Attached files
| file | filename |
|---|---|
| EX-32.2 - EX-32.2 - Loxo Oncology, Inc. | a18-1042_1ex32d2.htm |
| EX-32.1 - EX-32.1 - Loxo Oncology, Inc. | a18-1042_1ex32d1.htm |
| EX-31.2 - EX-31.2 - Loxo Oncology, Inc. | a18-1042_1ex31d2.htm |
| EX-31.1 - EX-31.1 - Loxo Oncology, Inc. | a18-1042_1ex31d1.htm |
| EX-23.2 - EX-23.2 - Loxo Oncology, Inc. | a18-1042_1ex23d2.htm |
| EX-23.1 - EX-23.1 - Loxo Oncology, Inc. | a18-1042_1ex23d1.htm |
| EX-10.20 - EX-10.20 - Loxo Oncology, Inc. | a18-1042_1ex10d20.htm |
| EX-10.19 - EX-10.19 - Loxo Oncology, Inc. | a18-1042_1ex10d19.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2017
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file number 000-36562
LOXO ONCOLOGY, INC.
(Exact name of Registrant as specified in its charter)
|
Delaware |
|
46-2996673 |
|
(State or Other Jurisdiction of |
|
(I.R.S. Employer |
|
|
|
|
|
281 Tresser Blvd., 9th Floor |
|
06901 |
|
(Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, including area code: (203) 653-3880
Securities registered pursuant to Section 12(b) of the Exchange Act:
|
Title of Each Class |
|
Name of Each Exchange on Which Registered |
|
Common Stock, $0.0001 Par Value Per Share |
|
The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Exchange Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No o
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Exchange Act. Yes o No x
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Rule 405 of Regulation S-K is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definite proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
|
x |
|
Accelerated filer |
o |
|
|
|
|
|
|
|
|
Non-accelerated filer |
|
o |
(Do not check if a smaller reporting company) |
Smaller reporting company |
o |
|
Emerging growth company |
|
o |
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
The aggregate market value of the voting stock held by non-affiliates of the Registrant on June 30, 2017 (the last business day of the Registrant’s second fiscal quarter), based upon the closing price of $80.19 of the Registrant’s common stock as reported on the Nasdaq Global Market, was approximately $1.4 billion.
Indicate the number of shares outstanding of each of the issuer’s classes of common stock, as of the latest practicable date.
|
Class |
|
Outstanding at February 23, 2018 |
|
Common stock, $0.0001 par value per share |
|
30,034,173 shares |
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the documents listed below have been incorporated by reference into the indicated parts of this report, as specified in the responses to the item numbers involved.
Designated portions of the Proxy Statement relating to the 2018 Annual Meeting of the Stockholders (the “Proxy Statement”): Part III (Items 10, 11, 12, 13 and 14), to be filed within 120 days of the Registrant’s fiscal year ended December 31, 2017. Except with respect to information specifically incorporated by reference in the Form 10-K, the Proxy Statement is not deemed to be filed as part hereof.
Except for historical financial information contained herein, the matters discussed in this Annual Report on Form 10-K may be considered forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and subject to the safe harbor created by the Securities Litigation Reform Act of 1995. Such statements include declarations regarding our intent, belief, or current expectations and those of our management. Prospective investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve a number of risks, uncertainties and other factors, some of which are beyond our control; actual results could differ materially from those indicated by such forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, but are not limited to: (i) that the information is of a preliminary nature and may be subject to further adjustment; (ii) those risks and uncertainties identified under “Risk Factors;” and (iii) the other risks detailed from time-to-time in our reports and registration statements filed with the Securities and Exchange Commission (“SEC”). Except as required by law, we undertake no obligation to revise or update publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
Overview
Loxo Oncology is a biopharmaceutical company innovating the development of highly selective medicines for patients with genetically defined cancers. Our pipeline focuses on cancers that are uniquely dependent on single gene abnormalities, such that a single drug has the potential to treat the cancer with dramatic effect. We believe that the most selective, purpose-built medicines have the highest probability of maximally inhibiting the intended target, thereby delivering best-in-class disease control and safety. Our management team seeks out experienced industry partners, world-class scientific advisors and innovative clinical-regulatory approaches to deliver new cancer therapies to patients as quickly and efficiently as possible.
As genetic testing in cancer becomes more routine, we are learning that cancers arising in diverse sites in the body may share common genetic alterations. Increasingly, tumors may be identified and treated according to their distinguishing genetic alterations, while in the past, the organ of origin was most important. Both research and clinical data suggest that some tumors, while having many identifiable genetic alterations, are primarily dependent on a single genetic alteration or signaling pathway for their proliferation and survival. This dependency, often referred to as oncogene addiction, renders such tumors highly susceptible to small molecule inhibitors targeting the relevant alteration or pathway.
We identify and prioritize our targets in two ways. First, we use reported clinical trial data to assess the response signals of drugs in development and identify those that show promise but also demonstrate drug-specific limitations such as poor absorption, poor distribution, unwanted side effects or limited efficacy against important patient subsets. Second, we monitor and/or sponsor academic research to quickly identify novel targets with emerging validation. Once an attractive target is identified, we launch internal discovery efforts, work with technology partners or identify business development opportunities to advance product candidates with the desired product profiles in a rapid, risk-mitigated, and cost-effective manner. After a compelling opportunity has been identified, we implement a stepwise approach to clinical development designed to reduce risk and identify response signals early in development. In early-stage trials, whenever possible, we seek to evaluate our product candidates in well-defined patient populations in order to maximize the likelihood of demonstrating a clinical benefit. This approach allows for the possibility of rapid clinical development and expedited regulatory strategies. We intend to develop companion diagnostics when appropriate, with the help of technology partners, to identify patients whose tumors harbor relevant genetic alterations.
Our Strategy
Our goal is to translate key scientific insights relating to underlying oncogenic drivers into the development of potent and highly selective therapeutics. To execute our strategy, we intend to:
· Rapidly advance our product candidates through clinical development and, if successful, commercialize them ourselves or with partners. Our product candidate pipeline currently consists of:
· Larotrectinib, an oral, potent and selective inhibitor of tropomyosin receptor kinases (TRK), a family of signaling proteins. In December 2017, we announced that we initiated submission of a rolling New Drug Application (“NDA”) to the U.S. Food and Drug Administration (“FDA”) for larotrectinib for the treatment of unresectable or metastatic solid tumors with NTRK-fusion proteins in adult and pediatric patients who require systemic therapy and who have either progressed following prior treatment or who have no acceptable alternative treatments. We expect to complete the NDA submission in the first quarter of 2018, and expect our partner Bayer Consumer Care AG (“Bayer”) to submit a Marketing Authorisation Application (“MAA”) to the European Medicines Agency in 2018.
· LOXO-195, an oral, potent and selective inhibitor of TRKthat retains inhibitory activity against certain mutated TRK fusion proteins no longer sensitive to larotrectinib or other TRK inhibitors. LOXO-195 is
currently being studied in a multi-center Phase 1/2 trial. We expect to provide a clinical data update on this program in a scientific forum in the second half of 2018. Preclinical data on our LOXO-195 preclinical program and clinical proof-of-concept data from the first two patients treated with LOXO-195 were published in an article in Cancer Discovery in June 2017.
In November 2017, we and Bayer entered into an exclusive global collaboration agreement for the development and commercialization of larotrectinib and LOXO-195, a next-generation TRK inhibitor. We lead worldwide development and U.S. regulatory activities. Bayer leads ex-U.S. regulatory activities and worldwide commercial activities. In the U.S., we and Bayer will co-promote the products.
· LOXO-292, an oral, potent and selective inhibitor of Rearranged during Transfection (“RET”) tyrosine kinase, a signaling protein. Following IND clearance by the FDA, we enrolled the first patient into our Phase 1 trial of LOXO-292 in May 2017. This first-in-human, global, multi-center Phase 1 trial is evaluating LOXO-292 as a single agent in patients with advanced solid tumors. In October 2017, we presented preliminary clinical data from the LOXO-292 program at the International Association for the Study of Lung Cancer (“IASLC”) 18th World Conference on Lung Cancer, in Yokohama, Japan. We expect to provide a more comprehensive trial update in a scientific forum in the first half of 2018.
· LOXO-305, an oral, potent and selective inhibitor of Bruton’s tyrosine (“BTK”) that retains inhibitory activity against certain mutated BTK proteins no longer sensitive to covalent BTK inhibitors. LOXO-305 is a novel, potent selective, and reversible BTK inhibitor with equivalent potency against wild-type and cysteine-481 (“C481”) mutated BTK. We believe that the widespread use of covalent BTK inhibitors, such as ibrutinib and acalabrutinib, will increasingly drive acquired resistance through a mutational event in BTK called C481, leading to a group of relapsing patients in need of new therapies. LOXO-305 was designed to reversibly bind BTK and preserve activity in the presence of the C481 acquired resistance mutation. Additionally, it was designed to avoid off-target kinases that have complicated the development of both covalent and other more promiscuous reversible BTK inhibitors, such as EGFR, ITK, BMX, TEC, ITK, BLK, LCK and SRC. LOXO-305 is expected to enter clinical development in the second half of 2018.
· Develop a pipeline of potent and highly selective targeted therapeutics. Our insights into cancer biology and target identification, coupled with our focus on purpose-built medicinal chemistry, have allowed us to build a proprietary pipeline of targeted product candidates. Our management team and Scientific Advisory Board have a history of success in the identification, translation and development of oncology therapeutics, and together we determine which programs merit advancement and continued development. We may engage in complementary activities to gain access to attractive drug candidates, including some or all of the following: 1) collaboration with third-party discovery partners to build new drugs that meet desired specifications; 2) internal discovery conducted by Loxo Oncology in its laboratory facilities in Boulder, CO; or 3) business development, such as licensing or acquisition.
· Increase the probability for clinical success by prioritizing targets for development that are believed to be oncogenic drivers. We attempt to select targets for drug development that behave as oncogenic drivers. If we are successful in inhibiting these targets with our product candidates, we may increase the likelihood of achieving tumor responses. This approach has been validated by others in the setting of epidermal growth factor (“EGFR”) mutated lung cancer, BRAF mutated melanoma, BCR-ABL translocated chronic myelogenous leukemia and ALK translocated lung cancer.
· Work with experienced third parties in the field of diagnostics. In initial clinical development, we will attempt to employ existing diagnostic tools to identify patient subsets with the highest likelihood of clinical benefit. In late-stage clinical development, once the most important screening criteria have been established, we will work with third-party technology partners to develop companion diagnostic assays that could support registration and marketing of our product candidates. Thus, relationships with established diagnostics partners are important to enrolling our clinical trials and identifying patients in the eventual commercial setting.
· Conduct international clinical and regulatory programs to support our global approval and commercialization strategy. Cancer is a global disease and we are pursuing clinical and regulatory programs for approval in the U.S. and internationally. As programs advance towards potential regulatory approvals and commercialization, we may license our drugs to partners, either in specific territories or globally, or build a focused commercial organization ourselves to launch the products.
Background on Cancer
Cancer is the second-leading cause of death in the U.S. The American Cancer Society estimates that in 2018 there will be approximately 1.74 million new cases and approximately 610,000 deaths from cancer in the U.S. Cancer originates from defects in the cell’s genetic code (“DNA”), which disrupt the mechanisms that normally prevent uncontrolled cell growth, invasion and programmed cell death. Increasingly, doctors are using diagnostic tests to identify these genetic defects and select better treatment options. As genetic
testing in cancer becomes more routine, we are learning that cancers arising in diverse sites in the body may share common genetic alterations.
The most common methods of treating patients with cancer are surgery, radiation and drug therapy. A cancer patient often receives treatment with a combination of these modalities. Surgery and radiation therapy are particularly effective in patients in whom the disease is localized. Physicians generally use systemic drug therapies in situations in which the cancer has spread beyond the primary site or cannot otherwise be treated through surgery. The goal of drug therapy is to damage and kill cancer cells or to interfere with the molecular and cellular processes that control the development, growth and survival of cancer cells. In many cases, drug therapy entails the administration of several different drugs in combination. Over the past several decades, drug therapy has evolved from non-specific drugs that kill both healthy and cancerous cells, to drugs that target specific molecular pathways involved in cancer and more recently to therapeutics that target specific oncogenic drivers. These therapies often require genetic testing of a cancer to identify the subsets of patients for whom a drug will most effectively impact tumor growth.
Cytotoxic Therapies. The earliest approach to cancer drug therapy was the development of cytotoxic drugs, commonly referred to as chemotherapy, designed to kill rapidly proliferating cancer cells. Cytotoxic drug therapies act in an indiscriminate manner, killing healthy as well as cancerous cells. Due to their mechanism of action, many cytotoxic drugs have a narrow therapeutic range; doses above this range cause unacceptable or even fatal levels of damage to normal organs, while doses below the range are not effective in eradicating the cancer cells. Examples of cytotoxic drugs include carboplatin (Paraplatin™), docetaxel (Taxotere™) and doxorubicin (Adriamycin™).
Targeted Therapies. A newer class of medicines targets specific biological signaling pathways that play a role in rapid cell growth or the spread of cancer. While these drugs have been effective in the treatment of some cancers, most of them do not address the genetic alterations that cause oncogenesis. As normal cells may also rely on these signaling pathways, there are often toxicities associated with inhibition of these pathways. In addition, targeted therapies may hit signaling pathways adjacent and in addition to the intended pathway, thus causing off-target toxicities. Examples of targeted therapies include sunitinib (Sutent™), sorafenib (Nexavar™), and cabozantinib (Cometriq™).
Targeted Therapies and Oncogene Addiction. The dissemination of approved and experimental targeted therapies into large populations revealed that some patients had particularly robust responses to these therapies. This observation was consistent with lab evidence that some tumors, despite having many measurable genetic alterations, are primarily dependent on a single activated kinase for their growth and survival advantage. Such cancers are highly susceptible to small molecule inhibitors. Often described as an oncogenic driver mutation or a dominant activating mutation, oncogene addiction is a term used to describe a tumor’s particular dependence on a specific pathway. Oncogene addiction has become an important concept in clinical development because patients with tumors governed by this behavior typically show rapid and measurable tumor shrinkage when exposed to drugs targeting the relevant alteration. These responses can be sufficiently dramatic in some cases to support expedited regulatory approval for the associated targeted therapy.
Kinase inhibitors against EGFR in stage IV lung cancer provide an informative case study regarding the importance of identifying oncogenic drivers in enabling more efficient drug development and improving clinical care. In 2004, researchers first discovered that a subset of lung cancer was caused by a genetic defect in EGFR. Drugs called erlotinib (Tarceva™ and gefitinib (Iressa™), which target EGFR, were in development for lung cancers of unknown mutation status. Investigators learned that patients with mutations in EGFR receiving Tarceva™ or Iressa™ experienced a much higher response rate, defined as the proportion of patients with meaningful tumor shrinkage on their clinical imaging scans, than unselected patients. Patients with EGFR mutations have a response rate in the 65% range, as opposed to the 10% range noted in unselected lung patients. Tarceva™ is approved in the U.S. as first-line treatment for non-small cell lung cancer patients with EGFR mutations, afatinib (Gilotrif™) is approved in the U.S. for patients with metastatic non-small cell lung cancer with specific EGFR mutations, and in November 2015, the U.S. FDA granted accelerated approval to osimertinib (Tagrisso™) for the treatment of patients with metastatic non-small cell lung cancer who have a specific genetic marker and have progressed on or after treatment with a prior EGFR inhibitor.
Inhibitors of other kinases associated with oncogenic driver events have shown compelling clinical effects in patients whose tumors harbor alterations in genes with acronyms such as ALK, BRAF, and ABL. Drugs such as crizotinib (Xalkori™), vemurafenib (Zelboraf™), imatinib (Gleevec™) and others have been successfully developed against these oncogenic drivers.
Researchers and clinical oncologists now often incorporate genetic assessments into clinical trials and routine care with the hope of directing patients to medicines that may have a greater chance of treating their cancers effectively. As more driver oncogenes in cancer are identified, clinicians and investigators are more willing to test routinely with an expanded panel genetic test. In turn, it is possible to develop drugs for defined subsets of patients, and to look for patients whose tumor types harbor genetically similar alterations. As such, doctors may begin to identify tumors and select therapies based on the type of mutations they share, rather than the part of the body from which they arise. Such a system should afford more efficient drug development, the opportunity for robust clinical responses and a better understanding of the underlying mechanisms of cancer.
The Loxo Approach
Step 1—Target Selection
Target selection requires an understanding of which genetic alterations are oncogenic drivers. Some targets have already been clinically validated as oncogenic drivers of cancer and offer increased likelihood of program success; these targets require improved chemistry or innovative development for competitive differentiation. Other targets are still emerging, so the understanding of oncogenic driver behavior requires careful analysis of new biological evidence. We believe we are well-positioned to exploit both types of opportunities.
Learning from Clinical Trial Data. Response signals for third-party drugs in clinical trials can point to the promise of a target that has been inadequately exploited. An important emerging concept in cancer drug development is that of target coverage, or the extent to which a drug fully engages its intended target. We believe that maximal target inhibition is required for maximal clinical effect. However, drugs often fail to reach sufficient concentrations in the human body because they are poorly absorbed, poorly distributed, rapidly cleared, or cause off-target toxicities at doses lower than those needed for optimal efficacy. Sometimes these limitations can be overcome with better chemistry, which improves drug exposure and reduces unwanted off-target effects. A second lesson that can be gleaned from clinical trial data relates to the concept of “acquired resistance.” Acquired resistance describes the clinical situation where a patient’s oncogene addicted tumor is at first sensitive to the relevant drug but then mutates again such that it becomes resistant. It is sometimes possible to understand how a new, acquired mutation has led to drug resistance. In turn, it is sometimes possible to develop a next-generation drug inhibitor that can re-induce a response in the patient. For example, the drug osimertinib (Tagrisso™) addresses acquired resistance in patients who have progressed on a prior EGFR inhibitor. Developing second-generation drugs against validated targets with acquired resistance can be of great clinical value to patients because it extends the time of durable disease control, while the well-characterized biology offers a compelling drug development opportunity.
Learning from Academic Research. Lab research can help identify and qualify emerging targets. Genetic studies across groups of patient tumor samples generate rich data sets, which are often publicly available. Bioinformatic approaches can help identify the alterations most likely to have clinical relevance. Lab experiments involving cell and animal models can be used to explore whether a novel target is an oncogenic driver and whether targeted drugs can be effective. Consistency of results across highly respected labs is especially important. We apply our judgment to synthesize these diverse data streams to identify the most promising targets.
Step 2—Drug Profiling
We translate target insight into a drug through the application of chemistry to a biologic problem. Whether our target of interest is fully validated or novel, we strive to understand, on a structural level, whether the relevant protein can be inhibited with a compound we can design and synthesize. We assess compounds based on their ability to fully engage their targets, avoid unwanted effects and have favorable pharmacologic properties. Target specificity also affords increased potential for combination therapy with other targeted agents. We have observed that better chemistry can unlock novel biology in the clinic. We rely on extensive experimental data from two domains to justify advancing a program.
Biologic Relevance. In consultation with our Scientific Advisory Board, we identify experiments with the intent to answer the underlying hypothesis of oncogenic driver potential, target validation, or a differentiated profile relative to competitive programs. These experiments may be conducted internally at our lab facilities in Boulder, CO, with third-party partners and vendors or with academic collaborators.
Chemistry Feasibility. Our internal discovery capability in Boulder, Colorado and our collaborations with leading third-party partners and vendors allow us to exploit structural biology and chemistry insights to solve problems that have historically plagued many cancer drugs. For each target under evaluation, we develop molecules with the specific pharmacokinetic properties that we have identified and are able to compile a comprehensive data review prior to advancing a molecule to preclinical toxicology studies. This understanding of how a molecule might behave in vivo prevents us from advancing products with undesirable physiochemical properties.
Step 3—Clinical Trial and Regulatory Execution
Our clinical development strategy employs a stepwise approach designed to identify response signals early in development and reduce development risks. In early stage development, we seek to explore one or more doses in well-defined patient populations and believe this gives us a higher likelihood of demonstrating a clinical benefit. This approach is intended to allow for early insight into the therapeutic potential of a product candidate and the possibility for rapid clinical development and expedited regulatory strategies, such as Breakthrough Therapy Designation, Fast Track Designation, Priority Review and Accelerated Approval. We intend to develop companion diagnostics as appropriate, with the help of technology partners, to identify patients whose tumors harbor the relevant genetic alterations.
Loxo Oncology Strengths
Management
Our management team is critical to our identification of suitable cancer targets, advancing compelling drug candidates through development, commercializing our products, and the financing of our activities. Our team has significant collective experience leading the discovery, development, regulatory approval, and commercialization of novel therapeutics in prior roles in emerging biotechnology and established pharmaceutical companies.
Scientific Advisory Board
Our Scientific Advisory Board is integral to our target qualification and drug development processes. These advisors are actively involved in development and candidate selection, and we leverage their insights, research and expertise. Their involvement in both academic research and clinical practice allows us to gain proprietary and early insight into emerging biology that conforms to our business strategy. We have assembled key opinion leaders within the oncology community to enable our target qualification approach.
Product Candidates
Larotrectinib (TRK Inhibitor)
Overview. Larotrectinib is an oral, selective inhibitor of the TRK family in development for the treatment of tumors with TRK fusions. TRK fusions have been implicated in diverse tumor types such as appendiceal cancer, breast cancer, cholangiocarcinoma, colorectal cancer, GIST, infantile fibrosarcoma, lung cancer, mammary analogue secretory carcinoma of the salivary gland, melanoma, pancreatic cancer, thyroid cancer, and various sarcomas. We selected larotrectinib from a portfolio of TRK inhibitors invented by our partner Array that had distinct chemical scaffolds and were initially developed to treat pain, a setting in which positive efficacy signals had been seen with therapeutic antibodies targeting the TRK pathway. As a result of the initial focus on a pain indication, the TRK inhibitors were designed for both potency and specificity. In purified enzyme inhibition studies, larotrectinib has demonstrated potent inhibition activity against TRKA, TRKB and TRKC at low nanomolar concentration levels. These studies also demonstrated that larotrectinib was highly selective, as it was not a strong inhibitor of any other tested kinase. Similarly, in cells expressing these TRK receptors, larotrectinib also demonstrated potent inhibition activity at low nanomolar concentrations. We believe that potent and selective inhibition of the TRK pathway may provide clinical benefit in patients whose tumors have relevant TRK alterations. Given larotrectinib’s specificity, we do not anticipate clinical activity in patients whose tumors are not driven by TRK.
TRK Biology. TRK signaling is important to neuronal development, including the growth and function of neuronal synapses, memory development and maintenance and the protection of neurons after ischemia or other injuries. TRK expression decreases after birth in most tissues and in adults expression is restricted primarily to cells of neural crest with a low level of expression under normal conditions. More recently, the role of TRK in non-neural tissues has also been recognized; these tissues include the kidney, prostate, B-lymphocytes, eosinophils, marrow-derived endothelial precursors (involving heart, muscle and ovary) and embryonic stem cells. Three high-affinity TRK receptors have been identified: TRKA, TRKB and TRKC. These proteins are encoded by the NTRK1, NTRK2 and NTRK3 genes, respectively.
The Role of TRK in Cancer. TRK has been widely implicated in multiple cancer types and research suggests that the genes that code TRK can be involved in fusion events, or the abnormal connection of two genes. Fusion events can result in pathologic activation of cellular growth and proliferation pathways. Multiple downstream pathways believed to be important in cancer are stimulated by activated TRK receptors, including the PI3-kinase, phospholipase C-gamma, and MAP-kinase pathways. Drugs targeting some of these pathways have demonstrated clinical activity in the treatment of cancer. The first TRK fusion kinases that were discovered in solid tumors involved the NTRK1 gene in colon cancer. In 2002, fusion kinases involving NTRK3 were identified in secretory breast carcinoma, a rare subtype of breast cancer. More recently, research has uncovered multiple other instances of fusions involving the genes that code TRK. For example, scientists identified NTRK1 gene fusions as oncogenic in lung adenocarcinomas in 2013. We believe that the growing body of scientific literature suggesting the presence of NTRK fusions suggests a possible dependency for cellular proliferation and survival, or an oncogenic addictive role, across multiple cancers.
Larotrectinib Clinical Development Program. We are evaluating larotrectinib in the following clinical trials:
· Phase 1 multicenter, open-label dose escalation trial in adult patients with advanced solid tumors refractory to standard therapy. The primary endpoints of this open label, multicenter, dose escalation trial include safety assessments, determining the maximum tolerated dose and identifying the appropriate dose for further clinical investigation. Secondary endpoints include pharmacokinetic assessments of orally administered larotrectinib, evaluation of tumor response and duration of response.
· Phase 2 basket trial, a multicenter, international open-label trial in adult cancer patients whose tumors harbor TRK fusions (NAVIGATE). The larotrectinib Phase 2 basket trial is enrolling patients with TRK fusions across all solid tumor types. A basket trial is a type of clinical study that seeks to enroll cancer patients with a common genetic feature, in this case, a TRK fusion, as opposed to patients with a particular type of cancer. During this trial, larotrectinib is administered orally as a single agent continuously in 28-day cycles. The 100 mg twice-daily dose was selected for this trial. The
primary endpoint of the trial is the objective response rate (“ORR”) to larotrectinib, as measured by the proportion of subjects with best overall confirmed response of complete response or partial response by RECIST v1.1, or Response Assessment in Neuro-Oncology RANO, criteria, as appropriate. Secondary endpoints include duration of response, the proportion of subjects that have any tumor regression as a best response, progression-free survival, overall survival, safety and tolerability.
· Phase 1/2 multicenter, open-label trial in pediatric patients with advanced solid or primary central nervous system (“CNS”) tumors (SCOUT). The trial utilizes the capsule dosage form as well as a liquid formulation of larotrectinib designed specifically for pediatric patients unable to swallow capsules. The primary objective of the trial is to explore the safety of larotrectinib. Secondary objectives include the characterization of pharmacokinetics, the identification of the maximum tolerated dose and/or the Phase 2 dose, and a description of antitumor activity.
In February 2017, we announced that we had completed clinical trial enrollment consistent with written FDA feedback which we believe affirmed a path forward for a tissue-type agnostic NDA filing strategy for adult and pediatric TRK fusion patients and that provided specific advice regarding the primary analysis set that could potentially support an NDA filing. The primary efficacy analysis for larotrectinib is based on RECIST v1.1 ORR, as determined by independent radiology review, for NTRK fusion patients enrolled across the three ongoing aforementioned larotrectinib clinical studies. Durability and magnitude of response and safety are also critical elements of the regulatory risk-benefit determination. The larotrectinib clinical trials remain open to continue long-term follow-up of enrolled patients and provide a mechanism for continued drug access to newly identified patients through trial enrollment during regulatory interactions.
In December 2017, we announced that we initiated the submission of a rolling NDA to the FDA for larotrectinib for the treatment of unresectable or metastatic solid tumors with NTRK-fusion proteins in adult and pediatric patients who require systemic therapy and who have either progressed following prior treatment or who have no acceptable alternative treatments. We expect to complete the NDA submission by the end of March 2018. However, we can make no assurances that the FDA will grant us approval for the indication that we are seeking, or at all. We expect our partner Bayer to submit a European MAA in 2018.
In February 2018, we announced a publication in the February 22, 2018 issue of the New England Journal of Medicine (“NEJM”) for larotrectinib in the treatment of pediatric and adult patients whose tumors harbor TRK gene fusions.
The NEJM publication provides additional clinical detail and patient follow-up from the 2017 American Society of Clinical Oncology (“ASCO”) Annual Meeting presentation. It includes the first 55 consecutively enrolled adult and pediatric patients with TRK fusion cancers treated across our Phase 1 adult trial, Phase 2 trial (NAVIGATE), and Phase 1/2 pediatric trial (SCOUT) and uses a July 17, 2017 data cutoff. The initial NDA submission utilized this same patient population and data cutoff.
The published data were based on the intent to treat (“ITT”) principle, using the first 55 TRK fusion patients with RECIST-evaluable disease enrolled to the three clinical trials, regardless of prior therapy or tumor tissue diagnostic method. The analysis included both adult and pediatric patients, ranging in age from four months to 76 years, who carried 17 unique TRK fusion-positive tumor diagnoses. Tumor types included salivary gland, infantile fibrosarcoma, thyroid, colon, lung, melanoma, gastrointestinal stromal tumor (“GIST”), and other cancers.
The primary endpoint for the analysis was ORR. Secondary endpoints included duration of response, progression-free survival, and safety. As shown below, as previously reported, the ORR was 75% by central assessment and 80% by investigator assessment.
|
|
|
Central Assessment (%) |
|
Investigator Assessment (%) |
|
Overall response rate (95% CI) |
|
75% (61–85%) |
|
80% (67–90%) |
|
Partial response |
|
62% |
|
64%* |
|
Complete response |
|
13% |
|
16% |
|
Stable disease |
|
13% |
|
9% |
|
Progressive disease |
|
9% |
|
11% |
|
Could not be evaluated |
|
4% |
|
0 |
* Data include one patient who had a partial response that was pending confirmation at the time of the July 17, 2017 data cut-off. The response was subsequently confirmed, and the patient’s treatment and response were ongoing as of the data cut-off date.
Median duration of response (“DOR”) and median progression-free survival (“PFS”) had not been reached after median follow-up durations of 8.3 months and 9.9, respectively. At 1 year, 71% of responses were ongoing. As of the July 17, 2017 data cutoff, 86% of responding patients remained on treatment or had undergone surgery with curative intent. The first patient treated with a TRK fusion tumor remained in response and on therapy at 27 months.
Larotrectinib was well tolerated with the majority of all adverse events being grade 1 or 2. Few grade 3 or 4 adverse events, regardless of attribution, were observed with the most common being anemia (11%), alanine or aspartate aminotransferase increase (7%), weight increase (7%), and neutrophil count decrease (7%) (all grade 3 events). There were no treatment-related grade 4 or 5 events, and no treatment-related grade 3 adverse events occurred in more than 5% of patients. Eight patients required larotrectinib dose reductions. Adverse events leading to dose reductions included AST/ALT elevation, dizziness, and neutrophil count decrease, all grade 2 or 3 events. In all cases, patients whose doses were reduced maintained their best response at the lower dose and none discontinued larotrectinib due to an adverse event.
Primary resistance was observed in six patients in the study. Of the six, one patient had been previously treated with another TRK inhibitor and tumor sequencing prior to larotrectinib dosing revealed a solvent front mutation, a known resistance mechanism. Tumor tissue was analyzed for three of the five remaining patients. In all three patients, TRK immunohistochemistry failed to demonstrate TRK expression, potentially implicating a false positive initial TRK fusion test result and therefore explaining the lack of response in these patients.
The publication also details mechanisms of acquired resistance to larotrectinib. Ten patients experienced disease progression while on treatment after a documented objective response or stable disease for at least six months, a phenomenon known as acquired resistance. Nine of the ten patients had assessments of post-progression tumor or plasma samples, and NTRK kinase domain mutations were identified in all of those samples tested. In seven of those assessed, investigators identified solvent front mutations as a convergent mechanism of acquired resistance; other NTRK kinase domain mutations were identified in the remaining two patients tested. Of the 10 patients who developed acquired resistance, 80% continued treatment with larotrectinib beyond progression due to ongoing clinical benefit.
Orphan Drug Status. In September 2015, we announced that the FDA granted larotrectinib orphan drug designation for the treatment of soft tissue sarcoma. In January 2016, we announced that the European Commission designated larotrectinib as an orphan medicinal product for treatment of patients with soft tissue sarcoma. In May 2017, we announced that the FDA granted orphan drug designation to larotrectinib for the “treatment of solid tumors with NTRK-fusion proteins.”
Breakthrough Therapy Designation. In July 2016, the FDA granted Breakthrough Therapy Designation to larotrectinib “for the treatment of unresectable or metastatic solid tumors with NTRK-fusion proteins in adult and pediatric patients who require systemic therapy and who have either progressed following prior treatment or who have no acceptable alternative treatments.” The larotrectinib Breakthrough Therapy Designation application, submitted approximately 60 days prior to designation receipt, included TRK fusion patient data from all three ongoing larotrectinib clinical trials.
Commercial Opportunity. To add to evidence from the literature, we have worked closely with tumor banks, third party labs, and various assay technology partners to better understand TRK fusion frequency and how to improve testing sensitivity. Our work required certain assumptions based on detection assays with known limitations with regard to sensitivity (i.e. ability to detect TRK fusions that truly exist) and specificity (i.e. ability to detect only TRK fusions). In light of these assay limitations, we provided an estimated range of patient numbers for TRK fusion cancers, which in the United States is 1,500 to 5,000 late-line eligible patients each year. We also believe there are similar numbers of patients in the large European countries and Japan relative to those population sizes, as has been shown for other targeted therapies.
The patient opportunity numbers we cite above reflect our best understanding of the peak addressable opportunity for larotrectinib. However, as patients with NTRK fusions must be identified in practice if they are to receive larotrectinib, two factors relating to the diagnostics market must continue to evolve for the full realization of the commercial potential of the drug. First, the diagnostic tools themselves must continue to demonstrate improving sensitivity for NTRK fusions. Available assays, especially those depending on next-generation sequencing, are imperfectly sensitive, though they are improving. Second, patients need better access to high quality diagnostic tests with the ability to comprehensively characterize the biology of their tumors. In large part, patient access is a byproduct of lab reimbursement—when financial incentives exist that encourage or discourage testing, testing volumes change accordingly. We believe that both assay sensitivity and patient access are improving and will continue to improve. The rate at which these trends evolve is key to finding the 1,500 to 5,000 patients who we believe exist each year in the United States. Our pre-launch preparations are therefore highly focused on the diagnostics and pathology lab communities.
In November 2017, we entered into a license, development and commercialization agreement (“Bayer Agreement”) with Bayer, pursuant to which we and Bayer will collaborate to develop and commercialize larotrectinib and LOXO-195, our franchise of highly selective TRK inhibitors for patients with TRK fusion cancers. Pursuant to the Agreement, we granted co-exclusive development and commercialization licenses to Bayer for both larotrectinib and LOXO-195. (See “Agreements” section below.)
LOXO-195 (TRK Inhibitor)
LOXO-195 is a drug candidate in clinical development. It was designed as a second-generation TRK inhibitor intended to address predicted acquired resistance mechanisms resulting from therapy with a first-generation TRK inhibitor such as larotrectinib, or multikinase inhibitors with significant anti-TRK activity. In preclinical models, LOXO-195 appears to be particularly active against so-called “solvent front” mutations, which are a class of structural mutations that have been described in the literature as relevant to TRK
acquired resistance, but also ALK and ROS1 acquired resistance. Given the limited clinical experience with TRK inhibitors, and the preliminary nature of the research describing mechanisms of acquired resistance in TRK fusion cancers, it is reassuring to see a resistance mechanism, i.e. solvent front mutations, which have been described in the setting of other oncogenic fusion events, namely those involving ALK and ROS1. Solvent front mutations often require novel medicinal chemistry solutions, and we believe LOXO-195 represents a structurally diverse solution to this mechanism of acquired resistance. With the LOXO-195 program, we hope to offer certain patients who progress on larotrectinib or other agents an opportunity to extend their time of durable disease control.
We presented preclinical data at the EORTC-NCI-AACR Molecular Targets and Cancer Therapeutics Symposium in December 2016 in Munich, Germany on our LOXO-195 preclinical program. In preclinical models, LOXO-195 demonstrated potent and selective inhibition of solvent front mutations, as well as other predicted resistance mutations reported in recent literature to date. LOXO-195 also exhibited favorable preclinical in vivo properties in relevant in vivo models.
Preclinical data on our LOXO-195 preclinical program and clinical proof-of-concept data from the first two patients treated with LOXO-195 were published in an article in Cancer Discovery in June 2017. An adult with colorectal cancer and a child with infantile fibrosarcoma were biopsied at the time of tumor progression and found to have a solvent front TRK mutation in the existing TRK fusion, which explained the diminished activity of larotrectinib. As no other treatment options exist to address TRK fusion solvent front mutations, the FDA permitted these patients to access LOXO-195 through emergency use Investigational New Drug Applications (“Emergency Use INDs”). Both patients had confirmed partial responses based on RECIST v1.1 ORR to LOXO-195, with minimal adverse events reported (grade 2 dizziness and grade 1 diarrhea reported, though neither interfered with dosing).
We submitted an Investigational New Drug (“IND”), application for a Phase 1/2 study of LOXO-195 in adults with NTRK fusion or non-fusion NTRK altered cancers and received clearance from the FDA to proceed in May 2017. LOXO-195 is currently being studied in a multi-center Phase 1/2 trial. The primary objective of the trial is to determine the maximum tolerated dose or recommended dose for further study. Key secondary objectives include measures of safety, pharmacokinetics, and anti-tumor activity (i.e. Objective Response Rate and Duration of Response, as determined by RECIST v1.1). The trial includes a dose escalation phase and a dose expansion phase. We expect to provide a clinical data update on this program in a scientific forum in the second half of 2018.
In November 2017, we entered into a license, development and commercialization agreement with Bayer pursuant to which we and Bayer will collaborate to develop and commercialize larotrectinib and LOXO-195, our franchise of highly selective TRK inhibitors for patients with TRK fusion cancers. Pursuant to the Agreement, we granted co-exclusive development and commercialization licenses to Bayer for both larotrectinib and LOXO-195. (See “Agreements” section below.)
LOXO-292 (RET Inhibitor)
LOXO-292 is a drug candidate in clinical development. It targets a cancer oncogene known as Rearranged during Transfection (“RET”). In normal physiology, RET is a tyrosine kinase receptor that binds the glial cell line-derived neurotrophic factor (GDNF) ligand family, a signaling pathway important to the development of the nervous system and kidneys. In cancer, activating fusions and mutations in RET have been identified across a range of cancer types, including lung, thyroid, breast and colon cancers. There are multiple peer-reviewed articles of patients with RET gene alterations demonstrating preliminary anti-tumor activity in experimental trials of multikinase inhibitors with anti-RET activity. LOXO-292 is designed to be a highly specific RET inhibitor that is intended to optimize on-target potency for RET fusions, activating mutations, and anticipated mechanisms of acquired resistance in these settings.
RET fusions account for approximately 2% of non-small cell lung cancer. RET fusions also account for 10-20% of papillary thyroid cancer, and may be found in other tumors such as colon cancer, where prevalence is not well understood. RET mutations account for approximately 60% of medullary thyroid cancer. Based on literature reports, we estimate that in lung and thyroid cancers, there are approximately 5,000 late-line eligible patients per year in the United States who might benefit from a targeted RET inhibitor such as LOXO-292. This estimate reflects our best understanding of the peak addressable opportunity for LOXO-292. However, as patients with activating RET alterations must be identified in practice if they are to receive LOXO-292, two factors relating to the diagnostics market must continue to evolve for the full realization of the commercial potential of the drug. First, the diagnostic tools themselves must continue to demonstrate improving sensitivity for activating RET alterations. Available assays, especially those depending on next-generation sequencing, are imperfectly sensitive, but improving. Second, patients need better access to high quality diagnostic tests with the ability to comprehensively characterize the biology of their tumors. In large part, patient access is a byproduct of lab reimbursement—when financial incentives exist that encourage or discourage testing, testing volumes change accordingly. We believe that both assay sensitivity and patient access are improving and will continue to improve. The rate at which these trends evolve is key to finding the approximately 5,000 patients who we believe exist each year in the United States.
We presented preclinical data at the EORTC-NCI-AACR Molecular Targets and Cancer Therapeutics Symposium in December 2016 in Munich, Germany on our LOXO-292 preclinical program. LOXO-292 demonstrated RET potency in enzyme and cellular assays with minimal activity against highly related kinases and other off-targets. In preclinical data, in vivo, LOXO-292 demonstrated its ability to drive tumor regressions in relevant RET-driven models, while having minimal effect on body weight, a proxy for toxicity. LOXO-292 also demonstrated potency against many anticipated mechanisms of acquired resistance, both in vitro and in vivo.
Following IND clearance by the FDA, we enrolled the first patient into our Phase 1 trial of LOXO-292 in May 2017. This first-in-human, global, multi-center Phase 1 trial is evaluating LOXO-292 as a single agent in patients with advanced solid tumors. The primary objective of the trial is to determine the maximum tolerated dose or recommended dose for further study. Key secondary objectives include measures of safety, pharmacokinetics, and anti-tumor activity (i.e. Objective Response Rate and Duration of Response, as determined by RECIST v1.1). The trial includes a dose escalation phase and dose expansion phase. During the dose escalation phase, patients with advanced solid tumors may be enrolled to inform the selection of a dose and schedule for the expansion phase. In the expansion phase, five cohorts are planned to allow for the characterization of preliminary activity of LOXO-292 in the following genetically-defined populations: 1) RET-fusion lung cancer patients with prior RET inhibitor experience; 2) RET-fusion lung cancer patients with no prior RET inhibitor experience; 3) RET-mutant medullary thyroid cancer patients with prior RET inhibitor experience; 4) RET-mutant medullary thyroid cancer patients with no prior RET inhibitor experience; and 5) other RET-altered solid tumors.
In October 2017, we presented preliminary clinical data from the LOXO-292 program at the International Association for the Study of Lung Cancer (“IASLC”) 18th World Conference on Lung Cancer, in Yokohama, Japan. The presentation, which employed a September 26, 2017 data cut-off date, described the first two patients with RET-fusion lung cancer with and without brain metastases treated with LOXO-292. Both patients had disease progression while receiving prior multi-kinase inhibitors (“MKIs”). On LOXO-292, both patients achieved partial responses. The first patient was previously treated with RXDX-105, enrolled on the first dose cohort of the Phase 1 trial, received LOXO-292 20 mg daily, and demonstrated a RECIST confirmed partial response. The second patient was previously treated with alectinib (starting at 600 mg twice daily and increased to 900 mg twice daily) and experienced disease progression systemically and in the brain. Due to the rapidly progressive nature of the brain metastases, the patient was ineligible for the Phase 1 trial and received LOXO-292 in doses ranging from 20-100 mg twice daily under an intra-patient dose escalation single patient protocol. The patient demonstrated a RECIST confirmed partial response, including a response in the brain. Both patients remained on LOXO-292 as of the data cut-off date. In this early two patient data set, LOXO-292 was well-tolerated, with no adverse events attributed to LOXO-292. Additionally, the presentation included pharmacology data from all patients enrolled at that time (n=28), providing evidence that the highest dose level shown (60mg twice daily) provides IC90 RET target coverage in patients. We expect to provide a more comprehensive trial update in a scientific forum in the first half of 2018.
LOXO-305 (BTK Inhibitor)
In July 2017, we announced that we entered into a definitive agreement to purchase the BTK inhibitor program from Redx Pharma Plc. Under the terms of the agreement, we made a $40 million payment to Redx Pharma Plc for the full acquisition of the BTK discovery program, including lead candidate LOXO-305 (formerly RXC005). We are not subject to milestone or royalty obligations.
LOXO-305 is a novel, potent selective, and reversible BTK inhibitor with equivalent potency against wild-type and cysteine-481 (“C481”) mutated BTK. We believe that the widespread use of covalent BTK inhibitors, such as ibrutinib and acalabrutinib, will increasingly drive acquired resistance through a mutational event in BTK called C481, leading to a group of relapsing patients in need of new therapies. Our work suggests that a highly selective, reversible BTK inhibitor can address this emerging unmet need in patients whose disease has progressed on a covalent BTK inhibitor.
LOXO-305 was designed to reversibly bind BTK and preserve activity in the presence of the C481 acquired resistance mutation. Additionally, it was designed to avoid off-target kinases that have complicated the development of both covalent and other more promiscuous reversible BTK inhibitors, such as EGFR, ITK, BMX, TEC, ITK, BLK, LCK and SRC. LOXO-305 is expected to enter clinical development in the second half of 2018.
FGFR
We are seeking a drug candidate capable of potently inhibiting fibroblast growth factor receptor (“FGFR”) isoforms 2 and/ or 3, while sparing isoforms 1 and 4. There are numerous FGFR1-3 inhibitors that have shown anti-tumor activity in Phase 1-2 human clinical trials, but they are associated with significant toxicities that limit dose intensity (see Lewin et al Journal of Clinical Oncology 33 (2015) 3372-3374). FGFR4 inhibitors are also in clinical development, tend to spare FGFR1-3, target different biologic hypotheses, and are not of interest to Loxo.
The FGFR family of receptors consists of four isoforms with tyrosine kinase domains, numbered one through four, which play important roles in embryonic development and adult angiogenesis, hormone regulation, and renal function. Fusions, point mutations, and gene amplifications in individual isoforms of the FGFR family have been associated with distinct cancer types in patients, and preliminary anti-tumor activity has been demonstrated in genitourinary, lung, and breast cancers in experimental trials of kinase inhibitors with anti-FGFR activity. However, most small molecule FGFR inhibitors are functionally equipotent against isoforms FGFR1, FGFR2 and FGFR3, and are associated with metabolic and systemic toxicities that limit dose, duration of therapy and target engagement.
We presented preclinical data at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics meeting in November 2015 in Boston, Massachusetts on our FGFR preclinical program. Data for our potent and selective FGFR inhibitor tool compounds show a relative sparing of FGFR1, while exhibiting high oral bioavailability and favorable PK properties in animal models.
Agreements
Bayer License, Development and Commercialization Agreement
In November 2017, we entered into a license, development and commercialization agreement with Bayer pursuant to which we and Bayer will collaborate to develop and commercialize larotrectinib and LOXO-195, our franchise of highly selective TRK inhibitors for patients with TRK fusion cancers. Pursuant to the Bayer Agreement, we granted co-exclusive development and commercialization licenses to Bayer for both larotrectinib and LOXO-195.
In addition to an upfront cash payment of $400.0 million, we are eligible to receive $450.0 million in milestone payments upon larotrectinib regulatory approvals and first commercial sale events in certain major markets and an additional $200.0 million in milestone payments upon LOXO-195 regulatory approvals and first commercial sale events in certain major markets.
We will lead global development activities and regulatory activities in the U.S. Bayer will lead regulatory activities outside the U.S. and global commercial activities. Globally, we will be responsible for 50% of development costs. In the U.S., where we and Bayer will co-promote the products, we will be responsible for 50% of the commercial costs and receive 50% of the profits. Bayer will pay us a $25.0 million milestone upon achieving a certain U.S. net sales threshold. We will have the right to opt-out of the U.S. co-promotion, in which case we would receive a royalty in the low thirties percent range on U.S. net sales, which is meant to approximate the economics of the 50/50 profit split.
Outside of the U.S., where Bayer will commercialize, Bayer will pay us tiered, double digit royalties on net sales, and sales milestones totaling $475.0 million.
The Bayer Agreement also includes a standstill provision that prevents Bayer from acquiring five percent or more of our voting securities.
The Bayer Agreement will terminate as to a product or country upon the expiration of the royalty term applicable to such product in such country. The Bayer Agreement may be terminated by either party for material breach or bankruptcy. In addition, Bayer may terminate the Bayer Agreement after the fourth anniversary of the effective date upon written notice to us, or in the event that we receive a “complete response letter” from the U.S. FDA with respect to larotrectinib, or if we do not receive marketing approval for larotrectinib by December 31, 2018.
Array Collaboration
Overview. On July 3, 2013, we entered into a Drug Discovery Collaboration Agreement with Array (“Array Agreement”) which was subsequently amended on November 26, 2013, April 10, 2014, October 13, 2014, March 31, 2015 and February 18, 2016. Pursuant to the Array Agreement, Array agreed to design, conduct and perform research and preclinical testing for certain compounds that we select, including larotrectinib, targeting TRKA, TRKB and TRKC, identify IND candidates for TRK and other targets, and undertake manufacturing activities sufficient to conduct Phase 1 clinical studies for a subset of these compounds. Array granted us exclusive licenses worldwide for clinical and commercial development of compounds that inhibit a defined number of targets. The Array Agreement also contains mechanisms for the replacement of a discovery program by substituting a new target for a target for which discovery activities were discontinued. As consideration, Array received or receives 1) shares of our capital stock; 2) ongoing cash payments proportionate to Array’s commitment of full-time equivalents to conduct preclinical research for our programs during a discovery research phase through July 2016, which we may extend for up to two additional one-year periods, with the Array Agreement now set to conclude in September 2018; 3) payments for certain other costs and research requirements related to the targets; 4) milestone payments of up to approximately (i) $223 million with respect to products related to TRK, including larotrectinib and its backup compounds, and (ii) $213 million with respect to product candidates directed to targets other than TRK, if certain clinical and sales milestones are achieved; and 5) single-digit royalties on sales of any resulting drugs.
In the October 2014 amendment to the Array Agreement, in addition to larotrectinib, the parties designated 12 discovery targets, of which seven were selected for additional study in January 2015, which was to be reduced to four on or before January 2016. The October 2014 amendment also created mechanisms through which we and Array could evaluate additional targets outside of the named targets to implement the originally contemplated potential substitution of one or more of the named targets. We had the option to maintain the total target number at five for an additional payment, and we exercised this option to maintain five discovery programs in January 2016. In the October 2014 and March 2015 amendments to the Array Agreement, we agreed to provide additional headcount support for Array’s research activities on our agreed-upon targets.
In the February 2016 amendment to the Array Agreement, we agreed to continue to support increased headcount for an additional interim period. Additionally, the parties extended the agreement term through September 30, 2017, with Loxo retaining an option to extend the term for up to one additional year. This option was exercised during the three-month period ended June 30, 2017. This headcount support for Array’s research activities on our agreed-upon targets currently expires on September 30, 2018. Also in the February 2016 amendment, the parties increased the discovery program list from five to six targets by adding a new target that was not previously worked on by either party, and further refined the original mechanism through which we and Array could evaluate additional targets outside of the named targets for potential substitution with one or more of the named targets. Lastly, the February 2016
amendment allowed Array to be eligible for milestones and royalties on any back-up compounds developed through the collaboration. This amendment also included an additional payment from us to Array, satisfying an obligation of the April 2014 amendment.
We and Array jointly own the intellectual property developed by the combined efforts of both our employees, and we each retain ownership of intellectual property that we develop independently pursuant to the collaboration. Array has granted us an exclusive license under all of Array’s intellectual property rights, including intellectual property rights developed in the collaboration, to research, develop and commercialize products resulting from the collaboration.
In October 2014, Array notified us in writing that it planned to begin substantial negotiations with third parties regarding the development and/or commercialization of compounds that selectively modulate TRKA for oncology indications. That notification triggered a 90-day period whereby we had the right to discuss the terms and conditions under which Array would grant such rights to us. This period has expired and Array is free to negotiate with, and grant such rights to, a third-party.
Governance. Our collaboration with Array is guided by a joint research committee (“JRC”). Decisions of the JRC are made by majority vote. If the votes required to approve a decision cannot be reached within the JRC, we have the deciding vote except with respect to matters that would cause Array to violate any obligation or agreement it may have with a third-party or unilaterally impose on Array any financial obligation that is beyond the scope of Array’s obligation under the Array Agreement.
Exclusivity Restrictions. Subject to exceptions specified in the Array Agreement, for so long as we have an active research or development program for a target selected by us or are commercializing a product for such a target, Array may not research, develop, manufacture or commercialize any product comprising a small molecule, whose primary mechanism of action for therapeutic or prophylactic effect, binds to or modulates the activity of such target, or binds or modulates at least two of TRKA, TRKB or TRKC. For any target added to the Array Agreement after the February 2016 amendment, whether by substitution or otherwise, Array may not conduct discovery research with respect to a product comprising a small molecule that, as a primary mechanism of action for therapeutic or prophylactic effect, binds to or modulates the activity of such target.
Term and Termination. The Array Agreement expires on a product-by-product and country-by-country basis on the date of the expiration of the applicable royalty term with respect to each licensed product in each country and in its entirety upon the expiration of all applicable royalty terms for all licensed products in all countries. The royalty term for each licensed product in each country is the period commencing with first commercial sale of the applicable licensed product in the applicable country and ending on the latest of (i) ten years following the date of the first commercial sale in the country and (ii) expiration of the last to expire of any patent specified by the Array Agreement that includes at least one valid claim covering the manufacture, use or sale of such product in such country. Following expiration of the Array Agreement, we will have a perpetual, fully paid-up, non-exclusive license to conduct research, develop and commercialize the products developed under the Array Agreement.
The Array Agreement may be terminated by either party upon the failure of the other party to cure any material breach of its obligations under the Array Agreement, provided that, so long as we are reasonably able to pay our debts as they are due, in the event of our breach after expiration of the discovery research phase, Array will only be entitled to seek monetary damages and will not have the right to terminate the Array Agreement. We also have the right to terminate the Array Agreement or to terminate discovery research with respect to any compounds under development on six months’ notice to Array. If we terminate the Array Agreement for convenience, all licenses granted to us will terminate and Array will receive a license under any intellectual property rights generated by us under the collaboration to further develop and commercialize the licensed programs. If we terminate the Array Agreement as a result of Array’s uncured breach, then the licenses granted by Array would continue and we would remain obligated to pay the milestone and royalty payments. For each specific compound for which we terminate research and development activities before the expiration of the research discovery phase, all licenses granted to us directed at that abandoned compound will concurrently terminate and Array will receive a license under any intellectual property rights generated by us under the collaboration to further develop and commercialize such abandoned compound. We and Array have each also agreed to indemnify the other party for breaches of representations and warranties under the Array Agreement, and we have agreed to indemnify Array against any claims relating to personal injury or death resulting from a compound developed, manufactured, used, sold or otherwise distributed under the Array Agreement.
Intellectual Property
Our intellectual property is critical to our business and we strive to protect it through the use of trade secrets and by seeking and maintaining patent protection in the U.S. and internationally for our product candidates, back-up compounds, and other inventions that are important to our business. For our product candidates, we generally strive for patent protection covering both composition of matter and methods of use. As more fully described below, we have an exclusive license from Array to issued composition of matter and method of use patents covering larotrectinib that expire in 2029, without taking into account any applicable extensions.
Through our internal discovery efforts and our work with third-parties, we patent wholly-owned, joint and/or exclusively licensed inventions in the U.S. and internationally. In addition, during the development of our product candidates, we may pursue patent protection to potentially enhance commercial success, such as method of use, formulation, treatment regimens, methods of making, synthetic intermediates, polymorphs, or other patent claims. We also rely on trade secrets relating to our discovery programs and product
candidates, and seek to protect and maintain the confidentiality of proprietary information to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection.
The patent positions of biotechnology companies like ours are generally uncertain and involve complex legal, scientific and factual questions. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted after issuance. Consequently, we may not obtain or maintain adequate patent protection for any of our product candidates. We cannot predict whether the patent applications we are currently pursuing will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide sufficient protection from competitors. Any patents that we hold may be challenged, circumvented or invalidated by third parties.
Our patent portfolio includes patents and patent applications we exclusively license from or jointly own with Array, exclusive worldwide licenses from Array for all therapeutic indications for new intellectual property developed in our Loxo/Array discovery programs, and Loxo-only owned patents and patent applications directed to formulations and methods of use of larotrectinib.
We also solely own patents acquired from Redx Pharma Plc when we purchased its BTK program. This patent portfolio includes issued patents and pending patent applications covering compositions of matter, methods of use, methods of making, and synthetic intermediates. The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing a non-provisional patent application.
Larotrectinib’s patent portfolio includes patents and applications exclusively licensed to us by Array for our product candidates on a worldwide basis for all therapeutic indications. Composition of matter patents for larotrectinib are issued in the U.S., Hong Kong, New Zealand, Australia, Chile, Gulf Cooperation Council (“GCC”), Indonesia, China, Columbia, Costa Rica, Korea, Japan, Russia, Taiwan, Ukraine, the Philippines, and Europe (which have been validated in all member and extension states). The issued U.S. and European patents expire in 2029, not taking into account any applicable extensions for patent office and/or regulatory delay in the relevant jurisdictions. The larotrectinib patent portfolio also includes separate US and foreign patent families directed to treatment regimens for larotrectinib, manufacturing processes for making larotrectinib, crystalline forms of larotrectinib, and pharmaceutical formulations of larotrectinib, which will expire in the range of 2035 to 2037, not taking into account any applicable extensions for patent office and/or regulatory delay in the relevant jurisdictions.
We have licensed from Array three other patent families that cover our next-generation selective TRK inhibitor program, including one patent family covering LOXO-195; some of the next-generation TRK inhibitors have activity against various mechanisms of acquired resistance to TRK inhibition, including LOXO-195. We have issued patents in Australia, the EPO (validated in all member states and extension states), Chile, China, Columbia, Hong Kong, Japan, Mexico, New Zealand, Philippines, Russia, Taiwan, Ukraine, and the U.S. providing composition of matter and method of use coverage for LOXO-195, with allowed cases in Korea and the GCC. The issued patents expire in 2031, not taking into account any applicable extensions for patent office and/or regulatory delay in the relevant jurisdiction. The LOXO-195 patent portfolio also includes pending U.S. and foreign applications related to methods of use for LOXO-195, manufacturing processes for making LOXO-195, crystalline forms of LOXO-195, and pharmaceutical formulations of LOXO-195, which will expire in the range of 2036 to 2038, not taking into account any applicable extensions for patent office and/or regulatory delay in the relevant jurisdictions.
In addition to the TRK inhibitor programs, we have pending patent applications directed to compositions of matter, methods of use, methods of making, and synthetic intermediates for our RET inhibitors, including LOXO-292, BTK inhibitors, including LOXO-305, and FGFR inhibitors programs. With respect to our RET inhibitors program, we have eight families of applications pending in the U.S. and foreign jurisdictions with terms ranging from 2036 to 2039, not taking into account any other term extensions that might be available for patent office and/or regulatory delay in the relevant jurisdiction. The BTK inhibitors program includes three families of applications pending in the U.S. and foreign jurisdictions with terms ranging from 2035 to 2036, not taking into account other term extensions that might be available for patent office and/or regulatory delay in the relevant jurisdiction. Finally, for the FGFR inhibitors program, we have pending applications in the U.S. and foreign jurisdictions with a term of 2035, not taking into account other term extensions that might be available for patent office and/or regulatory delay in the relevant jurisdiction.
In the U.S., the term of a patent that covers an FDA-approved drug may also be eligible for patent term extension, which permits patent term restoration as compensation for the patent term lost during FDA regulatory review process. The Drug Price Competition and Patent Term Restoration Act of 1984 (the “Hatch-Waxman Act”) permits a patent term extension of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time the drug is under regulatory review. Patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug may be extended. Similar provisions are available in Europe and other foreign jurisdictions to extend the term of a patent that covers an approved drug. In the future, if and when our products receive FDA approval, we expect to apply for patent term extensions on patents covering those products. We plan to seek patent term extensions to any of our issued patents in any jurisdiction where these are available, however there is no guarantee that the applicable authorities, including FDA in the U.S., will agree with our assessment of whether such extensions should be granted, and if granted, the length of such extensions.
We also rely on trade secret protection for our confidential and proprietary information. Although we take steps to protect our proprietary information and trade secrets, including through contractual means with our employees and consultants, third parties may
independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose our technology. Thus, we may not be able to meaningfully protect our trade secrets. It is our policy to require our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships with us. These agreements provide that all confidential information developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances. In the case of employees, the agreements provide that all inventions conceived by the individual shall be our exclusive property. There can be no assurance, however, that these agreements will provide meaningful protection or adequate remedies for our trade secrets in the event of unauthorized use or disclosure of such information.
Competition
Our industry is intensely competitive and subject to rapid and significant technological change. While we believe that our knowledge, experience and scientific resources provide us with competitive advantages, we face substantial competition from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. Many of our competitors have significantly greater financial, technical and human resources. Smaller and early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. As a result, our competitors may discover, develop, license or commercialize products before or more successfully than we do.
We face competition with respect to our current product candidates, and will face competition with respect to future product candidates, from segments of the pharmaceutical, biotechnology and other related markets that pursue targeted approaches to addressing activating molecular alterations in cancer. While there are currently no approved drugs targeting TRK, we are aware of a number of TRK RTK inhibitors, including Daiichi Sankyo and its subsidiary Plexxikon (PLX-7486), Tesaro (TSR-011), Roche (entrectinib), Novartis AG (dovitinib), Mirati (MGDC516), Ono Pharmaceutical (ONO-4474 and ONO-5390556), Chugai Pharmaceutical, a member of the Roche Group (CH7057288), Blueprint Medicines, TP Therapeutics (TPX-0005) and Deciphera. In addition, our collaboration partner, Array, has retained rights to development of compounds that target only one of the TRKA, TRKB or TRKC kinases, which if developed by Array or a licensee could be competitively significant. If larotrectinib or our future product candidates do not offer sustainable advantages over competing products, we may not be able to successfully compete against current and future competitors.
There are no selective RET inhibitors approved in RET-specific indications. Several multikinase inhibitors with anti-RET activity are part of ongoing RET-focused development programs: Eisai (lenvatinib), Exelixis (cabozantinib), AstraZeneca (vandetanib), Ariad (ponatinib), Novartis (dovitinib), Roche (alectinib), Pfizer (sunitinib) and Roche (RXDX-105). A compound from Blueprint Medicines (BLU-667) was developed to be a selective RET inhibitor and is currently in clinical development and there are preclinical selective RET inhibitor programs from Taiho Oncology, Nerviano Medical Sciences, and Cancer Research UK.
For LOXO-305, there are several covalent inhibitors that inhibit BTK, such as those from Abbvie/Pharmacyclics (ibrutinib), AstraZeneca/Acerta (acalabrutinib), Beigene (BGB-3111), Celgene (CC-292), Principia (PRN1008, PRN2246), and Gilead/Ono (GS-4059). They could be competitive if the LOXO-305 development program extends beyond the acquired resistance application. There are also inhibitors with reversible binding modes like LOXO-305, like those from ArQule (ARQ-531), Sunesis (SNS-062), Biogen (BIIB-068), Bristol-Myers Squibb (BMS-986142), Genentech (GDC-0853), Roche (RN983) and Impetis (PNQ-154). Lastly, drugs that work by different mechanisms, other than BTK inhibition, are available or could be developed in patient populations relevant to LOXO-305—these include classes such as BCL-2 inhibitors (e.g. Roche/Abbvie, venetoclax), anti-CD20 biologics (e.g. Roche, rituximab), PI3Kd inhibitors (e.g. Gilead, idelalisib) and cytotoxic chemotherapy.
While there are no approved selective FGFR inhibitors, we are aware of the following compounds with ongoing FGFR-focused programs: J&J (JNJ-42756493), QED Therapeutics (BGJ-398, dovitinib), AstraZeneca (AZD4547), Clovis Oncology (lucitinib), Chugai (CH5183284), Bayer (BAY 1163877, BAY 1179470), Lilly (LY2874455), Eisai (E7090), Taiho (TAS-120), BI (nintedanib), Ariad (ponatinib), FivePrime (FP-1039, FPA144), Incyte (INCB54828), ArQule (ARQ087), BioClinica (MFGR1877S) and Principia (PRN1371).
Our competitors may obtain regulatory approval of their products more rapidly than us or may obtain patent protection or other intellectual property rights that limit our ability to develop or commercialize our product candidates. Our competitors may also develop drugs that are more effective, more convenient, more widely used and less costly or have a better safety profile than our products and these competitors may also be more successful than us in manufacturing and marketing their products. In addition, we will need to develop our product candidates in collaboration with diagnostic companies, and we will face competition from other companies in establishing these collaborations. Our competitors will also compete with us in recruiting and retaining qualified scientific, management and commercial personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Furthermore, we also face competition more broadly across the market for cost-effective and reimbursable cancer treatments. The most common methods of treating patients with cancer are surgery, radiation and drug therapy, including chemotherapy, hormone therapy and targeted drug therapy or a combination of such methods. There are a variety of available drug therapies marketed for cancer. In many cases, these drugs are administered in combination to enhance efficacy. While our product candidates, if any are approved, may compete with these existing drug and other therapies, to the extent they are ultimately used in combination with or as an adjunct to these therapies, our product candidates may not be competitive with them. Some of these drugs are branded and subject to patent protection,
and others are available on a generic basis. Insurers and other third-party payers may also encourage the use of generic products or specific branded products. We expect that if our product candidates are approved, they will be priced at a significant premium over competitive generic, including branded generic, products. As a result, obtaining market acceptance of, and gaining significant share of the market for, any of our product candidates that we successfully introduce to the market will pose challenges. In addition, many companies are developing new therapeutics, and we cannot predict what the standard of care will be as our product candidates progress through clinical development.
The acquisition or licensing of pharmaceutical products is also very competitive. If we seek to acquire or license products, we will face substantial competition from a number of more established companies, some of which have acknowledged strategies to license or acquire products and many of which are bigger than us and have more institutional experience and greater cash flows than we have. These more established companies may have competitive advantages over us, as may other emerging companies taking similar or different approaches to product licenses and/or acquisitions. In addition, a number of established research-based pharmaceutical and biotechnology companies may acquire products in late stages of development to augment their internal product lines, which may provide those companies with an even greater competitive advantage.
Government Regulation
FDA Approval Process
In the U.S., pharmaceutical products are subject to extensive regulation by the FDA. The Federal Food, Drug, and Cosmetic Act and other federal and state statutes and regulations govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending NDAs, warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties and criminal prosecution.
Pharmaceutical product development for a new product or certain changes to an approved product in the U.S. typically involves preclinical laboratory and animal tests, the submission to the FDA of an IND, which must become effective before clinical testing may commence, and adequate and well-controlled clinical trials to establish the safety and effectiveness of the drug for each indication for which FDA approval is sought. Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease.
Preclinical tests include laboratory evaluation of product chemistry, formulation and toxicity, as well as animal trials to assess the characteristics and potential safety and efficacy of the product. The conduct of the preclinical tests must comply with federal regulations and requirements, including good laboratory practices. The results of preclinical testing are submitted to the FDA as part of an IND along with other information, including information about product chemistry, manufacturing and controls, and a proposed clinical trial protocol. Long-term preclinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted.
A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has neither commented on nor questioned the IND within this 30-day period, the clinical trial proposed in the IND may begin.
Clinical trials involve the administration of the investigational new drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted: (i) in compliance with federal regulations; (ii) in compliance with good clinical practices (“GCPs”), an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators and monitors; as well as (iii) under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to FDA as part of the IND.
The FDA may order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The study protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board (“IRB”) for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements, or may impose other conditions.
Clinical trials to support NDAs for marketing approval are typically conducted in three sequential phases, but the phases may overlap. In Phase 1, the initial introduction of the drug into healthy human subjects or patients, the drug is tested to assess metabolism, pharmacokinetics, pharmacological actions, side effects associated with increasing doses, and, if possible, early evidence of effectiveness. Phase 2 usually involves trials in a limited patient population to determine the effectiveness of the drug for a particular indication, dosage tolerance and optimum dosage, and to identify common adverse effects and safety risks. If a compound demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 trials are undertaken to obtain the additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to permit the FDA to evaluate the overall benefit-risk relationship of the drug and to provide adequate information for the labeling of the
drug. In most cases the FDA requires two adequate and well-controlled Phase 3 clinical trials to demonstrate the efficacy of the drug. A single Phase 3 or Phase 2 trial with other confirmatory evidence may be sufficient in rare instances where the study is a large multicenter trial demonstrating internal consistency and a statistically very persuasive finding of a clinically meaningful effect on mortality, irreversible morbidity or prevention of a disease with a potentially serious outcome and confirmation of the result in a second trial would be practically or ethically impossible.
Pursuant to the 21st Century Cures Act, which was enacted on December 13, 2016, the manufacturer of an investigational drug for a serious or life-threatening disease is required to make available, such as by posting on its website, its policy on evaluating and responding to requests for expanded access. This requirement applies on the later of 60 days after the date of enactment or the first initiation of a Phase 2 or Phase 3 trial of the investigational drug.
After completion of the required clinical testing, an NDA is prepared and submitted to the FDA. FDA approval of the NDA is required before marketing of the product may begin in the U.S. The NDA must include the results of all preclinical, clinical and other testing and a compilation of data relating to the product’s pharmacology, chemistry, manufacture and controls. The cost of preparing and submitting an NDA is substantial. The submission of most NDAs is additionally subject to a substantial application user fee, currently exceeding $2,421,000 for fiscal year 2018, and the manufacturer and/or sponsor under an approved new drug application are also subject to annual program fees, currently exceeding $304,000 per program for fiscal year 2018.
The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth review. The FDA has agreed to certain performance goals in the review of new drug applications to encourage timeliness. Applications under standard review are to be reviewed within ten months. Applications under priority review are to be reviewed within 6 months. Priority review can be applied to drugs that the FDA determines offer major advances in treatment, or provide a treatment where no adequate therapy exists. The review process for both standard and priority review may be extended by the FDA for three additional months to consider certain late-submitted information, or information intended to clarify information already provided in the submission.
The FDA may also refer applications for novel drug products, or drug products that present difficult questions of safety or efficacy, to an outside advisory committee—typically a panel that includes clinicians and other experts—for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations.
Before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCPs. Additionally, the FDA will inspect the facility or the facilities at which the drug is manufactured. The FDA will not approve the product unless compliance with current good manufacturing practices (“cGMPs”—a quality system regulating manufacturing) —is satisfactory and the NDA contains data that provide substantial evidence that the drug is safe and effective in the indication studied.
After the FDA evaluates the NDA and the manufacturing facilities, it issues either an approval letter or a complete response letter. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing, or information, in order for the FDA to reconsider the application. If, or when, those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included.
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. As a condition of NDA approval, the FDA may require a risk evaluation and mitigation strategy (“REMS”) to help ensure that the benefits of the drug outweigh the potential risks. REMS can include medication guides, communication plans for healthcare professionals, and elements to assure safe use (“ETASU”). ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring and the use of patient registries. The requirement for a REMS can materially affect the potential market and profitability of the drug. Moreover, product approval may require substantial post-approval testing and surveillance to monitor the drug’s safety or efficacy. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs.
Fast Track Designation and Accelerated Approval
The FDA is required to facilitate the development, and expedite the review, of drugs that are intended for the treatment of a serious or life-threatening disease or condition for which there is no effective treatment and which demonstrate the potential to address unmet medical needs for the condition. Under the Fast Track program, the sponsor of a new drug candidate may request that the FDA designate the drug candidate for a specific indication as a Fast Track drug concurrent with, or after, the filing of the IND for the drug
candidate. The FDA must determine if the drug candidate qualifies for Fast Track Designation within 60 days of receipt of the sponsor’s request.
Under the Fast Track program and the FDA’s Accelerated Approval regulations, the FDA may approve a drug for a serious or life-threatening illness that provides meaningful therapeutic benefit to patients over existing treatments based upon a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments.
In clinical trials, a surrogate endpoint is a measurement of laboratory or clinical signs of a disease or condition that substitutes for a direct measurement of how a patient feels, functions, or survives. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. A drug candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of post-approval clinical trials, often referred to as Phase 4 trials, to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or confirm a clinical benefit during post-marketing studies, will allow the FDA to withdraw the drug from the market on an expedited basis. All promotional materials for drug candidates approved under accelerated regulations are subject to priority review by the FDA.
If a submission is granted Fast Track Designation, the sponsor may engage in more frequent interactions with the FDA, and the FDA may review sections of the NDA before the application is complete. This rolling review is available if the applicant provides, and the FDA approves, a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, the FDA’s time period goal for reviewing an application does not begin until the last section of the NDA is submitted. Additionally, Fast Track Designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
Breakthrough Therapy Designation
The FDA is also required to expedite the development and review of the application for approval of drugs that are intended to treat a serious or life-threatening disease or condition where preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. Under the Breakthrough Therapy program, the sponsor of a new drug candidate may request that the FDA designate the drug candidate for a specific indication as a breakthrough therapy concurrent with, or after, the filing of the IND for the drug candidate. The FDA must determine if the drug candidate qualifies for Breakthrough Therapy designation within 60 days of receipt of the sponsor’s request.
Orphan Drugs
Under the Orphan Drug Act, the FDA may grant Orphan Drug Designation to drugs intended to treat a rare disease or condition—generally a disease or condition that affects fewer than 200,000 individuals in the U.S. Orphan Drug designation must be requested before submitting an NDA. After the FDA grants Orphan Drug Designation, the generic identity of the drug and its potential orphan use are disclosed publicly by the FDA. Orphan Drug Designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. The first NDA applicant to receive the FDA approval for a particular active ingredient to treat a particular disease with FDA Orphan Drug Designation is entitled to a seven-year exclusive marketing period in the U.S. for that product, for that indication. During the seven-year exclusivity period, the FDA may not approve any other applications to market the same drug for the same disease, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. Orphan drug exclusivity does not prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition. Among the other benefits of Orphan Drug Designation are tax credits for certain research and a waiver of the NDA application user fee.
Post-Approval Requirements
Once an NDA is approved, a product will be subject to certain post-approval requirements. For instance, the FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet. Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved labeling.
Adverse event reporting and submission of periodic reports are required following the FDA approval of an NDA. The FDA also may require post-marketing testing, known as Phase 4 testing, REMS, and surveillance to monitor the effects of an approved product, or the FDA may place conditions on an approval that could restrict the distribution or use of the product. In addition, quality control, drug manufacture, packaging and labeling procedures must continue to conform to cGMPs after approval. Drug manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies. Registration with the FDA subjects entities to periodic unannounced inspections by the FDA, during which the Agency inspects manufacturing facilities to assess compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money and effort in the areas of production and quality-control to maintain compliance with cGMPs. Regulatory authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered.
Pediatric Information
Under the Pediatric Research Equity Act (“PREA”), NDAs or supplements to NDAs must contain data to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the drug is safe and effective. The FDA may grant full or partial waivers, or deferrals, for submission of data. Unless otherwise required by regulation, PREA does not apply to any drug for an indication for which orphan designation has been granted.
The Best Pharmaceuticals for Children Act (“BPCA”), provides NDA holders a six-month extension of any exclusivity—patent or non-patent—for a drug if certain conditions are met. Conditions for exclusivity include the FDA’s determination that information relating to the use of a new drug in the pediatric population may produce health benefits in that population, the FDA making a written request for pediatric studies, and the applicant agreeing to perform, and reporting on, the requested studies within the statutory timeframe. Applications under the BPCA are treated as priority applications, with all of the benefits that designation confers.
FDA Regulation of Companion Diagnostics
Our drug products may rely upon in vitro companion diagnostics for use in selecting the patients that we believe will respond to our cancer therapeutics. If an in vitro diagnostic is essential to the safe and effective use of the therapeutic product, then the FDA generally will either require approval or clearance of the diagnostic at the same time that FDA approves the therapeutic product, or as a post-marketing commitment at the time of the therapeutic product’s approval. Companion diagnostics may have both regulatory and commercial importance for our cancer therapies.
Pursuing FDA approval of an in vitro companion diagnostic would require us or our partners to obtain a pre-market approval (“PMA”) for that diagnostic. Based on a final FDA guidance document, and the FDA’s past treatment of companion diagnostics, we believe that the FDA will require PMA approval of one or more in vitro companion diagnostics to identify patient populations suitable for our cancer therapies. The review of these in vitro companion diagnostics involves coordination of review by the FDA’s Center for Drug Evaluation and Research and by the FDA’s Center for Devices and Radiological Health. Approval of a companion diagnostic may be required at the time of new drug approval, or as a post-marketing commitment post-approval.
The PMA process, including the gathering of clinical and nonclinical data and the submission to and review by the FDA, can take several years or longer. It involves a rigorous premarket review during which the applicant must prepare and provide the FDA with reasonable assurance of the device’s safety and effectiveness and information about the device and its components regarding, among other things, device design, manufacturing and labeling. PMA applications are subject to an application fee, which exceeds $310,000 for most PMAs for fiscal year 2018. In addition, PMAs for devices must generally include the results from extensive preclinical and adequate and well-controlled clinical trials to establish the safety and effectiveness of the device for each indication for which FDA approval is sought. In particular, for a diagnostic, the applicant must demonstrate that the diagnostic produces reproducible results when the same sample is tested multiple times by multiple users at multiple laboratories. As part of the PMA review, the FDA will typically inspect the manufacturer’s facilities for compliance with the Quality System Regulation (“QSR”), which imposes elaborate testing, control, documentation and other quality assurance requirements.
PMA approval is not guaranteed, and the FDA may ultimately respond to a PMA submission with a not approvable determination based on deficiencies in the application and require additional clinical trial or other data that may be expensive and time-consuming to generate and that can substantially delay or prevent approval. If the FDA’s evaluation of the PMA application is favorable, the FDA typically issues an approvable letter requiring the applicant’s agreement to specific conditions, such as changes in labeling, or specific additional information, such as submission of final labeling, in order to secure final approval of the PMA. If the FDA concludes that the applicable criteria have been met, the FDA will issue a PMA for the approved indications, which can be more limited than those originally sought by the applicant. The PMA can include post-approval conditions that the FDA believes necessary to ensure the safety and effectiveness of the device, including, among other things, restrictions on labeling, promotion, sale and distribution.
After a device is placed on the market, it remains subject to significant regulatory requirements. Medical devices may be marketed only for the uses and indications for which they are cleared or approved. Device manufacturers must also establish registration and device listings with the FDA. A medical device manufacturer’s manufacturing processes and those of its suppliers are required to comply with the applicable portions of the QSR, which cover the methods and documentation of the design, testing, production, processes, controls, quality assurance, labeling, packaging and shipping of medical devices. Domestic facility records and manufacturing processes are subject to periodic unscheduled inspections by the FDA. The FDA also may inspect foreign facilities that export products to the U.S.
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions: warning letters, fines, injunctions, civil or criminal penalties, recall or seizure of current or future products, operating restrictions, partial suspension or total shutdown of production, denial of submissions for new products, or withdrawal of PMA approvals. Since we may not be the primary developers of the PMA product, our ability to ensure compliance with relevant regulatory requirements may be limited, even though our drug products depend on the successful development, commercialization and regulatory compliance of the product.
Disclosure of Clinical Trial Information
Sponsors of clinical trials of FDA regulated products, including drugs, are required to register and disclose certain clinical trial information. Information related to the product, patient population, phase of investigation, study sites and investigators, and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to discuss the results of their clinical trials after completion. Sponsors may also elect to provide interim disclosures of results for reasons such as the education of relevant stakeholders and accelerating clinical trial enrollment. Forums for such disclosures include, but are not limited to, scientific conferences, medical journals and press releases. Disclosure of the results of these trials can be delayed in certain circumstances for up to two years after the date of completion of the trial. Competitors may use this publicly available information to gain knowledge regarding the progress of development programs.
Review and Approval of Drug Products in the European Union
In order to market any product outside of the United States, a company must also comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of products. Whether or not it obtains FDA approval for a product, the company would need to obtain the necessary approvals by the comparable foreign regulatory authorities before it can commence clinical trials or marketing of the product in those countries or jurisdictions. The approval process ultimately varies between countries and jurisdictions and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries and jurisdictions might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others.
Procedures Governing Approval of Drug Products in the European Union
Pursuant to the European Clinical Trials Directive, a system for the approval of clinical trials in the E.U. has been implemented through national legislation of the member states. Under this system, an applicant must obtain approval from the competent national authority of an E.U. member state in which the clinical trial is to be conducted. Furthermore, the applicant may only start a clinical trial after a competent ethics committee has issued a favorable opinion. A clinical trial application (“CTA”) must be accompanied by an investigational medicinal product dossier (“IMPD”) with supporting information prescribed by the European Clinical Trials Directive and corresponding national laws of the member states and further detailed in applicable guidance documents.
To obtain marketing approval of a product under E.U. regulatory systems, an applicant must submit an MAA either under a centralized or decentralized procedure. The centralized procedure provides for the grant of a single marketing authorization by the European Commission that is valid for all E.U. member states. The centralized procedure is compulsory for specific products, including for medicines produced by certain biotechnological processes, products designated as orphan medicinal products, advanced therapy products and products with a new active substance indicated for the treatment of certain diseases. For products with a new active substance indicated for the treatment of other diseases and products that are highly innovative or for which a centralized process is in the interest of patients, the centralized procedure may be optional.
Under the centralized procedure, the Committee for Medicinal Products for Human Use (“CHMP”), established at the European Medicines Agency (“EMA”) is responsible for conducting the initial assessment of a product. The CHMP is also responsible for several post-authorization and maintenance activities, such as the assessment of modifications or extensions to an existing marketing authorization. Under the centralized procedure in the E.U., the maximum timeframe for the evaluation of an MAA is 210 days, excluding clock stops, when additional information or written or oral explanation is to be provided by the applicant in response to questions of the CHMP. Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is of major interest from the point of view of public health and in particular from the viewpoint of therapeutic innovation. In this circumstance, the EMA ensures that the opinion of the CHMP is given within 150 days.
The decentralized procedure is available to applicants who wish to market a product in various E.U. member states where such product has not received marketing approval in any E.U. member states before. The decentralized procedure provides for approval by one or more other, or concerned, member states of an assessment of an application performed by one member state designated by the applicant, known as the reference member state. Under this procedure, an applicant submits an application based on identical dossiers and related materials, including a draft summary of product characteristics, and draft labeling and package leaflet, to the reference member state and concerned member states. The reference member state prepares a draft assessment report and drafts of the related materials within 210 days after receipt of a valid application. Within 90 days of receiving the reference member state’s assessment report and related materials, each concerned member state must decide whether to approve the assessment report and related materials.
If a member state cannot approve the assessment report and related materials on the grounds of potential serious risk to public health, the disputed points are subject to a dispute resolution mechanism and may eventually be referred to the European Commission, whose decision is binding on all member states.
Within this framework, manufacturers may seek approval of hybrid medicinal products under Article 10(3) of Directive 2001/83/EC. Hybrid applications rely, in part, on information and data from a reference product and new data from appropriate preclinical tests and clinical trials. Such applications are necessary when the proposed product does not meet the strict definition of a generic medicinal product, or bioavailability studies cannot be used to demonstrate bioequivalence, or there are changes in the active substance(s), therapeutic indications, strength, pharmaceutical form or route of administration of the generic product compared to the reference medicinal product. In such cases the results of tests and trials must be consistent with the data content standards required in the Annex to the Directive 2001/83/EC, as amended by Directive 2003/63/EC.
Hybrid medicinal product applications have automatic access to the centralized procedure when the reference product was authorized for marketing via that procedure. Where the reference product was authorized via the decentralized procedure, a hybrid application may be accepted for consideration under the centralized procedure if the applicant shows that the medicinal product constitutes a significant therapeutic, scientific or technical innovation, or the granting of a community authorization for the medicinal product is in the interest of patients at the community level.
Clinical Trial Approval in the European Union
Requirements for the conduct of clinical trials in the E.U. including GCP are set forth in the Clinical Trials Directive 2001/20/EC and the GCP Directive 2005/28/EC. Pursuant to Directive 2001/20/EC and Directive 2005/28/EC, as amended, a system for the approval of clinical trials in the E.U. has been implemented through national legislation of the E.U. member states. Under this system, approval must be obtained from the competent national authority of each E.U. member state in which a study is planned to be conducted. To this end, a CTA is submitted, which must be supported by an IMPD and further supporting information prescribed by Directive 2001/20/EC and Directive 2005/28/EC and other applicable guidance documents. Furthermore, a clinical trial may only be started after a competent ethics committee has issued a favorable opinion on the CTA in that country.
In April 2014, the E.U. passed the new Clinical Trials Regulation, (EU) No 536/2014, which will replace the current Clinical Trials Directive 2001/20/EC. To ensure that the rules for clinical trials are identical throughout the E.U., the new E.U. clinical trials legislation was passed as a regulation that is directly applicable in all E.U. member states. All clinical trials performed in the E.U. are required to be conducted in accordance with the Clinical Trials Directive 2001/20/EC until the new Clinical Trials Regulation (EU) No 536/2014 becomes applicable. According to the current plans of the EMA, the new Clinical Trials Regulation will become applicable in 2019. The Clinical Trials Directive 2001/20/EC will, however, still apply three years from the date of entry into application of the Clinical Trials Regulation to (i) clinical trials applications submitted before the entry into application and (ii) clinical trials applications submitted within one year after the entry into application if the sponsor opts for old system.
The new Clinical Trials Regulation aims to simplify and streamline the approval of clinical trial in the E.U. The main characteristics of the regulation include: a streamlined application procedure via a single entry point, the E.U. portal; a single set of documents to be prepared and submitted for the application as well as simplified reporting procedures that will spare sponsors from submitting broadly identical information separately to various bodies and different member states; a harmonized procedure for the assessment of applications for clinical trials, which is divided in two parts (Part I is assessed jointly by all member states concerned, and Part II is assessed separately by each member state concerned); strictly defined deadlines for the assessment of CTAs; and the involvement of the ethics committees in the assessment procedure in accordance with the national law of the member state concerned but within the overall timelines defined by the Clinical Trials Regulation.
Periods of Authorization and Renewals
Marketing authorization is valid for five years in principle and the marketing authorization may be renewed after five years on the basis of a re-evaluation of the risk-benefit balance by the EMA or by the competent authority of the authorizing member state. To this end, the marketing authorization holder must provide the EMA or the competent authority with a consolidated version of the file in respect of quality, safety and efficacy, including all variations introduced since the marketing authorization was granted, at least six months before the marketing authorization ceases to be valid. Once renewed, the marketing authorization is valid for an unlimited period, unless the European Commission or the competent authority decides, on justified grounds relating to pharmacovigilance, to proceed with one additional five-year renewal. Any authorization which is not followed by the actual placing of the drug on the E.U. market (in case of centralized procedure) or on the market of the authorizing member state within three years after authorization ceases to be valid (the so-called sunset clause).
Data and Market Exclusivity in the European Union
In the E.U., new chemical entities qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity. This data exclusivity, if granted, prevents regulatory authorities in the E.U. from referencing the innovator’s data to assess a generic (abbreviated) application for eight years, after which generic marketing authorization can be submitted, and the innovator’s data may be referenced, but not approved for two years. The overall ten-year period will be extended to a maximum of eleven years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. Even if a compound is considered to be a new chemical entity and the
sponsor is able to gain the prescribed period of data exclusivity, another company nevertheless could also market another version of the product if such company can complete a full MAA with a complete database of pharmaceutical test, preclinical tests and clinical trials and obtain marketing approval of its product.
Orphan Drug Designation and Exclusivity in the European Union
Regulation 141/2000 provides that a drug shall be designated as an orphan drug if its sponsor can establish: that the product is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting not more than five in ten thousand persons in the European Community when the application is made, or that the product is intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition in the European Community and that without incentives it is unlikely that the marketing of the drug in the European Community would generate sufficient return to justify the necessary investment. For either of these conditions, the applicant must demonstrate that there exists no satisfactory method of diagnosis, prevention or treatment of the condition in question that has been authorized in the European Community or, if such method exists, the drug will be of significant benefit to those affected by that condition.
Regulation 847/2000 sets out criteria and procedures governing designation of orphan drugs in the E.U. Specifically, an application for designation as an orphan product can be made any time prior to the filing of an application for approval to market the product. Marketing authorization for an orphan drug leads to a ten-year period of market exclusivity. This period may, however, be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan drug designation, for example because the product is sufficiently profitable not to justify market exclusivity. Market exclusivity can be revoked only in very selected cases, such as consent from the marketing authorization holder, inability to supply sufficient quantities of the product, demonstration of “clinically relevant superiority” by a similar medicinal product, or, after a review by the Committee for Orphan Medicinal Products, requested by a member state in the fifth year of the marketing exclusivity period (if the designation criteria are believed to no longer apply). Medicinal products designated as orphan drugs pursuant to Regulation 141/2000 shall be eligible for incentives made available by the European Community and by the member states to support research into, and the development and availability of, orphan drugs.
Regulatory Requirements After Marketing Authorization in the European Union
As in the United States, both marketing authorization holders and manufacturers of medicinal products are subject to comprehensive regulatory oversight by the EMA and the competent authorities of the individual E.U. Member States both before and after grant of the manufacturing and marketing authorizations. The holder of an E.U. marketing authorization for a medicinal product must, for example, comply with E.U. pharmacovigilance legislation and its related regulations and guidelines which entail many requirements for conducting pharmacovigilance, or the assessment and monitoring of the safety of medicinal products. The manufacturing process for medicinal products in the E.U. is also highly regulated and regulators may shut down manufacturing facilities that they believe do not comply with regulations. Manufacturing requires a manufacturing authorization, and the manufacturing authorization holder must comply with various requirements set out in the applicable E.U. laws, including compliance with E.U. cGMP standards when manufacturing medicinal products and active pharmaceutical ingredients.
In the E.U., the advertising and promotion of approved products are subject to E.U. Member States’ laws governing promotion of medicinal products, interactions with clinicians, misleading and comparative advertising and unfair commercial practices. In addition, other legislation adopted by individual E.U. Member States may apply to the advertising and promotion of medicinal products. These laws require that promotional materials and advertising in relation to medicinal products comply with the product’s Summary of Product Characteristics (“SmPC”), as approved by the competent authorities. Promotion of a medicinal product that does not comply with the SmPC is considered to constitute off-label promotion, which is prohibited in the European Union.
Brexit and the Regulatory Framework in the United Kingdom
On June 23, 2016, the electorate in the United Kingdom, or U.K. voted in favor of leaving the E.U, which is commonly referred to as “Brexit”. Thereafter, on March 29, 2017, the country formally notified the E.U. of its intention to withdraw pursuant to Article 50 of the Lisbon Treaty. The withdrawal of the U.K. from the E.U. will take effect either on the effective date of the withdrawal agreement or, in the absence of agreement, two years after the U.K. provides a notice of withdrawal pursuant to the E.U. Treaty. Since the regulatory framework for pharmaceutical products in the U.K. covering quality, safety and efficacy of pharmaceutical products, clinical trials, marketing authorization, commercial sales and distribution of pharmaceutical products is derived from E.U. directives and regulations, Brexit could materially impact the future regulatory regime which applies to products and the approval of product candidates in the U.K. It remains to be seen how, if at all, Brexit will impact regulatory requirements for product candidates and products in the U.K.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of products approved by the FDA and other government authorities. Sales of products will depend, in part, on the extent to which third-party payors, including government health programs in the United States such as Medicare and Medicaid, commercial health insurers and managed care organizations, provide coverage, and establish adequate reimbursement levels for, such products. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the
product once coverage is approved. Third-party payors are increasingly challenging the prices charged, examining the medical necessity, and reviewing the cost-effectiveness of medical products and services and imposing controls to manage costs. Third-party payors may limit coverage to specific products on an approved list, or formulary, which might not include all of the approved products for a particular indication.
In order to secure coverage and reimbursement for any product that might be approved for sale, a company may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costs required to obtain FDA or other comparable regulatory approvals. Nonetheless, product candidates may not be considered medically necessary or cost effective. Additionally, a payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a drug product does not assure that other payors will also provide coverage for the drug product. Third-party reimbursement may not be sufficient to maintain price levels high enough to realize an appropriate return on investment in product development.
The containment of healthcare costs also has become a priority of federal, state and foreign governments and the prices of drugs have been a focus in this effort. Governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit a company’s revenue generated from the sale of any approved products. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which a company or its collaborators receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Outside the United States, ensuring adequate coverage and payment for our product candidates will face challenges. Pricing of prescription pharmaceuticals is subject to governmental control in many countries. Pricing negotiations with governmental authorities can extend well beyond the receipt of regulatory marketing approval for a product and may require us to conduct a clinical trial that compares the cost effectiveness of our product candidates or products to other available therapies. The conduct of such a clinical trial could be expensive and result in delays in our commercialization efforts.
In the E.U., pricing and reimbursement schemes vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular drug candidate to currently available therapies. For example, the E.U. provides options for its member states to restrict the range of drug products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. E.U. member states may approve a specific price for a drug product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the drug product on the market. Other member states allow companies to fix their own prices for drug products, but monitor and control company profits. The downward pressure on healthcare costs in general, particularly prescription drugs, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert competitive pressure that may reduce pricing within a country. Any country that has price controls or reimbursement limitations for drug products may not allow favorable reimbursement and pricing arrangements.
Manufacturing
We do not have any manufacturing facilities or personnel. We currently rely, and expect to continue to rely, on third parties for the manufacture of our product candidates undergoing preclinical and clinical testing, as well as for commercial manufacture if our product candidates receive marketing approval. We have identified, qualified and begun manufacturing with numerous manufacturers to provide active pharmaceutical ingredients, fill-and-finish services and commercial drug supply as our drug products progress through clinical development towards potential regulatory approvals, and future commercialization.
We expect to rely on third parties for the manufacture of our companion diagnostics. Depending on the technology solutions we choose, we may rely on multiple third parties to manufacture and sell a single test. For example, we may develop analyte specific reagents with one vendor, rely on another vendor for qualification and assembly in a finished in vitro diagnostic kit and rely on additional third parties for distribution and/or commercialization.
Commercialization
As programs advance towards potential regulatory approvals and commercialization, we may license our drugs to partners, in certain territories or globally, or build a focused commercial organization ourselves to launch the products, depending on risk-adjusted analyses of value and the larger strategic imperatives of the business
For larotrectinib and LOXO-195, in November 2017, we entered into the Bayer Agreement with Bayer as described above, pursuant to which the Company and Bayer will collaborate to develop and commercialize larotrectinib and LOXO-195. Pursuant to the Bayer Agreement, we granted co-exclusive development and commercialization licenses to Bayer for both larotrectinib and LOXO-195. We will lead global development activities and regulatory activities in the U.S. Bayer will lead regulatory activities outside the U.S. and
global commercial activities. For additional details regarding the Bayer Agreement see “—Agreements—Bayer License, Development and Commercialization Agreement”.
For LOXO-292, LOXO-305 and future product candidates not subject to a development or commercialization license, subject to receiving marketing approvals, we expect to commence commercialization activities by building a focused sales and marketing organization to sell our products. We believe that such an organization will be able to address the community of oncologists and pathologists who are the key specialists in diagnosing and treating the patient populations for which our product candidates are being developed. Outside the U.S., we plan to strategically evaluate building our own commercial organization, as well as commercial partnership opportunities for any of our product candidates that obtain marketing approval.
Employees
As of December 31, 2017, we had a total of 59 full-time employees, all located in the U.S. None of our employees is represented by a labor union or covered by a collective bargaining agreement. We have not experienced any work stoppages, and we consider our relations with our employees to be good. Of the 59 employees, 37 perform research and development activities and 22 serve in general and administrative functions.
Financial Information
We manage our operations and allocate resources as a single reporting segment. Financial information regarding our operations, assets and liabilities, including our net losses for the years ended December 31, 2017, 2016 and 2015 and our total assets as of December 31, 2017 and 2016, is included in our Consolidated Financial Statements in Item 8 of this Annual Report.
Corporate Information
We were incorporated under the laws of the State of Delaware in May 2013. Our principal executive offices are located at 281 Tresser Boulevard, 9th Floor, Stamford, CT 06901, and our telephone number is (203) 653-3880. Our website address is www.loxooncology.com.
Available Information
Our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and other filings with the SEC and all amendments to these filings, are available, free of charge, on our website at www.loxooncology.com as soon as reasonably practicable following our filing of any of these reports with the SEC. You can also obtain copies free of charge by contacting our Investor Relations department at our office address listed below. The public may read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street N.E., Room 1580, Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains a website that contains reports, proxy, and information statements, and other information regarding issuers that file electronically with the SEC at www.sec.gov. The information posted on or accessible through these websites are not incorporated into this filing.
This Form 10-K contains forward-looking information based on our current expectations. Because our actual results may differ materially from any forward-looking statements that we make or that are made on our behalf, this section includes a discussion of important factors that could affect our actual future results, including, but not limited to, our capital resources, the progress and timing of our clinical programs, the safety and efficacy of our product candidates, risks associated with regulatory filings, risks associated with determinations made by regulatory agencies, the potential clinical benefits and market potential of our product candidates, commercial market estimates, future development efforts, patent protection, effects of healthcare reform, reliance on third parties, and other risks set forth below.
Risks Related to Our Financial Position and Capital Needs
We have incurred significant losses since our inception. We expect to incur losses over the next several years and may never achieve or maintain profitability.
Since inception, we have incurred significant operating losses. Our net loss was $148.9 million for the fiscal year ended December 31, 2017. As of December 31, 2017, we had an accumulated deficit of $288.1 million. We have focused primarily on our drug discovery efforts and developing our product candidates. We have initiated clinical development of larotrectinib, LOXO-292 and LOXO-195. To date, we have financed our operations primarily through private placements of our convertible preferred stock, our initial public offering, our follow-on public offerings and our collaboration agreement with Bayer. We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. The net losses we incur may fluctuate significantly from quarter to quarter. We anticipate that our expenses will increase substantially if and as we:
· continue development of our product candidates;
· seek to identify additional product candidates;
· enter into additional collaboration arrangements with regards to product discovery, acquire or in-license other products and technologies, or develop internal drug discovery capabilities;
· enter into collaboration arrangements for companion diagnostics for our cancer therapies;
· maintain and leverage our collaborations with Array, Bayer or others;
· continue and initiate clinical trials for our product candidates;
· seek marketing approvals for our product candidates that successfully complete clinical trials;
· establish a sales, marketing and distribution infrastructure to commercialize any products for which we may obtain marketing approval;
· maintain, expand and protect our intellectual property portfolio;
· hire additional personnel;
· add operational, financial and management information systems and personnel, including personnel to support our product development and planned future commercialization efforts; and
· incur increased costs as a result of operating as a public company.
To become and remain profitable, we must, alone or with our collaborators, develop and eventually commercialize a product or products with significant market potential. This will require us to be successful in a range of challenging activities, including completing clinical trials of our product candidates, successfully developing companion diagnostics, obtaining marketing approval for these product candidates and manufacturing, marketing and selling those products for which we may obtain marketing approval. We may never succeed in these activities and, even if we do, may never generate revenues that are significant or large enough to achieve profitability. If we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable could impair our ability to raise capital, maintain our discovery and preclinical development efforts, expand our business or continue our operations and may require us to raise additional capital that may dilute your ownership interest. A decline in the value of Loxo Oncology could also cause you to lose all or part of your investment.
Our limited operating history may make it difficult to evaluate the success of our business to date and to assess our future viability.
We are a clinical development company. We were incorporated in May 2013 and commenced operations in the third quarter of 2013. We rely on collaborations with third parties for discovery, preclinical development, manufacturing, companion diagnostics development and other activities critical to our business. For larotrectinib and LOXO-195, we rely on our collaboration with Bayer to commercialize these products and secure regulatory approvals outside of the United States. Our operations to date have been limited to
organizing and staffing our Company, business planning, raising capital, acquiring and developing our technology, identifying and acquiring potential product candidates and conducting product development activities for larotrectinib, LOXO-292 and LOXO-195, which we have advanced into clinical trials, and other product candidates. We have not yet demonstrated our ability to successfully complete large-scale, pivotal clinical trials, develop companion diagnostics, obtain marketing approvals, manufacture a commercial scale product, or arrange for a third-party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. Medicines, on average, take ten to fifteen years to be developed from the time they are discovered to the time they are available for treating patients. Consequently, any predictions about our future success or viability based on our short operating history to date may not be as accurate as if we had a longer operating history.
In addition, as a new business, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors. We will need to transition from a company with a research focus to a company capable of supporting commercial activities. We may not be successful in such a transition.
We will need substantial additional funding. If we are unable to raise capital when needed, we would be compelled to delay, reduce or eliminate our product development programs or commercialization efforts.
We expect our expenses to increase in parallel with our ongoing activities, particularly as we continue our discovery and preclinical development collaborations to identify new clinical candidates and initiate clinical trials of, seek marketing approval for, and prepare for the commercial launch of our product candidates. In addition, if we obtain marketing approval for any of our product candidates, we expect to incur significant commercialization expenses related to product sales, marketing, diagnostics, manufacturing and distribution to the extent that such sales, marketing, manufacturing and distribution expenses are not the responsibility of Bayer or other collaborators. Furthermore, we continue to incur costs associated with operating as a public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we would be forced to delay, reduce or eliminate our discovery and preclinical development programs or any future commercialization efforts.
Our future capital requirements will depend on many factors, including:
· the scope, progress, results and costs of compound discovery, preclinical development, laboratory testing and clinical trials for our product candidates;
· the extent to which we enter into additional collaboration arrangements with regard to product discovery or acquire or in-license products or technologies;
· the extent to which we enter into collaboration arrangements for companion diagnostics for our cancer therapies;
· our ability to establish additional discovery collaborations on favorable terms, if at all;
· the extent to which we develop or expand internal drug discovery and development capabilities;
· the costs, timing and outcome of regulatory review of our product candidates;
· the costs of future commercialization activities, including product sales, marketing, manufacturing and distribution, for any of our product candidates for which we receive marketing approval;
· revenue, if any, received from commercial sales of our product candidates, should any of our product candidates receive marketing approval; and
· the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims.
Identifying potential product candidates and conducting preclinical testing and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve product sales. In addition, our product candidates, if approved, may not achieve commercial success. In addition, public policy around drug pricing, in the U.S., and outside of the U.S., may affect the commercial success of our product candidates. Our commercial revenues, if any, will be derived from sales of products that we do not expect to be commercially available for many years, if at all. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Adequate additional financing may not be available to us on acceptable terms, or at all.
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings or other sources, our collaboration with Bayer and other potential collaborations. We do not have any committed external source of funds, other than the $150 million receivable from Bayer as of December 31, 2017. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted,
and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through up-front payments or milestone payments pursuant to strategic collaborations with third parties, we may have to relinquish valuable rights to our product candidates, or grant licenses on terms that are not favorable to us.
We cannot be certain that additional funding will be available on acceptable terms, or at all. If we are unable to raise additional funds when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts.
Risks Related to the Discovery and Development of Our Product Candidates
Our discovery and preclinical development is focused on the development of targeted therapeutics for well-defined patient populations, which is a rapidly evolving area of science, and the approach we are taking to discover and develop drugs is relatively new and may never lead to marketable products.
The discovery and development of targeted therapeutics for well-defined patient populations is an emerging field, and the scientific discoveries that form the basis for our efforts to discover and develop product candidates are relatively new. The scientific evidence to support the feasibility of developing product candidates based on these discoveries is both preliminary and limited. The patient populations for our product candidates are not completely defined but are substantially smaller than the general treated cancer population, and we will need to screen and identify these patients. Successful identification of patients is dependent on several factors, including achieving certainty as to how specific genetic alterations respond to our product candidates and developing companion diagnostics to identify such genetic alterations as appropriate. Furthermore, even if we are successful in identifying patients, we cannot be certain that the resulting patient populations will be large enough to allow us to successfully commercialize our products and achieve profitability. Our estimates of the potential market opportunities for our products are informed by work that is not definitive and future analyses may lead to estimates that are higher or lower than these estimates than those provided at any given time, with respect to addressable patient populations. Therefore, we do not know if our approach will be successful, and if our approach is unsuccessful, our business will suffer.
We are early in our development efforts and are substantially dependent on the development of our product candidates. If we or our collaborators are unable to successfully develop and commercialize our product candidates or experience significant delays in doing so, our business will be materially harmed.
We currently do not have any products that have gained regulatory approval. We have invested significant financial resources in identifying potential product candidates, funding our collaboration agreement with Array to conduct preclinical studies, conducting clinical development of our product candidates, acquiring the Redx Pharma Plc BTK program, and opening scientific labs in Boulder, Colorado.
Our ability to generate product revenues will depend heavily on the successful development and eventual commercialization of our product candidates. We have not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the biopharmaceutical area. For example, to execute our business plan, we will need to successfully:
· execute and complete development activities;
· obtain required regulatory approvals for the development and commercialization of our product candidates;
· maintain, leverage and expand our intellectual property portfolio;
· build and maintain robust sales, distribution and marketing capabilities, either on our own or in collaboration with strategic partners;
· establish successful companion diagnostics collaborations;
· gain market acceptance;
· develop and maintain any strategic relationships we elect to enter into, including our collaborations with Bayer, Array and others; and
· manage our spending as costs and expenses increase due to drug discovery, preclinical development, clinical trials, regulatory approvals and commercialization.
If we are unsuccessful in accomplishing these objectives, we may not be able to successfully develop and commercialize larotrectinib or our other product candidates, and our business will suffer.
Difficulty in enrolling patients could delay or prevent clinical trials of our product candidates. We may find it difficult to enroll patients in our clinical trials given that we do not know how many patients harbor the relevant alteration each product candidate is designed to inhibit.
Identifying and qualifying patients to participate in clinical studies of our product candidates is critical to our success. The timing of our clinical studies depends in part on the speed at which we can recruit patients to participate in testing our product candidates, and we may experience delays in our clinical trials if we encounter difficulties in enrollment. The patient populations for our product candidates are not completely defined, but are substantially smaller than other cancer indications, because we are often looking for the same type of genetic alterations across different tumor types and the number of patients with these alterations may be small. We do not yet know exactly how many patients will have the targets that our product candidates are designed to inhibit. In addition, the adoption of genetic testing across large populations of patients with cancer will be required for us to identify patients appropriate for our trials that are restricted to genetically defined populations.
The number of patients suitable for trial enrollment and potential commercialization depends on a series of risks that are difficult to quantify based on available information. For example, in the case of TRK, there is significant uncertainty around the true number of patients with advanced cancer and a TRK fusion, the number of these patients who are referred for comprehensive genomic profiling, the sensitivity of the chosen comprehensive genomic assay for detecting TRK fusions, the ability of healthcare providers to recognize the importance of the presence of a TRK fusion, patient interest in seeking out a TRK inhibitor, and patient interest in larotrectinib instead of a competing program. Nevertheless, in the case of TRK fusion cancers, incidence appears to be low in the more common tumor types. Our proprietary work suggests that there are approximately 1,500-5,000 eligible advanced cancer patients addressable each year in the United States. However, the work that informed this estimate is not definitive and future analyses may lead to estimates that are higher or lower than this estimate. In addition, the broad utilization of sensitive diagnostic tests in routine clinical practice capable of identifying TRK fusion patients is as important to successful commercialization as the actual number of addressable patients. Similar issues apply to our RET and LOXO-305 programs as well.
In addition to potentially small populations, the eligibility criteria of our clinical trials will further limit the pool of available study participants as we will require that patients have specific characteristics that we can measure and/or that their disease is either severe enough or not too advanced to include them in a study. Additionally, the process of finding and diagnosing patients may prove costly. We also may not be able to identify, recruit, and enroll a sufficient number of patients to complete our clinical studies because of the perceived risks and benefits of the product candidate under study, the availability and efficacy of competing therapies and clinical trials, the proximity and availability of clinical study sites for prospective patients, and the patient referral practices of physicians. If patients are unwilling to participate in our studies for any reason, the timeline for recruiting patients, conducting studies, and obtaining regulatory approval of potential products may be delayed.
If we experience delays in the completion of, or termination of, any clinical trial of our product candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate product revenue from any of these product candidates could be delayed or prevented. In addition, any delays in completing our clinical trials will increase our costs, slow down our product candidate development and approval process, and jeopardize our ability to commence product sales and generate revenue. Any of these occurrences may harm our business, financial condition, and prospects significantly. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates, including:
· unforeseen safety issues or adverse side effects;
· failure of our companion diagnostics in identifying patients;
· modifications to protocols of our clinical trials resulting from FDA or institutional review board (“IRB”) decisions; and
· ambiguous or negative interim results of our clinical trials, or results that are inconsistent with earlier results.
Clinical drug development involves a lengthy and expensive process, with an uncertain outcome. We may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates.
We have commenced the clinical development of larotrectinib, LOXO-292, and LOXO-195, and there is significant risk that one or more of our product candidates will fail to reach commercialization. Before obtaining marketing approval from regulatory authorities for the sale of any product candidate, we must complete preclinical development and then conduct extensive clinical trials to demonstrate the safety and efficacy of our product candidates in humans. Clinical testing is expensive, difficult to design and implement and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. Further, the results of preclinical studies and early clinical trials of our product candidates may not be predictive of the results of later-stage clinical trials, and interim results of a clinical trial do not necessarily predict final results. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their products.
It is difficult to accurately predict when or if any of our product candidates will prove effective or safe in humans or will receive regulatory approval.
We may experience delays in our clinical trials and we do not know whether planned clinical trials will begin or enroll subjects on time, need to be redesigned or be completed on schedule, if at all. There can be no assurance that the FDA will not put any of our product candidates on clinical hold in the future. We may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent our ability to receive marketing approval or commercialize our product candidates. Clinical trials may be delayed, suspended or prematurely terminated because costs are greater than we anticipate or for a variety of reasons, such as:
· delay or failure in reaching agreement with the FDA or a comparable foreign regulatory authority on a trial design that we are able to execute;
· delay or failure in obtaining authorization to commence a trial or inability to comply with conditions imposed by a regulatory authority regarding the scope or design of a clinical trial;
· delays in reaching, or failure to reach, agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites;
· inability, delay, or failure in identifying and maintaining a sufficient number of trial sites, many of which may already be engaged in other clinical programs;
· delay or failure in recruiting and enrolling suitable subjects to participate in a trial;
· delay or failure in having subjects complete a trial or return for post-treatment follow-up;
· clinical sites and investigators deviating from trial protocol, failing to conduct the trial in accordance with regulatory requirements, or dropping out of a trial;
· lack of adequate funding to continue the clinical trial, including the incurrence of unforeseen costs due to enrollment delays, requirements to conduct additional clinical studies and increased expenses associated with the services of our clinical research organizations (“CROs”) and other third parties;
· clinical trials of our product candidates may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials or abandon product development programs;
· the number of patients required for clinical trials of our product candidates may be larger than we anticipate, enrollment in these clinical trials may be slower than we anticipate or participants may drop out of these clinical trials at a higher rate than we anticipate;
· we may experience delays or difficulties in the enrollment of patients whose tumors harbor the specific genetic alterations that our product candidates are designed to target;
· our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all;
· we may have difficulty partnering with experienced CROs that can screen for patients whose tumors harbor the applicable genetic alterations and run our clinical trials effectively;
· regulators or IRBs may require that we or our investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks;
· the supply or quality of our product candidates or other materials necessary to conduct clinical trials of our product candidates may be insufficient or inadequate; or
· there may be changes in governmental regulations or administrative actions.
If we are required to conduct additional clinical trials or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully complete clinical trials of our product candidates or other testing, if the results of these trials or tests are not positive or are only modestly positive or if there are safety concerns, we may:
· be delayed in obtaining marketing approval for our product candidates;
· not obtain marketing approval at all;
· obtain approval for indications or patient populations that are not as broad as intended or desired;
· obtain approval with labeling that includes significant use or distribution restrictions or safety warnings that would reduce the potential market for our products or inhibit our ability to successfully commercialize our products;
· be subject to additional post-marketing restrictions and/or testing requirements; or
· have the product removed from the market after obtaining marketing approval.
Our product development costs will also increase if we experience delays in testing or marketing approvals. We do not know whether any of our preclinical studies or clinical trials will need to be restructured or will be completed on schedule, or at all. Significant preclinical or clinical trial delays also could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do and impair our ability to successfully commercialize our product candidates and may harm our business and results of operations.
We may not be successful in advancing the clinical development of our product candidates, including larotrectinib, LOXO-292 and LOXO-195.
In order to execute on our strategy of advancing the clinical development of our product candidates, we have designed clinical trials for larotrectinib, LOXO-292, and LOXO-195, and expect to design future trials, to include patients whose tumors harbor the applicable genetic alterations that we believe contribute to cancer. Our goal is to enroll patients who have the highest probability of responding to the drug, in order to show early evidence of clinical efficacy. If we are unable to include patients whose tumors harbor the applicable genetic alterations, or if our product fails to work as we expect, our ability to assess the therapeutic effect, seek participation in FDA expedited review and approval programs, including Breakthrough Therapy, Fast Track Designation, Priority Review and Accelerated Approval, or otherwise to seek to accelerate clinical development and regulatory timelines, could be compromised, resulting in longer development times, larger trials and a greater likelihood of not obtaining regulatory approval.
We have initiated the submission of a rolling new drug application (“NDA”) for larotrectinib for the treatment of unresectable or metastatic solid tumors with NTRK-fusion proteins in adult and pediatric patients who require systemic therapy and who have either progressed following prior treatment or who have no acceptable alternative treatments. However, in order to obtain marketing approval from FDA, we may need to study our product candidates, including larotrectinib, in clinical trials specific for a given tumor type and this may result in increased time and cost. Even if our product candidate demonstrates efficacy in a particular tumor type, we cannot guarantee that any product candidate, including larotrectinib, will behave similarly in all tumor types, and we may be required to obtain separate regulatory approvals for each tumor type we intend a product candidate to treat. If any of our clinical trials are unsuccessful, our business will suffer. Furthermore, we do not yet know if the larotrectinib NDA will be considered for accelerated approval or full approval. If larotrectinib is granted accelerated approval, the FDA may impose significant post-marketing commitments that are challenging to satisfy. If these post-marketing commitments are not satisfied within an agreed-upon timeline with FDA, the larotrectinib approval could be rescinded.
Submission of MAAs are planned for the EU, Japan and other markets. Regulatory standards in these territories may differ from those in the U.S. There is risk that larotrectinib will not receive approvals in these territories.
If serious adverse events or unacceptable side effects are identified during the development of our product candidates, we may need to abandon or limit our development of some of our product candidates.
If our product candidates are associated with undesirable side effects in preclinical or clinical trials or have characteristics that are unexpected, we may need to interrupt, delay or abandon their development or limit development to more narrow uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective.
For example, toxicology studies for LOXO-292 and LOXO-195 have demonstrated potential side effects that could affect humans, but also may have failed to uncover additional side effects that could affect humans. In the case of larotrectinib, adverse events observed in ongoing clinical trials are discussed in more detail in “Business— Product Candidates—Larotrectinib (TRK Inhibitor)”.
Additional or more severe side effects may be identified in our ongoing clinical trials or in future clinical studies. These or other drug-related side effects could affect patient recruitment or the ability of enrolled subjects to complete the trial or result in potential product liability claims. Many compounds developed in the biopharmaceutical industry that initially showed promise in early-stage testing for treating cancer have later been found to cause side effects that prevented further development of the compound. Any of these occurrences may harm our business, financial condition and prospects significantly.
Investors should not place undue reliance on the results of preclinical experiments or our ongoing clinical trials since they are not necessarily predictive of the results that will form the basis of our global regulatory approval packages, and our product candidates may not receive regulatory approval.
Investors should not place undue reliance on the results from completed preclinical studies or data from our ongoing clinical trials since they do not ensure that other clinical trial data will be comparable, in terms of safety, ORR, DOR, or other factors the FDA and other regulators will consider in determining whether to approve our product candidates.
Final datasets, upon which global regulatory decisions will be based, will differ from interim datasets previously disclosed. Potential reasons for these differences include, but are not limited to:
· not all patients will demonstrate tumor regression, experience tumor regression that meets the measurement thresholds required under RECIST v1.1 for a partial response, or remain on study long enough for an initial or confirmatory response assessment;
· patients will discontinue our product candidates for a number of reasons, including an adverse event, tumor progression following a response, or a lack of tumor regression or clinical benefit and discontinuations will impact our product candidates’ reported duration of therapy and DOR;
· additional time and patient accrual provide new opportunities to capture new adverse events and further characterize the ORR and DOR;
· patient accrual beyond interim disclosed data will likely include study subjects with new tumor types, demographics (e.g. pediatric patients), and exposures to varying prior therapies. Thus, the inclusion of these subpopulations in the final dataset may alter the characterization of our product candidates’ overall safety, ORR and DOR; and
· the precise composition of the final dataset is subject to additional regulatory feedback, which is expected closer to the time of an NDA, or equivalent, and the advice may vary by regulatory authority.
As a result, the final efficacy and safety datasets for our product candidates have not been fully populated or established, and are expected to differ from any interim dataset publicly disclosed. Moreover, regulatory approvals will be based on the final efficacy and safety databases, and as such, we can give no assurance that our product candidates will receive regulatory approval.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we must focus on a limited number of research programs and product candidates and on specific indications. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future discovery and preclinical development programs and product candidates for specific indications may not yield any commercially viable products.
We may expand our business through the acquisition of drug products, companies or businesses or by entering into collaborations or in-licensing product candidates that could disrupt our business and harm our financial condition.
We have in the past and may in the future seek to expand our pipeline and capabilities by acquiring one or more companies, businesses or assets, entering into collaborations or in-licensing one or more product candidates. For example, in July 2017, we acquired a patent portfolio from Redx Pharma Plc and Redx Oncology Limited in connection with our acquisition of the Redx BTK discovery program. Any difficulties we experience in transitioning and integrating such product candidate into our operations may result in delays in clinical trials as well as problems in our development efforts and regulatory filings, particularly if we do not receive all of the necessary drug product, information, reports and data from third parties in a timely manner. More particularly, we have had no involvement with or control over the preclinical development of LOXO-305 prior to acquiring the rights to it. Furthermore, we did not get any representations or warranties with regards to the patents or associated rights. In November 2017, we entered into the Bayer Agreement pursuant to which we will collaborate with Bayer to develop and commercialize larotrectinib and LOXO-195. For more descriptions of the risks involved in this agreement, see the risk factor “Our existing collaboration with Bayer is important to our business. If we are unable to maintain this or any other collaboration, or if this or any other collaboration is not successful, our business could be adversely affected.”
Acquisitions, collaborations and in-licenses involve numerous risks, including:
· substantial cash expenditures;
· potentially dilutive issuance of equity securities;
· incurrence of debt and contingent liabilities, some of which may be difficult or impossible to identify at the time of acquisition;
· potential adverse consequences if the acquired assets are worth less than we anticipated or we are unable to successfully develop and commercialize the acquired assets for any reason;
· difficulties in assimilating the operations and technology of the acquired companies;
· potential disputes, including litigation, regarding contingent consideration for the acquired assets;
· the assumption of unknown liabilities of the acquired businesses;
· diverting our management’s attention away from other business concerns;
· entering markets in which we have limited or no direct experience; and
· potential loss of our key employees or key employees of the acquired companies or businesses.
Our experience in making acquisitions, entering collaborations and in-licensing product candidates is limited. We cannot assure you that any acquisition, collaboration or in-license will result in short-term or long-term benefits to us. We may incorrectly judge the value or worth of an acquired company or business or in-licensed product candidate. In addition, our future success may depend in part on our ability to manage the growth and technology integration associated with any of these acquisitions, collaborations and in-licenses. We cannot assure you that we will be able to successfully combine our business with that of acquired businesses, manage collaborations or integrate in-licensed product candidates or that such efforts would be successful. Furthermore, the development or expansion of our business or any acquired business or company or any collaboration or in-licensed product candidate may require a substantial capital investment by us. We may also seek to raise funds by selling shares of our capital stock, which could dilute our current stockholders’ ownership interest, or securities convertible into our capital stock, which could dilute current stockholders’ ownership interest upon conversion. We may also incur debt obligations, which could require us to comply with covenants which could restrict our ability to operate our business and negatively impact the value of our common stock.
Failure to successfully validate, develop and obtain regulatory approval for companion diagnostics for our product candidates could harm our drug development strategy and operational results.
As one of the central elements of our business strategy and clinical development approach, we often seek to identify subsets of patients with a genetic alteration who may derive meaningful benefit from our development product candidates. To achieve this, our product development programs can be dependent on the development and commercialization of a companion diagnostic by us or by third-party collaborators. Companion diagnostics are developed in conjunction with clinical programs for the associated product and are subject to regulation as medical devices. For example, for larotrectinib, we are working with collaborators to develop appropriate companion diagnostics to identify patients with tumors that harbor TRK fusions. The approval of a companion diagnostic as part of the product labeling may limit the use of the product candidate to only those patients who express the specific genetic alteration it was developed to detect. We may also experience delays in developing a sustainable, reproducible and scalable manufacturing process or transferring that process to commercial partners or negotiating insurance reimbursement plans, all of which may prevent us from completing our clinical trials or commercializing our products on a timely or profitable basis, if at all.
Companion diagnostics are subject to regulation by the FDA and comparable foreign regulatory authorities as medical devices and require separate clearance or approval prior to their commercialization. To date, the FDA has required premarket approval of all companion diagnostics for cancer therapies, either at the time of initial drug approval, or as a post-marketing commitment. We, and our third-party collaborators, may encounter difficulties in developing and obtaining approval for these companion diagnostics. Our third-party collaborators may de-prioritize, abandon or fail to execute against our development projects. Any delay or failure by us or third-party collaborators to develop or obtain regulatory approval of a companion diagnostic could delay or prevent approval of our related product candidates.
Failure by us or our third-party collaborators to successfully commercialize companion diagnostics developed for use with our product candidates could harm our ability to commercialize these product candidates.
Even if we or our companion diagnostic collaborators successfully obtain regulatory approval for the companion diagnostics for our product candidates, our collaborators:
· may not perform their obligations as expected;
· may not pursue commercialization of companion diagnostics for our therapeutic product candidates that achieve regulatory approval;
· may elect not to continue or renew commercialization programs based on changes in the collaborators’ strategic focus or available funding, or external factors, such as an acquisition, that divert resources or create competing priorities;
· may not commit sufficient resources to the marketing and distribution of such product or products;
· may fail to establish adequate reimbursement for their products, thus limiting the use of such product; and
· may terminate their relationship with us.
Additionally, we, or our collaborators, may encounter production difficulties that could constrain the supply of the companion diagnostics, affect the ease of use, affect the price or have difficulties gaining acceptance of the use of the companion diagnostics in the clinical community.
If companion diagnostics for use with our product candidates fail to gain market acceptance, our ability to derive revenues from sales of our product candidates could be harmed. If insurance reimbursement to the laboratories who perform the companion diagnostic
tests is inadequate, utilization may be low, and patient tumors may not be comprehensively screened for the presence of the genetic markers that predict response to our product candidates. If we or our collaborators fail to commercialize these companion diagnostics, we may not be able to enter into arrangements with another diagnostic company to obtain supplies of an alternative diagnostic test for use in connection with our product candidates or do so on commercially reasonable terms, which could adversely affect and delay the development or commercialization of our product candidates.
Risks Related to Regulatory Approval of Our Product Candidates and Other Legal Compliance Matters
If we or our collaborators are not able to obtain, or if there are delays in obtaining, required regulatory approvals, we will not be able to commercialize our product candidates, and our ability to generate revenue will be materially impaired.
Our product candidates must be approved by the FDA pursuant to an NDA in the United States and by the EMA and similar regulatory authorities outside the United States prior to commercialization. The process of obtaining marketing approvals, both in the United States and abroad, is expensive and takes many years, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. Failure to obtain marketing approval for a product candidate will prevent us from commercializing the product candidate. We have not received approval to market any of our product candidates from regulatory authorities in any jurisdiction. We have little experience in filing and supporting the applications necessary to gain marketing approvals and expect to rely on third-party CROs and our collaborators to assist us in this process. Securing marketing approval requires the submission of extensive preclinical and clinical data and supporting information to regulatory authorities for each therapeutic indication to establish the product candidate’s safety and efficacy. Securing marketing approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the regulatory authorities, among other requirements. Our product candidates may not be effective, may be only moderately effective, may not have an acceptable durability of response, may not have an acceptable risk-benefit profile, or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude our obtaining marketing approval or prevent or limit commercial use. Regulatory authorities have substantial discretion in the approval process and may refuse to accept any application or may decide that our data are insufficient for approval and require additional preclinical, clinical or other studies. For example, development programs that span many tumor types are relatively novel, and to date, the FDA has approved only one therapy to treat multiple tumor types based on a common biomarker. We cannot be sure that the FDA will accept our NDA for larotrectinib or our other product candidates. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent marketing approval of a product candidate. Changes in marketing approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application, may also cause delays in or prevent the approval of an application.
New cancer drugs frequently are indicated only for patient populations that have not responded to an existing therapy or have relapsed. If any of our product candidates receives marketing approval, the accompanying labeling may limit the approved use of our drug in this way, which could limit sales of the product.
Any marketing approval we or our collaborators ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
If we experience delays in obtaining approval or if we or our collaborators fail to obtain approval of our product candidates, the commercial prospects for our product candidates may be harmed and our ability to generate revenues will be materially impaired.
We may seek Orphan Drug Exclusivity for some of our product candidates, and we may be unsuccessful.
Regulatory authorities in some jurisdictions, including the United States and Europe, may designate drugs for relatively small patient populations as orphan drugs. Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a drug intended to treat a rare disease or condition, which is generally defined as a disease with a patient population of fewer than 200,000 individuals in the United States. In September 2015, we announced that the United States Food and Drug Administration, or the FDA, granted larotrectinib orphan drug designation for the treatment of soft tissue sarcoma. In January 2016, we announced that the European Commission designated larotrectinib as an orphan medicinal product for treatment of patients with soft tissue sarcoma. In May 2017, we announced that the FDA granted orphan drug designation to larotrectinib for the “treatment of solid tumors with NTRK-fusion proteins.” There can be no assurance that any of our other product candidates will be designated as an orphan drug.
Generally, if a product with an Orphan Drug Designation subsequently receives the first marketing approval for the indication for which it has such designation, the product is entitled to a period of marketing exclusivity, which precludes the EMA or the FDA from approving another marketing application for the same drug for the same indication during the period of exclusivity. The applicable period is seven years in the United States and ten years in Europe. The European exclusivity period can be reduced to six years if a drug no longer meets the criteria for Orphan Drug Designation or if the drug is sufficiently profitable so that market exclusivity is no longer justified. Orphan Drug Exclusivity may be lost if the FDA or EMA determines that the request for designation was materially defective, or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition.
Orphan Drug Exclusivity of larotrectinib or other product candidates, may not effectively protect the product candidate from competition because different drugs can be approved for the same orphan condition. In addition, after an orphan drug is approved and
granted exclusivity, the FDA can subsequently approve a different drug containing the same active moiety for the same condition if the FDA concludes that the later drug is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care. The FDA can also approve drugs containing the same active moiety for different indications.
A Fast Track Designation by the FDA, even if granted for any of our product candidates, may not lead to a faster development or regulatory review or approval process and does not increase the likelihood that our product candidates will receive marketing approval.
We do not currently have Fast Track Designation for any of our product candidates but we may seek such designation, if we believe such a designation is warranted. If a drug is intended for the treatment of a serious or life-threatening condition and the drug demonstrates the potential to address unmet medical needs for this condition, the drug sponsor may apply to the FDA for Fast Track Designation. The FDA has broad discretion whether or not to grant this designation. Even if we believe a particular product candidate is eligible for this designation, we cannot assure you that the FDA would decide to grant it. Even if we do receive Fast Track Designation, we may not experience a faster development process, review or approval compared to conventional FDA procedures. The FDA may withdraw Fast Track Designation if it believes that the designation is no longer supported by data from our clinical development program. Many drugs that have received Fast Track Designation have failed to obtain drug approval.
A Breakthrough Therapy Designation by the FDA may not lead to a faster development or regulatory review or approval process, and does not increase the likelihood that our product candidates will receive marketing approval.
In July 2016, we announced that the FDA granted Breakthrough Therapy Designation to larotrectinib “for the treatment of unresectable or metastatic solid tumors with NTRK-fusion proteins in adult and pediatric patients who require systemic therapy and who have either progressed following prior treatment or who have no acceptable alternative treatments.” There can be no assurance that larotrectinib will be approved by the FDA with this indication or at all. There can be no assurance that any of our other product candidates will receive Breakthrough Therapy Designation. A Breakthrough Therapy is defined as a drug that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. For drugs that have received a Breakthrough Therapy Designation, interaction and communication between the FDA and the sponsor can help to identify the most efficient path for development.
The receipt of a Breakthrough Therapy Designation for a product candidate may not result in a faster development process, review or approval compared to drugs considered for approval under conventional FDA procedures and does not assure ultimate approval by the FDA. In addition, even if our product candidates receive a Breakthrough Therapy Designation, the FDA may later decide that such product candidates no longer meet the conditions for qualification.
Failure to obtain marketing approval in international jurisdictions would prevent our product candidates from being marketed abroad.
In order to market and sell our products in the European Union and many other jurisdictions, we or our third-party collaborators, including Bayer, must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing and different criteria for approval. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The regulatory approval process outside the United States generally includes all of the risks associated with obtaining FDA approval. In addition, in many countries outside the United States, it is required that the product be approved for reimbursement before the product can be approved for sale in that country. We, or our third-party collaborators, may not obtain approvals from regulatory authorities outside the United States on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. However, failure to obtain approval in some countries or jurisdictions may compromise our ability to obtain approval elsewhere. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our products in any market.
Any product candidate for which we obtain marketing approval will be subject to extensive post-approval regulatory requirements and could be subject to post-approval restrictions or withdrawal from the market, and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our products, when and if any of them are approved.
Our product candidates and the activities associated with their development and commercialization, including their testing, manufacture, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-approval information and reports, registration and listing requirements, current good manufacturing practices (“cGMP”) requirements relating to manufacturing, quality control, quality assurance and corresponding maintenance of records and documents, including periodic
inspections by the FDA and other regulatory authority, restrictions or requirements regarding the distribution of samples to physicians and recordkeeping requirements.
The FDA may also impose requirements for costly post-marketing studies or clinical trials, diagnostic approval, and surveillance to monitor the safety or efficacy of the product. The FDA closely regulates the post-approval marketing and promotion of drugs to ensure drugs are marketed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA imposes stringent restrictions on manufacturers’ communications regarding use of their products and if we promote our products beyond their approved indications, we may be subject to enforcement action for off-label promotion. Violations of the FDC Act relating to the promotion of prescription drugs may lead to investigations alleging violations of federal and state healthcare fraud and abuse laws, as well as state consumer protection laws.
In addition, later discovery of previously unknown adverse events or other problems with our products, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:
· restrictions on such products, manufacturers or manufacturing processes;
· restrictions on the labeling or marketing of a product;
· restrictions on product distribution or use;
· requirements to conduct post-approval studies or clinical trials;
· warning or untitled letters;
· withdrawal of the products from the market;
· refusal to approve pending applications or supplements to approved applications that we submit;
· recall of products;
· fines, restitution or disgorgement of profits or revenues;
· suspension or withdrawal of marketing approvals;
· refusal to permit the import or export of our products;
· product seizure; or
· injunctions or the imposition of civil or criminal penalties.
Noncompliance with European Union requirements regarding safety monitoring or pharmacovigilance, and with requirements related to the development of products for the pediatric population, can also result in significant financial penalties. Similarly, failure to comply with the European Union’s requirements regarding the protection of personal information can also lead to significant penalties and sanctions.
Our relationships with customers and third-party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and third-party payors will play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute any products for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations include the following:
· the federal Anti-Kickback Statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid;
· the federal False Claims Act imposes criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
· the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
· HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information;
· federal law requires applicable manufacturers of covered drugs to report payments and other transfers of value to physicians and teaching hospitals, which includes data collection and reporting obligations. The information was to be made publicly available on a searchable website in September 2014; and
· analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers.
Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion of products from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Recently enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing approval.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (“MMA”) changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for physician-administered drugs. In addition, this legislation provided authority for limiting the number of drugs that will be covered in any therapeutic class. Cost reduction initiatives and other provisions of this legislation could decrease the coverage and price that we receive for any approved products. While the MMA only applies to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private payors.
In March 2010, former President Obama signed into law the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act (collectively the “PPACA”) a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms.
Among the provisions of the PPACA of importance to our potential product candidates are the following:
· an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic agents;
· an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program;
· expansion of healthcare fraud and abuse laws, including the False Claims Act and the Anti-Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance;
· a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices;
· extension of manufacturers’ Medicaid rebate liability;
· expansion of eligibility criteria for Medicaid programs;
· expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program;
· new requirements to report financial arrangements with physicians and teaching hospitals;
· a new requirement to annually report drug samples that manufacturers and distributors provide to physicians; and
· a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research.
In addition, other legislative changes have been proposed and adopted since the PPACA was enacted. These changes included aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, starting in 2013. In January 2013, former President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. The Tax Cuts and Jobs Act of 2017 includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the PPACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate”. These new laws may result in additional reductions in Medicare and other healthcare funding. In 2016, the U.S. Congress held hearings on the rising costs of prescription drugs and in October 2017, President Trump issued the Executive Order Promoting Healthcare Choice and Competition, directing certain federal agencies to modify their implementation of the PPACA. Future legislation could potentially change drug pricing dynamics.
We expect that the PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved product. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our products.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
Governments outside the United States tend to impose strict price controls, which may adversely affect our revenues, if any.
In some countries, particularly the countries of the European Union, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product candidate to other available therapies. In the absence of such data, reimbursement for our products may be negatively affected. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be harmed, possibly materially.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could harm our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties for failure to comply with such laws and regulations.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our discovery, preclinical development or production efforts. Our failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Risks Related to Our Dependence on Third Parties
Our existing collaboration with Bayer is important to our business. If we are unable to maintain this or any other collaboration, or if this or any other collaboration is not successful, our business could be adversely affected.
We have entered into collaborations with other companies to develop or commercialize several of our product candidates. We cannot predict the success of any current or future collaborations.
On November 14, 2017, we entered into a License, Development and Commercialization Agreement with Bayer pursuant to which we will collaborate with Bayer to develop and commercialize larotrectinib and LOXO-195. Pursuant to the Bayer Agreement, we have granted co-exclusive development and commercialization licenses to Bayer for both larotrectinib and LOXO-195. We will lead global development activities and U.S. regulatory activities. Bayer will lead ex-U.S. regulatory activities, and global commercial activities. We will co-promote the products with Bayer in the U.S. See “Business—Bayer Collaboration.”
Under the Bayer Agreement, we are eligible to receive $450.0 million in milestone payments upon larotrectinib regulatory approvals and first commercial sale events in certain major markets and an additional $200.0 million in milestone payments upon LOXO-195 regulatory approvals and first commercial sale events in certain major markets. We may not receive royalty or milestone revenue under the Bayer Agreement for several years, or at all.
Under the terms of the Bayer Agreement, Bayer will have significant discretion in determining the efforts and resources that they will apply to their marketing efforts and their management of the ex-U.S. regulatory activities and they may not perform their obligations as expected. Disputes may arise between the collaborators and us that result in the delay or termination of the research, development or commercialization of our medicines or product candidates or that result in costly litigation or arbitration that diverts management attention and resources. Furthermore, they may have changes in their strategic focus or available funding, or experience external factors, such as an acquisition, may divert resources or create competing priorities. Any of these events would have a material adverse effect on our results of operations and financial condition.
The Bayer Agreement may be terminated by either party for material breach or bankruptcy. In addition, Bayer may terminate the Bayer Agreement after the fourth anniversary of the effective date upon written notice to us, or in the event that we receive a “complete response letter” from the U.S. FDA with respect to larotrectinib, or if we do not receive marketing approval for larotrectinib by December 31, 2018.
If the Bayer Agreement is terminated, then, depending on the event:
· our cash expenditures could increase significantly if it is necessary for us to hire additional employees and allocate internal resources to the commercialization or regulatory activities that were previously shared by Bayer;
· we would bear all of the risks and costs related to the further development and commercialization, as well as regulatory activities, that were previously the subject of the Bayer Agreement;
· in order to fund further commercialization or regulatory activities, we may need to seek out and establish alternative strategic collaborations with third-party partners, which may not be possible; or
· we may not be able to do so on terms which are acceptable to us, in which case it may be necessary for us to limit the size or scope of one or more of our programs or increase our expenditures and seek additional funding by other means.
Any of these events would have a material adverse effect on our results of operations and financial condition.
Future collaborations may be important to us. If we are unable to maintain these collaborations, or if these collaborations are not successful, our business could be adversely affected.
For some of our product candidates, we may in the future determine to collaborate with pharmaceutical and biotechnology companies for development of products. For example, on July 3, 2013, we entered into the Array Agreement, pursuant to which Array agreed to design, conduct and perform research and preclinical testing for certain compounds that we select, including larotrectinib, targeted at TRKA, TRKB and TRKC, and identify Investigational New Drug candidates for TRK and other targets (including RET and FGFR), while undertaking manufacturing activities sufficient to conduct Phase 1 clinical trials for a subset of these programs. Array granted us exclusive licenses worldwide, for clinical and commercial development of these compounds. See “Business—Array Collaboration.”
The contractual end of the Array Agreement is currently scheduled for September 30, 2018, unless we otherwise mutually agree to extend. Array has an obligation to test targets during our discovery phase, but we cannot be certain that our collaboration will lead to the discovery of any additional product candidates beyond larotrectinib, LOXO-292, and LOXO-195, or that any of these product candidates will be successfully commercialized and developed. If the Array Agreement is terminated as scheduled, we will have to develop new ways to discover additional product candidates, as there can be no assurance that we will be successful.
Furthermore, if Array changes its strategic focus, or if it independently develops products that compete directly or indirectly with our product candidates using resources it acquires from our collaboration, our business and results of operations could suffer. For example, while Array has granted us a license for compounds designed to target at least two of the three known TRK kinases. Array has retained ownership and rights to development of compounds targeting only one TRK kinase. We were notified by Array regarding their efforts and use of third parties for the development and/or commercialization of compounds that selectively modulate TRKA for oncology indications. We have not elected to be the third-party partner for such efforts, as permitted under our collaboration agreement with Array. If Array or its partners were to develop such compounds in direct competition with our product candidates, our business could be adversely impacted.
We face significant competition in seeking appropriate collaborators. Our ability to reach a definitive agreement for any collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. If we are unable to reach agreements with suitable collaborators on a timely basis, on acceptable terms, or at all, we may have to curtail the development of a product candidate, reduce or delay its development program or one or more of our other development programs, delay its potential development schedule or reduce the scope of research activities, or increase our expenditures and undertake discovery or preclinical development activities at our own expense. If we fail to enter into collaborations and do not have sufficient funds or expertise to undertake the necessary development activities, we may not be able to further develop our product candidates or continue to develop our product candidates and our business may be materially and adversely affected.
Future development collaborations we may enter into may involve the following risks:
· collaborators may have significant discretion in determining the efforts and resources that they will apply to these collaborations;
· collaborators may not perform their obligations as expected;
· changes in the collaborators’ strategic focus or available funding, or external factors, such as an acquisition, may divert resources or create competing priorities;
· collaborators may delay discovery and preclinical development, provide insufficient funding for product development of targets selected by us, stop or abandon discovery and preclinical development for a product candidate, repeat or conduct new discovery and preclinical development for a product candidate;
· collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates if the collaborators believe that competitive products are more likely to be successfully developed than ours;
· product candidates discovered in collaboration with us may be viewed by our collaborators as competitive with their own product candidates or products, which may cause collaborators to cease to devote resources to the development of our product candidates;
· disagreements with collaborators, including disagreements over proprietary rights, contract interpretation or the preferred course of development, might cause delays or termination of the discovery, preclinical development or commercialization of product candidates, might lead to additional responsibilities for us with respect to product candidates, or might result in litigation or arbitration, any of which would be time-consuming and expensive;
· collaborators may not properly maintain or defend our intellectual property rights or intellectual property rights licensed to us or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation;
· collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability; and
· collaborations may be terminated for the convenience of the collaborator and, if terminated, we could be required to raise additional capital to pursue further development or commercialization of the applicable product candidates.
Additionally, subject to its contractual obligations to us, if a collaborator of ours is involved in a business combination, the collaborator might deemphasize or terminate the development of any of our product candidates. If one of our collaborators terminates its agreement with us, we may find it more difficult to attract new collaborators and the perception of our company by the business and financial communities could be adversely affected.
If we are unable to maintain our collaborations, development of our product candidates could be delayed and we may need additional resources to develop them. All of the risks relating to product development, regulatory approval and commercialization described in this filing also apply to the activities of our collaborators.
We expect to rely on third-party contractors and organizations to conduct our clinical trials, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials.
We will rely on third-party clinical research contractors and organizations to conduct our ongoing clinical trials of larotrectinib, LOXO-292, and LOXO-195, and we will rely on third-party contractors, clinical data management organizations, independent contractors, medical institutions and clinical investigators to conduct our clinical trials beyond our current trials, and for clinical trials for programs other than larotrectinib, LOXO-292, and LOXO-195. These agreements may terminate for a variety of reasons, including a failure to perform by the third parties. If we needed to enter into alternative arrangements, our product development activities could be delayed.
We compete with many other companies, some of which may be our business competitors, for the resources of these third parties. Large pharmaceutical companies often have significantly more extensive agreements and relationships with such third-party providers, and such third-party providers may prioritize the requirements of such large pharmaceutical companies over ours. The third parties on whom we rely may terminate their engagements with us at any time, which may cause delay in the development and commercialization of our product candidates. If any such third party terminates its engagement with us or fails to perform as agreed, we may be required to enter into alternative arrangements, which would result in significant cost and delay to our product development program. Moreover, our agreements with such third parties generally do not provide assurances regarding employee turnover and availability, which may cause interruptions in the research on our product candidates by such third parties.
Our reliance on these third parties to conduct our clinical trials reduces our control over these activities but does not relieve us of our responsibilities. For example, we remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA and other regulatory authorities require us to comply with GCPs for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. We are also required to register ongoing clinical trials and post the results of completed clinical trials on a government-sponsored database, ClinicalTrials.gov, within specified timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions.
Additionally, we expect to rely substantially on third-party data managers for our clinical trial data. There is no assurance that these third parties will not make errors in the design, management or retention of our data or data systems. There is no assurance that these third parties will pass FDA or other regulatory audits, which could delay or prevent regulatory approval.
If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, marketing approvals for our product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates.
We contract with third parties for the manufacture of our product candidates for preclinical and clinical testing and expect to continue to do so for commercialization. This reliance on third parties increases the risk that we will not have sufficient quantities of our product candidates or products at an acceptable cost and quality, which could delay, prevent or impair our development or commercialization efforts.
We do not own or operate facilities for the manufacture of our product candidates, and we rely on outside manufacturing personnel to operate these third-party facilities. We currently have no plans to build our own clinical or commercial scale manufacturing capabilities. We rely, and expect to continue to rely, on third parties for the manufacture of our product candidates for preclinical and clinical testing. We will rely on third parties as well for commercial manufacture if any of our product candidates receive marketing approval. This reliance on third parties increases the risk that we will not have sufficient quantities of our product candidates or products or such quantities at an acceptable cost or quality, which could delay, prevent or impair our development or commercialization efforts.
Any performance failure on the part of our existing or future manufacturers could delay clinical development or marketing approval. Arrangements for redundant supply or a source for bulk drug product may be infeasible, too costly, unavailable or inadequate to prevent a delay in clinical development or marketing approval should our existing or future manufacturers experience performance failure. The formulation used in early studies is not necessarily a final formulation for commercialization. Additional, changes may be required by the FDA or other regulatory authorities on specifications and storage conditions. These may require additional studies, and may delay our clinical trials.
We expect to rely on third-party manufacturers or third-party collaborators for the manufacture of commercial supply of any other product candidates for which our collaborators or we obtain marketing approval.
We also expect to rely on other third parties to store and distribute drug supplies for our clinical trials. Any performance failure on the part of our distributors could delay clinical development or marketing approval of our product candidates or commercialization of our products, producing additional losses and depriving us of potential product revenue.
We may be unable to establish any agreements with third-party manufacturers or to do so on acceptable terms. Even if we are able to establish agreements with third-party manufacturers, reliance on third-party manufacturers entails additional risks, including:
· reliance on the third party for regulatory compliance and quality assurance;
· the possible breach of the manufacturing agreement by the third party;
· the possible misappropriation of our proprietary information, including our trade secrets and know-how; and
· the possible termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us.
Third-party manufacturers may not be able to comply with cGMP regulations or similar regulatory requirements outside the United States. Our failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including clinical holds, fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of product candidates or products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of our products. In addition, if, during a preapproval inspection or other inspection of our third-party manufacturers’ facility or facilities, the FDA determines that the facility is not in compliance with cGMP, any of our marketing applications that lists such facility as a manufacturer may not be approved or approval may be delayed until the facility comes into compliance with cGMP and completes a successful reinspection by the FDA.
Our product candidates and any products that we may develop may compete with other product candidates and products for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that might be capable of manufacturing for us.
Our current and anticipated future dependence upon others for the manufacture of our product candidates or products may adversely affect our future profit margins and our ability to commercialize any products that receive marketing approval on a timely and competitive basis.
Risks Related to the Commercialization of Our Product Candidates
Even if any of our product candidates receives marketing approval, it may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success.
If any of our product candidates receives marketing approval, it may nonetheless fail to gain sufficient market acceptance by physicians, patients, third-party payors and others in the medical community. For example, current cancer treatments like chemotherapy and radiation therapy are well established in the medical community, and doctors may continue to rely on these treatments to the exclusion of our product candidates. In addition, physicians, patients and third-party payors may prefer other novel products to ours. If our product candidates do not achieve an adequate level of acceptance, we may not generate significant product revenues and we may not become profitable. The degree of market acceptance of our product candidates, if approved for commercial sale, will depend on a number of factors, including:
· the efficacy and safety and potential advantages and disadvantages compared to alternative treatments;
· our ability to offer our products for sale at competitive prices;
· the convenience and ease of administration compared to alternative treatments;
· the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;
· the strength of our marketing and distribution support;
· the availability of third-party coverage and adequate reimbursement, including patient cost-sharing programs such as copays and deductibles;
· our ability to develop or partner with third-party collaborators to develop companion diagnostics;
· the prevalence and severity of any side effects; and
· any restrictions on the use of our products together with other medications.
We currently have a limited commercial team. If we are unable to establish effective sales or marketing capabilities or enter into agreements with third parties to sell or market our product candidates, we may not be able to effectively sell or market our product candidates, if approved, or generate product revenues.
We currently have a limited commercial team for the marketing, sales and distribution of any of our product candidates that are able to obtain regulatory approval. Patient identification will be important in the commercial setting, much as it has been important in the clinical trial setting. Estimates for addressable patient populations relevant to our product candidates are uncertain. The work that informs these estimates is not definitive and future analyses may lead to estimates that are higher or lower than these estimates, making difficult the tasks of sizing of a marketing and sales force or evaluating the attractiveness of a commercial partnership. The utilization of sensitive diagnostic testing in routine clinical practice is likely an important variable in identifying all of the eligible patients that may
truly exist. This requirement may cause the potential launch of larotrectinib or our other product candidates to be slower than other commercialized oncology products.
In order to commercialize any product candidates, we, in collaboration with commercial partners as applicable, must build on a territory-by-territory basis marketing, sales, distribution, managerial and other non-technical capabilities or make arrangements with third parties to perform these services, and we may not be successful in doing so. If our product candidates receive regulatory approval, we, in collaboration with commercial partners as applicable, intend to establish an internal sales or marketing team with technical expertise and supporting distribution capabilities to commercialize our product candidates, which will be expensive and time consuming and will require significant attention of our executive officers to manage. Capable managers with commercial experience will need to be identified and successfully recruited to the company. Any failure or delay in the development of our internal sales, marketing and distribution capabilities would adversely impact the commercialization of any of our products that we obtain approval to market. With respect to the commercialization of all or certain of our product candidates, we may choose to collaborate, either globally or on a territory-by-territory basis, with third parties that have direct sales forces and established distribution systems, either to augment our own sales force and distribution systems or in lieu of our own sales force and distribution systems. Under our collaboration agreement with Bayer, Bayer will lead global commercial and marketing activities for larotrectinib and LOXO-195 outside of the United States, over which we will have limited control. Within the United States, Bayer will lead commercial and marketing activities for larotrectinib and LOXO-195, and we will co-promote the products with Bayer. If we are unable to enter into such arrangements when needed on acceptable terms or at all, or if Bayer breaches our collaboration agreement or is otherwise unsuccessful in marketing our products, we may not be able to successfully commercialize any of our product candidates that receive regulatory approval or any such commercialization may experience delays or limitations. If we are not successful in commercializing our product candidates, either on our own or through collaborations with one or more third parties, our future product revenue will suffer and we may incur significant additional losses.
We face substantial competition, which may result in others discovering, developing or commercializing competing products before or more successfully than we do.
The development and commercialization of new drug products is highly competitive. We face competition with respect to our current product candidates, and will face competition with respect to any product candidates that we may seek to develop or commercialize in the future, from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. There are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of products for the treatment of the disease indications for which we are developing our product candidates. Some of these competitive products and therapies are based on scientific approaches that are the same as or similar to our approach, and others are based on entirely different approaches. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
Specifically, there are a large number of companies developing or marketing treatments for cancer, including many major pharmaceutical and biotechnology companies. In addition, many companies are developing cancer therapeutics that work by inhibiting multiple kinases that may directly compete with larotrectinib and our other product candidates.
For larotrectinib and LOXO-195, examples of such potential competitors include Daiichi Sankyo and its subsidiary Plexxikon (PLX-7486), Tesaro (TSR-011), Roche (entrectinib), Novartis AG (dovitinib), Mirati (MGDC516), Ono Pharmaceutical (ONO-4474 and ONO-5390556), Chugai Pharmaceutical, a member of the Roche Group (CH7057288), Blueprint Medicines, TP Therapeutics (TPX-0005) and Deciphera.
For LOXO-292, examples of such potential competitors include Eisai (lenvatinib), Exelixis (cabozantinib), AstraZeneca (vandetanib), Ariad (ponatinib), Novartis (dovitinib), Roche (alectinib), Pfizer (sunitinib), Ignyta (RXDX-105) and Blueprint Medicines (BLU-667). There are no selective RET inhibitors approved in RET-specific indications. Several multikinase inhibitors with anti-RET activity are part of ongoing RET-focused development programs: Eisai (lenvatinib), Exelixis (cabozantinib), AstraZeneca (vandetanib), Ariad (ponatinib), Novartis (dovitinib), Roche (alectinib), pfizer (sunitinib) and Roche (RXDX-105). A compound from Blueprint Medicines (BLU-667) was developed to be a selective RET inhibitor and is currently in clinical development and there are preclinical selective RET inhibitor programs from Taiho Oncology, Nerviano Medical Sciences, and cancer Research UK.
For LOXO-305, examples of such potential competitors include Abbvie/Pharmacyclics (ibrutinib), AstraZeneca/Acerta (acalabrutinib), Beigene (BGB-3111), Gilead/Ono (GS-4059), ArQule (ARQ-531), Sunesis (SNS-062), Biogen (BIIB-068), Celgene (CC-292), Principia (PRN1008, PRN2246), Bristol-Myers Squibb (BMS-986142), Genentech (GDC-0853), Roche (RN983) and Impetis (PNQ-154). Also, drugs that work by different mechanisms, other than BTK inhibition, are available or could be developed in patient populations relevant to LOXO-305 —these include classes such as BCL-2 inhibitors (e.g. Roche/Abbvie, venetoclax), anti-CD20 biologics (e.g. Roche, rituximab), PI3kd inhibitors (e.g. Gilead, idelalisib) and cytotoxic chemotherapy.
For the FGFR program, examples of such potential competitors include J&J (JNJ- 42756493), QED Therapeutics (BGJ-398, dovitinib), AstraZeneca (AZD4547), Clovis Oncology (lucitinib), Chugai (CH5183284), Bayer (BAY 1163877, BAY 1179470), Lilly (LY2874455), Eisai (E7090), Taiho (TAS-120), BI (nintedanib), Ariad (ponatinib), FivePrime (FP-1039, FPA144), Incyte (INCB54828), ArQule (ARQ087), BioClinica (MFGR1877S) and Principia (PRN1371).
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market or slow our
regulatory approval. In addition, our ability to compete may be affected in many cases by insurers or other third-party payors seeking to encourage the use of generic products. Generic products are currently on the market for the indications that we are pursuing, and additional products are expected to become available on a generic basis over the coming years. If our product candidates achieve marketing approval, we expect that they will be priced at a significant premium over competitive generic products.
Many of the companies against which we are competing or against which we may compete in the future have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
The insurance coverage and reimbursement status of newly-approved products is uncertain. Failure to obtain or maintain adequate coverage and reimbursement for new or current products could limit our ability to market those products and decrease our ability to generate revenue.
The availability and extent of reimbursement by governmental and private payors is essential for most patients to be able to afford expensive treatments. Sales of our product candidates will depend substantially, both domestically and abroad, on the extent to which the costs of our product candidates will be paid by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or reimbursed by government health administration authorities, private health coverage insurers and other third-party payors. If reimbursement is not available, or is available only to limited levels, we may not be able to successfully commercialize our product candidates. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish or maintain pricing sufficient to realize a sufficient return on our investment.
There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products. In the United States, the principal decisions about reimbursement for new medicines are typically made by the Centers for Medicare & Medicaid Services (“CMS”) an agency within the U.S. Department of Health and Human Services, as CMS decides whether and to what extent a new medicine will be covered and reimbursed under Medicare. Private payors tend to follow CMS to a substantial degree. It is difficult to predict what CMS will decide with respect to reimbursement for fundamentally novel products such as ours, as there is no body of established practices and precedents for these new products. In 2016, the U.S. Congress held hearings on the rising costs of prescription drugs, and there is increased media attention on the issue. Future legislation could potentially change drug pricing dynamics. Reimbursement agencies in Europe may be more conservative than CMS. For example, a number of cancer drugs have been approved for reimbursement in the United States and have not been approved for reimbursement in certain European countries. Outside the United States, international operations are generally subject to extensive governmental price controls and other market regulations, and we believe the increasing emphasis on cost containment initiatives in Europe, Canada, and other countries has and will continue to put pressure on the pricing and usage of our product candidates. In many countries, the prices of medical products are subject to varying price control mechanisms as part of national health systems. In general, the prices of medicines under such systems are substantially lower than in the United States. Other countries allow companies to fix their own prices for medicines, but monitor and control company profits. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our product candidates. Accordingly, in markets outside the United States, the reimbursement for our products may be reduced compared with the United States and may be insufficient to generate commercially reasonable revenues and profits.
Moreover, increasing efforts by governmental and third-party payors, in the United States and abroad, to cap or reduce healthcare costs may cause such organizations to limit both coverage and level of reimbursement for new products approved and, as a result, they may not cover or provide adequate payment for our product candidates. We expect to experience pricing pressures in connection with the sale of any of our product candidates, due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription drugs and surgical procedures and other treatments, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products into the healthcare market.
In addition to CMS and private payors, professional organizations such as the National Comprehensive Cancer Network and the American Society of Clinical Oncology can influence decisions about reimbursement for new medicines by determining standards for care. In addition, many private payors contract with commercial vendors who sell software that provide guidelines that attempt to limit utilization of, and therefore reimbursement for, certain products deemed to provide limited benefit to existing alternatives. Such organizations may set guidelines that limit reimbursement or utilization of our products.
If insurance reimbursement to the laboratories who purchase the companion diagnostic tests is inadequate, utilization may be low, and patient tumors may not be comprehensively screened for the presence of the genetic markers that predict response to our product candidates.
Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any products that we may develop.
We and our collaborators face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials and will face an even greater risk if we commercially sell any products that we may develop. If we and our collaborators cannot successfully defend ourselves against claims that our product candidates or products caused injuries, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
· decreased demand for any product candidates or products that we may develop;
· injury to our reputation and significant negative media attention;
· withdrawal of clinical trial participants;
· significant costs to defend the related litigation;
· substantial monetary awards to trial participants or patients;
· loss of revenue;
· reduced resources of our management to pursue our business strategy; and
· the inability to commercialize any products that we may develop.
We currently hold $5 million in product liability insurance coverage in the aggregate, with a per incident limit of $5 million, which may not be adequate to cover all liabilities that we may incur. We may need to increase our insurance coverage as we expand our clinical trials or if we commence commercialization of our product candidates. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise. In addition, if one of our collaboration partners were to become subject to product liability claims or were unable to successfully defend themselves against such claims, any such collaboration partner could be more likely to terminate such relationship with us and therefore substantially limit the commercial potential of our products.
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain intellectual property protection for our technology and products, or if the scope of the intellectual property protection obtained is not sufficiently broad, our competitors could develop and commercialize technology and products similar or identical to ours, and our ability to successfully commercialize our technology and products may be impaired.
Our success depends in large part on our ability to obtain and maintain patent protection in the United States and other countries with respect to our proprietary technology and products, including any companion diagnostic developed by us or a third-party collaborator. We seek to protect our proprietary position by filing patent applications in the United States and abroad related to our novel technologies and product candidates. Our patent portfolio includes patents and patent applications we exclusively licensed from Array, exclusive worldwide licenses for all therapeutic indications for new intellectual property developed in our Array collaboration, and patents that we purchased from Redx. This patent portfolio includes issued patents and pending patent applications covering compositions of matter and methods of use.
The patent prosecution process is expensive and time-consuming, and we and our collaborators may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. We may choose not to seek patent protection for certain innovations and may choose not to pursue patent protection in certain jurisdictions, and under the laws of certain jurisdictions, patents or other intellectual property rights may be unavailable or limited in scope. It is also possible that we or our collaborators will fail to identify patentable aspects of our discovery and preclinical development output before it is too late to obtain patent protection. Moreover, in some circumstances, we do not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents, covering technology that we license from third parties. Therefore, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States. For example, India and China do not allow patents for methods of treating the human body. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot know with certainty whether we were the first to make the inventions claimed in our owned or licensed patents or pending patent applications, or that we were the first to file for patent protection of such inventions. As a result, the issuance, scope, validity, enforceability and commercial value of our and our collaborators’ patent rights are highly uncertain. Our and our collaborators’ pending and future patent applications may not result in patents being issued which protect our technology or products, in whole or in part, or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or
interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection.
Patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. On September 16, 2011, the Leahy-Smith America Invents Act (the “Leahy-Smith Act”) was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications are prosecuted and may also affect patent litigation. The U.S. Patent and Trademark Office (“U.S. PTO”) developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, only became effective on March 16, 2013. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business and financial condition.
Moreover, we may be subject to a third-party preissuance submission of prior art to the U.S. PTO, or become involved in opposition, derivation, reexamination, inter partes review, post-grant review or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.
Even if our owned and licensed patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
The risks described elsewhere pertaining to our patents and other intellectual property rights also apply to the intellectual property rights that we license, and any failure to obtain, maintain and enforce these rights could have a material adverse effect on our business. In some cases, we may not have control over the prosecution, maintenance or enforcement of the patents that we license, and our licensors may fail to take the steps that we believe are necessary or desirable in order to obtain, maintain and enforce the licensed patents. Any inability on our part to protect adequately our intellectual property may have a material adverse effect on our business, operating results and financial position.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will be due to be paid to the U.S. PTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and/or applications. We have systems in place to remind us to pay these fees, and we employ an outside firm and rely on our outside counsel to pay these fees due to non-U.S. patent agencies. The U.S. PTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. However, there are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to enter the market and this circumstance would have a material adverse effect on our business.
We may become involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive, time consuming and unsuccessful.
Because competition in our industry is intense, competitors may infringe or otherwise violate our issued or our collaborators’ patents, patents of our licensors or other intellectual property. To counter infringement or unauthorized use, we or our collaborators may
be required to file infringement claims, which can be expensive and time consuming. Any claims we or our collaborators assert against perceived infringers could provoke these parties to assert counterclaims against us or our collaborators alleging that we infringe their patents. In addition, in a patent infringement proceeding, a court may decide that a patent of ours or licensed to us is invalid or unenforceable, in whole or in part, construe the patent’s claims narrowly or refuse to stop the other party from using the technology at issue on the grounds that our or our licensors’ or collaborators’ patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our or our licensors’ or collaborators’ patents at risk of being invalidated or interpreted narrowly. We may also elect to enter into license agreements in order to settle patent infringement claims or to resolve disputes prior to litigation, and any such license agreements may require us to pay royalties and other fees that could be significant. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure.
We may need to license certain intellectual property from third parties, and such licenses may not be available or may not be available on commercially reasonable terms.
A third party may hold intellectual property, including patent rights, which are important or necessary to the development of our products. It may be necessary for us to use the patented or proprietary technology of third parties to commercialize our products, in which case we would be required to obtain a license from these third parties on commercially reasonable terms, or our business could be harmed, possibly materially. Although we believe that licenses to these patents are available from these third parties on commercially reasonable terms, if we were not able to obtain a license, or were not able to obtain a license on commercially reasonable terms, our business could be harmed, possibly materially.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our commercial success depends upon our ability, and the ability of our collaborators, to develop, manufacture, market and sell our product candidates and use our proprietary technologies without infringing the proprietary rights of third parties. There is considerable intellectual property litigation in the biotechnology and pharmaceutical industries. We or our collaborators may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our or our collaborators’ products and technology, including interference or derivation proceedings before the U.S. PTO. Third parties may assert infringement claims against us or our collaborators based on existing patents or patents that may be granted in the future.
If we or our collaborators are found to infringe a third party’s intellectual property rights, we or our collaborators could be required to obtain a license from such third party to continue developing and marketing our products and technology. However, we or our collaborators may not be able to obtain any required license on commercially reasonable terms or at all. Even if we or our collaborators were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We or our collaborators could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, we or our collaborators could be found liable for monetary damages, including treble damages and attorneys’ fees if we or our collaborators are found to have willfully infringed a patent. A finding of infringement could prevent us or our collaborators from commercializing our product candidates or force us or our collaborators to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
We may not be successful in obtaining or maintaining necessary rights for our development pipeline through acquisitions and in-licenses.
Presently we have rights to intellectual property to develop our product candidates, including patents and patent applications we exclusively licensed from Array, exclusive worldwide licenses for all therapeutic indications for new intellectual property developed in our Array collaboration, and patents that we purchased from Redx. Because our programs may involve additional product candidates that may require the use of proprietary rights held by third parties, the growth of our business may depend in part on our ability to acquire, in-license or use these proprietary rights. Additionally, a companion diagnostic may require that we or a third-party collaborator developing the diagnostic acquire use or proprietary rights held by third parties. We may be unable to acquire or in-license any compositions, methods of use, or other third-party intellectual property rights from third parties that we identify. The licensing and acquisition of third-party intellectual property rights is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire third-party intellectual property rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities.
For example, we may collaborate with U.S. and foreign academic institutions to accelerate our discovery and preclinical development work under written agreements with these institutions. Typically, these institutions provide us with an option to negotiate a license to any of the institution’s rights in technology resulting from the collaboration. Regardless of such right of first negotiation for intellectual property, we may be unable to negotiate a license within the specified time frame or under terms that are acceptable to us. If we are unable to do so, the institution may offer the intellectual property rights to other parties, potentially blocking our ability to pursue our program.
In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment. If we are unable to successfully obtain rights to required third-party intellectual property rights, our business, financial condition and prospects for growth could suffer.
We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property.
Many of our employees were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that these employees or we have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employer. Litigation may be necessary to defend against these claims.
In addition, while it is our policy to require our employees and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own. Our and their assignment agreements may not be self-executing or may be breached, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property.
If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to management.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for some of our technology and product candidates, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. We seek to protect our confidential proprietary information, in part, by entering into confidentiality and invention or patent assignment agreements with our employees and consultants, however, we cannot be certain that such agreements have been entered into with all relevant parties. Moreover, to the extent we enter into such agreements, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
Risks Related to Employee Matters, Managing Growth and Macroeconomic Conditions
We currently have a limited number of employees, are highly dependent on our Chief Executive Officer and our future success depends on our ability to retain key executives and to attract, retain and motivate qualified personnel.
We are an early-stage clinical development company with a limited operating history and, as of December 31, 2017, had 59 full-time employees. We are highly dependent on the research and development, clinical and business development expertise of Joshua H. Bilenker, M.D., our President and Chief Executive Officer, as well as the other principal members of our management, scientific and clinical team. Although we have entered into employment letter agreements with our executive officers, each of them may terminate their employment with us at any time. We do not maintain “key person” insurance for any of our executives or other employees.
Recruiting and retaining qualified scientific, clinical, manufacturing and sales and marketing personnel will also be critical to our success as we scale. The loss of the services of our executive officers or other key employees could impede the achievement of our research, development and commercialization objectives and seriously harm our ability to successfully implement our development or commercialization strategies. Furthermore, replacing executive officers and key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to successfully develop, gain regulatory approval of and commercialize products. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these key personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. Additionally, if we fail to provide an adequate amount of equity consideration to new and existing employees we may be unable to compete for new talent and retain our existing talent. We have a certain number of shares available for grant under our 2014 Equity Incentive Plan and it may not be adequate to enable us to continue to competitively compensate our employees in the future, which may prevent us from retaining our employees and could significantly impact our operating results.
We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our discovery and preclinical development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. If we are unable to continue to attract and retain high quality personnel, our ability to pursue our growth strategy will be limited.
We expect to expand our development and regulatory capabilities and potentially implement sales, marketing and distribution capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of medical affairs, drug development, regulatory affairs and, if any of our product candidates receives marketing approval, sales, marketing and distribution. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
Comprehensive tax reform bills could adversely affect our business and financial condition.
The U.S. government has recently enacted comprehensive tax legislation, the Tax Cuts and Jobs Act of 2017, that includes significant changes to the taxation of business entities. These changes include, among others, (i) a permanent reduction to the corporate income tax rate, (ii) a partial limitation on the deductibility of business interest expense, (iii) a shift of the U.S. taxation of multinational corporations from a tax on worldwide income to a territorial system (along with certain rules designed to prevent erosion of the U.S. income tax base) and (iv) a one-time tax on accumulated offshore earnings held in cash and illiquid assets, with the latter taxed at a lower rate.
Further, the newly enacted comprehensive tax legislation, among other things, reduces the orphan drug credit from 100% to 50% of qualifying expenditures. When and if we become profitable, this reduction in tax credits may result in an increased federal income tax burden on our orphan drug programs as it may cause us to pay federal income taxes earlier under the revised tax law than under the prior law and, despite being partially off-set by a reduction in the corporate tax rate from a top marginal rate of 35% to a flat rate of 21%, may increase our total federal tax liability attributable to such programs. The new tax law also eliminates entirely the carry back of net operating losses (“NOLs”). Companies may no longer carry back NOLs to receive refunds for taxes paid in the previous two years. The new law also changes the rules on the carry forward of NOLs. The previous 20-year limitation was eliminated, giving taxpayers the ability to carry forward losses indefinitely. However, NOL carry forward arising after January 1, 2018, will now be limited to 80 percent of taxable income.
Notwithstanding the reduction in the corporate income tax rate, the overall impact of this tax reform is uncertain, and our business and financial condition could be adversely affected. In addition, it is uncertain if and to what extent various states will conform to the newly enacted federal tax law.
Unfavorable global economic conditions could adversely affect our business, financial condition or results of operations.
Our results of operations could be adversely affected by general conditions in the global economy and in the global financial markets. A severe or prolonged economic downturn could result in a variety of risks to our business, including our ability to raise additional capital when needed on acceptable terms, if at all. A weak or declining economy could also strain our suppliers, possibly resulting in supply disruption. Any of the foregoing could harm our business and we cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact our business.
Failure to protect our information technology infrastructure against cyber-based attacks, network security breaches, service interruptions, or data corruption could significantly disrupt our operations and adversely affect our business and operating results.
We rely on information technology and telephone networks and systems, including the Internet, to process and transmit sensitive electronic information and to manage or support a variety of business processes and activities. We use enterprise information technology systems to record, process, and summarize financial information and results of operations for internal reporting purposes and to comply with regulatory financial reporting, legal, and tax requirements. Our and our collaborators’ information technology systems, some of which are managed by third-parties, such as those of our CROs, may be susceptible to damage, disruptions or shutdowns due to computer viruses, attacks by computer hackers, failures during the process of upgrading or replacing software, databases or components thereof, power outages, hardware failures, telecommunication failures, user errors or catastrophic events. Although we have developed systems and processes that are designed to protect proprietary or confidential information and prevent data loss and other security breaches, including systems and processes designed to reduce the impact of a security breach at a third-party vendor, such measures cannot provide absolute security. If our or our collaborators’ systems are breached or suffer severe damage, disruption or shutdown and we or our collaborators are unable to effectively resolve the issues in a timely manner, our business and operating results may significantly suffer and we may be subject to litigation, government enforcement actions or potential liability.
Security breaches could also cause us to incur significant remediation costs, result in product development delays, disrupt key business operations, including development of our product candidates, and divert attention of management and key information technology resources.
Risks Related to Our Common Stock
Provisions in our corporate charter documents and under Delaware law could make an acquisition of our Company, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our certificate of incorporation and our bylaws may discourage, delay or prevent a merger, acquisition or other change in control of our Company that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. In addition, because our board of directors is responsible for appointing the members of our management team, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Among other things, these provisions:
· establish a classified board of directors such that only one of three classes of directors is elected each year;
· allow the authorized number of our directors to be changed only by resolution of our board of directors;
· limit the manner in which stockholders can remove directors from our board of directors;
· establish advance notice requirements for stockholder proposals that can be acted on at stockholder meetings and nominations to our board of directors;
· require that stockholder actions must be effected at a duly called stockholder meeting and prohibit actions by our stockholders by written consent;
· limit who may call stockholder meetings;
· authorize our board of directors to issue preferred stock without stockholder approval, which could be used to institute a “poison pill” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors; and
· require the approval of the holders of at least two-thirds of the voting power of all of the then-outstanding shares of capital stock that would be entitled to vote generally in the election of directors to amend or repeal specified provisions of our certificate of incorporation or bylaws.
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
Future sales and issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plans or otherwise, could result in dilution to the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital will be needed in the future to continue our planned operations. To raise capital, we may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell additional common stock, convertible securities or other equity securities, investors in a prior transaction may be materially diluted. Additionally, new investors could gain rights, preferences and privileges senior to those of existing holders of our common stock. Further, any future sales of our common stock by us or resale of our common stock by our existing stockholders could cause the market price of our common stock to decline.
As of December 31, 2017, there were 549,726 shares of our common stock available for future grant under our 2014 Equity Incentive Plan. Additionally, as of December 31, 2017, there were outstanding options to purchase up to 3,225,356 shares of our common stock. Any future grants of options, warrants or other securities exercisable or convertible into our common stock, or the exercise or conversion of such shares, and any sales of such shares in the market, could have an adverse effect on the market price of our common stock.
The price of our common stock may be volatile and fluctuate substantially.
Our stock price is likely to be volatile. The stock market in general and the market for smaller biopharmaceutical companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of the underlying companies. As a result of this volatility, the market price of our common stock may fall. The market price for our common stock may be influenced by many factors, including:
· the success of competitive products or technologies;
· results of clinical trials of our product candidates or those of our competitors;
· events affecting our collaboration partners, including Bayer and Array;
· commencement or termination of collaborations for our development programs;
· regulatory or legal developments in the United States and other countries;
· developments or disputes concerning patent applications, issued patents or other proprietary rights;
· the recruitment or departure of key personnel;
· the level of expenses related to any of our product candidates or clinical development programs;
· the results of our efforts to discover, develop, acquire or in- license additional product candidates or products;
· actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts;
· variations in our financial results or those of companies that are perceived to be similar to us;
· changes in the structure of healthcare payment systems;
· market conditions in the pharmaceutical and biotechnology sectors;
· general economic, industry and market conditions; and
· the other factors described in this “Risk Factors” section.
We may be subject to securities litigation, which is expensive and could divert management attention.
Our share price may be volatile, and in the past companies that have experienced volatility in the market price of their stock have been subject to an increased incidence of securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business.
If securities or industry analysts do not publish research or reports about our business, or publish negative reports about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. There can be no assurance that analysts will cover us or provide favorable coverage. If one or more of the analysts who cover us downgrade our stock or change their opinion of our stock, our stock price would likely decline. If one or more of these analysts cease coverage of our Company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our stock price or trading volume to decline.
A significant portion of our total outstanding shares are eligible to be sold into the market, which could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. Sales of a substantial number of shares of our common stock in the public market could occur at any time.
We will continue to incur increased costs as a result of operating as a public company, and our management is required to devote substantial time to compliance initiatives and corporate governance practices.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of The NASDAQ Stock Market and other applicable securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance, which in turn could make it more difficult for us to attract and retain qualified members of our board of directors.
We are evaluating these rules and regulations, and cannot predict or estimate the amount of additional costs we may incur or the timing of such costs. These rules and regulations are often subject to varying interpretations, in many cases due to their lack of
specificity and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
Pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 (“Section 404”) we are required to furnish a report by our management on our internal control over financial reporting at the end of each fiscal year. This assessment includes disclosure of any material weaknesses identified by our management in our internal control over financial reporting. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting that results in more than a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis.
To achieve compliance with Section 404, we have been engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we have dedicated and will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude, that our internal control over financial reporting is effective as required by Section 404. If we identify one or more material weaknesses, it could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
Under Section 382 of the Internal Revenue Code of 1986, as amended (“Internal Revenue Code”), if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period, the corporation’s ability to use its pre-change NOLs and other pre-change tax attributes (such as research tax credits) to offset its post-change income or taxes may be limited. It is possible that we may have triggered an “ownership change” limitation. We may also experience ownership changes in the future as a result of subsequent shifts in our stock ownership (some of which are outside our control). As a result, if we earn net taxable income, our ability to use our pre-change NOL carryforwards to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us. In addition, at the state level, there may be periods during which the use of NOLs is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed.
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
Our corporate headquarters occupy approximately 36,400 square feet of leased office space in Stamford, Connecticut pursuant to a lease that expires in 2025. In November 2017, we amended our existing lease to add expansion space that commenced in February 2018 and to extend the lease term. Under the lease amendment, we have the option to extend the term of the lease for one additional five-year period. We have an option to terminate the original leased space in April 2020 subject to payment of an early termination fee and have an option to terminate the expansion space in December 2023 subject to payment of an early termination fee.
We also lease office space in South San Francisco, California, where we occupy approximately 9,300 square feet, under a lease which expires in 2023, and in Boulder, Colorado, where we occupy approximately 15,300 square feet, under a lease which commenced in October 2017 and expires in 2022. We have the option to terminate the Boulder, Colorado lease after 39 months subject to payment of an early termination fee.
We intend to add additional space if we add employees and expand geographically. We believe that our facilities are adequate to meet our needs for the immediate future, and that, should it be needed suitable additional space will be available on commercially reasonable terms to accommodate any such expansion of our operations.
We are not currently a party to any material legal proceedings and we are not aware of any pending or threatened legal proceeding against us that we believe could have a material adverse effect on our business, operating results or financial condition.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information and Holders
Our common stock is traded on the NASDAQ Global Select Market under the symbol “LOXO.” The table below summarizes the high and low sales prices of our common stock as reported on the Nasdaq Global Market.
|
|
|
High |
|
Low |
| ||
|
Year ended December 31, 2017 |
|
|
|
|
| ||
|
First Fiscal Quarter |
|
$ |
47.46 |
|
$ |
30.51 |
|
|
Second Fiscal Quarter |
|
$ |
83.12 |
|
$ |
41.83 |
|
|
Third Fiscal Quarter |
|
$ |
95.92 |
|
$ |
69.00 |
|
|
Fourth Fiscal Quarter |
|
$ |
95.00 |
|
$ |
71.45 |
|
|
|
|
|
|
|
| ||
|
Year ended December 31, 2016 |
|
|
|
|
| ||
|
First Fiscal Quarter |
|
$ |
28.39 |
|
$ |
16.36 |
|
|
Second Fiscal Quarter |
|
$ |
30.40 |
|
$ |
21.56 |
|
|
Third Fiscal Quarter |
|
$ |
30.19 |
|
$ |
22.62 |
|
|
Fourth Fiscal Quarter |
|
$ |
34.97 |
|
$ |
17.73 |
|
As of February 26, 2018, there were 9 registered holders of record of our common stock, based on information provided by our transfer agent. The actual number of stockholders is greater than this number of registered record holders, and includes stockholders who are beneficial owners, but whose shares are held in “street name” by brokers and other nominees.
Dividends
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business. We do not intend to pay cash dividends on our common stock for the foreseeable future.
Securities Authorized for Issuance under Equity Compensation Plans
The information required by this item will be included in an amendment to this Annual Report on Form 10-K or incorporated by reference from our definitive proxy statement to be filed pursuant to Regulation 14A.
Recent Sales of Unregistered Securities
None.
Stock Price Performance Graph
The graph below matches the Company’s cumulative 41-month total shareholder return on common shares with the cumulative total returns of the Nasdaq Composite Index and the Nasdaq Biotechnology Index. The graph tracks the performance of a $100 investment in Loxo Oncology’s common shares and in each index (with the reinvestment of all dividends) from August 1, 2014 to December 31, 2017.
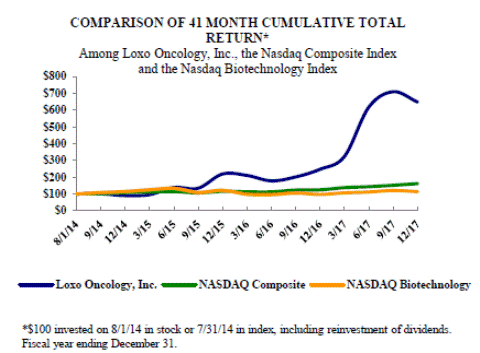
|
|
|
8/1/14 |
|
9/14 |
|
12/14 |
|
3/15 |
|
6/15 |
|
9/15 |
|
12/15 |
|
3/16 |
|
6/16 |
|
9/16 |
|
12/16 |
|
3/17 |
|
6/17 |
|
9/17 |
|
12/17 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loxo Oncology, Inc. |
|
100.00 |
|
101.00 |
|
90.38 |
|
95.77 |
|
138.69 |
|
134.46 |
|
218.85 |
|
210.31 |
|
178.31 |
|
201.38 |
|
247.04 |
|
323.69 |
|
616.85 |
|
708.62 |
|
647.54 |
|
|
Nasdaq Composite |
|
100.00 |
|
102.60 |
|
108.15 |
|
112.03 |
|
114.55 |
|
106.55 |
|
115.65 |
|
112.92 |
|
112.60 |
|
123.48 |
|
124.90 |
|
137.62 |
|
143.31 |
|
151.75 |
|
161.66 |
|
|
Nasdaq Biotechnology |
|
100.00 |
|
108.47 |
|
114.95 |
|
125.51 |
|
133.50 |
|
109.83 |
|
121.92 |
|
97.72 |
|
95.44 |
|
104.99 |
|
97.29 |
|
106.54 |
|
111.69 |
|
120.47 |
|
113.93 |
|
The stock price performance included in this graph is not necessarily indicative of future stock price performance.
ITEM 6. SELECTED FINANCIAL DATA
The following table sets forth certain selected consolidated financial data. The information should be read in conjunction with Management’s Discussion and Analysis of Financial Condition and Results of Operations and our financial statements and notes thereto appearing elsewhere in this report (in thousands, except per share data).
|
|
|
For the Years Ended December 31, |
|
Period From |
| |||||||||||
|
|
|
2017 |
|
2016 |
|
2015 |
|
2014 |
|
2013 |
| |||||
|
Statement of Operations Data: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Net loss attributable to common stockholders |
|
$ |
(148,876 |
) |
$ |
(72,398 |
) |
$ |
(35,876 |
) |
$ |
(20,706 |
) |
$ |
(10,302 |
) |
|
Net loss per share of common stock—basic and diluted |
|
$ |
(5.31 |
) |
$ |
(3.46 |
) |
$ |
(2.12 |
) |
$ |
(3.06 |
) |
$ |
(70.79 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Total assets |
|
$ |
783,442 |
|
$ |
145,312 |
|
$ |
157,458 |
|
$ |
114,459 |
|
$ |
15,022 |
|
|
Total stockholders’ equity (deficit) |
|
$ |
378,210 |
|
$ |
130,168 |
|
$ |
154,604 |
|
$ |
112,672 |
|
$ |
(10,231 |
) |
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and related notes appearing in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the “Risk Factors” section of this Annual Report on Form 10-K, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis. As used in this Form 10-K, unless the context suggests otherwise, “we,” “us,” “our,” “the Company” or “Loxo” refer to Loxo Oncology, Inc.
Overview
Loxo Oncology is a biopharmaceutical company innovating the development of highly selective medicines for patients with genetically defined cancers. Our pipeline focuses on cancers that are uniquely dependent on single gene abnormalities, such that a single drug has the potential to treat the cancer with dramatic effect. We believe that the most selective, purpose-built medicines have the highest probability of maximally inhibiting the intended target, thereby delivering best-in-class disease control and safety. Our management team seeks out experienced industry partners, world-class scientific advisors and innovative clinical-regulatory approaches to deliver new cancer therapies to patients as quickly and efficiently as possible.
With our scientific knowledge, collaborative partnerships and targeted approach, we are developing multiple small molecule therapeutics utilizing focused clinical development strategies in well-defined patient populations. Larotrectinib, a selective TRK inhibitor currently in clinical development, is being evaluated in three ongoing multi-center studies that include patients with solid tumors that harbor TRK gene fusions. In December 2017, we announced that we initiated the submission of a rolling NDA to the FDA for larotrectinib for “the treatment of unresectable or metastatic solid tumors with NTRK-fusion proteins in adult and pediatric patients who require systemic therapy and who have either progressed following prior treatment or who have no acceptable alternative treatments.” We expect to complete the NDA submission by the end of March 2018. We also have programs in development for TRK (LOXO-195), RET (LOXO-292), BTK (LOXO-305), FGFR and other targets.
Since inception, we have incurred significant operating losses. Our net loss for the year ended December 31, 2017 was $148.9 million, including approximately $140.0 million of total research and development expenses, and approximately $33.7 million of total general and administrative expenses. We expect to incur significant expenses and increasing operating losses for the foreseeable future as we continue the discovery, development and clinical trials of, and seek regulatory approval for and pursue potential commercialization of, our product candidates. In addition, we will also incur additional expenses if and as we enter into additional collaboration agreements, acquire or in-license products and technologies, expand our collaboration with Array, enter into companion diagnostics collaborations, establish sales, marketing and distribution infrastructure and/or expand and protect our intellectual property portfolio.
We will need to obtain substantial additional funding in connection with our continuing operations. We will seek to fund our operations through the sale of equity, debt financings or other sources, our collaboration with Bayer and other potential collaborations. We may be unable to raise additional funds or enter into such other agreements when needed on favorable terms, or at all. If we fail to raise capital or enter into such other arrangements as, and when, needed, we may have to significantly delay, scale back or discontinue the development and commercialization of one or more of our product candidates.
Bayer License, Development and Commercialization Agreement
In November 2017, we entered into a license, development and commercialization agreement with Bayer pursuant to which we and Bayer will collaborate to develop and commercialize larotrectinib and LOXO-195, our franchise of highly selective TRK inhibitors for patients with TRK fusion cancers. Pursuant to the Bayer Agreement, we granted co-exclusive development and commercialization licenses to Bayer for both larotrectinib and LOXO-195.
In addition to an upfront cash payment of $400.0 million, we are eligible to receive $450.0 million in milestone payments upon larotrectinib regulatory approvals and first commercial sale events in certain major markets and an additional $200.0 million in milestone payments upon LOXO-195 regulatory approvals and first commercial sale events in certain major markets.
We will lead global development activities and regulatory activities in the U.S. Bayer will lead regulatory activities outside the U.S. and global commercial activities. Globally, we will be responsible for 50% of development costs. In the U.S., where we and Bayer will co-promote the products, we will be responsible for 50% of the commercial costs and receive 50% of the profits. Bayer will pay us a $25.0 million milestone upon achieving a certain U.S. net sales threshold. We will have the right to opt-out of the U.S. co-promotion, in which case we would receive a royalty in the low thirties percent range on U.S. net sales, which is meant to approximate the economics of the 50/50 profit split.
Outside of the U.S., where Bayer will commercialize, Bayer will pay us tiered, double digit royalties on net sales, and sales milestones totaling $475.0 million.
The Bayer Agreement also includes a standstill provision that prevents Bayer from acquiring five percent or more of our voting securities.
The Bayer Agreement will terminate as to a product or country upon the expiration of the royalty term applicable to such product in such country. The Bayer Agreement may be terminated by either party for material breach or bankruptcy. In addition, Bayer may terminate the Bayer Agreement after the fourth anniversary of the effective date upon written notice to us, or in the event that we receive a “complete response letter” from the FDA with respect to larotrectinib, or if we do not receive marketing approval for larotrectinib by December 31, 2018.
Components of Operating Results
Revenue from Collaboration Agreement
Our revenue from collaboration agreement is derived from payments we receive under the license, development and commercialization collaboration agreement with Bayer. This currently includes a portion of the upfront payment, to be recognized as revenue over time using a proportional performance method as the related research and development activities are performed by us.
Research and Development Expenses with Related Party
Our research and development expenses with Array, a related party through December 31, 2015, relate to discovery, preclinical and manufacturing activities as defined within our collaboration agreement with Array. As of December 31, 2015, Array has indicated that it is no longer a holder of more than 5% of our capital stock; therefore, we will not report expenses with Array as a related party in future reporting periods, as applicable.
Research and Development Expenses
Research and development costs are charged to expense as incurred. These costs include, but are not limited to, employee-related expenses, including salaries, benefits, stock-based compensation and travel as well as expenses related to asset acquisitions of IPR&D, third-party collaborations, contract research arrangements and activities associated with the development of companion diagnostics for our product candidates.
Research and development activities are central to our business model. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. As we advance our product candidates, we expect the amount of external research and development will continue to increase for the foreseeable future, while our internal spending should increase at a slower and more controlled pace.
It is difficult to determine with certainty the duration and completion costs of our current or future preclinical programs and clinical trials of our product candidates, or if, when or to what extent we will generate revenue from the commercialization and sale of any of our product candidates that obtain regulatory approval. We may never succeed in achieving regulatory approval for any of our product candidates. The duration, costs and timing of clinical trials and development of our product candidates will depend on a variety of factors, including the uncertainties of future clinical and preclinical studies, uncertainties in clinical trial enrollment rate and significant and changing government regulation. In addition, the probability of success for each product candidate will depend on numerous factors, including competition, manufacturing capability and commercial viability. We will determine which programs to pursue and how much to fund each program in response to the scientific and clinical success of each product candidate, as well as an assessment of each product candidate’s commercial potential.
General and Administrative Expenses
General and administrative expenses consist principally of salaries and related costs for executive and other personnel, including stock-based compensation and travel expenses. General and administrative expenses also include facility-related costs, communication expenses and professional fees for legal, patent prosecution and maintenance, consulting and accounting services.
Interest Income, net
Interest income consists principally of the interest earned from our short-term and long-term investments, partially offset by the amortization of discounts recorded in connection with the purchase of certain investments.
Critical Accounting Policies and Significant Judgments and Estimates
This discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with generally accepted accounting principles in the U.S. (“GAAP”). The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the
reported period. In accordance with GAAP, we base our estimates on historical experience and on various other assumptions that we believe are reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 2 to the Financial Statements appearing elsewhere in this Form 10-K, we believe the following accounting policies are critical to the preparation of our financial statements.
Revenue Recognition
We entered into the Bayer Agreement in November 2017, which is within the scope of ASC 808, Collaborative Arrangements. Under the Bayer Agreement, we have licensed certain rights to our larotrectinib and LOXO-195 product candidates to Bayer. The terms include payment to us of one or more of the following: a non-refundable, up-front license fee, regulatory and commercial milestone payments, and royalties on net sales of licensed products.
Licenses of intellectual property: If the license of our intellectual property is determined to be a separate unit of accounting from the other performance obligations identified in the arrangement, we recognize revenue from non-refundable, up-front fees allocated to the license when the license is transferred to the collaborative partner and the collaborative partner is able to use and benefit from the license. For licenses that are bundled with other promises, such as development activities, we recognize revenue over time, using a proportional performance method as the related development activities are performed. Up-front payments are recorded as deferred revenue upon receipt and require deferral of revenue recognition to a future period until we perform our obligations under these arrangements. Amounts payable to us are recorded as accounts receivable when our right to consideration is unconditional.
Milestone payments: Regulatory or commercial milestone payments will be recognized as revenue in the period the milestone is achieved. To date, we have not recognized any milestone payments as revenue resulting from our collaboration arrangement.
Co-promote: In the United States, where the Company and Bayer will co-promote the products, the Company will be responsible for 50% of the commercial costs and receive 50% of the profits. Co-promote net cost/profit will be recognized when the related expenses and sales occur. To date, the Company has not recognized any co-promote net cost/profit resulting from its collaboration arrangement.
Royalties: Sales-based royalties, including milestone payments based on the level of sales, will be recognized when the related sales occur. To date, we have not recognized any royalty revenue resulting from our collaboration arrangement.
Research and Development Expenses
Research and development costs are charged to expense as incurred. These costs include, but are not limited to, expenses incurred under the Array Agreement, costs with contract service organizations for certain preclinical and clinical studies and chemistry, manufacturing and controls (“CMC”) related expenses based on our evaluation of the progress to completion of specific tasks using data such as information provided to us by our vendors on their actual costs incurred. Payments for these activities are based on the terms of the individual arrangements, which may differ from the pattern of costs incurred, and are reflected in the financial statements as prepaid or accrued research and development expense, as the case may be. Under the Bayer Agreement, we will receive reimbursement for 50% of our development activity expenses incurred for larotrectinib and LOXO-195 beginning January 1, 2018.
The following table shows, for each of the years ended, (i) collaboration expenses, (ii) third party expenses for research and development, on a project-by-project basis, and (iii) our unallocated research and development operating expenses. We use our employee and infrastructure resources across several projects, and many of our costs are not attributable to an individually named project, but are broadly applicable research projects. Accordingly, we do not account for internal research and development costs on a project-by-project basis.
|
|
|
Year Ended |
|
Year Ended |
|
Year Ended |
| |||
|
(in thousands) |
|
December 31, 2017 |
|
December 31, 2016 |
|
December 31, 2015 |
| |||
|
Array multi-target collaboration expenses |
|
$ |
10,812 |
|
$ |
17,457 |
|
$ |
11,611 |
|
|
Third party research and development expenses: |
|
|
|
|
|
|
| |||
|
Larotrectinib (TRK inhibitor) |
|
47,029 |
|
20,656 |
|
6,493 |
| |||
|
LOXO-292 (RET inhibitor) |
|
15,005 |
|
6,466 |
|
— |
| |||
|
LOXO-195 (TRK inhibitor) |
|
5,107 |
|
4,520 |
|
— |
| |||
|
LOXO-305 (BTK inhibitor) |
|
2,161 |
|
— |
|
— |
| |||
|
Acquisition of in process R&D (IPR&D) |
|
40,000 |
|
— |
|
— |
| |||
|
Other research programs and unallocated expenses |
|
19,925 |
|
9,176 |
|
7,463 |
| |||
|
Total research and development expenses |
|
$ |
140,039 |
|
$ |
58,275 |
|
$ |
25,567 |
|
Income Taxes
Income taxes are recorded in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codifications (“ASC”) Topic 740, Income Taxes (“ASC 740”), which provides for deferred taxes using an asset and liability approach. We recognize deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Deferred tax assets and liabilities are determined based on the differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Valuation allowances are provided if based upon the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
We account for uncertain tax positions in accordance with the provisions of ASC 740. When uncertain tax positions exist, we recognize the tax benefit of tax positions to the extent that the benefit will more likely than not be realized. The determination as to whether the tax benefit will more likely than not be realized is based upon the technical merits of the tax position as well as consideration of the available facts and circumstances.
We have incurred substantial losses during our history. To the extent that we continue to generate tax losses, unused losses will carry forward to offset future taxable income, if any, until such unused losses expire. Under Section 382 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change,” which is generally defined as a greater than 50% change, by value, in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes to offset its post-change income may be limited. See the “Income Taxes” footnote in our Consolidated Financial Statements.
On December 22, 2017, the Tax Cuts and Jobs Act (the “2017 Tax Act”) was enacted, which includes significant changes to existing U.S. tax laws including, among other things, a reduction of the U.S. corporate income tax rate from 35% to 21% effective for tax years beginning after December 31, 2017. We have calculated our reasonable estimate of the impact from the 2017 Tax Act in our year-end income tax provision. See the “Income Taxes” footnote in our Consolidated Financial Statements.
On December 22, 2017, the SEC issued Staff Accounting Bulletin No. 118 (“SAB 118”) to address the application of U.S. GAAP when a SEC registrant does not have the necessary information available, compiled, analyzed, or reviewed in sufficient detail to complete the accounting for certain income tax effects from the 2017 Tax Act. In accordance with SAB 118, we have made a reasonable estimate of the effects on our existing U.S. deferred tax balances. Additional work is necessary to produce more detailed analyses as well as evaluate potential correlative adjustments. See the “Income Taxes” footnote in our Consolidated Financial Statements.
Stock-Based Compensation
We apply the fair value recognition provisions of ASC Topic 718, Compensation—Stock Compensation (“ASC 718”). Determining the amount of stock-based compensation to be recorded requires us to develop estimates of the fair value of stock options as of their grant date. Calculating the fair value of share-based awards requires that we make highly subjective assumptions.
Stock-based compensation cost of our employees and members of our board of directors is measured at the date of grant based on the estimated fair value of the award, net of estimated forfeitures. We estimate the grant date fair value and the resulting stock-based compensation expense using the Black-Scholes option pricing model. The use of the Black-Scholes option pricing model requires us to make assumptions with respect to the expected term of the option, the expected volatility of the common stock consistent with the expected life of the option, risk-free interest rates, the value of the common stock and expected dividend yields of the common stock. The grant date fair value of a stock-based award is recognized as an expense over the requisite service period of the award on a straight-line basis. Forfeitures are required to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
We account for stock-based compensation arrangements with other non-employees using a fair value approach. The fair value of these options is measured using the Black-Scholes option pricing model reflecting an expected life that is assumed to be the remaining contractual life of the option. The compensation costs of these arrangements are subject to remeasurement over the vesting terms as earned.
Basic and Diluted Net Loss Per Share of Common Stock
Basic net loss per share of common stock is computed by dividing net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding during the period, excluding the dilutive effects of convertible preferred stock, unvested restricted stock and stock options. Diluted net loss per share of common stock is computed by dividing the net loss attributable to common stockholders by the sum of the weighted-average number of shares of common stock outstanding during the period plus the potential dilutive effects of convertible preferred stock, unvested restricted stock and stock options outstanding during the period calculated in accordance with the treasury stock method, although these shares and options are excluded if their effect is anti-
dilutive. Because the impact of these items is anti-dilutive during periods of net loss, there was no difference between basic and diluted net loss per share of common stock for the years ended December 31, 2017, 2016 and 2015.
Recently Adopted Accounting Pronouncements
In January 2017, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2017-01, which provides additional guidance on evaluating whether transactions should be accounted for as acquisitions of assets or businesses. ASU 2017-01 requires an entity to evaluate if substantially all of the fair value of the assets acquired is concentrated in a single identifiable asset or a group of similar identifiable assets. If this threshold is met, the new guidance would define this as an asset acquisition; otherwise, the entity then evaluates whether the asset meets the requirement that a business include, at a minimum, an input and substantive process that together significantly contribute to the ability to create outputs. We early adopted ASU 2017-01 in the third quarter of 2017. Refer to Note 12 for discussion on our acquisition of in process research and development (“IPR&D”) from Redx Pharma Plc and Redx Oncology Limited (collectively, “Redx”), which was accounted for as an asset acquisition under this new guidance.
In March 2016, the FASB issued ASU 2016-09, which provides for simplification of certain aspects of employee share-based payment accounting including income taxes, classification of awards as either equity or liabilities, accounting for forfeitures and classification on the statement of cash flows. ASU 2016-09 was effective for the Company in the first quarter of 2017. The standard requires the recognition of any pre-adoption date net operating loss (“NOL”) carryforwards from share-based compensation arrangements to be recognized on a modified retrospective basis, through an opening retained earnings adjustment on January 1, 2017. Any income tax effects from share-based compensation arrangements arising after January 1, 2017 will be recognized prospectively in the income statement. Upon adoption, we recognized all previously unrecognized tax benefits. These previously unrecognized tax benefits were recorded as a deferred tax asset, which was fully offset by a valuation allowance on January 1, 2017, thus there was no net impact from the adoption of ASU 2016-09 as of the same date. Our adoption of the standard did not have any impact to the consolidated statements of cash flows as no NOL carryforwards from share-based compensation arrangements were recognized prior to January 1, 2017. We have elected to continue to estimate forfeitures under the true-up provision of ASC 718.
In November 2015, the FASB issued ASU 2015-17, which eliminates the current requirement to present deferred tax liabilities and assets as current and noncurrent in a classified balance sheet. Instead, entities will be required to classify all deferred tax assets and liabilities as noncurrent. ASU 2015-17 was effective for us in the first quarter of 2017. The adoption of the ASU 2015-17 did not have a material impact on our financial statements.
Recent Accounting Pronouncements Not Yet Adopted
In November 2016, the FASB issued ASU No. 2016-18, Statement of Cash Flows (Topic 230): Restricted Cash (“ASU 2016-18”), which amended the existing accounting standards for the statement of cash flows by requiring restricted cash to be included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. ASU 2016-18 will be effective in fiscal years beginning after December 15, 2017, including interim periods within those fiscal years, and early adoption is permitted. The amendments should be applied retrospectively to all periods presented. We do not expect the adoption of ASU 2016-18 will have a material impact on our financial statements and related disclosures.
In August 2016, the FASB issued ASU No. 2016-15, Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments (“ASU 2016-15”), which amended the existing accounting standards for the statement of cash flows by providing guidance on eight classification issues related to the statement of cash flows. ASU 2016-15 will be effective in fiscal years beginning after December 15, 2017, including interim periods within those fiscal years, and early adoption is permitted. The amendments should be applied retrospectively to all periods presented. For issues that are impracticable to apply retrospectively, the amendments may be applied prospectively as of the earliest date practicable. We do not expect the adoption of ASU 2016-15 will have a material impact on our financial statements and related disclosures.
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842) (“ASU 2016-02”), which requires lessees to recognize assets and liabilities for the rights and obligations created by most leases on their balance sheet. The guidance is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. Early application is permitted. ASU 2016-02 requires modified retrospective adoption for all leases existing at, or entered into after, the date of initial application, with an option to use certain transition relief. We are currently evaluating the impact the standard may have on our financial statements and related disclosures.
In January 2016, the FASB issued ASU No. 2016-01, Financial Instruments - Overall (Subtopic 825-10), Recognition and Measurement of Financial Assets and Financial Liabilities (“ASU 2016-01”), which addresses certain aspects of recognition, measurement, presentation, and disclosure of financial instruments. ASU 2016-01 will be effective for annual periods and interim periods within those annual periods beginning after December 15, 2017 and early adoption is not permitted. We do not expect the adoption of ASU 2016-01 will have a material impact on our financial statements and related disclosures.
In May 2014, the FASB issued ASU No. 2014-09, which amends the guidance for accounting for revenue from contracts with customers. This ASU supersedes the revenue recognition requirements in ASC Topic 605, Revenue Recognition, and creates a new ASC Topic 606, Revenue from Contracts with Customers. Customers. Subsequent to May 2014, the FASB issued additional guidance
that delayed the effective date and clarified various aspects of the new guidance, including principal versus agent considerations, identifying performance obligations and licensing, and also included other improvements and practical expedients. ASU 2014-09 will be effective for annual periods and interim periods within those annual periods beginning after December 15, 2017, early adoption is permitted. We do not expect the adoption of ASU 2014-09 will have a material impact on our financial statements and related disclosures as we do not currently have any contracts with customers subject to the guidance in ASC 606.
Results of Operations
Comparison of Results of Operations (in thousands)
|
|
|
Year Ended |
|
Year Ended |
|
Year Ended |
| |||
|
|
|
December 31, 2017 |
|
December 31, 2016 |
|
December 31, 2015 |
| |||
|
Revenue from collaboration agreement |
|
$ |
21,300 |
|
$ |
— |
|
$ |
— |
|
|
|
|
|
|
|
|
|
| |||
|
Operating expenses: |
|
|
|
|
|
|
| |||
|
Research and development with related party |
|
— |
|
— |
|
11,611 |
| |||
|
Research and development |
|
140,039 |
|
58,275 |
|
13,956 |
| |||
|
General and administrative |
|
33,657 |
|
14,903 |
|
10,508 |
| |||
|
Total operating expenses |
|
(173,696 |
) |
(73,178 |
) |
(36,075 |
) | |||
|
Loss from operations |
|
$ |
(152,396 |
) |
$ |
(73,178 |
) |
$ |
(36,075 |
) |
Revenue from collaboration agreement
For the year ended December 31, 2017, we recognized $21.3 million of revenues under the Bayer Agreement. This amount represents the amount of the $400.0 million upfront license fee recognized as revenue using a proportional performance method based on actual research and development costs incurred from the effective date of the Bayer Agreement as a percentage of the current projection of total budgeted development costs. We did not recognize any revenue from collaboration agreements during the years ended December 31, 2016 and 2015.
Research and development expenses with related party
There were no research and development expenses with related party for the years ended December 31, 2017 or 2016. There were $11.6 million of related party research and development expenses in 2015. This decrease is due to the fact that subsequent to December 31, 2015, Array is not a related party as it no longer holds more than 5% of our capital stock.
Research and development expenses
Research and development expenses were $140.0 million for the year ended December 31, 2017, compared to $58.3 million for the year ended December 31, 2016. The increase was primarily due to the $40.0 million asset acquisition of the BTK inhibitor program from Redx, expanded development activities including clinical costs, chemistry, manufacturing and controls (CMC) related expenses and costs related to companion diagnostics development, as well as additional expenses related to our other programs. We also had higher headcount and employment-related costs, as well as higher stock-based compensation costs. As a result, we had increases in larotrectinib development expenses of $26.4 million, LOXO-292 development expenses of $8.5 million, LOXO-305 development costs of $2.2 million, LOXO-195 development expenses of $0.6 million, stock-based compensation of $6.0 million and employment and other costs of $4.6 million. This was offset by a net decrease in Array full-time equivalents and milestones of $6.6 million.
Research and development expenses were $58.3 million for the year ended December 31, 2016, compared to $14.0 million for the year ended December 31, 2015. The increase in research and development expense was primarily due to timing in which our clinical development efforts expanded during 2015 and into 2016 including site and patient enrollment in our Phase 1 and Phase 2 adult and Phase 1/2 pediatric clinical trials for larotrectinib, as well as additional expenses related to the preclinical pipeline. We also increased our internal headcount during 2015 and into 2016. As a result, we had increases in our clinical costs, preclinical costs, employment costs and stock compensation costs of $11.5 million, $19.4 million, $1.7 million and $0.2 million, respectively. The remaining increase was primarily due to the inclusion of Array expenditures totaling $17.4 million in research and development expense for the year ended December 31, 2016 rather than in research and development expense with related party. The increase in Array expenditures compared to the amount included in research and development expense with related party in the year ended December 31, 2015 was primarily related to $6.5 million in collaboration milestones which were achieved.
General and administrative expenses
General and administrative expenses were $33.7 million for the year ended December 31, 2017, compared to $14.9 million for the year ended December 31, 2016. The increase was primarily due to increases in preparation activities for the potential commercialization of larotrectinib of $5.4 million, general and administrative professional fees of $3.8 million, stock-based compensation expense of $5.4 million and employment costs and other costs of $4.2 million.
General and administrative expenses were $14.9 million for the year ended December 31, 2016, compared to $10.5 million for the year ended December 31, 2015. This increase was primarily attributable to employment costs, professional fees, stock-based compensation expense and rent expense of $0.5 million, $1.7 million, $1.6 million and $0.4 million, respectively.
Liquidity and Capital Resources
Our financial statements and related disclosures have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. Accordingly, the financial statements do not include any adjustments that might be necessary should we be unable to continue in existence. We have not generated any revenues and have not yet achieved profitable operations. There is no assurance that profitable operations, if ever achieved, could be sustained on a continuing basis. In addition, development activities, clinical and preclinical testing, and commercialization of our products will require significant additional financing. We incurred a net loss of $148.9 million for the year ended December 31, 2017. Net cash provided by operating activities was $107.8 million during the year ended December 31, 2017. At December 31, 2017, we had an accumulated deficit of $288.1 million and working capital of $560.2 million. Aggregate cash, cash equivalents, and short-term investments were $626.2 million as of December 31, 2017. Management expects to incur substantial and increasing losses in future periods.
Our ability to successfully pursue our business is subject to certain risks and uncertainties, including among others, uncertainty of product development, competition from third parties, uncertainty of capital availability, uncertainty in our ability to enter into agreements with collaborative partners, dependence on third parties, and dependence on key personnel. Historically, we have financed our operations principally through private placements of preferred stock, our initial public offering of common stock and follow-on offerings of common stock. We plan to finance future operations with a combination of proceeds from the issuance of equity, debt and other sources, our collaboration with Bayer and other potential collaborations and revenues from future product sales, if any. We have not generated positive cash flows from operations, and there are no assurances that we will be successful in obtaining an adequate level of financing for the development and commercialization of our planned products. We believe that our existing cash, cash equivalents and investments as of December 31, 2017, will be sufficient to enable us to continue as a going concern through at least March 1, 2019.
Cash Flows
The following table summarizes our cash flows for the years ended December 31, 2017, 2016 and 2015 (in thousands):
|
|
|
Year Ended |
|
Year Ended |
|
Year Ended |
| |||
|
Net cash provided by (used in): |
|
|
|
|
|
|
| |||
|
Operating activities |
|
$ |
107,763 |
|
$ |
(51,318 |
) |
$ |
(30,182 |
) |
|
Investing activities |
|
(374,161 |
) |
(26,489 |
) |
(17,177 |
) | |||
|
Financing activities |
|
378,047 |
|
40,006 |
|
71,606 |
| |||
|
Net increase (decrease) in cash and cash equivalents |
|
$ |
111,649 |
|
$ |
(37,801 |
) |
$ |
24,247 |
|
Net cash used in operating activities
Net cash provided by operating activities was $107.8 million for the year ended December 31, 2017 and consisted primarily of an increase in deferred revenues of $228.7 million, an increase in accrued expenses and other current liabilities of $8.5 million, an increase in accounts payable of $3.0 million and noncash expenses of $19.6 million, primarily attributable to stock-based compensation expense. This increase was offset by a net loss of $148.9 million and a decrease in prepaid expenses and other current assets of $3.1 million. The increase in deferred revenues represents the $400 million upfront payment related to the Bayer Agreement that will be recognized into revenue using a proportional performance method over time as development activities are performed by us, net of an $150 million receivable from Bayer which represents the second installment of the total $400 million upfront payment.
Net cash used in operating activities was $51.3 million for the year ended December 31, 2016 and consisted primarily of a net loss of $72.4 million. This was partially offset by noncash expenses of $8.6 million, primarily attributable to stock-based compensation expense and a $12.1 million increase in our net operating assets, related to the timing of payments for our clinical and preclinical activities.
Net cash used in operating activities was $30.2 million for the year ended December 31, 2015 and consisted primarily of a net loss of $35.9 million and a $0.9 million increase in our net operating assets. The increase in our net operating assets was primarily due to the timing of payments related to our clinical and preclinical activities. This activity was partially offset by noncash expenses of $6.6 million, primarily attributable to stock-based compensation expense.
Net cash used in investing activities
Net cash used in investing activities for the year ended December 31, 2017 totaled $374.2 million and consisted primarily of $574.5 million of available-for-sale security purchases partially offset by $201.1 million of proceeds from maturing available-for-sale securities.
Net cash used in investing activities for the year ended December 31, 2016 totaled $26.5 million and consisted primarily of $163.3 million of available-for-sale security purchases partially offset by $137.0 million of proceeds from maturing available-for-sale securities.
Net cash used in investing activities for the year ended December 31, 2015 totaled $17.2 million and consisted primarily of $133.7 million of available-for-sale security purchases partially offset by $116.7 million of proceeds from maturing available-for-sale securities.
Net cash provided by financing activities
Net cash provided by financing activities was $378.0 million for the year ended December 31, 2017, which was primarily due to $375.3 million in net proceeds from the sale and issuance of our common stock in January 2017 and June 2017. We also received $2.7 million in proceeds from the exercise of employee stock options.
Net cash provided by financing activities was $40 million for the year ended December 31, 2016, which was primarily due to $38.7 million in net proceeds from the sale and issuance of our common stock in May 2016. We also received $1.3 million in proceeds from the exercise of employee stock options.
Net cash provided by financing activities was $71.6 million for the year ended December 31, 2015, which was primarily due to $71.3 million in net proceeds from the issuance of our common stock in November 2015. We also received $0.3 million in proceeds from stock option exercises.
Operating and Capital Expenditure Requirements
We have not achieved profitability since our inception and we expect to continue to incur net losses for the foreseeable future. We expect our cash expenditures to increase in the near term as we fund the larotrectinib, LOXO-292 and LOXO-195 clinical trials, prepare for potential larotrectinib commercialization, establish companion diagnostics collaborations, fund clinical trials of our other preclinical product candidates and continue other preclinical activities.
As a publicly traded company, we incur significant legal, accounting and other expenses that we were not required to incur as a private company. In addition, the Sarbanes-Oxley Act of 2002, as well as rules adopted by the SEC and The Nasdaq Stock Market, requires public companies to implement specified corporate governance practices that were inapplicable to us as a private company. We expect these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly.
We anticipate that we will need to raise additional capital in the future to fund our operations. In order to meet these additional cash requirements, we may incur debt, license certain intellectual property and seek to sell additional equity or convertible securities that may result in dilution to our stockholders. If we raise additional funds through the issuance of equity or convertible securities, these securities could have rights or preferences senior to those of our common stock and could contain covenants that restrict our operations. There can be no assurance that we will be able to obtain additional equity or debt financing on terms acceptable to us, if at all. Our future capital requirements will depend on many factors, including:
· the progress and results of the clinical programs for larotrectinib, LOXO-292 and LOXO-195;
· the number and development requirements of any other product candidates that we pursue;
· our ability to enter into collaborative agreements for the development and commercialization of our product candidates;
· the scope, progress, results and costs of researching and developing our product candidates or any future product candidates, both in the U.S. and outside the U.S.;
· the costs, timing and outcome of regulatory review of our product candidates or any future product candidates, both in the U.S. and outside the U.S.;
· the costs and timing of future commercialization activities, including product manufacturing, marketing, sales and distribution, for any of our product candidates for which we receive marketing approval;
· the costs, timing and outcome of our companion diagnostics collaborations;
· any product liability or other lawsuits related to our products;
· the ability to achieve milestones associated with the Bayer collaboration;
· the expenses needed to attract and retain skilled personnel;
· the general and administrative expenses related to being a public company, including developing an internal accounting function;
· the revenue, if any, received from the commercialization of our product candidates for which we receive marketing approval; and
the costs involved in preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending our intellectual property-related claims, both in the U.S. and outside the U.S.
See Part I, Item 1A, “Risk Factors” for additional risks associated with our substantial capital requirements.
If we are unable to successfully raise sufficient additional capital, through future equity financings, product sales, debt or other sources, our collaboration with Bayer and other potential collaborations, we will not have sufficient cash flows and liquidity to fund our planned business operations. In that event, we might be forced to limit many, if not all, of our programs and consider other means of creating value for our stockholders, such as licensing to others the development and commercialization of products that we consider valuable and would otherwise likely develop internally. To the extent that we raise additional capital through marketing and distribution arrangements or other collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our product candidates, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us. If we do raise additional capital through public or private equity offerings, the ownership interest of our existing stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect our stockholders’ rights or restrict our operations. If we raise additional capital through debt financing, we may be subject to covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
Contractual Obligations and Commitments
The following is a summary of our long-term contractual cash obligations as of December 31, 2017 (in thousands):
|
|
|
Total |
|
Less |
|
2 - 3 Years |
|
4 - 5 Years |
|
More |
| |||||
|
Operating lease obligations (1) |
|
$ |
15,544 |
|
$ |
1,627 |
|
$ |
4,777 |
|
$ |
5,192 |
|
$ |
3,948 |
|
|
Collaboration obligations (2) |
|
7,208 |
|
7,208 |
|
— |
|
— |
|
— |
| |||||
|
Total contractual obligations |
|
$ |
22,752 |
|
$ |
8,835 |
|
$ |
4,777 |
|
$ |
5,192 |
|
$ |
3,948 |
|
(1) Operating lease obligations reflect our obligation to make payments in connection with the leases for our office spaces. We have the option to extend the term of the Stamford, CT lease for one additional five-year period, and we have options to terminate the original lease after 5.5 years and the amended lease after 5.5 years subject to payment of early termination fees both of which are excluded from this table. We have the option to extend the term of the South San Francisco, CA lease for one additional three-year period which is excluded from this table. We have the option to extend the term of the Boulder, CO lease for two additional five-year periods, and we have the option to terminate the lease after 39 months subject to payment of an early termination fee both of which are excluded from this table. (See Note 9 to the financial statements under Item 8 of this Form 10-K).
(2) The table includes non-cancelable obligations through December 2018 with Array for providing full-time equivalents and other support. In the second quarter of 2017, we extended the term of the collaboration agreement with Array for one year, through September 30, 2018. The table excludes milestones and royalties that may become payable under the Array Agreement. See “Array Collaboration” below for details.
Purchase Commitments
Other than amounts due for the leases of our locations in Stamford, Connecticut, South San Francisco, California and Boulder, Colorado offices (see Note 9 to the financial statements under Item 8 of this Form 10-K) and under the Array collaboration agreement, as described below, we have no material non-cancelable purchase commitments with contract manufacturers or service providers as we have generally contracted on a cancelable basis.
Array Collaboration
On July 3, 2013, the Company signed a multi-year strategic collaboration agreement with Array, and this agreement was subsequently amended on November 26, 2013, April 10, 2014, October 13, 2014, March 31, 2015 and February 18, 2016. Under the terms of the collaboration agreement, the Company obtained certain rights to Array’s tropomyosin receptor kinase inhibitor program, as well as additional novel oncology targets, including RET, and FGFR. The Company has worldwide commercial rights to each product candidate from the collaboration and Array participates in any potential successes through milestones and royalties.
With respect to the discovery and preclinical program, the collaboration agreement, as amended, runs through September 30, 2017, and the Company has the option to extend the term for up to one additional one-year renewal period by providing written notice
to Array at least three months before the end of the initial discovery and preclinical development programs term. This option was exercised during the three-month period ended June 30, 2017.
Before the February 2016 amendment, in addition to larotrectinib the parties designated 12 discovery targets, of which seven were selected for additional study in January 2015, which was to be reduced to four on or before January 2016. The Company had the option to maintain the total target number at five for an additional payment, and the Company exercised this option to maintain five discovery programs in January 2016. In the February 2016 amendment, the parties designated a total of six discovery targets. An additional payment was due at contract signing, satisfying a prior obligation of the April 2014 amendment
As part of the Array Agreement, as amended, we agreed to pay Array a fixed amount per month, based on Array’s commitment to provide full-time equivalents and other support relating to the conduct of the discovery and preclinical development programs. For the years ended December 31, 2017 and 2016, we recorded $8.5 million and $10.2 million of research and development expenses related to the collaboration agreement, respectively. We recorded related party research and development expenses for the year ended December 31, 2015 of $11.6 million for services provided by Array under the collaboration agreement. As of December 31, 2015, Array has indicated that it is no longer a holder of more than 5% of our capital stock; therefore, we will not report expenses with Array as a related party in future reporting periods, as applicable.
Milestones
With respect to product candidates directed to TRK, including larotrectinib and LOXO-195, we could be required to pay Array up to $223 million in milestone payments for each compound, the substantial majority of which are due upon the achievement of commercial milestones. We have made or accrued $7.0 million and $1.3 million in larotrectinib and LOXO-195 milestone payments, respectively, from inception through December 31, 2017. For the years ended December 31, 2017, 2016 and 2015, for larotrectinib, we recognized $0, $6.0 million and $1.0 million as Research and Development expense. For the years ended December 31, 2017, 2016 and 2015, for LOXO-195, we recognized $1.0 million, $0.3 million and $0 as Research and Development expense.
With respect to product candidates directed to targets other than TRK, including LOXO-292, we could be required to pay Array up to $213 million in milestone payments, the substantial majority of which are due upon the achievement of commercial milestones. We have made or accrued $1.3 million in LOXO-292 milestone payments from inception through December 31, 2017, of which $1.0 million, $0.3 million and $0 million relating to LOXO-292 was recognized as Research and Development expense for the years ended December 31, 2017, 2016 and 2015.
Royalties
We are required to pay Array mid-single digit royalties on worldwide net sales of products developed through the collaboration. With respect to the royalty on products directed to targets other than TRK, we have the right to credit certain milestone payments against royalties on sales of products directed to such target.
Other Commitments
In addition, in the course of normal business operations, we have agreements with contract service providers to assist in the performance of our research and development and manufacturing activities. We can generally elect to discontinue the work under these agreements. We could also enter into additional collaborative research, contract research, manufacturing and supplier agreements in the future, which may require upfront payments and even long-term commitments of cash.
Off-Balance Sheet Arrangements
Through December 31, 2017, we do not have any off-balance sheet arrangements, as defined by applicable SEC regulations.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to market risk related to changes in interest rates. As of December 31, 2017 and 2016, we had cash and cash equivalents and investments of $626.2 million and $141.8 million, respectively, consisting of money market funds, certificates of deposit, overnight repurchase agreements, government enterprise debt securities and U.S. Government debt securities. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because our investments are in marketable debt securities. Our available-for-sale securities are subject to interest rate risk and will fall in value if market interest rates increase. Due to the short-term duration of our investment portfolio and the low risk profile of our investments, an immediate 10% change in interest rates would not have a material effect on the fair market value of our portfolio. We have the ability to hold our available-sale-securities until maturity, and therefore, we would not expect our operating results or cash flows to be affected to any significant degree by the effect of a change in market interest rates on our investments. We do not currently have any auction rate securities.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of Loxo Oncology, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Loxo Oncology, Inc. (the Company) as of December 31, 2017, the related consolidated statements of operations, comprehensive loss, stockholders’ equity and cash flows for the year ended December 31, 2017, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2017, and the results of its operations and its cash flows for the year ended December 31, 2017, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework) and our report dated March 1, 2018, expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
|
/s/ Ernst & Young LLP |
|
|
|
|
|
We have served as the Company’s auditor since 2017. |
|
|
|
|
|
Hartford, Connecticut |
|
|
March 1, 2018 |
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders
Loxo Oncology, Inc.
We have audited the accompanying balance sheet of Loxo Oncology, Inc. as of December 31, 2016, and the related statements of operations, comprehensive loss, stockholders’ equity and cash flows for the years ended December 31, 2016 and 2015. Loxo Oncology, Inc.’s management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Loxo Oncology, Inc. as of December 31, 2016, and the results of its operations and its cash flows for the years ended December 31, 2016 and 2015, in conformity with accounting principles generally accepted in the United States of America.
|
/s/ CohnReznick LLP |
|
|
Roseland, New Jersey |
|
|
March 6, 2017 |
|
Loxo Oncology, Inc.
(in thousands, except share and per share amounts)
|
|
|
December 31, |
|
December 31, |
| ||
|
|
|
|
|
|
| ||
|
Assets |
|
|
|
|
| ||
|
Current assets: |
|
|
|
|
| ||
|
Cash and cash equivalents |
|
$ |
142,025 |
|
$ |
30,376 |
|
|
Short-term investments |
|
484,175 |
|
108,935 |
| ||
|
Receivable from collaboration partner |
|
150,000 |
|
— |
| ||
|
Other prepaid expenses and current assets |
|
5,607 |
|
2,483 |
| ||
|
Total current assets |
|
781,807 |
|
141,794 |
| ||
|
Long-term investments |
|
— |
|
2,499 |
| ||
|
Property and equipment, net |
|
912 |
|
248 |
| ||
|
Other assets |
|
723 |
|
771 |
| ||
|
Total assets |
|
$ |
783,442 |
|
$ |
145,312 |
|
|
Liabilities and stockholders’ equity |
|
|
|
|
| ||
|
Current liabilities: |
|
|
|
|
| ||
|
Accounts payable |
|
$ |
3,996 |
|
$ |
1,061 |
|
|
Accrued expenses and other current liabilities |
|
22,537 |
|
14,083 |
| ||
|
Current portion of deferred revenue |
|
195,037 |
|
— |
| ||
|
Total current liabilities |
|
221,570 |
|
15,144 |
| ||
|
Non-current portion of deferred revenue |
|
183,662 |
|
— |
| ||
|
Total liabilities |
|
405,232 |
|
15,144 |
| ||
|
Commitments and contingencies |
|
|
|
|
| ||
|
Stockholders’ equity: |
|
|
|
|
| ||
|
Preferred stock, $0.0001 par value; 5,000,000 shares authorized; no shares issued and outstanding at December 31, 2017 and 2016, respectively |
|
— |
|
— |
| ||
|
Common stock, $0.0001 par value; 125,000,000 shares authorized; 29,991,884 and 21,681,236 shares issued and outstanding at December 31, 2017 and 2016, respectively |
|
3 |
|
2 |
| ||
|
Additional paid-in capital |
|
666,891 |
|
269,423 |
| ||
|
Accumulated deficit |
|
(288,112 |
) |
(139,236 |
) | ||
|
Accumulated other comprehensive loss |
|
(572 |
) |
(21 |
) | ||
|
Total stockholders’ equity |
|
378,210 |
|
130,168 |
| ||
|
Total liabilities and stockholders’ equity |
|
$ |
783,442 |
|
$ |
145,312 |
|
See accompanying notes to consolidated financial statements.
Loxo Oncology, Inc.
Consolidated Statements of Operations
(in thousands, except share and per share amounts)
|
|
|
Year Ended |
|
Year Ended |
|
Year Ended |
| |||
|
|
|
December 31, 2017 |
|
December 31, 2016 |
|
December 31, 2015 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Revenue from collaboration agreement |
|
$ |
21,300 |
|
$ |
— |
|
$ |
— |
|
|
|
|
|
|
|
|
|
| |||
|
Operating expenses: |
|
|
|
|
|
|
| |||
|
Research and development with related party |
|
— |
|
— |
|
11,611 |
| |||
|
Research and development |
|
140,039 |
|
58,275 |
|
13,956 |
| |||
|
General and administrative |
|
33,657 |
|
14,903 |
|
10,508 |
| |||
|
Total operating expenses |
|
173,696 |
|
73,178 |
|
36,075 |
| |||
|
Loss from operations |
|
(152,396 |
) |
(73,178 |
) |
(36,075 |
) | |||
|
Interest income, net |
|
3,520 |
|
780 |
|
199 |
| |||
|
Net loss |
|
$ |
(148,876 |
) |
$ |
(72,398 |
) |
$ |
(35,876 |
) |
|
|
|
|
|
|
|
|
| |||
|
Per share information: |
|
|
|
|
|
|
| |||
|
Net loss per share of common stock—basic and diluted |
|
$ |
(5.31 |
) |
$ |
(3.46 |
) |
$ |
(2.12 |
) |
|
Weighted-average shares outstanding—basic and diluted |
|
28,035,697 |
|
20,905,448 |
|
16,894,549 |
| |||
See accompanying notes to consolidated financial statements.
Loxo Oncology, Inc.
Consolidated Statements of Comprehensive Loss
(in thousands)
|
|
|
Year Ended |
|
Year Ended |
|
Year Ended |
| |||
|
|
|
December 31, 2017 |
|
December 31, 2016 |
|
December 31, 2015 |
| |||
|
Net loss |
|
$ |
(148,876 |
) |
$ |
(72,398 |
) |
$ |
(35,876 |
) |
|
Other comprehensive income (loss) |
|
|
|
|
|
|
| |||
|
Unrealized (loss) gain on available-for-sale securities |
|
(551 |
) |
(5 |
) |
12 |
| |||
|
Comprehensive loss |
|
$ |
(149,427 |
) |
$ |
(72,403 |
) |
$ |
(35,864 |
) |
See accompanying notes to consolidated financial statements.
Loxo Oncology, Inc.
Consolidated Statements of Stockholders’ Equity
(in thousands, except share amounts)
|
|
|
Stockholders’ equity |
| |||||||||||||||
|
|
|
Common stock |
|
|
|
|
|
Accumulated |
|
|
| |||||||
|
|
|
|
|
$0.0001 |
|
Additional |
|
|
|
Other |
|
Total |
| |||||
|
|
|
|
|
Par |
|
Paid-in |
|
Accumulated |
|
Comprehensive |
|
Stockholders’ |
| |||||
|
|
|
Shares |
|
Value |
|
Capital |
|
Deficit |
|
Loss |
|
Equity |
| |||||
|
Balance at January 1, 2015 |
|
16,634,063 |
|
$ |
2 |
|
$ |
143,660 |
|
$ |
(30,962 |
) |
$ |
(28 |
) |
$ |
112,672 |
|
|
Stock-based compensation expense |
|
— |
|
— |
|
6,154 |
|
— |
|
— |
|
6,154 |
| |||||
|
Stock option exercises |
|
58,488 |
|
— |
|
307 |
|
— |
|
— |
|
307 |
| |||||
|
Reclassification of shares issued and previously subject to repurchase |
|
10,156 |
|
— |
|
37 |
|
— |
|
— |
|
37 |
| |||||
|
Issuance of common stock, net of offering costs |
|
2,875,000 |
|
— |
|
71,299 |
|
— |
|
— |
|
71,299 |
| |||||
|
Other comprehensive income |
|
— |
|
— |
|
— |
|
— |
|
12 |
|
12 |
| |||||
|
Net loss |
|
— |
|
— |
|
— |
|
(35,876 |
) |
— |
|
(35,876 |
) | |||||
|
Balance at December 31, 2015 |
|
19,577,707 |
|
$ |
2 |
|
$ |
221,457 |
|
$ |
(66,838 |
) |
$ |
(16 |
) |
$ |
154,605 |
|
|
Stock-based compensation expense |
|
— |
|
— |
|
7,960 |
|
— |
|
— |
|
7,960 |
| |||||
|
Stock option exercises |
|
177,279 |
|
— |
|
1,273 |
|
— |
|
— |
|
1,273 |
| |||||
|
Issuance of common stock, net of offering costs |
|
1,926,250 |
|
— |
|
38,733 |
|
— |
|
— |
|
38,733 |
| |||||
|
Other comprehensive loss |
|
— |
|
— |
|
— |
|
— |
|
(5 |
) |
(5 |
) | |||||
|
Net loss |
|
— |
|
— |
|
— |
|
(72,398 |
) |
— |
|
(72,398 |
) | |||||
|
Balance at December 31, 2016 |
|
21,681,236 |
|
$ |
2 |
|
$ |
269,423 |
|
$ |
(139,236 |
) |
$ |
(21 |
) |
$ |
130,168 |
|
|
Stock-based compensation expense |
|
— |
|
— |
|
19,422 |
|
— |
|
— |
|
19,422 |
| |||||
|
Stock option exercises |
|
237,648 |
|
— |
|
2,740 |
|
— |
|
— |
|
2,740 |
| |||||
|
Issuance of common stock, net of offering costs |
|
8,073,000 |
|
1 |
|
375,306 |
|
— |
|
— |
|
375,307 |
| |||||
|
Other comprehensive loss |
|
— |
|
— |
|
— |
|
— |
|
(551 |
) |
(551 |
) | |||||
|
Net loss |
|
— |
|
— |
|
— |
|
(148,876 |
) |
— |
|
(148,876 |
) | |||||
|
Balance at December 31, 2017 |
|
29,991,884 |
|
$ |
3 |
|
$ |
666,891 |
|
(288,112 |
) |
$ |
(572 |
) |
$ |
378,210 |
| |
See accompanying notes to consolidated financial statements.
Loxo Oncology, Inc.
Consolidated Statements of Cash Flows
(in thousands)
|
|
|
Year Ended |
|
Year Ended |
|
Year Ended |
| |||
|
Operating activities: |
|
|
|
|
|
|
| |||
|
Net loss |
|
$ |
(148,876 |
) |
$ |
(72,398 |
) |
$ |
(35,876 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
| |||
|
Amortization of premium and discounts on investments |
|
80 |
|
528 |
|
391 |
| |||
|
Depreciation of property and equipment |
|
125 |
|
77 |
|
17 |
| |||
|
Stock-based compensation |
|
19,422 |
|
7,960 |
|
6,154 |
| |||
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
| |||
|
Decrease (increase) in prepaid expenses and other assets |
|
(3,076 |
) |
224 |
|
(1,971 |
) | |||
|
Increase in accounts payable |
|
2,935 |
|
792 |
|
30 |
| |||
|
Increase in accrued expenses and other current liabilities |
|
8,454 |
|
11,499 |
|
1,073 |
| |||
|
Increase in deferred revenue |
|
228,699 |
|
— |
|
— |
| |||
|
Net cash provided by (used in) operating activities |
|
107,763 |
|
(51,318 |
) |
(30,182 |
) | |||
|
Investing activities: |
|
|
|
|
|
|
| |||
|
Purchases of available-for-sale securities |
|
(574,508 |
) |
(163,290 |
) |
(133,730 |
) | |||
|
Proceeds from maturing available-for-sale securities |
|
201,136 |
|
137,038 |
|
116,646 |
| |||
|
Purchase of property and equipment |
|
(789 |
) |
(237 |
) |
(93 |
) | |||
|
Net cash used in investing activities |
|
(374,161 |
) |
(26,489 |
) |
(17,177 |
) | |||
|
Financing activities: |
|
|
|
|
|
|
| |||
|
Proceeds from the issuance of common stock, net |
|
375,307 |
|
38,733 |
|
71,299 |
| |||
|
Proceeds from the exercise of stock options |
|
2,740 |
|
1,273 |
|
307 |
| |||
|
Net cash provided by financing activities |
|
378,047 |
|
40,006 |
|
71,606 |
| |||
|
Net increase (decrease) in cash and cash equivalents |
|
111,649 |
|
(37,801 |
) |
24,247 |
| |||
|
Cash and cash equivalents—beginning of year |
|
30,376 |
|
68,177 |
|
43,930 |
| |||
|
Cash and cash equivalents—end of year |
|
$ |
142,025 |
|
$ |
30,376 |
|
$ |
68,177 |
|
|
|
|
|
|
|
|
|
| |||
|
Supplemental schedule of noncash financing activities: |
|
|
|
|
|
|
| |||
|
Reclassification of share repurchase obligation |
|
$ |
— |
|
$ |
— |
|
$ |
37 |
|
See accompanying notes to consolidated financial statements.
Loxo Oncology, Inc.
1. Organization and Description of the Business
Loxo Oncology, Inc. (the “Company”) was incorporated on May 9, 2013 in the State of Delaware. The Company is a biopharmaceutical company innovating the development of highly selective medicines for patients with genetically defined cancers. Its pipeline focuses on cancers that are uniquely dependent on single gene abnormalities, such that a single drug has the potential to treat the cancer with dramatic effect. The Company operates in one segment and has its principal office in Stamford, Connecticut.
Liquidity
At December 31, 2017, the Company had working capital of $560.2 million, an accumulated deficit of $288.1 million and cash, cash equivalents and investments of $626.2 million. The Company has not generated any product revenues and has not achieved profitable operations. There is no assurance that profitable operations will ever be achieved, and, if achieved, could be sustained on a continuing basis. In addition, development activities, clinical and preclinical testing, and commercialization of the Company’s products will require significant additional capital.
The Company believes that its existing cash, cash equivalents and investments, will be sufficient to enable the Company to continue as a going concern through at least March 1, 2019. However, the Company will need to secure additional funding in the future, from one or more equity or debt financings, collaborations, or other sources, in order to carry out all of its planned research and development and commercialization activities. If the Company is unable to obtain additional financing or generate license, milestone or product revenue, the lack of liquidity could have a material adverse effect on the Company’s future prospects.
2. Summary of Significant Accounting Policies
Significant Accounting Policies
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”) and include the results of operations of the Company and its wholly owned subsidiary. All intercompany accounts and transactions have been eliminated in consolidation. Any reference in these notes to applicable guidance is meant to refer to GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Update (“ASU”) of the Financial Accounting Standards Board (“FASB”).
Principles of Consolidation
The consolidated financial statements include the accounts of Loxo Oncology, Inc. and its wholly owned subsidiary. All intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates
Management considers many factors in developing the estimates and assumptions that are used in the preparation of these financial statements. Management must apply significant judgment in this process. In addition, other factors may affect estimates, including expected business and operational changes, sensitivity and volatility associated with the assumptions used in developing estimates, and whether historical trends are expected to be representative of future trends. The estimation process often may yield a range of potentially reasonable estimates of the ultimate future outcomes and management must select an amount that falls within that range of reasonable estimates. This process may result in actual results differing materially from those estimated amounts used in the preparation of the financial statements if these results differ from historical experience, or other assumptions do not turn out to be substantially accurate, even if such assumptions are reasonable when made. In preparing these financial statements, management used significant estimates in the following areas, among others: revenue recognition, stock-based compensation expense, the determination of the fair value of stock-based awards, the accounting for research and development costs, and the recoverability of the Company’s net deferred tax assets and related valuation allowance.
Cash and Cash Equivalents
The Company considers all highly-liquid investments that have maturities of three months or less when acquired to be cash equivalents. As of December 31, 2017, and 2016, the Company’s cash and cash equivalents consisted of a business checking account, a certificate of deposit, repurchase agreements, money market account and government sponsored enterprise debt securities that had maturities of three months or less when acquired.
Restricted Cash
The Company had restricted cash of $0.3 million as of December 31, 2017 and 2016, respectively, which consisted of cash held to collateralize an outstanding letter of credit associated with the lease of its corporate office space in Connecticut. Restricted cash is included in other assets.
Investments
At the time of purchase, the Company classifies investments in marketable securities as either available-for-sale securities, held to maturity securities, or trading securities, depending on its intent at that time.
Investments available-for-sale are carried at fair value with unrealized holding gains and losses recorded within other comprehensive income (loss). Fair value is determined based on observable market quotes or valuation models using assessments of counterparty credit worthiness, credit default risk or underlying security and overall capital market liquidity. The Company reviews unrealized losses associated with available-for-sale investments to determine the classification as a “temporary” or “other-than-temporary” impairment. A temporary impairment results in an unrealized loss being recorded in other comprehensive income (loss). An impairment that is viewed as other-than-temporary is recognized in the statement of operations. The Company considers various factors in determining the classification, including the length of time and extent to which the fair value has been less than the Company’s cost basis, the financial condition and near-term prospects of the issuer or investee, and the Company’s ability to hold the investment for a period of time sufficient to allow for any anticipated recovery in market value. As of December 31, 2017 and 2016, the Company held $484.2 million and $108.9 million, respectively in short-term investments. As of December 31, 2017 and 2016, the Company held $0 and $2.5 million, respectively, in long-term investments.
Receivable from collaboration partner
Receivable from collaboration partner of $150.0 million at December 31, 2017 consists of a receivable from Bayer Consumer Care AG (“Bayer”) for the second and final installment of the upfront payment under the license, development and commercialization agreement with Bayer (“Bayer Agreement”), due in March 2018 (see Note 3). Bayer is a creditworthy entity that maintains an ongoing relationship with the Company, as such the Company did not have an allowance for estimated losses recorded related to this receivable.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist of cash and cash equivalents and available-for-sale securities, included in Short-term investments. At December 31, 2017 and 2016, the Company’s cash and cash equivalents were held by two financial institutions and the amounts on deposit were more than Federal Deposit Insurance Company insurance limits. The Company mitigates this risk by depositing its uninsured cash in major well capitalized financial institutions, and by investing excess operating cash in overnight repurchase agreements which are 100% collateralized by U.S. government backed securities with the Company’s bank. The Company has not recognized any losses on its cash and cash equivalents.
At December 31, 2017, the available-for-sale securities are invested in U.S. government sponsored enterprise debt securities and U.S. Government debt securities. As noted in Note 5 to the Financial Statements, the fair value of these securities was $484.2 million, $0.5 million less than their original par value purchase price.
Property and Equipment
Property and equipment are depreciated using the straight-line method over the estimated useful lives of the assets, which are generally three to seven years. Leasehold improvements are amortized over the shorter of the noncancelable term of operating leases or their economic useful lives. Maintenance and repairs are expensed as incurred. Upon disposal, retirement, or sale, the related cost and accumulated depreciation is removed from the accounts and any resulting gain or loss is included in the results of operations.
Collaboration Agreements
The Company evaluates whether an arrangement is a collaborative arrangement under the FASB ASC Topic 808, Collaborative Arrangements, at its inception based on the facts and circumstances specific to the arrangement. The Company also reevaluates whether an arrangement qualifies or continues to qualify as a collaborative arrangement whenever there is a change in either the roles of the participants or the participants’ exposure to significant risks and rewards dependent on the ultimate commercial success of the endeavor. For those collaborative arrangements where it is determined that the Company is the principal participant, costs incurred and revenue generated from third parties are recorded on a gross basis in the financial statements.
Revenue Recognition
The Company entered into a License, Development and Commercialization Agreement (the “Bayer Agreement”) in November 2017, which is within the scope of ASC 808. Under the Bayer Agreement, the Company has licensed certain rights to its larotrectinib and LOXO-195 product candidates to Bayer. The terms of the agreement include payment to the Company of one or more of the following: a non-refundable, up-front license fee, regulatory and commercial milestone payments, and royalties on net sales of licensed products.
Licenses of intellectual property: If the license of the Company’s intellectual property is determined to be a separate unit of accounting from the other performance obligations identified in the arrangement, the Company recognizes revenue from non-refundable, up-front fees allocated to the license when the license is transferred to the collaborative partner and the collaborative partner is able to use and benefit from the license. For licenses that are bundled with other promises, such as development activities, the Company recognizes revenue over time, using a proportional performance method as the related development activities are performed. Up-front
payments are recorded as deferred revenue upon receipt and require deferral of revenue recognition to a future period until the Company performs its obligations under these arrangements. Amounts payable to the Company are recorded as accounts receivable when the Company’s right to consideration is unconditional.
Milestone payments: Regulatory or commercial milestone payments will be recognized as revenue in the period the milestone is achieved. To date, the Company has not recognized any milestone payments as revenue resulting from its collaboration arrangement.
Co-promote: In the United States, where the Company and Bayer will co-promote the products, the Company will be responsible for 50% of the commercial costs and receive 50% of the profits. Co-promote net cost/profit will be recognized when the related expenses and sales occur. To date, the Company has not recognized any co-promote net cost/profit resulting from its collaboration arrangement.
Royalties: Sales-based royalties, including milestone payments based on the level of sales, will be recognized when the related sales occur. To date, the Company has not recognized any royalty revenue resulting from its collaboration arrangement.
Research and Development Expenses with a Related Party
Research and development expenses with a related party consisted of $11.6 million in expenses incurred in relation to the conduct of the discovery and preclinical development programs by Array BioPharma, Inc. (“Array”) for the year ended December 31, 2015 as part of the collaboration agreement (see Note 9). As of December 31, 2015, Array indicated that it was no longer a holder of more than 5% of the Company’s capital stock, therefore the Company will not report expenses with Array as a related party in future reporting periods, as applicable.
Research and Development Expenses
Research and development costs are charged to expense as incurred. These costs include, but are not limited to, employee-related expenses, including salaries, benefits, stock-based compensation and travel as well as expenses related to asset acquisitions of IPR&D, third-party collaborations, contract research arrangements, chemistry, manufacturing and controls (CMC) related expenses and activities associated with the development of companion diagnostics for our product candidates. Under the Bayer Agreement, the Company will receive reimbursement for 50% of its development activity expenses incurred for larotrectinib and LOXO-195 beginning January 1, 2018.
Research and development activities are central to our business model. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. As we advance our product candidates, we expect the amount of external research and development will continue to increase for the foreseeable future.
It is difficult to determine with certainty the duration and completion costs of our current or future preclinical programs and clinical trials of our product candidates, or if, when or to what extent we will generate revenue from the commercialization and sale of any of our product candidates that obtain regulatory approval. We may never succeed in achieving regulatory approval for any of our product candidates. The duration, costs and timing of clinical trials and development of our product candidates will depend on a variety of factors, including the uncertainties of future clinical and preclinical studies, uncertainties in clinical trial enrollment rate and significant and changing government regulation. In addition, the probability of success for each product candidate will depend on numerous factors, including competition, manufacturing capability and commercial viability. We will determine which programs to pursue and how much to fund each program in response to the scientific and clinical success of each product candidate, as well as an assessment of each product candidate’s commercial potential.
Comprehensive Loss
Comprehensive loss is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources. Comprehensive loss is comprised of net losses and unrealized gains or losses on investments.
Income Taxes
Income taxes are recorded in accordance with ASC Topic 740, Income Taxes (“ASC 740”), which provides for deferred taxes using an asset and liability approach. The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Deferred tax assets and liabilities are determined based on the differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Valuation allowances are provided, if based upon the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
The Company accounts for uncertain tax positions in accordance with the provisions of ASC 740. When uncertain tax positions exist, the Company recognizes the tax benefit of tax positions to the extent that the benefit will more likely than not be realized. The determination as to whether the tax benefit will more likely than not be realized is based upon the technical merits of the tax position as
well as consideration of the available facts and circumstances. As of December 31, 2017 and 2016, the Company did not have any uncertain tax positions.
Stock-Based Compensation
The Company’s stock-based compensation plans are more fully described in Note 8 to the Financial Statements. The Company accounts for stock-based compensation in accordance with the provisions of ASC Topic 718, Compensation-Stock Compensation (“ASC 718”), which requires the recognition of expense related to the fair value of stock-based compensation awards in the Statement of Operations.
For stock options issued to employees and members of the Board for their services on the Board, the Company estimates the grant date fair value of each option using the Black-Scholes option-pricing model. The use of the Black-Scholes option pricing model requires management to make assumptions with respect to the expected term of the option, the expected volatility of the common stock consistent with the expected term of the option, risk-free interest rates, the value of the common stock and expected dividend yield of the common stock. For awards subject to service-based vesting conditions, the Company recognizes stock-based compensation expense equal to the grant date fair value of stock options on a straight-line basis over the requisite service period, which is generally the vesting term. For awards subject to both performance and service-based vesting conditions, the Company recognizes stock-based compensation expense using the straight-line recognition method when it is probable that the performance condition will be achieved. Forfeitures are required to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
Share-based payments issued to non-employees are recorded at fair value, and are periodically revalued as the equity instruments vest and are recognized as expense over the related service period. See Note 8 for a discussion of the assumptions used by the Company in determining the grant date fair value of options granted under the Black-Scholes option pricing model, as well as a summary of the stock option activity under the Company’s stock-based compensation plan for the years ended December 31, 2017, 2016 and 2015.
Basic and Diluted Net Loss Per Share of Common Stock
Basic net loss per share of common stock is computed by dividing net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding during the period, excluding the dilutive effects of convertible preferred stock, unvested restricted stock and stock options. Diluted net loss per share of common stock is computed by dividing the net loss attributable to common stockholders by the sum of the weighted-average number of shares of common stock outstanding during the period plus the potential dilutive effects of convertible preferred stock, unvested restricted stock and stock options outstanding during the period calculated in accordance with the treasury stock method, although these shares and options are excluded if their effect is anti-dilutive. Because the impact of these items is anti-dilutive during periods of net loss, there was no difference between basic and diluted net loss per share of common stock for the years ended December 31, 2017, 2016 and 2015.
Recently Adopted Accounting Pronouncements
In January 2017, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2017-01, which provides additional guidance on evaluating whether transactions should be accounted for as acquisitions of assets or businesses. ASU 2017-01 requires an entity to evaluate if substantially all of the fair value of the assets acquired is concentrated in a single identifiable asset or a group of similar identifiable assets. If this threshold is met, the new guidance would define this as an asset acquisition; otherwise, the entity then evaluates whether the asset meets the requirement that a business include, at a minimum, an input and substantive process that together significantly contribute to the ability to create outputs. The Company has early adopted ASU 2017-01 in the third quarter of 2017. Refer to Note 12 for discussion on the Company’s acquisition of in process research and development (“IPR&D”) from Redx Pharma Plc and Redx Oncology Limited (collectively, “Redx”), which was accounted for as an asset acquisition under this new guidance.
In March 2016, the FASB issued ASU 2016-09, which provides for simplification of certain aspects of employee share-based payment accounting including income taxes, classification of awards as either equity or liabilities, accounting for forfeitures and classification on the statement of cash flows. ASU 2016-09 was effective for the Company in the first quarter of 2017. The standard requires the recognition of any pre-adoption date net operating loss (“NOL”) carryforwards from share-based compensation arrangements to be recognized on a modified retrospective basis, through an opening retained earnings adjustment on January 1, 2017. Any income tax effects from share-based compensation arrangements arising after January 1, 2017 will be recognized prospectively in the income statement. Upon adoption, the Company recognized all previously unrecognized tax benefits. These previously unrecognized tax benefits were recorded as a deferred tax asset, which was fully offset by a valuation allowance on January 1, 2017, thus there was no net impact from the adoption of ASU 2016-09 as of the same date. The Company’s adoption of the standard did not have any impact to the consolidated statements of cash flows as no NOL carryforwards from share-based compensation arrangements were recognized prior to January 1, 2017. The Company has elected to continue to estimate forfeitures under the true-up provision of ASC 718.
In November 2015, the FASB issued ASU 2015-17, which eliminates the current requirement to present deferred tax liabilities and assets as current and noncurrent in a classified balance sheet. Instead, entities will be required to classify all deferred tax assets and
liabilities as noncurrent. ASU 2015-17 was effective for the Company in the first quarter of 2017. The adoption of the ASU 2015-17 did not have an impact on the Company’s financial statements.
Recent Accounting Pronouncements Not Yet Adopted
In November 2016, the FASB issued ASU No. 2016-18, Statement of Cash Flows (Topic 230): Restricted Cash (“ASU 2016-18”), which amended the existing accounting standards for the statement of cash flows by requiring restricted cash to be included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. ASU 2016-18 will be effective in fiscal years beginning after December 15, 2017, including interim periods within those fiscal years, and early adoption is permitted. The amendments should be applied retrospectively to all periods presented. The Company does not expect the adoption of ASU 2016-18 will have a material impact on the financial statements and related disclosures.
In August 2016, the FASB issued ASU No. 2016-15, Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments (“ASU 2016-15”), which amended the existing accounting standards for the statement of cash flows by providing guidance on eight classification issues related to the statement of cash flows. ASU 2016-15 will be effective in fiscal years beginning after December 15, 2017, including interim periods within those fiscal years, and early adoption is permitted. The amendments should be applied retrospectively to all periods presented. For issues that are impracticable to apply retrospectively, the amendments may be applied prospectively as of the earliest date practicable. The Company does not expect the adoption of ASU 2016-15 will have a material impact on the financial statements and related disclosures.
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842) (“ASU 2016-02”), which requires lessees to recognize assets and liabilities for the rights and obligations created by most leases on their balance sheet. The guidance is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. Early application is permitted. ASU 2016-02 requires modified retrospective adoption for all leases existing at, or entered into after, the date of initial application, with an option to use certain transition relief. The Company is currently evaluating the impact the standard may have on the financial statements and related disclosures.
In January 2016, the FASB issued ASU No. 2016-01, Financial Instruments - Overall (Subtopic 825-10), Recognition and Measurement of Financial Assets and Financial Liabilities (“ASU 2016-01”), which addresses certain aspects of recognition, measurement, presentation, and disclosure of financial instruments. ASU 2016-01 will be effective for annual periods and interim periods within those annual periods beginning after December 15, 2017 and early adoption is not permitted. The Company does not expect the adoption of ASU 2016-01 to have a material impact on the financial statements and related disclosures.
In May 2014, the FASB issued ASU No. 2014-09, which amends the guidance for accounting for revenue from contracts with customers. This ASU supersedes the revenue recognition requirements in ASC Topic 605, Revenue Recognition, and creates a new ASC Topic 606, Revenue from Contracts with Customers. Customers. Subsequent to May 2014, the FASB issued additional guidance that delayed the effective date and clarified various aspects of the new guidance, including principal versus agent considerations, identifying performance obligations and licensing, and also included other improvements and practical expedients. ASU 2014-09 will be effective for annual periods and interim periods within those annual periods beginning after December 15, 2017, early adoption is permitted. The Company does not expect the adoption of ASU 2014-09 to have a material impact on the financial statements and related disclosures as the Company does not currently have any contracts with customers subject to the guidance in ASC 606.
Segment Information
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker, or decision-making group, in making decisions on how to allocate resources and assess performance. The Company’s chief operating decision maker is the chief executive officer. The Company and the chief executive officer view the Company’s operations and manage its business as one operating segment. All long-lived assets of the Company reside in the U.S.
3. Collaboration Agreement
Agreement Terms
On November 14, 2017, the Company entered into the Bayer Agreement pursuant to which the Company and Bayer will collaborate to develop and commercialize larotrectinib and LOXO-195, the Company’s franchise of highly selective TRK inhibitors for patients with TRK fusion cancers. Pursuant to the Bayer Agreement, Loxo has granted co-exclusive development and commercialization licenses to Bayer for both larotrectinib and LOXO-195. Upon the effective date, the Company became eligible for a non-refundable, upfront cash payment of $400 million from Bayer. In accordance with the terms of the Bayer Agreement, the Company received $250 million in November 2017 and recorded a receivable for the remaining $150 million, to be received in March 2018.
In addition to an upfront cash payment of $400 million, the Company is eligible to receive $450 million in milestone payments upon larotrectinib regulatory approvals and first commercial sale events in certain major markets and an additional $200 million in milestone payments upon LOXO-195 regulatory approvals and first commercial sale events in certain major markets.
The Company will lead global development activities and regulatory activities in the United States. Bayer will lead regulatory activities outside the United States and global commercial activities. Globally, the Company will be responsible for 50% of development costs. In the United States, where the Company and Bayer will co-promote the products, the Company will be responsible for 50% of the commercial costs and receive 50% of the profits. Bayer will pay the Company a $25 million milestone upon achieving a certain aggregate U.S. net sales threshold. The Company will have the right to opt-out of the U.S. co-promotion, in which case the Company would receive a royalty in the low thirties percentage range on U.S. net sales, which is meant to approximate the economics of the 50/50 profit split. Both parties will participate on a Global Steering Committee and a Joint Steering Committee and will participate in working groups established by the Committees.
Outside of the United States, where Bayer will commercialize, Bayer will pay the Company tiered, double digit royalties on net sales, and sales milestones totaling $475 million.
The Bayer Agreement will terminate as to a product or country upon the expiration of the royalty term applicable to such product in such country. The Bayer Agreement may be terminated by either party for material breach or bankruptcy. In addition, (i) Bayer may terminate the Bayer Agreement after the fourth anniversary of the effective date upon written notice to Loxo which termination shall be effective 18 months following the Company’s receipt of such notice, or (ii) Bayer shall have the right, but not the obligation, to terminate the Bayer Agreement with respect to the Co-Promotion Territory or in its entirety by written notice to Loxo with immediate effect in the event that Loxo receives a “complete response letter” from the U.S. Food and Drug Agency with respect to larotrectinib, or if Loxo does not receive marketing approval for larotrectinib by December 31, 2018.
The Agreement contains customary representations, warranties and covenants by the Company and Bayer. Each of the Company and Bayer is required to indemnify the other party against all losses and expenses related to breaches of its representations, warranties and covenants under the Agreement.
Revenue Recognition
To account for the Bayer Agreement, the Company applied the guidance in ASC 808 Collaborative Arrangements. ASC 808 does not contain prescriptive guidance on recognition of revenues. Therefore, there was significant judgment applied in determining a reasonable, rational method of recognizing revenue under the Bayer Agreement, with the Company considering the guidance in ASC 606 Revenue from Contracts with Customers. The Company identified the following performance obligations under the Bayer Agreement relating to the upfront payment: (1) the grant of licenses related to larotrectinib, (2) the grant of licenses related to LOXO-195, (3) research and development activities for larotrectinib, (4) research and development activities for LOXO-195. The Company determined that each of the licenses is not a separate unit of accounting from its research and development activities because they significantly increase the utility of the intellectual property transferred.
The Company concluded that it will utilize a proportional performance method to recognize revenue under the Bayer Agreement. In applying the proportional performance method of revenue recognition, revenue will be recognized based on actual development costs incurred as a percentage of the total budgeted development costs over the time period the Company completes its development activities, which the Company believes will be five years for larotrectinib and seven years for LOXO-195. A proportional performance method of revenue recognition requires management to make estimates of costs to complete the development activities. In making such estimates, significant judgment is required to evaluate assumptions related to cost estimates. The cumulative effect of revisions to estimated costs to complete the Company’s performance obligations will be recorded in the period in which changes are identified and amounts can be reasonably estimated. A significant change in these assumptions and estimates could have a material impact on the timing and amount of revenue recognized in future periods.
The Company has not yet recognized any revenues for milestone payments as the related regulatory or sales milestones have not yet been achieved.
The Company and Bayer will make quarterly cost-sharing payments to one another in amounts necessary to ensure that each party bears its contractual share of the overall shared costs incurred.
For the year ended December 31, 2017, the Company recognized $21.3 million of revenue under the Bayer Agreement related to the portion of the upfront payment earned during the period. No revenue was recognized for the years ended December 31, 2016 or 2015.
4. Net Loss Per Common Share
The following table sets forth the computation of basic and diluted net loss per share for the periods indicated (in thousands, except share and per share data):
|
|
|
Year Ended |
|
Year Ended |
|
Year Ended |
| |||
|
|
|
December 31, 2017 |
|
December 31, 2016 |
|
December 31, 2015 |
| |||
|
Basic and diluted net loss per common share calculation: |
|
|
|
|
|
|
| |||
|
|
|
|
|
|
|
|
| |||
|
Net loss attributable to common stockholders |
|
$ |
(148,876 |
) |
$ |
(72,398 |
) |
$ |
(35,876 |
) |
|
|
|
|
|
|
|
|
| |||
|
Weighted-average shares outstanding—basic and diluted |
|
28,035,697 |
|
20,905,448 |
|
16,894,549 |
| |||
|
Net loss per share of common stock—basic and diluted |
|
$ |
(5.31 |
) |
$ |
(3.46 |
) |
$ |
(2.12 |
) |
The following outstanding securities at December 31, 2017, 2016 and 2015 have been excluded from the computation of diluted weighted-average shares outstanding, as they would have been anti-dilutive:
|
|
|
Year Ended |
|
Year Ended |
|
Year Ended |
|
|
|
|
December 31, 2017 |
|
December 31, 2016 |
|
December 31, 2015 |
|
|
Unvested restricted stock |
|
— |
|
38,526 |
|
104,574 |
|
|
Stock options |
|
3,225,356 |
|
2,825,851 |
|
2,240,955 |
|
|
|
|
3,225,356 |
|
2,864,377 |
|
2,345,529 |
|
5. Fair Value Measurements
Financial Instruments
The financial instruments recorded in the Company’s balance sheets include cash and cash equivalents, investments, and accounts payable. Included in cash and cash equivalents are money market funds representing a type of mutual fund required by law to invest in low-risk securities (for example, U.S. government bonds, U.S. treasury bills and commercial paper) and overnight repurchase agreements. Money market funds are structured to maintain the fund’s net asset value at $1.00 per unit, which assists in providing adequate liquidity upon demand by the holder. Money market funds pay dividends that generally reflect short-term interest rates. Thus, only the dividend yield fluctuates. Due to their short-term maturity, the carrying amounts of cash and cash equivalents (including money market funds), and accounts payable approximate their fair values. The Company classifies its remaining investments as available-for-sale. Gains or losses on securities sold are based on the specific identification method
For investments classified as available-for-sale, the Company records unrealized gains or losses resulting from changes in fair value between measurement dates as a component of other comprehensive income (loss).
|
(amounts in thousands) |
|
Amortized |
|
Gross |
|
Gross |
|
Fair Value |
| ||||
|
December 31, 2017 |
|
|
|
|
|
|
|
|
| ||||
|
Overnight repurchase agreements |
|
$ |
51,750 |
|
$ |
— |
|
$ |
— |
|
$ |
51,750 |
|
|
Money market funds |
|
50,744 |
|
— |
|
— |
|
50,744 |
| ||||
|
Government enterprise debt securities |
|
23,444 |
|
— |
|
— |
|
23,444 |
| ||||
|
Total included in cash and cash equivalents |
|
125,938 |
|
— |
|
— |
|
125,938 |
| ||||
|
|
|
|
|
|
|
|
|
|
| ||||
|
U.S. Government debt securities |
|
192,473 |
|
1 |
|
(129 |
) |
192,345 |
| ||||
|
Government enterprise debt securities |
|
292,274 |
|
— |
|
(444 |
) |
291,830 |
| ||||
|
Short-term available-for-sale securities |
|
484,747 |
|
1 |
|
(573 |
) |
484,175 |
| ||||
|
Total fair value financial instruments |
|
$ |
610,685 |
|
$ |
1 |
|
$ |
(573 |
) |
$ |
610,113 |
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
December 31, 2016 |
|
|
|
|
|
|
|
|
| ||||
|
Overnight repurchase agreements |
|
$ |
10,000 |
|
$ |
— |
|
$ |
— |
|
$ |
10,000 |
|
|
Money market funds |
|
12,146 |
|
— |
|
— |
|
12,146 |
| ||||
|
Total included in cash and cash equivalents |
|
22,146 |
|
— |
|
— |
|
22,146 |
| ||||
|
|
|
|
|
|
|
|
|
|
| ||||
|
U.S. Government debt securities |
|
12,769 |
|
995 |
|
— |
|
13,764 |
| ||||
|
Government enterprise debt securities |
|
96,184 |
|
— |
|
(1,013 |
) |
95.171 |
| ||||
|
Short-term available-for-sale securities |
|
108,953 |
|
995 |
|
(1,013 |
) |
108.935 |
| ||||
|
|
|
|
|
|
|
|
|
|
| ||||
|
U.S. Government debt securities |
|
2,502 |
|
— |
|
(3 |
) |
2,499 |
| ||||
|
Long-term available-for-sale securities |
|
2,502 |
|
— |
|
(3 |
) |
2,499 |
| ||||
|
Total fair value financial instruments |
|
$ |
133,601 |
|
$ |
995 |
|
$ |
(1,016 |
) |
$ |
133,580 |
|
Fair value guidance establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. These tiers include:
· Level 1—Quoted prices in active markets for identical assets or liabilities.
· Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
· Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The Company’s financial assets measured at fair value on a recurring basis at December 31, 2017 were as follows (in thousands):
|
|
|
Fair Value Measurements at Measurement Date: |
| ||||||||||
|
|
|
Quoted Prices in Active |
|
Significant Other |
|
Significant Unobservable |
|
Total as of |
| ||||
|
|
|
|
|
|
|
|
|
|
| ||||
|
Assets: |
|
|
|
|
|
|
|
|
| ||||
|
Cash and cash equivalents |
|
|
|
|
|
|
|
|
| ||||
|
Cash |
|
$ |
16,087 |
|
$ |
— |
|
$ |
— |
|
$ |
16,087 |
|
|
Overnight repurchase agreements |
|
51,750 |
|
— |
|
— |
|
51,750 |
| ||||
|
Money market funds |
|
50,744 |
|
— |
|
— |
|
50,744 |
| ||||
|
Government enterprise debt securities |
|
— |
|
23,444 |
|
— |
|
23,444 |
| ||||
|
Total cash and cash equivalents |
|
118,581 |
|
23,444 |
|
— |
|
142,025 |
| ||||
|
Short-term investments |
|
|
|
|
|
|
|
|
| ||||
|
U.S. Government debt securities |
|
192,345 |
|
— |
|
— |
|
192,345 |
| ||||
|
Government enterprise debt securities |
|
— |
|
291,830 |
|
— |
|
291,830 |
| ||||
|
Total short-term investments |
|
192,345 |
|
291,830 |
|
— |
|
484,175 |
| ||||
|
Totals |
|
$ |
310,926 |
|
$ |
315,274 |
|
$ |
— |
|
$ |
626,200 |
|
The Company’s financial assets measured at fair value on a recurring basis at December 31, 2016 were as follows (in thousands):
|
|
|
Fair Value Measurements at Measurement Date: |
| ||||||||||
|
|
|
Quoted Prices in Active |
|
Significant Other |
|
Significant |
|
Total as of |
| ||||
|
|
|
|
|
|
|
|
|
|
| ||||
|
Assets: |
|
|
|
|
|
|
|
|
| ||||
|
Cash and cash equivalents |
|
|
|
|
|
|
|
|
| ||||
|
Cash |
|
$ |
8,230 |
|
$ |
— |
|
$ |
— |
|
$ |
8,230 |
|
|
Overnight repurchase agreements |
|
10,000 |
|
— |
|
— |
|
10,000 |
| ||||
|
Money market funds |
|
12,146 |
|
— |
|
— |
|
12,146 |
| ||||
|
Total cash and cash equivalents |
|
30,376 |
|
— |
|
— |
|
30,376 |
| ||||
|
Short-term investments |
|
|
|
|
|
|
|
|
| ||||
|
U.S. Government debt securities |
|
13,764 |
|
— |
|
— |
|
13,764 |
| ||||
|
Government enterprise debt securities |
|
— |
|
95,171 |
|
— |
|
95,171 |
| ||||
|
Total short-term investments |
|
13,764 |
|
95,171 |
|
— |
|
108,935 |
| ||||
|
Long-term investments |
|
|
|
|
|
|
|
|
| ||||
|
U.S. Government debt securities |
|
2,499 |
|
— |
|
— |
|
2,499 |
| ||||
|
Total long-term investments |
|
2,499 |
|
— |
|
— |
|
2,499 |
| ||||
|
Totals |
|
$ |
46,639 |
|
$ |
95,171 |
|
$ |
— |
|
$ |
141,810 |
|
There were no items that were accounted for at fair value on a non-recurring basis for the years ended December 31, 2017 and 2016. The Company’s Level 2 securities are typically valued utilizing third party pricing services or other observable market data. The pricing services utilize industry standard valuation models, including both income and market based approaches and observable market
inputs to determine value. These observable market inputs include reportable trades, benchmark yields, credit spreads, broker/dealer quotes, bids, offers, and other industry and economic events.
6. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consisted of the following (in thousands):
|
|
|
December 31, 2017 |
|
December 31, 2016 |
| ||
|
Research and development accrued expenses |
|
$ |
17,964 |
|
$ |
12,120 |
|
|
General and administrative accrued expenses |
|
4,573 |
|
1,963 |
| ||
|
|
|
$ |
22,537 |
|
$ |
14,083 |
|
Included in the above amounts is $2.5 million and $1.5 million of accrued bonuses at December 31, 2017 and 2016, respectively.
7. Capital Stock and Stockholders’ Equity
Capitalization
The Company completed its initial public offering in August 2014. As part of that offering, all of the Company’s outstanding convertible preferred stock was converted into an aggregate total of 9,932,042 shares of common stock. Upon the completion of the initial public offering, the Company’s authorized capital stock consisted of 125,000,000 shares of common stock, $0.0001 par value per share, and 5,000,000 shares of undesignated preferred stock, $0.0001 par value per share.
In November 2015, the Company sold 2,875,000 shares of common stock at a public offering price of $26.50 per share. Net proceeds from this offering, after deducting underwriting discounts and commissions and offering expenses, were approximately $71.3 million.
In May 2016, the Company sold 1,926,250 shares of common stock at a public offering price of $21.50 per share. Net proceeds from this offering, after deducting underwriting discounts and commissions and offering expenses, were approximately $38.7 million.
In January 2017, the Company sold 4,450,500 shares of common stock at a public offering price of $31.00 per share. Net proceeds from this offering, after deducting underwriting discounts and commissions and offering expenses, were approximately $129.4 million.
In June 2017, the Company sold 3,622,500 shares of common stock at a public offering price of $72.00 per share. Net proceeds from this offering, after deducting underwriting discounts and commissions and offering expenses, were approximately $245.9 million.
8. Stock-Based Compensation
Equity Incentive Plan (the “Plan”)
Effective July 2013, the Company adopted the 2013 Equity Incentive Plan, which was amended in November 2013 (the “2013 Plan”). The 2013 Plan provided for the granting of incentive stock options, non-statutory stock options and the issuance of restricted stock awards. The Company reserved 1,544,615 shares of common stock authorized for issuance in connection with the 2013 Plan. Certain options are eligible for exercise prior to vesting. Exercised but unvested shares are subject to repurchase by the Company at the initial exercise price. In connection with the Company’s initial public offering, no further grants will be made under this plan and all remaining shares available for grant were transferred to the 2014 Equity Incentive Plan.
The Company adopted the 2014 Equity Incentive Plan (the “2014 Plan”) that became effective on July 30, 2014 and serves as the successor to the 2013 Plan. The 2014 Plan provides for the grant of awards to employees, directors, consultants, independent contractors and advisors, provided the consultants, independent contractors, directors and advisors are natural persons that render services other than in connection with the offer and sale of securities in a capital-raising transaction. The exercise price of stock options must be at least equal to the fair market value of the Company’s common stock on the date of grant.
The Company has reserved 2,828,874 shares of its common stock to be issued under the 2014 Plan of which 549,726 shares were available for future issuance as of December 31, 2017. Shares authorized will increase automatically on January 1 of each of the years between 2015 through 2024 by the number of shares equal to 3.0% of the aggregate number of outstanding shares of the Company’s common stock as of the immediately preceding December 31. The Company’s Board may reduce the amount of the increase in any particular year. The 2014 Plan authorizes the award of stock options, restricted stock awards, or RSAs, stock appreciation rights, or SARs, restricted stock units, or RSUs, performance awards and stock bonuses.
The following table summarizes stock option activity for the year ended December 31, 2017:
|
|
|
|
|
|
|
Average |
|
|
| ||
|
|
|
|
|
Weighted- |
|
Remaining |
|
Aggregate |
| ||
|
|
|
Number |
|
Average |
|
Contractual |
|
Intrinsic Value |
| ||
|
|
|
of Shares |
|
Exercise Price |
|
Term (in years) |
|
(in thousands) |
| ||
|
Outstanding at December 31, 2016 |
|
2,825,851 |
|
15.35 |
|
8.34 |
|
|
| ||
|
Granted |
|
700,500 |
|
63.72 |
|
|
|
|
| ||
|
Exercised |
|
(237,648 |
) |
11.53 |
|
|
|
|
| ||
|
Forfeited and expired |
|
(63,347 |
) |
31.91 |
|
|
|
|
| ||
|
Outstanding at December 31, 2017 |
|
3,225,356 |
|
$ |
25.82 |
|
7.72 |
|
$ |
188,626 |
|
|
Vested and expected to vest at December 31, 2017 |
|
3,114,857 |
|
$ |
25.14 |
|
7.68 |
|
$ |
184,233 |
|
|
Exercisable at December 31, 2017 |
|
1,758,257 |
|
$ |
14.06 |
|
6.89 |
|
$ |
123,283 |
|
As of December 31, 2017, there was $40.4 million of total unrecognized compensation expense related to options granted but not yet vested of which $3.8 million is attributable to non-employee awards and subject to re-measurement until vested. The total unrecognized compensation expense of $40.4 million will be recognized as expense over a weighted-average period of 2.8 years.
The weighted-average grant-date fair value of stock options granted during the years ended December 31, 2017, 2016 and 2015 was $43.07, $17.61 and $16.83 per share, respectively. The total fair value of stock options vested during the years ended December 31, 2017, 2016 and 2015 was $21.5 million, $7.6 million and $6.1 million, respectively.
The Company uses the Black-Scholes option pricing model to estimate the fair value of option awards with the following weighted-average assumptions, certain of which are based on industry comparative information, for the period indicated:
|
|
|
Year Ended |
|
Year Ended |
|
Year Ended |
|
|
|
|
December 31, 2017 |
|
December 31, 2016 |
|
December 31, 2015 |
|
|
Risk-free interest rate |
|
2.00 |
% |
1.77 |
% |
1.78 |
% |
|
Expected dividend yield |
|
— |
% |
— |
% |
— |
% |
|
Expected stock price volatility |
|
77.79 |
% |
79.69 |
% |
74.76 |
% |
|
Expected term of options (in years) |
|
6.1 |
|
6.2 |
|
6.0 |
|
|
Expected forfeiture rate |
|
11.54 |
% |
12.05 |
% |
13.33 |
% |
The weighted-average valuation assumptions were determined as follows:
· Risk-free interest rate: The Company bases the risk-free interest rate on the interest rate payable on U.S. Treasury securities in effect at the time of grant for a period that is commensurate with the assumed expected option term.
· Expected annual dividends: The estimate for annual dividends is 0%, because the Company has not historically paid, and does not expect for the foreseeable future to pay, a dividend.
· Expected stock price volatility: The expected volatility used is based on historical volatilities of similar entities within the Company’s industry which were commensurate with the Company’s expected term assumption.
· Expected term of options: The expected term of options represents the period of time options are expected to be outstanding. The expected term of the options granted to employees is derived from the “simplified” method as described in Staff Accounting Bulletin 107 relating to stock-based compensation. The expected term for options granted to non-employees is equal to the contractual term of the awards.
· Expected forfeiture rate: The Company’s estimated forfeiture rate is based on historical forfeiture experience of its various employee groups.
· Estimated fair value of the Company’s stock-based awards: The estimated fair value of the Company’s stock-based awards is amortized on a straight-line basis over the awards’ service period for those awards with graded vesting and which contain only a service condition. For awards with graded vesting and a performance and service condition, when achievement of the performance condition is deemed probable, the Company recognizes compensation cost using the accelerated recognition method over the awards’ service period.
Share-based compensation expense recognized was as follows (in thousands):
|
|
|
Year Ended |
|
Year Ended |
|
Year Ended |
| |||
|
|
|
December 31, 2017 |
|
December 31, 2016 |
|
December 31, 2015 |
| |||
|
Research and development |
|
$ |
9,502 |
|
$ |
3,471 |
|
$ |
3,310 |
|
|
General and administrative |
|
9,920 |
|
4,489 |
|
2,844 |
| |||
|
|
|
$ |
19,422 |
|
$ |
7,960 |
|
$ |
6,154 |
|
Restricted Stock
The stock-based compensation expense for restricted stock is determined based on the estimated fair value of the Company’s common stock on the grant date of the awards applied to the total number of awards that are anticipated to vest. During 2013, the Company granted 264,189 restricted stock awards and as of December 31, 2015 there were 104,574 shares expected to vest over the next 3 years. All restricted stock is vested as of December 31, 2017. Stock-based compensation for restricted stock was de minimis at their original grant date.
9. Commitments and Contingencies
Operating Leases
The Company leases office space under operating leases for its locations in Stamford, Connecticut, South San Francisco, California, and Boulder, Colorado. The Company’s lease agreements contain escalation clauses; accordingly, the Company straight-lines the rent expense over the lease term. Rent expense under operating leases for the years ended December 31, 2017, 2016 and 2015 was $1.0 million, $0.7 million and $0.1 million, respectively.
Future minimum lease payments as of December 31, 2017 are as follows (in thousands):
|
|
|
Operating |
| |
|
2018 |
|
$ |
1,627 |
|
|
2019 |
|
2,272 |
| |
|
2020 |
|
2,505 |
| |
|
2021 |
|
2,565 |
| |
|
2022 |
|
2,627 |
| |
|
Thereafter |
|
3,948 |
| |
|
|
|
$ |
15,544 |
|
In August 2017, the Company signed the First Amendment to the San Francisco office space lease, in South San Francisco, CA, extending the end of the original lease term to January 31, 2023. In addition to extending the lease term, the First Amendment provides for additional space, beginning in November 2017. The Company expects to incur approximately $0.4 million of annual rent expense associated with the lease.
In May 2017, the Company entered into a lease agreement in Boulder, CO. The lease commencement date was October 1, 2017. The lease has a term of 63 months, ending December 31, 2022, after which the lease shall continue on a month to month basis. The Company has an option to renew the primary lease term for two additional periods of five years and has the one-time right to terminate the lease effective at the end of the 39th month following the commencement date by delivering six months prior written notice of such termination and upon payment of a termination fee equal to $30,000. The Company expects to incur approximately $0.3 million of annual rent expense associated with the lease.
In November 2017, the Company amended the lease for its Stamford, CT office space. The amendment extends the end of the lease term for the existing space from December 31, 2022 to January 31, 2025. In addition, the amendment provides for the leasing of an additional 22,987 of rentable square feet at the same location beginning in February 2018 and ending January 31, 2025. The Company expects to incur $1.6 million of aggregate annual rent expense associated with the amended lease.
Array Bio Pharma (“Array”) Collaboration
On July 3, 2013, the Company signed a multi-year strategic collaboration agreement with Array, and this agreement was subsequently amended on November 26, 2013, April 10, 2014, October 13, 2014, March 31, 2015 and February 18, 2016. Under the terms of the collaboration agreement, the Company obtained certain rights to Array’s tropomyosin receptor kinase (“TRK”) inhibitor program, as well as additional novel oncology targets, including rearranged during transfection (“RET”), and fibroblast growth factor receptor (“FGFR”). The Company has worldwide commercial rights to each product candidate from the collaboration and Array participates in any potential successes through milestones and royalties.
With respect to the discovery and preclinical program, the collaboration agreement, as amended, runs through September 30, 2017, and the Company has the option to extend the term for up to one additional one-year renewal period by providing written notice to Array at least three months before the end of the initial discovery and preclinical development programs term. This option was exercised during the three-month period ended June 30, 2017.
Before the February 2016 amendment, in addition to larotrectinib the parties designated 12 discovery targets, of which seven were selected for additional study in January 2015, which was to be reduced to four on or before January 2016. The Company
had the option to maintain the total target number at five for an additional payment, and the Company exercised this option to maintain five discovery programs in January 2016. In the February 2016 amendment, the parties designated a total of six discovery targets. An additional payment was due at contract signing, satisfying a prior obligation of the April 2014 amendment.
As part of the agreement the Company agreed to pay Array a fixed amount per month, based on Array’s commitment to provide full-time equivalents and other support relating to the conduct of the discovery and preclinical development programs. For the years ended December 31, 2017 and 2016, the Company recorded $8.5 million and $10.2 million of research and development expenses related to the collaboration agreement, respectively. See Note 11 for amounts the Company recorded for the year ended December 31, 2015 in related party research and development expenses.
Milestones
With respect to product candidates directed to TRK, including larotrectinib and LOXO-195, the Company could be required to pay Array up to $223 million in milestone payments for each compound, the substantial majority of which are due upon the achievement of commercial milestones. The Company has made or accrued $7.0 million and $1.3 million in larotrectinib and LOXO-195 milestone payments, respectively, from inception through December 31, 2017. For the years ended December 31, 2017, 2016 and 2015, for larotrectinib, the Company recognized $0, $6.0 million and $1.0 million as Research and Development expense. For the years ended December 31, 2017, 2016 and 2015, for LOXO-195, the Company recognized $1.0 million, $0.3 million and $0 as Research and Development expense.
With respect to product candidates directed to targets other than TRK, including LOXO-292, the Company could be required to pay Array up to $213 million in milestone payments, the substantial majority of which are due upon the achievement of commercial milestones. The Company has made or accrued $1.3 million in LOXO-292 milestone payments from inception through December 31, 2017, of which $1.0 million, $0.3 million and $0 million relating to LOXO-292 was recognized as Research and Development expense for the years ended December 31, 2017, 2016 and 2015.
Royalties
The Company is required to pay Array mid-single digit royalties on worldwide net sales of products that were discovered under the agreement. With respect to the royalty on products directed to targets other than TRK, the Company has the right to credit certain milestone payments against royalties on sales of products directed to such target.
Research and Development Arrangements
In the course of normal business operations, the Company enters into agreements with contract research organizations, or CROs, to assist in the performance of research and development and preclinical activities and contract manufacturers to assist with and chemistry, manufacturing and controls (CMC) related expenses. Expenditures to CROs may represent a significant cost in preclinical and clinical development for the Company in future periods. The Company can elect to discontinue the work under these agreements at any time. The Company also enters into agreements with third parties to develop and commercialize companion diagnostics. The Company could also enter into additional collaborative research, contract research, manufacturing, and supplier agreements in the future, which may require upfront payments and long-term commitments of cash.
Legal Proceedings
The Company is not involved in any legal proceeding that it expects to have a material effect on its business, financial condition, results of operations and cash flows.
10. Income Taxes
The Company provides for income taxes under ASC 740. Under ASC 740, the liability method is used in accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities, and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse.
The Company has not recorded a current or deferred income tax expense or benefit since its inception.
The Company’s loss before income taxes was $148.9 million, $72.4 million, and $35.9 million for the years ended December 31, 2017, 2016 and 2015, respectively, and was generated entirely in the U.S.
Deferred taxes are recognized for temporary differences between the basis of assets and liabilities for financial statement and income tax purposes. The significant components of the Company’s deferred tax assets are comprised of the following (in thousands):
|
|
|
Year Ended |
|
Year Ended |
|
Year Ended |
| |||
|
|
|
December 31, 2017 |
|
December 31, 2016 |
|
December 31, 2015 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Net operating losses |
|
$ |
48,220 |
|
$ |
44,927 |
|
$ |
20,086 |
|
|
Accrued expenses |
|
644 |
|
687 |
|
53 |
| |||
|
Research and development expenses |
|
9,639 |
|
2,103 |
|
2,344 |
| |||
|
Research and development tax credits |
|
20,665 |
|
3,929 |
|
1,899 |
| |||
|
Stock options |
|
7,178 |
|
5,186 |
|
3,065 |
| |||
|
Other temporary differences |
|
155 |
|
12 |
|
2 |
| |||
|
Gross deferred tax assets |
|
86,501 |
|
56,844 |
|
27,449 |
| |||
|
Deferred tax valuation allowance |
|
(86,501 |
) |
(56,844 |
) |
(27,449 |
) | |||
|
|
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
On December 22, 2017, the Tax Cuts and Jobs Act” (the “2017 Tax Act”) was enacted. The 2017 Tax Act lowered the U.S. federal corporate income tax rate from 35% to 21% effective January 1, 2018. As a result, the change in the U.S. federal tax rate required the Company to re-measure its federal deferred tax assets and liabilities. Effective for tax years beginning on January 1, 2018, the 2017 Tax Act repealed the performance exception permitting certain executive officer compensation greater than $1 million to be deducted.
On December 22, 2017, the SEC issued Staff Accounting Bulletin No. 118 (“SAB 118”) which also provides guidance on accounting for the impacts of the 2017 Tax Act. SAB 118 provides a measurement period of up to one year from enactment for a company to complete its tax accounting under ASC 740. Once a company is able to make a reasonable estimate and record a provisional amount for effects of the 2017 Tax Act, it is required to do so. Given the substantial uncertainties surrounding the Tax Act and the short period of time between December 22, 2017 and December 31, 2017 to calculate the impacts of the 2017 Tax Act, the Company is accounting for its impact on a provisional (estimated) basis as allowed by SAB 118. Such provisional measurement amounts are anticipated to change as remaining analysis and review are completed, until the Company records a final amount within the measurement period.
During the fourth quarter of 2017, the Company reduced its net deferred tax asset balance and offsetting valuation allowance by $38.8 million as a provisional amount for the re-measurement of its U.S. deferred tax balances. This amount represents the Company’s reasonable estimate of the impact from the 2017 Tax Act.
The Company has evaluated the positive and negative evidence bearing upon the realizability of its deferred tax assets. Based on the Company’s history of operating losses since inception, the Company has concluded that it is more likely than not that the benefit of its deferred tax assets will not be realized. Accordingly, the Company has provided a full valuation allowance for deferred tax assets as of December 31, 2017, 2016 and 2015. Although the Company expects that it will generate taxable income in 2018 to utilize some or all of the net operating loss carryforward, the Company does not expect to generate sufficient core earnings, and as a result, the expected level and character of future taxable income is not adequate to realize the benefit of previously recorded deferred tax assets. The valuation allowance increased by $29.7 million, $29.4 million, and $15.4 million for the years ended December 31, 2017, 2016 and 2015, respectively, due primarily to the generation of net operating losses during the periods.
A reconciliation of income tax benefit computed at the statutory federal income tax rate to income taxes as reflected in the financial statements is as follows:
|
|
|
Year Ended |
|
Year Ended |
|
Year Ended |
|
|
|
|
December 31, 2017 |
|
December 31, 2016 |
|
December 31, 2015 |
|
|
U.S. statutory income tax rate |
|
34.0 |
% |
34.0 |
% |
34.0 |
% |
|
State income taxes, net of federal benefit |
|
(2.5 |
) |
4.2 |
|
6.5 |
|
|
Stock options |
|
3.2 |
|
(0.4 |
) |
(0.7 |
) |
|
Provision to return true-up |
|
— |
|
(0.3 |
) |
(0.1 |
) |
|
2017 Tax Act |
|
(26.0 |
) |
— |
|
— |
|
|
R&D credit carryforwards |
|
11.2 |
|
3.1 |
|
3.3 |
|
|
Valuation allowance |
|
(19.9 |
) |
(40.6 |
) |
(43.0 |
) |
|
Effective tax rate |
|
— |
% |
— |
% |
— |
% |
As of December 31, 2017, 2016 and 2015, the Company had U.S. federal net operating loss carryforwards of $220.8 million, $116.7 million, and $52.1 million, respectively, which may be available to offset future income tax liabilities and will begin to expire at various dates starting in 2033. As of December 31, 2017, 2016 and 2015, the Company also had U.S. state net operating loss carryforwards of $209.3 million, $105.4 million and $40.2 million, respectively, which may be available to offset future income tax liabilities and will begin to expire at various dates starting in 2033.
As of December 31, 2017, 2016 and 2015, the Company had federal research and development tax credit carryforwards of $20.6 million, $3.9 million, and $1.9 million, respectively, available to reduce future tax liabilities which will begin to expire at various dates starting in 2033.
Under the provisions of the Internal Revenue Code, the NOL and tax credit carryforwards are subject to review and possible adjustment by the Internal Revenue Service and state tax authorities. NOL and tax credit carryforwards may become subject to an annual limitation in the event of certain cumulative changes in the ownership interest of significant shareholders over a three-year period in excess of 50%, as defined under Sections 382 and 383 of the Internal Revenue Code of 1986, respectively, as well as similar state tax provisions. This could limit the amount of tax attributes that the Company can utilize annually to offset future taxable income or tax liabilities. The amount of the annual limitation, will be determined based on the value of the Company immediately prior to the ownership change. Subsequent ownership changes may further affect the limitation in future years. The Company experienced ownership changes, as defined, in 2004 and 2017. Accordingly, the use of NOLs generated prior to these ownership changes is subject to an annual limitation. The Company does not expect the annual limitation will impact its ability to utilize some or all of the net operating loss carryforwards to offset the anticipated taxable income in 2018. If certain changes in ownership occur prospectively, there could be an additional annual limitation on the amount of utilizable NOLs.
Unrecognized income tax benefits represent income tax positions taken on income tax returns but not yet recognized in the consolidated financial statements. The company has unrecognized income tax benefits totaling $3.4 million as of December 31, 2017. If recognized, none of the unrecognized tax benefits would be recorded as a benefit to income tax expense on the consolidated statement of income.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
|
|
|
2017 |
| |
|
Unrecognized income tax benefits, January 1 |
|
$ |
— |
|
|
Additions for tax positions of prior years |
|
1.0 |
| |
|
Reductions for tax positions of prior years |
|
— |
| |
|
Additions for current year tax positions |
|
2.4 |
| |
|
Reductions for settlements with taxing authorities |
|
— |
| |
|
Reductions as a result of a lapse of an applicable statute of limitations |
|
— |
| |
|
Unrecognized income tax benefits, December 31 |
|
$ |
3.4 |
|
The Company files income tax returns in the U.S., and various state jurisdictions. The federal and state income tax returns are generally subject to tax examinations for the years ended December 31, 2017, 2016, 2015 and 2014. To the extent the Company has tax attribute carryforwards, the tax years in which the attribute was generated may still be adjusted upon examination by the Internal Revenue Service or state tax authorities to the extent utilized in a future period.
11. Related Party Transactions
As of December 31, 2015, Array indicated that it was no longer a holder of more than 5% of the Company’s common stock; therefore, the Company is not reporting expenses with Array as related party research and development expense subsequent to the year ended December 31, 2015. The Company recorded related party research and development expenses for services provided by Array under a collaboration agreement of $11.6 million for the year ended December 31, 2015.
Dr. Lori Kunkel, a board member, had a consulting agreement with the Company to assist in the Company’s drug development process which was modified effective as of October 31, 2015, to provide that she receives only the standard director compensation for her services. Dr. Kunkel also received stock option grants in 2013 and 2014 as compensation for her consulting services which continue to vest. Both cash compensation that was expensed as incurred and stock compensation are recorded as a component of research and development expenses. During the years ended December 31, 2017, 2016 and 2015, the Company recognized cash compensation expense of $0, $0 and $0.2 million and stock compensation expense of $1.8 million, $1.1 million, and $0 in accordance with the terms of the consulting agreement.
Dr. Keith Flaherty, a board member, has an agreement with the Company to serve as Scientific Advisor Board (SAB) Chair for which he receives cash compensation. Dr. Flaherty also received stock option grants in 2013 and 2014 as compensation for his SAB services which continue to vest. Both cash compensation that was expensed as incurred and stock compensation are recorded as a component of research and development expenses. During the years ended December 31, 2017, 2016 and 2015, the Company recognized cash compensation expense of $0.1 million, $0.1 million and $0.7 million and stock compensation expense of $2.5 million, $0.8 million, and $1.6 million in accordance with the terms of the SAB agreement.
12. Asset Acquisition
On July 28, 2017, the Company entered into and closed on an Agreement for the Assignment of Patents and other Rights and for the Novation of Certain Agreements, including for Product Manufacturing, with Redx Pharma Plc and Redx Oncology Limited (collectively “Redx”), pursuant to which the Company paid $40.0 million in cash to acquire IPR&D, including patents and other rights related to the Redx Bruton’s tyrosine kinase (“BTK”) inhibitor discovery program, including lead product candidate LOXO-305. The Company is not subject to any future milestone or royalty payments related to this transaction. The transaction was accounted for as an asset acquisition pursuant to ASU 2017-01 as the majority of the fair value of the assets acquired was concentrated in a group of similar assets. The total cost of $40.0 million was included in research and development expense in the Company’s consolidated statements of operations for the year ended December 31, 2017 because the IPR&D acquired did not have an alternative future use.
13. Unaudited Quarterly Data
The following table summarizes certain supplemental unaudited quarterly financial data for each of the quarters in the years ended December 31, 2017 and 2016, respectively. The operating results for any quarter are not indicative of results that may be expected for a full year or any future periods.
|
|
|
First |
|
Second |
|
Third |
|
Fourth |
| ||||
|
2017 |
|
|
|
|
|
|
|
|
| ||||
|
Net loss |
|
$ |
(24,528 |
) |
$ |
(30,401 |
) |
$ |
(73,319 |
) |
$ |
(20,628 |
) |
|
Net loss per share of common stock—basic and diluted |
|
$ |
(0.96 |
) |
$ |
(1.14 |
) |
$ |
(2.45 |
) |
$ |
(0.69 |
) |
|
|
|
|
|
|
|
|
|
|
| ||||
|
2016 |
|
|
|
|
|
|
|
|
| ||||
|
Net loss |
|
$ |
(11,597 |
) |
$ |
(15,916 |
) |
$ |
(17,691 |
) |
$ |
(27,194 |
) |
|
Net loss per share of common stock—basic and diluted |
|
$ |
(0.59 |
) |
$ |
(0.77 |
) |
$ |
(0.82 |
) |
$ |
(1.26 |
) |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and principal financial officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2017, the end of the period covered by this Annual Report on Form 10-K.
The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure.
Based on our evaluation, we have concluded that our disclosure controls and procedures as of December 31, 2017, the end of the period covered by this Annual Report on Form 10-K, have been designed and are effective to provide reasonable assurance that the information required to be disclosed by us in reports filed under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. We believe that a controls system, no matter how well designed and operated, cannot provide absolute assurance that the objectives of the controls system are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Management assessed the effectiveness of our internal control over financial reporting as of December 31, 2017. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control—Integrated Framework issued in 2013. Based upon the assessments, management has concluded that as of December 31, 2017 our internal control over financial reporting was effective to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with GAAP. Our independent registered public firm, Ernst & Young LLP, has issued an audit report with respect to our internal control over financial reporting, which appears in Part II, Item 9A of this Annual Report on Form 10-K.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting during our fourth fiscal quarter ended December 31, 2017 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of Loxo Oncology, Inc.
Opinion on Internal Control over Financial Reporting
We have audited Loxo Oncology’s internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, Loxo Oncology Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2017, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the 2017 consolidated financial statements of Loxo Oncology, Inc. and our report dated March 1, 2018 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
|
/s/ Ernst & Young LLP |
|
|
|
|
|
Hartford, Connecticut |
|
|
March 1, 2018 |
|
None.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Directors
Information required with respect to this Item 10 is set forth in the Proxy Statement for the 2018 Annual Meeting of Stockholders (“Proxy Statement”) under the headings “Election of Directors,” “Executive Officers,” “Section 16(a) Beneficial Ownership Reporting Compliance,” “Code of Ethics” and “Corporate Governance” and is incorporated herein by reference.
Our board of directors has adopted a Code of Business Conduct and Ethics applicable to all officers, directors and employees, including our principal executive officer and principal financial and accounting officer, which is available on our website (www.loxooncology.com).
The Proxy Statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item 11 is incorporated by reference to the information contained in our definitive Proxy Statement.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this Item 12 is incorporated by reference to the information contained in our Proxy Statement.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this Item 13 is incorporated by reference to the information contained in our Proxy Statement.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by this Item 14 is incorporated by reference to the information contained in our Proxy Statement.
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) DOCUMENTS FILED AS PART OF THIS REPORT
The following is a list of our financial statements included in this Annual Report on Form 10-K under Item 8 of Part II hereof:
1. FINANCIAL STATEMENTS AND SUPPLEMENTAL DATA
(b) EXHIBITS
|
|
|
|
|
Incorporated by Reference |
|
| ||||
|
Exhibit |
|
|
|
|
|
|
|
Date of First |
|
Exhibit |
|
Number |
|
Description of Document |
|
Form |
|
File No. |
|
Filing |
|
No. |
|
3.1 |
|
|
S-1/A |
|
333-197123 |
|
July 21, 2014 |
|
3.2 | |
|
3.2 |
|
|
S-1/A |
|
333-197123 |
|
July 21, 2014 |
|
3.4 | |
|
4.1 |
|
|
S-1/A |
|
333-197123 |
|
July 21, 2014 |
|
4.1 | |
|
4.2 |
|
|
S-1/A |
|
333-197123 |
|
July 21, 2014 |
|
4.2 | |
|
10.1 |
|
|
S-1/A |
|
333-197123 |
|
July 21, 2014 |
|
10.1 | |
|
10.2 |
|
|
S-1/A |
|
333-197123 |
|
July 30, 2014 |
|
10.2 | |
|
10.3 |
|
|
S-1/A |
|
333-197123 |
|
July 21, 2014 |
|
10.3 | |
|
10.4 |
|
|
S-1 |
|
333-197123 |
|
June 30, 2014 |
|
10.4 | |
|
10.5‡ |
|
|
10-K |
|
001-36562 |
|
March 7, 2017 |
|
10.5 | |
|
10.6 |
|
|
S-1 |
|
333-197123 |
|
June 30, 2014 |
|
10.6 | |
|
10.7† |
|
|
S-1 |
|
333-197123 |
|
June 30, 2014 |
|
10.7 | |
|
10.8‡ |
|
|
S-1 |
|
333-197123 |
|
June 30, 2014 |
|
10.8 | |
|
10.9‡ |
|
|
S-1/A |
|
333-197123 |
|
July 21, 2014 |
|
10.9 | |
|
10.10† |
|
Amendment No. 3 to Drug Discovery and Collaboration Agreement, dated October 13, 2014. |
|
10-K |
|
001-36562 |
|
March 27, 2015 |
|
10.11 |
|
10.11‡ |
|
Offer Letter by and between Loxo and Jennifer Burstein dated March 27, 2015. |
|
10-Q |
|
001-36562 |
|
May 14, 2015 |
|
10.2 |
|
10.12† |
|
Amendment No. 4 to Drug Discovery and Collaboration Agreement, dated March 31, 2015 |
|
10-Q |
|
001-36562 |
|
May 14, 2015 |
|
10.3 |
|
10.13 |
|
|
10-Q |
|
001-36562 |
|
November 10, 2015 |
|
10.1 | |
|
10.14† |
|
Amendment No. 5 to Drug Discovery and Collaboration Agreement, dated February 18, 2016 |
|
10-K |
|
001-36562 |
|
March 15, 2016 |
|
10.16 |
|
10.15† |
|
Separation and Consulting Agreements by and between Dr. Jennifer Low and the Registrant |
|
10-K |
|
001-36562 |
|
March 15, 2016 |
|
10.17 |
|
10.16‡ |
|
|
10-K |
|
001-36562 |
|
March 7, 2017 |
|
10.18 | |
|
10.17† |
|
|
10-Q |
|
001-36562 |
|
November 2, 2017 |
|
10.1 | |
|
10.18† |
|
|
10-Q |
|
001-36562 |
|
November 2, 2017 |
|
10.2 | |
|
10.19*± |
|
|
|
|
|
|
|
|
| |
|
10.20* |
|
|
|
|
|
|
|
|
| |
|
23.1* |
|
Consent of Ernst & Young LLP, independent registered public accounting firm. |
|
|
|
|
|
|
|
|
|
23.2* |
|
Consent of CohnReznick LLP, independent registered public accounting firm |
|
|
|
|
|
|
|
|
|
24.1* |
|
|
|
|
|
|
|
|
| |
|
31.1* |
|
|
|
|
|
|
|
|
| |
|
31.2* |
|
|
|
|
|
|
|
|
| |
|
32.1* |
|
|
|
|
|
|
|
|
| |
|
32.2* |
|
|
|
|
|
|
|
|
| |
|
101.INS* |
|
XBRL Instance Document |
|
|
|
|
|
|
|
|
|
101.SCH* |
|
XBRL Taxonomy Extension Schema Document |
|
|
|
|
|
|
|
|
|
101.CAL* |
|
XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
|
|
|
|
|
101.DEF* |
|
XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
|
|
|
|
|
|
101.LAB* |
|
XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
|
|
|
|
|
|
101.PRE* |
|
XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
|
|
|
|
|
† Confidential treatment request granted
± Confidential treatment requested
‡ Management compensation plan or agreement
* Filed herewith
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
LOXO ONCOLOGY, INC. | |
|
|
| |
|
Dated: March 1, 2018 |
| |
|
|
By: |
/s/Joshua H. Bilenker, M.D. |
|
|
|
Joshua H. Bilenker, M.D. |
|
|
|
President, Chief Executive Officer and Director |
|
|
|
(Principal Executive Officer) |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
LOXO ONCOLOGY, INC. | |
|
|
| |
|
Dated: March 1, 2018 |
| |
|
|
By: |
/s/ Jennifer Burstein |
|
|
|
Jennifer Burstein |
|
|
|
Senior Vice President of Finance |
|
|
|
(Principal Accounting Officer and |
|
|
|
Principal Financial Officer) |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Joshua H. Bilenker and Jennifer Burstein, jointly and severally, their attorneys-in-fact, each with the power of substitution, for them in any and all capacities, to sign any amendments to this Annual Report on Form 10-K and to file same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact, or his substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ Joshua H. Bilenker, M.D. |
|
President, Chief Executive Officer and |
|
March 1, 2018 |
|
Joshua H. Bilenker, M.D. |
|
Director (Principal Executive Officer) |
|
|
|
|
|
|
|
|
|
/s/ Jennifer Burstein |
|
Senior Vice President of Finance |
|
March 1, 2018 |
|
Jennifer Burstein |
|
(Principal Accounting Officer and |
|
|
|
|
|
Principal Financial Officer) |
|
|
|
|
|
|
|
|
|
/s/ Steven A. Elms |
|
Director |
|
March 1, 2018 |
|
Steven A. Elms |
|
|
|
|
|
|
|
|
|
|
|
/s/ Keith T. Flaherty, M.D. |
|
Director |
|
March 1, 2018 |
|
Keith T. Flaherty, M.D. |
|
|
|
|
|
|
|
|
|
|
|
/s/ Alan Fuhrman |
|
Director |
|
March 1, 2018 |
|
Alan Fuhrman |
|
|
|
|
|
|
|
|
|
|
|
/s/ Steve D. Harr, M.D. |
|
Director |
|
March 1, 2018 |
|
Steve D. Harr, M.D. |
|
|
|
|
|
|
|
|
|
|
|
/s/ Lori Kunkel, M.D. |
|
Director |
|
March 1, 2018 |
|
Lori Kunkel, M.D. |
|
|
|
|
|
|
|
|
|
|
|
/s/ Timothy Mayleben |
|
Director |
|
March 1, 2018 |
|
Timothy Mayleben |
|
|
|
|
|
|
|
|
|
|
|
/s/ Avi Z. Naider |
|
Director |
|
March 1, 2018 |
|
Avi Z. Naider |
|
|
|
|
