Attached files
| file | filename |
|---|---|
| EX-99.4 - EX-99.4 - Loxo Oncology, Inc. | a17-14662_1ex99d4.htm |
| 8-K - 8-K - Loxo Oncology, Inc. | a17-14662_18k.htm |
| EX-99.5 - EX-99.5 - Loxo Oncology, Inc. | a17-14662_1ex99d5.htm |
| EX-99.2 - EX-99.2 - Loxo Oncology, Inc. | a17-14662_1ex99d2.htm |
| EX-99.1 - EX-99.1 - Loxo Oncology, Inc. | a17-14662_1ex99d1.htm |
Exhibit 99.3
The efficacy of larotrectinib (LOXO-101), a selective tropomyosin receptor kinase (TRK) inhibitor, in adult and pediatric TRK fusion cancers Hyman DM,1 Laetsch TW, 2 Kummar S,3 DuBois SG,4 Farago AF,5 Pappo AS,6 Demetri GD,7 El-Deiry WS,8 Lassen UN,9 Dowlati A,10 Brose MS,11 Boni V,12 Turpin B,13 Nagasubramanian R,14 Cruickshank S,15 Cox MC,15 Ku NC,15 Hawkins DS,16 Hong DS,17 Drilon AE1 1Memorial Sloan Kettering Cancer Center, New York, NY; 2University of Texas Southwestern, Dallas, TX; 3Stanford University School of Medicine, Palo Alto, CA; 4Dana-Farber Cancer Institute/Boston Children's Cancer and Blood Disorders Center, Boston, MA; 5Massachusetts General Hospital, Boston, MA; 6St. Jude Children's Research Hospital, Memphis, TN; 7Dana-Farber Cancer Institute/Harvard Medical School, Boston, MA; 8Fox Chase Cancer Center, Philadelphia, PA; 9Rigshospitalet, Copenhagen, Denmark; 10UH Cleveland Medical Center, Cleveland, OH; 11Department of Otorhinolaryngology: Head and Neck Surgery, Abramson Cancer Center of the University of Pennsylvania, Philadelphia, PA; 12START Madrid CIOCC, Hospital HM Universitario Sanchinarro, Madrid, Spain; 13Cincinnati Children's Hospital Medical Center, Cincinnati, OH; 14Nemour's Children's Hospital, Orlando, FL; 15Loxo Oncology, Inc., San Francisco, CA; 16Seattle Children’s Hospital, University of Washington, Fred Hutchinson Cancer Research Center, Seattle, WA; 17The University of Texas MD Anderson Cancer Center, Houston, TX Hyman, LBA2501
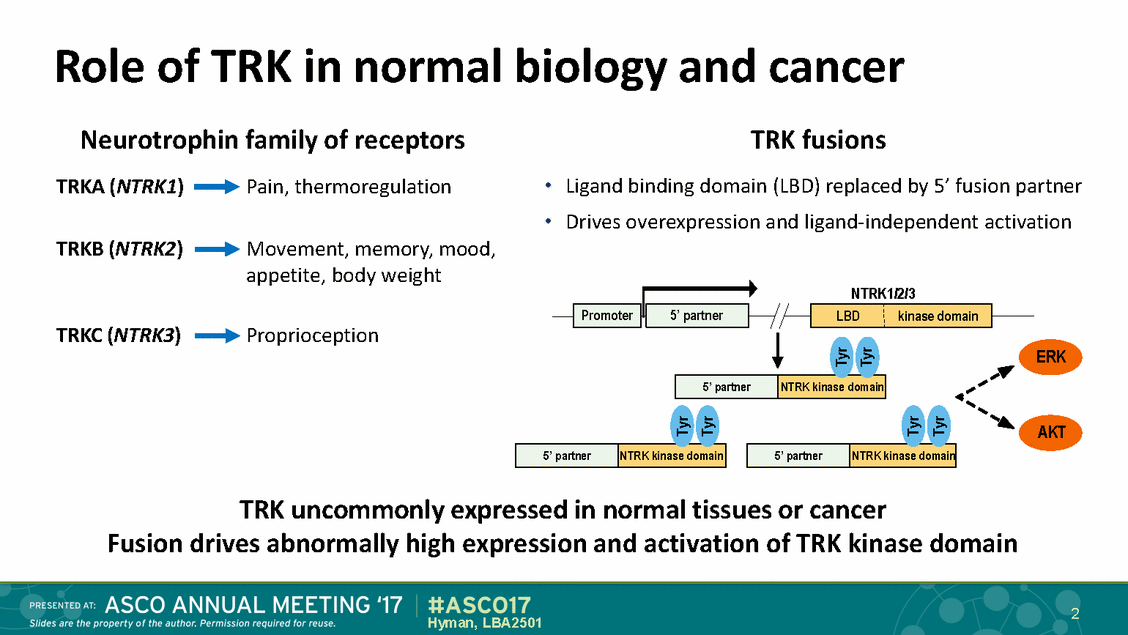
Role of TRK in normal Neurotrophin family of receptors biology and cancer TRK fusions • • TRKA (NTRK1) Pain, thermoregulation Ligand binding domain (LBD) replaced by 5’ fusion partner Drives overexpression and ligand-independent activation TRKB (NTRK2) Movement, memory, mood, appetite, body weight NTRK1/2/3 Proprioception TRKC (NTRK3) ERK AKT TRK uncommonly expressed in normal tissues or cancer Fusion drives abnormally high expression and activation of TRK kinase domain 2 Hyman, LBA2501 Tyr Tyr Tyr Tyr Tyr Tyr 5’ pa rtner NTRK kina se domain 5’ partner NTRK kinase domain 5’ partner NTRK kinase domain Promoter 5’ partner LBD kinase domain
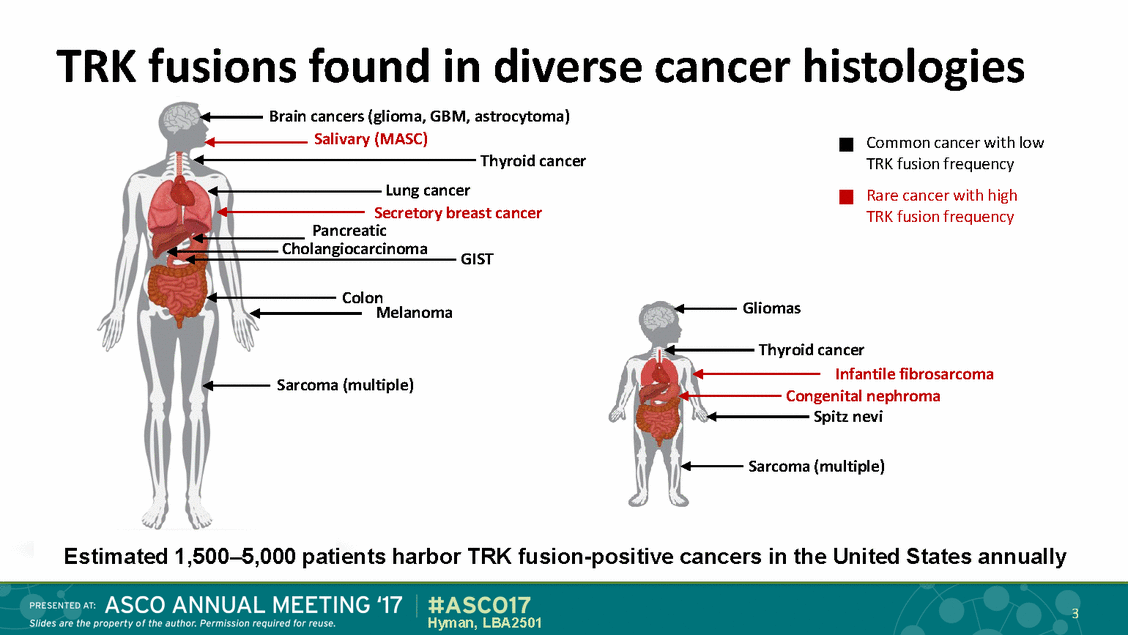
TRK fusions found in diverse Brain cancers (glioma, GBM, astrocytoma) Salivary (MASC) Thyroid cancer Lung cancer Secretory breast cancer Pancreatic cancer histologies Common cancer with low TRK fusion frequency Rare cancer with high TRK fusion frequency Cholangiocarcinoma GIST Colon Melanoma Gliomas Thyroid cancer Infantile fibrosarcoma Congenital nephroma Spitz nevi Sarcoma (multiple) Sarcoma (multiple) Estimated 1,500–5,000 patients harbor TRK fusion-positive cancers in the United States annually 3 Hyman, LBA2501
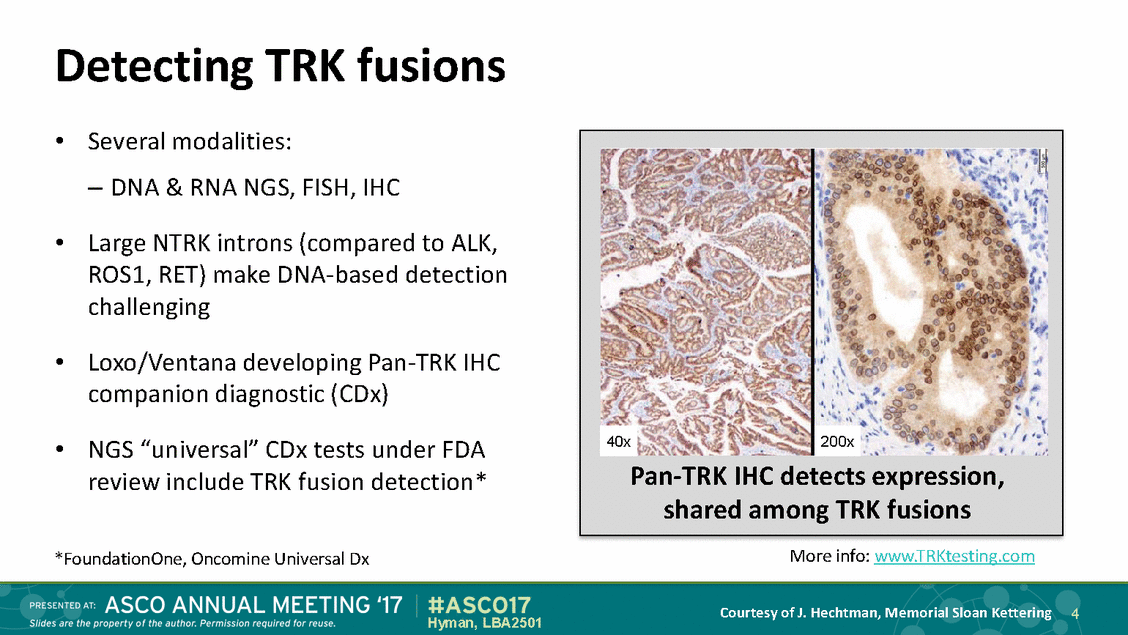
Detecting TRK fusions • Several modalities: – DNA & RNA NGS, FISH, IHC Large NTRK introns (compared to ALK, ROS1, RET) make DNA-based detection challenging • • Loxo/Ventana developing Pan-TRK IHC companion diagnostic (CDx) • NGS “universal” CDx tests under FDA review include TRK fusion detection* More info: www.TRKtesting.com *FoundationOne, Oncomine Universal Dx Courtesy of J. Hechtman, Memorial Sloan Kettering 4 Hyman, LBA2501 Pan-TRK IHC detects expression, shared among TRK fusions 200x 40x
Larotrectinib TRKA/B/C • Larotrectinib is the first and only selective pan-TRK inhibitor in clinical development • Highly potent against TRKA, TRKB, TRKC –5–11 nM IC50 in cellular assays Highly selective • • Development timeline – – – March 2015: 1st TRK-fusion patient treated July 2016: breakthrough therapy designation February 2017: pivotal enrollment complete 5 Hyman, LBA2501
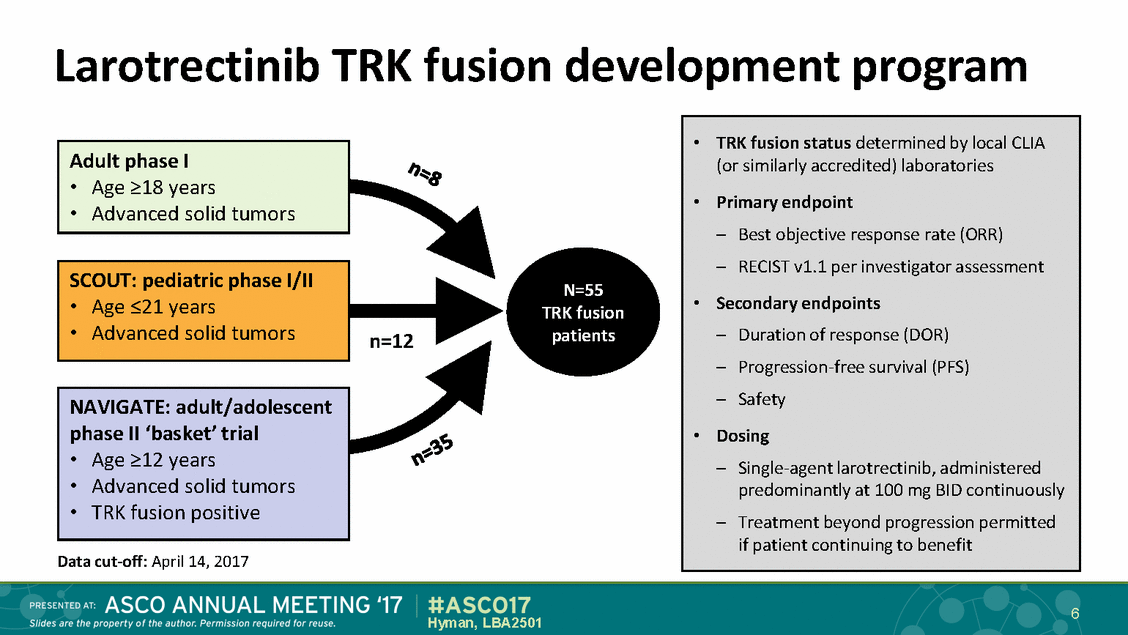
Larotrectinib TRK fusion development program • Age >18 years N=55 TRK fusion patients n=12 • Advanced solid tumors Data cut-off: April 14, 2017 6 Hyman, LBA2501 NAVIGATE: adult/adolescent phase II ‘basket’ trial • Age >12 years • TRK fusion positive SCOUT: pediatric phase I/II • Age <21 years • Advanced solid tumors Adult phase I • Advanced solid tumors •TRK fusion status determined by local CLIA (or similarly accredited) laboratories •Primary endpoint – Best objective response rate (ORR) – RECIST v1.1 per investigator assessment • Secondary endpoints – Duration of response (DOR) – Progression-free survival (PFS) – Safety • Dosing – Single-agent larotrectinib, administered predominantly at 100 mg BID continuously – Treatment beyond progression permitted if patient continuing to benefit
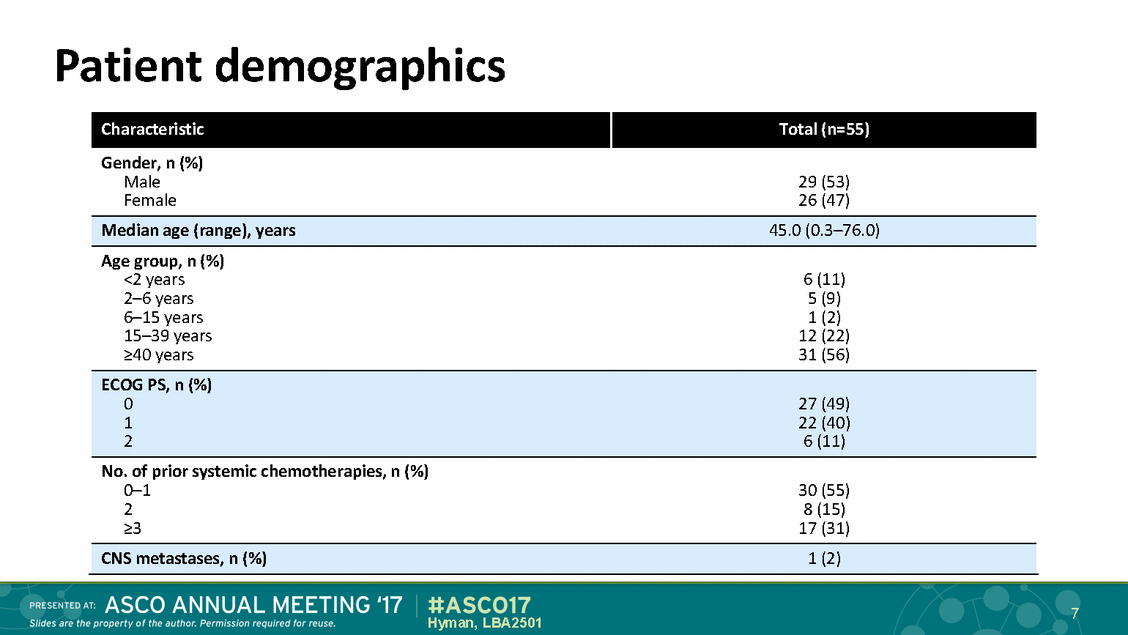
Patient demographics 7 Hyman, LBA2501 Characteristic Total (n=55) Gender, n (%) Male29 (53) Female26 (47) Median age (range), years45.0 (0.3–76.0) Age group, n (%) <2 years6 (11) 2–6 years5 (9) 6–15 years1 (2) 15–39 years12 (22) >40 years31 (56) ECOG PS, n (%) 027 (49) 122 (40) 26 (11) No. of prior systemic chemotherapies, n (%) 0–130 (55) 28 (15) >317 (31) CNS metastases, n (%)1 (2)
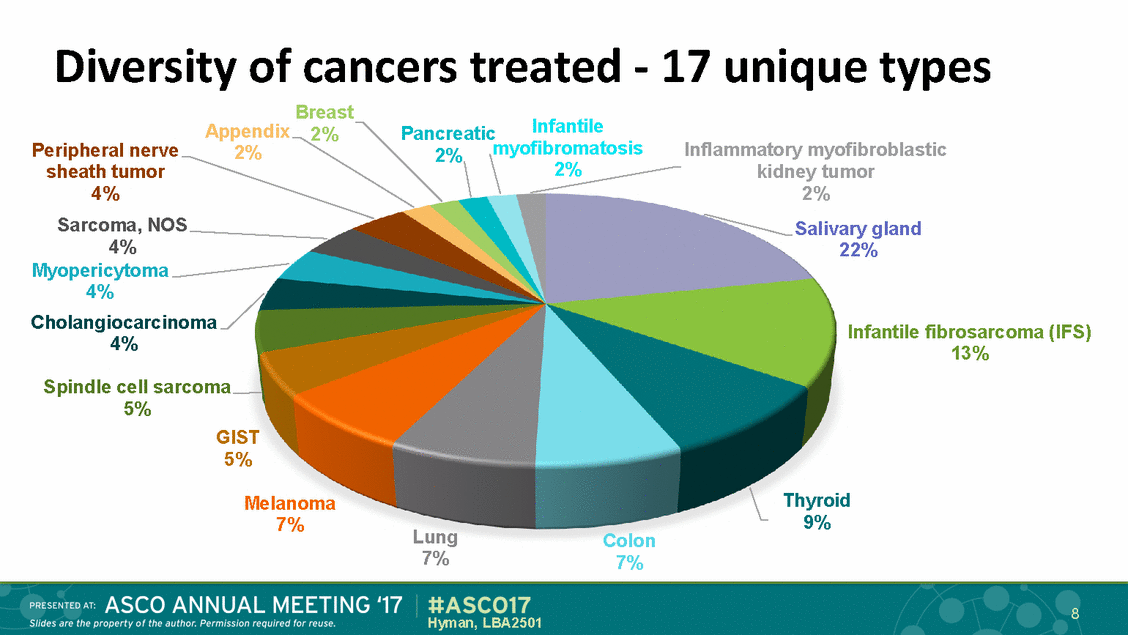
Diversity of cancers treated - 17 unique types Inflammatory myofibroblastic kidney tumor 2% Salivary gland 22% Breast 2% Infantile Appendix 2% Pancreatic myofibromatosis Peripheral nerve sheath tumor 4% Sarcoma, NOS 4% Myopericytoma 4% 2% 2% Cholangiocarcinoma 4% Infantile fibrosarcoma (IFS) 13% Spindle cell sarcoma 5% GIST 5% Thyroid 9% Melanoma 7% Lung 7% Colon 7% 8 Hyman, LBA2501
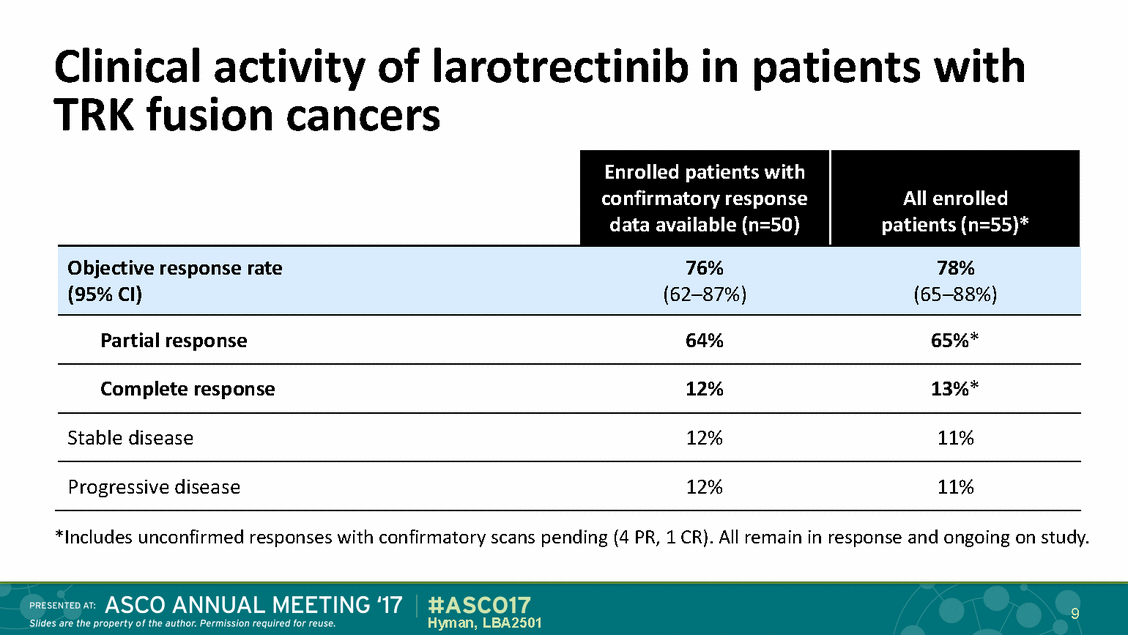
Clinical activity of larotrectinib TRK fusion cancers in patients with *Includes unconfirmed responses with confirmatory scans pending (4 PR, 1 CR). All remain in response and ongoing on study. 9 Hyman, LBA2501 Enrolled patients with confirmatory response data available (n=50) All enrolled patients (n=55)* Objective response rate76%78% (95% CI)(62–87%)(65–88%) Partial response64%65%* Complete response12%13%* Stable disease12%11% Progressive disease12%11%
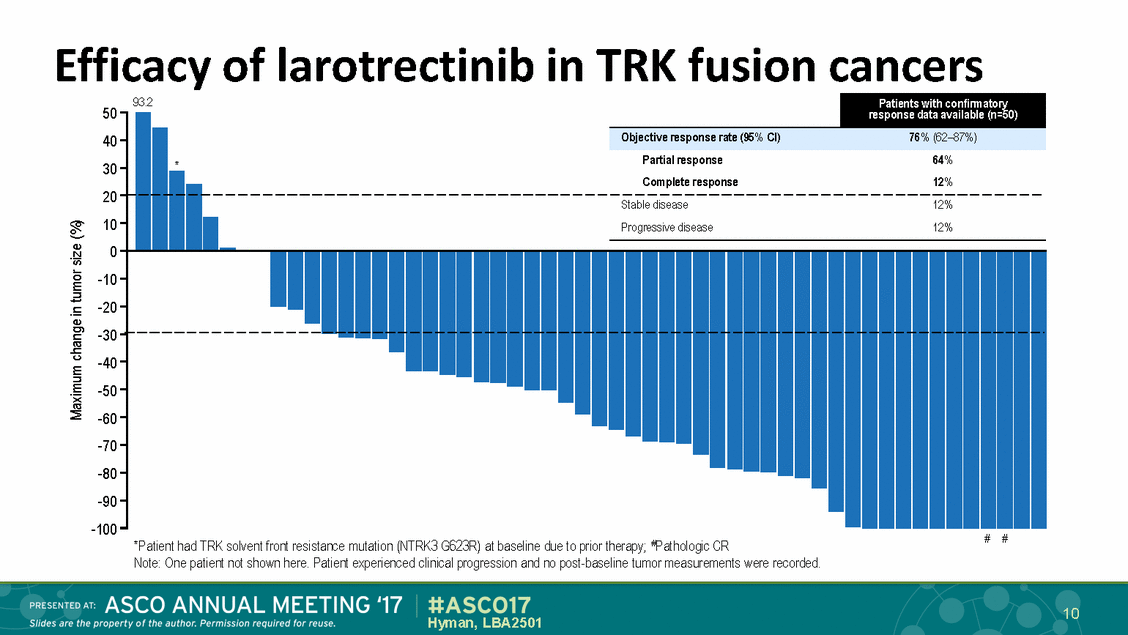
Efficacy of larotrectinib in TRK fusion cancers 93.2 50 40 30 20 10 0 -10 -20 -30 -40 -50 -60 -70 -80 -90 -100 Partial response Complete response 64% 12% * # # *Patient had TRK solvent front resistance mutation (NTRK3 G623R) at baseline due to prior therapy; #Pathologic CR Note: One patient not shown here. Patient experienced clinical progression and no post-baseline tumor measurements were recorded. 10 Hyman, LBA2501 Maximum change in tumor size (%) Stable disease12% Progressive disease12% Patients with confirmatory response data available (n=50) Objective response rate (95% CI)76% (62–87%)
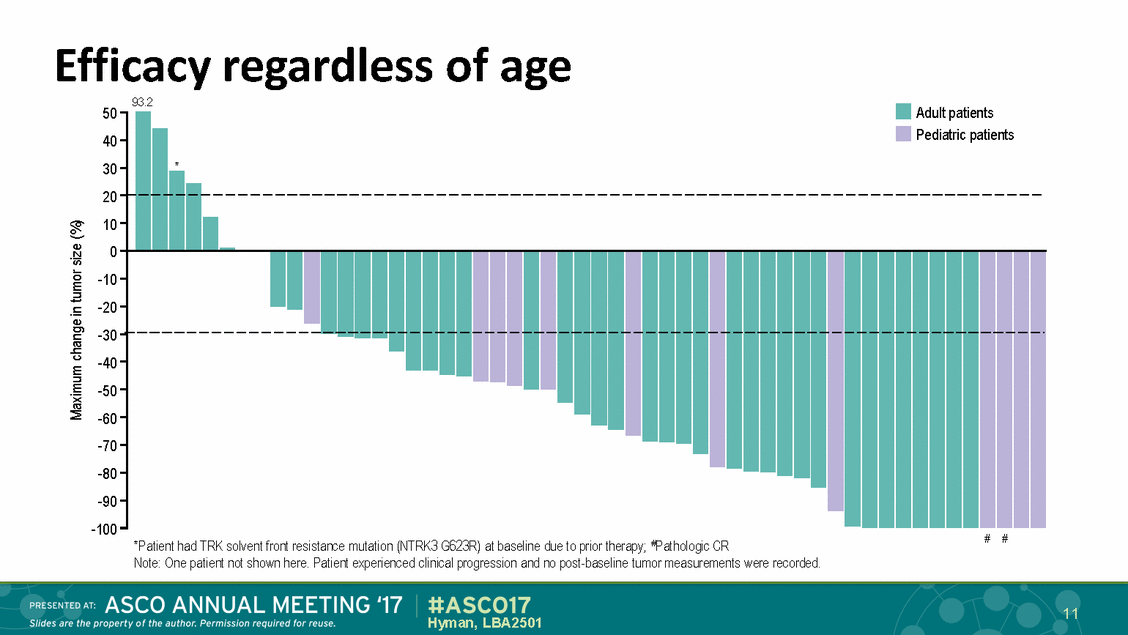
Efficacy 93.2 50-.- regardless of age •Adultpatients 40 30-20 - 10 -;-h cu ·u; 5 0 -10 -u---m----u-------;----.Ea .!: -20--30 -40 -50--60 -70--80 -90-cu Cl - ---r--c -;------------c:: (IJ ..r::. <> E ::::1 E ')( (IJ ........-........ .......... -100-.....n....n._.. # # 'Patient had TRK solvent front resistarce mutat1on (NTRK3 G623R) at baseline due to pr1or therapy; l?athologc CR Note One patient not shown here. Patient experienced clinicalprogression and no post-baseline tumor measurements were recorded. 1 11 EDAT: Hyman, LBA2501 Slides are the property of the author. Perm1ss1on required for reuse. PR ESENT #ASC017 17 ASCO ANNUAL MEETING . • Pediatric patients ,...., ......., -------------------------------------------------------------------In - .._.. .._ ._ r-----------.... r--c-----;-..._ ....... .................. .... .... ...... ...._. .........._ .._ ......
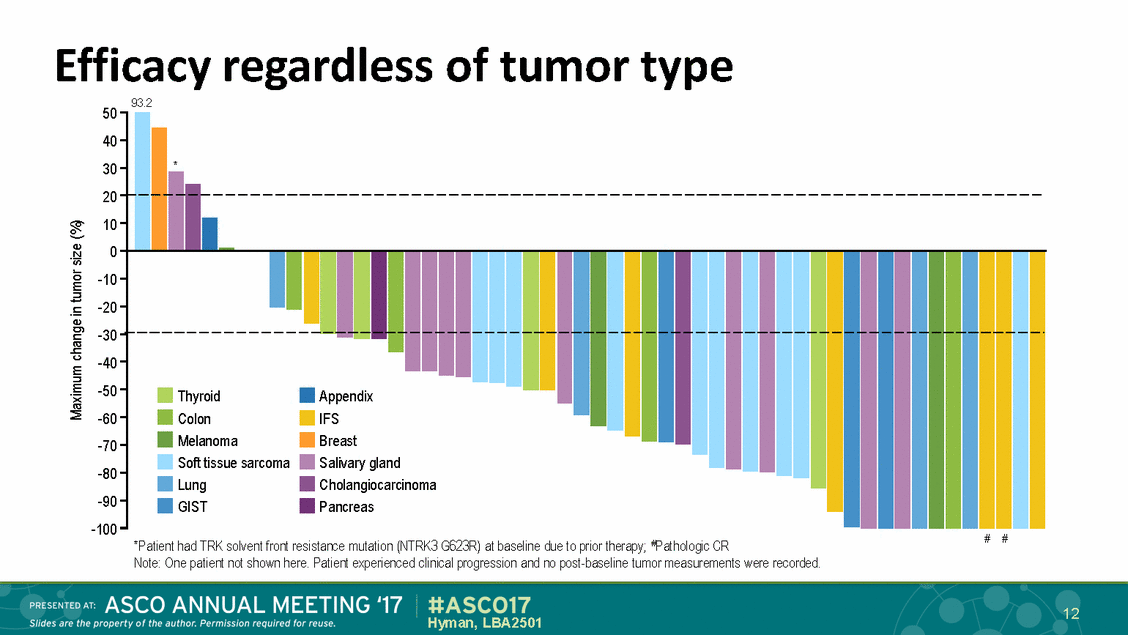
Efficacy regardless of tumor type 93.2 50 40 30 20 10 0 -10 -20 -30 -40 -50 -60 -70 -80 -90 -100 * Thyroid Colon Melanoma Soft tissue sarcoma Lung GIST Appendix IFS Breast Salivary gland Cholangiocarcinoma Pancreas # # *Patient had TRK solvent front resistance mutation (NTRK3 G623R) at baseline due to prior therapy; #Pathologic CR Note: One patient not shown here. Patient experienced clinical progression and no post-baseline tumor measurements were recorded. 12 Hyman, LBA2501 Maximum change in tumor size (%)
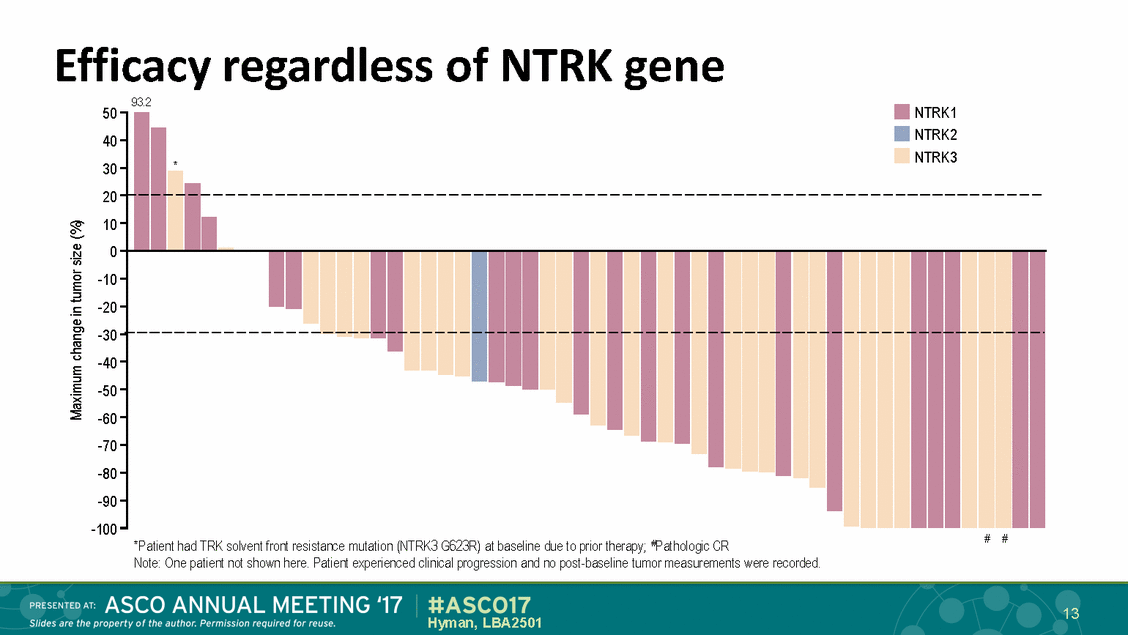
Efficacy regardless of NTRK gene 93.2 50-40 30-• NTRK1 • NTRK2 NTRK3 r-......., 20" ,_ ,_ ---------------------------------------------------------------------i<1) -30 r--------------------u---------E----------:---------------------E-----r-.... ......_ .... .., E ::::1 -40 -50--60 -70--80 -90--100-E ')( (IJ .... .... ............ .... .... # # *Patient had TRK solvent front resistarce mutat1on (NTRK3 G623R) at baseline due to pr1or therapy; l?athologc CR Note One patient not shown here. Patient experienced clinicalprogression and no post-baseline tumor measurements were recorded. 1 13 EDAT: Hyman, LBA2501 Slides are the property of the author. Perm1ss1on required for reuse. PR ESENT #ASC017 17 ASCO ANNUAL MEETING
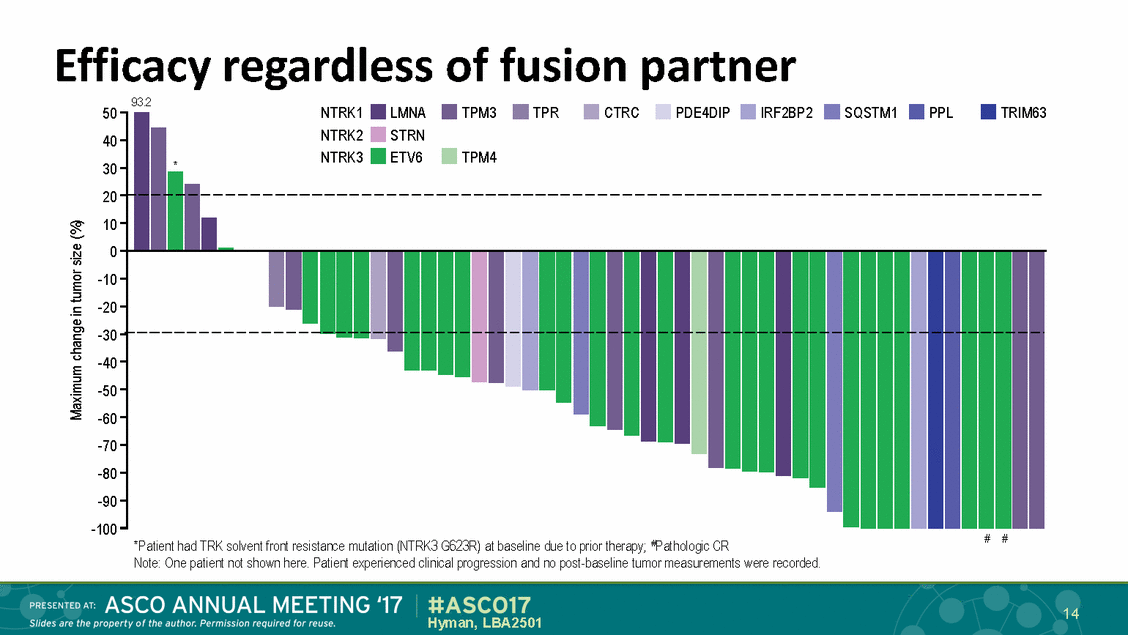
Efficacy regardless of fusion partner 93.2 NTRK1 NTRK2 NTRK3 LMNA STRN ETV6 TPM3 TPR CTRC PDE4DIP IRF2BP2 SQSTM1 PPL TRIM63 50 40 30 20 10 0 -10 -20 -30 -40 -50 -60 -70 -80 -90 -100 TPM4 * # # *Patient had TRK solvent front resistance mutation (NTRK3 G623R) at baseline due to prior therapy; #Pathologic CR Note: One patient not shown here. Patient experienced clinical progression and no post-baseline tumor measurements were recorded. 14 Hyman, LBA2501 Maximum change in tumor size (%)
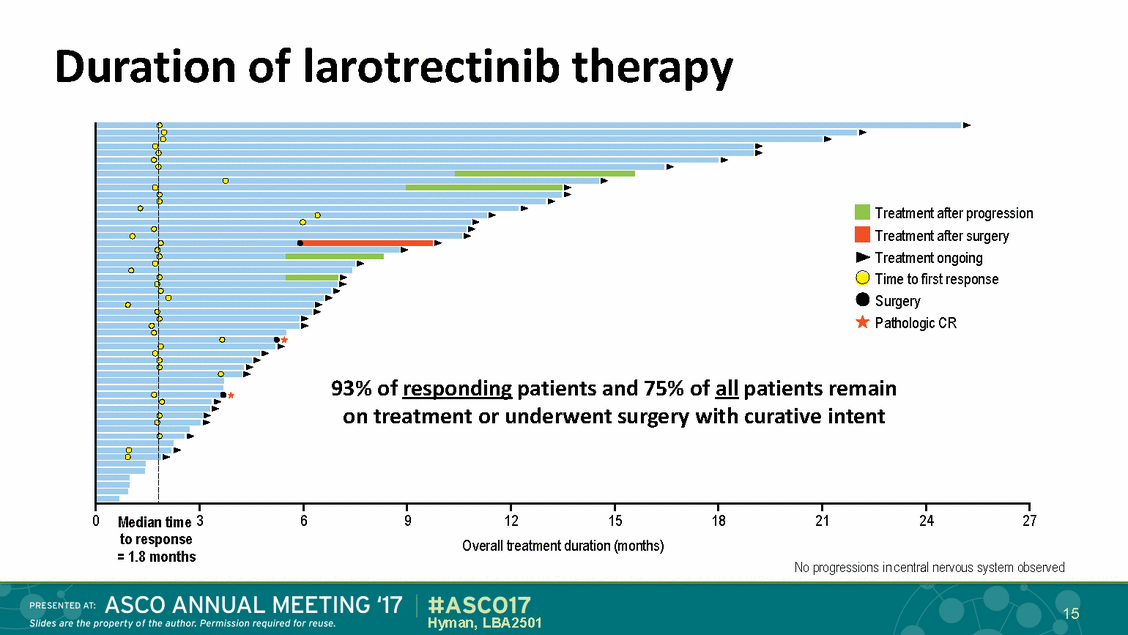
Duration of larotrectinib therapy progression surgery going esponse 93% of esponding tients and 75% of all patients remain n t atment or underwent surgery with curative ent 0 Median time 3 to response = 1.8 months 6 9 12 15 18 21 24 27 Overall treatment duration (months) No progressions in central nervous system observed 15 Hyman, LBA2501 Treatment after Treatment after Treatment on Time to first r Surgery Pathologic CR int pa r re o
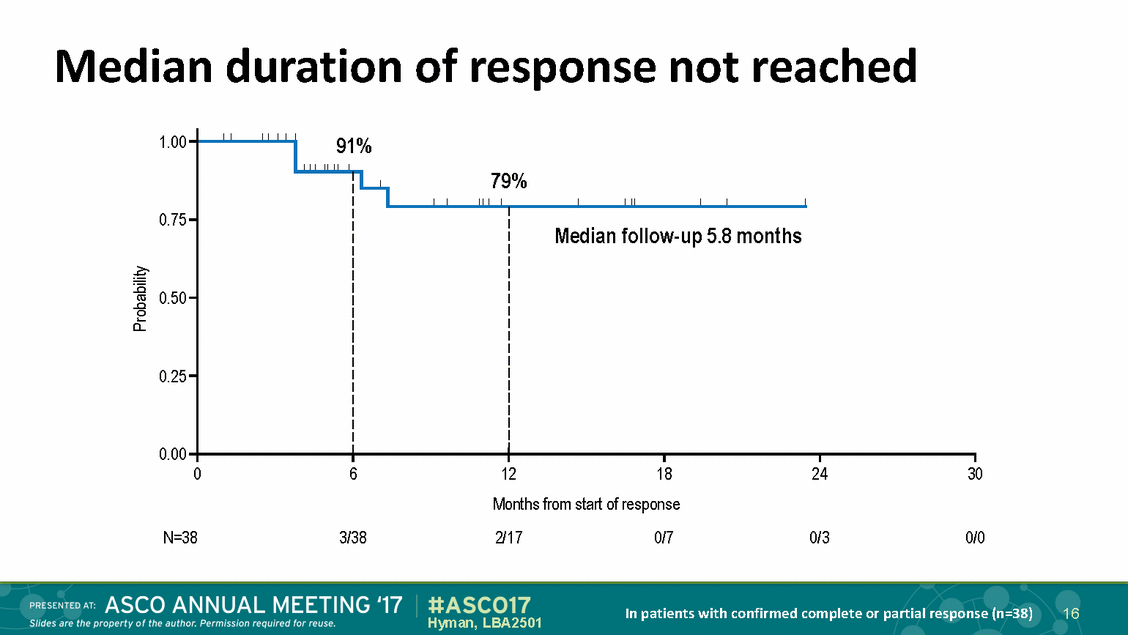
Median duration of response not reached 1.00 91% 0.75 dian follow-up 5.8 months 0.50 0.25 0.00 0 6 12 18 24 30 Months from start of response N=38 3/38 2/17 0/7 0/3 0/0 In patients with confirmed complete or partial response (n=38) 16 Hyman, LBA2501 Probability 79% Me
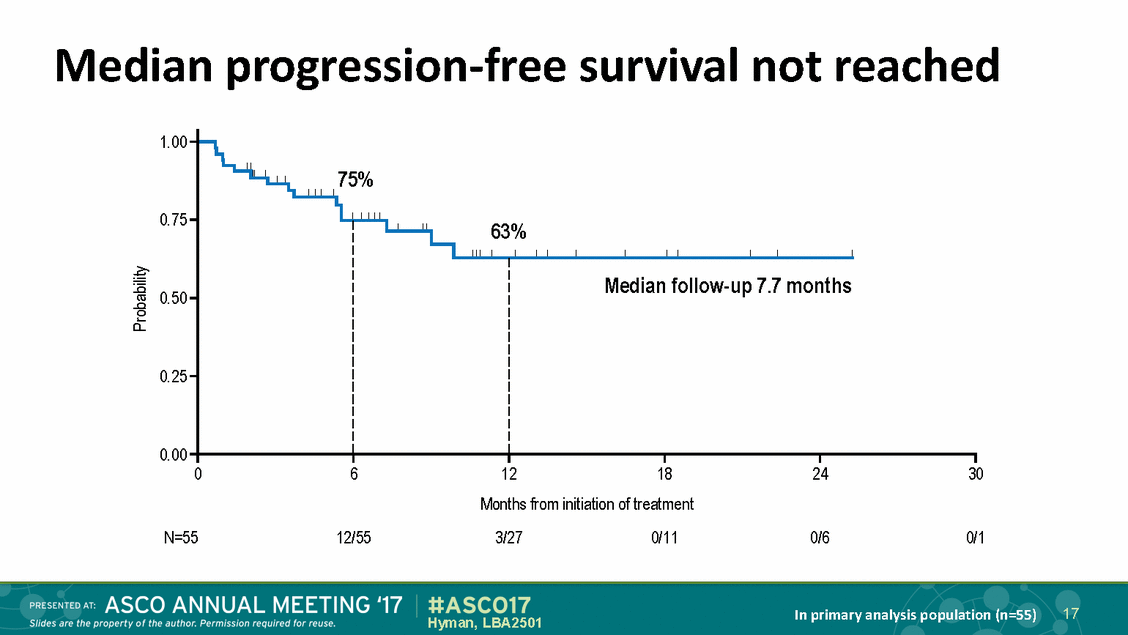
Median progression-free survival not reached 1.00 75% 0.75 63% Median follow-up 7.7 months 0.50 0.25 0.00 0 6 12 18 24 30 Months from initiation of treatment N=55 12/55 3/27 0/11 0/6 0/1 17 In primary analysis population (n=55) Hyman, LBA2501 Probability
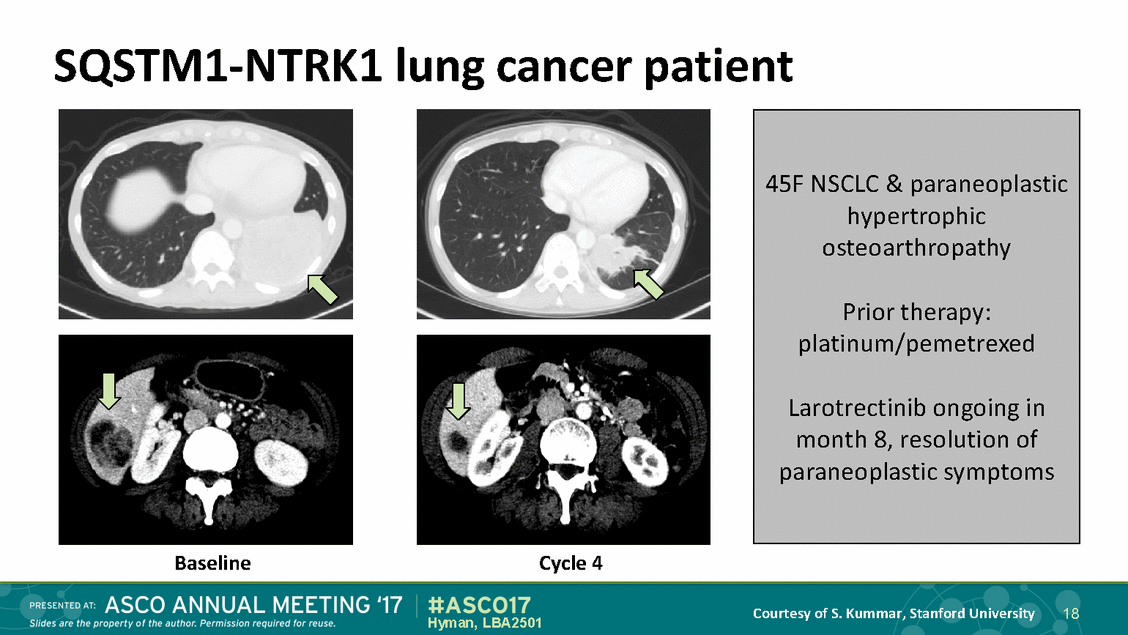
SQSTM1-NTRK1 lung cancer patient Baseline Cycle 4 Courtesy of S. Kummar, Stanford University 18 Hyman, LBA2501 45F NSCLC & paraneoplastic hypertrophic osteoarthropathy Prior therapy: platinum/pemetrexed Larotrectinib ongoing in month 8, resolution of paraneoplastic symptoms
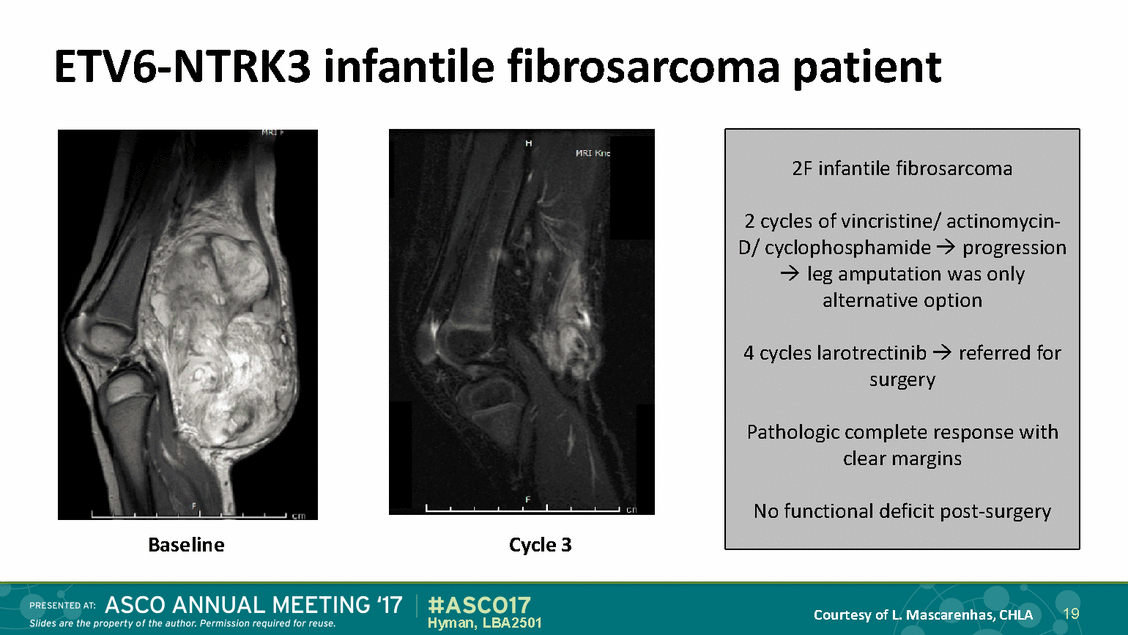
ETV6-NTRK3 infantile fibrosarcoma patient Baseline Cycle 3 19 Courtesy of L. Mascarenhas, CHLA Hyman, LBA2501 2F infantile fibrosarcoma 2 cycles of vincristine/ actinomycin-D/ cyclophosphamide progression leg amputation was only alternative option 4 cycles larotrectinib referred for surgery Pathologic complete response with clear margins No functional deficit post-surgery
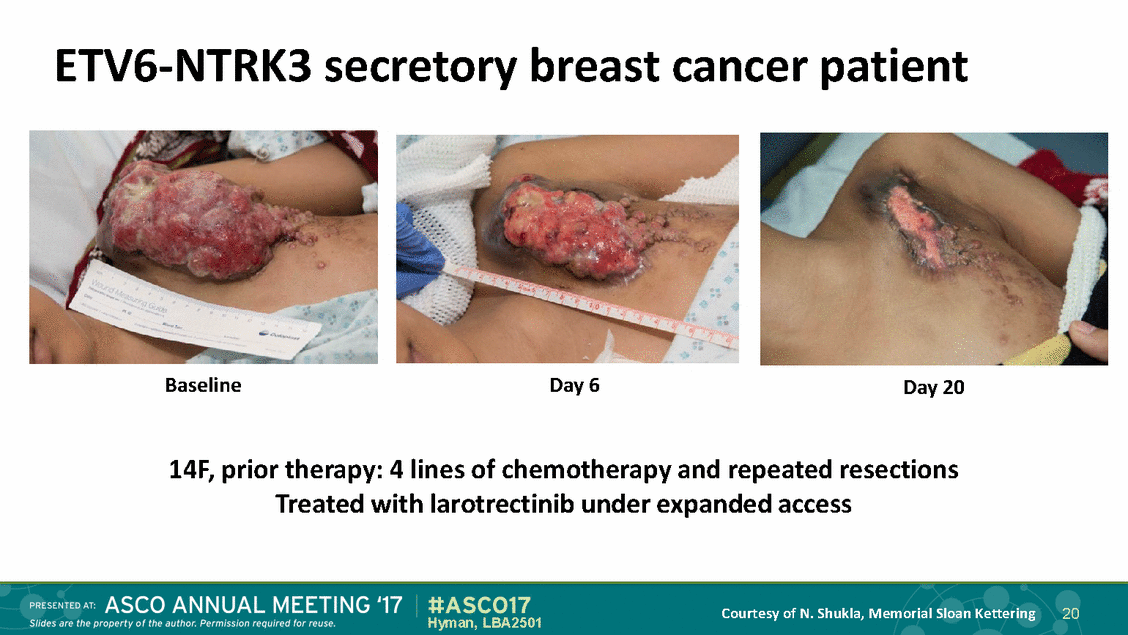
ETV6-NTRK3 secretory breast cancer patient Baseline Day 6 Day 20 14F, prior therapy: 4 lines of chemotherapy and repeated resections Treated with larotrectinib under expanded access Courtesy of N. Shukla, Memorial Sloan Kettering 20 Hyman, LBA2501
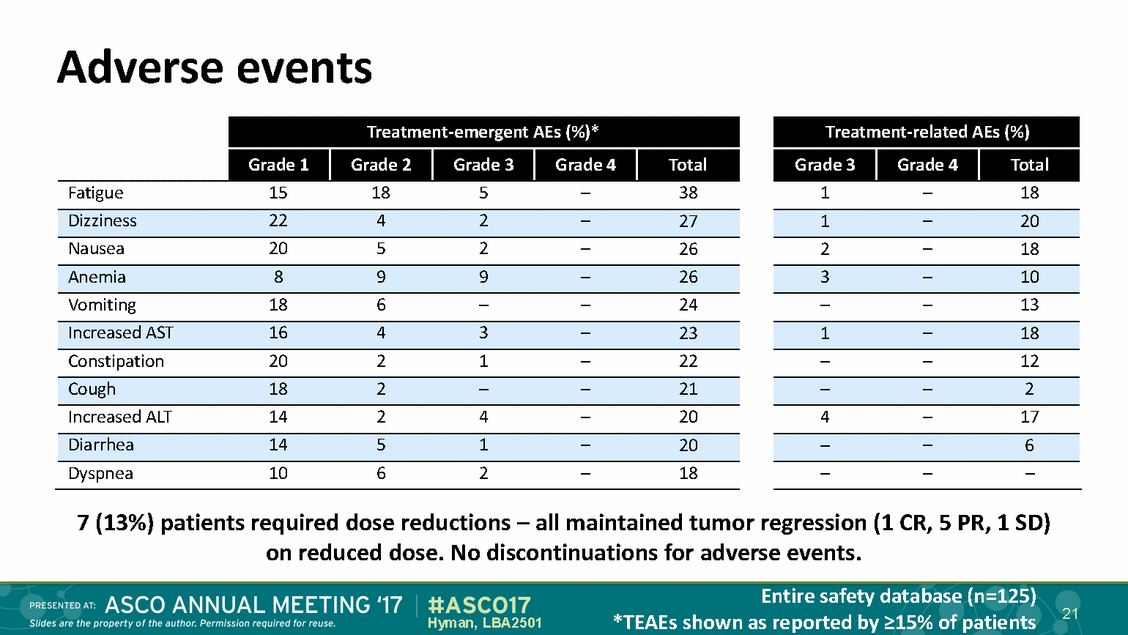
Adverse events 7 (13%) patients required dose reductions – all maintained tumor regression (1 CR, 5 PR, 1 SD) on reduced dose. No discontinuations for adverse events. Entire safety database (n=125) *TEAEs shown as reported by >15% of patients 21 Hyman, LBA2501 Treatment-related AEs (%) Grade 3 Grade 4 Total 1–18 1–20 2–18 3–10 ––13 1–18 ––12 ––2 4–17 ––6 ––– Treatment-emergent AEs (%)* Grade 1 Grade 2 Grade 3 Grade 4 Total Fatigue15185–38 Dizziness2242–27 Nausea2052–26 Anemia899–26 Vomiting186––24 Increased AST1643–23 Constipation2021–22 Cough182––21 Increased ALT1424–20 Diarrhea1451–20 Dyspnea1062–18
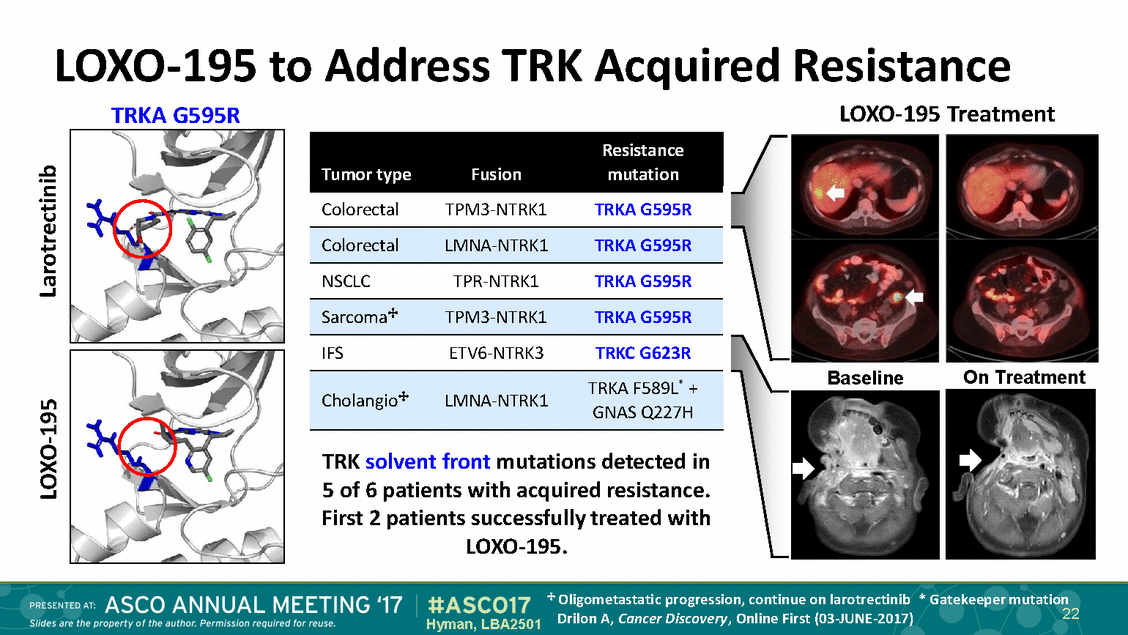
LOXO-195 TRKA G595R to Address TRK Acquired Resistance LOXO-195 Treatment Colorectal TPM3-NTRK1 TRKA G595R NSCLC TPR-NTRK1 TRKA G595R IFS ETV6-NTRK3 TRKC G623R On Treatment Baseline TRKA F589L + Cholangio LMNA-NTRK1 TRK solvent front mutations detected in 5 of 6 patients with acquired resistance. First 2 patients successfully treated with LOXO-195. Oligometastatic progression, continue on larotrectinib * Gatekeeper mutation 22 Drilon A, Cancer Discovery, Online First (03-JUNE-2017) Hyman, LBA2501 Larotrectinib LOXO-195 * GNAS Q227H Sarcoma TPM3-NTRK1TRKA G595R ColorectalLMNA-NTRK1TRKA G595R Resistance Tumor typeFusionmutation
Larotrectinib conclusions • Consistent and durable antitumor activity in TRK fusion cancers – – – ORR: 76% (12% CRs) 6-month landmark DOR: 91% Minimal side effects for patients • Notable elements of larotrectinib program – – – – – Potentially the first new targeted therapy developed in a tissue type-agnostic manner First new targeted therapy developed simultaneously in adults and pediatrics Rapid path from first patient to last patient enrolled (24 months) New Drug Application (NDA) to be submitted in late 2017 or early 2018 Real-time elucidation of convergent acquired resistance mechanism and treatment with LOXO-195 • For patients with TRK fusion cancer, larotrectinib may offer a potential new standard of care – Routine pan-cancer screening will be important to identify these patients, as well as those with other tumor-agnostic biomarkers (MSI-H) 23 Hyman, LBA2501
Acknowledgements • Patients and their families, many of whom traveled long distances to participate in these studies • National Institutes of Health P30 CA008748 • Marie-Josée and Henry R. Kravis Center for Molecular Oncology 24 Hyman, LBA2501