Attached files
| file | filename |
|---|---|
| 8-K - 8-K - GLAUKOS Corp | a17-8115_18k.htm |
Exhibit 99.1
March 2017 © 2017 Glaukos Corporation.

2 © 2017 Glaukos Corporation. DISCLAIMER All statements other than statements of historical facts included in this presentation that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements. Although we believe that we have a reasonable basis for forward-looking statements contained herein, we caution you that they are based on current expectations about future events affecting us and are subject to risks, uncertainties and factors relating to our operations and business environment, all of which are difficult to predict and many of which are beyond our control, that may cause our actual results to differ materially from those expressed or implied by forward-looking statements in this presentation. These potential risks and uncertainties include, without limitation, uncertainties about our ability to maintain profitability; our dependence on the success and market acceptance of the iStent®; our ability to leverage our sales and marketing infrastructure to increase market penetration and acceptance of our products; our dependence on a limited number of third-party suppliers for components of our products; the occurrence of a crippling accident or other disruption at our primary facility, which may materially affect our manufacturing capacity and operations; maintaining adequate coverage or reimbursement by third-party payors for procedures using the iStent or other products in development; our ability to properly train, and gain acceptance and trust from, ophthalmic surgeons in the use of our products; our ability to successfully develop and commercialize additional products; our ability to compete effectively in the highly competitive and rapidly changing medical device industry and against current and future competitors (including MIGS competitors) that are large public companies or divisions of publicly traded companies that have competitive advantages; the timing, effect and expense of navigating different regulatory approval processes as we develop additional products and penetrate foreign markets; the impact of any product liability claims against us and any related litigation; the effect of the extensive and increasing federal and state regulation in the healthcare industry on us and our suppliers; the lengthy and expensive clinical trial process and the uncertainty of outcomes from any particular clinical trial; our ability to protect, and the expense and time-consuming nature of protecting, our intellectual property against third parties and competitors that could develop and commercialize similar or identical products; the impact of any claims against us of infringement or misappropriation of third party intellectual property rights and any related litigation; and the market’s perception of our limited operating history as a public company. These and other known risks, uncertainties and factors are described in detail under the caption “Risk Factors” and elsewhere in our filings with the Securities and Exchange Commission, including our Quarterly Report on Form 10-Q for the quarter ended September 30, 2016 filed with the Securities and Exchange Commission on November 14, 2016 and our Annual Report on Form 10-K, which we expect to file on or before March 16, 2017. Our filings with the Securities and Exchange Commission are available in the Investor Section of our website at www.glaukos.com or at www.sec.gov. In addition, information about the risks and benefits of our products is available on our website at www.glaukos.com. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on the forward-looking statements in this press release, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise, except as may be required under applicable securities law.

glaucoma therapy and highly successful launch global opportunity1 of current treatments of micro-scale 3 © 2017 Glaukos Corporation. istransforming LARGE MARKETUNMET NEEDDEMONSTRATED EXECUTIONJUST THE BEGINNING Growing $5bnSignificant drawbacksBroad reimbursementAdvanced portfolio injectable therapies 1 Market Scope estimate for 2016 total global glaucoma market, Sept. 2016

4 © 2017 Glaukos Corporation. 2016Major Accomplishments 1.Reported 60% YoY global net sales growth versus 2015; exceeded U.S. surgeon training targets 2.Delivered 14th consecutive quarter of > 40% YoY growth since U.S. commercialization 3.Achieved gross margin in mid-80% range 4.Added Anthem and Tricare to reach 95+% U.S. reimbursement coverage 5.Secured new 2017 iStent device intensive offset Medicare payment for ASCs 6.Published 14 peer-reviewed clinical studies, bringing the company total to 56 7.Completed enrollment in initial phase of iStent inject® standalone clinical trial 8.Initiated iDose® Phase 2b clinical trial 9.Established direct sales operations in 8 new international markets1, bringing total number of global direct markets to 13 10. Secured Japanese iStent® regulatory approval (Mar) and formal reimbursement (Dec) 1 Brazil, UK, Ireland, Sweden, Netherlands, France, Spain, Switzerland
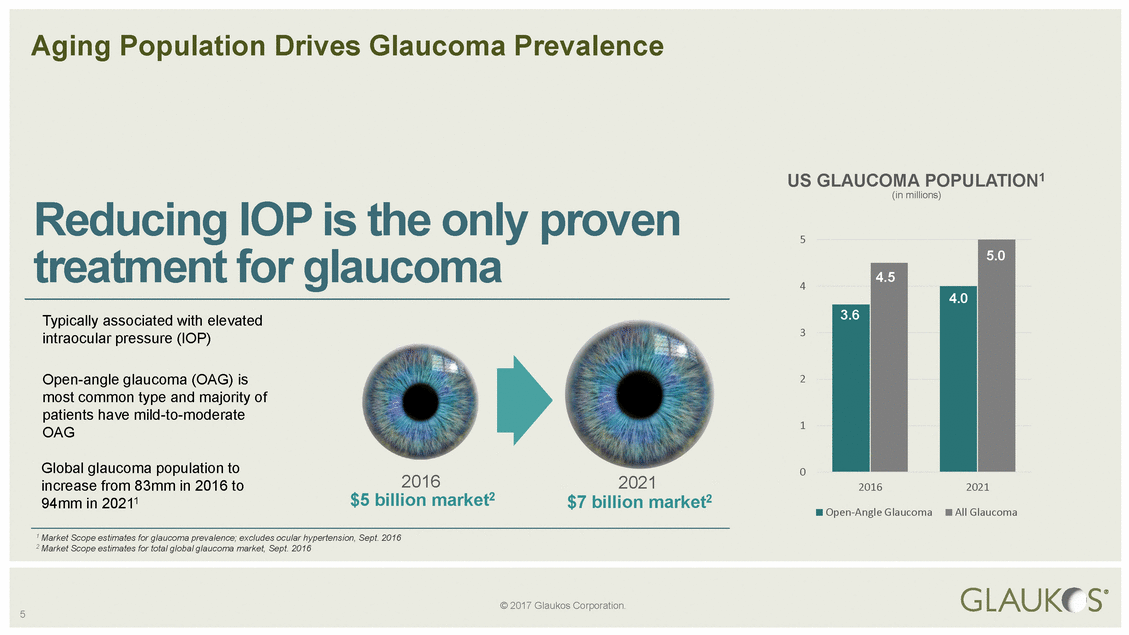
Reducing treatment IOP is the only proven for glaucoma 3 intraocular pressure (IOP) 1 OAG 2016 2021 increase from 83mm in 2016 to 2016 2021 Open-Angle Glaucoma All Glaucoma 5 © 2017 Glaukos Corporation. Aging Population Drives Glaucoma Prevalence US GLAUCOMA POPULATION1 (in millions) 5 4 Typically associated with elevated Open-angle glaucoma (OAG) is2 most common type and majority of patients have mild-to-moderate Global glaucoma population to0 94mm in 20211$5 billion market2$7 billion market2 1 Market Scope estimates for glaucoma prevalence; excludes ocular hypertension, Sept. 2016 2 Market Scope estimates for total global glaucoma market, Sept. 2016 5.0 4.5 4.0 3.6
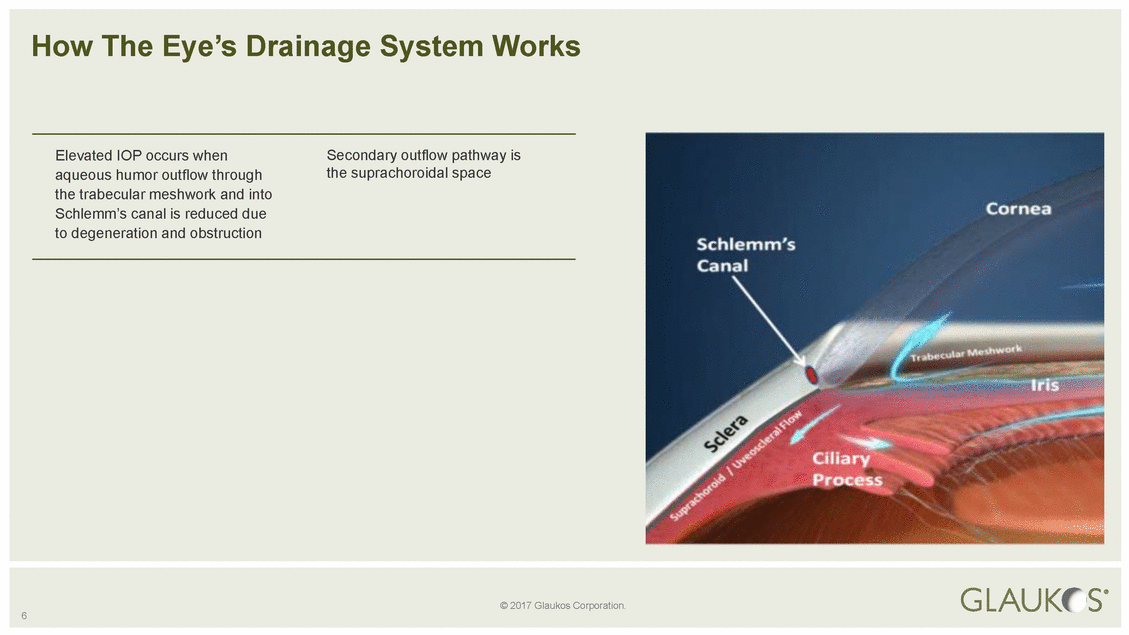
© 2017 Glaukos Corporation. 6 How The Eye’s Drainage System Works Elevated IOP occurs whenSecondary outflow pathway is aqueous humor outflow throughthe suprachoroidal space the trabecular meshwork and into Schlemm’s canal is reduced due to degeneration and obstruction
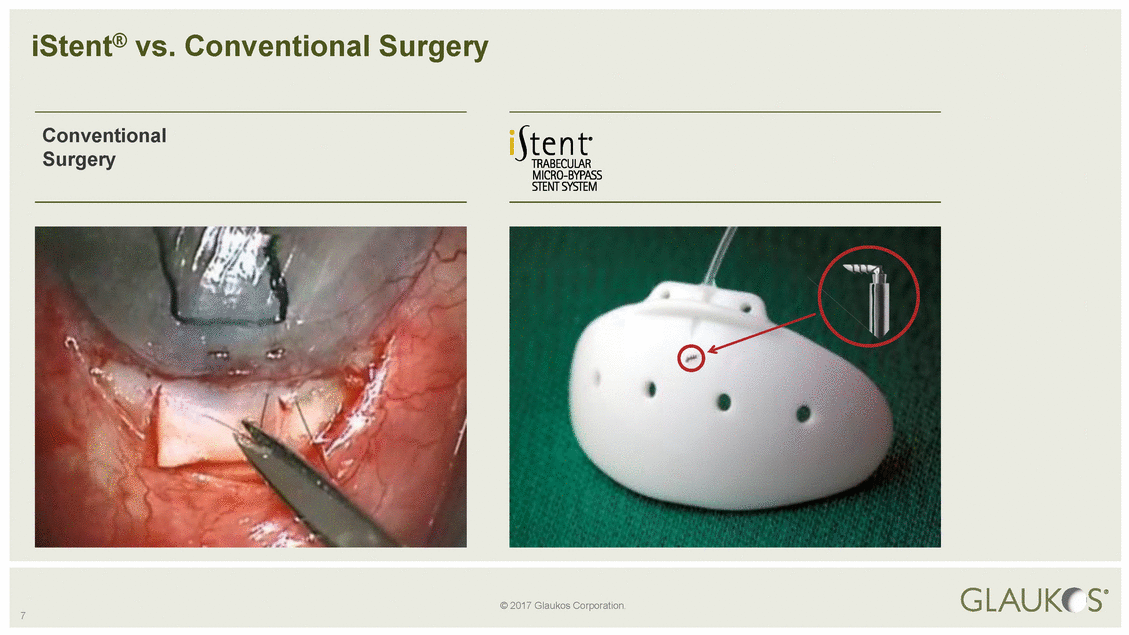
© 2017 Glaukos Corporation. 7 iStent® vs. Conventional Surgery Conventional Surgery
8 © 2017 Glaukos Corporation. Our Solution Portfolio: Transformational Shift to Micro-Scale Injectable Therapy Precision solutions forcomplete range of glaucoma disease states &progression iStent® iStent Inject® iStent Supra® iDoseTM Used in combinationInjectable 2-stent therapyDesigned to accessTargeted injectable drug-with cataract surgeryfor combo-cataract andsecondary outflowdelivery implant; sustained standalone procedurespathwaydrug therapy iStent Inject, iStent Supra and iDose are not approved by the FDA. iStent Inject and iStent Supra are currently being evaluated in IDE clinical trials; iDose is currently being evaluated in Phase II IND clinical trial. The iStent is approved in the European Union and certain other international markets for use either in combination with cataract surgery or as a standalone procedure in phakic and pseudophakic eyes. In the United States, the iStent is indicated for use in conjunction with cataract surgery for the reduction of IOP in adult patients with mild-to-moderate open-angle glaucoma currently treated with ocular hypotensive medication. RESTORE FLOWFURTHER ENHANCE FLOWCOMBINATION DRUG & FLOW Injectable Drug Delivery Injectable Flow Platform
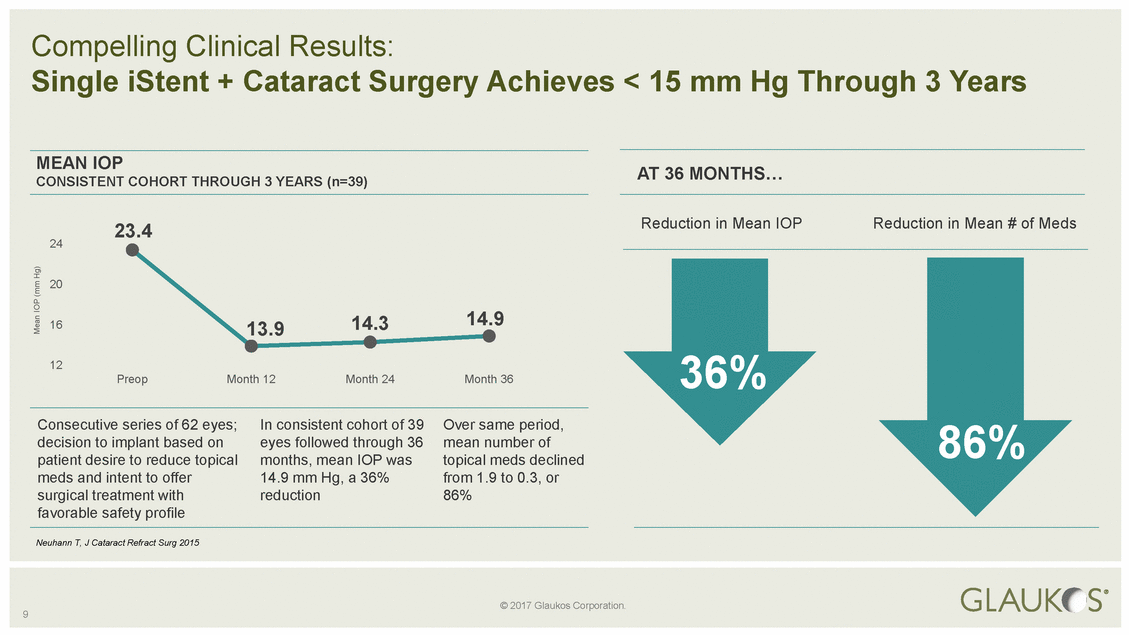
AT 36 MONTHS… CONSISTENT COHORT THROUGH 3 YEARS (n=39) 24 Preop Month 12 Month 24 Month 36 86% decision to implant based on eyes followed through 36 mean number of 9 Mean IOP (mm Hg) © 2017 Glaukos Corporation. Compelling Clinical Results: Single iStent + Cataract Surgery Achieves < 15 mm Hg Through 3 Years MEAN IOP 23.4Reduction in Mean IOPReduction in Mean # of Meds 20 1613.914.314.9 1236% Consecutive series of 62 eyes;In consistent cohort of 39Over same period, patient desire to reduce topicalmonths, mean IOP wastopical meds declined meds and intent to offer14.9 mm Hg, a 36%from 1.9 to 0.3, or surgical treatment withreduction86% favorable safety profile Neuhann T, J Cataract Refract Surg 2015
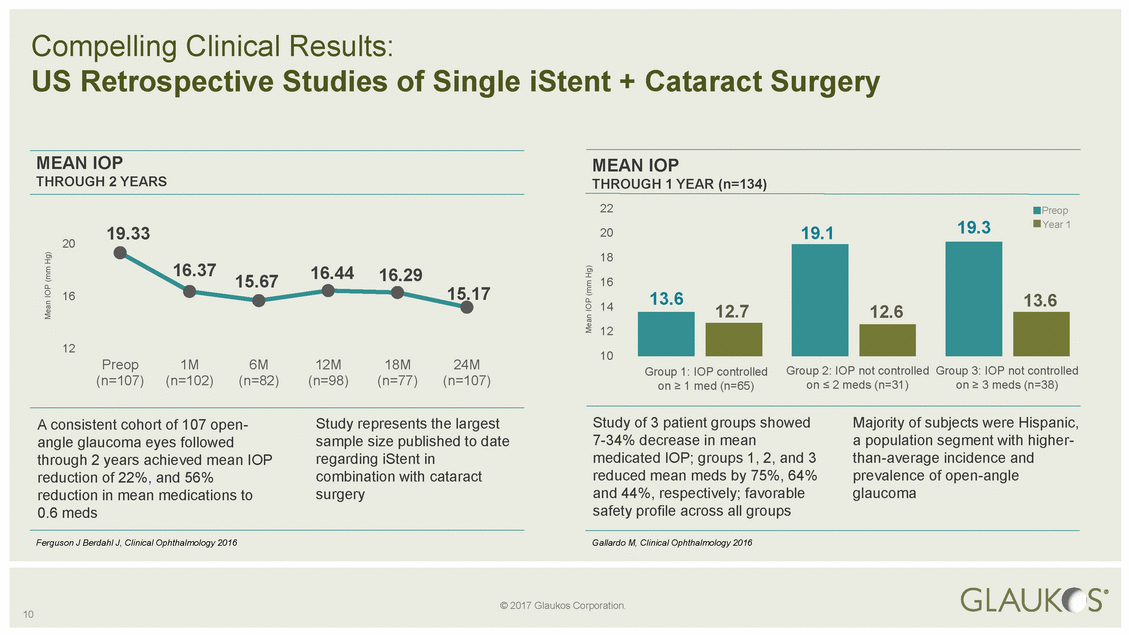
20 16.37 15.17 13.6 16 Preop 1M 6M 12M 18M 24M Group 3: IOP not controlled Group 2: IOP not controlled Group 1: IOP controlled 10 Mean IOP (mm Hg) Mean IOP (mm Hg) © 2017 Glaukos Corporation. Compelling Clinical Results: US Retrospective Studies of Single iStent + Cataract Surgery MEAN IOPMEAN IOP THROUGH 2 YEARSTHROUGH 1 YEAR (n=134) 22Preop 19.332019.119.3Year 1 18 15.6716.4416.2916 1412.712.613.6 12 1210 (n=107)(n=102)(n=82)(n=98)(n=77)(n=107)on > 1 med (n=65)on < 2 meds (n=31)on > 3 meds (n=38) A consistent cohort of 107 open-Study represents the largestStudy of 3 patient groups showedMajority of subjects were Hispanic, angle glaucoma eyes followedsample size published to date7-34% decrease in meana population segment with higher-through 2 years achieved mean IOPregarding iStent inmedicated IOP; groups 1, 2, and 3than-average incidence and reduction of 22%, and 56%combination with cataractreduced mean meds by 75%, 64%prevalence of open-angle reduction in mean medications tosurgeryand 44%, respectively; favorableglaucoma 0.6 medssafety profile across all groups Ferguson J Berdahl J, Clinical Ophthalmology 2016Gallardo M, Clinical Ophthalmology 2016
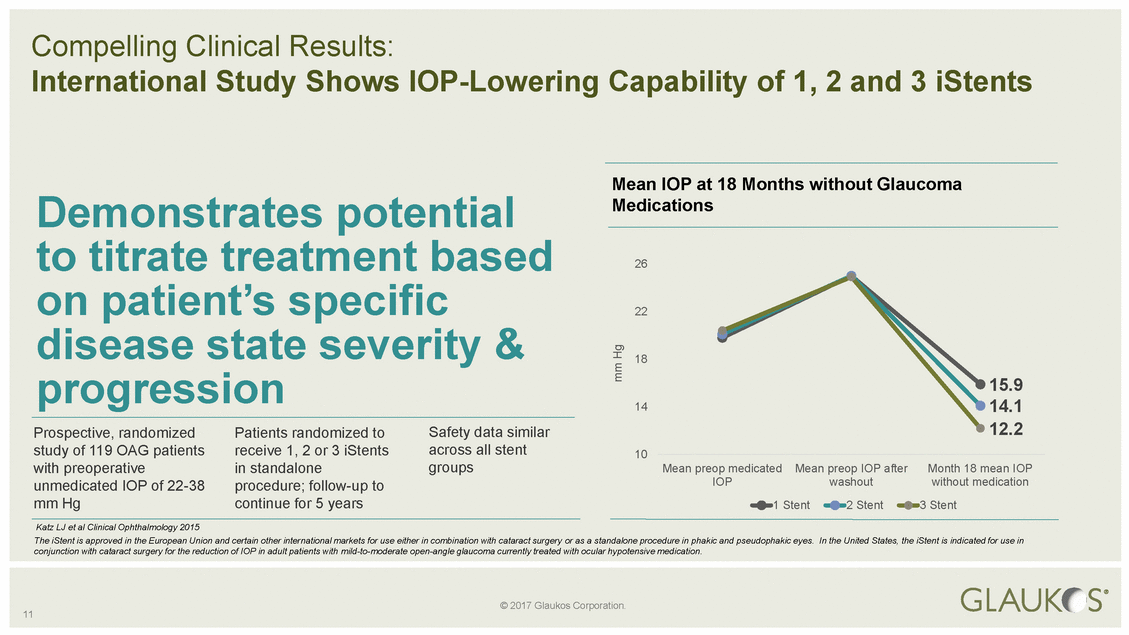
Demonstrates potential Medications 14.1 14 groups with preoperative in standalone Mean preop medicated Mean preop IOP after Month 18 mean IOP 11 mm Hg © 2017 Glaukos Corporation. Compelling Clinical Results: International Study Shows IOP-Lowering Capability of 1, 2 and 3 iStents Mean IOP at 18 Months without Glaucoma to titrate treatment based 26 on patient’s specific 22 disease state severity & 18 progression 15.9 Prospective, randomized Patients randomized to Safety data similar 12.2 study of 119 OAG patients receive 1, 2 or 3 iStents across all stent 10 unmedicated IOP of 22-38 procedure; follow-up to IOP washout without medication mm Hg continue for 5 years 1 Stent 2 Stent 3 Stent Katz LJ et al Clinical Ophthalmology 2015 The iStent is approved in the European Union and certain other international markets for use either in combination with cataract surgery or as a standalone procedure in phakic and pseudophakic eyes. In the United States, the iStent is indicated for use in conjunction with cataract surgery for the reduction of IOP in adult patients with mild-to-moderate open-angle glaucoma currently treated with ocular hypotensive medication.
21 Prospective clinical trials currently underway Articles published in journals 12 © 2017 Glaukos Corporation. Clinical Research Program Statistics 56 peer-reviewed
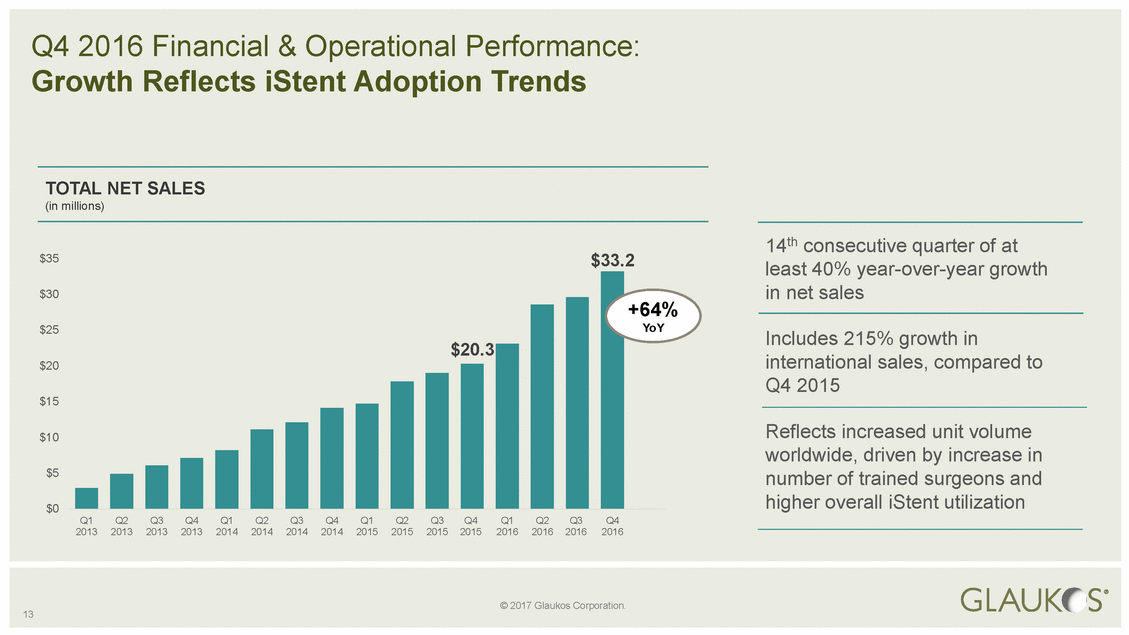
$33.2 $35 least 40% year-over-year growth +64% Includes 215% growth in 13 © 2017 Glaukos Corporation. Q4 2016 Financial & Operational Performance: Growth Reflects iStent Adoption Trends TOTAL NET SALES (in millions) 14th consecutive quarter of at $30in net sales $25YoY $20international sales, compared to Q4 2015 $15 $10Reflects increased unit volume worldwide, driven by increase in $5number of trained surgeons and $0higher overall iStent utilization Q1Q2Q3Q4Q1Q2Q3Q4Q1Q2Q3Q4Q1Q2Q3Q4 2013201320132013201420142014201420152015201520152016201620162016 $20.3
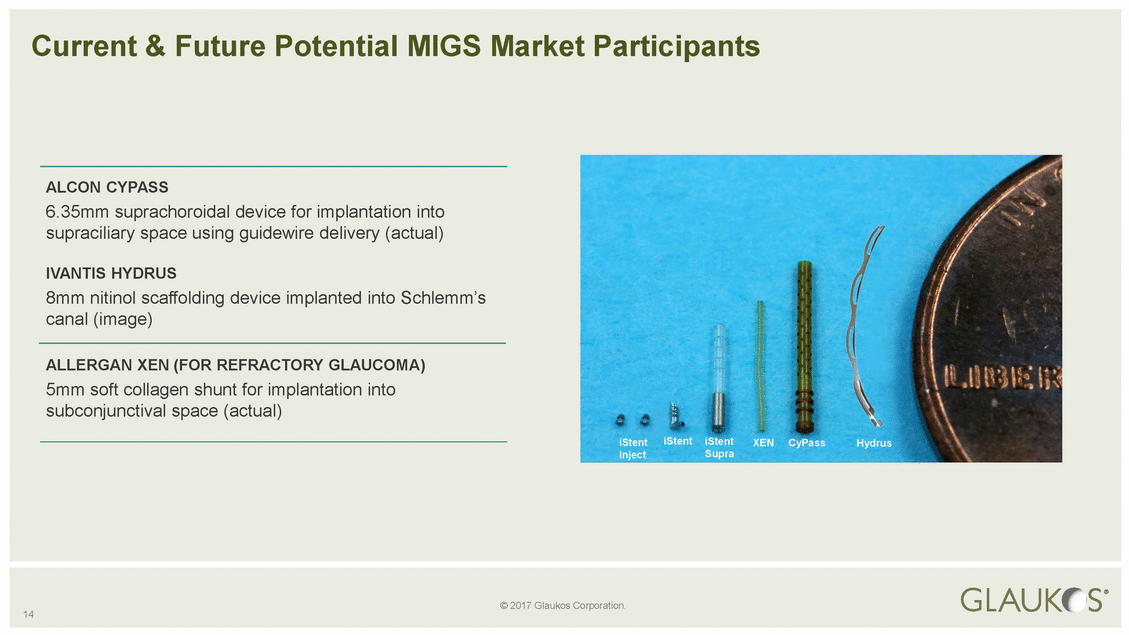
14 © 2017 Glaukos Corporation. Current & Future Potential MIGS Market Participants ALCON CYPASS 6.35mm suprachoroidal device for implantation into supraciliary space using guidewire delivery (actual) IVANTIS HYDRUS 8mm nitinol scaffolding device implanted into Schlemm’s canal (image) ALLERGAN XEN (FOR REFRACTORY GLAUCOMA) 5mm soft collagen shunt for implantation into subconjunctival space (actual) iStentiStentiStentXENCyPassHydrus InjectSupra
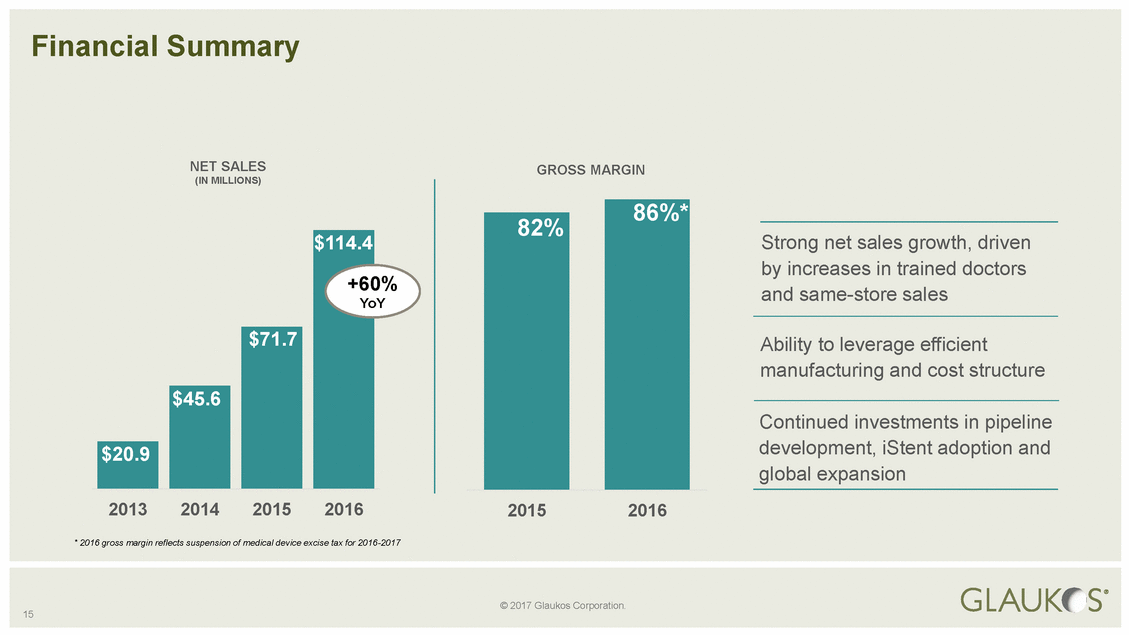
(IN MILLIONS) 0% and same-store sales global expansion 15 © 2017 Glaukos Corporation. Financial Summary NET SALESGROSS MARGIN Strong net sales growth, driven by increases in trained doctors oY Ability to leverage efficient manufacturing and cost structure Continued investments in pipeline development, iStent adoption and 201320142015201620152016 * 2016 gross margin reflects suspension of medical device excise tax for 2016-2017 $114.4 +6 Y $71.7 $45.6 $20.9 86%* 82%
16 © 2017 Glaukos Corporation. Growth Strategies: Establishing MIGS as OAG Standard of Care 01020304 Increase US iStentSecure FDA approvalsDevelop, secureExtend global reach of adoption with seasonedwith expandedregulatory approval forMIGS-based platform sales force, broadindications for iStentand commercializethrough targeted reimbursement coveragepipeline productsiDose drug-deliveryinternational expansion and compelling clinicalpipeline results
© 2017 Glaukos Corporation. 17

