Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - Asterias Biotherapeutics, Inc. | h10051697x1_ex32-1.htm |
| EX-31.2 - EXHIBIT 31.2 - Asterias Biotherapeutics, Inc. | h10051697x1_ex31-2.htm |
| EX-31.1 - EXHIBIT 31.1 - Asterias Biotherapeutics, Inc. | h10051697x1_ex31-1.htm |
| EX-23.1 - EXHIBIT 23.1 - Asterias Biotherapeutics, Inc. | h10051697x1_ex23-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2017
OR
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file number 001-36646
Asterias Biotherapeutics, Inc.
(Exact name of registrant as specified in its charter)
|
Delaware
|
46-1047971
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
6300 Dumbarton Circle
Fremont, California 94555
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code
(510) 456-3800
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
Name of exchange on which registered
|
|
Series A Common Stock, $0.0001 par value per share
|
NYSE American
|
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes o No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ☒ No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes ☒ No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer o
|
Accelerated filer ☒
|
|
Non-accelerated filer o (Do not check if a smaller reporting company)
|
Smaller reporting company o
|
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act):
Yes o No ☒
The aggregate market value of shares of voting and non-voting common equity held by non-affiliates of the registrant on June 30, 2017 (based on the closing price for shares of the registrant’s common stock as reported on the NYSE American under the symbol AST on that date) was approximately $88,204,340.
As of March 1, 2018, there were 54,201,162 outstanding shares of Series A Common Stock, par value $0.0001 per share.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of registrant’s proxy statement for its 2018 annual meeting of shareholders filed within 120 days after the end of the registrant’s fiscal year, are incorporated by reference to this annual report on Form 10-K
Certain statements contained herein are forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements pertaining to future financial and/or operating results, future growth in research, technology, clinical development, and potential opportunities for Asterias, along with other statements about the future expectations, beliefs, goals, plans, or prospects expressed by management. Any statements that are not historical fact (including, but not limited to statements that contain words such as “will,” “believes,” “plans,” “anticipates,” “expects,” “estimates”) should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. Actual results may differ materially from the results anticipated in these forward-looking statements and as such should be evaluated together with the many uncertainties that affect the businesses of Asterias, particularly those mentioned in the cautionary statements found in Asterias’ filings with the Securities and Exchange Commission. Asterias disclaims any intent or obligation to update these forward-looking statements.
References to “Asterias,” the “Company,” “we,” “our” or “us” means Asterias Biotherapeutics, Inc.
The description or discussion, in this Form 10-K, of any contract or agreement is a summary only and is qualified in all respects by reference to the full text of the applicable contract or agreement.
| Item 1. | Business |
Overview
We are a clinical-stage biotechnology company dedicated to developing cell-based therapeutics to treat neurological conditions associated with demyelination and cellular immunotherapies to treat cancer. We have industry-leading technology in two cell types, each with broad potential applicability: (1) oligodendrocyte progenitor cells, which become oligodendrocytes that have the potential to remyelinate axons within the central nervous system and perform other restorative functions, and (2) antigen-presenting dendritic cells, which train T-cells in the immune system to attack and destroy solid or liquid tumor cells across multiple types of cancer.
We currently have three clinical stage programs:
| • | AST-OPC1 is an oligodendrocyte progenitor cell population derived from pluripotent stem cells that is currently in a Phase 1/2a clinical trial for spinal cord injuries (“SCI”) that has been partially funded by the California Institute for Regenerative Medicine; |
| • | AST-VAC2 is a non-patient-specific (“off-the-shelf”) cancer immunotherapy derived from pluripotent stem cells for which a clinical trial in non-small cell lung cancer is being funded and sponsored by Cancer Research UK, the world’s largest independent cancer research charity; and |
| • | AST-VAC1 is a patient-specific cancer immunotherapy which has generated positive Phase 2 data in the treatment of Acute Myeloid Leukemia (“AML”). |
Like chimeric antigen receptor, or CAR-T, therapies, AST-VAC1 is an “autologous” therapy meaning that it is sourced from a patient’s own cells through a standard process known as leukapheresis. For AST-OPC1 and AST-VAC2, we use human embryonic stem (“hES”) cell lines, which were originally isolated in the 1990’s and which have almost unlimited capacity to expand and differentiate into the various cell types of the body. For AST-OPC1, hES cells are induced to become an oligodendrocyte progenitor cell population that supplements the body’s natural axon remyelination function, which can be damaged or otherwise insufficient as a result of certain neurological events or conditions including spinal cord injury. Likewise for AST-VAC2, the hES cells are differentiated to mature dendritic cells that educate the immune system to target telomerase, a protein produced by most tumor cells.
We believe that our experience, expertise, and intellectual property surrounding oligodendrocyte progenitor cells and dendritic cells provide us with two distinct technology platforms in neurology and cancer immunotherapy. Our neurology platform has the potential for application in additional indications, such as advanced multiple sclerosis and white matter stroke, and our immunotherapy platform potentially could be employed in any immunogenic cancer and could be designed to target antigens beyond telomerase.
2
Corporate History
We were incorporated in Delaware on September 24, 2012. In October 2013, we acquired intellectual property, cell lines, preclinical and clinical data, and other assets from Geron Corporation (“Geron”) and also acquired rights to use certain hES cell lines and to practice certain patents from BioTime, Inc. (“BioTime”). Additionally, in February 2016, we executed a broad, non-exclusive cross-license with BioTime and its subsidiary ES Cell International Pte Ltd. Our two core technology platforms are based upon and supported by assets acquired from these transactions.
Immediately following our 2013 transaction with Geron, we were approximately 72% owned by BioTime and, until May 13, 2016, we were a majority-owned and controlled subsidiary of BioTime. As of December 31, 2017, BioTime owned approximately 40% of our outstanding shares.
Our principal executive offices are located at 6300 Dumbarton Circle, Fremont, California 94555. Our telephone number is (510) 456-3800. Our corporate website is www.asteriasbiotherapeutics.com. The contents of our website are not part of this Annual Report on Form 10-K.
Products Under Development
AST-OPC1
About AST-OPC1
Our AST-OPC1 product candidate is an oligodendrocyte progenitor cell population, derived from a current Good Manufacturing Practice (“cGMP”) master cell bank of undifferentiated hES cells that has been qualified for human use. These cells, which are stored frozen until ready for use, are produced under cGMP conditions and screened for adventitious agents.
Oligodendrocytes are nature’s neural insulating cells. Like the insulation covering an electrical wire, oligodendrocytes enable the conduction of electrical impulses along nerve fibers throughout the central and peripheral nervous system. They are also known to promote neural growth, as well as induce blood vessel formation around nerve axons.
AST-OPC1 cells have been shown to reproduce the natural functions of oligodendrocytes in animals, including three potentially reparative functions that address the complex pathologies observed at the site of a spinal cord injury. These activities of AST-OPC1 include production of neurotrophic factors, stimulation of vascularization, and induction of remyelination of denuded axons, all of which are critical for survival, regrowth, and conduction of nerve impulses through axons at the injury site. In preclinical animal testing, AST-OPC1 administration led to remyelination of axons, improved hind limb and forelimb locomotor function, dramatic reductions in injury-related cavitation, and significant preservation of myelinated axons traversing the injury site.
Phase 1/2a Study in Patients with Complete Cervical Spinal Cord Injuries
Based on the results of a previously completed Phase 1 safety trial of AST-OPC1 in thoracic spinal cord injury, we obtained permission from the FDA in August 2014 to initiate a Phase 1/2a dose escalation trial in subjects with neurologically complete (AIS-A) cervical spinal cord injuries (the “SCiStar study”). In May 2016, we were granted FDA clearance to expand the SCiStar study to enroll additional subjects to include two additional cohorts of subjects with motor complete, sensory incomplete (AIS-B) cervical spinal cord injury. The SCiStar study is an open-label, single-arm trial testing three sequential escalating doses of AST-OPC1 administered at up to 20 million AST-OPC1 cells in twenty-five subjects with subacute, C-4 to C-7, motor complete (AIS-A or AIS-B) cervical spinal cord injuries (“SCI”). These individuals have essentially lost all movement below their injury site and experience severe paralysis of the upper and lower limbs. AIS-A patients have lost all motor and sensory function below their injury site, while AIS-B patients have lost all motor function but may retain some minimal sensory function below their injury site. AST-OPC1 was administered 21 to 42 days post-injury. Subjects will be followed by neurological exams and imaging procedures to assess the safety and activity of the product.
3
We completed enrollment of the entire SCiStar study in December 2017. The SCiStar study consists of five cohorts:
|
Cohort
|
Injury Type; AST-OPC1 Dose
|
# of Subjects
|
|
Cohort 1
|
AIS-A; 2M AST-OPC1 cells
(low dose for safety evaluation) |
3
|
|
Cohort 2
|
AIS-A; 10M AST-OPC1 cells
|
6
|
|
Cohort 3
|
AIS-A; 20M AST-OPC1 cells*
|
6
|
|
Cohort 4
|
AIS-B; 10M AST-OPC1 cells
|
6
|
|
Cohort 5
|
AIS-B; 20M AST-OPC1 cells*
|
4
|
|
Total
|
|
25
|
In October 2017, we reported 12-month data from the SCiStar study’s Cohort 2. At 12 months, 67% (4 out of 6) of Cohort 2 subjects recovered 2 or more motor levels on at least one side, exceeding the company’s 12-month tertiary target of 45-50% of subjects achieving this level of improvement. In February 2018, we provided a clinical trial update that highlighted, among other things, the following:
| • | Positive Safety Profile – We have dosed 25 subjects with AST-OPC1 in the SCiStar study and a total of 30 subjects including the five subjects from the previous Phase 1 safety trial who have been followed for as long as seven years. To date, there have been no serious adverse events (SAEs) related to the AST-OPC1 cells. |
| • | Cell Engraftment – 92% (11/12) of Cohort 3 and Cohort 4 subjects have magnetic resonance imaging (MRI) scans at six months consistent with the formation of a tissue matrix at the injury site, which is early and encouraging evidence that AST-OPC1 cells have engrafted at the injury site and helped to prevent cavitation. Together with the previously reported results from Cohort 2, the MRI results-to-date for 94% (17/18) of the Cohort 2-4 subjects provide supportive evidence that AST-OPC1 cells have durably engrafted at the injury site and helped to prevent cavitation. |
| • | Improved Motor Function – 75% (9/12) of Cohort 3 and Cohort 4 subjects have recovered at least one motor level on at least one side through six months, and 17% (2/12) of subjects have recovered two or more motor levels on at least one side through six months. At six months, 83% (15/18) of Cohort 2-4 subjects recovered at least 1 motor level on at least one side and 22% (4/18) of Cohort 2-4 subjects recovered two or more motor levels on at least one side. |
We will have various interim readouts in 2018 and expect to report the six-month top-line readout for the entire study late in the second quarter or early in the third quarter of 2018 and the 12-month top-line readout for the entire study late in the fourth quarter 2018 or early in the first quarter of 2019.
The SCiStar study followed a previous Phase 1 clinical trial, in which five subjects with neurologically complete, thoracic spinal cord injury were administered two million AST-OPC1 cells at the spinal cord injury site 7-14 days post-injury. They also received low levels of immunosuppression for the next 60 days. Delivery of AST-OPC1 was successful in all five subjects with no serious adverse events associated with the administration of the cells, with AST-OPC1 itself, or the immunosuppressive regimen. All five subjects have completed at least six years of follow-up. No evidence of rejection of AST-OPC1 was observed in detailed immune response monitoring of all subjects. In four of the five subjects, serial MRI scans indicated that reduced spinal cord cavitation may have occurred. There have been five minor adverse events possibly related to AST-OPC1 such as transient fever and nerve pain. There have been no unexpected neurological changes to date.
Regenerative Medicine Advanced Therapy Designation and Orphan Drug Designation
In September 2017, the FDA granted our request for AST-OPC1 to be designated a Regenerative Medicine Advanced Therapy (“RMAT”) under the 21st Century Cures Act. The RMAT designation is intended to facilitate expedited development, review and approval for important new regenerative medicine therapies for which preliminary clinical evidence indicates the potential to address a serious or life-threatening disease or condition. In addition to providing an avenue for increased and earlier interactions with the FDA, RMAT-designated products may be eligible for priority review and accelerated approval. We expect to have discussions with the FDA sometime in 2018 on the development of AST-OPC1 using the RMAT designation.
4
In February 2016, we announced that the FDA had granted our application for Orphan Drug Designation of AST-OPC1 for the treatment of acute spinal cord injury.
Manufacturing and Process Development
We are in the process of establishing additional cGMP master and working cell banks of undifferentiated hES cells of the H1 cell line for future clinical development and commercial use. We are also updating the manufacturing process for AST-OPC1 to improve yields and support a scalable process for eventually manufacturing on a larger scale. We will need to successfully complete the update of the manufacturing process in order to manufacture additional clinical-grade lots of AST-OPC1 to use for future clinical development.
Market Conditions
It is estimated that there are approximately 17,000 new spinal cord injuries annually in the United States (NSCISC SCI Facts and Figures at a Glance (2016)). As of 2016, the National Spinal Cord Injury Statistical Center reported that approximately 4,500 of these new spinal cord injuries annually in the United States are AIS-A, AIS-B or AIS-C patients with C-4 to C-7 spinal cord injuries (https://www.nscisc.uab.edu/). Individuals with neurologically complete cervical spinal cord injury are part of an orphan population with a severe unmet medical need due to the loss of function in all four limbs. These individuals frequently require significant assistance for their care and activities of daily living. A published study estimated the lifetime costs of care for a person who suffers a cervical SCI at age 25 to up to $5.4 million (Y. C. Cao and M. J. DeVivo (2011)).
There are currently no drugs approved by the United States Food and Drug Administration (“FDA”) specifically for the treatment of spinal cord injury, although methylprednisolone, a corticosteroid generally used as an anti-inflammatory drug, is sometimes prescribed on an off-label basis to reduce acute inflammation in the injured spinal cord immediately after injury. It is believed that in order to effect substantial benefit in treating this complex injury, multiple mechanisms of action are required, such as re-myelination of the demyelinated axons, generation of new blood vessels to repair the ischemic damage from injury, and the presence of biologics that cause neurite or new nerve growth to enable the severed axons to repair. In pre-clinical studies to date, AST-OPC1 cells have been shown to exhibit all three effects.
CIRM Grant and other Funding
In October 2014, we signed a Notice of Grant Award (“NGA”) with the California Institute for Regenerative Medicine (“CIRM”), effective October 1, 2014, with respect to a $14.3 million grant award for clinical development of AST-OPC1. The NGA, as amended, includes the terms under which CIRM will release grant funds to us. Under the NGA, as amended on March 2, 2016, CIRM pledged to disburse the grant funds to us based on our attainment of certain progress milestones and we have received the entire $14.3 million awarded to us under the NGA.
We will need to raise additional capital in order to conduct subsequent clinical trials and to complete product development work necessary for larger trials and commercialization, which may include additional non-dilutive funding through CIRM and other sources to develop this product.
AST-VAC2 and AST-VAC1
We are developing two experimental immunotherapeutic programs, AST-VAC2 and AST-VAC1, each designed to attack cancer cells by targeting the cancer cell’s expression of human telomerase (“hTERT”). Both product candidates use an immune cell type known as dendritic cells to stimulate immune responses to telomerase. Dendritic cells are antigen processing and presenting cells which are potent initiators of a cellular and antibody-mediated immune response. Telomerase is a pan-cancer antigen, expressed at high levels in nearly all human cancers, but at very low levels or not at all in normal human cells. Somewhat analogous to CAR-T therapies, the premise underlying these vaccines is to stimulate a T cell response to tumor cells. Our AST-VAC programs seek to “teach” the patient’s own immune system to attack cancer cells while sparing other normal healthy cells.
Our AST-VAC programs have the potential to be used as a monotherapy or in combination with other therapies. As a monotherapy, we believe our AST-VAC programs, which to date have shown a more favorable safety profile than many other approaches to treat cancer, have potential as a remission maintenance therapy in cancers that are susceptible to immune attack, express telomerase and have high rates of relapse. We believe our AST-VAC programs are also potentially complimentary with other therapies, such as CAR-T therapies, that program T cells to
5
aggressively attack cancer cells to induce remission but also have less favorable toxicity profiles and therefore are not well tolerated as a remission maintenance therapy. A CAR-T therapy that induces remission could potentially be used in combination with one of our AST-VAC programs to help a patient stay in remission.
We also believe our AST-VAC programs may be synergistic with other therapies such as immune checkpoint inhibitors, where the checkpoint inhibitor allows the immune system to again work naturally to attack cancer cells in a situation where the body’s own natural response is being suppressed by the cancer. We also believe that our AST-VAC programs have the potential to be applicable therapies across a wide range of cancers since telomerase is expressed at high levels in the vast majority of human cancers.
AST-VAC2
About AST-VAC2
AST-VAC2 is an allogeneic, or non-patient specific, cancer vaccine candidate designed to stimulate patient immune responses to telomerase. AST-VAC2 is produced from hES cells and can be modified with any antigen. The use of hES, as opposed to collecting and using the patient’s own blood, as the starting material for AST-VAC2 provides a scalable system for the production of a large number of vaccine doses in a single lot. Allogeneic vaccine production has the potential to have lower manufacturing costs, provide off-the-shelf availability which provides broader access to patients, and ensure greater product consistency. In addition, we believe that this approach has the potential to stimulate a more robust immune response through an adjuvant effect of the immune mismatch between the biological profile of AST-VAC2 and patients receiving the therapy.
Clinical Trial and Option Agreement with Cancer Research UK
During September 2014, we entered into a Clinical Trial and Option Agreement with Cancer Research UK (“CRUK”) and Cancer Research Technology Limited, a wholly-owned subsidiary of CRUK (the “CRUK Agreement”). Under the CRUK Agreement, CRUK has agreed to fund Phase 1 clinical development of our AST-VAC2 product candidate loaded with the same LAMP-telomerase construct we have used in AST-VAC1. Under the terms of the CRUK Agreement, we are responsible, at our own cost, for completing process development and manufacturing scale-up of the AST-VAC2 manufacturing process and transferring the resulting cGMP-compatible process to CRUK. CRUK is responsible, at its own cost, for manufacturing clinical grade AST-VAC2 and for carrying out the Phase 1 clinical trial of AST-VAC2. The study will administer AST-VAC2 to treat up to 24 patients with either advanced or resected NSCLC to examine the safety, immunogenicity, and activity of AST-VAC2 and position the immunotherapy for future clinical trials. We will continue to serve in a collaborative and advisory role with CRUK throughout this process. The CRUK Agreement is subject to termination if certain milestones relating to regulatory approvals are not achieved, if we are in material breach of the agreement and we do not cure such breach within 60 days, or if we are insolvent or commence liquidation proceedings. In addition, CRUK may terminate the CRUK Agreement if the clinical trial protocol, or an amendment thereto, is not approved by the regulatory authority, ethics committee or the Company, if CRUK is not satisfied with the product manufacturing process, if CRUK faces budget constraints, if certain problems arises during the clinical trial, or performance of the CRUK Agreement by CRUK would be unlawful.
In January 2016, we announced that we had completed the technology transfer of the AST-VAC2 manufacturing process to CRUK.
In September 2017, we announced that the Medicines and Healthcare Products Regulatory Agency (“MHRA”) and the NHS Research Ethics Committee (“REC”) had provided the necessary approvals to initiate the first-in-human (“FIH”) clinical trial of AST-VAC2 in the United Kingdom.
We anticipate the first subjects in this study to be dosed in the first half of 2018. Assuming the first subject is dosed in the first half of 2018, the Company expects to report initial safety readouts in the second half of 2018.
Upon completion of the Phase 1 study, we will have an exclusive first option to acquire the data generated in the trial. If we exercise that option we will be obligated to make payments upon the execution of the license agreement and, upon the achievement of various milestones, as well as make royalty payments on sales of products. In connection with the CRUK Agreement, we sublicensed to CRUK for use in the clinical trials and product manufacturing process certain patents that have been licensed or sublicensed to us by third parties. We would also be obligated to make payments to those licensors and sub licensors upon the achievement of various milestones, and then royalties on sales of products if AST-VAC2 is successfully developed and commercialized.
6
Services Agreement with Cell Therapy Catapult Services Limited
In October 2015, we entered into a Services Agreement (the “Services Agreement”) with Cell Therapy Catapult Services Limited (“Catapult”), a research organization specializing in the development of technologies which speed the growth of the cell and gene therapy industry. Under the Services Agreement, Catapult will license to us, certain background intellectual property and will develop a scalable manufacturing and differentiation process for our AST-VAC2 development program. In consideration for the license and Catapult’s performance of services, we agreed to make aggregate payments of up to GBP £4,350,000 over the five years after the execution of the Services Agreement. At our option of, up to GBP £3,600,000 of such payments may be settled in shares of our Series A Common Stock. As of December 31, 2017, we have incurred costs since commencement of the Services Agreement of GBP £3,200,000 under the Services Agreement.
The Services Agreement may be terminated by us for any reason upon 60 days prior written notice. Catapult may terminate the Services Agreement on 60 days prior written notice if it encounters a technical issue that would prevent it from completing the services at all or without obtaining additional resources or if the estimated time and cost of completing the services will be exceeded and both parties do not reach agreement on revised time and cost terms. Catapult may terminate the Services Agreement in the event we fail to pay any amount due under the Services Agreement 30 days after Catapult makes a written demand for payment. In addition, a non-breaching party may terminate the Services Agreement upon the occurrence a material breach that is not remedied within 30 days. Either party may terminate the Services Agreement in the event the other party becomes subject to insolvency, receivership, liquidation, or a similar event.
AST-VAC1
About AST-VAC1
AST-VAC1 is an autologous product candidate, manufactured from cells that come from the patient. AST-VAC1 consists of antigen-presenting mature dendritic cells pulsed with messenger RNA for the protein component of human telomerase (“hTERT”) and a portion of a lysosomal targeting signal (“LAMP”). LAMP directs the telomerase RNA to the lysosome, the subcellular organelle that directs the RNA to a particular part of the cell membrane. AST-VAC1 is injected into the patient’s skin and the dendritic cells travel to the lymph nodes and instruct cytotoxic T cells to kill cancerous tumor cells that express telomerase on their surface; helper T cells are also induced and are also theoretically capable of killing cancer cells.
Process Development
On August 3, 2016, we entered into a Development and Manufacturing Services Agreement (the “Services Agreement”) with Cognate BioServices, Inc. (“Cognate”), a fully-integrated contract bioservices organization providing development and cGMP manufacturing services to companies and institutions engaged in the development of cell-based products. On August 16, 2017, we amended certain statements of work to modify the timing of certain process development studies being performed by Cognate under the Services Agreement. Under the Services Agreement, as amended, Cognate performs process development studies in support of our clinical and commercial development activities of AST-VAC1. Most of the initial process development work that we intend to complete under this Services Agreement was completed in 2017.
Clinical Studies
A Phase 2 clinical trial of AST-VAC1 was conducted in subjects with AML in complete clinical remission. This trial completed subject enrollment in December 2009. Thirty-three subjects with AML entered the study in their first or second complete remission. Prior to or shortly after completing consolidation chemotherapy, subjects underwent leukapheresis, a process of collecting white blood cells directly from the subject. AST-VAC1 was produced at a centralized manufacturing facility from the subject-specific white blood cells. Subject blood cells were differentiated to dendritic cells in culture, modified to express telomerase linked to the LAMP targeting signal, aliquoted and cryopreserved. AST-VAC1 was released for subject dosing contingent on several product specifications that included identity of mature dendritic cells, confirmation of telomerase expression, and number of viable cells per dose after thawing, and product sterility.
Twenty-one subjects received AST-VAC1 in the study, including 19 in clinical remission and two in early relapse. AST-VAC1 was found to have a favorable safety and tolerability profile in this study over multiple
7
vaccinations, with up to 32 serial vaccinations administered (median=17). Idiopathic thrombocytopenic purpura (bleeding into the skin caused by low platelets in blood) (grade 3-4) was reported in one subject. Other toxicities (grade 1-2) included rash or headache. Subject immune response to telomerase after vaccination with AST-VAC1 was evaluated using a test called the enzyme-linked immunosorbent spot (“ELISPOT”) assay to measure the presence of activated T-cells specific to hTERT. Positive immune responses were detected in 55% of subjects.
We subsequently performed follow-up data collection on the 19 subjects treated in complete remission to determine the long-term effects of the AST-VAC1 administration on remission duration and disease-free survival. Eleven of 19 subjects (58%) remained in complete remission at a median follow-up of 52 months. These results compare to historical data suggesting that between 20-40% of subjects would be expected to be relapse free at 3-4 years. Additionally, of the 7 subjects in the higher risk over 60-year-old group, 4 (57%) remained relapse free at a median follow up of 54 months. Historically, relapse free survival rates in this population have been 10-20% at 3-4 years. We believe that the results of this trial provide encouraging evidence that by targeting hTERT, our AST-VAC programs may have the potential to be effective therapies in the treatment of a wide range of cancers.
The next major step in clinical development for AST-VAC1 would be to conduct a confirmatory Phase 2b study. We will need to receive additional sufficient funding or identify a partner for this program prior to the commencement of such a study.
Intellectual Property
The patent portfolio that we acquired pursuant to the Asset Contribution Agreement with Geron, dated January 4, 2013 (the “Asset Contribution Agreement”), currently includes approximately 420 patents and patent applications owned by or licensed to us that are directed to pluripotent stem (“pPS”) cell-, hES cell-, and dendritic cell-based product opportunities. The portfolio encompasses methods for making a number of cell types from hES or other pPS cells, including hepatocytes (liver cells), cardiomyocytes (heart muscle cells), neural cells (nerve cells, including dopaminergic neurons and oligodendrocytes), chondrocytes (cartilage cells), pancreatic islet β cells, osteoblasts (bone cells), hematopoietic cells (blood-forming cells) and dendritic cells, and related compositions. Also included in the patent portfolio are technologies for culture and scalable expansion of undifferentiated hES cells or other pPS cells, including methods for culturing without the need for cell feeder layers or conditioned media, and methods for culturing undifferentiated hES or pPS cells in suspension. The patent portfolio also encompasses novel synthetic surfaces for culturing certain hES or pPS-derived differentiated cell types, including oligodendrocytes and oligodendrocyte progenitor cells. In the past 12 months (during a period commencing on February 15, 2017 and ending on February 14, 2018), 46 new patents were issued that are either owned by us or exclusively licensed to us. Included in the 46 new patents issued in the United States, Europe, Japan, Canada, China, Australia, Israel and South Korea are patents for differentiating pPS cells to hematopoietic progenitors and immature and mature dendritic cells (supporting our AST-VAC2 program) as well as patents covering differentiation of pPS cells to various other lineages, including neural cells, cardiomyocytes and hepatocytes. Other patents that issued during the same period include patents related to various aspects of culture and expansion of undifferentiated hES or pPS cells. With respect to patents that are not specific to our core programs (AST-OPC1, AST-VAC1, and AST-VAC2), we are evaluating licensing and other partnering opportunities, including assignment of certain patents to third parties, on an ongoing basis. We are also evaluating maintenance costs relative to remaining patent term and may choose to no longer maintain certain of these patents. Collectively, these activities may result in a decrease in the total number of patents owned or controlled by us that are not specific to our core programs.
In February 2016, we executed a broad, non-exclusive cross-license (the “Cross-License”) with BioTime and its subsidiary ES Cell International Pte Ltd. (“ESI”). Under the Cross-License, we received: (i) non-exclusive worldwide rights in a range of therapeutic fields to over 30 patents and applications relating to hES cells, and (ii) non-exclusive worldwide rights for therapeutic applications of pluripotent stem cell-derived neural and cardiac cells to over 20 patents and applications relating to hydrogel formulations. Under the Cross-License, BioTime and ESI received a broad, non-exclusive license to certain of our patents and related patent rights for all purposes in the BioTime Licensed Field during the term of the Cross-License. The BioTime Licensed Field includes all fields of use except any and all applications (a) to treat disorders of the nervous system, and (b) utilizing the immune system to prevent, treat, or cure cancer, and (c) involving the use of cells comprising, derived from, or manufactured using, hES cells or human induced pluripotent stem cells for in vitro assay applications, including but not limited to drug discovery and development, drug monitoring, drug toxicology testing, and consumer products testing.
8
The patent positions for our core programs (AST-OPC1, AST-VAC1, and AST-VAC2) are summarized below.
Neural cells: This portfolio is related to our AST-OPC1 product. The patent rights relevant to neural cells, such as oligodendrocyte progenitor cells, include various patent families acquired by us from Geron that are directed to the differentiation of pluripotent stem cells (including hES cells) into various neural cell types, as well as various culture and purification methods. These patent rights also include rights licensed from the Regents of the University of California. There are issued patents in the United States, Australia, Canada, Europe, Japan, China, Hong Kong, India, Korea, Singapore and Israel. Additionally, there are five new pending patent families owned by us directed to improved methods of producing oligodendrocyte progenitor cells, oligodendrocyte progenitor cell compositions and methods of treatment of spinal cord injury and stroke using oligodendrocyte progenitor cells. The stroke family is jointly owned with the Regents of the University of California; the other four new pending families are solely owned by us. The expiration dates of the patents acquired from Geron and in-licensed from the Regents of the University of California will be within 2021 to 2030. The potential expiry dates of the four new patent families with applications pending will be within 2036 to 2038. The commercial success of our AST-OPC1 product depends, in part, upon our ability to exclude competition in this product with this patent portfolio, regulatory exclusivity, or a combination of both.
Dendritic cells: This portfolio is related to our AST-VAC1 and AST-VAC2 products. The patent rights relevant to dendritic cells include various patent families acquired by us from Geron or in-licensed from third parties that are directed to the differentiation of pluripotent stem cells (including hES cells) into hematopoietic progenitor cells and immature and mature dendritic cells. In addition, these patent rights include a patent family with claims directed to immunogenic compositions comprising antigen-presenting dendritic cells and methods of eliciting an anti-telomerase immune response in a subject by administering to the subject such compositions. There are issued patents in the United States, Australia, Europe, Canada, China, Hong Kong, Japan, Korea, Israel and Singapore. The expiration dates of the patents acquired from Geron and in-licensed to us range from 2019 to 2029. Additionally, there is a new pending patent family owned by us with claims directed to immunotherapeutic compositions comprising immunogenic peptides and methods of eliciting a cellular mediated immune response in a subject, with a provisional patent application filed in 2017. The potential expiry date of the new patent family with a pending provisional application will be in 2038. The commercial success of our AST-VAC1 and AST-VAC2 products depends, in part, upon our ability to exclude competition in these products with this patent portfolio, regulatory exclusivity, or a combination of both.
In addition, we have patent protection in the Unites States and various other jurisdictions for producing cardiomyocytes, pancreatic islet cells, hepatocytes, chondrocytes, and osteoblasts. The expiration dates of these patents range from 2020 to 2032. Should a competitor not be able to market a product covered by these patents or if we cannot license these patents before their expiration, the benefits for procurement and maintenance of these rights would not be fully realized and the associated costs would not be fully reimbursed.
Licensed Stem Cell Technology and Stem Cell Product Development Agreements
Telomerase Sublicense
We received the Telomerase Sublicense from Geron in connection with our acquisition of Geron’s stem cell assets. The Telomerase Sublicense grants us an exclusive sublicense under certain patents owned by the University of Colorado’s University License Equity Holdings, Inc. relating to telomerase and entitles us to use the technology covered by the patents in the development of AST-VAC1 and AST-VAC2 as immunological treatments for cancer. Under the Telomerase Sublicense, we paid Geron a one-time upfront license fee of $65,000, and we will pay Geron an annual license maintenance fee of $10,000 due on each anniversary of the effective date of the agreement, and a 1% royalty on sales of any products that we may develop and commercialize that are covered by the sublicensed patents. The Telomerase Sublicense will expire concurrently with the expiration of Geron’s license. That license will terminate in November 2018 when the last of the licensed patents expires. The Telomerase Sublicense may also be terminated by us by giving Geron 90 days written notice, by us or by Geron if the other party breaches its obligations under the sublicense agreement and fails to cure their breach within the prescribed time period, or by us or by Geron upon the filing or institution of bankruptcy, reorganization, liquidation or receivership proceedings, or upon an assignment of a substantial portion of the assets for the benefit of creditors by the other party.
9
We are obligated to indemnify Geron, Geron’s licensor, and certain other parties for certain liabilities, including those for personal injury, product liability, or property damage relating to or arising from the manufacture, use, promotion or sale of a product, or the use by any person of a product made, created, sold or otherwise transferred by us or our sublicensees that is covered by the patents sublicensed under this agreement.
License Agreement with University of California
Geron assigned to us its Exclusive License Agreement with The Regents of the University of California for patents covering a method for directing the differentiation of pPS cells to glial-restricted progenitor cells that generate pure populations of oligodendrocytes for remyelination and treatment of spinal cord injury. Pursuant to this agreement, we have an exclusive worldwide license under such patents, including the right to grant sublicenses, to create products for biological research, drug screening, and human therapy using the licensed patents.
Under the license agreement, we will be obligated to pay the university a royalty of 1% from sales of products that are covered by the licensed patent rights, and a minimum annual royalty of $5,000 starting in the year in which the first sale of a product covered by any licensed patent rights occurs, and continuing for the life of the applicable patent right under the agreement. The royalty payments due are subject to reduction, but not by more than 50%, to the extent of any payments that we may be obligated to pay to a third party for the use of patents or other intellectual property licensed from the third party in order to make, have made, use, sell, or import products or otherwise exercise our rights under the Exclusive License Agreement. We will be obligated to pay the university 7.5% of any proceeds, excluding debt financing and equity investments, and certain reimbursements, that we receive from sublicensees, other than our affiliates and joint ventures relating to the development, manufacture, purchase, and sale of products, processes, and services covered by the licensed patent.
The license agreement will terminate on the expiration of the last-to-expire of the university’s issued licensed patents. If no further patents covered by the license agreement are issued, the license agreement would terminate in 2024. The university may terminate the agreement in the event of our breach of the agreement. We can terminate the agreement upon 60 days’ notice.
World-Wide Non-Exclusive WARF License
On October 7, 2013, we entered into a Non-Exclusive License Agreement with the Wisconsin Alumni Research Foundation (“WARF”) under which we were granted a worldwide non-exclusive license under certain WARF patents and WARF-owned embryonic stem cell lines to develop and commercialize therapeutic, diagnostic and research products. The licensed patents include patents covering methods for growth and differentiation of primate embryonic stem cells. The licensed stem cell lines include the H1, H7, H9, H13, and H14 hES cell lines.
In consideration of the rights licensed to us, we have agreed to pay WARF an upfront license fee and have agreed to additional payments upon the attainment of specified clinical development milestones, royalties on sales of commercialized products, and, subject to certain exclusions, a percentage of any payments that we may receive from any sublicenses that we may grant to use the licensed patents or stem cell lines.
The license agreement will terminate with respect to licensed patents upon the expiration of the last licensed patent to expire; with respect to the licensed stem cell lines, the license agreement will remain in force until terminated by either party in accordance with the termination provisions. We may terminate the license agreement at any time by giving WARF prior written notice. WARF may terminate the license agreement if payments of earned royalties, once begun, cease for a specified period of time or if we and any third parties collaborating or cooperating with us in the development of products using the licensed patents or stem cell lines fail to spend a specified minimum amount on research and development of products relating to the licensed patents or stem cell lines for a specified period of time.
WARF also has the right to terminate the license agreement if we breach the license agreement or become bankrupt or insolvent or if any of the licensed patents or stem cell lines are offered to creditors.
We will indemnify WARF and certain other designated affiliated entities from liability arising out of or relating to the death or injury of any person or damage to property due to the sale, marketing, use, or manufacture of products that are covered by the licensed patents, or licensed stem cells, or inventions or materials developed or derived from the licensed patents or stem cell lines.
10
Royalty Agreement with Geron
In connection with our acquisition of Geron’s stem cell assets, we entered into a royalty agreement with Geron (the “Royalty Agreement”) pursuant to which we agreed to pay Geron a 4% royalty on net sales (as defined in the Royalty Agreement), by us or any of our affiliates or sales agents, of any products that we develop and commercialize that are covered by the patents Geron contributed to us. In the case of sales of such products by a person other than us or one of our affiliates or sales agents, we will be required to pay Geron 50% of all royalties and cash payments received by us or by our affiliate in respect of a product sale. Royalty payments will be subject to proration in the event that a product covered by a patent acquired from Geron is sold in combination with another product that is not covered by a patent acquired from Geron. The Royalty Agreement will terminate at the expiration or termination date of the last issued patent contributed by Geron under the Royalty Agreement. We estimate that the latest patent expiration date will be in 2032.
Protecting our Intellectual Property
We seek to protect our intellectual property (“IP”) by, among other methods, filing United States and foreign patent applications related to our patentable IP that we consider important to the development and implementation of our business and strategy. In addition to relying on patents, we rely on trade secrets, know-how, and contractual agreements to protect our IP.
Our success depends, in part, upon our ability to obtain and maintain patent and other intellectual property protection for our product candidates including compositions-of-matter, dosages, and formulations, manufacturing methods, and novel applications, uses and technological innovations related to our product candidates and core technologies. Our business would be negatively impacted if we are not successful in developing additional proprietary technologies that are protected either as trade secrets or by filing additional patent applications.
We cannot ensure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications that may be filed by us in the future, nor can we ensure that any of our existing or subsequently granted patents will be useful in protecting our drug candidates, technological innovations, and processes. The claims of any patents that are issued may not provide meaningful protection, may not provide a basis for commercially viable products or may not provide us with any competitive advantages. Because of the extensive time required for clinical development and regulatory review of a product candidate, certain patents related to our product candidates will expire before any of our product candidates can be commercialized, while other patents may remain in force for only a short period of time following commercialization, thereby reducing the advantage afforded by any such patent. In addition, others may independently develop similar or alternative technologies, duplicate any of our technologies and, if patents are licensed or issued to us, design around the patented technologies licensed to or developed by us. Therefore, our competitors may be able to commercialize similar products, or may be able to duplicate our business strategy, without infringing our patents or otherwise using our intellectual property.
The protection afforded by any particular patent depends upon many factors, including the type of patent, scope of coverage encompassed by the granted claims, availability of extensions of patent term and legal interpretation of patent laws in the United States and other countries that could diminish our ability to protect our inventions and to enforce our intellectual property rights. Furthermore, others may have patents that relate to our technology or business that may prevent us from marketing our product candidates unless we are able to obtain a license to those patents. Accordingly, while our ability to maintain and solidify our proprietary position for our products and core technologies will depend, in part, on our success in obtaining and enforcing valid patent claims, we cannot predict with certainty the enforceability of any granted patent claims or of any claims that may be granted from our patent applications.
The biotechnology and pharmaceutical industries are characterized by extensive litigation and other challenges regarding patents and other intellectual property rights that involve complex legal and factual questions making our patent position generally uncertain. Any existing or subsequently granted patents may be challenged, invalidated, found unenforceable, circumvented or infringed. We have been involved in the past in administrative proceedings with respect to our patents and patent applications and may, as a result of our extensive portfolio, be involved in such proceedings in the future. Additionally, in the future, we may claim that a third-party infringes our intellectual property or a third party may claim that we infringe its intellectual property. In any of the administrative proceedings or in litigation, we may incur significant expenses, damages, attorneys’ fees, costs of proceedings and experts’ fees, and management and employees may be required to spend significant time in connection with these actions.
11
A patent interference proceeding may be instituted with the United States Patent and Trademark Office (“USPTO”) when more than one person files a patent application covering the same technology, or if someone wishes to challenge the validity of an issued patent on patents and applications filed before March 16, 2013. At the completion of the interference proceeding, the USPTO will determine which competing applicant is entitled to the patent, or whether an issued patent is valid. Patent interference proceedings are complex, highly contested legal proceedings, and the USPTO’s decision is subject to appeal. This means that if an interference proceeding arises with respect to any of our patent applications, we may experience significant expenses and delay in obtaining a patent, and if the outcome of the proceeding is unfavorable to us, the patent could be issued to a competitor rather than to us. For patents and applications filed after March 16, 2013 a derivation proceeding may be initiated where the USPTO may determine if one patent was derived from the work of an inventor on another patent. Inventorship may also be challenged in litigation.
In addition to interference proceedings, the USPTO can reevaluate issued patents at the request of a third party seeking to have the patent invalidated. There are proceedings at the USPTO (ex parte reexamination, post grant review, or inter partes review proceeding), which allow third parties to challenge the validity of an issued patent where there is a reasonable likelihood of invalidity. As with the USPTO interference proceedings, these USPTO proceedings will be very expensive to contest and can result in the cancelation of a patent. This means that patents owned or licensed by us may be subject to further administrative challenges and may be lost if the outcome of the challenge is unfavorable to us.
There are also challenges to obtaining patents in countries outside of the United States. In particular, under European patent law and the patent laws of certain other countries, oppositions to the issuance of patents may be filed. These foreign proceedings can be very expensive to contest and can result in significant delays in obtaining a patent or can result in a denial of a patent application. Also, in certain countries, there is uncertainty about the eligibility of hES cell subject matter for a patent under so-called morality clauses. Following a December 2014 decision at the Court of Justice of the European Union, the European Patent Office now recognizes that human pluripotent stem cells (including hES cells) can be created without a destructive use of human embryos as of June 5, 2003. Consequently, patent applications relating to hES cell subject matter with a filing and priority date after this date are no longer automatically excluded from patentability under Article 53 (a) EPC and Rule 28(c) EPC. In other countries such as India and China, uncertainty about the eligibility of hES cell subject matter for patentability has not been resolved.
We may benefit from a variety of regulatory frameworks in the United States, Europe, China and other territories that provide periods of non-patent-based exclusivity for qualifying drug products. See “Government Regulation—FDA and Foreign Regulation.”
Manufacturing
We currently occupy a 44,000 square foot facility in Fremont, California which includes a cGMP compliant facility. We have used this facility to work on updating the manufacturing process for AST-OPC1 and we may eventually use this facility to manufacture AST-OPC1 or other product candidates to supply future trials. We will also rely on third-party manufacturers to manufacture certain product candidates. For example, CRUK will manufacture clinical grade AST-VAC2 for the upcoming Phase 1 clinical trial of AST-VAC2.
Competition
The industry for stem-cell derived therapeutics and other cell therapies is characterized by rapidly evolving technology and intense competition. Our competitors include major multinational pharmaceutical companies, specialty biotechnology companies, and chemical and medical products companies operating in the fields of regenerative medicine, cell therapy, tissue engineering, and tissue regeneration. Many of these companies are well-established and possess technical, research and development, financial, and sales and marketing resources significantly greater than ours. In addition, certain smaller biotech companies have formed strategic collaborations, partnerships and other types of joint ventures with larger, well established industry competitors that afford these companies’ potential research and development and commercialization advantages. Academic institutions, governmental agencies, and other public and private research organizations are also conducting and financing research activities which may produce products directly competitive to those we are developing. This intense competition leads to a rapidly evolving landscape on several fronts that creates risks for companies in this space, including changes in standard of care for certain disease states or conditions that we may be targeting for development and new intellectual property related to developing cell therapies that may impact our operations.
12
Some of our competitors may be trying to develop cell-based technologies and products that may compete with our potential products based on efficacy, safety, cost, and intellectual property positions.
We may also face competition from companies that have filed patent applications relating to the growth, differentiation and therapeutic use of stem cells and dendritic cells. We may be required to seek licenses from these competitors in order to commercialize certain of our proposed products, and such licenses may not be granted.
Government Regulation
Government authorities at the federal, state and local level, and in other countries, extensively regulate among other things, the development, testing, manufacture, quality, approval, distribution, labeling, packaging, storage, record keeping, marketing, import/export and promotion of drugs, biologics, and medical devices. Authorities also heavily regulate many of these activities for human cells, tissues and cellular and tissue-based products.
FDA and Foreign Regulation
We believe that the FDA will regulate most of our proposed products as biologics. In the United States, the FDA regulates drugs and biologics under the Federal Food, Drug and Cosmetic Act, the Public Health Service Act, and is responsible for implementing regulations. In addition, establishments that manufacture human cells, tissues, and cellular and tissue-based products are subject to additional registration and listing requirements, including current good tissue practice regulations. Many of our proposed products will be reviewed by the FDA staff in its Center for Biologics Evaluation and Research (“CBER”) Office of Tissues and Advanced Therapies (“OTAT”, formerly known as the Office of Cellular, Tissue and Gene Therapies, or “OCTGT”).
In the United States, biologic products like ours are subject to rigorous FDA review and approval procedures. After testing in animals to evaluate the potential efficacy and safety of the product candidate, an Investigational New Drug application (“IND”) must be submitted to the FDA to obtain authorization for human testing. Extensive clinical testing, which is generally done in three phases, must then be undertaken at one or more hospitals or medical centers to demonstrate optimal use, safety, and efficacy of each product in humans. The FDA closely monitors the progress of each of the three phases of clinical testing and may, at its discretion, re-evaluate, alter, suspend, or terminate the clinical trial based upon the data which have been accumulated to that point and its assessment of the risk/benefit ratio to the intended patient population. All adverse events must be reported to the FDA. Monitoring of all aspects of the study to minimize risks is a continuous process. The time and expense required to perform this clinical testing can far exceed the time and expense of the research and development required to create the product.
In additional to regulating the clinical development of our products, the FDA regulates in the United States other areas involving our product, including:
| • | Applications for Marketing Approval: No action can be taken to market any therapeutic product in the United States until an appropriate application, which in the case of a cell therapy or vaccine product will be a Biologics License Application (“BLA”), has been approved by the FDA. FDA regulations also restrict the export of therapeutic products for clinical use prior to BLA approval. To date, the FDA has not granted marketing approval to any hES-based therapeutic products and it is possible that the FDA or foreign regulatory agencies may subject our product candidates to additional or more stringent review than drugs or biologics derived from other technologies. |
| • | Combination Products: If we develop any products that are used with medical devices, they may be considered combination products, which are defined by the FDA to include products comprised of two or more regulated components or parts such as a biologic and a device. The regulatory requirements for a combination product comprised of a biologic administered with a delivery device can be more complex, because in addition to the individual regulatory requirements for each component, additional combination product regulatory requirements may apply. |
| • | Post-Approval Matters: Even after initial FDA approval has been obtained, further studies may be required to provide additional data on safety or to gain approval for the use of a product as a treatment for clinical indications other than those initially targeted. Data resulting from these clinical trials may result in expansions or restrictions to the labeled indications for which a product has already been approved. |
| • | Manufacturing: The FDA regulates the manufacturing process of pharmaceutical products, and human tissue and cell products, requiring that they be produced in compliance with cGMP. |
13
| • | FDA Regulation of Advertising and Product Promotion: The FDA also regulates the content of advertisements used to market pharmaceutical and biological products. Claims made in advertisements concerning the safety and efficacy of a product, or any advantages of a product over another product, must be supported by clinical data filed as part of a BLA or an amendment to a BLA, and must be consistent with the FDA approved labeling and dosage information for that product. |
Sales of pharmaceutical and biological products outside the United States are subject to foreign regulatory requirements that vary widely from country to country. Even if FDA approval has been obtained, approval of a product by comparable regulatory authorities of foreign countries must be obtained prior to the commencement of marketing the product in those countries. The time required to obtain such approval may be longer or shorter than that required for FDA approval.
California State Regulations
The state of California has adopted legislation and regulations that require institutions that conduct stem cell research to notify, and in certain cases obtain approval from, a Stem Cell Research Oversight Committee (“SCRO Committee”) before conducting the research. Under certain California regulations, all hES cell lines that will be used in our research must be acceptably derived.
We also comply with certain California regulations that require certain records to be maintained with respect to stem cell research and the materials used.
In compliance with state regulations, we have formed a SCRO Committee which reviews each of Asterias’ projects that involve the use of pluripotent stem cells. The committee reviews and confirms that we are using only hES cell lines that have been acceptably derived and that the research conducted using these cells lines is both scientifically and ethically justified. The AST-OPC1 and AST-VAC2 programs have been reviewed by the SCRO Committee and have been deemed to comply with federal and state guidelines. The hES cell lines that we use are all on the National Institutes of Health (“NIH”) registry of lines that have been reviewed and meet standards for federal funding grants.
California Proposition 71
During November 2004, California State Proposition 71 (“Prop. 71”), the California Stem Cell Research and Cures Initiative, was adopted by state-wide referendum. Prop. 71 provides for a state-sponsored program designed to encourage stem cell research in the State of California, and to finance such research with State funds totaling approximately $295,000,000 annually for an initially scheduled 10 years beginning in 2005. This initiative created CIRM, which will provide grants, primarily but not exclusively, to academic institutions to advance both hES cell research and adult stem cell research. On October 16, 2014 we signed a NGA with CIRM, effective October 1, 2014, with respect to a $14.3 million grant award for clinical development of our product, AST-OPC1. As of December 31, 2017, we have received the entire $14.3 million.
Employees
In 2017, we made adjustments to our operating expenses as appropriate by reducing staffing allocated to non-clinical activities as a part of a broader effort to more closely align operating expenses with the Company’s primary goal of continuing to generate clinical data in our clinical stage programs. The reduction in staffing reduced our number of employees by approximately 25 employees.
As of March 1, 2018, we employed 31 persons on a full-time basis, with 12 of our employees holding M.D. and/or Ph.D. degrees in one or more fields of medicine or science. None of our employees are subject to a collective bargaining agreement. All of our employees except for one individual located in the United Kingdom are based in the United States.
Research and Development
Our research and development expenses were $26.6 million, $25.5, million and $17.3 million for the years ended December 31, 2017, 2016, and 2015 respectively.
14
| Item 1A. | Risk Factors |
Our business is subject to various risks, including those described below. You should consider the following risk factors, together with all of the other information included in this report, which could materially adversely affect our proposed operations, our business prospects, and financial condition, and the value of an investment in our business. There may be other factors that are not mentioned here or of which we are not presently aware that could also affect our business operations and prospects.
Risks Related to Our Business Operations
We are attempting to develop cellular therapies from cells derived from human embryonic stem cells.
Our use of human embryonic stem cells (“hES cells”) to attempt to develop cell therapies such as oligodendrocyte progenitor cell populations in our AST-OPC1 Program and dendritic cells in our AST-VAC2 Program is at the leading edge of science. The novel nature of these therapies creates significant challenges in regard to product development and optimization, manufacturing, government regulation, third-party reimbursement, and market acceptance. For example, the pathway to regulatory approval for cell-based therapies, including our therapeutic product candidates, may be more complex and lengthy than the pathway for conventional drugs. Many of the risk factors described in greater detail below ultimately relate back to the fact that we are a hES cell research and development company. While each of these risk factors may be important to understanding other statements in this Annual Report on Form 10-K and our other filings, investors should also be mindful that we are attempting to do something that has never been done: to develop and obtain FDA approval of a cell therapy derived from human embryonic stem cells.
We have a history of operating losses and negative cash flows.
Since our inception in September 2012, we have incurred operating losses and negative cash flow, and we expect to continue to incur losses and negative cash flow in the future. Our net losses for the fiscal years ended December 31, 2017, 2016, and 2015 were $28.4 million, $35.5 million, and $15.0 million respectively, and we had an accumulated deficit of $112.1 million and $83.7 million as of December 31, 2017 and 2016, respectively. We have limited cash resources and will depend upon future equity financings, research grants, funding available through collaborations with third parties, and sales of BioTime and OncoCyte common shares that we have as a source of funding for our operations. There is no assurance that we will be able to obtain the financing we need from any of those sources, or that any such financing that may become available will be on terms that are favorable to us and our shareholders.
Our strategic business plan may not produce the intended growth in revenue and operating income.
Our strategies ultimately include making significant investments in our product development programs to achieve future revenues through partnering, collaboration, licensing or commercialization activities. If we do not achieve the expected benefits from these investments or otherwise fail to execute on our strategic initiatives, we may not achieve the milestones we are targeting and our results of operations may be adversely affected. We may also fail to secure the capital necessary to make investments in our product development programs, which will hinder our growth and ability to achieve future revenues.
In addition, from time to time, we may seek to make acquisitions, license our products, and/or enter into strategic alliances such as joint ventures and joint development agreements. However, we may not be able to identify suitable partners, and our strategic alliances may not prove to be successful. Such transactions involve numerous risks and, although we will endeavor to evaluate the risks inherent in any particular transaction, there can be no assurance that we will properly ascertain and mitigate all such risks. There can be no assurance that difficulties encountered with such transactions will not have a material adverse effect on our business, financial condition and results of operations.
Failure to attract and retain skilled personnel and key relationships could impair our research and development efforts.
We reduced our staffing in 2017. We will need to retain our existing workforce and at some point recruit and hire additional qualified research scientists, laboratory technicians, and clinical development and management personnel in order to continue to develop our programs. Competition for these types of personnel, especially in the San Francisco Bay area where our operations are located, is intense and we may experience delays in hiring the qualified people that we need. The inability to attract and retain sufficient qualified management, scientific, or
15
technical personnel, or to engage qualified third parties that could perform the necessary activities, may significantly delay or prevent the achievement of our product development and other business objectives and could have a material adverse effect on our business, operating results and financial condition. In light of our reduction in staffing in 2017, we will increasingly rely on consultants and advisors who are either self-employed or employed by other organizations to complete certain necessary activities, and they may have conflicts of interest or other commitments, such as consulting or advisory contracts with other organizations, that may affect their ability to perform services for us.
We will spend a substantial amount of our capital on research and development, but we might not succeed in developing products and technologies that are useful in medicine.
The product development work we plan to do is costly, time consuming and uncertain as to its results. We will attempt to develop new medical products and technologies that might not prove to be safe, efficacious or cost effective in human medical applications. Many of the products and technologies that we will seek to develop have not been applied in human medicine and have only been used in laboratory studies in vitro or in animals. Only two of our three current programs have been tested in humans, and those were Phase 1/2 trials involving only a small number of human patients. Even if we are ultimately successful in developing a new technology or product, development of the new technology or product will take years and require the expenditure of large sums of money.
The amount and pace of research and development work that we can do or sponsor, and our ability to commence and complete clinical trials required to obtain FDA and foreign regulatory approval of our products, depends upon the amount of funding available to us.
We have had to limit our laboratory research and development work, our process development work, and our clinical development of our product candidates, based on the amount of our cash resources. We plan to continue to seek research and development grants from government agencies, charitable organizations, and other sources and to attempt to enter into collaborative product development agreements through which third parties will provide funding or otherwise bear the cost of research and development or clinical trials of our product candidates. There is no assurance that the amount of any grants that we may receive will be adequate for our needs. The agreements we entered into to date with Cancer Research UK (“CRUK”) are subject to termination if certain milestones relating to regulatory approvals are not achieved, if we are in material breach of the agreement and we do not cure such breach within 60 days, or if we are insolvent or commence liquidation proceedings. Any future agreement with granting organizations will likewise be subject to termination based on the failure to achieve milestones or for other reasons set forth such agreements. Hence, there is no assurance that we will receive the full value of the agreement with CRUK or any other granting organization. Unless we are able to generate sufficient revenue or raise additional funds when needed, it is likely that we will be unable to continue our planned activities, even if we make progress in our research and development projects.
Our clinical trials on our product candidates are ongoing and our clinical trial results may not ultimately confirm initial positive indications, which would materially and adversely affect our business, financial condition and stock price.
Our efforts to commercialize our product programs are dependent on obtaining FDA or other non-U.S. regulatory agency approval of its use in patients. The process of obtaining approval of a drug product for use in humans is extremely lengthy and time-consuming, and numerous factors may prevent our successful development of our product candidates, including negative results in our current or future clinical trials, the development by competitors of other products with equal or better results, or inability to obtain sufficient additional funding to continue to pursue development. Failure to successfully develop our product candidates would have a material and adverse effect on our business, financial condition and stock price, and would threaten our ability to continue to operate our business.
At present, our ability to progress as a company is significantly dependent on our AST-OPC1 product and the data from its ongoing Phase 1/2a clinical trial. Any material safety issue or adverse side effect to any study participant participating in this trial, or the failure of this trial to show the results expected would likely depress our stock price significantly and could prevent us from obtaining sufficient additional funding to continue to pursue our planned development of our product programs.
16
Adverse events in our clinical trials may force us to stop development of our product candidates or prevent regulatory approval, if needed, of our product candidates.
The testing of our product candidates in human clinical trials may produce serious adverse events. These adverse events could interrupt, delay or halt clinical trials of product candidates, could result in the FDA or other regulatory authorities denying approval of, or adding black box warnings or other limitations to, our product candidates for any or all targeted indications, and could result in our inability to obtain sufficient funding to continue to pursue development. An independent data safety monitoring board, the FDA, other regulatory authorities or we may suspend or terminate clinical trials at any time. We cannot assure that any of our product candidates will be safe for human use.
We will need to issue additional equity or debt securities in order to raise additional capital needed to pay our operating expenses.
We plan to incur substantial research and product development expenses, and we will need to raise additional capital to pay operating expenses until we are able to generate sufficient revenues from product sales, royalties, and license fees. Additional sales of equity or debt securities will be required in the future to meet our capital needs. Sales of additional equity securities will result in the dilution of the interests of present shareholders.
The availability of cells will impact the time and cost of commencing our research and product development programs.
The cells, cell lines and other biological materials that we acquired are being stored under cryopreservation protocols intended to preserve their functionality. We have successfully completed the verification of the viability of the lots of AST-OPC1 cells that we have been using in our current SCiStar Phase 1/2a study. However, we do not currently have sufficient amounts of AST-OPC1 cells to complete a large randomized control trial or for future commercial activities. We are developing additional cell banks and modifying our process to generate sufficient amounts of AST-OPC1 cells for use in future trials. These process development activities increase the costs of our product development for AST-OPC1 and the ability to complete these activities will impact the ability to move forward the overall AST-OPC1 program.
Furthermore, we are currently relying on CRUK to manufacture AST-VAC2 for the product’s upcoming Phase 1 study in the UK. Any delays in CRUK being able to successfully manufacture AST-VAC2 for the upcoming Phase 1 study will delay the ability to enroll patients, generate data, and complete the study.
The manufacturing of cells for our clinical programs is difficult and costly.
We reduced our staffing in 2017 and may need to rely upon third parties to produce AST-OPC1 cells for future studies and commercialization. We are currently relying on CRUK to manufacture AST-VAC2 for its upcoming Phase 1 study in the UK. We cannot give any assurance that we or any third-party manufacturers that we use will be able to develop the manufacturing capabilities necessary to supply adequate amounts of product to support our future clinical trials or commercialization. Moreover, we cannot give any assurance that we or the contract manufacturers or suppliers that we select will be able to supply our products in a timely or cost-effective manner or in accordance with applicable regulatory requirements, our own specifications, or our clinical development timelines. The failure of us or any of our third-party manufactures or suppliers to comply with regulatory requirements could result in material manufacturing delays and product shortages, which could delay or otherwise negatively impact our clinical trials and product development plans.
Any products that receive regulatory approval may be difficult and expensive to manufacture on a commercial scale.
hES derived therapeutic cells have only been produced on a small scale and not in quantities and at levels of purity and viability that will be needed for larger registration trials or wide scale commercialization. If we are successful in advancing products that consist of hES cells or other cells or products derived from hES or other cells, we will need to develop, alone or in collaboration with one or more pharmaceutical companies or contract manufacturers, technology for the large-scale production of those products. Our hES cell or other cell-based products are likely to be more expensive to manufacture on a commercial scale than most other drugs on the market today. The high cost of manufacturing a product will require that we charge our customers a high price for the product in order to cover our costs and earn a profit. If the price of our products is too high, hospitals and physicians may be
17
reluctant to purchase our products, and third-party payers such as insurance companies may be reluctant to reimburse for our products, especially if lower priced alternative products are available, and we may not be able to sell our products in sufficient volumes to recover our costs of development and manufacture or to earn a profit.
New products and technological advances by our competitors may negatively affect our results of operations.
Any products that we are able to develop may face future competition from third parties. Competitors’ products may be safer, more effective; more effectively marketed or sold, or have lower prices or superior performance features than our products. We cannot predict with certainty the timing or impact of the introduction of our competitors’ products.
We do not have the ability or resources to independently conduct clinical trials required to obtain regulatory approvals for our therapeutic product candidates.
We will need to rely on third parties, such as CRUK, contract research organizations, data management companies, contract clinical research associates, medical institutions, clinical investigators and contract laboratories to conduct any clinical trials that we may undertake for our products. We may also rely on third parties to assist with our preclinical development of therapeutic product candidates. If we outsource clinical trials, we may be unable to directly control the timing, conduct and expense of our clinical trials. If we enlist third parties to conduct clinical trials and they fail to successfully carry out their contractual duties or regulatory obligations or fail to meet expected deadlines, if the third parties need to be replaced or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to clinical protocols or regulatory requirements or for other reasons, our preclinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for or successfully commercialize our therapeutic product candidates. We also may determine that, even following positive clinical results in an earlier stage trial, we do not have the ability or resources to continue the development of a product and move forward with efforts to license or partner such product for future development.
Delays in the commencement or completion of clinical testing of our current and potential product candidates could result in increased costs to us and delay our ability to generate revenues.
The commencement of clinical trials can be delayed for a variety of reasons, including delays in:
| • | the manufacturing activities needed to produce sufficient quantities of the product candidate that meets our quality standards for clinical testing; |
| • | regulatory approval needed to commence the planned clinical trials, including agreement with the FDA or other regulatory body on the clinical protocol and study design; |
| • | reaching agreement with our collaborators, including any contract research organizations (“CROs”) and the trial sites, on all aspects of the clinical trial; |
| • | securing the institutional review board approval needed to conduct the clinical trials at the prospective sites. |
Even after commencement, the completion of clinical trials can be delayed or prevented for a number of reasons. Clinical trials may be delayed due to insufficient patient enrollment, which is a function of many factors, including the size and nature of the relevant patient populations, the nature of the protocols, the proximity of patients to clinical sites, the availability of effective treatments for the relevant diseases, clinical testing alternatives available to patients interested in enrolling in our studies, and the eligibility criteria for our clinical trials. In addition, clinical trials may also be delayed by unforeseen events that we have little or no control over. Delays in clinical testing of any of our product candidates could prevent or delay us from obtaining the additional evidence of clinical efficacy we will need for the approval for our product candidate in any indication. Failure to move forward the clinical development of our product candidates in a timely manner could impact our ability to obtain sufficient levels of funding to develop these products and could ultimately have a material impact on our business.
We will have certain obligations and may incur liabilities arising from clinical trials, and we do not yet know the scope of any resulting expenses that might arise.
We face the risk of incurring liabilities to patients who participate in clinical trials of our product candidates if they incur any injuries as a result of their participation. We will also be obligated to obtain information and prepare reports about the health of the clinical trial patients. In addition, we have assumed Geron’s obligations to obtain
18
information and prepare reports about the health of patients, and we have assumed any liabilities to those patients that might arise from any injuries they may have incurred, as a result of their participation in the clinical trials of Geron’s GRNOPC1 (renamed AST-OPC1) cell replacement therapy for spinal cord damage and its GRNVAC1 (renamed AST-VAC1) immunological therapy for certain cancers. We are not aware of any claims by patients alleging injuries suffered as a result of any of those clinical trials, but if any claims are made and if liability can be established, the amount of any liability that we may incur, depending upon the nature and extent of any provable injuries, could exceed our insurance coverage, and the amount of the liability could be material to our financial condition.
We have no history of conducting large-scale, Phase 2 or 3 clinical trials or commercializing pharmaceutical products, which may make it difficult to evaluate the prospects for our future viability.
Our clinical trials thus far have been limited to conducting an ongoing Phase 1/2a clinical trial for AST-OPC1 and planning for a Phase 1 clinical trial for AST-VAC2. The Company has no prior experience in obtaining regulatory approval for a drug or commercializing an approved drug. Accordingly, we have not had experience designing or completing a large-scale or pivotal clinical trial, obtaining marketing approval, manufacturing product on a large scale or conducting sales and marketing activities. Consequently, predictions about our future success or viability may not be as accurate as they could be if we had a history of successfully developing and commercializing pharmaceutical products.
Our business could be adversely affected by the loss of key personnel that took place in 2017 and if we lose the services of other key personnel upon whom we depend.
In 2017 and early 2018, key personnel left the Company, including our Chief Operating Officer and Chief Scientific Officer. While we have entered into consulting arrangements with some of these individuals, we may not be able to replace their expertise without undue delay or additional cost or we may be unable to replace them at all. Our AST-OPC1 program is directed primarily by our Chief Medical Officer and the loss of our Chief Medical Officer or other key personnel could significantly impact our ability to move forward this program. In addition, our success depends to a large extent on our President and CEO and our Chief Financial Officer and General Counsel. If any of these key personnel should leave our employ we may be unable to locate and recruit sufficient replacement personnel without undue delay or additional cost or we may be unable to replace them at all. Any such delay or inability could delay or terminate some or all of our research programs, the commercialization of our products, or our ability to raise capital to fund our business. Even if we are able to attract suitable replacement personnel, we may incur delays during a transition period. Therefore, the loss of these key employees and others within our organization could have a material adverse effect on us.
Our business and operations could suffer in the event of system failures.
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage from computer viruses and bugs, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Such events could cause interruption of our operations. For example, the loss of data for our product candidates could result in delays in our regulatory filings and development efforts and significantly increase our costs. To the extent that any disruption or security breach was to result in a loss of or damage to our data or inappropriate disclosure of confidential or proprietary information, we could incur liability and the development of our product candidates could be delayed.
We could be subject to breaches of our information technology systems, which could damage our reputation and customer relationships. Such breaches could subject us to significant reputational, financial, legal, and operational consequences.
Our business relies on information systems to obtain, process, analyze and manage data concerning our clinical trials. A cyber-attack that bypasses our security, or employee error, malfeasance or other disruptions that cause a security breach could lead to a material disruption of our information systems and/or the loss of business information. Such an attack could result in, among other things:
| • | the theft, destruction, loss, misappropriation or release of confidential data and intellectual property; |
| • | operational or business delays; |
| • | liability for a breach of personal information belonging to our customers or our employees; and |
19
| • | damage to our reputation any of which could have a material adverse effect on our business, financial condition, and results of operations. In the event of an attack, we would be exposed to a risk of loss or litigation and possible liability, including under laws that protect the privacy of personal information. |
Failure of our internal control over financial reporting could harm our business and financial results.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting for external purposes in accordance with accounting principles generally accepted in the U.S. Internal control over financial reporting includes maintaining records that in reasonable detail accurately and fairly reflect our transactions; providing reasonable assurance that transactions are recorded as necessary for preparation of our financial statements; providing reasonable assurance that receipts and expenditures of our assets are made in accordance with management authorization; and providing reasonable assurance that unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements would be prevented or detected on a timely basis. Because of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of our financial statements would be prevented or detected. Our growth and entry into new products, technologies and markets will place significant additional pressure on our system of internal control over financial reporting. Any failure to maintain an effective system of internal control over financial reporting could limit our ability to report our financial results accurately and timely or to detect and prevent fraud.
Power shortages, natural disasters, terrorist acts or other calamities could disrupt our production and have a material adverse effect on our business, financial position and results of operations.
Substantially all of our internal operations are carried out in our headquarters in Fremont, California. A significant disruption at that facility, even on a short-term basis, could impair our ability to carry out our business, which could have a material adverse effect on our business, financial position and results of operations. Historically, the state of California has been vulnerable to many natural or man-made disasters, including earthquake, fire, floods, environmental accidents, power loss, communications failures and similar events. In recent years, terrorist activity has been on the rise, we may be the target of, or be affected by, terrorist activity. If any such disaster were to occur, our ability to operate our business at our facilities would be seriously impaired. Unexpected business interruptions resulting from disasters could disrupt our operations and thereby result in substantial costs and diversion of resources.
Transfers of our equity, or issuances of equity may impair our ability to utilize our federal income tax net operating loss carryforwards in future years.
Under federal income tax law, a corporation is generally permitted to deduct from taxable income net operating losses (“NOLs”) carried forward from prior years. We have net operating loss carryforwards for federal income tax purposes of approximately $62.3 million as of December 31, 2017. Our ability to utilize our net operating loss carryforwards to offset future taxable income and to reduce federal income tax liability is subject to certain requirements and restrictions. If we experience an “ownership change,” as defined in section 382 of the Internal Revenue Code, then our ability to use our net operating loss carryforwards may be substantially limited, which could have a negative impact on our financial position and results of operations. Generally, there is an “ownership change” if one or more stockholders owning 5% or more of a corporation’s common stock have aggregate increases in their ownership of such stock of more than 50 percentage points over the prior three-year period. Under section 382 of the Internal Revenue Code, absent an applicable exception, if a corporation undergoes an “ownership change,” the amount of its net operating losses that may be utilized to offset future taxable income generally is subject to an annual limitation. Further, future deductions for depreciation, depletion and amortization could be limited if the fair value of our assets is determined to be less than the tax basis. We have in the past, and we plan to, raise capital through sales of our common stock. Such sales of equity may cause or may have caused an “ownership change,” which could reduce our ability to offset our taxable income with losses, before such losses expire and therefore may cause us to incur a larger federal income tax liability.
20
Risks Related to Our Industry
We will face certain risks arising from regulatory, legal, and economic factors that affect our business and the business of other pharmaceutical and biological product development companies. Because we are a small company with limited revenues and limited capital resources, we may be less able to bear the financial impact of these risks than larger companies that have substantial income and available capital.
Before we are permitted to sell our products, we must meet requirements and receive approval from the FDA and other regulatory agencies.
The cell-based products that we are developing cannot be sold until the FDA or corresponding foreign regulatory authorities approve the products for medical use. The need to obtain regulatory approval to market a new product means that:
| • | we will have to conduct expensive and time consuming clinical trials of new products. The full cost of conducting and completing clinical trials necessary to obtain FDA and foreign regulatory approval of a new product cannot be presently determined, but could exceed our current financial resources; |
| • | clinical trials and the regulatory approval process for a cell-based product can take several years to complete. As a result, we will incur the expense and delay inherent in seeking FDA and foreign regulatory approval of new products, even if the results of clinical trials are favorable; |
| • | data obtained from preclinical and clinical studies is susceptible to varying interpretations that could delay, limit, or prevent regulatory agency approvals. Delays in the regulatory approval process or rejections of an application for approval of a new drug or cell-based product may be encountered as a result of changes in regulatory agency policy; |
| • | because the therapeutic products we plan to develop with hES technology involve the application of new technologies and approaches to medicine, the FDA or foreign regulatory agencies may subject those products to additional or more stringent review than drugs or biologics derived from other technologies. No therapeutic product based on hES technology has been approved by the FDA to date; |
| • | a product that is approved may be subject to restrictions on use; |
| • | the FDA can limit or withdraw approval of a product if problems arise; and |
| • | we will face similar regulatory issues in foreign countries. |
Clinical trial failures can occur at any stage of the testing and we may experience numerous unforeseen events during, or as a result of, the clinical trial process that could delay or prevent commercialization of our current or future therapeutic product candidates.
All of our product candidates are either at early stages of clinical development or at the preclinical or research stages of development. Clinical trial failures or delays can occur at any stage of the trials, and may be directly or indirectly caused by a variety of factors, including but not limited to:
| • | delays in securing clinical investigators or trial sites for our clinical trials; |
| • | delays in obtaining Institutional Review Board (“IRB”) and other regulatory approvals to commence a clinical trial; |
| • | slower than anticipated rates of patient recruitment and enrollment, or failing to reach the targeted number of patients due to competition for patients from other trials; |
| • | limited or no availability of coverage, reimbursement and adequate payment from health maintenance organizations (“HMOs”) and other third-party payers for the use of agents used in our clinical trials; |
| • | negative or inconclusive results from clinical trials; |
| • | unforeseen side effects interrupting, delaying, or halting clinical trials of our therapeutic product candidates, and possibly resulting in the FDA or other regulatory authorities denying approval of our therapeutic product candidates; |
| • | unforeseen safety issues; |
21
| • | uncertain dosing issues; |
| • | approval and introduction of new therapies or changes in standards of practice or regulatory guidance that render our clinical trial endpoints or the targeting of our proposed indications obsolete; |
| • | inability to monitor patients adequately during or after treatment or problems with investigator or patient compliance with the trial protocols; |
| • | inability to replicate in large controlled studies safety and efficacy data obtained from a limited number of patients in uncontrolled trials; |
| • | inability or unwillingness of medical investigators to follow our clinical protocols; and |
| • | unavailability of clinical trial product or other materials. |
Government imposed bans or restrictions, and religious, moral and ethical concerns on the use of hES cells could prevent us from developing and successfully marketing stem-cell derived products.
Government imposed bans or restrictions on the use of embryos or hES cells research and development in the United States and abroad could generally constrain stem cell research, thereby limiting the market and demand for any of our products that receive regulatory approval. In March 2009, President Barack Obama lifted certain restrictions on federal funding of research involving the use of hES cells, and in accordance with President Obama’s executive order, the NIH has adopted new guidelines for determining the eligibility of hES cell lines for use in federally funded research. The central focus of the proposed guidelines is to assure that hES cells used in federally funded research were derived from human embryos that were created for reproductive purposes, were no longer needed for this purpose, and were voluntarily donated for research purposes with the informed written consent of the donors. hES cells that were derived from embryos created for research purposes rather than reproductive purposes, and other hES cells that were not derived in compliance with the guidelines, are not eligible for use in federally funded research.
In May 2016, the Select Investigative Panel on Infant Lives of the United States House of Representatives Committee on Energy and Commerce (the “Panel”) submitted a formal request that we provide certain information relating to, among other things, whether we have used fetal tissue in our research. We fully complied with this request and have provided evidence, to the Panel’s full satisfaction, that we have never used fetal tissue in our research, as we only use specific hES cell lines that were deemed eligible for federal funding based on their original derivation by third parties according to ethical principles. We also provided evidence supporting that President George W. Bush in August 2001 signed an executive order approving, for research purposes, the use of these specific cell lines, among certain others, and approval for their use was subsequently reconfirmed under President Obama’s March 2009 executive order.
California law requires that stem cell research be conducted under the oversight of a SCRO Committee. Many kinds of stem cell research, including the derivation of new hES cell lines, may only be conducted in California with the prior written approval of the SCRO Committee. A SCRO Committee could prohibit or impose restrictions on the research we plan to do.
The use of hES cells gives rise to religious, moral and ethical issues regarding the appropriate means of obtaining the cells and the appropriate use and disposal of the cells. These considerations could lead to more restrictive government regulations or could generally constrain stem cell research thereby limiting the market and demand for any of our products that receive regulatory approval. From time to time, social views on religious, moral and ethical issues could change and could affect political viewpoints and government regulations. Therefore, it is difficult to forecast with certainty whether there will be additional government-imposed bans or restrictions, and religious, moral and ethical concerns on our use of hES cells that could potentially give rise to proceedings, litigation or disputes that could cause us to incur substantial expense, require significant time and attention from our management and result in civil penalties against us. The results of any such proceedings, litigation or disputes could have a material adverse effect on our business and results of operations. Furthermore, it is possible that such proceedings, litigation or disputes could negatively impact the ability of our vendors, suppliers or collaborators to conduct their operations, which could also have a material adverse effect on our business and results of operations.
22
Legislative actions and potential new accounting pronouncements may impact our future financial performance and results of operations
There have been regulatory enactments, including the Dodd–Frank Wall Street Reform and Consumer Protection Act and the Tax Cuts and Jobs Act of 2017, that have had an impact on our financial condition and results of operations. Tax Cuts and Jobs Act of 2017 will make significant changes to the U.S. Internal Revenue Code. Such changes include a reduction in the corporate tax rate and limitations on certain corporate deductions and credits, among other changes. Certain of these changes could have a negative impact on our business. Other potential future regulatory enactments are likely to increase our general and administrative costs and expenses. In addition, there could be new accounting pronouncements that could materially increase the expenses we report under generally accepted accounting principles, and adversely affect our operating results and financial condition.
The price and sale of any of our products that receive regulatory approval may be limited by health insurance coverage and government regulation.
Success in selling any of our products that receive regulatory approval may depend in part on the extent to which health insurance companies, HMOs, and government health administration authorities such as Medicare and Medicaid will pay for the cost of the products and related treatment. Until we actually introduce a new product into the medical market place we will not know with certainty whether adequate health insurance, HMO, and government coverage will be available to permit the product to be sold at a price high enough for us to generate a profit. In some foreign countries, pricing or profitability of health care products is subject to government control which may result in low prices for our products. In the United States, there have been a number of federal and state proposals to implement similar government controls, increased attention by Congress and others within the government regarding such proposals, and new proposals are likely to be made in the future.
There have been, and we expect there will continue to be, a number of federal legislative initiatives implemented to reform the U.S. healthcare system in ways that could adversely impact our business.
The FDA has established regulations, guidelines and policies to govern the drug development and approval process. Any change in regulatory requirements resulting from the adoption of new legislation, regulations or policies may require us to amend existing clinical trial protocols or add new clinical trials to comply with these changes. Such amendments to existing protocols or clinical trial applications or the need for new ones, may significantly and adversely affect the cost, timing and completion of the clinical trials for our drug candidates. In addition, the FDA’s policies may change and additional government regulations may be issued that could prevent, limit or delay regulatory approval of our drug candidates, or impose more stringent product labeling and post-marketing testing and other requirements. The recent elections in the U.S. could result in significant changes in, and uncertainty with respect to, legislation, regulation and government policy that could significantly impact our business and the health care industry.
In addition, we expect that the Affordable Care Act, which could be amended or repealed in the future, the 21st Century Cures Act, and other healthcare reform measures that may be adopted in the future, could have a material adverse effect on our industry generally and on our ability to commercialize our future products and establish and maintain product sales. If we are slow or unable to adapt to any such changes, our business, prospects and ability to achieve or sustain profitability would be adversely affected.
In 2017, our AST-OPC1 product obtained FDA designation as a Regenerative Medicine Advanced Therapy (“RMAT”) under the 21st Century Cures Act. The RMAT designation is intended to facilitate expedited development, review and approval for important new regenerative medicine therapies for which preliminary clinical evidence indicates the potential to address a serious or life-threatening disease or condition. While we view the RMAT designation as beneficial our AST-OPC1 program, in that RMAT status is intended to confer an accelerated approval process, the FDA has yet to finalize the full extent of benefits conferred upon a RMAT designee and the benefits may differ from what we initially expect.
23
Risks Related to Our Intellectual Property
If our efforts to protect our proprietary technologies are not adequate, we may not be able to compete effectively in our market.
We rely upon a combination of patents, trade secret protection and contractual arrangements to protect the intellectual property related to our technologies. We will only be able to protect our products and proprietary information and technology by preventing unauthorized use by third parties to the extent that our patents, trade secrets, and contractual position allow us to do so. Any disclosure to or misappropriation by third parties of our trade secrets or confidential information could compromise our competitive position. Moreover, we have in the past been involved in, and may in the future be involved in legal or administrative proceedings involving our intellectual property and initiated by third parties, which proceedings can result in significant costs and commitment of management time and attention. As our product candidates continue in development, third parties may attempt to challenge the validity and enforceability of our patents and proprietary information and technologies.
We also have in the past been involved in, and may in the future be involved in, initiating legal or administrative proceedings involving the product candidates and intellectual property of our competitors. These proceedings can result in significant costs and commitment of management time and attention, and there can be no assurance that our efforts would be successful in preventing or limiting the ability of our competitors to market competing products. Composition-of-matter patents relating to the active pharmaceutical ingredient are generally considered to be the strongest form of intellectual property protection for pharmaceutical products, as such patents provide protection not limited to any one method of use or manufacture. Method-of-use and method-of-manufacture patents protect the use or manufacture of a product for the specified method(s), and do not prevent a competitor from making and marketing a product that is identical to our product but produced using a method that is outside the scope of our patented method or for an indication that is outside the scope of our patented use. We rely on a combination of these and other types of patents to protect our product candidates, and there can be no assurance that our intellectual property will create and sustain the competitive position of our product candidates.
Biotechnology and pharmaceutical product patents involve highly complex legal and scientific questions and can be uncertain. Any patent applications that we own or license may fail to result in issued patents. Even if patents do successfully issue from our applications, third parties may challenge their validity or enforceability, which may result in such patents being narrowed, invalidated, or held unenforceable. Even if our patents and patent applications are not challenged by third parties, those patents and patent applications may not prevent others from designing around our claims and may not otherwise adequately protect our product candidates. If the breadth or strength of protection provided by the patents and patent applications we hold with respect to our product candidates is threatened, competitors with significantly greater resources could threaten our ability to commercialize our product candidates.
Subject to meeting other requirements for patentability, for United States patent applications filed prior to March 16, 2013, the first to invent the claimed invention is entitled to receive patent protection for that invention while, outside the United States, the first to file a patent application encompassing the invention is entitled to patent protection for the invention. The United States moved to a “first to file” system under the Leahy-Smith America Invents Act, or AIA, effective March 16, 2013. Discoveries are generally published in the scientific literature well after their actual development, and patent applications in the United States and other countries are typically not published until 18 months after filing and in some cases are never published. Accordingly, we cannot be certain that we or our licensors were the first to make the inventions claimed in our owned and licensed United States patents or patent applications filed prior to March 16, 2013, or that we or our licensors were the first to file for patent protection for inventions claimed in foreign patents or foreign patent applications and United States patents or patent applications filed on or after March 16, 2013. The AIA also includes new procedures for challenging issued patents and pending patent applications, which creates additional uncertainty. We may become involved in opposition or interference proceedings challenging our patents and patent applications or the patents and patent applications of others, and the outcome of any such proceedings are highly uncertain. An unfavorable outcome in any such proceedings could reduce the scope of, or invalidate our patent rights, allow third parties to commercialize our technology and compete directly with us, or result in our inability to manufacture, develop or commercialize our product candidates without infringing the patent rights of others.
In addition to the protection afforded by patents, we seek to rely on trade secret protection and confidentiality agreements to protect proprietary know-how, information, or technology that is not covered by our patents. Although our agreements require all of our employees to assign their inventions to us, and we require all of our employees,
24
consultants, advisors and any third parties who have access to our trade secrets, proprietary know-how and other confidential information and technology to enter into appropriate confidentiality agreements, we cannot be certain that our trade secrets, proprietary know-how and other confidential information and technology will not be subject to unauthorized disclosure or that our competitors will not otherwise gain access to or independently develop substantially equivalent trade secrets, proprietary know-how and other information and technology.
Furthermore, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property globally. If we are unable to prevent unauthorized disclosure of our intellectual property related to our product candidates and technology to third parties, we may not be able to establish or maintain a competitive advantage in our market, which could materially adversely affect our business and operations.
Intellectual property disputes with third parties and competitors may be costly and time consuming, and may negatively affect our competitive position.
Our commercial success may depend on our avoiding infringement of the patents and other proprietary rights of third parties as well as on enforcing our patents and other proprietary rights against third parties. Pharmaceutical and biotechnology intellectual property disputes are characterized by complex, lengthy and expensive litigation over patents and other intellectual property rights. We may initiate or become a party to, or be threatened with, future litigation or other proceedings regarding intellectual property rights with respect to our product candidates and competing products.
As our product candidates progress toward commercialization, we or our collaboration partners may be subject to patent infringement claims from third parties. The patent landscape in competitive product areas is highly complex, and there may be patents of third parties of which we are unaware that may result in claims of infringement. For example, as we optimize the manufacturing processes for AST-OPC1 and AST-VAC2 to improve yields and scalability, we will be implementing new methods and materials. While the optimization of these processes may result in new intellectual property for us, it may also result in us including methods and materials which may be subject to patents of third parties of which are unaware of at the time. This may lead to potential infringement claims from third parties or subject us to costly license arrangements if we do not remove and replace such methods or materials from the manufacturing process. There can be no assurance that our product candidates and processes do not infringe proprietary rights of third parties, and parties making claims against us may seek and obtain injunctive or other equitable relief, which could potentially block further efforts to develop and commercialize our product candidates. Any litigation involving defense against claims of infringement, regardless of the merit of such claims, would involve substantial litigation expense and would be a substantial diversion of management time.
We intend, if necessary, to vigorously enforce our intellectual property in order to protect the proprietary position of our product candidates. Efforts to enforce our patents may include litigation, administrative proceedings, or both, depending on the potential benefits that might be available from those actions and the costs associated with undertaking those efforts against third parties. We carefully review and monitor publicly available information regarding products that may be competitive with our product candidates and assert our intellectual property rights where appropriate.
We may consider administrative proceedings and other means for challenging third-party patents and patent applications. Third parties may also challenge our patents and patent applications, through interference, reexamination, inter partes review, and post-grant review proceedings before the USPTO or through other comparable proceedings, such as oppositions or invalidation proceedings, before foreign patent offices. An unfavorable outcome in any such challenge could require us to cease using the related technology and to attempt to license rights to it from the prevailing third party, which may not be available on commercially reasonable terms, if at all, in which case our business could be harmed. Even if we are successful, participation in administrative proceedings before the USPTO or a foreign patent office may result in substantial costs and time on the part of our management and other employees.
Furthermore, there is a risk that any public announcements concerning the status or outcomes of intellectual property litigation or administrative proceedings may adversely affect the price of our stock. If securities analysts or our investors interpret such status or outcomes as negative or otherwise creating uncertainty, our common stock price may be adversely affected.
25
Our reliance on third parties and agreements with collaboration partners requires us to share our trade secrets, which increases the possibility that a competitor may discover them or that our trade secrets will be misappropriated or disclosed.
Our reliance on third-party contractors to develop and manufacture our product candidates is based upon agreements that limit the rights of the third parties to use or disclose our confidential information, including our trade secrets and know-how. Despite the contractual provisions, the need to share trade secrets and other confidential information increases the risk that such trade secrets and information are disclosed or used, even if unintentionally, in violation of these agreements. In the highly competitive markets in which our product candidates are expected to compete, protecting our trade secrets, including our strategies for addressing competing products, is imperative, and any unauthorized use or disclosure could impair our competitive position and may have a material adverse effect on our business and operations.
In addition, our collaboration partners are larger, more complex organizations than ours, and the risk of inadvertent disclosure of our proprietary information may be increased despite their internal procedures and contractual obligations in place with our collaboration partners. Despite our efforts to protect our trade secrets and other confidential information, a competitor’s discovery of such trade secrets and information could impair our competitive position and have an adverse impact on our business.
We have an extensive worldwide patent portfolio. The cost of maintaining our patent protection is high and maintaining our patent protection requires continuous review and compliance in order to maintain worldwide patent protection. We may not be able to effectively maintain our intellectual property position throughout the major markets of the world.
The USPTO and foreign patent authorities require maintenance fees and payments as well as continued compliance with a number of procedural and documentary requirements. Noncompliance may result in abandonment or lapse of the subject patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance may result in reduced royalty payments for lack of patent coverage in a particular jurisdiction from our collaboration partners or may result in competition, either of which could have a material adverse effect on our business.
We have made, and will continue to make, certain strategic decisions in balancing costs and the potential protection afforded by the patent laws of certain countries. As a result, we may not be able to prevent third parties from practicing our inventions in all countries throughout the world, or from selling or importing products made using our inventions in and into the United States or other countries. Third parties may use our technologies in territories in which we have not obtained patent protection to develop their own products and, further, may infringe our patents in territories which provide inadequate enforcement mechanisms, even if we have patent protection. Such third-party products may compete with our product candidates, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
The laws of some foreign countries do not protect proprietary rights to the same extent as do the laws of the United States, and we may encounter significant problems in securing and defending our intellectual property rights outside the United States.
Many companies have encountered significant problems in protecting and defending intellectual property rights in certain countries. The legal systems of certain countries do not always favor the enforcement of patents, trade secrets, and other intellectual property rights, particularly those relating to pharmaceutical and biotechnology products, which could make it difficult for us to stop infringement of our patents, misappropriation of our trade secrets, or marketing of competing products in violation of our proprietary rights. Proceedings to enforce our intellectual property rights in foreign countries could result in substantial costs and divert our efforts and attention from other aspects of our business, and could put our patents in these territories at risk of being invalidated or interpreted narrowly, or our patent applications at risk of not granting, and could provoke third parties to assert claims against us. We may not prevail in all legal or other proceedings that we may initiate and, if we were to prevail, the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
26
Intellectual property rights do not address all potential threats to any competitive advantage we may have.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and intellectual property rights may not adequately protect our business or permit us to maintain our competitive advantage. The following examples are illustrative:
| • | Others may be able to make cellular treatments that are the same as or similar to our current or future product candidates but that are not covered by the claims of the patents that we own or have exclusively licensed. |
| • | We or any of our licensors or strategic partners might not have been the first to make the inventions covered by the issued patent or pending patent application that we own or have exclusively licensed. |
| • | We or any of our licensors or strategic partners might not have been the first to file patent applications covering certain of our inventions. |
| • | Others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights. |
| • | The prosecution of our pending patent applications may not result in granted patents. |
| • | Granted patents that we own or have exclusively licensed may not provide us with any competitive advantages, or may be held invalid or unenforceable, as a result of legal challenges by our competitors. |
| • | Patent protection on our product candidates may expire before we are able to develop and commercialize the product, or before we are able to recover our investment in the product. |
| • | Our competitors might conduct research and development activities in the United States and other countries that provide a safe harbor from patent infringement claims for such activities, as well as in countries in which we do not have patent rights, and may then use the information learned from such activities to develop competitive products for sale in markets where we intend to market our product candidates. |
If we fail to meet our obligations under license agreements, we may lose our rights to key technologies on which our business depends.
Our business will depend in part on certain technologies that are licensed from third parties, including the Regents of the University of California, and the Wisconsin Alumni Research Foundation. Those third-party license agreements impose obligations on us, including payment obligations and obligations to pursue development of commercial products under the licensed patents or technology. If a licensor believes that we have failed to meet our obligations under a license agreement, the licensor could seek to limit or terminate our license rights, which could lead to costly and time-consuming litigation and, potentially, a loss of the licensed rights. During the period of any such litigation our ability to carry out the development and commercialization of potential products, and our ability to raise capital, could be significantly and negatively affected. If our license rights were restricted or ultimately lost, we would not be able to continue to use the licensed technology in our business.
Risks Related to Our Relationship with BioTime
BioTime has a significant influence on our business operations.
As of December 31, 2017, BioTime owns approximately 40% of our issued and outstanding common stock. Because BioTime is by far our largest shareholder and owns close to a majority of the outstanding common stock, it has the voting power to significantly impact any matter that requires shareholder approval. Furthermore, four of the ten members of our Board of Directors, including our Chief Executive Officer, are also directors of BioTime, and another director is an employee of Broadwood Capital, Inc., which is the general partner of Broadwood Partners, L.P., the partnership that is the largest shareholder of BioTime. Some of our directors also serve on the Boards of Directors of one or more subsidiaries or affiliates of BioTime, including AgeX Therapeutics, Inc. As a result of the relationships described above, BioTime has significant influence over our business operations and capital raising activities, and therefore, BioTime could cause corporate actions to be taken even if the interests of BioTime conflict with the interests of our other shareholders. This concentration of voting power could have the effect of deterring or preventing institutional investor interest in Asterias or a change in control that might be beneficial to our other shareholders.
27
If BioTime should elect to sell or distribute all or a substantial part of its ownership in Asterias, it could, depending on the manner in which these shares are distributed or sold, have a depressing impact on the price of our common stock and impact our ability to raise capital to fund our operations or attract new investors.
We have historically relied upon BioTime for certain services and resources.
Although we have our own research facilities, scientific personnel, and management and administrative personnel and do not rely upon BioTime for any of these resources, we previously relied on BioTime to provide certain services, including certain administrative and financial services related to financial accounting and reporting. We have entered into a Shared Facilities and Services Agreement with BioTime under which we have agreed to bear costs allocated to us by BioTime for services and materials provided for our benefit by BioTime. We pay BioTime 105% of its costs of providing personnel and services to us, and for any use of its facilities by us, including an allocation of general overhead based on that use. We may also share the services of some research personnel with BioTime. Conflicts of interest may arise from our relationship with BioTime.
Our relationship with BioTime could give rise to certain conflicts of interest that could have an impact on our research and development programs, business opportunities, and operations generally.
We and BioTime or any of its other subsidiaries may determine to engage in research and development of the same or similar products or technologies, or products that would otherwise compete in the market place. Even if we utilize different technologies than BioTime or its other subsidiaries, we could find ourselves in competition with them for research scientists, financing and other resources, licensing, manufacturing, and distribution.
Because of our relationship with BioTime, BioTime could prevent us from engaging in research and development programs, investments, business ventures, or agreements to develop, license, or acquire products or technologies that would or might compete with those owned, licensed, or under development by BioTime or any of its other subsidiaries.
In February 2016, we entered into a Cross-License with BioTime and a subsidiary of BioTime, ES Cell (“ESI”). Under this Cross-License, we received a fully-paid, non-royalty-bearing, world-wide, non-exclusive, sub-licensable license under certain patents and related patent rights owned by BioTime and ESI, and in exchange, we granted BioTime and ESI a fully-paid, non-royalty-bearing, world-wide, non-exclusive, sub-licensable license certain patents and related patent rights we own. Certain of the patents and related patent rights that were made available to Asterias under the non-exclusive Cross-License were separately licensed by BioTime to its subsidiary AgeX Therapeutics, Inc., which was formed in 2017 and which may become a separate, independent company in the future. As of December 31, 2017, all three members of the board of directors of AgeX, including its Chief Executive Officer, serve on our Board of Directors. We may enter into additional license or sublicense agreements with BioTime or a BioTime subsidiary. Conflicts of interest could arise in determining the scope and financial terms of any such licenses or sublicenses, including the fields of use permitted, licensing fees, and royalties, if any, and other matters.
BioTime and its other subsidiaries will engage for their own accounts in research and product development programs, investments, and business ventures and we will not be entitled to participate or to receive an interest in those programs, investments, or business ventures. BioTime and its other subsidiaries will not be obligated to present any particular research and development, investment, or business opportunity to us, even if the opportunity would be within the scope of our research and development plans or programs, business objectives, or investment policies. These opportunities may include, for example, opportunities to acquire businesses or assets, including but not limited to patents and other intellectual property that could be used by us or by BioTime or by any of BioTime’s other subsidiaries. Our respective boards of directors will have to determine which company should pursue those opportunities, taking into account relevant facts and circumstances at the time, such as the financial and other resources of the companies available to acquire and utilize the opportunity, and the best “fit” between the opportunity and the business and research and development programs of the companies. However, by virtue of their significant voting power, BioTime has significant influence in decision making with respect to the allocation of opportunities.
Under the Cross-License, BioTime and ESI may have a conflict of interest in determining how and when they should enforce their rights under the Cross-License if they were to default or otherwise fail to perform any of their obligations under the Cross-License.
One of our significant assets is 3,481,085 BioTime common shares that we held as of December 31, 2017. We may sell the BioTime common shares from time to time, or pledge those shares as collateral for loans, or to raise
28
capital to finance our operations. Because a sale of those shares could have a depressing effect on the market value of BioTime common shares, BioTime will have a continuing interest in the number of shares that we sell, the prices at which we sell the shares, and time and manner in which the shares are sold. Further, we may need or find it desirable to sell BioTime common shares at the same time as BioTime, or other BioTime subsidiaries that hold BioTime common shares, also desire to sell some of their BioTime common shares. Concurrent sales of BioTime common shares by us, BioTime, or other BioTime subsidiaries could have a depressing effect on the market price of the BioTime common shares, lowering the price at which we and they are able to sell BioTime common shares and resulting in lower net proceeds from the sales. We may coordinate any future sales of our BioTime common shares with BioTime and its other subsidiaries in order to provide an orderly and controlled process for raising capital through the sale of BioTime shares. This may include an agreement as to the number of shares to be sold, the time period or “market window” for selling shares, the use of a common securities broker-dealer, and a fair allocation of net sales based on average sales prices during any trading day on which we and they sell BioTime shares.
Risks Related to Our Dependence on Third Parties
We may not be able to obtain additional non-dilutive funding to advance our programs.
We are hopeful that we will be able to obtain an additional CIRM grant or another source of funding as a source of financing the costs of conducting a subsequent trial of AST-OPC1. If we fail to obtain an additional grant from CIRM or another significant source of funding, it may force us to postpone the commencement of any subsequent trial.
Establishing and maintaining strategic alliances is a key component of our business strategy. If we are unable to establish and maintain strategic alliances for our therapeutic product candidates, we may have to reduce or delay our product development or increase our expenditures.
A key component of our current strategy for developing, manufacturing and commercializing our therapeutic product candidates will be entering into strategic alliances with pharmaceutical companies or other industry participants to advance our programs and enable us to maintain our financial and operational capacity. We will face significant competition in seeking appropriate alliances. We may not be able to negotiate alliances on acceptable terms, if at all. If our strategic alliances do not result in the successful development and commercialization of our product candidates or if one or more of our collaborators terminates its agreement with us, we may not receive any future research and development funding or milestone or royalty payments under the collaboration. If we do not receive the funding we expect under these agreements, our continued development of our product candidates could be delayed, and we may need to obtain additional funding, which may be unavailable or available only on unfavorable terms.
If we are able to enter into product development and marketing arrangements with pharmaceutical companies, we may license product development, manufacturing, and marketing rights to the pharmaceutical company or to a joint venture company formed with the pharmaceutical company. Under such arrangements we might receive only a royalty on sales of the products developed or an equity interest in a joint venture company that develops the product. As a result, our revenues from the sale of those products may be substantially less than the amount of revenues and gross profits that we might receive if we were to develop, manufacture, and market the products ourselves.
We may become dependent on possible future collaborations or licensing of our products to develop and commercialize many of our product candidates and to provide the manufacturing, regulatory compliance, sales, marketing and distribution capabilities required for the success of our business.
We may enter into various kinds of license, collaborative research and development, manufacturing, and product marketing agreements to develop and commercialize our products. Any future milestone payments and cost reimbursements from these agreements could provide an important source of financing for our research and development programs, but there are risks associated with entering into collaboration arrangements.
There is a risk that we could become dependent upon one or more arrangements for product development or manufacturing or as a source of revenues from the sale of any products that may be developed by us alone or through one of the arrangements. A collaborative or partnering arrangement upon which we might depend might be terminated by our partner or they might determine not to actively pursue the development or commercialization of our products. A partner also may not be precluded from independently pursuing competing products and drug delivery approaches or technologies.
29
There is a risk that a partner might fail to perform its obligations under an agreement or may be slow in performing its obligations. In addition, a partner may experience financial difficulties at any time that could prevent it from having available funds to contribute to develop a product. If a partner fails to conduct its product development, manufacturing, commercialization, regulatory compliance, sales and marketing or distribution activities successfully and in a timely manner, or if it terminates or materially modifies its agreements with us, the development and commercialization of one or more product candidates could be delayed, curtailed or terminated because we may not have sufficient financial resources or capabilities to continue product development, manufacturing, and commercialization on our own.
Industry and other market data used in our SEC filings, including market data undertaken by us or our engaged consultants, may prove to be unrepresentative of current and future market conditions or future results.
Our SEC filings include statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties, and surveys and studies we commissioned, regarding the market potential for our product candidates. Although we believe that such information has been obtained from sources believed to be reliable, neither the sources of such data, nor we, can guarantee the accuracy or completeness of such information. While we believe these industry publications and third-party research, surveys and studies are reliable, we have not independently verified such data. With respect to the information from third party-consultants, the results of that study represent the independent consultants’ own methodologies, assumptions, research, analysis, projections, estimations, composition of respondent pool, presentation of data, and adjustments, each of which may ultimately prove to be incorrect, and cause actual results and market viability to differ materially from those presented in such report. Readers should not place undue reliance on this information.
Risks Pertaining to Our Common Stock
Ownership of our common stock will entail certain risks associated with the volatility of prices for our shares and the fact that we do not pay dividends on our common stock.
The price of our common stock may rise and fall rapidly.
The market price of our common stock like that of the shares of many biotechnology companies is highly volatile. The price of our common stock may rise or fall rapidly as a result of a number of factors, including:
| • | sales or potential sales of substantial amounts of our common stock, including from our largest shareholder BioTime; |
| • | results of preclinical testing or clinical trials of our product candidates or those of our competitors; |
| • | announcements about us or about our competitors, including clinical trial results, regulatory approvals, new product introductions and commercial results; |
| • | the cost of our development programs; |
| • | the success of competitive products or technologies; |
| • | litigation and other developments relating to our issued patents or patent applications or other proprietary rights or those of our competitors; |
| • | conditions in the pharmaceutical or biotechnology industries; |
| • | actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; |
| • | variations in our financial results or those of companies that are perceived to be similar to us, including the failure of our earnings to meet analysts’ expectations; and |
| • | general economic, industry and market conditions. |
Many of these factors are beyond our control. The stock markets in general, and the market for pharmaceutical and biotechnological companies in particular, have been experiencing extreme price and volume fluctuations which have affected the market price of the equity securities without regard to the operating performance of the issuing companies. Broad market fluctuations, as well as industry factors and general economic and political conditions, may adversely affect the market price of our common stock.
30
The price of our common stock, and the value of our assets, will be affected by changes in the value of the BioTime common shares that we own.
As of December 31, 2017, we held 3,481,085 BioTime common shares. The value of our common stock will reflect, in part, the value of the BioTime common shares that we hold. The value of the BioTime common shares we hold will vary with the price at which BioTime common shares trade in the public market. The market price of BioTime common shares will be impacted by a number of factors, including the results of BioTime’s operations.
The JOBS Act allows us to postpone the date by which we must comply with certain laws and regulations intended to protect investors and to reduce the amount of information we provide in our reports filed with the Commission, which could undermine investor confidence in our company and adversely affect the market price of our securities.
The JOBS Act is intended to reduce the regulatory burden on “emerging growth companies.” As defined in the JOBS Act, a public company whose initial public offering of common equity securities occurred after December 8, 2011 and whose annual gross revenues are less than $1.0 billion will, in general, qualify as an emerging growth company. Under this definition, we are an emerging growth company and could remain an emerging growth company until as late as December 31, 2019. The JOBS Act provides that, so long as we qualify as an emerging growth company, we will, among other things:
| • | be exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that our independent registered public accounting firm provide an attestation report on the effectiveness of our internal control over financial reporting; |
| • | be exempt from the “say on pay” provisions (requiring a non-binding stockholder vote to approve compensation of certain executive officers) and the “say on golden parachute” provisions (requiring a non-binding stockholder vote to approve golden parachute arrangements for certain executive officers in connection with mergers and certain other business combinations) of the Dodd-Frank Act and certain disclosure requirements of the Dodd-Frank Act relating to compensation of our named executive officers; |
| • | be permitted to omit the detailed compensation discussion and analysis from proxy statements and reports filed under the Securities Exchange Act of 1934 and instead provide a reduced level of disclosure concerning executive compensation; and |
| • | be exempt from any rules that may be adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report on the financial statements. |
We currently take advantage of many of the reduced regulatory and reporting requirements that are available to us so long as we qualify as an “emerging growth company”
Among other things, this means that our independent registered public accounting firm will not be required to provide an attestation report on the effectiveness of our internal control over financial reporting so long as we qualify as an emerging growth company, which may increase the risk that weaknesses or deficiencies in our internal control over financial reporting go undetected. Likewise, so long as we qualify as an emerging growth company, we may elect not to provide you with certain information, including certain financial information and certain information regarding compensation of our named executive officers, that we would otherwise have been required to provide in filings we make with the Commission, which may make it more difficult for investors and securities analysts to evaluate our company. As a result, investor confidence in our company and the market price of our securities may be materially and adversely affected.
Our stock price could decline due to the large number of outstanding shares of our common stock eligible for future sale.
Sales of substantial amounts of our common stock in the public market, or the perception that those sales could occur, could cause the market price of our common stock to decline. Sales of substantial amounts of common stock could also make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem appropriate.
We do not currently intend to pay dividends on any of our classes of securities and, consequently, your ability to achieve a return on your investment will depend on the appreciation in the price of our securities.
We have never declared or paid any cash dividends on any class of our securities. We currently intend to retain any future earnings to fund our future growth and do not expect to declare or pay any dividend on any class of our
31
securities in the foreseeable future. As a result, you may only realize a gain on your investment in our securities if the market price of our securities appreciates and you sell your securities at a price above your cost after accounting for any taxes. The price of our securities may not appreciate in value or ever exceed the price that you paid for our securities.
If securities analysts do not publish research or reports about our business or if they downgrade our stock, the price of our securities could decline.
The current trading market for our common stock relies in part on the research and reports that industry or financial analysts publish about us, our business, our markets and our competitors. We do not control these analysts. If securities analysts do not cover us, the lack of research coverage may adversely affect the market price of our common stock. Furthermore, if one or more of the analysts who do cover us downgrade our stock or if those analysts issue other unfavorable commentary about us or our business, our stock price would likely decline. If one or more of these analysts cease coverage of us or fails to regularly publish reports on us, we could lose visibility in the market and interest in our stock could decrease, which in turn could cause our stock price or trading volume to decline.
The implementation of a new FASB accounting standard could increase the risk that our future financial statements could be qualified by going concern uncertainty.
In August 2014, the FASB issued ASU No. 2014-15, “Presentation of Financial Statements-Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern.” ASU No. 2014-15 defines management’s responsibility to assess an entity’s ability to continue as a going concern, and to provide related footnote disclosures. ASU No. 2014-15 is effective for us for the year ended December 31, 2016, and all annual and interim periods thereafter. In connection with preparing financial statements for each annual and interim reporting period, ASU No. 2014-15 requires that an entity’s management evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued (or within one year after the date that the financial statements are available to be issued when applicable). As a result of the implementation of ASU No. 2014-15, we will be required to have more cash, cash equivalents, and liquid investments on hand on the date we issue or file our financial statements than had been the case during prior years in order to avoid a going concern qualification in our auditor’s report and in the footnotes to our financial statements. If our financial statements were to become subject to a going concern qualification or uncertainty or if we are unable to alleviate substantial doubt as part of our going concern assessment, or both, the market price of our common stock could decline.
BioTime and OncoCyte will also be impacted by ASU No. 2014-15 in much the same manner as us. If the financial statements of BioTime, or OncoCyte, or both, were to become subject to a going concern qualification or uncertainty, the market price of their common stock could decline, resulting in a loss or decline in value of the BioTime shares we own, the OncoCyte shares we own, or both, as available-for-sale equity securities at fair value.
You will likely experience dilution of your ownership interests because of the future issuance of additional shares of our common stock and our preferred stock.
In the future, we will likely need to issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our present shareholders. We are likely to issue additional shares of Common Stock or other securities in order to raise additional capital, or in connection with hiring or retaining employees or consultants, or in connection with future acquisitions of licenses to technology or rights to acquire products, in connection with future business acquisitions, or for other business purposes. The future issuance of any such additional shares of common stock or other securities may create downward pressure on the trading price of our Common Stock.
32
| Item 1B. | Unresolved Staff Comments |
None
| Item 2. | Properties |
We entered into a lease for our Fremont office and research facility on December 30, 2013. This facility consists of a building with approximately 44,000 square feet of space. The building will be used by us primarily as a laboratory and production facility that can be used to produce hES cells and related products under cGMP. We completed construction of certain tenant improvements for our use, which cost approximately $4.9 million, of which a maximum of $4.4 million was paid to us by the landlord. We believe that our current facilities are adequate to meet our operational needs for 2018.
The lease is for a term of 96 months commencing on October 1, 2014, with two available five-year options to extend the term, upon one year written notice from us. During the first 15 months of the lease term, from October 1, 2014 through December 31, 2015, we paid monthly base rent of $51,000 representing 22,000 square feet rather than 44,000 square feet. Beginning on January 1, 2016, base rent increased to $105,000 per month and will increase by approximately 3% annually on every October 1 thereafter. As of October 1, 2017, our base rent increased to $108,000 per month.
In addition to monthly base rent, we pay all real estate taxes, insurance and the cost of maintenance, repair and replacement of the leased premises. During the first 15 months of the lease term, we paid only 50% of the real estate taxes assessed on the premises. Beginning January 1, 2016, we pay 100% of the real estate taxes assessed on the premises.
| Item 3. | Legal Proceedings |
From time to time, we may be involved in routine litigation incidental to the conduct of our business. We are not presently involved in any material litigation or proceedings, and to our knowledge no such litigation or proceedings are contemplated.
| Item 4. | Mine Safety Disclosures |
Not applicable
33
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities |
Our Series A Shares have been traded on the NYSE American under the symbol “AST” since October 7, 2014. The following table sets forth the range of high and low closing prices for our Series A Shares during 2017 and 2016 as reported by the NYSE American (formerly NYSE MKT). We do not have any shares of our Series B common stock outstanding.
|
Quarter Ended
|
High
|
Low
|
||||
|
Period
|
|
|
|
|
|
|
|
2016
|
|
|
|
|
|
|
|
March 31, 2016
|
$
|
5.49
|
|
$
|
2.60
|
|
|
June 30, 2016
|
$
|
4.75
|
|
$
|
2.35
|
|
|
September 30, 2016
|
$
|
4.75
|
|
$
|
2.58
|
|
|
December 31, 2016
|
$
|
5.65
|
|
$
|
3.00
|
|
|
2017
|
|
|
|
|
|
|
|
March 31, 2017
|
$
|
5.00
|
|
$
|
3.07
|
|
|
June 30, 2017
|
$
|
4.30
|
|
$
|
2.83
|
|
|
September 30, 2017
|
$
|
3.75
|
|
$
|
2.95
|
|
|
December 31, 2017
|
$
|
3.74
|
|
$
|
1.95
|
|
As of March 1, 2018, we had 482 holders of record of our Series A Shares.
Dividend Policy
We have never paid cash dividends on our capital stock and we do not anticipate paying cash dividends in the foreseeable future but intend to retain our capital resources for reinvestment in our business. Any future determination to pay cash dividends will be at the discretion of our Board of Directors and will be dependent upon our financial condition, results of operations, capital requirements and other factors as our Board of Directors deems relevant.
Performance Measurement Comparison(1)
The following graph compares total stockholder returns of Asterias Biotherapeutics, Inc. for the forty-two months beginning July 18, 2014and ending December 31, 2017 to two indices: the NYSE Amex Market Value – U.S. Companies (Amex Market Value) and the NYSE Arca Biotechnology Index (NYSE Arca Biotechnology Index). The total return for our stock and for each index assumes the reinvestment of dividends, although we have never declared dividends on our capital stock, and is based on the returns of the component companies weighted according to their capitalizations as of the end of each quarterly period. The NYSE Amex Market Value tracks the aggregate price performance of equity securities of U.S. companies listed therein. The NYSE Arca Biotechnology Index represents biotechnology companies, trading on NYSE American under the Standard Industrial Classification (SIC) Code Nos. 283 (Drugs) and 382 (Laboratory Apparatus and Analytical, Optical) main categories (2834:Pharmaceutical Preparations; 2835: Diagnostic Substances; 2836: Biological Products; 3826: Laboratory Analytical Instruments; and 3829: Measuring & Controlling Devices). Our common stock trades on the NYSE American and is a component of the NYSE Amex Market Value – US Companies.
34
Asterias Biotherapeutics, Inc., the Amex Market Value and Amex Biotechnology Index(2)
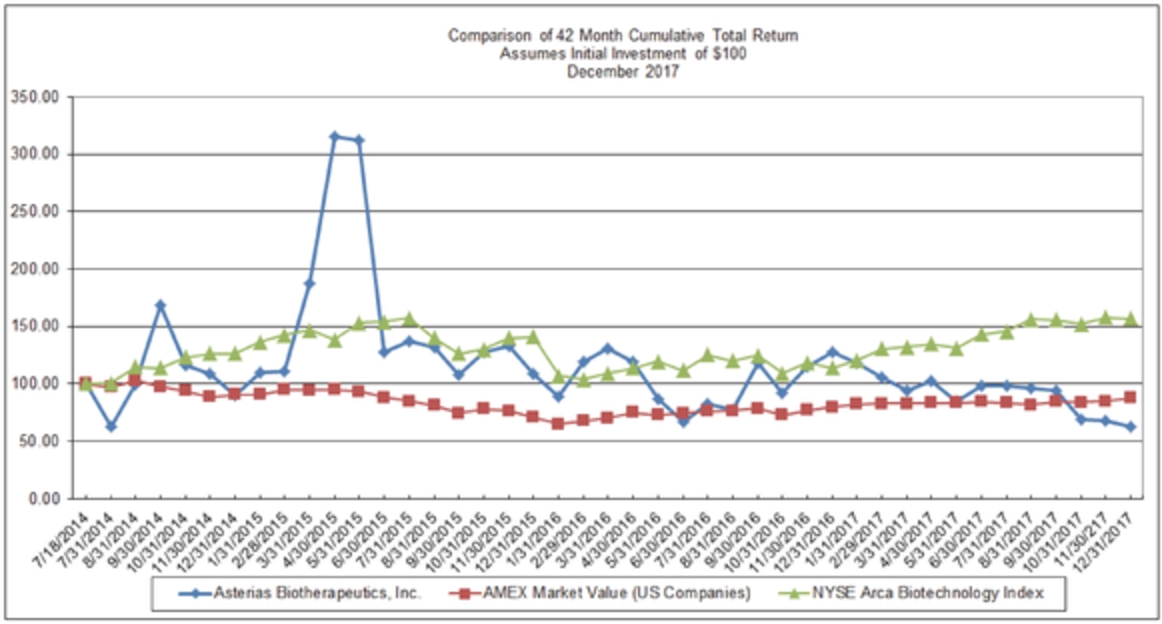
| (1) | This Section is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference in any filing of Asterias Biotherapeutics under the Securities Act of 1933, or the Securities Exchange Act of 1934, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing. |
| (2) | Shows the cumulative total return on investment assuming an investment of $100 in each of Asterias Biotherapeutics, Inc., the Amex Market Value and NYSE Arca Biotechnology Index on July 18, 2014. The cumulative total return on our common stock has been computed based on a price of $3.60 per share, the price at which our shares closed on July 18, 2014, which is the date our common stock was initially listed on the NYSE American. |
35
| Item 6. | Selected Financial Data |
The following Selected Financial Data is derived from and should be read in conjunction with “Item 7 – Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Item 8 – Financial Statements and Supplementary Data” included elsewhere in this Annual Report on Form 10-K.
|
|
Year Ended December 31,
|
||||||||||||||
|
Statement of Operations Data:
|
2017
|
2016
|
2015
|
2014
|
2013
|
||||||||||
|
(in thousands, except per share data)
|
|
|
|
|
|
||||||||||
|
REVENUES:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grant income
|
$
|
3,711
|
|
$
|
6,572
|
|
$
|
3,007
|
|
$
|
1,035
|
|
$
|
—
|
|
|
Sale of cell lines
|
|
—
|
|
|
—
|
|
|
40
|
|
|
—
|
|
|
—
|
|
|
License revenue
|
|
—
|
|
|
125
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Royalties from product sales
|
|
331
|
|
|
257
|
|
|
535
|
|
|
189
|
|
|
—
|
|
|
Total revenues
|
|
4,042
|
|
|
6,954
|
|
|
3,582
|
|
|
1,224
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales
|
|
(165
|
)
|
|
(128
|
)
|
|
(268
|
)
|
|
(95
|
)
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total gross profit
|
|
3,877
|
|
|
6,826
|
|
|
3,314
|
|
|
1,129
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
(26,640
|
)
|
|
(25,467
|
)
|
|
(17,321
|
)
|
|
(13,310
|
)
|
|
(4,319
|
)
|
|
Acquired in-process research and development(1)
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(17,459
|
)
|
|
General and administrative
|
|
(10,488
|
)
|
|
(15,482
|
)
|
|
(7,901
|
)
|
|
(5,280
|
)
|
|
(3,883
|
)
|
|
Total operating expenses
|
|
(37,128
|
)
|
|
(40,949
|
)
|
|
(25,222
|
)
|
|
(18,590
|
)
|
|
(25,661
|
)
|
|
Loss from operations
|
|
(33,251
|
)
|
|
(34,123
|
)
|
|
(21,908
|
)
|
|
(17,461
|
)
|
|
(25,661
|
)
|
|
OTHER INCOME/(EXPENSES):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gain/(loss) from change in fair value of warrant liability
|
|
5,908
|
|
|
(3,108
|
)
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Interest expense, net
|
|
(465
|
)
|
|
(546
|
)
|
|
(341
|
)
|
|
(10
|
)
|
|
(2
|
)
|
|
Other income (expense), net
|
|
(564
|
)
|
|
(37
|
)
|
|
(6
|
)
|
|
(2
|
)
|
|
2
|
|
|
Total other income (expenses), net
|
|
4,879
|
|
|
(3,691
|
)
|
|
(347
|
)
|
|
(12
|
)
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LOSS BEFORE INCOME TAX BENEFIT
|
|
(28,372
|
)
|
|
(37,814
|
)
|
|
(22,255
|
)
|
|
(17,473
|
)
|
|
(25,661
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred income tax benefit
|
|
—
|
|
|
2,325
|
|
|
7,252
|
|
|
7,376
|
|
|
3,281
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET LOSS
|
$
|
(28,372
|
)
|
$
|
(35,489
|
)
|
$
|
(15,003
|
)
|
$
|
(10,097
|
)
|
$
|
(22,380
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BASIC AND DILUTED NET LOSS PER SHARE
|
$
|
(0.56
|
)
|
$
|
(0.83
|
)
|
$
|
(0.42
|
)
|
$
|
(0.33
|
)
|
$
|
(2.90
|
)
|
|
WEIGHTED AVERAGE SHARES OUTSTANDING: BASIC AND DILUTED
|
|
50,271
|
|
|
42,934
|
|
|
35,443
|
|
|
30,720
|
|
|
7,726
|
|
| (1) | Represents the value of research and development projects acquired by Asterias from Geron under the Asset Contribution Agreement. |
|
|
As of December 31,
|
||||||||||||||
|
Balance Sheet Data(2):
|
2017
|
2016
|
2015
|
2014
|
2013
|
||||||||||
|
(in thousands)
|
|
|
|
|
|
||||||||||
|
Cash and cash equivalents
|
$
|
13,266
|
|
$
|
19,800
|
|
$
|
11,183
|
|
$
|
3,076
|
|
$
|
2,171
|
|
|
Total assets
|
|
43,092
|
|
|
61,010
|
|
|
57,234
|
|
|
44,114
|
|
|
80,354
|
|
|
Total long-term liabilities
|
|
6,028
|
|
|
12,447
|
|
|
7,155
|
|
|
4,986
|
|
|
8,278
|
|
|
Total liabilities
|
|
9,549
|
|
|
18,982
|
|
|
12,135
|
|
|
11,483
|
|
|
26,573
|
|
|
Accumulated deficit
|
|
(112,100
|
)
|
|
(83,728
|
)
|
|
(48,239
|
)
|
|
(33,236
|
)
|
|
(23,139
|
)
|
|
Total stockholders’ equity
|
|
33,543
|
|
|
42,028
|
|
|
45,099
|
|
|
32,631
|
|
|
53,780
|
|
| (2) | Balance Sheet Data as of December 31, 2015, 2014, and 2013 have been retroactively adjusted for the early adoption of ASU 2015-17, Income Taxes: Balance Sheet Classification of Deferred Taxes (see Note 10 to our financial statements included in Item 8). |
36
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations is intended to provide information necessary to understand our audited financial statements for the years ended December 31, 2017, 2016 and 2015, and highlight certain other information which, in the opinion of management, will enhance a reader’s understanding of our financial condition, changes in financial condition and results of operations. In particular, the discussion is intended to provide an analysis of significant trends and material changes in our financial position and the operating results of our business during the year ended December 31, 2017 as compared to 2016 and during the year ended December 31, 2015. This discussion should be read in conjunction with our financial statements for the three year period ended December 31, 2017 and related notes included elsewhere in this Annual Report on Form 10-K. These historical financial statements may not be indicative of our future performance. This Management’s Discussion and Analysis of Financial Condition and Results of Operations contains a number of forward - looking statements, all of which are based on our current expectations and could be affected by the uncertainties and risks described throughout this filing, particularly in “Item 1A. Risk Factors.”
Critical Accounting Policies and Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts in our financial statements and related notes. Our significant accounting policies are described in Note 2 to our financial statements included in Item 8 of this Annual Report. We have identified below our critical accounting policies and estimates that we believe require the greatest amount of judgment. On an ongoing basis, we evaluate estimates which are subject to significant judgment, including those related to the going concern assessment of our financial statements, useful lives associated with long-lived assets, including an evaluation of asset impairment, loss contingencies, deferred income taxes and tax reserves, including valuation allowances related to deferred income taxes, and assumptions used to value stock-based awards, liability or other equity instruments. Actual results could differ materially from those estimates. On an ongoing basis, we evaluate our estimates compared to historical experience and trends which form the basis for making judgments about the carrying value of assets and liabilities. To the extent that there are material differences between our estimates and our actual results, our future financial statement presentation, financial condition, results of operations and cash flows will be affected.
We believe the assumptions and estimates associated with the following have the greatest potential impact on our financial statements.
Going concern assessment – With the implementation of FASB’s new standard on going concern, ASU No. 2014-15, beginning with the year ended December 31, 2016 and all annual and interim periods thereafter, we will assess going concern uncertainty for our financial statements to determine if we have sufficient cash and cash equivalents on hand and working capital to operate for a period of at least one year from the date the financial statements are issued or are available to be issued, which is referred to as the “look - forward period” as defined by ASU No. 2014-15. As part of this assessment, based on conditions that are known and reasonably knowable to us, we will consider various scenarios, forecasts, projections, and estimates, and we will make certain key assumptions, including the timing and nature of projected cash expenditures or programs, and our ability to delay or curtail those expenditures or programs, among other factors, if necessary, within the look-forward period in accordance with ASU No. 2014-15.
Revenue recognition – We comply with ASC 605-10 and record revenue when persuasive evidence of an arrangement exists, delivery has occurred, or services have been rendered, the price is fixed or determinable, and collectability is reasonably assured. Grant income is recognized as revenue when the related research and development expenses are incurred. Royalty revenues consist of royalty payments on sales of products under license agreements. We recognize revenue in the quarter in which the royalty reports are received rather than the quarter in which the sales took place. When we are entitled to receive up - front nonrefundable licensing or similar fees pursuant to agreements under which we have no continuing performance obligations, the fees are recognized as revenues when collection is reasonably assured. When we receive up-front nonrefundable licensing or similar fees pursuant to agreements under which we do have continuing performance obligations, the fees are deferred and amortized ratably over the performance period. If the performance period cannot be reasonably estimated, we amortize nonrefundable fees over the life of the contract until such time that the performance period can be more reasonably estimated.
37
Milestone payments, if any, related to scientific or technical achievements, subject to substantial uncertainty are recognized in income when the milestone is accomplished if (a) substantive effort was required to achieve the milestone, (b) the amount of the milestone payment appears reasonably commensurate with the effort expended, and (c) collection of the payment is reasonably assured.
Available-for-sale securities, at fair value – Marketable equity and debt securities not classified as held-to-maturity are classified as available-for-sale. Available-for-sale securities are carried at fair value, with the unrealized gains and losses, net of tax, reported in other comprehensive income or loss. Realized gains and losses and declines in value judged to be other-than-temporary related to equity securities, are included in other income/(expense), net.
We account for the BioTime and OncoCyte shares we hold as available-for-sale equity securities in accordance with ASC 320-10-25, Investments-Debt and Equity Securities, as the shares have a readily determinable fair value quoted on the NYSE American and are held principally for future working capital purposes, as necessary. These shares are measured at fair value and reported as current assets on the balance sheet based on the closing trading price of the security as of the date being presented (see Note 4 to our financial statements included in Item 8). Unrealized holding gains, and losses are excluded from the statements of operations and reported in equity as part of other accumulated comprehensive income or loss until realized.
Realized gains and losses on sale of BioTime shares prior to May 13, 2016, were reclassified out of other comprehensive income or loss and included in equity, as an increase or decrease in additional paid - in capital consistent with, and pursuant to, ASC 805-50, Transactions Between Entities Under Common Control. Beginning on May 13, 2016, due to the deconsolidation of our financial statements from BioTime and loss of control experienced by BioTime over us, as discussed in Note 1 to our financial statements included in Item 8, realized gains and losses, and declines in value judged to be other-than-temporary related to equity securities, are included in other income/(expense), net. For OncoCyte shares we held, realized gains and losses, and declines in value judged to be other-than-temporary related to equity securities, are included in other income/(expense), net.
We review various factors in determining whether we should recognize an other-than-temporary impairment charge for our available-for-sale securities, including our intent and ability to hold the investment for a period of time sufficient for any anticipated recovery in market value, and the length of time and extent to which the fair value has been less than its cost basis. Based on consideration of these factors, as of December 31, 2017 and 2016, no other -than-temporary impairment was recognized.
Long-lived intangible assets – Long-lived intangible assets, consisting primarily of acquired patents, patent applications, and licenses to use certain patents are stated at acquired cost, less accumulated amortization. Amortization expense is computed using the straight-line method over the estimated useful lives of the assets, generally over 10 years.
Impairment of long-lived assets – Long-lived assets, including long - lived intangible assets, will be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be fully recoverable. If an impairment indicator is present, Asterias evaluates recoverability by a comparison of the carrying amount of the assets to future undiscounted net cash flows expected to be generated by the assets. If the assets are impaired, an impairment charge will be recognized and measured by the amount by which the carrying amount exceeds the estimated fair value of the assets.
Accounting for warrants – We determine the accounting classification of warrants that are issued, as either liability or equity, by first assessing whether the warrants meet liability classification in accordance with ASC 480-10, Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity, and then in accordance with ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock. Under ASC 480, warrants are considered liability classified if the warrants are mandatorily redeemable, obligate the issuer to settle the warrants or the underlying shares by paying cash or other assets, or warrants that must or may require settlement by issuing variable number of shares. If warrants do not meet liability classification under ASC 480-10, we assess the requirements under ASC 815-40, which states that contracts that require or may require the issuer to settle the contract for cash are liabilities recorded at fair value, irrespective of the likelihood of the transaction occurring that triggers the net cash settlement feature. If the warrants do not require liability classification under ASC 815-40, in order to conclude equity classification, we assess whether the warrants are indexed to our common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable GAAP. After all relevant assessments are made, we conclude whether the warrants are classified as liability or equity. Liability classified warrants are required to be accounted for at fair value both on the date of
38
issuance and on subsequent accounting period ending dates, with all changes in fair value after the issuance date recorded in the statements of operations as a gain or loss. Equity classified warrants are accounted for at fair value on the issuance date with no changes in fair value recognized subsequent to the issuance date.
Historically, we have issued warrants that are classified as equity and as a liability (see Note 6 to our financial statements included in Item 8).
Research and development – Research and development expenses consist of costs incurred for company- sponsored, collaborative and contracted research and development activities. These costs include direct and research -related overhead expenses including compensation and related benefits, stock-based compensation, consulting fees, research and laboratory fees, rent of research facilities, amortization of intangible assets, patent applications and prosecutions, license fees paid to third parties to acquire patents or licenses to use patents and other technology. We expense research and development costs as incurred. Research and development expenses incurred and reimbursed under grants approximate the grant income recognized in the statements of operations.
Income taxes – As of October 1, 2013, we filed our own U.S. federal tax returns. Operations prior to that period were included in BioTime’s consolidated U.S. federal tax return. For California purposes our activity through May 12, 2016 was included in BioTime’s combined tax return. Activity from May 13, 2016 on will be included in Asterias’ separate California income tax return filing due to the deconsolidation of the Company from BioTime as of that date. We account for income taxes in accordance with ASC 740, Income Taxes, which prescribes the use of the asset and liability method, whereby deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect. Valuation allowances are established when necessary to reduce deferred tax assets when it is more likely than not that a portion or all of the deferred tax assets will not be realized. The guidance also prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not sustainable upon examination by taxing authorities. For federal purposes we are no longer subject to tax examination for years before 2013. For California purposes we are subject to income tax examinations by tax authorities for all years since inception. Although the statute is closed for purposes of assessing additional income and tax in those years, the taxing authorities may still make adjustments to the net operating loss and credit carryforwards used in open years. Therefore, the statute should be considered open as it relates to the net operating loss and credit carryforwards. We recognize accrued interest and penalties related to unrecognized tax benefits as income tax expense. No amounts were accrued for the payment of interest and penalties as of December 31, 2017 and 2016.
Stock-based compensation – We account for share-based payments in accordance with ASC 718, Compensation – Stock Compensation, which require the measurement and recognition of compensation expense for all share-based payment awards made to directors and employees, including employee stock options, based on estimated fair values. Consistent with those guidelines, we utilize the Black-Scholes-Merton option pricing model. Our determination of fair value of share-based payment awards on the date of grant using that option-pricing model is affected by our stock price as well as by assumptions regarding a number of complex and subjective variables. These variables include, but are not limited to, our expected stock price volatility over the term of the awards; the expected term of options granted; and a risk-free rate based on the U.S. Treasury rates in effect during the corresponding expected term of the grant. The expected term is derived from a combination of our own historical experience, to the extent available, and using the simplified method under SEC Staff Accounting Bulletin Topic 14, as applicable. We recognize stock-based compensation on a straight-line basis over the requisite service period. Through January 1, 2017 we recorded stock-based compensation expense net of estimated forfeitures. Upon the adoption of ASU 2016-09, we account for forfeitures as they occur (See Accounting changes in Note 2).
We also, at times, issue restricted stock or restricted stock units (“RSUs”) to our executive officers, employees, and members of our Board of Directors (the Board), which are restricted and unvested common shares issued or shares issuable as RSUs vest. Restricted stock and RSU compensation expense is recognized on a straight-line basis over the requisite service period of generally four years, based on the grant-date fair value of the stock. Restricted stock is considered legally issued and outstanding on the grant date, while RSUs are not until RSUs vest. Once the RSUs are vested, equivalent common shares will be issued or issuable to the grantee and therefore the RSUs are not included in total common shares issued and outstanding until vested.
We account for share-based payments to non-employees in accordance with ASC 505-50, Equity-Based Payments to Non-Employees (“ASC 505-50”). Stock option awards issued to non-employees, principally consultants
39
and employees of BioTime or employees of BioTime subsidiaries who perform services for us, are accounted for at fair value using the Black-Scholes-Merton option pricing model. We believe that the fair value of the stock options is more reliably measured than the fair value of services received. We record compensation expense based on the then-current fair values of the stock options at each financial reporting date. Compensation expense recorded during the service period is adjusted in subsequent periods for changes in the fair value of the stock options until the earlier of the date at which the non-employee’s performance is complete or a performance commitment is reached, which is generally when the stock option award vests. Compensation expense for non-employee grants is recorded on a straight-line basis in the statements of operations.
Fair value of financial instruments – ASC 820, Fair Value Measurements, clarifies that fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability.
ASC 820 requires that the valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. ASC 820 establishes a three tier value hierarchy, which prioritizes inputs that may be used to measure fair value as follows:
| • | Level 1 – Observable inputs that reflect quoted prices for identical assets or liabilities in active markets. |
| • | Level 2 – Observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| • | Level 3 – Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The carrying amounts of current assets and current liabilities approximate their fair value because of the relatively short period until they mature or are required to be settled, except for money market funds and the investment in BioTime and OncoCyte shares, which are carried at fair value based on Level 1 inputs, and the warrant liability which is carried at fair value based on Level 3 inputs (see Note 6 to our financial statements included in Item 8 for a discussion on the valuation of warrants classified as liabilities).
Results of Operations
Comparison of Years Ended December 31, 2017, 2016 and 2015
For the years ended December 31, 2017, 2016 and 2015 we recorded net losses of $28.4 million, $35.5 million, and $15.0 million, respectively.
Revenues
The following table shows certain information about our revenues for the years ended December 31, 2017, 2016 and 2015 (in thousands):
|
|
Year Ended December 31,
|
||||||||
|
|
2017
|
2016
|
2015
|
||||||
|
Grant income
|
$
|
3,711
|
|
$
|
6,572
|
|
$
|
3,007
|
|
|
Sale of cell lines
|
|
—
|
|
|
—
|
|
|
40
|
|
|
License revenue
|
|
—
|
|
|
125
|
|
|
—
|
|
|
Royalties from product sales
|
|
331
|
|
|
257
|
|
|
535
|
|
|
Total revenues
|
|
4,042
|
|
|
6,954
|
|
|
3,582
|
|
|
Cost of sales
|
|
(165
|
)
|
|
(128
|
)
|
|
(268
|
)
|
|
Total gross profit
|
$
|
3,877
|
|
$
|
6,826
|
|
$
|
3,314
|
|
Grant income in 2017 is entirely from the NGA with CIRM which awarded us a $14.3 million grant for clinical development of our product, AST-OPC1. Asterias received $1.5 million, $6.2, million and $5.6 million in cash during fiscal years 2017, 2016, and 2015, respectively. Revenues pursuant to the NGA recognized during the fiscal years ended December 31, 2017, 2016 and 2015 were $3.7 million, $6.6 million, and $3.0 million, respectively. Although the cash payments from CIRM were dependent on achieving certain milestones pursuant to the contract with CIRM, we recognize grant income as related research expenses are incurred.
40
Our royalty revenues from product sales is entirely from non-exclusive license agreements with Ajinomoto, GE Healthcare, Stem Cell Technologies, Inc., Corning Life Science, Life Technologies, and Millipore which we assumed as part of the consideration received from Geron under the Asset Contribution Agreement. Our other licensing revenue in 2016 comes from the licensing of certain intellectual property that is unrelated to our core development programs to third parties.
Operating Expenses
The following table shows our operating expenses for the years ended December 31, 2017, 2016 and 2015 (in thousands):
|
|
Year Ended December 31,
|
||||||||
|
|
2017
|
2016
|
2015
|
||||||
|
Research and development expenses
|
$
|
26,640
|
|
$
|
25,467
|
|
$
|
17,321
|
|
|
General and administrative expenses
|
|
10,488
|
|
|
15,482
|
|
|
7,901
|
|
Research and development expenses – Research and development expenses increased by approximately $1.2 million to $26.6 million for the year ended December 31, 2017 compared to $25.5 million for the year ended December 31, 2016. The increase in research and development expenses for the fiscal year ended December 31, 2017 compared to the same period in 2016 is primarily attributed a $2.1 million increase in spending related to our AST -OPC1 clinical trial and AST-OPC1-related manufacturing planning expenses offset by decreased patent related legal spending of $0.6 million.
Research and development expenses increased by approximately $8.2 million to $25.5 million for the year ended December 31, 2016 compared to $17.3 million for the year ended December 31, 2015. The increase in research and development expenses for the fiscal year ended December 31, 2016 compared to the same period in 2015 is primarily attributed to the following: an increase of $4.4 million in salaries, bonuses and stock-based compensation expense due to an increase in headcount related to hiring personnel to support our AST-OPC1 program; a $0.9 million increase in scientific and related consulting services; a $0.8 million increase in clinical laboratory supplies for our laboratory and production facility in Fremont; a $0.7 million increase in outside service fees related to analytical testing; a $0.6 million increase in vendor fees related to process development activities for our AST-VAC1 program; a $0.5 million increase in clinical trial costs associated with our Phase 1/2a study for the AST-OPC1 program and a $0.5 million increase in depreciation expense.
General and administrative expenses – General and administrative expenses decreased by approximately $5.0 million to $10.5 million for the year ended December 31, 2017 compared to $15.5 million for the year ended December 31, 2016. The decrease in general and administrative expenses in 2017 is primarily attributed to a decrease of $3.8 million in shareholder warrant distribution expense related to revaluing warrants outstanding. Additionally, we had a decrease of $0.3 million in legal related expenses for the fiscal year ended December 31, 2017 offset by an increase in fourth quarter of 2017 of $0.4 million in connection with the reduction in staffing, consisting of severance and other employee termination benefits.
General and administrative expenses increased by approximately $7.6 million to $15.5 million for the year ended December 31, 2016 compared to $7.9 million for the year ended December 31, 2015. The increase in general and administrative expenses in 2016 is primarily attributed to the following: an increase of $5.3 in shareholder warrant distribution expense; an increase of $3.3 million in salaries, bonuses, stock-based compensation, severance and related expenses; and an increase of $0.2 million in depreciation expense. These increases were in part offset by a decrease of $1.5 million in general and administrative expenses related to consulting services and tax payments.
Other income and expenses, net
Other income and expenses, net, is mainly comprised of the non-cash gain (loss) pertaining to our liability classified warrants issued in May 2016, as discussed in Note 6 to our financial statements. These warrants are recorded at fair value with all changes in fair value included in our statements of operations. Increases in fair value are recognized as noncash losses and decreases are recognized as noncash gains included in other income and expenses, net. For the year ended December 31, 2017 we generated other income of $4.9 million as the fair value of these warrants decreased. For the year ended December 31, 2016 and 2015, we generated other expenses, net, of $3.7 million and $0.3 million, respectively, the increase principally due to the $3.1 million loss recognized from these warrants in 2016.
41
Income Taxes
We recorded a deferred income tax benefit of approximately $2.3 million for the year ended December 31, 2016 related to a federal tax benefit. No state tax provision or benefit was recorded for year ended December 31, 2016. A deferred income tax benefit of $7.3 million was recorded for the year ended December 31, 2015, of which $7.4 million was related to federal taxes and $0.1 million was related to state taxes.
As of December 31, 2015, we did not have any valuation allowance on our federal deferred tax assets since our deferred tax liabilities exceeded our deferred tax assets as of that date. Our deferred tax liabilities are primarily related to our acquisition of certain intellectual property and available for sale securities held in BioTime and OncoCyte common stock and are a source of taxable income for our deferred tax assets. During the year ended December 31, 2016, as we continued to generate net operating losses and our deferred tax assets exceeded our deferred tax liabilities, we established a full valuation allowance for federal deferred tax assets as of December 31, 2016. For the California deferred tax assets, we established a valuation allowance as of December 31, 2016 and 2015. Due to the full valuation allowance on our federal and California deferred tax assets as of December 31, 2017, we do not expect to record an income tax provision or benefit as long as our deferred tax assets continue to exceed our deferred tax liabilities.
Liquidity and Capital Resources
The following table shows our liquidity and capital resources for the years ended December 31, 2017 and 2016 (in thousands):
|
|
December 31,
|
|||||
|
|
2017
|
2016
|
||||
|
Cash and cash equivalents
|
$
|
13,266
|
|
$
|
19,800
|
|
|
Available-for-sale securities, at fair value
|
|
8,329
|
|
|
15,269
|
|
At December 31, 2017, we had $21.6 million of cash, cash equivalents and available-for-sale securities compared to $35.1 million at December 31, 2016. The decrease in cash, cash equivalents was primarily due the cash used to finance operations of $24.4 million which was partially offset by cash received from financing of approximately $17.2 million, which was primarily related to the sale of Asterias’ stock. The decrease in our available -for-sales securities of $6.9 million was largely related to the decline in the fair value of our BioTime and OncoCyte shares, combined with the sale of approximately 10% of our BioTime shares and 6% of our OncoCyte shares.
We may raise capital from time to time through the sale of our Series A common shares or other securities, or the sale of our BioTime or OncoCyte common shares. We may sell our Series A common shares or other securities in public offerings registered under the Securities Act of 1933, as amended (the “Securities Act”), by use of the at -the-market issuance sales agreement (“ATM” or “Sales Agreement”) in place with B. Riley FBR, Inc. (the “Sales Agent”), or in private placements to select investors. We may sell some or all of our BioTime common shares and OncoCyte common shares by any method permitted by law, including in privately negotiated transactions. The prices at which we may issue and sell our Series A common shares or other securities and our BioTime common shares in the future are not presently determinable and will depend upon many factors, including prevailing prices for those securities in the public market.
As of December 31, 2017, we have the following outstanding warrants to purchase shares of our Series A common stock:
|
|
Outstanding Warrants:
|
||||||||||
|
|
Quantity
|
Exercise Price
|
Expiration Date
|
||||||||
|
Warrants Group (Not publicly traded)
|
|
2,813,159
|
|
|
4.37
|
|
May 13, 2021
|
||||
There can be no assurance that any of these warrants will be exercised but to the extent warrants are exercised we will receive proceeds from the exercise of such warrants.
We have been awarded $14.3 million under the NGA from CIRM to help fund our clinical development of AST-OPC1. As of December 31, 2017, we have received the entire $14.3 million.
Pursuant to the CRUK Agreement, CRUK has agreed to fund Phase 1 clinical development of our AST-VAC2 product candidate. We have completed process development and manufacturing scale-up of the AST-VAC2
42
manufacturing process and transferred the resulting cGMP-compatible process to CRUK. CRUK will, at its own cost, manufacture clinical grade AST-VAC2 and will carry out the Phase 1 clinical trial of AST-VAC2 in cancer patients both resected early-stage and advanced forms of lung cancer.
We plan to use the cash we have available for general corporate purposes, including to fund our ongoing clinical programs, to develop certain of our product candidates and technology, to acquire new stem cell products and technology through licenses or similar agreements from other companies, and to defray overhead expenses and to pay general and administrative expenses. We expect to continue to incur operating losses and negative cash flows.
At December 31, 2017, we had an accumulated deficit of $112.1 million, working capital of $19.2 million and stockholders’ equity of $33.5 million. We have evaluated our projected cash flows and believe that our cash and cash equivalents of $13.3 million and our available-for-sale securities of $8.3 million as of December 31, 2017 will be sufficient to fund our operations through at least twelve months from the issuance date of the financial statements included in Item 8, or at least through March 15, 2019. If we are unable to obtain additional grant funding to support future trials, if the value of our available-for-sale securities were to decrease, or if we are unable to obtain future adequate financing for our clinical trials, then we may be required to delay, postpone, or cancel our clinical trials or limit the number of clinical trial sites, or otherwise reduce or curtail our operations. Future financings, if necessary, may not be available to us at acceptable terms, or if at all. Sales of additional equity securities would result in the dilution of interests of current shareholders.
Cash Flows
Cash used in operations
During the year ended December 31, 2017, our total research and development expenses were $26.6 million and our general and administrative expenses were $10.5 million. Net loss for the year ended December 31, 2017 amounted to $28.4 million. Our sources of cash from operations during 2017 primarily consisted of $0.3 million from royalty revenues on product sales by licensees, and grant payments of $1.5 million from CIRM. As of December 31, 2017 and 2016, we had a working capital of $19.2 million and $30.9 million and an accumulated deficit of $112.1 million and $83.7 million, respectively.
Net cash used in operating activities during the year ended December 31, 2017 amounted to $24.4 million. The difference between the net loss and net cash used in operating activities during the year ended December 31, 2017 was primarily attributable to the following noncash items: stock-based compensation paid to employees of $4.4 million, amortization of intangible assets of $2.7 million, costs associated with the extension of certain warrants of $2.0 million, depreciation expense of $1.1 million, and $1.2 million related to issuance of common stock for services, which was offset by noncash gain on warrant liability for mark to market adjustment of $5.9 million.
Net cash used in operating activities during the year ended December 31, 2016 amounted to $19.0 million. The difference between the net loss and net cash used in operating activities during the year ended December 31, 2016 was primarily attributable to the following noncash items: the distribution of warrants to our shareholders of $5.3 million, stock-based compensation paid to employees of $4.8 million, noncash loss on warrant liability for mark to market adjustment of $3.1 million, amortization of intangible assets of $2.7 million, depreciation expense of $1.2 million, and $0.9 million related to issuance of common stock for services, offset by the deferred tax benefit of $2.3 million.
Net cash used in operating activities during the year ended December 31, 2015 amounted to $12.4 million. The difference between the net loss and net cash used in operating activities during the year ended December 31, 2015 was primarily attributable to the following noncash items: stock-based compensation paid to employees of $3.6 million, amortization of intangible assets of $2.7 million, deferred grant income of $2.5 million, an increase in accrued expenses of $0.6 million, depreciation expense of $0.6 million, and shares issued to a vendor for services of $0.5 million. The difference was primarily offset by our deferred income tax benefit of $7.3 million and decreases in prepaid expenses and other current assets of $0.7 million.
Investing and financing activities
Net cash provided by investing activities during the year ended December 31, 2017 amounted to $0.7 million. In 2017, we received $1.0 million for sales of portion of our available-for-sale securities. These proceeds were partially offset by $0.3 million in payments made to acquire property, plant and equipment.
43
Net cash used in investing activities during the year ended December 31, 2016 amounted to $0.9 million. In 2016, we paid $894,000 for property, plant and equipment including tenant improvements and other fixed assets.
Net cash used in investing activities during the year ended December 31, 2015 amounted to $4.6 million. During the year ended December 31, 2015, we used $4.3 million in cash to pay for construction in progress for our Fremont facility and $0.3 million to purchase equipment.
Net cash provided by financing activities during the year ended December 31, 2017 amounted to $17.2 million and primarily resulted from the following:
| • | In October 2017, we completed the sale of 4,000,000 shares of our common stock through a public offering at $2.60 per share, or net proceeds of $9.9 million. |
| • | In 2017, we raised $7.8 million in net proceeds under our ATM from the sale of 2,005,784 shares of our common stock at a weighted average price of $3.99 per share. |
Net cash provided by financing activities during the year ended December 31, 2016 amounted to $28.5 million and primarily resulted from the following:
| • | In May 2016, we completed the sale and the underwriters’ exercise of the overallotment for 5,889,480 shares of our common stock and warrants to purchase 2,959,559 shares of our common stock at $3.40 per share, or net proceeds of $18.2 million. |
| • | In 2016, we raised approximately $7.6 million in net proceeds under our ATM from the sale of 1,811,522 shares of our common stock at a weighted average price of $4.40 per share. |
| • | During 2016, we received $567,000 from our landlord on reimbursable construction in progress financed by the landlord. In addition, we received $2.0 million from the exercise of stock options, offset by payments made for the landlord liability and capital lease obligations of $0.4 million. |
| • | In December 2016, we raised approximately $651,000 from the exercise of warrants to purchase our common stock. |
Net cash provided by financing activities during the year ended December 31, 2015 amounted to $25.1 million and primarily resulted from the following:
| • | In May 2015, we received $11.7 million from the exercise of 5,000,000 warrants to purchase our common stock. |
| • | In February 2015, we raised approximately $5.0 million in aggregate net proceeds from the sale of 1,410,255 shares of our common stock at a price of $3.90 per share through an underwritten public offering and a private placement. |
| • | During 2015, we raised approximately $4.7 million in net proceeds under our ATM from the sale of 685,465 shares of our common stock at a weighted average price of $7.01 per share. |
| • | We also received $3.8 million from our landlord on reimbursable construction in progress. |
44
Contractual Obligations
As of December 31, 2017, our contractual obligations for the next five years and thereafter were as follows (in thousands):
|
|
Payments Due by Period
|
||||||||||||||
|
Contractual Obligations(1)
|
Total
|
Less Than
1 Year |
1-3 Years
|
4-5 Years
|
After
5 Years |
||||||||||
|
Operating leases(2)
|
$
|
6,571
|
|
$
|
1,308
|
|
$
|
2,735
|
|
$
|
2,528
|
|
$
|
—
|
|
|
Capital lease(3)
|
|
21
|
|
|
7
|
|
|
14
|
|
|
—
|
|
|
—
|
|
|
Total Contractual Obligations
|
$
|
6,592
|
|
$
|
1,315
|
|
$
|
2,749
|
|
$
|
2,528
|
|
$
|
—
|
|
| (1) | This table does not include payments to key employees that could arise if they were involuntarily terminated or if their employment terminated following a change in control. |
| (2) | Includes the lease of our principal office and laboratory facilities in Fremont, California, including the lease liability (see Note 8 to our financial statements included in Item 8). |
| (3) | Includes one capital lease for phone equipment. |
Off-Balance Sheet Arrangements
As of December 31, 2017 and 2016, we did not have any off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of SEC Regulation S-K.
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk |
Foreign Currency Exchange Risk
We are not presently exposed in a significant degree to foreign exchange currency risks because we are not otherwise conducting international business at this time, and we do not engage in foreign currency hedging activities. If we engage in international transactions, we will need to translate foreign currencies into U.S. dollars for reporting purposes, and currency fluctuations could have a greater impact on our financial results.
Credit Risk
We place some of our cash in U.S. banks and invest most of our cash in money market funds. Deposits with banks may temporarily exceed the amount of insurance provided on such deposits. We will monitor the cash balances in the accounts and adjust the cash balances as appropriate, but if the amount of a deposit at any time exceeds the federally insured amount at a bank, the uninsured portion of the deposit could be lost, in whole or in part, if the bank were to fail. Our investments in money market funds are not insured or guaranteed by the United States government or any of its agencies.
Interest Rate Risk
We invest most of our cash in money market funds. The primary objective of our investments will be to preserve principal and liquidity while earning a return on our invested capital, without incurring significant risks. Our future investment income is not guaranteed and may fall short of expectations due to changes in prevailing interest rates, or we may suffer losses in principal if the net asset value of a money market fund falls below $1 per share.
Market Risk
At December 31, 2017 we held 3,481,085 shares of BioTime common stock and 181,756 shares of OncoCyte common stock. These shares are classified as available-for-sale securities and carried at fair value. As a result, the carrying values are subject to changes in the stock price of BioTime and OncoCyte shares. BioTime common stock trades on the NYSE American under the ticker “BTX” and OncoCyte common stock trades on the NYSE American under the ticker “OCX”. As of December 31, 2017, the 52-week high/low stock price per share range for BioTime and OncoCyte shares were $3.80 - $2.10 and $7.85 - $3.60, respectively.
45
Report of Independent Registered Public Accounting Firm
Stockholders and Board of Directors
Asterias Biotherapeutics, Inc.
Fremont, California
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Asterias Biotherapeutics, Inc. (the “Company”) as of December 31, 2017 and 2016, the related statements of operations, comprehensive loss, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2017, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2017, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ OUM & CO. LLP
San Francisco, California
March 15, 2018
We have served as the Company’s auditor since 2014.
46
| Item 8. | Financial Statements and Supplementary Data |
ASTERIAS BIOTHERAPEUTICS, INC.
BALANCE SHEETS
(IN THOUSANDS EXCEPT PAR VALUE AMOUNTS)
|
|
December 31,
|
|||||
|
|
2017
|
2016
|
||||
|
ASSETS
|
|
|
|
|
|
|
|
CURRENT ASSETS
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
$
|
13,266
|
|
$
|
19,800
|
|
|
Available-for-sale securities, at fair value
|
|
8,329
|
|
|
15,269
|
|
|
Prepaid expenses and other current assets
|
|
1,121
|
|
|
1,921
|
|
|
Total current assets
|
|
22,716
|
|
|
36,990
|
|
|
|
|
|
|
|
|
|
|
NONCURRENT ASSETS
|
|
|
|
|
|
|
|
Intangible assets, net
|
|
15,444
|
|
|
18,130
|
|
|
Property, plant and equipment, net
|
|
4,543
|
|
|
5,475
|
|
|
Other assets
|
|
389
|
|
|
415
|
|
|
TOTAL ASSETS
|
$
|
43,092
|
|
$
|
61,010
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|
|
|
|
|
|
|
CURRENT LIABILITIES
|
|
|
|
|
|
|
|
Amount due to BioTime, Inc.
|
$
|
—
|
|
$
|
288
|
|
|
Accounts payable
|
|
401
|
|
|
1,076
|
|
|
Accrued expenses
|
|
2,557
|
|
|
2,495
|
|
|
Capital lease liability, current
|
|
7
|
|
|
7
|
|
|
Lease liability, current
|
|
556
|
|
|
484
|
|
|
Deferred grant income
|
|
—
|
|
|
2,185
|
|
|
Total current liabilities
|
|
3,521
|
|
|
6,535
|
|
|
|
|
|
|
|
|
|
|
LONG-TERM LIABILITIES
|
|
|
|
|
|
|
|
Warrant liability
|
|
2,757
|
|
|
8,665
|
|
|
Capital lease liability, noncurrent
|
|
14
|
|
|
20
|
|
|
Deferred rent liability
|
|
316
|
|
|
266
|
|
|
Lease liability, noncurrent
|
|
2,941
|
|
|
3,496
|
|
|
TOTAL LIABILITIES
|
|
9,549
|
|
|
18,982
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies (see Note 8)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS’ EQUITY
|
|
|
|
|
|
|
|
Preferred Stock, $0.0001 par value, authorized 5,000 shares; none issued and outstanding
|
|
—
|
|
|
—
|
|
|
Common Stock, $0.0001 par value, authorized 75,000 Series A Common Stock and 75,000 Series B Common Stock; 54,051 and 47,467 shares Series A Common Stock issued and outstanding at December 31, 2017 and 2016, respectively; no Series B Common Stock issued and outstanding at December 31, 2017 and 2016
|
|
5
|
|
|
5
|
|
|
Additional paid-in capital
|
|
152,136
|
|
|
126,829
|
|
|
Accumulated other comprehensive loss
|
|
(6,498
|
)
|
|
(1,078
|
)
|
|
Accumulated deficit
|
|
(112,100
|
)
|
|
(83,728
|
)
|
|
Total stockholders’ equity
|
|
33,543
|
|
|
42,028
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY
|
$
|
43,092
|
|
$
|
61,010
|
|
The accompanying notes are an integral part of these financial statements.
47
ASTERIAS BIOTHERAPEUTICS, INC.
STATEMENTS OF OPERATIONS
(IN THOUSANDS, EXCEPT PER SHARE DATA)
|
|
Year Ended December 31,
|
||||||||
|
|
2017
|
2016
|
2015
|
||||||
|
REVENUES:
|
|
|
|
|
|
|
|
|
|
|
Grant income
|
$
|
3,711
|
|
$
|
6,572
|
|
$
|
3,007
|
|
|
Sale of cell lines
|
|
—
|
|
|
—
|
|
|
40
|
|
|
License revenue
|
|
—
|
|
|
125
|
|
|
—
|
|
|
Royalties from product sales
|
|
331
|
|
|
257
|
|
|
535
|
|
|
Total revenues
|
|
4,042
|
|
|
6,954
|
|
|
3,582
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales
|
|
(165
|
)
|
|
(128
|
)
|
|
(268
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
Total gross profit
|
|
3,877
|
|
|
6,826
|
|
|
3,314
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES:
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
(26,640
|
)
|
|
(25,467
|
)
|
|
(17,321
|
)
|
|
General and administrative
|
|
(10,488
|
)
|
|
(15,482
|
)
|
|
(7,901
|
)
|
|
Total operating expenses
|
|
(37,128
|
)
|
|
(40,949
|
)
|
|
(25,222
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
(33,251
|
)
|
|
(34,123
|
)
|
|
(21,908
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER INCOME/(EXPENSES):
|
|
|
|
|
|
|
|
|
|
|
Gain/(loss) from change in fair value of warrant liability
|
|
5,908
|
|
|
(3,108
|
)
|
|
—
|
|
|
Interest expense, net
|
|
(465
|
)
|
|
(546
|
)
|
|
(341
|
)
|
|
Other expense, net
|
|
(564
|
)
|
|
(37
|
)
|
|
(6
|
)
|
|
Total other income (expenses), net
|
|
4,879
|
|
|
(3,691
|
)
|
|
(347
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
LOSS BEFORE INCOME TAX BENEFIT
|
|
(28,372
|
)
|
|
(37,814
|
)
|
|
(22,255
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred income tax benefit
|
|
—
|
|
|
2,325
|
|
|
7,252
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET LOSS
|
$
|
(28,372
|
)
|
$
|
(35,489
|
)
|
$
|
(15,003
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
BASIC AND DILUTED NET LOSS PER SHARE
|
$
|
(0.56
|
)
|
$
|
(0.83
|
)
|
$
|
(0.42
|
)
|
|
WEIGHTED AVERAGE SHARES OUTSTANDING: BASIC AND DILUTED
|
|
50,271
|
|
|
42,934
|
|
|
35,443
|
|
The accompanying notes are an integral part of these financial statements.
48
ASTERIAS BIOTHERAPEUTICS, INC.
STATEMENTS OF COMPREHENSIVE LOSS
(IN THOUSANDS)
|
|
Years Ended December 31,
|
||||||||
|
|
2017
|
2016
|
2015
|
||||||
|
NET LOSS
|
$
|
(28,372
|
)
|
$
|
(35,489
|
)
|
$
|
(15,003
|
)
|
|
Other comprehensive income/(loss):
|
|
|
|
|
|
|
|
|
|
|
Unrealized (loss)/gain on available-for-sale securities, net of tax
|
|
(5,927
|
)
|
|
(1,512
|
)
|
|
937
|
|
|
Reclassification of realized loss on available-for-sale securities, net of taxes
|
|
508
|
|
|
—
|
|
|
—
|
|
|
Total other comprehensive income/(loss)
|
|
(5,419
|
)
|
|
(1,512
|
)
|
|
937
|
|
|
COMPREHENSIVE LOSS
|
$
|
(33,791
|
)
|
$
|
(37,001
|
)
|
$
|
(14,066
|
)
|
The accompanying notes are an integral part of these financial statements.
49
ASTERIAS BIOTHERAPEUTICS, INC.
STATEMENTS OF STOCKHOLDERS’ EQUITY
(IN THOUSANDS)
|
|
Common Stock
|
Additional
Paid-In Capital |
Accumulated
Other Comprehensive Income (Loss) |
|
|
|||||||||||||
|
|
Shares
|
Amount
|
Accumulated
Deficit |
Stockholders’
Equity |
||||||||||||||
|
Balance as of December 31, 2014
|
|
30,902
|
|
$
|
3
|
|
$
|
66,367
|
|
$
|
(503
|
)
|
$
|
(33,236
|
)
|
$
|
32,631
|
|
|
Stock-based compensation
|
|
145
|
|
|
—
|
|
|
3,625
|
|
|
—
|
|
|
—
|
|
|
3,625
|
|
|
Shares retired to pay employee taxes
|
|
(24
|
)
|
|
—
|
|
|
(98
|
)
|
|
—
|
|
|
—
|
|
|
(98
|
)
|
|
Unrealized gain on available-for-sale securities, net of taxes
|
|
—
|
|
|
—
|
|
|
—
|
|
|
937
|
|
|
—
|
|
|
937
|
|
|
Sale of common stock under at-the-market transactions
|
|
686
|
|
|
—
|
|
|
4,839
|
|
|
—
|
|
|
—
|
|
|
4,839
|
|
|
Financing costs to issue common stock
|
|
—
|
|
|
—
|
|
|
(665
|
)
|
|
—
|
|
|
—
|
|
|
(665
|
)
|
|
Issuance of common stock upon exercise of warrants
|
|
5,000
|
|
|
1
|
|
|
11,700
|
|
|
—
|
|
|
—
|
|
|
11,701
|
|
|
Common stock issued at Private Placement
|
|
1,026
|
|
|
—
|
|
|
4,000
|
|
|
—
|
|
|
—
|
|
|
4,000
|
|
|
Common stock issued in public offering
|
|
385
|
|
|
—
|
|
|
1,500
|
|
|
—
|
|
|
—
|
|
|
1,500
|
|
|
Issuance of common stock upon exercise of stock options
|
|
12
|
|
|
—
|
|
|
29
|
|
|
—
|
|
|
—
|
|
|
29
|
|
|
OncoCyte common stock received as a dividend from BioTime, net of taxes
|
|
—
|
|
|
—
|
|
|
1,117
|
|
|
—
|
|
|
—
|
|
|
1,117
|
|
|
Common stock issued for services
|
|
96
|
|
|
—
|
|
|
486
|
|
|
—
|
|
|
—
|
|
|
486
|
|
|
Net loss
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(15,003
|
)
|
|
(15,003
|
)
|
|
Balance as of December 31, 2015
|
|
38,228
|
|
|
4
|
|
|
92,900
|
|
|
434
|
|
|
(48,239
|
)
|
|
45,099
|
|
|
Stock-based compensation
|
|
457
|
|
|
—
|
|
|
4,797
|
|
|
—
|
|
|
—
|
|
|
4,797
|
|
|
Shares retired to pay employee taxes
|
|
(37
|
)
|
|
—
|
|
|
(168
|
)
|
|
—
|
|
|
—
|
|
|
(168
|
)
|
|
Unrealized loss on available-for-sale securities, net of taxes
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(1,512
|
)
|
|
—
|
|
|
(1,512
|
)
|
|
Sale of common stock under at-the-market transactions
|
|
1,812
|
|
|
—
|
|
|
7,969
|
|
|
—
|
|
|
—
|
|
|
7,969
|
|
|
Financing costs for at-the-market sales
|
|
—
|
|
|
—
|
|
|
(328
|
)
|
|
—
|
|
|
—
|
|
|
(328
|
)
|
|
Issuance of common stock upon exercise of stock options
|
|
827
|
|
|
—
|
|
|
2,026
|
|
|
—
|
|
|
—
|
|
|
2,026
|
|
|
Issuance of common stock upon exercise of warrants, including fair value of warrants
|
|
148
|
|
|
—
|
|
|
1,102
|
|
|
—
|
|
|
—
|
|
|
1,102
|
|
|
Issuance of common stock in public offering
|
|
5,889
|
|
|
1
|
|
|
14,014
|
|
|
—
|
|
|
—
|
|
|
14,015
|
|
|
Financing costs of public offering
|
|
—
|
|
|
—
|
|
|
(1,275
|
)
|
|
—
|
|
|
—
|
|
|
(1,275
|
)
|
|
Distribution of warrants to shareholders other than BioTime
|
|
—
|
|
|
—
|
|
|
5,285
|
|
|
—
|
|
|
—
|
|
|
5,285
|
|
|
Common stock issued for services
|
|
219
|
|
|
—
|
|
|
922
|
|
|
—
|
|
|
—
|
|
|
922
|
|
|
Cross-License and Share Transfer with BioTime, net
|
|
(76
|
)
|
|
—
|
|
|
(415
|
)
|
|
—
|
|
|
—
|
|
|
(415
|
)
|
|
Net loss
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(35,489
|
)
|
|
(35,489
|
)
|
|
Balance as of December 31, 2016
|
|
47,467
|
|
|
5
|
|
|
126,829
|
|
|
(1,078
|
)
|
|
(83,728
|
)
|
|
42,028
|
|
|
Stock-based compensation
|
|
251
|
|
|
—
|
|
|
4,444
|
|
|
—
|
|
|
—
|
|
|
4,444
|
|
|
Unrealized loss on available-for-sale securities, net of taxes
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(5,420
|
)
|
|
—
|
|
|
(5,420
|
)
|
|
Sale of common stock under at-the-market transactions
|
|
2,005
|
|
|
—
|
|
|
8,002
|
|
|
—
|
|
|
—
|
|
|
8,002
|
|
|
Financing costs for at-the-market sales
|
|
—
|
|
|
—
|
|
|
(238
|
)
|
|
—
|
|
|
—
|
|
|
(238
|
)
|
|
Issuance of common stock upon exercise of stock options
|
|
8
|
|
|
—
|
|
|
18
|
|
|
—
|
|
|
—
|
|
|
18
|
|
|
Costs associated with the extension of warrants
|
|
—
|
|
|
—
|
|
|
2,042
|
|
|
—
|
|
|
—
|
|
|
2,042
|
|
|
Issuance of common stock upon exercise of warrants, including fair value of warrants
|
|
1
|
|
|
—
|
|
|
5
|
|
|
—
|
|
|
—
|
|
|
5
|
|
|
Issuance of common stock in public offering
|
|
4,000
|
|
|
—
|
|
|
10,400
|
|
|
—
|
|
|
—
|
|
|
10,400
|
|
|
Financing costs of public offering
|
|
—
|
|
|
—
|
|
|
(517
|
)
|
|
—
|
|
|
—
|
|
|
(517
|
)
|
|
Common stock issued for services
|
|
319
|
|
|
—
|
|
|
1,151
|
|
|
—
|
|
|
—
|
|
|
1,151
|
|
|
Net loss
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(28,372
|
)
|
|
(28,372
|
)
|
|
Balance as of December 31, 2017
|
|
54,051
|
|
$
|
5
|
|
$
|
152,136
|
|
$
|
(6,498
|
)
|
$
|
(112,100
|
)
|
$
|
33,543
|
|
The accompanying notes are an integral part of these financial statements.
50
ASTERIAS BIOTHERAPEUTICS, INC.
STATEMENTS OF CASH FLOWS
(IN THOUSANDS)
|
|
Year Ended December 31,
|
||||||||
|
|
2017
|
2016
|
2015
|
||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
$
|
(28,372
|
)
|
$
|
(35,489
|
)
|
$
|
(15,003
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization expense
|
|
1,110
|
|
|
1,176
|
|
|
564
|
|
|
Stock-based compensation
|
|
4,444
|
|
|
4,797
|
|
|
3,625
|
|
|
Amortization of intangible assets
|
|
2,686
|
|
|
2,686
|
|
|
2,686
|
|
|
Realized loss on sale of available-for-sale securities
|
|
508
|
|
|
—
|
|
|
—
|
|
|
Amortization of prepaid rent
|
|
—
|
|
|
—
|
|
|
85
|
|
|
Deferred income tax benefit
|
|
—
|
|
|
(2,325
|
)
|
|
(7,252
|
)
|
|
Common stock issued for services in lieu of cash
|
|
1,151
|
|
|
922
|
|
|
486
|
|
|
Gain (loss) from change in fair value of warrant liability
|
|
(5,908
|
)
|
|
3,108
|
|
|
—
|
|
|
Distribution of Asterias warrants to shareholders other than BioTime
|
|
2,042
|
|
|
5,285
|
|
|
—
|
|
|
Loss on disposal of equipment
|
|
112
|
|
|
—
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
Grant receivable
|
|
—
|
|
|
—
|
|
|
118
|
|
|
Prepaid expenses and other current assets
|
|
13
|
|
|
(887
|
)
|
|
(680
|
)
|
|
Other assets
|
|
846
|
|
|
10
|
|
|
(95
|
)
|
|
Accounts payable
|
|
(675
|
)
|
|
329
|
|
|
(24
|
)
|
|
Accrued expenses
|
|
62
|
|
|
1,863
|
|
|
584
|
|
|
Deferred rent liability
|
|
50
|
|
|
87
|
|
|
85
|
|
|
Deferred grant income
|
|
(2,185
|
)
|
|
(328
|
)
|
|
2,513
|
|
|
Amount due to BioTime
|
|
(321
|
)
|
|
(242
|
)
|
|
(85
|
)
|
|
Net cash used in operating activities
|
|
(24,437
|
)
|
|
(19,008
|
)
|
|
(12,393
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
|
Purchase of property, plant and equipment, including leasehold improvements
|
|
(290
|
)
|
|
(894
|
)
|
|
(313
|
)
|
|
Payments on construction in progress
|
|
—
|
|
|
—
|
|
|
(4,279
|
)
|
|
Proceeds from the sale of available-for-sale securities
|
|
1,012
|
|
|
—
|
|
|
—
|
|
|
Reimbursement (payment) of security deposit, net
|
|
—
|
|
|
31
|
|
|
(1
|
)
|
|
Net cash provided by/(used in) investing activities
|
|
722
|
|
|
(863
|
)
|
|
(4,593
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
|
Proceeds from sale of common shares under at-the-market transactions
|
|
8,002
|
|
|
7,969
|
|
|
4,839
|
|
|
Financing costs for at-the-market sales
|
|
(238
|
)
|
|
(328
|
)
|
|
(157
|
)
|
|
Proceeds from sale of common shares in public offering
|
|
10,400
|
|
|
14,014
|
|
|
5,500
|
|
|
Proceeds allocated to warrants classified as liabilities
|
|
—
|
|
|
6,009
|
|
|
—
|
|
|
Proceeds from exercise of warrants
|
|
5
|
|
|
651
|
|
|
11,700
|
|
|
Financing costs for sale of common stock in public offering
|
|
(517
|
)
|
|
(1,275
|
)
|
|
(508
|
)
|
|
Financing costs allocated to warrants classified as liabilities
|
|
—
|
|
|
(550
|
)
|
|
—
|
|
|
Proceeds from exercises of stock options
|
|
18
|
|
|
2,026
|
|
|
29
|
|
|
Repayment of lease liability and capital lease obligation
|
|
(489
|
)
|
|
(427
|
)
|
|
(1
|
)
|
|
Shares retired to pay for employees’ taxes
|
|
—
|
|
|
(168
|
)
|
|
(98
|
)
|
|
Reimbursement from landlord on construction in progress
|
|
—
|
|
|
567
|
|
|
3,789
|
|
|
Net cash provided by financing activities
|
|
17,181
|
|
|
28,488
|
|
|
25,093
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET (DECREASE)/INCREASE IN CASH AND CASH EQUIVALENTS
|
|
(6,534
|
)
|
|
8,617
|
|
|
8,107
|
|
|
At beginning of year
|
|
19,800
|
|
|
11,183
|
|
|
3,076
|
|
|
At end of year
|
$
|
13,266
|
|
$
|
19,800
|
|
$
|
11,183
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL SCHEDULE OF NON-CASH FINANCING AND INVESTING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
|
OncoCyte common stock received as a dividend in kind from BioTime, net of taxes
|
$
|
—
|
|
$
|
—
|
|
$
|
1,117
|
|
|
Landlord receivable
|
$
|
—
|
|
$
|
—
|
|
$
|
(189
|
)
|
|
Lease liability
|
$
|
—
|
|
$
|
—
|
|
$
|
189
|
|
|
Cross-License and Share Transfer with BioTime Inc., net
|
$
|
—
|
|
$
|
415
|
|
$
|
—
|
|
The accompanying notes are an integral part of these financial statements.
51
ASTERIAS BIOTHERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
1. Organization, Basis of Presentation and Liquidity
Asterias Biotherapeutics, Inc. (“Asterias”) was incorporated in Delaware on September 24, 2012. Prior to May 13, 2016, Asterias was a majority-owned and controlled subsidiary of BioTime, Inc. (“BioTime”). As further discussed below, on May 13, 2016, BioTime deconsolidated Asterias’ financial statements due to BioTime’s loss of control of Asterias as defined by generally accepted accounting principles.
Asterias is a clinical-stage biotechnology company dedicated to developing cell-based therapeutics to treat neurological conditions associated with demyelination and cellular immunotherapies to treat cancer. The Company has industry-leading technology in two cell types, each with broad potential applicability: oligodendrocyte progenitor cells which, as oligodendrocytes, re-myelinate axons within the central nervous system and perform other restorative functions, and antigen presenting dendritic cells which train T-cells in the immune system to attack and destroy solid or liquid tumor cells across multiple types of cancer.
The financial statements and the notes thereto are presented in accordance with accounting principles generally accepted in the U.S. (“GAAP”) and with the accounting and reporting requirements to Form 10-K and Article 10 of Regulation S-X of the Securities and Exchange Commission (“SEC”).
Prior to May 13, 2016, BioTime consolidated the results of Asterias into BioTime’s consolidated results based on BioTime’s ability to control Asterias’ operating and financial decisions and policies through a majority ownership of Asterias common stock. On May 13, 2016, Asterias completed the sale and the underwriters’ exercise of the overallotment for 5,889,480 shares of its common stock and warrants to purchase 2,959,559 shares of its common stock, through an underwritten public offering (the “Asterias Offering”) (see Note 6). BioTime did not participate in the Asterias Offering. As a result of the sale of Asterias common stock in the Asterias Offering and the issuance of 708,333 shares of Asterias common stock upon the exercise of certain stock options by a former Asterias executive, BioTime’s percentage ownership of the outstanding common stock of Asterias declined to less than 50% on May 13, 2016. Under generally accepted accounting principles, loss of control of a subsidiary is deemed to have occurred when, among other things, a parent company owns less than a majority of the outstanding shares of common stock of the subsidiary, lacks a controlling financial interest in the subsidiary, and is unable to unilaterally control the subsidiary through other means such as having, or having the ability to obtain, a majority of the subsidiary’s Board of Directors. BioTime determined that all of these loss of control factors were present for BioTime as of May 13, 2016. Accordingly, BioTime deconsolidated Asterias’ financial statements and results of operations from those of BioTime, effective May 13, 2016, in accordance with ASC, 810-10-40-4(c), Consolidation.
BioTime continues to allocate expenses such as salaries and payroll related expenses incurred and paid on behalf of Asterias based on the amount of time that particular employees of BioTime devote to Asterias affairs. Other expenses such as legal, accounting, travel, and entertainment expenses are allocated to Asterias to the extent that those expenses are incurred by or on behalf of Asterias. These allocations are made based upon activity-based allocation drivers such as time spent, percentage of square feet of office or laboratory space used, if applicable, and percentage of personnel devoted to Asterias operations or management. These allocated and overhead expenses have decreased during 2017 as Asterias continued to hire its operations and management personnel. Management evaluates the appropriateness of the percentage allocations on a quarterly basis and believes that this basis for allocation is reasonable.
In connection with the services performed by employees of BioTime, or employees of other BioTime commonly controlled and consolidated subsidiaries within the BioTime group of affiliated entities, Asterias has in the past granted stock options to those performing services for Asterias, for which Asterias records stock-based compensation expense in its statements of operations for such services performed in the relevant periods (see Note 9).
Reclassifications – Certain reclassifications have been made to the 2016 financial statements to conform to the 2017 financial statement presentation. These reclassifications had no effect on net earnings or cash flows as previously reported.
Liquidity – Since inception, Asterias has incurred operating losses and has funded its operations primarily through issuance of equity securities, warrants, payments from research grants, and royalties from product sales, and the support from BioTime. At December 31, 2017, Asterias had an accumulated deficit of $112.1 million, working capital of $19.2 million and stockholders’ equity of $33.5 million. Asterias has evaluated its projected cash flows and
52
believes that its cash and cash equivalents of $13.3 million and available-for-sale securities of $8.3 million as of December 31, 2017, will be sufficient to fund Asterias’ operations through at least twelve months from the issuance date of these financial statements, or at least through March 15, 2019. Some of the clinical trials being conducted by Asterias have historically been funded in part with funds from the $14.3 million grant awarded in 2014 by the California Institute for Regenerative Medicine (“CIRM”) and not from cash on hand, and the value of our available-for-sale securities is subject to market risk. If Asterias were unable to obtain future grant funding from CIRM, the value of its available-for-sale securities decreases, or it is unable to obtain future adequate financing for its clinical trials, it may be required to delay, postpone, or cancel its clinical trials or limit the number of clinical trial sites, or otherwise reduce or curtail its operations. Future financings, if necessary, may not be available to Asterias at acceptable terms, or if at all. Sales of additional equity securities would result in the dilution of interests of current shareholders.
2. Summary of Significant Accounting Policies
Use of estimates – The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period with consideration given to materiality. Significant estimates and assumptions used include those related to the going concern assessment of our financial statements, useful lives associated with long-lived assets, including evaluation of asset impairment, loss contingencies, deferred income taxes and tax reserves, including valuation allowances related to deferred income taxes, and assumptions used to value stock-based awards, liability or other equity instruments. Actual results could differ materially from those estimates.
Going concern assessment – At each annual and interim period, Asterias will assess going concern uncertainty to determine if Asterias has sufficient cash and cash equivalents on hand and working capital to operate for a period of at least one year from the date the financial statements are issued or are available to be issued, which is referred to as the “look-forward period” as defined by ASU No. 2014-15. As part of this assessment, based on conditions that are known and reasonably knowable to Asterias, Asterias will consider various scenarios, forecasts, projections, and estimates, and Asterias will make certain key assumptions, including the timing and nature of projected cash expenditures or programs, and its ability to delay or curtail those expenditures or programs, among other factors, if necessary, within the look-forward period in accordance with ASU No. 2014-15.
Revenue recognition – Asterias complies with ASC 605-10 and records revenue when persuasive evidence of an arrangement exists, delivery has occurred, or services have been rendered, the price is fixed or determinable, and collectability is reasonably assured. Grant income is recognized as revenue when the related research and development expenses are incurred. Royalty revenues consist of royalty payments on sales of products under license agreements. Asterias recognizes revenue in the quarter in which the royalty reports are received rather than the quarter in which the sales took place. When Asterias is entitled to receive up-front nonrefundable licensing or similar fees pursuant to agreements under which Asterias has no continuing performance obligations, the fees are recognized as revenues when collection is reasonably assured. When Asterias receives up-front nonrefundable licensing or similar fees pursuant to agreements under which Asterias does have continuing performance obligations, the fees are deferred and amortized ratably over the performance period. If the performance period cannot be reasonably estimated, Asterias amortizes nonrefundable fees over the life of the contract until such time that the performance period can be more reasonably estimated. Milestone payments, if any, related to scientific or technical achievements, subject to substantial uncertainty, are recognized in income when the milestone is accomplished if (a) substantive effort was required to achieve the milestone, (b) the amount of the milestone payment appears reasonably commensurate with the effort expended, and (c) collection of the payment is reasonably assured.
Cash and cash equivalents – Asterias considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents. As of December 31, 2017 and 2016, Asterias had $5.3 million and $13.7 million in money market funds, respectively, considered to be cash equivalents.
Concentrations of credit risk – Financial instruments that potentially subject Asterias to significant concentrations of credit risk consist primarily of cash and cash equivalents. Asterias limits the amount of credit exposure of cash balances by maintaining its accounts in high credit quality financial institutions. Cash equivalent deposits with financial institutions may occasionally exceed the limits of insurance on bank deposits; however, Asterias has not experienced any losses on such accounts.
53
Comprehensive income/loss – ASC 220, Comprehensive Income, requires that an entity’s change in equity or net assets during a period from transactions and other events from non-owner sources be reported. Asterias reports unrealized gains and losses on its available-for-sale securities as other comprehensive income/(loss).
Available-for-sale securities, at fair value – Marketable equity and debt securities not classified as held-to-maturity are classified as available-for-sale. Available-for-sale securities are carried at fair value, with the unrealized gains and losses, net of tax, reported in other comprehensive income/(loss). Realized gains and losses and declines in value judged to be other-than-temporary related to equity securities, are included in other income/(expenses), net.
Asterias accounts for the BioTime and OncoCyte shares it holds as available-for-sale securities in accordance with ASC 320-10-25, Investments-Debt and Equity Securities, as the shares have a readily determinable fair value quoted on the NYSE American and are held principally for future working capital purposes, as necessary. These shares are measured at fair value and reported as current assets on the balance sheet based on the closing trading price of the security as of the date being presented (see Note 4). Unrealized holding gains and losses are excluded from the statements of operations and reported in equity as part of other comprehensive income/(loss) until realized.
Realized gains and losses on the sale of BioTime shares prior to May 13, 2016, were reclassified out of other comprehensive income/(loss) and included in equity, as an increase or decrease in additional paid-in capital consistent with, and pursuant to, ASC 805-50, Transactions Between Entities Under Common Control. Beginning on May 13, 2016, due to the deconsolidation of Asterias, financial statements from BioTime and loss of control experienced by BioTime in Asterias, as discussed in Note 1, realized gains and losses, and declines in value judged to be other-than-temporary related to equity securities, are included in other income/(expense), net. For OncoCyte shares that Asterias holds, realized gains and losses, and declines in value judged to be other-than-temporary related to equity securities, are included in other income/(expense), net.
Asterias reviews various factors in determining whether it should recognize an other-than-temporary impairment charge for its available-for-sale securities, including its intent and ability to hold the investment for a period of time sufficient for any anticipated recovery in market value, and the length of time and extent to which the fair value has been less than its cost basis. Based on consideration of these factors, as of December 31, 2017 and 2016, no other-than-temporary impairment loss was recognized on the available-for-sales securities held on these respective dates.
Property, plant and equipment – Property, plant and equipment includes equipment, fixtures and leasehold improvements stated at cost. Depreciation is calculated using the straight-line method over the period of their estimated useful lives ranging from 36 to 120 months. Leasehold improvements are amortized using the shorter of the useful life or the lease term.
Long-lived intangible assets – Long-lived intangible assets, consisting primarily of acquired patents, patent applications, and licenses to use certain patents are stated at acquired cost, less accumulated amortization. Amortization expense is computed using the straight-line method over the estimated useful lives of the assets, generally over 10 years.
Impairment of long-lived assets – Long-lived assets, including long-lived intangible assets, will be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be fully recoverable. If an impairment indicator is present, Asterias evaluates recoverability by a comparison of the carrying amount of the assets to future undiscounted net cash flows expected to be generated by the assets. If the assets are impaired, the impairment will be recognized and measured by the amount by which the carrying amount exceeds the estimated fair value of the assets.
Accounting for warrants – Asterias determines the accounting classification of warrants that it issues, as either liability or equity, by first assessing whether the warrants meet liability classification in accordance with ASC 480-10, Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity, and then in accordance with ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock. Under ASC 480, warrants are considered liability classified if the warrants are mandatorily redeemable, obligate the issuer to settle the warrants or the underlying shares by paying cash or other assets, or warrants that must or may require settlement by issuing variable number of shares. If warrants do not meet liability classification under ASC 480-10, Asterias assesses the requirements under ASC 815-40, which states that contracts that require or may require the issuer to settle the contract for cash are liabilities recorded at fair value, irrespective of the likelihood of the transaction occurring that triggers the net cash settlement feature. If the warrants
54
do not require liability classification under ASC 815-40, in order to conclude equity classification, Asterias assesses whether the warrants are indexed to its common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable GAAP. After all relevant assessments are made, Asterias concludes whether the warrants are classified as liability or equity. Liability classified warrants are required to be accounted for at fair value both on the date of issuance and on subsequent accounting period ending dates, with all changes in fair value after the issuance date recorded in the statements of operations as a gain or loss. Equity classified warrants are accounted for at fair value on the issuance date with no changes in fair value recognized subsequent to the issuance date.
Historically, Asterias has issued warrants that are classified as equity and as a liability (see Note 6).
Research and development – Research and development expenses consist of costs incurred for company-sponsored, collaborative and contracted research and development activities. These costs include direct and research-related overhead expenses including compensation and related benefits including stock-based compensation, consulting fees, research and laboratory fees, rent of research facilities, amortization of intangible assets, and license fees paid to third parties to acquire patents or licenses to use patents and other technology. Asterias expenses research and development costs as incurred. Research and development expenses incurred and reimbursed under grants approximate the grant income recognized in the statements of operations.
General and administrative – General and administrative expenses consist of compensation and related benefits, including stock-based compensation, for executive and corporate personnel; professional and consulting fees; and allocated overhead. General and administrative expenses also include costs allocated from BioTime pursuant to the Shared Facilities and Services Agreement (see Note 9).
Income taxes – As of October 1, 2013, Asterias has filed its own U.S. federal tax returns. Operations prior to that period were included in BioTime’s consolidated U.S. federal tax return. For California purposes Asterias’ activity through May 12, 2016 was included in BioTime’s combined tax return. Activity from May 13, 2016 on will be included in Asterias’ separate California income tax return filing due to the deconsolidation of Asterias from BioTime as of that date. Asterias accounts for income taxes in accordance with ASC 740, Income Taxes, which prescribes the use of the asset and liability method, whereby deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect. Valuation allowances are established when necessary to reduce deferred tax assets when it is more likely than not that a portion or all of the deferred tax assets will not be realized. The guidance also prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not sustainable upon examination by taxing authorities. For federal purposes Asterias is no longer subject to tax examination for years before 2013. For California purposes Asterias is subject to income tax examinations by tax authorities for all years since inception. Although the statute is closed for purposes of assessing additional income and tax in those years, the taxing authorities may still make adjustments to the net operating loss and credit carryforwards used in open years. Therefore, the statute should be considered open as it relates to the net operating loss and credit carryforwards. Asterias will recognize accrued interest and penalties related to unrecognized tax benefits as income tax expense. No amounts were accrued for the payment of interest and penalties as of December 31, 2017 and 2016.
Stock-based compensation – Asterias accounts for share-based payments in accordance with ASC 718, Compensation – Stock Compensation, which require the measurement and recognition of compensation expense for all share-based payment awards made to directors and employees, including employee stock options, based on estimated fair values. Consistent with those guidelines, Asterias utilizes the Black-Scholes-Merton option pricing model. Asterias’ determination of fair value of share-based payment awards on the date of grant using that option-pricing model is affected by Asterias’ stock price as well as by assumptions regarding a number of complex and subjective variables. These variables include, but are not limited to, Asterias’ expected stock price volatility over the term of the awards; the expected term of options granted; and a risk-free rate based on the U.S. Treasury rates in effect during the corresponding expected term of the grant. Expected term is derived from a combination of Asterias own, historical experience, to the extent available, and using the simplified method under SEC Staff Accounting Bulletin Topic 14, as applicable. Asterias recognizes stock-based compensation on a straight-line basis over the requisite service period. Through January 1, 2017 Asterias recorded stock-based compensation expense net of estimated forfeitures. Upon the adoption of ASU 2016-09, Asterias accounts for forfeitures as they occur.
Asterias also, at times, issues restricted stock or restricted stock units (“RSUs”) to its executive officers, employees, and members of its Board of Directors, which are restricted and unvested common shares issued or shares
55
issuable as RSUs vest. Restricted stock and RSU compensation expense is recognized on a straight-line basis over the requisite service period of generally four years, based on the grant-date fair value of the stock. Restricted stock is considered legally issued and outstanding on the grant date, while RSUs are not until RSUs vest. Once the RSUs are vested, equivalent common shares will be issued or issuable to the grantee and therefore the RSUs are not included in total common shares issued and outstanding until vested.
Stock-based compensation expense for non-employee stock-based awards is recognized in accordance with ASC 505-50, Equity-Based Payments to Non-Employees (“ASC 505-50”). Stock option awards issued to non-employees, principally consultants and employees of BioTime or employees of BioTime subsidiaries who perform services for Asterias, are accounted for at fair value using the Black-Scholes-Merton option pricing model. Management believes that the fair value of the stock options is more reliably measured than the fair value of services received. Asterias records compensation expense based on the then-current fair values of the stock options at each financial reporting date. Compensation expense recorded during the service period is adjusted in subsequent periods for changes in the fair value of the stock options until the earlier of the date at which the non-employee’s performance is complete or a performance commitment is reached, which is generally when the stock option award vests. Compensation expense for non-employee grants is recorded on a straight-line basis in the statements of operations.
Fair value of financial instruments – ASC 820, Fair Value Measurements, clarifies that fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability.
ASC 820 requires that the valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. ASC 820 establishes a three-tier value hierarchy, which prioritizes inputs that may be used to measure fair value as follows:
| • | Level 1– Observable inputs that reflect quoted prices for identical assets or liabilities in active markets. |
| • | Level 2– Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| • | Level 3– Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The carrying amounts of current assets and current liabilities approximate their fair value because of the relatively short period until they mature or are required to be settled, except for money market funds and the investment in BioTime and OncoCyte shares, which are carried at fair value based on Level 1 inputs, and the warrant liability which is carried at fair value based on Level 3 inputs (see Note 6).
The following table shows the activity in warrants classified as a liability discussed in Note 6 (in thousands):
|
|
Warrant
Liability |
Warrant
Shares |
||||
|
Fair value of warrants issued on May 13, 2016
|
$
|
6,009
|
|
|
2,959
|
|
|
Fair value of warrants exercised on December 2, 2016
|
|
(452
|
)
|
|
(146
|
)
|
|
Increase in fair value of warrants during 2016
|
|
3,108
|
|
|
—
|
|
|
Fair value of warrants at December 31, 2016
|
|
8,665
|
|
|
2,813
|
|
|
Decrease in fair value of warrants during 2017
|
|
(5,908
|
)
|
|
—
|
|
|
Fair value of warrants at December 31, 2017
|
$
|
2,757
|
|
|
2,813
|
|
Basic and diluted net loss per share – Basic net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding for the year. Diluted net loss per share reflects the weighted-average number of shares of common stock outstanding plus the potential effect of dilutive securities or contracts which are convertible to common stock, such as options and warrants (using the treasury stock method) and shares issuable in future periods, such as restricted stock or RSU awards, except in cases where the effect would be anti-dilutive.
56
The computations of basic and diluted net loss per share are as follows (in thousands, except per share data):
|
|
Year Ended December 31,
|
||||||||
|
|
2017
|
2016
|
2015
|
||||||
|
Net loss
|
$
|
(28,372
|
)
|
$
|
(35,489
|
)
|
$
|
(15,003
|
)
|
|
Weighted average common shares outstanding – basic and diluted
|
|
50,271
|
|
|
42,934
|
|
|
35,443
|
|
|
Net loss per share – basic and diluted
|
$
|
(0.56
|
)
|
$
|
(0.83
|
)
|
$
|
(0.42
|
)
|
The following common stock equivalents were excluded from the computation of diluted net loss per share of common stock for the periods presented because including them would have been antidilutive (in thousands):
|
|
Year Ended December 31,
|
||||||||
|
|
2017
|
2016
|
2015
|
||||||
|
Stock options and restricted stock units
|
|
7,066
|
|
|
6,432
|
|
|
5,123
|
|
|
Warrants
|
|
2,813
|
|
|
6,552
|
|
|
3,500
|
|
Recently Adopted Accounting Pronouncements – The following Accounting Standard(s) were effective during 2017:
In March 2016, the FASB issued ASU 2016-09, Compensation - Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting (“ASU 2016-09”), which simplifies several aspects of the accounting for share-based payment transactions, including the income tax consequences, forfeitures, classification of awards as either equity or liabilities, and classification on the statement of cash flows. Forfeitures are now accounted for as they occur instead of based on the number of awards that were expected to vest. Based on the nature and timing of Asterias equity grants, straight-line expense attribution of stock-based compensation for the entire award and the relatively low forfeiture rate on Asterias experience, the impact of adoption of ASU 2016-09 pertaining to forfeitures was not significant to Asterias’ financial statements (see Note 7).
In connection with the adoption of ASU 2016-09, Asterias changed how it accounts for excess tax benefits and deficiencies, if any, and forfeitures, as applicable. All excess tax benefits and tax deficiencies from stock-based compensation awards accounted for under ASC 718 are recognized as an income tax benefit or expense, respectively, in the statements of operations. Prior to the adoption of ASU 2016-09, Asterias recognized excess tax benefits, if any, in additional paid-in capital only if the tax deduction reduced cash income taxes payable and excess tax deficiencies were recognized either as an offset to accumulated excess tax benefits, if any, on Asterias’ statements of operations. An excess income tax benefit arises when the tax deduction of a share-based award for income tax purposes exceeds the compensation cost recognized for financial reporting purposes and, a tax deficiency arises when the compensation cost exceeds the tax deduction. Because Asterias has a full valuation allowance, there was no impact to Asterias’ statements of operations for any excess tax benefits or deficiencies, as any excess benefit or deficiency would be offset by the change in the valuation allowance (see Note 10). Asterias adopted ASU 2016-09 beginning on January 1, 2017.
Recently Issued Accounting Pronouncements – The following accounting standards, which are not yet effective, are presently being evaluated by Asterias to determine the impact that they might have on its financial statements.
On January 5, 2016, the FASB issued Accounting Standards Update 2016-01, Financial Instruments–Overall: Recognition and Measurement of Financial Assets and Financial Liabilities (ASU No. 2016-01). Changes to the current GAAP model primarily affect the accounting for equity investments, financial liabilities under the fair value option, and the presentation and disclosure requirements for financial instruments. In addition, ASU No. 2016-01 clarified guidance related to the valuation allowance assessment when recognizing deferred tax assets resulting from unrealized losses on available-for-sale debt securities. The accounting for other financial instruments, such as loans, investments in debt securities, and financial liabilities is largely unchanged. The more significant amendments are to equity investments in unconsolidated entities.
In accordance with ASU No. 2016-01, all equity investments in unconsolidated entities (other than those accounted for using the equity method of accounting) will generally be measured at fair value through earnings. There will no longer be an available-for-sale classification (changes in fair value reported in other comprehensive income) for equity securities with readily determinable fair values. The classification and measurement guidance will be effective for public business entities in fiscal years beginning after December 15, 2017, including interim periods within those fiscal years. Upon the adoption of ASU No. 2016-01, the Company will record a cumulative-effect
57
adjustment to the balance sheet as of January 1, 2018, the date of adoption. The Company has completed its assessment of the impact from adoption and estimates that an adjustment of approximately $6.5 between accumulated other comprehensive income and accumulated deficit will be recorded. The adjustment represents the cumulative unrealized holding loss from the date that the securities were acquired through the date of adoption. Refer to Note 4 for discussion regarding Asterias’ available-for-sale securities.
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842), which requires lessees to recognize assets and liabilities for leases with lease terms greater than twelve months in the statement of financial position. Leases will be classified as either financing or operating, with classification affecting the pattern of expense recognition in the statements of operations. ASU 2016-02 also requires improved disclosures to help users of financial statements better understand the amount, timing and uncertainty of cash flows arising from leases. The update is effective for fiscal years beginning after December 15, 2018, including interim reporting periods within that reporting period. Early adoption is permitted. Although Asterias has not completed its evaluation of the impact of the adoption of ASU 2016-02, Asterias currently holds a significant portion of its operating leases, related to tenant improvements on Asterias’ balance sheet (see Note 8), the adoption of ASU 2016-02 is expected to have a material impact to Asterias’ financial statements.
In May 2014, the FASB issued ASU 2014-09, Revenue from Contracts with Customers, which requires an entity to recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. In doing so, companies will need to use more judgment and make more estimates than under today’s guidance. These may include identifying performance obligations in the contract, estimating the amount of variable consideration to include in the transaction price and allocating the transaction price to each separate performance obligation. The ASU will replace most existing revenue recognition guidance in U.S. GAAP when it becomes effective. In August 2015, the FASB issued ASU No. 2015-14, Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date, which provided for the adoption of the new standard for fiscal years beginning after December 15, 2017. Accordingly, ASU No. 2014-09 is effective for the Company in the first quarter of 2018. Upon adoption, ASU No. 2014-09 can be applied retrospectively to all periods presented or only to the most current period presented with the cumulative effect of changes reflected in the opening balance of retained earnings in the most current period presented. The FASB has also issued the following standards which clarify ASU No. 2014-09 and have the same effective date as the original standard:
| • | ASU No. 2016-08, Revenue from Contracts with Customers (Topic 606): Principal versus Agent Considerations (Reporting Revenue Gross versus Net); |
| • | ASU No. 2016-10, Identifying Performance Obligations and Licensing (Topic 606); |
| • | ASU No. 2016-11, Revenue Recognition (Topic 605) and Derivatives and Hedging (Topic 815): Rescission of SEC Guidance Because of Accounting Standards Updates 2014-09 and 2014-16 Pursuant to Staff Announcements at the March 3, 2016, EITF Meeting; |
| • | ASU No. 2016-12, Revenue from Contracts with Customers (Topic 606): Narrow-Scope Improvements and Practical Expedients; and |
| • | ASU No. 2016-20, Revenue from Contracts with Customers (Topic 606): Technical Corrections and Improvements. |
The Company expects to adopt ASU 2014-09 effective January 1, 2018, using the modified retrospective transition method. The Company has completed a quantitative analysis of the impact to its customer contracts in transition at the adoption date and is currently evaluating the effect that the new standard will have on its internal processes, financial statements, and related disclosures.
In the third quarter of 2017, the Company recognized the final CIRM milestone payment under legacy GAAP. Therefore, the CIRM contract falls out of scope and is not considered in transition at adoption. The Company has also reviewed each of its license agreements and has determined that while there will be changes to its policies related primarily to the way that the Company classifies contract consideration, and when variable consideration is deemed probable, the quantitative impact from adoption of the new standard will not be material to the financial statements at adoption.
58
The Company will continue to monitor additional modifications, clarifications or interpretations undertaken by the FASB that may impact its current conclusions and will expand its analysis to include any new revenue arrangements initiated before adoption. As the Company completes its evaluation of the new standard, new information may arise that could change the Company’s understanding of the impact to its financial statements.
In May 2017, the FASB issued ASU 2017-09, Compensation – Stock Compensation: Scope of Modification Accounting to clarify the scope of modification accounting for share-based compensation. Under the new guidance, modification accounting is required only if the fair value, the vesting conditions, or the classification of the award (as equity or liability) changes as a result of the change in terms or conditions. The new guidance will reduce diversity in practice and result in fewer changes to the terms of an award being accounted for as modifications. The new authoritative guidance will be effective for public business entities in fiscal years beginning after December 15, 2017. The authoritative guidance will be effective for the Company beginning in fiscal year 2018. The Company does not anticipate that adoption of this guidance will have a material impact on its financial statements.
In February 2018, the FASB issued ASU 2018-02, Income Statement – Reporting Comprehensive Income (Topic 220). The amendments ASU 2018-02 allow a reclassification from accumulated other comprehensive income to retained earnings for stranded tax effects resulting from the Tax Cuts and Jobs Act. Consequently, the amendments eliminate the stranded tax effects resulting from the Tax Cuts and Jobs Act and will improve the usefulness of information reported to financial statement users. The new authoritative guidance will be effective for all entities for fiscal years beginning after December 15, 2018. For public business entities, early adoption is permitted at any time, including interim periods, for reporting periods for which financial statement have not yet been issued. The Company does not currently expect that the impact from the adoption of this guidance to have a material impact on its financial statements, as the adoption of ASU 2016-01 on January 1, 2018 will eliminate the current accumulated other comprehensive loss balance.
3. Balance Sheet Components
Property, plant and equipment, net
As of December 31, 2017 and 2016, property, plant and equipment, net were comprised of the following (in thousands):
|
|
December 31,
|
|||||
|
|
2017
|
2016
|
||||
|
Computers, machinery and equipment
|
$
|
2,112
|
|
$
|
2,545
|
|
|
Furniture, fixtures and leasehold improvements
|
|
5,275
|
|
|
5,421
|
|
|
|
|
7,387
|
|
|
7,966
|
|
|
Less - accumulated depreciation and amortization
|
|
(2,844
|
)
|
|
(2,491
|
)
|
|
Property, plant and equipment, net
|
$
|
4,543
|
|
$
|
5,475
|
|
Depreciation and amortization expense amounted to $1.1 million, $1.2 million, and $564,000 for the years ended December 31, 2017, 2016 and 2015, respectively.
Accrued expenses
As of December 31, 2017 and 2016, accrued expenses were comprised of the following (in thousands):
|
|
December 31,
|
|||||
|
|
2017
|
2016
|
||||
|
Accrued compensation and benefits
|
$
|
1,561
|
|
$
|
1,770
|
|
|
Other accrued expenses
|
|
996
|
|
|
725
|
|
|
Accrued expenses
|
$
|
2,557
|
|
$
|
2,495
|
|
On November 2, 2017, we began making adjustments to our operating expenses as appropriate by reducing staffing allocated to non-clinical activities as a part of a broader effort to more closely align operating expenses with the Company’s primary goal of continuing to generate clinical data in our clinical stage programs which we believe are the activities that have the greatest potential to create value for shareholders over the next several years. The
59
reduction in staffing affected approximately 25 employees and was completed in the fourth quarter of 2017. The Company recognized approximately $0.5 million of pre-tax restructuring charges in the fourth quarter of 2017 in connection with the reduction in staffing, consisting of severance and other employee termination benefits, substantially all of which are expected to be settled in cash. As of December 31, 2017, $0.3 million in severance and other termination benefits are still due to employees and included in accrued compensation and benefits.
4. Investments in BioTime and OncoCyte Common Stock
Investment in BioTime Common Stock
BioTime common shares (traded on NYSE American under the symbol “BTX”) are included at fair value in current assets on the balance sheets as the shares are available for use and could be sold at fair value for working capital purposes. During the year ended December 31, 2017, Asterias sold 371,795 of its BTX shares at a weighted-average price of $2.60. As of December 31, 2017 and 2016, Asterias held 3,481,085 and 3,852,880 BioTime shares, respectively. As of December 31, 2017 and 2016 these shares are valued at $7.5 million and $13.9 million, respectively, based on the closing price on those dates.
Investment in OncoCyte Common Stock
On December 31, 2015, in connection with BioTime’s distribution of OncoCyte common stock to BioTime shareholders, on a pro rata basis, Asterias received 192,644 shares of OncoCyte common stock from BioTime as a dividend in kind. On this date, BioTime shareholders, including Asterias, received one share of OncoCyte common stock for every twenty shares of BioTime common stock held. Asterias recorded the fair value of the OncoCyte common stock as contributed capital from BioTime. The OncoCyte common stock distribution resulted in a taxable gain to Asterias of $819,000 (see Note 10).
The OncoCyte shares are included in available-for-sale securities at fair value in current assets in Asterias’ balance sheets as the shares are traded on NYSE American (symbol “OCX”) and available for working capital purposes. As of December 31, 2017 and 2016, Asterias held 181,756 and 192,644 shares of OncoCyte, respectively. As of December 31, 2017 and 2016, the OncoCyte shares are valued at $0.8 million and $1.4 million, respectively, based on the OncoCyte closing price on those dates.
5. Intangible assets, net
As of December 31, 2017 and, 2016, Asterias had capitalized intangible assets acquired from Geron Corporation, primarily related to patents and other intellectual property rights related to hES cells. These assets are being amortized over their estimated useful lives of 10 years.
Intangible assets, net at December 31, 2017 and, 2016 are shown in the following table (in thousands):
|
|
December 31,
|
|||||
|
|
2017
|
2016
|
||||
|
Intangible assets
|
$
|
26,860
|
|
$
|
26,860
|
|
|
Less - accumulated amortization
|
|
(11,416
|
)
|
|
(8,730
|
)
|
|
Intangible assets, net
|
$
|
15,444
|
|
$
|
18,130
|
|
Asterias recognized $2.7 million in amortization expense of intangible assets for the years ended December 31, 2017, 2016 and 2015, respectively.
Amortization of intangible assets for periods subsequent to December 31, 2017 is as follows (in thousands):
|
Year Ending
December 31, |
Amortization
Expense |
||
|
2018
|
|
2,686
|
|
|
2019
|
|
2,686
|
|
|
2020
|
|
2,686
|
|
|
2021
|
|
2,686
|
|
|
2022
|
|
2,686
|
|
|
Thereafter
|
|
2,014
|
|
|
Total
|
$
|
15,444
|
|
60
6. Common Stock and Warrants
At December 31, 2017, Asterias had outstanding 54,051,142 Series A Shares and no Series B Shares. At December 31, 2016, Asterias had outstanding 47,466,596 Series A Shares and no Series B Shares. All outstanding Series B Shares were converted into Series A Shares on October 3, 2014.
Common Stock Issuance
On October 16, 2017, Asterias completed the sale, in a registered direct offering, of 4,000,000 shares of its common stock, at an offering price of $2.60 per share, or net proceeds of $9.9 million.
On May 13, 2016, Asterias completed the sale and the underwriters’ exercise of the overallotment for 5,889,480 shares of its common stock and warrants to purchase 2,959,559 shares of its common stock, through an underwritten public offering (the “Asterias Offering”), for $3.40 per unit, or net proceeds to Asterias of $18.2 million. Total financing costs were approximately $1.8 million, of which $1.3 million were allocated to the Asterias common stock (see Warrants classified as liability below). The net proceeds allocated to the common stock were $12.7 million and the net proceeds allocated to the warrants were $5.5 million. During the year ended December 31, 2016, Asterias received approximately $2.7 million in net proceeds from exercise of stock options and warrants.
During the year ended December 31, 2015, Asterias raised approximately $5.5 million in aggregate gross proceeds from the sale of 1,410,255 shares of common stock at a price of $3.90 per share through an underwritten public offering and a private placement. Broadwood Partners, L.P., British & American Investment Trust PLC and Pedro Lichtinger, related parties, purchased an aggregate of 1,025,640 of the shares.
On April 10, 2015, Asterias entered into an at-the-market (ATM) Sales Agreement with MLV & Co., which is now owned by B. Riley FBR, Inc., pursuant to which Asterias may sell up to a maximum of $20.0 million of its common stock from time to time through the Sales Agent under Asterias’ previously filed and currently effective shelf registration statement on Form S-3 (File No. 333-200745). On March 28, 2017, Asterias entered into an amendment to this Sales Agreement. Under the Sales Agreement, as amended, Asterias may issue and sell shares of its Series A common stock having an aggregate offering price of up to an additional $25.0 million. During the fiscal year ended December 31, 2017, Asterias raised approximately $8.0 million in gross proceeds under the ATM from the sale of 2,005,784 shares of its common stock at a weighted average price of $3.99. During the fiscal year ended December 31, 2016, Asterias raised approximately $8.0 million in gross proceeds under the ATM from the sale of 1,811,522 shares of its common stock at a weighted average price of $4.41 per share. During the fiscal year ended December 31, 2015, Asterias raised approximately $4.8 million in gross proceeds from the sale of 685,465 shares of its common stock at a weighted average price of $7.01 per share. As of December 31, 2017, up to approximately $22.7 million of shares of Asterias common stock are available for issuance and sale pursuant to the terms of the ATM Sales Agreement.
During 2017, 2016 and 2015, pursuant to a services agreement with Cell Therapy Catapult Services Limited, Asterias had issued 318,748 shares, 218,520 shares and 94,479 shares, respectively of Asterias Series A common stock with a fair value of $1.2 million, $922,000 and $486,000, respectively to pay for services in lieu of cash (see Note 13).
Asterias issued 148,594 shares of common stock for proceeds of $1.1 million, pursuant to the exercise of warrants in 2016. Asterias issued 5,000,000 shares of common stock pursuant to the exercise of warrants in 2015, for net proceeds of $11.7 million.
Warrants classified as a liability
On May 13, 2016, included in the Asterias Offering, Asterias issued 2,959,559 warrants (the “Asterias Offering Warrants”). The Asterias Offering Warrants have an exercise price $4.37 per share and expire in five years of the issuance date, or May 13, 2021. The Asterias Offering Warrants also contain certain provisions in the event of a Fundamental Transaction, as defined in the warrant agreement governing the Asterias Offering Warrants (“Warrant Agreement”), that Asterias or any successor entity will be required to purchase, at a holder’s option, exercisable at any time concurrently with or within thirty days after the consummation of the fundamental transaction, the Asterias Offering Warrants for cash. This cash settlement will be in an amount equal to the value of the unexercised portion of such holder’s warrants, determined in accordance with the Black Scholes-Merton option pricing model as specified in the Warrant Agreement.
61
In accordance with ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock, contracts that require or may require the issuer to settle the contract for cash are liabilities recorded at fair value, irrespective of the likelihood of the transaction occurring that triggers the net cash settlement feature. Changes to the fair value of those liabilities are recorded in the statements of operations. Accordingly, since Asterias may be required to net cash settle the Asterias Offering Warrants in the event of a Fundamental Transaction; the Asterias Offering Warrants are classified as noncurrent liabilities at fair value, with changes in fair value recorded in other income or expense, net, in the statements of operations.
The fair value of the Asterias Offering Warrants at the time of issuance was determined by using a combination of the Binomial Lattice and Black-Scholes-Merton option pricing models under various probability-weighted outcomes which take into consideration the probability of the fundamental transaction and net cash settlement occurring, using the contractual term of the warrants. In applying these models, the fair value is determined by applying Level 3 inputs, as defined by ASC 820; these inputs have included assumptions around the estimated future stock price of Asterias common stock, volatility and the timing of, and varying probabilities that certain events will occur. The Asterias Offering Warrants are revalued each reporting period using the same methodology described above. Changes in any of the key assumptions used to value the Asterias Offering Warrants could materially impact the fair value of the warrants and Asterias’ financial statements.
On May 13, 2016, the fair value of the Asterias Offering Warrants was approximately $6.0 million. Because the Asterias Offering Warrants are accounted for as liabilities, the total proceeds from the Asterias Offering were allocated first entirely to the Asterias Offering Warrants’ fair value and the remaining residual proceeds to the Asterias common stock. In addition, of the total $1.8 million of the Asterias Offering discounts and expenses incurred, $0.6 million were allocated to the Asterias Offering Warrants, based on the full fair value of the Asterias Offering Warrants and total gross proceeds, and immediately expensed as general and administrative expenses. Total net proceeds allocated to the Asterias Offering Warrants were $5.5 million.
On December 2, 2016, certain investors exercised 146,400 Asterias Offering Warrants for cash proceeds to Asterias of approximately $640,000 (see Note 2).
At December 31, 2017 and 2016, based on valuations performed by Asterias using the methodology described above, the fair value of the Asterias Offering Warrants liability was $2.8 million and $8.7 million, respectively, resulting in Asterias recording an unrealized gain of $5.9 million for the year ended December 31, 2017 and an unrealized loss of $3.1 million for the year ended December 31, 2016, which are included in other income and expenses, net, in the statements of operations.
Warrants classified as equity
On March 30, 2016, Asterias’ board of directors declared a distribution of Asterias common stock purchase warrants to all Asterias shareholders other than BioTime, in the ratio of one warrant for every five shares of Asterias common stock owned of record as of the close of business on April 11, 2016. On April 25, 2016, Asterias distributed 3,331,229 warrants (the “Distribution Warrants”). The distribution of the Distribution Warrants was treated as a disproportionate distribution since, in accordance with the terms of the Share Transfer with BioTime, no warrants were distributed to BioTime (see Note 15). The Distribution Warrants are classified as equity, have an exercise price of $5.00 per share, and were set to expire on September 30, 2016. Asterias recorded the Distribution Warrants at a fair value of approximately $3.1 million with a noncash charge to shareholder expense included in general and administrative expenses and a corresponding increase to equity as of March 30, 2016 as the Distribution Warrants were deemed to be issued for accounting purposes on that date.
On September 19, 2016 and February 3, 2017, Asterias extended the expiration date of the Distribution Warrants to February 15, 2017 and September 29, 2017, respectively, no other terms were changed. As a result of the extension of the expiration date of these warrants, Asterias recorded a $2.0 million and $1.7 million noncash charges to shareholder expense included in general and administrative expenses and a corresponding increase to equity for the years ended December 31, 2016 and 2017. These warrants expired unexercised on September 29, 2017.
In connection with the warrant distribution to shareholders discussed above, 350,000 warrants with an exercise price of $5.00 per share held by Romulus Films, Ltd. were adjusted to become exercisable into 409,152 shares at an exercise price of $4.28 per share (the “Romulus Warrants”). These warrants had an original expiration date of September 30, 2016. On September 19, 2016, Asterias extended the expiration date of the Romulus Warrants to February 15, 2017, no other terms were changed. As a result of the extension of the expiration date of these warrants,
62
Asterias recorded a $0.2 million noncash charge to shareholder expense included in general and administrative expenses and a corresponding increase to equity for the year ended December 31, 2016. On February 3, 2017, Asterias extended the expiration date of the Romulus Warrants to September 29, 2017. These warrants expired unexercised on September 29, 2017.
Warrants Outstanding in 2017, 2016 and 2015
At December 31, 2014, warrants to purchase 8,500,000 common shares with a weighted average exercise price of $3.44 and a weighted average remaining contractual life of 0.99 years were outstanding. At December 31, 2015, warrants to purchase 3,500,000 common shares with an exercise price of $5.00 and a weighted average remaining contractual life of 0.75 years were outstanding (see Note 15).
In February 2016, of the warrants to purchase 3,500,000 shares, 3,150,000 were returned to Asterias by BioTime as part of the Share Transfer between Asterias and BioTime (see Note 9). As of March 20, 2016, these warrants to purchase 3,150,000 shares were retired by Asterias. Asterias warrants outstanding ending December 31, 2016 was 6,552,479. In September 2017, 3,328,033 shares of unexercised Distribution Warrants and 409,152 shares of unexercised Romulus Warrants expired. Asterias warrants outstanding at December 31, 2017 was 2,813,159.
Activity related to equity and liability classified warrants in 2017 and 2016, is presented in the table below (in thousands, except per share and weighted average exercise prices):
|
|
Number of
Warrants |
Per share
exercise price |
Weighted
Average Exercise Price |
||||||
|
Outstanding, December 31, 2015
|
|
3,500
|
|
$
|
5.00
|
|
$
|
5.00
|
|
|
Issued in 2016
|
|
6,350
|
|
|
4.28-5.00
|
|
|
4.69
|
|
|
Exercised in 2016
|
|
(148
|
)
|
|
4.37-5.00
|
|
|
4.38
|
|
|
Retired in 2016
|
|
(3,150
|
)
|
|
5.00
|
|
|
5.00
|
|
|
Outstanding, December 31, 2016
|
|
6,552
|
|
$
|
4.28-5.00
|
|
$
|
4.68
|
|
|
Expired in 2017
|
|
(3,738
|
)
|
|
5.00
|
|
|
5.00
|
|
|
Exercised in 2017
|
|
(1
|
)
|
|
5.00
|
|
|
5.00
|
|
|
Outstanding, December 31, 2017
|
|
2,813
|
|
$
|
4.37
|
|
$
|
4.37
|
|
7. Equity Incentive Plan
During March 2013, Asterias’ Board of Directors approved an Equity Incentive Plan (the “Plan”) under which Asterias has reserved 4,500,000 shares of common stock for the grant of stock options or the sale of restricted stock. Initially, Asterias issued Series B Shares under the Plan. Since the date on which all of the outstanding Series B Shares were converted into Series A Shares, Asterias has issued Series A Shares under the Plan. The Plan also permits Asterias to issue such other securities as its Board of Directors or the Compensation Committee administering the Plan may determine. Asterias’ stockholders approved the Plan in September 2013.
During May 2015, Asterias’ Board of Directors approved an amendment to increase the number shares authorized for issuance under the Plan by 3,500,000 shares. This amendment was approved by the shareholders at the 2015 annual meeting of shareholders held on July 9, 2015.
During May 2016, Asterias’ Board of Directors approved an amendment to increase the number of shares authorized for issuance under the Plan by 3,000,000 shares. This amendment was approved by the shareholders at the 2016 annual meeting of shareholders held on June 9, 2016.
During May 2017, Asterias’ Board of Directors approved an amendment to increase the number of shares authorized for issuance under the Plan by 2,500,000 shares. This amendment was approved by the shareholders at the 2017 annual meeting of shareholders held on June 14, 2017.
No options may be granted under the Plan more than ten years after the date upon which the Plan was adopted by the Board of Directors, and no options granted under the Plan may be exercised after the expiration of ten years from the date of grant. Under the Plan, options to purchase common stock may be granted to employees, directors and certain consultants at prices not less than the fair market value at date of grant, subject to certain limited
63
exceptions for options granted in substitution of other options. Options may be fully exercisable immediately, or may be exercisable according to a schedule or conditions specified by the Board of Directors or the Compensation Committee. The Plan also permits Asterias to award restricted stock for services rendered or to sell common stock to employees, subject to vesting provisions under restricted stock agreements that provide for forfeiture of unvested shares upon the occurrence of specified events under a restricted stock award agreement. Asterias may permit employees or consultants, but not officers or directors, who purchase stock under restricted stock purchase agreements, to pay for their shares by delivering a promissory note that is secured by a pledge of their shares.
Asterias may also grant stock appreciation rights (“SARs”) and hypothetical units issued with reference to Asterias common stock (restricted stock units or “RSUs”) under the Plan. A SAR is the right to receive, upon exercise, an amount payable in cash or shares or a combination of shares and cash, as determined by the Board of Directors or the Compensation Committee, equal to the number of shares subject to the SAR that is being exercised multiplied by the excess of (a) the fair market value of a share of Asterias common stock on the date the SAR is exercised, over (b) the exercise price specified in the SAR Award agreement.
The terms and conditions of a grant of RSUs is determined by the Board of Directors or Compensation Committee. No shares of stock will be issued at the time a RSU is granted, and Asterias will not be required to set aside a fund for the payment of any such award. A recipient of RSUs will have no voting rights with respect to the Restricted Stock Units. Upon the expiration of the restrictions applicable to a RSU, Asterias will either issue to the recipient, without charge, one share of common stock per RSU or cash in an amount equal to the fair market value of one share of common stock.
Stock Options
As of December 31, 2017, Asterias had outstanding to certain officers, employees, and directors, options to purchase a total of 6,375,828 shares of common stock at a weighted average exercise price of $3.31 per share and 690,000 restricted stock/RSUs.
The following table summarizes the stock option activity related to shares of common stock under the Company’s Option Plan:
|
|
Number of
Options Outstanding |
Weighted
Average Exercise Price |
Weighted
Average Remaining Contractual Life (Years) |
Aggregate
Intrinsic Value (in thousands) |
||||||||
|
Options outstanding at December 31, 2014
|
|
3,101
|
|
$
|
2.42
|
|
|
5.64
|
|
$
|
2,724
|
|
|
Options granted
|
|
2,000
|
|
|
4.33
|
|
|
|
|
|
|
|
|
Options exercised
|
|
(12
|
)
|
|
2.34
|
|
|
|
|
|
|
|
|
Options forfeited/cancelled
|
|
(14
|
)
|
|
3.45
|
|
|
|
|
|
|
|
|
Options outstanding at December 31, 2015
|
|
5,075
|
|
$
|
3.17
|
|
|
6.37
|
|
$
|
4,835
|
|
|
Options granted
|
|
2,975
|
|
|
3.56
|
|
|
|
|
|
|
|
|
Options exercised
|
|
(827
|
)
|
|
2.45
|
|
|
|
|
|
|
|
|
Options forfeited/cancelled
|
|
(991
|
)
|
|
3.86
|
|
|
|
|
|
|
|
|
Options outstanding at December 31, 2016
|
|
6,232
|
|
$
|
3.34
|
|
|
6.96
|
|
$
|
8,183
|
|
|
Options granted
|
|
1,690
|
|
|
3.57
|
|
|
|
|
|
|
|
|
Options exercised
|
|
(8
|
)
|
|
2.34
|
|
|
|
|
|
|
|
|
Options forfeited/cancelled
|
|
(1,538
|
)
|
|
3.72
|
|
|
|
|
|
|
|
|
Options outstanding at December 31, 2017
|
|
6,376
|
|
$
|
3.31
|
|
|
6.01
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options vested and expected to vest at December 31, 2017
|
|
6,376
|
|
$
|
3.31
|
|
|
6.01
|
|
$
|
—
|
|
|
Options exercisable at December 31, 2017
|
|
4,219
|
|
$
|
3.17
|
|
|
4.61
|
|
$
|
—
|
|
The aggregate intrinsic value represents the difference between the exercise price of the awards and the Company’s fair value per share of $2.25, $4.60 and $3.93 as of December 31, 2017, 2016, and 2015, respectively.
64
Additional information regarding the Company’s outstanding stock options and vested and exercisable stock options is summarized below:
|
|
As of December 31, 2017
|
||||||||||||||
|
|
Options Outstanding
|
Options Exercisable
|
|||||||||||||
|
Exercise Prices
|
Number of
Shares |
Weighted-
Average Remaining Contractual Life (Years) |
Weighted-
Average Exercise Price |
Number of
shares |
Weighted-
Average Exercise Price per Share |
||||||||||
|
$2.34 - $3.60
|
|
2,794
|
|
|
4.09
|
|
$
|
2.58
|
|
|
2,238
|
|
$
|
2.47
|
|
|
$3.64 - $6.17
|
|
3,478
|
|
|
7.69
|
|
$
|
3.82
|
|
|
1,879
|
|
$
|
3.85
|
|
|
$6.22 - $6.25
|
|
104
|
|
|
1.69
|
|
$
|
6.23
|
|
|
102
|
|
$
|
6.23
|
|
|
|
|
6,376
|
|
|
6.01
|
|
$
|
3.31
|
|
|
4,219
|
|
$
|
3.17
|
|
Restricted Stock and Restricted Stock Units
The following table summarizes the restricted stock award and restricted stock unit activity under the Company’s Option Plan:
|
Restricted Stock Award/Unit
|
Number of
RSUs Outstanding |
Weighted
Average Grant Date Fair Value |
Weighted
Average Remaining Contractual Life (Years) |
Aggregate
Intrinsic Value (in thousands) |
||||||||
|
RSUs outstanding at December 31, 2014
|
|
100
|
|
$
|
3.64
|
|
|
0.23
|
|
$
|
324
|
|
|
RSUs granted
|
|
194
|
|
|
2.67
|
|
|
|
|
|
|
|
|
RSUs vested
|
|
(245
|
)
|
|
1.58
|
|
|
|
|
|
|
|
|
RSUs canceled
|
|
(1
|
)
|
|
2.62
|
|
|
|
|
|
|
|
|
RSUs outstanding at December 31, 2015
|
|
48
|
|
$
|
2.67
|
|
|
0.25
|
|
$
|
114
|
|
|
RSAs/RSUs granted
|
|
515
|
|
|
3.54
|
|
|
|
|
|
|
|
|
RSAs/RSUs vested
|
|
(360
|
)
|
|
3.51
|
|
|
|
|
|
|
|
|
RSUs canceled
|
|
(3
|
)
|
|
3.49
|
|
|
|
|
|
|
|
|
RSUs outstanding at December 31, 2016
|
|
200
|
|
$
|
3.39
|
|
|
1.22
|
|
$
|
380
|
|
|
RSUs granted
|
|
1,007
|
|
|
3.25
|
|
|
|
|
|
|
|
|
RSAs/RSUs vested
|
|
(351
|
)
|
|
3.53
|
|
|
|
|
|
|
|
|
RSUs canceled
|
|
(166
|
)
|
|
3.58
|
|
|
|
|
|
|
|
|
RSUs outstanding at December 31, 2017
|
|
690
|
|
$
|
3.06
|
|
|
2.31
|
|
$
|
1,553
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RSUs vested and expected to vest at December 31, 2017
|
|
690
|
|
$
|
3.06
|
|
|
2.31
|
|
$
|
1,553
|
|
The aggregate intrinsic value for RSUs represents the Company’s fair market value per share of the awards and the Company’s fair value per share of $2.25, $4.60, and $3.93 as of December 31, 2017, 2016, and 2015, respectively for the total number of underlying RSUs.
Stock-Based Compensation Expense
The weighted-average estimated fair value of stock options granted during the years ended December 31, 2017, 2016 and 2015 was $3.57, $3.56, and $4.33 per share respectively, using the Black-Scholes-Merton option pricing model with the following weighted-average assumptions:
|
|
Years Ended December 31,
|
||||||||
|
|
2017
|
2016
|
2015
|
||||||
|
Expected life (in years)
|
|
5.76
|
|
|
5.88
|
|
|
3.41
|
|
|
Risk-free interest rates
|
|
1.89
|
%
|
|
1.33
|
%
|
|
1.01
|
%
|
|
Volatility
|
|
74.64
|
%
|
|
75.6
|
%
|
|
34.67
|
%
|
|
Dividend yield
|
|
0
|
%
|
|
0
|
%
|
|
0
|
%
|
65
The risk-free rate is based on the rates in effect at the time of grant for zero coupon U.S. Treasury notes with maturities approximately equal to each grant’s expected life. A dividend yield of zero is applied since Asterias has not historically paid dividends and does not expect to pay dividends in the foreseeable future. The expected volatility is based upon the volatility of Asterias’ own trading stock and of a group of publicly traded industry peer companies. The expected term of options granted is derived from a combination of Asterias historical experience, to the extent available, and using the simplified method under SEC Staff Accounting Bulletin Topic 14.
Prior to the adoption of ASU 2016-09 in 2017 stock-based compensation expense was recognized based on awards that are ultimately expected to vest, and as a result, the amount has been reduced by estimated forfeitures. Forfeitures were estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differed from those estimates. Forfeitures were estimated based on Asterias’ historical experience and future expectations. Subsequent to the adoption of ASU 2016-09 on January 1, 2017, forfeitures are accounted for as they occur.
The determination of stock-based compensation is inherently uncertain and subjective and involves the application of valuation models and assumptions requiring the use of judgment. If Asterias had made different assumptions, its stock-based compensation expense, and net loss for years ended December 31, 2017, 2016 and 2015, may have been significantly different.
Asterias does not recognize deferred income taxes for incentive stock option compensation expense, and records a tax deduction only when a disqualified disposition has occurred.
Operating expenses include stock-based compensation expense as follows (in thousands):
|
|
Year Ended December 31,
|
||||||||
|
|
2017
|
2016
|
2015
|
||||||
|
Research and development
|
$
|
2,469
|
|
$
|
2,655
|
|
$
|
1,604
|
|
|
General and administrative
|
|
1,975
|
|
|
2,142
|
|
|
2,021
|
|
|
Total stock-based compensation expense
|
$
|
4,444
|
|
$
|
4,797
|
|
$
|
3,625
|
|
As of December 31, 2017, unrecognized compensation expense related to stock options and RSUs was $4.8 million and $1.7 million, respectively with a weighted-average remaining amortization period of 2.75 years and 2.31 years, respectively.
Common Stock Reserved for Future Issuance
The Company had the following shares of common stock reserved for future issuance under the Equity Incentive Plan:
|
|
As of December 31,
|
|||||
|
|
2017
|
2016
|
||||
|
Equity Incentive Plan:
|
|
|
|
|
|
|
|
Common stock subject to options outstanding
|
|
6,376
|
|
|
6,232
|
|
|
RSUs outstanding
|
|
690
|
|
|
200
|
|
|
Shares available for future grants
|
|
2,782
|
|
|
2,115
|
|
|
Common stock reserved for future issuances
|
|
9,848
|
|
|
8,547
|
|
8. Commitments and Contingencies
Development and Manufacturing Services Agreement
On August 3, 2016, Asterias entered into a Development and Manufacturing Services Agreement (the “Services Agreement”) with Cognate BioServices, Inc. (“Cognate”), a fully-integrated contract bioservices organization providing development and current Good Manufacturing Practice (“cGMP”) manufacturing services to companies and institutions engaged in the development of cell-based products.
Under the Services Agreement, Cognate is performing under an Initial Statement of Work process development studies in support of Asterias’ clinical and commercial development activities of AST-VAC1 and production and manufacturing services of AST-VAC1 under cGMP under the Second Statement of Work. In consideration for the
66
process development services set forth in the Initial Statement of Work, Asterias agreed to make aggregate payments of up to approximately $1.7 million in fees over the term of the Initial Statement of Work and pay for additional pass through costs for materials and equipment estimated by management to be approximately $0.5 million. In consideration of the production and manufacturing services set forth in the Second Statement of Work, once the services under the Initial Statement of Work are completed and if Asterias receives FDA concurrence on the clinical protocol for an AST-VAC1 trial, then Asterias will make an initial start-up payment, a monthly payment for dedicated manufacturing capacity, and certain other manufacturing fees.
On August 16, 2017, the Company amended SOW 1 (“Amended SOW 1”) and entered into a Statement of Work 1.5 (“SOW 1.5”) with Cognate to modify the timing of certain process development studies being performed by Cognate under the Services Agreement. Under Amended SOW 1 and SOW 1.5, Cognate will perform certain process development studies initially contemplated by SOW 1 under SOW 1.5 after Cognate has completed the activities under Amended SOW 1 and the Company provides written notice to commence the activities under SOW 1.5.
The Services Agreement will expire on the later of (a) August 3, 2019; or (b) the completion of all services contracted for by the parties in the Statements of Work under the Services Agreement prior to August 3, 2019. The term of the Services Agreement and any then pending Statements of Work thereunder may be extended by Asterias continuously for additional two-year periods upon written notice to Cognate with at least thirty days prior to the expiration of the then-current term.
The Services Agreement provides certain termination rights to each party and customary provisions relating to indemnity, confidentiality and other matters. Asterias incurred $1.1 million and $574,000 of expenses payable to Cognate pursuant to the Services Agreement for the years ended December 31, 2017 and 2016.
Fremont Lease
On December 30, 2013, Asterias entered into a lease for an office and research facility located in Fremont, California, consisting of an existing building with approximately 44,000 square feet of space. The building is being used by Asterias as a combined office, laboratory and production facility that can be used to produce hES and related products under current good manufacturing procedures. Asterias completed the tenant improvements in November 2015, which cost approximately $4.9 million, of which the maximum of $4.4 million was paid to Asterias by the landlord. Asterias placed the asset into service in November 2015 and is amortizing the leasehold improvements and the landlord liability over the remaining lease term through September 30, 2022.
As of December 31, 2017 and 2016, the landlord liability was $3.5 million and $4.0 million, respectively and the deferred rent liability was $316,000 and $266,000, respectively.
Beginning on October 1, 2016, base rent increased to $105,000 per month and will increase by approximately 3% annually on every October 1 thereafter. On October 1, 2017, the base rent increased to $108,000 per month.
In addition to monthly base rent, Asterias will pay all real estate taxes, insurance and the cost of maintenance, repair and replacement of the leased premises. During the first 15 months of the lease term, Asterias paid only 50% of the real estate taxes assessed on the premises. Beginning January 1, 2016, Asterias will pay 100% of the taxes levied on the excess assessed valuation.
Asterias is considered the owner of the asset for accounting purposes only under build-to-suit accounting under ASC 840-40-55, Accounting for Leases, Sale-leaseback transactions, as Asterias, among other things, has the primary obligation to pay for construction costs and Asterias will retain exclusive use of the building for its office and research facility requirements after construction is completed. In addition, the lease does not qualify for sale-leaseback accounting due to Asterias’ significant continuing involvement with the facility that Asterias considers to be other than a normal leaseback as defined by GAAP. In accordance with this guidance, amounts previously expended by Asterias for construction would continue to be reported as construction in progress in Asterias’ financial statements, and the landlord reimbursement proceeds received, including amounts earned by Asterias but not yet paid by the landlord at period end, are reported as a lease liability. The property was placed in service in November 2015 and Asterias commenced depreciating the property. Lease payments allocated to the landlord liability are accounted for as debt service payments on that liability using the finance method of accounting. As of December 31, 2015, Asterias had incurred $4.9 million of construction costs included in property, plant and equipment (see Note 3), of which $4.4 million was the lease liability included in long term liabilities at December 31, 2015. The lease liability is being amortized using the effective interest method.
67
Total rent expense for all rented facilities for the years ended December 31, 2017, 2016, and 2015 was $0.4 million, $0.5 million, and $1.0 million, respectively.
Future minimum annual lease payments, including the lease liability, under the Fremont Lease for the years ending after December 31, 2017 are as follows (in thousands):
|
Year Ending
December 31, |
Minimum Lease
Payments |
||
|
2018
|
$
|
1,308
|
|
|
2019
|
|
1,346
|
|
|
2020
|
|
1,389
|
|
|
2021
|
|
1,431
|
|
|
2022
|
|
1,097
|
|
|
Total
|
$
|
6,571
|
|
Litigation – General
Asterias is subject to various claims and contingencies in the ordinary course of its business, including those related to litigation, business transactions, employee-related matters, and others. When Asterias is aware of a claim or potential claim, it assesses the likelihood of any loss or exposure. If it is probable that a loss will result and an amount that can be reasonably estimated, Asterias will record a liability for the loss. If the loss is not probable or the amount of the loss cannot be reasonably estimated, Asterias discloses the claim if the likelihood of a potential loss is reasonably possible and the amount involved could be material. Asterias is not aware of any claims likely to have a material adverse effect on its financial condition or results of operations.
Employment Contracts
Asterias has entered into employment contracts with certain executive officers. Under the provisions of the contracts, Asterias may be required to incur severance obligations for matters relating to changes in control, as defined and involuntary terminations. In 2016, Asterias paid $309,000 in severance to two former executives in accordance with their respective separation agreements.
At December 31, 2017, total potential severance obligations in connection with the termination of employment contracts approximated $1.3 million for termination without cause and $2.0 million for termination due to a change in control.
Indemnification
In the normal course of business, Asterias may provide indemnifications of varying scope under Asterias’ agreements with its directors and executive employees or other companies or consultants, typically Asterias’ clinical research organizations, investigators, clinical sites, suppliers and others. Pursuant to these agreements, Asterias will generally agree to indemnify, hold harmless, and reimburse the indemnified parties for losses and expenses suffered or incurred by the indemnified parties arising from claims of third parties in connection with the use or testing of Asterias’ products and services. Indemnification provisions could also cover third-party infringement claims with respect to patent rights, copyrights, or other intellectual property pertaining to Asterias products and services. The term of these indemnification agreements will generally continue in effect after the termination or expiration of the particular research, development, services, or license agreement to which they relate. The potential future payments Asterias could be required to make under these indemnification agreements will generally not be subject to any specified maximum amount. Historically, Asterias has not been subject to any claims or demands for indemnification. Asterias maintains various liability insurance policies that limit Asterias’ exposure. As a result, Asterias believes the fair value of these indemnification agreements is minimal. Accordingly, Asterias has not recorded any liabilities for these agreements as of December 31, 2017 and 2016.
9. Shared Facilities and Service Agreement
On April 1, 2013, Asterias and BioTime executed a Shared Facilities and Services Agreement (“Shared Facilities Agreement”). Under the terms of the Shared Facilities Agreement, BioTime will allow Asterias to use its premises and equipment located at Alameda, California for the sole purpose of conducting business. BioTime will provide
68
basic accounting, billing, bookkeeping, payroll, treasury, collection of accounts receivable (excluding the institution of legal proceedings or taking of any other action to collect accounts receivable), payment of accounts payable, and other similar administrative services to Asterias. BioTime may also provide the services of attorneys, accountants, and other professionals who may also provide professional services to BioTime and its other subsidiaries. BioTime will also provide Asterias with the services of its laboratory and research personnel, including BioTime employees and contractors, for the performance of research and development work for Asterias at BioTime’s premises.
BioTime will charge Asterias a fee for the services and usage of facilities, equipment, and supplies aforementioned. For each billing period, BioTime will equitably prorate and allocate its employee costs, equipment costs, insurance costs, lease costs, professional costs, software costs, supply costs, and utilities costs, between BioTime and Asterias based upon actual documented use and cost by or for Asterias or upon proportionate usage by BioTime and Asterias, as reasonably estimated by BioTime. Asterias shall pay 105% of the allocated costs (the “Use Fee”). The allocated cost of BioTime employees and contractors who provide services will be based upon records maintained of the number of hours of such personnel devoted to the performance of services.
The Use Fee will be determined and invoiced to Asterias on a quarterly basis for each calendar quarter of each calendar year. If the Shared Facilities Agreement terminates prior to the last day of a billing period, the Use Fee will be determined for the number of days in the billing period elapsed prior to the termination of the Shared Facilities Agreement. Each invoice will be payable in full by Asterias within 30 days after receipt. Any invoice or portion thereof not paid in full when due will bear interest at the rate of 15% per annum until paid, unless the failure to make a payment is due to any inaction or delay in making a payment by BioTime employees from Asterias funds available for such purpose, rather than from the unavailability of sufficient funds legally available for payment or from an act, omission, or delay by any employee or agent of Asterias.
In addition to the Use Fees, Asterias will reimburse BioTime for any out of pocket costs incurred by BioTime for the purchase of office supplies, laboratory supplies, and other goods and materials and services for the account or use of Asterias, provided that invoices documenting such costs are delivered to Asterias with each invoice for the Use Fee. Furthermore, BioTime will have no obligation to purchase or acquire any office supplies or other goods and materials or any services for Asterias, and if any such supplies, goods, materials or services are obtained for Asterias, BioTime may arrange for the suppliers thereof to invoice Asterias directly.
Asterias in turn may charge BioTime or any Other Subsidiary for similar services provided by Asterias at the same rate and terms as aforementioned. “Other Subsidiary” means a subsidiary of BioTime other than Asterias and other than a subsidiary of Asterias.
The Shared Facilities Agreement’s term ended on December 31, 2017 but the Shared Facilities Agreement was automatically renewed for an additional year and will expire on December 31, 2018. Under the Shared Facilities Agreement, the term of the Shared Facilities Agreement will automatically be renewed and the termination date will be extended for an additional year each year, unless either party gives the other party written notice stating that the Shared Facilities Agreement will terminate on December 31 of that year.
General and administrative expenses include costs allocated from BioTime pursuant to the Shared Facilities Agreement. BioTime allocated $129,000, $265,000, and $282,000, of general overhead expenses to Asterias during the years ended December 31, 2017, 2016 and 2015, respectively. At December 31, 2017 the company had a receivable due from BioTime of $33,000. As of December 31, 2016, Asterias had a $288,000 payable to BioTime under the Shared Facilities Agreement.
69
10. Income Taxes
The primary components of the deferred tax assets and liabilities at December 31, 2017 and 2016 were as follows (in thousands):
|
|
December 31,
|
|||||
|
|
2017
|
2016
|
||||
|
Deferred tax assets:
|
|
|
|
|
|
|
|
Net operating loss carryforwards
|
$
|
15,110
|
|
$
|
16,844
|
|
|
Research and development credits
|
|
5,643
|
|
|
2,395
|
|
|
Stock based compensation and other
|
|
2,130
|
|
|
2,597
|
|
|
Valuation allowance
|
|
(17,691
|
)
|
|
(8,081
|
)
|
|
Total deferred tax assets
|
|
5,192
|
|
|
13,755
|
|
|
Deferred tax liabilities:
|
|
|
|
|
|
|
|
Patents and licenses
|
|
(3,488
|
)
|
|
(7,564
|
)
|
|
Securities held as available for sale
|
|
(1,704
|
)
|
|
(6,191
|
)
|
|
Total deferred tax liabilities
|
|
(5,192
|
)
|
|
(13,755
|
)
|
|
Net deferred tax liabilities
|
$
|
—
|
|
$
|
—
|
|
Income taxes differed from the amounts computed by applying the U.S. federal income tax of 34% to pretax losses from operations as a result of the following:
|
|
Years Ended December 31,
|
|||||
|
|
2017
|
2016
|
||||
|
Computed tax benefit at federal statutory rate
|
|
34
|
%
|
|
34
|
%
|
|
Permanent differences
|
|
3
|
%
|
|
(10
|
%)
|
|
State tax benefit, net of effect on federal income taxes
|
|
5
|
%
|
|
(3
|
%)
|
|
Change in valuation allowance
|
|
(29
|
%)
|
|
(16
|
%)
|
|
Research and development credits
|
|
7
|
%
|
|
1
|
%
|
|
Tax reform – tax rate change
|
|
(20
|
%)
|
|
—
|
|
|
|
|
—
|
%
|
|
6
|
%
|
As of December 31, 2017, Asterias has net operating loss carryforwards of approximately $62.3 million and $29.1 million, respectively, for federal and California tax purposes, which expire between 2032 and 2037 for federal and between 2033 and 2037 for California. In addition, as of December 31, 2017, Asterias has federal and California research tax credit carry forwards of $4.1 million and $2.0 million, respectively. The federal tax credits expire between 2033 and 2036, while the state tax credits have no expiration date.
No federal and state tax provision or benefit was recorded for year ended December 31, 2017. A deferred income tax benefit of approximately $2.3 million was recorded for the year ended December 31, 2016 related to federal taxes. No state tax provision or benefit was recorded for year ended December 31, 2016. A deferred income tax benefit of approximately $7.3 million was recorded for the year ended December 31, 2015, of which approximately $7.4 million was related to federal taxes and $0.1 million was related to state taxes.
Asterias established deferred tax liabilities primarily related to its acquisition of certain intellectual property and available for sale securities held in BioTime and OncoCyte common stock. Asterias has established a valuation allowance for California deferred tax assets as of December 31, 2015. In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon future generation of taxable income during the periods in which temporary differences representing net future deductible amounts become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment. After consideration of all the information available, management believes that significant uncertainty exists with respect to future realization of the deferred tax assets and has therefore established a full valuation allowance for federal and California deferred tax assets as of December 31, 2016 and 2017. For the years ended December 31, 2017 and 2016, the change in the valuation allowance was approximately $9.6 million and $5.2 million, respectively.
70
On February 16, 2016, Asterias entered into a Cross-License Agreement and Share Transfer Agreement with BioTime and BioTime’s wholly owned subsidiary ES Cell International Pte. Ltd. (“ESI”). The transfer of assets was a taxable transaction to Asterias generating a taxable gain of approximately $3.1 million. Asterias has sufficient current year losses from operations in 2016 to offset the entire gain resulting in no income taxes due. As the transfer of assets and the resulting taxable gain is due to a direct effect of transactions between Asterias and its then parent company, BioTime, Asterias recorded the tax effect of this gain through equity with a corresponding release of the valuation allowance, in accordance with ASC 740-20-45-11(g), during the year ended December 31, 2016.
On December 31, 2015, BioTime distributed 4.7 million shares of OncoCyte common stock to its shareholders, including Asterias, on a pro rata basis as a dividend in kind. As part of the distribution of OncoCyte common stock, Asterias, as it also holds BioTime common stock, received 192,644 shares of OncoCyte common stock as contributed capital from BioTime resulting in a taxable gain to Asterias of $819,000. Asterias has sufficient current year losses from operations in 2015 to offset the entire taxable gain resulting in no income taxes due. As the distribution was treated as contributed capital for financial reporting purposes, Asterias recorded the tax effect of this gain through equity consistent with BioTime’s treatment of the distribution in accordance with ASC 740-20-45-11(g).
Internal Revenue Code Section 382 places a limitation (“Section 382 Limitation”) on the amount of taxable income that can be offset by net operating loss carryforwards after an ownership change (generally greater than 50% change in ownership within a three-year-period) of a loss corporation. California has similar rules. Generally, after an ownership change, a loss corporation cannot deduct net operating loss carryforwards in excess of the Section 382 Limitation. Similar rules exist under Internal Revenue Code Section 383 that may limit the use of credits in the future. The future utilization of the net operating loss carryforwards and tax credits to offset future taxable income may be subject to an annual limitation, as a result of ownership changes that may have occurred previously of that could occur in the future. A Section 382 analysis to determine the limitation of the net operating loss carryforwards has not been performed.
On December 22, 2017 the Tax Cuts and Jobs Act (the “Act”) was signed into law. Among other provisions, the Act reduces the Federal statutory corporate income tax rate from 34% to 21%. This rate reduction has a significant impact on our provisions for income taxes for periods beginning after December 31, 2017, including a one-time impact resulting from the revaluation of our deferred tax assets and liabilities to reflect the new lower rate. However, we still maintain a full valuation allowance against our deferred taxes. Thus, the impact of the change is fully offset by our valuation allowance.
As of December 31, 2017, Asterias had no unrecognized tax benefits and has recorded no liability related to uncertain tax positions. Asterias did not record a change in its unrecorded tax benefits during the year ended December 31, 2017, and expects no change in its unrecorded tax benefits in the next 12 months.
Asterias files tax returns in the U.S. federal and state jurisdictions and is subject to examination by tax authorities. Asterias is not currently under examination by income tax authorities in federal or state. Due to net operating loss and research credit carryforwards, substantially all of the Company’s tax years, from 2012 through 2017, remain open to U.S. federal and state tax examinations.
11. Segment Information
Operating segments are defined as components of an enterprise that engage in business activities for which separate financial information is available and evaluated by the chief operating decision maker in deciding how to allocate resources and assess performance. Asterias’ executive management team represents its chief operating decision maker. The executive management team reviews financial information presented on a consolidated basis for purposes of allocating resources and evaluating financial performance and there are no managers who are held accountable for levels or components below the consolidated unit level. Asterias executive management views Asterias’ operations as one segment.
12. Selected Quarterly Financial Information (unaudited) (in thousands)
Asterias has derived this data from the unaudited interim financial statements that, in Asterias’ opinion, have been prepared on substantially the same basis as the audited financial statements contained in this report and include all normal recurring adjustments necessary for a fair presentation of the financial information for the periods presented. These unaudited quarterly results should be read in conjunction with the financial statements and notes thereto included in this report. The operating results in any quarter are not necessarily indicative of the results that may be expected for any future period.
71
|
Year Ended December 31, 2017
|
First
Quarter |
Second
Quarter |
Third
Quarter |
Fourth
Quarter |
||||||||
|
Revenues, net
|
$
|
1,958
|
|
$
|
298
|
|
$
|
1,607
|
|
$
|
14
|
|
|
Operating expenses
|
|
11,065
|
|
|
8,831
|
|
|
8,670
|
|
|
8,562
|
|
|
Loss from operations before deferred tax benefits
|
|
(6,287
|
)
|
|
(8,728
|
)
|
|
(6,809
|
)
|
|
(6,548
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per share
|
|
(0.13
|
)
|
|
(0.18
|
)
|
|
(0.14
|
)
|
|
(0.12
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, 2016
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues, net
|
$
|
1,541
|
|
$
|
1,526
|
|
$
|
2,017
|
|
$
|
1,742
|
|
|
Operating expenses
|
|
12,633
|
|
|
8,600
|
|
|
9,442
|
|
|
10,274
|
|
|
Loss from operations before deferred tax benefits
|
|
(11,239
|
)
|
|
(5,610
|
)
|
|
(11,550
|
)
|
|
(9,415
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per share
|
|
(0.27
|
)
|
|
(0.12
|
)
|
|
(0.24
|
)
|
|
(0.20
|
)
|
13. License and Royalty Obligations
Services Agreement with Cell Therapy Catapult Services Limited
In October 2015, Asterias entered into a Services Agreement (the “Services Agreement”) with Cell Therapy Catapult Services Limited (“Catapult”), a research organization specializing in the development of technologies which speed the growth of the cell and gene therapy industry. Under the Services Agreement, Catapult will license to Asterias, certain background intellectual property and will develop a scalable manufacturing and differentiation process for Asterias’ human embryonic stem cell derived dendritic cell cancer vaccine development program. In consideration for the license and Catapult’s performance of services, Asterias agreed to make aggregate payments of up to GBP £4,350,000 over the next five years (approximately $5.9 million based on the foreign currency exchange rate on December 31, 2017). At the option of Asterias, up to GBP £3,600,000 (approximately $4.9 million based on the foreign currency exchange rate on December 31, 2017) of such payments may be settled in shares of Asterias Series A Common Stock instead of cash. If Asterias elects to pay for the services in stock and Catapult is unable to sell the stock in the market within 60 days of issuance, after reasonable and diligent efforts through its broker, Catapult may request that the unsold portion of the stock payment, if any, be paid by Asterias in cash at a value equal to approximately 91% of the total amount that was issued in stock. This right by Catapult to put the unsold shares back to Asterias for cash expires the earlier to occur of the sale of the stock in the market or after 60 days of issuance. As of December 31, 2017, we have incurred costs since commencement of the Services Agreement of GBP £3,200,000 under the Services Agreement.
The Services Agreement may be terminated by Asterias for any reason upon 60 days prior written notice. Catapult may terminate the Services Agreement on 60 days prior written notice if it encounters a technical issue that would prevent it from completing the services at all or without obtaining additional resources, or if the estimated time and cost of completing the services will be exceeded and Catapult and Asterias do not reach agreement on revised time and cost terms. Catapult may terminate the Services Agreement in the event Asterias fails to pay any amount due under the Services Agreement 30 days after Catapult makes a written demand for payment. In addition, a non-breaching party may terminate the Services Agreement upon the occurrence a material breach that is not remedied within 30 days. Either party may terminate the Services Agreement in the event the other party becomes subject to insolvency, receivership, liquidation, or a similar event.
Advance payments for research and development services to be performed by Catapult are deferred and recognized as research and development expense ratably as the services are performed. Advance payments related to licenses will be expensed when paid due to the experimental nature of the project. Pursuant to the Services Agreement, if there are any issued, but unsold Asterias stock, to Catapult for payment of services and the 60-day put right has not expired as of the period end being reported on, Asterias will present that amount as “temporary” equity in accordance with ASC 480-10-S99 Distinguishing Liabilities from Equity – SEC Materials. Once the put right expires or the shares are sold by Catapult, the temporary equity amount will be reclassified by Asterias to permanent equity without adjustment to the carrying value of the stock
In the fiscal years ended December 31, 2017, 2016 and 2015 Asterias paid $1.6 million, $1.7 million and $1.2 million, respectively, for services pursuant to the Services Agreement. Asterias paid $385,000, $815,000, and $713,000, respectively, in cash for these services and the remainder was paid with Asterias Series A Common Stock.
72
Asterias issued 318,748, 218,520, and 96,479 shares of Asterias Series A Common Stock with fair market values of $1.2 million, $922,000, and $486,000 at the time of issuance which Asterias reclassified into permanent equity.
Royalty Agreement with Geron
In connection with our acquisition of Geron’s stem cell assets, Asterias entered into a royalty agreement with Geron (the “Royalty Agreement”) pursuant to which Asterias agreed to pay Geron a 4% royalty on net sales (as defined in the Royalty Agreement), by Asterias or any of its affiliates or sales agents, of any products that Asterias develops and commercializes that are covered by the patents Geron contributed to Asterias. In the case of sales of products covered by the patents Geron contributed to Asterias by a person other than Asterias or one of its affiliates or sales agents, Asterias will be required to pay Geron 50% of all royalties and cash payments received by Asterias or by its affiliate in respect of a product sale. Royalty payments will be subject to proration in the event that a product covered by a patent acquired from Geron is sold in combination with another product that is not covered by a patent acquired from Geron. The Royalty Agreement will terminate at the expiration or termination date of the last issued patent contributed by Geron under the Royalty Agreement. Asterias estimates that the latest patent expiration date will be in 2032. In 2017, 2016 and 2015 Asterias paid Geron $165,000, $134,000, and $281,000, respectively under this agreement in royalty fees.
Asterias License from WARF
Asterias has entered into a Non-Exclusive License Agreement with Wisconsin Alumni Research Foundation (“WARF”) under which Asterias was granted a worldwide non-exclusive license under certain WARF patents and WARF-owned embryonic stem cell lines to develop and commercialize therapeutic, diagnostic and research products.
In consideration of the rights licensed, Asterias has agreed to pay WARF an upfront license fee, payments upon the attainment of specified clinical development milestones, royalties on sales of commercialized products, and, subject to certain exclusions, a percentage of any payments that Asterias may receive from any sublicenses that it may grant to use the licensed patents or stem cell lines.
The license agreement will terminate with respect to licensed patents upon the expiration of the last licensed patent to expire; with respect to the licensed stem cell lines, the license agreement will remain in force until terminated by either party in accordance with the termination provisions. Asterias may terminate the license agreement at any time by giving WARF prior written notice. WARF may terminate the license agreement if payments of earned royalties, once begun, cease for a specified period of time or if Asterias and any third parties collaborating or cooperating with Asterias in the development of products using the licensed patents or stem cell lines fail to spend a specified minimum amount on research and development of products relating to the licensed patents or stem cell lines for a specified period of time. WARF also has the right to terminate the license agreement if Asterias breaches the license agreement or becomes bankrupt or insolvent or if any of the licensed patents or stem cell lines are offered to creditors. The payments to WARF were a recurring $25,000 license maintenance fee for each of the years 2017, 2016, and 2015.
Asterias License from the University of California
Geron assigned to Asterias its Exclusive License Agreement with The Regents of the University of California (the “University”) for patents covering a method for directing the differentiation of multipotential hES cells to glial-restricted progenitor cells that generate pure populations of oligodendrocytes for remyelination and treatment of spinal cord injury. Pursuant to this agreement, Asterias has an exclusive worldwide license under such patents, including the right to grant sublicenses, to create products for biological research, drug screening, and human therapy using the licensed patents. Under the license agreement, Asterias will be obligated to pay the University a royalty of 1% from sales of products that are covered by the licensed patent rights, and a minimum annual royalty of $5,000 starting in the year in which the first sale of a product covered by any licensed patent rights occurs, and continuing for the life of the applicable patent right under the agreement. The royalty payments due are subject to reduction, but not by more than 50%, to the extent of any payments that Asterias may be obligated to pay to a third party for the use of patents or other intellectual property licensed from the third party in order to make, have made, use, sell, or import products or otherwise exercise its rights under the Exclusive License Agreement. Asterias will be obligated to pay the University 7.5% of any proceeds, excluding debt financing and equity investments, and certain reimbursements, that its receives from sublicensees, other than Asterias’ affiliates and joint ventures relating to the development, manufacture, purchase, and sale of products, processes, and services covered by the licensed patent.
73
The Company had no expenses related to these fees in the years 2017, 2016, and 2015, respectively. The license agreement will terminate on the expiration of the last-to-expire of the university’s issued licensed patents. If no further patents covered by the license agreement are issued, the license agreement would terminate in 2024. The university may terminate the agreement in the event of Asterias’ breach of the agreement. Asterias can terminate the agreement upon 60 days’ notice.
Telomerase Sublicense from Geron
Asterias received the Telomerase Sublicense from Geron in connection with our acquisition of Geron’s stem cell assets. The Telomerase Sublicense grants Asterias an exclusive sublicense under certain patents owned by the University of Colorado’s University License Equity Holdings, Inc. relating to telomerase and entitles Asterias to use the technology covered by the patents in the development of AST-VAC1 and AST-VAC2 as immunological treatments for cancer. Under the Telomerase Sublicense, Asterias paid Geron a one-time upfront license fee of $65,000, and Asterias will pay Geron an annual license maintenance fee of $10,000 due on each anniversary of the effective date of the agreement, and a 1% royalty on sales of any products that Asterias may develop and commercialize that are covered by the sublicensed patents. The Telomerase Sublicense will expire concurrently with the expiration of Geron’s license. That license will terminate in November 2018 when the last of the licensed patents expires. The Telomerase Sublicense may also be terminated by Asterias by giving Geron 90 days written notice, by us or by Geron if the other party breaches its obligations under the sublicense agreement and fails to cure their breach within the prescribed time period, or by Asterias or by Geron upon the filing or institution of bankruptcy, reorganization, liquidation or receivership proceedings, or upon an assignment of a substantial portion of the assets for the benefit of creditors by the other party.
Asterias is obligated to indemnify Geron, Geron’s licensor, and certain other parties for certain liabilities, including those for personal injury, product liability, or property damage relating to or arising from the manufacture, use, promotion or sale of a product, or the use by any person of a product made, created, sold or otherwise transferred by us or our sublicensees that is covered by the patents sublicensed under this agreement.
14. Clinical Trial and Option Agreement with CRUK and CIRM Grant Award
During September 2014, Asterias entered into a Clinical Trial and Option Agreement (the “CRUK Agreement”) with Cancer Research UK (“CRUK”) and Cancer Research Technology Limited, a wholly-owned subsidiary of CRUK, pursuant to which CRUK has agreed to fund Phase 1 clinical development of Asterias’ human embryonic stem cell derived AST-VAC2 allogeneic (non-patient specific) dendritic cancer vaccine product candidate. Asterias, at its own cost, completed process development and manufacturing scale-up of the AST-VAC2 manufacturing process and transferred the resulting cGMP-compatible process to CRUK. CRUK will, at its own cost, manufacture clinical grade AST-VAC2 and will carry out the Phase 1 clinical trial of AST-VAC2 in cancer patients both resected early-stage and advanced forms of lung cancer. Asterias will have an exclusive first option to obtain a license to use the data from the clinical trial. If Asterias exercises that option, then Asterias will be obligated to make payments upon the execution of the License Agreement, upon the achievement of various milestones, and royalties on sales of products. In connection with the CRUK Agreement, Asterias sublicensed to CRUK for use in the clinical trials and product manufacturing process certain patents that have been licensed or sublicensed to us by third parties. Asterias would also be obligated to make payments to those licensors and sublicensors upon the achievement of various milestones, and then royalties on sales of products if AST-VAC2 is successfully developed and commercialized.
On October 16, 2014 Asterias signed a Notice of Grant Award (“NGA”) with CIRM, effective October 1, 2014, with respect to a $14.3 million grant award for clinical development of Asterias’ product, AST-OPC1. The NGA was subsequently amended effective November 26, 2014 and March 2, 2016. The NGA includes the terms under which CIRM will release grant funds to Asterias. Under the NGA as amended on March 2, 2016, CIRM will disburse the grant funds to Asterias based on Asterias’ attainment of certain progress milestones.
Asterias received $5.6 million under the NGA during 2015. During the fiscal year ended December 31, 2016, Asterias received an additional $6.2 million under the NGA grant. In September 2017, we received the final $1.5 million payment under the CIRM grant which was due upon achievement of certain clinical milestones. Revenues pursuant to the NGA recognized during the fiscal years ended December 31, 2017, 2016 and 2015 were $3.7 million, $6.6 million and $3.0 million, respectively. Although the cash payments from CIRM were dependent on achieving certain milestones pursuant to the contract with CIRM, Asterias recognized grant income as related research expenses are incurred. We had no deferred revenues related to the CIRM grant as of December 31, 2017. Deferred revenues relating to the CIRM grant were $2.2 million at December 31, 2016.
74
15. Cross-License and Share Transfer with BioTime and Subsidiaries
On February 16, 2016, Asterias entered into a Cross-License Agreement (the “Cross-License”) with BioTime and BioTime’s wholly owned subsidiary ESI. Under the terms of the Cross-License, Asterias received a fully-paid, non-royalty-bearing, world-wide, non-exclusive, sub-licensable license under certain BioTime patents and related patent rights and ESI patents and related patent rights specified in the Cross-License, for all purposes in the Asterias Licensed Field, as defined in the Cross-License agreement, during the term of the license.
Under the terms of the Cross-License, BioTime and ESI received a fully-paid, non-royalty-bearing, world-wide, non-exclusive, sub-licensable license in, to, and under the certain Asterias patents and related patent rights for all purposes in the BioTime/ESI Licensed Field, as defined in the Cross-License agreement, during the term of the license.
On February 16, 2016, Asterias also entered into a Share Transfer Agreement (“Share Transfer”) with BioTime and ESI pursuant to which (a) Asterias transferred to BioTime 2,100,000 shares of common stock of OrthoCyte Corporation (“OrthoCyte) and 21,925 ordinary shares of Cell Cure Neurosciences Ltd (“Cell Cure”), each a majority-owned subsidiary of BioTime, with an aggregate carrying value at the time of the transaction of approximately $416,000 and (b) BioTime transferred to Asterias 75,771 shares of Series A common stock of Asterias with a carrying value at the time of the transaction of approximately $197,000 and warrants to purchase 3,150,000 Series A common stock of Asterias at an exercise price of $5.00 per share, with a carrying value at the time of the transaction of approximately $2.0 million, as additional consideration for the license of patents and patent rights from Asterias under the Cross License. On March 20, 2016, the warrants to purchase 3,150,000 shares of Series A common stock were retired by Asterias in addition to 75,771 shares of Series A common stock retired.
The Cross-License and Share Transfer transaction was accounted for as a transfer of assets between entities under common control and recorded at carrying value, with the resulting gain on transfer of approximately $1.8 million recorded by Asterias in equity as contributed capital to BioTime in accordance with, and pursuant to ASC 805-50, Transactions Between Entities Under Common Control. Accordingly, the net financial reporting impact of the Cross-License and Share Transfer of $0.4 million charged to additional paid-in capital was comprised of the retirement of the aggregate $2.2 million carrying value of the warrants and the Series A Common Stock offset by the $1.8 million transfer gain.
The transfer of assets was also a taxable transaction to Asterias generating a taxable gain of approximately $3.1 million as further discussed in Note 10.
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
Not applicable.
| Item 9A. | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and our principal financial officer, have reviewed and evaluated the effectiveness of our disclosure controls and procedures as December 31, 2017. Following this review and evaluation, our principal executive officer and principal financial officer have concluded that our disclosure controls and procedures were effective to ensure that information required to be disclosed by us in reports that we file or submit under the Securities Exchange Act of 1934 (“Exchange Act”) (i) is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms; and (ii) is accumulated and communicated to management, including our principal executive officer and our principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting, as defined in Exchange Act Rule 13a-15(f), is a process designed by, or under the supervision of, our principal executive officer and our principal financial officer, and effected by our Board of Directors, management, and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| • | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; |
75
| • | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
| • | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2017, based on criteria established in the 2013 Internal Control - Integrated Framework issued by COSO. Based on this assessment, management believes that, as of that date, our internal control over financial reporting was effective.
This annual report does not include an attestation report of our registered public accounting firm due to a transition period established by the rules of the SEC for newly public companies status as an “emerging growth company” under the JOBS Act.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting identified in connection with the evaluation required by Exchange Act rules 13a-15(d) and 15d-15(d) that occurred during the quarter ended December 31, 2017 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | Other Information |
None.
76
| Item 10. | Directors, Executive Officers, and Corporate Governance |
Information regarding our directors, executive officers and corporate governance required under this Item 10. Directors, Executive Officers and Corporate Governance will be included in our definitive proxy statement for our annual general meeting of shareholders, which will be filed with the United States Securities and Exchange Commission within 120 days after the end of our fiscal year.
| Item 11. | Executive Compensation |
Compensation Committee Interlocks and Insider Participation in Compensation Decisions
Information regarding the compensation of our named executive officers and directors required under this Item 11. Executive Compensation will be included in our definitive proxy statement for our annual general meeting of shareholders, which will be filed with the United States Securities and Exchange Commission within 120 days after the end of our fiscal year.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management, and Related Stockholder Matters |
Information regarding individuals or groups which own more than 5% of our ordinary shares, as well as information regarding the security ownership of our executive officers and directors, and other shareholder matters required under this Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters will be included in our definitive proxy statement for our annual general meeting of shareholders, which will be filed with the United States Securities and Exchange Commission within 120 days after the end of our fiscal year.
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
Information regarding transactions with related parties and director independence required under this Item 13. Certain Relationships and Related Transactions, and Director Independence will be included in our definitive proxy statement for our annual general meeting of shareholders, which will be filed with the United States Securities and Exchange Commission within 120 days after the end of our fiscal year.
| Item 14. | Principal Accounting Fees and Services |
General
Information regarding the services provided by and the fees paid to our independent auditors required under this Item 14. Principal Accounting Fees and Services will be included in our definitive proxy statement for our annual general meeting of shareholders, which will be filed with the United States Securities and Exchange Commission within 120 days after the end of our fiscal year.
77
| Item 15. | Exhibits, Financial Statements and Schedules |
| (a-1) | Financial Statements. |
The following financial statements of Asterias Biotherapeutics, Inc. are filed in the Form 10-K:
Balance sheets
Statements of operations
Statements of comprehensive loss
Statements of stockholders’ equity
Statements of cash flows
Notes to Financial Statements
| (a-2) | Financial Statement Schedules |
All schedules are omitted because the required information is inapplicable or the information is presented in the financial statements or the notes thereto.
| (a-3) | Exhibits. |
78
ASTERIAS BIOTHERAPEUTICS, INC.
EXHIBIT INDEX TO ANNUAL REPORT ON FORM 10-K
FOR THE YEAR ENDED DECEMBER 31, 2017
|
Exhibit
No. |
Exhibit
|
Method of Filing
|
|
Asset Contribution Agreement, dated January 4, 2013, by and among BioTime, Inc., BioTime Acquisition Corporation, and Geron Corporation.(1)
|
Incorporated by reference to Exhibit 2.1 to the Current Report on Form 8-K filed by BioTime, Inc. with the Securities and Exchange Commission on January 8, 2013.
|
|
|
|
|
|
|
Amended and Restated Certificate of Incorporation
|
Incorporated by reference Exhibit 3.1 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on April 3, 2013.
|
|
|
|
|
|
|
Bylaws
|
Incorporated by reference Exhibit 3.2 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on April 3, 2013.
|
|
|
|
|
|
|
Specimen of Series A Common Stock Certificate
|
Incorporated by reference Exhibit 4.1 to Amendment No. 3 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on September 3, 2013
|
|
|
|
|
|
|
Form of Warrant Agreement by and between the Company and American Stock Transfer & Trust Company, including the form of Warrant.
|
Incorporated by reference to Exhibit 4.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on May 10, 2016.
|
|
|
|
|
|
|
Shared Facilities and Services Agreement, dated April 1, 2013, between Asterias Biotherapeutics, Inc. and BioTime, Inc.
|
Incorporated by reference to Exhibit 10.4 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on April 3, 2013.
|
|
|
|
|
|
|
Amended and restated 2013 Equity Incentive Plan
|
Incorporated by reference to Exhibit 10.1 to the Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 15, 2016.
|
|
|
|
|
|
|
Form of Employee Incentive Stock Option Agreement
|
Incorporated by reference to Exhibit 10.9 to Amendment No. 2 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on August 13, 2013
|
|
|
|
|
|
|
Form of Non-employee Director Stock Option Agreement
|
Incorporated by reference to Exhibit 10.10 to Amendment No. 2 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on August 13, 2013
|
|
|
|
|
|
|
Royalty Agreement, dated October 1, 2013 between Asterias Biotherapeutics, Inc. and Geron Corporation
|
Incorporated by reference to Exhibit 10.6 to Amendment No. 2 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on August 13, 2013
|
79
|
Exhibit
No. |
Exhibit
|
Method of Filing
|
|
Exclusive Sublicense Agreement between Geron Corporation and Asterias Biotherapeutics, Inc.
|
Incorporated by reference to Exhibit 10.7 to Amendment No. 2 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on August 13, 2013
|
|
|
|
|
|
|
Sublicense Agreement between BioTime, Inc. and Asterias Biotherapeutics, Inc.
|
Incorporated by reference to Exhibit 10.8 to Amendment No. 2 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on August 13, 2013
|
|
|
|
|
|
|
Exclusive License Agreement, dated February 20, 2003, and First Amendment thereto dated September 7, 2004, between The Regents of the University of California and Geron Corporation
|
Incorporated by reference to Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q as filed with the Securities and Exchange Commission on November 12, 2013
|
|
|
|
|
|
|
Non-exclusive License Agreement, dated October 7, 2013, between the Wisconsin Alumni Research Foundation and Asterias Biotherapeutics, Inc.
|
Incorporated by reference to Exhibit 10.5 to the Company’s Quarterly Report on Form 10-Q as filed with the Securities and Exchange Commission on November 12, 2013
|
|
|
|
|
|
|
Lease, dated December 30, 2013, by and between BMR 6300 Dumbarton Circle, LP, and Asterias Biotherapeutics, Inc.
|
Incorporated by reference to Exhibit 10.17 to the Company’s Annual Report on Form 10-K as filed with the Securities and Exchange Commission on March 17, 2014
|
|
|
|
|
|
|
Clinical Trial and Option Agreement, dated September 8, 2014, between Asterias Biotherapeutics, Inc. and Cancer Research UK and Cancer Research Technology Limited +
|
Incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q/A as filed with the Securities and Exchange Commission on January 13, 2015
|
|
|
|
|
|
|
At the Market Issuance Sales Agreement, dated April 10, 2015, by and between the Registrant and MLV & Co. LLC
|
Incorporated by reference to Exhibit 10.10 to the Current Report on Form 8-K as filed with the Securities and Exchange Commission on April 10, 2015
|
|
|
|
|
|
|
Employment Agreement with Edward D. Wirth III, dated June 16, 2013
|
Incorporated by reference to Exhibit 10.1 to the Quarterly Report on Form 10-Q, for the period ended June 30, 2015, filed August 10, 2015
|
|
|
|
|
|
|
Services Agreement, dated October 8, 2015 by Asterias and Catapult
|
Incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K, filed October 15, 2015
|
|
|
|
|
|
|
Notice of Award from the California Institute of Regenerative Medicine dated October 16, 2014
|
Incorporated by reference to Exhibit 10.26 to the Company’s Annual Report on Form 10-K as filed with the Securities and Exchange Commission on March 11, 2015
|
80
|
Exhibit
No. |
Exhibit
|
Method of Filing
|
|
|
|
|
|
Amendment to the Notice of Award from the California Institute of Regenerative Medicine dated November 26, 2014
|
Incorporated by reference to Exhibit 10.27 to the Company’s Annual Report on Form 10-K as filed with the Securities and Exchange Commission on March 11, 2015
|
|
|
|
|
|
|
Amendment to the Notice of Award from the California Institute of Regenerative Medicine dated March 2, 2016
|
Incorporated by reference to Exhibit 10.34 to the Company’s Annual Report on Form 10-K as filed with the Securities and Exchange Commission on March 29, 2016
|
|
|
|
|
|
|
Cross License Agreement between Asterias, BioTime and ES Cell International Pte Ltd
|
Incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K, filed February 18, 2016
|
|
|
|
|
|
|
Share Transfer Agreement between Asterias and BioTime
|
Incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K, filed February 18, 2016
|
|
|
|
|
|
|
Development and Manufacturing Services Agreement, dated August 3, 2016, between Asterias and Cognate BioServices, Inc.
|
Incorporated by reference to Exhibit 10.4 to Asterias’ Quarterly Report on Form 10-Q, for the period ended September 30, 2016, filed November 14, 2016
|
|
|
|
|
|
|
Employment Agreement of Ryan D. Chavez, dated July 18, 2016
|
Incorporated by reference to Exhibit 10.1 to Asterias’ Current Report on Form 8-K, filed November 17, 2016.
|
|
|
|
|
|
|
Employment Agreement of Michael H. Mulroy, dated May 23, 2017
|
Incorporated by reference to Exhibit 10.1 to Asterias’ Current Report on Form 8-K, filed May 23, 2017.
|
|
|
|
|
|
|
Amendment to the At the Market Sales Agreement, dated March 28, 2017, by and between the Company, MLV & Co. and FBR Capital Markets & Co.
|
Incorporated by reference to Exhibit 10.24 to the Company’s Annual Report on Form 10-K as filed with the Securities and Exchange Commission on March 28, 2017
|
|
|
|
|
|
|
Securities Purchase Agreement
|
Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K as filed with the Securities and Exchange Commission on October 16, 2017
|
|
|
|
|
|
|
Amendment No. 1 to the Securities Purchase Agreement
|
Incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q as filed with the Securities and Exchange Commission on November 14, 2017
|
|
|
|
|
|
|
Consent of OUM & Co. LLP
|
Filed herewith
|
|
|
|
|
|
|
Certification of Chief Executive Officer Pursuant to SEC Rule 13a-14
|
Filed herewith
|
|
|
|
|
|
|
Certification of Chief Financial Officer Pursuant to SEC Rule 13a-14
|
Filed herewith
|
|
|
|
|
|
81
|
Exhibit
No. |
Exhibit
|
Method of Filing
|
|
Certification of Chief Executive Officer and Chief Financial Officer Pursuant to Rule 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
Furnished herewith
|
|
|
|
|
|
|
101
|
Interactive Data File
|
Filed herewith
|
|
|
|
|
|
101.INS
|
XBRL Instance Document
|
Filed herewith
|
|
|
|
|
|
101 SCH
|
XBRL Taxonomy Extension Schema
|
Filed herewith
|
|
|
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase
|
Filed herewith
|
|
|
|
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase
|
Filed herewith
|
|
|
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase
|
Filed herewith
|
|
|
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Document
|
Filed herewith
|
|
|
|
|
| (1) | All exhibits to this exhibit have been omitted pursuant to Item 601(b)(2) of Regulation S-K. The Company will furnish the omitted exhibits to the SEC upon request by the SEC. |
| + | Confidential treatment has been granted with respect to redacted portions of this document. |
| * | Management contract or compensatory plan or arrangement required to be filed as an exhibit to this Annual Report on Form 10-K pursuant to Item 15(a). |
| Item 16. | Form 10-K Summary |
None.
82
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized on the 15th day of March, 2018.
|
|
ASTERIAS BIOTHERAPEUTICS, INC.
|
|
|
|
|
|
|
|
By:
|
/s/ Michael H. Mulroy
|
|
|
|
Michael H. Mulroy
|
|
|
|
President and Chief Executive Officer
|
|
Signature
|
Title
|
Date
|
|
/s/ Michael H. Mulroy
|
Chief Executive Officer and Director
(Principal Executive Officer) |
March 15, 2018
|
|
MICHAEL H. MULROY
|
||
|
|
|
|
|
/s/ Ryan Chavez
|
Chief Financial Officer (Principal
Financial and Accounting Officer) |
March 15, 2018
|
|
RYAN CHAVEZ
|
||
|
|
|
|
|
/s/ Andrew Arno
|
Director
|
March 15, 2018
|
|
ANDREW ARNO
|
||
|
|
|
|
|
/s/ Don M. Bailey
|
Director
|
March 15, 2018
|
|
DON M. BAILEY
|
||
|
|
|
|
|
/s/ Alfred D. Kingsley
|
Director
|
March 15, 2018
|
|
ALFRED D. KINGSLEY
|
||
|
|
|
|
|
/s/ Richard LeBuhn
|
Director
|
March 15, 2018
|
|
RICHARD LEBUHN
|
||
|
|
|
|
|
/s/ Howard Scher, M.D.
|
Director
|
March 15, 2018
|
|
HOWARD SCHER, M.D.
|
||
|
|
|
|
|
/s/ Natale Ricciardi
|
Director
|
March 15, 2018
|
|
NATALE RICCIARDI
|
||
|
|
|
|
|
/s/ Adi Mohanty
|
Director
|
March 15, 2018
|
|
ADI MOHANTY
|
||
|
|
|
|
|
/s/ Michael D. West, Ph.D.
|
Director
|
March 15, 2018
|
|
MICHAEL D. WEST, Ph.D.
|
83
