Attached files
| file | filename |
|---|---|
| 8-K - 8-K - MEI Pharma, Inc. | d75702d8k.htm |
| EX-99.3 - EX-99.3 - MEI Pharma, Inc. | d75702dex993.htm |
| EX-99.2 - EX-99.2 - MEI Pharma, Inc. | d75702dex992.htm |

Building a Leading Oncology Franchise NASDAQ: MEIP April 13, 2021 Exhibit 99.1

Forward-Looking Statements This presentation contains, and our officers and representatives may from time to time make, statements that are “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Examples of forward-looking statements include, among others, statements regarding our development strategy; potential advantages of our product candidates; the initiation and completion of preclinical and clinical studies and the reporting of the results thereof; the timing of regulatory submissions and actions; the sufficiency of our existing cash; and all other statements relating to our plans, objectives, expectations and beliefs regarding future performance, operations, financial condition and other future events (including assumptions underlying or relating to any of the foregoing). These forward-looking statements rely on a number of assumptions concerning future events and are subject to a number of risks, uncertainties, and other factors, many of which are outside of our control. Important factors that could cause our actual results and financial condition to differ materially from those indicated in forward-looking statements include, among others: uncertainties relating to the initiation and completion of preclinical and clinical studies; whether preclinical and clinical study results will validate and support the safety and efficacy of our product candidates; the outcome of regulatory reviews of our product candidates; varying interpretation of research and development and market data; the impact of the COVID-19 pandemic on our industry and individual companies, including on our counterparties, the supply chain, the execution of our clinical development programs, our access to financing and the allocation of government resources; risks and uncertainties relating to intellectual property and the other factors discussed under the caption “Item 1A. Risk Factors” in our most recent annual report on Form 10-K and our most recent quarterly report on Form 10-Q. Any forward-looking statement made by us in this presentation is based only on information currently available to us and speaks only as of the date on which it is made. In addition, we operate in a highly competitive and rapidly changing environment, and new risks may arise. Accordingly, you should not place any reliance on forward-looking statements as a prediction of actual results. We disclaim any intention to, and undertake no obligation to, update or revise any forward-looking statement. You are urged to carefully review and consider the various disclosures in our most recent annual report on Form 10-K, our most recent Form 10-Q and our other public filings with the SEC since the filing of our most recent annual report.

MEI Pharma: Who We Are Clinical Development Company Building a Leading Oncology Franchise with 4 Clinical-Stage Programs: Focus On HemOnc Zandelisib (f/k/a ME-401) Potential Best-in-Class PI3Kδ Inhibitor in Phase 2 Study Intended to Support Accelerated Approval Application with U.S. FDA Well Capitalized with ~$164.6 Million* * As of March 31, 2021, MEI had preliminary $164.6 million in cash, cash equivalents, and short-term investments with no outstanding debt (Unaudited).
Zandelisib Topline TIDAL Study Data on Track to be Reported in the Fourth Quarter of CY2021 Announced Today: Enrollment Complete in Follicular Lymphoma Primary Efficacy Population of Global Phase 2 TIDAL The complete Phase 2 TIDAL study data are intended to be submitted to FDA to support accelerated approval applications

Voruciclib: Oral CDK Inhibitor with Potent CDK9 Activity Preclinical Data
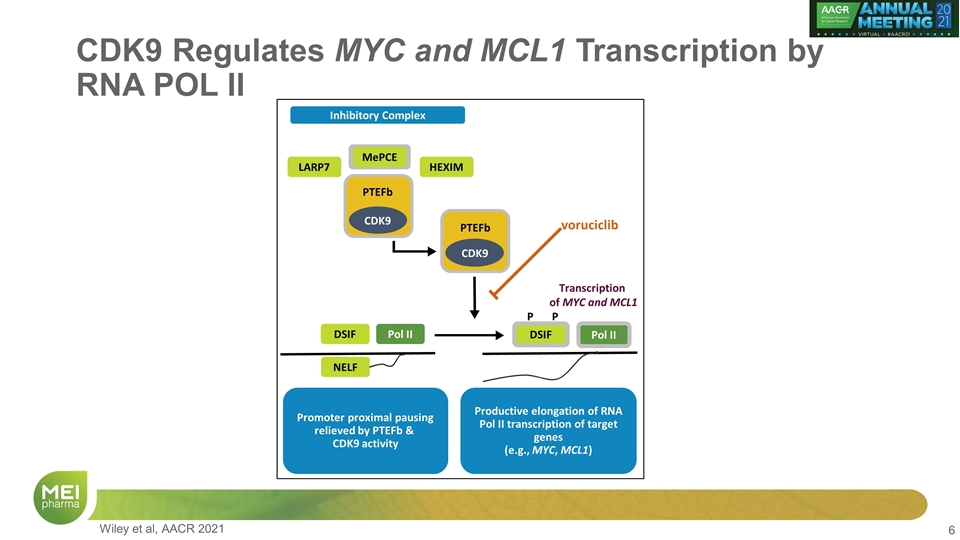
CDK9 Regulates MYC and MCL1 Transcription by RNA POL II DSIF HEXIM NELF Pol II DSIF Pol II Inhibitory Complex Productive elongation of RNA Pol II transcription of target genes (e.g., MYC, MCL1) Promoter proximal pausing relieved by PTEFb & CDK9 activity CDK9 P P PTEFb CDK9 PTEFb voruciclib Transcription of MYC and MCL1 MePCE LARP7 Wiley et al, AACR 2021
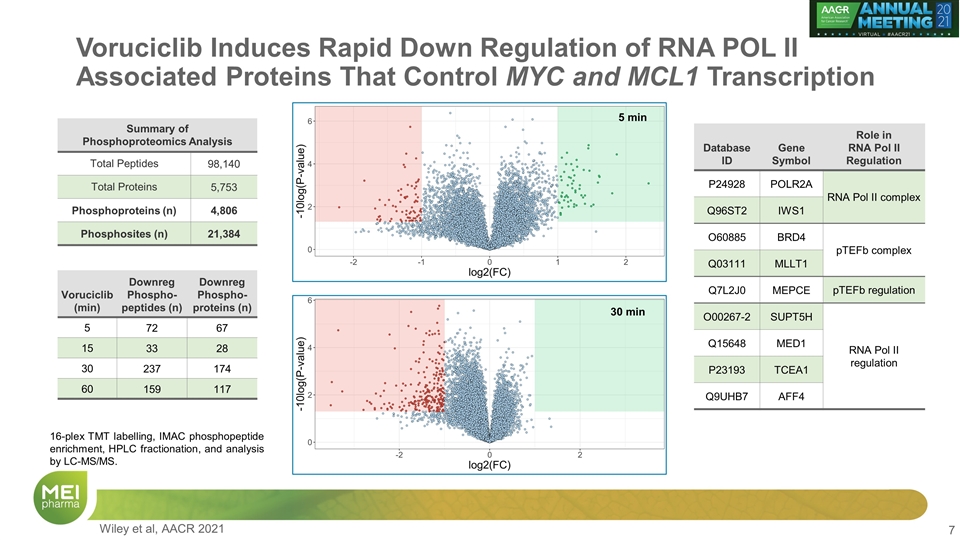
Voruciclib Induces Rapid Down Regulation of RNA POL II Associated Proteins That Control MYC and MCL1 Transcription Summary of Phosphoproteomics Analysis Total Peptides 98,140 Total Proteins 5,753 Phosphoproteins (n) 4,806 Phosphosites (n) 21,384 Voruciclib (min) Downreg Phospho-peptides (n) Downreg Phospho-proteins (n) 5 72 67 15 33 28 30 237 174 60 159 117 Database ID Gene Symbol Role in RNA Pol II Regulation P24928 POLR2A RNA Pol II complex Q96ST2 IWS1 RNA Pol II complex O60885 BRD4 pTEFb complex Q03111 MLLT1 pTEFb complex Q7L2J0 MEPCE pTEFb regulation O00267-2 SUPT5H RNA Pol II regulation Q15648 MED1 RNA Pol II regulation P23193 TCEA1 RNA Pol II transcription elongation Q9UHB7 AFF4 SEC regulation of RNA Pol II 5 min 30 min 16-plex TMT labelling, IMAC phosphopeptide enrichment, HPLC fractionation, and analysis by LC-MS/MS. Wiley et al, AACR 2021
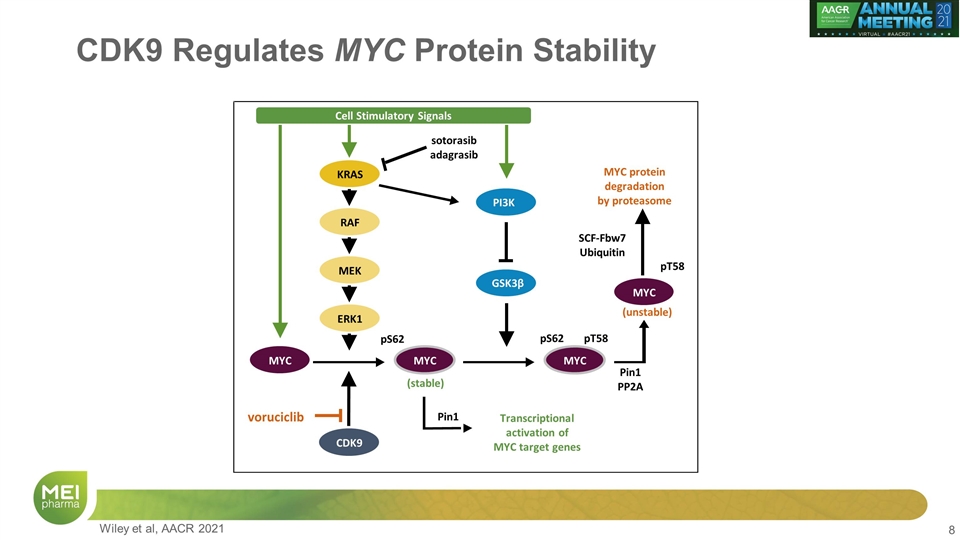
CDK9 Regulates MYC Protein Stability Cell Stimulatory Signals KRAS PI3K GSK3β CDK9 RAF MEK MYC MYC MYC protein degradation by proteasome SCF-Fbw7 Ubiquitin sotorasib adagrasib pT58 Pin1 Pin1 PP2A pS62 pS62 pT58 (unstable) voruciclib (stable) Transcriptional activation of MYC target genes MYC MYC ERK1 CDK9 Wiley et al, AACR 2021
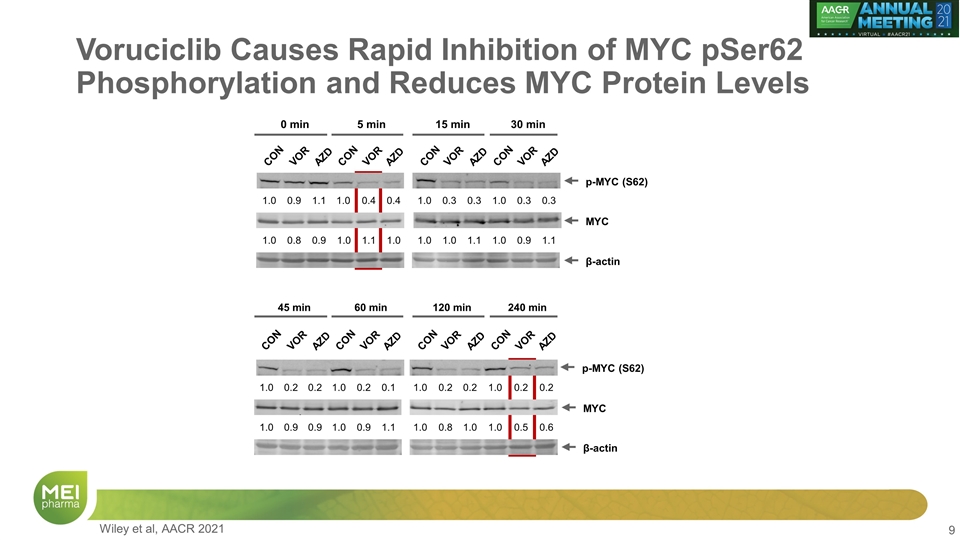
Voruciclib Causes Rapid Inhibition of MYC pSer62 Phosphorylation and Reduces MYC Protein Levels 0 min 5 min 15 min 30 min CON VOR AZD CON VOR AZD CON VOR AZD CON VOR AZD MYC β-actin p-MYC (S62) 1.0 0.9 1.1 1.0 0.4 0.4 1.0 0.8 0.9 1.0 1.1 1.0 1.0 0.3 0.3 1.0 0.3 0.3 1.0 1.0 1.1 1.0 0.9 1.1 β-actin MYC 45 min CON VOR AZD 60 min CON VOR AZD 120 min CON VOR AZD 240 min CON VOR AZD p-MYC (S62) 1.0 0.2 0.2 1.0 0.2 0.1 1.0 0.9 0.9 1.0 0.9 1.1 1.0 0.2 0.2 1.0 0.2 0.2 1.0 0.8 1.0 1.0 0.5 0.6 Wiley et al, AACR 2021
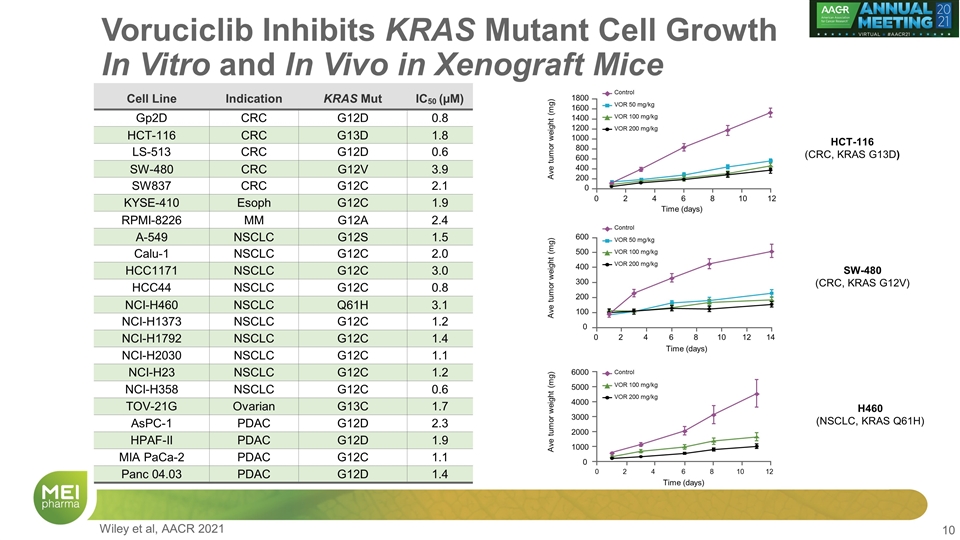
Voruciclib Inhibits KRAS Mutant Cell Growth In Vitro and In Vivo in Xenograft Mice Cell Line Indication KRAS Mut IC50 (µM) Gp2D CRC G12D 0.8 HCT-116 CRC G13D 1.8 LS-513 CRC G12D 0.6 SW-480 CRC G12V 3.9 SW837 CRC G12C 2.1 KYSE-410 Esoph G12C 1.9 RPMI-8226 MM G12A 2.4 A-549 NSCLC G12S 1.5 Calu-1 NSCLC G12C 2.0 HCC1171 NSCLC G12C 3.0 HCC44 NSCLC G12C 0.8 NCI-H460 NSCLC Q61H 3.1 NCI-H1373 NSCLC G12C 1.2 NCI-H1792 NSCLC G12C 1.4 NCI-H2030 NSCLC G12C 1.1 NCI-H23 NSCLC G12C 1.2 NCI-H358 NSCLC G12C 0.6 TOV-21G Ovarian G13C 1.7 AsPC-1 PDAC G12D 2.3 HPAF-II PDAC G12D 1.9 MIA PaCa-2 PDAC G12C 1.1 Panc 04.03 PDAC G12D 1.4 Time (days) Ave tumor weight (mg) 600 500 400 100 200 300 0 0 2 4 6 8 10 12 14 1800 1600 1400 1200 1000 800 600 400 200 0 0 2 4 6 Time (days) Ave tumor weight (mg) 8 10 12 Control VOR 100 mg/kg VOR 50 mg/kg VOR 200 mg/kg 6000 5000 4000 3000 2000 1000 0 0 2 4 6 Time (days) Ave tumor weight (mg) 8 10 12 Control VOR 100 mg/kg VOR 50 mg/kg VOR 200 mg/kg Control VOR 100 mg/kg VOR 200 mg/kg HCT-116 (CRC, KRAS G13D) SW-480 (CRC, KRAS G12V) H460 (NSCLC, KRAS Q61H) Wiley et al, AACR 2021
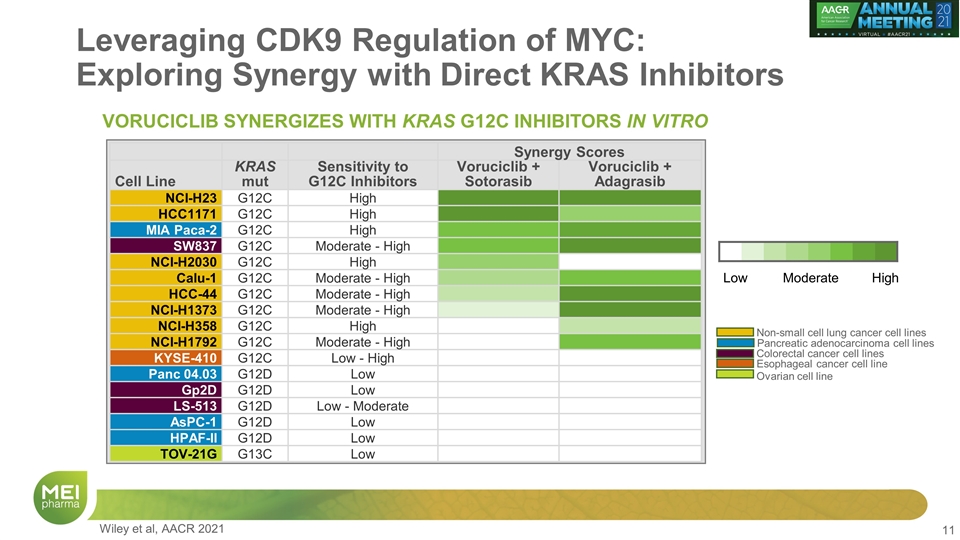
Leveraging CDK9 Regulation of MYC: Exploring Synergy with Direct KRAS Inhibitors Synergy Scores Cell Line KRAS mut Sensitivity to G12C Inhibitors Voruciclib + Sotorasib Voruciclib + Adagrasib NCI-H23 G12C High HCC1171 G12C High MIA Paca-2 G12C High SW837 G12C Moderate - High NCI-H2030 G12C High Calu-1 G12C Moderate - High HCC-44 G12C Moderate - High NCI-H1373 G12C Moderate - High NCI-H358 G12C High NCI-H1792 G12C Moderate - High KYSE-410 G12C Low - High Panc 04.03 G12D Low Gp2D G12D Low LS-513 G12D Low - Moderate AsPC-1 G12D Low HPAF-II G12D Low TOV-21G G13C Low Low High Moderate Esophageal cancer cell line Colorectal cancer cell lines Non-small cell lung cancer cell lines Pancreatic adenocarcinoma cell lines Ovarian cell line VORUCICLIB SYNERGIZES WITH KRAS G12C INHIBITORS IN VITRO Wiley et al, AACR 2021
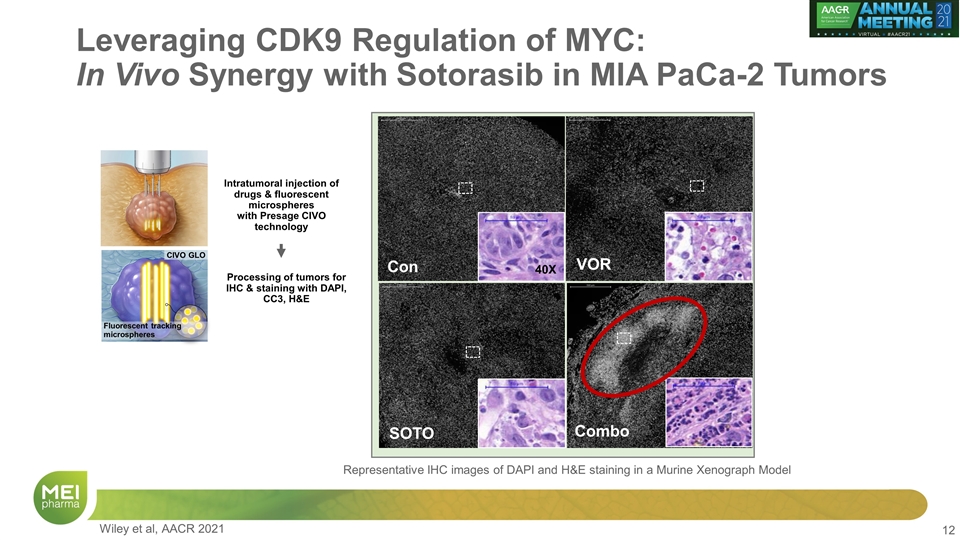
Leveraging CDK9 Regulation of MYC: In Vivo Synergy with Sotorasib in MIA PaCa-2 Tumors Con VOR SOTO Combo 40X Representative IHC images of DAPI and H&E staining in a Murine Xenograph Model CIVO GLO Fluorescent tracking microspheres Intratumoral injection of drugs & fluorescent microspheres with Presage CIVO technology Processing of tumors for IHC & staining with DAPI, CC3, H&E Wiley et al, AACR 2021
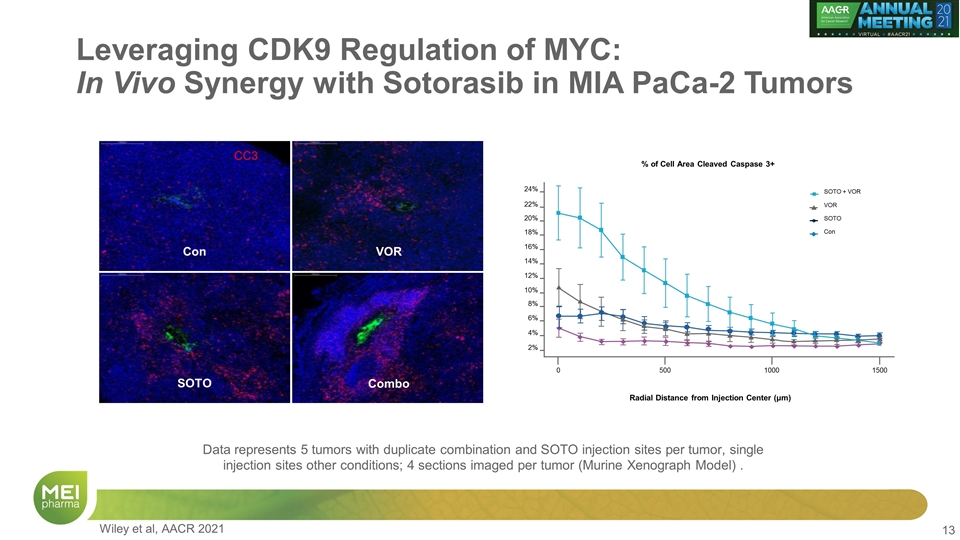
Leveraging CDK9 Regulation of MYC: In Vivo Synergy with Sotorasib in MIA PaCa-2 Tumors VOR Combo SOTO Con CC3 Radial Distance from Injection Center (μm) % of Cell Area Cleaved Caspase 3+ 24% 20% 16% 12% 8% 4% 0 500 1000 1500 22% 18% 14% 10% 6% 2% SOTO + VOR VOR SOTO Con Data represents 5 tumors with duplicate combination and SOTO injection sites per tumor, single injection sites other conditions; 4 sections imaged per tumor (Murine Xenograph Model) . Wiley et al, AACR 2021
CONCLUSIONS MYC is implicated in KRAS mutant tumors. CDK9 is a known regulator of MYC transcription and a modulator of MYC protein phosphorylation at Ser62 Treatment of KRAS G12C mutant MIA PaCa-2 pancreatic cancer cells with voruciclib resulted in a rapid decrease in both phosphorylation of proteins that regulate transcription of MYC, and in phosphorylation of MYC protein on Ser62 that was followed by a reduction in total MYC protein In in vitro and in vivo preclinical models, voruciclib demonstrated single agent activity against multiple KRAS mutant cancer cell lines harboring various G12, G13, and Q61 mutations Voruciclib acted synergistically with KRAS G12C inhibitors in killing KRAS G12C mutant cancer cell lines, both in vitro and in vivo Collectively, these experiments suggest that voruciclib could be an attractive therapeutic option for cancers driven by KRAS-MYC Wiley et al, AACR 2021

Voruciclib: Oral CDK Inhibitor with Potent CDK9 Activity Clinical Experience
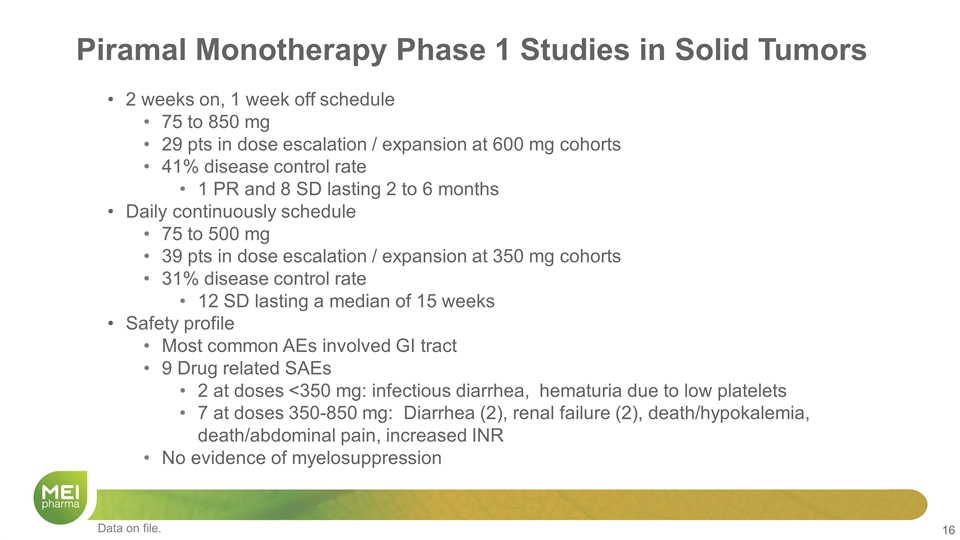
Piramal Monotherapy Phase 1 Studies in Solid Tumors 2 weeks on, 1 week off schedule 75 to 850 mg 29 pts in dose escalation / expansion at 600 mg cohorts 41% disease control rate 1 PR and 8 SD lasting 2 to 6 months Daily continuously schedule 75 to 500 mg 39 pts in dose escalation / expansion at 350 mg cohorts 31% disease control rate 12 SD lasting a median of 15 weeks Safety profile Most common AEs involved GI tract 9 Drug related SAEs 2 at doses <350 mg: infectious diarrhea, hematuria due to low platelets 7 at doses 350-850 mg: Diarrhea (2), renal failure (2), death/hypokalemia, death/abdominal pain, increased INR No evidence of myelosuppression Data on file.
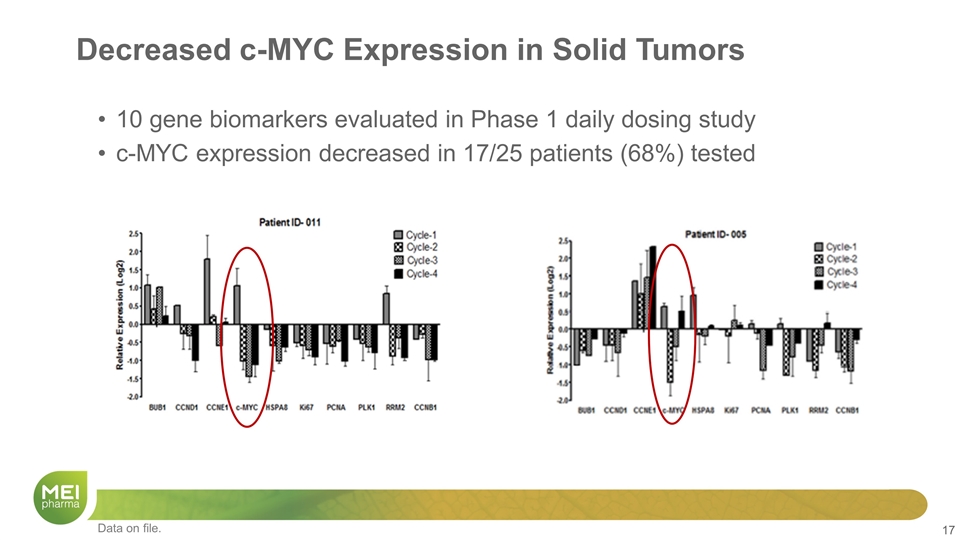
Decreased c-MYC Expression in Solid Tumors 10 gene biomarkers evaluated in Phase 1 daily dosing study c-MYC expression decreased in 17/25 patients (68%) tested Data on file.
Piramal Study of Voruciclib + Vemurafenib in BRAF-mut Advanced/Inoperable Malignant Melanoma Voruciclib 150 mg daily plus vemurafenib 720 mg or 960 mg BID in 28-day cycles 9 pts treated before study termination for business reasons 8 patients evaluable for efficacy 5 patients were BRAFi refractory Best Response = PD 3 patients were BRAF/MEK naïve 1 CR and 2 PR ongoing for 3 to 14 months Most common AEs were fatigue, constipation, diarrhea, arthralgia and headache 1 DLT = grade 3 fatigue No SAEs related to voruciclib Diab et al, ASCO 2015
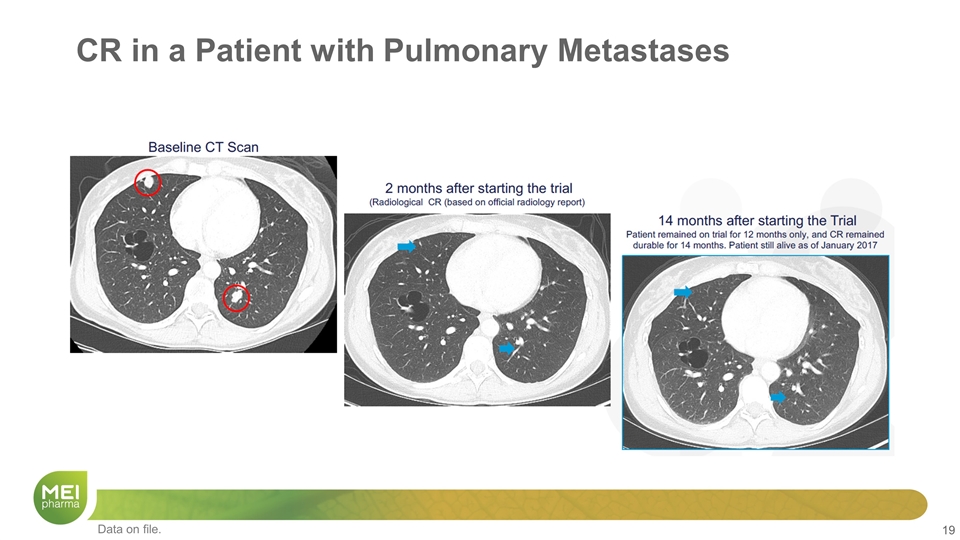
CR in a Patient with Pulmonary Metastases Data on file.
Leveraging CDK9 Regulation of MCL1: Phase 1 Study in R/R B-Cell Malignancies and AML Study population Relapsed/Refractory B-cell malignancies Relapsed/Refractory AML Dose escalation with standard 3+3 design Endpoints Safety and tolerability Pharmacokinetics Biologic correlative studies BH3 profiling, MCL-1 expression (Dana Farber) Molecular mutations analysis (City of Hope) Response rates Voruciclib single agent dose escalation/optimization - Enrolling 100 mg 150 mg 200 mg 50mg
Phase 1 Study in Hematologic Malignancies 24 pts treated in 3 dose levels 10 AML and 14 B-cell malignancies No drug related GI toxicity or neutropenia at doses studied Favorable PK profile across all voruciclib studies Half life 24-28 hours supports once-a-day dosing Dose proportional Cmax and AUC High volume of distribution indicates broad entry into tissues Doses of 150-200 mg projected to achieve plasma concentrations sufficient to inhibit molecular target Data on file.
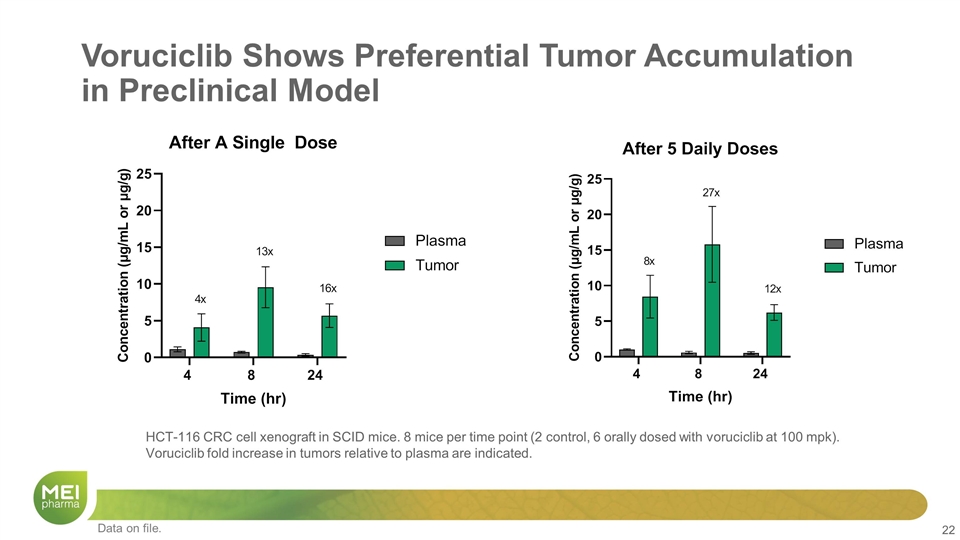
HCT-116 CRC cell xenograft in SCID mice. 8 mice per time point (2 control, 6 orally dosed with voruciclib at 100 mpk). Voruciclib fold increase in tumors relative to plasma are indicated. Voruciclib Shows Preferential Tumor Accumulation in Preclinical Model Data on file.
Evidence of Biologic Activity in AML Suspected Differentiation syndrome seen in 5 pts (50%) Increased WBC without increased in blasts, bone pain, and/or pulmonary symptoms Response to corticosteroids Differentiation syndrome reported with ATRA, IDHi, and other AML targeted therapies Data on file.
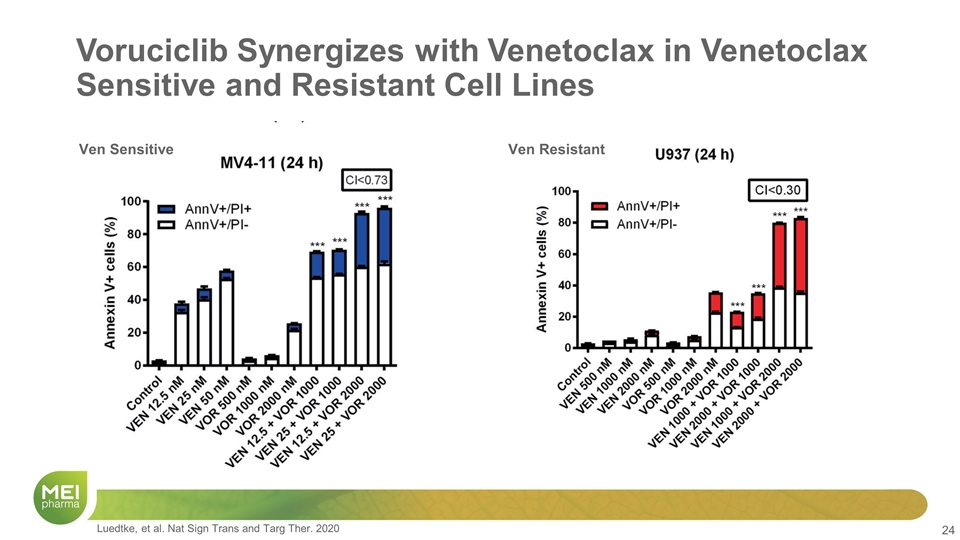
Voruciclib Synergizes with Venetoclax in Venetoclax Sensitive and Resistant Cell Lines Ven Sensitive Ven Resistant Luedtke, et al. Nat Sign Trans and Targ Ther. 2020
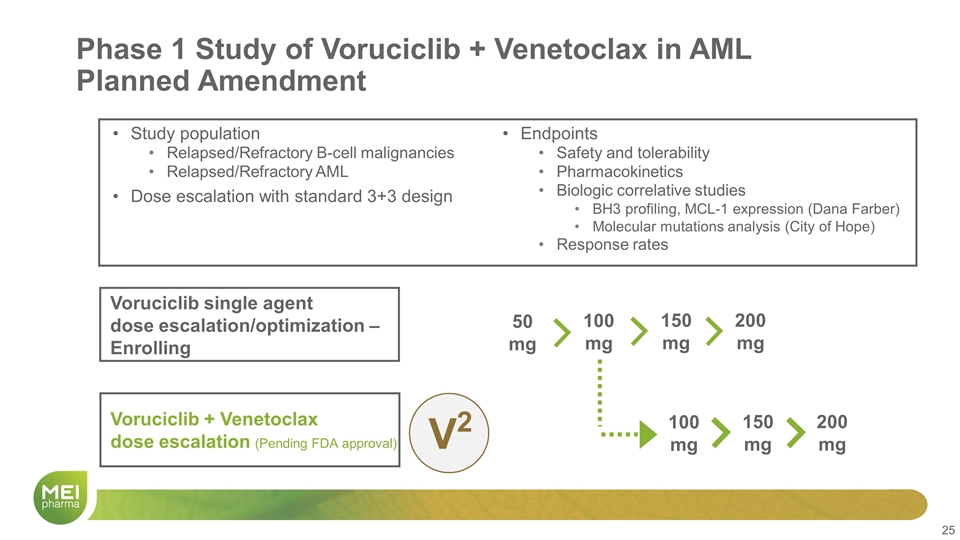
Phase 1 Study of Voruciclib + Venetoclax in AML Planned Amendment Study population Relapsed/Refractory B-cell malignancies Relapsed/Refractory AML Dose escalation with standard 3+3 design Endpoints Safety and tolerability Pharmacokinetics Biologic correlative studies BH3 profiling, MCL-1 expression (Dana Farber) Molecular mutations analysis (City of Hope) Response rates Voruciclib single agent dose escalation/optimization – Enrolling Voruciclib + Venetoclax dose escalation (Pending FDA approval) 100 mg 150 mg 200 mg 100 mg 150 mg 200 mg V2 50 mg

Key Upcoming 12 Month Milestones Across Portfolio Zandelisib TIDAL, study intended to support accelerated approval application for R/R follicular lymphoma: Announce top-line data fourth quarter CY2021 New clinical studies to expand development, including: COASTAL, intended confirmatory Phase 3 study + Rituxan® in 2L FL/MZL TIDAL study arm: 3L MZL 1L DLBCL + RCHOP (IIT) Initial data of Phase 1b evaluating zandelisib with zanubrutinib under clinical collaboration with BeiGene: Mid CY2021 Voruciclib Initial data, Phase 1 monotherapy and +BCL2i data updates ME-344 Institute plan to leverage clinically demonstrated anti-tumor activity in combination with anti-VEGF Pracinostat Program / Phase 2 MDS Helsinn Update All Timing subject to developments related to the COVID-19 pandemic

Q&A NASDAQ: MEIP

Building a Leading Oncology Franchise NASDAQ: MEIP April 13, 2021
