UNITED STATES SECURITIES AND
EXCHANGE COMMISSION
Washington, D.C.
20549
Form 10-K
| |
|
|
|
þ
|
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
For the fiscal year ended
December 31,
2009
|
|
Or
|
|
o
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
For the transition period
from to
|
Commission File Number:
000-28782
Spectrum Pharmaceuticals,
Inc.®
(Exact Name of Registrant as
Specified in its Charter)
| |
|
|
|
Delaware
|
|
93-0979187
|
(State or other jurisdiction
of
incorporation or organization)
|
|
(I.R.S. Employer
Identification No.)
|
157 Technology Drive
Irvine, California
(Address of principal
executive offices)
|
|
92618
(Zip
Code)
|
Registrant’s telephone number, including area code:
(949) 788-6700
Securities registered pursuant to Section 12(b) of the
Act:
| |
|
|
|
Title of Each Class
|
|
Name of Each Exchange on Which Registered
|
|
Common Stock, $0.001 par value
|
|
The NASDAQ Stock Market, LLC
|
|
Common Stock Purchase Warrants
|
|
|
|
Rights to Purchase Series B Junior Participating Preferred
Stock
|
|
|
Securities registered pursuant to Section 12(g) of the
Act:
None
Indicate by check mark if the registrant is a well-known
seasoned issuer, as defined in Rule 405 of the Securities
Act. Yes o No þ
Indicate by check mark if the registrant is not required to file
reports pursuant to Section 13 or Section 15(d) of the
Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed
all reports required to be filed by Section 13 or 15(d) of
the Securities Exchange Act of 1934 during the preceding
12 months (or for such shorter period that the registrant
was required to file such reports), and (2) has been
subject to such filing requirements for the past
90 days. Yes þ No o
Indicate by check mark whether the registrant has submitted
electronically and posted on its corporate Web site, if any,
every Interactive Data File required to be submitted and posted
pursuant to Rule 405 of
Regulation S-T
(§ 229.405 of this chapter) during the preceding
12 months (or for such shorter period that the registrant
was required to submit and post such
files). Yes o No þ
Indicate by check mark if disclosure of delinquent filers
pursuant to Item 405 of
Regulation S-K
is not contained herein, and will not be contained, to the best
of registrant’s knowledge, in definitive proxy or
information statements incorporated by reference in
Part III of this
Form 10-K
or any amendment to this
Form 10-K o
Indicate by check mark whether the registrant is a large
accelerated filer, an accelerated filer, a non-accelerated
filer, or a smaller reporting company. See the definitions of
“large accelerated filer,” “accelerated
filer” and “smaller reporting company” in Rule
12b-2 of the Exchange Act. (Check one):
| |
|
|
|
|
|
|
|
Large accelerated filer o
|
|
Accelerated filer þ
|
|
Non-accelerated
filer o
(Do not check if a smaller reporting company)
|
|
Smaller Reporting company þ
|
Indicate by check mark whether the Registrant is a shell company
(as defined in
Rule 12b-2
of the Exchange
Act). Yes o No þ
The aggregate market value of the voting and non-voting common
equity held by non-affiliates of the registrant as of
June 30, 2009 was $310,033,876 based on the closing sale
price of such common equity on such date.
As of March 29, 2010 there were 49,170,969 shares of
the registrant’s common stock outstanding.
DOCUMENTS
INCORPORATED BY REFERENCE
Portions of the Proxy Statement for the Registrant’s 2010
Annual Meeting of Stockholders, to be filed on or before
April 30, 2010, are incorporated by reference into
Part III of this
Form 10-K.
FORWARD-LOOKING
STATEMENTS
Spectrum Pharmaceuticals, Inc.’s Annual Report on
Form 10-K
contains certain forward-looking statements. These
forward-looking statements involve a number of risks and
uncertainties. These forward-looking statements can generally be
identified as such because the context of the statement will
include certain words, including but not limited to,
“believes,” “may,” “will,”
“expects,” “intends,” “estimates,”
“anticipates,” “plans,” “seeks,”
“continues,” “predicts,”
“potential,” “likely,” or
“opportunity,” and also contains predictions,
estimates and other forward-looking statements within the
meaning of Section 27A of the Securities Act of 1933, as
amended, and Section 21E of the Securities Exchange Act of
1934, as amended, and in reliance upon the safe harbor
provisions of the Private Securities Litigation Reform Act of
1995. Such forward-looking statements are based on the current
beliefs of the Company’s management, as well as assumptions
made by and information currently available to the
Company’s management. Readers of this Annual Report on
Form 10-K
should not put undue reliance on these forward-looking
statements, which speak only as of the time this Annual Report
on
Form 10-K
was filed with the Securities and Exchange Commission, or SEC.
Reference is made in particular to forward-looking statements
regarding the success, safety and efficacy of our drug products,
product approvals, product sales, revenues, development
timelines, product acquisitions, liquidity and capital resources
and trends. Forward-looking statements are inherently subject to
risks and uncertainties, some of which cannot be predicted or
quantified. Spectrum Pharmaceuticals, Inc.’s actual results
may differ materially from the results projected in the
forward-looking statements. Factors that might cause such a
difference include, but are not limited to, those discussed in
this Report, including the “Risk Factors” in
“Item 1A — Risk Factors”, and in
“Item 7 — Management’s Discussion and
Analysis of Financial Condition and Results of Operations”
included in Part II. In addition, past financial or
operating performance is not necessarily a reliable indicator of
future performance, and you should not use our historical
performance to anticipate results or future period trends. We
can give no assurances that any of the events anticipated by the
forward-looking statements will occur or, if any of them do,
what impact they will have on our results of operations and
financial condition. Except as required by law, we do not
undertake to update any such forward-looking statements and
expressly disclaim any duty to update the information contained
in this Annual Report on
Form 10-K.
Unless the context otherwise requires, all references in this
Annual Report on
Form 10-K
to the “Company”, “we,” “us,”
“our,” “Spectrum” and “Spectrum
Pharmaceuticals” refer to Spectrum Pharmaceuticals, Inc.
and its subsidiaries and other consolidated entities, as a
consolidated entity. We primarily conduct all our activities as
Spectrum Pharmaceuticals.
Spectrum Pharmaceuticals,
Inc.®,
Fusilev®,
Zevalin®
and
RenaZorb®
are registered trademarks of Spectrum Pharmaceuticals, Inc. and
its subsidiaries. Belinostat, Turning Insights Into
Hopetm,
RIT Oncology,
LLCtm,
RITtm,
and our logos are trademarks owned by Spectrum Pharmaceuticals,
Inc. and its subsidiaries.
EOquin®
is a registered trademark of Allergan, Inc. All other trademarks
and trade names are the property of their respective owners.
3
PART I
Overview
We are a commercial stage biopharmaceutical company committed to
developing and commercializing innovative therapies with a
primary focus in the areas of hematology-oncology and urology.
We have a fully developed commercial infrastructure that markets
and sells two drugs in the United States,
Zevalin®
and
Fusilev®.
We have several drug candidates in development, the most
advanced of which are apaziquone
(EOquin®),
which is presently being studied in two large Phase 3 clinical
trials for non-muscle invasive bladder cancer (NMIBC) under a
strategic collaboration with Allergan; and belinostat, a drug we
recently partnered with TopoTarget A/S to jointly develop.
Belinostat is being studied in multiple indications, including a
Phase 2 registrational trial for relapsed or refractory
Peripheral T-Cell Lymphoma (PTCL).
Our business strategy is comprised of the following initiatives:
|
|
|
| |
•
|
Maximizing the growth potential of our marketed drugs,
Zevalin and Fusilev. Our
near-term outlook largely depends on sales and marketing
successes for our two marketed drugs. For Zevalin, our initial
goal was to stabilize sales, which we believe we accomplished in
2009. With the approval by the U.S. Food and Drug
Administration (FDA) for a significantly larger indication in
non-Hodgkin’s lymphoma (NHL) in late 2009 and our success
in addressing historical hurdles associated with the uptake of
this drug, we believe we can grow sales in 2010 and beyond. For
Fusilev, which we launched in August 2008, we were able to
benefit from broad utilization in community clinics and
hospitals through mid-2009. Our focus now is to obtain approval
for Fusilev in advanced metastatic colorectal cancer, which
could potentially increase the patient pool substantially. As
part of its review of our supplemental new drug application
(sNDA) the FDA has requested additional data which we expect to
submit in the third quarter of 2010.
|
For both Zevalin and Fusilev, we initiated and continue to stage
appropriate infrastructure expansions and additional initiatives
to facilitate broad customer reach and to address other market
requirements, as appropriate. We have formed a dedicated
commercial organization comprised of highly experienced and
motivated sales representatives, account managers, medical
science liaisons and a complement of other support marketing
personnel to manage the sales and marketing of these drugs.
|
|
|
| |
•
|
Optimizing our development portfolio and maximizing the
asset values of its components. While over
the recent few years, we have evolved from a development-stage
to a commercial-stage pharmaceutical company, we have maintained
a highly focused development portfolio. Our strategy with regard
to our development portfolio is to focus on late-stage drugs and
to develop them rapidly to the point of regulatory approval. We
plan to develop some of these drugs ourselves or with our
subsidiaries and affiliates, or secure collaborations such that
we are able to suitably monetize these assets.
|
We have assembled a drug development infrastructure that is
comprised of highly experienced and motivated MDs, PhDs, medical
science liaisons and a complement of other support personnel to
rapidly develop these drugs. During 2009, this team achieved our
goal of completing enrollment in the two Phase 3 apaziquone
trials (with more than 1,600 patients enrolled). We expect
to continue to maximize the value of apaziquone through further
developmental efforts and initiation of additional trials, which
we aim to begin in 2010. In addition, this team will focus its
efforts in rapidly advancing the development of belinostat by
expediting the patient enrollment in the registrational trial
for PTCL and initiating additional studies in other indications
in 2010.
We have several other exciting compounds in earlier stages of
development in our portfolio. Based upon a criteria-based
portfolio review, we are in the process of streamlining our
pipeline drugs, allowing for greater focus and integration of
our development and commercial goals.
|
|
|
| |
•
|
Expanding commercial bandwidth through licensing and
business development. It is our goal to
identify new strategic opportunities that will create strong
synergies with our currently marketed drugs and identify and
pursue partnerships for out-licensing certain of our drugs in
development. To this end, we will continue
|
4
|
|
|
| |
|
to explore strategic collaborations as these relate to drugs
that are either in advanced clinical trials or are currently on
the market. We believe that such opportunistic collaborations
will provide synergies with respect to how we deploy our
internal resources. In this regard, we intend to identify and
secure drugs that have significant growth potential either
through enhanced marketing and sales efforts or through pursuit
of additional clinical development. We believe our recent
in-licensing of belinostat, a novel histone deacetylase (HDAC)
inhibitor, is demonstrative of such licensing and business
development efforts outlined above.
|
|
|
|
| |
•
|
Managing our financial resources
effectively. We remain committed to
fiscal discipline, a policy which has allowed us to become well
capitalized among our peers, despite a very challenging capital
markets environment in 2009. This policy includes the pursuit of
non-dilutive funding options, prudent expense management, and
the achievement of critical synergies within our operations in
order to maintain a reasonable burn rate. Even with the
continued
build-up in
operational infrastructure to facilitate the marketing of our
two commercial drugs, we intend to be fiscally prudent in any
expansion we undertake. In terms of revenue generation, we plan
to become more reliant on sales from currently marketed drugs
and intend to pursue out-licensing of select pipeline drugs in
select territories, as discussed above. When appropriate, we may
pursue other sources of financing, including non-dilutive
financing alternatives. While we are currently focused on
advancing our key drug development programs, we anticipate that
we will make regular determinations as to which other programs,
if any, to pursue and how much funding to direct to each program
on an ongoing basis, based on clinical success and commercial
potential, including termination of our existing development
programs, especially if we do not expect value being driven from
continued development. Our raising of over $100 million in
equity financing in 2009 in a difficult financing environment,
and our recent termination of the development of ozarelix in
benign prostate hypertrophy (BPH), which resulted in planned
development expense reduction, are recent examples of this
strategy.
|
| |
| |
•
|
Further enhancing the organizational structure to meet our
corporate objectives. We have highly
experienced staff in pharmaceutical operations, clinical
development, regulatory and commercial functions who previously
held positions at both small to mid-size biotech companies, as
well as large pharmaceutical companies. We recently strengthened
the ranks of our management team, and will continue to pursue
talent on an opportunistic basis. Finally, we remain committed
to running a lean and efficient organization, while effectively
leveraging our critical resources.
|
Restatement
of Previously Issued Consolidated Financial Statements
In this Annual Report on
Form 10-K,
we have restated our previously issued consolidated financial
statements and related disclosures for fiscal years ended
December 31, 2007 and 2008, and each of the quarterly
condensed consolidated financial statements on
Form 10-Q
for the periods ended March 31, 2008 through
September 30, 2009 to reclassify warrant contracts based on
a reassessment of the applicable accounting and classification.
The Company has historically accounted for warrants as equity
instruments, pursuant to its, and Kelly & Company’s
(Kelly & Co.), the predecessor independent registered
public accounting firm, interpretation and evaluation of
applicable accounting guidance contained in Accounting Standards
Codification (ASC) Topic 815 “Derivatives and
Hedging — Contracts in Entity’s Own Equity”
(ASC 815) (formerly known as Emerging Issues Task Force
Issue 00-19,
“Accounting for Derivative Financial Instruments Indexed
to, and Potentially Settled in, a Company’s Own
Stock”). Accordingly, in connection with warrants issued in
registered offerings during 2005 and 2009, the Company
classified the warrants as equity. In connection with the audit
for the fiscal year 2009, the Company, in consultation with
Ernst & Young LLP (“Ernst &
Young”), the Company’s current independent registered
public accounting firm, reassessed the accounting classification
of the warrants payment to ASC 815 based on certain terms of the
warrants. The warrants provide that in the event the Company is
unable to issue registered shares upon exercise, the warrant
holders are entitled, under securities laws, to receive freely
tradable shares pursuant to a “cashless exercise”
provision. However, based on interpretation of ASC 815, there is
a required presumption of net cash settlement, as it is not
within the control of the Company to provide registered shares,
no matter how remote the probability. After several extensive
discussions among the Company’s management, Ernst &
Young and the Company’s outside legal advisors, as well as
informal discussions with Staff of the Securities and Exchange
Commission by the Company’s management, it appears that the
interpretation and applicability of this particular accounting
pronouncement is complex and must be applied based on a strict
reading of the authoritative
5
literature without respect to probability. The Company’s
Audit Committee, together with management, in consultation with
the Company’s outside legal advisors, determined on
March 30, 2010 that, notwithstanding the highly remote
theoretical nature of the possibility of net cash settlement,
the warrants should have originally been recorded as
liabilities, measured at fair value, with changes in the fair
values being recognized in the statement of operations. In this
regard, the Company reassessed the accounting classification of
the warrants issued in September 2005 pursuant to ASC 815, and
in consultation with its predecessor auditor, Kelly &
Co., determined that there should be consistent treatment of the
warrants issued in September 2005 with the warrants issued in
2009, and concluded that such 2005 warrants should also be
reclassified as a liability.
During meetings held on March 30, 2010, the Audit
Committee, together with management, in consultation with
Kelly & Co., the Company’s independent registered
public accounting firm during the years ended December 31,
2008 and 2007, concluded that the Company’s previously
filed consolidated financial statements for the fiscal years
ended December 31, 2005, 2006, 2007 and 2008 on
Form 10-K,
each of the quarterly condensed consolidated financial
statements on
Form 10-Q
for the periods ended March 31, 2008 through
September 30, 2009, and the independent registered public
accounting firm’s reports on the financial statements and
the effectiveness of internal control over financial reporting
for the fiscal years ended December 31, 2007 and 2008, and
all related earnings releases and similar communications issued
by the Company, should no longer be relied upon.
The restatements reflect the reclassification of the warrants
from equity to a liability in the following amounts, which
represents the fair value of the warrants, as of the issuance
dates, calculated using the Black-Scholes option pricing model.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value
|
|
|
|
|
Number of
|
|
|
|
|
|
|
|
of Warrants
|
|
|
|
|
Warrants
|
|
|
Exercise
|
|
|
Expiration
|
|
at Issuance
|
|
|
Issuance Date
|
|
Issued
|
|
|
Price
|
|
|
of Warrants
|
|
Date
|
|
|
|
|
|
|
|
|
|
|
|
|
(In thousands)
|
|
|
|
|
September 14, 2005
|
|
|
4,000,000
|
|
|
$
|
6.62
|
|
|
September 14, 2011
|
|
$
|
15,472
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
May 27, 2009
|
|
|
1,956,947
|
|
|
$
|
5.11
|
|
|
February 25, 2010
|
|
$
|
2,881
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 15, 2009
|
|
|
857,633
|
|
|
$
|
5.83
|
|
|
March 15, 2010
|
|
$
|
1,847
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30, 2009
|
|
|
1,468,020
|
|
|
$
|
7.10
|
|
|
March 30, 2010
|
|
$
|
4,117
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 18, 2009
|
|
|
2,649,007
|
|
|
$
|
7.55
|
|
|
June 20, 2010
|
|
$
|
5,170
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The revaluation of the warrants at each subsequent balance sheet
date to fair value, results in a change in the carrying value of
the liability, which change is recorded as “Change in fair
value of common stock warrant liability” in the
consolidated statement of operations. The net effect of these
changes for fiscal years ended December 31, 2008 and 2007,
and for each of the quarterly condensed consolidated financial
statements on
Form 10-Q
for the periods ended March 31, 2008 through
September 30, 2009 are as follows:
| |
|
|
|
|
|
|
|
Income (Loss) Resulting
|
|
|
|
|
from Change in Fair
|
|
|
|
|
Value of Common
|
|
|
Reporting Period
|
|
Stock Warrant Liability
|
|
|
|
|
(In thousands)
|
|
|
|
|
Annual
|
|
|
|
|
|
Year ended December 31, 2007
|
|
$
|
12,055
|
|
|
|
|
|
|
|
|
Year ended December 31, 2008
|
|
$
|
1,271
|
|
|
|
|
|
|
|
|
Interim (unaudited)
|
|
|
|
|
|
Quarter ended March 31, 2008
|
|
$
|
520
|
|
|
|
|
|
|
|
|
Quarter ended June 30, 2008
|
|
$
|
916
|
|
|
|
|
|
|
|
|
Quarter ended September 30, 2008
|
|
$
|
45
|
|
|
|
|
|
|
|
|
Quarter ended December 31, 2008
|
|
$
|
(210
|
)
|
|
|
|
|
|
|
|
Quarter ended March 31, 2009
|
|
$
|
(509
|
)
|
|
|
|
|
|
|
6
| |
|
|
|
|
|
|
|
Income (Loss) Resulting
|
|
|
|
|
from Change in Fair
|
|
|
|
|
Value of Common
|
|
|
Reporting Period
|
|
Stock Warrant Liability
|
|
|
|
|
(In thousands)
|
|
|
|
|
Quarter ended June 30, 2009
|
|
$
|
(20,113
|
)
|
|
|
|
|
|
|
|
Quarter ended September 30, 2009
|
|
$
|
8,863
|
|
|
|
|
|
|
|
We have not amended our previously filed Annual Reports on Form
10-K for the
fiscal years ended December 31, 2005, 2006, 2007 and 2008,
or the Quarterly Reports on Form
10-Q for the
periods ended September 30, 2005 through September 30,
2009 to reflect the restatements described in this Annual Report
on
Form 10-K,
and thus the financial statements and related financial
statement information contained in those reports should no
longer be relied upon. Throughout this Annual Report on
Form 10-K,
all amounts presented from prior periods and prior period
comparisons that have been revised are labeled as
“restated” and reflect the balances and amounts on a
restated basis.
Recent
Developments
In 2009 and early 2010, we have executed on our business
strategy that we described above. We discuss below the key
developments during that period.
We recorded approximately $28.2 million in sales of our
products for the year 2009. We successfully increased Zevalin
sales to approximately $15.7 million in 2009 as compared to
approximately $11 million by the predecessor owner of the
product in 2008. We recorded approximately $0.3 million in
Zevalin sales since we acquired the rights to fifty percent of
the product in December 2008. We also recorded Fusilev sales of
$12.5 million in 2009, compared to approximately
$7.7 million in 2008. We believe that in 2010 and beyond,
revenues from these products have the potential to significantly
grow.
In September 2009, Zevalin received FDA approval for an expanded
label for the treatment of patients with previously untreated
follicular NHL who achieve a partial or complete response to
first-line chemotherapy. This new and expanded indication
supplements the 2002 FDA approval of Zevalin as treatment for
patients with relapsed or refractory, low-grade or follicular
B-cell NHL. Additionally, in November 2009, the Centers for
Medicare and Medicaid Services (CMS) decided that Zevalin should
be reimbursed under an Average Sales Price (ASP) methodology in
the Hospital Outpatient Prospective Payment System (HOPPS) and
issued a corresponding proposed rule, which became effective on
January 1, 2010. The ASP methodology is widely used for
injectable chemotherapy drugs and creates a consistent
reimbursement standard in the hospital setting.
In October 2009, the FDA issued a Complete Response letter
regarding the sNDA for Fusilev. In the Complete Response letter,
the FDA recommended that we meet with them to discuss options
for continuing to seek approval of Fusilev in advanced
metastatic colorectal cancer. We promptly requested such a
meeting, which occurred in January 2010. In that meeting, the
FDA requested additional data which we expect to submit in the
third quarter of 2010.
As for apaziquone, in November 2009, we entered into a
collaboration agreement with Nippon Kayaku Co. Ltd. for the
development and commercialization of apaziquone in Asia, with
the exception of North and South Korea. In exchange, Nippon
Kayaku paid Spectrum an up-front payment of $15 million and
agreed to make additional payments of up to $136.0 million
based on the achievement of certain regulatory and
commercialization milestones contained in the collaboration
agreement, as well as royalties on net sales. Nippon Kayaku
received exclusive rights to apaziquone for the treatment of
NMIBC in Asia, including Japan and China. Under the terms of the
Nippon Kayaku collaboration agreement, Nippon Kayaku will
conduct the apaziquone clinical trials pursuant to a development
plan, and will be responsible for all expenses relating to the
development and commercialization of apaziquone in the Nippon
Kayaku territory. As for South Korea, or the Republic of Korea,
and North Korea, or the Democratic People’s Republic of
Korea, (collectively, “Korea”), we entered into a
collaboration agreement with Handok Pharmaceuticals Co. Ltd. for
the development and commercialization of apaziquone for the
treatment of NMIBC. Under the terms of the Handok collaboration
agreement, Handok paid us an up-front payment of
7
$1.0 million and there are potential milestone payments of
approximately $19 million, as well as royalties on net
sales. The potential milestones will be based on the achievement
of certain regulatory and commercialization milestones.
Additionally, Handok will conduct the apaziquone clinical trials
pursuant to a development plan and will be responsible for all
expenses relating to the development and commercialization of
apaziquone in North and South Korea.
In addition, in the fourth quarter of 2009, we completed
enrollment of two Phase 3 pivotal clinical trials for
apaziquone. The two trials enrolled more than
1,600 patients with non-muscle invasive bladder cancer. We
received a $1.5 million milestone payment in January 2010
from Allergan for the completion of these clinical trials, per
the terms of the collaboration agreement, which we entered into
with Allergan on October 28, 2008.
In February 2010, we entered into a licensing and collaboration
agreement with TopoTarget, for the development and
commercialization of belinostat, a drug being studied in
multiple indications, including a Phase 2 registrational
trial for patients with PTCL. The licensing and collaboration
agreement provides that we have the exclusive right to make,
develop and commercialize belinostat in North America and India,
with an option for China. In consideration for the rights
granted under the licensing and collaboration agreement, we paid
TopoTarget an up-front fee of $30 million. In addition, we
will pay up to $313 million and one million shares of
Spectrum common stock based on the achievement of certain
development, regulatory and sales milestones, as well as
double-digit royalties on net sales of belinostat.
In 2009, we raised net proceeds of approximately
$95.8 million from the sale of 15,187,715 shares of
our common stock, despite adverse global financial market
conditions. We believe these funds, as well as the funds
generated through the sales of our products and other
non-dilutive funding in 2008, have resulted in our being well
capitalized. At the end of 2009, we had approximately
$125.0 million in cash, cash equivalents and marketable
securities, which we believe will be sufficient to finance our
anticipated capital and operating requirements for the next
twelve months and beyond.
In August 2009, we acquired 100% of the rights to RenaZorb (a
family of compounds represented by SP-014, also known as
RZB-014), a lanthanum-based nanotechnology compound with potent
and selective phosphate binding capabilities from Altair
Nanotechnologies. Our acquisition of RenaZorb expands upon our
2005 license agreement with Altair, pursuant to which Altair
granted us worldwide rights to Renazorb, but only for human
uses. The August 2009 acquisition provides us with access to all
uses of and intellectual property for the asset. In
consideration for the acquisition, we paid Altair a total of
$750,000 in restricted shares of common stock.
In January 2010, based upon the mixed results of our earlier
Phase 2 study of ozarelix for the treatment of BPH and the
recently announced failure of Aeterna Zentaris’s large,
Phase 3, registrational trial of cetrorelix (another LHRH
antagonist), we discontinued development of ozarelix in BPH. We
estimate that this discontinuation will result in a substantial
reduction in future clinical development expenses. We will
continue to look for alternative indications for the development
of ozarelix.
We continued our efforts to build a global pharmaceutical
organization in 2009. For two of our non-US business entities,
Spectrum Pharma Canada, Inc., a Canadian affiliate headquartered
in the Province of Quebec, Canada, and OncoRx Pharma Private
Ltd., a wholly-owned Indian subsidiary headquartered in Mumbai,
India, we continued to grow and establish these entities in an
effort to facilitate the opening of clinical trials sites in
these countries to continue the clinical development of our
products at a reduced cost.
Product
Portfolio
We have a product portfolio consisting of both commercial stage
and development stage products. While we are committed to
growing the sales of our marketed products, we strive to
maintain a robust pipeline of products under development to
bring to the market.
8
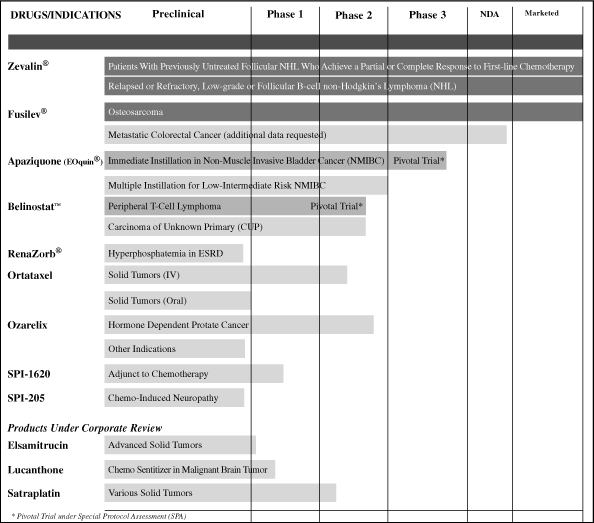
Our drug products, their approved
and/or
target indications, and status of development are summarized in
the following table, and discussed below in further detail:
Some of our drugs may prove to be beneficial in additional
disease indications as we continue their study and development.
In addition, we have intellectual property rights to neurology
compounds that we may out-license to third parties for further
development.
Overview
of Cancer
According to the American Cancer Society’s publication
Cancer Facts & Figures 2009, cancer is the
second leading cause of death in the United States, accounting
for approximately 25% of all deaths. In the United States,
approximately 1.5 million new cancer cases were expected to
be diagnosed in 2009 and over 562,000 persons were expected
to die from the disease in 2009. Accordingly, there is
significant demand for improved and novel cancer treatments.
Cancer develops when cells in a part of the body begin to grow
out of control. Although there are many kinds of cancer, they
all start because of
out-of-control
growth of abnormal cells. Normal body cells grow, divide, and
die in an orderly fashion. During the early years of a
person’s life, normal cells divide more rapidly until the
person becomes an adult. After that, cells in most parts of the
body divide only to replace worn-out or dying cells and to
9
repair injuries. Because cancer cells continue to grow and
divide, they are different from normal cells. Instead of dying,
they outlive normal cells and continue to form new abnormal
cells.
Cancer cells develop because of damage to DNA. Most of the time,
when DNA becomes damaged, the body is able to repair it. In
cancer cells, the damaged DNA is not repaired. People can
inherit damaged DNA, which accounts for inherited cancers. More
often, however, a person’s DNA becomes damaged by exposure
to something in the environment, such as smoking.
Cancer usually forms as a tumor. Some cancers, like leukemia, do
not form tumors. Instead, these cancer cells involve the blood
and blood-forming organs and circulate through other tissues
where they grow. Often, cancer cells travel to other parts of
the body where they begin to grow and replace normal tissue.
This process is called metastasis. Regardless of where a cancer
may spread, however, it is always named for the place it began.
For instance, breast cancer that spreads to the liver is still
called breast cancer, not liver cancer.
Different types of cancer can behave very differently. For
example, lung cancer and breast cancer are very different
diseases. They grow at different rates and respond to different
treatments. That is why people with cancer need treatment that
is aimed at their particular kind of cancer. Cancer is currently
treated by surgery, chemotherapy, radiation therapy, hormonal
therapy, biological therapy and immunotherapy. Cancer is
referred to as refractory when it has not responded, or is no
longer responding, to a treatment.
We are seeking novel drugs that address cancer or cancer related
indications with significant unmet medical need, that:
|
|
|
| |
•
|
are already approved for sale or have demonstrated initial
safety and efficacy in clinical trials
and/or we
believe have a higher probability of regulatory approval than
that of a typical compound at a similar stage of development;
|
| |
| |
•
|
target cancer indications with significant unmet medical need,
where current treatments either do not exist or are not deemed
to be effective; and
|
| |
| |
•
|
we believe we can acquire at a fair value based on our judgment
of clinical success and commercial potential.
|
Our drug
products
Zevalin ([90Y]-ibritumomab
tiuxetan): In December 2008, we acquired
rights to commercialize and develop Zevalin in the United
States, as the result of a transaction with Cell Therapeutics,
Inc., (CTI) further described below.
Zevalin is a prescribed form of cancer therapy called
radioimmunotherapy. Radioimmunotherapy combines a source of
radiation, called a radioisotope, with an antibody. As part of
the Zevalin therapeutic regimen, the Y-90 radioisotope is
combined with a monoclonal antibody (CD20 MAB) that specifically
recognizes a particular part of a B-cell (the cells of the
immune system that make antibodies to invading pathogens) called
the CD20 antigen. The CD20 antigen is found on malignant and
normal B-cells. As the patient is infused with Y-90 Zevalin and
it enters the bloodstream, the antibody portion recognizes and
attaches to the CD20 antigen on tumor cells, allowing the
radiation energy emitted from the Y-90 radioisotope
(i.e., beta emission) to penetrate and damage the
malignant B-cells as well as nearby neighboring cells, many of
which are also lymphoma cells.
The current Zevalin therapeutic regimen also requires a bioscan
(also known as an “imaging study”) of the prospective
patient prior to treatment with Y-90 Zevalin. For the bioscan,
the patient is infused with In-111 Zevalin, the In-111
radioisotope combined with the CD20 MAB. In-111 Zevalin produces
a kind of radiation called gamma emission, which is very similar
to the kind of radiation used to produce x-rays. Once infused
with In-111, the prospective patient goes through a bioscan. The
bioscan allows a physician to follow In-111 Zevalin as it
travels within the prospective patient’s body. Based upon
the distribution of In-111 Zevalin (whether the In-111 Zevalin
goes to certain unintended areas of the body), the physician may
elect to not infuse the patient with Y-90 Zevalin. Many
healthcare providers throughout the world who provide Zevalin
therapy do not believe that the In-111 bioscan is a necessary
part of the Zevalin therapeutic regimen. In the EU, most
countries do not perform the In-111 bioscan prior to the Y-90
Zevalin infusion. Currently, we are working with the FDA to
remove this bioscan requirement.
10
Zevalin was approved by the FDA in February of 2002 as the first
radioimmunotherapeutic agent for the treatment of NHL. Zevalin
was approved as part of a Zevalin therapeutic regimen for
treatment of relapsed or refractory, low-grade or follicular
B-cell NHL, including patients with rituximab-refractory
follicular NHL. For reference, the term refractory refers to
lymphoma that does not respond to a particular therapy. The term
relapsed refers to lymphoma that returns after initially
responding to therapy. The terms low-grade and follicular refer
to types of lymphoma cells as determined by laboratory tests,
which have an indolent (slow growing) clinical course. Rituximab
is a monoclonal antibody that specifically recognizes a
particular part of a B-cell also called the CD 20 antigen, and
is used as monotherapy or in combination with other agents for
the treatment of B-cell NHL.
NHL is caused by the abnormal proliferation of white blood cells
and normally spreads through the lymphatic system, a system of
vessels that drains fluid from the body. There are many
different types of NHL which can be divided into aggressive NHL,
a rapidly spreading acute form of the disease, and indolent NHL,
which progresses more slowly, and can be classified as either
B-cell or T-cell NHL. According to the National Cancer
Institute’s SEER database there were nearly
400,000 people in the U.S. with NHL in 2004. The
American Cancer Society estimated that in the United States
65,980 people were expected to be newly diagnosed with NHL
in 2009. Additionally, approximately 19,500 were expected to die
from this disease in 2009.
In December 2008, the FDA accepted for filing and review, and
granted priority review status for RIT’s supplemental
biologics license application (sBLA) for the use of Zevalin as
first-line therapy for patients with a previously untreated
follicular NHL who achieve a partial or complete response of
first-line chemotherapy.
The sBLA was based upon data from the multinational, randomized
Phase 3 First-line Indolent Trial (FIT) which evaluated the
efficacy and safety of a single infusion of Zevalin in
414 patients with CD20-positive follicular NHL who had
achieved a partial response or a complete response after
receiving one of the standard first-line chemotherapy regimens.
The FIT trial demonstrated that when used as a first-line
consolidation therapy for patients with follicular NHL, Zevalin
significantly improved the median progression-free survival time
from 18 months (control arm) to 38 months (Zevalin
arm) (p<0.0001).
The primary investigators of the study concluded that Zevalin
consolidation of first remission in advanced stage follicular
NHL is highly effective, resulting in a total complete response
(CR + CRu) rate of 87 percent and prolongation of median
progression-free survival by almost two years, with a toxicity
profile comparable to that seen with Zevalin’s use in
relapsed or refractory indications. Zevalin-treated patients had
reversible and manageable Grade 3 or 4 hematologic side effects
including neutropenia in 41 percent, thrombocytopenia in
51 percent, and anemia in 5 percent of patients.
Non-hematologic toxicities were 24 percent Grade 3,
5 percent Grade 4, and Grade 3 – 4 infections
were 8 percent.
In September 2009, we received FDA approval for the sBLA.
Additionally, in November 2009, the CMS decided that Zevalin
should be reimbursed under an ASP methodology in the HOPPS and
issued a corresponding proposed rule, which went into effect on
January 1, 2010. The ASP methodology is widely used for
injectable chemotherapy drugs and creates a consistent
reimbursement standard in the hospital setting.
The following describes the principal commercial terms relating
to Zevalin licensing and development:
|
|
|
| |
•
|
On December 15, 2008, we closed a transaction to enter into
a 50/50 owned joint venture called RIT, with CTI. CTI previously
acquired the U.S. rights to develop, market and sell
Zevalin from Biogen Idec, Inc. (Biogen) on December 21,
2007.
|
| |
| |
•
|
Upon entering into the joint venture arrangement, CTI
contributed the Zevalin product assets to RIT in exchange for a
50% membership interest in RIT and the cash payments to CTI
noted below. CTI received an initial cash payment of
$7.5 million at the closing of the joint venture
transaction on December 15, 2008, and received an
additional $7.5 million cash payment in early January 2009.
CTI also had the option to sell its remaining 50% membership
interest in RIT to us, subject to adjustment for any amounts
owed between RIT and CTI at the time of sale. CTI exercised this
“Put” option in February 2009. On March 15, 2009,
we entered into an agreement with CTI to complete such sale for
an aggregate amount of $16.5 million subject to certain
adjustments for, among other things, payables determined to be
owed between CTI and RIT. CTI
|
11
|
|
|
| |
|
disputed the adjustments, but in a May 2009 arbitration
proceeding, we were awarded approximately $4.3 million. As
a result of the sale, we own 100% of RIT and are its sole member
and therefore, we have, through licenses, all of the
U.S. rights to Zevalin.
|
|
|
|
| |
•
|
In connection with obtaining the required consent of Biogen to
the foregoing joint venture arrangement, we entered into certain
agreements with Biogen. Such agreements included:
|
|
|
|
| |
•
|
an amendment to the original asset purchase agreement between
CTI and Biogen (CTI/Biogen Agreement), modifying future
milestone payments, to provide that (i) concurrently with
the execution of the amendment CTI was required to pay Biogen
$0.2 million (which was reimbursed to CTI by RIT from the
initial capital contributions made by CTI and us),
(ii) upon the December 2008 closing of the joint venture
transaction, CTI was required to pay Biogen an additional
$2.0 million (which was paid by RIT as successor to CTI
under the amendment), (iii) upon the achievement of the
specified FDA approval milestone, RIT (as successor to CTI) was
required to pay Biogen an additional amount of $5.5 million
if the milestone event occurred in 2009 (provided that RIT may
elect to defer any such payment until January 1, 2010, but
upon such election the required payment will increase to
$6.0 million), $7.0 million if the milestone event
occurs in 2010, $9.0 million if the milestone event occurs
in 2011, or $10.0 million if the milestone event occurs in
2012 or later. As disclosed above, we received FDA approval for
the treatment of patients with previously untreated follicular
NHL who achieve a partial or complete response to first-line
chemotherapy and in accordance with the amendment, we paid
Biogen $5.5 million. No other material terms of the
CTI/Biogen Agreement were modified. CTI’s rights and
obligations, including its payment obligations to Biogen,
including royalties on net sales of Zevalin and an additional
regulatory milestone payment, under both the CTI/Biogen
Agreement and the amendment were assigned to and assumed by RIT
in connection with the closing of the joint venture transaction.
|
| |
| |
•
|
an amendment to the original supply agreement between Biogen and
CTI (CTI/Biogen Supply Agreement), modifying certain of the
pricing and manufacturing technology transfer terms contained in
the CTI/Biogen Supply Agreement and also providing that the term
of the agreement may be shortened in some instances in the event
of a mid-term manufacturing technology transfer. CTI’s
rights and obligations, including its payment obligations to
Biogen, under both the CTI/Biogen Supply Agreement and the
amendment were assigned to and assumed by RIT in connection with
the closing of the joint venture transaction.
|
| |
| |
•
|
a security agreement, by and between RIT and Biogen whereby RIT
granted to Biogen a first priority security interest in all of
RIT’s assets, including the assets contributed to RIT by
CTI in connection with the closing of the joint venture
transaction, to secure certain payment, indemnification and
other obligations of RIT to Biogen.
|
| |
| |
•
|
a guarantee, by us for the benefit of Biogen whereby we have,
among other things, guaranteed the payment and performance all
of RIT’s obligations to Biogen (including its obligations
as assignee of CTI under all contractual arrangements between
CTI and Biogen that were assigned to and assumed by RIT in
connection with the closing of the joint venture transaction).
|
| |
| |
•
|
pursuant to the transfer of Zevalin assets from CTI to RIT in
December 2008, RIT assumed certain license and sublicense
agreements with various third parties related to Zevalin
intellectual property under which RIT is required to make
certain payment obligations including milestone payments and
royalties.
|
Fusilev®
(levoleucovorin) for injection: On
March 7, 2008, our new drug application (NDA) for our
proprietary drug Fusilev was approved by the FDA. We
commercially launched Fusilev in August 2008, with an in-house
sales force and commercialization team. Subsequent to the
launch, in November 2008, we received a unique J-code for
Fusilev from CMS, which went into effect on January 1,
2009. The J-code is a unique, product-specific billing code that
assists providers (e.g., physicians that prescribe
Fusilev) in obtaining reimbursement for Fusilev.
Fusilev is a novel folate analog formulation and the
pharmacologically active isomer (the levo-isomer) of the
racemic compound, calcium leucovorin. Isomers are compounds with
the same molecular formula, but “mirror image” atomic
structures. Leucovorin is a mixture of equal parts of both
isomers: the pharmacologically active levo-isomer and the
inactive dextro-isomer. Preclinical studies have
demonstrated that the inactive dextro-isomer may
12
compete with the active levo-isomer for uptake at the
cellular level. By removing the inactive dextro form, the
dosage of Fusilev is one-half that of leucovorin and patients
are spared the administration of an inactive substance.
Fusilev rescue is indicated after high-dose methotrexate therapy
in patients with osteosarcoma, and to diminish the toxicity and
counteract the effects of impaired methotrexate elimination or
inadvertent overdose of folic acid antagonists. Fusilev has been
designated as an orphan drug for its approved indications.
Methotrexate is a widely used anti-cancer drug. It is a
therapeutic option in the treatment of solid tumors and
hematological malignancies, such as NHL. In addition,
methotrexate is also used to treat autoimmune diseases such as
rheumatoid arthritis, psoriasis and some rare opportunistic
infections.
In mid-year 2008, we filed an NDA amendment for Fusilev tablets.
Following the tablet submission, in October 2008, we filed a
sNDA for Fusilev (levoleucovorin) for injection in combination
with 5-FU-containing regimens in the treatment of colorectal
cancer. In October 2009, the FDA issued a Complete Response
letter regarding the sNDA for Fusilev. In the Complete Response
letter, the FDA recommended that we meet with them to discuss
options for continuing to seek approval of Fusilev in advanced
metastatic colorectal cancer. We promptly requested such a
meeting, which occurred in January 2010. In that meeting, the
FDA requested additional data which we expect to submit in the
third quarter of 2010.
Leucovorin is currently a standard combination agent with 5-FU
in various colorectal cancer treatment regimens. Leucovorin
potentiates the effects of 5-FU and its derivatives by
stabilizing the binding of the drug’s metabolite to its
target enzyme, thus prolonging drug activity. There are
peer-reviewed publications wherein Fusilev is used in place of
the leucovorin in combination with 5-FU containing regimens for
adjuvant and advanced colorectal cancer and in combination with
oxaliplatin
and/or
irinotecan for advanced disease. The National Comprehensive
Cancer Network Clinical Practice Guidelines in
Oncologytm
in colon cancer and rectal cancer have been updated to reflect
that Fusilev is available in the United States. Additionally, in
the fourth quarter of 2008, Fusilev was listed and continues to
be listed in the NCCN Drugs and Biologic Compendium for use in
combination with high-dose methotrexate for the treatment of
bone cancer (osteosarcoma and de-differentiated
chrondrosarcoma). The NCCN Drugs and Biologics Compendium is an
important reference that has been recognized by United
HealthCare as a formal guidance for coverage policy. In
addition, CMS announced in June 2008 that it would recognize the
NCCN Drugs & Biologics Compendium as a source of
information to determine which drugs may be covered under
Medicare Part B.
The following describes the principal commercial terms relating
to Fusilev licensing and development.
|
|
|
| |
•
|
In April 2006, we acquired all of the oncology drug product
assets of Targent, Inc. Targent is eligible to receive payments,
in the form of our common stock
and/or cash,
upon achievement of certain regulatory and sales milestones. At
our option, any amounts due in cash under the purchase agreement
may be paid by issuing shares of our common stock having a
value, determined as provided in the purchase agreement, equal
to the cash payment amount.
|
| |
| |
•
|
In May 2006, we amended and restated a license agreement with
Merck Eprova AG, a Swiss corporation, that we assumed in
connection with the acquisition of the assets of Targent.
Pursuant to the license agreement with Merck Eprova, we obtained
the exclusive license to use regulatory filings related to
Fusilev and a non-exclusive license under certain patents and
know-how related to Fusilev to develop, make, have made, use,
sell and have sold Fusilev in the field of oncology in North
America. In addition, we have the right of first opportunity to
negotiate an exclusive license to manufacture, have
manufactured, use and sell Fusilev products outside the field of
oncology in North America. Also, under the terms of the license
agreement, we paid Merck Eprova $100,000 for the achievement of
FDA approval of Fusilev. Eprova is also eligible to receive a
payment upon achievement of another regulatory milestone, in
addition to royalties on net sales. The term of the license
agreement is determined on a
product-by-product
and
country-by-country
basis until royalties are no longer owed under the license
agreement. The license agreement expires in its entirety after
the date that we no longer owe any royalties to Merck Eprova. We
have the unilateral right to terminate the license agreement, in
its entirety or on a
product-by-product
or
country-by-country
basis, at any time for any reason and either party may terminate
the license agreement due to material breach of the terms of the
license agreement by or insolvency of the other party.
|
13
Apaziquone (EOquin): Apaziquone is an
anti-cancer agent that becomes activated by certain enzymes
often present in higher amounts in cancer cells than in normal
cells. It is currently being investigated for the treatment of
NMIBC, which is a cancer that is only in the innermost layer of
the bladder and has not spread to deeper layers of the bladder.
The American Cancer Society estimated that the 2009 incidence
and prevalence of bladder cancer in the United States would be
approximately 70,980 and over 500,000, respectively. According
to Botteman et al., (PharmacoEconomics 2003), bladder cancer is
the most expensive cancer to treat on a lifetime basis.
The initial treatment of this cancer is complete surgical
removal of the tumor. However, bladder cancer is a highly
recurrent disease with approximately 75% of patients recurring
within 5 years, and a majority of patients recurring within
2 years. This high recurrence rate is attributed to:
1) the highly implantable nature of cancer cells that are
dispersed during surgery, 2) incomplete tumor resection,
and 3) tumors present in multiple locations in the bladder
which may be missed or too small to visualize at the time of
resection. Despite evidence in the published literature and
guidance from the American and European Urology Associations,
instillation of a chemotherapeutic agent immediately following
surgery is not a standard clinical practice. Currently, there
are no approved drugs for this indication which may, in part,
explain the difference between the literature and urology
guidelines and actual clinical management of this disease. For
more than 30 years, no new drugs have been introduced in
the market for treatment of NMIBC. An immediate instillation of
apaziquone may help by 1) reducing tumor recurrence by
destroying dispersed cancer cells that would otherwise
re-implant onto the inner lining of the bladder, 2) by
destroying remaining cancer cells at the site of tumor resection
(also known as chemo-resection), and 3) by destroying
tumors not observed during resection (also known as
chemo-ablation).
Apaziquone is a bio-reductive alkylating indoloquinone that is
enzymatically activated by enzymes that are over expressed by
bladder tumors. Pharmacokinetic studies have verified that
apaziquone is not detectable in the bloodstream of patients when
it is administered either after surgical resection or as a part
of a delayed multi-instillation protocol. The proposed dose
therefore carries a minimal risk of systemic toxicity which
could arise from absorption of a drug through the bladder wall
into the bloodstream. Additionally, the current proposed dose is
a fraction of the systemic toxic dose. These features of
apaziquone are distinct from other intravesical agents currently
in use for the treatment of recurrent bladder cancer.
A Phase 1 dose-escalation marker lesion (tumor) study
demonstrated that apaziquone had no systemic toxicity, and was
well tolerated at the dose level being used in the Phase 3
trials. Apaziquone also demonstrated anti-tumor activity against
NMIBC, as evidenced by eight of twelve patients showing a
complete response, defined as the complete disappearance of the
marker lesion as confirmed by biopsy, after receiving six
treatments with apaziquone over a period of six weeks.
Phase 2 data has confirmed anti-tumor activity in patients with
multiple, recurrent NMIBC, as evidenced by 31 of
46 patients (67%) showing a complete response after
receiving six weekly treatments with 4 mg of apaziquone
instilled into the urinary bladder in this marker lesion study.
Apaziquone was well-tolerated, with no significant systemic
toxicity, and local toxicity limited to temporary chemical
cystitis (inflammation of the urinary bladder) resulting in
increased urinary frequency, dysuria (painful urination) and
hematuria (blood in the urine) in a few patients. At the
two-year follow up, eighteen patients (38%) were disease free.
In September 2005, we initiated an open label, multi-center
clinical study in Europe in high-risk NMIBC in 53 patients.
Patients with high-risk NMIBC usually have more aggressive
bladder cancer with higher incidence of recurrence
and/or
progression to a more invasive stage, where the cancer invades
the muscle wall of the bladder, which may require total surgical
removal of the bladder. Enrollment has been completed and all
patients will be followed for twenty-four months or until
recurrence or disease progression is observed.
In 2006, we performed a 20 patient pilot safety study in
low-grade NMIBC. In this study, apaziquone was found to be well
tolerated when a single 4 mg dose is given to patients
immediately following surgery. In addition, there was no adverse
effect on wound healing and apaziquone was not detected in the
bloodstream.
In March 2007, we received concurrence from the FDA for the
design of a Phase 3 study protocol for the treatment of
non-invasive bladder cancer under a special protocol assessment
procedure. The development plan for apaziquone is two
randomized, double-blind, placebo-controlled Phase 3 clinical
trials, each with 562 patients with
14
TaG1-G2
(low-grade) NMIBC. Patients are being randomized in a
one-to-one
ratio to apaziquone or placebo. Under the protocol, the patients
are given a single 4 mg dose following surgical removal of
the tumors. The primary endpoint is a statistically significant
difference (p < 0.05) in the rate of tumor recurrence at
year two between the apaziquone patient group and the placebo
group. The first study began during the second quarter of 2007,
and the second study began during the third quarter of 2007. In
2008, we received scientific advice from the European Medicines
Agency (EMEA) whereby the EMEA agreed that the two Phase 3
studies as designed should be sufficient for a regulatory
decision regarding European registration. We continue to recruit
sites and enroll patients in these two studies. In December
2009, we achieved our goal of completing enrollment for both
Phase 3 clinical trials by year-end 2009.
We plan to begin a multiple instillation phase 3 study in low to
intermediate NMIBC by the end of 2010.
The following describes the principal commercial terms relating
to apaziquone licensing and development.
|
|
|
| |
•
|
In October 2008, we terminated our 2001 license agreement for
apaziquone with INC
Research®,
formerly NDDO Research
Foundation®
(INC) in the Netherlands, as the patents underlying the
agreement were all about to expire. Pursuant to the termination,
INC assigned to us all rights it had in the know-how or
intellectual property licensed under the agreement and all
rights in may have had in any know-how or intellectual property
created during the term of the agreement. In exchange, we paid
INC a nominal amount of cash and issued them a nominal number of
shares of our common stock. In addition, INC is entitled to up
to 25,000 additional shares of our common stock and an
additional payment of $300,000 upon achievement of certain
regulatory milestones.
|
| |
| |
•
|
In October, 2008, we entered into a license, development, supply
and distribution agreement with Allergan pursuant to which we
and Allergan agreed to a collaboration for the development and
commercialization of a formulation of apaziquone suitable for
use in treating cancer or precancerous conditions via
instillation. The agreement with Allergan also provides that
Allergan has the exclusive right to make, develop and
commercialize apaziquone for the treatment of bladder cancer, or
pre-bladder cancer conditions worldwide except for Asia (as is
defined in the agreement). We also entered into a co-promotion
agreement with Allergan providing for the joint
commercialization of apaziquone in the United States, whereby we
and Allergan will share equally all profits and
commercialization expenses. We also have the right, in our sole
discretion, to opt-out of the co-promotion agreement before
January 1, 2012. If we elect to opt-out of the co-promotion
agreement, our share of any future development costs shall be
significantly reduced. Part of the aggregate development costs
and marketing expenses incurred by us since January 1, 2009
shall be reimbursed by Allergan in the form of a one-time
payment. In addition, if we opt-out of the co-promotion
agreement, the co-promotion agreement will terminate and instead
of a sharing of profit and expenses, Allergan will pay us
royalties on a percentage of net sales of the apaziquone in the
United States that are slightly greater than the royalties paid
on net sales outside the United States. In addition, Allergan
will pay us up to $245 million in additional milestones
based upon the achievement of certain sales milestones in the
United States.
|
| |
| |
•
|
In consideration for the rights granted under our license,
development, supply and distribution agreements with Allergan,
Allergan paid us an up-front fee of $41.5 million. In
addition, Allergan will pay us up to $304.0 million based
on the achievement of certain development, regulatory and sales
milestones. For example, for completing enrollment of both
aforementioned Phase III trials by year-end 2009, Allergan
paid us a $1.5 million milestone payment. Also, Allergan
has agreed to pay us tiered royalties starting in the mid-teens
based on a percentage of net sales of the apaziquone outside of
the United States.
|
| |
| |
•
|
We will continue to conduct the current Phase 3 clinical trials
as well as certain future planned clinical trials pursuant to a
joint development plan, of which Allergan will fund 65% of the
development costs.
|
| |
| |
•
|
In November 2009, we entered into a collaboration agreement with
Nippon Kayaku Co., LTD. for the development and
commercialization of apaziquone in Asia, except North and South
Korea (Nippon Kayaku Territory). In exchange, Nippon Kayaku paid
Spectrum an up-front payment of $15 million and agreed to
make additional payments of up to $136.0 million based on
the achievement of certain regulatory and commercialization
milestones contained in the agreement. In addition, Nippon
Kayaku received exclusive
|
15
|
|
|
| |
|
rights to apaziquone for the treatment of NMIBC in Asia,
including Japan and China. Nippon Kayaku will conduct apaziquone
clinical trials in the Nippon Kayaku Territory pursuant to a
development plan. In addition, Nippon Kayaku will be responsible
for all expenses relating to the development and
commercialization of apaziquone in the Nippon Kayaku Territory.
|
|
|
|
| |
•
|
Also in November 2009, we entered into a collaboration agreement
with Handok Pharmaceuticals for the development and
commercialization of apaziquone in North and South Korea. Under
the terms of the Handok collaboration agreement, Handok paid us
an up-front payment of $1.0 million and potential milestone
payments totaling approximately $19 million. The potential
milestone payments will be based on the achievement of certain
regulatory and commercialization milestones. Handok received
rights to apaziquone for the treatment of NMIBC in North and
South Korea. Additionally, Handok will conduct the apaziquone
clinical trials in North and South Korea pursuant to a
development plan and will be responsible for all expenses
relating to the development and commercialization of apaziquone
in North and South Korea.
|
Belinostat: Belinostat is a histone
deacytelase (HDAC) inhibitor that is being studied in multiple
clinical trials, both as a single drug and in combination with
chemotherapeutic drugs for the treatment of various
hematological and solid tumors. HDACs catalyze the removal of
chemical groups known as acetyl groups from certain portions of
human DNA, and thus regulate gene expression. By inhibiting this
enzyme, belinostat induces cell cycle arrest, and leads to
inhibition of cancer cell proliferation and induction of
apoptosis, or cell death. Additional mechanisms of action
thought to be responsible for belinostat’s anti-cancer
effect include inhibition of angiogenesis, or blood vessel
growth, and the resensitization of cells that have overcome drug
resistance to anticancer drugs, such as platinums and taxanes.
Belinostat is currently the only HDAC inhibitor in clinical
development with multiple potential routes of administration,
including intravenous administration, continuous intravenous
infusion and oral administration, which we believe may afford
belinostat with a significant competitive advantage.
Belinostat is currently in a registrational trial, under a
special protocol assessment, as a monotherapy for Peripheral
T-Cell Lymphoma (PTCL) an indication which has been granted
Orphan Drug and Fast Track designation by the FDA. The
registrational trial is an open-label, multicenter, single arm
efficacy and safety study, in which we plan to enroll
approximately 120 patients with relapsed or refractory
peripheral T-cell lymphoma, who have failed at least one prior
systemic therapy. We expect to file an NDA for belinostat in
PTCL in 2011.
Belinostat is also currently in a randomized Phase 2 trial for
carcinoma of unknown primary (CUP) in combination with
carboplatin and paclitaxel. There are currently no approved
therapies or drugs for treatment of CUP, which is an indication
with a large patient population. The NCI estimated that for
2008, approximately 2 to 4% of all cancers are CUP.
Based on the data from past and ongoing studies, we believe
there are many potential attributes associated with belinostat
that separate it from other currently marketed HDACs, including
efficacy when used alone and in combination, less toxicities
(when compared to other currently-marketed HDACs), including
lack of or less bone marrow toxicity, and a lack of other severe
side effects, such as mucositis, that may enable full dose
combinations of this drug with several other cytotoxic agents.
Hence, belinostat is currently being investigated in multiple
indications, both as monotherapy and in combination with other
treatment regimens. Numerous studies have been conducted, and
are ongoing, through the NCI and other well-known oncologic
academic institutions. Additionally, we plan on a comprehensive
development program for belinostat, which includes both
hematologic indications, such as PTCL, and solid tumor
indications, such as lung cancer, ovarian cancer, and CUP. Based
upon the foregoing, we believe belinostat potentially has broad
applicability and hence, commercial potential beyond that of
currently marketed HDACs.
The following describes the principal commercial terms relating
to belinostat licensing and development.
|
|
|
| |
•
|
In February 2010, we entered into a licensing and collaboration
agreement with TopoTarget, for the development and
commercialization of belinostat, pursuant to which TopoTarget
and we agreed to a collaboration for the development and
commercialization of belinostat. The agreement provides that we
have the exclusive right to make, develop and commercialize
belinostat in North America and India, with an
|
16
|
|
|
| |
|
option for China. The agreement also grants TopoTarget a
co-promote option if and only if we do not maintain a minimum
number (subject to adjustment for certain events outside of our
control) of field personnel (as defined in the agreement) for a
certain number of years post-approval of the PTCL indication.
|
|
|
|
| |
•
|
In consideration for the rights granted to us under the license
and collaboration agreement with TopoTarget, we paid TopoTarget
an up-front fee of $30.0 million. In addition, we will pay
up to $313 million and one million shares of Spectrum
common stock based on the achievement of certain development,
regulatory and sales milestones. as well as certain royalties on
net sales of belinostat.
|
| |
| |
•
|
Under the terms of the agreement, all development, including
studies, will be conducted under a joint development plan (JDP)
and in accordance with a mutually agreed upon target product
profile (TPP) provided that we have final decision-making
authority for all developmental activities in North America and
India (and China upon exercise of the option for China) and
TopoTarget has final decision-making authority for all
developmental activities in all other jurisdictions, We will
assume all responsibility for and future costs of the ongoing
registrational PTCL trial while TopoTarget will assume all
responsibility for and future costs of the ongoing Phase 2 CUP
trial. We and TopoTarget will conduct future planned clinical
trials pursuant to the JDP, of which we will fund 70% of
the development costs and TopoTarget will fund 30% of the
development costs.
|
| |
| |
•
|
We and TopoTarget will each pay 50% of the costs for chemical,
pharmaceutical and other process development related to the
manufacturing of the Product that are incurred with a mutually
agreed upon budget in the JDP. TopoTarget is responsible for
supplying us with both clinical and commercial product.
|
Ozarelix: Ozarelix is a Luteinizing
Hormone Releasing Hormone (LHRH) antagonist (a substance that
blocks the effects of a natural hormone found in the body).
Mechanistically, LHRH antagonists exert rapid inhibition of
luteinizing hormone and follicle stimulating hormone with an
accompanying rapid decrease in sex hormones and would therefore
be expected to be effective in a variety of hormonally dependent
disease states including ovarian cancer, prostate cancer, BPH,
infertility, uterine myoma and endometriosis.
In January 2010, based upon the mixed results of our earlier
Phase 2 study of ozarelix for the treatment of BPH and the
recently announced failure of Aeterna Zentaris’s large,
Phase 3, registrational trial of cetrorelix (another LHRH
antagonist), we discontinued development of ozarelix in BPH. We
estimate that this discontinuation will result in substantial
reduction in future clinical development expenses. Currently, we
are considering the future development of Ozarelix in other
indications.
The following describes the principal commercial terms relating
to ozarelix licensing and development.
|
|
|
| |
•
|
In 2004, we entered into a license agreement with a subsidiary
of Aeterna Zentaris, Inc., Aeterna Zentaris GmbH, whereby we
acquired an exclusive license to develop and commercialize
ozarelix in North America (including Canada and Mexico) and
India. In addition, we have a 50% financial interest in any
income Aeterna Zentaris derives from ozarelix in Japan. We are
contingently obligated to pay amounts based upon achievement of
milestones and a royalty based on any future net sales.
|
| |
| |
•
|
With certain exceptions, we are required to purchase all
finished drug product from Aeterna Zentaris for the clinical
development of ozarelix at a set price. The parties agreed to
discuss entering into a joint supply agreement for commercial
supplies of finished drug product.
|
| |
| |
•
|
The term of the license agreement expires ten years after the
first commercial sale of a product in any country within the
territory or as long as any product is covered by a patent in
any country in the territory, whichever term is longer, although
some obligations survive termination. In addition, the agreement
may be terminated earlier by either party (in some cases either
in whole or on a
product-by-product
and/or
country-by-country
and/or
indication-by-indication
basis), based upon material breach or the commencement of
bankruptcy or insolvency proceedings involving the other, or by
us upon sixty days’ notice to Aeterna Zentaris.
|
Ortataxel: In July 2007, we entered
into an exclusive worldwide license agreement for ortataxel with
Indena S.p.A., a third-generation taxane. In clinical studies,
ortataxel has been shown to be bioavailable when administered
orally to patients with solid tumors. In addition, it belongs to
a new generation of taxanes with the potential to be active
against tumors resistant to paclitaxel (Bristol-Myers
Squibb’s
Taxol®)
and docetaxel (Sanofi-Aventis’
Taxotere®).
Phase 1 and 2 studies in more than 350 patients with solid
tumors have shown activity in patients that
17
were refractory to treatment with the available taxane drugs.
The safety profile of ortataxel is comparable to that of
paclitaxel and docetaxel.
While optimizing the oral formulation for better
bioavailability, we will consider future studies with the oral
formulation.
The following describes the principal commercial terms relating
to ortataxel licensing and development.
|
|
|
| |
•
|
Under the terms of the license agreement with Indena, we are
obligated to make payments based on the achievement of certain
development, regulatory filing and sales milestones. We will
also pay Indena certain royalties on worldwide sales of
ortataxel, if and when the product is approved.
|
| |
| |
•
|
Also, we are obligated to purchase all of our requirements of
ortataxel active pharmaceutical ingredient from Indena.
|
Satraplatin: Satraplatin, an orally
administered platinum-derived chemotherapy agent, is being
developed by our sublicensee, GPC Biotech AG. On
October 30, 2007, GPC announced that the Phase 3 trial
evaluating satraplatin for the treatment of hormone-refractory
prostate cancer failed to meet its primary efficacy endpoint
and, as a result, GPC stopped seeking approval for satraplatin
in this indication. In November 2009, GPC merged with
Houston-based Agennix Inc. Agennnix is evaluating the
development plan for satraplatin.
The following describes the principal commercial terms relating
to satraplatin licensing and development.
|
|
|
| |
•
|
In 2001, we in-licensed exclusive worldwide rights to
satraplatin from its developer, Johnson Matthey, PLC in exchange
for an up-front fee, additional payments to be made based upon
achievement of certain milestones and royalties based on any net
sales, if and when a commercial drug is approved and sales are
initiated.
|
| |
| |
•
|
In 2002, in exchange for an up-front license fee and future
milestones and royalties, we entered into a co-development and
license agreement with GPC for worldwide rights for further
development and commercialization of satraplatin. Under the
terms of the agreement, GPC agreed to fully fund the development
expenses for satraplatin. We are entitled to additional revenues
upon: achievement of specified milestones by GPC, which are
generally based on regulatory and sales milestones; and
royalties on worldwide sales, if any, of the product.
|
Elsamitrucin: Elsamitrucin is an
anti-tumor antibiotic that acts as a dual inhibitor of two key
enzymes involved in DNA replication, topoisomerase I and II. By
inhibiting the activity of these two key enzymes involved in DNA
replication, elsamitrucin is thought to lead to DNA breaks that
prevent the correct replication of DNA and ultimately result in
cancer cell death.
On the basis of previous studies conducted by our licensor,
Bristol-Myers Squibb Inc. (BMS) elsamitrucin has been shown to
have minimal toxicity to bone marrow while demonstrating
promising anti-tumor activity.
We conducted a Phase 2, single agent study in heavily
pre-treated patients with NHL. The level of activity seen did
not justify further development for this indication as a single
agent. However, elsamitrucin appears to have synergy with taxane
and platinum derivatives in experimental models. Also, minimal
toxicity to bone marrow may allow combinations with other drugs
without a need to significantly reduce doses, which may result
in improved therapeutic effects. We are currently reviewing all
pre-clinical and clinical data of this product to determine the
best path forward for its development.
The following describes the principal commercial terms relating
to elsamitrucin licensing and development.
|
|
|
| |
•
|
We in-licensed exclusive worldwide rights to elsamitrucin from
its developer BMS, in 2001, in exchange for a small up-front fee
and additional future payments based upon achievement of
development and regulatory milestones and a royalty based on net
sales, if and when a commercial drug is approved and sales are
initiated.
|
Lucanthone: Lucanthone is an orally
administered small-molecule which inhibits Topoisomerase II
and AP endonuclease. In preclinical tests, lucanthone was shown
to enhance the sensitivity of animals to an anticancer agent in
a time dependent and reversible manner.
18
Lucanthone was originally used as an antiparasitic agent for the
treatment of schistosomiasis in the 1950s and 1960s, and has a
demonstrated safety profile. It was later discontinued because
better anti-parasitic medications became available. We are
currently working on the development plan for lucanthone.
The following describes the principal commercial terms relating
to lucanthone licensing and development.
|
|
|
| |
•
|
We entered into a license agreement with Dr. Robert E.
Bases, the inventor of a method of treating cancer of the
central nervous system through the administration of lucanthone
and radiation, whereby we acquired worldwide exclusive rights to
develop and commercialize a product based upon his invention in
May 2005. Under the terms of the license agreement, we made a
small up-front payment and are obligated to make additional
periodic payments, a payment upon achievement of a certain
regulatory milestone and royalties on potential net sales, if
any.
|
SPI-1620: SPI-1620 is a highly
selective peptide agonist of endothelin B receptors, which can
stimulate receptors on endothelial cells, the innermost layer of
cells lining the blood vessels. This technology takes advantage
of the fact that the blood supply to tumors is different than
the blood supply to healthy organs. Blood vessels in the growing
part of tumors are relatively devoid of smooth muscle covering
and are rich in endothelial cells. Therefore, by stimulating the
endothelial B receptors present on the endothelial cells,
SPI-1620 should selectively increase tumor blood flow while
sparing healthy tissue.
Chemotherapy is one of the mainstays of therapy for solid
carcinomas, including breast, lung, and prostate. Chemotherapy
uses drugs called cytotoxic agents that are poisonous to cells
and kill cancer cells. Chemotherapy often fails because adequate
and uniform distribution of the cytotoxic agents is not achieved
in the tumor, and serious side effects can result from toxicity
to normal cells. Consequently, any means to increase the
delivery of a cytotoxic agent selectively to tumors, while
minimizing its concentration in normal tissues may be beneficial.
SPI-1620 is being developed as an adjunct to chemotherapy. In
pre-clinical studies, when anti-cancer drugs, such as
paclitaxel, are administered shortly after SPI-1620, the
anti-cancer drug concentration in the tumor is increased several
fold. This results in increased anti-tumor efficacy at a given
dose of a cytotoxic agent, and might allow physicians to
maximize efficacy with reduced cytotoxic agent doses with
resultant decreased toxicity to the normal organs.
In the first quarter of 2008, we initiated an open label,
dose-escalation Phase 1 study assessing the safety,
tolerability, pharmacokinetics and pharmacodynamics of SPI-1620
in patients with recurrent or progressive carcinoma. We enrolled
the first patient in this study in February 2008, and are
continuing to enroll patients in this study.
The following describes the principal commercial terms relating
to SPI-1620 licensing and development.
|
|
|
| |
•
|
We acquired an exclusive worldwide license to develop and
commercialize SPI-1620 for the prevention and treatment of
cancer from Chicago Labs, Inc. in February 2005. We paid Chicago
Labs a small up-front fee and are obligated to make future
payments contingent upon the successful achievement of certain
development and regulatory milestones. In addition, we will pay
royalties and sales milestones on net sales, after marketing
approval is obtained.
|
SPI-205: SPI-205, a lipid suspension of
leteprinim, has demonstrated, in experimental models, benefits
in treating chemotherapy induced peripheral neuropathy.
Chemotherapy drugs can damage the nervous system, especially the
peripheral nervous system, which are those nerves that carry
motor (movement) information for muscle contraction and those
that carry sensory information such as touch, vibration, pain
and temperature. Damage to the peripheral nerves is known as
neuropathy. Currently, there is no effective treatment for
chemotherapy induced neuropathy.
During 2010, we plan to continue preclinical evaluation of
SPI-205.
RenaZorb: RenaZorb, a second-generation
lanthanum-based nanoparticle phosphate binding agent, has the
potential to treat hyperphosphatemia, (high phosphate levels in
blood), in patients with stage 5 chronic kidney disease
(end-stage renal disease). Hyperphosphatemia affects patients
with chronic kidney disease, especially end-
19
stage kidney disease patients on dialysis. It can lead to
significant bone disease (including pain and fractures) and
cardiovascular disease, and is independently associated with
increased mortality.
According to The United States Renal Data System (USRDS) in
2010, there will be an estimated 600,000 patients with end-stage
renal disease in the United States. Treatment of
hyperphosphatemia is aimed at lowering blood phosphate levels
by: (1) restricting dietary phosphorus intake; and
(2) using, on a daily basis, and with each meal, oral
phosphate binding drugs that facilitate fecal elimination of
dietary phosphate before its absorption from the
gastrointestinal tract into the bloodstream. Restricting dietary
phosphorus intake has historically not been a successful means
of serum phosphate control, therefore phosphate binders are the
mainstay of hyperphosphatemia management.
Currently marketed therapies for treating hyperphosphatemia
include polymer-based and lanthanum-based phosphate binders,
aluminum-based phosphate binders, and calcium-based phosphate
binders. Under the National Kidney Foundation K/DOQI guidelines,
both calcium-based phosphate binders and non-calcium,
non-aluminum, non-magnesium phosphate binders are recommended as
first line or long-term therapy for the management of
hyperphosphatemia. However, the current therapies require use of
a large number of pills or large pills to be chewed or swallowed
along with each meal, leading to problems with patient
compliance with the treatment regimen.
We believe that RenaZorb has the opportunity, because of its
potentially higher capacity for binding phosphate on an equal
weight basis, to significantly improve patient compliance by
offering the
lowest-in-class
dosage to achieve the same therapeutic benefit as other
phosphate binders. We continue to perform preclinical
development work on RenaZorb.
The following describes the principal commercial terms relating
to RenaZorb licensing and development.
|
|
|
| |
•
|
We entered into a license agreement with Altair Nanomaterials,
Inc. and its parent Altair Nanotechnologies, Inc., whereby we
acquired an exclusive worldwide right to develop and
commercialize RenaZorb for all human therapeutic and diagnostic
uses in January 2005. Under the terms of the license agreement,
we made up-front and milestone payments and are obligated to
make additional payments upon achievement of certain clinical
development and regulatory and sales milestones, in addition to
royalties on potential net sales.
|
| |
| |
•
|
In August 2009, we entered into an acquisition agreement with
Altair, in which we acquired 100% of the rights to Renazorb and
all of Altair’s life science technology. Our acquisition of
RenaZorb expands upon our prior license agreement with Altair,
pursuant to which Altair granted us human uses. Our acquisition
of RenaZorb provides us with access to all uses of and
intellectual property for RenaZorb. In consideration for the
acquisition, we paid Altair a total of $750,000 in the form of
restricted shares of our common stock.
|
Manufacturing
We currently do not have internal manufacturing capabilities;
therefore, all of our products are manufactured on a contract
basis. We expect to continue to contract with third party
providers for manufacturing services, including active
pharmaceutical ingredient (API), finished-dosage product, as
well as packaging operations. We believe that our current
agreements with third party manufacturers provide for sufficient
operating capacity to support the anticipated commercial demand
for our products. However, we have only one approved contract
manufacturer for each aspect of the manufacturing process for
Zevalin and Fusilev. If we are unable to obtain a sufficient
supply of our required products, or if we should encounter
delays or difficulties in our relationships with our
manufacturers, we may lose potential sales.
We attempt to prevent disruption of supplies through supply
agreements, appropriate forecasting, maintaining stock levels
and other strategies. We believe that the market for such
manufacturers and suppliers is such that we could quickly enter
into another supply or manufacturing agreement, on substantially
similar terms, if we were required to do so. However, in the
event we are unable to manufacture our products, either directly
or indirectly through others or on commercially acceptable
terms, if at all, we may not be able to commercialize our
products as planned. Although we are taking these actions to
avoid a disruption in supply, we cannot provide assurance that
we may not experience a disruption in the future.
20
Sales,
Marketing and Distribution
We have built, and continue to build, a sales and marketing
infrastructure as part of our commercialization efforts for
Fusilev and Zevalin. While we maintain a relatively small sales
force, we believe that the size of our sales force is
appropriate to effectively reach our target audience for our two
commercial products.
For Fusilev, we utilize a third-party logistics company to store
and distribute this drug product. The same third party logistics
company also stores and ships Zevalin kits containing the CD20
MAB.
For Zevalin, we changed the supply and distribution model in
2009. Previously, we sold Zevalin kits containing the CD20 MAB
to radiopharmacies, who then in turn ordered the radioactive
isotope (Y-90 or In-111) separately and radiolabeled (or
attached) the radioactive isotope to the CD20 MAB. The
radiopharmacy then sold the end user product to the consumer.
Under the current model we do not sell the Zevalin kits
containing the CD20 MAB to the radiopharmacies, but instead
contract with them, as a
fee-for-service,
to radiolabel the individual components of the CD20 MAB to the
radioactive isotope, and then, also under a
fee-for-service
arrangement, have them distribute the end use product to the end
user, which are clinics, hospitals or other medical settings. In
this regard, we now sell the CD20 MAB together with the
radioactive isotope as the end user product directly to the
healthcare service provider.
Customers
Our product sales are concentrated in a limited number of
customers. For the year ended December 31, 2009,
approximately 44% of our Fusilev product sales were derived from
specialty distributors of oncology products as compared to 100%
for the year ended 2008; for Zevalin, we reaccorded 21% of
revenues from radiopharmacies as compared to 0% for the years
ended December 31, 2009 and 2008, respectively; and the
balance from end use customers. For Zevalin, not a single end
user customer constituted revenues over 10% individually. Due to
changes in market dynamics, these ratios are not indicative of
future concentrations. As of December 31, 2009, for
Fusilev, not a single specialty distributor owed us more than
10% of the total net accounts receivables. Three specialty
distributors owned us 100% of receivables at the end of 2008.
For Zevalin, no single end user customer owed us more than 10%
of net receivables as of December 31, 2009 or 2008. All
sales were to customers in the United States.
Due to changes in market dynamics, these ratios are not
indicative of future concentrations. We do not require
collateral or other security to support credit sales, but
provide an allowance for bad debts when warranted, based on
review of our receivables.
Competition
The pharmaceutical industry is characterized by rapidly evolving
biotechnology and intense competition. We expect
biotechnological developments and improvements in the fields of
our business to continue to occur at a rapid rate and, as a
result, expect competition to remain intense. Many companies are
engaged in research and development of compounds that are
similar to our research. Biotechnologies under development by
these and other pharmaceutical companies could result in
treatments for the diseases and disorders for which we are
developing our own treatments. In the event that one or more of
those programs are successful, the market for some of our drug
products could be reduced or eliminated. Any product for which
we obtain FDA approval must also compete for market acceptance
and market share.
Competing in the branded product business requires us to
identify and quickly bring to market new products embodying
therapeutic innovations. Successful marketing of branded
products depends primarily on the ability to communicate the
effectiveness, safety and value of the products to healthcare
professionals in private practice, group practices, hospitals
and academic institutions, and managed care organizations.
Competition for branded drugs is less driven by price and is
more focused on innovation in treatment of disease, advanced
drug delivery and specific clinical benefits over competitive
drug therapies. Unless our products are shown to have a better
safety profile, efficacy and cost-effectiveness as compared to
other alternatives, they may not gain acceptance by medical
professionals and may therefore never be successful commercially.
Companies that have products on the market or in research and
development that target the same indications as our products
target include, among others, Abraxis Bioscience, Inc., Astra
Zeneca LP, Bayer AG, Endo Pharmaceuticals, Eli Lilly and Co.,
Novartis Pharmaceuticals, Corporation, Genentech, Inc.,
Bristol-Myers Squibb Company, GlaxoSmithKline, Biogen-IDEC
Pharmaceuticals, Inc., OSI Pharmaceuticals, Inc., Cephalon,
Inc., Sanofi-Aventis,
21
Inc., Pfizer, Inc., Genta Incorporated, Merck, Celegen
Corporation, Allos Therapeutics, Inc., BiPar Sciences, Inc.,
Genzyme Corporation, Shire Pharmaceuticals, Abbott Laboratories,
Poniard Pharmaceuticals, Inc., Roche Pharmaceuticals and
Johnson & Johnson who may be more advanced in
development of competing drug products or are more established
and are currently marketing products for the treatment of
various indications that our drug products target. Many of our
competitors are large and well-capitalized companies focusing on
a wide range of diseases and drug indications, and have
substantially greater financial, research and development,
marketing, human and other resources than we do. Furthermore,
large pharmaceutical companies have significantly more
experience than we do in pre-clinical testing, human clinical
trials and regulatory approval procedures, among other things.
As noted above, we launched our proprietary product, Fusilev, in
August 2008. Fusilev is the levo-isomeric form of the racemic
compound calcium leucovorin, a product already approved for the
same indications our product is approved for. Leucovorin has
been sold as a generic product on the market for a number of
years. There are two generic companies currently selling the
leucovorin product and therefore we are competing against a low
cost alternative. Also, Fusilev will be offered as part of a
treatment regimen, and that regimen may change to exclude
Fusilev. For these reasons, we may not recognize the full
potential value of our investment in the product.
Regarding Zevalin, there are three products which are potential
competitors for the indications it is currently approved for.
Treanda®
(bendamustine hydrochloride) for Injection, for Intravenous
Infusion, marketed by Cephalon, is indicated for the treatment
of patients with indolent B-cell NHL that has progressed during
or within six months of treatment with rituximab or a
rituximab-containing regimen.
Also, the
Bexxar®
therapeutic regimen (Tositumomab and Iodine I 131 Tositumomab),
a radiopharmaceutical marketed by GlaxoSmithKline, is indicated
for the treatment of patients with CD20 antigen-expressing
relapsed or refractory, low-grade, follicular, or transformed
NHL, including patients with Rituximab-refractory NHL.
Finally,
Rituxan®
(rituximab), marketed by Genentech and Biogen, is indicated for
the treatment of patients with relapsed or refractory, low-grade
or follicular, CD20-positive, B-cell NHL as a single agent;
previously untreated follicular, CD20-positive, B-cell NHL in
combination with CVP (cyclophosphamide, vincristine and
prednisolone combination) chemotherapy; and non-progressing
(including stable disease), low-grade, CD20-positive B-cell NHL,
as a single agent, after first-line CVP chemotherapy. Rituxan is
administered as a part of various chemotherapy regimens and
schedules, the vast majority of which, could be used in concert
with other therapeutic agents, such as Zevalin, as part of a
treatment plan.
For more information regarding competition to our products,
please also read our discussion of competition matters in
Item 1A “Risk Factors” of this report.
Research
and Development
New drug development, which is the process whereby drug product
candidates are tested for the purpose of filing a NDA or BLA (or
similar filing in other countries) and eventually obtaining
marketing approval from the FDA or a similar marketing
authorization from other regulatory authorities outside of the
United States, is an inherently uncertain, lengthy and expensive
process that requires several phases of clinical trials to
demonstrate to the satisfaction of the appropriate regulatory
authorities that the products are both safe and effective for
their respective indications. Our development focus is primarily
based on acquiring and developing late-stage development drugs
as compared to new drug discovery, which is very uncertain and
lengthy.
22
Research and development expenses for such drug development are
comprised of the following types of costs incurred in performing
research and development activities: personnel expenses,
facility costs, contract services, license fees and milestone
payments, costs of clinical trials, laboratory supplies and drug
products, and allocations of corporate costs. Research and
development expenditures, including related stock-based charges
but not including amortization of intangibles or expensing of
in-process research and development costs, are expensed as we
incur them and were approximately $21.1 million in 2009,
$26.7 million in 2008 and $33.3 million in 2007 broken
out by product as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
($ in ’000’s)
|
|
|
|
|
Eoquin
|
|
$
|
10,915
|
|
|
$
|
5,477
|
|
|
$
|
6,348
|
|
|
Ozarelix
|
|
|
1,168
|
|
|
|
2,435
|
|
|
|
6,217
|
|
|
Ortataxel
|
|
|
311
|
|
|
|
150
|
|
|
|
3,719
|
|
|
Fusilev
|
|
|
940
|
|
|
|
1,791
|
|
|
|
1,368
|
|
|
Zevalin
|
|
|
563
|
|
|
|
151
|
|
|
|
—
|
|
|
Lucanthone
|
|
|
289
|
|
|
|
348
|
|
|
|
1,405
|
|
|
Other development drugs
|
|
|
496
|
|
|
|
956
|
|
|
|
2,046
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total — Direct Costs
|
|
|
14,682
|
|
|
|
11,308
|
|
|
|
21,103
|
|
|
Indirect Costs (including non-cash share-based compensation of
$4.1 million, $3.9 million and $3.6 million,
respectively)
|
|
|
6,376
|
|
|
|
15,375
|
|
|
|
12,182
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Research & Development
|
|
$
|
21,058
|
|
|
$
|
26,683
|
|
|
$
|
33,285
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Patents
and Proprietary Rights
Our
Patents and Proprietary Rights
We in-license from third parties certain patent and related
intellectual property rights related to our proprietary
products. In particular, we have licensed patent rights with
respect to Fusilev, Zevalin, ozarelix, ortataxel, satraplatin,
elsamitrucin, lucanthone, belinostat and SPI-1620, in each case
for the remaining life of the applicable patents. Except for
Zevalin, Fusilev, belinostat and ozarelix, our agreements
generally provide us with exclusive worldwide rights to, among
other things, develop, sublicense, and commercialize the drug
products. Under most of these license arrangements, we are
generally responsible for all development, patent filing and
maintenance costs, sales, marketing and liability insurance
costs related to the drug products. In addition, these licenses
and agreements may require us to make royalty and other payments
and to reasonably exploit the underlying technology of
applicable patents. If we fail to comply with these and other
terms in these licenses and agreements, we could lose the
underlying rights to one or more of our potential products,
which would adversely affect our product development and harm
our business. In addition, with regard to Zevalin, apaziquone,
RenaZorb and SPI-205, we own patent and related intellectual
property rights related to these products.
The protection, preservation and infringement-free commercial
exploitation of these patents and related intellectual property
rights is very important to the successful execution of our
strategy. However, the issuance of a patent is not conclusive as
to its validity nor as to the enforceable scope of the claims of
the patent. Accordingly, our patents and the patents we have may
not prevent other companies from developing similar or
functionally equivalent products or from successfully
challenging the validity of our patents. If our patent
applications are not allowed or, even if allowed and issued as
patents, if such patents or the patents we have in-licensed, are
circumvented or not upheld by the courts, our ability to
competitively exploit our patented products and technologies may
be significantly reduced. Also, such patents may or may not
provide competitive advantages for their respective products or
they may be challenged or circumvented by competitors, in which
case our ability to commercially exploit these products may be
diminished.
23
From time to time, we may need to obtain licenses to patents and
other proprietary rights held by third parties to develop,
manufacture and market our products. If we are unable to timely
obtain these licenses on commercially reasonable terms, our
ability to commercially exploit such products may be inhibited
or prevented.
As mentioned above, we own and in-license from third parties
certain patent rights related to our products. We believe that
our patents and licenses are important to our business, but that
with the exception of the United States and European patents
discussed in this paragraph, no one patent or license is
currently of material importance to our business. For Fusilev,
we have one United States formulation patent that covers Fusilev
that expires in 2019. For Zevalin, we have sublicensed United
States patents that cover the processes and tools for making
monoclonal anti-bodies (MABs), in general, licensed United
States patents that cover the CD-20 MAB in Zevalin as well as
the use of Zevalin to treat NHL, and acquired patent
applications covering the Zevalin compounding process
(i.e., process of linking the CD20 MAB to a radioactive
isotope to make the patient-ready dosage form of Zevalin). These
patents expire over a wide range of dates beginning in 2009, but
the licensed patents covering the CD-20 MAB itself do not begin
to expire until 2015. Additionally, we have pending United
States patent applications covering the compounding process, and
will consider filing more patent applications, if the
opportunity arises. For belinostat, there are composition of
matter patents that cover belinostat and related compounds that
do not begin to expire until 2021. Currently, there are multiple
United States and foreign patent applications pending that cover
belinostat formulations, uses and manufacturing and synthesis
processes. We plan to file additional United States and foreign
patent applications covering new formulations, uses and
manufacturing and synthesis processes, where appropriate. For
apaziquone, there is a United States formulation patent that
does not expire until 2022. We have filed and plan to file
additional United States and foreign patent applications
covering new formulations
and/or uses
for this product. For ozarelix, there is a United States
composition patent that will expire in 2020, and method of use
and formulation patent applications on file in the United
States. For ortataxel, there are two United States composition
patents that will expire in 2013 and multiple manufacturing and
synthesis patents that do not begin to expire till 2021, and the
corresponding European patents will expire in 2014. We
anticipate filing new method of use and formulation patent
applications for the ortataxel product in the future. There is
one United States patent covering satraplatin, a method of use
patent that expires in 2010. For elsamitrucin, we have filed
United States and foreign formulation and method of use patent
applications, and we anticipate filing future United States and
foreign patent applications covering new formulations
and/or uses
for this product. For lucanthone, there is a United States
method of use patent that expires in 2019. For RenaZorb, there
is one method of use patent that expires in 2024 and pending
United States and foreign patent applications covering
compositions of matter directed to treating hyperphosphatemia.
For SPI-1620, we have filed method of use patent applications in
the United States and Europe. For SPI-205, there is a United
States composition and method of use patent that expires in
2010. This patent expires in certain European countries in 2011.
We also have multiple United States method of use patents that
expire in 2021 and 2022, and there is ongoing prosecution for
their European counterparts. We have also filed another method
of use patent application in the United States and Europe and
anticipate filing future patent applications pending the
continued development of new methods of use and new
formulations. We are constantly evaluating our patent portfolio
and are currently prosecuting patent applications for our drug
products and are considering new patent applications in order to
maximize the life cycle of each of our products.
While the United States and the European Union are currently the
largest potential markets for most of our products, we also have
patents issued and patent applications pending outside of the
United States and Europe. Limitations on patent protection in
these countries, and the differences in what constitutes
patentable subject matter in countries outside the United
States, may limit the protection we have on patents issued or
licensed to us outside of the United States. In addition, laws
of foreign countries may not protect our intellectual property
to the same extent as would laws in the United States. To
minimize our costs and expenses and to maintain effective
protection, we usually focus our patent and licensing activities
within the United States, the European Union, Canada and Japan.
In determining whether or not to seek a patent or to license any
patent in a certain foreign country, we weigh the relevant costs
and benefits, and consider, among other things, the market
potential and profitability, the scope of patent protection
afforded by the law of the jurisdiction and its enforceability,
and the nature of terms with any potential licensees. Failure to
obtain adequate patent protection for our proprietary drugs and
technology would impair our ability to be commercially
competitive in these markets.
24
In addition to the specific intellectual property subjects
discussed above, we have trademark protection in the United
States for Spectrum Pharmaceuticals,
Inc.®,
Fusilev®,
Turning Insights Into
Hopetm,
Zevalin®
and
RenaZorb®.
Additionally, for some other of these and other works related to
our business, we have pending United States and
ex-United
States trademark applications.
EOquin®
is a registered trademark of Allergan.
In conducting our business generally, we rely upon trade
secrets, know-how, and licensing arrangements and use customary
practices for the protection of our confidential and proprietary
information such as confidentiality agreements and trade secret
protection measures, such as periodic internal and external
trade secret audits. It is possible that these agreements will
be breached or will not be enforceable in every instance, and
that we will not have adequate remedies for any such breach. It
is also possible that our trade secrets or know-how will
otherwise become known or independently developed by
competitors. The protection of know-how is particularly
important because the know-how is often the necessary or useful
information that allows us to practice the claims in the patents
related to our proprietary drug products.
We may find it necessary to initiate litigation to enforce our
patent rights, to protect our trade secrets or know-how or to
determine the scope and validity of the proprietary rights of
others. Litigation concerning patents, trademarks, copyrights
and proprietary technologies can often be protracted and
expensive and, as with litigation generally, the outcome is
inherently uncertain. See Item 1A “Risk Factors”
for more information.
The
Patent Process
The United States Constitution provides Congress with the
authority to provide inventors the exclusive right to their
discoveries. Congress codified this right in United States Code
Title 35, which gave the U.S. Patent and Trademark
Office (USPTO) the right to grant patents to inventors and
defined the process for securing a United States patent. This
process involves the filing of a patent application that teaches
a person having ordinary skill in the respective art how to make
and use the invention in clear and concise terms. The invention
must be novel (not previously known) and non-obvious (not an
obvious extension of what is already known). The patent
application concludes with a series of claims that specifically
describe the subject matter that the patent applicant considers
his invention.
The USPTO undertakes an examination process that can take from
one to seven years, or more, depending on the complexity of the
patent and the problems encountered during examination.
In exchange for disclosing the invention to the public, for all
United States patent applications filed after 1995, the
successful patent applicant is currently provided a right to
exclude others from making, using or selling the claimed
invention for a period of 20 years from the effective
filing date of the patent application.
Under certain circumstances, a patent term may be extended.
Patent extensions are most frequently granted in the
pharmaceutical and medical device industries under the Drug
Price Competition and Pricing Term Restoration Act of 1984
(Hatch-Waxman Act) to recover some of the time lost during the
FDA regulatory process, subject to a number of limitations and
exceptions. The patent term may be extended up to a maximum of
five years; however, as a general rule, the average extension
period granted for a new drug is approximately three years. Only
one patent can be extended per FDA approved product, and a
patent can only be extended once.
Product
Exclusivity
Under the Hatch-Waxman Act, drug products are provided
exclusivity whereby the FDA will not accept applications to
market a generic form of an innovator reference listed drug
product until the end of the prescribed period. A product is
granted a five-year period of exclusivity if it contains a
chemical entity never previously approved by the FDA either
alone or in combination, although generic applications may be
submitted after four years if they contain a certification of
patent invalidity or non-infringement as further discussed
below. A three-year period of exclusivity is granted to a
previously approved product based on certain changes, e.g.,
in strength, dosage form, route of administration or
conditions of use, where the application is supported by new
clinical investigations that are essential to approval. In
addition, in 1997 Congress amended the law to provide an
additional six months of exclusivity as a reward for studying
drugs in children. This pediatric exclusivity, which can be
obtained during the approval process or after approval,
effectively delays the approval of a generic application until
six months after the
25
expiration of any patent or other exclusivity that would
otherwise delay approval, thus providing an additional six
months free of generic competition. In order to qualify for
pediatric exclusivity, the FDA must make a written request for
pediatric studies, the application holder must agree to the
request and complete the studies with required timeframe, and
the studies must be accepted by the FDA based on a determination
that the studies fairly respond to the request. The provisions
were enacted with a five-year sunset date, and have been
reauthorized in 2002 and 2007. The current provisions are set to
expire in October 2012, and Congress is likely to consider
reauthorizing the statute again.
Generic
Approval and Patent Certification
The Hatch-Waxman Act also created the abbreviated new drug
application (ANDA) approval process, which permits the approval
of a generic version of a previously approved branded drug
without the submission of a full new drug application (NDA) and
based in part on the FDA’s finding of safety and
effectiveness for the reference listed drug. Applicants
submitting an NDA are required to list patents associated with
the drug product, which are published in the FDA Orange Book,
and the timing of an ANDA approval depends in part on patent
protection for the branded drug. When an ANDA is filed, the
applicant must file a certification for each of the listed
patents for the branded drug, stating one of the following:
(1) that there is no patent information listed;
(2) that such patent has expired; (3) that the patent
will expire on a particular date (indicating that the ANDA may
be approved on that date); or (4) that the drug for which
approval is sought either does not infringe the patent or the
patent is invalid, otherwise known as paragraph IV
certification. If an ANDA applicant files a paragraph IV
certification, it is required to provide the patent holder with
notice of that certification. If the patent holder brings suit
against the ANDA applicant for patent infringement within
45 days of receiving notice, the FDA may not approve the
ANDA until the earlier of (1) 30 months from the
patent holder’s receipt of the notice (the
30-month
stay) or (2) the issuance of a final, non-appealed, or
non-appealable court decision finding the patent invalid,
unenforceable or not infringed.
The Hatch-Waxman Act also provided an incentive for generic
manufacturers to file paragraph iv certifications challenging
patents that may be invalid unenforceable, or not infringed,
whereby the first company to successfully challenge a listed
patent and receive ANDA approval is protected from competition
from subsequent generic versions of the same drug product for
180 days after the earlier of (1) the date of the
first commercial marketing of the first-filed ANDA
applicant’s generic drug or (2) the date of a decision
of a court in an action holding the relevant patent invalid,
unenforceable, or not infringed. These
180-day
exclusivity provisions have been the subject of litigation and
administrative review, and the Medicare Prescription Drug,
Improvement, and Modernization Act of 2003 (MMA) amended the
provisions in several ways, including by providing that an ANDA
applicant entitled to
180-day
exclusivity may lose such exclusivity if any of the following
events occur: (1) failure to market; (2) withdrawal of
the ANDA; (3) change in patent certification;
(4) failure to obtain tentative approval; (5) illegal
settlement agreement; and (6) patent expiration.
With respect to the illegal settlement prong, the MMA amendments
require that certain types of settlement agreements entered into
between branded and generic pharmaceutical companies related to
the manufacture, marketing and sale of generic versions of
branded drugs are required to be filed with the Federal Trade
Commission and the Department of Justice for review of potential
anti-competitive practices. This requirement could affect the
manner in which generic drug manufacturers resolve intellectual
property litigation and other disputes with branded
pharmaceutical companies, and could result generally in an
increase in private-party litigation against pharmaceutical
companies. The impact of this requirement, and the potential
governmental investigations and private-party lawsuits
associated with arrangements between brand name and generic drug
manufacturers, remains uncertain and could adversely affect our
business. In addition, Congress has considered enacting
legislation that would prohibit such settlements between brand
name and generic drug manufacturers. Such a provision was
considered as part of the recently enacted healthcare reform,
the Patient Protection and Affordable Care Act (PPACA) signed
into law on March 23, 2010. However, Congress removed the
provision prior to passage. It is possible that Congress will
again consider a ban on such settlements between brand name and
generic drug manufacturers in the future.
With the passage of the PPACA, there are now exclusivity
protections for certain innovator biological products and a
framework for FDA review and approval of biosimilar and
interchangeable versions of innovator biologic products. The
PPACA provides that no application for a biosimilar product may
be approved until 12 years after the date on which the
innovator product was first licensed, and no application may be
submitted until four years after
26
the date of first licensure. Products deemed interchangeable (as
opposed to biosimilar) are also eligible for certain exclusivity.
Please also read our discussion of patent and intellectual
property matters in Item 1A “Risk Factors”
section of this report.
Orphan
Drug Designation
Some jurisdictions, including Europe and the United States, may
designate drugs for relatively small patient populations as
“orphan” drugs. The FDA may grant orphan drug
designation to a drug intended to treat a rare disease or
condition that affects fewer than 200,000 individuals in the
United States, and a drug may also be considered an orphan even
if the drug treats a disease or condition affecting more than
200,000 individuals in the United States where the drug has no
expected profitability. Orphan drug designation does not
necessarily convey any advantage in, or shorten the duration of,
the regulatory review and process for marketing approval. If a
product with an orphan drug designation subsequently receives
the first FDA approval for the indication for which it has such
designation, the product is entitled to seven years of orphan
drug exclusivity, during which time FDA will not approve any
other application to market the same drug for the same
indication except in limited circumstances, such as a showing of
clinical superiority to the product with orphan exclusivity.
Also, competitors are not prohibited from receiving approval to
market the same drug or biologic for a different indication than
that which received orphan approval.
Under European Union medicines laws, the criteria for
designating an “orphan medicinal product” are similar
in principle to those in the United States. Criteria for orphan
designation are set out in Article 3 of Regulation (EC)
141/2000 on the basis of two alternative conditions. A medicinal
product may be designated as orphan if it is intended for the
diagnosis, prevention or treatment of a life-threatening or
chronically debilitating condition affecting not more than five
in 10 thousand persons in the European Union (EU) when the
application is made. This is commonly known as the “disease
prevalence criterion” Alternatively, a product may be so
designated if it is intended for the diagnosis, prevention or
treatment of a life-threatening, seriously debilitating or
serious and chronic condition in the EU and if without
incentives it is unlikely that the marketing of the product in
the EU would generate sufficient return to justify the necessary
investment. This is commonly known as the “insufficient
return criterion.”
These two alternative criteria must cumulatively meet the second
condition that there exists no satisfactory method of diagnosis,
prevention or treatment of the condition in question that has
been authorized in the EU, or if such a method exists, the
product will be of significant benefit to those affected by the
condition. “Significant benefit” is defined in
Regulation (EC) 847/2000 as a clinically relevant advantage or a
major contribution to patient care.
Upon grant of a marketing authorization, orphan medicinal
products are entitled to ten years of market exclusivity in
respect of the approved therapeutic indication. Within the
period of market exclusivity, no competent authority in the EU
is permitted to accept an application for marketing
authorization, a variation or a line-extension for the same
approved therapeutic indication in respect of a similar
medicinal product pursuant to Article 8.1 of
Regulation 141/2000 unless one of derogations set out in
Article 8.3 of the same Regulation applies. In order to
determine whether two products are considered similar,
Regulation 847/2000 requires an assessment of the principal
molecular structure and the underlying mode of action. Any minor
variation or modification of the principal molecular structure
would not ordinarily render the second product dissimilar to the
first authorized product.
In order for the second applicant to break the market
exclusivity granted to the first authorized similar medicinal
product in respect of the same therapeutic indication, the
second applicant would principally rely upon data to demonstrate
that his product is safer, more efficacious or clinically
superior to the first product pursuant to Article 8.3(c) of
Regulation 141/2000. Ordinarily, such an assessment will
require a
head-to-head
comparative clinical trial for the purpose of demonstrating
clinical superiority.
The 10-year
market exclusivity may be reduced to 6 years if at the end
of the fifth year it is established that the product no longer
meets the criteria for orphan designation on the basis of
available evidence.
27
Fusilev has been granted orphan drug designations for its use in
conjunction with high dose methotrexate in the treatment of
osteosarcoma and for its use in combination chemotherapy with
the approved agent 5-fluorouracil in the palliative treatment of
metastatic adenocarcinoma of the colon and rectum (colorectal
cancer). In addition, belinostat has been granted an orphan drug
designation for PTCL. As discussed above, a drug with orphan
designation status may obtain orphan exclusivity upon marketing
approval under specified conditions set out in the applicable
laws and regulations.
Governmental
Regulation
The development, production and marketing of our proprietary and
generic drug products are subject to regulation for safety,
efficacy and quality by numerous governmental authorities in the
United States and other countries. In the United States, drugs
are subject to rigorous regulation. The Federal Food, Drug, and
Cosmetic Act, as amended from time to time, and the regulations
promulgated there under, as well as other federal and state
statutes and regulations, govern, among other things, the
development, approval, manufacture, safety, labeling, storage,
record keeping, distribution, promotion, and advertising of our
products. Product development and approval within this
regulatory framework, including for drugs already at a clinical
stage of development, can take many years and require the
expenditure of substantial resources, and to obtain FDA
approval, a product must satisfy mandatory procedures and safety
and efficacy requirements. In addition, each drug-manufacturing
establishment must be registered with the FDA. Domestic
manufacturing establishments must comply with the FDA’s
current good manufacturing practice (cGMP) regulations and are
subject to inspections by the FDA. To supply drug ingredients or
products for use in the United States, foreign manufacturing
establishments must also comply with cGMP and are subject to
inspections by the FDA or by other regulatory authorities in
certain countries under reciprocal agreements with the FDA.
General
Information about the Drug Approval Process and Post-Marketing
Requirements
The United States system of new drug approval is one of the most
rigorous in the world. Only a small percentage of compounds that
enter the pre-clinical testing stage are ever approved for
commercialization. Our strategy focuses on in-licensing clinical
stage drug products that are already in or about to enter human
clinical trials. A late-stage focus helps us to effectively
manage the high cost of drug development by focusing on
compounds that have already passed the many hurdles in the
pre-clinical and early clinical process.
The following general comments about the drug approval process
are relevant to the development activities we are undertaking
with our proprietary drugs.
Pre-clinical Testing: During the pre-clinical
testing stage, laboratory and animal studies are conducted to
show biological activity of a drug compound against the targeted
disease and the compound is evaluated for safety.
Investigational New Drug Application: After
pre-clinical testing, an Investigational New Drug, or IND,
Application is submitted to the FDA to request the ability to
begin human testing of the drug. An IND becomes effective thirty
days after the FDA receives the application (unless the FDA
notifies the sponsor of a clinical hold), or upon prior
notification by the FDA.
Phase 1 Clinical Trials: These trials,
typically involving small numbers of healthy volunteers or
patients, usually define a drug candidate’s safety profile,
including the safe dosage range.
Phase 2 Clinical Trials: In phase 2 clinical
trials, controlled studies of human patients with the targeted
disease are conducted to assess the drug’s effectiveness.
These studies are designed primarily to determine the
appropriate dose levels, dose schedules and route(s) of
administration, and to evaluate the effectiveness of the drug on
humans, as well as to determine if there are any side effects on
humans to expand the safety profile following phase 1. These
clinical trials, and phase 3 trials discussed below, are
designed to evaluate the drug’s overall benefit-risk
profile, and to provide information to inform physician labeling.
Phase 3 Clinical Trials: This phase usually
involves larger number of patients with the targeted disease.
Investigators (typically physicians) monitor the patients to
determine the drug candidate’s efficacy and to observe and
report any adverse reactions that may result from long-term use
of the drug on a large, more widespread, patient population.
During the phase 3 clinical trials, typically the drug candidate
is compared to either a placebo or a standard treatment for the
target disease.
28
New Drug Application: After completion of all
three clinical trial phases, if the data indicates that the drug
is safe and effective, a NDA is filed with the FDA requesting
FDA approval to market the new drug as a treatment for the
target disease.
Fast Track and Priority Review: The FDA has
established procedures for accelerating the approval of drugs to
be marketed for serious or life threatening diseases for which
the manufacturer can demonstrate the potential to address unmet
medical needs.
Abbreviated New Drug Application: An ANDA is
the abbreviated review and approval process created by the Drug
Price Competition and Patent Term Restoration Act of 1984 signed
into law in part for the accelerated approval of generic drugs.
When a company files an ANDA, it must make a patent
certification regarding the patents covering the branded product
listed in the FDA’s Orange Book. An ANDA applicant must
make one of four certifications: (1) that there is no
patent information listed in the Orange Book; (2) that the
listed patent has expired; (3) that the listed patent will
expire on a stated date and the applicant will not market the
product until the patent expires; or (4) that the listed
patent is invalid or will not be infringed by the generic
product. The ANDA drug development and approval process
generally takes less time than the NDA drug development and
approval process since the ANDA process usually does not require
new clinical trials establishing the safety and efficacy of the
drug product.
NDA and ANDA Approval: The FDA approves drugs
that are subject to NDA review based on data in the application
demonstrating the drug is safe and effective in its proposed
use(s) and that the drug’s benefits outweigh its risks. FDA
will also review the NDA applicant’s manufacturing process
and controls to ensure they are adequate to preserve the
drug’s identity, strength, quality, and purity, and FDA
will review and approve the drug’s proposed labeling. As
for the ANDA approval process, these “abbreviated”
applications are generally not required to include preclinical
or clinical data to establish safety and effectiveness. Rather,
an ANDA must demonstrate both chemical equivalence and
bio-equivalence (the rate and extent of absorption in the body)
to the innovator drug — unless a bio-equivalence
waiver is granted by the FDA.
Phase 4 Clinical Trials: After a drug has been
approved by the FDA, phase 4 studies may be conducted to explore
additional patient populations, compare the drug to a
competitor, or to further study the risks, benefits and optimal
use of a drug. These studies may be a requirement as a condition
of the initial approval of the NDA.
Post-Approval Studies Requirements under
FDAAA: The Food and Drug Administration
Amendments Act of 2007 (FDAAA), which President Bush signed into
law in September 2007, significantly added to the FDA’s
authority to require post-approval studies. Under the FDAAA, if
the FDA becomes aware of new safety information after approval
of a product, they may require us to conduct further clinical
trials to assess a known serious risk, signals of serious risk
or to identify an unexpected serious risk. If required to
conduct a post-approval study, periodic status reports must be
submitted to the FDA. Failure to conduct such post-approval
studies in a timely manner may result in administrative action
being taken by FDA, including substantial civil fines.
Risk Evaluation and Mitigation Strategy Authority under
FDAAA: The FDAAA also gave the FDA new authority
to require the implementation of a Risk Evaluation and
Mitigation Strategy (REMS) for a product when necessary to
minimize known and preventable safety risks associated with the
product. The FDA may require the submission of a REMS before a
product is approved, or after approval based on “new safety
information,” including new analyses of existing safety
information. A REMS may include a medication guide, patient
package insert, a plan for communication with healthcare
providers, or other elements as the FDA deems are necessary to
assure safe use of the product, which could include imposing
certain restrictions on distribution or use of a product. A REMS
must include a timetable for submission of assessments of the
strategy at specified time intervals. Failure to comply with a
REMS — including the submission of a required
assessment — may result in substantial civil or
criminal penalties.
Other Issues Related to Product
Safety: Adverse events that are reported after
marketing approval also can result in additional limitations
being placed on a product’s use and, potentially,
withdrawal of the product from the market. In addition, under
the FDAAA, the FDA has authority to mandate labeling changes to
products at any point in a product’s lifecycle based on new
safety information derived from clinical trials, post-approval
studies, peer-reviewed medical literature, or post-market risk
identification and analysis systems data.
29
FDA
Enforcement
The development of drug products, as well as the marketing of
approved drugs, is subject to substantial continuing regulation
by the FDA, including regulation of adverse event reporting,
manufacturing practices and the advertising and promotion of the
drug. Failure to comply with the FDA and other governmental
regulations can result in fines, unanticipated compliance
expenditures, recall or seizure of products, total or partial
suspension of production
and/or
distribution, suspension of the FDA’s review of NDAs, ANDAs
or other product applications, enforcement actions, injunctions
and criminal prosecution. Under certain circumstances, the FDA
also has the authority to revoke previously granted drug
approvals. Although we have internal compliance programs, if
these programs do not meet regulatory agency standards or if our
compliance is deemed deficient in any significant way, it could
have a material adverse effect on our business. See Item 1A
“Risks Factors — Our failure to comply with
governmental regulation may delay or prevent approval of our
products
and/or
subject us to penalties.”
With respect specifically to information submitted to FDA in
support of marketing applications, the FDA, under its Fraud,
Untrue Statements of Material Facts, Bribery and Illegal
Gratuities Policy, can significantly delay the approval of a
marketing application — or seek to withdraw an
approved application — where it identifies fraud or
discrepancies in regulatory submissions. Such actions by the FDA
may significantly delay or suspend substantive scientific review
of a pending application during validity assessment or remove
approved products from the market until the assessment is
complete and questions regarding reliability of the data are
resolved. In addition, the Generic Drug Enforcement Act of 1992
established penalties for wrongdoing in connection with the
development or submission of an ANDA. Under this Act, the FDA
has the authority to permanently or temporarily bar companies or
individuals from submitting or assisting in the submission of an
ANDA, and to temporarily deny approval and suspend applications
to market generic drugs. The FDA may also suspend the
distribution of all drugs approved or developed in connection
with certain wrongful conduct
and/or
withdraw approval of an ANDA and seek civil penalties.
Healthcare
Reform
Continuing studies of the proper utilization, safety and
efficacy of pharmaceuticals and other health care products are
being conducted by industry, government agencies and others.
Such studies, which increasingly employ sophisticated methods
and techniques, can call into question the utilization, safety
and efficacy of previously marketed products and in some cases
have resulted, and may in the future result, in the
discontinuance of their marketing.
The Patient Protection and Affordable Care Act (PPACA) signed
into law on March 23, 2010 creates the Patient Centered
Outcomes Research Institute, a private, non-profit corporation
that is tasked with assisting patients, clinician, purchasers,
and policy-makers in making informed health decisions. One of
the Institute’s initiatives will be to conduct comparative
clinical effectiveness research, which is defined as
“research evaluating and comparing health outcomes and the
clinical effectiveness, risks, and benefits of 2 or more medical
treatments, services, and items.” It is important to note
that the Institute would not be permitted to mandate coverage,
reimbursement, or other policies for any public or private payer.
Foreign
Regulation
Whether or not we obtain FDA approval for a product, we must
obtain approval of a product by the comparable regulatory
authorities of foreign countries before we can commence clinical
trials or marketing of the product in those countries. The
approval process varies from country/region to country/region,
and the time may be longer or shorter than that required for FDA
approval. The requirements governing the conduct of clinical
trials, product licensing, pricing and reimbursement also may
vary, sometimes significantly, from country/region to
country/region.
Under the European Union regulatory systems, we may submit
marketing authorization applications either under a centralized
procedure or decentralized procedure or the mutual recognition
procedure. The centralized procedure is mandatory for medicines
produced by a biotechnological process. The procedure is also
mandatory for new active substances which are indicated for
treatment of several diseases or conditions, including cancer
and orphan conditions. Companies may apply for centralized
assessment if the product contains a new active substance
30
or the product constitutes significant therapeutic, scientific
or technical innovation or the granting of authorization under
the centralized procedure is in the interests of the EU
patients. A centralized marketing authorization is valid in all
European Union member states. This marketing authorization is
issued in the form of a Commission decision which is legally
binding in its entirety to which it is addressed.
Directive 2004/27/EC introduced two Community parallel
procedures to the centralized procedure to allow a product to be
progressively authorized in each of the member states of the EU.
They are the decentralized procedure and the mutual recognition
procedure. The mutual recognition procedure applies where the
product has already been authorized in a member state of the EU
that will act as reference member state. The national marketing
authorization granted by the reference member state forms the
basis for mutual recognition in the member states chosen by the
applicant. In the decentralized procedure, the product in
question is not authorized in any one the EU member states. In
such a situation, the applicant company will request a member
state to act as the reference member state to lead the
scientific assessment for the benefit/risk balance for agreement
by the concerned member states. In both cases, the concerned
member states have up to 90 days to accept or raise
reasoned objections to the assessment made by the reference
member state.
In addition, pricing and reimbursement is subject to negotiation
and regulation in most countries outside the United States.
Increasingly, adoption of a new product for use in national
health services is subject to health technology assessment under
the national rules and regulations to establish the clinical
effectiveness and cost-effectiveness of a new treatment. In some
countries, in order to contain health care expenditures,
reference price is introduced in order for the national
healthcare providers to achieve a price comparable to the
reference price in the same therapeutic category. We may
therefore face the risk that the resulting prices would be
insufficient to generate an acceptable return to us.
Third
Party Reimbursement and Pricing Controls
In the United States and elsewhere, sales of pharmaceutical
products depend in significant part on the availability of
reimbursement to the consumer from third-party payers, such as
government and private insurance plans. Third-party payers are
increasingly challenging the prices charged for medical products
and services. It is time-consuming and expensive for us to go
through the process of seeking reimbursement from Medicare and
private payers. Our products may not be considered cost
effective, and coverage and reimbursement may not be available
or sufficient to allow us to sell our products on a competitive
and profitable basis.
The PPACA enacted significant reforms, including revising the
definition of “average manufacturer price” for
reporting purposes, increasing Medicaid rebates, expanding the
340B drug discount program, and making changes to affect the
Medicare Part D coverage gap, or “donut hole.” In
the coming years, additional significant changes could be made
to governmental healthcare programs, and the United States
healthcare system as a whole, that may result in significantly
increased rebates, decreased pricing flexibility, diminished
negotiating flexibility, coverage and reimbursement limitations
based upon comparative and cost-effectiveness reviews, and other
measures that could significantly impact the success of our
products.
In many foreign markets, including the countries in the EU,
pricing of pharmaceutical products is subject to governmental
control. In the United States, there have been, and we expect
that there will continue to be, a number of federal and state
proposals to implement similar governmental pricing control.
While we cannot predict whether such legislative or regulatory
proposals will be adopted, the adoption of such proposals could
have a material adverse effect on our business, financial
condition and profitability.
Employees
The efforts of our employees are critical to our success. We
believe that we have assembled a strong management team with the
experience and expertise needed to execute our business
strategy. We anticipate hiring additional personnel as needs
dictate to implement our growth strategy. As of
December 31, 2009, we had 158 employees, of which 8
held a M.D. degree, 16 held a Ph.D. degree and 3 held a Pharm. D
degree. We cannot be sure that we will be able to attract and
retain qualified personnel in sufficient numbers to meet our
needs. Our employees are not subject to any collective
bargaining agreements, and we regard our relations with our
employees to be good.
31
Corporate
Background and Available Information
We are a Delaware corporation that was originally incorporated
in Colorado as Americus Funding Corporation in December 1987,
became NeoTherapeutics, Inc. in August 1996, was reincorporated
in Delaware in June 1997, and was renamed Spectrum
Pharmaceuticals, Inc. in December 2002.
We also maintain websites located at
http://www.sppirx.com
and
http://www.spectrumpharm.com,
and electronic copies of our periodic and current reports, proxy
statements for our annual stockholder’s meetings, and any
amendments to those reports, are available, free of charge,
under the “Investor Relations” link on our website as
soon as practicable after such material is filed with, or
furnished to, the SEC.
For financial information regarding our business activities,
please see “Item 8 — Financial Statements
and Supplementary Data.”
An investment in our common stock involves a high degree of
risk. Our business, financial condition, operating results and
prospects can be impacted by a number of factors, any one of
which could cause our actual results to differ materially from
recent results or from our anticipated future results. As a
result, the trading price of our common stock could decline, and
you could lose part or all of your investment. You should
carefully consider the risks described below with all of the
other information included in this Annual Report on
Form 10-K. Failure to satisfactorily achieve any of our
objectives or avoid any of the risks below would likely have a
material adverse effect on our business and results of
operations.
Risks
Related to Our Business
Like
other early-stage biotech companies, we have a history of
operating losses and our losses may continue to increase as we
expand our commercialization and development efforts, and our
efforts may never result in profitability.
Our cumulative losses since our inception in 1987 through
December 31, 2009 were approximately $262.0 million.
Our net losses in 2009, 2008 and 2007 were approximately
$19.0 million, $14.2 million and $22.0 million,
respectively, after recording approximately $8.1 million,
$1.3 million and $12.1 million, respectively, of
warrant based income due to our restatement of previously issued
financial statements. We expect to continue to incur additional
losses as we implement our growth strategy of commercializing
our approved drug products and developing our pipeline products
for at least the next few years. We may never achieve
significant revenues from sales of products or become
profitable. Even if we eventually generate significant revenues
from sales, we will likely continue to incur losses over the
next several years.
Our
business does not generate sufficient cash to finance our
ongoing operations and therefore, we will likely need to
continue to raise additional capital.
Our current commercial operations do not generate sufficient
operating cash to finance the clinical development of all our
drug products, to commercialize our approved drug products and
to capitalize on growth opportunities. While we have been
successful recently in generating funds through the licensing
and sale of our assets, we have historically relied primarily on
raising capital through the sale of our securities and
out-licensing our drug products to meet our financial needs.
Although we began selling products in 2008, we believe that in
the near-term we will likely need to continue to raise funds in
order to continue drug product commercialization, development
and acquisition.
We may not be able to raise additional capital on favorable
terms, if at all, particularly with the current volatile
financial market conditions. Accordingly, we may be forced to
significantly change our business plans and restructure our
operations to conserve cash, which would likely involve
out-licensing or selling some or all of our intellectual,
technological and tangible property not presently contemplated
and at terms that we believe would not be favorable to us,
and/or
reducing the scope and nature of our currently planned drug
development and commercialization activities. An inability to
raise additional capital would also materially impact our
ability to expand operations.
32
Clinical
trials may fail to demonstrate the safety and efficacy of our
drug products, which could prevent or significantly delay
obtaining regulatory approval.
Prior to receiving approval to commercialize any of our drug
products, we must demonstrate with substantial evidence from
well-controlled clinical trials, and to the satisfaction of the
FDA, and other regulatory authorities in the United States and
other countries, that each of the products is both safe and
effective. For each drug product, we will need to demonstrate
its efficacy and monitor its safety throughout the process. If
such development is unsuccessful, our business and reputation
would be harmed and our stock price would be adversely affected.
All of our drug products are prone to the risks of failure
inherent in drug development. Clinical trials of new drug
products sufficient to obtain regulatory marketing approval are
expensive and take years to complete. We may not be able to
successfully complete clinical testing within the time frame we
have planned, or at all. We may experience numerous unforeseen
events during, or as a result of, the clinical trial process
that could delay or prevent us from receiving regulatory
approval or commercializing our drug products. In addition, the
results of pre-clinical studies and early-stage clinical trials
of our drug products do not necessarily predict the results of
later-stage clinical trials. Later-stage clinical trials may
fail to demonstrate that a drug product is safe and effective
despite having progressed through initial clinical testing. Even
if we believe the data collected from clinical trials of our
drug products is promising, such data may not be sufficient to
support approval by the FDA or any other United States or
foreign regulatory approval. Pre-clinical and clinical data can
be interpreted in different ways.
Accordingly, FDA officials could interpret such data in
different ways than we or our partners do, which could delay,
limit or prevent regulatory approval. The FDA, other regulatory
authorities, our institutional review boards, our contract
research organizations, or we may suspend or terminate our
clinical trials for our drug products. Any failure or
significant delay in completing clinical trials for our drug
products, or in receiving regulatory approval for the sale of
any drugs resulting from our drug products, may severely harm
our business and reputation. Even if we receive FDA and other
regulatory approvals, our drug products may later exhibit
adverse effects that may limit or prevent their widespread use,
may cause the FDA to revoke, suspend or limit their approval, or
may force us to withdraw products derived from those drug
products from the market.
If we
are unable to effectively maintain and expand our sales and
marketing capabilities, we may be unable to successfully
commercialize our approved products.
Historically, we have had limited internal experience in
selling, marketing or distributing pharmaceutical products.
However, we have recently built a commercial team to market our
approved products and we continue to seek top talent to add to
the team. If we are not able to effectively hire and maintain
qualified individuals as part of our commercial team, our
product sales and resulting revenues will be negatively impacted.
If we
are unable to maintain or obtain improved reimbursement rates
for Zevalin, the product’s operating results may be harmed,
which could adversely affect our financial and operating
results.
Effective January 1, 2010, the Centers for
Medicare & Medicaid Services (CMS) finalized a policy
to allow reimbursement for Zevalin in the Hospital Outpatient
Prospective Payment System (HOPPS), based on the Average Sales
Price (ASP) methodology applicable to other injectable drugs and
biologicals. We will seek a consistent reimbursement methodology
in the community clinic setting. If we are not able to maintain
this reimbursement methodology in the HOPPS setting or obtain
one in the community setting, we could face significant
difficulty in getting health care providers to prescribe
Zevalin, which will have an adverse impact on the product’s
expected operating results, and in turn adversely impact our
financial and operating results.
We may
face difficulties in achieving broader market acceptance of
Zevalin if we do not invest significantly in our sales and
marketing infrastructure.
United States sales of Zevalin have declined over the several
years prior to our acquisition of the Zevalin assets. We believe
that an enhanced sales and marketing strategy for Zevalin, in
conjunction with efforts to obtain approval by the FDA for
expanded uses of Zevalin, has significant potential to increase
sales of and revenue from Zevalin over the next few years.
However, implementation of the sales and marketing strategy for
Zevalin, and the efforts to expand approved usage of Zevalin,
will require a continued significant investment of financial and
other resources
33
by us for the foreseeable future and may not ultimately increase
Zevalin sales or allow us to realize the anticipated benefits
from our investment in the product. Additionally, our efforts to
establish an effective commercial team for Zevalin will require
significant commitments of both financial and management
resources by us, and may not ultimately be successful due a
variety of factors, including industry competition for effective
commercial personnel or the inability of us to dedicate the
necessary resources to those efforts.
Although chemotherapy is still the backbone to B-cell follicular
NHL, monoclonal antibody development has been paramount to the
success of therapeutic options for this tumor type. Rituximab, a
chimeric anti-CD20 monoclonal antibody, whether as monotherapy
or in combination with chemotherapy (CHOP-R, CVP-R) has been a
mainstay in the therapeutic options for low grade follicular
NHL. Much ongoing research has focused on optimizing monoclonal
antibody use, integrating them into multi-agent regimens, and
developing newer antibodies. Attempts to improve the efficacy of
monoclonal antibody-based therapy have included altering the
dosing schedule, optimizing patient selection, maintenance
therapy, and improving upon radioimmunotherapy, as well as
combinations with cytotoxic molecules and other novel agents.
The eventual goal of targeted therapies is to individualize
treatment to increase response and survival, while reducing
treatment-related toxicity.
There are many monoclonal antibodies in development for NHL,
including Ofatumumab, Veltuzumab, GA 101, AME-133 (all
targeting CD20), Galizimab (targeting CD80), Dacetuzumab
(targeting CD40), Lucatumumab (targeting CD40), Alemtuzumab
(targeting CD52). Furthermore, tumor necrosis factor-related
apoptosis ligand, small modular immunopharmaceuticals,
drug-antibody conjugates are also in the competitive landscape
and under development for low grade NHL.
In addition, Treanda (Bendamustine), a chemotherapeutic,
approved in the area of indolent B-cell NHL that has progressed
during or within six months of treatment with rituximab or a
rituximab-containing regimen, has emerged as a competitive force
for Zevalin in both the relapsed, refractory setting and in the
first line setting (BR — in combination with
Rituximab).
There are three key trials in first line therapy of follicular
lymphoma. The ECOG 4402/RESORT trial is comparing rituximab
maintenance and re-treatment on progression after a CR or PR to
initial rituximab treatment in patients with low tumor burden.
The GELA PRIMA trial is examining maintenance rituximab versus
observation in patients with high tumor burden achieving a CRor
PR with CHOP, CVP, or FCM plus rituximab. The SWOG 0016 trial is
comparing R-CHOP with CHOP followed by
Bexxar®
in patients with high tumor burden.
We are
aware of several competitors attempting to develop and market
products competitive to Zevalin, which may reduce or eliminate
our commercial opportunity.
The pharmaceutical and biotechnology industries are intensely
competitive and subject to rapid and significant technological
changes, and a number of companies are pursuing the development
of pharmaceuticals and products that target the same diseases
and conditions that Zevalin targets.
We cannot predict with accuracy the timing or impact of the
introduction of potentially competitive products or their
possible effect on our sales. Certain potentially competitive
products to Zevalin are in various stages of development, some
of which have been filed for approval with the FDA or have been
approved by regulatory authorities in other countries. Also,
there are many ongoing studies with currently marketed products
including
Rituxan®,
Treanda®
and other developmental products, which may yield new data that
could adversely impact the use of Zevalin in specific states for
which it has obtained FDA approval
Some of the companies developing competing technologies and
products have significantly greater financial resources and
expertise in development, manufacturing, obtaining regulatory
approvals, and marketing than we do. Other smaller companies may
also prove to be significant competitors, particularly through
collaborative arrangements with large and established companies.
The introduction of competitive products to Zevalin could
significantly reduce the sales of Zevalin, which, in turn would
adversely impact our financial and operating results.
34
The
intellectual property and assets owned by our subsidiary, RIT,
are subject to a security agreement with Biogen that secures the
entity’s payment and other obligations to Biogen, and we
have guaranteed all of those obligations.
In connection with the formation of RIT, RIT entered into a
security agreement with Biogen pursuant to which RIT granted to
Biogen a first priority security interest in all of its assets,
which consist of the Zevalin-related intellectual property and
other assets RIT. The security agreement secures certain
payment, indemnification and other obligations of RIT to Biogen
related to Zevalin. If RIT were to default on certain of its
obligations to Biogen, or in certain other circumstances
generally related to a bankruptcy or insolvency of RIT, Biogen
could seek to foreclose on the collateral under the security
agreement to obtain satisfaction of RIT’s obligations to
it. If RIT were to default on its obligations to Biogen, and
Biogen were to foreclose on the collateral under the security
agreement, RIT’s business could be materially and adversely
impacted, which could in turn materially and adversely impact
our investment in RIT and our financial condition and results of
operations.
Furthermore, in connection with the formation of RIT we
guaranteed all of RIT’s obligations to Biogen. If RIT were
to default on its obligations to Biogen, Biogen could require us
alone to satisfy all of those obligations under our guarantee.
The financial and other obligations that we would incur could
have a material and adverse effect on our financial condition
and results of operations.
If we
are unable to expand the approved usage of Fusilev, the
product’s operating results may be harmed, which could
adversely affect our financial and operating
results.
We have filed a supplemental new drug application for Fusilev
for use in combination with 5-FU-containing regimens in the
treatment of colorectal cancer. The greatest potential use of
this product is in this indication. If we are not able to obtain
approval for this indication, we may not recognize the full
anticipated value of our investment in the product and our
financial and operating results could be adversely affected.
Our
drug product Fusilev may not be more cost-effective than
competing drugs and otherwise may not have any competitive
advantage, which could hinder our ability to successfully
commercialize it.
Fusilev is a novel folate analog formulation and the
pharmacologically active isomer (the levo-isomer) of the racemic
compound calcium leucovorin, a product already approved for the
same indications our product is approved for. Leucovorin has
been sold as a generic product on the market for a number of
years. There are generic companies currently selling the product
and therefore, Fusilev competes against a low-cost alternative.
Also, Fusilev will be offered as part of a treatment regimen,
and that regimen may change to exclude Fusilev. Accordingly, it
may not gain acceptance by the medical field or become
commercially successful.
The
marketing and sale of Fusilev and Zevalin may be adversely
affected by the marketing and sales efforts of third parties who
sell these products outside the United States.
We have only licensed the rights to develop, market and sell
Fusilev in North America, and have licensed the rights to
develop, market and sell Zevalin in the United States. Other
companies market and sell the same products in other parts of
the world. If, as a result of their actions, negative publicity
is associated with the product, our own efforts to successfully
market and sell these products, may be adversely impacted.
The
development of our drug product, apaziquone, may be adversely
affected if the development efforts of Allergan, who retained
certain rights to the product, are not successful.
In 2008, we entered into a co-development and license agreement
with Allergan, Inc., or Allergan, for the worldwide development
and commercialization of our drug product, apaziquone. Allergan
has agreed to partially fund development and commercialization
expenses for apaziquone. We do not fully control the drug
development process under the license agreement. In addition, if
we do not achieve certain milestones under the license agreement
and it has been determined that failure to achieve these
milestones was a result of our actions or inactions, Allergan is
entitled to assume additional control over the development
process. As a result, success of this
35
product could depend, in part, upon the efforts of Allergan.
Allergan may not be successful in the clinical development of
the drug, obtaining approval of the product by regulatory
authorities, or the eventual commercialization of apaziquone.
The
development of our drug product, belinostat, may be adversely
affected if the development efforts of TopoTarget, who retained
certain rights to the product, are not successful.
TopoTarget licensed to us the rights to develop and market
belinostat in the United States, Canada, Mexico and India.
TopoTarget is currently fully funding and overseeing one
clinical study underway with Belinostat. In addition, TopoTarget
has agreed to partially fund other development expenses for
belinostat. We do not fully control the drug development process
under our agreement with TopoTarget. TopoTarget, or its
partners, may conduct their own clinical trials on belinostat
for regulatory approval in all other parts of the world. We will
not have control over such development activities and our
ability to attain regulatory approvals for belinostat may be
adversely impacted if its efforts are not successful.
The
development of our drug product, ozarelix, may be adversely
affected if the development efforts of Aeterna Zentaris, who
retained certain rights to the product, are not
successful.
Aeterna Zentaris licensed to us the rights to develop and market
ozarelix in the United States, Canada, Mexico and India. Aeterna
Zentaris, or its partners, may conduct their own clinical trials
on ozarelix for regulatory approval in all other parts of the
world. We will not have control over such development activities
and our ability to attain regulatory approvals for ozarelix may
be adversely impacted if its efforts are not successful.
The
development of our drug product, satraplatin, depends on the
efforts of a third party and, therefore, its eventual success or
commercial viability is largely beyond our
control.
In 2002, we entered into a co-development and license agreement
with GPC, for the worldwide development and commercialization of
our drug product, satraplatin. GPC has agreed to fully fund
development and commercialization expenses for satraplatin. We
do not have control over the drug development process and
therefore the success of this product depends upon the efforts
of GPC and any of its sublicensees. GPC may not be successful in
the clinical development of the drug, obtaining approval of the
product by regulatory authorities, or the eventual
commercialization of satraplatin.
Our
dependence on key executives, scientists and sales and marketing
personnel could impact the development and management of our
business.
We are highly dependent upon our ability to attract and retain
qualified scientific, technical sales & marketing and
managerial personnel. There is intense competition for qualified
personnel in the pharmaceutical and biotechnology industries,
and we cannot be sure that we will be able to continue to
attract and retain the qualified personnel necessary for the
development and management of our business. Although we do not
believe the loss of one individual would materially harm our
business, our business might be harmed by the loss of the
services of multiple existing personnel, as well as the failure
to recruit additional key scientific, technical and managerial
personnel in a timely manner. Much of the know-how we have
developed resides in our scientific and technical personnel and
is not readily transferable to other personnel. While we have an
employment agreement with our Chief Executive Officer, we do not
ordinarily enter into employment agreements with our other key
scientific, technical and managerial employees.
As we
evolve from a company primarily involved in development to a
company also involved in commercialization, we may encounter
difficulties in managing our growth and expanding our operations
successfully.
We only recently began commercial sales of our products and have
had to increase our personnel accordingly, including
establishing a direct sales force and complete commercial team.
In addition, as we advance our drug products through clinical
trials, we will need to expand our development, regulatory,
manufacturing, marketing and sales capabilities or contract with
third parties to provide these capabilities for us. As our
operations expand, we
36
expect that we will need to manage additional relationships with
such third parties, as well as additional collaborators and
suppliers. Maintaining these relationships and managing our
future growth will impose significant added responsibilities on
members of our management. We must be able to: manage our
development efforts effectively; manage our clinical trials
effectively; hire, train and integrate additional management,
development, administrative and sales and marketing personnel;
improve our managerial, development, operational and finance
systems and expand our facilities, all of which may impose a
strain on our administrative and operational infrastructure.
If we
acquire additional businesses, we may not successfully integrate
their operations.
We may acquire additional businesses that complement or augment
our existing business. Integrating any newly acquired business
could be expensive and time-consuming. We may not be able to
integrate any acquired business successfully or operate any
acquired business profitably. Our future financial performance
will depend, in part, on our ability to manage any future growth
effectively and our ability to integrate any acquired
businesses. We may not be able to accomplish these tasks, and
our failure to accomplish any of them could prevent us from
successfully growing our company.
Our
collaborations with outside scientists may be subject to change,
which could limit our access to their expertise.
We work with scientific advisors and collaborators at research
institutions. These scientists are not our employees and may
have other commitments that would limit their availability to
us. If a conflict of interest between their work for us and
their work for another entity arises, we may lose their
services, which could negatively impact our research and
development activities.
We may
rely on contract research organizations and other third parties
to conduct clinical trials and, in such cases, we are unable to
directly control the timing, conduct and expense of our clinical
trials.
We may rely, in full or in part, on third parties to conduct our
clinical trials. In such situations, we have less control over
the conduct of our clinical trials, the timing and completion of
the trials, the required reporting of adverse events and the
management of data developed through the trial than would be the
case if we were relying entirely upon our own staff.
Communicating with outside parties can also be challenging,
potentially leading to mistakes as well as difficulties in
coordinating activities. Outside parties may have staffing
difficulties, may undergo changes in priorities or may become
financially distressed, adversely affecting their willingness or
ability to conduct our trials. We may experience unexpected cost
increases that are beyond our control. Problems with the
timeliness or quality of the work of a contract research
organization may lead us to seek to terminate the relationship
and use an alternative service provider. However, making this
change may be costly and may delay our trials, and contractual
restrictions may make such a change difficult or impossible.
Additionally, it may be impossible to find a replacement
organization that can conduct our trials in an acceptable manner
and at an acceptable cost.
We are
subject to risks associated with doing business
internationally.
Since we conduct clinical trials and manufacture our drug
products internationally, our business is subject to certain
risks inherent in international business, many of which are
beyond our control. These risks include, among other things:
|
|
|
| |
•
|
maintaining compliance with foreign legal requirements,
including employment law;
|
| |
| |
•
|
unexpected changes in foreign regulatory requirements, including
quality standards and other certification requirements;
|
| |
| |
•
|
tariffs, customs, duties and other trade barriers;
|
| |
| |
•
|
changing economic conditions in countries where our products are
manufactured;
|
| |
| |
•
|
exchange rate risks;
|
| |
| |
•
|
product liability, intellectual property and other claims;
|
37
|
|
|
| |
•
|
political instability;
|
| |
| |
•
|
new export license requirements; and
|
| |
| |
•
|
difficulties in coordinating and managing foreign operations.
|
Any of these factors could have an adverse effect on our
business, financial condition and results of operations. We may
not be able to successfully manage these risks or avoid their
effects.
We may
have conflicts with our partners that could delay or prevent the
development or commercialization of our drug
products.
We may have conflicts with our partners, such as conflicts
concerning the interpretation of preclinical or clinical data,
the achievement of milestones, the interpretation of contractual
obligations, payments for services, development obligations or
the ownership of intellectual property developed during our
collaboration. If any conflicts arise with any of our partners,
such partner may act in a manner that is adverse to our best
interests. Any such disagreement could result in one or more of
the following, each of which could delay or prevent the
development or commercialization of our drug product, and in
turn prevent us from generating revenues:
|
|
|
| |
•
|
unwillingness on the part of a partner to pay us milestone
payments or royalties that we believe are due to us under a
collaboration;
|
| |
| |
•
|
uncertainty regarding ownership of intellectual property rights
arising from our collaborative activities, which could prevent
us from entering into additional collaborations;
|
| |
| |
•
|
unwillingness by the partner to cooperate in the development or
manufacture of the product, including providing us with product
data or materials;
|
| |
| |
•
|
unwillingness on the part of a partner to keep us informed
regarding the progress of its development and commercialization
activities or to permit public disclosure of the results of
those activities;
|
| |
| |
•
|
initiation of litigation or alternative dispute resolution
options by either party to resolve the dispute;
|
| |
| |
•
|
attempts by either party to terminate the collaboration;
|
| |
| |
•
|
our ability to maintain or defend our intellectual property
rights may be compromised by our partner’s acts or
omissions;
|
| |
| |
•
|
a partner may utilize our intellectual property rights in such a
way as to invite litigation that could jeopardize or invalidate
our intellectual property rights or expose us to potential
liability;
|
| |
| |
•
|
a partner may change the focus of their development and
commercialization efforts. As previously noted, pharmaceutical
and biotechnology companies historically have re-evaluated their
priorities following mergers and consolidations, which have been
common in recent years in these industries. The ability of our
products to reach their potential could be limited if future
partners decrease or fail to increase spending relating to such
products;
|
| |
| |
•
|
unwillingness of a partner to fully fund or commit sufficient
resources to the testing, marketing, distribution or development
of our products;
|
| |
| |
•
|
unwillingness or ability of a partner to fulfill their
obligations to us. A partner may develop alternative products
either on their own or in collaboration with others, or
encounter conflicts of interest or changes in business strategy
or other business issues; and/or
|
| |
| |
•
|
we may not be able to guarantee supplies of development or
marketed products.
|
Given these risks, it is possible that any collaborative
arrangements which we have or may enter into may not be
successful.
38
Our
efforts to acquire or in-license and develop additional drug
products may fail, which might limit our ability to grow our
business.
Our long-term strategy includes the acquisition or in-license of
additional drug products. We are actively seeking to acquire, or
in-license, additional commercial drug products as well as drug
products that have demonstrated positive pre-clinical
and/or
clinical data. We have certain criteria that we are looking for
in any drug product acquisition and we may not be successful in
locating and acquiring, or in-licensing, additional desirable
drug products on acceptable terms. In addition, many other large
and small companies within the pharmaceutical and biotechnology
industry seek to establish collaborative arrangements for
product research and development, or otherwise acquire products
in late-stage clinical development, in competition with us. We
face additional competition from public and private research
organizations, academic institutions and governmental agencies
in establishing collaborative arrangements for drug products in
late-stage clinical development. Many of the companies and
institutions that compete against us have substantially greater
capital resources, research and development staffs and
facilities than we have, and greater experience in conducting
business development activities. These entities represent
significant competition to us as we seek to expand our portfolio
through the in-license or acquisition of compounds. Moreover,
while it is not feasible to predict the actual cost of acquiring
additional drug products, that cost could be substantial and we
may need to raise additional financing, which may further dilute
existing stockholders, in order to acquire new drug products.
From
time to time we may need to license patents, intellectual
property and proprietary technologies from third parties, which
may be difficult or expensive to obtain.
We may need to obtain licenses to patents and other proprietary
rights held by third parties to successfully develop,
manufacture and market our drug products. As an example, it may
be necessary to use a third party’s proprietary technology
to reformulate one of our drug products in order to improve upon
the capabilities of the drug product. If we are unable to timely
obtain these licenses on reasonable terms, our ability to
commercially exploit our drug products may be inhibited or
prevented.
We are
a small company relative to our principal competitors, and our
limited financial resources may limit our ability to develop and
market our drug products.
Many companies, both public and private, including well-known
pharmaceutical companies and smaller niche-focused companies,
are developing products to treat many, if not all, of the
diseases we are pursuing or are currently distributing drug
products that directly compete with the drugs that we sell or
that we intend to develop, market and distribute. Many of these
companies have substantially greater financial, research and
development, manufacturing, marketing and sales experience and
resources than us. As a result, our competitors may be more
successful than us in developing their products, obtaining
regulatory approvals and marketing their products to consumers.
Competition for branded or proprietary drugs is less driven by
price and is more focused on innovation in the treatment of
disease, advanced drug delivery and specific clinical benefits
over competitive drug therapies. We may not be successful in any
or all of our current clinical studies; or if successful, and if
one or more of our drug products is approved by the FDA, we may
encounter direct competition from other companies who may be
developing products for similar or the same indications as our
drug products. Companies that have products on the market or in
research and development that target the same indications as our
products target include, among others, Abraxis Bioscience, Inc.,
Astra Zeneca LP, Bayer AG, Endo Pharmaceuticals, Eli Lilly and
Co., Novartis Pharmaceuticals Corporation, Genentech, Inc.,
Bristol-Myers Squibb Company, GlaxoSmithKline, Biogen-IDEC
Pharmaceuticals, Inc., OSI Pharmaceuticals, Inc., Cephalon,
Inc., Sanofi-aventis, Inc., Pfizer, Inc., Genta Incorporated,
Merck, Celegen Corporate, Allos Therapeutics, Inc., BiPar
Sciences, Inc., Genzyme Corporation, Shire Pharmaceuticals,
Abbott Laboratories, Poniard Pharmaceuticals, Inc., Roche
Pharmaceuticals and Johnson & Johnson who may be more
advanced in the development of competing drug products or are
more established. Many of our competitors are large and
well-capitalized companies focusing on a wide range of diseases
and drug indications, and have substantially greater financial,
research and development, marketing, human and other resources
than we do. Furthermore, large pharmaceutical companies have
significantly more experience than we do in pre-clinical
testing, human clinical trials and regulatory approval
procedures, among other things.
39
Our
supply of drug products will be dependent upon the production
capabilities of contract manufacturing organizations (CMOs) and
component and packaging supply sources, and, if such CMOs are
not able to meet our demands, we may be limited in our ability
to meet demand for our products, ensure regulatory compliance or
maximize profit on the sale of our products.
We have no internal manufacturing capacity for our drug
products, and, therefore, we have entered into agreements with
CMOs to supply us with active pharmaceutical ingredients and our
finished dose drug products. Consequently, we will be dependent
on our CMO partners for our supply of drug products. Some of
these manufacturing facilities are located outside the United
States. The manufacture of finished drug products, including the
acquisition of compounds used in the manufacture of the finished
drug product, may require considerable lead times. We will have
little or no control over the production process. Accordingly,
while we do not currently anticipate shortages of supply, there
could arise circumstances in which we will not have adequate
supplies to timely meet our requirements or market demand for a
particular drug product could outstrip the ability of our supply
source to timely manufacture and deliver the product, thereby
causing us to lose sales. In addition, our ability to make a
profit on the sale of our drug products depends on our ability
to obtain price arrangements that ensure a supply of product at
favorable prices.
Reliance on CMOs entails risks to which we would not be subject
if we manufactured products ourselves, including reliance on the
third party for regulatory compliance and adherence to the
FDA’s current Good Manufacturing Practice (cGMP)
requirements, the possible breach of the manufacturing agreement
by the CMO and the possibility of termination or non-renewal of
the agreement by the CMO, based on its own business priorities,
at a time that is costly or inconvenient for us. Before we can
obtain marketing approval for our drug products, our CMO
facilities must pass an FDA pre-approval inspection. In order to
obtain approval, all of the facility’s manufacturing
methods, equipment and processes must comply with cGMP
requirements. The cGMP requirements govern all areas of record
keeping, production processes and controls, personnel and
quality control. In addition, our CMOs will be subject to
on-going periodic inspection by the FDA and corresponding state
and foreign agencies for compliance with cGMP regulations,
similar foreign regulations and other regulatory standards. We
do not have control over our CMOs’ compliance with these
regulations and standards. Any failure of our third party
manufacturers or us to comply with applicable regulations,
including an FDA pre-approval inspection and cGMP requirements,
could result in sanctions being imposed on them or us, including
warning letters, fines, injunctions, civil penalties, failure of
regulatory authorities to grant marketing approval of our
products, delay, suspension or withdrawal of approvals, license
revocation, seizures or recalls of product, operation
restrictions and criminal prosecutions, any of which could
significantly and adversely affect our business.
We may
not be successful in establishing additional active
pharmaceutical ingredient or finished dose drug supply
relationships, which would limit our ability to develop and
market our drug products.
Success in the development and marketing of our drugs depends in
part upon our ability to maintain, expand and enhance our
existing relationships and establish new sources of supply for
active pharmaceutical ingredients (API) or for the manufacture
of our finished dose drug products. We do not presently intend
to focus our research and development efforts on developing APIs
or manufacturing of finished dosage form for our drugs. In
addition, we currently have no capacity to manufacture APIs or
finished dose drug products and do not intend to spend our
capital resources to develop the capacity to do so. Therefore,
we must rely on relationships with API suppliers and other CMOs,
to supply our APIs and finished dose drug products. We may not
be successful in maintaining, expanding or enhancing our
existing relationships or in securing new relationships with API
suppliers or CMOs. If we fail to maintain or expand our existing
relationships or secure new relationships, our ability to
develop and market our drug products could be harmed.
We rely on contract suppliers to supply our existing products,
and will likely do the same for other products that we may
develop, commercialize or acquire in the future. Contract
suppliers may not be able to meet our needs with respect to
timing, cost, quantity or quality. All of our suppliers are
sole-source suppliers, including for Zevalin and Fusilev, and no
currently qualified alternative suppliers exist. If problems
arise during the production of a batch of our products, that
batch of product may have to be discarded. This could, among
other things, lead to increased costs, lost revenue, damage to
customer relations, time and expense spent investigating the
cause and, depending on the cause, similar losses with respect
to other batches or products. If problems are not discovered
before the product
40
is released to the market, recall and product liability costs
may also be incurred. To the extent that one of our suppliers
experiences significant manufacturing problems, this could have
a material adverse effect on our revenues and profitability.
If we are unable to obtain a sufficient supply of our required
products and services on acceptable terms, or if we should
encounter delays or difficulties in our relationships with our
manufacturers, or if any required approvals by the FDA and other
regulatory authorities do not occur on a timely basis, we will
lose sales. Moreover, contract suppliers that we may use must
continually adhere to current good manufacturing practices
enforced by the FDA. If the facilities of these suppliers cannot
pass an inspection, we may lose FDA approval of our products.
Failure to obtain products for sale for any reason may result in
an inability to meet product demand and a loss of potential
revenues.
Our
drug products may not be more effective, safer or more
cost-efficient than a competing drug and otherwise may not have
any competitive advantage, which could hinder our ability to
successfully commercialize our drug products.
Any drug product for which we obtain FDA approval must compete
for market acceptance and market share. Drugs produced by other
companies are currently on the market for each disease type we
are pursuing. Even if one or more of our drug development
products ultimately receives FDA approval, our drug products may
not have better efficacy in treating the target indication than
a competing drug, may not have a more favorable side-effect
profile than a competing drug, may not be more cost-efficient to
manufacture or apply, or otherwise may not demonstrate a
competitive advantage over competing therapies. Accordingly,
even if FDA approval is obtained for one or more of our drug
development products, they may not gain acceptance by the
medical field or become commercially successful.
The
size of the market for our potential products is
uncertain.
We often provide estimates of the number of people who suffer
from the diseases that our drugs are targeting. However, there
is limited information available regarding the actual size of
these patient populations. In addition, it is uncertain whether
the results from previous or future clinical trials of drug
products will be observed in broader patient populations, and
the number of patients who may benefit from our drug products
may be significantly smaller than the estimated patient
populations.
If
actual future payments for allowances, discounts, returns,
rebates and chargebacks exceed the estimates we made at the time
of the sale of our products, our financial position, results of
operations and cash flows may be materially and negatively
impacted.
We recognize product revenue net of estimated allowances for
discounts, returns, rebates and chargebacks. Such estimates
require our most subjective and complex judgment due to the need
to make estimates about matters that are inherently uncertain.
Based on industry practice, pharmaceutical companies, including
us, have liberal return policies. Generally, we are obligated to
accept from customers the return of pharmaceuticals that have
reached their expiration date up to twelve months after
their expiration. We authorize returns for damaged products and
exchanges for expired products in accordance with our return
goods policy and procedures. In addition, like our competitors,
we also give credits for chargebacks to wholesale customers that
have contracts with us for their sales to hospitals, group
purchasing organizations, pharmacies or other retail customers.
A chargeback is the difference between the price the wholesale
customer (in our case, the GPOs) pays (wholesale acquisition
cost) and the price that the GPO’s end-customer pays for a
product (contracted customer). Since we have only recently begun
commercial distribution of our products, we do not have
historical data on returns and allowances. Although we have
estimated the allowances very conservatively, actual results may
differ significantly from our estimated allowances for
discounts, returns, rebates and chargebacks. Changes in
estimates and assumptions based upon actual results may have a
material impact on our results of operations
and/or
financial condition. Such changes to estimates will be made to
the financial statements in the year in which the estimate is
charged. In addition, our financial position, results of
operations and cash flows may be materially and negatively
impacted if actual future payments for allowances, discounts,
returns, rebates and chargebacks exceed the estimates we made at
the time of the sale of our products.
41
Risks
Related to Our Industry
If
third-party payors do not adequately reimburse providers for any
of our products, if approved for marketing, we may not be
successful in selling them.
Our ability to commercialize any products successfully will
depend in part on the extent to which reimbursement will be
available from governmental and other third-party payors, both
in the United States and in foreign markets. Even if we succeed
in bringing one or more products to the market, the amount
reimbursed for our products may be insufficient to allow us to
compete effectively and could adversely affect our profitability.
Reimbursement by a governmental and other third-party payors may
depend upon a number of factors, including a governmental or
other third-party payor’s determination that use of a
product is:
|
|
|
| |
•
|
a covered benefit under its health plan;
|
| |
| |
•
|
safe, effective and medically necessary;
|
| |
| |
•
|
appropriate for the specific patient;
|
| |
| |
•
|
cost-effective; and
|
| |
| |
•
|
neither experimental nor investigational.
|
Obtaining reimbursement approval for a product from each
third-party and governmental payor is a time-consuming and
costly process that could require us to provide supporting
scientific, clinical and cost-effectiveness data for the use of
our products to each payor. We may not be able to provide data
sufficient to obtain reimbursement.
In the United States, there have been, and we expect there will
continue to be, a number of state and federal proposals that
limit the amount that private insurance plans may pay to
reimburse the cost of drugs, including our products. We believe
the increasing emphasis on managed care in the United States has
and will continue to put pressure on the price and usage of our
products, which may also impact sales of our products. In
addition, current third-party reimbursement policies for our
products may change at any time. Negative changes in
reimbursement or our failure to obtain reimbursement for our
products may reduce the demand for, or the price of, products,
which could result in lower sales of our products, thereby
weakening our competitive position and negatively impacting our
results of operations.
Eligibility for coverage does not imply that any drug product
will be reimbursed in all cases or at a rate that allows us to
make a profit. Interim payments for new products, if applicable,
may also not be sufficient to cover our costs and may not become
permanent. Reimbursement rates may vary according to the use of
the drug and the clinical setting in which it is used, may be
based on payments allowed for lower-cost drugs that are already
reimbursed, may be incorporated into existing payments for other
products or services, and may reflect budgetary constraints
and/or
Medicare or Medicaid data used to calculate these rates. Net
prices for products also may be reduced by mandatory discounts
or rebates required by government health care programs or by any
future relaxation of laws that restrict imports of certain
medical products from countries where they may be sold at lower
prices than in the United States.
Wholesaler
actions could increase competitive and pricing pressures on
pharmaceutical manufacturers, including us.
We sell Fusilev primarily through wholesalers. These wholesale
customers comprise a significant part of the distribution
network for pharmaceutical products in the United States. A
small number of large wholesale distributors control a
significant share of the market, which can increase competitive
and pricing pressures on pharmaceutical manufacturers, including
us. In addition, wholesalers may apply pricing pressure through
fee-for-service
arrangements, and their purchases may exceed customer demand,
resulting in reduced wholesaler purchases in later quarters. We
cannot assure you that we can manage these pressures or that
wholesaler purchases will not decrease as a result of this
potential excess buying.
42
Rapid
bio-technological advancement may render our drug products
obsolete before we are able to recover expenses incurred in
connection with their development. As a result, our drug
products may never become profitable.
The pharmaceutical industry is characterized by rapidly evolving
biotechnology. Biotechnologies under development by other
pharmaceutical companies could result in treatments for diseases
and disorders for which we are developing our own treatments.
Several other companies are engaged in research and development
of compounds that are similar to our research. A competitor
could develop a new biotechnology, product or therapy that has
better efficacy, a more favorable side-effect profile or is more
cost-effective than one or more of our drug products and thereby
cause our drug products to become commercially obsolete. Some of
our drug products may become obsolete before we recover the
expenses incurred in their development. As a result, such
products may never become profitable.
Competition
for patients in conducting clinical trials may prevent or delay
product development and strain our limited financial
resources.
Many pharmaceutical companies are conducting clinical trials in
patients with the disease indications that our drug products
target. As a result, we must compete with them for clinical
sites, physicians and the limited number of patients who fulfill
the stringent requirements for participation in clinical trials.
Also, due to the confidential nature of clinical trials, we do
not know how many of the eligible patients may be enrolled in
competing studies and who are consequently not available to us
for our clinical trials. Our clinical trials may be delayed or
terminated due to the inability to enroll enough patients to
complete our clinical trials. Patient enrollment depends on many
factors, including the size of the patient population, the
nature of the trial protocol, the proximity of patients to
clinical sites and the eligibility criteria for the study. The
delay or inability to meet planned patient enrollment may result
in increased costs and delays or termination of the trial, which
could have a harmful effect on our ability to develop products.
Failure
to obtain regulatory approval outside the United States will
prevent us from marketing our product candidates
abroad.
We intend to market certain of our existing and future product
candidates in
non-U.S. markets.
In order to market our existing and future product candidates in
the European Union and many other
non-U.S. jurisdictions,
we must obtain separate regulatory approvals according to the
applicable domestic laws and regulations. We have had limited
interactions with
non-U.S. regulatory
authorities, and the approval procedures vary among countries
and can involve additional testing, and the time required to
obtain approval may differ from that required to obtain FDA
approval. Approval by the FDA does not guarantee approval by
regulatory authorities in other countries, and approval by one
or more
non-U.S. regulatory
authorities does not necessarily ensure approval by regulatory
authorities in other countries or by the FDA. The
non-U.S. regulatory
approval process may include all of the risks associated with
obtaining FDA approval as well as other risks specific to the
jurisdictions in which we may seek approval. We may not obtain
non-U.S. regulatory
approvals on a timely basis, if at all. We may not be able to
file for
non-U.S. regulatory
approvals and may not receive necessary approvals to
commercialize our existing and future product candidates in any
market.
Even
after we receive regulatory approval to market our drug
products, the market may not be receptive to our drug products
upon their commercial introduction, which would negatively
impact our ability to achieve profitability.
Our drug products may not gain market acceptance among
physicians, patients, healthcare payors and the medical
community. The degree of market acceptance of any approved drug
products will depend on a number of factors, including:
|
|
|
| |
•
|
the effectiveness of the drug product;
|
| |
| |
•
|
the prevalence and severity of any side effects;
|
| |
| |
•
|
Potential advantages or disadvantages over alternative
treatments;
|
43
|
|
|
| |
•
|
relative convenience and ease of administration;
|
| |
| |
•
|
the strength of marketing and distribution support;
|
| |
| |
•
|
the price of the drug product, both in absolute terms and
relative to alternative treatments; and
|
| |
| |
•
|
sufficient third-party coverage or reimbursement.
|
If our drug products receive regulatory approval but do not
achieve an adequate level of acceptance by physicians,
healthcare payors and patients, we may not generate drug product
revenues sufficient to attain profitability.
Guidelines
and recommendations published by various organizations can
reduce the use of our products.
Government agencies such as the Centers for Medicare &
Medicaid Services promulgate regulations, and issue guidelines,
directly applicable to us and to our products. In addition,
third parties such as professional societies, practice
management groups, insurance carriers, physicians, private
health/science foundations and organizations involved in various
diseases from time to time may publish guidelines or
recommendations to healthcare providers, administrators and
payers, and patient communities. Recommendations may relate to
such matters as usage, dosage, route of administration and use
of related therapies and reimbursement of our products by
government and private payers. Third-party organizations like
the above have in the past made recommendations about our
products. Recommendations or guidelines that are followed by
patients and healthcare providers could result in decreased use
and/or
dosage of our products.
Any recommendations or guidelines that result in decreased use,
dosage or reimbursement of our products could adversely affect
our product sales and operating results materially. In addition,
the perception by the investment community or stockholders that
such recommendations or guidelines will result in decreased use
and dosage of our products could adversely affect the market
price for our common stock.
Our
failure to comply with governmental regulations may delay or
prevent approval of our drug products and/or subject us to
penalties.
The FDA and comparable agencies in foreign countries impose many
requirements related to the drug development process through
lengthy and rigorous clinical testing and data collection
procedures, and other costly and time consuming compliance
procedures. While we believe that we are currently in compliance
with applicable FDA regulations, if our partners, the contract
research organizations or contract manufacturers with which we
have relationships, or we fail to comply with the regulations
applicable to our clinical testing, the FDA may delay, suspend
or cancel our clinical trials, or the FDA might not accept the
test results. The FDA, an institutional review board, third
party investigators, any comparable regulatory agency in another
country, or we, may suspend clinical trials at any time if the
trials expose subjects participating in such trials to
unacceptable health risks. Further, human clinical testing may
not show any current or future drug product to be safe and
effective to the satisfaction of the FDA or comparable
regulatory agencies, or the data derived from the clinical tests
may be unsuitable for submission to the FDA or other regulatory
agencies. Once we submit an application seeking approval to
market a drug product, the FDA or other regulatory agencies may
not issue their approvals on a timely basis, if at all. If we
are delayed or fail to obtain these approvals, our business and
prospects may be significantly damaged.
If we obtain regulatory approval for our drug products, we, our
partners, our manufacturers, and other contract entities will
continue to be subject to extensive requirements by a number of
national, foreign, state and local agencies. These regulations
will impact many aspects of our operations, including testing,
research and development, manufacturing, safety, effectiveness,
labeling, storage, quality control, adverse event reporting,
record keeping, approval, advertising and promotion of our
future products. Failure to comply with applicable regulatory
requirements could, among other things, result in:
|
|
|
| |
•
|
warning letters;
|
| |
| |
•
|
fines;
|
| |
| |
•
|
changes in advertising;
|
44
|
|
|
| |
•
|
revocation or suspension of regulatory approvals of products;
|
| |
| |
•
|
product recalls or seizures;
|
| |
| |
•
|
delays, interruption, or suspension of product distribution,
marketing and sale;
|
| |
| |
•
|
civil or criminal sanctions;
|
| |
| |
•
|
suspend or terminate any of our ongoing clinical trials;
|
| |
| |
•
|
impose restrictions on our operations;
|
| |
| |
•
|
close the facilities of our contract manufacturers; and
|
| |
| |
•
|
refusals to approve new products.
|
The
discovery of previously unknown safety risks with drug products
approved to go to market may raise costs or prevent us from
marketing such products or change the labeling of our products
or take other potentially limiting or costly actions if we or
others identify safety risks after our products are on the
market.
The later discovery of previously unknown safety risks with our
products may result in restrictions of the drug product,
including withdrawal from the market. The FDA may revisit and
change its prior determinations with regard to the safety and
efficacy of our products. If the FDA’s position changes, we
may be required to change our labeling or to cease manufacture
and marketing of the products at issue. Even prior to any formal
regulatory action, we could voluntarily decide to cease the
distribution and sale or recall any of our products if concerns
about their safety or effectiveness develop.
On September 27, 2007, President Bush signed into law the
Food and Drug Administration Amendments Act of 2007,
significantly adding to the FDA’s authority including
allowing the FDA to (i) require sponsors of marketed
products to conduct post-approval clinical studies to assess a
known serious risk, signals of serious risk or to identify an
unexpected serious risk; (ii) mandate labeling changes to
products, at any point in a product’s lifecycle, based on
new safety information and (iii) require sponsors to
implement a Risk Evaluation and Mitigation Strategy (REMS), for
a product which could include a medication guide, patient
package insert, a communication plan to healthcare providers, or
other elements as the FDA deems are necessary to assure safe use
of the drug (either prior to approval or post-approval as
necessary), which could include imposing certain restrictions on
distribution or use of a product. Failure to comply with a REMS
could result in significant civil monetary penalties or other
administrative actions by FDA. Further, regulatory agencies
could change existing, or promulgate new, regulations at any
time which may affect our ability to obtain or maintain approval
of our existing or future products or require significant
additional costs to obtain or maintain such approvals.
Our
failure to comply with FDA (and related) regulations applicable
to our business may subject us to sanctions, which could damage
our reputation and adversely affect our business
condition.
In the U.S., the FDA, and comparable state regulatory agencies
and enforcement authorities, impose requirements on us as a
manufacturer and marketer of prescription drug products. Drug
manufacturers are required to register with FDA, and are
required to comply with various regulatory requirements
regarding drug research, manufacturing, distribution, reporting
and recordkeeping. Most drug products must be approved by the
FDA prior to marketing, and companies are required to comply
with numerous post-marketing requirements. Drug manufacturing
establishments are subject to inspection by the FDA for
compliance with cGMP regulations and other applicable
regulations.
Further, drug manufacturers are required to comply with FDA
requirements for labeling and advertising, as well as other
Federal and state requirements for advertising. This includes a
prohibition on promotion for unapproved or “off-label”
uses, e.g., promotion of products for uses that are not
described in the product’s FDA-approved labeling. While a
physician may prescribe a medication for off-label uses where
appropriate, companies may not generally promote drug products
for off-label uses.
If FDA or other Federal and state agencies believe that a
company is not in compliance with applicable regulations, they
have various enforcement authorities to address violations. FDA
can issue a warning letter and seek voluntary compliance from a
company in the form of remedial or corrective action. FDA may
also impose civil
45
money penalties by administrative action, and through judicial
enforcement seek actions including injunctions, seizures, and
criminal penalties. FDA or other Federal and state authorities
may also seek operating restrictions on a company in order to
achieve compliance, including termination or suspension of
company activities. Such agencies and enforcement authorities
may also disseminate information to the public about their
enforcement actions.
If we were to become subject to any FDA or similar enforcement
action related to any of our drug products, our business
condition could be adversely affected, and the public release of
such information could be damaging to our reputation.
Legislative
or regulatory reform of the healthcare system and pharmaceutical
industry related to pricing or reimbursement may hurt our
ability to sell our products profitably or at all.
In both the United States and certain foreign jurisdictions,
there have been and may continue to be a number of legislative
and regulatory proposals related to pricing and reimbursement
that could impact our ability to sell our products profitably.
The Patient Protection and Affordable Care Act (PPACA) signed
into law on March 23, 2010 enacted provision including a
revision to the definition of “average manufacturer
price” for reporting purposes, increasing Medicaid rebates,
expanding the 340B drug discount program, and making changes to
affect the Medicare Part D coverage gap, or “donut
hole.” These reforms will significantly impact the
pharmaceutical industry; however, the full effects will take as
these laws are implemented and the Centers for
Medicare & Medicaid Services and other agencies issue
applicable regulations or guidance. Moreover, in the coming
years, additional changes could be made to governmental
healthcare programs that could significantly impact the success
of our products.
Moreover, in the coming years, additional changes could be made
to governmental healthcare programs, and the delivery of
healthcare generally, that could significantly impact the
success of our products. The sales of our products depend in
part on the availability of reimbursement from third-party
payors such as government health administration authorities,
private health insurers, health maintenance organizations
including pharmacy benefit managers and other health
care-related organizations. Both the Federal and state
governments in the U.S. and foreign governments continue to
propose and pass new legislation and regulations designed to
contain or reduce the cost of health care. Such legislation and
regulations may result in decreased reimbursement for
prescription drugs, which may further exacerbate industry-wide
pressure to reduce the prices charged for prescription drugs.
This could harm our ability to market our products and generate
revenues.
It is possible that proposals will be adopted, or existing
regulations that affect the coverage or pricing of
pharmaceutical and other medical products may change, before any
of our products are approved for marketing. Cost control
initiatives could decrease the price that we receive for any of
our products that we are developing. In addition, third-party
payors are increasingly challenging the price and
cost-effectiveness of medical products and services. Significant
uncertainty exists as to the reimbursement status of
newly-approved pharmaceutical products.
The high cost of pharmaceutical prices continues to generate
substantial government interest. Various governmental entities
may focus on pharmaceutical prices by holding hearings or
launching investigations regarding the pricing for drugs by
pharmaceutical companies such as ours and the ability of
patients to obtain drugs. In December 2009, the Government
Accounting Office released its report on the growing cost of
brand-name prescription drugs. In addition, in July 2008, the
Joint Economic Committee of Congress held hearings on the
pricing of drugs for rare conditions. Based on further
developments, we may be required to decrease the price that we
charge for our products, thereby negatively affecting our
financial results.
In some foreign countries, particularly in the European Union,
prescription drug pricing is subject to governmental control.
Drug pricing may be made against a reference price set by the
healthcare providers as a measure for healthcare cost
containment. Pricing negotiations with governmental authorities
can take considerable time after the receipt of marketing
approval for a product. To obtain reimbursement or pricing
approval in some countries, we may be required to conduct a
clinical trial that seeks to address the clinical effectiveness
and cost-effectiveness of our product candidate as compared with
other available therapies as part of the health technology
assessment. If reimbursement of our products is unavailable or
limited in scope or amount, or if pricing is set at
unsatisfactory levels for the purpose of adoption of these
products in the national health services in these jurisdictions,
our profitability will likely be negatively affected.
46
If we
market products in a manner that violates health care
anti-kickback or other anti-fraud and
anti-abuse
laws, we may be subject to civil or criminal penalties,
including exclusions from participation in Federal health care
programs.
The Federal health care program anti-kickback statute prohibits,
among other things, knowingly and willfully offering, paying,
soliciting, or receiving remuneration to induce or in return for
purchasing, leasing, ordering, or arranging for the purchase,
lease or order of any health care item or service reimbursable
under Medicare, Medicaid or other federally financed health care
programs. This statute applies to arrangements between
pharmaceutical manufacturers and prescribers, purchasers and
formulary managers. Although there are a number of statutory
exemptions and regulatory safe harbors protecting certain common
activities, the exemptions and safe harbors are drawn narrowly,
and practices that involve remuneration intended to induce
prescribing, purchases or recommendations may be subject to
scrutiny if they do not qualify for an exemption or safe harbor.
Federal false claims laws prohibit any person from knowingly
presenting, or causing to be presented, a false claim for
payment to the Federal government, or knowingly making, or
causing to be made, a false statement to get a false claim paid.
Pharmaceutical companies have been prosecuted under these laws
for a variety of alleged promotional and marketing activities,
such as providing free product to customers with the expectation
that the customers would bill Federal programs for the product;
reporting to pricing services inflated average wholesale prices
that were then used by Federal programs to set reimbursement
rates; engaging in off-label promotion that caused claims to be
submitted to Medicaid for non-covered off-label uses; and
submitting inflated best price information to the Medicaid
Rebate Program.
The Health Insurance Portability and Accountability Act of 1996
also created prohibitions against health care fraud and false
statements relating to health care matters. The health care
fraud statute prohibits knowingly and willfully executing a
scheme to defraud any health care benefit program, including
private payors. The false statements statute prohibits knowingly
and willfully falsifying, concealing or covering up a material
fact or making any materially false, fictitious or fraudulent
statement in connection with the delivery of or payment for
health care benefits, items or services.
The majority of states also have statutes or regulations similar
to these Federal laws, which apply to items and services
reimbursed under Medicaid and other state programs, or, in
several states, apply regardless of the payor. In addition, some
states have laws that require pharmaceutical companies to adopt
comprehensive compliance programs. For example, under California
law, pharmaceutical companies must comply with both the April
2003 Office of Inspector General Compliance Program Guidance for
Pharmaceutical Manufacturers and the PhRMA Code on Interactions
with Healthcare Professionals, as amended. We have adopted and
implemented a compliance program which we believe satisfies the
applicable requirements of California law.
Sanctions under these Federal and state laws may include civil
monetary penalties, exclusion of a manufacturer’s products
from reimbursement under government programs, criminal fines and
imprisonment. Because of the breadth of these laws and the
narrowness of the safe harbors, it is possible that some of our
business activities could be subject to challenge under one or
more of such laws. The PPACA makes several important changes to
the federal anti-kickback statute, false claims laws, and health
care fraud statute — for example by weakening the
intent requirement for under the anti-kickback and health care
fraud statutes — that may make it easier for the
government, or whistleblowers to charge such fraud and abuse
violations. In addition, the PPACA increase penalties for fraud
and abuse violations. If our past, present or future operations
are found to be in violation of any of the laws described above
or other similar governmental regulations to which we are
subject, we may be subject to the applicable penalty associated
with the violation which could adversely affect our ability to
operate our business and our financial results.
If we
are unable to adequately protect our technology or enforce our
patent rights, our business could suffer.
Our success with the drug products that we develop will depend,
in part, on our ability and the ability of our licensors to
obtain and maintain patent protection for these products. We
currently have a number of United States and foreign patents
issued and pending, however, we primarily rely on patent rights
licensed from others. Our license agreements generally give us
the right
and/or
obligation to maintain and enforce the subject patents. We may
not receive patents for any of our pending patent applications
or any patent applications we may file in the future. If our
pending and future patent applications are not allowed or, if
allowed and issued into patents, if such patents and
47
the patents we have licensed are not upheld in a court of law,
our ability to competitively exploit our drug products would be
substantially harmed. Also, such patents may or may not provide
competitive advantages for their respective products or they may
be challenged or circumvented by our competitors, in which case
our ability to commercially exploit these products may be
diminished.
The patent positions of pharmaceutical and biotechnology
companies can be highly uncertain and involve complex legal and
factual questions. No consistent policy regarding the breadth of
claims allowed in pharmaceutical and biotechnology patents has
emerged to date in the United States. The laws of many countries
may not protect intellectual property rights to the same extent
as United States laws, and those countries may lack adequate
rules and procedures for defending our intellectual property
rights. Filing, prosecuting and defending patents on all our
products or product candidates throughout the world would be
prohibitively expensive. Competitors may use our technologies in
jurisdictions and may not be covered by any of our patent claims
or other intellectual property rights.
Changes in either patent laws or in interpretations of patent
laws in the United States and other countries may diminish the
value of our intellectual property. We do not know whether any
of our patent applications will result in the issuance of any
patents, and we cannot predict the breadth of claims that may be
allowed in our patent applications or in the patent applications
we license from others.
The degree of future protection for our proprietary rights is
uncertain because legal means afford only limited protection and
may not adequately protect our rights or permit us to gain or
keep our competitive advantage. For example:
|
|
|
| |
•
|
in certain jurisdictions, we or our licensors might not have
been the first to make the inventions covered by each of our or
our licensors’ pending patent applications and issued
patents, and we may have to participate in expensive and
protracted interference proceedings to determine priority of
invention;
|
| |
| |
•
|
we or our licensors might not have been the first to file patent
applications for these inventions;
|
| |
| |
•
|
others may independently develop similar or alternative product
candidates or duplicate any of our or our licensors’
product candidates;
|
| |
| |
•
|
our or our licensors’ pending patent applications may not
result in issued patents;
|
| |
| |
•
|
our or our licensors’ issued patents may not provide a
basis for commercially viable products or may not provide us
with any competitive advantages or may be challenged by third
parties;
|
| |
| |
•
|
others may design around our or our licensors’ patent
claims to produce competitive products that fall outside the
scope of our or our licensors’ patents;
|
| |
| |
•
|
we may not develop or in-license additional patentable
proprietary technologies related to our product
candidates; or
|
| |
| |
•
|
the patents of others may prevent us from marketing one or more
of our product candidates for one or more indications that may
be valuable to our business strategy.
|
Moreover, an issued patent does not guarantee us the right to
practice the patented technology or commercialize the patented
product. Third parties may have blocking patents that could be
used to prevent us from commercializing our patented products
and practicing our patented technology. Our issued patents and
those that may be issued in the future may be challenged,
invalidated or circumvented, which could limit our ability to
prevent competitors from marketing related product candidates or
could limit the length of the term of patent protection of our
product candidates. In addition, our competitors may
independently develop similar technologies. Moreover, because of
the extensive time required for development, testing and
regulatory review of a potential product, it is possible that,
before any of our product candidates can be commercialized, any
related patent may expire or remain in force for only a short
period following commercialization, thereby reducing any
advantage of the patent.
We also rely on trade secret protection and contractual
protections for our unpatented, confidential and proprietary
technology. Trade secrets are difficult to protect. While we
enter into confidentiality agreements with our employees,
consultants and others, these agreements may not successfully
protect our trade secrets or other
48
confidential and proprietary information. It is possible that
these agreements will be breached, or that they will not be
enforceable in every instance, and that we will not have
adequate remedies for any such breach. Likewise, although we
conduct periodic trade secret audits of certain partners,
vendors and contract manufacturers, these trade secret audits
may not protect our trade secrets or other confidential and
proprietary information. It is possible that despite having
certain trade secret audited security measures in place, trade
secrets or other confidential and proprietary information may
still be leaked or disclosed to a third party. It is also
possible that our trade secrets will become known or
independently developed by our competitors.
If we are unable to adequately protect our technology, trade
secrets or proprietary know-how, or enforce our patents, our
business, financial condition and prospects could suffer.
Intellectual
property rights are complex and uncertain and therefore may
subject us to infringement claims.
The patent positions related to our drug products are inherently
uncertain and involve complex legal and factual issues. Although
we are not aware of any infringement by any of our drug products
on the rights of any third party, there may be third party
patents or other intellectual property rights, including
trademarks and copyrights, relevant to our drug products of
which we are not aware. Third parties may assert patent or other
intellectual property infringement claims against us with
products. This could draw us into costly litigation as well as
result in the loss of our use of the intellectual property that
is critical to our business strategy.
Intellectual
property litigation is increasingly common and increasingly
expensive and may result in restrictions on our business and
substantial costs, even if we prevail.
Patent and other intellectual property litigation is becoming
more common in the pharmaceutical industry. Litigation is
sometimes necessary to defend against or assert claims of
infringement, to enforce our patent rights, including those we
have licensed from others, to protect trade secrets or to
determine the scope and validity of proprietary rights of third
parties. Currently, no third party is asserting that we are
infringing upon their patent rights or other intellectual
property, nor are we aware or believe that we are infringing
upon any third party’s patent rights or other intellectual
property. We may, however, be infringing upon a third
party’s patent rights or other intellectual property, and
litigation asserting such claims might be initiated in which we
would not prevail, or we would not be able to obtain the
necessary licenses on reasonable terms, if at all. All such
litigation, whether meritorious or not, as well as litigation
initiated by us against third parties, is time-consuming and
very expensive to defend or prosecute and to resolve. In
addition, if we infringe the intellectual property rights of
others, we could lose our right to develop, manufacture or sell
our products or could be required to pay monetary damages or
royalties to license proprietary rights from third parties. An
adverse determination in a judicial or administrative proceeding
or a failure to obtain necessary licenses could prevent us from
manufacturing or selling our products, which could harm our
business, financial condition and prospects.
If our competitors prepare and file patent applications in the
United States or Europe that claim technology we also claim, we
may have to participate in interference proceedings required by
the USPTO to determine priority of invention or opposition
proceedings in Europe, both of which could result in substantial
costs, even if we ultimately prevail. Results of interference
and opposition proceedings are highly unpredictable and may
result in us having to try to obtain licenses in order to
continue to develop or market certain of our drug products.
We may
be subject to damages resulting from claims that we, or our
employees, have wrongfully used or disclosed alleged trade
secrets of our employees’ former employers.
Many of our employees were previously employed at universities
or biotechnology or pharmaceutical companies, including our
competitors or potential competitors. Although we have not
received any claim to date, we may be subject to claims that
these employees through their employment inadvertently or
otherwise used or disclosed trade secrets or other proprietary
information of their former employers. Litigation may be
necessary to defend against these claims. If we fail in
defending such claims, in addition to paying monetary damages,
we may lose valuable intellectual property rights or personnel.
49
We may
be subject to product liability claims, and may not have
sufficient product liability insurance to cover any such claims,
which may expose us to substantial liabilities.
We may be held liable if any product we or our partners develop
causes injury or is found otherwise unsuitable during product
testing, manufacturing, clinical trials, marketing or sale.
Regardless of merit or eventual outcome, product liability
claims could result in decreased demand for our product
candidates, injury to our reputation, withdrawal of patients
from our clinical trials, substantial monetary awards to trial
participants and the inability to commercialize any products
that we may develop. These claims might be made directly by
consumers, health care providers, pharmaceutical companies or
others selling or testing our products. Although we currently
carry product liability insurance in the amount of at least
$15.0 million in the aggregate, it is possible that this
coverage will be insufficient to protect us from future claims.
However, our insurance may not reimburse us or may not be
sufficient to reimburse us for expenses or losses we may suffer.
Moreover, if insurance coverage becomes more expensive, we may
not be able to maintain insurance coverage at a reasonable cost
or in sufficient amounts to protect us against losses due to
liability. Failure to maintain sufficient insurance coverage
could have a material adverse effect on our business, prospects
and results of operations if claims are made that exceed our
coverage.
On occasion, juries have awarded large judgments in class action
lawsuits for claims based on drugs that had unanticipated side
effects. In addition, the pharmaceutical and biotechnology
industries, in general, have been subject to significant medical
malpractice litigation. A successful product liability claim or
series of claims brought against us could harm our reputation
and business and would decrease our cash reserves.
The use of hazardous materials, including radioactive and
biological materials, in our research and development and
commercial efforts imposes certain compliance costs on us and
may subject us to liability for claims arising from the use or
misuse of these materials.
Our research and development, manufacturing (including a
radiolabeling step for Zevalin) and administration of our drugs
involves the controlled use of hazardous materials, including
chemicals, radioactive and biological materials, such as
radioactive isotopes, which is done by qualified third parties;
in essence. We do not physically handle these radioactive
isotopes or such hazardous materials. We are subject to federal,
state and local laws and regulations governing the storage, use
and disposal of these materials and some waste products. We
believe that our safety procedures for the storage, use and
disposal of these materials comply with the standards prescribed
by federal, state and local regulations. However, we cannot
completely eliminate the risk of accidental contamination or
injury from these materials. If there were to be an accident, we
could be held liable for any damages that result, which could
exceed our financial resources. We currently maintain insurance
coverage for injuries resulting from the hazardous materials we
use; however, future claims may exceed the amount of our
coverage. Also, we do not have insurance coverage for pollution
cleanup and removal. Currently the costs of complying with
federal, state and local regulations are not significant, and
consist primarily of waste disposal expenses, however, they
could become expensive, and current or future environmental
regulations may impair our research, development, production and
commercialization efforts.
Risks
Related to Our Common Stock
There
are a substantial number of shares of our common stock eligible
for future sale in the public market. The sale of these shares
could cause the market price of our common stock to fall. Any
future equity issuances by us may have dilutive and other
effects on our existing stockholders.
As of March 29, 2010, there were approximately
49.2 million shares of our common stock outstanding, and in
addition, security holders held options, warrants and preferred
stock which, if vested, exercised or converted, would obligate
us to issue up to approximately 19.1 million additional
shares of common stock. However, we would receive over
$104.0 million from the issuance of shares of common stock
upon the exercise of all of the options and warrants. A
substantial number of those shares, when we issue them upon
vesting, conversion or exercise, will be available for immediate
resale in the public market. In addition, we may sell additional
shares of common stock or securities convertible or exercisable
into common stock in public or private offerings, which would be
available for resale in the market. The market price of our
common stock could fall as a result of sales of any of these
shares of common stock due to the increased number of shares
available for sale in the market.
50
We have primarily financed our operations, and we anticipate
that we will have to finance a large portion of our operating
cash requirements, by issuing and selling our common stock or
securities convertible into or exercisable for shares of our
common stock. Any issuances by us of equity securities may be at
or below the prevailing market price of our common stock and may
have a dilutive impact on our existing stockholders. These
issuances or other dilutive issuances would also cause our net
income, if any, per share to decrease in future periods. As a
result, the market price of our common stock could drop.
The
market price and trading volume of our common stock fluctuate
significantly and could result in substantial losses for
individual investors.
The stock market from time to time experiences significant price
and trading volume fluctuations that are unrelated to the
operating performance of particular companies. These broad
market fluctuations may cause the market price and trading
volume of our common stock to decrease. In addition, the market
price and trading volume of our common stock is often highly
volatile.
Factors that may cause the market price and volume of our common
stock to decrease include:
|
|
|
| |
•
|
recognition on up-front licensing or other fees or revenues;
|
| |
| |
•
|
payments of non-refundable up-front or license fees, or payment
for cost-sharing expenses, to third parties;
|
| |
| |
•
|
adverse results or delays in our clinical trials;
|
| |
| |
•
|
fluctuations in our results of operations;
|
| |
| |
•
|
timing and announcements of our bio-technological innovations or
new products or those of our competitors;
|
| |
| |
•
|
developments concerning any strategic alliances or acquisitions
we may enter into;
|
| |
| |
•
|
announcements of FDA non-approval of our drug products, or
delays in the FDA or other foreign regulatory review process or
actions;
|
| |
| |
•
|
adverse actions taken by regulatory agencies with respect to our
drug products, clinical trials, manufacturing processes or sales
and marketing activities;
|
| |
| |
•
|
concerns about our products being reimbursed;
|
| |
| |
•
|
any lawsuit involving us or our drug products;
|
| |
| |
•
|
developments with respect to our patents and proprietary rights;
|
| |
| |
•
|
announcements of technological innovations or new products by
our competitors;
|
| |
| |
•
|
public concern as to the safety of products developed by us or
others;
|
| |
| |
•
|
regulatory developments in the United States and in foreign
countries;
|
| |
| |
•
|
changes in stock market analyst recommendations regarding our
common stock or lack of analyst coverage;
|
| |
| |
•
|
the pharmaceutical industry generally and general market
conditions;
|
| |
| |
•
|
failure of our results of operations to meet the expectations of
stock market analysts and investors;
|
| |
| |
•
|
sales of our common stock by our executive officers, directors
and five percent stockholders or sales of substantial amounts of
our common stock;
|
| |
| |
•
|
changes in accounting principles; and
|
| |
| |
•
|
loss of any of our key scientific or management personnel.
|
Also, certain dilutive securities such as warrants can be used
as hedging tools which may increase volatility in our stock and
cause a price decline. While a decrease in market price could
result in direct economic loss for an individual investor, low
trading volume could limit an individual investor’s ability
to sell our common stock, which could result in substantial
economic loss as well. Since January 1, 2009 through
March 29, 2010, the price of our
51
common stock ranged between $1.39 and $10.00, and the daily
trading volume was as high as 15,476,100 shares and as low
as 27,100 shares. In addition, due in large part to the
current global economic crisis many institutional investors that
historically had invested in specialty pharmaceutical companies
have ceased operations or further investment in these companies,
which has had negatively impacted trading volume for our stock.
Following periods of volatility in the market price of a
company’s securities, securities class action litigation
may be instituted against that company. Regardless of their
merit, these types of lawsuits generally result in substantial
legal fees and management’s attention and resources being
diverted from the operations of a business.
Provisions
of our charter, bylaws and stockholder rights plan may make it
more difficult for someone to acquire control of us or replace
current management even if doing so would benefit our
stockholders, which may lower the price an acquirer or investor
would pay for our stock.
Provisions of our certificate of incorporation and bylaws, both
as amended, may make it more difficult for someone to acquire
control of us or replace our current management. These
provisions include:
|
|
|
| |
•
|
the ability of our board of directors to amend our bylaws
without stockholder approval;
|
| |
| |
•
|
the inability of stockholders to call special meetings;
|
| |
| |
•
|
the ability of members of the board of directors to fill
vacancies on the board of directors;
|
| |
| |
•
|
the inability of stockholders to act by written consent, unless
such consent is unanimous; and
|
| |
| |
•
|
the establishment of advance notice requirements for nomination
for election to our board of directors or for proposing matters
that can be acted on by stockholders at stockholder meetings.
|
These provisions may make it more difficult for stockholders to
take certain corporate actions and could delay, discourage or
prevent someone from acquiring our business or replacing our
current management, even if doing so would benefit our
stockholders. These provisions could limit the price that
certain investors might be willing to pay for shares of our
common stock.
We have a stockholder rights plan pursuant to which we
distributed rights to purchase units of our series B junior
participating preferred stock. The rights become exercisable
upon the earlier of ten days after a person or group of
affiliated or associated persons has acquired 15% or more of the
outstanding shares of our common stock or ten business days
after a tender offer has commenced that would result in a person
or group beneficially owning 15% or more of our outstanding
common stock. These rights could delay or discourage someone
from acquiring our business, even if doing so would benefit our
stockholders. We currently have no stockholders who own 15% or
more of the outstanding shares of our common stock.
The
restatement of our historical financial statements has already
consumed, and may continue to consume, a significant amount of
our time and resources and may have a material adverse effect on
our business and stock price.
As described earlier, we have restated our consolidated
financial statements. The restatement process was highly time
and resource-intensive and involved substantial attention from
management and significant legal and accounting costs. Although
we have now completed the restatement, we cannot guarantee that
we will have no inquiries from the SEC or NASDAQ regarding our
restated financial statements or matters relating thereto.
Any future inquiries from the SEC as a result of the restatement
of our historical financial statements will, regardless of the
outcome, likely consume a significant amount of our resources in
addition to those resources already consumed in connection with
the restatement itself.
Further, many companies that have been required to restate their
historical financial statements have experienced a decline in
stock price and stockholder lawsuits related thereto.
52
If we
fail to maintain an effective system of internal control over
financial reporting, we may not be able to accurately report our
financial results, and current and potential stockholders may
lose confidence in our financial reporting.
We are required by the SEC to establish and maintain adequate
internal control over financial reporting that provides
reasonable assurance regarding the reliability of our financial
reporting and the preparation of financial statements in
accordance with generally accepted accounting principles. We are
likewise required, on a quarterly basis, to evaluate the
effectiveness of our internal controls and to disclose any
changes and material weaknesses in those internal controls.
As described in greater detail elsewhere in this Annual Report
on Form
10-K, in
connection with the restatement process, we identified a
material weakness with regard to accounting for warrant
instruments in our internal control over financial reporting,
specifically with regard to our prior interpretation of ASC 815
“Derivatives and Hedging — Contracts in
Entity’s Own Equity” (formerly known as Emerging
Issues Task Force (EITF)
00-19,
“Accounting for Derivative Financial Instruments Indexed
to, and Potentially Settled in, a Company’s Own
Stock”), as it related to the accounting for and
classification of certain warrant instruments dating back to
September 2005 that we had previously classified as equity. Upon
a reassessment of those financial instruments, in light of GAAP
as currently interpreted, we determined that we should have
accounted for certain warrant instruments as debt instead of
equity. Given this material weakness with regard to warrants,
management was unable to conclude that we maintained effective
internal control over financial reporting as of
December 31, 2009.
Since the determination regarding this material weakness, we
plan to devote significant effort and resources to the
remediation and improvement of our internal control over
financial reporting. While we have processes to identify and
intelligently apply developments in accounting, we plan to
enhance these processes to better evaluate and document our
research and understanding of the nuances of increasingly
complex accounting standards. Our plans include the following:
enhanced access to accounting literature, research materials and
documents; identification of third party professionals with whom
to consult regarding complex accounting applications; and the
consideration of involving additional staff with the requisite
experience and training to supplement our current accounting
professionals. The elements of our remediation plan can only be
accomplished over time and we can offer no assurance that these
initiatives will ultimately have the intended effects. Any
failure to maintain such internal controls could adversely
impact our ability to report our financial results on a timely
and accurate basis. If our financial statements are not
accurate, investors may not have a complete understanding of our
operations. Likewise, if our financial statements are not filed
on a timely basis as required by the SEC and NASDAQ, we could
face severe consequences from those authorities. In either case,
there could result a material adverse affect on our business.
Inferior internal controls could also cause investors to lose
confidence in our reported financial information, which could
have a negative effect on the trading price of our stock.
Our
publicly-filed SEC reports are reviewed by the SEC from time to
time and any significant changes required as a result of any
such review may result in material liability to us and have a
material adverse impact on the trading price of our common
stock.
The reports of publicly-traded companies are subject to review
by the SEC from time to time for the purpose of assisting
companies in complying with applicable disclosure requirements
and to enhance the overall effectiveness of companies’
public filings, and reviews of such reports are now required at
least every three years under the Sarbanes-Oxley Act of 2002.
SEC reviews may be initiated at any time, and we could be
required to modify or reformulate information contained in prior
filings as a result of an SEC review. Any modification or
reformulation of information contained in such reports could be
significant and could result in material liability to us and
have a material adverse impact on the trading price of our
common stock.
We
were unable to timely file this Annual Report on Form 10-K as
required by the Securities Exchange Act of 1934. Our continued
inability to file these reports on time could result in
investors not having access to important information about us
and the delisting of our common stock from NASDAQ.
We were late in filing this Annual Report on Form 10-K. As a
result, we may not be in compliance with the continued listing
requirements of the NASDAQ Global Market and with applicable SEC
rules under the Securities
53
Exchange Act of 1934 (Exchange Act). We are required to comply
with these rules as a condition of the continued listing of our
common stock on NASDAQ.
Although we have been timely with respect to other annual and
quarterly reports, there can be no assurance that we will be
able to timely file all such reports in the future. If we are
unable to timely file these reports in the future, you may not
receive important information about us in a timely manner. In
addition, our common stock could be delisted from NASDAQ, which
could materially adversely impact the liquidity and price of our
common stock.
Changes
in our effective income tax rate could adversely affect our
results of operations.
We are subject to federal and state income taxes in the United
States and our tax liabilities are dependent upon the
distribution of income among these different jurisdictions.
Various factors may have favorable or unfavorable effects on our
effective income tax rate. These factors include, but are not
limited to, interpretations of existing tax laws, the accounting
for stock options and other share-based compensation, changes in
tax laws and rates, future levels of research and development
spending, changes in accounting standards, changes in the mix of
earnings in the various tax jurisdictions in which we operate,
the outcome of examinations by the Internal Revenue Service and
other jurisdictions, the accuracy of our estimates for
unrecognized tax benefits and realization of deferred tax
assets, and changes in overall levels of pre-tax earnings. The
impact on our income tax provision resulting from the
above-mentioned factors may be significant and could have an
impact on our results of operations.
We do
not anticipate declaring any cash dividends on our common
stock.
We have never declared or paid cash dividends on our common
stock and do not plan to pay any cash dividends on our common
stock in the foreseeable future. Our current policy is to retain
all funds and any earnings for use in the operation and
expansion of our business.
|
|
|
Item 1B.
|
Unresolved
Staff Comments
|
None.
Our principle executive office is located at 157 Technology
Drive, Irvine, California 92618. The lease on this facility
expires on June 30, 2016. In addition, we also have an
office in Henderson, Nevada. We lease this space pursuant to an
agreement that expires on September 30, 2011. We also lease
small administrative offices in Zurich, Switzerland, Montreal,
Canada, and Mumbai, India on an expense-sharing basis. The
financial and other terms of these lease arrangements are not
material to our business. We believe that our leased facilities
are adequate to meet our needs at this time.
|
|
|
Item 3.
|
Legal
Proceedings
|
We are involved with various legal matters arising from the
ordinary course of business. Although the ultimate resolution of
these various matters cannot be determined at this time, we do
not believe that such matters, individually or in the aggregate,
will have a material adverse effect on our future consolidated
results of operations, cash flows or financial condition.
54
PART II
|
|
|
Item 5.
|
Market
for Registrant’s Common Equity, Related Stockholder Matters
and Issuer Purchases of Equity Securities
|
Common
Stock
As of March 29, 2010 there were 49,170,969 shares of
common stock outstanding and 359 shareholders of record. On
March 29, 2010, the closing sale price of our common stock
was $4.64 per share.
Market
for Securities
Our common stock is traded on the NASDAQ Global Market under the
symbol “SPPI.” The high and low sale prices of our
common stock reported by NASDAQ during each quarter ended in
2009 and 2008 were as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
High
|
|
Low
|
|
|
|
Year 2009
|
|
|
|
|
|
|
|
|
|
Quarter Ended
|
|
|
|
|
|
|
|
|
|
March 31
|
|
$
|
2.10
|
|
|
$
|
1.39
|
|
|
June 30
|
|
$
|
8.15
|
|
|
$
|
1.75
|
|
|
September 30
|
|
$
|
10.00
|
|
|
$
|
4.76
|
|
|
December 31
|
|
$
|
6.74
|
|
|
$
|
3.97
|
|
|
Year 2008
|
|
|
|
|
|
|
|
|
|
Quarter Ended
|
|
|
|
|
|
|
|
|
|
March 31
|
|
$
|
3.35
|
|
|
$
|
2.25
|
|
|
June 30
|
|
$
|
2.98
|
|
|
$
|
0.46
|
|
|
September 30
|
|
$
|
1.90
|
|
|
$
|
1.30
|
|
|
December 31
|
|
$
|
2.25
|
|
|
$
|
0.55
|
|
The high and low sales prices of our common stock, reported by
NASDAQ, reflect inter-dealer prices, without retail
mark-ups,
markdowns or commissions, and may not represent actual
transactions.
Dividends
We have never paid cash dividends on our common stock and we do
not intend to pay cash dividends of our common stock in the
foreseeable future. We currently intend to retain our earnings,
if any, to finance future growth.
|
|
|
Item 6.
|
Selected
Financial Data
|
The following table summarizes certain historical financial
information at the dates and for the periods indicated prepared
in accordance with U.S. Generally Accepted Accounting
Principles and gives effect to the restatements described in
“Management’s Discussion and Analysis of Financial
Condition and Results of Operations” and in Note 2 to
our consolidated financial statements. The consolidated
statement of operations data for the years ended
December 31, 2009, 2008 (as restated) and 2007 (as
restated), the consolidated balance sheet data as of
December 31, 2009 and 2008 (as restated), have been derived
from our audited consolidated financial statements included
elsewhere in this Annual Report on
Form 10-K.
Financial data for the years ended December 31, 2007, 2006
and 2005 (all as restated) and as of December 31, 2007,
2006 and 2005 (all as restated) has been derived from our
restated financial statements not included herein. Certain
reclassifications have been made to prior-years’
comparative financial statements to conform to the current year
presentation. These reclassifications had no effect on
previously reported results of operations or financial position.
The selected consolidated financial data should be read in
conjunction with “Management’s Discussion and Analysis
of Financial Condition and Results of
55
Operations” and the consolidated financial statements and
notes thereto, which are included elsewhere in this Annual
Report on
Form 10-K.
CONSOLIDATED
FINANCIAL INFORMATION
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Statement of Operations Data for the Years Ended December
31:
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
2005
|
|
|
|
|
|
|
|
(As Restated)
|
|
|
(As Restated)
|
|
|
(As Restated)
|
|
|
(As Restated)
|
|
|
|
|
(In thousands, except Share data)
|
|
|
|
|
Total revenues
|
|
$
|
38,025
|
|
|
$
|
28,725
|
|
|
$
|
7,672
|
|
|
$
|
5,673
|
|
|
$
|
577
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of product sales (excludes amortization of purchased
intangibles shown below)
|
|
|
8,148
|
|
|
|
1,193
|
|
|
|
—
|
|
|
|
97
|
|
|
|
397
|
|
|
Selling, general and administrative
|
|
|
33,607
|
|
|
|
15,156
|
|
|
|
11,577
|
|
|
|
7,736
|
|
|
|
6,615
|
|
|
Research and development
|
|
|
21,058
|
|
|
|
26,683
|
|
|
|
33,285
|
|
|
|
23,728
|
|
|
|
13,483
|
|
|
Amortization of purchased intangibles
|
|
|
3,720
|
|
|
|
158
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Acquired in-process research and development
|
|
|
—
|
|
|
|
4,700
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(28,508
|
)
|
|
|
(19,165
|
)
|
|
|
(37,190
|
)
|
|
|
(25,888
|
)
|
|
|
(19,918
|
)
|
|
Change in fair value of common stock warrant liability
|
|
|
8,075
|
|
|
|
1,271
|
|
|
|
12,055
|
|
|
|
(2,485
|
)
|
|
|
3,867
|
|
|
Other income, net
|
|
|
662
|
|
|
|
1,165
|
|
|
|
3,139
|
|
|
|
2,606
|
|
|
|
1,279
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pre-tax net loss
|
|
$
|
(19,771
|
)
|
|
$
|
(16,729
|
)
|
|
$
|
(21,996
|
)
|
|
$
|
(25,767
|
)
|
|
$
|
(14,772
|
)
|
|
Income tax expense
|
|
|
(421
|
)
|
|
|
(5
|
)
|
|
|
(5
|
)
|
|
|
(5
|
)
|
|
|
(4
|
)
|
|
Net loss attributable to non-controlling interest
|
|
|
1,146
|
|
|
|
2,538
|
|
|
|
20
|
|
|
|
3
|
|
|
|
1
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss — attributable to Spectrum
Pharmaceuticals, Inc. stockholders
|
|
$
|
(19,046
|
)
|
|
$
|
(14,196
|
)
|
|
$
|
(21,981
|
)
|
|
$
|
(25,769
|
)
|
|
$
|
(14,775
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per share — attributable to
Spectrum Pharmaceuticals, Inc. stockholders
|
|
$
|
(0.48
|
)
|
|
$
|
(0.45
|
)
|
|
$
|
(0.76
|
)
|
|
$
|
(1.06
|
)
|
|
$
|
(0.84
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash dividends on common stock
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance Sheet Data at December 31:
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
2005
|
|
|
|
|
|
|
|
(As Restated)
|
|
|
(As Restated)
|
|
|
(As Restated)
|
|
|
(As Restated)
|
|
|
|
|
(In thousands, except Share data)
|
|
|
|
|
Cash, cash equivalents and marketable securities
|
|
$
|
113,341
|
|
|
$
|
75,938
|
|
|
$
|
55,659
|
|
|
$
|
50,697
|
|
|
$
|
63,667
|
|
|
Other current assets
|
|
|
12,916
|
|
|
|
12,310
|
|
|
|
953
|
|
|
|
1,590
|
|
|
|
718
|
|
|
Property and equipment, net
|
|
|
1,928
|
|
|
|
1,782
|
|
|
|
716
|
|
|
|
625
|
|
|
|
562
|
|
|
Intangible assets and goodwill, net
|
|
|
33,325
|
|
|
|
37,042
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Other assets
|
|
|
11,623
|
|
|
|
2,437
|
|
|
|
212
|
|
|
|
205
|
|
|
|
128
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
173,133
|
|
|
$
|
129,509
|
|
|
$
|
57,540
|
|
|
$
|
53,117
|
|
|
$
|
65,075
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities
|
|
$
|
32,864
|
|
|
$
|
32,806
|
|
|
$
|
7,799
|
|
|
$
|
6,233
|
|
|
$
|
3,828
|
|
|
Common stock warrant liability
|
|
|
6,635
|
|
|
|
765
|
|
|
|
2,035
|
|
|
|
14,090
|
|
|
|
11,605
|
|
|
Other non-current-liabilities
|
|
|
25,310
|
|
|
|
42,822
|
|
|
|
992
|
|
|
|
1,035
|
|
|
|
241
|
|
|
Commitments and contingencies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity (including non-controlling interest)
|
|
|
108,324
|
|
|
|
53,116
|
|
|
|
46,714
|
|
|
|
31,759
|
|
|
|
49,401
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and equity
|
|
$
|
173,133
|
|
|
$
|
129,509
|
|
|
$
|
57,540
|
|
|
$
|
53,117
|
|
|
$
|
65,075
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
56
|
|
|
Item 7.
|
Management’s
Discussion and Analysis of Financial Condition and Results of
Operations
|
The following discussion and analysis should be read in
conjunction with our consolidated financial statements and
related notes included elsewhere in this Annual Report on
Form 10-K.
The information below has been adjusted solely to reflect the
impact of the restatement of our financial results which is more
fully described in Note 2 to the consolidated financial
statements contained in this Annual Report on Form 10-K and
under the paragraph “Restatement of Previously Issued
Consolidated Financial Statements” below and does not
reflect any subsequent information or events occurring after the
date of the filing of our reports originally presenting the
financial information being restated or update any disclosure
herein to reflect the passage of time since the date of such
filings. The following discussion contains forward-looking
statements that involve risks and uncertainties, such as
statements of our plans, objectives, expectations and
intentions. Reference is made in particular to forward-looking
statements regarding the success of our drug candidates, the
safety and efficacy of our drug candidates’ product
approvals, product sales, revenue development timelines, product
acquisitions, liquidity and capital resources and trends. Our
actual results could differ materially from those discussed
here. Factors that might cause such a difference include, but
are not limited to, those discussed below and elsewhere,
including under Item 1A “Risk Factors” of this
Annual Report on
Form 10-K.
The cautionary statements made in this Annual Report on Form
10-K should be read as applying to all related forward-looking
statements wherever they appear in this Annual Report on Form
10-K.
Restatement
of Previously Issued Consolidated Financial Statements
As discussed above under Item 1, “Restatement of
Privately Issued Consolidated Financial Statements” in this
Annual Report on Form 10-K, we have restated our previously
issued consolidated financial statements for fiscal years ended
December 31, 2007 and 2008, and each of the quarterly
condensed consolidated financial statements on Form 10-Q
for the periods ended March 31, 2008 through
September 30, 2009 to reclassify warrant contracts based on
a reassessment of the applicable accounting and classification.
In connection with the warrants issued in registered offerings
during 2005 and 2009, the Company had previously classified the
warrants as equity under its evaluation of applicable guidance
contained in ASC 815 “Derivatives and Hedging —
Contracts in Entity’s Own Equity” (formerly known as
Emerging Issues Task Force Issue
00-19,
“Accounting for Derivative Financial Instruments Indexed
to, and Potentially Settled in, a Company’s Own
Stock”), a highly complex area of accounting. In connection
with the audit for the fiscal year 2009, the Company, in
consultation with Ernst & Young, reassessed the accounting
classification of the warrants pursuant to ASC 815 based on
certain terms of the warrants. The restatement has no impact on
cash flows from operating activities.
Overview
We are a commercial-stage biopharmaceutical company committed to
developing and commercializing innovative therapies with a
primary focus in the areas of hematology-oncology and urology.
We have a fully developed commercial infrastructure that markets
and sells two drugs,
Zevalin®
and
Fusilev®,
in the United States. We have several drug candidates in
development, the most advanced of which are apaziquone
(EOquin®),
which is presently being studied in two large Phase 3 clinical
trials for non-muscle invasive bladder cancer (NMIBC) under a
strategic collaboration with Allergan; and belinostat, a drug we
recently partnered with TopoTarget to jointly develop.
Belinostat is being studied in a Phase 2 trial for relapsed or
refractory Peripheral
T-Cell
Lymphoma (PTCL).
Our business strategy is comprised of the following initiatives:
|
|
|
| |
•
|
Maximizing the growth potential of our marketed drugs,
Zevalin and Fusilev. Our
near-term outlook largely depends on sales and marketing
successes for our two marketed drugs. For Zevalin, our initial
goal was to stabilize sales, which we believe we accomplished in
2009. With the approval by the FDA for a significantly larger
indication in non-Hodgkin’s lymphoma (NHL) in late 2009 and
our success in addressing historical hurdles associated with the
uptake of this drug, we believe we can grow sales in 2010 and
beyond. For Fusilev, which we launched in August 2008, we were
able to benefit from broad utilization in community clinics and
hospitals through mid-2009. Our focus now is to obtain approval
for Fusilev in advanced metastatic colorectal cancer, which
could potentially increase the patient pool
|
57
|
|
|
| |
|
substantially. As part of its review of our supplemental new
drug application (sNDA), the FDA has requested additional data
which we expect to submit in the third quarter of 2010.
|
For both Zevalin and Fusilev, we initiated and continue to stage
appropriate infrastructure expansions and additional initiatives
to facilitate broad customer reach and to address other market
requirements, as appropriate. We have formed a dedicated
commercial organization comprised of highly experienced and
motivated sales representatives, account managers, medical
science liaisons and a complement of other support marketing
personnel to manage the sales and marketing of these drugs.
|
|
|
| |
•
|
Optimizing our development portfolio and maximizing the
asset values of its components. While over
the recent few years, we have evolved from a development-stage
company to a commercial-stage pharmaceutical company, we have
maintained a highly focused development portfolio. Our strategy
with regard to our development portfolio is to focus on
late-stage drugs and to develop them rapidly to the point of
regulatory approval. We plan to develop some of these drugs
ourselves or with our subsidiaries and affiliates, or secure
collaborations such that we are able to suitably monetize these
assets.
|
We have assembled drug development infrastructure that is
comprised of highly experienced and motivated MDs, PhDs, medical
science liaisons and a complement of other support personnel to
rapidly develop these drugs. During 2009, this team achieved our
goal of completing enrollment in the two Phase 3 apaziquone
trials (with more than 1,600 patients enrolled). We expect
to continue to maximize the value of apaziquone through further
developmental efforts and initiation of additional trials, which
we aim to begin in 2010. In addition, this team will focus its
efforts in rapidly advancing the development of belinostat by
expediting the patient enrollment in the registrational trial
for PTCL and initiating additional studies in other indications
in 2010.
We have several other exciting compounds in earlier stages of
development in our portfolio. Based upon a criteria-based
portfolio review, we are in the process of streamlining our
pipeline drugs, allowing for greater focus and integration of
our development and commercial goals.
|
|
|
| |
•
|
Expanding commercial bandwidth through licensing and
business development. It is our goal to
identify new strategic opportunities that will create strong
synergies with our currently marketed drugs and identify and
pursue partnerships for out-licensing certain of our drugs in
development. To this end, we will continue to explore strategic
collaborations as these relate to drugs that are either in
advanced clinical trials or are currently on the market. We
believe that such opportunistic collaborations will provide
synergies with respect to how we deploy our internal resources.
In this regard, we intend to identify and secure drugs that have
significant growth potential either through enhanced marketing
and sales efforts or through pursuit of additional clinical
development. We believe our recent in-licensing of belinostat, a
novel histone deacetylase (HDAC) inhibitor, is demonstrative of
such licensing and business development efforts outlined above.
|
| |
| |
•
|
Managing our financial resources
effectively. We remain committed to fiscal
discipline, a policy which has allowed us to become well
capitalized among our peers, despite a very challenging capital
markets environment in 2009. This policy includes the pursuit of
non-dilutive funding options, prudent expense management, and
the achievement of critical synergies within our operations in
order to maintain a reasonable burn rate. Even with the
continued
build-up in
operational infrastructure to facilitate the marketing of our
two commercial drugs, we intend to be fiscally prudent in any
expansion we undertake. In terms of revenue generation, we plan
to become more reliant on sales from currently marketed drugs
and intend to pursue out-licensing of select pipeline drugs in
select territories, as discussed above. When appropriate, we may
pursue other sources of financing, including non-dilutive
financing alternatives. While we are currently focused on
advancing our key drug development programs, we anticipate that
we will make regular determinations as to which other programs,
if any, to pursue and how much funding to direct to each program
on an ongoing basis, based on clinical success and commercial
potential, including termination of our existing development
programs, especially if we do not expect value being driven from
continued development. Our raising of over $100 million in
equity financing in 2009 in a difficult financing environment,
and our recent termination of the development of ozarelix in
2009 in benign prostate hypertrophy which resulted in planned
development expense reduction, are recent examples of this
strategy.
|
58
|
|
|
| |
•
|
Further enhancing the organizational structure to meet our
corporate objectives. We have highly
experienced staff in pharmaceutical operations, clinical
development, regulatory and commercial functions who previously
held positions at both small to mid-size biotech companies, as
well as large pharmaceutical companies. We recently strengthened
the ranks of our management team, and will continue to pursue
talent on an opportunistic basis. Finally, we remain committed
to running a lean and efficient organization, while effectively
leveraging our critical resources.
|
Financial
Condition
Liquidity
and Capital Resources
Our cumulative losses, since inception in 1987 through
December 31, 2009, are approximately $262 million. We
expect to continue to incur additional losses for at least the
next few years, as we implement our growth strategy of
commercializing marketed drugs, while continuing to develop our
portfolio of late-stage drug products. Our long-term strategy is
to generate profits from the sale and licensing of our drug
products. Accordingly, in the next several years, we expect to
supplement our cash position with sales of Zevalin and Fusilev
and generate licensing revenue from out-licensing our other drug
products.
While we believe that the approximately $125 million in
cash, cash equivalents and marketable securities, including some
long term marketable securities, which we had available on
December 31, 2009 will allow us to fund our current planned
operations for at least the next twelve to eighteen months, we
may, however, seek to obtain additional capital through the sale
of debt or equity securities, if necessary, especially in
conjunction with opportunistic acquisitions or license of drugs.
We may be unable to obtain such additional capital when needed,
or on terms favorable to us or our stockholders, if at all. If
we raise additional funds by issuing equity securities, the
percentage ownership of our stockholders will be reduced,
stockholders may experience additional dilution or such equity
securities may provide for rights, preferences or privileges
senior to those of the holders of our common stock. If
additional funds are raised through the issuance of debt
securities, the terms of such securities may place restrictions
on our ability to operate our business. If and when appropriate,
just as we have done in the past, we may pursue non-dilutive
financing alternatives as well.
Zevalin sales growth is largely dependent on the successful
launch of Zevalin for use as part of first-line therapy for
follicular NHL, continued use in its initial indication, and
establishing a consistent and accurate reimbursement standard.
As noted above, we recently obtained a CMS decision for a
reimbursement standard based on ASP methodology in the HOPPS
setting. Fusilev sales largely depend upon obtaining FDA
approval for use of Fusilev in combination with 5-FU containing
regimens for the treatment of colorectal cancer and favorable
reimbursement. As previously discussed, the FDA stated in their
October 2009 Complete Response letter that the submission did
not demonstrate that Fusilev is non-inferior to leucovorin; and
at our January 2010 meeting the FDA requested additional data,
which we expect to submit in the third quarter of 2010. We are
unable to reasonably estimate when, if ever, we will realize
sustainable net profit from sales of these two products or any
of our other products, if they are approved by the FDA.
Our expenditures for research and development consist of direct
product specific costs (such as up-front license fees, milestone
payments, active pharmaceutical ingredients, clinical trials,
patent related legal costs, and product liability insurance,
among others) and non-product specific, or indirect, costs. The
following summarizes our research and development expenses for
the periods indicated and include related stock-based charges
but not amortization of intangibles or expensing of in-process
research and development costs. To the extent that costs,
including personnel costs, are not tracked to a specific product
development program, they are included in the
59
“Indirect Costs” category in the table below. We
charge all research and development expenses to operations as
incurred.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
($ in ’000’s)
|
|
|
|
|
Eoquin
|
|
$
|
10,915
|
|
|
$
|
5,477
|
|
|
$
|
6,348
|
|
|
Ozarelix
|
|
|
1,168
|
|
|
|
2,435
|
|
|
|
6,217
|
|
|
Ortataxel
|
|
|
311
|
|
|
|
150
|
|
|
|
3,719
|
|
|
Fusilev
|
|
|
940
|
|
|
|
1,791
|
|
|
|
1,368
|
|
|
Zevalin
|
|
|
563
|
|
|
|
151
|
|
|
|
—
|
|
|
Lucanthone
|
|
|
289
|
|
|
|
348
|
|
|
|
1,405
|
|
|
Other development drugs
|
|
|
496
|
|
|
|
956
|
|
|
|
2,046
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total — Direct Costs
|
|
|
14,682
|
|
|
|
11,308
|
|
|
|
21,103
|
|
|
Indirect Costs (including non-cash share-based compensation of
$4.1 million, $3.9 million and $3.6 million,
respectively)
|
|
|
6,376
|
|
|
|
15,375
|
|
|
|
12,182
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Research & Development
|
|
$
|
21,058
|
|
|
$
|
26,683
|
|
|
$
|
33,285
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
While we are currently focused on advancing our key product
development programs, we anticipate that we will make regular
determinations as to which other programs, if any, to pursue and
how much funding to direct to each program on an on-going basis
in response to the scientific and clinical success of each
product candidate, as well as an ongoing assessment as to the
product candidate’s commercial potential.
Under our various existing licensing agreements, we are
contingently obligated to make various regulatory and business
milestone payments. In connection with the development of
certain in-licensed drug products, we anticipate the occurrence
of certain of these milestones during 2010. Upon successful
achievement of these milestones, we will likely become obligated
to pay up to approximately $0.2 million during 2010. The
FDA’s acceptance of our sNDA for Fusilev for CRC in March
2009, triggered the issuance of an aggregate of
125,000 shares of our common stock to Targent, or its
stockholders, with a fair market value of approximately
$185,000. In August 2009, we acquired 100% of the rights to
RenaZorb and
Renalan®,
a lanthanum-based nanotechnology compounds with potent and
selective phosphate binding properties, for all uses pursuant to
an amended and restated agreement that we entered into with
Altair Nanomaterials, Inc and Altair Nanotechnologies, Inc. In
2005, we had acquired the worldwide license from Altair to
develop and commercialize Altair’s lanthanum-based
nanotechnology compounds and related technology or all human
therapeutic uses. In consideration, we issued
113,809 shares of our common stock, with a then fair value
of approximately $750,000. Moving forward, we are responsible
for all development, commercialization and intellectual property
costs that accrue after the August 2009 execution date for the
amended and restated agreement.
Our anticipated net use of cash for operations in the fiscal
year ending December 31, 2010, excluding the cost of
in-licensing or acquisitions of additional drugs, if any, is
expected to range between approximately $30 and
$35 million. The programs that will represent a significant
part of our expenditures are the on-going clinical studies of
apaziquone and belinostat, the commercialization of Fusilev, and
the re-launch of Zevalin. The level of funding of our other
development projects is subject to the commercial success of our
marketed products, clinical progress with apaziquone and
belinostat and continued positive results from the preclinical
and clinical studies with these other products.
Further, while we do not receive any funding from third parties
for research and development that we conduct, co-development and
out-licensing agreements with other companies for any of our
drug products may reduce our expenses. In this regard, we
entered into a collaboration agreement with Allergan whereby,
commencing January 1, 2009, Allergan has borne 65% of the
development costs of apaziquone. Additionally, we entered into a
collaboration agreement with TopoTarget, whereby, commencing
February 2, 2010, TopoTarget bears, for belinostat, 100% of
the CUP trial costs and 30% of other development costs unrelated
to the PTCL study.
60
In addition to our present portfolio of drug product candidates,
we continually evaluate proprietary products for acquisition. If
we are successful in acquiring rights to additional products, we
may pay up-front licensing fees in cash
and/or
common stock and our research and development expenditures would
likely increase.
Net
Cash used in Operating Activities
During the year ended December 31, 2009 net cash used
in operations was approximately $17.6 million compared to
net cash used in operations of approximately $8.0 million
during 2008. The 2008 cash flows were favorably impacted by
revenues of approximately $20.7 million from the sale of
interests in certain non-core assets. The higher operating cash
outflows in 2009, are primarily attributable to higher selling,
general and administrative costs incurred due, in a large part,
to the marketing efforts associated with Zevalin and were
substantially mitigated by the revenues derived from Fusilev and
Zevalin, and the participation by Allergan in the development
activities for apaziquone.
Net
Cash used for Investing Activities
We used net cash in investing activities of approximately
$5.8 million for the year ended December 31, 2009 as
compared to net cash used in investing activities of
approximately $24.8 million for the year ended
December 31, 2008. The amounts were primarily as follows:
approximately $25.8 million of net disposition of
marketable securities as compared to approximately
$13.1 million invested in marketable securities and
approximately $0.7 million and $1.5 million, for
purchases of property and equipment in 2009 and 2008,
respectively. In addition, during 2009 and 2008, we invested
approximately $30.9 million and $10.2 million,
respectively, for the acquisition (including acquisition costs)
of the joint venture interest in Zevalin and certain milestone
payments upon approval of first line therapy of Zevalin
partially offset by amounts received in arbitration. At
December 31, 2008, we had acquired a 50% interest in the
joint venture in Zevalin and with a balance of $7.5 million
payable in January 2009. In March 2009, we completed the
acquisition of the full rights to Zevalin.
Net
Cash provided by Financing Activities
Net cash provided by financing activities totaled approximately
$95.9 million and $41.5 million for the years ended
December 31, 2009 and 2008, respectively. The 2009 amounts
were primarily from the sale of 15,187,715 shares of common
stock for net proceeds of approximately $95.8 million. In
2008, the $41.5 million up-front payment received from
Allergan was recorded as deferred revenue to be amortized over
future periods in accordance with our revenue recognition
policy. During 2009, pursuant to our revenue recognition policy,
we recognized $8.3 million of the $41.5 million
deferred in 2008 and expect that we will recognize the balance
over the period of the development work as defined in the
collaboration agreement with Allergan.
Results
of Operations
Our results of operations give effect to the restatement of our
previously issued consolidated financial statements as more
fully described above.
Results
of Operations for Fiscal 2009 Compared to Fiscal
2008
In 2009, we incurred a net loss of approximately
$19.0 million as compared to a net loss of
$14.2 million in 2008. The principal components of the
year-to-year
changes in line items are discussed below.
We recognized revenue of approximately $38.0 million in
2009 as compared to $28.7 million in 2008. During 2009, we
recorded approximately $28.2 million of revenue from the
sales of Zevalin and Fusilev as compared to approximately
$8.0 million in 2008. Zevalin and Fusilev revenues in 2009
were approximately $15.7 and $12.5 million respectively,
compared to approximately $0.3 million and
$7.7 million, respectively in 2008. While shipments of
Fusilev for the period ended December 31, 2008 were
approximately $10.8 million (net of estimates for
promotional, price and other adjustments), based on our revenue
recognition policy, we had deferred the recognition of
approximately $3.1 million of such revenue until we had
more experience with product returns. We also recognized
approximately $0.3 million net sales of Zevalin from the
consolidation of RIT Oncology, LLC (RIT) effective
December 15, 2008. We expect to continue to generate
revenue from the sales of these two products
61
in 2010, however, we are not able to provide any revenue
guidance at this time. During 2009, we also recognized
$8.3 million of licensing revenues from the amortization of
the $41.5 million up-front payment we received from
Allergan in 2008. We also recognized a milestone payment from
Allergan of $1.5 million on the completion of enrollment of
our two pivotal clinical trials for apaziquone. No similar
revenues were recognized in 2008. During 2008, we recognized
revenue from: (i) an agreement with Par Pharmaceutical, our
former marketing partner for sumatriptan injection, pursuant to
which we received a non-refundable $20 million cash payment
from Par for the transfer of our share of the profits from the
commercialization of sumatriptan injection; and (ii) the
transfer of rights to certain of our ANDAs to Sagent
Pharmaceuticals for $660,000. No similar revenues were generated
during 2009.
Selling, general and administrative expenses increased by
approximately $18.4 million, from approximately
$15.2 million in 2008 to approximately $33.6 million
in 2009, primarily due to approximately:
|
|
|
| |
•
|
$10.6 million increase attributable to sales and marketing
expenses, including payroll costs, incurred with the launch of
Zevalin and Fusilev.
|
| |
| |
•
|
$3.3 million increase in general and administrative costs
due to increased activities, including payroll costs and higher
professional costs due to business development activities
|
| |
| |
•
|
$1.6 million increase in non-cash compensation expenses.
|
We expect an increase in selling, general and administrative
expenses for 2010 primarily related to sales and marketing of
Zevalin and Fusilev.
Research and development expenses decreased by approximately
$5.7 million, from approximately $26.7 million in 2008
to approximately $21 million in 2009, which included
non-cash amortization and write off of Zevalin related
intangibles and
in-process
research, and development changes of approximately
$3.7 million and $4.9 million in 2009 and 2008,
respectively. Research and development expenses also reduced
primarily due to sharing of apaziquone related development costs
by our development partner, Allergan, of approximately
$11.2 million. In addition, we incurred reduced development
expense in other development products, including ozarelix.
During 2009, in line with the strategy outlined at the start of
the year, and in response to the global financial crisis we
focused on executing a successful launch of Zevalin and Fusilev
and prioritized our research and development efforts to complete
the rapid enrollment in the apaziquone clinical studies.
We anticipate research and development expense in 2010 to be
higher than 2009 primarily due to our recent partnership with
TopoTarget for the development of belinostat. Research and
development costs will be partially offset by our joint
development agreement with Allergan, under which Allergan funds
65% of the development costs of development costs related to
apaziquone and Topotarget will fund 100% of the development
costs of the CUP study and cover in 30% of the development costs
related to studies other than PTCL.
As reported above, we recorded approximately $3.7 million
of expense from amortization of Zevalin related intangibles for
the year ended December 31, 2009 as compared to
$0.2 million during the same period in 2008. This was a
full year’s amortization expense as compared to
15 day’s prorated amortization during 2008, since
Zevalin was acquired in December 2008. During the year 2008, we
recorded expense of $4.7 million for in-process research
and development, or IPRD, on the Zevalin related intangibles; no
similar expense was recorded in 2009.
We recorded approximately $8.1 million of income from
warrant obligations in 2009 as compared to $1.3 million in
2008, in connection with the restatement of our previously
reported financial statements.
Other income consisted of net interest income of approximately
$0.7 million and $1.2 million for the years ended
December 31, 2009 and 2008, respectively and in 2008
included approximately $200,000 realized investment gains. The
decrease in interest income was primarily due to lower
investment yields in 2009 due to the shift in our investment
strategy to more conservative US Treasury investments. We expect
similar yields going forward until such time the credit markets
improve.
62
Results
of Operations
Results
of Operations for Fiscal 2008 Compared to Fiscal
2007
In 2008, we incurred a net loss of approximately
$14.2 million compared to a net loss of approximately
$22.00 million in 2007. The principal components of the
year-to-year
changes in line items are discussed below.
We recognized revenue of approximately $28.7 million in
2008 as compared to approximately $7.7 million in 2007.
During 2008, we recorded approximately $7.7 million of
revenue from the August 2008 commercial launch of Fusilev, which
was approved by the FDA in March 2008. While shipments of
Fusilev for the period ended December 31, 2008 were
approximately $10.8 million (net of estimates for
promotional, price and other adjustments), based on our revenue
recognition policy, we have deferred the recognition of
approximately $3.1 million of such revenue until we have
more experience with the amount of product returns. We also
recognized approximately $0.3 million net sales of Zevalin
from the consolidation of RIT effective December 15, 2008.
In addition, during 2008, we recognized revenue from:
(i) an agreement with Par Pharmaceutical, our former
marketing partner for sumatriptan injection, pursuant to which
we received a non-refundable $20 million cash payment from
Par for the transfer of our share of the profits from the
commercialization of sumatriptan injection; and (ii) the
transfer of rights to certain of our ANDAs to Sagent
Pharmaceuticals for $660,000. No similar revenues were generated
during 2007. During 2007, we recognized approximately
$7.7 million in licensing milestone and related revenues,
pursuant to our agreement with GPC Biotech for satraplatin.
Research and development expenses decreased by approximately
$6.6 million, from approximately $33.3 million in 2007
to approximately $26.7 million in 2008. During 2008, in
line with the strategy outlined at the start of the year, and in
response to the global financial crisis we focused on executing
a successful launch of Fusilev and prioritized our R&D
efforts to the rapid enrollment of the apaziquone clinical
study. Principal components of the decrease in 2008 were as
follows. Approximately:
|
|
|
| |
•
|
$3.8 million and $3.6 million, respectively, due to
reductions in direct development costs related to ozarelix and
ortataxel; partially offset by,
|
| |
| |
•
|
$2.0 million increase in employee compensation expense
associated substantially with the hiring of personnel to advance
the apaziquone clinical study.
|
We recorded approximately $0.2 million of expense from
amortization of Zevalin related intangibles for the year ended
December 31, 2008 as compared to $0 million during the
same period in 2007. This was a 15 day’s prorated
amortization during 2008, since Zevalin was acquired in December
2008. During the year 2008, we recorded expense of approximately
$4.7 million for in-process research and development (IPRD)
on the Zevalin related intangibles; no similar expense was
recorded in 2007.
Selling, general and administrative expenses increased by
approximately $3.6 million, from approximately
$11.6 million in 2007 to approximately $15.2 million
in 2008, primarily due to approximately:
|
|
|
| |
•
|
$5.9 million increase attributable to sales and marketing
expenses, including payroll costs, incurred with the launch of
Fusilev.
|
| |
| |
•
|
$2.4 million decrease in legal expenses, largely
attributable to the non-recurrence of arbitration costs against
GPC Biotech incurred during 2007, partially offset by legal
expenses in connection with business development activities,
including the collaboration agreement with Allergan.
|
| |
| |
•
|
$1.2 million increase in employee compensation attributed
to the expanded scope of operations.
|
We recorded approximately $1.3 million of income from
warrant obligations in 2008 as compared to approximately
$12.1 million in 2007, in connection with the restatement
of our previously reported financial statements.
Other income consisted of net interest income of approximately
$1.2 million and $3.1 million for the years ended
December 31, 2008 and 2007 and in 2008 included
approximately $200,000 realized investment gains. The decrease
in interest income was primarily due to lower investment yields
in 2008 due to the shift in our investment strategy to more
conservative US Treasury investments.
63
Nature
of each accrual that reduces gross revenue to net
revenue
Provisions for product returns, sales discounts and rebates and
estimates for chargebacks are established as a reduction of
product sales revenue at the time revenues are recognized.
Management considers various factors in determination of such
provisions, which are described more in detail below. Such
estimated amounts are deducted from our gross sales to determine
our net revenues. Provisions for bad and doubtful accounts are
deducted from gross receivables to determine net receivables.
Changes in our estimates, if any, would be recorded in the
statement of operations in the period the change is determined.
If we materially over or under estimate the amount, there could
be a material impact on our consolidated financial statements.
For the periods ended December 31, 2009 and 2008, the
following is a roll forward of the provisions for return,
discounts and rebates and chargebacks allowances and estimated
doubtful account allowances.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chargebacks &
|
|
|
|
|
|
Doubtful
|
|
|
|
|
|
Schedule of Allowances for Receivables
|
|
Discounts
|
|
|
Returns
|
|
|
Accounts
|
|
|
Total
|
|
|
|
|
($ in ’000’s)
|
|
|
|
|
Year Ended December 31, 2009:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at beginning of the period
|
|
$
|
1,631
|
|
|
$
|
3,144
|
|
|
$
|
150
|
|
|
$
|
4,925
|
|
|
Add/(less) provisions:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Related to the sales of current fiscal year
|
|
|
3,760
|
|
|
|
95
|
|
|
|
—
|
|
|
|
3,855
|
|
|
Related to the sales of prior fiscal years
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Less : Credits or actual allowances:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Related to sales from current fiscal year
|
|
|
2,900
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,900
|
|
|
Related to sales from prior fiscal years
|
|
|
1,631
|
|
|
|
2,063
|
|
|
|
—
|
|
|
|
3,694
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at the close of the period
|
|
$
|
860
|
|
|
$
|
1,176
|
|
|
$
|
150
|
|
|
$
|
2,186
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, 2008:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at beginning of period
|
|
|
—
|
|
|
|
—
|
|
|
$
|
150
|
|
|
$
|
150
|
|
|
Provisions:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Related to the sales of current fiscal year
|
|
$
|
1,691
|
|
|
$
|
3,144
|
|
|
|
—
|
|
|
$
|
4,835
|
|
|
Related to the sales of prior fiscal years
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Credits or actual allowances:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Related to sales from current fiscal year
|
|
|
60
|
|
|
|
—
|
|
|
|
—
|
|
|
|
60
|
|
|
Related to sales from prior fiscal years
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at the close of the period
|
|
$
|
1,631
|
|
|
$
|
3,144
|
|
|
$
|
150
|
|
|
$
|
4,925
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amounts recorded as allowances on our consolidated balance
sheets for 2009 and 2008 are reflected in the table above. The
basis and methods of estimating these allowances, used by
management, are described below.
Chargebacks,
discounts and rebates
Chargebacks represent a provision against gross accounts
receivable and related reduction to gross revenue. A chargeback
is the difference between the price the wholesale customer, in
our case the wholesaler or distributor, pays (the wholesale
acquisition cost, or WAC) and the price (contracted price) that
a contracted customer (e.g., a Group Purchasing Organization
(GPO) member) pays for a product. We accrue for chargebacks in
the relevant period on the presumption that all units of product
sold to members of the GPOs will be charged back. We estimate
chargebacks at the time of sale of our products to the members
of the GPOs based on:
(1) volume of all products sold via distributors to members
of the GPOs and the applicable chargeback rates for the relevant
period;
(2) applicable WAC and the contract prices agreed with the
GPOs; and
64
(3) the information of inventories remaining on hand at the
wholesalers and distributors at the end of the period, actual
chargeback reports received from our wholesalers and
distributors as well as the chargebacks not yet billed (product
shipped less the chargebacks already billed back) in the
calculation and validation of our chargeback estimates and
reserves.
Discounts (generally prompt payment discounts) are accrued at
the end of every reporting period based on the gross sales made
to the customers during the period and based on their terms of
trade for a product. We generally review the terms of the
contracts, specifically price and discount structures, payment
terms in the contracts between the customer and the Company to
estimate the discount accrual.
Customer rebates are estimated at every period end, based on
direct purchases, depending on whether any rebates have been
offered. The rebates are recognized when products are purchased
and a periodic credit is given. Medicaid rebates are based on
the data we receive from the public sector benefit providers,
which is based on the final dispensing of our product by a
pharmacy to a benefit plan participant.
We record Medicaid and Medicare rebates based on estimates for
such expense. However, such amount have not been material to the
financial statements.
Product
returns allowances
Customers are typically permitted to return products within
thirty days after shipment, if incorrectly shipped or not
ordered, and within a window of time six months before and
twelve months after the expiration of product dating,
subject to certain restocking fees and preauthorization
requirements, as applicable. The returned product is destroyed
if it is damaged, quality is compromised or past its expiration
date. Based on our returns policy, we refund the sales price to
the customer as a credit and record the credit against
receivables. In general, returned product is not resold. As of
each balance sheet date, we estimate potential returns, based on
several factors, including: inventory held by distributors, sell
through data of distributor sales to end users, customer and
end-user ordering and re-ordering patterns, aging of accounts
receivables, rates of returns for directly substitutable
products and pharmaceutical products for the treatment of
therapeutic areas similar to indications served by our products,
shelf life of our products and based on experience of our
management with selling similar oncology products. We record an
allowance for future returns by debiting revenue, thereby
reducing gross revenues and crediting a reserve for returns to
reduce gross receivables.
Doubtful
Accounts
An allowance for doubtful accounts is estimated based on the
customer payment history and a review by management of the aging
of the accounts receivables as of the balance sheet date. We
accrue for doubtful accounts by recording an expense and
creating an allowance for such accounts. If we are privy to
information on the solvency of a customer or observe a payment
history change, we estimate the accrual for such doubtful
receivables or write the receivable off.
Off-Balance
Sheet Arrangements
We do not have any off balance sheet arrangements.
65
Contractual
and Commercial Obligations
The following table summarizes our contractual and other
commitments, including obligations under a facility lease and
equipment leases, as of December 31, 2009, approximately:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Less than
|
|
|
|
|
|
|
|
|
After
|
|
|
|
|
Total
|
|
|
1 Year
|
|
|
2-3 Years
|
|
|
4-5 Years
|
|
|
5 Years
|
|
|
|
|
Contractual Obligations(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital Lease Obligations(2)
|
|
$
|
147
|
|
|
$
|
50
|
|
|
$
|
97
|
|
|
|
—
|
|
|
|
—
|
|
|
Operating Lease Obligations(3)
|
|
|
3,284
|
|
|
|
428
|
|
|
|
939
|
|
|
|
1,054
|
|
|
$
|
863
|
|
|
Purchase Obligations(4)
|
|
|
8,955
|
|
|
|
7,278
|
|
|
|
1,677
|
|
|
|
—
|
|
|
|
—
|
|
|
Contingent Milestone Obligations(5)
|
|
|
75,749
|
|
|
|
200
|
|
|
|
3,519
|
|
|
|
2,109
|
|
|
|
69,921
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
88,135
|
|
|
$
|
7,956
|
|
|
$
|
6,232
|
|
|
$
|
3,163
|
|
|
$
|
70,784
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
The table of contractual and commercial obligations excludes
contingent payments that we may become obligated to pay upon the
occurrence of future events whose outcome is not readily
determinable. Such significant contingent obligations are
described below under “Employment Agreements.” |
| |
|
(2) |
|
The capital lease obligations are related to leased office
equipment. |
| |
|
(3) |
|
The operating lease obligations are primarily related to the
facility lease for our corporate office, which we renewed in
July 2009 and expires in June 2016. |
| |
|
(4) |
|
Purchase obligations represent the amount of open purchase
orders and contractual commitments to vendors for products and
services that have not been delivered, or rendered, as of
December 31, 2009. Approximately 90% of the purchase
obligations consist of expenses associated with clinical trials
and related costs for apaziquone and ozarelix for each of the
periods presented. Please see “Service Agreements”
below for further information. |
| |
|
(5) |
|
Milestone obligations are payable contingent upon successfully
reaching certain development and regulatory milestones as
further described below under “Licensing Agreements.”
While the amounts included in the table above represent all of
our potential cash development and regulatory milestone
obligations as of December 31, 2009, given the
unpredictability of the drug development process, and the
impossibility of predicting the success of current and future
clinical trials, the timelines estimated above do not represent
a forecast of when payment milestones will actually be reached,
if at all. Rather, they assume that all development and
regulatory milestones under all of our license agreements are
successfully met, and represent our best estimates of the
timelines. In the event that the milestones are met, we believe
it is likely that the increase in the potential value of the
related drug product will exceed the amount of the milestone
obligation. |
Licensing
Agreements
Almost all of our drug candidates are being developed pursuant
to license agreements that provide us with rights to certain
territories to, among other things, develop, sublicense, and
sell the drugs. We are required to use commercially reasonable
efforts to develop the drugs, are generally responsible for all
development, patent filing and maintenance costs, sales,
marketing and liability insurance costs, and are generally
contingently obligated to make milestone payments to the
licensors if we successfully reach development and regulatory
milestones specified in the agreements. In addition, we are
obligated to pay royalties and, in some cases, milestone
payments based on net sales, if any, after marketing approval is
obtained from regulatory authorities.
The potential contingent development and regulatory milestone
obligations under all our licensing agreements are generally
tied to progress through the FDA approval process, which
approval significantly depends on positive clinical trial
results. The following list is typical of milestone events
relevant for us: conclusion of Phase 2 or commencement of Phase
3 clinical trials; filing of new drug applications in each of
the United States, Europe and Japan; and approvals from each of
the regulatory agencies in those jurisdictions.
66
Service
Agreements
In connection with the research and development of our drug
products, we have entered into contracts with numerous third
party service providers, such as clinical trial centers,
clinical research organizations, data monitoring centers, and
with drug formulation, development and testing laboratories. The
financial terms of these agreements are varied and generally
obligate us to pay in stages, depending on achievement of
certain events specified in the agreements, such as contract
execution, reservation of service or production capacity, actual
performance of service, or the successful accrual and dosing of
patients.
At each period end, we accrue for all costs of goods and
services received, with such accruals based on factors such as
estimates of work performed, patient enrollment, completion of
patient studies and other events. As of December 31, 2009,
we were committed under such contracts for up to approximately
$9.0 million, for future goods and services, including
approximately $7.3 million due within one year. We are in a
position to accelerate, slow-down or discontinue any or all of
the projects that we are working on at any given point in time.
Should we decide to discontinue
and/or
slow-down the work on any project, the associated costs for
those projects would get limited to the extent of the work
completed. Generally, we are able to terminate these contracts
due to the discontinuance of the related project(s) and thus
avoid paying for the services that have not yet been rendered
and our future purchase obligations would reduce accordingly.
Employment
Agreement
We have entered into an employment agreement with
Dr. Shrotriya, our President and Chief Executive Officer,
which expires January 2, 2011. The employment agreement
automatically renews for a one-year calendar term unless either
party gives written notice of such party’s intent not to
renew the agreement at least ninety days prior to the
commencement of the next year. The employment agreement requires
Dr. Shrotriya to devote his full working time and effort to
the business and affairs of the Company during the term of the
agreement. The employment agreement provides for a minimum
annual base salary with annual increases, periodic bonuses and
option grants as determined by the Compensation Committee of the
Board of Directors.
Dr. Shrotriya’s employment may be terminated due to
non-renewal of his employment agreement by us, mutual agreement,
death or disability, or by us for cause (as that term is defined
in the employment agreement) or without cause, or by
Dr. Shrotriya for no reason, good reason (as defined in the
agreement) or non-renewal. The employment agreement provides for
various guaranteed severance payments and benefits if:
(i) the agreement is not renewed by us,
(ii) Dr. Shrotriya’s employment is terminated
without cause, (iii) Dr. Shrotriya resigns for good
reason, (iv) the agreement is terminated due to death or
disability of Dr. Shrotriya, (v) if Dr. Shrotriya
voluntarily resigns his employment for no reason or (vi) if
Dr. Shrotriya’s employment is terminated (other than
by Dr. Shrotriya) without cause within twelve months after
a change in control, or Dr. Shrotriya is adversely affected
in connection with a change in control and resigns within twelve
months. If the agreement is terminated due to mutual agreement,
Dr. Shrotriya’s non-renewal of the agreement, or by us
for cause, Dr. Shrotriya shall not be entitled to any
severance.
If any payment or distribution by us to or for the benefit of
Dr. Shrotriya is subject to the excise tax imposed by
Section 4999 of the Internal Revenue Code (IRC) or any
interest or penalties are incurred by Dr. Shrotriya with
respect to such excise tax, then Dr. Shrotriya shall be
entitled to receive an additional payment in an amount such that
after payment by Dr. Shrotriya of all taxes (including any
interest and penalties imposed with respect thereto) and excise
tax imposed upon such payment, Dr. Shrotriya retains an
amount of the payment equal to the excise tax imposed upon the
payment.
If we determine that any payments to Dr. Shrotriya under
the agreement fail to satisfy the distribution requirement of
Section 409A(a)(2)(A) of the IRC, the payment schedule of
that benefit shall be revised to the extent necessary so that
the benefit is not subject to the provisions of
Section 409A(a)(1) of the IRC. We may attach conditions to
or adjust the amounts so paid to preserve, as closely as
possible, the economic consequences that would have applied in
the absence of this adjustment; provided, however, that no such
condition or adjustment shall result in the payments being
subject to Section 409A(a)(1) of the IRC.
67
Critical
Accounting Policies, Estimates and Assumptions
Our discussion and analysis of our consolidated financial
condition and results of operations are based upon our
consolidated financial statements, which have been prepared in
conformity with accounting principles generally accepted in the
United States (GAAP). The preparation of these financial
statements requires us to make estimates and assumptions that
affect the reported amounts of assets, liabilities, revenues and
expenses, and related disclosure of contingent assets and
liabilities reported in our consolidated financial statements.
The estimation process requires assumptions to be made about
future events and conditions, and is consequently inherently
subjective and uncertain. Actual results could differ materially
from our estimates. We regularly evaluate our estimates,
including cash requirements, by assessing: planned research and
development activities and general and administrative
requirements; required clinical trial activity; market need for
our drug candidates; and other major business assumptions.
The SEC defines critical accounting policies as those that are,
in management’s view, most important to the portrayal of
our financial condition and results of operations and most
demanding of our judgment. We consider the following policies to
be critical to an understanding of our consolidated financial
statements and the uncertainties associated with the complex
judgments made by us that could impact our results of
operations, financial position and cash flows.
Cash,
Cash Equivalents and Marketable Securities
Cash, cash equivalents and marketable securities primarily
consist of bank checking deposits, short-term treasury
securities, and institutional money market funds, corporate debt
and equity, municipal obligations, including market auction debt
securities, government agency notes, and certificates of
deposit. We classify highly liquid short-term investments, with
insignificant interest rate risk and maturities of
ninety days or less at the time of acquisition, as cash and
cash equivalents. Other investments, which do not meet the above
definition of cash equivalents, are classified as either
“held-to-maturity”
or
“available-for-sale”
marketable securities. Investments that we intend to hold for
more than one year are classified as long-term investments. All
of our “available for sale securities” are classified
as current assets based on our intent and ability to use any and
all of these securities as necessary to satisfy our cash needs
as they arise, by redeeming them at par with short notice and
without a penalty. Investments with maturity dates over
one year from December 31, 2009 are classified as
held-to-maturity.
Revenue
Recognition
We sell our products to wholesalers and distributors of oncology
products and directly to the end user, directly or through GPOs
(e.g., certain hospitals or hospital systems and clinics with
whom we have entered into a direct purchase agreement). Our
wholesalers and distributors purchase our products and sell the
products directly to the end users, which include, but are not
limited to, hospitals, clinics, medical facilities, managed care
facilities and private oncology based practices etc. Revenue
from product sales is recognized upon shipment of product when
title and risk of loss have transferred to the customer, and the
following additional criteria specified by ASC
No. 605-15,
“Revenue Recognition: Products” are met:
(i) the price is substantially fixed and determinable;
(ii) our customer has economic substance apart from that
provided by us;
(iii) our customer’s obligation to pay us is not
contingent on resale of the product; and
(iv) we do not have significant obligations for future
performance to directly bring about the resale of our
product; and
(v) we have a reasonable basis to estimate future returns.
We also follow the provisions as set forth by current accounting
rules, which primarily include Staff Accounting
Bulletin ASC
No. 605-15
“Revenue Recognition,” “Revenue
Recognition,” and ASC
No. 605-25,
“Revenue Recognition: Multiple-Element Arrangements.”
68
Generally, revenue is recognized when all four of the following
criteria are met:
(i) persuasive evidence that an arrangement exists;
(ii) delivery of the products has occurred, or services
have been rendered;
(iii) the selling price is both fixed and
determinable; and
(iv) collectibility is reasonably assured.
Provisions for estimated product returns, sales discounts,
rebates and charge backs are established as a reduction of gross
product sales at the time such revenues are recognized. Thus,
revenue is recorded, net of such estimated provisions. Our
estimates for product returns are based our review of inventory
in the channels and review of historical rates of actual returns.
Consistent with industry practice, our product return policy
permits our customers to return products within thirty days
after shipment, if incorrectly shipped or not ordered, and
within a window of time six months before and
twelve months after the expiration of product dating,
subject to certain restocking fees and preauthorization
requirements, as applicable. Currently, our returns policy does
not allow for replacement of product. The returned product is
destroyed if it is damaged, it’s quality is compromised or
it is past its expiration date. Based on our returns policy, we
refund the sales price to the customer as a credit and record
the credit against receivables. In general returned product is
not resold. We generally reserve the right to decline granting a
return and to decide on product destruction. As of each balance
sheet date, we estimate potential returns, based on several
factors, including: inventory held by distributors, sell through
data of distributor sales to end users, customer and end-user
ordering and re-ordering patterns, aging of accounts
receivables, rates of returns for directly substitutable
products and other pharmaceutical products for the treatment of
therapeutic areas similar to indications served by our products,
shelf life of our products and the extensive experience of our
management with selling the same and similar oncology products.
We record an allowance for future returns by debiting revenue,
thereby reducing gross revenues and crediting a reserve for
returns to reduce gross receivables. If allowances exceed the
related accounts receivables, we reclassify such allowances to
accrued obligations.
We also state the related accounts receivable at net realizable
value, with any allowance for doubtful accounts charged to
general operating expenses. If revenue from sales is not
reasonably determinable due to provisions for estimates,
promotional adjustments, price adjustments, returns or any other
potential adjustments, we defer the revenue and recognize
revenue when the estimates are reasonably determinable, even if
the monies for the gross sales have been received.
Up-front fees representing non-refundable payments received upon
the execution of licensing or other agreements are recognized as
revenue upon execution of the agreements where we have no
significant future performance obligations and collectibility of
the fees is reasonably assured. Milestone payments, which are
generally based on developmental or regulatory events, are
recognized as revenue when the milestones are achieved,
collectibility is reasonably assured, and we have no significant
future performance obligations in connection with the milestone.
In those instances where we have collected fees or milestone
payments but have significant future performance obligations
related to the development of the drug product, we record
deferred revenue and recognize it over the period of our future
obligations.
Purchase
Price Allocation
The purchase price allocation for acquisitions of the tangible
and identifiable intangible assets acquired and liabilities
assumed based on their estimated fair values at the acquisition
date requires extensive accounting estimates and judgments,
including in process research and development. Based on the
provisions of ASC No. 805, “Business
Combinations,” for transactions that occurred prior to
December 31, 2008, we allocated the purchase price for the
identifiable intangibles. For each acquisition, we engaged an
independent third-party valuation firm to assist in determining
the fair value of in-process research and development and
identifiable intangible assets. Such a valuation requires
significant estimates and assumptions including but not limited
to: determining the timing and expected costs to complete the
in-process projects, projecting regulatory approvals, estimating
future cash flows from product sales resulting from in-process
projects, and developing appropriate discount rates and
probability
69
rates by project. We believe the fair values assigned to the
assets acquired and liabilities assumed are based on reasonable
assumptions. However, these assumptions may be inaccurate, and
unanticipated events and circumstances may occur. Additionally,
we must determine whether an acquired entity considered to be a
business or a set of net assets because a portion of the
purchase price can only be allocated to goodwill in a business
combination.
Research
and Development
Research and development expenses include salaries and benefits,
clinical trial and related manufacturing costs, contract and
other outside service fees, and facilities and overhead costs
related to our research and development efforts. Research and
development expenses also consist of costs incurred for
proprietary and collaboration research and development and
include activities such as product registries and
investigator-sponsored trials. Research and development costs
are expensed as incurred. In certain instances we enter into
agreements with third parties for research and development
activities, where we may prepay fees for services at the
initiation of the contract. We record such prepayment as a
prepaid asset and charge research and development expense over
the period of time the contracted research and development
services are performed. In connection with the October 2008
co-development agreement, Allergan bears 65% of the development
costs incurred for apaziquone in NMIBC, commencing
January 1, 2009. During the year ended December 31,
2009, approximately $11.2 million of development costs were
reimbursed by Allergan, and credited against total related
research and development expense.
As of each balance sheet date, we review purchase commitments
and accrue drug development expenses based on factors such as
estimates of work performed, patient enrollment, completion of
patient studies and other events. Accrued clinical study costs
are subject to revisions as trials progress to completion.
Revisions are recorded in the period in which the facts that
give rise to the revision become known.
Amortization
and impairment of intangible assets
Identifiable intangible assets with definite lives are amortized
on a straight-line basis over their estimated useful lives,
ranging from 1 to 10 years.
We evaluate the recoverability of intangible assets whenever
events or changes in circumstances indicate that an intangible
asset’s carrying amount may not be recoverable. Such
circumstances could include, but are not limited to the
following:
i a significant decrease in the market value of an asset;
ii a significant adverse change in the extent or manner in
which an asset is used; or
iii an accumulation of costs significantly in excess of the
amount originally expected for the acquisition of an asset.
We measure the carrying amount of the asset against the
estimated undiscounted future cash flows associated with it.
Should the sum of the expected future net cash flows be less
than the carrying value of the asset being evaluated, an
impairment loss would be recognized. The impairment loss would
be calculated as the amount by which the carrying value of the
asset exceeds its fair value. No impairment loss was recorded
during the years 2009, 2008 or 2007.
Share-Based
Compensation
We recognize compensation expenses for all share-based awards to
employees and directors. In estimating the fair value of
share-based compensation, we use the quoted closing market
price, based on the date prior to our grant date, of our common
stock for stock awards and the Black-Scholes option pricing
model for stock options and warrants. We estimate future
volatility based on historical volatility of our common stock,
and we estimate the expected life of options based on several
criteria, including the vesting period of the grant and the
expected volatility.
Share based compensation is recognized only for those awards
that are ultimately expected to vest, and we have applied or
estimated forfeiture rate to unvested awards for purposes of
calculating compensation costs. These
70
estimates will be revised in future periods if actual
forfeitures differ from the estimates. Changes in forfeiture
estimates impact compensation cost in the period in which the
change in estimate occurs.
Warrant
accounting
We account for registered common stock warrants pursuant to
applicable accounting guidance contained in ASC 815
“Deviratives and Hedging — Contracts in
Entity’s Own Equity” (formerly known as EITF
00-19) on
the understanding that in compliance with applicable securities
laws, the registered warrants require the issuance of registered
securities upon exercise and do not sufficiently preclude an
implied right to net cash settlement. We classify registered
warrants on the consolidated balance sheet as a current
liability which is revalued at each balance sheet date
subsequent to the initial issuance. Determining the appropriate
fair-value model and calculating the fair value of registered
warrants requires considerable judgment, including estimating
stock price volatility and expected warrant life. We develop our
estimates based on historical data. A small change in the
estimates used may have a relatively large change in the
estimated valuation. We use the Black-Scholes pricing model to
value the registered warrants. Changes in the fair market value
of the warrants are reflected in the consolidated statement of
operations as “Change in fair value of common stock warrant
liability.”
The following summarizes the activity of Level 3 inputs measured
on a recurring basis for the years ended December 31, 2009
and 2008:
| |
|
|
|
|
|
|
|
Fair Value Measurements of
|
|
|
|
|
Common Stock Warrants
|
|
|
|
|
Using Significant
|
|
|
|
|
Unobservable Inputs (Level 3)
|
|
|
|
|
($ in 000’s)
|
|
|
|
|
Balance at January 1, 2008
|
|
$
|
2,036
|
|
|
|
|
|
|
|
|
Transfers in / (out) of Level 3
|
|
|
—
|
|
|
Issuance of common stock warrants
|
|
|
—
|
|
|
Repurchase of Forfeitures
|
|
|
—
|
|
|
Expirations
|
|
|
—
|
|
|
Settlements associated with exercises
|
|
|
—
|
|
|
Adjustments resulting from change in value of warrants
recognized in earnings
|
|
|
(1,271
|
)
|
|
|
|
|
|
|
|
Balance at December 31, 2008
|
|
|
765
|
|
|
|
|
|
|
|
|
Transfers in / (out) of Level 3
|
|
|
—
|
|
|
Issuance of common stock warrants
|
|
|
14,016
|
|
|
Repurchases or forfeitures
|
|
|
(394
|
)
|
|
Gain on repurchase recognized in earnings
|
|
|
323
|
|
|
Expirations
|
|
|
—
|
|
|
Settlements associated with exercises
|
|
|
—
|
|
|
Adjustments resulting from change in value of warrants
recognized in earnings
|
|
|
(8,075
|
)
|
|
|
|
|
|
|
|
Balance at December 31, 2009
|
|
$
|
6,635
|
|
|
|
|
|
|
|
New
Accounting Pronouncements
See Note 3: Recent Accounting Pronouncements of our
accompanying consolidated financial statements for a description
of recent accounting pronouncements that have a potentially
significant impact on our financial reporting and our
expectations of their impact on our results of operations and
financial condition.
71
|
|
|
Item 7A.
|
Quantitative
and Qualitative Disclosures About Market Risk
|
The primary objective of our investment activities is to
preserve principal, while at the same time maximizing yields
without significantly increasing risk. We do not utilize hedging
contracts or similar instruments.
We are exposed to certain market risks. Our primary exposures
relate to (1) interest rate risk on our investment
portfolio, (2) credit risk of the companies’ bonds in
which we invest, (3) general credit market risks as have
existed since late 2007 and (4) the financial viability of
the institutions which hold our capital and through which we
have invested our funds. We manage such risks on our investment
portfolio by matching scheduled investment maturities with our
cash requirements and investing in highly rated instruments.
In response to the dislocation in the credit markets since the
latter part of 2007, in early 2008 we converted substantially
all of our investments, including all of our market auction debt
securities, into highly liquid and safe instruments. Our
investments, as of December 31, 2009, were primarily in
money market accounts, short-term corporate bonds, certificate
of deposits, U.S. Treasury bills and
U.S. Treasury-backed securities. We believe the financial
institutions through which we have invested our funds are
strong, well capitalized and our instruments are held in
accounts segregated from the assets of the institutions.
However, due to the current extremely volatile financial and
credit markets and liquidity crunch faced by most banking
institutions, the financial viability of these institutions, and
the safety and liquidity of our funds is being constantly
monitored.
Because of our ability to generally redeem these investments at
par at short notice and without penalty, changes in interest
rates would have an immaterial effect on the fair value of these
investments. If a 10% change in interest rates were to have
occurred on December 31, 2009, any decline in the fair
value of our investments would not be material in the context of
our consolidated financial statements. In addition, we are
exposed to certain market risks associated with credit ratings
of corporations whose corporate bonds we may purchase from time
to time. If these companies were to experience a significant
detrimental change in their credit ratings, the fair market
value of such corporate bonds may significantly decrease. If
these companies were to default on these corporate bonds, we may
lose part or all of our principal. We believe that we
effectively manage this market risk by diversifying our
investments, and investing in highly rated securities.
In addition, we are exposed to foreign currency exchange rate
fluctuations relating to payments we make to vendors, suppliers
and license partners using foreign currencies. In particular,
some of our obligations are incurred in Euros. We mitigate such
risk by maintaining a limited portion of our cash in Euros and
other currencies.
|
|
|
Item 8.
|
Financial
Statements and Supplementary Data
|
Our annual consolidated financial statements are included in
Item 15 of this report.
|
|
|
Item 9.
|
Changes
in and Disagreements with Accountants on Accounting and
Financial Disclosure
|
Effective December 3, 2009, our Audit Committee approved
the engagement of Ernst & Young to serve as our
independent registered public accounting firm. Our prior
auditors, Kelly & Co., were re-engaged by our audit
committee on March 30, 2010 to reopen the audit of the
years ended December 31, 2007 and 2008.
During the two fiscal years ended December 31, 2009 and
2008, there were no disagreements between us and Kelly &
Co. on any matter of accounting principles or practices,
financial statement disclosure, or auditing scope or procedure
which, if not resolved to Kelly & Co.’s satisfaction,
would have caused Kelly & Co. to make reference to the
subject matter of the disagreement in connection with its report
for such years within the meaning of Item 304(a)(1)(iv) of
Regulation S-K.
In addition, during the period identified above, there were no
reportable events as defined in Item 304(a)(1)(v) of
Regulation S-K.
|
|
|
Item 9A.
|
Controls
and Procedures
|
Our principal executive officer and principal financial officer
have provided certifications filed as Exhibits 31.1 and 32.1,
and 31.2 and 32.2, respectively. Such certifications should be
read in conjunction with the information contained in this
Item 9A for a more complete understanding of the matters
covered by such certifications.
72
(i) Disclosure
Controls and Procedures
We have established disclosure controls and procedures (as such
terms are defined in
Rules 13a-15(e)
and
15d-15(e)
under the Exchange Act, that are designed to ensure that
information required to be disclosed in our Exchange Act reports
is recorded, processed, summarized and reported within the time
periods specified in the SEC’s rules and forms, and that
such information is accumulated and communicated to our
management, including our Chief Executive Officer (our principal
executive officer) and Vice President Finance (our principal
financial officer), as appropriate, to allow for timely
decisions regarding required disclosure. In designing and
evaluating the disclosure controls and procedures, our
management is required to apply its judgment in evaluating the
cost-benefit relationship of possible controls and procedures.
Our disclosure controls and procedures are designed to provide a
reasonable level of assurance of reaching our desired disclosure
control objectives.
We carried out an evaluation, under the supervision and with the
participation of our management, including our Chief Executive
Officer and our Vice President of Finance, of the effectiveness
of the design and operation of our disclosure controls and
procedures as of December 31, 2009, the end of the period
covered by this annual report. Based on such evaluation, as of
December 31, 2009, our Chief Executive Officer and Vice
President of Finance concluded that, in light of the restatement
required for warrants pursuant to ASC 815 “Derivatives and
Hedging — Contracts in Entity’s Own Equity”
(formerly known as EITF
00-19) our
disclosure controls and procedures required improvement in order
to prevent such a recurrence and were thus not effective as of
December 31, 2009. While we have processes to identify and
intelligently apply developments in accounting, we plan to
enhance these processes to better understand nuances to
increasingly complex accounting standards. Our plans include the
following: enhanced access to accounting literature, research
materials and documents; identification of third party
professionals with whom to consult regarding complex accounting
applications; and consideration of additional staff with the
requisite experience and training to supplement our current
accounting professionals.
|
|
|
(ii)
|
Internal
Control Over Financial Reporting
|
|
|
|
(a)
|
Management’s
annual report on internal control over financial
reporting
|
Our management is responsible for establishing and maintaining
adequate internal control over financial reporting, as such term
is defined in Exchange Act
Rules 13a-15(f)
and
15d-15(f).
Our internal control system was designed to provide reasonable
assurance to our management and board of directors regarding the
reliability of financial reporting and the preparation of
financial statements for external purposes in accordance with
generally accepted accounting principles. Due to the small size
of our company and the limited number of employees, it is not
possible for us to fully segregate duties associated with the
financial reporting process; accordingly, we rely on mitigating
controls to reduce the risks from such lack of segregation of
duties. Further, all internal control systems, no matter how
well designed, have inherent limitations. Therefore, even those
systems determined to be effective can provide only reasonable
assurance with respect to financial statement preparation and
presentation. Because of such inherent limitations, internal
control over financial reporting may not prevent or detect
misstatements. Projections of any evaluation of effectiveness to
future periods are subject to the risk that controls may become
inadequate because of changes in conditions, or that the degree
of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of our
management, including our principal executive officer and
principal financial officer, we conducted an evaluation of the
effectiveness of our internal control over financial reporting
based on the framework in Internal Control —
Integrated Framework issued by the Committee of Sponsoring
Organizations of the Treadway Commission, or COSO. The objective
of this assessment was to determine whether our internal control
over financial reporting was effective as of December 31,
2009.
A material weakness is a deficiency, or a combination of
deficiencies, in internal control over financial reporting such
that there is a reasonable possibility that a material
misstatement of our annual or interim consolidated financial
statements will not be prevented or detected on a timely basis.
In our assessment of the effectiveness of internal control over
financial reporting as of December 31, 2009, we identified
a material weakness over the accounting for and disclosure of
derivatives associated with warrant instruments primarily
because we lacked technical expertise and adequate procedures to
develop and document our common stock
73
warrant analysis on the applicability of ASC 815
“Derivatives and Hedging — Contracts in
Entity’s Own Equity” (formerly known as
EITF 00-19)
to our warrant instruments. Because we lacked technical
expertise and adequate procedures to develop and document our
analysis of the applicability of ASC 815, and was
characterized as a material weakness with regard to accounting
for warrants, management has concluded that we did not maintain
effective internal control over financial reporting as of
December 31, 2009 based on the criteria in Internal
Control — Integrated Framework.
Ernst & Young, our independent registered public
accounting firm, audited the effectiveness of our internal
controls over financial reporting and, based on that audit,
issued an adverse opinion on their report as stated below.
|
|
|
(b)
|
Changes
in internal control over financial reporting
|
Based on the matters discussed above, we intend to develop and
implement a remediation plan to address the identified material
weakness as follows: enhanced access to accounting literature,
research materials and documents; identification of third party
professionals with whom to consult regarding complex accounting
applications; and consideration of involving additional staff
with the requisite experience and training to supplement our
current accounting professionals.
Other than with respect to the identification of the material
weakness over the accounting for warrants discussed above, there
have been no changes in our internal control over financial
reporting that occurred during our most recent fiscal quarter
that have materially affected, or are reasonably likely to
materially affect, our internal control over financial reporting.
74
Report of
Ernst & Young LLP, Independent Registered Public
Accounting Firm
The Board of Directors and
Stockholders of Spectrum Pharmaceuticals, Inc.
We have audited Spectrum Pharmaceuticals, Inc. and
Subsidiaries’ internal control over financial reporting as
of December 31, 2009, based on criteria established in
Internal Control — Integrated Framework issued by the
Committee of Sponsoring Organizations of the Treadway Commission
(the COSO criteria). Spectrum Pharmaceuticals, Inc. and
Subsidiaries’ management is responsible for maintaining
effective internal control over financial reporting, and for its
assessment of the effectiveness of internal control over
financial reporting included in the accompanying
Management’s Annual Report on Internal Control over
Financial Reporting. Our responsibility is to express an opinion
on the company’s internal control over financial reporting
based on our audit.
We conducted our audit in accordance with the standards of the
Public Company Accounting Oversight Board (United States). Those
standards require that we plan and perform the audit to obtain
reasonable assurance about whether effective internal control
over financial reporting was maintained in all material
respects. Our audit included obtaining an understanding of
internal control over financial reporting, assessing the risk
that a material weakness exists, testing and evaluating the
design and operating effectiveness of internal control based on
the assessed risk, and performing such other procedures as we
considered necessary in the circumstances. We believe that our
audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a
process designed to provide reasonable assurance regarding the
reliability of financial reporting and the preparation of
financial statements for external purposes in accordance with
generally accepted accounting principles. A company’s
internal control over financial reporting includes those
policies and procedures that (1) pertain to the maintenance
of records that, in reasonable detail, accurately and fairly
reflect the transactions and dispositions of the assets of the
company; (2) provide reasonable assurance that transactions
are recorded as necessary to permit preparation of financial
statements in accordance with generally accepted accounting
principles, and that receipts and expenditures of the company
are being made only in accordance with authorizations of
management and directors of the company; and (3) provide
reasonable assurance regarding prevention or timely detection of
unauthorized acquisition, use, or disposition of the
company’s assets that could have a material effect on the
financial statements.
Because of its inherent limitations, internal control over
financial reporting may not prevent or detect misstatements.
Also, projections of any evaluation of effectiveness to future
periods are subject to the risk that controls may become
inadequate because of changes in conditions, or that the degree
of compliance with the policies or procedures may deteriorate.
A material weakness is a deficiency, or combination of
deficiencies, in internal control over financial reporting, such
that there is a reasonable possibility that a material
misstatement of the company’s annual or interim financial
statements will not be prevented or detected on a timely basis.
The following material weakness has been identified and included
in management’s assessment. Management has identified a
material weakness in controls related to the accounting for, and
disclosure of, warrants to purchase common stock in accordance
with relevant generally accepted accounting principles. We also
have audited, in accordance with the standards of the Public
Company Accounting Oversight Board (United States), the
consolidated balance sheet of Spectrum Pharmaceuticals, Inc. and
Subsidiaries as of December 31, 2009 and the related
consolidated statements of operations, equity and cash flows for
the year ended December 31, 2009. This material weakness
was considered in determining the nature, timing, and extent of
audit tests applied in our audit of the 2009 consolidated
financial statements, and this report does not affect our report
dated April 2, 2010 which expressed an unqualified opinion
on those financial statements.
In our opinion, because of the effect of the material weakness
described above on the achievement of the objectives of the
control criteria, Spectrum Pharmaceuticals, Inc. and
Subsidiaries has not maintained effective internal control over
financial reporting as of December 31, 2009, based on the
COSO criteria.
Orange County, California
April 2, 2010
75
|
|
|
Item 9B.
|
Other
Information
|
|
|
|
Item 4.01. —
|
Changes
in Registrant’s Certifying Accountant.
|
(b)
On December 3, 2009, we engaged Ernst & Young as
the Company’s independent registered public accounting firm
with respect to the Company’s financial statements for the
fiscal year ended December 31, 2009, and discontinued using
Kelly & Co. who served as the independent registered
public accounting firm for the Company from December 23,
2002 to December 3, 2009. During such time,
Kelly & Co. rendered the audit opinions on the
Company’s consolidated financial statements included in the
Company’s annual reports on
Form 10-K
filed with the Securities and Exchange Commission for the fiscal
years ended December 31, 2007 and 2008.
In connection with the restatement of the Company’s
consolidated financial statements discussed below in
Item 4.02 and elsewhere in this document, on March 30,
2010, the Company’s Audit Committee re-engaged
Kelly & Co. to audit the restatement adjustments that
the Company made to its 2007 and 2008 consolidated financial
statements in this
Form 10-K.
With respect to Ernst & Young’s ongoing audit of the
Company’s financial statements for the fiscal year ended
December 31, 2009, the Audit Committee authorized
Kelly & Co. to respond fully to: (i) inquiries
from Ernst & Young regarding the restatement items in
the financial statements for the years ended December 31,
2007 and 2008, and (ii) any other inquiries from
Ernst & Young regarding any of the financial
statements of the Company.
The Company provided Kelly & Co. with a copy of the
foregoing disclosures in this Item 4.01(b) and requested
that Kelly & Co. review such disclosures. In addition,
Kelly & Co. has been given an opportunity to furnish
the Company with a letter addressed to the SEC containing any
new information, clarification of the Company’s expression
of its views, or the extent to which it does not agree with the
statements made by the Company in this Item 4.01(b).
Kelly & Co. informed the Company on April 2, 2010
that it agrees with the disclosure provided in this
Item 4.01(b) and has not furnished such a letter to the
Company or the SEC.
|
|
|
Item 4.02. —
|
Non-Reliance
on Previously Issued Financial Statements or a Related Audit
Report or Completed Interim Review.
|
In connection with warrants issued in registered offerings
during 2005 and 2009, the Company had previously classified the
warrants as equity under its evaluation of applicable guidance
contained in ASC 815 “Derivatives and Hedging —
Contracts in Entity’s Own Equity” (formerly known as
Emerging Issues Task Force Issue
00-19,
“Accounting for Derivative Financial Instruments Indexed
to, and Potentially Settled in, a Company’s Own
Stock”). In connection with the audit for the fiscal year
2009, the Company, in consultation with Ernst & Young,
the Company’s current independent registered public
accounting firm, reassessed the accounting classification of the
warrants under ASC 815 based on certain terms of the warrants.
The warrants provide that in the event the Company is unable to
issue registered shares upon exercise, the warrant holders are
entitled, under securities laws, to receive freely tradable
shares pursuant to a “cashless exercise” provision.
However, based on interpretation of ASC 815, there is a
required presumption of net cash settlement as it is not within
the control of the Company to provide registered shares, no
matter how remote the probability. The Company’s Audit
Committee, together with management, in consultation with the
Company’s outside legal advisors, determined on
March 30, 2010 that, notwithstanding the highly remote
theoretical nature of the possibility of net cash settlement,
the warrants should have originally been recorded as
liabilities, measured at fair value each reporting period, with
changes in the fair values being recognized in the statement of
operations.
76
The restatements reflect the reclassification of the warrants
from equity to a liability in the following amounts, which
represents the fair value of the warrants, as of the issuance
dates, calculated using the Black-Scholes option pricing model.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value
|
|
|
|
|
Number of
|
|
|
|
|
|
|
|
of Warrants
|
|
|
|
|
Warrants
|
|
|
Exercise
|
|
|
Expiration
|
|
at Issuance
|
|
|
Issuance Date
|
|
Issued
|
|
|
Price
|
|
|
of Warrants
|
|
Date
|
|
|
|
|
|
|
|
|
|
|
|
|
(In thousands)
|
|
|
|
|
September 14, 2005
|
|
|
4,000,000
|
|
|
$
|
6.62
|
|
|
September 14, 2011
|
|
$
|
15,472
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
May 27, 2009
|
|
|
1,956,947
|
|
|
$
|
5.11
|
|
|
February 25, 2010
|
|
$
|
2,881
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 15, 2009
|
|
|
857,633
|
|
|
$
|
5.83
|
|
|
March 15, 2010
|
|
$
|
1,847
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30, 2009
|
|
|
1,468,020
|
|
|
$
|
7.10
|
|
|
March 30, 2010
|
|
$
|
4,117
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 18, 2009
|
|
|
2,649,007
|
|
|
$
|
7.55
|
|
|
June 20, 2010
|
|
$
|
5,170
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The revaluation of the warrants at each subsequent balance sheet
date to fair value, results in a change in the carrying value of
the liability, which change is recorded as “Change in fair
value of common stock warrant liability” in the
consolidated statement of operations. The net effect of these
changes for fiscal years ended December 31, 2008 and 2007,
and for each of the quarterly condensed consolidated financial
statements on
Form 10-Q
for the periods ended March 31, 2008 through
September 30, 2009 are as follows:
| |
|
|
|
|
|
|
|
Income (Loss) Resulting
|
|
|
|
|
from Change in Fair
|
|
|
|
|
Value of Common
|
|
|
Reporting Period
|
|
Stock Warrant Liability
|
|
|
|
|
(In thousands)
|
|
|
|
|
Annual
|
|
|
|
|
|
Year ended December 31, 2007
|
|
$
|
12,055
|
|
|
|
|
|
|
|
|
Year ended December 31, 2008
|
|
$
|
1,271
|
|
|
|
|
|
|
|
|
Interim (unaudited)
|
|
|
|
|
|
Quarter ended March 31, 2008
|
|
$
|
520
|
|
|
|
|
|
|
|
|
Quarter ended June 30, 2008
|
|
$
|
916
|
|
|
|
|
|
|
|
|
Quarter ended September 30, 2008
|
|
|
45
|
|
|
|
|
|
|
|
|
Quarter ended December 31, 2008
|
|
$
|
(210
|
)
|
|
|
|
|
|
|
|
Quarter ended March 31, 2009
|
|
$
|
(509
|
)
|
|
|
|
|
|
|
|
Quarter ended June 30, 2009
|
|
$
|
(20,113
|
)
|
|
|
|
|
|
|
|
Quarter ended September 30, 2009
|
|
$
|
8,863
|
|
|
|
|
|
|
|
We have not amended our previously field Annual Reports on Form
10-K for the fiscal years ended December 31, 2005, 2006,
2007 and 2008, or the Quarterly Reports on Form 10-Q for the
periods ended September 30, 2005 through September 30,
2009 to reflect the restatements described in this Annual Report
on Form 10-K, and thus the financial statements and related
financial statement information contained in those reports
should no longer be relied upon.
The Audit Committee and management have discussed these matters
with Ernst & Young, the Company’s independent
registered public accounting firm.
77
PART III
|
|
|
Item 10.
|
Directors,
Executive Officers and Corporate Governance
|
The information required under this item is incorporated by
reference from our definitive proxy statement related to our
2010 Annual Meeting of Stockholders, or the Proxy Statement, to
be filed pursuant to Regulation 14A, on or before
April 30, 2010.
|
|
|
Item 11.
|
Executive
Compensation
|
The information required under this item is incorporated herein
by reference from the Proxy Statement.
|
|
|
Item 12.
|
Security
Ownership of Certain Beneficial Owners and Management and
Related Stockholder Matters
|
The information required under this item is incorporated herein
by reference from the Proxy Statement.
|
|
|
Item 13.
|
Certain
Relationships and Related Transactions, and Director
Independence
|
The information required under this item is incorporated herein
by reference from the Proxy Statement.
|
|
|
Item 14.
|
Principal
Accountant Fees and Services
|
The information required under this item is incorporated herein
by reference from the Proxy Statement.
PART IV
|
|
|
Item 15.
|
Exhibits
and Financial Statement Schedules
|
(a)(1) Consolidated Financial Statements:
| |
|
|
|
|
|
Page
|
|
|
|
Reports of Independent Registered Public Accounting Firms
|
|
F-2
|
|
Consolidated Balance Sheets as of December 31, 2009 and
2008 (as restated)
|
|
F-4
|
|
Consolidated Statements of Operations for each of the years in
the periods ended December 31, 2009, 2008 (as restated) and
2007 (as restated)
|
|
F-5
|
|
Consolidated Statements of Equity for each of the years in the
periods ended December 31, 2009, 2008 (as restated) and
2007 (as restated)
|
|
F-6
|
|
Consolidated Statements of Cash Flow for each of the years in
the periods ended December 31, 2009, 2008 (as restated) and
2007 (as restated)
|
|
F-7
|
|
Notes to Consolidated Financial Statements
|
|
F-8
|
(a)(2) Financial Statement Schedules: All
financial statement schedules are omitted because they are not
applicable or the required information is included in the
Consolidated Financial Statements or notes thereto.
(a)(3) Exhibits.
78
Index to
Exhibits
| |
|
|
|
|
Exhibit
|
|
|
|
No.
|
|
Description
|
|
|
|
|
2
|
.1
|
|
Asset Purchase Agreement by and between the Registrant, Targent
Inc. and Certain Stockholders of Targent, Inc., dated March 17
2006. (Filed as Exhibit 2.1 to
Form 10-K/A,
Amendment No. 1, as filed with the Securities and Exchange
Commission on May 1, 2006, and incorporated herein by
reference.)
|
|
|
2
|
.2
|
|
Asset Purchase Agreement by and between the Registrant and Par
Pharmaceutical, Inc., dated as of May 6, 2008. (Filed as
Exhibit 2.1 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 11, 2008, and incorporated herein by reference.)
|
|
|
2
|
.3#
|
|
Purchase and Formation Agreement, dated as of November 26,
2008, by and among the Registrant, Cell Therapeutics, Inc. and
RIT Oncology, LLC. (Filed as Exhibit 2.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
December 19, 2008, and incorporated herein by reference.)
|
|
|
2
|
.4#
|
|
Limited Liability Company Interest Assignment Agreement, dated
as of March 15, 2009, by and between the Registrant and
Cell Therapeutics, Inc. (Filed as Exhibit 2.1 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
May 15, 2009, and incorporated herein by reference.)
|
|
|
3
|
.1
|
|
Amended Certificate of Incorporation, as filed. (Filed as
Exhibit 3.1 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 8, 2006, and incorporated herein by reference.)
|
|
|
3
|
.2
|
|
Form of Amended and Restated Bylaws of the Registrant. (Filed as
Exhibit 3.1 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 16, 2004, and incorporated herein by reference.)
|
|
|
4
|
.1
|
|
Rights Agreement, dated as of December 13, 2000, between
the Registrant and ComputerShare Trust Company, N.A.
(formerly U.S. Stock Transfer Corporation), as Rights Agent,
which includes as Exhibit A thereto the form of Certificate
of Designation for the Series B Junior Participating
Preferred Stock, as Exhibit B thereto the Form of Rights
Certificate and as Exhibit C thereto a Summary of Terms of
Stockholder Rights Plan. (Filed as Exhibit 4.1 to
Form 8-A12G,
as filed with the Securities and Exchange Commission on
December 26, 2000, and incorporated herein by reference.)
|
|
|
4
|
.2
|
|
Amendment No. 1 to the Rights Agreement, dated as of
December 13, 2000 by and between the Registrant and
ComputerShare Trust Company, N.A. (formerly U.S. Stock
Transfer Corporation). (Filed as Exhibit 4.1 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 14, 2003, and incorporated herein by reference.)
|
|
|
4
|
.3
|
|
Registration Rights Agreement, dated as of September 26,
2003, by and among the Registrant and the persons listed on
Schedule 1 attached thereto. (Filed as Exhibit 4.4 to
Form 8-K,
as filed with the Securities and Exchange Commission on
September 30, 2003, and incorporated herein by reference.)
|
|
|
4
|
.4
|
|
Investor Rights Agreement, dated as of April 20, 2004, by
and among the Registrant and the persons listed on
Schedule 1 attached thereto. (Filed as Exhibit 4.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
April 23, 2004, and incorporated herein by reference.)
|
|
|
4
|
.5
|
|
Amendment No. 2 to the Rights Agreement, dated as of
December 13, 2000, by and between the Registrant and
ComputerShare Trust Company, N.A. (formerly U.S. Stock
Transfer Corporation). (Filed as Exhibit 4.1 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
May 17, 2004, and incorporated herein by reference.)
|
|
|
4
|
.6
|
|
Amendment No. 3 to the Rights Agreement, dated as of
December 13, 2000 by and between the Registrant and
ComputerShare Trust Company, N.A. (formerly U.S. Stock
Transfer Corporation). (Filed as Exhibit 4.2 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
May 17, 2004, and incorporated herein by reference.)
|
|
|
4
|
.7
|
|
Warrant issued by the Registrant to a Consultant, dated as of
September 17, 2003. (Filed as Exhibit 4.3 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
May 17, 2004, and incorporated herein by reference.)
|
|
|
4
|
.8
|
|
Amendment No. 1, dated as of November 2, 2005, to
Warrant issued by the Registrant to a Consultant, dated as of
September 17, 2003. (Filed as Exhibit 4.2 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
November 4, 2005, and incorporated herein by reference.)
|
|
|
4
|
.9
|
|
Warrant issued by the Registrant to a Consultant, dated as of
September 20, 2005. (Filed as Exhibit 4.3 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
November 4, 2005, and incorporated herein by reference.)
|
79
| |
|
|
|
|
Exhibit
|
|
|
|
No.
|
|
Description
|
|
|
|
|
4
|
.10
|
|
Form of Warrant, dated September 15, 2005. (Filed as
Exhibit 4.35 to
Form 10-K,
as filed with the Securities and Exchange Commission on
March 15, 2006, and incorporated herein by reference.)
|
|
|
4
|
.11
|
|
Registration Rights Agreement, dated as of April 20, 2006,
by and among the Registrant and Targent, Inc. (Filed as
Exhibit 4.2 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
May 8, 2006, and incorporated herein by reference.)
|
|
|
4
|
.12
|
|
Fourth Amendment to Rights Agreement, dated July 7, 2006.
(Filed as Exhibit 4.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
July 12, 2006, and incorporated herein by reference.)
|
|
|
4
|
.13
|
|
Amendment No. 5 to the Rights Agreement, dated as of
December 13, 2000, by and between the Registrant and
ComputerShare Trust Company, N.A. (formerly U.S. Stock
Transfer Corporation). (Filed as Exhibit 4.2 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
November 3, 2006, and incorporated herein by reference.)
|
|
|
4
|
.14
|
|
Amendment No. 2, dated as of March 26, 2007, to
Warrant issued by the Registrant to a Consultant, dated as of
September 17, 2003. (Filed as Exhibit 4.1 to
Form 10-K/A,
as filed with the Securities and Exchange Commission on
April 30, 2007, and incorporated herein by reference.)
|
|
|
4
|
.15
|
|
Warrant issued by the Registrant to a Consultant, dated as of
April 28, 2008. (Filed as Exhibit 4.1 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 11, 2008, and incorporated herein by reference.)
|
|
|
4
|
.16
|
|
Form of Common Stock Purchase Warrant. (Filed as
Exhibit 4.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
May 28, 2009, and incorporated herein by reference.)
|
|
|
4
|
.17
|
|
Form of Common Stock Purchase Warrant. (Filed as
Exhibit 4.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
June 18, 2009, and incorporated herein by reference.)
|
|
|
4
|
.18
|
|
Form of Common Stock Purchase Warrant. (Filed as
Exhibit 4.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
July 2, 2009, and incorporated herein by reference.)
|
|
|
4
|
.19
|
|
Form of Common Stock Purchase Warrant (Filed as Exhibit 4.1
to
Form 8-K,
as filed with the Securities and Exchange Commission on
September 23, 2009, and incorporated herein by reference).
|
|
|
10
|
.1
|
|
Industrial Lease Agreement, dated as of January 16, 1997,
between the Registrant and the Irvine Company. (Filed as
Exhibit 10.11 to
Form 10-KSB,
as filed with the Securities and Exchange Commission on
March 31, 1997, and incorporated herein by reference.)
|
|
|
10
|
.2
|
|
Preferred Stock and Warrant Purchase Agreement, dated as of
September 26, 2003, by and among the Registrant and the
purchasers listed on Schedule 1 attached thereto. (Filed as
Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
September 30, 2003, and incorporated herein by reference.)
|
|
|
10
|
.3
|
|
First Amendment, dated March 25, 2004, to Industrial Lease
Agreement dated as of January 16, 1997 by and between the
Registrant and the Irvine Company. (Filed as Exhibit 10.1
to
Form 10-Q,
as filed with the Securities and Exchange Commission on
May 17, 2004, and incorporated herein by reference.)
|
|
|
10
|
.4
|
|
Common Stock and Warrant Purchase Agreement, dated as of
April 20, 2004, by and among the Registrant and the
purchasers listed on Schedule 1 attached thereto. (Filed as
Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
April 23, 2004, and incorporated herein by reference.)
|
|
|
10
|
.5#
|
|
License and Collaboration Agreement by and between the
Registrant and Zentaris GmbH, dated as of August 12, 2004.
(Filed as Exhibit 10.1 to
Form S-3/A,
as filed with the Securities and Exchange Commission on
January 21, 2005, and incorporated herein by reference.)
|
|
|
10
|
.6*
|
|
Form of Stock Option Agreement under the 2003 Amended and
Restated Incentive Award Plan. (Filed as Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
December 17, 2004, and incorporated herein by reference.)
|
|
|
10
|
.7#
|
|
License Agreement by and between the Registrant and Chicago
Labs, Inc. (Filed as Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
February 25, 2005, and incorporated herein by reference.)
|
|
|
10
|
.8*
|
|
Form of Non-Employee Director Stock Option Agreement under the
2003 Amended and Restated Incentive Award Plan. (Filed as
Exhibit 10.5 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
May 10, 2005, and incorporated herein by reference.)
|
80
| |
|
|
|
|
Exhibit
|
|
|
|
No.
|
|
Description
|
|
|
|
|
10
|
.9*
|
|
Restricted Stock Award Grant Notice and Restricted Stock Award
Agreement under the 2003 Amended and Restated Incentive Award
Plan. (Filed as Exhibit 10.44 to
Form 10-K,
as filed with the Securities and Exchange Commission on
March 15, 2006, and incorporated herein by reference.)
|
|
|
10
|
.10#
|
|
License Agreement between the Registrant and Merck Eprova AG,
dated May 23, 2006. (Filed as Exhibit 10.1 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 8, 2006, and incorporated herein by reference.)
|
|
|
10
|
.11*
|
|
Third Amended and Restated 1997 Stock Incentive Plan. (Filed as
Exhibit 10.2 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
November 3, 2006, and incorporated herein by reference.)
|
|
|
10
|
.12#
|
|
Agreement by and between the Registrant and Glaxo Group Limited
(d/b/a GlaxoSmithKline), dated November 10, 2006. (Filed as
Exhibit 10.38 to
Form 10-K,
as filed with the Securities and Exchange Commission on
March 14, 2007, and incorporated herein by reference.)
|
|
|
10
|
.13*
|
|
2003 Amended and Restated Incentive Award Plan. (Filed as
Exhibit 10.3 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 9, 2007, and incorporated herein by reference.)
|
|
|
10
|
.14#
|
|
License Agreement by and between the Registrant and Indena,
S.p.A., dated July 17, 2007. (Filed as Exhibit 10.4 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
November 9, 2007, and incorporated herein by reference.)
|
|
|
10
|
.15*
|
|
Executive Employment Agreement by and between the Registrant and
Rajesh C. Shrotriya, M.D., entered into June 20, 2008
and effective as of January 2, 2008. (Filed as
Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
June 26, 2008, and incorporated herein by reference.)
|
|
|
10
|
.16
|
|
Consulting Agreement by and between the Registrant and Luigi
Lenaz, M.D., effective as of July 1, 2008. (Filed as
Exhibit 10.2 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 11, 2008, and incorporated herein by reference.)
|
|
|
10
|
.17*
|
|
Form of Indemnity Agreement of the Registrant. (Filed as
Exhibit 10.32 to
Form 10-K,
as filed with the Securities and Exchange Commission on
March 31, 2009, and incorporated herein by reference.)
|
|
|
10
|
.18#
|
|
License, Development, Supply and Distribution Agreement, dated
October 28, 2008, by and among the Registrant, Allergan
Sales, LLC, Allergan USA, Inc. and Allergan, Inc. (Filed as
Exhibit 10.33 to
Form 10-K,
as filed with the Securities and Exchange Commission on
March 31, 2009, and incorporated herein by reference.)
|
|
|
10
|
.19*
|
|
Form of Stock Purchase Agreement, dated May 6, 2009. (Filed
as Exhibit 1.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
May 7, 2009, and incorporated herein by reference.)
|
|
|
10
|
.20
|
|
Form of Securities Purchase Agreement, dated May 27, 2009.
(Filed as Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
May 28, 2009, and incorporated herein by reference.)
|
|
|
10
|
.21
|
|
Placement Agency Agreement between the Registrant and
Rodman & Renshaw, LLC, dated May 26, 2009. (Filed
as Exhibit 1.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
May 28, 2009, and incorporated herein by reference.)
|
|
|
10
|
.22
|
|
Form of Securities Purchase Agreement, dated June 15, 2009.
(Filed as Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
June 18, 2009, and incorporated herein by reference.)
|
|
|
10
|
.23
|
|
Placement Agency Agreement between the Registrant and
Rodman & Renshaw, LLC, June 15, 2009. (Filed as
Exhibit 1.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
June 18, 2009, and incorporated herein by reference.)
|
|
|
10
|
.24*
|
|
2009 Employee Stock Purchase Plan. (Filed as Exhibit 99.1
to
Form S-8,
as filed with the Securities and Exchange Commission on
June 29, 2009, and incorporated herein by reference.)
|
|
|
10
|
.25*
|
|
2009 Incentive Award Plan. (Filed as Exhibit 99.2 to
Form S-8,
as filed with the Securities and Exchange Commission on
June 29, 2009, and incorporated herein by reference.)
|
|
|
10
|
.26
|
|
Form of Securities Purchase Agreement, dated June 30, 2009.
(Filed as Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
July 2, 2009, and incorporated herein by reference.)
|
|
|
10
|
.27
|
|
Placement Agency Agreement between the Registrant and
Rodman & Renshaw, LLC, dated June 30, 2009.
(Filed as Exhibit 1.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
July 2, 2009, and incorporated herein by reference.)
|
81
| |
|
|
|
|
Exhibit
|
|
|
|
No.
|
|
Description
|
|
|
|
|
10
|
.28*
|
|
2003 Amended and Restated Incentive Award Plan. (Filed as
Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
July 2, 2009, and incorporated herein by reference.)
|
|
|
10
|
.29+
|
|
Fourth Amendment, dated July 29, 2009, to Industrial Lease
Agreement dated as of January 16, 1997 by and between the
Registrant and the Irvine Company.
|
|
|
10
|
.30*
|
|
Term Sheet for 2009 Incentive Award Plan Stock Option Award.
(Filed as Exhibit 10.8 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 13, 2009, and incorporated herein by reference.)
|
|
|
10
|
.31*
|
|
Term Sheet for 2009 Incentive Award Plan, Nonqualified Stock
Option Award Awarded to Non-Employee Directors. (Filed as
Exhibit 10.9 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 13, 2009, and incorporated herein by reference.)
|
|
|
10
|
.32*
|
|
Term Sheet for 2009 Incentive Award Plan, Restricted Stock
Award. (Filed as Exhibit 10.10 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 13, 2009, and incorporated herein by reference.)
|
|
|
10
|
.33*
|
|
Summary of Director Compensation. (Filed as Exhibit 10.11
to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 13, 2009, and incorporated herein by reference.)
|
|
|
10
|
.34
|
|
Placement Agency Agreement by and between the Registrant, and
Rodman & Renshaw, LLC, dated September 18, 2009
(Filed as Exhibit 1.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
September 23, 2009, and incorporated herein by reference.)
|
|
|
10
|
.35
|
|
Form of Securities Purchase Agreement, dated September 18,
2009 (Filed as Exhibit 1.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
September 23, 2009, and incorporated herein by reference.)
|
|
|
10
|
.36#+
|
|
License Agreement, dated November 6, 2009, by and between
the Registrant and Nippon Kayaku Co., Ltd.
|
|
|
10
|
.37#+
|
|
License and Collaboration Agreement, dated February 2,
2010, by and between the Registrant and TopoTarget A/S.
|
|
|
16
|
.1
|
|
Letter from Kelly and Company to the Securities and Exchange
Commission, dated December 3, 2009. (Filed as
Exhibit 16.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
December 8, 2009, and incorporated herein by reference.)
|
|
|
21
|
+
|
|
Subsidiaries of Registrant.
|
|
|
23
|
.1+
|
|
Consent of Ernst & Young LLP, Independent Registered
Public Accounting Firm.
|
|
|
23
|
.2+
|
|
Consent of Kelly & Company, Independent Registered
Public Accounting Firm.
|
|
|
24
|
.1
|
|
Power of Attorney (included in the signature page.)
|
|
|
31
|
.1+
|
|
Certification of Chief Executive Officer, pursuant to
Rule 13a-14(a)/15d-14(a)
of the Securities Exchange Act of 1934.
|
|
|
31
|
.2+
|
|
Certification of Vice President Finance, pursuant to
Rule 13a-14(a)/15d-14(a)
of the Securities Exchange Act of 1934.
|
|
|
32
|
.1+
|
|
Certification of Chief Executive Officer, pursuant to
Rule 13a-14(b)/15d-14(b)
of the Securities Exchange Act of 1934 and 18 U.S.C.
Section 1350.
|
|
|
32
|
.2+
|
|
Certification of Vice President Finance, pursuant to
Rule 13a-14(b)/15d-14(b)
of the Securities Exchange Act of 1934 and 18 U.S.C.
Section 1350.
|
|
|
|
|
* |
|
Indicates a management contract or compensatory plan or
arrangement. |
| |
|
# |
|
Confidential portions omitted and filed separately with the U.S.
Securities and Exchange Commission pursuant to
Rule 24b-2
promulgated under the Securities Exchange Act of 1934, as
amended. |
| |
|
+ |
|
Filed herewith. |
82
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the
Securities Exchange Act of 1934, the registrant has duly caused
this Annual Report on
Form 10-K
to be signed on its behalf by the undersigned, thereunto duly
authorized.
Spectrum Pharmaceuticals, Inc.
|
|
|
| |
By:
|
/s/ Rajesh
C. Shrotriya, M.D.
|
Rajesh C. Shrotriya, M.D.
Chief Executive Officer and President
Date: April 2, 2010
POWER OF
ATTORNEY
Each person whose signature appears below constitutes and
appoints each of Rajesh C. Shrotriya and Shyam K. Kumaria as his
attorney-in-fact, with full power of substitution, for him in
any and all capacities, to sign any amendments to this
Form 10-K,
and to file the same, with all exhibits thereto and other
documents in connection therewith, with the Securities and
Exchange Commission, hereby ratifying and confirming all that
each attorney-in-fact, or his substitute, may do or cause to be
done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of
1934, this Annual Report on
Form 10-K
has been signed below by the following persons on behalf of the
Registrant and in the capacities and on the dates indicated:
| |
|
|
|
|
|
|
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
|
|
/s/ Rajesh
C. Shrotriya, M.D.
Rajesh
C. Shrotriya, M.D.
|
|
Chairman of the Board, Chief Executive Officer, and President
(Principal Executive Officer)
|
|
April 2, 2010
|
|
|
|
|
|
|
|
/s/ Shyam
K. Kumaria
Shyam
K. Kumaria
|
|
Vice President Finance
(Principal Financial and Accounting Officer)
|
|
April 2, 2010
|
|
|
|
|
|
|
|
/s/ Mitchell
P. Cybulski
Mitchell
P. Cybulski
|
|
Director
|
|
April 2, 2010
|
|
|
|
|
|
|
|
/s/ Richard
D. Fulmer
Richard
D. Fulmer
|
|
Director
|
|
April 2, 2010
|
|
|
|
|
|
|
|
/s/ Stuart
M. Krassner, Sc.D., Psy.D.
Stuart
M. Krassner, Sc.D., Psy.D.
|
|
Director
|
|
April 2, 2010
|
|
|
|
|
|
|
|
/s/ Anthony
E. Maida, III
Anthony
E. Maida, III
|
|
Director
|
|
April 2, 2010
|
|
|
|
|
|
|
|
/s/ Julius
A. Vida, Ph.D.
Julius
A. Vida, Ph.D.
|
|
Director
|
|
April 2, 2010
|
83
Spectrum Pharmaceuticals, Inc. and Subsidiaries
Consolidated Financial Statements
As of December 31, 2009 and 2008 and
For Each of the Three Years in the Period Ended
December 31, 2009
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Consolidated
Financial Statements
INDEX TO
FINANCIAL STATEMENTS
| |
|
|
|
|
|
|
|
Page
|
|
|
|
|
|
|
F-2
|
|
|
|
|
|
F-4
|
|
|
|
|
|
F-5
|
|
|
|
|
|
F-6
|
|
|
|
|
|
F-7
|
|
|
|
|
|
F-8
|
|
F-1
Report of
Ernst & Young LLP, Independent Registered Public
Accounting Firm
The Board of Directors and
Stockholders of Spectrum Pharmaceuticals, Inc.
We have audited the accompanying consolidated balance sheet of
Spectrum Pharmaceuticals, Inc. and Subsidiaries as of
December 31, 2009, and the related consolidated statements
of operations, equity and cash flows for the year then ended.
These financial statements are the responsibility of Spectrum
Pharmaceuticals, Inc. and Subsidiaries’ management. Our
responsibility is to express an opinion on these financial
statements based on our audit.
We conducted our audit in accordance with the standards of the
Public Company Accounting Oversight Board (United States). Those
standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are
free of material misstatement. An audit includes examining, on a
test basis, evidence supporting the amounts and disclosures in
the financial statements. An audit also includes assessing the
accounting principles used and significant estimates made by
management, as well as evaluating the overall financial
statement presentation. We believe that our audit provides a
reasonable basis for our opinion.
In our opinion, the 2009 consolidated financial statements
referred to above present fairly, in all material respects, the
consolidated financial position of Spectrum Pharmaceuticals,
Inc. and Subsidiaries at December 31, 2009, and the
consolidated results of its operations and its cash flows for
the year then ended, in conformity with U.S. generally
accepted accounting principles.
As discussed in Note 1 to the consolidated financial
statements, the Company changed its method of accounting and
financial reporting for noncontrolling ownership interests in
subsidiaries held by parties other than the parent with the
adoption of FASB Accounting Standards Codification (ASC) Topic
810, Consolidation, effective January 1, 2009, and
retroactively adjusted all periods presented in the consolidated
financial statements for this change.
We also have audited, in accordance with the standards of the
Public Company Accounting Oversight Board (United States), the
Company’s internal control over financial reporting as of
December 31, 2009, based on criteria established in
Internal Control-Integrated Framework issued by the Committee of
Sponsoring Organizations of the Treadway Commission and our
report dated April 2, 2010 expressed an adverse opinion
thereon.
Orange County, California
April 2, 2010
F-2
REPORT OF
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders Of
Spectrum Pharmaceuticals, Inc.
We have audited the accompanying consolidated balance sheet of
Spectrum Pharmaceuticals, Inc., as of December 31, 2008 and
the related consolidated statements of operations,
stockholders’ equity, comprehensive loss and cash flows for
each of the two years in the period ended December 31,
2008. These consolidated financial statements are the
responsibility of the Company’s management. Our
responsibility is to express an opinion on these consolidated
financial statements based on our audits.
We conducted our audits in accordance with the standards of the
Public Company Accounting Oversight Board (United States). Those
standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are
free of material misstatement. An audit also includes examining,
on a test basis, evidence supporting the amounts and disclosures
in the financial statements, assessing the accounting principles
used and significant estimates made by management, and
evaluating the overall financial statement presentation. We
believe that our audits provide a reasonable basis for our
opinion.
In our opinion, the consolidated financial statements referred
to above present fairly, in all material respects, the
consolidated financial position of Spectrum Pharmaceuticals,
Inc. as at December 31, 2008 and the consolidated results
of its operations and its cash flows for each of the two years
in the period ended December 31, 2008 in conformity with
United States generally accepted accounting principles.
As discussed in Note 2, the consolidated balance sheet as
of December 31, 2008 and the related consolidated
statements of operations, stockholders’ equity,
comprehensive loss and cash flows for each of the two years in
the period ended December 31, 2008 have been restated to
record certain common stock warrants originally issued in 2005
as liabilities rather than as equity.
Kelly & Company
Costa Mesa, California
March 31, 2009 except for Note 2,
which is as of April 2, 2010
F-3
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Consolidated Balance Sheets
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
|
|
|
(As Restated)
|
|
|
|
|
(In thousands, except par value and share data)
|
|
|
|
|
ASSETS
|
|
Current Assets:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
82,336
|
|
|
$
|
9,860
|
|
|
Marketable securities
|
|
|
31,005
|
|
|
|
66,078
|
|
|
Accounts receivable, net
|
|
|
8,658
|
|
|
|
9,776
|
|
|
Inventories
|
|
|
3,230
|
|
|
|
1,841
|
|
|
Prepaid expenses and other current assets
|
|
|
1,028
|
|
|
|
693
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
126,257
|
|
|
|
88,248
|
|
|
Bank certificates of deposit & treasuries
|
|
|
11,438
|
|
|
|
2,148
|
|
|
Property and equipment, net
|
|
|
1,928
|
|
|
|
1,782
|
|
|
Zevalin related intangible assets, net
|
|
|
33,325
|
|
|
|
37,042
|
|
|
Other assets
|
|
|
185
|
|
|
|
289
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
173,133
|
|
|
$
|
129,509
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|
Current Liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable and other accrued obligations
|
|
$
|
16,606
|
|
|
$
|
10,401
|
|
|
Accrued compensation
|
|
|
3,360
|
|
|
|
2,956
|
|
|
Note payable in connection with Zevalin acquisition
|
|
|
—
|
|
|
|
7,500
|
|
|
Current portion of deferred revenue
|
|
|
8,300
|
|
|
|
8,500
|
|
|
Common stock warrant liability
|
|
|
6,635
|
|
|
|
765
|
|
|
Accrued drug development costs
|
|
|
4,598
|
|
|
|
3,449
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
39,499
|
|
|
|
33,571
|
|
|
Capital lease obligations, net of current portion
|
|
|
69
|
|
|
|
95
|
|
|
Deferred revenue and other credits, net of current portion
|
|
|
24,943
|
|
|
|
33,929
|
|
|
Zevalin related contingent obligations
|
|
|
298
|
|
|
|
8,798
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
64,809
|
|
|
|
76,393
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies
|
|
|
|
|
|
|
|
|
|
Equity:
|
|
|
|
|
|
|
|
|
|
Spectrum Pharmaceuticals, Inc. stockholders’ equity:
|
|
|
|
|
|
|
|
|
|
Preferred Stock, par value $0.001 per share,
5,000,000 shares authorized:
|
|
|
|
|
|
|
|
|
|
Series B Junior participating preferred stock,
1,000,000 shares authorized, no shares issued and
outstanding
|
|
|
—
|
|
|
|
—
|
|
|
Series E Convertible voting preferred stock,
2,000 shares authorized, stated value $10,000 per share,
$0.8 million aggregate liquidation value, issued and
outstanding, 68 shares at December 31, 2009 and 2008
|
|
|
419
|
|
|
|
419
|
|
|
Common stock, par value $0.001 per share,
100,000,000 shares authorized;
|
|
|
|
|
|
|
|
|
|
Issued and outstanding, 48,926,314 and 32,166,316 shares at
December 31, 2009 and December 31, 2008
|
|
|
49
|
|
|
|
32
|
|
|
Additional paid-in capital
|
|
|
369,482
|
|
|
|
281,059
|
|
|
Accumulated other comprehensive loss
|
|
|
(70
|
)
|
|
|
(146
|
)
|
|
Accumulated deficit
|
|
|
(261,556
|
)
|
|
|
(242,510
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
108,324
|
|
|
|
38,854
|
|
|
Non-controlling interest
|
|
|
—
|
|
|
|
14,262
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
108,324
|
|
|
|
53,116
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and equity
|
|
$
|
173,133
|
|
|
$
|
129,509
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying Notes to Consolidated Financial Statements are
an integral part of these statements.
F-4
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Consolidated Statements of Operations
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
|
|
|
(As Restated)
|
|
|
(As Restated)
|
|
|
|
|
(In thousands, except share and share data)
|
|
|
|
|
Revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product net sales
|
|
$
|
28,225
|
|
|
$
|
8,049
|
|
|
|
—
|
|
|
License and contract revenue
|
|
|
9,800
|
|
|
|
20,676
|
|
|
$
|
7,672
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
$
|
38,025
|
|
|
$
|
28,725
|
|
|
$
|
7,672
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of product sales (excludes amortization of purchased
intangibles shown below)
|
|
|
8,148
|
|
|
|
1,193
|
|
|
|
—
|
|
|
Selling, general and administrative
|
|
|
33,607
|
|
|
|
15,156
|
|
|
|
11,577
|
|
|
Research and development
|
|
|
21,058
|
|
|
|
26,683
|
|
|
|
33,285
|
|
|
Amortization of purchased intangibles
|
|
|
3,720
|
|
|
|
158
|
|
|
|
—
|
|
|
Acquired in-process research and development
|
|
|
—
|
|
|
|
4,700
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
66,533
|
|
|
|
47,890
|
|
|
|
44,862
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(28,508
|
)
|
|
|
(19,165
|
)
|
|
|
(37,190
|
)
|
|
Change in fair value of common stock warrant liability
|
|
|
8,075
|
|
|
|
1,271
|
|
|
|
12,055
|
|
|
Other income, net
|
|
|
662
|
|
|
|
1,165
|
|
|
|
3,139
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pre-tax net loss
|
|
|
(19,771
|
)
|
|
|
(16,729
|
)
|
|
|
(21,996
|
)
|
|
Income tax expense
|
|
|
(421
|
)
|
|
|
(5
|
)
|
|
|
(5
|
)
|
|
Net loss attributable to non-controlling interest
|
|
|
1,146
|
|
|
|
2,538
|
|
|
|
20
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss — attributable to Spectrum
Pharmaceuticals, Inc. stockholders
|
|
$
|
(19,046
|
)
|
|
$
|
(14,196
|
)
|
|
$
|
(21,981
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per share — attributable to
Spectrum Pharmaceuticals, Inc. stockholders
|
|
$
|
(0.48
|
)
|
|
$
|
(0.45
|
)
|
|
$
|
(0.76
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted weighted average common shares outstanding
|
|
|
39,273,905
|
|
|
|
31,551,152
|
|
|
|
29,013,850
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying Notes to Consolidated Financial Statements are
an integral part of these statements.
F-5
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Consolidated Statement of Equity
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other
|
|
|
|
|
|
Total
|
|
|
Non-
|
|
|
|
|
|
|
|
Preferred Stock
|
|
|
Common Stock
|
|
|
Additional
|
|
|
Comprehensive
|
|
|
Accumulated
|
|
|
Stockholders
|
|
|
Controlling
|
|
|
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
Shares
|
|
|
Amount
|
|
|
Paid-In Capital
|
|
|
Income (Loss)
|
|
|
Deficit
|
|
|
Equity
|
|
|
Interest
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(As Restated)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(In thousands, except share data)
|
|
|
|
|
Balance at December 2006 (restated)
|
|
|
219
|
|
|
$
|
1,281
|
|
|
|
25,217,793
|
|
|
$
|
25
|
|
|
$
|
236,408
|
|
|
$
|
357
|
|
|
$
|
(206,332
|
)
|
|
|
31,739
|
|
|
$
|
20
|
|
|
$
|
31,759
|
|
|
Net Loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(21,981
|
)
|
|
|
(21,981
|
)
|
|
|
(20
|
)
|
|
|
(22,001
|
)
|
|
Unrealized gain on investments
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
136
|
|
|
|
—
|
|
|
|
136
|
|
|
|
—
|
|
|
|
136
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive gain (loss), net
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
136
|
|
|
|
(21,981
|
)
|
|
|
(21,845
|
)
|
|
|
(20
|
)
|
|
|
(21,865
|
)
|
|
Conversion of Series D Preferred Stock into Common Stock
|
|
|
(49
|
)
|
|
|
(233
|
)
|
|
|
207,957
|
|
|
|
1
|
|
|
|
232
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Issuance of common stock and warrants for cash, net of issuance
costs
|
|
|
—
|
|
|
|
—
|
|
|
|
5,134,100
|
|
|
|
5
|
|
|
|
30,004
|
|
|
|
—
|
|
|
|
—
|
|
|
|
30,009
|
|
|
|
—
|
|
|
|
30,009
|
|
|
Fair value of common stock issued to Targent, Inc. for milestones
|
|
|
—
|
|
|
|
—
|
|
|
|
125,000
|
|
|
|
—
|
|
|
|
520
|
|
|
|
—
|
|
|
|
—
|
|
|
|
520
|
|
|
|
—
|
|
|
|
520
|
|
|
Share-based compensation expense and common stock issued (net of
forfeitures)
|
|
|
—
|
|
|
|
—
|
|
|
|
235,313
|
|
|
|
—
|
|
|
|
5,278
|
|
|
|
—
|
|
|
|
—
|
|
|
|
5,278
|
|
|
|
—
|
|
|
|
5,278
|
|
|
Issuance of common stock upon exercise of warrants
|
|
|
—
|
|
|
|
—
|
|
|
|
161,145
|
|
|
|
—
|
|
|
|
519
|
|
|
|
—
|
|
|
|
—
|
|
|
|
519
|
|
|
|
—
|
|
|
|
519
|
|
|
Issuance of common stock upon exercise of employee stock options
|
|
|
—
|
|
|
|
—
|
|
|
|
81,438
|
|
|
|
—
|
|
|
|
120
|
|
|
|
—
|
|
|
|
—
|
|
|
|
120
|
|
|
|
—
|
|
|
|
120
|
|
|
Issuance of common stock to 401(k) plan
|
|
|
—
|
|
|
|
—
|
|
|
|
44,118
|
|
|
|
—
|
|
|
|
211
|
|
|
|
—
|
|
|
|
—
|
|
|
|
211
|
|
|
|
—
|
|
|
|
211
|
|
|
Fair value of common stock issued to consultant for services
|
|
|
—
|
|
|
|
—
|
|
|
|
25,000
|
|
|
|
—
|
|
|
|
163
|
|
|
|
—
|
|
|
|
—
|
|
|
|
163
|
|
|
|
—
|
|
|
|
163
|
|
|
Fractional share adjustments
|
|
|
—
|
|
|
|
—
|
|
|
|
6
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Series D Preferred Stock dividend paid with common stock
|
|
|
—
|
|
|
|
—
|
|
|
|
1,928
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 2007 (restated)
|
|
|
170
|
|
|
$
|
1,048
|
|
|
|
31,233,798
|
|
|
$
|
31
|
|
|
$
|
273,455
|
|
|
$
|
493
|
|
|
$
|
(228,313
|
)
|
|
$
|
46,713
|
|
|
$
|
—
|
|
|
$
|
46,713
|
|
|
Net Loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(14,196
|
)
|
|
|
(14,196
|
)
|
|
|
(2,538
|
)
|
|
|
(16,734
|
)
|
|
Realized gains on investments
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(493
|
)
|
|
|
—
|
|
|
|
(493
|
)
|
|
|
—
|
|
|
|
(493
|
)
|
|
Unrealized loss on investments
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(146
|
)
|
|
|
—
|
|
|
|
(146
|
)
|
|
|
—
|
|
|
|
(146
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive loss, net
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(639
|
)
|
|
|
(14,196
|
)
|
|
|
(14,835
|
)
|
|
|
(2,538
|
)
|
|
|
(17,373
|
)
|
|
Conversion of Series E Preferred Stock into Common Stock
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
16,800
|
|
|
|
16,800
|
|
|
Fair value of common stock issued to Targent, Inc. for NDA
Approval
|
|
|
(102
|
)
|
|
|
(629
|
)
|
|
|
204,000
|
|
|
|
—
|
|
|
|
629
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Fair value of common stock issued to NDDO, University of
Bradford et al
|
|
|
—
|
|
|
|
—
|
|
|
|
125,000
|
|
|
|
—
|
|
|
|
305
|
|
|
|
—
|
|
|
|
—
|
|
|
|
305
|
|
|
|
—
|
|
|
|
305
|
|
|
Share-based compensation expense and common stock issued (net of
forfeitures)
|
|
|
—
|
|
|
|
—
|
|
|
|
75,000
|
|
|
|
—
|
|
|
|
74
|
|
|
|
—
|
|
|
|
—
|
|
|
|
74
|
|
|
|
—
|
|
|
|
74
|
|
|
Issuance of common stock to 401(k) plan
|
|
|
—
|
|
|
|
—
|
|
|
|
362,088
|
|
|
|
1
|
|
|
|
6,322
|
|
|
|
—
|
|
|
|
—
|
|
|
|
6,324
|
|
|
|
—
|
|
|
|
6,324
|
|
|
Fractional share adjustments
|
|
|
—
|
|
|
|
—
|
|
|
|
166,430
|
|
|
|
—
|
|
|
|
274
|
|
|
|
—
|
|
|
|
—
|
|
|
|
274
|
|
|
|
—
|
|
|
|
274
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2008 (restated)
|
|
|
68
|
|
|
$
|
419
|
|
|
|
32,166,316
|
|
|
$
|
32
|
|
|
$
|
281,059
|
|
|
$
|
(146
|
)
|
|
$
|
(242,510
|
)
|
|
$
|
38,854
|
|
|
$
|
14,262
|
|
|
$
|
53,116
|
|
|
Net Loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(19,046
|
)
|
|
|
(19,046
|
)
|
|
|
(1,146
|
)
|
|
|
(20,192
|
)
|
|
Realized gains on investments
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
101
|
|
|
|
—
|
|
|
|
101
|
|
|
|
—
|
|
|
|
101
|
|
|
Unrealized loss on investments
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(25
|
)
|
|
|
—
|
|
|
|
(25
|
)
|
|
|
—
|
|
|
|
(25
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive loss, net
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
76
|
|
|
|
(19,046
|
)
|
|
|
(18,970
|
)
|
|
|
(1,146
|
)
|
|
|
(20,116
|
)
|
|
Issuance of common stock and warrants for cash, net of issuance
costs
|
|
|
—
|
|
|
|
—
|
|
|
|
15,187,715
|
|
|
|
15
|
|
|
|
81,779
|
|
|
|
—
|
|
|
|
—
|
|
|
|
81,794
|
|
|
|
—
|
|
|
|
81,794
|
|
|
Contributions by non-controlling interest
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,067
|
|
|
|
2,067
|
|
|
Purchase of non controlling interest
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,798
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,798
|
)
|
|
|
(15,183
|
)
|
|
|
(16,981
|
)
|
|
Issuance of common stock to employees - shelf takedown
|
|
|
—
|
|
|
|
—
|
|
|
|
432,200
|
|
|
|
1
|
|
|
|
1,166
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,167
|
|
|
|
—
|
|
|
|
1,167
|
|
|
Stock Options tender offer
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(2,520
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(2,520
|
)
|
|
|
—
|
|
|
|
(2,520
|
)
|
|
Issuance of common stock to 401(k) plan
|
|
|
—
|
|
|
|
—
|
|
|
|
139,795
|
|
|
|
—
|
|
|
|
448
|
|
|
|
—
|
|
|
|
—
|
|
|
|
448
|
|
|
|
—
|
|
|
|
448
|
|
|
Issuance of common stock for ESPP
|
|
|
—
|
|
|
|
—
|
|
|
|
65,715
|
|
|
|
—
|
|
|
|
292
|
|
|
|
—
|
|
|
|
—
|
|
|
|
292
|
|
|
|
—
|
|
|
|
292
|
|
|
Issuance of common stock upon exercise of stock options
|
|
|
—
|
|
|
|
—
|
|
|
|
488,750
|
|
|
|
1
|
|
|
|
1,261
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,262
|
|
|
|
—
|
|
|
|
1,262
|
|
|
Share-based compensation expense and common stock issued (net of
forfeitures)
|
|
|
—
|
|
|
|
—
|
|
|
|
207,014
|
|
|
|
—
|
|
|
|
6,860
|
|
|
|
—
|
|
|
|
—
|
|
|
|
6,860
|
|
|
|
—
|
|
|
|
6,860
|
|
|
Fair value of common stock issued to Targent, Inc. for NDA
Approval
|
|
|
—
|
|
|
|
—
|
|
|
|
125,000
|
|
|
|
—
|
|
|
|
185
|
|
|
|
—
|
|
|
|
—
|
|
|
|
185
|
|
|
|
—
|
|
|
|
185
|
|
|
Fair value of common stock issued to Altair Inc. for Renazorb
rights
|
|
|
—
|
|
|
|
—
|
|
|
|
113,809
|
|
|
|
—
|
|
|
|
750
|
|
|
|
—
|
|
|
|
—
|
|
|
|
750
|
|
|
|
—
|
|
|
|
750
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009
|
|
|
68
|
|
|
$
|
419
|
|
|
|
48,926,314
|
|
|
$
|
49
|
|
|
$
|
369,482
|
|
|
$
|
(70
|
)
|
|
$
|
(261,556
|
)
|
|
$
|
108,324
|
|
|
$
|
—
|
|
|
$
|
108,324
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying Notes to Consolidated Financial Statements are
an integral part of these statements.
F-6
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
|
|
|
(As Restated)
|
|
|
(As Restated)
|
|
|
|
|
(In thousands, except share
|
|
|
|
|
and share data)
|
|
|
|
|
Cash Flows From Operating Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(19,046
|
)
|
|
|
(14,196
|
)
|
|
$
|
(21,981
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating
activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortization of deferred revenue
|
|
|
(9,186
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Depreciation and amortization
|
|
|
4,244
|
|
|
|
610
|
|
|
|
255
|
|
|
Fair value adjustments of common stock warrants
|
|
|
(8,075
|
)
|
|
|
(1,271
|
)
|
|
|
(12,055
|
)
|
|
Acquired in-process research and development
|
|
|
—
|
|
|
|
4,700
|
|
|
|
—
|
|
|
Share-based compensation expense
|
|
|
7,423
|
|
|
|
6,537
|
|
|
|
5,652
|
|
|
Fair value of common stock issued in connection with drug license
|
|
|
935
|
|
|
|
379
|
|
|
|
520
|
|
|
Non-controlling interest in consolidated entities
|
|
|
(1,146
|
)
|
|
|
(2,538
|
)
|
|
|
(20
|
)
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
1,118
|
|
|
|
(4,811
|
)
|
|
|
959
|
|
|
Inventories
|
|
|
(1,389
|
)
|
|
|
(1,841
|
)
|
|
|
—
|
|
|
Prepaid expenses and other current assets
|
|
|
(354
|
)
|
|
|
101
|
|
|
|
(268
|
)
|
|
Accounts payable and other accrued obligations
|
|
|
7,097
|
|
|
|
2,387
|
|
|
|
1,463
|
|
|
Other assets
|
|
|
104
|
|
|
|
—
|
|
|
|
—
|
|
|
Accrued compensation
|
|
|
404
|
|
|
|
1,845
|
|
|
|
103
|
|
|
Accrued drug development costs
|
|
|
1,149
|
|
|
|
|
|
|
|
|
|
|
Deferred revenue and other credits
|
|
|
(912
|
)
|
|
|
93
|
|
|
|
(43
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities
|
|
|
(17,634
|
)
|
|
|
(8,005
|
)
|
|
|
(25,415
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Investing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net sales (purchases) of marketable securities
|
|
|
25,783
|
|
|
|
(13,056
|
)
|
|
|
(4,265
|
)
|
|
Investment in Zevalin acquisition
|
|
|
(30,940
|
)
|
|
|
(10,202
|
)
|
|
|
—
|
|
|
Purchases of property and equipment
|
|
|
(673
|
)
|
|
|
(1,518
|
)
|
|
|
(346
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
|
(5,830
|
)
|
|
|
(24,776
|
)
|
|
|
(4,611
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock and warrants, net of
related offering costs and expenses
|
|
|
95,810
|
|
|
|
—
|
|
|
|
30,009
|
|
|
Proceeds from sale of common stock to employees —
shelf takedown
|
|
|
1,167
|
|
|
|
—
|
|
|
|
—
|
|
|
Proceeds from sale of common stock to employees — ESPP
|
|
|
292
|
|
|
|
—
|
|
|
|
—
|
|
|
Proceeds from (repurchase) exercise of warrants
|
|
|
(71
|
)
|
|
|
—
|
|
|
|
519
|
|
|
Proceeds from exercise of stock options
|
|
|
1,262
|
|
|
|
—
|
|
|
|
120
|
|
|
Repurchase of stock options pursuant to tender offer
|
|
|
(2,520
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Proceeds from Allergan, Inc. collaboration
|
|
|
—
|
|
|
|
41,500
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by financing activities
|
|
|
95,940
|
|
|
|
41,500
|
|
|
|
30,648
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase in cash and cash equivalents
|
|
|
72,476
|
|
|
|
8,719
|
|
|
|
622
|
|
|
Cash and cash equivalents, beginning of period
|
|
|
9,860
|
|
|
|
1,141
|
|
|
|
519
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
$
|
82,336
|
|
|
$
|
9,860
|
|
|
$
|
1,141
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental Cash Flow Information:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest paid
|
|
$
|
28
|
|
|
$
|
36
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income taxes paid
|
|
$
|
45
|
|
|
$
|
5
|
|
|
$
|
5
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Schedule of Non-Cash Investing and Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of common stock issued in connection with drug license
|
|
$
|
935
|
|
|
$
|
379
|
|
|
$
|
520
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of restricted stock granted employees and directors
|
|
$
|
488
|
|
|
$
|
606
|
|
|
$
|
1,308
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of stock issued to match employee 401k contributions
|
|
$
|
448
|
|
|
$
|
274
|
|
|
$
|
179
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of common stock warrants
|
|
$
|
14,016
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying Notes to Condensed Consolidated Financial
Statements are an integral part of these statements.
F-7
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Restatement
of Historical Financial Statements
The accompanying consolidated balance sheet as of
December 31, 2008, and the consolidated statements of
operations, equity, and cash flows for the years ended
December 31, 2007 and 2008, have been restated in this
report to reclassify warrant contracts based on a reassessment
of the applicable accounting and classification, as are more
fully discussed in Note 2.
We are a commercial stage biopharmaceutical company committed to
developing and commercializing innovative therapies with a focus
primarily in the areas of hematology-oncology and urology. We
have a fully developed commercial infrastructure that markets
and sells two drugs in the United States,
Zevalin®
and
Fusilev®.
We also have a portfolio of drugs under various stages of
development. Our lead developmental drug is apaziquone
(EOquin®),
which is presently being studied in two large Phase 3 clinical
trials for non-muscle invasive bladder cancer under a strategic
collaboration with Allergan, Inc. Subsequent to
December 31, 2009, we acquired development and
commercialization rights for North America and India for
belinostat, from TopoTarget A/S to jointly develop. Belinostat
is being studied in multiple indications, including a Phase 2
trial for relapsed or refractory Peripheral T-Cell Lymphoma
(PTCL).
|
|
|
2.
|
Restatement
of Financial Statements
|
The Company’s consolidated financial statements contained
herein include restatements of previously reported financial
statements and related disclosures for fiscal years ended
December 31, 2007 and 2008 and each of the quarterly
condensed consolidated financial statements on Form 10-Q
for the periods ended March 31, 2008 through
September 30, 2009 to record common stock warrants as a
liability based on a reassessment of the applicable accounting
and classification.
In connection with warrants issued in registered offerings
during 2005 and 2009 (the “Warrants”), the Company had
previously recorded the Warrants as equity under its evaluation
of applicable guidance contained in Accounting Standards
Codification (“ASC”) Topic 815 “Derivatives and
Hedging — Contracts in Entity’s Own Equity”
(“ASC 815”) (formerly known as Emerging Issues Task
Force Issue
00-19,
“Accounting for Derivative Financial Instruments Indexed
to, and Potentially Settled in, a Company’s Own
Stock”). In connection with the audit for the fiscal year
2009, the Company, in consultation with Ernst & Young
LLP (“Ernst & Young”), the Company’s
current independent registered public accounting firm,
reassessed the accounting classification of the Warrants payment
to ASC 815 based on certain terms of the Warrants. The Warrants
provide that in the event the Company is unable to issue
registered shares upon exercise, the Warrant holders are
entitled, under securities laws, to receive freely tradable
shares pursuant to a “cashless exercise” provision.
However, based on interpretation of ASC 815, there is a required
presumption of net cash settlement as it is not within the
control of the Company to provide registered shares, no matter
how remote the probability. The Company’s Audit Committee,
together with management, in consultation with the
Company’s outside legal advisors, determined on
March 30, 2010 that, notwithstanding the highly remote
theoretical nature of the possibility of net cash settlement,
the Warrants should have originally been recorded as
liabilities, measured at fair value with changes in the fair
values being recognized in the statement of operations.
We have not amended our previously filed Annual Reports on Form
10-K for the
fiscal years ended December 31, 2005, 2006, 2007 and 2008,
or the Quarterly Reports on Form
10-Q for the
periods ended September 30, 2005 through September 30,
2009 to reflect the restatements described in this Annual Report
on Form 10-K, and thus the financial statements and related
financial statement information contained in those reports
should no longer be relied upon. Throughout this Annual Report
on Form 10-K, all amounts presented from prior periods and prior
period comparisons have been revised and labeled as
“restated” and reflect the balances and amounts on a
restated basis.
F-8
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
The restatements reflect the reclassification of the warrants
from equity to a liability in the following amounts, which
represents the fair value of the warrants, as of the issuance
dates, calculated using the Black-Scholes option pricing model.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value
|
|
|
|
|
Number of
|
|
|
|
|
|
|
|
of Warrants
|
|
|
|
|
Warrants
|
|
|
Exercise
|
|
|
Expiration
|
|
at Issuance
|
|
|
Issuance Date
|
|
Issued
|
|
|
Price
|
|
|
of Warrants
|
|
Date
|
|
|
|
|
|
|
|
|
|
|
|
|
(In thousands)
|
|
|
|
|
September 14, 2005
|
|
|
4,000,000
|
|
|
$
|
6.62
|
|
|
September 14, 2011
|
|
$
|
15,472
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
May 27, 2009
|
|
|
1,956,947
|
|
|
$
|
5.11
|
|
|
February 25, 2010
|
|
$
|
2,881
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 15, 2009
|
|
|
857,633
|
|
|
$
|
5.83
|
|
|
March 15, 2010
|
|
$
|
1,847
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30, 2009
|
|
|
1,468,020
|
|
|
$
|
7.10
|
|
|
March 30, 2010
|
|
$
|
4,117
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 18, 2009
|
|
|
2,649,007
|
|
|
$
|
7.55
|
|
|
June 20, 2010
|
|
$
|
5,170
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The revaluation of the warrants at each subsequent balance sheet
date to fair value, results in a change in the carrying value of
the liability, which change is recorded as ‘‘Change in
fair value of common stock warrant liability” in the
consolidated statement of operations. The net effect of these
changes for fiscal years ended December 31, 2008 and 2007,
and for each of the quarterly condensed consolidated financial
statements on
Form 10-Q
for the periods ended March 31, 2008 through
September 30, 2009 are as follows:
| |
|
|
|
|
|
|
|
Income (Loss) Resulting
|
|
|
|
|
from Change in Fair
|
|
|
|
|
Value of Common
|
|
|
Reporting Period
|
|
Stock Warrant Liability
|
|
|
|
|
(In thousands)
|
|
|
|
|
Annual
|
|
|
|
|
|
Year ended December 31, 2007
|
|
$
|
12,055
|
|
|
|
|
|
|
|
|
Year ended December 31, 2008
|
|
$
|
1,271
|
|
|
|
|
|
|
|
|
Interim (unaudited)
|
|
|
|
|
|
Quarter ended March 31, 2008
|
|
$
|
520
|
|
|
|
|
|
|
|
|
Quarter ended June 30, 2008
|
|
$
|
916
|
|
|
|
|
|
|
|
|
Quarter ended September 30, 2008
|
|
$
|
45
|
|
|
|
|
|
|
|
|
Quarter ended December 31, 2008
|
|
$
|
(210
|
)
|
|
|
|
|
|
|
|
Quarter ended March 31, 2009
|
|
$
|
(509
|
)
|
|
|
|
|
|
|
|
Quarter ended June 30, 2009
|
|
$
|
(20,113
|
)
|
|
|
|
|
|
|
|
Quarter ended September 30, 2009
|
|
$
|
(8,863
|
)
|
|
|
|
|
|
|
F-9
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
The following tables summarize the effects of the restatements
on the specific line items presented in the Company’s
historical consolidated financial statements included in the
Company’s Annual Report on
Form 10-K
as of the fiscal year ended December 31, 2008 and for the
two years ended December 31, 2008:
| |
|
|
|
|
|
|
|
|
|
Consolidated Balance Sheet
|
|
December 31, 2008
|
|
|
December 31, 2008
|
|
|
|
|
(As previously reported)
|
|
|
(As Restated)
|
|
|
|
|
(In thousands)
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Common stock warrant liability
|
|
$
|
—
|
|
|
$
|
765
|
|
|
Total current liabilities
|
|
$
|
28,032
|
|
|
$
|
33,571
|
|
|
|
|
|
|
|
|
|
|
|
|
Spectrum Pharmaceuticals, Inc. stockholders’ equity:
|
|
|
|
|
|
|
|
|
|
Additional
paid-in-capital
|
|
$
|
296,531
|
|
|
$
|
281,059
|
|
|
Accumulated deficit
|
|
$
|
(257,217
|
)
|
|
$
|
(242,510
|
)
|
|
Total stockholders’ equity
|
|
$
|
39,619
|
|
|
$
|
38,854
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
|
|
|
Year Ended
|
|
|
Year Ended
|
|
|
Year Ended
|
|
|
Consolidated Statements of Operations
|
|
December 31, 2008
|
|
|
December 31, 2008
|
|
|
December 31, 2007
|
|
|
December 31, 2007
|
|
|
|
|
(As Previously
|
|
|
(As Restated)
|
|
|
(As Previously
|
|
|
(As Restated)
|
|
|
|
|
Reported)
|
|
|
|
|
|
Reported)
|
|
|
|
|
|
|
|
Change in fair value of common stock warrant liability
|
|
$
|
—
|
|
|
$
|
1,271
|
|
|
$
|
—
|
|
|
$
|
12,055
|
|
|
Net loss — attributable to Spectrum Pharmaceuticals,
Inc. stockholders
|
|
|
(15,467
|
)
|
|
|
(14,196
|
)
|
|
|
(34,036
|
)
|
|
|
(21,981
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Per share data — basic and diluted —
attributable to Spectrum Pharmaceuticals, Inc. stockholders:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per share — attributable to Spectrum
Pharmaceuticals, Inc.
|
|
$
|
(0.49
|
)
|
|
$
|
(0.45
|
)
|
|
$
|
(1.17
|
)
|
|
$
|
(0.76
|
)
|
The restatements resulted in changes to the opening balances of
accumulated defict, additional paid in capital and total
shareholders’ equity as of January 1, 2007 as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
January 1, 2007
|
|
|
January 1, 2007
|
|
|
|
|
(As Previously
|
|
|
(As Restated)
|
|
|
|
|
Reported)
|
|
|
|
|
|
|
|
(In Thousands)
|
|
|
|
|
Additional paid-in capital
|
|
$
|
251,880
|
|
|
$
|
27,345
|
|
|
Accumulated deficit
|
|
$
|
(207,714
|
)
|
|
$
|
(228,313
|
)
|
|
Stockholders’ equity
|
|
$
|
45,829
|
|
|
$
|
46,713
|
|
The restatements had no impact on the financial statement
amounts previously reported for the Company’s assets,
revenues and operating costs and expenses and cash flows from
operations other than the change in net income (loss) for the
years ended December 31, 2008 and 2007, or for the first
nine months ended September 30, 2009, or any quarterly
periods in the years ended December 31, 2009, 2008 and 2007.
|
|
|
3.
|
Summary
of Significant Accounting Policies
|
Principles
of Consolidation and Basis of Presentation
The consolidated financial statements include the accounts of
Spectrum Pharmaceuticals, Inc. (Spectrum or the Company), our
wholly-owned subsidiaries, and joint ventures the Company
controls, or of which it is the primary beneficiary. We evaluate
the need to consolidate joint ventures based on ASC
No. 810-10-15,
Consolidation, Variable Interest Entities. Investments by
outside parties in our consolidated entities are recorded as non-
F-10
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
controlling interest in consolidated entities in our
consolidated financial statements, and stated net after
allocation of income and losses in the entity.
As of December 31, 2009, we had three consolidated
subsidiaries: OncoRx Pharma Private Limited
(“OncoRx”), 100% owned, organized in Mumbai, India in
2008; Spectrum Pharmaceuticals GmbH, wholly-owned inactive
subsidiary, incorporated in Switzerland in April 1997; RIT
Oncology, LLC (“RIT”), 100% owned since March 15,
2009, organized in Delaware in October 2008; and one
consolidated joint venture, Spectrum Pharma Canada, organized in
Quebec, Canada in January 2008. We have eliminated all
significant intercompany accounts and transactions from the
consolidated financial statements.
Use of
Estimates
The preparation of financial statements in conformity with
accounting principles generally accepted in the United States
requires us to make estimates and assumptions that affect the
reported amounts of assets, liabilities, revenues and expenses
and disclosure of contingent obligations in the consolidated
financial statements and accompanying notes. The estimation
process requires assumptions to be made by management about
future events and conditions, and as such, is inherently
subjective and uncertain. Actual results could differ materially
from those estimates.
Subsequent
Events
In connection with the preparation of the consolidated financial
statements, we have evaluated subsequent events through the
filing date of this
Form 10-K.
Cash
and Cash Equivalents, Marketable Securities and Fair Value of
Financial Instruments
Cash and cash equivalents and marketable securities primarily
consist of bank checking deposits, short-term treasury
securities, institutional money market funds, corporate debt and
equity, municipal obligations, government agency notes, and
certificates of deposit. We classify highly liquid short-term
investments, with insignificant interest rate risk and
maturities of 90 days or less at the time of acquisition,
as cash and cash equivalents. Other investments, which do not
meet the above definition of cash equivalents, are classified as
either
“held-to-maturity”
or
“available-for-sale”
marketable securities. Investments that lack immediate
liquidity, or which we intend to hold for more than one year are
classified as long-term investments, and included in other
assets. All of our “available for sale securities” are
classified as current assets based on our intent and ability to
use any and all of these securities as necessary to satisfy our
cash needs as they arise, by redeeming them at par with short
notice and without penalty.
We have classified $11.4 million of our investments with
maturity dates over 1 year from December 31, 2009 as
long term based on held to maturity.
The Company measures fair value based on the prices that would
be received to sell an asset or paid to transfer a liability in
an orderly transaction between market participants at the
measurement date. Fair value measurements are based on a
three-tier hierarchy that prioritizes the inputs used to measure
fair value. These tiers include:
Level 1: Quoted prices (unadjusted) in
active markets that are accessible at the measurement date for
assets or liabilities. The fair value hierarchy gives the
highest priority to Level 1 inputs.
Level 2: Observable prices that are based
on inputs not quoted on active markets, but corroborated by
market data.
Level 3: Unobservable inputs are used
when little or no market data is available. The fair value
hierarchy gives the lowest priority to Level 3 inputs.
F-11
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
In determining fair value, we utilize valuation techniques that
maximize the use of observable inputs and minimize the use of
unobservable inputs to the extent possible, as well as consider
counterparty credit risk in the assessment of fair value.
The carrying values of our cash, cash equivalents, marketable
securities, other securities and common stock warrants, carried
at fair value as of December 31, 2009 and 2008, are
classified in the table below in one of the three categories
described above:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value Measurements at December 31,
|
|
|
|
|
2009 and 2008
|
|
|
|
|
(In ‘000’s)
|
|
|
|
|
Level 1
|
|
|
Level 2
|
|
|
Level 3
|
|
|
Total
|
|
|
|
|
2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash & Equivalents
|
|
$
|
82,336
|
|
|
|
—
|
|
|
|
—
|
|
|
$
|
82,336
|
|
|
U.S. Treasury T-Bills
|
|
|
3,501
|
|
|
|
—
|
|
|
|
—
|
|
|
|
3,501
|
|
|
Money Market Currency Funds
|
|
|
4,800
|
|
|
|
—
|
|
|
|
—
|
|
|
|
4,800
|
|
|
FDIC insured Bank CDs
|
|
|
20,948
|
|
|
|
—
|
|
|
|
—
|
|
|
|
20,948
|
|
|
Medium Term Corporate Notes
|
|
|
153
|
|
|
|
—
|
|
|
|
—
|
|
|
|
153
|
|
|
U.S. Treasury Backed Securities
|
|
|
13,041
|
|
|
|
—
|
|
|
|
—
|
|
|
|
13,041
|
|
|
Other Securities (included in other assets)
|
|
|
35
|
|
|
|
—
|
|
|
|
—
|
|
|
|
35
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
124,814
|
|
|
|
—
|
|
|
|
—
|
|
|
$
|
124,814
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock warrant liability
|
|
|
—
|
|
|
|
—
|
|
|
$
|
6,635
|
|
|
$
|
6,635
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash & Equivalents
|
|
$
|
9,860
|
|
|
|
—
|
|
|
|
—
|
|
|
$
|
9,860
|
|
|
U.S. Treasury T-Bills
|
|
|
12,217
|
|
|
|
—
|
|
|
|
—
|
|
|
|
12,217
|
|
|
Money Market Currency Funds
|
|
|
128
|
|
|
|
—
|
|
|
|
—
|
|
|
|
128
|
|
|
FDIC insured Bank CDs
|
|
|
10,509
|
|
|
|
—
|
|
|
|
—
|
|
|
|
10,509
|
|
|
Medium Term Corporate Notes
|
|
|
1,912
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,912
|
|
|
U.S. Treasury Backed Securities
|
|
|
43,650
|
|
|
|
—
|
|
|
|
—
|
|
|
|
43,650
|
|
|
Other Securities (included in other assets)
|
|
|
47
|
|
|
|
—
|
|
|
|
—
|
|
|
|
47
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
78,323
|
|
|
|
—
|
|
|
|
—
|
|
|
$
|
78,323
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock warrant liability
|
|
|
—
|
|
|
|
—
|
|
|
$
|
765
|
|
|
$
|
765
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following summarizes the activity of Level 3 inputs measured
on a recurring basis for the years ended December 31, 2009
and 2008:
| |
|
|
|
|
|
|
|
Fair Value Measurements of
|
|
|
|
|
Common Stock Warrants
|
|
|
|
|
Using Significant
|
|
|
|
|
Unobservable Inputs (Level 3)
|
|
|
|
|
($ in 000’s)
|
|
|
|
|
Balance at January 1, 2008
|
|
$
|
2,036
|
|
|
|
|
|
|
|
|
Transfers in / (out) of Level 3
|
|
|
—
|
|
|
Issuance of common stock warrants
|
|
|
—
|
|
|
Repurchase of Forfeitures
|
|
|
—
|
|
|
Expirations
|
|
|
—
|
|
F-12
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
Fair Value Measurements of
|
|
|
|
|
Common Stock Warrants
|
|
|
|
|
Using Significant
|
|
|
|
|
Unobservable Inputs (Level 3)
|
|
|
|
|
($ in 000’s)
|
|
|
|
|
Settlements associated with exercises
|
|
|
—
|
|
|
Adjustments resulting from change in value of warrants
recognized in earnings
|
|
|
(1,271
|
)
|
|
|
|
|
|
|
|
Balance at December 31, 2008
|
|
|
765
|
|
|
|
|
|
|
|
|
Transfers in / (out) of Level 3
|
|
|
—
|
|
|
Issuance of common stock warrants
|
|
|
14,016
|
|
|
Repurchases or forfeitures
|
|
|
(394
|
)
|
|
Gain on repurchase recognized in earnings
|
|
|
323
|
|
|
Expirations
|
|
|
—
|
|
|
Settlements associated with exercises
|
|
|
—
|
|
|
Adjustments resulting from change in value of warrants
recognized in earnings
|
|
|
(8,075
|
)
|
|
|
|
|
|
|
|
Balance at December 31, 2009
|
|
$
|
6,635
|
|
|
|
|
|
|
|
The fair value of common stock warrants are measured on their
respective origination dates and at the end of each reporting
period using Level 3 inputs in accordance with the accounting
guidance. The significant assumptions used in the calculations
under the Black-Scholes pricing model as of December 31,
2009 and 2008, included an expected term based on the remaining
contractual life of the warrants, a risk-free interest rate
based upon observed interest rates appropriate for the expected
term of the instruments, volatility based on the historical
volatility of the Company’s common stock, and a zero
dividend rate based on the Company’s past, current and
expected practices of granting dividends on common stock.
As of December 31, 2009, substantially all of our cash,
cash equivalents and marketable securities were held at major
financial institutions, which are required to invest our funds
in accordance with our investment policy with the principal
objectives of such policy being preservation of capital,
fulfillment of liquidity needs and above market returns
commensurate with preservation of capital. Our investment policy
also requires that investments in marketable securities be in
only highly rated instruments, which are primarily US treasury
bills or US treasury backed securities, with limitations on
investing in securities of any single issuer. To a limited
degree, these investments are insured by the Federal Deposit
Insurance Corporation and by third party insurance. However,
these investments are not insured against the possibility of a
complete loss of earnings or principal and are inherently
subject to the credit risk related to the continued credit
worthiness of the underlying issuer and general credit market
risks. We manage such risks on our portfolio by matching
scheduled investment maturities with our cash requirements and
investing in highly rated instruments.
The Company did not elect the fair value option, as allowed to
account for its financial assets and liabilities that were not
previously carried at fair value. Therefore, material financial
assets and liabilities that are not carried at fair value, such
as trade accounts receivable and payable, are still reported at
their historical carrying values.
Concentration
of credit risk
We are subject to concentration of credit risk primarily from
our cash investments. Under our investment guidelines, credit
risk is managed by diversification of the investment portfolio
and by the purchase of investment-grade securities.
Our product sales are concentrated in a limited number of
customers. For the year ended December 31, 2009,
approximately 44% of our product sales of Fusilev were derived
from specialty distributors of oncology products as
F-13
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
compared to 100% for the year ended 2008. For Zevalin, we
recorded 21% of revenues from radiopharmacies as compared to 0%
for the years ended December 31, 2009 and 2008,
respectively; and the balance from end user customers. For
Zevalin, no single end user customer constituted revenues over
10% individually. Due to changes in market dynamics, these
ratios are not indicative of future concentrations. As of
December 31, 2009, no single specialty distributor owed us
more than 10% of the total net accounts receivables. Three
specialty distributors owed us 100% at the end of 2008. No
single end user customer owed us more than 10% of net
receivables as of December 31, 2009 or 2008. We maintain
reserves for potential credit losses and such losses, in the
aggregate, have not exceeded our estimates. We do not require
collateral or other security to support credit sales, but
provide an allowance for bad debts when warranted.
Currently we have single source suppliers for raw materials, and
the manufacturing of finished product of Zevalin and Fusilev. A
disruption in supply could materially affect our sales.
Similarly, we have single source suppliers for raw materials,
and manufactured finished product for our development drug
candidates. If we are unable to obtain sufficient quantities of
such product, our research and development activities may be
adversely affected.
Inventories
Inventory is valued at the lower of cost
(first-in,
first-out method) or market. The lower of cost or market is
determined based on net estimated realizable value after
appropriate consideration is given to obsolescence, excessive
levels, deterioration, and other factors.
Property
and Equipment
Property and equipment is stated at cost. Equipment is
depreciated on a straight-line basis over its estimated useful
life (generally 5 to 7 years). Leasehold improvements are
amortized over the shorter of the estimated useful life or lease
term. Maintenance and repairs are expensed as incurred. Major
renewals and improvements that extend the life of the property
are capitalized.
All long-lived assets, including property and equipment, are
reviewed for impairment whenever events or changes in business
circumstances indicate that the carrying amount of the assets
may not be fully recoverable. If impairment is indicated, we
reduce the carrying value of the asset to fair value. Fair value
would be determined by the use of appraisals, discounted cash
flow analyses or comparable fair values of similar assets.
Patents
and Licenses
We expense all licensing and patent application costs as they
are incurred.
Intangible
Assets
As described in note 4 below, we acquired 50% of the rights
in RIT in December 2008 and the remaining 50% in March 2009.
The purchase price for the acquisition of Zevalin rights was
allocated to identifiable intangible assets acquired and
liabilities assumed based on their estimated fair values at the
acquisition date. Such a valuation requires significant
estimates and assumptions including but not limited to:
determining the timing and expected costs to complete the
in-process projects, projecting regulatory approvals, estimating
future cash flows from product sales resulting from in-process
projects, and developing appropriate discount rates and
probability rates by project. We believe the fair values
assigned to the assets acquired and liabilities assumed are
based on reasonable assumptions.
Identifiable intangible assets with definite lives are amortized
on a straight-line basis over their estimated useful lives,
ranging from 1 to 10 years.
F-14
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
We evaluate the recoverability of intangible assets whenever
events or changes in circumstances indicate that an intangible
asset’s carrying amount may not be recoverable. Such
circumstances could include, but are not limited to the
following:
i a significant decrease in the market value of an asset;
ii a significant adverse change in the extent or manner in
which an asset is used; or
iii an accumulation of costs significantly in excess of the
amount originally expected for the acquisition of an asset.
We measure the carrying amount of the asset against the
estimated undiscounted future cash flows associated with it.
Should the sum of the expected future net cash flows be less
than the carrying value of the asset being evaluated, an
impairment loss would be recognized. The impairment loss would
be calculated as the amount by which the carrying value of the
asset exceeds its fair value. No impairment loss was recorded
during the years 2009, 2008 or 2007.
Segment
and Geographic Information
We operate in one business segment, that is to acquire, develop
and commercialize prescription drug products. Accordingly, the
accompanying consolidated financial statements are reported in
the aggregate, including all our activities in one segment. Our
foreign operations were not significant for any of the years
presented herein.
Revenue
Recognition
Revenue from product sales is recognized upon shipment of
product when title and risk of loss have transferred to the
customer. We sell our products to wholesalers and distributors
of oncology products and directly to the end user, directly or
through GPOs (e.g., certain hospitals or hospital systems and
clinics with whom we have entered into a direct purchase
agreement). Our wholesalers and distributors purchase our
products and sell the products directly to end users, which
include, but are not limited to, hospitals, clinics, medical
facilities, managed care facilities and private oncology based
practices etc. Revenue from product sales is recognized upon
shipment of product when title and risk of loss have transferred
to the customer, and the following additional criteria specified
by ASC
No. 605-15,
“Revenue Recognition: Products”, are met:
(i) the price is substantially fixed and determinable;
(ii) our customer has economic substance apart from that
provided by us;
(iii) our customer’s obligation to pay us is not
contingent on resale of the product;
(iv) we do not have significant obligations for future
performance to directly bring about the resale of our product;
and
(v) we have a reasonable basis to estimate future returns.
We also follow the provisions as set forth by current accounting
rules, which primarily include ASC
No. 605-15,
“Revenue Recognition: Products,” and ASC
No. 605-25,
“Revenue Recognition: Multiple-Element Arrangements.”
Generally, revenue is recognized when all four of the following
criteria are met:
(i) persuasive evidence that an arrangement exists;
(ii) delivery of the products has occurred, or services
have been rendered;
(iii) the selling price is both fixed and determinable; and
(iv) collectibility is reasonably assured.
F-15
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
Provision for estimated product returns, sales discounts,
rebates and chargebacks are established as a reduction of gross
product sales at the time such revenues are recognized. Thus,
revenue is recorded, net of such estimated provisions.
Consistent with industry practice, our product return policy
permits our customers to return products within 30 days
after shipment, if incorrectly shipped or not ordered, and
within a window of time 6 months before and 12 months
after the expiration of product dating, subject to certain
restocking fees and preauthorization requirements, as
applicable. The returned product is destroyed if it is damaged,
it’s quality is compromised or it is past its expiration
date. Based on our returns policy, we refund the sales price to
the customer as a credit and record the credit against
receivables. In general, returned product is not resold. We
generally reserve the right to decline granting a return and to
decide on product destruction. As of each balance sheet date, we
estimate potential returns, based on several factors, including:
inventory held by distributors, sell through data of distributor
sales to end users, customer and end-user ordering and
re-ordering patterns, aging of accounts receivables, rates of
returns for directly substitutable products and other
pharmaceutical products for the treatment of therapeutic areas
similar to indications served by our products, shelf life of our
products and the extensive experience of our management with
selling the same and similar oncology products. We record an
allowance for future returns by reducing gross revenues and
increasing the allowance for returns. If allowances exceed the
related accounts receivables, we reclassify such allowances to
accrued obligations. Historical allowances for product returns
have been within estimated amounts reserved or accrued.
We record Medicaid and Medicare rebates based on estimates for
such expense. However, such amounts have not been material to
the financial statements.
We also state the related accounts receivable at net realizable
value, with any allowance for doubtful accounts charged to
general operating expenses. If revenue from sales is not
reasonably determinable due to provisions for estimates,
promotional adjustments, price adjustments, returns or any other
potential adjustments, we defer the revenue and recognize
revenue when the estimates are reasonably determinable, even if
the monies for the gross sales have been received.
Up-front fees representing non-refundable payments received upon
the execution of licensing or other agreements are recognized as
revenue upon execution of the agreements where we have no
significant future performance obligations and collectibility of
the fees is reasonably assured. Milestone payments, which are
generally based on developmental or regulatory events, are
recognized as revenue when the milestones are achieved,
collectibility is reasonably assured, and we have no significant
future performance obligations in connection with the milestone.
In those instances where we have collected fees or milestone
payments but have significant future performance obligations
related to the development of the drug product, we record
deferred revenue and recognize it over the period of our future
obligations.
Research
and Development
Research and development expenses include salaries and benefits,
clinical trial and related manufacturing costs, contract and
other outside service fees, and facilities and overhead costs
related to our research and development efforts. Research and
development expenses also consist of costs incurred for
proprietary and collaborative research and development and
include activities such as product registries and
investigator-sponsored trials. Research and development costs
are expensed as incurred. In certain instances, we enter into
agreements with third parties for research and development
activities, where we may prepay fees for services at the
initiation of the contract. In accordance with ASC
No. 730-20,
“Research and Development: Research & Development
Arrangements,” we record such prepayment as a prepaid asset
and charge research and development expense over the period of
time the contracted research and development services are
performed. Other types of arrangements with third parties may be
fixed fee or fee for service, and may include monthly payments
or payments upon the completion of milestones or receipt of
deliverables.
F-16
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
As of each balance sheet date, we review purchase commitments
and accrue drug development expenses based on factors such as
estimates of work performed, patient enrollment, completion of
patient studies and other events. Accrued clinical study costs
are subject to revisions as trials progress to completion.
Revisions are recorded in the period in which the facts that
give rise to the revision become known.
Basic
and Diluted Net Loss Per Share
We calculate basic and diluted net loss per share using the
weighted average number of common shares outstanding during the
periods presented, and adjust the amount of net loss, used in
this calculation, for preferred stock dividends declared during
the period.
We incurred net losses in each of the periods presented, and as
such, did not include the effect of potentially dilutive common
stock equivalents in the diluted net loss per share calculation,
as their effect would be anti-dilutive for all periods.
Potentially dilutive common stock equivalents would include the
common stock issuable upon conversion of preferred stock and the
exercise of warrants and stock options that have conversion or
exercise prices below the market value of our common stock at
the measurement date.
The following shows the amounts used in computing basic loss per
share for each of the three years in the period ended
December 31, 2009.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
|
|
|
(As Restated)
|
|
|
(As Restated)
|
|
|
|
|
(Amounts in thousands except share
|
|
|
|
|
and per share data)
|
|
|
|
|
Net loss — attributable to Spectrum Pharmaceuticals,
Inc. stockholders
|
|
$
|
(19,046
|
)
|
|
$
|
(14,196
|
)
|
|
$
|
(21,981
|
)
|
|
Less:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred dividends paid in cash or stock
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss attributable to Spectrum stockholders
|
|
$
|
(19,046
|
)
|
|
$
|
(14,196
|
)
|
|
$
|
(21,981
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares issued and outstanding
|
|
|
48,425,486
|
|
|
|
31,551,152
|
|
|
|
29,013,850
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per share
|
|
$
|
(0.39
|
)
|
|
$
|
(0.45
|
)
|
|
$
|
(0.76
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following table sets forth the number of shares excluded
from the computation of diluted earnings per share, as to do so
would have been anti-dilutive:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Series E Preferred Shares
|
|
|
136,000
|
|
|
|
136,000
|
|
|
|
340,000
|
|
|
Stock Options
|
|
|
4,451,733
|
|
|
|
5,097,835
|
|
|
|
4,185,273
|
|
|
Warrants
|
|
|
8,379,912
|
|
|
|
5,444,555
|
|
|
|
9,572,051
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12,967,645
|
|
|
|
10,678,390
|
|
|
|
14,097,324
|
|
Accounting
for Employee Share-Based Compensation
We measure compensation cost for all share-based awards at fair
value on the date of grant and recognize compensation expense in
our consolidated statements of operations over the service
period that the awards are expected to vest. We have elected to
recognize compensation expense for all options with graded
vesting on a straight-line basis over the vesting period of the
entire option.
The fair value of share-based compensation is estimated based on
the closing market price of our common stock on the day prior to
the award grants for stock awards, and the Black-Scholes Option
Pricing Model for stock
F-17
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
options and warrants. We estimate volatility based on historical
volatility of our common stock, and estimate the expected length
of options based on several criteria, including the vesting
period of the grant and the term of the award.
We recorded share-based employee compensation during each of the
three years in the period ended December 31, 2009 as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
($ in ‘000’s)
|
|
|
|
|
Research and development
|
|
$
|
3,192
|
|
|
$
|
3,925
|
|
|
$
|
3,555
|
|
|
Selling, general and administrative
|
|
|
4,231
|
|
|
|
2,612
|
|
|
|
2,097
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total employee pre-tax share-based compensation
|
|
$
|
7,423
|
|
|
$
|
6,537
|
|
|
$
|
5,652
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrant
accounting
We account for common stock warrants pursuant to the applicable
guidance on accounting for derivative financial instruments
indexed to, and potentially settled in, a company’s own
stock, on the understanding that in compliance with applicable
securities laws, registered warrants require the issuance of
registered securities upon exercise and do not sufficiently
preclude an implied right to net cash settlement. We classify
registered warrants on the consolidated balance sheet as a
current liability, which is revalued at each balance sheet date
subsequent to the initial issuance. Determining the appropriate
fair-value model and calculating the fair value of registered
warrants requires considerable judgment, including estimating
stock price volatility and expected warrant life. We develop our
estimates based on historical data. A small change in the
estimates used may have a relatively large change in the
estimated valuation. We use the Black-Scholes pricing model to
value the registered warrants. Changes in the fair market value
of the warrants are reflected in the consolidated statement of
operations as “Change in the fair value of common stock
warrant liability.”
Income
Taxes
Deferred tax assets and liabilities are recognized for the
future tax consequences attributable to differences between the
financial statement carrying amounts of existing assets and
liabilities and their respective tax bases. Deferred tax assets
and liabilities are measured using enacted tax rates expected to
apply to taxable income in the years in which those temporary
differences are expected to be recovered or settled. The effect
on the deferred tax assets and liabilities of a change in tax
rates is recognized in income in the period that includes the
enactment date. The Company has determined that the net deferred
tax asset does not meet the “more likely than not” to
be realized criteria and, accordingly, a valuation allowance has
been recorded to reduce the net deferred tax asset to zero.
Comprehensive
Income (loss)
Comprehensive loss is calculated in accordance with ASC
No. 220, “Comprehensive Income,” which requires
the disclosure of all components of comprehensive income,
including net income and changes in equity during a period from
transactions and other events and circumstances generated from
non-owner sources. Our accumulated other comprehensive loss at
December 31, 2009, 2008 and 2007, respectively consisted
primarily of net unrealized gains/losses on investments in
marketable securities as of that date.
Reclassification
of Accounts
Certain reclassifications of prior-year comparative financial
statements have been made to conform to the current year
presentation. These reclassifications had no effect on
previously reported consolidated results of operations or
financial position.
F-18
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
New
Accounting Pronouncements
In June 2009, the FASB issued authoritative guidance that
establishes the FASB Accounting Standards CodificationTM as the
single source of authoritative U.S. GAAP to be applied by
nongovernmental entities and modifies the U.S. GAAP
hierarchy to only two levels: authoritative and
nonauthoritative. This guidance became effective for interim
periods and fiscal years ending after September 15, 2009.
The Company adopted the provisions of the guidance in the third
quarter of 2009. The adoption did not have a material impact on
the Company’s consolidated financial statements.
In May 2009, the FASB issued authoritative guidance that
establishes general standards of accounting for and disclosure
of events that occur after the balance sheet date but before
financial statements are issued or are available to be issued.
This guidance became effective for interim periods and fiscal
years ending after June 15, 2009. The Company adopted the
provisions of the guidance in the second quarter of 2009. The
adoption did not have a material impact on the Company’s
consolidated financial statements.
In April 2009, the FASB issued authoritative guidance that
requires publicly traded companies to include in their interim
financial reports certain disclosures about the carrying value
and fair value of financial instruments previously required only
in annual financial statements and to disclose changes in
significant assumptions used to calculate the fair value of
financial instruments. This guidance became effective for
interim reporting periods ending after June 15, 2009, with
early adoption permitted for interim reporting periods ending
after March 15, 2009. The Company adopted the provisions of
the guidance in the first quarter of 2009. The adoption did not
have a material impact on the Company’s consolidated
financial statements.
In November 2008, the FASB issued authoritative guidance that
clarifies how to account for acquired intangible assets
subsequent to initial measurement in situations in which an
entity does not intend to actively use the assets but intends to
hold the asset to prevent others from obtaining access to the
asset (a defensive intangible asset), except for intangible
assets that are used in research and development activities.
This guidance requires that a defensive intangible asset be
accounted for as a separate unit of accounting and assigned a
useful life that reflects the entity’s consumption of the
expected benefits related to that asset. This guidance became
effective for intangible assets acquired on or after
December 15, 2008. The Company adopted the provisions of
the guidance in the first quarter of 2009. The adoption did not
have a material impact on the Company’s consolidated
financial statements.
In June 2008, the FASB issued authoritative guidance that
requires companies to determine if an instrument (or embedded
feature) is indexed to an entity’s own stock and provides
guidance in evaluating whether certain financial instruments or
embedded features can be excluded from the scope of the guidance
for accounting for derivatives and hedging activities. The
guidance sets forth a two-step approach that evaluates an
instrument’s contingent exercise and settlement provisions
for the purpose of determining whether such instruments are
indexed to an issuer’s own stock (a requirement necessary
to comply with the scope exception under the guidance for
accounting for derivatives). The Company adopted the guidance in
the first quarter of 2009. The adoption did not have a material
impact on the Company’s consolidated financial statements.
In April 2008, the FASB issued authoritative guidance that
amends the guidance for estimating the useful lives of
recognized intangible assets and requires additional disclosure
related to renewing or extending the useful lives of recognized
intangible assets. This guidance became effective for fiscal
years and interim periods beginning after December 15,
2008. The Company adopted the provisions of the guidance in the
first quarter of 2009. The adoption did not have a material
impact on the Company’s consolidated financial statements.
In December 2007, the FASB issued authoritative guidance that
significantly changes the accounting and reporting requirements
for business combination transactions, including capitalization
of in-process research and development assets and expensing
acquisition costs as incurred. This guidance became effective
for business combination transactions occurring in fiscal years
beginning after December 15, 2008. The Company adopted the
provisions of the guidance in the first quarter of 2009. The
adoption did not have a material impact on the Company’s
consolidated financial statements.
F-19
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
In December 2007, the FASB issued authoritative guidance that
changes the accounting and financial reporting of noncontrolling
ownership interests in subsidiaries held by parties other than
the parent, and the allocation of net income attributable to the
parent and the noncontrolling interest. This guidance also
establishes disclosure requirements to separately identify the
interests of the parent and the interests of the noncontrolling
owners. This guidance became effective for fiscal years
beginning after December 15, 2008. The Company adopted the
provisions of the guidance in the first quarter of 2009. The
adoption changed the presentation format of the Company’s
consolidated statements of operations and equity and
consolidated balance sheets, but did not have an impact on net
earnings or equity attributable to the Company’s
stockholders.
In December 2007, the FASB issued authoritative guidance that
defines collaborative arrangements and requires that
transactions with third parties that do not participate in the
arrangement be reported in the appropriate income statement line
items pursuant to existing authoritative accounting literature.
Income statement classification of payments made between
participants of a collaborative arrangement are to be based on
other applicable authoritative accounting literature. If the
payments are not within the scope or analogy of other
authoritative accounting literature, a reasonable, rational and
consistent accounting policy is to be elected. This guidance
became effective for fiscal years beginning after
December 15, 2008 and was applied as a change in accounting
principle to all prior periods retrospectively for all
collaborative arrangements existing as of the effective date.
The Company adopted the provisions of the guidance in the first
quarter of 2009. The adoption did not have a material impact on
the Company’s consolidated financial statements.
New
Accounting Standards Not Yet Adopted
In January 2010, the FASB issued new accounting guidance related
to the disclosure requirements for fair value measurements and
provides clarification for existing disclosures requirements.
More specifically, this update will require (a) an entity
to disclose separately the amounts of significant transfers in
and out of Levels 1 and 2 fair value measurements and to
describe the reasons for the transfers; and (b) information
about purchases, sales, issuances and settlements to be
presented separately (i.e. present the activity on a gross basis
rather than net) in the reconciliation for fair value
measurements using significant unobservable inputs (Level 3
inputs). This guidance clarifies existing disclosure
requirements for the level of disaggregation used for classes of
assets and liabilities measured at fair value and requires
disclosures about the valuation techniques and inputs used to
measure fair value for both recurring and nonrecurring fair
value measurements using Level 2 and Level 3 inputs.
The new disclosures and clarifications of existing disclosure
are effective for fiscal years beginning after December 15,
2009, except for the disclosure requirements for related to the
purchases, sales, issuances and settlements in the rollforward
activity of Level 3 fair value measurements. Those
disclosure requirements are effective for fiscal years ending
after December 31, 2010. The Company has not yet evaluated
the potential impact of adopting this guidance on the
Company’s consolidated financial statements.
In October 2009, the FASB issued an accounting standards update
that requires an entity to allocate arrangement consideration at
the inception of an arrangement to all of its deliverables based
on their relative selling prices, eliminates the use of the
residual method of allocation, and requires the
relative-selling-price method in all circumstances in which an
entity recognizes revenue of an arrangement with multiple
deliverables. This guidance will be effective for revenue
arrangements entered into or materially modified in fiscal years
beginning on or after June 15, 2010, which will be the
Company’s fiscal year 2011, with earlier application
permitted. The Company has not yet evaluated the potential
impact of adopting this guidance on the Company’s
consolidated financial statements.
In June 2009, the FASB issued authoritative guidance that
requires an enterprise to perform an analysis to determine
whether the enterprise’s variable interest or interests
give it a controlling financial interest in a variable interest
entity. This analysis identifies the primary beneficiary of a
variable interest entity as the enterprise that has both the
power to direct the activities of a variable interest entity
that most significantly impact the entity’s economic
performance, and the obligation to absorb losses or the right to
receive benefits of the entity that could
F-20
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
potentially be significant to the variable interest entity. This
guidance also requires ongoing reassessments of whether an
enterprise is the primary beneficiary of a variable interest
entity and eliminates the quantitative approach previously
required for determining the primary beneficiary. This guidance
will be effective for fiscal years beginning after
November 15, 2009, which will be the Company’s fiscal
year 2010. The Company does not expect that the adoption of the
guidance will have a material impact on the Company’s
consolidated financial statements.
|
|
|
4.
|
Commercial
and Development Drug Products
|
We currently market two products in the United States, Zevalin
and Fusilev. In addition, we have several products in clinical
development, primarily including apaziquone (EOquin) which has
completed patient enrollment for two Phase 3 clinical trials for
bladder cancer and has one multiple instillation study under
development and belinostat, a drug we recently partnered with
TopoTarget A/S to jointly develop. Belinostat is being studied
under a Special Protocol Assessment (SPA), in a Phase 2 trial
for relapsed or refractory Peripheral
T-Cell
Lymphoma (PTCL). The following is a brief description of our key
products as of December 31, 2009.
Zevalin: Zevalin is a prescribed form
of cancer therapy called radioimmunotherapy. Radioimmunotherapy
combines a source of radiation, called a radioisotope, with an
antibody.
During the year ended December 31, 2009 and 2008, we
recorded net revenues of $15.7 million and
$0.3 million, respectively from sales of Zevalin.
In December 2008, we partnered with Cell Therapeutics, Inc.
(CTI) to form a
50-50 owned
joint venture, RIT Oncology, LLC (RIT) to commercialize and
develop Zevalin, a CD20-directed radiotherapeutic antibody, in
the United States. We paid $15 million for our 50% interest
in RIT. Pursuant to provisions of the 2008 joint-venture
agreement, in March 2009, we acquired the remaining 50%
ownership of RIT for $16.5 million, resulting in RIT
becoming our wholly-owned subsidiary. In April 2009, we disputed
payment of an installment of $3.5 million of the
$16.5 million, on the grounds that CTI’s unpaid
liabilities pertaining to Zevalin, and CTI’s share of joint
venture expenses equaled or exceeded the installment amount. In
May 2009, we received an arbitration award of approximately
$4.3 million. The entire $3.5 million was released to
us and CTI additionally paid us approximately $0.8 million.
The award was final, binding and non-appealable by either party.
The assets contributed by CTI to RIT were all of its interests
in the Zevalin business, which included the following:
(i) assets acquired in the December 2007 agreement with
Biogen, which included the U.S. development, sales and
marketing rights to Zevalin. The assets acquired included the
Zevalin FDA registration, FDA dossier, U.S. trademark,
trade name and trade dress, customer list, certain patents and
the assignment of numerous contracts. There was no continuity of
physical facilities or personnel from the December 2007
transaction; (ii) assets acquired in the June 2008 Access
Agreement with Bayer Schering Pharma AG, which holds the rights
to Zevalin outside of the United States. Under the agreement,
Bayer gave CTI access to data from Bayer’s phase 3
first-line indolent trial (FIT Trial), of Zevalin; and
(iii) CTI’s September 30, 2008 submission of the
Zevalin sBLA for use in first-line consolidation therapy for
patients with B-cell follicular NHL. The joint venture also
assumed obligations of $2.2 million in current liabilities
and certain contingent obligations.
F-21
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
The allocation of the initial capitalization of the joint
venture, detailed below, was based on the relative fair values
of the intangible assets acquired, as determined by an
independent valuation consultant, and the obligations assumed by
the joint venture.
| |
|
|
|
|
|
|
|
|
|
Developed technology
|
|
|
|
|
|
$
|
23,100
|
|
|
Core technology
|
|
|
|
|
|
|
14,100
|
|
|
Acquired in-process research and development
|
|
|
|
|
|
|
4,700
|
|
|
Assumed obligation to pay Biogen
|
|
|
|
|
|
|
(2,200
|
)
|
|
Acquisition transaction costs
|
|
|
|
|
|
|
(902
|
)
|
|
Fair value of assumed contingent obligations
|
|
$
|
12,500
|
|
|
|
|
|
|
Less: Limitation based on excess of values of intangibles
acquired over initial capitalization
|
|
|
(1,898
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Maximum amount available
|
|
$
|
10,602
|
|
|
|
|
|
|
Contingent obligations, restricted out of $10,602 as recorded
|
|
|
|
|
|
|
(8,798
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total initial capitalization of joint venture
|
|
|
|
|
|
$
|
30,000
|
|
|
|
|
|
|
|
|
|
|
|
The total fair value of developed and core assets equals
$37.2 million. The developed technology asset relates to
intellectual property and rights thereon related to Zevalin as
approved by the FDA for relapsed or refractory, low-grade or
follicular B-cell NHL. The core technology asset represents the
value of the intellectual property and rights therein expected
to be leveraged in the development of label expansions for
Zevalin. Developed and core technologies are amortized over the
term of the patents related to such technologies. Identifiable
intangible assets with definite lives are amortized on a
straight-line basis over their estimated useful lives. The
developed and core technology assets will be amortized over
10 years, or approximately $3.7 million annually
through 2018. In addition, during 2008 an amount of
$4.7 million of IPR&D for a medical indication still
awaiting approval by the FDA was recorded to operating expenses.
Such amount was completely written off during the year ended
December 31, 2008. Amortization expense expected to be
recorded over the next five years is approximately
$18.5 million. In process research and development
(IPR&D) for RIT was evaluated utilizing the present value
of the estimated after- tax cash flows expected to be generated
by purchased undeveloped technology related to the Zevalin
business or label expansions for indications that have not been
approved by the FDA. Since, at the effective time of the
transaction establishing RIT, the IPR&D had not reached
technological feasibility, such amount was charged to operations
for the year ended December 31, 2008 as of the formation
date of RIT. The March 2009 50% acquisition of the
non-controlling interest in RIT included a premium of
$1.8 million, including certain acquisition related costs,
was charged to additional paid in capital in accordance with ASC
810, “Consolidation.”
The RIT transaction involved contingent consideration, therefore
we recognized $8.8 million as a Zevalin related contingent
obligation on the balance sheet, which is equal to the excess of
the fair value of the intangible assets over the initial
capitalization, and is less than the approximately
$12.5 million fair value of the contingent consideration,
as determined by the independent valuation consultant. Certain
contingencies were resolved during 2009 and $8.5 million of
the contingent consideration payable was charged to the recorded
amount, which reduced contingent liabilities to approximately
$0.3 million at December 31, 2009.
In December 2008, the United States Food and Drug Administration
(FDA) had accepted for filing and review, and granted priority
review status for a supplemental Biologics License Application
(sBLA) for the use of Zevalin as part of a first-line therapy
for patients with previously untreated follicular
non-Hodgkin’s lymphoma (NHL). The sBLA application was
approved by the FDA on September 3, 2009, which now allows
the use of Zevalin for a substantially larger patient
population. Zevalin is now FDA approved and marketed by Spectrum
for treatment of patients with previously untreated follicular
NHL who achieve a partial or complete response to chemotherapy
and with relapsed or refractory, low-grade or follicular B-cell
NHL, including patients who have rituximab-refractory follicular
NHL. In connection with the FDA approval, we became obligated to
pay $8.5 million in milestone
F-22
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
payments. Such amount was included in accrued liabilities as of
September 30, 2009 and paid in October 2009. In November
2009, the Centers for Medicare & Medicaid Services
(CMS) finalized a policy to allow reimbursement for
Zevalin®,
in the Hospital Outpatient Prospective Payment System, based on
the Average Sales Price (ASP) methodology applicable to other
injectable drugs and biologicals. This reimbursement methodology
will go into effect on January 1, 2010.
Fusilev for Injection: Fusilev is the
only commercially available drug containing only the pure active
L-isomer of racemic (L and R forms) leucovorin. Fusilev is
currently indicated after high-dose methotrexate therapy in
patients with osteosarcoma, and to diminish the toxicity and
counteract the effects of impaired methotrexate elimination or
inadvertent overdose of folic acid antagonists.
We commercially launched Fusilev in August 2008 and recorded net
revenues of approximately $12.5 million and
$7.7 million from Fusilev sales for the year ended
December 31, 2009 and 2008 respectively.
In April 2006, we acquired all of the oncology drug assets of
Targent, Inc. The principal asset in the transaction was a
license agreement to market Fusilev in the field of oncology in
North America. We paid an up-front fee in common stock, with a
fair market value of approximately $2.7 million, and are
contingently obligated to pay additional amounts based upon
achievement of milestones. At our option, cash payments for
milestones specified in the agreement may be paid in shares of
the Company’s common stock having a value determined as
provided in the asset purchase agreement, equal to the cash
payment amount. In 2009, 2008 and 2007, we recorded stock-based
research and development charges of $185,000, $305,000 and
$520,000, respectively, which represents the fair market value
of 125,000 shares of our common stock issued at each of
October 2007 and March 2008 as milestone payments to Targent,
LLC.
Apaziquone
(EOquin®): Apaziquone,
a synthetic drug which is activated by certain enzymes present
in higher amounts in cancer cells than in normal tissues, is
currently being developed for non-muscle invasive bladder cancer.
In October 2008, we signed an exclusive development and
commercialization collaboration agreement with Allergan for
apaziquone. Under the terms of the agreement, Allergan paid us
an up-front non-refundable $41.5 million at closing and
will make additional payments of up to $304 million based
on the achievement of certain development, regulatory and
commercialization milestones. We retained exclusive rights to
apaziquone in Asia, including Japan and China. Allergan received
exclusive rights to apaziquone for the treatment of bladder
cancer in the rest of the world, including the United States,
Canada and Europe.
In the United States, Allergan and we will co-promote apaziquone
and share equally in its profits and expenses. Allergan will
also pay us royalties on all of its apaziquone sales outside of
the United States. Under the terms of the agreement, we will
continue to conduct the development program, including the
manufacture of clinical supplies and the conduct of the current
and future phase 3 clinical trials, and will be jointly
responsible for obtaining regulatory approval for the product.
Both parties share development expenses with Allergan bearing
65% of the cost. Pursuant to our revenue recognition policy, we
expect that we will recognize the up front payment of
$41.5 million over the period of the development work,
estimated at 4 to 5 years. As of December 31, 2009 and
2008, we have classified $8.3 million of such amount
recorded on the consolidated balance sheet as current portion of
deferred revenue.
In December 2009, we completed enrollment of our two Phase 3
pivotal clinical trials enrolling more than 1,600 patients
with non-muscle invasive bladder cancer. As per the
collaboration agreement with Allergan, Spectrum recorded a
$1.5 million milestone payment from Allergan. Such amount
was received in January 2010.
We also have the right, in our sole discretion, to opt-out of
the co-promotion agreement before January 1, 2012. If we do
so, our share of any future development costs shall be
significantly reduced. Part of the aggregate development costs
and marketing expenses incurred by us since January 1, 2009
shall be reimbursed by Allergan in the form of a one-time
payment. The co-promotion agreement will terminate and instead
of a sharing of profit and
F-23
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
expenses, Allergan will pay us royalties on a percentage of net
sales of the apaziquone in the United States that are slightly
greater than the royalties paid on net sales outside the United
States. In addition, Allergan will pay us up to
$245 million in additional milestones based upon the
achievement of certain sales milestones in the United States.
In October 2008, we terminated our 2001 license agreement for
apaziquone with INC
Research®,
formerly NDDO Research Foundation (INC) in the Netherlands, as
the patents underlying the agreement were all about to expire.
Pursuant to the termination, INC assigned to us all rights it
had in the know-how or intellectual property licensed under the
agreement and all rights in may have had in any know-how or
intellectual property created during the term of the agreement.
In exchange we paid INC a nominal amount of cash and issued them
a nominal number of shares of our common stock. In addition, INC
is entitled to up to 25,000 additional shares of our common
stock and an additional payment of $300,000 upon achievement of
certain regulatory milestones.
In November 2009, we entered into a collaboration agreement with
Nippon Kayaku Co., LTD. for the development and
commercialization of apaziquone in Asia, except North and South
Korea (Nippon Kayaku Territory). In exchange, Nippon Kayaku is
required to pay Spectrum an up-front payment of
$15.0 million, which was received in January 2010, and
agreed to make additional payments of up to $136.0 million
based on the achievement of certain regulatory and
commercialization milestones. Nippon Kayaku received exclusive
rights to apaziquone for the treatment of non-muscle invasive
bladder cancer in Asia, including Japan and China. Under the
terms of the Nippon Kayaku collaboration agreement, Nippon
Kayaku will conduct the apaziquone clinical trials pursuant to a
development plan. In addition, Nippon Kayaku will be responsible
for all expenses relating to the development and
commercialization of apaziquone in the Nippon Kayaku Territory.
Also in November 2009, we entered into collaboration agreement
with Handok Pharmaceuticals of Korea for the development and
commercialization of apaziquone for the treatment of non-muscle
invasive bladder cancer in North and South Korea. Under the
terms of the Handok collaboration agreement, Handok is required
to pay us an up-front payment of $1.0 million, which was
received in January 2010, and potential milestone payments
totaling approximately $19 million. The potential
milestones will be based on the achievement of certain
regulatory and commercialization milestones. Additionally,
Handok will conduct the apaziquone clinical trials pursuant to a
development plan and will be responsible for all expenses
relating to the development and commercialization of apaziquone
in North and South Korea.
RenaZorb®: RenaZorb,
a second-generation lanthanum-based nanoparticle phosphate
binding agent, has the potential to treat hyperphosphatemia,
(high phosphate levels in blood), in patients with stage 5
chronic kidney disease (end-stage renal disease).
Hyperphosphatemia affects patients with chronic kidney disease,
especially end-stage kidney disease patients on dialysis. It can
lead to significant bone disease (including pain and fractures)
and cardiovascular disease, and is independently associated with
increased mortality.
In August 2009, we acquired 100% of the rights to RenaZorb and
Renalan®,
lanthanum-based nanotechnology compounds with potent and
selective phosphate binding properties, for all uses pursuant to
an amended and restated agreement that we entered into with
Altair Nanomaterials, Inc. and Altair Nanotechnologies. In 2005,
the Company had acquired the worldwide license from Altair to
develop and commercialize Altair’s lanthanum-based
nanotechnology compounds and related technology or all human
therapeutic uses. The August 2009 acquisition expanded the
worldwide, exclusive license to include all uses. In conjunction
with the expanded license, Altair assigned all intellectual
property associated with RenaZorb (associated with human uses),
Renalan®
(associated with animal or veterinarian use), its
lanthanum-based nanotechnology and all of its other life
sciences research and development to us. In consideration, we
issued 113,809 shares of our common stock, with a then fair
value of approximately $750,000, which was recorded to research
ad development in the consolidated statement of operations in
2009. We are responsible for all development, commercialization
and intellectual property costs that accrue after the August
2009 execution date for the amended and restated agreement.
Ozarelix: Ozarelix, a LHRH (Luteinizing
Hormone Releasing Hormone, also known as GnRH or Gonadotropin
Releasing Hormone) antagonist (a substance that blocks the
effects of a natural hormone found in the
F-24
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
body) is currently being investigated for its targeted
indications in hormone dependent prostate cancer, and
endometriosis.
In January 2010, subsequent to the close of the year, we
terminated a multi-center, randomized, double-blind,
placebo-controlled study to evaluate the efficacy of ozarelix
compared to placebo in the treatment of lower urinary tract
symptoms (LUTS) secondary to BPH in men. Currently, we are
considering the future development of ozarelix.
|
|
|
5.
|
Cash and
Cash Equivalents and Marketable Securities
|
Cash and cash equivalents, and investments in marketable
securities, including long term bank certificates of deposits,
totaled $124.8 million and $78.3 million as of
December 31, 2009 and 2008, respectively. The following is
a summary of such investments (in thousands):
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross
|
|
|
Gross
|
|
|
Estimated
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortized
|
|
|
Unrealized
|
|
|
Unrealized
|
|
|
Fair
|
|
|
|
|
|
Marketable Securities
|
|
|
|
|
Cost
|
|
|
Gains
|
|
|
Losses
|
|
|
Value
|
|
|
Cash
|
|
|
Current
|
|
|
Long Term
|
|
|
|
|
December 31, 2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
82,336
|
|
|
|
—
|
|
|
|
—
|
|
|
$
|
82,336
|
|
|
$
|
82,336
|
|
|
|
—
|
|
|
|
—
|
|
|
Bank certificates of deposit
|
|
|
20,948
|
|
|
|
—
|
|
|
|
—
|
|
|
|
20,948
|
|
|
|
—
|
|
|
$
|
12,260
|
|
|
$
|
8,688
|
|
|
Money Market Currency Funds
|
|
|
4,800
|
|
|
|
—
|
|
|
|
—
|
|
|
|
4,800
|
|
|
|
—
|
|
|
|
4,800
|
|
|
|
—
|
|
|
U.S. Government securities
|
|
|
16,542
|
|
|
|
—
|
|
|
|
—
|
|
|
|
16,542
|
|
|
|
—
|
|
|
|
13,792
|
|
|
|
2,750
|
|
|
Corporate debt securities
|
|
|
153
|
|
|
|
—
|
|
|
|
—
|
|
|
|
153
|
|
|
|
—
|
|
|
|
153
|
|
|
|
—
|
|
|
Other securities (included in other assets)
|
|
|
47
|
|
|
|
—
|
|
|
|
12
|
|
|
|
35
|
|
|
|
—
|
|
|
|
—
|
|
|
|
35
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total investments
|
|
$
|
124,826
|
|
|
$
|
—
|
|
|
$
|
12
|
|
|
$
|
124,814
|
|
|
$
|
82,336
|
|
|
$
|
31,005
|
|
|
$
|
11,473
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
9,860
|
|
|
|
—
|
|
|
|
—
|
|
|
$
|
9,860
|
|
|
$
|
9,860
|
|
|
|
—
|
|
|
|
—
|
|
|
Bank certificates of deposit
|
|
|
10,509
|
|
|
|
—
|
|
|
|
—
|
|
|
|
10,509
|
|
|
|
—
|
|
|
$
|
10,319
|
|
|
|
190
|
|
|
Money Market Currency Funds
|
|
|
128
|
|
|
|
—
|
|
|
|
—
|
|
|
|
128
|
|
|
|
—
|
|
|
|
128
|
|
|
|
—
|
|
|
U.S. Government securities
|
|
|
55,867
|
|
|
|
—
|
|
|
|
—
|
|
|
|
55,867
|
|
|
|
—
|
|
|
|
55,867
|
|
|
|
—
|
|
|
Corporate debt securities
|
|
|
2,000
|
|
|
|
—
|
|
|
|
88
|
|
|
|
1,912
|
|
|
|
—
|
|
|
|
1,912
|
|
|
|
—
|
|
|
Other securities (included in other assets)
|
|
|
104
|
|
|
|
—
|
|
|
|
57
|
|
|
|
47
|
|
|
|
—
|
|
|
|
—
|
|
|
|
47
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total investments
|
|
$
|
78,468
|
|
|
$
|
—
|
|
|
$
|
145
|
|
|
$
|
78,323
|
|
|
$
|
9,860
|
|
|
$
|
68,226
|
|
|
$
|
237
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
“Available-for-sale”
marketable securities are carried at fair value, with any
unrealized gains and losses included as a component of
accumulated other comprehensive income (loss) in
stockholders’ equity. Realized gains and losses and
declines in value judged to be
other-than-temporary,
as well as interest income and dividends on investments, are
included in other income and expense. We have classified
$11.4 million of our investments with maturity dates over
1 year from December 31, 2009 as long term based on
held to maturity.
|
|
|
6.
|
Accounts
Receivables, Related Allowances and Revenues
|
Our product sales are concentrated in a limited number of
customers. For the year ended December 31, 2009,
approximately 44% of our Fusilev product sales were derived from
specialty distributors of oncology products as compared to 100%
for the year ended 2008; for Zevalin, we accorded 21% of
revenues from radio pharmacies as
F-25
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
compared to 0% for the years ended December 31, 2009 and
2008, respectively; and the balance from end use customers. For
Zevalin, not a single end user customer constituted revenues
over 10% individually. Due to changes in market dynamics, these
ratios are not indicative of future concentrations. As of
December 31, 2009, for Fusilev, not a single specialty
distributor owed us more than 10% of the total net accounts
receivables. Three specialty distributors owned us 100% of
receivables at the end of 2008. For Zevalin, no single end user
customer owed us more than 10% of net receivables as of
December 31, 2009 or 2008. All sales were to customers in
the United States.
For Fusilev, we utilize a third-party logistics company to store
and distribute this drug product. The same third party logistics
company also stores and ships Zevalin kits containing the CD20
MAB.
During 2009, we changed the supply and distribution model for
Zevalin. Previously, we sold Zevalin kits containing the CD20
MAB to radiopharmacies, who in turn ordered the radioactive
isotope (Y-90 or In-111) separately and radiolabeled (or
attached) the radioactive isotope to the CD20 MAB. The
radiopharmacy then sold the end user product to the consumer.
Under the current model we do not sell the Zevalin kits
containing the CD20 MAB to the radiopharmacies, but instead
contract with them, as a
fee-for-service,
to radiolabel the individual components of the CD20 MAB to the
radioactive isotope, and then, also under a
fee-for-service
arrangement, have them distribute the end use product to the end
user; the clinics, hospitals or other medical settings. In this
regard, we now sell the CD20 MAB together with the radioactive
isotope as the end user product.
Accounts receivable, net of allowances for doubtful accounts,
consisted of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
($ in ‘000’s)
|
|
|
|
|
Accounts receivables gross
|
|
$
|
8,808
|
|
|
$
|
9,926
|
|
|
Allowances for doubtful accounts
|
|
|
(150
|
)
|
|
|
(150
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivables net of allowances
|
|
$
|
8,658
|
|
|
$
|
9,776
|
|
|
|
|
|
|
|
|
|
|
|
Allowances for chargebacks, discounts and rebates and returns as
of December 31, 2009 and 2008 are recorded as a part of
other accrued liabilities on the balance sheet. Allowances thus
recorded consisted of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
($ in ‘000’s)
|
|
|
|
|
Allowances for discounts, chargebacks and rebates
|
|
$
|
860
|
|
|
$
|
1,631
|
|
|
Allowances for returns
|
|
|
1,176
|
|
|
|
3,143
|
|
|
|
|
|
|
|
|
|
|
|
|
Total allowances
|
|
$
|
2,036
|
|
|
$
|
4,774
|
|
|
|
|
|
|
|
|
|
|
|
Shipments of Fusilev for the year ended December 31, 2008
were approximately $10.8 million (net of estimates for
promotional, price and other adjustments). We deferred the
recognition of approximately $3.1 million of such revenue
to allow for potential sales returns. In 2009, based on our
evaluation of return history to date combined with inventory
held by distributors, sell through data of distributor sales to
end users, customer and end-user ordering and re-ordering
patterns, aging of accounts receivables, rates of returns for
directly substitutable products and other pharmaceutical
products for the treatment of therapeutic areas similar to
indications served by our products, shelf life of our products
and the extensive experience of our management with selling the
same and similar oncology products, we reduced the reserve to
approximately $1.2 million. No returns reserve is recorded
for Zevalin since we invoice our end user customers and
recognize revenues only when a patient is treated with Zevalin.
F-26
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
As of December 31, 2009 and 2008, inventories, net, consisted of
the following:
| |
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
($ in ‘000’s)
|
|
|
|
|
Finished Goods
|
|
$
|
3,039
|
|
|
$
|
1,492
|
|
|
Work In Process
|
|
|
—
|
|
|
|
312
|
|
|
Raw Materials
|
|
|
280
|
|
|
|
68
|
|
|
Less: reserve for obsolescence
|
|
|
(89
|
)
|
|
|
(31
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
3,230
|
|
|
$
|
1,841
|
|
|
|
|
|
|
|
|
|
|
|
We continually review product inventories on hand, evaluating
inventory levels relative to product demand, remaining shelf
life, future marketing plans and other factors, and reserves for
obsolete and slow-moving inventories are recorded for amounts
which may not be realizable.
|
|
|
8.
|
Property
and Equipment
|
As of December 31, 2009 and 2008, property and equipment
consisted of:
| |
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
($ in 000’s)
|
|
|
|
|
Equipment
|
|
$
|
2,762
|
|
|
$
|
2,286
|
|
|
Leasehold improvements
|
|
|
1,255
|
|
|
|
1,255
|
|
|
|
|
|
|
|
|
|
|
|
|
Total property and equipment
|
|
|
4,017
|
|
|
|
3,541
|
|
|
Less: accumulated depreciation and amortization
|
|
|
(2,089
|
)
|
|
|
(1,759
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment, net
|
|
$
|
1,928
|
|
|
$
|
1,782
|
|
|
|
|
|
|
|
|
|
|
|
For the years ended December 31, 2009, 2008 and 2007, the
Company recorded depreciation expense of approximately $527,000,
$452,000 and $255,000, respectively.
Significant components of the income tax expense for each of the
three years ended December 31, 2009 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
(Amounts in thousands)
|
|
|
|
|
Current:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal
|
|
$
|
78
|
|
|
|
|
|
|
|
|
|
|
State
|
|
$
|
343
|
|
|
$
|
5
|
|
|
$
|
5
|
|
|
Foreign
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
421
|
|
|
$
|
5
|
|
|
$
|
5
|
|
|
Deferred:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
State
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Foreign
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Provision
|
|
$
|
421
|
|
|
$
|
5
|
|
|
$
|
5
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-27
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
The income tax provision differs from that computed using the
federal statutory rate applied to income before taxes as follows
(in thousands):
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
|
|
|
(As Restated)
|
|
|
(As Restated)
|
|
|
|
|
(Amounts in thousands)
|
|
|
|
|
Tax benefit computed at the federal statutory rate
|
|
$
|
(6,697
|
)
|
|
$
|
(6,086
|
)
|
|
$
|
(8,548
|
)
|
|
State tax, net of federal benefit
|
|
|
(981
|
)
|
|
|
(1,039
|
)
|
|
|
(1,462
|
)
|
|
Expired Tax Attributes
|
|
|
8,097
|
|
|
|
—
|
|
|
|
—
|
|
|
Credits
|
|
|
(1,644
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Common stock warrant liability
|
|
|
(2,745
|
)
|
|
|
(432
|
)
|
|
|
(4,099
|
)
|
|
Permanent items and other
|
|
|
796
|
|
|
|
—
|
|
|
|
—
|
|
|
Valuation allowance
|
|
|
3,595
|
|
|
|
7,562
|
|
|
|
14,114
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax provision
|
|
$
|
421
|
|
|
$
|
5
|
|
|
$
|
5
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Significant components of the Company’s deferred tax assets
as of December 31, 2009 and 2008 are shown below. A
valuation allowance has been recognized to offset the net
deferred tax assets as realization of such deferred tax assets
has not met the more likely than not threshold.
| |
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
|
|
|
(As Restated)
|
|
|
|
|
(Amounts in thousands)
|
|
|
|
|
Deferred tax assets:
|
|
|
|
|
|
|
|
|
|
Net operating loss carryforwards
|
|
$
|
58,597
|
|
|
$
|
70,468
|
|
|
Research Credits
|
|
|
10,230
|
|
|
|
9,468
|
|
|
Stock Compensation
|
|
|
2,641
|
|
|
|
2,755
|
|
|
Deferred Revenue
|
|
|
12,839
|
|
|
|
—
|
|
|
Depreciation and amortization differences
|
|
|
1,466
|
|
|
|
698
|
|
|
Other, Net
|
|
|
1,211
|
|
|
|
—
|
|
|
Valuation Allowance
|
|
|
(86,984
|
)
|
|
|
(83,389
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, 2009, the Company has federal and state net
operating loss carryforwards of approximately
$153.2 million and $94.1 million, respectively. The
Company has approximately $2.5 million of foreign loss
carryforwards that begin to expire in 2010. The federal and
state loss carryforwards begin to expire in 2018 and 2012,
respectively, unless previously utilized. At December 31,
2009, the Company has federal and state research and development
tax credits of approximately $10 million and
$0.2 million, respectively. The federal research tax credit
begins to expire in 2010 unless previously utilized.
The utilization of the net operating loss and research and
development tax credit carryforwards is subject to an annual
limitation under Sections 382 and 383 of the Internal
Revenue Code of 1986, and similar state tax provisions due to
the amount of the net operating loss and research and
development tax credits carryforwards and other deferred tax
assets that can be utilized to offset future taxable income and
tax, respectively. In general, an ownership change, as defined
by Sections 382 and 383, results form transactions
increasing ownership of certain stockholders or public groups in
the stock of the corporation by more than 50 percentage
points over a three-year period. An analysis was performed which
indicated that multiple ownership changes have occurred in
previous years which created an annual limitation on the
Company’s ability to utilize its net operating loss and
research and development tax carryovers.
F-28
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
The Company has not completed a study to assess whether an
ownership change has occurred. If the Company has experienced an
ownership change, utilization of the NOL or R&D credit
carryforwards would be subject to an annual limitation under
Section 382 of the Code, which is determined by first
multiplying the value of the Company’s stock at the time of
the ownership change by the applicable long-term, tax-exempt
rate, and then could be subject to additional adjustments, as
required. Any limitation may result in expiration of a portion
of the NOL or R&D credit carryforwards before utilization.
Further, until a study is completed and any limitation is known,
no amounts are being considered as an uncertain tax position or
disclosed as an unrecognized tax benefit under FIN 48. Due
to the existence of the valuation allowance, future changes in
the Company’s unrecognized tax benefits will not impact its
effective tax rate. Any carryforwards that will expire prior to
utilization as a result of such limitations will be removed from
deferred tax assets with a corresponding reduction of the
valuation allowance.
The Company is subject to the accounting guidance for uncertain
income tax positions as of January 1, 2007. The Company
believes that is income tax filing positions and deductions will
be sustained on audit and does not anticipate any adjustments
that will result in a material adverse effect on the
Company’s financial condition, results of operations, or
cash flow. Therefore, no reserves for uncertain income tax
positions have been recorded pursuant to the accounting guidance.
Management does not believe that the amounts of unrecognized tax
benefits will increase within the next twelve months. With a few
exceptions, the Company is no longer subject to U.S. federal,
state and local income tax examinations for years before 2005.
The Company policy is to recognize interest and/or penalties
related to unrecognized tax benefits in income tax expense.
|
|
|
10.
|
Commitments
and Contingencies
|
Facility
and Equipment Leases
The Company leases certain facilities and office equipment. As
of December 31, 2009, we primarily had obligations under a
facility lease in Irvine, California, which expires in
June 30, 2016, an office lease in Henderson, Nevada, which
expires in September 2011, and various operating and capital
equipment leases.
Minimum lease requirements for each of the next five years and
thereafter, under the property and equipment operating leases,
are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
Lease
|
|
|
Capital Lease
|
|
|
|
|
Commitments
|
|
|
Commitments
|
|
|
|
|
($ in ‘000’s)
|
|
|
|
|
Year ending December 31:
|
|
|
|
|
|
|
|
|
|
2010
|
|
$
|
428
|
|
|
$
|
50
|
|
|
2011
|
|
|
455
|
|
|
|
50
|
|
|
2012
|
|
|
484
|
|
|
|
47
|
|
|
2013
|
|
|
513
|
|
|
|
—
|
|
|
2014
|
|
|
542
|
|
|
|
—
|
|
|
Thereafter
|
|
|
863
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
3,285
|
|
|
$
|
147
|
|
|
|
|
|
|
|
|
|
|
|
Rent expense for the years ended December 31, 2009, 2008
and 2007 was approximately $593,000, $583,000 and $579,000,
respectively.
Licensing
Agreements
Almost all of our drug candidates are being developed pursuant
to license agreements that provide us with rights to certain
territories to, among other things, develop, sublicense, and
sell the drugs. We are required to use
F-29
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
commercially reasonable efforts to develop the drugs, are
generally responsible for all development, patent filing and
maintenance costs, sales, marketing and liability insurance
costs, and are generally contingently obligated to make
milestone payments to the licensors if we successfully reach
development and regulatory milestones specified in the
agreements. In addition, we are obligated to pay royalties and,
in some cases, milestone payments based on net sales, if any,
after marketing approval is obtained from regulatory authorities.
The potential contingent development and regulatory milestone
obligations under all our licensing agreements are generally
tied to progress through the FDA approval process, which
approval significantly depends on positive clinical trial
results. The following represents typical milestone events for
the Company: conclusion of Phase 2 or commencement of Phase 3
clinical trials; filing of new drug applications in each of the
United States, Europe and Asia; and approvals from each of the
regulatory agencies in those jurisdictions.
Given the uncertainty of the drug development and regulatory
approval process, we are unable to predict with any certainty
when any of the milestones will occur, if at all. Accordingly,
the milestone payments represent contingent obligations that
will be recorded as expense when the milestone is achieved.
While it is difficult to predict when milestones will be
achieved, we estimate that if all of our contingent milestones
are successfully achieved within our anticipated timelines, our
potential contingent cash development and regulatory milestone
obligations, aggregating to approximately $75.7 million as
of December 31, 2009, would be due approximately as
follows: $0.2 million within 12 months;
$3.5 million in 2 to 3 years; $2.1 million in 4
to 5 years; and $69.9 million after 5 years.
Service
Agreements
In connection with the research and development of our drug
products, we have entered into contracts with numerous third
party service providers, such as clinical trial centers,
clinical research organizations, data monitoring centers, and
with drug formulation, development and testing laboratories. The
financial terms of these agreements are varied and generally
obligate us to pay in stages, depending on achievement of
certain events specified in the agreements, such as contract
execution, reservation of service or production capacity, actual
performance of service, or the successful accrual and dosing of
patients.
At each period end, we accrue for all costs of goods and
services received, with such accruals based on factors such as
estimates of work performed, patient enrollment, completion of
patient studies and other events. As of December 31, 2009,
we were committed under such contracts for up to approximately
$9.0 million, for future goods and services, including
approximately $7.3 million due within one year. We are in a
position to accelerate, slow-down or discontinue any or all of
the projects that we are working on at any given point in time.
Should we decide to discontinue
and/or
slow-down the work on any project, the associated costs for
those projects would be limited to the extent of the work
completed. Generally, we are able to terminate these contracts
due to the discontinuance of the related project(s) and thus
avoid paying for the services that have not yet been rendered
and our future purchase obligations would reduce accordingly.
Supply
Agreements
In connection with our acquisition of Zevalin, RIT Oncology
assumed a supply agreement with Biogen Idec Inc.
(“Biogen”) to manufacture Zevalin for sale in the
United States pursuant to which we would purchase from Biogen,
and Biogen would provide to us, kits to make Zevalin doses for
sale to end-users in the United States at a “cost
plus” manufacturing price. RIT Oncology also assumed a
manufacturing and supply agreement with MDS (Canada) Inc., MDS
Nordion Division, or MDS (Canada), for yttrium-90, a
radioisotope used in connection with the administration of
Zevalin.
In connection with Fusilev, we have a single source API supplier
as well as a single source finished product manufacturer.
F-30
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
Employment
Agreement
We have entered into an employment agreement with
Dr. Shrotriya, our President and Chief Executive Officer,
which expires January 2, 2011. The employment agreement
automatically renews for a one-year calendar term unless either
party gives written notice of such party’s intent not to
renew the agreement at least 90 days prior to the
commencement of the next year. The employment agreement requires
Dr. Shrotriya to devote his full working time and effort to
the business and affairs of the Company during the term of the
agreement. The employment agreement provides for a minimum
annual base salary with annual increases, periodic bonuses and
option grants as determined by the Compensation Committee of the
Board of Directors.
Litigation
At December 31, 2009, we are involved with various legal
matters arising from the ordinary course of business. Although
the ultimate resolution of these various matters cannot be
determined at this time, we do not believe that such matters,
individually or in the aggregate, will have a material adverse
effect on our consolidated results of operations, cash flows or
financial condition.
Authorized
Stock
On July 6, 2006, our stockholders approved an amendment to
our Certificate of Incorporation to increase the authorized
number of shares of our common stock from 50 million shares
to 100 million shares. The amendment was filed with the
Delaware Secretary of State on July 7, 2006. Further, on
July 7, 2006, we amended the Certificate of Designation of
Rights, Preferences and Privileges of Series B Junior
Participating Preferred Stock filed with the Delaware Secretary
of State on December 18, 2000 to increase the authorized
number of Series B Junior Participating Preferred Stock
from 200,000 shares to 1,000,000 shares.
Preferred
Stock
In December 2000, we adopted a stockholder rights plan pursuant
to which we distributed rights to purchase units of our
Series B Junior Participating Preferred Stock
(“Series B Preferred Stock”). Under this plan, as
amended through December 31, 2008, the rights become
exercisable upon the earlier of ten days after a person or group
of affiliated or associated persons has acquired 15% or more of
the outstanding shares of our common stock or ten business days
after a tender offer has commenced that would result in a person
or group beneficially owning 15% or more of our outstanding
common stock. These rights could delay or discourage someone
from acquiring our business, even if doing so would benefit our
stockholders. We currently have no stockholders who own 15% or
more of the outstanding shares of our common stock. Five days
after the rights become exercisable, each right, other than
rights held by the person or group of affiliated persons whose
acquisition of more than 15% of our outstanding common stock
caused the rights to become exercisable, will entitle its holder
to buy, in lieu of shares of Series B Preferred Stock, a
number of shares of our common stock having a market value of
twice the exercise price of the rights. After the rights become
exercisable, if we are a party to certain merger or business
combination transactions or transfers 50% or more of our assets
or earnings power (as defined), each right will entitle its
holder to buy a number of shares of common stock of the
acquiring or surviving entity having a market value of twice the
exercise price of the right. The rights expire on
December 13, 2010 and may be redeemed by us at one-tenth of
one cent per right at any time up to ten days after a person has
announced that they have acquired 15% or more of our outstanding
common stock.
In May 2003, we received gross cash proceeds of $6,000,000 in
exchange for the issuance of 600 shares of our
Series D 8% Cumulative Convertible Voting Preferred Stock
(“Series D Preferred Stock”), convertible into
2,553,191 shares of common stock, and Series D
Warrants, exercisable for five years, to purchase up to a total
of 1,276,595 shares of our common stock at an exercise
price of $3.00 per share and up to a total of
1,276,595 shares of
F-31
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
our common stock at an exercise price of $3.50 per share. As of
December 31, 2007, all Series D Preferred Stock had
been converted to common stock. Dividends on the Series D
Preferred Stock were payable quarterly at an annual rate of 8%
either in cash or shares of our common stock at our discretion.
In September 2003, we received gross cash proceeds of
$20,000,000 in exchange for the issuance of 2,000 shares of
our Series E Convertible Voting Preferred Stock
(“Series E Preferred Stock”), convertible into
4,000,000 shares of common stock, and Series E
Warrants, exercisable for five years, to purchase up to a total
of 2,800,000 shares of our common stock at an exercise
price of $6.50 per share. As of December 31, 2009 and 2008,
68 shares of Series E Preferred Stock, convertible
into 136,000 shares of common stock are outstanding. No
dividends are payable on the Series E Preferred Stock.
Pursuant to certain provisions of the Certificate of
Designation, Rights and Preferences of the Series E
Preferred Stock, we have the option to redeem all of the
unconverted Series E Preferred Stock outstanding at the end
of a 20-day
trading period if, among other things, in that period the common
stock of the Company trades above $12.00 per share.
In the event of any voluntary or involuntary liquidation,
dissolution or winding up of the Corporation, before any
distribution of assets of the Corporation shall be made to the
common stockholders, the holders of the Series E Preferred
Stock shall be entitled to receive a liquidation preference in
an amount equal to 120% of the stated value per share plus any
declared and unpaid dividends thereon.
Common
Stock Issuances for Cash
In September 2005, we sold 8,000,000 shares of our common
stock at a purchase price of $5.25 per share, and six year
warrants purchasing up to a total of 4,000,000 shares of
our common stock at an exercise price of $6.62 per share,
for net cash proceeds of approximately $39.3 million after
offering costs of approximately $2.7 million.
In May 2007, we sold 5,134,100 shares of our common stock
at a purchase price of $6.25 per share for net cash proceeds of
approximately $30 million, after placement agent fees and
other offering costs of approximately $2 million. No
warrants were issued in connection with this offering.
On May 6, 2009, we sold an aggregate of 432,200 shares
of common stock to certain of our employees at a purchase price
of $2.70 per share, which was the closing price of our common
stock on May 6, 2009. This offering resulted in gross
proceeds to us of approximately $1.2 million. The investors
in this offering included Dr. Rajesh Shrotriya, M.D.,
our Chairman, President and Chief Executive Officer, and Shyam
Kumaria, our Vice President of Finance. Dr. Shrotriya
purchased 290,000 shares of common stock and
Mr. Kumaria purchased 85,000 shares of common stock.
We decided to conduct this offering with certain of our
employees to allow such employees to invest their personal cash
directly into the Company at the current fair market value of
our stock. The purchase agreements include provisions
prohibiting the investors from disposing of the shares of common
stock purchased in the offering for ninety days. The offering
was approved by the Placement Committee of the Board of
Directors. In addition, the Audit Committee of the Board of
Directors approved the offering pursuant to our Related Party
Transaction Policies and Procedures.
On May 26, 2009, we sold 3,913,895 shares of our
common stock at a purchase price of $5.11 per share for net cash
proceeds of approximately $19 million, after placement
agent fees and other offering costs of approximately
$1 million. In connection with this offering, 1,956,947
common stock warrants exercisable at $5.11 between
November 27, 2009 and February 25, 2010, were issued
to the investors.
On June 15, 2009, we sold 1,715,266 shares of our
common stock at a purchase price of $5.83 per share for net cash
proceeds of approximately $9.5 million, after placement
agent fees and other offering costs of approximately
$0.5 million. In connection with this offering, 857,633
common stock warrants exercisable at $5.83 between
December 15, 2009 and March 15, 2010, were issued to
the investors.
On June 30, 2009, we sold 2,936,037 shares of our
common stock at a purchase price of $7.15 per share for net cash
proceeds of approximately $20 million, after placement
agent fees and other offering costs of approximately
F-32
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
$1 million. In connection with this offering, 1,468,020
common stock warrants exercisable at $7.10 between
December 30, 2009 and March 30, 2010, were issued to
the investors.
On September 18, 2009, we sold 6,622,517 shares of our
common stock at a purchase price of $7.55 per share for net cash
proceeds of approximately $47.5 million, after placement
agent fees and other offering costs of approximately
$2.5 million. In connection with this offering, 2,649,007
common stock warrants exercisable at $7.55 between
March 22, 2010 and June 21, 2010, were issued to the
investors.
All the warrants issued in conjunction with the 2009 financings
were outstanding as of December 31, 2009. Of the 6,931,607
warrants outstanding, 4,282,600 were exercisable as of
December 31, 2009. Subsequent to the close of the year and
before the date of this filing, 4,282,600 of the 6,931,607
warrants issued in conjunction with the 2009 financing expired
and 2,649,007 of the warrants will expire on June 20, 2010
if not exercised.
Other
Equity Transactions
Pursuant to the terms of the April 2006 asset purchase agreement
with Targent, LLC, upon achievement of certain regulatory and
sales milestones Targent is eligible to receive payments in the
form of shares of the Company’s common stock
and/or cash.
At our option, cash payments specified in the agreement may be
paid in shares of the Company’s common stock having a value
determined as provided in the asset purchase agreement, equal to
the cash payment amount. During the three years ended
December 31, 2009, we issued shares of common stock, for
achievement of certain regulatory milestones, as follows. The
fair value of the issued stock was recorded as stock-based
research and development expense for the period in which the
milestone was achieved:
|
|
|
| |
•
|
October 2007: 125,000 shares with a fair value of $520,000.
|
| |
| |
•
|
March 2008: 125,000 shares with a fair value of $305,000.
|
| |
| |
•
|
March 2009: 125,000 shares with a fair value of $185,000.
|
In October 2008, we issued 75,000 shares of the
Company’s common stock in connection with the assignment to
us of certain intellectual property rights related to apaziqone.
The fair value of the stock, $74,000, was recorded as a
stock-based research and development expense for the year ended
December 31, 2008.
In August 2009, we acquired 100% of the rights to
RenaZorb®
and
Renalin®,
lanthanum-based nanotechnology compounds with potent and
selective phosphate binding properties, for all uses pursuant to
an amended and restated agreement that we entered into with
Altair Nanomaterials, Inc. and Altair Nanotechnologies. In 2005,
the Company had acquired the worldwide license from Altair to
develop and commercialize Altair’s lanthanum-based
nanotechnology compounds and related technology for all human
therapeutic uses. The August 2009 acquisition expanded the
worldwide, exclusive license to include all uses. In conjunction
with the expanded license, Altair assigned all intellectual
property associated with
RenaZorb®
(associated with human uses),
Renalin®
(associated with animal or veterinarian use), its
lanthanum-based nanotechnology and all of its other life
sciences research and development to us. In consideration, we
issued 113,809 shares of our common stock, with a then fair
value of approximately $750,000.
Common
Stock Reserved for Future Issuance
As of December 31, 2009, approximately 19.1 million
shares of common stock were issuable upon conversion or exercise
of rights granted under prior financing arrangements, stock
options and warrants, as follows:
| |
|
|
|
|
|
Conversion of Series E preferred shares
|
|
|
136,000
|
|
|
Exercise of stock options
|
|
|
7,945,245
|
|
|
Exercise of warrants
|
|
|
11,028,919
|
|
|
|
|
|
|
|
|
Total shares of common stock reserved for future issuances
|
|
|
19,110,164
|
|
|
|
|
|
|
|
F-33
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
Subsequent to the close of the year and before the date of this
filing, 4,282,600 of the 6,931,607 warrants issued in
conjunction with the 2009 financing expired and 2,649,007 of the
warrants will expire on June 20, 2010 if not exercised.
Warrant
Activity
We typically issue warrants to purchase shares of our common
stock to investors as part of a financing transaction or in
connection with services rendered by placement agents and
consultants. Our outstanding warrants expire on varying dates
through September 2013. Below is a summary of warrant activity
during each of the three years in the period ended
December 31, 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Common
|
|
|
Weighted
|
|
|
Common
|
|
|
Weighted
|
|
|
Common
|
|
|
Weighted
|
|
|
|
|
Stock
|
|
|
Average
|
|
|
Stock
|
|
|
Average
|
|
|
Stock
|
|
|
Average
|
|
|
|
|
Warrants
|
|
|
Exercise Price
|
|
|
Warrants
|
|
|
Exercise Price
|
|
|
Warrants
|
|
|
Exercise Price
|
|
|
|
|
Outstanding at beginning of year
|
|
|
5,444,555
|
|
|
$
|
7.28
|
|
|
|
9,652,051
|
|
|
$
|
6.51
|
|
|
|
9,917,077
|
|
|
$
|
6.71
|
|
|
Granted
|
|
|
6,931,607
|
|
|
|
3.67
|
|
|
|
50,000
|
|
|
|
1.79
|
|
|
|
—
|
|
|
|
—
|
|
|
Repurchased
|
|
|
(95,238
|
)
|
|
|
6.62
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Exercised
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(161,145
|
)
|
|
|
3.22
|
|
|
Forfeited
|
|
|
—
|
|
|
|
—
|
|
|
|
(157,450
|
)
|
|
|
6.62
|
|
|
|
—
|
|
|
|
—
|
|
|
Expired
|
|
|
(1,252,005
|
)
|
|
|
10.03
|
|
|
|
(4,100,046
|
)
|
|
|
5.43
|
|
|
|
(103,881
|
)
|
|
|
30.54
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding, at the end of year
|
|
|
11,028,919
|
|
|
$
|
6.52
|
|
|
|
5,444,555
|
|
|
$
|
7.28
|
|
|
|
9,652,051
|
|
|
$
|
6.51
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable, at the end of year
|
|
|
8,379,912
|
|
|
$
|
6.19
|
|
|
|
5,432,055
|
|
|
$
|
7.29
|
|
|
|
9,572,051
|
|
|
$
|
6.52
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following table summarizes information about warrants
outstanding at December 31, 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants
|
|
Weighted
|
|
Weighted
|
|
Warrants
|
|
Weighted
|
|
|
|
Outstanding
|
|
Average
|
|
Average
|
|
Exercisable
|
|
Average
|
|
Range of Exercise Price
|
|
12/31/2009
|
|
Remaining Life
|
|
Exercise Price
|
|
12/31/2009
|
|
Exercise Price
|
|
|
|
$1.00 - $2.50
|
|
|
50,000
|
|
|
|
3.25
|
|
|
$
|
1.79
|
|
|
|
50,000
|
|
|
$
|
1.79
|
|
|
$5.01 - $6.00
|
|
|
3,114,580
|
|
|
|
0.39
|
|
|
|
5.31
|
|
|
|
3,114,580
|
|
|
|
5.31
|
|
|
$6.01 - $7.00
|
|
|
3,747,312
|
|
|
|
1.71
|
|
|
|
6.62
|
|
|
|
3,747,312
|
|
|
|
6.62
|
|
|
$7.01 - $7.55
|
|
|
4,117,027
|
|
|
|
0.39
|
|
|
|
7.39
|
|
|
|
1,468,020
|
|
|
|
7.10
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
11,028,919
|
|
|
|
|
|
|
$
|
6.52
|
|
|
|
8,379,912
|
|
|
$
|
6.19
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As described above, subsequent to the close of the year and
before the date of this filing, 4,282,600 of the 6,931,607
warrants issued in conjunction with the 2009 financing expired
and 2,649,007 of the warrants will expire on June 20, 2010
if not exercised.
|
|
|
12.
|
Share-Based
Compensation
|
Stock
Options
We have three stock incentive plans: the 1997 Stock Incentive
Plan (the “1997 Plan”) , the 2003 Amended and Restated
Incentive Award Plan (the “2003 Plan”) and the 2009
Incentive Award Plan (the “2009 Plan”) which was
approved by our shareholders in June 2009 (collectively, the
“Plans”). The 2003 Plan authorizes the grant of
incentive awards, including stock options, for the purchase of
up to a total of 10,000,000 shares. Subsequent to the
adoption of the 2009 Plan, no new options have been granted
pursuant the 2003 Plan or 1997 Plan. The Board and
F-34
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
the shareholders approved 10,000,000 shares of common stock
available for issuance under the 2009 Plan. Beginning on
January 1, 2010, and each January 1st thereafter,
the number of shares of common stock available for issuance
under the 2009 Plan shall increase by the greater of
(i) 2,500,000 and (ii) a number of shares such that
the total number of shares of common stock available for
issuance under the Plan shall equal 30% of the then number of
shares of common stock issued and outstanding. As of
December 31, 2009, approximately 9.3 million incentive
awards were available for grant under the 2009 Plan.
During each of the three years in the period ended
December 31, 2009, we granted stock options at exercise
prices equal to or greater than the quoted price of our common
stock on the grant date. The fair value of each option grant was
estimated on the date of grant using the Black-Scholes option
pricing model with the following weighted average assumptions
used for grants in 2009, 2008 and 2007, respectively: risk-free
interest rates of 2.27% (2009), 2.66% (2008) and 4.57%
(2007); zero expected dividend yields; expected lives of
5 years; expected volatility of 72.4% (2009), 65.9%
(2008) and, 68.3% (2007). The risk-free interest rate
assumption is based upon observed interest rates appropriate for
the expected term of the Company’s employee stock options.
The expected volatility is based on the historical volatility of
the Company’s stock. The Company has not paid any dividends
on common stock since its inception and does not anticipate
paying dividends on its common stock in the foreseeable future.
The weighted average fair value of stock options, using the
Black-Scholes option pricing model, that were granted in 2009,
2008 and 2007, was $2,87, $1.19 and $3.54, respectively.
A summary of stock option activity for each of the three years
in the period ended December 31, 2009, is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
Weighted
|
|
|
|
|
Common
|
|
|
Average
|
|
|
Common
|
|
|
Average
|
|
|
Common
|
|
|
Average
|
|
|
|
|
Stock
|
|
|
Exercise
|
|
|
Stock
|
|
|
Exercise
|
|
|
Stock
|
|
|
Exercise
|
|
|
|
|
Options
|
|
|
Price
|
|
|
Options
|
|
|
Price
|
|
|
Options
|
|
|
Price
|
|
|
|
|
Outstanding at beginning of year
|
|
|
7,115,772
|
|
|
$
|
4.80
|
|
|
|
6,482,260
|
|
|
$
|
5.91
|
|
|
|
4,640,252
|
|
|
$
|
5.86
|
|
|
Granted
|
|
|
4,141,000
|
|
|
|
4.70
|
|
|
|
2,148,000
|
|
|
|
2.10
|
|
|
|
1,974,700
|
|
|
|
5.85
|
|
|
Exercised
|
|
|
(488,750
|
)
|
|
|
2.58
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(81,438
|
)
|
|
|
1.48
|
|
|
Forfeited
|
|
|
(551,130
|
)
|
|
|
5.35
|
|
|
|
(294,521
|
)
|
|
|
4.38
|
|
|
|
(39,425
|
)
|
|
|
5.04
|
|
|
Cancelled
|
|
|
(2,165,372
|
)
|
|
|
7.75
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Expired
|
|
|
(106,275
|
)
|
|
|
4.62
|
|
|
|
(1,219,967
|
)
|
|
|
6.08
|
|
|
|
(11,829
|
)
|
|
|
8.80
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding, at end of year
|
|
|
7,945,245
|
|
|
$
|
4.04
|
|
|
|
7,115,772
|
|
|
$
|
4.80
|
|
|
|
6,482,260
|
|
|
$
|
5.91
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable at end of year
|
|
|
4,451,733
|
|
|
$
|
4.06
|
|
|
|
5,097,835
|
|
|
$
|
5.22
|
|
|
|
4,185,273
|
|
|
$
|
5.89
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following table summarizes information about stock options
outstanding under all plans at December 31, 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
Weighted
|
|
|
|
Options
|
|
Weighted
|
|
Average
|
|
Options
|
|
Average
|
|
|
|
Outstanding
|
|
Average
|
|
Exercise
|
|
Exercisable
|
|
Exercise
|
|
Range of Exercise Price
|
|
12/31/09
|
|
Remaining Term
|
|
Price
|
|
12/31/09
|
|
Price
|
|
|
|
|
|
(In years)
|
|
|
|
|
|
|
|
|
|
$1.00 - $2.50
|
|
|
2,250,650
|
|
|
|
8.03
|
|
|
$
|
1.59
|
|
|
|
915,525
|
|
|
$
|
1.54
|
|
|
$2.51 - $5.00
|
|
|
2,500,270
|
|
|
|
7.67
|
|
|
|
3.66
|
|
|
|
1,829,770
|
|
|
|
3.51
|
|
|
$5.01 - $10.00
|
|
|
3,194,325
|
|
|
|
8.10
|
|
|
|
6.06
|
|
|
|
1,706,438
|
|
|
|
6.01
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7,945,245
|
|
|
|
|
|
|
|
|
|
|
|
4,451,733
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Due to our rapid growth over the past few years and a low
personnel turnover rate, in early 2009, we had a limited number
of shares available for future grant under the 2003 Plan.
Primarily in order to increase the pool of
F-35
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
shares available for future grant under such plan, we conducted
a tender offer to eligible employees to acquire options granted
to certain employees of the Company pursuant to the Third
Amended and Restated 1997 Stock Incentive Plan and 2003 Plan,
and which were outstanding at March 23, 2009. Eligible
employees were employees of Spectrum or its subsidiaries who
held options with exercise prices in excess of $5.00. The cash
amount offered to those employees was $0.01 for options with an
exercise price over $10.00 and $1.15 for the options with an
exercise price between $5.00 and $9.99.
On April 23, 2009, a total of 2,165,372 shares
underlying eligible options were tendered by eligible employees
and were accepted by us, representing 73% of the shares
underlying eligible options that were eligible to be tendered in
the offer. We made a cash payment in the aggregate of
approximately $2.5 million to the eligible employees
participating in the offer.
Presented below is the aggregate intrinsic value of the stock
options outstanding, vested and expected to vest, and
exercisable as of December 31, 2009. The intrinsic value
represents the total difference between $4.44, the
Company’s closing common stock price on December 31,
2009, and the exercise price, multiplied by the number of all
in-the-money
options, that would have been received by the option holders had
all option holders exercised their options on December 31,
2009. This amount changes based on the fair market value of the
Company’s common stock.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
Common
|
|
|
Average
|
|
|
Weighted
|
|
|
Aggregate
|
|
|
|
|
Stock
|
|
|
Exercise
|
|
|
Average
|
|
|
Intrinsic
|
|
|
|
|
Options
|
|
|
Price
|
|
|
Remaining Term
|
|
|
Value
|
|
|
|
|
|
|
|
|
|
|
(In years)
|
|
|
(In thousands)
|
|
|
|
|
Stock Options as of December 31, 2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding
|
|
|
7,945,245
|
|
|
$
|
4.04
|
|
|
|
7.97
|
|
|
$
|
8,632
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vested and expected to vest
|
|
|
7,735,634
|
|
|
$
|
4.04
|
|
|
|
7.93
|
|
|
$
|
8,383
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable
|
|
|
4,451,733
|
|
|
$
|
4.06
|
|
|
|
6.94
|
|
|
$
|
4,490
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
During the years ended December 31, 2009, 2008 and 2007,
the share-based charge in connection with the expensing of stock
options was approximately $6.6 million, $5.5 million
and $4.6 million, respectively. As of December 31,
2009, there was $7.0 million of unrecognized share-based
compensation cost related to stock options, which is expected to
be recognized over a weighted average period of 2.5 years.
Restricted
Stock
A summary of the status of the Company’s restricted stock
awards as of December 31, 2009 and of changes in unvested
shares outstanding is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Restricted
|
|
|
Average
|
|
|
Restricted
|
|
|
Average
|
|
|
Restricted
|
|
|
Average
|
|
|
|
|
Stock
|
|
|
Grant date
|
|
|
Stock
|
|
|
Grant date
|
|
|
Stock
|
|
|
Grant date
|
|
|
|
|
Awards
|
|
|
Fair Value
|
|
|
Awards
|
|
|
Fair Value
|
|
|
Awards
|
|
|
Fair Value
|
|
|
|
|
Nonvested at beginning of period
|
|
|
377,500
|
|
|
$
|
3.04
|
|
|
|
277,500
|
|
|
$
|
5.03
|
|
|
|
146,250
|
|
|
$
|
4.25
|
|
|
Granted
|
|
|
262,500
|
|
|
|
1.86
|
|
|
|
372,500
|
|
|
|
1.65
|
|
|
|
265,000
|
|
|
|
5.56
|
|
|
Vested
|
|
|
(284,375
|
)
|
|
|
2.82
|
|
|
|
(272,500
|
)
|
|
|
3.17
|
|
|
|
(133,750
|
)
|
|
|
5.22
|
|
|
Forfeited
|
|
|
(2,500
|
)
|
|
|
5.45
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nonvested at the end of period
|
|
|
353,125
|
|
|
$
|
2.32
|
|
|
|
377,500
|
|
|
$
|
3.04
|
|
|
|
277,500
|
|
|
$
|
5.03
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-36
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
The fair value of restricted stock awards is the quoted market
price of our stock on the grant date, and is charged to expense
over the period of vesting. These awards are subject to
forfeiture to the extent that the recipient’s service is
terminated prior to the shares becoming vested.
During the years ended December 31, 2009, 2008 and 2007,
the stock-based charge in connection with the expensing of
restricted stock awards was approximately $665,000, $862,000 and
$842,000, respectively. As of December 31, 2009, there was
approximately $0.6 million of unrecognized stock-based
compensation cost related to non-vested restricted stock awards,
which is expected to be recognized over a weighted average
period of 1.0 year.
401(k)
Plan Matching Contribution
During the years ended December 31, 2009, 2008 and 2007, we
issued 139,795, 166,430 and 44,118 shares of common stock
as the Company’s match of approximately $448,000, $274,000
and $211,000 on the 401(k) contributions of its employees during
those periods.
2009
Employee Stock Purchase Plan (ESPP)
There are 5,000,000 shares of common stock available for
issuance under the 2009 ESPP. Beginning on January 1, 2010,
and each January 1st thereafter, the number of shares
of common stock available for issuance under the 2009 ESPP shall
increase by an amount equal to the lesser of
(i) 1,000,000 shares or (ii) an amount determined
by the ESPP Administrator. However, in no event shall the number
of shares of common stock available for future sale under the
2009 ESPP exceed 10,000,000 shares, subject to
capitalization adjustments occurring due to dividends, splits,
dissolution, liquidation, mergers, or changes in control.
The 2009 ESPP provides that there shall be consecutive periods
during which an option to purchase common stock under the 2009
ESPP may be exercised (“Offering Periods”), each of
which will last approximately six months. The first Offering
Period shall commence on July 1, 2009 and shall terminate
on December 31, 2009. Thereafter, the first Offering Period
of a given year shall commence on January 1st of that
year and shall terminate on June 30th of the same
year. The second Offering Period of a given year shall commence
on July 1st of each year and shall terminate on
December 31st of each year.
The purchase price per share for which shares of common stock
will be sold pursuant to the 2009 ESPP is an amount equal to the
lesser of: (a) 85% of the fair market value of common stock
on the first day of the Offering Period or (b) 85% of the
fair market value of common stock on the last day of the
Offering Period.
The 2009 ESPP replaces our 2001 Employee Stock Purchase Program,
which was terminated by the Board effective June 26, 2009.
Total related stock based compensation expense for the year
ended December 31, 2009 was $0.3 million. No similar
expense was incurred in 2008 or 2007. The fair value of these
shares as of December 31, 2009 was $0.3 million.
|
|
|
13.
|
Quarterly
Financial Information (Unaudited)
|
As discussed in Note 2, the Company has restated its
consolidated financial statements for the years ended December
31, 2007 and 2008 and for each of the quarterly periods ended
March 31, 2008 through September 30, 2009 (the
“Affected Periods”) to reflect certain warrant-related
accounting adjustments identified in connection with its
reassessment of the accounting and classification of its warrant
contracts. The tables that follow provide quarterly information
and display the unaudited condensed consolidated financial
statements for each of the Affected Periods. The financial
statements are presented “As Previously Reported” and
“As Restated” to reflect the impact of the changes
resulting from the restatements of the Affected Periods. Certain
reclassifications, including
F-37
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
reclassification of all historical activities have been made to
the historical financial statements to conform to the fiscal
2009 presentation.
The following is a summary of the unaudited quarterly results of
consolidated operations for each of the calendar quarters in the
two-year period ended December 31, 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, 2008
|
|
|
Adjustments
|
|
|
March 31, 2008
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
4,037
|
|
|
|
—
|
|
|
$
|
4,037
|
|
|
Marketable securities
|
|
|
44,549
|
|
|
|
—
|
|
|
|
44,549
|
|
|
Accounts receivable, net of allowance for doubtful accounts
|
|
|
84
|
|
|
|
—
|
|
|
|
84
|
|
|
Inventory
|
|
|
562
|
|
|
|
—
|
|
|
|
562
|
|
|
Prepaid expenses and other current assets
|
|
|
692
|
|
|
|
—
|
|
|
|
692
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
49,924
|
|
|
|
—
|
|
|
|
49,924
|
|
|
Property and equipment, net
|
|
|
767
|
|
|
|
—
|
|
|
|
767
|
|
|
Other assets
|
|
|
155
|
|
|
|
—
|
|
|
|
155
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
50,846
|
|
|
|
—
|
|
|
$
|
50,846
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and Stockholders’ Equity
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
Current Liabilities:
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
Accounts payable and other accrued liabilities
|
|
$
|
1,864
|
|
|
|
—
|
|
|
$
|
1,864
|
|
|
Accrued compensation
|
|
|
1,004
|
|
|
|
—
|
|
|
|
1,004
|
|
|
Accrued drug development costs
|
|
|
4,654
|
|
|
|
—
|
|
|
|
4,654
|
|
|
Common stock warrant liability
|
|
|
—
|
|
|
|
1,516
|
|
|
|
1,516
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
7,522
|
|
|
|
1,516
|
|
|
|
9,038
|
|
F-38
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, 2008
|
|
|
Adjustments
|
|
|
March 31, 2008
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Deferred revenue and other credits
|
|
|
979
|
|
|
|
—
|
|
|
|
979
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
8,501
|
|
|
|
1,516
|
|
|
|
10,017
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Minority interest
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Stockholders’ Equity:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock, par value $0.001 per share,
5,000,000 shares authorized:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Series B Junior participating preferred stock,
1,000,000 shares authorized, no shares issued and
outstanding
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Series E convertible voting preferred stock,
2,000 shares authorized, stated value $10,000 per share,
$2.0 million aggregate liquidation value, issued and
outstanding, 170 shares at March 31, 2008
|
|
|
1,048
|
|
|
|
—
|
|
|
|
1,048
|
|
|
Common stock, par value $0.001 per share,
100,000,000 shares authorized:
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Issued and outstanding, 31,461,396 shares at March 31,
2008
|
|
|
31
|
|
|
|
—
|
|
|
|
31
|
|
|
Additional paid-in capital
|
|
|
290,947
|
|
|
|
(15,472
|
)
|
|
|
275,475
|
|
|
Deferred stock-based compensation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Accumulated other comprehensive income
|
|
|
735
|
|
|
|
—
|
|
|
|
735
|
|
|
Accumulated deficit
|
|
|
(250,416
|
)
|
|
|
13,956
|
|
|
|
(236,460
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
42,345
|
|
|
|
(1,516
|
)
|
|
|
40,829
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
$
|
50,846
|
|
|
|
—
|
|
|
$
|
50,846
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-39
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three-Months
|
|
|
|
|
|
Three-Months
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
March 31, 2008
|
|
|
Adjustments
|
|
|
March 31, 2008
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, except share and share data)
|
|
|
|
|
Revenues
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Licensing and milestone revenues
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
Other revenue
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
6,382
|
|
|
|
—
|
|
|
|
6,382
|
|
|
Selling, general and administrative
|
|
|
2,585
|
|
|
|
—
|
|
|
|
2,585
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
8,967
|
|
|
|
—
|
|
|
|
8,967
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(8,967
|
)
|
|
|
—
|
|
|
|
(8,967
|
)
|
|
Change in fair value of common stock warrant liability
|
|
|
—
|
|
|
|
520
|
|
|
|
520
|
|
|
Other income, net
|
|
|
301
|
|
|
|
—
|
|
|
|
301
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income
|
|
$
|
(8,666
|
)
|
|
$
|
520
|
|
|
$
|
(8,146
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted
|
|
$
|
(0.28
|
)
|
|
$
|
—
|
|
|
$
|
(0.26
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted
|
|
|
31,271,281
|
|
|
|
—
|
|
|
|
31,271,281
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-40
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quarter
|
|
|
|
|
|
Quarter
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
March 31, 2008
|
|
|
Adjustments
|
|
|
March 31, 2008
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, Except Share and Per Share Data)
|
|
|
|
|
Cash Flows From Operating Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income
|
|
$
|
(8,666
|
)
|
|
$
|
520
|
|
|
$
|
(8,146
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating
activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
87
|
|
|
|
—
|
|
|
|
87
|
|
|
Fair value adjustments of common stock warrants
|
|
|
—
|
|
|
|
(520
|
)
|
|
|
(520
|
)
|
|
Share-based compensation
|
|
|
1,731
|
|
|
|
—
|
|
|
|
1,731
|
|
|
Fair value of common stock issued in connection with drug license
|
|
|
305
|
|
|
|
—
|
|
|
|
305
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
Decrease in Accounts Receivable
|
|
|
107
|
|
|
|
—
|
|
|
|
107
|
|
|
Increase in Inventory
|
|
|
(562
|
)
|
|
|
—
|
|
|
|
(562
|
)
|
|
Decrease in other assets
|
|
|
188
|
|
|
|
—
|
|
|
|
188
|
|
|
Decrease in accounts payable and accrued expenses
|
|
|
(170
|
)
|
|
|
—
|
|
|
|
(170
|
)
|
|
Decrease in accrued compensation and related taxes
|
|
|
(107
|
)
|
|
|
—
|
|
|
|
(107
|
)
|
|
Decrease in deferred revenue and other credits
|
|
|
(30
|
)
|
|
|
—
|
|
|
|
(30
|
)
|
|
Net cash used in operating activities
|
|
|
(7,117
|
)
|
|
|
—
|
|
|
|
(7,117
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Investing Activities:
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
Sales of marketable securities
|
|
|
10,151
|
|
|
|
—
|
|
|
|
10,151
|
|
|
Purchases of marketable securities
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
Purchases of property and equipment
|
|
|
(138
|
)
|
|
|
—
|
|
|
|
(138
|
)
|
|
Net cash provided by investing activities
|
|
|
10,013
|
|
|
|
—
|
|
|
|
10,013
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
Proceeds from issuance of common stock and warrants, net of
related offering costs and expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from exercise of warrants
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Proceeds from exercise of stock options
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Net cash provided by financing activities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Net increase in cash and cash equivalents
|
|
|
2,896
|
|
|
|
—
|
|
|
|
2,896
|
|
|
Cash and cash equivalents, beginning of period
|
|
|
1,141
|
|
|
|
—
|
|
|
|
1,141
|
|
|
Cash and cash equivalents, end of period
|
|
|
4,037
|
|
|
|
—
|
|
|
|
4,037
|
|
F-41
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quarter
|
|
|
|
|
|
Quarter
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
March 31, 2008
|
|
|
Adjustments
|
|
|
March 31, 2008
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, Except Share and Per Share Data)
|
|
|
|
|
Supplemental Cash Flow Information:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest paid
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Income taxes paid
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Schedule of Non-Cash Investing and Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of common stock issued in connection with drug license
|
|
$
|
305
|
|
|
|
—
|
|
|
$
|
305
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of restricted stock granted employees and directors
|
|
$
|
223
|
|
|
|
—
|
|
|
$
|
223
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of stock issued to match employee 401k contributions
|
|
$
|
61
|
|
|
|
—
|
|
|
$
|
61
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock dividends paid with common stock
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-42
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30, 2008
|
|
|
Adjustments
|
|
|
June 30, 2008
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
1,725
|
|
|
|
—
|
|
|
$
|
1,725
|
|
|
Marketable securities
|
|
|
57,825
|
|
|
|
—
|
|
|
|
57,825
|
|
|
Accounts receivable, net of allowance for doubtful accounts
|
|
|
379
|
|
|
|
—
|
|
|
|
379
|
|
|
Inventory
|
|
|
1,197
|
|
|
|
—
|
|
|
|
1,197
|
|
|
Prepaid expenses and other current assets
|
|
|
781
|
|
|
|
—
|
|
|
|
781
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
61,907
|
|
|
|
—
|
|
|
|
61,907
|
|
|
Property and equipment, net
|
|
|
1,217
|
|
|
|
—
|
|
|
|
1,217
|
|
|
Other assets
|
|
|
112
|
|
|
|
—
|
|
|
|
112
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
|
63,236
|
|
|
|
—
|
|
|
|
63,236
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and Stockholders’ Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable and other accrued liabilities
|
|
|
2,910
|
|
|
|
—
|
|
|
|
2,910
|
|
|
Common stock warrant liability
|
|
|
—
|
|
|
|
600
|
|
|
|
600
|
|
|
Accrued compensation
|
|
|
1,062
|
|
|
|
—
|
|
|
|
1,062
|
|
|
Accrued drug development costs
|
|
|
3,782
|
|
|
|
—
|
|
|
|
3,782
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
7,754
|
|
|
|
600
|
|
|
|
8,354
|
|
|
Deferred revenue and other credits
|
|
|
966
|
|
|
|
—
|
|
|
|
966
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
8,720
|
|
|
|
600
|
|
|
|
9,320
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Equity:
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
Preferred stock, par value $0.001 per share,
5,000,000 shares authorized:
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Series B Junior participating preferred stock,
1,000,000 shares authorized, no shares issued and
outstanding
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Series E Convertible Voting Preferred Stock; 2,000 shares
authorized, stated value $10,000 per share, $2.0 million
aggregate liquidation value, issued and outstanding, 170 shares
at June 30, 2008
|
|
|
1,048
|
|
|
|
|
|
|
|
1,048
|
|
|
Common stock, par value $0.001 per share,
100,000,000 shares authorized:
|
|
|
|
|
|
|
|
|
|
|
—
|
|
F-43
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30, 2008
|
|
|
Adjustments
|
|
|
June 30, 2008
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
Issued and outstanding, 31,518,603 shares at June 30,
2008
|
|
|
32
|
|
|
|
—
|
|
|
|
32
|
|
|
Additional paid-in capital
|
|
|
292,332
|
|
|
|
(15,472
|
)
|
|
|
276,860
|
|
|
Accumulated other comprehensive income
|
|
|
843
|
|
|
|
—
|
|
|
|
843
|
|
|
Accumulated deficit
|
|
|
(239,739
|
)
|
|
|
14,872
|
|
|
|
(224,867
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
54,516
|
|
|
|
(600
|
)
|
|
|
53,916
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
|
63,236
|
|
|
$
|
—
|
|
|
|
63,236
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-44
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months
|
|
|
|
|
|
Three Months
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
June 30, 2008
|
|
|
Adjustments
|
|
|
June 30, 2008
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, except share and share data)
|
|
|
|
|
Revenues
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Licensing and milestone revenues
|
|
$
|
20,676
|
|
|
|
—
|
|
|
$
|
20,676
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
$
|
20,676
|
|
|
|
—
|
|
|
$
|
20,676
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
6,747
|
|
|
|
—
|
|
|
|
6,747
|
|
|
Selling, general and administrative
|
|
|
3,230
|
|
|
|
—
|
|
|
|
3,230
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
9,977
|
|
|
|
—
|
|
|
|
9,977
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income from operations
|
|
|
10,699
|
|
|
|
—
|
|
|
|
10,699
|
|
|
Change in fair value of common stock warrant liability
|
|
|
—
|
|
|
|
916
|
|
|
|
916
|
|
|
Other expense, net
|
|
|
(21
|
)
|
|
|
—
|
|
|
|
(21
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income before minority interest in consolidated subsidiary
|
|
|
10,678
|
|
|
|
916
|
|
|
|
11,594
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
10,678
|
|
|
$
|
916
|
|
|
$
|
11,594
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
|
|
|
Net income per share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
0.34
|
|
|
|
|
|
|
$
|
0.37
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted
|
|
$
|
0.34
|
|
|
|
|
|
|
$
|
0.36
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
|
31,462,522
|
|
|
|
|
|
|
|
31,462,522
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted
|
|
|
31,872,224
|
|
|
|
|
|
|
|
31,872,224
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-45
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months
|
|
|
|
|
|
Six Months
|
|
|
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
|
|
|
June 30, 2008
|
|
|
Adjustments
|
|
|
June 30, 2008
|
|
|
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
|
|
|
Cash Flows From Operating Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
2,012
|
|
|
$
|
1,435
|
|
|
$
|
3,447
|
|
|
|
|
|
|
Adjustments to reconcile net loss to net cash used in operating
activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
185
|
|
|
|
—
|
|
|
|
185
|
|
|
|
|
|
|
Fair value adjustments of common stock warrants
|
|
|
—
|
|
|
|
(1,435
|
)
|
|
|
(1,435
|
)
|
|
|
|
|
|
Stock-based compensation
|
|
|
3,117
|
|
|
|
—
|
|
|
|
3,117
|
|
|
|
|
|
|
Fair value of common stock issued in connection with drug license
|
|
|
305
|
|
|
|
—
|
|
|
|
305
|
|
|
|
|
|
|
Minority interest in subsidiary
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Increase in accounts receivable
|
|
|
(188
|
)
|
|
|
—
|
|
|
|
(188
|
)
|
|
|
|
|
|
Increase in inventory
|
|
|
(1,197
|
)
|
|
|
—
|
|
|
|
(1,197
|
)
|
|
|
|
|
|
Decrease in other assets
|
|
|
190
|
|
|
|
—
|
|
|
|
190
|
|
|
|
|
|
|
Increase in accounts payable and accrued expenses
|
|
|
4
|
|
|
|
—
|
|
|
|
4
|
|
|
|
|
|
|
Decrease in accrued compensation and related taxes
|
|
|
(49
|
)
|
|
|
—
|
|
|
|
(49
|
)
|
|
|
|
|
|
Decrease in deferred revenue and other credits
|
|
|
(43
|
)
|
|
|
—
|
|
|
|
(43
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by operating activities
|
|
|
4,336
|
|
|
|
—
|
|
|
|
4,336
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Investing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of marketable securities
|
|
|
(3,065
|
)
|
|
|
—
|
|
|
|
(3,065
|
)
|
|
|
|
|
|
Sales of marketable securities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
Purchases of property and equipment
|
|
|
(687
|
)
|
|
|
—
|
|
|
|
(687
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
|
(3,752
|
)
|
|
|
—
|
|
|
|
(3,752
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock and warrants, net of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
related offering costs and expenses
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
Proceeds from exercise of warrants
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
Repurchase of warrants
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
Proceeds from exercise of stock options
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
Payments made on capital lease obligations
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
Minority investment in subsidiary
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
F-46
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months
|
|
|
|
|
|
Six Months
|
|
|
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
|
|
|
June 30, 2008
|
|
|
Adjustments
|
|
|
June 30, 2008
|
|
|
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
|
|
|
Cash dividends paid on preferred stock
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by financing activities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase in cash and cash equivalents
|
|
|
584
|
|
|
|
—
|
|
|
|
584
|
|
|
|
|
|
|
Cash and cash equivalents, beginning of period
|
|
|
1,141
|
|
|
|
—
|
|
|
|
1,141
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
$
|
1,725
|
|
|
|
—
|
|
|
$
|
1,725
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental Cash Flow Information:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest paid
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income taxes paid
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Schedule of Non-Cash Investing and Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of common stock issued in connection with drug license
|
|
$
|
305
|
|
|
|
—
|
|
|
$
|
305
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of restricted stock granted employees and directors
|
|
$
|
223
|
|
|
|
—
|
|
|
$
|
223
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of stock issued to match employee 401k contributions
|
|
$
|
129
|
|
|
|
—
|
|
|
$
|
129
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock dividends paid with common stock
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of warrants issued to consultants and placement agents
|
|
$
|
69
|
|
|
|
—
|
|
|
$
|
69
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-47
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2008
|
|
|
Adjustments
|
|
|
September 30, 2008
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
4,679
|
|
|
|
—
|
|
|
$
|
4,679
|
|
|
Marketable securities
|
|
|
46,957
|
|
|
|
—
|
|
|
|
46,957
|
|
|
Accounts receivable, net of allowance for doubtful accounts
|
|
|
186
|
|
|
|
—
|
|
|
|
186
|
|
|
Inventory
|
|
|
1,446
|
|
|
|
—
|
|
|
|
1,446
|
|
|
Prepaid expenses and other current assets
|
|
|
254
|
|
|
|
—
|
|
|
|
254
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
53,522
|
|
|
|
—
|
|
|
|
53,522
|
|
|
Property and equipment, net
|
|
|
1,633
|
|
|
|
—
|
|
|
|
1,633
|
|
|
Other assets
|
|
|
143
|
|
|
|
—
|
|
|
|
143
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
55,298
|
|
|
|
—
|
|
|
$
|
55,298
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and Stockholders’ Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable and other accrued liabilities
|
|
$
|
3,217
|
|
|
|
—
|
|
|
$
|
3,217
|
|
|
Common stock warrant liability
|
|
|
—
|
|
|
|
556
|
|
|
|
556
|
|
|
Accrued compensation
|
|
|
1,145
|
|
|
|
—
|
|
|
|
1,145
|
|
|
Accrued drug development costs
|
|
|
3,572
|
|
|
|
—
|
|
|
|
3,572
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
7,934
|
|
|
|
556
|
|
|
|
8,490
|
|
|
Deferred revenue and other credits
|
|
|
1,026
|
|
|
|
—
|
|
|
|
1,026
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
8,960
|
|
|
|
556
|
|
|
|
9,516
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and Contingencies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Equity:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred Stock, par value $0.001 per share,
5,000,000 shares authorized:
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
Series E Convertible Voting Preferred Stock,
2,000 shares authorized, stated value $10,000 per share,
$2.0 million aggregate liquidation value, issued and
outstanding, 68 shares at September 30, 2008
|
|
|
419
|
|
|
|
—
|
|
|
|
419
|
|
|
Common stock, par value $0.001 per share,
100,000,000 shares authorized:
|
|
|
|
|
|
|
|
|
|
|
|
|
F-48
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2008
|
|
|
Adjustments
|
|
|
September 30, 2008
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Issued and outstanding, 31,771,876 shares at
September 30, 2008
|
|
|
32
|
|
|
|
—
|
|
|
|
32
|
|
|
Additional paid-in capital
|
|
|
294,051
|
|
|
|
(15,472
|
)
|
|
|
278,579
|
|
|
Accumulated other comprehensive income
|
|
|
390
|
|
|
|
—
|
|
|
|
390
|
|
|
Accumulated deficit
|
|
|
(248,554
|
)
|
|
|
14,916
|
|
|
|
(233,638
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
46,338
|
|
|
|
(556
|
)
|
|
|
45,782
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
$
|
55,298
|
|
|
$
|
—
|
|
|
$
|
55,298
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-49
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months
|
|
|
|
|
|
Three Months
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
September 30, 2008
|
|
|
Adjustments
|
|
|
September 30, 2008
|
|
|
|
|
(In thousands, except share and share data)
|
|
|
|
|
Revenues
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Licensing and milestone revenues
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
$
|
—
|
|
|
|
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
$
|
5,960
|
|
|
|
—
|
|
|
$
|
5,960
|
|
|
Selling, general and administrative
|
|
|
3,132
|
|
|
|
—
|
|
|
|
3,132
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
9,092
|
|
|
|
—
|
|
|
|
9,092
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
Loss from operations
|
|
|
(9,092
|
)
|
|
|
—
|
|
|
|
(9,092
|
)
|
|
Change in fair value of common stock warrant liability
|
|
|
—
|
|
|
|
45
|
|
|
|
45
|
|
|
Other income, net
|
|
|
276
|
|
|
|
—
|
|
|
|
276
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss before minority interest in consolidated subsidiary
|
|
|
(8,816
|
)
|
|
|
45
|
|
|
|
(8,771
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income
|
|
$
|
(8,816
|
)
|
|
$
|
45
|
|
|
$
|
(8,771
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted
|
|
$
|
(0.28
|
)
|
|
|
|
|
|
$
|
(0.28
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted
|
|
|
31,538,023
|
|
|
|
|
|
|
|
31,538,023
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-50
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months
|
|
|
|
|
|
Nine Months
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
September 30, 2008
|
|
|
Adjustments
|
|
|
September 30, 2008
|
|
|
|
|
(Previously Reported)
|
|
|
|
|
|
(As Restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Cash Flows From Operating Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income
|
|
$
|
(6,804
|
)
|
|
$
|
1,480
|
|
|
$
|
(5,324
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating
activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
146
|
|
|
|
—
|
|
|
|
146
|
|
|
Fair value adjustments of common stock warrants
|
|
|
—
|
|
|
|
(1,480
|
)
|
|
|
(1,480
|
)
|
|
Share-based compensation
|
|
|
4,207
|
|
|
|
—
|
|
|
|
4,207
|
|
|
Fair value of common stock issued in connection with drug license
|
|
|
305
|
|
|
|
—
|
|
|
|
305
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Decrease in accounts receivable
|
|
|
5
|
|
|
|
—
|
|
|
|
5
|
|
|
Increase in inventory
|
|
|
(1,446
|
)
|
|
|
—
|
|
|
|
(1,446
|
)
|
|
Decrease in prepaids and other assets
|
|
|
686
|
|
|
|
—
|
|
|
|
686
|
|
|
Increase in accounts payable and accrued expenses
|
|
|
101
|
|
|
|
—
|
|
|
|
101
|
|
|
Increase in accrued compensation and related taxes
|
|
|
34
|
|
|
|
—
|
|
|
|
34
|
|
|
Increase in deferred revenue and other credits
|
|
|
17
|
|
|
|
—
|
|
|
|
17
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities
|
|
|
(2,749
|
)
|
|
|
—
|
|
|
|
(2,749
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Investing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales of marketable securities
|
|
|
7,351
|
|
|
|
—
|
|
|
|
7,351
|
|
|
Sales of marketable securities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Purchases of property and equipment
|
|
|
(1,064
|
)
|
|
|
—
|
|
|
|
(1,064
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by investing activities
|
|
|
6,287
|
|
|
|
—
|
|
|
|
6,287
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock and warrants, net of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
related offering costs and expenses
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Proceeds from exercise of warrants
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Repurchase of warrants
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Proceeds from exercise of stock options
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Payments made on capital lease obligations
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Cash dividends paid on preferred stock
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by financing activities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-51
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months
|
|
|
|
|
|
Nine Months
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
September 30, 2008
|
|
|
Adjustments
|
|
|
September 30, 2008
|
|
|
|
|
(Previously Reported)
|
|
|
|
|
|
(As Restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Net increase in cash and cash equivalents
|
|
|
3,538
|
|
|
|
—
|
|
|
|
3,538
|
|
|
Cash and cash equivalents, beginning of period
|
|
|
1,141
|
|
|
|
—
|
|
|
|
1,141
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
$
|
4,679
|
|
|
|
—
|
|
|
$
|
4,679
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental Cash Flow Information:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest paid
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income taxes paid
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Schedule of Non-Cash Investing and Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of common stock issued in connection with drug license
|
|
$
|
305
|
|
|
|
—
|
|
|
$
|
305
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of restricted stock granted employees and directors
|
|
$
|
275
|
|
|
|
—
|
|
|
$
|
275
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of stock issued to match employee 401k contributions
|
|
$
|
208
|
|
|
|
—
|
|
|
$
|
208
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock dividends paid with common stock
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of warrants issued to consultants and placement agents
|
|
$
|
69
|
|
|
|
—
|
|
|
$
|
69
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-52
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2008
|
|
|
Adjustments
|
|
|
December 31, 2008
|
|
|
|
|
(Previously Reported)
|
|
|
|
|
|
(As Restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
9,860
|
|
|
|
—
|
|
|
|
9,860
|
|
|
Marketable securities
|
|
|
66,078
|
|
|
|
—
|
|
|
|
66,078
|
|
|
Accounts receivable-trade, net
|
|
|
9,776
|
|
|
|
—
|
|
|
|
9,776
|
|
|
Inventory
|
|
|
1,841
|
|
|
|
—
|
|
|
|
1,841
|
|
|
Prepaid expenses and other current assets
|
|
|
693
|
|
|
|
—
|
|
|
|
693
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
88,248
|
|
|
|
—
|
|
|
|
88,248
|
|
|
Bank certificates of deposit
|
|
|
2,148
|
|
|
|
|
|
|
|
2,148
|
|
|
Property and equipment, net
|
|
|
1,782
|
|
|
|
—
|
|
|
|
1,782
|
|
|
Zevalin related intangible assets, net
|
|
|
37,042
|
|
|
|
—
|
|
|
|
37,042
|
|
|
Other assets
|
|
|
289
|
|
|
|
—
|
|
|
|
289
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
129,509
|
|
|
|
—
|
|
|
|
129,509
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and Stockholders’ Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued obligations
|
|
$
|
10,401
|
|
|
|
—
|
|
|
|
10,401
|
|
|
Common stock warrant liability
|
|
|
—
|
|
|
|
765
|
|
|
|
765
|
|
|
Accrued compensation
|
|
|
2,956
|
|
|
|
—
|
|
|
|
2,956
|
|
|
Note payable in connection with Zevalin joint venture
|
|
|
7,500
|
|
|
|
—
|
|
|
|
7,500
|
|
|
Current portion of deferred revenue and other credits
|
|
|
8,500
|
|
|
|
—
|
|
|
|
8,500
|
|
|
Accrued drug development costs
|
|
|
3,449
|
|
|
|
—
|
|
|
|
3,449
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
32,806
|
|
|
|
765
|
|
|
|
33,571
|
|
|
Capital lease obligations, net of current portion
|
|
|
95
|
|
|
|
—
|
|
|
|
95
|
|
|
Deferred revenue and other credits, net of current portion
|
|
|
33,929
|
|
|
|
—
|
|
|
|
33,929
|
|
|
Zevalin related contingent obligations
|
|
|
8,798
|
|
|
|
—
|
|
|
|
8,798
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
75,628
|
|
|
|
765
|
|
|
|
76,393
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Minority interest in consolidated entity
|
|
|
14,262
|
|
|
|
—
|
|
|
|
14,262
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Equity:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock, par value $0.001 per share,
5,000,000 shares authorized:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Series B Junior participating preferred stock,
1,000,000 shares authorized, no shares issued and
outstanding
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
F-53
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2008
|
|
|
Adjustments
|
|
|
December 31, 2008
|
|
|
|
|
(Previously Reported)
|
|
|
|
|
|
(As Restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Series E convertible voting preferred stock,
2,000 shares authorized, stated value $10,000 per share,
$0.8 million aggregate liquidation value, issued and
outstanding, 68 shares at December 31, 2008
|
|
|
419
|
|
|
|
—
|
|
|
|
419
|
|
|
Common stock, par value $0.001 per share,
100,000,000 shares authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issued and outstanding, 32,166,316 shares at
December 31, 2008
|
|
|
32
|
|
|
|
—
|
|
|
|
32
|
|
|
Additional paid-in capital
|
|
|
296,531
|
|
|
|
(15,472
|
)
|
|
|
281,059
|
|
|
Accumulated other comprehensive loss
|
|
|
(146
|
)
|
|
|
—
|
|
|
|
(146
|
)
|
|
Accumulated deficit
|
|
|
(257,217
|
)
|
|
|
14,707
|
|
|
|
(242,510
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
39,619
|
|
|
|
(765
|
)
|
|
|
38,854
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
$
|
129,509
|
|
|
$
|
—
|
|
|
|
129,509
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-54
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months
|
|
|
|
|
|
Three Months
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
December 31, 2008
|
|
|
Adjustments
|
|
|
December 31, 2008
|
|
|
|
|
|
|
|
|
|
|
(As Restated)
|
|
|
|
|
(In thousands, except share and share data)
|
|
|
|
|
Revenues
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Licensing and milestone revenues
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
Other revenue
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Product sales
|
|
|
8,049
|
|
|
|
—
|
|
|
|
8,049
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
$
|
8,049
|
|
|
|
—
|
|
|
$
|
8,049
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
Cost of product sold
|
|
$
|
1,193
|
|
|
|
—
|
|
|
$
|
1,193
|
|
|
Research and development
|
|
|
7,594
|
|
|
|
—
|
|
|
|
7,594
|
|
|
Selling, general and administrative
|
|
|
6,209
|
|
|
|
—
|
|
|
|
6,209
|
|
|
Acquired in-process research and development
|
|
|
4,700
|
|
|
|
—
|
|
|
|
4,700
|
|
|
Amortization of purchased intangibles
|
|
|
158
|
|
|
|
—
|
|
|
|
158
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
19,854
|
|
|
|
—
|
|
|
|
19,854
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
Loss from operations
|
|
|
(11,805
|
)
|
|
|
—
|
|
|
|
(11,805
|
)
|
|
Change in fair value of common stock warrants
|
|
|
—
|
|
|
$
|
(210
|
)
|
|
|
(210
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
Other income, net
|
|
|
609
|
|
|
|
—
|
|
|
|
609
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pre-tax net loss
|
|
|
(11,196
|
)
|
|
|
(210
|
)
|
|
|
(11,406
|
)
|
|
Income tax expense
|
|
|
(5
|
)
|
|
|
—
|
|
|
|
(5
|
)
|
|
Net loss attributable to non-controlling interest
|
|
|
2,538
|
|
|
|
—
|
|
|
|
2,538
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income
|
|
$
|
(8,663
|
)
|
|
$
|
(210
|
)
|
|
$
|
(8,873
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted
|
|
$
|
(0.28
|
)
|
|
|
|
|
|
$
|
(0.28
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted
|
|
|
31,928,778
|
|
|
|
|
|
|
|
31,928,778
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-55
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Twelve Months
|
|
|
|
|
|
Twelve Months
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
December 31, 2008
|
|
|
Adjustment
|
|
|
December 31, 2008
|
|
|
|
|
(Previously Reported)
|
|
|
|
|
|
(As Restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Cash Flows From Operating Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income
|
|
$
|
(15,467
|
)
|
|
|
1,271
|
|
|
|
(14,196
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating
activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
610
|
|
|
|
—
|
|
|
|
610
|
|
|
Fair value adjustments of common stock warrants
|
|
|
—
|
|
|
|
(1,271
|
)
|
|
|
(1,271
|
)
|
|
Acquired in-process research and development
|
|
|
4,700
|
|
|
|
—
|
|
|
|
4,700
|
|
|
Share-based compensation expense
|
|
|
6,537
|
|
|
|
—
|
|
|
|
6,537
|
|
|
Fair value of common stock issued in connection with drug license
|
|
|
379
|
|
|
|
—
|
|
|
|
379
|
|
|
Minority interest in consolidated entities
|
|
|
(2,538
|
)
|
|
|
—
|
|
|
|
(2,538
|
)
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
(4,811
|
)
|
|
|
—
|
|
|
|
(4,811
|
)
|
|
Inventory
|
|
|
(1,841
|
)
|
|
|
—
|
|
|
|
(1,841
|
)
|
|
Prepaid expenses and other assets
|
|
|
101
|
|
|
|
—
|
|
|
|
101
|
|
|
Accounts payable and accrued obligations
|
|
|
2,387
|
|
|
|
—
|
|
|
|
2,387
|
|
|
Notes payable
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Accrued compensation and related taxes
|
|
|
1,845
|
|
|
|
—
|
|
|
|
1,845
|
|
|
Deferred revenue and other credits
|
|
|
93
|
|
|
|
—
|
|
|
|
93
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities
|
|
|
(8,005
|
)
|
|
|
—
|
|
|
|
(8,005
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Investing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net purchases of marketable securities
|
|
|
(13,056
|
)
|
|
|
—
|
|
|
|
(13,056
|
)
|
|
Investment in Zevalin joint venture
|
|
|
(10,202
|
)
|
|
|
—
|
|
|
|
(10,202
|
)
|
|
Purchases of property and equipment
|
|
|
(1,518
|
)
|
|
|
—
|
|
|
|
(1,518
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
|
(24,776
|
)
|
|
|
—
|
|
|
|
(24,776
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock and warrants, net of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
related offering costs and expenses
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Proceeds from exercise of warrants
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Proceeds from exercise of stock options
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Proceeds from Allergan Collaboration
|
|
|
41,500
|
|
|
|
—
|
|
|
|
41,500
|
|
|
Payments made on capital lease obligations
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Minority investment in subsidiary
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Cash dividends paid on preferred stock
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by financing activities
|
|
|
41,500
|
|
|
|
—
|
|
|
|
41,500
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase in cash and cash equivalents
|
|
|
8,719
|
|
|
|
—
|
|
|
|
8,719
|
|
F-56
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Twelve Months
|
|
|
|
|
|
Twelve Months
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
December 31, 2008
|
|
|
Adjustment
|
|
|
December 31, 2008
|
|
|
|
|
(Previously Reported)
|
|
|
|
|
|
(As Restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Cash and cash equivalents, beginning of period
|
|
|
1,141
|
|
|
|
—
|
|
|
|
1,141
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
$
|
9,860
|
|
|
|
—
|
|
|
|
9,860
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental Cash Flow Information:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest paid
|
|
$
|
36
|
|
|
|
—
|
|
|
|
36
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income taxes paid
|
|
$
|
5
|
|
|
|
—
|
|
|
|
5
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Schedule of Non-Cash Investing and Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of common stock issued in connection with drug license
|
|
$
|
379
|
|
|
|
—
|
|
|
|
379
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of restricted stock granted employees and directors
|
|
$
|
606
|
|
|
|
—
|
|
|
|
606
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of stock issued to match employee 401k contributions
|
|
$
|
274
|
|
|
|
—
|
|
|
|
274
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of equity awarded to consultants and placement agents
|
|
$
|
70
|
|
|
|
—
|
|
|
|
70
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-57
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, 2009
|
|
|
Adjustments
|
|
|
March 31, 2009
|
|
|
|
|
(Previously Reported)
|
|
|
|
|
|
(As Restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
4,065
|
|
|
|
—
|
|
|
$
|
4,065
|
|
|
Restricted cash in escrow
|
|
|
10,000
|
|
|
|
—
|
|
|
|
10,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total cash and cash equivalents
|
|
|
14,065
|
|
|
|
—
|
|
|
|
14,065
|
|
|
Marketable securities
|
|
|
49,833
|
|
|
|
—
|
|
|
|
49,833
|
|
|
Accounts receivable-trade, net
|
|
|
6,306
|
|
|
|
—
|
|
|
|
6,306
|
|
|
Inventory
|
|
|
1,894
|
|
|
|
—
|
|
|
|
1,894
|
|
|
Prepaid expenses and other current assets
|
|
|
736
|
|
|
|
—
|
|
|
|
736
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
72,834
|
|
|
|
—
|
|
|
|
72,834
|
|
|
Property and equipment, net
|
|
|
1,818
|
|
|
|
—
|
|
|
|
1,818
|
|
|
Zevalin related intangible assets, net
|
|
|
36,092
|
|
|
|
—
|
|
|
|
36,092
|
|
|
Other assets
|
|
|
104
|
|
|
|
—
|
|
|
|
104
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
110,848
|
|
|
|
—
|
|
|
$
|
110,848
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and Stockholders’ Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued obligations
|
|
$
|
8,221
|
|
|
|
—
|
|
|
$
|
8,221
|
|
|
Common stock warrant liability
|
|
|
—
|
|
|
|
1,274
|
|
|
|
1,274
|
|
|
Accrued compensation
|
|
|
1,921
|
|
|
|
—
|
|
|
|
1,921
|
|
|
Note payable in connection with Zevalin acquisition
|
|
|
10,000
|
|
|
|
—
|
|
|
|
10,000
|
|
|
Current portion of deferred revenue and other credits
|
|
|
8,500
|
|
|
|
—
|
|
|
|
8,500
|
|
|
Accrued drug development costs
|
|
|
4,798
|
|
|
|
—
|
|
|
|
4,798
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
33,440
|
|
|
|
1,274
|
|
|
|
34,714
|
|
|
Capital lease obligations, net of current portion
|
|
|
89
|
|
|
|
—
|
|
|
|
89
|
|
|
Deferred revenue and other credits, net of current portion
|
|
|
31,785
|
|
|
|
—
|
|
|
|
31,785
|
|
|
Zevalin related contingent obligations
|
|
|
4,998
|
|
|
|
—
|
|
|
|
4,998
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
70,312
|
|
|
|
1,274
|
|
|
|
71,586
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Minority interest in consolidated entity
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Equity:
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Preferred stock, par value $0.001 per share,
5,000,000 shares authorized:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Series B Junior participating preferred stock,
1,000,000 shares authorized, no shares issued and
outstanding
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
F-58
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, 2009
|
|
|
Adjustments
|
|
|
March 31, 2009
|
|
|
|
|
(Previously Reported)
|
|
|
|
|
|
(As Restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Series E convertible voting preferred stock,
2,000 shares authorized, stated value $10,000 per share,
$0.8 million aggregate liquidation value, issued and
outstanding, 68 shares at March 31, 2009
|
|
|
419
|
|
|
|
—
|
|
|
|
419
|
|
|
Common stock, par value $0.001 per share,
100,000,000 shares authorized.
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Issued and outstanding, 32,547,700 shares at March 31,
2009
|
|
|
33
|
|
|
|
—
|
|
|
|
33
|
|
|
Additional paid-in capital
|
|
|
297,208
|
|
|
|
(15,472
|
)
|
|
|
281,736
|
|
|
Accumulated other comprehensive loss
|
|
|
(531
|
)
|
|
|
—
|
|
|
|
(531
|
)
|
|
Accumulated deficit
|
|
|
(256,593
|
)
|
|
|
14,198
|
|
|
|
(242,395
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
40,536
|
|
|
|
(1,274
|
)
|
|
|
39,262
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
$
|
110,848
|
|
|
$
|
—
|
|
|
$
|
110,848
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-59
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months
|
|
|
|
|
|
Three Months
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
March 31, 2009
|
|
|
Adjustments
|
|
|
March 31, 2009
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Revenues
|
|
|
|
|
|
|
|
|
|
|
|
|
|
License and contract revenue
|
|
$
|
2,125
|
|
|
|
—
|
|
|
$
|
2,125
|
|
|
Product sales
|
|
|
12,038
|
|
|
|
—
|
|
|
|
12,038
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
$
|
14,163
|
|
|
|
—
|
|
|
$
|
38,025
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of product sold
|
|
$
|
1,834
|
|
|
|
—
|
|
|
$
|
1,834
|
|
|
Research and development
|
|
|
5,654
|
|
|
|
—
|
|
|
|
5,654
|
|
|
Amortization of purchased intangibles
|
|
|
950
|
|
|
|
—
|
|
|
|
950
|
|
|
Selling, general and administrative
|
|
|
6,351
|
|
|
|
—
|
|
|
|
6,351
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
14,789
|
|
|
|
—
|
|
|
|
66,533
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(626
|
)
|
|
|
—
|
|
|
|
(626
|
)
|
|
Change in fair value of common stock warrant liability
|
|
|
—
|
|
|
|
(509
|
)
|
|
|
(509
|
)
|
|
Other income, net
|
|
|
104
|
|
|
|
—
|
|
|
|
104
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss before minority interest in consolidated entities
|
|
|
(522
|
)
|
|
|
(509
|
)
|
|
|
(1,031
|
)
|
|
Minority interest in net loss of consolidated entities
|
|
|
1,146
|
|
|
|
—
|
|
|
|
1,146
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
$
|
624
|
|
|
$
|
(509
|
)
|
|
$
|
(19,046
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income per share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
0.02
|
|
|
|
|
|
|
$
|
0.00
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted
|
|
$
|
0.02
|
|
|
|
|
|
|
$
|
0.00
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
|
31,952,523
|
|
|
|
|
|
|
|
31,952,523
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted
|
|
|
32,157,425
|
|
|
|
|
|
|
|
32,157,425
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-60
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quarter
|
|
|
|
|
|
Quarter
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
March 31, 2009
|
|
|
Adjustments
|
|
|
March 31, 2009
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Cash Flows From Operating Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
$
|
624
|
|
|
$
|
(509
|
)
|
|
$
|
115
|
|
|
Adjustments to reconcile net income (loss) to net cash provided
by (used in) operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortization of deferred revenue
|
|
|
(2,125
|
)
|
|
|
—
|
|
|
|
(2,125
|
)
|
|
Depreciation and amortization
|
|
|
136
|
|
|
|
—
|
|
|
|
136
|
|
|
Fair value adjustments of common stock warrants
|
|
|
—
|
|
|
|
509
|
|
|
|
509
|
|
|
Share-based compensation expense
|
|
|
968
|
|
|
|
—
|
|
|
|
968
|
|
|
Fair value of common stock issued in connection with drug license
|
|
|
185
|
|
|
|
—
|
|
|
|
185
|
|
|
Minority interest in consolidated entities
|
|
|
(1,146
|
)
|
|
|
—
|
|
|
|
(1,146
|
)
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
(1,304
|
)
|
|
|
—
|
|
|
|
(1,304
|
)
|
|
Inventory
|
|
|
(53
|
)
|
|
|
—
|
|
|
|
(53
|
)
|
|
Prepaid expenses and other assets
|
|
|
148
|
|
|
|
—
|
|
|
|
148
|
|
|
Accounts payable and accrued obligations
|
|
|
3,942
|
|
|
|
—
|
|
|
|
3,942
|
|
|
Accrued compensation and related taxes
|
|
|
(1,035
|
)
|
|
|
—
|
|
|
|
(1,035
|
)
|
|
Deferred revenue and other credits
|
|
|
(25
|
)
|
|
|
—
|
|
|
|
(25
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by operating activities
|
|
|
315
|
|
|
|
—
|
|
|
|
315
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Investing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net sales of marketable securities
|
|
|
18,112
|
|
|
|
—
|
|
|
|
18,112
|
|
|
Investment in Zevalin acquisition
|
|
|
(14,050
|
)
|
|
|
—
|
|
|
|
(14,050
|
)
|
|
Restricted cash in escrow for Zevalin acquisition
|
|
|
(10,000
|
)
|
|
|
—
|
|
|
|
(10,000
|
)
|
|
Purchases of property and equipment
|
|
|
(172
|
)
|
|
|
—
|
|
|
|
(172
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
|
(6,110
|
)
|
|
|
—
|
|
|
|
(6,110
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock and warrants, net of
related offering costs and expenses
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Proceeds from exercise of warrants
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Proceeds from exercise of stock options
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Proceeds from Allergan Collaboration
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Payments made on capital lease obligations
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Minority investment in subsidiary
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Cash dividends paid on preferred stock
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Net cash provided by financing activities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-61
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quarter
|
|
|
|
|
|
Quarter
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
March 31, 2009
|
|
|
Adjustments
|
|
|
March 31, 2009
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Net decrease in cash and cash equivalents
|
|
|
(5,795
|
)
|
|
|
—
|
|
|
|
(5,795
|
)
|
|
Cash and cash equivalents, beginning of period
|
|
|
9,860
|
|
|
|
—
|
|
|
|
9,860
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
$
|
4,065
|
|
|
|
—
|
|
|
$
|
4,065
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental Cash Flow Information:
|
|
|
|
|
|
|
|
|
|
$
|
—
|
|
|
Interest paid
|
|
$
|
7
|
|
|
|
—
|
|
|
$
|
7
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income taxes paid
|
|
$
|
45
|
|
|
|
—
|
|
|
$
|
45
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Schedule of Non-Cash Investing and Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of common stock issued in connection with drug license
|
|
$
|
185
|
|
|
|
—
|
|
|
$
|
185
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of restricted stock granted employees and directors
|
|
$
|
182
|
|
|
|
—
|
|
|
$
|
182
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of stock issued to match employee 401k contributions
|
|
$
|
108
|
|
|
|
—
|
|
|
$
|
108
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock dividends paid with common stock
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of equity awarded to consultants and placement agents
|
|
$
|
111
|
|
|
|
—
|
|
|
$
|
111
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-62
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30, 2009
|
|
|
Adjustments
|
|
|
June 30, 2009
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
7,993
|
|
|
|
—
|
|
|
$
|
7,993
|
|
|
Marketable securities
|
|
|
77,062
|
|
|
|
—
|
|
|
|
77,062
|
|
|
Financing proceeds receivable
|
|
|
21,000
|
|
|
|
—
|
|
|
|
21,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash, cash equivalents, marketable securities and financing
proceeds receivable
|
|
|
106,055
|
|
|
|
—
|
|
|
|
106,055
|
|
|
Accounts receivable-trade, net
|
|
|
1,531
|
|
|
|
—
|
|
|
|
1,531
|
|
|
Inventory
|
|
|
2,355
|
|
|
|
—
|
|
|
|
2,355
|
|
|
Prepaid expenses and other current assets
|
|
|
661
|
|
|
|
—
|
|
|
|
661
|
|
|
Total current assets
|
|
|
110,602
|
|
|
|
—
|
|
|
|
110,602
|
|
|
Property and equipment, net
|
|
|
1,845
|
|
|
|
—
|
|
|
|
1,845
|
|
|
Zevalin related intangible assets, net
|
|
|
35,143
|
|
|
|
—
|
|
|
|
35,143
|
|
|
Other assets
|
|
|
99
|
|
|
|
—
|
|
|
|
99
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
147,689
|
|
|
|
—
|
|
|
$
|
147,689
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and Stockholders’ Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued obligations
|
|
$
|
13,985
|
|
|
|
—
|
|
|
$
|
13,985
|
|
|
Common stock warrant liability
|
|
|
—
|
|
|
|
30,232
|
|
|
|
30,232
|
|
|
Accrued compensation
|
|
|
2,278
|
|
|
|
—
|
|
|
|
2,278
|
|
|
Current portion of deferred revenue and other credits
|
|
|
8,500
|
|
|
|
—
|
|
|
|
8,500
|
|
|
Accrued drug development costs
|
|
|
3,929
|
|
|
|
—
|
|
|
|
3,929
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
28,692
|
|
|
|
30,232
|
|
|
|
58,924
|
|
|
Capital lease obligations, net of current portion
|
|
|
102
|
|
|
|
—
|
|
|
|
102
|
|
|
Deferred revenue and other credits, net of current portion
|
|
|
29,622
|
|
|
|
—
|
|
|
|
29,622
|
|
|
Zevalin related contingent obligations
|
|
|
6,755
|
|
|
|
—
|
|
|
|
6,755
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
65,171
|
|
|
|
30,232
|
|
|
|
95,403
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Minority interest in consolidated entity
|
|
|
|
|
|
|
|
|
|
|
|
|
F-63
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30, 2009
|
|
|
Adjustments
|
|
|
June 30, 2009
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Stockholders’ Equity:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock, par value $0.001 per share,
5,000,000 shares authorized:
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Series B Junior participating preferred stock,
1,000,000 shares authorized, no shares issued and
outstanding
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Series E convertible voting preferred stock,
2,000 shares authorized, stated value $10,000 per share,
$0.8 million aggregate liquidation value, issued and
outstanding, 68 shares at June 30, 2009
|
|
|
419
|
|
|
|
—
|
|
|
|
419
|
|
|
Common stock, par value $0.001 per share,
100,000,000 shares authorized:
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Issued and outstanding, 41,707,484 shares at June 30,
2009
|
|
|
42
|
|
|
|
—
|
|
|
|
42
|
|
|
Additional paid-in capital
|
|
|
348,521
|
|
|
|
(24,318
|
)
|
|
|
324,203
|
|
|
Accumulated other comprehensive loss
|
|
|
(166
|
)
|
|
|
—
|
|
|
|
(166
|
)
|
|
Accumulated deficit
|
|
|
(266,298
|
)
|
|
|
(5,914
|
)
|
|
|
(272,212
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
82,518
|
|
|
|
(30,232
|
)
|
|
|
52,286
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
$
|
147,689
|
|
|
$
|
—
|
|
|
$
|
147,689
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-64
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months
|
|
|
|
|
Three Months
|
|
|
|
|
|
Ended
|
|
|
|
|
Ended
|
|
|
|
|
|
June 30,
|
|
|
|
|
June 30, 2009
|
|
|
Adjustments
|
|
|
2009
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Revenues
|
|
|
|
|
|
|
|
|
|
|
|
|
|
License and contract revenue
|
|
$
|
2,125
|
|
|
|
—
|
|
|
$
|
2,125
|
|
|
Product sales
|
|
|
6,016
|
|
|
|
—
|
|
|
|
6,016
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
$
|
8,141
|
|
|
|
—
|
|
|
$
|
8,141
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of product sold
|
|
$
|
1,439
|
|
|
|
—
|
|
|
$
|
1,439
|
|
|
Research and development
|
|
|
6,391
|
|
|
|
—
|
|
|
|
6,391
|
|
|
Amortization of purchased intangibles
|
|
|
950
|
|
|
|
—
|
|
|
|
950
|
|
|
Selling, general and administrative
|
|
|
9,192
|
|
|
|
—
|
|
|
|
9,192
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
17,972
|
|
|
|
—
|
|
|
|
17,972
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(9,831
|
)
|
|
|
—
|
|
|
|
(9,831
|
)
|
|
Change in fair value of common stock warrant liability
|
|
|
—
|
|
|
|
(20,113
|
)
|
|
|
(20,113
|
)
|
|
Other income, net
|
|
|
125
|
|
|
|
—
|
|
|
|
125
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss before minority interest in consolidated entities
|
|
|
(9,706
|
)
|
|
|
(20,113
|
)
|
|
|
(29,819
|
)
|
|
Minority interest in net loss of consolidated entities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(9,706
|
)
|
|
$
|
(20,113
|
)
|
|
$
|
(29,819
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted
|
|
$
|
(0.28
|
)
|
|
|
|
|
|
$
|
(0.87
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted
|
|
|
34,137,640
|
|
|
|
|
|
|
|
34,137,640
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-65
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months
|
|
|
|
|
|
Six Months
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
June 30, 2009
|
|
|
Adjustments
|
|
|
June 30, 2009
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Cash Flows From Operating Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(9,081
|
)
|
|
$
|
(20,621
|
)
|
|
$
|
(29,702
|
)
|
|
Adjustments to reconcile net income (loss) to net cash provided
by operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortization of deferred revenue
|
|
|
(4,250
|
)
|
|
|
—
|
|
|
|
(4,250
|
)
|
|
Depreciation and amortization
|
|
|
2,178
|
|
|
|
—
|
|
|
|
2,178
|
|
|
Fair value adjustments of common stock warrants
|
|
|
—
|
|
|
|
20,621
|
|
|
|
20,621
|
|
|
Share-based compensation expense
|
|
|
4,793
|
|
|
|
—
|
|
|
|
4,793
|
|
|
Fair value of common stock issued in connection with drug license
|
|
|
185
|
|
|
|
—
|
|
|
|
185
|
|
|
Minority interest in consolidated entities
|
|
|
(1,146
|
)
|
|
|
—
|
|
|
|
(1,146
|
)
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
3,471
|
|
|
|
—
|
|
|
|
3,471
|
|
|
Inventory
|
|
|
(514
|
)
|
|
|
—
|
|
|
|
(514
|
)
|
|
Prepaid expenses and other assets
|
|
|
228
|
|
|
|
—
|
|
|
|
228
|
|
|
Accounts payable and accrued obligations
|
|
|
9,154
|
|
|
|
—
|
|
|
|
9,154
|
|
|
Accrued compensation and related taxes
|
|
|
(678
|
)
|
|
|
—
|
|
|
|
(678
|
)
|
|
Deferred revenue and other credits
|
|
|
(49
|
)
|
|
|
—
|
|
|
|
(49
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by operating activities
|
|
|
4,291
|
|
|
|
—
|
|
|
|
4,291
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Investing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net purchases of marketable securities
|
|
|
(8,862
|
)
|
|
|
—
|
|
|
|
(8,862
|
)
|
|
Investment in Zevalin Acquisition
|
|
|
(22,687
|
)
|
|
|
—
|
|
|
|
(22,687
|
)
|
|
Purchases of property and equipment
|
|
|
(344
|
)
|
|
|
—
|
|
|
|
(344
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
|
(31,893
|
)
|
|
|
—
|
|
|
|
(31,893
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock and warrants, net of
related offering costs and expenses
|
|
|
27,070
|
|
|
|
—
|
|
|
|
27,070
|
|
|
Proceeds from sale of common stock to employees —
shelf takedown
|
|
|
1,167
|
|
|
|
—
|
|
|
|
1,167
|
|
|
Repurchase of warrants
|
|
|
(71
|
)
|
|
|
—
|
|
|
|
(71
|
)
|
|
Proceeds from exercise of stock options
|
|
|
89
|
|
|
|
—
|
|
|
|
89
|
|
|
Repurchase of stock options pursuant to tender offer
|
|
|
(2,520
|
)
|
|
|
—
|
|
|
|
(2,520
|
)
|
|
Net cash provided by financing activities
|
|
|
25,735
|
|
|
|
—
|
|
|
|
25,735
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net decrease in cash and cash equivalents
|
|
|
(1,867
|
)
|
|
|
—
|
|
|
|
(1,867
|
)
|
|
Cash and cash equivalents, beginning of period
|
|
|
9,860
|
|
|
|
—
|
|
|
|
9,860
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
$
|
7,993
|
|
|
|
—
|
|
|
$
|
7,993
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-66
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months
|
|
|
|
|
|
Six Months
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
June 30, 2009
|
|
|
Adjustments
|
|
|
June 30, 2009
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Supplemental Cash Flow Information:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest paid
|
|
$
|
10
|
|
|
|
—
|
|
|
$
|
10
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income taxes paid
|
|
$
|
45
|
|
|
|
—
|
|
|
$
|
45
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Schedule of Non-Cash Investing and Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of common stock issued in connection with drug license
|
|
$
|
185
|
|
|
|
—
|
|
|
$
|
185
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of restricted stock granted employees and directors
|
|
$
|
226
|
|
|
|
—
|
|
|
$
|
226
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of stock issued to match employee 401k contributions
|
|
$
|
219
|
|
|
|
—
|
|
|
$
|
219
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of equity awarded to consultants and placement agents
|
|
$
|
111
|
|
|
|
—
|
|
|
$
|
111
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-67
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2009
|
|
|
Adjustments
|
|
|
September 30, 2009
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
9,686
|
|
|
|
—
|
|
|
$
|
9,686
|
|
|
Marketable securities
|
|
|
133,785
|
|
|
|
—
|
|
|
|
133,785
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash, cash equivalents and marketable securities
|
|
|
143,471
|
|
|
|
—
|
|
|
|
143,471
|
|
|
Accounts receivable-trade, net
|
|
|
4,441
|
|
|
|
—
|
|
|
|
4,441
|
|
|
Inventory
|
|
|
2,160
|
|
|
|
—
|
|
|
|
2,160
|
|
|
Prepaid expenses and other current assets
|
|
|
472
|
|
|
|
—
|
|
|
|
472
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
150,544
|
|
|
|
—
|
|
|
|
150,544
|
|
|
Property and equipment, net
|
|
|
1,771
|
|
|
|
—
|
|
|
|
1,771
|
|
|
Zevalin related intangible assets, net
|
|
|
35,941
|
|
|
|
—
|
|
|
|
35,941
|
|
|
Other assets
|
|
|
193
|
|
|
|
—
|
|
|
|
193
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
188,449
|
|
|
|
—
|
|
|
$
|
188,449
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and Stockholders’ Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued obligations
|
|
$
|
21,460
|
|
|
|
—
|
|
|
$
|
21,460
|
|
|
Common stock warrant liability
|
|
|
—
|
|
|
|
26,540
|
|
|
|
26,540
|
|
|
Accrued compensation
|
|
|
2,476
|
|
|
|
—
|
|
|
|
2,476
|
|
|
Current portion of deferred revenue and other credits
|
|
|
8,500
|
|
|
|
—
|
|
|
|
8,500
|
|
|
Accrued drug development costs
|
|
|
3,779
|
|
|
|
—
|
|
|
|
3,779
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
36,215
|
|
|
|
26,540
|
|
|
|
62,755
|
|
|
Capital lease obligations, net of current portion
|
|
|
102
|
|
|
|
—
|
|
|
|
102
|
|
|
Deferred revenue and other credits, net of current portion
|
|
|
27,512
|
|
|
|
—
|
|
|
|
27,512
|
|
|
Total liabilities
|
|
|
63,829
|
|
|
|
26,540
|
|
|
|
90,369
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies (Note 5)
|
|
|
|
|
|
|
|
|
|
|
|
|
F-68
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2009
|
|
|
Adjustments
|
|
|
September 30, 2009
|
|
|
|
|
(Previously reported)
|
|
|
|
|
|
(As restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Stockholders’ Equity:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock, par value $0.001 per share,
5,000,000 shares authorized:
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Series B Junior participating preferred stock,
1,000,000 shares authorized, no shares issued and
outstanding
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Series E convertible voting preferred stock,
2,000 shares authorized, stated value $10,000 per share,
$0.8 million aggregate liquidation value, issued and
outstanding, 68 shares at September 30, 2009
|
|
|
419
|
|
|
|
—
|
|
|
|
419
|
|
|
Common stock, par value $0.001 per share,
100,000,000 shares authorized:
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Issued and outstanding, 48,741,009 shares at
September 30, 2009
|
|
|
49
|
|
|
|
—
|
|
|
|
49
|
|
|
Additional paid-in capital
|
|
|
398,967
|
|
|
|
(29,489
|
)
|
|
|
369,478
|
|
|
Non-controlling interest
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive loss
|
|
|
(136
|
)
|
|
|
—
|
|
|
|
(136
|
)
|
|
Accumulated deficit
|
|
|
(274,679
|
)
|
|
|
2,949
|
|
|
|
(271,730
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
124,620
|
|
|
|
(26,540
|
)
|
|
|
98,080
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
$
|
188,449
|
|
|
$
|
—
|
|
|
$
|
188,449
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-69
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months
|
|
|
|
|
|
Three Months
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
September 30, 2009
|
|
|
Adjustments
|
|
|
September 30, 2009
|
|
|
|
|
(Previously Reported)
|
|
|
|
|
|
(As Restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Revenues
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product sales
|
|
$
|
4,976
|
|
|
|
—
|
|
|
$
|
4,976
|
|
|
License and contract revenue
|
|
|
2,125
|
|
|
|
—
|
|
|
|
2,125
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
$
|
7,101
|
|
|
|
—
|
|
|
$
|
7,101
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of product sold (excludes amortization of purchased
intangibles shown below)
|
|
$
|
2,429
|
|
|
|
—
|
|
|
$
|
2,429
|
|
|
Amortization of purchased intangibles
|
|
|
950
|
|
|
|
—
|
|
|
|
950
|
|
|
Research and development
|
|
|
5,488
|
|
|
|
—
|
|
|
|
5,488
|
|
|
Acquired in-process research and development
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Selling, general and administrative
|
|
|
6,995
|
|
|
|
—
|
|
|
|
6,995
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
15,862
|
|
|
|
—
|
|
|
|
15,862
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(8,761
|
)
|
|
|
—
|
|
|
|
(8,761
|
)
|
|
Change in fair value of common stock warrant liability
|
|
|
—
|
|
|
|
8,863
|
|
|
|
8,863
|
|
|
Other income, net
|
|
|
372
|
|
|
|
—
|
|
|
|
372
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated loss
|
|
|
(8,389
|
)
|
|
|
8,863
|
|
|
|
474
|
|
|
Net loss attributable to non-controlling interest
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income — attributable to Spectrum
|
|
$
|
(8,389
|
)
|
|
$
|
8,863
|
|
|
$
|
474
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income per share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
(0.20
|
)
|
|
|
|
|
|
$
|
0.01
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted
|
|
$
|
(0.19
|
)
|
|
|
|
|
|
$
|
0.01
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
|
42,364,983
|
|
|
|
|
|
|
|
42,364,983
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted
|
|
|
44,191,257
|
|
|
|
|
|
|
|
44,191,257
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-70
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine-Months
|
|
|
|
|
|
Nine-Months
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
September 30, 2009
|
|
|
Adjustments
|
|
|
September 30, 2009
|
|
|
|
|
(Previously Reported)
|
|
|
|
|
|
(As Restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Cash Flows From Operating Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(17,471
|
)
|
|
$
|
(11,759
|
)
|
|
$
|
(29,230
|
)
|
|
Adjustments to reconcile net income (loss) to net cash provided
by operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortization of deferred revenue
|
|
|
(6,375
|
)
|
|
|
—
|
|
|
|
(6,375
|
)
|
|
Depreciation and amortization
|
|
|
3,248
|
|
|
|
—
|
|
|
|
3,248
|
|
|
Fair value adjustments of common stock warrants
|
|
|
—
|
|
|
|
11,759
|
|
|
|
11,759
|
|
|
Share-based compensation expense
|
|
|
6,013
|
|
|
|
—
|
|
|
|
6,013
|
|
|
Fair value of common stock issued in connection with drug license
|
|
|
935
|
|
|
|
—
|
|
|
|
935
|
|
|
Minority interest in consolidated entities
|
|
|
(1,146
|
)
|
|
|
—
|
|
|
|
(1,146
|
)
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
561
|
|
|
|
—
|
|
|
|
561
|
|
|
Inventory
|
|
|
(319
|
)
|
|
|
—
|
|
|
|
(319
|
)
|
|
Prepaid expenses and other assets
|
|
|
314
|
|
|
|
—
|
|
|
|
314
|
|
|
Accounts payable and accrued obligations
|
|
|
7,663
|
|
|
|
—
|
|
|
|
7,663
|
|
|
Accrued compensation and related taxes
|
|
|
(480
|
)
|
|
|
—
|
|
|
|
(480
|
)
|
|
Deferred revenue and other credits
|
|
|
(35
|
)
|
|
|
—
|
|
|
|
(35
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities
|
|
|
(7,092
|
)
|
|
|
—
|
|
|
|
(7,092
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Investing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net purchases of marketable securities
|
|
|
(65,538
|
)
|
|
|
—
|
|
|
|
(65,538
|
)
|
|
Investment in Zevalin acquisition
|
|
|
(22,687
|
)
|
|
|
—
|
|
|
|
(22,687
|
)
|
|
Purchases of property and equipment
|
|
|
(388
|
)
|
|
|
—
|
|
|
|
(388
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
|
(88,613
|
)
|
|
|
—
|
|
|
|
(88,613
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock and warrants, net of
related offering costs and expenses
|
|
|
95,810
|
|
|
|
—
|
|
|
|
95,810
|
|
|
Proceeds from sale of common stock to employees —
shelf takedown
|
|
|
1,167
|
|
|
|
—
|
|
|
|
1,167
|
|
|
Repurchase of warrants
|
|
|
(71
|
)
|
|
|
—
|
|
|
|
(71
|
)
|
|
Proceeds from exercise of stock options
|
|
|
1,145
|
|
|
|
—
|
|
|
|
1,145
|
|
|
Repurchase of stock options pursuant to tender offer
|
|
|
(2,520
|
)
|
|
|
—
|
|
|
|
(2,520
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by financing activities
|
|
|
95,531
|
|
|
|
—
|
|
|
|
95,531
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase in cash and cash equivalents
|
|
|
(174
|
)
|
|
|
—
|
|
|
|
(174
|
)
|
F-71
Spectrum
Pharmaceuticals, Inc. and Subsidiaries
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine-Months
|
|
|
|
|
|
Nine-Months
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
September 30, 2009
|
|
|
Adjustments
|
|
|
September 30, 2009
|
|
|
|
|
(Previously Reported)
|
|
|
|
|
|
(As Restated)
|
|
|
|
|
(In thousands, except share and per share data)
|
|
|
|
|
Cash and cash equivalents, beginning of period
|
|
|
9,860
|
|
|
|
—
|
|
|
|
9,860
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
$
|
9,686
|
|
|
|
—
|
|
|
$
|
9,686
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental Cash Flow Information:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest paid
|
|
$
|
10
|
|
|
|
—
|
|
|
$
|
10
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income taxes paid
|
|
$
|
45
|
|
|
|
—
|
|
|
$
|
45
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Schedule of Non-Cash Investing and Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of common stock issued in connection with drug license
|
|
$
|
935
|
|
|
|
—
|
|
|
$
|
935
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of restricted stock granted employees and directors
|
|
$
|
226
|
|
|
|
—
|
|
|
$
|
226
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of stock issued to match employee 401k contributions
|
|
$
|
342
|
|
|
|
—
|
|
|
$
|
342
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of equity awarded to consultants and placement agents
|
|
$
|
111
|
|
|
|
—
|
|
|
$
|
111
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-72
| |
|
|
|
|
|
|
|
Three Months
|
|
|
|
|
Ended
|
|
|
|
|
December 31, 2009
|
|
|
|
|
Revenues
|
|
|
|
|
|
Product sales
|
|
$
|
5,195
|
|
|
License and contract revenue
|
|
|
3,425
|
|
|
|
|
|
|
|
|
Total revenues
|
|
$
|
8,620
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
Cost of product sold (excludes amortization of purchased
intangibles shown below)
|
|
|
2,446
|
|
|
Selling, general and administrative
|
|
|
11,069
|
|
|
Research and development
|
|
|
3,525
|
|
|
Amortization of purchased intangibles
|
|
|
870
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
17,910
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(9,290
|
)
|
|
Change in fair value of common stock warrant liability
|
|
|
19,834
|
|
|
Other income, net
|
|
|
61
|
|
|
|
|
|
|
|
|
Consolidated loss
|
|
|
10,605
|
|
|
Income tax expense
|
|
|
(421
|
)
|
|
Net income
|
|
$
|
10,184
|
|
|
|
|
|
|
|
|
Net income per share
|
|
|
|
|
|
Basic
|
|
$
|
0.21
|
|
|
|
|
|
|
|
|
Diluted
|
|
$
|
0.20
|
|
|
|
|
|
|
|
|
Weighted average common shares:
|
|
|
|
|
|
Basic
|
|
|
48,425,486
|
|
|
|
|
|
|
|
|
Diluted
|
|
|
49,704,126
|
|
|
|
|
|
|
|
In connection with the preparation of the Consolidated Financial
Statements, we have evaluated subsequent events through the
filing date of this form 10-K.
In February 2010, we entered into a licensing and collaboration
agreement with TopoTarget, for the development and
commercialization of belinostat, a drug being studied in
multiple indications, including a Phase 2 registrational trial
for patients with Peripheral T-Cell Lymphoma. The agreement
provides that we have the exclusive right to make, develop and
commercialize belinostat in North America and India, with an
option for China. In consideration for the rights granted under
the license agreement, we paid TopoTarget an up-front fee of
$30 million. In addition, we will pay up to
$313 million and one million shares of Spectrum common
stock based on the achievement of certain development,
regulatory and sales milestones, as well as royalties on net
sales of belinostat.
Index to
Exhibits
| |
|
|
|
|
Exhibit
|
|
|
|
No.
|
|
Description
|
|
|
|
|
2
|
.1
|
|
Asset Purchase Agreement by and between the Registrant, Targent
Inc. and Certain Stockholders of Targent, Inc., dated March 17
2006. (Filed as Exhibit 2.1 to
Form 10-K/A,
Amendment No. 1, as filed with the Securities and Exchange
Commission on May 1, 2006, and incorporated herein by
reference.)
|
| |
|
|
|
|
Exhibit
|
|
|
|
No.
|
|
Description
|
|
|
|
|
2
|
.2
|
|
Asset Purchase Agreement by and between the Registrant and Par
Pharmaceutical, Inc., dated as of May 6, 2008. (Filed as
Exhibit 2.1 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 11, 2008, and incorporated herein by reference.)
|
|
|
2
|
.3#
|
|
Purchase and Formation Agreement, dated as of November 26,
2008, by and among the Registrant, Cell Therapeutics, Inc. and
RIT Oncology, LLC. (Filed as Exhibit 2.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
December 19, 2008, and incorporated herein by reference.)
|
|
|
2
|
.4#
|
|
Limited Liability Company Interest Assignment Agreement, dated
as of March 15, 2009, by and between the Registrant and
Cell Therapeutics, Inc. (Filed as Exhibit 2.1 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
May 15, 2009, and incorporated herein by reference.)
|
|
|
3
|
.1
|
|
Amended Certificate of Incorporation, as filed. (Filed as
Exhibit 3.1 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 8, 2006, and incorporated herein by reference.)
|
|
|
3
|
.2
|
|
Form of Amended and Restated Bylaws of the Registrant. (Filed as
Exhibit 3.1 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 16, 2004, and incorporated herein by reference.)
|
|
|
4
|
.1
|
|
Rights Agreement, dated as of December 13, 2000, between
the Registrant and ComputerShare Trust Company, N.A.
(formerly U.S. Stock Transfer Corporation), as Rights Agent,
which includes as Exhibit A thereto the form of Certificate
of Designation for the Series B Junior Participating
Preferred Stock, as Exhibit B thereto the Form of Rights
Certificate and as Exhibit C thereto a Summary of Terms of
Stockholder Rights Plan. (Filed as Exhibit 4.1 to
Form 8-A12G,
as filed with the Securities and Exchange Commission on
December 26, 2000, and incorporated herein by reference.)
|
|
|
4
|
.2
|
|
Amendment No. 1 to the Rights Agreement, dated as of
December 13, 2000 by and between the Registrant and
ComputerShare Trust Company, N.A. (formerly U.S. Stock
Transfer Corporation). (Filed as Exhibit 4.1 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 14, 2003, and incorporated herein by reference.)
|
|
|
4
|
.3
|
|
Registration Rights Agreement, dated as of September 26,
2003, by and among the Registrant and the persons listed on
Schedule 1 attached thereto. (Filed as Exhibit 4.4 to
Form 8-K,
as filed with the Securities and Exchange Commission on
September 30, 2003, and incorporated herein by reference.)
|
|
|
4
|
.4
|
|
Investor Rights Agreement, dated as of April 20, 2004, by
and among the Registrant and the persons listed on
Schedule 1 attached thereto. (Filed as Exhibit 4.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
April 23, 2004, and incorporated herein by reference.)
|
|
|
4
|
.5
|
|
Amendment No. 2 to the Rights Agreement, dated as of
December 13, 2000, by and between the Registrant and
ComputerShare Trust Company, N.A. (formerly U.S. Stock
Transfer Corporation). (Filed as Exhibit 4.1 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
May 17, 2004, and incorporated herein by reference.)
|
|
|
4
|
.6
|
|
Amendment No. 3 to the Rights Agreement, dated as of
December 13, 2000 by and between the Registrant and
ComputerShare Trust Company, N.A. (formerly U.S. Stock
Transfer Corporation). (Filed as Exhibit 4.2 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
May 17, 2004, and incorporated herein by reference.)
|
|
|
4
|
.7
|
|
Warrant issued by the Registrant to a Consultant, dated as of
September 17, 2003. (Filed as Exhibit 4.3 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
May 17, 2004, and incorporated herein by reference.)
|
|
|
4
|
.8
|
|
Amendment No. 1, dated as of November 2, 2005, to
Warrant issued by the Registrant to a Consultant, dated as of
September 17, 2003. (Filed as Exhibit 4.2 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
November 4, 2005, and incorporated herein by reference.)
|
|
|
4
|
.9
|
|
Warrant issued by the Registrant to a Consultant, dated as of
September 20, 2005. (Filed as Exhibit 4.3 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
November 4, 2005, and incorporated herein by reference.)
|
|
|
4
|
.10
|
|
Form of Warrant, dated September 15, 2005. (Filed as
Exhibit 4.35 to
Form 10-K,
as filed with the Securities and Exchange Commission on
March 15, 2006, and incorporated herein by reference.)
|
|
|
4
|
.11
|
|
Registration Rights Agreement, dated as of April 20, 2006,
by and among the Registrant and Targent, Inc. (Filed as
Exhibit 4.2 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
May 8, 2006, and incorporated herein by reference.)
|
| |
|
|
|
|
Exhibit
|
|
|
|
No.
|
|
Description
|
|
|
|
|
4
|
.12
|
|
Fourth Amendment to Rights Agreement, dated July 7, 2006.
(Filed as Exhibit 4.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
July 12, 2006, and incorporated herein by reference.)
|
|
|
4
|
.13
|
|
Amendment No. 5 to the Rights Agreement, dated as of
December 13, 2000, by and between the Registrant and
ComputerShare Trust Company, N.A. (formerly U.S. Stock
Transfer Corporation). (Filed as Exhibit 4.2 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
November 3, 2006, and incorporated herein by reference.)
|
|
|
4
|
.14
|
|
Amendment No. 2, dated as of March 26, 2007, to
Warrant issued by the Registrant to a Consultant, dated as of
September 17, 2003. (Filed as Exhibit 4.1 to
Form 10-K/A,
as filed with the Securities and Exchange Commission on
April 30, 2007, and incorporated herein by reference.)
|
|
|
4
|
.15
|
|
Warrant issued by the Registrant to a Consultant, dated as of
April 28, 2008. (Filed as Exhibit 4.1 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 11, 2008, and incorporated herein by reference.)
|
|
|
4
|
.16
|
|
Form of Common Stock Purchase Warrant. (Filed as
Exhibit 4.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
May 28, 2009, and incorporated herein by reference.)
|
|
|
4
|
.17
|
|
Form of Common Stock Purchase Warrant. (Filed as
Exhibit 4.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
June 18, 2009, and incorporated herein by reference.)
|
|
|
4
|
.18
|
|
Form of Common Stock Purchase Warrant. (Filed as
Exhibit 4.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
July 2, 2009, and incorporated herein by reference.)
|
|
|
4
|
.19
|
|
Form of Common Stock Purchase Warrant (Filed as Exhibit 4.1
to
Form 8-K,
as filed with the Securities and Exchange Commission on
September 23, 2009, and incorporated herein by reference).
|
|
|
10
|
.1
|
|
Industrial Lease Agreement, dated as of January 16, 1997,
between the Registrant and the Irvine Company. (Filed as
Exhibit 10.11 to
Form 10-KSB,
as filed with the Securities and Exchange Commission on
March 31, 1997, and incorporated herein by reference.)
|
|
|
10
|
.2
|
|
Preferred Stock and Warrant Purchase Agreement, dated as of
September 26, 2003, by and among the Registrant and the
purchasers listed on Schedule 1 attached thereto. (Filed as
Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
September 30, 2003, and incorporated herein by reference.)
|
|
|
10
|
.3
|
|
First Amendment, dated March 25, 2004, to Industrial Lease
Agreement dated as of January 16, 1997 by and between the
Registrant and the Irvine Company. (Filed as Exhibit 10.1
to
Form 10-Q,
as filed with the Securities and Exchange Commission on
May 17, 2004, and incorporated herein by reference.)
|
|
|
10
|
.4
|
|
Common Stock and Warrant Purchase Agreement, dated as of
April 20, 2004, by and among the Registrant and the
purchasers listed on Schedule 1 attached thereto. (Filed as
Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
April 23, 2004, and incorporated herein by reference.)
|
|
|
10
|
.5#
|
|
License and Collaboration Agreement by and between the
Registrant and Zentaris GmbH, dated as of August 12, 2004.
(Filed as Exhibit 10.1 to
Form S-3/A,
as filed with the Securities and Exchange Commission on
January 21, 2005, and incorporated herein by reference.)
|
|
|
10
|
.6*
|
|
Form of Stock Option Agreement under the 2003 Amended and
Restated Incentive Award Plan. (Filed as Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
December 17, 2004, and incorporated herein by reference.)
|
|
|
10
|
.7#
|
|
License Agreement by and between the Registrant and Chicago
Labs, Inc. (Filed as Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
February 25, 2005, and incorporated herein by reference.)
|
|
|
10
|
.8*
|
|
Form of Non-Employee Director Stock Option Agreement under the
2003 Amended and Restated Incentive Award Plan. (Filed as
Exhibit 10.5 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
May 10, 2005, and incorporated herein by reference.)
|
|
|
10
|
.9*
|
|
Restricted Stock Award Grant Notice and Restricted Stock Award
Agreement under the 2003 Amended and Restated Incentive Award
Plan. (Filed as Exhibit 10.44 to
Form 10-K,
as filed with the Securities and Exchange Commission on
March 15, 2006, and incorporated herein by reference.)
|
|
|
10
|
.10#
|
|
License Agreement between the Registrant and Merck Eprova AG,
dated May 23, 2006. (Filed as Exhibit 10.1 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 8, 2006, and incorporated herein by reference.)
|
| |
|
|
|
|
Exhibit
|
|
|
|
No.
|
|
Description
|
|
|
|
|
10
|
.11*
|
|
Third Amended and Restated 1997 Stock Incentive Plan. (Filed as
Exhibit 10.2 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
November 3, 2006, and incorporated herein by reference.)
|
|
|
10
|
.12#
|
|
Agreement by and between the Registrant and Glaxo Group Limited
(d/b/a GlaxoSmithKline), dated November 10, 2006. (Filed as
Exhibit 10.38 to
Form 10-K,
as filed with the Securities and Exchange Commission on
March 14, 2007, and incorporated herein by reference.)
|
|
|
10
|
.13*
|
|
2003 Amended and Restated Incentive Award Plan. (Filed as
Exhibit 10.3 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 9, 2007, and incorporated herein by reference.)
|
|
|
10
|
.14#
|
|
License Agreement by and between the Registrant and Indena,
S.p.A., dated July 17, 2007. (Filed as Exhibit 10.4 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
November 9, 2007, and incorporated herein by reference.)
|
|
|
10
|
.15*
|
|
Executive Employment Agreement by and between the Registrant and
Rajesh C. Shrotriya, M.D., entered into June 20, 2008
and effective as of January 2, 2008. (Filed as
Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
June 26, 2008, and incorporated herein by reference.)
|
|
|
10
|
.16
|
|
Consulting Agreement by and between the Registrant and Luigi
Lenaz, M.D., effective as of July 1, 2008. (Filed as
Exhibit 10.2 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 11, 2008, and incorporated herein by reference.)
|
|
|
10
|
.17*
|
|
Form of Indemnity Agreement of the Registrant. (Filed as
Exhibit 10.32 to
Form 10-K,
as filed with the Securities and Exchange Commission on
March 31, 2009, and incorporated herein by reference.)
|
|
|
10
|
.18#
|
|
License, Development, Supply and Distribution Agreement, dated
October 28, 2008, by and among the Registrant, Allergan
Sales, LLC, Allergan USA, Inc. and Allergan, Inc. (Filed as
Exhibit 10.33 to
Form 10-K,
as filed with the Securities and Exchange Commission on
March 31, 2009, and incorporated herein by reference.)
|
|
|
10
|
.19*
|
|
Form of Stock Purchase Agreement, dated May 6, 2009. (Filed
as Exhibit 1.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
May 7, 2009, and incorporated herein by reference.)
|
|
|
10
|
.20
|
|
Form of Securities Purchase Agreement, dated May 27, 2009.
(Filed as Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
May 28, 2009, and incorporated herein by reference.)
|
|
|
10
|
.21
|
|
Placement Agency Agreement between the Registrant and
Rodman & Renshaw, LLC, dated May 26, 2009. (Filed
as Exhibit 1.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
May 28, 2009, and incorporated herein by reference.)
|
|
|
10
|
.22
|
|
Form of Securities Purchase Agreement, dated June 15, 2009.
(Filed as Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
June 18, 2009, and incorporated herein by reference.)
|
|
|
10
|
.23
|
|
Placement Agency Agreement between the Registrant and
Rodman & Renshaw, LLC, June 15, 2009. (Filed as
Exhibit 1.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
June 18, 2009, and incorporated herein by reference.)
|
|
|
10
|
.24*
|
|
2009 Employee Stock Purchase Plan. (Filed as Exhibit 99.1
to
Form S-8,
as filed with the Securities and Exchange Commission on
June 29, 2009, and incorporated herein by reference.)
|
|
|
10
|
.25*
|
|
2009 Incentive Award Plan. (Filed as Exhibit 99.2 to
Form S-8,
as filed with the Securities and Exchange Commission on
June 29, 2009, and incorporated herein by reference.)
|
|
|
10
|
.26
|
|
Form of Securities Purchase Agreement, dated June 30, 2009.
(Filed as Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
July 2, 2009, and incorporated herein by reference.)
|
|
|
10
|
.27
|
|
Placement Agency Agreement between the Registrant and
Rodman & Renshaw, LLC, dated June 30, 2009.
(Filed as Exhibit 1.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
July 2, 2009, and incorporated herein by reference.)
|
|
|
10
|
.28*
|
|
2003 Amended and Restated Incentive Award Plan. (Filed as
Exhibit 10.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
July 2, 2009, and incorporated herein by reference.)
|
|
|
10
|
.29+
|
|
Fourth Amendment, dated July 29, 2009, to Industrial Lease
Agreement dated as of January 16, 1997 by and between the
Registrant and the Irvine Company.
|
|
|
10
|
.30*
|
|
Term Sheet for 2009 Incentive Award Plan Stock Option Award.
(Filed as Exhibit 10.8 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 13, 2009, and incorporated herein by reference.)
|
| |
|
|
|
|
Exhibit
|
|
|
|
No.
|
|
Description
|
|
|
|
|
10
|
.31*
|
|
Term Sheet for 2009 Incentive Award Plan, Nonqualified Stock
Option Award Awarded to Non-Employee Directors. (Filed as
Exhibit 10.9 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 13, 2009, and incorporated herein by reference.)
|
|
|
10
|
.32*
|
|
Term Sheet for 2009 Incentive Award Plan, Restricted Stock
Award. (Filed as Exhibit 10.10 to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 13, 2009, and incorporated herein by reference.)
|
|
|
10
|
.33*
|
|
Summary of Director Compensation. (Filed as Exhibit 10.11
to
Form 10-Q,
as filed with the Securities and Exchange Commission on
August 13, 2009, and incorporated herein by reference.)
|
|
|
10
|
.34
|
|
Placement Agency Agreement by and between the Registrant, and
Rodman & Renshaw, LLC, dated September 18, 2009
(Filed as Exhibit 1.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
September 23, 2009, and incorporated herein by reference.)
|
|
|
10
|
.35
|
|
Form of Securities Purchase Agreement, dated September 18,
2009 (Filed as Exhibit 1.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
September 23, 2009, and incorporated herein by reference.)
|
|
|
10
|
.36#+
|
|
License Agreement, dated November 6, 2009, by and between
the Registrant and Nippon Kayaku Co., Ltd.
|
|
|
10
|
.37#+
|
|
License and Collaboration Agreement, dated February 2,
2010, by and between the Registrant and TopoTarget A/S.
|
|
|
16
|
.1
|
|
Letter from Kelly and Company to the Securities and Exchange
Commission, dated December 3, 2009. (Filed as
Exhibit 16.1 to
Form 8-K,
as filed with the Securities and Exchange Commission on
December 8, 2009, and incorporated herein by reference.)
|
|
|
21
|
+
|
|
Subsidiaries of Registrant.
|
|
|
23
|
.1+
|
|
Consent of Ernst & Young LLP, Independent Registered
Public Accounting Firm.
|
|
|
23
|
.2+
|
|
Consent of Kelly & Company, Independent Registered
Public Accounting Firm.
|
|
|
24
|
.1
|
|
Power of Attorney (included in the signature page.)
|
|
|
31
|
.1+
|
|
Certification of Chief Executive Officer, pursuant to
Rule 13a-14(a)/15d-14(a)
of the Securities Exchange Act of 1934.
|
|
|
31
|
.2+
|
|
Certification of Vice President Finance, pursuant to
Rule 13a-14(a)/15d-14(a)
of the Securities Exchange Act of 1934.
|
|
|
32
|
.1+
|
|
Certification of Chief Executive Officer, pursuant to
Rule 13a-14(b)/15d-14(b)
of the Securities Exchange Act of 1934 and 18 U.S.C.
Section 1350.
|
|
|
32
|
.2+
|
|
Certification of Vice President Finance, pursuant to
Rule 13a-14(b)/15d-14(b)
of the Securities Exchange Act of 1934 and 18 U.S.C.
Section 1350.
|
|
|
|
|
* |
|
Indicates a management contract or compensatory plan or
arrangement. |
| |
|
# |
|
Confidential portions omitted and filed separately with the U.S.
Securities and Exchange Commission pursuant to
Rule 24b-2
promulgated under the Securities Exchange Act of 1934, as
amended. |
| |
|
+ |
|
Filed herewith. |