Attached files
| file | filename |
|---|---|
| 8-K - 8-K - GLAUKOS Corp | f8-k.htm |
Exhibit 99.1
|
|
March 2018 1 © 2018 Glaukos Corporation 1 |
|
|
Disclaimer All statements other than statements of historical facts included in this presentation that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements. Although we believe that we have a reasonable basis for forward-looking statements contained herein, we caution you that they are based on current expectations about future events affecting us and are subject to risks, uncertainties and factors relating to our operations and business environment, all of which are difficult to predict and many of which are beyond our control, that may cause our actual results to differ materially from those expressed or implied by forward-looking statements in this presentation. These potential risks and uncertainties include, without limitation, uncertainties about our ability to maintain profitability; our dependence on the success and market acceptance of the iStent®; our ability to leverage our sales and marketing infrastructure to increase market penetration and acceptance both in the United States and internationally of our products; our dependence on a limited number of third-party suppliers for components of our products; the occurrence of a crippling accident, natural disaster or other disruption at our primary facility, which may materially affect our manufacturing capacity and operations; maintaining adequate coverage or reimbursement by third-party payors for procedures using the iStent or other products in development; our ability to properly train, and gain acceptance and trust from, ophthalmic surgeons in the use of our products; our ability to successfully develop and commercialize additional products; our ability to compete effectively in the highly competitive and rapidly changing medical device industry and against current and future competitors (including MIGS competitors) that are large public companies or divisions of publicly traded companies that have competitive advantages; the timing, effect and expense of navigating different regulatory approval processes as we develop additional products and penetrate foreign markets; the impact of any product liability claims against us and any related litigation; the effect of the extensive and increasing federal and state regulation in the healthcare industry on us and our suppliers; the lengthy and expensive clinical trial process and the uncertainty of outcomes from any particular clinical trial; our ability to protect, and the expense and time-consuming nature of protecting, our intellectual property against third parties and competitors that could develop and commercialize similar or identical products; the impact of any claims against us of infringement or misappropriation of third party intellectual property rights and any related litigation; and the market’s perception of our limited operating history as a public company. These and other known risks, uncertainties and factors are described in detail under the caption “Risk Factors” and elsewhere in our filings with the Securities and Exchange Commission, including our Annual Report on Form 10-K for 2017. Our filings with the Securities and Exchange Commission are available in the Investor Section of our website at www.glaukos.com or at www.sec.gov. In addition, information about the risks and benefits of our products is available on our website at www.glaukos.gov.All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on the forward-looking statements in this press release, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise, except as may be required under applicable securities law. 2 © 2018 Glaukos Corporation |
|
|
Glaukos is Transforming Glaucoma Therapy OUR MISSION To pioneer and lead the global glaucoma market micro-scale injectable therapies that advance the with standard-of-care and enrich the lives and treatment alternatives for glaucoma patients worldwide. 3 © 2018 Glaukos Corporation |
|
|
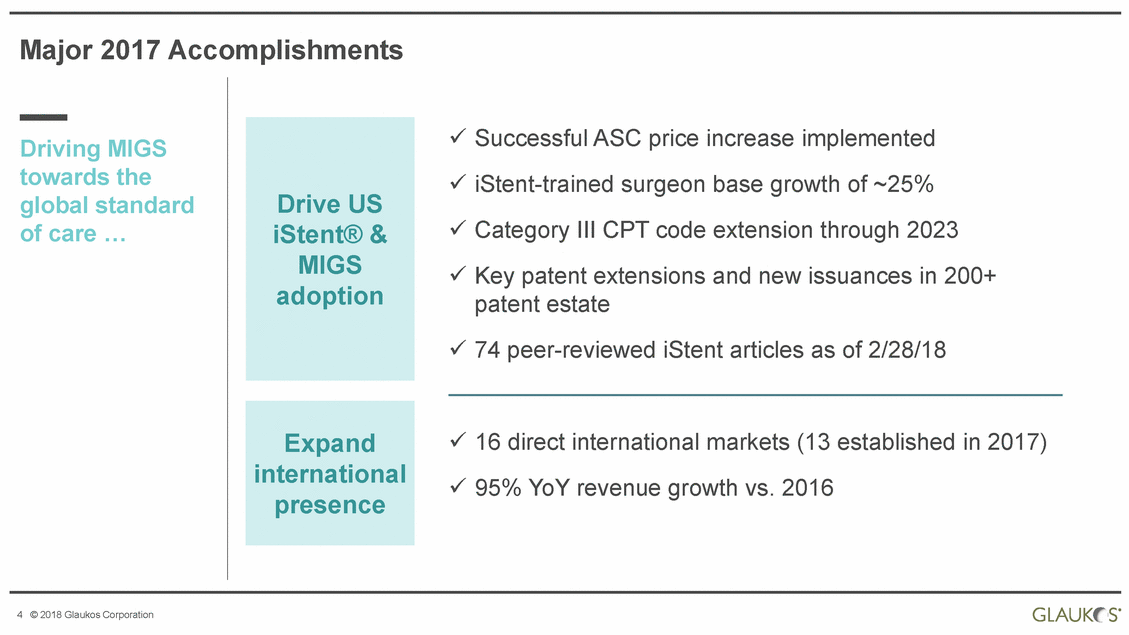
Major 2017 Accomplishments Successful ASC price increase implemented Driving MIGS towards the global standard iStent-trained surgeon Category III CPT code Key patent extensions patent estate base growth of ~25% extension through 2023 and new issuances in 200+ of care … 74 peer-reviewed iStent articles as of 2/28/18 16 direct international markets (13 established 95% YoY revenue growth vs. 2016 in 2017) 4 © 2018 Glaukos Corporation Expand international presence Drive US iStent® & MIGS adoption |
|
|
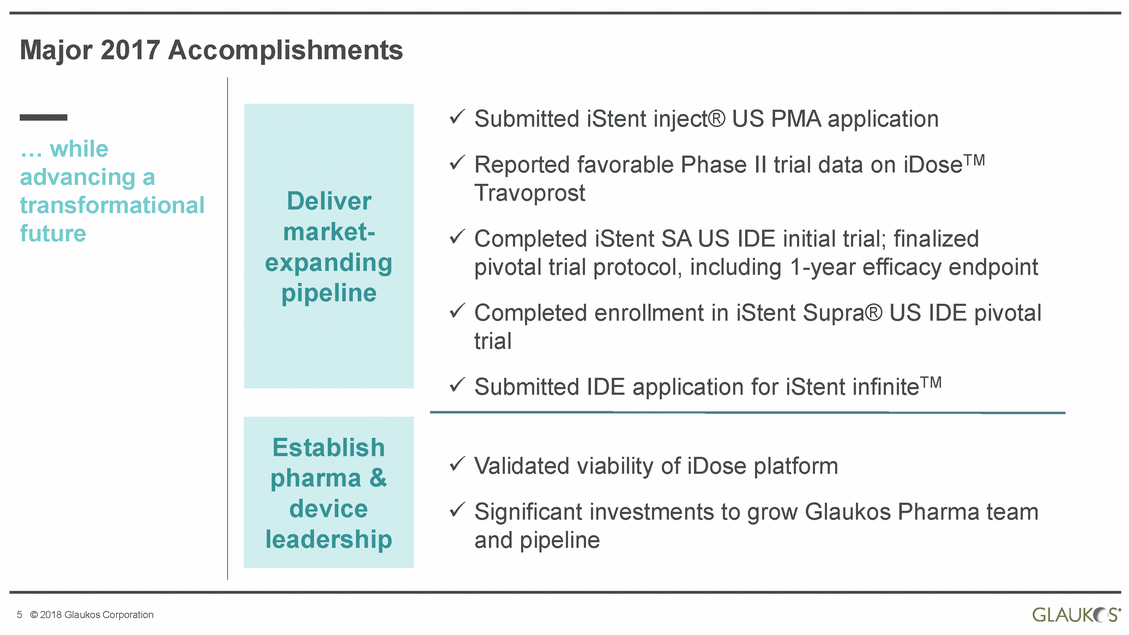
Major 2017 Accomplishments Submitted iStent inject® US PMA application Reported favorable Phase II trial data on iDoseTM Travoprost … while advancing a transformational future Completed pivotal trial Completed trial iStent SA US IDE initial trial; finalized protocol, including 1-year efficacy endpoint enrollment in iStent Supra® US IDE pivotal Submitted IDE application for iStent infiniteTM Validated viability of iDose platform Significant investments to grow Glaukos Pharma and pipeline team 5 © 2018 Glaukos Corporation Establish pharma & device leadership Deliver market-expanding pipeline |
|
|
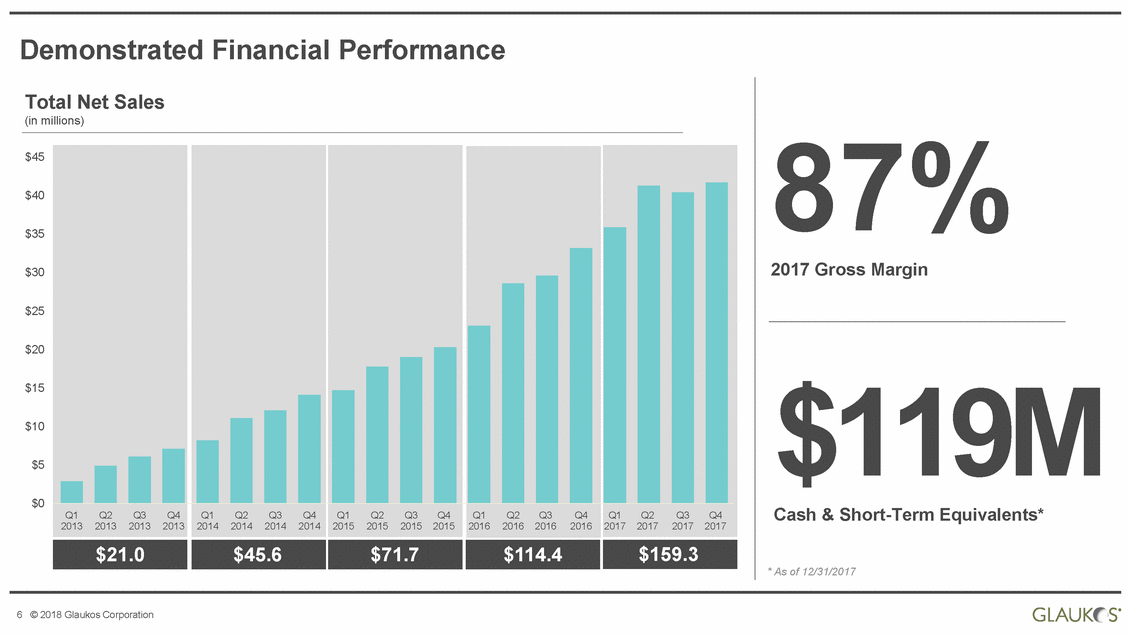
Demonstrated Financial Performance Total Net (in millions) Sales 87% 2017 Gross Margin $45 $40 $35 $30 $25 $20 $119M Cash & Short-Term Equivalents* $15 $10 $5 $0 * As of 12/31/2017 6 © 2018 Glaukos Corporation Q1Q2Q3Q4 2013201320132013 Q1Q2Q3Q4 2014201420142014 Q1Q2Q3Q4 2015201520152015 Q1Q2Q3Q4 2016201620162016 Q1Q2Q3Q4 2017201720172017 $45.6 $71.7 $114.4 $159.3 $21.0 |
|
|
M I G S A N D B E Y O N D Delivering Novel Surgical & Pharmaceutical Glaucoma Therapy 7 © 2018 Glaukos Corporation |
|
|
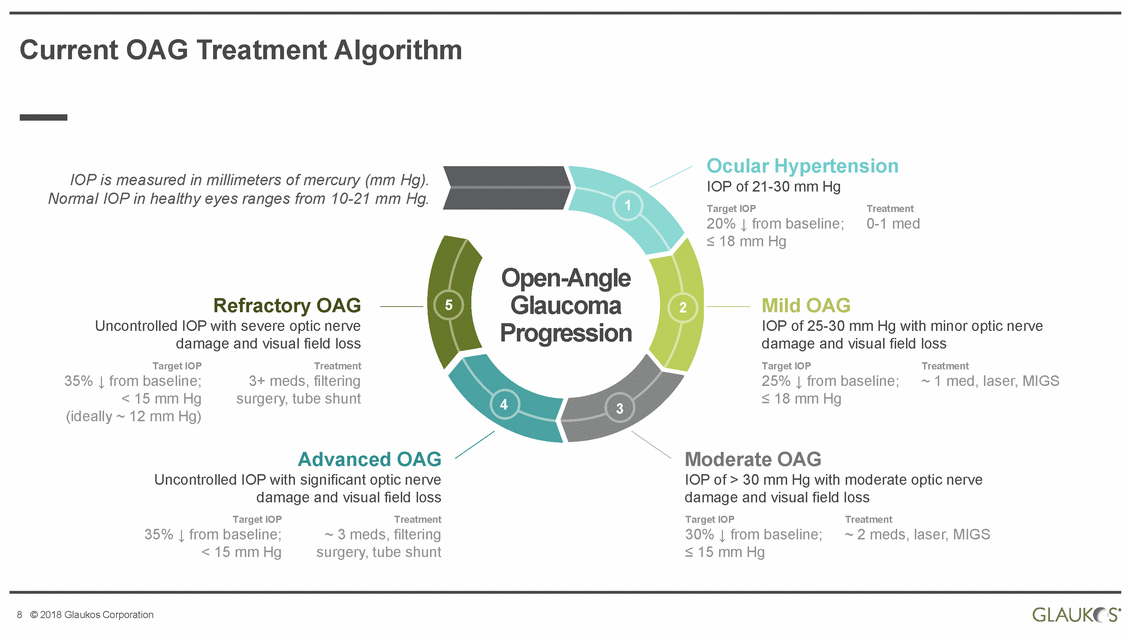
Current OAG Treatment Algorithm Ocular Hypertension IOP of 21-30 mm Hg IOP is measured in millimeters of mercury (mm Hg). Normal IOP in healthy eyes ranges from 10-21 mm Hg. 1 Target IOP 20% ↓ from baseline; ≤ 18 mm Hg Treatment 0-1 med Open-Angle Glaucoma Progression Refractory OAG Uncontrolled IOP with severe optic nerve damage and visual field loss Mild OAG IOP of 25-30 mm Hg with minor optic nerve damage and visual field loss 5 2 Target IOP 35% ↓ from baseline; < 15 mm Hg (ideally ~ 12 mm Hg) Treatment 3+ meds, filtering surgery, tube shunt Target IOP 25% ↓ from baseline; ≤ 18 mm Hg Treatment ~ 1 med, laser, MIGS 4 3 Advanced OAG Moderate OAG IOP of > 30 mm Hg with moderate optic nerve damage and visual field loss Uncontrolled IOP with significant optic nerve damage and visual field loss Target IOP 35% ↓ from baseline; < 15 mm Hg Treatment ~ 3 meds, filtering surgery, tube shunt Target IOP 30% ↓ from baseline; ≤ 15 mm Hg Treatment ~ 2 meds, laser, MIGS 8 © 2018 Glaukos Corporation |
|
|
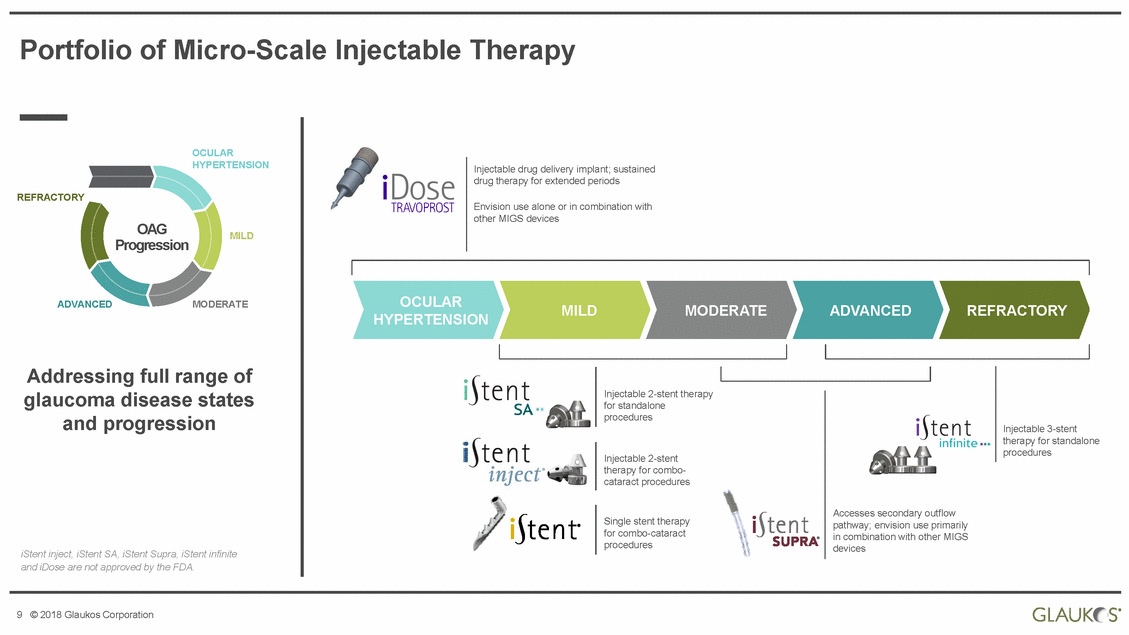
Portfolio of Micro-Scale Injectable Therapy OCULAR HYPERTENSION Injectable drug delivery implant; sustained drug therapy for extended periods REFRACTORY Envision use alone or in combination with other MIGS devices OAG Progression MILD OCULAR HYPERTENSION ADVANCED MODERATE MILD MODERATE ADVANCED REFRACTORY Addressing full range of glaucoma disease states and progression Injectable 2-stent therapy for standalone procedures Injectable 3-stent therapy for standalone procedures Injectable 2-stent therapy for combo-cataract procedures Accesses secondary outflow pathway; envision use primarily in combination with other MIGS devices Single stent therapy for combo-cataract procedures iStent inject, iStent SA, iStent Supra, iStent infinite and iDose are not approved by the FDA. 9 © 2018 Glaukos Corporation |
|
|
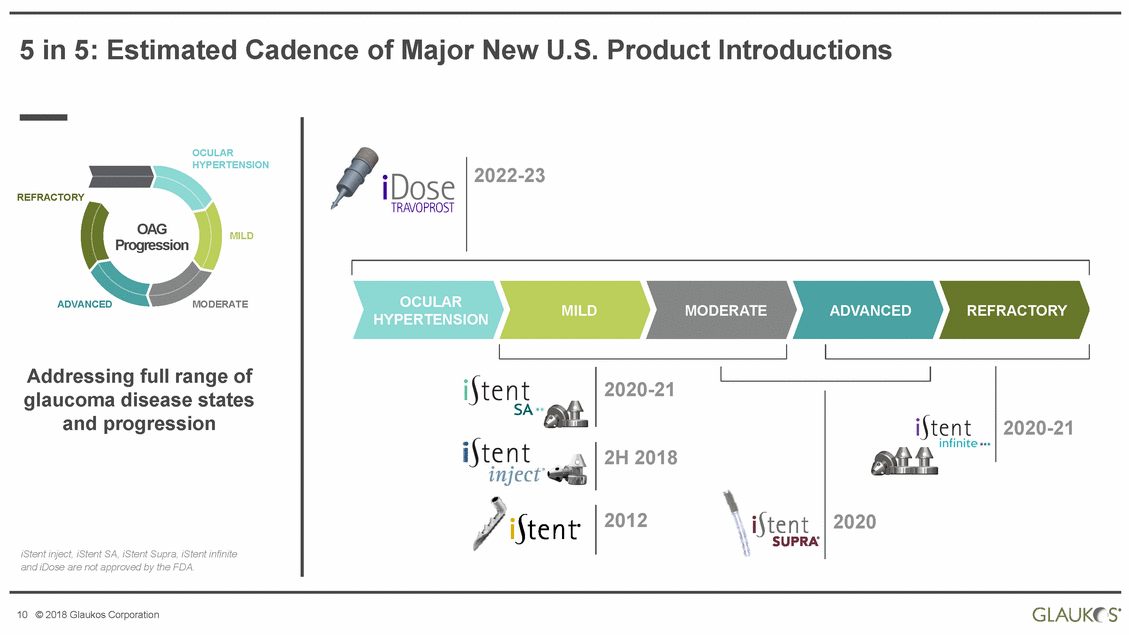
5 in 5: Estimated Cadence of Major New U.S. Product Introductions OCULAR HYPERTENSION 2022-23 REFRACTORY OAG Progression MILD OCULAR HYPERTENSION ADVANCED MODERATE MILD MODERATE ADVANCED REFRACTORY Addressing full range of glaucoma disease states and progression 2020-21 2020-21 2H 2018 2012 2020 iStent inject, iStent SA, iStent Supra, iStent infinite and iDose are not approved by the FDA. 10 © 2018 Glaukos Corporation |
|
|
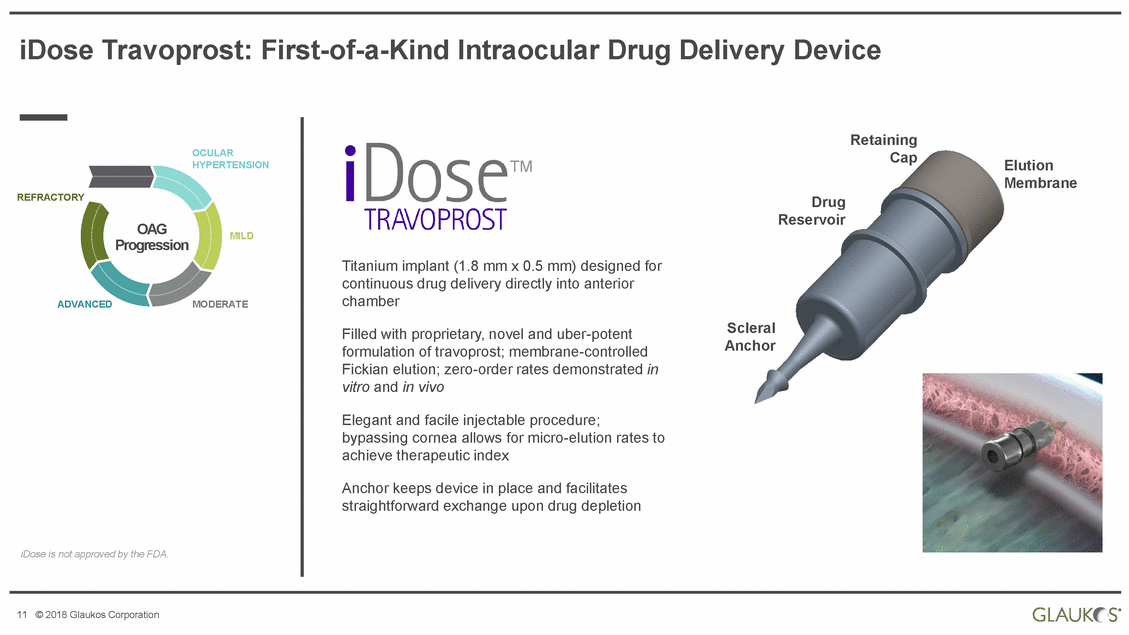
iDose Travoprost: First-of-a-Kind Intraocular Drug Delivery Device Retaining Cap OCULAR HYPERTENSION TM Elution Membrane REFRACTORY Drug Reservoir OAG Progression MILD Titanium implant (1.8 mm x 0.5 mm) designed for continuous drug delivery directly into anterior chamber ADVANCED MODERATE Scleral Anchor Filled with proprietary, novel and uber-potent formulation of travoprost; membrane-controlled Fickian elution; zero-order rates demonstrated in vitro and in vivo Elegant and facile injectable procedure; bypassing cornea allows for micro-elution rates to achieve therapeutic index Anchor keeps device in place and facilitates straightforward exchange upon drug depletion iDose is not approved by the FDA. 11 © 2018 Glaukos Corporation |
|
|
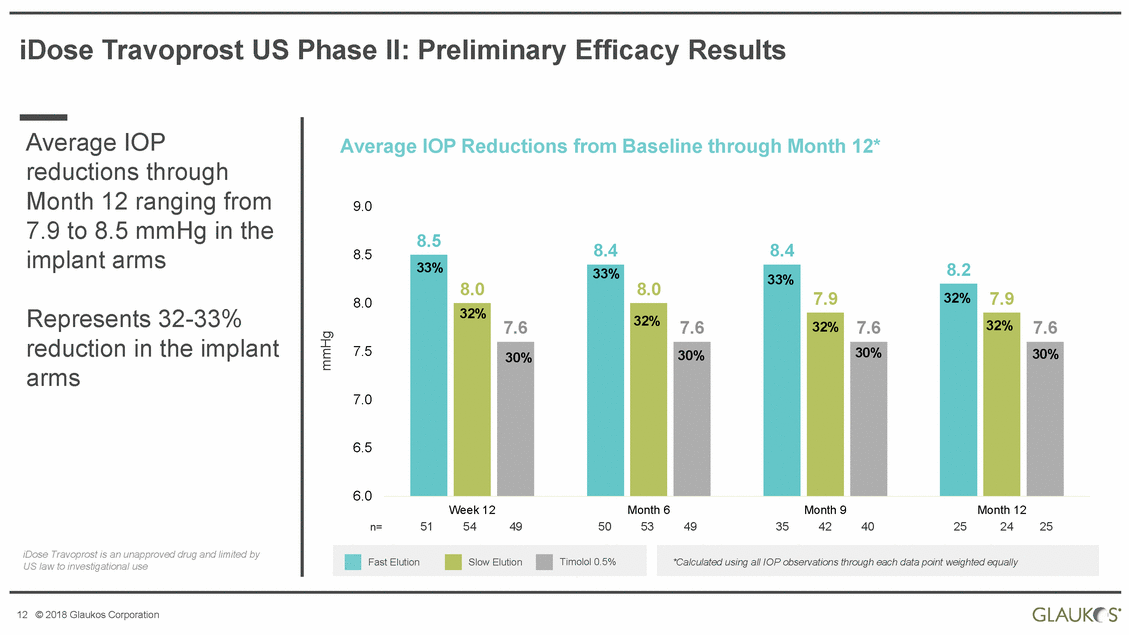
iDose Travoprost US Phase II: Preliminary Efficacy Results Average IOP reductions through Month 12 ranging from 7.9 to 8.5 mmHg in the implant arms Average IOP Reductions from Baseline through Month 12* 9.0 8.5 8.4 8.4 8.5 8.2 7.9 8.0 Represents 32-33% 7.6 reduction arms in the implant 7.5 7.0 6.5 6.0 Week 12 54 Month 6 53 Month 9 42 Month 12 24 n= 51 49 50 49 35 40 25 25 iDose Travoprost is an unapproved drug and limited by US law to investigational use 12 © 2018 Glaukos Corporation mmHg *Calculated using all IOP observations through each data point weighted equally Fast ElutionSlow ElutionTimolol 0.5% 33% 8.0 32%7.6 30% 33% 8.0 32%7.6 30% 33% 7.9 32%7.6 30% 32% 32% 30% |
|
|
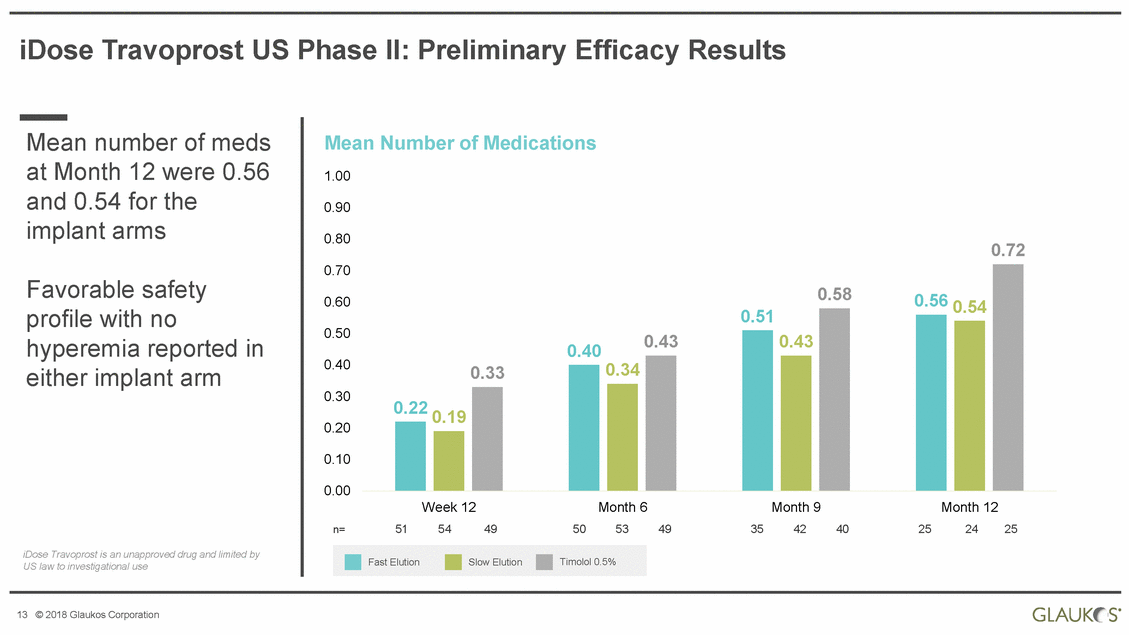
iDose Travoprost US Phase II: Preliminary Efficacy Results Mean number of meds Mean 1.00 Number of Medications at Month 12 were and 0.54 for the implant arms 0.56 0.90 0.80 0.72 0.70 Favorable safety profile with no hyperemia reported either implant arm 0.60 0.50 in 0.40 0.30 0.20 0.10 0.00 Week 12 54 Month 6 53 Month 9 42 Month 12 24 51 49 50 49 35 40 25 25 n= iDose Travoprost is an unapproved drug and limited by US law to investigational use 13 © 2018 Glaukos Corporation Fast ElutionSlow ElutionTimolol 0.5% 0.580.56 0.54 0.51 0.400.430.43 0.330.34 0.22 0.19 |
|
|
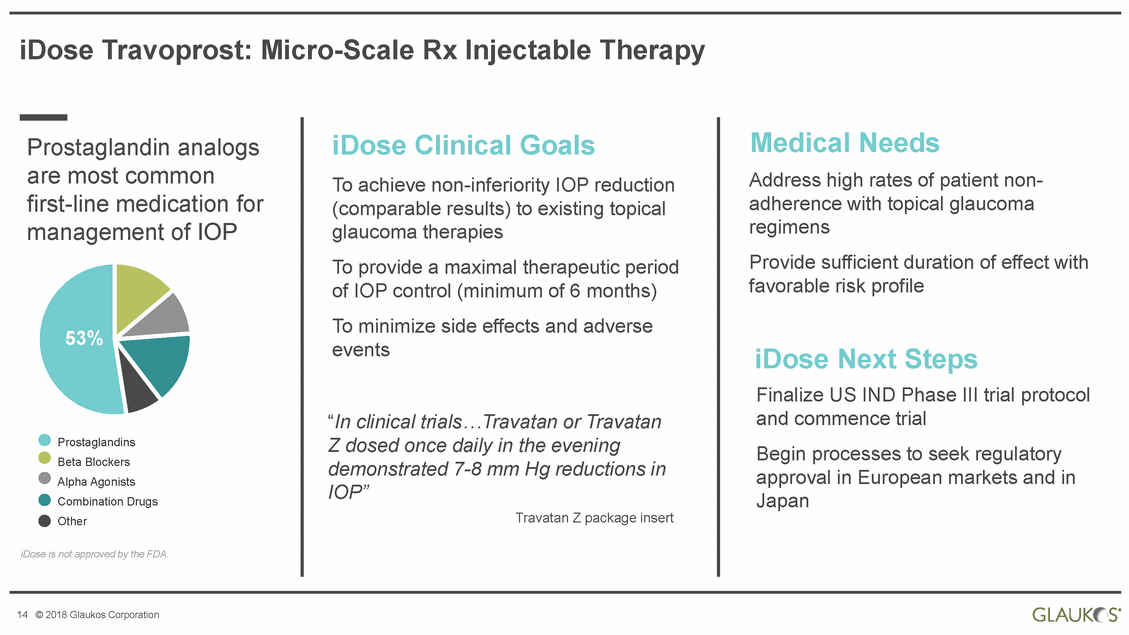
iDose Travoprost: Micro-Scale Rx Injectable Therapy Medical Needs Address high rates of patient non-adherence with topical glaucoma regimens Provide sufficient duration of effect favorable risk profile iDose Clinical Goals To achieve non-inferiority IOP reduction (comparable results) to existing topical glaucoma therapies To provide a maximal therapeutic period of IOP control (minimum of 6 months) To minimize side effects and adverse events Prostaglandin analogs are most common first-line medication for management of IOP with 53% iDose Next Steps Finalize US IND Phase III trial protocol and commence trial Begin processes to seek regulatory approval in European markets and in Japan “In clinical trials…Travatan or Travatan Z dosed once daily in the evening Prostaglandins Beta Blockers Alpha Agonists Combination Drugs Other demonstrated 7-8 mm Hg reductions IOP” in Travatan Z package insert iDose is not approved by the FDA. 14 © 2018 Glaukos Corporation |
|
|
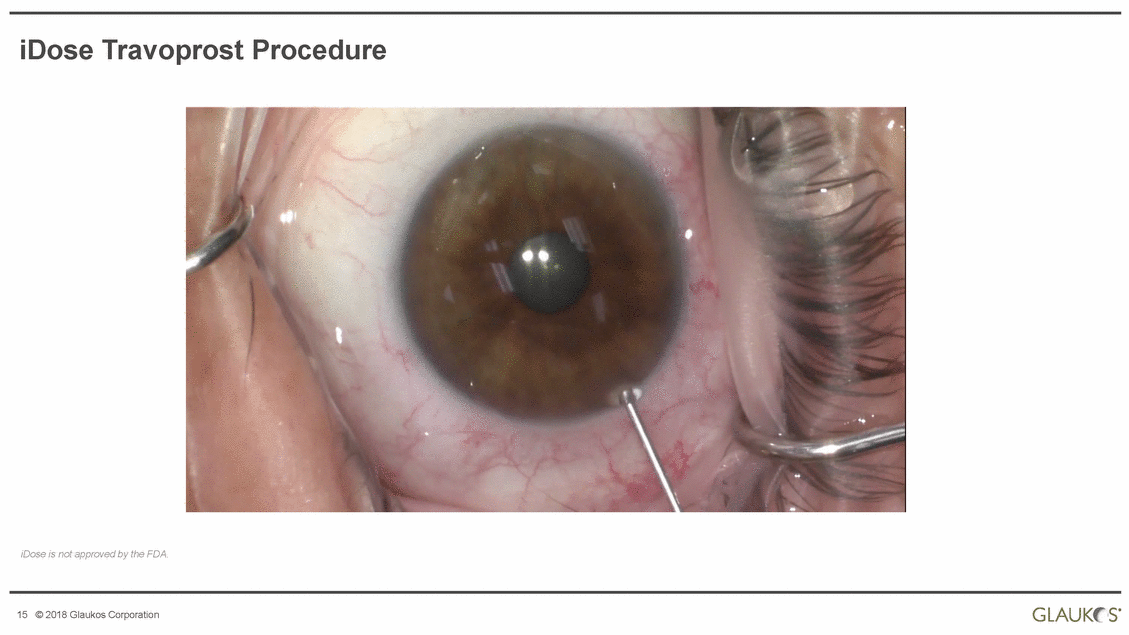
iDose Travoprost Procedure iDose is not approved by the FDA. s· GLAUK! 15 © 2018 Glaukos Corporation |
|
|
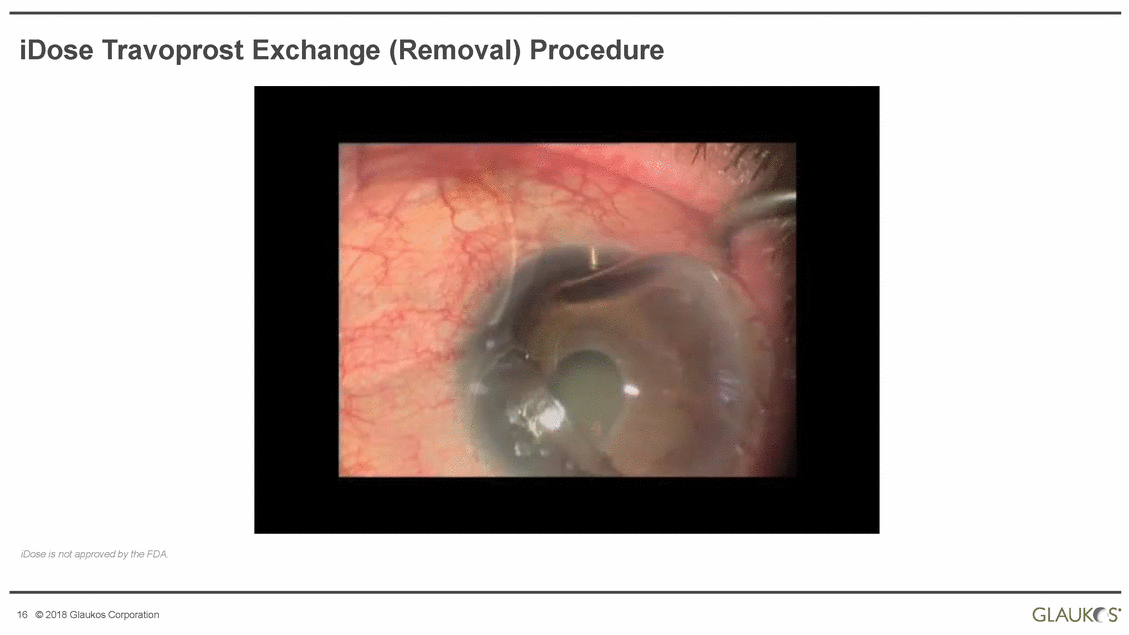
iDose Travoprost Exchange (Removal) Procedure iDose is not approved by the FDA. s· GLAUK! 16 © 2018 Glaukos Corporation |
|
|
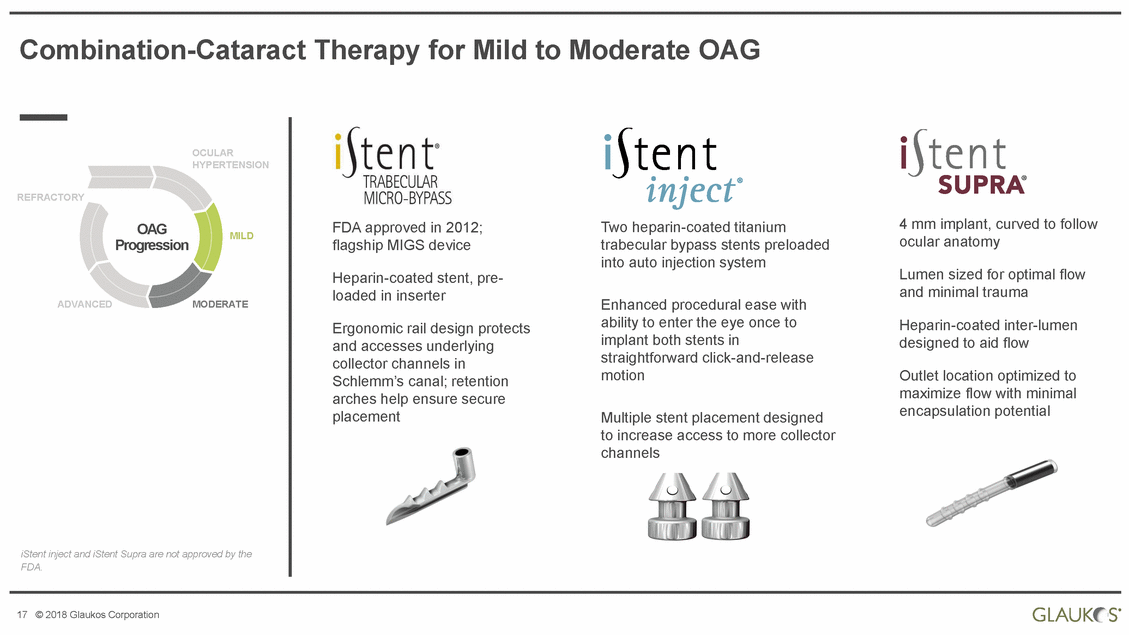
Combination-Cataract Therapy for Mild to Moderate OAG OCULAR HYPERTENSION REFRACTORY 4 mm implant, curved to follow ocular anatomy FDA approved in 2012; flagship MIGS device Two heparin-coated titanium trabecular bypass stents preloaded into auto injection system OAG Progression MILD Lumen sized for optimal flow and minimal trauma Heparin-coated stent, pre-loaded in inserter Enhanced procedural ease with ability to enter the eye once to implant both stents in straightforward click-and-release motion ADVANCED MODERATE Heparin-coated inter-lumen designed to aid flow Ergonomic rail design protects and accesses underlying collector channels in Schlemm’s canal; retention arches help ensure secure placement Outlet location optimized to maximize flow with minimal encapsulation potential Multiple stent placement designed to increase access to more collector channels iStent inject and iStent Supra are not approved by the FDA. 17 © 2018 Glaukos Corporation |
|
|
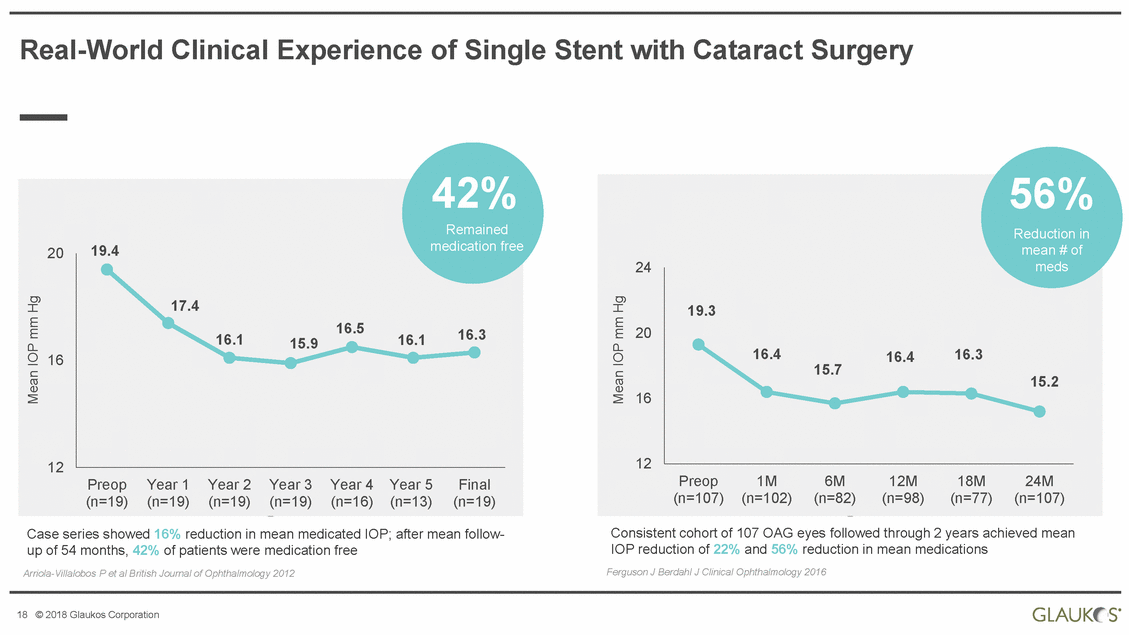
Real-World Clinical Experience of Single Stent with Cataract Surgery medication free 19.4 20 16 Preop Year 1 Year 2 Year 3 Year 4 Year 5 Final Consistent cohort of 107 OAG eyes followed through 2 years achieved mean IOP reduction of 22% and 56% reduction in mean medications Ferguson J Berdahl J Clinical Ophthalmology 2016 Case series showed 16% reduction in mean medicated IOP; after mean follow-up of 54 months, 42% of patients were medication free Arriola-Villalobos P et al British Journal of Ophthalmology 2012 18 © 2018 Glaukos Corporation Mean IOP mm Hg Mean IOP mm Hg 42% Remained 16.115.916.116.3 12 (n=19)(n=19)(n=19)(n=19)(n=16)(n=13)(n=19) 17.4 16.5 56% Reduction in mean # of 24meds 20 15.7 16 12 Preop1M6M12M18M24M (n=107)(n=102)(n=82)(n=98)(n=77)(n=107) 19.3 16.416.416.3 15.2 |
|
|
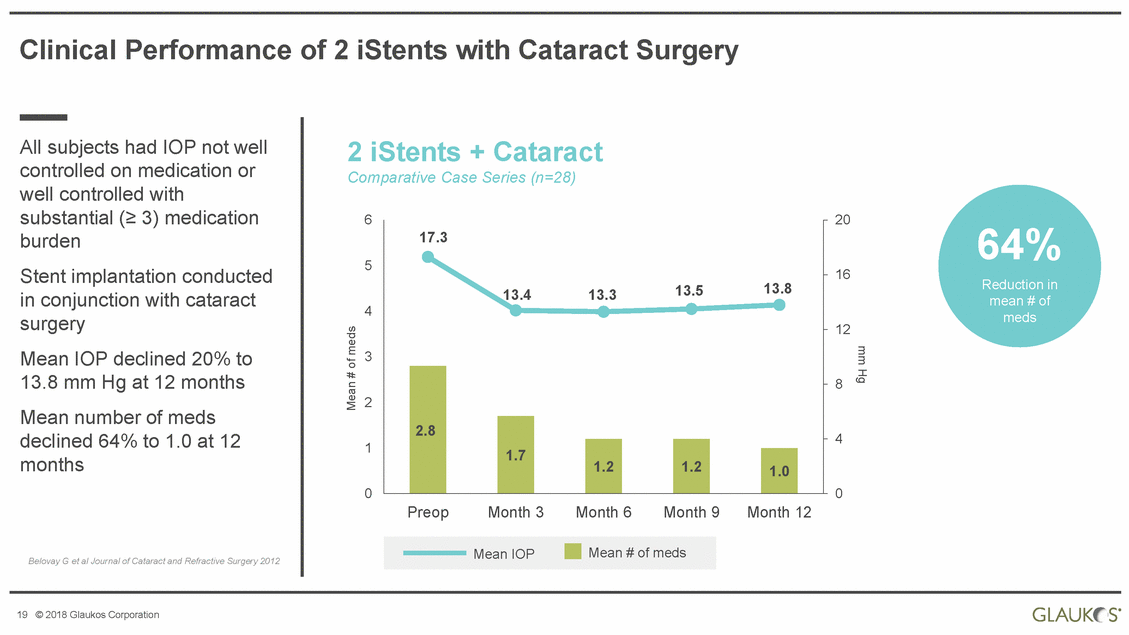
Clinical Performance of 2 iStents with Cataract Surgery All subjects had IOP not well controlled on medication or well controlled with substantial (≥ 3) medication burden Stent implantation conducted in conjunction with cataract surgery Mean IOP declined 20% to 13.8 mm Hg at 12 months Mean number of meds declined 64% to 1.0 at 12 months 2 iStents + Cataract Comparative Case Series (n=28) 6 20 64% Reduction in mean # of meds 5 16 4 12 3 8 2 4 1 0 0 Preop Month 3 Month 6 Month 9 Month 12 Belovay G et al Journal of Cataract and Refractive Surgery 2012 19 © 2018 Glaukos Corporation mm Hg Mean # of meds Mean IOPMean # of meds 17.3 13.413.313.513.8 2.8 1.7 1.2 1.2 1.0 |
|
|
Facile, Click-and-Release 2-Stent Procedure s· GLAUK! 20 © 2018 Glaukos Corporation |
|
|
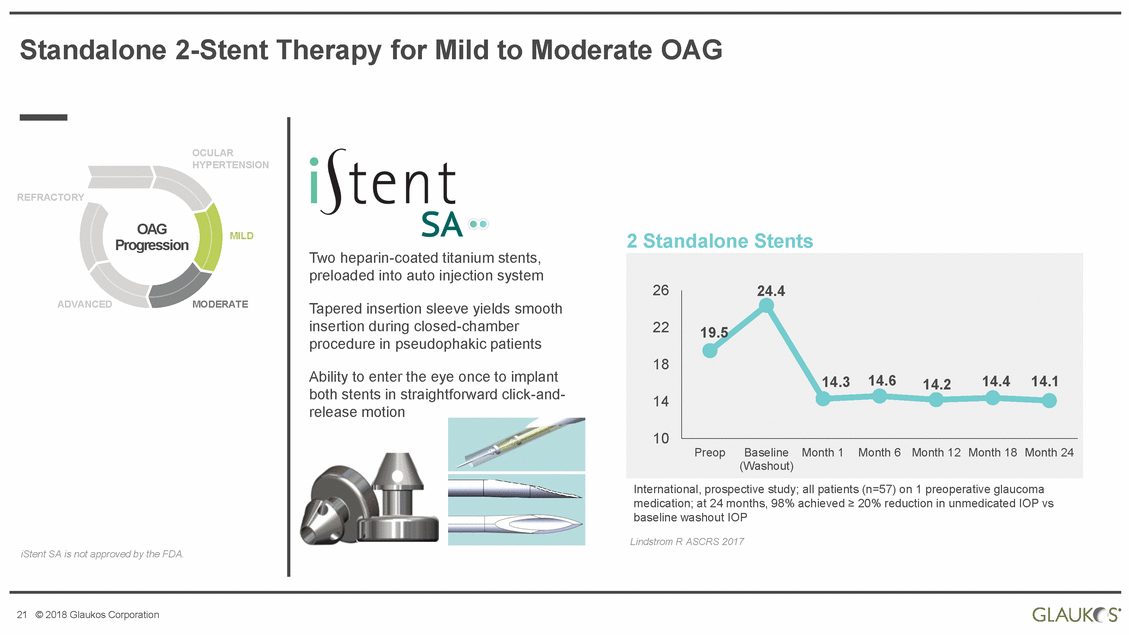
Standalone 2-Stent Therapy for Mild to Moderate OAG OCULAR HYPERTENSION REFRACTORY OAG Progression MILD 2 Standalone Stents Two heparin-coated titanium stents, preloaded into auto injection system ADVANCED MODERATE Tapered insertion sleeve yields smooth insertion during closed-chamber procedure in pseudophakic patients Ability to enter the eye once to implant both stents in straightforward click-and-release motion International, prospective study; all patients (n=57) on 1 preoperative glaucoma medication; at 24 months, 98% achieved ≥ 20% reduction in unmedicated IOP vs baseline washout IOP Lindstrom R ASCRS 2017 iStent SA is not approved by the FDA. 21 © 2018 Glaukos Corporation 2624.4 22 18 14 10 PreopBaselineMonth 1Month 6Month 12 Month 18 Month 24 (Washout) 19.5 14.314.614.214.414.1 |
|
|
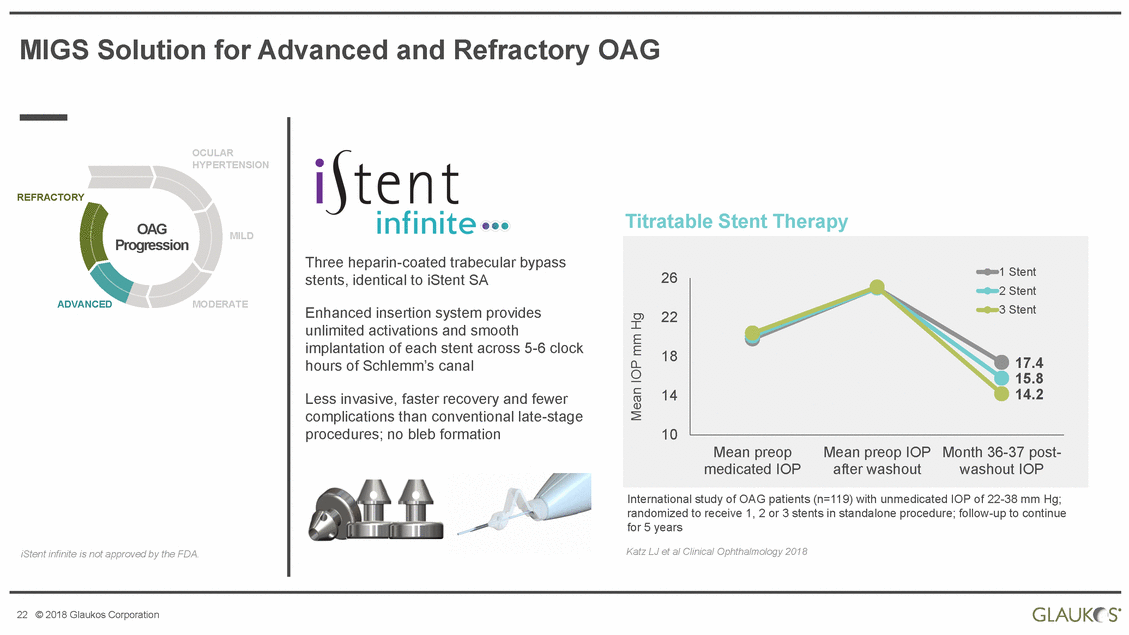
MIGS Solution for Advanced and Refractory OAG OCULAR HYPERTENSION REFRACTORY Titratable Stent Therapy OAG Progression MILD Three heparin-coated trabecular bypass stents, identical to iStent SA ADVANCED MODERATE Enhanced insertion system provides unlimited activations and smooth implantation of each stent across 5-6 clock hours of Schlemm’s canal Less invasive, faster recovery and fewer complications than conventional late-stage procedures; no bleb formation International study of OAG patients (n=119) with unmedicated IOP of 22-38 mm Hg; randomized to receive 1, 2 or 3 stents in standalone procedure; follow-up to continue for 5 years Katz LJ et al Clinical Ophthalmology 2018 iStent infinite is not approved by the FDA. 22 © 2018 Glaukos Corporation Mean IOP mm Hg 261 Stent 22 18 14 10 Mean preopMean preop IOPMonth 36-37 post-medicated IOPafter washoutwashout IOP 2 Stent 3 Stent 17.4 15.8 14.2 |
|
|
MIGS Solution for Advanced and Refractory OAG iStent infinite is not approved by the FDA. 23 © 2018 Glaukos Corporation |
|
|
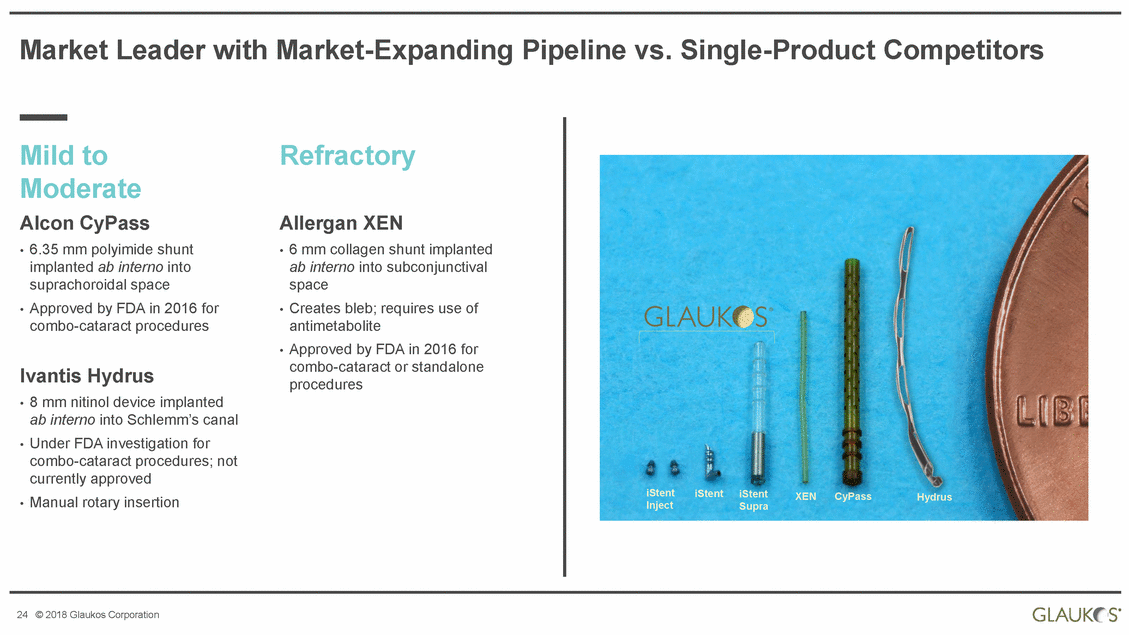
Market Leader with Market-Expanding Pipeline vs. Single-Product Competitors Mild to Moderate Alcon CyPass • 6.35 mm polyimide shunt implanted ab interno into suprachoroidal space • Approved by FDA in 2016 for combo-cataract procedures Refractory Allergan XEN 6 mm collagen shunt implanted ab interno into subconjunctival space Creates bleb; requires use of antimetabolite Approved by FDA in 2016 for combo-cataract or standalone procedures • • • Ivantis Hydrus • 8 mm nitinol device implanted ab interno into Schlemm’s canal • Under FDA investigation for combo-cataract procedures; not currently approved • Manual rotary insertion iStent Inject iStent iStent Supra XEN CyPass Hydrus 24 © 2018 Glaukos Corporation |
|
|
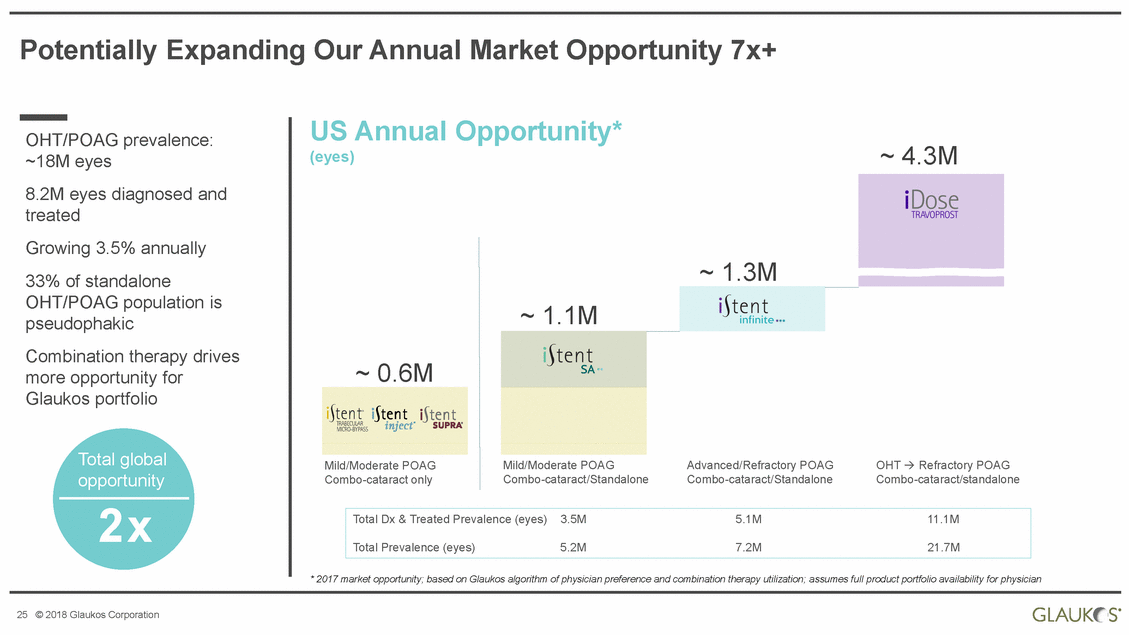
Potentially Expanding Our Annual Market Opportunity 7x+ US Annual (eyes) Opportunity* OHT/POAG prevalence: ~18M eyes 8.2M eyes diagnosed and treated Growing 3.5% annually 33% of standalone OHT/POAG population is pseudophakic ~ 4.3M Combination therapy more opportunity for Glaukos portfolio drives ~ 0.6M Total global opportunity Mild/Moderate POAG Combo-cataract only Mild/Moderate POAG Combo-cataract/Standalone Advanced/Refractory POAG Combo-cataract/Standalone OHT Refractory POAG Combo-cataract/standalone 2x * 2017 market opportunity; based on Glaukos algorithm of physician preference and combination therapy utilization; assumes full product portfolio availability for physician 25 © 2018 Glaukos Corporation Total Dx & Treated Prevalence (eyes)3.5M5.1M11.1M Total Prevalence (eyes)5.2M7.2M21.7M ~ 1.3M ~ 1.1M |
|
|
M I G S A N D B E Y O N D Platform Development 26 © 2018 Glaukos Corporation |
|
|
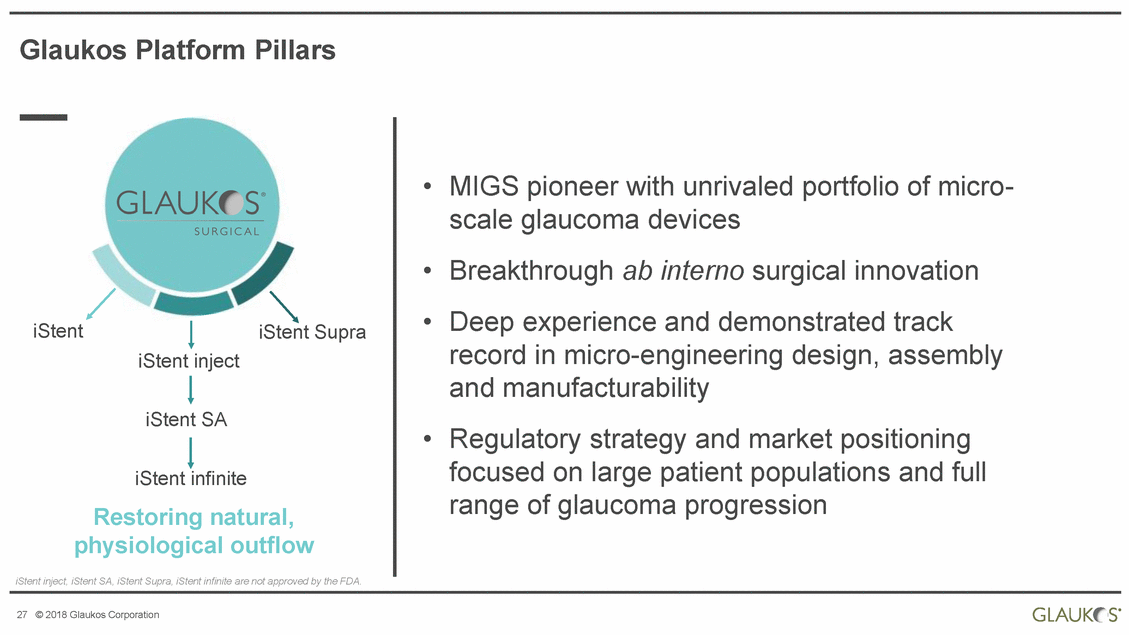
Glaukos Platform Pillars • MIGS pioneer with unrivaled portfolio of micro-scale glaucoma devices Breakthrough ab interno surgical innovation Deep experience and demonstrated track record in micro-engineering design, assembly and manufacturability Regulatory strategy and market positioning focused on large patient populations and full range of glaucoma progression • • iStent iStent Supra iStent inject iStent SA • iStent infinite Restoring natural, physiological outflow iStent inject, iStent SA, iStent Supra, iStent infinite are not approved by the FDA. 27 © 2018 Glaukos Corporation |
|
|
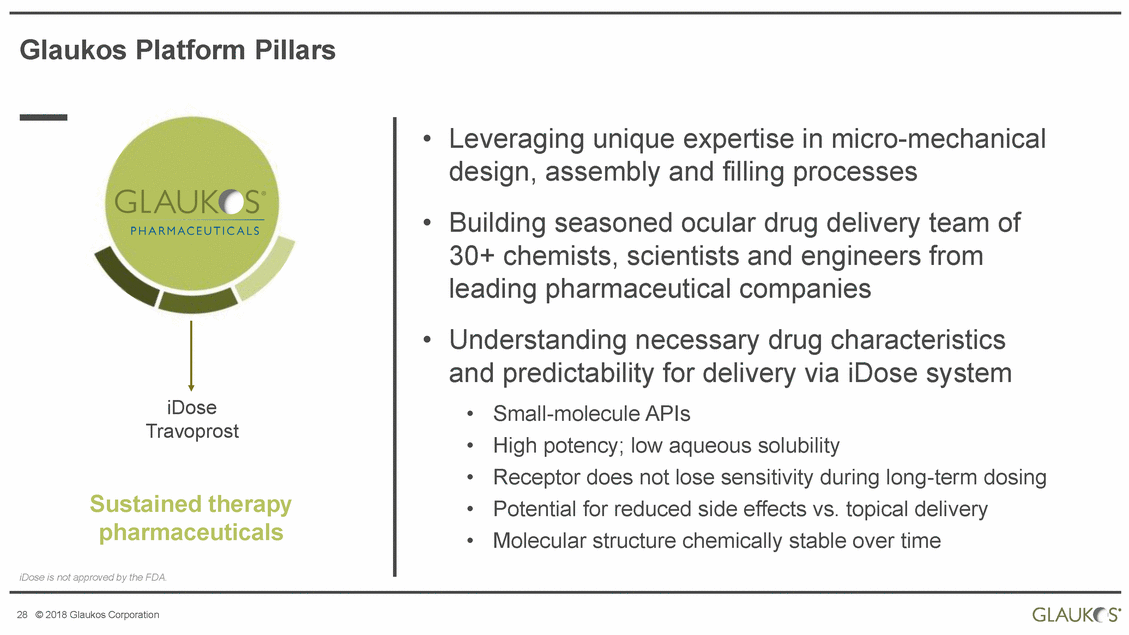
Glaukos Platform Pillars • Leveraging unique expertise in micro-mechanical design, assembly and filling processes Building seasoned ocular drug delivery team of 30+ chemists, scientists and engineers from leading pharmaceutical companies Understanding necessary drug characteristics and predictability for delivery via iDose system • • iDose Travoprost • • • • • Small-molecule APIs High potency; low aqueous solubility Receptor does not lose sensitivity during long-term dosing Potential for reduced side effects vs. topical delivery Molecular structure chemically stable over time Sustained therapy pharmaceuticals iDose is not approved by the FDA. 28 © 2018 Glaukos Corporation |
|
|
Glaukos: Key Takeaways Delivering novel surgical and pharmaceutical glaucoma therapy • Validating iDose drug delivery system and developing new sustained pharmaceuticals platform Extending leadership in MIGS treatment class with industry’s most comprehensive surgical offering Delivering solid cadence of market-expanding product introductions for next 5+ years • • • Addressing important unmet clinical needs in large growing markets Becoming multi-faceted organization capable of transforming glaucoma therapy and • 29 © 2018 Glaukos Corporation |
|
|
30 © 2018 Glaukos Corporation 3 |