Attached files
| file | filename |
|---|---|
| 8-K - 8-K - LANNETT CO INC | a15-2186_18k.htm |
Exhibit 99.1
|
|
Arthur Bedrosian, CEO Marty Galvan, CFO January 2015 |
|
|
2 Forward-Looking Statements Except for historical facts, the statements in this presentation, as well as oral statements or other written statements made or to be made by Lannett Company, Inc. (the “Company”), are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and involve risks and uncertainties. For example, statements about the expected positive FDA inspection results of the Company’s manufacturing facilities and product approvals, anticipated growth and future operations, the current or expected market size for its products, the success of current or future product offerings, continued relationships with the Company’s suppliers and customers, the research and development efforts, the Company’s ability to file for and obtain U.S. Food and Drug Administration (FDA) approvals for future products, and the Company’s ability to obtain and maintain necessary licenses and permits, are forward-looking statements. Forward-looking statements are merely the Company’s current prediction of future events. The statements are inherently uncertain and actual results could differ materially from the statements made herein. There is no assurance that the Company will achieve the sales levels that will keep its operations profitable or that FDA filings and approvals will be completed and obtained as anticipated. For a description of additional risks and uncertainties, please refer to the Company’s filings with the Securities and Exchange Commission, including its latest Annual Report on Form 10–K and its latest Quarterly Reports on Form 10-Q. The Company assumes no obligation to update its forward-looking statements to reflect new information and developments. |
|
|
3 U.S. Generic Pharmaceutical Industry Strong drivers for continued growth Cost: Often 80-85% less than the brand Supply: $86 billion of brand drugs are coming off patent through 2017 Demand: Aging baby boomers will continue to fuel market growth Account for over 83% of prescriptions* Same active ingredient, dosage form and route of administration as brand Similar safety, efficacy and quality as brand, at a lower price * Per IMS Sept. 2014 |
|
|
4 U.S. Generic Pharmaceutical Industry (con’t) Product approval process: Abbreviated New Drug Application (ANDA) Generally no preclinical/clinical data required; only need to demonstrate bioequivalence Less development time, money and risk compared to New Drug applications (NDA) for brands Distribution Model Brand products require marketing budgets and investment in sales reps to call directly on physicians / hospitals -- new products need detailing Generics are sold through 3rd party channels including: Wholesaler Distributors (McKesson, Cardinal, AmerisourceBergen) Chain Drug Stores (Walgreens, CVS, RiteAid) Mail-order Pharmacies (Express Scripts/Medco, OPTUMRx, Caremark) |
|
|
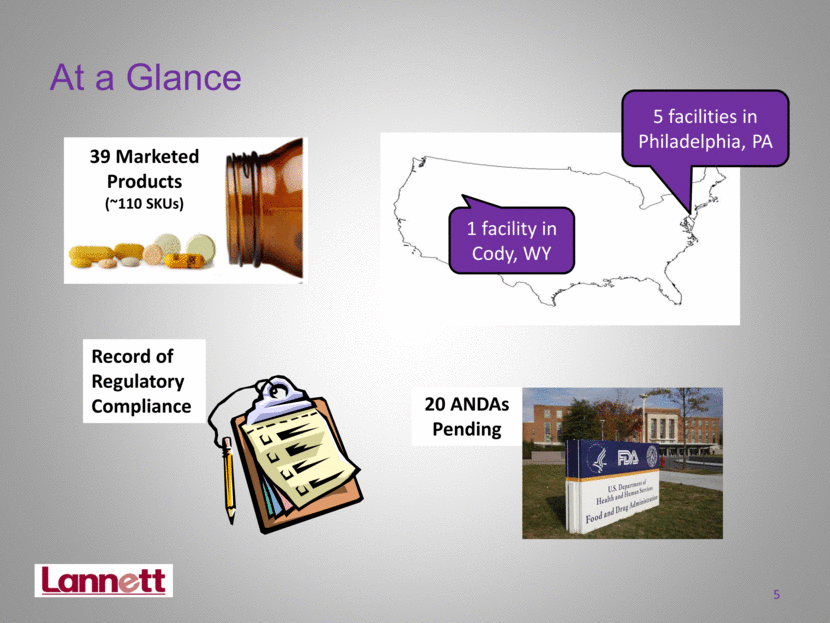
5 At a Glance 39 Marketed Products (~110 SKUs) 20 ANDAs Pending Record of Regulatory Compliance 1 facility in Cody, WY 5 facilities in Philadelphia, PA |
|
|
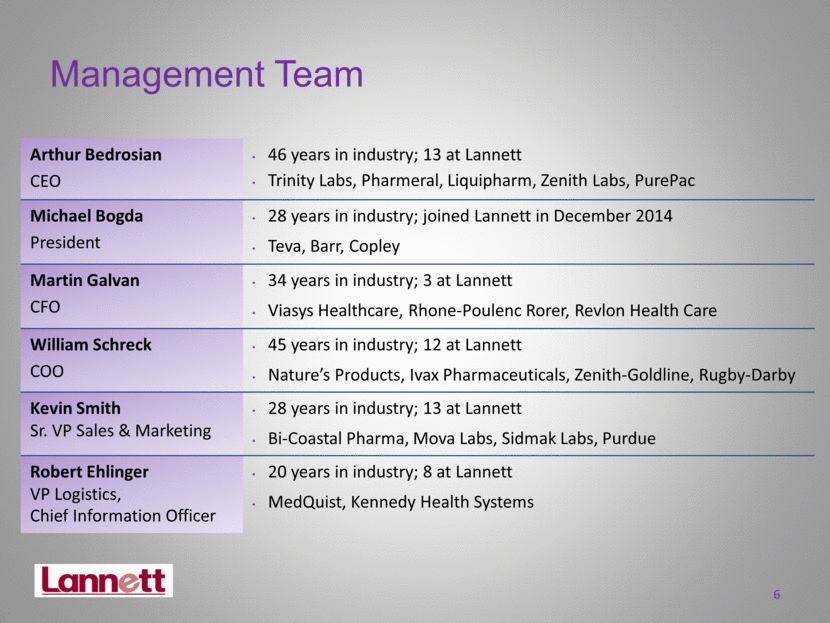
6 Management Team Arthur Bedrosian CEO 46 years in industry; 13 at Lannett Trinity Labs, Pharmeral, Liquipharm, Zenith Labs, PurePac Michael Bogda President 28 years in industry; joined Lannett in December 2014 Teva, Barr, Copley Martin Galvan CFO 34 years in industry; 3 at Lannett Viasys Healthcare, Rhone-Poulenc Rorer, Revlon Health Care William Schreck COO 45 years in industry; 12 at Lannett Nature’s Products, Ivax Pharmaceuticals, Zenith-Goldline, Rugby-Darby Kevin Smith Sr. VP Sales & Marketing 28 years in industry; 13 at Lannett Bi-Coastal Pharma, Mova Labs, Sidmak Labs, Purdue Robert Ehlinger VP Logistics, Chief Information Officer 20 years in industry; 8 at Lannett MedQuist, Kennedy Health Systems |
|
|
Our Strengths Talented management across all functional areas: product development, regulatory, manufacturing, compliance, sales, finance Strong customer relationships fostered over many years - business is personal Track record of: Selecting products with high profit potential and manageable competition Getting products approved and maintaining regulatory compliance Stability plus growth: Base generics business represents a solid financial foundation Controlled substances represent area for higher profit margins and growth rates One of seven DEA-licensed importers of concentrated poppy straw (CPS) 7 |
|
|
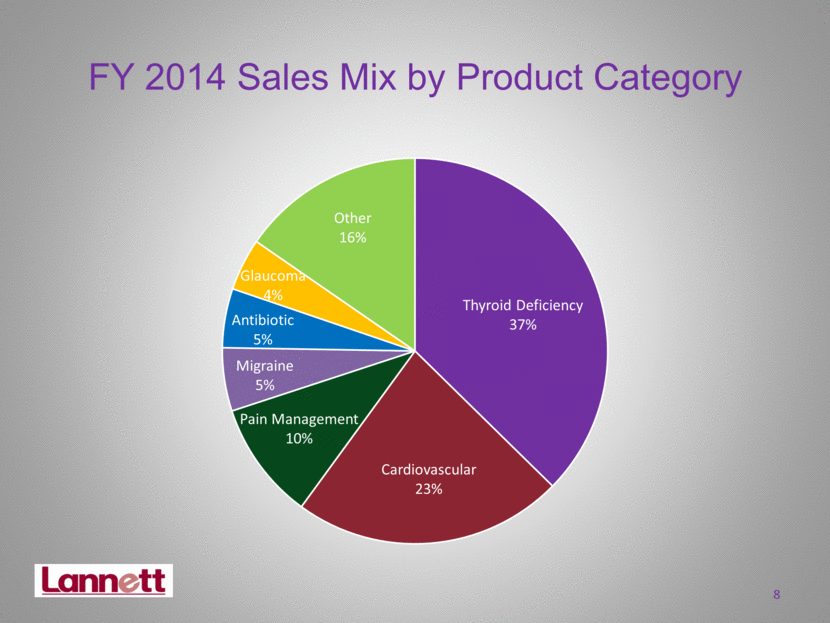
FY 2014 Sales Mix by Product Category 8 Thyroid Deficiency 37% Cardiovascular 23% Pain Management 10% Migraine 5% Antibiotic 5% Glaucoma 4% Other 16% |
|
|
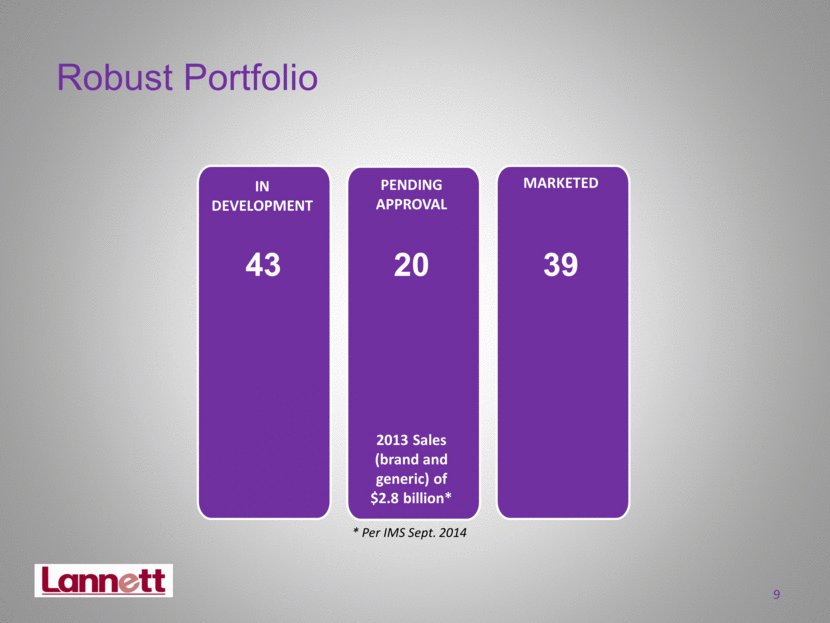
9 Robust Portfolio IN DEVELOPMENT MARKETED PENDING APPROVAL 39 43 2013 Sales (brand and generic) of $2.8 billion* * Per IMS Sept. 2014 20 |
|
|
Controlled Substance Market and Goal US market: $26 billion in 2013* Generic portion: $9.7 billion in 2013* High barrier to entry: DEA-required licenses and quotas 1 of 7 companies with DEA import license for CPS CPS = natural raw material from which APIs are manufactured Profitability and growth: Current gross margin on controlled substances: 60% 5-year goal: at least 50% of manufactured products to be controlled substances 10 * Per IMS Sept. 2014 |
|
|
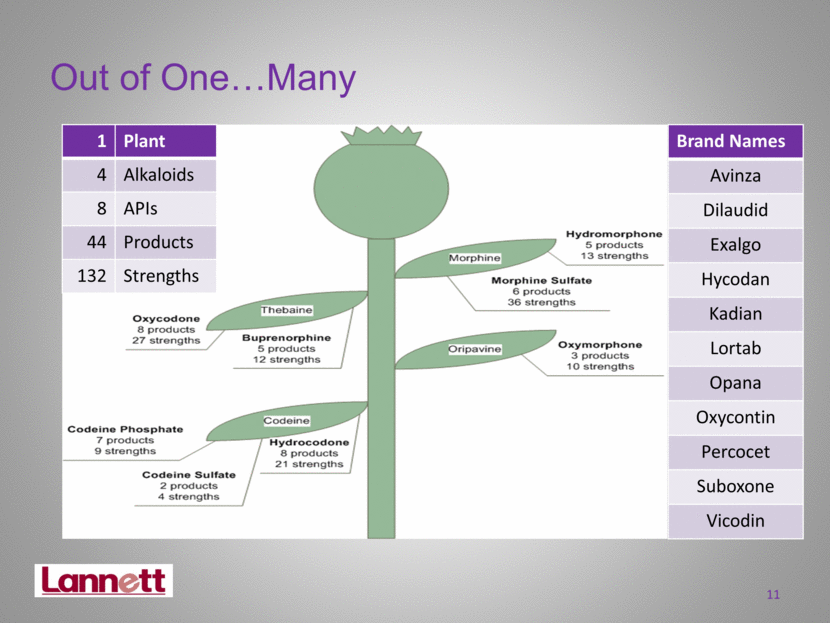
1 Plant 4 Alkaloids 8 APIs 44 Products 132 Strengths Brand Names Avinza Dilaudid Exalgo Hycodan Kadian Lortab Opana Oxycontin Percocet Suboxone Vicodin Out of One Many 11 |
|
|
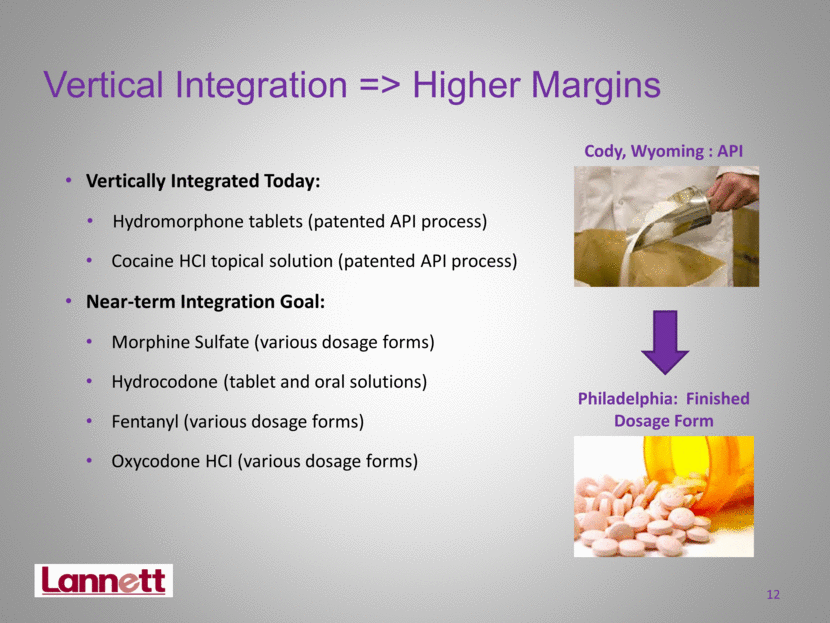
Vertical Integration => Higher Margins Vertically Integrated Today: Hydromorphone tablets (patented API process) Cocaine HCI topical solution (patented API process) Near-term Integration Goal: Morphine Sulfate (various dosage forms) Hydrocodone (tablet and oral solutions) Fentanyl (various dosage forms) Oxycodone HCI (various dosage forms) 12 Philadelphia: Finished Dosage Form Cody, Wyoming : API |
|
|
Growth Strategy Base generic products: Commercialize products upon FDA approval Acquire ANDAs and products that meet our expectations for sales potential, barriers to entry, limited competition and gross margin Expand product development partnerships to enhance internal efforts Monitor market for opportunities to increase prices File Paragraph IV challenges for products which meet target metric thresholds Controlled substance products: Become a dominant player and one-stop shop in the U.S. Grow percent of manufactured products to at least 50% by 2018 Continue to invest in development of higher margin products Develop novel (proprietary) forms of API delivery 13 M A |
|
|
Brand product: C-Topical® Cocaine HCl Requirements for commercialization: Clinical testing for FDA approval Marketing and dedicated sales force to call on surgeons Product Advantages: Increased number of procedures per day due to faster therapeutic onset Ease of use - one product versus current therapy of combining two products Potential to provide: Higher profit margins New Chemical Entity (NCE) designation = Market Exclusivity 14 Growth Strategy (cont’d) |
|
|
15 C-Topical® Sales Territories |
|
|
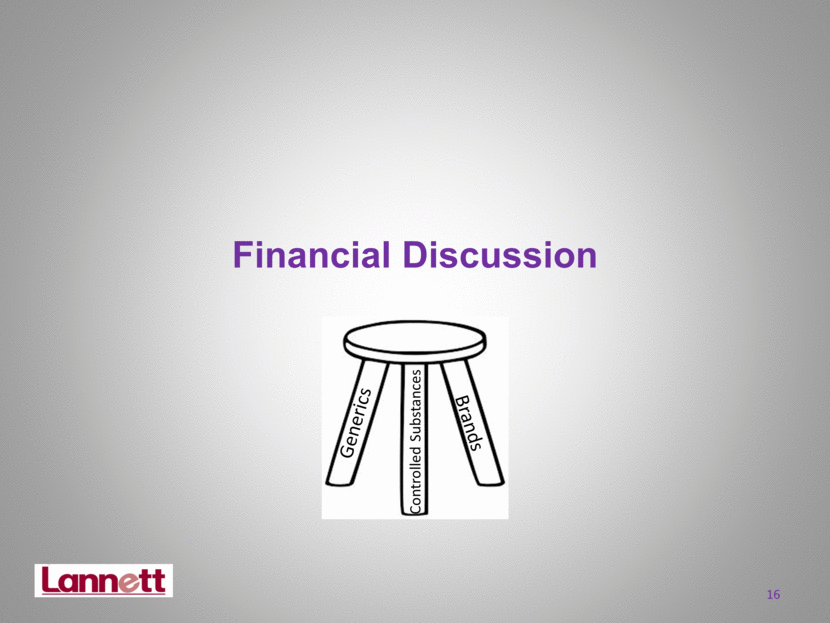
Financial Discussion 16 Generics Controlled Substances Brands |
|
|
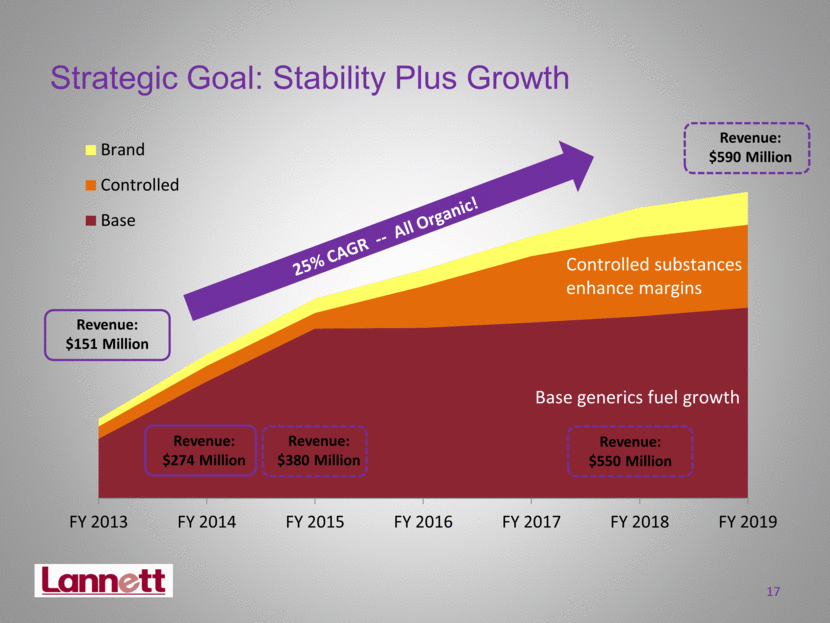
Strategic Goal: Stability Plus Growth 17 25% CAGR -- All Organic! Revenue: $151 Million Revenue: $380 Million Revenue: $590 Million Revenue: $550 Million FY 2013 FY 2014 FY 2015 FY 2016 FY 2017 FY 2018 FY 2019 Brand Controlled Base Base generics fuel growth Controlled substances enhance margins Revenue: $274 Million |
|
|
Achievements: Fiscal 2014 – Present Net sales increased 81% for FY 2014 Q4-FY14 was seventh consecutive quarter of record net sales Increased market share on Levothyroxine: the #2 most prescribed molecule in U.S.* Thyroid Deficiency and Cardiovascular both almost doubled Migraine nearly tripled from FY 2013 Gross margin up 26 percentage points in FY 2014, excluding $20.1 M non-recurring charge for JSP contract renewal, due to price increases and manufacturing efficiencies Adjusted EPS up more than four-fold to $1.98 in FY 2014, vs. $0.46 in FY 2013 Significantly increased investment in product development, expanded applications pending at FDA from 15 to 20 Received five product approvals, completed two product acquisitions and formed strategic relationships Advanced branded generics business 18 * Per IMS Sept. 2014 |
|
|
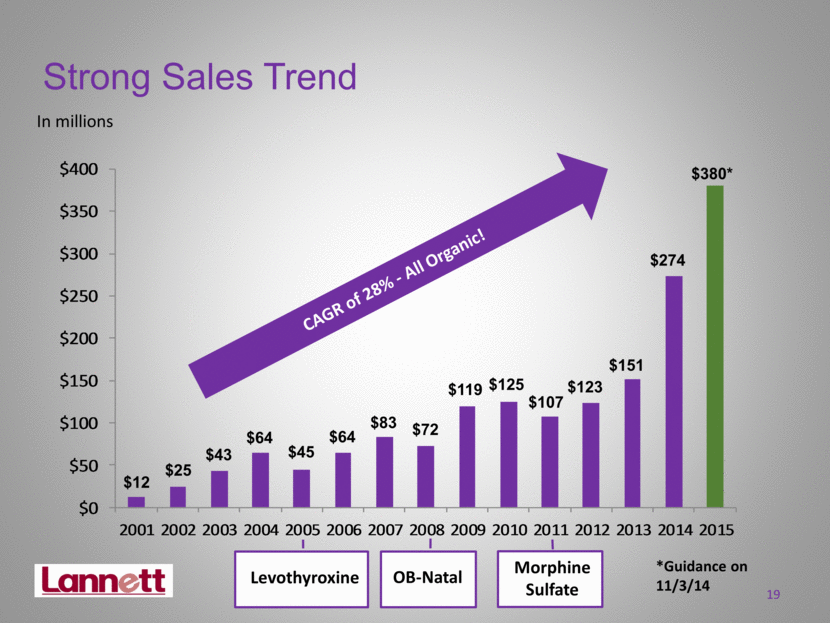
Strong Sales Trend In millions $12 $25 $43 $64 $45 $64 $83 $72 $119 $125 $107 CAGR of 28% - All Organic! $151 $274 19 Levothyroxine OB-Natal Morphine Sulfate $380* $123 *Guidance on 11/3/14 $0 $50 $100 $150 $200 $250 $300 $350 $400 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 |
|
|
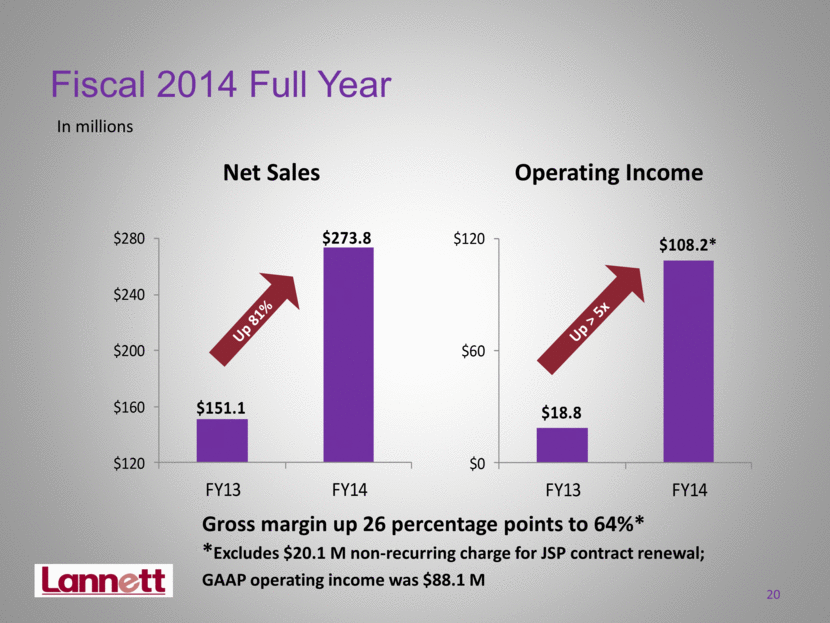
Fiscal 2014 Full Year In millions Operating Income Net Sales Gross margin up 26 percentage points to 64%* *Excludes $20.1 M non-recurring charge for JSP contract renewal; GAAP operating income was $88.1 M 20 Up 81% Up > 5x $18.8 $108.2* $0 $60 $120 FY13 FY14 $151.1 $273.8 $120 $160 $200 $240 $280 FY13 FY14 |
|
|
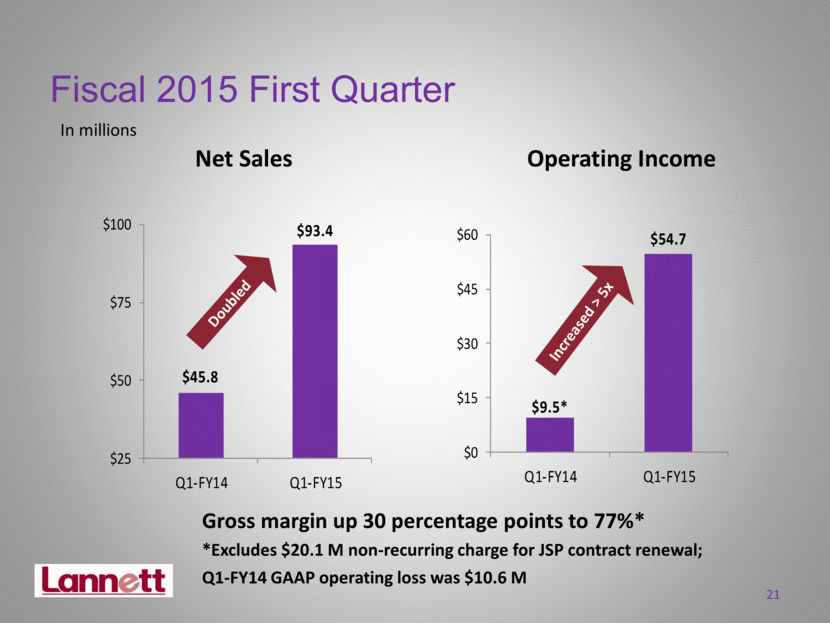
Operating Income Net Sales In millions Fiscal 2015 First Quarter 21 Doubled Increased > 5x Gross margin up 30 percentage points to 77%* *Excludes $20.1 M non-recurring charge for JSP contract renewal; Q1-FY14 GAAP operating loss was $10.6 M $45.8 $93.4 $25 $50 $75 $100 Q1-FY14 Q1-FY15 $9.5* $54.7 $0 $15 $30 $45 $60 Q1-FY14 Q1-FY15 |
|
|
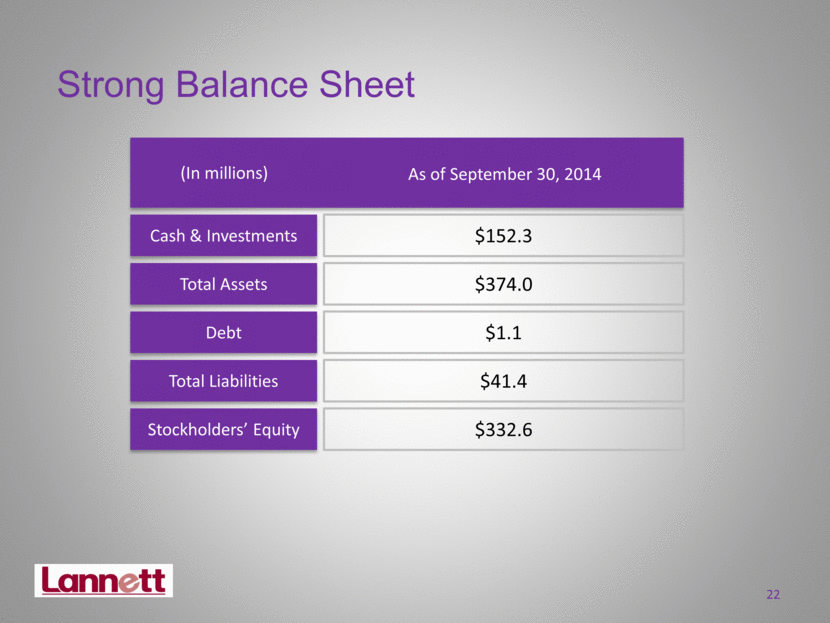
Strong Balance Sheet Cash & Investments Debt Stockholders’ Equity Total Assets Total Liabilities (In millions) As of September 30, 2014 $152.3 $374.0 $1.1 $41.4 $332.6 22 |
|
|
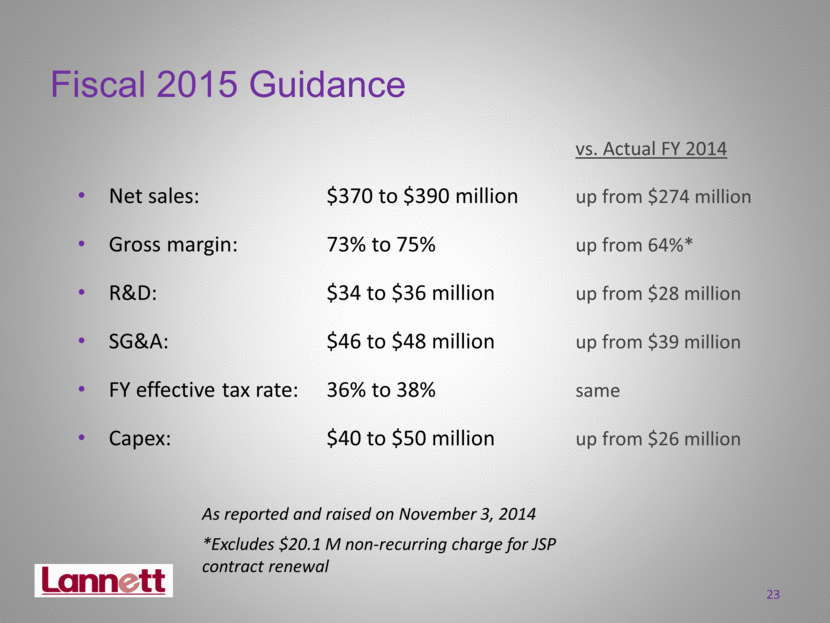
Fiscal 2015 Guidance vs. Actual FY 2014 Net sales: $370 to $390 million up from $274 million Gross margin: 73% to 75% up from 64%* R&D: $34 to $36 million up from $28 million SG&A: $46 to $48 million up from $39 million FY effective tax rate: 36% to 38% same Capex: $40 to $50 million up from $26 million As reported and raised on November 3, 2014 23 *Excludes $20.1 M non-recurring charge for JSP contract renewal |
|
|
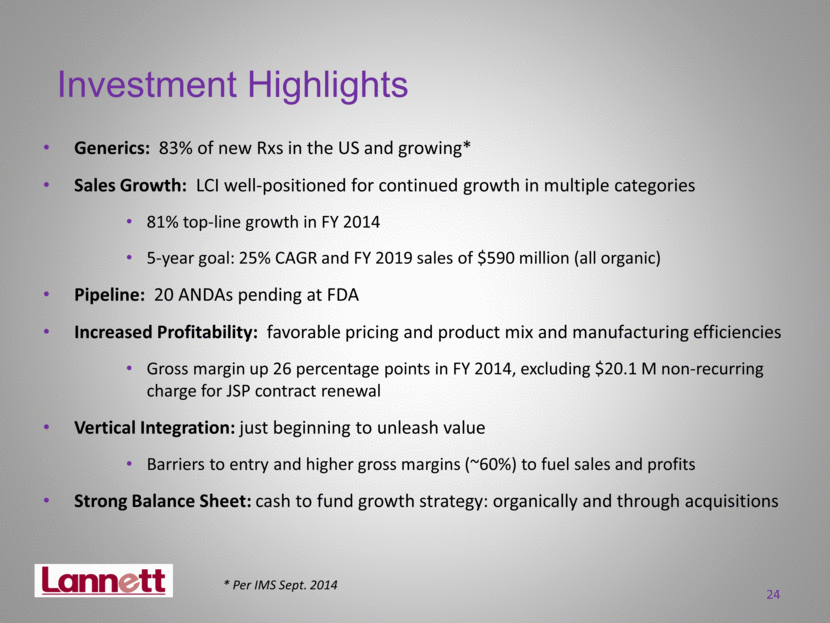
24 Investment Highlights Generics: 83% of new Rxs in the US and growing* Sales Growth: LCI well-positioned for continued growth in multiple categories 81% top-line growth in FY 2014 5-year goal: 25% CAGR and FY 2019 sales of $590 million (all organic) Pipeline: 20 ANDAs pending at FDA Increased Profitability: favorable pricing and product mix and manufacturing efficiencies Gross margin up 26 percentage points in FY 2014, excluding $20.1 M non-recurring charge for JSP contract renewal Vertical Integration: just beginning to unleash value Barriers to entry and higher gross margins (~60%) to fuel sales and profits Strong Balance Sheet: cash to fund growth strategy: organically and through acquisitions * Per IMS Sept. 2014 |
|
|
Thank You It has been a pleasure. NYSE: LCI www.Lannett.com 25 |