UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): September 16, 2014
ARATANA THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-35952 | 38-3826477 | ||
| (State or other jurisdiction of incorporation or organization) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
1901 Olathe Blvd., Kansas City, KS 66103
(Address of principal executive offices) (Zip Code)
(913) 353-1000
(Registrant’s telephone number, include area code)
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
| Item 8.01. | Other Events. |
Aratana Therapeutics, Inc. (the “Company” or “we”) recently updated its business information as follows:
For 2014, it is estimated that the annual United States pet market will be approximately $59 billion. Cats and dogs are the most popular pet species in the United States and Europe: there are approximately 96 million cats and 83 million dogs in the United States and 90 million cats and 75 million dogs in Europe. An estimated 68% of U.S. households have at least one pet. The U.S. veterinary care segment was $13.6 billion in 2012. We estimate that of this $13.6 billion, approximately $6.3 billion related to consumer spending in pet medicines, which included approximately $1.6 billion for pet therapeutics. The $1.6 billion U.S. pet therapeutics market represents less than $10 per year per pet. We believe that the pet market, driven in part by the expansion of the veterinary care segment, will continue to grow and that the introduction of novel pet therapeutics offering significant safety and efficacy benefits over existing products will result in pet therapeutics garnering a larger share of total consumer spending on pets. In addition, development of pet therapeutics is generally faster and less expensive than for human therapeutics because it requires fewer clinical studies, involves fewer subjects and is conducted directly in the target species. Tufts Center for the Study of Drug Development estimates that the cost of developing a new human drug is approximately $1.3 billion and takes about ten years to move from the lab to patients. In contrast, based on our internal evaluation of the development and regulatory approval process, we estimate that developing a pet therapeutic for U.S. Food and Drug Administration (the “FDA”) approval costs approximately $10 million and takes about five years to accomplish. Review and approval of biologics to a conditional and full license is done by the USDA with similar cost and time requirements.
In the United States there are approximately 300,000 dogs diagnosed annually with lymphoma, of which approximately 76%, or 228,000, is B-cell lymphoma, and 24%, or 72,000, is T-cell lymphoma. We estimate the average treatment costs to be approximately $5,000 per patient. Based on this information, we believe that the oncology market for pets is approximately $1 billion.
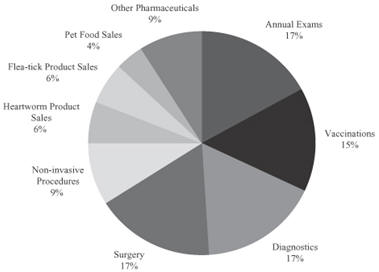
Given our estimates that on average pet owners are spending $10 per year on pet therapeutics, we believe that if safe and effective pet therapeutics products are available, veterinarians will prescribe them and pet owners will buy them. Based on our review of industry sources, the following chart depicts the average percentage of practice revenue that we believe veterinarians received for various services and medicines in 2012.
We expect to use approximately $35 million to $40 million in cash in 2014 to fund our operations.
AT-001
In May 2014, the Company commenced a pivotal field effectiveness study in dogs to confirm the safety and effectiveness of AT-001 (grapiprant) for the control of pain and inflammation associated with osteoarthritis. The Company plans to enroll approximately 300 client-owned dogs with osteoarthritis, which receive a once-daily dose of AT-001 or placebo. The Company expects top line data from this study in the fourth quarter of 2014, and expects to complete the FDA effectiveness review and receive the related technical section complete letter in the second quarter of 2015. In addition, the Company expects to complete the FDA chemistry, manufacturing and controls (“CMC”) review and receive the related technical section complete letter in the first quarter of 2016 and complete the FDA safety review and receive the related technical section complete letter in the first quarter of 2015, with FDA approval of AT-001 for dogs in 2016.
In addition, the Company is pursuing FDA approval for AT-001 for the control of pain and inflammation associated with degenerative joint disease in cats. The Company expects to complete the FDA CMC review and receive the related technical section complete letter in the second half of 2017, commence a pivotal field effectiveness study in the second half of 2015 with top line results expected in the first half of 2017, complete the FDA safety review and receive the related technical section complete letter in the first half of 2017 and complete the effectiveness review and receive the related technical section complete letter in the second half of 2017, with FDA approval of AT-001 for cats in 2018.
The Company is also pursuing approval in Europe and expects to submit a dossier to the European Medicines Agency (“EMA”) for use of AT-001 in dogs in 2016 with approval in 2017.
AT-002
The Company evaluated the effectiveness of AT-002 (capromorelin) compared to placebo for the treatment of inappetence in a pilot placebo-controlled, blinded, multi-veterinary clinic field study in client-dogs (17 treated, 12 placebo). The mean percentage change of the appetite score on day 6 for the dogs treated with AT-002 was 79% compared to 22% for the dogs given the placebo. The mean percentage change in body weight for the dogs treated with AT-002 was 3.2% compared to -0.5% for the dogs given the placebo.
In December 2013, the Company commenced a pivotal field effectiveness study for AT-002 for inappetance in dogs. The Company plans to enroll approximately 200 client-owned dogs, which receive a once-daily dose of AT-002 or placebo. The Company expects top line results from this study in the first quarter of 2015, to complete the effectiveness review and receive the related technical section complete letter in the second half of 2015, to complete the FDA safety review and receive the related technical section complete letter in the first quarter of 2015 and to complete the FDA CMC review and receive the related technical section complete letter in the second half of 2015, with FDA approval of AT-002 for dogs in 2016.
In addition, the Company is pursuing FDA approval for AT-002 for weight gain in cats. The Company expects to complete the FDA CMC and safety reviews and receive the related technical section complete letters by the second half of 2016 and expects to obtain FDA approval of AT-002 for cats by 2017. The Company also expects to pursue approval in Europe and to submit a dossier to the EMA for use of AT-002 in cats in 2017 with approval in 2018.
AT-003
On September 9, 2014, the Company announced the results from its pilot field study of AT-003 (bupivacaine) for managing post-operative pain in dogs following surgery. In the study, pain levels of 46 client-owned dogs were assessed repeatedly for 72 hours following knee surgery using the Glasgow Composite Measure Pain Scale. Treatment with AT-003 resulted in a statistically significant reduction in mean total pain scores over time compared to placebo (p<0.05). In addition, the percent of dogs treated with AT-003 that did not require additional pain medication was statistically greater (p<0.05) compared to placebo at each 24-hour interval for up to 72 hours after surgery. The Company anticipates discussing the design of a pivotal field effectiveness study with the Center for Veterinary Medicine and commencing a pivotal field effectiveness study in the fourth quarter of 2014 with top line data released in the second quarter of 2015. The Company expects to complete the FDA CMC review and receive the related technical section complete letter by the second half of 2015, complete the safety review and receive the related technical section complete letter by the first quarter of 2015 and complete the effectiveness review and receive the related technical section complete letter by the first half of 2016, and to obtain FDA approval of AT-003 in dogs in 2016.
In addition, the Company is pursuing FDA approval for AT-003 for managing post-operative pain in cats. The Company expects to complete the FDA CMC review by the second half of 2015 and the safety review by the first half of 2016 and expects to obtain FDA approval of AT-003 for cats by 2017.
The Company is also pursuing approval in Europe and expects to submit a dossier to the EMA for use of AT-003 for dogs in 2016 with approval in 2017 and for cats in 2017 with approval in 2018.
AT-004
The Company expects to receive a full United States Department of Agriculture (the “USDA”) license for AT-004 in the fourth quarter of 2014.
AT-005
The Company submitted a data package for AT-005 and expects to receive a full USDA license for AT-005 in the second half of 2015 with a full commercial launch in 2016. In May 2014, the Company commenced a study to evaluate AT-005 in combination with two different chemotherapy regimens used to treat dogs diagnosed with intermediate- to high-grade peripheral T-cell lymphoma. In addition, in the fourth quarter of 2014, the Company expects to make the product commercially available to study sites as well as other selected sites. The Company expects AT-005 to be used in approximately two dozen hospitals by the end of the third quarter of 2014, approximately three dozen by the end of the fourth quarter of 2014, approximately 50 hospitals by the end of the second quarter of 2015 and approximately 75 hospitals by the end of the fourth quarter of 2015.
AT-014
In March 2014, the Company licensed AT-014, a novel her2/neu-directed cancer immunotherapy for the treatment of canine osteosarcoma and other cancers, from Advaxis, Inc. The company received data from a clinical study in 30 client-owned dogs with osteosarcoma (17 treated, 13 placebo). The median survival time of the control group was less than 316 days. The median survival time of the treatment group had not been reached with 80% of dogs (15 dogs) alive (p<0.001). In July 2014, the Company submitted for a product license with the USDA. The Company expects to receive a conditional USDA license in the second half of 2015 and a full USDA license in 2017.
We believe that approximately 8,000 to 20,000 dogs in the United States are affected by osteosarcoma annually. The current standard of care is amputation and post-operative chemotherapy. We believe that the median survival time for dogs affected by osteosarcoma is approximately nine to twelve months, with only approximately 25% of dogs surviving two years.
AT-016
In June 2014, the Company entered into an exclusive license agreement with Vet-Stem, Inc. (“Vet-Stem”) for the rights to commercialize Vet-Stem’s novel, allogeneic stem cell therapy technology (“AT-016”). AT-016 may provide long-term relief of pain and disability caused by osteoarthritis through regeneration of joint damage. AT-016 could provide immediately available point-of-care and does not require daily administration. If approved, the Company believes that AT-016 would be the first FDA-regulated “off the shelf” regenerative cell therapy for the treatment of osteoarthritis in dogs.
Forward-Looking Statements
Some of the information contained in this report, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. In this report, the words “anticipates,” “believes,” “expects,” “intends,” “future,” “could,” “estimates,” “plans,” “would,” “should,” “potential,” “continues” and similar words or expressions (as well as other words or expressions referencing future events, conditions or circumstances) identify forward-looking statements. These forward-looking statements involve risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-
looking statements, including, but not limited to: our history of operating losses and our expectation that we will continue to incur losses for the foreseeable future; failure to obtain sufficient capital to fund our operations; market conditions and our ability to raise capital under the shelf registration statement from the sale of our securities; our substantial dependence upon the success of our product candidates; development of our biologic product candidates is dependent upon relatively novel technologies and uncertain regulatory pathways, and biologics may not be commercially viable; denial or delay of regulatory approval for our existing or future product candidates; failure of our product candidates that receive regulatory approval to obtain market approval or achieve commercial success; failure to realize anticipated benefits of our acquisitions and difficulties associated with integrating the acquired businesses; development of pet therapeutics is a lengthy and expensive process with an uncertain outcome; competition in the pet therapeutics market, including from generic alternatives to our product candidates, and failure to compete effectively; failure to identify, license or acquire, develop and commercialize additional product candidates; failure to attract and retain senior management and key scientific personnel; our reliance on third-party manufacturers, suppliers and partners; regulatory restrictions on the marketing of our product candidates; our lack of an internal sales organization, and any failure to create a sales force or partner with third-parties to commercialize our product candidates; difficulties in managing the growth of our company; significant costs of being a public company; our current exemption from the requirement to maintain internal control over financial reporting, and any failure to achieve and maintain effective internal control over financial reporting in the future; changes in distribution channels for pet therapeutics; consolidation of our veterinarian customers; limitations on our ability to use our net operating loss carryforwards; safety or efficacy concerns with respect to our product candidates; failure to obtain ownership of issued patents covering our product candidates or failure to prosecute or enforce licensed patents; failure to comply with our obligations under our license agreements; risks associated with our intellectual property rights; effects of patent or other intellectual property lawsuits; failure to protect our intellectual property; changing patent laws and regulations; non-compliance with any legal or regulatory requirements; the uncertainty of the regulatory approval process and the costs associated with government regulation of our product candidates; failure to obtain regulatory approvals in foreign jurisdictions; effects of legislative or regulatory reform with respect to pet therapeutics; the volatility of the price of our common stock; our status as an emerging growth company, which could make our common stock less attractive to investors; dilution of our common stock as a result of future financings; the influence of certain significant shareholders over our business; the eligibility of a significant portion of our total outstanding shares to be sold into the market in the near future, which could cause the market price of our common stock to drop significantly; and provisions in our charter documents and under Delaware law could delay or prevent a change in control. These and other important factors discussed under the caption “Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended December 31, 2013, filed with the Securities and Exchange Commission (the “SEC”) on March 26, 2014, along with our other reports filed with the SEC, could cause actual results to differ materially from those indicated by the forward-looking statements made in this report.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| ARATANA THERAPEUTICS, INC. | ||||
| Date: September 16, 2014 |
By: | /s/ Steven St. Peter | ||
|
| ||||
| Name: Steven St. Peter, M.D. | ||||
| Title: President and Chief Executive Officer | ||||
