Attached files
| file | filename |
|---|---|
| 8-K - 8-K - GLAUKOS Corp | f8-k.htm |
Exhibit 99.1
| 1 © 2018 Glaukos Corporation 1 November 2018 |
| 2 © 2018 Glaukos Corporation All statements other than statements of historical facts included in this presentation that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements. Although we believe that we have a reasonable basis for forward-looking statements contained herein, we caution you that they are based on current expectations about future events affecting us and are subject to risks, uncertainties and factors relating to our operations and business environment, all of which are difficult to predict and many of which are beyond our control, that may cause our actual results to differ materially from those expressed or implied by forward-looking statements in this presentation. These potential risks and uncertainties include, without limitation, uncertainties about our ability to maintain profitability; our dependence on the success and market acceptance of the iStent®; our ability to leverage our sales and marketing infrastructure to increase market penetration and acceptance both in the United States and internationally of our products; our dependence on a limited number of third-party suppliers, some of which are single-source, for components of our products; the occurrence of a crippling accident, natural disaster or other disruption at our primary facility, which may materially affect our manufacturing capacity and operations; maintaining adequate coverage or reimbursement by third-party payors for procedures using the iStent or other products in development; our ability to properly train, and gain acceptance and trust from, ophthalmic surgeons in the use of our products; our ability to successfully develop and commercialize additional products; our ability to compete effectively in the highly competitive and rapidly changing medical device industry and against current and future competitors (including MIGS competitors) that are large public companies or divisions of publicly traded companies that have competitive advantages; the timing, effect and expense of navigating different regulatory approval processes as we develop additional products and penetrate foreign markets; the impact of any product liability claims against us and any related litigation; the effect of the extensive and increasing federal and state regulation in the healthcare industry on us and our suppliers; the lengthy and expensive clinical trial process and the uncertainty of outcomes from any particular clinical trial; our ability to protect, and the expense and time- consuming nature of protecting, our intellectual property against third parties and competitors that could develop and commercialize similar or identical products; the impact of any claims against us of infringement or misappropriation of third party intellectual property rights and any related litigation; and the market’s perception of our limited operating history as a public company. These and other known risks, uncertainties and factors are described in detail under the caption “Risk Factors” and elsewhere in our filings with the Securities and Exchange Commission, including our Annual Report on Form 10-K for 2017 and Quarterly Report on Form 10-Q for the quarter ended September 30, 2018. Our filings with the Securities and Exchange Commission are available in the Investor Section of our website at www.glaukos.com or at www.sec.gov. In addition, information about the risks and benefits of our products is available on our website at www.glaukos.gov. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on the forward-looking statements in this press release, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise, except as may be required under applicable securities law. Disclaimer |
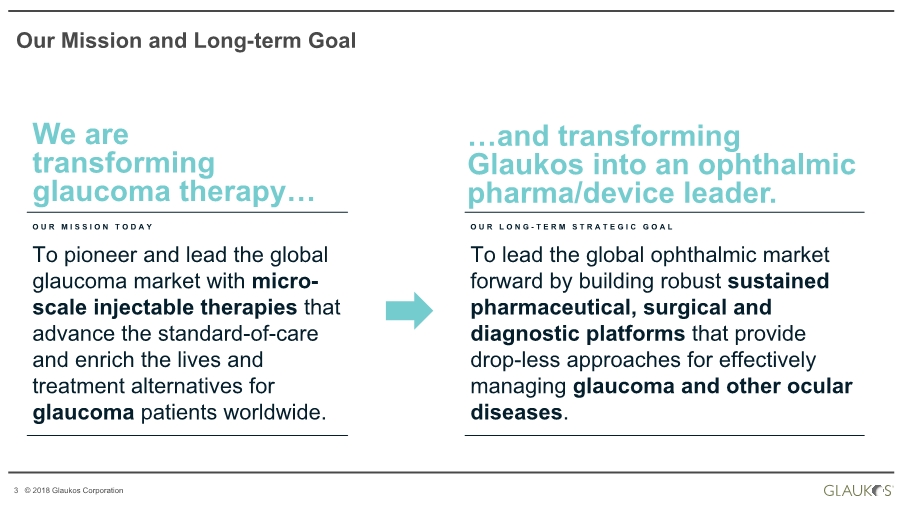
| 3 © 2018 Glaukos Corporation We are transforming glaucoma therapy… OUR MISSION TODAY To pioneer and lead the global glaucoma market with micro- scale injectable therapies that advance the standard-of-care and enrich the lives and treatment alternatives for glaucoma patients worldwide. O U R L O N G - TERM STRATEGIC GOAL To lead the global ophthalmic market forward by building robust sustained pharmaceutical, surgical and diagnostic platforms that provide drop-less approaches for effectively managing glaucoma and other ocular diseases. …and transforming Glaukos into an ophthalmic pharma/device leader. Our Mission and Long-term Goal |
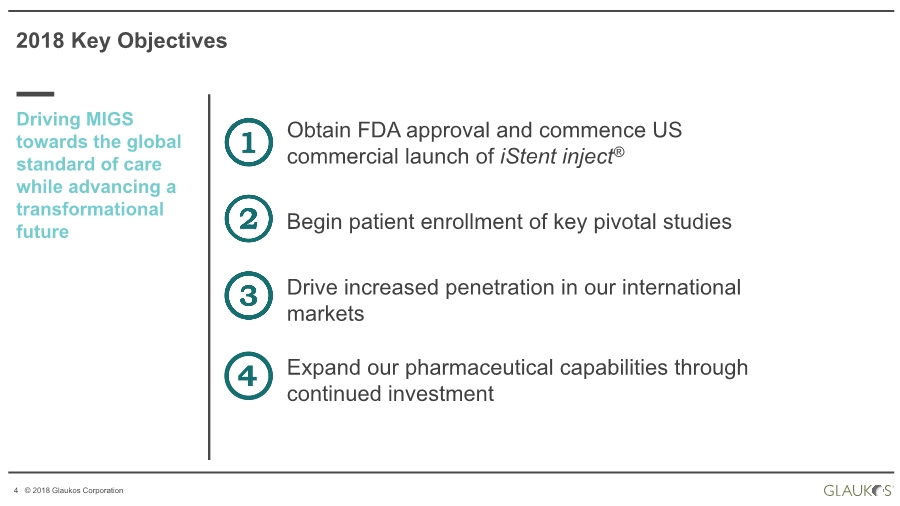
| 4 © 2018 Glaukos Corporation 2018 Key Objectives Driving MIGS towards the global standard of care while advancing a transformational future Obtain FDA approval and commence US commercial launch of iStent inject® Begin patient enrollment of key pivotal studies Drive increased penetration in our international markets Expand our pharmaceutical capabilities through continued investment |
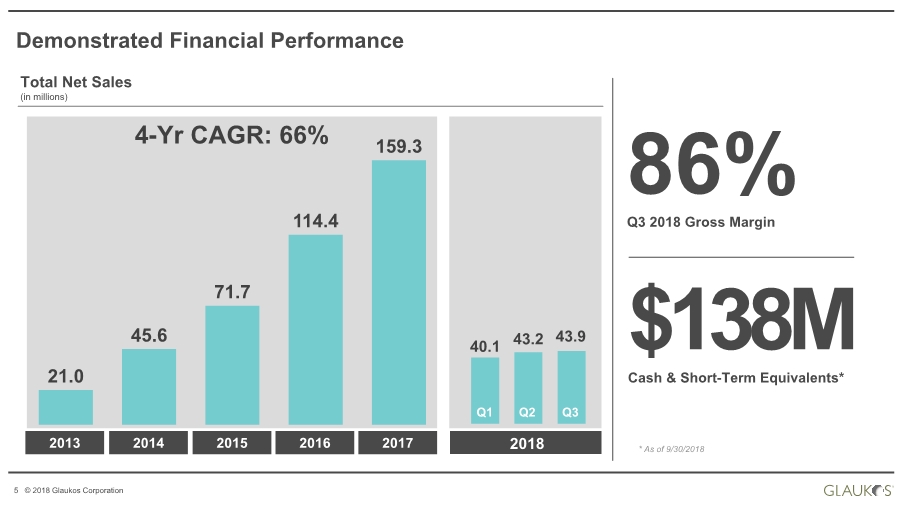
| 5 © 2018 Glaukos Corporation 2013 2014 2015 2016 Total Net Sales (in millions) 86% Q3 2018 Gross Margin $138M Cash & Short-Term Equivalents* * As of 9/30/2018 Demonstrated Financial Performance 21.0 45.6 71.7 114.4 159.3 20182017 40.1 43.2 43.9 Q3Q1 Q2 4-Yr CAGR: 66% |
| 6 © 2018 Glaukos Corporation MIGS AND BEYOND Delivering Novel Surgical & Pharmaceutical Glaucoma Therapy |
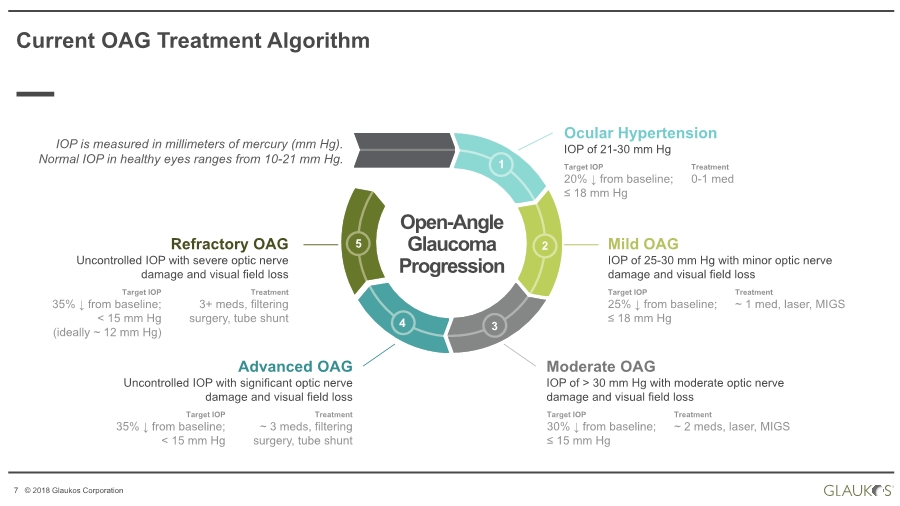
| 7 © 2018 Glaukos Corporation Ocular Hypertension IOP of 21-30 mm Hg Target IOP 20% ↓ from baseline; ≤ 18 mm Hg Treatment 0-1 med Mild OAG IOP of 25-30 mm Hg with minor optic nerve damage and visual field loss Moderate OAG IOP of > 30 mm Hg with moderate optic nerve damage and visual field loss Advanced OAG Uncontrolled IOP with significant optic nerve damage and visual field loss Refractory OAG Uncontrolled IOP with severe optic nerve damage and visual field loss IOP is measured in millimeters of mercury (mm Hg). Normal IOP in healthy eyes ranges from 10-21 mm Hg. Current OAG Treatment Algorithm 1 2 34 5 Open-Angle Glaucoma Progression Target IOP 25% ↓ from baseline; ≤ 18 mm Hg Treatment ~ 1 med, laser, MIGS Target IOP 30% ↓ from baseline; ≤ 15 mm Hg Treatment ~ 2 meds, laser, MIGS Target IOP 35% ↓ from baseline; < 15 mm Hg Treatment ~ 3 meds, filtering surgery, tube shunt Target IOP 35% ↓ from baseline; < 15 mm Hg (ideally ~ 12 mm Hg) Treatment 3+ meds, filtering surgery, tube shunt |
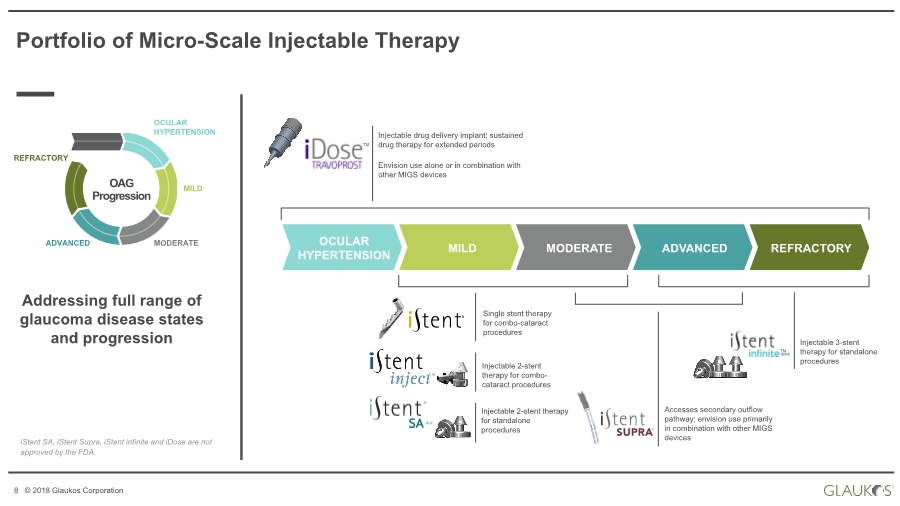
| 8 © 2018 Glaukos Corporation OAG Progression OCULAR HYPERTENSION MILD MODERATEADVANCED REFRACTORY Addressing full range of glaucoma disease states and progression Injectable drug delivery implant; sustained drug therapy for extended periods Envision use alone or in combination with other MIGS devices Injectable 2-stent therapy for standalone procedures Injectable 2-stent therapy for combo- cataract procedures Accesses secondary outflow pathway; envision use primarily in combination with other MIGS devices Injectable 3-stent therapy for standalone procedures Portfolio of Micro-Scale Injectable Therapy REFRACTORYADVANCEDMODERATEMILDOCULAR HYPERTENSION Single stent therapy for combo-cataract procedures TM TM ® iStent SA, iStent Supra, iStent infinite and iDose are not approved by the FDA. |
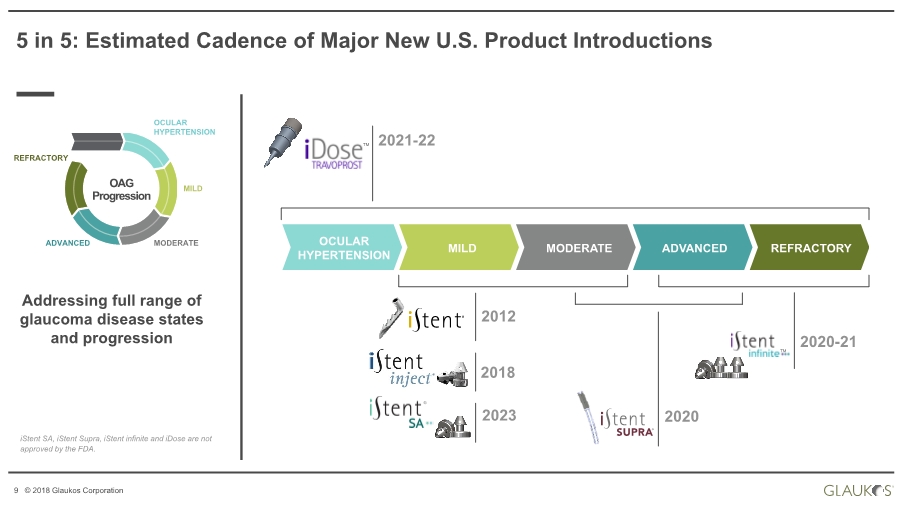
| 9 © 2018 Glaukos Corporation OAG Progression OCULAR HYPERTENSION MILD MODERATEADVANCED REFRACTORY 2021-22 2018 2020 2020-21 5 in 5: Estimated Cadence of Major New U.S. Product Introductions REFRACTORYADVANCEDMODERATEMILDOCULAR HYPERTENSION 2023 iStent SA, iStent Supra, iStent infinite and iDose are not approved by the FDA. Addressing full range of glaucoma disease states and progression TM TM 2012 ® |
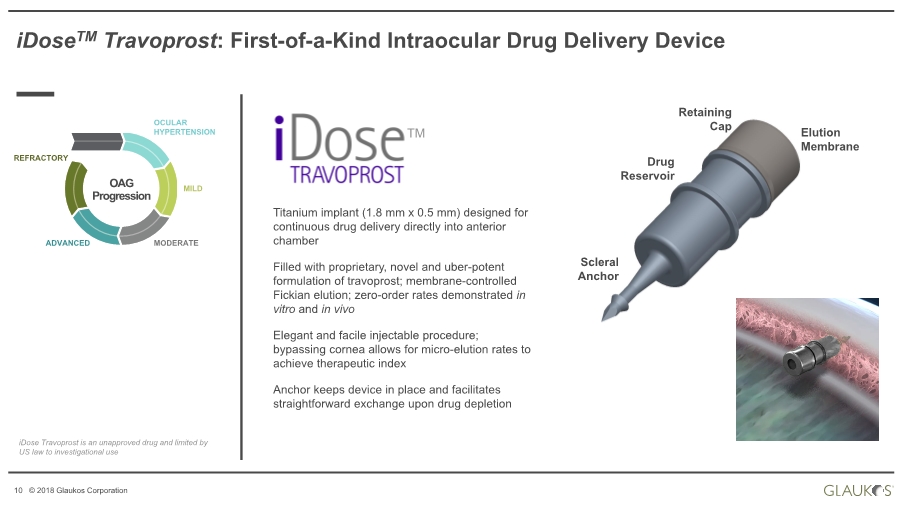
| 10 © 2018 Glaukos Corporation Drug Reservoir Scleral Anchor Retaining Cap Elution Membrane Titanium implant (1.8 mm x 0.5 mm) designed for continuous drug delivery directly into anterior chamber Filled with proprietary, novel and uber-potent formulation of travoprost; membrane-controlled Fickian elution; zero-order rates demonstrated in vitro and in vivo Elegant and facile injectable procedure; bypassing cornea allows for micro-elution rates to achieve therapeutic index Anchor keeps device in place and facilitates straightforward exchange upon drug depletion OAG Progression OCULAR HYPERTENSION MILD MODERATEADVANCED REFRACTORY iDoseTM Travoprost: First-of-a-Kind Intraocular Drug Delivery Device TM iDose Travoprost is an unapproved drug and limited by US law to investigational use |
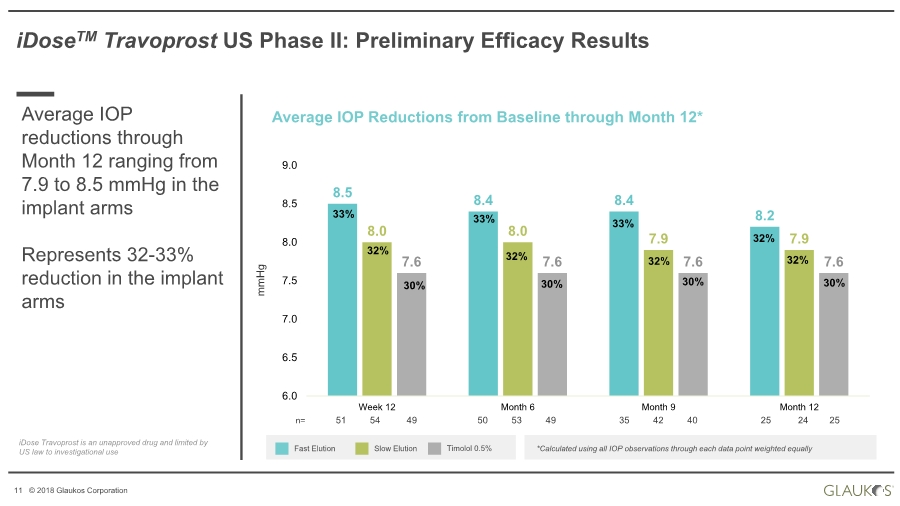
| 11 © 2018 Glaukos Corporation iDoseTM Travoprost US Phase II: Preliminary Efficacy Results Average IOP reductions through Month 12 ranging from 7.9 to 8.5 mmHg in the implant arms Represents 32-33% reduction in the implant arms Average IOP Reductions from Baseline through Month 12* Fast Elution Slow Elution *Calculated using all IOP observations through each data point weighted equally mmHg Timolol 0.5% 8.5 8.4 8.4 8.2 8.0 8.0 7.9 7.9 7.6 7.6 7.6 7.6 6.0 6.5 7.0 7.5 8.0 8.5 9.0 Week 12 Month 6 Month 9 Month 12 33% 30% n= 51 54 49 50 53 49 35 42 40 25 24 25 32% 33% 33% 32% 32% 32% 32% 30% 30% 30% iDose Travoprost is an unapproved drug and limited by US law to investigational use |
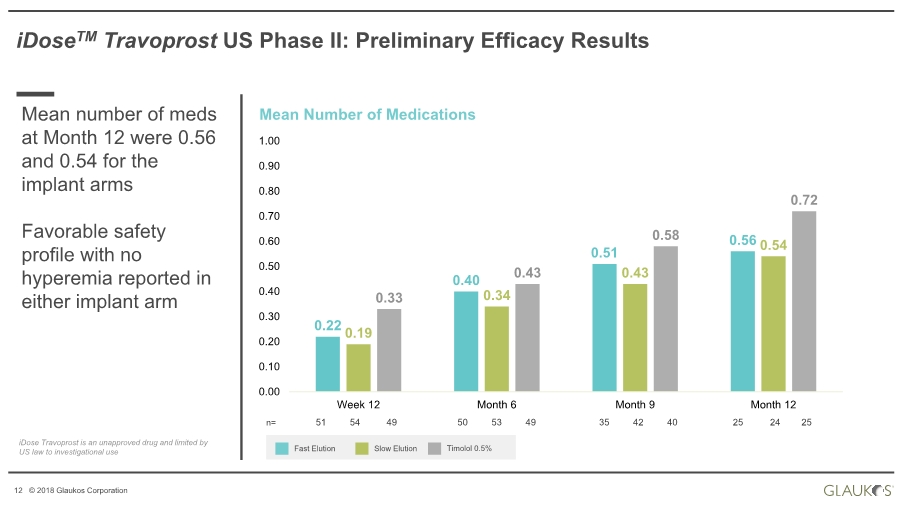
| 12 © 2018 Glaukos Corporation iDoseTM Travoprost US Phase II: Preliminary Efficacy Results Mean Number of Medications Fast Elution Slow Elution Timolol 0.5% n= 51 54 49 50 53 49 35 42 40 25 24 25 iDose Travoprost is an unapproved drug and limited by US law to investigational use 0.22 0.40 0.51 0.56 0.19 0.34 0.43 0.54 0.33 0.43 0.58 0.72 0.00 0.10 0.20 0.30 0.40 0.50 0.60 0.70 0.80 0.90 1.00 Week 12 Month 6 Month 9 Month 12 Mean number of meds at Month 12 were 0.56 and 0.54 for the implant arms Favorable safety profile with no hyperemia reported in either implant arm |
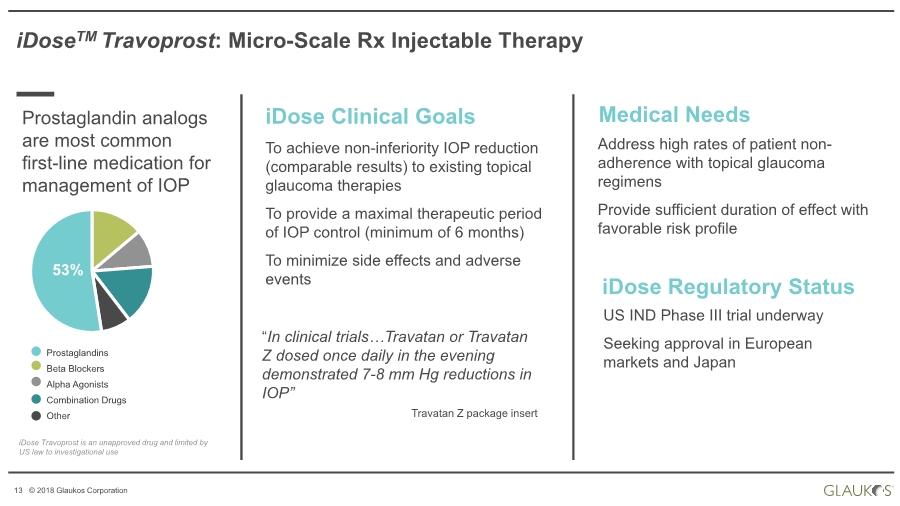
| 13 © 2018 Glaukos Corporation iDoseTM Travoprost: Micro-Scale Rx Injectable Therapy iDose Clinical Goals To achieve non-inferiority IOP reduction (comparable results) to existing topical glaucoma therapies To provide a maximal therapeutic period of IOP control (minimum of 6 months) To minimize side effects and adverse events Medical Needs Address high rates of patient non- adherence with topical glaucoma regimens Provide sufficient duration of effect with favorable risk profile iDose Regulatory Status US IND Phase III trial underway Seeking approval in European markets and JapanProstaglandins Beta Blockers Alpha Agonists Combination Drugs Other 53% Prostaglandin analogs are most common first-line medication for management of IOP “In clinical trials…Travatan or Travatan Z dosed once daily in the evening demonstrated 7-8 mm Hg reductions in IOP” Travatan Z package insert iDose Travoprost is an unapproved drug and limited by US law to investigational use |
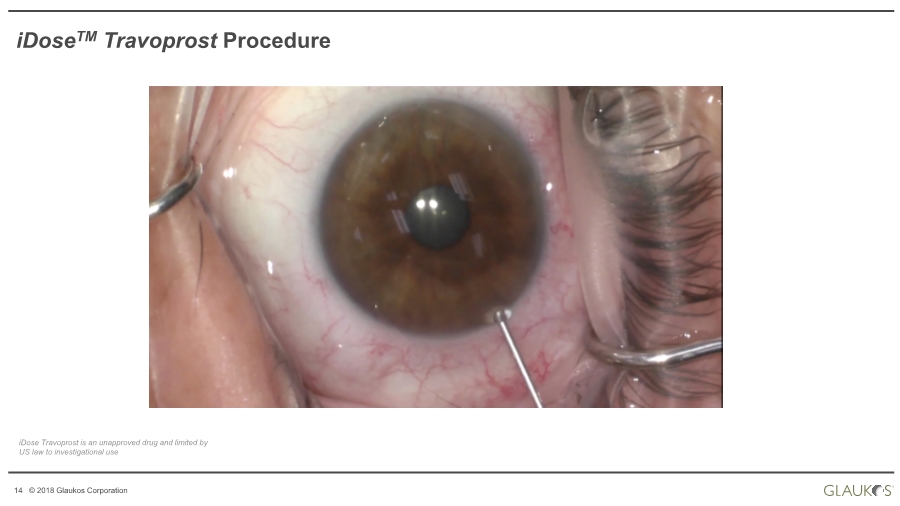
| 14 © 2018 Glaukos Corporation iDoseTM Travoprost Procedure iDose Travoprost is an unapproved drug and limited by US law to investigational use |
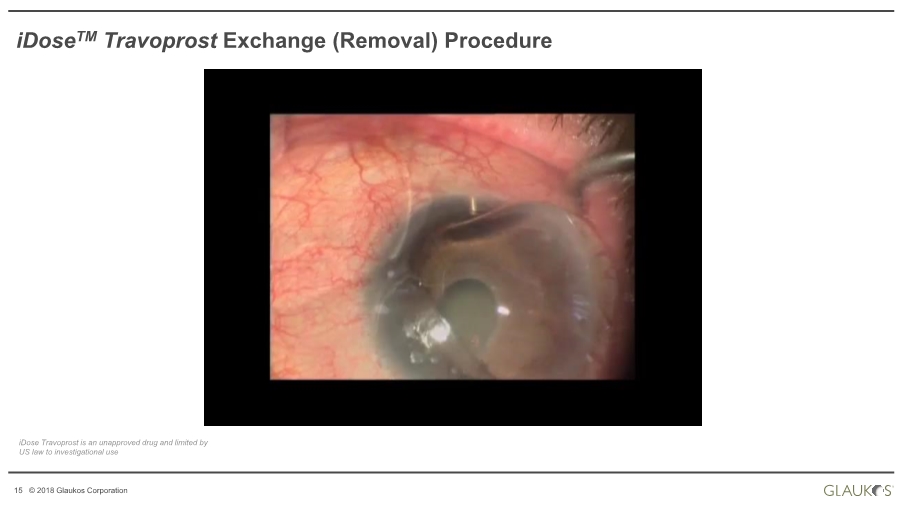
| 15 © 2018 Glaukos Corporation iDoseTM Travoprost Exchange (Removal) Procedure iDose Travoprost is an unapproved drug and limited by US law to investigational use |
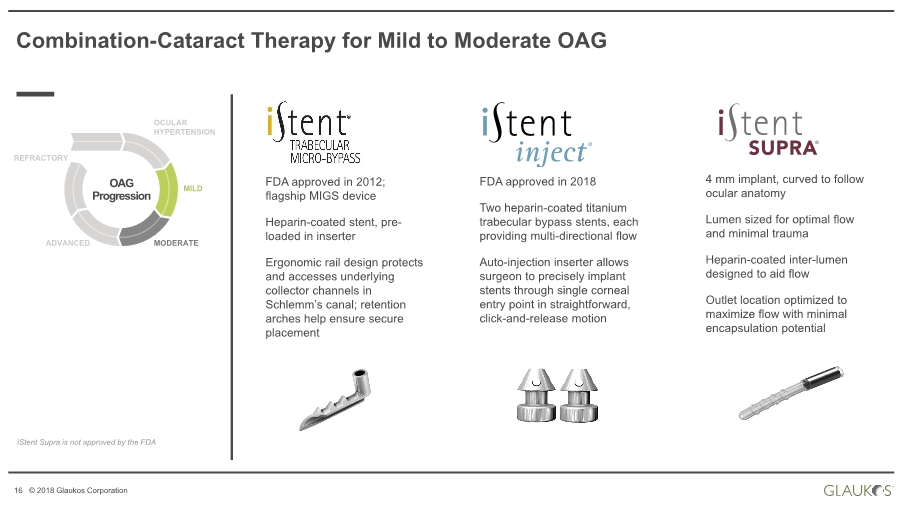
| 16 © 2018 Glaukos Corporation FDA approved in 2018 Two heparin-coated titanium trabecular bypass stents, each providing multi-directional flow Auto-injection inserter allows surgeon to precisely implant stents through single corneal entry point in straightforward, click-and-release motion Combination-Cataract Therapy for Mild to Moderate OAG OAG Progression OCULAR HYPERTENSION MILD MODERATEADVANCED REFRACTORY iStent Supra is not approved by the FDA 4 mm implant, curved to follow ocular anatomy Lumen sized for optimal flow and minimal trauma Heparin-coated inter-lumen designed to aid flow Outlet location optimized to maximize flow with minimal encapsulation potential FDA approved in 2012; flagship MIGS device Heparin-coated stent, pre- loaded in inserter Ergonomic rail design protects and accesses underlying collector channels in Schlemm’s canal; retention arches help ensure secure placement |
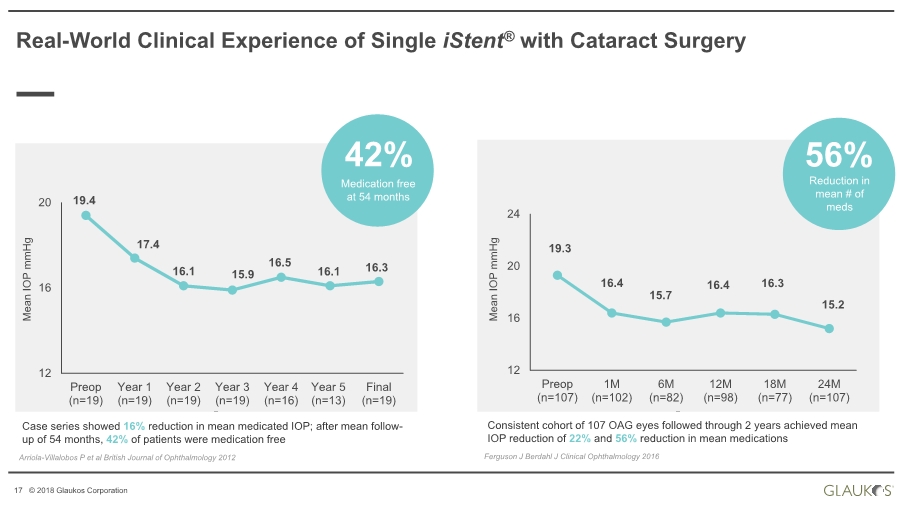
| 17 © 2018 Glaukos Corporation Real-World Clinical Experience of Single iStent® with Cataract Surgery Case series showed 16% reduction in mean medicated IOP; after mean follow- up of 54 months, 42% of patients were medication free Arriola-Villalobos P et al British Journal of Ophthalmology 2012 Mean IOP mmHg 19.4 17.4 16.1 15.9 16.5 16.1 16.3 12 16 20 Preop (n=19) Year 1 (n=19) Year 2 (n=19) Year 3 (n=19) Year 4 (n=16) Year 5 (n=13) Final (n=19) Medication free at 54 months 42% Mean IOP mmHg 19.3 16.4 15.7 16.4 16.3 15.2 12 16 20 24 Preop (n=107) 1M (n=102) 6M (n=82) 12M (n=98) 18M (n=77) 24M (n=107) Reduction in mean # of meds 56% Consistent cohort of 107 OAG eyes followed through 2 years achieved mean IOP reduction of 22% and 56% reduction in mean medications Ferguson J Berdahl J Clinical Ophthalmology 2016 |
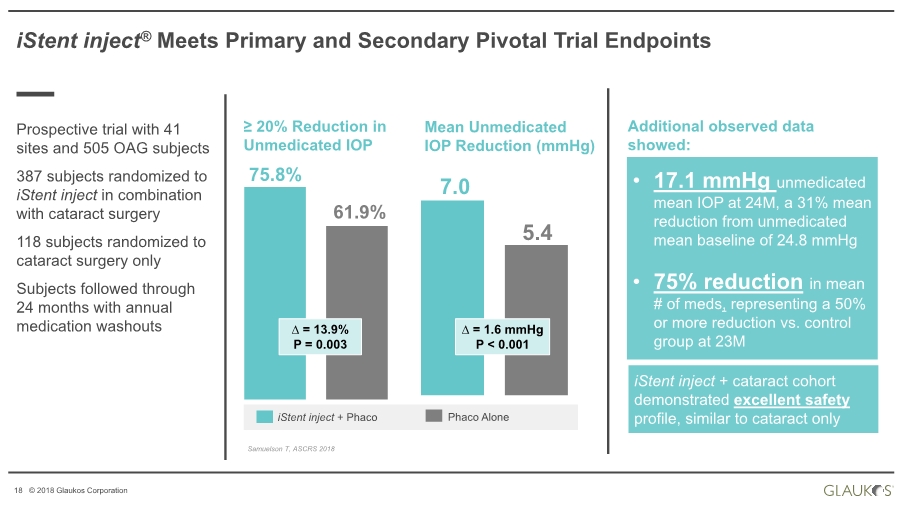
| 18 © 2018 Glaukos Corporation Samuelson T, ASCRS 2018 iStent inject® Meets Primary and Secondary Pivotal Trial Endpoints Prospective trial with 41 sites and 505 OAG subjects 387 subjects randomized to iStent inject in combination with cataract surgery 118 subjects randomized to cataract surgery only Subjects followed through 24 months with annual medication washouts 75.8% 61.9% ≥ 20% Reduction in Unmedicated IOP ∆ = 13.9% P = 0.003 7.0 5.4 Mean Unmedicated IOP Reduction (mmHg) ∆ = 1.6 mmHg P < 0.001 Phaco AloneiStent inject + Phaco • 17.1 mmHg unmedicated mean IOP at 24M, a 31% mean reduction from unmedicated mean baseline of 24.8 mmHg • 75% reduction in mean # of meds, representing a 50% or more reduction vs. control group at 23M Additional observed data showed: iStent inject + cataract cohort demonstrated excellent safety profile, similar to cataract only |
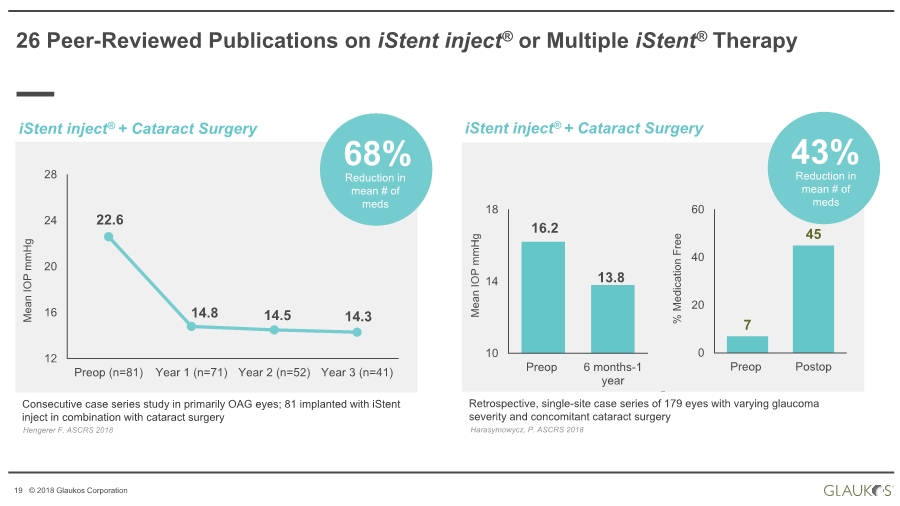
| 19 © 2018 Glaukos Corporation 26 Peer-Reviewed Publications on iStent inject® or Multiple iStent® Therapy Consecutive case series study in primarily OAG eyes; 81 implanted with iStent inject in combination with cataract surgery Hengerer F. ASCRS 2018 Mean IOP mmHg 22.6 14.8 14.5 14.3 12 16 20 24 28 Preop (n=81) Year 1 (n=71) Year 2 (n=52) Year 3 (n=41) Reduction in mean # of meds 68% Retrospective, single-site case series of 179 eyes with varying glaucoma severity and concomitant cataract surgery Harasymowycz, P. ASCRS 2018 Mean IOP mmHg 16.2 13.8 10 14 18 Preop 6 months-1 year Reduction in mean # of meds 43% 7 45 0 20 40 60 Preop Postop % Medication Free iStent inject® + Cataract Surgery iStent inject® + Cataract Surgery |
| 20 © 2018 Glaukos Corporation Facile, Click-and-Release 2-Stent Procedure |
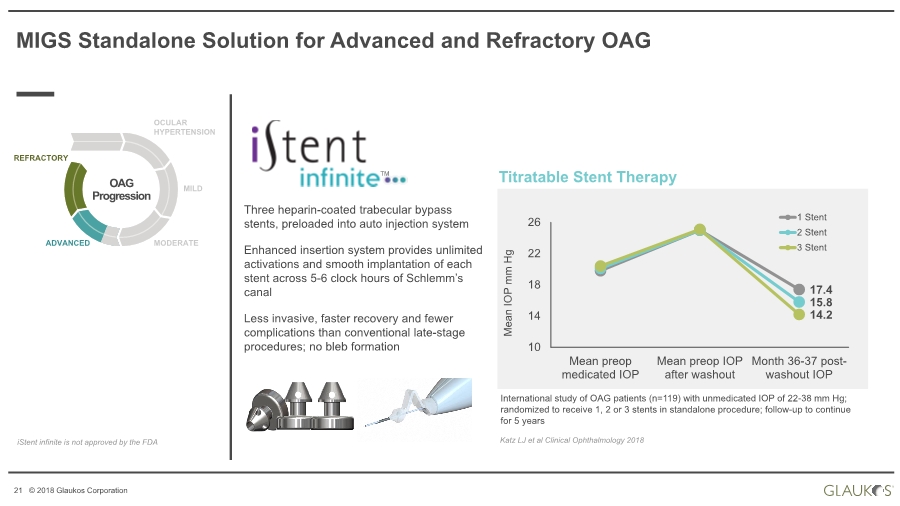
| 21 © 2018 Glaukos Corporation MIGS Standalone Solution for Advanced and Refractory OAG Three heparin-coated trabecular bypass stents, preloaded into auto injection system Enhanced insertion system provides unlimited activations and smooth implantation of each stent across 5-6 clock hours of Schlemm’s canal Less invasive, faster recovery and fewer complications than conventional late-stage procedures; no bleb formation OAG Progression OCULAR HYPERTENSION MILD MODERATEADVANCED REFRACTORY iStent infinite is not approved by the FDA Titratable Stent Therapy Mean IOP mm Hg 17.4 15.8 14.2 10 14 18 22 26 Mean preop medicated IOP Mean preop IOP after washout Month 36-37 post- washout IOP 1 Stent 2 Stent 3 Stent International study of OAG patients (n=119) with unmedicated IOP of 22-38 mm Hg; randomized to receive 1, 2 or 3 stents in standalone procedure; follow-up to continue for 5 years Katz LJ et al Clinical Ophthalmology 2018 TM |
| 22 © 2018 Glaukos Corporation MIGS Standalone Solution for Advanced and Refractory OAG iStent infinite is not approved by the FDA |
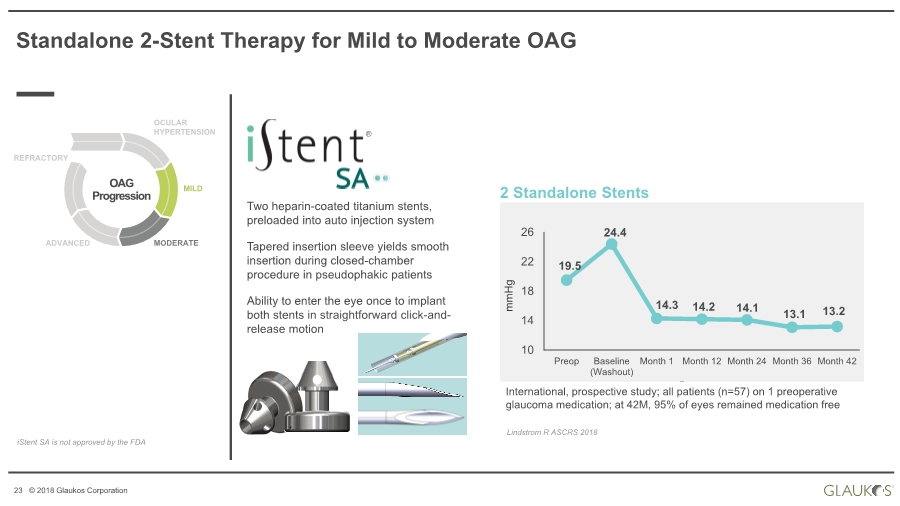
| 23 © 2018 Glaukos Corporation Two heparin-coated titanium stents, preloaded into auto injection system Tapered insertion sleeve yields smooth insertion during closed-chamber procedure in pseudophakic patients Ability to enter the eye once to implant both stents in straightforward click-and- release motion Standalone 2-Stent Therapy for Mild to Moderate OAG OAG Progression OCULAR HYPERTENSION MILD MODERATEADVANCED REFRACTORY iStent SA is not approved by the FDA 2 Standalone Stents 19.5 24.4 14.3 14.2 14.1 13.1 13.2 10 14 18 22 26 Preop Baseline (Washout) Month 1 Month 12 Month 24 Month 36 Month 42 International, prospective study; all patients (n=57) on 1 preoperative glaucoma medication; at 42M, 95% of eyes remained medication free Lindstrom R ASCRS 2018 mmHg ® |
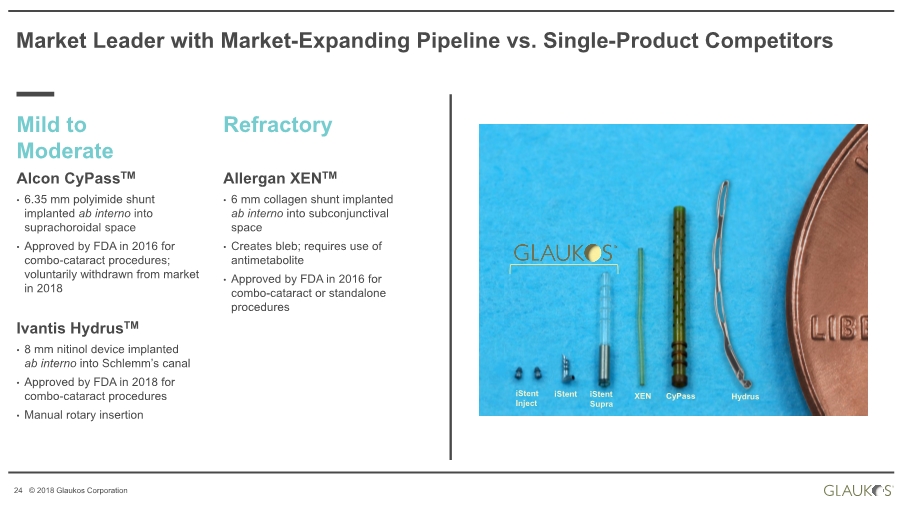
| 24 © 2018 Glaukos Corporation Alcon CyPassTM • 6.35 mm polyimide shunt implanted ab interno into suprachoroidal space • Approved by FDA in 2016 for combo-cataract procedures; voluntarily withdrawn from market in 2018 Ivantis HydrusTM • 8 mm nitinol device implanted ab interno into Schlemm’s canal • Approved by FDA in 2018 for combo-cataract procedures • Manual rotary insertion XEN Market Leader with Market-Expanding Pipeline vs. Single-Product Competitors Mild to Moderate Refractory Allergan XENTM • 6 mm collagen shunt implanted ab interno into subconjunctival space • Creates bleb; requires use of antimetabolite • Approved by FDA in 2016 for combo-cataract or standalone procedures iStent iStent Inject CyPass HydrusXENiStent Supra |
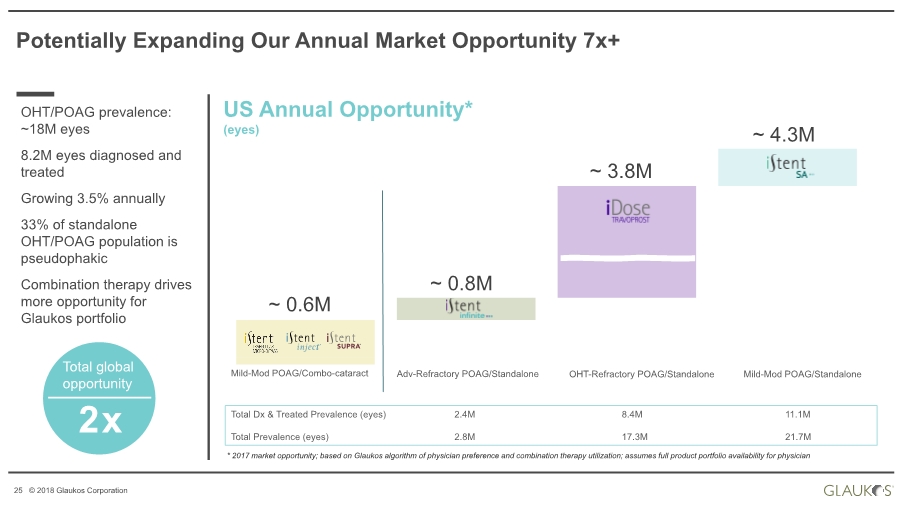
| 25 © 2018 Glaukos Corporation Potentially Expanding Our Annual Market Opportunity 7x+ OHT/POAG prevalence: ~18M eyes 8.2M eyes diagnosed and treated Growing 3.5% annually 33% of standalone OHT/POAG population is pseudophakic Combination therapy drives more opportunity for Glaukos portfolio * 2017 market opportunity; based on Glaukos algorithm of physician preference and combination therapy utilization; assumes full product portfolio availability for physician 2x Total global opportunity US Annual Opportunity* (eyes) Mild-Mod POAG/Combo-cataract Adv-Refractory POAG/Standalone Mild-Mod POAG/StandaloneOHT-Refractory POAG/Standalone ~ 0.6M ~ 0.8M ~ 3.8M ~ 4.3M Total Dx & Treated Prevalence (eyes) 2.4M 8.4M 11.1M Total Prevalence (eyes) 2.8M 17.3M 21.7M |
| 26 © 2018 Glaukos Corporation MIGS AND BEYOND Platform Development |
| 27 © 2018 Glaukos Corporation Glaukos Platform Pillars iStent inject® iStent® SA iStent infiniteTM iStent Supra® Restoring natural, physiological outflow iStent® • MIGS pioneer with unrivaled portfolio of micro- scale glaucoma devices • Breakthrough ab interno surgical innovation • Deep experience and demonstrated track record in micro-engineering design, assembly and manufacturability • Regulatory strategy and market positioning focused on large patient populations and full range of glaucoma progression iStent SA, iStent Supra and iStent infinite are not approved by the FDA |
| 28 © 2018 Glaukos Corporation Glaukos Platform Pillars iDoseTM Travoprost Sustained therapy pharmaceuticals • Leveraging unique expertise in micro-mechanical design, assembly and filling processes • Building seasoned ocular drug delivery team of 30+ chemists, scientists and engineers from leading pharmaceutical companies • Entered into pharmaceutical development agreement with D. Western Therapeutics Institute to explore novel rho kinase (ROCK) inhibitor compounds • Understanding necessary drug characteristics and predictability for delivery via iDoseTM system • Small-molecule APIs, high potency, low aqueous solubility • Receptor does not lose sensitivity during long-term dosing • Potential for reduced side effects vs. topical delivery • Molecular structure chemically stable over time iDose Travoprost is an unapproved drug and limited by US law to investigational use D. Western Development Agreement |
| 29 © 2018 Glaukos Corporation Glaukos: Key Takeaways Delivering novel surgical and pharmaceutical glaucoma therapy • Validating iDoseTM drug delivery system and developing new sustained pharmaceuticals platform • Extending leadership in MIGS treatment class with industry’s most comprehensive surgical offering • Delivering solid cadence of market-expanding product introductions for next 5+ years • Addressing important unmet clinical needs in large and growing markets • Becoming multi-faceted organization capable of transforming glaucoma therapy |
| 30 © 2018 Glaukos Corporation 3 0 |