Attached files
| file | filename |
|---|---|
| EX-32 - EX-32 - LANNETT CO INC | lci-20200630xex32.htm |
| EX-31.2 - EX-31.2 - LANNETT CO INC | lci-20200630ex312aaf0ad.htm |
| EX-31.1 - EX-31.1 - LANNETT CO INC | lci-20200630ex3111d1d02.htm |
| EX-23.1 - EX-23.1 - LANNETT CO INC | lci-20200630ex23174efe0.htm |
| EX-21.1 - EX-21.1 - LANNETT CO INC | lci-20200630ex211a682b4.htm |
| EX-10.63 - EX-10.63 - LANNETT CO INC | lci-20200630ex1063a66fd.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
⌧ ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended June 30, 2020
OR
◻ TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission File No. 001-31298
LANNETT COMPANY, INC.
(Exact name of registrant as specified in its charter)
State of Delaware | 23-0787699 |
State of Incorporation | I.R.S. Employer I.D. No. |
9000 State Road
Philadelphia, Pennsylvania 19136
Registrant’s telephone number, including area code: (215) 333-9000
(Address of principal executive offices and telephone number)
Securities registered under Section 12(b) of the Exchange Act:
Title of each class |
| Trading Symbol(s) |
| Name of each exchange on which registered |
Common Stock, $0.001 par value | | LCI | | New York Stock Exchange |
Securities registered under Section 12(g) of the Exchange Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ◻ No ⌧
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ◻ No ⌧
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes ⌧ No ◻
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ⌧ No ◻
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer ◻ | Accelerated filer ⌧ |
| |
Non-accelerated filer ◻ | Smaller reporting company ☐ |
| Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ◻
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ⌧
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12B-12 of the Exchange Act). Yes ◻ No ⌧
Aggregate market value of common stock held by non-affiliates of the registrant, as of December 31, 2019 was $277,527,086 based on the closing price of the stock on the NYSE.
As of July 31, 2020, there were 40,220,659 shares of the registrant’s common stock, $.001 par value, outstanding.
2
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements. Any statements made in this Annual Report that are not statements of historical fact or that refer to estimated or anticipated future events are forward-looking statements. We have based our forward-looking statements on management’s beliefs and assumptions based on information available to them at this time. Without limiting the generality of the foregoing, words such as “may,” “will,” “expect,” “believe,” “anticipate,” “intend,” “could,” “would,” “estimate,” “continue,” or “pursue,” or the negative other variations thereof or comparable terminology, are intended to identify forward-looking statements. Such forward-looking statements reflect our current perspective of our business, future performance, existing trends and information as of the date of this filing. These include, but are not limited to our beliefs about future revenue and expense levels, growth rates, prospects related to our strategic initiatives and business strategies, express or implied assumptions about government regulatory action or inaction, anticipated product approvals and launches, business initiatives and product development activities, assessments related to clinical trial results, product performance and competitive environment, anticipated financial performance, integration of acquisitions and the impact of the nonrenewal of the exclusive distribution agreement with Jerome Stevens Pharmaceuticals on our future business and prospects. The statements are not guarantees of future performance and involve certain risks, uncertainties and assumptions that are difficult to predict. We caution the reader that certain important factors may affect our actual operating results and could cause such results to differ materially from those expressed or implied by forward-looking statements. Lannett is under no obligation to, and expressly disclaims any such obligation to, update or alter its forward-looking statements, whether as a result of new information, future events or otherwise and other events or factors, many of which are beyond our control, including those resulting from such events, or the prospect of such events, such as public health issues including health epidemics or pandemics, such as the recent outbreak of the novel coronavirus (“COVID-19”), whether occurring in the United States or elsewhere, which could disrupt our operations, disrupt the operations of our suppliers and business development and other strategic partners, disrupt the global financial markets or result in political or economic instability. We believe the risks and uncertainties discussed under the “Item 1A - Risk Factors” and other risks and uncertainties detailed herein and from time to time in our SEC filings may affect our actual results.
We disclaim any obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise. We also may make additional disclosures in our Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and in other filings that we may make from time to time with the SEC. Other factors besides those listed here could also adversely affect us.
3
ITEM 1. DESCRIPTION OF BUSINESS
Business Overview
Lannett Company, Inc. and subsidiaries (the “Company,” “Lannett,” “we,” or “us”) was incorporated in 1942 under the laws of the Commonwealth of Pennsylvania and reincorporated in 1991 as a Delaware corporation. We primarily develop, manufacture, market and distribute generic versions of brand pharmaceutical products. We report financial information on a quarterly and fiscal year basis with the most recent being the fiscal year ended June 30, 2020. All references herein to a “fiscal year” or “Fiscal” refer to the applicable fiscal year ended June 30.
The Company has experienced total net sales growth at a compounded annual growth rate in excess of 22% over the past 18 years. In that time period, total net sales increased from $12.1 million in fiscal year 2001 to $545.7 million in fiscal year 2020. This growth has been achieved through filing and receiving approvals for abbreviated new drug applications (“ANDAs”), strategic partnerships and launches of additional manufactured drugs, opportunities resulting from our strong historical record of regulatory compliance, as well as the acquisitions of Silarx Pharmaceuticals, Inc. (“Silarx”) and Kremers Urban Pharmaceuticals Inc. (“KUPI”) in 2015.
Most products that we currently manufacture and/or distribute are prescription products. Our top five products in fiscal years 2020, 2019 and 2018 accounted for 45%, 52% and 58% of total net sales, respectively. On March 23, 2019, the Company’s distribution agreement with Jerome Stevens Pharmaceuticals (the “JSP Distribution Agreement”) expired and was not renewed. Accordingly, top product concentration rates declined in Fiscal 2020. Net sales of JSP products, primarily Levothyroxine Sodium Tablets USP, which was one of our top five products in fiscal 2019, totaled $202.5 million and $253.1 million in fiscal years 2019 and 2018, respectively, or 31% and 37% of total net sales, respectively.
Competitive Strengths
Management. We have been focused on maintaining and augmenting the quality of our management team in anticipation of continuing growth. As part of our growth, we have established corporate and non-corporate officer positions. We have hired experienced personnel from large, established, brand pharmaceutical companies as well as competing generic companies to complement the skills and knowledge of the existing management team. As we continue to grow, additional personnel may need to be added to our management team. We intend to hire the best people available to expand the knowledge base and expertise within our team.
Market Orientation. We believe that our success depends on our ability to properly assess the competitive market for new products, including customer interest, the number of competitors, market share opportunity and the generic unit price erosion. We intend to reduce our exposure to competitive influences that may negatively affect our sales and profits, including the potential saturation of the market for certain products, by continuing to emphasize a strong product selection process with an orientation to internal development in which we have technological and manufacturing expertise and external development partnerships to access other technologies and associated manufacturing capacity as well as risk-sharing.
Dependable U.S. Based Supplier to our Customers. We believe we are viewed by our customers as a strong, dependable supplier due in part to our agile and reliable operations network, as well as having a less complex manufacturing/supply chain based mostly within the U.S. We have cultivated productive customer relationships by focusing on what is important to them and their patients, along with maintaining adequate inventory levels, employing a responsive order filling system and prioritizing timely fulfillment of those orders. A majority of our orders are filled and shipped on or the day after we receive the order.
Reputation for Regulatory Compliance. We have a strong track record of regulatory compliance. We believe that we have effective regulatory compliance capabilities and practices due to: (1) the hiring of qualified individuals, (2) the implementation of comprehensive Standard Operating Procedures (“SOP”) and (3) adherence to current Good
4
Manufacturing Practices (“cGMP”). Our agility in responding quickly to market events and a reputation for regulatory compliance positions us to avail ourselves of market opportunities as they materialize.
We continue to pursue “Quality by Design” for improving and maintaining product quality in our pharmaceutical development and manufacturing facilities, which is outlined in the Food and Drug Administration (the “FDA”) report entitled, “Pharmaceutical Quality for the 21st Century: A Risk-Based Approach.” The FDA periodically inspects our operations to determine our compliance with applicable laws and regulations. During an inspection, the FDA may issue an inspection report , entitled a “Form 483,” containing potentially objectionable observations arising from an inspection. Additionally, at the close of each inspection, FDA will issue an Establishment Inspection Report (EIR) that details the final classification for each site, either No Action Indicated (NAI), Voluntary Action Indicated (VAI), or Official Action Indicated (OAI). The FDA’s observations may be minor or severe in nature and the degree of severity is generally determined by potential consequences to the consumer. By strictly complying with cGMPs and the various FDA guidelines as well as adherence to our Standard Operating Procedures, we have never received a cGMP Warning Letter in more than 70 years of business.
Leverage our Flexibility and Speed. We believe flexibility and speed in decision-making are critical success factors in the generic industry. Our mid-sized scale and relatively less complex organizational structure as a U.S. based organization results in a nimbler response to securing market opportunities.
Extensive Experience with Productive Partnerships. We continue to grow, diversify and strengthen our business by entering into partnerships to distribute both externally developed products and authorized generic equivalents of brand products. In fiscal year 2020, we successfully launched 18 new products, several of which are sourced from external parties, including Posaconazole (Noxafil®) and Amphetamine Salts ER (Adderall ER®). We believe that our success with these products, along with existing alliances, has established us as a strong development and marketing partner creating the foundation for continued productive partnership alliances in the future.
Strong Track Record of Obtaining Regulatory Approvals for New Products. During the past three fiscal years, we have received 1 NDA approval and 15 ANDA approvals from the FDA. Although the timing of ANDA approvals by the FDA is uncertain, we currently expect to continue to receive more during Fiscal 2021. These regulatory approvals will enable us to manufacture and supply a broader portfolio of generic pharmaceutical products.
Efficient Development Systems and Manufacturing Expertise for New Products. We believe that our U.S.-based manufacturing expertise, low overhead expenses and skilled product development capabilities will help us remain competitive in the generic pharmaceutical market. We intend to dedicate significant resources toward developing new products because we believe our success is linked to our ability to continually introduce new generic products into the marketplace.
Business Strategies
Focus on the large US Generic Market
We believe generics are the foundation of efficient pharmaceutical care and are estimated to be approximately 90% of all US pharmaceutical prescription volume with an IQVIA value of approximately $56 billion for the 12-month period ending June 30, 2020. While that estimate likely well exceeds actual market size, Lannett’s opportunity is significant relative to Lannett’s size.
We are focused on increasing our market share in the generic pharmaceutical industry while directing additional resources on the development of new products. We continue to improve our financial performance by expanding our line of generic products, increasing unit sales to current customers, creating manufacturing efficiencies and managing our overhead and administrative costs.
5
Emphasis on In-Line Execution
We have a broad portfolio of existing generics and we continually look to optimize the share and value of our existing portfolio. We look to capitalize on competitor supply disruptions which occur frequently in the industry of both a shorter and longer duration. We seek to reduce the cost of our products through various life cycle management approaches including increasing the efficiency of our plant, and our product manufacturing yields, and lowering incipient and API costs from third-party suppliers.
Strategic Expansion of our Product Offering
We have three primary strategies for expanding our product offerings: (1) deploying our experienced Research and Development (“R&D”) staff to develop products in-house; (2) entering into product development agreements or strategic alliances with third-party product developers and formulators; and (3) purchasing ANDAs or New Drug Applications (“NDA”) from other manufacturers. We expect that each strategy will facilitate our identification, selection and development of additional pharmaceutical products that we may sell to our existing network of customers.
Between January 2018 and June 2020, the number of alliances that our business development efforts have secured increased significantly and we have acquired or in-licensed over 60 ANDA products as a result of these efforts.
Opportunistically, we may increase our focus on specialty markets within the pharmaceutical industry. As a result, in Fiscal 2018, the Company filed its first NDA for Numbrino (cocaine hydrochloride solution), which was approved by the FDA in January 2020.
Similarly, in 2016, the Company announced a strategic partnership with YiChang HEC ChangJiang Pharmaceutical Co., Ltd, an HEC Group company, to co-develop a biosimilar insulin glargine pharmaceutical product for the U.S. market. The product is currently in development, and a healthy human Pharmacokinetic/Pharmacodynamic modeling (“PK/PD”) clinical trial was conducted in South Africa. It compared the Lannett/HEC insulin glargine to U.S. Lantus® as part of the effort to file a biosimilar Biologics License Application (“BLA”) with the U.S. FDA. The study met all of its primary inputs. Subsequently, Lannett held a Biosimilar Biological Product Development Type II meeting with the FDA. The feedback was consistent with our expectation. The Company plans to manage the clinical and regulatory steps specific for an FDA approval to market and will have the exclusive U.S. marketing rights to the product. We currently expect to file the product during our Fiscal 2023. In addition, we will market other generic products developed by HEC with several launches expected over the next few years.
In July 2019, the Company entered into an agreement with Cediprof, Inc. (“Cediprof”) to distribute Levothyroxine Sodium Tablets USP beginning not later than August 1, 2022. Levothyroxine is one of the largest generics sold in the USA. The product has several technical attributes that make receiving an FDA approval and continuous manufacturing challenging. The Cediprof product is already approved and has been sold in the U.S. by its current partner for the past several years. In August 2020, the Company announced it had commenced distributing Cediprof’s Levothyroxine product under an interim exclusive supply and distribution agreement.
In October 2019, the Company announced it had entered into an exclusive U.S. distribution agreement for the therapeutically equivalent generic of ADVAIR DISKUS® (Fluticasone Propionate – Salmeterol Xinafoate Powder Inhaler) of Respirent Pharmaceuticals Co. Ltd. ADVAIR DISKUS had U.S. sales of $3.6 billion for the 12 months ending July 2019, according to IQVIA, although actual generic market values are expected to be lower. The Company currently anticipates the product would be filed during Fiscal Year 2021. Under the agreement, the Company will commence U.S. distribution of the product after FDA approval. The Company will make an upfront payment, as well as future milestone payments, and receive a portion of the net profits once it commences distribution of the product. The term of the agreement is 12 years, which begins upon commencement of distribution. We have several other existing supply and development agreements with both international and domestic companies; in addition, we are currently in negotiations on similar agreements with other companies through which we can market and distribute future products. We intend to capitalize on our strong customer relationships to build our market share for such products.
6
Due to the expiration of the JSP Distribution Agreement in March 2019, management reassessed its overall business strategies to offset the impact of the loss on a short- and long-term basis. These plans currently include, among other things, an emphasis on reducing cost of sales, R&D and selling, general and administrative (“SG&A”) expenses; continuing to accelerate new product launches; increasing the level of strategic partnerships; and reducing capital expenditures. Management will also continue its emphasis on accelerating ANDA filings. In addition, management plans to attempt, at the appropriate time, to refinance all or a significant portion of its outstanding long-term debt to reduce principal repayment requirements and establish more flexibility around financial covenants. These actions may increase related interest expense, but are expected to positively impact cash flows.
Mergers and Acquisitions.
We evaluate potential mergers and acquisitions opportunities that are a strategic fit and accretive to our business. During Fiscal 2016, we completed the acquisition of KUPI, the former subsidiary of global biopharmaceuticals company UCB S.A. KUPI is a U.S. specialty pharmaceuticals manufacturer focused on the development of products that are difficult to formulate or utilize specialized delivery technologies. Strategic benefits of the acquisition include expanded manufacturing capacity, a diversified product portfolio and pipeline and complementary R&D expertise.
Key Products
Key products were selected based on current and future sales and profitability. In aggregate, the 11 products noted below accounts for approximately 57% of Lannett sales in Fiscal 2020. While these products are our top selling products, margins may vary well above or below average margins based on changing competitive circumstances as well as product partnership royalties, where applicable.
Fluphenazine Tablets
Fluphenazine tablets are used for the management of manifestations of psychotic disorders. Net sales of Fluphenazine tablets represented approximately 18% of total net sales in fiscal year 2020.
Posaconazole DR Tablets
Posaconazole DR tablets are used to prevent fungal infections in people who have a weak immune system resulting from certain treatments or conditions. The product is the generic version of Noxafil®. Net Sales of Posaconazole DR represented approximately 10% of total net sales in fiscal year 2020.
Verapamil SR Tablets
Verapamil SR tablets are a calcium channel blocker used in the treatment of high blood pressure, arrhythmia and angina. We market the authorized generic of Verelan PM.
Methylphenidate CD Capsules
Methylphenidate CD is a central nervous system (“CNS”) stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorder (“ADHD”). This product is the authorized generic version of the brand Metadate CD®.
Omeprazole Capsules
Omeprazole is a proton pump inhibitor. The product is a generic version of the branded drug Prilosec®. It is indicated for the treatment of certain diseases of the esophagus and stomach ulcers as well as pathologic hypersecretory conditions. KUPI produces Omeprazole DR capsules in 10mg, 20mg and 40mg dosages.
7
Pantoprazole Sodium DR Tablets
Pantoprazole is a proton pump inhibitor. The product is a generic version of the branded drug Nexium®. It is indicated for the treatment of certain diseases of the esophagus and pathological hypersecretory conditions. KUPI produces Pantoprazole tablets in 20mg and 40mg dosages.
Sumatriptan Nasal Spray
Sumatriptan Nasal Spray is indicated for the acute treatment of migraine attacks. This product is a generic version of Imitrex® Nasal Spray. The Company distributes the 5mg and 20mg dosages.
Metolazone Tablets
Metolazone is a diuretic medication. It is indicated for the treatment of hypertension, alone or in combination with other anti-hypertensives. We market the authorized generic version of Zaroxolyn®.
Amphetamine IR Tablets
Amphetamine IR Tablets are used to treat attention deficit hyperactivity disorder (ADHD) and narcolepsy. It is the generic version of Adderall.
Methylphenidate Hydrochloride ER
Methylphenidate ER is a CNS stimulant indicated for the treatment of ADHD in children six years of age and older, adolescents and adults up to the age of 65. The product is a generic version of the branded drug Concerta®.
The Company markets one form of the product which was designated “BX.” Per a teleconference on November 2014, the FDA informed KUPI that it was changing the therapeutic equivalence rating of its product from “AB” (therapeutically equivalent) to “BX.” A BX-rated drug is a product for which data are insufficient to determine therapeutic equivalence; it is still approved and can be prescribed, but the FDA does not recommend it as automatically substitutable for the brand-name drug at the pharmacy.
The Company has been working with the FDA to regain the “AB” rating, and in the meantime, maintain the drug on the U.S. market with a BX rating. However, there can be no assurance as to when or if the Company will regain the “AB” rating or be permitted to remain on the market. The Company also agreed to potential acquisition-related contingent payments to UCB related to Methylphenidate ER if the FDA reinstates the AB-rating and certain sales thresholds are met. Such potential contingent payments are set to expire after December 31, 2020.
In August 2018, the Company entered into an exclusive perpetual licensing agreement with Andor Pharmaceuticals, LLC for Methylphenidate Hydrochloride Extended Release (ER) tablets USP (CII) in 18 mg, 27 mg, 36 mg and 54 mg strengths. Andor’s ANDA of Methylphenidate was approved by the FDA on April 24, 2019 as an AB-rated generic equivalent to the brand Concerta®. Lannett commenced marketing of this product on May 29, 2019.
Under the licensing agreement, Lannett is providing sales, marketing and distribution support of Andor’s Methylphenidate ER product, for which it will receive a percentage of the net profits.
Cocaine Hydrochloride Solution
In December 2017, a competitor received approval from the FDA to market and sell a Cocaine Hydrochloride topical product. This approval affects the Company’s right to market and sell its unapproved cocaine hydrochloride solution product. According to FDA guidance, the FDA typically allows the marketing of unapproved products for up to one year following the approval of an NDA for the product. Upon the recent request of the FDA to cease manufacturing and distributing our unapproved cocaine hydrochloride solution product as a result of an approved product on the market, the Company committed to not manufacture or distribute cocaine hydrochloride 10% solution, which has not been sold
8
during Fiscal 2019, as of April 15, 2019. The Company also ceased manufacturing its unapproved cocaine hydrochloride 4% solution on June 15, 2019 and ceased distributing the product on August 15, 2019.
We filed a NDA for Numbrino® Nasal Solution in Fiscal 2018. We received approval in January 2020 and launched the product in March 2020.
The competitor filed a Citizen Petition with the FDA in February 2019, claiming that the grant of the NCE exclusivity blocks the approval of the Company’s application for five years and requesting that the FDA refuse to accept any further submissions in furtherance of the Company’s Section 505(b)(2) NDA application, treat as withdrawn any submissions made by the Company after December 2017 and withdraw the Company’s Section 505(b)(2) application. On April 24, 2019, the Company filed an opposition to the Citizen Petition requesting that it be denied. On July 3, 2019, the FDA denied the competitor’s Citizen Petition. Thereafter, the competitor filed a second Citizen Petition claiming that the FDA should rescind the acceptance of the Company’s Section 505(b)(2) application and only permit the Company to re-submit the application as an ANDA after the expiration of the competitor’s five-year exclusivity. The Company filed an opposition to the second Citizen Petition asserting, among other things, that the FDA should summarily deny the second Citizen Petition as an improper attempt to delay competition. On January 10, 2020, the FDA denied the second Citizen Petition and the FDA approved the Company’s Section 505(b)(2) NDA application. On January 27, 2020, the competitor filed a complaint against the FDA seeking an order invalidating the approval of the Company’s 505(b)(2) NDA, claiming the approval violates the competitor’s five-year exclusivity. On February 14, 2020, the Company filed a motion to intervene in the competitor’s lawsuit in order to argue that the request for relief be denied. On April 15, 2020, the competitor filed a motion for summary judgment. The Company and FDA filed responses in opposition and cross motions for summary judgment requesting dismissal of the complaint. The parties submitted further reply briefs and are awaiting a decision by the Court.
On June 6, 2020, the competitor filed a patent infringement complaint in the United States District Court for the District of Delaware, asserting that the Company’s approved cocaine hydrochloride product infringes three patents issued to the competitor. On June 19, 2020, the Company filed an answer and counterclaim, alleging that the Company either does not infringe or the three asserted patents are invalid. In addition, the Company sought a declaration that, as to the competitor’s three additional patents not asserted against the Company, they are either not infringed or invalid.
Sales & Marketing and Customers
We enter into contracts with Group Purchasing Organizations (“GPOs”) to sell our products to their members who are our direct and indirect customers. The largest GPOs are ClarusOne, Red Oak Sourcing and Walgreens Boots Alliance Development. Net sales to these GPOs accounted for 74% of total net sales in fiscal year 2020 and 63% in fiscal year 2019.
We sell our pharmaceutical products to generic pharmaceutical distributors, drug wholesalers, chain drug retailers, private label distributors, mail-order pharmacies, other pharmaceutical companies, managed care organizations, hospital buying groups, governmental entities and health maintenance organizations. The pharmaceutical industry’s largest wholesale distributors, Amerisource Bergen, McKesson and Cardinal Health, each associated with one of the GPOs mentioned above, accounted for 25%, 23% and 11%, respectively, of our total net sales in fiscal year 2020, 21%, 18% and 10%, respectively, of our total net sales in fiscal year 2019 and 29%, 17% and 6%, respectively, of our total net sales in fiscal year 2018.
Sales to wholesale customers include “indirect sales,” which represent sales to third-party entities, such as independent pharmacies, managed care organizations, hospitals, nursing homes and group purchasing organizations, collectively referred to as “indirect customers.”
We enter into definitive agreements with our indirect customers to establish pricing for certain covered products. Under such agreements, the indirect customers independently select a wholesaler from which to purchase the products at these agreed-upon prices. We will provide credit to the wholesaler for the difference between the agreed-upon price with the indirect customer and the wholesaler’s invoice price. This credit is called a “chargeback.” For more information on chargebacks, see the section entitled “Critical Accounting Policies” in Item 7, “Management’s Discussion and Analysis
9
of Financial Condition and Results of Operations” of this Form 10-K. These indirect sale transactions are recorded on our books as sales to wholesale customers.
We promote our products through direct sales, trade shows and group purchasing organizations’ bidding processes. We also have a limited number of products that are marketed as part of our customers’ “private label” programs. Private label products are manufactured by Lannett but distributed to the customer with a label typically containing the name and logo of the customer. Private label allows us to leverage our internal sales efforts by using the sales and marketing efforts of those customers.
Strong and dependable customer relationships have created a positive platform for us to increase our sales volumes. Historically and in fiscal years 2020, 2019 and 2018, our advertising expenses have been immaterial. When our sales representatives make contact with a customer, we will generally offer to supply the customer our products at fixed prices. If accepted, the customer’s purchasing department will coordinate the purchase, receipt and distribution of the products throughout its distribution centers and retail outlets. Once a customer accepts our supply of a product, the customer typically expects a high standard of service, including timely receipt of products ordered, availability of convenient, user-friendly and effective customer service functions and maintaining open lines of communication.
We believe that retail-level consumer demand dictates the total volume of sales for most of our various products. In the event that wholesale and retail customers adjust their purchasing volumes, we believe that consumer demand will be fulfilled by other wholesale or retail sources of supply. As a result, we attempt to develop and maintain strong relationships with most of the major retail chains, wholesale distributors and mail-order pharmacies in order to facilitate the supply of our products through whatever channel the consumer prefers. Although we have agreements with customers governing the transaction terms of our sales, generally there are no minimum purchase quantities applicable to these agreements. Our practice of maintaining adequate inventory levels, employing a responsive order filling system and prioritizing timely fulfillment of those orders have contributed to a strong reputation among our customers as a dependable supplier of high-quality generic pharmaceuticals.
Competition
The manufacturing and distribution of generic pharmaceutical products is a highly competitive industry. Competition is based primarily on a reliable supply and price. In addition to competitive pricing, our competitive advantages are our ability to provide strong and dependable customer service by maintaining adequate inventory levels, employing a responsive order filling system and prioritizing timely fulfillment of orders. We ensure that our products are available from national wholesale, chain drug and mail-order suppliers as well as our own warehouse. The modernization of our facilities, hiring of experienced staff and implementation of inventory and quality control programs have improved our competitive cost position. Our primary competitors across our product portfolio are Teva Pharmaceutical Industries Ltd., Mylan N.V., and Amneal Pharmaceuticals Inc.
Validated Pharmaceutical Capabilities
The Company’s 432,000 square foot Seymour, Indiana facility contains approximately 107,000 square feet of manufacturing space as well as a leased 116,000 square foot temperature/humidity-controlled storage warehouse. The Seymour facility has had satisfactory inspections conducted by the FDA and EMA and similar regulatory authorities of Japan, Taiwan, Brazil, China, Korea and Turkey. As of June 30, 2020, the facility has a production capacity of approximately 4.0 billion doses based on our current product mix and plant configuration.
The Company has an 110,000 square foot manufacturing facility located in Carmel, New York, which sits on 25.8 acres of land. The facility specializes in liquid products and currently houses manufacturing, packaging, quality and research and development and has capacity for additional manufacturing space, if needed.
Lannett owns two facilities in Philadelphia, Pennsylvania. The research and development facilities are located in a 31,000 square foot facility at 9000 State Road and a second, 63,000 square foot facility that is located within one mile of the State Road facility at 9001 Torresdale Avenue, Philadelphia, PA. The latter facility contains our analytical research and development and quality control laboratories. We have adopted many systems and processes to ensure adherence to
10
FDA requirements and we believe we are operating our facilities in substantial compliance with the FDA’s cGMP regulations.
Raw Materials and Finished Goods Suppliers
Our use of raw materials in the production process consists of pharmaceutical chemicals in various forms that are often available from several sources. In addition to the raw materials we purchase for the production process, we purchase certain finished dosage inventories. We sell these finished dosage form products directly to our customers along with the finished dosage form products manufactured in-house. We generally take precautionary measures to avoid a disruption in raw materials and finished goods, such as finding secondary suppliers for certain raw materials or finished goods when available and maintaining adequate inventory levels.
Over time, we have entered into supply and development agreements with Summit Bioscience LLC, HEC Pharm Group, Dexcel Pharma, Elite Pharmaceuticals, RivoPharm and various other international and domestic companies. The Company is currently in negotiations on similar agreements with other companies and is actively seeking additional strategic partnerships, through which it will market and distribute products manufactured in-house or by third parties. The Company plans to continue evaluating ways to improve its capital structure and consider potential merger and acquisition opportunities. The Company also continues to assess product acquisitions that are a strategic fit and accretive to the business.
Research and Development Process
Over the past several years, we have invested in R&D projects. The costs of these R&D efforts are expensed during the periods incurred. We believe that such costs may be recovered in future years when we receive approval from the FDA to manufacture and distribute such products. We have embarked on a plan to grow in future years, which includes organic growth to be achieved through our R&D efforts. We expect that our growing list of generic products under development will drive future growth. The following steps outline the numerous stages in the generic drug development process:
| 1.) | Formulation and Analytical Method Development. After a drug candidate is selected for future sale, product development scientists perform various experiments to incorporate excipients with the APIs to produce a robust, stable and bioequivalent dosage form that will be therapeutically equivalent to the brand name drug, and meet all FDA requirements for approval. These experiments will result in the creation of a number of product formulations to determine which formula will be most suitable for our subsequent development process. Various formulations are tested in the laboratory to measure results against the innovator brand drug. During this time, we may use reverse engineering methods on samples of the innovator drug to determine the type and quantity of inactive ingredients. During the formulation phase, our R&D chemists begin to develop an analytical, laboratory testing method. The successful development of this test method will allow us to test developmental and commercial batches of the product in the future. All of the information used in the final formulation, including the analytical test methods adopted for the generic drug candidate, will be included as part of the Chemistry, Manufacturing and Controls (“CMC”) section of the ANDA submitted to the FDA. |
| 2.) | Scale-up and Tech Transfer. After product development, our R&D formulators and our R&D chemists agree on a final formulation for use in moving the drug candidate forward in the developmental process, we then attempt to increase the batch size of the product. The batch size represents the standard magnitude to be used in manufacturing a batch of the product. The determination of batch size affects the amount of raw material that is used in the manufacturing process and the number of expected dosages to be created during the production cycle. We attempt to determine batch size based on the amount of active ingredient in each dosage, the available production equipment and unit sales projections. The scaled-up batch is then generally produced in our commercial manufacturing facilities. During this manufacturing process, we document the equipment used, the amount of time in each major processing step and any other steps needed to consistently produce a batch of that product. |
11
| 3.) | Bio equivalency and Clinical Testing. After a successful scale-up of the generic drug batch, we schedule and perform generally required bio equivalency testing on the product and in some cases, clinical testing, if required by the FDA. These procedures, which are generally outsourced to third parties, include testing the absorption rate and extent of the generic product in the human bloodstream compared to the absorption of the innovator drug. The results of this testing are then documented and reported to us to determine the “success” of the generic drug product. Success, in this context, means that we are able to demonstrate that our product is comparable to the innovator product in dosage form, strength, route of administration, quality, performance characteristics and intended use. |
Bioequivalence (meaning that the product has the same blood levels and dosage form as the innovator drug) and a stable formula are the primary requirements for a generic drug approval (assuming the manufacturing plant is in compliance with the FDA’s cGMP regulations). Lengthy and costly clinical trials proving safety and efficacy, which are required by the FDA for NDAs (and may include 505(b)(2)NDAs), are typically unnecessary for generic companies. If the results are successful, we will continue the collection of information and documentation for assembly of the drug application.
| 4.) | Submission of the ANDA for FDA Review and Approval. An ANDA is a comprehensive submission that contains, among other things, data and information pertaining to the proposed labeling, active pharmaceutical ingredient, excipients, container/closure, drug product formulation, drug product testing specification, methodology and results. Bioequivalence study reports are also included in the ANDA submission. |
Our ANDAs and NDAs are submitted to the FDA electronically using the most current Electronic Common Technical Document standards. Lannett strives to achieve a first cycle approval for each ANDA under the Generic Drug User Fee Amendments of 2012 (“GDUFA”) review metrics.
In fiscal year 2020, we launched several products from internal and external sources. The following summary contains more specific details regarding our latest product launches. Market data was obtained from IQVIA although actual generic market sizes are expected to be smaller.
| | |
| |
| | Total Market Size as of | |
Product Launch |
| Month of Launch |
| Equivalent Brand |
| May 2020 ($ in millions) | ||
1 | Posaconazole DR Tablets |
| September, 2019 |
| Noxafil® | | $ | 259.8 |
2 | Cyproheptadine Oral Solution |
| October, 2019 |
| Periactin® | | $ | 5.5 |
3 | Prednisone Tablets |
| October, 2019 |
| Deltasone® | | $ | 125.5 |
4 | Venlafaxine HCL ER Tablets |
| December, 2019 |
| Effexor® | | $ | 133.6 |
5 | Lidocaine HCl Topical Solution |
| December, 2019 |
| Lidocaine | | $ | 17.9 |
6 | Butalbital w/ Acetaminophen & Caffeine Caps - 300 mg |
| December, 2019 |
| Nexgen Generic | | $ | 20.1 |
7 | Butalbital w/ Acetaminophen & Caffeine Caps - 325 mg |
| December, 2019 |
| Mayne Generic | | $ | 54.5 |
8 | Propranolol HCL ER Capsules |
| February, 2020 |
| Inderal LA® | | $ | 120.7 |
9 | Numbrino (Cocaine Hydrochloride) Nasal Solution (CII) |
| March, 2020 |
| Numbrino® | | $ | 12.0 |
10 | Nystatin Oral Suspension |
| March, 2020 |
| Mycostatin® | | $ | 32.0 |
11 | Valproic Acid Oral Solution |
| March, 2020 |
| Depakene® | | $ | 9.5 |
12 | Dextroamphetamine-Amphetamine ER Capsule (CII) |
| April, 2020 |
| Adderall XR® | | $ | 1,488.1 |
13 | Lactulose Solution |
| April, 2020 |
| Chronulac® | | $ | 17.4 |
14 | Clobazam Tablets (CIV) |
| May, 2020 |
| Onfi® | | $ | 152.1 |
15 | Sulfamethoxazole + Trimethoprim Suspension |
| May, 2020 |
| Septra® | | $ | 23.6 |
16 | Clobazam Oral Suspension | | May, 2020 | | Onfi® | | $ | 90.0 |
17 | Amphetamine Sulfate IR Tablets (CII) | | June, 2020 | | Evekeo® | | $ | 31.4 |
18 | Brompheniramine-Pseudoephedrine-DM Syrup |
| June, 2020 |
| Bromfed DM® | | $ | 37.4 |
We have additional products of various dosage forms currently under development. Our developmental drug products are intended to treat a diverse range of indications. The products under development are at various stages in the development cycle—formulation, scale-up, clinical testing and/or FDA review.
12
The cost associated with each product that we are currently developing is dependent on numerous factors, including but not limited to, the complexity of the active ingredient’s chemical characteristics, the price of the raw materials and the FDA-mandated requirement of bioequivalence studies (depending on the FDA’s Product Specific Guidance). With the introduction of GDUFA and additional guidance issued by the FDA, the cost to develop a new generic product varies but can total several million dollars.
In addition, we currently own several ANDAs for products that are not currently marketed and noted as Discontinued in FDA’s Orange Book. Occasionally, we review such discontinued products to determine if the market potential for any of these products has recently changed to make it attractive for us to reconsider manufacturing and selling. If we decide to commercially market one of these products, we evaluate the requirements necessary for commercial launch, including a filing strategy to properly report the relaunch to the FDA so that the product is moved to the Active section of the Orange Book.
In addition to the efforts of our internal product development group, we have contracted with numerous outside firms for the formulation and development of several new generic drug products. These outsourced R&D products are at various stages in the development cycle—formulation, analytical method development and testing and manufacturing scale-up. These products include orally administered solid dosage products, injectables and nasal delivery products that are intended to treat a diverse range of medical indications.
We intend to ultimately transfer the formulation technology and manufacturing process for some of these R&D products to our own commercial manufacturing sites. We initiated these outsourced R&D efforts to complement the progress of our own internal R&D efforts.
We recorded R&D expenses of $30.0 million in fiscal year 2020, $38.3 million in fiscal year 2019 and $29.2 million in fiscal year 2018. These amounts included expenses associated with bioequivalence studies, internal development resources as well as outsourced development. While we manage all R&D from our principal executive office in Philadelphia, Pennsylvania, we have also been taking steps to capitalize on favorable development costs in other countries. We have strategic relationships with various companies that either act as contract research organizations or API suppliers as well as dosage form manufacturers. In addition, U.S.-based research organizations have been engaged for product development to enhance our internal development. Fixed payment arrangements are established between Lannett and these research organizations and in some cases include a royalty provision. Development payments are normally scheduled in advance, based on attaining development milestones.
Government Regulation
Pharmaceutical manufacturers are subject to extensive regulation by the federal government, including the FDA and, in cases of controlled substance products the DEA, as well as other federal regulatory bodies and state governments. The Federal Food, Drug and Cosmetic Act (the “FDCA”), the Controlled Substance Act (the “CSA”) and other federal statutes and regulations govern or influence the testing, manufacture, safety, labeling, storage, record keeping, approval, advertising and promotion of our generic drug products. Non-compliance with applicable regulations can result in fines, product recalls and seizure of products, total or partial suspension of production, personal and/or corporate prosecution and debarment and refusal of the government to approve applications. The FDA also has the authority to revoke previously approved drug applications.
Generally, FDA approval is required before a drug can be marketed. A new drug is one not generally recognized by qualified experts as safe and effective for its intended use and is submitted to the FDA as a NDA. The FDA review process for new drugs is very extensive and requires a substantial investment to research and test the drug candidate. A less burdensome approval pathway, the ANDA, is used for generic drug products. Typically, the investment required to develop a generic drug is less costly than the new drug. Some drug products may be submitted as a 505(b)(2) NDA, allowing some of the required research and testing to be waived by relying on FDA’s previous findings of safety and efficacy and literature. For additional information on the FDA approval pathways, refer to section 505(b)(1) and 505(b)(2) of the FD&C Act for NDAs, section 505(j) for ANDAs and resources available on the FDA website, www.fda.gov.
13
Manufacturing cGMP Requirements
Among the requirements for a new drug approval, facilities identified in each application that perform operations related to the drug product, including drug substance manufacturers and outside contract facilities, must conform to FDA cGMP regulations. The FDA may perform general GMP and/or pre-approval inspections to assess a company’s compliance with cGMP regulations. These inspections include reviews of procedures, operations, and data used to support the application and ongoing drug product manufacturing and testing. FDA’s cGMP regulations require, among other things, quality control and quality assurance systems as well as the corresponding records and documentation. In complying with the evolving standards set forth in the cGMP regulations, we must continue to expend time, money and effort in many areas to ensure compliance.
Failure to comply with statutory and regulatory requirements subject a manufacturer to possible legal or regulatory action, including but not limited to, warning letters, consent decrees placing significant restrictions on or suspending manufacturing operations, injunctions, the seizure of non-complying drug products and/or civil and criminal penalties.
Adverse experiences with the product and certain non-compliance events may need to be reported to the FDA and could result in regulatory actions such as labeling changes or FDA request for application withdrawal or product removal.
Other Regulatory Requirements
With respect to post-market product advertising and promotion, the FDA imposes a number of complex regulations on entities that advertise and promote pharmaceuticals, which include, among others, standards for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet. The FDA has very broad enforcement authority under the FDCA and failure to abide by these regulations can result in penalties, including the issuance of a warning letter directing entities to correct deviations from FDA standards, a requirement that future advertising and promotional materials be pre-cleared by the FDA and state and/or federal civil and criminal investigations and prosecutions. Some of our products require participation in Risk Evaluation and Mitigation Strategies (“REMS”) programs. A shared system REMS encompasses multiple prescription drug products and is developed and implemented jointly by two or more companies marketing the same products. These programs can add significant costs for the Company, depending on market share and complexity of the program.
Any one or a combination of FDA regulatory or enforcement actions against the Company could have a material adverse effect on our financial results.
DEA Regulation
We maintain registrations and quota (limitations on purchases of controlled substances) with the DEA that enable us to receive, manufacture, store, develop, test and distribute controlled substances in connection with our operations. Controlled substances are those drugs that appear on one of five schedules promulgated and administered by the DEA under the CSA. The CSA governs, among other things, the distribution, recordkeeping, quota, handling, security and disposal of controlled substances. We are subject to periodic and ongoing inspections by the DEA and similar state drug enforcement authorities to assess our ongoing compliance with the DEA’s regulations. Any failure to comply with these regulations could lead to a variety of sanctions, including the revocation or a denial of renewal of our DEA registration or quota, injunctions, or civil or criminal penalties. We are subject to an allocation of national (aggregate) quota for several products in our portfolio. Our quota requests require DEA approval in full for us to meet our forecasted customer demands. The DEA may or may not approve our quota requests in full based on factors that we do not control.
Fraud and Abuse Laws
Because of the significant federal and state funding involved in the provision of health care services, including Medicare and Medicaid funding, Congress and state legislatures have enacted and federal and state governments actively enforce, a number of laws whose purpose is to eliminate fraud and abuse in federal health care programs. Our business is subject to compliance with these laws, including both federal and state level Anti-Kickback Statutes, as well as other laws aimed at eliminating fraud and abuse such as the False Claims Act and the Foreign Corrupt Practices Act (“FCPA”).
14
Anti-Kickback Statutes and Federal False Claims Act
One of the primary federal laws aimed at curbing fraud and abuse in the federal health care programs is the federal Anti-Kickback Statute, 42 U.S.C. § 1320a–7b(b), which prohibits persons from knowingly and willfully soliciting, offering, receiving, or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal health care program such as Medicare, Medicaid or TRICARE. The definition of “remuneration” has been broadly interpreted to include anything of value, including for example gifts, certain discounts, the furnishing of free supplies, equipment or services, credit arrangements, payment of cash and waivers of payments, including copayments. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal health care covered business, the statute has been violated. Penalties for violations include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal health care programs. In addition, violations of the Anti-Kickback Statute can be considered violations of the Federal False Claims Act, discussed in more detail below.
The federal Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the health care industry. Recognizing that the Anti-Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements, Congress incorporated several statutory exceptions into the federal Anti-Kickback Statute’s framework, which protect certain types of business arrangements. Congress also authorized the Office of Inspector General of the U.S. Department of Health and Human Services (“OIG”) to issue a series of “regulatory safe harbors.” Both the statutory exceptions and regulatory safe harbors set forth provisions that, if all of their applicable requirements are met, will assure health care providers and other parties to the arrangement that the federal Anti-Kickback Statute has not been violated and that that they will not be prosecuted under the Anti-Kickback Statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy each applicable safe harbor may result in increased scrutiny by government enforcement authorities such as OIG.
Many states have adopted laws similar to the federal Anti-Kickback Statute. Some of these state prohibitions apply to referrals of patients for health care items or services reimbursed by any source, including commercial payers and private pay patients.
Government officials have focused their Anti-Kickback Statute enforcement efforts on marketing of health care services and products, among other activities and recently have brought cases against companies and certain sales, marketing and executive personnel, for allegedly offering unlawful inducements to potential or existing customers in an attempt to procure their business. Additionally, a number of courts have ruled that a transaction that violates the Anti-Kickback Statute is unenforceable as against public policy.
In addition to applying federal and state Anti-Kickback Statutes in enforcement actions involving the marketing of healthcare services and products, the federal government and various states also have enacted laws specifically regulating the sales and marketing practices of pharmaceutical companies. These laws and regulations may limit financial interactions between manufacturers and health care providers, require disclosure to the federal or state government and the public of such interactions (e.g. federal and state “Sunshine” laws), or require the adoption of compliance standards or programs. Many of these laws and regulations contain ambiguous requirements or require administrative guidance for implementation and, given the lack of clarity, our activities could be subject to the penalties under the pertinent laws and regulations.
Another development affecting the health care industry is the increased use of the Federal False Claims Act (“FFCA”) and in particular, action brought pursuant to the FFCA’s “Whistleblower” or “Qui Tam” provisions. The FFCA imposes liability on any person or entity who, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment by a federal health care program. The Qui Tam provisions of the FFCA allow a private individual to bring actions on behalf of the federal government alleging that the defendant has submitted a false claim to the federal government and to share in any monetary recovery. In recent years, the number of suits brought against health care providers by private individuals has increased dramatically, and in Fiscal 2019, the federal government recovered more than $3 billion in judgements and settlements related to FFCA violations in the health care industry. In
15
addition to the FFCA, various states have enacted false claims laws analogous to the FFCA, and many of these state laws apply where a claim is submitted to any third-party payer and not merely a federal health care program.
When an entity is determined to have violated the FFCA, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties in excess of $23,000 per claim, as adjusted annually. Liability arises, primarily, when an entity knowingly submits or causes another to submit a false claim for reimbursement to the federal government. The federal government has used the FFCA to assert liability on the basis of inadequate care, kickbacks and other improper referrals; improper use of Medicare numbers by the provider of services; as well as allegations regarding misrepresentations with respect to the services rendered. In addition, the federal government has prosecuted companies under the FFCA in connection with off-label promotion of products. Our future activities relating to the reporting of wholesale or estimated retail prices of our products, the reporting of discount and rebate information and other information affecting federal, state and third-party reimbursement of our products and the sale and marketing of our products may be subject to scrutiny under these laws. We are unable to predict whether we will be subject to actions under the FFCA or a similar state law, or the impact of such actions. However, the costs of defending such claims, as well as any sanctions imposed, could significantly affect our financial performance.
Travel Act
Recently, the Department of Justice has begun to use the 1961 federal Travel Act as a tool to pursue criminal charges in the case of health care kickback and commercial bribery allegations. This law was enacted as part of the Kennedy Administration’s war on organized crime. It formed the basis for a federal enforcement action against the Forest Park Medical Center, a Texas physician-owned specialty hospital, and a number of surgeons and administrators, who were convicted of conspiring to pay or receive bribes in exchange for referrals of patients in violation of a state commercial bribery law. Importantly, this case was not limited to claims covered under federal programs, and the failure of the state to bring charges under its own statute did not prevent the federal case from proceeding. The Travel Act may be used by the Justice Department as a way to expand its reach to penalize kickbacks and similar arrangements even when the Anti-Kickback Statute and FFCA would not apply. These efforts could increase our vulnerability to litigation and penalties if our past or present operations are found to be in violation of such act.
Foreign Corrupt Practices Act
The U.S. Foreign Corrupt Practices Act of 1977, as amended, and similar anti-bribery laws in other jurisdictions generally prohibit certain classes of persons and entities, and their intermediaries, from making payments to foreign government officials to assist in obtaining or retaining business. Specifically, the anti-bribery provisions of the FCPA prohibit the bribery of government officials. If we are found to be liable for FCPA or other violations, we could suffer from civil and criminal penalties or other sanctions, including contract cancellations or debarment, and loss of our reputation, any of which could have a significant impact on our business, financial condition and operations.
HIPAA and Other Fraud and Privacy Regulations
The Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) created two new federal crimes: health care fraud and false statements relating to health care matters. The HIPAA health care fraud statute prohibits, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any health care benefit program, including private payment programs. HIPAA’s extensive privacy and security regulations impose significant regulatory requirements on covered entities to acquire and implement information systems and to adopt business procedures and security measures designed to protect the privacy and security of patients’ protected health information. These particular HIPAA requirements have had a significant financial impact on many sectors of the health care industry because they impose extensive new requirements and restrictions on the use and disclosure of identifiable patient information, and the financial consequences of a data breach or unauthorized disclosure of patients’ protected health information, including data breaches caused by malicious third parties and inadvertent disclosures, can result in substantial civil fines, penalties and lawsuits, negative publicity, and costly remediation efforts imposed by the Office for Civil Rights of the U.S. Department of Health and Human Services. The HIPAA false statements statute prohibits knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious,
16
or fraudulent statement or representation in connection with the delivery of or payment for health care benefits, items, or services. A violation of this statute is a felony and may result in fines, imprisonment and/or exclusion from government-sponsored programs.
Pricing
In the United States, our sales are dependent upon the availability of coverage and reimbursement for our products from third-party payors, including federal and state programs such as Medicare and Medicaid and private organizations such as commercial health insurance and managed care companies. Such third-party payors challenge the price of medical products and services and continue to institute cost containment measures to control or significantly influence the purchase of medical products and services.
Over the past several years, the rising costs of providing health care services has triggered legislation to make certain changes to the way in which pharmaceuticals are covered and reimbursed, particularly by government programs. For instance, federal legislation and regulations have created a voluntary prescription drug benefit, Medicare Part D, which revised the formula used to reimburse health care providers and physicians under Medicare Part B and imposed significant revisions to the Medicaid Drug Rebate Program. These changes have resulted in and may continue to result in, coverage and reimbursement restrictions and increased rebate obligations by manufacturers.
In addition, there continues to be legislative and regulatory proposals at the federal and state levels directed at containing or lowering the cost of health care. Examples of how limits on drug coverage and reimbursement in the United States may cause reduced payments for drugs in the future include:
| ● | changing Medicare reimbursement methodologies; |
| ● | revising drug rebate calculations under the Medicaid program; |
| ● | reforming drug importation laws; |
| ● | fluctuating decisions on which drugs to include in formularies; and |
| ● | requiring pre-approval of coverage for new or innovative drug therapies. |
Also, over the last few years, several states have passed legislation or have proposed legislation that have imposed price reporting requirements for both generic and brand pharmaceutical products and that include price transparency, price increase notification and supplement rebate requirements.
We cannot predict the likelihood or pace of such additional changes or whether there will be significant legislative or regulatory reform impacting our products, nor can we predict with precision what effect such governmental measures would have if they were ultimately enacted into law. However, in general, we believe that legislative and regulatory reform activity likely will continue.
Current or future federal or state laws and regulations may influence the prices of drugs and, therefore, could adversely affect the prices that we receive for our products. Programs in existence in certain states seek to set prices of all drugs sold within those states through the regulation and administration of the sale of prescription drugs. Expansion of these programs, in particular, state Medicaid programs, or changes required in the way in which Medicaid rebates are calculated under such programs, could adversely affect the price we receive for our products and could have a material adverse effect on our business, results of operations and financial condition. Further, generic pharmaceutical drug prices have been the focus of increased scrutiny by certain states’ attorney generals, the U.S. Department of Justice and Congress. Decreases in health care reimbursements or prices of our prescription drugs could limit our ability to sell our products or could decrease our revenues, which could have a material adverse effect on our business, results of operations and financial condition.
17
The Company believes that under the current regulatory environment, the generic pharmaceutical industry as a whole will be the target of increased governmental scrutiny, especially with respect to state and federal anti-trust and price-fixing claims.
See Note 10 “Legal, Regulatory Matters and Contingencies” for a description of current state and federal anti-trust and price-fixing claims.
Other Applicable Laws
We are also subject to federal, state and local laws of general applicability, including laws regulating working conditions and the storage, transportation, or discharge of items that may be considered hazardous substances, hazardous waste, or environmental contaminants. We monitor our compliance with laws and we believe we are in substantial compliance with all regulatory bodies.
As a publicly-traded company, we are also subject to significant regulations and laws, including the Sarbanes-Oxley Act of 2002. Since its enactment, we have developed and instituted a corporate compliance program based on what we believe are the current best practices and we continue to update the program in response to newly implemented or changing regulatory requirements.
Employees
As of June 30, 2020, we had 954 full-time employees.
Securities and Exchange Act Reports
We maintain a website at www.lannett.com. We make available on or through our website our current and periodic reports, including any amendments to those reports, that are filed with the Securities and Exchange Commission (the “SEC”) in accordance with the Securities Exchange Act of 1934, as amended (the “Exchange Act”). These reports include Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. This information is available on our website free of charge as soon as reasonably practicable after we electronically file the information with, or furnish it to, the SEC.
The contents of our website are not incorporated by reference in this Form 10-K and shall not be deemed “filed” under the Exchange Act.
18
A relatively small group of products may represent a significant portion of our revenues, gross profit, or net earnings from time to time.
Sales of a limited number of our products from time to time represent a significant portion of our revenues, gross profit and net earnings. For Fiscal 2020, 2019 and 2018, our top five products in terms of sales, in the aggregate, represented approximately 45%, 52% and 58%, respectively, of our total net sales. If the volume or pricing of our largest selling products decline in the future, our business, financial condition, results of operations, cash flows and/or share price could be materially adversely affected. See Item 1. Description of Business for more information on our top products.
The generic pharmaceutical industry is highly competitive.
We face strong competition in our generic product business. Revenues and gross profit derived from the sales of generic pharmaceutical products tend to follow a pattern based on certain regulatory and competitive factors. Typically, as patents for brand-name products and related exclusivity periods expire or fall under patent challenges, the first generic manufacturer to receive regulatory approval for generic equivalents of such products is generally able to achieve significant market penetration. As competing off-patent manufacturers receive regulatory approvals on similar products or as brand manufacturers launch generic versions of such products (for which no separate regulatory approval is required), market share, revenues and gross profit typically decline, in some cases dramatically. Accordingly, the level of market share, revenue and gross profit attributable to a particular generic product is normally related to the number of competitors in that product’s market and the timing of that product’s regulatory approval and launch, in relation to competing approvals and launches. Consequently, we must continue to develop and introduce new products in a timely and cost-effective manner to maintain our revenues and gross margins.
If we are unable to successfully develop or commercialize new products, our operating results will suffer.
Our future results of operations will depend to a significant extent upon our ability to successfully commercialize new generic products in a timely manner. There are numerous difficulties in developing and commercializing new products, including:
| ● | developing, testing and manufacturing products in compliance with regulatory standards in a timely manner; |
| ● | receiving requisite regulatory approvals for such products in a timely manner; |
| ● | the availability, on commercially reasonable terms, of raw materials, including APIs and other key ingredients; |
| ● | developing and commercializing a new product is time consuming, costly and subject to numerous factors that may delay or prevent the successful commercialization of new products; and |
| ● | commercializing generic products may be substantially delayed by unexpired patents covering the brand drug. |
As a result of these and other difficulties, products currently in development by Lannett may or may not receive the regulatory approvals necessary for marketing. If any of our products, when developed and approved, cannot be successfully or timely commercialized, our operating results could be adversely affected. We cannot guarantee that any investment we make in developing products will be recouped, even if we are successful in commercializing those products.
Refer to the risk factor below related to the COVID-19 pandemic for further discussion of risks identified by the Company relating to the development and commercialization of new products.
19
Our gross profit may fluctuate from period to period depending upon our product sales mix, our product pricing and our costs to manufacture or purchase products.
Our future results of operations, financial condition and cash flows depend to a significant extent upon our product sales mix. Sales of certain products that we manufacture tend to create higher gross margins than the products we purchase and resell. As a result, our sales mix will significantly impact our gross profit from period to period.
Factors that may cause our sales mix to vary include:
| ● | the number of new product introductions; |
| ● | marketing exclusivity, if any, which may be obtained on certain new products; |
| ● | the level of competition in the marketplace for certain products; |
| ● | the availability of raw materials and finished products from our suppliers; and |
| ● | the scope and outcome of governmental regulatory action that may involve us. |
The Company is continuously seeking to keep product costs low, however there can be no guarantee that gross profit percentages will stay consistent in future periods. Pricing pressure from competitors, changes in product mix and the costs of producing or purchasing new drugs may also fluctuate in future periods.
Our substantial indebtedness may adversely affect our financial health.
We currently have substantial indebtedness. As of June 30, 2020, we had total outstanding debt of $708.0 million, of which $88.2 million is due within 12 months. The term loan A matures in November 2020 with $48.8 million outstanding as of June 30, 2020. As of June 30, 2020, we also have an undrawn $125.0 million revolving credit facility (the “Revolving Credit Facility”), which expires in November 2020. The Amended Term Loan Facility consists of an initial $910.0 million senior secured term loan facility (the “Senior Secured Term Loan Facility”), which was amended in June 2016 to include an additional $150.0 million incremental term loan (the “Incremental Term Loan”). The Amended Term Loan Facility, together with the Revolving Credit Facility comprises the amended senior secured credit facility (the “Amended Senior Secured Credit Facility”).
Our substantial indebtedness may have important consequences for us. For example, it may:
| ● | increase our vulnerability to general economic and industry conditions, including recessions and periods of significant inflation and financial market volatility; |
| ● | expose us to the risk of increased interest rates, because any borrowings we make under the Revolving Facility and other borrowings under the Term Loan Facility under certain circumstances, will bear interest at variable rates; |
| ● | require us to use a substantial portion of cash flow from operations to service our indebtedness, thereby reducing our ability to fund working capital, capital expenditures and other expenses; |
| ● | limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; |
| ● | place us at a competitive disadvantage compared to competitors that have less indebtedness; and |
| ● | limit our ability to borrow additional funds that may be needed to operate and expand our business. |
20
Due to many factors beyond our control, we may not be able to generate sufficient cash to service all of our indebtedness and meet our other ongoing liquidity needs and we may be forced to take other actions to satisfy our obligations under our debt agreements, which may not be successful.
Our ability to make payments on and to refinance, our indebtedness and to fund planned capital expenditures will depend on our ability to generate cash in the future. This is subject to general economic, financial, competitive, legislative, regulatory and other factors, many of which are beyond our control.
Our business may not generate sufficient cash flow from operations and we may not have available to us future borrowings in an amount sufficient, to enable us to pay our indebtedness or to fund our other liquidity needs. In these circumstances, we may need to refinance all or a portion of our indebtedness on or before maturity. Any refinancing of our debt could be at higher interest rates and may require us to comply with more onerous covenants, which could further restrict our business operations. Our ability to refinance our indebtedness or obtain additional financing will depend on, among other things:
| ● | our financial condition at the time; |
| ● | restriction in the agreements governing our indebtedness; |
| ● | the condition of the financial markets and the industry in which we operate; and |
| ● | our debt credit ratings. |
As a result, we may not be able to refinance any of our indebtedness on commercially reasonable terms or at all. In such a case, we could be forced to sell assets, reduce or delay capital expenditures or issue equity securities to make up for any shortfall in our payment obligations under unfavorable circumstances. The terms of the Amended Senior Secured Credit Facility limit our ability to sell assets. In addition, we may not be able to sell assets quickly enough or for sufficient amounts to enable us to meet our obligations. Any failure to make scheduled payments of interest and principal on our outstanding indebtedness when due would permit the holders of such indebtedness to declare an event of default and accelerate the indebtedness. This could result in the lenders under the Amended Senior Secured Credit Facility terminating their commitments to lend us money and foreclosing against the assets securing the borrowings and we could be forced into bankruptcy or other insolvency proceedings. In addition, any failure to make payments of interest and principal on our outstanding indebtedness on a timely basis would likely result in a reduction of our credit rating, which could harm our ability to incur additional indebtedness on acceptable terms. In August 2018, both Moody’s and S&P reduced the Company’s debt credit rating to “B3” and “B-”, respectively.
The Amended Senior Secured Credit Facility imposes operating and financial restrictions, which may prevent us from pursuing certain business opportunities and taking certain actions that may be potentially profitable or in our best interests.
The operating and financial restrictions and covenants in our Amended Senior Secured Credit Facility restrict and future debt instruments may restrict, subject to certain important exceptions and qualifications, our and our subsidiaries’ ability to, among other things:
| ● | incur or guarantee additional indebtedness; |
| ● | make certain investments or acquisitions; |
| ● | grant or permit certain liens on our assets; |
| ● | enter into certain transactions with affiliates; |
| ● | pay dividends, redeem our equity or make other restricted payments; |
21
| ● | prepay, repurchase or redeem contractually subordinated debt and certain other debt; |
| ● | merge, consolidate or transfer substantially all of our assets; |
| ● | transfer, sell or dispose of property and assets; and |
| ● | change the business we conduct or enter into new kinds of business. |
These covenants could adversely affect our ability to finance our future operations or capital needs, withstand a future downturn in our business or the economy in general, engage in business activities, including future opportunities that may be in our interest and plan for or react to market conditions or otherwise execute our business strategies. Our ability to comply with these covenants may be affected by events beyond our control. A breach of any of these covenants could result in a default in respect of the related indebtedness. If an event of default occurs, the relevant lenders or holders of such indebtedness could elect to declare the indebtedness, together with accrued interest, fees and other liabilities, to be immediately due and payable and proceed against any collateral securing that indebtedness. Acceleration of our other indebtedness could result in a default under the terms of the Amended Senior Secured Credit Facility. There is no guarantee that we would be able to satisfy our obligations if any of our indebtedness is accelerated.
In addition, the limitations imposed in the Amended Senior Secured Credit Facility on our ability to incur certain additional debt and to take other corporate actions might significantly impair our ability to obtain other financing. If, for any reason, we are unable to comply with the restrictions in the Amended Senior Secured Credit Facility, we may not be granted waivers or amendments to such restrictions or we may not be able to refinance our debt on terms acceptable to us, or at all. The lenders under the Amended Senior Secured Credit Facility also have the right in these circumstances to terminate any commitments they have to provide further borrowings. If we fail to meet any covenants in our Amended Senior Secured Credit Facility and cannot secure a waiver for such failure, the lenders under our Amended Senior Secured Credit Facility would be entitled to exercise various rights, including causing the amounts outstanding under the entire Amended Senior Secured Credit Facility to become immediately due and payable. If we were unable to pay such amounts, the lenders under the Amended Senior Secured Credit Facility could recover amounts owed to them by foreclosing against the collateral pledged to them. We have pledged a substantial portion of our assets to the lenders under the Amended Senior Secured Credit Facility, including the equity of our subsidiaries.
Our Amended Senior Secured Credit Facility contains financial covenants and other restrictive covenants that may limit our flexibility. We may not be able to comply with these covenants, which could result in the amounts outstanding under our Amended Senior Secured Credit Facility becoming immediately due and payable.
Our Revolving Credit Facility requires us to comply with a first lien net leverage ratio not to exceed 3.25:1.00 when there are outstanding loans and letters of credit (other than (i) drawn letters of credit that have been cash collateralized and (ii) up to $5.0 million of undrawn letters of credit) thereunder that exceed 30% of the aggregate commitment amount under the Revolving Credit Facility of $125.0 million as of the last day of the applicable fiscal quarter.
In addition, the Amended Senior Secured Credit Facility is subject to a financial performance covenant, which provides that the Company shall not permit its secured net leverage ratio as of the last day of any four consecutive fiscal quarters to be greater than to 4.25:1.00 (with a step-down, occurring as of September 30, 2020, to 4.00:1.00).
Lastly, the Amended Senior Secured Credit Facility is also subject to a minimum liquidity covenant, which provides that the Company shall not permit its liquidity as of the last day of any fiscal quarter to be less than $75.0 million. For purposes of this covenant liquidity is defined as the sum of availability under the Revolving Credit Facility (calculated in the manner described in the following sentence) plus unrestricted cash. For purposes of this covenant, availability under the Revolving Credit Facility is calculated as the amount available to be drawn other than any amount which if drawn would result in a breach of the Revolving Credit Facility financial covenant on a pro forma basis.
Accordingly, if our liquidity and performance significantly worsens, we could become non-compliant with such covenants.
22
As of June 30, 2020, the Company was in compliance with the financial and other covenants included in our debt agreements. See Note. 9 “Long-Term Debt.” Based on our current projections, the Company expects to have sufficient liquidity and cash flows to be able to meet our debt service requirements through June 30, 2021 and expects to be in compliance with its financial covenants through maturity of the Term Loan A in November 2020. If actual results for the year ending June 30, 2021 are less than the Company’s current projections and/or if management’s plans to offset the loss of the revenues and cash flows from the products distributed under the JSP Distribution Agreement are not successful, the Company could be in violation of its covenants, which may require significant accelerated payments of debt.
We are also subject to requirements to make mandatory prepayments, with the net proceeds of certain asset sales, excess cash flows and debt issuances. These requirements could limit our ability to obtain future financing, make acquisitions or needed capital expenditures, withstand any downturns in our business or the economy in general, conduct operations or otherwise take advantage of business opportunities that may arise, any of which could place us at a competitive disadvantage relative to our competitors that have less debt and are not subject to such restrictions.
Our variable rate indebtedness subjects us to interest rate risk, which could cause our debt service obligations to increase significantly.
Borrowings under the Amended Senior Secured Credit Facility are at variable rates of interest and expose us to interest rate risk. If interest rates increase, our debt service obligations on our variable rate indebtedness will increase even though the amount borrowed remained the same and our net income and cash flows, including cash available for servicing our indebtedness, will correspondingly decrease. Based on total outstanding debt as of June 30, 2020 and the assumption that interest rates are above the interest rate floor set forth in the Amended Senior Secured Credit Facility, each 1/8th percentage point change in interest rates would result in a $0.8 million change in annual interest expense on our outstanding debt under the Amended Senior Secured Credit Facility.
Management’s plans to address the impact of the nonrenewal of the JSP Distribution Agreement may not be successful.
Net sales of JSP products totaled $202.5 million in fiscal year 2019 and $253.1 million in fiscal year 2018. Our Distribution Agreement with JSP was not renewed when it expired on March 23, 2019. Management has implemented plans to offset the impact of the nonrenewal of the JSP Distribution Agreement. These plans currently include, among other things, an emphasis on reducing cost of sales, R&D and selling, general and administration (“SG&A”) expenses, continuing to accelerate new product launches, increasing its level of strategic partnerships and reducing capital expenditures. Management will also continue its emphasis on accelerating ANDA filings. However, the impact that these actions will have cannot be assured and they may not be sufficient to offset the impact, in whole or in part, of the loss of net sales, earnings or cash flows resulting from the nonrenewal of the JSP Distribution Agreement.
Public health threats, including a pandemic, epidemic or outbreak of an infectious disease in the United States or elsewhere may adversely affect our business and financial results.
Our business may be adversely affected by public health threats, including any pandemic, epidemic or outbreak of an infectious disease occurring in the United States or worldwide. In December 2019, a novel strain of coronavirus (“COVID-19”) was identified in Wuhan, China. This virus continues to spread globally and has spread to over 200 countries, including the United States, and has been declared a pandemic by the World Health Organization (“WHO”). The spread of COVID-19 has impacted the global economy and may impact our business, operations and financial results.
For example, COVID-19 has resulted in significant governmental “social-distancing” measures being implemented to control the spread of the virus, including quarantines, travel restrictions and business shutdowns. Any business shutdowns or other business interruptions affecting our suppliers or interruptions in global shipping affecting our suppliers could result in our inability to continue receiving sufficient amount of finished dosage products, API and other raw materials. Any business shutdowns or other business interruptions affecting our business development and other strategic partners could also cause delays in the regulatory approval process for and launching of some or all of our
23
pipeline drug candidates. We cannot presently predict the duration and severity of any potential business shutdowns or disruptions, but if we or any of the third parties with whom we engage, including the partners and other third parties with whom we conduct business, were to experience shutdowns or other business disruptions, our ability to conduct our business in the manner and on the timelines presently planned could be materially and adversely impacted.
Additionally, while the potential economic impact brought by, and the duration of, COVID-19 may be difficult to assess or predict, a widespread pandemic could result in significant disruption of global economies and financial markets, reducing our ability to access funding sources and capital, and our ability to refinance our existing indebtedness on reasonable terms or at all, which could in the future negatively affect our liquidity and our results of operations.
Infections and deaths related to COVID-19 may disrupt the United States’ healthcare and healthcare regulatory systems. Such disruptions could divert healthcare resources away from, or materially delay the FDA approval with respect to, our pending and future drug product applications. It is unknown how long these disruptions could continue, were they to occur. Additionally, subsequent to an initial stocking up of supplies at the start of the pandemic, the total volume of drug prescriptions written during the pandemic has decreased, causing less demand for our products. The length and severity of the pandemic may continue to affect the demand for our products in the future.
In line with the WHO recommendation to practice social distancing, individuals may be more reluctant to go to hospitals or see doctors, and hospitals and doctors may temporarily cease performing elective procedures, all of which could impact the number of overall prescriptions prescribed. Furthermore, we have taken temporary precautionary measures intended to help minimize the risk of the virus to our employees, including temporarily requiring all employees, other than employees in our manufacturing plants, distribution centers, and R&D facilities, who are able to work from home to work remotely. We have already suspended non-essential travel worldwide for our employees and are discouraging employee attendance at other gatherings. These measures could negatively affect our business. For instance, temporarily requiring many of our employees to work remotely may disrupt our operations or increase the risk of a cybersecurity incident.
The full extent to which COVID-19 may impact our business will depend on future developments, which are highly uncertain and cannot be predicted with confidence, such as the duration of the outbreak, the severity of COVID-19 or the effectiveness of actions to contain and treat COVID-19, particularly in the geographies where we or our third party suppliers or business development and other strategic partners operate. Given the speed and frequency of continuously evolving developments with respect to this pandemic, we cannot reasonably estimate the magnitude of any impact on our operations and the full extent to which COVID-19 may impact our business, results of operations, liquidity or financial position is uncertain.
Governmental investigations into sales and marketing practices in the generic pharmaceutical industry and claims by private parties relating to such investigations may result in substantial penalties or settlements.
There has been increased press coverage and increased scrutiny from regulatory and enforcement agencies and legislative bodies with respect to matters relating to the pricing of generic pharmaceuticals, including publicity and pressure resulting from prices charged by our competitors. We have experienced and may continue to experience downward pricing pressure on the price of our products due to competitive pressure to lower the cost of drugs to the ultimate consumer, which could reduce our revenue and future profitability. This increased press coverage and public scrutiny have resulted in, and may continue to result in, investigations, and calls for investigations, by governmental agencies at both the federal and state level and have resulted in, and may continue to result in, claims brought against us by private parties or by regulators taking other measures that could have a negative effect on our business. For a description of current, federal, and state investigations and claims by private parties, see Note 10 “Legal, Regulatory Matters and Contingencies.” Additional actions are possible. Responding to such investigations and claims is costly and involves a significant diversion of management attention. Such proceedings are unpredictable and may develop over lengthy periods of time. Future settlements may involve large monetary penalties. It is not possible at this time to predict the ultimate outcome of any such investigations or claims or what other investigations or lawsuits or regulatory responses may result from such assertions, or their impact on our business, financial condition, results of operations, cash flows, and/or our stock price. Any such investigation or claim could also result in reputational harm and reduced market acceptance and demand for our products, could harm our ability to market our products in the future, could cause
24
us to incur significant expense, could cause our senior management to be distracted from execution of our business strategy, and could have a material adverse effect on our business, financial condition, results of operations and growth prospects. Accompanying the press and media coverage of pharmaceutical pricing practices and public complaints about the same, there has been increasing U.S. federal and state legislative and enforcement interest with respect to drug pricing. In recent years, both the U.S. House of Representatives and the U.S. Senate have conducted numerous hearings with respect to pharmaceutical drug pricing practices, including in connection with the investigation of specific price increases by pharmaceutical companies. In addition to the effects of any investigations or claims brought against us described above, our revenue and future profitability could also be negatively affected if any such inquiries, of us or of other pharmaceutical companies or the industry more generally, were to result in legislative or regulatory proposals that limit our ability to increase the prices of our products. Any of the events or developments described above could have a material adverse impact on our business, financial condition or results of operations, as well as on our reputation.
The recent enactment of State laws affecting the pricing of our products could have the effect of reducing our profitability.
Since 2016, several state legislatures have enacted laws regulating the pricing of various types of pharmaceutical products, including generic pharmaceutical products. These laws vary in applicability and scope, and generally require manufacturers to notify various state agencies of price increases over a given threshold for a given period of time and to include a justification for any price increases. At least one state law (subsequently struck by the court) authorized the state attorney general to seek civil penalties and disgorgement in the event a price increase is deemed unconscionable. To the extent these laws apply to our products, they could limit the prices which the company may charge for its products and reduce the company’s profitability and could have a material adverse effect on our financial condition, results of operations and growth prospects.
The market price of our common stock has been volatile and may continue to be volatile in the future, and the value of any investment in our common stock could decline significantly.
The market price for our shares of common stock listed on the NYSE has fluctuated significantly from time to time, for example, varying between an intra-day high of $15.52 to an intra-day low of $5.46 during Fiscal 2020. Material amounts of short-selling of our common stock over the past few years has also increased the volatility of the market price for our common stock. The market price of our common stock is likely to continue to be volatile and subject to significant price and volume fluctuations in response to market, industry and other factors, including the risks described in this section. Further, the stock market for pharmaceutical companies has recently experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies. In particular, recent negative publicity regarding pricing and price increases by pharmaceutical companies has negatively impacted, and may continue to negatively impact, the market for pharmaceutical companies. These broad market and industry factors have negatively impacted, and in the future may seriously negatively impact, the market price of our common stock, regardless of our operating performance. Our stock market price may also be dependent upon the valuations and recommendations of the analysts who cover our business. If our results do not meet these analysts’ forecasts, the expectations of our investors or the financial guidance we provide to investors in any period, the market price of our common stock could decline. In the past, following periods of volatility in the market or significant price decline, securities class-action litigation has often been instituted against companies and we have been subject to one such suit, as further described in Note 10 “Legal, Regulatory Matters and Contingencies.” Such suits could result in substantial costs and diversion of management’s attention and resources, which could materially and adversely affect our business, results of operations and financial condition.
If our intangible assets become impaired, we may be required to record a significant charge to earnings.
Under accounting principles generally accepted in the U.S. (“U.S. GAAP”), we review our indefinite-lived intangible assets for impairment at least annually and when there are changes in circumstances. We may be required to record additional significant charges to earnings in our financial statements during the period in which any impairment of our indefinite-lived intangible assets is determined, resulting in a negative effect on our results of operations. Changes in market conditions or other changes in the future outlook of value may lead to further impairments in the future. In addition, we continue to review the potential divestment of certain assets as part of our future plans, which may lead to
25
additional impairments. Future events or decisions may lead to asset impairments and/or related charges. For assets that are not impaired, we may adjust the remaining useful lives. Certain non-cash impairments may result from a change in our strategic goals, business direction or other factors relating to the overall business environment. Any significant impairment could have a material adverse effect on our results of operations.
Extensive industry regulation has had and will continue to have, a significant impact on our business in the area of cost of goods, especially our product development, manufacturing and distribution capabilities.
All pharmaceutical companies, including Lannett, are subject to extensive, complex, costly and evolving regulation by the federal government, including the FDA and, in the case of controlled drugs, the DEA and state government agencies. The FDCA, the CSA and other federal statutes, regulations and guidance govern or influence the development, testing, manufacturing, packing, labeling, storing, record keeping, safety, approval, advertising, promotion, sale and distribution of our products.
The process for obtaining governmental approval to manufacture and market pharmaceutical products is rigorous, time-consuming and costly and we cannot predict the extent to which we may be affected by legislative and regulatory developments. We are dependent on receiving FDA and other governmental or third-party approvals prior to manufacturing, marketing and shipping our products. The FDA approval process for a particular product candidate can take several years and requires us to dedicate substantial resources to complete all activities necessary to secure approvals and we may not be able to obtain regulatory approval for our product candidates in a timely manner, or at all. In order to obtain approval of Abbreviated New Drug Applications (“ANDAs”) for our generic product candidates, we must demonstrate that our drug product is therapeutically equivalent and bioequivalent to a drug previously approved by the FDA through the drug approval process, known as the reference listed drug (“RLD”) or reference standard drug (“RS”). Bioequivalence may be demonstrated in vivo or in vitro by comparing the generic product candidate to the innovator drug product. Approval of our drug products that vary in certain ways from a brand name version of that drug may require a different FDA review process and application known as a 505(b)(2) NDA. Such 5050(b)(2) applications may require costly human clinical studies which may extend the time for approval of such drug product. Moreover, the FDA may request additional information and studies to support approval of an application, which could delay approval of the product and impair our ability to compete with other versions of the generic drug product.
Consequently, there is always the chance that we will not obtain FDA or other necessary approvals, or that the rate, timing and cost of such approvals will adversely affect our product introduction plans or results of operations. We carry inventories of certain products in anticipation of launch and if such products are not subsequently launched, we may be required to write-off the related inventory. Furthermore, the FDA also has the authority to withdraw drug approvals previously granted after a hearing and require a firm to remove these products from the market for a variety of reasons, including a failure to comply with applicable regulations or the discovery of previously unknown safety problems with the product.
Additionally, certain products marketed prior to the enactment of the 1962 amendments to the FDCA may be considered “generally recognized as safe and effective based on published scientific literature” or “GRASE.” GRASE products are those “old drugs that do not require prior approval from the FDA in order to be marketed.” Similarly, pursuant to 21 U.S.C. § 321(p)(1), also known as the “grandfather clause”, a product is exempted from the “effectiveness requirements [of the act] if its composition and labeling have not changed since 1962 and if, on the day before the 1962 amendments became effective, it was (1) used or sold commercially in the United States, (2) not a new drug as defined by the act at that time and (3) not covered by an effective application.” FDA’s current legal interpretation regarding GRASE status effectively limits the legal commercialization of such products. Moreover recently, the FDA has increased its efforts to force companies to file and seek FDA approval for Grandfathered products. FDA efforts included issuing notices to companies currently producing these products to cease its distribution of said products. Lannett currently manufactures and markets the unapproved product hyosyne solution/elixir.
In addition, facilities used to manufacture and/or test materials and drug products we market are subject to periodic inspection of facilities by the FDA, the DEA and other authorities to confirm that firms are in compliance with all applicable regulations. The FDA conducts pre-approval and/or post-approval inspections to determine whether systems and processes are in compliance with cGMP and other FDA regulations. A Form 483 notice is generally issued at the
26
conclusion of a FDA inspection and lists conditions the FDA inspectors believe may violate cGMP or other FDA regulations. If more serious violations are identified, the FDA may take additional action, such as issuing warning letters, import alerts, etc. The DEA and comparable state-level agencies also heavily regulate the manufacturing, holding, processing, security, record-keeping and distribution of drugs that are controlled substances. Lannett manufactures and/or distributes a variety of controlled substances. The DEA periodically inspects facilities for compliance with its regulations. If our manufacturing facilities or those of our suppliers fail to comply with applicable regulatory requirements, it could result in regulatory action and additional costs.
Our inability or the inability of our suppliers to comply with applicable FDA and other regulatory requirements can result in, among other things, delays in or denials of new product approvals, warning letters, import alerts, fines, consent decrees restricting or suspending manufacturing operations, injunctions, civil penalties, recall or seizure of products, total or partial suspension of sales and/or criminal prosecution. Any of these or other regulatory actions could materially harm our operating results and financial condition. Although we have instituted internal compliance programs, if these programs do not meet regulatory agency standards or if compliance is deemed deficient in any significant way, it could materially harm our business. Additionally, if the FDA were to undertake additional enforcement activities with Lannett’s Grandfathered products, their actions could result in, among other things, removal of some products from the market, seizure of the product and total or partial suspension of sales. Any of these regulatory actions could materially harm our operating results and financial condition.
Our manufacturing operations as well as our suppliers’ manufacturing operations are subject to establishment registration by the FDA and periodic inspections by the FDA to assure compliance regarding the manufacturing of our products. If we or our suppliers do not maintain the current registrations or if we or our partners receive notices of manufacturing and quality-related observations following inspections by the FDA, our operating results would be materially negatively impacted.
All of our facilities as well as applicable contract/supplier facilities, rely on maintaining current FDA registration and other licenses to produce and develop generic drugs. If the Company does not successfully renew its FDA registrations, the financial results of Lannett would be negatively impacted. We and our third-party manufacturers are subject to periodic inspection by the FDA to assure regulatory compliance regarding the manufacturing, distribution, and promotion of pharmaceutical products. The FDA imposes stringent mandatory requirements on the manufacture and distribution of pharmaceutical products to ensure their safety and efficacy. If we or our partners receive similar notices of manufacturing and quality-related observations and correspondence in the future, and if we are unable to resolve these observations and address the FDA’s concerns in a timely fashion, our business, financial results and/or stock price could be materially affected.
We have and will continue to enter into strategic alliances and collaborations with third parties for the commercialization of some of our drug candidates. If those collaborations are not successful, we may not be able to capitalize on the market potential of these drug candidates.
We previously have and will continue in the future to seek third-party collaborators for the commercialization of some of our drug candidates on a selected basis, which adds a level of complexity to our supply network. If we do enter into any such arrangements with any third parties, we will likely have limited control over the amount and timing of resources that our collaborators dedicate to the development of our drug candidates. Our ability to generate revenues from these arrangements will depend on our collaborators’ abilities and efforts to successfully perform the functions assigned to them in these arrangements. Many risks associated with relying on third-party collaborators for developing new products are beyond our control. For example, some of our collaboration partners may decide to make substantial changes to a product’s formulation or design, may experience supply interruptions or financial difficulties or may have limited financial resources. Any of the foregoing may delay the development of new products or interrupt their market supply. In addition, if a third-party collaborator on a new product terminates our collaboration agreement or does not perform under the agreement, we may experience delays and additional costs in developing or replacing that product.
Refer to the risk factor above related to the COVID-19 pandemic for further discussion of risks identified by the Company relating to third-party collaborators and the development of new products.
27
The loss of key personnel could cause our business to suffer.
The success of our present and future operations will depend, to a significant extent, upon the experience, abilities and continued services of our key personnel. If we lose the services of our key personnel, or if they are unable to devote sufficient attention to our operations for any other reason, our business may be significantly impaired. If the employment of any of our current key personnel is terminated, we cannot assure you that we will be able to attract and replace the employee with the same caliber of key personnel. As such, we have entered into employment agreements with all of our senior executive officers in order to help retain these key individuals.
If brand pharmaceutical companies are successful in limiting the use of generics through their legislative and regulatory efforts, our sales of generic products may suffer.
Many brand pharmaceutical companies have increasingly used state and federal legislative and regulatory means to delay generic competition. These efforts have included:
| ● | pursuing new patents for existing products which may be granted just before the expiration of one patent which could extend patent protection for additional years or otherwise delay the launch of generics; |
| ● | using the Citizen Petition process to request amendments to FDA standards; |
| ● | seeking changes to U.S. Pharmacopeia, an organization which publishes industry recognized compendia of drug standards; |
| ● | attaching patent extension amendments to non-related federal legislation; |
| ● | engaging in state-by-state initiatives to enact legislation that restricts the substitution of some generic drugs, which could have an impact on products that we are developing; |
| ● | persuading regulatory bodies to withdraw the approval of brand-name drugs for which the patents are about to expire and converting the market to another product of the brand company on which longer patent protection exists; |
| ● | limiting the availability of certain RLDs, with Risk Evaluation and Mitigation Strategies (“REMS”) distribution requirements, to generic companies for bioequivalence testing required for ANDA premarket approval for commercialization; |
| ● | entering into agreements whereby other generic companies will begin to market an AG, a generic equivalent of a branded product, at the same time or after generic competition initially enters the market; |
| ● | filing suits for patent infringement and other claims that may delay or prevent regulatory approval, manufacture and/or scale of generic products; and, |
| ● | introducing “next-generation” products prior to the expiration of market exclusivity for the reference product, which often materially reduces the demand for the generic or the reference product for which we seek regulatory approval. |
In the U.S., some pharmaceutical companies have lobbied Congress for amendments to the Hatch-Waxman Act that would give them additional advantages over generic competitors. For example, although the term of a company’s drug patent can be extended to reflect a portion of the time an NDA is under regulatory review, some companies have proposed extending the patent term by a full year for each year spent in clinical trials rather than the one-half year that is currently permitted.
28
If proposals like these were to become effective, or if any other actions by our competitors and other third parties to prevent or delay activities necessary to the approval, manufacture, or distribution of our products are successful, our entry into the market and our ability to generate revenues associated with new products may be delayed, reduced, or eliminated, which could have a material adverse effect on our business, financial condition, results of operations, cash flows and/or share price.
The generic pharmaceutical industry is characterized by intellectual property litigation and third parties may claim that we infringe on their proprietary rights which could result in litigation that could be costly, result in the diversion of management’s time and efforts, require us to pay damages or prevent us from marketing our existing or future products.
Our commercial success will depend in part on not infringing or violating the intellectual property rights of others. The manufacture, use and sale of new products that are the subject of conflicting patent rights have been the subject of substantial litigation in the pharmaceutical industry. These lawsuits relate to the validity and infringement of patents or proprietary rights of third parties. We may have to defend against charges that we violated patents or proprietary rights of third parties. This is especially true in the case of generic products on which the patent covering the brand product is expiring, an area where infringement litigation is prevalent and in the case of new brand products in which a competitor has obtained patents for similar products. Our competitors, some of which have substantially greater resources than we do and have made substantial intellectual property investments in competing technologies, may have applied for or obtained, or may in the future apply for and obtain, patent rights and other intellectual property that will prevent, limit or otherwise interfere with our ability to make, use and sell our products. We may not be aware of whether our products do or will infringe existing or future patents or the intellectual property rights of others. In addition, patent applications can be pending for many years and may be confidential for a number of months after filing and because pending patent claims can be revised before issuance, there may be applications of others now pending of which we are unaware that may later result in issued patents that will prevent, limit or otherwise interfere with our ability to make, use or sell our products. Even if we prevail, litigation may be costly and time-consuming and could divert the attention of our management and technical personnel. Any potential intellectual property litigation also could force us to do one or more of the following:
| ● | stop making, selling or using products or technologies that allegedly infringe the asserted intellectual property; |
| ● | lose the opportunity to license our technology to others or to collect royalty payments based upon successful protection and assertion of our intellectual property rights against others; |
| ● | incur significant legal expenses; |
| ● | pay substantial damages or royalties to the party whose intellectual property rights we may be found to be infringing; |
| ● | pay the attorney fees and costs of litigation to the party whose intellectual property rights we may be found to be infringing; |
| ● | redesign or rename, in the case of trademark claims, those products that contain the allegedly infringing intellectual property, which could be costly, disruptive and/or infeasible; or |
| ● | attempt to obtain a license to the relevant intellectual property from third parties, which may not be available on reasonable terms or at all. |
Any litigation or claim against us, even those without merit, may cause us to incur substantial costs and could place a significant strain on our financial resources, divert the attention of management from our core business and harm our reputation. If we are found to infringe the intellectual property rights of third parties, we could be required to pay substantial damages and/or substantial royalties and could be prevented from selling our products unless we obtain a
29
license or are able to redesign our products to avoid infringement. If we fail to obtain any required licenses or make any necessary changes to our products or technologies, we may have to withdraw existing products from the market or may be unable to commercialize one or more of our products, all of which could have a material adverse effect on our business, results of operations and financial condition.
Although the parties to patent and intellectual property disputes in the pharmaceutical industry have often settled their disputes through licensing or similar arrangements, the costs associated with these arrangements may be substantial and could include ongoing royalties. Any such license may not be available on reasonable terms, if at all and there can be no assurance that we would be able to redesign our products in a way that would not infringe the intellectual property rights of others. Even if we were able to obtain rights to the third-party’s intellectual property, these rights may be non-exclusive, thereby giving our competitors access to the same intellectual property. As a result, an adverse determination in a judicial or administrative proceeding or failure to obtain necessary licenses could prevent us from manufacturing and selling a number of our products, or force us to redesign or rename our products to avoid infringing the intellectual property rights of third parties, which, even if it is possible to so redesign or rename our products, which could harm our business, financial condition, results of operations and cash flows.
If we are unable to obtain sufficient supplies from key suppliers that in some cases may be the only source of finished products or raw materials, our ability to deliver our products to the market may be impeded.
We are required to identify the supplier(s) of all the raw materials for our products in our applications with the FDA. To the extent practicable, we attempt to identify more than one supplier in each drug application. However, some products and raw materials are available only from a single source and, in some of our drug applications, only one supplier of products and raw materials has been identified, even in instances where multiple sources exist. To the extent any difficulties experienced by our suppliers cannot be resolved within a reasonable time and at reasonable cost, or if raw materials for a particular product become unavailable from an approved supplier and we are required to qualify a new supplier with the FDA, our profit margins and market share for the affected product could decrease and our development and sales and marketing efforts could be delayed.
Our policies regarding returns, allowances and chargebacks and marketing programs adopted by wholesalers may reduce our revenues in future fiscal periods.
Based on industry practice, generic drug manufacturers have liberal return policies and have been willing to give customers post-sale inventory allowances. Under these arrangements, from time to time we give our customers credits on our generic products that our customers hold in inventory after we have decreased the market prices of the same generic products due to competitive pricing. Therefore, if new competitors enter the marketplace and significantly lower the prices of any of their competing products, we would likely reduce the price of our products. As a result, we would likely be obligated to provide credits to our customers who are then holding inventories of such products, which could reduce sales revenue and gross margin for the period the credit is provided. Like our competitors, we also give credits for chargebacks to wholesalers that have contracts with us for their sales to hospitals, group purchasing organizations, pharmacies or other customers.
A chargeback is the difference between the price the wholesaler pays and the price that the wholesaler’s end-customer pays for a product. Although we establish reserves based on our prior experience and our best estimates of the impact that these policies may have in subsequent periods, we cannot ensure that our reserves are adequate or that actual product returns, allowances and chargebacks will not exceed our estimates.
Health care initiatives and other third-party payor cost-containment pressures have and could continue to cause us to sell our products at lower prices, resulting in decreased revenues.
Some of our products are purchased or reimbursed by state and federal government authorities, private health insurers and other organizations, such as health maintenance organizations, or HMOs and managed care organizations, or MCOs. Third-party payors increasingly challenge pharmaceutical product pricing. There also continues to be a trend toward managed health care in the United States. Pricing pressures by third-party payors and the growth of organizations such as HMOs and MCOs could result in lower prices and a reduction in demand for our products.
30
One such governmental program, known as the 340B Program, requires pharmaceutical manufacturers to enter into an agreement, called a pharmaceutical pricing agreement (PPA), with the Secretary of Health and Human Services. Under the PPA, the manufacturer agrees to provide front-end discounts on covered outpatient drugs purchased by specified providers, called “covered entities,” that serve the nation’s most vulnerable patient populations. Outpatient prescription drugs, over the counter drugs (accompanied by a prescription), and clinic-administered drugs within eligible facilities are covered.
In addition, legislative and regulatory proposals and enactments to reform health care and government insurance programs could significantly influence the manner in which pharmaceutical products and medical devices are prescribed and purchased. We expect there will continue to be federal and state laws and/or regulations, proposed and implemented, that could limit the amounts that federal and state governments will pay for health care products and services. The extent to which future legislation or regulations, if any, relating to the health care industry or third-party coverage and reimbursement may be enacted or what effect such legislation or regulation would have on our business remains uncertain. For example, H.R.987, the “Strengthening Health Care and Lowering Prescription Drug Costs Act,” which incorporated a bipartisan effort to address prescription drug pricing combined with broader provisions protecting the Affordable Care Act, was passed by the House of Representatives on May 16, 2019, but it is not expected to pass in the Senate. The bill does represent bipartisan consensus on the need to reform the drug pricing system. Another bill, the Lower Drugs Now Act of 2019 would give Medicare the ability to negotiate drug prices. Other proposals include the Prescription Drug Pricing Reduction Act of 2019, and the Advanced Notice of Proposed Rulemaking Medicare Program, IPI Model for Medicare Part B Drugs, issued by the Centers for Medicare and Medicaid Services. Such measures or other health care system reforms that are adopted could have a material adverse effect on our industry generally and our ability to successfully commercialize our products or could limit or eliminate our spending on development projects and affect our ultimate profitability.
We may need to change our business practices to comply with changes to fraud and abuse laws.
We are subject to various federal and state laws pertaining to health care fraud and abuse, including the Medicare and Medicaid Anti-Kickback Statute (the “Anti-Kickback Statute”), which apply to our sales and marketing practices and our relationships with physicians and other referral sources. At the federal level, the Anti-Kickback Statute prohibits any person or entity from knowingly and willfully soliciting, receiving, offering, or paying any remuneration, including a bribe, kickback, or rebate, directly or indirectly, in return for or to induce the referral of patients for items or services covered by federal health care programs, or the furnishing, recommending, or arranging for products or services covered by federal health care programs. Federal health care programs have been defined to include plans and programs that provide health benefits funded by the federal government, including Medicare and Medicaid, among others. The definition of “remuneration” has been broadly interpreted to include anything of value, including, for example, gifts, discounts, the furnishing of supplies or equipment, credit arrangements, payments of cash and waivers of payments. Several courts have interpreted the federal Anti-Kickback Statute’s intent requirement to mean that if even one purpose in an arrangement involving remuneration is to induce referrals or otherwise generate business involving goods or services reimbursed in whole or in part under federal health care programs, the statute has been violated. The federal government has issued regulations, commonly known as safe harbors that set forth certain provisions which, if fully met, will assure parties that they will not be prosecuted under the federal Anti-Kickback Statute. The failure of a transaction or arrangement to fit within a specific safe harbor does not necessarily mean that the transaction or arrangement will be illegal or that prosecution under the federal Anti-Kickback Statute will be pursued, but such transactions or arrangements face an increased risk of scrutiny by government enforcement authorities and an ongoing risk of prosecution. If our sales and marketing practices or our relationships with physicians are considered by federal or state enforcement authorities to be knowingly and willfully soliciting, receiving, offering, or providing any remuneration in exchange for arranging for or recommending our products and services and such activities do not fit within a safe harbor, then these arrangements could be challenged under the federal Anti-Kickback Statute.
If our operations are found to be in violation of the federal Anti-Kickback Statute we may be subject to civil and criminal penalties including fines of up to $100,000 per violation, civil monetary penalties of up to $100,000 per violation, assessments of up to three times the amount of the prohibited remuneration, imprisonment and exclusion from participating in the federal health care programs. Violations of the Anti-Kickback Statute also may result in a finding of civil liability under the FFCA (as further discussed below) and the potential imposition of additional civil fines and
31
monetary penalties that could be substantial. Falsely certifying compliance with the Anti-Kickback Statute in connection with a claim submitted to a federally funded insurance program is actionable under the FFCA. In addition, HIPAA and its implementing regulations created two new federal crimes: health care fraud and false statements relating to health care matters. The HIPAA health care fraud statute prohibits, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any health care benefit program, including private payors. A violation of this statue is a felony and may result in fines, imprisonment and/or exclusion from government-sponsored programs. The HIPAA false statements statute prohibits, among other things, knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation in connection with the delivery of or payment for health care benefits, items, or services.
A number of states also have anti-fraud and anti-kickback laws similar to the federal Anti-Kickback Statute that prohibit certain direct or indirect payments if such arrangements are designed to induce or encourage the referral of patients or the furnishing of goods or services. Some states’ anti-fraud and anti-kickback laws apply only to goods and services covered by Medicaid. Other states’ anti-fraud and anti-kickback laws apply to all health care goods and services, regardless of whether the source of payment is governmental or private. Due to the breadth of these laws and the potential for changes in laws, regulations, or administrative or judicial interpretations, we may have to change our business practices or our existing business practices could be challenged as unlawful, which could materially adversely affect our business.
Certain federal and state governmental agencies, including the U.S. Department of Justice and the U.S. Department of Health and Human Services, have been investigating issues surrounding pricing information reported by drug manufacturers and used in the calculation of reimbursements as well as sales and marketing practices. For example, many government and third-party payors, historically including Medicare and Medicaid, reimburse doctors and others for the purchase of certain pharmaceutical products based on the product’s average wholesale price (“AWP”) reported by pharmaceutical companies, although the Company has not used the term AWP since 2000. Medicare currently uses average sales price (“ASP”) and wholesale acquisition cost (“WAC”) when ASP data is unavailable. The federal government, certain state agencies and private payors are investigating and have begun to file court actions related to pharmaceutical companies’ reporting practices with respect to AWP, alleging that the practice of reporting prices for pharmaceutical products has resulted in a false and overstated AWP, which in turn is alleged to have improperly inflated the reimbursement paid by Medicare beneficiaries, insurers, state Medicaid programs, medical plans and others to health care providers who prescribed and administered those products. In addition, some of these same payors are also alleging that companies are not reporting their “best price” to the states under the Medicaid program.
Furthermore, under the FDCA, it is illegal for pharmaceutical companies to promote their products for uses that are not approved by the FDA, and companies that market drugs for so-called “off-label” indications may be subject to civil liability under the FFCA (as further discussed below), as well as to criminal penalties. Over the past decade, numerous lawsuits have been filed against pharmaceutical companies challenging their off-label promotional activities, and pharmaceutical companies, in the aggregate, have paid billions of dollars to defend and settle these cases.
We may become subject to federal and state false claims litigation brought by private individuals and the government.
We are subject to state and federal laws that govern the submission of claims for reimbursement. The FFCA imposes civil liability on individuals or entities that knowingly submit, or cause to be submitted, false or fraudulent claims for payment to the government. Violations of the FFCA and other similar laws may result in criminal fines, imprisonment and substantial civil penalties for each false claim submitted (including civil penalties presently in excess of $23,330 per claim, plus treble damages, plus liability for attorney’s fees) and exclusion from federally funded health care programs, including Medicare and Medicaid. The FFCA also allows private individuals to bring a suit on behalf of the government against an individual or entity for violations of the FFCA. These suits, also known as Qui Tam or whistleblower actions, may be brought by, with only a few exceptions, any private citizen who has material information of a false claim that has not yet been previously disclosed. These suits have increased significantly in recent years because the FFCA allows an individual to share in the amounts paid to the federal government in fines or settlement as a result of a successful Qui Tam action, in addition to the recovery of legal fees in bringing such an action. If our past or present operations are found to be in violation of any of such laws or any other governmental regulations that may apply to us, we may be
32
subject to penalties, including civil and criminal penalties, damages, fines, exclusion from federal health care programs and/or the curtailment or restructuring of our operations. Any penalties, damages, fines, curtailment, or restructuring of our operations could adversely affect our ability to operate our business and our financial results. Action against us for violation of these laws, even if we successfully defend against them, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
Recently, the Department of Justice has begun to use the 1961 federal Travel Act as a tool to pursue criminal charges in the case of health care kickback and commercial bribery allegations. This law was enacted as part of the Kennedy administration’s war on organized crime. It formed the basis for a federal enforcement action against a Texas physician-owned specialty hospital and a number of surgeons and administrators, who were convicted of conspiring to pay or receive bribes in exchange for referrals of patients in violation of a state commercial bribery law. Importantly, this case was not limited to claims covered under federal programs, and the failure of the state to bring charges under its own statute did not prevent the federal case from proceeding. The Travel Act may be used by the Department of Justice as a way to expand its reach to penalize kickbacks and similar arrangements even when the Anti-Kickback Statute and FFCA would not apply. These efforts could increase our vulnerability to litigation and penalties if our past or present operations are found to be in violation of applicable law.
Sales of our products may continue to be adversely affected by the continuing consolidation of our distribution network and the concentration of our customer base.
Our principal customers are wholesale drug distributors, major retail drug store chains and mail order pharmacies. These customers comprise a significant part of the distribution network for pharmaceutical products in the U.S. This distribution network has undergone significant consolidation marked by mergers and acquisitions among wholesale distributors and the growth of large retail drug store chains. As a result, a small number of large wholesale distributors control a significant share of the market and the number of independent drug stores and small drug store chains has decreased. We expect that consolidation of drug wholesalers and retailers will increase pricing and other competitive pressures on drug manufacturers, including Lannett.
Our net sales may also be affected by fluctuations in the buying patterns of retail chains, mail order distributors, wholesalers and other trade buyers, whether resulting from pricing, wholesaler buying decisions or other factors.
Our three largest customers accounted for 25%, 23% and 11%, respectively, of our total net sales for Fiscal 2020 and 21%, 18% and 12%, respectively, of our total net sales for Fiscal 2019. The loss of any of these customers, any financial difficulties experienced by any of these customers or any delay in receiving payments from such customers could materially adversely affect our business, results of operations and financial condition and our cash flows. In addition, the Company generally does not enter into long-term supply agreements with its customers that would require them to purchase our products.
We are increasingly dependent on information technology and our systems and infrastructure face certain risks, including cybersecurity and data leakage risks.
Significant disruptions to our information technology systems or breaches of information security could adversely affect our business. We are increasingly dependent on information technology systems and infrastructure to operate our business. In the ordinary course of business, we collect, store and transmit large amounts of confidential information (including trade secrets or other intellectual property, proprietary business information and personal information) and it is critical that we do so in a secure manner to maintain the confidentiality and integrity of such confidential information. We could be susceptible to third-party attacks on our information technology systems, which attacks are of ever-increasing levels of sophistication and are made by groups and individuals with a wide range of motives and expertise, including state and quasi-state actors, criminal groups, “hackers” and others. Maintaining the security, confidentiality and integrity of this confidential information (including trade secrets or other intellectual property, proprietary, business information and personal information) is important to our competitive business position. There can be no assurance that we can prevent service interruptions or security breaches in our systems or the unauthorized or inadvertent wrongful use or disclosure of confidential information that could adversely affect our business operations or result in the loss, misappropriation and/or unauthorized access, use or disclosure of, or the prevention of access to, confidential
33
information. A breach of our security measures or the accidental loss, inadvertent disclosure, unapproved dissemination, misappropriation or misuse of trade secrets, proprietary information, or other confidential information, whether as a result of theft, hacking, fraud, trickery or other forms of deception, or for any other cause, could enable others to produce competing products, use our proprietary technology or information and/or adversely affect our business position. Further, any such interruption, security breach, or loss, misappropriation and/or unauthorized access, use or disclosure of confidential information could result in financial, legal, business and reputational harm to us and could have a material adverse effect on our business, financial condition and results of operations.
The design, development, manufacture and sale of our products involves the risk of product liability claims by consumers and other third parties and insurance against such potential claims is expensive and may be difficult to obtain.
The design, development, manufacture and sale of our products involve an inherent risk of product liability claims and the associated adverse publicity. Insurance coverage is expensive and may be difficult to obtain and may not be available in the future on acceptable terms, or at all. Although we currently maintain product liability insurance for our products in amounts we believe to be commercially reasonable, if the coverage limits of these insurance policies are not adequate, a claim brought against Lannett, whether covered by insurance or not, could have a material adverse effect on our business, results of operations, financial condition and cash flows.
Rising insurance costs, as well as the inability to obtain certain insurance coverage for risks faced by us, could negatively impact profitability.
The cost of insurance, including workers compensation, product liability and general liability insurance, has risen in recent years and may increase in the future. In response, we may increase deductibles and/or decrease certain coverage to mitigate these costs. These increases and our increased risk due to increased deductibles and reduced coverage, could have a negative impact on our results of operations, financial condition and cash flows.
Additionally, certain insurance coverage may not be available to us for risks faced by us. Sometimes the coverage we obtain for certain risks may not be adequate to fully reimburse the amount of damage that we could possibly sustain. Should either of these events occur, the lack of insurance to cover our entire cost would adversely affect our results of operations and financial condition.
Federal regulation of arrangements between manufacturers of brand and generic products could adversely affect our business.
As part of the Medicare Prescription Drug, Improvement and Modernization Act of 2003, companies are now required to file with the Federal Trade Commission (“FTC”) and the Department of Justice certain types of agreements entered into between brand and generic pharmaceutical companies related to the manufacture, marketing and sale of generic versions of brand drugs. This new requirement could affect the manner in which generic drug manufacturers resolve intellectual property litigation and other disputes with brand pharmaceutical companies and could result generally in an increase in private-party litigation against pharmaceutical companies or additional investigations or proceedings by the FTC or other governmental authorities. The impact of this new requirement and the potential private-party lawsuits associated with arrangements between brand-name and generic drug manufacturers is uncertain and could adversely affect our business.
We expend a significant amount of resources on research and development efforts that may not lead to successful product introductions.
We conduct R&D primarily to enable us to gain approval for, manufacture, and market pharmaceuticals in accordance with applicable laws and regulations. We also partner with third parties to develop products. We cannot be certain that any investment made in developing products will be recovered, even if we are successful in commercialization. To the extent that we expend significant resources on R&D efforts and are not able, ultimately, to introduce successful new and/or complex products as a result of those efforts, there could be a material adverse effect on our business, financial condition, results of operations, cash flows, and/or the price of our common stock.
34
Investigations of the calculation of average wholesale prices may adversely affect our business.
Many government and third-party payers, including Medicare, Medicaid, Health Maintenance Organization and Managed Care Organization, have historically reimbursed doctors, pharmacies and others for the purchase of certain prescription drugs based on a drug’s AWP or WAC. In the past several years, state and federal government agencies have conducted ongoing investigations of manufacturers’ reporting practices with respect to AWP and WAC, in which they have suggested that reporting of inflated AWP’s or WAC’s has led to excessive payments for prescription drugs. For a description of current and federal and state investigations and claims by private parties, see Note 10 “Legal, Regulatory Matters and Contingencies.” Additional actions are possible. These actions, if successful, could adversely affect us and may have a material adverse effect on our business, results of operations, financial condition and cash flows.
Other manufacturers and distributors of pain management products have had complaints filed against and investigations commenced on them, and if similar actions are taken against us, it could reduce our revenue and future profitability.
During the past few years, a number of complaints have been filed with respect to sales and distribution of various types of pain management medications against various pharmaceutical companies (not including Lannett), by a number of cities, counties and states across the country alleging among other things that such companies failed to develop and implement systems sufficient to identify suspicious orders of such products and prevent the diversion of such products to individuals who used them for other than legitimate medical purposes. The complaints generally contend that the defendants allegedly engaged in improper marketing of pain management products, and seek a variety of remedies, including restitution, civil penalties, disgorgement of profits, treble damages, attorneys’ fees and injunctive relief. In addition, a number of State Attorneys General, including a coordinated multistate effort, have initiated investigations into sales and marketing practices of various pharmaceutical companies (not including Lannett) with respect to such pain management products. If any similar investigations or claims are commenced against us, it could result in reputational harm and reduced market acceptance and demand for our products, could harm our ability to market our products in the future, could cause us to incur significant expense, could cause our senior management to be distracted from execution of our business strategy, and could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
Guidelines and recommendations published by various organizations can reduce the use of our pain management products.
Government agencies promulgate regulations and guidelines directly applicable to us and to our products. In addition, professional societies, practice management groups, private health and science foundations and organizations from time to time may also publish guidelines or recommendations to the healthcare and patient communities. Recommendations of government agencies or these other groups or organizations may relate to such matters as usage, dosage, route of administration and use of concomitant therapies. For example, the Centers for Disease Control and Prevention has issued guidelines about the use of pain management products for chronic pain, the FDA has issued an Opioid Action Plan and in 2017 President Trump signed an executive order establishing the President’s Commission on Combatting Drug Addiction. Additionally, the FDA has required all opioid products, including immediate release drugs, to join a shared REMS program that educates healthcare providers to reduce serious adverse outcomes resulting from inappropriate prescribing, misuse, and abuse of opioid analgesics while maintaining patient access to pain medications. REMS participation has added significant costs to the Company. Recommendations or guidelines suggesting the reduced use of our products or the use of competitive or alternative products as the standard of care to be followed by patients and healthcare providers could result in decreased use of our products and could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
Acquisitions could result in operating difficulties, dilution and other harmful consequences that may adversely impact our business and results of operations.
Acquisitions are an important element of our overall corporate strategy and use of capital. These transactions could be material to our financial condition and results of operations. We also expect to continue to evaluate and enter into
35
discussions regarding a wide array of potential strategic transactions. We may compete for certain acquisition targets with companies having greater financial resources than us or other advantages over us that may hinder or prevent us from acquiring a target company or completing another transaction, which could also result in significant diversion of management time, as well as substantial out-of-pocket costs. The process of integrating an acquired company, business, or technology may create unforeseen operating difficulties and expenditures. The areas where we may face risks include but are not limited to (i) diversion of management time and focus from operating our business to acquisition integration challenges, (ii) implementation or remediation of controls, procedures and policies at the acquired company, (iii) integration of the acquired company’s accounting, human resource and other administrative systems and coordination of product, engineering and sales and marketing functions, (iv) transition of operations, users and customers onto our existing platforms, (v) failure to obtain required approvals from governmental authorities under competition and antitrust laws on a timely basis, if at all, which could, among other things, delay or prevent us from completing a transaction, or otherwise restrict our ability to realize the expected financial or strategic goals of an acquisition, (vi) cultural challenges associated with integrating employees from the acquired company into our organization and retention of employees from the businesses we acquire and (vii) liability for activities of the acquired company before the acquisition, including infringement claims, violations of laws, commercial disputes, tax liabilities, claims from current and former employees and customers and other known and unknown liabilities.
Our failure to address these risks or other problems encountered in connection with our past or future acquisitions could cause us to fail to realize the anticipated benefits of such acquisitions, incur unanticipated liabilities and harm our business generally. Future acquisitions could also result in dilutive issuances of our equity securities, the incurrence of debt, contingent liabilities, or amortization expenses, or write-offs of goodwill, any of which could harm our financial condition. Also, the anticipated benefit of many of our acquisitions may not materialize.
The phase out of the London Interbank Offered Rate (LIBOR), or the replacement of LIBOR with a different reference rate, may adversely affect interest rates.
On July 27, 2017, the Financial Conduct Authority (the authority that regulates LIBOR) announced that it would phase out LIBOR by the end of 2021. It is unclear whether new methods of calculating LIBOR will be established such that it continues to exist after 2021, or if alternative rates or benchmarks will be adopted. Changes in the method of calculating LIBOR, or the replacement of LIBOR with an alternative rate or benchmark, may adversely affect interest rates and result in higher borrowing costs. This could materially and adversely affect the Company’s results of operations, cash flows and liquidity. We cannot predict the effect of the potential changes to LIBOR or the establishment and use of alternative rates or benchmarks.
ITEM 2. DESCRIPTION OF PROPERTY
The Company’s 432,000 square foot Seymour, Indiana facility contains approximately 107,000 square feet of manufacturing space as well as a leased 116,000 square foot temperature/humidity-controlled storage warehouse. The Seymour facility has had satisfactory inspections conducted by the FDA and EMA and similar regulatory authorities of Japan, Taiwan, Brazil, China, Korea and Turkey. As of June 30, 2020, the facility has a production capacity of approximately 4.0 billion doses based on our current product mix and plant configuration.
The Company has an 110,000 square foot manufacturing facility located in Carmel, New York, which sits on 25.8 acres of land. The facility specializes in liquid products and currently houses manufacturing, packaging, quality and research and development and has capacity for additional manufacturing space, if needed.
Lannett owns two facilities in Philadelphia, Pennsylvania. The research and development facilities are located in a 31,000 square foot facility at 9000 State Road and a second, 63,000 square foot facility that is located within one mile of the State Road facility at 9001 Torresdale Avenue, Philadelphia, PA. The latter facility contains our analytical research and development and quality control laboratories. We have adopted many systems and processes to ensure adherence to FDA requirements and we believe we are operating our facilities in substantial compliance with the FDA’s cGMP regulations.
36
Information pertaining to legal proceedings can be found in Note 10 “Legal, Regulatory Matters and Contingencies” under Item 15. Exhibits and Financial Statement Schedule and is incorporated by reference herein.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable
ITEM 5. MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information
The Company’s common stock trades on the NYSE. The following table sets forth certain information with respect to the intraday high and intraday low sales prices per share of the Company’s common stock during Fiscal 2020 and 2019, as quoted by the NYSE.
Fiscal Year Ended June 30, 2020
|
| High |
| Low | ||
| | | | | | |
First quarter | | $ | 15.52 | | $ | 5.46 |
| | | | | | |
Second quarter | | $ | 13.12 | | $ | 8.16 |
| | | | | | |
Third quarter | | $ | 10.34 | | $ | 5.91 |
| | | | | | |
Fourth quarter | | $ | 10.01 | | $ | 6.10 |
Fiscal Year Ended June 30, 2019
|
| High |
| Low | ||
| | | | | | |
First quarter |
| $ | 14.55 | | $ | 4.60 |
| |
| | | | |
Second quarter | | $ | 6.48 | | $ | 3.33 |
| | | | | | |
Third quarter | | $ | 10.45 | | $ | 4.80 |
| | | | | | |
Fourth quarter | | $ | 9.39 | | $ | 5.16 |
Holders
As of June 30, 2020, there were 1,115 holders of record of the Company’s common stock.
Dividends
The Company did not pay cash dividends in Fiscal 2020, Fiscal 2019 or Fiscal 2018. The Company intends to use available funds for working capital, to pay down outstanding debt, plant and equipment additions, various product extension ventures and merger and acquisition or other growth opportunities. In addition, the Company is subject to
37
certain restrictions on dividends under its Amended Senior Secured Credit Facility. The Company does not expect to pay, nor should stockholders expect to receive, cash dividends in the foreseeable future.
The following table sets forth certain information with respect to the Company’s share repurchase activity in the fourth quarter of Fiscal 2020.
ISSUER PURCHASES OF EQUITY SECURITIES
| | | | | | | | | (d) Maximum | |
| | | | | | | (c) Total | | Number (or | |
| | | | | | | Number of | | Approximate | |
| | | | | | | Shares (or | | Dollar Value) | |
| | | | | | | Units) | | of Shares (or | |
| | | | | | | Purchased as | | Units) that | |
| | (a) Total | | | | | Part of | | May Yet Be | |
| | Number of | | (b) Average | | Publicly | | Purchased | ||
| | Shares (or | | Price Paid | | Announced | | Under the | ||
Period | | Units) | | per Share (or | | Plans or | | Plans or | ||
(In thousands) |
| Purchased* |
| Unit) |
| Programs |
| Programs | ||
| | | | | | | | | | |
April 1 to April 30, 2020 |
| 2,083 | | $ | 8.15 |
| — | | $ | — |
May 1 to May 31, 2020 |
| 2,829 | |
| 7.58 |
| — | |
| — |
June 1 to June 30, 2020 |
| 2,302 | |
| 7.28 |
| — | |
| — |
Total |
| 7,214 | | $ | 7.65 |
| — | |
| — |
* Shares were repurchased to settle employee tax withholding obligations pursuant to equity award programs.
Stock Performance Chart
The following graph presents a comparison of the cumulative total stockholder return on the Company’s stock with the cumulative total return of various indexes for the period of five fiscal years commencing July 1, 2015 and ending June 30, 2020. The graph assumes that $100 was invested on July 1, 2015 in each of the various indexes.
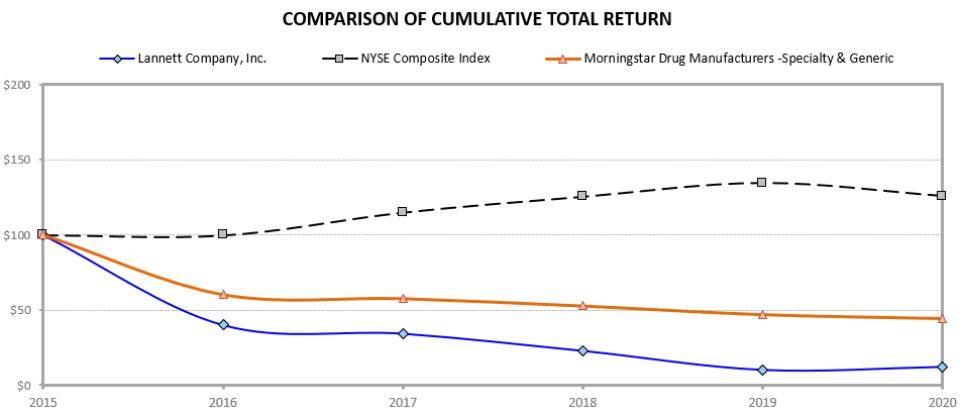
38
ITEM 6. SELECTED FINANCIAL DATA
The following financial information as of and for the five years ended June 30, 2020, has been derived from our Consolidated Financial Statements. This information should be read in conjunction with our Consolidated Financial Statements and related notes thereto included elsewhere herein.
Lannett Company, Inc. and Subsidiaries
Financial Highlights
(In thousands, except per share data) | |
| | |
| | |
| | |
| | |
| |
As of and for the Fiscal Year Ended June 30, |
| 2020 |
| 2019 |
| 2018 |
| 2017 |
| 2016 | |||||
Operating Highlights |
| |
|
| |
|
| |
|
| |
|
| |
|
| | | | | | | | | | | | | | | |
Net sales | | $ | 545,744 | | $ | 655,407 | | $ | 684,563 | | $ | 637,341 | | $ | 566,091 |
Settlement agreement | | $ | — | | $ | — | | $ | — | | $ | (4,000) | | $ | (23,598) |
Total net sales | | $ | 545,744 | | $ | 655,407 | | $ | 684,563 | | $ | 633,341 | | $ | 542,493 |
Gross profit | | $ | 165,220 | | $ | 243,610 | | $ | 288,706 | | $ | 301,213 | | $ | 286,493 |
Operating income (loss) | | $ | 19,556 | | $ | (262,321) | | $ | 129,696 | | $ | 86,446 | | $ | 130,758 |
Net income (loss) attributable to Lannett Company, Inc. | | $ | (33,366) | | $ | (272,107) | | $ | 28,690 | | $ | (581) | | $ | 44,782 |
Basic earnings (loss) per common share attributable to Lannett Company, Inc. | | $ | (0.86) | | $ | (7.20) | | $ | 0.77 | | $ | (0.02) | | $ | 1.23 |
Diluted earnings (loss) per common share attributable to Lannett Company, Inc. | | $ | (0.86) | | $ | (7.20) | | $ | 0.75 | | $ | (0.02) | | $ | 1.20 |
| | | | | | | | | | | | | | | |
Balance Sheet Highlights | |
|
| |
|
| |
|
| |
|
| |
|
|
| | | | | | | | | | | | | | | |
Total Assets | | $ | 1,136,555 | | $ | 1,187,413 | | $ | 1,575,304 | | $ | 1,603,312 | | $ | 1,764,018 |
Total Debt, net | | $ | 681,129 | | $ | 729,048 | | $ | 839,270 | | $ | 903,647 | | $ | 1,061,848 |
Long-Term Debt, net | | $ | 592,940 | | $ | 662,203 | | $ | 772,425 | | $ | 843,530 | | $ | 883,612 |
Total Stockholders’ Equity | | $ | 302,896 | | $ | 334,041 | | $ | 598,915 | | $ | 561,122 | | $ | 554,457 |
On November 25, 2015, the Company completed the acquisition of KUPI. The Company’s Consolidated Statements of Operations for Fiscal 2016 and thereafter includes the impact of KUPI from that date.
39
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis describes significant changes in the financial condition and results of operations, as well as liquidity and capital resources of the Company. Additionally, it addresses accounting policies that management has deemed are “critical accounting policies.” This discussion and analysis is intended as a supplement to and should be read in conjunction with the Consolidated Financial Statements, the Notes to the Consolidated Financial Statements and other sections of this Form 10-K.
The following discussion contains forward-looking statements. You should refer to the “Cautionary Statement Regarding Forward-Looking Statements” set forth in Part I of this Annual Report.
We report financial information on a quarterly and fiscal year basis with the most recent being the fiscal year ended June 30, 2020. All references herein to a “fiscal year” or “Fiscal” refer to the applicable fiscal year ended June 30.
Company Overview
Lannett Company, Inc. (a Delaware corporation) and its subsidiaries (collectively, the “Company”, “Lannett”, “we” or “us”) primarily develop, manufacture, package, market and distribute solid oral and extended release (tablets and capsules), topical, liquids, nasal and oral solution finished dosage forms of drugs, generic forms of both small molecule and biologic medications, that address a wide range of therapeutic areas. Certain of these products are manufactured by others and distributed by the Company. Additionally, the Company is pursuing partnerships, research contracts and internal expansion for the development and production of other dosage forms including: ophthalmic, nasal, patch, foam, buccal, sublingual, suspensions, soft gel, injectable and oral dosages.
The Company operates pharmaceutical manufacturing plants in Carmel, New York and Seymour, Indiana. The Company’s customers include generic pharmaceutical distributors, drug wholesalers, chain drug stores, private label distributors, mail-order pharmacies, other pharmaceutical manufacturers, managed care organizations, hospital buying groups, governmental entities and health maintenance organizations.
Impact of COVID-19 Pandemic
In December 2019, the COVID-19 virus emerged in Wuhan, China and spread to other parts of the world. In March 2020, the World Health Organization (“WHO”) designated COVID-19 a global pandemic. Governments on the national, state and local level in the United States, and around the world, have implemented lockdown and shelter-in-place orders, requiring many non-essential businesses to shut down operations for the time being. The Company’s business, however, is deemed “essential” and it has continued to operate and has continued to manufacture and distribute its medicines to customers. The Company has developed a comprehensive plan that enables it to maintain operational continuity with an emphasis on manufacturing, distribution and R&D facilities during this crisis, and to date, has not encountered any significant obstacles implementing its business continuity plans. However, the Company continually assesses COVID-19 related developments and adjusts its risk mitigation planning and business continuity activities as needed.
In mid-March, 2020, the Company instituted a work from home process for all employees, other than employees in our manufacturing plants, distribution center, and R&D facilities which support manufacturing. For employees who cannot perform their job remotely, the Company has implemented enhanced cleaning and sanitizing procedures, weekly fogging and provided additional personal hygiene supplies and personal protective equipment such as rubber gloves, N95 respirators and powered air-purifying respirator that are in line with Centers for Disease Control and Preventions (“CDC”) recommendations. The Company has also implemented thermal screening for all employees entering its plants. Employees are required to adhere to the CDC guidelines, social distancing and any employee experiencing any symptoms of COVID-19 is required to stay home and seek medical attention. Any employee who tests positive for COVID-19 is required to quarantine and is not allowed to return to the facilities without a physician’s release. The Company has closed its facilities to outside persons that are not critical to continuing our operations. In cases where they are essential, visitors undergo a pre-admittance check to include a thermal screening and risk evaluation. While the
40
Company has experienced some increased absenteeism, to date the rate of employee absenteeism has not had any material effect on the Company’s business or its ability to manufacture and distribute products and plants continue to operate at normal capacity. As the pandemic continues to spread over time, there is an increased risk of employee absenteeism which could materially impact the Company’s operations. To date, the Company’s work from home process has not materially impacted the Company’s financial reporting systems or controls over financial reporting and disclosures nor do we expect that the remote work arrangement will have a material impact in the future.
Currently and as anticipated for the near future, the supply chain supporting the Company’s products remains intact, enabling the Company to receive sufficient inventory of the key materials needed across the Company’s network. The Company is experiencing some delays and allocations for certain API and other raw materials of higher demand, which delays, to date, have not had a material impact on its results of operations. However, the Company is regularly communicating with its suppliers, third-party partners, customers, healthcare providers and government officials in order to respond rapidly to any issues as they arise. The longer the current situation continues, it is more likely that the Company may experience some sort of interruption to its supply chain, and such an interruption could materially affect its business, including but not limited to, our ability to timely manufacture and distribute its products as well as unfavorably impact our results of operations. Additionally, subsequent to an initial stocking up of supplies at the start of the pandemic, the total volume of drug prescriptions written during the pandemic has decreased causing less demand for our products.
As a result of the pandemic, certain clinical trials which were underway or scheduled to begin have been temporarily placed on hold. Such delays will impact the Company’s timing for filing applications for product approvals with the FDA as well as related timing of FDA approval of such filings. Additionally, the pandemic has slowed down the Company’s efforts to expand its product portfolio through acquisitions and distribution opportunities, impacting the speed with which the Company is able to bring additional products to market. While there have been some efforts by some of our customers to increase their inventory levels for the Company’s products in the near term, the Company has not seen significant increases in demand. The Company does not anticipate any significant changes in demand for its products in the future, however, depending on the duration and severity of the outbreak, levels of demand may change. The Company currently markets an HIV product, Lopinavir-Ritonavir, which is the subject of clinical trials by various health organizations to combat the virus. If those clinical trials show the product to be helpful in combating the virus, which some trials have not, the Company may see an increase in demand. There are other sources of the product including the original brand.
In light of the economic impacts of COVID-19, the Company performed a review of the assets on our Consolidated Balance Sheet as of June 30, 2020, including intangible and other long-lived assets. Based on our review, we continue to believe that we will be able to realize the full value of our assets and that a triggering event does not exist at this time. As such, no impairments or other write-downs were recorded during the fiscal year ended June 30, 2020 specifically related to COVID-19. Our assessment was based on information currently available and is highly reliant on various assumptions which include estimates of future cash flows and the probability of achieving the estimated cash flows. Changes in market conditions or other changes in the future outlook may lead to impairments in the future.
As of June 30, 2020, the Company’s outstanding debt balance was $708.0 million, of which $88.2 million represents the current portion. In addition, the Company’s existing revolving credit facility is scheduled to mature on November 25, 2020. The impacts of COVID-19 have adversely affected the capital markets and the ability for many companies to access capital and liquidity on favorable terms or at all. The Company believes it has sufficient liquidity and cash flows to meet its operating and debt service requirements for at least the next twelve months from the issuance of the June 30, 2020 Consolidated Financial Statements. The Company also expects to be in compliance with its financial covenants during the same period. However, the Company is currently unable to predict the precise impact that COVID-19 will have on its ability to access capital in the future. If the Company is unable to access additional capital and liquidity on acceptable terms, it could adversely impact the Company’s ability to meet its future obligations beyond the next twelve months subsequent to the issuance of the June 30, 2020 Consolidated Financial Statements as well as unfavorably impact our results of operations.
Based on the foregoing, the Company cannot reasonably predict the ultimate impact of COVID-19 on our future results of operations and cash flows due to the continued uncertainty around the duration and severity of the pandemic.
41
JSP Distribution Agreement
On March 23, 2004, the Company entered into an agreement with JSP (the “JSP Distribution Agreement”) for the exclusive distribution rights in the United States to four different JSP products. On August 19, 2013, the Company entered into an agreement with JSP to extend the JSP Distribution Agreement to continue as the exclusive distributor in the United States of three JSP products: Butalbital, Aspirin, Caffeine with Codeine Phosphate Capsules USP; Digoxin Tablets USP; and Levothyroxine Sodium Tablets USP. The amendment to the JSP Distribution Agreement extended the term of the initial contract, which was due to expire on March 22, 2014, for five years through March 23, 2019.
In August 2018, JSP notified the Company that it would not extend or renew the JSP Distribution Agreement. The Company determined that JSP’s decision represented a triggering event under U.S. GAAP to perform an analysis to determine the potential for impairment of goodwill. In October 2018, the Company completed the analysis based on market data and concluded that it would record a full impairment of goodwill totaling $339.6 million in Fiscal 2019. On March 23, 2019, the JSP Distribution Agreement expired and was not renewed or extended.
Net sales of JSP products totaled $202.5 million in Fiscal 2019 and $253.1 million in Fiscal 2018. Of that amount, Levothyroxine Sodium Tablets USP net sales totaled $197.5 million and $245.9 million, with gross margins of approximately 60%, in Fiscal 2019 and 2018, respectively.
Because products covered by the JSP Distribution Agreement generated a significant portion of our revenues and gross profits, JSP’s decision not to renew or extend its distribution agreement with us have materially adversely affected our operating results and cash flows. When announced on August 20, 2018, this resulted in a significant decline in the Company’s market capitalization.
As noted above, JSP’s decision not to renew or extend its distribution agreement with us have materially adversely affected our operating results, liquidity and cash flows, which could impact our ability to comply with the financial and other covenants in our Amended Senior Secured Credit Facility. On December 10, 2018, the Company entered into an amendment to the Senior Secured Credit Facility and the Credit and Guaranty Agreement. Pursuant to the amendment, the Secured Net Leverage Ratio applicable to the financial leverage ratio covenant was increased from 3.25:1.00 to 4.25:1.00 as of December 31, 2019 and prior to September 30, 2020, and then to 4.00:1:00 as of September 30, 2020. The Amended Senior Secured Credit Facility is also subject to a minimum liquidity covenant, which provides that the Company shall not permit its liquidity as of the last day of any fiscal quarter to be less than $75.0 million. On September 27, 2019, the Company issued $86,250,000 aggregate principal amount of its 4.50% convertible senior notes due 2026 (the “Notes”) and used the net proceeds to repay a portion of the outstanding Term Loan A balance. The Notes are senior unsecured obligations of the Company and therefore are not included within the calculation of the Secured Net Leverage Ratio under the existing Amended Senior Secured Credit Facility. As of June 30, 2020, the Company was in compliance with its financial covenants. As of June 30, 2020, cash and cash equivalents totaled $144.3 million in addition to availability under our undrawn Revolver totaling $125.0 million.
Although management cannot predict with certainty the precise impact its plans will have on offsetting the loss of the JSP Distribution Agreement, management is continuing to execute on plans to offset the impact of the loss on a short- and long-term basis. These plans currently include, among other things, an emphasis on reducing cost of sales, R&D and SG&A expenses; continuing to accelerate new product launches; increasing the level of strategic partnerships; and reducing capital expenditures. The Company has also signed several distribution and in-licensing agreements that will provide both immediate and longer-term contribution margins. Additionally, the Company continues to supplement existing in-process cost reduction plans with additional cost savings initiatives. Management also plans to attempt, at the appropriate time, to refinance a significant portion of its outstanding long-term debt to reduce principal repayment requirements and eliminate existing financial covenants, which we expect will increase related interest expense, but will positively impact short-term cash flows.
42
Cody API Restructuring Plan
On June 11, 2019, the Company approved a restructuring plan (the “Cody API Restructuring Plan”) with respect to Cody Labs. In September 2018, the Company approved a plan to sell the active pharmaceutical ingredient manufacturing distribution business of Cody Labs (the “Cody API business”) but the Company was unable to sell the Cody API business as an ongoing operation. Therefore, the Company decided to sell the equipment and real estate utilized by the Cody API business and to have Cody Labs cease all operations. In connection with the Cody API Restructuring Plan, the Company eliminated approximately 70 positions at Cody Labs. The restructuring activities under the Cody API Restructuring Plan are substantially complete as of June 30, 2020. During Fiscal 2020, the Company completed the sale of the equipment associated with the Cody API business for approximately $3.0 million and also signed a two-year agreement to lease a portion of the Cody Labs real estate to a third party.
The costs to implement the Cody API Restructuring Plan totaled approximately $6.2 million, including approximately $3.7 million of severance and employee-related costs and approximately $2.0 million of contract termination costs, as well as approximately $0.5 million of costs to be incurred in connection with moving equipment and other property to other Company-owned facilities that were originally anticipated to be incurred in connection with the Cody Restructuring Plan announced in June 2018.
2020 Restructuring Plan
On July 10, 2020, the Board of Directors authorized a restructuring and cost savings plan (the “2020 Restructuring Plan”). The purpose of the 2020 Restructuring Plan is to enhance manufacturing efficiencies, streamline operations and reduce the Company’s cost structure, and is being implemented, in part, as a result of previously anticipated near-term competition and pricing pressure with respect to certain key products. The 2020 Restructuring Plan will include the consolidation of the Company’s research and development (R&D) function into a single location in Philadelphia, PA, lowering operating costs and reducing the workforce by approximately 80 positions, equal to approximately 8.5% of the Company’s total number of employees. The 2020 Restructuring Plan was initiated on July 13, 2020.
The Company estimates that it will incur approximately $4.0 million in severance-related costs, primarily in the first quarter of Fiscal 2021, in connection with the 2020 Restructuring Plan. The Company expects the 2020 Restructuring Plan to result in annual cost savings in excess of $15.0 million.
The amounts are preliminary estimates based on the information currently available to management. It is possible that additional charges and future cash payments could occur in relation to the restructuring actions.
Financial Summary
For Fiscal 2020, net sales decreased to $545.7 million compared to $655.4 million in the same prior-year period. Gross profit decreased $78.4 million to $165.2 million compared to the prior-year period and gross profit percentage decreased to 30% compared to 37% in Fiscal 2019. R&D expenses decreased 23% to $30.0 million compared to the prior-year period while SG&A expenses decreased 9% to $79.5 million. Restructuring expenses decreased to $1.7 million compared to $4.1 million in the prior-year period. Operating income for Fiscal 2020, which included asset impairment charges totaling $34.4 million, was $19.6 million compared to operating loss of $262.3 million in the prior-year period, which included asset impairment charges totaling $375.4 million. Net loss for Fiscal 2020 was $33.4 million, or $(0.86) per diluted share. Comparatively, net loss in the prior-year period was $272.1 million, or $(7.20) per diluted share.
A more detailed discussion of the Company’s financial results can be found below.
43
Results of Operations — Fiscal 2020 compared to Fiscal 2019
Net sales decreased 17% to $545.7 million for the fiscal year ended June 30, 2020. The following table identifies the Company’s net product sales by medical indication for the fiscal years ended June 30, 2020 and 2019. The medical indication categories for the fiscal year ended June 30, 2019 were reclassified to better align with industry standards and the Company’s peers.
(In thousands) | | Fiscal Year Ended June 30, | ||||
Medical Indication |
| 2020 |
| 2019 | ||
Analgesic | | $ | 8,680 | | $ | 8,251 |
Anti-Psychosis | |
| 104,934 | |
| 73,453 |
Cardiovascular | |
| 88,576 | |
| 101,467 |
Central Nervous System | |
| 77,256 | |
| 59,019 |
Endocrinology | | | — | | | 197,522 |
Gastrointestinal | |
| 73,477 | |
| 63,043 |
Infectious Disease | | | 73,237 | | | 16,950 |
Migraine | |
| 44,266 | |
| 41,592 |
Respiratory/Allergy/Cough/Cold | |
| 11,576 | |
| 12,479 |
Urinary | |
| 4,225 | |
| 6,755 |
Other | |
| 35,013 | |
| 51,517 |
Contract manufacturing revenue | |
| 24,504 | |
| 23,359 |
Total net sales | | $ | 545,744 | | $ | 655,407 |
The decrease in net sales was driven by decreased volumes of $79.4 million and, to a lesser extent, decreased average selling price of products of $30.3 million. Overall volumes decreased primarily due to the loss of Levothyroxine sales associated with the expiration of the JSP Distribution Agreement, partially offset by additional volumes from product launches and increased market share in certain key products. Average selling prices were impacted by product mix and price decreases in certain key products due to competitive pricing pressures. Although the Company has benefited in the past from favorable pricing trends, these trends have reversed. Net sales within the infectious disease category increased significantly as a result of the distribution and supply agreement with Sinotherapeutics Inc., which was signed in August 2019, to distribute Posaconazole tablets.
In January 2017, a provision in the Bipartisan Budget Act of 2015 required drug manufacturers to pay additional rebates to state Medicaid programs if the prices of their generic drugs rise at a rate faster than inflation. The provision negatively impacted the Company’s net sales by $35.7 million and $30.8 million in Fiscal 2020 and Fiscal 2019, respectively, which contributed to the overall decreased average selling price.
The following chart details price and volume changes by medical indication between Fiscal 2020 and Fiscal 2019:
| | Sales volume |
| Sales price |
|
Medical indication |
| change % |
| change % | |
Analgesic | | 25 | % | (20) | % |
Anti-Psychosis |
| 33 | % | 10 | % |
Cardiovascular |
| (12) | % | (1) | % |
Central Nervous System |
| 47 | % | (16) | % |
Endocrinology | | (100) | % | — | % |
Gastrointestinal |
| 16 | % | 1 | % |
Infectious Disease | | 346 | % | (14) | % |
Migraine |
| 21 | % | (14) | % |
Respiratory/Allergy/Cough/Cold |
| (5) | % | (2) | % |
Urinary |
| (34) | % | (3) | % |
44
The Company sells its products to customers through various distribution channels. The table below presents the Company’s net sales to each distribution channel for the fiscal year ended June 30:
(In thousands) | | June 30, | | June 30, | ||
Customer Distribution Channel |
| 2020 |
| 2019 | ||
Wholesaler/Distributor | | $ | 429,824 | | $ | 529,717 |
Retail Chain | |
| 79,606 | |
| 80,944 |
Mail-Order Pharmacy | |
| 11,810 | |
| 21,387 |
Contract manufacturing revenue | |
| 24,504 | |
| 23,359 |
Total net sales | | $ | 545,744 | | $ | 655,407 |
Overall net sales decreased primarily due to the loss of the Levothyroxine sales associated with the expiration of the JSP Distribution Agreement, partially offset by additional volumes from product launches and increased market share in certain key products. The decrease in sales to wholesalers, as well as mail-order pharmacies, was also primarily due to the loss of Levothyroxine sales.
Cocaine Hydrochloride Solution
In December 2017, a competitor received approval from the FDA to market and sell a Cocaine Hydrochloride topical product. The approval affected the Company’s right to market and sell its unapproved cocaine hydrochloride solution product. According to FDA guidance, the FDA typically allows the marketing of unapproved products for up to one year following the approval of an NDA for the product. Upon the request of the FDA to cease manufacturing and distributing our unapproved cocaine hydrochloride solution product as a result of an approved product on the market, the Company committed to not manufacture or distribute cocaine hydrochloride 10% solution, which has not been sold during Fiscal 2019, as of April 15, 2019. The Company also ceased manufacturing its unapproved cocaine hydrochloride 4% solution on June 15, 2019 and ceased distributing the product on August 15, 2019.
The competitor filed a Citizen Petition with the FDA in February 2019, claiming that the grant of the NCE exclusivity blocks the approval of the Company’s application for five years and requesting that the FDA refuse to accept any further submissions in furtherance of the Company’s Section 505(b)(2) NDA application, treat as withdrawn any submissions made by the Company after December 2017 and withdraw the Company’s Section 505(b)(2) application. On April 24, 2019, the Company filed an opposition to the Citizen Petition requesting that it be denied. On July 3, 2019, the FDA denied the competitor’s Citizen Petition. Thereafter, the competitor filed a second Citizen Petition claiming that the FDA should rescind the acceptance of the Company’s Section 505(b)(2) application and only permit the Company to re-submit the application as an ANDA after the expiration of the competitor’s five-year exclusivity. The Company filed an opposition to the second Citizen Petition asserting, among other things, that the FDA should summarily deny the second Citizen Petition as an improper attempt to delay competition. On January 10, 2020, the FDA denied the second Citizen Petition and the FDA approved the Company’s Section 505(b)(2) NDA application. On January 27, 2020, the competitor filed a complaint against the FDA seeking an order invalidating the approval of the Company’s 505(b)(2) NDA, claiming the approval violates the competitor’s five-year exclusivity. On February 14, 2020, the Company filed a motion to intervene in the competitor’s lawsuit in order to argue that the request for relief be denied. On April 15, 2020, the competitor filed a motion for summary judgment. The Company and FDA filed responses in opposition and cross motions for summary judgement requesting dismissal of the complaint. The parties submitted further reply briefs and are awaiting a decision by the Court.
On June 6, 2020, the competitor filed a patent infringement complaint in the United States District Court for the District of Delaware, asserting that the Company’s approved cocaine hydrochloride product infringes three patents issued to the competitor. On June 19, 2020, the Company filed an answer and counterclaim, alleging that the Company either does not infringe or the three asserted patents are invalid. In addition, the Company sought a declaration that, as to the competitor’s three additional patents not asserted against the Company, they are either not infringed or invalid.
45
Thalomid®
The Company filed with the FDA an ANDA No. 206601, along with a paragraph IV certification, alleging that the fifteen patents associated with the Thalomid drug product are invalid, unenforceable and/or not infringed. On January 30, 2015, Celgene Corporation and Children’s Medical Center Corporation filed a patent infringement lawsuit in the United States District Court for the District of New Jersey, alleging that the Company’s filing of ANDA No. 206601 constitutes an act of patent infringement and seeking a declaration that the patents at issue are valid and infringed. A settlement agreement was reached, and the Court dismissed the lawsuit in October 2017. Pursuant to the settlement agreement, the Company entered into a license agreement that permitted Lannett to manufacture and market in the U.S. its generic thalidomide product as of August 1, 2019 or earlier under certain circumstances. In the second quarter of Fiscal 2019, the Company received a Major Complete Response Letter (“CRL”) related to issues at its API supplier. The Company filed a response to the CRL. The Company received a second Major CRL in the first quarter of Fiscal 2020 related to continued issues at the API supplier, as well as issues with the Risk Evaluation and Mitigation Strategy (“REMS”) program hosted by Celgene. The Company is working on addressing the FDA comments and expects its product launch could be delayed until Fiscal Year 2022.
Ranitidine Oral Solution, USP
As part of an industry-wide action, the Company issued a voluntary recall on all lots within expiry of Ranitidine Syrup (Ranitidine Oral Solution, USP), 15mg/mL to the consumer level due to levels of N-Nitrosodimethylamine (“NDMA”), a probable human carcinogen, above the levels recently established by the FDA. On September 17, 2019, the FDA notified the Company about the possible presence of NDMA in its Ranitidine Oral Solution product and the Company immediately commenced testing and analysis of the active pharmaceutical ingredient (“API”) and drug product and confirmed the presence of NDMA. The Company is in the process of switching its API supplier for its Ranitidine Oral Solution, USP product. The Company’s net sales of Ranitidine Oral Solution in the fourth quarter of fiscal year 2019 totaled $1.9 million. On April 1, 2020, the FDA ordered all Ranitidine products (including the Company’s product) withdrawn from the US market and provided guidance on the requirements for submitting additional information to the FDA in order to re-introduce the product to the market. Since initiating the voluntary recall, the Company has not been marketing its Ranitidine Oral Solution product and has no future plans to attempt to re-introduce the product at this time. The Company does not believe the recall will have a significant impact on our future expected financial position, results of operations and cash flows.
On June 1, 2020, a class action Complaint was served upon the Company and approximately forty five (45) other companies asserting claims for personal injury arising from the presence of NDMA in Ranitidine products. A similar complaint was served upon the Company by the State of Mexico in July 2020. The Company has learned that several other similar class action complaints naming the Company and others were filed but, to date, none of those complaints have been served upon the Company. The Company has placed its insurance carrier on notice of the claim and the carrier has appointed counsel to defend the Company. The Company has not yet responded to the Complaint.
Cost of Sales, including amortization of intangibles. Cost of sales, including amortization of intangibles, for Fiscal 2020 decreased 8% to $380.5 million from $411.8 million in the same prior-year period. The decrease was primarily attributable to the loss of Levothyroxine sales associated with the expiration of the JSP Distribution Agreement as well as lower cost of sales as a result of the Company’s decision to cease operations at Cody Labs, partially offset by additional volumes of other products sold as well as increased product royalties expense related to various distribution agreements. Product royalties expense included in cost of sales totaled $77.7 million for Fiscal 2020 and $43.6 million for Fiscal 2019. Amortization expense included in cost of sales totaled $32.0 million for Fiscal 2020 and $32.2 million for Fiscal 2019.
Gross Profit. Gross profit for Fiscal 2020 decreased 32% to $165.2 million or 30% of total net sales. In comparison, gross profit for Fiscal 2019 was $243.6 million or 37% of total net sales. The decrease in gross profit percentage was primarily attributable to the loss of Levothyroxine sales associated with the expiration of the JSP Distribution Agreement, which had higher than average gross profit margins, price decreases across our product portfolio as well as increased product royalties related to various distribution agreements, partially offset by manufacturing efficiencies as a
46
result of cost reduction initiatives and an increase in volumes of certain key products with higher than average gross margins.
Research and Development Expenses. Research and development expenses decreased 23% to $30.0 million in Fiscal 2020 from $38.8 million in Fiscal 2019. The decrease was primarily due to lower R&D expenses as a result of the Company’s decision to cease operations at Cody Labs as well as the timing of certain milestones related to product development projects.
Selling, General and Administrative Expenses. Selling, general and administrative expenses decreased 9% to $79.5 million in Fiscal 2020 compared with $87.6 million in Fiscal 2019. The decrease was primarily driven by lower financial advisory costs, a decrease in regulatory-related costs, lower expenses at the Company’s Cody Labs subsidiary and other cost reduction initiatives, partially offset by a branded prescription drug fee as well as increased legal costs.
The Company is focused on controlling operating expenses and has executed on its Cody Restructuring Plan and Cody API Restructuring Plan and, more recently, implemented the 2020 Restructuring Plan, as noted above; however, increases in personnel and other costs to facilitate enhancements in the Company’s infrastructure and expansion may impact operating expenses in future periods.
Asset impairment charges. In Fiscal 2020, the Company recorded various asset impairment charges totaling $34.4 million. The Company recorded a ROU lease asset totaling $1.2 million related to an existing lease at Cody Labs upon adoption of ASU No. 2016-02. The Company subsequently recorded a full impairment of the asset as a result of the decision to cease operations at Cody Labs.
In the third quarter of Fiscal 2020, the Company performed an impairment analysis of its AB-rated Methylphenidate Hydrochloride product, which is distributed under a license agreement with Andor, due to significant declines in the projected profitability of the distribution arrangement. As a result of the analysis, the Company recorded a $14.0 million impairment charge.
In the fourth quarter of Fiscal 2020, the Company performed an annual impairment analysis of our indefinite-lived intangible assets. As a result, the Company recorded a $9.0 million and an $8.0 million impairment charge to its KUPI IPR&D and Silarx IPR&D assets, respectively, due to the abandonment of several pipeline products within both portfolios. See Note 8 “Goodwill and Intangible Assets” for more information.
Other Income (Loss). Interest expense for the year ended June 30, 2020 totaled $66.8 million compared to $84.6 million for the year ended June 30, 2019. The decrease was due to a lower weighted-average debt balance in Fiscal 2020 as compared to the prior-year period as well as a lower weighted-average interest rate due to the partial repayment of the outstanding Term Loan A balance with proceeds from the issuance of the 4.50% Convertible Senior Notes. The weighted average interest rate for Fiscal 2020 and 2019 was 8.8% and 9.7%, respectively. Investment income totaled $1.6 million in Fiscal 2020 compared with $3.2 million in Fiscal 2019.
Income Tax. The Company recorded income tax benefit in Fiscal 2020 of $15.3 million compared to income tax benefit of $74.1 million in Fiscal 2019. The effective tax rate for Fiscal 2020 was 31.4%, compared to 21.4% for Fiscal 2019. The effective tax rate for the period ended June 30, 2020 was higher compared to the same prior-year period primarily due to the impact of the CARES Act which allowed the Company to carryback its current year taxable loss into its Fiscal 2015 tax year, where the statutory tax rate was 35%. The increase was slightly offset by excess tax shortfalls related to stock compensation as well as a non-deductible branded prescription drug fee.
Net Income (Loss). For the year ended June 30, 2020, the Company reported net loss of $33.4 million, or $(0.86) per diluted share. Comparatively, net loss in the corresponding prior-year period was $272.1 million, or $(7.20) per diluted share.
47
Results of Operations — Fiscal 2019 compared to Fiscal 2018
Total net sales decreased to $655.4 million from $684.6 million in the prior-year period.
Net sales decreased 4% to $655.4 million for the fiscal year ended June 30, 2019. The following table identifies the Company’s net product sales by medical indication for the fiscal years ended June 30, 2019 and 2018. The medical indication categories for the fiscal years ended June 30, 2019 and 2018 were reclassified to better align with industry standards and the Company’s peers.
(In thousands) | | Fiscal Year Ended June 30, | ||||
Medical Indication |
| 2019 |
| 2018 | ||
Analgesic | | $ | 8,251 | | $ | 3,809 |
Anti-Psychosis | |
| 73,453 | |
| 59,557 |
Cardiovascular | |
| 101,467 | |
| 64,011 |
Central Nervous System | |
| 59,019 | |
| 59,672 |
Endocrinology | |
| 197,522 | |
| 245,929 |
Gastrointestinal | |
| 63,043 | |
| 67,762 |
Infectious Disease | |
| 16,950 | |
| 17,685 |
Migraine | |
| 41,592 | |
| 54,015 |
Respiratory/Allergy/Cough/Cold | |
| 12,479 | |
| 25,284 |
Urinary | |
| 6,755 | |
| 8,068 |
Other | |
| 51,517 | |
| 58,936 |
Contract manufacturing revenue | |
| 23,359 | |
| 19,835 |
Total net sales | | $ | 655,407 | | $ | 684,563 |
The decrease in net sales was driven by decreased average selling price of products of $80.2 million, partially offset by increased volumes of $51.0 million. Average selling prices were negatively impacted by product mix, changes within distribution channels and competitive pricing pressures. Volumes were favorably impacted due to product launches during Fiscal 2019 and increased market share in several key products, partially offset by lower volumes of Levothyroxine due to the expiration of the JSP Distribution Agreement in March 2019.
In January 2017, a provision in the Bipartisan Budget Act of 2015 required drug manufacturers to pay additional rebates to state Medicaid programs if the prices of their generic drugs rise at a rate faster than inflation. The provision negatively impacted the Company’s net sales by $30.8 million in Fiscal 2019 and $31.0 million in Fiscal 2018 which contributed to the overall decreased average selling price.
The following chart details price and volume changes by medical indication between Fiscal 2019 and Fiscal 2018:
| | Sales volume | | Sales price | |
Medical indication |
| change % |
| change % | |
Analgesic |
| 269 | % | (152) | % |
Anti-Psychosis |
| 48 | % | (25) | % |
Cardiovascular |
| 50 | % | 9 | % |
Central Nervous System |
| 19 | % | (20) | % |
Endocrinology | | (10) | % | (10) | % |
Gastrointestinal |
| 3 | % | (10) | % |
Infectious Disease | | (1) | % | (3) | % |
Migraine | | (13) | % | (10) | % |
Respiratory/Allergy/Cough/Cold | | (42) | % | (9) | % |
Urinary |
| (26) | % | 10 | % |
48
The Company sells its products to customers through various distribution channels. The table below presents the Company’s net sales to each distribution channel for the fiscal year ended June 30:
(In thousands) | | June 30, | | June 30, | ||
Customer Distribution Channel |
| 2019 |
| 2018 | ||
Wholesaler/Distributor | | $ | 529,717 | | $ | 504,030 |
Retail Chain | |
| 80,944 | |
| 117,331 |
Mail-Order Pharmacy | |
| 21,387 | |
| 43,367 |
Contract manufacturing revenue | |
| 23,359 | |
| 19,835 |
Total net sales | | $ | 655,407 | | $ | 684,563 |
Net sales to wholesalers/distributors increased primarily due to the transition services agreement with Amneal, which shifted all sales of Levothyroxine in the third quarter of Fiscal 2019 from other distribution channels into a single distributor. Net sales to retail chains decreased significantly as a result of additional sales in the year ended June 30, 2018 to a customer that was unable to obtain supply from a competitor due to a temporary disruption in the competitor’s supply chain.
Cost of Sales, including amortization of intangibles. Cost of sales, including amortization of intangibles, for Fiscal 2019 increased 4% to $411.8 million from $395.9 million in the same prior-year period. The increase was primarily attributable to increased product royalties, and to a lesser extent, higher volumes of products sold, offset by manufacturing efficiencies as a result of cost reduction initiatives as well as product mix. Product royalties expense included in cost of sales totaled $43.6 million for Fiscal 2019 and $29.7 million for Fiscal 2018. Amortization expense included in cost of sales totaled $32.2 million for Fiscal 2019 and $32.1 million for Fiscal Year 2018.
Gross Profit. Gross profit for Fiscal 2019 decreased 16% to $243.6 million or 37% of total net sales. In comparison, gross profit for Fiscal 2018 was $288.7 million or 42% of total net sales. The decrease in gross profit percentage was primarily a result of the expiration of the JSP Distribution Agreement as well as lower average selling prices of certain key products and increased product royalties related to a distribution agreements.
Research and Development Expenses. Research and development expenses increased 33% to $38.8 million in Fiscal 2019 from $29.2 million in Fiscal 2018. The increase was primarily due to lower R&D expense in Fiscal 2018 related to a cancelled order for pre-launch inventory purchased in Fiscal 2017, and to a lesser extent, higher incentive-based compensation in Fiscal 2019.
Selling, General and Administrative Expenses. Selling, general and administrative expenses increased 7% to $87.6 million in Fiscal 2019 compared with $82.2 million in Fiscal 2018. The increase was primarily to higher incentive-based compensation, depreciation related to software integration costs, and increased legal and financial advisory costs, partially offset by a reduction of selling and marketing expenses related to headcount reductions in product salesforce.
The Company is focused on controlling operating expenses and has implemented its 2016 Restructuring Plan, Cody Restructuring Plan and Cody API Restructuring Plan as noted above; however increases in personnel and other costs to facilitate enhancements in the Company’s infrastructure and expansion may continue to impact operating expenses in future periods.
Restructuring Expenses. Restructuring expenses decreased $3.0 million to $4.1 million for Fiscal Year 2019 compared with $7.1 million in fiscal 2018 primarily due to lower facility closure costs associated with the completion of the 2016 Restructuring Program.
Asset impairment charges. In the first quarter of Fiscal 2019, the Company approved a plan to sell the Cody API business. As such, all assets and liabilities associated with the Cody API business are recorded in the assets and liabilities held for sale captions in the Consolidated Balance Sheet as of June 30, 2019. As part of the held for sale classification, the Company recorded the assets of the Cody API business at fair value less costs to sell. The Company performed a fair value analysis which resulted in a $29.9 million impairment of the Cody long-lived assets in Fiscal
49
2019. In the fourth quarter of Fiscal 2019, the Company recorded an additional $2.9 million impairment of the Cody API assets.
On August 17, 2018, JSP notified the Company that it would not extend or renew the JSP Distribution Agreement when the current term expires on March 23, 2019. The Company determined the JSP’s decision represented a triggering event under U.S. GAAP to perform an analysis to determine the potential for impairment of goodwill. On October 4, 2018, the Company completed the analysis based on market data and concluded that it would record a full impairment of goodwill totaling $339.6 million during Fiscal 2019.
Other Income (Loss). Interest expense for the period ended June 30, 2019 totaled $84.6 million compared to $85.6 million for the period ended June 30, 2018. The weighted average interest rate for Fiscal 2019 and 2018 was 9.7% and 8.7%, respectively. Investment income totaled $3.2 million in Fiscal 2019 compared with $4.8 million in Fiscal 2018.
Income Tax. The Company recorded income tax benefit in Fiscal 2019 of $74.1 million compared to income tax expense of $22.4 million in Fiscal 2018. The effective tax rate for Fiscal 2019 was 21.4%, compared to 43.8% for Fiscal 2018. The effective tax rate for the period ended June 30, 2019 was lower compared to the same prior-year period primarily due to the application of 2017 Tax Reform in Fiscal 2018, which resulted in a revaluation of the Company’s net long term deferred tax assets. In addition, the federal statutory rate for the Fiscal 2019 was 21% compared to a blended federal statutory tax rate of 28% in the prior-year period.
Net Income (Loss). For the period ended June 30, 2019, the Company reported net loss of $272.1 million, or $(7.20) per diluted share. Comparatively, net income in the corresponding prior-year period was $28.7 million, or $0.75 per diluted share.
Liquidity and Capital Resources
Cash Flow
The Company had historically financed its operations with cash flow generated from operations supplemented with borrowings from various government agencies and financial institutions. At June 30, 2020, working capital was $228.3 million as compared to $295.6 million at June 30, 2019, a decrease of $67.3 million. Current product portfolio sales as well as sales related to future product approvals are anticipated to continue to generate positive cash flow from operations.
Net cash from operating activities of $116.0 million for the fiscal year ended June 30, 2020 reflected net loss of $33.4 million, adjustments for non-cash items of $110.7 million, as well as cash provided by changes in operating assets and liabilities of $38.7 million. In comparison, net cash from operating activities of $176.3 million for the fiscal year ended June 30, 2019 reflected net loss of $272.1 million, adjustments for non-cash items of $375.0 million, as well as cash provided by changes in operating assets and liabilities of $73.4 million.
Significant changes in operating assets and liabilities from June 30, 2019 to June 30, 2020 are comprised of:
| ● | A decrease in accounts receivable of $39.1 million mainly due to the timing of sales and cash receipts, as well as adjustments to wholesale acquisition pricing to our customers. The Company’s days sales outstanding (“DSO”) at June 30, 2020, based on gross sales for the fiscal year ended June 30, 2020 and gross accounts receivable at June 30, 2020, was 61 days. The level of DSO at June 30, 2020 was significantly lower than the Company’s expectation that DSO will be in the 70 to 85-day range based on customer payment terms, due to |
50
| higher gross sales in the three months ended March 31, 2020 compared to the three months ended June 30, 2020. |
| ● | An increase in accounts payable totaling $19.0 million primarily due to the timing of vendor invoices and payments. |
Significant changes in operating assets and liabilities from June 30, 2018 to June 30, 2019 are comprised of:
| ● | A decrease in accounts receivable of $84.9 million primarily as a result of the collection of receivables related to Levothyroxine sales as well as of the timing of receipts. The Company’s days sales outstanding (“DSO”) at June 30, 2019, based on gross sales for the fiscal year ended June 30, 2019 and gross accounts receivable at June 30, 2019 was 78 days. The level of DSO at June 30, 2019 was comparable to the Company’s expectations that DSO will be in the 70 to 85-day range based on customer payment terms. |
| ● | A decrease in accounts payable totaling $43.3 million primarily due to a lower balance as of June 30, 2019 related to the expiration of the JSP Distribution Agreement. The timing of payments also contributed to the decrease in accounts payable. |
| ● | An increase in royalties payable of $10.3 million primarily due to an increase in business development deals and product revenues royalties. |
| ● | A decrease in prepaid income taxes totaling $18.3 million primarily due to receipt of approximately $15.2 million in tax refunds from the Internal Revenue Service (“IRS”). |
| ● | An increase in accrued payroll and payroll-related costs of $12.1 million primarily due to higher accrued incentive compensation-related costs and, to a lesser extent, the timing of payroll payments. |
Net cash used in investing activities of $40.0 million for the fiscal year ended June 30, 2020 was mainly the result of purchases of intangible assets of $28.8 million and purchases of property, plant and equipment of $18.3 million, partially offset by proceeds from the sale of property, plant and equipment of $7.4 million. Net cash used in investing activities of $7.3 million for the fiscal year ended June 30, 2019 was primarily due to purchases of property, plant and equipment of $24.3 million and purchases of intangible assets of $3.0 million, partially offset by proceeds from the sale of property, plant and equipment of $14.4 million and proceeds from the sale of an outstanding variable interest entity (“VIE”) loan to a third party of $5.6 million.
Net cash used in financing activities of $71.9 million for the fiscal year ended June 30, 2020 was due to debt repayments of $146.7 million, purchase of capped calls in connection with the 4.50% Convertible Senior Notes offering totaling $7.1 million, payments of debt issuance costs totaling $3.5 million, and purchases of treasury stock totaling $1.9 million, partially offset by proceeds from issuance of 4.50% Convertible Senior Notes of $86.3 million and proceeds from sale of stock pursuant to stock compensation plans of $1.0 million. Net cash used in financing activities of $127.3 million for the fiscal year ended June 30, 2019 was primarily due to debt repayments of $126.7 million, of which $59.9 million were open market repurchases of debt, payments of debt issuance costs totaling $1.1 million, and purchases of treasury stock totaling $0.6 million, partially offset by proceeds from issuance of stock pursuant to stock compensation plans of $1.1 million.
51
Credit Facility and Other Indebtedness
The Company has previously entered into and may enter future agreements with various government agencies and financial institutions to provide additional cash to help finance the Company’s acquisitions, various capital investments and potential strategic opportunities. These borrowing arrangements as of June 30, 2020 are as follows:
Amended Senior Secured Credit Facility
On November 25, 2015, in connection with its acquisition of KUPI, Lannett entered into a credit and guaranty agreement (the “Credit and Guaranty Agreement”) among certain of its wholly-owned domestic subsidiaries, as guarantors, Morgan Stanley Senior Funding, Inc., as administrative agent and collateral agent and other lenders providing for a senior secured credit facility (the “Senior Secured Credit Facility”). The Senior Secured Credit Facility consisted of Term Loan A in an aggregate principal amount of $275.0 million, Term Loan B in an aggregate principal amount of $635.0 million and a revolving credit facility providing for revolving loans in an aggregate principal amount of up to $125.0 million. As of June 30, 2020, there was no balance outstanding under the revolving credit facility.
On June 17, 2016, Lannett amended the Senior Secured Credit Facility and the Credit and Guaranty Agreement to raise an incremental term loan in the principal amount of $150.0 million (the “Incremental Term Loan”) and amended certain sections of the agreement (the “Amended Senior Secured Credit Facility”). The terms of this Incremental Term Loan are substantially the same as those applicable to the Term Loan B. The Company used the proceeds of the Incremental Term Loan and cash on hand to repurchase the outstanding $250.0 million aggregate principal amount of Lannett’s 12.0% Senior Notes due 2023 (the “Senior Notes”) issued in connection with the KUPI acquisition.
The Term Loan A Facility will mature on November 25, 2020. The Term Loan A Facility amortizes in quarterly installments from January 1, 2018 through September 30, 2020 in amounts equal to 2.50% of the original principal amount of the Term Loan A Facility, with the balance payable on November 25, 2020. The Term Loan B Facility will mature on November 25, 2022. The Term Loan B Facility amortizes in equal quarterly installments in amounts equal to 1.25% of the original principal amount of the Term Loan B Facility with the balance payable on November 25, 2022. Any outstanding Revolving Loans will mature on November 25, 2020.
The Amended Senior Secured Credit Facility is guaranteed by all of Lannett’s significant wholly-owned domestic subsidiaries (the “Subsidiary Guarantors”) and is collateralized by substantially all present and future assets of Lannett and the Subsidiary Guarantors.
The interest rates applicable to the Amended Term Loan Facility are based on a fluctuating rate of interest of the greater of an adjusted LIBOR and 1.00%, plus a borrowing margin of originally 4.75% (for Term Loan A Facility, 5.00% as amended on December 10, 2018) or 5.375% (for Term Loan B Facility). The interest rates applicable to the Revolving Credit Facility is based on a fluctuating rate of interest of an adjusted LIBOR plus a borrowing margin of 4.75%. The interest rate applicable to the unused commitment for the Revolving Credit Facility was initially 0.50%. Since March 2016, the interest margins and unused commitment fee on the Revolving Credit Facility have been subject to a leveraged based pricing grid.
The Amended Senior Secured Credit Facility contains a number of covenants that, among other things, limit the ability of Lannett and its restricted subsidiaries to: incur more indebtedness; pay dividends; redeem stock or make other distributions of equity; make investments; create restrictions on the ability of Lannett’s restricted subsidiaries that are not Subsidiary Guarantors to pay dividends to Lannett or make intercompany transfers; create negative pledges; create liens; transfer or sell assets; merge or consolidate; enter into sale leasebacks; enter into certain transactions with Lannett’s affiliates; and prepay or amend the terms of certain indebtedness.
The Amended Senior Secured Credit Facility contains a financial performance covenant that is triggered when the aggregate principal amount of outstanding Revolving Credit Facility and outstanding letters of credit as of the last day of the most recent fiscal quarter is greater than 30% of the aggregate commitments under the Revolving Credit Facility. The covenant provides that Lannett shall not permit its first lien net senior secured leverage ratio as of the last day of any four consecutive fiscal quarters (i) from and after December 31, 2017 to be greater than 3.75:1.00 and
52
(ii) from and after December 31, 2019 to be greater than 3.25:1.00. The Amended Senior Secured Credit Facility also contains a financial performance covenant for the benefit of the Term Loan A Facility lenders which provides that Lannett shall not permit its Net Senior Secured Leverage Ratio as of the last day of any four consecutive fiscal quarters (i) as of December 31, 2017 and prior to December 31, 2019 to be greater than 3.75:1.00 and (ii) as of December 31, 2019 and thereafter to be greater than 3.25:1.00. The Amended Senior Secured Credit Facility also contains certain affirmative covenants, including financial and other reporting requirements.
On December 10, 2018, the Company entered into an amendment to the Senior Secured Credit Facility and the Credit and Guaranty Agreement. Pursuant to the amendment, the Secured Net Leverage Ratio applicable to the financial leverage ratio covenant was increased from 3:25:1.00 to 4.25:1.00 as of December 31, 2019 and prior to September 30, 2020, and then to 4:00:1:00 as of September 30, 2020. In exchange, the Company agreed to include a minimum liquidity covenant of $75 million, a 25-basis point increase to the interest rate margin paid on the Term A Loans and pay a consent fee equal to 50 basis points, paid only to consenting lenders.
In order to reduce future cash interest payments, as well as future amounts due at maturity, Lannett may, from time to time, purchase our debt for cash in open market purchases and/or privately negotiated transactions. Lannett will evaluate any such transactions in light of then-existing market conditions, taking into account the Company’s current liquidity among other factors. The amounts involved in any such transactions, individually or in the aggregate, may be material.
4.50% Convertible Senior Notes due 2026
On September 27, 2019, the Company issued $86.3 million aggregate principal amount of the Notes in a private offering to qualified institutional buyers pursuant to Rule 144A under the Securities Act of 1933, as amended. The Notes are senior unsecured obligations of the Company and bear interest at an annual rate of 4.50% payable semi-annually in arrears on April 1 and October 1 of each year, beginning on April 1, 2020. The Notes will mature on October 1, 2026, unless earlier repurchased, redeemed or converted in accordance with their terms. The Notes are convertible into shares of the Company’s common stock at an initial conversion rate of 65.4022 shares per $1,000 principal amount of Notes (which is equivalent to an initial conversion price of approximately $15.29 per share), subject to adjustments upon the occurrence of certain events (but will not be adjusted for any accrued and unpaid interest). The Company may redeem all or a part of the Notes on or after October 6, 2023 at a redemption price equal to 100% of the principal amount of the Notes redeemed, plus accrued and unpaid interest, if any, up to, but excluding, the redemption date, subject to certain conditions relating to the Company’s stock price having been met. Following certain corporate events that occur prior to the maturity date or if the Company delivers a notice of redemption, the Company will, in certain circumstances, increase the conversion rate for a holder who elects to convert its Notes in connection with such corporate event or notice of redemption. The indenture covering the Notes contains certain other customary terms and covenants, including that upon certain events of default occurring and continuing, either the trustee or holders of at least 25% in principal amount of the outstanding Notes may declare 100% of the principal of, and accrued and unpaid interest on, all the Notes to be due and payable.
In connection with the offering of the Notes, the Company also entered into privately negotiated “capped call” transactions with several counterparties. The capped call transaction will initially cover, subject to customary anti-dilution adjustments, the number of shares of common stock that initially underlie the Notes. The capped call transactions are expected to generally reduce the potential dilutive effect on the Company’s common stock upon any conversion of the Notes with such reduction subject to a cap which is initially $19.46 per share.
53
Other Liquidity Matters
Refer to the “JSP Distribution Agreement” and “Impact of COVID-19 Pandemic” sections above for the impact of each on our future liquidity.
Future Acquisitions
We are continuously evaluating the potential for product and company acquisitions as a part of our future growth strategy. In conjunction with a potential acquisition, the Company may utilize current resources or seek additional sources of capital to finance any such acquisition, which could have an impact on future liquidity.
We may also from time to time depending on market conditions and prices, contractual restrictions, our financial liquidity and other factors, seek to prepay outstanding debt or repurchase our outstanding debt through open market purchases, privately negotiated purchases, or otherwise. The amounts involved in any such transactions, individually or in the aggregate, may be material and may be funded from available cash or from additional borrowings.
Contractual Obligations
The following table represents annual contractual obligations as of June 30, 2020:
| |
| | | Less than 1 | |
| | |
| | | More than 5 | ||
(In thousands) |
| Total |
| year |
| 1-3 years |
| 3-5 years |
| Years | |||||
Long-Term Debt (1) | | $ | 707,951 | | $ | 88,189 | | | 533,512 | | | — | | | 86,250 |
Operating Lease Obligations (3) | |
| 15,466 | |
| 1,101 | | | 4,269 | | | 4,347 | | | 5,749 |
Purchase Obligations (2) | |
| 3,255 | |
| 3,255 | | | — | | | — | | | — |
Asset Purchase Payment Obligations (2) | |
| 14,372 | |
| 2,713 | | | 11,659 | | | — | | | — |
Interest on Obligations (1) | |
| 106,894 | |
| 41,099 | | | 54,151 | | | 7,763 | | | 3,881 |
Total | | $ | 847,938 | | $ | 136,357 | | $ | 603,591 | | $ | 12,110 | | $ | 95,880 |
| (1) | Long-term debt and interest on obligations amounts above primarily relate to the Company’s Amended Senior Secured Credit Facility, as well as the 4.5% Convertible Senior Notes. Refer to Note 9 “Long-Term Debt” for additional information. Interest on obligations was calculated based on interest rates in effect at June 30, 2020. |
| (2) | The purchase obligations above are primarily related to noncancelable open purchase orders for API and ongoing capital expenditure projects. The asset purchase payment obligation above refers to the consideration due to Andor Pharmaceuticals, LLC for the AB-rated Methylphenidate Hydrochloride perpetual license agreement. |
| (3) | Operating lease obligations primarily relate to an eight year lease for the Company’s new headquarters in Trevose, Pennsylvania as well as a 116,000 square foot leased warehouse in Seymour, Indiana. |
Research and Development Arrangements
In the normal course of business, the Company has entered into certain research and development and other arrangements. As part of these arrangements, the Company has agreed to certain contingent payments which generally become due and payable only upon the achievement of certain developmental, regulatory, commercial and/or other milestones. In addition, under certain arrangements, we may be required to make royalty payments based on a percentage of future sales, or other metric, for products currently in development in the event that the Company begins to market and sell the product. Due to the inherent uncertainty related to these developmental, regulatory, commercial and/or other milestones, it is unclear if the Company will ever be required to make such payments.
54
Critical Accounting Policies
The preparation of our Consolidated Financial Statements in accordance with accounting principles generally accepted in the United States and the rules and regulations of the U.S. Securities & Exchange Commission requires the use of estimates and assumptions. A listing of the Company’s significant accounting policies are detailed in Note 2 “Summary of Significant Accounting Policies.” A subsection of these accounting policies have been identified by management as “Critical Accounting Policies.” Critical accounting policies are those which require management to make estimates using assumptions that were uncertain at the time the estimates were made and for which the use of different assumptions, which reasonably could have been used, could have a material impact on the financial condition or results of operations.
Management has identified the following as “Critical Accounting Policies”: Revenue Recognition, Inventories, Income Taxes, Business Combinations, Valuation of Long-Lived Assets, including Goodwill and Intangible Assets, In-Process Research and Development and Share-based Compensation.
Revenue Recognition
On July 1, 2018, the Company adopted Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers, which superseded ASC Topic 605, Revenue Recognition. Under ASC 606, the Company recognizes revenue when title and risk of loss of promised goods or services have transferred to the customer at an amount that reflects the consideration the Company is expected to be entitled. Our revenue consists almost entirely of sales of our pharmaceutical products to customers, whereby we ship product to a customer pursuant to a purchase order. Revenue contracts such as these do not generally give rise to contract assets or contract liabilities because: (i) the underlying contracts generally have only a single performance obligation and (ii) we do not generally receive consideration until the performance obligation is fully satisfied. The new revenue standard also impacts the timing of the Company’s revenue recognition by requiring recognition of certain contract manufacturing arrangements to change from “upon shipment or delivery” to “over time.” However, the recognition of these arrangements over time does not currently have a material impact on the Company’s consolidated results of operations or financial position. The Company adopted ASC 606 using the modified retrospective method.
When revenue is recognized, a simultaneous adjustment to gross sales is made for estimated chargebacks, rebates, returns, promotional adjustments and other potential adjustments. These provisions are primarily estimated based on historical experience, future expectations, contractual arrangements with wholesalers and indirect customers and other factors known to management at the time of accrual. Accruals for provisions are presented in the Consolidated Financial Statements as a reduction to gross sales with the corresponding reserve presented as a reduction of accounts receivable or included as rebates payable, depending on the nature of the reserve.
Provisions for chargebacks, rebates, returns and other adjustments require varying degrees of subjectivity. While rebates generally are based on contractual terms and require minimal estimation, chargebacks and returns require management to make more subjective assumptions. Each major category is discussed in detail below:
Chargebacks
The provision for chargebacks is the most significant and complex estimate used in the recognition of revenue. The Company sells its products directly to wholesale distributors, generic distributors, retail pharmacy chains and mail-order pharmacies. The Company also sells its products indirectly to independent pharmacies, managed care organizations, hospitals, nursing homes and group purchasing organizations, collectively referred to as “indirect customers.” The Company enters into agreements with its indirect customers to establish pricing for certain products. The indirect customers then independently select a wholesaler from which to purchase the products. If the price paid by the indirect customers is lower than the price paid by the wholesaler, the Company will provide a credit, called a chargeback, to the wholesaler for the difference between the contractual price with the indirect customers and the wholesaler purchase price. The provision for chargebacks is based on expected sell-through levels by the Company’s wholesale customers to the indirect customers and estimated wholesaler inventory levels. As sales to the large wholesale customers, such as Cardinal Health,
55
AmerisourceBergen and McKesson increase (decrease), the reserve for chargebacks will also generally increase (decrease). However, the size of the increase (decrease) depends on product mix and the amount of sales made to indirect customers with which the Company has specific chargeback agreements. The Company continually monitors the reserve for chargebacks and makes adjustments when management believes that expected chargebacks may differ from the actual chargeback reserve.
Rebates
Rebates are offered to the Company’s key chain drug store, distributor and wholesaler customers to promote customer loyalty and increase product sales. These rebate programs provide customers with credits upon attainment of pre-established volumes or attainment of net sales milestones for a specified period. Other promotional programs are incentive programs offered to the customers. Additionally, as a result of the Patient Protection and Affordable Care Act (“PPACA”) enacted in the U.S. in March 2010, the Company participates in a new cost-sharing program for certain Medicare Part D beneficiaries designed primarily for the sale of brand drugs and certain generic drugs if their FDA approval was granted under a NDA or 505(b) NDA versus an ANDA. Drugs purchased within the Medicare Part D coverage gap (commonly referred to as the “donut hole”) result in additional rebates. The Company estimates the reserve for rebates and other promotional credit programs based on the specific terms in each agreement when revenue is recognized. The reserve for rebates increases (decreases) as sales to certain wholesale and retail customers increase (decrease). However, since these rebate programs are not identical for all customers, the size of the reserve will depend on the mix of sales to customers that are eligible to receive rebates.
Returns
Consistent with industry practice, the Company has a product returns policy that allows customers to return product within a specified time period prior to and subsequent to the product’s expiration date in exchange for a credit to be applied to future purchases. The Company’s policy requires that the customer obtain pre-approval from the Company for any qualifying return. The Company estimates its provision for returns based on historical experience, changes to business practices, credit terms and any extenuating circumstances known to management. While historical experience has allowed for reasonable estimations in the past, future returns may or may not follow historical trends. The Company continually monitors the reserve for returns and makes adjustments when management believes that actual product returns may differ from the established reserve. Generally, the reserve for returns increases as net sales increase.
Other Adjustments
Other adjustments consist primarily of price adjustments, also known as “shelf-stock adjustments” and “price protections,” which are both credits issued to reflect increases or decreases in the invoice or contract prices of the Company’s products. In the case of a price decrease, a credit is given for product remaining in customer’s inventories at the time of the price reduction. Contractual price protection results in a similar credit when the invoice or contract prices of the Company’s products increase, effectively allowing customers to purchase products at previous prices for a specified period of time. Amounts recorded for estimated shelf-stock adjustments and price protections are based upon specified terms with direct customers, estimated changes in market prices and estimates of inventory held by customers. The Company regularly monitors these and other factors and evaluates the reserve as additional information becomes available. Other adjustments also include prompt payment discounts and “failure-to-supply” adjustments. If the Company is unable to fulfill certain customer orders, the customer can purchase products from our competitors at their prices and charge the Company for any difference in our contractually agreed upon prices.
Inventories
Inventories are stated at the lower of cost or net realizable value determined by the first-in, first-out method. Inventories are regularly reviewed and write-downs for excess and obsolete inventory are recorded based primarily on current inventory levels, expiration date and estimated sales forecasts.
56
Income Taxes
The Company uses the liability method to account for income taxes as prescribed by ASC 740, Income Taxes. Deferred taxes are recorded to reflect the tax consequences on future years of events that the Company has already recognized in the financial statement or tax returns. Deferred income tax assets and liabilities are adjusted to recognize the effect of changes in tax law or tax rates in the period during which the new law is enacted. Under ASC 740, Income Taxes, a valuation allowance is required when it is more likely than not that all or some portion of the deferred tax assets will not be realized through generating sufficient future taxable income. Failure to achieve forecasted taxable income in applicable tax jurisdictions could affect the ultimate realization of deferred tax assets and could result in an increase in the Company’s effective tax rate on future earnings.
The Company may recognize the tax benefit from an uncertain tax position claimed on a tax return only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement. The benefit from uncertain tax positions recorded in the financial statements was immaterial for all periods presented.
The Company’s future effective income tax rate is highly reliant on future projections of taxable income, tax legislation, and potential tax planning strategies. A change in any of these factors could materially affect the effective income tax rate of the Company in future periods.
Valuation of Long-Lived Assets, including Goodwill and Intangible Assets
The Company’s long-lived assets primarily consist of property, plant and equipment, definite and indefinite-lived intangible assets.
Property, plant and equipment are stated at cost less accumulated depreciation. Depreciation is computed on a straight-line basis over the assets’ estimated useful lives, generally for periods ranging from 5 to 39 years. Definite-lived intangible assets are stated at cost less accumulated amortization and are amortized on a straight-line basis over the assets’ estimated useful lives, generally for periods ranging from 5 to 15 years. The Company continually evaluates the reasonableness of the useful lives of these assets.
Property, plant and equipment and definite-lived intangible assets are reviewed for impairment whenever events or changes in circumstances (“triggering events”) indicate that the carrying amount of the asset may not be recoverable. The nature and timing of triggering events by their very nature are unpredictable; however, management regularly considers the performance of an asset as compared to its expectations, industry events, industry and economic trends, as well as any other relevant information known to management when determining if a triggering event occurred.
If a triggering event is determined to have occurred, the first step in the impairment test is to compare the asset’s carrying value to the undiscounted cash flows expected to be generated by the asset. If the carrying value exceeds the undiscounted cash flows of the asset, then an impairment exists. An impairment loss is measured as the excess of the asset’s carrying value over its fair value, which in most cases is calculated using a discounted cash flow model. Discounted cash flow models are highly reliant on various assumptions which are considered Level 3 inputs, including estimates of future cash flows (including long-term growth rates), discount rates and the probability of achieving the estimated cash flows. The judgments made in determining the estimated fair value can materially impact our results of operations. There can be no assurances as to when, or if, future impairments may occur.
Indefinite-lived intangible assets, including in-process research and development, are not amortized. Instead, indefinite-lived intangible assets are tested for impairment annually during the fourth quarter of each fiscal year, or more frequently whenever events or triggering events indicate that the asset might be impaired. The Company’s fair value assessments are highly reliant on various assumptions which are considered Level 3 inputs, including estimates of future cash flows (including long-term growth rates), discount rates and the probability of achieving the estimated cash flows. The judgments made in determining the estimated fair value of an indefinite-lived intangible asset can materially impact our results of operations. There can be no assurances as to when, or if, future impairments may occur.
57
In-Process Research and Development
Acquired businesses are accounted for using the acquisition method of accounting. The acquisition purchase price is allocated to the net assets of the acquired business at their respective fair values. Amounts allocated to in-process research and development are recorded at fair value and are considered indefinite-lived intangible assets subject to the impairment testing in accordance with the Company’s impairment testing policy for indefinite-lived intangible assets as described above. As products in development are approved for sale, amounts will be allocated to product rights and will be amortized over their estimated useful lives. Definite-lived intangible assets are amortized over the expected life of the asset. The Company’s fair value assessments are highly reliant on various assumptions which are considered Level 3 inputs, including estimates of future cash flows (including long-term growth rates), discount rates and the probability of achieving the estimated cash flows. The judgments made in determining the estimated fair value of in-process research and development, as well as asset lives, can materially impact our results of operations. There can be no assurances as to when, or if, future impairments may occur.
Share-based Compensation
Share-based compensation costs are recognized over the vesting period, using a straight-line method, based on the fair value of the instrument on the date of grant less an estimate for expected forfeitures. The Company uses the Black-Scholes valuation model to determine the fair value of stock options, the stock price on the grant date to value restricted stock and the Monte-Carlo simulation model to determine the fair value of performance-based shares. The Black-Scholes valuation and Monte-Carlo simulation models include various assumptions, including the expected volatility, the expected life of the award, dividend yield and the risk-free interest rate.
Expected volatility is based on the historical volatility of the price of our common shares during the historical period equal to the expected term of the option. The Company uses historical information to estimate the expected term, which represents the period of time that options granted are expected to be outstanding. The risk-free rate for the period equal to the expected life of the option is based on the U.S. Treasury yield curve in effect at the time of grant. The forfeiture rate assumption is the estimated annual rate at which unvested awards are expected to be forfeited during the vesting period. This assumption is based on our actual forfeiture rate on historical awards. Periodically, management will assess whether it is necessary to adjust the estimated rate to reflect changes in actual forfeitures or changes in expectations. Additionally, the expected dividend yield is equal to zero, as the Company has not historically issued and has no immediate plans to issue, a dividend. These assumptions involve inherent uncertainties based on market conditions which are generally outside the Company’s control. Changes in these assumptions could have a material impact on share-based compensation costs recognized in the financial statements.
Recent Accounting Pronouncements
In December 2019, the Financial Accounting Standards Board ("FASB") issued ASU 2019-12, Simplifying the Accounting for Income Taxes, which is meant to reduce complexity in the accounting for income taxes, eliminates certain exceptions within ASC 740, and clarifies certain aspects of the current guidance to promote consistency among reporting entities. ASU 2019-12 is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2020, with early adoption permitted for periods for which financial statements have not been issued as of December 15, 2019. The Company early adopted this guidance in the second quarter of Fiscal 2020. As a result of the adoption, the Company is no longer subject to the exception to the general methodology for calculating income taxes in an interim period when a year-to-date loss exceeds the anticipated loss for the year. The adoption of ASU 2019-12 did not have an impact on the Company’s income tax benefit for the fiscal year ended June 30, 2020.
In February 2016, the FASB issued ASU 2016-02, Leases. ASU 2016-02 requires an entity to recognize ROU assets and liabilities on its balance sheet for all leases with terms longer than 12 months. Lessees and lessors are required to disclose quantitative and qualitative information about leasing arrangements to enable a user of the financial statements to assess the amount, timing and uncertainty of cash flows arising from leases. ASU 2016-02 is effective for annual reporting periods beginning after December 15, 2018, including interim periods within that reporting period and requires a modified retrospective application, with early adoption permitted. The Company adopted ASU 2016-02 as of July 1, 2019 on a modified retrospective basis applying the guidance to leases existing as of this effective date. The Company
58
has determined that there was no cumulative-effect adjustment to beginning retained earnings on the Consolidated Balance Sheet. The Company will continue to report periods prior to July 1, 2019 in our financial statements under prior guidance as outlined in Topic 840. Refer to Note 11 "Commitments" for additional information.
The Company’s adoption of ASU No. 2016-02 resulted in an increase in the Company’s assets and liabilities of $7.9 million at July 1, 2019. The Company’s adoption of ASU No. 2016-02 did not have any impact to the Company’s Consolidated Statements of Operations, or its Consolidated Statements of Cash Flows. Further, there was no impact on the Company’s covenant compliance under its current debt agreements as a result of the adoption of ASU No. 2016-02. The Company elected the package of practical expedients included in this guidance, which allowed it to not reassess: (i) whether any expired or existing contracts contain leases; (ii) the lease classification for any expired or existing leases; and, (iii) the initial direct costs for existing leases. The Company does not recognize short-term leases of 12 months or less on its Consolidated Balance Sheets and will recognize those lease payments in the Consolidated Statements of Operations on a straight-line basis over the lease term.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
On November 25, 2015, in connection with the acquisition of KUPI, the Company entered into a Senior Secured Credit Facility, which was subsequently amended in June 2016 and December 2018. Based on the variable-rate debt outstanding at June 30, 2020, each 1/8% increase in interest rates would yield $0.8 million of incremental annual interest expense. The Company’s variable-rate debt is subject to a 1.0% London Inter-bank Offered Rate (“LIBOR”) floor.
The Company has historically invested in equity securities, U.S. government agency securities and corporate bonds, which are exposed to market and interest rate fluctuations. The market value, interest and dividends earned on these investments may vary based on fluctuations in interest rate and market conditions.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The Consolidated Financial Statements and Report of the Independent Registered Public Accounting Firm is set forth in Item 15 of this Annual Report on Form 10-K under the caption “Consolidated Financial Statements” and incorporated herein by reference.
59
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We carried out an evaluation under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as such term is defined under Rule 13a-15(e) promulgated under the Exchange Act, as amended, for financial reporting as of June 30, 2020. Based on that evaluation, our chief executive officer and chief financial officer concluded that these controls and procedures are effective to ensure that information required to be disclosed by the Company in reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported as specified in SEC rules and forms and is accumulated and communicated to our management to allow timely decisions regarding required disclosures. There were no changes in these controls or procedures identified in connection with the evaluation of such controls or procedures that occurred during our last fiscal quarter, or in other factors that have materially affected, or are reasonably likely to materially affect these controls or procedures.
Our disclosure controls and procedures are designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the rules and forms of the Securities and Exchange Commission. These disclosure controls and procedures include, among other things, controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file under the Exchange Act is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control over Financial Reporting
The report of management of the Company regarding internal control over financial reporting is set forth in Item 15 of this Annual Report on Form 10-K under the caption “Consolidated Financial Statements: Management’s Report on Internal Control Over Financial Reporting “and incorporated herein by reference.
Attestation Report of Independent Registered Public Accounting Firm
The attestation report of the Company’s independent registered public accounting firm regarding internal control over financial reporting is set forth in Item 15 of this Annual Report on Form 10-K under the caption “Consolidated Financial Statements: Report of Independent Registered Public Accounting Firm” and incorporated herein by reference.
Changes in Internal Control over Financial Reporting
During the quarter ended June 30, 2020, there were no changes in the Company’s internal control over financial reporting (as defined in Rule 13a-15(f) of the Exchange Act) that materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
None.
60
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Directors and Executive Officers
The directors and executive officers of the Company are set forth below:
|
| Age |
| Position |
Directors: |
|
|
|
|
| | | | |
Patrick G. LePore |
| 65 |
| Chairman of the Board |
| | | | |
John C. Chapman |
| 65 |
| Director |
| | | | |
Timothy C. Crew |
| 59 |
| Director |
| | | | |
David Drabik |
| 52 |
| Director |
| | | | |
Jeffrey Farber |
| 59 |
| Director |
| | | | |
Melissa Rewolinski |
| 50 |
| Director |
| | | | |
Paul Taveira |
| 60 |
| Director |
| | | | |
Officers: | | |
|
|
| | | | |
Timothy C. Crew |
| 59 |
| Chief Executive Officer |
| | | | |
John Kozlowski |
| 48 |
| Vice President of Finance, Chief Financial Officer |
| | | | |
John M. Abt |
| 55 |
| Vice President and Chief Quality and Operations Officer |
| | | | |
Maureen M. Cavanaugh |
| 60 |
| Senior Vice President and Chief Commercial Operations Officer |
| | | | |
Robert Ehlinger |
| 62 |
| Vice President and Chief Information Officer |
| | | | |
Samuel H. Israel |
| 58 |
| General Counsel and Chief Legal Officer |
| | | | |
Patrick G. LePore was appointed as a Director of the Company in July 2017. On July 1, 2018, Mr. LePore succeeded Mr. Farber as Chairman of the Board of Directors. Mr. LePore served as chairman, Chief Executive Officer and president of Par Pharmaceuticals, Inc., until the company’s acquisition by private equity investor TPG in 2012. He remained as chairman of the new company through the sale of the company to Endo Pharmaceuticals. Mr. LePore began his career with Hoffmann LaRoche. Later, he founded Boron LePore and Associates, a medical communications company, which he took public and was eventually sold to Cardinal Health. Mr. LePore is the Vice Chairman of the board of Matinas BioPharm and is a trustee of Villanova University. Mr. LePore earned his bachelor’s degree from Villanova University and Master of Business Administration from Fairleigh Dickinson University.
The Governance and Nominating Committee concluded that Mr. LePore is well qualified to serve as a Director due, in part, to his understanding and experience as a Chief Executive Officer and Director of highly regarded companies within the pharmaceutical industry. Mr. LePore is an independent director as defined by the rules of the NYSE.
61
John C. Chapman was appointed as a Director of the Company in July 2018. Mr. Chapman is a retired audit partner for KPMG, having specialized in providing audit services to large complex multinational pharmaceutical and consumer market companies. During his tenure at KPMG, he served for six years as a member of the firm’s board of directors and for several years as KPMG’s global chair of pharmaceuticals and chemicals. Mr. Chapman also served as global lead partner for some of KPMG’s largest clients, including Pfizer, Hoechst and PepsiCo, among others. Mr. Chapman, a certified public accountant (CPA), earned a Bachelor of Business Administration in accounting practice degree from Pace University, New York. On August 21, 2018, Mr. Chapman was appointed as Chairman of the Audit Committee, effective upon filing of the Company’s Fiscal 2018 Consolidated Financial Statements.
The Governance and Nominating Committee concluded that Mr. Chapman is well qualified to serve as a Director, due to his extensive experience in the public accounting profession. Additionally, Mr. Chapman has significant experience in dealing with acquisitions, divestitures, initial public offerings and secondary offerings. Mr. Chapman is an independent director as defined by the rules of the NYSE.
Timothy C. Crew was appointed as the Company’s Chief Executive Officer and a Director of the Company in January 2018. Mr. Crew has more than 25 years of experience in the generic and branded pharmaceutical industries. Previously, he served as Chief Executive Officer of Cipla North America, a global pharmaceutical company based in Mumbai, India. Before Cipla, he worked for eight years at Teva Pharmaceuticals Industries Ltd. (“Teva”), where he ultimately served as Senior Vice President and Commercial Operating Officer of the North American Generics division, the world’s largest generic operation with multibillion dollars of annual sales. Before that, he was Teva’s Vice President, Alliances and Business Development. Mr. Crew was also an Executive Vice President, North America, for Dr. Reddy’s Laboratories Ltd. Mr. Crew began his pharmaceutical career at Bristol-Myers Squibb, where he held a number of senior management positions in global marketing, managed healthcare, marketing, business development and strategic planning. Prior to his pharmaceutical roles, Mr. Crew served in the United States Army, where he rose to the rank of Captain. Mr. Crew earned a Bachelor of Arts degree in economics from Pomona College and a Masters of Business Administration degree from Columbia Business School.
The Governance and Nominating Committee concluded that Mr. Crew is well qualified to serve as a Director due, in part, to his understanding and experience as a Chief Executive Officer and Director of highly regarded companies within the pharmaceutical industry.
David Drabik was elected a Director of the Company in January 2011. Mr. Drabik is a National Association of Corporate Directors Governance Fellow. Since 2002, Mr. Drabik has been President of Cranbrook & Co., LLC (“Cranbrook”), an advisory firm primarily serving the private equity and venture capital community. At Cranbrook, Mr. Drabik assists and advises its clientele on originating, structuring and executing private equity and venture capital transactions. From 1995 to 2002, Mr. Drabik served in various roles and positions with UBS Capital Americas (and its predecessor UBS Capital LLC), a New York City based private equity and venture capital firm that managed $1.5 billion of capital. From 1992 to 1995, Mr. Drabik was a banker with Union Bank of Switzerland’s Corporate and Institutional Banking division in New York City. Mr. Drabik graduated from the University of Michigan with a Bachelor of Business Administration degree.
The Governance and Nominating Committee concluded that Mr. Drabik is well qualified to serve as a Director due, in part, to his understanding and involvement in investment banking. As a global investment bank professional with extensive experience advising senior management, his skills include business analytics, financing and a strong familiarity with SEC documentation. Mr. Drabik is an independent director as defined by the rules of the NYSE.
Jeffrey Farber was appointed a Director of the Company in May 2006 and was appointed Chairman of the Board of Directors in July 2012. On July 2018, Patrick LePore succeeded Jeffrey Farber as the Chairman of the Board. Jeffrey Farber joined the Company in August 2003 as Secretary. Since 1994, Mr. Farber has been President and the owner of Auburn Pharmaceutical (“Auburn”), a national generic pharmaceutical distributor. Prior to starting Auburn, Mr. Farber served in various positions at Major Pharmaceutical (“Major”), where he was employed for over 15 years. At Major, Mr. Farber was involved in sales, purchasing and eventually served as President of the Midwest division. Mr. Farber also spent time working at Major’s manufacturing division, Vitarine Pharmaceuticals, where he served on its Board of
62
Directors. Mr. Farber graduated from Western Michigan University with a Bachelors of Science Degree in Business Administration and participated in the Pharmacy Management Graduate Program at Long Island University.
The Governance and Nominating Committee concluded that Mr. Farber is qualified to serve, due, in part, to his significant experience in the generic drug industry and his ongoing role as the owner of a highly regarded and successful generic drug distributor. His skills include a thorough knowledge of the generic drug marketplace and drug supply chain management.
Melissa Rewolinski was appointed as a Director of the Company in July 2019. Dr. Rewolinski currently serves as principal of MVR Consulting, where she specializes in providing counsel to small and mid-size biotechnology and pharmaceutical companies. Earlier she held a number of senior level R&D positions for Intercept, rising to Senior Vice President, Head of Technical Operations, and member of the Executive Team. Previously, she served as Senior Director, Development for Amira Pharmaceuticals, and before that as a Chemical Development Group Leader and a Pharmaceutical Sciences Project Team Leader for Pfizer Global R&D. Dr. Rewolinski began her career at Pharmacia & Upjohn as a post-doctoral research scientist. Dr. Rewolinski earned a doctorate degree in organic chemistry and Bachelor of Science degree in chemistry, magna cum laude, from Rice University.
The Governance and Nominating Committee concluded that Dr. Rewolinski is well qualified to serve as a Director due, in part, to her significant experience in operational and drug development roles within the pharmaceutical industry. Dr. Rewolinski is an independent director as defined by the rules of the NYSE.
Paul Taveira was appointed a Director of the Company in May 2012. Mr. Taveira was the Chief Executive Officer of the National Response Corporation, an international firm specializing in environmental services, from June 2015 to February 2019. He previously served on the Board of Directors and as the Chief Executive Officer of A&D Environmental Services Inc., an environmental and industrial services company. From 2007 to 2009, Mr. Taveira was a Managing Partner of Precision Source LLC, a manufacturer of precision parts for various industries across the United States. From 1997 to 2007, Mr. Taveira held several positions at PSC Inc., a national provider of environmental services, including President, Vice President and Regional General Manager. From 1987 to 1997, Mr. Taveira held several management positions with Clean Harbors Inc., an international provider of environmental and energy services. Mr. Taveira graduated from Worcester State University with a Bachelor of Science degree in Biology.
The Governance and Nominating Committee concluded that Mr. Taveira is well qualified to serve as a Director due, in part, to his understanding and experience as a Chief Executive Officer and Director of various companies. Mr. Taveira is an independent director as defined by the rules of the NYSE.
John Kozlowski joined the Company in 2009 and was promoted in 2010 to Corporate Controller. In 2016, Mr. Kozlowski was promoted to Vice President Financial Operations & Corporate Controller. In October 2017, Mr. Kozlowski was promoted to Chief Operating Officer. In April 2018, Mr. Kozlowski was promoted to Chief of Staff and Strategy Officer. In August 2019, Mr. Kozlowski succeeded Martin Galvan as the Vice President of Finance and Chief Financial Officer. In July 2020, Mr. Kozlowski was also appointed the Principal Accounting Officer. Prior to joining the Company, Mr. Kozlowski served in senior finance and accounting roles for Optium Corporation and Finisar Australia. He earned a Bachelor of Arts degree in finance from James Madison University and a Masters of Business Administration degree from Rider University.
John M. Abt joined the Company in March 2015 as Vice President of Quality and was promoted to Vice President and Chief Quality and Operations Officer in April 2018. Prior to joining the Company, Mr. Abt held senior level positions in both quality and operations and has extensive knowledge in pharmaceutical manufacturing, quality, strategy, business improvement and site transformation. Prior to joining the Company, he most recently served as Teva Pharmaceuticals’ Vice President Global Quality Strategy, overseeing the development and implementation of strategy and associated initiatives for the global quality organization. Before that, he held a number of leadership positions of increasing responsibility in operations, continuous improvement, quality systems and compliance. He earned his Doctorate in Business Administration from Temple University, Masters of Administrative Science in Business Management from Johns Hopkins University and a Bachelor of Science in Biochemistry from Niagara University.
63
Maureen M. Cavanaugh joined the Company in May 2018 as Senior Vice President and Chief Commercial Operations Officer. Prior to joining the Company, Ms. Cavanaugh spent the past 11 years at Teva, most recently as Senior Vice President, Chief Commercial Officer, North American Generics. Earlier at Teva, Ms. Cavanaugh served as Senior Vice President and General Manager, US Generics and before that held a variety of positions in sales, marketing and customer operations. Ms. Cavanaugh also previously served as Senior Director of Marketing at PAR Pharmaceuticals, as Director, Product Management and Marketing Research at Sandoz Inc., and held a number of finance, sales and marketing operations positions at Bristol Myers-Squibb. Ms. Cavanaugh earned a Bachelor of Science in Business Administration degree from LaSalle University and a Masters of Business Administration degree from Rider University.
Robert Ehlinger joined the Company in July 2006 as Chief Information Officer. In June 2011, Mr. Ehlinger was promoted to Vice President of Logistics and Chief Information Officer. Prior to joining Lannett, Mr. Ehlinger was the Vice President of Information Technology at MedQuist, Inc., a healthcare services provider, where his career spanned 10 years in progressive operational and technology roles. Prior to MedQuist, Mr. Ehlinger was with Kennedy Health Systems as their Corporate Director of Information Technology supporting acute care and ambulatory care health information systems and biomedical support services. Earlier on, Mr. Ehlinger was with Dowty Communications where he held various technical and operational support roles prior to assuming the role of International Distribution Sales Executive managing the Latin America sales distribution channels. Mr. Ehlinger received a Bachelor’s of Arts degree in Physics from Gettysburg College in Gettysburg, PA.
Samuel H. Israel joined the Company in July 2017 as General Counsel and Chief Legal Officer. Prior to joining Lannett, Mr. Israel was a partner with Fox Rothschild LLP, a national, full-service law firm, with 26 offices that provide services in more than 60 practice areas, since 1998. He served as chair of the firm’s Pharmaceutical and Biotechnology Practice and handled a variety of commercial litigation matters. Mr. Israel earned a bachelor of science degree in chemical engineering from the University of Pennsylvania and a juris doctor degree with honors from Rutgers University School of Law.
To the best of the Company’s knowledge, there have been no events under any bankruptcy act, no criminal proceedings and no judgments or injunctions that are material to the evaluation of the ability or integrity of any director, executive officer, or significant employee during the past ten years.
Delinquent Section 16(a) Reports
Section 16(a) of the Exchange Act (“Section 16”) requires the Company’s directors, executive officers and persons who own more than ten percent of the common stock of the Company, to file with the SEC initial reports of beneficial ownership and reports of changes in beneficial ownership of common stock of the Company. Based solely on review of these reports, or written representations from these persons that no other reports were required to be filed with the SEC, the Company believes that all reports for the Company’s directors, executive officers and ten percent shareholders that were required to be filed under Section 16 during the fiscal year ended June 30, 2020 were timely filed, except for one Form 4 for Jeffrey Farber reporting a single sale of 142 shares. The transaction was subsequently reported on a Form 5 and Jeffrey Farber disclaims beneficial ownership of these shares.
Code of Ethics
The Company has adopted the Code of Professional Conduct (the “code of ethics”), a code of ethics that applies to the Company’s Chief Executive Officer and Chief Financial Officer, as well as all other company personnel. The code of ethics is publicly available on our website at www.lannett.com. If the Company makes any substantive amendments to the code of ethics or grants any waiver, including any implicit waiver, from a provision of the code to our Chief Executive Officer, Chief Financial Officer, or any other executive, we will disclose the nature of such amendment or waiver on our website or in a report on Form 8-K.
Audit Committee
The Audit Committee has responsibility for overseeing the Company’s financial reporting process on behalf of the Board. In addition, Audit Committee responsibilities include selection of the Company’s independent auditors,
64
conferring with the independent auditors regarding their audit of the Company’s Consolidated Financial Statements, pre-approving and reviewing the independent auditors’ fees and considering whether non-audit services are compatible with maintaining their independence and considering the adequacy of internal financial controls. The Audit Committee operates pursuant to a written charter adopted by the Board, which is available on the Company’s website at www.lannett.com. The charter describes the nature and scope of the Audit Committee’s responsibilities. The members of the Audit Committee are Paul Taveira, David Drabik, John Chapman, and Melissa Rewolinski. All members of the Audit Committee are independent directors as defined by the rules of the NYSE.
Financial Expert on Audit Committee: The Board has determined that John Chapman, a current Director and Chairman of the Audit Committee, is the Audit Committee financial expert as defined in section 3(a)(58) of the Exchange Act and the related rules of the Commission for the year ended June 30, 2020.
65
ITEM 11. EXECUTIVE COMPENSATION
Compensation Discussion and Analysis
This Compensation Discussion and Analysis (“CD&A”) describes our Fiscal 2020 Executive Compensation Program. It provides an overview of the compensation program for the following Named Executive Officers (“NEOs”) and how the Compensation Committee of the Board of Directors (“the Committee”) made its decisions for our 2020 fiscal year.
NEO |
| Title/Role |
Timothy C. Crew | | Chief Executive Officer (“CEO”) |
John Kozlowski | | Vice President of Finance, Chief Financial Officer and Principal Accounting Officer* |
Maureen Cavanaugh | | Senior Vice President and Chief Commercial Operations Officer |
Samuel H. Israel | | Chief Legal Officer and General Counsel |
John Abt | | Vice President and Chief Quality and Operations Officer |
Martin P. Galvan | | Former Vice President of Finance and Chief Financial Officer* |
| * | Mr. Galvan retired from the Company effective August 30, 2019 and Mr. Kozlowski assumed the role of Vice President, Finance and Chief Financial Officer effective August 31, 2019 and also assumed the Principal Accounting Officer role effective July 13, 2020. |
Say on Pay Results in 2020
At our annual shareholders meeting in January 2020, our shareholders approved the “say-on-pay” proposal, with approximately 95% of votes cast in support of our executive compensation program.
Although this vote is non-binding, its outcome, along with shareholder feedback and the competitive business environment, plays an important role in how the Committee makes decisions about the program’s structure. To this end, the Committee periodically conducts reviews of the Executive Compensation Program, monitors industry practices and seeks feedback from some of our largest investors. Based in part on this feedback, the Committee introduced performance shares tied to the Company’s three-year total shareholder returns relative to companies in the S&P Pharmaceuticals Select Industry Index as part of the long-term incentive program for NEOs in Fiscal 2018 and increased its weighting from 25% to 33.3% of the target award opportunity in Fiscal 2019 and 2020. Our executive compensation program for NEOs continues to place a significant emphasis on performance-based variable pay tied to key strategic objectives. We also maintain stock ownership requirements for executive officers and non-employee directors, and our Board of Directors recently approved an expanded compensation recovery or “clawback” provision amending all executive officer employment contracts in the event of the need for a restatement of a financial statement arising from fraud or misconduct. We believe these actions demonstrate our responsiveness to shareholder feedback and our ongoing commitment to aligning executive pay with performance and long-term value creation.
The following pages of this CD&A highlight performance results since Fiscal 2017 that have had a direct impact on the compensation paid to our NEOs over the same period of time. It looks specifically at the performance measures used in the short- and long-term incentive awards under the Executive Compensation Program that the Committee believes drive shareholder value. It also describes recently approved changes for Fiscal 2021 to further align our Executive Compensation Program with our objectives and best competitive practice.
COVID-19 Impact and Response
The COVID-19 pandemic poses serious health risks for all companies and the general public as well as financial challenges for many organizations. As a generic pharmaceutical company, we are considered to be an essential business and have maintained continuous operations throughout the pandemic. Over the past several years, we took a variety of actions to refocus our business on core generics, streamline our operations, strengthen our financial position and supply chain, and prioritize new product development to further diversify our portfolio and customer base. These initiatives, along with the hard work and dedication of our employees, has allowed us to successfully navigate through these challenging times. In March 2020, we implemented a variety of procedures and safeguards to help ensure the safety of
66
employees at our manufacturing plants, distribution center, and research and development facilities, while enabling other staff to work remotely. As a result, we have continued to operate profitably (excluding impairment charges and certain non-cash and non-recurring expenses), minimize supply chain disruptions, and produce and distribute more than 100 high quality pharmaceutical products that address a wide variety of therapeutic needs. Demand for many of our products remains strong, although our profitability and sales for certain products have been adversely impacted by the pandemic.
Due to the continued uncertainty within the current environment, the Committee decided to defer the timing of base salary merit increases, excluding certain promotional adjustments, from the first quarter to the second quarter of Fiscal 2021. Additionally, the Committee slightly increased the emphasis on restricted stock for Fiscal 2021 equity grants to NEOs and other executives to further enhance retention. Equity grants and total compensation opportunities for NEOs will continue to emphasize at-risk, variable pay. The Committee will continue to carefully monitor COVID-19’s impact on our business and may consider additional actions or modifications to our executive compensation program as needed to help ensure it continues to reinforce strategic priorities and incentivize and retain our NEOs, while also being mindful of the pandemic’s impact on all company stakeholders.
A Word About Risk
The Committee believes that incentive plans, along with the other elements of the Executive Compensation Program, provide appropriate rewards to our NEOs to keep them focused on our goals. The Committee also believes that the program’s structure, along with its oversight, continues to provide a setting that does not encourage the NEOs to take excessive risks in their business decisions.
Executive Summary
Business Highlights
Fiscal 2020 included various challenges relating to the COVID-19 pandemic, as noted above, as well as many achievements. Most importantly, the Company was able to maintain operations, produce and distribute critical medications to patients and customers while providing a safe working environment for our employees during an unprecedented public health pandemic and broad economic shutdown. We continued to successfully execute on our strategy of growing our core business, launching new products, building our R&D pipeline, expanding strategic alliances, and reducing costs. We launched a total of 18 new products during Fiscal 2020, most of which have limited or moderate competitors, matching the record number of launches in Fiscal 2019. During a very challenging environment, we achieved our budgeted revenue goal, with nearly 23% of sales derived from new products launched in Fiscal 2019 and 2020. We also continued to operate profitably, based on adjusted metrics for Operating Income and EPS which exclude impairments, amortization, restructuring and non-cash interest expenses, and certain other non-recurring items. During Fiscal 2020, we completed certain refinancing transactions to lower interest expense and reduced our total debt by approximately $60 million. We also continued to execute on our cost savings program, which generated net annualized cost savings of approximately $33.0 million at the end of Fiscal 2020 when compared to the Fiscal 2018 expenses. In July 2020, we announced a new restructuring and cost savings plan with expected annual savings of more than $15 million to help address ongoing competitive pricing pressure within the generic pharmaceuticals sector. We added a new non-employee director in July 2019 and continue to execute on a number of key strategic initiatives as discussed below. We believe these actions will better position the Company for long-term profitable growth and shareholder value creation.
In addition, we continued to make important advances in product development and mix, market share, and in our regulatory approval process, allowing us to efficiently and safely place our products that span a variety of categories on the market. As noted above, we have launched a total of 18 new products over each of the past two fiscal years, with additional launches planned in Fiscal 2021 and beyond. As of June 30, 2020, we had over 100 products available to the market. We also continue to capitalize on our strategic partnerships, both domestically and internationally. Since January 2018, we acquired or in-licensed approximately 60 ANDA products and entered into several new strategic alliance agreements which diversified and enhanced our revenue streams. In Fiscal 2020, we entered into commercialization agreements with several leading pharmaceutical companies that have the potential to significantly increase our future annual revenues. Included among these is an agreement with our strategic alliance partner, HEC
67
Group, for an insulin-based product with significant market potential to treat type 1 and type 2 diabetes, which impacts 34 million Americans. We continue to make progress advancing this and other product candidates towards commercial launches over the next several years.
Our financial performance in Fiscal 2020 was adversely impacted by the COVID-19 pandemic and ongoing competitive pressures within the generic pharmaceutical industry. Despite these challenges, our executive leadership and other employees made significant progress in executing our strategic plan and positioning the Company for future growth. Excluding Fiscal 2019 sales associated with a former distribution agreement with Jerome Stevens Pharmaceuticals, which expired in March 2019, our annual revenues grew by approximately 19% in Fiscal 2020. Our Fiscal 2020 sales mix shifted towards lower margin products due to the pandemic, adversely impacting our profitability. Our financial results for Fiscal 2020 slightly exceeded the target performance goal for net sales and were between threshold and target levels for profitability metrics under the Annual Bonus Plan. We achieved target results for refinancing goals under the strategic objectives component. Calculated award funding levels under our Annual Bonus Plan were equal to approximately 83% of target for most NEOs. In July 2020, our NEOs received target equity grants based on a target value mix of 45% for restricted stock, 20% for stock options, and 35% for performance shares tied to our relative TSR vs. companies in the S&P Pharmaceuticals Select Index for the three-year performance cycle running from July 1, 2020 through June 30, 2023. Many outstanding stock options held by our NEOs are currently “underwater” and the value of many other outstanding equity awards are below grant date target values. Based on our interim relative TSR results through June 30, 2020, performance shares granted in Fiscal 2018 are currently tracking below threshold levels which, if sustained over the applicable three-year performance periods, would result in no awards being earned by NEOs, while Fiscal 2019 grants are tracking slightly above threshold levels (and below-target) and those granted in Fiscal 2020 are currently tracking at above-target levels.
68
Key financial performance highlights, as reported in accordance with GAAP requirements, are shown below. GAAP-based results for Fiscal 2020 reflect asset impairments and certain other non-cash and/or non-recurring expenses that are excluded from adjusted profitability metrics. Year over year declines vs. Fiscal 2019 results reflect continued challenging market conditions within the generic pharmaceuticals industry as well as the non-renewal of the former distribution agreement with Jerome Stevens Pharmaceuticals (JSP), which expired in March 2019 and had significantly contributed to our prior net sales and profitability. See the section of our Form 10-K entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” for additional details and discussion of Company performance.
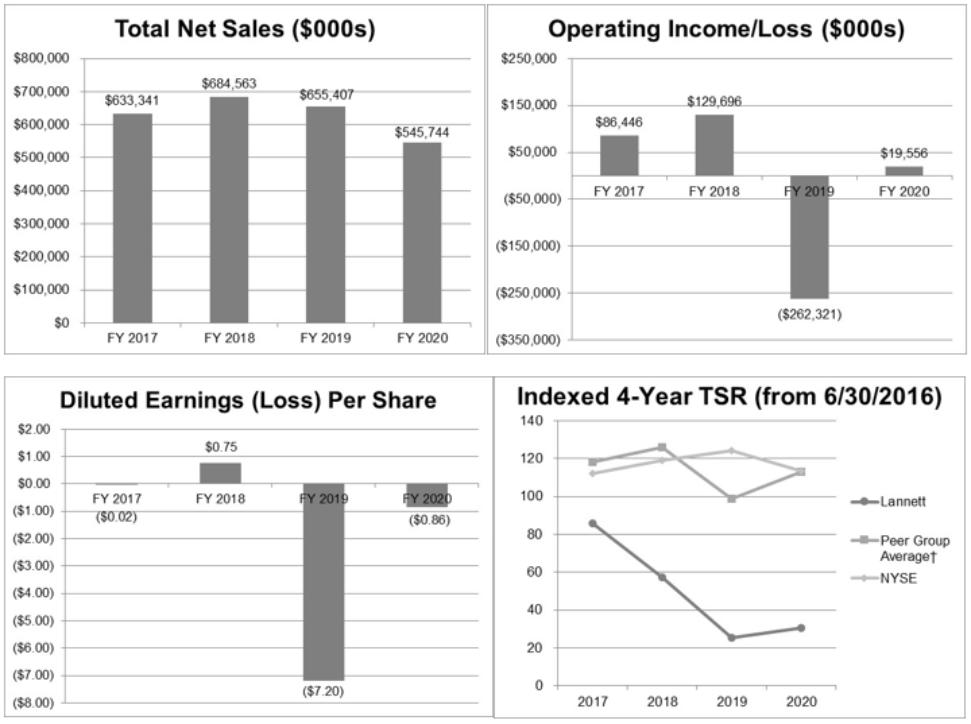
† Peer Group average pertains to the Fiscal 2020 peer group.
69
Comparison of Target Versus Actual CEO Pay (In Year Earned)
The following chart compares actual versus target CEO pay for Mr. Crew for the past three fiscal years. To more accurately demonstrate the impact of Company performance on executive pay, comparisons include annual equity grants in the year earned, as opposed to the year granted. Fiscal 2018 values for Mr. Crew include annualized base salary and short-term incentives (STI) and new hire equity grants. Actual pay for each of Fiscal 2019 and 2020 include the applicable pro-rata portions of a one-time retention incentive earned by Mr. Crew during the period of 12/1/18-12/1/19. As shown below, actual pay levels earned for Fiscal 2018 were well below target opportunities, even when new hire equity grants are included. Including the value of the one-time retention incentive, Mr. Crew’s actual pay was well above target for Fiscal 2019 and slightly above target for Fiscal 2020. Excluding the retention incentive award, actual pay was slightly above target for Fiscal 2019 and below target for Fiscal 2020. Equity-based long-term incentives reflect grant-date award values, with current actual or realizable values for Fiscal 2018 awards considerably lower based on our closing stock price as of June 30, 2020.
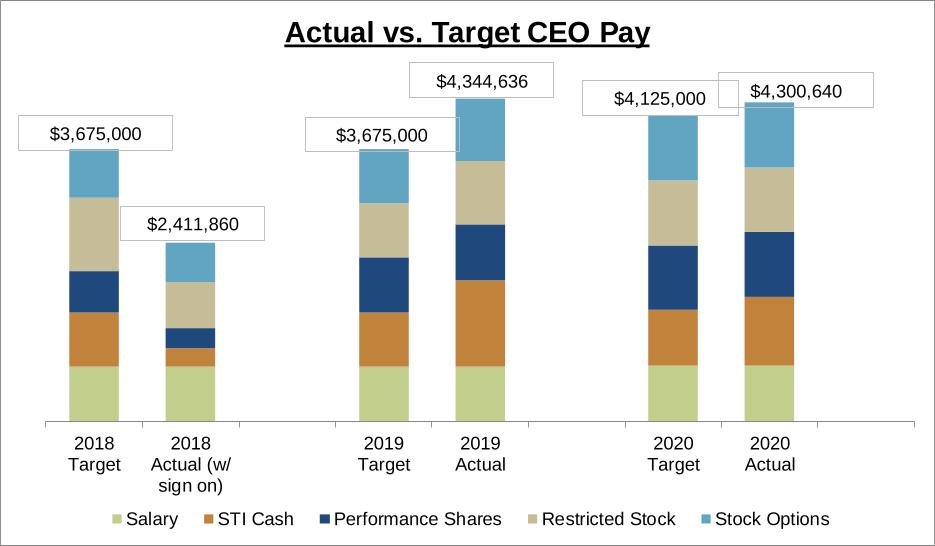
70
Comparison of Disclosed Versus Realizable CEO Pay for Mr. Crew (Based on Summary Compensation Table)
Compared with values reported in the Summary Compensation Table for Mr. Crew, current realizable values are 20% lower for Fiscal 2019 and 12% lower for Fiscal 2020. Mr. Crew’s reported compensation for Fiscal 2019 reflects actual base salary plus bonus (STI) earned plus equity awards granted in Fiscal 2019 (with stock options and restricted stock based on Fiscal 2018 performance). Fiscal 2020 reported compensation includes actual base salary plus STI earned plus the full value of a retention incentive earned in December 2019 plus equity awards granted in Fiscal 2020 (with stock options and restricted stock based on Fiscal 2019 performance). Realizable pay reflects current intrinsic values for equity grants based on our stock price as of June 30, 2020, with assumed performance share award funding at 63% of target for the Fiscal 2019 grant and at 158% of target for the Fiscal 2020 grant based on interim relative TSR results from date of grant through June 30, 2020.
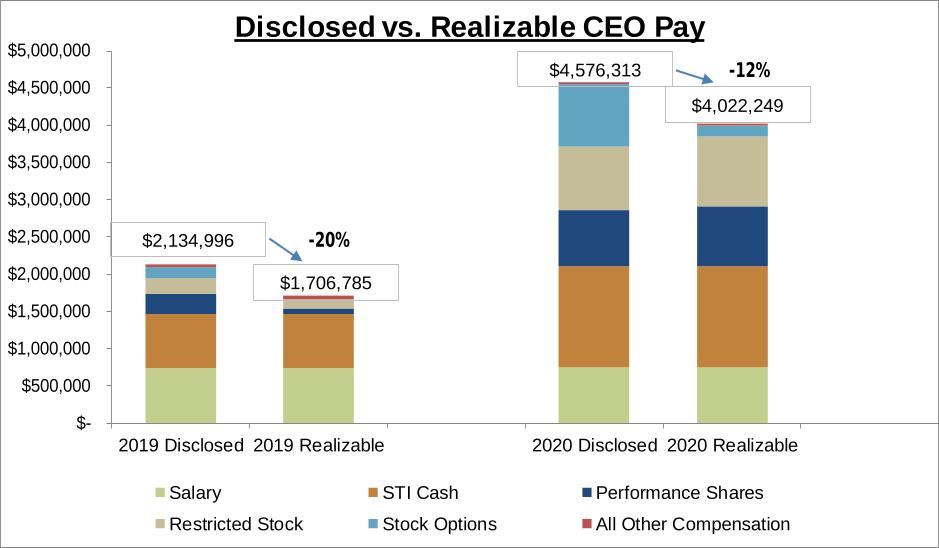
71
Fiscal 2020 Executive Compensation Program Changes
As our Company grows, the Committee is committed to the evolution and improvement of our Executive Compensation Program to ensure alignment with our business strategy and shareholder interests, as well as best competitive practices. The Committee made the following adjustments to the program’s core compensation elements for 2020:
What’s Changed |
| How It’s Changed |
| Explanation |
Short-Term Incentives (“Annual Bonus”) | | · Changed performance mix to increase the emphasis on the strategic objectives component with a corresponding decrease in the emphasis on Adjusted Operating Income, in each case by 10% of the total target award opportunity. | | No changes were made to performance metrics or award opportunities. Half of total award opportunities are tied to corporate profitability. Increased emphasis on strategic objectives allows for a more holistic assessment of performance, with Fiscal 2020 component tied to refinancing goals to strengthen our financial position. |
Long-Term Incentives | | · Increased target award opportunities for Messrs. Crew (from 300% to 350% of salary), Kozlowski (from 150% to 175% of salary) and Abt (from 100% to 150% of salary). · Eliminated former performance “look back” feature that impacted stock option and restricted stock grant levels. Fiscal 2020 target value mix was equally weighted between stock options, restricted stock, and performance shares tied to our 3-year relative total shareholder return (TSR) vs. industry comparators. · Increased full vesting period to four years for all award vehicles. | | Mr. Crew received a market adjustment to align his target total compensation more closely with 50th percentile market values. Mr. Kozlowski received a promotional adjustment and Mr. Abt’s increase recognizes the assumption of additional responsibilities. The performance lookback elimination allows for more consistency in terms of annual equity grant levels, to further enhance retention and align more closely with market practice. The time frame for full vesting for all awards was increased from three years to four years to further enhance retention. |
To address retention concerns and encourage NEOs to focus on achieving strategic objectives following the announced non-renewal of the JSP contract, the Committee adopted a retention bonus program in December 2018. NEOs were eligible to receive cash payments equal to 100% of their Fiscal 2019 base salary if they remained employed and performed duties and responsibilities in a satisfactory manner through December 1, 2019, or if they were terminated other than for Cause (as defined in employment agreements) during the one-year retention period. Our current NEOs earned retention incentives in December 2019 upon completing the service requirements and Mr. Galvan, our former Vice President Finance & CFO, received a pro-rated portion of his incentive following his termination without Cause on August 30, 2019 per his Separation Agreement. Retention incentive awards are reported in the “Bonus” column of the Summary Compensation Table for Fiscal 2020.
72
Our Commitment to Sound Corporate Governance
In order to align our executive compensation program with long-term shareholder interests, we have adopted a variety of sound corporate governance practices, as illustrated in the following table:
What We Do |
| What We Don’t Do |
· Emphasize variable incentives to align pay with performance | | · Provide multi-year pay guarantees within employment agreements |
· Tie incentive compensation to multiple performance metrics that reinforce key business objectives | | · Allow stock option repricing without shareholder approval |
· Place primary emphasis on equity compensation to align executive and shareholder interests | | · Permit stock hedging or pledging activities |
· Use stock ownership guidelines for executive officers and non-employee directors | | · Provide uncapped incentive awards |
· Maintain a clawback policy allowing for the recoupment of excess compensation to executive officers in the event of a material financial restatement and fraud or misconduct | | · Pay tax gross-ups on any awards |
· Engage an independent compensation consultant to advise the Compensation Committee | | · Provide excessive executive perquisites |
Executive Officer Stock Ownership Guidelines
To further encourage alignment with shareholder interests, the Board has established stock ownership and retention requirements for executive officers. Within five years of first being subject to guidelines in their current role, each executive officer is required to achieve and maintain ownership levels, based on a multiple of base salary, as noted in the following table.
Position | | Base Salary Multiple Ownership Requirement |
CEO | | 3.0X (300%) annual base salary |
All Other Executive Officers | | 1.5X (150%) annual base salary |
Until guidelines are met, executive officers must retain 50% of net after-tax shares received from equity grants, including net after-tax shares received from stock option exercise or vesting of restricted stock and performance shares, until they are in compliance. If guidelines are not met within the five year compliance period, the holding requirement increases to 100% of net after-tax shares from equity grants until achieved. Shares owned outright by executive officers or their spouse, as well as shares held in retirement plans and unvested time-based restricted stock count towards ownership requirements. Unearned performance shares and outstanding stock options do not count towards ownership. Non-employee directors are also subject to stock ownership and holding requirements, as described in the “Compensation of Directors” section of this proxy.
Overview of the Executive Compensation Program
Our Philosophy
A fundamental objective of our Executive Compensation Program is to focus our executives on creating long-term shareholder value — all aspects of our program are rooted in this goal and designed around the following guiding principles:
| ● | Pay for performance: A significant portion of compensation should be variable and directly linked to corporate and individual performance goals and results. |
| ● | Competitiveness: Compensation should be sufficiently competitive to attract, motivate and retain an executive team fully capable of driving exceptional performance. |
73
| ● | Alignment: The interests of executives should be aligned with those of our shareholders through equity-based compensation and performance measures that help to drive shareholder value over the long term. |
To support these guiding principles, our program includes the following compensation elements:
Pay Element |
| Form |
| Purpose |
Base Salary | | Cash | | Provides a competitive level of compensation that reflects position responsibilities, strategic importance of the position and individual experience. |
Short-Term Incentives (Annual Bonus) | | Cash | | Provides a cash-based award that recognizes the achievement of corporate goals in support of the annual business plan, as well as specific, qualitative and quantitative individual goals for the most recently completed fiscal year. |
Long-Term Incentives | | Equity | | Provides incentives for management to execute on financial and strategic goals that drive long-term shareholder value creation and support the Company’s retention strategy. |
Target Compensation Mix
The charts below show that most of our NEO’s target compensation for Fiscal 2020 is variable (82% for our CEO and an average of 69% for our other current NEOs). Variable pay includes the target value of short-term cash incentives (“STI”), performance shares, stock options, and restricted stock.
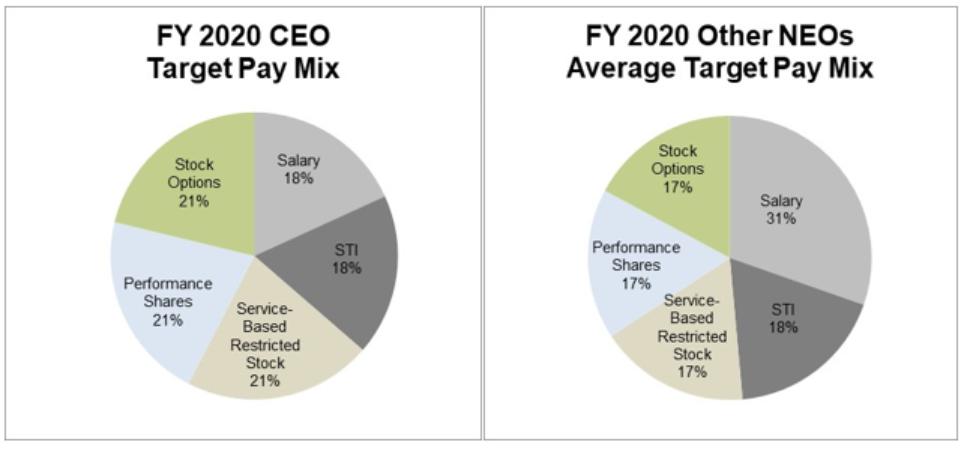
Based upon Fiscal 2020 compensation as reported in the Summary Compensation Table on page 87 of this Form 10-K, variable pay represents approximately 67% of total pay for our CEO and 52% of average total pay for our other current NEOs. This mix reflects below target annual incentives earned in Fiscal 2020 under the Annual Bonus Plan (shown as STI), the full value of one-time retention incentives earned in December 2019, target performance share grants in Fiscal 2020, and slightly above-target stock option and restricted stock grants in Fiscal 2020 based on Fiscal 2019 Company
74
performance. Excluding one-time cash retention incentives, the weighting on variable pay increases to 80% for the CEO and averages 68% for other current NEOs.
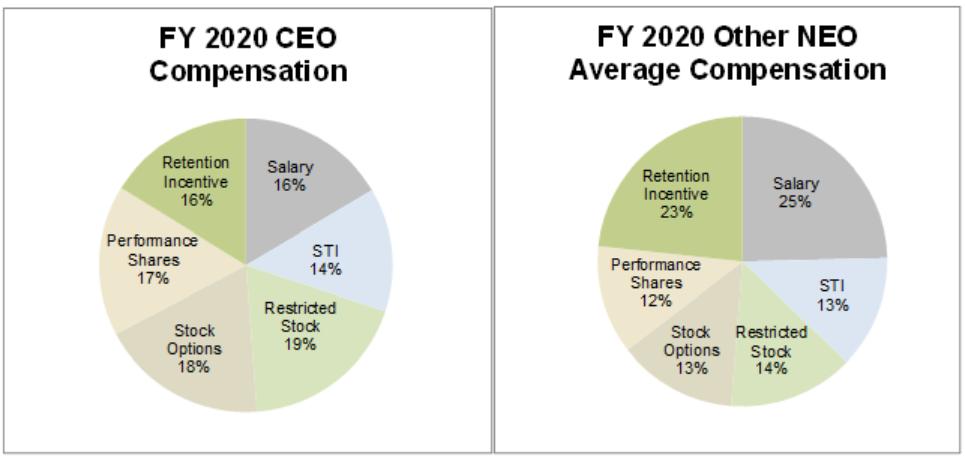
How Compensation Decisions Are Made
| ● | The Role of the Compensation Committee. The Committee, composed entirely of independent directors, is responsible for making executive compensation decisions for the NEOs. The Committee works closely with its independent compensation consultant, Pearl Meyer & Partners (“Pearl Meyer”), and management to examine pay and performance matters throughout the year. The Committee’s charter, which sets out its objectives and responsibilities, can be found at our website at www.lannett.com under the “Investors” section. |
The Committee has authority and responsibility to establish and periodically review our Executive Compensation Program and compensation philosophy. Importantly, the Committee also has the sole responsibility for approving the corporate performance goals upon which compensation for the CEO is based, evaluating the CEO’s performance and determining and approving the CEO’s compensation, including equity-based compensation, based on the achievement of his goals. The Committee also reviews and approves compensation levels for other NEOs, taking into consideration recommendations from the CEO.
In making its determinations, the Committee considers market data and advice from Pearl Meyer, as well as budgets, reports, performance assessments and other information provided by management. It also considers other factors, such as the experience, skill sets, and contributions of each NEO towards our overall success. However, the Committee is ultimately responsible for all compensation-related decisions for the NEOs and may exercise its own business judgment when evaluating performance results and making compensation decisions.
Timing of Committee Meetings and Grants; Option and Share Pricing
The Committee meets as necessary to fulfill its responsibilities, and the timing of these meetings is established during the year. The Committee holds special meetings from time to time as its workload requires. Annual equity grants occur after finalizing fiscal year end performance results, typically within the July/August time frame. Individual grants (for example, associated with the timing of a new NEO or promotion to an NEO position) and special recognition awards may occur at any time of year. The exercise price of each stock option and fair value of restricted stock awarded to our NEOs is the closing price of our common stock on the date of grant.
75
| ● | The Role of the CEO. The CEO does not play any role in the Committee’s determination of his own compensation. However, he presents the Committee with recommendations for each element of compensation including base salaries and short- and long-term incentive awards for the other NEOs, as well as non-executive employees who are eligible for equity grants. The CEO bases these recommendations upon his assessment of each individual’s performance, as well as market practice. The Committee has full discretion to modify the recommendations of the CEO in the course of its approvals. |
| ● | The Role of the Independent Consultant. The Committee consults, as needed, with an outside compensation consulting firm. As it makes decisions about executive compensation, the Committee reviews data and advice from its consultant about current compensation practices and trends among publicly-traded companies in general and comparable generic pharmaceutical companies in particular. The Committee also periodically reviews recommendations from its outside consultant and makes recommendations to the Board about the compensation for non-employee directors. |
In Fiscal 2019, Pearl Meyer was retained by the Committee, as its independent consultant, to review the competitiveness of the Executive Compensation Program. Pearl Meyer provided the Committee with compensation data with respect to similarly sized biopharmaceutical and life sciences companies and consulted with the Committee about a variety of issues related to competitive compensation practices and incentive plan designs. Pearl Meyer was also retained by the Committee in Fiscal 2020 to review the competitiveness of the Executive Compensation Program and to provide ongoing advice relating to the Executive Compensation Program. The Committee assessed the independence of Pearl Meyer pursuant to the SEC rules and concluded that no conflict of interest exists that would prevent Pearl Meyer from independently advising the Committee.
76
Peer Group & Benchmarking
The Committee evaluates industry-specific and general market compensation practices and trends to ensure the Executive Compensation Program is appropriately competitive. When making decisions about the program for Fiscal 2020, the Committee considered publicly-available data, as well as a market study conducted by Pearl Meyer in April 2019. The Pearl Meyer study developed market values using a blend of peer group proxy pay data for the companies shown below as well as published survey data for the broader life sciences industry. Using this information, the Committee compared our program to the compensation practices of other companies which the Committee believes are comparable to the Company in terms of size, scope and business complexity (the “peer group”). As shown below, the Company ranked in the upper half of the peer group in terms of employee headcount, operating income and net sales and between the 25th and 50th percentiles for enterprise value.
| | | | Enterprise | | Fiscal Year | | Fiscal | | | | | | |
| |||
| | Fiscal Year | | Value | | End Operating | | Year End | | Cumulative | | Cumulative | | Cumulative |
| |||
| | End # of | | 6/30/2020 | | Income | | Sales | | 1 YR TSR | | 3 YR TSR | | 5 YR TSR |
| |||
Company Name |
| Employees |
| ($mm) |
| ($mm) |
| ($mm) |
| 6/30/2020 |
| 6/30/2020 |
| 6/30/2020 |
| |||
Acorda Therapeutics, Inc. |
| 334 | | $ | 203 | | $ | (117) | | $ | 192 |
| (90.4) | % | (96.3) | % | (97.8) | % |
Akorn, Inc. |
| 2,227 | | $ | 843 | | $ | (61) | | $ | 682 |
| (94.6) | % | (99.2) | % | (99.4) | % |
Amneal Pharmaceuticals, Inc. |
| 5,500 | | $ | 3,719 | | $ | 19 | | $ | 1,626 |
| (33.6) | % | — | % | — | % |
Amphastar Pharmaceuticals, Inc. |
| 2,027 | | $ | 1,083 | | $ | — | | $ | 322 |
| 6.4 | % | 25.8 | % | 27.8 | % |
ANI Pharmaceuticals, Inc. | | 338 | | $ | 592 | | $ | 16 | | $ | 207 |
| (60.7) | % | (30.9) | % | (47.9) | % |
Assertio Therapeutics, Inc. |
| 125 | | $ | 24 | | $ | (4) | | $ | 230 |
| (75.2) | % | (92.0) | % | (96.0) | % |
Cambrex Corporation |
| 1,732 | | $ | — | | $ | 11 | | $ | 531 |
| — | % | — | % | — | % |
Catalent, Inc. | | 12,300 | | $ | 15,216 | | $ | 314 | | $ | 2,518 |
| 35.2 | % | 108.8 | % | 149.9 | % |
Momenta Pharmaceuticals, Inc. |
| 118 | | $ | 3,408 | | $ | (312) | | $ | 24 |
| 167.2 | % | 96.9 | % | 45.9 | % |
Prestige Consumer Healthcare Inc. |
| 520 | | $ | 3,564 | | $ | 300 | | $ | 963 |
| 18.6 | % | (28.9) | % | (18.8) | % |
Supernus Pharmaceuticals, Inc. |
| 464 | | $ | 1,230 | | $ | 149 | | $ | 393 |
| (28.2) | % | (44.9) | % | 39.9 | % |
United Therapeutics Corporation |
| 920 | | $ | 4,581 | | $ | (178) | | $ | 1,449 |
| 55.0 | % | (6.7) | % | (30.4) | % |
Lannett Company, Inc. |
| 954 | | $ | 889 | | $ | 20 | | $ | 546 |
| 19.8 | % | (64.4) | % | (87.8) | % |
Percentile Rank |
| 58 | % |
| 36 | % |
| 67 | % |
| 58 | % | 73 | % | 30 | % | 30 | % |
Subsequent to the 2019 study, Akorn, Inc. filed for bankruptcy and Cambrex Corporation was acquired. For purposes of a subsequent market pay analysis conducted by Pearl Meyer in May 2020, the Committee approved a revised peer group excluding Akorn, Inc. and Cambrex Corporation and including the 10 remaining companies from the 2019 peer group as shown above. The revised peer group aligns with us in terms of company size and industry focus.
The Committee uses external market data as a reference point to ensure the Company’s executive compensation program is sufficiently competitive to attract, retain, and motivate highly experienced and talented NEOs. The Committee generally seeks to position target total direct compensation for NEOs at or near 50th percentile market values for comparable positions but does not utilize a purely formulaic benchmarking approach. Based on the April 2019 Pearl Meyer study, target total direct compensation, including the sum of base salary plus target short-term and long-term incentives, was within the competitive range (defined as +/- 15%) of 50th percentile market values for all then-current NEOs other than Mr. Kozlowski, who was above the range based on market values for his then-current role (and below the range vs. his now-current CFO role), and equal to 102% of the 50th percentile in the aggregate. Actual total direct compensation was well-below 50th percentile market values for all then-current executive officers participating in the Fiscal 2019 short-term and long-term incentive programs and equal to 61% of the 50th percentile in the aggregate, reflecting below-target incentive awards based on actual vs. planned performance. As previously noted, when evaluating our executive compensation program, the Committee considers a variety of other factors in addition to
77
external market data, such as Company and individual performance, and each NEO’s qualifications, skill sets, and past and expected future contributions towards our success.
2020 Executive Compensation Program Decisions
Base Salary
We attribute much of our success to our highly-experienced executive management team, and the strength of their leadership has been clearly demonstrated by our exceptional long-term performance results and growth. In order to remain competitive among our industry peers, the Committee believes it should set compensation at market-competitive levels that reflect the executive’s experience, role and responsibilities. Based on Pearl Meyer’s 2019 study, current salaries were below 50th percentile market values for 3 of our 5 current NEOs and within a competitive range (+/- 10%) of the 50th percentile for all incumbents. The Committee approved merit increases equal to 2% of base salary for Mr. Crew and 3% base salary for all of our other current NEOs for Fiscal 2020, plus an additional 15% market adjustment effective August 5, 2019 for Mr. Kozlowski upon his promotion of the VP, Finance and Chief Financial Officer role. The following table summarizes annualized salaries for Fiscal 2019 and 2020 for our NEOs. Annualized salaries differ from actual values received as reported in the Summary Compensation Table for certain incumbents with less than a full year of service or promotions.
NEO |
| 2019 Base Salary |
| 2020 Base Salary | | % Change | |||
Timothy C. Crew | | $ | 735,000 | | $ | 750,000 | | 2 | % |
John Kozlowski | | $ | 325,000 | | $ | 385,000 | | 18 | % |
Maureen Cavanaugh | | $ | 425,000 | | $ | 438,000 | | 3 | % |
Samuel H. Israel | | $ | 400,000 | | $ | 412,000 | | 3 | % |
John Abt | | $ | 344,500 | | $ | 354,500 | | 3 | % |
Short-Term Incentives (Annual Bonus)
The Company’s NEOs participate in an annual bonus program, which is designed to reinforce the annual business plan and budgeted goals and to recognize yearly performance achievements focused primarily on financial and operating results. Actual payouts can range from 0% (below threshold) to 200% (superior performance) of target awards and are paid in cash. The Committee sets each NEO’s threshold, target and superior bonus opportunity as a percentage of base salary, as follows:
| | Annual Bonus Opportunity As a % of Salary |
| ||||
| | Threshold | | Target | | Superior |
|
NEO |
| (25% of Target) |
| (100% of Target) |
| (200% of Target) |
|
Timothy C. Crew |
| 25 | % | 100 | % | 200 | % |
All Other NEOs |
| 15 | % | 60 | % | 120 | % |
Expressed as percentages of salary, Fiscal 2020 award opportunities were the same as those established in Fiscal 2019 for all NEOs who were employed during both years.
78
The overall annual bonus plan for Fiscal 2020 was comprised of two components:
| ● | Corporate Financial & Operational Goals: 70% of the total target award opportunity is tied to operating results versus targets established by the Committee to promote a focus on Company-wide profitable growth and collaboration: |
Performance Metric |
| Weighting (out of 100%) | |
Adjusted Operating Income | | 30 | % |
Adjusted Earnings Per Share (“EPS”) | | 20 | % |
Net Sales | | 20 | % |
Strategic Objectives | | 20 | % |
Individual Objectives | | 10 | % |
Fiscal 2020 performance metrics and weightings were similar to those established in Fiscal 2019, , except that weightings were decreased for the Adjusted Operating Income component (from 40% to 30% of the total target mix) and increased for the strategic objectives component (from 10% to 20% of the total target mix). These changes were made to allow for a more holistic assessment of performance and increased emphasis on key strategic objectives.
Adjusted Operating Income and Adjusted EPS are defined as GAAP Operating Income and diluted EPS, respectively, excluding bonus and stock-based compensation expense, as further adjusted for certain non-recurring items.
| ● | Strategic / Individual Objectives: 30% of the total target award opportunity is based on the achievement of pre-established quantitative and qualitative strategic and individual goals, to reinforce key strategic objectives and to promote individual accountability and “line of sight.” For Fiscal 2020, the strategic objectives component for all NEOs was tied to various refinancing objectives. The individual objectives component for each NEO is tied to various other strategic, financial and operational objectives, taking into consideration each NEO’s job function and responsibilities. For competitive harm reasons, the Company does not disclose specific details on individual goals and other strategic objectives. |
2020 Short-Term Incentives (Annual Bonus): Results and Payouts
| ● | Corporate Financial & Operational Results (Collectively Weighted 70% of Total Target Award). Fiscal 2020 Target goals for Adjusted Operating Income, Adjusted EPS, and Net Sales were set below Fiscal 2019 actual levels based on our 2020 internal budgets which accounted for the non-renewal of the former JSP agreement and anticipated continued challenging market conditions within the generic pharmaceuticals sector. The Committee viewed the Fiscal 2020 performance hurdles as very challenging in light of then-current internal forecasts and industry and economic conditions. The Committee established Threshold performance hurdles at 85% of Target goals and Superior hurdles at 120% of Target to account for stretch goals, challenging market conditions, and to align more closely with our historical performance range spreads. Fiscal 2020 financial performance goals and actual results are shown in the following table: |
| | Weighting | | Performance Goals | ||||||||||
Performance Metric |
| (Out of 70%) |
| Threshold |
| Target |
| Superior |
| Actual | ||||
Adjusted Operating Income ($ millions) |
| 30 | % | $ | 117.2 | | $ | 137.9 | | $ | 165.4 | | $ | 125.5 |
Adjusted EPS |
| 20 | % | $ | 1.26 | | $ | 1.49 | | $ | 1.78 | | $ | 1.41 |
Net Sales ($ millions) |
| 20 | % | $ | 457.4 | | $ | 538.1 | | $ | 645.7 | | $ | 545.7 |
Actual Fiscal 2020 performance results were slightly above the Target goal level for Net Sales and between Threshold and Target levels for both profitability metrics. Actual Adjusted Operating Income for Fiscal 2020 excluded pre-tax items totaling approximately $105.9 million, including restructuring expenses, impairments, and other non-recurring items. Actual Adjusted EPS excluded the same $105.9 million in pre-tax items plus $16.5 million primarily related to
79
non-cash interest expense and a loss on extinguishment of debt as well as the related tax effects for all of these items. For Fiscal 2020, the Net Sales result was the same as the GAAP-reported value, with no adjustments applied.
| ● | Strategic and Individual Performance Results (Collectively Weighted 30% of Total Target Award). For Fiscal 2020, the strategic objectives component was primarily tied to various refinancing goals, which were met at the Target level. The Committee also considered each NEO’s contributions towards a variety of other company-wide strategic and function-specific objectives, including 18 new product launches, matching the prior year’s record setting level. While no specific weightings were assigned to these other objectives, the Committee considered each NEO’s contributions towards the Company’s response to the COVID-19 pandemic, ongoing success with restructuring activities, the continued strengthening of our balance sheet, maintaining operational discipline within a challenging market environment, and achievement of various other strategic growth milestones. Based on the Committee’s overall assessment, each NEO met or exceeded all goals for the strategic objectives and individual performance components. |
Total Annual Bonus
Based on our Fiscal 2020 performance results, calculated award funding levels were equal to approximately 83% of target levels for each NEO other than Mr. Israel, who earned a maximum payout for his individual performance component and whose actual total award was equal to 93% of target. In evaluating these results, the Committee chose to not apply any discretion to calculated performance outcomes and award funding levels. Total Fiscal 2020 payouts for current NEOs are summarized in the following table:
| | Corporate Financial / | | Strategic / Individual | | Total Actual Bonus for | |||
Current NEO |
| Operational Component |
| Objectives Component |
| Fiscal 2020 | |||
Timothy C. Crew | | $ | 394,390 | | $ | 225,000 | | $ | 619,390 |
John Kozlowski | | $ | 119,768 | | $ | 68,328 | | $ | 188,096 |
Maureen Cavanaugh | | $ | 138,194 | | $ | 78,840 | | $ | 217,034 |
Samuel H. Israel | | $ | 129,991 | | $ | 98,880 | | $ | 228,871 |
John Abt | | $ | 111,849 | | $ | 63,810 | | $ | 175,659 |
Long-Term Incentives
NEOs participate in a performance-based long-term incentive program. Target award opportunities, expressed as percentages of base salary, for Fiscal 2020 are summarized in the following table:
NEO |
| Target Award as % of Base Salary |
|
Timothy C. Crew |
| 350 | % |
John Kozlowski |
| 175 | % |
Maureen Cavanaugh |
| 175 | % |
Samuel H. Israel |
| 175 | % |
John Abt |
| 150 | % |
80
The target value mix for our NEOs in Fiscal 2020 is summarized below:
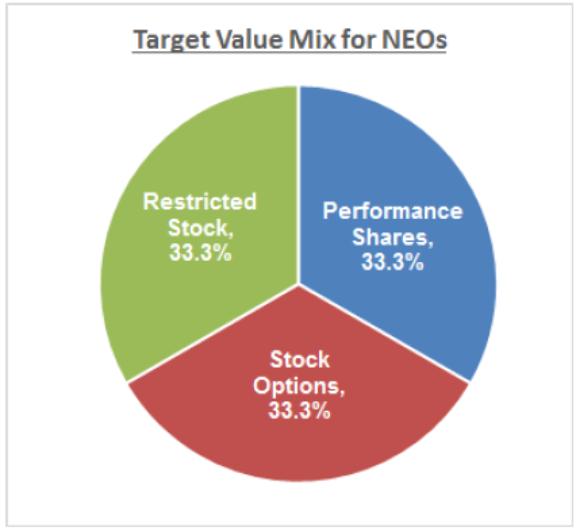
All equity grants made in Fiscal 2020 were tied to performance. For the stock option and restricted stock components, grant levels were tied to Company and individual performance, using the same metrics and weightings as under the Annual Bonus Plan. Actual grants could range from 0% (for below Threshold results) to 150% (for Superior performance) of target award levels, as shown in the following table:
| | Percentage of Target Equity Grants Earned |
Performance Result |
| (as % of Target Grant) |
Below Threshold |
| 0% (subject to Committee discretion) |
Threshold |
| 50% |
Target |
| 100% |
Superior |
| 150% |
For equity grants in Fiscal 2020 or prior years, any earned stock option and restricted stock grants were made following the end of the fiscal year in which performance was measured. These grants typically occurred in the first quarter of the next fiscal year.
For the performance share component, award opportunities can range from 0% to 200% of target levels, based on our three-year TSR relative to companies in the S&P Pharmaceuticals Select Industry Index, as follows:
Lannett Three-Year Relative TSR vs. S&P | | Percentage of Target Grant |
|
Pharmaceuticals Select Index |
| Earned |
|
Below 40th Percentile |
| — | |
40th Percentile |
| 50 | % |
50th Percentile |
| 100 | % |
80th Percentile or Higher |
| 200 | % |
Beginning in Fiscal 2021, all equity grants will be made at target levels, to align more closely with market practice, provide for more consistent and predictable awards, and further enhance retention. Grants will continue to occur during
81
the first quarter of each fiscal year, with stock options and restricted stock tied to continued service over the applicable vesting period and performance shares tied to 3-year relative TSR vs. comparator companies..
Grants Made in Fiscal 2020 (Based on Fiscal 2019 Performance)
In Fiscal 2019, the Company achieved financial performance results between Target and Superior levels for Net Sales, Adjusted Operating Income, and Adjusted EPS. Based on Company financial and strategic / individual objective performance results, the Committee approved the following stock option and restricted stock grants, effective as of July 29, 2019:
| | Equity Grants Earned Based on Fiscal 2019 Performance | ||
NEO |
| # of Stock Options |
| # of Restricted Shares |
Timothy C. Crew |
| 208,070 |
| 129,590 |
John Kozlowski |
| 46,000 |
| 28,650 |
Maureen Cavanaugh |
| 68,600 |
| 42,720 |
Samuel H. Israel |
| 64,570 |
| 40,210 |
John Abt |
| 32,510 |
| 25,250 |
These stock options vest in four equal annual increments, beginning on the first anniversary of the grant date and expire on the tenth anniversary from the date of grant. Each stock option has an exercise price of $6.57, equal to our closing stock price on the date of grant. Restricted stock granted in Fiscal 2020 also vests in four equal annual increments, beginning on the first anniversary of grant.
Our NEOs also received the following TSR performance share grants:
NEO |
| Target Number of Performance Shares Granted |
Timothy C. Crew |
| 70,390 |
John Kozlowski |
| 15,560 |
Maureen Cavanaugh |
| 23,740 |
Samuel H. Israel |
| 22,350 |
John Abt |
| 11,000 |
Grants were made on July 29, 2019 and were determined by dividing target award values by the grant date fair value of $10.71 per share, based on a Monte-Carlo binomial modeling valuation tool, as discussed in Note 14 “Share-based Compensation” of our Consolidated Financial Statements. Award vesting will be based on the Company’s TSR relative to companies in the S&P Pharmaceuticals Index for the three-year period ending July 29, 2022, with no awards earned for below-Threshold results and maximum awards of up to 200% of target grants for Superior performance. For this grant, any earned performance shares are payable after one additional year of continued service beyond the end of the three-year performance cycle.
Target Equity Grants Made in Fiscal 2021
Beginning in Fiscal 2021, all equity grants are made at target award levels. For Fiscal 2021 grants, the Committee approved a target value mx equal to 35% for performance shares, 20% for stock options, and 45% for service-based restricted shares . The Committee approved the following performance share, stock option and restricted stock target grants, effective as of July 31, 2020
82
| | Target Equity Grants | ||||||
NEO |
| # of Performance Shares |
| # of Stock Options | | # of Restricted Shares | ||
Timothy C. Crew |
| 158,679 |
| 144,628 | | 204,016 | ||
John Kozlowski |
| 40,728 |
| 37,121 | | 52,364 | ||
Maureen Cavanaugh |
| 46,334 |
| 42,231 | | 59,573 | ||
Samuel H. Israel |
| 43,584 |
| 39,725 | | 56,036 | ||
John Abt |
| 32,144 |
| 29,298 | | 41,328 | ||
Retention Bonus Program
To address retention concerns and to further encourage NEOs to focus on achieving strategic objectives following the announced non-renewal of the JSP contract, the Committee adopted a retention bonus program in December 2018. NEOs were eligible to receive cash payments equal to 100% of their Fiscal 2019 base salary if they remained employed and performed duties and responsibilities in a satisfactory manner through December 1, 2019, or if they were terminated other than for Cause (as defined in employment agreements) during the one-year retention period. All current NEOs earned cash retention incentives in December 2019. Mr. Galvan, our former Vice President, Finance and Chief Financial Officer who retired from the Company on August 30, 2019, received a pro-rata payout of $311,250 per the terms of his Separation Agreement. Retention incentives are reported in the “Bonus” column of the Summary Compensation Table for Fiscal 2020 in this proxy.
Compensation Recoupment (Clawback) Policy for Executive Officers
In early Fiscal 2021, our Board of Directors approved an expanded compensation recovery or “clawback” provision that will be incorporated into all executive officer employment contracts. Under the revised contracts, if the Company is required to issue a material financial restatement as a result of fraud or other misconduct, the Board may, in its discretion, seek to recoup any excess performance-based short-term or long-term incentive compensation awarded during the three-year period following the originally filed financial statement(s). The recoupment provision applies to any executive officer who is found to have participated in or knew or should have known about such fraud or misconduct and took no action to prevent it. In determining the amount of any excess performance-based incentives, the Board will compare the award received based on the original financial statement(s) against the amount that would have been earned based on the restated financial results. Prior to this new policy, the Company maintained a clawback policy under the Sarbanes-Oxley Act, with incentive awards for the CEO and CFO subject to recoupment in the event of a material financial restatement triggered by fraud or misconduct. Additionally, any employee who violates the provisions of the Company’s Code of Business Conduct and Ethics is subject to disciplinary penalties that may include termination of employment. The Committee intends to comply with any regulatory requirements pertaining to clawback provisions under the Dodd-Frank Act once rules are finalized by the SEC and New York Stock Exchange.
Other Policies, Programs and Guidelines
NEOs, like all other employees, have retirement programs and other benefits as part of their overall compensation package. The Committee believes that these programs and benefits support our compensation philosophy, part of which is to provide compensation that is sufficiently competitive to attract, motivate and retain an executive team fully capable of driving exceptional performance. The Committee periodically reviews these programs to validate that they are reasonable and consistent with market practice. Attributed costs of the personal benefits available to the NEOs are included in column (h) of the Summary Compensation Table on page 87.
| ● | Retirement Benefits. Each of our NEOs is eligible to participate in a 401(k) plan that is available to all employees. The Company provides matching contributions on a $0.50 basis up to 8% of the contributing employee’s base salary, subject to limitations of the 401(k) plan and applicable law. |
| ● | Other Benefits. Our NEOs are eligible to participate in the same health benefits available to all other employees. They also participate in a wellness program which provides a comprehensive annual physical examination and up to $1,000 in optional preventive health screening benefits. Lannett provides life insurance for NEOs which would, in the event of death, pay up to $250,000 to designated beneficiaries. Premiums paid for coverage above $50,000 are |
83
| treated as imputed income. Lannett also provides short- and long-term disability insurance which would, in the event of disability, pay the NEO 100% of his base salary up to the plan limits of $10,000 per week for short-term disability and $15,000 per month for long-term disability. The NEOs are also provided with car allowances. |
| ● | Post-Termination Pay. The Committee believes that reasonable severance and change-in-control benefits are necessary in order to recruit and retain qualified senior executives and are generally required by the competitive recruiting environment within our industry and the marketplace in general. These severance benefits reflect the fact that it may be difficult for our NEOs to find comparable employment within a short period of time and are designed to alleviate concerns about the loss of his or her position without cause. The Committee also believes that a change-in-control arrangement will provide security that will likely reduce the reluctance of an NEO to pursue a change in control transaction that could be in the best interest of our shareholders. Lannett’s severance plan is designed to pay severance benefits to a NEO for a qualifying separation. For the CEO, the severance plan provides for payment of three times base salary, plus a pro-rated annual cash bonus for the current year calculated as if all targets and goals are achieved. For the other NEOs, the severance plan provides for a payment of 18-months of base salary, plus a pro-rated annual cash bonus for the current year calculated as if all targets and goals are achieved. Employment agreements with NEOs do not have any tax gross-up provisions, and include non-compete, non-solicitation, and other restrictive covenants for designated time frames. As previously noted, Mr. Crew’s employment agreement was amended during Fiscal 2018 to eliminate a “walk away” provision that would have entitled him to severance benefits upon a voluntary resignation within thirty days of a Change in Control of the Company. This change was made based on the 2018 say on pay vote and concerns raised by shareholder advisory groups and further demonstrates our commitment to sound corporate governance practices. None of the agreements with our other NEOs contain any type of “walk away” provision, with severance benefits only payable upon a qualifying termination of employment by the Company without “Cause” (as defined in the agreements) or a voluntary resignation for “Good Reason” (as defined in the agreements). |
| ● | Tax and Accounting Implications. Section 162(m) of the Internal Revenue Code of 1986, as amended, precludes the deductibility of an NEO’s compensation that exceeds $1,000,000 per year. The Tax Cuts and Jobs Act, which became effective as of January 1, 2018, modified Section 162(m) provisions, including the elimination of the “performance-based exception” that previously allowed certain performance-based compensation meeting specific requirements to qualify for full tax deductibility by the Company. The changes to Section 162(m) do not apply to certain compensation paid pursuant to a binding written contract that was in effect as of November 2, 2017. As a result of the tax law changes, compensation paid to designated “covered executives”, including current and former NEOs, in excess of $1,000,000 per individual will generally not be deductible, whether or not it is performance-based. Although the Committee has historically attempted to structure executive compensation to preserve deductibility, it also reserves the right to provide compensation that may not be fully deductible, in order to maintain flexibility in compensating NEOs in a manner consistent with our compensation philosophy, as deemed appropriate. The Committee believes that shareholder interests are best served by not restricting the Committee’s discretion in this regard, even though such compensation may result in non-deductible compensation expenses to the Company. |
84
Looking Ahead: Executive Compensation Program Changes for Fiscal 2021
For Fiscal 2021, the Committee decided to increase base salaries for all NEOs, modify the short-term incentive (Annual Bonus) design, and to modify the long-term incentive plan design, as shown below.
| ● | Base Salaries. For Fiscal 2021, the Committee approved the following market adjustments to position NEO salaries at or near 50th percentile market values. Due to the COVID-19 pandemic, the effective date for all salary increases, other than a market adjustment for Mr. Kozlowski, whose salary remains below 50th percentile market values for the CFO position, was delayed from the first quarter to the second quarter of Fiscal 2021. |
NEO |
| 2020 Base Salary |
| 2021 Base Salary* |
| % Change |
| ||
Timothy C. Crew | | $ | 750,000 | | $ | 772,500 | | 3.0 | % |
John Kozlowski | | $ | 385,000 | | $ | 410,000 | | 6.5 | % |
Maureen Cavanaugh | | $ | 438,000 | | $ | 451,000 |
| 3.0 | % |
Samuel H. Israel | | $ | 412,000 | | $ | 424,400 | | 3.0 | % |
John Abt | | $ | 354,500 | | $ | 365,000 |
| 3.0 | % |
| * | Reflects full-year annualized salaries; as noted above, all increases become effective October 1, 2020 except for Mr. Kozlowski, whose increase became effective July 1, 2020 |
85
| ● | Short-Term Incentives (Annual Bonus). For Fiscal 2021, target award opportunities, expressed as percentages of base salary, are the same as in Fiscal 2020. Performance metrics and mix will be similar to Fiscal 2020. Award funding for Threshold performance will increase from 25% of Target to 50% of Target to align more closely with market practice and account for the use of challenging performance hurdles. |
|
| Weighting (out of |
|
Performance Metric | | 100%) |
|
Adjusted Operating Income |
| 30 | % |
Adjusted Earnings Per Share (“EPS”) |
| 20 | % |
Net Sales |
| 20 | % |
Strategic Objectives |
| 20 | % |
Individual Objectives |
| 10 | % |
| ● | Long-Term Incentives. Expressed as percentages of base salary, target long-term incentive award opportunities are the same as in Fiscal 2020 for all NEOs. To further enhance retention during an uncertain environment and help manage equity plan share reserves, the target value mix changed slightly from the prior year, with assigned weightings of 45% restricted stock, 35% for performance shares, and 20% for stock options. All equity grants will continue to be made at target award levels, with the majority of award opportunities “at risk”. Vesting periods for all equity grants in Fiscal 2021 were set at three years, consistent with vesting periods for grants prior to Fiscal 2020, and to align more closely with market practice. |
Stock option and restricted stock grants were made at target award levels in July 2020, vesting in three equal annual increments based on continued service.
For the performance share component, award opportunities can range from 0% to 200% of target levels, based on our three-year TSR relative to companies in the S&P Pharmaceuticals Select Industry Index, as follows:
Lannett Three-Year Relative TSR vs. S&P | | Percentage of Target Award Opportunity |
|
Pharmaceuticals Select Index |
| Earned | |
Below 40th Percentile | | — | |
40th Percentile | | 50 | % |
50th Percentile | | 100 | % |
80th Percentile or Higher | | 200 | % |
Target performance shares were granted in July 2020. Any earned shares will vest following the end of the three-year performance period.
REPORT OF THE COMPENSATION COMMITTEE
The Compensation Committee has reviewed, discussed and approved the CD&A as set forth above with management. Taking this review and discussion into account, the undersigned Committee members recommend to the Board of Directors that the CD&A be included in the annual report on Form 10-K.
Paul Taveira, Chairman
John Chapman
David Drabik
86
COMPENSATION OF EXECUTIVE OFFICERS
Overview
The tables and narratives set forth below provide specified information concerning the compensation of our Named Executive Officers (NEOs) for the fiscal year ended June 30, 2020.
Summary Compensation Table
This table summarizes all compensation paid to or earned by our Fiscal 2020 NEOs for the years indicated to the extent they were serving as NEOs.
|
| |
| | |
| | |
| | |
| | |
| Non-equity |
| | |
| | | |
| | | | | | | | | Restricted | | | | | incentive plan | | All Other | | | | ||||
Name and Principal Position | | Fiscal Year | | Salary | | Bonus | | Stock Awards | | Options Awards | | compensation | | Compensation | | Total | |||||||
(a) |
| (b) |
| (c) |
| (d) |
| (d) |
| (e) |
| (f) |
| (g) |
| (h) | |||||||
Timothy Crew (1) | | 2020 | | $ | 748,269 | | $ | 735,000 | | $ | 1,605,283 | | $ | 840,603 | | $ | 619,390 | | $ | 27,768 | | $ | 4,576,314 |
Chief Executive Officer | | 2019 | | | 735,000 | | | — | | | 483,359 | | | 141,002 | | | 735,000 | | | 40,635 | | | 2,134,996 |
| | 2018 | | | 350,539 | | | — | | | 400,016 | | | 400,003 | | | 126,252 | | | 52,971 | | | 1,329,781 |
| | | | | | | | | | | | | | | | | | | | | | | |
John Kozlowski (2) |
| 2020 | | $ | 378,077 | | $ | 325,000 | | $ | 354,878 | | $ | 185,840 | | $ | 188,096 | | $ | 35,582 | | $ | 1,467,474 |
Vice President of Finance and |
| 2019 | |
| 325,000 | |
| — | |
| 219,228 | |
| 64,894 | |
| 195,000 | |
| 47,199 | |
| 851,320 |
Chief Financial Officer |
| 2018 | |
| 325,000 | |
| — | |
| 171,624 | |
| — | |
| 67,921 | |
| 31,769 | |
| 596,314 |
| | | | | | | | | | | | | | | | | | | | | | | |
Maureen Cavanaugh (3) |
| 2020 | | $ | 436,500 | | $ | 425,000 | | $ | 534,926 | | $ | 277,144 | | $ | 217,034 | | $ | 25,206 | | $ | 1,915,810 |
Senior VP and Chief Commercial |
| 2019 | |
| 425,000 | |
| — | |
| — | |
| — | |
| 255,000 | |
| 31,799 | |
| 711,799 |
Operations Officer |
| 2018 | |
| 57,212 | |
| — | |
| 368,753 | |
| — | |
| — | |
| 52,554 | |
| 478,518 |
| | | | | | | | | | | | | | | | | | | | | | | |
Samuel Israel (4) |
| 2020 | | $ | 410,616 | | $ | 400,000 | | $ | 503,548 | | $ | 260,863 | | $ | 228,871 | | $ | 24,896 | | $ | 1,828,794 |
Chief Legal Officer and |
| 2019 | |
| 400,000 | |
| — | |
| 300,969 | |
| 89,096 | |
| 240,000 | |
| 27,122 | |
| 1,057,186 |
General Counsel |
| 2018 | |
| 376,923 | |
| — | |
| 585,010 | |
| 24,997 | |
| 79,931 | |
| 16,980 | |
| 1,083,841 |
| | | | | | | | | | | | | | | | | | | | | | | |
John Abt | | 2020 | | | 353,346 | | | 344,500 | | | 283,703 | | | 131,340 | | | 175,659 | | | 25,080 | | | 1,313,628 |
Vice President and Chief Quality | | 2019 | | | 344,500 | | | — | | | 144,249 | | | 42,080 | | | 206,700 | | | 24,221 | | | 761,750 |
Operations Officer | | 2018 | | | 299,539 | | | — | | | 153,505 | | | 24,997 | | | 67,817 | | | 19,155 | | | 565,012 |
| | | | | | | | | | | | | | | | | | | | | | | |
Martin P. Galvan (5) |
| 2020 | | $ | 79,808 | | $ | 311,250 | | $ | 597,073 | | $ | 309,302 | | $ | 41,500 | | $ | 428,231 | | $ | 1,767,164 |
Former Vice President of Finance and |
| 2019 | |
| 415,000 | |
| — | |
| 364,823 | |
| 104,874 | |
| 249,000 | |
| 50,477 | |
| 1,184,174 |
Chief Financial Officer |
| 2018 | |
| 415,000 | |
| — | |
| 207,505 | |
| 24,997 | |
| 86,730 | |
| 29,513 | |
| 763,745 |
| (1) | Mr. Crew joined the Company as CEO effective January 2, 2018. |
| (2) | Mr. Kozlowski became an NEO in Fiscal 2018. He was appointed to the role of Vice President of Finance and Chief Financial Officer effective August 31, 2019. |
| (3) | Ms. Cavanaugh joined the Company as Senior Vice President & Chief Commercial Operations Officer effective May 7, 2018. |
| (4) | Mr. Israel joined the Company as Chief Legal Officer and General Counsel effective July 15, 2017. |
| (5) | Mr. Galvan retired from the Company effective August 30, 2019. |
87
All Other Compensation
The following summarizes the components of column (g) of the Summary Compensation Table above:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| |
| Company |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | | |
| | | | Match | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Contributions | | Auto | | Pay in Lieu of | | Separation | | Wellness | | Excess Life | | Relocation | | Signing | | | | ||||||||
Name and Principal Position | | Fiscal Year | | 401(k) Plan | | Allowance | | Vacation | | Payments | | Benefit | | Insurance | | Reimbursement | | Bonus | | Total | |||||||||
Timothy Crew | | 2020 | | $ | 9,750 | | $ | 13,500 | | $ | — | | $ | — | | $ | 4,250 | | $ | 268 | | $ | — | | $ | — | | $ | 27,768 |
Chief Executive Officer | | 2019 | | | 5,655 | | | 13,500 | | | 16,962 | | | — | | | 4,250 | | | 268 | | | — | | | — | | | 40,635 |
| | 2018 | | | 6,463 | | | 6,439 | | | — | | | — | | | — | | | 69 | | | 40,000 | | | — | | | 52,971 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
John Kozlowski | | 2020 | | $ | 13,035 | | $ | 10,800 | | $ | 7,404 | | $ | — | | $ | 4,250 | | $ | 93 | | $ | — | | $ | — | | $ | 35,582 |
Vice President of Finance and |
| 2019 |
| | 9,556 |
| | 10,800 |
| | 22,500 |
| | — |
| | 4,250 |
| | 93 |
| | — |
| | — |
| | 47,200 |
Chief Financial Officer |
| 2018 |
| | 9,770 |
| | 7,062 |
| | 14,844 |
| | — |
| | — |
| | 93 |
| | — |
| | — |
| | 31,768 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Maureen Cavanaugh |
| 2020 | | $ | 9,760 | | $ | 10,800 | | $ | — | | $ | — | | $ | 4,250 | | $ | 396 | | $ | — | | $ | — | | $ | 25,206 |
Senior VP and Chief Commercial |
| 2019 | |
| 16,442 | |
| 10,800 | |
| — | |
| — | |
| 4,250 | |
| 307 | |
| — | |
| — | |
| 31,800 |
Operations Officer |
| 2018 | |
| 1,308 | |
| 1,246 | |
| — | |
| — | |
| — | |
| — | |
| — | |
| 50,000 | |
| 52,554 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Samuel Israel |
| 2020 | | $ | 9,588 | | $ | 10,800 | | $ | — | | $ | — | | $ | 4,250 | | $ | 258 | | $ | — | | $ | — | | $ | 24,896 |
Chief Legal Officer and |
| 2019 | |
| 8,727 | |
| 10,800 | |
| 3,077 | |
| — | |
| 4,250 | |
| 268 | |
| — | |
| — | |
| 27,122 |
General Counsel |
| 2018 | |
| 6,615 | |
| 10,177 | |
| — | |
| — | |
| — | |
| 188 | |
| — | |
| — | |
| 16,981 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
John Abt | | 2020 | | $ | 9,832 | | $ | 10,800 | | $ | — | | $ | — | | $ | 4,250 | | $ | 198 | | $ | — | | $ | — | | $ | 25,080 |
Vice President and Chief Quality | | 2019 | |
| 9,033 | |
| 10,800 | |
| — | |
| — | |
| 4,250 | |
| 138 | |
| — | |
| — | |
| 24,221 |
Operations Officer | | 2018 | |
| 8,217 | |
| 10,800 | |
| — | |
| — | |
| — | |
| 138 | |
| — | |
| — | |
| 19,155 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Martin P. Galvan |
| 2020 | | $ | 210 | | $ | 2,077 | | $ | 20,351 | | $ | 401,167 | | $ | 4,250 | | $ | 176 | | $ | — | | $ | — | | $ | 428,231 |
Former Vice President of Finance and |
| 2019 | |
| 9,896 | |
| 10,800 | |
| 24,740 | |
| — | |
| 4,250 | |
| 791 | |
| — | |
| — | |
| 50,478 |
Chief Financial Officer |
| 2018 | |
| 9,343 | |
| 10,800 | |
| 8,579 | |
| — | |
| — | |
| 791 | |
| — | |
| — | |
| 29,514 |
88
Grants of Plan-Based Awards in Fiscal 2020
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | All Other Stock | | All Other | | | | | | |
| | | | Estimated Future Payouts | | Estimated Future Payouts | | Awards: | | Option Awards: | | | | | Grant Date | |||||||||
| | | | Under Non-Equity Incentive | | Under Equity Incentive Plan | | Number of | | Number of | | Exercise or | | Fair Value of | ||||||||||
| | | | Plan Awards | | Awards | | Shares of | | Securities | | Base Price | | Stock and | ||||||||||
| | | | | | | | | | Threshold | | Target | | Maximum | | Stocks or Units | | Underlying | | of Option | | Options | ||
Name | | Grant Date | | Threshold | | Target | | Maximum | | ($) | | ($) | | ($) | | (#) (1) (2) | | Options (#) (1) | | Awards | | Awards (4) | ||
(a) |
| (b) |
| (c) |
| (d) |
| (e) |
| (f) |
| (g) |
| (h) |
| (i) |
| (j) |
| ($/sh) (3) |
| (i) | ||
Timothy Crew | |
| | 187,500 | | 750,000 | | 1,500,000 | |
| |
| |
| |
| | | | | | |
| |
Chief Executive Officer | | 7/29/2019 | | | |
| |
| | 35,195 |
| 70,390 |
| 140,780 | |
| | | | | | | $ | 753,877 |
| | 7/29/2019 | | | | | | | | | | | | | | 129,590 | | | | | | | $ | 851,406 |
| | 7/29/2019 | | | | | | | | | | | | | | | | 208,070 | | $ | 6.57 | | $ | 840,603 |
| | | | | | | | | | | | | | | | | | | | | | | | |
John Kozlowski | |
| | 56,887 | | 227,548 | | 455,096 | | |
| |
| | | | | | | | | |
| |
Vice President of Finance and | | 7/29/2019 | | | |
| |
| | 7,780 |
| 15,560 |
| 31,120 | | | | | | | | | $ | 166,648 |
Chief Financial Officer | | 7/29/2019 | | | | | | | | | | | | | | 28,650 | | | | | | | $ | 188,231 |
| | 7/29/2019 | | | | | | | | | | | | | | | | 46,000 | | $ | 6.57 | | $ | 185,840 |
| | | | | | | | | | | | | | | | | | | | | | | | |
Maureen Cavanaugh | |
| | 65,700 | | 262,800 | | 525,600 | | |
| |
| | | | | | | | | |
| |
Senior VP and Chief Commercial | | 7/29/2019 | | | |
| |
| | 11,870 |
| 23,740 |
| 47,480 | | | | | | | | | $ | 254,255 |
Operations Officer | | 7/29/2019 | | | | | | | | | | | | | | 42,720 | | | | | | | $ | 280,670 |
| | 7/29/2019 | | | | | | | | | | | | | | | | 68,600 | | $ | 6.57 | | $ | 277,144 |
| | | | | | | | | | | | | | | | | | | | | | | | |
Samuel Israel | |
| | 61,800 | | 247,200 | | 494,400 | | |
| |
| | | | | | | | | |
| |
Chief Legal Officer and | | 7/29/2019 | | | |
| |
| | 11,175 |
| 22,350 |
| 44,700 | | | | | | | | | $ | 239,369 |
General Counsel | | 7/29/2019 | | | | | | | | | | | | | | 40,210 | | | | | | | $ | 264,180 |
| | 7/29/2019 | | | | | | | | | | | | | | | | 64,570 | | $ | 6.57 | | $ | 260,863 |
| | | | | | | | | | | | | | | | | | | | | | | | |
John Abt | | | | 53,175 | | 212,700 | | 425,500 | | | | | | | | | | | | | | | | |
Vice President and Chief Quality | | 7/29/2019 | | | | | | | | 5,500 | | 11,000 | | 22,000 | | | | | | | | | | 117,810 |
Operations Officer | | 7/29/2019 | | | | | | | | | | | | | | 25,250 | | | | | | | | 165,893 |
| | 7/29/2019 | | | | | | | | | | | | | | | | 32,510 | | | 6.57 | | | 131,340 |
| | | | | | | | | | | | | | | | | | | | | | | | |
Martin P. Galvan | |
| | | |
| |
| | |
| |
| | | | | | | | | |
| |
Former Vice President of Finance and | | 7/29/2019 | | | |
| |
| | 13,250 |
| 26,500 |
| 53,000 | | | | | | | | | $ | 283,815 |
Chief Financial Officer | | 7/29/2019 | | | | | | | | | | | | | | 47,680 | | | | | | | $ | 313,258 |
| | 7/29/2019 | | | | | | | | | | | | | | | | 76,560 | | $ | 6.57 | | $ | 309,302 |
| (1) | Reflects grants made in Fiscal 2020 based on Fiscal 2019 performance. All stock option and restricted stock grants vest in four equal annual increments. |
| (2) | The exercise price was equal to the Company’s closing stock price on the date of grant. |
| (3) | Stock options were valued using the Black-Scholes option pricing model. Performance shares were valued using a Monte Carlo binomial model. The assumptions used in fair value calculations are described in Note 14 “Share-based Compensation,” in the Form 10-K. The grant date fair value for other stock grants reflects the number of shares multiplied by the Company’s closing stock price on the applicable date of grant. |
| (4) | Grants to Mr. Galvan fully vested upon his termination of employment on August 30, 2019 per the terms of his Separation Agreement. |
89
Outstanding Equity Awards at 2020 Fiscal Year End
The following table sets forth information concerning the outstanding stock awards held at June 30, 2020 by each of the NEOs. The options were granted ten years prior to the option expiration date and vest over three or four years from that grant date. Restricted shares vest over three or four years from the date of grant.
| | | | | | | | | | | | | | | | | | | | |
Option Awards | | | | | | | | | | | | | Stock Awards | |||||||
| | | | | | | | | | | | | | | | | | Equity | | Equity |
| | | | | | Equity | | | | | | | | | | | | Incentive Plan | | Incentive Plan |
| | | | | | Incentive Plan | | | | | | | | | | | | Awards: | | Awards: |
| | | | | | Awards: | | | | | | | | | | | | Number of | | Market or |
| | Number of | | Number of | | Number of | | | | | | | Number of | | | | | Unearned | | Payout Value |
| | Securities | | Securities | | Securities | | | | | | | Shares or | | Market Value | | Shares, Units | | of Unearned | |
| | Underlying | | Underlying | | Underlying | | | | | | | Units of | | of Shares or | | or Other | | Shares, Units | |
| | Unexercised | | Unexercised |
| Unexercised | | Option | | Option | | Stock That | | Units of Stock |
| Rights That |
| or Other | ||
| | Options (#) | | Options (#) | | Unearned | | Exercise | | Expiration | | Have Not | | That Have Not |
| Have Not |
| Rights That | ||
Name | | Exercisable | | Unexercisable | | Options (#) | | Price ($) | | Date | | Vested (#) | | Vested ($) | | Vested (#) |
| Have Not | ||
(a) |
| (b) |
| (c) |
| (d) |
| (e) |
| (f) |
| (g) |
| (h) |
| (i) |
| Vested ($) | ||
Timothy Crew |
| 21,402 |
| 10,701 |
| — | | $ | 23.65 |
| 1/1/2028 |
| |
| | |
|
|
|
|
Chief Executive Officer |
| 7,208 |
| 14,418 |
| — | | $ | 12.20 |
| 7/29/2028 |
| |
| | |
|
|
|
|
| | — | | 208,070 | | — | | $ | 6.57 | | 7/28/2029 | | | | | | | | | |
| | | | | | | | | | | |
| 146,786 | | $ | 1,065,666 | | 85,758 | $ | 622,603 |
| | | | | | | | | | | | | | | | | | | | |
John Kozlowski |
| 4,000 |
| — |
| — | | $ | 4.16 |
| 10/25/2022 |
| | |
| |
| |
| |
Vice President of Finance and |
| 9,334 |
| — |
| — | | $ | 13.86 |
| 9/4/2023 |
| | |
| |
| |
| |
Chief Financial Officer |
| 4,200 |
| — |
| — | | $ | 34.77 |
| 8/11/2024 | | | | | | | | | |
|
| 3,317 |
| 6,636 |
| — | | $ | 12.20 |
| 7/29/2028 | | | | | | | | | |
|
| — |
| 46,000 |
| — | | $ | 6.57 |
| 7/28/2029 | | | | | | | | | |
|
| |
| |
| | | | |
| | | 36,585 | | $ | 265,607 | | 22,450 | $ | 162,987 |
| | | | | | | | | | | | | | | | | | | | |
Maureen Cavanaugh |
| — |
| 68,600 |
| — | | $ | 6.57 |
| 7/28/2029 |
| | |
| |
| |
| |
Senior VP and Chief Commercial |
| | | | | | | | | | |
| 50,600 | | $ | 367,356 |
| 23,740 | $ | 172,352 |
Operations Officer | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Samuel Israel |
| 1,839 |
| 920 |
| — | | $ | 17.40 |
| 9/21/2027 |
| | |
| |
| |
| |
Chief Legal Officer and |
| 4,555 |
| 9,110 |
| — | | $ | 12.20 |
| 7/29/2028 |
| | |
| |
| |
| |
General Counsel |
| — |
| 64,570 |
| — | | $ | 6.57 |
| 7/28/2029 | | | | | | | | | |
| | | | | | | | | | | |
| 53,588 | | $ | 389,049 | | 38,650 | $ | 280,599 |
| | | | | | | | | | | | | | | | | | | | |
John Abt | | 1,970 | | — | | — | | $ | 59.20 | | 7/21/2025 | | | | | | | | | |
Vice President and Chief Quality | | 1,155 | | — | | — | | $ | 31.30 | | 7/26/2026 | | | | | | | | | |
Operations Officer | | 1,839 | | 920 | | — | | $ | 17.40 | | 9/21/2027 | | | | | | | | | |
| | 2,151 | | 4,303 | | — | | $ | 12.20 | | 7/29/2028 | | | | | | | | | |
| | — | | 32,510 | | — | | $ | 6.57 | | 7/28/2029 | | | | | | | | | |
| | | | | | | | | | | | | 30,431 | | $ | 220,929 | | 18,420 | $ | 133,729 |
| | | | | | | | | | | | | | | | | | | | |
Martin P. Galvan |
| 32,000 |
| — |
| — | | $ | 4.16 |
| 8/30/2020 |
| | |
| |
| |
| |
Former Vice President of Finance and | | 50,000 |
| — |
| — | | $ | 13.86 |
| 8/30/2020 | | | | | | | | | |
Chief Financial Officer |
| 30,000 |
| — |
| — | | $ | 34.77 |
| 8/30/2020 | | | | | | | | | |
| | 8,990 | | — | | — | | $ | 59.20 | | 8/30/2020 | | | | | | | | | |
| | 1,769 | | — | | — | | $ | 31.30 | | 8/30/2020 | | | | | | | | | |
| | 2,759 | | — | | — | | $ | 17.40 | | 8/30/2020 | | | | | | | | | |
| | 16,085 | | — | | — | | $ | 12.20 | | 8/30/2020 | | | | | | | | | |
|
| 76,560 |
| — |
| — | | $ | 6.57 |
| 8/30/2020 | | | | | | | | | |
| | | | | | | | | | | |
| — | | $ | — | | — | $ | — |
90
Options Exercised and Stock Vested During the Fiscal Year Ended June 30, 2020
The following table sets forth information concerning stock options exercised and stock awards that vested during Fiscal 2020 for each of the NEOs.
| | Options | | Stock Awards | ||||||
|
| Number of Shares |
| Value |
| Number of |
| Value | ||
Name and Principal Position | | Acquired | | Realized | | Shares Acquired | | Realized | ||
(a) | | On Exercise | | on Exercise | | on Vesting | | on Vesting | ||
Timothy Crew |
| — | | $ | — |
| 11,416 | | $ | 89,550 |
Chief Executive Officer |
|
| |
|
|
| | |
| |
| | | | | | | | | | |
John Kozlowski |
| — | | $ | — |
| 7,884 | | $ | 67,918 |
Vice President of Finance and Chief Financial Officer |
|
| |
|
|
| | |
| |
| | | | | | | | | | |
Maureen Cavanaugh |
| — | | $ | — |
| 7,879 | | $ | 59,723 |
Senior VP and Chief Commercial Operations Officer |
|
| |
|
|
| | |
| |
| | | | | | | | | | |
Samuel Israel |
| — | | $ | — |
| 9,725 | | $ | 60,895 |
Chief Legal Officer and General Counsel |
|
| |
|
|
| | |
| |
| | | | | | | | | | |
John Abt |
| — | | $ | — |
| 4,699 | | $ | 38,823 |
Vice President and Chief Quality Operations Officer |
|
| |
|
|
| | |
| |
| | | | | | | | | | |
Martin P. Galvan |
| 40,000 | | $ | — |
| 108,348 | | $ | 1,099,701 |
Former Vice President of Finance and Chief Financial Officer |
|
| |
|
|
| | |
| |
Employment and Separation Agreements
The Company has entered into employment agreements with its current NEOs. Each of the agreements provides for an annual base salary and eligibility to receive a bonus. The salary and bonus amounts of these executives are determined by the review and approval of the Compensation Committee in accordance with the Committee’s charter as approved by the Board of Directors. Additionally, these executives are eligible to receive stock options and restricted stock awards. In 2018, the Company amended each of the employment agreements it has entered into with its current NEOs and with other employees to confirm and clarify that nothing in the employment agreements prohibits or limits the right of any employee from providing confidential information to or otherwise communicating with the SEC or any other governmental entity or self-regulatory organization or from accepting financial awards from the SEC or any other governmental entity or self-regulatory organization. Under the terms of the employment agreements, these executive employees may be terminated at any time with or without cause, or by reason of death or disability. In certain termination situations, the Company is liable to pay these executives severance compensation as discussed in the table below.
Effective August 30, 2019, the Company entered into a Separation Agreement and General Release with Mr. Galvan, our former Vice President of Finance and Chief Financial Officer, upon his termination of employment. The agreement provided for separation payments totaling $622,500, equal to eighteen months of Mr. Galvan’s final base salary plus a pro rata target cash bonus for Fiscal 2020 of $41,500, payable in twelve monthly installments, beginning on November 28, 2019. Mr. Galvan also received a pro rata cash retention bonus of $311,250, paid in December 2019. The agreement also provided for full vesting of all unvested stock options and all other equity awards, plus health benefits continuation (via reimbursement of COBRA premiums) for up to eighteen months from the date of termination. Mr. Galvan agreed to release the Company from any claims and to cooperate in the resolution of any issues pertaining to filings, investigations, or claims relating to events that occurred during his tenure with the Company. He also agreed to various restrictive covenants during the eighteen-month period following his termination of employment.
91
Potential Payments upon Termination or Change in Control
The following table summarizes potential payments or benefits upon various termination of employment scenarios for our current NEOs as of fiscal year end and assumes that the relevant triggering event occurred on June 30, 2020. The fair market values of share-based compensation (i.e. Stock Options and Restricted Stock) were calculated using the closing price of Lannett Company, Inc. stock ($7.26) on June 30, 2020, which was the last trading day of Fiscal 2020. The “spread” or difference between the fair market value of Lannett Company’s stock on June 30, 2020, and the option exercise price, was used for valuing stock options.
| | | | | | | | | | | | | | | | | | | | | |
|
| | |
| | |
| Acceleration and |
| | |
| | |
| | |
| | | |
| | | | | | | | Exercisability | | Acceleration | | | | | | | | | | ||
| | Base | | Annual | | Of Unvested | | Of Unvested | | Insurance | | | | | | | |||||
| | Salary | | Cash | | Stock Option | | Restricted | | Benefit | | Other | | | | ||||||
Name | | Continuation | | Bonus | | Awards | | Stock | | Continuation | | Benefits | | Total | |||||||
Timothy Crew | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
Without Cause/With Good Reason (1) (2) | | $ | 2,250,000 | | $ | 619,390 | | $ | 143,568 | | $ | 1,688,269 | | $ | 26,827 | | $ | 4,008 | | $ | 4,732,062 |
| | | | | | | | | | | | | | | | | | | | | |
For Cause (3) (4) | | $ | — | | $ | 619,390 | | $ | — | | $ | — | | $ | — | | $ | 4,008 | | $ | 623,398 |
| | | | | | | | | | | | | | | | | | | | | |
Retirement / Death / Disability (3) | | $ | — | | $ | 619,390 | | $ | — | | $ | — | | $ | — | | $ | 4,008 | | $ | 623,398 |
| | | | | | | | | | | | | | | | | | | | | |
Change in Control (5) | | $ | 2,250,000 | | $ | 619,390 | | $ | 143,568 | | $ | 1,688,269 | | $ | 26,827 | | $ | 4,008 | | $ | 4,732,062 |
| | | | | | | | | | | | | | | | | | | | | |
John Kozlowski | |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
Without Cause/With Good Reason (1) (2) | | $ | 577,500 | | $ | 188,096 | | $ | 31,740 | | $ | 428,594 | | $ | 34,542 | | $ | 6,004 | | $ | 1,266,476 |
| | | | | | | | | | | | | | | | | | | | | |
For Cause (3) (4) | | $ | — | | $ | 188,096 | | $ | — | | $ | — | | $ | — | | $ | 6,004 | | $ | 194,100 |
| | | | | | | | | | | | | | | | | | | | | |
Retirement / Death / Disability (3) | | $ | — | | $ | 188,096 | | $ | — | | $ | — | | $ | — | | $ | 6,004 | | $ | 194,100 |
| | | | | | | | | | | | | | | | | | | | | |
Change in Control (5) | | $ | 577,500 | | $ | 188,096 | | $ | 31,740 | | $ | 428,594 | | $ | 34,542 | | $ | 6,004 | | $ | 1,266,476 |
| | | | | | | | | | | | | | | | | | | | | |
Maureen Cavanaugh | |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
Without Cause/With Good Reason (1) (2) | | $ | 657,000 | | $ | 217,034 | | $ | 47,334 | | $ | 539,708 | | $ | 26,827 | | $ | 3,104 | | $ | 1,491,007 |
| | | | | | | | | | | | | | | | | | | | | |
For Cause (3) (4) | | $ | — | | $ | 217,034 | | $ | — | | $ | — | | $ | — | | $ | 3,104 | | $ | 220,138 |
| | | | | | | | | | | | | | | | | | | | | |
Retirement / Death / Disability (3) | | $ | — | | $ | 217,034 | | $ | — | | $ | — | | $ | — | | $ | 3,104 | | $ | 220,138 |
| | | | | | | | | | | | | | | | | | | | | |
Change in Control (5) | | $ | 657,000 | | $ | 217,034 | | $ | 47,334 | | $ | 539,708 | | $ | 26,827 | | $ | 3,104 | | $ | 1,491,007 |
| | | | | | | | | | | | | | | | | | | | | |
Samuel Israel | |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
Without Cause/With Good Reason (1) (2) | | $ | 618,000 | | $ | 228,871 | | $ | 44,553 | | $ | 669,648 | | $ | 4,507 | | $ | 3,092 | | $ | 1,568,671 |
| | | | | | | | | | | | | | | | | | | | | |
For Cause (3) (4) | | $ | — | | $ | 228,871 | | $ | — | | $ | — | | $ | — | | $ | 3,092 | | $ | 231,963 |
| | | | | | | | | | | | | | | | | | | | | |
Retirement / Death / Disability (3) | | $ | — | | $ | 228,871 | | $ | — | | $ | — | | $ | — | | $ | 3,092 | | $ | 231,963 |
| | | | | | | | | | | | | | | | | | | | | |
Change in Control (5) | | $ | 618,000 | | $ | 228,871 | | $ | 44,553 | | $ | 669,648 | | $ | 4,507 | | $ | 3,092 | | $ | 1,568,671 |
| | | | | | | | | | | | | | | | | | | | | |
John Abt | |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
Without Cause/With Good Reason (1) (2) | | $ | 531,750 | | $ | 175,659 | | $ | 22,432 | | $ | 354,658 | | $ | 50,223 | | $ | 4,508 | | $ | 1,139,230 |
| | | | | | | | | | | | | | | | | | | | | |
For Cause (3) (4) | | $ | — | | $ | 175,659 | | $ | — | | $ | — | | $ | — | | $ | 4,508 | | $ | 180,167 |
| | | | | | | | | | | | | | | | | | | | | |
Retirement / Death / Disability (3) | | $ | — | | $ | 175,659 | | $ | — | | $ | — | | $ | — | | $ | 4,508 | | $ | 180,167 |
| | | | | | | | | | | | | | | | | | | | | |
Change in Control (5) | | $ | 531,750 | | $ | 175,659 | | $ | 22,432 | | $ | 354,658 | | $ | 50,223 | | $ | 4,508 | | $ | 1,139,230 |
92
| (1) | Each employment agreement ranges from 1-3 years and is automatically renewed unless notice is given by either party. Any non-renewal of the existing employment agreements by the Company and any resignation of the Executive with Good Reason both constitute a termination without Cause. Under the existing employment agreements base salary continuation for a period of 18-36 months, pro-rated cash bonus as if all targets and goals were achieved subject to any applicable cap on cash payments, acceleration of exercisability of unvested stock option awards, acceleration of unvested restricted stock, and insurance benefit continuation for a period of 18 months (collectively “Severance Compensation”) will only be made if the Executive executes and delivers to the Company, in a form prepared by the Company, a release of all claims against the Company and other appropriate parties, excluding the Company’s performance obligation to pay Severance Compensation and the Executive’s vested rights under the Company sponsored retirement plans, 401(k) plans and stock ownership plans (“General Release”). Severance Compensation is paid in equal monthly installments over a 12 month period to commence on the 90th day following the Termination Date provided the Executive has not revoked the General Release prior to that date. Earned but unpaid base salary, accrued but unpaid annual bonus (if the Executive otherwise meets the eligibility requirements) and accrued but unpaid paid time off and other miscellaneous items are to be paid in a single lump sum in cash no later than the earlier of: (1) the date required under applicable law; or (2) 60 days following the Termination Date. |
| (2) | Under the existing employment agreements, Good Reason is defined as giving written notice of his resignation within thirty (30) days after Executive has actual knowledge of the occurrence, without the written consent of Executive, of one of the following events: (A) the assignment to Executive of duties materially and adversely inconsistent with Executive’s position or a material and adverse alteration in the nature of his duties, responsibilities and/or reporting obligations, (B) a reduction in Executive’s Base Salary or a failure to pay any such amounts when due; or (C) the relocation of Company headquarters more than 100 miles from its current location. |
| (3) | Under the existing employment agreements, if the Executive is terminated For Cause; by death; by disability; resigns without Good Reason; or retires; earned but unpaid base salary, accrued but unpaid annual bonus (if the Executive otherwise meets the eligibility requirements) and accrued but unpaid paid time off and other miscellaneous items are to be paid in a single lump sum in cash no later than the earlier of: (1) the date required under applicable law; or (2) 60 days following the Termination Date. |
| (4) | For Cause generally means Executive’s willful commission of an act constituting fraud, embezzlement, breach of fiduciary duty, material dishonesty with respect to the Company, gross negligence or willful misconduct in performance of Executive duties, willful violation of any law, rule or regulation relating to the operation of the Company, abuse of illegal drugs or other controlled substances or habitual intoxication, willful violation of published business conduct guidelines, code of ethics, conflict of interest or other similar policies, and Executive becoming under investigation by or subject to any disciplinary charges by any regulatory agency having jurisdiction over the Company (including but not limited to the Drug Enforcement Administration (DEA), Food and Drug Administration (FDA) or the Securities and Exchange Commission (SEC)) or if any complaint is filed against the Executive by any such regulatory agency. |
| (5) | Under the existing employment agreements, a Change in Control is defined as a “change in ownership of the Company”, “a change in effective control of the Company”, or “a change in ownership of a substantial portion of the Company’s assets.” If the Executive is terminated by the Company without Cause or resigns with Good Reason within 24 months of a Change in Control event, the Executive shall be entitled to earned but unpaid base salary, accrued but unpaid annual bonus (if the Executive otherwise meets the eligibility requirements) and accrued but unpaid paid time off and other miscellaneous items. These items are to be paid in a single lump sum in cash no later than the earlier of: (1) the date required under applicable law; or (2) 60 days following the Termination Date. Additionally, the Executive shall be entitled to Severance Compensation to be paid in equal monthly installments over a 12 month period to commence on the 90th day following the Termination Date provided the Executive has not revoked the General Release prior to that date. A written notice that the Executive’s employment term is not extended within the 24-month period after a Change in Control shall be deemed a termination without Cause, unless the Executive and the Company execute a new employment agreement. |
93
CEO Pay Ratio Disclosure
As required by the Dodd-Frank Wall Street Reform and Consumer Protection Act and the regulations of the SEC, we are providing the following information about the annual total compensation of our employees and the annual total compensation of our current CEO, Timothy Crew. For the year ended June 30, 2020, Mr. Crew’s total compensation, as reported in the Summary Compensation Table of this proxy, was $4,576,313 and total compensation for our median employee, as calculated in accordance with the requirements of Regulation S-K, was $59,289, resulting in a ratio of 77.2 to 1. This pay ratio information has been calculated in a manner consistent with SEC regulations.
For purposes of determining the median employee for the fiscal year ending June 30, 2020, we determined that as of May 31, 2020, our employee population consisted of 951 individuals working at our company and its consolidated subsidiaries. For each of the 950 U.S.-based employees (other than Mr. Crew), we used their annualized base salary and target cash and equity incentive awards as of May 31, 2020 as a consistently applied compensation measure to identify the median employee. We used target cash and equity incentives since actual awards for Fiscal 2020 for each employee were not yet determined. We annualized values for employees hired after July 1, 2019, the start of our fiscal year.
Because the SEC rules permit significant flexibility in terms of approaches used to calculate compensation and identify the median employee, comparisons among companies may not be very meaningful, even for companies within the same industry.
94
COMPENSATION OF DIRECTORS
Our Board of Directors is actively involved in providing strategic direction and fiduciary oversight to the Company. During Fiscal 2020, we had a total of eight Board members (seven at fiscal year-end), which resulted in a significant workload for our directors, with our five independent directors serving on an average of three committees each. Our Board of Directors held numerous meetings and teleconferences in Fiscal 2020 in carrying out its responsibilities. The Board is actively involved in transactional due diligence, management succession planning, on-going reviews of business development activities and strategic initiatives to position the Company for future growth. The Board has also been actively involved in addressing the COVID-19 pandemic.
For Fiscal 2020, our non-employee directors received a cash retainer of $90,000, unchanged from Fiscal 2019, payable in monthly increments of $7,500, for Board and committee service. Mr. LePore also received an additional retainer of $30,000 for serving as our Independent Non-Employee Board Chairman, and Mr. Drabik received an additional retainer of $24,000 for his central role and work on the special committees and for continued, board leadership work. Certain directors also received an extra $1,000 per meeting for attending special meetings during the year. No other cash retainers or meeting fees were provided during Fiscal 2020. As an executive director, Mr. Crew does not participate in the non-employee director compensation program.
Board members receive annual equity grants to recognize their service during the prior fiscal year. Grant levels may vary from year to year based on Company performance. Based on the Company’s performance and the significant efforts and contributions of our directors in Fiscal 2019, in July 2019, each then-current non-employee Board member, other than Dr. Rewolinski, received an award of 33,445 common shares with a grant date value of $200,001, immediately vested at grant. Dr. Rewolinski received a grant of 5,785 common shares upon joining the Board on July 1, 2019. These grants are shown in the table below, since they occurred in Fiscal 2020. Beginning with equity awards in Fiscal 2021, the Board moved the annual grant date from July to September 1, which is not during a “blackout” period, to allow directors to immediately sell shares to fund tax liabilities.
Effective in July 2014, the Board of Directors approved stock ownership guidelines for non-employee directors equal to three times their cash retainer. Non-employee directors must meet required ownership levels within five years of first becoming subject to the guidelines and must hold 50% of all net after-tax shares from equity grants until ownership requirements are met (or 100% of such shares if ownership levels are not met by the end of the five-year compliance period). All directors other than Dr. Rewolinski, who joined the board in Fiscal 2020, met required ownership levels as of the end of Fiscal 2020.
We maintain policies that prohibit Directors from pledging Lannett stock or engaging in activity considered hedging of our common stock, and none of our Directors has pledged Lannett stock as collateral for a personal loan or other obligations.
95
The following table shows compensation information for Fiscal 2020 for non-employee members of our Board of Directors.
DIRECTOR COMPENSATION
|
| | |
| | |
| |
| |
| Change in |
| |
| | |
| | | | | | | | | | | | Pension Value | | | | | |
| | | | | | | | | | Non-Equity | | and Nonqualified | | | | | |
| | | | | | | | Options | | Incentive Plan | | Deferred | | All Other | | | |
Name | | Fees Earned | | Stock Awards | | Awards | | Compensation | | Compensation | | Compensation | | Total | |||
(a) | | (b) | | (c) | | (d) | | (e) | | (f) | | (g) | | (h) | |||
Jeffrey Farber | | $ | 90,000 | | $ | 200,001 | | — | | — | | — | | — | | $ | 290,001 |
| | | | | | | | | | | | | | | | | |
David Drabik | | $ | 120,000 | | $ | 200,001 |
| — |
| — |
| — |
| — | | $ | 320,001 |
| | | | | | | | | | | | | | | | | |
Paul Taveira | | $ | 96,000 | | $ | 200,001 |
| — |
| — |
| — |
| — | | $ | 296,001 |
| | | | | | | | | | | | | | | | | |
Albert Paonessa III | | $ | 52,500 | | $ | 200,001 |
| — |
| — |
| — |
| — | | $ | 252,501 |
| | | | | | | | | | | | | | | | | |
Patrick LePore | | $ | 120,000 | | $ | 200,001 |
| — |
| — |
| — |
| — | | $ | 320,001 |
| | | | | | | | | | | | | | | | | |
John Chapman | | $ | 96,000 | | $ | 200,001 |
| — |
| — |
| — |
| — | | $ | 296,001 |
| | | | | | | | | | | | | | | | | |
Melissa Rewolinski | | $ | 90,000 | | $ | 34,999 |
| — |
| — |
| — |
| — | | $ | 124,999 |
| (1) | Reflects grant date award value for equity grants received in Fiscal 2020 to recognize Board service in Fiscal 2019. Dr. Rewolinski received a grant of 5,785 common shares upon joining the Board on July 1, 2019. |
| (2) | Mr. Paonessa retired from the Board in January 2020. |
| (3) | Dr. Rewolinski joined the Board in July 2019. |
96
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth, as of July 31, 2020, information regarding the security ownership of the directors and certain executive officers of the Company and persons known to the Company to be beneficial owners of more than five (5%) percent of the Company’s common stock. Although grants of restricted stock under the Company’s 2011 and 2014 Long Term Incentive Plans (“LTIPs”) generally vest equally over time from the grant date, the restricted shares are included below because the voting rights with respect to such restricted stock are acquired immediately upon grant.
Name and Address of | | | |
| | | | | | | |
| | |
|
Beneficial Owner / | |
| | Excluding Options (*) | | Including Options (**) |
| ||||||||
Director / Executive |
|
|
| Shares Held |
| Shares Held |
| Total |
| Percent of |
| Number of |
| Percent of |
|
Officer | | Office | | Directly | | Indirectly | | Shares | | Class | | Shares | | Class |
|
| | | | | | | | | | | | | | |
|
John M. Abt | | | | | | | | | | | | | | | |
9000 State Road | | VP and Chief Quality | | | | | | | | | | | | | |
Philadelphia, PA 19136 | | and Operations Officer | | 87,012 | | — | | 87,012 | | 0.22 | % | 105,325 | | 0.26 | % |
| | | | | | | | | | | | | | | |
Maureen Cavanaugh |
| Senior VP & Chief |
| | | | | | | | | | | | |
9000 State Road | | Commercial Operations | | | | | | | | | | | | | |
Philadelphia, PA 19136 | | Officer | | 118,120 | | — | | 118,120 | | 0.29 | % | 135,270 | | 0.33 | % |
| | | | | | | | | | | | | | | |
John Chapman |
| | | | | | | | | | | | | | |
9000 State Road | | | | | | | | | | | | | | | |
Philadelphia, PA 19136 | | Director |
| 46,019 | | — | | 46,019 | | 0.11 | % | 46,019 | | 0.11 | % |
| | | | | | | | | | | | | | | |
Timothy Crew |
| | | | | | | | | | | | | | |
9000 State Road | | | | | | | | | | | | | | | |
Philadelphia, PA 19136 | | Chief Executive Officer |
| 396,232 | | — | | 396,232 | | 0.99 | % | 484,068 | | 1.20 | % |
| | | | | | | | | | | | | | | |
David Drabik |
| | | | | | | | | | | | | | |
9000 State Road | | | | | | | | | | | | | | | |
Philadelphia, PA 19136 | | Director |
| 45,972 | | — | | 45,972 | | 0.11 | % | 45,972 | | 0.11 | % |
| | | | | | | | | | | | | | | |
Robert Ehlinger |
| |
| | | | | | | | | | | | |
9000 State Road | | VP and Chief | | | | | | | | | | | | | |
Philadelphia, PA 19136 | | Information Officer | | 70,191 | | — | | 70,191 | | 0.17 | % | 112,054 | | 0.28 | % |
| | | | | | | | | | | | | | | |
Jeffrey Farber |
| | | | | | | | | | | | | | |
9000 State Road | | | | | | | | | | | | | | | |
Philadelphia, PA 19136 | | Director |
| 2,343,614 | | 2,552,140 | | 4,895,754 | | 12.17 | % | 4,895,754 | | 12.10 | % |
| | | | | | | | | | | | | | | |
David Farber |
|
|
| | | | | | | | | | | | |
9000 State Road | | | | | | | | | | | | | | | |
Philadelphia, PA 19136 | | | | 1,885,870 | | 1,772,787 | | 3,658,657 | | 9.10 | % | 3,658,657 | | 9.04 | % |
| | | | | | | | | | | | | | | |
Samuel H. Israel |
| | | | | | | | | | | | | | |
9000 State Road | | | | | | | | | | | | | | | |
Philadelphia, PA 19136 | | Chief Legal Officer and General Counsel |
| 120,888 | | — | | 120,888 | | 0.30 | % | 148,899 | | 0.37 | % |
| | | | | | | | | | | | | | | |
John Kozlowski |
| VP of Finance, | | | | | | | | | | | | | |
9000 State Road | | Chief Financial Officer and | | | | | | | | | | | | | |
Philadelphia, PA 19136 | | Principal Accounting Officer |
| 100,025 | | — | | 100,025 | | 0.25 | % | 135,694 | | 0.34 | % |
| | | | | | | | | | | | | | | |
Patrick G. Lepore |
| | | | | | | | | | | | | | |
9000 State Road | | | | | | | | | | | | | | | |
Philadelphia, PA 19136 | | Chairman of the Board, Director |
| 201,340 | | — | | 201,340 | | 0.50 | % | 201,340 | | 0.50 | % |
| | | | | | | | | | | | | | | |
Melissa Rewolinski |
| | | | | | | | | | | | | | |
9000 State Road | | | | | | | | | | | | | | | |
Philadelphia, PA 19136 | | Director |
| 5,785 | | — | | 5,785 | | 0.01 | % | 5,785 | | 0.01 | % |
| | | | | | | | | | | | | | | |
Paul Taveira |
| | | | | | | | | | | | | | |
9000 State Road | | | | | | | | | | | | | | | |
Philadelphia, PA 19136 | | Director |
| 51,415 | | — | | 51,415 | | 0.13 | % | 51,415 | | 0.13 | % |
| | | | | | | | | | | | | | | |
All directors and executive officers as a group |
|
|
| | | | | | | | | | | | |
(12 persons) | | | | 3,586,613 | | 2,552,140 | | 6,138,753 | | 15.25 | % | 6,367,595 | | 15.74 | % |
| (1) | Includes 22,394 unvested shares received pursuant to restricted stock awards granted in April 2018, July 2018 and July 2019. |
| (2) | Includes 1,970 vested options to purchase common stock at an exercise price of $59.20 per share, 1,155 vested options to purchase common stock at an exercise price of $31.30 per share, 2,759 vested options to purchase common stock at an exercise price of $17.40 per share, 4,304 vested options to purchase common stock at an exercise price of $12.20 per share, and 8,127 vested options to purchase common stock at an exercise price of $6.57 per share. |
97
| (3) | Includes 39,920 unvested shares received pursuant to restricted stock awards granted in May 2018 and July 2019. |
| (4) | Includes 17,150 vested options to purchase common stock at an exercise price of $6.57 per share. |
| (5) | Includes 108,610 unvested shares received pursuant to restricted stock awards granted in January 2018, July 2018 and July 2019. |
| (6) | Includes 21,402 vested options to purchase common stock at an exercise price of $23.65 per share, 14,417 vested options to purchase common stock at an exercise price of $12.20 per share, and 51,017 vested options to purchase common stock at an exercise price of $6.57 per share. |
| (7) | Includes 13,588 unvested shares received pursuant to restricted stock awards granted in July 2018 and July 2019. |
| (8) | Includes 11,667 vested options to purchase common stock at an exercise price of $13.86 per share, 10,000 vested options to purchase common stock at an exercise price of $34.77 per share, 6,300 vested options to purchase common stock at an exercise price of $59.20 per share, 968 vested options to purchase common stock at an exercise price of $31.30 per share, 2,759 vested options to purchase common stock at an exercise price of $17.40 per share 3,712 vested options to purchase common stock at an exercise price of $12.20 per share, and 6,457 vested options to purchase common stock at an exercise price of $6.57 per share. |
| (9) | Includes 994,412 shares held by the Jeffrey Farber Family Foundation which is managed by Jeffrey Farber. Jeffrey Farber disclaims beneficial ownership of these shares. Includes 30,000 shares held by the Jeffrey and Jennifer Farber Family Foundation which is managed by Jeffrey Farber. Jeffrey Farber disclaims beneficial ownership of these shares. Includes 528,122 shares held by Farber Family LLC (“FFLLC”) which is managed by Jeffrey and David Farber. David Farber and Jeffrey Farber each disclaim beneficial ownership of these shares. Includes 73,408 shares held by Jeffrey Farber as custodian for his children, 17,279 shares held as joint custodian with David Farber for a relative, and also includes 38,000 shares held by Farber Investment Company (“FIC”). Jeffrey Farber and David Farber each beneficially own 25% of FIC and each disclaim beneficial ownership of all but 9,500 shares held by FIC. Includes 870,919 shares held by a Grantor Retained Annuity Trust, in which Jeffrey Farber is the trustee. |
| (10) | Includes 864,443 shares held by the David and Nancy Family Foundation and 524,760 shares held by the David and Nancy Philanthropic foundation. David Farber disclaims beneficial ownership of these shares. Includes 528,122 shares held by FFLLC which is managed by Jeffrey and David Farber. David Farber and Jeffrey Farber each disclaim beneficial ownership of these shares. Includes 180,145 shares held by David Farber as joint custodian with his children, 148,160 shares held as trustee for his children and 17,279 shares held as joint custodian with Jeffrey Farber for a relative. David Farber disclaims beneficial ownership of these shares. Also includes 38,000 shares held by FIC. Jeffrey Farber and David Farber each beneficially own 25% of FIC and each disclaim beneficial ownership of all but 9,500 shares held by FIC. |
| (11) | Includes 33,810 unvested shares received pursuant to restricted stock awards granted in July 2017, July 2018 and July 2019. |
| (12) | Includes 2,759 vested options to purchase common stock at an exercise price of $17.40 per share, 9,110 vested options to purchase common stock at an exercise price of $12.20 per share, and16,142 vested options to purchase common stock at an exercise price of $6.57 per share. |
| (13) | Includes 26,763 unvested shares received pursuant to restricted stock awards granted in September 2017, October 2017, July 2018 and July 2019. |
| (14) | Includes 4,000 vested options to purchase common stock at an exercise price of $4.16 per share, 9,334 vested options to purchase common stock at an exercise price of $13.86 per share, 4,200 vested options to purchase common stock at an exercise price of $34.77 per share, 6,635 vested options to purchase common stock at an exercise price of $12.20 per share, and 11,500 vested options to purchase common stock at an exercise price of $6.57 per share. |
98
* Percent of class calculation is based on 40,220,659 outstanding shares of common stock at July 31, 2020.
** Assumes that all options exercisable within sixty days after July 31, 2020 have been exercised.
The following table sets forth, as of July 31, 2020, information regarding the names and addresses of the shareholders known to the Company to be beneficial owners of more than five (5%) percent of the Company’s common stock.
|
| Number of |
| Percent of |
|
Name and Address of Beneficial Owner | | Shares | | Class |
|
| | | | | |
BlackRock, Inc. |
| | | | |
55 East 52nd Street | | | | | |
New York, NY 10055 | | 5,227,912 | (1) | 13.0 | % |
| | | | | |
D.E. Shaw & Co., L.P. | | | | | |
1166 avenue of the Americas, 9th Floor, 6th Ave | | | | | |
New York, NY 10036 | | 3,412,246 | (2) | 8.50 | % |
| | | | | |
JP Morgan Chase & Co. |
| | | | |
383 Madison Avenue | | | | | |
New York, NY 10179 | | 2,765,513 | (3) | 6.80 | % |
| | | | | |
The Vanguard Group |
| | | | |
100 Vanguard Blvd. | | | | | |
Malvern, PA 19355 | | 2,360,972 | (4) | 5.85 | % |
| | | | | |
LSV Asset Management |
| | | | |
155 N Wacker Dr, Suite 4600 | | | | | |
Chicago, IL 60606 | | 2,099,152 | (5) | 5.21 | % |
| | | | | |
Highbridge Capital Management, LLC |
| | | | |
277 Park Avenue, 23rd Floor | | | | | |
New York, New York 10172 | | 2,128,841 | (6) | 5.01 | % |
| (1) | Based on Schedule 13G/A filed by Blackrock, Inc. with the SEC on February 4, 2020, Blackrock, Inc. has sole voting power over 5,172,055 shares, shared voting power over 0 shares, sole dispositive power over 5,227,912 shares and shared dispositive power over 0 shares. |
| (2) | Based on Schedule 13G/A filed by D.E. Shaw & Co., L.P. with the SEC on February 14, 2020, D.E. Shaw & Co., L.P. has sole voting power over 0 shares, shared voting power over 3,320,872 shares, sole dispositive power over 0 shares and shared dispositive power over 3,412,246 shares. |
| (3) | Based on a Schedule 13G filed by JP Morgan Chase & Co. with the SEC on January 24, 2020, JP Morgan Chase & Co. has sole voting power over 2,492,538 shares, shared voting power over 0 shares, sole dispositive power over 2,735,913 shares and shared dispositive power over 0 shares. |
| (4) | Based on a Schedule 13G filed by The Vanguard Group with the SEC on February 11, 2020, The Vanguard Group has sole voting power over 34,000 shares, shared voting power over 3,946 shares, sole dispositive power over 2,330,532 shares and shared dispositive power over 30,440 shares. |
| (5) | Based on Schedule 13G filed by LSV Asset Management with the SEC on February 11, 2020, LSV Asset Management has sole voting power over 1,309,713 shares, shared voting power over 0 shares, sole dispositive power over 2,099,152 shares and shared dispositive power over 0 shares. |
99
| (6) | Based on Schedule 13G filed by Highbridge Capital Management, LLC with the SEC on July 20, 2020, Highbridge Capital Management, LLC has sole voting power over 0 shares, shared voting power over 2,128,841 shares, sole dispositive power over 0 shares and shared dispositive power over 2,128,841 shares. |
Equity Compensation Plan Information
The following table summarizes the equity compensation plans as of June 30, 2020:
|
| |
| | |
| Number of securities |
| | | | Weighted average | | remaining available for | |
| | Number of securities to | | exercise price of | | future issuance under | |
|
| be issued upon exercise |
| outstanding |
| equity compensation plans | |
|
| of outstanding options, |
| options, warrants |
| (excluding securities | |
(In thousands, except for weighted average exercise price) | | warrants and rights |
| and rights |
| reflected in column (a)) | |
Plan Category | | (a) | | (b) |
| (c) | |
Equity Compensation plans approved by security holders |
| 991 | | $ | 12.11 |
| 1,298 |
Equity Compensation plans not approved by security holders |
| — | |
| — |
| — |
Total |
| 991 | | $ | 12.11 |
| 1,298 |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
Review and Approval of Transactions with Related Persons
The responsibility for the review of transactions with “related persons” (as defined below) has been assigned to the Audit Committee of the Board of Directors, which is comprised of three independent directors. “Related persons” are defined as directors and executive officers or their immediate family members or stockholders owning more than five percent of the Company’s common stock. The Audit Committee annually reviews related party transactions with any related person in which the amount exceeds $120,000.
The Company had net sales of $3.0 million, $3.8 million and $3.9 million during the fiscal years ended June 30, 2020, 2019 and 2018, respectively, to a generic distributor, Auburn Pharmaceutical Company (“Auburn”). Jeffrey Farber, a current board member, is the owner of Auburn, which is a member of the Premier Buying Group. Accounts receivable includes amounts due from Auburn of $0.7 million and $1.2 million at June 30, 2020 and 2019, respectively.
The Company also had net sales of $2.6 million, $2.4 million and $1.9 million during the fiscal years ended June 30, 2020, 2019 and 2018 to a generic distributor, KeySource Medical (“KeySource”), which is a member of the OptiSource Buying Group. Albert Paonessa, a board member until the date of the Company’s 2020 Annual Meeting of Stockholders, was appointed the CEO of KeySource in May 2017. Accounts receivable includes amounts due from KeySource of $0.6 million and $0.7 million as of June 30, 2020 and 2019.
As part of its review, the Audit Committee noted that the amount of net sales to Auburn approximated 0.6% of total net sales during the fiscal years ended June 30, 2020, 2019 and 2018, respectively. The Audit Committee also noted that the amount of net sales to KeySource approximated 0.5%, 0.4% and 0.3% of total net sales during each of the fiscal years ended June 30, 2020, 2019 and 2018, respectively.
The Audit Committee reviewed an analysis of sales prices charged to Auburn and KeySource, which compared the average sales prices by product for Auburn and KeySource sales to the average sales prices by product to other Lannett customers during the same period. As a result of this analysis, the Audit Committee ratified the net sales made to Auburn and KeySource during the fiscal years ended June 30, 2020, 2019 and 2018.
100
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Grant Thornton LLP served as the independent auditors of the Company during Fiscal 2020, 2019 and 2018. No relationship exists, other than the usual relationship between independent public accountant and client. The following table identifies the fees incurred for services rendered by Grant Thornton LLP in Fiscal 2020, 2019 and 2018.
(In thousands) |
| Audit Fees |
| Audit-Related (1) |
| Tax Fees (2) |
| All Other Fees (3) |
| Total Fees | |||||
| | | | | | | | | | | | | | | |
Fiscal 2020: | | $ | 1,383 | | $ | 127 | | $ | 211 | | $ | — | | $ | 1,721 |
Fiscal 2019: | | $ | 1,409 | | $ | — | | $ | 193 | | $ | 7 | | $ | 1,609 |
Fiscal 2018: | | $ | 1,586 | | $ | — | | $ | 180 | | $ | 26 | | $ | 1,792 |
| (1) | Audit-related fees primarily include fees paid for comfort letters procedures. |
| (2) | Tax fees include fees paid for preparation of annual federal, state and local income tax returns, quarterly estimated income tax payments and various tax planning services. |
| (3) | Other fees include fees paid for review of various correspondences including IRS audit assistance, miscellaneous studies, etc. |
The non-audit services provided to the Company by Grant Thornton LLP were pre-approved by the Company’s Audit Committee. Prior to engaging its auditor to perform non-audit services, the Company’s Audit Committee reviews the particular service to be provided and the fee to be paid by the Company for such service and assesses the impact of the service on the auditor’s independence.
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULE
1. Consolidated Financial Statements:
See accompanying Index to Consolidated Financial Statements.
2. Consolidated Financial Statement Schedule:
Lannett Company, Inc.
Schedule II - Valuation and Qualifying Accounts
For the years ended June 30:
| | Balance at | | Charged to | | | | | Balance at | |||
Description | | Beginning of | | (Reduction of) | | | | | End of Fiscal | |||
(In thousands) |
| Fiscal Year |
| Expense |
| Deductions |
| Year | ||||
| | | | | | | | | | | | |
Allowance for Doubtful Accounts | | | | | | | | | | | | |
2020 | | $ | 1,223 | | $ | 386 | | $ | (506) | | $ | 1,103 |
2019 | |
| 1,308 | | | 870 | | | (955) | | | 1,223 |
2018 | |
| 796 | |
| 1,560 | |
| (1,048) | |
| 1,308 |
| | | | | | | | | | | | |
Deferred Tax Asset Valuation Allowance | | | | | | | | | | | | |
2020 | | $ | 13,549 | | $ | 1,073 | | $ | — | | $ | 14,622 |
2019 | |
| 8,120 | | | 5,429 | | | — | | | 13,549 |
2018 | |
| 6,391 | |
| 1,729 | |
| — | |
| 8,120 |
101
3. Exhibits:
Those exhibits marked with a (*) refer to management contracts or compensatory plans or arrangements.
Exhibit |
| Description |
| Method of Filing |
|---|---|---|---|---|
| | | | |
2.1 | | | Incorporated by reference to Exhibit 2.1 on Form 8-K dated May 18, 2015 | |
| | | | |
2.2 | | | Incorporated by reference to Exhibit 2.2 on Form 8-K dated September 4, 2015 | |
| | | | |
2.3 | | | Incorporated by reference to Exhibit 2.3 on Form 8-K dated December 2, 2015 | |
| | | | |
3.1 | | Certificate of Incorporation | | Incorporated by reference to the Proxy Statement filed with respect to the Annual Meeting of Shareholders held on December 6, 1991 (the “1991 Proxy Statement”). |
| | | | |
3.2 | | By-Laws, as amended | | Incorporated by reference to the 1991 Proxy Statement. |
| | | | |
3.3 | | | Incorporated by reference to Exhibit 3.3 on Form 8-K dated January 16, 2014 | |
| | | | |
3.4 | | | Incorporated by reference to Exhibit 3.4 on Form 8-K dated July 17, 2014 | |
| | | | |
3.5 | | | Incorporated by reference to Exhibit 3.5 to the Annual Report on 2014 Form 10-K | |
| | | | |
3.6 | | | Incorporated by reference to Exhibit 3.6 to the Annual Report on 2014 Form 10-K | |
| | | | |
3.7 | | Amended and Restated Bylaws of Lannett Company Inc., as amended through January 21, 2015. | | Incorporated by reference to Exhibit 3.7 on Form 8-K dated April 3, 2015 |
| | | | |
3.8 | | Amended and Restated Bylaws of Lannett Company Inc., as amended through July 6, 2015. | | Incorporated by reference to Exhibit 3.8 on Form 8-K dated July 9, 2015 |
| | | | |
4 | | Specimen Certificate for Common Stock | | Incorporated by reference to Exhibit 4(a) to Form 8 dated April 23, 1993 (Amendment No. 3 to Form 10-KSB for Fiscal 1992) (“Form 8”) |
| | | | |
4.1 | | | Incorporated by reference to Exhibit 4.1 on Form 8-K dated December 2, 2015 | |
| | | | |
102
Exhibit |
| Description |
| Method of Filing |
|---|---|---|---|---|
4.2 | | | Incorporated by reference to Exhibit 4.2 on Form 8-K dated December 2, 2015 | |
| | | | |
4.3 | | | Incorporated by reference to Exhibit 4.3 on Form 8-K dated December 2, 2015 | |
| | | | |
4.4 | | | Incorporated by reference to Exhibit 4.4 on Form 10-K dated August 28, 2019 | |
| | | | |
10.1 | | Line of Credit Note dated March 11, 1999 between the Company and First Union National Bank | | Incorporated by reference to Exhibit 10(ad) to the Annual Report on 1999 Form 10-KSB |
| | | | |
10.2 | | | Incorporated by reference to Exhibit 10(ae) to the Annual Report on 1999 Form 10-KSB | |
| | | | |
10.3 | | | Incorporated by reference to Exhibit 10(af) to the Annual Report on 1999 Form 10-KSB | |
| | | | |
10.4 | | | Incorporated by reference to Exhibit 10(ag) to the Annual Report on 1999 Form 10-KSB | |
| | | | |
10.5* | | | Incorporated by reference to the Proxy Statement for Fiscal Year Ending June 30, 2002 | |
| | | | |
10.6* | | | Incorporated by reference to Exhibit 10.6 to the Annual Report on 2003 Form 10-KSB | |
| | | | |
10.7* | | | Incorporated by reference to Exhibit 10 to the Quarterly Report on Form 10-Q dated May 12, 2004. | |
| | | | |
10.9 | | Agreement between Lannett Company, Inc and Siegfried (USA), Inc. | | Incorporated by reference to Exhibit 10.9 to the Annual Report on 2003 Form 10-KSB |
| | | | |
10.10 | | Agreement between Lannett Company, Inc and Jerome Stevens Pharmaceuticals, Inc. | | Incorporated by reference to Exhibit 2.1 to Form 8-K dated May 5, 2004 |
| | | | |
10.11* | | | Incorporated by reference to Exhibit 10.11 to the Annual Report on 2009 Form 10-K | |
| | | | |
10.12 | | Agreement of Sale Between Anvil Construction Company, Inc. and Lannett Company, Inc. | | Incorporated by reference to Exhibit 10.12 to the Annual Report on 2009 Form 10-K |
| | | | |
10.13* | | | Incorporated by reference to the Proxy Statement dated January 5, 2007 | |
| | | | |
10.15* | | | Incorporated by reference to the Proxy Statement dated January 19, 2011 | |
| | | | |
103
Exhibit |
| Description |
| Method of Filing |
|---|---|---|---|---|
10.16* | | | Incorporated by reference to Exhibit 10.1 on Form 8-K dated August 11, 2011 | |
| | | | |
10.17 | | | Incorporated by reference to Exhibit 10.17 to the Annual Report on 2011 Form 10-K | |
| | | | |
10.18 | | | Incorporated by reference to Exhibit 10.18 to the Annual Report on 2011 Form 10-K | |
| | | | |
10.19* | | Second Amended and Restated Employment Agreement of Arthur P. Bedrosian | | Incorporated by reference to Exhibit 10.19 on Form 8-K dated January 3, 2013 |
| | | | |
10.20* | | Amended and Restated Employment Agreement of Martin P. Galvan | | Incorporated by reference to Exhibit 10.20 on Form 8-K dated January 3, 2013 |
| | | | |
10.21* | | Amended and Restated Employment Agreement of William F. Schreck | | Incorporated by reference to Exhibit 10.21 on Form 8-K dated January 3, 2013 |
| | | | |
10.22* | | | Incorporated by reference to Exhibit 10.22 on Form 8-K dated January 3, 2013 | |
| | | | |
10.23* | | | Incorporated by reference to Exhibit 10.23 on Form 8-K dated January 3, 2013 | |
| | | | |
10.24* | | Amended and Restated Employment Agreement of Robert Ehlinger | | Incorporated by reference to Exhibit 10.24 on Form 8-K dated January 3, 2013 |
| | | | |
10.25 | | | Incorporated by reference to Exhibit 10.25 on Form 8-K dated August 19, 2013 | |
| | | | |
10.26 | | | Incorporated by reference to Exhibit 10.26 on Form 8-K dated December 19, 2013 | |
| | | | |
10.27 | | | Incorporated by reference to Exhibit 10.27 on Form 8-K dated December 19, 2013 | |
| | | | |
10.28* | | Employment Agreement of Michael Bogda dated December 1, 2014 | | Incorporated by reference to Exhibit 10.28 on Form 8-K dated December 5, 2014 |
| | | | |
10.29 | | | Incorporated by reference to Exhibit 10.29 on Form 8-K dated April 24, 2015 | |
| | | | |
10.30* | | | Incorporated by reference to Exhibit 10.30 on Form 10-Q dated May 8, 2015 | |
| | | | |
104
Exhibit |
| Description |
| Method of Filing |
|---|---|---|---|---|
10.31* | | | Incorporated by reference to Exhibit 10.31 to the Annual Report on 2015 Form 10-K | |
| | | | |
10.32* | | | Incorporated by reference to Exhibit 10.32 to the Annual Report on 2015 Form 10-K | |
| | | | |
10.33 | | | Incorporated by reference to Exhibit 10.33 on Form 8-K dated September 4, 2015 | |
| | | | |
10.34* | | | Incorporated by reference to Exhibit 10.34 on Form 8-K dated September 15, 2015 | |
| | | | |
10.35 | | | Incorporated by reference to Exhibit 10.35 on Form 8-K dated September 25, 2015 | |
| | | | |
10.36 | | | Incorporated by reference to Exhibit 10.36 on Form 8-K dated December 2, 2015 | |
| | | | |
10.37 | | | Incorporated by reference to Exhibit 10.37 on Form 8-K dated December 2, 2015 | |
| | | | |
10.38 | | | Incorporated by reference to Exhibit 10.38 on Form 8-K dated December 2, 2015 | |
| | | | |
10.39 | | | Incorporated by reference to Exhibit 10.39 on Form 8-K dated December 2, 2015 | |
| | | | |
10.40 | | | Incorporated by reference to Exhibit 10.40 on Form 8-K dated December 2, 2015 | |
| | | | |
10.41 | | | Incorporated by reference to Exhibit 10.41 on Form 8-K dated December 2, 2015 | |
| | | | |
10.42* | | | Incorporated by reference to Exhibit 10.42 on Form 8-K dated April 12, 2016 | |
| | | | |
10.43 | | Amendment No. 1 to Credit and Guaranty Agreement dated June 17, 2016 | | Incorporated by reference to Exhibit 10.43 on Form 8-K dated June 20, 2016 |
| | | | |
10.44 | | Amendment No. 2 to Credit and Guaranty Agreement dated June 17, 2016 | | Incorporated by reference to Exhibit 10.44 on Form 8-K dated June 20, 2016 |
| | | | |
10.45* | | | Incorporated by reference to Exhibit 10.45 on Form 8-K dated July 19, 2017 | |
| | | | |
10.46* | | Restated Employment Agreement of John Kozlowski dated October 26, 2017 | | Incorporated by reference to Exhibit 10.46 on Form 8-K dated November 1, 2017 |
| | | | |
10.47* | | Employment Agreement of Timothy C. Crew effective as of January 2, 2018 | | Incorporated by reference to Exhibit 10.47 on Form 8-K dated December 21, 2017 |
| | | | |
105
Exhibit |
| Description |
| Method of Filing |
|---|---|---|---|---|
10.48* | | | Incorporated by reference to Exhibit 10.48 on Form 8-K dated January 24, 2018 | |
| | | | |
10.49* | | Addendum to Employment Agreement of Timothy C. Crew dated March 28, 2018 | | Incorporated by reference to Exhibit 10.49 on Form 8-K dated April 2, 2018 |
| | | | |
10.50* | | Employment Agreement of Maureen M. Cavanaugh effective as of May 7, 2018 | | Incorporated by reference to Exhibit 10.50 on Form 8-K dated April 23, 2018 |
| | | | |
10.51* | | | Incorporated by reference to Exhibit 10.51 on Form 8-K dated June 22, 2018 | |
| | | | |
10.52 | | | Incorporated by reference to Exhibit 10.52 on Form 8-K dated December 12, 2018 | |
| | | | |
10.53* | | | Incorporated by reference to Exhibit 10.53 on Form 8-K dated December 18, 2018 | |
| | | | |
10.54 | | | Incorporated by reference to Exhibit 10.54 on Form 10-Q dated February 7, 2019 | |
| | | | |
10.55* | | | Incorporated by reference to Exhibit 10.55 on Form 8-K dated May 24, 2019 | |
| | | | |
10.56* | | Second Amendment to Restated Employment Agreement of John Kozlowski, dated as of July 31, 2019 | | Incorporated by reference to Exhibit 10.56 on Form 8-K dated August 1, 2019 |
| | | | |
10.57 | | | Incorporated by reference to Exhibit 10.57 on Form 8-K dated September 27, 2019 | |
| | | | |
10.58 | | | Incorporated by reference to Exhibit 10.58 on Form 10-Q dated November 7, 2019 | |
10.59 | | | Incorporated by reference to Exhibit 10.59 on Form 10-Q dated November 7, 2019 | |
10.60* | | Lannett Company, Inc. Non-Qualified Deferred Compensation Plan | | Incorporated by reference to Exhibit 10.60 on Form 10-Q dated November 7, 2019 |
10.61 | | | | Incorporated by reference to Exhibit 10.61 on Form 10-Q dated February 6, 2020 |
10.62 | | | | Incorporated by reference to Exhibit 10.62 on Form 10-Q dated February 6, 2020 |
10.63 | | Amendment to Sinotherapeutics Distribution and Supply Agreement | | Filed Herewith |
| | | | |
106
Exhibit |
| Description |
| Method of Filing |
|---|---|---|---|---|
21.1 | | | Filed Herewith | |
| | | | |
23.1 | | | Filed Herewith | |
| | | | |
31.1 | | Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | | Filed Herewith |
| | | | |
31.2 | | Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | | Filed Herewith |
| | | | |
32 | | | Filed Herewith | |
| | | | |
101.INS | | XBRL Instance Document | | |
| | | | |
101.SCH | | XBRL Extension Schema Document | | |
| | | | |
101.CAL | | XBRL Calculation Linkbase Document | | |
| | | | |
101.DEF | | XBRL Definition Linkbase Document | | |
| | | | |
101.LAB | | XBRL Label Linkbase Document | | |
| | | | |
101.PRE | | XBRL Presentation Linkbase Document | | |
107
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | LANNETT COMPANY, INC. | |
| | | | |
Date: | August 27, 2020 | | By: | /s/ Timothy C. Crew |
| | | | Timothy C. Crew, |
| | | | Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Date: | August 27, 2020 | | By: | /s/ John Kozlowski |
| | | | John Kozlowski, |
| | | | Vice President of Finance, Chief Financial Officer and Principal Accounting Officer |
| | | | |
Date: | August 27, 2020 | | By: | /s/ Patrick G. LePore |
| | | | Patrick G. LePore, |
| | | | Director, Chairman of the Board of Directors |
| | | | |
Date: | August 27, 2020 | | By: | /s/ Timothy C. Crew |
| | | | Timothy C. Crew, |
| | | | Director, Chief Executive Officer |
| | | | |
Date: | August 27, 2020 | | By: | /s/ David Drabik |
| | | | David Drabik, |
| | | | Director, Chairman of Governance and Nominating Committee |
| | | | |
Date: | August 27, 2020 | | By: | /s/ Paul Taveira |
| | | | Paul Taveira, |
| | | | Director, Chairman of Compensation Committee |
| | | | |
Date: | August 27, 2020 | | By: | /s/ Melissa Rewolinski |
| | | | Melissa Rewolinski, |
| | | | Director |
| | | | |
Date: | August 27, 2020 | | By: | /s/ John C. Chapman |
| | | | John C. Chapman, |
| | | | Director, Chairman of Audit Committee |
| | | | |
Date: | August 27, 2020 | | By: | /s/ Jeffrey Farber |
| | | | Jeffrey Farber, |
| | | | Director |
108
Index to Consolidated Financial Statements
109
Management’s Report on Internal Control over Financial Reporting
Management of Lannett Company Inc. (the “Company”) is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended. The Company’s internal control framework was designed to provide the Company’s management and Board of Directors, reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with policies or procedures may deteriorate.
Management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control — Integrated Framework (2013) in conducting its assessment as of June 30, 2020. As a result of this assessment, management has concluded that, as of June 30, 2020, the Company’s internal control over financial reporting is effective.
The Company’s independent registered public accounting firm, Grant Thornton, LLP, has issued its report on the effectiveness of the Company’s internal control over financial reporting as of June 30, 2020. Grant Thornton LLP’s opinion on the Company’s internal control over financial reporting appears on page 112 of this Form 10-K.
110
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
Lannett Company Inc.
Opinion on the financial statements
We have audited the accompanying consolidated balance sheets of Lannett Company, Inc. (a Delaware corporation) and subsidiaries (the “Company”) as of June 30, 2020 and 2019, the related consolidated statements of operations, comprehensive income (loss), changes in stockholders’ equity, and cash flows for each of the three years in the period ended June 30, 2020, and the related notes and financial statement schedule included under Item 15 (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2020, and 2019, and the results of its operations and its cash flows for each of the three years in the period ended June 30, 2020, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the Company’s internal control over financial reporting as of June 30, 2020, based on criteria established in the 2013 Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”), and our report dated August 27, 2020 expressed an unqualified opinion.
Change in accounting principle
As discussed in Note 2 to the consolidated financial statements, the Company has changed its method of accounting for leases effective July 1, 2019 due to the adoption of Accounting Standards Codification (ASC) Topic 842, Leases.
Basis for opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ GRANT THORNTON LLP
We have served as the Company’s auditor since 2000.
Iselin, New Jersey
August 27, 2020
111
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
Lannett Company, Inc.
Opinion on internal control over financial reporting
We have audited the internal control over financial reporting of Lannett Company, Inc. (a Delaware corporation) and subsidiaries (the “Company”) as of June 30, 2020, based on criteria established in the 2013 Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of June 30, 2020, based on criteria established in the 2013 Internal Control—Integrated Framework issued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the consolidated financial statements of the Company as of and for the year ended June 30, 2020, and our report dated August 27, 2020 expressed an unqualified opinion on those financial statements.
Basis for opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and limitations of internal control over financial reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ GRANT THORNTON LLP
Iselin, New Jersey
August 27, 2020
112
LANNETT COMPANY, INC.
(In thousands, except share and per share data)
|
| June 30, 2020 |
| June 30, 2019 | ||
ASSETS | | | | | | |
Current assets: | | | | | | |
Cash and cash equivalents | | $ | 144,329 | | $ | 140,249 |
Accounts receivable, net | |
| 125,688 | |
| 164,752 |
Inventories | |
| 142,867 | |
| 143,971 |
Income taxes receivable | | | 14,419 | | | — |
Assets held for sale | |
| 2,678 | |
| 9,671 |
Other current assets | |
| 13,227 | |
| 13,606 |
Total current assets | |
| 443,208 | |
| 472,249 |
Property, plant and equipment, net | |
| 179,518 | |
| 186,670 |
Intangible assets, net | |
| 374,735 | |
| 411,229 |
Operating lease right-of-use assets | | | 9,343 | | | — |
Deferred tax assets | |
| 117,890 | |
| 109,305 |
Other assets | |
| 11,861 | |
| 7,960 |
TOTAL ASSETS | | $ | 1,136,555 | | $ | 1,187,413 |
| | | | | | |
LIABILITIES | | | | | | |
Current liabilities: | | | | | | |
Accounts payable | | $ | 32,535 | | $ | 13,493 |
Accrued expenses | |
| 14,962 | |
| 5,805 |
Accrued payroll and payroll-related expenses | |
| 16,304 | |
| 19,924 |
Rebates payable | |
| 38,175 | |
| 46,175 |
Royalties payable | | | 20,863 | | | 16,215 |
Restructuring liability | | | 27 | | | 2,315 |
Income taxes payable | | | — | | | 2,198 |
Current operating lease liabilities | | | 1,097 | | | — |
Short-term borrowings and current portion of long-term debt | |
| 88,189 | |
| 66,845 |
Other current liabilities | | | 2,713 | | | 3,652 |
Total current liabilities | |
| 214,865 | |
| 176,622 |
Long-term debt, net | |
| 592,940 | |
| 662,203 |
Long-term operating lease liabilities | | | 9,844 | | | — |
Other liabilities | | | 16,010 | | | 14,547 |
TOTAL LIABILITIES | |
| 833,659 | |
| 853,372 |
Commitments (Note 11) | | | | | | |
| | | | | | |
STOCKHOLDERS’ EQUITY | | | | | | |
Common stock ($0.001 par value, 100,000,000 shares authorized; 39,963,127 and 38,969,518 shares issued; 38,798,787 and 38,010,714 shares outstanding at June 30, 2020 and June 30, 2019, respectively) | |
| 40 | |
| 39 |
Additional paid-in capital | |
| 321,164 | |
| 317,023 |
Retained earnings (accumulated deficit) | |
| (1,291) | |
| 32,075 |
Accumulated other comprehensive loss | |
| (627) | |
| (615) |
Treasury stock (1,164,340 and 958,804 shares at June 30, 2020 and June 30, 2019, respectively) | |
| (16,390) | |
| (14,481) |
Total stockholders’ equity | |
| 302,896 | |
| 334,041 |
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | | $ | 1,136,555 | | $ | 1,187,413 |
The accompanying notes are an integral part of the Consolidated Financial Statements.
113
LANNETT COMPANY, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except share and per share data)
| | | | | | | | | |
| | Fiscal Year Ended June 30, | |||||||
| | 2020 |
| 2019 |
| 2018 | |||
Net sales | | $ | 545,744 | | $ | 655,407 | | $ | 684,563 |
Cost of sales | |
| 348,508 | |
| 379,601 | |
| 363,729 |
Amortization of intangibles | | | 32,016 | | | 32,196 | | | 32,128 |
Gross profit | |
| 165,220 | |
| 243,610 | |
| 288,706 |
Operating expenses: | | | | | | | | | |
Research and development expenses | |
| 29,978 | |
| 38,807 | |
| 29,196 |
Selling, general and administrative expenses | |
| 79,467 | |
| 87,648 | |
| 82,196 |
Acquisition and integration-related expenses | | | — | | | — | | | 83 |
Restructuring expenses | | | 1,771 | | | 4,095 | | | 7,061 |
Loss on sale of intangible asset | | | — | | | — | | | 15,514 |
Asset impairment charges | | | 34,448 | | | 375,381 | | | 24,960 |
Total operating expenses | |
| 145,664 | |
| 505,931 | |
| 159,010 |
Operating income (loss) | |
| 19,556 | |
| (262,321) | |
| 129,696 |
Other income (loss): | | | | | | | | | |
Loss on extinguishment of debt | | | (2,145) | | | (448) | | | — |
Investment income | | | 1,646 | | | 3,166 | | | 4,753 |
Interest expense | |
| (66,845) | |
| (84,624) | |
| (85,634) |
Other | | | (840) | | | (2,018) | | | 2,278 |
Total other loss | |
| (68,184) | |
| (83,924) | |
| (78,603) |
Income (loss) before income tax | |
| (48,628) | |
| (346,245) | |
| 51,093 |
Income tax expense (benefit) | |
| (15,262) | |
| (74,138) | |
| 22,403 |
Net income (loss) | | $ | (33,366) | | $ | (272,107) | | $ | 28,690 |
| | | | | | | | | |
Earnings (loss) per common share: | | | | | | | | | |
Basic | | $ | (0.86) | | $ | (7.20) | | $ | 0.77 |
Diluted (1) | | $ | (0.86) | | $ | (7.20) | | $ | 0.75 |
| | | | | | | | | |
Weighted average common shares outstanding: | | | | | | | | | |
Basic | |
| 38,592,618 | |
| 37,779,812 | |
| 37,127,306 |
Diluted (1) | |
| 38,592,618 | |
| 37,779,812 | |
| 38,162,514 |
________________________________
(1) See Note 14 "Earnings (Loss) Per Common Share" for details on calculation.
The accompanying notes are an integral part of the Consolidated Financial Statements.
114
LANNETT COMPANY, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
(In thousands)
| | | | | | | | | |
| | Fiscal Year Ended June 30, | |||||||
| | 2020 |
| 2019 |
| 2018 | |||
Net income (loss) | | $ | (33,366) | | $ | (272,107) | | $ | 28,690 |
Other comprehensive income (loss), before tax: | | | | | | | | | |
Foreign currency translation loss | |
| (12) | |
| (100) | |
| (293) |
Total other comprehensive loss, net of taxes | |
| (12) | |
| (100) | |
| (293) |
Comprehensive income (loss) | | $ | (33,378) | | $ | (272,207) | | $ | 28,397 |
The accompanying notes are an integral part of the Consolidated Financial Statements.
115
LANNETT COMPANY, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
(In thousands)
| | Stockholders’ Equity Attributable to Lannett Company Inc. | | | ||||||||||||||||
|
| |
| | |
| | |
| Retained |
| Accumulated |
| | |
| | | ||
| | Common Stock | | Additional | | Earnings | | Other | | | | | Total | |||||||
| | Shares | | | | | Paid-In | | (Accumulated | | Comprehensive | | Treasury | | Stockholders’ | |||||
|
| Issued |
| Amount |
| Capital |
| Deficit) |
| Loss |
| Stock |
| Equity | ||||||
| | | | | | | | | | | | | | | | | | | | |
Balance, June 30, 2017 |
| 37,528 | | $ | 37 | | $ | 292,780 | | $ | 277,774 | | $ | (222) | | $ | (9,247) | | $ | 561,122 |
| | | | | | | | | | | | | | | | | | | | |
Shares issued in connection with share-based compensation plans |
| 729 | |
| 1 | |
| 4,141 | |
| — | |
| — | |
| — | |
| 4,142 |
Share-based compensation |
| — | |
| — | |
| 9,896 | |
| — | |
| — | |
| — | |
| 9,896 |
Purchase of treasury stock |
| — | |
| — | |
| — | |
| — | |
| — | |
| (4,642) | |
| (4,642) |
Other comprehensive income, net of income tax | | — | | | — | | | — | | | — | | | (293) | | | — | | | (293) |
Net income |
| — | |
| — | |
| — | |
| 28,690 | |
| — | |
| — | |
| 28,690 |
Balance, June 30, 2018 |
| 38,257 | | $ | 38 | | $ | 306,817 | | $ | 306,464 | | $ | (515) | | $ | (13,889) | | $ | 598,915 |
| | | | | | | | | | | | | | | | | | | | |
Shares issued in connection with share-based compensation plans |
| 713 | |
| 1 | |
| 1,179 | |
| — | |
| — | |
| — | |
| 1,180 |
Share-based compensation |
| — | |
| — | |
| 9,027 | |
| — | |
| — | |
| — | |
| 9,027 |
Purchase of treasury stock |
| — | |
| — | |
| — | |
| — | |
| — | |
| (592) | |
| (592) |
Other comprehensive loss, net of income tax | | — | | | — | | | — | | | — | | | (100) | | | — | | | (100) |
ASC 606 adjustment | | — | | | — | | | — | | | (2,282) | | | — | | | — | | | (2,282) |
Net loss |
| — | |
| — | |
| — | |
| (272,107) | |
| — | |
| — | |
| (272,107) |
Balance, June 30, 2019 |
| 38,970 | | $ | 39 | | $ | 317,023 | | $ | 32,075 | | $ | (615) | | $ | (14,481) | | $ | 334,041 |
| | | | | | | | | | | | | | | | | | | | |
Shares issued in connection with share-based compensation plans |
| 993 | |
| 1 | |
| 997 | |
| — | |
| — | |
| — | |
| 998 |
Share-based compensation |
| — | |
| — | |
| 10,216 | |
| — | |
| — | |
| — | |
| 10,216 |
Purchase of treasury stock |
| — | |
| — | |
| — | |
| — | |
| — | |
| (1,909) | |
| (1,909) |
Other comprehensive loss, net of income tax |
| — | |
| — | |
| — | |
| — | |
| (12) | |
| — | |
| (12) |
Capped call transaction | | — | | | — | | | (7,072) | | | | | | — | | | — | | | (7,072) |
Net loss |
| — | |
| — | |
| — | |
| (33,366) | |
| — | |
| — | |
| (33,366) |
| | | | | | | | | | | | | | | | | | | | |
Balance, June 30, 2020 |
| 39,963 | | $ | 40 | | $ | 321,164 | | $ | (1,291) | | $ | (627) | | $ | (16,390) | | $ | 302,896 |
The accompanying notes are an integral part of the Consolidated Financial Statements.
116
LANNETT COMPANY, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | Fiscal Year Ended June 30, | |||||||
|
| 2020 |
| 2019 |
| 2018 | |||
OPERATING ACTIVITIES: | | | | | | | | | |
Net income (loss) | | $ | (33,366) | | $ | (272,107) | | $ | 28,690 |
Adjustments to reconcile net income (loss) to net cash provided by operating activities: | | | | | | | | | |
Depreciation and amortization | |
| 56,309 | |
| 55,594 | |
| 55,115 |
Deferred income tax expense (benefit) | |
| (8,585) | |
| (87,242) | |
| 30,690 |
Share-based compensation | |
| 10,216 | |
| 9,027 | |
| 9,896 |
Asset impairment charges | | | 34,448 | | | 375,381 | | | 24,960 |
Loss (gain) on sale/disposal of assets | |
| (159) | |
| 1,559 | |
| 848 |
Gain on investment securities | |
| — | |
| — | |
| (3,313) |
Loss on extinguishment of debt | | | 2,145 | | | 448 | | | — |
Loss on sale of intangible asset | | | — | | | — | | | 15,514 |
Amortization of debt discount and other debt issuance costs | | | 14,336 | | | 17,641 | | | 21,866 |
Other noncash expenses | |
| 1,969 | |
| 2,579 | |
| 5 |
Changes in assets and liabilities which provided (used) cash: | | | | | | | | | |
Accounts receivable, net | |
| 39,064 | |
| 84,949 | |
| (48,585) |
Inventories | |
| 1,104 | |
| (2,336) | |
| (19,031) |
Prepaid income taxes/income taxes payable | |
| (14,465) | |
| 18,319 | |
| 2,174 |
Other assets | |
| 4,095 | |
| 2,643 | |
| (2,287) |
Rebates payable | |
| (8,000) | |
| (3,225) | |
| 4,807 |
Royalties payable | | | 4,648 | | | 10,260 | | | 2,940 |
Restructuring liability | | | (2,288) | | | (4,391) | | | 1,275 |
Operating lease liability | | | (1,464) | | | — | | | — |
Accounts payable | |
| 19,042 | |
| (43,274) | |
| (4,953) |
Accrued expenses | |
| 2,213 | |
| (1,620) | |
| (5,074) |
Accrued payroll and payroll-related expenses | | | (3,620) | | | 12,105 | | | 2,986 |
Other liabilities | | | (1,628) | | | — | | | — |
Net cash provided by operating activities | |
| 116,014 | |
| 176,310 | |
| 118,523 |
INVESTING ACTIVITIES: | | | | | | | | | |
Purchases of property, plant and equipment | |
| (18,330) | |
| (24,340) | |
| (52,316) |
Proceeds from sale of property, plant and equipment | |
| 7,380 | |
| 14,450 | |
| 28 |
Proceeds from sale of outstanding loan to Variable Interest Entity (“VIE”) | | | — | | | 5,600 | | | — |
Advance to VIE | | | (250) | | | — | | | (10,254) |
Purchases of intangible assets | | | (28,800) | | | (3,000) | | | (19,038) |
Proceeds from sale of investment securities | |
| — | |
| — | |
| 94,047 |
Purchase of investment securities | |
| — | |
| — | |
| (63,643) |
Net cash used in investing activities | |
| (40,000) | |
| (7,290) | |
| (51,176) |
FINANCING ACTIVITIES: | | | | | | | | | |
Proceeds from issuance of long-term debt | | | 86,250 | | | — | | | — |
Purchase of capped call | | | (7,072) | | | — | | | — |
Repayments of short-term borrowings and long-term debt | |
| (146,700) | |
| (126,743) | |
| (85,705) |
Proceeds from issuance of stock | |
| 998 | |
| 1,180 | |
| 4,142 |
Payment of debt issuance costs | | | (3,489) | | | (1,102) | | | — |
Purchase of treasury stock | |
| (1,909) | |
| (592) | |
| (4,642) |
Net cash used in financing activities | |
| (71,922) | |
| (127,257) | |
| (86,205) |
Effect on cash and cash equivalents of changes in foreign exchange rates | |
| (12) | |
| (100) | |
| (293) |
NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | |
| 4,080 | |
| 41,663 | |
| (19,151) |
CASH AND CASH EQUIVALENTS, BEGINNING OF PERIOD | |
| 140,249 | |
| 98,586 | |
| 117,737 |
CASH AND CASH EQUIVALENTS, END OF PERIOD | | $ | 144,329 | | $ | 140,249 | | $ | 98,586 |
| | | | | | | | | |
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | | | | | | | | | |
Interest paid (net of amounts capitalized of $0, $0 and $1.6 million for the years ended June 30, 2020, 2019 and 2018, respectively) | | $ | 51,928 | | $ | 66,750 | | $ | 63,563 |
Income taxes paid (refunded) | | $ | 7,787 | | $ | (4,641) | | $ | (6,559) |
Credits issued pursuant to Settlement Agreement | | $ | — | | $ | — | | $ | 17,000 |
Andor Pharmaceuticals, LLC (“Andor”) License Agreement acquisition | | $ | — | | $ | 16,000 | | $ | — |
Accrued purchases of property, plant and equipment | | $ | 2,295 | | $ | 765 | | $ | 3,572 |
The accompanying notes are an integral part of the Consolidated Financial Statements.
117
LANNETT COMPANY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1. The Business and Nature of Operations
Lannett Company, Inc. (a Delaware corporation) and its subsidiaries (collectively, the “Company” or “Lannett”) primarily develop, manufacture, package, market and distribute solid oral and extended release (tablets and capsules), topical, nasal and oral solution finished dosage forms of drugs that address a wide range of therapeutic areas. Certain of these products are manufactured by others and distributed by the Company.
The Company operates pharmaceutical manufacturing plants in Carmel, New York and Seymour, Indiana. The Company’s customers include generic pharmaceutical distributors, drug wholesalers, chain drug stores, private label distributors, mail-order pharmacies, other pharmaceutical manufacturers, managed care organizations, hospital buying groups, governmental entities and health maintenance organizations.
COVID-19 Update
In December 2019, the COVID-19 virus emerged in Wuhan, China and spread to other parts of the world. In March 2020, the World Health Organization (“WHO”) designated COVID-19 a global pandemic. Governments on the national, state and local level in the United States, and around the world, implemented lockdown and shelter-in-place orders, requiring many non-essential businesses to shut down operations. The Company’s business, however, is deemed “essential” and it has continued to operate and it has continued to manufacture and distribute its medicines to customers.
In light of the economic impacts of COVID-19, the Company performed a review of the assets on our Consolidated Balance Sheet as of June 30, 2020, including intangible and other long-lived assets. Based on our review, we believe that we will be able to realize the full value of our assets and that a triggering event does not exist at this time. As such, no impairments or other write-downs were recorded during Fiscal 2020 specifically related to COVID-19. Our assessment is based on information currently available and is highly reliant on various assumptions. Changes in market conditions or other changes in the future outlook may lead to impairments in the future.
While COVID-19 has thus far not had a material impact on the Company’s operations, subsequent to an initial stocking up of supplies at the start of the pandemic the total volume of drug prescriptions being written in the country has decreased causing less demand for our products. We cannot reasonably predict the ultimate impact of COVID-19 on our future results of operations and cashflows due to the continued uncertainty around the duration and severity of the pandemic.
Note 2. Summary of Significant Accounting Policies
Basis of Presentation
The Consolidated Financial Statements have been prepared in conformity with U.S. GAAP.
Principles of consolidation
The Consolidated Financial Statements include the accounts of Lannett Company, Inc. and its wholly-owned subsidiaries. All intercompany accounts and transactions have been eliminated.
Business Combinations
Acquired businesses are accounted for using the acquisition method of accounting, which requires that the assets acquired and liabilities assumed be recorded at the date of acquisition at their respective estimated fair values. The fair values and useful lives assigned to each class of assets acquired and liabilities assumed are based on, among other factors, the expected future period of benefit of the asset, the various characteristics of the asset and projected future cash
118
flows. Significant judgment is employed in determining the assumptions utilized as of the acquisition date and for each subsequent measurement period. Accordingly, changes in assumptions described above could have a material impact on our consolidated results of operations.
Reclassifications
Certain prior year amounts have been reclassified to conform to the current year financial statement presentation.
Use of estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant estimates and assumptions are required in the determination of revenue recognition and sales deductions for estimated chargebacks, rebates, returns and other adjustments including a provision for the Company’s liability under the Medicare Part D program. Additionally, significant estimates and assumptions are required when determining the value of inventories and long-lived assets, including intangible assets, income taxes, contingencies and share-based compensation.
Because of the inherent subjectivity and complexity involved in these estimates and assumptions, actual results could differ from those estimates.
Foreign currency translation
The Consolidated Financial Statements are presented in U.S. dollars, the reporting currency of the Company. The financial statements of the Company’s foreign subsidiary are maintained in local currency and translated into U.S. dollars at the end of each reporting period. Assets and liabilities are translated at period-end exchange rates, while revenues and expenses are translated at average exchange rates during the period. The adjustments resulting from the use of differing exchange rates are recorded as part of stockholders’ equity in accumulated other comprehensive income (loss). Gains and losses resulting from transactions denominated in foreign currencies are recognized in the Consolidated Statements of Operations under other income (loss). Amounts recorded due to foreign currency fluctuations are immaterial to the Consolidated Financial Statements.
Cash and cash equivalents
The Company considers all highly liquid investments with original maturities less than or equal to three months at the date of purchase to be cash and cash equivalents. Cash and cash equivalents are stated at cost, which approximates fair value, and consist of bank deposits and certificates of deposit that are readily convertible into cash. The Company maintains its cash deposits and cash equivalents at well-known, stable financial institutions. Such amounts frequently exceed insured limits.
Allowance for doubtful accounts
The Company continuously monitors collections and payments from its customers and maintains a provision for estimated credit losses. The Company determines its allowance for doubtful accounts by considering a number of factors, including the length of time balances are past due, the Company’s previous loss history, the customer’s current ability to pay its obligations to the Company and the condition of the general economy and the industry as a whole. The Company writes off accounts receivable when they are determined to be uncollectible.
Inventories
Inventories are stated at the lower of cost or net realizable value by the first-in, first-out method. Inventories are regularly reviewed and write-downs for excess and obsolete inventory are recorded based primarily on current inventory levels, expiration date and estimated sales forecasts.
119
Property, Plant and Equipment
Property, plant and equipment are stated at cost less accumulated depreciation. Depreciation is computed on a straight-line basis over the assets’ estimated useful lives. Repairs and maintenance costs that do not extend the useful life of the asset are expensed as incurred.
Intangible Assets
Definite-lived intangible assets are stated at cost less accumulated amortization. Amortization of definite-lived intangible assets is computed on a straight-line basis over the assets’ estimated useful lives which commences upon shipment of the product, generally for periods ranging from 5 to 15 years. The Company continually evaluates the reasonableness of the useful lives of these assets. Indefinite-lived intangible assets are not amortized, but instead are tested at least annually for impairment. Costs to renew or extend the term of a recognized intangible asset are expensed as incurred.
Valuation of Long-Lived Assets, including Intangible Assets other than Goodwill
The Company’s long-lived assets primarily consist of property, plant and equipment and definite and indefinite-lived intangible assets. Property, plant and equipment and definite-lived intangible assets are reviewed for impairment whenever events or changes in circumstances (“triggering events”) indicate that the carrying amount of the asset may not be recoverable. If a triggering event is determined to have occurred, the asset’s carrying value is compared to the future undiscounted cash flows expected to be generated by the asset. If the carrying value exceeds the undiscounted cash flows of the asset, then impairment exists. Indefinite-lived intangible assets are tested for impairment at least annually during the fourth quarter of each fiscal year or more frequently if events or triggering events indicate that the asset might be impaired.
An impairment loss is measured as the excess of the asset’s carrying value over its fair value, which in most cases is calculated using a discounted cash flow model. Discounted cash flow models are highly reliant on various assumptions which are considered Level 3 inputs, including estimates of future cash flows (including long-term growth rates), discount rates and the probability of achieving the estimated cash flows.
In-Process Research and Development
Amounts allocated to in-process research and development in connection with a business combination are recorded at fair value and are considered indefinite-lived intangible assets subject to impairment testing in accordance with the Company’s impairment testing policy for indefinite-lived intangible assets. As products in development are approved for sale, amounts will be allocated to product rights and will be amortized over their estimated useful lives. Definite-lived intangible assets are amortized over the expected lives of the related assets. The judgments made in determining the estimated fair value of in-process research and development, as well as asset lives, can materially impact our results of operations. The Company’s fair value assessments are highly reliant on various assumptions which are considered Level 3 inputs, including estimates of future cash flows (including long-term growth rates), discount rates and the probability of achieving the estimated cash flows.
120
Segment Information
The Company operates in one reportable segment, generic pharmaceuticals. As such, the Company aggregates its financial information for all products. The following table identifies the Company’s net sales by medical indication for fiscal years ended June 30, 2020, 2019 and 2018. The medical indication categories for the fiscal years ended June 30, 2019 and 2018 were reclassified to better align with industry standards and the Company’s peers.
| | | | | | | | | |
| | | | | | | | | |
(In thousands) | | Fiscal Year Ended June 30, | |||||||
Medical Indication | | 2020 |
| 2019 |
| 2018 | |||
Analgesic | | $ | 8,680 | | $ | 8,251 | | $ | 3,809 |
Anti-Psychosis | | | 104,934 | | | 73,453 | | | 59,557 |
Cardiovascular | |
| 88,576 | |
| 101,467 | |
| 64,011 |
Central Nervous System | | | 77,256 | | | 59,019 | | | 59,672 |
Endocrinology | | | — | | | 197,522 | | | 245,929 |
Gastrointestinal | | | 73,477 | | | 63,043 | | | 67,762 |
Infectious Disease | | | 73,237 | | | 16,950 | | | 17,685 |
Migraine | |
| 44,266 | |
| 41,592 | |
| 54,015 |
Respiratory/Allergy/Cough/Cold | | | 11,576 | | | 12,479 | | | 25,284 |
Urinary | | | 4,225 | | | 6,755 | | | 8,068 |
Other | |
| 35,013 | |
| 51,517 | |
| 58,936 |
Contract manufacturing revenue | | | 24,504 | | | 23,359 | | | 19,835 |
Total net sales | | $ | 545,744 | | $ | 655,407 | | $ | 684,563 |
Customer, Supplier and Product Concentration
The following table presents the percentage of total net sales, for the fiscal years ended June 30, 2020, 2019 and 2018, for certain of the Company’s products, defined as products containing the same active ingredient or combination of ingredients, which accounted for at least 10% of total net sales in any of those periods:
| | | | | | | |
| | June 30, | | June 30, | | June 30, |
|
| | 2020 |
| 2019 |
| 2018 |
|
| | | | | | | |
Product 1 |
| 18 | % | 10 | % | 8 | % |
Product 2 |
| 10 | % | — | % | — | % |
Product 3 | | — | % | 30 | % | 36 | % |
The following table presents the percentage of total net sales, for the fiscal years ended June 30, 2020, 2019 and 2018, for certain of the Company’s customers which accounted for at least 10% of total net sales in any of those periods:
| | | | | | | |
| | | | | | | |
|
| June 30, |
| June 30, |
| June 30, |
|
| | 2020 |
| 2019 |
| 2018 |
|
| | | | | | | |
Customer A |
| 25 | % | 21 | % | 29 | % |
Customer B |
| 23 | % | 18 | % | 17 | % |
Customer C | | 11 | % | 10 | % | 5 | % |
Customer D |
| — | % | 12 | % | — | % |
The Company’s primary finished goods inventory supplier through March 23, 2019 was Jerome Stevens Pharmaceuticals, Inc. (“JSP”), in Bohemia, New York. Purchases of finished goods inventory from JSP accounted for 29% and 37% of the Company’s inventory purchases in fiscal years 2019 and 2018, respectively. There were no purchases of finished goods inventory from JSP in the fiscal year ended June 30, 2020.
121
Revenue Recognition
On July 1, 2018, the Company adopted Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers, which superseded ASC Topic 605, Revenue Recognition. Under ASC 606, the Company recognizes revenue when (or as) we satisfy our performance obligations by transferring a promised good or service to a customer at an amount that reflects the consideration the Company is expected to be entitled. Our revenue consists almost entirely of sales of our pharmaceutical products to customers, whereby we ship product to a customer pursuant to a purchase order. Revenue contracts such as these do not generally give rise to contract assets or contract liabilities because: (i) the underlying contracts generally have only a single performance obligation and (ii) we do not generally receive consideration until the performance obligation is fully satisfied. The new revenue standard impacts the timing of the Company’s revenue recognition by requiring recognition of certain contract manufacturing arrangements to change from “upon shipment or delivery” to “over time.” However, the recognition of these arrangements over time does not currently have a material impact on the Company’s consolidated results of operations or financial position. The Company adopted ASC 606 using the modified retrospective method.
When revenue is recognized, a simultaneous adjustment to gross sales is made for estimated chargebacks, rebates, returns, promotional adjustments and other potential adjustments. These provisions are primarily estimated based on historical experience, future expectations, contractual arrangements with wholesalers and indirect customers and other factors known to management at the time of accrual. Accruals for these provisions are presented in the Consolidated Financial Statements as a reduction to gross sales with the corresponding reserve presented as a reduction of accounts receivable or included as rebates payable, depending on the nature of the reserve.
Provisions for chargebacks, rebates, returns and other adjustments require varying degrees of subjectivity. While rebates generally are based on contractual terms and require minimal estimation, chargebacks and returns require management to make more subjective assumptions. Each major category is discussed in detail below:
Chargebacks
The provision for chargebacks is the most significant and complex estimate used in the recognition of revenue. The Company sells its products directly to wholesale distributors, generic distributors, retail pharmacy chains and mail-order pharmacies. The Company also sells its products indirectly to independent pharmacies, managed care organizations, hospitals, nursing homes and group purchasing organizations, collectively referred to as “indirect customers.” The Company enters into agreements with its indirect customers to establish pricing for certain products. The indirect customers then independently select a wholesaler from which to purchase the products. If the price paid by the indirect customers is lower than the price paid by the wholesaler, the Company will provide a credit, called a chargeback, to the wholesaler for the difference between the contractual price with the indirect customers and the wholesaler purchase price. The provision for chargebacks is based on expected sell-through levels by the Company’s wholesale customers to the indirect customers and estimated wholesaler inventory levels. As sales to the large wholesale customers, such as Cardinal Health, AmerisourceBergen and McKesson increase (decrease), the reserve for chargebacks will also generally increase (decrease). However, the size of the increase (decrease) depends on product mix and the amount of sales made to indirect customers with which the Company has specific chargeback agreements. The Company continually monitors the reserve for chargebacks and makes adjustments when management believes that expected chargebacks may differ from the actual chargeback reserve.
Rebates
Rebates are offered to the Company’s key chain drug store, distributor and wholesaler customers to promote customer loyalty and increase product sales. These rebate programs provide customers with credits upon attainment of pre-established volumes or attainment of net sales milestones for a specified period. Other promotional programs are incentive programs offered to the customers. Additionally, as a result of the Patient Protection and Affordable Care Act (“PPACA”) enacted in the U.S. in March 2010, the Company participates in a cost-sharing program for certain Medicare Part D beneficiaries designed primarily for the sale of brand drugs and certain generic drugs if their Food and Drug Administration (“FDA”) approval was granted under a New Drug Application (“NDA”) or 505(b) NDA versus an Abbreviated New Drug application (“ANDA’). Drugs purchased within the Medicare Part D coverage gap (commonly
122
referred to as the “donut hole”) result in additional rebates. The Company estimates the reserve for rebates and other promotional credit programs based on the specific terms in each agreement when revenue is recognized. The reserve for rebates increases (decreases) as sales to certain wholesale and retail customers increase (decrease). However, since these rebate programs are not identical for all customers, the size of the reserve will depend on the mix of sales to customers that are eligible to receive rebates.
Returns
Consistent with industry practice, the Company has a product returns policy that allows customers to return product within a specified time period prior to and subsequent to the product’s expiration date in exchange for a credit to be applied to future purchases. The Company’s policy requires that the customer obtain pre-approval from the Company for any qualifying return. The Company estimates its provision for returns based on historical experience, changes to business practices, credit terms and any extenuating circumstances known to management. While historical experience has allowed for reasonable estimations in the past, future returns may or may not follow historical trends. The Company continually monitors the reserve for returns and makes adjustments when management believes that actual product returns may differ from the established reserve. Generally, the reserve for returns increases as net sales increase.
Other Adjustments
Other adjustments consist primarily of “price adjustments,” also known as “shelf-stock adjustments” and “price protections,” which are both credits issued to reflect increases or decreases in the invoice or contract prices of the Company’s products. In the case of a price decrease, a credit is given for product remaining in customer’s inventories at the time of the price reduction. Contractual price protection results in a similar credit when the invoice or contract prices of the Company’s products increase, effectively allowing customers to purchase products at previous prices for a specified period of time. Amounts recorded for estimated shelf-stock adjustments and price protections are based upon specified terms with direct customers, estimated changes in market prices and estimates of inventory held by customers. The Company regularly monitors these and other factors and evaluates the reserve as additional information becomes available. Other adjustments also include prompt payment discounts and “failure-to-supply” adjustments. If the Company is unable to fulfill certain customer orders, the customer can purchase products from our competitors at their prices and charge the Company for any difference in our contractually agreed upon prices.
Leases
On July 1, 2019, the Company adopted ASC Topic 842, Leases, which superseded ASC Topic 840, Leases. Refer to the “Recent Accounting Pronouncements” section of this footnote for further discussion on the impact of the adoption. Under ASC 842, when the Company enters into a new arrangement, it must determine, at the inception date, whether the arrangement is or contains a lease. This determination generally depends on whether the arrangement conveys to the Company the right to control the use of an explicitly or implicitly identified asset for a period of time in exchange for consideration. Control of an underlying asset is conveyed to the Company if the Company obtains the rights to direct the use of and to obtain substantially all of the economic benefits from using the underlying asset. Once a lease has been identified, the Company must determine the lease term, the present value of lease payments and the classification of the lease as either operating or financing.
The lease term is determined to be the non-cancelable period plus any lessee renewal options which are considered to be reasonably certain of exercise. Our lease agreements do not contain any material residual value guarantees or material restrictive covenants
The present value of lease payments includes fixed and certain variable payments, less lease incentives, together with amounts probable of being owed by the Company under residual value guarantees and, if reasonably certain of being paid, the cost of certain renewal options and early termination penalties set forth in the lease arrangement. To calculate the present value of lease payments, we use our incremental borrowing rate based on the information available at commencement date, as the rate implicit in the lease is generally not readily available.
123
In making the determination of whether a lease is an operating lease or a finance lease, the Company considers the lease term in relation to the economic life of the leased asset, the present value of lease payments in relation to the fair value of the leased asset and certain other factors.
Upon the commencement of the lease, the Company will record a lease liability and right-of-use (“ROU”) asset based on the present value of the future minimum lease payments over the lease term at commencement date. The ROU asset also includes any lease payments made and excludes lease incentives and initial direct costs incurred.
For operating leases, a single lease cost is generally recognized in the Consolidated Statements of Operations on a straight-line basis over the lease term unless an impairment has been recorded with respect to a leased asset. For finance leases, amortization expense and interest expense are recognized separately in the Consolidated Statements of Operations, with amortization expense generally recorded on a straight-line basis and interest expense recorded using the effective interest method. Variable lease costs not initially included in the lease liability and ROU asset impairment charges are expensed as incurred.
Cost of Sales, including Amortization of Intangibles
Cost of sales includes all costs related to bringing products to their final selling destination, which includes direct and indirect costs, such as direct material, labor and overhead expenses. Additionally, cost of sales includes product royalties, depreciation, amortization and costs to renew or extend recognized intangible assets, freight charges and other shipping and handling expenses.
Research and Development
Research and development costs are expensed as incurred, including all production costs until a drug candidate is approved by the FDA. Research and development expenses include costs associated with internal projects as well as costs associated with third-party research and development contracts.
Contingencies
Loss contingencies, including litigation-related contingencies, are included in the Consolidated Statements of Operations when the Company concludes that a loss is both probable and reasonably estimable. Legal fees for litigation-related matters are expensed as incurred and included in the Consolidated Statements of Operations under the Selling, general and administrative expense line item.
Restructuring Costs
The Company records charges associated with approved restructuring plans to remove duplicative headcount and infrastructure associated with business acquisitions or to simplify business processes. Restructuring charges can include severance costs to eliminate a specified number of employees, infrastructure charges to vacate facilities and consolidate operations and contract cancellation costs. The Company records restructuring charges based on estimated employee terminations, site closure and consolidation plans. The Company accrues severance and other employee separation costs under these actions when it is probable that a liability exists and the amount is reasonably estimable.
Share-Based Compensation
Share-based compensation costs are recognized over the vesting period, using a straight-line method, based on the fair value of the instrument on the date of grant less an estimate for expected forfeitures. The Company uses the Black-Scholes valuation model to determine the fair value of stock options, the stock price on the grant date to value restricted stock and the Monte-Carlo simulation model to determine the fair value of performance-based shares. The Black-Scholes valuation and Monte-Carlo simulation models include various assumptions, including the expected volatility, the expected life of the award, dividend yield and the risk-free interest rate as well as performance assumptions of peer companies. These assumptions involve inherent uncertainties based on market conditions which are generally outside
124
the Company’s control. Changes in these assumptions could have a material impact on share-based compensation costs recognized in the Consolidated Financial Statements.
Self-Insurance
The Company self-insures for certain employee medical and prescription benefits. The Company also maintains stop loss coverage with third party insurers to limit its total liability exposure. The liability for self-insured risks is primarily calculated using independent third party actuarial valuations which take into account actual claims, claims growth and claims incurred but not yet reported. Actual experience, including claim frequency and severity as well as health-care inflation, could result in different liabilities than the amounts currently recorded. The liability for self-insured risks under this plan was not material to the consolidated financial position of the Company as of June 30, 2020 and 2019.
Income Taxes
The Company uses the liability method to account for income taxes as prescribed by ASC 740, Income Taxes. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities as measured by the enacted tax rates which will be in effect when these differences reverse. Deferred tax expense (benefit) is the result of changes in deferred tax assets and liabilities. Deferred income tax assets and liabilities are adjusted to recognize the effects of changes in tax laws or enacted tax rates in the period during which they are signed into law. The factors used to assess the likelihood of realization are the Company’s forecast of future taxable income and available tax planning strategies that could be implemented to realize the net deferred tax assets. Under ASC 740, Income Taxes, a valuation allowance is required when it is more likely than not that all or some portion of the deferred tax assets will not be realized through generating sufficient future taxable income. Failure to achieve forecasted taxable income in applicable tax jurisdictions could affect the ultimate realization of deferred tax assets and could result in an increase in the Company’s effective tax rate on future earnings.
The Company may recognize the tax benefit from an uncertain tax position claimed on a tax return only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement. The authoritative accounting standards also provide guidance on de-recognition, classification, interest and penalties on income taxes, accounting in interim periods and requires increased disclosures.
On December 22, 2017, President Trump signed the Tax Cut and Jobs Act legislation (“2017 Tax Reform”) into law, which included a broad range of tax reform provisions affecting businesses, including corporate tax rates, business deductions and international tax provisions. Many of these provisions significantly differ from current U.S. tax law, resulting in pervasive financial reporting implications. As a result of the new law, the SEC issued Staff Accounting Bulletin No. 118 (“SAB 118”) to address the application of U.S. GAAP in situations when a registrant does not have the necessary information available, prepared, or analyzed in reasonable detail to complete the accounting for certain income tax effects of 2017 Tax Reform. SAB 118 required registrants to report the tax effects of 2017 Tax Reform, inclusive of provisional amounts for which the accounting is incomplete but a reasonable estimate can be determined. In the second quarter of Fiscal 2019, the Company finalized the provisional amounts without any further adjustments.
On March 27, 2020, in response to COVID-19 and its detrimental impact to the global economy, President Trump signed the Coronavirus Aid, Relief, and Economic Security Act (“CARES Act”) into law, which provides a stimulus to the U.S. economy in the form of various individual and business assistance programs as well as temporary changes to existing tax law. Among the changes to the provision in business tax laws include a five-year net operating loss carryback for the Fiscal 2019 - 2021 tax years, a deferral of the employer’s portion of certain payroll tax, and an increase in the interest expense deductibility limitation for the Fiscal 2020 and 2021 tax years. ASC 740 requires the tax effects of changes in tax laws or rates to be recorded in the period of enactment. As a result of the CARES Act, the Company will carry back its Fiscal 2020 taxable loss into the Fiscal 2015 tax year. The Company also reviewed its existing deferred tax assets in light of COVID-19 and determined that no additional valuation allowance is required at this time. However, the Company will continue to monitor the status of the COVID-19 pandemic and its impact on our results of operations.
125
Earnings (Loss) Per Common Share
A dual presentation of basic and diluted earnings (loss) per common share is required on the face of the Company's Consolidated Statement of Operations as well as a reconciliation of the computation of basic earnings (loss) per common share to diluted earnings (loss) per common share. Basic earnings (loss) per common share excludes the dilutive impact of potentially dilutive securities and is computed by dividing net income (loss) by the weighted average number of shares outstanding during the period. Beginning in the first quarter of Fiscal 2020, the Company's diluted earnings (loss) per common share is computed using the "if-converted" method by dividing the adjusted "if-converted" net income by the adjusted weighted average number of shares of common stock outstanding during the period. The adjusted "if-converted" net income is adjusted for interest expense and amortization of debt issuance costs, both net of tax, associated with the Company’s 4.50% Convertible Senior Notes due 2026. The weighted average number of diluted shares is adjusted for the potential dilutive effect of the exercise of stock options, treats unvested restricted stock and performance-based shares as if it were vested, and assumes the conversion of the 4.50% Convertible Senior Notes. Anti-dilutive securities are excluded from the calculation. Dilutive shares are also excluded in the calculation in periods of net loss because the effect of including such securities would be anti-dilutive.
Comprehensive Income (Loss)
Comprehensive income (loss) includes all changes in equity during a period except those that resulted from investments by or distributions to the Company’s stockholders. Other comprehensive income (loss) refers to gains and losses that are included in comprehensive income (loss), but excluded from net income (loss) as these amounts are recorded directly as an adjustment to stockholders’ equity.
Recent Accounting Pronouncements
In December 2019, the Financial Accounting Standards Board ("FASB") issued ASU 2019-12, Simplifying the Accounting for Income Taxes, which is meant to reduce complexity in the accounting for income taxes, eliminates certain exceptions within ASC 740, and clarifies certain aspects of the current guidance to promote consistency among reporting entities. ASU 2019-12 is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2020, with early adoption permitted for periods for which financial statements have not been issued as of December 15, 2019. The Company early adopted this guidance in the second quarter of Fiscal 2020. As a result of the adoption, the Company is no longer subject to the exception to the general methodology for calculating income taxes in an interim period when a year-to-date loss exceeds the anticipated loss for the year. The adoption of ASU 2019-12 did not have an impact on the Company’s income tax benefit for the fiscal year ended June 30, 2020.
In February 2016, the FASB issued ASU 2016-02, Leases. ASU 2016-02 requires an entity to recognize ROU assets and liabilities on its balance sheet for all leases with terms longer than 12 months. Lessees and lessors are required to disclose quantitative and qualitative information about leasing arrangements to enable a user of the financial statements to assess the amount, timing and uncertainty of cash flows arising from leases. ASU 2016-02 is effective for annual reporting periods beginning after December 15, 2018, including interim periods within that reporting period and requires a modified retrospective application, with early adoption permitted. The Company adopted ASU 2016-02 as of July 1, 2019 on a modified retrospective basis applying the guidance to leases existing as of this effective date. The Company has determined that there was no cumulative-effect adjustment to beginning retained earnings on the Consolidated Balance Sheet. The Company will continue to report periods prior to July 1, 2019 in our financial statements under prior guidance as outlined in Topic 840. Refer to Note 11 "Commitments" for additional information.
The Company’s adoption of ASU No. 2016-02 resulted in an increase in the Company’s assets and liabilities of $7.9 million at July 1, 2019. The Company’s adoption of ASU No. 2016-02 did not have any impact to the Company’s Consolidated Statements of Operations, or its Consolidated Statements of Cash Flows. Further, there was no impact on the Company’s covenant compliance under its current debt agreements as a result of the adoption of ASU No. 2016-02. The Company elected the package of practical expedients included in this guidance, which allowed it to not reassess: (i) whether any expired or existing contracts contain leases; (ii) the lease classification for any expired or existing leases; and, (iii) the initial direct costs for existing leases. The Company does not recognize short-term leases of 12 months or
126
less on its Consolidated Balance Sheets and will recognize those lease payments in the Consolidated Statements of Operations on a straight-line basis over the lease term.
Recent Accounting Pronouncements, Not Yet Adopted
In June 2016, the FASB issued ASU 2016-13, Measurement of Credit Losses on Financial Instruments, which changes the impairment model used to measure credit losses for most financial assets. We will be required to use a new forward-looking expected credit loss model that will replace the existing incurred credit loss model for our accounts receivables and loans. The guidance is effective for fiscal years beginning after December 15, 2019, including interim periods within those fiscal years, with early adoption permitted. The Company does not anticipate that the adoption will have a material impact on its Consolidated Financial Statements.
Note 3. Restructuring Charges
Cody Restructuring Program
On June 29, 2018, the Company announced a restructuring plan related to the future of Cody Laboratories, Inc. (“Cody Labs”) and the Company’s operations (the “Cody Restructuring Plan”). The plan focused on a more select set of opportunities which would result in streamlined operations, improved efficiencies and a reduced cost structure. The Company incurred approximately $2.5 million of severance and employee-related costs under this plan. The restructuring activities under the Cody Restructuring Program were completed as of June 30, 2019.
The expenses associated with the Cody Restructuring Plan included in restructuring expenses during the fiscal year ended June 30, 2019 were as follows:
| | Fiscal Year Ended | |
(In thousands) | | June 30, 2019 | |
Employee separation costs (credits) | | $ | (585) |
Facility closure costs | |
| — |
Total | | $ | (585) |
A reconciliation of the changes in restructuring liabilities associated with the Cody Restructuring Plan from June 30, 2019 through June 30, 2020 is set forth in the following table:
| | | | | | | | | |
|
| Employee |
| Facility Closure |
| | |||
(In thousands) |
| Separation Costs |
| Costs |
| Total | |||
Balance at June 30, 2019 | | $ | 108 | | $ | — | | $ | 108 |
Restructuring Charges | |
| — | |
| — | |
| — |
Payments | |
| (108) | |
| — | |
| (108) |
Balance at June 30, 2020 | | $ | — | |
| — | | $ | — |
Cody API Restructuring Plan
In September 2018, the Company approved a plan to sell the active pharmaceutical ingredient manufacturing distribution business of Cody Labs (the “Cody API business”). The Company was unable to sell the Cody API business as an ongoing operation and decided to sell the real estate utilized by the Cody API business and to have Cody Labs cease all operations. In June 2019, the Company approved the Cody API Restructuring Plan. In connection with the Cody API Restructuring Plan, the Company eliminated approximately 70 positions at Cody Labs. The restructuring activities under the Cody API Restructuring Plan are substantially complete as of June 30, 2020. During Fiscal 2020, the Company completed the sale of the equipment associated with the Cody API business for $3.0 million. In the second quarter of Fiscal 2020, the Company signed a two-year agreement to lease a portion of the real estate to a third party.
127
The costs to implement the Cody API Restructuring Plan totaled approximately $6.2 million, including approximately $3.7 million of severance and employee-related costs and approximately $2.0 million of contract termination costs, as well as approximately $0.5 million of costs incurred in connection with moving equipment and other property to other Company-owned facilities that were originally anticipated to be incurred in connection with the Cody Restructuring Plan announced in June 2018.
The expenses associated with the Cody API Restructuring Plan included in restructuring expenses during the fiscal years ended June 30, 2020 and 2019 were as follows:
| | | | | | |
| | Fiscal Year Ended | | Fiscal Year Ended | ||
(In thousands) | | June 30, 2020 | | June 30, 2019 | ||
Employee separation costs | | $ | 1,275 | | $ | 2,430 |
Facility closure costs | |
| 496 | |
| — |
Total | | $ | 1,771 | | $ | 2,430 |
A reconciliation of the changes in restructuring liabilities associated with the Cody API Restructuring Plan from June 30, 2018 through June 30, 2020 is set forth in the following table:
| | | | | | | | | | | | |
| | Employee | | Contract | | Facility | | | | |||
| | Separation | | Termination | | Closure | | | | |||
(In thousands) |
| Costs |
| Costs |
| Costs |
| Total | ||||
Balance at June 30, 2018 | | $ | — |
| $ | — | | $ | — | | $ | — |
Restructuring Charges | |
| 2,430 | | | — | |
| — | |
| 2,430 |
Payments | |
| (223) | | | — | |
| — | |
| (223) |
Balance at June 30, 2019 | | $ | 2,207 |
| $ | — | | $ | — | | $ | 2,207 |
Restructuring Charges | |
| 1,275 | | | — | |
| 496 | |
| 1,771 |
Payments | |
| (3,455) | | | — | |
| (496) | |
| (3,951) |
Balance at June 30, 2020 | | $ | 27 |
| $ | — | | | — | | $ | 27 |
2016 Restructuring Program
On February 1, 2016, in connection with the acquisition of Kremers Urban Pharmaceuticals Inc. (“KUPI”), the Company announced a plan related to the future integration of KUPI and the Company’s operations (the “2016 Restructuring Program”). The plan focused on the closure of KUPI’s corporate functions and the consolidation of manufacturing, sales, research and development and distribution functions. The restructuring activities under the 2016 Restructuring Program were completed as of March 31, 2019. The Company incurred an aggregate of approximately $21.0 million in restructuring charges for actions that have been announced or communicated since the 2016 Restructuring Program began. Of this amount, approximately $11.0 million related to employee separation costs, approximately $1.0 million relates to contract termination costs and approximately $9.0 million related to facility closure costs and other actions.
The expenses associated with the restructuring program included in restructuring expenses during the fiscal year ended June 30, 2019 were as follows:
| | | | | | |
| | Fiscal Year Ended | | Fiscal Year Ended | ||
(In thousands) | | June 30, 2019 | | June 30, 2018 | ||
Employee separation costs | | $ | 1,084 | | $ | 246 |
Facility closure costs | |
| 1,166 | |
| 3,723 |
Total | | $ | 2,250 | | $ | 3,969 |
128
Note 4. Accounts Receivable
Accounts receivable consisted of the following components at June 30, 2020 and 2019:
| | | | | | |
| | June 30, |
| June 30, | ||
(In thousands) |
| 2020 |
| 2019 | ||
Gross accounts receivable | | $ | 271,557 | | $ | 361,323 |
Less: Chargebacks reserve | |
| (61,877) | |
| (89,567) |
Less: Rebates reserve | |
| (24,536) | |
| (32,099) |
Less: Returns reserve | |
| (40,796) | |
| (55,554) |
Less: Other deductions | |
| (17,557) | |
| (18,128) |
Less: Allowance for doubtful accounts | |
| (1,103) | |
| (1,223) |
Accounts receivable, net | | $ | 125,688 | | $ | 164,752 |
For the fiscal year ended June 30, 2020, the Company recorded a provision for chargebacks, rebates, returns and other deductions of $761.8 million, $223.9 million, $16.9 million and $88.5 million, respectively. For the fiscal year ended June 30, 2019, the Company recorded a provision for chargebacks, rebates, returns and other deductions of $1.0 billion, $250.6 million, $42.0 million and $67.3 million, respectively. For the fiscal year ended June 30, 2018, the Company recorded a provision for chargebacks, rebates, returns and other deductions of $1.1 billion, $296.8 million, $24.0 million and $69.9 million, respectively.
The following table identifies the activity and ending balances of each major category of revenue-related reserve for fiscal years 2020, 2019 and 2018:
| | | | | | | | | | | | | | | |
Reserve Category | | | | | | | | | | | | | | | |
(In thousands) |
| Chargebacks |
| Rebates |
| Returns |
| Other |
| Total | |||||
Balance at June 30, 2017 | | $ | 79,537 | | $ | 87,616 | | $ | 42,135 | | $ | 11,096 | | $ | 220,384 |
Current period provision | | | 1,141,995 | | | 296,784 | | | 24,024 | | | 69,898 | | | 1,532,701 |
Credits issued during the period | |
| (1,068,498) | |
| (301,898) | |
| (23,100) | |
| (60,973) | |
| (1,454,469) |
Balance at June 30, 2018 | |
| 153,034 | |
| 82,502 | |
| 43,059 | |
| 20,021 | |
| 298,616 |
Adjustment related to adoption of ASC 606 | |
| — | |
| — | |
| — | |
| 3,536 | |
| 3,536 |
Current period provision | |
| 1,047,192 | |
| 250,555 | |
| 41,982 | |
| 67,344 | |
| 1,407,073 |
Credits issued during the period | |
| (1,110,659) | |
| (254,783) | |
| (29,487) | |
| (72,773) | |
| (1,467,702) |
Balance at June 30, 2019 | | | 89,567 | | | 78,274 | | | 55,554 | | | 18,128 | | | 241,523 |
Current period provision | |
| 761,787 | | | 223,932 | | | 16,863 | | | 88,468 | |
| 1,091,050 |
Credits issued during the period | |
| (789,477) | | | (239,495) | | | (31,621) | | | (89,039) | |
| (1,149,632) |
Balance at June 30, 2020 | | $ | 61,877 | | $ | 62,711 | | $ | 40,796 | | $ | 17,557 | | $ | 182,941 |
For the fiscal years ended June 30, 2020, 2019 and 2018, as a percentage of gross sales the provision for chargebacks was 47.2%, 51.4% and 52.0%, respectively, the provision for rebates was 13.9%, 12.3% and 13.5%, respectively, the provision for returns was 1.0%, 2.1% and 1.1%, respectively and the provision for other adjustments was 5.5%, 3.3% and 3.2%, respectively.
On July 1, 2018, the Company adopted ASC 606 which resulted in a $3.2 million pre-tax adjustment to opening retained earnings and accounts receivable, of which $3.5 million related to “failure-to-supply” reserves offset by $0.3 million related to the timing of recognition of certain contract manufacturing arrangements.
The decrease in total reserves from June 30, 2019 to June 30, 2020 was primarily attributable to a decrease in total net sales as well as product sales mix in the fiscal year ended June 30, 2020 as compared to the fiscal year ended June 30, 2019. The decrease in the chargebacks reserve was primarily due to decreases to wholesale acquisition pricing to our customers, which decrease the expected chargeback submitted by the wholesaler. The rebates reserve decreased primarily as a result of a $9.4 million rebate payment to the Department of Veteran's Affairs related to pricing overcharges, of which $8.1 million was indemnified by UCB, the former parent company of KUPI. The decrease in the returns reserve was primarily due to higher returns associated with Levothyroxine sales during the fiscal year ended June
129
30, 2019. Historically, we have not recorded any material amounts in the current period related to reversals or additions of prior period reserves.
Note 5. Inventories
Inventories at June 30, 2020 and 2019 consisted of the following:
| | June 30, | | June 30, | ||
(In thousands) |
| 2020 |
| 2019 | ||
Raw Materials | | $ | 59,703 | | $ | 56,740 |
Work-in-process | |
| 12,235 | |
| 18,988 |
Finished Goods | |
| 70,929 | |
| 68,243 |
Total | | $ | 142,867 | | $ | 143,971 |
Inventory balances were written-down by $13.6 million and $20.7 million at June 30, 2020 and 2019, respectively, for excess and obsolete inventory amounts. During the fiscal years ended June 30, 2020, 2019 and 2018, the Company recorded write-downs to net realizable value for excess and obsolete inventory of $10.3 million, $21.8 million and $12.2 million, respectively.
Note 6. Property, Plant and Equipment
Property, plant and equipment at June 30, 2020 and 2019 consisted of the following:
| | | | June 30, |
| June 30, | ||
(In thousands) |
| Useful Lives |
| 2020 |
| 2019 | ||
Land |
| — | | $ | 1,783 | | $ | 1,783 |
Building and improvements |
| 10 - 39 years | |
| 100,285 | |
| 87,609 |
Machinery and equipment |
| 5 - 10 years | |
| 164,704 | |
| 156,166 |
Furniture and fixtures |
| 5 - 7 years | |
| 3,116 | |
| 3,105 |
Less accumulated depreciation | | | | | (102,983) | | | (83,424) |
| | | | | 166,905 | | | 165,239 |
Construction in progress |
| | |
| 12,613 | |
| 21,431 |
Property, plant and equipment, net | | | | $ | 179,518 | | $ | 186,670 |
Depreciation expense for the fiscal years ended June 30, 2020, 2019 and 2018 was $24.3 million, $23.4 million and $22.4 million, respectively.
In the first quarter of Fiscal 2019, the Company approved a plan to sell the Cody API business and performed a fair value analysis which resulted in a $29.9 million impairment of the Cody API property, plant and equipment assets. The Company was unable to sell the Cody API business as an ongoing operation and decided to sell the equipment and real estate utilized by the Cody API business and to have Cody Labs cease all operations. As such, Cody Labs’ property, plant and equipment totaling $6.7 million, were recorded in the assets held for sale caption in the Consolidated Balance Sheet as of June 30, 2019. As of June 30, 2020, the Company has a remaining balance of $2.7 million recorded in the assets held for sale caption in the Consolidated Balance Sheet. See Note 18 “Assets Held for Sale” for more information.
Property, plant and equipment, net included amounts held in foreign countries in the amount of $0.6 million and $1.0 million at June 30, 2020 and June 30, 2019, respectively.
Note 7. Fair Value Measurements
The Company’s financial instruments recorded in the Consolidated Balance Sheets include cash and cash equivalents, accounts receivable, accounts payable, accrued expenses and debt obligations. The Company’s cash and cash equivalents include money market funds. The carrying value of certain financial instruments, primarily cash and cash
130
equivalents, accounts receivable, accounts payable and accrued expenses, approximate their estimated fair values based upon the short-term nature of their maturity dates.
The Company follows the authoritative guidance of ASC Topic 820 “Fair Value Measurements and Disclosures.” Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The authoritative guidance also establishes a fair value hierarchy which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The Company’s financial assets and liabilities measured at fair value are entirely within Level 1 of the hierarchy as defined below:
Level 1 — Quoted prices (unadjusted) in active markets for identical assets or liabilities that the reporting entity can access at the measurement date.
Level 2 — Directly or indirectly observable inputs, other than quoted prices, such as quoted prices for similar assets or liabilities; quoted prices for identical or similar instruments in markets that are not active; or model-derived valuations whose inputs are observable or whose significant value drivers are observable.
Level 3 — Unobservable inputs that are supported by little or no market activity and that are material to the fair value of the asset or liability. Financial instruments whose values are determined using pricing models, discounted cash flow methodologies, or similar techniques, as well as instruments for which the determination of fair value requires significant judgment or estimation are examples of Level 3 assets and liabilities.
If the inputs used to measure the financial assets and liabilities fall within more than one level described above, the categorization is based on the lowest level input that is significant to the fair value measurement of the instrument.
Financial Instruments Disclosed, But Not Reported, at Fair Value
We estimate the fair value of our debt utilizing market quotations for debt that have quoted prices in active markets. Since our debt does not trade on a daily basis in an active market, the fair value estimates are based on market observable inputs based on borrowing rates currently available for debt with similar terms and average maturities (Level 2). The estimated fair value of our term loan debt was approximately $608 million and $724 million as of June 30, 2020 and June 30, 2019, respectively. The estimated fair value of our 4.5% Convertible Senior Notes was approximately $58 million as of June 30, 2020, which was lower than the carrying value primarily due to the Company’s stock price at June 30, 2020 as compared to the $15.29 conversion price.
Note 8. Goodwill and Intangible Assets
On August 17, 2018, JSP notified the Company that it would not extend or renew the JSP Distribution Agreement when the current term expired on March 23, 2019. The Company determined that JSP’s decision represented a triggering event under U.S. GAAP to perform an analysis to determine the potential for impairment of goodwill. On October 4, 2018, the Company completed the analysis based on market data and concluded a full impairment of goodwill, totaling $339.6 million, was required.
131
Intangible assets, net as of June 30, 2020 and June 30, 2019, consisted of the following:
| | Weighted | | Gross Carrying Amount | | Accumulated Amortization | | Intangible Assets, Net | ||||||||||||
|
| Avg. Life |
| June 30, |
| June 30, |
| June 30, |
| June 30, |
| June 30, |
| June 30, | ||||||
(In thousands) |
| (Yrs.) |
| 2020 |
| 2019 |
| 2020 |
| 2019 |
| 2020 |
| 2019 | ||||||
Definite-lived: | | | | | | | | | | | | | | | | | | | | |
KUPI product rights | | 15 | | | 416,154 | | | 416,154 | | | (125,327) | | | (97,583) | | | 290,827 | | | 318,571 |
KUPI trade name | | 2 | | | 2,920 | | | 2,920 | | | (2,920) | | | (2,920) | | | — | | | — |
KUPI other intangible assets | | 15 | | | 19,000 | | | 19,000 | | | (5,828) | | | (4,562) | | | 13,172 | | | 14,438 |
Silarx product rights | | 15 | | | 20,000 | | | 10,000 | | | (3,556) | | | (2,722) | | | 16,444 | | | 7,278 |
Other product rights | | 10 | | | 50,718 | | | 39,160 | | | (5,426) | | | (4,667) | | | 45,292 | | | 34,493 |
Total definite-lived | | | | $ | 508,792 | | $ | 487,234 | | $ | (143,057) | | $ | (112,454) | | $ | 365,735 | | $ | 374,780 |
| | | | | | | | | | | | | | | | | | | | |
Indefinite-lived: | | | | | | | | | | | | | | | | | | | | |
KUPI in-process research and development | | — | | $ | 9,000 | | $ | 18,000 | | $ | — | | $ | — | | $ | 9,000 | | $ | 18,000 |
Silarx in-process research and development | | — | | | — | | | 18,000 | | | — | | | — | | | — | | | 18,000 |
Other product rights | | — | | | — | | | 449 | | | — | | | — | | | — | | | 449 |
Total indefinite-lived | | | | | 9,000 | | | 36,449 | | | — | | | — | | | 9,000 | | | 36,449 |
Total intangible assets, net | | | | $ | 517,792 | | $ | 523,683 | | $ | (143,057) | | $ | (112,454) | | $ | 374,735 | | $ | 411,229 |
For the fiscal years ended June 30, 2020, 2019 and 2018, the Company recorded amortization expense of $32.0 million, $32.2 million and $32.7 million, respectively.
In the fourth quarter of Fiscal 2020, the Company performed its annual impairment test of our indefinite-lived intangible assets. As a result, the Company recorded a $9.0 million and an $8.0 million impairment charge to its KUPI IPR&D and Silarx IPR&D assets, respectively, due to the abandonment of several pipeline products within both portfolios. The Company also reclassed $10.0 million of Silarx IPR&D assets into Silarx product rights and the Company began amortization of the assets over a useful life of 15 years in Fiscal 2020.
In the third quarter of Fiscal 2020, the Company performed an impairment analysis of its AB-rated Methylphenidate Hydrochloride product, which is distributed under a license agreement with Andor Pharmaceuticals, LLC (“Andor”), due to additional competition resulting in a significant decline in sales and overall profitability of the distribution arrangement. The analysis resulted in the Company recording a $14.0 million impairment charge. The remaining carrying value of the intangible asset as of June 30, 2020, totaling $2.1 million, is recorded within the definite-lived “other product rights” caption in the table above and will continue to be amortized over its remaining useful life.
The Company previously entered into a distribution and supply agreement with Dexcel Pharma Technologies to distribute Venlafaxine XR upon FDA approval. In the second quarter of Fiscal 2020, Dexcel Pharma Technologies received FDA approval and the Company initiated commercial launch of the product. The Company paid total consideration of $3.0 million upon reaching certain milestones, which is included within the “Other product rights” category of definite-lived intangible assets.
In July 2019, the Company entered into a distribution and supply agreement with Cediprof, Inc. The Company made an upfront payment of $20.0 million to distribute Levothyroxine Sodium Tablets USP, commencing no later than August 1, 2022, which is included within the “Other product rights” category of definite-lived intangible assets. In August 2019, the Company entered into a distribution and supply agreement with Sinotherapeutics Inc. to distribute Posaconazole Delayed-Release Tablets 100mg. The Company paid $2.0 million upon FDA approval of the product and $1.5 million upon the first commercial sale of the product, which is included within the “Other product rights” category of definite-lived intangible assets.
132
Future annual amortization expense consists of the following:
(In thousands) |
| Amortization | |
Fiscal Year Ending June 30, |
| Expense | |
2021 | | $ | 34,068 |
2022 | |
| 35,668 |
2023 | |
| 35,367 |
2024 | |
| 35,067 |
2025 | |
| 34,667 |
Thereafter | |
| 190,898 |
| | $ | 365,735 |
Note 9. Long-Term Debt
Long-term debt, net consisted of the following:
| | | | | | |
| | June 30, | | June 30, | ||
(In thousands) |
| 2020 |
| 2019 | ||
Term Loan A due 2020; 6.00% as of June 30, 2020 | | $ | 48,844 | | $ | 153,933 |
Unamortized discount and other debt issuance costs | |
| (433) | |
| (4,722) |
Term Loan A, net | |
| 48,411 | |
| 149,211 |
Term Loan B due 2022; 6.38% as of June 30, 2020 | |
| 572,857 | |
| 614,468 |
Unamortized discount and other debt issuance costs | |
| (23,278) | |
| (34,631) |
Term Loan B, net | |
| 549,579 | |
| 579,837 |
4.50% Convertible Senior Notes due 2026 | | | 86,250 | | | — |
Unamortized discount and other debt issuance costs | | | (3,111) | | | — |
4.50% Convertible Senior Notes, net | | | 83,139 | | | — |
$125 million Revolving Credit Facility due 2020 | |
| — | |
| — |
Total debt, net | |
| 681,129 | |
| 729,048 |
Less short-term borrowings and current portion of long-term debt | |
| (88,189) | |
| (66,845) |
Total long-term debt, net |
| $ | 592,940 |
| $ | 662,203 |
The weighted average interest rate for Fiscal 2020 and 2019 was 8.8% and 9.7%, respectively.
On September 27, 2019, the Company issued $86,250,000 aggregate principal amount of its 4.50% convertible senior notes due 2026 (the “Notes”) in a private offering to qualified institutional buyers pursuant to Rule 144A under the Securities Act of 1933, as amended. The Notes are senior unsecured obligations of the Company and bear interest at an annual rate of 4.50% payable semi-annually in arrears on April 1 and October 1 of each year, beginning on April 1, 2020. The Notes will mature on October 1, 2026, unless earlier repurchased, redeemed or converted in accordance with their terms. The Notes are convertible into shares of the Company’s common stock at an initial conversion rate of 65.4022 shares per $1,000 principal amount of Notes (which is equivalent to an initial conversion price of approximately $15.29 per share), subject to adjustments upon the occurrence of certain events (but will not be adjusted for any accrued and unpaid interest). The Company may redeem all or a part of the Notes on or after October 6, 2023 at a redemption price equal to 100% of the principal amount of the Notes redeemed, plus accrued and unpaid interest, if any, up to, but excluding, the redemption date, subject to certain conditions relating to the Company's stock price having been met.
Following certain corporate events that occur prior to the maturity date or if the Company delivers a notice of redemption, the Company will, in certain circumstances, increase the conversion rate for a holder who elects to convert its Notes in connection with such corporate event or notice of redemption. The indenture covering the Notes contains certain other customary terms and covenants, including that upon certain events of default occurring and continuing, either the trustee or holders of at least 25% in principal amount of the outstanding Notes may declare 100% of the principal of, and accrued and unpaid interest on, all the Notes to be due and payable.
133
In connection with the offering of the Notes, the Company also entered into privately negotiated “capped call” transactions with several counterparties. The capped call transaction will initially cover, subject to customary anti-dilution adjustments, the number of shares of common stock that initially underlie the Notes. The capped call transactions are expected to generally reduce the potential dilutive effect on the Company’s common stock upon any conversion of the Notes with such reduction subject to a cap which is initially $19.46 per share. The capped call transactions are recorded in stockholders' equity and are not accounted for as derivatives. The fees associated with the capped call transactions totaled $7.1 million and were recorded as a reduction to additional paid-in capital on the Consolidated Balance Sheet. The form of capped call confirmations was filed as Exhibit 10.57 to the Form 8-K filed with the SEC on September 27, 2019.
A portion of the net proceeds received from the offering of the Notes was used to pay the cost of the capped call transactions. The remaining net proceeds, totaling $77.0 million, was used to repay a portion of the outstanding Term Loan A balance on September 27, 2019. As a result of the repayment, the Company recorded a loss on extinguishment of debt of $2.1 million in the Consolidated Statement of Operations in Fiscal 2020.
Long-term debt amounts due, for the twelve month periods ending June 30 were as follows:
| | | |
| | Amounts Payable | |
(In thousands) |
| to Institutions | |
2021 | | $ | 88,189 |
2022 | |
| 39,345 |
2023 | |
| 494,167 |
2024 | |
| — |
Thereafter | |
| 86,250 |
Total | | $ | 707,951 |
The outstanding Term Loan A, Term Loan B and Revolving Credit Facility amounts above are guaranteed by all of Lannett’s significant wholly-owned domestic subsidiaries and are collateralized by substantially all present and future assets of the Company.
Note 10. Legal, Regulatory Matters and Contingencies
State Attorneys General Inquiry into the Generic Pharmaceutical Industry
In July 2014, the Company received interrogatories and a subpoena from the State of Connecticut Office of the Attorney General concerning its investigation into the pricing of digoxin. According to the subpoena, the Connecticut Attorney General is investigating whether anyone engaged in any activities that resulted in (a) fixing, maintaining or controlling prices of digoxin or (b) allocating and dividing customers or territories relating to the sale of digoxin in violation of Connecticut antitrust law. In June 2016, the Connecticut Attorney General issued interrogatories and a subpoena to an employee of the Company in order to gain access to documents and responses previously supplied to the Department of Justice pursuant to the federal investigation described below. In December 2016, the Connecticut Attorney General, joined by numerous other State Attorneys General, filed a civil complaint alleging that six pharmaceutical companies engaged in anti-competitive behavior. The Company was not named in the action and does not compete on the products that formed the basis of the complaint. The complaint was later transferred for pretrial purposes to the United States District Court for the Eastern District of Pennsylvania as part of a multidistrict litigation captioned In re: Generic Pharmaceuticals Pricing Antitrust Litigation. On October 31, 2017, the State Attorneys General filed a motion in the District Court for leave to amend their complaint to add numerous additional defendants, including the Company, and claims relating to 13 additional drugs. The Court granted that motion on June 5, 2018. The State Attorneys General filed their amended complaint on June 18, 2018. The claim relating to Lannett involves alleged price-fixing for one drug, doxycycline monohydrate, but does not involve the pricing for digoxin. The State Attorneys General also allege that all defendants were part of an overarching, industry-wide conspiracy to allocate markets and fix prices generally. On August 15, 2019, the Court denied the defendants' joint motion to dismiss the overarching conspiracy claims, but has yet to decide an individual motion filed by the Company to dismiss the overreaching conspiracy claims as to it.
134
On May 10, 2019, the State Attorneys General filed a new lawsuit naming the Company, and one of its employees as defendants, along with 33 other corporations and individuals. The new complaint again alleges an overarching conspiracy and contains claims for price fixing and market allocation under the Sherman Act and related state laws. The complaint focuses on the conduct of another generic pharmaceutical company, and the relationships that company had with other generic companies and their employees. The specific allegations in the new complaint against Lannett relate to the Company’s sales of baclofen and levothyroxine. The new complaint also names another current employee as a defendant, however the allegations pertain to conduct that occurred prior to their employment by Lannett. The Company has not responded to the new complaint as of the date of this report.
Based on internal investigations performed to date, the Company currently believes that it has acted in compliance with all applicable laws and regulations.
Federal Investigation into the Generic Pharmaceutical Industry
In November and December 2014, the Company and certain affiliated individuals and customers were served with grand jury subpoenas relating to a federal investigation of the generic pharmaceutical industry into possible violations of the Sherman Act. The subpoenas request corporate documents of the Company relating to corporate, financial and employee information, communications or correspondence with competitors regarding the sale of generic prescription medications and the marketing, sale, or pricing of certain products, generally for the period of 2005 through the dates of the subpoenas.
The Company received a Civil Investigative Demand (“CID”) from the Department of Justice on May 14, 2018. The CID requests information regarding allegations that the generic pharmaceutical industry engaged in market allocation, price fixing, payment of illegal remuneration and submission of false claims. The CID requests information from 2009-present. The Company is in the process of responding to the CID.
Based on internal investigations performed to date, the Company believes that it has acted in compliance with all applicable laws and regulations.
Government Pricing
During the quarter ended December 31, 2016, the Company completed a contract compliance review, for the period January 1, 2012 through June 30, 2016, for one of KUPI’s government-entity customers. As a result of the review, the Company identified certain commercial customer prices and other terms that were not properly disclosed to the government-entity resulting in potential overcharges. For the period January 1, 2012 through November 24, 2015 (“the pre-acquisition period”), the Company is fully indemnified per the Stock Purchase Agreement.
On May 22, 2019, the Department of Veterans Affairs issued a Contracting Officer’s Final Decision and Demand for Payment, assessing the sum of $9.4 million for overpayments by the Veteran’s Administration for the period of January 1, 2012 through June 30, 2016. In August 2019, the Company remitted payment to the VA and received reimbursement from UCB for the indemnified portion of the payment in the amount of $8.1 million.
Private Antitrust and Consumer Protection Litigation
In 2016 and 2017, the Company and certain competitors were named as defendants in a number of lawsuits alleging that the Company and certain generic pharmaceutical manufacturers have conspired to fix prices of generic digoxin, levothyroxine, ursodiol and baclofen. These cases are part of a larger group of more than 100 lawsuits generally alleging that over 30 generic pharmaceutical manufacturers and distributors conspired to fix prices for multiple different generic drugs in violation of the federal Sherman Act, various state antitrust laws, and various state consumer protection statutes. The United States also has been granted leave to intervene in the cases. On April 6, 2017, the Judicial Panel on Multidistrict Litigation (the “JPML”) ordered that all of the cases alleging price-fixing for generic drugs be consolidated for pretrial proceedings in the United States District Court for the Eastern District of Pennsylvania under the caption In re: Generic Pharmaceuticals Pricing Antitrust Litigation (the “MDL”). The various plaintiffs are grouped into three
135
categories - Direct Purchaser Plaintiffs, End Payer Plaintiffs, and Indirect Reseller Purchasers - and filed Consolidated Amended Complaints (“CACs”) against the Company and the other defendants on August 15, 2017.
The CACs naming the Company as a defendant involve generic digoxin, levothyroxine, ursodiol and baclofen. Pursuant to a court-ordered schedule grouping the 18 different drug cases into three separate tranches, the Company and other generic pharmaceutical manufacturer defendants on October 6, 2017 filed joint and individual motions to dismiss the CACs involving the six drugs in the first tranche, including digoxin. On October 16, 2018, the Court (with one exception) denied defendants’ motions to dismiss plaintiffs’ Sherman Act claims with respect to the drugs in the first tranche. On March 15, 2019, the Company and other defendants filed answers to the Sherman Act claims. In addition, on February 15, 2019, the Court granted defendants’ motions to dismiss certain of the plaintiffs’ state law claims brought under the laws of Illinois, Rhode Island, Georgia, South Carolina, Montana, West Virginia, Alabama, New Jersey, Michigan and Nevada, but denied the remainder of defendants’ motions to dismiss. The Court set a deadline of April 1, 2019 for certain plaintiffs to amend their existing complaints to reflect the rulings set forth in the Court’s February 15, 2019 ruling on the state law motions to dismiss. Those plaintiffs amended their complaints, but further motions to dismiss the state-law claims have been deferred until the Court decides pending motions to dismiss with respect to the plaintiffs’ various overarching-conspiracy claims.
In addition to lawsuits brought by private plaintiffs, the Attorneys General of 49 states, the District of Columbia, Puerto Rico and various territories have filed their own lawsuits alleging overarching, industry-wide price-fixing conspiracies by the Company and various other generic pharmaceutical manufacturers. Those suits have been consolidated in the MDL. The first suit alleges that the Company was involved in price-fixing for one drug, doxycycline monohydrate. Defendants’ joint motion to dismiss the overarching conspiracy claims in that suit was denied on August 15, 2019, but the Company’s individual motion to dismiss the overarching conspiracy claims as to it remains outstanding. The Attorneys General also filed a second overarching conspiracy complaint on May 10, 2019 involving dozens of different drugs, including alleged price-fixing by the Company for baclofen and levothyroxine. On June 10, 2020, the Attorneys General filed a third overarching conspiracy complaint involving scores of different drugs, including alleged price-fixing by the Company for acetazolamide.
Following the lead of the state Attorneys General, the Direct Purchaser Plaintiffs, End Payer Plaintiffs and Indirect Reseller Plaintiffs filed their own complaints in June 2018 alleging an overarching conspiracy, making similar allegations to those of the state Attorneys General, relating to 14 generic drugs in the End Payer complaint and 15 generic drugs in the Indirect Reseller complaint. Although the complaints allege an overarching conspiracy with respect to all of the drugs identified, the specific allegations related to drugs the Company manufactures involve acetazolamide and doxycycline monohydrate.
The Company and the other defendants filed motions to dismiss the overarching conspiracy claims. On August 15, 2019, the Court denied the defendants' joint motion to dismiss the overarching conspiracy claims, but has yet to decide an individual motion filed by the Company to dismiss the overarching conspiracy claims as to it. In addition, between December 2019 and February 2020, the End Payer Plaintiffs, Indirect Reseller Purchasers, and Direct Purchaser Plaintiffs filed separate complaints alleging overarching, industry-wide price-fixing conspiracies modeled on the second one filed by the state Attorneys General. The new complaint involve 135 new drugs in addition to those named in previous complaints. As to the Company, the new drugs involved are Pilocarpine HCL, Triamterence HCTZ capsules, Amantadine HCL, and Oxycodone HCL. None of the defendants, including the Company, has responded yet to these new complaints.
Between January 2018 and June 2020, a number of opt-out parties have filed individual complaints or otherwise commenced actions against the Company and dozens of other companies alleging an overarching conspiracy and individual conspiracies to fix the prices and allocate markets on scores of different drug products, including digoxin, doxycycline, levothyroxine, ursodiol and baclofen. The opt-out parties include various retailers, insurers and county governments, which have filed federal suits in Pennsylvania, New York, California, and Texas. All of those complaints have been added to the MDL but none of the defendants, including the Company, has responded to any of the complaints. Other groups of insurers have commenced actions in Pennsylvania state court against the Company and other drug companies by filing writs of summons, which are not complaints but can serve to toll the running of statutes
136
of limitations. Those state-court cases have not been added to the MDL, although the parties have agreed to stay those cases pending further developments in the MDL.
In June 2020, the Company was named as a Defendant in a Statement of Claim, along with a number of other generic pharmaceutical manufacturers, in a proposed class proceeding in federal court in Toronto, Ontario, Canada. The case alleges a violation of Canada’s Competition Act. The allegations are similar to those contained in the antitrust litigation pending in the United States, including claims that the generic pharmaceutical manufacturers engaged in an overarching, industry-wide conspiracy to allocate markets and fix the price of generic drugs. That alleged conspiracy reached Canada because these same manufacturers also allegedly sell the majority of generic drugs in Canada. The Statement of Claim alleges that the conspiracy extends to the entire generic pharmaceutical market. The specific drugs identified with respect to the Company are: Acetazolamide, Baclofen, Digoxin, Doxycycline Monohydrate, Levothyroxine, and Ursodiol. The Company has not yet responded to the Statement of Claim.
On July 13, 2020, the court overseeing the MDL overruled the objections of the Company and various other defendants and adopted a report and recommendation of a special master regarding the selection of certain “bellwether” cases in the MDL to be placed first on a trial track. The court selected the second overarching conspiracy case filed by the state Attorneys General on May 10, 2019 as well as individual-conspiracy cases filed by the Direct Purchaser Plaintiffs, End Payer Plaintiffs, and Indirect Reseller Purchasers involving the drugs clobetasol, clomipramine and pravastatin. The Company is a defendant in the overarching conspiracy case, but not in the clobetasol, clomipramine and pravastatin cases. To date, none of the “bellwether” cases has been scheduled for trial.
The Company believes that it acted in compliance with all applicable laws and regulations. Accordingly, the Company disputes the allegations set forth in these class actions and plans to vigorously defend itself from these claims.
Shareholder Litigation
In November 2016, a putative class action lawsuit was filed against the Company and two of its former officers in the federal court for the Eastern District of Pennsylvania, alleging that the Company, its former Chief Executive Officer, and its former Chief Financial Officer damaged the purported class by including in its securities filings false and misleading statements regarding the Company’s drug pricing methodologies and internal controls. An amended complaint was filed in May 2017, and the Company filed a motion to dismiss the amended complaint in September 2017. In December 2017, counsel for the putative class filed a second amended complaint, and the Court denied as moot the Company’s motion to dismiss the first amended complaint. The Company filed a motion to dismiss the second amended complaint in February 2018. In July 2018, the court granted the Company’s motion to dismiss the second amended complaint . In September 2018, counsel for the putative class filed a third amended complaint. The Company filed a motion to dismiss the third amended complaint in November 2018. In May 2019, the court denied the Company’s motion to dismiss the third amended complaint. In July 2019, the Company filed an answer to the third amended complaint. The Company believes it acted in compliance with all applicable laws and plans to vigorously defend itself from these claims. The Company cannot reasonably predict the outcome of the suit at this time.
In May 2019, a shareholder derivative lawsuit was filed against certain of the Company’s current and former officers and certain of the current and former members of the Company’s Board of Directors in the federal court for the District of Delaware. The Company was also named as a nominal defendant in the suit. The suit alleges that the defendants breached their fiduciary duties as directors and/or officers of the Company, that certain of the defendants caused the Company to issue false and misleading proxy statements in violation of Section 14(a) of the Securities Exchange Act of 1934, that the defendants were unjustly enriched at the expense of the Company, and that the defendants wasted corporate assets belonging to the Company. On December 4, 2019 the court entered a stipulation consolidating the suit with a separate shareholder derivative suit filed in July 2019, as described below. On December 6, 2019, the Company filed a motion to dismiss the consolidated cases. On January 14, 2020, the parties reached an agreement in principle to resolve the consolidated cases, subject to the execution of a mutually acceptable settlement document and Court approval.
In July 2019, a shareholder derivative lawsuit was filed against certain of the Company’s current and former officers and directors in the federal court for the Eastern District of Pennsylvania. The Company was also named as a nominal
137
defendant in the suit. The suit alleges that the defendants breached their fiduciary duties as directors and/or officers of the Company and that certain of the defendants caused the Company to violate Sections 10(b), 14(a), and 29(b) of the Securities Exchange Act of 1934. In October 2019, this suit was transferred to the federal court for the District of Delaware and is pending before the same judge presiding over the shareholder derivative suit that was filed in May 2019. On December 4, 2019, the Court entered a stipulation consolidating the suit with a separate shareholder derivative suit filed in May 2019, as described above. On December 6, 2019, the Company filed a motion to dismiss the consolidated cases. On January 14, 2020, the parties reached an agreement in principle to resolve the consolidated cases, subject to the execution of a mutually agreeable settlement document and Court approval.
The settlement of the two consolidated cases, if approved by the Court, will require the Company to implement certain new corporate policies and pay the plaintiffs’ counsel in the consolidated cases, collectively, the sum of $600,000 in exchange for a release of all liability with respect to both of the consolidated cases. On March 27, 2020, the parties jointly submitted a letter to the Court enclosing an executed Memorandum of Understanding setting forth the terms of their agreement and stating that the parties intend to execute and file a formal settlement stipulation for approval by the Court. On May 22, 2020, the plaintiffs filed an unopposed motion for preliminary approval of the proposed settlement. On June 1, 2020, in view of the plaintiffs’ unopposed motion for preliminary approval of the proposed settlement, the Court denied the defendants’ motions to dismiss the consolidated cases, without prejudice to the defendants’ ability to renew those motions if the proposed settlement is not approved. The Company cannot reasonably predict the outcome of the consolidated suits at this time.
In September 2019, a shareholder derivative lawsuit was filed against certain of the Company’s current and former officers, directors, and employees in the federal court for the District of Delaware. The Company was also named as a nominal defendant in the suit. The suit alleges that the defendants breached their fiduciary duties as directors and/or officers of the Company, alleges waste of corporate assets and gross mismanagement, and alleges that certain of the defendants caused the Company to violate Section 14(a) of the Securities and Exchange Act of 1934. On November 22, 2019, the Company filed a motion to dismiss the complaint. On January 16, 2020, the Court entered the parties’ stipulation to stay the case pending the resolution of the defendants’ motion to dismiss the two earlier filed consolidated shareholder derivative cases referenced above. On February 18, 2020, the Court entered the parties’ stipulation to withdraw the Company’s motion to dismiss without prejudice to the Company’s ability to refile a renewed motion to dismiss after the stay is lifted. On March 11, 2020, following notice that Plaintiffs no longer consented to the stay, the Court lifted the stay. On April 6, 2020, certain of the defendants, including the Company, filed a renewed motion to dismiss or, in the alternative, to stay the account. On April 29, 2020, the Court entered the parties’ stipulation to stay the action, pending a decision from the Court regarding the settlement in the consolidated derivative actions discussed above. On May 14, 2020, the parties filed a joint letter and a stipulation to withdraw the defendants’ motion to dismiss without prejudice to the defendants’ ability to refile a renewed motion to dismiss after the stay is lifted. On May 15, 2020, the Court entered the parties’ stipulation. The Company cannot reasonably predict the outcome of the suit at this time.
In February 2020, a shareholder derivative lawsuit was filed against certain of the Company’s current and former officers, directors and employees in the Court of Chancery of the State of Delaware. The Company was also named as a nominal defendant in the suit. The suit alleges that the defendants breached their fiduciary duties as directors and/or officers of the Company, and were unjustly enriched. On March 16, 2020, the Company filed a motion to dismiss the complaint, and a motion to stay the proceedings. On March 27, 2020, the Company filed its opening brief in support of its motion to stay the proceedings. On April 6, 2020, the parties entered into a stipulation and proposed order to stay the action. The Court granted the stipulation and proposed order that same day. Under the terms of the stipulated stay, the action shall be stayed pending a decision from the Court in the shareholder derivative lawsuit filed in May 2019 on the plaintiffs’ unopposed motion for approval of the proposed settlement. The Company cannot reasonably predict the outcome of the suit at this time.
Genus Life Sciences
In December 2018, Genus Lifesciences, Inc. (“Genus”) sued the Company, Cody Labs, and others in California federal court, alleging violations of the Lanham Act, Sherman Act, and California false advertising law. Genus received FDA approval for a cocaine hydrochloride product in December 2018, and its claims are premised in part on allegations that
138
the Company falsely advertises its unapproved cocaine hydrochloride solution product. The Company denies that it is falsely advertising its cocaine hydrochloride solution product and continues to market its unapproved product relying on the Guidance for FDA Staff and Industry, Marketed Unapproved Drugs — Compliance Policy Guide, pending approval of its Section 505(b)(2) application. In January 2019, the Company filed a motion to dismiss the complaint. On May 3, 2019, the Court issued a written decision granting in part and denying in part the motion to dismiss. On June 6, 2019, Genus filed an Amended Complaint. On June 27, 2019, the Company filed a motion to dismiss the amended complaint. By Order dated September 3, 2019, the Court granted in part and denied in part the Company's motion to dismiss. On November 20, 2019, Genus filed a second amended complaint. On December 17, 2019, the Company filed an answer to the second amended complaint. The Company believes it acted in compliance with all applicable laws and regulations and plans to vigorously defend itself from these claims. Discovery is ongoing and the Company cannot reasonably predict the outcome of this suit at this time.
Sandoz, Inc.
On July 20, 2020, Sandoz, Inc. (“Sandoz”) filed a complaint in federal court in Philadelphia, alleging claims for tortious interference with contract, unfair competition and conversion of confidential information, arising out of the Cediprof, Inc.’s (“Cediprof”) termination of Sandoz’ contract to distribute levothyroxine tablets in the United States and certain territories. Along with the complaint, Sandoz filed a motion for a temporary restraining order and preliminary injunction, seeking to enjoin the Company from commencing the distribution of levothyroxine tablets on August 3, 2020. On the same day, Sandoz filed a separate complaint and application for a temporary restraining order and preliminary injunction against Cediprof in federal court in New York, seeking to prevent Cediprof from selling its levothyroxine tablets in the United States and certain of its territories to anyone other than Sandoz. On July 27, 2020, the New York court held a hearing and denied Sandoz’s application for a temporary restraining order, ruling Sandoz had failed to establish irreparable harm. On July 28, 2020, the Philadelphia court held a hearing and denied Sandoz’s application for a temporary restraining order, ruling that Sandoz had failed to establish irreparable harm and failed to establish that it is likely to succeed on the merits of its claim against Lannett. The Company denies that it tortiously interfered with Sandoz’s contract or that it converted any of Sandoz’s alleged confidential information. The Company has not yet responded to the complaint.
Other Litigation Matters
The Company is also subject to various legal proceedings arising out of the normal course of its business including, but not limited to, product liability, intellectual property, patent infringement claims and antitrust matters. It is not possible to predict the outcome of these various proceedings. An adverse determination in any of these proceedings in the future could have a significant impact on the financial position, results of operations and cash flows of the Company.
Note 11. Commitments
Leases
The Company’s adoption of ASU No. 2016-02 resulted in an increase in the Company’s assets and liabilities of $7.9 million at July 1, 2019. In the first quarter of Fiscal 2020, the Company recorded a ROU lease asset totaling $1.2 million related to an existing lease at Cody Labs upon adoption of ASU No. 2016-02. The Company subsequently recorded a full impairment of the asset as a result of the decision to cease operations at Cody Labs. At June 30, 2020, the Company has a ROU lease asset of $9.3 million and a ROU liability of $10.9 million, of which $1.1 million and $9.8 million represent the current and non-current balance, respectively.
In November 2019, the Company signed an eight year lease for its new headquarters in Trevose, Pennsylvania. The Company is currently providing lease improvements and met the lease commencement date criteria under ASC Topic 842 Leases as of March 31, 2020. Accordingly, the Company recorded a ROU lease asset and liability totaling $4.3 million, respectively, in the third quarter of Fiscal 2020.
139
Components of lease costs are as follows:
| | Fiscal Year Ended | |
(In thousands) |
| June 30, 2020 | |
Operating lease cost | | $ | 2,246 |
Variable lease cost | |
| 153 |
Short-term lease cost (a) | |
| 579 |
Total | | $ | 2,978 |
______________________
(a) Not recorded on the Consolidated Balance Sheet
Supplemental cash flow information and non-cash activity related to our operating leases are as follows:
| | | |
| | Fiscal Year Ended | |
(In thousands) |
| June 30, 2020 | |
Cash paid for amounts included in the measurement of lease liabilities: | | | |
Operating cash flows from operating leases |
| $ | 2,086 |
Non-cash activity: | | | |
ROU assets obtained in exchange for new operating lease liabilities |
| $ | 4,317 |
Weighted average remaining lease term and discount rate for our operating leases are as follows:
| | Fiscal Year Ended | |
|
| June 30, 2020 | |
Weighted-average remaining lease term | | 9 | years |
Weighted-average discount rate |
| 7.91 | % |
Maturities of lease liabilities by fiscal year for our operating leases are as follows:
(In thousands) |
| Amounts Due | |
2021 | | $ | 1,101 |
2022 | | | 2,125 |
2023 | |
| 2,144 |
2024 | |
| 2,164 |
2025 | |
| 2,183 |
Thereafter | |
| 5,749 |
Total lease payments | |
| 15,466 |
Less: Imputed interest | |
| 4,525 |
Present value of lease liabilities |
| $ | 10,941 |
As of June 30, 2019, future minimum lease payments under noncancelable operating leases (with initial or remaining lease terms in excess of one year) for the twelve-month periods ending June 30 thereafter are as follows:
(In thousands) |
| Amounts Due | |
2021 |
| $ | 1,450 |
2022 | |
| 1,123 |
2023 | |
| 1,123 |
2024 | |
| 1,123 |
2025 | |
| — |
Thereafter | |
| 3,839 |
Total |
| $ | 8,658 |
140
Other Commitments
During Fiscal 2017, the Company signed an agreement with a company operating in the pharmaceutical business, under which the Company agreed to provide up to $15.0 million in revolving loans, which expires in seven years from the loan origination date and bears interest at 2.0%, for the purpose of expansion and other business needs. The decision to provide any portion of the revolving loan is at the Company’s sole discretion.
In Fiscal 2019, the Company sold 50% of the outstanding loan to a third party for $5.6 million, in addition to assigning 50% of all rights, title and interest in the loan and loan documents. As of June 30, 2020, $6.5 million was outstanding under the revolving loan and is included in other assets. Based on the guidance set forth in ASC 810-10 Consolidation, the Company has concluded that it has a variable interest in the entity. However, the Company is not the primary beneficiary to the entity and as such, is not required to consolidate the entity’s results of operations.
In Fiscal 2020, the Company executed a License and Collaboration Agreement with North South Brother Pharmacy Investment Co., Ltd. and HEC Group PTY, Ltd. (collectively, “HEC”) to develop an insulin glargine product that would be biosimilar to Lantus Solostar. Under the terms of the deal, among other things, the Company shall fund up to the initial $32 million of the development costs and split 50/50 any development costs in excess thereof. Lannett shall receive an exclusive license to distribute and market the product in the United States upon FDA approval under the 50/50 profit split for the first ten years following commercialization, followed by a 60/40 split in favor of HEC for the following five years. To date, the COVID-19 pandemic has not had a material impact on the development of the insulin glargine product. The longer countries around the world remain on lockdown, the more likely the timing of the product development and approval will be delayed.
Note 12. Accumulated Other Comprehensive Loss
The Company’s Accumulated Other Comprehensive Loss was comprised of the following components as of June 30, 2020 and 2019:
| | June 30, | ||||
(In thousands) |
| 2020 |
| 2019 | ||
Foreign Currency Translation | | | | | | |
Beginning Balance | | $ | (615) | | $ | (515) |
Net (loss) on foreign currency translation (net of tax of $0 and $0) | |
| (12) | |
| (100) |
Other comprehensive (loss), net of tax | |
| (12) | |
| (100) |
Ending Balance | | | (627) | | | (615) |
Total Accumulated Other Comprehensive Loss | | $ | (627) | | $ | (615) |
141
Note 13. Earnings (Loss) Per Common Share
A reconciliation of the Company’s basic and diluted earnings (loss) per common share was as follows:
| | | | | | | | | |
| | For Fiscal Year Ended June 30, | |||||||
(In thousands, except share and per share data) |
| 2020 |
| 2019 |
| 2018 | |||
Numerator: | | | | | | | | | |
Net income (loss) | | $ | (33,366) | | $ | (272,107) | | $ | 28,690 |
Interest expenses applicable to the Notes, net of tax | | | — | | | — | | | — |
Amortization of debt issuance costs applicable to the Notes, net of tax | | | — | | | — | | | — |
Adjusted "if-converted" net income (loss) | | $ | (33,366) | | $ | (272,107) | | $ | 28,690 |
| | | | | | | | | |
Denominator: | | | | | | | | | |
Basic weighted average common shares outstanding | |
| 38,592,618 | |
| 37,779,812 | |
| 37,127,306 |
Effect of potentially dilutive options and restricted stock awards | |
| — | |
| — | |
| 1,035,208 |
Effect of conversion of the Notes | | | — | | | — | | | — |
Diluted weighted average common shares outstanding | |
| 38,592,618 | |
| 37,779,812 | |
| 38,162,514 |
| | | | | | | | | |
Earnings (loss) per common share: | | | | | | | | | |
Basic | | $ | (0.86) | | $ | (7.20) | | $ | 0.77 |
Diluted | | $ | (0.86) | | $ | (7.20) | | $ | 0.75 |
The number of anti-dilutive shares that have been excluded in the computation of diluted earnings per share for the fiscal years ended June 30, 2020, 2019 and 2018 were 6.6 million, 1.9 million and 3.0 million, respectively. The effect of potentially dilutive shares was excluded from the calculation of diluted loss per share in the fiscal years ended June 30, 2020 and 2019 because the effect of including such securities would be anti-dilutive.
Note 14. Share-based Compensation
At June 30, 2020, the Company had two share-based employee compensation plans (the 2011 Long-Term Incentive Plan “LTIP” and the 2014 “LTIP”). Together these plans authorized an aggregate total of 6.5 million shares to be issued. As of June 30, 2020, the plans have a total of 1.3 million shares available for future issuances.
Historically, the Company has issued share-based compensation awards with a vesting period ranging up to 3 years and a maximum contractual term of 10 years. The Company issues new shares of stock when stock options are exercised. As of June 30, 2020, there was $9.5 million of total unrecognized compensation cost related to non-vested share-based compensation awards. That cost is expected to be recognized over a weighted average period of 2.2 years.
Stock Options
The Company measures share-based compensation costs for options using the Black-Scholes option pricing model. The following table presents the weighted average assumptions used to estimate fair values of the stock options granted, the
142
estimated annual forfeiture rates used to recognize the associated compensation expense and the weighted average fair value of the options granted during the fiscal years ended June 30:
| | 2020 | | 2019 | | 2018 | ||||||
Risk-free interest rate | | | 1.9 | % | | | 2.9 | % | | | 2.1 | % |
Expected volatility | | | 73.7 | % | | | 58.4 | % | | | 57.6 | % |
Expected dividend yield | | | — | % | | | — | % | | | — | % |
Forfeiture rate | | | — | % | | | 6.5 | % | | | 6.5 | % |
Expected term | | | 5.1 | years | | | 5.3 | years | | | 5.4 | years |
Weighted average fair value | | $ | 4.0 | | | $ | 6.52 | | | $ | 11.25 | |
Expected volatility is based on the historical volatility of the price of our common shares during the historical period equal to the expected term of the option. The Company uses historical information to estimate the expected term, which represents the period of time that options granted are expected to be outstanding. The risk-free rate for the period equal to the expected life of the option is based on the U.S. Treasury yield curve in effect at the time of grant. The forfeiture rate assumption is the estimated annual rate at which unvested awards are expected to be forfeited during the vesting period. This assumption is based on our actual forfeiture rate on historical awards. Periodically, management will assess whether it is necessary to adjust the estimated rate to reflect changes in actual forfeitures or changes in expectations. Additionally, the expected dividend yield is equal to zero, as the Company has not historically issued and has no immediate plans to issue a dividend.
A stock option summary as of June 30, 2020, 2019 and 2018 and changes during the years then ended, is presented below:
|
|
|
| | |
| | |
| Weighted |
| | | | Weighted- | | | | | Average | |
| | | | Average | | Aggregate | | Remaining | ||
| | | | Exercise | | Intrinsic | | Contractual | ||
(In thousands, except for weighted average price and life data) |
| Awards |
| Price |
| Value |
| Life (yrs.) | ||
| | | | | | | | | | |
Outstanding at June 30, 2017 |
| 1,475 | | $ | 18.02 | | $ | 12,212 | | 5.7 |
Granted |
| 50 | | $ | 21.43 | | | | | |
Exercised |
| (445) | | $ | 7.23 | | $ | 4,243 | | |
Forfeited, expired or repurchased |
| (23) | | $ | 30.83 | | | | | |
Outstanding at June 30, 2018 |
| 1,057 | | $ | 22.46 | | $ | 2,584 | | 5.4 |
Granted |
| 73 | | $ | 12.20 | | | | | |
Exercised |
| (94) | | $ | 4.06 | | $ | 311 | | |
Forfeited, expired or repurchased |
| (464) | | $ | 30.61 | | | | | |
Outstanding at June 30, 2019 |
| 572 | | $ | 17.56 | | $ | 273 | | 5.0 |
Granted |
| 522 | | $ | 6.57 | | | | | |
Exercised |
| (56) | | $ | 5.42 | | $ | 237 | | |
Forfeited, expired or repurchased |
| (47) | | $ | 24.73 | | | | | |
Outstanding at June 30, 2020 |
| 991 | | $ | 12.11 | | $ | 678 |
| 5.6 |
| | | | | | | | | | |
Vested and expected to vest at June 30, 2020 |
| 989 | | $ | 12.10 | | $ | 678 |
| 5.6 |
Exercisable at June 30, 2020 |
| 493 | | $ | 16.82 | | $ | 371 |
| 2.3 |
Restricted Stock
The Company measures restricted stock compensation costs based on the stock price at the grant date less an estimate for expected forfeitures. The annual forfeiture rate used to calculate compensation expense was 6.5%, 6.5% and 5.7% for fiscal years ended June 30, 2020, 2019 and 2018, respectively.
143
A summary of restricted stock awards as of June 30, 2020, 2019 and 2018 and changes during the fiscal years then ended, is presented below:
| | | | Weighted | | | | |
| | | | Average Grant - | | Aggregate | ||
(In thousands, except for weighted average price data) |
| Awards |
| date Fair Value |
| Intrinsic Value | ||
| | | | | | | | |
Non-vested at June 30, 2017 |
| 334 | | $ | 30.71 | | | |
Granted |
| 641 | |
| 18.01 | | | |
Vested |
| (191) | |
| 31.30 | | $ | 4,104 |
Forfeited |
| (80) | |
| 20.95 | | | |
Non-vested at June 30, 2018 |
| 704 | | $ | 20.06 | | | |
Granted |
| 1,176 | |
| 9.90 | | | |
Vested |
| (434) | |
| 19.75 | | $ | 4,107 |
Forfeited |
| (158) | |
| 14.00 | | | |
Non-vested at June 30, 2019 |
| 1,288 | | $ | 11.63 | | | |
Granted |
| 941 | |
| 6.45 | | | |
Vested |
| (773) | |
| 10.54 | | $ | 6,401 |
Forfeited |
| (112) | |
| 10.75 | | | |
Non-vested at June 30, 2020 |
| 1,344 | | $ | 8.70 | | | |
Performance-Based Shares
In September 2017, the Company approved a plan to begin granting performance-based awards to certain key executives. The stock-settled awards will cliff vest based on relative Total Shareholder Return (“TSR”) over a three-year performance period. The Company measures share-based compensation cost for TSR awards using a Monte-Carlo simulation model.
A summary of performance-based share awards as of June 30, 2020, 2019 and 2018 and changes during the current fiscal years then ended, is presented below:
| | | | Weighted | | | | |
| | | | Average Grant - | | Aggregate | ||
(In thousands, except for weighted average price and life data) |
| Awards |
| date Fair Value |
| Intrinsic Value | ||
|
|
|
| |
|
| |
|
Non-vested at June 30, 2017 | | — | | $ | — | | | |
Granted | | 47 | | | 25.58 | | | |
Vested | | (27) | | | 25.58 | | $ | 574 |
Forfeited | | — | | | — | | | |
Non-vested at June 30, 2018 |
| 20 | | $ | 25.58 |
| |
|
Granted |
| 52 | | | 17.69 |
| |
|
Vested |
| — | | | — | | $ | — |
Forfeited |
| — | | | — | |
|
|
Non-vested at June 30, 2019 |
| 72 | | $ | 19.92 | |
|
|
Granted |
| 178 | | $ | 10.71 | |
|
|
Vested |
| (46) | | $ | 15.08 | | $ | 477 |
Forfeited |
| — | | $ | — | |
|
|
Non-vested at June 30, 2020 |
| 204 | | $ | 12.99 | |
|
|
Employee Stock Purchase Plan
In February 2003, the Company’s stockholders approved an Employee Stock Purchase Plan (“ESPP”). Employees eligible to participate in the ESPP may purchase shares of the Company’s stock at 85% of the lower of the fair market value of the common stock on the first day of the calendar quarter, or the last day of the calendar quarter. Under the ESPP, employees can authorize the Company to withhold up to 10% of their compensation during any quarterly offering
144
period, subject to certain limitations. The ESPP was implemented on April 1, 2003 and is qualified under Section 423 of the Internal Revenue Code. The Board of Directors authorized an aggregate total of 1.1 million shares of the Company’s common stock for issuance under the ESPP. During the fiscal years ended June 30, 2020, 2019 and 2018, 118 thousand shares, 185 thousand shares and 66 thousand shares were issued under the ESPP, respectively. As of June 30, 2020, 910 thousand total cumulative shares have been issued under the ESPP.
The following table presents the allocation of share-based compensation costs recognized in the Consolidated Statements of Operations by financial statement line item:
| | | | | | | | | |
| | For Fiscal Year Ended June 30, | |||||||
(In thousands) | | 2020 |
| 2019 |
| 2018 | |||
Selling, general and administrative expenses | | $ | 7,087 | | $ | 5,715 | | $ | 7,570 |
Research and development expenses | |
| 801 | |
| 750 | |
| 680 |
Cost of sales | |
| 2,328 | |
| 2,562 | |
| 1,646 |
Total | | $ | 10,216 | | $ | 9,027 | | $ | 9,896 |
| | | | | | | | | |
Tax benefit at statutory rate | | $ | 2,299 | | $ | 2,031 | | $ | 2,919 |
Note 15. Employee Benefit Plan
The Company has a 401(k) defined contribution plan (the “Plan”) covering substantially all employees. Pursuant to the Plan provisions, the Company is required to make matching contributions equal to 50% of each employee’s contribution, not to exceed 4% of the employee’s compensation for the Plan year. Contributions to the Plan during the fiscal years ended June 30, 2020, 2019 and 2018 were $2.2 million, $2.3 million and $2.3 million, respectively.
In Fiscal 2020, the Company implemented a non-qualified deferred compensation plan for certain senior-level management and executives. The non-qualified deferred compensation plan allows certain eligible employees to defer additional pre-tax earnings for retirement, beyond the IRS limits in place under the Plan. Contributions to the non-qualified deferred compensation plan during Fiscal 2020 were not material.
Note 16. Income Taxes
The Company uses the liability method to account for income taxes. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities as measured by the enacted tax rates which will be in effect when these differences reverse. Deferred tax expense (benefit) is the result of changes in deferred tax assets and liabilities.
On December 22, 2017, the 2017 Tax Reform was enacted into law, which significantly revised the Internal Revenue Code of 1986, as amended. The 2017 Tax Reform includes, among other items, permanent reduction of the corporate tax rate from a top marginal rate of 35% to a flat rate of 21%; and modifying or repealing many other business deductions and credits.
On March 27, 2020, in response to COVID-19 and its detrimental impact to the global economy, President Trump signed the Coronavirus Aid, Relief, and Economic Security Act (“CARES Act”) into law, which provides a stimulus to the U.S. economy in the form of various individual and business assistance programs as well as temporary changes to existing tax law. Among the changes to the provision in business tax laws include a five-year net operating loss carryback for the Fiscal 2019 - 2021 tax years, a deferral of the employer’s portion of certain payroll tax, and an increase in the interest expense deductibility limitation for the Fiscal 2020 and 2021 tax years. ASC 740 requires the tax effects of changes in tax laws or rates to be recorded in the period of enactment. As a result of the CARES Act, the Company will carry back its Fiscal 2020 taxable loss into the Fiscal 2015 tax year, which resulted in an approximately $2.8 million tax rate benefit in the current year. The Company also reviewed its existing deferred tax assets in light of COVID-19 and determined that no valuation allowance is required at this time. However, the Company will continue to monitor the status of the COVID-19 pandemic and its impact on our results of operations.
145
The following table summarizes the components of the provision for income taxes for the fiscal years ended June 30:
| | | | | | | | | |
|
| June 30, |
| June 30, |
| June 30, | |||
(In thousands) | | 2020 | | 2019 | | 2018 | |||
Current Income Tax Expense (Benefit) | | | | | | | | | |
Federal | | $ | (7,082) | | $ | 13,185 | | $ | (9,439) |
State and Local | |
| 405 | |
| (81) | |
| 1,152 |
Total Current Income Tax Expense (Benefit) | |
| (6,677) | |
| 13,104 | |
| (8,287) |
Deferred Income Tax Expense (Benefit) | | | | | | | | | |
Federal | |
| (6,525) | |
| (85,022) | |
| 31,263 |
State and Local | |
| (2,060) | |
| (2,220) | |
| (573) |
Total Deferred Income Tax Expense (Benefit) | |
| (8,585) | |
| (87,242) | |
| 30,690 |
Total Income Tax Expense (Benefit) | | $ | (15,262) | | $ | (74,138) | | $ | 22,403 |
A reconciliation of the differences between the effective rates and federal statutory rates was as follows:
| | | | | | | |
|
| June 30, |
| June 30, |
| June 30, |
|
| | 2020 | | 2019 | | 2018 |
|
| | | | | | | |
Federal income tax at statutory rate |
| 21.0 | % | 21.0 | % | 28.1 | % |
State and local income tax, net |
| 2.7 | % | 0.5 | % | 0.6 | % |
Nondeductible expenses |
| (1.1) | % | (0.1) | % | 0.2 | % |
Nondeductible drug fee | | (1.6) | % | — | % | — | % |
Foreign rate differential |
| (0.1) | % | (0.4) | % | 0.4 | % |
Income tax credits |
| 2.5 | % | 0.5 | % | (1.4) | % |
Domestic production activity deduction | | — | % | — | % | (1.5) | % |
Unrecognized tax benefits | | (5.0) | % | 0.1 | % | (6.7) | % |
Change in tax laws |
| 15.4 | % | — | % | 25.6 | % |
Excess tax benefits on share-based compensation | | (0.8) | % | (0.3) | % | (0.3) | % |
Other |
| (1.6) | % | 0.1 | % | (1.2) | % |
Effective income tax rate |
| 31.4 | % | 21.4 | % | 43.8 | % |
The principal types of differences between assets and liabilities for financial statement and tax return purposes are accruals, reserves, impairment of intangibles, accumulated amortization, accumulated depreciation and share-based compensation expense. A deferred tax asset is recorded for the future benefits created by the timing of accruals and reserves and the application of different amortization lives for financial statement and tax return purposes. The Company’s deferred tax liability is mainly attributable to different depreciation methods for financial statement and tax return purposes. A deferred tax asset valuation allowance is established if it is more likely than not that the Company
146
will be unable to realize certain of the deferred tax assets. As of June 30, 2020 and 2019, temporary differences which give rise to deferred tax assets and liabilities were as follows:
| | | | | | |
|
| June 30, |
| June 30, | ||
(In thousands) | | 2020 | | 2019 | ||
Deferred tax assets: | | | | | | |
Share-based compensation expense | | $ | 2,661 | | $ | 4,134 |
Reserve for returns | |
| 11,022 | |
| 12,014 |
Reserves for accounts receivable and inventory | |
| 5,526 | |
| 8,208 |
Federal net operating loss | |
| 273 | |
| 324 |
State net operating loss | | | 8,387 | | | 6,479 |
Impairment on Cody note receivable | |
| 1,171 | |
| 1,161 |
Accumulated amortization on intangible assets | |
| 79,939 | |
| 76,401 |
Foreign net operating loss | |
| 1,822 | |
| 1,792 |
Interest Carryforward | | | 25,392 | | | 11,008 |
Operating lease | | | 3,439 | | | — |
Other | |
| 2,747 | |
| 2,506 |
Total deferred tax asset | |
| 142,379 | |
| 124,027 |
Valuation allowance | |
| (14,622) | |
| (13,549) |
Total deferred tax asset less valuation allowance | |
| 127,757 | |
| 110,478 |
Deferred tax liabilities: | | | | | | |
Prepaid expenses | |
| 681 | |
| 182 |
Property, plant and equipment | |
| 5,383 | |
| 991 |
Operating lease | | | 3,803 | | | — |
Total deferred tax liability | |
| 9,867 | |
| 1,173 |
Net deferred tax asset | | $ | 117,890 | | $ | 109,305 |
The net deferred tax asset as of June 30, 2020 and 2019 is reduced by a valuation allowance of $14.6 million and $13.5 million, respectively, which are primarily related to deferred tax assets for various states and foreign net operating losses. The federal and state and local tax deferred tax assets begin to expire in fiscal years 2026 and 2036, respectively. The increase in the valuation allowance in Fiscal 2020 primarily related to an increase of state deferred tax assets.
The Company may recognize the tax benefit from an uncertain tax position claimed on a tax return only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement.
A reconciliation of the beginning and ending amount of gross unrecognized tax benefits (exclusive of interest and penalties) was as follows:
(In thousands) |
| Balance | |
Balance at June 30, 2018 | | $ | 2,537 |
Additions for tax positions of the current year | |
| 244 |
Additions for tax positions of prior years | |
| 36 |
Lapse of statute of limitations | |
| (618) |
Balance at June 30, 2019 | | $ | 2,199 |
Additions for tax positions of the current year | |
| 2,467 |
Additions for tax positions of prior years | |
| (51) |
Lapse of statute of limitations | |
| (24) |
Balance at June 30, 2020 | | $ | 4,591 |
The amount of unrecognized tax benefits at June 30, 2020, 2019 and 2018 was $4.6 million, $2.2 million and $2.5 million, respectively, of which $4.5 million, $2.1 million and $2.3 million would impact the Company’s effective tax rate, respectively, if recognized.
147
The Company has not recorded any interest and penalties for the periods ended June 30, 2020, 2019 and 2018 in the statement of operations and no cumulative interest and penalties have been recorded either in the Company’s Consolidated Balance Sheet as of June 30, 2020 and 2019. The Company will recognize interest accrued on unrecognized tax benefits in interest expense and any related penalties in operating expenses.
The Company files income tax returns in the United States federal jurisdiction and various states. The Company’s tax returns for Fiscal Year 2014 and prior generally are no longer subject to review as such years generally are closed. The Company’s Fiscal Year 2015 through 2017 federal returns are currently under examination by the Internal Revenue Service (“IRS”). In October 2018, the Company was notified that the Commonwealth of Pennsylvania will conduct a routine field audit of the Company’s Fiscal 2016 and Fiscal 2017 corporate tax returns. In December 2019, the Company was notified that the Florida Department of Revenue will conduct a routine field audit of the Company’s Fiscal 2016, 2017 and 2018 corporate tax returns. The Company has received preliminary assessments from the IRS and the Florida Department of Revenue; however, we cannot reasonably predict the final outcome of the examinations at this time.
Note 17. Related Party Transactions
The Company had sales of $3.0 million, $3.8 million and $3.9 million during the fiscal years ended June 30, 2020, 2019 and 2018, respectively, to a generic distributor, Auburn Pharmaceutical Company (“Auburn ”), which is a member of the Premier Buying Group. Jeffrey Farber, a current board member, is the owner of Auburn. Accounts receivable includes amounts due from Auburn of $0.7 million and $1.2 million at June 30, 2020 and 2019, respectively.
The Company also had net sales of $2.6 million, $2.4 million, and $1.9 million during the fiscal years ended June 30, 2020, 2019 and 2018, respectively, to a generic distributor, KeySource Medical (“KeySource), which is a member of the OptiSource Buying Group. Albert Paonessa, a board member until the date of the Company’s 2020 Annual meeting of Stockholders, was appointed the CEO of KeySource in May 2017. Accounts receivable includes amounts due from KeySource of $0.6 million and $0.7 million as of June 30, 2020 and 2019.
Note 18. Assets Held for Sale
In the first quarter of Fiscal 2019, the Company approved a plan to sell the Cody API business, which includes the manufacturing and distribution of active pharmaceutical ingredients for use in finished goods production. As a result of the plan, the Company recorded the assets of the Cody API business at fair value less costs to sell. The Company performed a fair value analysis which resulted in a $29.9 million impairment of the Cody Labs long-lived assets in Fiscal 2019.
The Company was unable to sell the Cody API business as an ongoing operation and intended to sell the equipment utilized by the Cody API business as well as the real estate upon receiving approval of the Company’s cocaine hydrochloride solution Section 505(b)(2) NDA application and to have Cody Labs cease all operations. During Fiscal 2020, the Company completed the sale of the equipment associated with the Cody API business for approximately $3.0 million. In the second quarter of Fiscal 2020, the Company signed a two year agreement to lease a portion of the real estate to a third party. As of June 30, 2020, the real estate associated with the Cody API business, totaling $2.7 million, is recorded in the assets held for sale caption in the Consolidated Balance Sheet.
The following table summarizes the financial results of the Cody API business for the fiscal years ended June 30, 2020 and 2019:
| | Fiscal Year Ended | | ||||
| | June 30, | | ||||
(In thousands) |
| 2020 |
| 2019 | | ||
Net sales | | $ | 1,067 | | $ | 3,139 | |
Pretax loss attributable to Cody API business | |
| (6,549) | | | (51,509) | |
148
The pretax loss attributable to the Cody API business during the fiscal year ended June 30, 2020 includes a full impairment of a $1.2 million ROU lease asset that was recorded upon adoption of ASU No. 2016-02 on July 1, 2019.
The pretax loss attributable to the Cody API business during the fiscal year ended June 30, 2019 includes impairment charges totaling $32.8 million to adjust the long-lived assets to their fair value less costs to sell.
Note 19. Subsequent Events
2020 Restructuring Plan
On July 10, 2020, the Board of Directors authorized a restructuring and cost savings plan (the “2020 Restructuring Plan”). The purpose of the 2020 Restructuring Plan is to enhance manufacturing efficiencies, streamline operations and reduce the Company’s cost structure, and is being implemented, in part, as a result of previously anticipated near-term competition and pricing pressure with respect to certain key products. The 2020 Restructuring Plan will include the consolidation of the Company’s R&D function into a single location in Philadelphia, PA, lowering operating costs and reducing the workforce by approximately 80 positions, equal to approximately 8.5% of the Company’s total number of employees. The 2020 Restructuring Plan was initiated on July 13, 2020.
The Company estimates that it will incur approximately $4.0 million in severance-related costs, primarily in the first quarter of Fiscal 2021, in connection with the 2020 Restructuring Plan. The Company expects the 2020 Restructuring Plan to result in annual cost savings in excess of $15.0 million.
The amounts are preliminary estimates based on the information currently available to management. It is possible that additional charges and future cash payments could occur in relation to the restructuring actions.
Note 20. Quarterly Financial Information (Unaudited)
Lannett’s quarterly consolidated results of operations are shown below:
|
| Fourth |
| Third |
| Second |
| First | ||||
(In thousands, except per share data) | | Quarter | | Quarter | | Quarter | | Quarter | ||||
Fiscal 2020 | | | | | | | | | | | | |
Net sales | | $ | 137,920 | | $ | 144,372 | | $ | 136,110 | | $ | 127,342 |
Cost of sales | |
| 98,328 | |
| 102,696 | |
| 94,816 | |
| 84,684 |
Gross profit | |
| 39,592 | |
| 41,676 | |
| 41,294 | |
| 42,658 |
Operating expenses | |
| 44,123 | |
| 43,768 | |
| 24,519 | |
| 33,254 |
Operating income (loss) | |
| (4,531) | |
| (2,092) | |
| 16,775 | |
| 9,404 |
Other loss | |
| (15,247) | |
| (16,164) | |
| (16,999) | |
| (19,774) |
Income tax expense (benefit) | |
| (10,077) | |
| (1,664) | |
| (5,308) | |
| 1,787 |
Net income (loss) | | $ | (9,701) | | $ | (16,592) | | $ | 5,084 | | $ | (12,157) |
Earnings (loss) per common share (1) | | | | | | | | | | | | |
Basic | | $ | (0.25) | | $ | (0.43) | | $ | 0.13 | | $ | (0.32) |
Diluted | | $ | (0.25) | | $ | (0.43) | | $ | 0.13 | | $ | (0.32) |
149
| | | | | | | | | | | | |
|
| Fourth |
| Third |
| Second |
| First | ||||
(In thousands, except per share data) | | Quarter | | Quarter | | Quarter | | Quarter | ||||
Fiscal 2019 | | | | | | | | | | | | |
Net sales | | $ | 133,841 | | $ | 172,794 | | $ | 193,718 | | $ | 155,054 |
Cost of sales | |
| 84,499 | |
| 107,477 | |
| 123,908 | |
| 95,913 |
Gross profit | |
| 49,342 | |
| 65,317 | |
| 69,810 | |
| 59,141 |
Operating expenses | |
| 39,940 | |
| 31,939 | |
| 33,133 | |
| 400,919 |
Operating income (loss) | |
| 9,402 | |
| 33,378 | |
| 36,677 | |
| (341,778) |
Other loss | |
| (19,532) | |
| (21,374) | |
| (21,668) | |
| (21,350) |
Income tax expense (benefit) | |
| (2,544) | |
| 1,359 | |
| 2,647 | |
| (75,600) |
Net income (loss) | | $ | (7,586) | | $ | 10,645 | | $ | 12,362 | | $ | (287,528) |
Earnings (loss) per common share (1) | | | | | | | | | | | | |
Basic | | $ | (0.20) | | $ | 0.28 | | $ | 0.33 | | $ | (7.65) |
Diluted | | $ | (0.20) | | $ | 0.27 | | $ | 0.32 | | $ | (7.65) |
| | | | | | | | | | | | |
|
| Fourth |
| Third |
| Second |
| First | ||||
(In thousands, except per share data) | | Quarter | | Quarter | | Quarter | | Quarter | ||||
Fiscal 2018 | | | | | | | | | | | | |
Net sales | | $ | 170,911 | | $ | 174,386 | | $ | 184,305 | | $ | 154,961 |
Cost of sales | |
| 104,383 | |
| 107,329 | |
| 96,855 | |
| 87,290 |
Gross profit | |
| 66,528 | |
| 67,057 | |
| 87,450 | |
| 67,671 |
Operating expenses | |
| 57,926 | |
| 33,777 | |
| 40,315 | |
| 26,992 |
Operating income | |
| 8,602 | |
| 33,280 | |
| 47,135 | |
| 40,679 |
Other loss | |
| (20,844) | |
| (22,785) | |
| (14,975) | |
| (19,999) |
Income tax expense (benefit) | |
| (883) | |
| (2,275) | |
| 18,138 | |
| 7,423 |
Net income (loss) | | $ | (11,359) | | $ | 12,770 | | $ | 14,022 | | $ | 13,257 |
Earnings (loss) per common share (1) | | | | | | | | | | | | |
Basic | | $ | (0.30) | | $ | 0.34 | | $ | 0.38 | | $ | 0.36 |
Diluted | | $ | (0.30) | | $ | 0.33 | | $ | 0.37 | | $ | 0.35 |
(1)Due to differences in weighted average common shares outstanding, quarterly earnings per share may not add up to the totals reported for the full fiscal year.
The increase in operating expenses in the third and fourth quarters of Fiscal 2020 is primarily driven by asset impairment charges related to the abandonment of several pipeline products within the KUPI IPR&D and Silarx IPR&D asset portfolios, as well as a significant decline in sales and overall profitability of the distribution agreement with Andor for the AB-rated Methylphenidate Hydrochloride product.
The increase in net sales and gross profit in the second and third quarters of Fiscal 2019 is primarily due to increased sales of Levothyroxine as customer demand increased in anticipation of the transition of the product to Amneal. The subsequent decrease in net sales and gross profit in the fourth quarter of Fiscal 2019 is primarily due to the expiration of the JSP Distribution Agreement on March 23, 2019. The significant operating loss in the first quarter of Fiscal 2019 was mainly attributable to the full impairment of goodwill totaling $339.6 million as a result of JSP’s decision not to renew the JSP Distribution Agreement.
The increase in net sales and gross profit in the second quarter of Fiscal 2018 is primarily due to a temporary disruption of our competitor’s supplies in the Thyroid Deficiency and Migraine medical indications. The declines in operating income in the third and fourth quarters of Fiscal 2018 were primarily related to a loss on sale of an intangible asset and asset impairment charges as a result of the Cody Restructuring Plan as well as other activities related to the consolidation of the Company’s manufacturing facilities. Income tax expense in the second quarter of Fiscal 2018 was negatively impacted due to the adoption of 2017 Tax Reform which resulted in a revaluation of the Company’s net long term deferred tax assets.
150
