Attached files
| file | filename |
|---|---|
| EX-99.1 - PRESS RELEASE - Simulations Plus, Inc. | simulations_ex9901.htm |
| 8-K - FORM 8-K - Simulations Plus, Inc. | simulations_8k.htm |
Exhibit 99.2

( NASDAQ:SLP ) Investor Conference Call July 14, 2016

2 With the exception of historical information, the matters discussed in this presentation are forward - looking statements that involve a number of risks and uncertainties . The actual results of the Company could differ significantly from those statements . Factors that could cause or contribute to such differences include, but are not limited to : continuing demand for the Company’s products, competitive factors, the Company’s ability to finance future growth, the Company’s ability to produce and market new products in a timely fashion, the Company’s ability to continue to attract and retain skilled personnel, and the Company’s ability to sustain or improve current levels of productivity . Further information on the Company’s risk factors is contained in the Company’s quarterly and annual reports and filed with the Securities and Exchange Commission . Safe Harbor Statement

3 Highlights Walt Woltosz Chairman and Chief Executive Officer

4 • Software renewal rates: 88% (accounts); 96%(fees) • 16 new software client sites added • Product development • PKPlus™ Beta test version released this week • new software product for noncompartmental analysis and compartmental analysis for regulatory submissions • potential to become significant contributor to revenues and earnings • Finalizing Version 9.5 of GastroPlus ™ • Intramuscular dosing and enhanced ocular dosing model (2 FDA RCAs helping) • Antibody - drug conjugates (ADCs) • New animal physiologies for Additional Dosing Routes • Finalizing Version 8.0 of ADMET Predictor ™ • major overhaul of user interface and code refactoring – extensive QA testing • greater integration with MedChem Studio™ • Released Version 5.0 of DDDPlus ™ • several new dosage forms • tablet disintegration addition • biorelevant solubilities • tablet compression model • Consulting services continue to grow • Announcement of $4.7MM/5 - year contract 3QFY16 Highlights

5 • Completed first year of up to 3 - year, $200,000/year collaboration for improved ocular dosing simulations – contract renewed for 2nd year • Met all milestones during first full year • Established consortium of leading pharmaceutical companies • The global ophthalmic drugs market was valued at $16 billion in 2012, and was expected to reach an estimated value of $21.6 billion in 2018 • Prevalence of eye disorders is increasing as the population ages (e.g., diabetic retinopathy, macular degeneration) FDA Office of Generic Drugs (OGD) Funded Collaborations • Began first year of up to 3 - year, $200,000/year collaboration for simulation of long - acting injectable microspheres • Formed consortium of industry partners, FDA scientists, and Simulations Plus • Added intramuscular dosing to GastroPlus (V. 9.5) • Developed enhancements to DDDPlus to simulate in vitro dissolution/release from polymer microspheres

6 • $250,000/year for up to two years to combine PBPK modeling with population statistical methods used in Phase 2 & 3 trial data analysis • Proposals submitted May 16 • Combined expertise of our Lancaster and Buffalo divisions – excellent synergy • “Up to two” contracts to be awarded • We believe our proposal was very strong. Award announcement should be sometime before September FDA Office of Generic Drugs (OGD) New Requests for Applications (RFAs) • $250,000/year for up to two years to develop software to predict in vivo pharmacokinetic profiles of supersaturating formulations • Proposals submitted June 3 • Our GastroPlus™ software already incorporates some of the requested modeling • “Up to two” contracts to be awarded • We believe this proposal was also very strong. Award announcement should be sometime before September

7 • AEROModeler™ • Application of our artificial neural network ensemble (ANNE) technology to: • Predict aerodynamic force coefficients for missiles • Classify missiles from radar tracking data • Discriminate between warheads and decoys from sensor data • Recent USAF contact (June) has identified another potential application • Exploring several government and industry contacts • Put on temporary hold to allow development team to focus on PKPlus • MRIModeler™ • Application of our ANNE technology to analysis of magnetic resonance imaging (MRI) data to classify patients as healthy or likely to experience various disease states • Put on temporary hold to allow development team to focus on PKPlus Exploring Business Opportunities Outside of Pharmaceutical Industry

8 • Major provider of software and consulting services for pharma R&D • Earliest drug discovery – when a chemist first draws a molecule • Preclinical development (lab and animals) through first - in - human trials • Phase 2 and 3 clinical trials • Beyond patent life to supporting generic companies • Enhancing every existing software product • Finalizing PKPlus – exciting new product • Developing new applications in aerospace and general healthcare based on our machine - learning technologies (on temporary hold for PKPlus) • 3 QFY16 revenues were up by $ 7 0,000 (1.2%) to $6.01 million • 3 QFY16 net income was up by $57,000 (3.1%) to $1.91 million • 3 QFY16 diluted EPS up 2.10% to $0.111 (11¢) per share compared to $ 0.108 (11¢) in 3QFY15 . • 9 moFY16 revenues were up by $1.41 Million (9.7%) to $16.01 Million • 9moFY16 net income was up by $810,000 (24.2%) to $4.16 million • 9moFY16 diluted EPS up 23.1% to $ 0.242 (24¢) per share compared to $ 0.196 (20¢) in 9moFY15 . Overview

9 • Continued Growth • Eight - year - plus profitable trend • Successful strategic acquisition • Customer base increased: • 16 new software customers in 3QFY16 • 96% renewal rate (fees) • Expecting continued compounded growth • 3rd Quarter earnings per share increased 2.1% • Year - to - date (9 month) earnings per share increased 23.1% • Strong cash position; returning cash to shareholders • Company has continued to pay dividend of $0.05 per quarter • Over $15 million in cash dividends distributed since 2012, yet cash remains at $8.9 million as of yesterday Summary

10 Financial Overview John Kneisel Chief Financial Officer

11 Income Statement 3 QFY16 Compared to 3QFY15 (in millions) Lancaster Buffalo 3QFY16 3QFY15 Diff % chg Net sales $ 4.664 $ 1.348 $ 6.012 $ 5.942 $0.070 1.2% Gross profit 4.023 0.794 4.817 4.809 0.008 0.2% Gross profit margin 86.26% 58.90% 80.12% 80.93% - 0.81% - 1.0% SG&A 1.131 0.550 1.681 1.607 0.074 4.6% R&D 0.316 0.033 0.349 0.348 0.001 0.3% Total operating expenses 1.446 0.583 2.029 1.956 0.073 3.7% Income from operations 2.577 0.211 2.788 2.854 - 0.066 - 2.3% Other income (expense) 0.012 - 0.012 (0.031) 0.043 - 138.7% Income from operations before income taxes 2.589 0.211 2.800 2.823 - 0.023 - 0.8% Net income $ 1.778 $ 0.131 $ 1.909 $ 1.852 0.057 3.1% Diluted earnings per share (in dollars) $ 0.111 $ 0.108 $ 0.0023 2.1% EBITDA 2.971 0.309 3.28 3.326 - 0.046 - 1.4%

12 Income Statement 9moFY16 Compared to 9moFY15 (in millions) Lancaster Buffalo 9moFY16 9moFY15 Diff % chg Net sales $ 11.722 $ 4.292 $ 16.014 $ 14.602 $1.412 9.7% Gross profit 9.935 2.538 12.473 $ 11.294 1.179 10.4% Gross profit margin 84.76% 59.13% 77.89% 77.35% 0.54% 0.7% SG&A 3.376 1.704 5.080 $ 5.234 - 0.154 - 2.9% R&D 1.087 0.074 1.161 $ 0.982 0.179 18.2% Total operating expenses 4.463 1.778 6.241 $ 6.216 0.025 0.4% Income from operations 5.471 0.760 6.231 5.078 1.153 22.7% Other income (expense) ( 0.023) 0 (0.022) (0.065) 0.043 - 66.2% Income from operations before income taxes 5.449 0.761 6.210 5.014 1.196 23.9% Net income $ 3.691 $ 0.471 $ 4.162 $ 3.352 0.81 24.2% Diluted earnings per share (in dollars) $ 0.242 $ 0.196 $ 0.045 23.1% EBITDA 6.62 1.040 7.66 6.497 1.163 17.9%
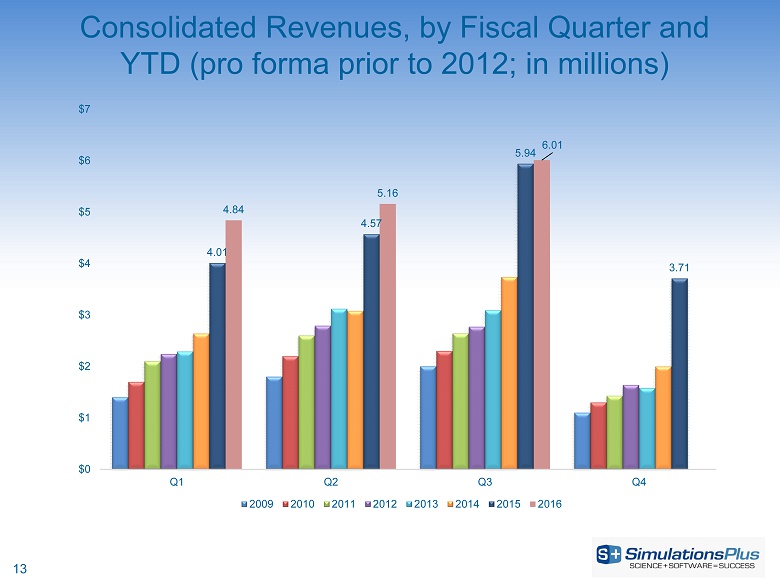
13 Consolidated Revenues, by Fiscal Quarter and YTD (pro forma prior to 2012; in millions) 4.01 4.57 5.94 3.71 4.84 5.16 6.01 $0 $1 $2 $3 $4 $5 $6 $7 Q1 Q2 Q3 Q4 2009 2010 2011 2012 2013 2014 2015 2016

14 Consolidated Gross Profit, by Fiscal Quarter & YTD (pro forma prior to 2012; in millions) 3.09 3.45 4.83 2.64 3.76 3.9 4.82 $0 $1 $2 $3 $4 $5 $6 Q1 Q2 Q3 Q4 2009 2010 2011 2012 2013 2014 2015 2016

15 Consolidated Net Income, by Fiscal Quarter and YTD (pro forma prior to 2012; in millions) $0.53 $0.97 $1.85 $0.49 $1.11 $1.15 $1.91 $- $0.50 $1.00 $1.50 $2.00 $2.50 Q1 Q2 Q3 Q4 2009 2010 2011 2012 2013 2014 2015 2016

16 Consolidated Diluted Earnings Per Share 0 0.02 0.04 0.06 0.08 0.1 0.12 Q1 Q2 Q3 Q4 $0.03 $0.06 $0.11 $0.03 $0.06 $0.07 $0.11 Quarterly EPS FY09 FY10 FY11 FY12 FY13 FY14 FY15 FY16

17 Consolidated EBITDA, by Fiscal Quarter & YTD (in millions) 1.22 1.97 3.33 1.19 2.2 2.18 3.28 $0 $1 $2 $3 $4 Q1 Q2 Q3 Q4 2009 2010 2011 2012 2013 2014 2015 2016

18 Returning Cash to Shareholders (in millions) 1.6 0.8 0.8 2.2 0 0.5 0.5 0.6 0.81 0.81 0.81 0.84 0.84 0.84 0.85 0.85 0.85 0.85 13.2 12.9 12.7 11.4 9.3 9.8 10 10.1 10.6 11 7.8 8.6 5.8 6.1 6.4 8.6 7.2 7.1 8.8 8.9 $0 $1 $1 $2 $2 $3 $0 $2 $4 $6 $8 $10 $12 $14 Dividend Paid Cash on Hand Cash paid $2.5M TSRL Cash paid $2.1M for Cognigen

19 Selected Balance Sheet Items (in millions, except where indicated) May 31, 2016 August 31, 2015 Cash and cash equivalents $ 7.280* $ 8.551 Cash per share ( in Dollars ) $ 0.43 $ 0.50 Total current assets 13.589 11.533 Total assets 28.798 27.344 Total current liabilities 4.028 3.613 Total liabilities 7.292 7.812 Current ratio 3.37x 3.19x Shareholders’ equity 20.311 19.532 Total liabilities and shareholders’ equity 28.798 27.344 Shareholders’ equity per diluted share( in Dollars ) $1.25 $1.15 * Cash as of July 12, 2016 was ~$8.9 million

20 Marketing and Sales John DiBella VP of Marketing and Sales

21 End - to - end M&S Solutions Provider N H O OH O CH 3 CH 3 CH 3 ADMET Predictor™ GastroPlus ™ MedChem Studio™ MedChem Designer™ DDDPlus ™ MembranePlus™ Consulting Services and Collaborations Discovery Preclinical C linical COMING SOON: PKPlus™ KIWI™

22 • Version 9.5 scheduled for summer 2016 ‒ Intramuscular dosing model – optional add - on model ‒ Antibody - drug conjugate (ADC) models for biologics • Version 8.0 scheduled for summer 2016 ‒ Significant refresh of the graphical user interface ‒ New ‘MedChem’ module – optional add - on incorporating items from MedChem Studio • Version 4 .0 still licensed by clients ‒ Many features merged into ADMET Predictor 8.0 as optional add - on to consolidate under one GUI Software Product News • Version 5.0 released in April 2016 ‒ Integration of models from ADMET Predictor™ – optional add - on ‒ New dosage form options for immediate & controlled release formulations • Version 1.5 scheduled for 2016 ‒ Ability to model multiple compounds to optimize in vitro drug - drug interaction parameters • New product: v ersion 1.0 scheduled for summer 2016 ‒ Validated software for noncompartmental (NCA) & compartmental PK modeling ‒ Reports in user - customized formats for regulatory submission ‒ Large potential market
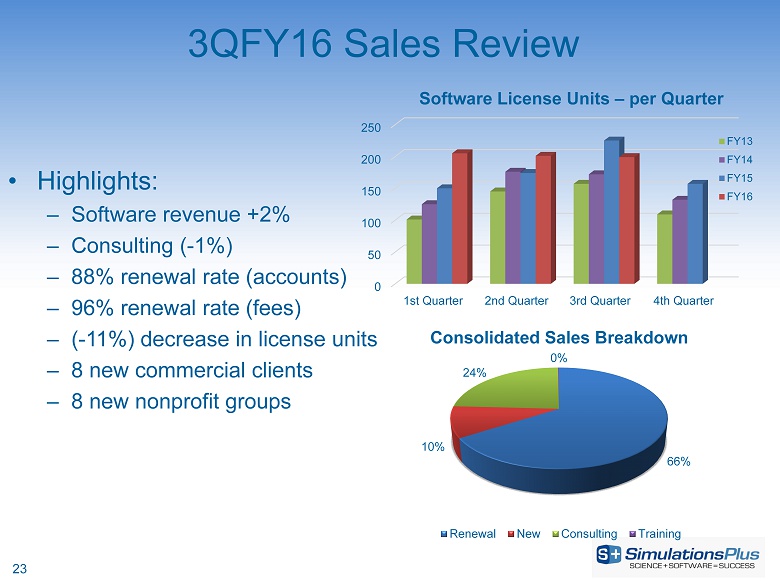
23 3 QFY16 Sales Review • Highlights: – Software revenue + 2 % – C onsulting ( - 1%) – 88% renewal rate (accounts) – 96% renewal rate (fees) – ( - 11%) decrease in license units – 8 new commercial clients – 8 new nonprofit groups 66% 10% 24% 0% Consolidated Sales Breakdown Renewal New Consulting Training 0 50 100 150 200 250 1st Quarter 2nd Quarter 3rd Quarter 4th Quarter Software License Units – per Quarter FY13 FY14 FY15 FY16

24 9 moFY16 Sales Review • Highlights: – Software revenue + 9 % – C onsulting +12% – 87% renewal rate (accounts) – 93% renewal rate (fees) – 10% increase in license units – 28 new commercial clients – 27 new nonprofit groups – Significant expansion of licenses at US FDA, US EPA, and China FDA 58% 12% 30% 0% Consolidated Sales Breakdown Renewal New Consulting Training 0 50 100 150 200 250 1st Quarter 2nd Quarter 3rd Quarter 4th Quarter Software License Units – per Quarter FY13 FY14 FY15 FY16

25 3 QFY16 Software Revenue by Region Europe 27% North America 52% Asia 21% South America <1% Japan = 56% India = 23% China = 15% Korea = 6 %

26 Marketing Activities – 3QFY16 • Website redesign – Design finalized and new content being developed – summer launch • Conferences and scientific meetings – 16 scientific meetings attended; co - authors on 1 2 presentations – Invited speaker at FDA meeting on oral absorption modeling • Trainings and workshops – Workshops hosted in China, Europe and US • 2 - day seminar with China FDA drew >80 FDA scientists – Workshops scheduled in US and Japan in fall – PKPlus™ roadshow in Boston drew global audience at 2 large pharma companies • Strategic digital marketing initiatives – Hosted 4 webinars on our software updates and applications – Active updates: LinkedIn, Twitter, YouTube, and Facebook accounts – Continued web - based advertising for all programs – 14 peer - reviewed publications citing use of software

27 Buffalo Division Update Re - imagining the future of science - based research and development Ted Grasela President

28 Consulting Services • Strategic and synergistic benefits of the Buffalo (Cognigen) acquisition are being realized • Strong collaborations between Buffalo and Lancaster scientists have identified new and innovative ways of using modeling and simulation to bringing value to our clients • Consulting projects help shape management and regulatory decision - making process • Successful projects help drive additional consulting and software sales

29 Status Report - Consulting • In FY2016 working with 27 companies on 48 drugs, 85 projects – 8 new companies in FY2016 – 11 new projects in FY2016Q3 – 8 projects expanded scope in FY2016Q3 – 2 projects delayed because of slow enrollment – 3 projects cancelled because of failed studies – 39 outstanding proposals with 20 different companies • In FY2016, presented 15 posters and published 2 papers – Working on 15 publications and 4 conference abstracts • Most common therapeutic area is oncology, followed by neurology and immunology – ~25% of projects result directly in regulatory interaction. • First - time presentation of ADMET Predictor™ at clinical pharmacology meeting opened up conversations with new community of users.

30 • Analysis in R&D can be messy • Data and inputs coming from many groups • Careful synthesis and communication is essential • The industry has enormous computing power • The industry lacks tools for harnessing that power Behind the scenes in R&D Coping with complexity and chaos

31 KIWI™ – A Software Platform for Accessing Simulations Plus’ Validated Cloud Processing Capabilities

32 • Signed a $4.7 million contract with a major foundation to implement a KIWI platform for global teams engaged in model - based drug development; 5 - year term contingent on satisfactory completion of milestones • Project builds on our extensive process - related research; enables substantial enhancements to the KIWI platform • Enhancements will provide a scaffold with broad applicability and will be available to academic and industry clients of KIWI • We expect to close on 3 KIWI licenses in FY2016Q4 • Have several KIWI demonstrations ongoing with research groups ranging from academics to large pharma . KIWI Update

33 Summary Buffalo division is strong and growing • Consulting activities continuing to expand, and realizing synergies with Lancaster division for PBPK modeling in clinical pharmacology • Foundation contract to enhance the KIWI platform is a major step forward in our long - term vision for the product • Continued interest in the academic and industry communities regarding licensing the product

34 Final Summary Walt Woltosz Chairman and Chief Executive Officer

