Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - Asterias Biotherapeutics, Inc. | ex32_1.htm |
| EX-31.2 - EXHIBIT 31.2 - Asterias Biotherapeutics, Inc. | ex31_2.htm |
| EX-31.1 - EXHIBIT 31.1 - Asterias Biotherapeutics, Inc. | ex31_1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
| ☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the quarterly period ended June 30, 2017
OR
| ☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the transition period from _________ to ________
Commission file number 001-36646
Asterias Biotherapeutics, Inc.
(Exact name of registrant as specified in its charter)
|
Delaware
|
46-1047971
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
6300 Dumbarton Circle
Fremont, California 94555
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code
(510) 456-3800
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). ☒Yes☐ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer
|
☐
|
Accelerated filer
|
☐
|
|
|
Non-accelerated filer
|
☒
|
(Do not check if a smaller reporting company)
|
Smaller reporting company
|
☐
|
|
Emerging growth company
|
☒
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ☐ Yes ☒ No
Indicate the number of shares outstanding of each of the issuer’s classes of common stock, as of the latest practicable date: 49,978,791 shares of Series A Common Stock, $0.0001 par value, as of August 8, 2017.
PART I--FINANCIAL INFORMATION
Statements made in this Report that are not historical facts may constitute forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those discussed. Such risks and uncertainties include but are not limited to those discussed in this Report under Item 1 of the Notes to Financial Statements, and under Risk Factors in this Report. Words such as “expects,” “may,” “will,” “anticipates,” “intends,” “plans,” “believes,” “seeks,” “estimates,” and similar expressions identify forward-looking statements.
References to “Asterias,” “our” or “we” means Asterias Biotherapeutics, Inc.
The description or discussion, in this Form 10-Q, of any contract or agreement is a summary only and is qualified in all respects by reference to the full text of the applicable contract or agreement.
2
| Item 1. |
Financial Statements
|
ASTERIAS BIOTHERAPEUTICS, INC.
CONDENSED BALANCE SHEETS
(IN THOUSANDS EXCEPT PAR VALUE AMOUNTS)
|
June 30,
2017
(unaudited)
|
December 31,
2016
|
|||||||
|
ASSETS
|
||||||||
|
CURRENT ASSETS
|
||||||||
|
Cash and cash equivalents
|
$
|
11,875
|
$
|
19,800
|
||||
|
Available-for-sale securities, at fair value
|
13,141
|
15,269
|
||||||
|
Prepaid expenses and other current assets
|
1,349
|
1,921
|
||||||
|
Total current assets
|
26,365
|
36,990
|
||||||
|
NONCURRENT ASSETS
|
||||||||
|
Intangible assets, net
|
16,787
|
18,130
|
||||||
|
Property, plant and equipment, net
|
4,999
|
5,475
|
||||||
|
Other assets
|
411
|
415
|
||||||
|
TOTAL ASSETS
|
$
|
48,562
|
$
|
61,010
|
||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
CURRENT LIABILITIES
|
||||||||
|
Amount due to BioTime, Inc.
|
$
|
-
|
$
|
288
|
||||
|
Accounts payable
|
408
|
1,076
|
||||||
|
Accrued expenses
|
1,604
|
2,495
|
||||||
|
Capital lease liability, current
|
7
|
7
|
||||||
|
Deferred grant income
|
-
|
2,185
|
||||||
|
Total current liabilities
|
2,019
|
6,051
|
||||||
|
LONG-TERM LIABILITIES
|
||||||||
|
Warrant liability
|
5,767
|
8,665
|
||||||
|
Capital lease liability, noncurrent
|
18
|
20
|
||||||
|
Deferred rent liability
|
296
|
266
|
||||||
|
Lease liability
|
3,747
|
3,980
|
||||||
|
TOTAL LIABILITIES
|
11,847
|
18,982
|
||||||
|
Commitments and contingencies (see Note 9)
|
||||||||
|
STOCKHOLDERS’ EQUITY
|
||||||||
|
Preferred Stock, $0.0001 par value, authorized 5,000 shares; none issued and outstanding
|
-
|
-
|
||||||
|
Common Stock, $0.0001 par value, authorized 75,000 Series A Common Stock and 75,000 Series B Common Stock; 49,556 and 47,567 shares Series A Common Stock issued and outstanding at June 30, 2017 and December 31, 2016, respectively; no Series B Common Stock issued and outstanding at June 30, 2017 and December 31, 2016
|
5
|
5
|
||||||
|
Additional paid-in capital
|
138,659
|
126,829
|
||||||
|
Accumulated other comprehensive loss
|
(3,206
|
)
|
(1,078
|
)
|
||||
|
Accumulated deficit
|
(98,743
|
)
|
(83,728
|
)
|
||||
|
Total stockholders’ equity
|
36,715
|
42,028
|
||||||
|
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY
|
$
|
48,562
|
$
|
61,010
|
||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
3
ASTERIAS BIOTHERAPEUTICS, INC.
CONDENSED STATEMENTS OF OPERATIONS
(IN THOUSANDS, EXCEPT PER SHARE DATA)
(UNAUDITED)
|
Three Months Ended
June 30,
|
Six Months Ended
June 30,
|
|||||||||||||||
|
2017
|
2016
|
2017
|
2016
|
|||||||||||||
|
REVENUE
|
||||||||||||||||
|
Grant income
|
$
|
291
|
$
|
1,520
|
$
|
2,185
|
$
|
3,007
|
||||||||
|
Royalties from product sales
|
25
|
12
|
141
|
119
|
||||||||||||
|
Total revenue
|
316
|
1,532
|
2,326
|
3,126
|
||||||||||||
|
Cost of sales
|
(18
|
)
|
(6
|
)
|
(70
|
)
|
(59
|
)
|
||||||||
|
Gross profit
|
298
|
1,526
|
2,256
|
3,067
|
||||||||||||
|
EXPENSES
|
||||||||||||||||
|
Research and development
|
(6,984
|
)
|
(6,019
|
)
|
(13,582
|
)
|
(12,362
|
)
|
||||||||
|
General and administrative
|
(1,847
|
)
|
(2,581
|
)
|
(6,314
|
)
|
(8,871
|
)
|
||||||||
|
Total operating expenses
|
(8,831
|
)
|
(8,600
|
)
|
(19,896
|
)
|
(21,233
|
)
|
||||||||
|
Loss from operations
|
(8,533
|
)
|
(7,074
|
)
|
(17,640
|
)
|
(18,166
|
)
|
||||||||
|
OTHER INCOME/(EXPENSE)
|
||||||||||||||||
|
Gain/(loss) from change in fair value on warrant liability
|
(56
|
)
|
1,628
|
2,898
|
1,628
|
|||||||||||
|
Interest expense, net
|
(114
|
)
|
(139
|
)
|
(239
|
)
|
(285
|
)
|
||||||||
|
Other expense, net
|
(25
|
)
|
(25
|
)
|
(34
|
)
|
(26
|
)
|
||||||||
|
Total other income (expense), net
|
(195
|
)
|
1,464
|
2,625
|
1,317
|
|||||||||||
|
LOSS BEFORE INCOME TAX BENEFIT
|
(8,728
|
)
|
(5,610
|
)
|
(15,015
|
)
|
(16,849
|
)
|
||||||||
|
Deferred income tax benefit
|
-
|
451
|
-
|
1,353
|
||||||||||||
|
NET LOSS
|
$
|
(8,728
|
)
|
$
|
(5,159
|
)
|
$
|
(15,015
|
)
|
$
|
(15,496
|
)
|
||||
|
BASIC AND DILUTED NET LOSS PER SHARE
|
$
|
(0.18
|
)
|
$
|
(0.12
|
)
|
$
|
(0.31
|
)
|
$
|
(0.39
|
)
|
||||
|
WEIGHTED AVERAGE SHARES OUTSTANDING: BASIC AND DILUTED
|
48,511
|
41,777
|
48,129
|
40,201
|
||||||||||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
4
ASTERIAS BIOTHERAPEUTICS, INC.
CONDENSED STATEMENTS OF COMPREHENSIVE LOSS
(IN THOUSANDS)
(UNAUDITED)
|
Three Months Ended
June 30,
|
Six Months Ended
June 30,
|
|||||||||||||||
|
2017
|
2016
|
2017
|
2016
|
|||||||||||||
|
NET LOSS
|
$
|
(8,728
|
)
|
$
|
(5,159
|
)
|
$
|
(15,015
|
)
|
$
|
(15,496
|
)
|
||||
|
Unrealized loss on available-for-sale securities, net of taxes
|
(1,300
|
)
|
(664
|
)
|
(2,128
|
)
|
(5,497
|
)
|
||||||||
|
COMPREHENSIVE LOSS
|
$
|
(10,028
|
)
|
$
|
(5,823
|
)
|
$
|
(17,143
|
)
|
$
|
(20,993
|
)
|
||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
5
ASTERIAS BIOTHERAPEUTICS, INC.
CONDENSED STATEMENTS OF CASH FLOWS
(IN THOUSANDS)
(UNAUDITED)
|
Six Months Ended
June 30,
|
||||||||
|
2017
|
2016
|
|||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||||||
|
Net loss
|
$
|
(15,015
|
)
|
$
|
(15,496
|
)
|
||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||
|
Depreciation and amortization expense
|
555
|
605
|
||||||
|
Stock-based compensation
|
2,709
|
2,477
|
||||||
|
Amortization of intangible assets
|
1,343
|
1,343
|
||||||
|
Deferred income tax benefit
|
-
|
(1,353
|
)
|
|||||
|
Common stock issued for services in lieu of cash
|
562
|
644
|
||||||
|
Gain from change in fair value of warrant liability
|
(2,898
|
)
|
(1,628
|
)
|
||||
|
Distribution of Asterias warrants to shareholders other than BioTime, Inc.
|
2,042
|
3,125
|
||||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Prepaid expenses and other current assets
|
647
|
(572
|
)
|
|||||
|
Other assets
|
5
|
5
|
||||||
|
Accounts payable
|
(668
|
)
|
(496
|
)
|
||||
|
Accrued expenses and other current liabilities
|
(1,254
|
)
|
1,291
|
|||||
|
Deferred rent liability
|
29
|
48
|
||||||
|
Lease liability
|
-
|
(203
|
)
|
|||||
|
Deferred grant income
|
(2,185
|
)
|
736
|
|||||
|
Amount due to BioTime, Inc.
|
-
|
(540
|
)
|
|||||
|
Net cash used in operating activities
|
(14,128
|
)
|
(10,014
|
)
|
||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||
|
Purchase of property, plant and equipment
|
(79
|
)
|
(499
|
)
|
||||
|
Reimbursement of security deposit
|
-
|
32
|
||||||
|
Net cash used in investing activities
|
(79
|
)
|
(467
|
)
|
||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
||||||||
|
Proceeds from sale of common stock and warrants
|
-
|
20,017
|
||||||
|
Financing costs for sale of common stock and warrants
|
-
|
(1,815
|
)
|
|||||
|
Proceeds from sale of common shares under at-the-market transactions
|
6,696
|
177
|
||||||
|
Financing costs for at-the-market sales
|
(198
|
)
|
(6
|
)
|
||||
|
Proceeds from exercise of stock options
|
20
|
1,933
|
||||||
|
Repayment of lease liability and capital lease obligation
|
(236
|
)
|
(4
|
)
|
||||
|
Shares retired to pay for employees’ taxes
|
-
|
(102
|
)
|
|||||
|
Reimbursement from landlord on construction in progress
|
-
|
567
|
||||||
|
Net cash provided by financing activities
|
6,282
|
20,767
|
||||||
|
NET INCREASE/(DECREASE) IN CASH AND CASH EQUIVALENTS CASH AND CASH EQUIVALENTS:
|
(7,925
|
)
|
10,286
|
|||||
|
At beginning of period
|
19,800
|
11,183
|
||||||
|
At end of period
|
$
|
11,875
|
$
|
21,469
|
||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
6
ASTERIAS BIOTHERAPEUTICS, INC.
NOTES TO CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
| 1. |
Organization, Basis of Presentation and Liquidity
|
Asterias Biotherapeutics, Inc. (“Asterias”) is a biotechnology company focused on the emerging fields of cell therapy and regenerative medicine. Asterias has two core technology platforms. The first is a type of stem cell capable of becoming all of the cell types in the human body, a property called pluripotency. The second is a type of cell called “dendritic cells” used to teach cancer patients’ immune systems to attack their tumors. Asterias currently has three clinical stage programs based on these platforms: AST-OPC1 is a therapy derived from pluripotent stem cells that is currently in a Phase 1/2a clinical trial for spinal cord injuries; AST-VAC1 is a patient-specific cancer immunotherapy for Acute Myeloid Leukemia (AML); and AST-VAC2 is a non-patient specific cancer immunotherapy for which the initiation of a Phase 1/2a clinical trial in non-small cell lung cancer is planned for 2017. Asterias’ technology platforms have the potential for application in additional indications, such as advanced multiple sclerosis and white matter stroke for AST-OPC1 and other additional cancer indications for our cancer immunotherapy platform. Asterias was incorporated in Delaware on September 24, 2012.
The accompanying unaudited condensed financial statements have been prepared in accordance with accounting principles generally accepted in the United States (“GAAP”) for interim financial information and with the instructions to Form 10-Q and Article 10 of Regulation S-X of the Securities Exchange Commission. In accordance with those rules and regulations certain information and footnote disclosures normally included in comprehensive financial statements have been condensed or omitted pursuant to such rules and regulations. The balance sheet as of December 31, 2016 was derived from the audited financial statements at that date, but does not include all the information and footnotes required by GAAP. These financial statements should be read in conjunction with the audited financial statements and notes thereto included in Asterias’ Annual Report on Form 10-K for the year ended December 31, 2016.
The accompanying interim condensed financial statements, in the opinion of management, include all adjustments, consisting only of normal recurring adjustments, necessary for a fair presentation of Asterias’ financial condition and results of operations. The condensed results of operations are not necessarily indicative of the results to be expected for any future interim period or for the entire year.
Liquidity – Since inception, Asterias has incurred operating losses and has funded its operations primarily through issuance of equity securities, warrants, payments from research grants, and royalties from product sales. At June 30, 2017, Asterias had an accumulated deficit of $98.7 million, working capital of $24.3 million and stockholders’ equity of $36.7 million. Asterias has evaluated its projected cash flows and believes that its cash and cash equivalents of $11.9 million and available for sale securities of $13.1 million as of June 30, 2017, will be sufficient to fund Asterias’ operations through at least twelve months from the issuance date of these financial statements. If the value of Asterias’ available-for-sale securities decreases or it is unable to obtain future adequate financing for its clinical trials, it may be required to delay, postpone, or cancel its clinical trials, limit the number of clinical trial sites, or otherwise reduce or curtail its operations. Future financings may not be available to Asterias at acceptable terms, or at all. Sales of additional equity securities would result in the dilution of interests of current shareholders.
| 2. |
Summary of Significant Accounting Policies
|
Basic and diluted net loss per share – The computations of basic and diluted net loss per share are as follows (in thousands, except per share data):
|
Three Months Ended
June 30,
(Unaudited)
|
Six Months Ended
June 30,
(Unaudited)
|
|||||||||||||||
|
2017
|
2016
|
2017 | 2016 | |||||||||||||
|
Net loss
|
$
|
(8,728
|
)
|
$
|
(5,159
|
)
|
$
|
(15,015
|
)
|
$
|
(15,496
|
)
|
||||
|
Weighted average common shares outstanding – basic and diluted
|
48,511
|
41,777
|
48,129
|
40,201
|
||||||||||||
|
Net loss per share – basic and diluted
|
$
|
(0.18
|
)
|
$
|
(0.12
|
)
|
$
|
(0.31
|
)
|
$
|
(0.39
|
)
|
||||
The following common stock equivalents were excluded from the computation of diluted net loss per share of common stock for the periods presented because including them would have been antidilutive (in thousands):
|
As of
June 30,
(Unaudited)
|
||||||||
|
2017
|
2016
|
|||||||
|
Stock options and restricted stock units
|
7,459
|
6,235
|
||||||
|
Warrants
|
6,551
|
6,700
|
||||||
Adoption of ASU 2016-09, Improvements to Employee Share-Based Payment Accounting
7
In March 2016, the FASB issued ASU 2016-09, Compensation - Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting (“ASU 2016-09”), which simplifies several aspects of the accounting for share-based payment transactions, including the income tax consequences, forfeitures, classification of awards as either equity or liabilities, and classification on the statement of cash flows. Asterias adopted ASU 2016-09 beginning on January 1, 2017.
In connection with the adoption of ASU 2016-09, Asterias changed how it accounts for excess tax benefits and deficiencies, if any, and forfeitures, as applicable. All excess tax benefits and tax deficiencies from stock-based compensation awards accounted for under ASC 718 are recognized as an income tax benefit or expense, respectively, in the statements of operations. Prior to the adoption of ASU 2016-09, Asterias recognized excess tax benefits, if any, in additional paid-in capital only if the tax deduction reduced cash income taxes payable and excess tax deficiencies were recognized either as an offset to accumulated excess tax benefits, if any, on Asterias’ statements of operations. An excess income tax benefit arises when the tax deduction of a share-based award for income tax purposes exceeds the compensation cost recognized for financial reporting purposes and, a tax deficiency arises when the compensation cost exceeds the tax deduction. Because Asterias has a full valuation allowance, there was no impact to Asterias’ statements of operations for any excess tax benefits or deficiencies, as any excess benefit or deficiency would be offset by the change in the valuation allowance (see Note 11).
Forfeitures are now accounted for as they occur instead of based on the number of awards that were expected to vest. Based on the nature and timing of Asterias equity grants, straight-line expense attribution of stock-based compensation for the entire award and the relatively low forfeiture rate on Asterias experience, the impact of adoption of ASU 2016-09 pertaining to forfeitures was not significant to Asterias’ financial statements (see Note 8).
| 3. |
Balance Sheet Components
|
Property, plant and equipment, net
As of June 30, 2017 and December 31, 2016, property, plant and equipment consisted of the following (in thousands):
|
June,
2017
(Unaudited)
|
December 31,
2016
|
|||||||
|
Furniture, fixtures and leasehold improvements
|
$
|
5,421
|
$
|
5,421
|
||||
|
Computers, machinery and equipment
|
2,625
|
2,545
|
||||||
|
8,046
|
7,966
|
|||||||
|
Less - accumulated depreciation and amortization
|
(3,047
|
)
|
(2,491
|
)
|
||||
|
Property, plant and equipment, net
|
$
|
4,999
|
$
|
5,475
|
||||
Depreciation expense for the three and six months ended June 30, 2017 was $278,000 and $555,000, respectively. Depreciation expense for the three and six months ended June 30, 2016 was $303,000 and $605,000, respectively.
| 4. |
Investments in BioTime and OncoCyte
|
Investment in BioTime
BioTime common shares are included in available-for-sale securities at fair value in current assets in Asterias’ balance sheet as the shares are traded on NYSE: MKT (symbol “BTX”) and available for working capital purposes. As of June 30, 2017 and December 31, 2016, Asterias held 3,852,880 BioTime shares which were valued at $12.1 million and $13.9 million based on the closing price on those respective dates.
Investment in OncoCyte
On December 31, 2015, in connection with BioTime’s distribution of OncoCyte common stock to BioTime shareholders, on a pro rata basis, Asterias received 192,644 shares of OncoCyte common stock from BioTime as a dividend in kind. On that date, BioTime shareholders, including Asterias, received one share of OncoCyte common stock for every twenty shares of BioTime common stock held. Asterias recorded the fair value of the OncoCyte common stock as contributed capital from BioTime.
The OncoCyte shares are included in available-for-sale securities at fair value in current assets in Asterias’ balance sheet as the shares are traded on NYSE: MKT (symbol “OCX”) and available for working capital purposes. As of June 30, 2017 and December 31, 2016, the OncoCyte shares were valued at $1.0 million and $1.4 million based on the OncoCyte closing prices on those respective dates.
| 5. |
Cross-License and Share Transfer with BioTime and Subsidiaries
|
On February 16, 2016, Asterias entered into a Cross-License Agreement (the “Cross-License”) with BioTime and BioTime's wholly owned subsidiary ES Cell International Pte Ltd (“ESI”). Under the terms of the Cross-License, Asterias received a fully-paid, non-royalty-bearing, world-wide, non-exclusive, sub-licensable license under certain BioTime patents and related patent rights and ESI patents and related patent rights specified in the Cross-License, for all purposes in the Asterias Licensed Field, as defined in the Cross-License agreement, during the term of the license.
8
Under the terms of the Cross-License, BioTime and ESI received a fully-paid, non-royalty-bearing, world-wide, non-exclusive, sub-licensable license in, to, and under the certain Asterias patents and related patent rights for all purposes in the BioTime/ESI Licensed Field, as defined in the Cross-License agreement, during the term of the license.
On February 16, 2016, Asterias also entered into a Share Transfer Agreement (“Share Transfer”) with BioTime and ESI pursuant to which (a) Asterias transferred to BioTime 2,100,000 shares of common stock of OrthoCyte Corporation (“OrthoCyte) and 21,925 ordinary shares of Cell Cure Neurosciences Ltd (“Cell Cure”), each a majority-owned subsidiary of BioTime, with an aggregate carrying value at the time of the transaction of approximately $416,000 and (b) BioTime transferred to Asterias 75,771 shares of Series A Common Stock of Asterias with a carrying value at the time of the transaction of approximately $197,000 and warrants to purchase 3,150,000 Series A common stock of Asterias at an exercise price of $5.00 per share, with a carrying value at the time of the transaction of approximately $2.0 million, as additional consideration for the license of patents and patent rights from Asterias under the Cross License. On March 20, 2016, the warrants to purchase 3,150,000 shares of Series A common stock were retired by Asterias.
The Cross-License and Share Transfer transaction was accounted for as a transfer of assets between entities under common control and recorded at carrying value, with the resulting gain on transfer of approximately $1.8 million recorded by Asterias in equity as contributed capital from BioTime in accordance with, and pursuant to ASC 805-50, Transactions Between Entities Under Common Control. The transfer of assets was also a taxable transaction to Asterias generating a taxable gain of approximately $3.1 million as further discussed in Note 11.
| 6. |
Intangible Assets
|
Intangible assets net of accumulated amortization at June 30, 2017 and December 31, 2016 are shown in the following table (in thousands):
|
June 30,
2017
(Unaudited)
|
December 31,
2016
|
|||||||
|
Intangible assets
|
$
|
26,860
|
$
|
26,860
|
||||
|
Less- accumulated amortization
|
(10,073
|
)
|
(8,730
|
)
|
||||
|
Intangible assets, net
|
$
|
16,787
|
$
|
18,130
|
||||
Asterias recognized $672,000 and $1.3 million in amortization expense of intangible assets during the three and six months ended June 30, 2017 and 2016, respectively.
| 7. |
Common Stock and Warrants
|
As of June 30, 2017 and December 31, 2016, Asterias had outstanding 49,555,959 and 47,566,596 Series A Shares and no Series B Shares, respectively.
Common Stock Issuance
On March 28, 2017, Asterias entered into an amendment to its at-the-market (ATM) Sales Agreement, dated April 10, 2015, with MLV. The amendment to the Sales Agreement was entered into by Asterias, MLV and FBR Capital Markets & Co. (“FBR” and together with MLV, the “Agents”), which acquired MLV. Under the Sales Agreement, as amended, Asterias may issue and sell shares of its Series A common stock having an aggregate offering price of up to $25 million from time to time on or after March 28, 2017, through the Agents, subject to certain limitations, including the number of shares registered and available under the Company’s previously filed and currently effective shelf registration statement on Form S-3 (File No. 333-215154) (the “Registration Statement”). For the six months ended June 30, 2017, Asterias has sold approximately 1.6 million shares of Series A common stock for gross proceeds of $6.7 million. For the six months ended June 30, 2016, Asterias sold approximately 41,211 shares of Series A common stock for gross proceeds of $0.2 million.
For the six months ended June 30, 2017 and 2016, pursuant to a services agreement with Cell Therapy Catapult Services Limited, Asterias issued 134,766 and 142,020 shares of Asterias Series A common stock with a fair value of $562,000 and $644,000 respectively (see Note 12).
Warrants classified as a liability
On May 13, 2016, as part of the Asterias Series A Common Stock Offering, Asterias issued 2,959,559 warrants (the “Asterias Offering Warrants”). The Asterias Offering Warrants have an exercise price of $4.37 per share and expire in five years of the issuance date, or May 13, 2021. The Asterias Offering Warrants also contain certain provisions in the event of a Fundamental Transaction, as defined in the warrant agreement governing the Asterias Offering Warrants (“Warrant Agreement”), that Asterias or any successor entity will be required to purchase, at a holder’s option, exercisable at any time concurrently with or within thirty days after the consummation of the fundamental transaction, the Asterias Offering Warrants for cash. This cash settlement will be in an amount equal to the value of the unexercised portion of such holder’s warrants, determined in accordance with the Black Scholes-Merton option pricing model as specified in the Warrant Agreement.
9
In accordance with ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock, contracts that require or may require the issuer to settle the contract for cash are liabilities recorded at fair value, irrespective of the likelihood of the transaction occurring that triggers the net cash settlement feature. Changes to the fair value of those liabilities are recorded in the statements of operations. Accordingly, since Asterias may be required to net cash settle the Asterias Offering Warrants in the event of a Fundamental Transaction; the Asterias Offering Warrants are classified as noncurrent liabilities at fair value, with changes in fair value recorded in other income or expense, net, in the statements of operations.
The fair value of the Asterias Offering Warrants at the time of issuance was determined by using a combination of the Binomial Lattice and Black-Scholes-Merton option pricing models under various probability-weighted outcomes which take into consideration the probability of the fundamental transaction and net cash settlement occurring, using the contractual term of the warrants. In applying these models, the fair value is determined by applying Level 3 inputs, as defined by ASC 820; these inputs have included assumptions around the estimated future stock price of Asterias common stock, volatility and the timing of, and varying probabilities that certain events will occur. The Asterias Offering Warrants are revalued each reporting period using the same methodology described above. Changes in any of the key assumptions used to value the Asterias Offering Warrants could materially impact the fair value of the warrants and Asterias’ financial statements.
At June 30, 2017, based on a valuation performed on the Asterias Offering Warrants using the methodology described above, the fair value of the Asterias Offering Warrants liability was $5.8 million, resulting in Asterias recording an unrealized gain of $2.9 million for the six months ended June 30, 2017, included in other income and expenses, net, in the statements of operations.
Warrants classified as equity
On March 30, 2016, Asterias’ board of directors declared a distribution of Asterias common stock purchase warrants to all Asterias shareholders other than BioTime, in the ratio of one warrant for every five shares of Asterias common stock owned of record as of the close of business on April 11, 2016. On April 25, 2016, Asterias distributed 3,331,229 warrants (the “Distribution Warrants”). The distribution of the Distribution Warrants was treated as a disproportionate distribution since, in accordance with the terms of the Share Transfer with BioTime, no warrants were distributed to BioTime. The Distribution Warrants are classified as equity, have an exercise price of $5.00 per share, and were set to expire on September 30, 2016. Asterias recorded the Distribution Warrants at a fair value of approximately $3.1 million with a noncash charge to shareholder expense included in general and administrative expenses and a corresponding increase to equity as of March 30, 2016 as the Distribution Warrants were deemed to be issued for accounting purposes on that date.
On September 19, 2016, Asterias extended the expiration date of the Distribution Warrants to February 15, 2017, no other terms were changed. As a result of the extension of the expiration date of these warrants, Asterias recorded a $2.0 million noncash charge to shareholder expense included in general and administrative expenses and a corresponding increase to equity for the year ended December 31, 2016. On February 3, 2017, Asterias extended the expiration date of the Distribution Warrants to September 29, 2017. As a result of this extension, Asterias recorded a $1.7 million noncash charge to shareholder expense included in general and administrative expenses and a corresponding increase to equity for the quarter ended March 31, 2017.
In connection with the warrant distribution to shareholders discussed above, 350,000 warrants with an exercise price of $5.00 per share held by Romulus Films, Ltd. were adjusted to become exercisable into 409,152 shares at an exercise price of $4.28 per share (the “Romulus Warrants”). These warrants had an original expiration date of September 30, 2016. On September 19, 2016, Asterias extended the expiration date of the Romulus Warrants to February 15, 2017, no other terms were changed. As a result of the extension of the expiration date of these warrants, Asterias recorded a $0.2 million noncash charge to shareholder expense included in general and administrative expenses and a corresponding increase to equity for the year ended December 31, 2016. On February 3, 2017, Asterias extended the expiration date of the Romulus Warrants to September 29, 2017. As a result of this extension of the expiration date of these warrants, Asterias recorded a $0.3 million noncash charge to shareholder expense included in general and administrative expenses and a corresponding increase to equity for the quarter ended March 31, 2017.
| 8. |
Stock-Based Compensation
|
The following table shows the stock-based compensation expenses included in the operating expenses for the three and six months ended June 30, 2017 and 2016 (in thousands):
|
Three Months Ended
June 30,
(Unaudited)
|
Six Months Ended
June 30,
(Unaudited)
|
|||||||||||||||
|
2017
|
2016
|
2017
|
2016
|
|||||||||||||
|
Research and development
|
$
|
502
|
$
|
631
|
$
|
1,427
|
$
|
1,356
|
||||||||
|
General and administrative
|
489
|
237
|
1,282
|
1,121
|
||||||||||||
|
Total stock-based compensation expense
|
$
|
991
|
$
|
868
|
$
|
2,709
|
$
|
2,477
|
||||||||
The fair value of each option award is estimated on the date of grant using a Black-Scholes option valuation model applying the weighted-average assumptions in the following table:
10
|
Three Months Ended
June 30,
(Unaudited)
|
Six Months Ended
June 30,
(Unaudited)
|
|||||||||||||||
|
2017
|
2016
|
2017
|
2016 | |||||||||||||
|
Expected life (in years)
|
6.06
|
5.37
|
5.74
|
5.76
|
||||||||||||
|
Risk-free interest rates
|
1.85
|
%
|
1.28
|
%
|
1.88
|
%
|
1.39
|
%
|
||||||||
|
Volatility
|
74.13
|
%
|
76.62
|
%
|
74.80
|
%
|
74.99
|
%
|
||||||||
|
Dividend yield
|
0
|
0
|
0
|
%
|
0
|
%
|
||||||||||
The risk-free rate is based on the rates in effect at the time of grant for zero coupon U.S. Treasury notes with maturities approximately equal to each grant’s expected term. A dividend yield of zero is applied since Asterias has not historically paid dividends and does not expect to pay dividends in the foreseeable future. The expected volatility is based upon the volatility of Asterias’ own trading stock and a group of publicly traded industry peer companies. The expected term of options granted is derived from using the simplified method under SEC Staff Accounting Bulletin Topic 14.
The determination of stock-based compensation is inherently uncertain and subjective and involves the application of valuation models and assumptions requiring the use of judgment. If Asterias had made different assumptions, its stock-based compensation expense, and net loss for the three and six months ended June 30, 2017 and 2016, may have been significantly different.
| 9. |
Commitments and Contingencies
|
Development and Manufacturing Services Agreement
On August 3, 2016, Asterias entered into a Development and Manufacturing Services Agreement (the “Services Agreement”) with Cognate BioServices, Inc. (“Cognate”), a fully-integrated contract bioservices organization providing development and current Good Manufacturing Practice (“cGMP”) manufacturing services to companies and institutions engaged in the development of cell-based products.
Under the Services Agreement, Cognate is performing under an Initial Statement of Work process development studies in support of Asterias’ clinical and commercial development activities of AST-VAC1 and production and manufacturing services of AST-VAC1 under cGMP under the Second Statement of Work. In consideration for the process development services set forth in the Initial Statement of Work, Asterias agreed to make aggregate payments of up to approximately $1.7 million in fees over the term of the Initial Statement of Work and pay for additional pass through costs for materials and equipment estimated by management to be approximately $0.5 million. In consideration of the production and manufacturing services set forth in the Second Statement of Work, once the services under the Initial Statement of Work are completed and if Asterias receives FDA concurrence on the clinical protocol for an AST-VAC1 trial, then Asterias will make an initial start-up payment, a monthly payment for dedicated manufacturing capacity, and certain other manufacturing fees.
The Services Agreement will expire on the later of (a) August 3, 2019; or (b) the completion of all services contracted for by the parties in the Statements of Work under the Services Agreement prior to August 3, 2019. The term of the Services Agreement and any then pending Statements of Work thereunder may be extended by Asterias continuously for additional two-year periods upon written notice to Cognate with at least thirty days prior to the expiration of the then-current term.
The Services Agreement provides certain termination rights to each party and customary provisions relating to indemnity, confidentiality and other matters. Asterias incurred $216,000 and $424,000 of expense to Cognate pursuant to the Services Agreement for the three and six months ended June 30, 2017.
Fremont Lease
On December 30, 2013, Asterias entered into a lease for an office and research facility located in Fremont, California, consisting of an existing building with approximately 44,000 square feet of space. The building is being used by Asterias as a combined office, laboratory and production facility that can be used to manufacture its product using current good manufacturing procedures. Asterias completed the tenant improvements in November 2015, which cost approximately $4.9 million, of which the maximum of $4.4 million was paid to Asterias by the landlord. Asterias placed the asset into service in November 2015 and is amortizing the leasehold improvements and the landlord liability over the remaining lease term through September 30, 2022.
As of June 30, 2017 and December 31, 2016, the landlord lease liability was $3.7 million and $4.0 million and the deferred rent liability was $296,000 and $266,000, respectively.
Litigation – General
Asterias is subject to various claims and contingencies in the ordinary course of its business, including those related to litigation, business transactions, employee-related matters, and others. When Asterias is aware of a claim or potential claim, it assesses the likelihood of any loss or exposure. If it is probable that a loss will result and the amount of the loss can be reasonably estimated, Asterias will record a liability for the loss. If the loss is not probable or the amount of the loss cannot be reasonably estimated, Asterias discloses the claim if the likelihood of a potential loss is reasonably possible and the amount involved could be material. Asterias is not aware of any claims likely to have a material adverse effect on its financial condition or results of operations.
11
Employment Contracts
Asterias has entered into employment contracts with certain executive officers. Under the provisions of the contracts, Asterias may be required to incur severance obligations for matters relating to changes in control, as defined and involuntary terminations.
Indemnification
In the normal course of business, Asterias may provide indemnifications of varying scope under Asterias’ agreements with other companies or consultants, typically Asterias’ clinical research organizations, investigators, clinical sites, suppliers and others. Pursuant to these agreements, Asterias will generally agree to indemnify, hold harmless, and reimburse the indemnified parties for losses and expenses suffered or incurred by the indemnified parties arising from claims of third parties in connection with the use or testing of Asterias’ products and services. Indemnification provisions could also cover third party infringement claims with respect to patent rights, copyrights, or other intellectual property pertaining to Asterias products and services. The term of these indemnification agreements will generally continue in effect after the termination or expiration of the particular research, development, services, or license agreement to which they relate. The potential future payments Asterias could be required to make under these indemnification agreements will generally not be subject to any specified maximum amount. Historically, Asterias has not been subject to any claims or demands for indemnification. Asterias also maintains various liability insurance policies that limit Asterias’ exposure. As a result, Asterias believes the fair value of these indemnification agreements is minimal. Accordingly, Asterias has not recorded any liabilities for these agreements as of June 30, 2017 and December 31, 2016.
| 10. |
Shared Facilities and Services Agreement
|
On April 1, 2013, Asterias and BioTime executed a Shared Facilities and Services Agreement (“Shared Services Agreement”). Under the terms of the Shared Services Agreement, Asterias has the right to use BioTime's premises and equipment located at Alameda, California, for the sole purpose of conducting Asterias' business. BioTime also may provide certain services, including basic accounting, billing, bookkeeping, payroll, treasury, collection of accounts receivable (excluding the institution of legal proceedings or taking of any other action to collect accounts receivable), payment of accounts payable, and other similar administrative services to Asterias and services of its laboratory and research personnel. BioTime may also provide the services of attorneys, accountants, and other professionals who may also provide professional services to BioTime and its other subsidiaries.
BioTime charges Asterias a fee for the services and usage of facilities, equipment, and supplies aforementioned. For each billing period, BioTime equitably prorates and allocates its employee costs, equipment costs, insurance costs, lease costs, professional costs, software costs, supply costs, and utilities costs, if any, between BioTime and Asterias based upon actual documented use and cost by or for Asterias or upon proportionate usage by BioTime and Asterias, as reasonably estimated by BioTime. Asterias pays 105% of the allocated costs (the “Use Fee”). The allocated cost of BioTime employees and contractors who provide services is based upon records maintained of the number of hours of such personnel devoted to the performance of services.
The Use Fee is determined and invoiced to Asterias on a quarterly basis for each calendar quarter of each calendar year. If the Shared Services Agreement terminates prior to the last day of a billing period, the Use Fee will be determined for the number of days in the billing period elapsed prior to the termination of the Shared Services Agreement. Each invoice is payable in full by Asterias within 30 days after receipt. Any invoice or portion thereof not paid in full when due will bear interest at the rate of 15% per annum until paid, unless the failure to make a payment is due to any inaction or delay in making a payment by BioTime employees from Asterias funds available for such purpose, rather than from the unavailability of sufficient funds legally available for payment or from an act, omission, or delay by any employee or agent of Asterias.
Asterias in turn may charge BioTime or any Other Subsidiary for similar services provided by Asterias at the same rate and terms as aforementioned. “Other Subsidiary” means a subsidiary of BioTime other than a subsidiary of Asterias.
The Shared Services Agreement was renewed through December 31, 2017. The term of the Shared Services Agreement will automatically be renewed and the termination date will be extended for an additional year each year, unless either party gives the other party written notice stating that the Shared Services Agreement will terminate on December 31 of that year.
BioTime allocated $74,000 and $437,000 of general overhead expenses to Asterias during the six months ended June 30, 2017, and 2016, respectively. At June 30, 2017 Asterias had no net payable to BioTime under the Shared Services Agreement.
| 11. |
Income Taxes
|
The provision for income taxes is determined using an estimated annual effective tax rate. The effective tax rate may be subject to fluctuations during the year as new information is obtained, which may affect the assumptions used to estimate the annual effective tax rate, including factors such as valuation allowances against deferred tax assets, the recognition or de-recognition of tax benefits related to uncertain tax positions, if any, and changes in or the interpretation of tax laws in jurisdictions where Asterias conducts business.
Management believes that the Asterias net operating losses generated during the three and six months ended June 30, 2017 will result in no income tax benefit or provision in the current year due to the full valuation allowance on its net deferred tax assets for the year ended December 31, 2016 and a full valuation allowance expected on its net deferred tax assets for the year ending December 31, 2017.
12
A valuation allowance is provided when it is more likely than not that some portion of the deferred tax assets will not be realized. Asterias established a full valuation allowance as of December 31, 2016 due to the uncertainty of realizing future tax benefits from its net operating loss carryforwards and other deferred tax assets.
A deferred federal income tax benefit of approximately $1.4 million was recorded for the six months ended June 30, 2016 as Asterias’ deferred tax liabilities exceeded their deferred tax assets and recorded no valuation allowance on its deferred tax assets. Asterias established deferred tax liabilities primarily related to its acquisition of certain intellectual property and available for sale securities held in BioTime and OncoCyte common stock. For state income tax purposes Asterias has a full valuation allowance on its state deferred tax assets as of June 30, 2017 and December 31, 2016 and, accordingly, no state tax provision or benefit was recorded for any period presented.
As discussed in Note 5, in connection with the Cross-License and Share Transfer transaction completed on February 16, 2016, the transfer of assets was a taxable transaction to Asterias generating a taxable gain of approximately $3.1 million. Asterias had sufficient current year losses from operations to offset the entire gain resulting in no income taxes due. As the transfer of assets and the resulting taxable gain is due to a direct effect of transactions between the former parent company, BioTime, and its former subsidiary, Asterias recorded the tax effect of this gain through equity in accordance with ASC 740-20-45-11(g).
| 12. |
License and Royalty Obligations
|
Services Agreement with Cell Therapy Catapult Services Limited
In October 2015, Asterias entered into a Services Agreement (the “Services Agreement”) with Cell Therapy Catapult Services Limited (“Catapult”), a research organization specializing in the development of technologies which speed the growth of the cell and gene therapy industry. Under the Services Agreement, Catapult will license to Asterias, certain background intellectual property and will develop a scalable manufacturing and differentiation process for Asterias’ human embryonic stem cell derived dendritic cell cancer vaccine development program. In consideration for the license and Catapult’s performance of services, at the time of the Services Agreement Asterias agreed to make aggregate payments of up to GBP £4,350,000 over the next five years (approximately $5.6 million based on the foreign currency exchange rate on June 30, 2017). At the option of Asterias, up to GBP £3,600,000 (approximately $4.7 million based on the foreign currency exchange rate on June 30, 2017) of such payments may be settled in shares of Asterias Series A Common Stock instead of cash. If Asterias elects to pay for the services in stock and Catapult is unable to sell the stock in the market within 60 days of issuance, after reasonable and diligent efforts through its broker, Catapult may request that the unsold portion of the stock payment, if any, be paid by Asterias in cash at a value equal to approximately 91% of the total amount that was issued in stock. This right by Catapult to put the unsold shares back to Asterias for cash expires the earlier to occur of the sale of the stock in the market or after 60 days of issuance.
Advance payments for research and development services to be performed by Catapult are deferred and recognized as research and development expense ratably as the services are performed. Advance payments related to licenses will be expensed when paid due to the experimental nature of the project. Pursuant to the Services Agreement, if there are any issued, but unsold Asterias stock, to Catapult for payment of services and the 60-day put right has not expired as of the period end being reported on, Asterias will present that amount as “temporary” equity in accordance with ASC 480-10-S99. Once the put right expires or the shares are sold by Catapult, the temporary equity amount will be reclassified by Asterias to permanent equity without adjustment to the carrying value of the stock.
In the six months ended June 30, 2017 and 2016, pursuant to the Services Agreement, Asterias issued 134,766 and 142,020 shares of Asterias Series A Common Stock with a fair market values of $562,000 and $644,000 at the time of issuance which Asterias reclassified into permanent equity. For the six months ended June 30, 2017 and 2016, in connection with payments under the Services Agreement, Asterias expensed as stock-based compensation for services in lieu of cash of $562,000 and $644,000, respectively.
| 13. |
Clinical Trial and Option Agreement and CIRM Grant Award
|
On October 16, 2014 Asterias signed a Notice of Grant Award (“NGA”) with CIRM, effective October 1, 2014, with respect to a $14.3 million grant award for clinical development of Asterias’ product, AST-OPC1. The NGA was subsequently amended effective November 26, 2014 and March 2, 2016. The NGA includes the terms under which CIRM will release grant funds to Asterias. Under the NGA as amended on March 2, 2016, CIRM will disburse the grant funds to Asterias based on Asterias’ attainment of certain progress milestones.
Asterias received initial payment from CIRM in the amount of $917,000 during October 2014 and had received $12.8 million through December 31, 2016. For the three and six months ended June 30, 2017, we have not received any payment under the CIRM grant with approximately $1.5 million expected upon further clinical milestone achievements. We had no deferred grant income relating to the CIRM grant as of June 30, 2017 and deferred grant income relating to the CIRM grant was $2.2 million at December 31, 2016.
13
| Item 2. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
The matters addressed in this Item 2 that are not historical information constitute “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, including statements about any of the following: any projections of earnings, revenue, gross profit, cash, effective tax rate, use of net operating losses, or any other financial items; the plans, strategies and objectives of management for future operations or prospects for achieving such plans, and any statements of assumptions underlying any of the foregoing. Any statements contained herein that are not statements of historical fact may be deemed to be forward-looking statements. Without limiting the foregoing, the words “believes,” “anticipates,” “plans,” “expects,” “seeks,” “estimates,” and similar expressions are intended to identify forward-looking statements. While Asterias may elect to update forward-looking statements in the future, it specifically disclaims any obligation to do so, even if the Asterias’ estimates change and readers should not rely on those forward-looking statements as representing Asterias’ views as of any date subsequent to the date of the filing of this Quarterly Report. Although we believe that the expectations reflected in these forward-looking statements are reasonable, such statements are inherently subject to risks and Asterias can give no assurances that its expectations will prove to be correct. Actual results could differ materially from those described in this report because of numerous factors, many of which are beyond the control of Asterias. A number of important factors could cause the results of the company to differ materially from those indicated by such forward-looking statements, including those detailed under the heading “Risk Factors” in Part I, Item 1A of Asterias’ Form 10-K for the year ended December 31, 2016, and the additional risk factors contained in this Quarterly Report on Form 10-Q.
The following discussion should be read in conjunction with Asterias’ interim condensed financial statements and the related notes provided under “Item 1- Financial Statements” above.
Company Overview
Asterias is a biotechnology company focused on the emerging fields of cell therapy and regenerative medicine. Asterias has two core technology platforms. The first is a type of stem cell capable of becoming all of the cell types in the human body, a property called pluripotency. The second is the use of a cell type called “dendritic cells” to teach cancer patients’ immune systems to attack their tumors.
Asterias currently has three clinical stage programs based on these platforms: AST-OPC1 is a therapy derived from pluripotent stem cells that is currently in a Phase 1/2a clinical trial for spinal cord injuries; AST-VAC1 is a patient-specific cancer immunotherapy using dendritic cells being evaluated by Asterias in Acute Myeloid Leukemia (AML); and AST-VAC2 is a non-patient specific cancer immunotherapy using dendritic cells for which the initiation of a Phase 1/2a clinical trial in non-small cell lung cancer is planned for the first half of 2017. Asterias’ technology platforms have the potential for application in additional indications, such as advanced multiple sclerosis and white matter stroke for AST-OPC1 and other additional cancer indications for our cancer immunotherapy platform.
Recent Developments
Below are recent updates regarding Asterias’ clinical programs:
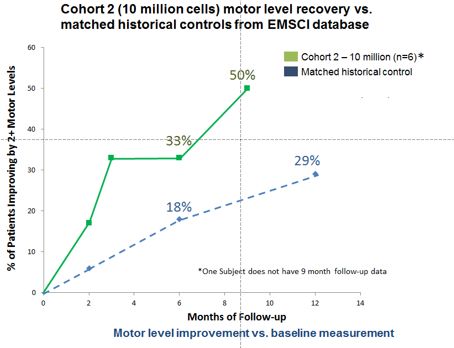
In June 2017, Asterias announced new 9-month follow-up data from the AIS-A 10 million cell cohort in its SCiStar Phase 1/2a clinical trial (the “SCiStar study”), and included the following observations:
14
| · |
Motor Level Improvement – Additional motor level improvement was seen in the AIS-A 10 million cell cohort at 9 months.
|
| o |
Three of six patients (50%) achieved two motor levels of improvement over baseline on at least one side as of their latest follow-up visit through 9 months. This compares to two of six patients (33%) that had improved two motor levels on at least one side through 3- and 6-months of follow-up.
|
| o |
In addition, all six patients (100%) achieved at least one motor level of improvement on at least one side as of their latest follow-up through 9-months.
|
| · |
Upper Extremity Motor Score (UEMS) – Additional improvement in the average UEMS score for this cohort was observed at 9 months. The average UEMS improvement at 9 months was 11.2 points, compared to 9.7 points at 6 months.
|
| · |
Matched Historical Control Data - The 9-month results show a meaningful improvement in the motor function recovery in the AIS-A patients receiving 10 million AST-OPC1 cells compared to a historical control group of 62 closely matched patients from the EMSCI database.
|
| · |
Safety - The trial results to date continue to indicate a positive safety profile for AST-OPC1.
|
On July 10, 2017, Asterias announced that the U.S. Food and Drug Administration accepted the company's amendment to the clinical research protocol for the SCiStar study. The amendment expands the eligibility criteria to include patients with a C-4 spinal cord injury and extends the dosing window from 14 to 30 days to 21 to 42 days post-injury.
In July 2017, Asterias announced completion of enrollment and dosing of the AIS-A 20 million cell cohort and the AIS-B 10 million cell cohort in the SCiStar study. The enrollment of the fifth patient in the AIS-A 20 million cell cohort triggered the final $1.5 million grant payment from CIRM under the existing $14.3 million Strategic Partnerships Award grant awarded to Asterias. Asterias expects to receive this $1.5 million grant payment in the third quarter of 2017.
In July 2017, Asterias announced that two additional clinical sites have opened to enroll subjects for the SCiStar study, providing additional geographical reach and previous experience with spinal cord injury trials. Asterias now has eight clinical sites throughout the country enrolling patients in the study.
In August, Asterias announced it enrolled and dosed the first patient in the fifth and final cohort in the SCiStar study. Asterias has now completed enrollment and dosing in four of the five planned SCiStar study cohorts and enrolled twenty-two patients in the SCiStar study. Twenty-seven patients have been administered AST-OPC1 after including patients from a previous Phase 1 safety trial and results-to-date continue to support the safety of AST-OPC1. Asterias intends to complete enrollment of the entire SCiStar study later this year, with multiple safety and efficacy readouts anticipated during the remainder of 2017 and 2018.
In August, Asterias announced that Cancer Research UK, supported by Asterias technical personnel, has successfully completed manufacture of the first cGMP (current Good Manufacturing Practice) clinical grade lot of AST-VAC2, which meets all release specifications. This lot will provide clinical trial material for patients enrolling in the upcoming Phase 1/2a study evaluating AST-VAC2 in non-small cell lung cancer.
Critical Accounting Policies
This Management's Discussion and Analysis of Financial Condition and Results of Operations discusses and analyzes data in our unaudited Condensed Financial Statements, which we have prepared in accordance with U.S. generally accepted accounting principles. Preparation of these financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue and expenses, and related disclosure of contingent assets and liabilities. Management bases its estimates on historical experience and on various other assumptions that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Senior management has discussed the development, selection and disclosure of these estimates with the Audit Committee of our Board of Directors. Actual conditions may differ from our assumptions and actual results may differ from our estimates.
An accounting policy is deemed critical if it requires an accounting estimate to be made based on assumptions about matters that are highly uncertain at the time the estimate is made, if different estimates reasonably could have been used, or if changes in the estimate that are reasonably likely to occur could materially impact the financial statements. Management believes that there have been no significant changes during the three and six months ended June 30, 2017 to the items that we disclosed as our critical accounting policies and estimates in Management's Discussion and Analysis of Financial Condition and Results of Operations in our Annual Report on Form 10-K for the year ended December 31, 2016.
Results of Operations
Comparison of three and six months ended June 30, 2017 and 2016.
For the three months ended June 30, 2017 and 2016 we recorded net loss of $8.7 million and $5.2 million, respectively. For the six months ended June 30, 2017 and 2016 we recorded net losses of $15.0 million and $15.5 million, respectively.
15
Revenues
The following table shows certain information about our revenues for the three and six months ended June 30, 2017 and 2016 (in thousands, except for percentages):
|
Three Months Ended
June 30,
|
$
Increase
(Decrease)
|
%
Increase
(Decrease)
|
||||||||||||||
|
2017
|
2016
|
|||||||||||||||
|
Grant income
|
$
|
291
|
$
|
1,520
|
$
|
-1,229
|
-81
|
%
|
||||||||
|
Royalties from product sales
|
25
|
12
|
+13
|
+108
|
%
|
|||||||||||
|
Total revenues
|
316
|
1,532
|
-1,216
|
-79
|
%
|
|||||||||||
|
Cost of sales
|
(18
|
)
|
(6
|
)
|
-12
|
-200
|
%
|
|||||||||
|
Gross profit
|
$
|
298
|
$
|
1,526
|
$
|
-1,228
|
- 80
|
%
|
||||||||
|
Six Months Ended
June 30,
|
$
Increase
(Decrease)
|
%
Increase
(Decrease)
|
||||||||||||||
|
2017
|
2016
|
|||||||||||||||
|
Grant income
|
$
|
2,185
|
$
|
3,007
|
$
|
-822
|
- 27
|
%
|
||||||||
|
Royalties from product sales
|
141
|
119
|
+22
|
+ 18
|
%
|
|||||||||||
|
Total revenues
|
2,326
|
3,126
|
-800
|
- 26
|
%
|
|||||||||||
|
Cost of sales
|
(70
|
)
|
(59
|
)
|
-11
|
-19
|
%
|
|||||||||
|
Gross profit
|
$
|
2,256
|
$
|
3,067
|
$
|
- 811
|
- 26
|
%
|
||||||||
Our royalty revenues from product sales is entirely from non-exclusive license agreements with Stem Cell Technologies, Inc., Corning Life Science, Life Tech, and Millipore each of which we assumed as part of the consideration received from Geron under the 2013 Asset Contribution Agreement.
Grant income in 2016 was entirely from CIRM, which awarded us a $14.3 million grant for clinical development of AST-OPC1. We received our first payment from CIRM in the amount of $917,000 during October 2014 and had received $12.8 million through December 31, 2016. For the six months ended June 30, 2017, we have not received any payment under the CIRM grant with approximately $1.5 million expected in the third quarter upon further clinical milestone achievements. Revenues recognized under the CIRM grant during the six months ended June 30, 2017 and 2016 were $2.2 and $3.0 million, respectively.
Operating Expenses
The following table shows our operating expenses for the three and six months ended June 30, 2017 and 2016 (in thousands, except for percentages):
|
Three Months Ended
June 30,
|
$
Increase/
(Decrease)
|
%
Increase/
Decrease
|
||||||||||||||
|
2017
|
2016
|
|||||||||||||||
|
Research and development expenses
|
$
|
6,984
|
$
|
6,019
|
$
|
+965
|
+16
|
%
|
||||||||
|
General and administrative expenses
|
1,847
|
2,581
|
-734
|
-28
|
%
|
|||||||||||
|
Six Months Ended
June 30,
|
$
Increase/
(Decrease)
|
%
Increase/
Decrease
|
||||||||||||||
|
2017
|
2016
|
|||||||||||||||
|
Research and development expenses
|
$
|
13,582
|
$
|
12,362
|
$
|
+1,220
|
+10
|
%
|
||||||||
|
General and administrative expenses
|
6,314
|
8,871
|
-2,557
|
-29
|
%
|
|||||||||||
Research and development expenses – Research and development expenses increased $1.0 million to $7.0 million for the three months ended June 30, 2017 compared to $6.0 million for the three months ending June 30, 2016. This increase was largely associated with our AST-OPC1 clinical trial and AST-OPC1-related manufacturing planning expenses. Research and development expenses increased $1.2 million to $13.6 million for the six months ended June 30, 2017 compared to $12.4 million for the six months ending June 30, 2016. This increase was largely associated with our AST-OPC1 clinical trial and AST-OPC1-related manufacturing planning expenses.
16
General and administrative expenses – General and administrative expenses decreased by approximately $0.7 million to $1.8 million for the three months ended June 30, 2017 compared to $2.6 million for the same period in 2016. The decrease in general and administrative expense is primarily attributable to the following: a decrease of $0.6 million in shareholder warrant distribution expense related to revaluing warrants outstanding. General and administrative expenses decreased by approximately $2.6 million to $6.3 million for the six months ended June 30, 2017 compared to $8.9 million for the same period in 2016. The decrease in general and administrative expense is primarily attributable to the following: a decrease of $1.7 million in shareholder warrant distribution expense related to revaluing warrants outstanding, a decrease of $0.4 million in salaries due to severance paid to two executives in 2016, and a decrease of $0.3 million due lower legal related expenses.
Other income/(expense), net
Other income/(expense), net – Other expense, net, in 2017 and 2016 consists primarily of the change in fair value of the warrants classified as liabilities.
Income Taxes
Management believes that our net operating losses incurred during the three and six months ended June 30, 2017 will result in no income tax benefits in the current year due to the full valuation allowance as of December 31, 2016 and a full valuation allowance expected on its net deferred tax assets for the year ending December 31, 2017.
A deferred federal income tax benefit of approximately $451,000 and $1.4 million was recorded for the three and six months ended June 30, 2016 as Asterias had no valuation allowance on its deferred tax assets as of December 31, 2015. Asterias established deferred tax liabilities primarily related to its acquisition of certain intellectual property and available for sale securities held in BioTime and OncoCyte common stock.
Liquidity and Capital Resources
At June 30, 2017, we had $11.9 million of cash and cash equivalents on hand, held 3,852,880 BioTime common shares and 192,644 shares of OncoCyte common stock, with a market value of $12.1 million and $1.0 million, respectively. We may raise capital from time to time through the sale of our Series A Shares or other securities, and our BioTime or OncoCyte common shares. We may sell our Series A Shares or other securities in public offerings registered under the Securities Act of 1933, as amended (the “Securities Act”), including in at-the-market transactions, or in private placements to select investors. We may sell our BioTime common shares, from time to time, by any method that is deemed to be an “at-the-market” equity offering as defined in Rule 415 promulgated under the Securities Act, including sales made directly on or through the NYSE MKT or any other existing trading market for the common shares in the U.S. or to or through a market maker, at prices related to the prevailing market price, or through block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction, or through one more of the foregoing transactions. We may also sell some or all of our BioTime common shares and OncoCyte common shares by any other method permitted by law, including in privately negotiated transactions. We will bear all broker-dealer commissions payable in connection with the sale of our Series A Shares, our BioTime common shares, OncoCyte common shares or other securities. Broker-dealers may receive commissions or discounts from us (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated. The prices at which we may issue and sell our Series A Shares, our BioTime common shares, OncoCyte common shares or other securities in the future are not presently determinable and will depend upon many factors, including prevailing prices for those securities in the public market.
On March 28, 2017, Asterias entered into an amendment to its at-the-market (ATM) Sales Agreement, dated April 10, 2015, with MLV. The amendment to the Sales Agreement was entered into by Asterias, MLV and FBR Capital Markets & Co. (“FBR” and together with MLV, the “Agents”), which acquired MLV. Under the Sales Agreement, as amended, Asterias may issue and sell shares of its Series A common stock having an aggregate offering price of up to $25 million from time to time on or after March 28, 2017, through the Agents, subject to certain limitations, including the number of shares registered and available under the Company’s previously filed and currently effective shelf registration statement on Form S-3 (File No. 333-215154) (the “Registration Statement”). For the six months ended June 30, 2017, Asterias has sold approximately 1.6 million shares of Series A common stock for gross proceeds of $6.7 million. For the six months ended June 30, 2016, Asterias sold approximately 41,211 shares of Series A common stock for gross proceeds of $0.2 million.
We plan to use the proceeds and other cash we have available for general corporate purposes, including to fund our ongoing clinical programs, to develop certain of our product candidates and technology, to acquire new stem cell products and technology through licenses or similar agreements from other companies, and to defray overhead expenses and to pay general and administrative expenses.
Since inception, we have incurred net losses and have funded our operations primarily through the issuance of equity securities, warrants, payments from research grants, and royalties from product sales. At June 30, 2017 we had an accumulated deficit of $98.7 million, working capital of $24.3 million and stockholders’ equity of $36.7 million. We have evaluated our projected cash flows and believe that our cash and cash equivalents of $11.9 million as of June 30, 2017 and our available-for-sale securities of $13.1 million as of June 30, 2017 will be sufficient to fund our operations through at least the next twelve months from the issuance date of these financial statements. If the value of Asterias’ available-for-sale securities decreases or it is unable to obtain future adequate financing for its clinical trials, it may be required to delay, postpone, or cancel its clinical trials or limit the number of clinical trial sites, or otherwise reduce or curtail its operations. Future financings may not be available to Asterias at acceptable terms, or at all. Sales of additional equity securities would result in the dilution of interests of current shareholders.
17
During the six months ended June 30, 2017, our total research and development expenditures were $13.6 million and our general and administrative expenses were $6.3 million. Our sources of cash during 2017 primarily consisted of $6.7 million from sales of our equity securities. As of June 30, 2017 and December 2016, we had a working capital surplus of $24.3 million and $30.9 million, respectively.
Cash used in operations
Net cash used in operating activities during the six months ended June 30, 2017 amounted to $14.1 million. The difference between the net loss and net cash used in operating activities during the period was primarily attributable to the following noncash items: Asterias Warrants classified as equity noncash expense in the amount of $2.0 million related to the modification of expiration date, stock-based compensation of $2.7 million, $1.3 million in amortization of intangible assets, $562,000 of stock issued in lieu of cash to a contract vendor and $555,000 in depreciation and amortization expense offset by $2.9 million in noncash decrease on the Asterias Offering Warrants classified as a liability. The remaining $3.4 million is associated with changes in our operating assets and liabilities, of which $2.2 million is associated with decreases in our deferred grant income and $1.8 million is associated with decreases in accounts payable and other accrued liabilities.
Net cash used in operating activities during the six months ended June 30, 2016 amounted to $10.0 million. The difference between the net loss and net cash used in operating activities during the period was primarily attributable to the following noncash items: Asterias warrants noncash expense to its shareholders in the amount of $3.1 million, stock-based compensation of $2.5 million, $1.3 million in amortization of intangible assets, $644,000 of stock issued in lieu of cash to a contract vendor and $605,000 in depreciation expense. The noncash increases were offset by a $1.6 million gain on change in fair value to the Asterias Offering Warrants and $1.4 million in deferred income tax benefit. Changes in working capital contributed by $0.3 million as a source of cash.
Investing and financing activities
During the six months ended June 30, 2017, we used $79,000 to purchase equipment.
During the six months ended June 30, 2016, we paid $499,000 for property, plant and equipment including tenant improvements and other fixed assets. In March 2016, we received $32,000 of the security deposit back from our previous office location in Menlo Park.
During the six months ended June 30, 2017, Asterias raised approximately $6.7 million in gross proceeds under its ATM from the sale of 1,645,549 shares of its common stock at a weighted average price of $4.07 per share.
During the six months ended June 30, 2016, we completed an underwritten public offering and issued 5,147,059 shares of common stock, and warrants to purchase an aggregate of 2,573,530 shares of common stock at an exercise price of $4.37 per share; we issued an additional 742,421 shares of common stock upon the full exercise of the over-allotment option by the underwriters for aggregate net proceeds of $18.2 million from the Asterias Offering. We received $567,000 from our landlord on reimbursable construction in progress financed by the landlord and received $2.0 million from the exercise of stock options.
Contractual Obligations
As of June 30, 2017, there were no material changes to the contractual obligations information in Item 7 in our Annual Report on Form 10-K filed with the SEC on March 28, 2017.
Off-Balance Sheet Arrangements
As of June 30, 2017 and December 31, 2016, we did not have any off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of SEC Regulation S-K.
| Item 3. |
Quantitative and Qualitative Disclosures about Market Risk
|
There have been no material changes in Asterias’ qualitative and quantitative market risk since the disclosure in Asterias’ Annual Report on Form 10-K for the year ended December 31, 2016, except as follows.
Available-for-sale securities at fair value
We hold 3,852,880 BioTime common shares and 192,644 shares of OncoCyte common stock at fair value; therefore, our available-for-sale investment values are subject to changes in the stock price of BioTime and OncoCyte. BioTime common stock trades on the NYSE MKT under the ticker “BTX” and OncoCyte common stock trades on the NYSE MKT under the ticker “OCX”. As of June 30, 2017, the 52-week high/low stock price per share range for BioTime and OncoCyte shares were $2.59 - $4.01 and $3.10 - $7.95, respectively.
18
| Item 4. |
Controls and Procedures
|
Evaluation of Disclosure Controls and Procedures
Our management, including our principal executive officer and principal financial officer, have reviewed and evaluated the effectiveness of our disclosure controls and procedures as of the end of the period covered by this report. Following this review and evaluation , the principal executive officer and principal financial officer determined that our disclosure controls and procedures are effective to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act (i) is recorded, processed, summarized, and reported within the time periods specified in SEC rules and forms, and (ii) is accumulated and communicated to management, including our principal executive officer, and principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Changes in Internal Controls
There were no changes in our internal control over financial reporting that occurred during the period covered by this Quarterly Report on Form 10-Q that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II - OTHER INFORMATION
| Item 1. |
Legal Proceedings.
|
From time to time, we may be involved in routine litigation incidental to the conduct of our business. We are not presently involved in any other material litigation or proceedings, and to our knowledge no such litigation or proceedings are contemplated.
| Item 1A. |
Risk Factors
|
The following risk factors under the header "Risks Related to Our Business Operations" includes any changes to, and supersedes the risk factors associated with the Company previously disclosed in Part I, Item 1A of the 2016 Form 10-K under the heading "Risks Related to Our Business Operations." The business, financial condition and operating results of the Company can be affected by a number of factors, whether currently known or unknown, including but not limited to those described below, any one or more of which could, directly or indirectly, cause the Company’s actual financial condition and operating results to vary materially from past, or from anticipated future, financial condition and operating results. Any of these factors, in whole or in part, could materially and adversely affect the Company’s business, financial condition, operating results and stock price.
We are also supplementing the risk factors associated with the Company previously disclosed in Part I, Item 1A of the 2016 Form 10-K under the heading "Risks Related to our Industry," "Risks Related to Our Relationship with BioTime," "Risks Related to Our Dependence on Third Parties" and "Risks Pertaining to Our Common Stock" by revising or adding the risk factors set forth below. You should read these risk factors in conjunction with the risk factors in the risk factors 2016 Form 10-K under the heading "Risks Related to our Industry," "Risks Related to Our Relationship with BioTime," "Risks Related to Our Dependence on Third Parties" and "Risks Pertaining to Our Common Stock."
The following discussion of risk factors contains forward-looking statements. These risk factors may be important to understanding other statements in this Form 10-Q. The following information should be read in conjunction with the condensed consolidated financial statements and related notes in Part I, Item 1, “Financial Statements” and Part I, Item 2, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of this Form 10-Q.
Because of the following factors, as well as other factors affecting the Company’s financial condition and operating results, past financial performance should not be considered to be a reliable indicator of future performance, and investors should not use historical trends to anticipate results or trends in future periods.
Risks Related to Our Business Operations
We are attempting to develop cellular therapies from cells derived from human embryonic stem cells.
Our use of human embryonic stem cells (“hES cells”) to attempt to develop cell therapies such as oligodendrocyte progenitor cells in our AST-OPC1 Program and dendritic cells in our AST-VAC2 Program is at the leading edge of science, and results in numerous risks and uncertainties described in greater detail in the other risk factors below. Many of these risk factors ultimately relate back to the fact that we are a hES cell research and development company. While each of these risk factors may be important to understanding other statements in this Form 10-Q and our other filings, investors should also be mindful that we are attempting to do something that has never been done: to develop and obtain FDA approval of a cell therapy derived from human embryonic stem cells.
We have a history of operating losses and negative cash flows.
Since our inception in September 2012, we have incurred operating losses and negative cash flow, and we expect to continue to incur losses and negative cash flow in the future. Our net losses for the fiscal years ended December 31, 2016, 2015, and 2014 were $35.5 million, $15.0 million, and $10.1 million respectively, and we had an accumulated deficit of $83.7 million and $48.2 million as of December 31, 2016 and 2015, respectively. As of June 30, 2017, we had an accumulated deficit of $98.7 million. We have limited cash resources and will depend upon future equity financings, research grants, funding available through collaborations with third parties, and sales of BioTime and OncoCyte common shares that we have as a source of funding for our operations. There is no assurance that we will be able to obtain the financing we need from any of those sources, or that any such financing that may become available will be on terms that are favorable to us and our shareholders.
19
Our strategic business plan may not produce the intended growth in revenue and operating income.
Our strategies ultimately include making significant investments in our product development programs to achieve future revenues through licensing or commercialization activities. If we do not achieve the expected benefits from these investments or otherwise fail to execute on our strategic initiatives, we may not achieve the milestones we are targeting and our results of operations may be adversely affected. We may also fail to secure the capital necessary to make investments in our product development programs, which will hinder our growth.
In addition, from time to time, we may make acquisitions, license our products, and/or enter into strategic alliances such as joint ventures and joint development agreements. However, we may not be able to identify suitable partners, and our strategic alliances may not prove to be successful. Such transactions involve numerous risks and, although we will endeavor to evaluate the risks inherent in any particular transaction, there can be no assurance that we will properly ascertain and mitigate all such risks. There can be no assurance that difficulties encountered with such transactions will not have a material adverse effect on our business, financial condition and results of operations.
Failure to attract and retain skilled personnel and key relationships could impair our research and development efforts.
We will need to retain our existing workforce and to recruit and hire additional qualified research scientists, laboratory technicians, clinical development and management personnel as we continue to develop our programs. Competition for these types of personnel, especially in the San Francisco Bay area where our operations are located, is intense and we may experience delays in hiring the qualified people that we need. The inability to attract and retain sufficient qualified management, scientific, or technical personnel may significantly delay or prevent the achievement of our product development and other business objectives and could have a material adverse effect on our business, operating results and financial condition. We will rely on consultants and advisors who are either self-employed or employed by other organizations, and they may have conflicts of interest or other commitments, such as consulting or advisory contracts with other organizations, that may affect their ability to perform services for us.
We will spend a substantial amount of our capital on research and development but we might not succeed in developing products and technologies that are useful in medicine.
The product development work we plan to do is costly, time consuming and uncertain as to its results. We will attempt to develop new medical products and technologies that might not prove to be safe and efficacious in human medical applications. Many of the products and technologies that we will seek to develop have not been applied in human medicine and have only been used in laboratory studies in vitro or in animals. Only two of our three current programs have been tested in humans, and those were early stage trials involving only a small number of patients. Even if we are successful in developing a new technology or product, refinement of the new technology or product and definition of the practical applications and limitations of the technology or product may take years and require the expenditure of large sums of money.
The amount and pace of research and development work that we can do or sponsor, and our ability to commence and complete clinical trials required to obtain FDA and foreign regulatory approval of our products, depends upon the amount of funding available to us.
We may have to limit our laboratory research and development work, our process development work, and our clinical development of our product candidates, based on the amount of our cash resources. We plan to continue to seek research and development grants from government agencies, charitable organizations, and other sources and to attempt to enter into collaborative product development agreements through which third parties will provide funding or otherwise bear the cost of research and development or clinical trials of our product candidates. There is no assurance that the amount of any grants that we may receive will be adequate for our needs. The agreements we entered into to date with Cancer Research UK (CRUK) are subject to termination if certain milestones are not achieved, and any future agreement with granting organizations will likewise be subject to termination based on the failure to achieve milestones. Hence, there is no assurance that we will receive the full value of the agreement with either entity. Unless we are able to generate sufficient revenue or raise additional funds when needed, it is likely that we will be unable to continue our planned activities, even if we make progress in our research and development projects.
Our clinical trials on our product candidates are ongoing and our clinical trial results may not ultimately confirm initial positive indications, which would materially and adversely affect our business, financial condition and stock price.
Our efforts to commercialize AST-OPC1, AST-VAC1 and AST-VAC2 are dependent on obtaining FDA or other non-U.S. regulatory agency approval of its use in patients. Although test results have been positive thus far for some of our programs, the process of obtaining approval of a drug product for use in humans is extremely lengthy and time-consuming, and numerous factors may prevent our successful development of our product candidates, including negative results in future clinical trials, the development by competitors of other products with equal or better results, or inability to obtain sufficient additional funding to continue to pursue development. Failure to successfully develop our product candidates would have a material and adverse effect on our business, financial condition and stock price, and would threaten our ability to continue to operate our business.
20
Adverse events in our clinical trials may force us to stop development of our product candidates or prevent regulatory approval, if needed, of our product candidates.
The eventual testing of our product candidates in human clinical trials may produce serious adverse events. These adverse events could interrupt, delay or halt clinical trials of product candidates and could result in the FDA or other regulatory authorities denying approval of, or adding black box warnings or other limitations to, our product candidates for any or all targeted indications. An independent data safety monitoring board, the FDA, other regulatory authorities or we may suspend or terminate clinical trials at any time. We cannot assure that any of our product candidates will be safe for human use.
We will need to issue additional equity or debt securities in order to raise additional capital needed to pay our operating expenses.
We plan to incur substantial research and product development expenses, and we will need to raise additional capital to pay operating expenses until we are able to generate sufficient revenues from product sales, royalties, and license fees. Additional sales of equity or debt securities will be required in the future to meet our capital needs. Sales of additional equity securities will result in the dilution of the interests of present shareholders.
The availability of cells could impact the time and cost of commencing our research and product development programs.
The cells, cell lines and other biological materials that we acquired are being stored under cryopreservation protocols intended to preserve their functionality. We have successfully completed the verification of the viability of the lots of AST-OPC1 cells that we have been using in our current SCiStar Phase 1/2a study. However, we do not currently have sufficient amounts of AST-OPC1 cells to complete a larger randomized control trial or for future commercial activities. We are developing additional cell banks and modifying and scaling up our process to generate sufficient amounts of AST-OPC1 cells for use in a larger trial and any future commercial activities. These process development and manufacturing-related activities increase the costs of our product development for AST-OPC1 and any delays in these activities could delay the overall AST-OPC1 program.
The manufacturing of cells for our clinical programs is difficult and costly.
We intend to use our internal manufacturing facilities to produce AST-OPC1 cells for future studies and commercialization and we are currently relying on CRUK to manufacture AST-VAC2 for its upcoming Phase 1/2a study in the UK. We cannot give any assurance that we or any third-party manufacturers that we use will be able to develop the manufacturing capabilities necessary to supply adequate amounts of product to support our future clinical trials or commercialization. Moreover, we cannot give any assurance that we or the contract manufacturers or suppliers that we select will be able to supply our products in a timely or cost effective manner or in accordance with applicable regulatory requirements or our own specifications. The failure of us or any of our third-party manufactures or suppliers to comply with regulatory requirements could result in material manufacturing delays and product shortages, which could delay or otherwise negatively impact our clinical trials and product development plans.
Any products that receive regulatory approval may be difficult and expensive to manufacture on a commercial scale.
hES derived therapeutic cells have only been produced on a small scale and not in quantities and at levels of purity and viability that will be needed for larger registration trials or wide scale commercialization. If we are successful in advancing products that consist of hES cells or other cells or products derived from hES or other cells, we will need to develop, alone or in collaboration with one or more pharmaceutical companies or contract manufacturers, technology for the large-scale production of those products. Our hES cell or other cell-based products are likely to be more expensive to manufacture on a commercial scale than most other drugs on the market today. The high cost of manufacturing a product will require that we charge our customers a high price for the product in order to cover our costs and earn a profit. If the price of our products is too high, hospitals and physicians may be reluctant to purchase our products, and third-party payers such as insurance companies may be reluctant to reimburse for our products, especially if lower priced alternative products are available, and we may not be able to sell our products in sufficient volumes to recover our costs of development and manufacture or to earn a profit.
New products and technological advances by our competitors may negatively affect our results of operations.
Any products that we are able to develop may face future competition from third parties. Competitors’ products may be safer, more effective; more effectively marketed or sold, or have lower prices or superior performance features than our products. We cannot predict with certainty the timing or impact of the introduction of our competitors’ products.
We do not have the ability to independently conduct clinical trials required to obtain regulatory approvals for our therapeutic product candidates.
We will need to rely on third parties, such as CRUK, contract research organizations, data management companies, contract clinical research associates, medical institutions, clinical investigators and contract laboratories to conduct any clinical trials that we may undertake for our products. We may also rely on third parties to assist with our preclinical development of therapeutic product candidates. If we outsource clinical trials, we may be unable to directly control the timing, conduct and expense of our clinical trials. If we enlist third parties to conduct clinical trials and they fail to successfully carry out their contractual duties or regulatory obligations or fail to meet expected deadlines, if the third parties need to be replaced or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to clinical protocols or regulatory requirements or for other reasons, our preclinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for or successfully commercialize our therapeutic product candidates.
21
We will have certain obligations and may incur liabilities arising from clinical trials, and we do not yet know the scope of any resulting expenses that might arise.
We face the risk of incurring liabilities to patients who participate in clinical trials of our product candidates if they incur any injuries as a result of their participation. We will also be obligated to obtain information and prepare reports about the health of the clinical trial patients. In addition, we have assumed Geron’s obligations to obtain information and prepare reports about the health of patients, and we have assumed any liabilities to those patients that might arise from any injuries they may have incurred, as a result of their participation in the clinical trials of Geron’s GRNOPC1 cell replacement therapy for spinal cord damage and its GRNVAC1 immunological therapy for certain cancers. We are not aware of any claims by patients alleging injuries suffered as a result of any of those clinical trials, but if any claims are made and if liability can be established, the amount of any liability that we may incur, depending upon the nature and extent of any provable injuries, could exceed our insurance coverage, and the amount of the liability could be material to our financial condition.
We have no history of conducting large-scale, pivotal Phase 2 or 3 clinical trials or commercializing pharmaceutical products, which may make it difficult to evaluate the prospects for our future viability.
Our clinical trials thus far have been limited to conducting an ongoing Phase 1/2(a) clinical trial for AST-OPC1 and planning for a Phase 1/2(a) clinical trial for AST-VAC2. The Company has no prior experience in obtaining regulatory approval for a drug or commercializing an approved drug. Accordingly, we have not had experience completing a large-scale or pivotal clinical trial, obtaining marketing approval, manufacturing product on a large scale or conducting sales and marketing activities. Consequently, predictions about our future success or viability may not be as accurate as they could be if we had a history of successfully developing and commercializing pharmaceutical products.
Our business could be adversely affected if we lose the services of the key personnel upon whom we depend.
Our research and development programs are directed primarily by our Executive Vice President and Chief Operating Officer, Dr. Katharine E. Spink, our Chief Medical Officer, Dr. Edward D. Wirth, III and our Chief Scientific Officer, Dr. Jane S. Lebkowski. In addition, our success depends to a large extent on our President and CEO, Michael Mulroy, and our Chief Financial Officer and General Counsel, Ryan D. Chavez. If any of these key personnel should leave our employ we may be unable to locate and recruit sufficient replacement personnel without undue delay or additional cost or we may be unable to replace them at all. Any such delay or inability could delay or terminate some or all of our research programs, the commercialization of our products, or our ability to raise capital to fund our business. Even if we are able to attract suitable replacement personnel, we may incur delays during a transition period. Therefore, the loss of these key employees and others within our organization could have a material adverse effect on us.
Our business and operations could suffer in the event of system failures.
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Such events could cause interruption of our operations. For example, the loss of data for our product candidates could result in delays in our regulatory filings and development efforts and significantly increase our costs. To the extent that any disruption or security breach was to result in a loss of or damage to our data or inappropriate disclosure of confidential or proprietary information, we could incur liability and the development of our product candidates could be delayed.
We could be subject to breaches of our information technology systems, which could damage our reputation and customer relationships. Such breaches could subject us to significant reputational, financial, legal, and operational consequences.
Our business relies on information systems to obtain, process, analyze and manage data concerning our clinical trials. A cyber-attack that bypasses our security, or employee error, malfeasance or other disruptions that cause a security breach could lead to a material disruption of our information systems and/or the loss of business information. Such an attack could result in, among other things:
• the theft, destruction, loss, misappropriation or release of confidential data and intellectual property;
• operational or business delays;
• liability for a breach of personal information belonging to our customers or our employees; and
• damage to our reputation any of which could have a material adverse effect on our business, financial condition, and results of operations. In the event of an attack, we would be exposed to a risk of loss or litigation and possible liability, including under laws that protect the privacy of personal information.
Failure of our internal control over financial reporting could harm our business and financial results.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting for external purposes in accordance with accounting principles generally accepted in the U.S. Internal control over financial reporting includes maintaining records that in reasonable detail accurately and fairly reflect our transactions; providing reasonable assurance that transactions are recorded as necessary for preparation of our financial statements; providing reasonable assurance that receipts and expenditures of our assets are made in accordance with management authorization; and providing reasonable assurance that unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements would be prevented or detected on a timely basis. Because of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of our financial statements would be prevented or detected. Our growth and entry into new products, technologies and markets will place significant additional pressure on our system of internal control over financial reporting. Any failure to maintain an effective system of internal control over financial reporting could limit our ability to report our financial results accurately and timely or to detect and prevent fraud.
22
Power shortages, natural disasters, terrorist acts or other calamities could disrupt our production and have a material adverse effect on our business, financial position and results of operations.
Substantially all of our operations are carried out in our headquarters in Fremont, California. A significant disruption at that facility, even on a short-term basis, could impair our ability to carry out our business, which could have a material adverse effect on our business, financial position and results of operations. Historically, the state of California has been vulnerable to many natural or man-made disasters, including earthquake, fire, floods, environmental accidents, power loss, communications failures and similar events. In recent years, terrorist activity has been on the rise, we may be the target of, or be affected by, terrorist activity. If any such disaster were to occur, our ability to operate our business at our facilities would be seriously impaired. Unexpected business interruptions resulting from disasters could disrupt our operations and thereby result in substantial costs and diversion of resources.
Risks Related to Our Industry
Legislative actions and potential new accounting pronouncements may impact our future financial and results of operations
There have been regulatory enactments, including the Dodd–Frank Wall Street Reform and Consumer Protection Act, that have had an impact on our financial condition and results of operations. Other potential future regulatory enactments are likely to increase our general and administrative costs and expenses. In addition, there could be new accounting pronouncements that could materially increase the expenses we report under generally accepted accounting principles, and adversely affect our operating results and financial condition.
Risks Related to Our Relationship With BioTime
BioTime has a significant influence on our business operations.
As of June 30, 2017, BioTime owns approximately 44% of our issued and outstanding common stock. Because BioTime is by far our largest shareholder and owns close to a majority of the outstanding common stock, it has the voting power to significantly impact any matter that requires shareholder approval. Furthermore, four of the nine members of our Board of Directors are also directors of BioTime, and another director is an employee of Broadwood Capital, Inc., which is the general partner of Broadwood Partners, L.P., the partnership that is the largest shareholder of BioTime. Some of our directors also serve on the Boards of Directors of one or more of BioTime’s other subsidiaries. As a result of the relationships described above, BioTime has significant influence over our business operations, and therefore, BioTime could cause corporate actions to be taken even if the interests of BioTime conflict with the interests of our other shareholders. This concentration of voting power could have the effect of deterring or preventing a change in control that might be beneficial to our other shareholders.
If BioTime should elect to sell or distribute all or a substantial part of its ownership in Asterias, it could, depending on the manner in which these shares are distributed or sold, have a depressing impact on the price of our common stock and impact our ability to raise capital to fund our operations or attract new investors.
Risks Related to Our Dependence on Third Parties
We may not be able to obtain additional non-dilutive funding to advance our programs.
We are hopeful that we will be able to obtain an additional CIRM grant or another source of funding as a source of financing the costs of conducting a subsequent trial of AST-OPC1. If we fail to obtain an additional grant from CIRM or another significant source of funding, it may force us to postpone the commencement of any subsequent trial.
Risks Pertaining to Our Common Stock
Ownership of our common stock will entail certain risks associated with the volatility of prices for our shares and the fact that we do not pay dividends on our common stock.
The price of our common stock may rise and fall rapidly.
The market price of our common stock like that of the shares of many biotechnology companies is highly volatile. The price of our common stock may rise or fall rapidly as a result of a number of factors, including:
• sales or potential sales of substantial amounts of our common stock, including from our largest shareholder Biotime;
• results of preclinical testing or clinical trials of our product candidates or those of our competitors;
• announcements about us or about our competitors, including clinical trial results, regulatory approvals, new product introductions and commercial results;
• the cost of our development programs;
23
• the success of competitive products or technologies;
• litigation and other developments relating to our issued patents or patent applications or other proprietary rights or those of our competitors;
• conditions in the pharmaceutical or biotechnology industries;
• actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts;
• variations in our financial results or those of companies that are perceived to be similar to us, including the failure of our earnings to meet analysts’ expectations; and
• general economic, industry and market conditions.
Many of these factors are beyond our control. The stock markets in general, and the market for pharmaceutical and biotechnological companies in particular, have been experiencing extreme price and volume fluctuations which have affected the market price of the equity securities without regard to the operating performance of the issuing companies. Broad market fluctuations, as well as industry factors and general economic and political conditions, may adversely affect the market price of our common stock.
The price of our common stock, and the value of our assets, will be affected by changes in the value of the BioTime common shares that we own.
As of June 30, 2017, we held 3,852,880 BioTime common shares. The value of our common stock will reflect, in part, the value of the BioTime common shares that we hold. The value of the BioTime common shares we hold will vary with the price at which BioTime common shares trade in the public market. The market price of BioTime common shares will be impacted by a number of factors, including the results of BioTime’s operations.
| Item 2. |
Unregistered Sales of Equity Securities and Use of Proceeds
|
None.
| Item 3. |
Default Upon Senior Securities
|
None.
| Item 4. |
Mine Safety Disclosures
|
Not applicable.
| Item 5. |
Other Information
|
None.
| Item 6. |
Exhibits
|
|
Exhibit Number
|
Description
|
|
|
*
|
Certification of Chief Executive Officer pursuant to Exchange Act Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
|
*
|
Certification of Chief Financial Officer pursuant to Exchange Act Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
|
**
|
Certifications of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
|
|
101
|
*
|
XBRL Instance Document
|
|
101.INS
|
*
|
XBRL Taxonomy Extension Schema
|
|
101.CAL
|
*
|
XBRL Taxonomy Extension Calculation Linkbase
|
|
101.DEF
|
*
|
XBRL Taxonomy Extension Definition Linkbase
|
|
101.LAB
|
*
|
XBRL Taxonomy Extension Label Linkbase
|
|
101.PRE
|
*
|
XBRL Taxonomy Extension Presentation Linkbase
|
|
*
|
Filed herewith.
|
|
**
|
Furnished herewith.
|
24
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
ASTERIAS BIOTHERAPEUTICS, INC.
|
||
|
Date: August 14, 2017
|
/s/ Michael H. Mulroy
|
|
|
Michael H. Mulroy
|
||
|
President and Chief Executive Officer
|
||
|
Date: August 14, 2017
|
/s/ Ryan Chavez
|
|
|
Ryan Chavez
|
||
|
Chief Financial Officer
|
||
25
