Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - GLAUKOS Corp | tm212991d1_ex99-1.htm |
| 8-K - FORM 8-K - GLAUKOS Corp | tm212991d1_8k.htm |
Exhibit 99.2

1 © 2021 Glaukos Corporation January 2021

2 © 2021 Glaukos Corporation Disclaimer All statements other than statements of historical facts included in this presentation that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward - looking statements . These statements are based on management’s current expectations, assumptions, estimates and beliefs . Although we believe that we have a reasonable basis for forward - looking statements contained herein, we caution you that they are based on current expectations about future events affecting us and are subject to risks, uncertainties and factors relating to our operations and business environment, all of which are difficult to predict and many of which are beyond our control, that may cause our actual results to differ materially from those expressed or implied by forward - looking statements in this presentation . These potential risks and uncertainties that could cause actual results to differ materially from those described in forward - looking statements include, without limitation, uncertainties regarding the duration and severity of the COVID - 19 pandemic and its impact on our business or the economy generally ; uncertainties about our dependence on the success and market acceptance of the iStent, iStent inject, and iStent inject W ; our ability to reach sustained profitability ; our ability to leverage our sales and marketing infrastructure to increase market penetration and acceptance of our products both in the United States and internationally ; our ability to bring our pipeline products to market ; our dependence on a limited number of third - party suppliers, some of which are single - source, for components of our products ; the occurrence of a crippling accident, natural disaster, pandemic (including an outbreak of COVID - 19 ) or other disruption at our primary facility, which may materially affect our manufacturing capacity and operations ; maintaining adequate coverage or reimbursement by third - party payors for procedures using the iStent, the iStent inject, our corneal cross - linking products or other products in development ; our ability to properly train, and gain acceptance and trust from, ophthalmic surgeons in the use of our products ; our ability to successfully develop and commercialize investigational products and the continued efficacy and safety profile once commercialized ; our ability to compete effectively in the highly competitive and rapidly changing medical device industry and against current and future competitors (including MIGS competitors) that are large public companies or divisions of publicly traded companies that have competitive advantages ; the timing, effect, expense and uncertainty of navigating different regulatory approval processes as we develop additional products and penetrate foreign markets ; the impact of any product liability claims against us and any related litigation ; the effect of the extensive and increasing federal and state regulation in the healthcare industry on us and our suppliers ; the lengthy and expensive clinical trial process and the uncertainty of timing and outcomes from any particular clinical trial ; the risk of recalls or serious safety issues with our products and the uncertainty of patient outcomes ; our ability to protect, and the expense and time - consuming nature of protecting, our intellectual property against third parties and competitors that could develop and commercialize similar or identical products ; the impact of any claims against us of infringement or misappropriation of third party intellectual property rights and any related litigation ; the market’s perception of our limited operating history as a public company ; our ability to service our indebtedness ; and potential disruptions from the acquisition of Avedro that may divert management attention from other important business objectives . These and other known risks, uncertainties and factors are described in detail under the caption “Risk Factors” and elsewhere in our filings with the Securities and Exchange Commission, including our Quarterly Report on Form 10 - Q for the quarter ended June 30 , 2020 , which was filed with the SEC on August 7 , 2020 and our Quarterly Report on Form 10 - Q for the quarter ended September 30 , 2020 , which was filed with the SEC on November 5 , 2020 . Our filings with the SEC are available in the Investor Section of our website at www . glaukos . com or at www . sec . gov . In addition, information about the risks and benefits of our products is available on our website at www . glaukos . com . All forward - looking statements included in this presentation are expressly qualified in their entirety by the foregoing cautionary statements . You are cautioned not to place undue reliance on the forward - looking statements in this presentation, which speak only as of the date hereof . We do not undertake any obligation to update, amend or clarify these forward - looking statements whether as a result of new information, future events or otherwise, except as may be required under applicable securities law . In addition, with respect to COVID - 19 , we are currently unable to reasonably estimate the specific extent, or duration, of the impact of the COVID - 19 outbreak on our financial and operating results . We are also unable to predict how the outbreak will continue to affect restrictions and advisories on elective procedures and therapies, the availability of physicians and/or their treatment prioritizations or the impact of the outbreak on the overall healthcare infrastructure . In addition to an impact on procedure volumes, we are experiencing and may experience other disruptions as a result of the COVID - 19 outbreak . For example, it is possible our suppliers will incur challenges supplying the materials needed for the manufacture of our product . In addition, our clinical trials may be adversely affected . Other disruptions or potential disruptions include restrictions on the ability of Company personnel to travel and access customers for training and case support ; delays in approvals by regulatory bodies ; delays in product development efforts ; and additional government requirements to “shelter at home” or other incremental mitigation efforts that may further impact our capacity to manufacture, sell and support the use of our products . The total impact of these disruptions could have a material impact on the Company’s financial condition, cash flows and results of operations .
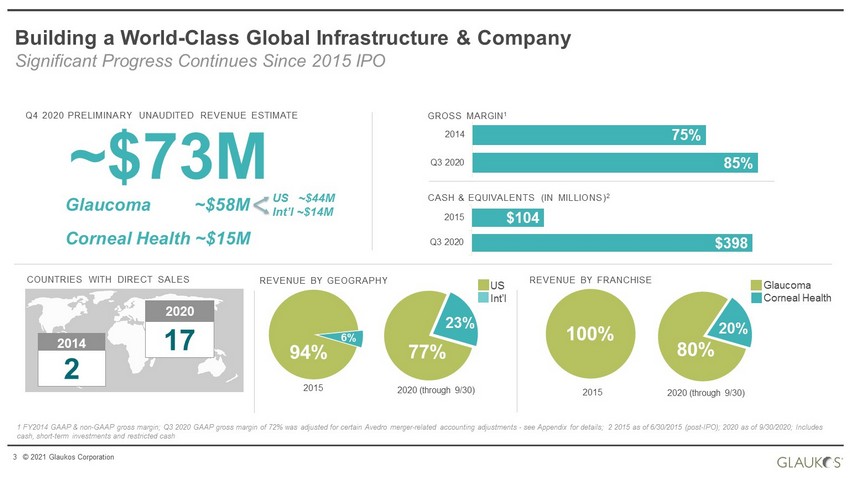
3 © 2021 Glaukos Corporation 1 FY2014 GAAP & non - GAAP gross margin; Q3 2020 GAAP gross margin of 72% was adjusted for certain Avedro merger - related accounting adjustments - see Appendix for details; 2 2015 as of 6/30/2015 (post - IPO); 2020 as of 9/30/2020; Includes cash, short - term investments and restricted cash Building a World - Class Global Infrastructure & Company Significant Progress Continues Since 2015 IPO 85% 75% Q3 2020 2014 GROSS MARGIN 1 CASH & EQUIVALENTS (IN MILLIONS) 2 2014 2020 COUNTRIES WITH DIRECT SALES 2 17 $398 $104 Q3 2020 2015 REVENUE BY GEOGRAPHY US Int’l 77% 23% 2020 (through 9/30) REVENUE BY FRANCHISE 2015 94% 6% 100% 80% 20% 2015 2020 (through 9/30) Glaucoma Corneal Health Q4 2020 PRELIMINARY UNAUDITED REVENUE ESTIMATE ~$73M Glaucoma ~$58M Corneal Health ~$15M US ~$44M Int’l ~$14M

4 © 2021 Glaukos Corporation 2020: Major Accomplishments that Advanced our Growth Strategy ESTABLISHED CORNEAL HEALTH FRANCHISE SUCCESSFUL LAUNCH OF ISTENT INJECT W SWIFT & EFFECTIVE COVID RESPONSE STRENGTHENED BALANCE SHEET COMPLETED GLOBAL SYSTEMS TRANFORMATION INTERNATIONAL SALES GROWTH & APPROVALS ADVANCED MARKET - EXPANDING PIPELINE CONTINUED TO OPTIMIZE REIMBURSEMENT Completed seamless integration of Avedro business, delivering record Photrexa revenue and KXL placements Providing surgeons optimized stent visualization, streamlined implantation and procedural predictability with iStent inject W Executing plans designed to ensure safe work environments for employees while meeting customer needs and reducing annual costs by ~$25M Reported strong international sales related to recent expansion efforts; received new regulatory approvals in Australia and Japan Worked proactively with multiple entities to ensure reimbursement coverage and payment, including upcoming combo - cataract MIGS transition to Category I Completed iStent infinite FDA trial, continued enrollment in Phase 3 iDose TR trials and made progress on multiple pre - clinical R&D programs Completed $288M convertible debt offering, further strengthening our balance sheet and ability to invest in future growth Enterprise - wide upgrades designed to enhance business activities and support long - term growth

5 © 2021 Glaukos Corporation Our Strategy for Long - Term Growth & Profitability Creating a Unique Vision Care Leader Micro - Scale Surgical Devices Pharmaceuticals Biosensors Ocular Hypertension to Refractory Disease Glaucoma Corneal Health Keratoconus Ocular Surface Disease Refractive Conditions Retinal Disease Wet AMD DME RVO MAJOR PLATFORMS THERAPEUTIC CLASSES

6 © 2021 Glaukos Corporation Our Strategy for Long - Term Growth & Profitability Serving the Clinical Needs of Large Patient Populations 1 Estimate based on Glaukos algorithm of physician preference and combination therapy, utilization; assumes full product port fol io availability to physician, except pre - clinical products (iDose TREX, iDose Rock, IOP Sensor); 2 Company estimates of US opportunity; 3 Market Scope estimates Ocular Hypertension to Refractory Disease Glaucoma Corneal Health Keratoconus Ocular Surface Disease Refractive Conditions Retinal Disease Wet AMD DME RVO $13B Est. of global opportunity 1 $23B Est. of US opportunity for keratoconus ($3B) and iLink for presbyopia ($15B), and est. of US annual dry eye market ($2.4B) 2,3 $13B Est. of global annual market size 3 THERAPEUTIC CLASSES
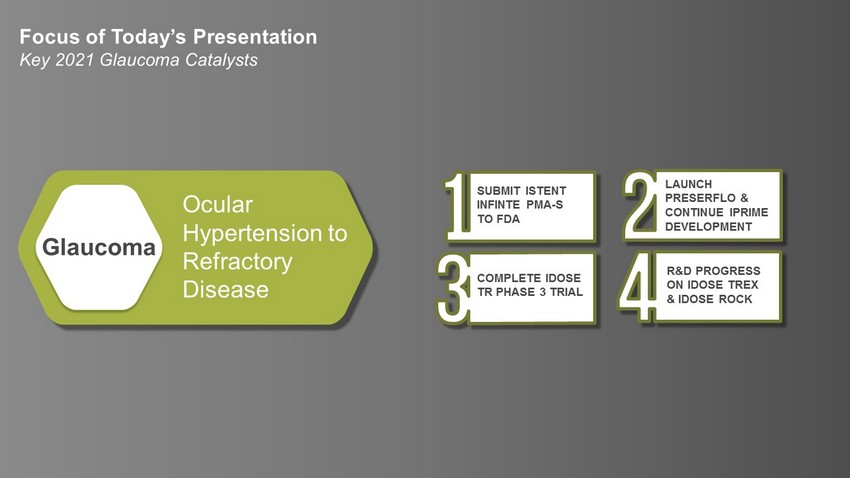
7 © 2021 Glaukos Corporation Ocular Hypertension to Refractory Disease Glaucoma Focus of Today’s Presentation Key 2021 Glaucoma Catalysts SUBMIT ISTENT INFINTE PMA - S TO FDA COMPLETE IDOSE TR PHASE 3 TRIAL R&D PROGRESS ON IDOSE TREX & IDOSE ROCK LAUNCH PRESERFLO & CONTINUE IPRIME DEVELOPMENT

8 © 2021 Glaukos Corporation iStent infinite: Powerful Standalone MIGS Indication FDA Submission Set for 1H 2021; Targeting Late 2021 Approval Three wide - flange stents preloaded in injector system that facilitates placement across ~6 clock hours of Schlemm’s canal US IDE open - label, single - arm study in a standalone procedure 72 subjects with open - angle glaucoma uncontrolled by prior surgical or medical therapy; enrollment completed Oct 2019 Our focus is on patient achievement of 20% or greater reduction in mean diurnal IOP from baseline at 12 months on same or lower ocular hypotensive medication burden US CLINICAL TRIAL iStent infinite is not approved by the FDA Photo courtesy of George R. Reiss MD

9 © 2021 Glaukos Corporation iStent infinite: Significant IOP Reduction in Severe Patients FDA Submission Set for 1H 2021; Targeting Late 2021 Approval 76% of subjects achieved 20% or greater reduction in mean diurnal IOP from baseline on same or lower ocular hypotensive medication burden Highly favorable safety profile (no explants, infections or device - related interventions or hypotony) iStent infinite is not approved by the FDA 13% Most difficult to treat glaucoma patients Average of 3.1 IOP lowering medications at baseline Already failed average of 2 prior glaucoma surgeries ≥ 50% of subjects achieved 30% or greater reduction in mean diurnal IOP from baseline mean reduction in medication burden from baseline

10 © 2021 Glaukos Corporation iStent infinite: Viable Alternative to More Complex Procedures Three - stent injectable will offer new compelling treatment option in standalone procedure with highly favorable safety profile Clinical data suggest iStent infinite may help patients with open - angle glaucoma uncontrolled by prior surgical or medical therapy Expect surgeons – glaucoma specialists and comprehensive ophthalmologists – to gravitate to iStent infinite before proceeding to tissue - destructive procedures iStent infinite is not approved by the FDA

11 © 2021 Glaukos Corporation Santen PreserFlo MicroShunt An Alternative to Trabeculectomy & Tube Shunts 8.5 mm tube made of biocompatible material (SIBS); standalone, ab - externo procedure to filter fluid from anterior chamber to subconjunctival space Designed to treat late - stage, refractory glaucoma Attractive alternative to trabeculectomy, tubes, XEN and ExPress • Demonstrated IOP reduction (30+%) and medication burden reduction (2.4 meds) in pivotal study 1 Favorable safety, efficacy and post - op care profile Glaukos is exclusive partner in US and Australia • FDA approval pending • Anticipate US launch in Q2 2021 • Will utilize established Category I CPT code (66183) Santen PreserFlo is not approved by the FDA 1 Santen press release issued Aug 30, 2019

12 © 2021 Glaukos Corporation Introducing iPRIME™ A New Viscodelivery System from Glaukos iPRIME is a minimally invasive viscoelastic delivery system that further supports the needs of physicians and patients This complementary technology further expands Glaukos’ broad portfolio of innovative ophthalmic solutions Development - stage program, not FDA - approved iPRIME is not approved by the FDA or commercially available A viscoelastic delivery system for use in Schlemm’s canal

13 © 2021 Glaukos Corporation Glaucoma Micro - Surgical Portfolio Comprehensive, Tissue - Sparing Offering Designed to Address Full Range of Disease Progression & Facilitate Combination Therapies Creates 2 pathways for fluid outflow Creates 3 pathways for fluid outflow across 6 clock hours of Schlemm’s canal Like iStent inject W; but for use in pseudophakic patients Ab - externo device for late - stage glaucoma Santen’s exclusive US partner INTENDED DISEASE STATE Mild to Moderate Advanced to Refractory flexible microcatheter along with inserting viscoelastic to disconnect potential adhesions and debris in the canal and distal co llector channels Creates pathway for fluid outflow Combo - Cataract Standalone ™ ™ Creates 2 pathways for fluid outflow; wider flange enhances ease of use and visibility Viscoelastic delivery system for use in Schlemm’s canal iStent infinite, iStent SA, iPRIME and PreserFlo are not approved by the FDA

14 © 2021 Glaukos Corporation Sustained - Release Pharmaceuticals The Next Stage in Glaucoma Therapy • Designed to provide longest duration intracameral pharmaceutical; secure and anchored design; facile implantation and exchange • Membrane designed to elute specially formulated travoprost, a commonly prescribed topical prostaglandin • Phase 3 clinical trials underway iDose 1 Market opportunity estimates based on Glaukos algorithm of physician preference and combination therapy, utilization; assum es full product portfolio availability to physician, except pre - clinical products (iDose TREX, iDose Rock, IOP Sensor) Topical drugs subject to significant issues of patient non - adherence, impositions to quality - of - life and ocular surface disease and toxicity Unmet need and appetite among glaucoma specialists and ophthalmologists for sustained - release pharmaceutical alternatives Current mobile, intracameral bio - erodible implants limited by endothelial cell loss, labeling restrictions and relatively short durations of activity CLINICAL NEED • AMA approved Cat III codes for implantation, removal and re - implantation of drug delivery system into anterior chamber, effective 7/21 • Estimated annual US opportunity of 3M eyes 1 Ocular Hypertension to Refractory OAG iDose TR is not approved by the FDA

15 © 2021 Glaukos Corporation Sustained - Release Pharmaceuticals: iDose TR Alternative to Topical Medications, Addressing Non - Adherence & Other Drawbacks iDose TR is not approved by the FDA Average Reduction in IOP PHASE 2 CLINICAL DATA AT 24 MONTHS* 154 - patient, multi - center, randomized, double - blind trial Evaluated 2 iDose models with two different travoprost elution rates, compared to topical timolol ophthalmic solution, 0.5% Primary efficacy endpoint of non - inferiority to topical timolol Subjects diagnosed with mild to moderate OAG or ocular hypertension, on 0 to 3 meds with baseline IOP between 21 mmHg and 36 mmHg Additional medications were added if IOP was above 18 mmHg Month 3 Month 6 Month 9 Month 12 Month 18 Month 24 5.0 6.0 7.0 8.0 9.0 7.5 7.5 7.6 7.6 7.6 7.8 7.7 7.7 7.6 7.5 7.5 7.4 8.3 8.1 8.0 7.8 7.7 7.9 m m H g iDose FE iDose SE Timolol 0.5% BID *Calculated using all IOP observations through each data point weighted equally, no imputations for mandated medications PHASE 2 CLINICAL TRIAL n = 51 54 49 51 54 49 49 54 49 49 53 48 49 52 48 47 50 46
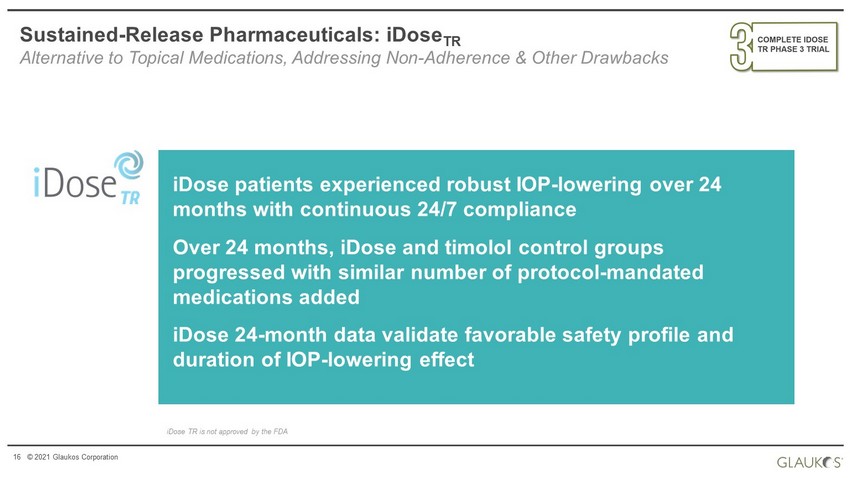
16 © 2021 Glaukos Corporation Sustained - Release Pharmaceuticals: iDose TR Alternative to Topical Medications, Addressing Non - Adherence & Other Drawbacks iDose patients experienced robust IOP - lowering over 24 months with continuous 24/7 compliance Over 24 months, iDose and timolol control groups progressed with similar number of protocol - mandated medications added iDose 24 - month data validate favorable safety profile and duration of IOP - lowering effect iDose TR is not approved by the FDA
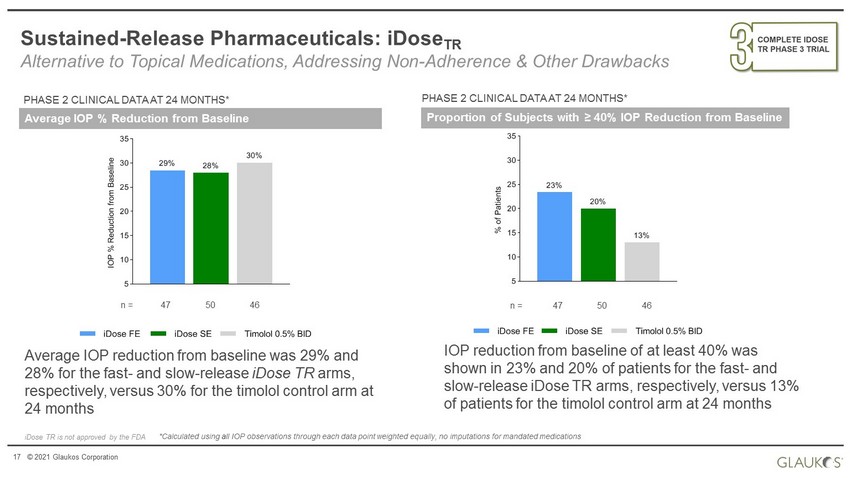
17 © 2021 Glaukos Corporation Sustained - Release Pharmaceuticals: iDose TR Alternative to Topical Medications, Addressing Non - Adherence & Other Drawbacks Average IOP % Reduction from Baseline PHASE 2 CLINICAL DATA AT 24 MONTHS* iDose TR is not approved by the FDA 5 10 15 20 25 30 35 30% 28% 29% I O P % R e d u c t i o n f r o m B a s e l i n e iDose FE iDose SE Timolol 0.5% BID *Calculated using all IOP observations through each data point weighted equally, no imputations for mandated medications Average IOP reduction from baseline was 29% and 28% for the fast - and slow - release iDose TR arms, respectively, versus 30% for the timolol control arm at 24 months n = 47 50 46 5 10 15 20 25 30 35 13% 20% 23% % o f P a t i e n t s iDose FE iDose SE Timolol 0.5% BID n = 47 50 46 Proportion of Subjects with ≥ 40% IOP Reduction from Baseline PHASE 2 CLINICAL DATA AT 24 MONTHS* IOP reduction from baseline of at least 40% was shown in 23% and 20% of patients for the fast - and slow - release iDose TR arms, respectively, versus 13% of patients for the timolol control arm at 24 months

18 © 2021 Glaukos Corporation Sustained - Release Pharmaceuticals: iDose TR Alternative to Topical Medications, Addressing Non - Adherence & Other Drawbacks Designed to address primary shortcomings of existing glaucoma topical and intracameral therapeutics Patients are generally poorly compliant with IOP - lowering eye drops iDose design implies 100% compliance once administered Improved compliance with glaucoma medications has been associated with better outcomes in glaucoma disease iDose offers fixed placement to improve the safety for corneal endothelium 1,460 Subjects received one administration of iDose vs approximately eye drops per eye in the control arm with twice - a - day timolol over 24 months per protocol greater* IOP reduction Subjects who were on one pre - study IOP - lowering medication at screening had over 24 months on iDose TR vs the pre - study IOP - lowering eye drops iDose subjects in fast - and slow - elution arms had clinically significant corneal endothelial cell loss, no serious corneal adverse events, and no conjunctival hyperemia adverse events to date no iDose TR is not approved by the FDA * For subjects on a single pre - study medication, iDose TR demonstrated incremental IOP reduction at 24 months of 1.1 mmHg and 1.5 mmHg versus pre - study IOP for slow and fast eluting arms, respectively
19 © 2021 Glaukos Corporation iDose TR, iDose TREX and iDose ROCK are not approved by the FDA ® Sustained - Release Pharmaceuticals: iDose Platform Next Generation iDose TREX & iDose ROCK INTENDED DISEASE STATE Ocular Hypertension to Refractory OAG Substantial increase in drug payload, with potential to double the duration - of - effect vs first - generation iDose TR Same external dimensions, titanium material, elution rate, anchor design and implantation procedure as first - generation iDose TR Uses travoprost formulation identical to first - generation iDose TR J - code structure provides opportunity for additional reimbursement vs iDose TR ® Leveraging iDose platform technology to develop sustained - release system for rho kinase inhibitors Evaluating multiple compounds with plan to select candidate for advancement into clinical trials ®

20 © 2021 Glaukos Corporation Creating a Vision Care Leader

21 © 2021 Glaukos Corporation Ocular Hypertension to Refractory Disease Glaucoma Corneal Health Keratoconus Ocular Surface Disease Refractive Conditions Retinal Disease Wet AMD DME RVO SUBMIT ISTENT INFINTE PMA - S TO FDA LAUNCH PRESERFLO & CONTINUE IPRIME DEVELOPMENT COMPLETE IDOSE TR PHASE 3 TRIAL R&D PROGRESS ON IDOSE TREX & IDOSE ROCK PREPARE FOR EPI - ON NDA FILING IN 2022 BEGIN CLINCAL TRIALS FOR EPI - ON NEXT GEN GENERATE FIRST CLINICAL DRY EYE CANDIDATE MOVE FIRST RETINAL DISEASE PROGRAM INTO CLINIC 2021: Key Catalysts to Drive the Business Forward

22 © 2021 Glaukos Corporation Corneal Health Platforms Epi - on (Epioxa) Photrexa (Epi - off) Single - application, bio - activated topical pharmaceutical solution shown to halt keratoconus progression Uses photo - activation to create bonds between corneal collagen fibers Epi - on Next - Gen Second - generation treatment for keratoconus Designed to reduce treatment time and complexity, improving patient comfort and recovery time Uses stronger UVA irradiation protocol and “boost” goggles to increase oxygen availability FDA trial enrollment completed; targeting NDA filing in 2022 New laser system, personalized treatment algorithm and proprietary chemical entity Designed to further enhance iLink therapy for keratoconus Evaluating potential for treating other conditions Patented, cream - based formulation designed to be applied to upper eyelid for delivery at the lacrimal functional unit for treating dry eye Easier administration than topical eye drops, potentially better patient compliance Currently investigating possible applications for glaucoma and other ocular disorders Dry Eye / Ocular Surface Disease Not approved by the FDA Not approved by the FDA Not approved by the FDA

23 © 2021 Glaukos Corporation Triamcinolone Acetonide SR RVO & DME Formulations developed to release triamcinolone acetonide for up to 6 months Microscope and SEM images of implants 1 Multi - Kinase Inhibitor SR Wet AMD, RVO & DME Developing a multi - kinase inhibitor small molecule sustained - release delivery system Formulations developed for 6 to 12 months release Prototype 4 - month implant demonstrated 4 months of promising efficacy in a translational model of persistent retinal vessel leakage following a single intravitreal injection 2 % Released Anti - VEGF SR Wet AMD, RVO & DME Developing sustained release, hydrogel - based, erodible implant with protein stabilization technology Release rate of anti - VEGF protein verified in animal model of persistent retinal vascular leakage Optimization of delivery system is underway Released Cumulative Released (ug) 3 Retinal Disease Programs Retina pipeline not approved by the FDA

24 © 2021 Glaukos Corporation PRODUCT/CANDIDATE PLATFORM / PRODUCT TYPE PATIENT STATUS Micro - Surgical Devices iStent Trabecular Bypass Stents Approved iStent inject / iStent inject W Trabecular Bypass Stents Approved iStent SA Trabecular Bypass Stents Pivotal IDE Trial iStent infinite Trabecular Bypass Stents PMA - S Pending iPRIME Viscodelivery Class II (TBD) PreserFlo (Santen) Microshunt PMA Submitted Pharmaceuticals iDose TR Sustained - Release System Phase 3 iDose TREX Sustained - Release System Pre - clinical iDose ROCK Sustained - Release System Pre - clinical iLink Epi - off (Photrexa) Bio - Activated Approved iLink Epi - on (Epioxa) Bio - Activated Phase 3 iLink Epi - on Next Generation Bio - Activated Pre - clinical Dry Eye Candidate Eyelid Drug Delivery Pre - clinical Multi - Kinase Inhibitor SR Bio - Erodible Pre - clinical Triamcinolone Acetonide SR Bio - Erodible Pre - clinical Anti - VEGF SR Bio - Erodible Pre - clinical Biosensors IOP Sensor Implantable sensor Pre - clinical Our Strategy for Long - Term Growth & Profitability Pipeline Currently Includes 14 Candidates, Plus Additional Undisclosed Programs Mild - to - moderate glaucoma w/cataract Mild - to - moderate glaucoma pseudophakic Advanced - to - refractory glaucoma Keratoconus Ocular surface disease OHT - to - moderate glaucoma Refractive disorders Wet AMD DME RVO

25 © 2021 Glaukos Corporation MARKET OPPORTUNITY 2015 - 2020 2021 - 22 2023 - 24 2025+ Delivering a Deep Pipeline of Disruptive Therapies Leveraging Multiple Proprietary Platforms to Serve Large Ophthalmic Markets iStent iStent inject iStent inject W iLink Epi - off PreserFlo iStent infinite Dry Eye Therapy iDose Rock IOP Sensor iLink Epi - on Next Gen Retinal Disease Programs iDose TR iDose TREX iLink Epi - on iStent SA

26 © 2021 Glaukos Corporation

27 © 2021 Glaukos Corporation Appendix Rollout of Amort. Of Stock-based Reversal of Inventory Dev Tech comp exp on restructuring Fair Value Adj Intangibles replacement expenses 3Q 2020 GAAP net of COVID awards 3Q 2020 Non-GAAP Gross Margin E&O reversal Gross Margin Net Sales 64,831$ 64,831$ COGS 17,932$ (2,331)$ (5,523)$ (581)$ 291$ 9,789$ Gross Profit 46,899$ 2,331$ 5,523$ 581$ (291)$ 55,042$ Gross Margin 72% 85% GAAP to Non-GAAP Reconciliation - 3Q 2020
