Attached files
| file | filename |
|---|---|
| EX-32.2 - EX-32.2 - ARATANA THERAPEUTICS, INC. | petx-20160630xex32_2.htm |
| EX-32.1 - EX-32.1 - ARATANA THERAPEUTICS, INC. | petx-20160630xex32_1.htm |
| EX-31.2 - EX-31.2 - ARATANA THERAPEUTICS, INC. | petx-20160630xex31_2.htm |
| EX-31.1 - EX-31.1 - ARATANA THERAPEUTICS, INC. | petx-20160630xex31_1.htm |
| EX-10.4 - EX-10.4 - ARATANA THERAPEUTICS, INC. | petx-20160630xex10_4.htm |
| EX-10.3 - EX-10.3 - ARATANA THERAPEUTICS, INC. | petx-20160630xex10_3.htm |
| EX-10.2 - EX-10.2 - ARATANA THERAPEUTICS, INC. | petx-20160630xex10_2.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended June 30, 2016
or
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number 001-35952
ARATANA THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
38-3826477 |
|
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification Number) |
11400 Tomahawk Creek Parkway
Suite 340
Leawood, KS 66211
(913) 353-1000
(Address of principal executive offices, zip code and telephone number, including area code)
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes: ☒ No: ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes: ☒ No: ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
Accelerated filer |
|||||
|
|
|
|
|
|||
|
Non-accelerated filer |
Smaller reporting company |
|||||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes: ☐ No: ☒
As of August 1, 2016, there were 35,415,772 shares of common stock outstanding.
ARATANA THERAPEUTICS, INC.
|
|
|
Page |
|
|
|
|||
|
Item 1. |
3 | ||
|
|
Consolidated Balance Sheets as of June 30, 2016 and December 31, 2015 |
3 | |
|
|
Consolidated Statements of Operations for the Three and Six Months Ended June 30, 2016 and 2015 |
4 | |
|
|
5 | ||
|
|
Consolidated Statements of Cash Flows for the Six Months Ended June 30, 2016 and 2015 |
6 | |
|
|
7 | ||
|
Item 2. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
24 | |
|
Item 3. |
37 | ||
|
Item 4. |
37 | ||
|
|
|||
|
Item 1. |
38 | ||
|
Item 1A. |
38 | ||
|
Item 2. |
39 | ||
|
Item 3. |
39 | ||
|
Item 4. |
39 | ||
|
Item 5. |
39 | ||
|
Item 6. |
39 | ||
| 40 | |||
2
Consolidated Balance Sheets (Unaudited)
(Amounts in thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30, 2016 |
|
December 31, 2015 |
||
|
Assets |
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
108,759 |
|
$ |
26,755 |
|
Short-term investments |
|
|
747 |
|
|
59,447 |
|
Accounts receivable, net |
|
|
17 |
|
|
60 |
|
Inventories |
|
|
2,566 |
|
|
1,306 |
|
Prepaid expenses and other current assets |
|
|
1,568 |
|
|
1,451 |
|
Total current assets |
|
|
113,657 |
|
|
89,019 |
|
Property and equipment, net |
|
|
2,273 |
|
|
2,555 |
|
Goodwill |
|
|
40,006 |
|
|
39,781 |
|
Intangible assets, net |
|
|
12,294 |
|
|
15,067 |
|
Restricted cash |
|
|
350 |
|
|
350 |
|
Other long-term assets |
|
|
280 |
|
|
294 |
|
Total assets |
|
$ |
168,860 |
|
$ |
147,066 |
|
Liabilities and Stockholders’ Equity |
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
Accounts payable |
|
$ |
7,272 |
|
$ |
1,400 |
|
Accrued expenses |
|
|
4,505 |
|
|
4,247 |
|
Licensing and collaboration commitment |
|
|
7,000 |
|
|
— |
|
Current portion – loans payable |
|
|
2,333 |
|
|
— |
|
Other current liabilities |
|
|
11 |
|
|
37 |
|
Total current liabilities |
|
|
21,121 |
|
|
5,684 |
|
Loans payable, net |
|
|
37,616 |
|
|
39,710 |
|
Other long-term liabilities |
|
|
502 |
|
|
122 |
|
Total liabilities |
|
|
59,239 |
|
|
45,516 |
|
Commitments and contingencies (Notes 8 and 10) |
|
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
|
Common stock, $0.001 par value; 100,000,000 shares authorized at June 30, 2016 and December 31, 2015, 34,798,120 and 34,563,816 issued and outstanding at June 30, 2016 and December 31, 2015, respectively |
|
|
35 |
|
|
35 |
|
Treasury stock |
|
|
(1,088) |
|
|
(1,088) |
|
Additional paid-in capital |
|
|
268,410 |
|
|
263,941 |
|
Accumulated deficit |
|
|
(148,889) |
|
|
(152,018) |
|
Accumulated other comprehensive loss |
|
|
(8,847) |
|
|
(9,320) |
|
Total stockholders’ equity |
|
|
109,621 |
|
|
101,550 |
|
Total liabilities and stockholders’ equity |
|
$ |
168,860 |
|
$ |
147,066 |
The accompanying notes are an integral part of these unaudited interim consolidated financial statements.
3
Consolidated Statements of Operations (Unaudited)
(Amounts in thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended |
|
Six Months Ended |
||||||||
|
|
|
June 30, |
|
June 30, |
||||||||
|
|
|
2016 |
|
2015 |
|
2016 |
|
2015 |
||||
|
Revenues |
|
|
|
|
|
|
|
|
|
|
|
|
|
Licensing and collaboration revenue |
|
$ |
38,000 |
|
$ |
— |
|
$ |
38,151 |
|
$ |
— |
|
Product sales |
|
|
47 |
|
|
230 |
|
|
68 |
|
|
386 |
|
Total revenues |
|
|
38,047 |
|
|
230 |
|
|
38,219 |
|
|
386 |
|
Costs and expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of product sales |
|
|
1,741 |
|
|
109 |
|
|
1,760 |
|
|
219 |
|
Royalty expense |
|
|
20 |
|
|
23 |
|
|
38 |
|
|
43 |
|
Research and development |
|
|
5,303 |
|
|
6,081 |
|
|
16,052 |
|
|
12,302 |
|
Selling, general and administrative |
|
|
6,148 |
|
|
4,879 |
|
|
12,699 |
|
|
9,064 |
|
Amortization of acquired intangible assets |
|
|
95 |
|
|
483 |
|
|
190 |
|
|
966 |
|
Impairment of acquired intangible assets |
|
|
2,780 |
|
|
— |
|
|
2,780 |
|
|
— |
|
Total costs and expenses |
|
|
16,087 |
|
|
11,575 |
|
|
33,519 |
|
|
22,594 |
|
Income (loss) from operations |
|
|
21,960 |
|
|
(11,345) |
|
|
4,700 |
|
|
(22,208) |
|
Other income (expense) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income |
|
|
83 |
|
|
43 |
|
|
160 |
|
|
114 |
|
Interest expense |
|
|
(846) |
|
|
(217) |
|
|
(1,695) |
|
|
(435) |
|
Other income (expense), net |
|
|
(1) |
|
|
3,176 |
|
|
(36) |
|
|
5,141 |
|
Total other income (expense) |
|
|
(764) |
|
|
3,002 |
|
|
(1,571) |
|
|
4,820 |
|
Income (loss) before income taxes |
|
|
21,196 |
|
|
(8,343) |
|
|
3,129 |
|
|
(17,388) |
|
Income tax benefit |
|
|
— |
|
|
360 |
|
|
— |
|
|
631 |
|
Net income (loss) |
|
|
21,196 |
|
|
(7,983) |
|
|
3,129 |
|
|
(16,757) |
|
Net income attributable to participating securities |
|
|
(20) |
|
|
— |
|
|
(3) |
|
|
— |
|
Net income (loss) attributable to common stockholders |
|
$ |
21,176 |
|
$ |
(7,983) |
|
$ |
3,126 |
|
$ |
(16,757) |
|
Net income (loss) per share attributable to common stockholders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per share, basic and diluted |
|
$ |
0.61 |
|
$ |
(0.23) |
|
$ |
0.09 |
|
$ |
(0.49) |
|
Weighted average shares outstanding, basic |
|
|
34,762,533 |
|
|
34,278,105 |
|
|
34,708,006 |
|
|
34,236,282 |
|
Weighted average shares outstanding, diluted |
|
|
34,938,455 |
|
|
34,278,105 |
|
|
34,779,786 |
|
|
34,236,282 |
The accompanying notes are an integral part of these unaudited interim consolidated financial statements.
4
Consolidated Statements of Comprehensive Income (Unaudited)
(Amounts in thousands)
|
|
||||||||||||
|
|
Three Months Ended |
Six Months Ended |
||||||||||
|
|
June 30, |
June 30, |
||||||||||
|
|
2016 |
2015 |
2016 |
2015 |
||||||||
|
Net income (loss) |
$ |
21,196 |
$ |
(7,983) |
$ |
3,129 |
$ |
(16,757) | ||||
|
|
||||||||||||
|
Other comprehensive income (loss): |
||||||||||||
|
Foreign currency translation adjustment |
(704) | (1,390) | 473 | (3,191) | ||||||||
|
Unrealized gain on available-for-sale securities |
— |
958 |
— |
2,622 | ||||||||
|
Net gain reclassified into income on sale of |
— |
(2,864) |
— |
(3,874) | ||||||||
|
Other comprehensive income (loss) |
(704) | (3,296) | 473 | (4,443) | ||||||||
|
Comprehensive income (loss) |
$ |
20,492 |
$ |
(11,279) |
$ |
3,602 |
$ |
(21,200) | ||||
The accompanying notes are an integral part of these unaudited interim consolidated financial statements.
5
Consolidated Statements of Cash Flows (Unaudited)
(Amounts in thousands)
|
|
|||||
|
|
Six Months Ended |
||||
|
|
June 30, |
||||
|
|
2016 |
2015 |
|||
|
Cash flows from operating activities |
|||||
|
Net income (loss) |
$ |
3,129 |
$ |
(16,757) | |
|
Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: |
|||||
|
Stock-based compensation expense |
4,449 | 4,389 | |||
|
Depreciation and amortization expense |
493 | 1,056 | |||
|
Impairment of acquired intangible assets |
2,780 |
— |
|||
|
Gain on sale of marketable securities |
— |
(3,874) | |||
|
Non-cash interest expense |
239 | 20 | |||
|
Market value adjustments to inventories |
1,552 |
— |
|||
|
Change in fair value of contingent consideration |
— |
(1,248) | |||
|
Change in fair value of derivative instruments |
— |
(1,274) | |||
|
Deferred tax benefit |
— |
(631) | |||
|
Changes in operating assets and liabilities: |
|||||
|
Accounts receivable, net |
43 | 194 | |||
|
Inventories |
(2,812) | (455) | |||
|
Prepaid expenses |
(101) | (57) | |||
|
Other assets |
17 | 15 | |||
|
Accounts payable |
5,874 | 213 | |||
|
Accrued expenses and other liabilities |
624 | 603 | |||
|
Licensing and collaboration commitment |
7,000 |
— |
|||
|
Net cash provided by (used in) operating activities |
23,287 | (17,806) | |||
|
Cash flows from investing activities |
|||||
|
Purchases of property and equipment, net |
(19) | (1,098) | |||
|
Proceeds from sales of marketable securities |
— |
7,456 | |||
|
Purchase of investments |
(227,346) | (905,000) | |||
|
Proceeds from maturities of investments |
286,046 | 922,249 | |||
|
Net cash provided by investing activities |
58,681 | 23,607 | |||
|
Cash flows from financing activities |
|||||
|
Proceeds from stock option exercises |
1 | 47 | |||
|
Cash paid for contingent consideration |
— |
(3,000) | |||
|
Net cash provided by (used in) financing activities |
1 | (2,953) | |||
|
Effect of exchange rate on cash |
35 | (80) | |||
|
Net increase in cash and cash equivalents |
82,004 | 2,768 | |||
|
Cash and cash equivalents, beginning of period |
26,755 | 9,823 | |||
|
Cash and cash equivalents, end of period |
$ |
108,759 |
$ |
12,591 | |
|
Supplemental disclosure of cash flow information |
|||||
|
Cash paid for interest |
$ |
1,447 |
$ |
415 | |
|
Supplemental disclosure of noncash investing and financing activities: |
|||||
|
Non-cash exercise of warrant |
$ |
— |
$ |
750 | |
The accompanying notes are an integral part of these unaudited interim consolidated financial statements.
6
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements (Unaudited)
(Amounts in thousands, except share and per share data)
1. Summary of Significant Accounting Policies
Business Overview
Aratana Therapeutics, Inc., including its subsidiaries (the “Company” or “Aratana”), is a pet therapeutics company focused on licensing, developing and commercializing innovative biopharmaceutical products for companion animals. The Company has one operating segment: pet therapeutics.
Basis of Presentation
The accompanying unaudited consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”) for interim financial information. Accordingly, they do not include all of the information and footnotes required by GAAP for complete financial statements. These unaudited consolidated financial statements should be read in conjunction with the audited consolidated financial statements of the Company for the year ended December 31, 2015 and the notes thereto in the Company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 15, 2016. In the opinion of management, all adjustments, consisting of a normal and recurring nature, considered necessary for a fair presentation, have been included.
The Company has incurred recurring losses and negative cash flows from operations and has an accumulated deficit of $148,889 as of June 30, 2016. The Company expects to continue to generate operating losses for the foreseeable future. The Company believes that its cash, cash equivalents and short-term investments on hand will be sufficient to fund operations at least through June 30, 2017. As disclosed in Note 8 to the consolidated financial statements, the Company has a term loan and a revolving credit facility with an aggregate principal balance of $40,000 as of June 30, 2016. The terms of this agreement require the Company to receive unrestricted net cash proceeds of at least $45,000 from partnering transactions and/or the issuance of equity securities from October 16, 2015 to October 16, 2016. The loan agreements also require that the Company have at least three products fully United States Department of Agriculture (“USDA”) or U.S. Food and Drug Administration (“FDA”) approved for commercialization by December 31, 2016. With the FDA approval of GALLIPRANT in March 2016 and the receipt of the upfront payment of $45,000 under the Elanco Animal Health, Inc. (“Elanco”) agreement (Note 10) entered into in April 2016, the Company has met both conditions. Additionally, the loan agreement requires that the Company maintain certain minimum liquidity at all times, which as of June 30, 2016, was approximately $20,000.
Consolidation
The Company’s consolidated financial statements include its financial statements, and those of its wholly-owned subsidiaries and a consolidated variable interest entity. Intercompany balances and transactions are eliminated in consolidation.
To determine if the Company holds a controlling financial interest in an entity, the Company first evaluates if it is required to apply the variable interest entity (“VIE”) model to the entity. Where the Company holds current or potential rights that give it the power to direct the activities of a VIE that most significantly impact the VIE’s economic performance combined with a variable interest that gives it the right to receive potentially significant benefits or the obligation to absorb potentially significant losses, the Company is the primary beneficiary of that VIE. When changes occur to the design of an entity, the Company reconsiders whether it is subject to the VIE model. The Company continuously evaluates whether it is the primary beneficiary of a consolidated VIE.
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates, judgments and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Estimates are periodically reviewed in light of changes in circumstances, facts and experience. Actual results could differ from those estimates.
Revenue Recognition
The Company recognizes revenue when all of the following conditions are met:
• persuasive evidence of an arrangement exists;
• delivery has occurred or services have been rendered;
• the seller’s price to the buyer is fixed or determinable; and
• collectibility is reasonably assured.
7
The Company’s principal revenue streams and their respective accounting treatments are discussed below:
(i) Product sales - Revenue for the sale of products is recognized when delivery has occurred and substantially all the risks and rewards of ownership have been transferred to the customer. Revenue for the sale of products are recorded net of sales returns, allowances and discounts.
(ii) Royalty revenue - Royalty revenue relating to the Company’s out-licensed technology is recognized when reasonably estimable. The revenues are recorded based on estimates of the licensee’s sales that occurred during the relevant period. Differences between actual and estimated royalty revenues are adjusted for in the period in which they become known, typically in the following quarter. If the Company is unable to reasonably estimate royalty revenue or does not have access to the information, then the Company records royalty revenue on a cash basis.
(iii) Licensing and collaboration revenues - Revenues derived from product out-licensing arrangements typically consist of an initial up-front payment at inception of the license and subsequent milestone payments contingent on the achievement of certain regulatory, development and commercial milestones.
Product out-licensing arrangements with multiple elements are divided into separate units of accounting if certain criteria are met. The up-front payment received is allocated among the separate units of accounting based on their respective fair values, and the applicable revenue recognition criteria are applied to each of the separate units of accounting. The application of the multiple element guidance requires subjective determinations, and requires the Company to make judgments about the individual deliverables and whether such deliverables are separable from the other aspects of the contractual relationship. Deliverables are considered separate units of accounting provided that:
(1) the delivered item(s) has value to the customer on a stand-alone basis and
(2) if the arrangement includes a general right of return relative to the delivered item(s), delivery or performance of the undelivered item(s) is considered probable and substantially in the Company's control.
In determining the units of accounting, the Company evaluates certain criteria, including whether the deliverables have stand-alone value, based on the consideration of the relevant facts and circumstances for each arrangement. In addition, the Company considers whether the buyer can use the other deliverable(s) for their intended purpose without the receipt of the remaining element(s), whether the value of the deliverable is dependent on the undelivered item(s), and whether there are other vendors that can provide the undelivered element(s).
Arrangement consideration that is fixed or determinable is allocated among the separate units of accounting using the relative selling price method, and the applicable revenue recognition criteria. The Company determines the estimated selling price for deliverables within each agreement using vendor-specific objective evidence (“VSOE”) of selling price, if available, third-party evidence (“TPE”) of selling price if VSOE is not available, or management's best estimate of the selling price (“BESP”) if neither VSOE nor TPE is available. Determining the BESP for a unit of accounting requires significant judgment. In developing the BESP for a unit of accounting, the Company considers applicable market conditions and relevant entity-specific factors, including factors that were contemplated in negotiating the agreement with the customer and estimated costs.
If there are deliverables in an arrangement that are not separable from other aspects of the contractual relationship, they are treated as a combined unit of accounting, with the allocated revenue for the combined unit recognized in a manner consistent with the revenue recognition applicable to the final deliverable in the combined unit.
Amounts received prior to satisfying all relevant revenue recognition criteria are recorded as deferred revenue in the consolidated balance sheets and recognized as revenue when the related revenue recognition criteria are met. Amounts not expected to be recognized as revenue within the next twelve months of the consolidated balance sheet date are classified as long-term deferred revenue.
The Company recognizes revenue contingent upon the achievement of a milestone in its entirety in the period in which the milestone is achieved only if the milestone meets all the criteria to be considered substantive. At the inception of each arrangement that includes milestone payments, the Company evaluates each contingent payment on an individual basis to determine whether they are considered substantive milestones, specifically reviewing factors such as the degree of certainty in achieving the milestone, the research and development risk and other risks that must be overcome to achieve the milestone, as well as the level of effort and investment required and whether the milestone consideration is reasonable relative to all deliverables and payment terms in the arrangement. This evaluation includes an assessment of whether (a) the consideration is commensurate with either (1) the entity’s performance to achieve the milestone, or (2) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the entity’s performance to achieve the milestone, (b) the consideration relates solely to past performance and (c) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement.
8
Milestone payments which are non-refundable and deemed substantive, non-creditable and contingent on achieving certain development, regulatory, or commercial milestones are typically recognized as revenues either on achievement of such milestones or over the period the Company has continuing substantive performance obligations. The Company recognizes revenue associated with the non-substantive milestones upon achievement of the milestone if there are no undelivered elements and the Company has no remaining performance obligations. Revenues from commercial milestone payments are recorded as revenue upon achievement of the milestone, assuming all other revenue recognition criteria are met.
In the event that an agreement is terminated and the Company then has no further performance obligations, the Company recognizes as revenue any amounts that had not previously been recorded as revenue but were classified as deferred revenue at the date of such termination.
Cash considerations (including a sales incentive) given by the Company to a licensee/collaborator/customer is presumed to be a reduction of the selling prices of the Company’s products or services and is recognized as a reduction of revenue unless both of the following conditions are met:
a. The Company receives, or will receive, an identifiable benefit (goods or services) in exchange for the consideration. In order to meet this condition, the identified benefit must be sufficiently separable from the recipient’s purchase of the Company’s products such that the Company could have entered into an exchange transaction with a party other than a purchaser of its products or services in order to receive that benefit.
b. The Company can reasonably estimate the fair value of the benefit identified under the preceding condition. If the amount of consideration paid by the Company exceeds the estimated fair value of the benefit received, that excess amount shall be characterized as a reduction of revenue when recognized in the Company’s income statement.
If both conditions are met, the cash consideration is recognized as a cost incurred.
Pre-Launch Inventory
The Company may scale-up and make commercial quantities of certain of its product candidates prior to the date it anticipates that such products will receive final FDA/USDA approval. The scale-up and commercial production of pre-launch inventories involves the risk that such products may not be approved for marketing by the FDA/USDA on a timely basis, or ever. Inventory costs associated with product candidates that have not yet received regulatory approval are capitalized if the Company believes there is probable future commercial use and future economic benefit. If the probability of future commercial use and future economic benefit cannot be reasonably determined, then pre-launch inventory costs associated with such product candidates are expensed as research and development expense during the period the costs are incurred. Specifically, the Company has determined that for FDA-regulated product candidates there is a probable future commercial use and future economic benefit upon the receipt of the three major technical section complete letters from the FDA’s Center for Veterinary Medicine (“CVM”). For USDA product candidates, the Company has determined there is a probable future commercial use and future economic benefit upon the receipt of a conditional license from the USDA’s Center for Veterinary Biologics. The Company makes at least quarterly reassessments of the probability of regulatory approval and useful life of the pre-launch inventory, and determines whether such inventory continues to have a probable future economic benefit.
Property and Equipment, net
Property and equipment is recorded at historical cost, net of accumulated depreciation and amortization of $733 and $430 as of June 30, 2016, and December 31, 2015, respectively.
Goodwill
In the first quarter of 2016, the Company completed an interim impairment assessment due to a decline in its market capitalization. In performing step one of the assessment, the Company determined that as of March 31, 2016, its fair value exceeded its carrying value by 121%. Based on this result, step two of the assessment was not required to be performed, and the Company determined there was no impairment of goodwill during the first quarter of 2016. The Company did not identify any potential indicators of goodwill impairment during the second quarter of 2016, and, accordingly, no interim assessment was performed.
9
New Accounting Standards
Revenue from Contracts with Customers
In May 2014, the Financial Accounting Standards Board (“FASB”) issued guidance on recognizing revenue in contracts with customers. The guidance affects any entity that either enters into contracts with customers to transfer goods or services or enters into contracts for the transfer of nonfinancial assets unless those contracts are within the scope of other standards (e.g., insurance contracts or lease contracts). This guidance will supersede the revenue recognition requirements in topic, Revenue Recognition, and most industry-specific guidance. This guidance also supersedes certain cost guidance included in subtopic, Revenue Recognition – Construction-Type and Production-Type Contracts. In addition, the existing requirements for the recognition of a gain or loss on the transfer of nonfinancial assets that are not in a contract with a customer (e.g., assets within the scope of topic, Property, Plant, and Equipment, and tangible assets within the scope of topic, Intangibles – Goodwill and Other) are amended to be consistent with the guidance on recognition and measurement (including the constraint on revenue) in this guidance.
The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services.
In July 2015, the FASB approved a one-year delay in the effective date of the new revenue standard. These changes become effective for the Company on January 1, 2018. Early adoption is permitted but not before the original effective date of January 1, 2017. The standard permits the use of either the retrospective or cumulative effect transition method. The Company is currently assessing the impact, if any, this new guidance will have on its consolidated financial statements.
Inventory – Simplifying the Measurement of Inventory
In July 2015, the FASB issued guidance that requires entities to measure most inventory “at lower of cost and net realizable value” thereby simplifying the current guidance under which an entity must measure inventory at the lower of cost or market. This guidance is effective for financial statements issued for fiscal years beginning after December 15, 2016, and interim periods within those fiscal years. Early adoption is permitted and is to be applied using a prospective basis. The Company does not expect that this new guidance will have a material impact on its consolidated financial statements.
Leases
In February 2016, the FASB issued guidance that requires, for operating leases, a lessee to recognize a right-of-use asset and a lease liability, initially measured at the present value of the lease payments, in its balance sheet. The standard also requires a lessee to recognize a single lease cost, calculated so that the cost of the lease is allocated over the lease term, on a generally straight-line basis. This guidance is effective for financial statements issued for fiscal years beginning after December 15, 2018, and interim periods within those fiscal years. Early adoption is permitted and is to be applied using a modified retrospective transition method. The Company is currently assessing the effect that adoption of this guidance will have on its consolidated financial statements.
Compensation – Stock Compensation
In March 2016, the FASB issued guidance that simplifies several aspects of the accounting for employee share-based payment transactions including accounting for income taxes, forfeitures and statutory tax withholding requirements, as well as classification in the statement of cash flows. This guidance is effective for financial statements issued for fiscal years beginning after December 15, 2016, and interim periods within those fiscal years. Early adoption is permitted and is to be applied using a modified retrospective transition method by means of a cumulative-effect adjustment to equity as of the beginning of the period in which the guidance is adopted. The Company is currently assessing the effect that adoption of this guidance will have on its consolidated financial statements.
10
2. Fair Value of Financial Assets and Liabilities
Financial Assets and Liabilities Measured at Fair Value on a Recurring Basis
The following financial assets are measured at fair value on a recurring basis using quoted prices in active markets for identical assets (Level 1); significant other observable inputs (Level 2); and significant unobservable inputs (Level 3).
|
|
|||||||||||||||
|
|
Fair Value Measurements as of |
||||||||||||||
|
|
Carrying |
June 30, 2016 Using: |
|||||||||||||
|
|
Value |
Level 1 |
Level 2 |
Level 3 |
Total |
||||||||||
|
Assets: |
|||||||||||||||
|
Cash equivalents: |
|||||||||||||||
|
Certificates of deposit |
$ |
7,221 |
$ |
— |
$ |
7,221 |
$ |
— |
$ |
7,221 | |||||
|
Short-term investments: |
|||||||||||||||
|
Short-term marketable securities - certificates of deposit |
747 |
— |
747 |
— |
747 | ||||||||||
|
|
$ |
7,968 |
$ |
— |
$ |
7,968 |
$ |
— |
$ |
7,968 | |||||
|
|
|||||||||||||||
|
|
Fair Value Measurements as of |
||||||||||||||
|
|
Carrying |
December 31, 2015 Using: |
|||||||||||||
|
|
Value |
Level 1 |
Level 2 |
Level 3 |
Total |
||||||||||
|
Assets: |
|||||||||||||||
|
Cash equivalents: |
|||||||||||||||
|
Certificates of deposit |
$ |
6,972 |
$ |
— |
$ |
6,972 |
$ |
— |
$ |
6,972 | |||||
|
Money market fund |
35 | 35 |
— |
— |
35 | ||||||||||
|
Short-term investments: |
|||||||||||||||
|
Short-term marketable securities - certificates of deposit |
747 |
— |
747 |
— |
747 | ||||||||||
|
Reverse repurchase agreements |
58,700 |
— |
58,700 |
— |
58,700 | ||||||||||
|
|
$ |
66,454 |
$ |
35 |
$ |
66,419 |
$ |
— |
$ |
66,454 | |||||
Certain estimates and judgments are required to develop the fair value amounts shown above. The fair value amounts shown above are not necessarily indicative of the amounts that the Company would realize upon disposition, nor do they indicate the Company’s intent or ability to dispose of the financial instrument.
The following methods and assumptions were used to estimate the fair value of each material class of financial instrument:
|
· |
Marketable securities (short-term) – the fair value of marketable securities has been determined to be amortized cost given the short duration of the securities. |
|
· |
Reverse repurchase agreements – the fair value of the reverse repurchase agreements has been determined to be amortized cost given the short duration of the agreements. |
|
· |
Cash equivalents – the fair value of the cash equivalents has been determined to be amortized cost or has been based on the quoted prices in active markets or exchanges for identical assets. |
Financial Assets and Liabilities that are not Measured at Fair Value on a Recurring Basis
The carrying amounts and estimated fair value of the Company’s financial liabilities which are not measured at fair value on a recurring basis was as follows:
|
|
||||||
|
|
June 30, 2016 |
|||||
|
|
Carrying Value |
Fair Value |
||||
|
Liabilities: |
||||||
|
Loans payable (Level 2) |
$ |
39,949 |
$ |
39,938 | ||
11
|
|
||||||
|
|
December 31, 2015 |
|||||
|
|
Carrying Value |
Fair Value |
||||
|
Liabilities: |
||||||
|
Loans payable (Level 2) |
$ |
39,710 |
$ |
40,569 | ||
Certain estimates and judgments were required to develop the fair value amounts. The fair value amount shown above is not necessarily indicative of the amounts that the Company would realize upon disposition, nor does it indicate the Company’s intent or ability to dispose of the financial instrument.
The fair value of loans payable was estimated using discounted cash flow analysis discounted at current rates.
Fair value information about the intangible assets that were impaired during the six months ended June 30, 2016 (Note 7) was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value |
||||||||||
|
|
|
Carrying |
|
June 30, 2016 Using: |
|||||||||||
|
|
|
Value |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Impairment |
|||||
|
Intellectual property rights for currently marketed products |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
551 |
|
Intellectual property rights acquired for in-process research and development |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
2,229 |
|
|
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
2,780 |
The fair value amount is presented as of the date of impairment, as these assets are not measured at fair value on a recurring basis (Note 7). The fair value reflects intangible assets written down to fair value during the six months ended June 30, 2016. Fair-value was determined using the income approach, specifically the multi-period excess earnings method, a form of a discounted cash flow method. The Company started with a forecast of all the expected net cash flows associated with the asset and then it applied an asset-specific discount rate to arrive at a net present value amount. Some of the more significant estimates and assumptions inherent in this approach include: the amount and timing of the projected net cash flows, which includes the expected impact of competitive legal and/or regulatory forces on the product and the impact of technological risk associated with IPR&D assets; the discount rate, which seeks to reflect the various risks inherent in the projected cash flows; and the tax rate, which seeks to incorporate the geographic diversity of the projected cash flows.
3. Investments
Marketable Securities
Marketable securities consisted of the following:
|
|
||||||||||||
|
|
June 30, 2016 |
|||||||||||
|
|
Gross |
Gross |
||||||||||
|
|
Amortized |
Unrealized |
Unrealized |
Fair |
||||||||
|
|
Cost |
Losses |
Losses |
Value |
||||||||
|
Short-term marketable securities: |
||||||||||||
|
Certificates of deposit |
$ |
747 |
$ |
— |
$ |
— |
$ |
747 | ||||
|
Total |
$ |
747 |
$ |
— |
$ |
— |
$ |
747 | ||||
At June 30, 2016, short-term marketable securities consisted of investments that mature within one year. Short-term marketable securities are recorded as short-term investments in the consolidated balance sheets.
12
|
|
||||||||||||
|
|
December 31, 2015 |
|||||||||||
|
|
Gross |
Gross |
||||||||||
|
|
Amortized |
Unrealized |
Unrealized |
Fair |
||||||||
|
|
Cost |
Losses |
Losses |
Value |
||||||||
|
Short-term marketable securities: |
||||||||||||
|
Certificates of deposit |
$ |
747 |
$ |
— |
$ |
— |
$ |
747 | ||||
|
Total |
$ |
747 |
$ |
— |
$ |
— |
$ |
747 | ||||
At December 31, 2015, short-term marketable securities consisted of investments that mature within one year. Short-term marketable securities are recorded as short-term investments in the consolidated balance sheets.
Reverse Repurchase Agreements
The Company, as part of its cash management strategy, may invest excess cash in reverse repurchase agreements. All reverse repurchase agreements are tri-party and have maturities of three months or less at the time of investment. The underlying collateral is U.S. government securities including U.S. treasuries, agency debt and agency mortgage securities. The underlying collateral posted by each counterparty is required to cover 102% of the principal amount and accrued interest after the application of a discount to fair value. The Company was not invested in any reverse repurchase agreements as of June 30, 2016.
4. Derivative Financial Instruments
In 2015, the Company’s derivative financial instrument, the warrant the Company received in connection with the license agreement with Advaxis, Inc. (“Advaxis”), was not designated as a hedging instrument and was adjusted to fair value through earnings in other income (expense). During the year ended December 31, 2015, the Company exercised the Advaxis warrant and subsequently sold the shares of common stock received upon exercise.
The following table shows the Company’s gain recognized in other income (expense) for the three and six months ended:
|
|
||||||||||||
|
|
Gain Recognized in |
Gain Recognized in |
||||||||||
|
|
Other Income (expense) |
Other Income (expense) |
||||||||||
|
|
Three Months Ended |
Six Months Ended |
||||||||||
|
|
June 30, |
June 30, |
||||||||||
|
|
2016 |
2015 |
2016 |
2015 |
||||||||
|
Derivative assets: |
||||||||||||
|
Warrant |
$ |
— |
$ |
316 |
$ |
— |
$ |
1,274 | ||||
As the Company exercised the warrant and subsequently sold the shares of common stock received upon exercise resulting in a gain of $341 recognized in other income (expense) during the second quarter of 2015, no gain was recorded during the three and six months ended June 30, 2016.
Inventories are stated at the lower of cost or market and comprised of the following:
|
|
||||||
|
|
June 30, 2016 |
December 31, 2015 |
||||
|
Raw materials |
$ |
88 |
$ |
120 | ||
|
Work-in-process |
2,233 | 441 | ||||
|
Finished goods |
245 | 745 | ||||
|
|
$ |
2,566 |
$ |
1,306 | ||
Work-in-process inventories at June 30, 2016, included approximately $1,738 of pre-launch GALLIPRANT® (grapiprant tablets) product costs capitalized due to anticipated benefit from future commercialization of this product. GALLIPRANT was approved by the CVM for the control of pain and inflammation associated with osteoarthritis in dogs in the first quarter of 2016. As of June 30, 2016, the Company has accrued $1,819 for pre-launch GALLIPRANT inventories received but not invoiced.
13
During the three and six months ended June 30, 2016, the Company recognized inventory valuation adjustment losses in the amount $1,552 from application of lower of cost or market, in cost of product sales. The losses related to TACTRESS™ inventories that were written off and pre-launch GALLIPRANT inventories marked to market due to terms agreed upon in the Elanco collaboration agreement (Note 10).
Goodwill is recorded as an indefinite-lived asset and is not amortized for financial reporting purposes but is tested for impairment on an annual basis or when indications of impairment exist. No goodwill impairment losses have been recognized to date. Goodwill is not expected to be deductible for income tax purposes. The Company performs its annual impairment test of the carrying value of the Company’s goodwill during the third quarter of each year.
Goodwill as of June 30, 2016, was as follows:
|
|
|||||||||
|
|
Gross |
Impairment |
Net |
||||||
|
|
Carrying Amount |
Losses |
Carrying Value |
||||||
|
Goodwill |
$ |
40,006 |
$ |
— |
$ |
40,006 | |||
The change in the net book value of goodwill for the six months ended June 30, 2016, was as follows:
|
|
|||
|
|
2016 |
||
|
As of January 1, |
$ |
39,781 | |
|
Effect of foreign currency exchange |
225 | ||
|
As of the end of the period, |
$ |
40,006 | |
7. Intangible Assets, Net
The change in the net book value of intangible assets for the six months ended June 30, 2016, was as follows:
|
|
|||
|
|
2016 |
||
|
As of January 1, |
$ |
15,067 | |
|
Amortization expense |
(190) | ||
|
Effect of foreign currency exchange |
197 | ||
|
Impairment |
(2,780) | ||
|
As of the end of the period, |
$ |
12,294 | |
The estimated useful lives of the individual categories of intangible assets were based on the nature of the applicable intangible asset and the expected future cash flows to be derived from the intangible asset. Amortization of intangible assets with finite lives is recognized over the shorter of the respective term of the agreement or the period of time the intangible assets are expected to contribute to future cash flows. The Company amortizes finite-lived intangible assets using the straight-line method. The Company recognized amortization expense of $95 and $190 for the three and six months ended June 30, 2016, respectively, and $483 and $966 for the three and six months ended June 30, 2015, respectively. Indefinite-lived in-process research and development (“IPR&D”) intangible assets are not amortized until a product reaches its first conditional license or approval, and then they are amortized over their estimated useful lives.
Unamortized Intangible Assets
Unamortized intangible assets as of June 30, 2016, were as follows:
|
|
|||
|
|
Net |
||
|
|
Carrying |
||
|
|
Value |
||
|
Unamortized intangible assets: |
2016 |
||
|
Intellectual property rights acquired for in-process research and development |
$ |
6,979 | |
14
The net carrying value above includes asset impairment charges to date of $10,946 and $5,819 for AT-007 and AT-011, respectively.
Return of AT-006 Global Rights
On May 11, 2016, the Company and Elanco agreed to terminate the Exclusive License, Development, and Commercialization Agreement with Elanco (the “Elanco AT-006 Agreement”) (Note 10) that granted Elanco global rights for development and commercialization of licensed animal health products for an anti-viral for the treatment of feline herpes virus induced ophthalmic conditions. As a result of the termination of the Elanco AT-006 Agreement, the Company conducted an impairment assessment of the AT-006 intangible asset to assess the impact of the termination agreement. As part of the assessment, the Company considered development timing and expenses, manufacturing expenses, technology royalties and royalties due to Elanco, anticipated timing of commercial availability, as well as marketing, and selling expenses to commercialize the product. The Company concluded that the AT-006 intangible asset was not impaired due to the termination of the Elanco AT-006 Agreement.
Impairment of Unamortized Intangible Assets
AT-007 (Feline immunodeficiency virus)
The Company has been considering out-licensing or internally advancing the AT-007 program for feline immunodeficiency virus since an impairment expense was recorded in the third quarter of 2015. Due to the return of the AT-006 global rights from Elanco in May 2016 (Note 10) and ensuing development program portfolio prioritization, including consideration of the Company’s focus on commercial launch activities to support its recently approved products, the Company decided to discontinue the development of AT-007 during the second quarter of 2016. This resulted in an impairment charge of $2,229, which was recorded during the three months ended June 30, 2016, reducing the carrying value of AT-007 to $0.
Unfavorable outcomes of the Company’s development activities or the Company’s estimates of the market opportunities for the product candidates could result in additional impairment charges in future periods.
Amortized Intangible Assets
Amortized intangible assets as of June 30, 2016, were as follows:
|
|
Gross |
Net |
||||||||||
|
|
Carrying |
Accumulated |
Carrying |
Average |
||||||||
|
Amortized intangible assets: |
Value |
Amortization |
Value |
Useful Life |
||||||||
|
Intellectual property rights for currently marketed products: |
||||||||||||
|
BLONTRESS® |
$ |
28,572 |
$ |
23,257 |
$ |
5,315 | 20 |
Years |
||||
Accumulated amortization includes both amortization expense and asset impairment charges. Asset impairment charges for BLONTRESS to date are $20,228.
Impairment of Amortized Intangible Assets
TACTRESS
Since the acquisition of Vet Therapeutics, Inc. (October 2013), the Company has been performing various scientific and clinical activities to gain further knowledge around the science and efficacy of TACTRESS. In the third quarter of 2015, the Company’s interim analysis of the clinical results indicated that TACTRESS did not seem to be adding significant progression free survival in canine T-cell lymphoma; those results were confirmed in the final study results in July 2016. In addition, scientific studies suggested that TACTRESS was not as specific to the target as expected. Given those clinical and scientific results, the Company no longer believed that TACTRESS would capture the desired T-cell lymphoma market opportunity and recorded an impairment expense.
While TACTRESS remains commercially available, the use by oncologists has been more limited than the Company anticipated, resulting in sales during the quarter ended June 30, 2016, being significantly lower than forecasted. The Company deemed the events and market projections described above to be indicators of potential impairment of its finite-lived intangible asset TACTRESS during the second quarter of 2016. The Company performed impairment testing for the intangible asset TACTRESS as of June 30, 2016, and recorded an impairment expense of $551, which was recorded during the three months ended June 30, 2016, resulting in a net carrying value of $0 for TACTRESS.
Unfavorable outcomes of the Company’s development activities or the Company’s estimates of the market opportunities for the product candidates could result in additional impairment charges in future periods. For example, we anticipate the results of the mini B-CHOMP study in the fall of 2016.
15
8. Debt
Loan and Security Agreements
Effective as of October 16, 2015, the Company and Vet Therapeutics, Inc., (the “borrowers”), entered into a Loan and Security Agreement (“Loan Agreement”), with Pacific Western Bank, or Pacific Western, as a collateral agent and Oxford Finance, LLC, (the “Lenders”). The loan is secured by substantially all of the borrowers’ personal property other than intellectual property. The outstanding principal balance under the Loan Agreement was $35,000 under the term loan facility and $5,000 under the revolving facility at June 30, 2016. The interest rate on the term loan and revolving credit facility as of June 30, 2016, was 7.16%. During the three and six months ended June 30, 2016, the Company recognized interest expense of $843 and $1,687, respectively and during the three and six months ended June 30, 2015, the Company recognized interest expense of $219 and $435, respectively.
The Loan Agreement contains customary representations and warranties and customary affirmative and negative covenants, including, among others, limits or restrictions on the borrowers’ ability to incur liens, incur indebtedness, make certain restricted payments, make certain investments, merge, consolidate, make an acquisition, enter into certain licensing arrangements and dispose of certain assets. In addition, the Loan Agreement contains customary events of default that entitle the Lenders to cause the borrowers’ indebtedness under the Loan Agreement to become immediately due and payable. The events of default, some of which are subject to cure periods, include, among others, a non-payment default, a covenant default, the occurrence of a material adverse change, the occurrence of an insolvency, a material judgment default, defaults regarding other indebtedness and certain actions by governmental authorities. Upon the occurrence and for the duration of an event of default, an additional default interest rate equal to 4% per annum will apply to all obligations owed under the Loan Agreement.
The Loan Agreement requires that the Company receive unrestricted net cash proceeds of at least $45,000 from partnering transactions and/or the issuance of equity securities from October 16, 2015 to October 16, 2016. The Loan Agreement also requires that the Company has at least three products fully USDA- or FDA-approved for commercialization by December 31, 2016. With the FDA approval of GALLIPRANT in March 2016 and the receipt of the upfront payment of $45,000 under the Elanco collaboration agreement (Note 10) entered into in April 2016, the Company has met both conditions. Additionally, the Loan Agreement requires that the Company maintain certain minimum liquidity at all times, which as of June 30, 2016 was approximately $20,000. At June 30, 2016, the Company was in compliance with all financial covenants. If the minimum liquidity covenant is not met, the Company may be required to repay the loan prior to scheduled maturity date.
Loans payable as of June 30, 2016, were as follows:
|
Principal amounts |
|||
|
Term loan, 7.16%, due October 16, 2019 |
$ |
35,000 | |
|
Revolving line, 7.16%, due October 16, 2017 |
5,000 | ||
|
Add: accretion of final payment and termination fees |
262 | ||
|
Less: unamortized debt issuance costs |
(313) | ||
|
As of the end of the period, |
$ |
39,949 |
As of June 30, 2016, $2,333 related to the term loan was reclassified as Current portion – loans payable.
9. Accrued Expenses
Accrued expenses consisted of the following:
|
|
||||||
|
|
June 30, 2016 |
December 31, 2015 |
||||
|
Accrued expenses: |
||||||
|
Payroll and related expenses |
$ |
1,376 |
$ |
1,922 | ||
|
Professional fees |
201 | 388 | ||||
|
Royalty expense |
37 | 1 | ||||
|
Interest expense |
239 | 238 | ||||
|
Research and development costs |
444 | 1,111 | ||||
|
Goods received but not invoiced |
1,819 |
— |
||||
|
Milestone |
139 | 500 | ||||
|
Other |
250 | 87 | ||||
|
Total accrued expenses |
$ |
4,505 |
$ |
4,247 | ||
16
10. Agreements
RaQualia Pharma Inc. (“RaQualia”)
On December 27, 2010, the Company entered into two Exclusive License Agreements with RaQualia (the “RaQualia Agreements”) that granted the Company global rights, subject to certain exceptions for injectables in Japan, Korea, China and Taiwan for development and commercialization of licensed animal health products for compounds RQ-00000005 (ENTYCE, also known as AT-002) and RQ-00000007 (GALLIPRANT, also known as AT-001). The Company will be required to pay RaQualia milestone payments associated with GALLIPRANT and ENTYCE of up to $10,000 and $8,500, respectively, upon the Company’s achievement of certain development, regulatory and commercial milestones, as well as mid-single digit royalties on the Company’s or the Company’s sublicensee’s product sales, if any.
The Company achieved milestones totaling $0 and $5,000 which were expensed within research and development expenses during the three and six months ended June 30, 2016, respectively, and included in accounts payable in the consolidated balance sheets. As of June 30, 2016, the Company had not paid any milestone or royalty payments since execution of the RaQualia Agreements.
It is possible that multiple additional milestones related to the RaQualia Agreements are achieved within the next 12 months totaling $6,500.
Elanco
BLONTRESS
On January 2, 2015, the Company was granted a full product license from the USDA for BLONTRESS. The approval resulted in a $3,000 milestone payment being earned and due to the Company per the terms of the Exclusive Commercial License Agreement with Elanco (formerly Novartis Animal Health, Inc.) (the “Elanco Agreement”). During the first quarter of 2015, the Company recognized $3,000 of licensing revenue related to the milestone payment.
On February 24, 2015, the Company and Elanco agreed to terminate the Elanco Agreement. In consideration for the return of the commercial license granted to Elanco, the Company paid Elanco $2,500 in March 2015, and will be required to pay an additional $500 upon the first commercial sale by the Company. At that time the Company determined that it was probable that the $500 payment will be paid, and recorded the $500 as a current liability in the first quarter of 2015. The first commercial sale occurred in March 2016. The Company recorded the $3,000 paid to Elanco as a reduction in revenues received from Elanco as the payment was to re-acquire rights that the Company had previously licensed to Elanco.
On February 25, 2016, the Company and Elanco agreed to amend the terms related to the $500 payment due upon the first commercial sale by the Company. Under the amended terms, upon the first commercial sale, the Company will be required to pay quarterly, a royalty per vial sold until $500 in royalties are paid or the end of two years. After two years, the Company will be required to pay Elanco $500 plus 10% interest, compounded annually against any unpaid balance, less any royalties paid during the two years. If during the two years following the first commercial sale the Company withdraws BLONTRESS from the market and ceases all commercialization, the remaining royalty and related interest are no longer payable. As of June 30, 2016, $355 of the remaining $494 accrued milestone was included in other long-term liabilities in the consolidated balance sheets.
GALLIPRANT
On April 22, 2016, the Company entered into a Collaboration, License, Development and Commercialization Agreement (the “Collaboration Agreement”) with Elanco pursuant to which the Company granted Elanco rights to develop, manufacture, market and commercialize the Company’s products based on licensed grapiprant rights and technology (the “Product”), including GALLIPRANT (grapiprant tablets), an FDA-approved therapeutic for the control of pain and inflammation associated with osteoarthritis in dogs. Pursuant to the Collaboration Agreement, Elanco will have exclusive rights globally outside the United States and co-exclusive rights with the Company in the United States during the term of the Collaboration Agreement.
Under the terms of the Collaboration Agreement, the Company received a non-refundable, non-creditable upfront payment of $45,000. The Company is entitled to a $4,000 milestone payment upon European approval of GALLIPRANT for the treatment of pain and inflammation, another $4,000 payment upon achievement of a development milestone related to the manufacturing of GALLIPRANT, and payments up to $75,000 upon the achievement of certain sales milestones. The sales milestone payments are subject to a one-third reduction for each year the occurrence of the milestone is not achieved beyond December 31, 2021, with any non-occurrence beyond December 31, 2023 cancelling out the applicable milestone payment obligation entirely.
The Collaboration Agreement also provides that Elanco will pay the Company royalty payments on a percentage of net sales in the mid-single to low-double digits. The Company is responsible for all development activities required to obtain the first registration or regulatory approval for the Product for use in dogs in each of the European Union (“the EU Product Registration”) and the United States, and Elanco is responsible for all other development activities. First registration for the Product in the U.S. was achieved before the completion of the Collaboration Agreement. In addition, the Company and Elanco have agreed to pay 25% and 75%, respectively, of all third-party development fees and expenses through December 31, 2018 in connection with preclinical and clinical trials necessary for any additional registration or regulatory approval of the Products, provided that the Company’s contribution to such development fees and expenses is capped at $7,000 (“R&D Cap”). Commencing on the effective date of the Collaboration Agreement,
17
the Company is responsible for the manufacture and supply of all of Elanco’s reasonable requirements of GALLIPRANT under the supply terms agreed upon pursuant to the Collaboration Agreement. However, Elanco retains the ability to assume all or a portion of the manufacturing responsibility during the term of the Collaboration Agreement. The parties have agreed under the Collaboration Agreement to negotiate and enter into a supply agreement formalizing the terms of supply of active product ingredients and/or finished GALLIPRANT by the Company to Elanco.
On April 22, 2016, in connection with the Collaboration Agreement, the Company entered into a Co-Promotion Agreement (the “Co-Promotion Agreement”) with Elanco to co-promote the Product in the United States.
Under the terms of the Co-Promotion Agreement, Elanco has agreed to pay the Company, as a fee for promotional services performed and expenses incurred by the Company under the Co-Promotion Agreement, (i) 25% of the gross margin on net sales of Product sold in the United States under the Collaboration Agreement prior to December 31, 2018 (unless extended by mutual agreement), and (ii) a mid-single digit percentage of net sales of the Product in the United States after December 31, 2018 through 2028 (unless extended by mutual agreement).
The Company concluded that the Collaboration Agreement and Co-Promotion Agreement represent a multiple-element arrangement, and evaluated if deliverables in the arrangement represent separate units of accounting. The Company identified the following deliverables under the agreement: (i) a royalty-bearing, sub-licensable, development, manufacturing and commercialization license; (ii) manufacturing and supply services; (iii) participation in a joint manufacturing subcommittee; and (iv) services associated with obtaining the EU Product Registration. The Company performed an assessment and concluded that the license had stand-alone value from the other undelivered elements in the arrangement. The Company’s best estimate of the selling price for the manufacturing subcommittee and the EU Product Registration services were immaterial and, therefore, no consideration was allocated to these deliverables. Under the manufacturing and supply services terms, Elanco will be obligated to pay for any future orders at a price per unit representative of market value, and, therefore, no upfront consideration was allocated to this deliverable. The Company allocated $38,000 of the $45,000 upfront payment to the license, and recognized $38,000 of licensing and collaboration revenue during the quarter ended June 30, 2016. The Company allocated $7,000 of upfront consideration to the R&D Cap, which was recorded as licensing and collaboration commitment liability in the consolidated balance sheet at June 30, 2016. The Company classified the licensing and collaboration commitment liability as a current liability due to the Company having no control over when R&D Cap expenses will be incurred and the expected timing of R&D Cap expenses being unknown as of June 30, 2016. The licensing and collaboration commitment liability will be reduced in future periods as the related expenses are incurred by Elanco and paid for by the Company. Any remaining balance not paid to Elanco will be recognized as licensing and collaboration revenue on December 31, 2018 when the Company’s obligation to fund 25% of Elanco’s development efforts expires.
The Company evaluated if the sales and other milestones per the Collaboration Agreement are substantive. The Company determined that the milestones are non-substantive, and, therefore, these milestones will be allocated amongst the delivered, and any undelivered elements at the time the milestones are earned. If there are no undelivered elements, the milestone payments will be recognized as revenue in their entirety upon achievement of each milestone. For the three and the six months ended June 30, 2016, no milestones were achieved, and accordingly, no revenues were recognized from the milestones.
AT-006
On May 11, 2016, the Company and Elanco agreed to terminate the Elanco AT-006 Agreement that granted Elanco global rights for development and commercialization of licensed animal health products for an anti-viral for the treatment of feline herpes virus induced ophthalmic conditions. In consideration for the return of the Elanco AT-006 Agreement global rights, the Company is required to pay Elanco a low single-digit royalty on any product sales, if any, up to an amount in the low-single digit millions.
Pacira Pharmaceuticals, Inc. (“Pacira”)
On December 5, 2012, the Company entered into an Exclusive License, Development, and Commercialization Agreement with Pacira (the “Pacira Agreement”) that granted the Company global rights for development and commercialization of licensed animal health products for NOCITA® (also known as AT-003). The Company is required to pay Pacira milestone payments of up to $41,000 upon the Company’s achievement of certain development, regulatory ($1,000) and commercial ($40,000) milestones, as well as tiered royalties on the Company’s product sales, if any. The commercial milestones owed to Pacira under the Pacira Agreement begin to be triggered once NOCITA annual net sales reach $100,000 with the final tier being owed to Pacira once NOCITA annual net sales reach $500,000.
The Company achieved milestones totaling $1,000 and $1,000 which were expensed within research and development expenses during the three and six months ended June 30, 2016, respectively, and included in accounts payable in the consolidated balance sheets. As of June 30, 2016, the Company had paid $500 in milestone payments and no royalty payments since execution of the Pacira Agreement.
It is possible that multiple additional milestones related to the Pacira Agreement are achieved within the next 12 months totaling $1,000.
18
11. Common Stock
As of June 30, 2016, there were 34,798,120 shares of the Company’s common stock outstanding, net of 579,132 shares of unvested restricted common stock.
Sales Agreement
On October 16, 2015, the Company entered into a Sales Agreement with Barclays Capital Inc. (“Barclays”) pursuant to which the Company may sell from time to time, at its option, up to an aggregate of $52,000 of shares of its common stock (the “Shares”) through Barclays, as sales agent. Sales of the Shares, if any, will be made under the Company’s previously filed and currently effective Registration Statement on Form S-3 (Reg. No. 333-197414), by means of ordinary brokers’ transactions on the NASDAQ Global Market or otherwise. Additionally, under the terms of the Sales Agreement, the Shares may be sold at market prices, at negotiated prices or at prices related to the prevailing market price. The Company will pay Barclays a commission of 2.75% of the gross proceeds from the sale of the Shares, if any. As of the date of this filing, the Company has not sold any shares pursuant to the Sales Agreement.
12. Stock-Based Awards
2010 Equity Incentive Plan
Activity related to stock options under the 2010 Equity Incentive Plan (the “2010 Plan”) for the six months ended June 30, 2016, was as follows:
|
|
||||||||||
|
|
Weighted |
|||||||||
|
|
Shares |
Weighted |
Average |
|||||||
|
|
Issuable |
Average |
Remaining |
Aggregate |
||||||
|
|
Under |
Exercise |
Contractual |
Intrinsic |
||||||
|
|
Options |
Price |
Term |
Value |
||||||
|
|
(In Years) |
|||||||||
|
|
||||||||||
|
Outstanding as of December 31, 2015 |
86,490 |
$ |
2.95 | 7.09 |
$ |
228 | ||||
|
Granted |
— |
— |
||||||||
|
Exercised |
(1,500) | 0.40 | ||||||||
|
Forfeited |
— |
— |
||||||||
|
Expired |
— |
— |
||||||||
|
Outstanding as of June 30, 2016 |
84,990 |
$ |
2.99 | 6.59 |
$ |
283 | ||||
As of June 30, 2016, 23,112 shares of common stock granted from early exercised options are unvested and subject to repurchase. For the six months ended June 30, 2016, the total intrinsic value of options exercised was $8 and the total received from stock option exercises was $1.
Activity related to restricted stock under the 2010 Plan for the six months ended June 30, 2016, was as follows:
|
|
|||||
|
|
Weighted |
||||
|
|
Average Grant |
||||
|
|
Shares |
Date Fair Value |
|||
|
Unvested restricted common stock as of December 31, 2015 |
37,078 |
$ |
0.36 | ||
|
Issued |
— |
— |
|||
|
Vested |
(27,129) | 0.37 | |||
|
Forfeited |
— |
— |
|||
|
Unvested restricted common stock as of June 30, 2016 |
9,949 |
$ |
0.32 | ||
For the six months ended June 30, 2016, the total fair value of restricted common stock vested was $134.
19
2013 Incentive Award Plan
Activity related to stock options under the 2013 Incentive Award Plan (the “2013 Plan”) for the six months ended June 30, 2016, was as follows:
|
|
||||||||||
|
|
Weighed |
|||||||||
|
|
Shares |
Weighted |
Average |
|||||||
|
|
Issuable |
Average |
Remaining |
Aggregate |
||||||
|
|
Under |
Exercise |
Contractual |
Intrinsic |
||||||
|
|
Options |
Price |
Term |
Value |
||||||
|
|
(in years) |
|||||||||
|
|
||||||||||
|
Outstanding as of December 31, 2015 |
1,728,199 |
$ |
16.57 | 8.31 |
$ |
— |
||||
|
Granted |
626,600 | 3.62 | ||||||||
|
Exercised |
— |
— |
||||||||
|
Forfeited |
(49,790) | 12.07 | ||||||||
|
Expired |
(9,748) | 10.39 | ||||||||
|
Outstanding as of June 30, 2016 |
2,295,261 |
$ |
13.16 | 8.19 |
$ |
1,735 | ||||
For the six months ended June 30, 2016, the weighted average grant date fair value of stock options granted was $2.42.
Activity related to restricted stock under the 2013 Plan for the six months ended June 30, 2016, was as follows:
|
|
|||||
|
|
|||||
|
|
Weighted |
||||
|
|
Average Grant |
||||
|
|
Shares |
Date Fair Value |
|||
|
Unvested restricted common stock as of December 31, 2015 |
333,263 |
$ |
17.77 | ||
|
Issued |
385,650 | 3.42 | |||
|
Vested |
(157,328) | 13.43 | |||
|
Forfeited |
(15,514) | 9.54 | |||
|
Unvested restricted common stock as of June 30, 2016 |
546,071 |
$ |
9.12 | ||
For the six months ended June 30, 2016, the total fair value of restricted common stock vested was $700. The Company did not receive cash proceeds for any of the restricted common stock issued during the six months ended June 30, 2016.
Stock-Based Compensation
The Company recognizes compensation expense for only the portion of awards that are expected to vest. In developing a forfeiture rate estimate, the Company has considered its historical experience to estimate pre-vesting forfeitures for service-based awards. The impact of a forfeiture rate adjustment will be recognized in full in the period of adjustment, and if the actual forfeiture rate is materially different from the Company’s estimate, the Company may be required to record adjustments to stock-based compensation expense in future periods.
The Company recorded stock-based compensation expense related to stock options and restricted stock as follows:
|
|
||||||||||||
|
|
Three Months Ended |
Six Months Ended |
||||||||||
|
|
June 30, |
June 30, |
||||||||||
|
|
2016 |
2015 |
2016 |
2015 |
||||||||
|
Cost of product sales and inventories |
$ |
19 |
$ |
30 |
$ |
50 |
$ |
67 | ||||
|
Research and development |
264 | 394 | 629 | 999 | ||||||||
|
Selling, general and administrative |
1,913 | 1,605 | 3,770 | 3,323 | ||||||||
|
|
$ |
2,196 |
$ |
2,029 |
$ |
4,449 |
$ |
4,389 | ||||
The Company had an aggregate of $8,822 and $4,011 of unrecognized stock-based compensation expense for options outstanding and restricted stock awards, respectively, as of June 30, 2016, which is expected to be recognized over a weighted average period of 2.18 years and 1.55 years, respectively.
20
13. Net Income (Loss) Per Share
Basic and diluted net income (loss) per share attributable to common stockholders was calculated as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended |
|
Six Months Ended |
||||||||
|
|
|
June 30, |
|
June 30, |
||||||||
|
|
|
2016 |
|
2015 |
|
2016 |
|
2015 |
||||
|
Basic net income (loss) per share attributable to common stockholders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Numerator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
21,196 |
|
$ |
(7,983) |
|
$ |
3,129 |
|
$ |
(16,757) |
|
Net income attributable to participating securities |
|
|
(20) |
|
|
— |
|
|
(3) |
|
|
— |
|
Net income (loss) attributable to common stockholders |
|
$ |
21,176 |
|
$ |
(7,983) |
|
$ |
3,126 |
|
$ |
(16,757) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding – basic |
|
|
34,762,533 |
|
|
34,278,105 |
|
|
34,708,006 |
|
|
34,236,282 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per share attributable to common stockholders – basic |
|
$ |
0.61 |
|
$ |
(0.23) |
|
$ |
0.09 |
|
$ |
(0.49) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted net income (loss) per share attributable to common stockholders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Numerator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
21,196 |
|
$ |
(7,983) |
|
$ |
3,129 |
|
$ |
(16,757) |
|
Net income attributable to participating securities |
|
|
(20) |
|
|
— |
|
|
(3) |
|
|
— |
|
Net income (loss) attributable to common stockholders |
|
$ |
21,176 |
|
$ |
(7,983) |
|
$ |
3,126 |
|
$ |
(16,757) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding – basic |
|
|
34,762,533 |
|
|
34,278,105 |
|
|
34,708,006 |
|
|
34,236,282 |
|
Dilutive effect of outstanding stock awards |
|
|
175,922 |
|
|
— |
|
|
71,780 |
|
|
— |
|
Weighted average shares outstanding – diluted |
|
|
34,938,455 |
|
|
34,278,105 |
|
|
34,779,786 |
|
|
34,236,282 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per share attributable to common stockholders – diluted |
|
$ |
0.61 |
|
$ |
(0.23) |
|
$ |
0.09 |
|
$ |
(0.49) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Company’s participating securities consist of unvested restricted common stock issued from early exercised stock options and restricted common stock awards granted under the 2010 Plan, due to the shares having non-forfeitable dividend rights.
Stock options for the purchase of 1,705,396 and 1,941,521 weighted average shares of common stock and 551,709 and 557,149 of unvested restricted stock awards were excluded from the computation of diluted net income per share attributable to common stockholders for the three and six months ended June 30, 2016, respectively, because those awards had an anti-dilutive impact on the net income attributable to common stockholders.
Stock options for the purchase of 1,671,270 shares of common stock were excluded from the computation of diluted net loss per share attributable to common stockholders for the three and six months ended June 30, 2015, because those options had an anti-dilutive impact due to the net loss attributable to common stockholders incurred for the period.
21
14. Income Taxes
The Company recorded no income tax expense or benefit during the three and six months ended June 30, 2016, compared to an income tax benefit of $360 and $631 during the three and six months ended June 30, 2015, respectively, which was recognized for losses incurred that would reduce the amount of deferred tax liability related to intangible assets. The Company projects a loss before income taxes for 2016 and an annual effective tax rate of zero percent for 2016. The Company’s income for the three and six months ended June 30, 2016, was fully offset by net operating loss carryforwards, and no deferred tax expense was recognized due to a full valuation allowance recognized against its deferred tax assets.
15. Accumulated Other Comprehensive Loss
The changes in accumulated other comprehensive loss, net of their related tax effects, were as follows:
|
|
||||||
|
|
Foreign |
Accumulated |
||||
|
|
Currency |
Other |
||||
|
|
Translation |
Comprehensive |
||||
|
|
Adjustment |
Loss |
||||
|
As of December 31, 2015 |
$ |
(9,320) |
$ |
(9,320) | ||
|
Foreign currency translation adjustments |
473 | 473 | ||||
|
As of June 30, 2016 |
$ |
(8,847) |
$ |
(8,847) | ||
Amounts reclassified from accumulated other comprehensive loss were as follows:
|
|
|||||||||||||
|
|
Amounts Reclassified from |
Amounts Reclassified from |
|||||||||||
|
|
Accumulated Other |
Accumulated Other |
|||||||||||
|
|
Comprehensive Loss |
Comprehensive Loss |
|||||||||||
|
|
Three Months Ended |
Six Months Ended |
|||||||||||
|
|
Location in |
June 30, |
June 30, |
||||||||||
|
|
Statements of Operations |
2016 |
2015 |
2016 |
2015 |
||||||||
|
Gain on sale of securities available-for-sale |
Other income (expense) |
$ |
— |
$ |
2,864 |
$ |
— |
$ |
3,874 | ||||
|
|
$ |
— |
$ |
2,864 |
$ |
— |
$ |
3,874 | |||||
16. Variable Interest Entity
ViroVet BVBA (“ViroVet”)
During the third quarter of 2015, the Company reviewed certain operations of its wholly owned subsidiary, Aratana Therapeutics NV (“Aratana NV”). As a result, the Company made a strategic decision to wind down pre-clinical discovery efforts being performed at Aratana NV and focus future efforts of Aratana NV on clinical assets, the development of core legacy programs, i.e. AT-001, AT-002 and AT-003 for EU approval and business development and monetization of production animal assets and know-how obtained in the acquisition of Okapi Sciences. To facilitate this reorganization, the Company, via Aratana NV, along with the former General Manager of Aratana NV, the current General Manager of Aratana NV and a consultant to the Company, formed ViroVet BVBA. During the third quarter of 2015 the Company began to transition some of its employees from Aratana NV to ViroVet. The Company plans to transition selected Aratana NV employees, assets and liabilities throughout 2016 to ViroVet to further pursue the research and development of production animal products. These employees will be focused on the advancement of production animal assets/know-how and the securing of additional funding for future operations.
Except for the financing matters described below, the Company will have little to no involvement in the operations of ViroVet.
22
Equity Investment
In July 2015, the Company paid $2 and committed another $4 for 28% ownership interest in ViroVet. The Company has no further obligation to provide any further capital.
Convertible Loan Agreement
On September 11, 2015, Aratana NV and ViroVet executed a convertible loan agreement in which Aratana NV agreed to loan ViroVet $335 (€300) on September 15, 2015. The proceeds from the loan require ViroVet to use the monies towards the development and operations of ViroVet in accordance with the budget prepared by ViroVet. The loan bears annual interest of 7% and is unsecured.
Primary Beneficiary
The Company determined ViroVet was a VIE and it had a controlling financial interest in ViroVet due to the Company having the power to direct the activities of ViroVet that most significantly impact ViroVet’s economic performance and having the obligation to absorb losses or receive benefits. The Company will continue to consolidate ViroVet unless a reconsideration event occurs, for example, an equity financing.
Total assets and liabilities of the Company’s consolidated VIE were not material as of June 30, 2016.
For the three and six months ended June 30, 2016, ViroVet’s net loss and non-controlling interest were not material and are included in the Company’s consolidated statement of operations. Creditors in ViroVet only have recourse to the assets owned by the VIE and not to the Company’s general credit. The Company currently does not have implicit support arrangements with ViroVet.
23
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and the related notes and other financial information included elsewhere in this Quarterly Report on Form 10-Q. Some of the statements contained in this discussion and analysis or set forth elsewhere in this Quarterly Report on Form 10-Q that are not statements of historical fact are forward-looking statements within the meaning of the Private Securities Litigation Reform Act. In this Quarterly Report on Form 10-Q, the words “anticipates,” “believes,” “expects,” “intends,” “future,” “could,” “estimates,” “plans,” “would,” “should,” “potential,” “continues” and similar words or expressions (as well as other words or expressions referencing future events, conditions or circumstances) identify forward-looking statements. The forward-looking statements herein include without limitation, statements with respect to our plans and strategy for our business, anticipated timing of regulatory submissions and approvals, anticipated timing of availability and announcement of study results, anticipated timing of launch and commercialization of product candidates, beliefs regarding market opportunities for our products and product candidates; and anticipated milestone payments. These and other forward-looking statements included in this Quarterly Report on Form 10-Q involve risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to: our history of operating losses and our expectation that we will continue to incur losses for the foreseeable future; failure to obtain sufficient capital to fund our operations; risks relating to the impairment of intangible assets AT-004, AT-005, AT-007 and AT-011; unstable market and economic conditions; restrictions on our financial flexibility due to the terms of our credit facility; our substantial dependence upon the success of our product candidates; development of our biologic product candidates is dependent upon relatively novel technologies and uncertain regulatory pathways, and biologics may not be commercially viable; denial or delay of regulatory approval for our existing or future product candidates; failure of our product candidates that receive regulatory approval to obtain market approval or achieve commercial success; failure to realize anticipated benefits of our acquisitions and difficulties associated with integrating the acquired businesses; development of pet therapeutics is a lengthy and expensive process with an uncertain outcome; competition in the pet therapeutics market, including from generic alternatives to our product candidates, and failure to compete effectively; failure to identify, license or acquire, develop and commercialize additional product candidates; failure to attract and retain senior management and key scientific personnel; our reliance on third-party manufacturers, suppliers and collaborators; regulatory restrictions on the marketing of our product candidates; our small commercial sales organization, and any failure to create a sales force or collaborate with third-parties to commercialize our product candidates; difficulties in managing the growth of our company; significant costs of being a public company; risks related to the restatement of our financial statements for the year ended December 31, 2013, and the identification of a material weakness in our internal control over financial reporting; changes in distribution channels for pet therapeutics; consolidation of our veterinarian customers; limitations on our ability to use our net operating loss carryforwards; impacts of generic products; safety or efficacy concerns with respect to our product candidates; effects of system failures or security breaches; failure to obtain ownership of issued patents covering our product candidates or failure to prosecute or enforce licensed patents; failure to comply with our obligations under our license agreements; effects of patent or other intellectual property lawsuits; failure to protect our intellectual property; changing patent laws and regulations; non-compliance with any legal or regulatory requirements; litigation resulting from the misuse of our confidential information; the uncertainty of the regulatory approval process and the costs associated with government regulation of our product candidates; failure to obtain regulatory approvals in foreign jurisdictions; effects of legislative or regulatory reform with respect to pet therapeutics; the volatility of the price of our common stock; our status as an emerging growth company, which could make our common stock less attractive to investors; dilution of our common stock as a result of future financings; the influence of certain significant stockholders over our business; and provisions in our charter documents and under Delaware law could delay or prevent a change in control. These and other important factors discussed under the caption “Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended December 31, 2015, filed with the Securities and Exchange Commission (the “SEC”) on March 15, 2016, and the “Risk Factors” section of this Quarterly Report on Form 10-Q, could cause actual results to differ materially from those indicated by the forward-looking statements made in this Quarterly Report on Form 10-Q.
Overview
We are a pet therapeutics company focused on licensing, developing and commercializing innovative biopharmaceutical products for companion animals. We operate in one business segment: pet therapeutics, which sits at the intersection of the more than $60 billion annual U.S. pet market, and more than $23 billion annual worldwide animal health market. Our current product portfolio includes multiple therapeutic products and product candidates in development consisting of small molecule pharmaceuticals and large molecule biologics that target large opportunities in serious medical conditions in pets.
Our lead products and product candidates in development include small molecules directed at treating osteoarthritis pain and inflammation (GALLIPRANT®), appetite stimulation (ENTYCE®) and post-operative pain (NOCITA®).
We have incurred significant net losses since our inception. These losses have resulted principally from costs incurred in connection with in-licensing our product candidates, research and development activities and selling, general and administrative costs associated with our operations. As of June 30, 2016, we had a deficit accumulated since inception of $148.9 million, and cash, cash equivalents and short-term investments of $109.5 million.
24
We expect to continue to incur operating losses as we work to commercialize our products and develop and commercialize our product candidates. As a result, we expect to seek to fund our operations through corporate collaborations and licensing arrangements, as well as public or private equity offerings or further debt financings. We cannot assure you that such funds will be available on terms favorable to us, if at all. Arrangements with collaborators or others may require us to relinquish rights to certain of our technologies or product candidates. In addition, there is a risk that we may never successfully complete development of our product candidates, obtain adequate patent protection for our technology, obtain necessary regulatory approval for our product candidates or achieve commercial viability for any approved product candidates. If we are not able to raise additional capital on terms acceptable to us, or at all, as and when needed, we may be required to curtail our operations which could include delaying the commercial launch of our products, discontinuing product development programs, or granting rights to develop and market products or product candidates that we would otherwise prefer to develop and market ourselves. As disclosed in Note 8 to the consolidated financial statements, we have a term loan and a revolving credit facility with an aggregate principal balance of $40.0 million as of June 30, 2016. The terms of this agreement require us to receive unrestricted net cash proceeds of at least $45.0 million from partnering transactions and/or the issuance of equity securities from October 16, 2015 to October 16, 2016. The loan agreement also requires that we have at least three products fully United States Department of Agriculture (“USDA”) or U.S. Food and Drug Administration (“FDA”)-approved for commercialization by December 31, 2016. With the FDA approval of GALLIPRANT in March 2016 and the receipt of the upfront payment of $45.0 million under our agreement with Elanco Animal Health, Inc., a division of Eli Lilly & Co. (“Elanco”) entered into in April 2016, we have met both conditions. Additionally, the loan agreement requires that we maintain certain minimum liquidity at all times, which as of June 30, 2016, was approximately $20.0 million. As of the date of the filing of this Quarterly Report on Form 10-Q, we believe that our existing cash, cash equivalents and short-term investments of $109.5 million, will allow us to fund our operations, at least through June 30, 2017.
Business Updates
During the six months ended June 30, 2016, we continued to make progress towards our objective of becoming a fully integrated and commercial-stage company in 2016. During the second quarter of 2016, we received our second FDA approval, filed for our third FDA approval, entered into a collaboration, license, development and commercialization agreement and a co-promotion agreement with Elanco, continued to progress on the manufacturing of commercial supply for upcoming product launches and prepare ourselves to have a commercial presence in the pet therapeutic market.
Research and Development
The following tables identify the most advanced product candidates being developed under the FDA’s Center for Veterinary Medicine (“CVM”) regulations and their current regulatory status:
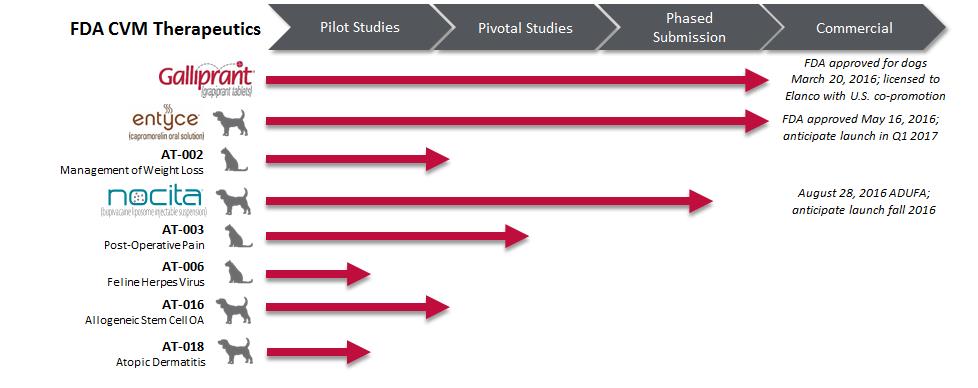
25
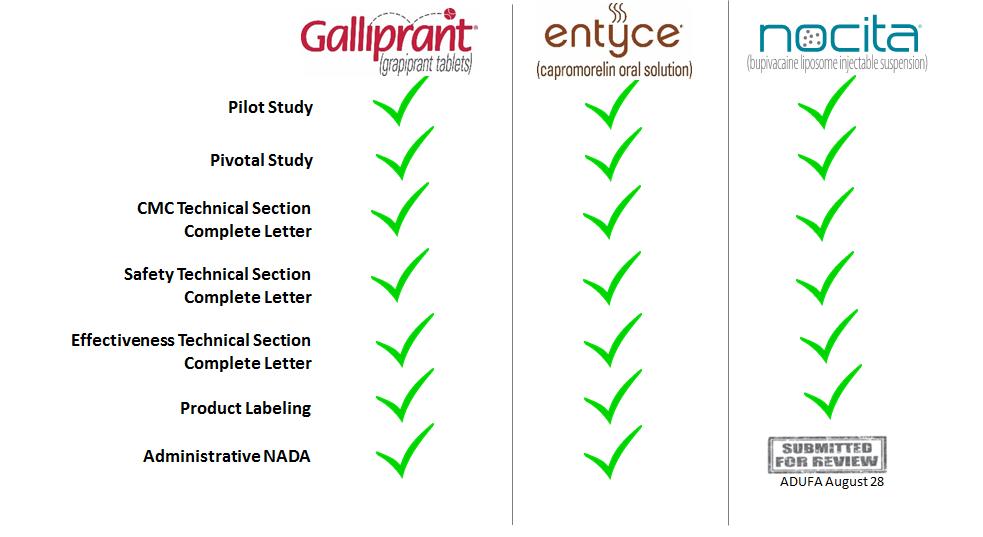
GALLIPRANT
Collaboration, License, Development and Commercialization Agreement
On April 22, 2016, we entered into a Collaboration, License, Development and Commercialization Agreement (the “Collaboration Agreement”) with Elanco that granted Elanco rights to develop, manufacture and commercialize our products based upon the licensed grapiprant rights and technology (the “Product”), including GALLIPRANT (grapiprant tablets), an FDA-approved therapeutic for the control of pain and inflammation associated with osteoarthritis in dogs. Elanco will have exclusive rights globally outside the United States and co-exclusive rights with us in the United States during the term of the Collaboration Agreement.
During the second quarter of 2016, Elanco paid us an upfront payment of $45.0 million. Elanco has also agreed to pay us a $4.0 million milestone payment upon European approval of GALLIPRANT for the treatment of pain and inflammation, a $4.0 million payment upon achievement of a development milestone related to the manufacturing of GALLIPRANT and payments up to $75.0 million upon the achievement of certain sales milestones. The sales milestone payments are subject to a one-third reduction for each year the occurrence of the milestone is not achieved beyond December 31, 2021, with any non-occurrence beyond December 31, 2023, cancelling out the applicable milestone payment obligation entirely. All milestone payments are non-refundable.
Elanco also has agreed to pay us royalty payments on a percentage of net sales in the mid-single to low-double digits. We are responsible for all development activities required to obtain the first registration for the product for use in dogs in each of the European Union and the United States, and Elanco is responsible for all other development activities. In addition, we and Elanco have agreed to pay 25% and 75%, respectively, of all third-party development fees and expenses through December 31, 2018, in connection with preclinical and clinical trials necessary for any additional registration or regulatory approval of the products, provided that our contribution to such development fees and expenses is capped at $7.0 million.
The term of the collaboration will continue throughout the development and commercialization of the product candidates, on a Product-by-Product and country-by-country basis, until the latest of (i) the date on which no valid claim of certain issued or granted patents specified in the Collaboration Agreement in the respective country exists, (ii) the expiration of any regulatory exclusivity in such country covering such Product, and (iii) the tenth anniversary of the first commercial sale of such product in such country.
The Collaboration Agreement may be terminated by Elanco at any time upon 90 days’ written notice to us. The Collaboration Agreement may also be terminated by either party (i) for the other party’s material breach, where such breach is not cured within the timeframe specified by the agreement, (ii) upon the bankruptcy, insolvency or dissolution of the other party, or (iii) for certain activities involving the challenge of certain patents licensed by us to Elanco. Upon Elanco’s voluntary termination or termination for Elanco’s breach, among other things, (a) all licenses and rights granted to Elanco will terminate and revert to us, and (b) Elanco has agreed to assign to us all registrations and trademarks obtained in connection with the Product. Upon termination for our breach, among other things, Elanco may elect to retain its rights to the licenses granted by us under the Collaboration Agreement subject to specified payment obligations.
26
Co-Promotion Agreement
On April 22, 2016, in connection with the Collaboration Agreement, we entered into a Co-Promotion Agreement (“Co-Promotion Agreement”) with Elanco to co-promote the Product in the United States.
Under the terms of the Co-Promotion Agreement, Elanco has agreed to pay us, as a fee for services performed and expenses incurred by us under the Co-Promotion Agreement, (i) 25% of the gross margin on net sales of Product sold in the United States under the Collaboration Agreement prior to December 31, 2018 (unless extended by mutual agreement), and (ii) a mid-single digit percentage of net sales of the Product in the United States after December 31, 2018 through 2028 (unless extended by mutual agreement).
The Co-Promotion Agreement expires on December 31, 2028, unless extended by mutual agreement. In addition, the Co-Promotion Agreement provides that it will automatically terminate if the Collaboration Agreement is terminated early.
ENTYCE
On May 16, 2016, the FDA’s CVM approved ENTYCE (capromorelin oral solution) for appetite stimulation in dogs. We intend to commercially launch ENTYCE in the United States in the first quarter of 2017 in conjunction with the North American Veterinary Conference and other major veterinary conferences.
NOCITA
In June 2016, we received the final remaining major technical section complete letter for Chemistry, Manufacturing and Controls (“CMC”) from the CVM for NOCITA (bupivacaine liposome injectable suspension) for post-operative pain management in dogs undergoing knee surgery and filed an administrative new animal drug application (“NADA”) with the CVM for NOCITA as a local post-operative analgesia for cranial cruciate ligament surgery in dogs. The Animal Drug User Fee Act (“ADUFA”) date for approval has been set for August 28, 2016. If approved, we expect to commence commercialization of NOCITA in the United States in the fall of 2016.
AT-002 for cats
During the second quarter of 2016, we received concurrence from the FDA on the protocol for a pivotal field effectiveness study. We anticipate initiating this pivotal study in late 2016 with a cat specific formulation.
AT-003 for cats
In July 2016, we initiated the pivotal field effectiveness study for post-operative pain management in cats under a FDA concurred protocol.
AT-006 for cats
During 2014, we completed a field study of AT-006 as an anti-viral for the treatment of feline herpes virus induced ophthalmic conditions. We have subsequently been working on a refined formulation to meet the regulatory standards for both the U.S. and Europe and exploring how to move the product candidate into a pivotal field effectiveness study. We had been collaborating and sharing the cost of developing AT-006 with Elanco but on May 11, 2016, we and Elanco agreed to terminate the Exclusive License, Development, and Commercialization Agreement that granted Elanco global rights for development and commercialization of licensed animal health products for AT-006. In consideration for the return of the global rights for AT-006, we will be required to pay Elanco a low single-digit royalty on any product sales, if any, up to an amount in the low-single digit millions. We now fully control the global rights to AT-006, and we are planning to conduct a pilot study and eventually move forward with pivotal work that we anticipate will enable us to submit for regulatory approval for the product candidate.
AT-016 for dogs
As of April 2016, our exclusive license partner responsible for development, VetStem Biopharma, Inc. (“VetStem”), received concurrence from the FDA on the protocol for a pivotal field effectiveness and safety study. We anticipate VetStem will initiate the pivotal field effectiveness study for dogs with pain associated with osteoarthritis in 2016.
AT-018 for dogs
At the end of the first quarter of 2016, we closed enrollment of a multi-center, masked, placebo-controlled, randomized pilot field study in client-owned dogs with atopic dermatitis. We and our license partner, Atopix Therapies, Ltd, expect high-level results in the third quarter of 2016. In July 2016, we met with the FDA to discuss the regulatory path forward, and in preparation for the pivotal program, we intend to initiate an additional pilot field study in 2016.
27
Products in our pipeline regulated by the USDA are:
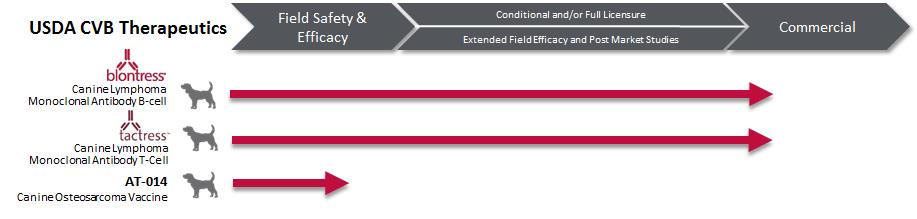
BLONTRESS and TACTRESS
We anticipate the results of the BLONTRESS post-approval study, mini B-CHOMP, in the fall of 2016. In the third quarter of 2015, our interim analysis of clinical results indicated that TACTRESS did not seem to be adding significant progression free survival in canine T-cell lymphoma; those results were confirmed in the final study results in July 2016.
AT-014 for dogs
During the second quarter of 2016, we initiated a pivotal field safety study for AT-014, a potential canine osteosarcoma vaccine that we licensed from Advaxis, Inc. We anticipate conditional licensure in late-2016 or shortly thereafter.
Manufacturing and Supply Chain
During the second quarter of 2016, we continued to transfer the manufacturing technology processes for GALLIPRANT and ENTYCE to our identified active pharmaceutical ingredient (“API”) and formulated product contract manufacturers to provide commercial supplies.
GALLIPRANT
We continue to complete the required manufacturing validation work in regards to API, formulated product and packaging to produce launch inventories. We also continue to work with an API manufacturer towards the achievement of the manufacturing milestone under the Collaboration Agreement.
ENTYCE
We intend to make filings to the FDA in the third quarter of 2016, in support of the transfer of API and formulated contract manufacturing to the respective contract manufacturing organizations to be used for the production of launch inventories. FDA approval of these filings are required for product launch.
Sales and Marketing
To prepare for the expected commercial introduction of our products, we are building our commercial organization to target companion animal veterinarians in the U.S. who typically act as not only the prescriber of product, but as the pharmacist as well. In June, we announced we appointed a Chief Operating Officer, a newly created role to emphasize our commitment to sales operations and distribution. In June and July 2016, we also hired National Account Managers and Regional Sales Leaders.
With the sales leadership team and the support of approximately two dozen therapeutic specialists covering key territories throughout the U.S, we aim to meet the needs of both general practitioners and specialists who we believe want to provide innovative veterinary products to their clients. In achieving our objectives, we believe our go-to-market strategy should be based on the competitive landscape, as well as the adoption and relevance to general practitioners or specialists on a product-by-product basis.
28
According to independent market research commissioned by us, there are approximately 25,000 veterinary clinics in the U.S. The research indicates that to target general practitioners with products like flea and tick medicine or therapies for osteoarthritis pain, a company would need to call on approximately 10,000 clinics to cover 50 percent of the revenue opportunity, whereas for highly specialized products like oncology, a company can expect to cover 60-70 percent of the revenue opportunity in approximately 100-200 clinics across the U.S. Therefore, we have evaluated and will continue to assess our approved, licensed products and late-stage pipeline product candidates to map the relevance to specialists, how likely general practitioners are to adopt the product or product candidate, as well as taking into account the competitive landscape to define the sales strategy. Our strategy is intended to leverage a combination of direct sales, distribution, co-promotion agreements or contract selling agreements, as well as direct sales to corporate customers, such as the approximately 1,500 locations of the two largest corporate hospitals, and an eCommerce platform for veterinarians to purchase products directly from us.
Specifically, the commercial strategy for GALLIPRANT is a global strategic collaboration with Elanco in order to access its large sales organization. We have chosen this approach because the therapeutic is more likely to be highly relevant to general practitioners and is in a competitive category. GALLIPRANT may also be used by specialists as part of a multi-modal approach in treating osteoarthritis pain, but we believe many of those cases are referred back to the general practitioner. In contrast, ENTYCE is highly relevant to specialists as a tool for treating inappetence while the veterinarian diagnoses and addresses the underlying acute or chronic condition. We believe ENTYCE is likely to be adopted by general practitioners especially for acute cases since there are no FDA-approved products in veterinary medicine and it is in what we believe to be an important market opportunity. Therefore, our strategy for ENTYCE will likely utilize direct sales with approximately two dozen therapeutic specialists covering key territories throughout the U.S., as well as national and regional veterinary distributors that cover approximately 90 percent of veterinary hospitals and have as many as 300 field representatives per distributor. We believe NOCITA will primarily be adopted by specialists, such as surgeons, but eventually may be used by general practitioners. Thus, we currently plan on a direct sales approach for NOCITA to educate veterinary specialists on how the long-acting analgesia fits into the multi-modal approach, which may be complemented through contract selling. BLONTRESS®, TACTRESS™ and AT-014 are likely to be best suited for specialists and we therefore believe direct sales is most effective. For therapeutics in the pipeline, we believe the go-to-market strategy for AT-016 will be similar to NOCITA and AT-018 is anticipated to be similar to ENTYCE.
As we introduce our lead therapeutics, we will continue to measure the success of our commercialization efforts and evaluate, on a product-by-product basis, how to continue to best to target general practitioners and/or specialists.
Recent Developments
Properties
Effective July 1, 2016, we renewed our lease of approximately 6,600 square feet of office, laboratory and manufacturing space in San Diego, California, that now expires on June 30, 2021.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of our consolidated financial statements and related disclosures requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, and revenues, costs and expenses and related disclosures during the reporting periods. On an ongoing basis, we evaluate our estimates and judgments. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
There have been no material changes to our critical accounting policies through June 30, 2016, from those discussed in our Annual Report on Form 10-K for the fiscal year ended December 31, 2015, filed with the SEC on March 15, 2016.
29
Results of Operations
Comparison of the Three and Six Months Ended June 30, 2016 and 2015
|
|
||||||||||||||||||
|
|
||||||||||||||||||
|
|
Three Months Ended |
Six Months Ended |
||||||||||||||||
|
|
June 30, |
June 30, |
||||||||||||||||
|
|
2016 |
2015 |
% Change |
2016 |
2015 |
% Change |
||||||||||||
|
|
(Dollars in thousands) |
(Dollars in thousands) |
||||||||||||||||
|
Revenues: |
||||||||||||||||||
|
Licensing and collaboration revenue |
$ |
38,000 |
$ |
— |
NA |
$ |
38,151 |
$ |
— |
NA |
||||||||
|
Product sales |
47 | 230 | (79.6) |
% |
68 | 386 | (82.4) |
% |
||||||||||
|
Total revenues |
38,047 | 230 |
>100.0 |
% |
38,219 | 386 |
>100.0 |
% |
||||||||||
|
Costs and expenses: |
||||||||||||||||||
|
Cost of product sales |
1,741 | 109 |
>100.0 |
% |
1,760 | 219 |
>100.0 |
% |
||||||||||
|
Royalty expense |
20 | 23 | (13.0) |
% |
38 | 43 | (11.6) |
% |
||||||||||
|
Research and development |
5,303 | 6,081 | (12.8) |
% |
16,052 | 12,302 | 30.5 |
% |
||||||||||
|
Selling, general and administrative |
6,148 | 4,879 | 26.0 |
% |
12,699 | 9,064 | 40.1 |
% |
||||||||||
|
Amortization of acquired intangible assets |
95 | 483 | (80.3) |
% |
190 | 966 | (80.3) |
% |
||||||||||
|
Impairment of acquired intangible assets |
2,780 |
— |
NA |
2,780 |
— |
NA |
||||||||||||
|
Other income (expense): |
||||||||||||||||||
|
Interest income |
83 | 43 | 93.0 |
% |
160 | 114 | 40.4 |
% |
||||||||||
|
Interest expense |
(846) | (217) |
>100.0 |
% |
(1,695) | (435) |
>100.0 |
% |
||||||||||
|
Other income (expense), net |
(1) | 3,176 |
<(100.0) |
% |
(36) | 5,141 |
<(100.0) |
% |
||||||||||
|
Income tax benefit |
$ |
— |
$ |
360 | (100.0) |
% |
$ |
— |
$ |
631 | (100.0) |
% |
||||||
Revenues
During the three and six months ended June 30, 2016, total revenues increased by $37.8 million, as compared to the corresponding 2015 periods. These increases were primarily due to an increase of $38.0 million in licensing and collaboration revenue related to the Collaboration Agreement, partially offset by a decrease of $0.2 million and $0.3 million, for the three and six months ended June 30, 2016, respectively, in product sales. During the three and six months ended June 30, 2016, product sales consisted of sales of BLONTRESS and TACTRESS. During the three and six months ended June 30, 2015, we recorded $0.2 million and $0.4 million, respectively, in product sales of TACTRESS, which was granted conditional approval in early 2014. We believe that BLONTRESS and TACTRESS product sales will continue to be modest for 2016, and product sales from other products will depend on the timing of their commercial launch. Any future licensing and collaboration revenue for 2016 will be substantially dependent on Elanco’s ability to successfully commercialize GALLIPRANT in accordance with the Collaboration Agreement.
Cost of product sales
During the three and the six months ended June 30, 2016, cost of product sales increased by $1,632 and $1,541, respectively, as compared to the corresponding 2015 periods. The increases were primarily due to inventory valuation adjustment losses in the amount $1,552 from the write off of TACTRESS inventories and pre-launch GALLIPRANT inventories marked to market due to terms agreed upon in the Collaboration Agreement.
Research and development
|
|
||||||||||||||||||
|
|
||||||||||||||||||
|
|
Three Months Ended |
Six Months Ended |
||||||||||||||||
|
|
June 30, |
June 30, |
||||||||||||||||
|
|
2016 |
2015 |
% Change |
2016 |
2015 |
% Change |
||||||||||||
|
|
(Dollars in thousands) |
(Dollars in thousands) |
||||||||||||||||
|
|
||||||||||||||||||
|
Contracted development costs |
$ |
2,735 |
$ |
3,940 | (30.6) |
% |
$ |
6,788 |
$ |
8,102 | (16.2) |
% |
||||||
|
Milestones |
1,200 |
— |
NA |
6,200 |
— |
NA |
||||||||||||
|
Personnel costs |
1,181 | 1,704 | (30.7) |
% |
2,677 | 3,416 | (21.6) |
% |
||||||||||
|
Other costs |
187 | 437 | (57.2) |
% |
387 | 784 | (50.6) |
% |
||||||||||
|
Total research and development |
$ |
5,303 |
$ |
6,081 | (12.8) |
% |
$ |
16,052 |
$ |
12,302 | 30.5 |
% |
||||||
30
During the three and six months ended June 30, 2016, research and development expense decreased by $0.8 million and increased by $3.8 million, respectively, as compared to the corresponding 2015 periods. The decrease for the three months ended June 30, 2016, was due to a decrease of $1.2 million in contracted development costs as a result of the completion of several clinical studies for our lead programs, $0.3 million decrease in licensing fees related to option programs, and $0.5 million decrease in personnel costs relating to a lower headcount in 2016, partially offset by an increase of $1.2 million in milestones due to achievement of ENTYCE, NOCITA and AT-016 milestones. The increase for the six months ended June 30, 2016, was primarily due to achievement of $6.2 million in milestones relating to GALLIPRANT, ENTYCE, NOCITA, and AT-016. This increase was partially offset by a decrease of $0.7 million in personnel costs relating to a lower headcount in the 2016 six month period, a decrease of $1.3 million in contracted development costs as a result of the completion of several clinical studies for our lead programs, and a $0.4 million decrease in other costs related to supporting multiple facilities.
In addition, based on our development plans as of June 30, 2016, we have committed to make potential future milestone payments to third parties of up to approximately $113.4 million as part of our various collaborations, including licensing and development programs. Payments under these agreements generally become due and payable only upon achievement of certain development, regulatory or commercial milestones. Of the $113.4 million, $80.4 million are commercial milestones. Of the $80.4 million, we anticipate paying $6.0 million in the next 12 months, up to an additional $10.0 million in the next five years as a series of first commercial sales milestones for AT-001 and AT-002 if those products are launched in the U.S. and Europe, and the remainder if certain net revenue thresholds are achieved thereafter. Of the remaining $33.0 million, we anticipate paying an additional $7.8 million during the next 12 months, provided various development and regulatory milestones are achieved. The $33.0 million in milestones is spread across AT-001, AT-002, AT-003, AT-014, AT-016, AT-017, AT-018, and other pre-development candidates. Amounts related to contingent milestone payments are not considered contractual obligations as they are contingent on the successful achievement of certain development, regulatory approval and commercial milestones.
In 2016, we expect to incur research and development expenses related to technology transfer to contract manufacturing suppliers for commercial supply for our lead programs and regulatory expenses related to product filings.
We expect to fund our research and development expenses from our cash, cash equivalents and short-term investments and any future collaboration or financing arrangements. We cannot forecast with any degree of certainty which product candidates may be subject to future collaborations or contracts, when such arrangements will be secured, if at all, and to what degree such arrangements would affect our development plans and capital requirements.
Selling, general and administrative
During the three and six months ended June 30, 2016, selling, general and administrative expense increased by $1.3 million and $3.6 million, respectively, as compared to the corresponding 2015 periods. For the three months and six months ended June 30, 2016, these increases were due to increases of $0.6 and $1.0 million, respectively, in personnel expenses primarily as a result of higher sales and marketing headcount, and $0.7 million and $1.4 million increases, respectively, in expenses incurred in preparation for the commercialization of product candidates for which we have received or anticipate regulatory approval in 2016. The increases in the commercialization expenses were primarily due to product branding, sponsorship expenses in conjunction with our participation in several key veterinary conferences to build awareness of Aratana and our product candidates, and expenses related to corporate technology and infrastructure. For the six months ended June 30, 2016, the $3.6 million increase in selling, general and administrative expenses was also due to a credit of $1.2 million recorded in the first quarter of 2015 to reduce the fair value of the contingent consideration to zero, which had originally been due under the Vet Therapeutics, Inc. merger agreement.
We expect selling, general and administrative expense to continue to increase as we build out our sales organization and corporate infrastructure in support of the expected commercialization of GALLIPRANT, ENTYCE, NOCITA, AT-014, and other development programs.
Royalty expense
During the three and six months ended June 30, 2016, royalty expense decreased by $3,000 and $5,000, respectively, as compared to the corresponding 2015 periods. The decreases in both the three and six month periods were primarily a result of the decreased sales of TACTRESS.
Amortization of acquired intangible assets
During the three and six months ended June 30, 2016, amortization of acquired intangible assets decreased by $0.4 million and $0.8 million, respectively, as compared to the corresponding 2015 periods. The decreases in both the three and six month periods reflect the impact of the impairment of BLONTRESS and TACTRESS in 2015. The lower carrying value due to the impairment charge resulted in the lower expense being recognized. Lower carrying values due to the 2016 TACTRESS impairment charge will also result in lower amortization of acquired intangible assets for the impaired intangibles in future periods.
31
Impairment of acquired intangible assets
During both the three and six months ended June 30, 2016, impairment of acquired intangible assets increased by $2.8 million, as compared to the corresponding 2015 periods. The impairment of acquired intangible assets in 2016 was related to impairment charges of TACTRESS ($0.6 million) and AT-007 ($2.2 million). The impairment charge related to TACTRESS resulted from updated sales expectations and resulted in a carrying value of $0 for TACTRESS. The impairment charge related to AT-007 was the result of our decision to discontinue the development of AT-007 due to the return of global rights of AT-006 and ensuing development program portfolio prioritization, including consideration of our focus on commercial launch activities to support our recently approved products, and resulted in a carrying value of $0 for AT-007. For more information regarding the impairment charges, see Note 7 to the consolidated financial statements. Unfavorable outcomes of our development activities or our estimates of the market opportunities for the product candidates could result in additional impairment charges in future periods. For example, we anticipate the results of the mini B-CHOMP study in the fall of 2016.
Interest income
During the three and six months ended June 30, 2016, interest income increased by $40,000 and $46,000, respectively, as compared to the corresponding 2015 periods. The increases primarily related to interest earned at higher interest rates and on higher deposits held at Square 1 Bank and short-term investments in reverse repurchase agreements.
Interest expense
During the three and six months ended June 30, 2016, interest expense increased by $0.6 million and $1.3 million, respectively, as compared to the corresponding 2015 periods. These increases were due to interest expense related to our Loan Agreement, as discussed below in “Financial Condition, Liquidity and Capital Resources – Indebtedness”, which was entered into during October 2015. During the three and six months ended June 30, 2016, accretion of the debt discount and amortization of deferred financing costs totaled $0.1 and $0.2 million, respectively, which is non-cash interest included in our interest expense above.
Other income (expense), net
During the three and six months ended June 30, 2016, other income (expense), net decreased by $3.2 million and $5.2 million, as compared to the corresponding 2015 periods. The decrease during the three month period was primarily due to the $2.5 million gain on the sale of Advaxis stock and $0.6 million gain related to the increase in fair value of the Advaxis warrant and subsequent sale of Advaxis common stock that occurred in the corresponding period in 2015. The decrease during the six month period was primarily related to a $3.5 million gain on the sale of Advaxis stock, a $1.3 million increase in the fair value of the Advaxis warrant, and a $0.3 million gain on the sale of shares received from the exercise of the Advaxis warrant that occurred in the corresponding period in 2015.
Income tax benefit
During the three and six months ended June 30, 2016, income tax benefit decreased by $0.4 million and $0.6 million, respectively, as compared to the corresponding 2015 periods. The tax benefit in 2015 was recognized for losses incurred that would reduce the amount of deferred tax liability related to intangible assets. We project a loss before income taxes in 2016 and an annual effective tax rate of zero percent for 2016. Our income for the three and six months ended June 30, 2016, was fully offset by net operating loss carryforwards, and no deferred tax expense was recognized in 2016 due to a full valuation allowance recognized against our deferred tax assets.
32
Financial Condition, Liquidity and Capital Resources
Our financial condition is summarized as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
June 30, 2016 |
|
December 31, 2015 |
|
Change % |
|||
|
|
|
(Dollars in thousands) |
|
|
|
||||
|
Financial assets: |
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
108,759 |
|
$ |
26,755 |
|
>100.0 |
% |
|
Marketable securities - short-term |
|
|
747 |
|
|
747 |
|
— |
% |
|
Reverse repurchase agreements |
|
|
— |
|
|
58,700 |
|
(100.0) |
% |
|
Total cash, cash equivalents, marketable securities and reverse repurchase agreements |
|
$ |
109,506 |
|
$ |
86,202 |
|
27.0 |
% |
|
Borrowings: |
|
|
|
|
|
|
|
|
|
|
Loans payable, net |
|
$ |
39,949 |
|
$ |
39,710 |
|
0.6 |
% |
|
Working capital: |
|
|
|
|
|
|
|
|
|
|
Current assets |
|
$ |
113,657 |
|
$ |
89,019 |
|
27.7 |
% |
|
Current liabilities |
|
|
21,121 |
|
|
5,684 |
|
>100.0 |
% |
|
Total working capital |
|
$ |
92,536 |
|
$ |
83,335 |
|
11.0 |
% |
We have incurred significant net losses since our inception. These losses have resulted principally from costs incurred in connection with in-licensing our product candidates, research and development activities and selling, general and administrative costs associated with our operations. As of June 30, 2016, we had an accumulated deficit of $148.9 million and cash, cash equivalents and short-term investments of $109.5 million.
We expect to continue to incur operating losses for the next several years as we work to develop and commercialize our product candidates. As a result, we expect to seek to fund our operations through corporate collaborations and licensing arrangements, as well as public or private equity offerings or further debt financings. If we are not able to raise additional capital on terms acceptable to us, or at all, as and when needed, we may be required to curtail our operations which could include delaying the commercial launch of our products, discontinuing product development programs, or granting rights to develop and market products or product candidates that we would otherwise prefer to develop and market ourselves. Our failure to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategies as this capital is necessary for us to perform the research and development and commercial activities required to generate future revenue streams. Arrangements with collaborators or others may require us to relinquish rights to certain of our technologies or product candidates. In addition, there is a risk that we may never successfully complete development of our product candidates, obtain adequate patent protection for our technology, obtain necessary regulatory approval for our product candidates or achieve commercial viability for any approved product candidates. As disclosed in Note 8 to the consolidated financial statements, we have a term loan and a revolving credit facility with an aggregate principal balance of $40.0 million as of June 30, 2016. The terms of the agreement require us to receive unrestricted net cash proceeds of at least $45.0 million from partnering transactions and/or the issuance of equity securities from October 16, 2015 to October 16, 2016. The loan agreement also requires that we have at least three products fully USDA-or FDA-approved for commercialization by December 31, 2016. With the FDA approval of GALLIPRANT in March 2016 and the receipt of the upfront payment of $45.0 million under the Collaboration Agreement with Elanco entered into in April 2016, we have met both conditions. Additionally, the loan agreement requires that we maintain certain minimum liquidity at all times, which as of June 30, 2016, was approximately $20.0 million. As of the date of the filing of this Quarterly Report on Form 10-Q, we believe that our existing cash and cash equivalents and short-term investments of $109.5 million will allow us to fund our operations at least through June 30, 2017.
Cash, Cash Equivalents and Investments
Until required for another use in our business, we typically invest our cash reserves in bank deposits, certificates of deposit, and other interest bearing debt instruments in accordance with our investment policy. It is our policy to mitigate credit risk in our cash reserves and investments by maintaining a well-diversified portfolio that limits the amount of exposure as to institution, maturity, and investment type. The value of our investments, however, may be adversely affected by increases in interest rates, instability in the global financial markets that reduces the liquidity of securities included in our portfolio, and by other factors which may result in declines in the value of the investments. Each of these events may cause us to record charges to reduce the carrying value of our investment portfolio if the declines are other-than-temporary or sell investments for less than our acquisition cost which could adversely impact our financial position and our overall liquidity.
33
Sales Agreement
On October 16, 2015, we entered into a Sales Agreement with Barclays Capital Inc. (“Barclays”) pursuant to which we may sell from time to time, at our option, up to an aggregate of $52.0 million of shares of our common stock (the “Shares”) through Barclays, as sales agent. Sales of the Shares, if any, will be made under our previously filed and currently effective Registration Statement on Form S-3 (Reg. No. 333-197414), by means of ordinary brokers’ transactions on the NASDAQ Global Market or otherwise. Additionally, under the terms of the Sales Agreement, the Shares may be sold at market prices, at negotiated prices or at prices related to the prevailing market price. We will pay Barclays a commission of 2.75% of the gross proceeds from the sale of the Shares, if any. As of the date of this filing, we have not sold any shares pursuant to the Sales Agreement.
Indebtedness
On October 16, 2015, we and Vet Therapeutics (together the “Borrowers”) entered into a Loan and Security Agreement (“Loan Agreement”) with Pacific Western Bank (“Pacific Western Bank”) as collateral agent (“Collateral Agent”) and a lender and Oxford Finance LLC as a lender (“Oxford” and together with Pacific Western Bank, the “Lenders”), pursuant to which the Lenders agreed to make available to the Borrowers, term loans in an aggregate principal amount up to $35.0 million (the “Term Loan”), and a revolving credit facility in an aggregate principal amount up to $5.0 million (the “Revolving Line” and together with the Term Loan, the “Credit Extensions”), subject to certain conditions to funding. A term loan was made on October 16, 2015 in an aggregate principal amount equal to $35.0 million, and an advance under the Revolving Line was made on October 16, 2015 in an aggregate principal amount equal to $5.0 million. The Borrowers are required to make interest-only payments on the Term Loan for 18 months, and beginning on May 1, 2017, are required to make payments of principal and accrued interest on the Term Loan in equal monthly installments over a term of 30 months. The interest-only period can be extended by one year to May 1, 2018 if the Borrowers have at least four products fully USDA- or FDA-approved, plus another product conditionally- or fully-approved, in each case for commercialization by December 31, 2016, and agree to certain other financial covenants with the Lenders. The Credit Extensions bear interest per annum at the greater of (i) 6.91% or (ii) 3.66% plus the prime rate, which is customarily defined. All principal and accrued interest on the Term Loan are due on October 16, 2019 (the “Term Loan Maturity Date”), and all principal and accrued interest on the Revolving Line are due on October 16, 2017 (the “Revolving Maturity Date”).
The Borrowers used approximately $15.0 million of the proceeds from the Credit Extensions to repay all the amounts owed under their Loan and Security Agreement, dated as of March 4, 2013, as amended, with Pacific Western Bank (as successor in interest to Square 1 Bank) and the lenders party thereto.
As security for their obligations under the Loan Agreement, the Borrowers granted a security interest in substantially all of their existing and after-acquired assets except for their intellectual property and certain other customary exclusions. Subject to customary exceptions, the Borrowers are not permitted to encumber their intellectual property.
Upon execution of the Loan Agreement, the Borrowers were obligated to pay a facility fee to the Lenders of $0.2 million, and an agency fee to the Collateral Agent of $0.1 million. In addition, the Borrowers are or will be obligated to pay a final payment fee equal to 3.30% of such Term Loan being prepaid or repaid with respect to the Term Loans upon the earliest to occur of the Term Loan Maturity Date, the acceleration of any Term Loan or the prepayment of a Term Loan. The Borrowers will also be obligated to pay a termination fee equal to 3.30% of the highest outstanding amount of the Revolving Line with respect to the Revolving Line upon the earliest to occur of the Revolving Maturity Date, the acceleration of the Revolving Line or the termination of the Revolving Line. The Borrowers will also be obligated to pay an unused-line fee equal to 0.25% per annum of the average unused portion of the Revolving Line.
The Loan Agreement contains customary representations and warranties and customary affirmative and negative covenants, including, among others, limits or restrictions on the Borrowers’ ability to incur liens, incur indebtedness, make certain restricted payments, make certain investments, merge, consolidate, make an acquisition, enter into certain licensing arrangements and dispose of certain assets. In addition, the Loan Agreement contains customary events of default that entitle the Lenders to cause the Borrowers’ indebtedness under the Loan Agreement to become immediately due and payable. The events of default, some of which are subject to cure periods, include, among others, a non-payment default, a covenant default, the occurrence of a material adverse change, the occurrence of an insolvency, a material judgment default, defaults regarding other indebtedness and certain actions by governmental authorities. Upon the occurrence and for the duration of an event of default, an additional default interest rate equal to 4% per annum will apply to all obligations owed under the Loan Agreement.
The Loan Agreement requires that the Borrowers receive unrestricted net cash proceeds of at least $45.0 million from partnering transactions and/or the issuance of equity securities from October 16, 2015 to October 16, 2016. The Loan Agreement also requires that the Borrowers have at least three products fully USDA- or FDA approved for commercialization by December 31, 2016. With the FDA approval of GALLIPRANT in March 2016 and the receipt of the upfront payment of $45.0 million under the Collaboration Agreement with Elanco entered into in April 2016, we have met both conditions. Additionally, the Loan Agreement requires that we maintain certain minimum liquidity at all times, which as of June 30, 2016, was approximately $20.0 million. At June 30, 2016, the Borrowers were in compliance with all financial covenants.
34
Working Capital
We define working capital as current assets less current liabilities. The increase in working capital from December 31, 2015, reflects an increase in total current assets of $24.6 million and an increase in current liabilities of $15.4 million. The increase in total current assets was primarily driven by an increase in cash and cash equivalents due to the receipt of the $45.0 million upfront payment under the Collaboration Agreement. The increase in total current liabilities was primarily a result of the achieved research and development milestones relating to GALLIPRANT, ENTYCE and NOCITA, an increase in the current portion of loans payable and an increase in the licensing and collaboration commitment.
Cash Flows
Summary of our cash flows for the six months ended June 30, 2016 and 2015, was as follows:
|
|
Six Months Ended |
|||||
|
|
June 30, |
|||||
|
|
2016 |
2015 |
||||
|
|
(Dollars in thousands) |
|||||
|
Net cash provided by (used in) operating activities |
$ |
23,287 |
$ |
(17,806) | ||
|
Net cash provided by investing activities |
$ |
58,681 |
$ |
23,607 | ||
|
Net cash provided by (used in) financing activities |
$ |
1 |
$ |
(2,953) | ||
Net cash provided by (used in) operating activities
During the six months ended June 30, 2016, net cash provided by operating activities was $23.3 million. We had a pretax income of $3.1 million which includes an adjustment of a non-cash expense for stock-based compensation of $4.5 million, a non-cash depreciation and amortization expense of $0.5 million, a non-cash impairment of acquired intangible assets expense of $2.8 million, and a lower of cost or market adjustment to inventories of $1.6 million. Our net income was primarily attributed to licensing and collaboration revenues of $38.0 million from the Collaboration Agreement, research and development activities related to our programs and our selling, general and administrative expenses. Net cash provided by changes in our working capital consisted primarily of an increase in accounts payable of $5.9 million, an increase of $7.0 million in licensing and collaboration commitment under the Collaboration Agreement and an increase in accrued expenses and other liabilities of $0.6 million, partially offset by an increase in prepaid expenses of $0.1 million and an increase in inventories of $2.8 million. The increase in inventories was primarily related to GALLIPRANT pre-launch inventories. The increase in accounts payable was primarily related to the achieved milestones relating to GALLIPRANT, ENTYCE and NOCITA. The increase in accrued expenses and other current liabilities was primarily due to the licensing and collaboration commitment related to the Collaboration Agreement.
During the six months ended June 30, 2015, net cash used in operating activities was $17.8 million. We had a pretax loss of $17.4 million which includes a gain on sale of marketable securities of $3.9 million, an adjustment of a non-cash expense for stock-based compensation of $4.4 million, a non-cash depreciation and amortization expense of $1.1 million, off-set by a non-cash change in fair value of contingent consideration of $1.2 million, a non-cash change in fair value of derivative instruments of $1.3 million, and a non-cash deferred income tax benefit of $0.6 million. Our net losses were primarily attributed to research and development activities related to our programs and our selling, general and administrative expenses. Net cash provided by changes in our working capital consisted primarily of an increase in accounts payable, accrued expenses and other liabilities of $0.8 million, and a decrease in accounts receivable of $0.2 million, partially offset by an increase in inventories of $0.4 million. The increase in accounts payable primarily related to the timing of payments made for our outsourced research and development activities.
Net cash provided by investing activities
During the six months ended June 30, 2016, net cash provided by investing activities was $58.7 million, which related to the proceeds from the maturities and sales of marketable securities of $286.0 million, partially offset by the purchases of investments of $227.3 million.
During the six months ended June 30, 2015, net cash provided by investing activities was $23.6 million, which related to the proceeds from the maturities and sales of marketable securities of $929.7 million, partially offset by the purchases of investments of $905 million. This was partially offset by $1.1 million for purchases of property and equipment.
Net cash provided by (used in) financing activities
During the six months ended June 30, 2016, net cash provided by financing activities consisted solely of proceeds from stock option exercises of $1,000.
35
During the six months ended June 30, 2015, net cash used in financing activities was $3.0 million. Net cash used in financing activities primarily resulted from cash paid for contingent consideration of $3.0 million to the former shareholders of Vet Therapeutics.
Contractual Obligations and Off-Balance Sheet Arrangements
Contractual Obligations
Our contractual obligations primarily consist of our obligations under our loans payable, non-cancellable operating leases, minimum royalties and other purchase obligations, excluding amounts related to other funding commitments, contingent development, regulatory and commercial milestone payments, contract manufacturer commitments and off-balance sheet arrangements as described below. As of June 30, 2016, there were no material changes in our contractual obligations since December 31, 2015, except for the contract manufacturer commitments described below.
Other Funding Commitments
As of June 30, 2016, we have several on-going development programs in various stages of the regulatory process. Our most significant expenditures are to clinical research and contract manufacturing organizations. The contracts are generally cancellable, with notice, at our option.
Contingent Development, Regulatory and Commercial Milestone Payments
Based on our development plans as of June 30, 2016, we have committed to make potential future milestone payments to third parties of up to approximately $113.4 million, of which $80.4 million are for commercial milestones, as part of our various collaborations, including licensing and development programs. Approximately $68.9 million of the commercial milestones relate to the achievement of various sales thresholds. During the first six months of 2016, we achieved milestones in the amount of $6.0 million that as of the date of this filing had not been paid and are not included in the $113.4 million amount. Payments under these agreements generally become due and payable only upon achievement of certain development, regulatory or commercial milestones. Because the achievement of these milestones had not occurred or was not considered probable as of June 30, 2016, such contingencies have not been recorded in our consolidated financial statements, except for $0.5 million due to our former commercial licensee of BLONTRESS.
Including the $6.0 million of unpaid milestones achieved year to date, we anticipate that we may pay approximately $10.8 million and $13.8 million of milestone payments during the remainder of 2016, and the next 12 months, respectively, provided various development, regulatory or commercial milestones are achieved. Amounts related to contingent milestone payments are not considered contractual obligations as they are contingent on the successful achievement of certain development, regulatory approval and commercial milestones that may not be achieved.
Contract Manufacturer Commitments
Our independent contract manufacturers manufacture our products and product components based on our forecasts. These forecasts are based on estimates of future demand for our products, which are in turn based on available historical trends and an analysis from sales and product marketing organizations, adjusted for overall market conditions. In order to reduce manufacturing lead times and plan for adequate supply, we may issue forecasts and orders for components and products that are non-cancelable. As of June 30, 2016 and December 31, 2015, we had non-cancellable open orders of $0.3 million and $0 million, respectively.
Off-Balance Sheet Arrangements
We have not engaged in the use of any off-balance sheet arrangements, such as structured finance entities or special purpose entities.
Recently Issued and Adopted Accounting Pronouncements
For a discussion of new accounting standards please read Note 1, Summary of Significant Accounting Policies – New Accounting Standards to our consolidated financial statements included within this report.
36
Item 3. Quantitative and Qualitative Disclosures About Market Risk
Our market risks, and the ways we manage them are summarized in Part II, Item 7A, “Quantitative and Qualitative Disclosures About Market Risk” of our Annual Report on Form 10-K for the fiscal year ended December 31, 2015, filed with the SEC on March 15, 2016. As of June 30, 2016, there were no material changes to our market risks or management of such risks since December 31, 2015.
Item 4. Controls and Procedures
Limitations on Effectiveness of Controls and Procedures
In designing and evaluating our disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply judgment in evaluating the benefits of possible controls and procedures relative to their costs.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated, as of the end of the period covered by this Quarterly Report on Form 10-Q, the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)). Based on that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level as of June 30, 2016.
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) identified in connection with the evaluation of our internal control performed during the fiscal quarter ended June 30, 2016, that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
37
We are not currently a party to any material legal proceedings.
Our business faces significant risks and uncertainties, which may have a material adverse effect on our business prospects, financial condition and results of operations, and you should carefully consider them.
Apart from the below, there have been no material changes in the six months ended June 30, 2016, to the risk factors described in Part I, Item 1A of our Annual Report on Form 10-K for the fiscal year ended December 31, 2015.
Our Collaboration Agreement and Co-Promotion Agreement with Elanco are important to our business. If we or Elanco fail to adequately perform under the Collaboration Agreement and/or the Co-Promotion Agreement, or if we or Elanco terminate the Collaboration Agreement and/or the Co-Promotion Agreement, the development and commercialization of GALLIPRANT and any other grapiprant product candidates would be delayed or terminated and our business would be adversely affected.
The Collaboration Agreement and Co-Promotion Agreement are important to our business, and our ability to develop and commercialize GALLIPRANT and any grapiprant product candidates is dependent upon these agreements.
The Collaboration Agreement may be terminated by Elanco at any time upon 90 days’ written notice to us. The Collaboration Agreement may also be terminated by either party:
|
· |
for the other party’s material breach, where such breach is not cured within the timeframe specified by the agreement; |
|
· |
upon the bankruptcy, insolvency or dissolution of the other party; or |
|
· |
for certain activities involving the challenge of certain patents licensed by us to Elanco. |
Upon Elanco’s voluntary termination or termination for Elanco’s breach, among other things, all licenses and rights granted to Elanco will terminate and revert to us, and Elanco has agreed to assign to us all registrations and trademarks obtained in connection with the products covered by the agreement. Upon termination for our breach, among other things, Elanco may elect to retain its rights to the licenses granted by us under the Collaboration Agreement subject to specified payment obligations.
Elanco may terminate the Co-Promotion Agreement in the event Elanco substantially stops marketing the products covered by the Collaboration Agreement, and either party may terminate the Co-Promotion Agreement upon the other party’s material breach, where such breach is not cured within the timeframe specified by the Co-Promotion Agreement. In addition, the Co-Promotion Agreement provides that it will automatically terminate if the Collaboration Agreement is terminated early.
Termination of the Collaboration Agreement and/or the Co-Promotion Agreement could cause significant delays in our product development and commercialization efforts that could prevent us from commercializing our grapiprant product candidates, including GALLIPRANT, without first expanding our internal capabilities, securing additional financing or entering into another agreement with a third party. Any alternative collaboration or license could also be on less favorable terms to us.
Under the Collaboration Agreement, among other things, we are responsible for the manufacture and supply of all of Elanco’s reasonable requirements of the products covered by the agreement. If we are unable to meet our manufacture and supply obligations, Elanco may claim that we have materially breached the Collaboration Agreement and terminate such agreement, which could adversely affect our business and our ability to successfully develop and commercialize any products covered by the agreement, including GALLIPRANT.
Under the Collaboration Agreement, Elanco has agreed to provide funding for certain clinical development activities. If the Collaboration Agreement were terminated, we may need to seek additional financing to support the research and development of any terminated products or discontinue any terminated products, which could adversely affect our business. In addition, under the Collaboration Agreement, Elanco is solely responsible for commercializing products outside the United States. We cannot directly control Elanco’s commercialization activities or the resources it allocates to our product candidates. Our interests and Elanco’s interests may differ or conflict from time to time, or we may disagree with Elanco’s level of effort or resource allocation. Elanco may internally prioritize our product candidates differently than we do or it may not allocate sufficient resources to effectively or optimally commercialize them. If these events were to occur, our business would be adversely affected.
38
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
Unregistered Sales of Equity Securities
None.
Repurchases of Common Stock
There were no share repurchases during the three months ended June 30, 2016.
Item 3. Defaults Upon Senior Securities
None.
Item 4. Mine Safety Disclosures
Not applicable.
None.
A list of exhibits is set forth on the Exhibit Index immediately following the signature page of this Quarterly Report on Form 10-Q, and is incorporated herein by reference.
39
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
ARATANA THERAPEUTICS, INC. |
||||||
|
Date: August 5, 2016 |
By: |
/s/ Steven St. Peter |
||||
|
Steven St. Peter, M.D. |
||||||
|
President and Chief Executive Officer |
||||||
|
(Principal Executive Officer) |
||||||
|
Date: August 5, 2016 |
By: |
/s/ Craig Tooman |
||||
|
Craig Tooman |
||||||
|
Chief Financial Officer |
||||||
|
(Principal Financial and Accounting Officer) |
||||||
40
Exhibit Index
|
|
|||||||
|
|
|
Incorporated by Reference |
|
||||
|
Exhibit Number |
Exhibit Description |
Form |
File No. |
Exhibit |
Filing Date |
Filed/ Furnished Herewith |
|
|
3.1 |
Restated Certificate of Incorporation |
8-K |
001-35952 |
3.1 |
7/3/13 |
|
|
|
3.2 |
Amended and Restated Bylaws |
8-K |
001-35952 |
3.2 |
7/3/13 |
|
|
|
10.1 |
Employment Agreement, dated as of June 24, 2016, between Aratana Therapeutics, Inc. and Brent Standridge |
8-K |
001-35952 |
10.11 |
6/30/16 |
|
|
|
10.2 |
Non-Employee Director Compensation Program, as amended |
|
|
|
|
* |
|
|
10.3† |
Collaboration, License, Development and Commercialization Agreement, dated April 22, 2016, by and between Aratana Therapeutics, Inc. and Eli Lilly and Company, acting on behalf of its Elanco Animal Health Division |
|
|
|
|
* |
|
|
10.4† |
Co-Promotion Agreement, dated April 22, 2016, by and between Aratana Therapeutics, Inc. and Eli Lilly and Company, acting on behalf of its Elanco Animal Health Division |
|
|
|
|
* |
|
|
31.1 |
Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
|
|
|
|
* |
|
|
31.2 |
Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
|
|
|
|
* |
|
|
32.1 |
Certification of Principal Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
|
|
|
|
** |
|
|
32.2 |
Certification of Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
|
|
|
|
** |
|
|
101.INS |
XBRL Instance Document |
|
|
|
|
|
|
|
101.SCH |
XBRL Taxonomy Extension Schema Document |
|
|
|
|
|
|
|
101.CAL |
XBRL Taxonomy Extension Calculation Linkbase |
|
|
|
|
|
|
|
101.DEF |
XBRL Taxonomy Extension Definition Linkbase |
|
|
|
|
|
|
|
101.LAB |
XBRL Taxonomy Extension Label Linkbase |
|
|
|
|
|
|
|
101.PRE |
XBRL Taxonomy Extension Presentation Linkbase |
|
|
|
|
|
|
* Filed herewith.
** Furnished herewith and not “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended.
† Confidential treatment has been requested with respect to certain portions of this exhibit, which portions have been filed separately with the Securities and Exchange Commission
41
