Attached files
| file | filename |
|---|---|
| EX-10.3 - SETTLEMENT AGREEMENT, DATED JANUARY 1, 2018, BETWEEN DELMAR PHARMACEUTICALS, INC - Kintara Therapeutics, Inc. | f10q1217ex10-3_delmarpharma.htm |
| EX-32.2 - CERTIFICATION - Kintara Therapeutics, Inc. | f10q1217ex32-2_delmarpharma.htm |
| EX-32.1 - CERTIFICATION - Kintara Therapeutics, Inc. | f10q1217ex32-1_delmarpharma.htm |
| EX-31.2 - CERTIFICATION - Kintara Therapeutics, Inc. | f10q1217ex31-2_delmarpharma.htm |
| EX-31.1 - CERTIFICATION - Kintara Therapeutics, Inc. | f10q1217ex31-1_delmarpharma.htm |
| EX-10.4 - DELMAR PHARMACEUTICALS, INC. 2017 OMNIBUS EQUITY INCENTIVE PLAN (AS AMENDED AND - Kintara Therapeutics, Inc. | f10q1217ex10-4_delmarpharma.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-Q
(Mark One)
þ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended December 31, 2017
or
☐ TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from __________________ to __________________________
Commission file number: 001-37823
DelMar Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
| Nevada | 99-0360497 | |
| (State
or other jurisdiction of incorporation or organization) |
(I.R.S.
Employer Identification No.) |
Suite 720-999 West Broadway Vancouver, British Columbia, Canada |
V5Z 1K5 | |
| (Address of principal executive offices) | (zip code) |
| (604) 629-5989 |
| (Registrant's telephone number, including area code) |
| N/A |
| (Former name, former address and former fiscal year, if changed since last report) |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes þ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes þ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company”, and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ | |
Non-accelerated filer ☐ (Do not check if smaller reporting company) Emerging growth company ☐ |
Smaller reporting company þ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act)
Yes ☐ No þ
Indicate the number of shares outstanding of each of the issuer's classes of common stock, as of the latest practicable date, 21,927,517 shares of common stock are issued and outstanding as of February 13, 2018.
TABLE OF CONTENTS
| Page No. | ||||
| PART I. - FINANCIAL INFORMATION | ||||
| Item 1. | Financial Statements. | 1 | ||
| Item 2. | Management's Discussion and Analysis of Financial Condition and Results of Operations. | 22 | ||
| Item 3. | Quantitative and Qualitative Disclosures About Market Risk. | 49 | ||
| Item 4 | Controls and Procedures. | 49 | ||
| PART II - OTHER INFORMATION | ||||
| Item 1. | Legal Proceedings. | 49 | ||
| Item 1A. | Risk Factors. | 49 | ||
| Item 2. | Unregistered Sales of Equity Securities and Use of Proceeds. | 49 | ||
| Item 3. | Defaults Upon Senior Securities. | 49 | ||
| Item 4. | Mine Safety Disclosures. | 49 | ||
| Item 5. | Other Information. | 50 | ||
| Item 6. | Exhibits. | 50 | ||
PART 1. - FINANCIAL INFORMATION
Item 1. Financial Statements.
DelMar Pharmaceuticals, Inc.
Consolidated Condensed Interim Financial Statements
(Unaudited)
For the six months ended December 31, 2017
(expressed in US dollars unless otherwise noted)
| 1 |
DelMar Pharmaceuticals, Inc.
Consolidated Condensed Interim Balance Sheets
(Unaudited)
(expressed in US dollars unless otherwise noted)
| Note | December 31, 2017 $ | June 30, 2017 $ | ||||||||||
| Assets | ||||||||||||
| Current assets | ||||||||||||
| Cash | 11,021,568 | 6,586,014 | ||||||||||
| Prepaid expenses and deposits | 1,140,801 | 1,208,122 | ||||||||||
| Taxes and other receivables | 24,667 | 76,595 | ||||||||||
| 12,187,036 | 7,870,731 | |||||||||||
| Intangible assets - net | 29,080 | 40,290 | ||||||||||
| 12,216,116 | 7,911,021 | |||||||||||
| Liabilities | ||||||||||||
| Current liabilities | ||||||||||||
| Accounts payable and accrued liabilities | 1,829,137 | 1,182,312 | ||||||||||
| Related party payables | 4 | 397,856 | 88,957 | |||||||||
| Current portion of derivative liability | 5 | 95 | 33,091 | |||||||||
| 2,227,088 | 1,304,360 | |||||||||||
| Derivative liability | 5 | 5,454 | 28,137 | |||||||||
| 2,232,542 | 1,332,497 | |||||||||||
| Stockholders’ accumulated equity | ||||||||||||
| Preferred stock | ||||||||||||
| Authorized | ||||||||||||
| 5,000,000 shares, $0.001 par value | ||||||||||||
| Issued and outstanding | ||||||||||||
| 278,530 Series A shares at December 31, 2017 (June 30, 2017 – 278,530) | 3,6 | 278,530 | 278,530 | |||||||||
| 881,113 Series B shares at December 31, 2017 (June 30, 2017 – 881,113) | 6 | 6,146,880 | 6,146,880 | |||||||||
| 1 special voting share at December 31, 2017 (June 30, 2017 – 1) | - | - | ||||||||||
| Common stock | ||||||||||||
| Authorized | ||||||||||||
| 50,000,000 shares, $0.001 par value | ||||||||||||
| 22,608,837 issued at December 31, 2017 (June 30, 2017 – 14,509,633) | 6 | 22,609 | 14,510 | |||||||||
| Additional paid-in capital | 6 | 43,238,880 | 36,665,285 | |||||||||
| Warrants | 6 | 7,321,844 | 4,570,574 | |||||||||
| Accumulated deficit | (47,046,347 | ) | (41,118,433 | ) | ||||||||
| Accumulated other comprehensive income | 21,178 | 21,178 | ||||||||||
| 9,983,574 | 6,578,524 | |||||||||||
| 12,216,116 | 7,911,021 | |||||||||||
Nature of operations, corporate history, and liquidity risk (note 1) | ||||||||||||
Subsequent events (note 9) | ||||||||||||
The accompanying notes are an integral part of these consolidated condensed interim financial statements
| 2 |
DelMar Pharmaceuticals, Inc.
Consolidated Condensed Interim Statement of Loss and Comprehensive Loss
(Unaudited)
(expressed in US dollars unless otherwise noted)
Note | Three months ended December 31, 2017 $ | Three months ended December 31, 2016 $ | Six months ended December 31, 2017 $ | Six months ended December 31, 2016 $ | ||||||||||||||||
| Expenses | ||||||||||||||||||||
| Research and development | 4 | 2,141,945 | 1,120,910 | 4,076,588 | 1,853,639 | |||||||||||||||
| General and administrative | 4 | 1,011,879 | 571,286 | 1,756,500 | 1,887,925 | |||||||||||||||
| 3,153,824 | 1,692,196 | 5,833,088 | 3,741,564 | |||||||||||||||||
| Other loss (income) | ||||||||||||||||||||
| Change in fair value of stock option and derivative liabilities | 5, 6 | 889 | (361,668 | ) | (55,679 | ) | (135,980 | ) | ||||||||||||
| Foreign exchange loss (gain) | 7,120 | (8,495 | ) | 50,986 | 6,829 | |||||||||||||||
| Interest income | (235 | ) | (60 | ) | (391 | ) | (101 | ) | ||||||||||||
| 7,774 | (370,223 | ) | (5,084 | ) | (129,252 | ) | ||||||||||||||
| Net and comprehensive loss for the period | 3,161,598 | 1,321,973 | 5,828,004 | 3,612,312 | ||||||||||||||||
| Computation of basic loss per share | ||||||||||||||||||||
| Net and comprehensive loss for the period | 3,161,598 | 1,321,973 | 5,828,004 | 3,612,312 | ||||||||||||||||
| Series B Preferred stock dividend | 54,066 | 159,756 | 95,732 | 467,054 | ||||||||||||||||
| Net and comprehensive loss available to common stockholders | 3,215,664 | 1,481,729 | 5,923,736 | 4,079,366 | ||||||||||||||||
| Basic and fully diluted loss per share | 0.14 | 0.13 | 0.31 | 0.36 | ||||||||||||||||
| Basic weighted average number of shares | 22,559,234 | 11,424,485 | 18,882,259 | 11,363,237 | ||||||||||||||||
The accompanying notes are an integral part of these consolidated condensed interim financial statements.
| 3 |
DelMar Pharmaceuticals, Inc.
Consolidated Condensed Interim Statement of Cash Flows
(Unaudited)
(expressed in US dollars unless otherwise noted)
| Six months ended December 31, | ||||||||||||
| 2017 | 2016 | |||||||||||
| Note | $ | $ | ||||||||||
| Cash flows from operating activities | ||||||||||||
| Loss for the period | (5,828,004 | ) | (3,612,312 | ) | ||||||||
| Items not affecting cash | ||||||||||||
| Amortization of intangible assets | 11,210 | 7,716 | ||||||||||
| Change in fair value of stock option and derivative liabilities | 5,6 | (55,679 | ) | (135,980 | ) | |||||||
| Shares issued for services | 6 | - | 564,000 | |||||||||
| Warrants issued for services | 6 | (1,481 | ) | 50,244 | ||||||||
| Stock option expense (income) | 6 | 293,377 | (43,384 | ) | ||||||||
| Changes in non-cash working capital | ||||||||||||
| Taxes and other receivables | 51,928 | 399 | ||||||||||
| Prepaid expenses | 67,321 | 13,341 | ||||||||||
| Accounts payable and accrued liabilities | 646,825 | (68,369 | ) | |||||||||
| Related party payables | 4 | 308,899 | 161,937 | |||||||||
| (4,505,604 | ) | (3,062,408 | ) | |||||||||
| Cash flows from financing activities | ||||||||||||
| Net proceeds from the issuance of shares and warrants | 6 | 8,945,336 | - | |||||||||
| Proceeds from the exercise of warrants | 6 | - | 326,699 | |||||||||
| Series A preferred stock dividend | 6 | (4,178 | ) | (4,178 | ) | |||||||
| 8,941,158 | 322,521 | |||||||||||
| Increase (decrease) in cash | 4,435,554 | (2,739,887 | ) | |||||||||
| Cash - beginning of period | 6,586,014 | 6,157,264 | ||||||||||
| Cash - end of period | 11,021,568 | 3,417,377 | ||||||||||
Supplementary information (note 8) | ||||||||||||
The accompanying notes are an integral part of these consolidated condensed interim financial statements.
| 4 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2017
(expressed in US dollars unless otherwise noted)
| 1 | Nature of operations, corporate history, and liquidity risk |
Nature of operations
DelMar Pharmaceuticals, Inc. (the “Company”) is a clinical stage drug development company with a focus on the treatment of cancer. We are conducting clinical trials in the United States with VAL-083 as a potential new treatment for glioblastoma multiforme, the most common and aggressive form of brain cancer. We have also acquired certain exclusive commercial rights to VAL-083 in China where it is approved as a chemotherapy for the treatment of chronic myelogenous leukemia and lung cancer. In order to accelerate our development timelines, we leverage existing clinical and commercial data from a wide range of sources. We may seek marketing partnerships to potentially generate future royalty revenue.
The address of the Company’s administrative offices is Suite 720 - 999 West Broadway, Vancouver, British Columbia, V5Z 1K5 with clinical operations located at 3485 Edison Way, Suite R, Menlo Park, California, 94025.
Corporate history
The Company is a Nevada corporation formed on June 24, 2009 under the name Berry Only, Inc. On January 25, 2013, the Company entered into and closed an exchange agreement (the “Exchange Agreement”), with Del Mar Pharmaceuticals (BC) Ltd. (“DelMar (BC)”), 0959454 B.C. Ltd. (“Callco”), and 0959456 B.C. Ltd. (“Exchangeco”) and the security holders of DelMar (BC). Upon completion of the Exchange Agreement, DelMar (BC) became a wholly-owned subsidiary of the Company (the “Reverse Acquisition”).
DelMar Pharmaceuticals, Inc. is the parent company of DelMar (BC), a British Columbia, Canada corporation incorporated on April 6, 2010, which is a clinical stage company with a focus on the development of drugs for the treatment of cancer. The Company is also the parent company to Callco and Exchangeco which are British Columbia, Canada corporations. Callco and Exchangeco were formed to facilitate the Reverse Acquisition.
References to the Company refer to the Company and its wholly-owned subsidiaries, DelMar (BC), Callco and Exchangeco.
Liquidity risk
For the six months ended December 31, 2017, the Company reported a loss of $5,828,004 and the Company had an accumulated deficit of $47,046,347 at that date. As at December 31, 2017, the Company had cash on hand of $11,021,568. During the six months ended December 31, 2017, the Company received $8,945,336 in net proceeds from a registered direct offering. We believe, based on our current estimates, that we will be able to fund our operations beyond the next twelve months.
The Company does not have the prospect of achieving revenues in the near future and the Company will require additional funding for its clinical trials, to maintain its research and development projects, and for general operations. There is a great degree of uncertainty with respect to the expenses the Company will incur in executing its business plan. Consequently, management continually evaluates various financing alternatives to fund the Company’s operations so it can continue as a going concern in the medium to longer term.
There is no assurance that our cost estimates will prove to be accurate or that unforeseen events, problems or delays will not occur with respect thereto. The ability of the Company to meet its obligations and continue the research and development of its product candidate is dependent on its ability to continue to raise adequate financing. There can be no assurance that such financing will be available to the Company in the amount required at any time or for any period or, if available, that it can be obtained on terms satisfactory to the Company. The Company may tailor its drug candidate development program based on the amount of funding the Company raises.
| 5 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2017
(expressed in US dollars unless otherwise noted)
| 2 | Significant accounting policies |
Basis of presentation
The consolidated condensed interim financial statements of the Company have been prepared in accordance with United States Generally Accepted Accounting Principles (“U.S. GAAP”) and are presented in United States dollars. The functional currency of the Company and each of its subsidiaries is the United States dollar.
The accompanying consolidated condensed interim financial statements include the accounts of the Company and its wholly-owned subsidiaries, DelMar BC, Callco, and Exchangeco. All intercompany balances and transactions have been eliminated.
The principal accounting policies applied in the preparation of these financial statements are set out below and have been consistently applied to all periods presented.
Unaudited interim financial data
The accompanying unaudited December 31, 2017 consolidated condensed interim balance sheet, the consolidated condensed interim statements of loss and comprehensive loss for the three and six months ended December 31, 2017 and 2016, and consolidated condensed cash flows for the six months ended December 31, 2017 and 2016, and the related interim information contained within the notes to the consolidated condensed interim financial statements have been prepared in accordance with the rules and regulations of the Securities and Exchange Commission for interim financial information. Accordingly, they do not include all of the information and the notes required by U.S. GAAP for complete financial statements. These consolidated condensed interim financial statements should be read in conjunction with the audited financial statements of the Company as at June 30, 2017 included in the Company’s Form 10-K filed with the Securities and Exchange Commission on September 27, 2017. In the opinion of management, the unaudited consolidated condensed interim financial statements reflect all adjustments, consisting of normal and recurring adjustments, necessary for the fair statement of the Company’s financial position at December 31, 2017 and results of its operations for the three and six months ended December 31, 2017 and 2016, and its cash flows for the six months ended December 31, 2017 and 2016. The results for six months ended December 31, 2017 are not necessarily indicative of the results to be expected for the fiscal year ending June 30, 2018 or for any other future annual or interim period.
| 6 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2017
(expressed in US dollars unless otherwise noted)
Use of estimates
The preparation of consolidated condensed interim financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions about future events that affect the reported amounts of assets, liabilities, expenses, contingent assets and contingent liabilities as at the end of, or during, the reporting period. Actual results could significantly differ from those estimates. Significant areas requiring management to make estimates include the derivative liability, the valuation of equity instruments issued for services, and clinical trial accruals. There have been no changes to the methodology used in determining these estimates from the fiscal year ended June 30, 2017.
Loss per share
Loss per share is calculated based on the weighted average number of common shares outstanding. For the three-month period ended December 31, 2017 and 2016 diluted loss per share does not differ from basic loss per share since the effect of the Company’s warrants and stock options are anti-dilutive. At December 31, 2017, potential common shares of 15,028,906 (2016 – 4,505,852) relating to warrants, 1,420,850 (2016 – 896,250) relating to stock options, and 2,202,792 (2016 – 2,224,668) relating to the Series B convertible preferred stock were excluded from the calculation of net loss per common share because their inclusion would be anti-dilutive.
Recent accounting pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (“FASB”) or other standard setting bodies that are adopted by the Company as of the specified effective date.
Accounting Standards Update (“ASU”) 2017-11 — I. Accounting for Certain Financial Instruments with Down Round Features, II. Replacement of the Indefinite Deferral for Mandatorily Redeemable Financial Instruments of Certain Non-public Entities and Certain Mandatorily Redeemable Noncontrolling Interests with a Scope Exception
The amendments in this update are intended to reduce the complexity associated with the accounting for certain financial instruments with characteristics of liabilities and equity. Specifically, a down round feature would no longer cause a freestanding equity-linked financial instrument (or an embedded conversion option) to be accounted for as a derivative liability at fair value with changes in fair value recognized in current earnings. In addition, the indefinite deferral of certain provisions of Topic 480 have been re-characterized to a scope exception. The re-characterization has no accounting effect. ASU 2017-11 is effective for public business entities for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019, and interim periods within fiscal years beginning after December 15, 2020. Early adoption is permitted. The Company is currently evaluating the potential impact of the adoption of this standard. The adoption of this guidance is not expected to have a material impact on the consolidated, condensed financial statements.
| 7 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2017
(expressed in US dollars unless otherwise noted)
ASU 2016-09 —Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting.
The amendments in this update change existing guidance related to accounting for employee share-based payments affecting the income tax consequences of awards, classification of awards as equity or liabilities, and classification on the statement of cash flows. ASU 2016-09 is effective for annual reporting periods beginning after December 15, 2016, including interim periods within those annual periods, with early adoption permitted. The Company has adopted this standard as of its September 30, 2017 quarter end.
ASU 2016-02 — Leases (Topic 842).
The new standard establishes a right-of-use (“ROU”) model that requires a lessee to record a ROU asset and a lease liability on the consolidated balance sheet for all leases with terms longer than 12 months. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the consolidated income statement. ASU 2016-02 is effective for annual periods beginning after December 15, 2018, including interim periods within those annual periods, with early adoption permitted. A modified retrospective transition approach is required for lessees for capital and operating leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements, with certain practical expedients available. The Company is currently evaluating the potential impact of the adoption of this standard.
ASU No. 2016-01 — Financial Instruments - Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities.
The updated guidance enhances the reporting model for financial instruments and requires entities to use the exit price notion when measuring the fair value of financial instruments for disclosure purposes, and the separate presentation of financial assets and financial liabilities by measurement category and form of financial asset (i.e., securities or loans and receivables) on the balance sheet or the accompanying notes to the financial statements. The guidance is effective for annual and interim reporting periods beginning after December 15, 2017. The Company is currently assessing this standard for its impact on future reporting periods.
| 3 | Valent Technologies, LLC |
On September 30, 2014, the Company entered into an exchange agreement (the “Valent Exchange Agreement”) with Valent Technologies, LLC (“Valent”), an entity owned by Dr. Dennis Brown, the Company’s Chief Scientific Officer and director, and DelMar (BC). Pursuant to the Valent Exchange Agreement, Valent exchanged its loan payable in the outstanding amount of $278,530 (including aggregate accrued interest to September 30, 2014 of $28,530), issued to Valent by DelMar (BC), for 278,530 shares of the Company’s Series A Preferred Stock. The Series A Preferred Stock has a stated value of $1.00 per share (the “Series A Stated Value”) and is not convertible into common stock. The holder of the Series A Preferred Stock is entitled to dividends at the rate of 3% of the Series A Stated Value per year, payable quarterly in arrears.
For the three months ended December 31, 2017 and 2016 respectively, the Company recorded $2,089 related to the dividend payable to Valent. For the six months ended December 31, 2017 and 2016 respectively, the Company recorded $4,178 related to the dividend payable to Valent. The dividends have been recorded as a direct increase in accumulated deficit.
| 8 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2017
(expressed in US dollars unless otherwise noted)
| 4 | Related party transactions |
Pursuant to employment and consulting agreements with the Company’s officers the Company recognized a total of $226,667 (2016 - $275,000) in expenses for the three months ended December 31, 2017 and $369,167 (2016 - $395,000) for the six months ended December 31, 2017. In addition, at December 31, 2017, the Company also recognized a total of $311,683 relating to the settlement agreement with the Company’s former President and Chief Operating Officer. Amounts owed to related parties, including to the Company’s former President and Chief Operating Officer, are non-interest bearing and payable on demand.
The Company recognized $43,750 (2016 – $37,000) in directors’ fees during the three months ended December 31, 2017 and $96,250 (2016 - $82,000) during the six months ended December 31, 2017.
As part of the Series B preferred stock dividend (note 6) the Company issued 1,511 (2016 – 1,511) shares of common stock to officers and directors of the Company and recognized an amount of $1,647 (2016 - $4,819) for the three months ended December 31, 2017. For the six months ended December 31, 2017, the Company issued 3,022 (2016 – 3,022) shares of common stock and recognized $2,916 (2016 - $13,960). All of the dividends have been recognized as a direct increase to deficit.
The Company recorded $2,089 (2016 - $2,089) in dividends related to the Series A preferred stock issued to Valent (note 3) for the three months ended December 31, 2017 and $4,178 (2016 - $4,178) for the six months ended December 31, 2017.
During the six months ended December 31, 2017, the Company granted a total of 180,000 stock options to the Company’s independent directors. The stock options are exercisable at a price of $2.11 and have a term of 10 years. One-third of the options vest on June 30, 2018 and 15,000 options vest on a quarterly basis thereafter commencing September 30, 2018. In addition, during the three and six months ended December 31, 2017, the Company granted 120,000 stock options at an exercise price of $0.87 to its Interim President and Chief Executive Officer. The stock options have a term of 10 years and vest pro rata monthly during the year following grant. The Company also modified certain stock options held by its former President and Chief Operating Officer (note 6).
| 9 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2017
(expressed in US dollars unless otherwise noted)
| 5 | Derivative liability |
The Company has issued common stock purchase warrants. Based on the terms of certain of these warrants the Company determined that the warrants were a derivative liability which is recognized at fair value at the date of the transaction and re-measured at fair value each reporting period with the changes in fair value recorded in the consolidated condensed interim statement of loss and comprehensive loss.
2013 Investor Warrants
During the quarter ended March 31, 2013 the Company issued an aggregate of 3,281,250 units at a purchase price of $3.20 per unit, for aggregate gross proceeds of $10,500,000 (the “Private Offering”). Each unit consisted of one share of common stock and one five-year warrant (the “2013 Investor Warrants”) to purchase one share of common stock at an initial exercise price of $3.20. The exercise price of the 2013 Investor Warrants is subject to adjustment in the event that the Company issues common stock at a price lower than the exercise price, subject to certain exceptions. The 2013 Investor Warrants are redeemable by the Company at a price of $0.004 per 2013 Investor Warrant at any time subject to the conditions that (i) the Company’s common stock has traded for twenty (20) consecutive trading days with a closing price of at least $6.40 per share with an average trading volume of 50,000 shares per day, and (ii) the underlying shares of common stock are registered for resale.
As a result of the financing completed by the Company during the three months ended September 30, 2015, the exercise price of all of the 2013 Investor Warrants was reduced from $3.20 to $3.14. As a result of the financing completed by the Company during the three months ended September 30, 2017 the exercise price of the un-amended 2013 Investor Warrants was further reduced from $3.14 to $2.68. The change in exercise price did not result in a material change in the fair value of the derivative liability.
2013 Investor Warrant exercises
During the three months ended December 31, 2016, 22,188 of the 2013 Investor Warrants were exercised for 22,188 shares of common stock at an exercise price of $3.14 per share. The Company received proceeds of $69,759 from these exercises. The warrants that have been exercised were revalued at their respective exercise dates and then the reclassification to equity was recorded resulting in $57,466 of the derivative liability being reclassified to equity.
During the six months ended December 31, 2016, 65,095 of the 2013 Investor Warrants were exercised for 65,095 shares of common stock at an exercise price of $3.14 per share. The Company received proceeds of $204,659 from these exercises. The warrants that have been exercised were revalued at their respective exercise dates and then the reclassification to equity was recorded resulting in $238,474 of the derivative liability being reclassified to equity.
There were no exercises of 2013 Investor Warrants during the three or six months ended December 31, 2017.
2013 Investor Warrant amendments
During the year ended June 30, 2016, the Company entered into amendments (the “2013 Investor Warrant Amendments”) with the holders of certain of the 2013 Investor Warrants to extend the expiration date to March 31, 2019 and remove the provision requiring an adjustment of the warrant exercise price in the event the Company sells common stock at a purchase price lower than the current warrant exercise price. During the six months ended December 31, 2016, 15,944 of the 2013 Investor Warrants were amended. The warrants that have been amended were revalued at their respective amendment dates and then the reclassification to equity was recorded resulting in $53,006 of the derivative liability being reclassified to equity.
There were no amendments of the 2013 Investor Warrants during the three months ended December 31, 2017 and 2016 or during the six months ended December 31, 2017.
| 10 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2017
(expressed in US dollars unless otherwise noted)
2015 Agent Warrants
As part of a financing completed by the Company in a prior period, the Company issued warrants to purchase 23,477 shares of common stock to certain placement agents (“2015 Agent Warrants”) and recognized them as a derivative liability of $29,594 at the time of issuance. The 2015 Agent Warrants are exercisable at a per share price equal to $3.00 until July 15, 2020. During the six months ended December 31, 2016, 680 of the 2015 Agent Warrants were exercised for cash proceeds of $2,040 and 1,000 of the 2015 Agent Warrants were exercised on a cashless basis for 594 shares of common stock. The total reclassification to equity subsequent to revaluation at the respective exercise dates was $9,935.
There were no exercises of the 2015 Agent Warrants during the three and six months ended December 31, 2017.
The Company’s derivative liability is summarized as follows:
| Three months ended | |||||||||
| December 31, | |||||||||
| 2017 | 2016 | ||||||||
| $ | $ | ||||||||
| Opening balance | 4,660 | 590,345 | |||||||
| Change in fair value of warrants | 889 | (361,668 | ) | ||||||
| Reclassification to equity upon exercise of warrants | - | (57,466 | ) | ||||||
| Closing balance | 5,549 | 171,211 | |||||||
| Less current portion | (95 | ) | - | ||||||
| Long term portion | 5,454 | 171,211 | |||||||
| Six months ended | |||||||||
| December 31, | |||||||||
| 2017 | 2016 | ||||||||
| $ | $ | ||||||||
| Opening balance | 61,228 | 693,700 | |||||||
| Change in fair value of warrants | (55,679 | ) | (221,074 | ) | |||||
| Reclassification to equity upon amendment of warrants | - | (53,006 | ) | ||||||
| Reclassification to equity upon exercise of warrants | - | (248,409 | ) | ||||||
| Closing balance | 5,549 | 171,211 | |||||||
| Less current portion | (95 | ) | - | ||||||
| Long term portion | 5,454 | 171,211 | |||||||
| 11 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2017
(expressed in US dollars unless otherwise noted)
The derivative liability consists of the following warrants:
| December 31, 2017 | |||||||||
| Number of warrants | $ | ||||||||
| 2013 Investor Warrants | 105,129 | 23 | |||||||
| Warrants issued for services | 43,750 | 72 | |||||||
| 2015 Agent Warrants | 21,768 | 5,454 | |||||||
| Closing balance | 170,647 | 5,549 | |||||||
| Less current portion | (148,879 | ) | (95 | ) | |||||
| Long-term portion | 21,768 | 5,454 | |||||||
| 6 | Stockholders’ equity |
Preferred stock
Authorized
5,000,000 preferred shares, $0.001 par value
Issued and outstanding
Special voting shares – at December 31, 2017 – 1 (June 30, 2017 – 1)
Series A shares – at December 31, 2017 – 278,530 (June 30, 2017 – 278,530)
Series B shares – at December 31, 2017 – 881,113 (June 30, 2017 – 881,113)
Series B Preferred Shares
During the year ended June 30, 2016 the Company issued an aggregate of 902,238 shares of Series B Preferred Stock at a purchase price of at $8.00 per share. Each share of Series B Preferred Stock is convertible into 2.5 shares of common stock equating to a conversion price of $3.20 (the “Conversion Price”) and will automatically convert to common stock at the earlier of 24 hours following regulatory approval of VAL-083 with a minimum closing bid price of $8.00 or five years from the final closing date. The holders of the Series B Preferred Stock are entitled to an annual cumulative, in arrears, dividend at the rate of 9% payable quarterly. The 9% dividend accrues quarterly commencing on the date of issue and is payable quarterly on September 30, December 31, March 31, and June 30 of each year commencing on June 30, 2016. Dividends are payable solely by delivery of shares of common stock (the “PIK Shares”), in an amount for each holder equal to the aggregate dividend payable to such holder with respect to the shares of Series B Preferred Stock held by such holder divided by the Conversion Price. The Series B Preferred Stock does not contain any repricing features.
| 12 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2017
(expressed in US dollars unless otherwise noted)
In addition, the Company and the holders entered into a royalty agreement, pursuant to which the Company will pay the holders of the Series B Preferred Stock, in aggregate, a low, single-digit royalty based on their pro rata ownership of the Series B Preferred Stock on products sold directly by the Company or sold pursuant to a licensing or partnering arrangement (the “Royalty Agreement”).
Upon conversion of a holder’s Series B Preferred Stock to common stock, such holder shall no longer receive ongoing royalty payments under the Royalty Agreement but will be entitled to receive any residual royalty payments that have vested. Rights to the royalties shall vest during the first three years following the applicable closing date, in equal thirds to holders of the Series B Preferred Stock on each of the three vesting dates, upon which vesting dates such royalty amounts shall become “Vested Royalties”.
Pursuant to the Series B Preferred Stock dividend, during the three months ended December 31, 2017, the Company issued 49,602 (2016 – 50,096) shares of common stock and recognized a total of $54,066 (2016 – $159,756). During the six months ended December 31, 2017, the Company issued 99,204 (2016 – 100,889) shares of common stock and recognized a total of $95,732 (2016 – $467,054). All dividends have been recognized as a direct increase in accumulated deficit.
A total of 881,113 (2016 – 889,863) shares of Series B Preferred Stock are outstanding as of December 31, 2017, such that a total of 2,202,792 (2016 – 2,224,668) shares of common stock are issuable upon conversion of the Series B Preferred Stock as at December 31, 2017. Converted shares are rounded up to the nearest whole share.
No shares of the Series B Preferred Stock were converted to common stock during the three and six months ended December 31, 2017. During the three and six months ended December 31, 2016, a total of 12,375 shares of Series B preferred stock were converted for an aggregate 30,938 shares of common stock.
Series A Preferred Shares
Effective September 30, 2014 pursuant to the Company’s Valent Exchange Agreement (note 3), the Company filed a Certificate of Designation of Series A Preferred Stock (the “Series A Certificate of Designation”) with the Secretary of State of Nevada. Pursuant to the Series A Certificate of Designation, the Company designated 278,530 shares of preferred stock as Series A Preferred Stock. The shares of Series A Preferred Stock have a stated value of $1.00 per share (the “Series A Stated Value”) and are not convertible into common stock. The holder of the Series A Preferred Stock is entitled to dividends at the rate of 3% of the Series A Stated Value per year, payable quarterly in arrears. Upon any liquidation of the Company, the holder of the Series A Preferred Stock will be entitled to be paid, out of any assets of the Company available for distribution to stockholders, the Series A Stated Value of the shares of Series A Preferred Stock held by such holder, plus any accrued but unpaid dividends thereon, prior to any payments being made with respect to the common stock.
| 13 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2017
(expressed in US dollars unless otherwise noted)
Common stock
Authorized - 50,000,000 common shares, $0.001 par value
Issued and outstanding - December 31, 2017 – 22,608,837 (June 30, 2017 – 14,509,633)
| Shares of common stock | Common stock | Additional paid-in capital | Warrants | Accumulated deficit | |||||||||||||||||
| outstanding | $ | $ | $ | $ | |||||||||||||||||
| Balance – June 30, 2017 | 14,509,633 | 14,510 | 36,665,285 | 4,570,574 | (41,118,433 | ) | |||||||||||||||
| Issuance of shares and warrants | 8,000,000 | 8,000 | 6,184,585 | 2,752,751 | - | ||||||||||||||||
| Series B Preferred stock dividend | 99,204 | 99 | 95,633 | - | (95,732 | ) | |||||||||||||||
| Stock option expense | - | - | 293,377 | - | - | ||||||||||||||||
| Warrants issued for services | - | - | - | (1,481 | ) | - | |||||||||||||||
| Series A Preferred cash dividend | - | - | - | - | (4,178 | ) | |||||||||||||||
| Loss for the period | - | - | - | - | (5,828,004 | ) | |||||||||||||||
| Balance – December 31, 2017 | 22,608,837 | 22,609 | 43,238,880 | 7,321,844 | (47,046,347 | ) | |||||||||||||||
The issued and outstanding common shares at December 31, 2017 include 932,761 (June 30, 2017 – 982,761) shares of common stock on an as-exchanged basis with respect to the shares of Exchangeco that can be exchanged for shares of common stock of the Company.
Six months ended December 31, 2017
During the six months ended December 31, 2017 the Company completed a registered direct offering (the “2018 Registered Offering”) of an aggregate of 8,000,000 shares of common stock and warrants to purchase an additional 8,000,000 shares of common stock at a price of $1.25 per share and related warrant for gross proceeds of $10.0 million. The warrants have an exercise price of $1.25 per share, are immediately exercisable and have a term of exercise of five years (the “2018 Investor Warrants”).
The Company engaged a placement agent for the 2018 Registered Offering. Under the Company’s engagement agreement with the placement agent, the Company paid $800,000 in cash commission and other fees to the placement agent and issued warrants to purchase 400,000 shares of common stock to the placement agent (the “2018 Agent Warrants”). The 2018 Agent Warrants are exercisable at a per share price of $1.25 and have a term of exercise of five years.
In addition to the cash commission and other placement agent fees, the Company also incurred additional cash issue costs of $254,664 resulting in net cash proceeds of $8,945,336.
2017 Omnibus Incentive Plan
On July 7, 2017, as amended on February 9, 2018, and subject to approval by the Company’s stockholders, the Company’s board of directors approved adoption of the Company’s 2017 Omnibus Equity Incentive Plan (the “2017 Plan”). The board of directors also approved a form of Performance Stock Unit Award Agreement to be used in connection with grants of performance stock units (“PSUs”) under the 2017 Plan.
| 14 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2017
(expressed in US dollars unless otherwise noted)
Under the 2017 Plan, 7,800,000 shares of Company common stock are reserved for issuance, less the number of shares of common stock issued under the Del Mar Pharmaceuticals (BC) Ltd. 2013 Amended and Restated Stock Option Plan (the “Legacy Plan”) or that are subject to grants of stock options made, or that may be made, under the Legacy Plan. A total of 1,420,850 shares of common stock have been issued under the Legacy Plan and/or are subject to outstanding stock options granted under the Legacy Plan, leaving a potential 6,379,150 shares of common stock available for issuance under the 2017 Plan if all such options under the Legacy Plan were exercised and no new grants are made under the Legacy Plan. The number of shares of Company common stock available for issuance under the 2017 Plan has been set at approximately 20% of the Company’s fully diluted shares of common stock as of February 9, 2018 (excluding the number of shares of common stock issued under the 2017 Plan and/or the Legacy Plan or subject to outstanding awards granted under the 2017 Plan and/or the Legacy Plan). The maximum number of shares of Company common stock with respect to which any one participant may be granted awards during any calendar year is 8% of the Company’s fully diluted shares of common stock on the date of grant (excluding the number of shares of common stock issued under the 2017 Plan and/or the Legacy Plan or subject to outstanding awards granted under the 2017 Plan and/or the Legacy Plan). No award will be granted under the 2017 Plan on or after July 7, 2027, but awards granted prior to that date may extend beyond that date.
Performance Stock Unit grants
Subject to approval by the Company’s stockholders of the 2017 Plan, the Company’s board of directors granted a total of 1,000,000 PSUs under the 2017 Plan to the Company’s independent directors. In total, the awards represent the right to receive an aggregate of 1,000,000 shares of the Company’s common stock upon vesting of the PSU based on targets approved by the Company’s board of directors related to the Company’s fully diluted market capitalization. The PSUs will vest in full upon the later of one year from the grant date and the Company achieving a fully diluted market capitalization of at least $500 million for five consecutive business days. The PSUs expire on July 7, 2022.
Stock Options (granted under the Legacy Plan)
The following table sets forth the stock options outstanding under the Legacy Plan:
| Number of stock options outstanding | Weighted average exercise price $ | ||||||||
| Balance – June 30, 2017 | 1,120,850 | 4.18 | |||||||
| Granted | 300,000 | 1.61 | |||||||
| Balance – December 31, 2017 | 1,420,850 | 3.64 | |||||||
| 15 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2017
(expressed in US dollars unless otherwise noted)
The following table summarizes stock options currently outstanding and exercisable at December 31, 2017 under the Legacy Plan:
| Exercise price $ | Number Outstanding at December 31, 2017 | Weighted average remaining contractual life (years) | Number exercisable at December 31, 2017 | ||||||||||
| 0.87 | 120,000 | 9.84 | 10,000 | ||||||||||
| 1.59 | 25,000 | 3.87 | 25,000 | ||||||||||
| 2.00 | 131,250 | 3.87 | 131,250 | ||||||||||
| 2.11 | 180,000 | 9.52 | - | ||||||||||
| 2.96 | 45,000 | 7.09 | 45,000 | ||||||||||
| 3.20 | 30,000 | 7.25 | 30,000 | ||||||||||
| 3.76 | 45,000 | 8.11 | 27,495 | ||||||||||
| 4.00 | 12,500 | 1.75 | 12,500 | ||||||||||
| 4.10 | 40,000 | 8.86 | 14,443 | ||||||||||
| 4.20 | 412,500 | 5.62 | 412,500 | ||||||||||
| 4.48 | 30,000 | 8.11 | 18,332 | ||||||||||
| 4.95 | 224,600 | 6.56 | 129,990 | ||||||||||
| 5.32 | 80,000 | 8.35 | 42,223 | ||||||||||
| 6.16 | 15,000 | 5.25 | 15,000 | ||||||||||
| 9.20 | 30,000 | 5.42 | 30,000 | ||||||||||
| 1,420,850 | 943,733 | ||||||||||||
Included in the number of stock options outstanding are 25,000 stock options granted at an exercise price of CDN $2.00. The exercise prices shown in the above table have been converted to $1.59 using the period ending closing exchange rate. Certain stock options have been granted to non-employees and will be revalued at each reporting date until they have fully vested. The stock options have been re-valued using a Black-Scholes pricing model using the following assumptions:
| December 31, 2017 | |||||
| Dividend rate | 0 | % | |||
| Volatility | 76.4% to 80.0 | % | |||
| Risk-free rate | 1.90% to 2.17 | % | |||
| Term - years | 1.0 to 2.0 | ||||
| 16 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2017
(expressed in US dollars unless otherwise noted)
The Company has recognized the following amounts as stock option expense for the periods noted:
| Three months ended December 31, | Six months ended December 31, | ||||||||||||||||
| 2017 | 2016 | 2017 | 2016 | ||||||||||||||
| $ | $ | $ | $ | ||||||||||||||
| Research and development | 126,375 | (65,727 | ) | 121,401 | (35,012 | ) | |||||||||||
| General and administrative | 102,132 | (9,475 | ) | 171,976 | (8,372 | ) | |||||||||||
| 228,507 | (75,202 | ) | 293,377 | (43,384 | ) | ||||||||||||
All of the stock option expense for the periods ended December 31, 2017 and 2016 has been recognized as additional paid in capital. The aggregate intrinsic value of stock options outstanding at December 31, 2017 was $26,400 (2016 - $208,846) and the aggregate intrinsic value of stock options exercisable at December 31, 2017 was $2,200 (2016 - $208,846). As of December 31, 2017, there was $257,895 in unrecognized compensation expense that will be recognized over the next 2.50 years. No stock options granted under the Plan have been exercised to December 31, 2017. Upon the exercise of stock options new shares will be issued.
A summary of status of the Company’s unvested stock options under the Legacy Plan is presented below:
| Number of Options | Weighted average exercise price $ | Weighted average grant date fair value $ | |||||||||||
| Unvested at June 30, 2017 | 318,033 | 4.81 | 2.57 | ||||||||||
| Granted | 300,000 | 1.61 | 0.86 | ||||||||||
| Vested | (140,916 | ) | 4.58 | 2.47 | |||||||||
| Unvested at December 31, 2017 | 477,117 | 2.87 | 1.54 | ||||||||||
Stock option modification
During the three and six months ended December 31, 2017, certain stock options were modified pursuant to a separation agreement with the Company’s former President and Chief Operating Officer. A total of 67,600 options had their vesting accelerated such that they became fully vested on December 22, 2017, resulting in additional stock option expense of $93,777. In addition, a total of 218,600 options were modified such that their remaining exercise period was increased from one year to three years, resulting in additional stock option expense of $28,561.
| 17 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2017
(expressed in US dollars unless otherwise noted)
Stock option liability
Certain of the Company’s stock options have been issued in $CDN. Of these, a portion were classified as a stock option liability which is revalued at each reporting date. During the six months ended December 31, 2016, the Company amended 43,750 of these stock options held by five optionees such that the exercise price of the options was adjusted to be denominated in $USD. No other terms of the stock options were amended. As a result of the amendment, the Company recognized $85,094 in stock option liability expense and $260,969 was reclassified to equity during the three months ended September 30, 2016.
Warrants
Certain of the Company’s warrants have been recognized as a derivative liability (note 5). The following table summarizes changes in the Company’s outstanding warrants as of December 31, 2017:
| Description | Number | ||||
| Balance – June 30, 2017 | 6,628,906 | ||||
| Issuance of 2018 Investor Warrants (i) | 8,000,000 | ||||
| Issuance of 2018 Agent Warrants (ii) | 400,000 | ||||
| Balance - December 31, 2017 | 15,028,906 | ||||
| i) | The 2018 Investor Warrants are exercisable at $1.25 per share until September 22, 2022. |
| ii) | The 2018 Agent Warrants are exercisable at $1.25 per share until September 20, 2022. |
The following table summarizes the Company’s outstanding warrants as of December 31, 2017:
| Description | Number | Exercise price $ | Expiry date | ||||||||
| 2017 Investor | 2,076,924 | 3.50 | April 19, 2022 | ||||||||
| 2013 Placement Agent | 1,262,500 | 3.14 | June 30, 2019 | ||||||||
| 2018 Investor | 8,000,000 | 1.25 | September 22, 2022 | ||||||||
| 2015 Investor | 979,003 | 3.00 | July 31, 2020 | ||||||||
| 2013 Investor – Amended | 778,504 | 3.14 | March 31, 2019 | ||||||||
| 2013 Investor – Un-amended (note 5) | 105,129 | 2.68 | January 25 to March 6, 2018 | ||||||||
| Dividend | 812,502 | 5.00 | January 24, 2018 | ||||||||
| Issued for services | 265,000 | 3.00 | March 1, 2020 to February 1, 2021 | ||||||||
| Issued for services | 43,750 | 7.04 | September 12, 2018 | ||||||||
| Issued for services | 41,400 | 5.93 | February 27, 2020 | ||||||||
| 2018 Agent | 400,000 | 1.25 | September 20, 2022 | ||||||||
| 2017 Agent | 138,462 | 4.06 | April 12, 2022 | ||||||||
| 2016 Agent | 103,964 | 4.00 | May 12, 2021 | ||||||||
| 2015 Agent | 21,768 | 3.00 | July 15, 2020 | ||||||||
| 15,028,906 | 2.25 | ||||||||||
| 18 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2017
(expressed in US dollars unless otherwise noted)
| 7 | Financial instruments |
The Company has financial instruments that are measured at fair value. To determine the fair value, we use the fair value hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use to value an asset or liability and are developed based on market data obtained from independent sources. Unobservable inputs are inputs based on assumptions about the factors market participants would use to value an asset or liability. The three levels of inputs that may be used to measure fair value are as follows:
| ● | Level one - inputs utilize quoted prices (unadjusted) in active markets for identical assets or liabilities; |
| ● | Level two - inputs are inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly such as interest rates, foreign exchange rates, and yield curves that are observable at commonly quoted intervals; and |
| ● | Level three - unobservable inputs developed using estimates and assumptions, which are developed by the reporting entity and reflect those assumptions that a market participant would use. |
Assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurements. Changes in the observability of valuation inputs may result in a reclassification of levels for certain securities within the fair value hierarchy.
The Company’s financial instruments consist of cash and cash equivalents, other receivables, accounts payable, related party payables and derivative liability. The carrying values of cash and cash equivalents, other receivables, accounts payable and related party payables approximate their fair values due to the immediate or short-term maturity of these financial instruments.
Derivative liability
The Company accounts for certain warrants under the authoritative guidance on accounting for derivative financial instruments indexed to, and potentially settled in, a company’s own stock, on the understanding that in compliance with applicable securities laws, the warrants require the issuance of securities upon exercise and do not sufficiently preclude an implied right to net cash settlement. The Company classifies these warrants on its balance sheet as a derivative liability which is fair valued at each reporting period subsequent to the initial issuance. The Company has used a simulated probability valuation model to value the warrants. Determining the appropriate fair-value model and calculating the fair value of warrants requires considerable judgment. Any change in the estimates (specifically probabilities) used may cause the value to be higher or lower than that reported. The estimated volatility of the Company’s common stock at the date of issuance, and at each subsequent reporting period, is based on the historical volatility of similar life sciences companies. The risk-free interest rate is based on rates published by the government for bonds with a maturity similar to the expected remaining life of the warrants at the valuation date. The expected life of the warrants is assumed to be equivalent to their remaining contractual term.
| 19 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2017
(expressed in US dollars unless otherwise noted)
| a) | Fair value of derivative liability |
The derivative is not traded in an active market and the fair value is determined using valuation techniques. The Company uses judgment to select a variety of methods to make assumptions that are based on specific management plans and market conditions at the end of each reporting period. The Company uses a fair value estimate to determine the fair value of the derivative liability. The carrying value of the derivative liability would be higher or lower as management estimates around specific probabilities change. The estimates may be significantly different from those recorded in the consolidated financial statements because of the use of judgment and the inherent uncertainty in estimating the fair value of these instruments that are not quoted in an active market. All changes in the fair value are recorded in the consolidated statement of operations and comprehensive loss each reporting period. This is considered to be a Level 3 financial instrument as volatility is considered a level 3 input.
The Company has the following liabilities under the fair value hierarchy:
| December 31, 2017 | |||||||||||||
| Liability | Level 1 | Level 2 | Level 3 | ||||||||||
| Derivative liability | - | - | $ | 5,549 | |||||||||
| June 30, 2017 | |||||||||||||
| Liability | Level 1 | Level 2 | Level 3 | ||||||||||
| Derivative liability | - | - | $ | 61,228 | |||||||||
| 8 | Supplementary statement of cash flows information |
| Six months ended | |||||||||
| December 31, | |||||||||
| 2017 | 2016 | ||||||||
| $ | $ | ||||||||
| Reclassification of derivative liability to equity upon the exercise of warrants (note 5) | - | 248,409 | |||||||
| Reclassification of derivative liability to equity upon the amendment of warrants (note 5) | - | 53,006 | |||||||
| Reclassification of stock option liability to equity upon settlement (note 6) | - | 260,969 | |||||||
| Series B Preferred share common stock dividend (note 6) | 95,732 | 467,054 | |||||||
| Income taxes paid | - | - | |||||||
| Interest paid | - | - | |||||||
| 20 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
December 31, 2017
(expressed in US dollars unless otherwise noted)
| 9 | Subsequent events |
Exercise of warrants
Subsequent to December 31, 2017, 250,000 share purchase warrants were exercised at $1.25 per share for gross proceeds of $312,500.
Issuance of warrants
Subsequent to December 31, 2017, 60,000 share purchase warrants were issued for services. Each is warrant exercisable at $1.78 per share until January 25, 2023.
Expiry of warrants
Subsequent to December 31, 2017, 812,502 share purchase warrants exercisable at $5.00 per share and 21,564 share purchase warrants exercisable at $2.68 expired.
| 21 |
Item 2. Management's Discussion and Analysis of Financial Condition and Results of Operations.
This Management’s Discussion and Analysis (“MD&A”) contains “forward-looking statements”, within the meaning of the Private Securities Litigation Reform Act of 1995, which represent our projections, estimates, expectations, or beliefs concerning, among other things, financial items that relate to management’s future plans or objectives or to our future economic and financial performance. In some cases, you can identify these statements by terminology such as “may”, “should”, “plans”, “believe”, “will”, “anticipate”, “estimate”, “expect” “project”, or “intend”, including their opposites or similar phrases or expressions. You should be aware that these statements are projections or estimates as to future events and are subject to a number of factors that may tend to influence the accuracy of the statements. These forward-looking statements should not be regarded as a representation by the Company or any other person that the events or plans of the Company will be achieved. You should not unduly rely on these forward-looking statements, which speak only as of the date of this report. Except as may be required under applicable securities laws, we undertake no obligation to publicly revise any forward-looking statement to reflect circumstances or events after the date of this report or to reflect the occurrence of unanticipated events.
You should review the factors and risks we describe under “Risk Factors” in our report on Form 10-K for the year ended June 30, 2017 and the Company’s other filings with the Securities and Exchange Commission (the “SEC”), available at www.sec.gov. Actual results may differ materially from any forward-looking statement.
Changing Landscape and Recent Highlights
| ● | We believe the evaluation of MGMT promotor methylation status has increasingly become common practice in the diagnostic assessment of GBM providing us with an enhanced ability to leverage MGMT methylation as a biomarker to optimize patient selection for our novel DNA-targeting agent in the treatment of GBM. |
| ● | In September 2017, the National Comprehensive Cancer Network (“NCCN”), provided updated guidelines for the standard treatment of glioblastoma multiforme (“GBM”) based on MGMT methylation status. We believe these recently published guidelines provide for enhanced opportunities for us to capitalize on VAL-083’s unique mechanism of action to target the majority of GBM patients who are diagnosed with MGMT-unmethylated tumors. |
| ● | In September 2017, the US Food and Drug Administration (“FDA”) allowed a second Investigational New Drug Application ("IND") for our lead drug candidate, VAL-083, as a potential treatment for platinum-resistant ovarian cancer. |
| ● | In September 2017, we completed an offering of common stock and warrants for aggregate gross proceeds of $10.0 million. We intend to use the proceeds to support our research, clinical trials and for general corporate purposes. |
| ● | In October 2017, we announced: |
| o | The first patient dosing for our STAR-3 registration study for refractory patients with recurrent GBM (“rGBM”). This was a part of a planned U.S.-based 180 patient, 25 site study initiated in July 2017; and |
| o | The first patient dosing of our open label Phase 2 clinical trial of VAL-083 in newly diagnosed patients with MGMT-unmethylated GBM, which is being conducted at Sun Yat-sen University Cancer Center with funding support through our collaboration with Guangxi Wuzhou Pharmaceutical (Group) Co. Ltd. |
| ● | In November 2017, at the annual meeting of the Society for NeuroOncology (“SNO”) we presented a positive interim update from our ongoing open label Phase 2 clinical trial in patients with MGMT-unmethylated rGBM whose tumors have recurred following treatment with temozolomide (Avastin naïve). This study was initiated in February 2017 and is being conducted at the University of Texas MD Anderson Cancer Center. |
| 22 |
| ● | In December 2017, the FDA fully approved Avastin (bevacizumab) which may impact our ability to recruit suitable patients for our STAR-3 Phase 3 clinical trial. |
| ● | In December 2017, the FDA granted Fast Track designation for the company's lead product candidate, VAL-083, in recurrent glioblastoma. Fast Track designation is designed to expedite the review of drugs that show promise in treating life-threatening diseases and address unmet medical needs, with the goal of getting new treatments to patients earlier. Fast Track designation provides sponsors with an opportunity for increased frequency of communication with the FDA to ensure an optimal development plan and to collect appropriate data needed to support drug approval. |
| ● | Update on clinical trial status as of December 31, 2017: |
| ○ | Phase 3 STAR-3 Refractory trial: 4 sites activated with one (1) patient enrolled in our registration study for rGBM patients. As further described below, we have decided to suspend further enrollment in this trial until a full analysis of the potential impact of the Avastin approval can be undertaken. |
| ○ | Phase 2 MGMT-unmethylated rGBM: 17 patients have been enrolled in our ongoing open label Phase 2 clinical trial in patients with MGMT-unmethylated (Avastin naïve) rGBM. Patients in this trial have rGBM following standard-of-care chemo-radiation treatment with temozolomide. This study was initiated in February 2017 and is designed to enroll up to 48 patients and is being conducted in collaboration with the University of Texas MD Anderson Cancer Center (“MDACC”); and |
| ○ | Phase 2 trial in MGMT-unmethylated newly diagnosed GBM: One (1) patient has been enrolled in our single site, open label Phase 2 clinical trial of newly diagnosed MGMT-unmethylated GBM patients. Patients in this trial will be treated with VAL-083 in combination with radiotherapy as a potential alternative to the current standard-of-care chemo-radiation regimen. This study was initiated in September 2017, and is designed to enroll up to 30 patients at Sun Yat-sen University Cancer Center in Guangzhou, China and is being conducted under the terms of our collaboration with Guangxi Wuzhou Pharmaceutical (Group) Co. Ltd. |
| ● | During the three months ended December 31, 2017, we strengthened our management team by appointing Saiid Zarrabian as interim president and chief executive officer. Mr. Zarrabian’s experience in overseeing the growth of multiple companies will augment our management team as we continue to efficiently advance our product candidates to maximize shareholder value. |
| ● | During the three months ended December 31, 2017, we presented promising research results supporting the potential of VAL-083 in the treatment of cancers for patients whose tumors exhibit features that make them resistant to, or unlikely to respond to, currently available therapies. For example: |
| ● | At the American Association for Cancer Research (“AACR”)-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics we presented incremental VAL-083 data suggesting the potential for synergy with treatments that depend on a cancer cell to be in the S-phase for activity. Such agents include topoisomerase inhibitors, commonly used in the treatment of brain cancer and other solid tumors, and PARP inhibitors, commonly used in the treatment of platinum resistant ovarian cancer; and |
| ● | At AACR’s Special Conference: Addressing Critical Questions in Ovarian Cancer Research and Treatment, we presented data demonstrating how VAL-083 targets the DNA of cancer cells in a mechanistically different fashion than platinum (Pt)-based chemotherapeutic agents or PARP inhibitors, and how these differences position VAL-083 as a potential new therapeutic option in the treatment of platinum-resistant ovarian cancer, a significant and well recognized unmet medical need. |
| ● | Based on our updated strategy, as described below, we believe we have cash available to fund operations into the second quarter of calendar 2019. |
| 23 |
VAL-083 Clinical Trials and Updated Strategic Direction
Our recent research has highlighted the opportunities afforded by VAL-083’s unique mechanism of action and its potential to address unmet medical needs by focusing our development efforts on patients whose tumors exhibit biological features that make them resistant to, or unlikely to respond to, currently available therapies. For example, our research demonstrating VAL-083’s activity in GBM independent of the expression of the MGMT methylation status allows us to focus patient selection based on this important biomarker. Our current priority is to leverage this research and VAL-083’s unique mechanism of action to efficiently advance our drug candidate for the most promising indications, including:
| ● | MGMT-unmethylated GBM, currently comprising two ongoing separate Phase 2 clinical trials for: |
| ○ | rGBM patients (ongoing study at MDACC); and |
| ○ | Newly diagnosed GBM patients (ongoing study at Sun Yat-sen University); and |
| ● | Platinum-resistant ovarian cancer; and | |
| ● | Undertaking an assessment of the full approval of Avastin on our STAR-3 program and patient selection criteria in order to reach a formal decision on the future of this program within the next 12 months. |
Phase 3: VAL-083 STAR-3 GBM Trial
In July 2017, we initiated our VAL-083 STAR-3 GBM trial as an adaptive, randomized, controlled pivotal Phase 3 clinical trial in patients with GBM whose tumor has progressed following treatment with Avastin (bevacizumab).
Based on a number of factors, including low patient enrollment to-date, and our belief that the recent full approval of Avastin for rGBM may negatively impact the timely recruitment of suitable patients for this trial, we have made the decision to park this trial for up to 12 months while we undertake a detailed assessment of the trial. We are suspending additional enrollment in the STAR-3 trial until:
| ● | Further information is available regarding the potential impact of the recent FDA approval of Avastin on this patient population; |
| ● | Further data is available from our ongoing, open label clinical trials in MGMT-unmethylated GBM; |
| ● | We have evaluated whether possible protocol amendments designed to increase patient enrollment can be implemented without negatively impacting our ability to recruit subjects with the chance for a measurable clinical benefit following treatment; and/or |
| ● | We potentially find a partner to support the costs of the STAR-3 trial. |
During this interim evaluation period, we will continue to provide treatment to patients already enrolled in the STAR-3 trial but we will not be recruiting further patients for this trial. During this interim period, we will also consider, on a case-by-case basis, and subject to required institutional and regulatory approvals, providing VAL-083 to patients in this population in accordance with our expanded access policy.
| 24 |
A detailed description of the STAR-3 trial and DelMar’s expanded access program can be found at clinicaltrials.gov, Identifier Number: NCT03149575 and NCT03138629, respectively.
MGMT-unmethylated GBM
GBM is the most common and the most lethal form of glioma. According to the World Health Organization, GBM occurs with an incidence of 3.17 per 100,000 person-years. Approximately 18,000 new cases of GBM are expected to be diagnosed in the United States and 26,000 in Europe during 2017.
Measurement of MGMT methylation status has become routine in clinical practice as a biomarker that correlates with resistance to the standard-of-care chemotherapy with temozolomide (Temodar® “TMZ”), and patient outcomes in GBM. The majority of GBM patient’s tumors are characterized as “MGMT-unmethylated” and exhibit a high expression of O6-methyl guanine methyltransferase (“MGMT”), a naturally occurring DNA-repair enzyme, the activity of which nullifies the chemotherapeutic activity of TMZ. The development of new therapies for MGMT-unmethylated GBM is a significant unmet medical need. Importantly, the most recent update to NCCN guidelines states that the treatment benefit of TMZ is likely to be lower in GBM patients with an unmethylated MGMT promoter and therefore allows for withholding of TMZ in the treatment of newly diagnosed GBM patients with MGMT-unmethylated tumors due to lack of efficacy.
We have demonstrated that VAL-083’s anti-tumor mechanism is active independent from the MGMT status in vitro. We believe this suggests the potential of VAL-083 as a replacement for the current standard-of-care chemotherapy, temozolomide, in MGMT-unmethylated GBM. We are therefore utilizing MGMT-methylation status to identify GBM patients who are unlikely to respond to temozolomide and instead treat them with VAL-083.
We believe that our research, in the context of the recent amendment to NCCN guidelines, highlights this unmet need and the opportunity for VAL-083 as a potential new standard-of-care in the treatment of MGMT-unmethylated GBM.
Phase 2 Study in MGMT-unmethylated rGBM in Collaboration with University of Texas MD Anderson Cancer Center
In February 2017, we initiated a biomarker driven, open-label, single-arm Phase 2 study in collaboration with the University of Texas MD Anderson Cancer Center. This trial will enroll up to 48 MGMT-unmethylated GBM patients whose tumors have recurred following treatment with temozolomide. These patients will not have been treated previously with Avastin.
The primary endpoint of the trial is overall survival. Safety data from this trial will become part of the overall safety dossier to support future filings with the FDA and other regulatory agencies.
As of December 31, 2017, seventeen (17) patients had been enrolled in this trial. We believe a positive outcome from this trial will establish a strong position for VAL-083 in the treatment of MGMT-unmethylated GBM.
Data from the trial will be used to help form potential future clinical trial designs with VAL-083 in MGMT-unmethylated rGBM. We anticipate providing updates regarding the progress of this trial, including safety data and observations regarding outcomes, at scientific meetings during 2018. A detailed description of this trial can be found at clinicaltrials.gov, Identifier Number: NCT03050736.
Phase 2 Trial in Newly Diagnosed MGMT-unmethylated GBM
In September 2017, we initiated a single arm, biomarker driven, open-label Phase 2 study in newly diagnosed MGMT-unmethylated GBM patients at Sun Yat-sen University Cancer Center in Guangzhou, China. The trial is being conducted in the context of our 2012 collaboration agreement with Guangxi Wuzhou Pharmaceutical (Group) Co. Ltd. Under the terms of this agreement, Guangxi Wuzhou Pharmaceutical (Group) Co. Ltd. is responsible for funding VAL-083 clinical trials that we conduct in China.
| 25 |
In this study, VAL-083 is combined with radiotherapy as a potential replacement for standard-of-care chemoradiation with temozolomide in patients with MGMT-unmethylated GBM. The main goal of the trial will be to confirm the safety of DelMar’s optimized dosing regimen in combination with radiotherapy and to investigate outcomes of the combination of VAL-083 and radiotherapy in MGMT-unmethylated GBM patients.
We plan to enroll up to 30 newly diagnosed MGMT-unmethylated GBM patients in this trial. The primary efficacy endpoint is the determination of tumor response in patients measured by progression free survival (“PFS”). Assessments of safety and tolerability will be used to support further clinical development of VAL-083 in combination with radiotherapy. Pharmacokinetic assessments of VAL-083 in plasma and cerebral spinal fluid (“CSF”) will be used to correlate drug exposure in the central nervous system with patient outcomes.
We plan to use data from the trial to establish a dosing regimen and trial design for advanced registration-directed clinical trials with VAL-083 in newly diagnosed MGMT-unmethylated GBM.
We anticipate providing updates regarding the progress of this trial, including safety data and observations regarding outcomes, at scientific meetings during 2018. A detailed description of this trial can be found at clinicaltrials.gov, Identifier Number: NCT02717962.
Ovarian Cancer Summary
Ovarian cancer is the fifth most common cancer in women and is the leading cause of death among women diagnosed with gynecological malignancies. In 2016, approximately 22,300 women in the US were diagnosed with ovarian cancer and 14,300 died from their disease.
Platinum-based chemotherapy is the standard-of-care in the treatment of advanced ovarian cancer. Ovarian cancer patients whose tumors are sensitive to Pt-based chemotherapy have the most favorable outcome. Recently, the approval of PARP inhibitors in the treatment of ovarian cancer patients demonstrated improved outcomes, particularly in patients whose tumors remain sensitive to Pt-based treatments.
Unfortunately, the development of resistance to Pt-based agents is nearly inevitable, leading to disease recurrence and increased mortality. Ultimately, most women with advanced ovarian cancer develop recurrent disease with progressively shorter disease-free intervals. Those whose tumors recur within 6 months of Pt-based therapy are considered Pt-resistant/refractory and have a very poor prognosis.
Currently, there are no high-efficacy therapeutic options for Pt-resistant ovarian tumors, leaving these cancer patients with a very poor prognosis. The response rate to second line therapy for Pt-resistant ovarian cancer patients is in the 10-15% range and overall survival is approximately 12 months. The development of new chemotherapies and targeted agents to overcome Pt resistance in ovarian cancer is a significant unmet medical need.
Phase 1-2 Study in Platinum-resistant Ovarian Cancer
In April 2016, the FDA granted orphan drug designation for the use of VAL-083 in the treatment of ovarian cancer.
In September 2017, we received notice of allowance from the FDA of an IND to initiate a Phase 1/2, open-label, multicenter, study of VAL-083 in patients with Recurrent Platinum Resistant Ovarian Cancer (the REPROVe trial).
The Phase 1 portion of the trial will enroll approximately 24 patients with Pt-resistant ovarian cancer to evaluate the response to treatment with VAL-083.
Ovarian cancer patients enrolled in the trial will have been previously treated with at least two lines of Pt-based chemotherapy and up to two other cytotoxic regimens, and whose cancer has recurred within 6 months of prior Pt-based chemotherapy.
The primary efficacy of the trial will be overall response rate (“ORR”) based on Response Evaluation Criteria in Solid Tumors (RECIST) criteria. RECIST is a set of published rules that define when tumors in cancer patients improve (“respond”), stay the same (“stabilize”), or worsen (“progress”) during treatment.
We plan to request a meeting with FDA following completion of the Phase 1 portion of the REPROVe trial. If successful, data from this trial may lead to a confirmatory Phase 2 study of approximately 60 patients, which if successful, and subject to feedback from the FDA, may position us to potentially file an application for accelerated approval or to advance to a pivotal Phase 3 trial.
| 26 |
Subject to negotiating acceptable clinical research agreements and budgets with clinical investigators and their institutions and obtaining IRB approvals we anticipate initiating the REPROVe trial in the first half of calendar 2018.
A detailed description of the REPROVe trial can be found at clinicaltrials.gov, Identifier Number: NCT03281681.
VAL-083 Overview
Our lead product candidate, VAL-083, is a first-in-class small molecule chemotherapeutic. “First-in-class” means that VAL-083 embodies a unique molecular structure of VAL-083, which is not an analogue or derivative of any approved product, or product under development for the treatment of cancer. Prior VAL-083 clinical trials supported by the US National Cancer Institute (“NCI”) demonstrated activity against a range of cancers including lung, brain, cervical, ovarian tumors, and leukemia. As part of our business strategy, we leverage and build upon these prior NCI investments and data from more than 40 NCI- Phase 1 and Phase 2 clinical trials with our own research to identify and target unmet medical needs in modern cancer care.
DNA-targeting agents are among the most successful and widely used treatments for cancer. Their efficacy is based on the ability to bind with cancer cell’s DNA and interfere e with the process of protein production required for growth and survival of cancer cells.
Our research demonstrates the unique mechanism of action of VAL-083 is distinct from other DNA-targeting agents. VAL-083 exhibits its anti-cancer activity by forming DNA-cross links at the N7 position of guanine leading to DNA double strand breaks, cell-cycle arrest, and cancer cell death. We have presented research at peer-reviewed scientific meetings demonstrating that VAL-083 is active in patient-derived tumor cell lines and cancer stem cells that are resistant to other chemotherapies. These data, combined with clinical activity demonstrated against various cancers in prior NCI-sponsored clinical trials enhance our confidence that VAL-083 may offer an opportunity as a new therapeutic option for patients whose tumors exhibit biological features that cause them to be resistant or unlikely to respond to currently available treatments.
We are currently studying VAL-083 in multiple clinical trials for the treatment of GBM, the most common and aggressive form of brain cancer. We have also received notice of allowance from the FDA for an IND to initiate clinical trials with VAL-083 in the treatment of platinum-resistant ovarian cancer.
The FDA Office of Orphan Products Development has granted orphan drug designations to VAL-083 for the treatment of glioma, ovarian cancer and medulloblastoma. VAL-083 has also been granted an orphan drug designation for the treatment of glioma in Europe. Orphan diseases are defined in the United States under the Rare Disease Act of 2002 as “any disease or condition that affects fewer than 200,000 persons in the United States”. The Orphan Drug Act of 1983 is a federal law that provides financial and other incentives including a seven-year period of market exclusivity in the United States to encourage the development of new treatments for orphan diseases.
Fast Track Designation
In December 2017, the FDA granted Fast Track designation for our lead product candidate, VAL-083, in rGBM.
Fast Track designation is designed to expedite the review of drugs that show promise in treating life-threatening diseases and address unmet medical needs, with the goal of getting new treatments to patients earlier. Fast Track designation provides sponsors with an opportunity for increased frequency for communication with the FDA to ensure an optimal development plan and to collect appropriate data needed to support drug approval. Additional benefits of the Fast Track designation may include an Accelerated Approval, a Priority Review, and a Rolling Review. Accelerated Approval is granted to drugs that demonstrate an effect on a surrogate, or intermediate endpoints, reasonably likely to predict clinical benefit. Priority Review shortens the FDA review process for a new drug from ten months to six months and is appropriate for drugs that demonstrate significant improvements in both safety and efficacy of an existing therapy. Rolling Review provides a drug company the opportunity to submit completed sections of its New Drug Application (“NDA”) for review by the FDA. Typically, NDA reviews do not commence until the drug company has submitted the entire application to the FDA. Through the Fast Track designation, the FDA attempts to ensure that questions raised during the drug development process are resolved quickly, often leading to earlier approval and increased access for patients.
| 27 |
VAL-083 Mechanism of Action and the Opportunity in the Treatment of Cancer
Chemotherapy forms the basis of treatment in nearly all cancers. We believe that VAL-083 may be effective in treating tumors exhibiting biological features that cause resistance to currently available chemotherapy, particularly for patients who have failed, or become resistant to, other treatment regimens.
Based on published research and our own data, the cytotoxic functional groups, and the mechanism of action of VAL-083 are functionally different from alkylating agents commonly used in the treatment of cancer. VAL-083 has previously demonstrated activity in cell-lines that are resistant to other types of chemotherapy. No evidence of cross-resistance has been reported in published clinical studies.
Our research suggests that VAL-083 attacks cancer cells via a unique mechanism of action which is distinct from other chemotherapies used in the treatment of cancer. Our data indicate that VAL-083 forms interstrand crosslinks at the N7 position of guanine on the DNA of cancer cells. Our data also indicate that this crosslink forms rapidly and is not easily repaired by the cancer cell resulting in cell-cycle arrest and lethal double-strand DNA breaks in cancer cells. VAL-083 readily crosses the blood brain barrier. Published preclinical and clinical research demonstrate that VAL-083 is absorbed more readily in tumor cells than in normal cells.
In vitro, our data also demonstrate that VAL-083’s distinct mechanism may be able to overcome drug resistance against a range of cancers. For example, VAL-083 is active against MGMT-unmethylated GBM cells which are resistant to treatment with temozolomide and nitrosoureas. VAL-083 also retains a high level of activity in p53 mutated non-small cell lung cancer (“NSCLC”), ovarian cancer and medulloblastoma cell lines that are resistant to platinum-based chemotherapy.
Importantly, clinical activity against each of the tumors mentioned above was established in prior NCI-sponsored Phase 2 clinical trials. We believe that these historical clinical data and our own research support the development of VAL-083 as a potential new treatment for multiple types of cancer.
The main dose-limiting toxicity (“DLT”) related to the administration of VAL-083 in previous NCI-sponsored clinical studies and our own clinical trials is myelosuppression, particularly thrombocytopenia. Myelosuppression and thrombocytopenia are common side effects of chemotherapy. Myelosuppression is the decrease in cells responsible for providing immunity, carrying oxygen, and causing normal blood clotting. Thrombocytopenia is a reduction in platelet counts, which assist in blood clotting. Modern medicine allows for better management of myelosuppressive side effects. We believe this offers the potential opportunity to improve upon the drug’s already established efficacy profile by substantially increasing the dose of VAL-083 that can be safely administered to cancer patients.
There is no evidence of lung, liver, or kidney toxicity even with prolonged treatment by VAL-083. Data from the Chinese market where the drug has been approved for more than 15 years supports the safety findings of the NCI studies.
Gliomas and Glioblastoma Multiforme (“GBM”)
Gliomas are a type of Central Nervous System (“CNS”) tumor that arises from glial cells in the brain or spine. Glial cells are the cells surrounding nerves. Their primary function is to provide support and protection for neurons in the CNS.
GBM is the most common and the most lethal form of glioma. According to the World Health Organization, GBM occurs with an incidence of 3.17 per 100,000 person-years. Approximately 18,000 new cases of GBM are expected to be diagnosed in the United States and 26,000 in Europe during 2017.
Common symptoms of GBM include headaches, seizures, nausea, weakness, paralysis and personality or cognitive changes such as loss of speech or difficulty in thinking clearly. GBM progresses quickly and patients’ conditions deteriorate rapidly progressing to death. The outlook for GBM patients is generally poor. The median survival in newly diagnosed patients with best available treatments is less than 15 months, and one-year and five-year survival rates are approximately 25% and less than 3%, respectively.
| 28 |
In September 2017, the National Comprehensive Cancer Network (“NCCN”), updated treatment guidelines for GBM. The recommended treatment regimen for GBM includes surgical resection to remove as much of the tumor as possible (“debulking”) followed by radiotherapy with concomitant and adjuvant chemotherapy with temozolomide with or without tumor treating fields (“TTF”). GBM patients whose tumors exhibit an unmethylated promotor for the gene encoding the DNA enzyme O6-DNA methylguanine methyl-transferase (“MGMT”), a biomarker correlated with resistance to temozolomide, may be treated with radiation alone following surgery.
Patients with an unmethylated MGMT promotor have high levels of MGMT, a naturally-occurring DNA repair enzyme that repairs tumor-fighting lesions induced by TMZ thus allowing a patient’s tumor to continue to grow despite treatment which leads to poor outcomes. Measurement of MGMT methylation status has become routine in clinical practice as biomarker that correlates with response to TMZ and patient outcomes in GBM.
Probability of GBM Patient Survival Correlated to Expression of MGMT Enzyme
(Unmethylated promoter = High MGMT Expression and Significantly Shorter Survival)
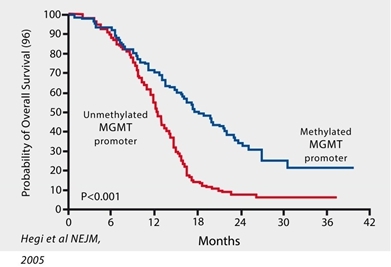
TTF (Optune®) is a non-invasive technique for adults with GBM. TTF uses alternating electrical fields to disrupt tumor cell division, or cause cell death, thereby preventing the tumor from growing or spreading as quickly. A clinical trial reported that GBM patients treated with TTF combined with TMZ experienced longer survival than those treated with TMZ alone.
The majority of GBM patients’ tumors recur within 6 – 12 months of initial treatment. Experimental therapy through clinical trials is recommended under NCCN guidelines for eligible patients. NCCN guidelines also recommend treatment with systemic chemotherapy, such as lomustine (“CCNU”). For patients who are eligible for additional surgical debulking, local chemotherapy with carmustine (“BCNU”) wafers may be employed. CCNU and BCNU target the same DNA-site as TMZ and are also subject to MGMT-related resistance.
Avastin (Avastin®, an anti-VEGF antibody) recently received full FDA approval as a single agent for patients with recurrent GBM following prior therapy in the US, Canada, Australia, and Japan. Avastin carries an FDA “black-box warning” related to severe, sometimes fatal, side effects such as gastrointestinal perforations, wound healing complications and hemorrhage. There are no data demonstrating an improvement in disease-related symptoms or increased survival for GBM patients treated with Avastin.
| 29 |
Recurrent GBM patients, especially those whose tumors progress following treatment with Avastin, have limited or no treatment options and a very poor prognosis. According to published literature, the median survival for GBM patients whose tumors progress following Avastin is less than four months.
VAL-083 Historical Data and Our Research in GBM
VAL-083 is first-in-class DNA targeting agent that readily crosses the blood-brain-barrier. Data from prior NCI-sponsored clinical trials with VAL-083 demonstrate activity against GBM and other CNS tumors. In general, historical NCI-sponsored trials demonstrate that tumor regression in brain cancer was achieved in 40% of patients treated and stabilization was achieved in an additional 20% to 30% of brain tumor patients following treatment with VAL-083.
VAL-083 demonstrated statistically significant improvement in the median survival of high grade glioma brain tumors, including GBM when combined with radiation versus radiation alone (p < 0.05) with results similar, or superior to, other chemotherapies approved for the treatment of GBM.
A Summary of Published Data adapted from Separate Sources Comparing the Efficacy of VAL-083
and Other Therapies in the Treatment of GBM
| Comparative Therapy | Median Survival Benefit vs. | |||||
| Chemotherapy | Radiation (XRT) Alone | Radiation + Chemotherapy | XRT alone | |||
VAL-083 (Eagan 1979) |
8.4 months | 16.8 months | 8.4 months | |||
Temozolomide (Temodar®) (Stupp 2005) |
12.1 months | 14.6 months | 2.5 months | |||
| Lomustine
(CCNU) (Walker 1976) |
11.8 months | 13 months | 1.2 months | |||
| Carmustine
(BCNU) (Reagan 1976) |
10 months | 12.5 months | 2.5 months | |||
| Semustine
(ACNU) (Takakura 1986) |
12 months | 14 months | 2.0 months | |||
VAL-083 is Active Independent of MGMT
We have presented data at several peer reviewed meetings demonstrating that VAL-083 is active independent of MGMT resistance in GBM cell lines and other CNS tumor cells. Our research, along with that of others, demonstrates that VAL-083’s unique cytotoxic mechanism forms DNA cross-links at the N7 position of guanine and retains cytotoxic activity independent of MGMT expression in vitro. Our studies demonstrate that VAL-083 has more potent activity against brain tumor cells in comparison to TMZ and overcomes resistance associated with MGMT, suggesting the potential to surpass the current standard-of-care in the treatment of GBM.
| 30 |
A
Summary of Our Data Demonstrating that VAL-083’s Anti-Tumor Mechanism is Distinct from, and can
Overcome, MGMT-Related Chemo resistance in the Treatment of GBM
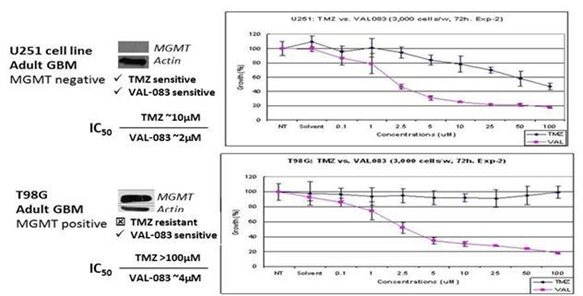
In addition, historical NCI clinical trial data and our own research support the activity of VAL-083 as a potentiator of radiotherapy. Radiotherapy in combination with temozolomide is the current standard of care in the treatment of newly diagnosed GBM. Our research demonstrates that temozolomide and radiotherapy are ineffective against GBM cells exhibiting a high expression of MGMT, whereas VAL-083 potentiates the tumor-killing effect of radiation independent of MGMT expression. Furthermore, the combination of VAL-083 and radiation has been demonstrated to be active against GBM cancer stem cells (“CSCs”) in vitro. CSCs are often resistant to chemotherapy and form the basis for tumor recurrence and metastasis. GBM CSCs display strong resistance to TMZ, even where MGMT expression is low. However, our data demonstrates that GBM CSCs are susceptible to VAL-083 independent of MGMT expression.
| 31 |
A Summary of Our Data Demonstrating that VAL-083 Maintains Activity in Both Temozolomide-resistant GBM Cell Lines and Matched Cancer Stem Cells and Potentiates Radiotherapy
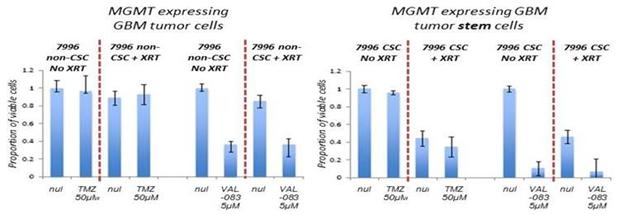
We believe that VAL-083’s more potent activity against brain tumor cells in comparison to TMZ, VAL-083’s ability to overcome MGMT-mediated resistance, and its activity against GBM CSCs suggests the potential of VAL-083 to surpass the current standard-of-care in the treatment of GBM.
Phase 1 – 2 Clinical Trial Overview and Summary of Results
Forty-eight GBM patients whose disease has progressed following prior treatment with temozolomide and Avastin were enrolled in an open-label, single arm dose-escalation study designed to evaluate the safety, tolerability, pharmacokinetics, and anti-cancer activity of VAL-083. The trial was conducted at five centers in the United States: The Mayo Clinic in Rochester, Minnesota; the Brain Tumor Center at University of California, San Francisco; the Sarah Cannon Cancer Research Center in Nashville, Tennessee, Denver, Colorado; and the SCRI affiliate site at the Florida Cancer Specialist Research Institute in Sarasota, Florida.
Patients received VAL-083 on days 1, 2 and 3 on a 21-day treatment cycle. The Phase 1 portion of the study involved dose escalation cohorts until a maximum tolerated dose (“MTD”) was established at 40mg/m2. A further 14-patient, Phase 2 expansion was then enrolled at the MTD to gather further safety data at our chosen therapeutic dose and to further explore the outcomes in this patient population.
In May 2016, we held an end of Phase 2 meeting with the FDA in which we discussed with the FDA the design of a Phase 3, registration-directed clinical program for VAL-083 in refractory GBM. Based on the input we received from the FDA, the agency confirmed that it would consider the totality of data available, including data obtained from DelMar’s other planned clinical trials in related GBM populations, when assessing the NDA. The FDA also noted that DelMar can rely on prior NCI studies and historical literature to support nonclinical data required for an NDA filing under a 505(b)(2) strategy which allows a sponsor to rely on already established safety and efficacy data in support of an NDA.
We reported updated results of our Phase 1/2 clinical trial at the 2016 ASCO annual meeting. In summary, these data are as follows:
Safety and Tolerability
In the Phase 1 dose escalation regimen, no serious adverse events (“SAE”) related to VAL-083 were encountered at doses up to 40 mg/m2/day.
Increasing frequency of, and higher grade, hematologic toxicities were observed at doses above 40 mg/m2/day. Consistent with the published literature, the observed dose limiting toxicity for VAL-083 is primarily thrombocytopenia (low platelets). Observed platelet nadir occurred at approximately day 18, and recovery was rapid and spontaneous following treatment.
Based on Phase 1 observations, fourteen additional patients were enrolled in a Phase 2 expansion cohort at 40mg/m2, which was established as the MTD. Consistent with Phase 1, the dose of VAL-083 of 40 mg/m2 on days 1, 2 and 3 of a 21-day cycle was generally well tolerated in Phase 2. At this dose, one subject previously treated with CCNU, a nitrosourea agent, reported severe (Grade 4) thrombocytopenia. As a result of this observation, the protocol inclusion criterion for platelet count was increased from 100,000/μL to 150,000/μL for patients receiving prior nitrosoureas within 12 weeks preceding enrollment. No other dose limiting toxicities were observed.
| 32 |
VAL-083 Safety Observations from Phase 1/2 Clinical Trial
| Hematologic parameter and CTCAE grade | dose | ≤30 mg/m2 | 40 mg/m2 | 45 mg/m2 | 50 mg/m2 | |||||||||||||||||||||||||||||
| n = | 20 | 17 | 4 | 7 | ||||||||||||||||||||||||||||||
| Anemia | ≤G2 | 11 | 55 | % | 2 | 12 | % | 2 | 50 | % | 6 | 86 | % | |||||||||||||||||||||
| G3 | 2 | 10 | % | - | 0 | % | - | 0 | % | - | 0 | % | ||||||||||||||||||||||
| G4 | - | 0 | % | - | 0 | % | - | 0 | % | - | 0 | % | ||||||||||||||||||||||
| Leukopenia | ≤G2 | 5 | 25 | % | 2 | 12 | % | - | 0 | % | 5 | 71 | % | |||||||||||||||||||||
| G3 | 1 | 5 | % | - | 0 | % | - | 0 | % | 3 | 43 | % | ||||||||||||||||||||||
| G4 | - | 0 | % | - | 0 | % | 2 | 50 | % | - | 0 | % | ||||||||||||||||||||||
| Neutropenia | ≤G2 | 4 | 20 | % | - | 0 | % | - | 0 | % | - | 0 | % | |||||||||||||||||||||
| G3 | - | 0 | % | - | 0 | % | - | 0 | % | 3 | 43 | % | ||||||||||||||||||||||
| G4 | - | 0 | % | - | 0 | % | 2 | 50 | % | 1 | 14 | % | ||||||||||||||||||||||
| Thrombocytopenia | ≤G2 | 9 | 45 | % | 3 | 18 | % | - | 0 | % | 3 | 43 | % | |||||||||||||||||||||
| G3 | - | 0 | % | - | 0 | % | 1 | 25 | % | 3 | 43 | % | ||||||||||||||||||||||
| G4 | - | 0 | % | 1 | 6 | % | 2 | 50 | % | 1 | 14 | % | ||||||||||||||||||||||
| DLT Observed | nil | 1 | 2 | 2 | ||||||||||||||||||||||||||||||
Doses Achieved
We confirmed that we achieved doses of VAL-083 that are substantially higher than were utilized in the original published NCI-sponsored clinical trials. A summary in comparison to the NCI’s historical regimen is as follows:
Dosing Regimen & Study |
Single Dose | Acute Regimen (single cycle) | Comparative Cumulative Dose (@ 35 days) |
Dose Intensity (dose per week) | ||||||||||
NCI GBM historical regimen (Eagan et al) daily x 5 q 5wks (cycle = 35 days) |
25 mg/m2 | x5 days = | 125 mg/m2 | 125 mg/m2 | 25 mg/m2/wk. | |||||||||
| DelMar VAL-083 optimized regimen daily x 3 q 3wks (cycle = 21 days) | 40 mg/m2 | x3 days = | 120 mg/m2 | 240 mg/m 2 | 40 mg/m2/wk. | |||||||||
Daily x 5 q 5wks refers to a dosing regimen of once per day for five consecutive days every five weeks (35-day cycle); while daily x 3 q 3wks refers to a dosing regimen of once per day for three consecutive days every three weeks (21-day cycle).
Our optimized dosing regimen increases the amount of VAL-083 delivered to the CNS by 60% over historical regimens without increased toxicity. Thus, the DelMar regimen achieves both a higher maximum concentration and higher overall exposure, which we believe may increase the likelihood of successful treatment outcomes in glioblastoma and other brain tumors.
Pharmacokinetics
Pharmacokinetic (“PK”) analyses showed dose-dependent linear systemic exposure with a short (1-2h) plasma terminal half-life; average Cmax at 40 mg/m2/day was 781 ng/mL (5.3µM). The observed PK profile is comparable to published literature. Prior NCI-sponsored studies demonstrated that VAL-083 readily crosses the blood brain barrier and has a long (>20 hour) half-life in the central nervous system (“CNS”).
We believe that this PK profile is optimal for the treatment of brain tumors: A long CNS half-life is expected to maximize exposure of the drug in the brain increasing the likelihood of successful treatment outcomes, while a short plasma half-life is desirable to minimize systemic side effects.
Observed pharmacokinetics from VAL-083 Phase 1 clinical trial dose vs. AUC
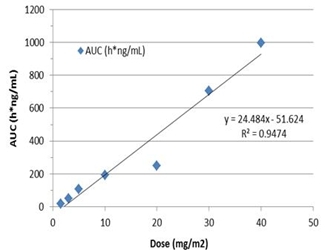
Based on observed and previously published pharmacokinetics, DelMar believes that therapeutic doses equal to, or above, 20 mg/m2 daily on days 1, 2 and 3 of a 21-day cycle should deliver sufficient levels of VAL-083 to brain tumors to achieve a therapeutic benefit.
MGMT & IDH1
High expression of MGMT and wild-type form of the enzyme isocitrate dehydrogenase (“IDH1”) have been previously shown to be diagnostic markers that correlate with resistance to currently available chemotherapies (e.g. temozolomide or nitrosourea) in the treatment of GBM and poor patient outcomes. Measurement of these biomarkers has become routine in clinical practice.
Notably, we have previously demonstrated that VAL-083’s anti-tumor mechanism is active independent from the MGMT status in vitro. We believe we will ultimately be able to use such biomarkers in a prognostic fashion to select the patients most likely to respond to treatment as we expand the clinical development of VAL-083.
| 33 |
MGMT expression was characterized by PCR and/or ELISA for nineteen GBM patients enrolled in our Phase 1/2 study. IDH1 status was reported in eleven patients; both MGMT and IDH1 status were reported in four patients. Notably, all patients whose samples were tested for both markers were MGMT-unmethylated by PCR and wild-type IDH1, a phenotype that is correlated with particularly poor prognosis.
| Biomarker | Observation in Phase 1/2 clinical trial | ||
| High MGMT (n=19) | 84% | ||
| IDH-WT (n=11) | 90% |
Tumor Response and Outcomes
rGBM patients in our Phase 1/2 clinical trial were not re-resected prior to treatment with VAL-083 and therefore had a growing recurrent GBM tumor at the time of enrollment. Patients were monitored for tumor response by MRI.
Consistent with un-resected rGBM, median progression free survival (“PFS”) was short at 1.2 months (range: 0.2 – 20.1 months). Five rGBM patients treated with VAL-083 were reported to have stable disease as their best response following treatment; the remainder reported progressive disease.
Disease progression is typical in a refractory GBM population with non-resected tumors. However, we believe that slowed progression may provide meaningful clinical benefit in this patient population through prolonged overall survival and improved quality of life.
According to published literature, GBM patients failing Avastin have a poor prognosis with expected survival under five months. Ad-hoc subgroup analysis of the Phase 1 dose-escalation data indicated a dose response trend and potential for improved survival. Increased survival was observed following initiation of treatment in a high dose (30 and 40mg/m2, n=9) sub-group vs. a low dose (≤5mg/m2, n=6) sub-group with median survival of >9 months vs. 4.4 months for the high and low dose groups, respectively. At the time of the analysis, more than half of patients receiving an assumed therapeutic dose survived more than six months following Avastin failure; more than 40% survived for nine months and more than 20% survived for twelve months or more.
Observed Survival Based on Phase 1 Sub-Group Analysis
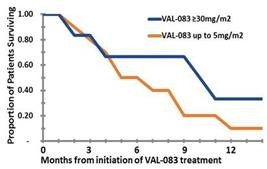
Analysis of twenty-two (22) patients receiving an assumed therapeutic dose of VAL-083 (≥20mg/m2) demonstrated median survival of 8.35 months following Avastin failure.
| 34 |
ASCO 2016: VAL-083 compared to published literature
| Reference | Post Avastin Salvage Therapy | Median Survival following Avastin Failure | |||
| Shih (2016) | VAL-083 | 8.35 months | |||
| Rahman (2014) | nitrosourea | 4.3 months | |||
| Mikkelson (2011) | TMZ + irinotecan | 4.5 months | |||
| Lu (2011) | dasatinib | 2.6 months | |||
| Reardon (2011) | etoposide | 4.7 months | |||
| Reardon (2011) | TMZ | 2.9 months | |||
| Iwomoto (2009) | various | 5.1 months |
While recognizing these data are representative of a relatively small, non-controlled Phase 1/2 clinical trial, we believe these outcomes support the potential of VAL-083 to offer meaningful clinical benefit to GBM patients who have failed Avastin, compared to currently available therapy.
VAL-083 Historical Data and DelMar Research in Ovarian Cancer
Ovarian cancer is the fifth most common cancer in women and is the leading cause of death among women diagnosed with gynecological malignancies. In 2016, approximately 22,300 women in the US were diagnosed with ovarian cancer and 14,300 died from their disease.
Without treatment, ovarian cancer spreads within the pelvic region and metastasizes to distant sites such as the lungs, liver, spleen and, rarely, the brain. The initial symptoms of ovarian cancer such as abdominal bloating, indigestion, pelvic pain, or nausea are often attributed to symptoms caused by a less serious condition. Therefore, in most cases, ovarian cancer is not diagnosed until it has progressed to an advanced stage when it is no longer possible to surgically remove all tumor tissue.
When diagnosed at an advanced stage the 5-year survival rate is less than 40%. Women with ovarian cancer receive chemotherapy following surgery to treat residual disease.
Activity against ovarian epithelial adenocarcinoma (“OEA”) and squamous cell carcinoma of the cervix (“SCC”) was reported in in prior NCI-sponsored clinical trials. Importantly, NCI-researchers recommended VAL-083 for further advanced studies in the treatment of ovarian cancer.
Pt-based chemotherapy is employed in the treatment of nearly 50% of all cancer patients and is employed in the treatment regimen or nearly all advanced-stage ovarian cancer patients. Ovarian cancer patients whose tumors are sensitive to Pt-based chemotherapy have the most favorable outcome. Recently, the approval of PARP inhibitors in the treatment of ovarian cancer patients demonstrated improved outcomes, particularly patients whose tumors remain sensitive to Pt-based treatments.
Pt-based chemotherapies function by causing extensive damage to a cancer cell’s DNA. Cancer cells are adept at overcoming DNA damage or employing mechanisms to repair DNA damage induced by Pt-based chemotherapy. One of the most common obstacles to DNA-damaging chemotherapy is mutations to a gene called p53. Cellular processes governed by the p53 gene are critical in assessing DNA damage and determining if a cell should cease from dividing or self-destruct. When p53 does not function properly, cancer cells continue to divide despite the treatment with DNA-damaging chemotherapy, making these drugs ineffective and leading to treatment resistance. This occurs in nearly all cases of the most difficult ovarian cancer to treat – high grade serous ovarian cancer (HGSOC) – which accounts for up to 70% of ovarian cancer cases and approximately 90% of ovarian cancer deaths. P53 mutations are associated with resistance to Pt-based chemotherapy, which leads to treatment failure and increased mortality. Solving this problem is a major goal in the development of new treatments for ovarian cancer.
Unfortunately, the development of resistance to Pt-based agents is nearly inevitable, leading to disease recurrence and increased mortality. Ultimately, most women with advanced ovarian cancer develop recurrent disease with progressively shorter disease-free intervals. Those whose tumors recur within 6 months of Pt-based therapy are considered Pt-resistant/refractory and have a very poor prognosis.
The response rate to second line therapy for Pt-resistant ovarian cancer patients is in the 10-15% range and overall survival is approximately 12 months. The development of new chemotherapies and targeted agents to overcome Pt resistance in ovarian cancer is a significant unmet medical need.
| 35 |
We have presented data demonstrating that VAL-083’s distinct mechanism of action allows activity in tumors that are resistant to other therapies. We have shown that cytotoxicity of VAL-083 against ovarian cancer is independent of sensitivity to cisplatin or p53 status in vitro. We have demonstrated that VAL-083 is active in Pt-resistant ovarian cells harboring a range of p53-mutations.
Our research has demonstrated that VAL-083 not only overcomes Pt resistance, but the combination of VAL-083 with Pt-based chemotherapy displays synergy in multiple models in vitro and in vivo. This further suggests a distinct mechanism of action and potential use as part of a VAL-083/Pt-combination therapy.
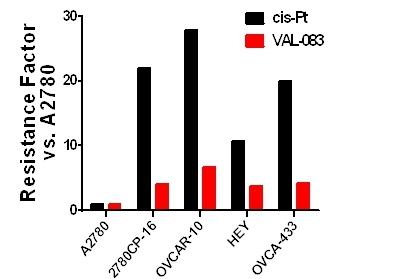
The combination of VAL-083 with either cisplatin (A) or oxaliplatin (B) in the human H460 (WT p53) NSCLC model demonstrated significant super additivity (p≤0.05) and/or synergism (CI<1) for both combinations. This cytotoxic effect of VAL-083 in combination with either platinum drug was observed also in A549 (WT p53) and H1975 (mutant p53) NSCLC cells, independently of p53 status (not shown). Data, where applicable, are shown as mean ± SE; N=7.
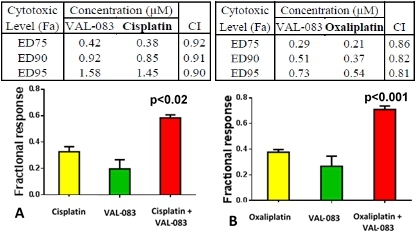
| 36 |
While Pt-based chemotherapy is the standard treatment for ovarian cancer, PARP inhibitors have recently provided a new treatment option for a subset of patients with platinum-sensitive recurrent ovarian cancer. VAL-083 also demonstrates synergistic activity with the PARP inhibitor olaparib in vitro, suggesting VAL-083 may have utility in the treatment of ovarian cancer in combination with PARP inhibitors.
We believe that these data demonstrate the potential of VAL-083 to treat platinum-resistant ovarian cancers as a single-agent against platinum-resistant tumors in combination with platinum-based chemotherapeutic regimens or in combination with PARP inhibitors.
Other Indications for VAL-083 – Potential Future Opportunities
VAL-083 in Lung Cancer
Lung cancer is a leading cause of cancer death around the world and effective treatment for lung cancer remains a significant global unmet need despite advances in therapy. Incidence of lung cancer in the United States is approximately 59 per 100,000 with the majority (52: 100,000) being NSCLC, the most common type of lung cancer. Globally, the market for lung cancer treatment may exceed $24 billion by 2033 according to a report published by Evaluate Pharma.
The activity of VAL-083 against solid tumors, including lung cancer, has been established in both preclinical and human clinical trials conducted by the NCI. DelMar has developed new nonclinical data to support the utility of VAL-083 in the modern treatment of lung cancer. In an established murine xenograft model of NSCLC, the activity of VAL-083 was compared to standard platinum-based therapy with cisplatin against human NSCLC cell lines A549 (TKI-sensitive) and H1975 (TKI-resistant). In the study, VAL-083 demonstrated superior efficacy and safety in the treatment of TKI-susceptible (A549) tumors and in TKI-resistant (H1975) tumors.
Central Nervous System Metastases of Solid Tumors
The successful management of systemic tumors by modern targeted therapies has led to increased incidence of mortality due to CNS metastases of lung cancer and other solid tumors. In June 2013, we split our Phase 1/2 clinical trial protocol into two separate studies: one focusing solely on refractory GBM and the other focusing on secondary brain cancers caused by other tumors that have spread to the brain.
Based on historical clinical activity and our own research, we believe that VAL-083 may be suitable for the treatment of patients with CNS metastases who currently have limited treatment options. Subject to the availability of financial and operating resources, we plan to develop a separate protocol for the continued exploration of VAL-083 in patients with secondary brain cancer caused by a solid tumor spreading to the brain.
Pediatric Brain Tumors
Tumors of the brain and spine make up approximately 20 percent of all childhood cancers and they are the second most common form of childhood cancer after leukemia.
The activity of VAL-083 against childhood and adolescent brain tumors has been established in both preclinical and human clinical trials conducted by the NCI. We have presented data indicating that VAL-083 offers potential therapeutic alternatives for the treatment of pediatric brain tumors including SHH-p53 mutated medulloblastoma. In March 2016, the FDA granted orphan drug designation for the use of VAL-083 in the treatment of medulloblastoma. Subject to the availability of resources, we intend to collaborate with leading academic researchers for the continued exploration of VAL-083 as a potential treatment of childhood brain tumors.
Additional Indications for VAL-083
In historical studies sponsored by the NCI in the United States, VAL-083 exhibited clinical activity against a range of tumor types including central nervous system tumors, solid tumors, and hematologic malignancies. We have established new nonclinical data supporting the activity of VAL-083 in different types of cancer that are resistant to modern targeted therapies and we believe that the unique cytotoxic mechanism of VAL-083 may provide benefit to patients in a range of indications. We intend to continue to research these opportunities, and if appropriate, expand our clinical development efforts to include additional indications.
| 37 |
Corporate History
We are a Nevada corporation formed on June 24, 2009 under the name Berry Only, Inc. Prior to a reverse acquisition undertaken on January 25, 2013 Berry did not have any significant assets or operations. The Company is the parent company of Del Mar Pharmaceuticals (BC) Ltd. (“DelMar (BC)”), a British Columbia, Canada corporation incorporated on April 6, 2010, that is focused on the development of drugs for the treatment of cancer. The Company is also the parent company to 0959454 B.C. Ltd., a British Columbia corporation (“Callco”), and 0959456 B.C. Ltd., a British Columbia corporation (“Exchangeco”). Callco and Exchangeco were formed to facilitate the reverse acquisition.
References to the Company, “we”, “us”, and “our” refer to the Company and its wholly-owned subsidiaries, DelMar (BC), Callco and Exchangeco.
Outstanding Securities
As of February 14, 2018, the Company has 21,927,517 shares of common stock issued and outstanding, 932,761 shares of common stock issuable upon exchange of the Exchangeable Shares of Exchangeco (which entitle the holder to require Exchangeco to redeem (or, at the option of the Company or Callco, to have the Company or Callco purchase) the Exchangeable Shares, and upon such redemption or purchase to receive an equal number of shares of common stock of the Company) (the Exchangeable Shares are recognized on an as-exchanged for common stock basis for financial statement purposes), outstanding warrants to purchase 14,004,840 shares of common stock, 881,113 outstanding shares of Series B Preferred Stock that are convertible into 2,202,792 shares of common stock, and outstanding stock options to purchase 1,420,850 shares of common stock. All Exchangeable Shares, warrants, and stock options are convertible, or exercisable into, one share of common stock. Each Series B convertible preferred share is convertible into 2.5 shares of common stock.
Related Parties
The Company acquired its initial patents and technology rights from Valent, an entity owned by Dr. Dennis Brown, the Company’s Chief Scientific Officer and Director. As a result, Valent is a related party to the Company.
Pursuant to employment and consulting agreements with the Company’s officers the Company recognized a total of $226,667 (2016 - $275,000) in expenses for the three months ended December 31, 2017 and $369,167 (2016 - $395,000) in expenses for the six months ended December 31, 2017. In addition, at December 31, 2017, the Company also recognized a total of $311,683 relating to the settlement agreement with the Company’s former President and Chief Operating Officer. Amounts owed to related parties, including to the Company’s former President and Chief Operating Officer, are non-interest bearing and payable on demand.
The Company recognized $43,750 (2016 – $37,000) in directors’ fees during the three months ended December 31, 2017 and $96,250 (2016 - $82,000) during the six months ended December 31, 2017.
As part of the Series B preferred stock dividend, the Company issued 1,511 (2016 – 1,511) shares of common stock to officers and directors of the Company and recognized an amount of $1,647 (2016 - $4,819) for the three months ended December 31, 2017. For the six months ended December 31, 2017, the Company issued 3,022 (2016 – 3,022) shares of common stock and recognized $2,916 (2016 - $13,960). All of the dividends have been recognized as a direct increase to deficit.
The Company recorded $2,089 (2016 - $2,089) in dividends related to the Series A preferred stock issued to Valent (note 3) for the three months ended December 31, 2017 and $4,178 (2016 - $4,178) for the six months ended December 31, 2017.
During the six months ended December 31, 2017, the Company granted a total of 180,000 stock options to the Company’s independent directors. The stock options are exercisable at a price of $2.11 and have a term of 10 years. One-third of the options vest on June 30, 2018 and 15,000 options vest on a quarterly basis thereafter commencing September 30, 2018. In addition, during the three and six months ended December 31, 2017, the Company granted 120,000 stock options at an exercise price of $0.87 to its Interim President and Chief Executive Officer. The stock options have a term of 10 years and vest pro rata monthly during the year following grant. The Company also modified certain stock options held by its former President and Chief Operating Officer.
| 38 |
Derivative Liability
The Company has issued common stock purchase warrants. Based on the terms of certain of these warrants the Company determined that the warrants were a derivative liability which is recognized at fair value at the date of the transaction and re-measured at fair value each reporting period with the changes in fair value recorded in the consolidated condensed interim statement of loss and comprehensive loss.
2013 Investor Warrants
During the quarter ended March 31, 2013 the Company issued an aggregate of 3,281,250 units at a purchase price of $3.20 per unit, for aggregate gross proceeds of $10,500,000 (the “Private Offering”). Each unit consisted of one share of common stock and one five-year warrant (the “2013 Investor Warrants”) to purchase one share of common stock at an initial exercise price of $3.20. The exercise price of the 2013 Investor Warrants is subject to adjustment in the event that the Company issues common stock at a price lower than the exercise price, subject to certain exceptions. The 2013 Investor Warrants are redeemable by the Company at a price of $0.004 per 2013 Investor Warrant at any time subject to the conditions that (i) the Company’s common stock has traded for twenty (20) consecutive trading days with a closing price of at least $6.40 per share with an average trading volume of 50,000 shares per day, and (ii) the underlying shares of common stock are registered for resale.
As a result of the financing completed by the Company during the three months ended September 30, 2015 the exercise price of all of the 2013 Investor Warrants was reduced from $3.20 to $3.14. As a result of the financing completed by the Company during the three months ended September 30, 2017 the exercise price of the un-amended 2013 Investor Warrants was further reduced from $3.14 to $2.68. The change in exercise price did not result in a material change in the derivative liability.
2013 Investor Warrant exercises
During the three months ended December 31, 2016, 22,188 of the 2013 Investor Warrants were exercised for 22,188 shares of common stock at an exercise price of $3.14 per share. The Company received proceeds of $69,759 from these exercises. The warrants that have been exercised were revalued at their respective exercise dates and then the reclassification to equity was recorded resulting in $57,466 of the derivative liability being reclassified to equity.
During the six months ended December 31, 2016, 65,095 of the 2013 Investor Warrants were exercised at an exercise price of $3.14 per share. The Company received proceeds of $204,659 from these exercises. The warrants that have been exercised were revalued at their respective exercise dates and then the reclassification to equity was recorded resulting in $238,474 of the derivative liability being reclassified to equity.
There were no exercises of 2013 Investor Warrants during the three or six months ended December 31, 2017.
2013 Investor Warrant amendments
During the year ended June 30, 2016, the Company entered into amendments (the “2013 Investor Warrant Amendments”) with the holders of certain of the 2013 Investor Warrants to extend the expiration date to March 31, 2019 and remove the provision requiring an adjustment of the warrant exercise price in the event the Company sells common stock at a purchase price lower than the current warrant exercise price. During the six months ended December 31, 2016, 15,944 of the 2013 Investor Warrants were amended. The warrants that have been amended were revalued at their respective amendment dates and then the reclassification to equity was recorded resulting in $53,006 of the derivative liability being reclassified to equity.
There were no amendments of the 2013 Investor Warrants during the three or six months ended December 31, 2017.
| 39 |
2015 Agent Warrants
As part of the Company’s financing completed during the year ended September 30, 2016, the Company issued warrants to purchase 23,477 shares of common stock to certain placement agents (“2015 Agent Warrants”) and recognized them as a derivative liability of $29,594 at the time of issuance. The 2015 Agent Warrants are exercisable at a per share price equal to $3.00 until July 15, 2020. During the three months ended December 31, 2016, 680 of the 2015 Agent Warrants were exercised for cash proceeds of $2,040 and 1,000 of the 2015 Agent Warrants were exercised on a cashless basis for 594 shares of common stock. The total reclassification to equity subsequent to revaluation at the respective exercise dates was $9,935.
There were no exercises of the 2015 Agent Warrants during the three months ended December 31, 2017.
The Company’s derivative liability is summarized as follows:
| Three months ended | ||||||||
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| $ | $ | |||||||
| Opening balance | 4,660 | 590,345 | ||||||
| Change in fair value of warrants | 889 | (361,668 | ) | |||||
| Reclassification to equity upon exercise of warrants | - | (57,466 | ) | |||||
| Closing balance | 5,549 | 171,211 | ||||||
| Less current portion | (95 | ) | - | |||||
| Long term portion | 5,454 | 171,211 | ||||||
| Six months ended | ||||||||
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| $ | $ | |||||||
| Opening balance | 61,228 | 693,700 | ||||||
| Change in fair value of warrants | (55,679 | ) | (221,074 | ) | ||||
| Reclassification to equity upon amendment of warrants | - | (53,006 | ) | |||||
| Reclassification to equity upon exercise of warrants | - | (248,409 | ) | |||||
| Closing balance | 5,549 | 171,211 | ||||||
| Less current portion | (95 | ) | - | |||||
| Long term portion | 5,454 | 171,211 | ||||||
The derivative liability consists of the following warrants:
| December 31, 2017 | ||||||||
| Number of warrants | $ | |||||||
| 2013 Investor Warrants | 105,129 | 23 | ||||||
| Warrants issued for services | 43,750 | 72 | ||||||
| 2015 Agent Warrants | 21,768 | 5,454 | ||||||
| Closing balance | 170,647 | 5,549 | ||||||
| Less current portion | (148,879 | ) | (95 | ) | ||||
| Long-term portion | 21,768 | 5,454 | ||||||
| 40 |
Selected Quarterly Information
The financial information reported herein has been prepared in accordance with accounting principles generally accepted in the United States. The Company’s functional currency at December 31, 2017 is the US$. The following tables represent selected financial information for the Company for the periods presented.
Selected Balance Sheet Data
December 31, 2017 $ | June 30, 2017 $ | |||||||
| Cash | 11,021,568 | 6,586,014 | ||||||
| Working capital | 9,959,948 | 6,566,371 | ||||||
| Total assets | 12,216,116 | 7,911,021 | ||||||
| Derivative liability | 5,549 | 61,228 | ||||||
| Total stockholders’ equity | 9,983,574 | 6,578,524 | ||||||
Selected Statement of operations data
For the three months ended:
| December 31, | December 31, | |||||||
| 2017 | 2016 | |||||||
| $ | $ | |||||||
| Research and development | 2,141,945 | 1,120,910 | ||||||
| General and administrative | 1,011,879 | 571,286 | ||||||
| Change in fair value of stock option and derivative liabilities | 889 | (361,668 | ) | |||||
| Foreign exchange loss (gain) | 7,120 | (8,495 | ) | |||||
| Interest income | (235 | ) | (60 | ) | ||||
| Net and comprehensive loss for the period | 3,161,598 | 1,321,973 | ||||||
| Series B preferred stock dividend | 54,066 | 159,756 | ||||||
| Net and comprehensive loss available to common stockholders | 3,215,664 | 1,481,729 | ||||||
| Basic weighted average number of shares outstanding | 22,559,234 | 11,424,845 | ||||||
| Basic loss per share | 0.14 | 0.13 | ||||||
For the six months ended:
| December 31, | December 31, | |||||||
| 2017 | 2016 | |||||||
| $ | $ | |||||||
| Research and development | 4,076,588 | 1,853,639 | ||||||
| General and administrative | 1,756,500 | 1,887,925 | ||||||
| Change in fair value of stock option and derivative liabilities | (55,679 | ) | (135,980 | ) | ||||
| Foreign exchange loss | 50,986 | 6,829 | ||||||
| Interest income | (391 | ) | (101 | ) | ||||
| Net and comprehensive loss for the period | 5,828,004 | 3,612,312 | ||||||
| Series B Preferred stock dividend | 95,732 | 467,054 | ||||||
| Net and comprehensive loss available to common stockholders | 5,923,736 | 4,079,366 | ||||||
| Basic weighted average number of shares outstanding | 18,882,259 | 11,363,237 | ||||||
| Basic loss per share | 0.31 | 0.36 | ||||||
| 41 |
Expenses net of share-based payments
The following table discloses research and development, and general and administrative expenses net of share-based payment expenses.
For the three months ended:
December 31, 2017 $ | December 31, 2016 $ | |||||||
| Research and development | 2,141,945 | 1,120,910 | ||||||
| Share-based (expenses) recovery included in research and development | (126,375 | ) | 65,727 | |||||
| Research and development net of non-cash | 2,015,570 | 1,186,637 | ||||||
| General and administrative | 1,011,879 | 571,286 | ||||||
| Share-based (expenses) recovery included in general and administrative | (102,132 | ) | 9,475 | |||||
| General and administrative net of non-cash | 909,747 | 580,761 | ||||||
For the six months ended:
December 31, 2017 $ | December 31, 2016 $ | |||||||
| Research and development | 4,076,588 | 1,853,639 | ||||||
| Share-based (expenses) recovery included in research and development | (121,401 | ) | 9,890 | |||||
| Research and development net of non-cash | 3,955,187 | 1,863,529 | ||||||
| General and administrative | 1,756,500 | 1,887,925 | ||||||
| Share-based (expenses) recovery included in general and administrative | (170,495 | ) | (580,750 | ) | ||||
| General and administrative net of non-cash | 1,586,005 | 1,307,175 | ||||||
Results of Operations
Comparison of the three months ended December 31, 2017 and December 31, 2016
| Three Months Ended | ||||||||||||||||
December 31, 2017 $ | December 31, 2016 $ |
Change $ | Change % | |||||||||||||
| Research and development | 2,141,945 | 1,120,910 | 1,021,035 | 91 | ||||||||||||
| General and administrative | 1,011,879 | 571,286 | 440,593 | 77 | ||||||||||||
| Change in fair value of stock option and derivative liabilities | 889 | (361,668 | ) | 362,557 | 100 | |||||||||||
| Foreign exchange gain (loss) | 7,120 | (8,495 | ) | 15,615 | (184 | ) | ||||||||||
| Interest income | (235 | ) | (60 | ) | (175 | ) | 292 | |||||||||
| Net loss and comprehensive loss | 3,161,598 | 1,321,973 | 1,839,625 | |||||||||||||
| 42 |
Research and Development
Research and development expenses increased to $2,141,945 for the three months ended December 31, 2017 from $1,120,910 for the three months ended December 31, 2016. The increase was largely attributable to an increase in clinical development costs, personnel, preclinical research, and non-cash expenses. Non-cash expense for the three months ended December 31, 2017 was the result of stock option expense of $126,375 while non-cash expense for the three months ended December 31, 2016 was a reversal of stock option expense of $65,727. During the quarter ended December 31, 2017, the Company entered into a separation agreement with the Company’s former President and Chief Operating Officer that required the accelerated vesting of certain stock options. The full expense of the accelerated vesting was recognized during the current quarter.
Excluding the impact of non-cash expense, research and development expenses increased to $2,015,570 during the three months ended December 31, 2017 from $1,186,637 for the three months ended December 31, 2016. The increase in clinical development costs for the current quarter compared to the prior quarter was primarily due to drug manufacturing costs and ongoing study expenses for site initiation and patient enrollment for the Company’s STAR-3 study which commenced in the quarter ended September 30, 2017. The current quarter also includes ongoing enrollment in the Company’s Phase II GBM trial in unmethylated patients being conducted at the MD Anderson Cancer Center. During the quarter ended December 31, 2016, neither the STAR-3 nor the Phase II study at MD Anderson had commenced. Personnel costs increased during the current quarter compared to the prior quarter primarily due to payments owing to the Company’s former President and Chief Operating Officer pursuant to the settlement agreement. Preclinical research increased primarily due to an increase in the ongoing mechanism of action research that the Company has undertaken in the current period.
General and Administrative
General and administrative expenses were $1,011,879 for the three months ended December 31, 2017 compared to $571,286 for the three months ended December 31, 2016. The increase was partially due to an increase in non-cash expenses in the current quarter compared to the prior quarter. In relation to general and administrative expenses during the three months ended December 31, 2017, the Company incurred non-cash expenses of $102,132 relating primarily to the accelerated vesting of certain options held by the Company’s former President and Chief Operating Officer pursuant to his settlement agreement.
Excluding the impact of non-cash expenses, general and administrative expenses increased in the three months ended December 31, 2017 to $909,747 from $580,761 for the three months ended December 31, 2016 largely due to increases in professional fees and personnel. Professional fees increased as a result of costs associated with various matters including preparation for the Company’s annual meeting of stockholders, regulatory filings, corporate governance matters, and increased public relations and business development costs. Personnel costs increased during the three months ended December 31, 2017 compared to the three months ended December 31, 2016 as a result of costs accrued to the former President and Chief Operating Officer pursuant to the settlement agreement.
Change in fair value of stock option and derivative liabilities
Based on the terms of certain warrants issued by the Company, the Company determined that the warrants were a derivative liability which is recognized at fair value at the date of the transaction and re-measured at fair value every reporting period with gains or losses on the changes in fair value recorded in the consolidated condensed statement of loss and comprehensive loss. The balances recognized during the three months ended December 31, 2017 and 2016 were primarily due to changes in the Company’s common stock price between the date the warrants were last valued on September 30, 2017 and 2016, respectively, which are the valuation dates used for the quarters ended December 31.
| 43 |
The Company recognized a loss of $889 from the change in fair value of the derivative liability for the three months ended December 31, 2017 and a gain of $361,668 for the three months ended December 31, 2016.
Changes in the Company’s common stock price can result in significant volatility in the Company’s reported net loss due to its impact on the fair value of the derivative liability. As a result of revaluation gains and losses, the Company expects that its reported net income or loss will continue to fluctuate significantly.
Foreign Exchange
The Company’s functional currency at December 31, 2017 is the US$ but the Company incurs a portion of its expenses in CA$. The foreign exchange gains and losses are reported in other loss (income) in the consolidated condensed interim statement of loss and comprehensive loss.
The Company recognized a foreign exchange loss of $7,120 for the three months ended December 31, 2017 and a gain of $8,495 for the three months ended December 31, 2016. The losses were due to changes in the exchange rate between the CA$ and the US$ and to varying levels of CA$ cash and accounts payable.
Preferred Share Dividends
For each of the three months ended December 31, 2017 and 2016, the Company recorded $2,089 related to the dividend payable to Valent on the Series A preferred stock. The dividend has been recorded as a direct increase in accumulated deficit for both periods.
The Company issued 49,602 (2016 – 50,096) shares of common stock on December 31, 2017 as a dividend on the Series B Preferred stock and recognized $54,066 (2016 - $159,756) as a direct increase in accumulated deficit.
Comparison of the six months ended December 31, 2017 and December 31, 2016
| Six months ended | ||||||||||||||||
December 31, 2017 $ | December 31, 2016 $ |
Change $ | Change % | |||||||||||||
| Research and development | 4,076,588 | 1,853,639 | 2,222,949 | 120 | ||||||||||||
| General and administrative | 1,756,500 | 1,887,925 | (131,425 | ) | (7 | ) | ||||||||||
| Change in fair value of stock option and derivative liabilities | (55,679 | ) | (135,980 | ) | 80,301 | (59 | ) | |||||||||
| Foreign exchange loss | 50,986 | 6,829 | 44,157 | 647 | ||||||||||||
| Interest income | (391 | ) | (101 | ) | (290 | ) | 287 | |||||||||
| Net loss and comprehensive loss | 5,828,004 | 3,612,312 | 2,215,692 | |||||||||||||
Research and Development
Research and development expenses increased to $4,076,588 for the six months ended December 31, 2017 from $1,853,639 for the six months ended December 31, 2016. The increase was largely attributable to an increase in clinical development costs, personnel, preclinical research, and non-cash expenses. For the six months ended December 31, 2017, non-cash expense related to stock option expense only while for the six months ended December 31, 2016, non-cash expense related to warrants issued for services as well as stock option expense. Non-cash expense for the six months ended December 31, 2017 was $121,401 while non-cash expense for the six months ended December 31, 2016 was $9,890. The change was primarily due to the accelerated vesting of certain options held by the Company’s former President and Chief Operating Officer pursuant to his settlement agreement.
| 44 |
Excluding the impact of non-cash expense, research and development expenses increased to $3,955,187 during the current period from $1,863,529 for the prior period. The increase in clinical development costs for the six months ended December 31, 2017 compared to the six months ended December 31, 2016 was primarily due to manufacturing costs and site initiation expenses for the commencement of patient enrollment for the Company’s STAR-3 study. Also, during the current period, enrollment in the Company’s Phase II GBM trial in unmethylated patients being conducted at the MD Anderson Cancer Center was ongoing. In the six months ended December 31, 2016, neither of these studies had commenced. Personnel costs increased during the current quarter compared to the prior quarter primarily due to payments owing to the Company’s former President and Chief Operating Officer pursuant to the settlement agreement. Preclinical research increased primarily due to an increase in the ongoing mechanism of action research that the Company has undertaken in the current period.
General and Administrative
General and administrative expenses were $1,756,500 for the six months ended December 31, 2017 compared to $1,887,925 for the six months ended December 31, 2016. The decrease was primarily due to a decrease in non-cash expenses in the current quarter compared to the prior quarter. In relation to general and administrative expenses during the six months ended December 31, 2017, the Company incurred non-cash expenses of $170,495 relating to warrants issued for services and stock option expense while during the six months ended December 31, 2016, the Company incurred non-cash expenses of $580,750 relating to shares and warrants issued for services, and stock option expense.
Excluding the impact of non-cash expenses, general and administrative expenses increased in the six months ended December 31, 2017 to $1,586,005 from $1,307,175 for the six months ended December 31, 2016. The increase was due to higher personnel and professional fees. Personnel costs increased during the current quarter compared to the prior quarter primarily due to payments owing to the Company’s former President and Chief Operating Officer pursuant to the settlement agreement. Professional fees incurred during the six months ended December 31, 2017 relate to various matters including preparation for the Company’s annual meeting of stockholders, completing the Company’s 2017 Omnibus Incentive Plan, regulatory filings, and corporate governance matters. In the six months ended December 31, 2016, the costs were incurred related to preparing for the Company’s uplisting of its common stock on the Nasdaq Stock Market as well as fees associated with one-time listing activities, and the filing of three registration statements with the SEC that were all declared effective in September 2016.
Change in fair value of stock option and derivative liabilities
Based on the terms of certain warrants issued by the Company, the Company determined that the warrants were a derivative liability which is recognized at fair value at the date of the transaction and re-measured at fair value every reporting period with gains or losses on the changes in fair value recorded in the consolidated condensed statement of loss and comprehensive loss. The balances recognized during the six months ended December 31, 2017 and 2016 were primarily due to changes in the Company’s common stock price between the date the warrants were last valued on June 30, 2017 and 2016, respectively, which are the valuation dates used for the quarters ended December 31.
The Company recognized a gain of $55,679 from the change in fair value of the derivative liability for the six months ended December 31, 2017 and a gain of $135,980 for the six months ended December 31, 2016.
Changes in the Company’s common stock price can result in significant volatility in the Company’s reported net loss due to its impact on the fair value of the derivative liability. As a result of revaluation gains and losses, the Company expects that its reported net income or loss will continue to fluctuate significantly.
Certain of the Company’s stock options have been issued in $CDN. Of these, a portion were classified as a stock option liability which is revalued at each reporting date. During the six months ended December 31, 2016, the Company amended 43,750 of these stock options held by five optionees such that the exercise price of the options was adjusted to be denominated in $USD. No other terms of the stock options were amended. As a result of the amendment, the Company recognized $85,094 in stock option liability expense and $260,969 was reclassified to equity during the six months ended December 31, 2016.
| 45 |
Foreign Exchange
The Company’s functional currency at December 31, 2017 was the US$, but the Company incurs a portion of its expenses in CA$. The foreign exchange gains and losses are reported in other loss (income) in the consolidated condensed interim statement of loss and comprehensive loss.
The Company recognized foreign exchange losses of $50,986 and $6,829 for the six months ended December 31, 2017 and 2016, respectively. The losses were due to changes in the exchange rate between the CA$ and the US$ and to varying levels of CA$ cash and accounts payable.
Preferred Share Dividends
For each of the six months ended December 31, 2017 and 2016, the Company recorded $4,718 related to the dividend payable to Valent on the Series A preferred stock. The dividend has been recorded as a direct increase in accumulated deficit for both periods.
The Company issued 99,204 (2016 – 100,889) shares of common stock on December 31, 2017 as a dividend on the Series B Preferred stock and recognized $95,732 (2016 - $467,054) as a direct increase in accumulated deficit.
Liquidity and Capital Resources
Six months ended December 31, 2017 compared to the six months ended December 31, 2016
December 31, 2017 $ |
December 31, 2016 $ |
Change $ |
Change % |
|||||||||||||
| Cash flows from operating activities | (4,505,604 | ) | (3,062,408 | ) | (1,443,196 | ) | 47 | |||||||||
| Cash flows from financing activities | 8,941,158 | 322,521 | 8,618,367 | 2,672 | ||||||||||||
Operating Activities
Net cash used in operating activities increased to $4,505,604 for the six months ended December 31, 2017 from $3,062,408 for the six months ended December 31, 2016. During the six months ended December 31, 2017 and 2016 the Company reported net losses of $5,828,004 and $3,612,312, respectively. During the six months ended December 31, 2017, the Company recorded a gain from the revaluation of the derivative and stock option liabilities of $55,679 compared to a gain of $135,980 for the six months ended December 31, 2016. Excluding the impact of changes in the fair value of the derivative and stock option liabilities, non-cash items relating to amortization, warrants issued for services, and stock option expense totaled $303,106 for the six months ended December 31, 2017. Non-cash items relating to amortization, shares and warrants issued for services, and stock option expense totaled $578,576 for the six months ended December 31, 2016. The most significant change in non-cash working capital for the six months ended December 31, 2017 was cash from an increase in accounts payable and accrued liabilities of $646,825 and from an increase in related party payables of $308,899. The most significant change in non-cash working capital for the six months ended December 31, 2016 was cash from an increase in related party payables of $161,937.
Financing Activities
During the six months ended December 31, 2017, the Company received $8,945,336 in net proceeds from the completion of a registered direct offering by the Company of common stock and common stock purchase warrants. During the six months ended December 31, 2016 the Company received $326,699 from the exercise of warrants. In addition, the Company recorded $4,178 related to the dividend payable to Valent during each of the six months ended December 31, 2017 and 2016, respectively.
| 46 |
Operating Capital and Capital Expenditure Requirements
Liquidity Risk
(See note 1 to the consolidated condensed interim financial statements)
For the six months ended December 31, 2017, the Company reported a loss of $5,828,004 and the Company had an accumulated deficit of $47,046,347 at that date. As at December 31, 2017, the Company had cash on hand of $11,021,568. During the six months ended December 31, 2017, the Company received $8,945,336 in net proceeds from a registered direct offering. We believe, based on our current estimates, that we will be able to fund our operations beyond the next twelve months.
The Company does not have the prospect of achieving revenues in the near future and the Company will require additional funding for its clinical trials, to maintain its research and development projects, and for general operations. There is a great degree of uncertainty with respect to the expenses the Company will incur in executing its business plan. Consequently, management continually evaluates various financing alternatives to fund the Company’s operations so it can continue as a going concern in the medium to longer term.
There is no assurance that our cost estimates will prove to be accurate or that unforeseen events, problems or delays will not occur with respect thereto. The ability of the Company to meet its obligations and continue the research and development of its product candidate beyond the next twelve months is dependent on its ability to continue to raise adequate financing. There can be no assurance that such financing will be available to the Company in the amount required at any time or for any period or, if available, that it can be obtained on terms satisfactory to the Company. The Company may tailor its drug candidate development program based on the amount of funding the Company raises.
Our future funding requirements will depend on many factors, including but not limited to:
| ● | the rate of progress and cost of our clinical trials, preclinical studies and other discovery and research and development activities; | |
| ● | the costs associated with establishing manufacturing and commercialization capabilities; | |
| ● | the costs of acquiring or investing in businesses, product candidates and technologies; | |
| ● | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; | |
| ● | the costs and timing of seeking and obtaining FDA and other regulatory approvals; | |
| ● | the effect of competing technological and market developments; and | |
| ● | the economic and other terms and timing of any collaboration, licensing or other arrangements into which we may enter. |
Until we can generate a sufficient amount of product revenue to finance our cash requirements, which we may never do, we expect to finance future cash needs primarily through public or private equity offerings, debt financings or strategic collaborations. Economic conditions may affect the availability of funds and activity in equity markets. We do not know whether additional funding will be available on acceptable terms, or at all. If we are not able to secure additional funding when needed, we may have to delay, reduce the scope of or eliminate one or more of our clinical trials or research and development programs or make changes to our operating plan. In addition, we may have to seek a partner for one or more of our product candidate programs at an earlier stage of development, which would lower the economic value of those programs to us.
Critical Accounting Policies
The preparation of financial statements, in conformity with generally accepted accounting principles in the United States, requires companies to establish accounting policies and to make estimates that affect both the amount and timing of the recording of assets, liabilities, revenues and expenses. Some of these estimates require judgments about matters that are inherently uncertain and therefore actual results may differ from those estimates.
| 47 |
A detailed presentation of all of the Company’s significant accounting policies and the estimates derived there from is included in Note 2 to the Company’s consolidated financial statements for the year ended June 30, 2017 contained in our Form 10-K filed with the SEC on September 27, 2017. While all of the significant accounting policies are important to the Company’s consolidated financial statements, the following accounting policies and the estimates derived therefrom are critical:
| ● | Warrants and shares issued for services | |
| ● | Stock options | |
| ● | Derivative liability | |
| ● | Clinical trial accruals | |
Warrants and shares issued for services
Periodically, the Company has issued equity instruments for services provided by employees and nonemployees. The equity instruments are valued at the fair value of the instrument granted.
Stock options
The Company accounts for these awards under ASC 718, “Compensation - Stock Compensation” (“ASC 718”). ASC 718 requires measurement of compensation cost for all stock-based awards at fair value on the date of grant and recognition of compensation over the requisite service period for awards expected to vest. Compensation expense for unvested options to non-employees is revalued at each period end and is being amortized over the vesting period of the options. The determination of grant-date fair value for stock option awards is estimated using the Black-Scholes model, which includes variables such as the expected volatility of the Company’s share price, the anticipated exercise behavior of its grantee, interest rates, and dividend yields. These variables are projected based on the Company’s historical data, experience, and other factors. Changes in any of these variables could result in material adjustments to the expense recognized for non-cash expenses. Such value is recognized as expense over the requisite service period, net of estimated forfeitures, using the straight-line attribution method. The estimation of stock awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from current estimates, such amounts are recorded as a cumulative adjustment in the period estimates are revised. The Company considers many factors when estimating expected forfeitures, including type of awards granted, employee class, and historical experience. Actual results and future estimates may differ substantially from current estimates.
Derivative liability
The Company accounts for certain warrants under the authoritative guidance on accounting for derivative financial instruments indexed to, and potentially settled in, a company’s own stock, on the understanding that in compliance with applicable securities laws, the warrants require the issuance of securities upon exercise and do not sufficiently preclude an implied right to net cash settlement. The Company classifies warrants in its balance sheet as a derivative liability which is fair valued at each reporting period subsequent to the initial issuance. As quoted prices for the derivative liability are not available, the Company uses a simulated probability valuation model to value the warrants. Determining the appropriate fair-value model and calculating the fair value of warrants requires considerable judgment. Any change in the estimates used may cause the value to be higher or lower than that reported. The estimated volatility of the Company’s common stock at the date of issuance, and at each subsequent reporting period, is based on the historical volatility of similar life sciences companies. The risk-free interest rate is based on rates published by the government for bonds with a maturity similar to the expected remaining life of the warrants at the valuation date. The expected life of the warrants is assumed to be equivalent to their remaining contractual term.
Clinical trial accruals
Clinical trial expenses are a component of research and development costs and include fees paid to contract research organizations, investigators and other service providers who conduct specific research for development activities on behalf of the Company. The amount of clinical trial expenses recognized in a period related to service agreements is based on estimates of the work performed on an accrual basis. These estimates are based on patient enrollment, services provided and goods delivered, contractual terms and experience with similar contracts. The Company monitors these factors by maintaining regular communication with the service providers. Differences between actual expenses and estimated expenses recorded are adjusted for in the period in which they become known. Prepaid expenses or accrued liabilities are adjusted if payments to service providers differ from estimates of the amount of service completed in a given period.
Off-Balance Sheet Arrangements
The Company does not have any off balance sheet arrangements.
| 48 |
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
Not required for a smaller reporting company.
Item 4. Controls and Procedures.
Disclosure Controls and Procedures
Management, with the participation of the Interim Chief Executive Officer and Chief Financial Officer, conducted an evaluation (as required by Rule 13a-15 under the Exchange Act) of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this report. Based on that evaluation, our Interim Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were not effective as of the end of the period covered by this report, due to the material weakness in internal control over financial reporting as discussed in the Company’s Annual Report on Form 10-K for the year ended June 30, 2017, filed with the SEC on September 27, 2017.
Changes in internal controls
There have been no changes in our internal control over financial reporting that occurred during the quarter ended December 31, 2017 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II - OTHER INFORMATION
Item 1. Legal Proceedings.
There are no legal proceedings the Company is party to or any of its property is subject to.
Item 1A. Risk Factors.
Not required for a smaller reporting company.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
During the three months ended December 31, 2017, we issued 49,602 shares of common stock as dividends on our outstanding shares of Series B Preferred Stock.
In connection with the foregoing, we relied upon the exemption from registration provided by Section 4(a)(2) under the Securities Act of 1933, as amended, for transactions not involving a public offering.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
Not applicable.
| 49 |
Item 5. Other Information.
On February 9, 2018, our Board of Directors amended and restated the DelMar Pharmaceuticals, Inc. 2017 Omnibus Equity Incentive Plan (the “2017 Plan”), which was initially adopted by the Board on July 7, 2017, to (i) eliminate certain provisions that were intended to allow for grants of awards that would be treated as “performance-based compensation” under Section 162(m) of the Internal Revenue Code of 1986, as amended, or the Code, since such types of awards were eliminated by federal tax reform legislation signed into law on December 22, 2017, (ii) increase the number of shares of our common stock that may be granted under the 2017 Plan from 3,487,785 to 7,800,000 shares, (iii) eliminate the 2017 Plan’s “evergreen” provision that would have maintained the number of shares of common stock available for issuance with respect to awards at any time under the 2017 Plan to thirteen percent (13%) of our fully diluted shares of common stock, and (iv) limit the number of shares of our common stock that may be subject to awards made to any person in any calendar year to 8% of our fully diluted shares of common stock on the date of grant (excluding the number of shares of common stock issued under the 2017 Plan and/or the Del Mar Pharmaceuticals (BC) Ltd. 2013 Amended and Restated Stock Option Plan (the “Legacy Plan”) or subject to outstanding awards granted under the 2017 Plan or Legacy Plan). The Company intends to seek shareholder approval of the 2017 Plan, as amended and restated, at the next annual meeting of shareholders.
Item 6. Exhibits.
* Filed herewith.
| 50 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| DelMar Pharmaceuticals, Inc. | ||
| Date: February 14, 2018 | By: | /s/ Saiid Zarrabian |
| Saiid Zarrabian | ||
| Interim President and Chief Executive Officer (Principal Executive Officer) | ||
| Date: February 14, 2018 | By: | /s/ Scott Praill |
| Scott Praill | ||
| Chief Financial Officer (Principal Financial and Accounting Officer) | ||
51
