Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Loxo Oncology, Inc. | a17-14662_18k.htm |
| EX-99.5 - EX-99.5 - Loxo Oncology, Inc. | a17-14662_1ex99d5.htm |
| EX-99.3 - EX-99.3 - Loxo Oncology, Inc. | a17-14662_1ex99d3.htm |
| EX-99.2 - EX-99.2 - Loxo Oncology, Inc. | a17-14662_1ex99d2.htm |
| EX-99.1 - EX-99.1 - Loxo Oncology, Inc. | a17-14662_1ex99d1.htm |
Exhibit 99.4
A pediatric phase 1 study of larotrectinib, a highly selective inhibitor of the tropomyosin receptor kinase (TRK) family Laetsch TW, 1 DuBois SG,2 Nagasubramanian R,3 Turpin B,4 Mascarenhas L,5 Federman N,6 Reynolds M,7 Smith S,7 Cruickshank S,7 Cox MC,7 Pappo AS,8 Hawkins DS9 1University of Texas Southwestern/Children’s Health, Dallas, TX; 2Dana-Farber/Boston Children's Cancer and Blood Disorders Center, Boston, MA; 3Nemours Children's Hospital, Orlando, FL; 4Cincinnati Children's Hospital Medical Center, Cincinnati, OH; 5Children’s Hospital Los Angeles, Keck School of Medicine, University of Southern California, Los Angeles, CA; 6University of California, Los Angeles, Los Angeles, CA; 7Loxo Oncology, Inc., South San Francisco, CA; 8St. Jude Children's Research Hospital, Memphis, TN; 9Seattle Children’s Hospital, University of Washington, Fred Hutchinson Cancer Research Center, Seattle, WA Laetsch, 10510
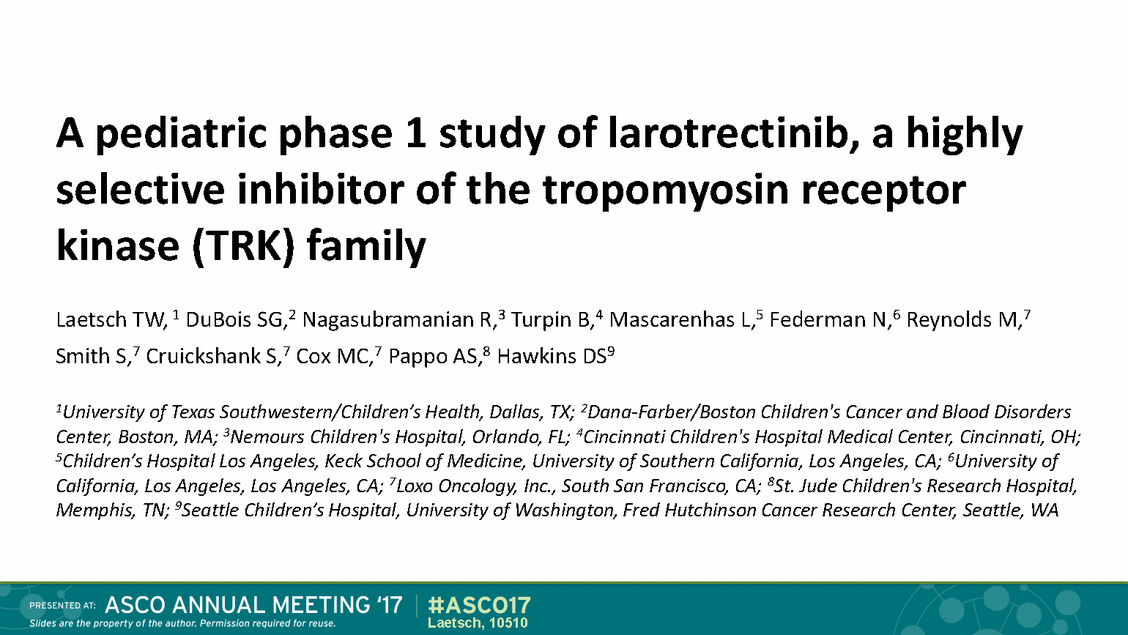
Role of TRK in normal Neurotrophin family of receptors biology and cancer TRK fusions • • TRKA (NTRK1) Pain, thermoregulation Ligand binding domain (LBD) replaced by 5’ fusion partner Drives overexpression and ligand-independent activation TRKB (NTRK2) Movement, memory, mood, appetite, body weight NTRK1/2/3 Proprioception TRKC (NTRK3) ERK AKT TRK uncommonly expressed in normal tissues or cancer Fusion drives abnormally high expression and activation of TRK kinase domain 2 Laetsch, 10510 Tyr Tyr Tyr Tyr Tyr Tyr 5’ pa rtner NTRK kina se domain 5’ partner NTRK kinase domain 5’ partner NTRK kinase domain Promoter 5’ partner LBD kinase domain
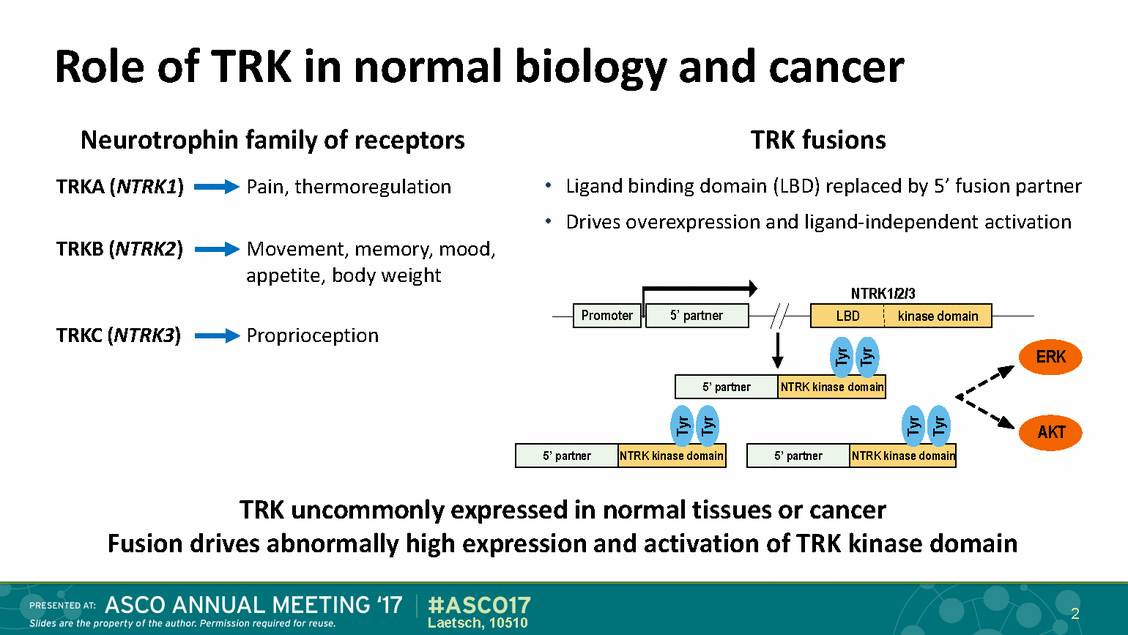
Pediatric cancers and TRK Gliomas fusions Rare neoplasm with high TRK fusion frequency Thyroid cancer Secretory breast carcinoma Infantile fibrosarcoma Spitz nevi Congenital mesoblastic nephroma Various sarcomas 3 Laetsch, 10510
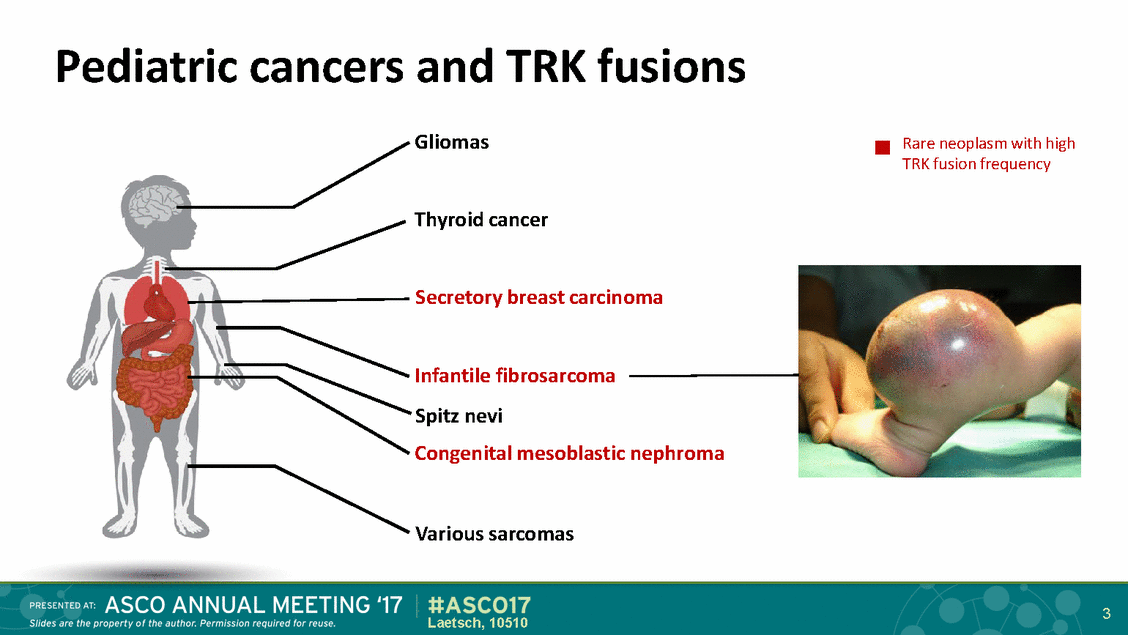
Larotrectinib (LOXO-101) TRKA/B/C • Larotrectinib is the first and only selective pan-TRK inhibitor in clinical development • Highly potent against TRKA, TRKB, TRKC (5–11 nM IC50 in cellular assays) Highly selective • • Responses seen in adult patients with TRK fusions • Recommended phase 2 dose in adults is 100 mg BID continuously • Liquid formulation for pediatric patients 4 Laetsch, 10510
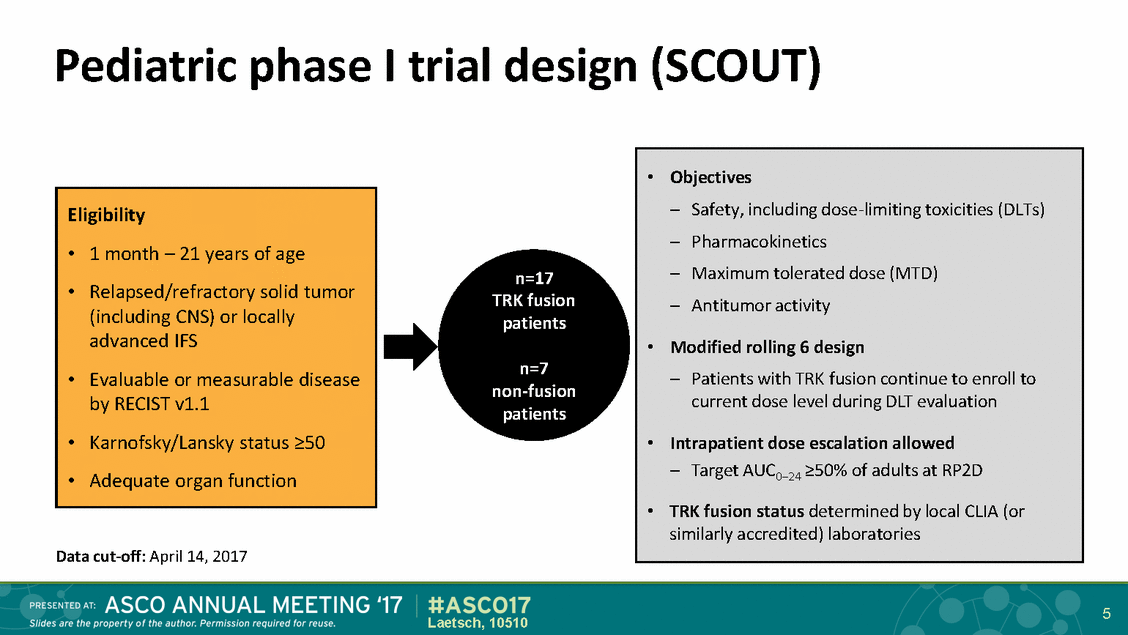
Pediatric phase I trial design (SCOUT) n=17 TRK fusion patients n=7 non-fusion patients Data cut-off: April 14, 2017 5 Laetsch, 10510 Eligibility • 1 month – 21 years of age • Relapsed/refractory solid tumor (including CNS) or locally advanced IFS • Evaluable or measurable disease by RECIST v1.1 • Karnofsky/Lansky status >50 • Adequate organ function •Objectives – Safety, including dose-limiting toxicities (DLTs) – Pharmacokinetics – Maximum tolerated dose (MTD) – Antitumor activity •Modified rolling 6 design – Patients with TRK fusion continue to enroll to current dose level during DLT evaluation •Intrapatient dose escalation allowed – Target AUC0–24 >50% of adults at RP2D • TRK fusion status determined by local CLIA (or similarly accredited) laboratories
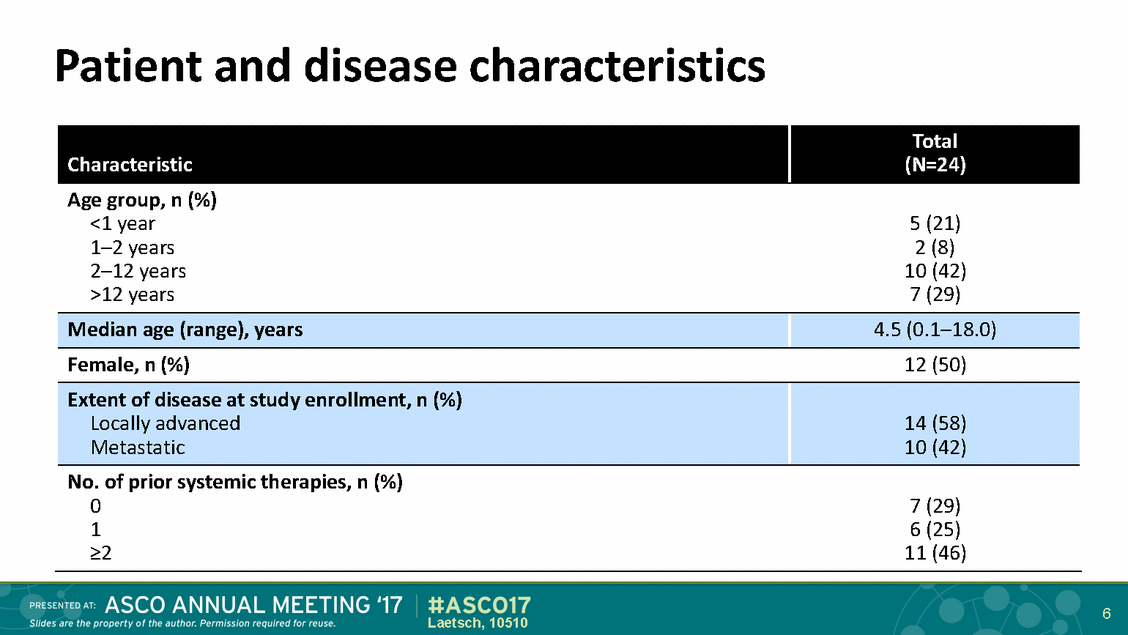
Patient and disease characteristics 6 Laetsch, 10510 Characteristic Total (N=24) Age group, n (%) <1 year 1–2 years 2–12 years >12 years 5 (21) 2 (8) 10 (42) 7 (29) Median age (range), years 4.5 (0.1–18.0) Female, n (%) 12 (50) Extent of disease at study enrollment, n (%) Locally advanced Metastatic 14 (58) 10 (42) No. of prior systemic therapies, n (%) 0 1 >2 7 (29) 6 (25) 11 (46)
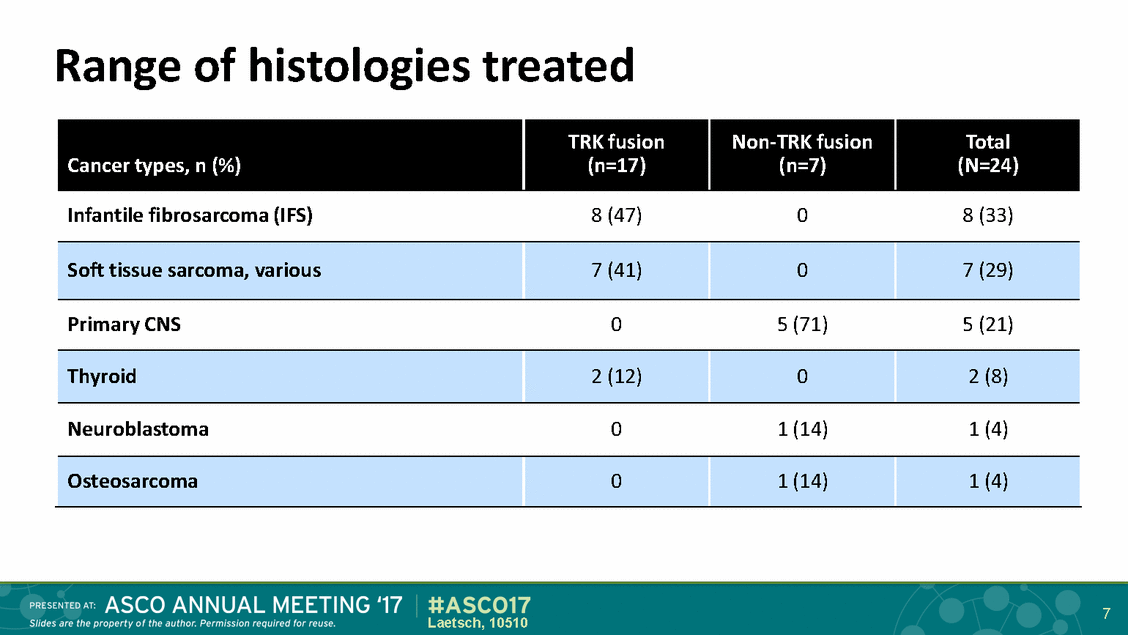
Range of histologies treated 7 Laetsch, 10510 Cancer types, n (%) TRK fusion (n=17) Non-TRK fusion (n=7) Total (N=24) Infantile fibrosarcoma (IFS) 8 (47) 0 8 (33) Soft tissue sarcoma, various 7 (41) 0 7 (29) Primary CNS 0 5 (71) 5 (21) Thyroid 2 (12) 0 2 (8) Neuroblastoma 0 1 (14) 1 (4) Osteosarcoma 0 1 (14) 1 (4)
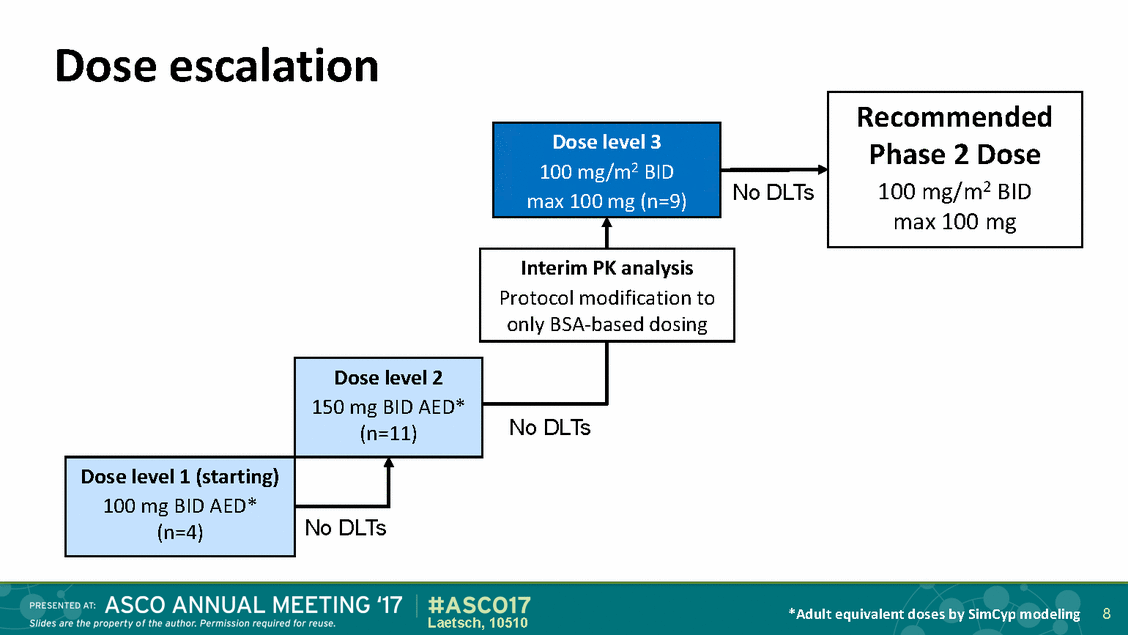
Dose escalation 100 mg/m2 BID No DLTs No DLTs *Adult equivalent doses by SimCyp modeling 8 Laetsch, 10510 Dose level 2 150 mg BID AED* (n=11) Dose level 1 (starting) 100 mg BID AED* (n=4) No DLTs Interim PK analysis Protocol modification to only BSA-based dosing Dose level 3 max 100 mg (n=9) Recommended Phase 2 Dose 100 mg/m2 BID max 100 mg
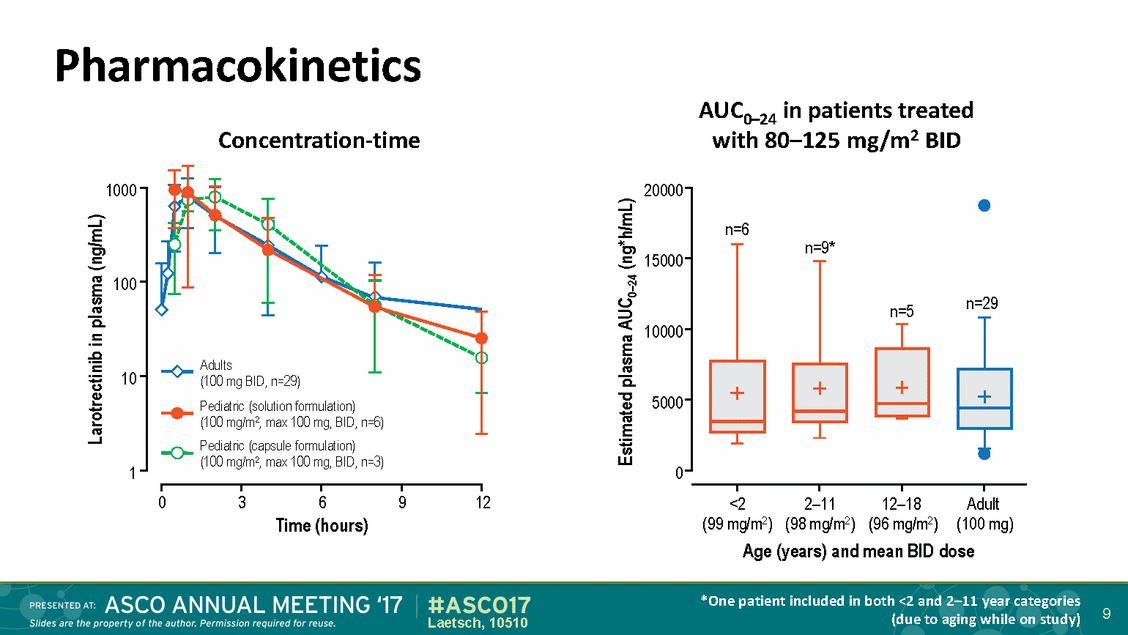
Pharmacokinetics Concentration-time AUC0–24 in patients treated with 80–125 mg/m2 BID 1000 20000 n=6 n=9* 15000 100 n=29 n=5 10000 Adults (100 mg BID, n=29) Pediatric (solution formulation) 10 5000 (100 mg/m2, max 100 mg, BID, n=6) Pediatric (capsule formulation) (100 mg/m2, max 100 mg, BID, n=3) 1 0 0 3 6 Time (hours) 9 12 <2 (99 mg/m2) 2–11 (98 mg/m2) 12–18 (96 mg/m2) Adult (100 mg) Age (years) and mean BID dose *One patient included in both <2 and 2–11 year categories (due to aging while on study) 9 Laetsch, 10510 Larotrectinib in plasma (ng/mL) Estimated plasma AUC0–24 (ng*h/mL)
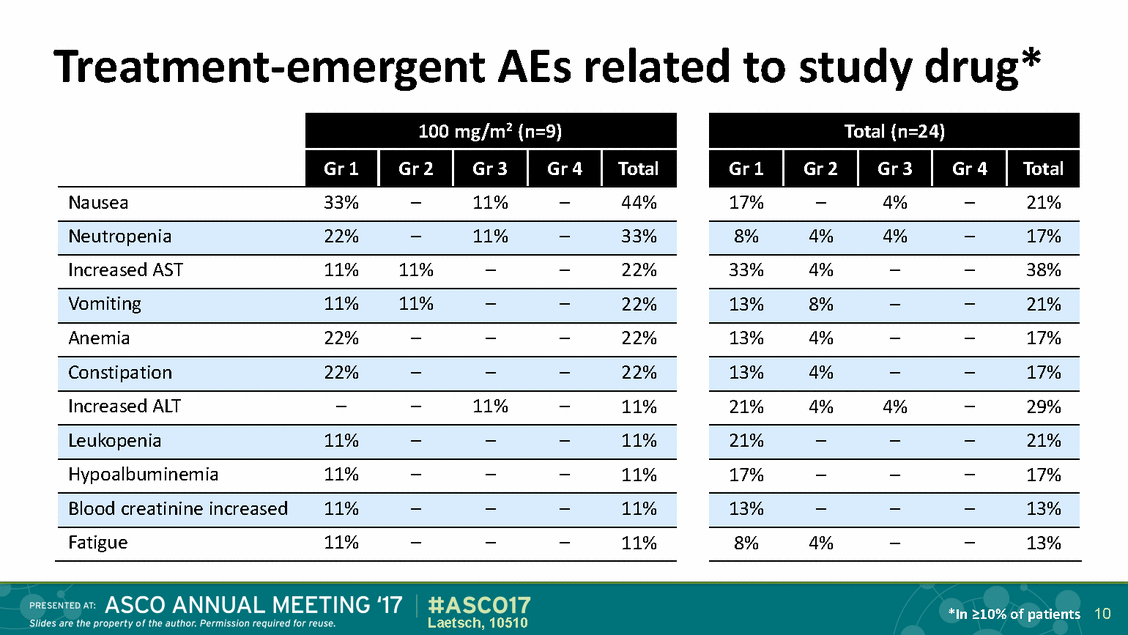
Treatment-emergent AEs related to study drug* *In >10% of patients 10 Laetsch, 10510 Total (n=24) Gr 1 Gr 2 Gr 3 Gr 4 Total 17%–4%–21% 8%4%4%–17% 33%4%––38% 13%8%––21% 13%4%––17% 13%4%––17% 21%4%4%–29% 21%–––21% 17%–––17% 13%–––13% 8%4%––13% 100 mg/m2 (n=9) Gr 1 Gr 2 Gr 3 Gr 4 Total Nausea33%–11%–44% Neutropenia22%–11%–33% Increased AST11%11%––22% Vomiting11%11%––22% Anemia22%–––22% Constipation22%–––22% Increased ALT––11%–11% Leukopenia11%–––11% Hypoalbuminemia11%–––11% Blood creatinine increased11%–––11% Fatigue11%–––11%
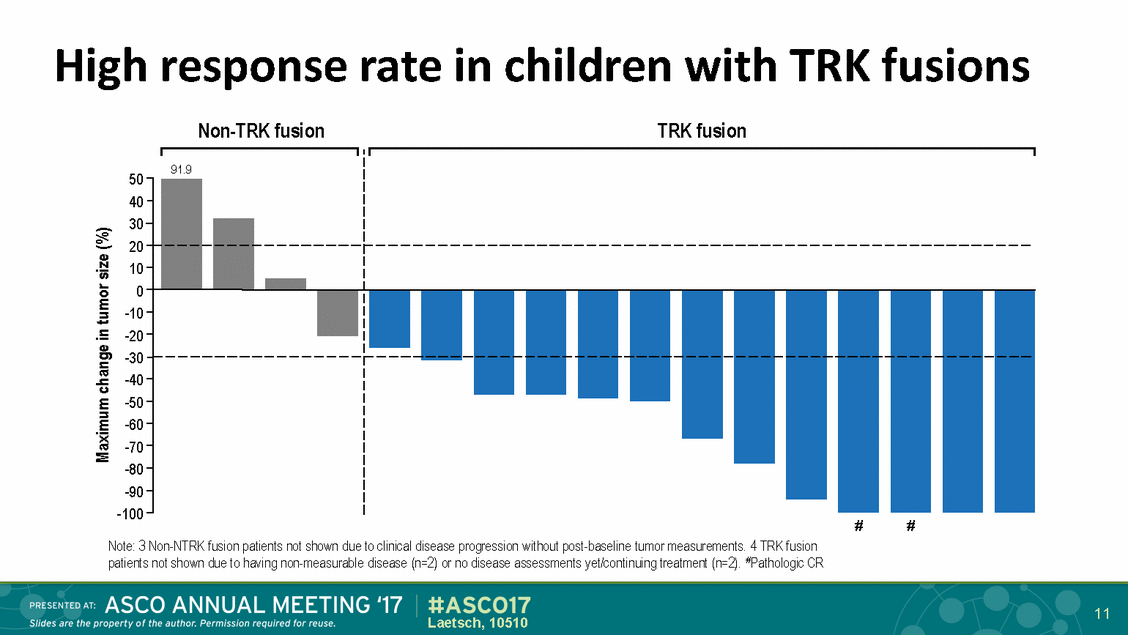
High response Non-TRK fusion rate in children with TRK fusion TRK fusions 50 40 30 20 10 0 -10 -20 -30 -40 -50 -60 -70 -80 -90 -100 # # Note: 3 Non-NTRK fusion patients not shown due to clinical disease progression without post-baseline tumor measurements. 4 TRK fusion patients not shown due to having non-measurable disease (n=2) or no disease assessments yet/continuing treatment (n=2). #Pathologic CR 11 Laetsch, 10510 Maximum change in tumor size (%) 91.9
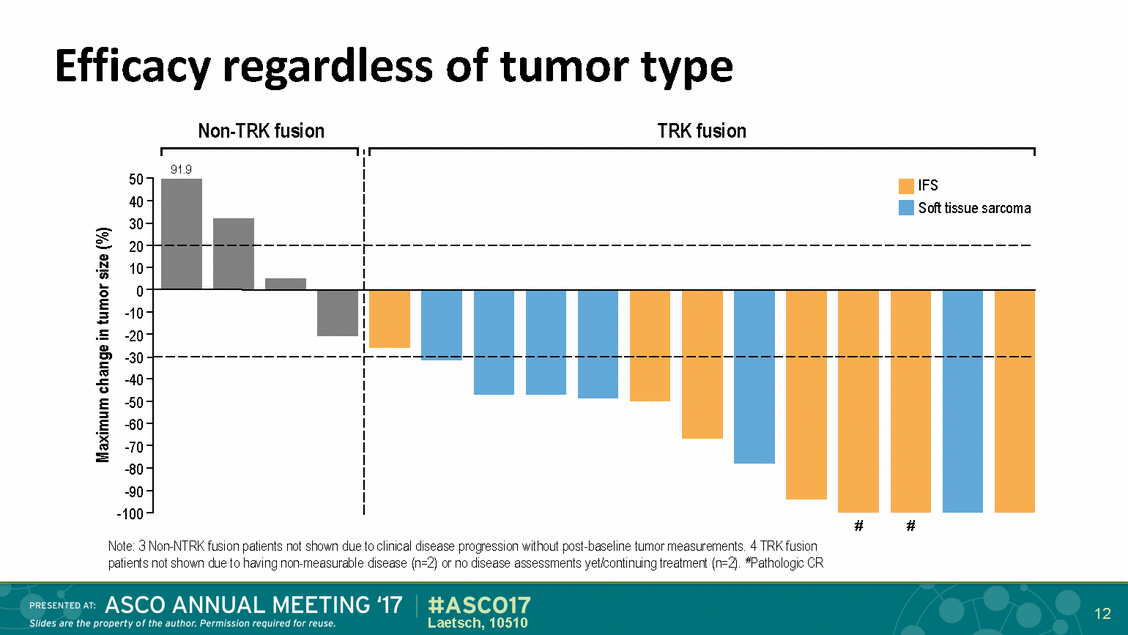
Efficacy regardless Non-TRK fusion of tumor type TRK fusion 50 40 30 20 10 0 -10 -20 -30 -40 -50 -60 -70 -80 -90 -100 # # Note: 3 Non-NTRK fusion patients not shown due to clinical disease progression without post-baseline tumor measurements. 4 TRK fusion patients not shown due to having non-measurable disease (n=2) or no disease assessments yet/continuing treatment (n=2). #Pathologic CR 12 Laetsch, 10510 Maximum change in tumor size (%) 91.9 IFS Soft tissue sarcoma
Efficacy regardless Non-TRK fusion of TRK gene TRK fusion 50 40 30 20 10 0 -10 -20 -30 -40 -50 -60 -70 -80 -90 -100 # # Note: 3 Non-NTRK fusion patients not shown due to clinical disease progression without post-baseline tumor measurements. 4 TRK fusion patients not shown due to having non-measurable disease (n=2) or no disease assessments yet/continuing treatment (n=2). #Pathologic CR 13 Laetsch, 10510 Maximum change in tumor size (%) 91.9 None NTRK1 NTRK2 NTRK3
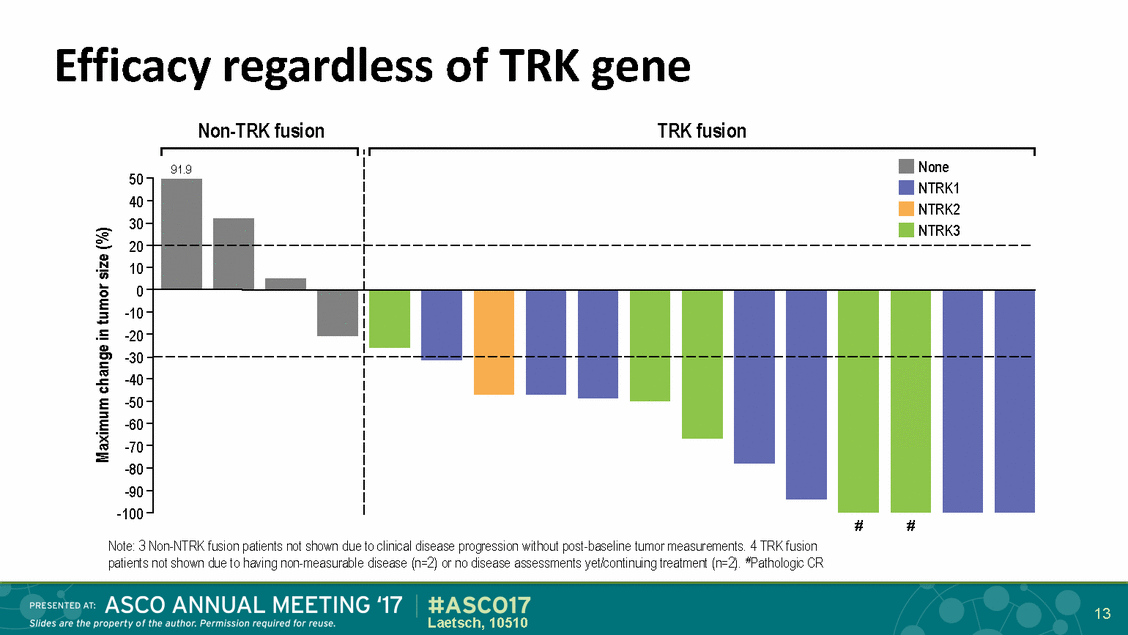
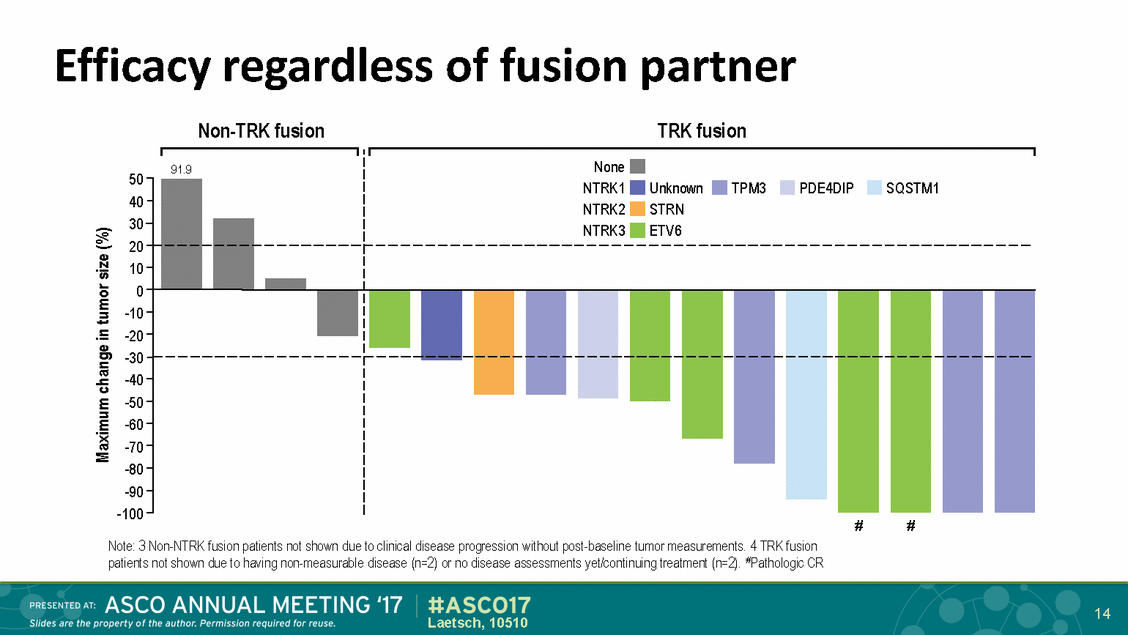
Efficacy regardless Non-TRK fusion of fusion partner TRK fusion 50 40 30 20 10 0 -10 -20 -30 -40 -50 -60 -70 -80 -90 -100 # # Note: 3 Non-NTRK fusion patients not shown due to clinical disease progression without post-baseline tumor measurements. 4 TRK fusion patients not shown due to having non-measurable disease (n=2) or no disease assessments yet/continuing treatment (n=2). #Pathologic CR 14 Laetsch, 10510 Maximum change in tumor size (%) 91.9 None NTRK1UnknownTPM3PDE4DIPSQSTM1 NTRK2STRN NTRK3ETV6
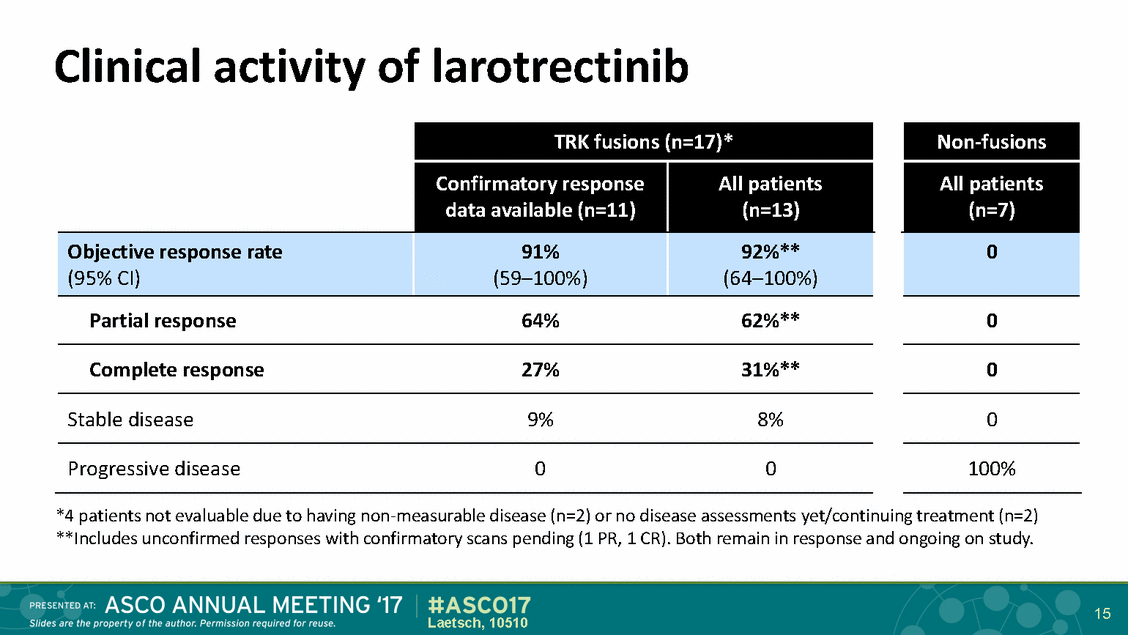
Clinical activity of larotrectinib *4 patients not evaluable due to having non-measurable disease (n=2) or no disease assessments yet/continuing treatment (n=2) **Includes unconfirmed responses with confirmatory scans pending (1 PR, 1 CR). Both remain in response and ongoing on study. 15 Laetsch, 10510 TRK fusions (n=17)* Non-fusions Confirmatory response data available (n=11) All patients (n=13) All patients (n=7) Objective response rate (95% CI) 91% (59–100%) 92%** (64–100%) 0 Partial response 64% 62%** 0 Complete response 27% 31%** 0 Stable disease 9% 8% 0 Progressive disease 0 0 100%
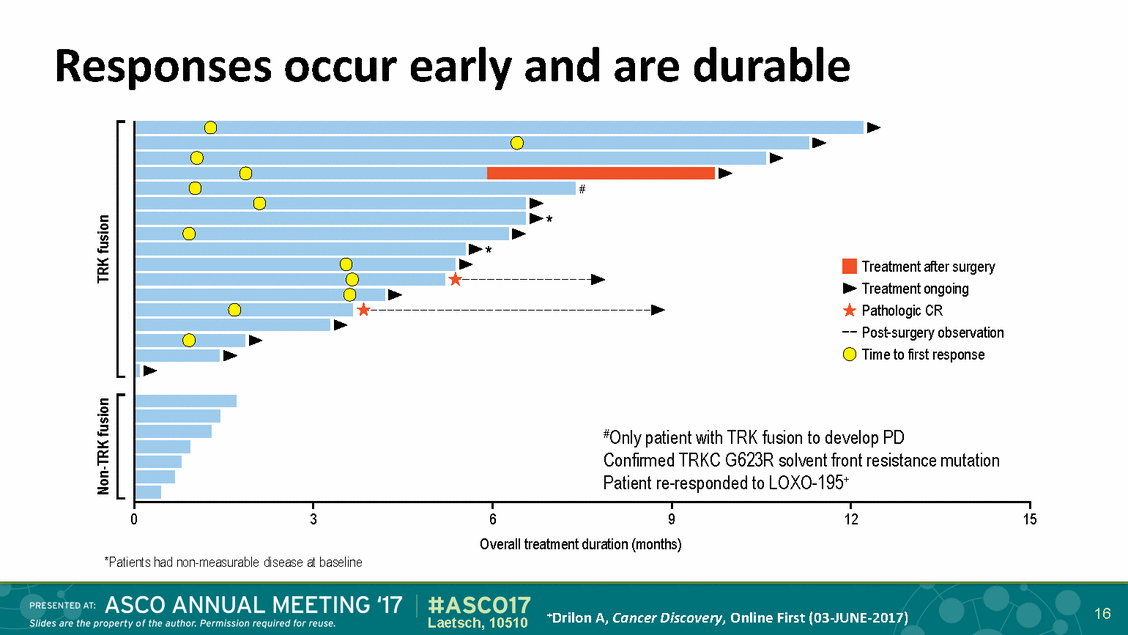
Responses occur early and are durable * * Treatment after surgery Treatment ongoing Pathologic CR Post-surgery observation Time to first response #Only patient with TRK fusion to develop PD Confirmed TRKC G623R solvent front resistance mutation Patient re-responded to LOXO-195+ 0 3 6 9 12 15 Overall treatment duration (months) *Patients had non-measurable disease at baseline 16 +Drilon A, Cancer Discovery, Online First (03-JUNE-2017) Laetsch, 10510 Non-TRK fusion TRK fusion #
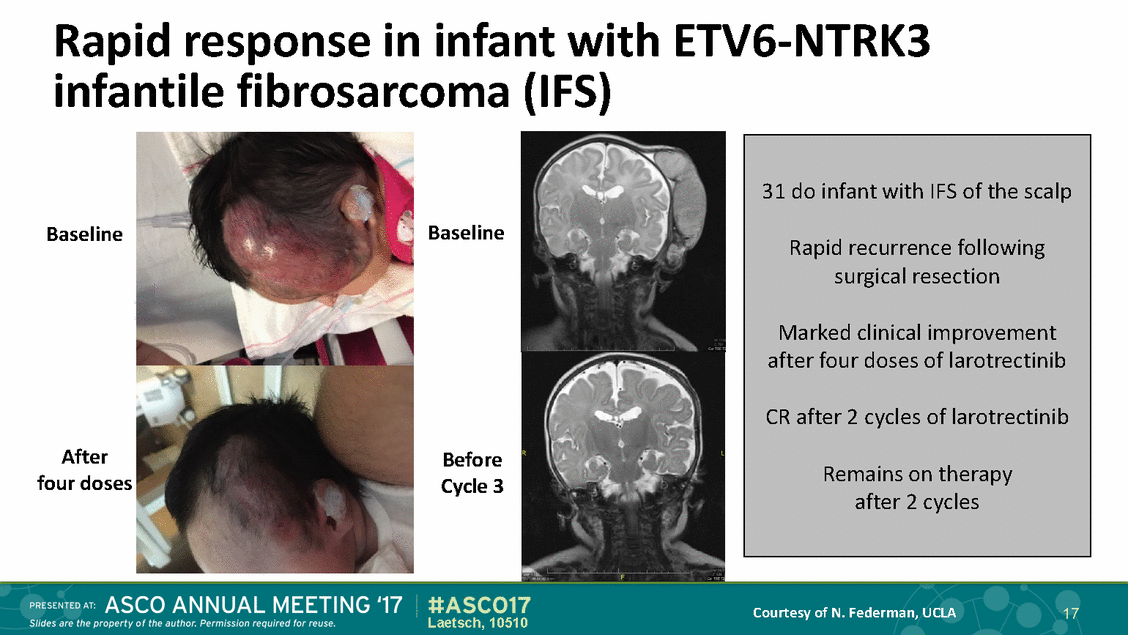
Rapid response in infant with ETV6-NTRK3 infantile fibrosarcoma (IFS) Baseline Baseline After four doses Before Cycle 3 Courtesy of N. Federman, UCLA 17 Laetsch, 10510 31 do infant with IFS of the scalp Rapid recurrence following surgical resection Marked clinical improvement after four doses of larotrectinib CR after 2 cycles of larotrectinib Remains on therapy after 2 cycles
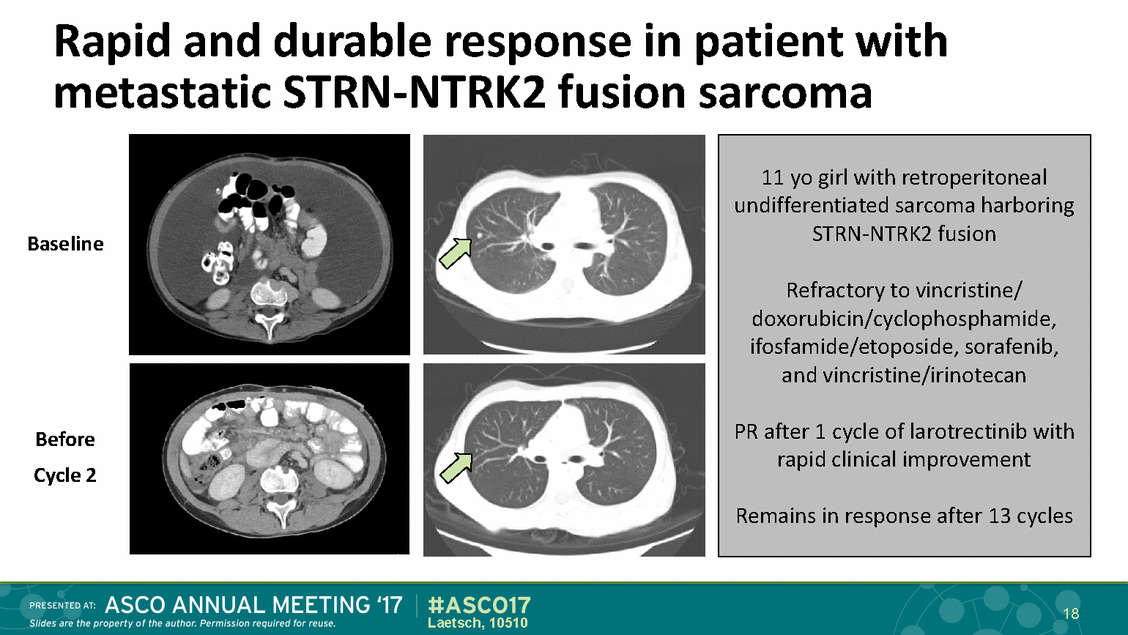
Rapid and durable response in patient with metastatic STRN-NTRK2 fusion sarcoma Baseline Before Cycle 2 18 Laetsch, 10510 11 yo girl with retroperitoneal undifferentiated sarcoma harboring STRN-NTRK2 fusion Refractory to vincristine/ doxorubicin/cyclophosphamide, ifosfamide/etoposide, sorafenib, and vincristine/irinotecan PR after 1 cycle of larotrectinib with rapid clinical improvement Remains in response after 13 cycles
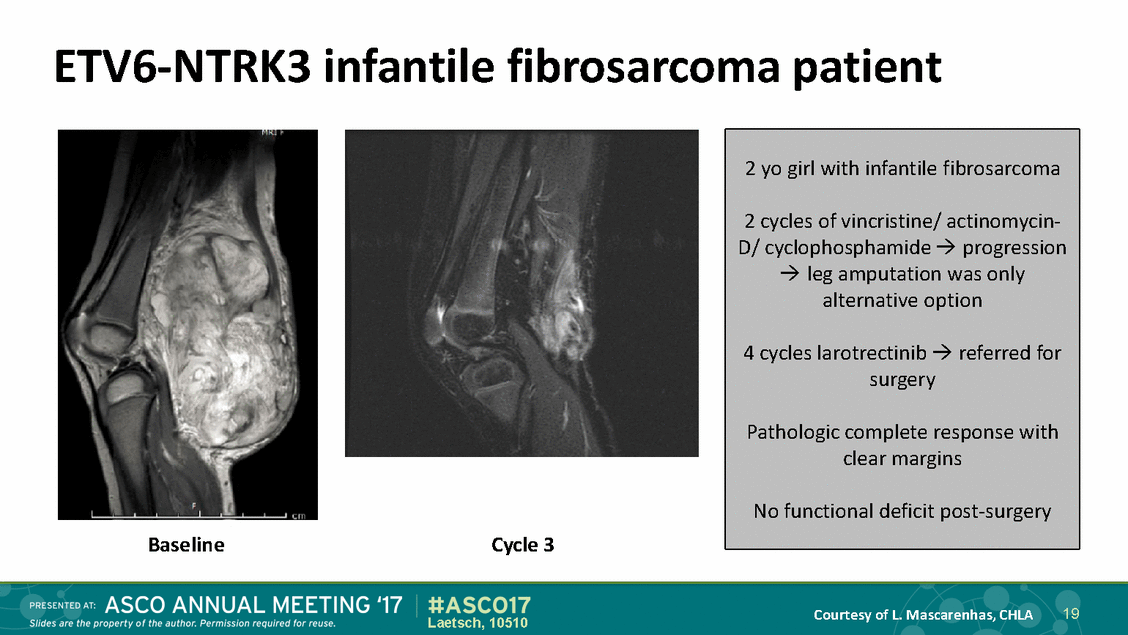
ETV6-NTRK3 infantile fibrosarcoma patient Baseline Cycle 3 19 Courtesy of L. Mascarenhas, CHLA Laetsch, 10510 2 yo girl with infantile fibrosarcoma 2 cycles of vincristine/ actinomycin-D/ cyclophosphamide progression leg amputation was only alternative option 4 cycles larotrectinib referred for surgery Pathologic complete response with clear margins No functional deficit post-surgery
Larotrectinib is active and well-tolerated in children with TRK fusion cancers • Larotrectinib demonstrated a favorable tolerability profile and histology-independent activity in pediatric patients harboring TRK fusions Recommended phase 2 dose in children: 100 mg/m2 BID continuously, cap 100 mg/dose • – – – No DLTs at any dose level Similar exposure to adults at RP2D Highly active • Phase 2 portion of trial is enrolling globally (Abstract TPS10577) – – Infantile fibrosarcoma Other CNS and extracranial TRK fusion solid cancers 20 Laetsch, 10510
Acknowledgements The authors would like to thank • Patients and their families • Research staff Funded by Loxo Oncology, Inc 21 Laetsch, 10510

