Attached files
Exhibit 99.2

Design, Objectives, and Status ofAsterias’ Current Spinal Cord InjuryClinical Trial Edward Wirth, III, M.D., Ph.D. Chief Medical Officer Investor DayMay 8, 2015 *

AST-OPC1: Phase 1 Safety Study in Complete Thoracic SCI * MRI MRI MRI MRI MRI MRI MRI MRI MRI MRI Primary Assessment: SafetySecondary Assessment: ISNCSCI examsExploratory AssessmentsUAB-IMRSCIMSCI Pain Basic Data SetBowel and Bladder Basic Data Set Open Label TrialMulti-Center (7 sites)8-10 SubjectsSubacute, Neurologically Complete T3-T11 Lesions2x106 CellsTransplant 7-14 Days Post InjuryTemporary Immunosuppression
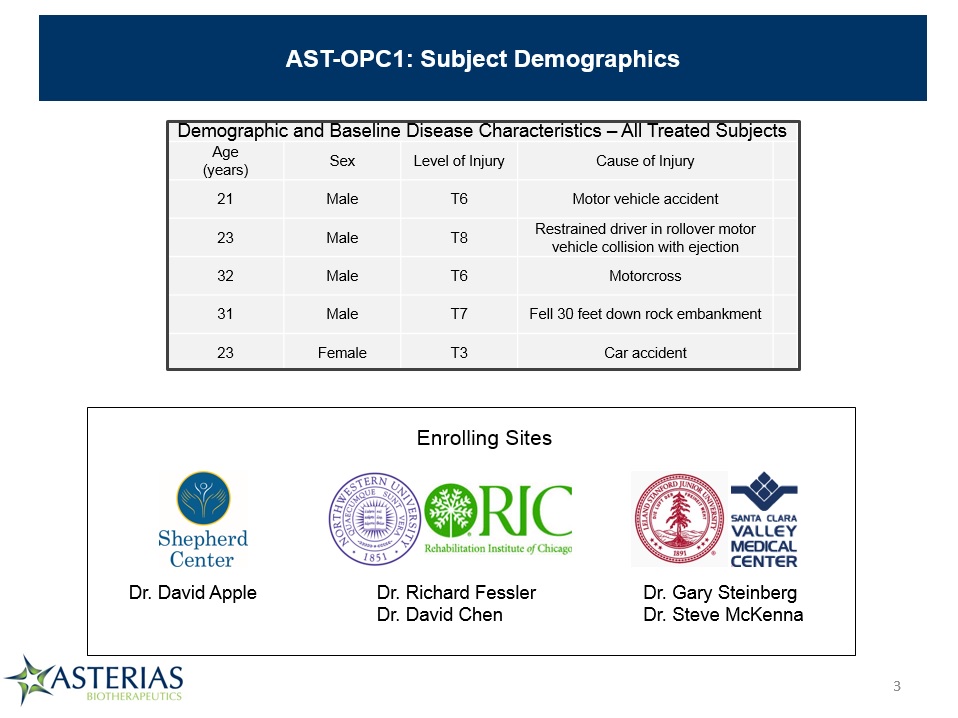
AST-OPC1: Subject Demographics Demographic and Baseline Disease Characteristics – All Treated Subjects Demographic and Baseline Disease Characteristics – All Treated Subjects Demographic and Baseline Disease Characteristics – All Treated Subjects Demographic and Baseline Disease Characteristics – All Treated Subjects Demographic and Baseline Disease Characteristics – All Treated Subjects Age(years) Sex Level of Injury Cause of Injury 21 Male T6 Motor vehicle accident 23 Male T8 Restrained driver in rollover motor vehicle collision with ejection 32 Male T6 Motorcross 31 Male T7 Fell 30 feet down rock embankment 23 Female T3 Car accident Dr. David Apple Dr. Gary SteinbergDr. Steve McKenna Dr. Richard FesslerDr. David Chen Enrolling Sites *

Delivery of AST-OPC1 in Complete Thoracic SCI Was Feasible and Safe * All Subjects Received AST-OPC1 (2 x 106 cells) at 1-2 weeks of post-injuryInjections performed using a Syringe Positioning Device (SPD)No intraoperative complicationsNo SAEs Associated with Delivery of Cells9 Adverse Events Possibly Related to Injection Procedure All Grade 1 or 2: Post-Operative Pain, Transient Fever (1) or Urinary Tract Infection (1)

Immunosuppression Regimen Was Well-Tolerated All Subjects Completed Tacrolimus Immunosuppression RegimenNo SAE’s Associated with Immunosuppression16 Grade 1 or 2 Adverse Events Possibly Associated with ImmunosuppressionNausea, Urinary Tract Infection, Low Magnesium Blood Levels *

No Evidence of AST-OPC1 Directed Immune Responses One Year After Administration Immune monitoring shows no evidence of antibodies or cellular immune responses to AST-OPC1 through 1 year in all subjects Some subjects complete mismatch with AST-OPC1: Closest match was 5 of 10 alleles PRA Assay ELISpot Assay Day Post AST-OPC1 Transplant Day Post AST-OPC1 Transplant *

AST-OPC1 Was Well Tolerated No SAE’s Associated with AST-OPC1No Evidence of adverse findings on MRI scans5 Adverse Events Possibly Associated with AST-OPC1Transient Low Grade Fever (1)Burning Sensation in Trunk and Lower Extremities (4 in one subject) Subject SAE Timeframe Related to AST-OPC1 1101 Pyelonephritis: Grade 2 Day 215 Not Related 1204 Urinary tract infection: Grade 3 Day 325 Not Related 1204 Autonomic Dysreflexia/Dyspnea: Grade 3 Year 2 Not Related * Three SAEs to date
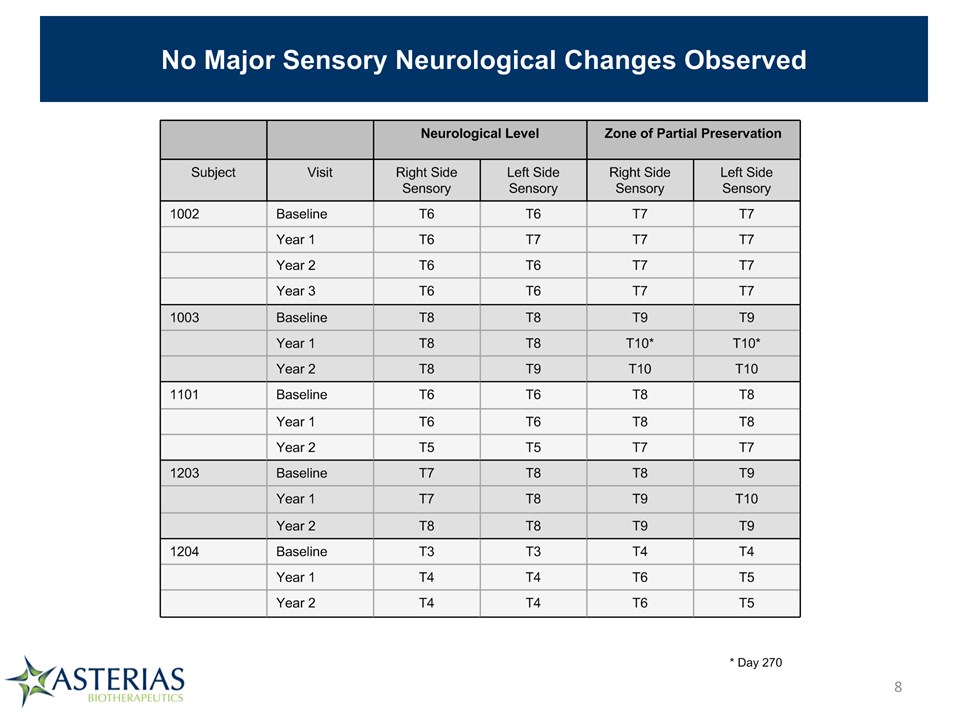
No Major Sensory Neurological Changes Observed Neurological Level Neurological Level Zone of Partial Preservation Zone of Partial Preservation Subject Visit Right Side Sensory Left Side Sensory Right Side Sensory Left Side Sensory 1002 Baseline T6 T6 T7 T7 Year 1 T6 T7 T7 T7 Year 2 T6 T6 T7 T7 Year 3 T6 T6 T7 T7 1003 Baseline T8 T8 T9 T9 Year 1 T8 T8 T10* T10* Year 2 T8 T9 T10 T10 1101 Baseline T6 T6 T8 T8 Year 1 T6 T6 T8 T8 Year 2 T5 T5 T7 T7 1203 Baseline T7 T8 T8 T9 Year 1 T7 T8 T9 T10 Year 2 T8 T8 T9 T9 1204 Baseline T3 T3 T4 T4 Year 1 T4 T4 T6 T5 Year 2 T4 T4 T6 T5 * Day 270 *

MRI Results: Evidence Consistent with Prevention of Lesion Cavity Formation No adverse findings on primary MRI safety readsIn 4 of 5 subjects, graft sites are hyperintense on T2, but signal intensity is < CSFSuggests lesion cavity formation may have been prevented by formation of a tissue matrix Image on right is axial T2 at 3 years post-grafting through center of lesion/graft site – Slice 22 on sagittal image above) *

Summary of Findings from First in Man Study of AST-OPC1 AST-OPC1 is extremely well tolerated, with no SAEs to date deemed related to the cells, delivery method, or immunosuppressive regimen4 of 5 patients have completed 3 year follow up visit, one has completed 4 years of follow-up5th patient will complete 3 year follow up in early NovemberImmune response monitoring shows no evidence of rejection of AST-OPC1, even 10 months after removal of all immunosuppressionDespite significant HLA mismatches between AST-OPC1 and subjectsSuggests well tolerated low dose, transient immunosuppressive regimen likely sufficient to enable long term engraftment of cellsMRI results consistent with continued, stable engraftment in 4 of 5 subjects at 2-3 years post-transplantNo evidence of significant changes in neurological functionNo evidence for ascending loss of function from cells or deliveryEfficacy not anticipated in this study due to low dose (5-10x below predicted efficacious range) and suboptimal patient population (complete thoracic injuries) *

AST-OPC1: Scientific Rationale for Evaluation in Cervical SCI Patients Repair/regeneration of axons only required over a short distance to reinnervate motor neurons for arms & handsFor example, with an SCI at C6 (last intact motor level at C5), repair of axons to C7 could yield return of 2 motor levels Cervical SCIRepair Thoracic SCIRepair *

AST-OPC1: Medical Rationale for Evaluation in Cervical SCI Patients Few complete cervical SCI patients recover >= 2 motor levels with current standard of careRecovery of >= 2 motor levels leads to significant improvement in self-care abilitySelf-care ability could be a clinically meaningful endpoint for a pivotal trial and BLA approval Analysis by SCOPE (Spinal Cord Outcomes Partnership Endeavor)Steeves et al., 2012. Top Spinal Cord Inj Rehabil 18:1-14 *

Design of Phase 1/2a Study of AST-OPC1 in Complete Cervical SCI 6m efficacy data 10M cell cohort (N=5) 20M cell cohort (N=5) 2M cell cohort (N=3) Key Design ElementsAST-OPC1 Injection 14-30 days post-injury10 day stagger within cohortsDMC review prior to dose escalation Efficacy TargetRecovery of >= 2 motor levels at 6 months or 12 months *

AST-OPC1 Phase 1/2a Study Schema * Acute completecervical SCI Protocol AST-OPC1-01 Protocol AST-OPC1-02 Day 0 Day 7 Day 30 Day 60 Day 90 Day 180 1 Year 5 Years 15 Years In personvisits Phone f/u DiscontinueImmunosuppression Days 46-60ImmunosuppressionTaper AST-OPC1Injection14-30 DaysPost-SCI Day -1 Screening Baseline Day -3 Day -11 MRI MRI MRI MRI MRI Primary Assessment: SafetySecondary Assessment: ISNCSCI examsExploratory Assessments: SCIM, GRASSP Open Label TrialMulti-Center (8 sites)Complete cervical SCI (C5-C7)Temporary Immunosuppression

Operational Update First two clinical sites are open for enrollment (Shepherd Center, Atlanta; Rush University, Chicago)Third clinical site expected to open in May (Stanford/Santa Clara Valley Medical Center)Patient recruitment efforts initiatedStudy website landing page to go live in MayBranding and messaging for health care providers developedOutreach to referral centers for open sites has begun *

AST-OPC1 Phase 1/2a Trial: Design and Milestones * Cohort 1:3 subjects2 million cells Cohort 2:5 subjects10 million cells Cohort 3:5 subjects20 million cells Q1’15: Study initiates Q3’15: Dose escalation Q1’16: Dose escalation 1H’16: 6 mo efficacy, cohort 2

