Attached files
| file | filename |
|---|---|
| 8-K - 8-K - GLAUKOS Corp | gkos-20170110x8k.htm |
Exhibit 99.1
|
|
January 2017 |
|
|
2 DISCLAIMER All statements other than statements of historical facts included in this presentation that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements. Although we believe that we have a reasonable basis for forward-looking statements contained herein, we caution you that they are based on current expectations about future events affecting us and are subject to risks, uncertainties and factors relating to our operations and business environment, all of which are difficult to predict and many of which are beyond our control, that may cause our actual results to differ materially from those expressed or implied by forward-looking statements in this presentation. These potential risks and uncertainties include, without limitation, uncertainties about our ability to maintain profitability; our dependence on the success and market acceptance of the iStent®; our ability to leverage our sales and marketing infrastructure to increase market penetration and acceptance of our products; our dependence on a limited number of third-party suppliers for components of our products; the occurrence of a crippling accident or other disruption at our primary facility, which may materially affect our manufacturing capacity and operations; maintaining adequate coverage or reimbursement by third-party payors for procedures using the iStent or other products in development; our ability to properly train, and gain acceptance and trust from, ophthalmic surgeons in the use of our products; our ability to successfully develop and commercialize additional products; our ability to compete effectively in the highly competitive and rapidly changing medical device industry and against current and future competitors (including MIGS competitors) that are large public companies or divisions of publicly traded companies that have competitive advantages; the timing, effect and expense of navigating different regulatory approval processes as we develop additional products and penetrate foreign markets; the impact of any product liability claims against us and any related litigation; the effect of the extensive and increasing federal and state regulation in the healthcare industry on us and our suppliers; the lengthy and expensive clinical trial process and the uncertainty of outcomes from any particular clinical trial; our ability to protect, and the expense and time-consuming nature of protecting, our intellectual property against third parties and competitors that could develop and commercialize similar or identical products; the impact of any claims against us of infringement or misappropriation of third party intellectual property rights and any related litigation; and the market’s perception of our limited operating history as a public company. These and other known risks, uncertainties and factors are described in detail under the caption “Risk Factors” and elsewhere in our filings with the Securities and Exchange Commission, including our Quarterly Report on Form 10-Q for the quarter ended September 30, 2016 filed with the Securities and Exchange Commission on November 14, 2016. Our filings with the Securities and Exchange Commission are available in the Investor Section of our website at www.glaukos.com or at www.sec.gov. In addition, information about the risks and benefits of our products is available on our website at www.glaukos.com. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on the forward-looking statements in this press release, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise, except as may be required under applicable securities law. |
|
|
3 is transforming glaucoma therapy LARGE MARKET Growing $5bn global opportunity1 UNMET NEED Significant drawbacks of current treatments DEMONSTRATED EXECUTION Broad reimbursement and highly successful launch JUST THE BEGINNING Advanced portfolio of micro-scale injectable therapies 1 Market Scope estimate for 2016 total global glaucoma market, Sept. 2016 |
|
|
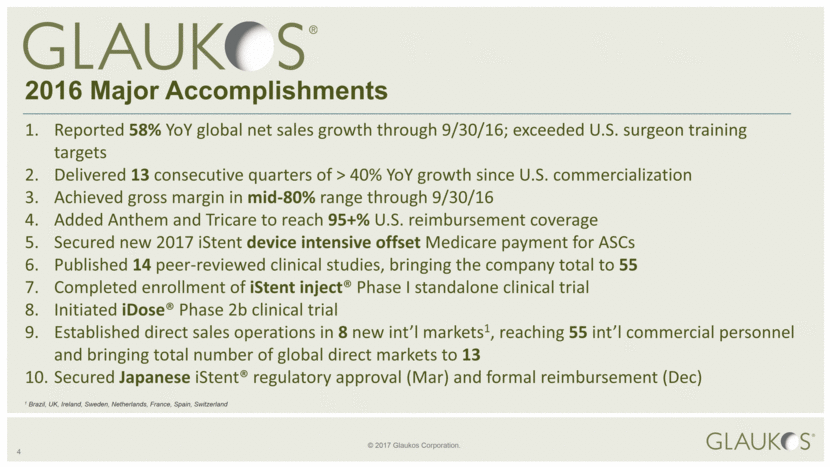
1. Reported 58% YoY global net sales growth through 9/30/16; exceeded U.S. surgeon training targets 2. Delivered 13 consecutive quarters of > 40% YoY growth since U.S. commercialization 3. Achieved gross margin in mid-80% range through 9/30/16 4 Added Anthem and Tricare to reach 95+% U.S. reimbursement coverage 5. Secured new 2017 iStent device intensive offset Medicare payment for ASCs 6. Published 14 peer-reviewed clinical studies, bringing the company total to 55 7. Completed enrollment of iStent inject® Phase I standalone clinical trial 8. Initiated iDose® Phase 2b clinical trial 9. Established direct sales operations in 8 new int’l markets1, reaching 55 int’l commercial personnel and bringing total number of global direct markets to 13 10. Secured Japanese iStent® regulatory approval (Mar) and formal reimbursement (Dec) 2016 Major Accomplishments 1 Brazil, UK, Ireland, Sweden, Netherlands, France, Spain, Switzerland |
|
|
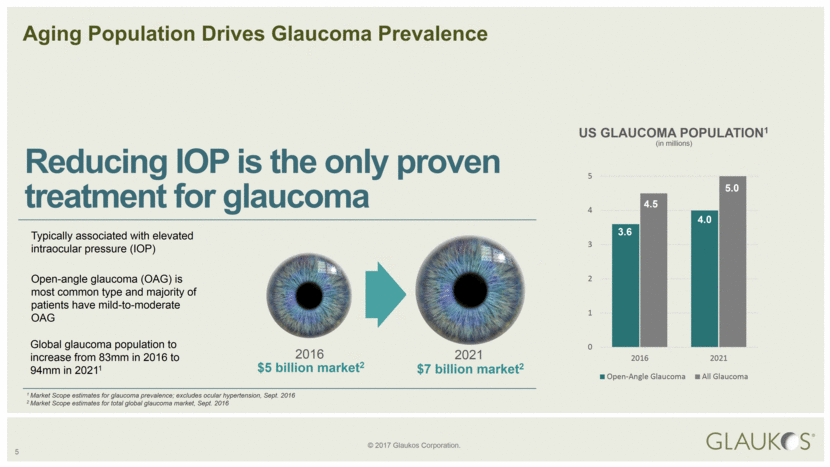
US GLAUCOMA POPULATION1 Reducing IOP is the only proven treatment for glaucoma Aging Population Drives Glaucoma Prevalence 1 Market Scope estimates for glaucoma prevalence; excludes ocular hypertension, Sept. 2016 2 Market Scope estimates for total global glaucoma market, Sept. 2016 Typically associated with elevated intraocular pressure (IOP) Open-angle glaucoma (OAG) is most common type and majority of patients have mild-to-moderate OAG Global glaucoma population to increase from 83mm in 2016 to 94mm in 20211 2016 2021 $5 billion market2 $7 billion market2 (in millions) 5 3.6 4.0 4.5 5.0 0 1 2 3 4 5 2016 2021 Open-Angle Glaucoma All Glaucoma |
|
|
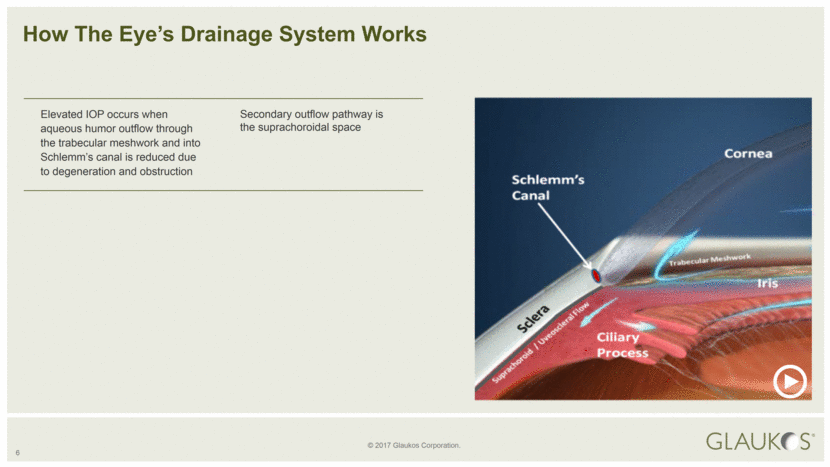
6 How The Eye’s Drainage System Works Elevated IOP occurs when aqueous humor outflow through the trabecular meshwork and into Schlemm’s canal is reduced due to degeneration and obstruction Secondary outflow pathway is the suprachoroidal space |
|
|
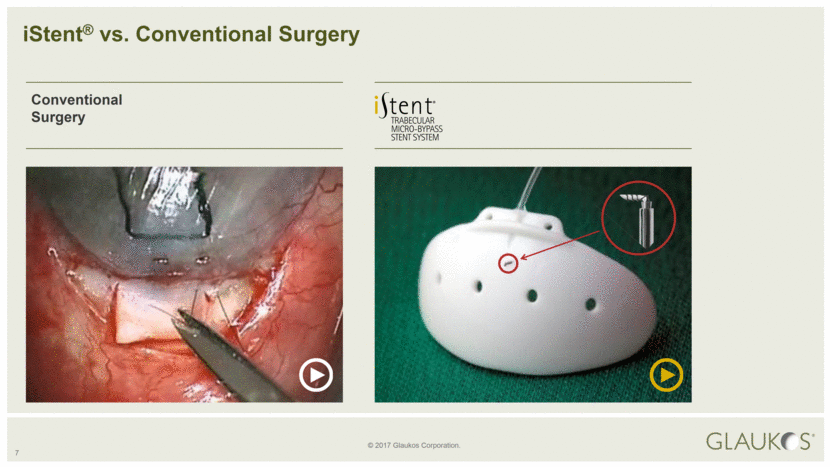
7 Conventional Surgery iStent® vs. Conventional Surgery |
|
|
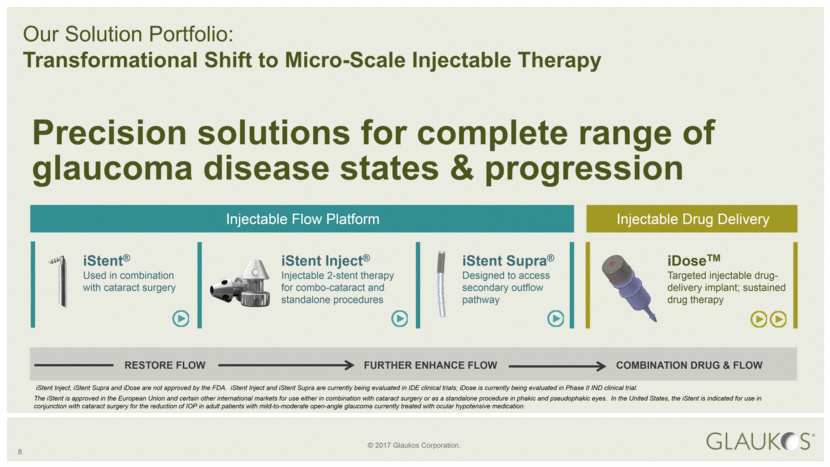
8 Our Solution Portfolio: Transformational Shift to Micro-Scale Injectable Therapy RESTORE FLOW FURTHER ENHANCE FLOW COMBINATION DRUG & FLOW iStent Inject, iStent Supra and iDose are not approved by the FDA. iStent Inject and iStent Supra are currently being evaluated in IDE clinical trials; iDose is currently being evaluated in Phase II IND clinical trial. iStent Inject® Injectable 2-stent therapy for combo-cataract and standalone procedures iStent® Used in combination with cataract surgery iStent Supra® Designed to access secondary outflow pathway iDoseTM Targeted injectable drug-delivery implant; sustained drug therapy Injectable Flow Platform Injectable Drug Delivery Precision solutions for complete range of glaucoma disease states & progression The iStent is approved in the European Union and certain other international markets for use either in combination with cataract surgery or as a standalone procedure in phakic and pseudophakic eyes. In the United States, the iStent is indicated for use in conjunction with cataract surgery for the reduction of IOP in adult patients with mild-to-moderate open-angle glaucoma currently treated with ocular hypotensive medication. |
|
|
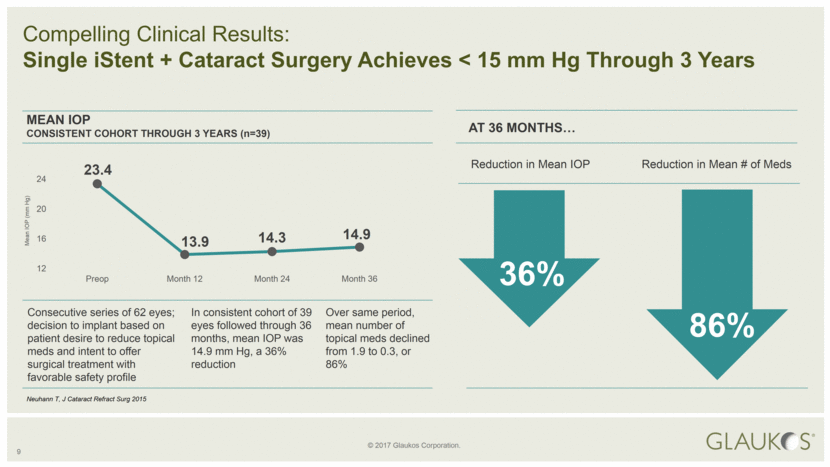
9 Compelling Clinical Results: Single iStent + Cataract Surgery Achieves < 15 mm Hg Through 3 Years Consecutive series of 62 eyes; decision to implant based on patient desire to reduce topical meds and intent to offer surgical treatment with favorable safety profile In consistent cohort of 39 eyes followed through 36 months, mean IOP was 14.9 mm Hg, a 36% reduction Over same period, mean number of topical meds declined from 1.9 to 0.3, or 86% MEAN IOP CONSISTENT COHORT THROUGH 3 YEARS (n=39) AT 36 MONTHS Reduction in Mean IOP Reduction in Mean # of Meds 36% 86% Neuhann T, J Cataract Refract Surg 2015 Mean IOP (mm Hg) 23.4 13.9 14.3 14.9 12 16 20 24 Preop Month 12 Month 24 Month 36 |
|
|
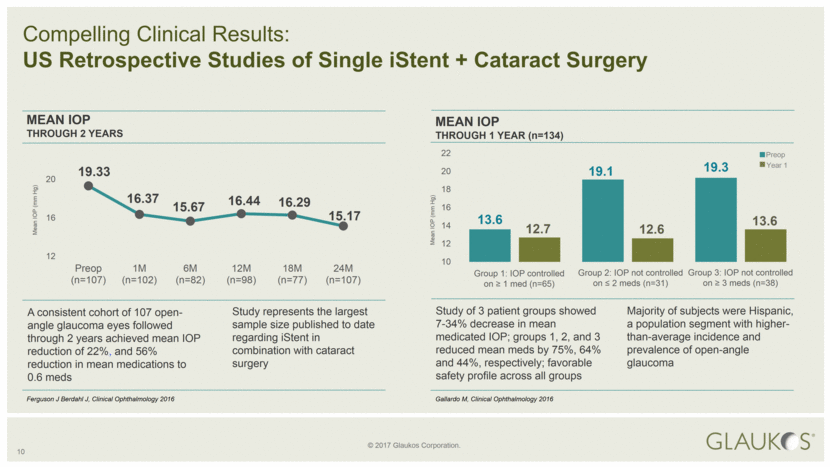
10 Compelling Clinical Results: US Retrospective Studies of Single iStent + Cataract Surgery A consistent cohort of 107 open-angle glaucoma eyes followed through 2 years achieved mean IOP reduction of 22%, and 56% reduction in mean medications to 0.6 meds Study represents the largest sample size published to date regarding iStent in combination with cataract surgery MEAN IOP THROUGH 2 YEARS Ferguson J Berdahl J, Clinical Ophthalmology 2016 Mean IOP (mm Hg) Study of 3 patient groups showed 7-34% decrease in mean medicated IOP; groups 1, 2, and 3 reduced mean meds by 75%, 64% and 44%, respectively; favorable safety profile across all groups Preop Year 1 Group 1: IOP controlled on ≥ 1 med (n=65) Group 2: IOP not controlled on ≤ 2 meds (n=31) Group 3: IOP not controlled on ≥ 3 meds (n=38) MEAN IOP THROUGH 1 YEAR (n=134) Majority of subjects were Hispanic, a population segment with higher-than-average incidence and prevalence of open-angle glaucoma Gallardo M, Clinical Ophthalmology 2016 Mean IOP (mm Hg) 13.6 19.1 19.3 12.7 12.6 13.6 10 12 14 16 18 20 22 19.33 16.37 15.67 16.44 16.29 15.17 12 16 20 Preop (n=107) 1M (n=102) 6M (n=82) 12M (n=98) 18M (n=77) 24M (n=107) |
|
|
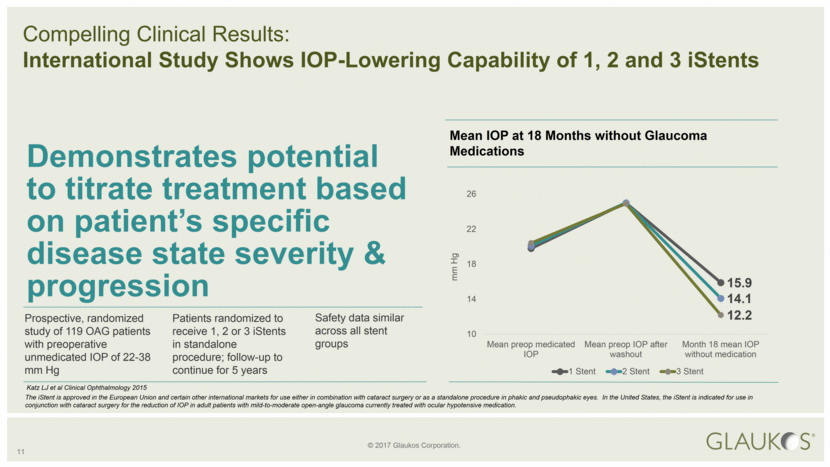
11 Compelling Clinical Results: International Study Shows IOP-Lowering Capability of 1, 2 and 3 iStents Prospective, randomized study of 119 OAG patients with preoperative unmedicated IOP of 22-38 mm Hg Safety data similar across all stent groups Demonstrates potential to titrate treatment based on patient’s specific disease state severity & progression Patients randomized to receive 1, 2 or 3 iStents in standalone procedure; follow-up to continue for 5 years Mean IOP at 18 Months without Glaucoma Medications Katz LJ et al Clinical Ophthalmology 2015 The iStent is approved in the European Union and certain other international markets for use either in combination with cataract surgery or as a standalone procedure in phakic and pseudophakic eyes. In the United States, the iStent is indicated for use in conjunction with cataract surgery for the reduction of IOP in adult patients with mild-to-moderate open-angle glaucoma currently treated with ocular hypotensive medication. mm Hg 15.9 14.1 12.2 10 14 18 22 26 Mean preop medicated IOP Mean preop IOP after washout Month 18 mean IOP without medication 1 Stent 2 Stent 3 Stent |
|
|
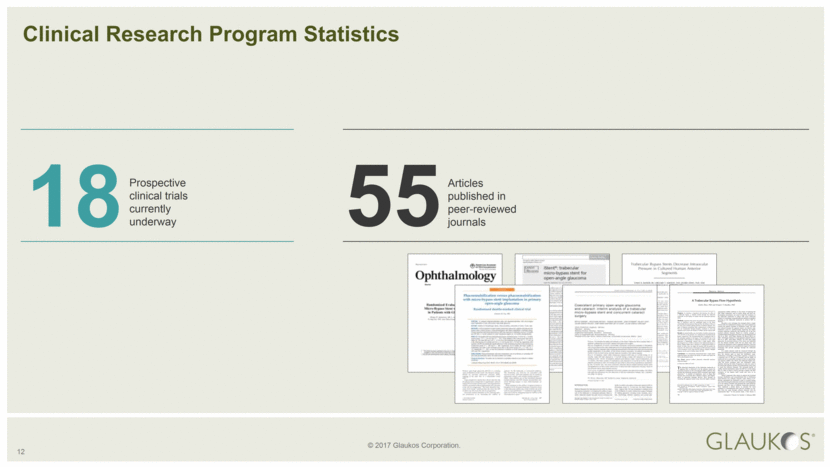
18 12 Prospective clinical trials currently underway Clinical Research Program Statistics 55 Articles published in peer-reviewed journals |
|
|
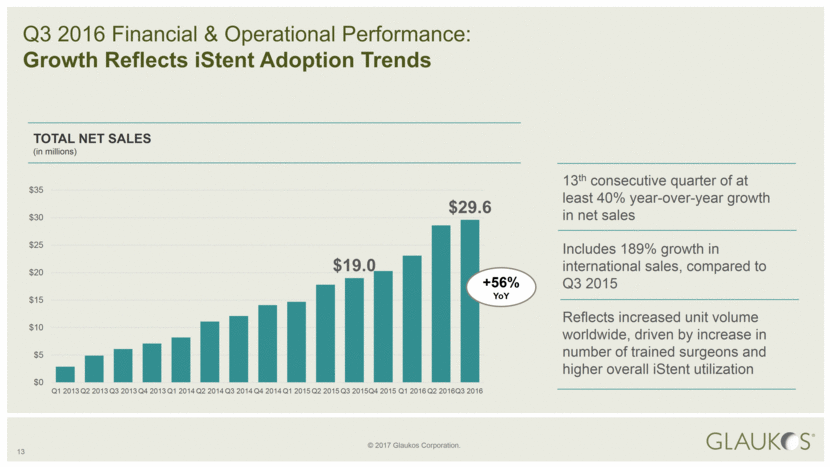
13 TOTAL NET SALES (in millions) Q3 2016 Financial & Operational Performance: Growth Reflects iStent Adoption Trends 13th consecutive quarter of at least 40% year-over-year growth in net sales Includes 189% growth in international sales, compared to Q3 2015 Reflects increased unit volume worldwide, driven by increase in number of trained surgeons and higher overall iStent utilization +56% YoY $19.0 $29.6 $0 $5 $10 $15 $20 $25 $30 $35 Q1 2013 Q2 2013 Q3 2013 Q4 2013 Q1 2014 Q2 2014 Q3 2014 Q4 2014 Q1 2015 Q2 2015 Q3 2015 Q4 2015 Q1 2016 Q2 2016 Q3 2016 |
|
|
14 Current & Future Potential MIGS Market Participants ALCON cypass 6.35mm suprachoroidal device for implantation into supraciliary space using guidewire delivery (actual) Ivantis hydrus 8mm nitinol scaffolding device implanted into Schlemm’s canal (image) Allergan XEN (for Refractory glaucoma) 5mm soft collagen shunt for implantation into subconjunctival space (actual) iStent iStent Inject CyPass Hydrus XEN iStent Supra |
|
|
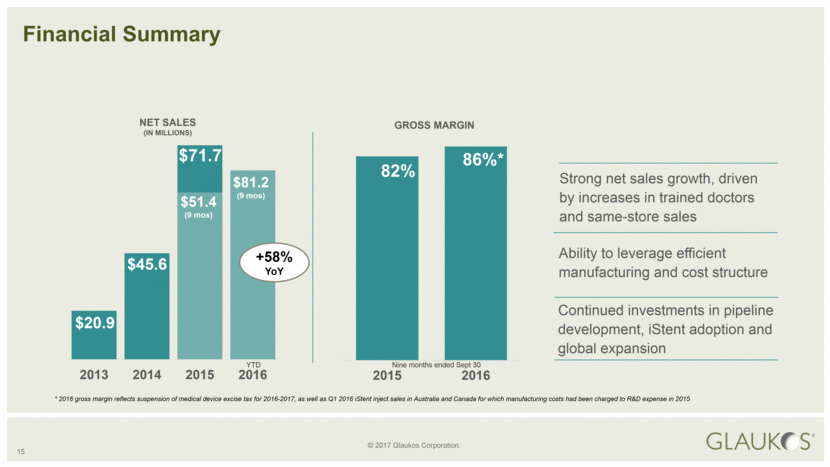
15 Strong net sales growth, driven by increases in trained doctors and same-store sales Financial Summary $20.9 $45.6 $71.7 NET SALES (IN MILLIONS) $81.2 (9 mos) Nine months ended Sept 30 GROSS MARGIN 82% 86%* Ability to leverage efficient manufacturing and cost structure Continued investments in pipeline development, iStent adoption and global expansion * 2016 gross margin reflects suspension of medical device excise tax for 2016-2017, as well as Q1 2016 iStent inject sales in Australia and Canada for which manufacturing costs had been charged to R&D expense in 2015 +58% YoY YTD 2013 2014 2015 2016 $51.4 (9 mos ) 2015 2016 |
|
|
16 Increase US iStent adoption with seasoned sales force, broad reimbursement coverage and compelling clinical results Growth Strategies: Establishing MIGS as OAG Standard of Care 01 Secure FDA approvals with expanded indications for iStent pipeline products 02 Develop, secure regulatory approval for and commercialize iDose drug-delivery pipeline 03 Extend global reach of MIGS-based platform through targeted international expansion 04 |
|
|
17 |