Attached files
| file | filename |
|---|---|
| 8-K - 8-K - MEI Pharma, Inc. | d75702d8k.htm |
| EX-99.2 - EX-99.2 - MEI Pharma, Inc. | d75702dex992.htm |
| EX-99.1 - EX-99.1 - MEI Pharma, Inc. | d75702dex991.htm |
Exhibit 99.3

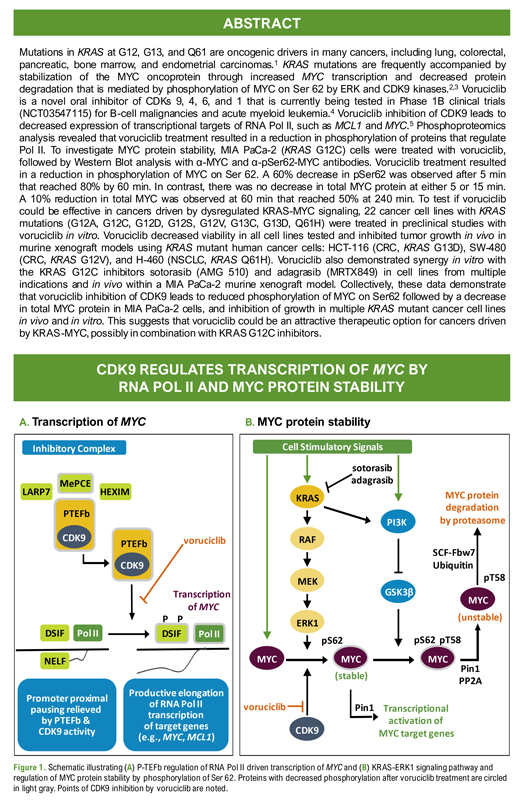
Exhibit 99.3 Poster: # 1962 Voruciclib, a CDK9 inhibitor, downregulates MYC and inhibits proliferation of KRAS mutant cancers in preclinical models Sandra Wiley*, Yongwei Su**, Yubin Ge** *MEI Pharma Inc, 11455 El Camino Real, Suite 250, San Diego, CA , 92130 **Department of Oncology and Molecular Therapeutics Program, Karmanos Cancer Institute, Wayne State University School of Medicine. ABSTRACT Mutations in KRAS at G12, G13, and Q61 are oncogenic drivers in many cancers, including lung, colorectal, pancreatic, bone marrow, and endometrial carcinomas.1 KRAS mutations are frequently accompanied by stabilization of the MYC oncoprotein through increased MYC transcription and decreased protein degradation that is mediated by phosphorylation of MYC on Ser 62 by ERK and CDK9 kinases.2,3 Voruciclib is a novel oral inhibitor of CDKs 9, 4, 6, and 1 that is currently being tested in Phase 1B clinical trials (NCT03547115) for B-cell malignancies and acute myeloid leukemia.4 Voruciclib inhibition of CDK9 leads to decreased expression of transcriptional targets of RNA Pol II, such as MCL1 and MYC.5 Phosphoproteomics analysis revealed that voruciclib treatment resulted in a reduction in phosphorylation of proteins that regulate Pol II. To investigate MYC protein stability, MIA PaCa-2 (KRAS G12C) cells were treated with voruciclib, followed by Western Blot analysis with α-MYC and α-pSer62-MYC antibodies. Voruciclib treatment resulted in a reduction in phosphorylation of MYC on Ser 62. A 60% decrease in pSer62 was observed after 5 min that reached 80% by 60 min. In contrast, there was no decrease in total MYC protein at either 5 or 15 min. A 10% reduction in total MYC was observed at 60 min that reached 50% at 240 min. To test if voruciclib could be effective in cancers driven by dysregulated KRAS-MYC signaling, 22 cancer cell lines with KRAS mutations (G12A, G12C, G12D, G12S, G12V, G13C, G13D, Q61H) were treated in preclinical studies with voruciclib in vitro. Voruciclib decreased viability in all cell lines tested and inhibited tumor growth in vivo in murine xenograft models using KRAS mutant human cancer cells: HCT-116 (CRC, KRAS G13D), SW-480 (CRC, KRAS G12V), and H-460 (NSCLC, KRAS Q61H). Voruciclib also demonstrated synergy in vitro with the KRAS G12C inhibitors sotorasib (AMG 510) and adagrasib (MRTX849) in cell lines from multiple indications and in vivo within a MIA PaCa-2 murine xenograft model. Collectively, these data demonstrate that voruciclib inhibition of CDK9 leads to reduced phosphorylation of MYC on Ser62 followed by a decrease in total MYC protein in MIA PaCa-2 cells, and inhibition of growth in multiple KRAS mutant cancer cell lines in vivo and in vitro. This suggests that voruciclib could be an attractive therapeutic option for cancers driven by KRAS-MYC,possibly in combination with KRAS G12C inhibitors. CDK9 REGULATES TRANSCRIPTION OF MYC BY RNA POL II AND MYC PROTEIN STABILITY A. Transcription of MYC B. MYC protein stability MePCE LARP7 HEXIM PTEFb voruciclib PTEFb Transcription of MYC P P DSIF DSIF NELF sotorasib adagrasib KRAS MYC protein degradation by proteasome RAF SCF-Fbw7 Ubiquitin pT58 MEK (unstable) ERK1 pS62 pS62 pT58 Pin1 (stable) PP2A voruciclib Pin1 Transcriptional activation of MYC target genes Figure 1. Schematic illustrating (A) P-TEFb regulation of RNA Pol II driven transcription of MYC and (B) KRAS-ERK1 signaling pathway and bility by phosphorylation of Ser 62. Proteins with decreased phosphorylation after voruciclib treatment are circled hibition by voruciclib are noted.

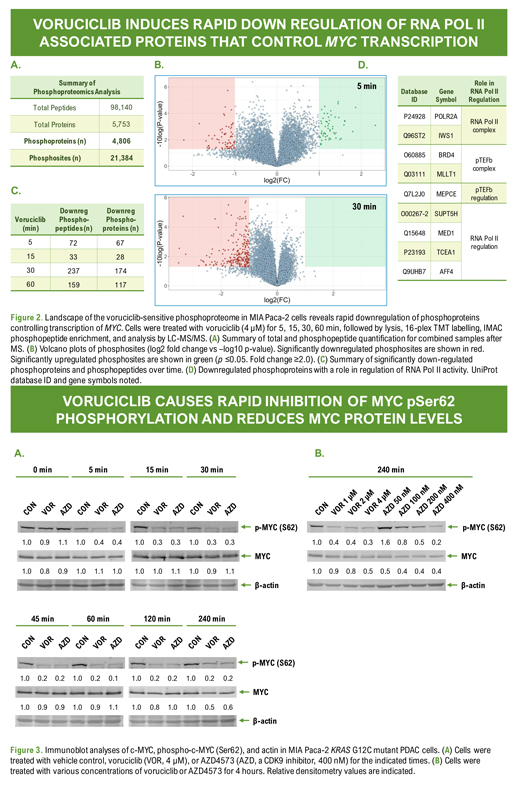
Poster: # 1962 Voruciclib, a CDK9 inhibitor, downregulates MYC and inhibits proliferation of KRAS mutant cancers in preclinical models Sandra Wiley*, Yongwei Su**, Yubin Ge** *MEI Pharma Inc, 11455 El Camino Real, Suite 250, San Diego, CA , 92130 **Department of Oncology and Molecular Therapeutics Program, Karmanos Cancer Institute, Wayne State University School of Medicine. VORUCICLIB INDUCES RAPID DOWN REGULATION OF RNA POL II ASSOCIATED PROTEINS THAT CONTROL MYC TRANSCRIPTION A. Summary of PhosphoproteomicsAnalysis Total Peptides 98,140 Total Proteins 5,753 Phosphoproteins (n) 4,806 Phosphosites (n) 21,384 C. Downreg Downreg Voruciclib Phospho- Phospho-(min) peptides (n) proteins (n) 5 72 67 15 33 28 30 237 174 60 159 117 Role in Database Gene RNA Pol II ID Symbol Regulation P24928 POLR2A RNA Pol II complex Q96ST2 IWS1 O60885 BRD4 pTEFb complex Q03111 MLLT1 pTEFb Q7L2J0 MEPCE regulation O00267-2 SUPT5H Q15648 MED1 RNA Pol II regulation P23193 TCEA1 Q9UHB7 AFF4 Figure 2. Landscape of the voruciclib-sensitive phosphoproteome in MIA Paca-2 cells reveals rapid downregulation of phosphoproteins controlling transcription of MYC. Cells were treated with voruciclib (4 µM) for 5, 15, 30, 60 min, followed by lysis, 16-plex TMT labelling, IMAC phosphopeptide enrichment, and analysis by LC-MS/MS. (A) Summary of total and phosphopeptide quantification for combined samples after MS. (B) Volcano plots of phosphosites (log2 fold change vs –log10 p-value). Significantly downregulated phosphosites are shown in red. Significantly upregulated phosphosites are shown in green (p £0.05. Fold change ³2.0). (C) Summary of significantly down-regulated phosphoproteins and phosphopeptides over time. (D) Downregulated phosphoproteins with a role in regulation of RNA Pol II activity. UniProt database ID and gene symbols noted. A. 0 min 5 min 15 min 30 min p-MYC (S62) 1.0 0.9 1.1 1.0 0.4 0.4 1.0 0.3 0.3 1.0 0.3 0.3 MYC 1.0 0.8 0.9 1.0 1.1 1.0 1.0 1.0 1.1 1.0 0.9 1.1 β-actin 45 min 60 min 120 min 240 min p-MYC (S62) 1.0 0.2 0.2 1.0 0.2 0.1 1.0 0.2 0.2 1.0 0.2 0.2 MYC 1.0 0.9 0.9 1.0 0.9 1.1 1.0 0.8 1.0 1.0 0.5 0.6 β-actin B. 240 min p-MYC (S62) 1.0 0.4 0.4 0.3 1.6 0.8 0.5 0.2 MYC 1.0 0.9 0.8 0.5 0.5 0.4 0.4 0.4 β-actin Figure 3. Immunoblot analyses of c-MYC, phospho-c-MYC (Ser62), and actin in MIA Paca-2 KRAS G12C mutant PDAC cells. (A) Cells were treated with vehicle control, voruciclib (VOR, 4 µM), or AZD4573 (AZD, a CDK9 inhibitor, 400 nM) for the indicated times. (B) Cells were treated with various concentrations of voruciclib or AZD4573 for 4 hours. Relative densitometry values are indicated.

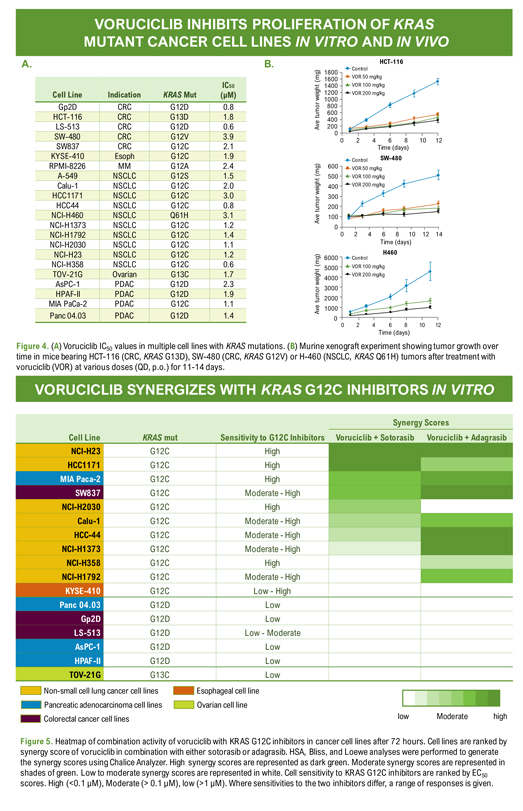
Poster: # 1962 Voruciclib, a CDK9 inhibitor, downregulates MYC and inhibits proliferation of KRAS mutant cancers in preclinical models Sandra Wiley*, Yongwei Su**, Yubin Ge** *MEI Pharma Inc, 11455 El Camino Real, Suite 250, San Diego, CA , 92130 **Department of Oncology and Molecular Therapeutics Program, Karmanos Cancer Institute, Wayne State University School of Medicine. VORUCICLIB INHIBITS PROLIFERATION OF KRAS MUTANT CANCER CELL LINES IN VITRO AND IN VIVO A. IC50 Cell Line Indication KRAS Mut (µM) Gp2D CRC G12D 0.8 HCT-116 CRC G13D 1.8 LS-513 CRC G12D 0.6 SW-480 CRC G12V 3.9 SW837 CRC G12C 2.1 KYSE-410 Esoph G12C 1.9 RPMI-8226 MM G12A 2.4 A-549 NSCLC G12S 1.5 Calu-1 NSCLC G12C 2.0 HCC1171 NSCLC G12C 3.0 HCC44 NSCLC G12C 0.8 NCI-H460 NSCLC Q61H 3.1 NCI-H1373 NSCLC G12C 1.2 NCI-H1792 NSCLC G12C 1.4 NCI-H2030 NSCLC G12C 1.1 NCI-H23 NSCLC G12C 1.2 NCI-H358 NSCLC G12C 0.6 TOV-21G Ovarian G13C 1.7 AsPC-1 PDAC G12D 2.3 HPAF-II PDAC G12D 1.9 MIA PaCa-2 PDAC G12C 1.1 Panc 04.03 PDAC G12D 1.4 B. HCT-116 Control 1800 (mg) 1600 VOR 50 mg/kg 1400 VOR 100 mg/kg weight 1200 VOR 200 mg/kg 1000 800 tumor 600 400 Ave 200 0 0 2 4 6 8 10 12 Time (days) Control SW-480 (mg) 600 VOR 50 mg/kg 500 VOR 100 mg/kg weight 400 VOR 200 mg/kg 300 tumor 200 Ave 100 0 0 2 4 6 8 10 12 14 Time (days) Control H460 (mg) 6000 5000 VOR 100 mg/kg VOR 200 mg/kg weight 4000 3000 tumor 2000 Ave 1000 0 0 2 4 6 8 10 12 Time (days) Time (days) Figure 4. (A) Voruciclib IC50 values in multiple cell lines with KRAS mutations. (B) Murine xenograft experiment showing tumor growth over time in mice bearing HCT-116 (CRC, KRAS G13D), SW-480 (CRC, KRAS G12V) or H-460 (NSCLC, KRAS Q61H) tumors after treatment with voruciclib (VOR) at various doses (QD, p.o.) for 11-14 days. VORUCICLIB SYNERGIZES WITH KRAS G12C INHIBITORS IN VITRO Synergy Scores Cell Line KRAS mut Sensitivity to G12C Inhibitors Voruciclib + Sotorasib Voruciclib + Adagrasib NCI-H23 G12C High HCC1171 G12C High G12C High G12C Moderate—High NCI-H2030 G12C High Calu-1 G12C Moderate—High HCC-44 G12C Moderate—High NCI-H1373 G12C Moderate—High NCI-H358 G12C High NCI-H1792 G12C Moderate—High G12C Low—High G12D Low G12D Low G12D Low—Moderate G12D Low G12D Low TOV-21G G13C Low Non-small cell lung cancer cell lines Esophageal cell line Pancreatic adenocarcinoma cell lines Ovarian cell line Colorectal cancer cell lines low Moderate high Figure 5. Heatmap of combination activity of voruciclib with KRAS G12C inhibitors in cancer cell lines after 72 hours. Cell lines are ranked by synergy score of voruciclib in combination with either sotorasib or adagrasib. HSA, Bliss, and Loewe analyses were performed to generate the synergy scores using Chalice Analyzer. High synergy scores are represented as dark green. Moderate synergy scores are represented in shades of green. Low to moderate synergy scores are represented in white. Cell sensitivity to KRAS G12C inhibitors are ranked by EC50 scores. High (<0.1 µM), Moderate (> 0.1 µM), low (>1 µM). Where sensitivities to the two inhibitors differ, a range of responses is given.

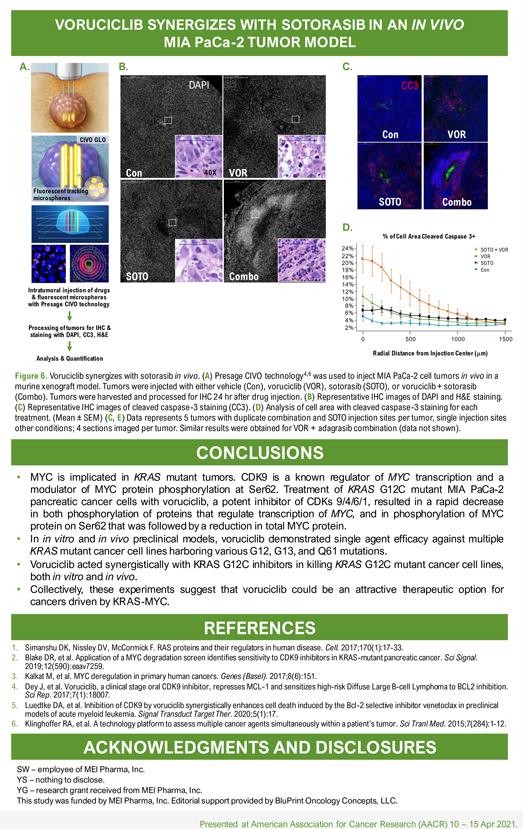
Poster: # 1962 Voruciclib, a CDK9 inhibitor, downregulates MYC and inhibits proliferation of KRAS mutant cancers in preclinical models Sandra Wiley*, Yongwei Su**, Yubin Ge** *MEI Pharma Inc, 11455 El Camino Real, Suite 250, San Diego, CA , 92130 **Department of Oncology and Molecular Therapeutics Program, Karmanos Cancer Institute, Wayne State University School of Medicine. VORUCICLIB SYNERGIZES WITH SOTORASIB IN AN IN VIVO MIA PaCa-2 TUMOR MODEL Intratumoral injection of drugs & fluorescent microspheres with Presage CIVO technology Processing of tumors for IHC & staining with DAPI, CC3, H&E Analysis & Quantification Figure 6. Voruciclib synergizes with sotorasib in vivo. (A) Presage CIVO technology4,6 was used to inject MIA PaCa-2 cell tumors in vivo in a murine xenograft model. Tumors were injected with either vehicle (Con), voruciclib (VOR), sotorasib (SOTO), or voruciclib + sotorasib (Combo). Tumors were harvested and processed for IHC 24 hr after drug injection. (B) Representative IHC images of DAPI and H&E staining. (C) Representative IHC images of cleaved caspase-3 staining (CC3). (D) Analysis of cell area with cleaved caspase-3 staining for each treatment. (Mean ± SEM) (C, E) Data represents 5 tumors with duplicate combination and SOTO injection sites per tumor, single injection sites other conditions; 4 sections imaged per tumor. Similar results were obtained for VOR + adagrasib combination (data not shown). CONCLUSIONS • MYC is implicated in KRAS mutant tumors. CDK9 is a known regulator of MYC transcription and a modulator of MYC protein phosphorylation at Ser62. Treatment of KRAS G12C mutant MIA PaCa-2 pancreatic cancer cells with voruciclib, a potent inhibitor of CDKs 9/4/6/1, resulted in a rapid decrease in both phosphorylation of proteins that regulate transcription of MYC, and in phosphorylation of MYC protein on Ser62 that was followed by a reduction in total MYC protein. • In in vitro and in vivo preclinical models, voruciclib demonstrated single agent efficacy against multiple KRAS mutant cancer cell lines harboring various G12, G13, and Q61 mutations. • Voruciclib acted synergistically with KRAS G12C inhibitors in killing KRAS G12C mutant cancer cell lines, both in vitro and in vivo. • Collectively, these experiments suggest that voruciclib could be an attractive therapeutic option for cancers driven by KRAS-MYC. REFERENCES 1. Simanshu DK, Nissley DV, McCormick F. RAS proteins and their regulators in human disease. Cell. 2017;170(1):17-33. 2. Blake DR, et al. Application of a MYC degradation screen identifies sensitivity to CDK9 inhibitors in KRAS-mutant pancreatic cancer. Sci Signal. 2019;12(590):eaav7259. 3. Kalkat M, et al. MYC deregulation in primary human cancers. Genes (Basel). 2017;8(6):151. 4. Dey Sci Rep. J, et 2017;7(1):18007. al. Voruciclib, a clinical stage oral CDK9 inhibitor, represses MCL-1 and sensitizes high-risk Diffuse Large B-cell Lymphoma to BCL2 inhibition. 5. Luedtke DA, et al. Inhibition of CDK9 by voruciclib synergistically enhances cell death induced by the Bcl-2 selective inhibitor venetoclax in preclinical models of acute myeloid leukemia. Signal Transduct Target Ther. 2020;5(1):17. 6. Klinghoffer RA, et al. A technology platform to assess multiple cancer agents simultaneously within a patient’s tumor. Sci Tranl Med. 2015;7(284):1-12. ACKNOWLEDGMENTS AND DISCLOSURES SW – employee of MEI Pharma, Inc. YS – nothing to disclose. YG – research grant received from MEI Pharma, Inc. This study was funded by MEI Pharma, Inc. Editorial support provided by BluPrint Oncology Concepts, LLC. Presented at American Association for Cancer Research (AACR) 10 – 15 Apr 2021.
