Attached files
Exhibit 99.1

AST-OPC1 Development Program for Spinal Cord Injury Asterias Biotherapeutics Inc. NYSE MKT: ASTInvestor DayMay 8, 2015 *

Forward Looking Statements Statements pertaining to future financial and/or operating results, future growth in research, technology, clinical development, and potential opportunities for Asterias, along with other statements about the future expectations, beliefs, goals, plans, or prospects expressed by management constitute forward-looking statements. Any statements that are not historical fact (including, but not limited to statements that contain words such as “will,” “believes,” “plans,” “anticipates,” “expects,” “estimates”) should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. Actual results may differ materially from the results anticipated in these forward-looking statements and as such should be evaluated together with the many uncertainties that affect the businesses of Asterias, particularly those mentioned in the cautionary statements found in Asterias’ Registration Statement on Form S-3 and Prospectus, as well as its other periodic reports, filed with the Securities and Exchange Commission. Asterias disclaims any intent or obligation to update these forward-looking statements. *

* Asterias Biotherapeutics, Inc Issuer: Asterias Biotherapeutics, Inc. Exchange / Tickers: NYSE MKT / AST Shares outstanding: 32.3 M Market cap: $341M (based on share price of $10.54 as of May 1, 2015) Major institutional holders: Romulus, Fidelity, Scarsdale Equities, First Eagle Key financials (as of February 2015): ~$7.4M cash1~$19M marketable securities2Potential near-term proceeds of $11.7M from 5M “in the money” warrants at $2.34 which expire June 15, 2015 Guidance for 2015: Expected net cash burn of $15-17M 1 $4.1M (unaudited) as of January 31, 2015 plus estimated $5.3M net proceeds from financing closed February 5, 20152 3.8M shares of BioTime (NYSE MKT: BTX) as of Mar 31, 2015

Two transformative platforms: Industry-leading Pluripotent Stem Cells Dendritic Cell ImmunotherapyBoth lead products entering the clinicClinical development focused on de-risked indications with robust proof-of concept dataPartnerships with leading institutions $14.3mm in non-dilutive funding from California Institute of Regenerative Medicine (CIRM) to launch AST-OPC1 Phase 1/2a for spinal cord injuryPartnered with Cancer Research UK (CRUK) to conduct AST-VAC2 Phase 1/2a for lung cancer with ~$20-30mm funded by CRUKAddressing large markets with significant unmet medical needs – multiple milestones over the next 24 monthsAST-OPC1 Phase 1/2a trial and FDA pending potential expansion cohort may result in path to registration study * Key Highlights of Asterias

* Today’s Agenda: AST-OPC1 Development Program for SCI 9:30AM: Welcome and Introduction to Asterias: Pedro Lichtinger (10’)9:40AM: Introduction to Spinal Cord Injury and AST-OPC1: Jane Lebkowski (25’) 10:05AM: Design, Objectives and Status of Asterias’ Current Spinal Cord Injury Clinical Trial: Ed Wirth (25’) 10:30AM: Clinical Foundations for the Endpoints and Outcome Measures in Asterias’ Spinal Cord Injury Trials: John Steeves. (30’) 11:00AM: Future Clinical Development Plan for AST-OPC1: Ed Wirth (30’)11:30AM: Summary and Questions: Jane Lebkowski: Moderator (30’)

Introduction to Spinal Cord Injury and AST-OPC1 Jane Lebkowski Ph.D. President of R&D Investor DayMay 8, 2015 *

* Spinal Cord Injury: Multiple Devastating Life-Changing Effects Loss of limb functionImpaired cardiovascular controlImpaired bowel and bladder controlChronic neurgenic painIncrease pain sensationDecreased sexual functionIncreased frequency of pressure soresUrinary tract infections SpasticityDebilitating burning/tingling sensations

Spinal Cord Injury: Incidence and Costs Devastating Condition with High Unmet Medical Need and High Costs to the SystemAffects 12,000 patients per year in US1 alonePrimarily affects young and previously healthy males in their 20s and 30s at time of injuryNo currently approved therapiesLifetime cost of care per patient of $2-4mm1Healthcare costs to system of $14.5bn per year in US alone, plus $5.5bn in lost productivity2Multi-billion dollar annual market opportunity3 * 1 National Spinal Cord Injury Statistical Center, Facts and Figures 20132 Berkowitz et al, Spinal Cord Injury: An Analysis of Medical and Social Costs, 19983 Internal estimates Cervical SCI has highest incidence and is most prevalent SCI typeFirst year cost of care is ~$1 million for cervical complete SCI and ongoing care and support thereafter exceeds $150,000/yearIntegration back into a work environment for patients with cervical complete injuries is rare. Average life expectancy is now 75% of able bodied population, which means a person injured as a young adult could live with SCI for ~ 50 years Lifetime costs for ongoing care and support for a severe cervical injury can exceed $8.5 million/person Cervical Spinal Cord injury is a High Priority Target

Human Spinal Cord Injury Causes Tissue Destruction and Ectopic Tissue Formation in the Spinal Cord Trauma to the spinal cord causes hemorrhagic necrosisSecondary damage includes cell death, cavity formation, demyelination, and scarringChronic stage: gray matter replaced by either a lesion cavity or collagenous scarTypical spared rim of white matter Kakulas, Paraplegia, 25:212-216, 1987 Cervical SCI at C5; 10 days post-injury Normal Spinal Cord Solid Cord Injury Contusion Cavity Norenberg et al., J. Neurotrauma, 21(4):429-440, 2004 *

* Rationale for Oligodendrocyte Progenitor Cells Cavitation Aubourg, P., Nature Genetics 2007 Obermair, Schröter and Thallmair, Physiology 2008 Pathology of the lesion provides rationale for oligodendrocyte progenitor transplantation

AST-OPC1: hESC-Derived Oligodendrocyte Progenitor Cells (OPCs) Cryopreserved Allogeneic Cell PopulationDerived from Human Embryonic Stem Cells (hESCs)Characterized Composition of Cells:Oligodendrocyte progenitorsNeural progenitorsInfrequent mature neural cells and Rare other characterized cell typesThree identified functionsProduces neurotrophic factorsInduces remyelinationInduces vascularization“Off the shelf” administrationFirst indication: spinal cord injuryPotential line extensions in other neurodegenerative diseases AST-OPC1 *

AST-OPC1: Three Major Physiologically Relevant Functional Activities 2. Produces neurotrophic factors and stimulates neurite outgrowth Promote increased neurite outgrowth 1. Wraps host neuronsand forms compact myelin sheaths Host Endothelial Cells AST-OPC1 Rat spinal cord 9 months post transplantation Rag2-/- γc-/- /shi mouse + AST-OPC1 TubIII * Zhang et al Stem Cells and Development (2006) 15: 943; Manuscript in preparation 3. Stimulates neovascularization

Models of Spinal Cord Injury Used to Evaluate Safety/Activity of AST-OPC1 MidlineContusion Injury at T10 Unilateral ContusionInjury at C5/C6 Thoracic Injury Cervical Injury Transplant AST-OPC1 7 days post- injury at injury site EvaluateEfficacy & ActivityHistological EffectsCell SurvivalCell PhenotypeCell MigrationToxicity T10 C5 *
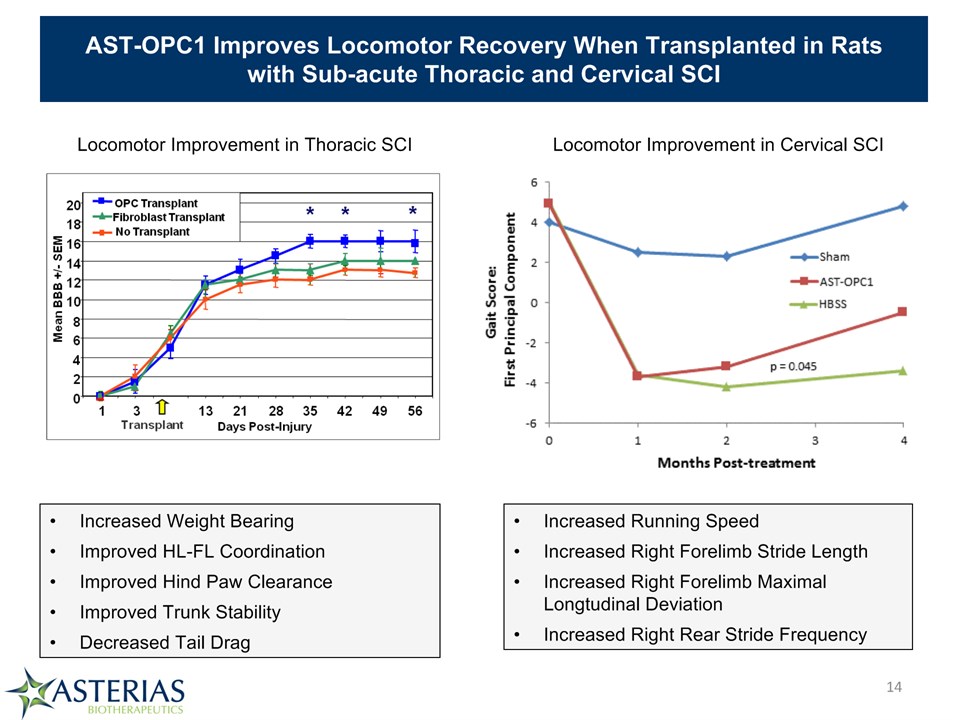
AST-OPC1 Improves Locomotor Recovery When Transplanted in Rats with Sub-acute Thoracic and Cervical SCI Locomotor Improvement in Thoracic SCI Locomotor Improvement in Cervical SCI Increased Running SpeedIncreased Right Forelimb Stride LengthIncreased Right Forelimb Maximal Longtudinal DeviationIncreased Right Rear Stride Frequency Increased Weight BearingImproved HL-FL Coordination Improved Hind Paw Clearance Improved Trunk StabilityDecreased Tail Drag *

AST-OPC1: Improved Locomotor Activity * Control (no cells) AST-OPC1 Treated

AST-OPC1 Decreases Parenchymal Cavitation In Injured Rats AST-OPC1 HBSS Area of Parenchymal Cavitation (mm2) p<0.05 AST-OPC1 HBSS *

AST-OPC1 Reduces SCI Cavity Formation and Induces Persistent Myelination Brown: antibody to human nuclear antigen labels AST-OPC1; Blue: Eriochrome Cyanine stains myelin hNucEC 1 mm 100 µm 50 µm AST-OPC1 in SCI Lesion; Significantly Reduced Cavity Formation Robust AST-OPC1 survival (brown) Myelinated Fibers (blue) Rat Thoracic Spinal Cord Injury Model hNucEC 1 mm 100 µm 50 µm Cavity forms in untreated SCI lesion Myelinated axons do not extend across cavity *

Activity/ Efficacy Biodistribution Dosing/Delivery Toxicity Tumorigenicity Ectopic Tissue Immune Rejection Survives in the Spinal CordPredominantly Neural Cell TypesGreatest Activity in Subacute InjuryImproves Locomotor ActivityReduces Parenchymal CavitationActive Doses EstablishedMigrates Up 5cm in Spinal CordNo Distribution Outside CNSDoes Not Increase MortalityDoes Not Induce AllodyniaDoes Not Induce Systemic ToxicityDoes Not Produce TeratomasProduces Low Frequency (1-2%) Small Ectopic Tissue at Injury Site Not Highly Susceptible to Direct Immune Responses 28 Animal Studies >3000 Rodents and Pigs Safety/Efficacy Profile of AST-OPC1 in Nonclinical Studies *

* Overall Development Path for AST-OPC1 and Regulatory Status IND Potential for:Orphan Drug StatusBreakthrough Designation Safety in Complete Thoracic SCI Safety and Activity in Complete Cervical SCI Dose Escalation Safety and Efficacy in Complete Cervical SCI Pivotal Clinical Trial INDAmendment Long-term Follow-up Open for Enrollment Future Expansion Trial INDAmendment
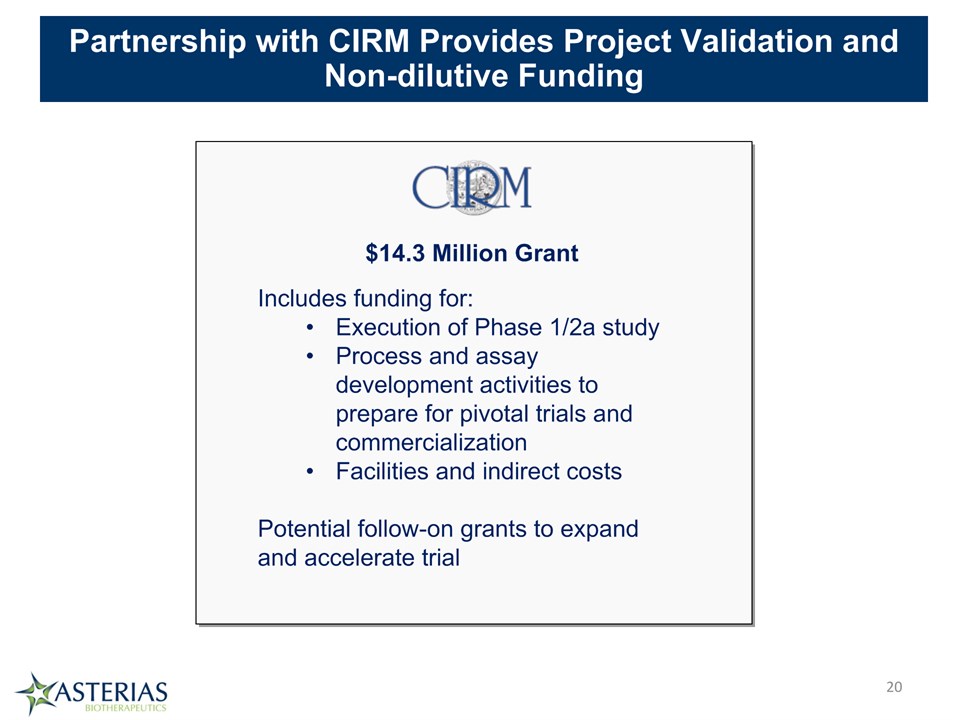
* Partnership with CIRM Provides Project Validation and Non-dilutive Funding $14.3 Million Grant Includes funding for:Execution of Phase 1/2a studyProcess and assay development activities to prepare for pivotal trials and commercializationFacilities and indirect costsPotential follow-on grants to expand and accelerate trial
