Attached files
| file | filename |
|---|---|
| EX-99.4 - EX-99.4 - Loxo Oncology, Inc. | a17-14662_1ex99d4.htm |
| 8-K - 8-K - Loxo Oncology, Inc. | a17-14662_18k.htm |
| EX-99.3 - EX-99.3 - Loxo Oncology, Inc. | a17-14662_1ex99d3.htm |
| EX-99.2 - EX-99.2 - Loxo Oncology, Inc. | a17-14662_1ex99d2.htm |
| EX-99.1 - EX-99.1 - Loxo Oncology, Inc. | a17-14662_1ex99d1.htm |
Exhibit 99.5
2017 ASCO Conference Call June 4, 2017 1
Forward Looking Statements This presentation and the accompanying oral presentation contain “forward-looking” statements within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as: "anticipate," "intend," "plan," "goal," "seek," "believe," "project," "estimate," "expect," "strategy," "future, "likely," "may," "should," "will" and similar references to future periods. Examples of forward-looking statements include, among others, statements we make regarding our future financial performance, business plans and objectives, timing and success of our clinical trials, our ability to obtain regulatory approval or the timing of regulatory filings, the potential therapeutic benefits and economic value of our lead product candidate and second generation product candidate, potential growth opportunities, the timing or success of commercialization, financing plans, competitive position and industry environment. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: those related to our future financial performance, our ability to raise additional funding when needed, our ability to develop and maintain partnerships, our ability to identify and develop new products in a timely manner, the outcome, cost and timing of our product development activities and clinical trials, market size and acceptance of our targeted small molecule therapeutics and diagnostics, our ability to maintain, protect and enhance our brand and intellectual property, our ability to continue to stay in compliance with applicable laws and regulations, our ability to scale our business and make key hires and such other factors as discussed under the section titled “Risk Factors” and elsewhere in our annual report on Form 10-K and quarterly reports on Form 10-Q that we filed with the Securities and Exchange Commission (“SEC”) as well as our other filings and the documents incorporated by reference therein, with the SEC. Any forward-looking statement made by us in this presentation and the accompanying oral presentation is based only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. Certain information in this slide deck on larotrectinib is derived from the presentation made at the 2017 ASCO Annual Meeting by Dr. Hyman, an independent third-party investigator. 2

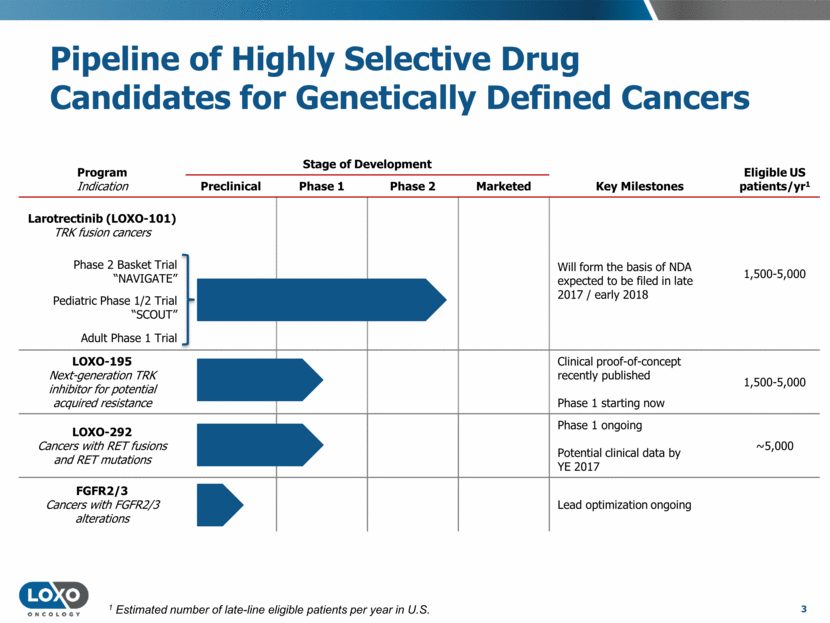
Pipeline of Highly Selective Drug Candidates for Genetically Defined Cancers 3 Program Indication Stage of Development Eligible US patients/yr1 Preclinical Phase 1 Phase 2 Marketed Key Milestones Larotrectinib (LOXO-101) TRK fusion cancers Will form the basis of NDA expected to be filed in late 2017 / early 2018 1,500-5,000 Phase 2 Basket Trial “NAVIGATE” Pediatric Phase 1/2 Trial “SCOUT” Adult Phase 1 Trial LOXO-195 Next-generation TRK inhibitor for potential acquired resistance Clinical proof-of-concept recently published Phase 1 starting now 1,500-5,000 LOXO-292 Cancers with RET fusions and RET mutations Phase 1 ongoing Potential clinical data by YE 2017 ~5,000 FGFR2/3 Cancers with FGFR2/3 alterations Lead optimization ongoing 1 Estimated number of late-line eligible patients per year in U.S.
Agenda Review of larotrectinib data presented at ASCO Dr. David Hyman, MSKCC Dr. Alex Drilon, MSKCC Dr. Theodore Laetsch, UT Southwestern Ongoing larotrectinib development activities LOXO-195 LOXO-292 Upcoming milestones / Q&A 4

5
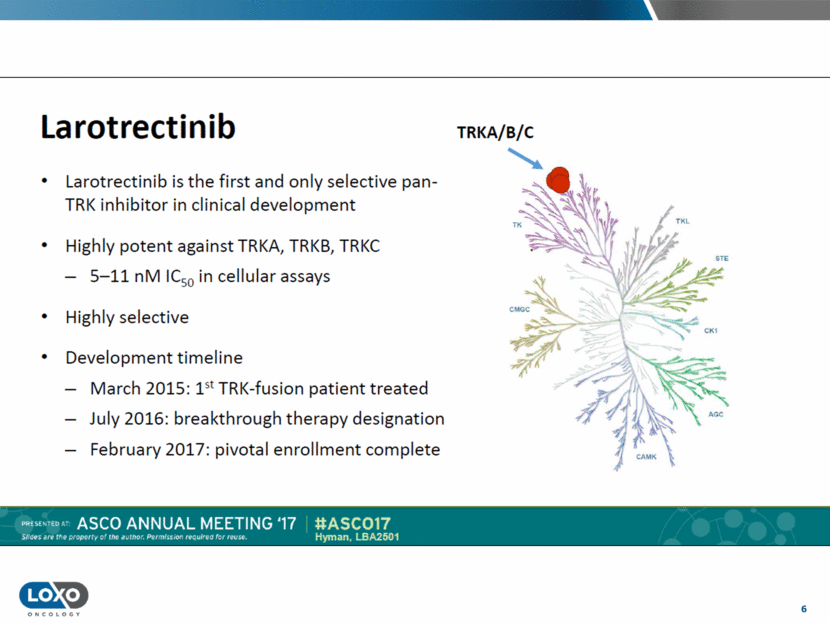
6
7
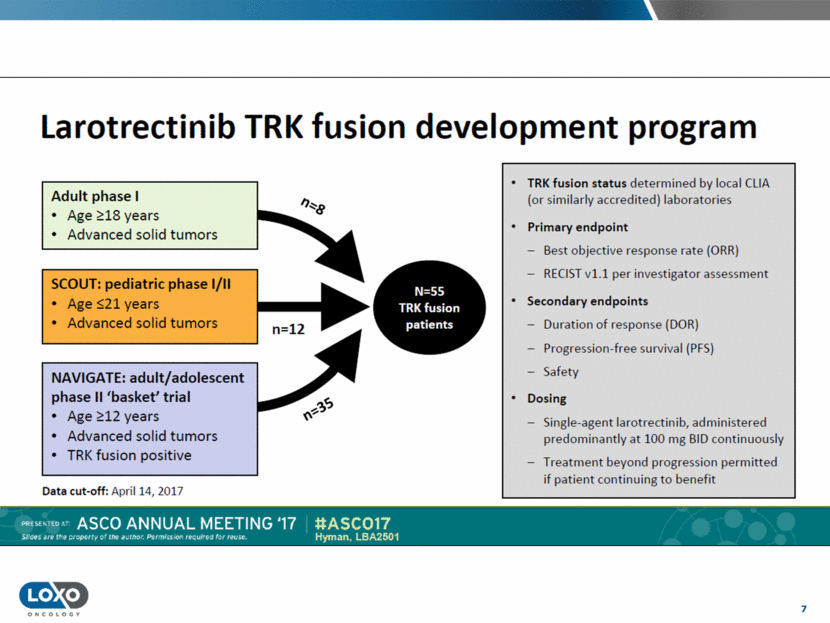
8
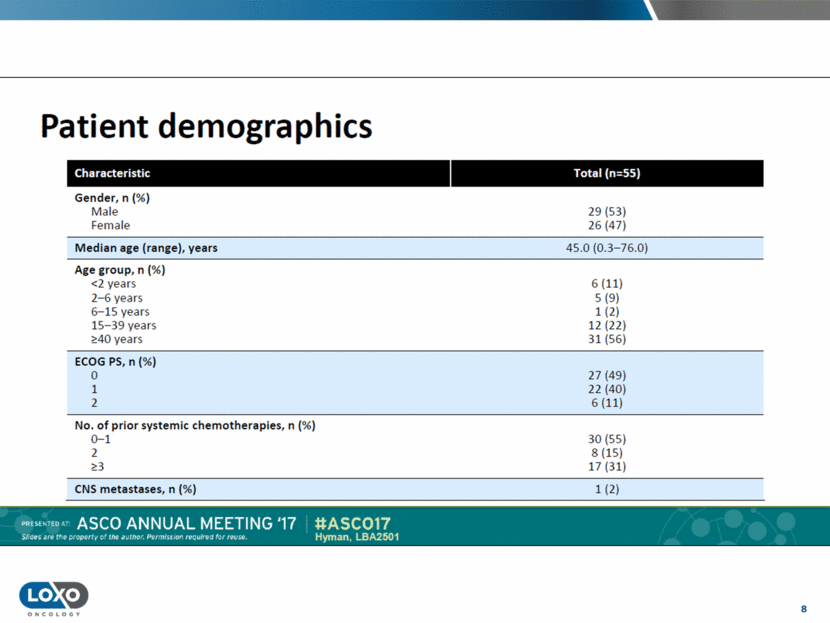
9
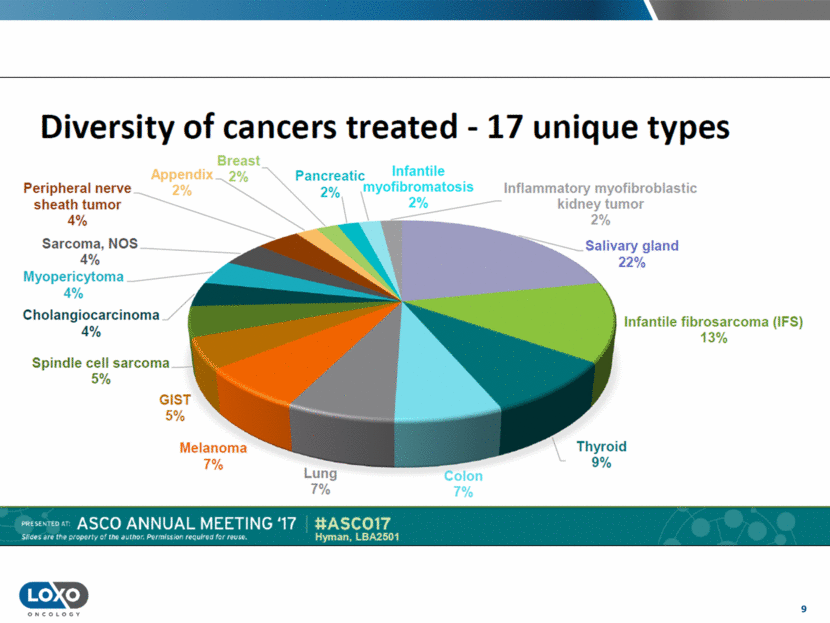
10
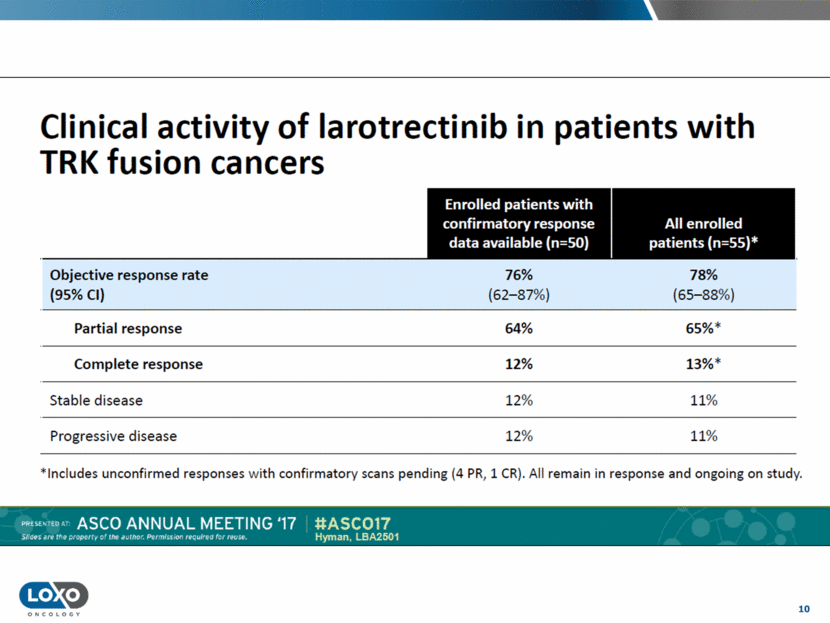
11
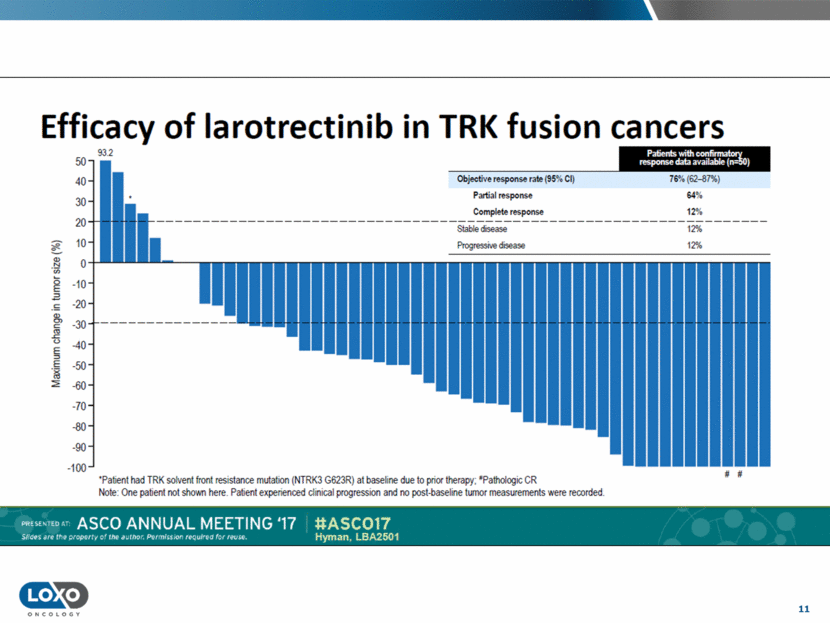
12
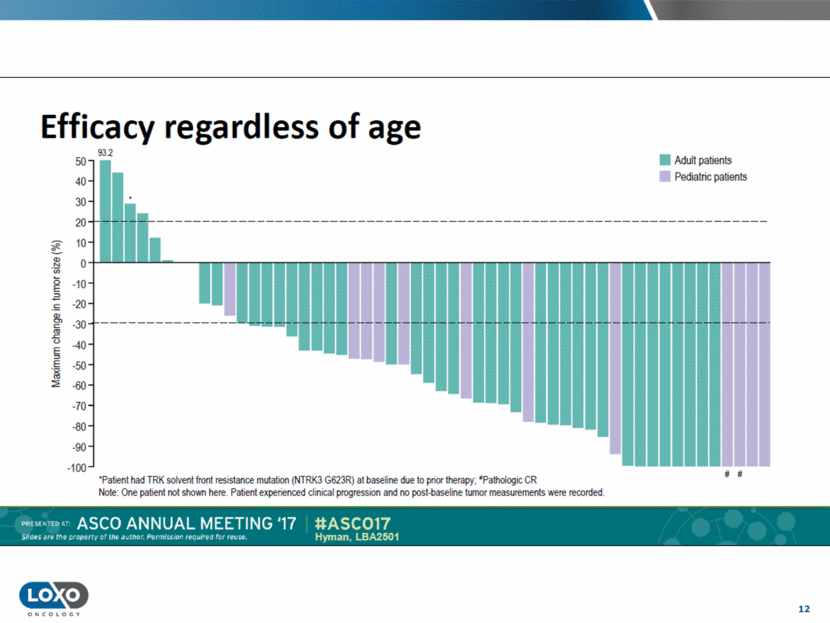
13
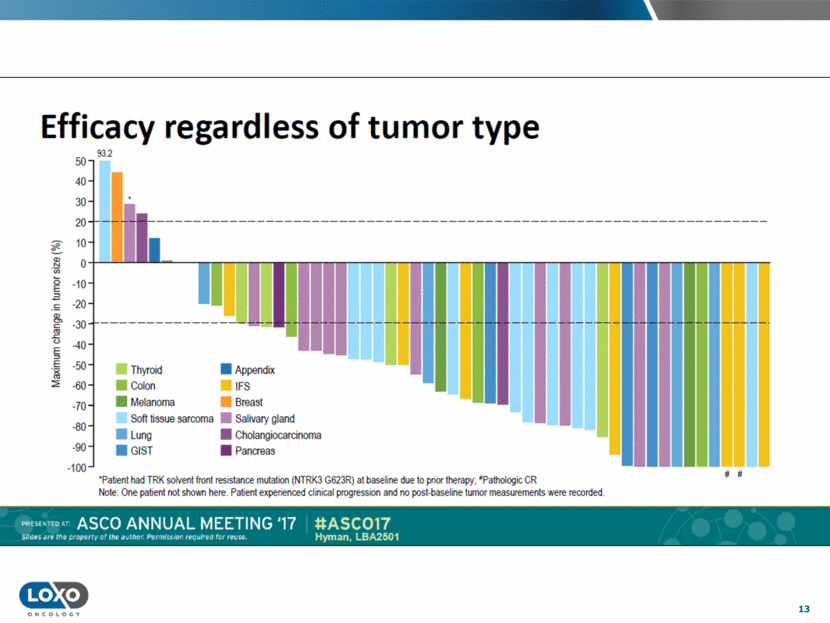
14
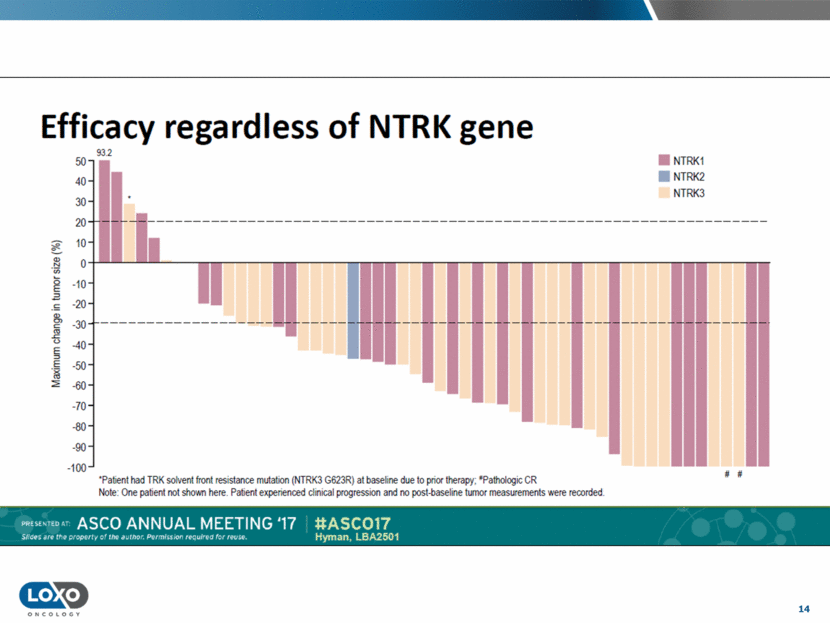
15
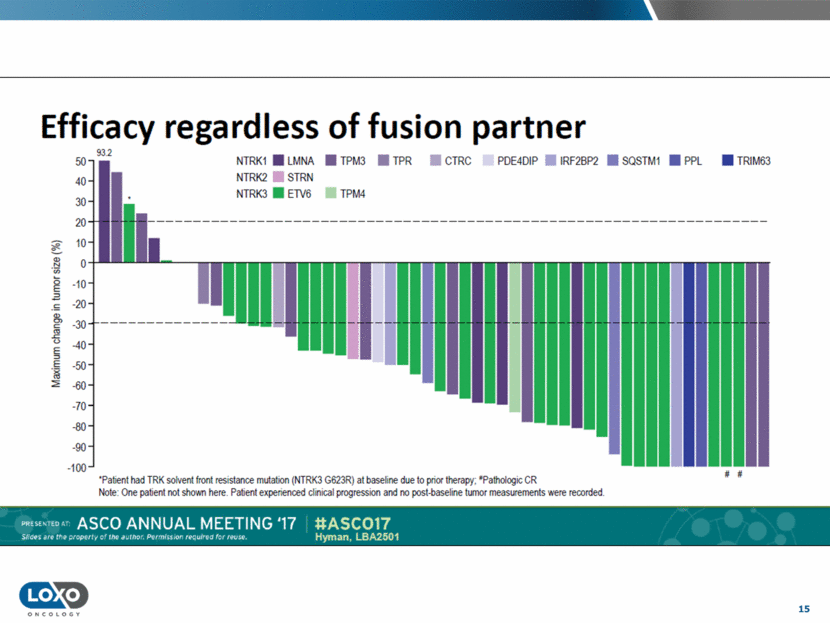
16
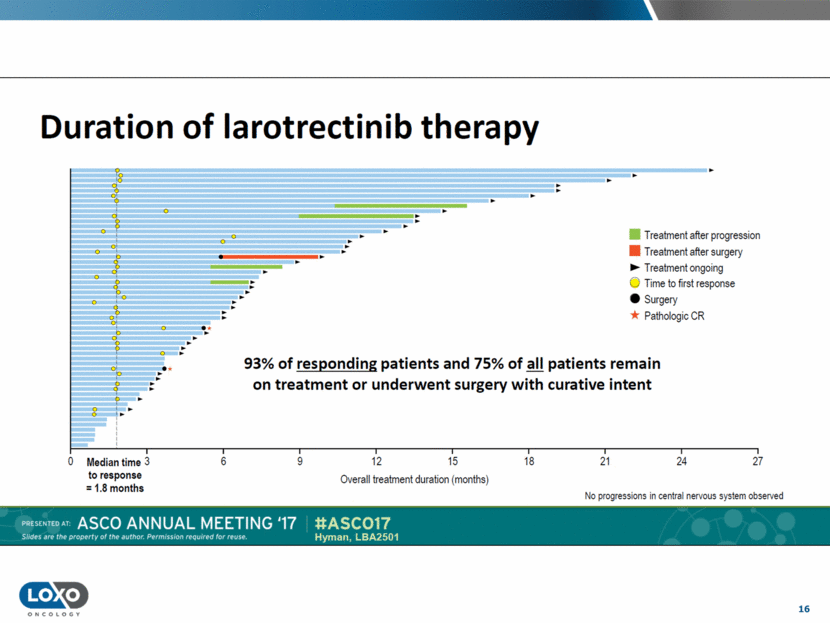
17
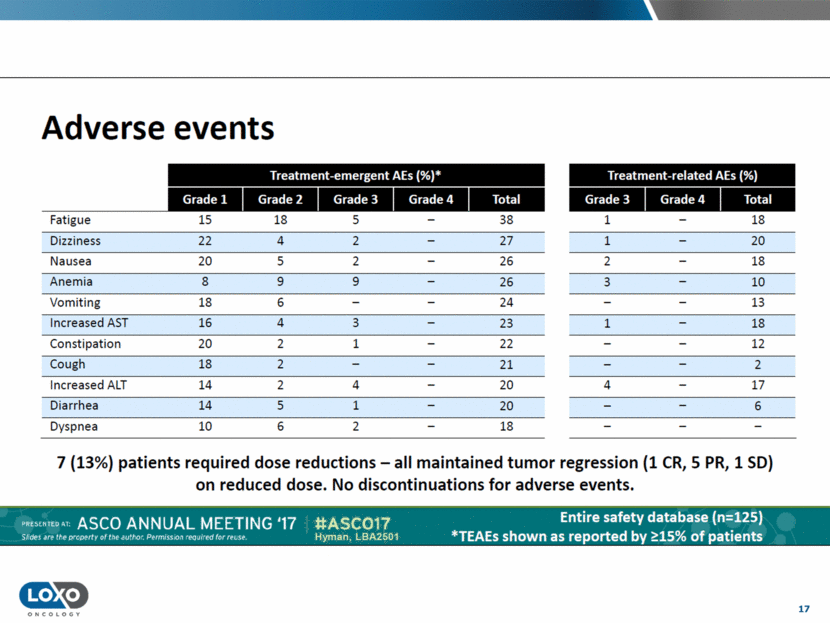
18
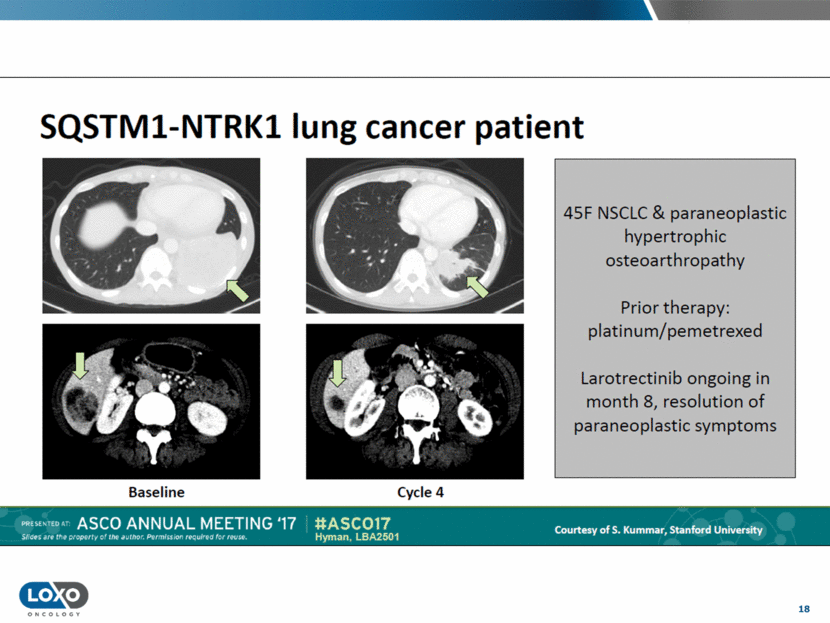
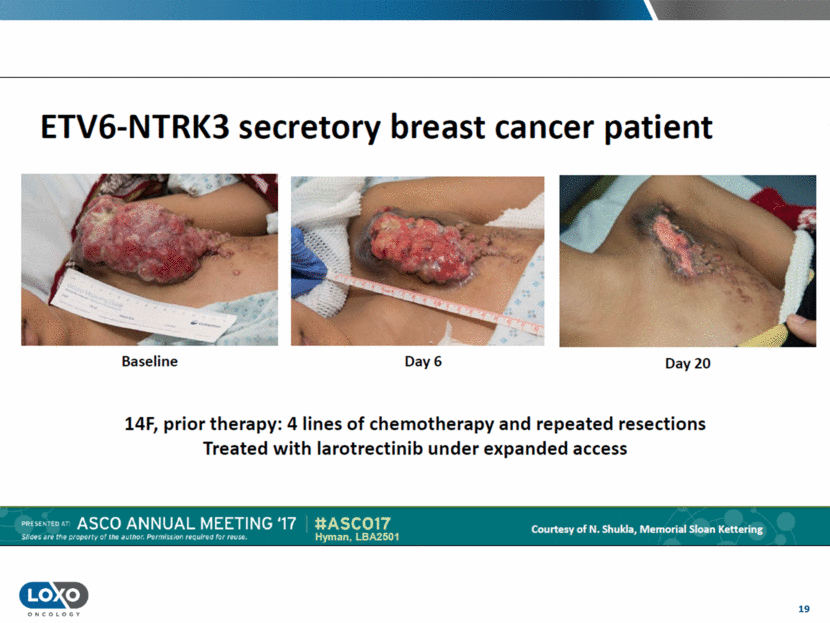
19
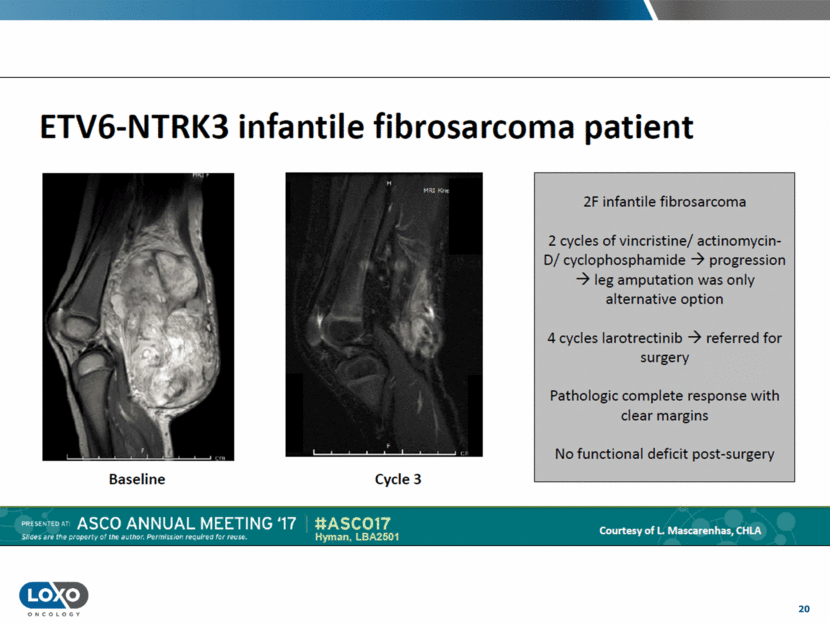
20
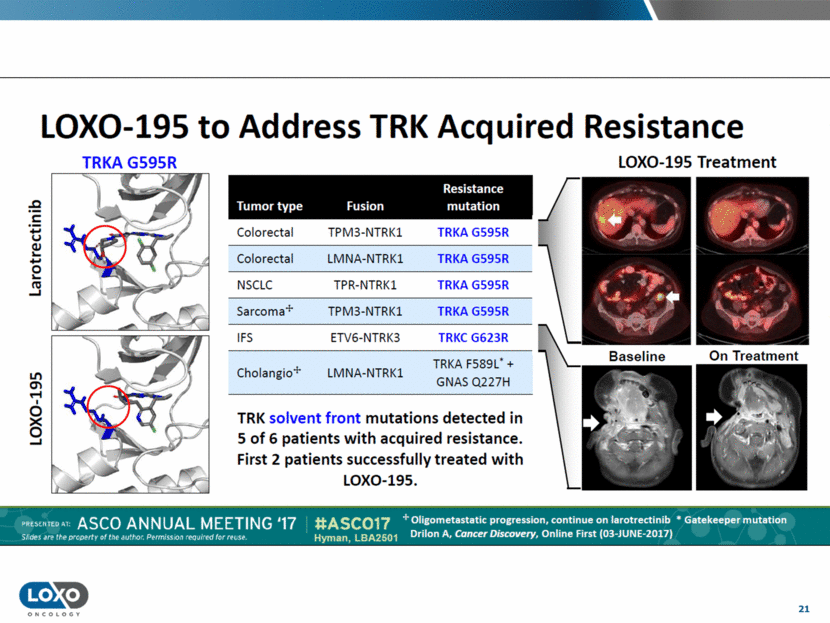
21
22
Path to Regulatory Filings Clinical Independent radiology review to be conducted 2H 2017 NDA filing will utilize a later data cut-off date relative to ASCO data Trials continue to enroll as mechanism of expanded access CMC scale-up and stability testing ongoing Dictates the expiration date of the commercial product Long-term toxicology ongoing Clinical pharmacology studies ongoing Drug-drug interactions Food effect ADME 23 – NDA submission expected in late 2017 or early 2018 – – MAA submission expected in 2018 –
Larotrectinib Diagnostics Strategy Multiple diagnostic relationships are likely Focused on immunohistochemistry (IHC) and next-generation sequencing (NGS) IHC Collaboration announced with Roche Ventana Loxo/Ventana are creating pan-TRK test 8,500+ installed base of Ventana instruments worldwide NGS Multiple players in an evolving space Keys for Loxo: (1) TRK fusion panel inclusion, (2) FDA path, (3) Payor traction 24
LOXO-195 25

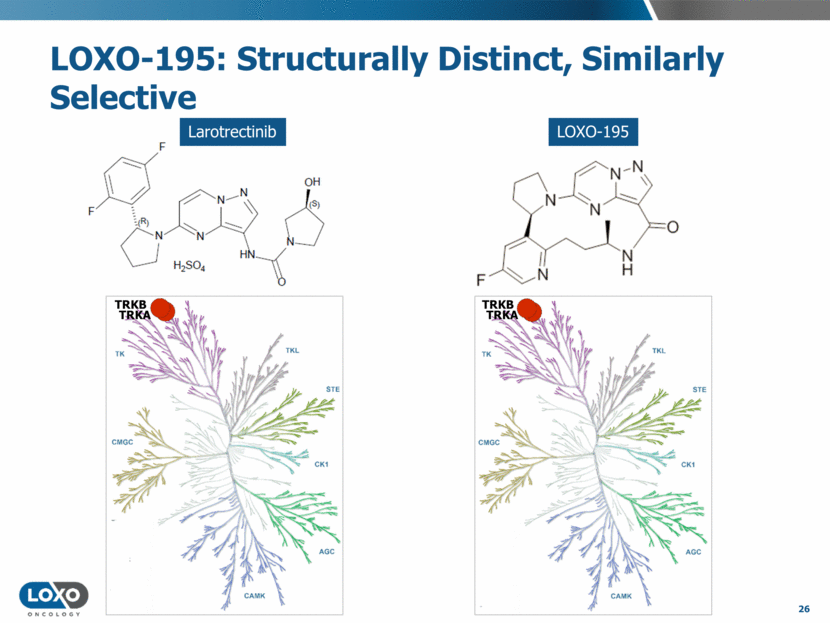
LOXO-195: Structurally Distinct, Similarly Selective 26 LOXO-195 Larotrectinib TRKB TRKB TRKA TRKA
LOXO-195 Clinical Development Clinical proof-of-concept established in Cancer Discovery publication First 2 emergency use patients with PRs 1 additional emergency use patient (unpublished) with limited duration of treatment amidst clinical deterioration 3 remaining larotrectinib RECIST progressions: 2 patients remain on larotrectinib post-progression, 1 patient with clinical deterioration too rapid for LOXO-195 initiation IND cleared by FDA Phase 1/2 trial startup in process Co-locate at high enrolling larotrectinib sites Co-locate at other sites with TRK fusion patients Plan to address patients with TRK fusion cancers harboring solvent front mutations, xDFG mutations, and gatekeeper mutations 27

LOXO-292 (RET) 28

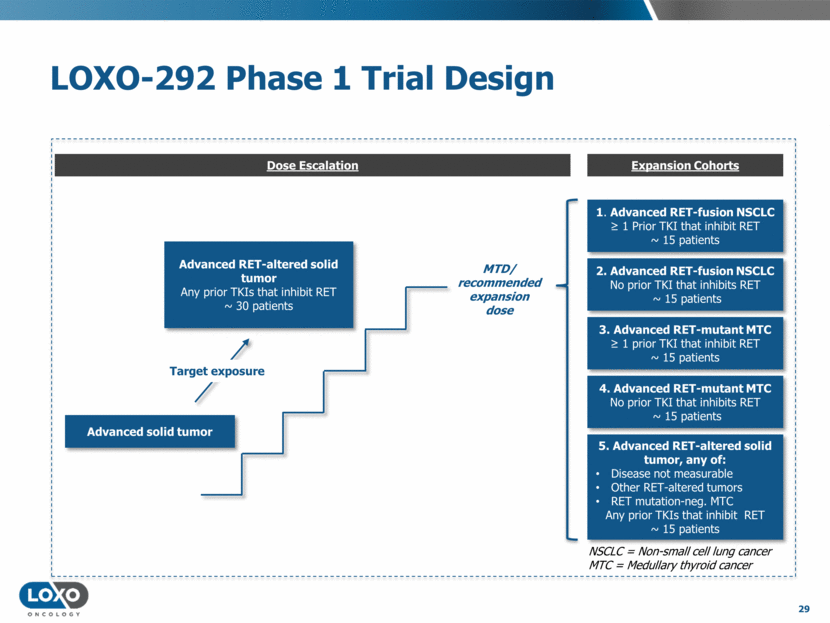
LOXO-292 Phase 1 Trial Design 29 Advanced RET-altered solid tumor Any prior TKIs that inhibit RET ~ 30 patients MTD/ recommended expansion dose 5. Advanced RET-altered solid tumor, any of: Disease not measurable Other RET-altered tumors RET mutation-neg. MTC Any prior TKIs that inhibit RET ~ 15 patients 1. Advanced RET-fusion NSCLC 1 Prior TKI that inhibit RET ~ 15 patients 2. Advanced RET-fusion NSCLC No prior TKI that inhibits RET ~ 15 patients 3. Advanced RET-mutant MTC 1 prior TKI that inhibit RET ~ 15 patients 4. Advanced RET-mutant MTC No prior TKI that inhibits RET ~ 15 patients Target exposure Advanced solid tumor NSCLC = Non-small cell lung cancer MTC = Medullary thyroid cancer Dose Escalation Expansion Cohorts
LOXO-292 Clinical Development First patient enrolled in Phase 1 clinical trial in May 2017 Early human pharmacokinetic data is in line with preclinical predictions Opportunity for patient enrichment in Phase 1 Growing target awareness Detection technology is relatively straightforward Expect single agent activity at biologically relevant doses Potential for initial clinical data by YE 2017 30

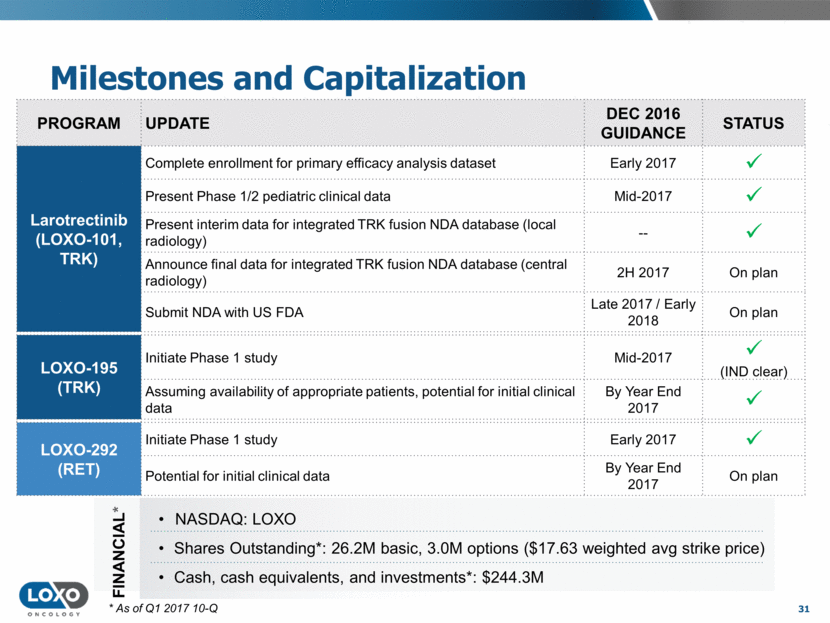
Milestones and Capitalization 31 FINANCIAL* NASDAQ: LOXO Shares Outstanding*: 26.2M basic, 3.0M options ($17.63 weighted avg strike price) Cash, cash equivalents, and investments*: $244.3M * As of Q1 2017 10-Q PROGRAM UPDATE DEC 2016 GUIDANCE STATUS Larotrectinib (LOXO-101, TRK) Complete enrollment for primary efficacy analysis dataset Early 2017 Present Phase 1/2 pediatric clinical data Mid-2017 Present interim data for integrated TRK fusion NDA database (local radiology) -- Announce final data for integrated TRK fusion NDA database (central radiology) 2H 2017 On plan Submit NDA with US FDA Late 2017 / Early 2018 On plan LOXO-195 (TRK) Initiate Phase 1 study Mid-2017 (IND clear) Assuming availability of appropriate patients, potential for initial clinical data By Year End 2017 LOXO-292 (RET) Initiate Phase 1 study Early 2017 Potential for initial clinical data By Year End 2017 On plan
Q&A 32

