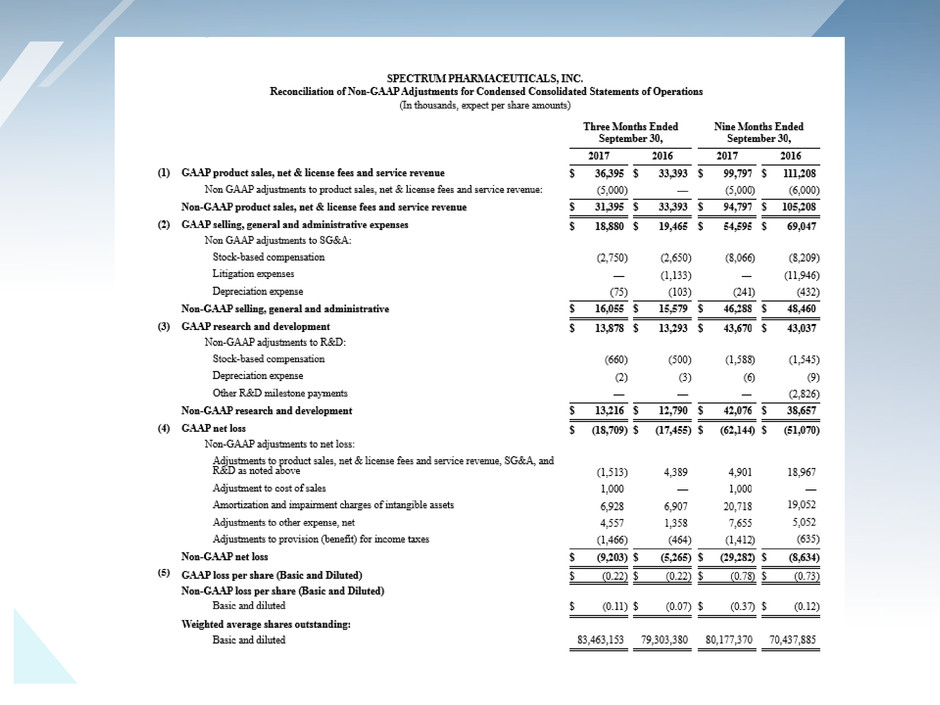
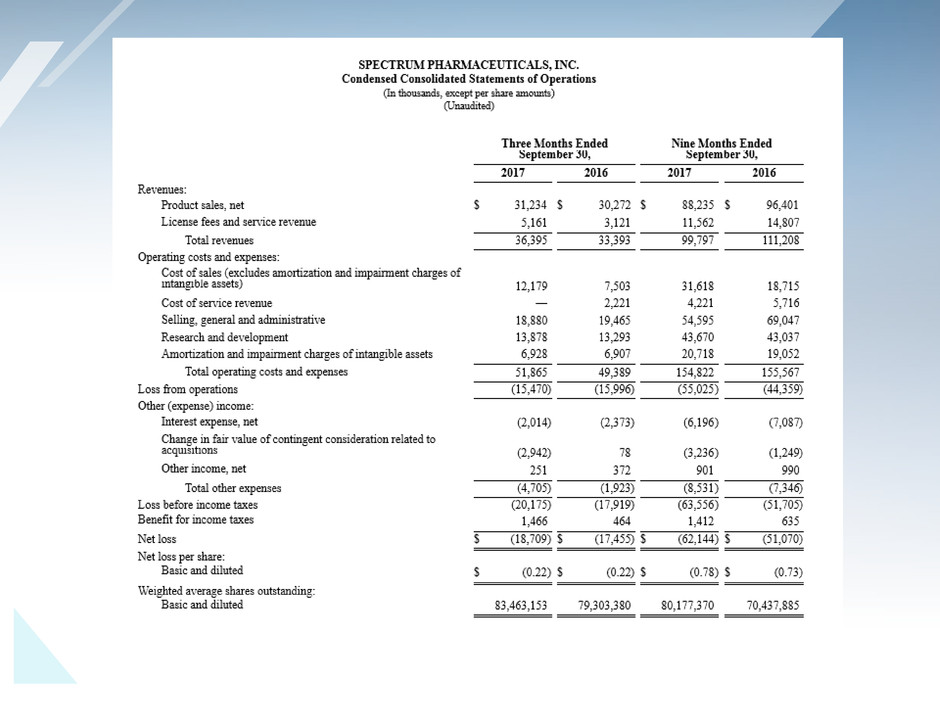
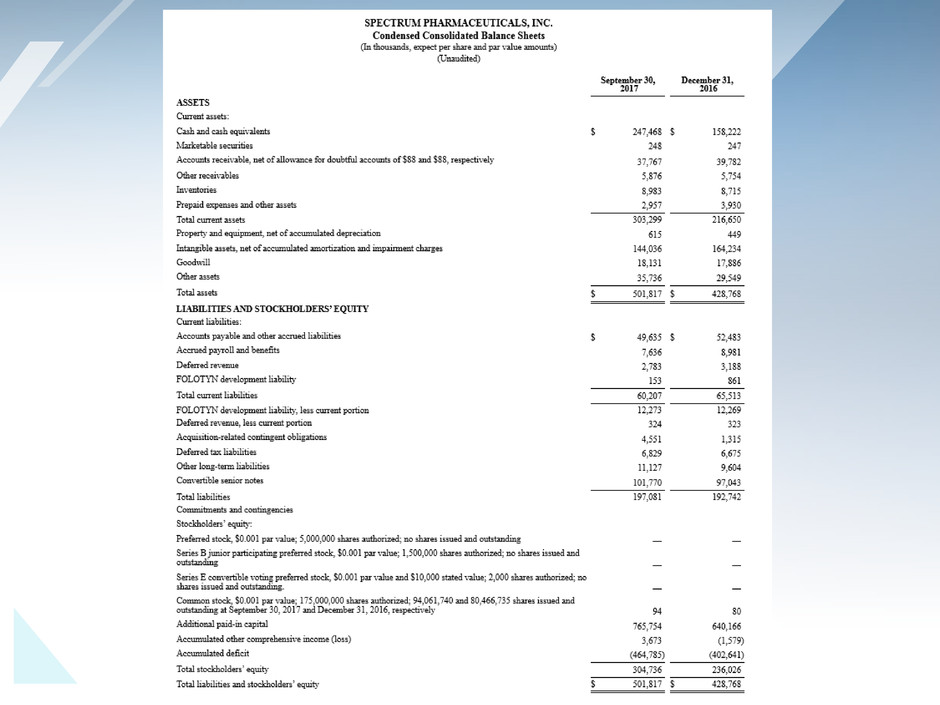
Attached files
| file | filename |
|---|---|
| 8-K - 8-K ITEM 7.01 - SPECTRUM PHARMACEUTICALS INC | item7011-8x2018.htm |

Spectrum
Pharmaceuticals
Joseph Turgeon
President and Chief Executive Officer
January 2018
Investor Presentation

2
Safe Harbor Statement
This presentation contains forward-looking statements regarding future events and the future performance
of Spectrum Pharmaceuticals that involve risks and uncertainties that could cause actual results to differ
materially. These statements are based on management’s current beliefs and expectations. These
statements include but are not limited to statements that relate to our business and its future, our strategy,
the success of our drug candidates, the safety and efficacy of our drug products, product approvals, market
potential, product sales, revenue, development, regulatory and approval timelines, product launches,
product acquisitions, capital resources and any statements that relate to the intent, belief, plans or
expectations of Spectrum or its management, or that are not a statement of historical fact.
Risks that could cause actual results to differ include the possibility that our existing and new drug candidates
may not prove safe or effective, the possibility that our existing and new drug candidates may not receive
approval from the FDA and other regulatory agencies in a timely manner or at all, the possibility that our
existing and new drug candidates, if approved, may not be more effective, safer or more cost efficient than
competing drugs, the possibility that price and other competitive pressures may make the marketing and sale
of our drugs not commercially feasible, the possibility that our efforts to acquire or in-license and develop
additional drug candidates may fail, our lack of sustained revenue history, our limited experience in
establishing strategic alliances, our limited marketing experience, our customer concentration, the possibility
for fluctuations in customer orders, evolving market dynamics, our dependence on third parties for clinical
trials, manufacturing, distribution, information and quality control and other risks that are described in
further detail in the Company's reports filed with the Securities and Exchange Commission. We do not plan
to update any such forward-looking statements and expressly disclaim any duty to update the information
contained in this presentation except as required by law.
Mission
At Spectrum Pharmaceuticals,
Inc., we bring the expertise and
passion for excellence of our
team to acquire, develop and
commercialize pharmaceuticals
for unmet medical needs while
building value for our
shareholders.
Spectrum’s

POZIOTINIB
Irreversible Tyrosine
Kinase Inhibitor
5
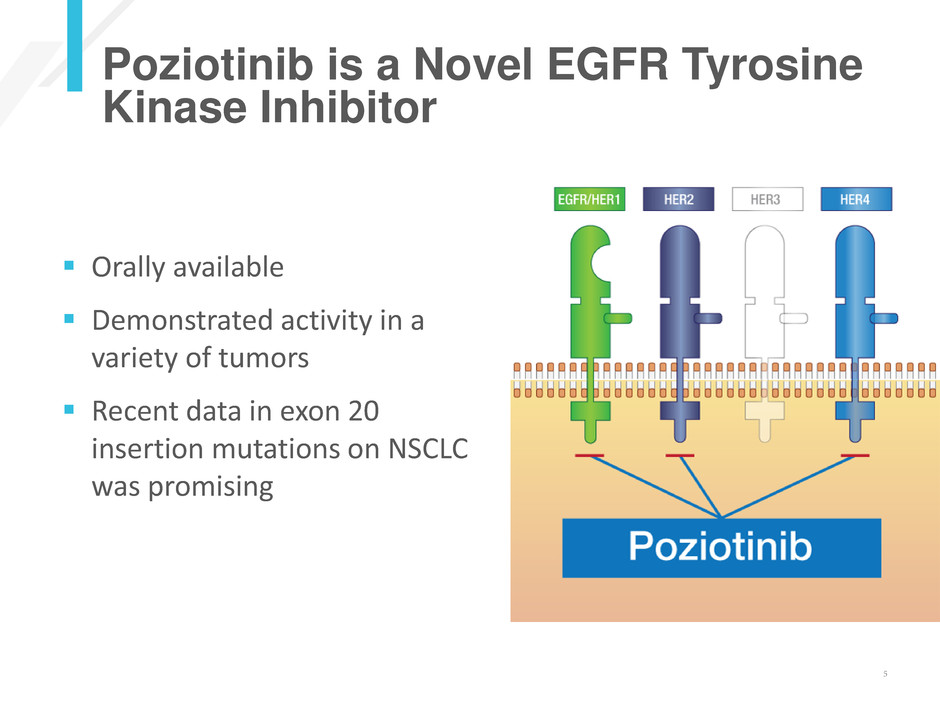
Poziotinib is a Novel EGFR Tyrosine
Kinase Inhibitor
Orally available
Demonstrated activity in a
variety of tumors
Recent data in exon 20
insertion mutations on NSCLC
was promising
6
Text here
0 10 20 30 40 50
0
20
40
60
80
100
Time (Months)
Pr
og
re
ss
io
n
fre
e
su
rv
iv
al
(%
o
f p
at
ie
nt
s)
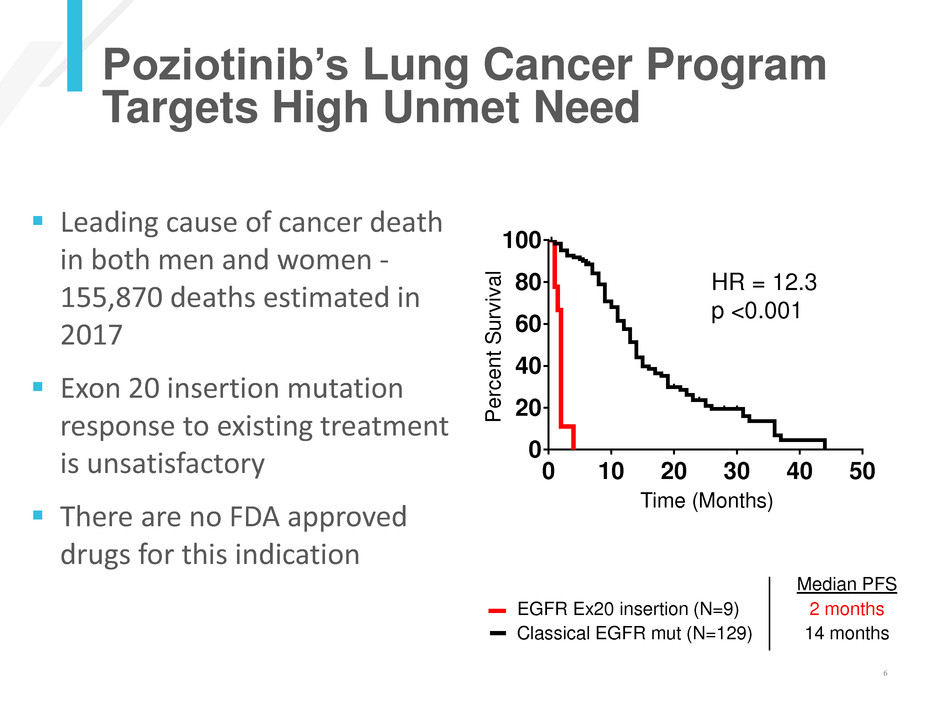
Classical EGFR mutations (n=129)
EGFR exon 20 insertions (n=9)
Median PFS (mo)
Classical EGFR Mutaions 14
EGFR exon 20 insertions 2
P<0.0001 (Log-rank)
Per
c
ent
Sur
v
iv
a
l
Time (Months)
HR = 12.3
p <0.001
Leading cause of cancer death
in both men and women -
155,870 deaths estimated in
2017
Exon 20 insertion mutation
response to existing treatment
is unsatisfactory
There are no FDA approved
drugs for this indication
Median PFS
EGFR Ex20 insertion (N=9) 2 months
Classical EGFR mut (N=129) 14 months
Poziotinib’s Lung Cancer Program
Targets High Unmet Need
7
Text here
0.001 0.01 0.1 1 10 100
0
25
50
75
100
125
EGFR Exon20 Insertion Mutations
log[Inhibitor], µM
%
V
ia
bi
lit
y AZD9291
EGF816
CO-1686
Afatinib
Erlotinib
Poziotinib
0.001 0.01 0.1 1 10 100
0
25
50
75
100
125
EGFR Exon20 Insertion Mutations
log[Inhibitor], µM
%
V
ia
b
il
it
y
AZD9291
EGF816
CO-1686
Afatinib
Erlotinib
Poziotinib
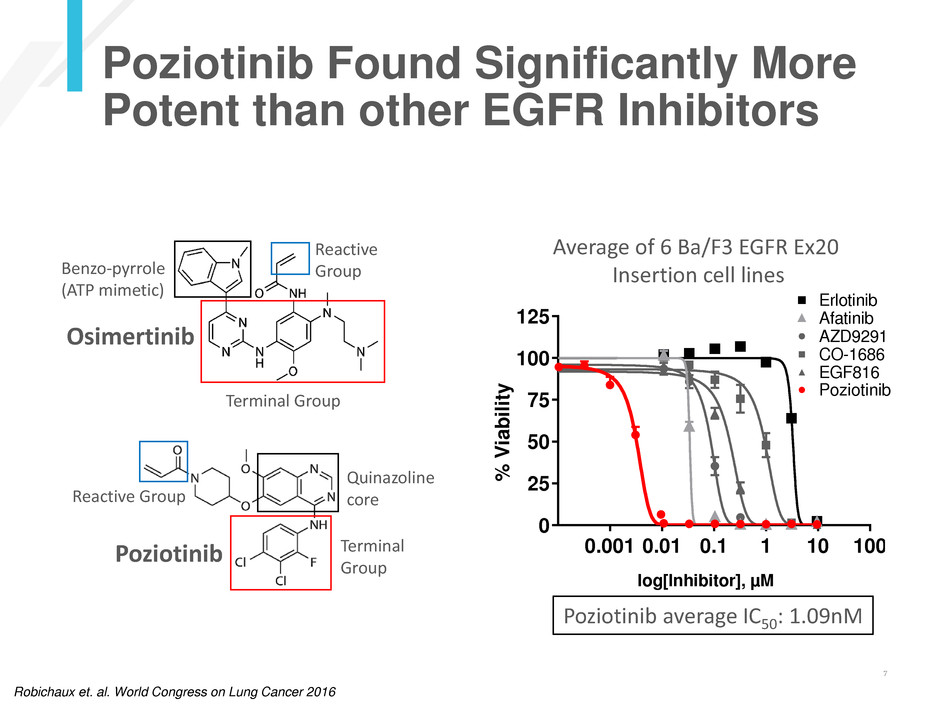
Average of 6 Ba/F3 EGFR Ex20
Insertion cell lines
0.001 0.01 0.1 1 10 100
0
25
50
75
100
125
EGFR Exon20 Insertion Mutations
log[Inhibitor], µM
% Viability
AZD9291
EGF816
CO-1686
fatinib
Erlotinib
Poziotinib average IC50: 1.09nM
EGFR A763insFQEA
0.01 0.1 1 10 100
0
25
50
75
100
125 Erlotinib
Gefitinib
log[Inhibitor], µM
%
V
i
a
b
i
l
i
t
y
Poziotinib
Reactive Group
Quinazoline
core
Terminal
Group
Osimertinib
Terminal Group
Benzo-pyrrole
(ATP mimetic)
Reactive
Group
Robichaux et. al. World Congress on Lung Cancer 2016
Poziotinib Found Significantly More
Potent than other EGFR Inhibitors
8
Text here
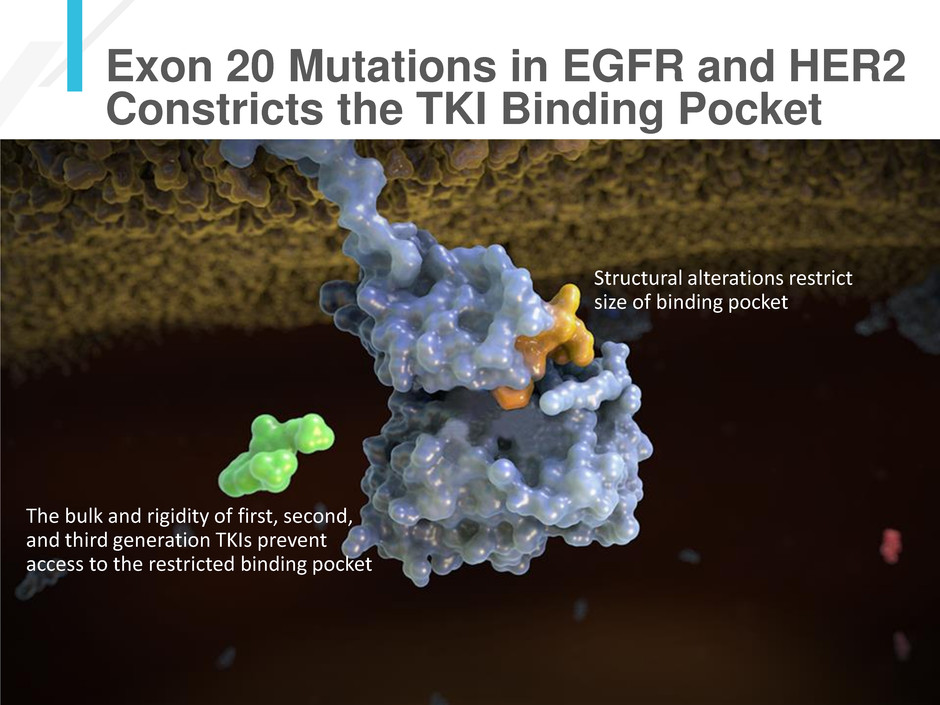
The bulk and rigidity of first, second,
and third generation TKIs prevent
access to the restricted binding pocket
Structural alterations restrict
size of binding pocket
Exon 20 Mutations in EGFR and HER2
Constricts the TKI Binding Pocket
9
Text here
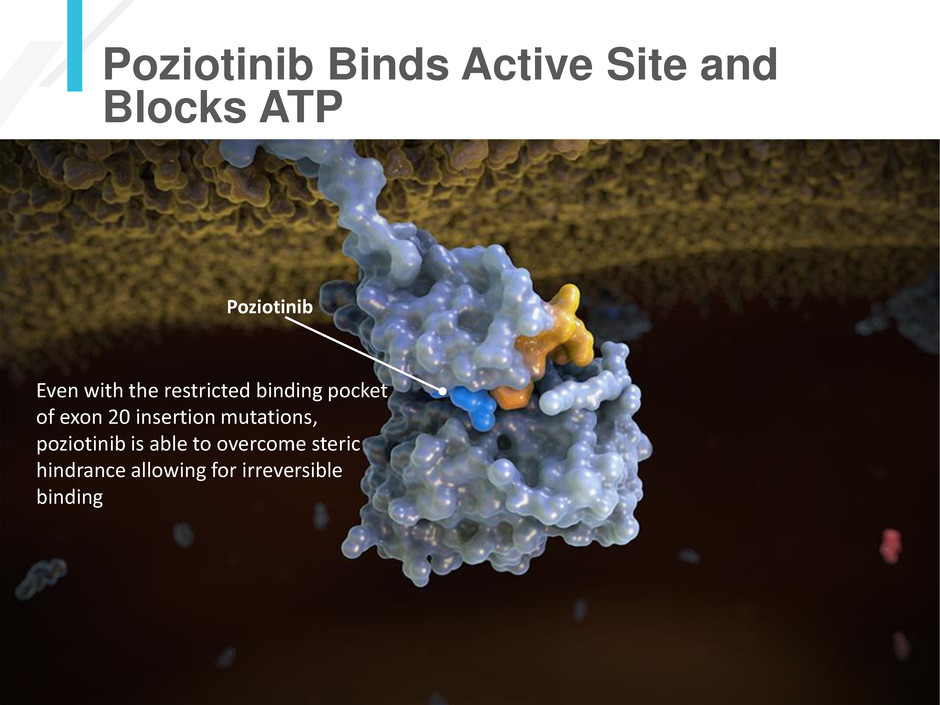
Poziotinib Binds Active Site and
Blocks ATP
Poziotinib
Even with the restricted binding pocket
of exon 20 insertion mutations,
poziotinib is able to overcome steric
hindrance allowing for irreversible
binding
1 0
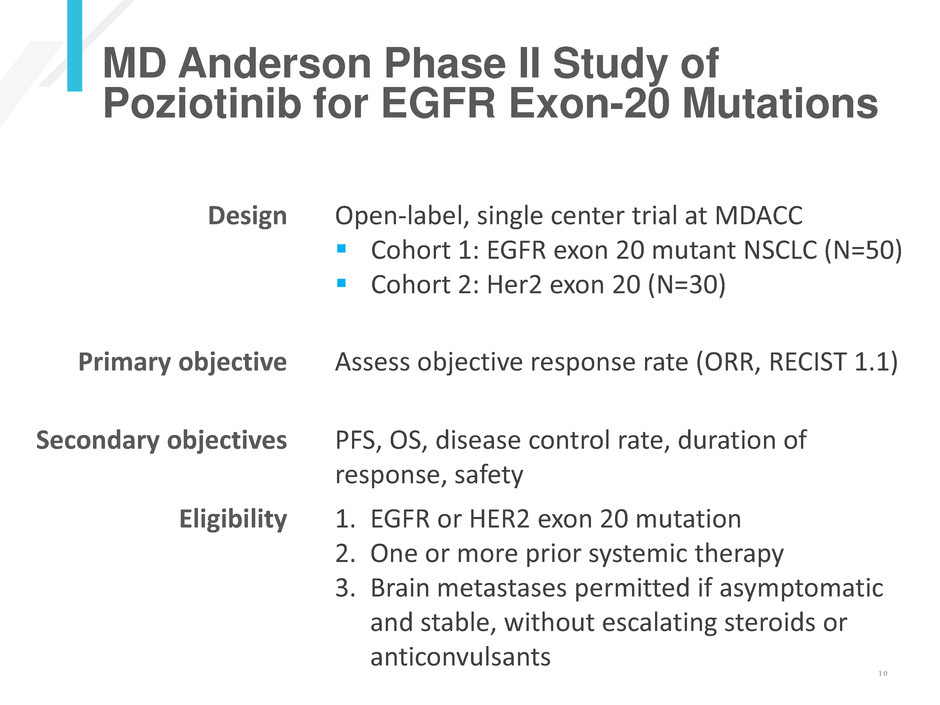
MD Anderson Phase II Study of
Poziotinib for EGFR Exon-20 Mutations
Design Open-label, single center trial at MDACC
Cohort 1: EGFR exon 20 mutant NSCLC (N=50)
Cohort 2: Her2 exon 20 (N=30)
Primary objective Assess objective response rate (ORR, RECIST 1.1)
Secondary objectives PFS, OS, disease control rate, duration of
response, safety
Eligibility 1. EGFR or HER2 exon 20 mutation
2. One or more prior systemic therapy
3. Brain metastases permitted if asymptomatic
and stable, without escalating steroids or
anticonvulsants
1 1
Poziotinib
-6 0
-4 5
-3 0
-1 5
0
1 5
3 0
4 5
M
a
x
im
u
m
R
e
s
p
o
n
s
e
f
ro
m
B
a
s
e
lin
e
V
7
6
9
in
s
G
S
V
H
7
7
3
in
s
A
H
H
7
7
3
d
u
p
P
R
N
7
7
1
in
s
H
H
D
7
7
0
in
s
G
P
7
7
2
in
s
D
N
P
A
7
6
7
d
u
p
A
S
V
S
7
6
8
I
S
7
6
8
d
u
p
S
V
D
D
7
7
0
d
e
l
in
s
G
Y
D
7
7
0
in
s
Y
H
7
7
3
Y
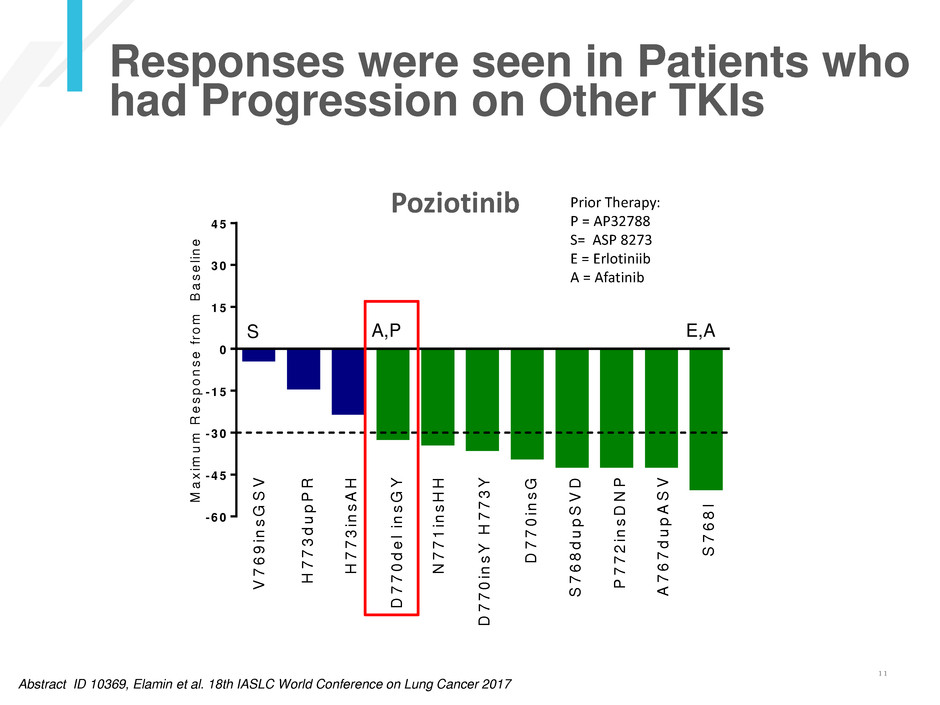
S E,A
Prior Therapy:
P = AP32788
S= ASP 8273
E = Erlotiniib
A = Afatinib
A,P
Responses were seen in Patients who
had Progression on Other TKIs
Abstract ID 10369, Elamin et al. 18th IASLC World Conference on Lung Cancer 2017
1 2
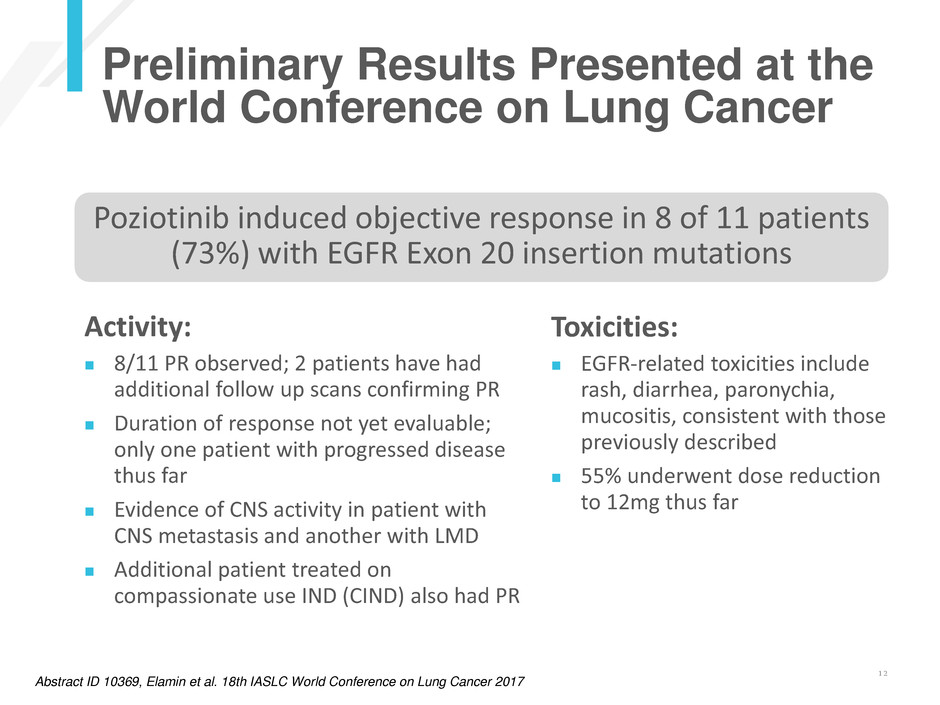
Preliminary Results Presented at the
World Conference on Lung Cancer
Activity:
8/11 PR observed; 2 patients have had
additional follow up scans confirming PR
Duration of response not yet evaluable;
only one patient with progressed disease
thus far
Evidence of CNS activity in patient with
CNS metastasis and another with LMD
Additional patient treated on
compassionate use IND (CIND) also had PR
Abstract ID 10369, Elamin et al. 18th IASLC World Conference on Lung Cancer 2017
Poziotinib induced objective response in 8 of 11 patients
(73%) with EGFR Exon 20 insertion mutations
Toxicities:
EGFR-related toxicities include
rash, diarrhea, paronychia,
mucositis, consistent with those
previously described
55% underwent dose reduction
to 12mg thus far
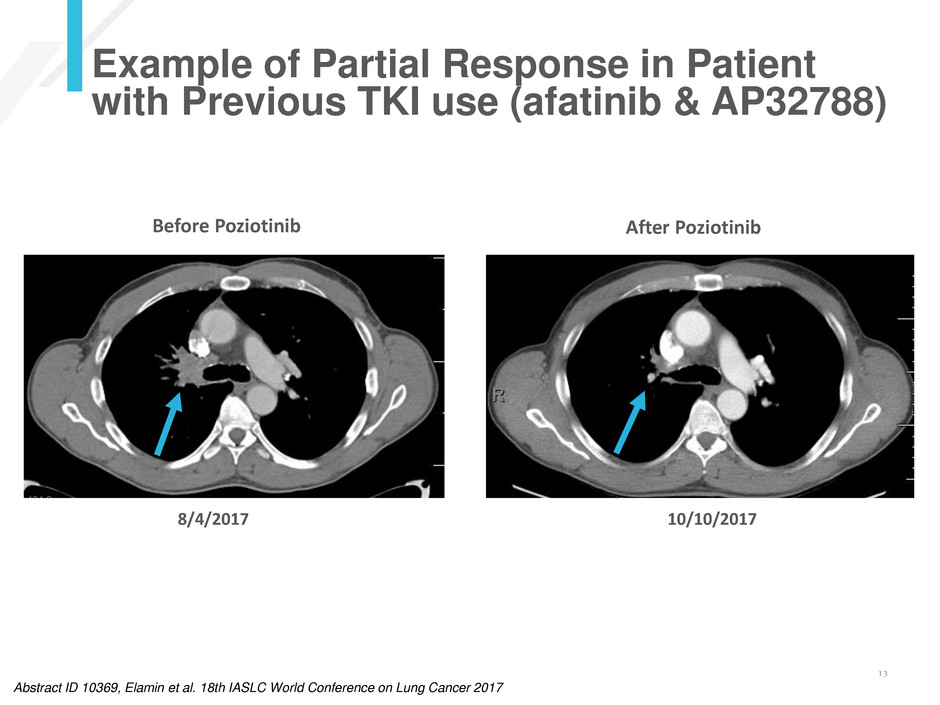
1 3
Text here
Example of Partial Response in Patient
with Previous TKI use (afatinib & AP32788)
8/4/2017 10/10/2017
Abstract ID 10369, Elamin et al. 18th IASLC World Conference on Lung Cancer 2017
Before Poziotinib After Poziotinib

1 4
Text here
We have initiated and are enrolling patients in a Phase 2
multi-center study at several leading cancer institutions
in the U.S.
The study is enrolling up to 87 patients with EGFR exon
20 insertion mutations and up to 87 patients with HER2
exon 20 insertion mutations
The study will evaluate Objective Response Rate (ORR) as
the primary endpoint and PFS and OS as secondary
endpoints
Multicenter Trial Enrolling Patients in
Phase 2 Poziotinib Study
1 5
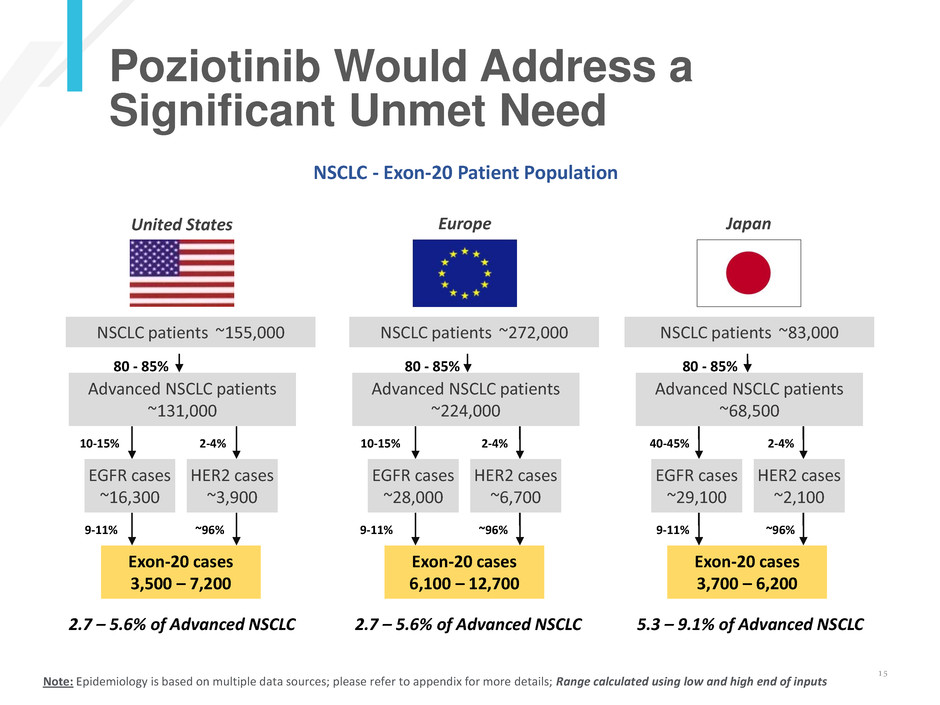
Text here
Poziotinib Would Address a
Significant Unmet Need
Advanced NSCLC patients
~131,000
EGFR cases
~16,300
10-15%
9-11%
HER2 cases
~3,900
2-4%
~96%
Advanced NSCLC patients
~224,000
EGFR cases
~28,000
HER2 cases
~6,700
2-4%
~96%
Advanced NSCLC patients
~68,500
EGFR cases
~29,100
40-45%
9-11%
HER2 cases
~2,100
~96%
Exon-20 cases
3,500 – 7,200
Exon-20 cases
6,100 – 12,700
Exon-20 cases
3,700 – 6,200
2.7 – 5.6% of Advanced NSCLC 2.7 – 5.6% of Advanced NSCLC 5.3 – 9.1% of Advanced NSCLC
NSCLC - Exon-20 Patient Population
United States Europe Japan
10-15%
9-11%
2-4%
NSCLC patients ~155,000 NSCLC patients ~272,000 NSCLC patients ~83,000
80 - 85% 80 - 85% 80 - 85%
Note: Epidemiology is based on multiple data sources; please refer to appendix for more details; Range calculated using low and high end of inputs
1 6
Open Label Phase 2 Study is
enrolling patients
Entry Criteria include HER2-
positive, metastatic breast cancer
patients with at least 2 prior
HER2-directed treatments
Primary Endpoint: Objective
Response Rate (ORR)
Approximately 75 patients
Poziotinib Phase 2 Ongoing in HER2+
Breast Cancer Patients
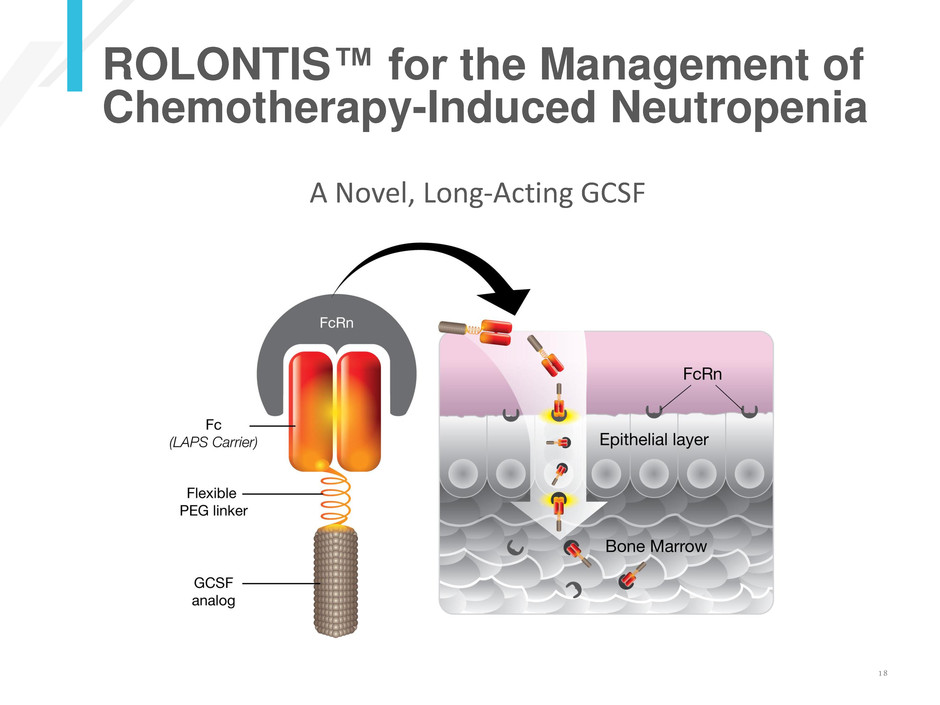
for the Management of
Chemotherapy-Induced
Neutropenia
ROLONTIS™
1 8
ROLONTIS™ for the Management of
Chemotherapy-Induced Neutropenia
A Novel, Long-Acting GCSF
1 9
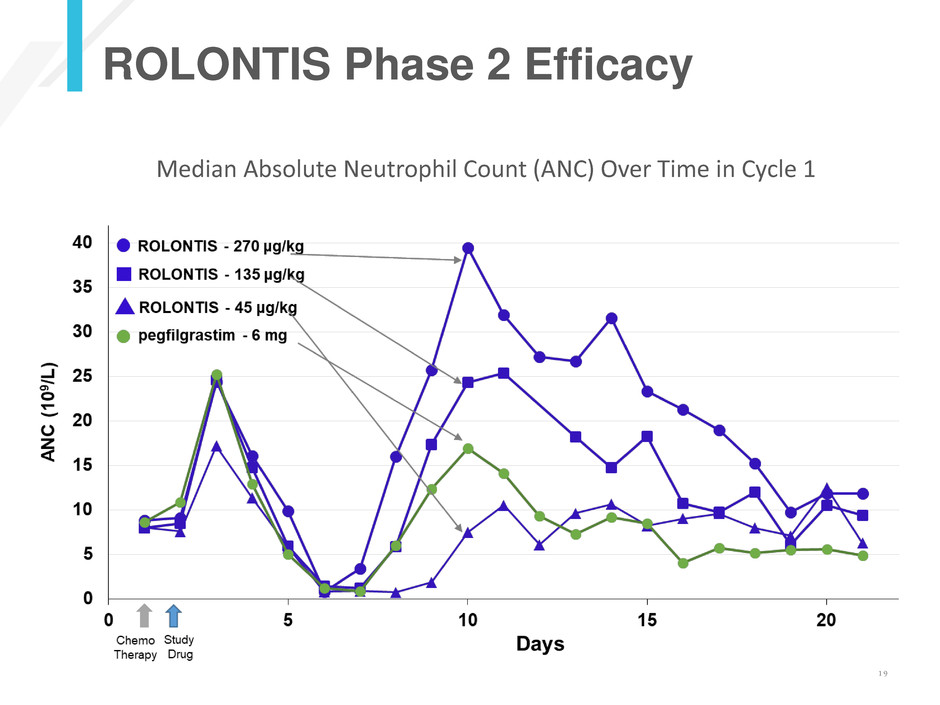
ROLONTIS Phase 2 Efficacy
Median Absolute Neutrophil Count (ANC) Over Time in Cycle 1
2 0
Text here
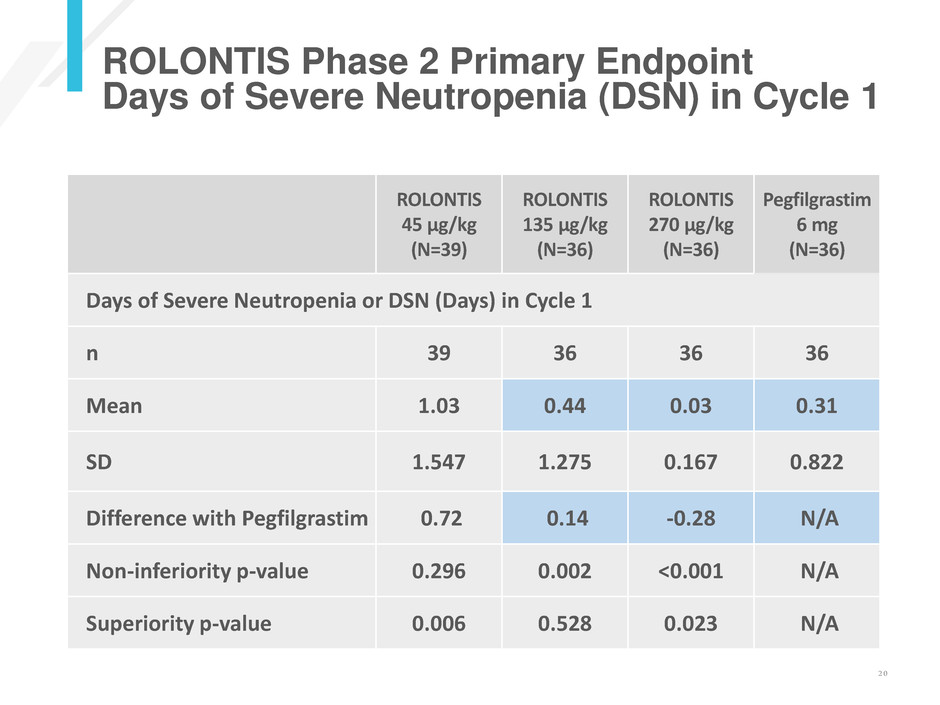
ROLONTIS Phase 2 Primary Endpoint
Days of Severe Neutropenia (DSN) in Cycle 1
ROLONTIS
45 µg/kg
(N=39)
ROLONTIS
135 µg/kg
(N=36)
ROLONTIS
270 µg/kg
(N=36)
Pegfilgrastim
6 mg
(N=36)
Days of Severe Neutropenia or DSN (Days) in Cycle 1
n 39 36 36 36
Mean 1.03 0.44 0.03 0.31
SD 1.547 1.275 0.167 0.822
Difference with Pegfilgrastim 0.72 0.14 -0.28 N/A
Non-inferiority p-value 0.296 0.002 <0.001 N/A
Superiority p-value 0.006 0.528 0.023 N/A

2 1
ADVANCE Registrational Study (n= ~400):
Enrollment completed August 2017
Topline results expected 1Q 2018
RECOVER International Study (n= ~218):
Similar study design
Enrolling in Europe and U.S.
ROLONTIS Registration Program
Includes Two Studies
Expect to file U.S. BLA in 4Q 2018
and file with EMA in 2019
A Potent Tumor-activated
Drug for Bladder Cancer
QAPZOLA®
2 3
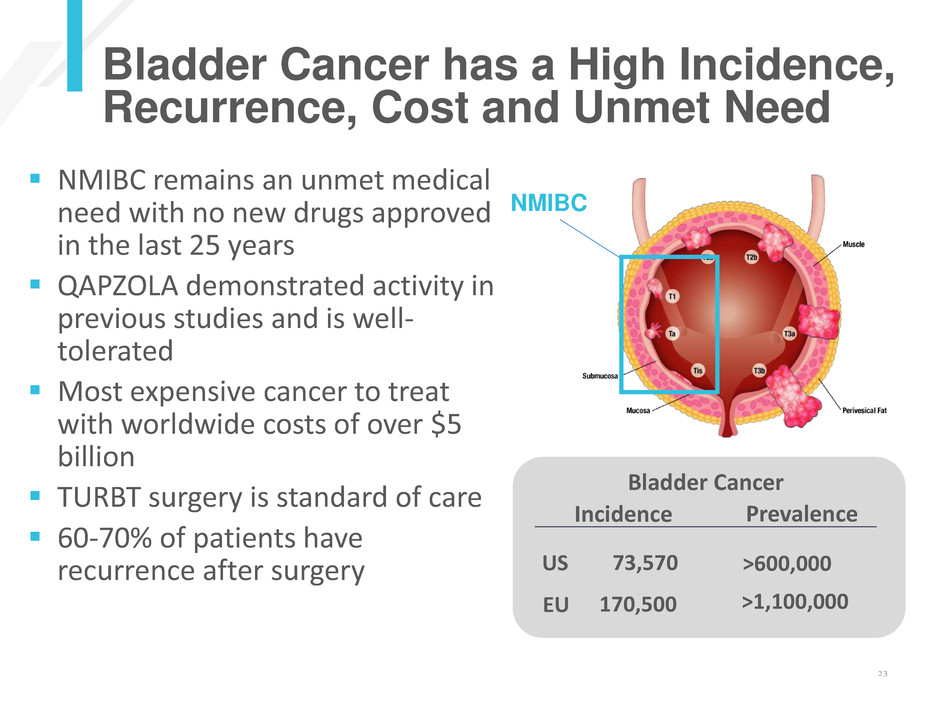
NMIBC remains an unmet medical
need with no new drugs approved
in the last 25 years
QAPZOLA demonstrated activity in
previous studies and is well-
tolerated
Most expensive cancer to treat
with worldwide costs of over $5
billion
TURBT surgery is standard of care
60-70% of patients have
recurrence after surgery
Bladder Cancer has a High Incidence,
Recurrence, Cost and Unmet Need
NMIBC
Incidence Prevalence
US
EU
73,570
170,500
>600,000
>1,100,000
Bladder Cancer

2 4
New study design incorporates learnings from previous studies
and recommendations from FDA
Study Design
Approximately 425 patients
Single dose: 8mg
Intravesical Instillation at 60 +/- 30 minutes post TURBT
Double blind, placebo controlled Phase 3 study with 2:1
randomization
Low to intermediate risk
End Point: Time to Recurrence
QAPZOLA SPA from FDA on New
Study Design
First Patient Dosed in August 2017
2 5
POZIOTINIB
A Irreversible Tyrosine Kinase Inhibitor
Promising Activity in Lung Cancer Patients with Exon 20
Insertion Mutations
Multicenter Clinical Trial Initiated to Expedite Development
Advanced Stage Pipeline
ROLONTIS™
A Novel Long-acting GCSF for Chemotherapy-Induced Neutropenia
Top-Line Data 1Q 2018
BLA Filing 4Q 2018
QAPZOLA®
A Potent Tumor-activated Drug for Bladder Cancer
• Phase 3 Initiated August 2017

Thank You