Attached files
| file | filename |
|---|---|
| EX-32 - EX-32 - HANGER, INC. | a18-8429_2ex32.htm |
| EX-31.2 - EX-31.2 - HANGER, INC. | a18-8429_2ex31d2.htm |
| EX-31.1 - EX-31.1 - HANGER, INC. | a18-8429_2ex31d1.htm |
| EX-21 - EX-21 - HANGER, INC. | a18-8429_2ex21.htm |
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934.
For the fiscal year ended December 31, 2017
OR
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934.
For the transition period from
to
Commission File Number 1-10670
HANGER, INC.
(Exact name of registrant as specified in its charter.)
|
Delaware |
|
84-0904275 |
|
|
|
|
|
10910 Domain Drive, Suite 300, Austin, TX |
|
78758 |
Registrant’s phone number, including area code: (512) 777-3800
Securities registered pursuant to Section 12(b) of the Act:
|
Title of class |
|
Name of exchange on which registered |
|
Common Stock, par value $0.01 per share |
|
OTC Pink (operated by OTC Markets Group Inc.) |
Securities registered pursuant to Section 12(g) of the Act: None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days: Yes o No x
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No x
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer o |
|
Accelerated filer x |
|
Non-accelerated filer o |
|
Smaller reporting company o |
|
|
|
Emerging growth company o |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter which was June 30, 2017, $425.8 million.
As of May 1, 2018 the registrant had 36,692,863 shares of its Common Stock issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE: None
|
Hanger, Inc. |
|
|
ii | |
|
| |
|
1 | |
|
13 | |
|
27 | |
|
28 | |
|
29 | |
|
31 | |
|
| |
|
32 | |
|
34 | |
|
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations |
36 |
|
Item 7A. Quantitative and Qualitative Disclosures about Market Risk |
83 |
|
84 | |
|
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
85 |
|
86 | |
|
94 | |
|
| |
|
Item 10. Directors, Executive Officers and Corporate Governance |
95 |
|
102 | |
|
134 | |
|
Item 13. Certain Relationships and Related Transactions, and Director Independence |
137 |
|
138 | |
|
| |
|
140 | |
|
144 | |
|
145 | |
|
F-1 | |
|
E-1 |
We filed our Annual Report on Form 10-K for the year ended December 31, 2016 (the “2016 Form 10-K”) on January 19, 2018. The 2016 Form 10-K contained our consolidated financial statements and related footnotes for the years ended December 31, 2015 and 2016, as well as consolidated financial information for each of the quarterly and year-to-date periods occurring within those two years. The filings of our 2016 Form 10-K and this Annual Report on Form 10-K for the year ended December 31, 2017 were delayed due to a number of factors including our need to restate certain previously issued financial statements and related footnotes as discussed further below, as well as the necessity of our undertaking additional accounting procedures as a result of the material weaknesses in our internal controls over financial reporting. See “Item 9A. Controls and Procedures” in this Annual Report on Form 10-K for information regarding these material weaknesses.
We have not filed our Quarterly Reports on Form 10-Q for 2017. In lieu of filing Quarterly Reports for 2017, we have included in this Annual Report on Form 10-K all material information required to be included in the Quarterly Reports on Form 10-Q for 2017.
Previously, on May 12, 2017, we filed our Annual Report on Form 10-K for the year ended December 31, 2014 (the “2014 Form 10-K”). The 2014 10-K contained our consolidated financial information and related footnotes for the year ended December 31, 2014, as well as consolidated financial statements for the third and fourth quarters of 2014. The 2014 Form 10-K also included a restatement of our previously issued consolidated financial statements and related footnotes for (i) the fiscal years ended December 31, 2013 and 2012; (ii) the first two quarters of fiscal year 2014 and (iii) each of the quarterly periods in fiscal year 2013 (the “Restatement”). The 2014 Form 10-K also contained restated financial results for the fiscal years ended December 31, 2011 and 2010 (each unaudited), as summarized in “Item 6. Selected Financial Data” to the 2014 Form 10-K. The Restatement resulted in a cumulative reduction to our previously reported income before taxes through June 30, 2014 of approximately $175.1 million due to prior misstatements.
We have made continued progress in our efforts to remediate the material weaknesses that have prevented us from reporting our financial results on a timely basis. To date, we have taken and continue to take the actions described in the section titled “Remediation Plans” included in “Item 9A. Controls and Procedures” in this Annual Report on Form 10-K to address these previously identified material weaknesses. Our remediation efforts are ongoing. As we continue to evaluate and improve our internal control over financial reporting, we may implement additional measures or modify the currently identified remedial actions to remediate our material weaknesses.
Despite the substantial time and resources we have directed at our remediation efforts, we are unable to estimate at this time when these remediation efforts will be completed. Until the remediation efforts, including any additional remediation efforts that our management identifies as necessary, are completed, the material weaknesses described in “Item 9A. Controls and Procedures” will continue to exist.
We intend to provide additional information regarding our remediation efforts with respect to the material weaknesses in future filings with the U.S. Securities and Exchange Commission (the “SEC”).
Business Overview
General
Hanger, Inc. (“the Company,” “we,” “our,” or “us”) is a leading national provider of products and services that assist in enhancing or restoring the physical capabilities of patients with disabilities or injuries. Built on the legacy of James Edward Hanger, the first amputee of the American Civil War, we and our predecessor companies have provided orthotic and prosthetic (“O&P”) services for over 150 years. We provide O&P services, distribute O&P devices and components, manage O&P networks and provide therapeutic solutions to patients and businesses in acute, post-acute and clinic settings. We operate through two segments - Patient Care and Products & Services.
Our Patient Care segment is primarily comprised of Hanger Clinic, which specializes in the design, fabrication and delivery of custom O&P devices through 682 patient care clinics and 112 satellite locations in 44 states and the District of Columbia, as of December 31, 2017. We also provide payor network contracting services to other O&P providers through this segment.
Our Products & Services segment is comprised of our distribution and therapeutic solutions businesses. As a leading provider of O&P products in the United States, we coordinate through our distribution business the procurement and distribution of a broad catalog of O&P parts, componentry and devices to independent O&P providers nationwide. To facilitate speed and convenience, we deliver these products through our five distribution facilities that are located in Nevada, Georgia, Illinois, Pennsylvania and Texas. The other business in our Products & Services segment is our therapeutic solutions business, which provides specialized rehabilitation technologies and evidence-based clinical programs for post-acute rehabilitation patients at approximately 4,000 skilled nursing and post-acute providers nationwide.
For the years ended December 31, 2017, 2016 and 2015, our net revenues were $1,040.8 million, $1,042.1 million and $1,067.2 million, respectively. We recorded a loss from continuing operations of $104.7 million, $107.4 million and $319.1 million for the years ended December 31, 2017, 2016 and 2015, respectively.
The following table summarizes the percentage of net revenues derived from each of our two operating segments:
|
|
|
For the Years Ended December 31, |
| ||||
|
|
|
2017 |
|
2016 |
|
2015 |
|
|
Patient Care |
|
81.9 |
% |
80.6 |
% |
82.0 |
% |
|
Products & Services |
|
18.1 |
% |
19.4 |
% |
18.0 |
% |
See Note R - “Segment and Related Information” to our consolidated financial statements in this Annual Report on Form 10-K for additional information about our segments.
Industry Overview
We estimate that approximately $4.0 billion is spent in the United States each year for prescription-based O&P products and services through O&P clinics. Orthotic devices, or “orthoses” are externally applied devices used to modify the structural and functional characteristics of the neuromuscular and skeletal system. These devices typically are provided to patients suffering from musculoskeletal disorders, such as ailments of the back, extremities or joints; injuries from sports; or conditions such as cerebral palsy, scoliosis and stroke. Prosthetic devices, or “prostheses” are artificial devices that replace a missing limb or portion of a limb. These devices are provided to patients with amputated or congenitally absent limbs to replace the function and appearance of a limb so that patients can resume activities of daily living and work. The most prevalent causes for amputations are from complications due to diabetes, trauma associated with accidents, physical injury or infection.
The industry derives its primary revenue from the evaluation, fabrication and fitting of custom O&P devices to serve patients needing both new and replacement devices. Additionally, O&P clinics typically provide patients with other non-custom orthotic products, diabetic shoes and inserts, and support patients through the repair and adjustment of their devices.
We believe our Patient Care segment currently serves approximately 20% of the O&P clinic market. We estimate that the next largest provider of O&P services in the United States is the U.S. Department of Veterans Affairs (the “VA”), which operates 79 O&P clinics on behalf of its covered veteran patients. In addition to serving veterans through their own facilities, in certain markets the VA is also a client of Hanger Clinic. Approximately 9% of Hanger Clinic’s revenue is derived from services provided to veteran patients through contracts with the VA.
The O&P patient care market is highly fragmented and is typically characterized by regional and local independent O&P businesses. We estimate that our top ten competitors have an average of approximately 25 clinics each, with the smallest having 16 and the largest having 36 clinics. The remainder of the market is served by individual practitioners and smaller regional or market-based firms with approximately ten or fewer clinics. Based on this, we do not believe that any single competitor accounts for more than approximately 2% of the nation’s total estimated O&P clinic revenues.
We anticipate that the demand for O&P services will continue to grow as the nation’s population increases, and as a result of several trends, including the aging of the U.S. population, there will be an increase in the prevalence of disease related disability and the demand for new and advanced devices. We believe the typical replacement time for prosthetic devices is three to five years, while the typical replacement time for orthotic devices varies, depending on the device.
We estimate that approximately $1.7 billion is spent in the United States each year by providers of O&P patient care services for the O&P products, components, devices and supplies used in their businesses. Our Products & Services segment distributes to independent providers of O&P services and to our own patient care clinics. We estimate that our distribution sales account for approximately 8% of the market for O&P products, components, devices and supplies (excluding sales to our Patient Care segment).
We estimate the market for rehabilitation technologies, integrated clinical programs and therapist training in skilled nursing facilities (“SNFs”) to be approximately $150 million annually. We currently provide these products and services to approximately 25% of the estimated 15,000 SNFs located in the U.S. We estimate the market for rehabilitation technologies, clinical programs and training within the broader post-acute rehabilitation markets to be approximately $400 million annually. We do not currently provide a meaningful amount of products and services to this broader market.
Business Strategy
Our goal is to be the provider of choice for patients, referring physicians and customers seeking products and services that enhance human physical capabilities. Our strategy is to pursue the creation of an integrated therapeutic solutions model that will have a strong focus in custom O&P and immediately adjacent markets to provide our patients and customers with a spectrum of services that address their individual needs. To foster growth, we intend to focus on initiatives that will differentiate Hanger from our competitors.
Government led health care reform is driving significant changes to our business environment, with focus on lowering health care costs while improving patient outcomes and satisfaction. As a result, our strategy is focused on enhancing the quality of care to elevate patient satisfaction, investing in processes and technologies to measure and report on patient outcomes and satisfaction, and further increasing our profile with referring health care providers and payors. In addition, we are committed to reducing the cost of this care by undertaking several initiatives that include establishing device standards that provide the highest function, durability and comfort at the lowest cost, reconfiguring our supply chain and fabrication processes, streamlining internal administrative processes and reducing back-office functions performed within patient care clinics.
Business Description
Patient Care
Our Patient Care segment employs approximately 1,500 clinical prosthetists, orthotists and pedorthists, which we refer to as clinicians, substantially all of which are certified by either the American Board for Certification (“ABC”) or the Board of Certification of Orthotists and Prosthetists, which are the two boards that certify O&P clinicians. To facilitate timely service to our patients, we also employ technicians, fitters and other ancillary providers to assist its clinicians in the performance of their duties. Through this segment, we additionally provide network contracting services to independent providers of O&P through our “Linkia” business.
Patients are typically referred to Hanger Clinic by an attending physician who determines a patient’s treatment and writes a prescription. Our clinicians then consult with both the referring physician and the patient with a view toward assisting in the design of an orthotic or prosthetic device to meet the patient’s needs. O&P devices are increasingly technologically advanced and custom designed to add functionality and comfort to patients’ lives, shorten the rehabilitation process and lower the cost of rehabilitation.
Based on the prescription written by a referring physician, our clinicians examine and evaluate the patient and either design a custom device or, in the case of certain orthotic needs, utilize a non-custom device, including, in appropriate circumstances, an “off the shelf” device, to address the patient’s needs. When fabricating a device, our clinicians ascertain the specific requirements, componentry and measurements necessary for the construction of the device. Custom devices are constructed using componentry provided by a variety of third party manufacturers who specialize in O&P, coupled with sockets and other elements that are fabricated by our clinicians and technicians, to meet the individual patient’s physical and ambulatory needs. Our clinicians and technicians typically utilize castings, electronic scans and other techniques to fabricate items that are specialized for the patient. After fabricating the device, a fitting process is undertaken and adjustments are made to ensure the achievement of proper alignment, fit and patient comfort. The fitting process often involves several stages to successfully achieve desired functional and cosmetic results.
Given the differing physical weight and size characteristics, location of injury or amputation, capability for physical activity and mobility, cosmetic and other needs of each individual patient, each fabricated prosthesis and orthosis is customized for each particular patient. These custom devices are commonly fabricated at one of our regional or national fabrication facilities.
We have earned a reputation within the O&P industry for the development and use of innovative technology in our products, which has increased patient comfort and capability and can significantly enhance the rehabilitation process. Frequently, our proprietary Insignia scanning system is used in the fabrication process. The Insignia system scans the patient and produces an accurate computer generated image, resulting in a faster turnaround for the patient’s device and a more professional overall experience.
In recent years, we have established a centralized revenue cycle management organization that assists our clinics in pre-authorization, patient eligibility, denial management, collections, payor audit coordination and other accounts receivable processes.
The principal reimbursement sources for our services are:
· Commercial private payors and other non-governmental organizations, which consist of individuals, rehabilitation providers, commercial insurance companies, health management organizations (“HMOs”), preferred provider organizations (“PPOs”), hospitals, vocational rehabilitation centers, workers’ compensation programs, third party administrators and similar sources;
· Medicare, a federally funded health insurance program providing health insurance coverage for persons aged 65 or older and certain disabled persons;
· Medicaid, a health insurance program jointly funded by federal and state governments providing health insurance coverage for certain persons based upon financial need, regardless of age, which may supplement Medicare benefits for financially needy persons aged 65 or older; and
· the U.S. Department of Veterans Affairs.
We typically enter into contracts with third party payors that allow us to perform O&P services for a referred patient and to be paid under the contract with the third party payor. These contracts usually have a stated term of one to three years. These contracts generally may be terminated without cause by either party on 60 to 90 days’ notice or on 30 days’ notice if we have not complied with certain licensing, certification, program standards, Medicare or Medicaid requirements or other regulatory requirements. Reimbursement for services is typically based on a fee schedule negotiated with the third party payor that reflects various factors, including market conditions, geographic area and number of persons covered. Many of our commercial contracts are indexed to the commensurate Medicare fee schedule that relates to the products or services being provided.
Government reimbursement, comprised of Medicare, Medicaid and the U.S. Department of Veterans Affairs, in the aggregate, accounted for approximately, 54.8%, 54.1% and 53.4% of our net revenue in 2017, 2016 and 2015, respectively. These payors set maximum reimbursement levels for O&P services and products. Medicare prices are adjusted each year based on the Consumer Price Index for All Urban Consumers (“CPI-U”) unless Congress acts to change or eliminate the adjustment. The CPI-U is adjusted further by an efficiency factor (the “Productivity Adjustment” or the “Multi-Factor Productivity Adjustment”) in order to determine the final rate adjustment each year. The Medicare price adjustments for 2017, 2016, 2015, 2014 and 2013 were 0.7%, (0.4%), 1.5%, 1.0% and 0.8%, respectively. There can be no assurance that future adjustments will not reduce reimbursements for O&P services and products from these sources.
We, and the O&P industry in general, are subject to various Medicare compliance audits, including Recovery Audit Contractor (“RAC”) audits, Comprehensive Error Rate Testing (“CERT”) audits, Targeted Probe and Educate (“TPE”) audits and Zone Program Integrity Contractor (“ZPIC”) audits. TPE audits are generally pre-payment audits, while RAC, CERT and ZPIC audits are generally post-payment audits. The recently implemented TPE audits have replaced the previous Medicare Administrative Contractor (“MAC”) audits. Adverse post-payment audit determinations generally require Hanger to reimburse Medicare for payments previously made, while adverse pre-payment audit determinations generally result in the denial of payment. In either case, we can request a redetermination or appeal, if we believe the adverse determination is unwarranted, which can take an extensive period of time to resolve, currently up to six years or more.
Products & Services
Through our wholly-owned subsidiary, Southern Prosthetic Supply, Inc. (“SPS”), we distribute O&P components to both independent customers and our own clinics in the Patient Care segment. SPS purchases, warehouses and distributes over 400,000 SKUs from more than 300 different manufacturers. Through our warehousing and distribution facilities in Nevada, Georgia, Illinois, Pennsylvania and Texas, we are able to deliver products to the vast majority of our customers in the United States within two business days. Through its SureFit subsidiary, SPS also manufactures and sells therapeutic footwear for diabetic patients in the podiatric market, and through its National Labs subsidiary, it is a fabricator of O&P devices both for our patient care clinics and competitor clinics.
Our distribution business enables us to:
· centralize our purchasing and thus lower our material costs by negotiating purchasing discounts from manufacturers;
· better manage our patient care clinic inventory levels and improve inventory turns;
· improve inventory quality control;
· encourage our patient care clinics to use the most clinically appropriate products; and
· coordinate new product development efforts with key vendors.
Through our wholly-owned subsidiaries, Accelerated Care Plus Corp. and Accelerated Care Plus Leasing, Inc. (together, “ACP”), our therapeutic solutions business is a leading provider of rehabilitation technologies and integrated clinical programs to rehabilitation providers. Our unique value proposition is to provide our customers with a full-service “total solutions” approach encompassing proven medical technology, evidence based clinical programs, and ongoing consultative education and training. Our services support increasingly advanced treatment options for a broader patient population and more medically complex conditions. We serve approximately 4,000 skilled nursing and post-acute providers nationwide.
Competition
The business of providing O&P patient care services is highly competitive in the markets in which we operate. In the prosthetic business, we compete with numerous small independent O&P providers for referrals from physicians, therapists, employers, HMOs, PPOs, hospitals, rehabilitation centers, out-patient clinics and insurance companies on both a local and regional basis. In the orthotic business, we compete with other patient care service providers, including device manufacturers that have independent sales forces, on the basis of quality and timeliness of patient care, location of patient care clinics and pricing for services.
Although we serve a significant portion of the O&P patient care market, referral decisions made by surgeons, physicians and other medical providers are generally made on a local basis, based on their individual evaluation of the relative quality of care provided by us and our local market competitors. Therefore, our national scale may not provide a competitive advantage in any particular market in which we operate.
We also compete with independent O&P providers for the retention and recruitment of qualified O&P clinicians. In some markets, the demand for clinicians exceeds the supply of qualified personnel.
Our Products & Services segment competes with other distributors, manufacturers who sell their products directly and providers of equipment and services on a regional and national basis that have similar sales forces and products. Some of our distributor competitors are also dedicated to the O&P industry, but many others are large medical product distributors who also distribute O&P products, particularly orthotic products.
Competitive Strengths
We believe that the combination of the following competitive strengths will help us to grow our businesses by increasing our net revenues, net income and market share:
· Leading market position in both the O&P market place and the post-acute rehabilitation markets;
· National scale of operations, which better enables us to:
· establish our brand name and generate economies of scale;
· identify and implement best practices throughout our organization;
· consistently apply the rigorous claims documentation standards required for reimbursement and facilitate reimbursement through a revenue cycle management organization;
· collect, aggregate and publish our statistically significant clinical outcomes and patient satisfaction data and metrics;
· offer a single network solution to national and regional shared fabrication facilities;
· identify, test and deploy emerging technology; and
· increase our influence on, and input into, regulatory trends;
· Distribution of, and purchasing power for, O&P components and finished O&P products, which better enables us to:
· negotiate greater purchasing discounts from manufacturers and freight providers;
· reduce patient care clinic inventory levels and improve inventory turns through centralized purchasing control;
· access prefabricated and finished O&P products;
· promote the usage by our patient care clinics of clinically appropriate products that also enhance our profit margins; and
· expand the external client base of the distribution business in our Products & Services segment;
· Proven ability to rapidly incorporate technological advances in the fitting and fabrication of O&P devices;
· History of integrating small and medium sized O&P business acquisitions, including 139 O&P businesses between 1997 and 2015, representing over 365 patient care clinics;
· Highly trained clinicians, whom we provide with the highest level of continuing education and training through programs designed to inform them of the latest technological developments in the O&P industry;
· Experienced and committed management team; and
· Beneficial government relations efforts, which enable us to educate legislators on the medical benefits and cost effectiveness of O&P services.
Suppliers
We purchase prefabricated O&P devices, components and materials from hundreds of suppliers across the country, which are utilized by our clinicians and technicians in the fabrication of O&P products. These devices, components and materials are
used in the products we offer in our patient care clinics throughout the United States. As of December 31, 2017, two suppliers accounted for 10% or more of our annual purchases, representing 17.6% and 10.8% respectively.
Sales and Marketing
In our Patient Care segment, primarily through their interaction with and provision of prosthetic or orthotic services to the patients of referring surgeons, physicians and other providers, our individual clinicians in local patient care clinics historically have conducted our sales and marketing efforts. Due primarily to the fragmented nature of the O&P industry, the success of a particular patient care clinic has been largely a function of its local reputation for quality of care, responsiveness and length of service in the local communities.
To augment the efforts of the business segment personnel, we have developed a centralized sales and marketing department, whose efforts target the following:
· Marketing and Public Relations. Our objective is to increase the visibility of the “Hanger” brand by building relationships with major referral sources. We also continue to explore creating alliances with certain vendors to market products and services on a nationwide basis.
· Business Development. We have dedicated personnel in most of our operating regions who are responsible for arranging seminars, clinics and forums to educate and consult with patients and to increase the local community’s awareness of the “Hanger” brand. These business development managers also meet with local referral and contract sources to help our clinicians develop new relationships in their markets.
· Insurance Contracts. Our specialty health care company, Linkia, works with national insurance companies to help manage their O&P networks. Linkia is a network management organization dedicated solely to the O&P industry to improve the interface between payors and O&P providers by simplifying network management and administration, in-depth industry expertise and scalability to payors.
Marketing of our services is conducted on a national basis through a dedicated sales force, print and e-commerce catalogs and exhibits at industry and medical meetings and conventions. We use directed marketing to segments of the health care industry, such as orthopedic surgeons, physical and occupational therapists, patient care managers and podiatrists, by providing specialized catalogs focused on their medical specialty.
In our Products & Services segment, we employ dedicated sales professionals that generally are responsible for a geographic region or specific product line.
Acquisition Strategy
Our strategy is to achieve long-term growth through disciplined diversification of our revenue streams, including geographic expansion or the broadening of our continuum of care through acquisitions. One of the primary drivers in executing our acquisition strategy is expanding our ability to serve new patients in new geographic markets.
Once an acquisition is consummated, we integrate and generally centralize certain key functions including IT, marketing, sales, finance and administration to ensure that we can optimize cross-selling opportunities and realize cost efficiencies.
In some of our historical acquisitions, in addition to cash paid at closing, the purchase price has included unsecured subordinated promissory notes (“Seller Notes”) and contingent consideration terms (“earnouts”) associated with the achievement of certain designated collection targets for the acquired business. Earnouts can be used to compromise between
our valuation and seller’s expectations regarding purchase price, while providing protection from our overpayment if historical collections are not an accurate indicator of post-closing financial performance of the acquired business.
Our evaluation of the acquired business is based on various factors, including specialized know-how, reputation, geographic coverage, competitive position and service and product offerings, as well as our experience and judgment.
Acquisition Activity
We have not made any acquisitions since the first quarter of 2015 due to the necessity of utilizing available operating cash flow to fund accounting, legal and other professional fees in connection with the preparation and review of our financial statements, efforts to remediate our material weaknesses, related legal matters, and due to the effect of our prior non-compliance (under the terms of previously existing debt instruments) with certain of our debt covenants relating to our failure to meet financial statement reporting requirements. In connection with refinancing of our debt in 2018, and the resolution of the primary factors which led us to halt our acquisitions, we currently intend to recommence acquisitions of O&P businesses similar to those that we have consummated in prior years.
In 2015, we acquired three O&P businesses with approximately $11.8 million in revenue, operating a total of 15 patient care clinics located in three states. The aggregate purchase price for these businesses was approximately $15.3 million, including approximately $10.2 million in cash, approximately $4.7 million in Seller Notes, approximately $0.4 million working capital adjustments and no contingent consideration.
Government Regulation
The operations of our business are subject to a variety of federal, state and local governmental regulations. We make every effort to comply with all applicable regulations through compliance programs, policies and procedures, manuals and personnel training. Despite these efforts, we cannot guarantee that we will be in absolute compliance with all regulations at all times. Failure to comply with applicable governmental regulations may result in significant penalties, including exclusion from the Medicare and Medicaid programs, which would have a material adverse effect on our business and financial results.
Fraud and Abuse. Violations of fraud and abuse laws are punishable by criminal and/or civil sanctions, including, in some instances, False Claims Act liability (discussed below), imprisonment and exclusion from participation in federal health care programs, including Medicare, Medicaid, U.S. Department of Veterans Affairs health programs and the Department of Defense’s TRICARE program, formerly known as CHAMPUS. These laws, which include but are not limited to federal and state anti-kickback laws, false claims laws, physician self-referral laws and federal criminal health care fraud laws, are discussed in further detail below. We believe our billing practices, operations and compensation and financial arrangements with referral sources and others materially comply with applicable federal and state requirements. However, we cannot assure that such requirements will always be interpreted by a governmental authority in a manner consistent with our interpretation and application. The failure to comply, even if inadvertent, with any of these requirements could require us to alter our operations with and/or refund payments to the government. Such refunds could be significant and could also lead to the imposition of significant penalties. Even if we successfully defend against any action against us for violation of these laws or regulations, we would likely be forced to incur significant legal expenses and divert our management’s attention from the operation of our business. Any of these actions, individually or in the aggregate, could have a material adverse effect on our business and financial results.
Anti-Kickback Laws. Our operations are subject to federal and state anti-kickback laws. The federal Anti-Kickback Statute (Section 1128B(b) of the Social Security Act) prohibits persons or entities from knowingly and willfully soliciting, offering, receiving or paying any remuneration in any form (including any kickback, bribe or rebate) in return for, or to induce, the referral of persons eligible for benefits under a federal health care program (including Medicare, Medicaid, the U.S. Department of Veterans Affairs health programs and TRICARE), or the ordering, purchasing, leasing, or arranging for, or the
recommendation of purchasing, leasing or ordering of, items or services that may be paid for, in whole or in part, by a federal health care program. Courts have held that the statute may be violated when even one purpose (as opposed to a primary or sole purpose) of the remuneration is to induce referrals or other business.
Recognizing that the Anti-Kickback Statute is broad and may technically prohibit beneficial commercial arrangements, the Office of Inspector General of the Department of Health and Human Services has developed regulations addressing certain business arrangements that will offer protection from scrutiny under the Anti-Kickback Statute. These “Safe Harbors” describe activities which may be protected from prosecution under the Anti-Kickback Statute, provided that they meet all of the requirements of the applicable Safe Harbor regulation. For example, the Safe Harbors cover activities such as offering discounts to health care providers and contracting with physicians or other individuals or entities that have the potential to refer business to us that would ultimately be billed to a federal health care program, so long as the discount is properly disclosed and appropriately reflected in any claims or charges.
Failure to qualify for Safe Harbor protection does not automatically mean that an arrangement is illegal. Rather, the facts and circumstances of the arrangement must be analyzed to determine whether there is improper intent to pay or receive remuneration in return for referrals. Conduct and business arrangements that do not fully satisfy one of the Safe Harbors may result in increased scrutiny by government enforcement authorities. In addition, some states have anti-kickback laws that vary in scope and may apply regardless of whether a federal health care program is involved.
Our operations and business arrangements include, for example, discount programs or other financial arrangements with individuals and entities, such as lease arrangements with hospitals and certain participation agreements. Therefore, our operations and business arrangements are required to comply with the anti-kickback laws. Although our business arrangements and operations may not always satisfy all the criteria of a Safe Harbor, we believe that our operations are in material compliance with federal and state anti-kickback statutes. Nonetheless, we cannot ensure that the government’s interpretation of a Safe Harbor provision will always be consistent with our own, and our arrangements may be subject to scrutiny under anti-kickback laws. Noncompliance with such laws can result in a number of enforcement actions, including the imposition of civil monetary penalties and exclusion from federal health care programs.
Medical Device Regulation. We provide, distribute and lease products that are subject to regulation as medical devices by the U.S. Food and Drug Administration (“FDA”) under the Federal Food, Drug and Cosmetic Act (“FDCA”) and accompanying regulations. In our Patient Care segment, with the exception of two products which have been cleared for marketing as prescription medical devices under section 510(k) of the FDCA, we believe that the products we provide, including O&P medical devices, accessories and components, are not Class III devices and thus are exempt from the FDA’s regulations for pre-market clearance or approval requirements and from most requirements relating to the quality system regulation (except for certain record keeping and complaint handling requirements). In our Products & Services segment, ACP manufactures, leases and sells a number of rehabilitation devices that have been cleared or approved for marketing under section 510(k) of the FDCA, and are subject to the requirements of the quality system regulation. All of our device businesses are required to adhere to regulations for medical devices regarding adverse event reporting, establishment registration and product listing, and we are subject to inspection by the FDA for compliance with all applicable requirements. Labeling and promotional materials also are subject to scrutiny by the FDA and, in certain circumstances, by the Federal Trade Commission. Our medical device operations are subject to inspection by the FDA for compliance with applicable FDA requirements, and the FDA has in the past raised compliance concerns in connection with these investigations. We have addressed these concerns and believe we are in compliance with applicable FDA requirements, but we cannot assure that we will be found to be in compliance at all times. Non-compliance could result in a variety of civil and/or criminal enforcement actions, including issuance of a Warning Letter, seizure, examination and inspection of our products and a civil injunction or criminal prosecution, which could have a material adverse effect on our business and results of operations.
Physician Self-Referral Laws. We are also subject to federal and state physician self-referral laws. With certain exceptions, the federal Medicare physician self-referral law (the “Stark Law”) (Section 1877 of the Social Security Act) prohibits a
physician from referring Medicare beneficiaries to an entity for “designated health services” including durable medical equipment and supplies, and prosthetic and orthotic devices and supplies, if the physician or the physician’s immediate family member has a financial relationship with the entity. A financial relationship includes both ownership or investment interests and compensation arrangements. An entity that furnishes designated health services pursuant to a prohibited referral may not present or cause to be presented a claim or bill for such designated health services. Penalties for violating the Stark Law include denial of payment for the service, an obligation to refund any payments received, civil monetary penalties, potential False Claims Act litigation and the possibility of being excluded from the Medicare or Medicaid programs.
Despite the general prohibition on such physician financial relationships, the Stark Law does provide a number of exceptions from liability. For example, with respect to ownership/investment interests, there is an exception under the Stark Law for referrals made to a publicly traded entity in which the physician or the physician’s immediate family member has an investment interest if the entity’s shares are generally available to the public at the time of the designated health service referral, and are traded on certain exchanges, including among others the New York Stock Exchange (“NYSE”) as well as over-the-counter quotation systems including the OTC Markets Group, Inc. (“OTC”) and/or the investment entity had shareholders’ equity exceeding $75.0 million for its most recent fiscal year or as an average during the three previous fiscal years. We meet these tests and, therefore, believe that referrals from physicians who have ownership interests in our stock, or whose immediate family members have ownership interests in our stock, should not result in liability under the Stark Law.
With respect to compensation arrangements, there are exceptions under the Stark Law that permit physicians to maintain certain business arrangements, such as personal service contracts and equipment or space leases, with health care entities to which they refer patients for designated health services. All of the elements of a Stark Law exception must be met in order for the exception to apply. Further, unlike the Anti-Kickback Statute, under the Stark Law, liability can result without specific intent to induce referrals. We believe that our compensation arrangements with physicians comply with the Stark Law, either because the physician’s relationship fits fully within a Stark Law exception or because the physician does not generate prohibited referrals. If, however, we receive a prohibited referral, our submission of a bill for services rendered pursuant to such a referral could subject us to sanctions under the Stark Law and applicable state self-referral laws, including false claims liability, potential exclusion and imposition of civil monetary penalties. State self-referral laws may extend the prohibitions of the Stark Law to Medicaid beneficiaries, and there are some indications that the federal government may similarly expand the reach of the law.
False Claims Laws. We are also subject to federal and state laws prohibiting individuals or entities from knowingly presenting, or causing to be presented, claims for payment to third party payors (including Medicare and Medicaid) that are false or fraudulent, are for items or services not provided as claimed, or otherwise contain misleading information. Each of our patient care clinics is responsible for the preparation of documents for the submission of reimbursement claims to third party payors for items and services furnished to patients. In addition, our personnel may, in some instances, provide advice on billing and reimbursement to purchasers of our products. Also, prosecutors and so-called “qui tam” relators (whistleblowers) may claim that a regulatory violation or wrongfully-retained overpayment may be the basis of False Claims Act litigation. Successful relators can receive a share of the recovery in a False Claims Act case ranging from 15% to 30%, depending on whether the government “intervenes” in the case. Penalties in a False Claims Act case may include double or triple damages plus penalties ranging from $11,181 to $22,363 per claim. These penalties are nearly double what they were in prior years. While we endeavor to assure that our billing practices comply with applicable laws, if claims submitted to payors are deemed to be false, fraudulent or for items or services not provided as claimed, we may face liability for presenting or causing to be presented such claims.
Certification and Licensure. Our clinicians and/or certain operating units may be subject to certification or licensure requirements under the laws of some states. Most states do not require separate licensure for clinicians. However, several states currently require clinicians to be certified by an organization such as the ABC. The ABC conducts a certification program for clinicians and an accreditation program for patient care clinics. The minimum requirements for new certified clinicians are a college degree, completion of an accredited master’s degree program, residency at a patient care clinic under
the supervision of a certified clinician and successful completion of certain examinations. Certified clinicians are required to participate in a prescribed number of hours of specialized continuing education courses to maintain their certifications. Minimum requirements for an accredited patient care clinic include the presence of a certified clinician and specific plant and equipment requirements.
While we endeavor to comply with all state licensure requirements, we cannot assure that we will be in compliance at all times with these requirements, or how they may be interpreted or re-interpreted by the various state and local agencies. Failure to comply with state licensure requirements could result in suspension or termination of licensure, civil penalties, termination of our Medicare and Medicaid agreements, and repayment of amounts received from Medicare and Medicaid for services and supplies furnished by an unlicensed individual or entity.
HIPAA Violations. The Health Insurance Portability and Accountability Act (“HIPAA”) provides criminal penalties for, among other offenses: health care fraud; theft or embezzlement with respect to a health care benefit program; false statements in connection with the delivery of or payment for health care benefits, items or services; and obstruction of criminal investigation of health care offenses. Unlike other federal laws, these offenses are not limited to federal health care programs.
In addition, HIPAA authorizes the imposition of civil monetary penalties where a person offers or pays remuneration to any individual eligible for benefits under a federal health care program that such person knows or should know is likely to influence the individual to order or receive covered items or services from a particular provider, clinician or supplier. Excluded from the definition of “remuneration” are incentives given to individuals to promote the delivery of preventive care (excluding cash or cash equivalents), incentives of nominal value and certain differentials in or waivers of coinsurance and deductible amounts.
These laws may apply to certain of our operations. As noted above, we have established various types of discount programs and other financial arrangements with individuals and entities. We also bill third party payors and other entities for items and services provided at our patient care clinics. While we endeavor to ensure that our discount programs and other financial arrangements and billing practices comply with applicable laws, such programs, arrangements and billing practices could be subject to scrutiny and challenge under HIPAA.
Confidentiality and Privacy Laws. The Administrative Simplification Provisions of HIPAA, and their implementing regulations, set forth privacy standards and implementation specifications concerning the use and disclosure of individually identifiable health information (referred to as “protected health information”) by health plans, health care clearinghouses and health care providers that transmit health information electronically in connection with certain standard transactions (“Covered Entities”). HIPAA further requires Covered Entities to protect the confidentiality of protected health information by meeting certain security standards and implementation specifications. In addition, under HIPAA, Covered Entities that electronically transmit certain administrative and financial transactions must utilize standardized formats and data elements (the “transactions/code sets standards”). HIPAA imposes civil monetary penalties for non-compliance, and, with respect to knowing violations of the privacy standards, or violations of such standards committed under false pretenses or with the intent to sell, transfer or use protected health information for commercial advantage, criminal penalties. Certain agents of Covered Entities (“business associates”) also have HIPAA responsibilities and liabilities. We have business associates and are business associates to other Covered Entities. We believe that we are subject to the Administrative Simplification Provisions of HIPAA and are taking steps to meet applicable standards and implementation specifications. The new requirements have had a significant effect on the manner in which we handle health data and communicate with payors.
In addition, state confidentiality and privacy laws may impose civil and/or criminal penalties for certain unauthorized or other uses or disclosures of protected health information. We are also subject to these laws. While we endeavor to assure that our operations comply with applicable laws governing the confidentiality and privacy of protected health information, we could face liability in the event of a use or disclosure of protected health information in violation of one or more of these laws.
Personnel and Training
None of our employees are subject to a collective bargaining agreement. We believe that we have satisfactory relationships with our approximately 4,600 employees and strive to maintain these relationships by offering competitive benefit packages, training programs and opportunities for advancement.
We provide a series of ongoing training programs to improve the professional knowledge of our clinicians. For example, we have an annual education fair that is attended by our clinicians, leaders and other employees. This annual meeting consists of lectures and seminars covering many clinical topics including the latest technology and process improvements, business courses and other courses that allow the clinicians to fulfill their ongoing continuing education requirements.
Insurance
We currently maintain insurance coverage for professional liability, product liability, general liability, directors’ and officers’ liability, workers’ compensation, executive protection, property damage and other lines of insurance. Our general liability insurance coverage is $1.0 million per occurrence, with a $25.0 million umbrella insurance policy. The coverage for professional liability, product liability and workers’ compensation is self-insured with both individual specific claim and aggregate stop-loss policies to protect us from either significant individual claims or dramatic changes in our loss experience. Based on our experience and prevailing industry practices, we believe our coverage is adequate as to risks and amount.
Our Website
Our website is http://www.hanger.com. We make available free of charge, on or through our website, our Annual Report on Form 10-K, Current Reports on Form 8-K, Section 16 filings (i.e., Forms 3, 4 and 5), proxy statements and other documents as required by applicable law and regulations as soon as reasonably practicable after electronically filing such reports with the SEC at http://www.sec.gov. The public may read and copy any materials that we file with the SEC at the SEC’s Public Reference Room at 100 F Street N.E., Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330 (1-800-732-0330). The SEC maintains an Internet site (http://www.sec.gov) that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. Our website also contains the charters of the Audit Committee, Corporate Governance and Nominating Committee, Compensation Committee and Quality, Technology, Compliance and Outcomes Committee of our Board of Directors; our Code of Business Conduct and Ethics for Directors and Employees, which includes our principal executive, financial and accounting officers; as well as our Corporate Governance Guidelines. Information contained on our website is not part of this report.
Set forth below are certain risk factors that could adversely affect our business, results of operations and financial condition. You should carefully read the following risk factors, together with the consolidated financial statements, related notes and other information contained in this Annual Report on Form 10-K. This Annual Report on Form 10-K contains forward-looking statements that contain risks and uncertainties. Please read the cautionary notice regarding forward-looking statements in Item 7. under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in connection with your consideration of the risk factors and other important factors that may affect future results described below.
The restatement of our previously issued consolidated financial statements was time-consuming and expensive and could expose us to additional risks that would adversely affect our financial position, results of operations and cash flows and, as a result, the value of our common stock.
As described in our 2014 Form 10-K and our 2016 Form 10-K, within our 2014 Form 10-K we restated our previously issued consolidated financial statements for the first two quarters in fiscal year 2014, for the fiscal years ended December 31, 2013 and 2012 and each of the quarters in fiscal year 2013. We also restated our financial results for the fiscal years ended December 31, 2011 and 2010 (each unaudited), as summarized in “Item 6. Selected Financial Data” to our 2014 Form 10-K. The Restatement was time-consuming and expensive and could continue to expose us to a number of additional risks that would adversely affect our financial position, results of operations and cash flows as well as investor confidence and, as a result, the value of our common stock.
In particular, we incurred, and continue to incur, significant expense, including audit, legal, consulting and other professional fees, in connection with the Restatement and the ongoing remediation of material weaknesses in our internal control over financial reporting. We have taken a number of steps that we have deemed appropriate and reasonable to strengthen our accounting function and to reduce the risk of future restatements, including adding internal personnel and hiring outside consultants, as described in more detail in “Item 9A. Controls and Procedures” contained in this Annual Report on Form 10-K. To the extent these steps are not successful, we may need to incur additional time and expense to address accounting issues that could arise in the future. Our management’s attention has also been, and may further be, diverted from the operation of our business as a result of the time and attention required to address the ongoing remediation of material weaknesses in our internal controls.
We are also subject to claims and proceedings arising out of the misstatements contained in our previously issued financial statements. For additional information regarding this litigation, see “Item 3. Legal Proceedings” in this Annual Report on Form 10-K.
We have identified material weaknesses in our internal control over financial reporting which could, if not remediated, adversely affect our ability to report our financial condition and results of operations in a timely and accurate manner, negatively impacting investor confidence and, as a result, the value of our common stock.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rule 13a-15(f) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and is required to evaluate the effectiveness of these controls and procedures on a periodic basis and publicly disclose the results of these evaluations and related matters in accordance with the requirements of Section 404 of the Sarbanes-Oxley Act of 2002. Management has identified numerous material weaknesses that existed as of December 31, 2015, December 31, 2016 and December 31, 2017, including material weaknesses relating to the ineffectiveness of the control environment. See “Item 9A. Controls and Procedures” in this Annual Report on Form 10-K. As a result of these material weaknesses, our management concluded that our internal controls and procedures were not effective as of December 31, 2015, December 31, 2016 and December 31, 2017.
A “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim consolidated financial statements will not be prevented or detected on a timely basis. We are actively engaged in developing and implementing remedial measures designed to address these material weaknesses. Our remedial measures are not complete and are ongoing. Although we are working to remedy the ineffectiveness of our internal control over financial reporting, there can be no assurance as to when the remedial measures will be fully developed, the timing and effectiveness of our implementation of such remedial measures or the aggregate cost of implementation. Until our remedial measures are fully implemented, our management will continue to devote significant time and attention to these efforts. If we do not complete our remediation in a timely fashion, or at all, or if our remedial measures are inadequate, there will continue to be an increased risk that we will be unable to timely file future periodic reports with the SEC and that our future consolidated financial statements could contain misstatements that will be undetected. If we are unable to report our results in a timely and accurate manner, then we may not be able to comply with the applicable covenants in our Credit Agreement, and may be required to seek amendments or waivers under the Credit Agreement, which could adversely impact our liquidity and financial condition. Further and continued determinations that there are material weaknesses in the effectiveness of our internal control over financial reporting could reduce our ability to obtain financing or could increase the cost of any financing we obtain and require additional expenditures of both money and our management’s time to comply with applicable requirements.
Any failure to implement or maintain required new or improved controls, or any difficulties we encounter in their implementation, could result in additional material weaknesses or material misstatements in our consolidated financial statements. Any new misstatement could result in a further restatement of our consolidated financial statements, cause us to fail to meet timely our periodic reporting obligations with the SEC, cause us to violate debt covenants, reduce our ability to obtain financing or cause investors to lose confidence in our reported financial information, leading to a decline in the value of our common stock. We cannot assure you that we will not discover additional weaknesses in our internal control over financial reporting.
Furthermore, as we grow our business, our disclosure controls and internal controls over financial reporting will become more complex, and we may require significantly more resources to ensure the effectiveness of these controls. If we are unable to continue upgrading our internal controls, reporting systems and IT in a timely and effective fashion, then we may require additional management time and attention and other resources to be devoted to assist in compliance with the disclosure and financial reporting requirements and other rules that apply to public companies, which could adversely affect our business, financial position and results of operations.
The restatement of our previously issued financial results has resulted in private litigation and could result in private litigation judgments that could have a material adverse impact on our results of operations and financial condition.
We are subject to shareholder derivative litigation relating to certain of our previous public disclosures. For additional discussion of this litigation, see “Item 3. Legal Proceedings” in this Annual Report on Form 10-K. Our management has been and may be required in the future to devote significant time and attention to this litigation, and this and any additional matters that arise could have a material adverse impact on our results of operations and financial condition as well as on our reputation. While we cannot estimate our potential exposure in these matters at this time, we have already incurred significant expense defending this litigation and expect to continue to need to incur significant expense in the defense.
The existence of the litigation may have an adverse effect on our reputation with referral sources and our patients themselves, which could have an adverse effect on our results of operations and financial condition.
Our failure to prepare and timely file our periodic reports with the SEC limits our access to the public markets to raise debt or equity capital, impacts our ability to obtain alternative financing and could have negative consequences under the terms of our existing credit agreement.
We have not made timely periodic reporting filings with the SEC since the filing of our Quarterly Report on Form 10-Q for the quarter ended June 30, 2014. We did not file our Annual Reports on Form 10-K for 2014, 2015, 2016 or 2017, or our Quarterly Reports on Form 10-Q for 2015, 2016 or 2017, within the time frame required by the SEC. As a result of our late SEC filings, we are limited in our ability to access the public markets to raise debt or equity capital, which could prevent us from pursuing transactions or implementing business strategies that we believe would be beneficial to our business.
On March 6, 2018, we entered into a new Credit Agreement that provides for (i) a revolving credit facility with an initial maximum aggregate amount of availability of $100 million that matures in March 2023 and (ii) a $505 million term loan facility due in quarterly principal installments commencing June 29, 2018, with all remaining outstanding principal due at maturity in March 2025. Proceeds from the borrowings under the new Credit Agreement were used in part to repay in full all previously existing loans under our prior Credit Agreement and Term B Credit Agreement.
The Credit Agreement contains various restrictions and covenants, including requirements that we maintain certain financial ratios at prescribed levels and requirements that we make timely filings with the SEC.
If we fail to comply with the terms of our Credit Agreement, or if we are unsuccessful at amending or waiving the Credit Agreement if such amendments or waivers become necessary, then we may be subject to numerous penalties, including but not limited to the acceleration of all of our debt outstanding under to the Credit Agreement. In the event that the debt was to be accelerated, then we may need to seek alternative financing to satisfy our financial obligations. This alternative financing may not be available to us on terms that are favorable to us, or at all.
See Note N - “Long-Term Debt” to our consolidated financial statements for additional information regarding the Credit Agreement and our long-term debt.
We have substantial indebtedness, and our failure to comply with the covenants and payment requirements of that indebtedness may subject us to increased interest expenses, lender consent and amendment costs or adverse financial consequences.
As of March 31, 2018, we had approximately $518.5 million in indebtedness. This current level of indebtedness is comprised of approximately $494.8 million of borrowings under the term loan facility under our Credit Agreement, no borrowings under the revolving credit facility of our Credit Agreement, and approximately $23.7 million of indebtedness related to other financing obligations and seller notes. Under our currently existing Credit Agreement, we are required to comply with certain financial covenants and other provisions. In addition to other requirements, these provisions include requirements that we timely prepare our financial statements and timely receive audits on our annual financial statements, meet certain financial ratio requirements and timely pay interest and principal when due.
Due to our material weaknesses and other factors, we did not file our 2014, 2015 or 2016 annual financial statements timely. Additionally, we have previously failed in our compliance with certain of our financial covenants. These failures on our part resulted in defaults under our previously existing debt agreements. To remedy these defaults, we had to provide lenders with consent and amendment fees, experienced increasing constraints on our ability to borrow under our debt agreements, have been required to pay higher interest costs and have been required to adhere to increased restrictions on the use of the funds we borrow. To the extent that we fail to meet our financial statement requirements in future periods, our operating trends do not enable us to meet our financial covenant requirements, we are unable to pay interest or principal when due or we are unable to meet other covenants and requirements contained within our currently existing Credit Agreement, we may default under the Credit Agreement. A default could result in increases in consent or amendment fees to lenders, increases in interest costs, the imposition of additional constraints on borrowing by our lenders or potentially more serious liquidity constraints and adverse financial consequences, including reductions in the value of our common stock or the necessity of seeking protection from creditors under bankruptcy laws. See the “Liquidity and Capital Resources” section in this Management’s Discussion and Analysis for further discussion.
Additionally, our current Credit Agreement includes variable interest rates. In the event that interest rates rise, we will be required to pay greater interest expenses, which will have an adverse effect on our income from operations and financial condition.
To remedy issues we may encounter with meeting our debt obligations, or for other purposes, we may find it necessary to seek further refinancing of our indebtedness, and may do so with debt instruments that are more costly than our existing instruments (and which will rank senior to our equity securities), or we may issue additional equity securities which may dilute the ownership interests or value of our existing shareholders. These actions may decrease the value of our equity securities.
Health care reform has initiated significant changes to the United States health care system and we expect to see further changes in the health care system in the future.
Various health care reform provisions became law upon enactment of the Patient Protection and Affordable Care Act, Pub. L. No. 111-148, on March 23, 2010 (the “Affordable Care Act”). The reforms contained in the Affordable Care Act have impacted our business. Continued political, economic and regulatory influences are subjecting the health care industry in the United States to fundamental change. Further changes relating to the health care industry and in health care spending may adversely affect our revenue. We anticipate that Congress will continue to review and assess alternative health care delivery and payment systems and may in the future propose and adopt legislation effecting additional fundamental changes in the health care system. Although efforts at replacing the Affordable Care Act and overhauling the health care system have currently stalled in Congress, health care reform remains a priority for the Trump Administration and for many members of Congress. We cannot assure you as to the ultimate content, timing or effect of changes, nor is it possible at this time to estimate the impact of potential legislation on our business. However, although the specific reforms to the current health care system cannot be accurately predicted at this time, such changes could have a considerable impact on how health care is reimbursed, particularly on the coverage for certain types of services and on the reimbursement levels provided by government sources.
Changes in government reimbursement levels could adversely affect our Patient Care segment’s net revenue, cash flows and profitability.
We derived approximately 54.8%, 54.1% and 53.4% of our net revenue for the years ended December 31, 2017, 2016 and 2015, respectively, from reimbursements for O&P services and products from programs administered by Medicare, Medicaid and the U.S. Department of Veterans Affairs (“VA”). Each of these programs set reimbursement levels for the O&P services and products provided under their program. If these agencies reduce reimbursement levels for O&P services and products in the future, our net revenues could substantially decline. In addition, the percentage of our net revenues derived from these sources may increase as the portion of the U.S. population over age 65 continues to grow, making us more vulnerable to reimbursement reductions by these organizations. Reduced government reimbursement levels could result in reduced private payor reimbursement levels because fee schedules of certain third party payors are indexed to Medicare reimbursement levels. Furthermore, the health care industry is experiencing a trend towards cost containment as government and other third party payors seek to impose lower reimbursement rates and negotiate reduced contract rates with service providers. This trend could adversely affect our net revenues. For example, a number of states have reduced their Medicaid reimbursement rates for O&P services and products, or have reduced Medicaid eligibility, and others are in the process of reviewing Medicaid reimbursement policies generally, including for prosthetic and orthotic devices.
Medicare provides for reimbursement for O&P products and services based on prices set forth in fee schedules for ten regional service areas. Medicare prices are adjusted each year based on the CPI-U unless Congress acts to change or eliminate the adjustment. The Medicare price changes for 2017, 2016, and 2015 were 0.7%, (0.4)%, and 1.5%, respectively. The Affordable Care Act (“ACA”) changed the Medicare inflation factors applicable to O&P (and other) suppliers. The annual updates for years subsequent to 2011 are based on the percentage increase in the CPI-U for the 12-months ended in
June of the previous year. Section 3401(m) of the ACA required that for 2011 and each subsequent year, the fee schedule update factor based on the CPI-U for the 12-months ended in June of the previous year is to be adjusted by the annual change in economy-wide private nonfarm business multifactor productivity (the “MFP Adjustment”). The MFP Adjustment may result in the percentage increase being less than zero for a year and may result in payment rates for a year being less than such payment rates for the preceding year. Although the decrease in the Medicare O&P fee schedule for 2016 is not unprecedented, it is the first time that there has been a decrease since 2011, when the Productivity Adjustment was first introduced following the ACA. The Centers for Medicare & Medicaid Services (“CMS”) has not yet issued a final rule implementing these adjustments for years beyond 2011, but has indicated in a proposed rule that it will do so as part of the annual program instructions to the O&P fee schedule updates. See 75 Fed. Reg. 40040, 40122-25 (July 13, 2010). If the U.S. Congress were to legislate additional modifications to the Medicare fee schedules, our net revenues from Medicare and other payors could be adversely and materially affected.
Alternative models of reimbursement for durable medical equipment, prosthetics, orthotics and supplies (“DMEPOS”) may also affect our business. The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 requires that Medicare replace the current fee schedule payment methodology for certain DMEPOS items and services with “single payment amounts” determined through a competitive bidding process, and CMS has issued regulations finalizing the methodology for adjusting fee schedule amounts for such items. See 79 Fed, Reg. 66120, 66123 (November 6, 2014). The types of DMEPOS subject to competitive bidding under the statute include: oxygen and oxygen equipment; continuous positive airway pressure devices, single and bi-level; standard manual and power wheelchairs, scooters and walkers; Group 2 complex rehabilitative power wheelchairs; hospital beds, commode chairs, patient lifts and seat lifts; support surfaces or pressure reducing mattresses and overlays; enteral nutrients, supplies and equipment; negative pressure wound therapy pumps; infusion pumps; transcutaneous electrical nerve stimulation devices; standard nebulizers; and certain mail-order diabetic testing supplies. Under the DMEPOS Competitive Bidding Program, suppliers compete to submit bids for selected products, and the Medicare suppliers offering the best price, in addition to meeting applicable quality and financial standards, are awarded contracts to supply the designated products and services to Medicare beneficiaries in specified competitive bidding areas. Although our product offerings currently subject to competitive bidding do not comprise a significant portion of our business, it is possible that the DMEPOS Competitive Bidding Program may expand to include other types of products we offer, or that other payors will adopt similar models for reimbursement, which may negatively affect our net revenue.
The Budget Control Act of 2011 required, among other things, mandatory across-the-board reductions in Federal spending, or “sequestration”. While delayed by the American Taxpayer Relief Act of 2012, President Obama issued a sequestration order on March 1, 2013. For services provided on or after April 1, 2013, Medicare fee-for-service claim payments, including those for DMEPOS as well as claims under the DMEPOS Competitive Bidding Program, are reduced by 2%. On November 2, 2015, President Obama signed the Bipartisan Budget Act of 2015 into law, which provided for two years of increases to discretionary spending to be offset by an additional year of Medicare sequestration, through 2025. This is a claims payment adjustment with limited impact on us; no permanent reductions in the Medicare DMEPOS fee schedule have been made as a result of sequestration, therefore additional reimbursements from Medicaid, the VA and commercial payors who use the Medicare fee schedule as a basis for reimbursement have not been impacted.
CMS may also develop policies to limit Medicare coverage of specific products and services. Medical administrative contractors may issue local coverage determinations (“LCD”) that limit coverage for a particular item or service in their jurisdiction only. This can lead to state-by-state variation in Medicare coverage for some items and services. Any LCD that negatively impacts orthotic or prosthetic reimbursement would negatively affect our revenue.
Finally, patients may continue to move to Medicare Advantage plans from traditional Medicare plans, which will change the nature of the reimbursement received by us from the traditional Medicare program and negatively affect our net revenue.
If the average rates that commercial payors pay us decline significantly, then it would have a material adverse effect on our Patient Care segment’s net revenues, earnings and cash flows.
We derived approximately 38.2%, 39.2% and 39.6% of our net revenue for the years ended December 31, 2017, 2016 and 2015, respectively, from reimbursements for O&P services and products for patients who have commercial payors as their primary payor. We continue to experience downward pressure on some of our commercial payment rates as a result of general conditions in the market, recent and future consolidations among commercial payors, increased focus on O&P services and products and other factors. There is no guarantee that commercial payment rates will not be materially lower in the future, particularly given the fluctuations in government reimbursement rates.
We are continuously in the process of negotiating new agreements and renegotiating agreements that are up for renewal with commercial payors, who tend to be aggressive in their negotiations with us. Sometimes many significant agreements are up for renewal or being renegotiated at the same time. In the event that our continual negotiations result in overall commercial rate reductions in excess of overall commercial rate increases, the cumulative effect could have a material adverse effect on our financial results. Consolidations in the commercial payor market have significantly increased the negotiating leverage of commercial payors. Our negotiations with payors are also influenced by competitive pressures, and we may experience decreased contracted rates with commercial payors or experience decreases in patient volume as our negotiations with commercial payors continue. If the average rates that commercial payors pay us decline significantly, or if we see a decline in commercial patients, it would have a material adverse effect on our revenues, earnings and cash flows.
Changes in government reimbursement levels could adversely affect our Products & Services segment’s net revenues, cash flows and profitability.
In addition to the risks to our Patient Care segment businesses discussed previously, changes in government reimbursement levels could also adversely affect the net revenues, cash flows and profitability of the businesses in our Products & Services segment. In particular, a significant majority of our therapeutic services sales involve devices and related services provided to SNFs and similar businesses. Reductions in government reimbursement levels to SNFs have caused, and could continue to cause, such SNFs to reduce or cancel their use of our therapeutic service equipment and related consultative services, negatively impacting net revenues, cash flows and profitability. For example, in July 2011 CMS announced an across the board reduction of approximately 11% in SNF reimbursement levels, which negatively impacted the demand for our devices and treatment modalities. Although CMS has announced increases in SNF reimbursement levels in the years since (the agency announced an increase of 1.0% for FY 2018, 2.4% for FY 2017, 1.4% for FY 2016, 2.0% for FY 2015, and 1.3% for FY 2014), we cannot predict whether any other changes to reimbursement levels will be implemented, or if implemented what form any changes might take. In May 2018 CMS announced a proposed replacement called Patient-Driven Payment Model (“PDRM”) for the current Resource Utilization Group IV (“RUG IV”) SNF payment system under Medicare Part A. PDRM is a further update to the Resident Classification System Version 1 (RCS-1) proposed by CMS in May 2017. CMS has proposed an effective date of October 1, 2019 for PDPM. PDPM as proposed is subject to change and revision before it becomes effective, and its effective date could be postponed. Additionally, its potential impact on SNF reimbursement levels is not clear.
We depend on reimbursements by third party payors, as well as payments by individuals, which could lead to delays and uncertainties in the Patient Care segment’s reimbursement process.
We receive a substantial portion of our payments for health care services on a fee for service basis from third party payors, including Medicare and Medicaid, private insurers and managed care organizations. We estimate that we have received approximately 93.0%, 93.3% and 93.0% of our net revenues from such third party payors during 2017, 2016 and 2015, respectively. We estimate that such amounts included approximately 30.5%, 30.5% and 30.3% from Medicare in 2017, 2016 and 2015, respectively, 15.6%, and 14.8% and 14.2% from Medicaid programs in 2017, 2016 and 2015, respectively. In addition, we estimate net revenues from the VA were 8.7%, 8.8% and 8.9% in 2017, 2016 and 2015, respectively.
The reimbursement process is complex and can involve lengthy delays. Third party payors continue their efforts to control expenditures for health care, including proposals to revise reimbursement policies. While we recognize revenue when health care services are provided, there can be delays before we receive payment. In addition, third party payors may disallow, in
whole or in part, requests for reimbursement based on determinations that certain amounts are not reimbursable under plan coverage, that services provided were not medically necessary or that additional supporting documentation is necessary. Retroactive adjustments may change amounts realized from third party payors. Third party payors may require pre-authorizations for certain services and/or devices, which may result in a delay in our ability to provide services or to provide services at all. Additionally, we may see an increase in bundled payment models, which can result in delays before we receive payment or no payment at all for certain services.
Changes in government reimbursement levels and policies such as those described above may also contribute to uncertainties surrounding the reimbursement process. We are subject to governmental audits of our reimbursement claims under Medicare, Medicaid, the VA and other governmental programs and may be required to repay these agencies if found that we were incorrectly reimbursed. Delays and uncertainties in the reimbursement process may adversely affect accounts receivable, increase the overall costs of collection and cause us to incur additional borrowing costs.
We also may not be paid with respect to co-payments and deductibles that are the patient’s financial responsibility. Many of the plans offered on the state health insurance exchanges have high deductibles and require coinsurance that patients cannot afford to pay. Amounts not covered by third party payors are the obligations of individual patients from whom we may not receive whole or partial payment. We also may not receive whole or partial payments from uninsured and underinsured individuals. In such an event, our earnings and cash flow would be adversely affected, potentially affecting our ability to maintain our restrictive debt covenant ratios and meet our financial obligations.
Additionally, employer based plans and other individual plans are increasingly relying on “high deductible” plan designs. As their participation in health plans with these high deductible designs increases, our patients will face greater financial burdens and participatory costs that may affect their decisions regarding the timing of their replacement of their devices. Due to cost considerations, they may seek to repair or refurbish their existing devices and delay the purchase of new replacement devices, which will adversely affect our revenues and our profitability.
The risks associated with third party payors, co-payments and deductibles and the inability to monitor and manage accounts receivable successfully could still have a material adverse effect on our business, financial condition and results of operations. Furthermore, our collection policies or our provisions for allowances for Medicare, Medicaid and contractual discounts and doubtful accounts receivable may not be adequate.
Due to constraints in the growth of our rates of reimbursement, we may face cost pressures that adversely affect our profitability.
Due to increased pressures on governmental and commercial payors to seek ways of reducing the costs of care, those payors have and may continue to seek ways to reduce growth in the rate of our reimbursement for the services we provide. This constraint in the rate of growth in reimbursement may adversely affect our profitability as we experience increases in the wages, materials and other costs necessary to the conduct of our business. These cost increases may adversely affect our profitability and our profit margins.
Given the complexities and demands related to reimbursement, we may fail to adequately provide the staffing and systems necessary to ensure we effectively manage our reimbursement processes.
The nature of our business requires that we are effective in the assessment of patient eligibility, the process of pre-authorization, the recordation and collection of provider documentation, the timely and complete submission of claims for reimbursement, the application of cash receipts to patient accounts, the timely response to payor denials and the conduct of collection activities. If we fail to provide adequate or qualified staffing, we could incur reductions in the amount of reimbursement we receive for the O&P services that we provide.
We face periodic reviews, audits and investigations under our contracts with federal and state government agencies, and these audits could have adverse findings that may negatively impact our business.
We contract with various federal and state governmental agencies to provide O&P services. Pursuant to these contracts, we are subject to various governmental reviews, audits and investigations to verify our compliance with the contracts and applicable laws and regulations. Any adverse review, audit or investigation could result in:
· refunding of amounts we have been paid pursuant to our government contracts;
· imposition of fines, penalties and other sanctions on us;
· loss of our right to participate in various federal programs;
· damage to our reputation in various markets; or
· material and/or adverse effects on our business, financial condition and results of operations.
In recent years we have seen a significant increase in Medicare audits, including RAC audits, CERT audits and MAC prepayment audits which have been replaced with the recently implemented TPE prepayment audits. In addition, ZPICs are responsible for the identification of suspected fraud through medical record review. We believe that Medicare audits, inquiries and investigations will continue to occur from time to time in the ordinary course of our business. Medicare audits could have a material and adverse effect on our business financial condition and results of operations, particularly if we are unsuccessful at final adjudication.
Consolidation of manufacturers within the O&P industry may adversely affect our business by increasing prices we pay for certain devices and components.
We depend on a limited number of manufacturers who supply us with certain key devices and components used in the prostheses we provide to our patients, particularly with respect to high technology components. These manufacturers are subject to a consolidation trend within the O&P industry. To the extent this trend continues, consolidation amongst certain manufacturers could result in a sole or limited source for certain high technology devices and components used in the devices we provide to patients. Any such consolidation could require us to pay increased prices for such devices and components, which could significantly reduce our gross margin and profitability and have a material adverse effect on our business.
We are subject to numerous federal, state and local governmental regulations, noncompliance with which could result in significant penalties that could have a material adverse effect on our business.
A failure by us to comply with the numerous federal, state and/or local health care and other governmental regulations to which we are subject, including the regulations discussed under “Government Regulation” in “Item 1. Business” above, could result in significant penalties and adverse consequences, including exclusion from the Medicare and Medicaid programs, which could have a material adverse effect on our business.
We could be subject to adverse changes in tax laws, regulations and interpretations or challenges to our tax positions.
We are subject to tax laws and regulations of the U.S. federal, state and local governments. We compute our income tax provision based on enacted tax rates in the jurisdictions in which we operate. As the tax rates vary among jurisdictions, a change in earnings attributable to the various jurisdictions in which we operate could result in an unfavorable change in our overall tax provision.
From time to time, changes in tax laws or regulations may be proposed or enacted that could adversely affect our overall tax liability. For example, the Tax Cuts and Jobs Act (the “Tax Act”) signed into law on December 22, 2017 represents a significant overhaul of the U.S. federal tax code. The Tax Act significantly reduced the U.S. statutory corporate tax rate and made other changes that we expect will reduce our effective U.S. federal tax rate in future periods. However, the Tax Act also included a number of provisions, including, but not limited to, the limitation or elimination of various deductions or credits (including for interest expense and for performance-based compensation under Section 162(m)), the changing of the timing of the recognition of certain income and deductions or their character, and the limitation of asset basis under certain circumstances, any of which could significantly and adversely affect our U.S. federal income tax position. The Tax Act also made significant changes to the tax rules applicable to insurance companies and other entities with which we do business. The estimated impact of the new law is based on management’s current knowledge and assumptions. We are continuing to evaluate the overall impact of this tax legislation on our operations and U.S. federal and state income tax position. The actual impact of the Tax Act could be materially different from our current estimates based on our actual results and our further analysis of the new law. There can be no assurance that future changes in tax laws or regulations will not materially and adversely affect our effective tax rate, tax payments, financial condition and results of operations. Similarly, changes in tax laws and regulations that impact our patients, business partners and counterparties or the economy generally may also impact our financial condition and results of operations.
In addition, tax laws and regulations are complex and subject to varying interpretations, and any significant failure to comply with applicable tax laws and regulations in all relevant jurisdictions could give rise to substantial penalties and liabilities. We are regularly subject to audits by tax authorities and, although we believe our tax estimates are appropriate, the final determination of tax audits and any related litigation could be materially different from our historical income tax provisions and accruals. Any changes in enacted tax laws (such as the recent U.S. tax legislation), rules or regulatory or judicial interpretations; any adverse outcome in connection with tax audits in any jurisdiction; or any change in the pronouncements relating to accounting for income taxes could materially and adversely impact our effective tax rate, tax payments, financial condition and results of operations.
Within our Products & Services segment, we provide certain equipment and consultative services to SNFs, who, due to reimbursement pressures, may choose to discontinue our services or seek alternative arrangements for the provision of this equipment.
Approximately $55.2 million of our net revenue in 2017 related to recurring revenues derived from providing therapeutic equipment and related consultative services to SNFs. SNFs have been experiencing reimbursement pressures which could adversely impact our business with them. To reduce costs, these facilities could choose to forgo our services, or seek alternative arrangements for the provision of the equipment we provide them, thereby reducing our revenue, earnings and could adversely impact the carrying value of our goodwill and other intangible assets.
Completing the implementation of NextGen, our comprehensive clinic management system, could interfere with our patient care clinic operations and adversely affect our business, financial condition and results of operations.
We depend on our IT infrastructure to achieve our business objectives. Beginning in 2014, we commenced the roll-out of a new patient management and electronic health record system in our patient care clinics. In the third quarter of 2014, we halted the roll-out due to the negative impact the roll-out had on revenue cycle management. We have revised, updated and reduced the amount of customizations made to the system, which we now refer to as NextGen. We have also substantially improved our revenue cycle management function, and are currently in the process of completing the roll-out of NextGen, and anticipate the roll-out to continue through the first half of 2019. Any disruptions, delays or complications in the implementation process, or any deficiencies in the design, operation or expected performance of the NextGen system, could result in higher than expected implementation costs, the diversion of management’s and other employees’ attention from the day-to-day operations of our patient care clinics, including scheduling patient visits, and other disruptions to our patient care business. Any of these consequences could have an adverse impact on our revenue or costs, billing and related accounts
receivable collections, all of which impacts cash flow and could materially and adversely affect our business, financial condition and results of operations.
To address our business needs and weaknesses in financial controls, we will likely be required to upgrade certain of our operational and financial systems in future years. If we fail in the selection or implementation of such systems, or fail to maintain our existing systems, our business and financial results could be adversely affected.
We are highly dependent on our ability to procure materials and componentry, manage our inventories, support our patient encounters and to otherwise support the administrative requirements associated with our human resource, financial and other needs. We may not have sufficient financial capacity to implement such systems, or may fail in our selection and implementation of such systems. The failure to implement systems in a timely manner may adversely affect our ability to establish an effective control environment. If we are delayed in the implementation of systems, and existing systems are not adequately maintained, we could experience adverse interruptions in our ability to operate, or experience excessive costs associated with the remediation of consequential systems issues. Additionally, our failure to correctly select and implement systems could cause operational disruptions, delays, duplicative operating costs or other financial burdens which could adversely affect our business and financial condition.
Our products and services face the risk of technological obsolescence, which, if realized, could have a material adverse effect on our business.
The medical device industry is characterized by rapid and significant technological change. There can be no assurance that third parties will not succeed in developing and marketing technologies, products or services that are more effective than ours or that would render our products and services obsolete or noncompetitive. Additionally, new surgical procedures and medications could be developed for diabetes, trauma associated with accidents or physical injury, tumors, infection or musculoskeletal disorders of the back, extremities or joints that would replace or reduce the importance of our prosthetic and orthotic products and services. Accordingly, our success will depend upon our ability to respond to future medical and technological changes that may impact the demand for our prosthetic and orthotic products and services.
Our failure to economically procure necessary components and to conduct timely and effective inventories of the materials and components we use in our business could result in an adverse effect on our business, financial condition and results of operations.
Our business involves the use of materials and componentry we acquire from third party manufacturers. If manufacturers critical to our business substantially increase the cost of the components they sell to us, then our inability to acquire the necessary materials and components on a cost effective basis may adversely affect revenues and earnings. Additionally, to successfully perform our business, it is necessary that we conduct timely and thorough inventories of our raw materials and Work in Process (“WIP”). The conduct of these inventories are costly and time consuming. If we encounter issues in their conduct, given that our clinicians oversee the inventory processes which occur in our clinics, remedial procedures can disrupt our ability to see and treat patients, and thereby adversely affect our revenues and profitability.
Our common stock was delisted from the NYSE and moved to the OTC, affecting the trading of our common stock and reputation.
Our common stock was delisted from the NYSE in February 2016 as a result of our failure to file our Annual Report on Form 10-K for the year ended December 31, 2014 within the extended compliance period required by the NYSE. After the delisting, our common stock began trading on the OTC. There can be no assurance whether or when our common stock will be relisted for trading on the NYSE or another national securities exchange. Our shareholders may continue to face material adverse consequences as a result of our trading on the OTC rather than a national securities exchange, including, but not limited to, a decrease in the price of our common stock, increased volatility, decreased trading activity or liquidity, a lack of
analyst research and a further decline in institutional holders whose charters do not allow them to hold securities in unlisted companies. In addition, the delisting from the NYSE may have had and may continue to have a negative impact on our reputation and, as a consequence, our business and the price of our common stock.
If we are unable to retain our senior management and key employees, then our business and results of operations and financial position could be harmed.
Our ability to maintain our competitive position is largely dependent on the services of our senior management, clinicians and other key employees. Although we have employment agreements with our senior management, these agreements do not prevent those individuals from ceasing their employment with us at any time. Additionally, adverse publicity and increased demands associated with our noncurrent filing status, material weaknesses and the Restatement associated litigation and regulatory investigations could increase our key employee retention risks. If we are unable to retain existing senior management, clinicians and other key employees, or to attract other such qualified employees on terms satisfactory to us, then our business could be adversely affected.
Our non-compete agreements and other restrictive covenants involving clinicians may not be enforceable.
We have contracts with clinicians in many states. Some of these contracts include provisions preventing these clinicians from competing with us both during and after the term of our relationship with them. The law governing non-compete agreements and other forms of restrictive covenants varies from state to state. Some states are reluctant to strictly enforce non-compete agreements and restrictive covenants applicable to health care providers. There can be no assurance that our non-compete agreements related to affiliated clinicians will not be successfully challenged as unenforceable in certain states. In such event, we would be unable to prevent former affiliated clinicians from competing with us, potentially resulting in the loss of some of our patients, reducing our revenues and earnings.
Cyber-attacks, system security risks, data breaches and other technology failures could adversely affect our ability to conduct business, our results of operations and our financial position.
A technology failure could occur and potentially disrupt our business, damage our reputation and adversely affect our profitability. Our information technology (“IT”) systems are subject to the risk of computer viruses or other malicious codes, unauthorized access or cyber-attacks. The administrative and technical controls and other preventive actions that we take to reduce the risk of cyber incidents and protect our IT systems may be insufficient to prevent physical and electronic break-ins, cyber-attacks or other security breaches to our computer systems. In addition, disruptions or breaches could occur as a result of natural disasters, man-made disasters, epidemic/pandemic, industrial accident, blackout, criminal activity, technological changes or events, terrorism or other unanticipated events beyond our control. While we have insurance intended to provide coverage from certain losses related to such incidents and a variety of preventative security measures such as risk management, information protection, disaster recovery and business continuity plans, we cannot predict the method or outcome of every possible cyber incident or ensure that we have protected ourselves against every possible cyber threat in light of the varied and increasingly complex breaches faced by companies on a regular basis. Unanticipated problems with our systems or recovery plans could have a material adverse impact on our ability to conduct business, our results of operations and our financial position.
A cybersecurity incident could cause a violation of HIPAA and other privacy laws and regulations or result in a loss of confidential data.
A cyber-attack that bypasses our IT security systems causing an IT security breach, loss of protected health information or other data subject to privacy laws, loss of proprietary business information, or a material disruption of our IT business systems, could have a material adverse impact on our business, financial condition or results of operations. In addition, our
future results of operations, as well as our reputation, could be adversely impacted by theft, destruction, loss, or misappropriation of protected health information, other confidential data or proprietary business information.
Insurance coverage for some of our losses may be inadequate and may be subject to the credit risk of commercial insurance companies.
Some of our insurance coverage is through various third-party insurers. To the extent we hold policies to cover certain groups of claims or rely on insurance coverage obtained by third parties to cover such claims, but either we or such third parties did not obtain sufficient insurance limits, did not buy an extended reporting period policy, where applicable, or the issuing insurance company is unable or unwilling to pay such claims, we may be responsible for those losses. Furthermore, for our losses that are insured or reinsured through commercial insurance companies, we are subject to the “credit risk” of those insurance companies. While we believe our commercial insurance company providers currently are creditworthy, there can be no assurance that such insurance companies will remain so in the future.
We have made and may continue to make acquisitions, which could divert the attention of management and which may not be integrated successfully into our existing business. We may not find suitable acquisitions in the future, which could adversely affect our ability to penetrate new markets and achieve our growth objectives.
In past years we have pursued, and we intend to continue to pursue, acquisitions to enter new geographic markets and expand the scope of services we provide. We cannot assure you that we will identify suitable acquisition candidates, acquisitions will be completed on acceptable terms or at all, our due diligence process will uncover all potential liabilities or issues affecting our integration process, we will not incur breakup, termination or similar fees and expenses, or we will be able to integrate successfully the operations of any acquired business. Furthermore, acquisitions in new geographic markets and services may require us to comply with new and unfamiliar legal and regulatory requirements, which could impose substantial obligations on us and our management, cause us to expend additional time and resources and increase our exposure to penalties or fines for noncompliance with such requirements. The acquisitions could be of significant size and involve operations in multiple jurisdictions. The acquisition and integration of another business could divert management attention from other business activities. This diversion, together with other difficulties we may incur in integrating an acquired business, could have a material adverse effect on our business, financial condition and results of operations. In addition, we may incur debt to finance acquisitions. Such borrowings may not be available on terms as favorable to us as our current borrowing terms and may increase our leverage.
In order to remain competitive, we are required to make capital expenditures relating to our leaseholds and our equipment.
A substantial portion of our capital expenditure requirements relate to maintaining and upgrading the appearance and function of our patient care clinic and satellite locations. If we do not maintain our facilities, their relative appearance to that of our competitors could adversely affect our ability to attract and retain patients. In addition, changing competitive conditions or the emergence of any significant advances in O&P technology or the delivery of O&P technology could require us to invest significant capital in additional equipment or capacity in order to remain competitive. If we are unable to fund any such investment or otherwise fail to invest in such items, our business, financial condition or results of operations could be materially and adversely affected.
We may not be able to adequately protect our intellectual property and other proprietary rights that are material to our business or to defend successfully against intellectual property infringement claims by third parties.
Our ability to compete effectively depends in part upon our intellectual property rights, including but not limited to our trademarks and copyrights, and our proprietary technology. Our use of contractual provisions, confidentiality procedures and agreements, and trademark, copyright, unfair competition, trade secret and other laws to protect our intellectual property
rights and proprietary technology may not be adequate. Litigation may be necessary to enforce our intellectual property rights and protect our proprietary technology, or to defend against claims by third parties that the conduct of our businesses or our use of intellectual property infringes upon such third-party’s intellectual property rights. Any intellectual property litigation or claims brought against us, whether or not meritorious, could result in substantial costs and diversion of our resources, and there can be no assurances that favorable final outcomes will be obtained in all cases. The terms of any settlement or judgment may require us to pay substantial amounts to the other party or cease exercising our rights in such intellectual property, including ceasing the use of certain trademarks used by us to distinguish our services from those of others or ceasing the exercise of our rights in copyrightable works. In addition, we may have to seek a license to continue practices found to be in violation of a third-party’s rights, which may not be available on reasonable terms, or at all. Our business, financial condition or results of operations could be adversely affected as a result.
The market price of our common stock may fluctuate significantly.
The market price of our common stock may fluctuate significantly. Among the factors that could affect our stock price are:
· industry or general market conditions;
· domestic and international economic factors unrelated to our performance;
· changes in our referral sources’ or customers’ preferences;
· new regulatory pronouncements and changes in regulatory guidelines;
· lawsuits, enforcement actions and other claims by third parties or governmental authorities;
· actual or anticipated fluctuations in our quarterly operating results;
· changes in securities analysts’ estimates of our financial performance or lack of research and reports by industry analysts;
· action by institutional shareholders or other large shareholders, including future sales of our common stock;
· speculation in the press or investment community;
· investor perception of us and our industry;
· changes in market valuations or earnings of similar companies;
· announcements by us or our competitors of significant contracts, acquisitions or strategic partnerships;
· any future sales of our common stock or other securities;
· additions or departures of key personnel; and
· ability to get current and file future SEC filings timely.
The stock markets have experienced extreme volatility in recent years that has been unrelated to the operating performance of particular companies. These broad market fluctuations may adversely affect the market price of our common stock. In the past, following periods of volatility in the market price of a company’s securities, class action litigation has often been
instituted against such company. Any litigation of this type brought against us could result in substantial costs and a diversion of management’s attention and resources, which would harm our business, results of operations and financial condition.
If securities or industry analysts do not publish research or publish misleading or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business, especially after our stock is no longer traded on the OTC and is once again traded on a national securities exchange. If one or more analysts downgrade our stock or publishes misleading or unfavorable research about our business, our stock price would likely decline. If one or more of these analysts ceases coverage of us or fails to publish reports on us regularly, demand for our stock could decrease, which could cause our stock price or trading volume to decline.
We do not intend to pay dividends on our common stock and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common stock.
We do not intend to declare and pay dividends on our common stock for the foreseeable future. We currently intend to invest our future earnings, if any, to fund our growth, to develop our business, and to potentially fund future share repurchases. Therefore, you are not likely to receive any dividends on your common stock for the foreseeable future, and the success of an investment in shares of our common stock will depend upon any future appreciation in their value. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which shareholders have purchased their shares.
Disruptions in our disaster recovery systems, management continuity planning or information systems could limit our ability to operate our business effectively, or adversely affect our financial condition and results of operations.
Our IT systems facilitate our ability to conduct our business. While we have disaster recovery systems and business continuity plans in place, any disruptions in our disaster recovery systems or the failure of these systems to operate as expected could, depending on the magnitude of the problem, adversely affect our operating results by limiting our capacity to effectively monitor and control our operations. Despite our implementation of a variety of security measures, our technology systems could be subject to physical or electronic break-ins and similar disruptions from unauthorized tampering. In addition, in the event that a significant number of our management personnel were unavailable in the event of a disaster, our ability to effectively conduct business could be adversely affected.
As of December 31, 2017, we operated or leased 794 patient care locations, comprised of 682 patient care clinics and 112 satellite locations, in 44 states and the District of Columbia. We own eleven buildings, including ten buildings that house a patient care clinic and one building that is currently unoccupied. Our patient care clinics occupied under leases have terms expiring between 2018 and 2027. Our patient care clinics average approximately 3,100 square feet in size. In total, including locations relating to our non-patient care businesses, administrative and fabrication locations, as well as storage and other non-occupied space, we currently have 908 locations, of which, 897 are under lease.
We believe our leased and owned facilities are adequate for carrying out our current and anticipated future O&P operations. We believe we will be able to renew such leases as they expire or find comparable or alternative space on commercially suitable terms. See Note M - “Leases” to our consolidated financial statements in this Annual Report on Form 10-K for additional information regarding our facilities leases.
The following table sets forth the number of our patient care clinics in each state as of December 31, 2017:
|
State |
|
Patient |
|
State |
|
Patient |
|
State |
|
Patient |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Alabama |
|
12 |
|
Maine |
|
9 |
|
North Dakota |
|
5 |
|
|
Arizona |
|
37 |
|
Maryland |
|
13 |
|
Ohio |
|
40 |
|
|
Arkansas |
|
6 |
|
Massachusetts |
|
6 |
|
Oklahoma |
|
10 |
|
|
California |
|
71 |
|
Michigan |
|
14 |
|
Oregon |
|
10 |
|
|
Colorado |
|
27 |
|
Minnesota |
|
21 |
|
Pennsylvania |
|
41 |
|
|
Connecticut |
|
14 |
|
Mississippi |
|
12 |
|
South Carolina |
|
15 |
|
|
District of Columbia |
|
2 |
|
Missouri |
|
27 |
|
South Dakota |
|
3 |
|
|
Florida |
|
47 |
|
Montana |
|
3 |
|
Tennessee |
|
20 |
|
|
Georgia |
|
40 |
|
Nebraska |
|
13 |
|
Texas |
|
45 |
|
|
Illinois |
|
21 |
|
Nevada |
|
5 |
|
Utah |
|
7 |
|
|
Indiana |
|
11 |
|
New Hampshire |
|
1 |
|
Virginia |
|
14 |
|
|
Iowa |
|
17 |
|
New Jersey |
|
7 |
|
Washington |
|
20 |
|
|
Kansas |
|
18 |
|
New Mexico |
|
13 |
|
West Virginia |
|
6 |
|
|
Kentucky |
|
8 |
|
New York |
|
30 |
|
Wisconsin |
|
13 |
|
|
Louisiana |
|
14 |
|
North Carolina |
|
21 |
|
Wyoming |
|
5 |
|
Other leased real estate holdings include our distribution facilities in Texas, Nevada, Georgia, Illinois and Pennsylvania, our corporate headquarters in Austin, Texas; the headquarters for our therapeutic solutions in Reno, Nevada, which is located within our Nevada distribution facility, and the headquarters for our distribution business in Alpharetta, Georgia, which is located within our Georgia distribution facility. We additionally operate eleven separate leased fabrication facilities that assist our patient care locations in the fabrication of devices. The fabrication facilities are located in the states of Alabama, Arizona, California, Colorado, Connecticut, Florida, Kansas, Tennessee and Texas. Substantially all of our owned properties are pledged to collateralize bank indebtedness. See Note N - “Long-Term Debt” to our consolidated financial statements in this Annual Report on Form 10-K for additional information regarding our outstanding debt and related collateral.
Securities and Derivative Litigation
In November 2014, a securities class action complaint, City of Pontiac General Employees’ Retirement System v. Hanger, et al., C.A. No. 1:14-cv-01026-SS, was filed against us in the United States District Court for the Western District of Texas. The complaint named us and certain of our current and former officers for allegedly making materially false and misleading statements regarding, inter alia, our financial statements, RAC audit success rate, the implementation of new financial systems, same-store sales growth, and the adequacy of our internal processes and controls. The complaint alleged violations of Sections 10(b) and 20(a) of the Exchange Act and Rule 10b-5 promulgated thereunder. The complaint sought unspecified damages, costs, attorneys’ fees, and equitable relief.
On April 1, 2016, the court granted our motion to dismiss the lawsuit for failure to state a claim upon which relief can be granted, and permitted plaintiffs to file an amended complaint. On July 1, 2016, plaintiffs filed an amended complaint. On September 15, 2016, we and certain of the individual defendants filed motions to dismiss the lawsuit. On January 26, 2017, the court granted the defendants’ motions and dismissed with prejudice all claims against all defendants for failure to state a claim. On February 24, 2017, plaintiffs filed a notice of appeal to the United States Court of Appeals for the Fifth Circuit. Appellate briefing was completed on August 18, 2017 and the appeal remains pending. The Court of Appeals held oral argument for the appeal on March 5, 2018. We are now awaiting a ruling from the Court of Appeals.
In February and August of 2015, two separate shareholder derivative suits were filed in Texas state court against us related to the announced restatement of certain of our financial statements. The cases were subsequently consolidated into Judy v. Asar, et. al., Cause No. D-1-GN-15-000625. On October 25, 2016, plaintiffs in that action filed an amended complaint, and the case is currently pending before the 345th Judicial District Court of Travis County, Texas.
The amended complaint in the consolidated derivative action names us and certain of our current and former officers and directors as defendants. It alleges claims for breach of fiduciary duty based, inter alia, on the defendants’ alleged failure to exercise good faith to ensure that we had in place adequate accounting and financial controls and that disclosures regarding our business, financial performance and internal controls were truthful and accurate. The complaint seeks unspecified damages, costs, attorneys’ fees, and equitable relief.
As disclosed in our Current Report on Form 8-K filed with the SEC on June 6, 2016, the Board of Directors appointed a Special Litigation Committee of the Board (the “Special Committee”). The Board delegated to the Special Committee the authority to (1) determine whether it is in our best interests to pursue any of the allegations made in the derivative cases filed in Texas state court (which cases were consolidated into the Judy case discussed above), (2) determine whether it is in our best interests to pursue any remedies against any of our current or former employees, officers or directors as a result of the conduct discovered in the Audit Committee investigation concluded on June 6, 2016 (the “Investigation”), and (3) otherwise resolve claims or matters relating to the findings of the Investigation. The Special Committee retained independent legal counsel to assist and advise it in carrying out its duties and reviewed and considered the evidence and various factors relating to our best interests. In accordance with its findings and conclusions, the Special Committee determined that it is not in our best interest to pursue any of the claims in the Judy derivative case. Also in accordance with its findings and conclusions, the Special Committee determined that it is not in our best interests to pursue legal remedies against any of our current or former employees, officers, or directors.
On April 14, 2017, we filed a motion to dismiss the consolidated derivative action based on the resolution by the Special Committee that it is not in our best interest to pursue the derivative claims. Counsel for the derivative plaintiffs opposed that motion and moved to compel discovery. In a hearing held on June 12, 2017, the Travis County court denied plaintiffs’ motion to compel, and held that the motion to dismiss would be considered only after appropriate discovery was concluded.
The plaintiffs have since subpoenaed counsel for the Special Committee, seeking a copy of the full report prepared by the Special Committee and its independent counsel. Counsel for the Special Committee, as well as our counsel, take the position that the full report is not discoverable under Texas law. Plaintiffs’ counsel has filed a motion to compel the Special Committee’s counsel to produce the report. We intend to vigorously oppose the motion to compel. Upon resolution of the discovery dispute and completion of discovery, we intend to file a motion to dismiss the consolidated derivative action.
Management intends to continue to vigorously defend against the shareholder derivative action and the appeal in the securities class action. At this time, we cannot predict how the Courts will rule on the merits of the claims and/or the scope of the potential loss in the event of an adverse outcome. Should we ultimately be found liable, the resulting damages could have a material adverse effect on our consolidated financial position, liquidity or results of our operations.
Other Matters
In May 2015 one of our clinics received a civil investigative demand for records relating to a sample of claims submitted to Medicare and Medicaid for reimbursement, and we provided records in response to the subpoena. In May 2017, we were informed by an Assistant United States Attorney that it was investigating whether we properly provided and claimed reimbursement for prosthesis skins and covers from July 2013 (after an industry announcement) to the present. We have reviewed the claims, and have cooperated with the government’s investigation. This matter was resolved in March 2018 and did not have a material impact on the first quarter of 2018 or on any financial period in 2017.
From time to time we are subject to legal proceedings and claims which arise in the ordinary course of our business, including additional payments under business purchase agreements. In the opinion of management, the amount of ultimate liability, if any, with respect to these actions will not have a materially adverse effect on our consolidated financial position, liquidity or results of our operations.
We are in a highly regulated industry and receive regulatory agency inquiries from time to time in the ordinary course of our business, including inquiries relating to our billing activities. No assurance can be given that any discrepancies identified during a regulatory review will not have a material adverse effect on our consolidated financial statements.
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
The following information in this Item 5 of this Annual Report on Form 10-K is not deemed to be “soliciting material” or to be “filed” with the SEC or subject to Regulation 14A or 14C under the Exchange Act or to the liabilities of Section 18 of the Exchange Act, and will not be deemed to be incorporated by reference into any filing under the Securities Act of 1933 or the Exchange Act, except to the extent we specifically incorporate it by reference into such a filing.
Market Information
Our common stock was listed and traded on the NYSE from December 15, 1998 to February 26, 2016 under the symbol “HGR.” On February 29, 2016, our common stock began trading on the OTC under the symbol “HNGR” after the NYSE notified us on February 26, 2016 of immediate suspension of trading of and the initiation of delisting procedures against our common stock for failing to file our 2014 Form 10-K within the extended compliance period granted by the NYSE. The following table sets forth the high and low closing sale prices for our common stock for the periods indicated as reported on the NYSE (through February 26, 2016) and the OTC (beginning on February 29, 2016):
|
Year ended December 31, 2017 |
|
High |
|
Low |
| ||
|
First Quarter |
|
$ |
15.15 |
|
$ |
11.45 |
|
|
Second Quarter |
|
13.45 |
|
11.30 |
| ||
|
Third Quarter |
|
12.00 |
|
10.87 |
| ||
|
Fourth Quarter |
|
18.00 |
|
10.99 |
| ||
|
|
|
|
|
|
| ||
|
Year ended December 31, 2016 |
|
High |
|
Low |
| ||
|
First Quarter |
|
$ |
15.67 |
|
$ |
2.49 |
|
|
Second Quarter |
|
7.97 |
|
6.25 |
| ||
|
Third Quarter |
|
11.00 |
|
7.43 |
| ||
|
Fourth Quarter |
|
11.50 |
|
7.75 |
| ||
Holders
At May 1, 2018, there were approximately 170 holders of record of our 36,692,863 shares of outstanding common stock.
Dividend Policy
We have never paid cash dividends on our common stock and our Board of Directors intends to continue this policy for the foreseeable future. We plan to retain earnings for use in our business. The terms of our credit agreements and certain other agreements limit the payment of dividends on our common stock and such agreements are expected to continue to limit the payment of dividends in the future.
Any future determination to pay cash dividends will be at the discretion of our Board of Directors and will be dependent on our results of operations, financial condition, contractual and legal restrictions and any other factors deemed to be relevant.
Sales of Unregistered Securities
During the year ended December 31, 2017, we did not sell any securities that were unregistered under the Securities Act of 1933.
Issuer Purchases of Equity Securities
During the year ended December 31, 2017, we have not made any purchases of our common stock.
STOCK PERFORMANCE CHART
The annual changes in the cumulative total shareholder return on our common stock for the five-year period shown in the graph below are based on the assumption that $100 had been invested in our common stock, the Standard & Poor’s 500 Stock Index, the Standard & Poor’s Small Cap 600 Stock Index, the Russell 2000 Stock Index, the Standard & Poor’s 500 Health Care Services Index and the Standard & Poor’s 500 Health Care Facilities Index on December 31, 2012, and that all quarterly dividends were reinvested at the average of the closing stock prices at the beginning and end of the quarter. The total cumulative dollar returns shown on the graph represent returns that such investments would have had on December 31, 2017.
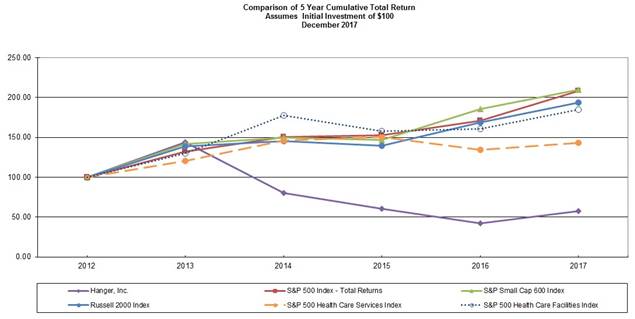
|
|
|
As of December 31, |
| ||||||||||||||||
|
|
|
2012 |
|
2013 |
|
2014 |
|
2015 |
|
2016 |
|
2017 |
| ||||||
|
Hanger, Inc. |
|
$ |
100.00 |
|
$ |
143.79 |
|
$ |
80.04 |
|
$ |
60.12 |
|
$ |
42.03 |
|
$ |
57.57 |
|
|
S&P 500 Index - Total Returns |
|
$ |
100.00 |
|
$ |
132.39 |
|
$ |
150.51 |
|
$ |
152.59 |
|
$ |
170.84 |
|
$ |
208.14 |
|
|
S&P Small Cap 600 Index |
|
$ |
100.00 |
|
$ |
141.31 |
|
$ |
149.45 |
|
$ |
146.50 |
|
$ |
185.40 |
|
$ |
209.94 |
|
|
Russell 2000 Index |
|
$ |
100.00 |
|
$ |
138.82 |
|
$ |
145.62 |
|
$ |
139.19 |
|
$ |
168.85 |
|
$ |
193.58 |
|
|
S&P 500 Health Care Services Index |
|
$ |
100.00 |
|
$ |
120.71 |
|
$ |
145.83 |
|
$ |
150.59 |
|
$ |
134.60 |
|
$ |
143.29 |
|
|
S&P 500 Health Care Facilities Index |
|
$ |
100.00 |
|
$ |
129.72 |
|
$ |
177.61 |
|
$ |
157.84 |
|
$ |
160.60 |
|
$ |
184.84 |
|
Our stock price in 2016 was negatively impacted by our common stock’s suspension on February 26, 2016 and subsequent delisting from trading on the NYSE and the commencement of trading on February 29, 2016 on the OTC.
ITEM 6. SELECTED FINANCIAL DATA
The following tables set forth certain selected consolidated financial data for each of the years in the five-year period ended December 31, 2017, and is derived from the consolidated financial statements of Hanger, Inc. and its subsidiaries. The Consolidated Financial Statements for each of the years in the three-year period ended December 31, 2017 are included in this Annual Report on Form 10-K. The selected consolidated balance sheet data as of December 31, 2015, 2014 and 2013 and the consolidated statements of operations data for the years ended December 31, 2014 and 2013 are derived from our consolidated financial statements, which are not included in this Annual Report on Form 10-K. The selected consolidated financial data set forth below is qualified in its entirety by, and should be read in conjunction with, the consolidated financial statements and notes thereto and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this Annual Report on Form 10-K.
|
Consolidated Statements of Operations and |
|
Year Ended December 31, |
| |||||||||||||
|
Comprehensive (Loss) Income: |
|
2017 |
|
2016 |
|
2015 |
|
2014 |
|
2013 |
| |||||
|
|
|
(in thousands, except per share amounts) |
| |||||||||||||
|
Net revenue |
|
$ |
1,040,769 |
|
$ |
1,042,054 |
|
$ |
1,067,172 |
|
$ |
1,012,100 |
|
$ |
975,769 |
|
|
Material costs |
|
329,223 |
|
332,071 |
|
336,283 |
|
324,284 |
|
302,003 |
| |||||
|
Personnel costs |
|
361,090 |
|
363,537 |
|
367,094 |
|
353,586 |
|
325,780 |
| |||||
|
Other operating costs |
|
129,831 |
|
139,024 |
|
140,839 |
|
136,885 |
|
115,767 |
| |||||
|
General and administrative expenses |
|
110,078 |
|
107,224 |
|
111,761 |
|
86,115 |
|
78,658 |
| |||||
|
Professional accounting and legal fees |
|
36,239 |
|
41,233 |
|
28,647 |
|
44,798 |
|
5,821 |
| |||||
|
Depreciation and amortization |
|
39,259 |
|
44,887 |
|
46,343 |
|
38,929 |
|
34,185 |
| |||||
|
Impairment of intangible assets |
|
54,735 |
|
86,164 |
|
385,807 |
|
223 |
|
— |
| |||||
|
(Loss) income from operations |
|
(19,686 |
) |
(72,086 |
) |
(349,602 |
) |
27,280 |
|
113,555 |
| |||||
|
Interest expense, net |
|
57,688 |
|
45,199 |
|
29,892 |
|
28,277 |
|
30,576 |
| |||||
|
Loss on extinguishment of debt |
|
— |
|
6,031 |
|
7,237 |
|
— |
|
6,645 |
| |||||
|
(Loss) income from continuing operations before income taxes |
|
(77,374 |
) |
(123,316 |
) |
(386,731 |
) |
(997 |
) |
76,334 |
| |||||
|
Provision (benefit) for income taxes |
|
27,297 |
|
(15,910 |
) |
(67,614 |
) |
2,023 |
|
30,455 |
| |||||
|
(Loss) income from continuing operations |
|
(104,671 |
) |
(107,406 |
) |
(319,117 |
) |
(3,020 |
) |
45,879 |
| |||||
|
Income (loss) from discontinued operations, net of income taxes |
|
— |
|
935 |
|
(7,974 |
) |
(15,946 |
) |
(5,368 |
) | |||||
|
Net (loss) income |
|
$ |
(104,671 |
) |
$ |
(106,471 |
) |
$ |
(327,091 |
) |
$ |
(18,966 |
) |
$ |
40,511 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Other comprehensive (loss) income, net |
|
(246 |
) |
(26 |
) |
474 |
|
(868 |
) |
899 |
| |||||
|
Comprehensive (loss) income |
|
$ |
(104,917 |
) |
$ |
(106,497 |
) |
$ |
(326,617 |
) |
$ |
(19,834 |
) |
$ |
41,410 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Basic Per Common Share Data: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
(Loss) income from continuing operations |
|
$ |
(2.89 |
) |
$ |
(2.99 |
) |
$ |
(8.96 |
) |
$ |
(0.09 |
) |
$ |
1.32 |
|
|
Income (loss) from discontinued operations, net of income taxes |
|
— |
|
0.03 |
|
(0.22 |
) |
(0.45 |
) |
(0.16 |
) | |||||
|
Basic (loss) income per share |
|
$ |
(2.89 |
) |
$ |
(2.96 |
) |
$ |
(9.18 |
) |
$ |
(0.54 |
) |
$ |
1.16 |
|
|
Shares used to compute basic per common share amounts |
|
36,271 |
|
35,933 |
|
35,635 |
|
35,309 |
|
34,826 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Diluted Per Common Share Data: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
(Loss) income from continuing operations |
|
$ |
(2.89 |
) |
$ |
(2.99 |
) |
$ |
(8.96 |
) |
$ |
(0.09 |
) |
$ |
1.30 |
|
|
Income (loss) from discontinued operations, net of income taxes |
|
— |
|
0.03 |
|
(0.22 |
) |
(0.45 |
) |
(0.15 |
) | |||||
|
Diluted (loss) income per share |
|
$ |
(2.89 |
) |
$ |
(2.96 |
) |
$ |
(9.18 |
) |
$ |
(0.54 |
) |
$ |
1.15 |
|
|
Shares used to compute diluted per common share amounts |
|
36,271 |
|
35,933 |
|
35,635 |
|
35,309 |
|
35,209 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
|
|
Year Ended December 31, |
| |||||||||||||
|
Consolidated Balance Sheet Data: |
|
2017 |
|
2016 |
|
2015 |
|
2014 |
|
2013 |
| |||||
|
(in thousands) |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Cash and cash equivalents |
|
$ |
1,508 |
|
$ |
7,157 |
|
$ |
58,753 |
|
$ |
11,699 |
|
$ |
1,613 |
|
|
Working capital |
|
$ |
78,666 |
|
$ |
55,014 |
|
$ |
139,824 |
|
$ |
75,197 |
|
$ |
112,910 |
|
|
Total assets |
|
$ |
640,423 |
|
$ |
755,104 |
|
$ |
973,084 |
|
$ |
1,235,733 |
|
$ |
1,141,163 |
|
|
Total debt |
|
$ |
450,264 |
|
$ |
472,650 |
|
$ |
566,433 |
|
$ |
522,336 |
|
$ |
479,050 |
|
|
Shareholders’ (deficit) equity |
|
$ |
(28,051 |
) |
$ |
65,414 |
|
$ |
165,246 |
|
$ |
483,536 |
|
$ |
491,313 |
|
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Forward Looking Statements
This Annual Report on Form 10-K including this “Management’s Discussion and Analysis of Financial Condition and Results of Operations” (or “Management’s Discussion and Analysis”) contains statements that are forward-looking statements within the meaning of the federal securities laws. Forward-looking statements include information concerning our liquidity and our possible or assumed future results of operations, including descriptions of our business strategies. These statements often include words such as “believe,” “expect,” “project,” “potential,” “anticipate,” “intend,” “plan,” “estimate,” “seek,” “will,” “may,” “would,” “should,” “could,” “forecasts” or similar words. These statements are based on certain assumptions that we have made in light of our experience in the industry as well as our perceptions of historical trends, current conditions, expected future developments and other factors we believe are appropriate in these circumstances. We believe these judgments are reasonable, but you should understand that these statements are not guarantees of performance or results, and our actual results could differ materially from those expressed in the forward-looking statements due to a variety of important factors, both positive and negative, that may be revised or supplemented in subsequent reports.
These statements involve risks, estimates, assumptions and uncertainties that could cause actual results to differ materially from those expressed in these statements, including but not limited the risk that additional information may arise during the course of the Company’s ongoing financial statement preparation and closing processes that would require the Company to make additional adjustments or revisions to its estimates or financial statements and other financial data, to identify additional material weaknesses, or to take any other necessary action relating to the Company’s accounting practices; the time required to complete the Company’s financial statements and other financial data and accounting review; the time required to prepare its periodic reports for filings with the Securities and Exchange Commission; the impact of the Tax Cuts and Jobs Act on the Company’s financial statements; and any regulatory review of, or litigation relating to, the Company’s accounting practices, financial statements and other financial data, periodic reports or other corporate actions; changes in the demand for the Company’s orthotic and prosthetic (“O&P”) products and services; uncertainties relating to the results of operations or recently acquired O&P patient care clinics; the Company’s ability to enter into and derive benefits from managed-care contracts; the Company’s ability to successfully attract and retain qualified O&P clinicians; federal laws governing the health care industry; uncertainties inherent in investigations and legal proceedings; governmental policies affecting O&P operations; and other risks and uncertainties generally affecting the health care industry.
Readers are cautioned that all forward-looking statements involve known and unknown risks and uncertainties including, without limitation, those described in Item 1A. “Risk Factors” contained in this Annual Report on Form 10-K, some of which are beyond our control. Although we believe that the assumptions underlying the forward-looking statements contained herein are reasonable, any of the assumptions could be inaccurate. Therefore, there can be no assurance that the forward-looking statements included in this report will prove to be accurate. Actual results could differ materially and adversely from those contemplated by any forward-looking statement. In light of the significant risks and uncertainties inherent in the forward-looking statements included herein, the inclusion of such information should not be regarded as a representation by us or any other person that our objectives and plans will be achieved. We undertake no obligation to publicly release any revisions to any forward-looking statements in this discussion to reflect events and circumstances occurring after the date hereof or to reflect unanticipated events. Forward-looking statements and our liquidity, financial condition and results of operations may be affected by the risks set forth in Item 1A. “Risk Factors” or by other unknown risks and uncertainties.
Effect of Delay in Financial Filings
The delay in our completion of this filing relates primarily to the effects of our delay in the completion of our Annual Reports on Form 10-K for the years ending December 31, 2014 and 2016. We filed our Annual Report on Form 10-K for the year ended December 31, 2016 on January 19, 2018. The 2016 Form 10-K contained our consolidated financial statements and related footnotes for the years ended December 31, 2015 and December 31, 2016, as well as consolidated financial statements
for each of the quarterly and year-to-date periods occurring within those two years. Previously, on May 12, 2017, we filed our Annual Report on Form 10-K for the year ended December 31, 2014. The 2014 10-K contained our consolidated financial statements and related footnotes for the year ended December 31, 2014, as well as consolidated financial statements for the third and fourth quarters of 2014. The 2014 Form 10-K also included a restatement of our previously issued consolidated financial statements and related footnotes for (i) the fiscal years ended December 31, 2013 and 2012; (ii) the first two quarters of fiscal year 2014 and (iii) each of the quarterly periods in fiscal year 2013. The 2014 Form 10-K also contained restated financial results for the fiscal years ended December 31, 2011 and 2010 (each unaudited).
Our efforts to remediate our material weaknesses, restate our historical financial statements, prepare this Annual Report on Form 10-K and other factors have come at a cost in excess of the amount we estimate we would otherwise have incurred in a typical fiscal year. The estimated professional fees associated with these efforts are as follows:
|
( in thousands) |
|
|
|
|
|
Balance to be Paid |
| |||
|
Year |
|
Expensed |
|
Paid |
|
in Future Periods |
| |||
|
2014 |
|
$ |
37,930 |
|
$ |
(1,792 |
) |
$ |
36,138 |
|
|
2015 |
|
23,475 |
|
(25,981 |
) |
33,632 |
| |||
|
2016 |
|
37,244 |
|
(47,975 |
) |
22,901 |
| |||
|
2017 |
|
32,301 |
|
(44,917 |
) |
10,285 |
| |||
We currently estimate that we will incur and pay an additional $13.0 million of such excess fees during 2018. Estimated payments for excess fees in 2018 will total $23.3 million, which includes $10.3 million related to prior periods. See the “Liquidity and Capital Resources” section in this Management’s Discussion and Analysis for further discussion.
Unless otherwise stated, this Management’s Discussion and Analysis has been written to provide you with pertinent information regarding our performance during the periods encompassed by this report. Accordingly, we have not provided information regarding our performance during subsequent periods. Nevertheless, for certain information and events, which relate primarily to our indebtedness, capital structure and liquidity, we have provided disclosure regarding subsequent periods or have included further information in Note U - “Subsequent Events” to our consolidated financial statements. Additionally, we have referenced certain trends and events occurring subsequent to December 31, 2017 as forward-looking items within this Management’s Discussion and Analysis.
Non-GAAP Measures
We refer to certain financial measures and statistics that are not prescribed under generally accepted accounting principles (“GAAP”) as applied in the United States. We utilize these non-GAAP measures in order to evaluate the underlying factors that affect our business performance and trends. These non-GAAP measures should not be considered in isolation and should not be considered superior to, or as a substitute for, financial measures calculated in accordance with GAAP. We have defined and provided a reconciliation of these non-GAAP measures to their most comparable GAAP measures. The non-GAAP measures used in this Management’s Discussion and Analysis are as follows:
Adjusted Gross Revenue and Disallowed Revenue - “Adjusted gross revenue” reflects our gross billings after their adjustment to reflect estimated discounts established in our contracts with payors of health care claims. As discussed in “Reimbursement Trends” below, pursuant to our contracts with payors, a portion of our adjusted gross billings may be disallowed based on factors including physician documentation, patient eligibility, plan design, prior authorization, timeliness of filings or appeal, coding selection, failure by certain patients to pay their portion of claims, computational errors associated with sequestration and other factors. We refer to these and other amounts as being “disallowed revenue.” Our net revenue reflects adjusted gross revenue after reduction for the estimated aggregate amount of disallowed revenue for the applicable period. To facilitate analysis of the comparability of our results, we provide these non-GAAP measures due to the significant changes
that we have experienced in recent years in disallowed revenue which are further discussed below. In addition, we provide measures of material costs, personnel costs, other operating costs, general and administrative expenses, professional accounting and legal fees, depreciation and amortization and operating expenses as a percentage of adjusted gross revenue because we believe these percentages provide an investor with another meaningful measure to compare our results with prior periods. These measures are non-GAAP and unaudited.
Same Clinic Revenue and Same Clinic Revenue per Day - Same clinic revenue measures revenue from clinics that have been operating for a full calendar year or more. Examples of clinics not included in the same center population are closures and acquisitions. Same clinic revenue per day normalizes sales for the number of days a clinic was open in each comparable period. These measures are both non-GAAP and unaudited.
Overview
We are a leading national provider of products and services that assist in enhancing or restoring the physical capabilities of patients with disabilities or injuries. Built on the legacy of James Edward Hanger, the first amputee of the American Civil War, we and our predecessor companies have provided O&P services for over 150 years. We provide O&P services, distribute O&P devices and components, manage O&P networks and provide therapeutic solutions to patients and businesses in acute, post-acute and clinic settings. We operate through two segments - Patient Care and Products & Services.
Our Patient Care segment is primarily comprised of Hanger Clinic, which specializes in the design, fabrication and delivery of custom O&P devices through 682 patient care clinics and 112 satellite locations in 44 states and the District of Columbia, as of December 31, 2017. We also provide payor network contracting services to other O&P providers through this segment.
Our Products & Services segment is comprised of our distribution and our therapeutic solutions businesses. As a leading provider of O&P products in the United States, we coordinate through our distribution business the procurement and distribution of a broad catalog of O&P parts, componentry and devices to independent O&P providers nationwide. To facilitate speed and convenience, we deliver these products through our five distribution facilities that are located in Nevada, Georgia, Illinois, Pennsylvania and Texas. The other business in our Products & Services segment is our therapeutic solutions business, which provides specialized rehabilitation technologies and evidence-based clinical programs for post-acute rehabilitation to patients at approximately 4,000 skilled nursing and post-acute providers nationwide.
In each of 2017, 2016 and 2015, we incurred a material impairment of our goodwill. These non-cash charges were the most significant contributing factor to our reported loss from operations and net loss in each period. We discuss the causes and manner of our determination of these impairment charges in Note H - “Goodwill and Other Intangible Assets” to our consolidated financial statements in this Annual Report on Form 10-K.
See Note R - “Segment and Related Information” to our consolidated financial statements in this Annual Report on Form 10-K for disclosure of financial information by operating segment for 2017, 2016 and 2015.
Reimbursement Trends
In our Patient Care segment, we are reimbursed primarily through employer-based plans offered by commercial insurance carriers, Medicare, Medicaid and the VA. The following is a summary of our payor mix, expressed as an approximate percentage of net revenues for the periods indicated:
|
|
|
For the Years Ended December 31, |
| ||||
|
|
|
2017 |
|
2016 |
|
2015 |
|
|
|
|
|
|
|
|
|
|
|
Medicare |
|
30.5 |
% |
30.5 |
% |
30.3 |
% |
|
Medicaid |
|
15.6 |
% |
14.8 |
% |
14.2 |
% |
|
Commercial Insurance/Managed Care (excluding Medicare and Medicaid Managed Care) |
|
38.2 |
% |
39.2 |
% |
39.6 |
% |
|
Veterans Affairs |
|
8.7 |
% |
8.8 |
% |
8.9 |
% |
|
Private Pay |
|
7.0 |
% |
6.7 |
% |
7.0 |
% |
|
Patient Care |
|
100.0 |
% |
100.0 |
% |
100.0 |
% |
Patient Care constitutes 81.9%, 80.6% and 82.0% of our net revenue for 2017, 2016 and 2015, respectively. Our remaining net revenue is produced in our Products & Services segment which derives its net revenue from commercial transactions with independent O&P providers, healthcare facilities and other customers. In contrast to net revenues from our Patient Care segment, payment for these products and services are not directly subject to third party reimbursement from health care payors.
The amount of our reimbursement varies based on the nature of the O&P device we fabricate for our patients. Given the particular physical weight and size characteristics, location of injury or amputation, capability for physical activity and mobility, cosmetic and other needs of each individual patient, each fabricated prostheses and orthoses is customized for each particular patient. The nature of this customization and the manner by which our claims submissions are reviewed by payors makes our reimbursement process administratively difficult.
To receive reimbursement for our work, we must ensure that our clinical, administrative and billing personnel receive and verify certain medical and health plan information, record detailed documentation regarding the services we provide and accurately and timely perform a number of claims submission and related administrative tasks. Traditionally, we have performed these tasks in a manual fashion and on a decentralized basis. In recent years, due to increases in payor pre-authorization processes, documentation requirements, pre-payment reviews and pre- and post-payment audits, our ability to successfully undertake these tasks using our traditional approach has become increasingly challenging. We believe these changes in industry trends have been brought about in part by increased nationwide efforts to reduce health care costs.
A measure of our effectiveness in securing reimbursement for our services can be found in the degree to which payors ultimately disallow payment of our claims. Payors can deny claims due to their determination that a physician who referred a patient to us did not sufficiently document that a device was medically necessary or clearly establish the ambulatory (or “activity”) level of a patient. Claims can also be denied based on our failure to ensure that a patient was currently eligible under a payor’s health plan, that the plan provides full O&P benefits, that we received prior authorization, that we filed or appealed the payor’s determination timely, on the basis of our coding, failure by certain classes of patients to pay their portion of a claim and for various other reasons. If any portion of, or administrative factor within, our claim is found by the payor to be lacking, then the entirety of the claim amount may be denied reimbursement. Due to the increasing demands of these processes, the level and capability of our staffing, as well as our material weaknesses and other considerations, our consolidated disallowed revenue and bad debt expense, and their relationship to consolidated adjusted gross revenue increased over historical levels to a peak level in 2014. In 2015, 2016 and 2017, through the initiatives discussed below, we achieved decreases in our disallowed revenue. Disallowed revenue and bad debt expense over the past five years has been as follows:
|
|
|
For the Years Ended December 31, |
| |||||||||||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
2015 |
|
2014 |
|
2013 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Net revenue |
|
$ |
1,040,769 |
|
$ |
1,042,054 |
|
$ |
1,067,172 |
|
$ |
1,012,100 |
|
$ |
975,769 |
|
|
Disallowed revenue |
|
36,962 |
|
49,387 |
|
60,669 |
|
82,330 |
|
65,629 |
| |||||
|
Adjusted gross revenue |
|
$ |
1,077,731 |
|
$ |
1,091,441 |
|
$ |
1,127,841 |
|
$ |
1,094,430 |
|
$ |
1,041,398 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Disallowed revenue |
|
$ |
36,962 |
|
$ |
49,387 |
|
$ |
60,669 |
|
$ |
82,330 |
|
$ |
65,629 |
|
|
Bad debt expense |
|
9,423 |
|
13,727 |
|
12,854 |
|
11,639 |
|
5,053 |
| |||||
|
Disallowed revenue & bad debt expense |
|
$ |
46,385 |
|
$ |
63,114 |
|
$ |
73,523 |
|
$ |
93,969 |
|
$ |
70,682 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Disallowed revenue % |
|
3.4 |
% |
4.5 |
% |
5.4 |
% |
7.5 |
% |
6.3 |
% | |||||
|
Bad debt expense % |
|
0.9 |
% |
1.3 |
% |
1.1 |
% |
1.1 |
% |
0.5 |
% | |||||
|
Disallowed revenue & bad debt expense % |
|
4.3 |
% |
5.8 |
% |
6.5 |
% |
8.6 |
% |
6.8 |
% | |||||
Adjusted gross revenue in the above chart reflects our gross billings after reduction for estimated contractual discounts. The percentage of our gross billings that have been disallowed increased to a high of 7.5% in 2014 from 2.9% in 2010. Due to industry trends and our specific administrative factors, our collection experience degraded and disallowed revenue increased during that period of time. These adverse industry trends included an increased level of payor audits and more stringent requests by payors that referring physician documentation be provided in connection with claims. During that period of time, we utilized a decentralized billing and collections approach, where invoicing and collections were undertaken at individual patient care locations. Our typical locations have an average of two office administrators who are required to handle patient administration, purchasing, and clinician support tasks. Due to increasing payor documentation demands and budgetary limitations on staffing, administrative staff were increasingly unable to successfully address the growing levels of payor denials. In 2014, our accounts receivable trends were further complicated due to issues encountered with our implementation of a new patient management and electronic health record system. Due to system customizations that were subsequently determined to not be adequately tested, staffing deficiencies in cash application functions and other related procedural issues, billing and collections were further adversely affected due to this system implementation during that year as can be seen by the increase in the disallowed revenue rate and bad debt expense in that year. Throughout this period, our processes were also impeded due to the subsequently identified underlying material control weakness in the administration of our contracts. As contracts were negotiated or amended with payors, our procedures did not provide adequate assurance of timely documented reconciliation of updated terms and conditions with those loaded into our remote billing systems.
Commencing in late 2014 and continuing through 2015, 2016 and 2017, we took a number of actions to halt and reverse these disallowed revenue and bad debt trends. These initiatives included: (i) the retention of consultants and constitution of a central revenue cycle management function; (ii) the temporary halting of the roll-out of our new patient management and electronic health record system to address the identified issues; and (iii) the establishment of new clinic-level procedures and training regarding the collection of supporting documentation and the importance of diligence in our claims submission processes. The percentage of our gross billings that have been disallowed decreased to 3.4% in 2017 from the high of 7.5% in 2014. These initiatives are each discussed more fully in sections provided for each of them below. While we intend to continue to work towards further improvements in our procedures and use of technology within our clinic and revenue cycle functions, we do not currently foresee that future reductions in disallowed revenue will be achievable or substantial as the improvements realized from 2014 through 2017.
In 2016, we experienced a $2.7 million increase in bad debt expense in our Products & Services segment resulting from the bankruptcy of one large customer of our distribution business and financial difficulties encountered by another.
These adverse trends also resulted in increases to our consolidated accounts receivable allowances during the period of 2010 through 2014. In a manner similar to the improvements achieved in disallowance trends, the initiatives undertaken in
establishing a revenue cycle management function, addressing weaknesses in our new patient management and electronic health record system and in improving our procedures and standards for clinic-level documentation have had a favorable effect on our accounts receivable balances in 2016 and 2017. Our accounts receivable balances for 2013 through 2017 were as follows:
|
|
|
As of December 31, |
| |||||||||||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
2015 |
|
2014 |
|
2013 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Accounts receivable, before allowance |
|
$ |
216,644 |
|
$ |
221,220 |
|
$ |
270,925 |
|
$ |
271,384 |
|
$ |
213,488 |
|
|
Allowance for disallowed revenue |
|
(56,233 |
) |
(61,137 |
) |
(81,306 |
) |
(87,192 |
) |
(52,277 |
) | |||||
|
Accounts receivable, gross |
|
160,411 |
|
160,083 |
|
189,619 |
|
184,192 |
|
161,211 |
| |||||
|
Allowance for doubtful accounts |
|
(14,065 |
) |
(15,521 |
) |
(15,027 |
) |
(9,944 |
) |
(6,472 |
) | |||||
|
Accounts receivable, net |
|
$ |
146,346 |
|
$ |
144,562 |
|
$ |
174,592 |
|
$ |
174,248 |
|
$ |
154,739 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Allowance for disallowed revenue % |
|
26.0 |
% |
27.6 |
% |
30.0 |
% |
32.1 |
% |
24.5 |
% | |||||
|
Allowance for doubtful accounts % |
|
6.5 |
% |
7.0 |
% |
5.5 |
% |
3.6 |
% |
3.0 |
% | |||||
|
Total allowance % |
|
32.5 |
% |
34.6 |
% |
35.5 |
% |
35.7 |
% |
27.5 |
% | |||||
Revenue Cycle Management
Prior to 2014, we performed our eligibility, patient pre-authorization, patient documentation, claims coding, claims submission, collection, cash application and claims audit support activities (our “revenue cycle management” functions) primarily on a decentralized location by location basis. Due to the increases experienced in disallowed revenue, as well as to address certain procedural requirements of our new patient management and electronic health record system and to otherwise improve the effectiveness of our revenue cycle management functions, during 2014 we commenced the process of establishing a centralized revenue cycle management organization with the strategy to gradually transition these functions from our decentralized clinics to a centralized organization. We continued and expanded this initiative in 2015, 2016 and 2017.
As discussed in the “Reimbursement Trends” section above, we experienced decreases in our disallowed revenue in 2015, 2016 and 2017, as compared with 2014. In addition to other training and claims documentation initiatives, we believe that decreases we have experienced in disallowed revenue (as well as our overall accounts receivables balances) are due in part to our revenue cycle management initiative.
New System Implementation
In 2014, in our Patient Care segment, we commenced the implementation of a new patient management and electronic health record system at our patient care clinics. A key purpose of the system was to automate clinician documentation, claims coding and other increasingly complex clinic administrative requirements. In connection with the system implementation, we customized certain templates and software code within a system developed by NextGen. In 2014, as the system was installed at increasing numbers of clinics, we encountered difficulties in clinic workload, were unable to timely apply cash we received from payors to patient accounts and experienced a marked increase in our accounts receivable balance. Due to these issues, we halted the implementation at the end of the third quarter of 2014, after the system had been installed at approximately one-third of our sites.
We believe the implementation issues we encountered related primarily to inadequate testing of the system, a failure to successfully establish and effectively staff a central cash applications function, insufficient training and other difficulties associated with our customizations of the software code.
We subsequently resolved the issues we encountered with the system and our implementation process, and recommenced implementation in 2016. In 2017 and 2016, we expensed $4.3 million and $2.7 million, respectively, in training, travel and related implementation costs. We currently anticipate that we will incur approximately $4.6 million in training, travel and related implementation costs in 2018. Approximately two-thirds of our clinics were utilizing this system as of the end of 2017, and we plan to convert the remaining locations to this system by mid-2019.
Clinic-Level Claims Documentation
In addition to our revenue cycle management initiatives and resolution of the aforementioned issues associated with our implementation of our new electronic health record and patient management system, in 2016 we commenced more intensive training and increased our internal clinic-level emphasis on the importance of adherence to procedural and documentation standards. The absence of sufficient documentation establishing medical necessity and a patient’s degree of ability for future activity is a key factor utilized by payors when denying our claims for reimbursement. Irrespective of a patient’s need and the existence of a referral from the treating physician, we have found it increasingly necessary to retrieve other supporting documentation and notes from referring physicians themselves to further justify and document their medical determinations relating to the patients they refer to us. Given that these referring physicians do not work for us, the retrieval of this additional information to suit payors can be difficult and time-consuming.
We believe our efforts to increase our discipline through this clinic-level claims documentation initiative assisted us in further reducing the level of our disallowed sales. However, we also believe these efforts had a one-time indirect effect of reducing our overall revenue growth rate. In addition to other factors affecting our same clinic sales trends in 2016 and early 2017, as clinicians and their office administrators increased their attention on achieving higher documentation standards, we believe we were able to see and treat fewer patients, thereby contributing to our reduced same clinic patient care net revenue.
We continued to apply these procedural and documentation standards throughout 2017 and plan to continue to do so in 2018. With the initial implementation impact behind us, we do not believe the use of these standards will be a significant factor in our year-over-year net revenue growth trends for 2018.
Increasing Patient Responsibility for the Cost of Devices
The majority of our devices are provided as replacement devices to patients with devices that are broken or have become worn with age. Prosthetic devices are typically replaced every three to five years. In recent years, an increasing number of employers have been shifting the cost burdens in their health plans to employees through use of “high deductible” or “consumer-driven” health plans. These plan designs typically require the patient to bear a greater portion of the cost of their care in exchange for a lower monthly premium. We believe the increased use of these plans has and will continue to have the effect of causing patients to delay the replacement of their devices and could accordingly adversely impact our net revenue.
Products & Services Segment Trends
During 2017, several of the larger independent O&P providers we serve through the distribution of componentry encountered financial difficulties which resulted in our discontinuing distribution services to them. Generally, we believe our distribution customers are encountering reimbursement pressures similar to those we have experienced in our own Patient Care services and, depending on their ability to adapt to the increased claims documentation standards that have emerged in our industry, that this may either limit the rate of growth of some of our customers, or otherwise affect the rate of growth we experience in our distribution of O&P componentry to independent providers.
Within our Products & Services segment, in addition to our distribution of products, we provide therapeutic equipment and services to patients at SNFs and other healthcare provider locations. In late 2016, a number of our clients, including several of our larger SNF clients elected to discontinue their use of our therapeutic services. We believe these discontinuances relate primarily to their overall efforts to reduce the costs they bear for therapy-related services within their facilities. As a part of
those terminations of service, in a number of cases, we elected to sell terminating clients the equipment that we had utilized for their locations, which resulted in our recognition of $3.1 million in equipment sales in 2017 as compared with $6.7 million in 2016 and $2.9 million in 2015. In 2017, due to customer discontinuances, we experienced a decrease of $10.7 million in therapeutic services and supplies revenue, and $3.6 million in therapeutic equipment sales, for a total reduction of $14.3 million in revenues we received from therapeutic equipment and services. We recognized a total of $60.1 million in revenues from therapeutic equipment and services in 2017. In 2018, we currently anticipate that we will experience a further decline of approximately $8.0 million in revenue from these services associated with customer discontinuances. Within this portion of our business, we have responded to these trends through increases in our marketing programs which convey the value we believe our services have to patients at SNFs and have begun to increase our focus on sales of our therapeutic services to other adjacent health services provider markets.
Discontinuance of the Dosteon and CARES Businesses
On November 5, 2014, the Audit Committee of our Board of Directors approved a plan to sell or otherwise dispose of Dosteon and CARES, both part of our Patient Care segment. This action was taken as a result of our strategic evaluation of these businesses. As of December 31, 2014, Dosteon qualified as assets held for sale and discontinued operations. As such the assets, operating results and cash flows of the Dosteon disposal group have been presented separately as discontinued operations within our consolidated financial statements. The CARES business did not qualify as assets held for sale and was ultimately wound down in 2015. Accordingly, the CARES business has been classified as a continuing operation in our consolidated financial statements for all financial periods through 2015, the year of its cessation of operations. The information provided herein is for continuing operations, unless otherwise indicated. See Note S - “Discontinued Operations” to our consolidated financial statements in this Annual Report on Form 10-K for additional discussion of our discontinued operations.
Acquisitions
We have not made any acquisitions since the first quarter of 2015 due to the necessity of utilizing available operating cash flow to fund accounting, legal and other professional fees in connection with the preparation and review of our financial statements, efforts to remediate our material weaknesses, related legal matters, and due to the effect of our non-compliance with certain of our debt covenants relating to our failure to meet financial statement reporting requirements. In connection with refinancing of our debt in 2018, and the resolution of the primary factors which led us to halt our acquisitions, we currently intend to recommence acquisitions of O&P businesses similar to those that we have consummated in prior years.
In the first quarter of 2015, we acquired three O&P businesses with approximately $11.8 million in revenue, operating a total of 15 patient care clinics located in three states. The aggregate purchase price for these businesses was $15.3 million, including $10.2 million in net cash, $4.7 million of Seller Notes and $0.4 million of working capital adjustments and other.
Seasonality
We believe our business is affected by the degree to which patients have otherwise met the deductibles for which they are responsible in their medical plans during the course of the year. The first quarter is normally our lowest relative net revenue quarter, followed by the second and third quarters, which are somewhat higher and consistent with one another, and, due to the general fulfillment by patients of their health plan co-payments and deductible requirements towards the year’s end, our fourth quarter is normally our highest revenue producing quarter.
Our results are also affected, to a lesser extent, by our holding of an education fair in the first quarter of each year. This one week event is conducted to assist our clinicians in maintaining their training and certification requirements and to facilitate a national meeting with our clinical leaders. We also invite manufacturers of the componentry for the devices we fabricate to these annual events so they can demonstrate their products and otherwise assist in our training process. During the first quarters of 2017, 2016 and 2015, we spent approximately $2.0 million, $2.1 million and $2.0 million, respectively, on travel
and other costs associated with this one week event. In addition to the costs we incur associated with this annual event, we also lose the productivity of a significant portion of our clinicians during the one week period in which this event occurs, which contributes to the lower seasonal revenue level we experience during the first quarter of each year.
Business Environment and Outlook
In our Patient Care segment, we have a positive view of the long-term need for prosthetic and orthotic devices and services within the markets that we serve. To address the debilitating effects of injuries and medical conditions such as diabetes, vascular disease, cancer and congenital disorders, we believe patients will have a continuing need for the O&P services that we provide. As the population grows and ages, we also believe there will be a gradual underlying increase in market demand.
To ensure we maintain and grow our share of this market, we believe that it will be necessary for us to find effective means to automate and better organize our business processes, further improve our reimbursement capabilities and lower our cost structure in the longer term. Our size may afford us the ability to achieve economies of scale through purchasing and process automation initiatives that would be difficult for our smaller competitors. However, our size can work against us if we do not succeed in effectively serving our referring physicians and in competing with our individual competitors in each of the markets that we serve.
See the “Products & Services Segment Trends” section in this Management’s Discussion and Analysis for information regarding the business environment and outlook of our Products & Services segment.
Critical Accounting Policies
Our analysis and discussion of our financial condition and results of operations is based upon the consolidated financial statements that have been prepared in accordance with GAAP. The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. GAAP provides the framework from which to make these estimates, assumptions and disclosures. We have chosen accounting policies within GAAP that management believes are appropriate to fairly present, in all material respects, our operating results and financial position. Our significant accounting policies are stated in Note B - “Significant Accounting Policies” to the consolidated financial statements included in this Annual Report on Form 10-K. We believe the following accounting policies are critical to understanding our results of operations and the more significant judgments and estimates used in the preparation of our consolidated financial statements.
Revenue Recognition
Patient Care Segment
Revenues in our Patient Care segment are primarily derived from the sale of O&P devices and are recognized when the patient has received the device or service. At or subsequent to delivery, we issue an invoice to a third party payor, which primarily consists of commercial insurance companies, Medicare, Medicaid, the VA and private or patient pay (“Private Pay”). We recognize revenue for the amounts we expect to receive from payors based on expected contractual reimbursement rates, which are net of estimated contractual discounts. Government reimbursement, comprised of Medicare, Medicaid and the U.S. Department of Veterans Affairs, in the aggregate, accounted for approximately, 54.8%, 54.1% and 53.4% of our net revenue in 2017, 2016 and 2015, respectively.
These revenue amounts are further revised as claims are adjudicated, which may result in disallowances, or decreases to revenue. We believe that adjustments related to write-offs of receivables should predominantly be recorded as a reduction of revenues, which we refer to as disallowed revenue. This is due to the majority of our revenues being collected from commercial insurance companies, Medicare, Medicaid and the VA, most of which are under contractual reimbursement rates.
As such, adjustments do not relate to an inability to pay, but to contractual allowances, lack of timely claims submission, insufficient medical documentation or other administrative errors. Amounts recorded to bad debt expense, which are presented within “Other operating costs,” generally relate to commercial payor bankruptcies and private pay balances for which there was an assessment of collectability and collection attempts were made. At the end of each period, we establish allowances for estimated disallowances relating to that period based on prior adjudication experience and record such amounts as an adjustment to revenue. In a similar fashion, we estimate and record allowances for doubtful accounts on unpaid receivables at each period end. We also record a liability, with a corresponding adjustment to revenue, for refunds expected to be paid to our patients or third party payors.
Medicare and Medicaid regulations and the various agreements we have with other third party payors, including commercial healthcare providers under which these contractual adjustments and disallowed revenue are calculated, are complex and are subject to interpretation and adjustment and may include multiple reimbursement mechanisms for different types of services. Therefore, the particular O&P devices and related services authorized and provided, and the related reimbursement, are subject to interpretation and adjustment that could result in payments that differ from our estimates. Additionally, updated regulations and reimbursement schedules, and contract renegotiations occur frequently, necessitating regular review and assessment of the estimation process by management. As a result, there is a reasonable possibility that recorded estimates could change and any related adjustments will be recorded as changes in estimates when they become known.
For more information on our use of estimates to calculate allowances for disallowed revenue and doubtful accounts, refer to the “Accounts Receivable, Net” section below.
We often invoice patients or payors after a device is delivered. To account for this delay, we record an estimated revenue accrual for devices delivered but not yet invoiced at period end. This estimate is based on a historical look-back analysis of lag times between delivery and invoicing that occur over a period end.
Products & Services Segment
Revenues in our Products & Services segment are derived from the distribution of O&P components and the leasing and sale of rehabilitation equipment and ancillary consumable supplies combined with equipment maintenance, education, and training. Distribution revenues are recorded upon the delivery of products, net of estimated returns.
Equipment leasing and related services revenue are recognized over the applicable term as the customer has the right to use the equipment and as the services are provided. Equipment sales revenue is recognized upon delivery, with any related services revenue deferred and recognized as the services are performed. Sales of consumables are recognized upon delivery.
Accounts Receivable, Net
Patient Care Segment
We establish allowances for accounts receivable to reduce the carrying value of such receivables to their estimated net realizable value. The Patient Care segment’s accounts receivables are recorded net of unapplied cash, estimated allowance for disallowed revenue and estimated allowance for doubtful accounts, as described in the Revenue Recognition accounting policy above.
Both the allowance for disallowed revenue and the allowance for doubtful accounts estimates consider historical collection experience by each of the Medicare and non-Medicare (commercial insurance, Medicaid, Veteran’s Administration and Private Pay) primary payor class groupings. For each payor class grouping, liquidation analysis of historical period end receivable balances are performed to ascertain collections experience by aging category. We believe the use of historical collection experience applied to current period end receivable balances is reasonable. In the absence of an evident adverse
trend, we use historical experience rates calculated using an average of four quarters of data with at least twelve months of adjudication. We believe the time periods analyzed provide sufficient time for most balances to adjudicate in the normal course of operations. We will modify the time periods analyzed when significant trends indicate that adjustments should be made. In addition, estimates are adjusted when appropriate for information available up through the issuance of the consolidated financial statements.
Products & Services Segment
Products & Services segment’s allowance for doubtful accounts is estimated based on the analysis of the segment’s historical write-offs experience, accounts receivable aging and economic status of its customers. Accounts receivable that are deemed uncollectible are written-off to the allowance for doubtful accounts. Accounts receivable are also recorded net of an allowance for estimated sales returns.
Inventories
Inventories are valued at the lower of estimated cost or net realizable value, with cost determined on a first-in, first-out (“FIFO”) basis. Provisions have also been made to reduce the carrying value of inventories for excess, obsolete, or otherwise impaired inventory on hand at period-end.
Patient Care Segment
Substantially all of our Patient Care segment inventories are recorded through a periodic approach whereby inventory quantities are adjusted on the basis of a quarterly physical count. Segment inventories relate primarily to raw materials and WIP at Hanger Clinics. Inventories at Hanger Clinics totaled $27.7 million and $29.1 million at December 31, 2017 and 2016, respectively, with WIP inventory representing $9.0 million and $9.0 million of the total inventory, respectively.
Raw materials consists of purchased parts, components, and supplies which are used in the assembly of O&P devices for delivery to patients. In some cases, purchased parts and components are also sold directly to patients. Raw materials are valued based on recent vendor invoices, reduced by estimated vendor rebates. Such rebates are recognized as a reduction of cost of materials in the consolidated statements of operations and comprehensive loss when the related devices or components are delivered to the patient. Approximately 71% and 69% of materials at December 31, 2017 and 2016, respectively were purchased from our Products & Services segment. Raw material inventory was $18.7 million and $20.1 million at December 31, 2017 and 2016, respectively.
WIP consists of devices which are in the process of assembly at our clinics or fabrication centers. WIP quantities were determined by the physical count of patient orders at the end of every quarter of 2017 and 2016 while the related stage of completion of each order was established by clinic personnel. We do not have an inventory costing system and as a result, the identified WIP quantities were valued on the basis of estimated raw materials, labor, and overhead costs. To estimate such costs, we develop bills of materials for certain categories of devices that we assemble and deliver to patients. Within each bill of material, we estimate (i) the typical types of component parts necessary to assemble each device; (ii) the points in the assembly process when such component parts are added; (iii) the estimated cost of such parts based on historical purchasing data; (iv) the estimated labor costs incurred at each stage of assembly; and (v) the estimated overhead costs applicable to the device.
Products & Services Segment
Product & Service segment inventories consist primarily of finished goods at its distribution centers as well as raw materials at fabrication facilities, and totaled $41.4 million and $39.1 million as of December 31, 2017 and 2016, respectively. Finished goods include products that are available for sale to third party customers as well as to our Patient Care segment as
described above. Such inventories were determined on the basis of perpetual records and a physical count at year end. Inventories in connection with therapeutic services are valued at a weighted average cost.
Business Combinations
We record tangible and intangible assets acquired and liabilities assumed in business combinations under the acquisition method of accounting. For consideration of the net assets acquired, we typically pay cash and issue a Seller Note. We may also include contingent consideration with payment terms associated with the achievement of designated collection targets of the acquired business. Amounts paid for each acquisition are allocated to the assets acquired and liabilities assumed based on their estimated fair values at the date of acquisition inclusive of identifiable intangible assets. The estimated fair value of identifiable assets and liabilities are based on detailed valuations performed internally or by external valuation specialists that use information and assumptions provided by management. We allocate any excess purchase price over the fair value of the net tangible and identifiable assets acquired and liabilities assumed to goodwill. Significant management judgments and assumptions are required in determining the fair value of acquired assets and liabilities, particularly acquired intangible assets, including estimated useful lives. The valuation of purchased intangible assets is based upon estimates of the future performance and discounted cash flows from the acquired business. Each asset acquired or liability assumed is measured at estimated fair value from the perspective of a market participant. Subsequent changes in estimated fair value of contingent consideration are recognized as “General and administrative expenses” within the consolidated statements of operations and comprehensive loss.
Goodwill and Other Intangible Assets, Net
Goodwill represents the excess of the purchase price over the estimated fair value of net identifiable assets acquired and liabilities assumed from purchased businesses. We assess goodwill for impairment annually during the fourth quarter, and between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. We have the option to first assess qualitative factors for a reporting unit to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount as a basis for determining whether it is necessary to perform the quantitative goodwill impairment test. If we choose to bypass this qualitative assessment or alternatively determine that a quantitative goodwill impairment test is required, our annual goodwill impairment test is performed by comparing the estimated fair value of a reporting unit with its carrying amount (including attributed goodwill). We will measure the fair value of the reporting units using a combination of income and market approaches. Any impairment would be recognized by a charge to income from operations and a reduction in the carrying value of the goodwill.
We apply judgment in determining the fair value of our reporting units and the implied fair value of goodwill which is dependent on significant assumptions and estimates regarding expected future cash flows, terminal value, changes in working capital requirements, and discount rates.
In January 2017, the Financial Accounting Standards Board issued Accounting Standards Update No. 2017-04 that sought to simplify the accounting for goodwill impairments by eliminating Step 2 from the goodwill impairment test. We early adopted this standard in 2017 and as a result, our impairment tests as of our October 1, 2017 annual impairment testing date compared the carrying values of our reporting units to their respective fair values with any necessary impairment charge recorded in an amount equal to the excess of carrying value over fair value.
The fair value of acquired customer intangibles was estimated using an excess earnings model. Key assumptions utilized in the valuation model included pro-forma projected cash flows adjusted for market-participant assumptions, forecasted customer retention curve, and discount rate. Customer intangibles are amortized, using the straight-line method over an estimated useful life of four to ten years. The fair value of non-compete agreements are estimated using a discounted cash flow model. The related intangible assets are amortized, using the straight-line method, over their term which ranges from
two to five years. Other definite-lived intangible assets are recorded at cost and are amortized, using the straight-line method, over their estimated useful lives of up to seventeen years. The fair value associated with trade names is estimated using the relief-from-royalty method with the primary assumptions being the royalty rate and expected revenues associated with the trade names. These assets, some of which have indefinite lives, are primarily included in the Products & Services segment. Indefinite lived trade name intangible assets are assessed for impairment in the fourth quarter of each year, or more frequently if events or changes in circumstances indicate that the asset might be impaired. Trade name intangible assets with definite lives are amortized over their estimated useful lives of one to ten years.
For the years ended December 31, 2017, 2016 and 2015, we recorded impairments of our goodwill totaling $53.3 million, $86.0 million and $382.9 million, respectively. See Note H - “Goodwill and Other Intangible Assets” to our consolidated financial statements in this Annual Report on Form 10-K for additional information regarding these charges.
In conjunction with our Goodwill impairment testing at December 31, 2015, we reevaluated the estimated useful life of our customer list intangibles. In the fourth quarter of 2015, the estimated useful lives of our customer list intangibles were reduced from 10 years to four years in our Patient Care segment and from 14 years to 10 years in our Products & Services segment. This change in the estimated useful lives increased amortization for the years ended December 31, 2017, 2016 and 2015 by approximately $3.0 million $7.0 million and $6.0 million, respectively.
As described, we apply judgment in the selection of key assumptions used in the goodwill impairment test and as part of our evaluation of intangible assets tested annually and at interim testing dates as necessary. If these assumptions differ from actual, we could incur additional impairment charges and those charges could be material.
Income Taxes
We recognize deferred tax assets and liabilities for net operating loss and other credit carry forwards and the expected tax consequences of temporary differences between the tax basis of assets and liabilities and their reported amounts using enacted tax rates in effect for the year the differences are expected to reverse. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. The evaluation of deferred tax assets requires judgment in assessing the likely future tax consequences of events that have been recognized in our financial statements or tax returns, and future profitability by tax jurisdiction.
We provide a valuation allowance to reduce our deferred tax assets to the amount that is more likely than not to be realized. We have experienced losses in the past four years due to impairments of our intangible assets, increased professional fees in relation to our restatement and related remediation procedures for identified material weaknesses, and increased interest and bank fees. These losses have necessitated that we evaluate the sufficiency of our valuation allowance. Federal net operating loss as of December 31, 2017 can only be carried forward. We have $24.2 million and $8.9 million of U.S. federal and $195.0 million and $185.3 million of state net operating loss carryforwards available at December 31, 2017 and 2016, respectively. These carryforwards will be used to offset future income but may be limited by the change in ownership rules in Section 382 of the Internal Revenue Code. These net operating loss carryforwards will expire in varying amounts between 2018 and 2037. We have $68.1 million of net deferred tax assets as of December 31, 2017. We expect to generate income before taxes in future periods at a level that would allow for the full realization of the majority of our net deferred tax assets. We continue to maintain a valuation allowance of approximately $8.8 million as of December 31, 2017, against net deferred tax assets, primarily related to various state jurisdictions where we do not currently believe that the full realization of our deferred tax assets is more likely than not.
We evaluate our deferred tax assets quarterly to determine whether adjustments to the valuation allowance are appropriate in light of changes in facts or circumstances, such as changes in expected future pre-tax earnings, tax law, interactions with taxing authorities and developments in case law. In making this evaluation, we rely on our history of pre-tax earnings. Our material assumptions are our forecasts of future pre-tax earnings and the nature and timing of future deductions and income represented by the deferred tax assets and liabilities, all of which involve the exercise of significant judgment.
Although we believe our estimates are reasonable, the ultimate determination of the appropriate amount of valuation allowance involves significant judgment. If expected future taxable income is not achieved a larger valuation allowance against our deferred tax assets could be required and could be significant, which could materially increase our expenses in the period the allowance is recognized and materially adversely affect our results of operations and statement of financial condition.
We believe that our tax positions are consistent with applicable tax law, but certain positions may be challenged by taxing authorities. In the ordinary course of business, there are transactions and calculations where the ultimate tax outcome is uncertain. In addition, we are subject to periodic audits and examinations by the Internal Revenue Service and other state and local taxing authorities. In these cases, we record the financial statement effects of a tax position when it is more-likely-than-not, based on the technical merits, that the position will be sustained upon examination. We record the largest amount of tax benefit that is greater than fifty percent likely of being realized upon settlement with a taxing authority that has full knowledge of all relevant information. If not paid, the liability for uncertain tax positions is generally recorded as a reduction of income tax expense at the earlier of the period when the position is effectively settled or when the statute of limitations has expired. Although we believe our estimates are reasonable, actual results could differ from these estimates.
As a result of the Tax Act, the U.S. statutory tax rate was lowered from 35% to 21% effective January 1, 2018, among other changes. ASC Topic 740 requires us to recognize the effect of tax law changes in the period of enactment; therefore, we were required to revalue our deferred tax assets and liabilities in the period ended December 31, 2017 at the new rate. The SEC issued Staff Accounting Bulletin No. 118 (“SAB 118”) to address the application of GAAP in situations when a registrant does not have the necessary information available, prepared, or analyzed (including computations) in reasonable detail to complete the accounting for certain tax effects of the Tax Act. The ultimate impact may differ from this provisional amount, possibly materially, as a result of additional analysis, changes in interpretations and assumptions we have made, additional regulatory guidance that may be issued, and actions we may take as a result of the Tax Act. The accounting for the tax effects of the Tax Act will be completed in 2018.
Recent Accounting Pronouncements
Refer to the “Recent Accounting Pronouncements” section in Note B - “Significant Accounting Policies” in this Annual Report on Form 10-K for disclosure of recent accounting pronouncements that are either expected to have more than a minimal impact on our consolidated financial position and results of operation, or that we are still assessing to determine their impact.
Results of Operations - Year Ended December 31, 2017 Compared to Year Ended December 31, 2016
For the years ended December 31, 2017 and 2016, our consolidated results of operations were as follows:
|
|
|
For the Years Ended |
|
Percent |
| ||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
2017 v 2016 |
| ||
|
|
|
|
|
|
|
|
| ||
|
Net revenue |
|
$ |
1,040,769 |
|
$ |
1,042,054 |
|
(0.1 |
)% |
|
Material costs |
|
329,223 |
|
332,071 |
|
(0.9 |
)% | ||
|
Personnel costs |
|
361,090 |
|
363,537 |
|
(0.7 |
)% | ||
|
Other operating costs |
|
129,831 |
|
139,024 |
|
(6.6 |
)% | ||
|
General and administrative expenses |
|
110,078 |
|
107,224 |
|
2.7 |
% | ||
|
Professional accounting and legal fees |
|
36,239 |
|
41,233 |
|
(12.1 |
)% | ||
|
Depreciation and amortization |
|
39,259 |
|
44,887 |
|
(12.5 |
)% | ||
|
Impairment of intangible assets |
|
54,735 |
|
86,164 |
|
(36.5 |
)% | ||
|
Loss from operations |
|
(19,686 |
) |
(72,086 |
) |
(72.7 |
)% | ||
|
|
|
|
|
|
|
|
| ||
|
Interest expense, net |
|
57,688 |
|
45,199 |
|
27.6 |
% | ||
|
Extinguishment of debt |
|
— |
|
6,031 |
|
(100.0 |
)% | ||
|
Loss from continuing operations before income taxes |
|
(77,374 |
) |
(123,316 |
) |
(37.3 |
)% | ||
|
Provision (benefit) for income taxes |
|
27,297 |
|
(15,910 |
) |
(271.6 |
)% | ||
|
Loss from continuing operations |
|
(104,671 |
) |
(107,406 |
) |
(2.5 |
)% | ||
|
Income from discontinued operations, net of income taxes |
|
— |
|
935 |
|
(100.0 |
)% | ||
|
Net loss |
|
$ |
(104,671 |
) |
$ |
(106,471 |
) |
(1.7 |
)% |
Material costs, personnel costs and other operating costs reflect expenses we incur in connection with our delivery of care through our clinics and other patient care operations, or through the distribution of products and services, and exclude general and administrative activities. General and administrative activities reflect expenses we incur that are not directly related to the operation of our clinics or provision of products and services.
Due to the substantial amount we have incurred for professional accounting and legal services, we separately disclose these expenses within operating expenses. We have incurred these increases primarily in connection with the Restatement, the Investigation and in connection with our accounting and remediation activities associated with the material weaknesses. We currently anticipate that these expenses will remain significant in comparison to a typical level of expenditure at least through 2018.
During 2017 and 2016, our operating expenses as a percentage of net revenue were as follows:
|
|
|
For the Years Ended December 31, |
| ||
|
|
|
2017 |
|
2016 |
|
|
|
|
|
|
|
|
|
Material costs |
|
31.6 |
% |
31.9 |
% |
|
Personnel costs |
|
34.7 |
% |
34.9 |
% |
|
Other operating costs |
|
12.4 |
% |
13.2 |
% |
|
General and administrative expenses |
|
10.6 |
% |
10.3 |
% |
|
Professional accounting and legal fees |
|
3.5 |
% |
4.0 |
% |
|
Depreciation and amortization |
|
3.8 |
% |
4.3 |
% |
|
Impairment of intangible assets |
|
5.3 |
% |
8.3 |
% |
|
Operating expenses |
|
101.9 |
% |
106.9 |
% |
Due to the significance of disallowed revenue as discussed above in “Reimbursement Trends”, the rate of disallowed revenue experienced during the periods encompassed by this Annual Report on Form 10-K and to assist in evaluating the comparability of expense trends, the following table provides our adjusted gross revenue, disallowed revenue and net revenue for each year as well as our expenses as a percentage of adjusted gross revenue:
|
|
|
For the Years Ended December 31, |
| ||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
| ||
|
|
|
|
|
|
| ||
|
Net revenue |
|
$ |
1,040,769 |
|
$ |
1,042,054 |
|
|
Disallowed revenue |
|
36,962 |
|
49,387 |
| ||
|
Adjusted gross revenue |
|
$ |
1,077,731 |
|
$ |
1,091,441 |
|
|
|
|
|
|
|
| ||
|
Material costs |
|
30.5 |
% |
30.4 |
% | ||
|
Personnel costs |
|
33.5 |
% |
33.3 |
% | ||
|
Other operating costs |
|
12.1 |
% |
12.8 |
% | ||
|
General and administrative expenses |
|
10.2 |
% |
9.8 |
% | ||
|
Professional accounting and legal fees |
|
3.4 |
% |
3.8 |
% | ||
|
Depreciation and amortization |
|
3.6 |
% |
4.1 |
% | ||
|
Impairment of intangible assets |
|
5.1 |
% |
7.9 |
% | ||
|
Operating expenses |
|
98.4 |
% |
102.1 |
% | ||
During the previous two years, the number of patient care clinics and satellite locations we operated or leased have been as follows:
|
|
|
As of December 31, |
| ||
|
|
|
2017 |
|
2016 |
|
|
|
|
|
|
|
|
|
Patient care clinics |
|
682 |
|
706 |
|
|
Satellite locations |
|
112 |
|
115 |
|
|
Total |
|
794 |
|
821 |
|
Patient care clinics reflect locations that are licensed as a primary location to provide O&P services and which are fully staffed and open throughout a typical operating week. To facilitate patient convenience, we also operate satellite clinics. These are remote locations associated with a primary care clinic, utilized to see patients and are open for operation on less than a full-time basis during a typical operating week.
Net revenue. Net revenue for the year ended December 31, 2017 was $1,040.8 million, a decrease of $1.3 million, or 0.1%, from $1,042.1 million for the year ended December 31, 2016. Net revenue by operating segment, after elimination of intersegment activity, was as follows:
|
|
|
For the Years Ended December 31, |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
851,973 |
|
$ |
840,130 |
|
$ |
11,843 |
|
1.4 |
% |
|
Products & Services |
|
188,796 |
|
201,924 |
|
(13,128 |
) |
(6.5 |
)% | |||
|
Net revenue |
|
$ |
1,040,769 |
|
$ |
1,042,054 |
|
$ |
(1,285 |
) |
(0.1 |
)% |
Patient Care net revenue for the year ended December 31, 2017 was $852.0 million, an increase of $11.8 million or 1.4% from $840.1 million for the year ended December 31, 2016. During 2017, same clinic revenue increased $3.0 million, or 0.8% per day, excluding the favorable effects of improvements in our rates of disallowance. Disallowed Patient Care revenue decreased by $12.0 million in 2017 compared to 2016 due to initiatives and actions taken in 2015 and 2016 to address previous increases in disallowed revenue trends. Including the favorable effect of improvements in the rate of disallowances, same clinic revenue grew by $15.0 million, or 2.2% per day. During the year, our revenue from prosthetics increased to approximately 53% of our total Patient Care revenue as compared with approximately 52% in the prior year. In addition to underlying growth in prosthetic revenue, this change in mix was in part due to a reduction in revenue from certain off-the-shelf orthotics and diabetic shoes. Growth in same clinic revenue was partially offset by a $3.2 million decrease in revenue associated with clinic closures. During the year, we had a net reduction of 27 clinic locations due primarily to their marginal performance and profitability.
Products & Services net revenue for the year ended December 31, 2017 was $188.8 million, a decrease of $13.1 million, or 6.5%, from $201.9 million for the year ended December 31, 2016. Within the Products & Services segment, due primarily to customer cancellations (as discussed in “Products and Services Segment Trends” above), revenue from therapeutic services declined $11.0 million and sales of therapeutic equipment declined $3.6 million. These adverse trends were partially offset by a $1.5 million increase in other Products & Services net revenue, primarily related to our distribution of orthotic and prosthetic componentry to independent providers.
Material costs. Material costs for the year ended December 31, 2017 were $329.2 million, a decrease of $2.8 million, or 0.9%, from $332.1 million for the year ended December 31, 2016. Due primarily to favorable changes in our Patient Care segment product mix, total material costs as a percentage of net revenue decreased slightly from 31.9% in 2016 to 31.6% in 2017. Material costs by operating segment, after elimination of intersegment activity, were as follows:
|
|
|
For the Years Ended December 31, |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
251,899 |
|
$ |
256,012 |
|
$ |
(4,113 |
) |
(1.6 |
)% |
|
Products & Services |
|
77,324 |
|
76,059 |
|
1,265 |
|
1.7 |
% | |||
|
Material costs |
|
$ |
329,223 |
|
$ |
332,071 |
|
$ |
(2,848 |
) |
(0.9 |
)% |
Patient Care material costs decreased $4.1 million for 2017 compared to 2016, and decreased as a percent of net revenue from 30.5% in 2016 to 29.6% in 2017. This underlying reduction in cost of materials related primarily to favorable changes in our underlying mix of orthotic and prosthetic devices during the year. In particular, reductions in revenue relating to certain off-the-shelf orthotics and diabetic shoes, which carry higher relative costs of materials than custom orthotic and prosthetic devices, contributed to reductions in Patient Care material costs as a percentage of net revenue. Additionally, we also benefited from an average aggregate reduction in the cost of components utilized in the fabrication of devices.
Favorable reductions in the cost of materials for Patient Care were partially offset by increases in the relative cost of components distributed through our Products & Services segment. In this segment, material costs increased $1.3 million for 2017 compared to 2016, and reflected an underlying increase on a percent of net revenue basis, growing from 37.7% in 2016 to 41.0% in 2017, inclusive of the benefit of intersegment cost allocations to the Patient Care segment. These increases in materials costs arose primarily due to the loss of certain of our larger, higher margin, independent O&P provider accounts which were offset by growth in sales to other customers with lower margins.
Personnel costs. Personnel costs for the year ended December 31, 2017 were $361.1 million, a decrease of $2.4 million, or 0.7%, from $363.5 million for the year ended December 31, 2016. Personnel costs by operating segment were as follows:
|
|
|
For the Years Ended December 31, |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
312,695 |
|
$ |
315,892 |
|
$ |
(3,197 |
) |
(1.0 |
)% |
|
Products & Services |
|
48,395 |
|
47,645 |
|
750 |
|
1.6 |
% | |||
|
Personnel costs |
|
$ |
361,090 |
|
$ |
363,537 |
|
$ |
(2,447 |
) |
(0.7 |
)% |
Personnel costs for our Patient Care segment for the year ended December 31, 2017 were $312.7 million, a decrease of $3.2 million, or 1.0%, from $315.9 million for the year ended December 31, 2016. Patient Care salaries, benefits and payroll taxes decreased $13.1 million from the closure and restructuring of clinics, partially offset by $9.9 million increase in incentive compensation expense. Personnel costs for our Products & Services segment for the year ended December 31, 2017 were $48.4 million, an increase of $0.8 million, or 1.6%, from $47.6 million for the year ended December 31, 2016. The increase in Products & Services personnel costs were from higher incentive compensation.
Other operating costs. Other operating costs for the year ended December 31, 2017 were $129.8 million, a decrease of $9.2 million, or 6.6%, from $139.0 million for the year ended December 31, 2016. Bad debt expense decreased $4.3 million due to improvements in our collection efforts, rent and related office and occupancy costs decreased $3.7 million from the closure and restructuring of clinics, telephone and data transmission costs decreased $2.1 million and other expenses decreased $0.8 million. These decreases were partially offset by $1.7 million increase in professional fees.
General and administrative expenses. General and administrative expenses for the year ended December 31, 2017 were $110.1 million, an increase of $2.9 million, or 2.7%, from $107.2 million for the year ended December 31, 2016. Incentive compensation expense increased $3.6 million, partially offset by a $1.6 million decrease in salaries, benefits and payroll taxes. The increase in incentive compensation was due in part to a discretionary employee bonus and 401(k) match awarded based on our performance during the year. Other office and related expenses increased $0.9 million.
Professional accounting and legal fees. Professional accounting and legal fees for the year ended December 31, 2017 were $36.2 million, a decrease of $5.0 million, or 12.1%, from $41.2 million for the year ended December 31, 2016. Legal fees decreased $8.6 million due to costs associated with the prior Investigation and Restatement, advisory and other fees decreased $0.1 million, partially offset by a $3.7 million increase in audit related fees.
Depreciation and amortization. Depreciation and amortization for the year ended December 31, 2017 was $39.3 million, a decrease of $5.6 million, or 12.5%, from $44.9 million for the year ended December 31, 2016. The decrease included $4.1 million lower amortization due to fully amortized customer list intangibles for prior acquisitions, $1.2 million lower depreciation of program equipment either sold or fully depreciated, $0.4 million lower amortization of tradenames, non-compete agreements and asset retirement obligations and $0.2 million lower depreciation of software. These decreases were partially offset by $0.3 million increase in depreciation of leasehold improvements.
Impairment of intangible assets. As more fully explained in Note H - “Goodwill and Intangible Assets” to our consolidated financial statements in this Annual Report on Form 10-K, due to the continued decline in our Therapeutic and Distribution reporting units forecasted outlook, we recorded an impairment of intangible assets of $54.7 million for the year ended December 31, 2017 compared with $86.2 million for the year ended December 31, 2016. See the “Products & Services Segment Trends” section in this Management’s Discussion and Analysis for information regarding the business environment and outlook of our Products & Services segment. In 2017, we recorded a goodwill impairment charge of $53.3 million, of which $32.8 million related to our Therapeutic reporting unit and $20.5 million related to our Distribution reporting unit, and other intangible asset impairment of $1.4 million related to our Therapeutic reporting unit’s indefinite life tradename. In 2016, we recorded a goodwill impairment charge of $86.0 million, of which $64.9 million related to our Therapeutic reporting unit and $21.1 million related to our Distribution reporting unit. In addition, we recorded other intangible asset impairment of $0.2 million related to our Therapeutic reporting unit’s indefinite life tradename.
Interest expense, net. Interest expense for the year ended December 31, 2017 was $57.7 million, an increase of $12.5 million, or 27.6%, from $45.2 million for the year ended December 31, 2016. The increase was primarily due to $10.7 million higher interest expense associated with our debt refinancing in the third quarter of 2016 and $2.8 million related to increased interest rates and revolver activity, partially offset by a net decrease of $1.0 million related primarily to reductions in seller notes, escheat liabilities and lease obligations.
Extinguishment of debt. In August 2016, we entered into a new Term B Credit Agreement providing for a new $280.0 million senior unsecured term loan facility. We used approximately $205.3 million of the proceeds from the Term B Credit Agreement to redeem our $200 million in senior notes (“Senior Notes”) which were scheduled to mature in November 2018. As a result, we recorded a loss on extinguishment of debt of $6.0 million for year ended December 31, 2016. We incurred no similar expense in 2017.
Provision (benefit) for income taxes. An income tax expense of $27.3 million was recognized for the year ended December 31, 2017, compared to a benefit of $15.9 million for the year ended December 31, 2016. The increase in tax expense was primarily due to a decrease in the Federal tax rate as a result of the Tax Act which decreased tax-affected net deferred tax asset balances by $35.0 million and therefore increased deferred tax expenses significantly. The decrease in losses from continuing operations before income taxes also contributed to the increase in tax expense. Our effective tax rate from continuing operations was (35.3)% and 12.9% for 2017 and 2016, respectively. The effective tax rates differ from the statutory rate primarily due to tax rate change impact on the deferred balance and other non-deductible expenses.
Income from discontinued operations, net of income taxes. Income from discontinued operations for the year ended December 31, 2016 was $0.9 million which related to contingent consideration received in 2016 from the disposal of Dosteon in 2015.
Net loss. Our net loss for year ended December 31, 2017 was $104.7 million as compared to a net loss of $106.5 million for year ended December 31, 2016.
Results of Operations - Quarterly Periods December 31, 2017 Compared to Quarterly Periods December 31, 2016
Quarterly results of operations for 2017 and 2016 were as follows:
|
|
|
For the Quarters Ended, |
| ||||||||||||||||||||||
|
|
|
Unaudited |
| ||||||||||||||||||||||
|
|
|
2017 |
|
2016 |
| ||||||||||||||||||||
|
(dollars in thousands) |
|
Dec 31 |
|
Sep 30 |
|
Jun 30 |
|
Mar 31 |
|
Dec 31 |
|
Sep 30 |
|
Jun 30 |
|
Mar 31 |
| ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
Net revenue |
|
$ |
285,736 |
|
$ |
257,966 |
|
$ |
263,386 |
|
$ |
233,681 |
|
$ |
281,053 |
|
$ |
260,084 |
|
$ |
264,456 |
|
$ |
236,461 |
|
|
Material costs |
|
88,816 |
|
82,345 |
|
83,657 |
|
74,405 |
|
86,963 |
|
85,437 |
|
82,971 |
|
76,700 |
| ||||||||
|
Personnel costs |
|
95,239 |
|
90,065 |
|
87,831 |
|
87,955 |
|
96,880 |
|
89,113 |
|
88,406 |
|
89,138 |
| ||||||||
|
Other operating costs |
|
32,097 |
|
33,184 |
|
31,861 |
|
32,689 |
|
36,154 |
|
34,139 |
|
31,970 |
|
36,761 |
| ||||||||
|
General and administrative expenses |
|
33,557 |
|
25,540 |
|
25,411 |
|
25,570 |
|
23,770 |
|
25,726 |
|
30,170 |
|
27,558 |
| ||||||||
|
Professional accounting and legal fees |
|
7,224 |
|
7,844 |
|
8,521 |
|
12,650 |
|
9,829 |
|
9,023 |
|
10,692 |
|
11,689 |
| ||||||||
|
Depreciation and amortization |
|
9,665 |
|
9,632 |
|
9,825 |
|
10,137 |
|
10,160 |
|
11,339 |
|
11,660 |
|
11,728 |
| ||||||||
|
Impairment of intangible assets |
|
54,735 |
|
— |
|
— |
|
— |
|
86,164 |
|
— |
|
— |
|
— |
| ||||||||
|
Operating expenses |
|
321,333 |
|
248,610 |
|
247,106 |
|
243,406 |
|
349,920 |
|
254,777 |
|
255,869 |
|
253,574 |
| ||||||||
|
(Loss) income from operations |
|
(35,597 |
) |
9,356 |
|
16,280 |
|
(9,725 |
) |
(68,867 |
) |
5,307 |
|
8,587 |
|
(17,113 |
) | ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
Interest expense, net |
|
14,491 |
|
15,097 |
|
14,091 |
|
14,009 |
|
13,734 |
|
12,809 |
|
9,818 |
|
8,838 |
| ||||||||
|
Extinguishment of debt |
|
— |
|
— |
|
— |
|
— |
|
— |
|
6,041 |
|
(10 |
) |
— |
| ||||||||
|
(Loss) income from continuing operations before income taxes |
|
(50,088 |
) |
(5,741 |
) |
2,189 |
|
(23,734 |
) |
(82,601 |
) |
(13,543 |
) |
(1,221 |
) |
(25,951 |
) | ||||||||
|
Provision (benefit) for income taxes |
|
34,325 |
|
(1,580 |
) |
552 |
|
(6,000 |
) |
(1,488 |
) |
(5,687 |
) |
(321 |
) |
(8,414 |
) | ||||||||
|
(Loss) income from continuing operations |
|
(84,413 |
) |
(4,161 |
) |
1,637 |
|
(17,734 |
) |
(81,113 |
) |
(7,856 |
) |
(900 |
) |
(17,537 |
) | ||||||||
|
(Loss) income from discontinued operations, net of income taxes |
|
— |
|
— |
|
— |
|
— |
|
(15 |
) |
378 |
|
572 |
|
— |
| ||||||||
|
Net (loss) income |
|
$ |
(84,413 |
) |
$ |
(4,161 |
) |
$ |
1,637 |
|
$ |
(17,734 |
) |
$ |
(81,128 |
) |
$ |
(7,478 |
) |
$ |
(328 |
) |
$ |
(17,537 |
) |
During these periods, our operating expenses as a percentage of net revenue were as follows:
|
|
|
For the Quarters Ended, |
| ||||||||||||||
|
|
|
Unaudited |
| ||||||||||||||
|
|
|
2017 |
|
2016 |
| ||||||||||||
|
|
|
Dec 31 |
|
Sep 30 |
|
Jun 30 |
|
Mar 31 |
|
Dec 31 |
|
Sep 30 |
|
Jun 30 |
|
Mar 31 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Material costs |
|
31.1 |
% |
31.9 |
% |
31.8 |
% |
31.8 |
% |
30.9 |
% |
32.8 |
% |
31.4 |
% |
32.4 |
% |
|
Personnel costs |
|
33.3 |
% |
34.9 |
% |
33.3 |
% |
37.6 |
% |
34.5 |
% |
34.3 |
% |
33.4 |
% |
37.7 |
% |
|
Other operating costs |
|
11.3 |
% |
13.0 |
% |
12.2 |
% |
14.2 |
% |
12.8 |
% |
13.1 |
% |
12.2 |
% |
15.5 |
% |
|
General and administrative expenses |
|
11.7 |
% |
9.9 |
% |
9.6 |
% |
10.9 |
% |
8.5 |
% |
9.9 |
% |
11.4 |
% |
11.7 |
% |
|
Professional accounting and legal fees |
|
2.5 |
% |
3.0 |
% |
3.2 |
% |
5.4 |
% |
3.5 |
% |
3.5 |
% |
4.0 |
% |
4.9 |
% |
|
Depreciation and amortization |
|
3.4 |
% |
3.7 |
% |
3.7 |
% |
4.3 |
% |
3.6 |
% |
4.4 |
% |
4.4 |
% |
5.0 |
% |
|
Impairment of intangible assets |
|
19.2 |
% |
— |
% |
— |
% |
— |
% |
30.7 |
% |
— |
% |
— |
% |
— |
% |
|
Operating expenses |
|
112.5 |
% |
96.4 |
% |
93.8 |
% |
104.2 |
% |
124.5 |
% |
98.0 |
% |
96.8 |
% |
107.2 |
% |
The following table provides our adjusted gross revenue, disallowed revenue and net revenue for each quarter of 2017 and 2016, as well as our expenses as a percentage of adjusted gross revenue:
|
|
|
For the Quarters Ended, |
| ||||||||||||||||||||||
|
|
|
Unaudited |
| ||||||||||||||||||||||
|
|
|
2017 |
|
2016 |
| ||||||||||||||||||||
|
(dollars in thousands) |
|
Dec 31 |
|
Sep 30 |
|
Jun 30 |
|
Mar 31 |
|
Dec 31 |
|
Sep 30 |
|
Jun 30 |
|
Mar 31 |
| ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
Net revenue |
|
$ |
285,736 |
|
$ |
257,966 |
|
$ |
263,386 |
|
$ |
233,681 |
|
$ |
281,053 |
|
$ |
260,084 |
|
$ |
264,456 |
|
$ |
236,461 |
|
|
Disallowed revenue |
|
8,711 |
|
9,557 |
|
11,578 |
|
7,116 |
|
12,193 |
|
14,233 |
|
13,243 |
|
9,718 |
| ||||||||
|
Adjusted gross revenue |
|
$ |
294,447 |
|
$ |
267,523 |
|
$ |
274,964 |
|
$ |
240,797 |
|
$ |
293,246 |
|
$ |
274,317 |
|
$ |
277,699 |
|
$ |
246,179 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
Material costs |
|
30.2 |
% |
30.8 |
% |
30.4 |
% |
30.9 |
% |
29.7 |
% |
31.1 |
% |
29.9 |
% |
31.2 |
% | ||||||||
|
Personnel costs |
|
32.3 |
% |
33.7 |
% |
31.9 |
% |
36.5 |
% |
33.0 |
% |
32.5 |
% |
31.8 |
% |
36.2 |
% | ||||||||
|
Other operating costs |
|
10.8 |
% |
12.4 |
% |
11.7 |
% |
13.6 |
% |
12.2 |
% |
12.5 |
% |
11.4 |
% |
14.9 |
% | ||||||||
|
General and administrative expenses |
|
11.4 |
% |
9.5 |
% |
9.2 |
% |
10.6 |
% |
8.1 |
% |
9.4 |
% |
10.9 |
% |
11.2 |
% | ||||||||
|
Professional accounting and legal fees |
|
2.5 |
% |
2.9 |
% |
3.1 |
% |
5.3 |
% |
3.4 |
% |
3.3 |
% |
3.9 |
% |
4.7 |
% | ||||||||
|
Depreciation and amortization |
|
3.3 |
% |
3.6 |
% |
3.6 |
% |
4.2 |
% |
3.5 |
% |
4.1 |
% |
4.2 |
% |
4.8 |
% | ||||||||
|
Impairment of intangible assets |
|
18.6 |
% |
— |
% |
— |
% |
— |
% |
29.4 |
% |
— |
% |
— |
% |
— |
% | ||||||||
|
Operating expenses |
|
109.1 |
% |
92.9 |
% |
89.9 |
% |
101.1 |
% |
119.3 |
% |
92.9 |
% |
92.1 |
% |
103.0 |
% | ||||||||
Results of operations - three months ended March 31, 2017 compared to three months ended March 31, 2016
Net revenue. Net revenue for the three months ended March 31, 2017 was $233.7 million, a decrease of $2.8 million, or 1.2%, from $236.5 million for the three months ended March 31, 2016. Net revenue by operating segment, after elimination of intersegment activity was as follows:
|
|
|
For the Three Months Ended March 31, |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
187,637 |
|
$ |
188,073 |
|
$ |
(436 |
) |
(0.2 |
)% |
|
Products & Services |
|
46,044 |
|
48,388 |
|
(2,344 |
) |
(4.8 |
)% | |||
|
Net revenue |
|
$ |
233,681 |
|
$ |
236,461 |
|
$ |
(2,780 |
) |
(1.2 |
)% |
Patient Care net revenue decreased $0.4 million, or 0.2% for the three months ended March 31, 2017 compared to the same period in the prior year. Excluding the effect of changes in disallowed revenue, same clinic Patient Care revenue decreased $1.7 million for the three months ended March 31, 2017 compared to the same quarter in the prior year, reflecting an underlying decline in same clinic revenue per day of 0.9%. This decrease was partially offset by a $2.2 million improvement in disallowed revenue due to initiatives and actions taken from 2015 through 2016 to address previous increases in disallowed revenue trends. Including improvements in disallowed revenue, same clinic revenue increased by $0.5 million and reflected a growth rate of 0.3% per day. This growth was offset by the effect of clinic closures which reflected decreased revenue of $0.9 million as compared with the prior year.
Products & Services net revenue decreased $2.3 million or 4.8% for the three months ended March 31, 2017 to $46.0 million from $48.4 million for the three months ended March 31, 2016. The net revenue decrease in the Products & Services segment was comprised of $3.1 million decrease in net revenue from therapeutic services, which related primarily to client cancellations, partially offset by $0.8 million increase from the distribution of O&P componentry to independent providers.
Material costs. Material costs for the three months ended March 31, 2017 were $74.4 million, a decrease of $2.3 million or 3.0%, from $76.7 million for the three months ended March 31, 2016. Due primarily to favorable changes in our Patient Care segment product mix, total material costs as a percentage of net revenue decreased slightly from 32.4% in 2016 to 31.8% in 2017. Material costs by operating segment, after elimination of intersegment activity, were as follows:
|
|
|
For the Three Months Ended March 31, |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
56,433 |
|
$ |
59,586 |
|
$ |
(3,153 |
) |
(5.3 |
)% |
|
Products & Services |
|
17,972 |
|
17,114 |
|
858 |
|
5.0 |
% | |||
|
Material costs |
|
$ |
74,405 |
|
$ |
76,700 |
|
$ |
(2,295 |
) |
(3.0 |
)% |
Patient Care material costs decreased $3.2 million for the three months ended March 31, 2017 compared to the three months ended March 31, 2016, and decreased as a percent of net revenue from 31.7% in 2016 to 30.1% in 2017. This underlying reduction in cost of materials related primarily to favorable changes in our underlying mix of orthotic and prosthetic devices during the year. In particular, reductions in revenue relating to certain off-the-shelf orthotics and diabetic shoes, which carry higher relative costs of materials than custom orthotic and prosthetic devices, contributed to reductions in Patient Care material costs as a percentage of net revenue. Additionally, we also benefited from an average aggregate reduction in the cost of components utilized in the fabrication of devices.
Products & Services material costs increased $0.9 million for the three months ended March 31, 2017 compared to the three months end March 31, 2016 and reflected an underlying increase on a percent of revenue basis, growing from 35.4% in 2016 to 39.0% in 2017. This increase in material costs arose primarily due to the loss of certain of our larger, higher margin, independent O&P provider accounts which were offset by growth in sales to other customers with lower margins.
Personnel costs. Personnel costs for the three months ended March 31, 2017 was $88.0 million, a decrease of $1.2 million, or 1.3% from $89.1 million for the three months ended March 31, 2016. Personnel costs by operating segment were as follows:
|
|
|
For the Three Months Ended March 31, |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
75,515 |
|
$ |
76,616 |
|
$ |
(1,101 |
) |
(1.4 |
)% |
|
Products & Services |
|
12,440 |
|
12,522 |
|
(82 |
) |
(0.7 |
)% | |||
|
Personnel costs |
|
$ |
87,955 |
|
$ |
89,138 |
|
$ |
(1,183 |
) |
(1.3 |
)% |
For the three months ended March 31, 2017, personnel costs for our Patient Care segment decreased $1.1 million or 1.4% to $75.5 million from $76.6 million, for the three months ended March 31, 2016. Patient Care salaries and payroll taxes decreased $3.7 million from the closure and restructuring of clinics, partially offset by $2.6 million increase in incentive compensation and benefits expense. Personnel costs in the Products & Services segment decreased $0.1 million, or 0.7% for the three months ended March 31, 2017 compared to the three months ended March 31, 2016.
Other operating costs. Other operating costs for the three months ended March 31, 2017 was $32.7 million, a decrease of $4.1 million, or 11.1% from $36.8 million for the three months ended March 31, 2016. Bad debt expense decreased $1.9 million, rent and related office and occupancy costs decreased $1.9 million, telephone and data transmission costs decreased $0.5 million and other expenses decreased $0.6 million, partially offset by $0.8 million increased professional fees.
General and administrative expenses. General and administrative expenses for the three months ended March 31, 2017 was $25.6 million, a decrease of $2.0 million, or 7.2% from $27.6 million for the three months ended March 31, 2016. The decrease of $2.0 million included decreases in compliance training of $0.5 million, insurance expense of $0.5 million, professional fees of $0.3 million, loss on disposal of assets of $0.3 million and other decreases of $0.6 million, partially offset by an increase in salaries, bonus expense, payroll taxes and benefits of $0.2 million.
Professional accounting and legal fees. Professional accounting and legal fees for the three months ended March 31, 2017 were $12.7 million, an increase of $1.0 million from $11.7 million for the three months ended March 31, 2016. Advisory and other fees increased $2.7 million and audit related fees increased $2.4 million, partially offset by $4.1 million decrease in legal fees. Legal fees decreased from costs associated with the prior Investigation and Restatement.
Depreciation and amortization. Depreciation and amortization for the three months ended March 31, 2017 was $10.1 million, a decrease of $1.6 million, or 13.6%, from $11.7 million for the three months ended March 31, 2016. The decrease included lower amortization of $1.7 million as a result of fully amortized assets, partially offset by $0.1 million higher depreciation for leasehold improvements.
Interest expense, net. Interest expense increased to $14.0 million from $8.8 million for the three months ended March 31, 2017 compared with the three months ended March 31, 2016. The $5.2 million increase was from increased borrowings and the higher interest rates on our credit facility related to the refinancing in 2016.
Benefit for income taxes. The benefit for income taxes for the three months ended March 31, 2017 was $6.0 million, or 25.3% of loss from continuing operations before taxes, compared to a benefit of $8.4 million, or 32.4% of loss from continuing operations before taxes for the three months ended March 31, 2016. The effective tax rate consists principally of the 35% federal statutory tax rate in addition to state income taxes, less permanent tax differences. The decreased benefit was largely driven by a decrease in the loss from continuing operations before taxes and a decrease in the estimated effective tax rate. The decrease in the estimated effective tax rate was driven by a decrease in the annual forecasted loss from continuing operations before taxes and an increase in non-deductible expenses.
Net loss. Net loss for the three months ended March 31, 2017 was $17.7 million compared to a net loss of $17.5 million for the three months ended March 31, 2016.
Results of operations - three months ended June 30, 2017 compared to three months ended June 30, 2016
Net revenue. Net revenue for the three months ended June 30, 2017 was $263.4 million, a decrease of $1.1 million, or 0.4% from $264.5 million for the three months ended June 30, 2016. Net revenue by operating segment, after elimination of intersegment activity was as follows:
|
|
|
For the Three Months Ended June 30, |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
216,221 |
|
$ |
214,264 |
|
$ |
1,957 |
|
0.9 |
% |
|
Products & Services |
|
47,165 |
|
50,192 |
|
(3,027 |
) |
(6.0 |
)% | |||
|
Net revenue |
|
$ |
263,386 |
|
$ |
264,456 |
|
$ |
(1,070 |
) |
(0.4 |
)% |
Patient Care net revenue increased $2.0 million, or 0.9% for the three months ended June 30, 2017 compared to the same period in the prior year. Excluding the favorable effect of $1.6 million in improved disallowances, same clinic revenue increased $1.3 million, or 0.6% per day during the quarter. Including the favorable effects of improvements in disallowed revenue, same clinic revenue grew by $2.9 million, or 1.4% per day. Decreased revenue from clinic closures was $0.9 million during the quarter.
Products & Services net revenue decreased $3.0 million or 6.0% for the three months ended June 30, 2017 to $47.2 million from $50.2 million for the three months ended June 30, 2016. The net revenue decrease in the Products & Services segment was comprised of $2.6 million decrease in net revenue from therapeutic services, resulting primarily from customer cancellations, and a $0.4 million decrease from the distribution of O&P componentry to independent providers.
Material costs. Material costs for the three months ended June 30, 2017 was $83.7 million, an increase of $0.7 million, or 0.8%, from $83.0 million for the three months ended June 30, 2016. Material costs by operating segment, after elimination of intersegment activity, were as follows:
|
|
|
For the Three Months Ended June 30, |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
64,245 |
|
$ |
63,061 |
|
$ |
1,184 |
|
1.9 |
% |
|
Products & Services |
|
19,412 |
|
19,910 |
|
(498 |
) |
(2.5 |
)% | |||
|
Material costs |
|
$ |
83,657 |
|
$ |
82,971 |
|
$ |
686 |
|
0.8 |
% |
Due primarily to unfavorable changes in our Patient Care segment product mix, Patient Care material costs increased $1.2 million, or 1.9%, for the three months ended June 30, 2017 compared to the three months ended June 30, 2016, and increased slightly as a percent of net revenue from 29.4% in 2016 to 29.7% in 2017.
Products & Services material costs decreased $0.5 million for the three months ended June 30, 2017 compared to the three months end June 30, 2016, but increased on a percent of net revenue basis, growing from 39.7% in 2016 to 41.2% in 2017. The increase in materials costs as a percent of net revenue arose primarily due to the loss of certain of our larger, higher margin, independent O&P provider accounts which were partially offset by sales to other customers with lower margins.
Personnel costs. Personnel costs for the three months ended June 30, 2017 was $87.8 million, a decrease of $0.6 million, or 0.7% from $88.4 million for the three months ended June 30, 2016. Personnel costs by operating segment were as follows:
|
|
|
For the Three Months Ended June 30, |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
75,906 |
|
$ |
77,149 |
|
$ |
(1,243 |
) |
(1.6 |
)% |
|
Products & Services |
|
11,925 |
|
11,257 |
|
668 |
|
5.9 |
% | |||
|
Personnel costs |
|
$ |
87,831 |
|
$ |
88,406 |
|
$ |
(575 |
) |
(0.7 |
)% |
For the three months ended June 30, 2017, personnel costs for our Patient Care segment decreased $1.2 million or 1.6% to $75.9 million from $77.1 million, for the three months ended June 30, 2016. Patient Care salaries, benefits, casual labor, commissions and payroll taxes decreased $3.3 million from the closure and restructuring of clinics, partially offset by $2.1 million increase in incentive compensation expense. Personnel costs in the Products & Services segment increased $0.7 million, or 5.9%, for the three months ended June 30, 2017 compared to the three months ended June 30, 2016 from higher payroll taxes, casual labor and incentive compensation expense.
Other operating costs. Other operating costs for the three months ended June 30, 2017 was $31.9 million, a decrease of $0.1 million, or 0.3% from $32.0 million for the three months ended June 30, 2016.
General and administrative expenses. General and administrative expenses for the three months ended June 30, 2017 was $25.4 million, a decrease of $4.8 million, or 15.8% from $30.2 million for the three months ended June 30, 2016. The decrease of $4.8 million included decreases in incentive compensation costs and casual labor of $2.8 million, professional fees of $1.2 million and travel, facility and other office related expenses of $0.8 million.
Professional accounting and legal fees. Professional accounting and legal fees for the three months ended June 30, 2017 were $8.5 million, a decrease of $2.2 million, or 20.3% from $10.7 million for the three months ended June 30, 2016. Legal fees decreased $1.6 million, advisory and other fees decreased $0.9 million, partially offset by $0.3 million increase in audit related fees.
Depreciation and amortization. Depreciation and amortization for the three months ended June 30, 2017 was $9.8 million, a decrease of $1.8 million, or 15.7%, from $11.7 million for the three months ended June 30, 2016. The decrease included lower amortization of $1.4 million from fully amortized customer list intangibles for prior acquisitions and decreases of $0.4 million in therapeutic services depreciation due to sales of equipment previously leased to customers and assets becoming fully depreciated.
Interest expense, net. Interest expense for the three months ended June 30, 2017 increased to $14.1 million from $9.8 million for the three months ended June 30, 2016, an increase of $4.3 million resulting from higher borrowings on our credit facility and higher interest rates compared with the prior year period.
Provision (benefit) for income taxes. The provision for income taxes for the three months ended June 30, 2017 was $0.6 million or 25.2% of income from continuing operations before taxes, compared to a benefit for income taxes of $0.3 million, or 26.3% of loss from continuing operations before taxes for the three months ended June 30, 2016. The effective tax rate consists principally of the 35% federal statutory tax rate in addition to state income taxes, less permanent tax differences. The increase in tax expense was largely driven by increased income from continuing operations before taxes coupled with a decrease in the estimated effective tax rate. The decrease in the estimated effective tax rate resulted from increased annual forecasted income from continuing operations before taxes and an increase in non-deductible expenses.
Income from discontinued operations, net of income taxes. For the three months ended June 30, 2016, net income from discontinued Dosteon operations was $0.6 million which related to contingent consideration received in 2016 from the disposal in 2015.
Net income (loss). For the three months ended June 30, 2017, we incurred net income of $1.6 million compared with net loss of $0.3 million for the three months ended June 30, 2016.
Results of operations - three months ended September 30, 2017 compared to three months ended September 30, 2016
Net revenue. Net revenue for the three months ended September 30, 2017 was $258.0 million, a decrease of $2.1 million, or 0.8% from $260.1 million for the three months ended September 30, 2016. Net revenue by operating segment, after elimination of intersegment activity was as follows:
|
|
|
For the Three Months Ended |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
210,637 |
|
$ |
208,374 |
|
$ |
2,263 |
|
1.1 |
% |
|
Products & Services |
|
47,329 |
|
51,710 |
|
(4,381 |
) |
(8.5 |
)% | |||
|
Net revenue |
|
$ |
257,966 |
|
$ |
260,084 |
|
$ |
(2,118 |
) |
(0.8 |
)% |
Patient Care net revenue increased $2.3 million, or 1.1%, for the three months ended September 30, 2017 compared to the same period in the prior year. Excluding the favorable effects of $4.5 million of improvements in disallowed revenue, Patient Care same clinic revenue declined $1.4 million. Due to there being one less business day in the quarter as compared with the prior year, same clinic revenue grew at a rate of 0.9% per day. Same clinic revenue growth was adversely affected in the
quarter due to the temporary suspension of operations in certain clinics and other disruption of patient volumes caused by the two hurricanes which affected Texas and Florida. Including the favorable effects of improvements in disallowed revenue, same clinic revenue grew by $3.1 million, or 3.1% per day, during the quarter. This same clinic growth was partially offset by $0.8 million in reduced revenue associated with clinic closures.
Products & Services net revenue decreased $4.4 million or 8.5% for the three months ended September 30, 2017 to $47.3 million from $51.7 million for the three months ended September 30, 2016. The net revenue decrease in the Products & Services segment was comprised of $3.1 million decrease in net revenue from therapeutic services, caused primarily by customer cancellations, and $1.3 million decrease from the distribution of O&P componentry to independent providers.
Material costs. Material costs for the three months ended September 30, 2017 were $82.3 million, a decrease of $3.1 million, or 3.6%, from $85.4 million for the three months ended September 30, 2016. Due primarily to favorable changes in our Patient Care segment product mix, total material costs as a percentage of net revenue decreased slightly from 32.8% in 2016 to 31.9% in 2017. Material costs by operating segment, after elimination of intersegment activity, were as follows:
|
|
|
For the Three Months Ended |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
62,745 |
|
$ |
64,595 |
|
$ |
(1,850 |
) |
(2.9 |
)% |
|
Products & Services |
|
19,600 |
|
20,842 |
|
(1,242 |
) |
(6.0 |
)% | |||
|
Material costs |
|
$ |
82,345 |
|
$ |
85,437 |
|
$ |
(3,092 |
) |
(3.6 |
)% |
Patient Care material costs decreased $1.9 million for the three months ended September 30, 2017 compared to the three months ended September 30, 2016 and reflected an underlying decrease on a percent of net revenue basis, from 31.0% in 2016 to 29.8% in 2017. This underlying reduction in cost of materials related primarily to favorable changes in our underlying mix of orthotic and prosthetic devices during the year. In particular, reductions in revenue relating to certain off-the-shelf orthotics and diabetic shoes, which carry higher relative costs of materials than custom orthotic and prosthetic devices, contributed to reductions in Patient Care material costs as a percentage of net revenue. Additionally, we benefited from an average aggregate reduction in the cost of components utilized in the fabrication of devices.
Products & Services material costs decreased $1.2 million for the three months ended September 30, 2017 compared to the three months end September 30, 2016 but reflected an underlying increase on a percent of net revenue basis, growing from 40.3% in 2016 to 41.4% in 2017. This increase in material costs arose primarily due to the loss of certain of our larger, higher margin, independent O&P provider accounts, which were partially offset by sales to other customers with lower margins.
|
|
|
For the Three Months Ended |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
78,161 |
|
$ |
77,466 |
|
$ |
695 |
|
0.9 |
% |
|
Products & Services |
|
11,904 |
|
11,647 |
|
257 |
|
2.2 |
% | |||
|
Personnel costs |
|
$ |
90,065 |
|
$ |
89,113 |
|
$ |
952 |
|
1.1 |
% |
Personnel costs. Personnel costs for the three months ended September 30, 2017 was $90.1 million, an increase of $1.0 million, or 1.1%, from $89.1 million for the three months ended September 30, 2016. Personnel costs for our Patient Care segment increased $0.7 million, or 0.9% to $78.2 million for the three months ended September 30, 2017 from $77.5 million, for the three months ended September 30, 2016. Patient Care incentive compensation expense increased $2.4 million, partially offset by a decrease in personnel costs of $1.7 million from the closure and restructuring of clinics. Personnel costs
in the Products & Services segment increased $0.3 million, or 2.2% for the three months ended September 30, 2017 compared to the three months ended September 30, 2016. Products & Services bonus expense increased $0.4 million, partially offset by a net decrease in other incentive compensations of $0.1 million.
Other operating costs. Other operating costs for the three months ended September 30, 2017 was $33.2 million, a decrease of $1.0 million, or 2.8% from $34.1 million for the three months ended September 30, 2016 primarily due to a $1.0 million reduction in telephone and data transmission costs.
General and administrative expenses. General and administrative expenses for the three months ended September 30, 2017 was $25.5 million, a decrease of $0.2 million, or 0.7% from $25.7 million for the three months ended September 30, 2016. The decrease included $1.9 million in incentive compensation costs and professional fees, partially offset by $1.7 million of increased other expenses.
Professional accounting and legal fees. Professional accounting and legal fees for the three months ended September 30, 2017 were $7.8 million, a decrease of $1.2 million, or 13.1% from $9.0 million for the three months ended September 30, 2016. Legal fees decreased $1.1 million, advisory and other fees decreased $1.0 million, partially offset by $0.9 million increase in audit related fees.
Depreciation and amortization. Depreciation and amortization for the three months ended September 30, 2017 was $9.6 million, a decrease of $1.7 million, or 15.1% from $11.3 million for the three months ended September 30, 2016. Amortization of customer list intangibles decreased $1.2 million due to fully amortized customer list intangibles for prior acquisitions, depreciation of therapeutic services program equipment decreased $0.4 million and amortization of trade name decreased $0.1 million.
Interest expense, net. Interest expense for the three months ended September 30, 2017 was $15.1 million, an increase of $2.3 million from $12.8 million for the three months ended September 30, 2016 primarily due to higher borrowings on our credit facility and higher interest rates compared with the prior year period.
Extinguishment of debt. In August, 2016, we entered into a new Term B Credit Agreement providing for a new $280.0 million senior unsecured term loan facility. We used approximately $205.3 million of the proceeds from the Term B Credit Agreement to redeem our Senior Notes. As a result of the issuance of the Term B Credit Agreement and repayment of the Senior Notes, we recorded a loss on extinguishment of debt of $6.0 million for the three months ended September 30. 2016. There was no debt extinguishment in the three months ended September 30, 2017.
Benefit for income taxes. The benefit for income taxes for the three months ended September 30, 2017 was $1.6 million, or 27.5% of loss from continuing operations before taxes, compared to a tax benefit of $5.7 million, or 42.0% of loss from continuing operations before taxes, for the three months ended September 30, 2016. The effective tax rate consists principally of the 35% federal statutory tax rate in addition to state income taxes coupled with permanent tax differences. The decreased tax benefit was largely driven by a decrease in the loss from continuing operations before taxes, and a decrease in the estimated effective tax rate. The decrease in the estimated effective tax rate resulted from a decrease in the annual forecasted loss from continuing operations before taxes and an increase in non-deductible expenses.
Income from discontinued operations, net of income taxes. Net income from discontinued Dosteon operations was $0.4 million for the three months ended September 30, 2016 which related to contingent consideration received in 2016 from the disposal in 2015.
Net loss. For the three months ended September 30, 2017, we incurred a net loss of $4.2 million compared with net loss of $7.5 million for the three months ended September 30, 2016.
Results of operations - three months ended December 31, 2017 compared to three months ended December 31, 2016
Net revenue. Net revenue for the three months ended December 31, 2017 was $285.7 million, an increase of $4.7 million or 1.7%, from $281.1 million for the three months ended December 31, 2016. Net revenue by operating segment, after elimination of intersegment activity, was as follows:
|
|
|
For the Three Months Ended |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
237,478 |
|
$ |
229,418 |
|
$ |
8,060 |
|
3.5 |
% |
|
Products & Services |
|
48,258 |
|
51,635 |
|
(3,377 |
) |
(6.5 |
)% | |||
|
Net revenue |
|
$ |
285,736 |
|
$ |
281,053 |
|
$ |
4,683 |
|
1.7 |
% |
Patient Care net revenue increased $8.1 million, or 3.5% to $237.5 million for the three months ended December 31, 2017 from $229.4 million compared to the same period in the prior year. Excluding the favorable effects of $3.6 million in reduced disallowances, same clinic revenue grew by $4.9 million, or 2.1% per day. Including this favorable change in disallowances, same clinic revenue grew by $8.5 million, or 3.7% per day. This revenue growth was partially offset by $0.4 million in decreased revenue associated with clinic closures.
Products & Services net revenue decreased $3.4 million, or 6.5% to $48.3 million for the three months ended December 31, 2017 from $51.6 million compared to the same period in the prior year. Revenue from sales of therapeutic equipment declined by $3.0 million and therapeutic services declined by $2.8 million and sales of therapeutic equipment during the quarter. In the fourth quarter of 2016, our sales of therapeutic equipment were higher than the levels we normally experience due primarily to our sale of the therapeutic equipment we used to service to certain large customers to those customers at the time of their cancellation of services with us. These declines in Products and Services revenue were partially offset by increased revenue of $2.4 million from the distribution of O&P components to independent providers.
Material costs. Material costs for the three months ended December 31, 2017 was $88.8 million, an increase of $1.9 million, or 2.1%, from $87.0 million for the three months ended December 31, 2016. Material costs by operating segment, after elimination of intersegment activity, were as follows:
|
|
|
For the Three Months Ended |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
68,477 |
|
$ |
68,770 |
|
$ |
(293 |
) |
(0.4 |
)% |
|
Products & Services |
|
20,339 |
|
18,193 |
|
2,146 |
|
11.8 |
% | |||
|
Material costs |
|
$ |
88,816 |
|
$ |
86,963 |
|
$ |
1,853 |
|
2.1 |
% |
Patient Care material costs decreased $0.3 million for the three months ended December 31, 2017 as compared to the three months ended December 31, 2016, and decreased as a percent of net revenue from 30.0% in 2016 to 28.8% in 2017. This underlying reduction in cost of materials related primarily to favorable changes in our underlying mix of orthotic and prosthetic devices during the year. In particular, reductions in revenue relating to certain off-the-shelf orthotics and diabetic shoes, which carry higher relative costs of materials than custom orthotic and prosthetic devices, contributed to reductions in Patient Care material costs as a percentage of net revenue. Additionally, we also benefited from an average aggregate reduction in the cost of components utilized in the fabrication of devices.
Products & Services material costs increased $2.1 million for the three months ended December 31, 2017 as compared to the three months ended December 31, 2016, and reflected an underlying increase on a percent of net revenue basis, growing from 35.2% in 2016 to 42.1% in 2017. These increases in material costs arose primarily due to the loss of certain of our larger, higher margin, independent O&P provider accounts which were partially offset by sales to other customers with lower margins.
Personnel costs. Personnel costs for the three months ended December 31, 2017 was $95.2 million, a decrease of $1.6 million from $96.9 million for the three months ended December 31, 2016. Personnel costs by operating segment were as follows:
|
|
|
For the Three Months Ended |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
83,113 |
|
$ |
84,661 |
|
$ |
(1,548 |
) |
(1.8 |
)% |
|
Products & Services |
|
12,126 |
|
12,219 |
|
(93 |
) |
(0.8 |
)% | |||
|
Personnel costs |
|
$ |
95,239 |
|
$ |
96,880 |
|
$ |
(1,641 |
) |
(1.7 |
)% |
Patient Care personnel costs decreased $1.5 million to $83.1 million for the three months ended December 31, 2017 compared to the same period in 2016 primarily due to decreases of $2.3 million in salaries and $3.9 million in benefits, partially offset by increases of $4.2 million in incentive compensation and $0.5 million in taxes. Products and Services decreased slightly for the three months ended December 31, 2017 compared to the same period in 2016.
Other operating costs. Other operating costs for the three months ended December 31, 2017 was $32.1 million, a decrease of $4.1 million, or 11.2% from $36.2 million for the three months ended December 31, 2016 primarily due to decreases of $3.6 million in bad debt expense and $0.5 million in other operating costs.
General and administrative expenses. General and administrative expenses for the three months ended December 31, 2017 was $33.6 million, an increase of $9.8 million, or 41.2% from $23.8 million for the three months ended December 31, 2016 primarily due to increases of $7.7 million for incentive compensation costs, $1.4 million for salaries, benefits and other personnel related costs, $0.6 million for other expenses, $0.3 million for rent and other occupancy costs, $0.3 million for office expenses and $0.1 million for travel and meals costs. These increases were partially offset by decreases of $0.6 million of professional fees.
Professional accounting and legal fees. Professional accounting and legal fees for the three months ended December 31, 2017 were $7.2 million, a decrease of $2.6 million, or 26.5% from $9.8 million for the three months ended December 31, 2016 primarily due to a decrease of $1.8 million in legal fees and $0.8 million in accounting related fees.
Depreciation and amortization. Depreciation and amortization for the three months ended December 31, 2017 was $9.7 million, a decrease of $0.5 million, or 4.9% from $10.2 million for the three months ended December 31, 2016. Selling therapeutic equipment to customers who discontinued our services and therapeutic assets becoming fully depreciated, reduced depreciation $0.2 million. Amortization of customer list intangibles decreased $0.1 million. Depreciation of software and other assets decreased $0.2 million.
Impairment of intangible assets. As more fully explained in Note H - “Goodwill and Intangible Assets” to our consolidated financial statements in this Annual Report on Form 10-K, for the three months ended December 31, 2017, we recorded impairment of intangible assets of $54.7 million. We recorded a total goodwill impairment charge of $53.3 million, of which $32.8 million related to our Therapeutic reporting unit and $20.5 million related to our Distribution reporting unit. For the three months ended December 31, 2017, we recorded intangible asset impairment of $1.4 million related to our Therapeutic reporting unit’s indefinite life tradename. For the three months ended December 31, 2016, we recorded impairment of
intangible assets of $86.2 million. We recorded a total goodwill impairment charge of $86.0 million, of which $64.9 million related to our Therapeutic reporting unit and $21.1 million related to our Distribution reporting unit. For the three months ended December 31, 2016, we recorded intangible asset impairment of $0.2 million related to our Therapeutic reporting unit’s indefinite life tradename.
Interest expense, net. Interest expense for the three months ended December 31, 2017 increased to $14.5 million from $13.7 million for the three months ended December 31, 2016. An increase in interest expense of $0.8 million in the fourth quarter resulted from higher borrowings on our revolving credit.
Provision (benefit) for income taxes. The provision for income taxes for the three months ended December 31, 2017 was $34.3 million, or (68.5)% of loss from continuing operations before taxes, compared to a benefit of $1.5 million, or 1.8% of loss from continuing operations before taxes for the three months ended December 31, 2016. The effective tax rate consists principally of the 35% federal statutory tax rate in addition to state income taxes, less permanent tax differences, and the revaluation of deferred tax balances due to the federal rate change enacted during the period. The increase in tax expense was largely driven by the impact of Federal tax rate change from 35% to 21% as a result of the Tax Act, which significantly decreased deferred tax balances; a decrease in goodwill impairment and an increase in other non-deductible expenses; coupled with change in uncertain tax positions and change in valuation allowance.
Net loss. For the three months ended December 31, 2017, we incurred a net loss of $84.4 million compared with a net loss of $81.1 million for the three months ended December 31, 2016.
Results of Operations - Year-to-Date Periods 2017 Compared to 2016
Our year-to-date results of operations for 2017 and the comparative periods in 2016 were as follows:
|
|
|
For the Six Months Ended |
|
Percent |
|
For the Nine Months Ended |
|
Percent |
| ||||||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
2017 v 2016 |
|
2017 |
|
2016 |
|
2017 v 2016 |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Net revenue |
|
$ |
497,067 |
|
$ |
500,917 |
|
(0.8 |
)% |
$ |
755,033 |
|
$ |
761,001 |
|
(0.8 |
)% |
|
Material costs |
|
158,062 |
|
159,671 |
|
(1.0 |
)% |
240,407 |
|
245,108 |
|
(1.9 |
)% | ||||
|
Personnel costs |
|
175,786 |
|
177,544 |
|
(1.0 |
)% |
265,851 |
|
266,657 |
|
(0.3 |
)% | ||||
|
Other operating costs |
|
64,550 |
|
68,731 |
|
(6.1 |
)% |
97,734 |
|
102,870 |
|
(5.0 |
)% | ||||
|
General and administrative expenses |
|
50,981 |
|
57,728 |
|
(11.7 |
)% |
76,521 |
|
83,454 |
|
(8.3 |
)% | ||||
|
Professional accounting and legal fees |
|
21,171 |
|
22,381 |
|
(5.4 |
)% |
29,015 |
|
31,404 |
|
(7.6 |
)% | ||||
|
Depreciation and amortization |
|
19,962 |
|
23,388 |
|
(14.6 |
)% |
29,594 |
|
34,727 |
|
(14.8 |
)% | ||||
|
Income (loss) from operations |
|
6,555 |
|
(8,526 |
) |
(176.9 |
)% |
15,911 |
|
(3,219 |
) |
(594.3 |
)% | ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Interest expense, net |
|
28,100 |
|
18,656 |
|
50.6 |
% |
43,197 |
|
31,465 |
|
37.3 |
% | ||||
|
Extinguishment of debt |
|
— |
|
(10 |
) |
(100.0 |
)% |
— |
|
6,031 |
|
(100.0 |
)% | ||||
|
Loss from continuing operations before income taxes |
|
(21,545 |
) |
(27,172 |
) |
(20.7 |
)% |
(27,286 |
) |
(40,715 |
) |
(33.0 |
)% | ||||
|
Benefit for income taxes |
|
(5,448 |
) |
(8,735 |
) |
(37.6 |
)% |
(7,028 |
) |
(14,422 |
) |
(51.3 |
)% | ||||
|
Loss from continuing operations |
|
(16,097 |
) |
(18,437 |
) |
(12.7 |
)% |
(20,258 |
) |
(26,293 |
) |
(23.0 |
)% | ||||
|
Income from discontinued operations, net of income taxes |
|
— |
|
572 |
|
(100.0 |
)% |
— |
|
950 |
|
(100.0 |
)% | ||||
|
Net loss |
|
$ |
(16,097 |
) |
$ |
(17,865 |
) |
(9.9 |
)% |
$ |
(20,258 |
) |
$ |
(25,343 |
) |
(20.1 |
)% |
During these periods, our operating expenses as a percentage of net revenue were as follows:
|
|
|
For the Six Months Ended |
|
For the Nine Months Ended |
| ||||
|
|
|
2017 |
|
2016 |
|
2017 |
|
2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Material costs |
|
31.8 |
% |
31.9 |
% |
31.8 |
% |
32.2 |
% |
|
Personnel costs |
|
35.4 |
% |
35.4 |
% |
35.2 |
% |
35.0 |
% |
|
Other operating costs |
|
12.9 |
% |
13.7 |
% |
13.1 |
% |
13.5 |
% |
|
General and administrative expenses |
|
10.3 |
% |
11.5 |
% |
10.1 |
% |
11.0 |
% |
|
Professional accounting and legal fees |
|
4.3 |
% |
4.5 |
% |
3.8 |
% |
4.1 |
% |
|
Depreciation and amortization |
|
4.0 |
% |
4.7 |
% |
3.9 |
% |
4.6 |
% |
|
Operating expenses |
|
98.7 |
% |
101.7 |
% |
97.9 |
% |
100.4 |
% |
The following table provides our adjusted gross revenue, disallowed revenue and net revenue for each period, as well as our expenses as a percentage of adjusted gross revenue:
|
|
|
For the Six Months Ended |
|
For the Nine Months Ended |
| ||||||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
2017 |
|
2016 |
| ||||
|
|
|
|
|
|
|
|
|
|
| ||||
|
Net revenue |
|
$ |
497,067 |
|
$ |
500,917 |
|
$ |
755,033 |
|
$ |
761,001 |
|
|
Disallowed revenue |
|
18,694 |
|
22,961 |
|
28,251 |
|
37,194 |
| ||||
|
Adjusted gross revenue |
|
$ |
515,761 |
|
$ |
523,878 |
|
$ |
783,284 |
|
$ |
798,195 |
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Material costs |
|
30.6 |
% |
30.5 |
% |
30.7 |
% |
30.7 |
% | ||||
|
Personnel costs |
|
34.1 |
% |
33.9 |
% |
33.9 |
% |
33.4 |
% | ||||
|
Other operating costs |
|
12.5 |
% |
13.0 |
% |
12.5 |
% |
12.8 |
% | ||||
|
General and administrative expenses |
|
9.9 |
% |
11.0 |
% |
9.8 |
% |
10.5 |
% | ||||
|
Professional accounting and legal fees |
|
4.1 |
% |
4.3 |
% |
3.7 |
% |
3.9 |
% | ||||
|
Depreciation and amortization |
|
3.9 |
% |
4.5 |
% |
3.8 |
% |
4.4 |
% | ||||
|
Operating expenses |
|
95.1 |
% |
97.2 |
% |
94.4 |
% |
95.7 |
% | ||||
Results of operations - six months ended June 30, 2017 compared to six months ended June 30, 2016
Net revenue. Net revenue for the six months ended June 30, 2017 was $497.1 million, a decrease of $3.9 million, or 0.8%, from $500.9 million for the six months ended June 30, 2016. Net revenue by operating segment, after elimination of intersegment activity was as follows:
|
|
|
For the Six Months Ended June 30, |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
403,858 |
|
$ |
402,337 |
|
$ |
1,521 |
|
0.4 |
% |
|
Products & Services |
|
93,209 |
|
98,580 |
|
(5,371 |
) |
(5.4 |
)% | |||
|
Net revenue |
|
$ |
497,067 |
|
$ |
500,917 |
|
$ |
(3,850 |
) |
(0.8 |
)% |
Patient Care net revenue increased $1.5 million or 0.4% to $403.9 million for the six months ended June 30, 2017 from $402.3 million for the six months ended June 30, 2016. Excluding the favorable effect of a $3.8 million reduction in year-to-date disallowed revenue, same clinic revenue decreased by $0.4 million, or 0.1% per day. Including the effect of favorable disallowance trends, same clinic revenue increased by $3.4 million, or 0.9% per day. This revenue growth was partially offset by $1.9 million in reduced revenue from clinic closures and consolidation.
Products & Services net revenue for the six months ended June 30, 2017 was $93.2 million, a decrease of $5.4 million, or 5.4%, from $98.6 million for the six months ended June 30, 2016. Products & Services revenue from therapeutic services declined $5.7 million due primarily to the effects of customer cancellations of these services. These declines were partially offset by $0.3 million in increased revenue associated with the distribution of O&P componentry to independent providers.
Material costs. Material costs for the six months ended June 30, 2017 were $158.1 million, a decrease of $1.6 million, or 1.0%, from the six months ended June 30, 2016, and reflected a slight decrease as a percent of net revenue from 31.9% in 2016 to 31.8% in 2017. Material costs by operating segment, after elimination of intersegment activity, were as follows:
|
|
|
For the Six Months Ended June 30, |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
120,678 |
|
$ |
122,647 |
|
$ |
(1,969 |
) |
(1.6 |
)% |
|
Products & Services |
|
37,384 |
|
37,024 |
|
360 |
|
1.0 |
% | |||
|
Material costs |
|
$ |
158,062 |
|
$ |
159,671 |
|
$ |
(1,609 |
) |
(1.0 |
)% |
Patient Care material costs decreased $2.0 million for the six months ended June 30, 2017 as compared to the same period 2016, and decreased as a percent of net revenue from 30.5% in 2016 to 29.9% in 2017. This underlying reduction in cost of materials related primarily to favorable changes in our underlying mix of orthotic and prosthetic devices during the year. In particular, reductions in revenue relating to certain off-the-shelf orthotics and diabetic shoes, which carry higher relative costs of materials than custom orthotic and prosthetic devices, contributed to reductions in Patient Care material costs as a percentage of net revenue. Additionally, we also benefited from an average aggregate reduction in the cost of components utilized in the fabrication of devices.
Products & Services material costs increased $0.4 million for the six months ended June 30, 2017 as compared to the six months ended June 30, 2016, and reflected an underlying increase on a percent of net revenue basis, growing from 37.6% in 2016 to 40.1% in 2017. These increases in material costs arose primarily due to the loss of certain of our larger, higher margin, independent O&P provider accounts which were partially offset by sales to other customers with lower margins.
Personnel costs. Personnel costs for the six months ended June 30, 2017 was $175.8 million, a decrease of $1.8 million or 1.0%, from $177.5 million for the six months ended June 30, 2016. Personnel costs by operating segment were as follows:
|
|
|
For the Six Months Ended June 30, |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
151,421 |
|
$ |
153,765 |
|
$ |
(2,344 |
) |
(1.5 |
)% |
|
Products & Services |
|
24,365 |
|
23,779 |
|
586 |
|
2.5 |
% | |||
|
Personnel costs |
|
$ |
175,786 |
|
$ |
177,544 |
|
$ |
(1,758 |
) |
(1.0 |
)% |
Personnel costs in our Patient Care segment decreased $2.3 million, or 1.5% for the six months ended June 30, 2017 as compared to the same period in the prior year due primarily to decreases of $4.8 million in salaries and casual labor, $1.0 million in taxes and $0.3 million in commissions, partially offset by increases of $3.2 million in incentive compensation, $0.4 million in benefits and $0.2 million in other personnel costs. The Products & Services segment’s personnel costs increased $0.6 million, or 2.5% for the six months ended June 30, 2017 compared to the same period in the prior year due primarily to increases of $0.2 million in salaries, $0.1 million in incentive compensation, $0.1 million in benefits and $0.4 million in taxes partially offset by $0.2 million in lower equity compensation.
Other operating costs. Other operating costs for the six months ended June 30, 2017 was $64.6 million, a decrease of $4.2 million, or 6.1%, from $68.7 million for the six months ended June 30, 2016 due primarily to decreases of $1.4 million in rent, $1.3 million in bad debt expense, $0.8 million in telephone and data transmission costs, $0.7 million in occupancy cost,$0.4 million in office costs, and $0.7 million in other costs, partially offset by $1.1 million increased professional fees.
General and administrative expenses. General and administrative expenses for the six months ended June 30, 2017 was $51.0 million, a decrease of $6.7 million, or 11.7%, from $57.7 million for the six months ended June 30, 2016 due primarily to decreases of $2.5 million for salaries, bonus expense, payroll taxes and other personnel costs, $1.7 million for advertising, office and other expenses, $1.4 million for professional fees, $0.9 million for travel and meals, $0.3 million for credit card and other fees and $0.2 million for professional education, partially offset by $0.3 million increase in rent, utilities and other occupancy costs.
Professional accounting and legal fees. Professional accounting and legal fees in the first six months of 2017 were $21.2 million, a decrease of $1.2 million, or 5.4% from $22.4 million for the six months ended June 30, 2016. Legal costs decreased by $5.7 million, partially offset by increased accounting, advisory and other professional fees of $4.5 million in 2017.
Depreciation and amortization. Depreciation and amortization for the six months ended June 30, 2017 was $20.0 million, a decrease of $3.4 million, or 14.6%, from $23.4 million for the six months ended June 30, 2016. Amortization of customer list intangibles decreased $2.9 million due to fully amortized customer list intangibles associated with prior acquisitions coupled with a decrease of $0.6 million in depreciation from equipment sold to customers. These decreases were partially offset by $0.1 million increase in depreciation and amortization on all other fixed assets.
Interest expense, net. Interest expense for the six months ended June 30, 2017 was $28.1 million, an increase of $9.4 million from $18.7 million for the six months ended June 30, 2016. This increase resulted from an increase in average outstanding balances on our debt and higher interest rates during the six months ended June 30, 2017 compared with the same period in the prior year.
Benefit for income taxes. The benefit for income taxes for the six months ended June 30, 2017 was $5.4 million or 25.3% of loss from continuing operations, compared to a benefit of $8.7 million for the six months ended June 30, 2016, or 32.1% of loss from continuing operations. The effective tax rate consists principally of the 35% federal statutory tax rate in addition to state income taxes, less permanent tax differences. The decreased tax benefit was largely driven by a decrease in the loss from continuing operations before taxes and a decrease in the estimated effective tax. The decrease in the estimated effective tax rate was driven by a decrease in the annual forecasted loss from continuing operations before taxes and an increase in non-deductible expenses.
Income from discontinued operations, net of income taxes. For the six months ended June 30, 2016, net income from discontinued Dosteon operations was $0.6 million which related to contingent consideration received in 2016 from the disposal in 2015.
Net loss. For the six months ended June 30, 2017, we incurred a net loss of $16.1 million compared with a net loss of $17.9 million for the six months ended June 30, 2016.
Results of operations - nine months ended September 30, 2017 compared to nine months ended September 30, 2016
Net revenue. Net revenue for the nine months ended September 30, 2017 was $755.0 million, a decrease of $6.0 million, or 0.8%, from $761.0 million for the nine months ended September 30, 2016. Net revenue by operating segment, after elimination of intersegment activity was as follows:
|
|
|
For the Nine Months Ended |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
614,495 |
|
$ |
610,711 |
|
$ |
3,784 |
|
0.6 |
% |
|
Products & Services |
|
140,538 |
|
150,290 |
|
(9,752 |
) |
(6.5 |
)% | |||
|
Net revenue |
|
$ |
755,033 |
|
$ |
761,001 |
|
$ |
(5,968 |
) |
(0.8 |
)% |
Patient Care net revenue increased $3.8 million or 0.6% to $614.5 million for the nine months ended September 30, 2017 from $610.7 million for the nine months ended September 30, 2016. Excluding the favorable effect of an $8.3 million year-to-date reduction in disallowed revenue, same clinic revenue decreased by $1.8 million. Due to there being one less business day in the year-to-date period for 2017, same clinic revenue growth on a per day basis was slightly positive, reflecting a 0.2% per day rate of growth. Including the favorable effect of improvements in disallowed revenue, same clinic revenue increased by $6.5 million, or 1.6% per day. Same clinic revenue growth was partially offset by a decrease of $2.7 million in revenue associated primarily with clinic closures.
Products & Services net revenue decreased $9.8 million or 6.5% for nine months ended September 30, 2017 to $140.5 million from $150.3 million for nine months ended September 30, 2016. Products & Services revenue from therapeutic services declined by $8.8 million for the year-to-date, primarily due to customer cancellations. We also experienced a decline of $1.0 million in revenue derived from the distribution of O&P componentry to independent providers.
Material costs. Material costs for the nine months ended September 30, 2017 was $240.4 million, a decrease of $4.7 million, or 1.9%, from $245.1 million for the nine months ended September 30, 2016, and reflected a slight decrease as a percent of net revenue from 32.2% in 2016 to 31.8% in 2017. Material costs by operating segment, after elimination of intersegment activity, were as follows:
|
|
|
For the Nine Months Ended |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
183,423 |
|
$ |
187,242 |
|
$ |
(3,819 |
) |
(2.0 |
)% |
|
Products & Services |
|
56,984 |
|
57,866 |
|
(882 |
) |
(1.5 |
)% | |||
|
Material costs |
|
$ |
240,407 |
|
$ |
245,108 |
|
$ |
(4,701 |
) |
(1.9 |
)% |
Patient Care material costs decreased $3.8 million for the nine months ended September 30, 2017 as compared to the same period 2016, and decreased as a percent of net revenue from 30.7% in 2016 to 29.8% in 2017. This underlying reduction in cost of materials related primarily to favorable changes in our underlying mix of orthotic and prosthetic devices during the year. In particular, reductions in revenue relating to certain off-the-shelf orthotics and diabetic shoes, which carry higher relative costs of materials than custom orthotic and prosthetic devices, contributed to reductions in Patient Care material costs as a percentage of net revenue. Additionally, we also benefited from an average aggregate reduction in the cost of components utilized in the fabrication of devices.
Products & Services material costs decreased $0.9 million for the nine months ended September 30, 2017 as compared to the nine months ended September 30, 2016, but reflected an underlying increase on a percent of net revenue basis, growing from
38.5% in 2016 to 40.5% in 2017. These increases in materials costs arose primarily due to the loss of certain of our larger, higher margin, independent O&P provider accounts which were partially offset by sales to other customers with lower margins.
Personnel costs. Personnel costs for the nine months ended September 30, 2017 was $265.9 million, a decrease of $0.8 million, or 0.3% from $266.7 million for the nine months ended September 30, 2016. Personnel costs by operating segment were as follows:
|
|
|
For the Nine Months Ended |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2017 |
|
2016 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
229,582 |
|
$ |
231,231 |
|
$ |
(1,649 |
) |
(0.7 |
)% |
|
Products & Services |
|
36,269 |
|
35,426 |
|
843 |
|
2.4 |
% | |||
|
Personnel costs |
|
$ |
265,851 |
|
$ |
266,657 |
|
$ |
(806 |
) |
(0.3 |
)% |
Patient Care segment personnel costs decreased $1.6 million, or 0.7% to $229.6 million for the nine months ended September 30, 2017 compared with $231.2 million in the same period in the prior year due primarily to $7.2 million in lower salaries partially offset by $5.6 million in higher incentive compensation. Personnel costs in the Products & Services segment increased $0.8 million, or 2.4% to $36.3 million for the nine months ended September 30, 2017 compared with $35.4 million in the same period in the prior year due primarily to increases of $0.5 million in incentive compensation, $0.4 million in taxes and $0.2 million in benefits, partially offset by $0.3 million in decreased equity based compensation.
Other operating costs. Other operating costs for the nine months ended September 30, 2017 was $97.7 million, a decrease of $5.1 million, or 5.0%, from $102.9 million for the nine months ended September 30, 2016 due primarily to decreases of $1.8 million in telephone and data charges, $1.2 million in rent, $1.0 million in other costs, $0.7 million in bad debt expense, $0.7 million in occupancy costs, $0.7 million in office and utilities costs, $0.3 million in advertising and $0.1 million in professional education, partially offset by $1.4 million in increased professional fees and $0.1 million in credit card and other fees.
General and administrative expenses. General and administrative expenses for the nine months ended September 30, 2017 was $76.5 million, a decrease of $6.9 million, or 8.3% from $83.5 million for the nine months ended September 30, 2016 due primarily to decreases of $3.5 million in salaries, $2.2 million in incentive compensation, $1.8 million in professional fees and $0.9 million in travel and meals, partially offset by increases of $1.0 million in equity based compensation, $0.3 million in payroll taxes and $0.2 million in rent expense.
Professional accounting and legal fees. Professional accounting and legal fees for the nine months ended September 30, 2017 were $29.0 million, a decrease of $2.4 million, or 7.6% from $31.4 million for the nine months ended September 30, 2016 due primarily to $6.8 million in lower legal fees partially offset by $4.4 million in increased accounting related fees.
Depreciation and amortization. Depreciation and amortization for the nine months ended September 30, 2017 was $29.6 million, a decrease of $5.1 million, or 14.8%, from $34.7 million for the nine months ended September 30, 2016. Amortization of customer list intangibles decreased $4.0 million, primarily due to a change in the estimated useful lives in the fourth quarter of 2015. Depreciation in therapeutic services from selling equipment to customers and equipment becoming fully depreciated resulted in overall depreciation expense decreasing $0.9 million. Amortization of non-compete agreement and trade names decreased $0.2 million.
Interest expense, net. Interest expense for the nine months ended September 30, 2017 was $43.2 million, an increase of $11.7 million from $31.5 million for the nine months ended September 30, 2016. The increase resulted from an increase in average outstanding balances on our debt and higher interest rates.
Extinguishment of debt. Fees of $6.0 million were incurred in the nine months ended September 30, 2016 in connection with the redemption of our Senior Notes. There was no similar expense incurred in the nine months ended September 30, 2017. See Note N - “Long-Term Debt” to our consolidated financial statements in this Annual Report on Form 10-K for further details.
Benefit for income taxes. The benefit for income taxes for the nine months ended September 30, 2017 was $7.0 million, or 25.8% of loss from continuing operations before taxes compared to a benefit of $14.4 million, or 35.4% of loss from continuing operations before taxes for the nine months ended September 30, 2016. The effective tax rate consists principally of the 35% federal statutory tax rate in addition to state income taxes, less permanent tax differences. The decreased benefit was largely driven by decreased loss from continuing operations before taxes and decreased estimated effective tax rate. The decrease in the estimated effective tax rate was driven by a decrease in the annual forecasted loss from continuing operations before taxes and an increase in non-deductible expenses.
Income from discontinued operations, net of income taxes. For the nine months ended September 30, 2016, net income from discontinued operations, net of income taxes was $1.0 million and related to contingent consideration received in 2016 from the disposal in 2015.
Net loss. For the nine months ended September 30, 2017, we incurred a net loss of $20.3 million compared with a net loss of $25.3 million for the nine months ended September 30, 2016.
Results of Operations - Year Ended December 31, 2016 Compared to Year Ended December 31, 2015
From 2015 through 2016, our annual consolidated results of operations were as follows:
|
|
|
For the Years Ended |
|
Percent |
| ||||
|
(dollars in thousands) |
|
2016 |
|
2015 |
|
2016 v 2015 |
| ||
|
|
|
|
|
|
|
|
| ||
|
Net revenue |
|
$ |
1,042,054 |
|
$ |
1,067,172 |
|
(2.4 |
)% |
|
Material costs |
|
332,071 |
|
336,283 |
|
(1.3 |
)% | ||
|
Personnel costs |
|
363,537 |
|
367,094 |
|
(1.0 |
)% | ||
|
Other operating costs |
|
139,024 |
|
140,839 |
|
(1.3 |
)% | ||
|
General and administrative expenses |
|
107,224 |
|
111,761 |
|
(4.1 |
)% | ||
|
Professional accounting and legal fees |
|
41,233 |
|
28,647 |
|
43.9 |
% | ||
|
Depreciation and amortization |
|
44,887 |
|
46,343 |
|
(3.1 |
)% | ||
|
Impairment of intangible assets |
|
86,164 |
|
385,807 |
|
(77.7 |
)% | ||
|
Loss from operations |
|
(72,086 |
) |
(349,602 |
) |
(79.4 |
)% | ||
|
|
|
|
|
|
|
|
| ||
|
Interest expense, net |
|
45,199 |
|
29,892 |
|
51.2 |
% | ||
|
Extinguishment of debt |
|
6,031 |
|
7,237 |
|
(16.7 |
)% | ||
|
Loss from continuing operations before income taxes |
|
(123,316 |
) |
(386,731 |
) |
(68.1 |
)% | ||
|
Benefit for income taxes |
|
(15,910 |
) |
(67,614 |
) |
(76.5 |
)% | ||
|
Loss from continuing operations |
|
(107,406 |
) |
(319,117 |
) |
(66.3 |
)% | ||
|
Income (loss) from discontinued operations, net of income taxes |
|
935 |
|
(7,974 |
) |
(111.7 |
)% | ||
|
Net loss |
|
$ |
(106,471 |
) |
$ |
(327,091 |
) |
(67.4 |
)% |
Material costs, personnel costs and other operating costs reflect expenses we incur in connection with our delivery of care through our clinics and other patient care operations, or distribution of products and services, and exclude expenses incurred in connection with general and administrative activities. General and administrative expenses reflect expenses we incur in the general management and administration of our businesses that are not directly attendant to the operation of our clinics or provision of products and services.
Due to the substantial amount and increase in the expenses we incur for professional accounting and legal services, we separately disclose these expenses within operating expenses. We have incurred increases in these expenses primarily in connection with the Restatement, the Investigation and in connection with our accounting and remedial activities associated with the material weaknesses. We currently anticipate that these expenses will remain significant in comparison to a normal level of expenditure at least through 2018.
When the financial statement carrying amount of a long-lived asset or asset group exceeds its fair value and is not recoverable, an asset impairment is recognized. The significant decline in the trading value of our stock impaired assets in the Patient Care, Distribution and Therapeutic reporting units. Impairment losses are separately disclosed within (Loss) income from operations in this Annual Report on Form 10-K.
During the years 2015 through 2016, our operating expenses as a percentage of net revenue were as follows:
|
|
|
For the Years Ended December 31, |
| ||
|
|
|
2016 |
|
2015 |
|
|
|
|
|
|
|
|
|
Material costs |
|
31.9 |
% |
31.5 |
% |
|
Personnel costs |
|
34.9 |
% |
34.4 |
% |
|
Other operating costs |
|
13.2 |
% |
13.2 |
% |
|
General and administrative expenses |
|
10.3 |
% |
10.5 |
% |
|
Professional accounting and legal fees |
|
4.0 |
% |
2.7 |
% |
|
Depreciation and amortization |
|
4.3 |
% |
4.3 |
% |
|
Impairment of intangible assets |
|
8.3 |
% |
36.2 |
% |
|
Operating expenses |
|
106.9 |
% |
132.8 |
% |
Due to the significance of disallowed revenue as discussed above in Reimbursement Trends, the rate of disallowed revenue experienced during the periods encompassed by this Annual Report on Form 10-K and to assist in evaluating the comparability of expense trends, the following table provides our adjusted gross revenue, disallowed revenue and net revenue for each year as well as our expenses as a percentage of adjusted gross revenue:
|
|
|
For the Years Ended December 31, |
| ||||
|
(dollars in thousands) |
|
2016 |
|
2015 |
| ||
|
|
|
|
|
|
| ||
|
Net revenue |
|
$ |
1,042,054 |
|
$ |
1,067,172 |
|
|
Disallowed revenue |
|
49,387 |
|
60,669 |
| ||
|
Adjusted gross revenue |
|
$ |
1,091,441 |
|
$ |
1,127,841 |
|
|
|
|
|
|
|
| ||
|
Material costs |
|
30.4 |
% |
29.8 |
% | ||
|
Personnel costs |
|
33.3 |
% |
32.6 |
% | ||
|
Other operating costs |
|
12.8 |
% |
12.5 |
% | ||
|
General and administrative expenses |
|
9.8 |
% |
9.9 |
% | ||
|
Professional accounting and legal fees |
|
3.8 |
% |
2.5 |
% | ||
|
Depreciation and amortization |
|
4.1 |
% |
4.1 |
% | ||
|
Impairment of intangible assets |
|
7.9 |
% |
34.2 |
% | ||
|
Operating expenses |
|
102.1 |
% |
125.6 |
% | ||
During the previous two years, the number of patient care clinics and satellite clinics we operated have been as follows:
|
|
|
As of December 31, |
| ||
|
|
|
2016 |
|
2015 |
|
|
|
|
|
|
|
|
|
Patient Care Clinics |
|
706 |
|
721 |
|
|
Satellite Clinics |
|
115 |
|
116 |
|
|
Total Clinics |
|
821 |
|
837 |
|
Patient care clinics reflect locations that are licensed as a primary location to provide O&P services and which are fully-staffed and open throughout a typical operating week. To facilitate patient convenience, we also operate satellite clinics. These are remote locations associated with a primary care clinic, utilized to see patients and are open for operation on less than a full-time basis during a typical operating week.
Net revenue. Net revenue for the year ended December 31, 2016 was $1,042.1 million, a decrease of $25.1 million, or 2.4%, from $1,067.2 million for the year ended December 31, 2015. Net revenue by operating segment, after elimination of intersegment activity, was as follows:
|
|
|
For the Years Ended December 31, |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2016 |
|
2015 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
840,130 |
|
$ |
874,960 |
|
$ |
(34,830 |
) |
(4.0 |
)% |
|
Products & Services |
|
201,924 |
|
192,212 |
|
9,712 |
|
5.1 |
% | |||
|
Net revenue |
|
$ |
1,042,054 |
|
$ |
1,067,172 |
|
$ |
(25,118 |
) |
(2.4 |
)% |
Patient Care net revenue decreased $34.8 million or 4.0% to $840.1 million for the year ended December 31, 2016 from $875.0 million for the year ended December 31, 2015. Patient care clinic net revenue declined $27.9 million primarily due to increased time spent processing submissions of claims coupled with the consolidation and closure of lower performing clinics. CARES net revenue declined $6.4 million in 2016 as the business unit was closed in 2015, and network contract management revenue declined $0.5 million for the year ended December 31, 2016 compared with the year ended December 31, 2015. Products & Services net revenue increased $9.7 million or 5.1% for year ended December 31, 2016 to $201.9 million from $192.2 million for year ended December 31, 2015. Net revenue from our distribution products increased $6.3
million from higher prosthetic sales, and therapeutic services net revenue increased $3.4 million primarily from sale of equipment to customers who discontinued our services as described in “Products & Services Segment Trends” section above.
Material costs. Material costs for the year ended December 31, 2016 were $332.1 million, a decrease of $4.2 million, or 1.2%, from $336.3 million for the year ended December 31, 2015. Material costs by operating segment, after elimination of intersegment activity, were as follows:
|
|
|
For the Years Ended December 31, |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2016 |
|
2015 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
256,012 |
|
$ |
262,327 |
|
$ |
(6,315 |
) |
(2.4 |
)% |
|
Products & Services |
|
76,059 |
|
73,956 |
|
2,103 |
|
2.8 |
% | |||
|
Material costs |
|
$ |
332,071 |
|
$ |
336,283 |
|
$ |
(4,212 |
) |
(1.3 |
)% |
|
|
|
|
|
|
|
|
|
|
| |||
|
Percent of net revenue |
|
31.9 |
% |
31.5 |
% |
|
|
|
| |||
|
Percent of adjusted gross revenue |
|
30.4 |
% |
29.8 |
% |
|
|
|
| |||
Material costs increased to 31.9% of net revenue in 2016 compared to 31.5% in 2015, and increased to 30.4% of adjusted gross revenue in 2016 compared to 29.8% in 2015. With the shutdown of the CARES business in 2015, material costs were lower by $3.6 million in 2016. In addition, Hanger Clinic’s material costs decreased $2.7 million for the year ended December 31, 2016 from lower revenue. Material costs in Products & Services increased $2.1 million from higher net revenue for distribution products for the year ended December 31, 2016 compared to the year ended December 31, 2015. Material costs were also impacted by product mix and vendor rebates.
Personnel costs. Personnel costs for the year ended December 31, 2016 decreased $3.6 million to $363.5 million from $367.1 million for the year ended December 31, 2015. Personnel costs by operating segment were as follows:
|
|
|
For the Years Ended December 31, |
|
|
|
Percent |
| |||||
|
(dollars in thousands) |
|
2016 |
|
2015 |
|
Change |
|
Change |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
Patient Care |
|
$ |
315,892 |
|
$ |
317,927 |
|
$ |
(2,035 |
) |
(0.6 |
)% |
|
Products & Services |
|
47,645 |
|
49,167 |
|
(1,522 |
) |
(3.1 |
)% | |||
|
Personnel costs |
|
$ |
363,537 |
|
$ |
367,094 |
|
$ |
(3,557 |
) |
(1.0 |
)% |
|
|
|
|
|
|
|
|
|
|
| |||
|
Percent of net revenue |
|
34.9 |
% |
34.4 |
% |
|
|
|
| |||
|
Percent of adjusted gross revenue |
|
33.3 |
% |
32.5 |
% |
|
|
|
| |||
Although we experienced an overall decline in personnel costs of $3.6 million in 2016 as compared to 2015, due to the decline in net revenue, personnel costs increased to 34.9% of net revenue in 2016 compared to 34.4% in 2015, and increased to 33.3% of adjusted gross revenue in 2016 compared to 32.5% in 2015. For the year ended December 31, 2016, personnel costs for our Patient Care segment decreased $2.0 million, or 0.6%, as compared to the year ended December 31, 2015. The shutdown of the CARES business decreased personnel costs $3.4 million. To address the increased time demand on processing revenue reimbursements, additional billing and collection personnel were added. Excluding CARES, this increase in personnel, coupled with an annual merit increase for employees added $10.5 million in higher salary, benefits and payroll taxes costs. Within patient care personnel costs, bonus and commissions were $8.9 million lower for 2016 compared to 2015 driven by lower segment revenue. Excluding CARES, temporary labor and other costs were $0.2 million lower in 2016 compared to the prior year. Personnel costs in the Products & Services segment declined $1.5 million, or 3.1%, primarily from lower bonuses and commissions of $3.6 million, partially offset by $2.1 million of increases in salary, benefit and other employee compensation for 2016 compared to 2015.
Other operating costs. Other operating costs were $139.0 million in 2016, a $1.8 million, or 1.3%, decrease compared to the $140.8 million incurred in 2015. The shutdown of the CARES business decreased other operating costs by $4.4 million. Rent, utilities, occupancy, office and other operating costs increased $1.5 million in 2016 as compared to 2015. Bad debt expense increased $1.1 million primarily from the bankruptcy of one large customer in our distribution area.
General and administrative expenses. For the year ended December 31, 2016, general and administrative expenses decreased $4.6 million, or 4.1% to $107.2 million from $111.8 million for the year ended December 31, 2015. Decreases in bonus and other personnel costs lowered personnel compensation by $3.3 million in 2016 compared to 2015, partially offset by $3.0 million of additional salary, benefits and payroll taxes that included an annual merit increase coupled with additional personnel hired in the accounting and finance function to help mitigate the material weakness and support the Restatement effort. Facility, other related office personnel costs and casual labor were lower by $4.3 million for 2016 compared to 2015.
Professional accounting and legal fees. Professional accounting and legal fees increased 43.9% to $41.2 million for the year ended December 31, 2016 from $28.6 million for the year ended December 31, 2015 from the restatement of prior financial periods and costs related to our financial accounting remediation process. Accounting related fees increased $10.7 million, legal fees increased $2.6 million and other related expenses decreased $0.7 million for the comparative twelve months.
Depreciation and amortization. Depreciation and amortization for the year ended December 31, 2016 decreased to $44.9 million from $46.3 million for the year ended December 31, 2015. The $1.4 million, or 3.1%, decrease included a $1.0 million decline in depreciation from therapeutic program equipment sold to customers as discussed above in “Products & Services Segment Trends” and assets becoming fully depreciated. In addition, the decline in depreciation and amortization expenses in 2016 compared to the 2015 included $0.5 million lower leasehold improvements and building depreciation from the closing and consolidation of low performing clinics, a decline of $0.4 million in amortization from the complete amortization in 2015 of non-compete agreements in therapeutic services, and $0.3 million lower software depreciation. Declines in depreciation and amortization were partially offset by a $0.8 million increase in customer list intangible amortization primarily from the change in estimated useful lives during the fourth quarter of 2015.
Impairment of intangible assets. As more fully explained in Note H - “Goodwill and Intangible Assets” to our consolidated financial statements in this Annual Report on Form 10-K, we recorded an impairment of intangible assets of $86.2 million for the year ended December 31, 2016 and $385.8 million for the year ended December 31, 2015. In 2016, we recorded a total goodwill impairment charge of $86.0 million, of which $64.9 million related to our Therapeutic reporting unit and $21.1 million related to our Distribution reporting unit. In 2015, we recorded a goodwill impairment charge of $382.9 million related to our Patient Care reporting unit. Other intangible asset impairments of $0.2 million in 2016 and $2.9 million in 2015 related to our Therapeutic reporting unit’s indefinite life tradename.
Loss from operations. Loss from operations was $72.1 million for the year ended December 31, 2016 compared with a loss from operations of $349.6 million for the year ended December 31, 2015, a decrease of $277.5 million or 79.4%. The decrease in the loss from continuing operations was the result of lower impairment of intangible assets partially offset by higher professional accounting and legal fees.
Interest expense, net. Interest expense for the year ended December 31, 2016 increased to $45.2 million from $29.9 million for the year ended December 31, 2015. The $15.3 million increase included $10.8 million of additional interest cost associated with early extinguishment of the Senior Notes and $4.8 million of higher interest expense associated with debt refinancing in the third quarter of 2016 as more fully disclosed in Note N - “Long-term Debt” in this Annual Report on Form 10-K. This is offset by $0.3 million lower interest mainly associated with a reduction in outstanding borrowings.
Extinguishment of debt. We recorded charges of $6.0 million in 2016 in conjunction with covenant violations, amendments and waivers related to two debt agreements. Charges for extinguishment of debt for the year ended December 31, 2016 were
$1.2 million lower than the year ended December 31, 2015 from the write-off of previously unamortized debt issuance costs. In 2015, seven agreements were entered into that included debt waivers and amendments resulting in charges of $7.2 million. See Note N - “Long-Term Debt” in this Annual Report on Form 10-K for more details.
Benefit for income taxes. An income tax benefit of $15.9 million was recognized for the year ended December 31, 2016, compared to a benefit of $67.6 million for the year ended December 31, 2015. This reduction in tax benefit was primarily due to lower losses from continuing operations before income taxes. Our effective tax rate from continuing operations was 12.9% and 17.5% for 2016 and 2015, respectively. The effective tax rates differ from the statutory rate due primarily to non-deductible goodwill impairments and other non-deductible expenses.
Income (loss) from discontinued operations, net of income taxes. Net income from the discontinued operations of Dosteon for the year ended December 31, 2016 was $0.9 million compared to a net loss of $8.0 million for the year ended December 31, 2015. Components of the Dosteon business were either sold or ceased operations by the end of the second quarter of 2015. Net income recognized in 2016 relates to contingent consideration resulting from the disposal.
Net loss. Our net loss for year ended December 31, 2016 was $106.5 million as compared to a net loss of $327.1 million for year ended December 31, 2015. Changes in net loss from period to period were driven by a lower impairment charge in 2016 as compared to 2015, offset by lower tax benefits and higher expenses in 2016 primarily including professional fees and interest expense.
Liquidity and Capital Resources
In this section, we provide a discussion of our liquidity and capital resources as of December 31, 2017 and 2016. Due to the passage of time since December 31, 2017, we additionally provide certain information regarding our liquidity as of March 31, 2018.
Liquidity and Capital Resources as of December 31, 2017 and 2016
In 2017 and 2016, due primarily to our inability to meet financial reporting covenant requirements within our Credit Agreement and Senior Notes Indenture, as well as other difficulties we encountered in meeting certain other financial covenants within those agreements, and in order to maintain sufficient liquidity to operate through continued access to our revolving credit facility and to remedy or avoid defaults under our credit agreements, it became necessary that we enter into various amendments and waivers with our lenders. These modifications to our agreements, and other information regarding our indebtedness and our liquidity is provided in Note N - “Long-Term Debt” to our consolidated financial statements in this Annual Report on Form 10-K.
Liquidity
To provide cash for our operations and capital expenditures, our immediate source of liquidity is our cash and investment balances and any amounts we have available for borrowing under our revolving credit facility. We refer to the sum of these two amounts as our “liquidity.” If we are not compliant with our debt covenants in any period, absent a waiver or amendment of our Credit Agreement, we may be unable to access funds in our revolving credit facility. As discussed below, during 2016 and 2017, due to the issues we encountered in preparing our financial statements and other factors, we reached an agreement with our lenders to decrease the amount of our available revolving credit facility by $48.7 million, which had a significant bearing on our overall liquidity. The nature of this decrease and our corresponding management of our liquidity are discussed below.
As of December 31, 2017, we had total liquidity of $87.9 million, which was comprised of $1.5 million in cash and $86.4 million under our revolving credit facility. During 2017, based on an amendment to our Credit Agreement we entered into in July 2016, access to our revolving credit facility was reduced from $101.0 million to $97.6 million, which had the effect of
reducing our liquidity by $3.4 million. Our net uses of cash during the year also contributed to further reductions in our liquidity during 2017. While we produced $30.1 million of net cash from operating activities, we utilized $1.9 million for capital expenditures and other investing activities, and $33.9 million for net reductions in our long-term term indebtedness, payments to lenders and other financing activities, and increased our letters of credit by $0.1 million. The net effect of these items was a further net reduction of $9.7 million in our liquidity. In total, due to the reduction in the size of our revolving facility and the net effect of our operating, investing and financing activities, our liquidity declined by $14.2 million during 2017, to $87.9 million.
In 2016, our liquidity increased by $33.3 million from $68.8 million to $102.1 million. In the aggregate, this improvement in liquidity was the result of an increase in net proceeds from our long-term indebtedness of $44.3 million, which was partially offset by $11.0 million in net cash flows from operating activities, investing activities, debt issuance costs and fees and further reductions in the borrowing capacity under our revolving credit facility. More specifically, excluding the proceeds from our long-term indebtedness, the $33.3 million net increase in liquidity was comprised of $69.1 million in operating cash flow (of which $34.1 million related to net refunds of previously remitted federal taxes) offset by a further reduction of $45.3 million in the total available size of our revolving credit facility, $17.2 million in capital expenditures and other investing activities, the payment of $15.8 million in debt issuance costs and fees and a $1.8 million increase in our outstanding letters of credit. Our resulting liquidity of $102.1 million as of December 31, 2016, was comprised of $7.2 million in cash and cash equivalents, and $94.9 million in available borrowing capacity under our reduced $101.0 million total revolving facility.
We discuss the sufficiency of our liquidity to fund our future operations and capital needs in the “Liquidity Outlook and Going Concern Evaluation” section provided below.
Working Capital and Days Sales Outstanding
At December 31, 2017, we had a working capital of $78.7 million compared to working capital of $55.0 million as of December 31, 2016. Our working capital increased $23.7 million in 2017 compared to 2016 primarily due to decreases in current liabilities of $25.0 million and current assets of $1.3 million. Our current liabilities decreased primarily due to the decreases in the current portion of long-term debt of $26.6 million and accrued expenses and other current liabilities of $13.1 million, partially offset by increased accrued compensation related costs of $16.8 million.
Our working capital decreased by $84.8 million in 2016 compared to 2015, due to several factors, including the reduction of cash of $51.6 million primarily due to repayments of long-term debt and a reduction of accounts receivable of $30.0 million. The decrease in accounts receivable was primarily the result of improved rates of collection, increases in our coordination of collections efforts on accounts receivable through our use of our newly established revenue cycle management group and through the remediation of issues we had encountered in our implementation of the patient management and electronic health record system.
Days sales outstanding (“DSO”) is a calculation that approximates the average number of days between the billing for our services and the date of our receipt of payment, which we estimate using a 90 day rolling period of net revenue. This computation can provide a relative measure of the effectiveness of our billing and collections activities. As of December 31, 2017, 2016 and 2015, our DSO was 46, 46 and 55 days, respectively. The DSO reduction of nine days from 2015 to 2016 primarily related to changes in our management of accounts receivable through our increased centralization of our revenue cycle management responsibilities, and the positive effects resulting from our remediation of issues we encountered in 2014 relating to the implementation of our new patient management and electronic health record system.
Sources and Uses of Cash in 2017 Compared to 2016
Cash flows provided by operating activities decreased $39.0 million, or 56.4%, from $69.1 million for the year ended December 31, 2016 to $30.1 million for the year ended December 31, 2017. This was due primarily to changes in working capital in 2017 compared to 2016.
Cash flows used in investing activities decreased $15.3 million, or 89.0%, from $17.2 million for the year ended December 31, 2016 to $1.9 million for the year ended December 31, 2017. The decrease for cash used in investing activities included $4.8 million decline in purchases of property, plant and equipment, $17.1 million of cash surrender value proceeds received from our termination of certain company-owned life insurance policies and a net increase of $6.6 million in other investing activities.
Cash flows used in financing activities decreased $69.6 million, or 67.2%, from $103.5 million for the year ended December 31, 2016 to $33.9 million for the year ended December 31, 2017. This decrease included $200.0 million related to 2016 redemption of Senior Notes, $128.2 million reduction in long-term debt associated with the term loan and revolving credit agreement, $13.0 million payment of debt issuance costs and fees and $3.9 million payment on seller note and other contingent consideration, partially offset by $274.0 primarily related to 2016 borrowings under the term loan.
Sources and Uses of Cash in 2016 Compared to 2015
Cash flows provided by operating activities increased $9.6 million, or 16.1%, from $59.5 million for the year ended December 31, 2015 to $69.1 million for the year ended December 31, 2016. This was due to an increase in the amount of cash provided by working capital in 2016 compared to 2015 with improved accounts receivable balances.
Cash flows used in investing activities decreased $18.0 million, or 51.1%, from $35.2 million for the year ended December 31, 2015 to $17.2 million for the year ended December 31, 2016. This decrease included $6.5 million decline in purchases of property, plant and equipment, a decrease in net cash expended on acquisitions of $10.2 million, and a $1.3 million net decrease in other investing activities.
Cash flows used in financing activities for the year ended December 31, 2016 totaled $103.5 million. On August 31, 2016, we used approximately $205.3 million of proceeds from the a new Term B Credit Agreement and existing cash on hand to (i) redeem $200.0 million of Senior Notes, (ii) to pay down approximately $81.0 million outstanding under the revolving credit facility and (iii) to pay approximately $7.9 million of Term B Credit Agreement issuance costs and bank consent fees. This compares with a net cash flows provided by financing activities of $22.7 million for the year ended December 31, 2015 from additional borrowings under our revolving credit facility.
Effect of Indebtedness
Due to the imminent maturity of our previously existing Credit Agreement on June 17, 2018, which had $151.9 million outstanding at December 31, 2017, and given that we would not have produced operating cash flow sufficient to retire this obligation through cash sources arising from our normal operations, and requirements under our Credit Agreement that we provide lenders with our audited financial statements for the year ended December 31, 2017 and our financial statements for the quarter ended March 31, 2018 no later than July 1, 2018, we entered into a new $605 million Senior Credit Facility on March 6, 2018 (the “New Credit Agreement”). These changes to our indebtedness are disclosed in note N - “Long-Term Debt”, in the notes to the consolidated financial statements (“Note N”).
The New Credit Agreement provides for (i) a revolving credit facility with an initial maximum aggregate amount of availability of $100 million that matures in March 2023 and (ii) a $505 million term loan facility due in quarterly principal installments commencing June 29, 2018, with all remaining outstanding principal due at maturity in March 2025. Availability under the revolving credit facility is reduced by outstanding letters of credit, which were approximately $5.93 million as of March 6, 2018. We may (a) increase the aggregate principal amount of any outstanding tranche of term loans or add one or more additional tranches of term loans under the loan documents, and/or (b) increase the aggregate principal amount of revolving commitments or add one or more additional revolving loan facilities under the loan documents by an
aggregate amount of up to the sum of (1) $125 million and (2) an amount such that, after giving effect to such incurrences of such amount (but excluding the cash proceeds of such incremental facilities and certain other indebtedness, and treating all commitments in respect of revolving indebtedness as fully drawn), the consolidated first lien net leverage ratio is equal to or less than 3.80 to 1.00, if certain conditions are satisfied, including the absence of a default or an event of default under the New Credit Agreement at the time of the increase and that we obtain the consent of each lender providing any incremental facility. On March 6, 2018, we had $505 million outstanding under the term loan facility and did not have any borrowings under the revolving credit facility.
Proceeds from the borrowings under the New Credit Agreement were used in part to repay in full all previously existing loans under (i) the Credit Agreement, and (ii) the Term B Credit Agreement. Proceeds were also used to pay various transaction costs of $25.1 including fees of $9.8 paid to respective lenders and accrued and unpaid interest. We expect that the remainder of the proceeds will be used to provide ongoing working capital and capital for other general corporate purposes.
Borrowings under the New Credit Agreement bear interest at a variable rate equal to (i) LIBOR plus a specified margin, or (ii) the base rate (which is the highest of (a) Bank of America, N.A.’s prime rate, (b) the federal funds rate plus 0.50% or (c) the sum of 1% plus one-month LIBOR) plus a specified margin; provided, however, that, in each case, such margins shall be increased by 0.25% per annum if our audited financial statements for the fiscal year ending December 31, 2017 are not delivered to the lenders on or prior to July 1, 2018 and filed with the SEC within five business days after such delivery, with such increase to remain in effect until the first business day following the date upon which we have both delivered such audited financial statements to the lenders and filed the same with the SEC.
Under the New Credit Agreement we are subject to a number of covenants, including covenants that (i) limit the relative amount of our debt as compared to Adjusted EBITDA (a “leverage ratio” limitation) and (ii) provide a minimum threshold for our Adjusted EBITDA relative to our interest expense (an “interest coverage ratio” minimum).
The New Credit Agreement also contains customary events of default. If an event of default under the New Credit Agreement occurs and is continuing, then the lenders may declare any outstanding obligations under the Credit Agreement to be immediately due and payable; provided, however, that the occurrence of an event of default as a result of a breach of a financial covenant under the New Credit Agreement does not constitute a default or event of default with respect to any term facility under the New Credit Agreement unless and until the required revolving lenders shall have terminated their revolving commitments and declared all amounts outstanding under the revolving credit facility to be due and payable.
Liquidity and Availability of Capital as of March 31, 2018
Since December 31, 2017, due to the passage of time and the refinancing that occurred on March 6, 2018, as discussed above and disclosed in note N - “Long-Term Debt”, in the notes to the consolidated financial statements we provide additional discussion regarding the effects of these changes, and our current liquidity in this section of our Management’s Discussion and Analysis.
Current Liquidity and Capital Obligations
At March 31, 2018 we had total liquidity of $127.0 million, which reflected an increase of $39.1 million, from the $87.9 million in liquidity we had as of December 31, 2017. Our liquidity at March 31, 2018 was comprised of cash and cash equivalents of $32.9 million and $94.1 million in available borrowing capacity under our $100.0 million revolving credit facility. This increase in liquidity relates primarily to the net proceeds of $49.7 million from the refinancing of our indebtedness.
In addition to our typical requirements for operating capital and capital expenditures, we have continued to expend a significant amount of professional accounting and legal fees in excess of the amount we estimate we would otherwise incur in a typical fiscal year due to the remediation of our financial statements and legal activities incurred in connection with the
Restatement. For the years ended December 31, 2017 and 2016, we paid $44.9 million and $48.0 million, respectively, in excess professional accounting and legal fees related to these activities. During 2018, to meet our financial reporting obligations, we believe it likely that we will continue to incur substantial fees for these services in connection with our financial statement preparation. We currently estimate that our cash payments for these professional fees during 2018 will be approximately $23.3 million.
Due to the substantial costs of the professional accounting and legal fees that we have been incurring, and anticipate continuing to incur, in connection with our financial reporting remediation activities, and due to the status of our covenant compliance with our lenders, we halted our acquisition activity after the first quarter of 2015. As of the date of this report, we do not currently have any pending acquisitions for which we anticipate the need to expend capital. In connection with refinancing of our debt in 2018 and the resolution of the primary factors which led us to halt our acquisitions, we currently intend to recommence acquisitions of O&P businesses similar to those that we have consummated in prior years.
Our capital expenditures are primarily comprised of the replacement of furniture, fixtures and equipment in our clinics and other facilities, the construction of leasehold improvements, and the purchase of computer equipment and related software. In 2018, to replace certain older equipment reaching the end of its useful life, we currently believe that it may be necessary for us to expend amounts additional to our normal levels in connection with the purchase of replacement therapeutic equipment for use in our therapy business in the approximate amount of $5.5 million. We may choose to delay or defer these or other capital expenditures in the event that business or financial conditions warrant.
Liquidity Outlook and Going Concern Evaluation
Our New Credit Agreement has a term loan facility with $505 million in principal outstanding at March 31, 2018, due in quarterly principal installments equal to 0.25% of the original aggregate principal amount of $505 million, commencing June 29, 2018, with all remaining outstanding principal due at maturity in March 2025 and a revolving credit facility with no borrowings and a maximum aggregate amount of availability of $100 million at March 31, 2018 that matures in March 2023.
ASU 2014-15 Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern requires that we evaluate whether there is substantial doubt about our ability to meet our financial obligations when they become due during the twelve month period from the date these financial statements are available to be issued. We have performed such an evaluation and, based on the results of that assessment, we are not aware of any relevant conditions or events that raise substantial doubt regarding our ability to continue as a going concern within one year of the date the financial statements are issued.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that may or could have a current or future material effect on our financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources, except for $6.2 million of letters of credit outstanding as of December 31, 2017.
Contractual Obligations
The following table sets forth our contractual obligations and commercial commitments as of December 31, 2017 for each of the indicated periods:
|
(in thousands) |
|
2018 |
|
2019 |
|
2020 |
|
2021 |
|
2022 |
|
Thereafter |
|
Total |
| |||||||
|
Debt principal payments (1) |
|
$ |
161,382 |
|
$ |
283,216 |
|
$ |
2,191 |
|
$ |
856 |
|
$ |
1,014 |
|
$ |
12,297 |
|
$ |
460,956 |
|
|
Interest payments on debt (1) |
|
40,411 |
|
18,481 |
|
2,120 |
|
1,949 |
|
1,777 |
|
7,015 |
|
71,753 |
| |||||||
|
Operating leases |
|
38,567 |
|
29,888 |
|
21,418 |
|
14,721 |
|
9,417 |
|
11,903 |
|
125,914 |
| |||||||
|
Other obligations (2) |
|
8,789 |
|
5,539 |
|
3,988 |
|
2,242 |
|
2,167 |
|
11,374 |
|
34,099 |
| |||||||
|
Total contractual cash obligations |
|
$ |
249,149 |
|
$ |
337,124 |
|
$ |
29,717 |
|
$ |
19,768 |
|
$ |
14,375 |
|
$ |
42,589 |
|
$ |
692,722 |
|
(1) Projections were based on the assumptions that the Revolving Credit Facility and Term Loans would remain in place but on March 6, 2018, we entered into a new Senior Credit Facility. See Note N - “Long-Term Debt” to our consolidated financial statements for additional information regarding the Credit Agreement and our long-term debt.
(2) Other long-term obligations include commitments under our DB SERP plan, a non-cancellable purchase commitment related to our Southern Prosthetic Supplies (“SPS”) subsidiary and IT related and telephone contracts. Refer to Note K - “Employee Benefits” for additional disclosure on the DB SERP plan and Note Q - “Commitments and Contingent Liabilities” for additional disclosure on the SPS non-cancellable purchase commitment to our consolidated financial statements in this Annual Report on Form 10-K.
Dividends
It is our policy to not pay cash dividends on our common stock, and given our capital needs we currently do not foresee a change in this policy. Certain of our agreements relating to indebtedness limit our ability to pay dividends, and we currently anticipate that these restrictions will continue to exist in future agreements that we may enter.
Defined Benefit Supplemental Executive Retirement Plan
In 2004, we implemented an unfunded noncontributory defined benefit plan that covers certain of our current and former senior executives (“DB SERP”). We have engaged an actuary to calculate the benefit obligation and net benefits cost as of December 31, 2017, 2016 and 2015 and utilized such to establish our benefit obligation liability.
The following weighted average assumptions were used to determine the benefit obligation and net benefit cost at December 31:
|
|
|
2017 |
|
2016 |
|
2015 |
|
|
Discount rate |
|
3.27 |
% |
3.54 |
% |
3.64 |
% |
|
Average rate of increase in compensation |
|
3.00 |
% |
3.00 |
% |
3.00 |
% |
The discount rate at December 31, 2017 of 3.27% decreased 0.27 basis points compared to the discount rate used at December 31, 2016 due to changes in the pension discount curve rate available on the open market at December 31, 2016. The discount rate at December 31, 2016 of 3.54% decreased 0.10 basis points compared to the 3.64% used at December 31, 2015 due to changes in the pension discount curve rate available on the open market at December 31, 2015. The average rate of increase in compensation was 3.00% at December 31, 2017, 2016 and 2015.
Future payments under the DB SERP as of December 31, 2017 are as follows:
|
(in thousands) |
|
|
| |
|
2018 |
|
$ |
1,913 |
|
|
2019 |
|
1,913 |
| |
|
2020 |
|
1,913 |
| |
|
2021 |
|
1,913 |
| |
|
2022 |
|
1,913 |
| |
|
Thereafter |
|
11,228 |
| |
|
|
|
$ |
20,793 |
|
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Our future financial results are subject to a variety of risks, including interest rate risk. As of December 31, 2017, the interest expense arising from the $156.9 million of outstanding borrowings under both our term loan facility under our Credit Agreement and our revolving credit facility under our Credit Agreement was subject to variable interest rates, partially offset by interest income subject to variable interest rates generated from our $1.5 million of cash equivalents as of that date. As of December 31, 2017, we had $304.1 million of fixed rate debt which included subordinated Seller Notes and financing leases.
Set forth below is an analysis of our financial instruments as of December 31, 2017 that were sensitive to changes in interest rates. The table demonstrates the changes in estimated annual cash flow related to the outstanding balance under the revolving and term loan facilities, calculated for an instantaneous shift in interest rates, plus or minus 50 BPS, 100 BPS and 150 BPS. As of December 31, 2017, the interest rate on the revolving and term loan facilities was 7.32% based on a LIBOR rate of 1.57% and the applicable margin of 5.75%.
|
Cash Flow Risk |
|
Annual Interest Expense Given an |
|
No Change in |
|
Annual Interest Expense Given an |
| ||||||||
|
(in thousands) |
|
(150 BPS) |
|
(100 BPS) |
|
(50 BPS) |
|
|
|
50 BPS |
|
100 BPS |
|
150 BPS |
|
|
Term Loan and Revolver |
|
9,129 |
(a) |
9,913 |
(a) |
10,697 |
|
11,482 |
|
12,266 |
|
13,050 |
|
13,835 |
|
(a) The term loan facility and the revolving credit facility under our prior Credit Agreement are subject to a LIBOR margin of 5.75%, which will serve as the floor on the applicable interest rate. The prior Credit Agreement was replaced in March 2018 with our New Credit Agreement.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The information required by this Item is incorporated herein by reference to the financial statements set forth in Item 15 “Exhibits and Financial Statement Schedules” of Part IV of this Form 10-K.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) are designed to ensure that information required to be disclosed in reports filed or submitted under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC rules and forms, and that such information is accumulated and communicated to management, including the Chief Executive Officer and the Chief Financial Officer, to allow timely decisions regarding required disclosures.
Management, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, conducted an evaluation of the effectiveness of the design and effectiveness of our disclosure controls and procedures as of December 31, 2017. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures were not effective as of December 31, 2017 because of the material weaknesses in our internal control over financial reporting described below.
Management’s Report on Internal Control over Financial Reporting
Management, under the supervision of our Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) is a process designed by, or under the supervision of our Chief Executive Officer and Chief Financial Officer and effected by our board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP.
Internal control over financial reporting includes those policies and procedures which (a) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of assets, (b) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, (c) provide reasonable assurance that receipts and expenditures are being made only in accordance with appropriate authorization of management and the board of directors, and (d) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on the financial statements.
Internal control over financial reporting has inherent limitations. Internal control over financial reporting is a process that involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper management override. Because of such limitations, there is a risk that material misstatements will not be prevented or detected on a timely basis. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate in the future.
Our management, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2017, based on the criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, and has concluded that we did not maintain effective internal control over financial reporting as of December 31, 2017 because of the material weaknesses identified below.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis.
Material Weaknesses
Control Environment
We did not design and maintain effective controls with respect to establishing and assigning authority and responsibility over accounting operations, including the consolidation process, and the preparation and review of financial statements.
Risk Assessment
We did not design and maintain effective internal controls to identify, assess and address risks that significantly impact our financial statements or the effectiveness of our internal controls over financial reporting.
Information and Communication
We did not design and maintain effective controls to obtain, generate and communicate relevant and accurate information to support the function of internal control over financial reporting. Specifically, we did not implement or maintain sufficient information systems in support of our accounting and financial reporting processes.
Monitoring
We did not design and maintain effective monitoring of compliance with established accounting policies, procedures and controls. This weakness included our failure to design and operate effective procedures and controls whose purpose is to evaluate and monitor the effectiveness of our individual control activities.
The material weaknesses in our control environment, risk assessment, information and communication, and monitoring controls contributed to the following additional material weaknesses.
Control Activities
· Inventory
We did not design and maintain effective controls over the accounting for inventory. Specifically, we did not operate effective controls over:
· raw materials to ensure items are priced using the FIFO method, resulting from the identification of inaccurate prices utilized in the valuation of our inventory quantities on hand based on physical observation;
· certain key assumptions used in the valuation of WIP, resulting from the identification of inaccurate or imprecise data used in the development of these assumptions; and
· the completeness, accuracy, valuation and presentation and disclosure of raw materials and WIP.
· Accounting for Leases
We did not design and maintain effective controls over our accounting for leases. Specifically, we did not design and maintain effective controls over the completeness, accuracy, existence, presentation and disclosure of our real property leases.
· Revenue
We did not design and maintain effective controls over our accounting for revenue. Specifically, we did not design and maintain effective controls over the completeness, accuracy, occurrence, and valuation of revenue.
· Accounts Receivable and Allowances
We did not design and maintain effective controls over accounts receivable and allowances. Specifically, we did not design and maintain effective controls over the completeness, accuracy, existence and valuation of amounts recorded to accounts receivable, including allowances.
· Property, Plant and Equipment and Depreciation
We did not design and maintain effective controls over property, plant and equipment, including depreciation. Specifically, we did not design and maintain effective controls over the completeness, accuracy, existence, valuation, presentation and disclosure over property, plant and equipment including capitalized software and related depreciation expense.
· Accounts Payable and Accruals
We did not design and maintain effective controls over accounts payable and accruals. Specifically, we did not design and maintain effective controls over the completeness, accuracy, existence, and rights and obligations related to purchased goods and services and liabilities for other items, accurately reflecting the receipt of such goods or services and the related liability in the proper period.
· Account Reconciliations
We did not design and maintain effective controls over the preparation, review and approval of account reconciliations. Specifically, we did not design and maintain controls to ensure that account reconciliations were completed timely and accurately, and that reconciling items were properly resolved on a timely basis.
· Business Combinations, Goodwill and Intangible Assets
There were no acquisitions in 2017 and therefore controls related to the initial recording of Business Combinations did not operate in 2017. We will evaluate the design and operating effectiveness over controls related to Business Combinations when and if we enter into another acquisition.
We did not design and maintain effective controls over the accounting for goodwill and intangible assets. Specifically, we did not design and maintain effective controls over the completeness, accuracy, existence, valuation and presentation and disclosure related to our accounting for goodwill and intangible assets.
· Share-based Compensation
We did not design and maintain effective controls over the completeness, accuracy, valuation and presentation and disclosure of our accounting for share-based compensation.
· Income Taxes
We did not design and maintain effective controls over our accounting for income taxes. Specifically, we did not design and maintain effective controls over the completeness, existence, accuracy and presentation of our accounting for income taxes, including the income tax provision and related assets and liabilities.
· Information Technology General Controls
We did not design and maintain effective controls over certain IT systems, which could result in misstatements potentially impacting all financial statement accounts and disclosures. Specifically, we did not design and maintain (i) user access controls to appropriately segregate duties and adequately restrict user and privileged access to
financial applications and data to the appropriate personnel, (ii) effective controls to monitor, document and approve data changes, and (iii) effective controls related to monitoring of critical jobs.
These material weaknesses could result in a misstatement of the aforementioned account balances or disclosures that would result in a material misstatement to the annual or interim financial statements that would not be prevented or detected.
The effectiveness of our internal control over financial reporting as of December 31, 2017 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which appears herein.
Remediation Plans
Since the end of 2013, under the oversight of our Audit Committee and Board of Directors, we have been, and continue to be, actively engaged in the design and implementation of remedial measures to address the material weaknesses in our internal control over financial reporting. We are committed to improving our internal control processes and resolving our control deficiencies, including the material weaknesses we have presented above, and will continue to review our effectiveness in accomplishing this critical objective.
To date, we have taken and continue to take the actions described below to remediate the identified material weaknesses. Our remediation efforts are ongoing. As we continue to evaluate and work to improve our internal control over financial reporting, we may implement additional measures or modify the remedial actions described below, as considered appropriate, to remediate our material weaknesses.
Control Environment
We made significant strides in improving our overall control environment. However, we believe the evidence that we have appropriately established and assigned authority and responsibility over accounting operations, including the consolidation process, and the preparation and review of financial statements will be that we have the ability to file our financial statements on a timely basis and have remediated substantially all other material weaknesses. The combination of these factors will indicate that our internal controls over financial reporting are designed and operating effectively as a whole.
Risk Assessment
In our efforts toward remediation of our material weakness in risk assessment, we intend to strengthen our annual risk assessment and to develop programmatic approaches towards the mitigation of risks identified through this process.
Information and Communication
In our efforts toward remediation of our material weakness in information and communication, we have implemented a new lease accounting system, a new payroll and time keeping system, and a new accounting controls administration system. We have also commenced an evaluation of a potential change in our primary general ledger system and accounting subsystems.
Monitoring
In our efforts toward remediation of our material weakness in monitoring and oversight we have:
· Changed the leadership of and expanded our Internal Audit organization.
· Realigned the reporting structure of our accounting organization to have divisional accounting personnel report into our corporate accounting group.
Control Activities
· Inventory
In our efforts toward remediation of our material weaknesses in inventory accounting, we have:
· Hired a Vice President of Inventory and Product Accounting and additional personnel with appropriate technical knowledge to support our inventory accounting requirements;
· Begun implementing measures to strengthen controls, including:
· The introduction of new controls for the aggregation and review of inventory information collected in connection with our physical inventory processes. This included the implementation of controls to ensure that the physical count results are recorded accurately and remediated the component of our inventory material weakness related to the accuracy of the stage of completion on our valuation of WIP.
· The implementation of a new inventory valuation process to establish FIFO valuation of our raw materials.
· The design of new processes and controls to establish and govern estimates of the value of our WIP.
· The development of procedures and controls to ensure reserves are appropriately recorded and intercompany profits in ending inventories are appropriately eliminated at the end of each period
· Accounting for Leases
In our efforts toward remediation of our material weakness related to our real property leases, we have:
· Hired a controller devoted to lease accounting and have added additional personnel with appropriate technical knowledge to support our lease accounting requirements under GAAP;
· Implemented a new lease accounting software system;
· Adopted new procedures and controls with respect to our determination of whether leases should be considered operating or capital for financial reporting purposes;
· Established a process and controls for evaluating new leases and lease renewals for build-to-suit accounting treatment;
· Established a policy for the consistent determination and application of lease terms; and
· Developed new lease accounting procedures and controls in order to calculate deferred rent, asset retirement obligations, and tenant improvement allowances.
We have also adopted procedures to promote effective communication between our real estate group and our lease accounting group to ensure timely, accurate and complete exchange of information.
· Revenue
In our efforts toward remediation of our material weakness in revenue accounting, we have:
· Improved our contract management function and controls to ensure adherence to procedures relating to the updating of our payor contract information in our billing systems;
· Commenced the migration of legacy billing systems utilized by acquired clinics to one of our two primary billing platforms, enabling these sites to be incorporated into our standard contract administration and other controls and processes;
· Established processes and controls to improve the accuracy of invoices;
· Commenced the design and implementation of controls to monitor data input and transfers of data across systems and reporting tools;
· Commenced the evaluation and establishment of policies and procedures, including various controls, to ensure that revenue is recorded in the appropriate period, consistent with the timing of delivery of products and services and the retention of certain risks of ownership; and
· Established a separate, centralized revenue cycle management function to oversee critical aspects of our claims submission and payor reimbursement processes.
· Accounts Receivable and Allowances
In our efforts toward remediation of our material weakness in accounts receivable allowances, we have commenced new preparation and review procedures and controls for allowances for disallowed revenue, bad debts and sales returns.
· Property, Plant and Equipment and Depreciation
In our efforts toward remediation of our material weakness in fixed asset accounting, we have:
· Centralized and updated our fixed asset procedures and controls, including the manner in which we establish the in-service date of assets, our asset capitalization policies and thresholds, and the manner in which we record fixed asset disposals;
· Refined our internal use software development processes and controls to more precisely define and monitor the capitalization of labor, including the enhancement of our timekeeping tools;
· Implemented a formal communications process between real estate, lease accounting, and fixed asset functions to ensure proper accounting and recording of fixed asset related transactions; and
· Commenced procedures and controls to periodically validate the existence of fixed assets.
· Accounts Payable and Accruals
In our efforts toward remediation of our material weakness in our accounts payable process, we have revised our procedures and controls to improve our manner of recording accounts payable and estimating certain period-end accrual balances.
· Account Reconciliations
In our efforts toward remediation of our material weakness around account reconciliations, we have developed policies, procedures and controls regarding the timely preparation and documentation of reconciliations of our general ledger accounts. In connection with the implementation of these policies, procedures and controls, we have improved our training regarding our account reconciliation processes and have adopted review procedures in accordance with job responsibilities.
· Business Combinations, Goodwill and Intangible Assets
There were no acquisitions in 2017 and therefore controls related to the initial recording of Business Combinations did not operate in 2017. We will evaluate the design and operating effectiveness over controls related to Business Combinations when and if the we enter into another acquisition.
Additionally, in our efforts toward remediation of our material weakness in business combinations, goodwill and intangible assets, we have:
· Hired new personnel to support our accounting for these transactions;
· Adopted improved policies, procedures and controls regarding the manner in which we identify and value working capital assets and liabilities assumed at the time of acquisitions, as well as measurement period adjustments; and
· Incorporated controls designed to improve the coordination between our business development group and our acquisition accounting personnel.
· Share-based Compensation
In our efforts toward remediation of our material weakness in share-based compensation, we have developed new policies, procedures and controls regarding the accounting for our grants of restricted stock units and performance based restricted stock units. In connection with these new policies, procedures and controls, we have revised and strengthened our processes and controls over grant information, assumptions, expense recognition, modifications and forfeitures.
· Income Taxes
In our efforts toward remediation of our material weakness in income taxes, we intend to improve our controls over accounting for income taxes by redesigning control procedures to create a more structured and uniform process including enhancing the formality and rigor of review and reconciliation procedures.
· Information Technology General Controls
In our efforts toward remediation of our material weaknesses in IT, we have:
· Updated IT policies and procedures and conducted training with process and control owners to clearly communicate IT general control requirements;
· Begun implementing measures to strengthen controls, including:
· The enforcement of adequate segregation of duties and implementing associated monitoring controls;
· The implementation of a more robust process for provisioning, terminating and periodically reviewing user and privileged access;
· The design of new processes and controls to monitor, document and approve direct data changes; and
· The development of procedures and controls to monitor and review critical jobs.
Despite the substantial time and resources we have directed at remediation efforts discussed in this section, we are unable to estimate at this time when these remediation efforts will be completed. Until the remediation efforts, including any additional remediation efforts that our management identifies as necessary, are completed, the material weaknesses described above will continue to exist.
We intend to provide additional information regarding our remediation efforts with respect to the material weaknesses in future filings with the SEC.
Changes in Internal Control over Financial Reporting
As outlined above, during the year ended December 31, 2017, management remediated the material weakness that existed related to journal entries and inventory, as it relates to WIP staging and existence. As such, certain changes related to our processes for journal entries and inventory have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Therefore, in accordance with Rule 13a-15(d) of the Exchange Act, management, with the participation of our Chief Executive Officer and our Chief Financial Officer, determined that elements of the changes listed above to our internal control over financial reporting occurred during the year ended December 31, 2017 and have materially affected or are reasonably likely to materially affect our internal control over financial reporting.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Directors
As of the date of this filing, our Board of Directors consisted of nine members (ages provided as of December 31, 2017):
|
Name |
|
Position with our Company |
|
Age |
|
Became |
|
|
Vinit K. Asar |
|
Chief Executive Officer and Director |
|
51 |
|
2012 |
|
|
Asif Ahmad |
|
Director |
|
50 |
|
2014 |
|
|
Christopher B. Begley |
|
Chairman of the Board |
|
65 |
|
2013 |
|
|
John T. Fox |
|
Director |
|
65 |
|
November 2017 |
|
|
Thomas C. Freyman |
|
Director |
|
63 |
|
November 2017 |
|
|
Stephen E. Hare |
|
Director |
|
64 |
|
2010 |
|
|
Cynthia L. Lucchese |
|
Director |
|
57 |
|
2015 |
|
|
Richard R. Pettingill |
|
Director |
|
69 |
|
2014 |
|
|
Kathryn M. Sullivan |
|
Director |
|
61 |
|
2015 |
|
Mr. Fox and Mr. Freyman joined our Board of Directors on November 14, 2017. Thomas P. Cooper, M.D. and Cynthia L. Feldmann retired from our Board of Directors effective January 1, 2018. At the time of his retirement, Dr. Cooper became Director Emeritus. As Director Emeritus, Dr. Cooper is invited to attend meetings of the Board, but is not entitled to vote on any matter presented to the Board. Dr. Cooper is not an employee of Hanger.
Vinit K. Asar has been our Chief Executive Officer and President since May 2012, and served as our President and Chief Operating Officer from September 2011 to May 2012. Mr. Asar also served as our Executive Vice President and Chief Growth Officer from December 2008 to September 2011. Mr. Asar came to Hanger from the Medical Device & Diagnostic sector at Johnson & Johnson, having worked at the Ethicon, Ethicon Endo Surgery, Cordis and Biosense Webster franchises. During his eighteen year career at Johnson & Johnson, Mr. Asar held various roles of increasing responsibility in Finance, Product Development, Manufacturing, and Marketing and Sales in the United States and in Europe. Prior to joining Hanger, Mr. Asar was the Worldwide Vice President at Biosense Webster, the Electrophysiology division of Johnson & Johnson, responsible for the Worldwide Sales, Marketing and Services organizations. Mr. Asar has a B.S.B.A from Aquinas College and a M.B.A. from Lehigh University. Mr. Asar’s service as our Chief Executive Officer, extensive knowledge and diverse experience in health care finance, product development, manufacturing, marketing and sales, led to the conclusion he should serve as a director.
Asif Ahmad, an independent healthcare consultant, served as the Chief Executive Officer of Anthelio Healthcare Solutions, Inc. from July 2013 to April 2017. Anthelio is one of the largest providers of information technology and business process services to hospitals, physician practice groups and other health care providers. Mr. Ahmad served as a Senior Vice President and General Manager of Information and Technology Services at McKesson Specialty Health between 2010 and 2013. From 2003 to 2010, he served as the Vice President of Diagnostic Services for the Duke University Health System and Medical Center, and he also held various faculty appointments at Duke University from 2004 to 2011. Prior to that, Mr. Ahmad served in various positions with Ohio State University Health System and Medical Center between 1992 and 2003, serving as Administrator and Chief Information Officer, Chief Technology Officer and the Chair of the Clinical Technology Council at the time of his departure. Mr. Ahmad earned a bachelor’s degree in electrical engineering from the University of Engineering and Technology in Pakistan, a master’s degree in biomedical engineering from Ohio State University, and a M.B.A. from Ohio State University. Mr. Ahmad’s experience as the former Chief Executive Officer of Anthelio Healthcare Solutions, Inc., a large independent health care company, as well as his background in information technology and business processes, led to the conclusion that he should serve as a director.
Christopher B. Begley serves as our non-executive Chairman of the Board, a role he has held since January 2018. He is the retired Executive Chairman and Chief Executive Officer of Hospira, Inc., a global provider of injectable drugs and infusion technology. Mr. Begley served as Executive Chairman of the Board of Hospira from May 2007 until January 2012, and served as Chief Executive Officer from April 30, 2004, when Hospira was spun off from Abbott Laboratories, to March 2011. Prior to that, Mr. Begley served in various positions at Abbott from 1986 to April 2004, leaving Abbott as Senior Vice President of its Hospital Products division. Mr. Begley also serves as a director of Zimmer/Biomet Holdings Inc., a medical device company. Mr. Begley served as non-executive Chairman of the Board of Adtalem Global Education Inc. (formerly known as DeVry Education Group Inc.), from November 2014 to November 2017, and as a director of Adtalem from November 2011 to November 2017. Mr. Begley previously served as non-executive Chairman of the Board of The Hillshire Brands Company from June 2012 to August 2014, and served as a director of the Sara Lee Corporation from October 2006 to May 2012. Mr. Begley earned a bachelor’s degree from Western Illinois University and an M.B.A. from Northern Illinois University. Mr. Begley’s experience as a former Chief Executive Officer of Hospira, Inc., a publicly traded health care company, as well as his experience as a director of other public companies and his background in health care companies generally, led to the conclusion that he should serve as our Chairman of the Board.
John T. Fox, since 2015, has held the position of Chief Executive Officer and President of Beaumont Health, Michigan’s largest healthcare system. Mr. Fox also serves as a director of Beaumont Health. Prior to joining Beaumont Health, Mr. Fox held positions at Emory Healthcare, the largest and most comprehensive health system in Georgia. He joined Emory as Chief Operating Officer in 1999 and assumed the role of Chief Executive Officer and President in 2002. Throughout his 30+ year career, Mr. Fox has held various roles within the healthcare sector, including Executive Vice President of IU Health (formerly Clarian Health), a large health system and academic medical center in Indianapolis, and Vice President and Chief Financial Officer at The John Hopkins Hospital in Baltimore, Maryland. After obtaining a bachelor’s degree and a master’s degree in business administration, Mr. Fox began his career as an MBA/CPA at Coopers & Lybrand. Mr. Fox was a director at HealthSpring, Inc. from 2010 until its acquisition by Cigna in 2012. Mr. Fox’s extensive experience in the healthcare industry, particularly as an executive officer of multiple healthcare systems and hospitals, his accounting and finance background including experience in public accounting, as well as his experience as a director for another public company in the healthcare industry, led to the conclusion that he should serve as a director.
Thomas C. Freyman retired from Abbott Laboratories in February 2017 after serving as Executive Vice President of Finance and Administration, and prior to that, Chief Financial Officer. Mr. Freyman served in a number of other key roles throughout his 38-year tenure at Abbott Laboratories, a publicly held company that engages in the discovery, development, manufacture and sale of a broad and diversified line of healthcare products. Earlier in his career, Mr. Freyman held various accounting, financial planning, treasury and controllership roles. Since 2013, Mr. Freyman has been a member of the Board of Directors and a member of the Audit Committee of Tenneco, Inc. Mr. Freyman earned a bachelor’s degree in accounting from University of Illinois, Urbana-Champaign and a master’s degree in management from Kellogg Graduate School of Management, Northwestern University. Mr. Freyman’s extensive experience in the healthcare industry, including as a senior executive officer at a publicly traded company, as well as his significant background in accounting and finance and his experience as a director for another large public company, led to the conclusion that he should serve as a director.
Stephen E. Hare served as Executive Vice President and Chief Financial Officer of Office Depot, Inc. from December 2013 to January 2018. Prior to that, he served as Senior Vice President and Chief Financial Officer of The Wendy’s Company from July 2011 to September 2013, and prior to that served as Senior Vice President and Chief Financial Officer of Wendy’s/Arby’s Group, Inc. from 2008 to July 2011. Mr. Hare served as Senior Vice President and Chief Financial Officer of Triarc Companies, Inc. from 2007 to the 2008 merger of Triarc and Wendy’s in 2008, and as Chief Financial Officer of Arby’s Restaurant Group, Inc. from 2006 until July 2011. Previously, Mr. Hare served as Executive Vice President of Cadmus Communications Corporation and as the President of Publisher Services Group, a division of Cadmus, from 2003 to 2006. Mr. Hare served as Executive Vice President and Chief Financial Officer of Cadmus from 2001 to 2003. From 1996 to 2001, Mr. Hare was Executive Vice President and Chief Financial Officer of AMF Bowling Worldwide, where he was also a member of the board of directors. From 1990 to 1996, Mr. Hare was Senior Vice President and Chief Financial Officer of James River Corporation. Mr. Hare was also a member of the board of directors of Pasta Pomodoro, Inc., the
operator of Pasta Pomodoro restaurants, from 2008 to 2009, and was a member of the board of directors and chair of the audit committee of Wolverine Tube Inc. from 2005 to 2007. Mr. Hare’s accounting and auditing expertise, including his experience as Chief Financial Officer of large, public companies, as well as his extensive experience in distributed retailing business models, led to the conclusion he should serve as a director.
Cynthia L. Lucchese is the Chief Administrative Officer and Chief Financial Officer of Hulman & Company, a privately held company headquartered in Indianapolis, Indiana, which owns and operates the Indianapolis Motor Speedway, INDYCAR racing league, Indianapolis Motor Speedway Productions, Clabber Girl Corporation and various real estate holdings. Prior to joining Hulman, Ms. Lucchese was Senior Vice President and Chief Financial Officer of Hillenbrand, Inc., where from 2008 to 2014 she was a key member of the leadership team that transformed Hillenbrand from a $650 million North American business to a $1.6 billion global diversified industrial company. She also has extensive experience in the medical device industry, including serving from 2005 to 2007 as Senior Vice President and Chief Financial Officer of Thoratec Corporation, a medical device company focused on treating advanced stage heart failure. Ms. Lucchese also held various senior financial positions with Guidant Corporation, now a part of Boston Scientific Corporation, from 1994 to 2005, including Vice President and Treasurer, Vice President of Finance and Administration - Guidant Sales Corporation, and Vice President - Controller and Chief Accounting Officer. Since July 2014, Ms. Lucchese has served as a member of the Board of Directors, a member of the Nominating & Corporate Governance Committee and Chairman of the Audit Committee of Intersect ENT, Inc. In June 2017, she joined the Board of Directors of Beaver-Visitec, Inc., and serves as Chairman of the Audit Committee. She previously served as a member of the Board of Directors of Brightpoint, Inc. from 2009 to 2012, serving as Chairman of the Audit Committee. In addition, she currently also serves as a member of the Dean’s Council for the Kelley School of Business of Indiana University. Ms. Lucchese, a Certified Public Accountant, earned a bachelor’s degree in accounting and a master’s degree in business administration from Kelley School of Business of Indiana University. Ms. Lucchese’s extensive experience in accounting and financial leadership roles at public companies, and her experience in the medical device industry, as well as her experience as a director of other public companies, led to the conclusion she should serve as a director.
Richard R. Pettingill is the retired President and Chief Executive Officer of Allina Hospitals and Clinics, a network of health care providers in Minneapolis, Minnesota, serving in this role from 2002 until 2009. During that time he also served on the board of directors of the Minnesota Hospital Association and the Minnesota Business Partnership. Prior to joining Allina Hospitals and Clinics, Mr. Pettingill served as Executive Vice President and Chief Operating Officer of Kaiser Foundation Health Plans and Hospitals from 1996 to 2002. From 1991 to 1995, Mr. Pettingill served as President and Chief Executive Officer of Camino Healthcare. Mr. Pettingill serves on the board of directors of two other public companies, Accuray Incorporated, a radiation oncology company, and Tenet Healthcare Corporation, a medical services provider. Mr. Pettingill received a bachelor’s degree from San Diego State University and a master’s degree in health care administration from San Jose State University. Mr. Pettingill’s experience as a former Chief Executive Officer of Allina, a not for profit health care company, as well as his experience as a director of other public companies and his background in health care companies generally, led to the conclusion he should serve as a director.
Kathryn M. Sullivan is the Chief Executive Officer of UnitedHealthcare Employer and Individual, Local Markets, which is an operating division of UnitedHealth Group, since March 2015. She joined UnitedHealthcare in July 2008, as Chief Executive Officer of UnitedHealthcare, Central Region. Prior to joining UnitedHealthcare, Ms. Sullivan served from 2004 to 2008 as Senior Vice President and Chief Financial Officer of Blue Cross Blue Shield Association, and from 2000 to 2004 as President and Chief Executive Officer of Blue Cross and Blue Shield of Louisiana. Presently, Ms. Sullivan serves as a director for UnitedHealthcare Children’s Foundation, for the Executives’ Club of Chicago, the YMCA of Metro Chicago and The Chicago Women’s Network. Ms. Sullivan, a Certified Public Accountant, earned a bachelor’s degree in business administration from Northeast Louisiana University and a master’s degree in business administration from Louisiana State University. Ms. Sullivan’s experience in the health care industry, especially with respect to the payor perspective, led to the conclusion she should serve as a director. Ms. Sullivan was originally recommended as a director nominee by a third party search firm.
There are no family relationships between any of the members of the Board of Directors.
Executive Officers
The following tables set forth information regarding our current and certain former executive officers. The ages listed for all executive officers are as of December 31, 2017.
|
Name |
|
Age |
|
Office with the Company |
|
Vinit K. Asar |
|
51 |
|
President and Chief Executive Officer |
|
Samuel M. Liang |
|
55 |
|
Executive Vice President of Hanger, Inc., President and Chief Operating Officer of Hanger Prosthetics & Orthotics, Inc. (dba Hanger Clinic) |
|
Thomas E. Kiraly |
|
57 |
|
Executive Vice President and Chief Financial Officer |
|
Kenneth W. Wilson |
|
54 |
|
Former Executive Vice President of Hanger, Inc., President and Chief Operating Officer of Southern Prosthetic Supply, Inc. |
|
Lucinda M. Baily |
|
54 |
|
Senior Vice President and Chief Compliance Officer |
|
Thomas E. Hartman |
|
55 |
|
Senior Vice President, Secretary and General Counsel |
|
Scott Ranson |
|
53 |
|
Senior Vice President and Chief Information Officer |
|
Rebecca J. Hast |
|
67 |
|
Senior Vice President, Strategic Initiatives |
|
Andrew C. Morton |
|
52 |
|
Former Senior Vice President and Chief Human Resources Officer |
|
Gabrielle B. Adams |
|
49 |
|
Vice President and Chief Accounting Officer |
Mr. Wilson resigned effective April 16, 2018. Mr. Morton resigned effective March 19, 2018.
Vinit K. Asar has been our Chief Executive Officer and President since May 2012, and served as our President and Chief Operating Officer from September 2011 to May 2012. Mr. Asar also served as our Executive Vice President and Chief Growth Officer from December 2008 to September 2011. Mr. Asar came to Hanger from the Medical Device & Diagnostic sector at Johnson & Johnson, having worked at the Ethicon, Ethicon-Endo-Surgery, Cordis and Biosense Webster franchises. During his eighteen year career at Johnson & Johnson, Mr. Asar held various roles of increasing responsibility in Finance, Product Development, Manufacturing, and Marketing and Sales in the United States and in Europe. Prior to joining Hanger, Mr. Asar was the Worldwide Vice-President at Biosense Webster, the Electrophysiology division of Johnson & Johnson, responsible for the Worldwide Sales, Marketing and Services organizations. Mr. Asar has a B.S.B.A from Aquinas College and a M.B.A. from Lehigh University.
Lucinda M. Baily is our Senior Vice President and Chief Compliance Officer, a role she has had since January 2018. Prior to joining Hanger, Ms. Baily held the role of Chief Compliance Officer for Alere Inc., a medical diagnostic and device company, from October 2014 to January 2018. Prior to joining Alere, Ms. Baily served as Senior Vice President and Chief Compliance Officer for WellCare Health Plans, Inc., a company that provides managed care services for government-sponsored health care programs, from July 2012 to May 2014. She also held the position of Vice President and Assistant General Counsel at WellCare Health Plans, and oversaw the operations and regulatory legal group. Ms. Baily has also served in legal and compliance roles throughout her twenty year career, at companies and associations such as Pacific Northwest National Laboratory, operated for the Department of Energy by Battelle Memorial Institute, King Abdullah University of Science and Technology, a graduate-level science and technology university located in Saudi Arabia and the country’s first co-ed institution, and Baylor College of Medicine in Houston, Texas. Ms. Baily received her Juris Doctorate with honors from the University of Houston Law Center, her Master of Public Health degree from the University of Texas School of Public Health, and a Bachelor of Science degree in biology from Allegheny College.
Thomas E. Hartman is our Senior Vice President, General Counsel and Secretary. He was appointed Senior Vice President in 2015 and Secretary in 2014, and has served as Vice President and General Counsel since 2009. Mr. Hartman joined Hanger from Foley & Lardner, LLP where he was a partner in Foley’s Business Law Department. Mr. Hartman’s practice at Foley was focused on securities transactions, securities law compliance, mergers and acquisitions, and corporate
governance. Prior to joining Foley in 1995, Mr. Hartman was a business law associate at Jones Day. Mr. Hartman received his J.D. from the University of Wisconsin in Madison, and a Bachelor of Science in Engineering (Industrial & Operations Engineering) from the University of Michigan in Ann Arbor.
Rebecca J. Hast has been our Senior Vice President, Strategic Initiatives, since January 2018. She served as Chief Compliance Officer from June 2015 until January 2018, and as President of Linkia, LLC from 2005 to June 2016. Prior to joining Linkia, Ms. Hast was with United Health Group Incorporated, a health care company that offers a broad spectrum of products and services through two distinct platforms: UnitedHealthcare, which provides health care coverage and benefits services; and Optum, which provides information and technology-enabled health services. Ms. Hast held a variety of positions from 1999 to 2004, leaving United Healthgroup as Senior Vice President, Account Development for the Dental Benefit Providers, a wholly-owned subsidiary of United Healthgroup’s Specialty Services Division (now Optum). Prior to joining United Healthgroup, Ms. Hast held management and leadership positions with Magellan Health Services, Inc., a specialty health care management company, and other health care insurance and services providers. Ms. Hast holds a Bachelor of Science degree from University of Pittsburgh.
Thomas E. Kiraly has been our Executive Vice President and Chief Financial Officer since January 2015. Mr. Kiraly joined Hanger in October 2014 as Executive Vice President. Prior to joining Hanger, Mr. Kiraly served as the Executive Vice President, Chief Financial Officer and Treasurer of Sheridan Healthcare, Inc., a provider of anesthesia, radiology, emergency department, and neonatology services from 2013 to 2014. From 1999 to 2011, Mr. Kiraly served as Executive Vice President, Chief Financial Officer and Treasurer and led the financial accounting, procurement and real estate functions of Concentra, Inc., a provider of urgent care, occupational health care, and other health care services. In 2010, when Concentra, Inc. was acquired by Humana, Inc., a Fortune 100 provider of insurance, health and wellbeing and related health care services, Mr. Kiraly transitioned to the position of Vice President of Finance for Humana, responsible for corporate financial forecasting, analysis, internal reporting, and accounting operations. From 1988 to 1999, Mr. Kiraly served as Executive Vice President and Chief Financial Officer of BRC Holdings, Inc., where he led the financial accounting, human resources and legal functions of this publicly-traded provider of information technology services to health care firms and local governments. Mr. Kiraly earned his Master of Business Administration from the University of Texas in Austin, Texas and his Bachelor of Arts in Speech Communication from California State University in Northridge, California.
Samuel M. Liang has been our Executive Vice President since May 2016, and has been the President and Chief Operating Officer of Hanger Prosthetics & Orthotics, Inc. (dba Hanger Clinic), our patient care subsidiary, since September 2014. Mr. Liang joined Hanger in May 2014. Between May 2010 and May 2014, Mr. Liang was a Senior Vice President of Bayer HealthCare where he served as President and CEO of MEDRAD, Inc. and Head of the Radiology & Interventional business. Prior to that, he served as President and Chief Executive Officer of Vascular Therapies, LLC, a company that created a combination drug and device product for vascular surgery. Mr. Liang also held numerous leadership positions over 24 years at Cordis Corporation, a Johnson & Johnson company. Mr. Liang earned a B.S.E. degree in mechanical engineering and material sciences from Duke University, North Carolina, and a master’s degree in management from the Kellogg Graduate School of Management, Northwestern University, Illinois.
Andrew C. Morton (former officer) was our Senior Vice President and Chief Human Resources Officer. He served as Senior Vice President from 2015 to March 2018, and served as Vice President and Chief Human Resources Officer since 2010. Prior to joining Hanger, Mr. Morton worked for Freescale Semiconductor since 2006 in two capacities; first as Vice President Talent and Corporate Services, and then Vice President Human Resources Supply Chain. From 1992 to 2006, Mr. Morton worked at IBM and held various global field and corporate HR executive roles of increasing responsibility across its software, hardware and sales businesses. Mr. Morton has a B.S. degree in Finance from the University of Colorado at Boulder, and an MBA from Syracuse University. In between degrees, he worked for Baxter Healthcare in Finance roles from 1988 to 1989.
Scott Ranson has been our Senior Vice President and Chief Information Officer since July 2015. Mr. Ranson joined Hanger after 14 years of service as the Chief Information Officer for Brookdale Senior Living Inc., a publicly traded senior housing solution provider, from 2001 to June 2015. Previously, Mr. Ranson served as the Director of Software for Marketing
Specialists Company, where he led the successful implementation of an ERP system and e-commerce strategies, and as Vice President of Information Technology for Atlas Marketing Company, Inc. Mr. Ranson earned his Bachelor of Science degree in Business Administration, Business Management, Computer Information Systems from Ashland University in Ohio.
Kenneth W. Wilson (former officer) was Executive Vice President from May 2016 to April 2018, and was President and Chief Operating Officer of Southern Prosthetic Supply, Inc. since September 2011. Mr. Wilson was previously employed by Cardinal Health Inc., the largest distributor of pharmaceuticals and medical products in the United States, for 22 years, serving as Senior Vice President/General Manager of its Ambulatory Care business from 2008 until September 2011, as Vice President/General Manager of its Onsite business from 2006 to 2008, and as Senior Vice President-Group Purchasing Accounts from 2004 to 2006. Prior to joining Cardinal Health, he worked at Allegiance Healthcare Corporation, a manufacturer of medical products, including surgical apparel and drapes, surgical instruments and respiratory care products. Allegiance Healthcare was a 1997 spin-off of Baxter Healthcare Corporation that was acquired by Cardinal Health in 1999. Mr. Wilson served Allegiance Healthcare as Head of Allegiance National Account and Health Systems from 2002 to 2004, and as Vice President-Health Systems from 1997 to 2002. From 1988 to 1997, Mr. Wilson was with Baxter Healthcare, a manufacturer of a wide variety of medical products across three divisions, including drugs and vaccines, dialysis equipment and intravenous (IV) supplies. Mr. Wilson left Baxter in 1997 as a Regional Director in Encinitas, California. Prior to joining Baxter, he also worked for PepsiCo, USA and Proctor & Gamble in a variety of sales roles. Mr. Wilson received his Bachelor of Science degree in Economics and Social Science from Davidson College in 1984.
Gabrielle B. Adams has been our Vice President and Chief Accounting Officer since April 2017. Ms. Adams joined Hanger as its Vice President - Accounting in February 2015. Prior to joining Hanger Ms. Adams served as Chief Financial Officer at the Texas Bankers Association, a trade association supporting the banking industry in Texas, from 2012 to 2015. Previously, Ms. Adams served in various roles of increasing responsibility at EZCorp, Inc., a publicly traded provider of pawn loans and operator of pawn stores, from 1999 to 2012, including serving as Vice President of Financial Planning and Analysis, Director of Internal Audit, and Assistant Controller. Ms. Adams holds a degree in accounting from the University of Texas at Austin and is a licensed CPA in the State of Texas.
There are no family relationships between any of the executive officers.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act, as amended, requires our officers and directors, and persons who beneficially own more than 10% of a registered class of our equity securities, to file initial reports of securities ownership on Form 3 and reports of changes in such ownership on Forms 4 and 5 with the SEC. Based solely on a review of the copies of such reports furnished to us during the fiscal year ended December 31, 2017, we believe that all reports of securities ownership and changes in such ownership required to be filed during 2017 were timely filed.
Corporate Governance Guidelines and Code of Business Conduct and Ethics for Directors and Employees
Our Board of Directors has adopted Corporate Governance Guidelines and a Code of Business Conduct and Ethics for directors, officers and employees in accordance with NYSE corporate governance listing standards. Copies of these documents are set forth on our website, www.hanger.com.
Policy Regarding Director Nominating Process
The Corporate Governance and Nominating Committee has adopted a policy pursuant to which a shareholder who has owned at least 2% of our outstanding shares of Common Stock for at least one year may recommend a director candidate that the Corporate Governance and Nominating Committee will consider when there is a vacancy on our Board of Directors either as a result of a director resignation or an increase in the size of our Board of Directors. Such recommendation must be made in writing addressed to the Chairperson of the Corporate Governance and Nominating Committee at our principal executive offices and must be received by the Chairperson at least 120 days prior to the anniversary date of the release of the prior
year’s proxy statement. Although the Corporate Governance and Nominating Committee has not formulated any specific minimum qualifications that it believes must be met by a nominee that the Corporate Governance and Nominating Committee recommends to our Board of Directors, the factors it will take into account will include strength of character, mature judgment, career specialization, relevant technical skills or financial acumen, diversity of viewpoint and industry knowledge, as set forth in the Corporate Governance and Nominating Committee’s charter. There will not be any difference between the manner in which the committee evaluates a nominee recommended by a shareholder and the manner in which the committee evaluates any other nominee.
While the Corporate Governance and Nominating Committee does not have a formal policy relating specifically to the consideration of diversity in its process to select and evaluate director nominees, the Corporate Governance and Nominating Committee does consider diversity as part of its overall evaluation of candidates for director nominees. Specifically, the “Selection of Board Members” section of our Corporate Governance Guidelines provides that the selection of potential directors should be based on all the factors the Corporate Governance and Nominating Committee considers appropriate, which may include diversity of viewpoints.
Audit Committee
The Audit Committee (as used in this section, the “Committee”) of our Board of Directors was formed in accordance with Section 3(a)(58)(A) of the Exchange Act, as amended. It is governed by its charter, a copy of which is available on our website, www.hanger.com.
The current members of the Committee are Stephen E. Hare (Chair), Christopher B. Begley, Cynthia L. Lucchese and Thomas C. Freyman.
All the members of the Committee were “independent” under the rules of the SEC and the listing standards of the NYSE, which means that they did not and have not received any consulting, advisory or other compensatory fee from other than board or committee fees, they were not “affiliated persons” of us and they had no relationship to us that may have interfered with the exercise of their independence from our management. Furthermore, each Committee member has been deemed by our Board of Directors to be financially literate and at least one member has accounting or related financial management expertise, as called for by NYSE listing standards. Our Board of Directors has determined that Mr. Hare and Mr. Begley are considered to be an “audit committee financial expert” within the meaning of the rules of the SEC.
ITEM 11. EXECUTIVE COMPENSATION
2017 COMPENSATION DISCUSSION & ANALYSIS
Overview
The following narrative and tables provide the required disclosures with respect to our fiscal year ended December 31, 2017.
Objectives of Our Executive Compensation Program
The compensation program covering our named executive officers is designed to drive our Company’s success, which will be achieved primarily through the actions of talented employees. Our executive compensation program covering named executive officers has specific primary objectives which include:
· attracting qualified and talented executives who are capable of providing the appropriate leadership to our Company;
· retaining executives who have the critical skills our Company needs to meet our strategic and operational objectives; and
· appropriately motivating our executives to drive outstanding Company performance.
These objectives reflect our belief, that programs that support the attraction and retention of a highly qualified executive management team-coupled with appropriate incentive programs to motivate performance-serve the long-term interests of our investors.
Compensation arrangements for our named executive officers are designed to reward long-term commitment to our Company’s success. The following principles guide our compensation decisions:
|
Guiding Principle |
|
Hanger Philosophy / Approach |
|
|
|
|
|
Pay for performance |
|
Our compensation program is designed to align executive compensation with the Company’s overall performance and business strategy. The design of our short- and long-term compensation programs is driven by business objectives and performance measures which we believe provide a direct link to the creation of shareholder value. We support a structural pay for performance philosophy by significantly emphasizing variable or at-risk compensation in the overall executive pay program. |
|
Facilitate alignment with shareholders |
|
Our long-term incentives are delivered in the form of equity to provide executives with a direct interest in the performance of our stock. We have adopted and implemented stock ownership guidelines for our executives which reinforce this principle. |
|
Be internally equitable |
|
Our executive compensation programs are designed to provide compensation that is fair and equitable based on the performance of the executive and the Company. In addition to conducting analyses of market pay levels, we consider the pay of the named executive officers relative to one another and relative to other members of the management team. |
|
Promote sound corporate governance objectives |
|
We seek broad compliance with all applicable legal, regulatory and financial regulations and requirements in the context of our executive compensation program. In addition, when designing and implementing our executive compensation programs, we give due consideration to the impact such programs have on shareholders and any relevant tax and accounting implications that may arise in connection with such programs. |
|
Provide leadership stability and continuity |
|
Our executive programs are designed to reward both long-term contributions to the Company, as well as attract new executive talent and reward commitment of our executives to our Company regardless of their length of service with the Company. We recognize that the stability of the leadership team enhances our business. |
|
Be competitive |
|
We conduct market pay analyses to ensure the compensation we pay our executives is competitive in terms of the elements and mix of pay, program design and resulting actual levels of pay. |
|
Reflect factors of role and individual |
|
We use the information from market pay analyses and apply it to the individual situation of each of our executives to ensure we are compensating for the officer’s responsibilities and the individual’s skills and performance. |
|
Encourage long-term executive service |
|
We provide our named executive officers with tax effective savings opportunities. Our savings and retirement plans, along with a market competitive offering of other pay elements, encourage employees to join and remain at our Company. In addition, the vesting provisions established for all of our long-term incentive vehicles support this objective. |
Named Executive Officers
Based on their compensation for the fiscal year ended December 31, 2017, the following individuals have been identified as the named executive officers for purposes of disclosure in this Annual Report on Form 10-K:
· Vinit K. Asar, our President and Chief Executive Officer;
· Thomas E. Kiraly, our Executive Vice President and Chief Financial Officer;
· Samuel M. Liang, our President of Hanger Clinic;
· Kenneth W. Wilson, our former President of Products & Services. Mr. Wilson resigned from the Company April 16, 2018; and
· Thomas E. Hartman, our Senior Vice-President, General Counsel and Secretary.
Overview of Our Executive Compensation Programs
Below are the purpose and key elements of our executive compensation program.
|
Element |
|
Purpose and Characteristics |
|
|
|
|
|
Base Salary |
· |
Fixed pay element to compensate for an individual’s competencies, skills and experience as valued in the marketplace and within the Company and to reward continued performance. |
|
|
· |
Base salary may be adjusted annually/periodically based on changes in job responsibilities, market conditions and individual performance. |
|
|
|
|
|
Short-Term Incentives |
· |
Performance-based annual cash opportunity to motivate and reward the achievement of annual financial results relative to business specific targets and individual goals tied to strategic initiatives. |
|
|
· |
Incentive goals are aligned with stakeholders’ interests. |
|
|
· |
Awards, if earned, are payable based on actual results. |
|
|
|
|
|
Long-Term Incentives |
· |
Performance-based equity opportunity to motivate and reward financial performance and stock price appreciation. |
|
|
· |
Amounts earned and realized will vary from the grant date fair value based on actual stock price performance. |
|
|
|
|
|
Retirement Benefits |
· |
Component of compensation that accrues each year to encourage employment stability of our executive leadership. |
|
|
· |
Benefits are payable upon or after retirement. |
|
|
|
|
|
Other Benefits and Perquisites |
· |
Generally certain pay elements which provide for life and income security needs; the actual cost to the Company is based on participation/usage. |
|
|
|
|
|
Severance Benefits |
· |
Contingent component to provide a bridge to future employment in the event an executive’s employment is terminated. |
|
|
· |
Payable only if an executive’s employment is terminated in certain predefined situations. |
Consideration of Advisory Shareholder Vote on Executive Compensation
As a result of the Company’s delay in filing periodic reports with the SEC, we have not held an annual meeting of shareholders since May 2014. Nevertheless, our Compensation Committee appreciates and values the compensation views of shareholders, and we intend to hold an annual meeting of shareholders later in 2018. Without the ability to respond to shareholders’ vote on our pay proposals, our Compensation Committee relied more heavily on the benchmarking and advice provided by the Committee’s independent compensation advisors for input into the Company’s pay practices. Our Compensation Committee concluded that the compensation paid to our executive officers and the Company’s overall pay practices continue to align with competitive marketplace practices and ranges. As a result, our Compensation Committee decided to retain our general approach to executive compensation, with an emphasis on performance-based annual and long-term incentive compensation that rewards our most senior executives when they successfully implement our business plan and, in turn, deliver value for our shareholders.
Our Compensation Committee recognizes that executive pay practices and notions of sound governance principles continue to evolve. Consequently, our Compensation Committee intends to continue paying close attention to competitive market practices and the advice and counsel of its independent compensation advisors and invites our shareholders to communicate any concerns or opinions on executive pay directly to our Compensation Committee or our Board of Directors.
At the annual meeting of shareholders held on May 12, 2011, our shareholders expressed a preference that advisory votes on executive compensation occur every year. In accordance with the results of this vote, our Board of Directors determined to implement an advisory vote on executive compensation every year until the next vote on the frequency of shareholder votes on executive compensation, which is scheduled to occur at our 2018 annual meeting of shareholders.
Pay Setting Process
Our Compensation Committee has used Korn Ferry Hay Group (“KFHG”) as its compensation consultant on executive compensation matters. Our Compensation Committee assessed the independence of KFHG and concluded that the work performed by the consultants for our Compensation Committee did not raise any conflict of interest under NYSE listing standards or SEC rules. For 2017, KFHG was paid $106,997 for executive compensation consulting services. For other board and executive search and management consulting services provided to our Company in 2017, KFHG was paid $522,675.
Our Compensation Committee works closely with our compensation consultant to understand general market practices, prevalence and trend information on the levels of salary, target annual incentives and long-term incentives, the relative mix of short- and long-term incentives and the mix of cash and stock-based pay for our executive roles. To determine competitive market pay, our Compensation Committee periodically analyzes the annual proxy statements of a peer group of companies and published survey data. In setting pay for our named executive officers, our Compensation Committee has established the target for compensation, by element and in the aggregate, as the competitive market pay median (50th percentile). Our competitive market pay median (the “Median”) is determined by averaging the in-depth peer group analysis and the published survey data. The design of our annual and long-term incentive plans provides our executives with the opportunity to exceed the Median for total direct compensation (the sum of base salary, annual bonus and long term incentives) based on Company performance. Actual compensation on a yearly basis, based on Company and individual performance, can vary widely, as our history has demonstrated.
Peer Group
Our Compensation Committee reviews and approves annually a peer group that it believes best reflects the competitive market for talent in our industry. In August 2016, our Compensation Committee approved the peer group listed below (the “2017 Peer Group”) to benchmark the overall executive pay program for our named executive officers. Our peer group construction methodology generally utilizes the following selection parameters to select peer companies: (1) company revenue size within a specified range of our historic revenue; (2) healthcare services, facilities or services industry sector; (3) similar healthcare payment models; (4) similar executive talent market; (5) national or global presence of business; and (6) demographics of customers.
When our Compensation Committee re-examined the peer group for 2017, it used a number of specific factors as criteria for inclusion including organization size and scope, industry and sector competitors, business model and executive talent market. The Compensation Committee chose to add one company to the peer group for 2017: BioScrip. The 2017 Peer Group does not include AmSurg, which was included in the 2016 Peer Group. AmSurg was removed because it merged with Envision Healthcare, resulting in a company with significantly more revenue than ours.
Our Compensation Committee believes that the 2017 Peer Group was reflective of the market we faced in 2017 and positioned our executive compensation benchmarking appropriately. Our Compensation Committee believes that, while they are not specific to the orthotics and prosthetics area of health care, the companies in the peer group reflect the range of business sectors where we are active. The sub-industry mix of these companies is 25% in Health Care Equipment, 25% in Health Care Facilities and 50% in Health Care Services. Our Compensation Committee further believes that these companies have executive talent who possess comparable skills and face similar business challenges common to our industry.
|
Peer Companies (1) |
|
Sub-Industry |
|
Acadia Healthcare Co. Inc. |
|
Health Care Facilities |
|
Air Methods Corp. |
|
Health Care Services |
|
Amedisys Inc. |
|
Health Care Services |
|
BioScrip |
|
Health Care Services |
|
Civitas Solutions Inc. |
|
Health Care Services |
|
Five Star Quality Care Inc. |
|
Health Care Facilities |
|
Healthsouth Corp. |
|
Health Care Facilities |
|
Healthways Inc. (rebranded to become Tivity Health) |
|
Health Care Services |
|
Integra LifeSciences Holdings Corporation |
|
Health Care Equipment |
|
LHC Group Inc. |
|
Health Care Services |
|
MEDNAX, Inc. |
|
Health Care Services |
|
National Healthcare Corp. |
|
Health Care Facilities |
|
Orthofix International N.V. |
|
Health Care Equipment |
|
Providence Service Corp. |
|
Health Care Services |
|
Teleflex Incorporated |
|
Health Care Equipment |
|
Wright Medical Group Inc. |
|
Health Care Equipment |
(1) The 2017 Peer Group’s 50th percentile revenue for fiscal year 2015, which was the data available to the Compensation Committee at the time that the peer group was selected, was $1,183 million.
Our Compensation Committee reviewed and considered KFHG’s analysis of the 2017 Peer Group pay practices for similarly situated executives to each named executive officer. KFHG’s analysis included, but was not limited to, a review of pay levels (base salary, annual incentives, total cash compensation, long-term incentives and total direct compensation) and pay structure (allocation of pay among base salary, annual incentives and long-term incentives).
Compensation Survey Data
In addition to the 2017 Peer Group data, our Compensation Committee also considered published survey data for a broader market perspective on executive compensation pay levels and practices. Our Committee believes that an alternative lens into the executive labor market is appropriate and meaningful in that survey data provides a robust data set, can be utilized for other executives who are not named executive officers and is consistent with our holistic approach to benchmarking executive compensation. For purposes of this year’s assessment, our Committee used KFHG’s 2016 Industrial Industry Survey. The cash compensation data was aged to November 1, 2016. Our Compensation Committee was provided a specific list of companies underlying the survey data, but did not select or otherwise have input on the companies participating in the survey.
Factors to Set or Adjust Pay
For each named executive officer, our Compensation Committee considers the relevant data regarding our peer group and the salary survey data. For each individual, we also focus specifically on:
· The transferability of professional and managerial skills;
· The depth of knowledge and experience in orthotics and prosthetics and related industries;
· The relevance of the named executive officer’s experience to other potential employers; and
· The readiness of the named executive officer to assume a different or more significant role either within our Company or with another organization.
The following factors are also considered in setting and adjusting pay for our named executive officers:
· The Company’s financial performance;
· The individual’s past and expected future performance;
· Peer group pay practices and broader market developments/trends; and
· Our business and people needs.
Focus on Pay-for-Performance
Our Compensation Committee sets each officer’s total direct compensation to approximate Median practices.
Consistent with our compensation philosophy and objectives, our Compensation Committee emphasizes performance-based incentive opportunities, particularly long-term incentives, over base salary when determining the mix of elements that constitute an officer’s total direct compensation. The following tables show the targeted and actual 2017 pay mix for our Chief Executive Officer and for our other named executive officers as a group.
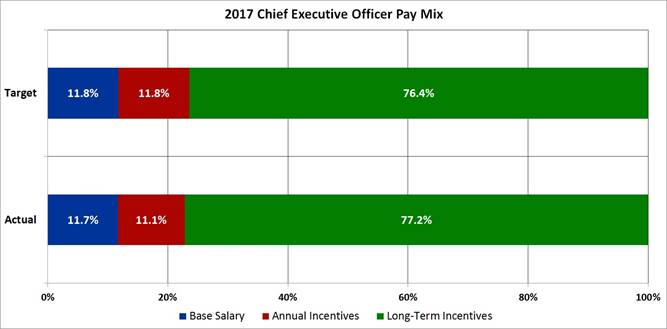
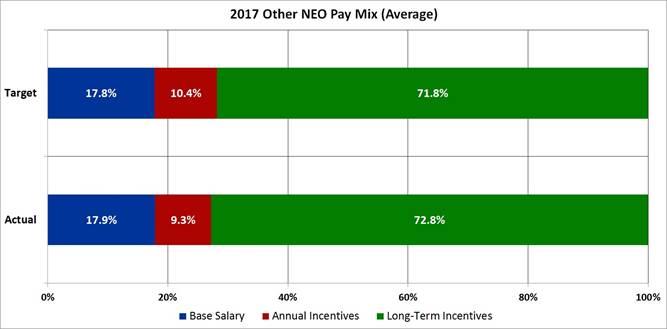
Determination of Pay Elements
In developing the pay programs and levels for our named executive officers, our Compensation Committee reviews peer group pay practices, survey data and other relevant benchmarks provided by the compensation consultant. Any changes to base salary and annual incentive target amounts generally become effective in March of each year.
Annually, our Chief Executive Officer reviews the performance of each of the other named executive officers and shares his perspective with our Compensation Committee. Our Compensation Committee considers this performance information in setting the pay for our named executive officers other than our Chief Executive Officer. All decisions regarding any adjustment to the compensation of our Chief Executive Officer are made solely by our Compensation Committee based on both competitive pay practices as well as our Compensation Committee’s assessment of his performance.
Our Compensation Committee considers previous compensation earned by the named executive officers and their current holdings of Company common stock when making compensation decisions. We believe that our named executive officers should be fairly and competitively compensated, both for annual and long-term compensation opportunities, based on the Company’s performance and each individual’s performance.
Our Compensation Committee may meet in executive session without the presence of any member of management and/or the consultant in making its decisions regarding the compensation of any of our named executive officers.
When making any executive compensation decision, our Compensation Committee follows a deliberate, multiple-step process:
1. Information review;
2. Evaluation and deliberation; and
3. Decision making.
First, our Compensation Committee collects all essential information that may be necessary to make an educated decision from our compensation consultant, our Chief Executive Officer or other sources. Next, our Compensation Committee members discuss the information and a deliberation of possible options ensues. After discussion, our Compensation Committee takes time for reflection and, where appropriate, consultation with other members of our Board of Directors.
Finally, our Compensation Committee reconvenes for additional discussion, if needed, before a final decision is made. As a result, some compensation decisions require two or more Compensation Committee meetings before any final decisions are made.
Additional information about the role and processes of our Compensation Committee is outlined in our Compensation Committee charter, which is available on the Company’s website at www.hanger.com.
Base Salary
As discussed above, our Compensation Committee targets base salary levels for our named executive officers at the Median. We generally aim for our named executive officers’ base salaries to fall within the competitive range of the Median, which we broadly define as within 85% to 115% of the Median for each position. For 2017, each of our named executive officers’ base salaries fell within the competitive range of the Median. Individual increases to base salary are based upon several considerations, including individual performance and contributions, internal equity considerations, as well as competitive market factors and practices.
Base salary compensates a named executive officer for the individual’s competencies, skills, experience and performance. When considering a candidate for a named executive officer role, our Compensation Committee considers all of these factors. For annual adjustments to the base salary of a named executive officer, our Compensation Committee primarily considers the Median, information set forth in general industry surveys, the Company’s performance, the individual’s performance and internal equity amongst our officers. Changes in the scope of a named executive officer’s role and responsibilities could result in an adjustment being considered and approved by our Compensation Committee at any time during the year.
For 2018 base salary adjustments, our Compensation Committee considered the peer group analysis and published survey data described earlier. Our Compensation Committee increased the base salaries of our named executive officers effective March 2018 by an average of 1.2%. Individual adjustments ranged from 0% to 3%, based on an analysis of past individual performance as well as market position of the base salary compared to the Median for the position. Our Compensation Committee believes that appropriate adjustments to base salary should continue to position the pay of our named executive officers within the competitive range of the Median.
Short-Term Incentive Compensation
Our Compensation Committee designs the short-term incentive compensation program to motivate and reward the achievement of annual financial results and individual goals. Currently, our philosophy for short-term incentive compensation is to generally target the Median, to provide the opportunity to earn in the range of the 75th percentile compared to peer group and published survey data with the achievement of exceptional Company and individual performance, and to reduce or even eliminate short-term incentive compensation when Company or individual performance is below expectations. In other words, when we reach target performance for the goals discussed below, then short-term incentive compensation should be close to the Median. If our Company and our named executive officers have exceptional performance based on the established performance goals, then short-term incentive compensation should exceed the Median and could approach the 75th percentile compared to the peer group and published survey data. If our Company or named executive officers performance is less than expected based on the established performance goals, then short-term incentive compensation should be less than Median and could be eliminated entirely.
In 2017, our Compensation Committee approved the continuation of the short-term incentive program comprised of two financial metrics which are leveraged by individual operational and strategic performance. The two metrics are revenue and “Adjusted EBITDA” which is defined as earnings before interest, taxes, depreciation, amortization, impairment of intangible assets, fees paid to third parties for Remediation and other miscellaneous non-recurring items. Revenue is weighted 35% and Adjusted EBITDA is weighted 65% to provide more emphasis on profitability to align with the current strategic initiatives of the Company while maintaining an appropriate emphasis on top-line growth. To receive any payment, the minimum threshold must be met for Adjusted EBITDA. If the results of the financial metrics are within the performance
ranges established for the plan, the amounts actually earned by the named executive officer are determined on the basis of individual performance against individual goals. Each financial metric is calculated independently with a potential funding for each metric that ranges from 20% of the target at threshold to 100% if financial goals are achieved at the target level and 200% if the financial goals are achieved at or above the maximum levels. The financial performance measures for our 2017 annual incentive program were:
|
Performance Measure |
|
Percentage |
|
|
Revenue |
|
35 |
% |
|
Earnings Before Interest, Taxes, Depreciation, Amortization and other items (“Adjusted EBITDA”) |
|
65 |
% |
The financial results used for the short-term incentive are for all of Hanger (enterprise) for all named executive officers except the President of Hanger Clinic. The table below shows the business unit mix for each executive.
Business Unit Mix for Financial Measures
|
Named Executive Officers |
|
Enterprise |
|
Hanger Clinic |
|
|
Vinit K. Asar |
|
100 |
% |
|
|
|
Thomas E. Kiraly |
|
100 |
% |
|
|
|
Samuel M. Liang |
|
75 |
% |
25 |
% |
|
Kenneth W. Wilson |
|
100 |
% |
|
|
|
Thomas E. Hartman |
|
100 |
% |
|
|
The financial goals for our 2017 short-term incentive program at threshold, target and maximum are presented in the table below. For the 2017 short-term incentive program, our Compensation Committee lowered the percentage of incentive payable if threshold performance goals are met from 35% to 20% to better align our short-term incentive payouts with the Company’s overall budget.
|
|
|
Threshold |
|
Target |
|
Maximum |
|
Actual Results |
| ||||
|
Enterprise Measures |
|
|
|
|
|
|
|
|
| ||||
|
Revenue |
|
$ |
1,025,100 |
|
$ |
1,071,400 |
|
$ |
1,129,100 |
|
$ |
1,041,000 |
|
|
Adjusted EBITDA |
|
$ |
120,000 |
|
$ |
130,000 |
|
$ |
148,800 |
|
$ |
125,400 |
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Hanger Clinic Measures |
|
|
|
|
|
|
|
|
| ||||
|
Revenue |
|
$ |
838,900 |
|
$ |
870,400 |
|
$ |
913,300 |
|
$ |
851,800 |
|
|
Adjusted EBITDA |
|
$ |
148,300 |
|
$ |
158,700 |
|
$ |
178,600 |
|
$ |
148,600 |
|
Our Compensation Committee sets the performance measure targets for a given year based on the Company’s strategic budgeting and goal setting process that begins in October of the previous year and is finalized in February of the year for which the targets will apply. In the first quarter of each year, our Compensation Committee approves the specific objectives for threshold, target and maximum levels for each of the performance measures used for the short-term incentive program for our named executive officers.
In addition to these financial goals, our named executive officers have individual goals that they must achieve for their individual performance which are focused on the Company’s strategic and operational initiatives. Individual performance is measured on initiatives such as cost reductions, process improvement, business development opportunities and people initiatives. An executive’s individual objectives may be qualitative and/or quantitative. The individual goals are typically developed to be stretch goals that are challenging for the executive to achieve. The overall results took into consideration our unaudited financial results where applicable.
|
Named Executive Officer |
|
2017 Individual Performance Goals/Results |
|
|
|
|
|
Mr. Asar |
|
Goals: financial growth objectives, strategic growth, accounting and shareholder credibility, culture advancement, and key leadership development. |
|
|
|
Results: in total, Mr. Asar’s results were at target. |
|
|
|
|
|
Mr. Kiraly |
|
Goals: accounting and shareholder credibility, data intelligence, financial growth objectives, cash flows and borrowings, accounting and finance organizational strength and culture |
|
|
|
Results: in total, Mr. Kiraly’s results were above target. |
|
|
|
|
|
Mr. Liang |
|
Goals: financial growth objectives, field process improvements, clinic strategic value initiatives, talent engagement and retention, reimbursement and outcomes |
|
|
|
Results: in total, Mr. Liang’s results were at target. |
|
|
|
|
|
Mr. Wilson |
|
Goals: financial growth objectives, supply chain transformation, strategic growth pivot plan, customer satisfaction and quality improvements. |
|
|
|
Results: in total, Mr. Wilson’s results were below target. |
|
|
|
|
|
Mr. Hartman |
|
Goals: regulatory and corporate governance initiatives, securities litigation, material weakness remediation and compliance programs. |
|
|
|
Results: in total, Mr. Hartman’s results were at target. |
After year end, our Compensation Committee assesses the attainment of the performance measures for the most recently completed year for the short-term incentive program against both financial and individual goals. Typically, the Compensation Committee makes the final assessment of the year-end results in February, at which time bonuses, if any, are approved for payment by March 15th. In February 2018, we did not have final, audited financial results for the 2017 performance year as a result of our ongoing financial restatement process, and so the Compensation Committee used unaudited financial results to assess attainment of bonuses. In February 2018, we reviewed 2017 results related to corporate and individual performance. The final assessment provided incentive funding attainment of between 51% and 58% for the financial metrics set under the short-term incentive plans. Our Compensation Committee decided to provide additional discretionary funding to bring the overall funding to 95% in recognition of the financial performance of the Patient Care segment and overall financial performance improvement of the Company as a whole compared to the prior year, certain significant achievements in 2017, and as an employee retention tool. The significant achievements included the filing on May 12, 2017 of our 2014 Form 10-K, which included restated financial information for 2012, 2013 and the first two quarters of 2014, and the substantial preparation of our 2016 Form 10-K, which included financial information for 2015 and 2016, and which was subsequently filed on January 19, 2018. Although the overall payout was set at 95%, the actual payout to each individual executive officer was adjusted to reflect that individual’s performance against their individual performance goals. As a result, our named executive officers received payouts ranging from 56% to 99% of their respective targets.
The target and maximum annual incentive awards for 2017 expressed as a percentage of base salary for our named executive officers are included in the below table. Our Compensation Committee sets these targets for annual incentives based on the Median of the annual incentives of our peer group and published survey data provided by our compensation consultant as
discussed previously. Our Compensation Committee used the same percentages of base salary for our continuing officers in the 2017 short-term incentive plan.
|
Incentive Awards Expressed as a Percentage of Base Salary |
|
Target |
|
Maximum |
|
|
Vinit K. Asar |
|
100 |
% |
200 |
% |
|
President and Chief Executive Officer |
|
|
|
|
|
|
Thomas E. Kiraly |
|
70 |
% |
140 |
% |
|
Executive Vice President and Chief Financial Officer |
|
|
|
|
|
|
Samuel M. Liang |
|
60 |
% |
120 |
% |
|
President, Hanger Clinic |
|
|
|
|
|
|
Kenneth W. Wilson |
|
50 |
% |
100 |
% |
|
Former President, Products & Services |
|
|
|
|
|
|
Thomas E. Hartman |
|
50 |
% |
100 |
% |
|
Senior Vice President, General Counsel and Secretary |
|
|
|
|
|
In February 2018, the Compensation Committee increased the target short-term incentive for Mr. Asar from 100% to 120%, effective March 2018, to further improve the competitive position of his total compensation package as compared to the Median.
Long Term Incentive Compensation
Long term incentive compensation opportunities are provided to our named executive officers to encourage the executives’ continued commitment to our Company by motivating and rewarding financial performance and stock price appreciation. Our Compensation Committee believes this is an important component of their pay which directly aligns the interests of our executives with the interests of our shareholders since amounts granted, earned and realized are dependent on actual stock price performance.
Our Compensation Committee approved the 2017 grants in March 2017 using a 50/50 split between performance-based and time-based grants for all named executive officers with the exception of Mr. Asar, who received his grants using a 60/40 split because of the Compensation Committee’s desire to have a higher proportion of his long-term incentive grant to be performance-based. With the assistance of the Committee’s independent compensation advisors, the Compensation Committee determined grant sizes taking into account factors including value on grant date, dilution, burn rate, and the Company’s recent and expected financial performance. The Compensation Committee approved grants for management and employees, and when combined with the non-employee director grants in May, the grants did not exceed a 2.5% annual burn rate. The grant date for management and employees was March 8, 2017. The time-based restricted share units granted to our named executive officers vest 25% annually over four years on the anniversary of the grant date commencing on the first anniversary. The performance-based restricted share units are only earned if the Company achieves the adjusted Earnings Per Share (“EPS”) performance goal established at the time of the grant and the related service conditions are met. If performance-based restricted share units are earned, the earned shares will vest 25% annually over four years on the anniversary of the grant date commencing on the first anniversary. The adjusted EPS goal for the 2017 grant was to achieve $0.44 per share for the twelve month period from January 2017 through December 2017. Our Compensation Committee created an additional incentive for the named executive officers if this adjusted EPS target was exceeded. Specifically, if the Company achieved an adjusted EPS goal of $0.54, then the named executive officers would receive 150% of their target performance-based awards. Based on the actual results and adjustments to the performance goal, the adjusted EPS is $0.45, resulting in a 105% attainment of the target performance-based awards. The adjustments made pursuant to the Plan under the terms of the performance-based restricted share unit grants were in the categories of asset write-downs, discontinued operations, changes in tax or accounting principles, regulations or laws, extraordinary, unusual and/or non-recurring items of gain or loss, and certain accruals of amounts for payment under the Plan, as well as a discretionary adjustment applied by the Compensation Committee to eliminate the benefit otherwise provided by the Company’s actual federal income tax rate in 2017.
In the first and second quarters of 2017, our Compensation Committee had multiple discussions with its independent compensation advisors about additional long-term retention strategies beyond the annual long-term incentive program for key leaders, including the named executive officers, given the contributions made and still required through this stabilization and preparation for growth phases of the Company. Ultimately, the Compensation Committee decided to create a Special Equity Plan and make a special one-time equity grant to certain key employees, including each of our named executive officers, for both recognition and retention after the Company filed its 2014 Form 10-K in May 2017. This special equity grant, awarded on May 19, 2017, was comprised of a mixture of two types of stock. The first was nonqualified stock options, which vest 33.3% annually over three years on the anniversary of the grant date commencing on the first anniversary. The second was performance-based restricted stock units which, if earned, cliff vest 100% on the third anniversary of the grant date. The financial target for the special equity grant of performance-based restricted stock units is to achieve a compounded annual growth rate (“CAGR”) of the Company’s common stock price of 20% as of market close on May 18, 2020. This equates to a share price on that date of $22.07 compared to the closing price on the eve of grant of $12.77. The grant provides for the vesting of 50% of the original targeted shares if a CAGR of 10% (a stock price of $17.00) is achieved. The grant also provides for the vesting of up to 200% of the original targeted shares if a CAGR of 30% (a stock price of $28.06) or more is achieved. The percentage of vested shares will be interpolated on a linear basis between 50% and 200% for a CAGR between 10% and 30%. The Special Equity Plan was created for these grants only, and no future grants are permitted or will be made under the plan.
Our restricted share unit awards are generally taxable income to the named executive officer when the award vests in the amount equal to the number of share units vested multiplied by our stock price on the vesting date. We generally receive a tax deduction in the same amount at the same time. The grants are valued as of the grant date for accounting purposes in accordance with FASB Accounting Standards Codification 718 (“ASC 718”).
Other Pay Elements
General Employee Benefits
Our Compensation Committee provides our executives, and all of our employees who qualify, with a benefits program that includes health, dental, disability and life insurance as well as a 401k savings plan with a Company match. This basic yet comprehensive approach provides our named executive officers with a broad umbrella of coverage.
Employment Agreements
Our Company has entered into employment agreements with all of our named executive officers. The agreements generally provide for compensation and benefits such as:
· Base salary;
· Annual and long-term incentive opportunities;
· Benefits that are provided to all of our employees who meet the eligibility requirements;
· Various executive benefits such as a company provided automobile;
· Severance benefits; and
· Change in control severance protection which may only be triggered upon a change in control and a material change in the terms of employment or responsibilities.
Our Company currently provides no other special benefits not outlined in the agreements. In January 2012, our Compensation Committee amended the agreements to eliminate all excise tax gross-ups for executive benefits. The excise
tax gross-up provisions were replaced with a provision that provides that the payments made to an executive officer under the agreement, and any other payments made in connection with the change of control of the Company, will either be capped as necessary to avoid the officer incurring any excess parachute payment excise tax or be paid in full (with the officer paying any excise taxes due), whichever places him or her in the best after-tax position.
We believe these employment agreements provide clarity as to the terms and conditions of employment as well as protect the Company’s interests through the non-compete provisions. Further, we intend for the change in control benefits to provide some economic stability to our named executive officers to enable them to focus on the performance of their duties without undue concern over their personal circumstances if there is a potential change of control of our Company.
The employment agreement of each named executive officer is described below.
Employment Agreement with Mr. Vinit K. Asar
The employment and non-compete agreement between the Company and Vinit K. Asar, our President and Chief Executive Officer, as amended and restated August 27, 2012, provides for the continued employment of Mr. Asar unless the employment agreement is terminated by either party pursuant to the terms therein.
The employment agreement entitles Mr. Asar to certain perquisites that were offered to him to complete his overall annual compensation package. These benefits include:
· Premiums for supplemental life insurance equal to two times his salary; and
· An automobile allowance in the amount of $1,250 per month and the provision of, or reimbursement for, parking of such automobile at our main office.
Mr. Asar is a participant in our Supplemental Executive Retirement Plan. Pursuant to the agreement, his benefit under this plan is equal to 65% of his final average base salary based on the three highest years of the last five years of his employment assuming normal retirement age of 65.
Mr. Asar’s employment agreement contains a severance provision which provides that upon the termination of his employment without cause, Mr. Asar will receive severance compensation equal to 24 months of his base salary then in effect plus two years of his annual target bonus, as well as continuation of certain welfare and perquisite benefits for a period of eighteen months. In addition, Mr. Asar will be eligible for outplacement services commensurate with those available to other senior corporate officers of the Company for a period of 24 months following such termination.
Mr. Asar’s employment agreement further provides that upon the occurrence of a material and negative alteration of the scope of Mr. Asar’s position, duties or title, or upon the occurrence of a material reduction of his compensation or benefits, Mr. Asar may provide the Company with notice of his intent to resign and, if the Company does not cure such alteration or reduction within 30 days thereafter, Mr. Asar may resign and receive severance compensation equal to 24 months of his base salary then in effect plus two years of his annual target bonus, as well as continuation of certain welfare and perquisite benefits for a period of eighteen months. In addition, Mr. Asar will be eligible for outplacement services commensurate with those available to other senior corporate officers of the Company for a period of 24 months following such resignation.
Mr. Asar’s employment agreement further provides that if his employment is terminated within two years after a change in control of the Company and the occurrence of a material diminution of his responsibilities, a reduction of his compensation or benefits, a relocation of his principal site of employment more than 50 miles from his then current location or any material breach of his employment agreement by the Company, then within 90 days after the occurrence of any such triggering events, Mr. Asar may resign and receive a continuation of certain welfare and perquisite benefits for a period of eighteen months and severance compensation equal to 24 months of his base pay then in effect plus two years of his annual target bonus. In addition, Mr. Asar will be eligible for outplacement services commensurate with those available to other senior corporate officers of the Company for a period of 24 months following such termination.
All restricted share units granted to Mr. Asar will immediately vest on the date of his termination, if such termination is by reason of his death or disability, termination without cause, a voluntary termination following the occurrence of certain material alterations or reductions which are not timely corrected by the Company, retirement upon or after age 65 or following a change in control.
Mr. Asar’s agreement also contains non-compete and non-solicitation provisions that provide that upon the termination of his employment, he will be unable to engage in any business that is competitive with the Company anywhere in the continental United States, and he will be unable to solicit any of the Company’s employees or customers for a period of 24 months.
Employment Agreement with Mr. Thomas E. Kiraly
The employment and non-compete agreement between the Company and Thomas E. Kiraly, our Executive Vice President and Chief Financial Officer, dated as of September 5, 2014, provides for the continued employment of Mr. Kiraly unless the employment agreement is terminated by either party pursuant to the terms therein.
The employment agreement entitles Mr. Kiraly to certain perquisites that were offered to him to complete his overall annual compensation package. These benefits include:
· Life insurance equal to whatever the Company provides to its employees, plus additional life insurance in an amount equal to $450,000;
· The option to participate in the Company’s supplemental life and accidental death and dismemberment insurance policies; and
· An automobile allowance in the amount of $1,000 per month and the provision of, or reimbursement for, parking of such automobile at our main office.
Mr. Kiraly is also entitled to participate in our Supplemental Executive Retirement Plan.
Mr. Kiraly’s employment agreement contains a severance provision which provides that upon the termination of his employment without cause, Mr. Kiraly will receive severance compensation equal his base salary at the annual amount then in effect and vacation accrued through the termination date and his bonus for the termination year. In addition, on the date that is six months and one day after the termination date, a lump sum equal to eighteen months his base salary then in effect plus an additional bonus payment equal to one and one-half times the target bonus for the termination year, as well as continuation of certain welfare and perquisite benefits for a period of eighteen months. In addition, Mr. Kiraly will be eligible for outplacement services commensurate with those available to other senior corporate officers of the Company for a period of eighteen months following such termination.
Mr. Kiraly’s employment agreement further provides that if his employment is terminated within two years after a change in control of the Company and the occurrence of a material diminution of his responsibilities, a reduction of his compensation or benefits, a relocation of his principal site of employment more than 50 miles from his then current location or any material breach of his employment agreement by the Company, then within 90 days after the occurrence of any such triggering events, Mr. Kiraly may resign and receive a continuation of certain welfare and perquisite benefits for a period of eighteen months and his severance compensation. In addition, Mr. Kiraly will be eligible for outplacement services commensurate with those available to other senior corporate officers of the Company for a period of eighteen months following such termination. In the event of his death or disability, Mr. Kiraly or his estate will receive a payment equal to his base salary at the annual rate in effect and vacation as accrued through the termination date and his bonus.
All restricted share units granted to Mr. Kiraly will immediately vest on the date of his termination, if such termination is by reason of his death or disability, termination without cause, a voluntary termination following the occurrence of certain
material alterations or reductions which are not timely corrected by the Company, retirement upon or after age 65 or following a change in control.
Mr. Kiraly’s agreement also contains non-compete and non-solicitation provisions that provide that upon the termination of his employment, he will be unable to engage in any business that is competitive with the Company anywhere in the continental United States, and he will be unable to solicit any of the Company’s employees or customers for a period of 24 months.
Employment Agreement with Mr. Samuel M. Liang
The employment and non-compete agreement between the Company and Samuel M. Liang, President of Hanger Clinic, dated September 1, 2014, provides for the continued employment of Mr. Liang unless the employment agreement is terminated by either party pursuant to the terms therein.
The employment agreement entitles Mr. Liang to certain perquisites that have been offered to him to complete his overall annual compensation package. These benefits include:
· Premiums for supplemental life insurance equal to $450,000; and
· An automobile allowance in the amount of $1,000 per month and the provision of, or reimbursement for, parking of such automobile at our headquarters.
Mr. Liang is entitled to participate in our Supplemental Executive Retirement Plan. Mr. Liang’s contribution in the Supplemental Executive Retirement Plan shall be determined annually by the Board of Directors or a committee thereof.
Mr. Liang’s employment agreement contains a severance provision which provides that upon the termination of his employment without cause, Mr. Liang will receive severance compensation equal to eighteen months of his base salary then in effect plus a bonus payment of one and one-half times his annual target bonus plus reimbursement for the costs of his benefits for a period of eighteen months. In addition, Mr. Liang will be eligible for outplacement services commensurate with those available to other senior corporate officers of the Company for a period of eighteen months following such termination.
Mr. Liang’s employment agreement further provides that if his employment is terminated within two years after a change in control of the Company and the occurrence of any termination, a material diminution of his responsibilities, a reduction of his compensation, a failure to provide benefits, a relocation of his principal place of employment more than 50 miles from his then current location, or any material breach of his employment agreement by the Company, then within 90 days after the occurrence of any such triggering events, Mr. Liang may resign and receive a payment equal to his base salary then in effect, his accrued vacation and his annual target bonus. In addition, he will receive reimbursement for the costs of his benefits for a period of eighteen months and severance compensation equal to eighteen months of his base salary then in effect plus a bonus payment of one and one-half times his annual target bonus.
All restricted share units granted to Mr. Liang will immediately vest on the date of his termination, if such termination is by reason of his death or disability, termination without cause, retirement upon or after age 65 or following a change in control.
Mr. Liang’s agreement also contains non-compete and non-solicitation provisions that provide that during Mr. Liang’s employment and for a period of 24 months thereafter, he will be unable to engage in any business that is competitive with the Company at any location within the contiguous United States and he will be unable to solicit any of the Company’s employees or customers during such period.
Employment Agreement with Mr. Kenneth W. Wilson
The employment and non-compete agreement between the Company and Kenneth W. Wilson, our former President of Products & Services, as amended and restated February 25, 2013, provided for the continued employment of Mr. Wilson unless the employment agreement is terminated by either party pursuant to the terms therein. This agreement has now terminated effective upon Mr. Wilson’s departure from the Company on April 16, 2018, due to his voluntary resignation. Mr. Wilson did not receive any amounts payable under the terms of his employment agreement at the time of his departure.
Mr. Wilson’s agreement contained non-compete and non-solicitation provisions that provide that during Mr. Wilson’s employment and for a period of two years thereafter, he will be unable to engage in any business that is competitive with the Company at any location within the contiguous United States at which he performed services or had oversight management responsibility and unable to solicit any of the Company’s employees or customers during such period.
Employment Agreement with Mr. Thomas E. Hartman
The employment and non-compete agreement between Hanger and Thomas E. Hartman, our Senior Vice President, General Counsel and Secretary, as amended and restated as of March 30, 2012, provides for the continued employment of Mr. Hartman unless the employment agreement is terminated by either party pursuant to the terms therein.
The employment agreement entitles Mr. Hartman to certain perquisites that were offered to him to complete his overall annual compensation package. These benefits include an automobile allowance in the amount of $700 per month and the provision of, or reimbursement for, parking of such automobile at our main office. Mr. Hartman is a participant in our Supplemental Executive Retirement Plan. Pursuant to the agreement, his benefit under this plan is equal to 40% of his final average base salary on the three highest years of the last five years of his employment, assuming normal retirement age of 65.
Mr. Hartman’s employment agreement contains a severance provision that provides that upon the termination of his employment without cause, Mr. Hartman will receive severance compensation equal to one year of his base salary then in effect, plus an additional bonus payment equal to his target bonus for the year in which his employment is terminated, as well as continuation of certain welfare and perquisite benefits for a period of one year. Mr. Hartman’s employment agreement further provides that if his employment is terminated within two years after a change in control of the Company and the occurrence of a material diminution of his responsibilities, a reduction of his compensation or benefits, a relocation of his principal site of employment more than 50 miles from his then current location, or any material breach of his employment agreement by the Company, then within 90 days after the occurrence of any such triggering events, Mr. Hartman may resign and receive the severance compensation and continuation of benefits described above for a period of one year.
Retirement Benefits
Messrs. Asar and Hartman participate in the Company’s nonqualified defined benefit Supplemental Executive Retirement Plan (the “DB SERP”). This benefit is intended to encourage and reward the long-term commitment of our named executive officers to the Company. The DB SERP is a nonqualified, unfunded plan that provides retirement benefits for executive officers and key employees of the Company as designated by our Compensation Committee. The plan contains provisions to ensure its compliance with Internal Revenue Code Section 409A. An outline of the plan provisions is included in the narrative following the Pension Benefits table.
The estimated present value of these benefits at age 65 for each of our named executive officers is shown in the Pension Benefits Table. The projected change (December 2017 versus December 2016) in the present value of this benefit is shown in the Summary Compensation Table.
In May 2013, our Board of Directors, upon the recommendation of our Compensation Committee, adopted the Hanger, Inc. Defined Contribution Supplemental Retirement Plan (the “DC SERP”). The DC SERP is a nonqualified defined contribution plan in which certain executive officers and other senior employees are eligible to participate. Under the terms of the DC SERP, we may credit a participant’s account with either an amount equal to a specified percentage of the
participant’s base salary or a stated flat dollar amount. Participants’ accounts generally vest after five continuous years of employment and participation in the DC SERP, five continuous years of employment and the participant having reached age 60, or a termination of employment without cause or good reason within one year after the Company undergoes a change of ownership or effective control. Although contributions are discretionary, we currently intend to contribute annually an amount to each participant’s account equal to 10-20% of the participant’s base salary. Our Compensation Committee recommended establishing the DC SERP as a means of providing a retirement benefit for certain executive officers who are not covered by the DB SERP. Messrs. Liang, Wilson and Kiraly were during 2017 the only named executive officers currently participating in the DC SERP. The first credits under the DC SERP for Mr. Kiraly and Mr. Liang were made in the first quarter of 2015. Mr. Wilson was not vested in the DC SERP at the time of his departure from the Company in April 2018, and he forfeited the amounts attributable to him under the DC SERP accordingly.
Other Compensation Related Policies
Securities Trading Policy
Our Company has a policy that executive officers and directors may not purchase or sell our stock when they may be in possession of nonpublic material information. In addition, this policy provides that no director or officer may sell short or engage in transactions in put or call options relating to our securities.
Stock Ownership Guidelines
Our Compensation Committee adopted formal stock ownership guidelines for the named executive officers and other key senior managers at the end of 2007, and amended those stock ownership guidelines in 2009 and 2013. These guidelines require the executives to hold a multiple of their base salary in company shares. The President and Chief Executive Officer is required to hold five times his base salary, the Chief Financial Officer and those named executive officers managing a P&L are required to hold three times their base salary, and the named executive officers in staff executive positions other than the Chief Financial Officer are required to hold one time their base salary. Individuals who are newly promoted or newly hired into a named executive officer position have up to five years to reach this level of ownership. Individuals who do not meet this requirement are subject to an evaluation by our Compensation Committee to review individual circumstances, including but not limited to retirement needs of individual officers.
Our named executive officers met the stock ownership requirements as of December 31, 2017.
Impact of Tax and Accounting Considerations
When determining compensation packages for 2017, we considered all factors that may impact financial performance, including tax and accounting rules and regulations under Section 162(m) of the Internal Revenue Code, or “Code.” For the 2017 fiscal year, the Code limited the Company from deducting compensation in excess of $1 million paid to the Chief Executive Officer or to the other three highest-paid executive officers (other than the Chief Financial Officer) for those fiscal years. However, compensation paid during 2017 that qualified as performance-based compensation under Section 162(m) was fully deductible. Our compensation philosophy for fiscal year 2017 emphasized performance-based compensation for executive officers, thus minimizing the consequences of the Section 162(m) limitation as described above. Nevertheless, certain of our performance-based awards, including awards granted under our 2016 Omnibus Incentive Plan, do not qualify for deductibility under Section 162(m) because the plan was not approved by our shareholders.
As a result of changes made by the Tax Cuts and Jobs Act, starting with compensation paid in 2018, Section 162(m) will limit us from deducting compensation, including performance-based compensation, in excess of $1 million paid to anyone who, starting in 2018, serves as the Chief Executive Officer, Chief Financial Officer, or who is among the three most highly compensated executive officers for any fiscal year. The only exception to this rule is for compensation that is paid in the future pursuant to a binding contract in effect on November 2, 2017 that would have otherwise been deductible under the prior Section 162(m) rules. Accordingly, any compensation paid in the future pursuant to new compensation arrangements entered into after November 2, 2017, even if performance-based, will count towards the $1 million fiscal year deduction
limit if paid to a covered executive. Because many different factors influence a well-rounded, comprehensive executive compensation program, and as a result of the changes made to Code Section 162(m) by the Tax Cuts and Jobs Act, some of the compensation we provide to our executive officers may not be deductible as a result of Code section 162(m) if our Committee believes it will contribute to the achievement of our business objectives.
Our Compensation Committee considers the impact of other tax provisions, such as Internal Revenue Code Section 409A’s restrictions on deferred compensation, and attempts to structure compensation in a tax-efficient manner for both the named executive officers and for our Company.
In adopting various executive compensation plans and packages as well as in making certain executive compensation decisions, particularly with respect to grants of equity based long-term incentive awards, our Compensation Committee considers the accounting treatment and the anticipated financial statement impact of such decisions, as well as the anticipated dilutive impact to our shareholders.
Compensation Committee Report
Our Compensation Committee of our Board of Directors has reviewed and discussed the above 2017 Compensation Discussion & Analysis with management and, based on such review and discussion, has recommended to the Board of Directors that the 2017 Compensation Discussion & Analysis be included in our Annual Report on Form 10-K for the year ended December 31, 2017.
|
Christopher B. Begley (Chair) |
|
|
|
Asif Ahmad |
|
|
|
John T. Fox |
|
|
|
Stephen E. Hare |
EXECUTIVE COMPENSATION
SUMMARY COMPENSATION TABLE FOR 2017
The following table sets forth for each of the named executive officers: (i) the dollar value of base salary and bonus earned during the year indicated; (ii) the grant date fair value of stock and option awards granted in the years indicated; (iii) the dollar value of awards granted during the year under non-equity incentive plans; (iv) the change in the actuarial present value of the accumulated pension benefit during the year; (v) all other compensation for the year; and, finally, (vi) the dollar value of total compensation for the year.
|
Name and Principal Position |
|
Year |
|
Salary |
|
Bonus (1) |
|
Stock |
|
Option |
|
Non-Equity |
|
Change in |
|
All Other |
|
Total |
| ||||||||
|
Vinit K. Asar |
|
2017 |
|
$ |
721,692 |
|
$ |
266,810 |
|
$ |
3,299,925 |
|
$ |
1,387,129 |
|
$ |
415,695 |
|
$ |
331,943 |
|
$ |
31,248 |
|
$ |
6,454,442 |
|
|
Chief Executive Officer of the Company |
|
2016 |
|
$ |
711,311 |
|
$ |
— |
|
$ |
1,035,000 |
|
$ |
— |
|
$ |
— |
|
$ |
264,017 |
|
$ |
31,092 |
|
$ |
2,041,420 |
|
|
|
2015 |
|
$ |
695,195 |
|
$ |
— |
|
$ |
2,750,025 |
|
$ |
— |
|
$ |
— |
|
$ |
168,055 |
|
$ |
30,791 |
|
$ |
3,644,066 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
Thomas E. Kiraly |
|
2017 |
|
$ |
467,959 |
|
$ |
136,319 |
|
$ |
1,415,096 |
|
$ |
816,478 |
|
$ |
188,681 |
|
$ |
— |
|
$ |
115,129 |
|
$ |
3,139,662 |
|
|
Executive Vice President and Chief Financial Officer of the Company |
|
2016 |
|
$ |
461,843 |
|
$ |
25,000 |
|
$ |
386,650 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
113,169 |
|
$ |
986,662 |
|
|
|
2015 |
|
$ |
453,635 |
|
$ |
— |
|
$ |
825,002 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
120,746 |
|
$ |
1,399,383 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
Samuel M. Liang |
|
2017 |
|
$ |
472,312 |
|
$ |
122,480 |
|
$ |
1,176,977 |
|
$ |
626,281 |
|
$ |
145,519 |
|
$ |
— |
|
$ |
129,636 |
|
$ |
2,673,205 |
|
|
Executive Vice President of the Company and Chief Operating Officer of Hanger Prosthetics & Orthotics, Inc. and HPO, Inc. |
|
2016 |
|
$ |
465,062 |
|
$ |
75,000 |
|
$ |
352,150 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
120,263 |
|
$ |
1,012,475 |
|
|
|
2015 |
|
$ |
445,602 |
|
$ |
— |
|
$ |
715,026 |
|
$ |
— |
|
$ |
200,000 |
|
$ |
— |
|
$ |
113,371 |
|
$ |
1,473,999 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
Kenneth W. Wilson |
|
2017 |
|
$ |
359,512 |
|
$ |
— |
|
$ |
726,047 |
|
$ |
475,440 |
|
$ |
100,000 |
|
$ |
— |
|
$ |
51,134 |
|
$ |
1,712,133 |
|
|
Former President of Products and Services |
|
2016 |
|
$ |
353,689 |
|
$ |
25,000 |
|
$ |
193,450 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
57,803 |
|
$ |
629,942 |
|
|
|
2015 |
|
$ |
339,728 |
|
$ |
— |
|
$ |
330,032 |
|
$ |
— |
|
$ |
142,000 |
|
$ |
— |
|
$ |
46,571 |
|
$ |
858,331 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
Thomas E. Hartman |
|
2017 |
|
$ |
355,213 |
|
$ |
65,661 |
|
$ |
821,828 |
|
$ |
614,030 |
|
$ |
102,301 |
|
$ |
129,277 |
|
$ |
10,084 |
|
$ |
2,098,394 |
|
|
Senior Vice President, General Counsel and Secretary |
|
2016 |
|
$ |
346,064 |
|
$ |
100,000 |
|
$ |
179,650 |
|
$ |
— |
|
$ |
— |
|
$ |
96,978 |
|
$ |
9,854 |
|
$ |
732,546 |
|
|
|
2015 |
|
$ |
328,273 |
|
$ |
— |
|
$ |
295,019 |
|
$ |
— |
|
$ |
105,000 |
|
$ |
65,310 |
|
$ |
10,022 |
|
$ |
803,624 |
| |
(1) All named executive officers other than Mr. Wilson received a discretionary addition to their bonus earned under the non-equity incentive plan in the following amounts: Mr. Asar: $266,810, Mr. Kiraly: $136,319, Mr. Liang: $122,480, Mr. Hartman: $65,661.
(2) In 2017, there were two distinct stock programs which comprised the amounts shown in this column: the annual stock cycle and a Special Equity award (as described in the Compensation Discussion and Analysis section of this annual report on Form 10-K). The amount reported in this column represents the aggregate grant date fair value of all time-based and performance-based awards granted during each calendar year as calculated in accordance with ASC 718.
The annual stock cycle was completed in March 2017. For this cycle, all restricted stock units vest 25% per year, commencing one year after the date of issuance. For this 2017 cycle, the executives received 150% of performance-based restricted stock units subject to the award: see “Grants of Plan-Based Awards”. For awards of performance-based restricted stock units, amounts shown in the column are the grant date fair values calculated based on the probable outcome of the performance conditions on the date of grant. For 2017, the probable outcome on the date of grant was the target outcome. The value of the performance-based restricted stock units based on the probable outcome was for Mr. Asar: $1,239,300, Mr. Kiraly: $344,250, Mr. Liang: $309,825, Mr. Wilson: $151,470 and Mr. Hartman: $137,700. The value of the performance-based restricted stock units assuming the highest level of performance was for Mr. Asar: $1,858,950, Mr. Kiraly: $516,375, Mr. Liang: $464,738, Mr. Wilson: $227,205, and Mr. Hartman: $206,550.
In May 2017, a Special Equity grant was completed, comprised of a mixture of nonqualified stock options and performance-based restricted stock units (which cliff vest 100% on the third anniversary of the grant date) that were a part of such grant. The amounts shown are the grant date fair values calculated based on the probable outcome of the performance conditions on the date of grant. For 2017, the probable outcome on the date of grant was the target outcome. The value of the performance-based restricted stock units based on the probable outcome was for Mr. Asar: $1,234,425, Mr. Kiraly: $726,596, Mr. Liang: $557,327, Mr. Wilson: $423,107, and Mr. Hartman: $546,428. The value of the performance-based restricted stock units assuming the highest level of performance was for Mr. Asar: $2,468,850, Mr. Kiraly: $1,453,193, Mr. Liang: $1,114,653, Mr. Wilson: $846,214, and for Mr. Hartman: $1,092,856.
(3) The amounts in this column represent the value of nonqualified stock options (vest 33.3% per year, commencing one year after the date of issuance) that were granted as part of our special equity grant. The options are valued using a Black-Scholes model.
(4) The annual short-term incentive awards for 2017 were based on 2017 performance and paid on March 15, 2018.
(5) The above amounts represent the change in actuarial present value of the accumulated pension benefit for each named executive officer who participates in our DB SERP. Details of the DB SERP are described after the Pension Benefits table below and in the Compensation Discussion and Analysis section. We did not provide above-market earnings in our DC SERP, and therefore we have not included any earnings on the DC SERP in this table.
(6) For Mr. Asar, this total includes: premiums for additional life and disability insurance ($9,768), Company contributions to the Company’s defined contribution plan ($6,480), and non-business related automobile expenses ($15,000). For Messrs. Kiraly, Liang, Wilson and Hartman, these totals include: premiums for additional life and disability insurance, non-business related automobile expenses, Company contributions to the individual’s health savings account, and/or Company contributions to the Company’s defined contribution plan. Additionally, Mr. Liang received relocation of $10,593 and contributions were made by the Company to non-qualified deferred compensation plans for Messrs. Kiraly ($92,369), Liang ($93,012) and Wilson ($45,980).
GRANTS OF PLAN-BASED AWARDS IN 2017
The following table sets forth information regarding all incentive plan awards that were granted to the named executive officers during 2017, including incentive plan awards (equity based and non-equity based) and other plan-based awards. Disclosure on a separate line item is provided for each grant of an award made to a named executive officer during the year. Non-equity incentive plan awards are awards that are not subject to ASC 718 and are intended to serve as an incentive for
performance to occur over a specified period. Non-Equity Awards are prorated for changes in base salary and/or target bonus percentages that occur throughout the year.
|
|
|
Grant |
|
Estimated Future Payouts |
|
Estimated Future Payouts |
|
All |
|
All Other |
|
Exercise |
|
Grant |
| |||||||||||||
|
Name |
|
Date |
|
Threshold |
|
Target |
|
Maximum |
|
Threshold |
|
Target |
|
Maximum |
|
Units |
|
Options |
|
Awards |
|
Awards |
| |||||
|
Vinit K. Asar (2) |
|
5/19/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
159,982 |
|
$ |
12.77 |
|
$ |
1,387,129 |
| |||
|
Vinit K. Asar (3) |
|
5/19/2017 |
|
|
|
|
|
|
|
— |
|
63,933 |
|
127,986 |
|
|
|
|
|
|
|
$ |
1,234,425 |
| ||||
|
Vinit K. Asar (4) |
|
3/8/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
60,000 |
|
|
|
|
|
$ |
826,200 |
| ||||
|
Vinit K. Asar (5) |
|
3/8/2017 |
|
|
|
|
|
|
|
— |
|
90,000 |
|
135,000 |
|
|
|
|
|
|
|
$ |
1,239,300 |
| ||||
|
Vinit K. Asar |
|
1/1/2017 |
|
$ |
— |
|
$ |
721,692 |
|
$ |
1,443,385 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Thomas E. Kiraly (2) |
|
5/19/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
94,167 |
|
$ |
12.77 |
|
$ |
816,478 |
| |||
|
Thomas E. Kiraly (3) |
|
5/19/2017 |
|
|
|
|
|
|
|
— |
|
37,667 |
|
75,334 |
|
|
|
|
|
|
|
$ |
726,596 |
| ||||
|
Thomas E. Kiraly (4) |
|
3/8/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
25,000 |
|
|
|
|
|
$ |
344,250 |
| ||||
|
Thomas E. Kiraly (5) |
|
3/8/2017 |
|
|
|
|
|
|
|
— |
|
25,000 |
|
37,500 |
|
|
|
|
|
|
|
$ |
344,250 |
| ||||
|
Thomas E. Kiraly |
|
1/1/2017 |
|
$ |
— |
|
$ |
327,572 |
|
$ |
655,143 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Samuel M. Liang (2) |
|
5/19/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
72,231 |
|
$ |
12.77 |
|
$ |
626,281 |
| |||
|
Samuel M. Liang (3) |
|
5/19/2017 |
|
|
|
|
|
|
|
— |
|
28,892 |
|
57,784 |
|
|
|
|
|
|
|
$ |
557,327 |
| ||||
|
Samuel M. Liang (4) |
|
3/8/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
22,500 |
|
|
|
|
|
$ |
309,825 |
| ||||
|
Samuel M. Liang (5) |
|
3/8/2017 |
|
|
|
|
|
|
|
— |
|
22,500 |
|
33,750 |
|
|
|
|
|
|
|
$ |
309,825 |
| ||||
|
Samuel M. Liang |
|
1/1/2017 |
|
$ |
— |
|
$ |
283,387 |
|
$ |
566,774 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Kenneth W. Wilson (2) |
|
5/19/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
54,824 |
|
$ |
12.77 |
|
$ |
475,440 |
| |||
|
Kenneth W. Wilson (3) |
|
5/19/2017 |
|
|
|
|
|
|
|
— |
|
21,934 |
|
43,868 |
|
|
|
|
|
|
|
$ |
423,107 |
| ||||
|
Kenneth W. Wilson (4) |
|
3/8/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
11,000 |
|
|
|
|
|
$ |
151,470 |
| ||||
|
Kenneth W. Wilson (5) |
|
3/8/2017 |
|
|
|
|
|
|
|
— |
|
11,000 |
|
16,500 |
|
|
|
|
|
|
|
$ |
151,470 |
| ||||
|
Kenneth W. Wilson |
|
1/1/2017 |
|
$ |
— |
|
$ |
179,756 |
|
$ |
359,512 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Thomas E. Hartman (2) |
|
5/19/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
70,818 |
|
$ |
12.77 |
|
$ |
614,030 |
| |||
|
Thomas E. Hartman (3) |
|
5/19/2017 |
|
|
|
|
|
|
|
— |
|
28,327 |
|
56,654 |
|
|
|
|
|
|
|
$ |
546,428 |
| ||||
|
Thomas E. Hartman (4) |
|
3/8/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10,000 |
|
|
|
|
|
$ |
137,700 |
| ||||
|
Thomas E. Hartman (5) |
|
3/8/2017 |
|
|
|
|
|
|
|
— |
|
10,000 |
|
15,000 |
|
|
|
|
|
|
|
$ |
137,700 |
| ||||
|
Thomas E. Hartman |
|
1/1/2017 |
|
$ |
— |
|
$ |
177,607 |
|
$ |
355,213 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
(1) Terms of compensation under the Non-Equity Incentive Plan are discussed in detail in the Compensation Discussion and Analysis section.
(2) These non-qualified stock options were granted on May 19, 2017 and vest 33.3% annually. The Black-Scholes fair value was calculated at $8.67 per share.
(3) The restricted stock detailed above is awarded as performance-based shares. This restricted stock was granted on May 19, 2017 and vests 100% three years after the date of issuance, assuming the performance goal is achieved. The stock price at the time of the award was $12.77, but given market condition performance criteria the Monte Carlo Simulation valuation was used to calculate a fair value of $19.29 per share. Release of the restrictions on this award will be subject to achieving compounded annual growth rate in the Common Stock price for the performance period of May 19, 2017 through May 18, 2020 per the schedule below. Results in between Threshold and Target, and between Target and Maximum, will use straight line calculations for payouts:
|
CAGR Result (May 19, 2017 |
|
Percent of Performance Shares |
|
|
10 |
% |
25 |
% |
|
20 |
% |
100 |
% |
|
30 |
% |
200 |
% |
(4) The time-based restricted stock detailed above was awarded on March 8, 2017. The share price at time of award was $13.77. All shares of restricted stock vest 25% per year, commencing one year after the date of issuance.
(5) The restricted stock detailed above is awarded as performance-based shares. The restricted stock was awarded on March 8, 2017 and vests to the extent of 25% per year, commencing approximately one year after the date of issuance, assuming the pro forma performance goal is achieved. Release of the restrictions on this award was subject to achieving pro forma EPS targets for the performance period of January 1, 2017 through December 31, 2017 per the schedule below. Results in between Threshold and Target, and between Target and Maximum will use straight line calculations for payouts:
|
EPS Result (Q1 2017 through |
|
Percent of Performance Shares |
| |
|
$ |
0.34 |
|
25 |
% |
|
$ |
0.44 |
|
100 |
% |
|
$ |
0.54 |
|
150 |
% |
Our Company’s adjusted EPS was $0.45 for purposes of the calculation of the attainment of the performance goal.
OUTSTANDING EQUITY AWARDS AT 2017 FISCAL YEAR-END
The following table sets forth information on outstanding equity awards held by the named executive officers at December 31, 2017, including the number and market value of restricted stock units and performance based restricted stock units that have not vested.
|
|
|
Option Awards |
|
Stock Awards |
| |||||||||||||||||
|
Name |
|
Number of |
|
Number of |
|
Equity |
|
Option |
|
Option |
|
Number of |
|
Market |
|
Equity |
|
Equity |
| |||
|
Vinit K. Asar (1) |
|
|
|
|
|
159,982 |
|
$ |
12.77 |
|
5/19/2027 |
|
|
|
|
|
|
|
|
| ||
|
Vinit K. Asar (2) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
127,986 |
|
$ |
2,015,780 |
| ||
|
Vinit K. Asar (3) |
|
|
|
|
|
|
|
|
|
|
|
60,000 |
|
$ |
945,000 |
|
|
|
|
| ||
|
Vinit K. Asar (4) |
|
|
|
|
|
|
|
|
|
|
|
94,500 |
|
$ |
1,488,375 |
|
|
|
|
| ||
|
Vinit K. Asar (6) |
|
|
|
|
|
|
|
|
|
|
|
45,000 |
|
$ |
708,750 |
|
|
|
|
| ||
|
Vinit K. Asar (8) |
|
|
|
|
|
|
|
|
|
|
|
21,195 |
|
$ |
333,821 |
|
|
|
|
| ||
|
Vinit K. Asar (9) |
|
|
|
|
|
|
|
|
|
|
|
47,688 |
|
$ |
751,086 |
|
|
|
|
| ||
|
Vinit K. Asar (12) |
|
|
|
|
|
|
|
|
|
|
|
4,494 |
|
$ |
70,781 |
|
|
|
|
| ||
|
Thomas E. Kiraly (1) |
|
|
|
|
|
94,167 |
|
$ |
12.77 |
|
5/19/2027 |
|
|
|
|
|
|
|
|
| ||
|
Thomas E. Kiraly (2) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
75,334 |
|
$ |
1,186,511 |
| ||
|
Thomas E. Kiraly (3) |
|
|
|
|
|
|
|
|
|
|
|
25,000 |
|
$ |
393,750 |
|
|
|
|
| ||
|
Thomas E. Kiraly (4) |
|
|
|
|
|
|
|
|
|
|
|
26,250 |
|
$ |
413,438 |
|
|
|
|
| ||
|
Thomas E. Kiraly (5) |
|
|
|
|
|
|
|
|
|
|
|
3,750 |
|
$ |
59,063 |
|
|
|
|
| ||
|
Thomas E. Kiraly (6) |
|
|
|
|
|
|
|
|
|
|
|
18,750 |
|
$ |
295,313 |
|
|
|
|
| ||
|
Thomas E. Kiraly (8) |
|
|
|
|
|
|
|
|
|
|
|
7,948 |
|
$ |
125,181 |
|
|
|
|
| ||
|
Thomas E. Kiraly (9) |
|
|
|
|
|
|
|
|
|
|
|
11,922 |
|
$ |
187,772 |
|
|
|
|
| ||
|
Thomas E. Kiraly (10) |
|
|
|
|
|
|
|
|
|
|
|
7,449 |
|
$ |
117,322 |
|
|
|
|
| ||
|
Thomas E. Kiraly (10) |
|
|
|
|
|
|
|
|
|
|
|
4,966 |
|
$ |
78,215 |
|
|
|
|
| ||
|
Samuel M. Liang (1) |
|
|
|
|
|
72,231 |
|
$ |
12.77 |
|
5/19/2027 |
|
|
|
|
|
|
|
|
| ||
|
Samuel M. Liang (2) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
57,784 |
|
$ |
910,098 |
| ||
|
Samuel M. Liang (3) |
|
|
|
|
|
|
|
|
|
|
|
22,500 |
|
$ |
354,375 |
|
|
|
|
| ||
|
Samuel M. Liang (4) |
|
|
|
|
|
|
|
|
|
|
|
23,625 |
|
$ |
372,094 |
|
|
|
|
| ||
|
Samuel M. Liang (5) |
|
|
|
|
|
|
|
|
|
|
|
3,750 |
|
$ |
59,063 |
|
|
|
|
| ||
|
Samuel M. Liang (6) |
|
|
|
|
|
|
|
|
|
|
|
16,875 |
|
$ |
265,781 |
|
|
|
|
| ||
|
Samuel M. Liang (8) |
|
|
|
|
|
|
|
|
|
|
|
6,889 |
|
$ |
108,502 |
|
|
|
|
| ||
|
Samuel M. Liang (9) |
|
|
|
|
|
|
|
|
|
|
|
10,333 |
|
$ |
162,745 |
|
|
|
|
| ||
|
Samuel M. Liang (11) |
|
|
|
|
|
|
|
|
|
|
|
1,475 |
|
$ |
23,231 |
|
|
|
|
| ||
|
Samuel M. Liang (11) |
|
|
|
|
|
|
|
|
|
|
|
2,212 |
|
$ |
34,839 |
|
|
|
|
| ||
|
Kenneth W. Wilson (1) |
|
|
|
|
|
54,834 |
|
$ |
12.77 |
|
5/19/2027 |
|
|
|
|
|
|
|
|
| ||
|
Kenneth W. Wilson (2) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
43,868 |
|
$ |
690,921 |
| ||
|
Kenneth W. Wilson (3) |
|
|
|
|
|
|
|
|
|
|
|
11,000 |
|
$ |
173,250 |
|
|
|
|
| ||
|
Kenneth W. Wilson (4) |
|
|
|
|
|
|
|
|
|
|
|
11,550 |
|
$ |
181,913 |
|
|
|
|
| ||
|
Kenneth W. Wilson (5) |
|
|
|
|
|
|
|
|
|
|
|
3,750 |
|
$ |
59,063 |
|
|
|
|
| ||
|
Kenneth W. Wilson (6) |
|
|
|
|
|
|
|
|
|
|
|
8,250 |
|
$ |
129,938 |
|
|
|
|
| ||
|
Kenneth W. Wilson (8) |
|
|
|
|
|
|
|
|
|
|
|
3,180 |
|
$ |
50,085 |
|
|
|
|
| ||
|
Kenneth W. Wilson (9) |
|
|
|
|
|
|
|
|
|
|
|
4,770 |
|
$ |
75,128 |
|
|
|
|
| ||
|
Kenneth W. Wilson (12) |
|
|
|
|
|
|
|
|
|
|
|
916 |
|
$ |
14,427 |
|
|
|
|
| ||
|
Thomas E. Hartman (1) |
|
|
|
|
|
70,818 |
|
$ |
12.77 |
|
5/19/2027 |
|
|
|
|
|
|
|
|
| ||
|
Thomas E. Hartman (2) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
56,654 |
|
$ |
892,301 |
| ||
|
Thomas E. Hartman (3) |
|
|
|
|
|
|
|
|
|
|
|
10,000 |
|
$ |
157,500 |
|
|
|
|
| ||
|
Thomas E. Hartman (4) |
|
|
|
|
|
|
|
|
|
|
|
10,500 |
|
$ |
165,375 |
|
|
|
|
| ||
|
Thomas E. Hartman (5) |
|
|
|
|
|
|
|
|
|
|
|
3,750 |
|
$ |
59,063 |
|
|
|
|
| ||
|
Thomas E. Hartman (6) |
|
|
|
|
|
|
|
|
|
|
|
7,500 |
|
$ |
118,125 |
|
|
|
|
| ||
|
Thomas E. Hartman (7) |
|
|
|
|
|
|
|
|
|
|
|
2,479 |
|
$ |
39,044 |
|
|
|
|
| ||
|
Thomas E. Hartman (8) |
|
|
|
|
|
|
|
|
|
|
|
2,120 |
|
$ |
33,390 |
|
|
|
|
| ||
|
Thomas E. Hartman (9) |
|
|
|
|
|
|
|
|
|
|
|
3,180 |
|
$ |
50,085 |
|
|
|
|
| ||
|
Thomas E. Hartman (12) |
|
|
|
|
|
|
|
|
|
|
|
433 |
|
$ |
6,820 |
|
|
|
|
| ||
(1) These non-qualified stock options were granted on May 19, 2017 and vest 33.3% annually.
(2) These performance-based restricted stock units (conditions discussed in the Compensation Discussion and Analysis section of this Annual Report on Form 10-K) were granted on May 19, 2017 and vest 100% upon the third anniversary of the award.
(3) These time-based restricted stock units were granted on March 8, 2017 and vest 25% annually.
(4) These performance-based restricted stock units (conditions discussed in the Compensation Discussion and Analysis section of this Annual Report on Form 10-K) were granted on March 8, 2017 and vest 25% annually as the performance goal was achieved as of December 31, 2017.
(5) These time-based restricted stock units were granted on October 11, 2016 and vest 25% annually.
(6) These time-based restricted stock units were granted on April 29, 2016 and vest 25% annually.
(7) These time-based restricted stock units were granted on November 10, 2015 and vest 25% annually.
(8) These time-based restricted stock units were granted on March 6, 2015 and vest 25% annually.
(9) These performance-based restricted stock units (conditions discussed in the Compensation Discussion and Analysis section of this Annual Report on Form 10-K) were granted on March 6, 2015 and vest 25% annually as the performance goal was achieved as of December 31, 2015.
(10) These time-based restricted stock units were granted on October 1, 2014 and vest 25% annually.
(11) These time-based restricted stock units were granted on May 19, 2014 and vest 25% annually.
(12) These time-based restricted stock units were granted on March 7, 2014 and vest 25% annually.
(13) The market value of stock reported was computed by multiplying the closing market price of the stock on December 29, 2017 ($15.75) by the number of unvested restricted stock units.
OPTION EXERCISES AND STOCK VESTED IN 2017
The following table sets forth information regarding stock options exercised and restricted share units vested during 2017 for each of the named executive officers on an aggregated basis:
|
|
|
Option Awards |
|
Stock Awards |
| |||||
|
Name |
|
Number of Shares |
|
Value Realized on |
|
Number of Shares |
|
Value Realized on |
| |
|
Vinit K. Asar |
|
|
|
|
|
37,414 |
|
$ |
516,105 |
|
|
Thomas E. Kiraly |
|
|
|
|
|
23,889 |
|
$ |
291,386 |
|
|
Samuel M. Liang |
|
|
|
|
|
14,005 |
|
$ |
185,984 |
|
|
Kenneth W. Wilson |
|
|
|
|
|
8,524 |
|
$ |
114,044 |
|
|
Thomas E. Hartman |
|
|
|
|
|
7,295 |
|
$ |
94,352 |
|
(1) The value of restricted stock units was calculated by multiplying the number of shares vesting by the closing market price of our common stock on the date of vesting.
(2) The 2015 PSU attainment calculation was not finalized until 2018. Although the shares were earned in 2017, the executives did not take possession of the shares during 2017, and therefore those shares are not included in this table. For Mr. Asar, 23,844 shares were earned but did not release in 2017. For Messrs. Kiraly, Liang, Wilson and Hartman, those numbers are 5,961 shares, 5,166 shares, 2,384 shares and 1,589 shares, respectively.
2017 PENSION BENEFITS
The following table sets forth the actuarial present value of each named executive officer’s accumulated benefit under our DB SERP, if any, assuming benefits are paid at normal retirement age based on current levels of compensation. The table also shows the number of years of credited service under each such plan, computed as of the same pension plan measurement date used in the Company’s audited financial statements for the year ended December 31, 2017. Only Messrs. Asar and Hartman were participants in our DB SERP during 2017.
|
Name |
|
Plan Name |
|
Number of Years |
|
Present Value of |
|
Payments During Last |
| ||
|
Vinit K. Asar |
|
SERP |
|
9 |
|
$ |
1,609,550 |
|
$ |
— |
|
|
Thomas E. Hartman |
|
SERP |
|
7 |
|
$ |
509,497 |
|
$ |
— |
|
The DB SERP is a nonqualified, unfunded plan that provides retirement benefits for executive officers; it contains provisions to ensure its compliance with Internal Revenue Code Section 409A.
Benefits accrue pro rata over the number of years (not to exceed 20) from a participant’s initial coverage by the DB SERP until the participant reaches the age of 65. The DB SERP was implemented in January 2004; credited service for the benefit accrual started at that time.
The DB SERP benefit is determined by the benefit percentage assigned by the Compensation Committee to an executive and is not primarily determined on the basis of average base compensation and years of service. The current benefit percentage for each named executive officer is: Vinit Asar-65%; Tom Hartman-40%.
Vesting is at the rate of 20% per year of employment with the Company. All named executive officers who are participants in the plan are fully vested.
The present value of the accumulated benefit was determined using the following assumptions, which are the same as used for financial reporting, except where noted:
· Measurement date: December 31, 2017 (December 31, 2016 for amounts calculated to determine year-over-year increase in actuarial present values)
· Fiscal year end: December 31, 2017
· Discount rate: 3.27% (3.54% for present values calculated as of December 31, 2016)
· Mortality table (pre-retirement): None
· Mortality table (post-retirement): Not applicable
· Normal retirement age for DB SERP: Age 65
· Withdrawal rates: None*
· Retirement rates: None prior to normal retirement age, 100% at normal retirement date*
· Accumulated benefit is calculated based on retirement percentage, credited service and pay as of the respective measurement dates
· Present value is the present value of fifteen years certain annuity payable at normal retirement date
*Assumes executive will not terminate, become disabled, die or retire prior to normal retirement age.
The DB SERP benefit, once calculated, is paid out annually for a fifteen year period, commencing after a participant’s retirement at age 65 from the Company, with no social security reduction or other offset. Upon the death of a participant, any unpaid vested benefits will be paid to the designated beneficiary of the participant. If a participant retires from the Company before reaching the age of 65, then the benefits of such participant under the DB SERP will be subject to a reduction for early commencement.
Upon the occurrence of a change in control of the Company, as defined in the DB SERP, all actively employed participants will be deemed to be 100% vested and the vested, accrued benefit will be funded via a Rabbi Trust in an amount equal to the present value of the accrued benefits. Periodic payments may be made to the trust so the trust’s assets continue to equal the present value of the accrued benefits. The trust is subject to the Company’s creditors’ claims in the event of the Company’s insolvency. Alternatively, the Company may, in its discretion, pay the present value of the DB SERP in a lump sum following a change in control.
2017 NONQUALIFIED DEFERRED COMPENSATION
The following table sets forth the contributions, earnings and aggregate balances under our DC SERP for those executive officers who participated in the plan in 2017 and received credits under our DC SERP in 2017. Messrs. Kiraly, Liang and
Wilson were the only named executive officers currently participating in our DC SERP in 2017. Messrs. Asar and Hartman do not participate in our DC SERP.
|
Name |
|
Executive |
|
Registrant |
|
Aggregate |
|
Aggregate |
|
Aggregate |
| |||||
|
Thomas E. Kiraly |
|
$ |
— |
|
$ |
92,369 |
|
$ |
— |
|
$ |
— |
|
$ |
315,362 |
|
|
Samuel M. Liang |
|
$ |
— |
|
$ |
93,012 |
|
$ |
— |
|
$ |
— |
|
$ |
328,828 |
|
|
Kenneth W. Wilson |
|
$ |
— |
|
$ |
45,980 |
|
$ |
— |
|
$ |
— |
|
$ |
215,306 |
|
(1) Amounts included in this column are reflected in the Summary Compensation Table.
(2) The Aggregate Earnings are not “above-market or preferential earnings” and are therefore not required to be reported in the Summary Compensation Table.
(3) Amounts included in this column that have been reported in the Summary Compensation Table since 2014 for each named executive officer are: Mr. Kiraly - $273,096; Mr. Liang - $267,132; Mr. Wilson - $171,086. Mr. Wilson was not vested in the DC SERP at the time of his departure from the Company in April 2018 and he forfeited his benefits under the DC SERP accordingly.
In May 2013, our Board of Directors, upon the recommendation of our Compensation Committee, adopted the DC SERP. The DC SERP is a nonqualified defined contribution plan in which certain executive officers and other senior employees are eligible to participate. Under the terms of the DC SERP, we may credit a participant’s account with either an amount equal to a specified percentage of the participant’s base salary or a stated flat dollar amount. Our Compensation Committee recommended establishing the DC SERP as a means of providing a retirement benefit for certain executive officers who are not covered by the DB SERP. The first credits under the DC SERP were made in 2014.
Unless specified otherwise in writing to a participant, a participant becomes 100% vested in his or her account upon the earlier of: (a) death; (b) disability; (c) five years of participation: (d) becoming retirement eligible (age 60 or greater with at least five years of service; or (e) if the participant’s employment is terminated upon or following a change in control and (1) the participant becomes entitled to severance benefits under any applicable employment, severance or similar agreement with the Company, or (2) within one year of the change in control, the Company terminates the participant for reasons other than cause, death, or disability, or the participant terminates employment because of the occurrence of a material diminution of his or her responsibilities, a reduction of his or her base salary or bonus plan targets, or a relocation of his or her principal place of employment more than 25 miles from his or her current location.
Benefits under the DC SERP are payable upon a termination from employment in either a lump sum or in annual installments (up to fifteen years), as previously elected by the participant, or upon death or disability as soon as administratively practicable thereafter (but in no event more than 90 days later).
TERMINATION AND CHANGE OF CONTROL PROVISIONS
The following tables set forth potential payments upon any termination of employment, including resignation, other types of separation or retirement of the named executive officer or change in control of the Company, assuming the triggering event took place on December 31, 2017 and the price per share of the Company’s common stock was $15.75, which was the closing market price as of December 29, 2017, the last trading day of the year. To the extent that the form and amount of any payment or benefit that would be provided in connection with any triggering event is fully disclosed in the foregoing Pension Benefits table, footnote reference is made to that disclosure.
As discussed in our 2017 Compensation Discussion and Analysis section, our Company has entered into employment and non-compete agreements with each of our named executive officers. The tables below show the amount of compensation and benefits that each of our named executive officers, other than Mr. Wilson, would receive upon certain terminations of employment or a change in control. Mr. Wilson voluntarily resigned from our Company effective April 16, 2018, and upon such termination, he received no post-termination compensation or benefits and forfeited all equity awards that were unvested as of his departure date. As such, we have not included a table below for Mr. Wilson.
Vinit K. Asar
|
|
|
Voluntary |
|
Retirement |
|
Involuntary |
|
Involuntary |
|
Death |
|
Disability |
| ||||||
|
Death Benefit (including life insurance) (1) |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
1,456,566 |
|
$ |
— |
|
|
Severance Payments (2) |
|
$ |
— |
|
$ |
— |
|
$ |
2,913,132 |
|
$ |
2,913,132 |
|
$ |
— |
|
$ |
— |
|
|
Restricted Share Units (Unvested and Accelerated) (3) |
|
$ |
— |
|
$ |
5,305,703 |
|
$ |
5,305,703 |
|
$ |
5,305,703 |
|
$ |
5,305,703 |
|
$ |
5,305,703 |
|
|
Options (Unvested and Accelerated) |
|
$ |
— |
|
$ |
476,746 |
|
$ |
476,746 |
|
$ |
476,746 |
|
$ |
476,746 |
|
$ |
476,746 |
|
|
DB SERP Benefit (4) |
|
$ |
— |
|
$ |
— |
|
$ |
370,979 |
|
$ |
370,979 |
|
$ |
— |
|
$ |
— |
|
|
DC SERP Benefit (5) |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
Benefits Continuation (6) |
|
$ |
— |
|
$ |
— |
|
$ |
74,318 |
|
$ |
74,318 |
|
$ |
— |
|
$ |
— |
|
|
Outplacement (7) |
|
$ |
— |
|
$ |
— |
|
$ |
30,000 |
|
$ |
30,000 |
|
$ |
— |
|
$ |
— |
|
|
Cutback (8) |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
(1,096,462 |
) |
$ |
— |
|
$ |
— |
|
(1) The death benefit includes a supplemental life insurance benefit equal to 2 times base salary. Mr. Asar is also eligible for the Company’s standard life insurance.
(2) The severance benefit is equal to 2 times base salary and target bonus.
(3) This calculation is based on the accelerated vesting of all unvested restricted share units and performance share units, as shown in the Outstanding Equity Awards at Fiscal Year-End table, except that all performance share units for which the performance period has not yet expired are shown in this table assuming that the target performance goals had been met.
(4) This amount reflects the present value of the additional benefit which would accrue based on providing additional credited service for the duration of any severance period. This is in addition to the present value of the DB SERP benefit as of December 31, 2016 as shown in the Pension Benefits table.
(5) Mr. Asar is not a participant in the DC SERP.
(6) This amount represents the cost of providing the continuation of certain benefits (e.g., health insurance, life and disability insurance, financial planning).
(7) Assumed value for providing outplacement services following a termination.
(8) Based on an estimated calculation, Mr. Asar’s separation payments upon termination following a change in control would trigger an excise tax payment in accordance with Internal Revenue Code Sections 280G and 4999. As a result, Mr. Asar would be in a better after-tax position by having his payments reduced by the cutback amount shown so that the excise tax would not apply than he would be if he received his payments and had to pay the excise tax.
Thomas E. Kiraly
|
|
|
Voluntary |
|
Retirement |
|
Involuntary |
|
Involuntary |
|
Death |
|
Disability |
| ||||||
|
Death Benefit (including life insurance) (1) |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
450,000 |
|
$ |
— |
|
|
Severance Payments (2) |
|
$ |
— |
|
$ |
— |
|
$ |
1,202,845 |
|
$ |
1,202,845 |
|
$ |
— |
|
$ |
— |
|
|
Restricted Share Units (Unvested and Accelerated) (3) |
|
$ |
— |
|
$ |
2,263,307 |
|
$ |
2,263,307 |
|
$ |
2,263,307 |
|
$ |
2,263,307 |
|
$ |
2,263,307 |
|
|
Options (Unvested and Accelerated) |
|
$ |
— |
|
$ |
280,618 |
|
$ |
280,618 |
|
$ |
280,618 |
|
$ |
280,618 |
|
$ |
280,618 |
|
|
DB SERP Benefit (4) |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
DC SERP Benefit (5) |
|
$ |
— |
|
$ |
315,362 |
|
$ |
— |
|
$ |
315,362 |
|
$ |
315,362 |
|
$ |
315,362 |
|
|
Benefits Continuation (6) |
|
$ |
— |
|
$ |
— |
|
$ |
61,047 |
|
$ |
61,047 |
|
$ |
— |
|
$ |
— |
|
|
Outplacement (7) |
|
$ |
— |
|
$ |
— |
|
$ |
22,500 |
|
$ |
22,500 |
|
$ |
— |
|
$ |
— |
|
|
Cutback (8) |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
(601,617 |
) |
$ |
— |
|
$ |
— |
|
(1) Mr. Kiraly is also eligible for the Company’s standard life insurance.
(2) The severance benefit is equal to 1.5 times base salary and target bonus.
(3) This calculation is based on the accelerated vesting of all unvested restricted share units and performance share units, as shown in the Outstanding Equity Awards at Fiscal Year-End table, except that all performance share units for which the performance period has not yet expired are shown in this table assuming that the target performance goals had been met.
(4) Mr. Kiraly is not a participant in the DB SERP.
(5) This amount reflects the full amount Mr. Kiraly would be entitled to on the corresponding event.
(6) This amount represents the cost of providing the continuation of certain benefits (e.g., health insurance, life and disability insurance, financial planning).
(7) Assumed value for providing outplacement services following a termination.
(8) Based on an estimated calculation, Mr. Kiraly’s separation payments upon termination following a change in control would trigger an excise tax payment in accordance with Internal Revenue Code Sections 280G and 4999. As a result, Mr. Kiraly would be in a better after-tax position by having his payments reduced by the cutback amount shown so that the excise tax would not apply than he would be if he received his payments and had to pay the excise tax.
Samuel M. Liang
|
|
|
Voluntary |
|
Retirement |
|
Involuntary |
|
Involuntary |
|
Death |
|
Disability |
| ||||||
|
Death Benefit (including life insurance) (1) |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
450,000 |
|
$ |
— |
|
|
Severance Payments (2) |
|
$ |
— |
|
$ |
— |
|
$ |
1,141,332 |
|
$ |
1,141,332 |
|
$ |
— |
|
$ |
— |
|
|
Restricted Share Units (Unvested and Accelerated) (3) |
|
$ |
— |
|
$ |
1,835,678 |
|
$ |
1,835,678 |
|
$ |
1,835,678 |
|
$ |
1,835,678 |
|
$ |
1,835,678 |
|
|
Options (Unvested and Accelerated) |
|
$ |
— |
|
$ |
215,248 |
|
$ |
215,248 |
|
$ |
215,248 |
|
$ |
215,248 |
|
$ |
215,248 |
|
|
DB SERP Benefit (4) |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
DC SERP Benefit (5) |
|
$ |
— |
|
$ |
328,828 |
|
$ |
— |
|
$ |
328,828 |
|
$ |
328,828 |
|
$ |
328,828 |
|
|
Benefits Continuation (6) |
|
$ |
— |
|
$ |
— |
|
$ |
69,248 |
|
$ |
69,348 |
|
$ |
— |
|
$ |
— |
|
|
Outplacement (7) |
|
$ |
— |
|
$ |
— |
|
$ |
22,500 |
|
$ |
22,500 |
|
$ |
— |
|
$ |
— |
|
|
Cutback (8) |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
(93,068 |
) |
$ |
— |
|
$ |
— |
|
(1) Mr. Liang is also eligible for the Company’s standard life insurance.
(2) The severance benefit is equal to 1.5 times base salary and target bonus.
(3) This calculation is based on the accelerated vesting of all unvested restricted share units and performance share units, as shown in the Outstanding Equity Awards at Fiscal Year-End table, except that all performance share units for which the performance period has not yet expired are shown in this table assuming that the target performance goals had been met.
(4) Mr. Liang is not a participant in the DB SERP.
(5) This amount reflects the full amount Mr. Liang would be entitled to on the corresponding event.
(6) This amount represents the cost of providing the continuation of certain benefits (e.g., health insurance, life and disability insurance, financial planning).
(7) Assumed value for providing outplacement services following a termination.
(8) Based on an estimated calculation, Mr. Liang’s separation payments upon termination following a change in control would trigger an excise tax payment in accordance with Internal Revenue Code Sections 280G and 4999. As a result, Mr. Liang would be in a better after-tax position if he received his full benefits and paid the excise tax than he would be if his benefits had been reduced to a level where the excise tax would not apply.
Thomas E. Hartman
|
|
|
Voluntary |
|
Retirement |
|
Involuntary |
|
Involuntary |
|
Death |
|
Disability |
| ||||||
|
Death Benefit (including life insurance) (1) |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
Severance Payments (2) |
|
$ |
— |
|
$ |
— |
|
$ |
532,073 |
|
$ |
532,073 |
|
$ |
— |
|
$ |
— |
|
|
Restricted Share Units (Unvested and Accelerated) (3) |
|
$ |
— |
|
$ |
1,075,552 |
|
$ |
1,075,552 |
|
$ |
1,075,552 |
|
$ |
1,075,552 |
|
$ |
1,075,552 |
|
|
Options (Unvested and Accelerated) |
|
$ |
— |
|
$ |
211,038 |
|
$ |
211,038 |
|
$ |
211,038 |
|
$ |
211,038 |
|
$ |
211,038 |
|
|
DB SERP Benefit (4) |
|
$ |
— |
|
$ |
— |
|
$ |
102,021 |
|
$ |
102,021 |
|
$ |
— |
|
$ |
— |
|
|
DC SERP Benefit (5) |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
Benefits Continuation (6) |
|
$ |
— |
|
$ |
— |
|
$ |
29,041 |
|
$ |
29,041 |
|
$ |
— |
|
$ |
— |
|
|
Outplacement (7) |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
Cutback (8) |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
(1) Mr. Hartman is eligible for the Company’s standard life insurance.
(2) The severance benefit is equal to 1.0 times base salary and target bonus.
(3) This calculation is based on the accelerated vesting of all unvested restricted share units and performance share units, as shown in the Outstanding Equity Awards at Fiscal Year-End table, except that all performance share units for which the performance period has not yet expired are shown in this table assuming that the target performance goals had been met.
(4) This amount reflects the present value of the additional benefit which would accrue based on providing additional credited service for the duration of any severance period. This is in addition to the present value of the DB SERP benefit as of December 31, 2016 as shown in the Pension Benefits table.
(5) Mr. Hartman is not a participant in the DC SERP.
(6) This amount represents the cost of providing the continuation of certain benefits (e.g., health insurance, life and disability insurance, financial planning).
(7) Mr. Hartman does not receive outplacement services following a termination.
(8) Based on an estimated calculation, Mr. Hartman’s separation payments upon termination following a change in control would not trigger an excise tax payment in accordance with Internal Revenue Code Sections 280G and 4999.
CEO PAY RATIO
As required by Section 953(b) of the Dodd-Frank Wall Street Reform and Consumer Protection Act, and Item 402(u) of Regulation S-K, we are providing the following information about the relationship of the annual total compensation of our employees and the annual total compensation of Mr. Vinit Asar, President and Chief Executive Officer:
For 2017, our last completed fiscal year:
· the annual total compensation of the employee identified at median of our company (other than our CEO), was $57,005;
· the annual total compensation of Mr. Asar was $6,454,442;
· based on this information, for 2017, the ratio of the annual total compensation of Mr. Asar, our Chief Executive Officer, to the median of the annual total compensation of all employees was estimated to be 113 to 1.
This pay ratio is a reasonable estimate calculated in a manner consistent with SEC rules based on our payroll and employment records and the methodology described below. The SEC rules for identifying the median compensated employee and calculating the pay ratio based on that employee’s annual total compensation allow companies to adopt a variety of methodologies, to apply certain exclusions, and to make reasonable estimates and assumptions that reflect their compensation practices. As such, the pay ratio reported by other companies may not be comparable to the pay ratio reported above, as other companies may have different employment and compensation practices and may utilize different methodologies, exclusions, estimates and assumptions in calculating their own pay ratios.
Under the relevant rules, we were required to identify the median employee by use of a “consistently applied compensation measure,” or CACM, as of a specific measurement date. For 2017: the methodology that we used was as follows:
· We identified the median employee using our employee population on December 15, 2017. We determined that, as of December 15, 2017, our employee population consisted of 4,743 individuals. All our employees work and reside within the United States.
· To identify the “median employee” from our employee population, we collected information regarding all wages, over-time pay, equity compensation, commission and bonus paid to each employee for services rendered during 2017. We annualized the compensation of all newly hired permanent employees during this period.
· Once we identified the median employee, we calculated the median, employee’s total annual compensation in accordance with the requirements of the Summary Compensation Table.
COMPENSATION RISK ASSESSMENT
We monitor and assess periodically our enterprise risks, including risks from our compensation policies and practices for our employees. Based on our periodic assessments, we believe that risks arising from our compensation policies and practices for our employees, including our named executive officers, are not reasonably likely to have a material adverse effect on our Company. We believe our compensation policies and practices provide an appropriate balance between short-term and long-term incentives, encourage our employees to produce superior results for our company without having to take excessive or inappropriate risks to do so, and continue to serve the best interests of our Company and our shareholders.
DIRECTOR COMPENSATION
The compensation structure for 2017 for non-employee directors included the following:
· An annual cash retainer of $60,000 paid in four equal installments.
· An annual grant of restricted stock units valued at $140,000. These units have a one-year vesting cycle. These restricted stock units, awarded in May 2017, will vest in full on May 19, 2018.
· A $1,000 honorarium for any committee meeting, whether attended in person or via conference call.
· A $20,000 cash retainer for the chairperson of the Audit Committee, a $15,000 cash retainer for the chairperson of the Compensation Committee and a $10,000 cash retainer for the chairpersons of the Corporate Governance & Nominating and Quality, Compliance, Technology & Outcomes Committees, paid at the same time as the first installment of the annual cash retainer.
· A $25,000 one-time payment was approved for Directors appointed to the Special Litigation Committee established by the Board of Directors on June 6, 2016. The committee concluded its duties and responsibilities in February 2017.
· A $75,000 cash retainer for the non-employee Chairman of the Board.
· A substantial target for stock ownership by each director, in a pre-determined time frame, has been established. Each director is expected to own at least $300,000 of the Company’s common stock within five years of joining our Board of Directors. As of December 31, 2017 all of the directors were in compliance with the policy based on their stock ownership or were within five years of joining our Board of Directors.
The following table sets forth information regarding the compensation received by each of our Company’s non-employee directors for their services as a director during the year ended December 31, 2017.
|
Name |
|
Fees Earned |
|
Stock |
|
Option |
|
Total |
| ||||
|
Asif Ahmad |
|
$ |
71,000 |
|
$ |
140,010 |
|
$ |
— |
|
$ |
211,010 |
|
|
Christopher B. Begley |
|
$ |
85,000 |
|
$ |
140,010 |
|
$ |
— |
|
$ |
225,010 |
|
|
Thomas P. Cooper |
|
$ |
155,630 |
|
$ |
140,010 |
|
$ |
— |
|
$ |
295,640 |
|
|
Cynthia L. Feldmann |
|
$ |
72,630 |
|
$ |
140,010 |
|
$ |
— |
|
$ |
212,640 |
|
|
John T. Fox |
|
$ |
14,260 |
|
$ |
63,095 |
|
$ |
— |
|
$ |
77,355 |
|
|
Thomas C. Freyman |
|
$ |
14,260 |
|
$ |
63,095 |
|
$ |
— |
|
$ |
77,355 |
|
|
Stephen E. Hare |
|
$ |
91,000 |
|
$ |
140,010 |
|
$ |
— |
|
$ |
231,010 |
|
|
Cynthia L. Lucchese |
|
$ |
92,000 |
|
$ |
140,010 |
|
$ |
— |
|
$ |
232,010 |
|
|
Richard R. Pettingill |
|
$ |
77,000 |
|
$ |
140,010 |
|
$ |
— |
|
$ |
217,010 |
|
|
Kathryn M. Sullivan |
|
$ |
88,000 |
|
$ |
140,010 |
|
$ |
— |
|
$ |
228,010 |
|
(1) Amounts shown include all fees earned for services as a director, including annual retainer fees, committee and/or chairmanship fees, and meeting fees.
(2) The restricted shares for the annual award had a grant date fair value of $12.77 based on the May 18, 2017 closing price of our common stock. John Fox and Thomas Freyman received prorated awards on November 15, 2017 for their initial service on our Board of Directors as non-employee Directors. Their award was 5,347 shares, with a grant date fair value of $11.80 based on the November 14, 2017 closing price of our common stock. The amount reported in this column represents the aggregate grant date fair value of all restricted stock awards granted to each Director during the 2017 calendar year as calculated in accordance with ASC 718.
Aggregate number of unvested restricted share units as of December 31, 2017 for each non-employee director in office as of such date is as follows:
|
Name |
|
Aggregate Number of Unvested Restricted Shares |
|
|
Asif Ahmad |
|
10,964 |
|
|
Christopher B. Begley |
|
10,964 |
|
|
Thomas P. Cooper |
|
10,964 |
|
|
Cynthia L. Feldmann |
|
10,964 |
|
|
John T. Fox |
|
5,347 |
|
|
Thomas C. Freyman |
|
5,347 |
|
|
Stephen E. Hare |
|
10,964 |
|
|
Cynthia L. Lucchese |
|
10,964 |
|
|
Richard R, Pettingill |
|
10,964 |
|
|
Kathryn M. Sullivan |
|
10,964 |
|
(3)No stock options were awarded to any directors during the 2017 calendar year.
As of December 31, 2017, none of our non-employee directors had any outstanding option awards.
COMPENSATION COMMITTEE INTERLOCKS AND INSIDER PARTICIPATION
None of our executive officers or directors had relationships in the year ended December 31, 2017 that would require disclosure as a “compensation committee interlock” or “insider participation.”
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Equity Compensation Plans
|
Plan Category |
|
Number of securities to be |
|
Weighted average exercise |
|
Number of securities |
| |
|
|
|
(a) |
|
(b) |
|
(c) |
| |
|
|
|
|
|
|
|
|
| |
|
Equity Compensation Plans Approved by Security Holders: |
|
|
|
|
|
|
| |
|
Restricted Stock |
|
120,178 |
|
— |
|
— |
| |
|
Stock Options |
|
— |
|
— |
|
— |
| |
|
|
|
|
|
|
|
|
| |
|
Equity Compensation Plans Not Approved by Security Holders: |
|
|
|
|
|
|
| |
|
Restricted Stock |
|
512,458 |
|
— |
|
1,190,964 |
| |
|
Stock Options |
|
798,020 |
|
$ |
12.77 |
|
— |
|
Principal Shareholders
As of March 31, 2018, Hanger had a total of 36,744,543 shares of common stock issued and outstanding. The following table sets forth the number of shares of common stock beneficially owned as of March 31, 2018 by: (i) each director and nominee for director of Hanger, (ii) each of the named executive officers; (iii) all directors, nominees and executive officers of Hanger as a group; and (iv) each person known by Hanger to be the beneficial owner of 5% or more of Hanger’s common stock. The list of beneficial owners of 5% or more of Hanger’s common stock is derived from filings of Schedule 13G and Schedule 13D, which we have assumed represent all current 5% or more beneficial owners.
|
Directors and Officers: |
|
Number of |
|
Percent of |
|
|
Asif Ahmad (2) |
|
32,623 |
|
* |
|
|
Vinit K. Asar (3) |
|
286,653 |
|
* |
|
|
Christopher B. Begley (4) |
|
38,200 |
|
* |
|
|
John T. Fox |
|
— |
|
* |
|
|
Thomas J. Freyman |
|
— |
|
* |
|
|
Stephen E. Hare (5) |
|
36,218 |
|
* |
|
|
Thomas E. Hartman (6) |
|
44,235 |
|
* |
|
|
Thomas E. Kiraly (7) |
|
99,763 |
|
* |
|
|
Samuel M. Liang (8) |
|
80,178 |
|
* |
|
|
Cynthia L. Lucchese (9) |
|
29,637 |
|
* |
|
|
Richard R. Pettingill (10) |
|
39,921 |
|
* |
|
|
Kathryn M. Sullivan (11) |
|
23,812 |
|
* |
|
|
Kenneth W. Wilson (12) |
|
54,717 |
|
* |
|
|
All directors, nominees and officers as a group (17 persons) (13) |
|
846,375 |
|
1.8 |
% |
|
5% Shareholders: |
|
|
|
|
|
|
FMR LLC (14) |
|
4,919,592 |
|
13.4 |
% |
|
Hotchkis and Wiley Capital Management, LLC (15) |
|
3,781,359 |
|
10.3 |
% |
|
Invesco Ltd. (16) |
|
4,910,580 |
|
13.4 |
% |
|
KKR North America Fund XI L.P. (17) |
|
3,220,476 |
|
8.8 |
% |
|
BlueMountain Capital Management, LLC (18) |
|
3,056,027 |
|
8.3 |
% |
|
Welsh, Carson, Anderson & Stowe XII, L.P. (19) |
|
2,399,385 |
|
6.5 |
% |
|
The Vanguard Group (20) |
|
2,498,217 |
|
6.8 |
% |
*Less than 1%
(1) Assumes in the case of each shareholder listed above that all shares of restricted stock vesting within 60 days were vested, and the related shares were owned by such shareholder. With respect to each company listed above, the amounts represent the number of shares beneficially owned, as disclosed in company reports regarding beneficial ownership filed with the SEC. To our knowledge, except as noted above, no person or entity is the beneficial owner of more than 5% of the voting power of the Company’s stock.
(2) Includes 32,623 shares owned directly by Mr. Ahmad.
(3) Includes 233,326 shares owned directly by Mr. Asar and 53,327 shares subject to acquisition by Mr. Asar pursuant to the exercise of stock options that will vest within 60 days of the Record Date. Does not include 176,958 shares subject to unvested restricted stock.
(4) Includes 38,200 shares owned directly by Mr. Begley.
(5) Includes 36,218 shares owned directly by Mr. Hare. Does not include 32,577 shares subject to vested restricted stock units that Mr. Hare has elected to defer. Such deferred restricted stock units will be delivered to Mr. Hare in the form of whole shares of common stock on or about January 15th of the year following the calendar year in which Mr. Hare’s service as a director terminates.
(6) Includes 20,629 shares owned directly by Mr. Hartman and 23,606 shares subject to acquisition by Mr. Hartman pursuant to the exercise of stock options that will vest within 60 days of the Record Date. Does not include 32,526 shares subject to unvested restricted stock.
(7) Includes 68,374 shares owned directly by Mr. Kiraly and 31,389 shares subject to acquisition by Mr. Kiraly pursuant to the exercise of stock options that will vest within 60 days of the Record Date. Does not include 80,758 shares subject to unvested restricted stock.
(8) Includes 56,101 shares owned directly by Mr. Liang and 24,077 shares subject to acquisition by Mr. Liang pursuant to the exercise of stock options that will vest within 60 days of the Record Date. Does not include 61,188 shares subject to unvested restricted stock.
(9) Includes 29,637 shares owned directly by Ms. Lucchese.
(10) Includes 39,921 shares owned directly by Mr. Pettingill.
(11) Includes 23,812 shares owned directly by Ms. Sullivan.
(12) Includes 36,439 shares owned directly by Mr. Wilson and 18,278 shares subject to acquisition by Mr. Wilson pursuant to the exercise of stock options that will vest within 60 days of the Record Date. Does not include 31,826 shares subject to unvested restricted stock. Mr. Wilson resigned from the Company effective April 16, 2018.
(13) Includes 669,555 shares owned directly or controlled by directors and officers of our Company and 176,820 shares subject to acquisition by officers pursuant to the exercise of stock options that will vest within 60 days of the Record Date. Does not include 500,309 shares subject to unvested restricted stock, or to unvested or deferred restricted stock units, issued to directors and officers of our Company.
(14) The address of FMR LLC is 245 Summer Street, Boston, Massachusetts 02210. FMR LLC has sole voting power with respect to 2,221,817 of these shares and sole dispositive power with respect to 4,919,592 shares. Fidelity Low-Priced Stock Fund has sole voting power with respect to 2,571,910 of these shares. Abigail P. Johnson, the Director, Chairman and Chief Executive Officer of FMR LLC, has sole dispositive power with respect to 4,919,592 shares.
(15) The address of Hotchkis and Wiley Capital Management, LLC (“Hotchkis”) is 725 South Figueroa Street, 39th Floor, Los Angeles, California 90017. Hotchkis has sole voting power with respect to 3,037,176 of these shares and sole dispositive power with respect to 3,781,359 shares. Hotchkis and Wiley Small Cap Value Fund has sole voting power and sole dispositive power with respect to 1,373,800 shares.
(16) The address of Invesco Ltd. is 1555 Peachtree Street NE, Atlanta, Georgia 30309. Invesco Ltd. has sole voting power and sole dispositive power with respect to all of these shares.
(17) The address of KKR North America Fund XI L.P. (“KKR”) is c/o Kohlberg Kravis Roberts & Co. L.P., 9 West 57th Street, Suite 4200, New York, New York 10019. KKR has sole voting power and sole dispositive power with respect to 3,220,476 shares
(18) The address of BlueMountain Capital Management, LLC (“BlueMountain”) is 280 Park Avenue, 12th Floor, New York, New York 10017. Blue Mountain has shared voting power and shared dispositive power with respect to 3,056,027 shares.
(19) The address of Welsh, Carson, Anderson & Stowe XII, L.P. (“WCAS XII”) is c/o Welsh, Carson, Anderson & Stowe, 320 Park Avenue, Suite 2500, New York, New York 10022. WCAS XII has sole voting and sole dispositive power with respect to 1,710,282 of these shares. Welsh, Carson, Anderson & Stowe XII Delaware, L.P. has sole voting and sole dispositive power with respect to 301,484 of these shares. Welsh Carson, Anderson & Stowe XII Delaware II, L.P. has sole voting and sole dispositive power with respect to 50,026 of these shares. Welsh, Carson, Anderson & Stowe XII Cayman, L.P. has sole voting and sole dispositive power with respect to 312,039 of these shares. WCAS XII Co-Investors LLC has sole voting and sole dispositive power with respect to 25,554 of these shares.
(20) The address of The Vanguard Group is 100 Vanguard Boulevard, Malvern, Pennsylvania 19355. The Vanguard Group has sole voting power with respect to 44,423 of these shares, and shared voting power with respect to 4,600 of these shares. The Vanguard Group has sole dispositive power with respect to 2,451,294 of these shares and shared dispositive power with respect to 46,923 of these shares.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Policies and Procedures Regarding Related Person Transactions
Our Board of Directors has adopted written policies and procedures, which were in effect in their current form as of December 31, 2017 regarding related person transactions. For purposes of these policies and procedures:
· a “related person” means any of our directors, executive officers or nominees for director, and any of their immediate family members; and
· a “related person transaction” generally is a transaction in which we were or are to be a participant and the amount involved exceeds $120,000, and in which any related person had or will have a direct or indirect interest.
The related person, or the director, executive officer or nominee who is an immediate family member of a related person, must notify our Corporate Governance and Nominating Committee of certain information relating to proposed related person transactions. The Corporate Governance and Nominating Committee will consider all of the relevant facts and circumstances available regarding the proposed related person transaction and will ratify or approve only those related person transactions that are in, or are not inconsistent with, the best interests of our company and our shareholders.
In the 2017 fiscal year, there were no proposed, pending or ongoing related person transactions subject to review by the Corporate Governance and Nominating Committee under the policy.
Board Independence
For the fiscal year ended December 31, 2017 and as of the current date, our Board of Directors has determined that all of the members of the Board of Directors except for Vinit K. Asar, including each of the members of the Audit Committee, Compensation Committee, and Corporate Governance & Nominating Committee, were independent directors within the meaning of NYSE listing standards and rules even though the Company was no longer listed on the NYSE as of December 31, 2017. Further, for the fiscal year ended December 31, 2017 and as of the current date, our Board of Directors had determined that each of the members of the Audit Committee qualified as “independent” under Rule 10A-3 of the Exchange Act, as amended, and that each of Messrs. Hare and Begley (and, for the year ended December 31, 2017, our former director Ms. Feldmann) qualified as an “audit committee financial expert” as defined in the U.S. Securities and Exchange Commission’s (“SEC”) rules. For a director to be deemed independent under NYSE rules, our Board of Directors must affirmatively determine that the director has no material relationship with our Company (either directly or as a partner, shareholder, or officer of an organization that has a relationship with our Company). In addition, the director (and any member of his or her immediate family) must meet the technical independence requirements of the NYSE’s listing standards.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Audit and Non-Audit Fees
|
(in thousands) |
|
2017 |
|
2016 |
|
2015 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Audit fees |
|
$ |
6,977 |
|
$ |
7,229 |
|
$ |
6,417 |
|
|
Audit-related fees |
|
— |
|
— |
|
— |
| |||
|
Tax fees |
|
58 |
|
— |
|
— |
| |||
|
All other fees |
|
4 |
|
4 |
|
3 |
| |||
|
|
|
$ |
7,039 |
|
$ |
7,233 |
|
$ |
6,420 |
|
Audit Fees
The aggregate fees by PricewaterhouseCoopers LLP (“PwC”) for each of the 2017, 2016 and 2015 fiscal years for professional services rendered for audit services totaled $7.0 million in 2017, $7.2 million in 2016 and $6.4 million in 2015, including fees associated with the audit of our annual financial statements and the audit of our internal control over financial reporting.
Audit-Related Fees
There were no aggregate fees billed by PwC for the 2017, 2016 and 2015 fiscal years for assurance and related services reasonably related to the performance of audit or review of our financial statements.
Tax Fees
In 2017, PwC was engaged to perform a U.S. transfer price study which was approved by the Audit Committee. In each of the 2016 and 2015 fiscal years, PwC was not engaged to supply any professional services for tax compliance, tax advice and tax planning.
All Other Fees
The aggregate fees billed by PwC in 2017, 2016 and 2015 for all other services were related to accounting research tools obtained by us.
The following describes the Audit Committee’s policies and procedures regarding pre-approval of the engagement of our independent auditor to perform audit as well as permissible non-audit services, all of which were in effect in their current form as of December 31, 2017, 2016 and 2015. For audit services, the independent auditor was to provide the Audit Committee with an engagement letter during the second calendar quarter of each year outlining the scope and cost of the audit services proposed to be performed in connection with the audit of the current fiscal year. If agreed to by the Audit Committee, the engagement letter will be formally accepted by the Audit Committee at an Audit Committee meeting held as practicably as possible following receipt of the engagement letter and fee estimate.
For non-audit services, our management may submit to the Audit Committee for approval the list of non-audit services that it recommends the Audit Committee allow us to engage the independent auditor to provide for the fiscal year. The list of services must be detailed as to the particular service and may not call for broad categorical approvals. Our management and the independent auditor will each confirm to the Audit Committee that each non-audit service on the list is permissible under all applicable legal requirements. In addition to the list of planned non-audit services, a budget estimating non-audit service spending for the fiscal year may be provided. The Audit Committee will consider for approval both the list of permissible
non-audit services and the budget for such services. The Audit Committee will be informed routinely as to the non-audit services actually provided by the independent auditor pursuant to this pre-approval process.
To ensure prompt handling of unexpected matters, the Audit Committee delegates to its Chairperson the authority to approve the auditor’s engagement for non-audit services with fees that do not exceed 5% of total fees paid to the independent auditors during the fiscal year, and to amend or modify the list of approved permissible non-audit services and fees of up to 5% of total fees paid to the independent auditors during the fiscal year. The Chairperson will report any action taken pursuant to this delegation to the Audit Committee at its next Audit Committee meeting.
All audit and non-audit services provided to us are required to be pre-approved by the Audit Committee. Our Chief Financial Officer will be responsible for tracking all independent auditor fees against the budget for such services and report at least annually to the Audit Committee.
All of the audit and non-audit services during the years ended December 31, 2017, 2016 and 2015 and the related professional engagement periods were pre-approved by the Audit Committee.
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) Financial Statements and Financial Statement Schedules:
(1) Financial Statements:
|
Hanger, Inc. |
|
|
|
|
F-2 | |
|
Consolidated Balance Sheets as of December 31, 2017 and 2016 |
|
F-4 |
|
|
F-5 | |
|
|
F-6 | |
|
Consolidated Statements of Cash Flows for the Three Years Ended December 31, 2017 |
|
F-7 |
|
|
F-8 |
(2) Exhibits:
See Part (b) of this Item 15.
(b) Exhibits: The following exhibits are filed herewith or incorporated herein by reference:
|
Exhibit |
|
Document |
|
3.1 |
|
Restated Certificate of Incorporation of Hanger, Inc., dated August 27, 2012. (Incorporated herein by reference to Exhibit 3.1 to the Current Report on Form 8-K filed by the Registrant on August 29, 2012.) |
|
3.2 |
|
Amended and Restated By-Laws of Hanger Orthopedic Group, Inc., as amended effective February 2, 2012. (Incorporated herein by reference to Exhibit 3.1 to the Current Report on Form 8-K filed by the Registrant on February 6, 2012.) |
|
3.3 |
|
Certificate of Designations, Preferences and Rights of Series A Junior Participating Preferred Stock of Hanger, Inc., effective February 28, 2016. (Incorporated herein by reference to Exhibit 3.1 to the Current Report on Form 8-K filed by the Registrant on March 2, 2016.) |
|
4.1 |
|
Credit Agreement, dated June 17, 2013, among Hanger, Inc. and the lenders and agents party thereto. (Incorporated herein by reference to Exhibit 4.1 to the Current Report on Form 8-K filed by the Registrant on June 19, 2013.) |
|
4.2 |
|
Waiver No. 1 to the Credit Agreement, dated December 12, 2014, among Hanger, Inc. and the lenders and agents party thereto. (Incorporated herein by reference to Exhibit 4.8 to the Annual Report on Form 10-K for the year ended December 31, 2014.) |
|
4.3 |
|
Waiver No. 2 to the Credit Agreement, dated January 14, 2015, among Hanger, Inc. and the lenders and agents party thereto. (Incorporated herein by reference to Exhibit 4.9 to the Annual Report on Form 10-K for the year ended December 31, 2014.) |
|
4.4 |
|
Waiver No. 3 to the Credit Agreement, dated March 17, 2015, among Hanger, Inc. and the lenders and agents party thereto. (Incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8-K filed by the Registrant on March 23, 2015.) |
|
4.5 |
|
First Amendment and Waiver, dated June 19, 2015, by and among Hanger, Inc., the guarantors party thereto and the lenders and agents party thereto (Incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8-K filed by the Registrant on June 22, 2015.) |
|
4.6 |
|
Second Amendment and Waiver, dated September 11, 2015, by and among Hanger, Inc., the guarantors party thereto and the lenders and agents party thereto (Incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8-K filed by the Registrant on September 14, 2015.) |
|
4.7 |
|
Third Amendment and Waiver, dated November 13, 2015, by and among Hanger, Inc., the guarantors party thereto and the lenders and agents party thereto (Incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8-K filed by the Registrant on November 13, 2015.) |
|
4.8 |
|
Fourth Amendment and Waiver, dated February 10, 2016, by and among Hanger, Inc., the guarantors party thereto and the lenders and agents party thereto (Incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8-K filed by the Registrant on February 10, 2016.) |
|
4.9 |
|
Fifth Amendment and Waiver, dated July 15, 2016, by and among Hanger, Inc., the guarantors party thereto and the lenders and agents party thereto (Incorporated herein by reference to Exhibit 10.2 to the Current Report on Form 8-K filed by the Registrant on August 2, 2016.) |
|
4.10 |
|
Sixth Amendment and Waiver, dated June 22, 2017, by and among Hanger, Inc., the guarantors party thereto and the lenders and agents party thereto (Incorporated herein by reference to Exhibit 10.2 to the Current Report on Form 8-K filed by the Registrant on June 23, 2017.) |
|
4.11 |
|
Rights Agreement, dated February 28, 2016, by and among Hanger, Inc. and Computershare, Inc. as rights agent. (Incorporated herein by reference to Exhibit 4.1 to the Current Report on Form 8-K filed by the Registrant on March 2, 2016.) |
|
4.12 |
|
Amendment No. 1 to Rights Agreement, dated June 23, 2017, by and among Hanger, Inc. and Computershare, Inc. as rights agent. (Incorporated herein by reference to Exhibit 4.1 to the Current Report on Form 8-K filed by the Registrant on June 23, 2017.) |
|
4.13 |
|
Credit Agreement, dated August 1, 2016, by and among Hanger, Inc. and the lenders and agents party thereto. (Incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8-K filed by the Registrant on August 2, 2016.) |
|
4.14 |
|
Amendment No. 1 to Credit Agreement, dated June 2, 2017, by and among Hanger, Inc. and the lenders and agents party thereto. (Incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8-K filed by the Registrant on June 23, 2017.) |
|
4.15 |
|
Credit Agreement, dated March 6, 2018, among Hanger, Inc. and the lenders and agents party thereto. (Incorporated herein by reference to Exhibit 4.1 to the Current Report on Form 8-K filed by the Registrant on March 6, 2018.) |
|
10.1 |
|
Amended and Restated 2002 Stock Incentive Plan, as amended through May 10, 2007. (Incorporated herein by reference to Appendix 1 to the Registrant’s Proxy Statement, dated April 10, 2007, relating to the Registrant’s Annual Meeting of Stockholders held on May 10, 2007.)* |
|
10.2 |
|
Amended and Restated 2003 Non-Employee Directors’ Stock Incentive Plan, as amended through May 10, 2007. (Incorporated herein by reference to Appendix 2 to the Registrant’s Proxy Statement, dated April 10, 2007, relating to the Registrant’s Annual Meeting of Stockholders held on May 10, 2007.) |
|
10.3 |
|
Form of Stock Option Agreement (Non-Executive Employees), Stock Option Agreement (Executive Employees), Restricted Stock Agreement (Non-Executive Employees) and Restricted Stock Agreement (Executive Employees). (Incorporated herein by reference to Exhibits 10.1, 10.2, 10.3 and 10.4, respectively, to the Registrant’s Current Report on Form 8-K filed on February 24, 2005.)* |
|
10.4 |
|
Supplemental Executive Retirement Plan, as amended and restated effective January 1, 2011 (Incorporated herein by reference to Exhibit 10.4 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2010.)* |
|
10.5 |
|
Hanger Orthopedic Group, Inc. 2010 Omnibus Incentive Plan. (Incorporated herein by reference to Annex A to Registrant’s Proxy Statement, dated April 2, 2010, relating to the Registrant’s Annual Meeting of Stockholders held on May 13, 2010.)* |
|
10.6 |
|
Form of Restricted Stock Agreement for Non-Employee Directors. (Incorporated herein by reference to Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2010.)* |
|
10.7 |
|
Form of Restricted Stock Agreement for Executives. (Incorporated herein by reference to Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2010.)* |
|
10.8 |
|
Form of Restricted Stock Agreement for Employees. (Incorporated herein by reference to Exhibit 10.4 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2010.)* |
|
10.9 |
|
Form of Non-Employee Director Non-Qualified Stock Option Agreement. (Incorporated herein by reference to Exhibit 10.5 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2010.)* |
|
10.10 |
|
Form of Executive Non-Qualified Stock Option Agreement. (Incorporated herein by reference to Exhibit 10.6 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2010.)* |
|
10.11 |
|
Form of Non-Qualified Stock Option Agreement. (Incorporated herein by reference to Exhibit 10.7 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2010.)* |
|
10.12 |
|
Amended and Restated Employment Agreement, dated as of March 30, 2012, between Thomas E. Hartman and Hanger Prosthetics & Orthotics, Inc. (Incorporated herein by reference to Exhibit 10.22 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2012.)* |
|
10.13 |
|
Second Amended and Restated Employment Agreement, dated August 27, 2012, by and between Vinit K. Asar and Hanger, Inc. (Incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8-K filed by the Registrant on August 29, 2012.)* |
|
10.14 |
|
Amended and Restated Employment Agreement, dated as of February 25, 2013, by and between Kenneth W. Wilson and Southern Prosthetic Supply, Inc. (Incorporated herein by reference to Exhibit 10.24 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2012.)* |
|
10.15 |
|
Defined Contribution Supplemental Retirement Plan, dated May 1, 2013. (Incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8-K filed by the Registration on May 13, 2013.) |
|
10.16 |
|
Employment Agreement, dated September 1, 2014, by and between Samuel M. Liang and Hanger Prosthetics & Orthotics, Inc. (Incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8-K filed by the Registrant on September 2, 2014.) |
|
10.17 |
|
Employment Agreement, dated September 5, 2014, by and between Thomas E. Kiraly and Hanger Prosthetics & Orthotics, Inc. (Incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8-K filed by the Registrant on January 1, 2015.) |
|
10.18 |
|
Assignment of Employment Agreement, effective March 1, 2017, by and among Hanger Prosthetics & Orthotics, Inc., Hanger, Inc. and Vinit K. Asar. (Incorporated herein by reference to Exhibit 10.22 to the Annual Report on Form 10-K for the year ended December 31, 2014.)* |
|
10.19 |
|
Assignment of Employment Agreement, effective March 1, 2017, by and among Hanger Prosthetics & Orthotics, Inc., Hanger, Inc. and Thomas E. Kiraly. (Incorporated herein by reference to Exhibit 10.23 to the Annual Report on Form 10-K for the year ended December 31, 2014.)* |
|
10.20 |
|
Hanger, Inc. 2016 Omnibus Incentive Plan. (Incorporated herein by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed by the Registrant on April 18, 2016.)* |
|
10.21 |
|
Form of Executive Non-Qualified Stock Option Agreement under the 2016 Omnibus Incentive Plan. (Incorporated herein by reference to Exhibit 10.2 to the Registrant’s Current Report on Form 8-K filed by the Registrant on April 18, 2016.)* |
|
10.22 |
|
Form of Non-Qualified Stock Option Agreement under the 2016 Omnibus Incentive Plan. (Incorporated herein by reference to Exhibit 10.3 to the Registrant’s Current Report on Form 8-K filed by the Registrant on April 18, 2016.)* |
|
10.23 |
|
Form of Non-Employee Director Non-Qualified Stock Option Agreement under the 2016 Omnibus Incentive Plan. (Incorporated herein by reference to Exhibit 10.4 to the Registrant’s Current Report on Form 8-K filed by the Registrant on April 18, 2016.)* |
|
10.24 |
|
Form of Restricted Stock Unit Agreement for Executives under the 2016 Omnibus Incentive Plan. (Incorporated herein by reference to Exhibit 10.5 to the Registrant’s Current Report on Form 8-K filed by the Registrant on April 18, 2016.)* |
|
10.25 |
|
Form of Restricted Stock Unit Agreement for Employees under the 2016 Omnibus Incentive Plan. (Incorporated herein by reference to Exhibit 10.6 to the Registrant’s Current Report on Form 8-K filed by the Registrant on April 18, 2016.)* |
|
10.26 |
|
Form of Restricted Stock Unit Agreement for Non-Employee Directors under the 2016 Omnibus Incentive Plan. (Incorporated herein by reference to Exhibit 10.7 to the Registrant’s Current Report on Form 8-K filed by the Registrant on April 18, 2016.)* |
|
10.27 |
|
Hanger, Inc. 2017 Special Equity Plan. (Incorporated herein by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed by the Registrant on May 23, 2017.)* |
|
10.28 |
|
Form of Non-Qualified Stock Option Agreement for Executives under the 2017 Special Equity Plan. (Incorporated herein by reference to Exhibit 10.2 to the Registrant’s Current Report on Form 8-K filed by the Registrant on May 23, 2017.)* |
|
10.29 |
|
Form of Non-Qualified Stock Option Agreement for Employees under the 2017 Special Equity Plan. (Incorporated herein by reference to Exhibit 10.3 to the Registrant’s Current Report on Form 8-K filed by the Registrant on May 23, 2017.)* |
|
10.30 |
|
Form of Performance Share Unit Agreement for Executives under the 2017 Special Equity Plan. (Incorporated herein by reference to Exhibit 10.4 to the Registrant’s Current Report on Form 8-K filed by the Registrant on May 23, 2017.)* |
|
10.31 |
|
Form of Performance Share Unit Agreement for Employees under the 2017 Special Equity Plan. (Incorporated herein by reference to Exhibit 10.5 to the Registrant’s Current Report on Form 8-K filed by the Registrant on May 23, 2017.)* |
|
21 |
|
List of Subsidiaries of the Registrant. (Filed herewith.) |
|
31.1 |
|
Written Statement of the Chief Executive Officer Pursuant to Section 302 of the Sarbanes Oxley Act of 2002. (Filed herewith.) |
|
31.2 |
|
Written Statement of the Chief Financial Officer Pursuant to Section 302 of the Sarbanes Oxley Act of 2002. (Filed herewith.) |
|
32 |
|
Written Statement of the Chief Executive Officer and Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as adopted Pursuant to Section 906 of the Sarbanes Oxley Act of 2002. (Filed herewith.) |
|
101.INS |
|
XBRL Instance Document. (Filed herewith.) |
|
101.SCH |
|
XBRL Taxonomy Extension Schema. (Filed herewith.) |
|
101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase. (Filed herewith.) |
|
101.LAB |
|
XBRL Taxonomy Extension Label Linkbase. (Filed herewith.) |
|
101.PRE |
|
XBRL Taxonomy Extension Presentation Linkbase. (Filed herewith.) |
|
101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase. (Filed herewith.) |
* Management contract or compensatory plan
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
HANGER, INC. | |
|
|
|
|
|
Dated: May 14, 2018 |
By: |
/s/ VINIT K. ASAR |
|
|
|
Vinit K. Asar |
|
|
|
Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
|
Dated: May 14, 2018 |
/s/ VINIT K. ASAR |
|
|
Vinit K. Asar |
|
|
Chief Executive Officer and Director |
|
|
(Principal Executive Officer) |
|
|
|
|
Dated: May 14, 2018 |
/s/ THOMAS E. KIRALY |
|
|
Thomas E. Kiraly |
|
|
Executive Vice President and |
|
|
Chief Financial Officer |
|
|
(Principal Financial Officer) |
|
|
|
|
Dated: May 14, 2018 |
/s/ GABRIELLE B. ADAMS |
|
|
Gabrielle B. Adams |
|
|
Vice President — Chief Accounting Officer |
|
|
(Principal Accounting Officer) |
|
|
|
|
Dated: May 14, 2018 |
/s/ ASIF AHMAD |
|
|
Asif Ahmad |
|
|
Director |
|
|
|
|
Dated: May 14, 2018 |
/s/ CHRISTOPHER B. BEGLEY |
|
|
Christopher B. Begley |
|
|
Director |
|
|
|
|
Dated: May 14, 2018 |
/s/ JOHN T. FOX |
|
|
John T. Fox |
|
|
Director |
|
|
|
|
Dated: May 14, 2018 |
/s/ THOMAS C. FREYMAN |
|
|
Thomas C. Freyman |
|
|
Director |
|
|
|
|
Dated: May 14, 2018 |
/s/ STEPHEN E. HARE |
|
|
Stephen E. Hare |
|
|
Director |
|
|
|
|
Dated: May 14, 2018 |
/s/ CYNTHIA L. LUCCHESE |
|
|
Cynthia L. Lucchese |
|
|
Director |
|
|
|
|
Dated: May 14, 2018 |
/s/ RICHARD R. PETTINGILL |
|
|
Richard R. Pettingill |
|
|
Director |
|
|
|
|
Dated: May 14, 2018 |
/s/ KATHRYN M. SULLIVAN |
|
|
Kathryn M. Sullivan |
|
|
Director |
|
Hanger, Inc. |
|
|
F-2 | |
|
Consolidated Balance Sheets as of December 31, 2017 and 2016 |
F-4 |
|
F-5 | |
|
F-6 | |
|
Consolidated Statements of Cash Flows for the Three Years Ended December 31, 2017 |
F-7 |
|
F-8 |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Hanger, Inc.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Hanger, Inc. and its subsidiaries as of December 31, 2017 and 2016, and the related consolidated statements of operations and comprehensive loss, of changes in shareholders’ (deficit) equity and of cash flows for each of the three years in the period ended December 31, 2017, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company’s internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2017 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company did not maintain, in all material respects, effective internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO because material weaknesses in internal control over financial reporting existed as of that date. The material weaknesses related to (1) an ineffective control environment due to ineffective controls with respect to establishing and assigning authority and responsibility over accounting operations; (2) risk assessment as the Company did not design and maintain effective internal controls to identify, assess and address risks that significantly impact the financial statements; (3) information and communication as the Company did not design and maintain effective controls to obtain, generate and communicate relevant and accurate information necessary for the function of internal control, including not implementing or maintaining sufficient information systems; (4) monitoring as the Company did not design and maintain effective controls to monitor compliance with established accounting policies, procedures and controls. The material weaknesses in control environment, risk assessment, information and communication and monitoring contributed to additional material weaknesses as the Company did not design and maintain effective controls over (5) the preparation, review and approval of account reconciliations; (6) certain information technology systems that are relevant to the preparation of the consolidated financial statements; and (7) the accounting for (a) inventory, (b) leases, (c) revenue, (d) accounts receivable, and related accounts, (e) property, plant and equipment, including capitalized software and depreciation expense, (f) accounts payable and related accruals, (g) business combinations, goodwill and intangible assets, (h) share-based compensation and (i) income taxes.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. The material weaknesses referred to above are described in Management’s Report on Internal Control over Financial Reporting appearing under Item 9A. We considered these material weaknesses in determining the nature, timing, and extent of audit tests applied in our audit of the 2017 consolidated financial statements, and our opinion regarding the effectiveness of the Company’s internal control over financial reporting does not affect our opinion on those consolidated financial statements.
Basis for Opinions
The Company’s management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in management’s report referred to above. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are
required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
|
/s/ PricewaterhouseCoopers LLP |
|
|
|
|
|
Austin, Texas |
|
|
May 14, 2018 |
|
We have served as the Company’s auditor since 1987.
HANGER, INC.
(dollars in thousands, except par value and share amounts)
|
|
|
As of December 31, |
| ||||
|
|
|
2017 |
|
2016 |
| ||
|
ASSETS |
|
|
|
|
| ||
|
Current assets: |
|
|
|
|
| ||
|
Cash and cash equivalents |
|
$ |
1,508 |
|
$ |
7,157 |
|
|
Net accounts receivable, less allowance for doubtful accounts of $14,065 and $15,521 in 2017 and 2016, respectively |
|
146,346 |
|
144,562 |
| ||
|
Inventories |
|
69,138 |
|
68,225 |
| ||
|
Income taxes receivable |
|
13,079 |
|
13,200 |
| ||
|
Other current assets |
|
20,888 |
|
19,137 |
| ||
|
Total current assets |
|
250,959 |
|
252,281 |
| ||
|
Non-current assets: |
|
|
|
|
| ||
|
Property, plant and equipment, net |
|
93,615 |
|
100,467 |
| ||
|
Goodwill |
|
196,343 |
|
249,678 |
| ||
|
Other intangible assets, net |
|
21,940 |
|
32,941 |
| ||
|
Deferred income taxes |
|
68,126 |
|
94,223 |
| ||
|
Other assets |
|
9,440 |
|
25,514 |
| ||
|
Total assets |
|
$ |
640,423 |
|
$ |
755,104 |
|
|
LIABILITIES AND SHAREHOLDERS’ (DEFICIT) EQUITY |
|
|
|
|
| ||
|
Current liabilities: |
|
|
|
|
| ||
|
Current portion of long-term debt |
|
$ |
4,336 |
|
$ |
30,944 |
|
|
Accounts payable |
|
48,269 |
|
50,549 |
| ||
|
Accrued expenses and other current liabilities |
|
65,838 |
|
78,950 |
| ||
|
Accrued interest payable |
|
845 |
|
662 |
| ||
|
Accrued compensation related costs |
|
53,005 |
|
36,162 |
| ||
|
Total current liabilities |
|
172,293 |
|
197,267 |
| ||
|
Long-term liabilities: |
|
|
|
|
| ||
|
Long-term debt, less current portion |
|
445,928 |
|
441,706 |
| ||
|
Other liabilities |
|
50,253 |
|
50,717 |
| ||
|
Total liabilities |
|
668,474 |
|
689,690 |
| ||
|
Commitments and contingent liabilities (Note Q) |
|
|
|
|
| ||
|
Shareholders’ (Deficit) Equity: |
|
|
|
|
| ||
|
Common stock, $.01 par value; 60,000,000 shares authorized; 36,515,232 shares issued and 36,372,411 shares outstanding in 2017, and 36,183,894 shares issued and 36,041,073 shares outstanding in 2016 |
|
365 |
|
362 |
| ||
|
Additional paid-in capital |
|
333,738 |
|
322,191 |
| ||
|
Accumulated other comprehensive loss |
|
(1,686 |
) |
(1,440 |
) | ||
|
Retained deficit |
|
(359,772 |
) |
(255,003 |
) | ||
|
Treasury stock, at cost; 142,821 shares at 2017 and 2016, respectively |
|
(696 |
) |
(696 |
) | ||
|
Total shareholders’ (deficit) equity |
|
(28,051 |
) |
65,414 |
| ||
|
Total liabilities and shareholders’ (deficit) equity |
|
$ |
640,423 |
|
$ |
755,104 |
|
The accompanying notes are an integral part of the consolidated financial statements.
HANGER, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
AND COMPREHENSIVE LOSS
(dollars in thousands, except share and per share amounts)
|
|
|
For the Years Ended December 31, |
| |||||||
|
|
|
2017 |
|
2016 |
|
2015 |
| |||
|
Net revenue |
|
$ |
1,040,769 |
|
$ |
1,042,054 |
|
$ |
1,067,172 |
|
|
Material costs |
|
329,223 |
|
332,071 |
|
336,283 |
| |||
|
Personnel costs |
|
361,090 |
|
363,537 |
|
367,094 |
| |||
|
Other operating costs |
|
129,831 |
|
139,024 |
|
140,839 |
| |||
|
General and administrative expenses |
|
110,078 |
|
107,224 |
|
111,761 |
| |||
|
Professional accounting and legal fees |
|
36,239 |
|
41,233 |
|
28,647 |
| |||
|
Depreciation and amortization |
|
39,259 |
|
44,887 |
|
46,343 |
| |||
|
Impairment of intangible assets |
|
54,735 |
|
86,164 |
|
385,807 |
| |||
|
Loss from operations |
|
(19,686 |
) |
(72,086 |
) |
(349,602 |
) | |||
|
Interest expense, net |
|
57,688 |
|
45,199 |
|
29,892 |
| |||
|
Loss on extinguishment of debt |
|
— |
|
6,031 |
|
7,237 |
| |||
|
Loss from continuing operations before income taxes |
|
(77,374 |
) |
(123,316 |
) |
(386,731 |
) | |||
|
Provision (benefit) for income taxes |
|
27,297 |
|
(15,910 |
) |
(67,614 |
) | |||
|
Loss from continuing operations |
|
(104,671 |
) |
(107,406 |
) |
(319,117 |
) | |||
|
Income (loss) from discontinued operations, net of income taxes |
|
— |
|
935 |
|
(7,974 |
) | |||
|
Net loss |
|
$ |
(104,671 |
) |
$ |
(106,471 |
) |
$ |
(327,091 |
) |
|
|
|
|
|
|
|
|
| |||
|
Other comprehensive (loss) income: |
|
|
|
|
|
|
| |||
|
Unrealized (loss) gain on DB SERP, net of income tax (benefit) provision of $(151), $(16) and $81 for 2017, 2016 and 2015, respectively |
|
$ |
(246 |
) |
$ |
(26 |
) |
$ |
474 |
|
|
Comprehensive loss |
|
$ |
(104,917 |
) |
$ |
(106,497 |
) |
$ |
(326,617 |
) |
|
|
|
|
|
|
|
|
| |||
|
Basic and Diluted Per Common Share Data: |
|
|
|
|
|
|
| |||
|
Loss from continuing operations |
|
$ |
(2.89 |
) |
$ |
(2.99 |
) |
$ |
(8.96 |
) |
|
Income (loss) from discontinued operations, net of income taxes |
|
— |
|
0.03 |
|
(0.22 |
) | |||
|
Basic and diluted loss per common share |
|
$ |
(2.89 |
) |
$ |
(2.96 |
) |
$ |
(9.18 |
) |
|
Shares used to compute basic and diluted per common share amounts |
|
36,270,920 |
|
35,933,222 |
|
35,635,448 |
| |||
The accompanying notes are an integral part of the consolidated financial statements.
HANGER, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ (DEFICIT) EQUITY
For the Three Years Ended December 31, 2017
(dollars and share amounts in thousands)
|
|
|
Common |
|
Common |
|
Additional |
|
Accumulated |
|
Retained |
|
Treasury |
|
Total |
| ||||||
|
Balance, December 31, 2014 |
|
35,399 |
|
$ |
355 |
|
$ |
307,166 |
|
$ |
(1,888 |
) |
$ |
178,559 |
|
$ |
(656 |
) |
$ |
483,536 |
|
|
Net loss |
|
— |
|
— |
|
— |
|
— |
|
(327,091 |
) |
— |
|
(327,091 |
) | ||||||
|
Issuance of common stock upon vesting of restricted stock units |
|
306 |
|
3 |
|
(3 |
) |
— |
|
— |
|
— |
|
— |
| ||||||
|
Issuance of common stock in connection with the exercise of stock options |
|
8 |
|
1 |
|
— |
|
— |
|
— |
|
— |
|
1 |
| ||||||
|
Purchase of treasury stock |
|
(2 |
) |
— |
|
40 |
|
— |
|
— |
|
(40 |
) |
— |
| ||||||
|
Stock-based compensation expense |
|
— |
|
— |
|
11,134 |
|
— |
|
— |
|
— |
|
11,134 |
| ||||||
|
Tax expense associated with vesting of restricted stock units |
|
— |
|
— |
|
(596 |
) |
— |
|
— |
|
— |
|
(596 |
) | ||||||
|
Effect of shares withheld to cover taxes |
|
— |
|
— |
|
(2,212 |
) |
— |
|
— |
|
— |
|
(2,212 |
) | ||||||
|
Tax provision on unrealized gain on DB SERP |
|
— |
|
— |
|
— |
|
(81 |
) |
— |
|
— |
|
(81 |
) | ||||||
|
Total other comprehensive income |
|
— |
|
— |
|
— |
|
555 |
|
— |
|
— |
|
555 |
| ||||||
|
Balance, December 31, 2015 |
|
35,711 |
|
359 |
|
315,529 |
|
(1,414 |
) |
(148,532 |
) |
(696 |
) |
165,246 |
| ||||||
|
Net loss |
|
— |
|
— |
|
— |
|
— |
|
(106,471 |
) |
— |
|
(106,471 |
) | ||||||
|
Issuance of common stock upon vesting of restricted stock units |
|
330 |
|
3 |
|
(3 |
) |
— |
|
— |
|
— |
|
— |
| ||||||
|
Stock-based compensation expense |
|
— |
|
— |
|
9,763 |
|
— |
|
|
|
— |
|
9,763 |
| ||||||
|
Tax expense associated with vesting of restricted stock units |
|
— |
|
— |
|
(2,810 |
) |
— |
|
— |
|
— |
|
(2,810 |
) | ||||||
|
Effect of shares withheld to cover taxes |
|
— |
|
— |
|
(288 |
) |
— |
|
— |
|
— |
|
(288 |
) | ||||||
|
Tax benefit on unrealized loss on DB SERP |
|
— |
|
— |
|
— |
|
16 |
|
— |
|
— |
|
16 |
| ||||||
|
Total other comprehensive loss |
|
— |
|
— |
|
— |
|
(42 |
) |
— |
|
— |
|
(42 |
) | ||||||
|
Balance, December 31, 2016 |
|
36,041 |
|
362 |
|
322,191 |
|
(1,440 |
) |
(255,003 |
) |
(696 |
) |
65,414 |
| ||||||
|
Net loss |
|
— |
|
— |
|
— |
|
— |
|
(104,671 |
) |
— |
|
(104,671 |
) | ||||||
|
Issuance of common stock upon vesting of restricted stock units |
|
331 |
|
3 |
|
(3 |
) |
— |
|
— |
|
— |
|
— |
| ||||||
|
Stock-based compensation expense |
|
— |
|
— |
|
12,929 |
|
— |
|
— |
|
— |
|
12,929 |
| ||||||
|
Cumulative effect of a change in accounting for stock-based payments (Note B) |
|
— |
|
— |
|
98 |
|
— |
|
(98 |
) |
— |
|
— |
| ||||||
|
Effect of shares withheld to cover taxes |
|
— |
|
— |
|
(1,477 |
) |
— |
|
— |
|
— |
|
(1,477 |
) | ||||||
|
Tax benefit on unrealized loss on DB SERP |
|
— |
|
— |
|
— |
|
151 |
|
— |
|
— |
|
151 |
| ||||||
|
Total other comprehensive loss |
|
— |
|
— |
|
— |
|
(397 |
) |
— |
|
— |
|
(397 |
) | ||||||
|
Balance, December 31, 2017 |
|
36,372 |
|
$ |
365 |
|
$ |
333,738 |
|
$ |
(1,686 |
) |
$ |
(359,772 |
) |
$ |
(696 |
) |
$ |
(28,051 |
) |
The accompanying notes are an integral part of the consolidated financial statements.
HANGER, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(dollars in thousands)
|
|
|
For the Years Ended December 31, |
| |||||||
|
|
|
2017 |
|
2016 |
|
2015 |
| |||
|
Cash flows from operating activities: |
|
|
|
|
|
|
| |||
|
Net loss |
|
$ |
(104,671 |
) |
$ |
(106,471 |
) |
$ |
(327,091 |
) |
|
Income (loss) from discontinued operations, net of income taxes |
|
— |
|
935 |
|
(7,974 |
) | |||
|
Loss from continuing operations |
|
(104,671 |
) |
(107,406 |
) |
(319,117 |
) | |||
|
Adjustments to reconcile net loss to net cash provided by operating activities: |
|
|
|
|
|
|
| |||
|
Depreciation and amortization |
|
39,259 |
|
44,887 |
|
46,343 |
| |||
|
Provision for doubtful accounts |
|
9,422 |
|
13,727 |
|
12,854 |
| |||
|
Impairment of long-lived and intangible assets |
|
54,735 |
|
86,164 |
|
385,807 |
| |||
|
Stock-based compensation expense |
|
12,930 |
|
9,763 |
|
11,134 |
| |||
|
Provision (benefit) for deferred income taxes |
|
26,248 |
|
4,031 |
|
(48,926 |
) | |||
|
Amortization of debt issuance costs |
|
8,876 |
|
4,921 |
|
3,371 |
| |||
|
Loss on extinguishment of debt |
|
— |
|
6,031 |
|
7,237 |
| |||
|
Gain on sale and disposal of fixed assets |
|
(2,059 |
) |
(5,055 |
) |
(2,384 |
) | |||
|
Changes in operating assets and liabilities, net of effects of acquired companies: |
|
|
|
|
|
|
| |||
|
Net accounts receivable |
|
(12,585 |
) |
17,612 |
|
(13,625 |
) | |||
|
Inventories |
|
(913 |
) |
253 |
|
2,520 |
| |||
|
Other current assets |
|
661 |
|
849 |
|
3,913 |
| |||
|
Income taxes |
|
121 |
|
18,725 |
|
(40,152 |
) | |||
|
Accounts payable |
|
(3,562 |
) |
(3,133 |
) |
8,084 |
| |||
|
Accrued expenses and accrued interest payable |
|
(12,929 |
) |
(3,045 |
) |
(6,264 |
) | |||
|
Accrued compensation related costs |
|
16,843 |
|
(12,006 |
) |
6,877 |
| |||
|
Other liabilities |
|
(2,271 |
) |
(5,797 |
) |
6,940 |
| |||
|
Net cash provided by operating activities - continuing operations |
|
30,105 |
|
70,521 |
|
64,612 |
| |||
|
Net cash used in operating activities - discontinued operations |
|
— |
|
(1,425 |
) |
(5,098 |
) | |||
|
Net cash provided by operating activities |
|
30,105 |
|
69,096 |
|
59,514 |
| |||
|
Cash flows from investing activities: |
|
|
|
|
|
|
| |||
|
Purchase of property, plant and equipment |
|
(16,355 |
) |
(21,148 |
) |
(27,620 |
) | |||
|
Purchase of equipment leased to third parties under operating leases |
|
(6,000 |
) |
(2,476 |
) |
(4,632 |
) | |||
|
Acquisitions, net of cash acquired |
|
— |
|
— |
|
(10,215 |
) | |||
|
Restricted cash |
|
(1,016 |
) |
1,615 |
|
(54 |
) | |||
|
Proceeds from company-owned life insurance investment |
|
17,135 |
|
— |
|
— |
| |||
|
Purchase of company-owned life insurance investment |
|
(555 |
) |
(2,543 |
) |
(2,544 |
) | |||
|
Proceeds from sale of property, plant and equipment |
|
4,909 |
|
5,960 |
|
4,954 |
| |||
|
Other investing activities, net |
|
— |
|
(10 |
) |
(50 |
) | |||
|
Net cash used in investing activities - continuing operations |
|
(1,882 |
) |
(18,602 |
) |
(40,161 |
) | |||
|
Net cash provided by investing activities - discontinued operations |
|
— |
|
1,425 |
|
4,987 |
| |||
|
Net cash used in investing activities |
|
(1,882 |
) |
(17,177 |
) |
(35,174 |
) | |||
|
Cash flows from financing activities: |
|
|
|
|
|
|
| |||
|
Borrowings under term loan |
|
420 |
|
274,400 |
|
— |
| |||
|
Repayment of term loan |
|
(28,545 |
) |
(19,688 |
) |
(14,063 |
) | |||
|
Borrowings under revolving credit agreement |
|
156,965 |
|
23,000 |
|
155,000 |
| |||
|
Repayments under revolving credit agreement |
|
(151,965 |
) |
(155,000 |
) |
(93,000 |
) | |||
|
Payment of senior notes |
|
— |
|
(200,000 |
) |
— |
| |||
|
Payment of employee taxes on stock-based compensation |
|
(1,477 |
) |
(288 |
) |
(2,212 |
) | |||
|
Payment on seller notes and other contingent consideration |
|
(5,197 |
) |
(9,128 |
) |
(13,561 |
) | |||
|
Payment of capital lease obligations |
|
(1,210 |
) |
(979 |
) |
(1,110 |
) | |||
|
Payment of debt issuance costs and fees |
|
(2,863 |
) |
(15,832 |
) |
(8,340 |
) | |||
|
Net cash (used in) provided by financing activities - continuing operations |
|
(33,872 |
) |
(103,515 |
) |
22,714 |
| |||
|
(Decrease) increase in cash and cash equivalents |
|
(5,649 |
) |
(51,596 |
) |
47,054 |
| |||
|
Cash and cash equivalents, at beginning of year |
|
7,157 |
|
58,753 |
|
11,699 |
| |||
|
Cash and cash equivalents, at end of year |
|
$ |
1,508 |
|
$ |
7,157 |
|
$ |
58,753 |
|
Supplemental cash flow information is disclosed in Note T to the consolidated financial statements.
The accompanying notes are an integral part of the consolidated financial statements.
HANGER, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
As of and for the Years Ended December 31, 2017, 2016 and 2015
NOTE A — THE COMPANY
Hanger, Inc. (“the Company,” “we,” “our,” or “us”) is a leading national provider of products and services that assist in enhancing or restoring the physical capabilities of patients with disabilities or injuries. We provide orthotic and prosthetic (“O&P”) services, distribute O&P devices and components, manage O&P networks and provide therapeutic solutions to patients and businesses in acute, post-acute and clinic settings. We operate through two segments, Patient Care and Products & Services.
Our Patient Care segment is primarily comprised of Hanger Clinic, which specializes in the design, fabrication and delivery of custom O&P devices through 682 patient care clinics and 112 satellite locations in 44 states and the District of Columbia as of December 31, 2017. On a regular basis, we have been opening, closing and merging patient care locations and satellite locations. During the year ended December 31, 2017, we have opened 31 and closed or consolidated 58 patient care locations.
Our Products & Services segment is comprised of our distribution and therapeutic solutions businesses. As a leading provider of O&P products in the United States, we coordinate through our distribution business the procurement and distribution of a broad catalog of O&P parts, componentry and devices to independent O&P providers nationwide. The other business in our Products & Services segment is our therapeutic solutions business, which develops specialized rehabilitation technologies and provides evidence-based clinical programs for post-acute rehabilitation to patients at approximately 4,000 skilled nursing and post-acute providers nationwide.
NOTE B — SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation
Our consolidated financial statements include our accounts and those of our wholly-owned subsidiaries. All material intercompany transactions and balances have been eliminated in the accompanying consolidated financial statements.
Use of Estimates and Assumptions
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America (“GAAP”) requires the use of estimates and assumptions that affect the reported amounts of revenues, expenses, assets, liabilities and contingencies. Although actual results in subsequent periods may differ from these estimates, such estimates are developed based on the best information available to management and based on management’s best judgments at the time. We base our estimates on historical experience, observable trends and various other assumptions that we believe are reasonable under the circumstances. All significant assumptions and estimates underlying the amounts reported in the consolidated financial statements and accompanying notes are regularly reviewed and updated when necessary. Changes in estimates are reflected prospectively in the consolidated financial statements based upon on-going actual trends, or subsequent settlements and realizations depending on the nature and predictability of the estimates and contingencies. Interim changes in estimates related to annual operating costs are applied prospectively within annual periods. Although we believe that our estimates are reasonable, actual results could differ from these estimates.
The most significant assumptions and estimates underlying these consolidated financial statements and accompanying notes involve revenue recognition and accounts receivable valuation, inventories, accounts payable and accrued liabilities (including self-insurance reserves and contingencies), impairments of long-lived assets including goodwill, income taxes, business combinations, leases and stock-based compensation.
Reclassifications
We have reclassified certain amounts in the 2016 and 2015 consolidated financial statements to be consistent with the 2017 presentation. These principally relate to classifications within the consolidated statements of cash flows.
Revenue Recognition
Patient Care Segment
Revenues in our Patient Care segment are primarily derived from the sale of O&P devices and are recognized when the patient has received the device or service. At or subsequent to delivery, we issue an invoice to a third party payor, which primarily consists of commercial insurance companies, Medicare, Medicaid, the U.S. Department of Veterans Affairs and private or patient pay (“Private Pay”). We recognize revenue for the amounts we expect to receive from payors based on expected contractual reimbursement rates, which are net of estimated contractual discounts. Government reimbursement, comprised of Medicare, Medicaid and the U.S. Department of Veterans Affairs, in the aggregate, accounted for approximately, 54.8%, 54.1% and 53.4% of our net revenue in 2017, 2016 and 2015, respectively.
These revenue amounts are further revised as claims are adjudicated, which may result in disallowances, or decreases to revenue. We believe that adjustments related to write-offs of receivables should predominantly be recorded as a reduction of revenues, which we refer to as disallowed revenue. This is due to the majority of our revenues being collected from commercial insurance companies, Medicare, Medicaid and the Veterans Administration, most of which are under contractual reimbursement rates. As such, adjustments do not relate to an inability to pay, but to contractual allowances, lack of timely claims submission, insufficient medical documentation or other administrative errors. Amounts recorded to bad debt expense, which are presented within “Other operating costs,” generally relate to commercial payor bankruptcies and private pay balances for which there was an assessment of collectability and collection attempts were made. At the end of each period, we establish allowances for estimated disallowances relating to that period based on prior adjudication experience and record such amounts as an adjustment to revenue. In a similar fashion, we estimate and record allowances for doubtful accounts on unpaid receivables at each period end. We also record a liability, with a corresponding adjustment to revenue, for refunds expected to be paid to our patients or third party payors.
Medicare and Medicaid regulations and the various agreements we have with other third party payors, including commercial healthcare payors under which these contractual adjustments and disallowed revenue are calculated, are complex and are subject to interpretation and adjustment and may include multiple reimbursement mechanisms for different types of services. Therefore, the particular O&P devices and related services authorized and provided, and the related reimbursement, are subject to interpretation and adjustment that could result in payments that differ from our estimates. Additionally, updated regulations and reimbursement schedules, and contract renegotiations occur frequently, necessitating regular review and assessment of the estimation process by management. As a result, there is a reasonable possibility that recorded estimates could change and any related adjustments will be recorded as changes in estimates when they become known.
For more information on our use of estimates to calculate allowances for disallowed revenue and doubtful accounts, refer to the “Accounts Receivable, Net” section below.
We often invoice patients or payors after a device is delivered. To account for this delay, we record an estimated revenue accrual for devices delivered but not yet invoiced at period end. This estimate is based on a historical look-back analysis of lag times between delivery and invoicing that occur over a period end.
Products & Services Segment
Revenues in our Products & Services segment are derived from the distribution of O&P components and the leasing and sale of rehabilitation equipment and ancillary consumable supplies combined with equipment maintenance, education, and training. Distribution revenues are recorded upon the delivery of products, net of estimated returns.
Equipment leasing and related services revenue are recognized over the applicable term as the customer has the right to use the equipment and as the services are provided. Equipment sales revenue is recognized upon delivery, with any related services revenue deferred and recognized as the services are performed. Sales of consumables are recognized upon delivery.
Material Costs
Material costs in our Patient Care segment reflect purchases of orthotics and prosthetic componentry and other related costs in connection with the delivery of care through our clinics and other patient care operations. Material costs in our Products & Services segment reflect purchases of orthotics and prosthetic materials and other related costs in connection with the distribution of products and services to third party customers.
Personnel Costs
Personnel costs reflect salaries, benefits, incentive compensation, contract labor, and other personnel costs we incur in connection with our delivery of care through our clinics and other patient care operations, or distribution of products and services, and exclude similar costs incurred in connection with general and administrative activities.
Other Operating Costs
Other operating costs reflect costs we incur in connection with our delivery of care through our clinics and other patient care operations or distribution of products and services. Marketing costs, including advertising, are expensed as incurred and are presented within this financial statement caption. We incurred approximately $3.8 million, $4.0 million, and $3.9 million in advertising costs during the years ended December 31, 2017, 2016 and 2015, respectively. Other costs include rent, utilities, and other occupancy costs, general office expenses, bad debt expense, and travel and clinical professional education costs, and exclude similar costs incurred in connection with general and administrative activities.
General and Administrative Expenses
General and administrative expenses reflect costs we incur in the management and administration of our businesses that are not directly related to the operation of our clinics or provision of products and services. These include personnel costs and other operating costs supporting our general and administrative functions. We incurred approximately $0.7 million, $0.6 million, and $0.6 million in advertising costs during the years ended December 31, 2017, 2016 and 2015, respectively.
Professional Accounting and Legal Fees
We recognize fees associated with audits of our financial statements in the fiscal period to which the audit relates. All other professional fees are generally recognized as expense in the periods in which services are performed. Please see the “Accounts Payable and Accrued Liabilities” section for legal fees associated with legal contingencies.
Depreciation and Amortization
Depreciation and amortization expenses reflect all depreciation and amortization expenses, whether incurred in connection with our delivery of care through our clinics, our distribution of products and services, or in the general management and administration of our business.
Cash and Cash Equivalents
We consider all highly liquid investments with original maturities of three months or less at the date of purchase to be cash equivalents. We maintain cash balances in excess of Federal Deposit Insurance Corporation (“FDIC”) limits at certain financial institutions. We manage this credit risk by concentrating our cash balances in high quality financial institutions and by periodically evaluating the credit quality of the primary financial institutions holding such deposits. With short maturities, the investments present insignificant risk of changes in value because of interest rate changes and are readily convertible to cash. Historically, no losses have been incurred due to such cash concentrations. Restricted cash balances are presented within “Other current assets” in the consolidated balance sheets. See Note I - “Other Current Assets and Other Assets” within these consolidated financial statements.
Accounts Receivable, Net
Patient Care Segment
We establish allowances for accounts receivable to reduce the carrying value of such receivables to their estimated net realizable value. The Patient Care segment’s accounts receivables are recorded net of unapplied cash, estimated allowance for disallowed revenue and estimated allowance for doubtful accounts, as described in the revenue recognition accounting policy above.
Both the allowance for disallowed revenue and the allowance for doubtful accounts estimates consider historical collection experience by each of the Medicare and non-Medicare (commercial insurance, Medicaid, U.S. Department of Veteran’s Affairs and Private Pay) primary payor class groupings. For each payor class grouping, liquidation analysis of historical period end receivable balances are performed to ascertain collections experience by aging category. We believe the use of historical collection experience applied to current period end receivable balances is reasonable. In the absence of an evident adverse trend, we use historical experience rates calculated using an average of four quarters of data with at least twelve months of adjudication. We believe the time periods analyzed provide sufficient time for most balances to adjudicate in the normal course of operations. We will modify the time periods analyzed when significant trends indicate that adjustments should be made. In addition, estimates are adjusted when appropriate for information available up through the issuance of the consolidated financial statements.
Products & Services Segment
Products & Services segment’s allowance for doubtful accounts is estimated based on the analysis of the segment’s historical write-offs experience, accounts receivable aging and economic status of its customers. Accounts receivable that are deemed uncollectible are written-off to the allowance for doubtful accounts. Accounts receivable are also recorded net of an allowance for estimated sales returns.
Inventories
Inventories are valued at the lower of estimated cost or net realizable value with cost determined on a first-in, first out (“FIFO”) basis. Provisions have also been made to reduce the carrying value of inventories for excess, obsolete, or otherwise impaired inventory on hand at period-end.
Patient Care Segment
Substantially all of our Patient Care segment inventories are recorded through a periodic approach whereby inventory quantities are adjusted on the basis of a quarterly physical count. Segment inventories relate primarily to raw materials and work-in-process (“WIP”) at Hanger Clinics. Inventories at Hanger Clinics totaled $27.7 million and $29.1 million at
December 31, 2017 and 2016, respectively, with WIP inventory representing $9.0 million and $9.0 million of the total inventory, respectively.
Raw materials consists of purchased parts, components, and supplies which are used in the assembly of O&P devices for delivery to patients. In some cases, purchased parts and components are also sold directly to patients. Raw materials are valued based on recent vendor invoices, reduced by estimated vendor rebates. Such rebates are recognized as a reduction of cost of materials in the consolidated statements of operations and comprehensive loss when the related devices or components are delivered to the patient. Approximately 71% and 69% of raw materials at December 31, 2017 and 2016, respectively were purchased from our Products & Services segment. Raw material inventory was $18.7 million and $20.1 million at December 31, 2017 and 2016, respectively.
WIP consists of devices which are in the process of assembly at our clinics or fabrication centers. WIP quantities were determined by the physical count of patient orders at the end of every quarter of 2017 and 2016 while the related stage of completion of each order was established by clinic personnel. We do not have an inventory costing system and as a result, the identified WIP quantities were valued on the basis of estimated raw materials, labor, and overhead costs. To estimate such costs, we develop bills of materials for certain categories of devices that we assemble and deliver to patients. Within each bill of material, we estimate (i) the typical types of component parts necessary to assemble each device; (ii) the points in the assembly process when such component parts are added; (iii) the estimated cost of such parts based on historical purchasing data; (iv) the estimated labor costs incurred at each stage of assembly; and (v) the estimated overhead costs applicable to the device.
Products & Services Segment
Product & Service segment inventories consist primarily of finished goods at its distribution centers as well as raw materials at fabrication facilities, and totaled $41.4 million and $39.1 million as of December 31, 2017 and 2016, respectively. Finished goods include products that are available for sale to third party customers as well as to our Patient Care segment as described above. Such inventories were determined on the basis of perpetual records and a physical count at year end. Inventories in connection with therapeutic services are valued at a weighted average cost.
Fair Value Measurements
We follow the authoritative guidance for financial assets and liabilities, which establishes a framework for measuring fair value and requires enhanced disclosures about fair value measurements. The authoritative guidance requires disclosure about how fair value is determined for assets and liabilities and establishes a hierarchy by which these assets and liabilities must be categorized, based on significant levels of inputs as follows:
Level 1 consists of securities for which there are quoted prices in active markets for identical securities;
Level 2 consists of securities for which observable inputs other than Level 1 inputs are used, such as quoted prices for similar securities in active markets or quoted prices for identical securities in less active markets and model-derived valuations for which the variables are derived from, or corroborated by, observable market data; and
Level 3 consists of securities for which there are no observable inputs to the valuation methodology that are significant to the measurement of the fair value.
The determination of where assets and liabilities fall within this hierarchy is based upon the lowest level of input that is significant to the fair value measurement.
Financial Instruments
We hold investments in money market funds which are measured at fair value on a recurring basis. As of December 31, 2017 and 2016, $3.3 million and $2.3 million, respectively of money market funds which are restricted from general use are presented within “Other current assets.” The fair values of our money market funds are based on Level 1 observable market prices and are equivalent to one dollar per share. The carrying value of accounts receivable and accounts payable, approximate their fair values based on the short-term nature of these instruments.
The carrying value of our outstanding term loan as of December 31, 2017 and 2016, was $151.9 million and $180.0 million compared to its fair value of $149.4 million and $172.6 million, respectively. The carrying values of our outstanding Term Loan B as of December 31, 2017 and 2016 was $280.0 million and $280.0 million compared to its fair value of $283.5 million and $278.6 million, respectively. Our estimates of fair value are based on a discounted cash flow model and indicative quote using unobservable inputs, primarily, our risk-adjusted credit spread, which represents a Level 3 measurement.
The carrying value of the amount outstanding on our revolving credit facilities as of December 31, 2017, was $5.0 million compared to its fair value of $4.9 million. We had no balances outstanding under revolving credit facilities as of December 31, 2016. Our estimates of fair value are based on a discounted cash flow model using unobservable inputs, primarily, our risk-adjusted credit spread, which represents a Level 3 measurement.
The carrying value of our outstanding subordinated promissory notes issued in connection with acquisitions (“Seller Notes”) as of December 31, 2017 and 2016 was $5.9 million and $11.1 million, respectively. We believe that the carrying value of the Seller Notes approximates their fair values based on a discounted cash flow model using unobservable inputs, primarily, our credit spread for subordinated debt, which represents a Level 3 measurement.
Insurance Recoveries Receivable
We incur legal and other costs with respect to a variety of issues on an ongoing basis. We record a related receivable when costs are reimbursable under applicable insurance policies, we believe it is probable such costs will be reimbursed and such reimbursements can be reasonably estimated. We record the benefit of related receivables from the insurer as a reduction of costs in the same financial statement caption in which the related loss was recognized in our consolidated statements of operations and comprehensive loss. Loss contingency reserves, which are recorded within accrued liabilities, are not reduced by estimated insurance recoveries.
Property, Plant and Equipment, Net
Property, plant and equipment are recorded at cost less accumulated depreciation and amortization. Equipment acquired under a capital lease is recorded at the present value of the future minimum lease payments. The cost and related accumulated depreciation of assets sold, retired or otherwise disposed of are removed from the respective accounts, and any resulting gains or losses are included in the consolidated statements of operations and comprehensive loss. Depreciation is computed for financial reporting purposes using the straight-line method over the useful lives of the related assets estimated as follows: furniture and fixtures, equipment and information systems, principally five years, buildings ten to forty years, capital leases over the shorter of the useful life or lease term, and leasehold improvements over the shorter of ten years or the lease term. We record maintenance and repairs, including the cost of minor replacements, to maintenance expense. Costs of major repairs that extend the effective useful life of property are capitalized and depreciated accordingly.
We capitalize the costs of obtaining or developing internal use software, including external direct costs of materials and services and directly related payroll costs. Amortization begins when the internal use software is ready for its intended use. Costs incurred during the preliminary project and post-implementation stages, as well as maintenance and training costs, are expensed as incurred.
Business Combinations
We record tangible and intangible assets acquired and liabilities assumed in business combinations under the acquisition method of accounting. Acquisition consideration typically includes cash payments, the issuance of Seller Notes and in certain instances contingent consideration with payment terms associated with the achievement of designated collection targets of the acquired business. Amounts paid for each acquisition are allocated to the assets acquired and liabilities assumed based on their estimated fair values at the date of acquisition inclusive of identifiable intangible assets. The estimated fair value of identifiable assets and liabilities, including intangibles, are based on detailed valuations that use information and assumptions available to management. We allocate any excess purchase price over the fair value of the tangible and identifiable intangible assets acquired and liabilities assumed to goodwill. Significant management judgments and assumptions are required in determining the fair value of assets acquired and liabilities assumed, particularly acquired intangible assets, including estimated useful lives. The valuation of purchased intangible assets is based upon estimates of the future performance and discounted cash flows from the acquired business. Each asset acquired or liability assumed is measured at estimated fair value from the perspective of a market participant. Subsequent changes in the estimated fair value of contingent consideration are recognized as “General and administrative expenses” within the consolidated statements of operations and comprehensive loss.
Goodwill and Other Intangible Assets, Net
Goodwill represents the excess of the purchase price over the estimated fair value of net identifiable assets acquired and liabilities assumed from purchased businesses. We assess goodwill for impairment annually during the fourth quarter, and between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. We have the option to first assess qualitative factors for a reporting unit to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount as a basis for determining whether it is necessary to perform the quantitative goodwill impairment test. If we choose to bypass this qualitative assessment or alternatively determine that a quantitative goodwill impairment test is required, our annual goodwill impairment test is performed by comparing the estimated fair value of a reporting unit with its carrying amount (including attributed goodwill). We will measure the fair value of the reporting units using a combination of income and market approaches. Any impairment would be recognized by a charge to income from operations and a reduction in the carrying value of the goodwill.
We apply judgment in determining the fair value of our reporting units and the implied fair value of goodwill which is dependent on significant assumptions and estimates regarding expected future cash flows, terminal value, changes in working capital requirements, and discount rates.
In January 2017, the Financial Accounting Standards Board issued Accounting Standards Update No. 2017-04 (“ASU 2017-04”) that sought to simplify the accounting for goodwill impairments by eliminating Step 2 from the goodwill impairment test. As a result, our impairment tests performed as of our October 1, 2017 annual impairment testing date compared the carrying values of our reporting units to their respective fair values with any necessary impairment charge recorded in an amount equal to the excess of carrying value over fair value. As required prior to the adoption of ASU 2017-04, impairments were recorded in an amount equal to the excess of the carrying value of a reporting unit’s goodwill over the implied fair value of goodwill.
The fair value of acquired customer intangibles was estimated using an excess earnings model. Key assumptions utilized in the valuation model included pro-forma projected cash flows adjusted for market-participant assumptions, forecasted customer retention curve, and discount rate. Customer intangibles are amortized, using the straight-line method over an estimated useful life of four to ten years. The fair value of non-compete agreements are estimated using a discounted cash flow model. The related intangible assets are amortized, using the straight-line method, over their term which ranges from two to five years. Other definite-lived intangible assets are recorded at cost and are amortized, using the straight-line method, over their estimated useful lives of up to seventeen years. The fair value associated with trade names is estimated using the relief-from-royalty method with the primary assumptions being the royalty rate and expected revenues associated with the trade names. These assets, some of which have indefinite lives, are primarily included in the Products & Services segment.
Indefinite lived trade name intangible assets are assessed for impairment in the fourth quarter of each year, or more frequently if events or changes in circumstances indicate that the asset might be impaired. Trade name intangible assets with definite lives are amortized over their estimated useful lives of one to ten years.
For the years ended December 31, 2017, 2016 and 2015, we recorded impairments of our goodwill totaling $53.3 million, $86.0 million and $382.9 million, respectively. See Note H - “Goodwill and Other Intangible Assets” to our consolidated financial statements in this Annual Report on Form 10-K for additional information regarding these charges.
In conjunction with our Goodwill impairment testing at December 31, 2015, we reevaluated the estimated useful life of our customer list intangibles. In the fourth quarter of 2015, the estimated useful lives of our customer list intangibles were reduced from 10 years to four years in our Patient Care segment and from 14 years to 10 years in our Products & Services segment. This change in the estimated useful lives increased amortization for the years ended December 31, 2017, 2016 and 2015 by approximately $3.0 million $7.0 million and $6.0 million, respectively.
As described, we apply judgment in the selection of key assumptions used in the goodwill impairment test and as part of our evaluation of intangible assets tested annually and at interim testing dates as necessary. If these assumptions differ from actual, we could incur additional impairment charges and those charges could be material.
Long-Lived Asset Impairment
We evaluate the carrying value of long-lived assets to be held and used for impairment whenever events or changes in circumstance indicate that the carrying amount may not be recoverable. The carrying value of a long-lived asset group is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposition of the asset group. We measure impairment as the amount by which the carrying value exceeds the estimated fair value. Estimated fair value is determined primarily using the projected future cash flows discounted at a rate commensurate with the risk involved. Long-lived assets to be disposed of by sale are classified as held for sale when the applicable criteria are met, and recognized within the consolidated balance sheet at the lower of carrying value or fair value less cost to sell. Depreciation on such assets is ceased.
Debt Issuance Costs, Net
Debt issuance costs incurred in connection with long-term debt are amortized utilizing the effective interest method, through the maturity of the related debt instrument. Debt issuance costs are classified as a reduction of debt in the consolidated balance sheets. Amortization of these costs is included within “Interest expense, net” in the consolidated statements of operations and comprehensive loss.
Accounts Payable and Accrued Liabilities
Accounts payable relating to goods or services received is based on various factors including payments made subsequent to period end, vendor invoice dates, shipping terms confirmed by certain vendors or other third party documentation. Accrued liabilities are recorded based on estimates of services received or amounts expected to be paid to third parties. Accrued legal costs for legal contingencies are recorded when they are probable and estimable.
Self-Insurance Reserves
We maintain insurance programs which include employee health insurance, workers’ compensation, product, professional and general liability. Our employee health insurance program is self-funded, with a stop-loss coverage on claims that exceed $0.4 million for any individually covered claim. We are responsible for workers’ compensation, product, professional and general liability claims up to $0.5 million per individual incident. The insurance and self-insurance accruals reflect the estimate of incurred but not reported losses, historical claims experience and expected costs to settle unpaid claims and are undiscounted. We record amounts due from insurance policies in “Other assets” while recording the estimated liability in “Accrued expenses and other current liabilities” in our consolidated balance sheets.
Leases
We lease a majority of our patient care clinics under lease arrangements, certain of which contain renewal options, rent escalation clauses, and/or landlord incentives. Rent expense for noncancellable leases with scheduled rent increases and/or landlord incentives is recognized on a straight-line basis over the lease term, including any applicable rent holidays, beginning on the earlier of the lease commencement date or the date we take control of the leased space.
We have certain building leases that are accounted for as financing transactions. In these instances, pursuant to ASC 840-40-55, The Effect of Lessee Involvement in Asset Construction, we are the deemed owner of the property during the construction phase and the associated building assets and financing obligations are recognized on our consolidated balance sheet. Subsequent to construction, the arrangement is evaluated in accordance with ASC 840-40-25 to determine whether the arrangement qualifies as a sale leaseback. Sale leasebacks of real estate require an analysis to identify indicators of continuing involvement and other factors. If no indicators of continuing involvement are found, the lease is considered to have passed the sales-leaseback criteria and both the asset and the related financing obligation are derecognized. These leases are then assessed for classification at lease inception and reported in accordance with ASC 840.
If indicators of continuing involvement are present, these transactions do not qualify for sale accounting and are accounted for as a failed sale-leaseback. In accordance with ASC 840-40, Leases - Sale-Leaseback Transactions, the buildings and related assets, as well as their associated financing obligations, continue to be reflected in our consolidated balance sheet, with the assets depreciated over their remaining useful lives. Payments required under the arrangement are recognized as reductions of the financing obligation and interest expense. At the end of the lease term, the corresponding financing obligation and the remaining net book value of the building are derecognized. When applicable, any associated gain is recognized within “Other operating costs” in our consolidated statements of operations and comprehensive loss.
Income Taxes
We use the liability method of accounting for income taxes as set forth in the authoritative guidance for accounting for income taxes. Under this method, we recognize deferred tax liabilities and assets for the expected future tax consequences of temporary differences between the respective carrying amounts and tax basis of our assets and liabilities. We recognize a valuation allowance on deferred tax assets if it is more likely than not that the assets will not be realized in future years. Significant accounting judgment is required in determining the provision for income taxes and related consolidated balance sheet accounts.
On December 22, 2017 the U.S. Tax Cuts and Jobs Act of 2017 (the “Tax Act”) was signed into law. As a result of the Tax Act, the U.S. statutory tax rate was lowered from 35% to 21% effective January 1, 2018, among other changes. ASC Topic 740 requires us to recognize the effect of tax law changes in the period of enactment; therefore, we were required to revalue our deferred tax assets and liabilities at December 31, 2017 at the new rate. The SEC issued SAB 118 to address the application of GAAP in situations when a registrant does not have the necessary information available, prepared, or analyzed (including computations) in reasonable detail to complete the accounting for certain tax effects of the Tax Act. The ultimate impact may differ from this provisional amount, possibly materially, as a result of additional analysis, changes in interpretations and assumptions we have made, additional regulatory guidance that may be issued, and actions we may take as a result of the Tax Act.
We believe that our tax positions are consistent with applicable tax law, but certain positions may be challenged by taxing authorities. In the ordinary course of business, there are transactions and calculations where the ultimate tax outcome is uncertain. In addition, we are subject to periodic audits and examinations by the Internal Revenue Service and other state and local taxing authorities. In these cases, we record the financial statement effects of a tax position when it is more-likely-than-not, based on the technical merits, that the position will be sustained upon examination. We record the largest amount of tax benefit that is greater than fifty percent likely of being realized upon settlement with a taxing authority that has full knowledge of all relevant information. If not paid, the liability for uncertain tax positions is reversed as a reduction of income tax expense at the earlier of the period when the position is effectively settled or when the statute of limitations has expired. Although we believe that our estimates are reasonable, actual results could differ from these estimates. Interest and penalties, when applicable, are recorded within the income tax provision.
Interest Expense, Net
We record interest expense net of interest income which was $0.1 million in each of the years ended December 31, 2017, 2016 and 2015 in our consolidated statements of operations and comprehensive loss.
Stock-Based Compensation
We primarily issue restricted common stock units under one active stock-based compensation plan. Shares of common stock issued under this plan are issued from our authorized and unissued shares.
We measure and recognize compensation expense, net of actual forfeitures, for all stock-based payments at fair value. Prior to the adoption of ASU 2016-09, compensation expense was measured and recognized net of estimated forfeitures. Our outstanding awards are comprised of restricted stock units, performance-based restricted stock units, and stock options. The restricted stock units are subject to a service condition or vesting period ranging from one to four years. The performance-based restricted stock units include performance or market and service conditions. The performance conditions are primarily based on annual earnings per share targets and the market condition utilized in the Special Equity Plan is based on the three year absolute Common Stock price compounded annual growth rate (“CAGR”).
Compensation expense associated with restricted stock units is recognized on a straight-line basis over the requisite service period. Compensation expense associated with performance-based restricted stock units is primarily recognized on a graded vesting over the requisite service period when the performance condition is probable of being achieved. The compensation expense associated with the performance-based restricted stock subject to market conditions is recognized on a straight-line basis over the requisite service period.
Segment Information
We have two segments, Patient Care and Products & Services. Except for the segment specific policies described above, the segments follow the same accounting policies as followed in the consolidated financial statements. We apply the “management approach” to disclosure of segment information. The management approach designates the internal organization that is used by management for making operating decisions and assessing performance as the basis of our reportable segments. The description of our reportable segments and the disclosure of segment information are presented in Note R - “Segment and Related Information” to these consolidated financial statements.
Intersegment revenue represents sales of O&P components from our Products & Services segment to our Patient Care segment and are recorded at prices that approximate material cost plus overhead.
Recent Accounting Pronouncements
In February 2018, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update No. 2018-02, Income Statement-Reporting Comprehensive Income (Topic 220): Reclassification of Certain Tax Effects from Accumulated Other Comprehensive Income (ASU 2018-02), which allows companies to reclassify stranded tax effects resulting from the Tax Act, from accumulated other comprehensive income to retained earnings. The new standard is effective for us beginning January 1, 2019, with early adoption permitted. We are currently evaluating the effects that the adoption of this guidance will have on our consolidated financial statements and the related disclosures.
In May 2017, the FASB issued Accounting Standards Update (“ASU”) No. 2017-09, Compensation - Stock Compensation (Topic 718): Scope of Modification Accounting, which clarifies what constitutes a modification of a share-based payment award. The ASU is intended to provide clarity and reduce both diversity in practice and cost and complexity when applying the guidance in Topic 718 to a change to the terms or conditions of a share-based payment award. ASU 2017-09 is effective for public entities for annual periods beginning after December 15, 2017, and interim periods within those fiscal years. We do not anticipate that the adoption of ASU 2017-09 will have a material impact on our financial conditions or results of operations.
In January 2017, the FASB issued ASU No. 2017-04, Intangibles-Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment. This ASU simplifies how an entity is required to test goodwill for impairment by eliminating Step Two from the goodwill impairment test. Step Two measures a goodwill impairment loss by comparing the implied fair value of a reporting unit’s goodwill with the carrying amount of that goodwill. Under this standard, an entity will recognize an impairment charge for the amount by which the carrying value of a reporting unit exceeds it fair value. The amendments in this ASU are effective for us in fiscal year 2020 with early adoption permitted beginning in 2017. We early adopted this ASU during the fourth quarter of 2017 and applied the amended standard to our 2017 annual goodwill impairment test.
In January 2017, the FASB issued ASU No. 2017-03, Accounting Changes and Error Corrections (Topic 250) and Investments-Equity Method and Joint Ventures (Topic 323): Amendments to SEC Paragraphs Pursuant to Staff Announcements at the September 22, 2016 and November 17, 2016 EITF Meetings. This ASU expands disclosures regarding potential material effects to our consolidated financial statements that may occur when adopting ASU’s in the future. When a company cannot reasonably estimate the impact of adopting an ASU, disclosures are to be expanded to include qualitative disclosures including a description to the effect to the company’s accounting policies, a comparison to the existing policies, the status of its process to implement the new standard and any significant implementation matters yet to be addressed. This ASU is effective upon issuance and will generally require more disclosure in the consolidated financial statements.
In January 2017, the FASB issued ASU No. 2017-01, Business Combinations (Topic 805): Clarifying the Definition of a Business. This ASU clarifies the definition of a business with the objective of adding guidance to assist entities with evaluating whether transactions should be accounted for as acquisitions (or disposals) of assets or businesses. This ASU is effective for our fiscal year 2018, including interim periods. The adoption of this standard is not expected to have a material impact on our consolidated financial statements, but may have an impact to the conclusion of future acquisitions.
In November 2016, the FASB issued ASU No. 2016-18, Statement of Cash Flows (Topic 230): Restricted Cash. This ASU provides guidance on presenting restricted cash in the statement of cash flows. Restricted cash and cash equivalents are to be included in cash and cash equivalents when reconciling the changes during the period, while separately identifying the changes in restricted cash and cash equivalents. This ASU is effective for our fiscal year 2018, including interim periods and will require a retrospective transition. Early adoption is permitted. The adoption of this standard will result in restricted cash being included in cash and cash equivalents within the Consolidated Statements of Cash Flows.
In October 2016, the FASB issued ASU No. 2016-16, Income Taxes (Topic 740): Intra-Entity Transfers of Assets Other Than Inventory. This ASU requires the recognition of the income tax consequences of an intra-entity transfer of an asset other than inventory when the transfer occurs. This ASU is effective for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years, with early adoption permitted. The amendments in this ASU should be applied on a modified retrospective basis through a cumulative-effect adjustment directly to retained earnings as of the beginning of the period of adoption. The adoption of this standard is not expected to have a material impact on our consolidated financial statements.
In August 2016, the FASB issued ASU 2016-15, Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments. The purpose of this ASU is to reduce the diversity in practice regarding how certain cash receipts and cash payments are presented and classified in the statement of cash flows. This ASU is effective for fiscal year 2018. Early adoption is permitted. A retrospective transition method is to be used in the application of this amendment. The adoption of this standard is not expected to have a material impact on our consolidated financial statements.
In March 2016, the FASB issued ASU No. 2016-09, Compensation-Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting. This ASU simplifies several aspects of the accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities and classification on the statement of cash flows. This ASU is effective for fiscal years beginning after December 15, 2016, although early adoption is permitted. We adopted ASU No. 2016-09 on January 1, 2017. The primary impact of adopting ASU 2016-09 is the recognition of excess tax benefits and tax shortfalls resulting from our stock awards being included in our provision for income taxes, whereas previously these amounts were adjusted directly to additional paid-in capital. Additionally, these amounts are required to appear in the statement of cash flow under operating activities, whereas previously these amounts were reported as financing activities. There was no impact to our classification of awards as either equity or liabilities. Upon the adoption of this ASU, we elected to account for forfeitures as they occur with the cumulative catch-up recorded to retained earnings. Additionally, in connection with the adoption of this ASU, for 2015 and 2016, we reclassified payments for employee taxes incurred upon the vesting of stock-based compensation from an operating cash flow to a financing cash flow. This resulted in approximately $2.2 million and $0.3 million for 2015 and 2016, respectively, to be reclassified in the consolidated statements of cash flows.
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842). The amendments in this ASU revise the accounting for leases. Under the new guidance, lessees will be required to recognize a lease liability and a right-of-use asset for all leases that extend beyond 12 months. The asset and liability will initially be measured at the present value of the lease payments. The new lease guidance also simplified the accounting for sale and leaseback transactions primarily because lessees must recognize lease assets and lease liabilities. The amendments in this ASU are effective for fiscal year 2019 and will be applied through a modified retrospective transition approach which includes a number of practical expedients for
leases existing at, or entered into after, the beginning of the earliest comparative period presented in the consolidated financial statements. Early adoption is permitted. We are currently evaluating the effects that the adoption of this ASU will have on our consolidated financial statements. We have not yet concluded how the new standard will impact the consolidated financial statements. Nonetheless, it is anticipated that there will be a material increase to assets and lease liabilities for existing property leases representing our nationwide retail locations that are not already included on our consolidated balance sheet through failed sale-leaseback accounting treatment.
In January 2016, the FASB issued ASU No. 2016-01, Financial Instruments - Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities. The amendments in this ASU revise the accounting related to (i) the classification and measurement of investments in equity securities and (ii) the presentation of certain fair value changes for financial liabilities at fair value. The amendments in this ASU are effective for us beginning on January 1, 2018 and should be applied through a cumulative-effect adjustment to the consolidated balance sheet. Early adoption is permitted under certain circumstances. The adoption of this standard is not expected to have a material impact on our consolidated financial statements.
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606). This ASU provides a comprehensive new revenue recognition model that requires a company to recognize revenue to depict the transfer of goods or services to a customer in an amount that reflects the consideration it expects to receive in exchange for those goods or services. Additional disclosures are required regarding the nature, amount, timing and uncertainty of revenue and cash flows arising from customer contracts, including significant judgments and changes in judgments and assets recognized from costs incurred to obtain or fulfill a contract. The FASB issued additional related ASU’s providing guidance on principal versus agent considerations, identification of performance obligations and the implementation guidance for licensing. The two permitted transition methods under the new standard are the full retrospective method, in which case the standard would be applied to each prior reporting period presented, or the modified retrospective method, in which case the cumulative effect of applying the standard would be recognized at the date of initial adoption. In August 2015, the FASB issued ASU No. 2015-14, Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date which deferred the effective date until fiscal year 2018. Our current revenue recognition policy materially complies with this ASU. The majority of our contracts are with patients and other customers and are generally short term in nature. Revenue is recognized at the point of time when the company transfers control of the good or service to the patient. When estimating the variable consideration, we use historical collection experience to estimate amounts not expected to be collected and record these amounts as a disallowed revenue which is presented as part of our net revenue in the consolidated statement of operations. Conversely, subsequent changes in collectability due to a change in the financial condition of the payers will be recognized as bad debt expense. We adopted this ASU on January 1, 2018, following the modified retrospective approach and do not expect our adoption to have a material impact on our consolidated financial statements, nor any significant changes to our systems, processes or controls. The cumulative effect of implementing this guidance will result in an immaterial decrease to the opening balance of retained earnings from establishing a contract liability for certain performance obligations that must be recognized over time.
NOTE C — EARNINGS PER SHARE
Basic earnings per common share is computed using the weighted average number of common shares outstanding during the period. Diluted earnings per common share is computed using the weighted average number of common shares outstanding during the period plus any potentially dilutive common shares, such as stock options, restricted stock units and performance-based units calculated using the treasury stock method. Total anti-dilutive shares excluded from the diluted earnings per share were 473,037 of as December 31, 2017 and 342,369 and 46,870 as of December 31, 2016 and 2015, respectively.
Our credit agreement restricts the payment of dividends or other distributions to our shareholders with respect to the parent company or any of its subsidiaries. See Note N - “Long-Term Debt” within these consolidated financial statements.
The reconciliation of the numerators and denominators used to calculate basic and diluted net (loss) income per share are as follows:
|
|
|
Year Ended December 31, |
| |||||||
|
(in thousands, except share and per share data) |
|
2017 |
|
2016 |
|
2015 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Loss from continuing operations applicable to common shareholders |
|
$ |
(104,671 |
) |
$ |
(107,406 |
) |
$ |
(319,117 |
) |
|
Income (loss) from discontinued operations, net of income taxes |
|
— |
|
935 |
|
(7,974 |
) | |||
|
Net loss applicable to common shareholders |
|
$ |
(104,671 |
) |
$ |
(106,471 |
) |
$ |
(327,091 |
) |
|
|
|
|
|
|
|
|
| |||
|
Shares of common stock outstanding used to compute basic per common share amounts |
|
36,270,920 |
|
35,933,222 |
|
35,635,448 |
| |||
|
Effect of dilutive restricted stock units and options (1) |
|
— |
|
— |
|
— |
| |||
|
Shares used to compute diluted per common share amounts |
|
36,270,920 |
|
35,933,222 |
|
35,635,448 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Basic and Diluted: |
|
|
|
|
|
|
| |||
|
Loss from continuing operations per share applicable to common stock |
|
$ |
(2.89 |
) |
$ |
(2.99 |
) |
$ |
(8.96 |
) |
|
Income (loss) from discontinued operations per share applicable to common stock |
|
— |
|
0.03 |
|
(0.22 |
) | |||
|
Net loss per share applicable to common shareholders |
|
$ |
(2.89 |
) |
$ |
(2.96 |
) |
$ |
(9.18 |
) |
(1) Given that we are recognizing a loss from continuing operations, shares used to compute diluted per common share amounts excludes 295,718 shares for 2017, 145,497 shares for 2016, and 102,288 shares for 2015 of potentially dilutive shares related to unvested restricted stock units and unexercised options in accordance with ASC 260 - Earnings Per Share.
NOTE D — ACCOUNTS RECEIVABLE, NET
Accounts receivable are principally from Medicare and Medicaid programs and commercial insurance plans. Our accounts receivables are recorded net of contractual discounts and net of estimated allowances for disallowed revenue and sales returns. These allowances are presented as a reduction of gross accounts receivable. We also record an allowance for doubtful accounts which is deducted from gross accounts receivable to arrive at “Accounts receivable, net.” Accounts receivable, net as of December 31, 2017, and 2016 is comprised of the following:
|
|
|
As of December 31, 2017 |
|
As of December 31, 2016 |
| ||||||||||||||
|
(in thousands) |
|
Patient Care |
|
Products & |
|
Consolidated |
|
Patient Care |
|
Products & |
|
Consolidated |
| ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Accounts receivable, before allowances |
|
$ |
193,150 |
|
$ |
23,494 |
|
$ |
216,644 |
|
$ |
193,835 |
|
$ |
27,385 |
|
$ |
221,220 |
|
|
Allowance for disallowed revenue |
|
(56,233 |
) |
— |
|
(56,233 |
) |
(61,137 |
) |
— |
|
(61,137 |
) | ||||||
|
Accounts receivable, gross |
|
136,917 |
|
23,494 |
|
160,411 |
|
132,698 |
|
27,385 |
|
160,083 |
| ||||||
|
Allowance for doubtful accounts |
|
(9,894 |
) |
(4,171 |
) |
(14,065 |
) |
(10,575 |
) |
(4,946 |
) |
(15,521 |
) | ||||||
|
Accounts receivable, net |
|
$ |
127,023 |
|
$ |
19,323 |
|
$ |
146,346 |
|
$ |
122,123 |
|
$ |
22,439 |
|
$ |
144,562 |
|
Approximately 49.2% and 48.3% of accounts receivable, before allowances, is due from the Federal Government (Medicare, Medicaid and U.S. Veterans Affairs) at December 31, 2017 and 2016, respectively.
The following tables represent accounts receivable, before allowances, by major payor classification and by aging categories reduced by the allowance for disallowed revenue and allowance for doubtful accounts to accounts receivable, net as of December 31, 2017 and 2016, respectively:
December 31, 2017
|
(in thousands) |
|
0-60 |
|
61-120 |
|
121-180 |
|
Over 180 |
|
Total |
| |||||
|
Patient Care |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Commercial insurance (excluding Medicare and Medicaid Managed Care) |
|
$ |
50,310 |
|
$ |
11,649 |
|
$ |
6,302 |
|
$ |
15,279 |
|
$ |
83,540 |
|
|
Private pay |
|
880 |
|
447 |
|
381 |
|
1,311 |
|
3,019 |
| |||||
|
Medicaid |
|
13,785 |
|
3,561 |
|
1,832 |
|
5,684 |
|
24,862 |
| |||||
|
VA |
|
4,578 |
|
1,193 |
|
552 |
|
694 |
|
7,017 |
| |||||
|
Non-Medicare |
|
69,553 |
|
16,850 |
|
9,067 |
|
22,968 |
|
118,438 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Medicare |
|
34,197 |
|
5,725 |
|
3,396 |
|
31,394 |
|
74,712 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Products & Services |
|
14,316 |
|
5,075 |
|
1,219 |
|
2,884 |
|
23,494 |
| |||||
|
Accounts receivable, before allowances |
|
118,066 |
|
27,650 |
|
13,682 |
|
57,246 |
|
216,644 |
| |||||
|
Allowance for disallowed revenue |
|
|
|
|
|
|
|
|
|
(56,233 |
) | |||||
|
Allowance for doubtful accounts |
|
|
|
|
|
|
|
|
|
(14,065 |
) | |||||
|
Accounts receivable, net |
|
|
|
|
|
|
|
|
|
$ |
146,346 |
| ||||
December 31, 2016
|
(in thousands) |
|
0-60 |
|
61-120 |
|
121-180 |
|
Over 180 |
|
Total |
| |||||
|
Patient Care |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Commercial insurance (excluding Medicare and Medicaid Managed Care) |
|
$ |
48,568 |
|
$ |
11,677 |
|
$ |
6,050 |
|
$ |
17,453 |
|
$ |
83,748 |
|
|
Private pay |
|
897 |
|
547 |
|
441 |
|
1,034 |
|
2,919 |
| |||||
|
Medicaid |
|
13,937 |
|
3,554 |
|
2,110 |
|
5,415 |
|
25,016 |
| |||||
|
VA |
|
3,638 |
|
875 |
|
395 |
|
721 |
|
5,629 |
| |||||
|
Non-Medicare |
|
67,040 |
|
16,653 |
|
8,996 |
|
24,623 |
|
117,312 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Medicare |
|
32,980 |
|
4,813 |
|
3,055 |
|
35,675 |
|
76,523 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Products & Services |
|
16,126 |
|
6,690 |
|
3,031 |
|
1,538 |
|
27,385 |
| |||||
|
Accounts receivable, before allowances |
|
116,146 |
|
28,156 |
|
15,082 |
|
61,836 |
|
221,220 |
| |||||
|
Allowance for disallowed revenue |
|
|
|
|
|
|
|
|
|
(61,137 |
) | |||||
|
Allowance for doubtful accounts |
|
|
|
|
|
|
|
|
|
(15,521 |
) | |||||
|
Accounts receivable, net |
|
|
|
|
|
|
|
|
|
$ |
144,562 |
| ||||
The following table summarizes activities by year for the allowance for disallowed revenue and the allowance for doubtful accounts:
|
(in thousands) |
|
Allowance for |
|
Allowance for |
| ||
|
|
|
|
|
|
| ||
|
Balance at December 31, 2014 |
|
$ |
87,192 |
|
$ |
9,944 |
|
|
Additions (1) |
|
60,076 |
|
14,515 |
| ||
|
Reductions |
|
(65,962 |
) |
(9,432 |
) | ||
|
Balance at December 31, 2015 |
|
81,306 |
|
15,027 |
| ||
|
Additions (1) |
|
48,961 |
|
13,727 |
| ||
|
Reductions |
|
(69,130 |
) |
(13,233 |
) | ||
|
Balance at December 31, 2016 |
|
61,137 |
|
15,521 |
| ||
|
Additions (1) |
|
36,962 |
|
9,423 |
| ||
|
Reductions |
|
(41,866 |
) |
(10,879 |
) | ||
|
Balance at December 31, 2017 |
|
$ |
56,233 |
|
$ |
14,065 |
|
(1)The accounts receivables associated with the Dosteon businesses, which are classified as discontinued operations as of each respective date of the consolidated financial statements, were not a part of the disposal transactions. Therefore the associated allowances, additions, and reductions are included in the above table. Dosteon’s bad debt expense included in “Income (loss) from discontinued operations, net of income taxes” were $0 million in 2017 and 2016, and $1.7 million in 2015. Dosteon’s disallowed revenue included in “Income (loss) from discontinued operations, net of income taxes” were $0 million in 2017 and 2016, and $(0.2) million in 2015.
NOTE E — INVENTORIES
Our inventories are comprised of the following:
|
|
|
As of December 31, |
| ||||
|
(in thousands) |
|
2017 |
|
2016 |
| ||
|
Raw materials |
|
$ |
19,929 |
|
$ |
21,277 |
|
|
Work in process |
|
8,996 |
|
9,009 |
| ||
|
Finished goods |
|
40,213 |
|
37,939 |
| ||
|
Total inventories |
|
$ |
69,138 |
|
$ |
68,225 |
|
NOTE F — PROPERTY PLANT AND EQUIPMENT, NET
Property, plant and equipment, net were comprised of the following:
|
|
|
December 31, |
| ||||
|
(in thousands) |
|
2017 |
|
2016 |
| ||
|
Land |
|
$ |
644 |
|
$ |
704 |
|
|
Buildings |
|
28,180 |
|
28,160 |
| ||
|
Furniture and fixtures |
|
12,968 |
|
12,312 |
| ||
|
Machinery and equipment |
|
26,838 |
|
26,173 |
| ||
|
Equipment leased to third parties under operating leases |
|
31,100 |
|
32,669 |
| ||
|
Leasehold improvements |
|
100,999 |
|
95,376 |
| ||
|
Computers and software |
|
65,455 |
|
87,147 |
| ||
|
Total property, plant, and equipment, gross |
|
266,184 |
|
282,541 |
| ||
|
Less: Accumulated depreciation |
|
(172,569 |
) |
(182,074 |
) | ||
|
Total property, plant, and equipment, net |
|
$ |
93,615 |
|
$ |
100,467 |
|
Total depreciation expense was approximately $29.7 million, $31.0 million, and $32.5 million for the years ended December 31, 2017, 2016 and 2015, respectively.
Included within Buildings was $24.1 million and $23.8 million recorded as an asset for certain build-to-suit leases as of December 31, 2017 and 2016, respectively. Accumulated depreciation on these assets was $8.9 million and $7.7 million as of December 31, 2017 and 2016, respectively.
The following table summarizes our investment in equipment leased to third parties under operating leases:
|
|
|
December 31, |
| ||||
|
(in thousands) |
|
2017 |
|
2016 |
| ||
|
Program equipment |
|
$ |
31,100 |
|
$ |
32,669 |
|
|
Less: Accumulated depreciation |
|
(19,954 |
) |
(22,850 |
) | ||
|
|
$ |
11,146 |
|
$ |
9,819 |
| |
NOTE G — ACQUISITIONS
In the first quarter of 2015, we acquired three O&P businesses operating a total of 15 patient care clinics located in three states. The aggregate purchase price for these businesses was $15.3 million, including $10.2 million in cash, $4.7 million in Seller Notes and $0.4 million of working capital adjustments and other.
The assets acquired and liabilities assumed for all acquisitions were recorded at their estimated fair values at the dates of the acquisitions and the results of their operations are included in our consolidated financial statements from their effective dates. The excess of purchase price over the estimated fair values of assets acquired and liabilities assumed was recorded as goodwill. The value of goodwill from acquisitions can be attributed to a number of business factors including, but not limited to, synergies associated with combining the acquired businesses with our existing business. We have made an election to treat the majority of these acquisitions as asset purchases for income tax purposes resulting in approximately $8.2 million of acquired goodwill being deductible for income tax purposes for acquisitions completed in 2015.
Acquisition-related expenses for the year ended December 31, 2015 which are included in “General and administrative expenses” in our consolidated statements of operations and comprehensive loss are not significant.
We made no acquisitions in 2017 or 2016.
The following table summarizes for 2015 acquisitions, the components of the aggregated purchase price the assets acquired and liabilities assumed in the above transactions and recognized at their respective acquisition dates at estimated fair value:
|
|
|
Year Ended |
| |
|
|
|
December 31, |
| |
|
(in thousands) |
|
2015 |
| |
|
|
|
|
| |
|
Net cash |
|
$ |
10,215 |
|
|
Issuance of seller notes |
|
4,662 |
| |
|
Other working capital adjustments |
|
376 |
| |
|
Aggregate purchase price |
|
15,253 |
| |
|
|
|
|
| |
|
Net accounts receivable |
|
1,045 |
| |
|
Inventories |
|
481 |
| |
|
Intangible assets, excluding goodwill |
|
5,455 |
| |
|
Other assets |
|
112 |
| |
|
Liabilities assumed |
|
(15 |
) | |
|
Net assets acquired |
|
7,078 |
| |
|
|
|
|
| |
|
Goodwill |
|
$ |
8,175 |
|
NOTE H — GOODWILL AND OTHER INTANGIBLE ASSETS
Goodwill Impairment Testing
Under the provisions of ASC 350-10, Intangibles-Goodwill and Other, goodwill is not amortized. Rather, an entity’s goodwill is subject to periodic impairment testing. ASC 350 requires that an entity assign its goodwill to reporting units and test each reporting unit’s goodwill for impairment at least on an annual basis and between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. Accordingly, we perform our goodwill test annually as of October 1 and between annual tests whenever we identify certain triggering events or circumstances that would more likely than not reduce the fair value of any of our reporting units below its respective carrying value. Additionally, we consider income tax effects from any tax deductible goodwill on the carrying amount of the reporting unit when measuring the goodwill impairment loss, if applicable.
Prior to our adoption of ASU 2017-04, our goodwill impairment testing for 2015 and 2016 was based on a two-step approach, with the second step of the goodwill impairment test requiring an assignment of the reporting unit’s fair value to the reporting unit’s assets and liabilities, including any unrecognized intangible assets, using the acquisition method accounting guidance in ASC 805, to determine the implied fair value of the reporting unit’s goodwill. The difference between the reporting unit’s fair value and the fair values assigned to the reporting unit’s individual assets and liabilities, is the implied fair value of the reporting unit’s goodwill. The implied fair value of the reporting unit’s goodwill is then compared with the carrying amount of the reporting unit’s goodwill to determine the goodwill impairment loss to be recognized, if any. An impairment charge is recognized for the excess of the carrying value of goodwill over its implied fair value.
The goodwill impairment test compares a reporting unit’s fair value to its carrying amount to identify any potential impairment. We apply judgment in determining the fair value of our reporting units for purposes of performing the goodwill impairment test. We rely on widely accepted valuation techniques, including discounted cash flow and market multiple analysis approaches, which capture both the future income potential of the reporting unit and the market behaviors and actions of market participants in the industry that includes the reporting unit. These types of analyses require us to make
assumptions and estimates regarding future cash flows, industry-specific economic factors and the profitability of future business strategies. The discounted cash flow approach uses a projection of estimated operating results and cash flows that are discounted using a weighted average cost of capital. Under the discounted cash flow approach, the projection uses management’s best estimates of the amount and timing of expected future cash flows impacted by economic and market conditions over the projected period for each reporting unit. Significant estimates and assumptions include terminal value growth rates, changes in working capital requirements and weighted average cost of capital. The market multiple analysis estimates fair value by applying revenue and earnings multiples to the reporting unit’s operating results. The multiples are derived from comparable publicly traded companies with similar operating and investment characteristics to the reporting units.
We evaluate the reasonableness of the estimated fair value of our reporting units by reconciling the aggregate fair value of all three of our reporting units to our total market capitalization as of our impairment testing date, taking into account an appropriate control premium. The determination of a control premium requires the use of judgment and is based upon control premiums observed in comparable market transactions.
We first assess qualitative factors for a reporting unit to determine if the quantitative goodwill impairment test is necessary. If we choose to bypass this qualitative assessment or alternatively determine that a quantitative goodwill impairment test is required, our annual goodwill impairment test is performed by comparing the estimated fair value of a reporting unit with its carrying amount (including attributed goodwill).
The changes in the carrying value of goodwill for the years ended December 31, 2017 and 2016 are as follows:
|
|
|
Patient Care |
|
Products & Services |
|
|
| |||||||||
|
(in thousands) |
|
Gross |
|
Accumulated |
|
Gross |
|
Accumulated |
|
Total |
| |||||
|
Balance at December 31, 2015 |
|
$ |
625,011 |
|
$ |
(428,668 |
) |
$ |
139,299 |
|
$ |
— |
|
$ |
335,642 |
|
|
Goodwill impairment |
|
— |
|
— |
|
— |
|
(85,964 |
) |
(85,964 |
) | |||||
|
Balance at December 31, 2016 |
|
625,011 |
|
(428,668 |
) |
139,299 |
|
(85,964 |
) |
249,678 |
| |||||
|
Goodwill impairment |
|
— |
|
— |
|
— |
|
(53,335 |
) |
(53,335 |
) | |||||
|
Balance at December 31, 2017 |
|
$ |
625,011 |
|
$ |
(428,668 |
) |
$ |
139,299 |
|
$ |
(139,299 |
) |
$ |
196,343 |
|
2017 Goodwill
At October 1, 2017, we tested each of our three reporting units as part of our annual goodwill impairment test. Due to the nature and magnitude of events adversely impacting the reimbursement environment within the skilled nursing facility industry (our primary customer source for our Therapeutic solutions business) and the O&P industry (our primary source for our Distribution services business), combined with customer losses and related margin pressures, which increased in the fourth quarter of 2017, our evaluation of our Therapeutic and Distribution reporting units’ long-term outlook resulted in our conclusion that the carrying amounts of these two reporting units exceeded their respective estimated fair values. Consistent with the provisions of ASU 2017-04, which we adopted in 2017, we recorded non-cash goodwill impairment charges of $32.8 million for our Therapeutic reporting unit and $20.5 million for our Distribution reporting unit which is included in “Impairment of intangible assets” in the consolidated statements of operations and comprehensive loss. The fair value of our Patient Care reporting unit exceeded its carrying amount. These goodwill impairment charges had no impact on our cash flow or compliance with debt covenants for 2017.
2016 Goodwill
As of October 1, 2016, we tested each of our three reporting units as part of our annual goodwill impairment test. We concluded that the carrying amounts of the Therapeutic and Distribution reporting units within our Products & Services
segment exceeded their respective estimated fair values. The second step of the test was then performed to measure the impairment loss, resulting in non-cash goodwill impairment charges of $64.9 million for our Therapeutic reporting unit and $21.1 million for our Distribution reporting unit which is included in “Impairment of intangible assets” in the consolidated statements of operations and comprehensive loss. The fair value of our Patient Care reporting unit exceeded its carrying amount.
These goodwill impairment charges had no impact on our cash flow or compliance with debt covenants for 2016.
2015 Goodwill
In the fourth quarter of 2015, it became likely that our 2014 financial statements would not be filed by March 19, 2016, our extended due date granted to us by the NYSE. Upon informing the NYSE of a further delay, we were delisted in February 2016. In addition to the decrease in market value due to the delisting, we also anticipated a significant increase in the time and cost for us to recover from these adverse events, and considered this to be a triggering event. We tested each of our three reporting units as of December 31, 2015. We concluded that the carrying amount of the Patient Care reporting unit exceeded its estimated fair value. The second step of the test was then performed to measure the impairment loss, resulting in a non-cash goodwill impairment charge for our Patient Care reporting unit of $382.9 million as of December 31, 2015, which is included in “Impairment of intangible assets” in the consolidated statements of operations and comprehensive loss. The fair value of our Distribution and Therapeutic reporting units exceeded their respective carrying amounts.
This goodwill impairment charge had no impact on our cash flow or compliance with debt covenants for 2015.
During the third quarter of 2015, we noted a significant decline in our stock price and market capitalization coupled with changes in our earnings expectations that were identified during our 2016 budget process which we considered to be a triggering event. We tested each of our three reporting units as of September 30, 2015. The fair value of each of our three reporting units exceeded their respective carrying amounts.
Intangible Asset Impairment Testing
Under the provisions of ASC 360-10, Property, plant, and equipment, an intangible asset that has a finite life should be amortized over its estimated useful life and should be tested for recoverability by comparing the net carrying value of the asset or asset group to the undiscounted net cash flows to be generated from the use and eventual disposition of that asset or asset group when events or changes in circumstances indicate that its carrying amount may not be recoverable. We perform our annual test for recoverability on October 1 of each fiscal year. If the carrying amount of a definite-lived asset or asset group is not recoverable, the fair value of the asset or asset group is measured and if the carrying amount exceeds the fair value, an impairment loss is recognized.
Under the provisions of ASC 350, Intangibles-goodwill and other, an indefinite-lived intangible asset is not amortized but should be tested for impairment annually and between annual tests if events or changes in circumstances indicate that it is more likely than not that the asset is impaired. The indefinite-lived intangible asset impairment standard allows an entity first to assess qualitative factors to determine if a quantitative impairment test is necessary. Further testing is only required if the entity determines, based on the qualitative assessment, that it is more likely than not that an indefinite-lived intangible asset’s fair value is less than its carrying amount. We perform our annual test for recoverability on October 1 of each fiscal year.
The fair value of acquired customer list intangibles is estimated using an excess earnings model. Key assumptions utilized in the valuation model include pro-forma projected cash flows adjusted for market-participant assumptions, forecasted customer retention curve, and discount rate. Customer intangibles are amortized, using the straight-line method over an estimated useful life of four to ten years. The fair value of non-compete agreements are estimated using a discounted cash flow model. The related intangible assets are amortized, using the straight-line method, over their term which ranges from two to five
years. Other definite-lived intangible assets are recorded at cost and are amortized, using the straight-line method, over their estimated useful lives of up to seventeen years. The fair value associated with trade names is estimated using the relief-from-royalty method with the primary assumptions being the royalty rate and expected revenues associated with the trade names. These assets, some of which have indefinite lives, are primarily included in the Products & Services segment. Indefinite lived trade name intangible assets are assessed for impairment in the fourth quarter of each year, or more frequently if events or changes in circumstances indicate that the asset might be impaired. Trade name intangible assets with definite lives are amortized over their estimated useful lives of one to ten years.
The balances related to intangible assets as of December 31, 2017 and 2016 are as follows:
|
|
|
December 31, 2017 |
| ||||||||||
|
(in thousands) |
|
Gross Carrying |
|
Accumulated |
|
Accumulated |
|
Net Carrying |
| ||||
|
Customer Lists |
|
$ |
36,439 |
|
$ |
(24,267 |
) |
$ |
— |
|
$ |
12,172 |
|
|
Trade Name |
|
462 |
|
(302 |
) |
— |
|
160 |
| ||||
|
Patents and Other Intangibles |
|
15,358 |
|
(10,050 |
) |
— |
|
5,308 |
| ||||
|
Definite-lived intangible assets |
|
52,259 |
|
(34,619 |
) |
— |
|
17,640 |
| ||||
|
Indefinite life - Trade Name |
|
9,070 |
|
— |
|
(4,770 |
) |
4,300 |
| ||||
|
Total other intangible assets |
|
$ |
61,329 |
|
$ |
(34,619 |
) |
$ |
(4,770 |
) |
$ |
21,940 |
|
|
|
|
December 31, 2016 |
| ||||||||||
|
(in thousands) |
|
Gross Carrying |
|
Accumulated |
|
Accumulated |
|
Net Carrying |
| ||||
|
Customer Lists |
|
$ |
43,380 |
|
$ |
(23,051 |
) |
$ |
— |
|
$ |
20,329 |
|
|
Trade Name |
|
462 |
|
(248 |
) |
— |
|
214 |
| ||||
|
Patents and Other Intangibles |
|
15,358 |
|
(8,660 |
) |
— |
|
6,698 |
| ||||
|
Definite-lived intangible assets |
|
59,200 |
|
(31,959 |
) |
— |
|
27,241 |
| ||||
|
Indefinite life - Trade Name |
|
9,070 |
|
— |
|
(3,370 |
) |
5,700 |
| ||||
|
Total other intangible assets |
|
$ |
68,270 |
|
$ |
(31,959 |
) |
$ |
(3,370 |
) |
$ |
32,941 |
|
2017 Intangible Assets
As of October 1, 2017, we tested our Therapeutic reporting unit’s indefinite lived tradename as part of our annual impairment test which compared the estimated fair value with the carrying amount of the tradename. The fair value of the intangible asset was estimated using an income approach, specifically the relief-from-royalty method. The cash flows used contain management’s best estimates using appropriate assumptions and projections as of the testing date. The royalty rate was estimated using rates applicable to similar business acquisition transactions. Due to the continued decline in our Therapeutic reporting unit as presented in our annual forecast, the fair value of the tradename was determined to be less than the carrying amount, resulting in a $1.4 million impairment charge recorded in the fourth quarter of 2017. This charge is included in “Impairment of intangible assets” in the consolidated statements of operations and comprehensive loss.
This intangible asset impairment charge had no impact on our cash flow or compliance with debt covenants for 2017.
2016 Intangible Assets
As of October 1, 2016, we tested our Therapeutic reporting unit’s indefinite lived tradename as part of our annual impairment test which compared the estimated fair value with the carrying amount of the tradename. The fair value of the intangible asset was estimated using an income approach, specifically the relief-from-royalty method. The cash flows used contain
management’s best estimates using appropriate assumptions and projections as of the testing date. The royalty rate was estimated using rates applicable to similar business acquisition transactions. The fair value of the tradename was determined to be less than the carrying amount, resulting in a $0.2 million impairment charge recorded in the fourth quarter of 2016. This charge is included in “Impairment of intangible assets” in the consolidated statements of operations and comprehensive loss.
This intangible asset impairment charge had no impact on our cash flow or compliance with debt covenants for 2016.
2015 Intangible Assets
In connection with our goodwill impairment testing as of September 30, 2015 and December 31, 2015 due to the triggering events discussed above, we tested our Therapeutic reporting unit’s indefinite-lived tradename intangible asset for impairment as of those dates. The fair value of the tradename was determined to be less than the carrying amount at both dates, resulting in a $0.8 million impairment charge recorded in the third quarter of 2015 and a $2.1 million impairment charge in the fourth quarter of 2015. These charges are included in “Impairment of intangible assets” in the consolidated statements of operations and comprehensive loss.
These intangible asset impairment charges had no impact on our cash flow or compliance with debt covenants for 2015.
In conjunction with our Goodwill impairment testing at December 31, 2015, we reevaluated the estimated useful life of our customer list intangibles. In the fourth quarter of 2015, the estimated useful life of our customer list intangibles was reduced from 10 years to four years in our Patient Care segment and from 14 years to 10 years in our Products & Services segment. This change in the estimated useful lives increased amortization for the years ended December 31, 2017, 2016 and 2015 by approximately $3.0 million, $7.0 million and $6.0 million, respectively.
Total intangible amortization expense was approximately $9.5 million, $13.9 million, and $13.8 million for the years ended December 31, 2017, 2016 and 2015, respectively, and reflects the impact of our change in the estimated useful lives of our customer list intangible assets beginning in the fourth quarter of 2015.
Estimated aggregate amortization expense for definite lived intangible assets for each of the next five years ended December 31 and thereafter is as follows:
|
(in thousands) |
|
December 31, |
| |
|
2018 |
|
$ |
6,690 |
|
|
2019 |
|
3,714 |
| |
|
2020 |
|
3,457 |
| |
|
2021 |
|
879 |
| |
|
2022 |
|
817 |
| |
|
Thereafter |
|
2,083 |
| |
|
Total |
|
$ |
17,640 |
|
As described, we apply judgment in the selection of key assumptions used in the goodwill impairment test and as part of our evaluation of intangible assets tested annually and at interim testing dates as necessary. If these assumptions differ from actual, we could incur additional impairment charges and those charges could be material.
NOTE I — OTHER CURRENT ASSETS AND OTHER ASSETS
Other current assets consists of the following:
|
|
|
As of December 31, |
| ||||
|
(in thousands) |
|
2017 |
|
2016 |
| ||
|
|
|
|
|
|
| ||
|
Non-trade receivables |
|
$ |
7,668 |
|
$ |
6,223 |
|
|
Prepaid rent |
|
4,248 |
|
4,070 |
| ||
|
Prepaid maintenance |
|
3,134 |
|
3,914 |
| ||
|
Restricted cash |
|
3,271 |
|
2,255 |
| ||
|
Prepaid other |
|
1,436 |
|
1,542 |
| ||
|
Prepaid education and training |
|
582 |
|
398 |
| ||
|
Prepaid insurance |
|
271 |
|
447 |
| ||
|
Other |
|
278 |
|
288 |
| ||
|
Total other current assets |
|
$ |
20,888 |
|
$ |
19,137 |
|
Non-trade receivables primarily relate to vendor rebate receivables, tenant improvement allowance receivables, and other non-trade receivables. Prepaid rent relates to amounts of future rent expense paid in advance of the rental period. Prepaid maintenance primarily relates to prepaid software and hardware maintenance and software license fees. Restricted cash relates to funds held by our captive insurance subsidiary and whose use for general purposes is restricted by Nevada state insurance regulations. Prepaid other includes the employer’s portion of health savings accounts, board member fees and tax and accounting services. Prepaid education and training is for our annual Education Fair event held in the first quarter of each fiscal year. Prepaid insurance is for product and general liability insurance. Other includes prepaid expenses for telecommunication, broker fees and other miscellaneous prepaid expenses.
Other assets consists of the following:
|
|
|
As of December 31, |
| ||||
|
(in thousands) |
|
2017 |
|
2016 |
| ||
|
|
|
|
|
|
| ||
|
Cash surrender value of COLI |
|
$ |
2,340 |
|
$ |
17,573 |
|
|
Non-trade receivables |
|
2,407 |
|
3,401 |
| ||
|
Deposits |
|
2,193 |
|
2,038 |
| ||
|
Other |
|
2,500 |
|
2,502 |
| ||
|
Total other assets |
|
$ |
9,440 |
|
$ |
25,514 |
|
The cash surrender value of company owned life insurance (“COLI”) funded our Defined Contribution Supplemental Executive Retirement Plan (“DC SERP”) at December 31, 2017 and funded both our Defined Benefit Supplemental Executive Retirement Plan (“DB SERP”) and our DC SERP at December 31, 2016. During 2017, we received the cash surrender value from the DB SERP COLI, which totaled $17.1 million. See Note K - “Employee Benefits” for additional information. Non-trade receivables primarily relate to estimated receivables due from our various business insurance policies. Deposits primarily relate to security deposits made in connection with property leases. Other relates to cash collateral posted for surety bonds.
NOTE J — INCOME TAXES
Components of provision (benefit) for income taxes are as follows:
|
|
|
Year Ended December 31, |
| |||||||
|
(in thousands) |
|
2017 |
|
2016 |
|
2015 |
| |||
|
Current: |
|
|
|
|
|
|
| |||
|
Federal |
|
$ |
541 |
|
$ |
(18,812 |
) |
$ |
(21,721 |
) |
|
State |
|
574 |
|
694 |
|
1,720 |
| |||
|
Total current |
|
1,115 |
|
(18,118 |
) |
(20,001 |
) | |||
|
Deferred: |
|
|
|
|
|
|
| |||
|
Federal |
|
28,905 |
|
3,008 |
|
(41,372 |
) | |||
|
State |
|
(2,723 |
) |
(800 |
) |
(6,241 |
) | |||
|
Total deferred |
|
26,182 |
|
2,208 |
|
(47,613 |
) | |||
|
Provision (benefit) for income taxes from continuing operations |
|
$ |
27,297 |
|
$ |
(15,910 |
) |
$ |
(67,614 |
) |
|
|
|
|
|
|
|
|
| |||
|
Income tax provision (benefit) attributable to discontinued operations |
|
$ |
— |
|
$ |
490 |
|
$ |
(3,249 |
) |
A reconciliation of the federal statutory tax rate to our effective tax rate applicable to continuing operations is as follows:
|
|
|
Year Ended December 31, |
| ||||
|
|
|
2017 |
|
2016 |
|
2015 |
|
|
Federal statutory tax rate- (benefit) provision |
|
(35.0 |
)% |
(35.0 |
)% |
(35.0 |
)% |
|
State and local income taxes |
|
(0.7 |
)% |
(0.4 |
)% |
(1.6 |
)% |
|
Change in valuation allowance |
|
0.7 |
% |
— |
% |
0.3 |
% |
|
Federal statutory tax rate change effect on deferred balance |
|
45.0 |
% |
— |
% |
— |
% |
|
Change in uncertain tax positions |
|
0.3 |
% |
(0.9 |
)% |
(0.2 |
)% |
|
Goodwill impairment |
|
21.1 |
% |
22.3 |
% |
18.4 |
% |
|
Other |
|
3.9 |
% |
1.1 |
% |
0.6 |
% |
|
Effective tax rate applicable to continuing operations |
|
35.3 |
% |
(12.9 |
)% |
(17.5 |
)% |
The significant components of the net deferred income tax asset are as follows:
|
|
|
As of December 31, |
| ||||
|
(in thousands) |
|
2017 |
|
2016 |
| ||
|
Deferred tax liabilities: |
|
|
|
|
| ||
|
Goodwill |
|
$ |
3,883 |
|
$ |
4,962 |
|
|
Intangible |
|
4 |
|
3,580 |
| ||
|
Prepaid expenses |
|
1,029 |
|
1,581 |
| ||
|
Sec. 481(a) adjustments |
|
56 |
|
172 |
| ||
|
|
|
4,972 |
|
10,295 |
| ||
|
Deferred tax assets: |
|
|
|
|
| ||
|
Deferred benefit plan compensation |
|
5,786 |
|
8,816 |
| ||
|
Provision for doubtful accounts and disallowed revenues |
|
18,243 |
|
30,203 |
| ||
|
Property, plant and equipment |
|
12,216 |
|
14,596 |
| ||
|
Net operating loss carryforwards |
|
15,191 |
|
11,157 |
| ||
|
Accrued expenses |
|
17,974 |
|
30,307 |
| ||
|
Inventory reserves |
|
2,123 |
|
3,318 |
| ||
|
Restricted stock |
|
3,972 |
|
4,286 |
| ||
|
Capital leases |
|
210 |
|
439 |
| ||
|
Deferred rent |
|
1,265 |
|
2,117 |
| ||
|
Refund liabilities |
|
2,895 |
|
3,456 |
| ||
|
Interest on seller notes |
|
1,010 |
|
1,408 |
| ||
|
Other |
|
967 |
|
1,310 |
| ||
|
|
|
81,852 |
|
111,413 |
| ||
|
Valuation allowance |
|
(8,754 |
) |
(6,895 |
) | ||
|
|
|
73,098 |
|
104,518 |
| ||
|
Net deferred tax asset |
|
$ |
68,126 |
|
$ |
94,223 |
|
We have $24.2 million and $8.9 million of U.S. federal and $195.0 million and $185.3 million of state net operating loss carryforwards available at December 31, 2017 and 2016, respectively. These carryforwards will be used to offset future income but may be limited by the change in ownership rules in Section 382 of the Internal Revenue Code. These net operating loss carryforwards will expire in varying amounts between 2018 and 2037.
As of December 31, 2017, we had approximately $68.1 million in net deferred tax assets (“DTAs”). These DTAs can be used to offset taxable income in future periods and reduce our income taxes payable in those future periods. At this time, we consider it more likely than not that we will have sufficient taxable income in the future that will allow us to realize these DTAs. However, it is possible that some or all of these DTAs may not be realized unless we are able to generate sufficient taxable income from our operations. If we do not generate sufficient taxable income in the future a substantial valuation allowance to reduce our DTAs may be required, which could materially increase our expenses in the period the allowance is recognized and materially adversely affect our results of operations and statement of financial condition. As of December 31, 2017, we had a valuation allowance of approximately $8.8 million, related primarily to certain state loss carryforwards, which are expected to expire before utilization. We monitor our cumulative loss position and other evidence each quarter to determine the appropriateness of our valuation allowance. Although we believe our estimates are reasonable, the ultimate determination of the appropriate amount of valuation allowance involves significant judgment.
The following schedule presents the activity in the valuation allowance:
|
(in thousands) |
Year |
|
Balance at |
|
Acquisitions |
|
Provision |
|
Released |
|
Balance at |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
|
2017 |
|
$ |
6,895 |
|
$ |
— |
|
$ |
2,306 |
|
$ |
447 |
|
$ |
8,754 |
|
|
|
2016 |
|
$ |
6,853 |
|
$ |
— |
|
$ |
377 |
|
$ |
335 |
|
$ |
6,895 |
|
|
|
2015 |
|
$ |
5,692 |
|
$ |
— |
|
$ |
1,195 |
|
$ |
34 |
|
$ |
6,853 |
|
A reconciliation of our liability for unrecognized tax benefits is as follows:
|
(in thousands) |
|
2017 |
|
2016 |
|
2015 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Unrecognized tax benefits, at beginning of the year |
|
$ |
4,664 |
|
$ |
7,567 |
|
$ |
7,605 |
|
|
Additions for tax positions related to the current year |
|
196 |
|
47 |
|
279 |
| |||
|
Additions for tax positions of prior years |
|
— |
|
— |
|
1,415 |
| |||
|
Decrease related to prior year positions |
|
— |
|
— |
|
(1,472 |
) | |||
|
Decrease for lapse of applicable statute of limitations |
|
— |
|
(2,950 |
) |
(260 |
) | |||
|
Unrecognized tax benefits, at end of the year |
|
$ |
4,860 |
|
$ |
4,664 |
|
$ |
7,567 |
|
As of December 31, 2017, the total amount of unrecognized tax benefits that, if recognized, would affect the effective tax rate is approximately $2.9 million. We do not expect the amount of unrecognized tax benefits to change within the next twelve months due to the lapse of statute limitations. We recognize accrued interest and penalties related to unrecognized tax benefits as a component of income tax expense. As of December 31, 2017, 2016 and 2015, the amount of accrued interest and penalties was approximately $0.6 million, $0.4 million and $0.5 million, respectively.
We are subject to income tax in the U.S. federal, state and local jurisdictions. With few exceptions, we are no longer subject to U.S. federal income tax examinations for years prior to 2013, as the statute of limitations has lapsed for 2012 and all preceding years. However, due to acquired net operating losses, tax authorities have the ability to adjust those net operating losses related to closed years. We are currently under income tax audits in various U.S. jurisdictions for the originally filed tax returns for tax years ended 2013 through 2015. Certain of these returns will be amended, and we believe we have adequate accruals for additional taxes and related interest expense which could result. We believe the ultimate resolution of income tax examinations will not have a material adverse effect on our consolidated financial position, results of operations, or liquidity.
The Tax Act reduces the U.S. federal corporate tax rate from 35% to 21% beginning in 2018. Based on a reduced U.S. federal corporate tax rate of 21% from the Tax Act, we re-measured deferred tax assets and liabilities at the tax rates at which they are expected to reverse in the future. Due to the limited time to consider the Tax Act and its various interpretations, we are still analyzing and refining our calculations, which could potentially affect the measurement of these balances or give rise to new deferred tax amounts, however, in certain cases, we have made a reasonable estimate of the effects on our existing deferred tax balances. For the items for which we were able to determine a reasonable estimate, we recognized a provisional amount, in accordance with Staff Accounting Bulletin 118, of approximately $35 million of tax expense related to re-measurement of our deferred tax assets and liabilities, which is included as a component of income tax expense from continuing operations resulting in the above impact to our 2017 effective income tax rate. As we continue to interpret the Tax Act and review any additional guidance issued by the U.S. Treasury Department, state taxation authorities and other standard-setting bodies, we may make adjustments to the provisional amounts noted above which may materially impact our provision for income taxes from continuing operations in the period in which the adjustments are made. The accounting for the tax effects of the Tax Act will be completed in 2018. We will continue to make and refine our calculations as additional analysis is completed. Our estimates may also be affected as we gain a more thorough understanding of the tax law.
NOTE K — EMPLOYEE BENEFITS
Savings Plan
We maintain a 401(k) Savings and Retirement plan that covers all of our employees. Under the plan, employees may defer a portion of their compensation up to the levels permitted by the Internal Revenue Service. We recorded matching contributions of approximately $5.9 million, $6.7 million and $6.6 million under this plan during 2017, 2016 and 2015, respectively, which were included within “Personnel costs” and “General and administrative expenses” in our consolidated statements of operations and comprehensive loss.
Defined Benefit Supplemental Executive Retirement Plan
Effective January 2004, we implemented an unfunded noncontributory defined benefit plan (“DB SERP”) for certain senior executives. The DB SERP, which we administer, calls for fifteen annual payments upon retirement with the payment amount based on years of service and final average salary. Benefit costs and liability balances are calculated based on certain assumptions including benefits earned, discount rates, interest costs, mortality rates and other factors. We engaged an actuary to calculate the related benefit obligation at December 31, 2017 and 2016 as well as net periodic benefit plan expense for the years ended December 31, 2017, 2016, and 2015. As of December 31, 2017 and 2016, the average remaining service period of plan participants is 11.5 and 12.5 years, respectively. We believe the assumptions used are appropriate; however, changes in assumptions or differences in actual experience may affect our benefit obligation and future expenses. Actual results that differ from the assumptions are accumulated and amortized over future periods, affecting the recorded obligation and expense in future periods.
The DB SERP’s net benefit obligation is as follows:
|
Change in Benefit Obligation |
|
Benefit |
| |
|
(in thousands) |
|
Obligation |
| |
|
Benefit obligation at December 31, 2014 |
|
$ |
23,054 |
|
|
Service cost |
|
386 |
| |
|
Interest cost |
|
703 |
| |
|
Payments |
|
(1,853 |
) | |
|
Actuarial gain |
|
(405 |
) | |
|
Benefit obligation at December 31, 2015 |
|
21,885 |
| |
|
Service cost |
|
390 |
| |
|
Interest cost |
|
740 |
| |
|
Amortization of loss |
|
— |
| |
|
Payments |
|
(1,847 |
) | |
|
Actuarial gain |
|
136 |
| |
|
Benefit obligation at December 31, 2016 |
|
21,304 |
| |
|
Service cost |
|
340 |
| |
|
Interest cost |
|
711 |
| |
|
Amortization of loss |
|
— |
| |
|
Payments |
|
(1,913 |
) | |
|
Actuarial loss |
|
351 |
| |
|
Benefit obligation at December 31, 2017 |
|
$ |
20,793 |
|
|
|
|
|
|
|
|
Unfunded status |
|
$ |
20,793 |
|
|
Unamortized net (gain) loss |
|
— |
| |
|
Net amount recognized |
|
$ |
20,793 |
|
Amounts Recognized in the Consolidated Balance Sheets:
|
|
|
As of December 31, |
| ||||
|
(in thousands) |
|
2017 |
|
2016 |
| ||
|
Current accrued expenses and other current liabilities |
|
$ |
1,913 |
|
$ |
1,913 |
|
|
Non-current other liabilities |
|
18,880 |
|
19,391 |
| ||
|
Total accrued liabilities |
|
$ |
20,793 |
|
$ |
21,304 |
|
We recorded gross actuarial (losses) gains under the DB SERP of approximately $(0.4) million, $(0.1) million, and $0.4 million in 2017, 2016, and 2015, respectively, in other comprehensive (loss) income. There were no other components such as prior service costs or transition obligations relating to the DB SERP costs recorded within other comprehensive (loss) income during 2017, 2016 or 2015.
The following weighted average assumptions were used to determine the benefit obligation as of December 31 of each year. Net periodic benefit cost for each year was determined using the weighted average assumptions as of the prior year. We used a third party actuarial specialist to assist in determining, among other things, the discount rate for all three years presented.
The cash surrender value of our COLI funded our DB SERP at December 31, 2016. We received the cash surrender value of the DB SERP COLI in the amount of $17.1 million in 2017 resulting in the benefit obligation being unfunded at December 31, 2017.
Our assumed weighted average discount rate for the defined benefit plan reflects the hypothetical rate at which the projected benefit obligation could be effectively settled or paid out to participants. We determine our discount rate based on a range of factors, including a yield curve composed of rates of return on high-quality, fixed income corporate bonds.
|
|
|
2017 |
|
2016 |
|
2015 |
|
|
Discount rate |
|
3.27 |
% |
3.54 |
% |
3.64 |
% |
|
Average rate of increase in compensation |
|
3.00 |
% |
3.00 |
% |
3.00 |
% |
At December 31, 2017, the estimated accumulated benefit obligation is $20.8 million. Future payments under the Plan are as follows:
|
(in thousands) |
|
|
| |
|
2018 |
|
$ |
1,913 |
|
|
2019 |
|
1,913 |
| |
|
2020 |
|
1,913 |
| |
|
2021 |
|
1,913 |
| |
|
2022 |
|
1,913 |
| |
|
Thereafter |
|
11,228 |
| |
|
Total |
|
$ |
20,793 |
|
Defined Contribution Supplemental Executive Retirement Plan
In 2013, we established a defined contribution plan (“DC SERP”) that covers certain of our senior executives. Each participant is given a notional account to manage his or her annual distributions and allocate the funds among various investment options (e.g. mutual funds). These accounts are tracking accounts only for the purpose of calculating the participant’s benefit. The participant does not have ownership of the underlying mutual funds. When a participant initiates or changes the allocation of his or her notional account, we will generally make an allocation of our investments, to match those chosen by the participant. While the allocation of our sub accounts is generally intended to mirror the participant’s account records (i.e. the distributions and gains or losses on those funds), the employee does not have legal ownership of any funds until payout upon retirement. The underlying investments are owned by the insurance company (and we own an insurance policy).
As of December 31, 2017 and 2016, the estimated accumulated obligation benefit is $3.0 million and $2.0 million, respectively, of which $2.3 million and $1.4 million is funded and $0.7 million and $0.6 million is unfunded at December 31, 2017 and 2016, respectively.
In connection with the DC SERP benefit obligation, we maintain a COLI policy. The carrying value of the COLI is measured at its cash surrender value and is presented within “Other assets” in our consolidated balance sheets. See Note I - “Other Current Assets and Other Assets” for additional information.
NOTE L — STOCK-BASED COMPENSATION
On May 19, 2017, the Board of Directors approved the Hanger, Inc. Special Equity Plan (the “Special Equity Plan”). The Special Equity Plan authorized up to 1.5 million shares of Common Stock and operates completely independent from our 2016 Omnibus Incentive Plan. All awards under the Special Equity Plan were made on May 19, 2017 which consisted of 0.8 million stock options and 0.3 million performance-based stock awards. No further grants of awards will be authorized or issued under the Special Equity Plan.
On April 15, 2016, our Board of Directors approved the Hanger, Inc. 2016 Omnibus Incentive Plan (the “2016 Plan”). The 2016 Plan authorizes the issuance of (a) up to 2.3 million shares of Common Stock, plus (b) 0.4 million shares available for issuance under the 2010 Plan that had not been subject to outstanding awards as of the effective date of the 2016 Plan and (c) any shares that would have become available again for new grants under the terms of the 2010 Omnibus Plan (“2010 Plan”) if such plan were still in effect.
Upon approval of the 2016 Plan, our 2010 Plan was no longer available for future awards.
As of December 31, 2017, approximately 1.2 million shares were available for future issuance. The available shares consisted of (a) 2.3 million shares of common stock authorized for issuance under the 2016 Plan plus (b) 0.4 million shares rolled forward from the 2010 Plan plus (c) 0.4 million shares forfeited and added back to the pool less (d) 1.9 million shares issued for awards. In 2017, shares issued under equity plans are issued from authorized and unissued shares. Total unrecognized stock-based compensation cost related to unvested restricted stock unit awards is approximately $11.8 million as of December 31, 2017, and is expected to be recognized as compensation expense over approximately 2.3 years.
On May 13, 2010, our shareholders approved the 2010 Plan and prohibited future awards under the Amended and Restated 2002 Stock Incentive and Bonus Plan (the “2002 Plan”) and 2003 Non-Employee Directors’ Stock Incentive Plan (the “2003 Plan”).
For the years ended December 31, 2017, 2016 and 2015, we recognized a total of approximately $12.9 million, $9.8 million and $11.1 million, respectively, of stock-based compensation expense for the 2002, 2003, 2010 and 2016 plans. Stock compensation expense, net of forfeitures, relates to restricted stock units, performance-based restricted stock units and options.
Restricted Stock Units
The summary of restricted stock units, performance-based stock units, and weighted average grant date fair values are as follows:
|
|
|
Employee Service-Based |
|
Employee Performance- |
|
Director Awards |
| |||||||||
|
|
|
Units |
|
Weighted |
|
Units |
|
Weighted |
|
Units |
|
Weighted |
| |||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||
|
Nonvested at December 31, 2015 |
|
810,840 |
|
$ |
26.90 |
|
145,451 |
|
$ |
25.83 |
|
94,169 |
|
$ |
18.25 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||
|
Granted |
|
631,011 |
|
6.90 |
|
192,600 |
|
6.90 |
|
71,000 |
|
6.53 |
| |||
|
Vested |
|
(279,421 |
) |
26.64 |
|
(19,722 |
) |
23.99 |
|
(93,704 |
) |
18.16 |
| |||
|
Forfeited |
|
(95,832 |
) |
20.69 |
|
(192,600 |
) |
6.90 |
|
— |
|
— |
| |||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||
|
Nonvested at December 31, 2016 |
|
1,066,598 |
|
16.30 |
|
125,729 |
|
26.11 |
|
71,465 |
|
6.72 |
| |||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||
|
Granted |
|
555,280 |
|
13.74 |
|
512,458 |
|
17.21 |
|
98,406 |
|
12.66 |
| |||
|
Vested |
|
(363,834 |
) |
19.27 |
|
(5,551 |
) |
29.66 |
|
(71,465 |
) |
6.72 |
| |||
|
Forfeited |
|
(75,005 |
) |
14.58 |
|
— |
|
— |
|
— |
|
— |
| |||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||
|
Nonvested at December 31, 2017 |
|
1,183,039 |
|
$ |
14.30 |
|
632,636 |
|
$ |
18.87 |
|
98,406 |
|
$ |
12.66 |
|
During the years ended December 31, 2017, 2016 and 2015, approximately 0.4 million, 0.4 million, and 0.4 million of restricted common stock units with an intrinsic value of $5.9 million, $2.1 million and $9.0 million, respectively, became fully vested. As of December 31, 2017, total unrecognized compensation expense related to unvested restricted stock units and unvested performance based restricted stock units for which we have concluded the performance condition was probable of achievement was approximately $19.4 million and the related weighted-average period over which it is expected to be recognized is approximately 2.4 years. The aggregate granted units have vesting dates through May 2020. The 2017, 2016 and 2015 aggregate grants had total estimated grant date fair values of $17.7 million, $6.2 million and $16.1 million, respectively.
A special equity grant of performance-based restricted stock units was granted on May 19, 2017 and vests 100% three years after the date of issuance, assuming the performance goal is achieved. The financial target for this grant is to achieve a compounded annual growth rate (“CAGR”) of our common stock price of 20% as of market close on May 18, 2020. This equates to a share price on that date of $22.07 compared to the closing price on the eve of grant of $12.77. The grant provides for the vesting of 50% of the original targeted shares if a CAGR of 10% (a stock price of $17.00) is achieved. The grant also provides for the vesting of up to 200% of the original targeted shares if a CAGR of 30% (a stock price of $28.06) or more is achieved. The percentage of vested shares will be interpolated on a linear basis between 50% and 200% for a CAGR between 10% and 30%. The stock price at time of award was $12.77, but given market condition performance criteria the Monte Carlo Simulation valuation was used to calculate a fair value of $19.29 per share.
Options
The summary of option activity and weighted average exercise prices are as follows:
|
|
|
Director Awards |
| ||||||||
|
Weighted Average Exercise Price |
|
Shares |
|
Weighted Average |
|
Aggregate |
|
Weighted Average |
| ||
|
|
|
|
|
|
|
|
|
|
| ||
|
Outstanding at December 31, 2016 |
|
— |
|
$ |
— |
|
$ |
— |
|
— |
|
|
Granted |
|
798,020 |
|
12.77 |
|
2,378,100 |
|
9.4 |
| ||
|
Outstanding at December 31, 2017 |
|
798,020 |
|
$ |
12.77 |
|
$ |
2,378,100 |
|
|
|
No options were exercisable under our stock-based compensation plans at December 31, 2016 and 2015. At December 31, 2017, 0.8 million options were granted but not yet exercisable with a weighted average exercise price of $12.77, average remaining contractual terms of 9.4 years and aggregate intrinsic values of approximately $2.4 million. As of December 31, 2017, there was unrecognized compensation cost related to stock option awards of $5.5 million. As of December 31, 2016 and 2015, there was no unrecognized compensation cost related to stock option awards.
There were 0.8 million options outstanding as of December 31, 2017 and no options outstanding as of December 31, 2016 and 2015.
NOTE M — LEASES
Rent expense under operating leases was approximately $47.3 million, $48.1 million, and $50.1 million, for the years ended December 31, 2017, 2016 and 2015, respectively, which was included within “Other operating costs” and “General and administrative expenses” in our consolidated statements of operations and comprehensive loss. Sublease rental income is not material. The net book value of office equipment under capital leases was approximately $0.7 million and $0.8 million at December 31, 2017 and 2016, respectively. Equipment capital lease obligations are included in long-term debt as a part of “Financing leases and other” in Note N - “Long-Term Debt.”
Future minimum rental payments, by year and in the aggregate, under operating and financing obligations with terms of one year or more at December 31, 2017 are as follows:
|
(in thousands) |
|
Operating |
|
Capital |
| ||
|
|
|
|
|
|
| ||
|
2018 |
|
$ |
38,567 |
|
$ |
343 |
|
|
2019 |
|
29,888 |
|
198 |
| ||
|
2020 |
|
21,418 |
|
121 |
| ||
|
2021 |
|
14,721 |
|
50 |
| ||
|
2022 |
|
9,417 |
|
— |
| ||
|
Thereafter |
|
11,903 |
|
— |
| ||
|
Total |
|
$ |
125,914 |
|
$ |
712 |
|
Future minimum rental payments, by year and in the aggregate, under operating and financing obligations with terms of one year or more at December 31, 2016 are as follows:
|
(in thousands) |
|
Operating |
|
Capital |
| ||
|
|
|
|
|
|
| ||
|
2017 |
|
$ |
39,581 |
|
$ |
467 |
|
|
2018 |
|
32,507 |
|
268 |
| ||
|
2019 |
|
25,192 |
|
121 |
| ||
|
2020 |
|
17,226 |
|
39 |
| ||
|
2021 |
|
11,556 |
|
— |
| ||
|
Thereafter |
|
16,147 |
|
— |
| ||
|
Total |
|
$ |
142,209 |
|
$ |
895 |
|
NOTE N — LONG-TERM DEBT
Long-term debt as of December 31 was as follows:
|
(in thousands) |
|
2017 |
|
2016 |
| ||
|
Term loan |
|
$ |
151,875 |
|
$ |
180,000 |
|
|
Term loan B |
|
280,000 |
|
280,000 |
| ||
|
Revolving credit facility |
|
5,000 |
|
— |
| ||
|
Seller notes |
|
5,912 |
|
11,110 |
| ||
|
Financing leases and other |
|
18,169 |
|
18,245 |
| ||
|
Total debt before unamortized discount and debt issuance costs |
|
460,956 |
|
489,355 |
| ||
|
Unamortized discount |
|
(5,556 |
) |
(7,511 |
) | ||
|
Debt issuance costs, net |
|
(5,136 |
) |
(9,194 |
) | ||
|
Total debt |
|
$ |
450,264 |
|
$ |
472,650 |
|
|
Reported as: |
|
|
|
|
| ||
|
Current portion of long-term debt |
|
$ |
4,336 |
|
$ |
30,944 |
|
|
Long-term debt |
|
445,928 |
|
441,706 |
| ||
|
Total debt |
|
$ |
450,264 |
|
$ |
472,650 |
|
We apply ASC No. 470-50, Debt - Modifications and Extinguishments (ASC 470-50), which defines a debt modification.
Credit Agreement - Revolving Credit Facility and Term Loan
On June 17, 2013, we entered into a five year credit agreement (as amended from time to time, the “Credit Agreement”) that provided senior secured facilities of up to $425.0 million. The Credit Agreement originally included a $200.0 million revolving credit facility and a $225.0 million term loan facility both of which mature on June 17, 2018 and were subject to a leverage-based pricing grid in which the applicable interest rate is dependent on our leverage ratio.
From January 1, 2016 through December 31, 2017, we entered into three agreements relating to our Credit Agreement that waived certain actual and potential events of default and amended various covenants and other provisions including, among other things, raising the interest rate and reducing the amounts available pursuant to the revolving credit facility.
The Credit Agreement (giving effect to all amendments and waivers) provides for (i) a revolving credit facility with aggregate revolving commitments of $118.3 million (subject to the mandatory commitment reductions and usage limitations described below) that matures in June 2018, and (ii) a $225.0 million term loan facility due in quarterly principal installments that began at 0.625% of the initial $225.0 million borrowed and then escalated to 1.25% on September 30, 2014, to 1.875% on September 30, 2015, to 2.5% on September 30, 2016, and escalated to 3.75% on September 30, 2017. A final principal installment of approximately $143.4 million is due at maturity in June 2018. From time to time, mandatory prepayments may be required as a result of the incurrence of certain types of debt, certain asset sales, or other events as defined in the Credit Agreement. No such mandatory prepayments were required during 2017 and 2016.
We previously received $34.1 million in federal income tax refunds with respect to tax year 2015 or earlier, and the effect of those previous receipts has been incorporated into the determination of our $118.3 million in aggregate revolving commitments. If we receive additional federal income tax refunds related to tax year 2015 or earlier, then 50% of our net cash proceeds in respect of those refunds will be applied as a further permanent reduction of the aggregate revolving commitments under the Credit Agreement, except that in no event shall the commitment be reduced to less than $108.0 million as a result of such refunds.
Until such time as (a) we have achieved a leverage ratio (as described below) for our then most recently ended fiscal quarter, of less than or equal to 4.00 to 1.00, and (b) we have delivered financial information and certain related materials for the fiscal
periods ended March 31, 2015, June 30, 2015, September 30, 2015, December 31, 2015 and December 31, 2016, the amount that we can borrow under the Credit Agreement in the form of revolving loans, swing line loans and/or letters of credit is reduced by specified revolving usage limitation amounts depending on the fiscal quarter. The aggregate revolving credit commitment of $118.3 million is reduced by $13.9 million for periods from July 15, 2016 to September 30, 2016; $17.3 million for the three months ending December 31, 2016; $10.7 million for the three months ending March 31, 2017; $20.7 million for the nine months ending December 31, 2017; $10.7 million for the three months ending March 31, 2018; and $20.7 million for periods subsequent to March 31, 2018.
Borrowings under the Credit Agreement bear interest at a variable rate per annum equal to (i) LIBOR plus 5.75%, or (ii) the base rate (which is the highest of (a) the administrative agent’s prime rate, (b) the federal funds rate plus 0.50% or (c) the sum of 1% plus one-month LIBOR) plus 4.75%. Due to various amendments to the debt agreement as mentioned above, the rates have increased from LIBOR plus 2.00% at December 31, 2016 and December 31, 2015. Interest rates on our debt were 7.32%, 5.52%, and 2.43% at December 31, 2017, 2016 and 2015, respectively. Upon (a) our delivering the financial information and certain related materials for the fiscal periods ended March 31, 2015, June 30, 2015, September 30, 2015, December 31, 2015 and December 31, 2016, and (b) achievement of a leverage ratio (as described below), for our then, most recently ended fiscal quarter, of less than or equal to 4.00 to 1.00, the margin for borrowings based on LIBOR will decrease to 4.00% per annum and the margin for borrowings based on the base rate will decrease to 3.00% per annum.
The Credit Agreement as amended on June 23, 2017 requires us to maintain a maximum consolidated leverage ratio (defined as, with certain adjustments, the ratio of our consolidated indebtedness to consolidated net income before interest, taxes, depreciation, amortization, non-cash charges and certain other items as of the end of any period of four consecutive fiscal quarters, as follows:
· 5.00 to 1.00 as of the last day of the fiscal quarter ended June 30, 2016;
· 5.75 to 1.00 as of the last day of the fiscal quarter ended September 30, 2016;
· 5.00 to 1.00 as of the last day of each fiscal quarter thereafter.
The minimum interest coverage ratio is, as of the end of our fiscal quarter ending:
· 3.50:1.00 as of the last day of the fiscal quarter ended June 30, 2016;
· 2:25:1:00 as of the last day of the fiscal quarter ended September 30, 2016;
· 2:25:1:00 as of the last day of the fiscal quarter ended December 31, 2016;
· 2:25:1:00 as of the last day of the fiscal quarter ended March 31, 2017;
· 2:25:1:00 as of the last day of the fiscal quarter ended June 30, 2017;
· 2:25:1:00 as of the last day of the fiscal quarter ended September 30, 2017;
· 2:25:1:00 as of the last day of the fiscal quarter ended December 31, 2017;
· 2:00:1:00 as of the last day of each quarter thereafter.
The Credit Agreement also contains other customary events of default and related remedies. Loans outstanding under the Credit Agreement will bear interest at a rate of 2.00% per annum in excess of the otherwise applicable rate (i) upon acceleration of such loans, (ii) while a payment event of default exists or (iii) upon the lenders’ request, during the continuance of any other event of default.
Subject to certain exceptions, the facilities under the Credit Agreement are senior obligations and are secured by first priority perfected liens and security interests in substantially all our personal property and each subsidiary guarantor.
We had approximately $86.4 million and $94.9 million available under the revolving credit facility as of December 31, 2017 and 2016, respectively. We had outstanding letters of credit against the revolving credit facility of $6.2 million and $6.1 million as of December 31, 2017 and 2016, respectively.
We incur an unused commitment fee on the amount of unused commitments under the Credit Agreement in the amount of 0.375% based on average quarterly utilization. The amounts incurred were $0.4 million, $0.3 million and $0.2 million for the years ended December 31, 2017, 2016 and 2015, respectively.
The unamortized loan discount is being recorded as additional interest expense on a quarterly basis over the term of the credit agreement.
Term B Credit Agreement
On August 1, 2016, we entered into a new Term B Credit Agreement providing for a new $280.0 million senior unsecured term loan facility due at maturity on August 1, 2019 and bearing interest at 11.5% per annum payable quarterly in arrears. On August 31, 2016, we used approximately $205.3 million of the proceeds from the Term B Credit Agreement and from existing cash on hand to redeem all of our $200 million senior notes (“Senior Notes”) which were scheduled to mature in November 2018, and satisfy and discharge the indenture related to the Senior Notes of approximately $81.0 million to pay down the revolving credit facility under the Credit Agreement, approximately $7.9 million to pay Term B Credit Agreement issuance costs and bank consent fees, and approximately $1.9 million to pay related legal and professional fees. As a result of the repayment of the Senior Notes and the issuance of the Term B Credit Agreement debt, we capitalized $6.7 million, which is being amortized to interest expense over the term of the Term B Credit Agreement debt and recorded a loss on extinguishment of debt of $6.0 million for the year ended December 31, 2016.
We may prepay borrowings under the Term B Credit Agreement in whole or in part at any time. Any voluntary prepayment, certain mandatory prepayments and prepayments in connection with certain repricing transactions of the loans will be subject to the following prepayment premiums: (i) if such prepayment is made before February 1, 2018, an amount equal to the discounted present value as of the date of prepayment, utilizing a comparable U.S. Treasury note yield plus 50 basis points, of the sum of (A) the remaining payments of interest on the principal amount prepaid through February 1, 2018, plus (B) 3.00% of the principal amount prepaid, (ii) if such prepayment is made on or after February 1, 2018, but prior to February 1, 2019, an amount equal to 3.00% of the principal amount prepaid, and (iii) if such prepayment is made on or after February 1, 2019, an amount equal to 1.50% of the principal amount prepaid.
The Term B Credit Agreement contains various restrictions and covenants, including restrictions on our ability and certain of our subsidiaries to consolidate or merge, create liens, incur additional indebtedness, dispose of assets, consummate acquisitions, make investments and pay dividends and other distributions. The covenants in the Term B Credit Agreement are similar to those contained in the Credit Agreement, except that the Term B Credit Agreement does not contain any separate financial covenants. Subject to a 90-day grace period, an event of default under the Credit Agreement will cause an event of default under the Term B Credit Agreement. An event of default under the Credit Agreement that results in acceleration of the indebtedness thereunder will cause an immediate event of default under the Term B Credit Agreement.
The Term B Credit Agreement also contains customary events of default and related remedies. Loans outstanding under the Term B Credit Agreement will bear interest at a rate of 2.00% per annum in excess of the otherwise applicable rate (i) upon acceleration of such loans, (ii) while a payment event of default exists or (iii) upon the lenders’ request, during the continuance of any other event of default.
Subsidiary Guarantees
The obligations under the Credit Agreement and the Term B Credit Agreement are guaranteed by our material domestic subsidiaries, which incorporates subsidiaries that both make up no less than 90% of our total net revenues and make up no less
than 90% of our total assets. Separate condensed consolidating information is not included as the parent company does not have independent assets or operations, and the guarantees are full and unconditional and joint and several.
Other Restrictions
The Credit Agreement and the Term B Credit Agreement limits our ability to, among other things, purchase capital assets, incur additional indebtedness, create liens, pay dividends on or redeem capital stock, make certain investments, make restricted payments, make certain dispositions of assets, engage in transactions with affiliates, engage in certain business activities, and engage in mergers, consolidations and certain sales of assets.
Seller Notes
We typically issue subordinated promissory notes (“Seller Notes”) as a part of the consideration transferred when making acquisitions. The Seller Notes are unsecured and are presented net of unamortized discount of $0.3 million and $0.6 million as of December 31, 2017 and 2016, respectively. In accordance with ASC 805, Accounting for Business Combinations, we measure these instruments at their estimated fair values as of the respective acquisition dates. The stated interest rates on these instruments range from 2.00% to 4.00% while the effective interest rate is 6.50%. Principal and interest are payable in monthly, quarterly or annual installments and mature through January 2020.
Financing Leases and Other
Financing leases relate to agreements when we are deemed the owner of a leased building, typically due to significant involvement during the construction period, and which do not qualify for de-recognition under the sale-leaseback accounting guidance due to one or more prohibited forms of continuing involvement in the property. Such forms of continuing involvement include us paying for a more than insignificant portion of project construction costs, us providing a security interest in the tenant’s personal property located at the premises, and/or we have renewal options for a term that comprises 90% or more of the remaining economic life of the property at a price other than estimated fair value. These liabilities have remaining terms ranging from 1 to 17 years with an average inherent interest rate of approximately 15%. Other obligations include equipment under capital leases.
The following table summarizes for the year ended December 31, 2017, the aggregate contractual payments associated with the financing leases and other obligations over the next 5 years and thereafter, including both principal and interest. Included in these amounts are payments for optional renewal periods for which management believes we will exercise our rights to renew, as well as the final non-monetary payment made with the return of the property at the end of the financing term:
|
(in thousands) |
|
December 31, 2017 |
| |
|
2018 |
|
$ |
3,209 |
|
|
2019 |
|
4,886 |
| |
|
2020 |
|
2,602 |
| |
|
2021 |
|
2,322 |
| |
|
2022 |
|
2,394 |
| |
|
Thereafter |
|
14,373 |
| |
|
Less: amount representing interest |
|
(11,617 |
) | |
|
Total |
|
$ |
18,169 |
|
Maturities of long-term debt at December 31, 2017 and the years thereafter are as follows:
|
(in thousands) |
|
December 31, |
| |
|
2018 |
|
$ |
161,382 |
|
|
2019 |
|
283,216 |
| |
|
2020 |
|
2,191 |
| |
|
2021 |
|
856 |
| |
|
2022 |
|
1,014 |
| |
|
Thereafter |
|
12,297 |
| |
|
Total debt before unamortized discount and debt issuance costs, net |
|
460,956 |
| |
|
Unamortized discount |
|
(5,556 |
) | |
|
Debt issuance costs, net |
|
(5,136 |
) | |
|
Total long-term debt |
|
$ |
450,264 |
|
Subsequent Refinancing of Credit Agreement and Term B Credit Agreement
Due to the maturity of the Credit Agreement on June 17, 2018, which had $151.9 million outstanding at December 31, 2017, and given that we do not produce operating cash flow sufficient to retire this obligation through cash sources arising from our normal operations, and requirements under our Credit Agreement that we provide lenders with our audited financial statements for the year ended December 31, 2017 no later than March 31, 2018, we entered into a new $605 million Senior Credit Facility on March 6, 2018 (the “New Credit Agreement”).
The New Credit Agreement provides for (i) a revolving credit facility with an initial maximum aggregate amount of availability of $100 million that matures in March 2023 and (ii) a $505 million term loan facility due in quarterly principal installments commencing June 29, 2018, with all remaining outstanding principal due at maturity in March 2025. Availability under the revolving credit facility is reduced by outstanding letters of credit, which were approximately $5.93 million as of March 6, 2018. We may (a) increase the aggregate principal amount of any outstanding tranche of term loans or add one or more additional tranches of term loans under the loan documents, and/or (b) increase the aggregate principal amount of revolving commitments or add one or more additional revolving loan facilities under the loan documents by an aggregate amount of up to the sum of (1) $125 million and (2) an amount such that, after giving effect to such incurrences of such amount (but excluding the cash proceeds of such incremental facilities and certain other indebtedness, and treating all commitments in respect of revolving indebtedness as fully drawn), the consolidated first lien net leverage ratio is equal to or less than 3.80 to 1.00, if certain conditions are satisfied, including the absence of a default or an event of default under the New Credit Agreement at the time of the increase and that we obtain the consent of each lender providing any incremental facility. On March 6, 2018, we had $505 million outstanding under the term loan facility and did not have any borrowings under the revolving credit facility.
Proceeds from the borrowings under the New Credit Agreement were used in part to repay in full all previously existing loans under (i) the Credit Agreement, and (ii) the Term B Credit Agreement. Proceeds were also used to pay various transaction costs of $25.1 including fees of $9.8 paid to respective lenders and accrued and unpaid interest. We expect that the remainder of the proceeds will be used to provide ongoing working capital and capital for other general corporate purposes.
Our obligations under the New Credit Agreement are currently guaranteed by certain of our domestic subsidiaries and will from time to time be guaranteed by, subject to certain exceptions, any domestic subsidiaries that may become material in the future. Subject to certain exceptions, the New Credit Agreement is secured by first-priority perfected liens and security interests in substantially all of our personal property and each subsidiary guarantor.
Borrowings under the New Credit Agreement bear interest at a variable rate equal to (i) LIBOR plus a specified margin, or (ii) the base rate (which is the highest of (a) Bank of America, N.A.’s prime rate, (b) the federal funds rate plus 0.50% or (c) the sum of 1% plus one-month LIBOR) plus a specified margin; provided, however, that, in each case, such margins shall be increased by 0.25% per annum if our audited financial statements for the fiscal year ending December 31, 2017 and financial statements for the quarter ending March 31, 2018 are not delivered to the lenders on or prior to July 1, 2018 and filed
with the U.S. Securities and Exchange Commission (the “SEC”) within five business days after such delivery, with such increase to remain in effect until the first business day following the date upon which we have both delivered such audited financial statements to the lenders and filed the same with the SEC.
We must also pay (i) an unused commitment fee ranging from 0.375% to 0.500% per annum of the average daily unused portion of the aggregate revolving credit commitments under the New Credit Agreement, and (ii) a per annum fee equal to (a) for each performance standby letter of credit outstanding under the New Credit Agreement with respect to nonfinancial contractual obligations, 50% of the applicable margin over LIBOR under the revolving credit facility in effect from time to time multiplied by the daily amount available to be drawn under such letter of credit, and (b) for each other letter of credit outstanding under the New Credit Agreement, the applicable margin over LIBOR under the revolving credit facility in effect from time to time multiplied by the daily amount available to be drawn for such letter of credit.
The New Credit Agreement contains various restrictions and covenants, including requirements that we maintain certain financial ratios at prescribed levels and restrictions on our ability and certain of our subsidiaries to consolidate or merge, create liens, incur additional indebtedness, dispose of assets, consummate acquisitions, make investments and pay dividends and other distributions. The New Credit Agreement includes the following financial covenants applicable for so long as any revolving loans and/or revolving commitments remain outstanding under the New Credit Agreement: (i) a maximum consolidated first lien net leverage ratio (defined as, with certain adjustments and exclusions, the ratio of consolidated first-lien indebtedness to consolidated net income before interest, taxes, depreciation, amortization, non-cash charges and certain other items (“EBITDA”) for the most recently ended period of four fiscal quarters for which financial statements are available) of 5.00 to 1.00 for the fiscal quarters ended June 30, 2018, September 30, 2018, December 31, 2018 and March 31, 2019; 4.75 to 1.00 for the fiscal quarters ended June 30, 2019, September 30, 2019, December 31, 2019 and March 31, 2020; 4.50 to 1.00 for the fiscal quarters ended June 30, 2020, September 30, 2020, December 31, 2020 and March 31, 2021; 4.25 to 1.00 for the fiscal quarters ended June 30, 2021, September 30, 2021, December 31, 2021 and March 31, 2022; and 3.75 to 1.00 for the fiscal quarter ended June 30, 2022 and the last day of each fiscal quarter thereafter; and (ii) a minimum interest coverage ratio (defined as, with certain adjustments, the ratio of our EBITDA to consolidated interest expense to the extent paid or payable in cash) of 2.75 to 1.00 as of the last day of any fiscal quarter.
The New Credit Agreement also contains customary events of default. If an event of default under the New Credit Agreement occurs and is continuing, then the lenders may declare any outstanding obligations under the New Credit Agreement to be immediately due and payable; provided, however, that the occurrence of an event of default as a result of a breach of a financial covenant under the New Credit Agreement does not constitute a default or event of default with respect to any term facility under the New Credit Agreement unless and until the required revolving lenders shall have terminated their revolving commitments and declared all amounts outstanding under the revolving credit facility to be due and payable. In addition, if we or any subsidiary guarantor becomes the subject of voluntary or involuntary proceedings under any bankruptcy, insolvency or similar law, then any outstanding obligations under the New Credit Agreement will automatically become immediately due and payable. Loans outstanding under the New Credit Agreement will bear interest at a rate of 2.00% per annum in excess of the otherwise applicable rate (i) upon acceleration of such loans, (ii) while a payment event of default exists or (iii) upon the lenders’ request, during the continuance of any other event of default.
NOTE O — ACCRUED EXPENSES, OTHER CURRENT LIABILITIES AND OTHER LIABILITIES
Accrued expenses and other current liabilities consists of:
|
|
|
As of December 31, |
| ||||
|
(in thousands) |
|
2017 |
|
2016 |
| ||
|
|
|
|
|
|
| ||
|
Patient prepayments deposits and refunds payable |
|
$ |
30,194 |
|
$ |
28,895 |
|
|
Accrued sales taxes and other taxes |
|
6,335 |
|
5,716 |
| ||
|
Accrued professional fees |
|
11,612 |
|
25,912 |
| ||
|
Insurance and self-insurance accruals |
|
8,901 |
|
9,866 |
| ||
|
Other current liabilities |
|
8,796 |
|
8,561 |
| ||
|
Total |
|
$ |
65,838 |
|
$ |
78,950 |
|
Patient prepayment deposits and refunds includes funds received for devices not yet delivered to a patient and refunds for overpayments. Taxes primarily includes accrued sales tax liabilities and other taxes payable. Accrued professional fees primarily relate to accruals for professional accounting and legal fees. Accrued insurance primarily relates to accruals for estimated losses for certain self-insured risks including property, professional liability, general liability and employee health care costs. Other current liabilities are primarily related to accruals for deferred revenue and warranty liabilities.
Other liabilities consists of:
|
|
|
As of December 31, |
| ||||
|
(in thousands) |
|
2017 |
|
2016 |
| ||
|
|
|
|
|
|
| ||
|
Supplemental executive retirement plan obligations |
|
$ |
21,842 |
|
$ |
21,478 |
|
|
Unrecognized tax benefits |
|
5,219 |
|
5,015 |
| ||
|
Long-term insurance accruals |
|
9,531 |
|
9,088 |
| ||
|
Deferred tenant improvement allowances |
|
7,361 |
|
7,345 |
| ||
|
Deferred rent |
|
4,909 |
|
5,433 |
| ||
|
Asset retirement obligations |
|
1,180 |
|
1,464 |
| ||
|
Other |
|
211 |
|
894 |
| ||
|
Total |
|
$ |
50,253 |
|
$ |
50,717 |
|
Supplemental executive retirement plan obligations includes obligations due on both the DB SERP and DC SERP. See Note K - “Employee Benefits” within these consolidated financial statements. Unrecognized tax benefits represent the difference between tax positions that we expect to take, or take on our income tax returns and the benefit we recognize on our financial statements. Deferred tenant improvement allowance represents deferred credits associated with receiving lease incentives. Deferred rent represents net deferred credits associated with recognizing rent expense on a straight-line basis for property operating leases whose lease payments escalate over the life of the lease. Both deferred credits are recognized as reductions of rent expense over the term of the associated lease. Asset retirement obligations is the liability to return a leased building to the state before it was occupied. Other includes fair market value lease differential liability, build-to-suit tenant interest accrual and other long-term accrued expenses.
NOTE P — SHAREHOLDERS’ (DEFICIT) EQUITY
Shareholder’s Rights Plan
On February 28, 2016, the Board of Directors declared a dividend of one preferred share purchase right (a “Right”) for each outstanding share of common stock, par value $0.01 per share (the “Common Stock”). The dividend is payable to the shareholders of record on March 10, 2016 (the “Record Date”). The Rights will not be exercisable until after the public announcement that a person or group of affiliated or associated persons has acquired or obtained the right or obligation to acquire beneficial ownership of 10% or more of our outstanding Common Stock (“Acquiring Person”) or following the commencement of a tender offer or exchange offer that, if consummated, would result in a person or group becoming an Acquiring Person. If a shareholder’s beneficial ownership of our Common Stock as of the time of the public announcement of the Rights Agreement and associated dividend declaration is at or above the applicable threshold, as defined by the Rights Agreement (including through entry into certain derivative positions), that shareholder’s then-existing ownership percentage would be grandfathered, but the rights would become exercisable if at any time after such announcement, the shareholder increases its ownership percentage.
Once exercisable, each Right will allow its holder to purchase one one-thousandth of a share of Series A Junior Participating Preferred Stock, par value $0.01 per share (the “Preferred Stock”), for $65.00 (the “Purchase Price”), subject to adjustment. Prior to exercise, the Right does not give its holder any dividend, voting, or liquidation rights. The description and terms of the Rights are set forth in a Rights Agreement, dated as of February 28, 2016, between us and Computershare Inc., as the Rights Agent.
The Rights have certain anti-takeover effects. The Rights will cause a substantial dilution to any person or group that attempts to acquire us without the approval of our Board of Directors. As a result, the overall effect of the Rights may be to render more difficult or discourage any attempt to acquire us even if such acquisition may be favorable to the interests of our shareholders. Because our Board of Directors can redeem the Rights and amend the Rights Agreement in any respect prior to a person or group becoming an Acquiring Person, the Rights should not interfere with a merger or other business combination approved by the Board of Directors. The rights were to expire on August 28, 2017.
Rights Agreement Amendment
On June 23, 2017, we entered into an amendment (the “Rights Agreement Amendment”) to the Rights Agreement to extend the “Final Expiration Date” under the Rights Agreement to December 31, 2018. Pursuant to the terms of the Rights Agreement as amended, we have the ability to redeem the rights prior to the “Final Expiration Date” or to further amend the Rights Agreement to provide for an earlier “Final Expiration Date”.
The “Final Expiration Date” under the Rights Agreement was not extended in response to any specific takeover bid or other proposal to acquire control.
NOTE Q — COMMITMENTS AND CONTINGENT LIABILITIES
Commitments
In April 2014, in connection with the settlement of a patent infringement dispute, our wholly-owned subsidiary, Southern Prosthetic Supply, Inc. (“SPS”), entered into a non-cancellable agreement to purchase a total of $4.5 million of prosthetic gel liners in five installments. We determined that a portion of the prosthetic gel liners should be reserved as excess and slow-moving inventory, and we accrued a liability and expensed $3.4 million in 2014. As of December 31, 2017, our reserve associated with the non-cancellable purchase commitment was $2.8 million. As of December 31, 2017, $1.5 million of the non-cancellable purchase commitment was outstanding with $1.0 million, and $0.5 million of purchases due by April of 2018, and 2019, respectively.
Contingencies
Legal Proceedings
In November 2014, a securities class action complaint was filed in federal district court in Texas against us. The case, City of Pontiac General Employees’ Retirement System v. Hanger, et al., C.A. No. 1:14-cv-01026-SS, is currently pending before the United States District Court for the Western District of Texas. The complaint names as defendants us and certain of our current and former officers and directors for allegedly making materially false and misleading statements regarding, among other things, our financial statements, Recovery Audit Contractor (“RAC”) audit success rate, our implementation of new financial systems, same-store sales growth, and the adequacy of our internal processes and controls. The complaint alleges violations of Sections 10(b) and 20(a) of the Exchange Act and Rule 10b-5 promulgated thereunder. The complaint seeks unspecified damages, costs and attorneys’ fees, and equitable relief.
On April 1, 2016, the court granted us motion to dismiss the lawsuit for failure to state a claim upon which relief can be granted, and permitted plaintiffs to file an amended complaint. On July 1, 2016, plaintiffs filed an amended complaint. On September 15, 2016, we and certain of the individual defendants filed motions to dismiss the lawsuit. On January 26, 2017, the court granted the defendants’ motions and dismissed with prejudice all claims against all defendants for failure to state a claim. On February 24, 2017, plaintiffs filed a notice of appeal to the United States Court of Appeals for the Fifth Circuit. Appellate briefing was completed on August 18, 2017 and the appeal remains pending. The Court of Appeals held oral argument for the appeal on March 5, 2018. We are now awaiting a ruling from the Court of Appeals.
In February and August of 2015, two separate shareholder derivative suits were filed against us in Texas state court related to the announced restatement of our certain financial statements. The cases were subsequently consolidated into Judy v. Asar, et. al., Cause No. D-1-GN-15-000625. On October 25, 2016, plaintiffs in that action filed an amended complaint, and the case is currently pending before the 345th Judicial District Court of Travis County, Texas.
The amended complaint in the consolidated derivative action names as defendants us and certain of our current and former officers and directors. It alleges claims for breach of fiduciary duty based, inter alia, on the defendants’ alleged failure to exercise good faith to ensure that adequate accounting and financial controls were in place and that disclosures regarding our business, financial performance and internal controls were truthful and accurate. The complaint seeks unspecified damages, costs, attorneys’ fees, and equitable relief.
As disclosed in our Current Report on Form 8-K filed with the SEC on June 6, 2016, the Board of Directors appointed a Special Litigation Committee of the Board (the “Special Committee”). The Board delegated to the Special Committee the authority to (1) determine whether it is in our best interests to pursue any of the allegations made in the derivative cases filed in Texas state court (which cases were consolidated into the Judy case discussed above), (2) determine whether it is in our best interests to pursue any remedies against any of our current or former employees, officers or directors as a result of the
conduct discovered in the Audit Committee investigation concluded on June 6, 2016 (the “Investigation”), and (3) otherwise resolve claims or matters relating to the findings of the Investigation. The Special Committee retained independent legal counsel to assist and advise it in carrying out its duties and reviewed and considered the evidence and various factors relating to our best interests. In accordance with its findings and conclusions, the Special Committee determined that it is not in our best interest to pursue any of the claims in the Judy derivative case. Also in accordance with its findings and conclusions, the Special Committee determined that it is not in our best interests to pursue legal remedies against any of our current or former employees, officers, or directors.
On April 14, 2017, we filed a motion to dismiss the consolidated derivative action based on the resolution by the Special Committee that it is not in our best interest to pursue the derivative claims. Counsel for the derivative plaintiffs opposed that motion and moved to compel discovery. In a hearing held on June 12, 2017, the Travis County court denied plaintiffs’ motion to compel, and held that the motion to dismiss would be considered only after appropriate discovery was concluded.
The plaintiffs have since subpoenaed counsel for the Special Committee, seeking a copy of the full report prepared by the Special Committee and its independent counsel. Counsel for the Special Committee, as well as our counsel, take the position that the full report is not discoverable under Texas law. Plaintiffs’ counsel has indicated it will file a motion to compel the Special Committee’s counsel to produce the report, but it has not yet done so. Upon resolution of the discovery dispute and completion of discovery, we intend to file a motion to dismiss the consolidated derivative action.
Management intends to vigorously defend against the shareholder derivative action and the appeal in the securities class action. At this time, we cannot predict how the Courts will rule on the merits of the claims and/or the scope of the potential loss in the event of an adverse outcome. Should we ultimately be found liable, the resulting damages could have a material adverse effect on our consolidated financial position, liquidity or results of operations.
Other Matters
In May 2015 one of our clinics received a civil investigative demand for records relating to a sample of claims submitted to Medicare and Medicaid for reimbursement, and we provided records in response to the subpoena. In May 2017, we were informed by an Assistant United States Attorney that it was investigating whether we properly provided and claimed reimbursement for prosthesis skins and covers from July 2013 (after an industry announcement) to the present. We have reviewed the claims, and have cooperated with the government’s investigation. This matter was resolved in March 2018 and did not have a material impact on the first quarter of 2018 or on any financial period in 2017.
From time to time we are subject to legal proceedings and claims which arise in the ordinary course of our business, including additional payments under business purchase agreements. In the opinion of management, the amount of ultimate liability, if any, with respect to these actions will not have a materially adverse effect on our consolidated financial position, liquidity or results of our operations.
We are in a highly regulated industry and receive regulatory agency inquiries from time to time in the ordinary course of our business, including inquiries relating to our billing activities. No assurance can be given that any discrepancies identified during a regulatory review will not have a material adverse effect on our consolidated financial statements.
Guarantees and Indemnifications
In the ordinary course of our business, we may enter into service agreements with service providers in which we agree to indemnify or limit the service provider against certain losses and liabilities arising from the service provider’s performance of the agreement. We have reviewed our existing contracts containing indemnification or clauses of guarantees and do not believe that our liability under such agreements is material.
NOTE R — SEGMENT AND RELATED INFORMATION
We have identified two operating segments and both performance evaluation and resource allocation decisions are determined based on each operating segment’s income from operations. The operating segments are described further below:
Patient Care - This segment consists of (i) our owned and operated patient care clinics, Dosteon, and CARES, and (ii) our contracting and network management business. Dosteon is presented as a discontinued operation and has therefore been excluded from the summarized financial information below. See Note S - “Discontinued Operations” within these consolidated financial statements. CARES was closed in 2015. The patient care clinics provide services to design and fit O&P devices to patients. These clinics also instruct patients in the use, care and maintenance of the devices. The principal reimbursement sources for our services are:
· Commercial private payors and other, which consist of individuals, rehabilitation providers, commercial insurance companies, HMOs, PPOs, hospitals, vocational rehabilitation, workers’ compensation programs and similar sources;
· Medicare, a federally funded health insurance program providing health insurance coverage for persons aged 65 or older and certain disabled persons, which provides reimbursement for O&P products and services based on prices set forth in published fee schedules with 10 regional pricing areas for prosthetics and orthotics and by state for durable medical equipment;
· Medicaid, a health insurance program jointly funded by federal and state governments providing health insurance coverage for certain persons in financial need, regardless of age, which may supplement Medicare benefits for financially needy persons aged 65 or older; and
· U.S. Department of Veterans Affairs.
Our contract and network management business, known as Linkia, is the only network management company dedicated solely to serving the O&P market and is focused on managing the O&P services of national and regional insurance companies. We partner with healthcare insurance companies by securing a national or regional contract either as a preferred provider or to manage their O&P network of providers.
Products & Services - This segment consists of our distribution business, which distributes and fabricates O&P products and components to sell to both the O&P industry and our own patient care clinics, and our therapeutic solutions business. The therapeutic solutions business leases and sells rehabilitation equipment and ancillary consumable supplies combined with equipment maintenance, education, and training. This segment also develops emerging neuromuscular technologies for the O&P and rehabilitation markets.
Corporate & Other - This consists of corporate overhead and includes unallocated expense such as personnel costs, professional fees and corporate offices expenses.
The accounting policies of the segments are the same as those described in Note B - “Significant Accounting Policies.”
Intersegment revenue primarily relates to sales of O&P components from the Products & Services segment to the Patient Care segment. The sales are priced at the cost of the related materials plus overhead.
We had no foreign and export sales and assets for the years ended December 31, 2017, 2016 and 2015.
For the Patient Care segment, government reimbursement, comprised of Medicare, Medicaid and the U.S. Department of Veterans Affairs, in the aggregate, accounted for approximately, 54.8%, 54.1% and 53.4% of their net revenue in 2017, 2016 and 2015, respectively.
Additionally, for the Products & Services segment, no single customer accounted for more than 10% of net revenues in 2017, 2016 or 2015, respectively.
Summarized financial information concerning our reporting segments is shown in the following tables.
|
(in thousands) |
|
Patient Care |
|
Products & |
|
Corporate & |
|
Consolidating |
|
Total |
| |||||
|
2017 |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Net revenue |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Third party |
|
$ |
851,973 |
|
$ |
188,796 |
|
$ |
— |
|
$ |
— |
|
$ |
1,040,769 |
|
|
Intersegments |
|
— |
|
178,768 |
|
— |
|
(178,768 |
) |
— |
| |||||
|
Total net revenue |
|
851,973 |
|
367,564 |
|
— |
|
(178,768 |
) |
1,040,769 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Material costs |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Third party suppliers |
|
228,091 |
|
101,132 |
|
— |
|
— |
|
329,223 |
| |||||
|
Intersegments |
|
23,808 |
|
154,960 |
|
— |
|
(178,768 |
) |
— |
| |||||
|
Total material costs |
|
251,899 |
|
256,092 |
|
— |
|
(178,768 |
) |
329,223 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Personnel costs |
|
312,695 |
|
48,395 |
|
— |
|
— |
|
361,090 |
| |||||
|
Other expenses |
|
143,598 |
|
25,855 |
|
106,695 |
|
— |
|
276,148 |
| |||||
|
Depreciation & amortization |
|
21,363 |
|
10,163 |
|
7,733 |
|
— |
|
39,259 |
| |||||
|
Impairment of intangible assets |
|
— |
|
54,735 |
|
— |
|
— |
|
54,735 |
| |||||
|
Income (loss) from operations |
|
122,418 |
|
(27,676 |
) |
(114,428 |
) |
— |
|
(19,686 |
) | |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Interest expense, net |
|
32,068 |
|
13,196 |
|
12,424 |
|
— |
|
57,688 |
| |||||
|
Income (loss) from continuing operations before income taxes |
|
90,350 |
|
(40,872 |
) |
(126,852 |
) |
— |
|
(77,374 |
) | |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Provision for income taxes |
|
— |
|
— |
|
27,297 |
|
— |
|
27,297 |
| |||||
|
Income (loss) from continuing operations |
|
$ |
90,350 |
|
$ |
(40,872 |
) |
$ |
(154,149 |
) |
$ |
— |
|
$ |
(104,671 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Total assets |
|
$ |
413,759 |
|
$ |
97,536 |
|
$ |
129,128 |
|
$ |
— |
|
$ |
640,423 |
|
|
Purchase of property, plant and equipment |
|
8,163 |
|
2,153 |
|
6,039 |
|
— |
|
16,355 |
| |||||
|
(in thousands) |
|
Patient Care |
|
Products & |
|
Corporate |
|
Consolidating |
|
Total |
| |||||
|
2016 |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Net revenue |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Third party |
|
$ |
840,130 |
|
$ |
201,924 |
|
$ |
— |
|
$ |
— |
|
$ |
1,042,054 |
|
|
Intersegments |
|
— |
|
175,539 |
|
— |
|
(175,539 |
) |
— |
| |||||
|
Total net revenue |
|
840,130 |
|
377,463 |
|
— |
|
(175,539 |
) |
1,042,054 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Material costs |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Third party suppliers |
|
230,957 |
|
101,114 |
|
— |
|
— |
|
332,071 |
| |||||
|
Intersegments |
|
25,055 |
|
150,484 |
|
— |
|
(175,539 |
) |
— |
| |||||
|
Total material costs |
|
256,012 |
|
251,598 |
|
— |
|
(175,539 |
) |
332,071 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Personnel costs |
|
315,892 |
|
47,645 |
|
— |
|
— |
|
363,537 |
| |||||
|
Other expenses |
|
150,604 |
|
32,228 |
|
104,649 |
|
— |
|
287,481 |
| |||||
|
Depreciation and amortization |
|
24,873 |
|
11,600 |
|
8,414 |
|
— |
|
44,887 |
| |||||
|
Impairment of intangible assets |
|
— |
|
86,164 |
|
— |
|
— |
|
86,164 |
| |||||
|
Income (loss) from operations |
|
92,749 |
|
(51,772 |
) |
(113,063 |
) |
— |
|
(72,086 |
) | |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Interest expense (income), net |
|
33,081 |
|
13,097 |
|
(979 |
) |
— |
|
45,199 |
| |||||
|
Extinguishment of debt |
|
— |
|
— |
|
6,031 |
|
— |
|
6,031 |
| |||||
|
Income (loss) from continuing operations before income taxes |
|
59,668 |
|
(64,869 |
) |
(118,115 |
) |
— |
|
(123,316 |
) | |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Benefit for income taxes |
|
— |
|
— |
|
(15,910 |
) |
— |
|
(15,910 |
) | |||||
|
Income (loss) from continuing operations |
|
$ |
59,668 |
|
$ |
(64,869 |
) |
$ |
(102,205 |
) |
$ |
— |
|
$ |
(107,406 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Total assets |
|
$ |
419,895 |
|
$ |
159,354 |
|
$ |
175,855 |
|
$ |
— |
|
$ |
755,104 |
|
|
Purchase of property, plant and equipment |
|
14,581 |
|
820 |
|
5,747 |
|
— |
|
21,148 |
| |||||
|
(in thousands) |
|
Patient |
|
Products & |
|
Corporate |
|
Consolidating |
|
Total |
| |||||
|
2015 |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Net revenue |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Third party |
|
$ |
874,960 |
|
$ |
192,212 |
|
$ |
— |
|
$ |
— |
|
$ |
1,067,172 |
|
|
Intersegments |
|
— |
|
137,282 |
|
— |
|
(137,282 |
) |
— |
| |||||
|
Total net revenue |
|
874,960 |
|
329,494 |
|
— |
|
(137,282 |
) |
1,067,172 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Material costs |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Third party suppliers |
|
242,714 |
|
93,569 |
|
— |
|
— |
|
336,283 |
| |||||
|
Intersegments |
|
19,613 |
|
117,669 |
|
— |
|
(137,282 |
) |
— |
| |||||
|
Total material costs |
|
262,327 |
|
211,238 |
|
— |
|
(137,282 |
) |
336,283 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Personnel costs |
|
317,927 |
|
49,167 |
|
— |
|
— |
|
367,094 |
| |||||
|
Other expenses |
|
163,240 |
|
25,487 |
|
92,520 |
|
— |
|
281,247 |
| |||||
|
Depreciation and amortization |
|
25,674 |
|
11,883 |
|
8,786 |
|
— |
|
46,343 |
| |||||
|
Impairment of intangible assets |
|
382,860 |
|
2,947 |
|
— |
|
— |
|
385,807 |
| |||||
|
(Loss) income from operations |
|
(277,068 |
) |
28,772 |
|
(101,306 |
) |
— |
|
(349,602 |
) | |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Interest expense (income), net |
|
33,677 |
|
13,114 |
|
(16,899 |
) |
— |
|
29,892 |
| |||||
|
Extinguishment of debt |
|
— |
|
— |
|
7,237 |
|
— |
|
7,237 |
| |||||
|
(Loss) income from continuing operations before income taxes |
|
(310,745 |
) |
15,658 |
|
(91,644 |
) |
— |
|
(386,731 |
) | |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Benefit for income taxes |
|
— |
|
— |
|
(67,614 |
) |
— |
|
(67,614 |
) | |||||
|
(Loss) income from continuing operations |
|
$ |
(310,745 |
) |
$ |
15,658 |
|
$ |
(24,030 |
) |
$ |
— |
|
$ |
(319,117 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Total assets |
|
$ |
468,575 |
|
$ |
257,640 |
|
$ |
246,869 |
|
$ |
— |
|
$ |
973,084 |
|
|
Purchase of property, plant and equipment |
|
16,505 |
|
2,937 |
|
8,178 |
|
— |
|
27,620 |
| |||||
NOTE S — DISCONTINUED OPERATIONS
On November 5, 2014, the Audit Committee of the Board of Directors approved a plan to sell and/or otherwise dispose of the Dosteon distribution product group (“Dosteon”), a component of our Patient Care segment. This action was taken following the conclusion of our strategic evaluation of this business in the fourth quarter of 2014. In accordance with ASC 205-20, Presentation of Financial Statements - Discontinued Operations, ASC 360-10, Property, Plant and Equipment - Overall, and ASC 350-20, Intangibles - Goodwill and Other - Goodwill, the operating results and cash flows of Dosteon have been presented separately as discontinued operations in the consolidated statements of operations and comprehensive loss and the consolidated statements of cash flows, respectively, for the years ended December 31, 2016 and 2015. We had no activities related to discontinued operations in 2017.
The remaining portions of Dosteon businesses were sold in 2015 for aggregate cash proceeds of approximately $4.9 million. Associated with the disposal of these businesses, we recorded a $1.3 million loss on disposal and a $0.6 million inventory impairment loss associated with writing down the inventory to expected fair value within “Income (loss) from discontinued operations, net of income taxes” in 2015. Costs associated with exit and disposal related to Dosteon were immaterial in 2015 and 2016.
In 2016, $1.4 million of contingent consideration gains resulting from the disposal of Dosteon in prior years was recorded in “Income (loss) before income taxes from discontinued operations” in our consolidated statements of operations and comprehensive loss.
The following is a summary of our operating results for discontinued operations:
|
|
|
Year Ended December 31, |
| |||||||
|
(in thousands) |
|
2017 |
|
2016 |
|
2015 |
| |||
|
Net revenue |
|
$ |
— |
|
$ |
— |
|
$ |
5,547 |
|
|
|
|
|
|
|
|
|
| |||
|
Income (loss) before income taxes from discontinued operations |
|
— |
|
1,425 |
|
(11,223 |
) | |||
|
Income tax provision (benefit) |
|
— |
|
490 |
|
(3,249 |
) | |||
|
Income (loss) from discontinued operations, net of income taxes |
|
$ |
— |
|
$ |
935 |
|
$ |
(7,974 |
) |
NOTE T — SUPPLEMENTAL CASH FLOW INFORMATION
The supplemental disclosure requirements for the statements of cash flows are as follows:
|
|
|
Year Ended December 31, |
| |||||||
|
(in thousands) |
|
2017 |
|
2016 |
|
2015 |
| |||
|
Cash paid during the period for: |
|
|
|
|
|
|
| |||
|
Interest paid |
|
$ |
48,437 |
|
$ |
42,345 |
|
$ |
26,070 |
|
|
Income taxes paid (refunds received) |
|
725 |
|
(35,092 |
) |
18,106 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Non-cash financing and investing activities: |
|
|
|
|
|
|
| |||
|
Issuance of seller notes in connection with acquisitions |
|
— |
|
— |
|
4,662 |
| |||
|
Additions to property, plant and equipment acquired through financing obligations |
|
1,484 |
|
374 |
|
3,743 |
| |||
|
Retirements of financed property, plant and equipment and related financing obligations |
|
811 |
|
2,381 |
|
1,434 |
| |||
|
Purchase of property, plant and equipment in accounts payable |
|
2,119 |
|
728 |
|
2,746 |
| |||
NOTE U — SUBSEQUENT EVENTS
On March 6, 2018, we entered into the New Credit Agreement by and among us, the various financial institutions party thereto as lenders and issuers, and Bank of America, N.A., as agent. The New Credit Agreement provides for (i) a revolving credit facility with an initial maximum aggregate amount of availability of $100 million that matures in March 2023 and (ii) a $505 million term loan facility due in quarterly principal installments commencing June 29, 2018, with all remaining outstanding principal due at maturity in March 2025.
See Note N - “Long-term Debt” within these consolidated financial statements.
NOTE V — QUARTERLY FINANCIAL INFORMATION (UNAUDITED)
The tables below present summarized unaudited quarterly financial statements for the years ended December 31, 2017 and 2016. In lieu of filing Quarterly Reports on Form 10-Q for each quarter of 2017 and 2016, quarterly financial information is included in this report in the tables that follow. Amounts are computed independently each quarter, therefore, the sum of the quarterly amounts may not equal the total amount for the respective year due to rounding.
HANGER, INC.
QUARTERLY CONSOLIDATED BALANCE SHEETS
(dollars in thousands, except par value and share amounts)
(Unaudited)
|
|
|
As of |
|
As of |
|
As of |
| |||
|
|
|
March 31, |
|
June 30, |
|
September 30, |
| |||
|
|
|
2017 |
|
2017 |
|
2017 |
| |||
|
ASSETS |
|
|
|
|
|
|
| |||
|
Current assets: |
|
|
|
|
|
|
| |||
|
Cash and cash equivalents |
|
$ |
1,414 |
|
$ |
911 |
|
$ |
460 |
|
|
Net accounts receivable, less allowance for doubtful accounts of $13,611 at March 31, 2017, $13,109 at June 30, 2017, and $13,472 at September 30, 2017 |
|
125,776 |
|
133,646 |
|
134,424 |
| |||
|
Inventories |
|
72,878 |
|
72,338 |
|
74,095 |
| |||
|
Income taxes receivable |
|
12,351 |
|
12,855 |
|
13,784 |
| |||
|
Other current assets |
|
18,465 |
|
19,066 |
|
19,176 |
| |||
|
Total current assets |
|
230,884 |
|
238,816 |
|
241,939 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Non-current assets: |
|
|
|
|
|
|
| |||
|
Property, plant and equipment, net |
|
96,540 |
|
93,381 |
|
93,346 |
| |||
|
Goodwill |
|
249,678 |
|
249,678 |
|
249,678 |
| |||
|
Other intangible assets, net |
|
30,471 |
|
28,027 |
|
25,666 |
| |||
|
Deferred income taxes |
|
100,273 |
|
99,771 |
|
101,402 |
| |||
|
Other assets |
|
27,433 |
|
26,785 |
|
26,547 |
| |||
|
Total assets |
|
$ |
735,279 |
|
$ |
736,458 |
|
$ |
738,578 |
|
|
LIABILITIES AND SHAREHOLDERS’ EQUITY |
|
|
|
|
|
|
| |||
|
Current liabilities: |
|
|
|
|
|
|
| |||
|
Current portion of long-term debt |
|
$ |
27,398 |
|
$ |
21,476 |
|
$ |
13,129 |
|
|
Accounts payable |
|
57,491 |
|
49,654 |
|
50,337 |
| |||
|
Accrued expenses and other current liabilities |
|
78,482 |
|
70,731 |
|
69,383 |
| |||
|
Accrued interest payable |
|
447 |
|
393 |
|
539 |
| |||
|
Accrued compensation related costs |
|
16,991 |
|
29,928 |
|
29,220 |
| |||
|
Total current liabilities |
|
180,809 |
|
172,182 |
|
162,608 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Long-term liabilities: |
|
|
|
|
|
|
| |||
|
Long-term debt, less current portion |
|
456,663 |
|
462,339 |
|
474,434 |
| |||
|
Other liabilities |
|
49,316 |
|
48,911 |
|
49,103 |
| |||
|
Total liabilities |
|
686,788 |
|
683,432 |
|
686,145 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Shareholders’ Equity: |
|
|
|
|
|
|
| |||
|
Common stock, $.01 par value; 60,000,000 shares authorized, 36,402,093 shares, 36,478,597 shares, and 36,486,490 shares issued and 36,259,272 shares, 36,335,776 shares and 36,343,669 shares outstanding at March 31, June 30, and September 30, 2017, respectively |
|
364 |
|
365 |
|
365 |
| |||
|
Additional paid-in capital |
|
323,113 |
|
326,027 |
|
329,613 |
| |||
|
Accumulated other comprehensive loss |
|
(1,458 |
) |
(1,475 |
) |
(1,492 |
) | |||
|
Retained deficit |
|
(272,832 |
) |
(271,195 |
) |
(275,357 |
) | |||
|
Treasury stock, at cost 142,821 shares at March 31, June 30, and September 30, 2017, respectively |
|
(696 |
) |
(696 |
) |
(696 |
) | |||
|
Total shareholders’ equity |
|
48,491 |
|
53,026 |
|
52,433 |
| |||
|
Total liabilities and shareholders’ equity |
|
$ |
735,279 |
|
$ |
736,458 |
|
$ |
738,578 |
|
HANGER, INC.
QUARTERLY CONSOLIDATED BALANCE SHEETS
(dollars in thousands, except par value and share amounts)
(Unaudited)
|
|
|
As of |
|
As of |
|
As of |
| |||
|
|
|
March 31, |
|
June 30, |
|
September 30, |
| |||
|
|
|
2016 |
|
2016 |
|
2016 |
| |||
|
ASSETS |
|
|
|
|
|
|
| |||
|
Current assets: |
|
|
|
|
|
|
| |||
|
Cash and cash equivalents |
|
$ |
19,962 |
|
$ |
49,011 |
|
$ |
1,003 |
|
|
Net accounts receivable, less allowance for doubtful accounts of $16,296 at March 31, 2016, $15,241 at June 30, 2016, and $14,418 at September 30, 2016 |
|
149,816 |
|
145,605 |
|
139,203 |
| |||
|
Inventories |
|
69,163 |
|
71,092 |
|
74,563 |
| |||
|
Income taxes receivable |
|
41,066 |
|
41,736 |
|
10,999 |
| |||
|
Other current assets |
|
24,014 |
|
19,199 |
|
18,102 |
| |||
|
Total current assets |
|
304,021 |
|
326,643 |
|
243,870 |
| |||
|
Non-current assets: |
|
|
|
|
|
|
| |||
|
Property, plant and equipment, net |
|
109,124 |
|
105,286 |
|
102,485 |
| |||
|
Goodwill |
|
335,642 |
|
335,642 |
|
335,642 |
| |||
|
Other intangible assets, net |
|
43,178 |
|
39,232 |
|
35,630 |
| |||
|
Deferred income taxes |
|
98,254 |
|
98,254 |
|
98,254 |
| |||
|
Other assets |
|
23,680 |
|
25,413 |
|
25,582 |
| |||
|
Total assets |
|
$ |
913,899 |
|
$ |
930,470 |
|
$ |
841,463 |
|
|
LIABILITIES AND SHAREHOLDERS’ EQUITY |
|
|
|
|
|
|
| |||
|
Current liabilities: |
|
|
|
|
|
|
| |||
|
Current portion of long-term debt |
|
$ |
29,447 |
|
$ |
29,713 |
|
$ |
28,833 |
|
|
Accounts payable |
|
40,813 |
|
68,909 |
|
59,113 |
| |||
|
Accrued expenses and other current liabilities |
|
81,934 |
|
78,026 |
|
75,443 |
| |||
|
Accrued interest payable |
|
7,636 |
|
5,255 |
|
554 |
| |||
|
Accrued compensation related costs |
|
25,131 |
|
23,772 |
|
22,193 |
| |||
|
Total current liabilities |
|
184,961 |
|
205,675 |
|
186,136 |
| |||
|
Long-term liabilities: |
|
|
|
|
|
|
| |||
|
Long-term debt, less current portion |
|
525,521 |
|
519,952 |
|
457,021 |
| |||
|
Other liabilities |
|
55,304 |
|
54,925 |
|
53,803 |
| |||
|
Total liabilities |
|
765,786 |
|
780,552 |
|
696,960 |
| |||
|
Shareholders’ Equity: |
|
|
|
|
|
|
| |||
|
Common stock, $.01 par value; 60,000,000 shares authorized, 36,044,247 shares, 36,144,560 shares, and 36,158,347 shares issued and 35,901,426 shares, 36,001,739 shares and 36,015,526 shares outstanding at March 31, June 30, and September 30, 2015, respectively |
|
361 |
|
362 |
|
362 |
| |||
|
Additional paid-in capital |
|
315,908 |
|
318,022 |
|
320,065 |
| |||
|
Accumulated other comprehensive loss |
|
(1,394 |
) |
(1,376 |
) |
(1,356 |
) | |||
|
Retained earnings |
|
(166,066 |
) |
(166,394 |
) |
(173,872 |
) | |||
|
Treasury stock, at cost 142,821 shares at March 31, June 30, and September 30, 2016, respectively |
|
(696 |
) |
(696 |
) |
(696 |
) | |||
|
Total shareholders’ equity |
|
148,113 |
|
149,918 |
|
144,503 |
| |||
|
Total liabilities and shareholders’ equity |
|
$ |
913,899 |
|
$ |
930,470 |
|
$ |
841,463 |
|
HANGER, INC.
QUARTERLY CONSOLIDATED STATEMENTS OF OPERATIONS
AND COMPREHENSIVE (LOSS) INCOME
(dollars and shares in thousands, except per share amounts)
(Unaudited)
|
|
|
Three Months |
|
Three Months |
|
Six Months |
|
Three Months |
|
Nine Months |
|
Three Months |
| ||||||
|
|
|
March 31, |
|
June 30, |
|
June 30, |
|
September 30, |
|
September 30, |
|
December 31, |
| ||||||
|
|
|
2017 |
|
2017 |
|
2017 |
|
2017 |
|
2017 |
|
2017 |
| ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Net revenue |
|
$ |
233,681 |
|
$ |
263,386 |
|
$ |
497,067 |
|
$ |
257,966 |
|
$ |
755,033 |
|
$ |
285,736 |
|
|
Material costs |
|
74,405 |
|
83,657 |
|
158,062 |
|
82,345 |
|
240,407 |
|
88,816 |
| ||||||
|
Personnel costs |
|
87,955 |
|
87,831 |
|
175,786 |
|
90,065 |
|
265,851 |
|
95,239 |
| ||||||
|
Other operating costs |
|
32,689 |
|
31,861 |
|
64,550 |
|
33,184 |
|
97,734 |
|
32,097 |
| ||||||
|
General and administrative expenses |
|
25,570 |
|
25,411 |
|
50,981 |
|
25,540 |
|
76,521 |
|
33,557 |
| ||||||
|
Professional accounting and legal fees |
|
12,650 |
|
8,521 |
|
21,171 |
|
7,844 |
|
29,015 |
|
7,224 |
| ||||||
|
Depreciation and amortization |
|
10,137 |
|
9,825 |
|
19,962 |
|
9,632 |
|
29,594 |
|
9,665 |
| ||||||
|
Impairment of intangible assets |
|
— |
|
— |
|
— |
|
— |
|
— |
|
54,735 |
| ||||||
|
(Loss) income from operations |
|
(9,725 |
) |
16,280 |
|
6,555 |
|
9,356 |
|
15,911 |
|
(35,597 |
) | ||||||
|
Interest expense, net |
|
14,009 |
|
14,091 |
|
28,100 |
|
15,097 |
|
43,197 |
|
14,491 |
| ||||||
|
(Loss) income from continuing operations before income taxes |
|
(23,734 |
) |
2,189 |
|
(21,545 |
) |
(5,741 |
) |
(27,286 |
) |
(50,088 |
) | ||||||
|
(Benefit) provision for income taxes |
|
(6,000 |
) |
552 |
|
(5,448 |
) |
(1,580 |
) |
(7,028 |
) |
34,325 |
| ||||||
|
(Loss) income from continuing operations |
|
(17,734 |
) |
1,637 |
|
(16,097 |
) |
(4,161 |
) |
(20,258 |
) |
(84,413 |
) | ||||||
|
Net (loss) income |
|
$ |
(17,734 |
) |
$ |
1,637 |
|
$ |
(16,097 |
) |
$ |
(4,161 |
) |
$ |
(20,258 |
) |
$ |
(84,413 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Other comprehensive (loss) income: |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Unrealized loss on DB SERP, net of tax |
|
$ |
(17 |
) |
$ |
(17 |
) |
$ |
(34 |
) |
$ |
(17 |
) |
$ |
(51 |
) |
$ |
(195 |
) |
|
Comprehensive (loss) income |
|
$ |
(17,751 |
) |
$ |
1,620 |
|
$ |
(16,131 |
) |
$ |
(4,178 |
) |
$ |
(20,309 |
) |
$ |
(84,608 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Basic Per Common Share Data: |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Basic (loss) income per share |
|
$ |
(0.49 |
) |
$ |
0.05 |
|
$ |
(0.44 |
) |
$ |
(0.11 |
) |
$ |
(0.56 |
) |
$ |
(2.32 |
) |
|
Shares used to compute basic per common share amounts |
|
36,085 |
|
36,287 |
|
36,187 |
|
36,340 |
|
36,239 |
|
36,410 |
| ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Diluted Per Common Share Data: |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Diluted (loss) income per share |
|
$ |
(0.49 |
) |
$ |
0.04 |
|
$ |
(0.44 |
) |
$ |
(0.11 |
) |
$ |
(0.56 |
) |
$ |
(2.32 |
) |
|
Shares used to compute diluted per common share amounts |
|
36,085 |
|
36,544 |
|
36,187 |
|
36,340 |
|
36,239 |
|
36,410 |
| ||||||
HANGER, INC.
QUARTERLY CONSOLIDATED STATEMENTS OF OPERATIONS
AND COMPREHENSIVE LOSS
(dollars and shares in thousands, except per share amounts)
(Unaudited)
|
|
|
Three Months |
|
Three Months |
|
Six Months |
|
Three Months |
|
Nine Months |
|
Three Months |
| ||||||
|
|
|
March 31, |
|
June 30, |
|
June 30, |
|
September 30, |
|
September 30, |
|
December 31, |
| ||||||
|
|
|
2016 |
|
2016 |
|
2016 |
|
2016 |
|
2016 |
|
2016 |
| ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Net revenue |
|
$ |
236,461 |
|
$ |
264,456 |
|
$ |
500,917 |
|
$ |
260,084 |
|
$ |
761,001 |
|
$ |
281,053 |
|
|
Material costs |
|
76,700 |
|
82,971 |
|
159,671 |
|
85,437 |
|
245,108 |
|
86,963 |
| ||||||
|
Personnel costs |
|
89,138 |
|
88,406 |
|
177,544 |
|
89,113 |
|
266,657 |
|
96,880 |
| ||||||
|
Other operating costs |
|
36,761 |
|
31,970 |
|
68,731 |
|
34,139 |
|
102,870 |
|
36,154 |
| ||||||
|
General and administrative expenses |
|
27,558 |
|
30,170 |
|
57,728 |
|
25,726 |
|
83,454 |
|
23,770 |
| ||||||
|
Professional accounting and legal fees |
|
11,689 |
|
10,692 |
|
22,381 |
|
9,023 |
|
31,404 |
|
9,829 |
| ||||||
|
Depreciation and amortization |
|
11,728 |
|
11,660 |
|
23,388 |
|
11,339 |
|
34,727 |
|
10,160 |
| ||||||
|
Impairment of intangible assets |
|
— |
|
— |
|
— |
|
— |
|
— |
|
86,164 |
| ||||||
|
(Loss) income from operations |
|
(17,113 |
) |
8,587 |
|
(8,526 |
) |
5,307 |
|
(3,219 |
) |
(68,867 |
) | ||||||
|
Interest expense, net |
|
8,838 |
|
9,818 |
|
18,656 |
|
12,809 |
|
31,465 |
|
13,734 |
| ||||||
|
Loss on extinguishment of debt |
|
— |
|
(10 |
) |
(10 |
) |
6,041 |
|
6,031 |
|
— |
| ||||||
|
Loss from continuing operations |
|
(25,951 |
) |
(1,221 |
) |
(27,172 |
) |
(13,543 |
) |
(40,715 |
) |
(82,601 |
) | ||||||
|
Benefit for income taxes |
|
(8,414 |
) |
(321 |
) |
(8,735 |
) |
(5,687 |
) |
(14,422 |
) |
(1,488 |
) | ||||||
|
Loss from continuing operations |
|
(17,537 |
) |
(900 |
) |
(18,437 |
) |
(7,856 |
) |
(26,293 |
) |
(81,113 |
) | ||||||
|
Income (loss) from discontinued operations, net of income taxes |
|
— |
|
572 |
|
572 |
|
378 |
|
950 |
|
(15 |
) | ||||||
|
Net loss |
|
$ |
(17,537 |
) |
$ |
(328 |
) |
$ |
(17,865 |
) |
$ |
(7,478 |
) |
$ |
(25,343 |
) |
$ |
(81,128 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Other comprehensive loss: |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Unrealized gain (loss) on DB SERP, net of tax |
|
$ |
19 |
|
$ |
19 |
|
$ |
38 |
|
$ |
19 |
|
$ |
57 |
|
$ |
(84 |
) |
|
Comprehensive loss |
|
$ |
(17,518 |
) |
$ |
(309 |
) |
$ |
(17,827 |
) |
$ |
(7,459 |
) |
$ |
(25,286 |
) |
$ |
(81,212 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Basic and Diluted Per Common Share Data: |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Loss from continuing operations |
|
$ |
(0.49 |
) |
$ |
(0.03 |
) |
$ |
(0.51 |
) |
$ |
(0.22 |
) |
$ |
(0.73 |
) |
$ |
(2.25 |
) |
|
Income from discontinued operations, net of income taxes |
|
— |
|
0.02 |
|
0.01 |
|
0.01 |
|
0.02 |
|
— |
| ||||||
|
Basic loss per share |
|
$ |
(0.49 |
) |
$ |
(0.01 |
) |
$ |
(0.50 |
) |
$ |
(0.21 |
) |
$ |
(0.71 |
) |
$ |
(2.25 |
) |
|
Shares used to compute basic per common share amounts |
|
35,742 |
|
35,949 |
|
35,846 |
|
36,008 |
|
35,900 |
|
36,032 |
| ||||||
HANGER, INC.
QUARTERLY CONSOLIDATED STATEMENTS OF CASH FLOWS
(dollars in thousands)
(Unaudited)
|
|
|
Three Months Ended |
|
Six Months Ended |
|
Nine Months Ended |
| |||
|
|
|
March 31, 2017 |
|
June 30, 2017 |
|
September 30, 2017 |
| |||
|
Cash flows from operating activities: |
|
|
|
|
|
|
| |||
|
Loss from continuing operations |
|
$ |
(17,734 |
) |
$ |
(16,097 |
) |
$ |
(20,258 |
) |
|
|
|
|
|
|
|
|
| |||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
| |||
|
Depreciation and amortization |
|
10,137 |
|
19,962 |
|
29,594 |
| |||
|
Provision for doubtful accounts |
|
2,360 |
|
4,517 |
|
6,850 |
| |||
|
Stock-based compensation expense |
|
2,164 |
|
5,080 |
|
8,693 |
| |||
|
Benefit for deferred income taxes |
|
(6,050 |
) |
(5,548 |
) |
(7,179 |
) | |||
|
Amortization of debt issuance costs |
|
1,952 |
|
3,874 |
|
6,348 |
| |||
|
Gain on sale and disposal of fixed assets |
|
(672 |
) |
(1,196 |
) |
(1,664 |
) | |||
|
|
|
|
|
|
|
|
| |||
|
Changes in operating assets and liabilities, net of effects of acquired companies: |
|
|
|
|
|
|
| |||
|
Net accounts receivable |
|
15,367 |
|
5,061 |
|
1,772 |
| |||
|
Inventories |
|
(4,653 |
) |
(4,113 |
) |
(5,870 |
) | |||
|
Other current assets |
|
39 |
|
947 |
|
1,510 |
| |||
|
Income taxes |
|
849 |
|
345 |
|
(584 |
) | |||
|
Accounts payable |
|
6,333 |
|
(1,267 |
) |
(348 |
) | |||
|
Accrued expenses and accrued interest payable |
|
(683 |
) |
(8,488 |
) |
(9,690 |
) | |||
|
Accrued compensation related costs |
|
(19,171 |
) |
(6,234 |
) |
(6,942 |
) | |||
|
Other liabilities |
|
(1,901 |
) |
(3,176 |
) |
(3,240 |
) | |||
|
Net cash used in operating activities - continuing operations |
|
(11,663 |
) |
(6,333 |
) |
(1,008 |
) | |||
|
|
|
|
|
|
|
|
| |||
|
Cash flows from investing activities: |
|
|
|
|
|
|
| |||
|
Purchase of property, plant and equipment |
|
(2,348 |
) |
(6,433 |
) |
(11,237 |
) | |||
|
Purchase of equipment leased to third parties under operating leases |
|
(629 |
) |
(1,333 |
) |
(3,714 |
) | |||
|
Restricted cash |
|
12 |
|
24 |
|
56 |
| |||
|
Purchase of company-owned life insurance investment |
|
(555 |
) |
(555 |
) |
(555 |
) | |||
|
Proceeds from sale of property, plant and equipment |
|
2,179 |
|
3,216 |
|
4,185 |
| |||
|
Net cash used in investing activities - continuing operations |
|
(1,341 |
) |
(5,081 |
) |
(11,265 |
) | |||
|
|
|
|
|
|
|
|
| |||
|
Cash flows from financing activities: |
|
|
|
|
|
|
| |||
|
Repayment of term loan |
|
(5,625 |
) |
(11,250 |
) |
(19,688 |
) | |||
|
Borrowings under revolving credit agreement |
|
49,500 |
|
110,000 |
|
140,965 |
| |||
|
Repayments under revolving credit agreement |
|
(31,500 |
) |
(85,000 |
) |
(105,965 |
) | |||
|
Payment of employee taxes on stock-based compensation |
|
(1,338 |
) |
(1,339 |
) |
(1,367 |
) | |||
|
Payment on seller’s note and other contingent consideration |
|
(3,498 |
) |
(3,808 |
) |
(4,633 |
) | |||
|
Payment of capital lease obligations |
|
(278 |
) |
(572 |
) |
(873 |
) | |||
|
Payment of debt issuance costs and fees |
|
— |
|
(2,863 |
) |
(2,863 |
) | |||
|
Net cash provided by financing activities - continuing operations |
|
7,261 |
|
5,168 |
|
5,576 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Decrease in cash and cash equivalents |
|
(5,743 |
) |
(6,246 |
) |
(6,697 |
) | |||
|
Cash and cash equivalents, at beginning of year |
|
7,157 |
|
7,157 |
|
7,157 |
| |||
|
Cash and cash equivalents, at end of period |
|
$ |
1,414 |
|
$ |
911 |
|
$ |
460 |
|
|
|
|
|
|
|
|
|
| |||
|
SUPPLEMENTAL CASH FLOW FINANCIAL INFORMATION: |
|
|
|
|
|
|
| |||
|
|
|
|
|
|
|
|
| |||
|
Cash paid during the period for: |
|
|
|
|
|
|
| |||
|
Interest |
|
$ |
12,218 |
|
$ |
24,387 |
|
$ |
36,807 |
|
|
Income taxes (refunds received) paid |
|
(849 |
) |
(345 |
) |
584 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Non-cash financing and investing activities: |
|
|
|
|
|
|
| |||
|
Additions to property, plant and equipment acquired through finance obligations |
|
623 |
|
669 |
|
1,299 |
| |||
|
Retirements of financed property, plant and equipment |
|
— |
|
135 |
|
135 |
| |||
|
Purchase of property, plant and equipment in accounts payable |
|
1,337 |
|
1,100 |
|
865 |
| |||
HANGER, INC.
QUARTERLY CONSOLIDATED STATEMENTS OF CASH FLOWS
(dollars in thousands)
(Unaudited)
|
|
|
Three Months Ended |
|
Six Months Ended |
|
Nine Months Ended |
| |||
|
|
|
March 31, 2016 |
|
June 30, 2016 |
|
September 30, 2016 |
| |||
|
Cash flows from operating activities: |
|
|
|
|
|
|
| |||
|
Net loss |
|
$ |
(17,537 |
) |
$ |
(17,865 |
) |
$ |
(25,343 |
) |
|
Income from discontinued operations, net of income taxes |
|
— |
|
572 |
|
950 |
| |||
|
Loss from continuing operations |
|
(17,537 |
) |
(18,437 |
) |
(26,293 |
) | |||
|
|
|
|
|
|
|
|
| |||
|
Adjustments to reconcile net loss to net cash (used in) provided by operating activities: |
|
|
|
|
|
|
| |||
|
Depreciation and amortization |
|
11,728 |
|
23,388 |
|
34,727 |
| |||
|
Provision for doubtful accounts |
|
4,249 |
|
5,780 |
|
7,546 |
| |||
|
Stock-based compensation expense |
|
2,948 |
|
5,425 |
|
7,552 |
| |||
|
Amortization of debt issuance costs |
|
766 |
|
1,618 |
|
2,845 |
| |||
|
Loss (gain) on extinguishment of debt |
|
— |
|
(10 |
) |
6,031 |
| |||
|
Gain on sale and disposal of fixed assets |
|
(704 |
) |
(1,213 |
) |
(1,901 |
) | |||
|
|
|
|
|
|
|
|
| |||
|
Changes in operating assets and liabilities, net of effects of acquired companies: |
|
|
|
|
|
|
| |||
|
Net accounts receivable |
|
20,522 |
|
23,102 |
|
28,336 |
| |||
|
Inventories |
|
(685 |
) |
(2,614 |
) |
(6,085 |
) | |||
|
Other current assets |
|
(2,055 |
) |
(602 |
) |
421 |
| |||
|
Income taxes |
|
(8,640 |
) |
(9,671 |
) |
20,994 |
| |||
|
Accounts payable |
|
(13,287 |
) |
14,608 |
|
4,259 |
| |||
|
Accrued expenses and accrued interest payable |
|
6,560 |
|
393 |
|
(6,881 |
) | |||
|
Accrued compensation related costs |
|
(23,037 |
) |
(24,396 |
) |
(25,975 |
) | |||
|
Other liabilities |
|
(223 |
) |
(1,230 |
) |
(2,060 |
) | |||
|
Net cash (used in) provided by operating activities - continuing operations |
|
(19,395 |
) |
16,141 |
|
43,516 |
| |||
|
Net cash used in operating activities - discontinued operations |
|
— |
|
(850 |
) |
(1,425 |
) | |||
|
Net cash (used in) provided by operating activities |
|
(19,395 |
) |
15,291 |
|
42,091 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Cash flows from investing activities: |
|
|
|
|
|
|
| |||
|
Purchase of property, plant and equipment |
|
(6,364 |
) |
(9,963 |
) |
(13,943 |
) | |||
|
Purchase of equipment leased to third parties under operating leases |
|
(608 |
) |
(1,190 |
) |
(1,914 |
) | |||
|
Restricted cash |
|
4 |
|
2,676 |
|
2,716 |
| |||
|
Purchase of company-owned life insurance investment |
|
(2,543 |
) |
(2,543 |
) |
(2,543 |
) | |||
|
Proceeds from sale of property, plant and equipment |
|
1,136 |
|
1,922 |
|
2,647 |
| |||
|
Other investing activities, net |
|
(10 |
) |
(10 |
) |
(10 |
) | |||
|
Net cash used in investing activities - continuing operations |
|
(8,385 |
) |
(9,108 |
) |
(13,047 |
) | |||
|
Net cash provided by investing activities - discontinued operations |
|
— |
|
850 |
|
1,425 |
| |||
|
Net cash used in investing activities |
|
(8,385 |
) |
(8,258 |
) |
(11,622 |
) | |||
|
|
|
|
|
|
|
|
| |||
|
Cash flows from financing activities: |
|
|
|
|
|
|
| |||
|
Borrowings under term loan |
|
— |
|
— |
|
274,400 |
| |||
|
Repayment of term loan |
|
(4,219 |
) |
(8,438 |
) |
(14,063 |
) | |||
|
Borrowings under revolving credit agreement |
|
— |
|
— |
|
20,000 |
| |||
|
Repayments under revolving credit agreement |
|
— |
|
— |
|
(144,000 |
) | |||
|
Payment of senior notes |
|
— |
|
— |
|
(200,000 |
) | |||
|
Payment of employee taxes on stock-based compensation |
|
(259 |
) |
(259 |
) |
(271 |
) | |||
|
Payment on seller notes and other contingent consideration |
|
(4,536 |
) |
(5,817 |
) |
(7,751 |
) | |||
|
Payment of capital lease obligations |
|
(230 |
) |
(494 |
) |
(702 |
) | |||
|
Payment of debt issuance costs and fees |
|
(1,767 |
) |
(1,767 |
) |
(15,832 |
) | |||
|
Net cash used in financing activities - continuing operations |
|
(11,011 |
) |
(16,775 |
) |
(88,219 |
) | |||
|
Decrease in cash and cash equivalents |
|
(38,791 |
) |
(9,742 |
) |
(57,750 |
) | |||
|
Cash and cash equivalents, at beginning of year |
|
58,753 |
|
58,753 |
|
58,753 |
| |||
|
Cash and cash equivalents, at end of period |
|
$ |
19,962 |
|
$ |
49,011 |
|
$ |
1,003 |
|
|
|
|
|
|
|
|
|
| |||
|
SUPPLEMENTAL CASH FLOW FINANCIAL INFORMATION: |
|
|
|
|
|
|
| |||
|
|
|
|
|
|
|
|
| |||
|
Cash paid during the period for: |
|
|
|
|
|
|
| |||
|
Interest |
|
$ |
3,759 |
|
$ |
15,111 |
|
$ |
30,850 |
|
|
Income taxes paid (refunds received) |
|
168 |
|
1,077 |
|
(33,821 |
) | |||
|
|
|
|
|
|
|
|
| |||
|
Non-cash financing and investing activities: |
|
|
|
|
|
|
| |||
|
Additions to property, plant and equipment acquired through finance obligations |
|
162 |
|
213 |
|
269 |
| |||
|
Retirements of financed property, plant and equipment |
|
1,663 |
|
2,157 |
|
2,228 |
| |||
|
Purchase of property, plant and equipment in accounts payable |
|
747 |
|
998 |
|
1,900 |
| |||
|
Exhibit |
|
Document |
|
3.1 |
|
|
|
3.2 |
|
|
|
3.3 |
|
|
|
4.1 |
|
|
|
4.2 |
|
|
|
4.3 |
|
|
|
4.4 |
|
|
|
4.5 |
|
|
|
4.6 |
|
|
|
4.7 |
|
|
|
4.8 |
|
|
|
4.9 |
|
|
|
4.10 |
|
|
|
4.11 |
|
|
|
4.12 |
|
|
|
4.13 |
|
|
|
4.14 |
|
|
|
4.15 |
|
|
10.1 |
|
|
|
10.2 |
|
|
|
10.3 |
|
|
|
10.4 |
|
|
|
10.5 |
|
|
|
10.6 |
|
|
|
10.7 |
|
|
|
10.8 |
|
|
|
10.9 |
|
|
|
10.10 |
|
|
|
10.11 |
|
|
|
10.12 |
|
|
|
10.13 |
|
|
|
10.14 |
|
|
|
10.15 |
|
|
|
10.16 |
|
|
|
10.17 |
|
|
|
10.18 |
|
|
|
10.19 |
|
|
|
10.20 |
|
|
|
10.21 |
|
|
|
10.22 |
|
|
10.23 |
|
|
|
10.24 |
|
|
|
10.25 |
|
|
|
10.26 |
|
|
|
10.27 |
|
|
|
10.28 |
|
|
|
10.29 |
|
|
|
10.30 |
|
|
|
10.31 |
|
|
|
21 |
|
|
|
31.1 |
|
|
|
31.2 |
|
|
|
32 |
|
|
|
101.INS |
|
XBRL Instance Document. (Filed herewith.) |
|
101.SCH |
|
XBRL Taxonomy Extension Schema. (Filed herewith.) |
|
101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase. (Filed herewith.) |
|
101.LAB |
|
XBRL Taxonomy Extension Label Linkbase. (Filed herewith.) |
|
101.PRE |
|
XBRL Taxonomy Extension Presentation Linkbase. (Filed herewith.) |
|
101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase. (Filed herewith.) |
* Management contract or compensatory plan
