042 shares of common stock on an as-exchanged basis with respect to the shares of Exchangeco that can be exchanged for shares of common stock of the Company.
| Shares of common stock outstanding | Common stock | Additional paid-in capital | Warrants | ||||||||||||||
| $ | $ | $ | |||||||||||||||
| June 30, 2015 – as previously reported | 39,455,931 | 39,456 | 17,500,008 | 6,138,426 | |||||||||||||
| Restatement adjustments | - | - | (136,800 | ) | (6,048,994 | ) | |||||||||||
| June 30, 2015 – as restated | 39,455,931 | 39,456 | 17,363,208 | 89,432 | |||||||||||||
| Issuance of shares and warrants – net of issue costs (i) | 4,277,667 | 4,278 | 1,198,453 | 671,189 | |||||||||||||
| Reclassification of warrants (ii) | - | - | 2,277,550 | - | |||||||||||||
| Exercise of warrants for cash (iii) | 515,500 | 515 | 652,108 | - | |||||||||||||
| Warrants issued for services (iv) | - | - | - | 211,114 | |||||||||||||
| Shares issued for services (v) | 60,000 | 60 | 80,340 | - | |||||||||||||
| Stock-based compensation | - | - | 114,868 | - | |||||||||||||
| December 31, 2015 | 44,309,098 | 44,309 | 21,686,527 | 971,735 | |||||||||||||
(i) On July 15, 2015 the Company’s Registration Statement on Form S-1 relating to a public offering by the Company of common stock and common stock purchase warrants (the “Offering”) was declared effective by the Securities and Exchange Commission. Pursuant to the Offering, the Company issued 4,277,667 shares of common stock at $0.60 per share and 4,277,667 warrants (the “2015 Offering Warrants”) to purchase shares of common stock at $0.001 per warrant for total gross proceeds of $2,566,660. The 2015 Offering Warrants are exercisable at $0.75 per share for a period of five years until they expire on July 31, 2020.
The Company engaged certain placement agents for the sale of a portion of the shares and 2015 Offering Warrants. Under the Company’s engagement agreements with these placement agents, the Company agreed to pay up to a 7% cash commission and issue warrants to purchase shares of common stock (the “2015 Agent Warrants”) up to the number of shares of our common stock equal to 5% of the aggregate number of shares sold in the Offering by such placement agent. Pursuant to the placement agent agreements the Company paid a total cash commission of $80,575 and issued 93,908 2015 Agent Warrants (note 6). The 2015 Agent Warrants are exercisable at a per share price equal to $0.75 during the five-year period commencing six months from the effective date of the Offering, which period shall not extend further than five years from the effective date of the 2015 Offering. Therefore, all 2015 Agent Warrants expire on July 15, 2020.
In addition to the cash commission of $80,575 the Company also incurred additional cash issue and closing costs of $582,511 (including costs deferred at June 30, 2015 of $550,119) resulting in net cash proceeds of $1,903,514. The 2015 Agent Warrants have been recognized as non-cash issue costs of $29,594.
| 17 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2015
(expressed in US dollars unless otherwise noted)
(ii) Upon the amendment of the 2013 Placement Agent Warrants, the Company reclassified the related derivative liability to equity (note 6).
(iii) During the period ended December 31, 2015, 515,500 Investor Warrants were exercised for proceeds of $405,183 (note 6).
(iv) During the period ended December 31, 2015, the Company issued 560,000 warrants for services. All warrants have an exercise price of $0.75. Of these warrants, 60,000 expire on July 31, 2020 and 500,000 expire December 1, 2020.
(v) During the period ended December 31, 2015, the Company issued 60,000 shares of common stock for services.
Stock Options
The following table sets forth the stock options outstanding:
Number of stock options outstanding | Weighted average exercise price $ | ||||||||
| June 30, 2015 | 3,595,000 | 0.94 | |||||||
| Granted | 90,000 | 1.00 | |||||||
| Cancelled | (50,000 | ) | 1.05 | ||||||
| December 31, 2015 | 3,635,000 | 0.93 | |||||||
| 18 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2015
(expressed in US dollars unless otherwise noted)
The following table summarizes stock options currently outstanding and exercisable at December 31, 2015:
| Exercise price $ | Number outstanding at December 31, 2015 | Weighted average remaining contractual life (years) | Weighted average exercise price $ | Number exercisable at December 31, 2015 | Weighted average exercise price $ | ||||||||||||||||||
| 0.36 | 825,000 | 6.12 | 0.36 | 825,000 | 0.36 | ||||||||||||||||||
| 0.74 | 180,000 | 9.09 | 0.74 | 126,778 | 0.74 | ||||||||||||||||||
| 0.80 | 120,000 | 9.25 | 0.80 | 90,000 | 0.80 | ||||||||||||||||||
| 1.00 | 390,000 | 5.17 | 1.00 | 117,500 | 1.00 | ||||||||||||||||||
| 1.05 | 1,820,000 | 7.62 | 1.05 | 1,687,259 | 1.05 | ||||||||||||||||||
| 1.54 | 180,000 | 7.25 | 1.54 | 180,000 | 1.54 | ||||||||||||||||||
| 2.30 | 120,000 | 7.42 | 2.30 | 120,000 | 2.30 | ||||||||||||||||||
| 3,635,000 | 0.93 | 3,146,537 | 0.92 | ||||||||||||||||||||
Included in the number of stock options outstanding are 825,000 stock options granted at an exercise price of CDN $0.50. The exercise prices shown in the above table have been converted to $0.36 using the period ending closing exchange rate.
Certain stock options have been granted to non-employees and will be revalued at each reporting date until they have fully vested. The stock options have been re-valued using a Black-Scholes pricing model using the following assumptions:
| December 31, 2015 | |||||
| Dividend rate | 0 | % | |||
| Volatility | 112% to 118 | % | |||
| Risk-free rate | 1.00 | % | |||
Term – years | 0.5 to 1.0 | ||||
The Company has recognized the following amounts as stock-based compensation expense for the periods noted:
| Three months ended December 31, | Six months ended December 31, | ||||||||||||||||
2015 $ | 2014 $ | 2015 $ | 2014 $ | ||||||||||||||
| Research and development | 9,775 | (8,077 | ) | 16,230 | 13,056 | ||||||||||||
| General and administrative | 65,187 | 38,460 | 98,638 | 66,267 | |||||||||||||
| 74,962 | 30,383 | 114,868 | 79,323 | ||||||||||||||
All of the total stock option expense of $114,868 for the six months ended December 31, 2015 has been recognized as additional paid in capital. Of the stock option expense of $79,323 for the six months ended December 31, 2014 $78,694 has been recognized as additional paid in capital and $629 has been recognized as a stock option liability. The aggregate intrinsic value of stock options outstanding at December 31, 2015 was $530,269 (December 31, 2014 - $312,675) and the aggregate intrinsic value of stock options exercisable at December 31, 2015 was $515,424 (December 31, 2014 - $304,495). As of December 31, 2015 there was $12,263 in unrecognized compensation expense that will be recognized over the next year. No stock options granted under the Plan have been exercised to December 31, 2015. Upon the exercise of stock options new shares will be issued.
| 19 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2015
(expressed in US dollars unless otherwise noted)
A summary of status of the Company’s unvested stock options under all plans is presented below:
| Number of Options | Weighted average exercise price $ | Weighted average grant date fair value $ | |||||||||||
| Unvested at June 30, 2015 | 722,361 | 0.95 | 0.41 | ||||||||||
| Granted | 90,000 | 1.00 | 1.34 | ||||||||||
| Vested | (273,898 | ) | 0.90 | 0.68 | |||||||||
| Cancelled | (50,000 | ) | 1.05 | 0.57 | |||||||||
| Unvested at December 31, 2015 | 488,463 | 0.97 | 0.52 | ||||||||||
Warrants
Certain of the Company’s warrants have been recognized as a derivative liability (note 6). The following table summarizes all of the Company’s outstanding warrants as of December 31, 2015:
| Description | Number | ||||
| Balance – June 30, 2015 | 13,472,870 | ||||
| 2015 Offering Warrants (i) | 4,277,667 | ||||
| 2015 Agent Warrants (ii) | 93,908 | ||||
| Warrants issued for services (iii) | 560,000 | ||||
| Warrants exercised for cash (iv) | (515,500 | ) | |||
| Balance - December 31, 2015 | 17,888,945 | ||||
| 20 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2015
(expressed in US dollars unless otherwise noted)
| i) | Issued as part of the Company’s financing completed in August 2015. Warrants are exercisable at $0.75 until July 31, 2020. |
| ii) | Issued as part of the Company’s financing completed in August 2015. The 2015 Agent Warrants are exercisable at a price of $0.75 during the period commencing January 15, 2016 until their expiry on July 15, 2020. |
| iii) | Warrants have an exercise price of $0.75. Of the total, 60,000 vested in tranches of 20,000 warrants each on November 30, 2015, December 31, 2015, and January 31, 2016 and are exercisable commencing January 1, 2016 until they expire on July 31, 2020. In addition, 500,000 of the warrants vest in tranches of 150,000 on December 1, 2015, and 50,000 on each of December 31, 2015, January 31, 2016, February 29, 2016, March 31, 2016, April 30, 2016, May 31, 2016, and June 30, 2016 until they expire on December 1, 2020. |
| iv) | 515,500 Investor Warrants were exercised for cash at $0.786 per share for proceeds of $405,183. |
| 8 | Financial instruments |
The Company has financial instruments that are measured at fair value. To determine the fair value, we use the fair value hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use to value an asset or liability and are developed based on market data obtained from independent sources. Unobservable inputs are inputs based on assumptions about the factors market participants would use to value an asset or liability. The three levels of inputs that may be used to measure fair value are as follows:
| ● | Level one - inputs utilize quoted prices (unadjusted) in active markets for identical assets or liabilities; |
| ● | Level two - inputs are inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly such as interest rates, foreign exchange rates, and yield curves that are observable at commonly quoted intervals; and |
| ● | Level three - unobservable inputs developed using estimates and assumptions, which are developed by the reporting entity and reflect those assumptions that a market participant would use. |
Assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurements. Changes in the observability of valuation inputs may result in a reclassification of levels for certain securities within the fair value hierarchy.
The Company’s financial instruments consist of cash and cash equivalents, other receivables, accounts payable, related party payables and derivative liability. The carrying values of cash and cash equivalents, other receivables, accounts payable and related party payables approximate their fair values due to the immediate or short-term maturity of these financial instruments.
| 21 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2015
(expressed in US dollars unless otherwise noted)
Derivative liability
The Company accounts for certain warrants under the authoritative guidance on accounting for derivative financial instruments indexed to, and potentially settled in, a company’s own stock, on the understanding that in compliance with applicable securities laws, the warrants require the issuance of securities upon exercise and do not sufficiently preclude an implied right to net cash settlement. The Company classifies these warrants on its balance sheet as a derivative liability which is fair valued at each reporting period subsequent to the initial issuance. The Company has used a simulated probability valuation model to value the warrants. Determining the appropriate fair-value model and calculating the fair value of warrants requires considerable judgment. Any change in the estimates (specifically probabilities) used may cause the value to be higher or lower than that reported. The estimated volatility of the Company’s common stock at the date of issuance, and at each subsequent reporting period, is based on the historical volatility of similar life sciences companies. The risk-free interest rate is based on rates published by the government for bonds with a maturity similar to the expected remaining life of the warrants at the valuation date. The expected life of the warrants is assumed to be equivalent to their remaining contractual term.
| a) | Fair value of derivative liability |
The derivative is not traded in an active market and the fair value is determined using valuation techniques. The Company uses judgment to select a variety of methods to make assumptions that are based on specific management plans and market conditions at the end of each reporting period. The Company uses a fair value estimate to determine the fair value of the derivative liability. The carrying value of the derivative liability would be higher or lower as management estimates around specific probabilities change. The estimates may be significantly different from those recorded in the consolidated financial statements because of the use of judgment and the inherent uncertainty in estimating the fair value of these instruments that are not quoted in an active market. All changes in the fair value are recorded in the consolidated statement of operations and comprehensive loss each reporting period. This is considered to be a Level 2 financial instrument.
The Company has the following liabilities under the fair value hierarchy:
| December 31, 2015 | |||||||||||||
| Liability | Level 1 | Level 2 | Level 3 | ||||||||||
| Derivative liability | - | 1,352,584 | - | ||||||||||
| June 30, 2015 | |||||||||||||
| (as restated) | |||||||||||||
| Liability | Level 1 | Level 2 | Level 3 | ||||||||||
| Derivative liability | - | 2,364,381 | - | ||||||||||
| 22 |
Item 2. Management's Discussion and Analysis of Financial Condition and Results of Operations.
This Management’s Discussion and Analysis (“MD&A”) contains “forward-looking statements”, which represent our projections, estimates, expectations or beliefs concerning, among other things, financial items that relate to management’s future plans or objectives or to our future economic and financial performance. In some cases, you can identify these statements by terminology such as “may”, “should”, “plans”, “believe”, “will”, “anticipate”, “estimate”, “expect” “project”, or “intend”, including their opposites or similar phrases or expressions. You should be aware that these statements are projections or estimates as to future events and are subject to a number of factors that may tend to influence the accuracy of the statements. These forward-looking statements should not be regarded as a representation by the Company or any other person that the events or plans of the Company will be achieved. You should not unduly rely on these forward-looking statements, which speak only as of the date of this report. Except as may be required under applicable securities laws, we undertake no obligation to publicly revise any forward-looking statement to reflect circumstances or events after the date of this report or to reflect the occurrence of unanticipated events.
You should review the factors and risks we describe under “Risk Factors” in our report on Form 10-K/A for the year ended June 30, 2015 and in the Company’s other filings with the Securities and Exchange Commission, available at www.sec.gov. Actual results may differ materially from any forward-looking statement.
Overview
DelMar Pharmaceuticals, Inc. (the “Company”) is a clinical stage drug development company with a focus on the treatment of cancer. We are conducting clinical trials in the United States with our product candidate, VAL-083, as a potential new treatment for glioblastoma multiforme (“GBM”), the most common and aggressive form of brain cancer. We have also acquired certain exclusive commercial rights to VAL-083 in China where it is approved as a chemotherapy for the treatment of chronic myelogenous leukemia (“CML”) and lung cancer. In order to accelerate our development timelines and reduce technical risk, we leverage existing clinical and commercial data from a wide range of sources. We plan to seek marketing partnerships in China and elsewhere in order to supplement our own commercialization efforts and potentially generate future royalty revenue.
Recent Highlights
| ● | We reported the completion of enrollment in the 14-patient expansion cohort of our Phase II clinical study of VAL-083 in patients with refractory GBM. In addition, we confirmed 40mg/m2 as the maximum tolerated dose (“MTD”) for advancement into registration-directed clinical trials. This optimized dosing regimen delivers substantially higher doses compared to previous clinical trials conducted by the National Cancer Institutes (“NCI”) in the United States. We believe that such higher doses may enhance the potential of VAL-083 to impact a patient’s tumor and as well as to improve patient outcomes; | |
| ● | We also reported the observation of a promising dose-response trend in the Phase I portion of the clinical trial. A subset analysis of GBM patients whose tumors have recurred following both front-line therapy with temozolomide and second-line bevacizumab (Avastin®) treatment in dose cohorts receiving ≥30mg/m2 had a median survival of approximately nine (9) months vs. approximately five (5) months in dose cohorts ≤5mg/m2; | |
| ● | At the Society for NeuroOncology (“SNO”) Annual Meeting in November 2015 we reported interim survival data from the 14-patient Phase II expansion cohort. A Kaplan Meyer survival estimate, based on these preliminary interim data, is consistent with that observed during the Phase I portion of the study; |
| 23 |
| ● | We announced that we entered into a collaboration with the University of Texas MD Anderson Cancer Center (“MDACC”) to accelerate the clinical development of VAL-083 for the treatment of GBM. As part of the collaboration, MDACC will initiate a new Phase II clinical study with VAL-083 in patients with GBM at first recurrence/progression, prior to Avastin® exposure; | |
| ● | At the American Association for Cancer Research (“AACR”)-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics we reported additional non-clinical data supporting the favorable differentiation of VAL-083 versus standard of care in the treatment of GBM, non-small cell lung cancer (“NSCLC”) and other solid tumors. We believe these data support the potential of VAL-083 to address the modern unmet medical needs in the treatment of a range of cancers, especially where other therapies have failed or are predicted to give sub-optimal outcomes; | |
| ● | At AACR – Advances in Pediatric Research: From Mechanisms and Models to Treatment and Survivorship, we presented data indicating that VAL-083 offers potential therapeutic alternatives in difficult-to-treat pediatric brain tumors; | |
| ● | At AACR’s Advances in Ovarian Cancer Research: Exploiting Vulnerabilities Conference, we presented data supporting the effectiveness of VAL-083 against cisplatin-resistant ovarian cancers and raised the potential for VAL-083 as a treatment for ovarian cancers as a single-agent against platinum-resistant tumors or in combination with platinum-based chemotherapeutic regimens; and | |
| ● | We continued to strengthen our intellectual property portfolio. DelMar now holds six issued US patents and four issued international patents. In addition, we have filed eleven patent applications across eight patent families. |
VAL-083
Clinical Developments
We anticipate advancing VAL-083 across a variety of cancer indications, including both GBM and NSCLC, in calendar 2016.
| Indication | Trial Phase | Planned Initiation | |||||
| Refractory GBM | II/III | 2H 2016*** | |||||
| 2nd line GBM* | II | 1H 2016 | |||||
| 1st line GBM** | II | 1H 2016 | |||||
| NSCLC** | II | 1H 2016 | |||||
* Study conducted by MDACC.
** Study to be conducted in China in collaboration with Guangxi Wuzhou Pharmaceutical (Group) Co. Ltd.
*** Initiation of registration-directed Phase II/III trial subject to end of Phase II meeting with FDA and additional funding.
Background on VAL-083
Our product candidate,VAL-083, represents a “first-in-class” small molecule chemotherapeutic which means that the molecular structure of VAL-083 is not an analogue or derivative of other small molecule chemotherapeutics approved for the treatment of cancer. VAL-083 was originally discovered in the 1960’s and has been assessed in 42 Phase 1 and Phase 2 clinical trials sponsored by the NCI in the United States as a treatment against various cancers including lung, brain, cervical, ovarian tumors and leukemia. Published pre-clinical and clinical data suggest that VAL-083 may be active against a range of tumor types. VAL-083 is approved as a cancer chemotherapeutic in China for the treatment of CML and lung cancer. VAL-083 has not been approved for any indications outside of China.
Upon obtaining regulatory approval, we intend to commercialize VAL-083 for the treatment of orphan and other cancer indications where patients have failed other therapies or have limited medical options. Orphan diseases are defined in the United States under the Rare Disease Act of 2002 as “any disease or condition that affects fewer than 200,000 persons in the United States”. The Orphan Drug Act of 1983 is a federal law that provides financial and other incentives including a seven year period of market exclusivity in the United States to encourage the development of new treatments for orphan diseases. In February 2012, we announced that VAL-083 has been granted protection under the Orphan Drug Act by the U.S. Food and Drug Administration (“FDA”) for the treatment of glioma, including GBM. In January 2013, the European Medicines Agency (“EMA”) also granted orphan drug protection to VAL-083 for the treatment of glioma. In Europe, the period of market exclusivity is for a 10 year period.
We research the mechanism of action of potential product candidates to determine the clinical indications best suited for therapy and seek to rapidly advance into human clinical trials and toward commercialization. The mechanism of action of VAL-083 is understood to be a bi-functional alkylating agent. Alkylating agents are a commonly used class of chemotherapy drugs. They work by binding to DNA and interfering with normal processes within the cancer cell, which prevents the cell from making the proteins needed to grow and survive. After exposure to alkylating agents, the cancer cell becomes dysfunctional and dies. There are a number of alkylating agents on the market that are used by physicians to treat different types of cancer.
Based on published research and our own data, the cytotoxic functional groups and the mechanism of action of VAL-083 are understood to be functionally different from alkylating agents commonly used in the treatment of cancer. VAL-083 has previously demonstrated activity in cell-lines that are resistant to other types of chemotherapy. No evidence of cross-resistance has been reported in published clinical studies. Therefore, we believe that VAL-083 may be effective in treating tumors that have failed or become resistant to other chemotherapies.
We have presented new research at peer-reviewed scientific meetings demonstrating that VAL-083 is active in patient-derived tumor cell lines and cancer stem cells that are resistant to other chemotherapies.
| 24 |
VAL-083 readily crosses the blood brain barrier where it maintains a long half-life in comparison to the plasma. Published pre-clinical and clinical research demonstrates that VAL-083 is selective for brain tumor tissue.
The main dose-limiting toxicity (“DLT”) related to the administration of VAL-083 in previous NCI-sponsored clinical studies was myelosuppression. Myelosuppression is the decrease in cells responsible for providing immunity, carrying oxygen, and those responsible for normal blood clotting. Myelosuppression is a common side effect of chemotherapy. There is no evidence of lung, liver or kidney toxicity even with prolonged treatment by VAL-083. Commercial data from the Chinese market where the drug has been approved for more than 15 years supports the safety findings of the NCI studies.
Modern medicine allows for better management of myelosuppressive side effects. We believe this offers the potential opportunity to improve upon the drug’s already established efficacy profile by substantially increasing the dose of VAL-083 that can be safely administered to GBM patients.
Background on GBM
Worldwide, there are an estimated 240,000 new cases of brain and central nervous system (“CNS”) tumors each year. Gliomas are a type of CNS tumor that arises from glial cells in the brain or spine. Glial cells are the cells surrounding nerves. Their primary function is to provide support and protection for neurons in the CNS.
GBM, also known as Grade IV astrocytoma, is the most common and the most lethal form of glioma. According to the World Health Organization, GBM occurs with an incidence of 3.17 per 100,000 person-years. Approximately 15,000 new cases of GBM are expected to be diagnosed in the United States during 2015.
GBM progresses quickly and patients deteriorate rapidly. Common symptoms include headaches, seizures, nausea, weakness, paralysis and personality or cognitive changes such as loss of speech or difficulty in thinking clearly.
The majority of GBM patients do not survive for more than two years following diagnosis, and the median survival in newly diagnosed patients with best available treatments is 14.6 months.
Standard treatment following diagnosis includes surgical resection to remove as much of the tumor as possible (“debulking”) followed by radiotherapy with concomitant and adjuvant chemotherapy with Temodar® (temozolomide, “TMZ”). Nearly all patients diagnosed with GBM will relapse following first-line treatment, with a 1-year survival rate of approximately 25% following failure of front-line therapy, with average 5-year survival rate less than 3%.
Avastin® (bevacizumab - an anti-VEGF antibody) is approved as a single agent for patients with recurrent GBM following prior therapy as an alternative to corticosteroids to relieve disease symptoms in the US, Canada, Australia and Japan. Avastin® carries a “black-box warning” related to severe, sometimes fatal, side effects related to gastrointestinal perforations, wound healing complications and hemorrhage. There are no data demonstrating an improvement in disease-related symptoms or increased survival in refractory GBM with Avastin®.
TMZ and the nitrosoureas, including carmustine, lomustine, and nimustine, are alkylating agents that readily cross the blood-brain-barrier (“BBB”) and are used in the treatment of CNS cancers, including GBM. Alkylating agents are among the oldest type of cancer chemotherapies in use today. Alkylating agents bind to DNA to cause damage to cancer cells. Their anti-tumor mechanism is via alkylation of DNA resulting in base-pair mismatch or strand-mediated cross links between base pairs. The DNA damage caused by alkylating agents mimics naturally occurring errors, resulting in apoptosis and tumor cell death.
The primary anti-cancer mechanism of TMZ and the nitrosoureas is to attack the tumor’s DNA via alkylation of the O6 position of the DNA base residue, guanine. TMZ treatment causes DNA damage mainly by methylation at the O6 position of guanine resulting in guanine-thymine base pair mismatches during replication. Nitrosoureas mediate their cytotoxic effect by ethylation at the O6 position of guanine which produces a cross-link to cytosine residues resulting in double-strand DNA breaks during mitosis.
| 25 |
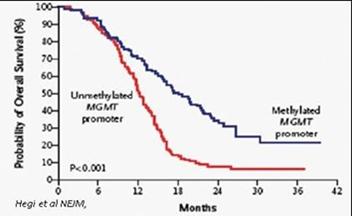
A majority of GBM patients’ tumors are resistant to TMZ or nitrosourea therapy due to high expression of a naturally occurring enzyme called O6-DNA methylguanine methyl-transferase (“MGMT”) enzyme which repairs O6-guanine lesions. MGMT repair in turn inhibits the activity of TMZ and nitrosoureas and allows a patients’ GBM tumor to continue to grow in spite of treatment.
Consistent with the importance of its repair activity, high expression of MGMT is strongly correlated with poor patient outcomes. Several clinical studies have established that MGMT is an important prognostic indicator of response to TMZ and patient survival.
Probability of GBM Patient Survival Correlated to Expression of MGMT Enzyme
(Unmethylated promoter = High MGMT Expression and Significantly Shorter Survival)
VAL-083 in GBM
VAL-083 is an alkylating agent which readily crosses the BBB. Its primary cytotoxic mechanism, epoxide derived DNA cross-links at the N7 position of guanine, is distinct from TMZ or the nitrosoureas.
Our research demonstrates that VAL-083’s unique cytotoxic mechanism forms DNA cross-links at the N7 position of guanine and retains cytotoxic activity independent of MGMT expression in vitro. We have presented new research at peer-reviewed scientific meetings demonstrating that VAL-083 is active in patient-derived tumor cell lines and cancer stem cells that are resistant to other chemotherapies. Of particular importance is resistance to Temodar® due to activity of the repair enzyme known as MGMT, which results in chemoresistance in many GBM patients. At AACR in 2012, we presented data demonstrating that VAL-083 is active independent of MGMT resistance in laboratory studies. VAL-083 has more potent activity against brain tumor cells in comparison to TMZ and overcome resistance associated with MGMT suggesting the potential to surpass the current standard-of-care in the treatment of GBM.
| 26 |
A Summary of Our Data Demonstrating that VAL-083’s Anti-Tumor Mechanism is Distinct from, and can Overcome, MGMT-Related Chemoresistance in the Treatment of GBM
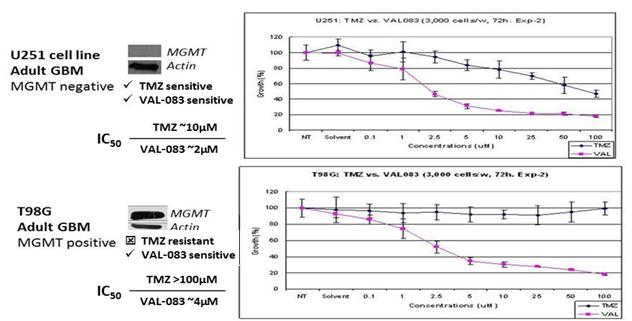
VAL-083 has been assessed in multiple historical NCI-sponsored clinical studies as chemotherapy in the treatment of newly diagnosed and recurrent brain tumors and other cancers. In general, tumor regression in brain cancer was achieved following therapy in greater than 40% of patients treated and stabilization was achieved in an additional 20% to 30%. In published clinical studies VAL-083 has previously been shown to have a statistically significant impact on median survival in high grade glioma brain tumors when combined with radiation versus radiation alone with results similar or superior to other chemotherapies approved for use in GBM.
A Summary of Published Data adapted from Separate Sources Comparing the Efficacy of VAL-083
and Other Therapies in the Treatment of GBM
| Comparative Therapy | Median Survival Benefit | |||||
| Chemotherapy | Radiation (XRT) | Radiation + Chemotherapy | vs. XRT alone | |||
| Temodar | 12.1 months | 58 weeks (14.6 months) |
2.5 months | |||
| VAL-083 | 8.8 months | 67 weeks (16.8 months) |
8.0 months | |||
| Lomustine | 52 weeks | |||||
| Carmustine | 40-50 weeks | |||||
| Semustine | 35 weeks | |||||
| Avastin | n.a. | |||||
| 27 |
Additional support for the differentiated profile of VAL-083 and TMZ comes from the results of studies with GBM cancer stem cells (“CSCs”). GBM CSCs display strong resistance to TMZ, even where MGMT expression is low. However, our data demonstrates that GBM CSCs are susceptible to VAL-083 independent of MGMT expression.
Based on historical data and our own research, we believe that VAL-083 has the potential to offer physicians and patients a new paradigm in the treatment of GBM that will address significant unmet medical needs. In addition, the profile of VAL-083 offers the potential of additive or synergistic benefit as a future combination therapy with existing chemotherapeutic agents or novel vaccines or immunotherapy approaches currently under investigation.
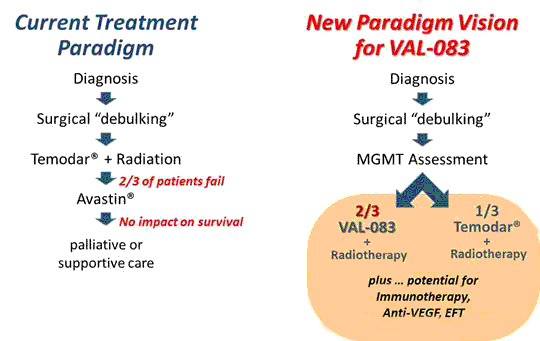
Interim Phase I/II Results in Refractory GBM
Our initial clinical research has been in refractory GBM, for patients who have failed currently approved therapies. These patients currently have no viable treatment options.
In 2011, we filed an investigational new drug (“IND”) application with the FDA and initiated human clinical trials with VAL-083 as a potential treatment for GBM. Details of the study, including enrollment estimates, are available at http://www.clinicaltrials.gov/ct2/show/NCT01478178?term=VAL-083&rank=1)
Our clinical trial is a Phase I/II, open-label, single arm dose-escalation study designed to evaluate the safety, tolerability, pharmacokinetics and anti-cancer activity of VAL-083 in patients with GBM. To be eligible for our clinical trial, patients must have been previously treated for GBM with surgery and/or radiation, if appropriate, and must have failed both bevacizumab (Avastin®) and temozolomide (Temodar®), unless either or both are contra-indicated.
Response to treatment with VAL-083 is measured prior to each treatment cycle. An initial phase of the study involves dose escalation cohorts until a maximum tolerated dose (“MTD”) is established in the context of modern care. The goal of our Phase I/II clinical trial is to determine a modernized dosing regimen for advancement into a registration directed clinical trial.
In the Phase I portion of the trial, we enrolled 30 GBM patients across 8 dose cohorts ranging from 1.5 to 50 mg/m2/d. Dose limiting toxicity (“DLT”) consisting of thrombocytopenia (low platelet counts) was observed at doses above 40 mg/m2/d. In accordance with the protocol, 40 mg/m2/d was defined as the maximum tolerated dose (MTD). Based on these observations, we have now enrolled an additional 14 patients in a Phase II expansion cohort at the MTD.
We have presented interim data from our Phase I/II clinical trial at peer-reviewed scientific meetings including most recently at the annual meetings of SNO in November 2015, ASCO in June 2015, and AACR in April 2015. We anticipate presenting additional data at upcoming scientific meetings during 2016.
In summary, at doses tested to date, our interim clinical data is as follows:
Safety and Tolerability
In the Phase I portion of the study, we confirmed that no drug-related severe adverse events were reported and myelosuppression was mild at doses ≤40mg/ m2/d. Dose limiting toxicity (DLT) defined by thrombocytopenia (low platelet counts) was observed at a dose of 50mg/ m2/d and at an interim dose of 45mg/ m2/d, which was added to the protocol to further explore the therapeutic window above the MTD. Notably, the low point of platelet counts (nadir) observed at doses above the MTD occurred around day 20 and generally DLT-related symptoms resolved rapidly and spontaneously without concomitant treatment.
Interim safety data from the 14-patient Phase II expansion cohort are consistent with observations from the Phase I portion of the study. Therefore, we have confirmed 40mg/m2 as the dose for advancement into registration-directed clinical trials. This optimized dosing regimen delivers substantially higher doses compared to previous clinical trials conducted by the NCI in the United States (see Doses Achieved and Pharmacokinetics below). We believe that such higher doses may enhance the potential of VAL-083 to impact a patent’s tumor and as well as to improve patient outcomes.
| 28 |
Therapeutic Observations
In the Phase I portion of the study, one of three GBM patients in cohort 6 (30mg/m2) and one of three GBM patients in cohort 7 (40 mg/m2) exhibited stable disease after one cycle of treatment. In earlier cohorts, we reported that two patients exhibited a response (stable disease or partial response) with a maximum response of 84 weeks and improved clinical signs prior to discontinuing due to adverse events unrelated to the study.
In the Phase 1 portion of the study, we reported that the progression free survival following treatment with VAL-083 was short (1.2 - 1.4 months) as expected since patients were not re-resected. However, preliminary analysis shows favorable increasing dose-dependent median survival after only two cycles of treatment with VAL-083: Median overall survival (“OS”) of approximately nine (9) months for patients enrolled in cohorts 6 & 7 (VAL-083 dose ≥30 mg/m2/day) vs. approximately five (5) months for patients enrolled in cohorts 1 - 3 (VAL-083 doses ≤5mg/m2/day). Based on pharmacokinetic analyses, doses above ≥30 mg/m2/day are projected to achieve sufficient drug concentrations at the tumor to achieve therapeutic benefit in patients.
At the Society for NeuroOncology Annual Meeting (November 2015), we reported interim survival data from the 14-patient Phase II expansion cohort that is consistent with that observed during the Phase I portion of the study. A Kaplan Meyer survival estimate, based on these preliminary interim data, projects a greater than nine-month median survival in refractory GBM patients whose tumors have recurred following both front-line therapy with temozolomide and second-line bevacizumab (Avastin®) treatment. We anticipate confirming the median overall survival for patients receiving a therapeutic dose of VAL-083 during the first half of 2016. These data will be used to inform the design of registration-directed Phase II/III clinical trials.
Doses Achieved
We confirmed that we achieved doses of VAL-083 that are substantially higher than were utilized in the original published NCI-sponsored clinical trials. A summary of doses completed in our dose-escalation phase of our clinical trial in comparison to the NCI’s historical regimen is as follows:
|
Dosing Regimen & Study |
Single Dose |
Acute Regimen (single cycle) |
Comparative Cumulative Dose (@ 35 days) |
Dose Intensity (dose per week) | ||||||
|
NCI GBM historical regimen (Eagan etal) daily x 5 q 5wks (cycle = 35 days) |
25 mg/m2 | x5 days = | 125 mg/m2 | 125 mg/m2 | 25mg/m2/wk | |||||
|
DelMar VAL-083 regimen daily x 3 q 3wks (cycle = 21 days) |
30 mg/m2 40 mg/m2 50 mg/m2 |
x3 days = |
90 mg/m2 120 mg/m2 150 mg/m2 |
180 mg/m2 240 mg/m 2 300 mg/m 2 |
30mg/m2/wk 40mg/m2/wk 50mg/m2/wk | |||||
Daily x 5 q 5wks refers to a dosing regimen of once per day for five consecutive days every five weeks (35 day cycle); while daily x 3 q 3wks refers to a dosing regimen of once per day for three consecutive days every three weeks (21 day cycle)
| 29 |
Pharmacokinetics
We reported that observed pharmacokinetics are linear and consistent with previous published data suggesting that concentrations of VAL-083 at a dose of 40mg/m2 achieve tissue levels in the central nervous system that have shown to be effective against glioma cell lines in vitro.
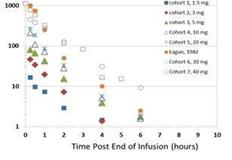 |
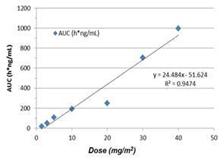 |
Observed pharmacokinetics measured by plasma concentration over time in escalating dose cohorts compared to historically literature (Eagan 1982) relationship between plasma exposure measured by area under the curve (AUC) vs. dose of VAL-083)
The estimated tissue concentration in brain tumor tissue following three consecutive daily doses of VAL-083 was calculated based on observed concentration of VAL-083 in the plasma and historical observations from the literature
| Dose | Plasma Cmax | Estimated Maximum Tumor Concentration in Brain (day 3) b |
IC50 in GBM Cell Linesd | |||||
| Current Trial | (g/mL) a | (g/g tissue) | µMc | μM | ||||
| 30mg/m2 | 0.46 | 0.35 | 2.4 | ~ 2 - 4 | ||||
| 40mg/m2 | 0.78 | 0.56 | 3.9 | |||||
aPK is measured only on Day 1, given the short t-1/2 of ~1h Cmax is assumed to be same for day 2 & 3*Volume of 1 g tissue assumed to be 1 mL
bPercent of plasma drug concentration in brain tumor = 44%, Eckhardt, 1977
cHalf-life of drug in human brain tumor tissue = 20h, Eckhardt, 1977
dIC50 range for low MGMT (U251 and SF188) and high MGMT (T98G) GBM cells treated with VAL-083 in vitro
Patient History
GBM patients enrolled in our current Phase I/II clinical trial have a growing brain tumor that has failed to respond to any other approved treatment.
GBM patients enrolled in the Phase I portion of our refractory GBM clinical trial failed prior treatment with standard front-line (temozolomide plus radiation) and 92% also failed Avastin®. In addition, 77% percent of GBM patients enrolled had also failed one or more courses of additional salvage therapy beyond temozolomide and Avastin® prior to treatment with VAL-083. Patients were not re-resected prior to treatment with VAL-083 and therefore had a growing refractory GBM tumor at the time of enrollment in our clinical trial and were considered salvage patients with an expected poor prognosis.
We also reported all patients in our current trial whose tumors were characterized exhibited high expression of MGMT, suggesting that these patients would be expected to have a poor prognosis and further highlighting the promising dose-dependent survival trend observed in the Phase I dose escalation portion of our clinical trial.
The correlation between tumor progression and impending death in this patient population is well-documented. Therefore we believe that our interim results demonstrating that VAL-083 can either stabilize disease progression by slowing or halting tumor growth or by shrinking the tumor is expected to result in longer patient survival and improved quality of life.
| 30 |
Based on these interim results, we believe that our modernized dosing regimen takes advantage of improved side-effect management and new knowledge of the pharmacokinetic, toxicity profile and anti-cancer mechanism of VAL-083. Our strategy to “hit the tumor harder more often” has allowed us to achieve higher levels of drug at the tumor-site, which we believe will result in significant and meaningful clinical benefit for GBM patients who have failed both temozolomide and Avastin® and increased survival via slowed tumor growth or tumor regression. These patients currently have no viable treatment options.
Observed Survival based on sub-group analysis
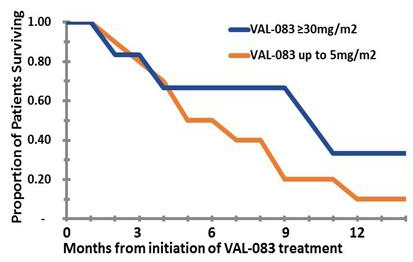
| Dose Cohort Subgroups | 6 months | 9 months | 12 months | |||
| High (30 & 40 mg/m2 n=6) | 67% | 67% | 33% | |||
| Low (up to 5mg/m2 n=10) | 44% | 33% | 22% |
Observed survival in the dose escalation phase of our Phase I/II clinical trial in comparison to
historical outcomes for GBM patients following Avastin® failure as described in the scientific literature
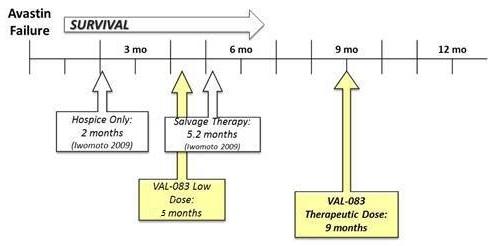
| 31 |
While these data are interim in nature and based on a small number of patients, we believe they support the further development of VAL-083. We anticipate presenting additional data, including data from the Phase II expansion cohort of our current clinical trial at scientific meetings during 2016. The purpose of the 14 patient Phase II expansion is to gather further safety data at our chosen therapeutic dose and to further clarify median overall survival in this patient population.
GBM patients were enrolled in our Phase I/II clinical trial at five centers: the Mayo Clinic in Rochester, Minnesota (“Mayo”), the Brain Tumor Center at University of California, San Francisco (“UCSF”), the Sarah Cannon Cancer Research Center (“SCRI”) in Nashville, Tennessee, Denver, Colorado, and the SCRI affiliate site at the Florida Cancer Specialist Research Institute in Sarasota, Florida. We plan to add additional clinical sites in order to accelerate enrollment as we advance to registration-directed clinical trials.
Registration-directed Trial
We anticipate that the registration-directed Phase II/III trial will be an open-label trial with overall survival as the primary endpoint. We plan to request a guidance meeting with the FDA during the first quarter of 2016 to discuss our proposed clinical trial design. The dose chosen, size, design and timing of initiation of the registration-directed clinical trial will depend on review of the data from the Phase II expansion phase of our current study and discussions with the FDA and our clinical advisors. Having announced full enrollment of the 14-patient expansion portion of the study and taking into account guidance from the FDA and our expected timelines, subject to funding, we believe it is possible that we will initiate registration-directed Phase II/III studies within the second half of calendar 2016. We will provide a formal update, including any adjustment to our projected timelines based on our discussions with the FDA and our clinical advisors.
Based on historical development of other products in GBM, we believe that we may be able to obtain FDA approval to commercialize VAL-083 to treat patients who have failed other therapies from an open-label registration-directed Phase II/III clinical trial, which will save significant costs of a large randomized Phase III clinical trial. We also believe that the FDA may grant Breakthrough Therapy, Fast Track, Accelerated Approval and/or Priority Review status to VAL-083, which will enable us to begin filing for commercial approval during the clinical trial process. Breakthrough Therapy, Fast Track, Accelerated Approval and Priority Review are expedited drug development designations established by the FDA that are intended to make therapeutically important drugs available at an earlier time.
Data from our planned registration-directed Phase II/III trial will form the basis of our application for FDA approval. Our overall goal remains to complete the registration-directed clinical trial with VAL-083 and to seek FDA approval as a new therapy for refractory glioblastoma in the timeliest manner possible. Based on our current financial resources, initiation of the registration-directed trial will require additional funding to support the expanded clinical operations necessary to conduct and manage the study.
We also believe that VAL-083 may be a potentially superior alternative to currently approved chemotherapies used in the treatment of newly diagnosed GBM patients. Subject to the availability of financial resources, we plan to investigate VAL-083 in clinical trials for newly diagnosed GBM patients whose tumors exhibit molecular features suggesting that they are unlikely to respond to currently available chemotherapies.
In February 2012, VAL-083 was granted protection under the Orphan Drug Act by the FDA for the treatment of glioma. In January 2013, the European Union also granted orphan drug protection to VAL-083. Orphan drugs generally follow the same regulatory development path as any other pharmaceutical product. However, incentives such as scientific advice and reduction or waiver of registration fees and access to specialized grant funding may be available to support and accelerate development of orphan drug candidates. In addition, we may sell VAL-083 as a treatment for glioma without competition for seven years in the U.S. and for ten years in the EU following market approval, due to the orphan drug protection afforded - meaning that the neither the FDA nor the EU regulatory authority will approve a medicinal product containing a similar active substance for the same indication during that time.
| 32 |
As part of our ASCO presentation on June 1, 2013, we also announced that we plan to split our current Phase I/II clinical trial protocol into two separate studies: one focusing solely on refractory GBM and the other focusing on secondary brain cancers caused by other tumors that have spread to the brain. Due to prior chemotherapy and radiation therapy, patients with secondary brain tumors are likely more prone to myelosuppression and may have a different toxicity and MTD than patients with GBM. We believe the strategy of splitting the trial into two separate studies will enable us to focus on accelerating the development of VAL-083 as a potential new treatment for GBM while appropriately exploring the potential of the drug to treat patients with solid tumors that have spread to the brain. In the future, we may develop a separate protocol for the continued exploration of VAL-083 in patients with secondary brain cancer caused by a solid tumor spreading to the brain.
Additional Clinical Trials in GBM
Based on our determination of the MTD and modernized dosing regimen in our current Phase I/II clinical trial in refractory GBM, we plan to initiate new clinical trials with the ultimate goal of seeking regulatory approval as an alternative and potentially superior chemotherapy for GBM patients whose tumors exhibit features that make them unlikely to respond to currently available chemotherapy regimens.
As previously noted, we have presented data at peer reviewed scientific meetings demonstrating that the cytotoxic activity of VAL-083 is distinct from standard-of-care in GBM. Specifically, our data shows that the tumor-killing activity of VAL-083 is independent of MGMT-mediated resistance. MGMT is believed to cause resistance to the current front-line therapy in the treatment of GBM, and is correlated with poor patient outcomes. MGMT is routinely measured in clinical practice, and we plan to utilize this measurement of a biomarker to identify patients for enrollment in new clinical trials.
GBM Study in Newly Diagnosed Patients
We plan to initiate a single-arm open label Phase II clinical trial in newly diagnosed GBM patients who are known to have a high expression of MGMT under the terms of our collaboration with Guangxi Wuzhou Pharmaceutical (Group) Co. Ltd (See Guangxi Wuzhou Pharmaceutical Company).
MGMT promoter methylation status will be used as a validated biomarker for enrollment and tumors must exhibit an unmethylated MGMT promoter for patients to be eligible for the trial. Patients will be treated with a combination of radiotherapy and VAL-083 followed by adjuvant (maintenance) therapy with VAL-083. This dosing regimen mirrors how temozolomide is currently used in the treatment of GBM, but substitutes VAL-083 as the chemotherapy in the treatment regimen. The study will assess the safety of DelMar’s modernized dosing regimen in combination with radiotherapy in the treatment of GBM. The study endpoints will also assess patient outcomes based on survival and disease progression.
Prior NCI-sponsored clinical trials demonstrated that the addition of VAL-083 to radiotherapy was superior to radiation alone in the treatment of GBM (Eagan, 1979). This study is expected to begin in the first half of 2016.
Second Line Study in GBM
In January 2016, we entered into a collaboration with the University of Texas MD Anderson Cancer Center (“MDACC”) to accelerate the clinical development of VAL-083 for the treatment of GBM. As part of the collaboration, MDACC will initiate a new randomized controlled Phase II clinical study with VAL-083 in patients with GBM at first recurrence/progression, prior to Avastin® (bevacizumab) exposure. The planned trial will randomize patients into two arms, one arm will be treated with VAL-083 and the second control arm will be treated with CCNU, a chemotherapy with a cytotoxic mechanism similar to temozolomide. The study endpoints will assess patient outcomes based on survival and disease progression.
Patients eligible for the study will have recurrent GBM characterized by a high expression of MGMT. MGMT promoter methylation status will be used as a validated biomarker for enrollment and tumors must exhibit an unmethylated MGMT promoter for patients to be eligible for the trial. This study is expected to begin in the first half of 2016.
Based on our research and historical clinical data, we believe that VAL-083 has the potential to offer physicians and patients a new paradigm in the treatment of GBM that will address significant unmet medical needs. As described above, we anticipate advancing VAL-083 into registration-directed Phase II/III clinical trials for the treatment of GBM in patients who have failed currently approved therapies during the coming year. There are no approved treatments for these patients.
| 33 |
We also anticipate initiating clinical trials to position VAL-083 as an alternative to standard-of-care to temozolomide therapy in patients whose tumors are known to exhibit high-expression of MGMT, a DNA repair enzyme correlated with resistance to temozolomide and poor patient outcomes in the treatment of GBM. Taken together, we believe that favorable results from these clinical trials would create a potential new survival paradigm for GBM patients and would lay the foundation for global development to address >$1 billion market opportunity for VAL-083 as a chemotherapy of choice in the treatment of GBM
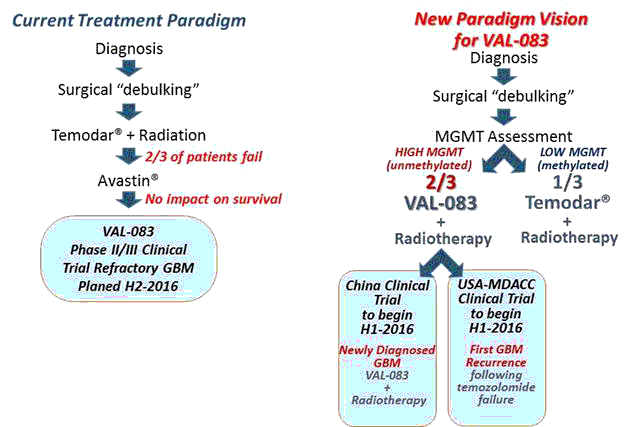
In addition, we believe that the mechanism of VAL-083 offers the potential of additive or synergistic benefit as a future combination therapy with existing chemotherapeutic agents or novel vaccines or immunotherapy approaches currently under investigation in the treatment of GBM.
VAL-083 in Lung Cancer
Lung cancer is a leading cause of cancer-related mortality around the world and effective treatment for lung cancer remains a significant global unmet need despite advances in therapy. In general, prognosis for lung cancer patients remains poor, with a 5-year survival rate of less than 14% among males and less than 18% among females in most countries. Globally, the market for lung cancer treatment may exceed $7 billion by 2019 according to a report published by Transparency Market research.
Non-small cell lung cancer (“NSCLC”) is the most common type of lung cancer. There are three common forms of NSCLC: adenocarcinomas are often found in an outer area of the lung; squamous cell carcinomas are usually found in the center of the lung next to an air tube (bronchus); and large cell carcinomas, which can occur in any part of the lung and tend to grow and spread faster than adenocarcinoma. NSCLC accounts for 85% of all lung cancer cases in the United States and approximately 90% of lung cancer cases diagnosed in China.
Smoking is the most important risk factor in the development of lung cancer. According to the World Cancer Report (2008), 21% of cancer deaths are related to smoking, especially lung cancer. Additionally, high levels of air pollution have been implicated as significant causes of lung cancer. Incidence of lung cancer in the United States is approximately 59 per 100,000 with the majority (52:100,000) being NSCLC.
According to The Nationwide Nutrition and Health Survey (2002), China has the world’s largest smoking population, with a smoking rate of 24.0% on average (50.2% for men and 2.8% for women), and a total number of 350 million smokers. The World Health Organization reports that the incidence of lung cancer in China is 34 per 100,000 population. However, some estimates are much higher exceeding 120 per 100,000 population for males aged 55-60 in urban areas.
According to a survey conducted by the Chinese Ministry of Health and the Ministry of Science and Technology, smoking, poor diet, water pollution and environmental problems have caused the nation's cancer death rate to rise 80 percent in the past 30 years and cancer is now accountable for 25 percent of all urban deaths and 21 percent of all rural deaths. Based on these trends, the World Health Organization projects that the incidence of lung cancer in China is expected to exceed one million (1,000,000) new cases per year by 2025.
The activity of VAL-083 against solid tumors, including lung cancer, has been established in both pre-clinical and human clinical trials conducted by the NCI. VAL-083 has been approved by the CFDA for the treatment of lung cancer. However, sales of VAL-083 in China have been limited by a lack of modern data, poor distribution, and preference for targeted therapies such as tyrosine kinase inhibitors (“TKIs”) in the modern era.
The current standard of care for newly diagnosed NSCLC is platinum-based combination therapy or TKI therapy for patients whose cancer exhibits epidermal growth factor receptor (“EGFR”) mutations. Patients exhibiting EGFR mutations have shown an initial response rate to TKIs which exceeds the response rate for conventional chemotherapy. However, TKI resistance has emerged as an important unmet medical need.
| 34 |
We believe VAL-083’s unique bi-functional alkylating mechanism of action could make it a valuable drug of choice in NSCLC patients who are or become resistant to TKI therapy. In addition, VAL-083 readily crosses the blood brain barrier suggesting that it may be possible for VAL-083 to treat patients whose lung cancer has spread to the brain.
Based on these beliefs, we have acquired certain commercial rights to VAL-083 in China where it is approved for the treatment of lung cancer. We have begun to establish a strong scientific and clinical rationale to support the development of VAL-083 as a potential treatment for NSCLC.
We plan to work with leading oncologists to develop new clinical and non-clinical data which will demonstrate the clinical utility of VAL-083 in NSCLC patients who are resistant to TKIs. We believe this strategy will result in sales growth for VAL-083 in China and generate future revenue for the Company through sales and marketing partnerships as well as position VAL-083 for global development in lung cancer.
In April 2014 at AACR we announced results of a pre-clinical study designed to evaluate the activity of VAL-083 in in vivo models of drug-resistant NSCLC in comparison to cisplatin. In an established murine xenograft model of NSCLC, the activity of VAL-083 was compared to standard platinum-based therapy with cisplatin against human NSCLC cell lines A549 (TKI-sensitive) and H1975 (TKI-resistant). In the study, VAL-083 demonstrated superior efficacy and safety in the treatment of TKI-susceptible (A549) tumors and in TKI-resistant (H1975) tumors.
| ● | Treatment of TKI-sensitive (A549) NSCLC with 3 mg/kg of VAL-083 resulted in tumor growth delay of 26 days compared to untreated controls. Cisplatin (5 mg/kg) resulted in tumor growth delay of just four days. In addition, mean tumor volume on day 68 was significantly reduced in animals treated with 3 mg/kg VAL-083 (p=0.001) compared to untreated controls. | |
| ● | Treatment of TKI-resistant (H1975) NSCLC with 4 mg/kg of VAL-083 resulted in a statistically significant reduction in tumor volume (p=0.01) versus untreated control after 27 days. In the same model, treatment with 5 mg/kg of cisplatin failed to achieve statistically significant reduction in tumor volume (p=0.23) versus untreated control after 27 days. Longer-term safety assessments are ongoing in this model. |
In April 2015, we presented new non-clinical data at the AACR annual meeting. These data demonstrated that VAL-083’s mechanism is distinct from platinum-based chemotherapy, the current standard of care for NSCLC. VAL-083 retains its high level of anti-cancer activity in p53 mutated NSCLC cell lines compared to cisplatin or oxaliplatin.
The p53 gene plays a central role in the protection of the human body from cancer and is responsible for initiating the process of programmed cell death, or apoptosis, which directs a cell to commit suicide if it becomes damaged or cancerous. The p53 pathway is also integral to the activity of many chemotherapy drugs. p53 is frequently mutated in NSCLC and p53 mutations are highly correlated with resistance to chemotherapy and poor patient outcomes in NSCLC.
In November 2015 at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics we presented data on the benefit of VAL-083 in combination with platinum-based chemotherapy regimens in the treatment of NSCLC. The results of the study presented provide support for VAL-083 as a viable treatment option for NSCLC patients who fail to respond to standard-of-care platinum-based therapy or TKI therapy, and also support potential therapeutic benefits of a VAL-083 along with platinum combination regimens in newly diagnosed patients. We demonstrated that the combination of VAL-083 with either cisplatin or oxaliplatin demonstrated a superadditive (synergistic) effect against NSCLC cell lines, including those resistant to TKI therapy in vitro.
| 35 |
In October 2014, we presented non-clinical data at the AACR New Horizon’s in Cancer Research Meeting. These data also support superior activity of VAL-083 compared to standard platinum-based treatment in both TKI-sensitive and TKI-resistant tumor models. Further, our data demonstrate that VAL-083 may have a synergistic effect in combination with cisplatin. These data suggest the potential of VAL-083 to be used in combination with platinum-based chemotherapy and to address modern unmet medical needs in the treatment of TKI-resistant NSCLC, especially where platinum-based therapy has already failed or is predicted to give sub-optimal outcomes.
These results may have immediate implications in the treatment of NSCLC in China, where VAL-083 is approved for as a chemotherapy for the treatment of lung cancer. The data also support exploring future clinical development of VAL-083 as a lung cancer therapy in the rest of the world thereby providing DelMar with a potential opportunity to expand our clinical development focus beyond glioblastoma.
As a next step in the investigation of VAL-083 as a potential treatment for NSCLC, we have developed a protocol for a post-market clinical study to be conducted by a leading cancer clinician in the context of the current approval in China.
We plan to conduct this trial in collaboration with Guangxi Wuzhou Pharmaceutical Group Co. Ltd. (Guangxi Wuzhou Pharma). Under the terms of our collaboration agreement with Guangxi Wuzhou Pharma, we are responsible for establishing protocols for and conducting clinical trials and Guangxi Wuzhou Pharma is responsible for the costs associated with clinical trials conducted in China. Our goal is to initiate this clinical trial during the first half of 2016, with the aim to develop new data to support product growth in China and to establish clinical proof of concept to expand our drug development efforts with VAL-083.
Conducting this clinical trial in China under our collaboration agreement with Guangxi Wuzhou Pharma will allow us to enhance the potential value of VAL-083 without significantly increasing our own planned cash expenditures. We also believe that these new data will support the potential to establish global partnerships and collaborations with larger pharmaceutical companies who have the resources and commercial infrastructure to effectively develop and commercialize VAL-083 as a treatment for NSCLC on a world-wide basis.
VAL-083 in Leukemia and Hematologic Cancers
The NCI studied VAL-083 extensively in laboratory and animal models of hematological malignancies (blood cancers). VAL-083 has been approved for the treatment of chronic myeloid leukemia, or CML, in China.
CML, also known as chronic myeloid leukemia is a cancer of the white blood cells. The incidence of CML in the United States is approximately two per 100,000 of population.
We believe that our pre-clinical research and data from NCI-sponsored studies and commercial evidence from the Chinese market support that there exists a substantive clinical benefit of VAL-083 in CML. We also believe that the unique mechanism of action of VAL-083, in combination with newly developed data positions the drug as a valuable therapy for patients who have failed other treatments, including TKIs. This represents a significant clinical and commercial opportunity for large subsets of patient populations in the existing-approved China market as well as for global development in CML.
Based on these beliefs, we have acquired certain commercial rights to VAL-083 in China, where it is approved for the treatment of CML and lung cancer. We have also developed new non-clinical data demonstrating that VAL-083 is active against TKI-resistant CML.
VAL-083 in Pediatric Brain Cancers
In November 2015 at AACR’s - Advances in Pediatric Research: From Mechanisms and Models to Treatment and Survivorship we presented data indicating that VAL-083 offers potential therapeutic alternatives in the treatment of pediatric brain tumors.
VAL-083 in Ovarian Cancers
In October 2015 at the AACR’s Advances in Ovarian Cancer Research: Exploiting Vulnerabilities Conference, the Company presented data from its collaboration with researchers at MD Anderson Cancer Center. The data demonstrate the effectiveness of VAL-083 against cisplatin-resistant ovarian cancers and raise the potential for VAL-083 as a treatment for ovarian cancers as a single-agent against platinum-resistant tumors or in combination with platinum-based chemotherapeutic regimens.
| 36 |
Additional Indications
In historical studies sponsored by the NCI in the United States, VAL-083 exhibited clinical activity against a range of tumor types including central nervous system tumors, solid tumors and hematologic malignancies. We have established new non-clinical data supporting the activity of VAL-083 in different types of cancer that are resistant to modern targeted therapies and we believe that the unique cytotoxic mechanism of VAL-083 may provide benefit to patients in a range of indications. We intend to continue to research these opportunities, and if appropriate, expand our clinical development efforts to include additional indications.
Guangxi Wuzhou Pharmaceutical Company
Pursuant to a memorandum of understanding and collaboration agreement, dated October 25, 2012, we have established a strategic collaboration with Guangxi Wuzhou Pharmaceutical Company (“Guangxi Wuzhou Pharmaceuticals”), a subsidiary of publicly traded Guangxi Wuzhou Zhongheng Group Co., Ltd. (SHG: 600252) (the “Guangxi Agreement”). VAL-083 is approved for the treatment of chronic myelogenous leukemia (“CML”) and lung cancer in China and Guangxi Wuzhou Pharmaceuticals is the only manufacturer licensed by the CFDA to produce the product for the China market. Through the Guangxi Agreement, we have obtained drug product for our VAL-083 clinical trials in the United States and we have also secured certain commercial rights in China.
Pursuant to the Guangxi Agreement, we granted to Guangxi Wuzhou Pharmaceuticals a royalty-free license to certain of our intellectual property, as it relates to quality control and drug production methods for VAL-083, and we agreed that Guangxi Wuzhou Pharmaceuticals will be our exclusive supplier of VAL-083 for clinical trials and commercial sales, subject to Guangxi Wuzhou Pharmaceuticals obtaining and maintaining cGMP certification by the FDA, EMA or other applicable regulatory agencies, and Guangxi Wuzhou Pharmaceuticals being able to meet volumes ordered by us. The Company and Guangxi Wuzhou Pharmaceuticals will work together to ensure the product specifications meet global standards in order to accelerate international development and regulatory approval. Guangxi Wuzhou Pharmaceuticals will be our exclusive supplier of VAL-083 for clinical development and commercial sales, subject to its meeting and maintaining required regulatory certification.
This Guangxi Agreement also provides us with certain exclusive commercial rights related to drug supply. Specifically, the Guangxi Agreement establishes an exclusive supply relationship between us and Guangxi Wuzhou Pharmaceuticals for the Chinese market and all markets outside China. Guangxi Wuzhou Pharmaceuticals agreed that it may not sell VAL-083 for markets outside of China to any other purchaser other than us. In addition, Guangxi Wuzhou Pharmaceuticals granted us a pre-emptive right in China (subject to our acceptance of proposed sales volume and prices) to purchase VAL-083 produced by Guangxi Wuzhou Pharmaceuticals.
Our collaboration with Guangxi Wuzhou Pharmaceuticals positions us with the potential to generate revenue through product sales or royalties for its approved indications in China while we seek global approval in new indications.
Our strategy in China is to work in collaboration with Guangxi Wuzhou Pharmaceuticals and globally recognized clinical investigators to develop new clinical and non-clinical data in collaboration with leading cancer researchers. We believe these data, if favorable, will allow the repositioning and sales growth of VAL-083 in the China market under its approved indications and provide us with clinical proof-of-concept to support global development of VAL-083 for the treatment of hematologic cancers and lung cancer.
We and Guangxi Wuzhou Pharmaceuticals have formed a clinical advisory board to oversee clinical studies. Under the terms of the Guangxi Agreement, Guangxi Wuzhou Pharmaceuticals will provide funding support for clinical trials conducted in China and we are responsible for development and commercialization. We anticipate establishing sales channels in China through a third-party marketing partner in collaboration with Guangxi Wuzhou Pharmaceuticals in order to obtain sales or royalty revenue from that market.
| 37 |
The term of the Guangxi Agreement (except as it relates to the exclusive rights in the China market) is indefinite, subject to termination upon written agreement of all parties, or if either party breaches any material term and fails to remedy such breach within 30 days of receipt of notice of the breach, or if any action to be taken thereunder is not agreed to by both parties, provided that such matter is referred to the chief executive officer of both parties, and they are unable to resolve such matter within 90 days. No payments have been made to date under the Guangxi Agreement.
Pursuant to our agreement with Guangxi Wuzhou Pharmaceuticals we anticipate the phase IV trials in NSCLC and GBM will commence in China during the first half of calendar 2016.
The protection of intellectual property rights in China (where VAL-083 is manufactured pursuant to the Guangxi Agreement with the only manufacturer presently licensed by the CFDA to produce the product for the China market, and where VAL-083 is approved for the treatment of CML and lung cancer) is relatively weak compared to the United States, which may negatively affect our ability to generate revenue from VAL-083.
Corporate History
We are a Nevada corporation formed on June 24, 2009 under the name Berry Only, Inc. Prior to a reverse acquisition undertaken on January 25, 2013 (see note 1 to the consolidated condensed financial statements) Berry did not have any significant assets or operations. The Company is the parent company of Del Mar Pharmaceuticals (BC) Ltd. (“DelMar (BC)”), a British Columbia, Canada corporation incorporated on April 6, 2010, that is focused on the development of drugs for the treatment of cancer. The Company is also the parent company to 0959454 B.C. Ltd., a British Columbia corporation (“Callco”), and 0959456 B.C. Ltd., a British Columbia corporation (“Exchangeco”). Callco and Exchangeco were formed to facilitate the reverse acquisition.
Pursuant to the reverse acquisition, the Company acquired (either directly or indirectly (through Exchangeco)) all of the issued and outstanding shares of DelMar (BC) on January 25, 2013. As a result of the shareholders of DelMar (BC) owning a controlling interest in the Company subsequent to the reverse acquisition, for accounting purposes the transaction is a capital transaction with DelMar (BC) being the accounting acquirer even though the legal acquirer is Berry. Therefore, the historic financial statements of DelMar (BC) are presented as the comparative balances for the periods prior to the reverse acquisition.
References to the Company, “we”, “us”, and “our” refer to the Company and its wholly-owned subsidiaries, DelMar (BC), Callco and Exchangeco. References to “Berry” relate to the Company prior to the reverse acquisition.
Outstanding Securities
As of February 12, 2016, we have 40,253,056 shares of common stock issued and outstanding, 4,056,042 shares of common stock issuable upon exchange of the Exchangeable Shares of Exchangeco (which entitle the holder to require Exchangeco to redeem (or, at the option of the Company or Callco, to have the Company or Callco purchase) the Exchangeable Shares, and upon such redemption or purchase to receive an equal number of shares of common stock of the Company) (the Exchangeable Shares are recognized on an as-exchanged for common stock basis for financial statement purposes), outstanding warrants to purchase 17,888,945 shares of common stock, and outstanding options to purchase 3,635,000 shares of common stock. All Exchangeable Shares, warrants, and options are convertible or exercisable into one share of common stock.
Related Parties
The Company acquired its VAL-083 prototype drug, patents and technology rights from Valent Technologies, LLC, (“Valent”), an entity owned by Dr. Dennis Brown, the Company’s Chief Scientific Officer. As a result Valent is a related party to the Company.
The following related party transactions and balances have been recorded by the Company.
| 38 |
During the six months ended December 31, 2015
Pursuant to consulting agreements with the Company’s officers the Company recognized a total of $240,000 in compensation expense for the six months ended December 31, 2015.
The Company paid $85,000 in directors’ fees during the six months ended December 31, 2015.
Included in accounts payable at December 31, 2015 is an aggregate amount owing of $56,734 (June 30, 2015 - $90,820) to the Company’s officers and directors for fees and expenses. The Company pays related party payables incurred for fees and expenses under normal commercial terms.
The Company recorded $4,178 in dividends related to the preferred stock issued to Valent.
During the six months ended December 31, 2014
Effective September 30, 2014, the Company entered into and closed an agreement with Valent to exchange its loan with Valent for 278,530 shares of preferred stock of the Company.
Pursuant to consulting agreements with the Company’s officers the Company recognized a total of $265,000 in compensation expense for the six months ended December 31, 2014.
The Company recognized $48,500 in directors’ fees during the six months ended December 31, 2014.
Derivative Liability
The Company has issued common stock purchase warrants. Based on the terms of certain of these warrants the Company determined that the warrants were a derivative liability which is recognized at fair value at the date of the transaction and re-measured at fair value each reporting period with the changes in fair value recorded in the consolidated condensed statement of loss and comprehensive loss.
Investor Warrants
In connection with the reverse acquisition during the quarter ended March 31, 2013 the Company issued units consisting of one share of common stock and one five-year warrant (the “Investor Warrants”) to purchase one share of common stock at an exercise price of $0.80. The exercise price of the Investor Warrants is subject to adjustment in the event that the Company issues common stock at a price lower than the exercise price, subject to certain exceptions. As a result of the financing completed by the Company during the three months ended September 30, 2015 the exercise price of the Investor Warrants was reduced from $0.80 to $0.786. As a result of the price being reduced, the Company has recognized a loss of $8,098.
| 39 |
Investor Warrant exercises
During the six months ended December 31, 2015, 515,500 Investor Warrants were exercised for 515,500 shares of common stock at an exercise price of $0.786 per share. The Company received proceeds of $405,183 from these exercises. The warrants that have been exercised were revalued at their exercise date and then the reclassification to equity was recorded resulting in $247,440 of the derivative liability being reclassified to equity.
During the six months ended December 31, 2014 the Company concluded a tender offer whereby the holders of the Investor Warrants had the opportunity to exercise their warrants at an exercise price of $0.65. Under the tender offer, a total of 762,227 warrants were exercised for net proceeds of $470,676 after payment by the Company of a 5% warrant agent fee of $24,772.
In addition, during the six months ended December 31, 2014, 1,223,847 warrants were exercised for 1,223,847 shares of common stock at an exercise price of $0.65 per warrant. The Company received proceeds of $795,501 from these exercises.
As a result of all of the warrant exercises during the six months ended December 31, 2014, the Company received net proceeds of $1,266,177 from the exercise of 1,986,074 warrants. The warrants that have been exercised were revalued at their exercise date and then the reclassification to equity was recorded resulting in $391,422 of the derivative liability being reclassified to equity.
Investor Warrant exchange
On December 31, 2014, the Company issued 414,889 shares of common stock in exchange for 1,244,666 Investor Warrants. The Investor Warrants that have been exchanged were revalued at their exchange date and then a reclassification to equity was recorded. The reclassification to equity upon the exchange was $305,112. The Company recognized a loss of $92,843 at the time of the exchange.
The 3,857,363 Investor Warrants outstanding at December 31, 2015 all have an exercise price of $0.786 and have been re-valued at December 31, 2015 using the adjusted exercise price of $0.786 and using a probability valuation model using the following assumptions: dividend rate - 0%, volatility – 99%, risk free rate – 1.18% and a term of approximately 2.25 years.
2013 Placement Agent Warrants
On March 6, 2013 the Company issued 5,250,000 warrants (the “2013 Placement Agent Warrants”) that are exercisable at $0.80 per share until March 6, 2018 but can be exercised on a cashless basis. The exercise price of the 2013 Placement Agent Warrants is subject to adjustment in the event that the Company sells common stock at a price lower than the exercise price, subject to certain exceptions. As a result of the financing completed by the Company during the quarter ended September 30, 2015 the exercise price of the Placement Agent Warrants was reduced from $0.80 to $0.786. As a result of the exercise price being reduced, the Company has recognized a loss of $13,467.
On December 30, 2015, the Company entered into amendments (the “2013 Placement Agent Warrant Amendments”) with the holders of the 2013 Placement Agent Warrants. Pursuant to the 2013 Placement Agent Warrant Amendments, 5,050,000 2013 Placement Agent Warrants were amended to extend the expiration date to June 30, 2019 and remove the provision requiring an adjustment of the exercise price in the event the Company sells common stock at a purchase price lower than the exercise price. As a result of the 2013 Placement Agent Warrant Amendments, the Company has recognized a loss of $242,400 and has reclassified $2,277,550 from the derivative liability to equity resulting in an increase in equity of $2,035,150. The 2013 Placement Agent Warrants were revalued to the date of the amendment and were then reclassified to equity.
Dividend Warrants
In connection with the reverse acquisition, effective January 24, 2013, the Company effected a warrant dividend (the “Warrant Dividend”) pursuant to which the Company issued one five-year warrant to purchase one share of common stock at an exercise price of $1.25 for each outstanding share of common stock (the “Dividend Warrants”). Pursuant to the Warrant Dividend, the Company issued an aggregate of 3,250,007 Dividend Warrants.
On October 31, 2014, the Company and all of its Dividend Warrant holders entered into amendments to the Dividend Warrants such that the Company’s redemption rights and certain provisions of the Dividend Warrant agreements relating to potential cash settlement of the Dividend Warrants were removed. The Dividend Warrants were revalued to the date of the amendment on October 31, 2014 which resulted in a reclassification to equity of $975,278.
2015 Agent Warrants
As part of the Company’s financing completed during the quarter ended September 30, 2015 (the “Offering”), the Company issued 93,908 warrants to certain placement agents (“2015 Agent Warrants”). The 2015 Agent Warrants are exercisable at a per share price equal to $0.75 during the five-year period commencing six months from the effective date of the Offering, which period shall not extend further than five years from the effective date of the Offering. Therefore, all Agent Warrants expire on July 15, 2020.
The 93,908 2015 Agent Warrants outstanding at December 31, 2015 have been re-valued at December 31, 2015 using a simulated probability valuation model using the following assumptions: dividend rate - 0%, volatility – 88%, risk free rate – 1.63% and a term of approximately 4.75 years.
| 40 |
Warrants issued for services
The Company has issued 300,000 warrants for services. The warrants were issued on September 12, 2013 and are exercisable on a cashless basis at an exercise price of $1.76 for five years. The warrants have been measured at December 31, 2015 using a simulated probability valuation model using the following assumptions: dividend rate - 0%, volatility – 96%, risk free rate – 1.31% and a term of approximately 2.75 years.
The Company’s derivative liability is summarized as follows:
December 31, 2015 $ | June 30, 2015 $ | |||||||
| (as restated) | ||||||||
| Opening balance | 2,364,381 | 5,111,007 | ||||||
| Issuance of 2015 Agent Warrants | 29,594 | – | ||||||
| Change in fair value of warrants | 1,219,634 | (627,433 | ) | |||||
| Change in fair value due to change in warrant terms | 263,965 | (23,658 | ) | |||||
| Reclassification to equity upon amendment of warrants | (2,277,550 | ) | (975,278 | ) | ||||
| Reclassification to equity upon exchange of warrants | – | (728,835 | ) | |||||
| Reclassification to equity upon exercise of warrants | (247,440 | ) | (391,422 | ) | ||||
| Closing balance | 1,352,584 | 2,364,381 | ||||||
Selected Quarterly Information
The financial information reported here in has been prepared in accordance with accounting principles generally accepted in the United States. The Company’s functional currency at December 31, 2015 is the USD. The following table represents selected financial information for the Company as of December 31, 2015 and 2014.
Selected Balance Sheet Data
December 31, 2015 $ | June 30, 2015 $ | |||||||
| (as restated) | ||||||||
| Cash and cash equivalents | 1,957,009 | 1,754,433 | ||||||
| Working capital | 1,605,025 | 1,722,336 | ||||||
| Total Assets | 2,184,593 | 2,575,421 | ||||||
| Derivative liability | 1,352,584 | 2,364,381 | ||||||
| Total stockholders’ equity (deficit) | 116,729 | (821,490 | ) | |||||
Selected Statement of Operations Data
| 41 |
For the Three months Ended:
| December 31, | December 31, | |||||||
| 2015 | 2014 | |||||||
| $ | $ | |||||||
| (as restated) | ||||||||
| Research and development | 789,187 | 612,169 | ||||||
| General and administrative | 890,672 | 656,229 | ||||||
| Change in fair value of derivative liability | 680,188 | (892,326 | ) | |||||
| Change in fair value of derivative liability due to change in warrant terms | 242,400 | 143,532 | ||||||
| Loss on exchange of warrants | – | 92,843 | ||||||
| Foreign exchange loss | 44,253 | 7,295 | ||||||
| Interest income | (10 | ) | (109 | ) | ||||
| Net loss from operations | 2,646,690 | 619,633 | ||||||
| Basic weighted average number of shares outstanding | 43,979,516 | 37,798,183 | ||||||
| Basic loss per share | 0.06 | 0.02 | ||||||
For the Six months Ended:
| December 31, | December 31, | |||||||
| 2015 | 2014 | |||||||
| $ | $ | |||||||
| (as restated) | ||||||||
| Research and development | 1,393,032 | 1,283,796 | ||||||
| General and administrative | 1,364,697 | 1,101,229 | ||||||
| Change in fair value of derivative liability | 1,219,634 | (329,357 | ) | |||||
| Change in fair value of derivative liability due to change in warrant terms | 263,965 | (23,658 | ) | |||||
| Loss on exchange of warrants | - | 92,843 | ||||||
| Foreign exchange loss | 26,780 | 9,686 | ||||||
| Interest expense | - | 2,091 | ||||||
| Interest income | (30 | ) | (261 | ) | ||||
| Net loss from operations | 4,268,078 | 2,136,369 | ||||||
| Basic weighted average number of shares outstanding | 43,234,639 | 37,125,074 | ||||||
| Basic loss per share | 0.10 | 0.06 | ||||||
Expenses net of share-based payments
The following table discloses research and development, and general and administrative expenses net of share-based payment expenses.
For the Three months Ended:
December 31, 2015 $ | December 31, 2014 $ | |||||||
| Research and development | 789,187 | 612,169 | ||||||
| Share-based payments included in research and development | (42,186 | ) | 8,077 | |||||
| Research and development net of share-based compensation | 747,001 | 620,246 | ||||||
| General and administrative | 890,672 | 656,229 | ||||||
| Share-based payments included in general and administrative | (288,093 | ) | (219,647 | ) | ||||
| General and administrative net of share-based compensation | 602,579 | 436,582 | ||||||
| 42 |
For the Six months Ended:
December 31, 2015 $ | December 31, 2014 $ | |||||||
| Research and development | 1,393,032 | 1,283,796 | ||||||
| Share-based payments included in research and development | (48,641 | ) | (13,056 | ) | ||||
| Research and development net of share-based compensation | 1,344,391 | 1,270,740 | ||||||
| General and administrative | 1,364,697 | 1,101,229 | ||||||
| Share-based payments included in general and administrative | (328,198 | ) | (247,454 | ) | ||||
| General and administrative net of share-based compensation | 1,036,499 | 853,775 | ||||||
Comparison of the three months ended December 31, 2015 and December 31, 2014
| Three Months Ended | ||||||||||||||||
|
December 31, 2015 $ |
December 31, 2014 $ |
Change $ |
Change % |
|||||||||||||
| (as restated) | ||||||||||||||||
| Research and development | 789,187 | 612,169 | 177,018 | 29 | ||||||||||||
| General and administrative | 890,672 | 656,229 | 234,443 | 36 | ||||||||||||
| Change in fair value of derivative liability | 680,188 | (892,326 | ) | 1,572,514 | 176 | |||||||||||
| Change in fair value of derivative liability due to change in warrant terms | 242,400 | 143,532 | 98,868 | 69 | ||||||||||||
| Loss on exchange of warrants | - | 92,843 | (92,843) | (100 | ) | |||||||||||
| Foreign exchange loss | 44,253 | 7,295 | 36,958 | 507 | ||||||||||||
| Interest income | (10 | ) | (109 | ) | 99 | (91 | ) | |||||||||
| Net loss | 2,646,690 | 619,633 | 2,027,057 | |||||||||||||
Research and Development
Research and development expenses increased to $789,187 for the three months ended December 31, 2015 from $612,169 for the three months ended December 31, 2014. The increase was largely attributable to an increase in clinical development, travel, and share-based expenses partially offset by a decrease in pre-clinical research and intellectual property costs. Share-based expenses included in research and development for the three months ended December 31, 2015 were $42,186 compared to a reversal of expense of $8,077 in the three months ended December 31, 2014. In the current quarter, the Company recognized share-based expenses related to stock option expense and warrants issued for services. In the prior quarter, the Company recognized a reversal of stock option expense only.
Excluding the impact of share-based payments, research and development expenses increased to $747,001 from $620,246 during the three months ended December 31, 2015 compared to the three months ended December 31, 2014. Clinical development costs have increased in the current quarter compared to the prior quarter due to higher monitoring and data costs as the Company continued the 14-patient expansion portion of its phase II study in refractory GBM. Travel costs increased during the three months ended December 31, 2015 compared to the three months ended December 31, 2014 as the Company attended several conferences in the current quarter in support of the release of data in new indications including pediatric brain tumors and ovarian cancer. The reduction in pre-clinical costs in the current quarter compared to the prior quarter is partially due to timing and partially due to grant funding. During the three months ended December 31, 2014, several studies were on-going at the B.C. Cancer Agency, MDACC, and the University of British Columbia. During the three months ended December 31, 2015, studies were on-going at the University of British Columbia but the balance of the pre-clinical study plan for the current period was still being finalized. In addition, the Company made more claims under its grant funding with the Government of Canada in the current quarter than in the prior quarter. Intellectual property costs decreased in the three months ended December 31, 2015 compared to the three months ended December 31, 2014 as the Company filed new patent applications in the prior quarter which resulted in higher expenses. Patent costs can vary considerably depending on the timing of the filing of new patents, conversion of the provisional applications to PCT applications, and actual filing costs.
| 43 |
General and Administrative
General and administrative expenses were $890,672 for the three months ended December 31, 2015 compared to $656,229 for the three months ended December 31, 2014. Overall, professional fees, travel, facilities costs and share-based expenses were higher in the current quarter than in the prior quarter. Share-based payments increased to $288,093 in the three months ended December 31, 2015 from $219,647 for the three months ended December 31, 2014. In relation to general and administrative expenses during the three months ended December 31, 2015 the Company incurred share-based payments related to stock option expense, and shares and warrants issued for services while during the three months ended December 31, 2014 the Company incurred share-based payments relating to stock options and for shares issued for services. The increase in stock option expense in the current quarter was due to the granting of new stock options and an increase in the Company’s share price in the current quarter compared to the corresponding quarter in the prior year.
Excluding the impact of share-based payments, general and administrative expenses increased to $602,579 during the three months ended December 31, 2015 from $436,582 for the three months ended December 31, 2014. Professional fees increased during the three months ended December 31, 2015 compared to the three months ended December 31, 2014 due to higher fees related to business development, investor relations, and directors’ fees. The Company’s Board of Directors has expanded by two seats as the Company has strengthened its corporate governance. Travel costs have increased during the three months ended December 31, 2015 compared to the three months ended December 31, 2014 as the Company followed up on its financing completed during the quarter ended September 30, 2015. Facilities costs increased in the current quarter compared to the prior quarter largely due an increase in promotion costs as the Company launched its social media channels.
Change in fair value of derivative liability
Based on the terms of certain warrants issued by the Company, the Company determined that the warrants were a derivative liability which is recognized at fair value at the date of the transaction and re-measured at fair value every reporting period with gains or losses on the changes in fair value recorded in the consolidated condensed interim statement of loss and comprehensive loss. The balances recognized during the three months ended December 31, 2015 and 2014 were primarily due to changes in the Company’s common stock price between the date the warrants were last valued on September 30, 2015 and 2014 respectively and December 31, 2015 and 2014 respectively which are the revaluation dates used during the quarters ended December 31.
The Company recognized a loss of $680,188 from the change in fair value of the derivative liability for the three months ended December 31, 2015. In addition, the Company recognized a loss of $242,400 which resulted from the change in fair value of the 2013 Placement Agent Warrants when the warrant expiry date was extended as part of the amendment of the 2013 Placement Agent Warrants. For the three months ended December 31, 2014 the Company recognized a gain of $892,326 due to the change in fair value of the derivative liability. In addition, as result of amending the Investor Warrants and Dividend Warrants during the quarter ended December 31, 2014, the Company also recognized a loss of $143,532. Also, during the quarter ended December 31, 2014, the Company exchanged certain Investor Warrants for shares of common stock resulting in the recognition of a loss of $92,843 on the exchange.
Changes in the Company’s common stock price can result in significant volatility in the Company’s reported net loss due to its impact on the fair value of the derivative liability. As a result of revaluation gains and losses, the Company expects that its reported net income or loss will continue to fluctuate significantly.
| 44 |
Foreign Exchange Gain
The Company’s functional currency at December 31, 2015 is the USD but the Company incurs a portion of its expenses in CDN. The foreign exchange gains and losses are reported in other (income) loss in the Consolidated Condensed Interim Statement of Loss and Comprehensive Loss.
The Company recognized a foreign exchange loss of $44,253 for the quarter ended December 31, 2015 compared to a loss of $7,295 for the quarter ended December 31, 2014. The change was due to changes in the exchange rate between the CDN and the USD and to varying levels of CDN cash and accounts payable.
Preferred Share Dividend
Pursuant to a loan agreement dated February 3, 2011, the Company received a loan from Valent in the amount of $250,000 for the purchase of the prototype drug product. The loan was payable on demand, unsecured and bore interest at 3% per year. Effective September 30, 2014 the loan balance, including accumulated interest to September 30, 2014, was exchanged for 278,530 shares of the Company’s Series A Preferred Stock. The Series A Preferred Stock pays an annual dividend of 3%.
For the three-months ended December 31, 2015 and 2014, the Company recorded $2,089 related to the dividend payable to Valent. The dividend has been recorded as a direct increase in accumulated deficit and was paid subsequent to quarter end.
Comparison of the six months ended December 31, 2015 and December 31, 2014
| Six Months Ended | ||||||||||||||||
|
December 31, 2015 $ |
December 31, 2014 $ |
Change $ |
Change % |
|||||||||||||
| (as restated) | ||||||||||||||||
| Research and development | 1,393,032 | 1,283,796 | 109,236 | 9 | ||||||||||||
| General and administrative | 1,364,697 | 1,101,229 | 263,468 | 24 | ||||||||||||
| Change in fair value of derivative liability | 1,219,634 | (329,357 | ) | 1,548,991 | 470 | |||||||||||
| Change in fair value of derivative liability due to change in warrant terms | 263,965 | (23,658 | ) | 287,623 | 1,216 | |||||||||||
| Loss on exchange of warrants | - | 92,843 | (92,843) | (100) | ||||||||||||
| Foreign exchange loss | 26,780 | 9,686 | 17,094 | 176 | ||||||||||||
| Interest expense | - | 2,091 | (2,091 | ) | (100 | ) | ||||||||||
| Interest income | (30 | ) | (261 | ) | 231 | (89 | ) | |||||||||
| Net loss | 4,268,078 | 2,136,369 | 2,131,709 | |||||||||||||
Research and Development
Research and development expenses increased to $1,393,032 for the six months ended December 31, 2015 from $1,283,796 for the six months ended December 31, 2014. The increase was largely attributable to an increase in clinical development, travel, and share-based expenses partially offset by decreases in pre-clinical research and intellectual property costs. Share-based expenses included in research and development for the six months ended December 31, 2015 were $48,641 compared to $13,056 in the six months ended December 31, 2014. In the current period, the Company recognized share-based expenses related to stock option expense and warrants issued for services. In the prior period, the Company recognized stock option expense only.
| 45 |
Excluding the impact of share-based payments, research and development expenses increased to $1,344,391 from $1,270,740 during the six months ended December 31, 2015 compared to the six months ended December 31, 2014.
Clinical development costs increased in the current period compared to the prior period due to higher monitoring and data costs as the Company continued the 14-patient expansion portion of its phase II study in refractory GBM. During the three months ended September 30, 2015 we announced full enrollment of the 14-patient expansion portion of our study. In addition, we are enrolling patients at five clinics which involves increased monitoring and data costs. In the prior period, we were enrolling single cohorts of three patients at a time which resulted in lower direct clinical, monitoring, and data costs. Travel costs increased during the six months ended December 31, 2015 compared to the six months ended December 31, 2014 as the Company attended several conferences in the current period in support of the release of data in new indications including pediatric brain tumors and ovarian cancer. The reduction in pre-clinical costs in the current period compared to the prior period is partially due to timing and partially due to grant funding. During the six months ended December 31, 2014, several studies were on-going at the B.C. Cancer Agency, MDACC, and the University of British Columbia. During the six months ended December 31, 2015, studies were on-going at the University of British Columbia but the balance of the pre-clinical study plan for the current year was still being finalized. The Company made more claims under its grant funding with the Government of Canada in the current six months than in the prior six months. Intellectual property costs have decreased in the six months ended December 31, 2015 compared to the six months ended December 31, 2014 as the Company filed new patent applications in the prior period which resulted in higher expenses.
General and Administrative
General and administrative expenses were $1,364,697 for the six months ended December 31, 2015 compared to $1,101,229 for the six months ended December 31, 2014. The increase was partially attributable to an increase in share-based payments to $328,198 in the six months ended December 31, 2015 from $247,454 for the six months ended December 31, 2014. In relation to general and administrative expenses during the six months ended December 31, 2015 the Company incurred share-based payments related to stock options, and shares and warrants issued for services while during the six months ended December 31, 2014 the Company incurred share-based payments relating to stock options and for shares issued for services. The increase in stock option expense in the current period was due to the granting of new stock options and an increase in the Company’s share price in the current period compared to the corresponding period in the prior year.
Excluding the impact of share-based payments, general and administrative expenses increased to $1,036,499 during the six months ended December 31, 2015 from $853,775 for the six months ended December 31, 2014. The principal reasons for the increase were higher professional fees, travel, and facilities costs. Professional fees increased during the six months ended December 31, 2015 compared to the six months ended December 31, 2014 due to higher fees related to partnering and business development activities as well as investor relations, and directors’ fees. The Company’s Board of Directors has expanded by two seats as the Company has strengthened its corporate governance. Travel costs increased during the six months ended December 31, 2015 compared to the six months ended December 31, 2014 due to travel related to the Company’s financing completed during the quarter ended September 30, 2015 as well as follow up with investors subsequent to the closing of the financing. Facilities costs increased in the period compared to the prior period largely due an increase in promotion costs as the Company launched its social media channels.
Change in fair value of derivative liability
Based on the terms of certain warrants issued by the Company, the Company determined that the warrants were a derivative liability which is recognized at fair value at the date of the transaction and re-measured at fair value every reporting period with gains or losses on the changes in fair value recorded in the consolidated condensed interim statement of loss and comprehensive loss. The balances recognized during the six months ended December 31, 2015 and 2014 were primarily due to changes in the Company’s common stock price between the date the warrants were last valued on June 30, 2015 and 2014 respectively and December 31, 2015 and 2014 respectively which are the revaluation dates used during the six months ended December 31.
The Company recognized a loss of $1,219,634 from the change in fair value of the derivative liability for the six months ended December 31, 2015. In addition, the Company also recognized a total loss of $263,965 which resulted from the change in fair value of the 2013 Placement Agent Warrants when the warrant expiry date was extended as part of the amendment of the 2013 Placement Agent Warrants as well as from the Investor Warrants and the 2013 Placement Agent Warrants when their respective exercise prices decreased as a result of the Company issuing shares below $0.80 per share during the financing the Company completed during the quarter ended September 30, 2015.
| 46 |
For the six months ended December 31, 2014 the Company recognized a gain of $329,357 due to the change in fair value of the derivative liability. In addition, as result of amending the Investor Warrants and Dividend Warrants during the period ended December 31, 2014, the Company also recognized a gain of $23,658. Also, during the six months ended December 31, 2014, the Company exchanged certain Investor Warrants for shares of common stock resulting in the recognition of a loss of $92,843 on the exchange.
Changes in the Company’s common stock price can result in significant volatility in the Company’s reported net loss due to its impact on the fair value of the derivative liability. As a result of revaluation gains and losses, the Company expects that its reported net income or loss will continue to fluctuate significantly.
Foreign Exchange Gain
The Company’s functional currency at December 31, 2015 is the USD but the Company incurs a portion of its expenses in CDN. The foreign exchange gains and losses are reported in other (income) loss in the Consolidated Condensed Interim Statement of Loss and Comprehensive Loss.
The Company recognized a foreign exchange loss of $26,780 for the six months ended December 31, 2015 compared to a loss of $9,686 for the six months ended December 31, 2014. The change was due to changes in the exchange rate between the CDN and the USD and to varying levels of CDN accounts payable.
Preferred Share Dividend and Interest Expense
For the six months ended December 31, 2015 the Company recorded $4,178 related to the dividend payable to Valent. The dividend has been recorded as a direct increase in accumulated deficit and was paid subsequent to quarter end.
For the six months ended December 31, 2014, the Company recognized $2,089 related to the dividend payable to Valent and $2,091 related to accrued interest from June 30, 2014 to September 30, 2014 when the loan was converted to preferred shares. The dividend has been recorded as a direct increase in accumulated deficit and the $2,091 has been recognized as interest expense.
Liquidity and Capital Resources
Six months ended December 31, 2015 compared to the six months ended December 31, 2014
December 31, 2014 $ | December 31, 2013 $ | Change $ | Change % | |||||||||||||
| Cash flows from operating activities | (2,635,300 | ) | (2,065,360 | ) | (569,940 | ) | 28 | |||||||||
| Cash flows from investing activities | (16,762 | ) | – | (16,762 | ) | (100 | ) | |||||||||
| Cash flows from financing activities | 2,854,638 | 1,264,088 | 1,590,550 | 126 | ||||||||||||
Operating Activities
Net cash used in operating activities increased to $2,635,300 for the six months ended December 31, 2015 from $2,065,360 for the six months ended December 31, 2014. During the six months ended December 31, 2015 and 2014 the Company reported net losses of $4,268,078 and $2,136,369 respectively. The loss from the revaluation of the derivative liability was $1,219,634 for the six months ended December 31, 2015 compared to a gain of $329,357 for the six months ended December 31, 2014. Excluding the impact of changes in the fair value of the derivative liability, non-cash items relating to amortization, loss due to changes in warrant terms, warrants and shares issued for services, and stock option expense totaled $643,376 for the six months ended December 31, 2015. Non-cash items relating to the accrued interest, gain due to changes in warrant terms, loss on exchange of warrants, and share-based compensation totaled $331,786 for the six months ended December 31, 2014. The most significant changes in non-cash working capital for the six months ended December 31, 2015 were cash used in a reduction of accounts payable and accrued liabilities of $283,164, cash used in a reduction of related party payables of $34,086, and cash flow from a decrease in prepaid expenses of $77,536. In the six months ended December 31, 2014 the most significant item was cash from a decrease in prepaid expenses of $78,770.
| 47 |
Investing Activities
During the six months ended December 31, 2015 the Company incurred $16,762 in cash costs for the development of its website. There were no investing activities during the six months ended December 31, 2014.
Financing Activities
During the six months ended December 31, 2015 the Company received $405,183 from the exercise of warrants and $2,453,633 in net proceeds from the completion of a public offering by the Company of common stock and common stock purchase warrants. Including deferred costs recorded by the Company at June 30, 2015, the total net cash proceeds of the offering was $1,903,514.
The Company received net proceeds of $1,266,177 from the exercise of warrants during the six months ended December 31, 2014.
In addition, the Company recorded $4,178 and $2,089 related to the dividend payable to Valent during the six months ended December 31, 2015 and 2014 respectively.
Operating Capital and Capital Expenditure Requirements
Going concern
(See note 1 to the Consolidated Condensed Interim Financial Statements)
The financial statements have been prepared on a going concern basis which assumes that the Company will continue its operations for the foreseeable future and contemplates the realization of assets and the settlement of liabilities in the normal course of business.
For the six-month period ended December 31, 2015, the Company reported a loss of $4,268,078 and the Company had an accumulated deficit of $22,885,550 at that date. As at December 31, 2015, the Company has cash and cash equivalents on hand of $1,957,009. The Company does not have the prospect of achieving revenues in the near future and the Company will require additional funding to maintain its research and development projects and for general operations. There is a great degree of uncertainty with respect to the expenses the Company will incur in executing its business plan. In addition, the Company has not begun to commercialize or generate revenues from any product candidate.
Consequently, management is pursuing various financing alternatives to fund the Company’s operations so it can continue as a going concern. During the six months ended December 31, 2015 the Company received net proceeds of $2,453,633 from a public offering of common stock and common stock purchase warrants and $405,183 from the exercise of warrants. However, the net proceeds from these sources are not enough to fund all of the Company’s planned activities. Management plans to secure the necessary additional financing through the issue of new equity and/or entering into strategic partnership arrangements. Nevertheless, there is no assurance that these initiatives will be successful.
The financial statements do not give effect to any adjustments to the amounts and classification of assets and liabilities that may be necessary should the Company be unable to continue as a going concern. Such adjustments could be material.
| 48 |
Our future funding requirements will depend on many factors, including but not limited to:
| ● | the rate of progress and cost of our clinical trials, preclinical studies and other discovery and research and development activities; | |
| ● | the costs associated with establishing manufacturing and commercialization capabilities; | |
| ● | the costs of acquiring or investing in businesses, product candidates and technologies; | |
| ● | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; | |
| ● | the costs and timing of seeking and obtaining FDA and other regulatory approvals; | |
| ● | the effect of competing technological and market developments; and | |
| ● | the economic and other terms and timing of any collaboration, licensing or other arrangements into which we may enter. |
Until we can generate a sufficient amount of product revenue to finance our cash requirements, which we may never do, we expect to finance future cash needs primarily through public or private equity offerings, debt financings or strategic collaborations. Although we are not reliant on institutional credit finance and therefore not subject to debt covenant compliance requirements or potential withdrawal of credit by banks, the current economic climate has also affected the availability of funds and activity in equity markets. We do not know whether additional funding will be available on acceptable terms, or at all. If we are not able to secure additional funding when needed, we may have to delay, reduce the scope of or eliminate one or more of our clinical trials or research and development programs or make changes to our operating plan. In addition, we may have to seek a partner for one or more of our product candidate programs at an earlier stage of development, which would lower the economic value of those programs to us.
Critical Accounting Policies
The preparation of financial statements, in conformity with generally accepted accounting principles in the United States, requires companies to establish accounting policies and to make estimates that affect both the amount and timing of the recording of assets, liabilities, revenues and expenses. Some of these estimates require judgments about matters that are inherently uncertain and therefore actual results may differ from those estimates.
A detailed presentation of all of the Company’s significant accountings policies and the estimates derived therefrom is included in Note 4 to the Company’s consolidated financial statements for the year ended June 30, 2015 contained in our Form 10-K/A filed with the SEC on November 16, 2015. While all of the significant accounting policies are important to the Company’s consolidated financial statements, the following accounting policies and the estimates derived therefrom have been identified as being critical:
| ● | Shares for services | |
| ● | Stock options | |
| ● | Derivative liability |
Shares for services
Periodically, the Company has issued equity instruments for services provided by employees and nonemployees. The equity instruments are valued at the fair value of the instrument granted.
| 49 |
Stock options
The Company accounts for these awards under ASC 718, “Compensation - Stock Compensation” (“ASC 718”). ASC 718 requires measurement of compensation cost for all stock-based awards at fair value on the date of grant and recognition of compensation over the requisite service period for awards expected to vest. Compensation expense for unvested options to non-employees is revalued at each period end and is being amortized over the vesting period of the options. The determination of grant-date fair value for stock option awards is estimated using the Black-Scholes model, which includes variables such as the expected volatility of the Company’s share price, the anticipated exercise behavior of its grantee, interest rates, and dividend yields. These variables are projected based on the Company’s historical data, experience, and other factors. Changes in any of these variables could result in material adjustments to the expense recognized for share-based compensation expense. Such value is recognized as expense over the requisite service period, net of estimated forfeitures, using the straight-line attribution method. The estimation of stock awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from current estimates, such amounts are recorded as a cumulative adjustment in the period estimates are revised. The Company considers many factors when estimating expected forfeitures, including type of awards granted, employee class, and historical experience. Actual results and future estimates may differ substantially from current estimates.
Derivative liability
The Company accounts for certain warrants under the authoritative guidance on accounting for derivative financial instruments indexed to, and potentially settled in, a company’s own stock, on the understanding that in compliance with applicable securities laws, the warrants require the issuance of securities upon exercise and do not sufficiently preclude an implied right to net cash settlement. The Company classifies warrants in its balance sheet as a derivative liability which is fair valued at each reporting period subsequent to the initial issuance. As quoted prices for the derivative liability are not available, the Company uses a simulated probability valuation model to value the warrants. Determining the appropriate fair-value model and calculating the fair value of warrants requires considerable judgment. Any change in the estimates used may cause the value to be higher or lower than that reported. The estimated volatility of the Company’s common stock at the date of issuance, and at each subsequent reporting period, is based on the historical volatility of similar life sciences companies. The risk-free interest rate is based on rates published by the government for bonds with a maturity similar to the expected remaining life of the warrants at the valuation date. The expected life of the warrants is assumed to be equivalent to their remaining contractual term.
Off-Balance Sheet Arrangements
We do not have any off balance sheet arrangements.
Recent Developments
Effective February 10, 2016 the Board of Directors accepted the resignation of William Garner from the Board of Directors.
| 50 |
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
Not required for a smaller reporting company.
Item 4. Controls and Procedures.
Disclosure Controls and Procedures
At the time that our Annual Report on Form 10-K for the year ended June 30, 2015 was filed on September 3, 2015, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of June 30, 2015. Subsequent to that evaluation, our management, including our Chief Executive Officer and Chief Financial Officer, concluded that our disclosure controls and procedures were not effective as of June 30, 2015, because of the material weakness in our internal control over financial reporting discussed in our Annual Report on Form 10-K/A filed on November 16, 2015.
Remediation Plan for the Material Weakness
Management has been actively engaged in developing remediation plans to address the above material weakness. The remediation efforts in process or expected to be implemented include the following:
- Engaging an industry expert to assist with the identification and assessment of non-routine, financial instrument related transactions; and
- Re-designing controls to identify, research, evaluate and review the appropriate accounting related to non-routine complex transactions and technical accounting matters. These include matters in the areas of share purchase warrants, derivative liability and other equity transactions.
We believe that the controls that we are, and will be, implementing will improve the effectiveness of our internal control over financial reporting. As we continue to evaluate and work to improve our internal control over financial reporting, we may determine to take additional measures to address the material weakness or determine to supplement or modify certain of the remediation measures described above. The remediation of the material weakness was completed in the quarter ended December 31, 2015.
Management, with the participation of the Chief Executive Officer and Chief Financial Officer, conducted an evaluation (as required by Rule 13a-15 under the Exchange Act) of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this report. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this report.
Changes in internal controls
Other than as described above, there have been no changes in our internal control over financial reporting that occurred during the quarter ended December 31, 2015 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| 51 |
PART II - OTHER INFORMATION
There are no legal proceedings to which the Company or any of its property is the subject.
Not required for a smaller reporting company.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
During the three months ended December 31, 2015, we issued 60,000 shares of common stock for services, 515,500 shares of common stock upon exercise of warrants at an exercise price of $0.786, and 200,000 shares of common stock upon exchange of Exchangeable Shares.
In connection with the foregoing, we relied upon the exemption from registration provided by Section 4(a)(2) under the Securities Act of 1933, as amended, for transactions not involving a public offering.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
Not applicable.
None.
| No. | Description | |
| 31.1 | Rule 13a-14(a)/ 15d-14(a) Certification of Chief Executive Officer | |
| 31.2 | Rule 13a-14(a)/ 15d-14(a) Certification of Chief Financial Officer | |
| 32.1 | Section 1350 Certification of Chief Executive Officer | |
| 32.2 | Section 1350 Certification of Chief Financial Officer |
| EX-101.INS | XBRL INSTANCE DOCUMENT | |
| EX-101.SCH | XBRL TAXONOMY EXTENSION SCHEMA DOCUMENT | |
| EX-101.CAL | XBRL TAXONOMY EXTENSION CALCULATION LINKBASE | |
| EX-101.LAB | XBRL TAXONOMY EXTENSION LABELS LINKBASE | |
| EX-101.PRE | XBRL TAXONOMY EXTENSION PRESENTATION LINKBASE |
| 52 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| DelMar Pharmaceuticals, Inc. | ||
| Date: February 12, 2016 | By: | /s/ Jeffrey Bacha |
| Jeffrey
Bacha Chief Executive Officer (Principal Executive Officer) | ||
| Date: February 12, 2016 | By: | /s/ Scott Praill |
| Scott
Praill Chief Financial Officer (Principal Financial and Accounting Officer) | ||
| 53 |
