Attached files
| file | filename |
|---|---|
| 8-K - 8-K - SPECTRUM PHARMACEUTICALS INC | form8-k.htm |

Spectrum
Pharmaceuticals
Rajesh C. Shrotriya, MD
Chairman and Chief Executive Officer
Investor Presentation
January 2017
Safe Harbor Statement
This presentation contains forward-looking statements regarding future events and the future performance of
Spectrum Pharmaceuticals that involve risks and uncertainties that could cause actual results to differ
materially. These statements are based on management’s current beliefs and expectations. These statements
include but are not limited to statements that relate to our business and its future, our strategy, the success of
our drug candidates, the safety and efficacy of our drug products, product approvals, market potential, product
sales, revenue, development, regulatory and approval timelines, product launches, product acquisitions, capital
resources and any statements that relate to the intent, belief, plans or expectations of Spectrum or its
management, or that are not a statement of historical fact.
Risks that could cause actual results to differ include the possibility that our existing and new drug candidates
may not prove safe or effective, the possibility that our existing and new drug candidates may not receive
approval from the FDA and other regulatory agencies in a timely manner or at all, the possibility that our
existing and new drug candidates, if approved, may not be more effective, safer or more cost efficient than
competing drugs, the possibility that price and other competitive pressures may make the marketing and sale of
our drugs not commercially feasible, the possibility that our efforts to acquire or in-license and develop
additional drug candidates may fail, our lack of sustained revenue history, our limited experience in establishing
strategic alliances, our limited marketing experience, our customer concentration, the possibility for fluctuations
in customer orders, evolving market dynamics, our dependence on third parties for clinical trials, manufacturing,
distribution, information and quality control and other risks that are described in further detail in the Company's
reports filed with the Securities and Exchange Commission. We do not plan to update any such forward-
looking statements and expressly disclaim any duty to update the information contained in this presentation
except as required by law.
2

Poziotinib
A promising
Phase 2
pan-HER
inhibitor
3
ROLONTIS
TM
Late-stage drug
targeting blockbuster
market
QAPZOLA
TM
Late-stage drug
targeting unmet
need in bladder
cancer
Spectrum Advancing Three Strong
Oncology Focused Drugs
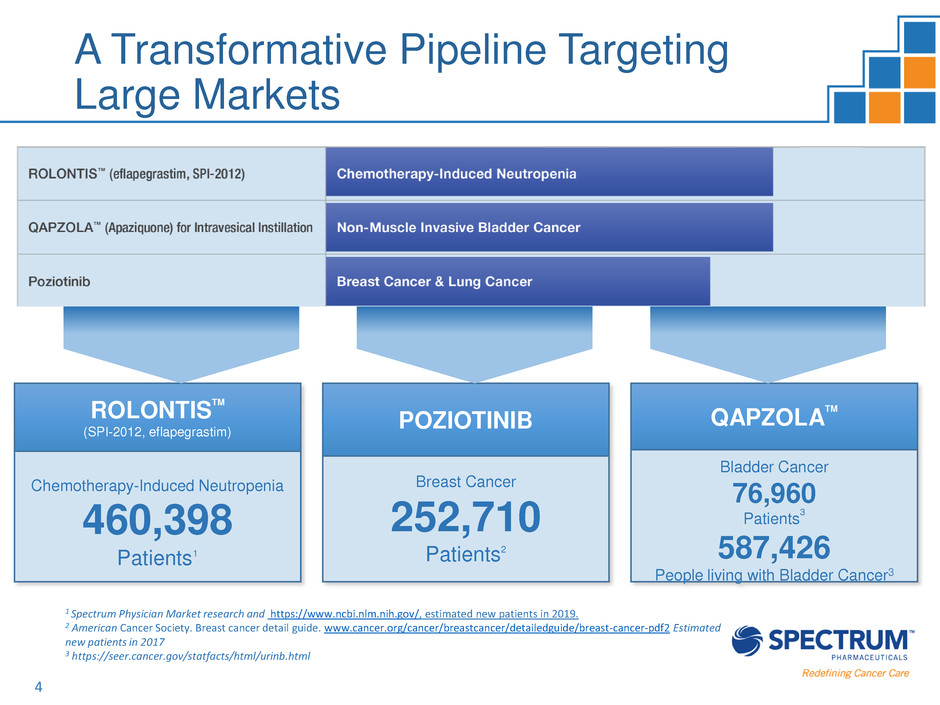
4
ROLONTIS
TM
(SPI-2012, eflapegrastim)
Chemotherapy-Induced Neutropenia
460,398
Patients1
POZIOTINIB
Breast Cancer
252,710
Patients2
QAPZOLA
TM
Bladder Cancer
76,960
Patients
3
587,426
People living with Bladder Cancer3
A Transformative Pipeline Targeting
Large Markets
1 Spectrum Physician Market research and https://www.ncbi.nlm.nih.gov/, estimated new patients in 2019.
2 American Cancer Society. Breast cancer detail guide. www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-pdf2 Estimated
new patients in 2017
3 https://seer.cancer.gov/statfacts/html/urinb.html

ROLONTISTM (eflapegrastim):
A Long-Acting Granulocyte-Colony Stimulating
Factor (G-CSF)
5
• Phase 3
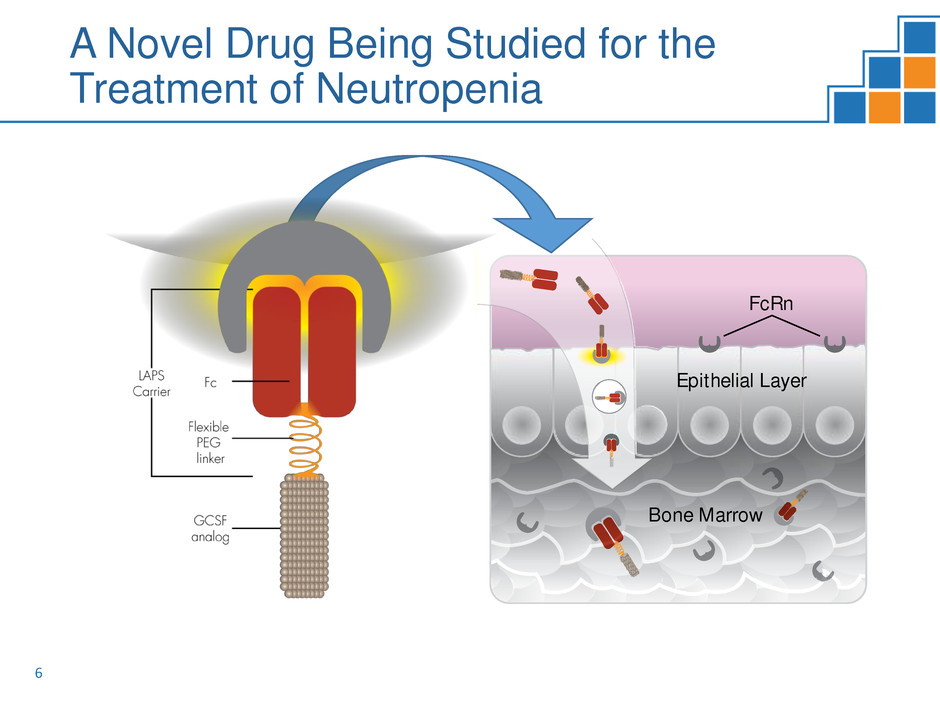
GCSF
Analog
Fc
FcRn
Flexible
PEG
Linker
LAPS
Carrier
FcRn
Bone Marrow
Epithelial Layer
A Novel Drug Being Studied for the
Treatment of Neutropenia
6
0
5
10
15
20
25
30
35
40
0 5 10 15 20
A
N
C
(1
0
9
/L
)
Days
ROLONTIS - 270 μg/kg
ROLONTIS - 45 μg/kg
ROLONTIS - 135 μg/kg
pegfilgrastim - 6 mg
Study
Drug
Chemo
Therapy
7
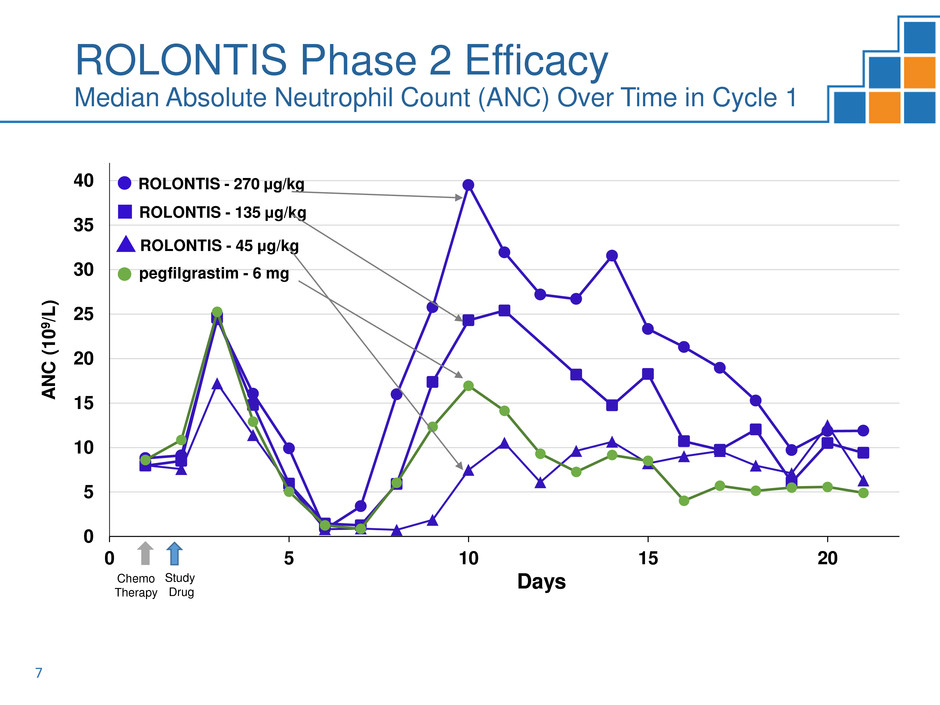
ROLONTIS Phase 2 Efficacy
Median Absolute Neutrophil Count (ANC) Over Time in Cycle 1
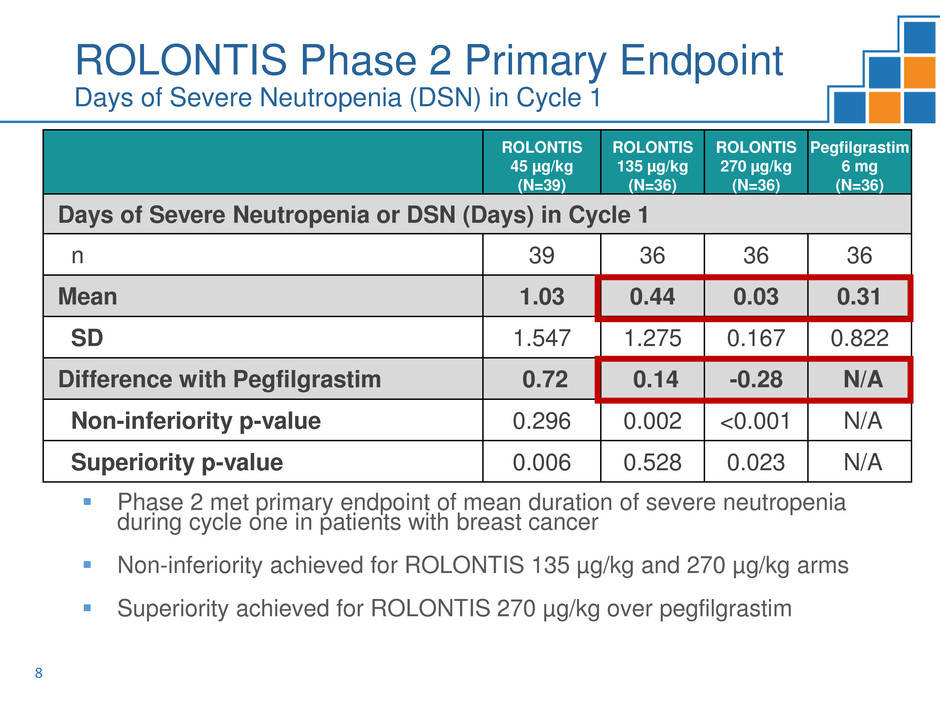
Phase 2 met primary endpoint of mean duration of severe neutropenia
during cycle one in patients with breast cancer
Non-inferiority achieved for ROLONTIS 135 µg/kg and 270 µg/kg arms
Superiority achieved for ROLONTIS 270 µg/kg over pegfilgrastim
ROLONTIS
45 µg/kg
(N=39)
ROLONTIS
135 µg/kg
(N=36)
ROLONTIS
270 µg/kg
(N=36)
Pegfilgrastim
6 mg
(N=36)
Days of Severe Neutropenia or DSN (Days) in Cycle 1
n 39 36 36 36
Mean 1.03 0.44 0.03 0.31
SD 1.547 1.275 0.167 0.822
Difference with Pegfilgrastim 0.72 0.14 -0.28 N/A
Non-inferiority p-value 0.296 0.002 <0.001 N/A
Superiority p-value 0.006 0.528 0.023 N/A
8
ROLONTIS Phase 2 Primary Endpoint
Days of Severe Neutropenia (DSN) in Cycle 1

Screening
Period
End of Treatment
Visit
30
Days
R
a
ndomi
z
atio
n
Treatment Period Four 21-day Cycles
Day 1 Day 2
Pegfilgrastim
ROLONTIS
(eflapegrastim)
Docetaxel +
Cyclophosphamide
~30 Days
after the end
of cycle 4
2 Randomized, Non-inferiority studies with duration of severe neutropenia
(DSN) as primary endpoint
ADVANCE is conducted under SPA from the FDA
RECOVER to enroll patients primarily from Europe
Pegfilgrastim sales in 2015 were $3.9 billion in US and $.8 billion in ROW
9
ROLONTIS Phase 3 Program:
Expected to File BLA in 2018

Poziotinib: A Novel, pan-HER Inhibitor
10
• Breast Cancer Phase 2
• Non-Small Cell Lung Cancer (NSCLC)
Phase 1
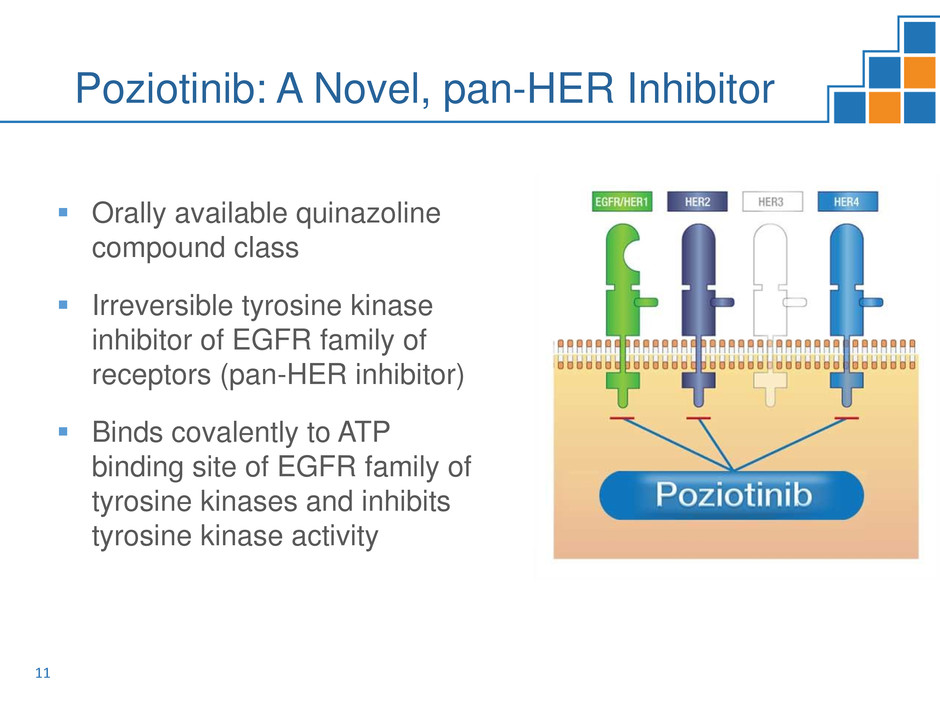
Orally available quinazoline
compound class
Irreversible tyrosine kinase
inhibitor of EGFR family of
receptors (pan-HER inhibitor)
Binds covalently to ATP
binding site of EGFR family of
tyrosine kinases and inhibits
tyrosine kinase activity
11
Poziotinib: A Novel, pan-HER Inhibitor
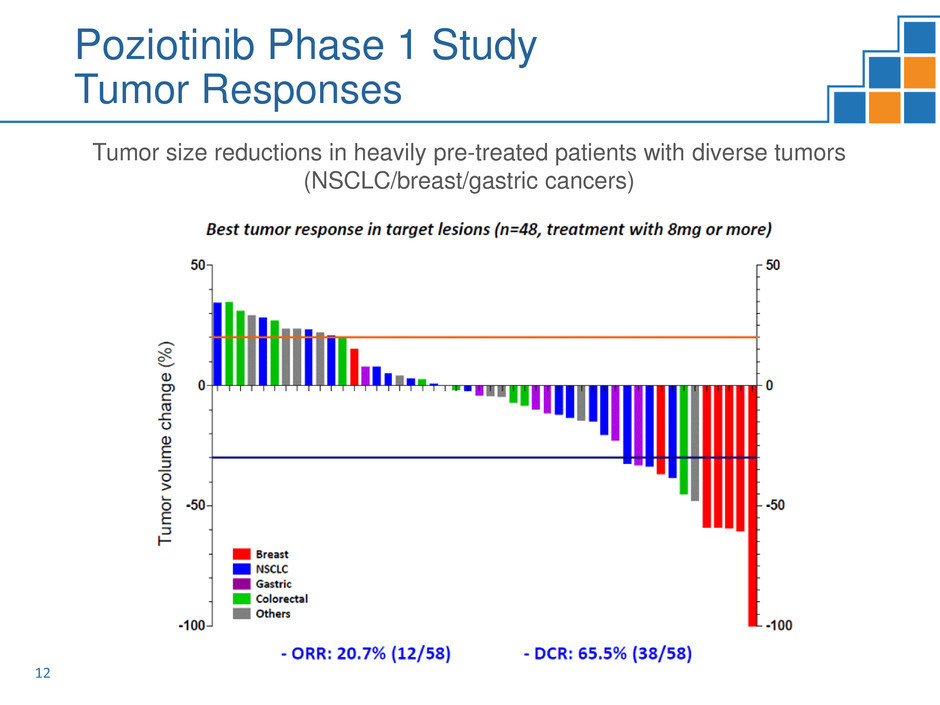
Poziotinib Phase 1 Study
Tumor Responses
Tumor size reductions in heavily pre-treated patients with diverse tumors
(NSCLC/breast/gastric cancers)
12
13
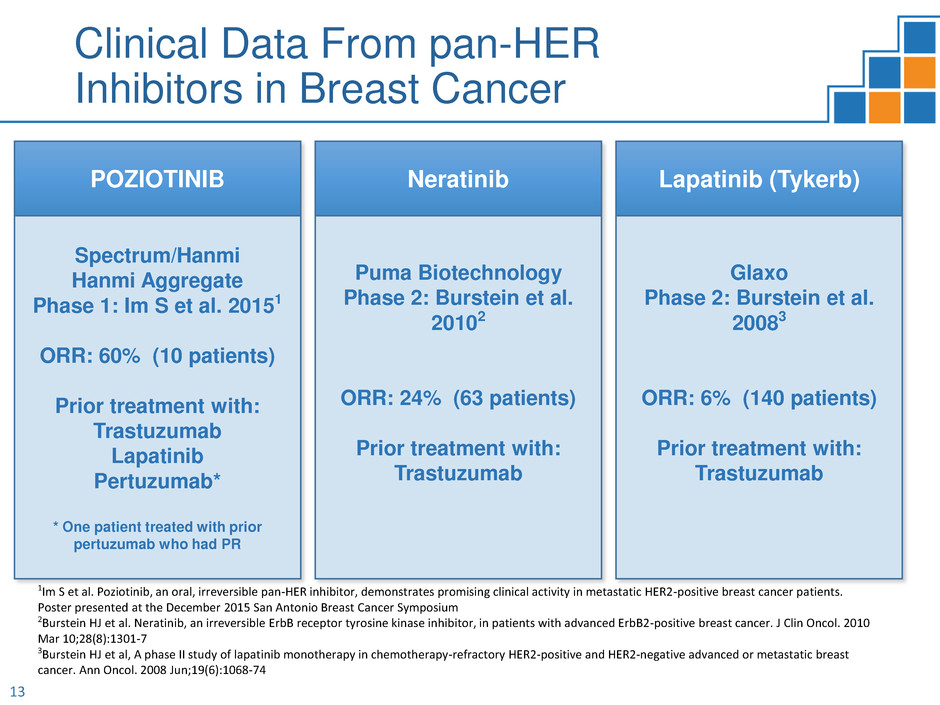
Clinical Data From pan-HER
Inhibitors in Breast Cancer
POZIOTINIB
Spectrum/Hanmi
Hanmi Aggregate
Phase 1: Im S et al. 20151
ORR: 60% (10 patients)
Prior treatment with:
Trastuzumab
Lapatinib
Pertuzumab*
* One patient treated with prior
pertuzumab who had PR
Neratinib
Puma Biotechnology
Phase 2: Burstein et al.
20102
ORR: 24% (63 patients)
Prior treatment with:
Trastuzumab
Lapatinib (Tykerb)
Glaxo
Phase 2: Burstein et al.
20083
ORR: 6% (140 patients)
Prior treatment with:
Trastuzumab
1Im S et al. Poziotinib, an oral, irreversible pan-HER inhibitor, demonstrates promising clinical activity in metastatic HER2-positive breast cancer patients.
Poster presented at the December 2015 San Antonio Breast Cancer Symposium
2Burstein HJ et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010
Mar 10;28(8):1301-7
3Burstein HJ et al, A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast
cancer. Ann Oncol. 2008 Jun;19(6):1068-74

Phase 2, Open Label
Indication: HER2-positive, recurrent, metastatic breast cancer
who have received at least 2 prior HER2-directed treatment
regimens.
Primary objective is to evaluate the Objective Response Rate
(ORR) of poziotinib in patients with human epidermal growth
factor receptor 2 (HER2)-positive MBC.
Approximately 70 patients
14
Poziotinib Phase 2 Design in
Breast Cancer

15
Poziotinib: Promising Activity in Non-
Small Cell Lung Cancer (NSCLC)
Lung cancer is by far the leading cause of cancer death
among both men and women, estimated 155,870 deaths in
20171
Existing therapies have very limited activity in NSCLC Exon 20
mutations
MD Anderson Cancer Center has screened multiple EGFR
inhibitors in preclinical models and has found poziotinib to be a
highly potent inhibitor of Exon 20 mutations
Data were presented in December 2016 at the World
Conference on Lung Cancer in Vienna
Investigator sponsored trial of poziotinib and EGFR/HER2
Exon 20 insertions scheduled to begin at MD Anderson
Cancer Center in first half of 2017
1http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-key-statistics
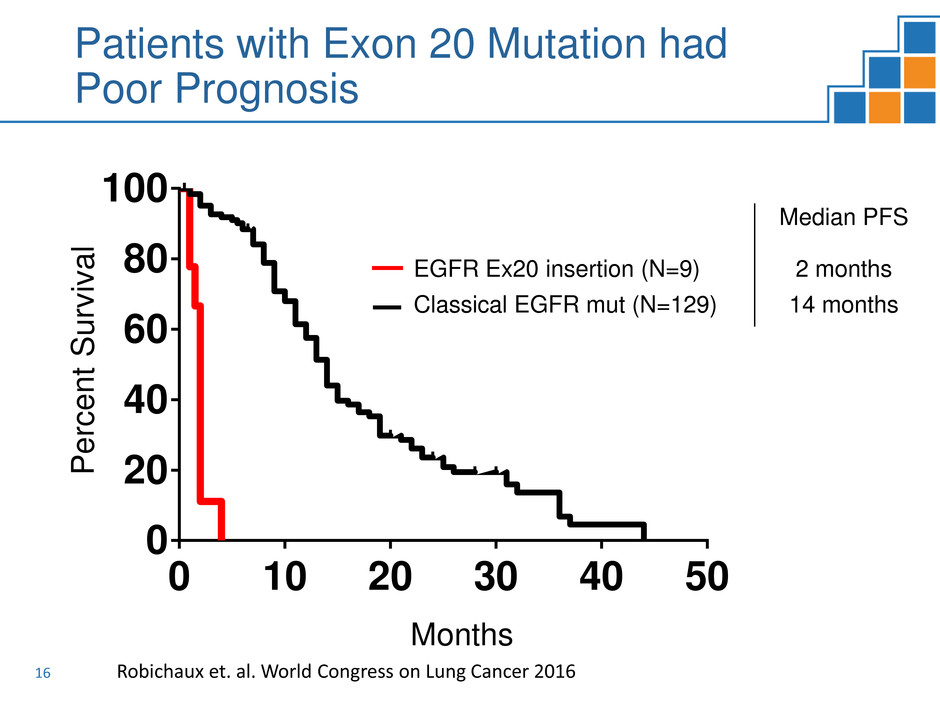
16
Patients with Exon 20 Mutation had
Poor Prognosis
Median PFS
EGFR Ex20 insertion (N=9) 2 months
Classical EGFR mut (N=129) 14 months
0 10 20 30 40 50
0
20
40
60
80
100
Time (Months)
Pr
og
re
ss
io
n
fre
e
su
rv
iv
al
(%
o
f p
at
ie
nt
s)
Classical EGFR mutations (n=129)
EGFR exon 20 insertions (n=9)
Median PFS (mo)
Classical EGFR Mutaions 14
EGFR exon 20 insertions 2
P<0.0001 (Log-rank)
P
e
rcen
t
S
u
rviv
a
l
Months
Robichaux et. al. World Congress on Lung Cancer 2016
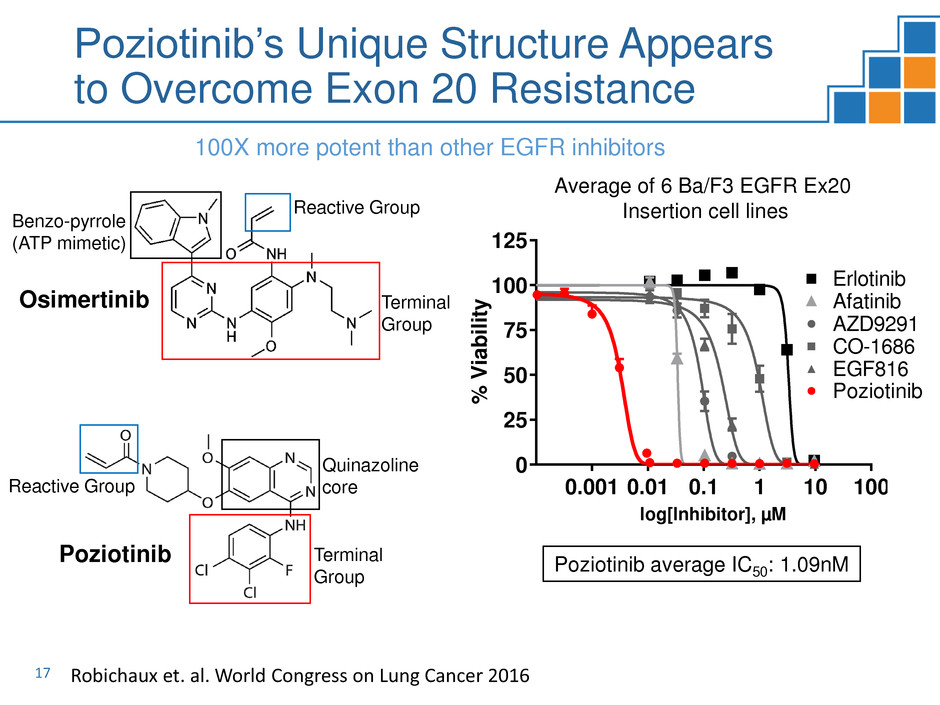
0.001 0.01 0.1 1 10 100
0
25
50
75
100
125
EGFR Exon20 Insertion Mutations
log[Inhibitor], µM
%
V
ia
bi
lit
y AZD9291
EGF816
CO-1686
Afatinib
Erlotinib
Poziotinib
0.001 0.01 0.1 1 10 100
0
25
50
75
100
125
EGFR Exon20 Insertion Mutations
log[Inhibitor], µM
%
V
ia
b
il
it
y
AZD9291
EGF816
CO-1686
Afatinib
Erlotinib
Poziotinib
Average of 6 Ba/F3 EGFR Ex20
Insertion cell lines
0.001 0.01 0.1 1 10 100
0
25
50
75
100
125
EGFR Exon20 Insertion Mutations
log[Inhibitor], µM
% Viability
AZD9291
EGF816
CO-1686
fatinib
Erlotinib
Poziotinib average IC50: 1.09nM
Poziotinib
Reactive Group
Quinazoline
core
Terminal
Group
Osimertinib Terminal
Group
Benzo-pyrrole
(ATP mimetic)
Reactive Group EGFR A763insFQEA
0.01 0.1 1 10 100
0
25
50
75
100
125 Erlotinib
Gefitinib
log[Inhibitor], µM
%
V
i
a
b
i
l
i
t
y
Robichaux et. al. World Congress on Lung Cancer 2016 17
Poziotinib’s Unique Structure Appears
to Overcome Exon 20 Resistance
100X more potent than other EGFR inhibitors

18
Poziotinib Summary
Orally available, Irreversible pan-
EGFR/HER inhibitor
Phase 2 Breast Cancer Study ongoing
Investigator sponsored trial of
poziotinib and EGFR/HER2 Exon 20
insertions scheduled to begin at MD
Anderson Cancer Center in first half of
2017
Hanmi studies in gastric and colorectal
continuing

QAPZOLATM (apaziquone) for
Intravesical Installation:
A potent tumor-activated drug for non-
muscle invasive bladder cancer
19

20
NMIBC
Non-muscle invasive bladder cancer remains an unmet
medical need with no new drugs approved in the last 25 years
QAPZOLA demonstrated activity in previous studies
Safety profile shows that QAPZOLA is well-tolerated
QAPZOLATM Targets Surface Tumors
in the Bladder

21
Spectrum is in Discussions with FDA
on New Study Design for QAPZOLA
Seeking SPA for proposed Phase 3 study design
New study design incorporates learnings from previous studies
Study Design
• Approximately 500 patients with non-muscle-invasive bladder
cancer (NMIBC)
• Single dose: 8mg
• Intravesical installation at 30 – 90 minutes post TURBT
• Double blind, placebo controlled Phase 3 study with 2:1
randomization to either QAPZOLA or placebo
• Primary endpoint: time to disease recurrence
Currently Marketing 6 Niche Anti-Cancer Drugs
Contributing to a Pipeline Targeting Large Markets
22
Proven Track Record to Pave the
Path to the Future
23
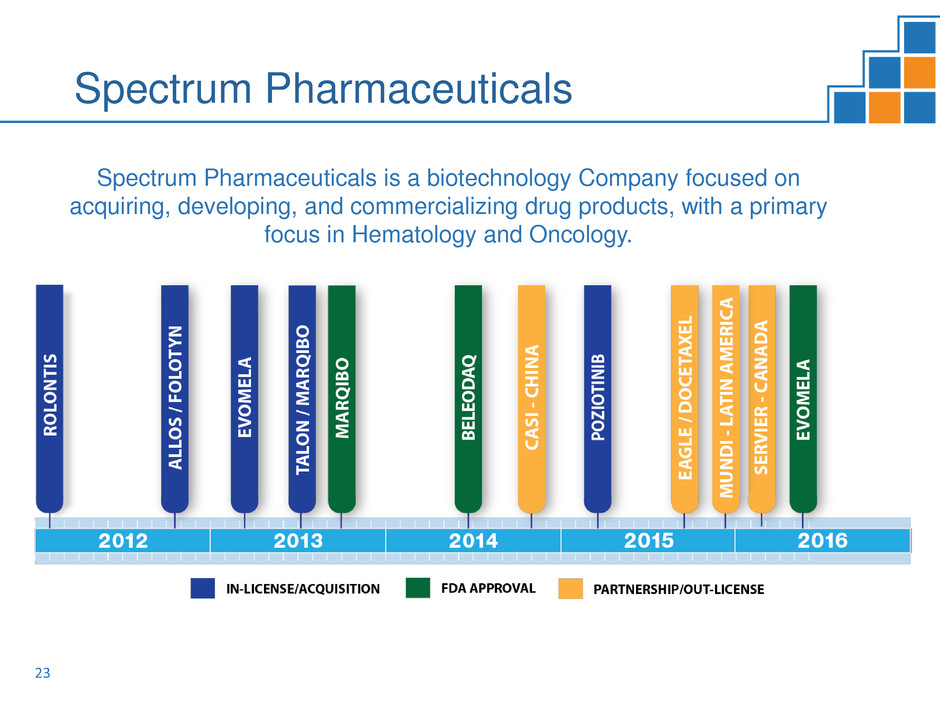
Spectrum Pharmaceuticals
Spectrum Pharmaceuticals is a biotechnology Company focused on
acquiring, developing, and commercializing drug products, with a primary
focus in Hematology and Oncology.

24
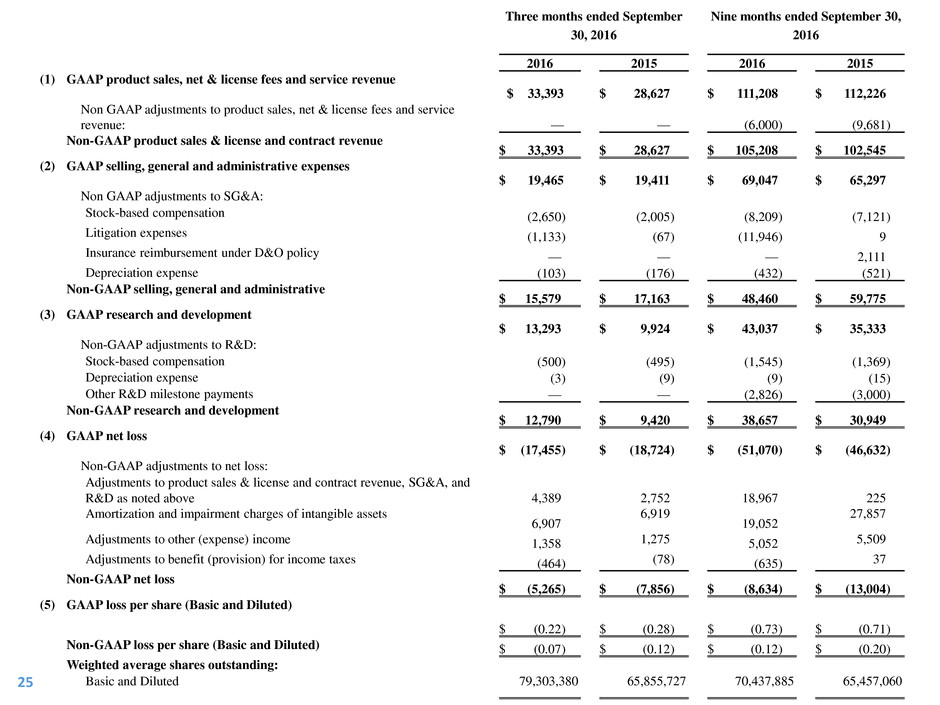
Three months ended September
30, 2016
Nine months ended September 30,
2016
2016 2015 2016 2015
(1) GAAP product sales, net & license fees and service revenue
$ 33,393
$ 28,627
$ 111,208
$ 112,226
Non GAAP adjustments to product sales, net & license fees and service
revenue: —
—
(6,000 ) (9,681 )
Non-GAAP product sales & license and contract revenue
$ 33,393
$ 28,627
$ 105,208
$ 102,545
(2) GAAP selling, general and administrative expenses
$ 19,465
$ 19,411
$ 69,047
$ 65,297
Non GAAP adjustments to SG&A:
Stock-based compensation (2,650 ) (2,005 ) (8,209 ) (7,121 )
Litigation expenses (1,133 ) (67 ) (11,946 ) 9
Insurance reimbursement under D&O policy — — — 2,111
Depreciation expense (103 ) (176 ) (432 ) (521 )
Non-GAAP selling, general and administrative
$ 15,579
$ 17,163
$ 48,460
$ 59,775
(3) GAAP research and development
$ 13,293
$ 9,924
$ 43,037
$ 35,333
Non-GAAP adjustments to R&D:
Stock-based compensation (500 ) (495 ) (1,545 ) (1,369 )
Depreciation expense (3 ) (9 ) (9 ) (15 )
Other R&D milestone payments — — (2,826 ) (3,000 )
Non-GAAP research and development
$ 12,790
$ 9,420
$ 38,657
$ 30,949
(4) GAAP net loss
$ (17,455 ) $ (18,724 ) $ (51,070 ) $ (46,632 )
Non-GAAP adjustments to net loss:
Adjustments to product sales & license and contract revenue, SG&A, and
R&D as noted above 4,389
2,752
18,967
225
Amortization and impairment charges of intangible assets
6,907
6,919
19,052
27,857
Adjustments to other (expense) income 1,358 1,275 5,052 5,509
Adjustments to benefit (provision) for income taxes (464 ) (78 ) (635 ) 37
Non-GAAP net loss
$ (5,265 ) $ (7,856 ) $ (8,634 ) $ (13,004 )
(5)
GAAP loss per share (Basic and Diluted)
$ (0.22 ) $ (0.28 ) $ (0.73 ) $ (0.71 )
Non-GAAP loss per share (Basic and Diluted) $ (0.07 ) $ (0.12 ) $ (0.12 ) $ (0.20 )
Weighted average shares outstanding:
Basic and Diluted 79,303,380
65,855,727
70,437,885
65,457,060 25

26
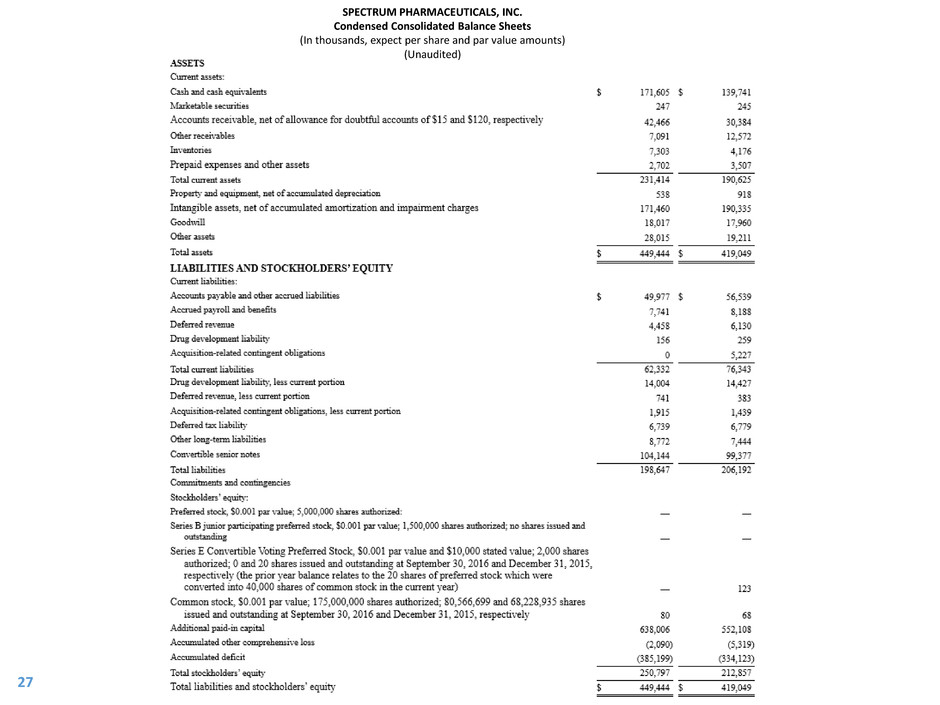
SPECTRUM PHARMACEUTICALS, INC.
Condensed Consolidated Balance Sheets
(In thousands, expect per share and par value amounts)
(Unaudited)
27
