Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - SELLAS Life Sciences Group, Inc. | gale-20161109ex991.htm |
| 8-K - 8-K - SELLAS Life Sciences Group, Inc. | gale-201611098xk.htm |

Q3, 2016
Financial Results &
Corporate Update

FORWARD LOOKING STATEMENT
This presentation contains forward-looking statements within the meaning of the
Private Securities Litigation Reform Act of 1995. Such statements include, but
are not limited to, statements about the divestiture of the commercial operations
including the two commercial products, the issuance and exclusivity of patents,
and the progress of development of Galena’s product candidates, including
patient enrollment in our clinical trials, interim analysis, time to complete the trials,
and expected time periods for results. These forward-looking statements are
subject to a number of risks, uncertainties and assumptions, including those
identified under “Risk Factors” in Galena’s Annual Report on Form 10-K for the
year ended December 31, 2015 and most recent Quarterly Reports on Form 10-Q
filed with the SEC. Actual results may differ materially from those contemplated
by these forward-looking statements. Galena does not undertake to update any of
these forward-looking statements to reflect a change in its views or events or
circumstances that occur after the date of this press release.
2

EARNINGS CALL PARTICIPANTS
Presenters
Mark W. Schwartz, Ph.D.
President & Chief Executive
Officer
Bijan Nejadnik, M.D.
Executive Vice President,
Chief Medical Officer
John T. Burns, CPA
Vice President, Finance and
Corporate Controller
Stephen Ghiglieri
Executive Vice President,
Chief Financial Officer
Other Participants
Remy Bernarda, IRC
SVP, Investor Relations &
Corporate Communications
Thomas J. Knapp, Esq
Interim General Counsel
3

OPENING
REMARKS
Mark W. Schwartz, Ph.D.
President and
Chief Executive Officer
4
Pseudo-Progression with Immunotherapy
Definition: Appearance of progression on radiographic imaging due to
increased tumor size (swelling) from tumor infiltrating lymphocytes
(TIL’s) and other immune cells
• A growing or new tumor detected via imaging isn't always progressing
cancer
Discovery of pseudoprogression in the context of cancer
immunotherapy in the last few years has changed the criteria for use of
imaging as a method for diagnosing tumor progression
• Image of a new tumor may simply represent an immune system
response to undetectable micrometastasis that would not otherwise
be seen on the scan
irRECIST (Immune-related Response Evaluation Criteria In Solid
Tumors) adapted rules that provide better assessment of the effect of
immunotherapeutic agents
Reference: https://www.lungevity.org/about-lung-cancer/experts-blog/immunotherapy-and-concept-of-pseudo-progression 5

CLINICAL
DEVELOPMENT
Bijan Nejadnik, M.D.
Executive Vice President,
Chief Medical Officer
6

PHASE 3 PRESENT
TOP-LINE INTERIM
RESULTS

PRESENT Top-Line Interim DFS Results
NeuVax Arm
Control Arm
Data based on interim data cut.
p-value = 0.07
Median time on trial
19.7 months

Imaging in the PRESENT Trial
NeuVax Arm
Control Arm
1st Imaging
~Month 12 2nd Imaging
~Month 24
3rd Imaging
~Month 36
Pre-treatment
Imaging
DFS Adjudicated Events
Diagnosed by
Clinical Presentation
Proactive Imaging 66% of DFS events
found via Proactive
Imaging 24 47
Data based on interim data cut.
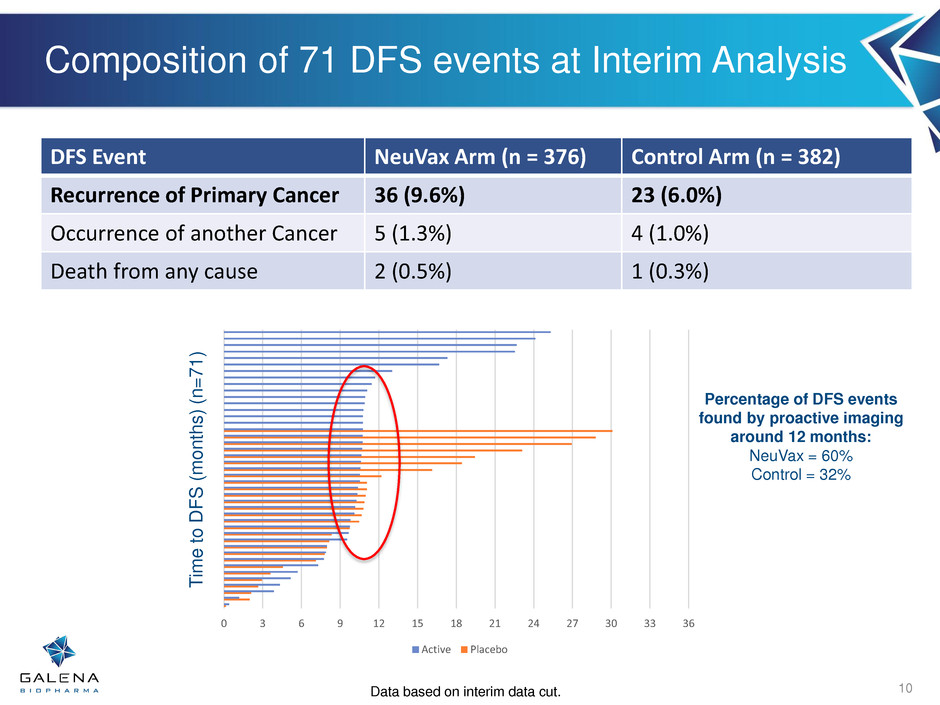
Composition of 71 DFS events at Interim Analysis
10
DFS Event NeuVax Arm (n = 376) Control Arm (n = 382)
Recurrence of Primary Cancer 36 (9.6%) 23 (6.0%)
Occurrence of another Cancer 5 (1.3%) 4 (1.0%)
Death from any cause 2 (0.5%) 1 (0.3%)
0 3 6 9 12 15 18 21 24 27 30 33 36
Active Placebo
T
im
e
t
o
D
F
S
(
m
on
th
s
)
(n=
7
1
)
Percentage of DFS events
found by proactive imaging
around 12 months:
NeuVax = 60%
Control = 32%
Data based on interim data cut.

Pseudo-Progression in Cancer Immunotherapy &
The PRESENT Trial
In adjuvant setting, immunological therapies require extended clinical monitoring over time to
evaluate their effectiveness in preventing recurrences of clinical significance
This inflammation can be potentially beneficial, but paradoxically show as worsening of a tumor or
be assessed inaccurately as a recurrence in the case of micrometasteses
Inflammation changes the consistency (more opaque to XRay) and the volume (swelling), which
makes a micrometastasis visible that otherwise would not have been detected, or a tumor appears
to be enlarged on a CT Scan
In the period of immunostimulation, immune inflammatory cells (NeuVax T-Cells) enter the tumor
or micrometastases and create inflammation which includes edema, and attracts inflammatory cells
11

Summary
Low rate of recurrence seen in the control arm may have resulted from
an overall improvement in the standard of care
Existing lesions were discovered by proactive imaging
• May not have had any clinical significance at the time of identification
• Lesions changed by inflammation and may never have progressed to
clinical tumors
The inflammation caused by NeuVax induced cytotoxic T-cells (TILs)
within the micrometastases made the lesions visible on scans in the
NeuVax group and not the control group
Pseudo-Recurrence
12

IMMUNOTHERAPY
PROGRAMS
13
NeuVax™ (nelipepimut-S)
GALE-301/GALE-302

DEVELOPMENT PIPELINE
PRODUCT THERAPETIC AREA PHASE 1 PHASE 2 PHASE 3 BLA / NDA
Hematology
GALE-401 (Anagrelide CR) Essential Thrombocythemia
Immunotherapy: Breast Cancer
NeuVax™ + Herceptin®
Node-positive or node negative/triple
negative, HER2 IHC 1+/2+
NeuVax™ + Herceptin®
High risk, node-positive or negative,
HER2 IHC 3+
NeuVax™ Ductal Carcinoma in Situ (DCIS)
Immunotherapy: Gastric Cancer
NeuVax™ Gastric, HER2 IHC 1+/2+/3+
Immunotherapy: Gynecological Cancer
GALE-301 Ovarian & Endometrial
GALE-301 + GALE-302 Ovarian & Breast
*NeuVax is an investigational product. Efficacy has not been established. Herceptin is a registered trademark of Genentech.
Ongoing Planned
VADIS
14
2b

GALE-401
Anagrelide Controlled
Release (CR)
15

Essential Thrombocythemia (ET)
Diagnosis
• Chronic
hematologic
malignancy with
no known cause
• Symptoms
• Diagnostic tools
• Blood test
• Bone marrow
biopsy
• Gene
mutation test
Common
Symptoms
• Headache
• Vision
disturbances or
migraines
• Dizziness or
lightheadedness
• Coldness or
blueness of
fingers or toes
• Burning,
redness, and
pain in the
hands and feet
Thrombotic
Complications
• Stroke
• Transient
ischemic attack
(TIA)
• Heart attack
• DVT or
pulmonary
embolus
• Blood clotting in
unusual
locations
Risk Factors
•Women 1.5x
more likely
• Patients >60
years old, with
20% <40 years
• Mutations
• JAK2 - 50%
• CALR ~25%
16 Source: MPN Research Foundation

Current ET Treatment Options
Hydroxyurea
• Generally first line therapy
• Cytotoxic Myelosuppressive drug
(reduces other blood cells as well)
• Increased risk of developing acute
leukemia after long term
• Avoided in younger patients
• ~25% of patients intolerant/refractory
Anagrelide IR
• Poor tolerability and compliance
thought to be related to blood
concentrations
• Non cytotoxic drug
• Not associated with increased risk of
leukemia
• Significant side effects
Aspirin
• Given to reduce the risk of blood
clotting
• May help relieve the burning
sensation in patient’s hands and feet
(erythromelagia)
Other Therapies
• Interferon
• Busulfan
• Retry hydroxyurea
• Observation
17 Sources: Leukemia and Lymphoma Society: Essential Thrombocythemia Facts Cervantes, F. Hematology 2011; 215-221

GALE-401 Development Summary
Potential Clinical Benefits from Phase 2 trial
• Potentially faster onset of action
• Consistent efficacy
• Indication of improved tolerability vs anagrelide IR
• Twice a day dosing with a PK profile supportive of once-a-day dosing
Strong Development Rational
• Novel proprietary formulation of FDA approved product with known mechanism of
action
• 505(b)(2) regulatory pathway for approval (to be confirmed with the FDA)
• Intellectual property allowing market exclusivity through 2029
18
GALE-401 ET Development Opportunity
Diagnosed patient population for ET
• US Prevalence: 135,000 - 175,000
Limited competition with very few agents in development
Multiple life cycle management opportunities
Next steps
• End of Phase 2 meeting with the FDA
• Finalize the Phase 3 clinical trial design
• Initiate pivotal trial in Q2, 2017
19
Sources: Harrison et al N Engl J Med 2005;353:33-45; Mehta et al, (2014) Epidemiology of myeloproliferative
neoplasms in the United States, Leukemia & Lymphoma, 55:3, 595-600, DOI: 10.3109/10428194.2013.813500

FINANCE
John Burns
Vice President, Finance and
Corporate Controller
20
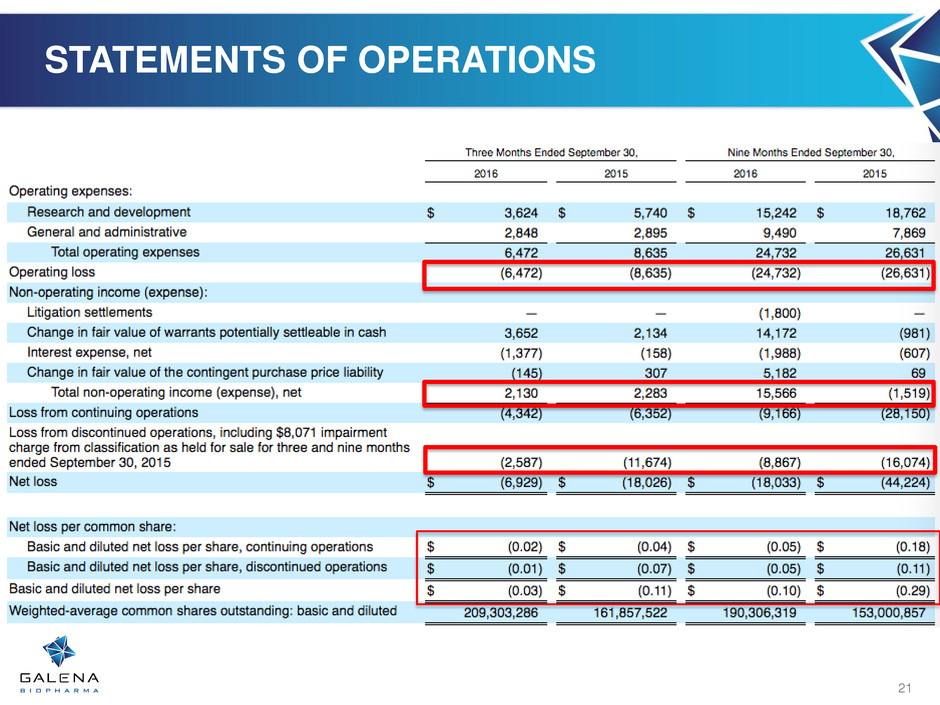
STATEMENTS OF OPERATIONS
21

Q3 2016 CASH BURN
Beginning Cash Position (as of June 30, 2016) $19.6 million
Net proceeds from equity financing $11.7 million
Reduction in restricted cash $5.5 million
Cash burn from continuing operations ($7.7 million)
Cash burn from discontinued operations ($2.1 million)
Cash burn on litigation settlements ($2.5 million)
Ending Cash Position (as of September 30, 2016) $24.5 million
22

FINANCIAL OVERVIEW
Cash Position (as of 30 Sept 16) $24.5 million
Restricted Cash (as of 30 Sept 16 - $18.5 relating to
Debenture)
$18.9 million
Projected Q4 Cash Burn from Operations $7 - $9 million
Shares Outstanding (as of 31 Oct 16) 217 million
23

FINANCE
Stephen Ghiglieri
Executive Vice President,
Chief Financial Officer
24

MILESTONES &
CLOSING
REMARKS
Mark W. Schwartz, Ph.D.
President and
Chief Executive Officer
25
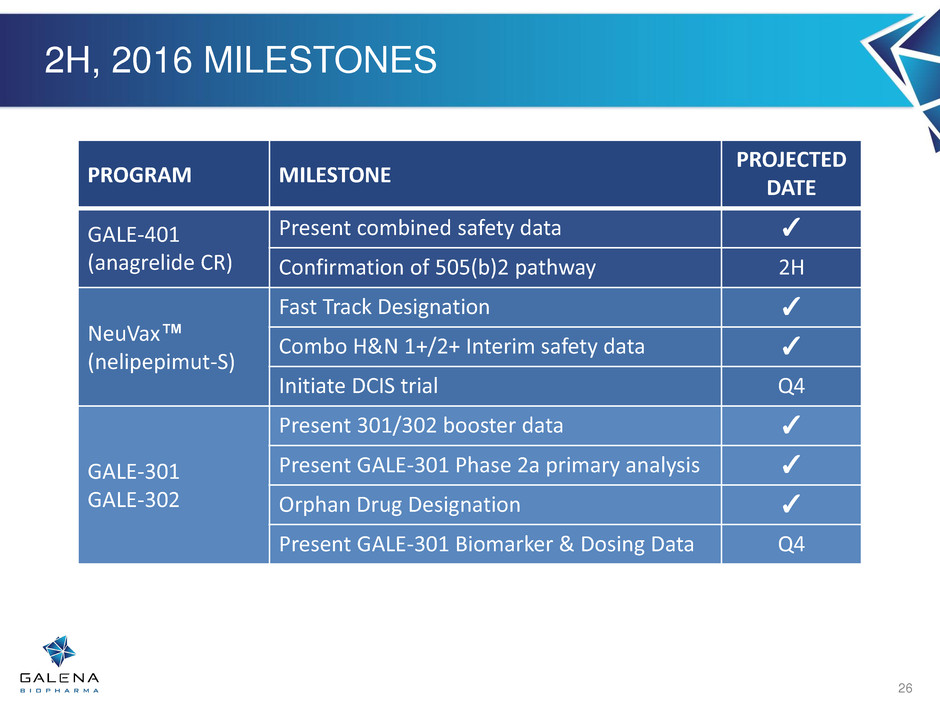
2H, 2016 MILESTONES
26
PROGRAM MILESTONE
PROJECTED
DATE
GALE-401
(anagrelide CR)
Present combined safety data ✓
Confirmation of 505(b)2 pathway 2H
NeuVax™
(nelipepimut-S)
Fast Track Designation ✓
Combo H&N 1+/2+ Interim safety data ✓
Initiate DCIS trial Q4
GALE-301
GALE-302
Present 301/302 booster data ✓
Present GALE-301 Phase 2a primary analysis ✓
Orphan Drug Designation ✓
Present GALE-301 Biomarker & Dosing Data Q4
