Attached files
| file | filename |
|---|---|
| EX-32.2 - EX-32.2 - ARATANA THERAPEUTICS, INC. | petx-20171231xex32_2.htm |
| EX-32.1 - EX-32.1 - ARATANA THERAPEUTICS, INC. | petx-20171231xex32_1.htm |
| EX-31.2 - EX-31.2 - ARATANA THERAPEUTICS, INC. | petx-20171231xex31_2.htm |
| EX-31.1 - EX-31.1 - ARATANA THERAPEUTICS, INC. | petx-20171231xex31_1.htm |
| EX-23.1 - EX-23.1 - ARATANA THERAPEUTICS, INC. | petx-20171231xex23_1.htm |
| EX-21.1 - EX-21.1 - ARATANA THERAPEUTICS, INC. | petx-20171231xex21_1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
____________________________________________
FORM 10-K
____________________________________________
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended December 31, 2017
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 12 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number: 001-35952
____________________________________________
ARATANA THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
____________________________________________
|
Delaware |
38-3826477 |
|
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification Number) |
11400 Tomahawk Creek Parkway, Suite 340
Leawood, KS 66211
(913) 353-1000
(Address of principal executive offices, zip code and telephone number, including area code)
Securities Registered Pursuant to Section 12(b) of the Act:
|
Title of Each Class |
Name of Exchange on Which Registered |
|
|
Common Stock, par value $0.001 per share |
The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
____________________________________________
Indicate by check mark whether the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act of 1933 Yes: ☐ No: ☒
Indicate by check if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes: ☐ No: ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 and 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes: ☒ No: ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes: ☒ No: ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer |
☐ |
Accelerated filer |
☒ |
|||
|
Non-accelerated filer |
☐ |
Smaller reporting company |
☐ |
|||
|
|
Emerging growth company |
☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes: ☐ No: ☒
The approximate aggregate market value of the common stock held by non-affiliates of the registrant based upon the closing price of the registrant’s common stock on the Nasdaq Global Market on June 30, 2017 was $225,560,521.
As of March 9, 2018, there were 45,854,403 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Specified portions of the registrant’s definitive proxy statement to be filed in connection with the registrant’s 2018 annual meeting of stockholders are incorporated by reference into Part III of this Form 10-K.
FORM 10-K
For the Year Ended December 31, 2017
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Page |
|
|
|
|
2 | |
|
Item 1. |
|
|
2 | |
|
Item 1A. |
|
|
25 | |
|
Item 1B. |
|
|
46 | |
|
Item 2. |
|
|
46 | |
|
Item 3. |
|
|
46 | |
|
Item 4. |
|
|
46 | |
|
|
|
|
47 | |
|
Item 5. |
|
|
47 | |
|
Item 6. |
|
|
49 | |
|
Item 7. |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
51 |
|
Item 7A. |
|
|
67 | |
|
Item 8. |
|
|
67 | |
|
Item 9. |
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
|
67 |
|
Item 9A. |
|
|
67 | |
|
Item 9B. |
|
|
68 | |
|
|
|
|
69 | |
|
Item 10. |
|
|
69 | |
|
Item 11. |
|
|
69 | |
|
Item 12. |
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
69 |
|
Item 13. |
|
Certain Relationships and Related Transactions, and Director Independence |
|
69 |
|
Item 14. |
|
|
69 | |
|
|
|
|
70 | |
|
Item 15. |
|
|
70 | |
|
Item 16. |
|
|
72 | |
|
|
|
|
73 |
1
Aratana Therapeutics and our logo are two of our trademarks that are used in this filing. This filing also includes trademarks, tradenames and service marks that are the property of other organizations. Solely for convenience, trademarks and tradenames referred to in this filing appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or that the applicable owner will not assert its rights, to these trademarks and tradenames.
Cautionary Note Regarding Forward-Looking Statements
Except for historical information, the matters discussed in this Annual Report on Form 10-K for the fiscal year ended December 31, 2017 (“2017 Annual Report”) are forward-looking statements that involve risks, uncertainties and assumptions that, if they never materialize or if they prove incorrect, could cause our consolidated results to differ materially from those expressed or implied by such forward-looking statements. The Company makes such forward-looking statements under the “Safe Harbor” section of the Private Securities Litigation Reform Act of 1995. Actual future results may vary materially from those projected, anticipated, or indicated in any forward-looking statements as a result of various important factors, including those set forth in Item 1A of this 2017 Annual Report under the heading “Risk Factors.” Readers should also carefully review the risk factors described in the other documents that we file from time to time with the SEC. In this 2017 Annual Report, the words “anticipates,” “believes,” “expects,” “intends,” “future,” “could,” “estimates,” “plans,” “would,” “should,” “potential,” “continues” and similar words or expressions (as well as other words or expressions referencing future events, conditions or circumstances) identify forward-looking statements. Forward-looking statements also include the assumptions underlying or relating to any of the foregoing statements. The forward-looking statements contained in this 2017 Annual Report include, but are not limited to, statements related to: industry trends; market conditions; management’s plans, objectives and expectations regarding product development and commercialization; expectations regarding regulatory submissions and approvals, and anticipated timing thereof; the stockholder class action lawsuits and any additional litigation; customer trends and demand for our current or potential products; investments in research and development; business prospects; our collaboration partners and our relationships and arrangements therewith; anticipated achievement of milestones; anticipated financial performance, including future revenues; expected liquidity and capitalization; our ability to protect our intellectual property from third-party claims; changes in accounting principles; changes in actual or assumed tax liabilities; expectations regarding tax exposures; anticipated reinvestment of future earnings; ability to repay our indebtedness; and our intentions regarding the use of cash. All forward-looking statements included in this document are based on information available to us on the date hereof. We will not undertake and specifically decline any obligation to update any forward-looking statements, except as required under applicable law.

ARATANA THERAPEUTICS ®
Our Company
Aratana Therapeutics, Inc. is a pet therapeutics company focused on licensing, developing and commercializing innovative therapeutics for dogs and cats. As a pioneer in pet therapeutics, Aratana’s mission is to deliver safe and effective therapeutics that elevate the standard of care in veterinary medicine. We work with companion animal veterinarians to bring new therapeutics to market that support the needs of pets and their owners.
We were incorporated on December 1, 2010 under the laws of the State of Delaware. We have completed several licensing transactions and acquisitions to build our pipeline. The address of our principal executive offices is 11400 Tomahawk Creek Parkway, Suite 340, Leawood, Kansas 66211. Unless the context requires otherwise, references to “Aratana,” the “Company,” “we,” “us” or “our” in this 2017 Annual Report refer to Aratana Therapeutics, Inc., a Delaware corporation, and its subsidiaries.
We have three marketed therapeutics, including ENTYCE® (capromorelin oral solution) for appetite stimulation in dogs; NOCITA® (bupivacaine liposome injectable suspension) as a local post-operative analgesia for cranial cruciate ligament surgery in dogs; and GALLIPRANT® (grapiprant tablets) for the control of pain and inflammation associated with osteoarthritis in dogs, which we co-promote under an agreement with Elanco Animal Health, Inc., a division of Eli Lilly & Co. (“Elanco”). Our Canine Osteosarcoma Vaccine, Live Listeria Vector (AT-014) is conditionally licensed and is being made available at approximately two dozen study sites across the United States. Our pipeline has multiple therapeutic candidates in development for the potential treatment of pain, allergy, viral disease and cancer for dogs and cats.
2
Our Goal and Strategies
Our goal is to develop and commercialize therapeutics for unmet or underserved medical needs in pets. We plan to accomplish this by:
Deploying a management team with established experience in human pharmaceutical and animal health industries.
In order to successfully execute our plan, we have assembled an experienced management team consisting of veterinarians, physicians, scientists and other professionals. The members of our senior management team have nearly 300 years of combined experience in animal health and human pharmaceutical industries, as well as a strong track record of successfully developing and commercializing therapeutics for dogs and cats. Our senior management team is complemented by our seasoned staff members, most of whom are veterans in the animal health or human health industries.
Advancing our therapeutic candidates to achieve regulatory approval or licensure.
We received three United States Food and Drug Administration (“FDA”) approvals of our lead therapeutic candidates in 2016, several United States Department of Agriculture (“USDA”) licensures in the past few years and we maintain a portfolio of therapeutic candidates, including small molecule pharmaceuticals and biologics. These therapeutic candidates are in various stages of development for the treatment of cats or dogs, or both.
Continuing to grow our therapeutic pipeline by in-licensing additional therapeutic candidates.
We believe the pet therapeutics market is significantly underserved and have identified more than 20 therapeutic areas that overlap with areas of human pharmaceutical development. Pursuant to our corporate strategy, we seek to identify these candidates and when appropriate, to seek exclusive, worldwide rights to these compounds in animal health. Each of our current candidates is covered by patents and/or other intellectual property that provide for a multi-year period of market exclusivity. Additionally, we intend to seek opportunities to collaborate with companies where we can provide commercialization for their approved or close-to-approved pet therapeutics.
Using a direct sales organization, distributors, co-promotion, corporate sales and/or e-commerce to make therapeutics commercially available in the United States.
Our sales team includes approximately two dozen therapeutic specialists and a sales leadership team. Our marketing, sales operations and veterinary services teams round out our unique, therapeutic-focused commercial organization. We extend our reach through strategic distributor relationships and other corporate selling arrangements. In addition, we have an agreement with Elanco to co-promote GALLIPRANT in the United States. Veterinarians typically sell therapeutics to pet owners or administer in-clinic at a mark-up. We believe our sales efforts align with a veterinarian’s goal of improving the health and quality of life of pets, and generating revenue from sales of our therapeutics. In 2017, we directly marketed three FDA-approved therapeutics to veterinarians, including GALLIPRANT in collaboration with Elanco.
Executing on global initiatives.
We have licensed the rights to certain of our therapeutics in geographies outside the United States. We intend to seek regulatory approval for our pet therapeutics in Europe and potentially other countries over time. In April 2016, we announced a global collaboration agreement with Elanco and in 2018, we and Elanco received marketing authorization for GALLIPRANT in Europe for the treatment of pain associated with mild to moderate osteoarthritis in dogs. We believe there continues to be a desire from large animal health companies to collaborate on the commercialization of innovative pet therapeutics in countries outside the United States.
Our strategy is to in-license proprietary technology from human pharmaceutical companies, academia or animal health companies that is applicable to dogs and cats with the intention to develop innovative pet therapeutics to solve unmet or underserved medical needs in companion pets. We seek to identify human therapeutic candidates that have demonstrated proof of safety in the target species, proof of efficacy in at least two mammalian species and a well-defined manufacturing process for the active pharmaceutical ingredients (“API”). We also seek to identify therapeutics already in development or being made commercially available for pets in an effort to license or acquire these products.
In addition, the Company entered into a collaboration, license, development and commercialization agreement (the “Collaboration Agreement”) and co-promotion agreement (the “Co-Promotion Agreement,” and together with the Collaboration Agreement, the “Elanco Agreements”) with Elanco, in April 2016, granting Elanco exclusive rights globally outside the United States to develop, manufacture, market and commercialize our products based on licensed grapiprant rights and technology, including GALLIPRANT (collectively, “Grapiprant Products”), and co-promotion rights in the United States with regards to such products.
To date, we have in-licensed, are further developing pharmaceutical compounds and/or have collaboration agreements with the following companies: Advaxis, Inc. (“Advaxis”), Atopix Therapeutics Ltd. acquired by Chiesi Farmaceutici Spa in November 2016 (“Atopix”), Elanco, Pacira Pharmaceuticals, Inc. (“Pacira”), RaQualia Pharma Inc. (“RaQualia”), Ajinomoto Pharmaceuticals Co., Ltd. (“Ajinomoto”), AskAt Inc. (“AskAt”), and Katholieke University Leuven Research and Development (“KU-Leuven”).
3
Key Developments
During 2017, Aratana had significant achievements that affected the business, including newly marketed therapeutics, progress with therapeutic candidates and other corporate updates.
|
|
|
|
entyce (capromorelin oral solution) |
In October 2017, Aratana made ENTYCE commercially available for appetite stimulation in dogs through distribution and direct purchase. As of December 31, 2017, approximately two months after the ENTYCE launch, Aratana had recorded $1.3 million in ENTYCE net product sales, which were primarily stocking orders to distribution. Initial uptake in the fourth quarter of 2017 exceeded Aratana’s original objective of placement in 1,800 clinics during the quarter by more than double. The Company plans to continue to focus on growing the number of accounts, driving re-orders and increasing days of use. |
|
nocita (bupivacaine liposome injectable suspension) |
In the full year 2017, NOCITA net product sales were $2.8 million as compared to $147 thousand in the fourth quarter and full year 2016 when the therapeutic was launched, and as of December 31, 2017, net product sales have increased sequentially in each quarter since launch. The Company believes the sequential increase in sales is primarily a result of steady, incremental growth of new accounts and strong re-order rates, including a re-order rate of approximately 65% in 2017 and more than 30% of revenues stemming from corporate accounts. |
|
Galliprant (grapiprant tablets) |
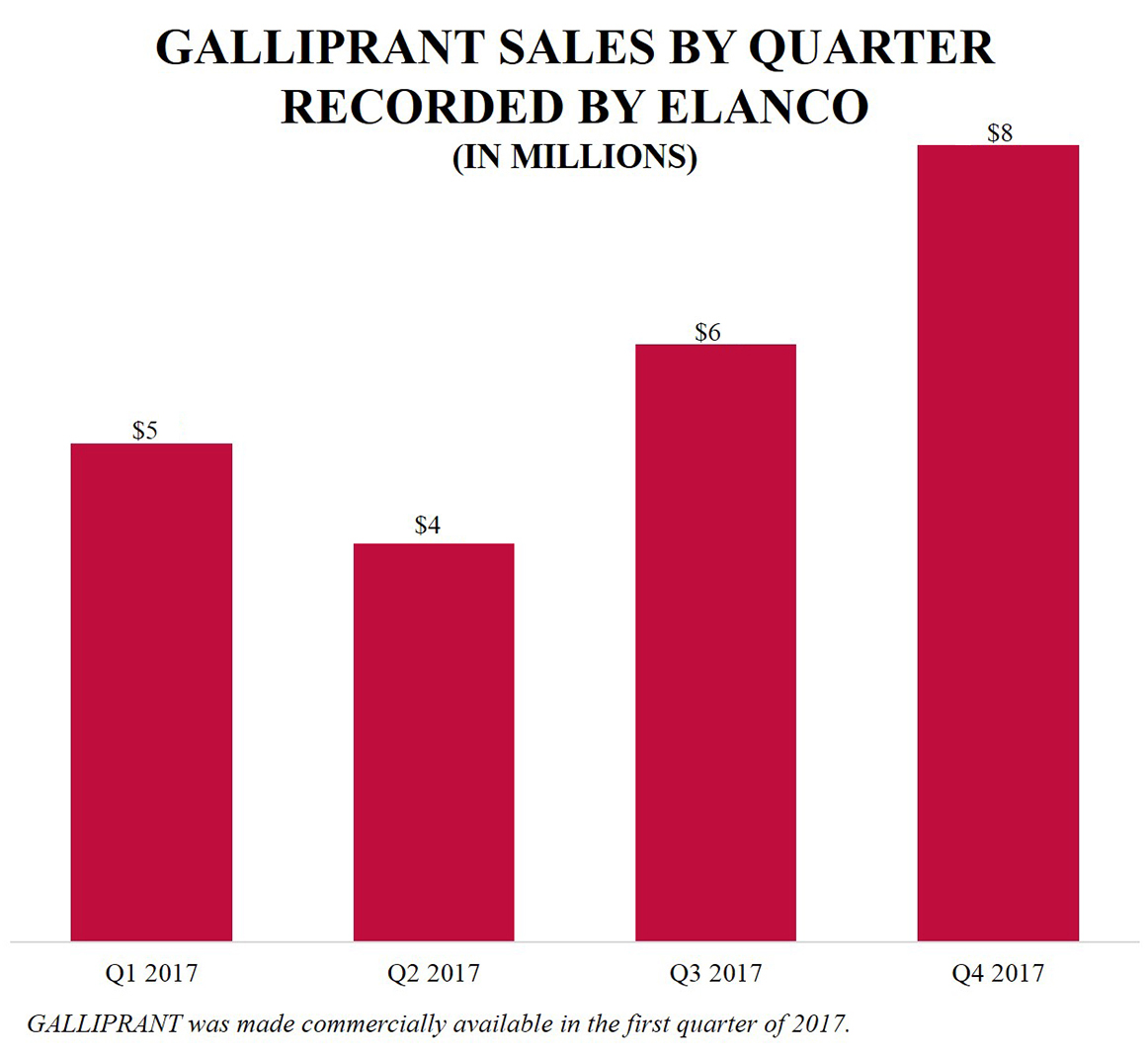
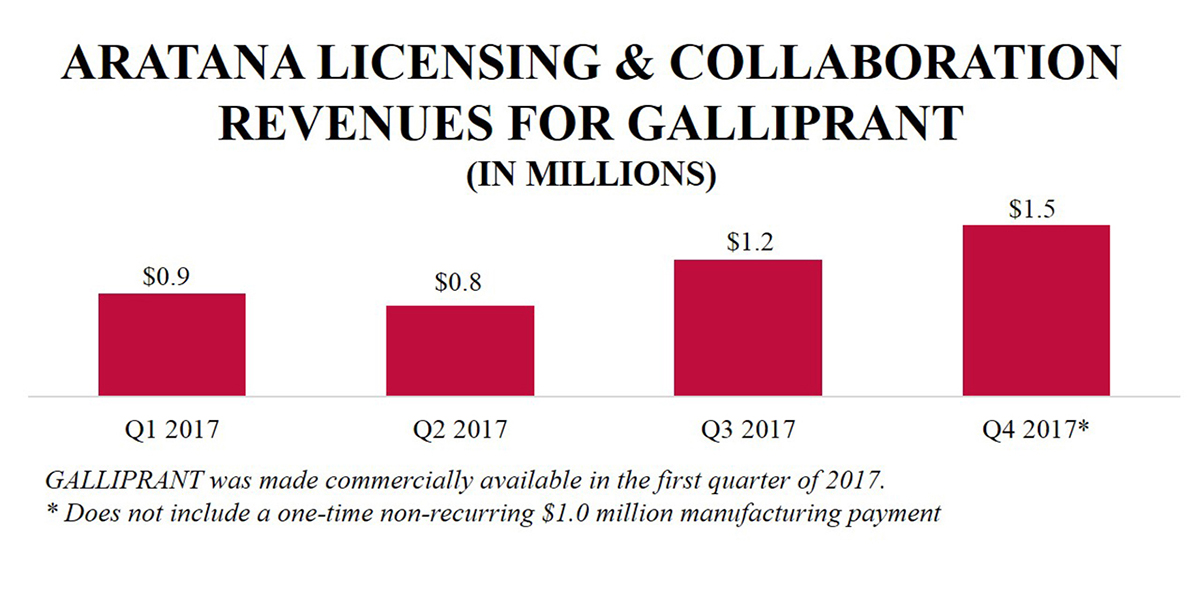
In the first quarter of 2017, in collaboration with Elanco, Aratana made GALLIPRANT commercially available for the control of pain and inflammation in dogs with osteoarthritis. In December 2017, Elanco and Aratana announced the European Medicine Agency's (“EMA”) Committee for Medicinal Products for Veterinary Use (“CVMP”) adopted a positive opinion to recommend the marketing authorization of GALLIPRANT in the European Union (“EU”) and in January 2018, the EMA granted marketing authorization. In the full year 2017, Aratana recorded $20.9 million in total revenues related to GALLIPRANT, which included $4.4 million in licensing and collaboration revenue from Elanco, a one-time non-recurring $1.0 million manufacturing payment and $15.5 million in product sales of finished goods prior to the assumption of manufacturing responsibility by Elanco in the third quarter of 2017. |
Canine Osteosarcoma Vaccine, Live Listeria Vector (AT-014). In December 2017, the USDA Center for Veterinary Biologics (“CVB”) granted Aratana conditional licensure for Canine Osteosarcoma Vaccine, Live Listeria Vector for the treatment of dogs diagnosed with osteosarcoma, one year of age or older. As required by USDA to progress from conditional licensure to full licensure, Aratana is conducting an extended field study, which started enrolling at approximately two dozen veterinary oncology practice groups across the United States in the first quarter of 2018.
AT-003 (bupivacaine liposome injectable suspension) for post-operative pain in cats. In the second quarter of 2017, the Company submitted results from the pivotal safety study to the FDA’s Center for Veterinary Medicine (“CVM”) for AT-003 in cats with post-operative pain. In December 2017, the Company received the target animal safety technical section complete letter for AT-003 from CVM. In the third quarter of 2017, Aratana announced positive results of a pivotal field effectiveness study evaluating AT-003 in client-owned cats undergoing an elective onychectomy. The Company submitted results from the pivotal efficacy study to CVM for AT-003, which if approved, would support the filing of a supplemental New Animal Drug Application (“NADA”) anticipated in the second quarter of 2018 to expand the NOCITA label to include cats.
Corporate Recognition and Awards. In the first quarter of 2017, Aratana was named Animal Pharm's Best Company in North America 2016 based on the FDA approvals of GALLIPRANT, ENTYCE and NOCITA, respectively, as well as the Company's evolution to a fully integrated commercial company. In the second quarter of 2017, the Kansas City Business Journal listed Aratana as the fastest growing Kansas City metro company in 2016 based on annual revenue growth, which was driven by the Elanco collaboration agreement upfront payment in 2016. In November 2017, Deloitte released its 2017 North America Technology Fast-500 and ranked Aratana fourth in revenue growth when measuring the rate of growth from 2013 to 2016.
Registered Direct Offering. In the second quarter of 2017, Aratana entered into a securities purchase agreement with several institutional investors providing for the issuance and sale of an aggregate of 5,000,000 shares of its common stock at a price of $5.25 per share in a registered direct offering.
4
Sales and Marketing
Our mission is to deliver safe and effective therapeutics that elevate the standard of care in veterinary medicine by working with companion animal veterinarians to bring new therapeutics to market that support pets and their owners. We reach companion animal veterinarians by utilizing a variety of tactics depending on the specific business situation for a particular therapeutic. We can market to companion animal veterinarians directly, which includes utilizing a sales force, telesales, e-commerce and selling to key accounts. Key accounts include corporate veterinary entities and group purchasing organizations. Our therapeutics also reach companion animal veterinarians indirectly, which entails selling our therapeutics to national or regional distributors, as well as via commercial collaboration partners who in turn sell to companion animal veterinarians. Typically, direct selling and indirect selling are complementary efforts aimed at raising awareness of the product, generating customer interest and supporting a good customer experience. In certain circumstances, for instance, where a therapeutic would require broad geographic sales coverage or faces established competition, we may choose to co-promote with one of the larger incumbent animal health companies.
In 2017, we gained significant sales experience with three FDA-approved therapeutics, ENTYCE, NOCITA and GALLIPRANT, and we recorded our first significant revenues from the commercialization of our therapeutics. We reported $9.5 million in net revenues in 2017 related to ENTYCE sales, NOCITA sales, GALLIPRANT licensing and collaboration (including a one-time $1.0 million manufacturing payment from Elanco) and other therapeutic sales. In addition, we reported $15.5 million in GALLIPRANT product sales to Elanco of finished goods prior to the assumption of manufacturing responsibility by Elanco in the third quarter of 2017. This compares to 2016 revenues, when NOCITA and other net product sales totaled $0.3 million and licensing and collaboration revenue was $38.2 million (almost all of which, $38.0 million, was related to the one-time upfront payment from Elanco), and to 2015, which had $0.7 million in net product sales.
|
|
|
|
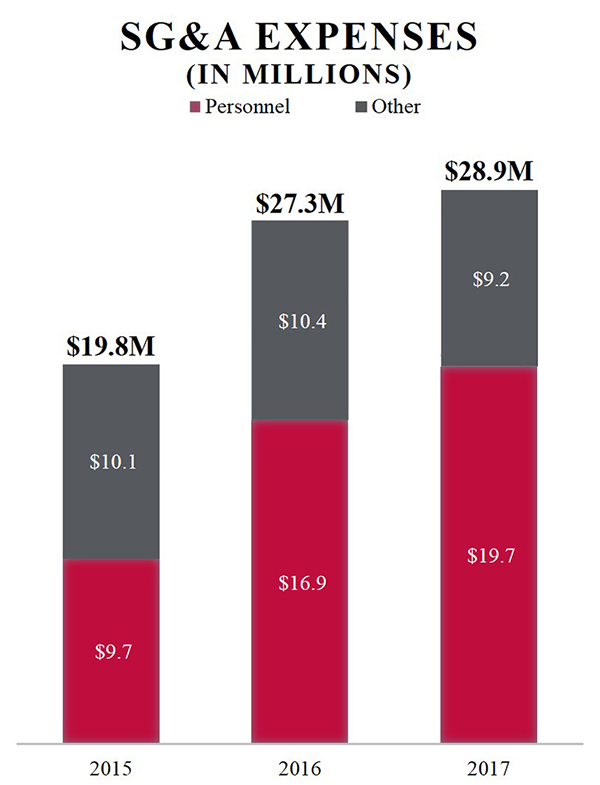
Our selling, general and administrative expenses were $28.9 million, $27.3 million and $19.8 million for the years ended December 31, 2017, 2016 and 2015, respectively. The focus of our current commercial activities is the United States, and we generally attempt to out-license the commercial rights outside the United States. According to independent market research commissioned by us, there are approximately 25,000 to 30,000 veterinary clinics in the United States. We believe we have direct access to approximately 50% of the revenue opportunity for our pet therapeutics through our sales organization of approximately 35 field sales team members detailing the top-40 metropolitan statistical areas (“MSAs”), including sales leadership and our veterinary medical liaisons. We will continue to review opportunities for incremental expansion of the sales organization where growth is expected to be significantly funded by sales. In addition, we believe we have indirect access to the remainder of the revenue opportunity through our relationships with distributors, corporate accounts and as in the case of GALLIPRANT, through a co-promotion arrangement. In 2018, we intend to remain focused on marketing our three FDA-approved therapeutics as we work to expand our product portfolio through business development and collaboration relationships. |
SG&A EXPENSES (IN MILLIONS) Personnel Other 2015 $9.7 $10.1 $19.8M 2016 $16.9 $10.4 $27.3M 2017 $19.7 $9.2 $28.9M |
5
ENTYCE® (capromorelin oral solution)
|
|
|
|
ENTYCE is the first FDA-approved appetite stimulant for dogs. It was made commercially available to veterinarians in the United States in October 2017 through our direct sales organization and our network of national and regional distributors. We currently market ENTYCE in three bottle sizes, 10mL, 15mL and 30mL. ENTYCE is administered as a flavored oral solution at a dose of 3 mg/kg body weight once daily. The cost per dose depends on the weight of the dog and as an example, the 15mL size bottle contains approximately ten doses for a 30 lbs. dog. |
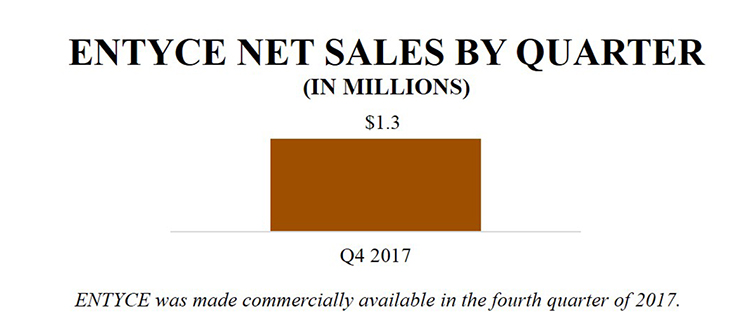
ENTYCE NET SALES BY QUARTER (IN MILLIONS) Q4 2017 $1.3 ENTYCE was made commercially available in the fourth quarter of 2017. |
As of December 31, 2017, approximately two months after its launch, we had recorded $1.3 million in ENTYCE net product sales, which was approximately evenly split between initial stocking orders to distribution and subsequent move-out from distribution representing product moving into the clinics to satisfy orders. ENTYCE had strong uptake in the fourth quarter by exceeding our original objective (placement in 1,800 clinics) by more than double. Placement in clinics continues to grow as of early 2018. And as of the date of the filing of this 2017 Annual Report, we continue to see subsequent move-out of ENTYCE from distribution into the clinics at approximately the same rate as what we saw in the first few months of the launch. As we get further into 2018, we anticipate that inventory levels across the sales channel will stabilize and we believe we will see sequential growth and a tighter correlation between shipments to distributors and move-out to veterinary clinics. Our confidence is bolstered by the rate at which we have been able to access clinics for their initial orders and the anecdotal reports that we are getting from the field. We are focused on growing the number of accounts, driving re-orders, increasing the days of use and succeeding in both acute and chronic inappetence. We believe that initial orders will be smaller as veterinarians gain trial experience and become familiar with the product. We believe that the long-term success of ENTYCE will be driven by veterinarians becoming increasingly comfortable with its use. We anticipate that as the therapeutic establishes itself in clinical practice, we will see a shift towards chronic use and larger orders at the clinic level. Currently, all package sizes are being ordered consistently, however we anticipate a shift toward the larger sizes if veterinarians’ dispensing patterns shift as expected.
Medical need. The control of hunger and satiety involves a complex system in mammals. In many acute and chronic disease states, as well as with aging, lack of appetite is a problem and can fuel a downward spiral. Malnutrition and decreased muscle mass can result from inadequate food intake regardless of the underlying condition. Until ENTYCE veterinarians did not have an FDA approved therapeutic specifically designed to treat inappetence in dogs, a frustrating clinical situation for the veterinarian and pet owner. In a survey of veterinarians 81% believed that stimulation of appetite and weight gain in dogs represented a significant unmet need and 68% of veterinarians reported they were unsatisfied with products being used to treat inappetence prior to ENTYCE. There are numerous chronic and acute conditions that can cause inappetence, some of which include: behavioral or environmental changes; gastroenteritis; psychological; medications; pain; post-surgery; aging; cancer; and various diseases, including autoimmune, endocrine, gastrointestinal, heart, infectious, nasal, neurological and respiratory.
Mechanism of Action. ENTYCE is a first-in-class ghrelin receptor agonist that works by mimicking the effect of ghrelin, the hunger hormone. Like naturally occurring ghrelin, ENTYCE binds to specific receptors in the hypothalamus and causes the feeling of hunger.
Efficacy & Safety. The FDA approved ENTYCE based on a pivotal clinical field study that demonstrated a significantly higher proportion of inappetent dogs receiving ENTYCE had increased appetite in the single question assessment and owner appetite assessment as compared to dogs receiving placebo. ENTYCE-treated dogs in the study also demonstrated an increase in body weight. A 12-month laboratory safety study demonstrated capromorelin was well-tolerated in dogs at daily doses up to 40 mg/kg (17.5x labeled dose).
Treatment options. One of the first goals of therapy for inappetence is to correct the underlying cause. Often veterinarians will begin treatment of inappetence by recommending a change to a highly palatable diet such as tuna for cats and chicken or beef for dogs. Depending on the severity of the condition, the animal may be supported with fluids and electrolytes until the diagnosis of the underlying condition is made and effective treatment is initiated where possible. Prolonged or severe inappetence may require hospitalization and feeding tube placement. Drug therapy to address inappetence has focused primarily on human drugs affecting the central nervous system such as benzodiazepines, cyproheptadine and mirtazapine. However, these drugs are not approved for veterinary use, are believed to have limited effectiveness in pets and are contraindicated for cats with hepatic lipidosis. We believe some veterinarians use Cerenia (maropitant), which is indicated for the control of vomiting, to determine whether the dog is inappetent due to nausea. We believe a significant number of veterinarians are not prescribing these various therapies due to their limited safety and efficacy.
Market opportunity. We believe there is a significant market opportunity for a therapeutic that can safely and effectively stimulate appetite in pets. Almost any disease can manifest or develop decreased appetite (hyporexia), complete lack of appetite (anorexia) or changes in appetite (dysrexia). Inappetence can be the first sign, and may be the only sign, that a dog is sick or has an underlying health condition. Prolonged inappetence, if left untreated, can become even more detrimental to the patient than the underlying primary disease. In addition to being detrimental to the pet, this can cause distress to pet owners and can be perceived as suffering or decreased quality of life. According to market research, approximately 9.8 million dogs in the United States are inappetent each year and approximately 4.1 million of such dogs are treated for the condition (2.3 million dogs are being treated for acute inappetence and 1.8 million dogs are being treated for chronic inappetence). The large majority of veterinarians (87%) we surveyed stated that they are
6
extremely likely or very likely to use ENTYCE in chronic conditions, which is 40 days of therapy per year on average, assuming competitive pricing. By contrast 63% of veterinarians surveyed said they would use the product in acute conditions, which is 4 days of therapy per year, assuming competitive pricing.
NOCITA® (bupivacaine liposome injectable suspension)
|
|
|
|
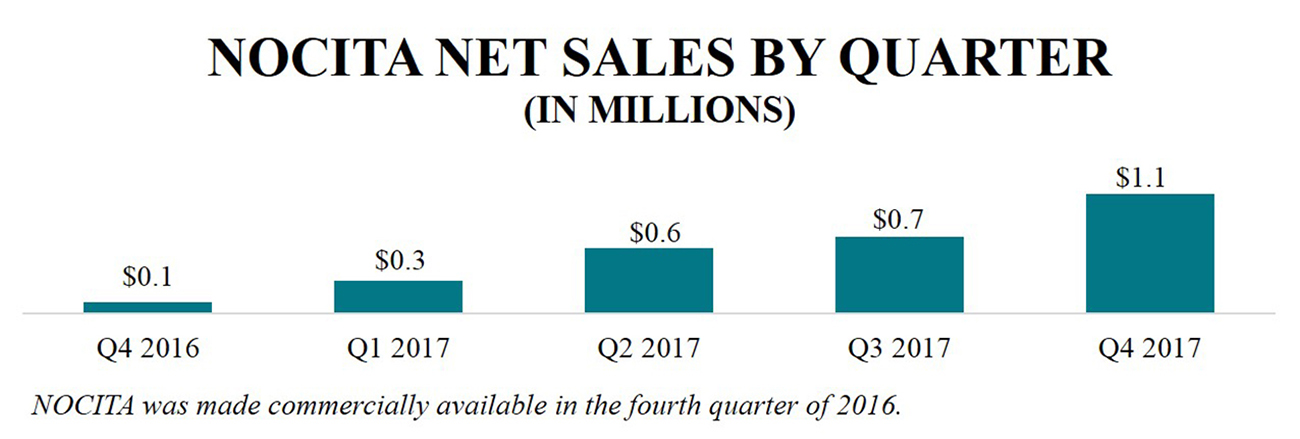
NOCITA is a long-acting local post-operative anesthetic providing 72 hours of post-operative pain relief for dogs undergoing cranial cruciate ligament surgery in one dose at closure. NOCITA was made commercially available to veterinarians in the United States in October 2016 through our direct sales organization. In 2017, we recorded approximately $2.8 million in NOCITA net product sales as compared to approximately $0.1 million in 2016, and as of December 31, 2017, net product sales have increased sequentially in each quarter since launch. |
NOCITA NET SALES BY QUARTER (IN MILLIONS) Q4 2016 $0.1 Q1 2017 $0.3 Q2 2017 $0.6 Q3 2017 $0.7 Q4 2017 $1.1 NOCITA was made commercially available in the fourth quarter of 2016. |
We believe the quarterly sequential increase in sales is primarily a result of steady, incremental growth of new accounts each month and strong re-order rates, including a re-order rate of approximately 65% in 2017 and more than 30% of revenues stemming from corporate accounts. In 2017, we had more than 13,000 interactions with veterinary customers and accounts, suppliers, and/or vendors to educate and discuss NOCITA. Based on third party market research, we have garnered more than 90% aided awareness about the therapeutic among surgeon targets. In 2018, we look to continue to market NOCITA as the standard of care for cranial cruciate ligament surgeries, and anticipate a supplemental NADA in 2018 to expand the NOCITA label to include cats. We are also exploring potentially making NOCITA available in smaller vial sizes, which we believe would increase the target addressable market.
Medical need. Veterinarians perform approximately 20 million dog surgeries each year. Approximately 50% of dog surgeries are spays and neuters, while other common surgeries include cruciate repairs, fracture repairs and cancer surgery. There is not one established protocol for the use of pain medications in these surgeries and pain management practices have traditionally been based on the veterinarian’s views on the level of pain associated with a specific surgical procedure and the perceived pain tolerance of the pets. Pain management has become more important to pet owners and they have begun requesting analgesia for their pets’ painful conditions. Furthermore, animal research demonstrates that pain can have a detrimental effect on healing, and pain experts in academia and specialty practices are advocating more use of local anesthesia for pain control.
Mechanism of Action. NOCITA is a long-acting, local anesthetic that lasts up to 72 hours post-surgery by releasing bupivacaine over time from multi-vesicular liposomes deposited in the tissue. The therapeutic is administered as a single dose by tissue infiltration during closure of cranial cruciate ligament surgery in dogs. This potentially prevents analgesia gaps up to the first 72 hours post-surgery and provides consistent pain control after the patient is discharged.
Efficacy & Safety. FDA approval was based on a pivotal clinical field study in dogs undergoing knee surgery and measured pain using the Glasgow Composite Measure Pain Scale-Short Form (“CMPS-SF”). NOCITA-treated dogs had statistically significant reduction in pain scores compared to placebo at 24, 48 and 72 hours following surgical closure. A four-week laboratory study at up to five times the labeled dose demonstrated there were no clinically relevant treatment-related effects.
Treatment options. We believe the most widely used drugs approved for treatment of post-operative pain are COX-inhibiting nonsteroidal anti-inflammatory drugs (“NSAIDs”) and opioids in dogs. In 2017, the United States Drug Enforcement Administration implemented its initiative to reduce opioids manufactured in the United States by 25%, including opioids sold to veterinarians. We believe this may lead to a decrease in the availability of opioids and, as a result, in surgeries associated with the most severe post-operative pain there may be a shift to using non-opioids. Currently, fentanyl is commonly used (dispensed as a patch), but is not FDA-approved in dogs, and pets are often kept in the hospital while receiving the narcotic. We believe that there are unmet needs in pets undergoing these more painful surgeries, especially if effective and extended pain relief could be achieved with a non-narcotic medicine. COX-inhibiting NSAIDs are used as part of the pain protocol and have demonstrated significant side effects that result in prescribed monitoring of dog health during their use due to concerns with effects on the liver, gastrointestinal system and kidneys.
Market opportunity. We believe that there is a significant market opportunity for the treatment of post-operative pain in dogs. According to market research, approximately 20 million dogs in the United States undergo surgery per year and of such dogs, approximately 5.8 million have very painful expensive surgeries, including cranial cruciate ligament knee surgery. Our initial product indication covered in the product label for NOCITA is for post-operative analgesia for cranial cruciate ligament surgery. In 2017, we made submissions with CVM with the intention of advancing our efforts to expand the label to include cats.
7
GALLIPRANT® (grapiprant tablets)
As part of our Collaboration Agreement with Elanco on Grapiprant Products, Elanco has the lead responsibility for all commercial activities globally. We believe that our commercial organization will continue to participate in the commercial activities in the United States via our Co-Promotion Agreement, which is effective through 2028. We recorded revenues related to our supply arrangement with Elanco in 2017 and we will continue to record certain co-promotion fees, royalties and, if achieved, regulatory, manufacturing and commercial milestones. Hence, the commercial success of Grapiprant Products is very meaningful to us. See “GALLIPRANT Collaboration, License, Development and Commercialization and Co-Promotion Agreements with Elanco” for additional information.
Canine Osteosarcoma Vaccine, Live Listeria Vector (AT-014) for dogs.
In December 2017, the USDA’s CVB granted Aratana conditional licensure for Canine Osteosarcoma Vaccine, Live Listeria Vector for the treatment of dogs diagnosed with osteosarcoma, one year of age or older. The therapeutic expresses a tumor-associated antigen and directs the T-cells to fight cancer cells, even after the bacteria are cleared. Thus, the therapeutic capitalizes on the dog's immune system and its ability to attack bacterial infections, redirecting it to fight cancer cells. As of the first quarter of 2018, we made the therapeutic initially available for purchase to approximately two dozen veterinary oncology practice groups participating in an extended field study across the United States. The goal is to gain more experience with the therapeutic and enroll dogs in the extended field study that is required by USDA to progress from conditional licensure to full licensure. The therapeutic is commercially available to those veterinarians participating in the clinical study. While the study is on-going, any product purchased for use in the clinical study will offset R&D expenses.
8
Competition
The development and commercialization of new animal health medicines is highly competitive, and we expect considerable competition from major pharmaceutical, biotechnology and specialty animal health medicines companies. As a result, there are, and likely will continue to be, extensive research and substantial financial resources invested in the discovery and development of new animal health medicines. Our potential competitors include large animal health companies, such as Zoetis; Merck Animal Health, the animal health division of Merck & Co., Inc.; Elanco; Bayer Animal Health, the animal health division of Bayer AG; Boehringer Ingelheim Animal Health, the animal health division of Boehringer Ingelheim GmbH; Virbac Group; Ceva Animal Health; Vetoquinol and Dechra Pharmaceuticals PLC. We are also aware of several smaller early stage animal health companies, such as Nexvet (acquired in 2017 by Zoetis); Kindred Biosciences; Zomedica Pharmaceuticals; Parnell Pharmaceuticals; and VetDC that are developing products for use in the pet therapeutics market.
Osteoarthritis is a competitive marketplace and Elanco has taken the lead on commercial activities for Grapiprant Products. ENTYCE entered a new market where it is the only product approved for veterinary use to stimulate appetite in dogs. However, we are aware that some veterinarians utilize mirtazapine, a human generic antidepressant with known side effects and limited effectiveness, to treat inappetence, and we are aware that a company is pursuing FDA approval of mirtazapine for weight gain in cats. NOCITA competes primarily with existing analgesics that are part of multi-modal pain protocols, including local anesthetics, opioids and cox-inhibiting NSAIDs. Regarding AT-014, we are aware that veterinarians often utilize off-label chemotherapy to treat this disease and we are aware of a variety of investigational candidates for osteosarcoma.
We are an emerging commercial company with a limited history of operations and many of our competitors have substantially more resources than we do, including both financial and technical resources. In addition, many of our competitors have more experience than we have in the development, manufacture, regulation and worldwide commercialization of animal health medicines. We are also competing with academic institutions, governmental agencies and private organizations that are conducting research in the field of animal health medicines.
Our competitive position will be determined in part by the potential indications for which our products are developed and ultimately approved by regulatory authorities. Additionally, the timing of market introduction of some of our potential products or of competitors’ products may be an important competitive factor. Accordingly, the speed with which we can develop our compounds, complete target animal studies and approval processes, and supply commercial quantities to market are expected to be important competitive factors. We expect that competition among products approved for sale will be based on various factors, including product efficacy, safety, reliability, availability, price and patent position.
|
|
|
|
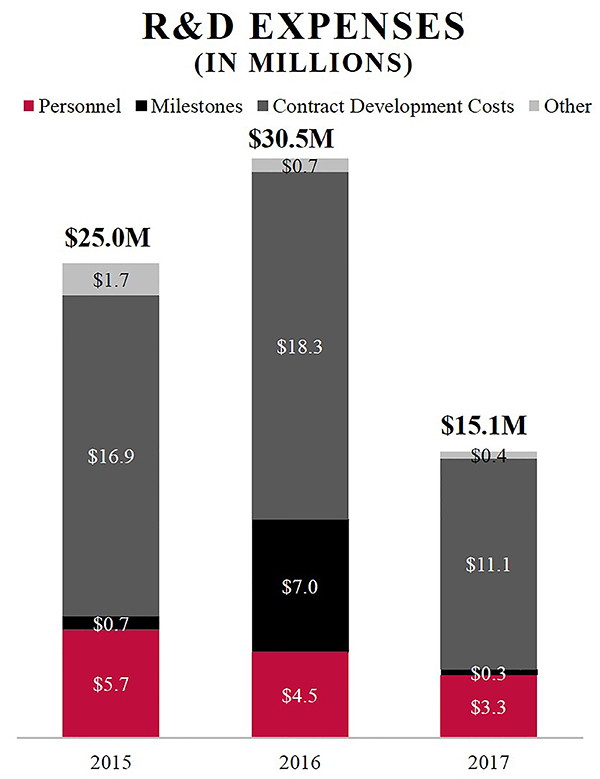
Research and Development Our drug development programs focus on the development of novel compounds with the intention of capturing opportunities that will raise the standard of care for dogs and cats. We are building a development pipeline, both through the application of early-stage in-licensed therapeutic candidates and through strategic agreements. Our development approach provides access to promising therapeutic development opportunities within our focus areas. Even after a therapeutic is commercially available, we may conduct additional clinical studies for life cycle management purposes (supplemental indications) or for scientific exchange. Our current therapeutic candidates are animal pharmaceuticals regulated by the FDA and immune-mediated biologics, including cancer vaccines, regulated by the USDA. We are focused on our core strengths of clinical development and navigating the regulatory environment with respect to our in-licensed therapeutic candidates. We continue to prioritize our development and commercial efforts with a primary focus on the United States. We have incurred and will continue to incur research and development expense as we develop our business. Our research and development expenses were $15.1 million, $30.5 million and $25.0 million for the years ended December 31, 2017, 2016 and 2015, respectively. |
R&D EXPENSES (IN MILLIONS) Personnel Milestones Contract Development Costs Other 2015 $5.7 $0.7 $16.9 $1.7 $25.0M 2016 $4.5 $7.0 $18.3 $0.7 $30.5M 2017 $3.3 $0.3 $11.1 $0.4 $15.1M |
9
Development Programs at the FDA and USDA
FDA: To begin the development process for our product candidates in the United States, we establish an Investigational New Animal Drug (“INAD”) file with the CVM. We then hold a pre-development meeting with the CVM to reach a general agreement on the plans for providing the data necessary to fulfill requirements for a NADA. During development, we submit pivotal protocols to the CVM for review and concurrence prior to conducting the required studies. We gather and submit data on manufacturing, safety and effectiveness to the CVM for review, and this review is conducted according to timelines specified in the Animal Drug User Fee Act (“ADUFA”). Once all data have been submitted and reviewed for each technical section – safety, effectiveness and Chemistry, Manufacturing and Controls (“CMC”) – the CVM issues us a technical section complete letter as each section review is completed, and when the three letters have been issued, we compile a draft of the Freedom of Information Summary, the proposed labeling, and all other relevant information, and submit these as an administrative NADA for CVM review. Generally, if there are no deficiencies in the submission, the NADA is issued within 60 days after submission of the administrative NADA, as was the case with all three of our therapeutics that received FDA approval in 2016. A separate approval either as an original or supplemental NADA is required for each species. In addition, additional indications and additional formulations to extend the lifecycle of our product candidates require separate approvals. By exploring new uses and methods, we may potentially be able to extend the patent life of our product candidates and achieve further differentiation in the marketplace.
USDA: There are many parallels between the requirements to receive FDA approval for a veterinary pharmaceutical candidate and certain veterinary biologics candidates. The terminology differs, but the three main components are consistent, including efficacy, manufacturing and safety. USDA regulations are designed to ensure that veterinary biologics are pure, safe, potent and effective. The differences compared to FDA regulations are based on the immunological nature of the mode of action in biologics and the manufacturing process involving living organisms. In cases of emergencies, which means there is no licensed option available, the USDA will issue a time-limited conditional license after the manufacturing and safety requirements have been substantially fulfilled and a reasonable expectation of efficacy has been established. The applicant is expected to continue the pivotal efficacy study and the testing of the validation of the therapeutic. The conditional license can be extended if reasonable progress toward full licensure can be demonstrated. A unique requirement for veterinary biologics in the United States is that manufacturers must hold a United States Veterinary Biologics Establishment License to produce licensed veterinary biologics. An establishment license will only be issued if at least one biologic qualifies for a license. Applications for veterinary biologics establishments include: articles of incorporation for the applicant; qualifications of veterinary biologics personnel for key employees; water quality statement; facility blueprints; plot plans; and legends.
We are developing the following therapeutic candidates under FDA regulations:
|
Pilot Studies |
Pivotal Studies |
Phased Submission |
Commercial |
||||
|
AT-003 (bupivacaine liposome injectable suspension) for post-operative pain in cats |
|
|
|||||
|
AT-002 (capromorelin) for the management of weight loss in CKD cats |
|
|
|
||||
|
AT-018 (timapiprant) for dogs |
|
|
|
|
|||
|
AT-006 for cats |
|
|
|
|
|||
We are developing the following therapeutic candidates under USDA regulations:
|
|
|
|
|
|
|
Field Safety & Efficacy |
Conditional and/or Full Licensure |
Commercial |
||
|
Canine Osteosarcoma Vaccine, Live Listeria Vector (AT-014) for dogs |
|
|
||
|
AT-017 for dogs |
|
|
|
|
10
The following summarizes our regulatory and development advances in 2017 and early 2018 for therapeutic candidates being developed under FDA and USDA regulations:
ENTYCE® (capromorelin oral solution) for dogs.
ENTYCE was approved by the FDA for appetite stimulation in dogs in 2016. In October 2017, we announced that we received CVM approval of the prior-approval supplement (“PAS”) submission for the transfer and scale-up of manufacturing of ENTYCE, which allowed us to introduce the product into the market in late-2017.
AT-003 (bupivacaine liposome injectable suspension) for cats.
In June 2017, we submitted results from the pivotal safety study showing the therapeutic candidate was well-tolerated for the safety technical section filing with CVM for AT-003 (in-licensed from Pacira) in cats. In December 2017, we received the target animal safety technical section complete letter for AT-003 from CVM. In July 2017, we announced positive results of a pivotal field effectiveness study evaluating AT-003 in 200 client-owned cats undergoing an elective onychectomy. Data showed AT-003 met protocol-defined efficacy success criteria, which were statistically significant (p < 0.05), and the data were submitted to CVM in October. We have submitted all major technical sections to CVM and if we receive a technical section complete letter for efficacy, we plan to file a supplemental NADA with CVM to expand the NOCITA label to include cats in 2018.
AT-002 (capromorelin) for cats.
AT-002 (in-licensed from RaQualia) is a cat-specific formulation of capromorelin with a flavor that differs from the therapeutic approved for use in dogs. In 2017, we completed pivotal target animal safety study under FDA-concurred protocol. Currently, AT-002 is being evaluated in an ongoing, FDA-concurred field effectiveness study for weight management in cats with chronic kidney disease. The study is anticipated to complete enrollment in late-2018 and we anticipate that results will be available in 2019.
AT-018 (timapiprant) for dogs.
AT-018, which we in-licensed from Atopix following an option period between the parties, is an oral CRTH2 antagonist for the potential treatment of atopic dermatitis in dogs. In April 2017, we initiated a pilot study evaluating timapiprant for the prevention of clinical signs of atopic dermatitis in at-risk dogs and anticipate completing enrollment of the study in 2018.
AT-006 (eprociclovir) for cats.
AT-006 is an anti-viral for the treatment of feline herpes virus-induced ophthalmic conditions in-licensed from Ajinomoto. We plan to procure supply to be prepared to initiate a pilot study in 2018.
Canine Osteosarcoma Vaccine, Live Listeria Vector (AT-014) for dogs.
Canine Osteosarcoma Vaccine is a novel immunotherapy developed by us using a listeria based antigen delivery system in-licensed from Advaxis and is a lyophilized formulation of a modified live, attenuated strain of listeria that activates cytotoxic T-cells. In 2017, we submitted pivotal field safety study data and in December 2017, the USDA granted Aratana conditional licensure for the treatment of dogs diagnosed with osteosarcoma, one year of age or older. As required by USDA to progress from conditional licensure to full licensure, Aratana has initiated an extended field study. Initially, the therapeutic will be made available for purchase at the approximately two dozen veterinary oncology practice groups across the United States which participate in the study.
AT-017 for dogs.
AT-017 is an investigational therapeutic using a listeria based antigen delivery system in-licensed from Advaxis with potential in canine lymphoma. In 2018, we anticipate initiating a laboratory safety study.
AT-016 (allogeneic adipose-derived stem cells) for dogs.
In December 2017, our license partner responsible for the development of AT-016, VetStem BioPharma (“VetStem”), shared results of a pivotal study, that did not achieve protocol-defined efficacy success criteria. We do not anticipate committing future resources to the program and in January 2018, we exercised our right to terminate the license agreement with VetStem effective as of mid-April 2018.
AT-019.
In February 2018, we licensed exclusive, worldwide rights to develop and commercialize AT-019 from AskAt. AT-019 is a potent and innovative EP4 receptor antagonist therapeutic candidate with potential in pain, inflammation and other indications. We intend to start development work in 2018.
11
Regulatory and development advances in 2017 and early 2018 for therapeutic candidates outside the United States:
Grapiprant Products.
Under the Collaboration Agreement, Elanco has exclusive rights to Grapiprant Products globally outside the United States for development, manufacturing, marketing and commercialization in additional species and/or indications. Aratana was responsible under the Collaboration Agreement for all development activities required to obtain the first regulatory approval for grapiprant for use in dogs in the EU and Elanco is responsible for all other development activities going forward. In February 2016, we filed a marketing authorization application with the EMA for grapiprant in dogs in the EU. In cooperation with Elanco, in October 2017, we submitted additional information to the EMA. In November 2017, Elanco and Aratana announced the EMA’s CVMP adopted a positive opinion to recommend the marketing authorization of GALLIPRANT in the EU. In January 2018, Elanco and Aratana announced that the European Commission had adopted the decision to grant marketing authorization of GALLIPRANT in the EU.
AT-008 (rabacfosadine) for dogs in Europe.
AT-008 is a potential therapeutic candidate for canine lymphoma we sub-licensed from KU-Leuven and we have rights to develop and market the therapeutic candidate outside North America. VetDC has rights to the therapeutic candidate in North America, and it was conditionally approved by the FDA as Tanovea®-CA1 in late-2016. As Tanovea®-CA1 is established in the United States, we continue to evaluate if and how to move the therapeutic forward in Europe.
Other therapeutics for dogs and cats in Europe.
We have started to develop a dossier for EMA regulatory authorities on bupivacaine liposome injectable suspension in Europe. Separately, based on conversations with the EMA, we believe the path forward for capromorelin may be informed by studies investigating weight gain in cats.
12
Manufacturing and Supply Chain
We manage third-party manufacturers to supply API, drug product and packaged product for the development and commercialization of our small molecule product candidates. To ensure dependable and high-quality supply, we have chosen to rely on Current Good Manufacturing Practices (“cGMP”) compliant contract manufacturer organizations (“CMO”) rather than devote capital and manpower toward developing or acquiring internal manufacturing facilities. We utilize CMOs that have established track records of quality product supply and significant experience with regulatory requirements of both CVM and EMA. We believe we have or will have sufficient supply to conduct each of our currently contemplated studies and for the continued commercial supply of our products.
As our products move from development-stage to commercial-stage, we work with the relevant CMOs to make changes in the manufacturing process as required to complete process validation, scale-up capacity and to implement process improvements. Depending on the nature of the changes, we make supplemental manufacturing filings to obtain the required approval to manufacture and sell products. As has been the case with each of our commercialized small molecule products, such transition and supplemental approval process can cause delays in making the first commercial sale following the initial approval.
|
|
|
|
entyce (capromorelin oral solution) |
In February 2017, we announced the FDA requested additional information on our PAS to transfer the manufacturing of the API of ENTYCE to a different vendor than included on our original NADA in order to produce ENTYCE at commercial scale. We met with the FDA in April 2017 and in late-June 2017, we re-submitted the PAS with the FDA’s CVM. Subsequent to the re-submission of the PAS, we resumed manufacturing additional commercial inventory of ENTYCE, including API from the manufacturer for which we were seeking approval from CVM. In October 2017, we received approval of our PAS from CVM for the transfer and commercial scale-up of the API of ENTYCE, and we made ENTYCE commercially available in late-October 2017. With the alternate API vendor in place, we believe that we can maintain sufficient inventory to continuously supply ENTYCE to the market. In addition, we are investing to establish redundant commercial supply such that we can continue to improve our costs of goods and mitigate any potential supply issues. |
|
nocita (bupivacaine liposome injetable suspension) |
For NOCITA, Pacira is our exclusive supplier and under our December 2012 supply agreement, Pacira is responsible for supplying us with finished drug product in vials. We are responsible for the labeling, packaging and shipping of NOCITA. We must submit rolling forecasts to Pacira with a portion of each forecast constituting a binding commitment. The term of the supply agreement extends for as long as the license agreement with Pacira continues in force. The license agreement has a term of fifteen years, until December 5, 2027, after which we have the option to renew the term for an additional five years. Pacira may terminate the supply agreement if we fail to make an undisputed payment, if we breach a material provision of the agreement, or if Pacira ceases manufacture of the drug product. Pacira also has the unilateral right to change its manufacturing process for the drug product. In this case, if we cannot reach agreement on the terms of continued supply of NOCITA meeting current specifications and Pacira decides that it is no longer commercially reasonable to supply us with drug product meeting such specifications, then Pacira may terminate the supply agreement. Our NOCITA supply is labeled with a 12-month shelf life. In 2018, we anticipate investigating opportunities with Pacira for making the therapeutic available in additional vial sizes. |
|
Galliprant (grapiprant tablets) |
GALLIPRANT has been available to customers since January 2017, and as part of the Collaboration Agreement, we agreed to provide commercial supply of GALLIPRANT to Elanco for a specified period. In July 2017, Elanco provided us with notice of its intent to assume responsibility for manufacturing of the Grapiprant Products and its intent to assume the regulatory approvals pursuant to the Collaboration Agreement. As of September 2017, Elanco assumed ownership of the NADA and manufacturing responsibility for GALLIPRANT. Prior to Elanco assuming manufacturing responsibility, we charged Elanco for bulk API at a fixed price that was above or below the actual API costs that we incurred in manufacturing the product. Hence, in aggregate we captured a modest manufacturing margin. Elanco is also working on a technology transfer at its expense to secure redundant supply and capture process improvements. Upon the successful establishment of this alternate supply source, we are eligible for a $4.0 million milestone payment from Elanco. GALLIPRANT is currently available in 20 mg and 60 mg tablets in a variety of packaging configurations. The 100 mg tablets of GALLIPRANT remain on backorder to resolve isolated reports of 100 mg tablets breaking in the bottle. With the transfer of manufacturing responsibility, Elanco is responsible for the timing and availability of the 100 mg tablets. |
13
Canine Osteosarcoma Vaccine. Regarding AT-014, we transferred the manufacturing from Advaxis to a third-party USDA-licensed CMO. We have the ability to expand the relationship with the CMO, subject to our needs and growth of current and/or future biologic therapeutics.
BLONTRESS (canine lymphoma monoclonal antibody, B-cell) and TACTRESS (canine lymphoma monoclonal antibody, T-cell).
As previously announced, we determined in the third quarter of 2017 that customer demand did not justify manufacturing additional BLONTRESS or TACTRESS and therefore, we discontinued the therapeutics in November 2017. We also closed our USDA-licensed research and manufacturing facility in San Diego by the year ended 2017, which resulted in us no longer holding a USDA establishment license and voiding the BLONTRESS and TACTRESS licenses with the USDA.
Intellectual Property and License Agreements
We seek to protect our products and technologies through a combination of patents, regulatory exclusivity, and proprietary know-how. Our goal is to obtain, maintain and enforce patent protection for our products, formulations, processes, methods and other proprietary technologies, preserve our trade secrets, and operate without infringing on the proprietary rights of other parties, both in the United States and in other countries. Our policy is to actively seek to obtain, where appropriate, the broadest intellectual property protection possible for our current compounds and any future compounds for development, proprietary information and proprietary technology through a combination of contractual arrangements and patents, both in the United States and abroad. However, even patent protection may not always afford us with complete protection against competitors who seek to circumvent our patents.
We depend upon the skills, knowledge and experience of our scientific and technical personnel, as well as that of our advisors, consultants and other contractors, none of which is patentable. To help protect our proprietary know-how, which is not patentable, and inventions for which patents may be difficult to obtain or enforce, we rely on trade secret protection and confidentiality agreements to protect our interests. To this end, we generally require all of our employees, consultants, advisors and other contractors to enter into confidentiality agreements that prohibit the disclosure of confidential information and, where applicable, require disclosure and assignment to us of the ideas, developments, discoveries and inventions important to our business.
Exclusive License Agreements with RaQualia
In December 2010, we entered into two agreements with RaQualia pursuant to which we exclusively licensed intellectual property rights relating to AT-001 and AT-002 in the animal health field. Pursuant to these agreements we obtained the rights to certain patents in the United States and other jurisdictions. The patents relating to AT-001 include composition of matter claims as well as claims to methods of use of AT-001. The patent rights relating to the use of AT-001 further include methods of preparing the compounds of interest and salts, polymorphs and intermediates thereof, as well as certain combination therapies. Additionally, we licensed from RaQualia additional patent rights relating to AT-002 that include composition of matter claims as well as claims to methods of use of AT-002. Under these agreements, we were granted exclusive, worldwide licenses to develop, manufacture and commercialize AT-001 and AT-002 in the field of animal health, except that we cannot develop, manufacture or commercialize injectable AT-001 products in Japan, South Korea, China or Taiwan. We have the right to grant sublicenses to third parties under these agreements. Under our agreement with RaQualia, we are responsible for using commercially reasonable efforts to develop and commercialize AT-001 and AT-002. The patent that we believe covers the crystalline form of the AT-001 compound expires on February 21, 2027 and is expected to be eligible for a patent term extension to August 2029. Certain of the AT-002 patents and applications licensed under the agreement are expected to extend out to 2034. In addition, the use of AT-002 in companion animals is protected by an Aratana patent, which expires in January 2036.
We are responsible for contingent milestone payments upon achievement of development and regulatory milestones and royalties on net sales of licensed products, subject to certain potential offsets and deductions, under each of the AT-001 and AT-002 agreements, and the royalty percentage is in the mid-single digits. We must also pay to RaQualia a portion of royalties we receive from any sublicensees, subject to a minimum royalty on net sales by such sublicensees. Our royalty obligations apply on a country-by-country and licensed product-by-licensed product basis, and end upon the expiration or abandonment of all patents with valid claims covering a licensed product in a given country.
Each of the AT-001 and AT-002 agreements continues until terminated. RaQualia may terminate the AT-001 agreement or the AT-002 agreement if we fail to pay any undisputed fee under the relevant agreement and do not cure such failure within 60 days after RaQualia notifies us of such failure. We may terminate the AT-001 agreement or the AT-002 agreement, or any license granted under either agreement, on a patent-by-patent and country-by-country basis at will, upon 30 days’ prior written notice to RaQualia. Once all of the patents licensed under the AT-001 agreement or the AT-002 agreement have expired or been abandoned, the licenses granted under the relevant agreement become fully-paid and irrevocable.
GALLIPRANT Collaboration, License, Development and Commercialization and Co-Promotion Agreements with Elanco
On April 22, 2016, we entered into a Collaboration Agreement with Elanco that granted Elanco rights to develop, manufacture and commercialize Grapiprant Products, an FDA-approved therapeutic for the control of pain and inflammation associated with osteoarthritis in dogs. Elanco will have exclusive rights globally outside the United States and co-promotion rights with us in the United States during the term of the Collaboration Agreement.
14
Elanco paid us an upfront payment of $45.0 million. Elanco has also agreed to pay us a $4.0 million milestone related to European approval of a Grapiprant Product for the treatment of pain and inflammation, a $4.0 million milestone related to the manufacturing of a Grapiprant Product from an alternate supply source and up to $75.0 million upon the achievement of certain sales milestones. The sales milestone payments are subject to a one third reduction for each year the occurrence of the milestone is not achieved beyond December 31, 2021, with any non-occurrence beyond December 31, 2023, cancelling out the applicable milestone payment obligation entirely.
Elanco will also pay us royalty payments on a percentage of net sales in the mid-single to low-double digits. In addition, we and Elanco have agreed to pay 25% and 75%, respectively, of all third-party development fees and expenses through December 31, 2018, in connection with preclinical and clinical trials necessary for any registration or regulatory approval of the products (“Registration”), provided that our contribution to such development fees and expenses is capped at $7.0 million. We were responsible for all development activities required to obtain the first Registration for Grapiprant Products for use in dogs in each of the European Union and the United States, and Elanco is responsible for all other development activities.
The term of the collaboration will continue throughout the development and commercialization of the product candidates, on a product-by-product and country-by-country basis, until the latest of (i) the date on which no valid claim of certain issued or granted patents specified in the Collaboration Agreement in the respective country exists, (ii) the expiration of any regulatory exclusivity in such country covering such Grapiprant Product, and (iii) the tenth anniversary of the first commercial sale of such product in such country.
The Collaboration Agreement may be terminated by Elanco at any time upon 90 days’ written notice to us. The Collaboration Agreement may also be terminated by either party (i) for the other party’s material breach, where such breach is not cured within the timeframe specified by the agreement, (ii) upon the bankruptcy, insolvency or dissolution of the other party, or (iii) for certain activities involving the challenge of certain patents licensed by us to Elanco. Upon Elanco’s voluntary termination or termination for Elanco’s breach, among other things, (a) all licenses and rights granted to Elanco will terminate and revert to us, and (b) Elanco has agreed to assign to us all registrations and trademarks obtained in connection with the Grapiprant Products. Upon termination for our breach, among other things, Elanco may elect to retain its rights to the licenses granted by us under the Collaboration Agreement subject to specified payment obligations.
On April 22, 2016, in connection with the Collaboration Agreement, we entered into the Co-Promotion Agreement with Elanco to co-promote the Grapiprant Products in the United States.
Under the terms of the Co-Promotion Agreement, Elanco has agreed to pay us, as a fee for services performed and expenses incurred by us under the Co-Promotion Agreement, (i) 25% of the gross margin on net sales of Grapiprant Products sold in the United States under the Collaboration Agreement prior to December 31, 2018 (unless extended by mutual agreement), and (ii) a mid-single digit percentage of net sales of the Grapiprant Products in the United States after December 31, 2018 through 2028 (unless extended by mutual agreement).
The Co-Promotion Agreement expires on December 31, 2028, unless extended by mutual agreement. In addition, the Co-Promotion Agreement provides that it will automatically terminate if the Collaboration Agreement is terminated early.
Exclusive License Agreement with Pacira
In December 2012, we entered into an exclusive license agreement and related exclusive supply agreement with Pacira. Under the license agreement, we were granted an exclusive, worldwide license to develop and commercialize, but not to manufacture, AT-003 in the veterinary field. Pursuant to this agreement we obtained the rights to certain patent rights relating to AT-003 including composition of matter claims and methods of use thereof. Patents relating to the AT-003 formulation extend out to 2031.
We have the right to grant sublicenses to third parties outside the United States upon Pacira’s approval. Any sublicenses we wish to grant to third parties within the United States must be discussed with Pacira and approved by Pacira in its sole discretion and good faith reasonable business judgment. We are responsible for using commercially reasonable efforts to develop and commercialize AT-003, and for launching AT-003 within a specified time period following regulatory approval in certain countries.
We paid Pacira an upfront fee and are responsible for contingent milestone payments upon the achievement of certain development and commercial milestones and for royalties on net sales of AT-003 by us and our affiliates, with a tiered royalty percentage in the low- to mid-20s. We must pay Pacira a royalty on net sales of AT-003 by us and our affiliates, subject to certain reductions. We must also pay to Pacira a percentage of all payments we receive from any sublicensee, subject to certain offsets, and under certain circumstances, share a portion of Pacira’s royalty payment obligations to its third-party licensors. We are responsible for meeting minimum annual revenue requirements for AT-003 beginning the fifth year after the first commercial sale of AT-003. If we fail to meet these requirements, either we or Pacira may terminate the license agreement.
The term of the license agreement extends for 15 years, until December 5, 2027, after which we have the option to renew the term for an additional five years. Pacira may terminate the agreement in its entirety if we fail to pay any amount due within a specified time period, or on a country-by-country basis if we fail to achieve certain regulatory, clinical and commercial milestones within certain timeframes. We may terminate the agreement on a country-by-country basis either upon the entry of a generic competitor, or at will outside the United States or the European Union. Either we or Pacira may terminate the agreement if the other party materially breaches or files for bankruptcy and fails to cure such breach within a specified time period, or if we do not pay the minimum annual
15
revenue requirements referenced above. The agreement automatically terminates if Pacira terminates the related supply agreement and if certain circumstances involving a United States sublicensee occur and we do not meet certain financial obligations to Pacira.
Other
As part of our January 2014 acquisition of Okapi Sciences, we acquired certain patent rights that cover formulations of AT-006 and methods of making the active ingredient of AT-006. Issued patents relating to AT-006 expire in 2031 and 2032. There are also pending applications relating to the issued patents. We also have a license to certain patent rights that covers composition and methods of use of AT-008 outside of North America. These patent rights will expire between 2024 and 2027.
As part of our October 2013 acquisition of Vet Therapeutics, we acquired a patent family related to the speciesization of antibodies that cover all Vet Therapeutics products with an issued patent expiring in 2029. We also acquired a patent family related to antibody constant domain regions and uses thereof, which also covers all Vet Therapeutics products and has an issued United States patent expiring in 2032.
Regulatory
The development, approval and sale of animal health products are governed by the laws and regulations of each country in which we intend to sell our products. To comply with these regulatory requirements, we have established processes and resources to provide oversight of the development and launch of our products and their maintenance in the market.
Requirements for Approval of Veterinary Pharmaceuticals for Pets
As a condition to regulatory approval for sale of animal products, regulatory agencies worldwide require that a product to be used for pets be demonstrated to be safe for the intended use in the intended species; have substantial evidence of effectiveness for the intended use; have a defined manufacturing process that ensures that the product can be made with high quality consistency; and be safe for humans handling the product and for the environment.
Safety. To determine that a new veterinary drug is safe for use, regulatory bodies will require us to provide data from a safety study generated in laboratory cats and dogs tested at doses higher than the intended label dose, over a period of time determined by the intended length of dosing of the product. In the case of the CVM, the design and review of the safety study and the study protocol are completed prior to initiation of the study to help assure that the data generated will meet FDA requirements. These studies are conducted under rigorous quality control, including Good Laboratory Practice (“GLP”), to assure integrity of the data. They are designed to clearly define a safety margin, identify any potential safety concerns, and establish a safe dose for the product. This dose and effectiveness is evaluated in the pivotal field effectiveness study where the product is studied in the animal patient population in which the product is intended to be used. Field safety data, obtained in a variety of breeds and animals kept under various conditions, are evaluated to assure that the product will be safe in the target population. Safety studies are governed by regulations and regulatory pronouncements that provide the parameters of required safety studies and are utilized by regulatory bodies in the United States, the European Union, Japan and other countries.
Chemistry, Manufacturing and Controls. To assure that the product can be manufactured consistently, regulatory agencies will require us to provide documentation of the process by which the API is made and the controls applicable to that process that assure the API and the formulation of the final commercial product meet certain criteria, including purity and stability. For FDA and EMA approvals, both pharmaceutical API and commercial formulations are required to be manufactured at facilities that practice cGMP. After a product is approved, we will be required to communicate with the regulatory bodies any changes in the procedures or manufacturing site. For example, with regard to FDA-regulated products, different reporting requirements apply depending on the scope and extent of post-approval changes to the CMC. Generally, “major changes” (as defined in the FDA’s guidance documents) require a PAS filing, which has a 120-day review period by the FDA and must be approved by the FDA before distribution or sale of the product. “Moderate changes” (as defined in the FDA’s guidance documents) can be filed as a Supplement Changes Being Effected in 30 Days (“CBE30”) or as a Supplement Changes Being Effected (“CBE0”). Products manufactured involving changes filed as a CBE30 can be distributed and/or sold within 30 days of receipt of the CBE30 by the FDA or immediately if filed with the FDA as a CBE0. No affirmative approval is required by the FDA for those categories of changes. Finally, “minor changes” (as defined in the FDA’s guidance documents) are simply required to be provided to the FDA by companies in their annual reports on CMC application matters titled Minor Changes and Stability Reports.
Effectiveness. Early pilot studies may be done in laboratory cats or dogs to establish effectiveness and the dose range for each product. Data on how well the drug is absorbed when dosed by different routes and the relationship of the dose to the effectiveness are studied. When an effective dose is established, a study protocol to test the product in real world conditions is developed prior to beginning the study. In the case of the CVM, the pivotal effectiveness field study protocol is submitted for review and concurrence prior to study initiation, to help assure that the data generated will meet requirements. The pivotal field effectiveness study must be conducted with the formulation of the product that is intended to be commercialized, and is a multi-site, randomized, controlled study, generally with a placebo control. To reduce bias in the study, individuals doing the assessment are not told whether the subject is in the group receiving the treatment being tested or the placebo group. For pharmaceuticals, in both the United States and the European Union, the number of patients enrolled in the pivotal field effectiveness studies is required to be approximately 100 to 150 animal subjects treated
16
with the test product and a comparable number of subjects in the control group that receive the placebo. In many cases, a pivotal field study may be designed with clinical sites in both the European Union and the United States, and this single study may satisfy regulatory requirements in both the European Union and the United States.
Environmental and Human Safety. We will not be required under United States law to provide an environment impact statement for products currently in development if the products are given at the home of the pet’s owner or in a veterinary hospital. If products might result in some type of environmental exposure or release, the environmental impact must be assessed. For approval in the EU, a risk assessment for potential human exposure will be required.
Labeling, All Other Information, and Freedom of Information Summary. We also will be required to submit the intended label for the product, and also any information regarding additional research that has been conducted with the drug, to the CVM and other regulatory bodies for review. We will draft, and submit for regulatory review, the Freedom of Information Summary for use in the United States. This summary outlines the studies and provides substantial information that CVM uses to assess the drug’s safety and effectiveness and then publishes on its website.
Post-Approval Product Monitoring and Maintenance. Post-approval monitoring of products is required by law, with reports being provided to the CVM's Surveillance and Compliance group. Reports of product quality defects, adverse events or unexpected results are produced in accordance with the law. Additional regulatory filings include the Periodic Drug Experience Report and the Minor Changes and Stability Report.
United States
Three federal regulatory agencies regulate the health aspects of animal health products in the United States: the FDA; the USDA; and the Environmental Protection Agency (“EPA”).
The CVM at the FDA regulates animal pharmaceuticals under the Food, Drug and Cosmetics Act. The CVB at the USDA regulates veterinary vaccines and some biologics pursuant to the Virus, Serum, Toxin Act. The EPA regulates veterinary pesticides under the Federal Insecticide, Fungicide and Rodenticide Act. Many topical products used for treatment of flea and tick infestations are regulated by the EPA.
Our current product candidates are animal pharmaceuticals regulated by the CVM and animal biologics regulated by the USDA. Manufacturers of animal health pharmaceuticals, including us, must show their products to be safe, effective and produced by a consistent method of manufacture. The CVM’s basis for approving a drug application is documented in a Freedom of Information Summary. We will be required to conduct post-approval monitoring of FDA- and EMA-approved pharmaceutical products and to submit reports of product quality defects, adverse events or unexpected results to the CVM’s Surveillance and Compliance group.
Regulatory Process at the FDA
To begin the development process for our products in the United States, we establish an INAD file with the CVM. We then hold a pre-development meeting with the CVM to reach a general agreement on the plans for providing the data necessary to fulfill requirements for an NADA. During development, we submit pivotal protocols to the CVM for review and concurrence prior to conducting the required studies. We gather and submit data on manufacturing, safety and effectiveness to the CVM for review, and this review is conducted according to timelines specified in the ADUFA. Once all data have been submitted and reviewed for each technical section – safety, effectiveness and CMC – the CVM issues us a technical section complete letter as each section review is completed, and when the three letters have been issued, we compile a draft of the Freedom of Information Summary, the proposed labeling, and all other relevant information, and submit these as an administrative NADA for CVM review. Generally, if there are no deficiencies in the submission, the NADA is issued within 60 days after submission of the administrative NADA. After approval, we will be required to collect reports of adverse events and submit them on a regular basis to the CVM.
The CVM has an alternative approval process for drugs used in minor species, or for drugs that are used for a ‘minor use’ in a major species. This process is called MUMS which stands for minor use, minor species. For example, if it can be documented that the population of cats or dogs that contract a specific condition is below a specified number, a company can apply to the CVM for MUMS designation. Once designation has been granted, then we must submit the same safety and CMC data as required for a full NADA, and also submit some evidence of effectiveness. After a review period, the CVM can then grant a conditional approval. This approval allows for the commercialization of the product, while completing the pivotal effectiveness study required for a full NADA. Because in many cases the CMC section of the submission takes the longest, MUMS conditional approval may not shorten the time to commercialization. Following submission, review and approval of the pivotal field effectiveness study, the CVM may grant a full NADA.
17
Requirements for Approval of Certain Veterinary Biologics for Pets
There are many parallels between the requirements to receive approvals for a veterinary pharmaceutical product candidates and certain veterinary biologics product candidates. The terminology differs, but the three main components are the same: efficacy, manufacturing, and safety. USDA regulations are designed to ensure that veterinary biologics are pure, safe, potent and effective. The differences compared to pharmaceutical product regulations are based on the immunological nature of the mode of action of the product and the manufacturing process involving living organisms.
Efficacy. Documentation requirements depend significantly on product type and typically include data from preliminary dose determination studies and master seed immunogenicity/efficacy studies.
Safety. Typical safety documentation includes safety data from laboratory animal studies, typically rodents, studies in host animals, typically laboratory dogs or cats, in biocontainment, and field safety studies conducted in client-owned animals.
Manufacturing. The required documentation must include an Outline of Production, Master Seed Reports, and Summary Information Formats, or SIFs, for novel live biological products and products based on recombinant DNA technology. SIFs contain additional safety and identity data to establish proper biocontainment requirements and to conduct confirmatory testing. Other supportive documentation is product-type specific and includes in-process procedures and corresponding validation reports, potency test development report, stability reports, and veterinary biologics production and test for satisfactory three consecutive prelicensing serials (numbered lots) of product.
Other information. This includes labels or label sketches.
A unique requirement for veterinary biologics in the United States is that manufacturers must hold a United States Veterinary Biologics Establishment License to produce licensed veterinary biologicals. An establishment license will only be issued if at least one biological product qualifies for a license. Applications for veterinary biologics establishments include articles of incorporation for the applicant, qualifications of veterinary biologics personnel for key employees, water quality statement, facility blueprints, plot plans, and legends.
Regulatory Process at the USDA
Applicants are encouraged to contact the CVB early in the product development process. A licensing reviewer will be assigned to help with the regulatory process. Initially, the CVB will confirm that the proposed product meets the definition of a veterinary biologic and is subject to regulation by the CVB. The CVB then recommends that applicants submit a licensing plan, including pivotal study protocols, to the CVB for review and comment prior to initiating work that will be used to support product licensure. The USDA provides a complete list of guidance documents named “Veterinary Services Memorandums” that lay out the data requirements and regulatory process. Applicants that do not hold a United States Veterinary Biologics Establishment License need to submit the required documentation for the establishment and the product concurrently.
Study protocols and reports can be submitted any time after the initial applications have been made. The administrative process is facilitated by forms (APHIS Forms) that accompany the submissions and capture regulatory actions. There is no requirement to submit parts of dossiers or entire dossiers. The CVB provides official responses to submissions in hard copy mail indicating if more data are needed or that the submission was satisfactory to support licensure. When master seed and master cell reports have been found to be satisfactory, samples have to be submitted to the CVB laboratory for confirmatory testing. Once all requirements have been satisfactorily met, the CVB will issue a veterinary biological product license.
In cases of emergencies, which means there is no approved product available, the USDA will issue a time-limited conditional license after the manufacturing and safety requirements have been substantially fulfilled and a reasonable expectation of efficacy has been established. The applicant has to continue the pivotal efficacy program and product testing validation. The conditional license can be extended if reasonable progress towards full licensure can be demonstrated.
There are no statutory review times. Submissions enter the review queue in chronological order. Hence predictions of development timelines and time to approval are difficult to make. However, we believe the typical time to achieve conditional licensure is approximately three years and the typical time to achieve full licensure is approximately five years.
Furthermore, while the CVB regulates certain biologics (for instance, based on the immunological nature of the mode of action) the CVM regulates other biologics in a manner described above under “Regulatory Process at the FDA.”
Other Regulatory Considerations
Regulatory rules relating to human food safety, food additives, or drug residues in food will not apply to the products we currently are developing because our products are not intended for use in food production animals.
Advertising and promotion of animal health products is controlled by regulations in many countries. These rules generally restrict advertising and promotion to those claims and uses that have been reviewed and endorsed by the applicable agency. We will conduct a review of advertising and promotional material for compliance with the local and regional requirements in the markets where we sell pet therapeutics.
18
European Regulatory Process
The EMA regulates the scientific evaluation of applications for marketing authorizations via the centralized procedure for medicines developed by pharmaceutical companies for use in the EU. Its veterinary review section is distinct from the review section for human drugs. The CVMP is responsible for scientific review of the submissions for animal pharmaceuticals and vaccines. However, the European Commission is responsible for the grant of EU marketing authorizations. Once a centralized marketing authorization is granted by the European Commission, it is valid throughout the European Economic Area (“EEA”) (meaning the 28 member states of the EU plus, by extension pursuant to the EEA Agreement, Norway, Iceland and Liechtenstein). The centralized procedure is mandatory for approval of certain veterinary medicines, including those derived from biotechnology processes and veterinary medicines for use as growth or yield enhancers. Other veterinary medicines may be approved centrally if the product contains a new active substance or if the applicant can demonstrate to the CVMP that the product is sufficiently innovative. We believe our current product candidates contain new active substances or are sufficiently innovative and thus will be subject to central approval.
For all other products, the competent authorities of the EU Member States are responsible for granting marketing authorizations for products that are sold in their markets. Applicants who intend to market such products in more than one Member State may seek marketing authorizations under the mutual recognition procedure or the decentralized procedure, which are procedures designed to streamline and harmonize approval in multiple EU Member States. If the product has already been authorized in one Member State, the mutual recognition procedure facilitates mutual recognition of the existing authorization, so called reference Member State approval, in another Member State. The decentralized procedure, on the other hand, may be used in cases where the product has not received a marketing authorization in any Member State. Under this procedure, the applicant submits an identical dossier to each relevant Member State, and one, known as the reference Member State, takes the lead in reviewing the application. Under both procedures, other Member States are required to accept the reference Member State’s view on the approvability of the product unless they can identify significant public health reasons not to do so.
In general, the requirements for regulatory approval of an animal health product in the EU are similar to those in the United States, requiring demonstrated evidence of purity, safety, efficacy and consistency of manufacturing processes.
The EMA is responsible for coordinating scientific evaluation of applications for marketing approval via the centralized procedure for pet therapeutics in the EU. To perform these evaluations, the EMA established a specific scientific committee, the CVMP. The CVMP considers applications submitted by companies for the marketing approval of individual pet therapeutics and evaluates whether or not the medicines meet the necessary quality, safety and efficacy requirements. Assessments conducted by the CVMP are based on scientific criteria and are intended to ensure that pet therapeutics reaching the marketplace have a positive benefit-risk balance in favor of the pet population they are intended for. Based on the CVMP’s recommendation, a centralized marketing authorization is granted by the European Commission, which allows the product to be marketed throughout the EEA. The CVMP is also responsible for various post-authorization and maintenance activities, including the assessment of modifications or extensions to an existing marketing authorization.
To obtain a centralized marketing authorization from the European Commission, we must submit a marketing authorization application called a dossier. The dossier is the EMA’s equivalent of the FDA’s NADA and includes data from studies showing the quality, safety and efficacy of the product. The CVMP reviews and evaluates the dossier. For any dossier, a rapporteur and co-rapporteur are appointed from the members of the CVMP. Their role is to lead the scientific evaluation and prepare the assessment report. The rapporteur can utilize experts to assist it in performing its assessment. The report is critiqued by the co-rapporteur and other members of the CVMP before the CVMP makes its determination. The final opinion of the CVMP is generally given within 210 days of the submission of a dossier.
For products that are not eligible for centralized approval, the competent authorities of the EU Member States are responsible for granting marketing authorizations for products that are sold in their markets. Such products may be approved nationally in one Member State, or in multiple Member States via the mutual recognition procedure or the decentralized procedure.
In the EU, products for MUMS are eligible for regulatory incentives such as free scientific advice and fee reductions. These incentives may apply, for example, if it can be documented that the population of cats or dogs that contract a specific condition is below a specified number in Europe. However, the EMA recently announced that fee reductions are only applicable to products indicated for food-producing species. An applicant may apply to the EMA for MUMS classification for any product irrespective of the intended route of approval (i.e. centralized, decentralized or national approval) and incentives may be requested for all routes of authorization. The CVMP has established guidelines specific to MUMS for data requirements, which apply to all sections of the application, i.e. quality, safety and efficacy. Consequently, there may be scope for a reduced quality data package. Similarly, the safety and efficacy sections might be abridged to a certain extent (more flexibility for the combination of dose-determination, dose-confirmation and field studies) provided reasonable evidence of safety and effectiveness are submitted. However, the CVMP and national veterinary medicines regulators have significant discretion in this respect. Overall, data requirements for demonstrating quality, efficacy and safety in the target species for minor use indications of a new medicine will be determined on a case-by-case basis and any potential applicant should seek scientific advice on specific data requirements to guide its research and development activities.
19
There are three different procedures to receive a marketing authorization (regulatory approval) in Europe, the decentralized procedure (“DCP”), the mutual recognition procedure (“MRP”), and the centralized procedure (“CP”). The centralized procedure is mandatory for certain products and technologies, for example biopharmaceuticals, gene therapy products, somatic cell therapeutic products or certain therapeutic areas, for example oncology or neurodegenerative disorders. Otherwise the sponsor can opt between CP and DCP.
An application for CP is submitted to the EMA which coordinates the scientific evaluation. To perform these evaluations, the EMA established a specific scientific committee, the CVMP. The CVMP evaluates whether the medicines meet the necessary quality, safety and efficacy requirements. Assessments conducted by the CVMP are based on scientific criteria and are intended to ensure that pet therapeutics reaching the marketplace have a positive benefit-risk balance in favor of the pet population they are intended for. Based on the CVMP’s recommendation, a centralized marketing authorization is granted by the European Commission, which allows the product to be marketed in any of the EU states. The CVMP is also responsible for various post-authorization and maintenance activities, including the assessment of modifications or extensions to an existing marketing authorization.
To obtain authorization from the EMA through CP, we must submit a marketing authorization application called a dossier, which consists of four parts. Part 1 includes administrative information, part 2 quality documentation, part 3 safety documentation, and part 4 efficacy documentation. For any dossier, a rapporteur and co-rapporteur are appointed from the members of the CVMP. Their role is to lead the scientific evaluation and prepare the assessment report. The rapporteur can utilize experts to assist it in performing its assessment. The entire regulatory assessment period is limited to 210 days, which is divided in three periods. After an initial review period of 120 days the sponsor receives a list of questions and the clock is stopped. With the submission on the response the clock starts again and after a 60-days review period the CVMP discusses a draft opinion and the clock is stopped. If felt necessary, an oral explanation is offered. In the last 30 days of the review period the CVMP finalizes the opinion and the assessment report.
A DCP can be used for products that have not been approved in any of the EU member states and do not fall under mandatory CP. The sponsor selects one Reference Member State (“RMS”) and one or more Concerned Member States (“CMS”). The RMS leads the scientific evaluation and with the input from CMS issues the initial and final assessment report. The regulatory assessment period is similar to the CP and divided into two periods of 120 and 90 days, respectively. The procedure ends with a consensus decision and leads to products approval in the RMS and CMS.
The MRP must be used for products that have been approved in at least one EU member state either by national procedure or DCP. The MRP uses an existing and if needed updated assessment report to extend marketing authorizations to more EU member states.
Rest of World
Each other country has its own regulatory requirements for approving and marketing veterinary pharmaceuticals. For example, in Brazil, the Ministry of Agriculture, Livestock Products and Supply (“MAPA”), is responsible for the regulation and control of pharmaceuticals, biologicals and feed additives for animal use. MAPA’s regulatory activities are conducted through the Secretary of Agricultural Defense and its Livestock Products Inspection Department. In addition, regulatory activities are conducted at a local level through the Federal Agriculture Superintendence. These activities include the inspection and licensing of both manufacturing and commercial establishments for veterinary products, as well as the submission, review and approval of pharmaceuticals, biological and feed additives.
In Australia, the Australian Pesticides and Veterinary Medicines Authority (“APVMA”), is the Australian government statutory authority for the registration of all agricultural and veterinary products. The APVMA assesses applications from manufacturers of veterinary pharmaceuticals and related products.
Many country specific regulatory laws contain provisions that include requirements for labeling, safety, efficacy and manufacturers’ quality control procedures to assure the consistency of the products, as well as company records and reports. With the exception of the EU, the regulatory agencies of most other countries generally refer to the FDA, USDA, EMA, and other international animal health entities, including the World Organization for Animal Health and the Codex Alimentarius Commission, in establishing standards and regulations for veterinary pharmaceuticals and vaccines.
20
Segment and Geographic Information
We operate in one business segment and have operations in the United States and Belgium. See our consolidated financial statements for further information regarding our segment, including revenues and loss from operations. See Note 2 “Summary of Significant Accounting Policies” to our consolidated financial statements for total assets, and geographic information including revenues and long-lived assets.
Employees
As of December 31, 2017, we had a total of 85 employees, including 84 full-time employees.
As of March 9, 2018, we have a total of 88 employees, including 87 full-time employees. We have a total of 14 employees with D.V.M., V.M.D., M.D. or Ph.D. degrees. Within our workforce, 18 employees are engaged in research and development and 70 in manufacturing and supply chain, business development, marketing and sales, finance, legal, human resources, facilities, information technology, and general management and administration.
Available Information
We maintain a website at www.aratana.com. We make available free of charge on our website our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to these reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to the Securities and Exchange Commission.
Executive Officers of the Registrant
The executive officers of Aratana Therapeutics, Inc. as of March 9, 2018, are as follows:
Steven St. Peter, M.D., age 51, is one of our founders and has served as our President and Chief Executive Officer since September 2012. He has been a member of our Board of Directors since December 2010 and served as the Chairman of our Board of Directors from December 2010 to September 2012. Dr. St. Peter was a Managing Director of MPM Asset Management LLC from January 2004 to May 2012, where he focused his investments on both venture and buyout transactions across the pharmaceuticals and medical technology industries. He has previous investment experience from Apax Partners and The Carlyle Group, two private equity firms. Dr. St. Peter was previously an Assistant Clinical Professor of medicine at Columbia University. He received his M.D. from Washington University and completed his residency and fellowship at the Hospital of the University of Pennsylvania. Prior to his medical training, he was an investment banker at Merrill Lynch. Dr. St. Peter also holds an M.B.A. from the Wharton School of Business at the University of Pennsylvania and a B.A. in Chemistry from the University of Kansas. Dr. St. Peter previously served as a director of PharmAthene, Inc., a publicly-traded biodefense company, from August 2007 through May 2017 as a member of its governance and nominating committee. Dr. St. Peter has also served as a member of the Board of Directors of the Kansas City Area Life Sciences Institute since March 2014 and as a member of the Board of Directors of the Greater Kansas City Foundation since November 2015. Dr. St. Peter’s previous board experience includes MPM Acquisition Corp., Proteon Therapeutics, Inc., Rhythm Pharmaceuticals, Inc. and Syndax Pharmaceuticals, Inc. As noted above, Dr. St. Peter is a member of our Board of Directors and as such, we believe Dr. St. Peter is qualified to serve on our Board because of his diverse background as a venture capital investor, investment banker, physician and director of several healthcare companies, which brings a unique perspective to our Board.
Ernst Heinen, D.V.M., Ph.D., age 55, has served as our Chief Development Officer since March 5, 2014. In addition, he served as our Head of Drug Evaluation and Development from June 2012 until March 5, 2014. From 1990 to 2012, Dr. Heinen held positions of increasing responsibility at Bayer Animal Health, the animal health division of Bayer AG, where he ultimately served as Vice President of Research & Development and Veterinary Technical Services, Pets. Dr. Heinen currently serves on the Kansas State University Olathe Advisory Board and previously served on the boards of the Kansas City Area Development Council and the Center for Animal Health Innovation, and he is the author of dozens of scientific articles and presentations focused on the animal health industry. Dr. Heinen received a veterinary degree and a D.V.M. in veterinary microbiology from the Justus-Liebig-University of Giessen Veterinary School in Giessen, Germany, and is a certified specialist in veterinary microbiology.
Brent A. Standridge, age 60, has served as our Chief Operating Officer since July 1, 2016. Prior to accepting the Chief Operating Officer role, Mr. Standridge served as a consultant to the Company from January 2016 through June 2016. Previously, Mr. Standridge formed a consulting business, providing commercial and operations-related services to animal health companies, including from July 2010 to May 2014, serving as a commercial consultant for Putney, Inc., a privately-held pet pharmaceutical company. Prior to that, he worked for Fort Dodge Animal Health, a global manufacturer of animal health products and division of Wyeth, from 1982 until February 2010, where he held numerous sales and marketing positions including Senior Vice President of Sales and Marketing, North America from 1999 until February 2010. During his career with Fort Dodge, Mr. Standridge was responsible for developing and building species-specific sales forces to maximize company sales, fully leverage marketing initiatives and provide optimal customer service as well as being instrumental in the acquisition and integration of several companies and business units. Mr. Standridge earned a Bachelor of Science in Animal Science and Agricultural Economics from The Ohio State University.
21
Craig A. Tooman, age 52, has served as our Chief Financial Officer since November 2013 and our Treasurer since January 2014. He was a member of our Board of Directors from April 2012 to November 2013, before accepting the CFO role. Mr. Tooman previously served as the Chief Executive Officer of Avanzar Medical, Inc., a privately-held company focused on commercial oncology opportunities, from February 2012 until November 2014. Mr. Tooman was also the founder and principal of Stockbourne LLC, a firm that provides strategic business and financial advisory services, a position he held from January 2011 to November 2013. From July 2010 to January 2011, Mr. Tooman was the Senior Vice President of Finance and Chief Financial Officer of Ikaria Inc., a biotherapeutics company. From January 2005 to July 2010, Mr. Tooman was the Executive Vice President of Finance and Chief Financial Officer at Enzon Pharmaceuticals, a biopharmaceutical company. Prior to that, Mr. Tooman was the Senior Vice President of Strategic Planning and Corporate Communications at ILEX Oncology, Inc. and the Vice President of Investor Relations at Pharmacia Corporation. Mr. Tooman previously served on the Board of Directors of Insite Vision Incorporated, a publicly-traded ophthalmological company, from September 2011 to November 2015. Mr. Tooman also served on the Board of Directors and as chair of the audit committee of Xanodyne Pharmaceuticals Inc., a privately-held specialty pharmaceutical company, from October 2007 until it was acquired in June 2013 upon the sale of its commercial assets. He has a B.A. in Economics from Kalamazoo College and M.B.A. in Finance from the University of Chicago.
Non-Employee Directors
The non-employee directors of Aratana Therapeutics, Inc. as of March 9, 2018, are as follows:
Wendy L. Yarno, age 63, has been a member of our Board of Directors since October 2013 and since August 2015 has served as the Chairperson of the Board. Ms. Yarno retired in September 2008 from Merck & Co., Inc. following a 26-year career there in commercial and human resource positions of increasing seniority, most recently Chief Marketing Officer before she retired. In that role, Ms. Yarno led a global organization charged with all aspects of supporting pre-and post-launch commercialization of pharmaceuticals in more than 20 therapeutic areas. Prior to this role, she served as General Manager, Cardiovascular/Metabolic United States Business Unit, where she had P&L responsibility for Merck’s largest therapeutic area, and as Senior Vice President, Human Resources. From September 2010 through September 2011, Ms. Yarno was the Chief Marketing Officer of HemoShear LLC, a biotechnology research company and leading developer of human cell-based surrogate systems for discovery and assessment of new drug compounds. Ms. Yarno currently serves on the board of directors of publicly-traded biopharmaceutical companies MyoKardia, Inc., Global Blood Therapeutics, Inc., Inovio Therapeutics, Inc. and Alder Biopharmaceuticals, Inc. Ms. Yarno has served as a Director and a member of the compensation committee for both Myokardia and Alder Biopharmaceuticals since March 2017. Since December 2017, she has served as a Director and a member of the compensation committee for both Global Blood Therapeutics and Inovio Therapeutics. Ms. Yarno served as a Director and member of the governance and nominating committee and compensation committee of St. Jude Medical, Inc., a Fortune 500 medical device company, from April 2002 until January 2017 when St. Jude Medical was acquired by Abbott Laboratories. She served as a Director and member of the governance and nominating committee and audit committee as well as the chair of the compensation committee of Medivation, Inc., a publicly-traded biopharmaceutical company, from April 2013 until September 2016 when Medivation was acquired by Pfizer Inc. Ms. Yarno also served as a Director and member of the compensation committee of Durata Therapeutics, Inc., a publicly-traded pharmaceutical company, from August 2014 until November 2014 when Durata was acquired by Actavis plc. Ms. Yarno received a B.S. in Business Administration from Portland State University and an M.B.A from Temple University. We believe Ms. Yarno is qualified to serve on our Board based on her extensive experience in commercialization of pharmaceutical products and in human resource management in the pharmaceutical industry and her service on the boards of multiple life sciences companies.
Laura A. Brege, age 60, has been a member of our Board of Directors since February 2014. In September 2015, Ms. Brege became managing director of Cervantes Life Sciences Partners, LLC, a healthcare advisory and consulting company. She also served as President and Chief Executive Officer of Nodality, Inc., a privately-held life sciences company, from September 2012 to July 2015. Prior to joining Nodality, from January 2011 to January 2012, Ms. Brege was the Executive Vice President, Corporate Affairs of Onyx Pharmaceuticals, Inc., a biopharmaceutical company. From October 2007 to January 2011, she was the Chief Operating Officer, and from June 2006 to October 2007, she was the Executive Vice President and Chief Business Officer of Onyx Pharmaceuticals. From 1999 to 2006, Ms. Brege was a General Partner at Red Rock Capital Management, a venture capital firm. Previously, Ms. Brege served as Chief Financial Officer at companies such as COR Therapeutics, Inc., a biotechnology company, and Flextronics, Inc., a supply-chain solutions company. Ms. Brege currently also serves on the Board of Directors of publicly-traded Acadia Pharmaceuticals, Inc., Dynavax Technologies Corporation, Pacira Pharmaceuticals, Inc. and Portola Pharmaceuticals, Inc. Ms. Brege has served as a Director of Acadia since May 2008 and is currently a member of its audit committee and has served as a Director and chair of the audit committee of Dynavax since February 2015. Ms. Brege has served as a Director of Pacira since June 2011 and is currently the chair of its audit committee and a member of its nominating and governance committee and has served as a Director and member of the audit committee of Portola since January 2015. Ms. Brege previously served as a member of the Board of Directors of publicly-traded Angiotech Pharmaceuticals, Inc. from 2007 to 2011 and Delcath Systems, Inc. from 2012 to December 2014. Ms. Brege earned her undergraduate degrees from Ohio University and has an M.B.A. from the University of Chicago. We believe Ms. Brege is qualified to serve on our Board based on her strong background in finance and her extensive executive leadership experience in the life sciences and biotechnology industries, including her service as a public company director and in various executive officer roles.
22
David L. Brinkley, age 60, has been a member of our Board of Directors since March 2014. Mr. Brinkley worked for Theravance, Inc., a publicly-traded biopharmaceutical company, from 2000 to 2013, most recently as the Head of Business Development from November 2008 to July 2013. Mr. Brinkley had previously served as Senior Vice President, Commercial Development at Theravance from September 2000 through December 2007, when he left to start a consulting practice. From 1996 to 2000 he served as Worldwide Team Leader for Viagra at Pfizer Inc., leading the team that had full responsibility for the global launch and marketing of Viagra. Mr. Brinkley joined Pfizer in 1995 through its acquisition of SmithKline Beecham’s Animal Health operations and was Director of New Product Planning before leading the Viagra launch team. Mr. Brinkley held various management positions with SmithKline Animal Health from 1983 to 1995. Mr. Brinkley previously served on the Board of Directors of Ziarco Pharma Ltd., a privately-held pharmaceutical company. Mr. Brinkley holds an M.A. with honors in International Economics from the School of Advanced International Studies of the Johns Hopkins University and a B.A. in International Relations from Kent State University, where he graduated with University Honors. We believe Mr. Brinkley is qualified to serve on our Board due to his extensive leadership experience in the biopharmaceutical industry, including his roles at Theravance and Pfizer.
Robert “Rip” B. Gerber, age 55, has been a member of our Board of Directors since October 2012. Since January 2015, Mr. Gerber has served as an executive at Vlocity, Inc., a software company, including as Chief Marketing Officer and Head of Alliances since June 2015. From July 2009 to January 2015, he served as the President and Chief Executive Officer of Locaid Technologies, Inc., a telecommunications software company. From June 2006 to June 2009, Mr. Gerber served as a member of the advisory board of SignalDemand Inc., a private firm focused on producing margin optimization software. From May 2004 to May 2006, Mr. Gerber served as Chief Marketing Officer and Senior Vice President of Intellisync Corporation, a public company and provider of data synchronization software to consumer mobile devices. Prior to that role, he served as Senior Vice President at Carlson Companies, Inc., one of the largest family-held corporations in the United States. Mr. Gerber was also on the founding executive team of Commtouch Software, Inc., where, as Chief Marketing Officer, he was a lead executive in taking the Company public in 1999. Earlier in his career, Mr. Gerber was a consultant for Deloitte & Touche LLP, a public accounting firm. Mr. Gerber serves on the Board of Directors of LocationSmart, a privately-held location software company. He holds an M.B.A. from Harvard Business School and a B.S. in Chemical Engineering from the University of Virginia. We believe Mr. Gerber is qualified to serve on our Board because of his experience as an entrepreneur and his extensive background in operational, marketing and strategic planning.
Irvine “Irv” O. Hockaday, Esq., age 81, has been a member of our Board of Directors since August 2014. Mr. Hockaday is the retired President and Chief Executive Officer of Hallmark Cards, Inc. Prior to joining Hallmark in 1983, Mr. Hockaday served as President and Chief Executive Officer of Kansas City Southern Industries, Inc. He was a member of the Hallmark Board of Directors from 1978 through 2001. Mr. Hockaday has been on the Board of Directors of the Estee Lauder Companies, Inc. since 2001 and is currently lead Director and chair of its audit committee. Mr. Hockaday is a former Director or Lead Director of Crown Media Holdings, Inc., Dow Jones & Company, Inc., Ford Motor Company and Sprint Nextel Corporation. He currently holds various civic positions including trustee of the Hall Family Foundation and board member of Kansas City Area Life Sciences Institute and has previously served as chairman of the board of the Tenth District Federal Reserve Bank. He graduated with an A.B. in English from Princeton University in 1958 and from the University of Michigan Law School with a J.D. in 1961. We believe Mr. Hockaday is qualified to serve on our Board due to his extensive experience as a Chief Executive Officer and board member of public companies.
Merilee Raines, age 62, has been a member of our Board of Directors since February 2014. Ms. Raines served as Chief Financial Officer of IDEXX Laboratories, Inc., a publicly-traded company providing diagnostic and IT products and services primarily to the companion animal health market, from October 2003 until her retirement in May 2013. Ms. Raines also served as Executive Vice President of IDEXX Laboratories from July 2012 to May 2013, and as Corporate Vice President, Finance of IDEXX Laboratories from May 1995 to July 2012. Ms. Raines has served as a Director of Watts Water Technologies, Inc., a publicly-traded manufacturer of products and systems focused on control, conservation and quality of water, since 2011, and is currently a member of its nominating and corporate governance committee and chair of its audit committee. Ms. Raines previously served as a Director of Affymetrix, Inc., a publicly-traded provider of life sciences products and molecular diagnostic products, from January 2015 until April 2016 when Affymetrix was acquired by Thermo Fisher Scientific, Inc. Ms. Raines also served as a Director of PetVet Care Centers, a privately-held operator of a network of veterinary hospitals from April 2016 until February 2018 when PetVet Care was acquired by KKR. Ms. Raines earned a bachelor’s degree in mathematics from Bowdoin College and an M.B.A. from the University of Chicago. We believe Ms. Raines is qualified to serve on our Board based on her experience as an executive of a public company in the animal health industry and her extensive financial expertise, including her role as Chief Financial Officer of IDEXX Laboratories and her service on the audit committee of Watts Water Technologies.
Robert P. Roche, age 62, has been a member of our Board of Directors since June 2014. Mr. Roche is the founding member of Robert Roche Associates, LLC, a consulting firm providing guidance to the pharmaceutical and healthcare industries. Mr. Roche created this firm upon his retirement from Cephalon, Inc., a biopharmaceutical company, in February 2010. Mr. Roche joined Cephalon in January 1995 as the Vice President of Sales and Marketing and was named Executive Vice President, Worldwide Pharmaceutical Operations of Cephalon in 2005. Before joining Cephalon, Mr. Roche served as Director and Vice President, Worldwide Strategic Product Development, for SmithKline Beecham’s central nervous system and gastrointestinal products business. Mr. Roche also was Managing Director of SmithKline’s pharmaceutical operations in the Philippines. Prior to that, he held senior marketing positions in Canada and Spain and had product planning responsibilities for SmithKline in Latin America. Mr. Roche began his pharmaceutical career in 1982 with SmithKline as a United States pharmaceutical sales representative. Mr. Roche has served as a Director of Antares Pharma, Inc., a publicly-traded specialty pharmaceutical company, since July 2013 and is currently a member of its governance and
23
nominating committee and audit committee. In December 2016, Mr. Roche was appointed as a Director of Egalet Corporation, a publicly-traded specialty pharmaceutical company focused on innovative treatments of pain and other conditions, and is currently a member of its compensation committee and nominating and corporate governance committee. Mr. Roche is also currently a Director of Paragon Bioservices, Inc., a privately-held contract development and manufacturing organization. He formerly served as a Director of EKR Therapeutics until its acquisition in 2012, NuPathe Inc. until its acquisition in February 2014 and Civitas Therapeutics until its acquisition in November 2014. He also serves on the boards of Bryn Mawr Hospital and Westtown School. Mr. Roche earned his B.A. from Colgate University and his M.B.A. from The Wharton School at the University of Pennsylvania. We believe Mr. Roche is qualified to serve on our Board due to his executive and board leadership experience in the global pharmaceutical industry and his extensive commercial operations and product launch background.
John Vander Vort, Esq., age 53, has been a member of our Board of Directors since September 2012. Mr. Vander Vort is currently a Managing Director at Pilot House Associates, LLC, a family investment office based in Boston which he joined in September 2014. Prior to this role, Mr. Vander Vort was a Managing Director and the Chief Operating Officer of Charlesbank Capital Partners, a private equity firm. Mr. Vander Vort joined Charlesbank in September 2013 from MPM Asset Management LLC, a venture capital firm, where he served as a Managing Director, the Chief Operating Officer and the Chief Compliance Officer since May 2005, and he served on the Board of Directors of MPM Acquisition Corp., a public shell company, from February 2008 to November 2010. Prior to joining MPM Asset Management, from May 2003 until May 2005, he worked as Portfolio Manager for DuPont Capital Management. Prior to that, he was a General Partner and co-founder of BlueStream Ventures, a venture capital firm. Previously, he was a Managing Director at Dain Rauscher Wessels (now the Royal Bank of Canada), where he was the head of the West Coast networking and communications investment banking group and served as an advisor to leading venture-backed technology companies. Mr. Vander Vort began his career as a corporate transaction attorney in the San Francisco office of Cooley Godward, where he represented venture capital firms and venture-backed companies. Mr. Vander Vort earned his B.A. from Amherst College and his J.D. from The University of Chicago Law School. We believe Mr. Vander Vort is qualified to serve on our Board because of his background in venture capital, significant legal experience and business acumen.
24
Investing in our common stock involves a high degree of risk. You should carefully consider the important risks described below, as well as the other information contained in or incorporated by reference into our public filings with the Securities and Exchange Commission, before deciding whether to invest in our common stock. The occurrence of any of the events or developments described below could harm our business, financial condition, results of operations and growth prospects. In such an event, the market price of our common stock could decline, and you may lose all or part of your investment.
Risks Related to Our Business
We have a limited operating history and have incurred significant losses since our inception and we anticipate that we will continue to incur losses for the foreseeable future, and our limited operating history makes it difficult to assess our future viability.
We are a fully integrated pet therapeutics company in the animal health industry that transitioned into a commercial enterprise in 2016, but we have a limited operating history. The development of pet therapeutics is a highly speculative undertaking and involves a substantial degree of risk. We currently have a product pipeline with multiple therapeutics under development, and in 2016, we received FDA approval of three therapeutics, GALLIPRANT, ENTYCE and NOCITA. We also have a biologic, Canine Osteosarcoma Vaccine, Live Listeria Vector (AT-014), for which we received a conditional license from the USDA in December 2017, but for which we do not expect to receive significant revenues during the ongoing clinical study.
We are not profitable and have incurred losses in each year since our inception in December 2010. In addition, we have limited experience and have not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the animal health industry. We continue to incur significant research and development expenses, selling expenses and other expenses related to our ongoing operations. Our net loss for the year ended December 31, 2017 was $47.5 million, for the year ended December 31, 2016 was $33.6 million and for the year ended December 31, 2015 was $84.1 million. As of December 31, 2017, we had an accumulated deficit of $233.3 million and we had $67.6 million in cash, cash equivalents and short-term investments. We expect to continue to incur losses for the foreseeable future, and we expect these losses to continue as we commercialize our FDA-approved therapeutics and continue our development of, and seek regulatory approvals for, our therapeutic candidates by the FDA, or for our biologic therapeutics, the USDA. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Our prior losses, combined with expected future losses, have had and will continue to have an adverse effect on our stockholders’ equity and working capital.
We may require substantial additional financing to achieve our goals, and a failure to obtain this necessary capital when needed on acceptable terms, or at all, could force us to delay, limit, reduce or terminate our therapeutics portfolio expansion, product development, other operations or commercialization efforts.
Since our inception, a majority of our resources have been dedicated to the in-licensing, acquisition and research and development of our therapeutics and current therapeutic candidates. We believe that we will expend substantial resources for the foreseeable future for the commercialization of our FDA-approved therapeutics and the continued development of, and obtaining regulatory approval for, our therapeutic candidates and any future therapeutic candidates we may choose to pursue. We also have an active in-licensing effort focused on identifying human therapeutics for development and commercialization as pet therapeutics. Expenditures related to the foregoing efforts will include costs associated with identifying potential therapeutic candidates, licensing or acquisition payments, conducting target animal studies, completing other research and development, obtaining regulatory approvals and manufacturing and supply, as well as marketing and selling any therapeutics approved for sale. In addition, other unanticipated costs may arise. Because the outcome of any target animal study is uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development and commercialization of any of our current or future therapeutic candidates. As of the date of the filing of this 2017 Annual Report, we believe that our existing cash, cash equivalents and short-term investments on hand at December 31, 2017, together with the proceeds of the January 2018 at-the-market offering, will allow us to fund our operations and our debt obligations at least through March 31, 2019. However, our operating plan may change as a result of many factors currently unknown to us, and we may seek additional funds sooner than planned through public or private equity or further debt (re)financings or other sources, such as strategic collaborations. Such (re)financings may result in dilution to stockholders, imposition of debt covenants and repayment obligations, or other restrictions that may affect our business. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
Our future capital requirements depend on many factors, including, but not limited to:
|
|
• |
|
the cost of commercialization activities for any of our current therapeutics, current therapeutic candidates or future therapeutic candidates, including marketing, sales and distribution costs; |
|
|
• |
|
the cost of manufacturing our current therapeutics, current therapeutic candidates and future therapeutic candidates and any therapeutics we successfully commercialize as well as the cost of minimum purchase commitments and the potential for funding time lags between purchase commitment and payment from the sale of the therapeutic; |
|
|
• |
|
the scope, progress, results and costs of researching and developing our current or future therapeutic candidates and conducting target animal studies; |
25
|
|
• |
|
the timing of, and the costs involved in, obtaining regulatory approvals for any of our current or future therapeutic candidates, or for our therapeutics, if any follow-up approval is required; |
|
|
• |
|
the upfront and other payments, and associated costs, related to identifying, acquiring and in-licensing new therapeutic candidates; |
|
|
• |
the number and characteristics of the therapeutic candidates we pursue; |
|
|
• |
whether we acquire any other companies, assets, intellectual property or technologies in the future; |
||
|
|
• |
|
our ability to collaborate with companies with an established commercial presence in Europe and/or other countries to provide our therapeutics in that market; |
|
|
• |
|
our ability to establish and maintain strategic collaborations, licensing or other arrangements and the financial terms of such arrangements, and the potential costs and other financial terms of amending or terminating such arrangements, including litigation costs and the outcome of such litigation; |
|
|
• |
|
whether we are required to repay grant amounts that we received from foreign, United States and/or state governments; |
|
|
• |
|
the expenses needed to attract and retain skilled personnel; |
|
|
• |
|
the costs associated with being a public company; |
|
|
• |
|
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent rights, including litigation costs; |
|
|
• |
|
any litigation we may be involved in from time to time; and |
|
|
• |
|
demand for our commercialized therapeutics. |
Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to delay, limit, reduce or terminate:
|
|
• |
|
our target animal studies or other development activities for our current or future therapeutic candidates; |
|
|
• |
|
our establishment of sales and marketing capabilities or other activities that may be necessary to launch and/or commercialize any of our current therapeutics, current therapeutic candidates or future therapeutic candidates; or |
|
|
• |
|
our in-licensing and acquisition efforts and expansion of our therapeutics portfolio. |
We have recognized substantial intangible asset impairment losses and may be required to recognize additional non-cash impairment losses in the future.
During the third quarter of 2015, we recorded a non-cash impairment charge of $43.4 million related to our intangible assets BLONTRESS, TACTRESS, AT-007 and AT-011. During the second quarter, fourth quarter and for the year ended 2016, we recorded non-cash impairment charges of $2.7 million, $5.2 million and $7.9 million, respectively, related to our intangible assets BLONTRESS, TACTRESS and AT-007. During the fourth quarter of 2017, we recorded non-cash impairment charges of $7.4 million related to our intangible assets AT-006 and AT-008. At December 31, 2017, we had $6.6 million of remaining intangible assets on our balance sheet, compared to $7.6 million at December 31, 2016. We could experience material impairment losses in the future. Certain factors, including negative pre-clinical or clinical study results and reduced market potential, might have a negative impact on the carrying value of our intangible assets. For example, with respect to BLONTRESS and TACTRESS, which as of December 31, 2016 had both been fully impaired, clinical results reviewed in 2016 indicated that neither BLONTRESS or TACTRESS was adding significant progression free survival indicating smaller commercial opportunities. The process of testing intangible assets for impairment involves numerous judgments, assumptions and estimates made by management including expected future profitability, cash flows and the fair values of assets and liabilities, which inherently reflect a high degree of uncertainty and may be affected by significant variability. If the business climate deteriorates, then actual results may not be consistent with these judgments, assumptions and estimates, and our intangible assets may become further impaired in future periods. This would in turn have an adverse impact on our business, financial condition and results of operations.
We have been named as a party in stockholder class action lawsuits, and we may be named in additional litigation, which will require significant management time and attention, and may result in significant legal expenses and an unfavorable outcome, which could have a material adverse effect on our business, operating results and financial condition.
We are and may become subject to legal proceedings and claims that arise in or outside the ordinary course of business, including those related to patents, product liability and government investigations. Also, we are and may become subject to purported class action lawsuits filed against us on behalf of certain purchasers of our common stock. Securities class action suits and derivative suits are often brought against companies following periods of volatility in the market price of their securities.
26
In February 2017, two purported class action lawsuits were filed in the United States District Court for the Southern District of New York against the Company and two of our current officers. Those cases have been consolidated into one purported class action lawsuit under the caption, In re Aratana Therapeutics, Inc., et al., Case No. 1:17-cv-00880. The consolidated lawsuit asserts claims under Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended, and is premised on allegedly false and/or misleading statements, and alleged non-disclosure of material facts, regarding our business, operations, prospects and performance during the proposed class period of March 16, 2015 to March 13, 2017. The complaints seek monetary damages, costs, attorney’s fees and other equitable and injunctive relief. See Part I, Item 3. “Legal Proceedings.”
We are vigorously defending all lawsuits and claims asserted, including by filing a motion to dismiss. We cannot assure you, however, that we will be successful. Also, our insurance coverage may be insufficient, our assets may be insufficient to cover any amounts that exceed our insurance coverage, and we may have to pay damage awards or otherwise may enter into settlement arrangements in connection with such claims. Any such payments or settlement arrangements in this current litigation or any future litigation could have material adverse effects on our business, operating results or financial condition. Even if the plaintiffs’ claims are not successful, this or future litigation could result in substantial costs and significantly and adversely impact our reputation and divert management’s attention and resources, which could have a material adverse effect on our business, operating results or financial condition. In addition, such lawsuits may make it more difficult to finance our operations.
Unstable market and economic conditions may have serious adverse consequences on our business.
Our business may be adversely affected by the recent unpredictable and unstable market conditions. If the equity and credit markets deteriorate, it may make any necessary debt or equity financing more difficult to obtain and more costly. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our development programs, commercialization efforts, financial performance and stock price and could require us to delay or abandon plans for our target animal studies and/or the commercialization of any approved therapeutics. In addition, difficult economic conditions may limit pet owners’ discretionary funds, which could in turn limit their ability to purchase pet therapeutics. A tight spending climate for pet owners could negatively affect our ability to generate revenues from any approved therapeutics. Further, we rely on third-parties for several aspects of our business, including contract manufacturers for the manufacture of our therapeutics, licensors of pharmaceutical compounds, national and regional distributors and national third-party logistics providers. During challenging and uncertain economic times and in difficult credit markets, there may be a disruption or delay in the performance of our third-party contractors and other collaborators. If such third parties are unable to satisfy their commitments to us, or if they become bankrupt or insolvent, our agreements with such parties may terminate, and our business and results of operations would likely be adversely affected.
The terms of our credit facility place restrictions on our operating and financial flexibility.
Effective as of October 16, 2015, we and Vet Therapeutics, Inc., and together with us, the borrowers, entered into a Loan and Security Agreement, with Pacific Western Bank, or Pacific Western, as collateral agent and the lenders party thereto from time to time, or the lenders, including Pacific Western and Oxford Finance, LLC, that is secured by substantially all of the borrowers’ personal property other than intellectual property. The outstanding principal balance under the loan agreement was $31.5 million under the term loan facility and $5.0 million under the revolving facility at December 31, 2017.
The loan agreement contains customary representations and warranties and customary affirmative and negative covenants, including, among others, limits or restrictions on the borrowers’ ability to incur liens, incur indebtedness, make certain restricted payments, make certain investments, merge, consolidate, make an acquisition, enter into certain licensing arrangements and dispose of certain assets. The loan agreement also contains customary events of default that entitle the lenders to cause the borrowers’ indebtedness under the loan agreement to become immediately due and payable. The events of default, some of which are subject to cure periods, include, among others, a non-payment default, a covenant default, the occurrence of a material adverse change in our business, the occurrence of an insolvency, a material judgment default, defaults regarding other indebtedness and certain actions by governmental authorities. Upon the occurrence and for the duration of an event of default, an additional default interest rate equal to 4% per annum will apply to all obligations owed under the loan agreement and would provide Pacific Western, as collateral agent, with the right to exercise remedies against us and the collateral securing the loan agreement, including foreclosure against our properties securing the loan agreement, including our cash.
The loan agreement, as amended, requires that the borrowers maintain certain minimum liquidity at all times, which as of December 31, 2017, was approximately $18.3 million. At December 31, 2017, the borrowers were in compliance with the minimum liquidity covenant. Our ability to make scheduled payments on or to refinance our indebtedness depends on our future performance and ability to raise additional sources of cash, which is subject to economic, financial, competitive and other factors beyond our control. If we are unable to generate sufficient cash to service our debt, we may be required to adopt one or more alternatives, such as selling assets, restructuring our debt or obtaining additional equity capital on terms that may be onerous or highly dilutive. If we desire to refinance our indebtedness, our ability to do so will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations.
27
We are substantially dependent on the commercial success of our therapeutics GALLIPRANT, ENTYCE and NOCITA.
To date, we have invested substantial efforts and financial resources in the in-licensing, research and development, and commercialization of GALLIPRANT, ENTYCE and NOCITA. Our collaboration partner began commercializing GALLIPRANT in early-2017. We began commercializing NOCITA in late-2016 and began commercializing ENTYCE in late-2017.
Our near-term prospects, including our ability to finance our company and to enter into future strategic collaborations and generate revenue, will depend heavily on the successful development and commercialization of GALLIPRANT, ENTYCE and NOCITA. The commercial success of our current therapeutics will depend on a number of factors, including the following:
|
|
• |
|
the effectiveness of our commercialization efforts, including the effectiveness of marketing, sales and distribution strategy and operations, whether performed solely by us or in collaboration with others; |
|
|
• |
|
the ability of us or our third-party manufacturers to manufacture supplies of any of our therapeutics to meet the market demand and to develop, validate and maintain commercially viable manufacturing processes that are compliant with cGMP and to manufacture such therapeutics at an acceptable cost as well as the ability to sell such therapeutics at an acceptable price with reasonable margins; |
|
|
• |
|
our ability to successfully commercialize our therapeutics whether alone or in collaboration with others; |
|
|
• |
|
our ability to demonstrate to the satisfaction of the CVM, the USDA and the European Medicines Agency, or EMA, or the applicable EU Member State national competent authorities, the safety and efficacy of our therapeutics and therapeutic candidates, and for biologics, the potency and purity of our fully licensed biologics and therapeutic candidates and to obtain regulatory approval in the United States and Europe; |
|
|
• |
|
our ability to establish and maintain strategic collaborations, licensing or other arrangements, the financial terms of such agreements and the potential costs and financial terms to amend or terminate such relationships or other arrangements, including litigation costs; |
|
|
• |
|
the availability, perceived advantages, relative cost, relative safety and relative efficacy of alternative and competing treatments; |
|
|
• |
|
achieving and maintaining compliance with all regulatory requirements applicable to our therapeutics, including related to regulatory requirements for manufacturing by our third-party manufacturers; |
|
|
• |
|
the prevalence and severity of adverse side effects and our ability to maintain a continued acceptable safety profile of the therapeutic following approval; |
|
|
• |
|
our ability to obtain supplemental indications for our therapeutics; |
|
|
• |
|
our ability to enforce our intellectual property rights in and to our therapeutics and therapeutic candidates, and avoid third-party patent interference, third-party initiated and United States Patent and Trademark Office (“PTO”)-initiated administrative patent proceedings or patent infringement claims; |
|
|
• |
|
our success in educating veterinarians and pet owners about the benefits, administration and use of our therapeutics; |
|
|
• |
|
acceptance of our therapeutics as safe and effective by veterinarians, pet owners and the animal health community; and |
|
|
• |
|
any product liability claim or lawsuit we may be involved in from time to time with regards to our therapeutics. |
Many of these factors are beyond our control. Accordingly, we cannot assure you that we will ever be able to generate significant revenues through the sale of our therapeutics. If we are not successful in commercializing one or more of our therapeutics, or are significantly delayed in doing so, such as when the commercialization of ENTYCE was delayed in 2017 while we worked with the FDA to approve the transfer of the API manufacturing of ENTYCE to a new vendor, our business will be materially harmed and the value of your investment could substantially decline.
The development of our biologic therapeutic candidates is dependent upon relatively novel technologies and compliance with complex regulatory requirements.
We are developing biologics for pets, such as vaccines, and may in the future develop other biologics such as animal antibodies. Identification, optimization and manufacturing of therapeutic animal biologics is a relatively new field in which unanticipated difficulties or challenges could arise. While many biologics have been approved for use in humans, very few have been approved for use in animals, except for vaccines. There are unique risks and uncertainties with biologics, the development, manufacturing and sale of which are subject to regulations that are often as complex and extensive as the regulations applicable to other small molecule therapeutics. We anticipate that our animal biologics will continue to be regulated by the USDA, rather than CVM, and the regulatory standards that the USDA may require for novel biologics may be more difficult to satisfy than we anticipate.
28
We may be unable to obtain regulatory approval for our existing or future therapeutic candidates under applicable regulatory requirements. The denial or delay of any such approval would delay commercialization efforts and adversely impact our potential to generate revenue, our business and our results of operations.
Our therapeutic candidates are in various stages of development. The research, testing, manufacturing, labeling, approval, sale, marketing and distribution of pet therapeutics are subject to extensive regulation by the CVM, the USDA, the EMA and other regulatory authorities in the United States and other countries, whose regulations differ from country to country. We are not permitted to market our therapeutics in the United States until we receive approval of a NADA from the CVM or a product license from the USDA with respect to our biologic therapeutics, or in Europe until we receive approval from the European Commission or applicable EU State national competent authorities.
Even if we receive approval of an NADA, USDA product license or foreign regulatory filing for our therapeutic candidates, the CVM, the USDA or the applicable foreign regulatory body may approve our therapeutic candidates for a more limited indication than we originally requested, and the CVM or the USDA may not approve the labeling that we believe is necessary or desirable for the successful commercialization of our therapeutic candidates.
We also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action, either in the United States or abroad. For example, the current United States presidential administration has taken several executive actions, including the issuance of a number of Executive Orders, that could potentially impose significant burdens on, or otherwise materially delay, FDA’s ability to engage in routine regulatory and oversight activities such as implementation of statutes through rulemaking, issuance of guidance, and review and approval of marketing applications. If these executive actions impose constraints on FDA’s ability to engage in oversight and implementation activities in the normal course, our customers may be affected. Any delay in obtaining, or inability to obtain, applicable regulatory approval, or any uncertainties around related government regulation, would delay or prevent commercialization of our therapeutic candidates and would materially adversely impact our business and prospects.
Our therapeutics, and our current or future therapeutic candidates that may obtain regulatory approval, may never achieve market acceptance or commercial success.
Even if we obtain CVM, USDA, EMA or other regulatory approvals, our current or future therapeutics may not achieve market acceptance among veterinarians and pet owners, and may not be commercially successful. For example, our two canine-specific monoclonal antibody (MAb) therapies, BLONTRESS and TACTRESS, both received a full license from the USDA, however, we impaired the value of both of these assets during the third quarter of 2015 and again in 2016 (in the second quarter of 2016 for TACTRESS and in the fourth quarter of 2016 for BLONTRESS). Based on the results of the final clinical study and resulting market demand, the therapeutics are no longer being made commercially available and were discontinued as of the fourth quarter of 2017. In addition, to date, NOCITA has taken a longer sales cycle than what we would expect for a general practice product, and we believe the long-term success of ENTYCE will be driven by veterinarians becoming comfortable with its use and shifting towards chronic use settings, which may never occur or may take longer than we would expect for a general practice product. Market acceptance of any of our current or future therapeutics will depend on a number of factors, including:
|
|
• |
|
the effectiveness of our sales and marketing efforts and those of our collaborators; |
|
|
• |
|
the consistent and reliable supply and manufacture of the therapeutics; |
|
• |
the acceptance by veterinarians and pet owners of the therapeutics as safe and effective treatments; |
||
|
|
• |
|
the indications for which our therapeutics are approved; |
|
|
• |
|
the proper training and administration of our therapeutics by veterinarians; |
|
|
• |
|
the actual, potential and perceived advantages of our therapeutics over alternative treatments, including generic medicines and therapeutics approved for use by humans that are used off label; |
|
|
• |
|
the cost of alternative treatments and willingness to pay for our therapeutics, if approved, on the part of veterinarians and pet owners; |
|
|
• |
|
the willingness of pet owners to pay for our treatments, relative to other discretionary items, especially during economically challenging times; |
|
|
• |
|
the relative convenience and ease of administration; |
|
• |
the prevalence and severity of adverse side effects; |
||
|
|
• |
|
the safety of our therapeutics as demonstrated in our target animal studies; and |
|
|
• |
|
any negative publicity surrounding our Company, therapeutics or current or future therapeutic candidates, including any negative perception that may result from product liability lawsuit or other litigation. |
29
Because we expect sales of GALLIPRANT, ENTYCE and NOCITA to generate substantially all of our product revenues for the foreseeable future, the failure of these therapeutics to gain market acceptance or achieve commercial success would adversely affect our financial results and require us to seek additional financing.
Product liability lawsuits against us could cause us to incur substantial liabilities and limit commercialization of our therapeutics and any therapeutics that we may develop.
We face an inherent risk of product liability exposure related to the testing of our therapeutic candidates in clinical trials and will face an even greater risk for our therapeutics under commercialization. If we cannot successfully defend ourselves against claims that our therapeutic candidates or therapeutics caused injuries, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
|
|
• |
|
regulatory investigations, product recalls or withdrawals, or labeling, marketing or promotional restrictions; |
|
|
• |
|
decreased demand for any product candidates or products that we may develop; |
|
|
• |
|
injury to our reputation and significant negative media attention; |
|
|
• |
|
significant costs to defend the related litigation; |
|
|
• |
|
substantial monetary awards to consumers of our therapeutics; |
|
|
• |
|
loss of revenue; |
|
|
• |
|
reduced resources of our management to pursue our business strategy; and; |
|
|
• |
|
the inability to commercialize any therapeutics that we develop. |
We currently maintain product liability insurance at limits of $3.0 million per occurrence and in the policy aggregate. Those limits may not be adequate to cover all liabilities that we may incur. We may need to increase our insurance coverage as we expand our clinical trials and commercialize our therapeutics and therapeutic candidates. Product liability insurance is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liabilities that might arise.
We may not realize all of the anticipated benefits of acquisitions, or those benefits may take longer to realize than expected. We may also encounter significant unexpected difficulties in integrating acquired businesses.
We have made, and may continue to make, acquisitions. The overall integration of any acquired businesses may result in material unanticipated problems, expenses, liabilities, competitive responses, and diversion of management’s attention. The difficulties of combining the operations of acquired companies include, among others:
|
|
• |
|
the diversion of management’s attention to integration matters; |
|
|
• |
|
difficulties in achieving anticipated cost savings, synergies, business opportunities and growth prospects from combining any acquired businesses with our company; |
|
|
• |
|
difficulties in the integration of operations and systems; |
|
|
• |
|
difficulties in the assimilation of employees; |
|
|
• |
|
challenges in attracting and retaining key personnel; and |
|
|
• |
|
challenges in maintaining previously-established relationships with licensors and licensees. |
Many of these factors will be outside of our control and any one of them could result in increased costs and diversion of management’s time and energy, which could materially impact our business, financial condition and results of operations. In addition, even if the operations of any acquired businesses are integrated successfully, we may not realize the full benefits of the transaction, including the synergies or growth opportunities that we expect. For example, we acquired BLONTRESS and TACTRESS in connection with our acquisition of Vet Therapeutics, Inc., and since the acquisition, have fully impaired the value of these assets, discontinued the products and closed a USDA-licensed facility in San Diego where they had been produced. Other expected benefits of our acquisitions may not be achieved within the anticipated time frame, or at all.
In addition, through acquisitions, we may assume liabilities, losses or costs for which we are not indemnified or insured or for which our indemnity or insurance is inadequate. Any such liabilities may have a material adverse effect on our financial position or results of operations.
30
Development of pet therapeutics is an expensive and lengthy process with an uncertain outcome, and results of earlier studies may not be predictive of future study results.
Development of pet therapeutics is expensive and can take many years to complete, and its outcome is inherently uncertain. To gain approval to market a pet therapeutic for a particular species of pet, we must provide the CVM, the USDA or foreign regulatory authorities, as applicable, with data from animal safety and effectiveness studies that adequately demonstrate the safety and efficacy of that product in the target animal for the intended indication applied for in the NADA, product license or other regulatory filing. We rely on contract research organizations, or CROs, and other third parties to ensure the proper and timely conduct of most of our studies and development efforts and, while we have agreements governing their committed activities, we have limited influence over their actual performance. Failure can occur at any time during the development process. Success in prior target animal studies or in the treatment of human beings with a therapeutic candidate does not ensure that our target animal studies will be successful and the results of development efforts by other parties may not be indicative of the results of our target animal studies and other development efforts. For example, in December 2017, VetStem Biopharma, our collaborator responsible for the development of AT-016, shared the results of a pivotal study that did not achieve protocol-defined efficacy success criteria and as a result, we ultimately made the decision to exercise our rights to terminate the license agreement effective April 2018. Product candidates in our studies may fail to show the desired safety and efficacy despite showing such results in initial data or previous human or animal studies conducted by other parties. Even if our studies and other development efforts are completed, the results may not be sufficient to obtain regulatory approval for our therapeutic candidates.
Once our target animal studies commence, we may experience delays in such studies and other development efforts and we do not know whether planned studies will begin on time, need to be redesigned or be completed on schedule, if at all. Pet therapeutics studies can be delayed or discontinued for a variety of reasons, including delay or failure to:
|
|
• |
|
reach agreement on acceptable terms with prospective CROs and study sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
|
|
• |
|
complete target animal studies due to deviations from study protocol; |
|
|
• |
|
address any safety concerns that arise during the course of testing; |
|
|
• |
|
address any conflicts with new or existing laws or regulations; |
|
|
• |
|
add new study sites and/or enroll patients; or |
|
|
• |
|
manufacture sufficient quantities of formulated drug for use in studies. |
If we experience delays in the completion of, or terminate any development efforts for our therapeutic candidates, the commercial prospects of our therapeutic candidates will be harmed, and our ability to generate product revenues from any of these therapeutic candidates will be delayed. In addition, any delays in completing our development efforts will increase our costs, slow down our therapeutic candidate development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may harm our business, financial condition and prospects significantly. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of our development efforts may also ultimately lead to the denial of regulatory approval of our therapeutic candidates.
Our therapeutics, and therapeutic candidates, if approved, will face significant competition and our failure to effectively compete may prevent us from achieving significant market penetration.
The development and commercialization of new animal health medicines is highly competitive, and we expect considerable competition from major pharmaceutical, biotechnology and specialty animal health medicines companies. As a result, there are, and likely will continue to be, extensive research and substantial financial resources invested in the discovery and development of new animal health medicines. Our potential competitors include large animal health companies, such as Zoetis; Merck Animal Health, the animal health division of Merck & Co., Inc.; Elanco, the animal health division of Eli Lilly and Company; Bayer Animal Health, the animal health division of Bayer AG; Boehringer Ingelheim Animal Health, the animal health division of Boehringer Ingelheim GmbH; Virbac Group; Ceva Animal Health; Vetoquinol and Dechra Pharmaceuticals PLC. We are also aware of several smaller early stage animal health companies, such as Nexvet (acquired by Zoetis in 2017), Parnell Pharmaceuticals, Zomedica Pharmaceuticals, VetDC and Kindred Biosciences that are developing products for use in the pet therapeutics market.
Osteoarthritis is a competitive marketplace and Elanco will take the lead on commercial activities for Grapiprant Products. ENTYCE entered a new market where it is currently the only product FDA approved for veterinary use to stimulate appetite. However, we are aware that some veterinarians utilize mirtazapine, a human generic antidepressant with known side effects and limited effectiveness, to treat inappetence, and we are aware that a company is pursuing FDA approval of mirtazapine for weight gain in cats. We expect NOCITA in dogs and cats will compete primarily with existing analgesics that are part of multi-modal pain protocols, including local anesthetics, opioids and cox-inhibiting NSAIDs. Regarding AT-014, we are aware of investigational candidates for osteosarcoma.
31
We are an emerging commercial company with a limited history of operations and many of our competitors have substantially more resources than we do, including both financial and technical resources. In addition, many of our competitors have more experience than we have in the development, manufacture, regulation and worldwide commercialization of animal health medicines. We are also competing with academic institutions, governmental agencies and private organizations that are conducting research in the field of animal health medicines.
Our competition will be determined in part by the potential indications for which our therapeutics are developed and ultimately approved by regulatory authorities. Additionally, the timing of market introduction of some of our potential products or of competitors’ products may be an important competitive factor. Accordingly, the speed with which we can develop our compounds, complete target animal studies and approval processes, and supply commercial quantities to market are expected to be important competitive factors. We expect that competition among products approved for sale will be based on various factors, including product efficacy, safety, reliability, availability, price and patent position.
If we are not successful in identifying, licensing or acquiring, developing and commercializing additional therapeutic candidates, our ability to expand our business and achieve our strategic objectives would be impaired.
A key element of our strategy is to identify, license or acquire, develop and commercialize a portfolio of therapeutics to serve the pet therapeutics market. We derive potential pet therapeutic candidates from molecules and compounds discovered or developed as part of human biopharmaceutical research. We expect to enter into license arrangements with third parties to provide us with rights to human health compounds for purposes of our business. Such agreements are typically complex and require time to negotiate and implement. If we enter into these arrangements, we may not be able to maintain these relationships or establish new ones in the future on acceptable terms or at all. If we are unable to access human health-generated molecules and compounds to conduct research and development on cost-effective terms, our ability to develop new products could be limited. In some instances, human biopharmaceutical companies may be unwilling to license us their products or compounds for development as pet therapeutics because of perceived regulatory and commercial risks, including the risk that the FDA could delay or halt an ongoing human development trial if the same compound, when studied in animals, produces an unexplained adverse event or death, and the risk that, if the same compound is developed for humans and pets, and the human version is priced significantly higher than the pet version, which is usually the case, human patients would attempt to use the cheaper animal version of the drug. Even if we successfully identify and license potential therapeutic candidates, we may still fail to yield therapeutic candidates for development and commercialization for many reasons, including the following:
|
|
• |
|
competitors may develop alternatives that render our therapeutic candidates obsolete; |
|
|
|
• |
|
therapeutic candidates we develop may nevertheless be covered by third parties’ patents or other exclusive rights; |
|
|
|
• |
|
a therapeutic candidate may on further study be shown to have harmful side effects in pets or other characteristics that indicate it is unlikely to be effective or otherwise does not meet applicable regulatory criteria; |
|
|
|
• |
|
a therapeutic candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and |
|
|
|
• |
|
a therapeutic candidate may not be accepted as safe and effective by veterinarians, pet owners and the pet therapeutics community. |
|
If we fail to develop and successfully commercialize other therapeutic candidates, our business and future prospects may be harmed and our business will be more vulnerable to any problems that we encounter in developing and commercializing our current and future therapeutic candidates.
If we fail to attract and keep senior management and key scientific and commercial personnel, we may be unable to successfully develop any of our current or future therapeutic candidates, conduct our in-licensing and development efforts and commercialize any of our therapeutics or current or future therapeutic candidates.
Our success depends in part on our continued ability to attract, retain and motivate highly qualified management and scientific personnel. We are highly dependent upon our senior management team as well as our senior scientists and sales and marketing team. The loss of services of any of these individuals could delay or prevent the successful development of our current or future therapeutic pipeline, completion of our planned development efforts or the commercialization of our therapeutics and therapeutic candidates.
In addition, we could experience difficulties attracting and retaining qualified employees in the future. For example, competition for qualified personnel in the animal health fields is intense due to the limited number of individuals who possess the skills and experience required by our industry. We will need to hire additional personnel as we expand our development and commercial activities. We may not be able to attract and retain quality personnel on acceptable terms, or at all. In addition, to the extent we hire personnel from competitors, we may be subject to allegations that they have been improperly solicited or that they have divulged proprietary or other confidential information, or that their former employers own their research output. Failure to attract and retain highly qualified personnel could have an adverse effect on our business and our ability to develop our therapeutic candidates and commercialize our therapeutics.
32
We rely completely on third-party manufacturers to manufacture the supplies for the development of therapeutic candidates and to produce commercial quantities of our therapeutics.
We do not currently have, nor do we currently plan to acquire, the internal infrastructure or capability to manufacture the formulated drug for use in the conduct of our target animal studies. We also lack the resources and the capability to manufacture any of our therapeutics or therapeutic candidates on a scale necessary for commercialization. We rely on contract manufacturers to provide commercial supplies of the formulated drugs. For example, for NOCITA, we have entered into a commercial supply agreement with Pacira to supply the formulated drug. If this supply agreement terminates for any reason, or Pacira does not produce the necessary quantities, or demand of the product unexpectedly exceeds forecasts, we may be unable to arrange for alternative supply of NOCITA in a timely manner, on commercially reasonable terms, or at all, which could result in the product being unavailable. Our agreement with Pacira may terminate due to factors outside of our control, including if Pacira ceases to manufacture, for any reason, the formulated drug. With respect to NOCITA and our other therapeutics, as well as our therapeutic candidates, any delay in our ability to identify and contract with a replacement or an initial third-party contract manufacturer, as applicable, on commercially reasonable terms, or at all, would have an adverse impact upon our business.
The facilities used by our contract manufacturers to manufacture the active pharmaceutical ingredients and formulated drugs may be subject to inspections by one or more regulatory bodies. We do not control the manufacturing processes used by, and we are completely dependent on, our contract manufacturers to comply with cGMP, as applicable, for the manufacture of active pharmaceutical ingredients and/or finished drug products. In addition, we have no control over the ability of our contract manufacturers to maintain adequate quality control and quality assurance practices and to engage qualified personnel. If our contract manufacturers cannot successfully manufacture material that conforms to our specifications and complies with regulatory requirements, they will not be able to secure and/or maintain regulatory approval for their manufacturing facilities. For example, in February 2017, we received a response from the CVM in connection with our PAS to transfer the manufacturing of ENTYCE to a new vendor in order to produce ENTYCE at commercial scale. The CVM requested additional information regarding the proposed transfer in order to complete the supplemental application, which was ultimately approved in October 2017 but delayed our commercialization of ENTYCE. We may also decide to add additional redundant contract manufacturers or replace current contract manufacturers, which could require regulatory approval for the new manufacturing facilities. If the CVM, the USDA or the EMA does not approve our contract manufacturers’ facilities used for the manufacture of our therapeutics or therapeutic candidates, or if any such agency withdraws any such approval in the future, we may need to find alternative manufacturing facilities, which would adversely impact our ability to develop and obtain regulatory approval for or market our therapeutics or therapeutic candidates, if approved.
We and our third-party contractors are continuing to refine and improve the manufacturing process for our therapeutics and therapeutic candidates, certain aspects of which are complex and unique. We may encounter difficulties with new or existing manufacturing processes. In addition, to manufacture our therapeutics and therapeutic candidates in the quantities that we believe would be required to meet anticipated market demand, our third-party manufacturers may need to increase manufacturing capacity, which could involve significant challenges and may require additional regulatory approvals (including, for example, Grapiprant Products for Elanco pursuant to the Collaboration Agreement). Neither we nor our third-party manufacturers may successfully complete any manufacturing scale-up activities required to increase existing manufacturing capabilities in a timely manner, or at all. In certain instances, we may have to switch our third-party manufacturer to meet the scale of demand, which may require PAS and may result in regulatory action, additional costs incurred, delay in commercialization of our therapeutics and lawsuits.
Furthermore, we rely on our contract manufacturers to obtain any raw materials necessary to manufacture our therapeutics, and we do not have any control over the process or timing of the acquisition of these materials. If there is a disruption to our or our third-party manufacturers’ relevant operations, we will have no other means of producing our therapeutics or therapeutic candidates until they restore the affected facilities or we or they procure alternative manufacturing facilities or raw materials. Additionally, any damage to or destruction of our third-party manufacturers’ facilities or equipment may significantly impair our ability to manufacture our therapeutics and therapeutic candidates on a timely basis.
We currently rely on third parties to conduct our target animal studies and certain other development efforts. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may be unable to obtain regulatory approval for current or future therapeutic candidates or commercialize our current therapeutic candidates or future therapeutic candidates.
We currently do not conduct our target animal studies, and we rely on CROs and/or academic institutions to conduct these studies. The third parties with whom we contract for the execution of our studies play a significant role in the conduct of these studies and the subsequent collection and analysis of data. However, these third parties are not our employees, and except for contractual duties and obligations, we have limited ability to control the amount or timing of resources that they devote to our programs. Although we rely on these third parties to conduct our studies, we remain responsible for ensuring that each of our studies is conducted in accordance with the development plan and protocol. Moreover, the CVM, the USDA and EMA require us to comply with regulations and standards, commonly referred to as current good clinical practices, or cGCPs, or GLPs for conducting, monitoring, recording and reporting the results of our studies to ensure that the data and results are scientifically credible and accurate.
33
In addition, the execution of target animal studies and the subsequent compilation and analysis of the data produced requires coordination among various parties. If the third parties conducting our target animal studies do not perform their contractual duties or obligations, experience work stoppages, do not meet expected deadlines, terminate their agreements with us or need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our development protocols or cGCPs, or for any other reason, we may need to enter into new arrangements with alternative third parties, which could be difficult and costly, and our target animal studies may be extended, delayed or terminated or may need to be repeated. If any of the foregoing were to occur, the regulatory approval for the therapeutic candidate being tested in any such study or commercialization of our approved therapeutics may be delayed or require us to utilize additional resources.
Our ability to market our approved therapeutics and therapeutic candidates, if approved, will be limited to use for the treatment of the indications for which they are approved, and if we want to expand the indications for which we may market our therapeutics and therapeutic candidates, we will need to obtain additional CVM, USDA or EMA approvals, which may not be granted.
We received CVM approval in the United States for GALLIPRANT for the control of pain and inflammation associated with osteoarthritis in dogs, ENTYCE for appetite stimulation in dogs and NOCITA as a local post-operative analgesia for cranial cruciate ligament surgery in dogs. We and Elanco also received marketing authorization in the European Union for GALLIPRANT for the treatment of pain associated with mild to moderate osteoarthritis in dogs. In addition, we have received a conditional license from the USDA for Canine Osteosarcoma Vaccine, Live Listeria Vector (AT-014) for the treatment of dogs diagnosed with osteosarcoma, one year of age or older. We may market or advertise our therapeutics only for the treatment of indications for which they are approved, which could limit their adoption by veterinarians and pet owners. We may attempt to develop, promote and commercialize new treatment indications and protocols for these therapeutics or other therapeutic candidates in the future, but we cannot predict when or if we will receive the approvals required to do so. In addition, we would be required to conduct additional target animal studies to support our applications, which would utilize additional resources and may produce results that do not result in CVM, USDA or EMA approvals. If we do not obtain additional CVM, USDA or EMA approvals, our ability to expand our business will be limited.
We currently have a small commercial organization. If we are unable to expand sales capabilities on our own or through third parties, we may not be able to market and sell significant amounts of our approved therapeutics or current or future therapeutic candidates, if approved, or generate product revenue.
We currently have a small commercial organization. In order to commercialize any of our approved therapeutics in the United States and any jurisdictions outside the United States, including GALLIPRANT, ENTYCE and NOCITA, we must continue to build our marketing, sales, distribution, managerial and other non-technical capabilities or make arrangements with third parties to perform these services, and we may not be successful in doing so. We expanded our direct sales organization in the United States in 2016 and expect to continue to expand over time, complemented by distributors, to commercialize our therapeutics, which will be expensive and time-consuming. Because we have limited prior experience in the marketing, sale and distribution of pet therapeutics, there are significant risks involved in building and managing a sales organization, including our ability to hire, retain and motivate qualified individuals, generate sufficient sales leads, provide adequate training to sales and marketing personnel, and effectively oversee a geographically dispersed sales and marketing team. Any failure or delay in the development of our internal sales, marketing and distribution capabilities would adversely impact the commercialization of our therapeutics. Outside of the United States we intend to collaborate with companies with an established commercial presence to market our therapeutics in those locations. If we are unable to enter into such arrangements on acceptable terms or at all, we may not be able to successfully commercialize our therapeutics. If we are not successful in commercializing any of our therapeutics, either on our own or through collaborations with one or more distributors, our future product revenue will suffer and we would incur significant additional losses.
We will need to increase the size of our organization, and we may experience difficulties in managing growth.
Since our initial public offering in June 2013, we have grown from approximately 16 full-time employees to approximately 87 full-time employees as of March 9, 2018. We will need to continue to expand our managerial, operational, financial and other resources in order to manage our operations and target animal studies, continue our development activities and commercialize any of our therapeutics, current therapeutic candidates or future therapeutic candidates. Our management and personnel, systems and facilities currently in place may not be adequate to support this future growth. Our need to effectively execute our growth strategy requires that we:
|
|
• |
|
manage our target animal studies and other development efforts effectively; |
|
|
• |
|
identify, recruit, maintain, motivate and integrate additional employees; |
|
|
• |
|
manage our internal development efforts effectively while carrying out our contractual obligations to third parties; and |
|
|
• |
|
continue to improve our operational, financial and management controls, reporting systems and procedures. |
Any failure to successfully manage our growth could have a material adverse effect on our ability to effectively carry out our target animal studies, continue our development of our therapeutic candidates and commercialize our therapeutics.
34
We are incurring significant costs as a result of operating as a public company, and our management is expected to devote substantial time to new compliance initiatives.
As a publicly-traded company, we have incurred and will continue to incur significant legal, accounting and other expenses that we did not incur when we were a private company, particularly after we are no longer an “emerging growth company” as defined under the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. In addition, new and changing laws, regulations and standards relating to corporate governance and public disclosure, including the Dodd-Frank Wall Street Reform and Consumer Protection Act and the rules and regulations promulgated and to be promulgated thereunder, as well as under the Sarbanes-Oxley Act, the JOBS Act, and the rules and regulations of the United States Securities and Exchange Commission, or SEC, and the Nasdaq Global Market, have created uncertainty for public companies and increased our costs and time that our Board of Directors and management must devote to complying with these rules and regulations. We expect these rules and regulations to continue to increase our legal and financial compliance costs and lead to a diversion of management time and attention from revenue-generating activities.
Furthermore, the need to establish and maintain the corporate infrastructure demanded of a public company may divert management’s attention from implementing our growth strategy, which could prevent us from improving our business, results of operations and financial condition.
Any failure to maintain effective internal control over financial reporting could have a significant adverse effect on our business and the price of our common stock.
Our management is required to report annually on the effectiveness of our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act, or Section 404. The rules governing the standards that must be met for our management to assess our internal control over financial reporting are complex and require significant documentation, testing and possible remediation.
In the future, we may identify material weaknesses or significant deficiencies in our internal control over financial reporting, and we may not be able to remediate them in time to meet the deadline imposed by the Sarbanes-Oxley Act for compliance with the requirements of Section 404. In addition, we may encounter problems or delays in completing the implementation of any requested improvements and receiving a favorable attestation report from our independent registered public accounting firm, if such a report is required. We will be unable to issue securities in the public markets through the use of a shelf registration statement if we are not in compliance with Section 404. Furthermore, failure to achieve and maintain an effective internal control environment could materially adversely affect our business, reduce the market’s confidence in our common stock, adversely affect the price of our common stock and limit our ability to report our financial results accurately and timely.
Changes in distribution channels for pet therapeutics could negatively impact our market share, margins and distribution of our therapeutics.
In most markets, pet owners typically purchase their pet therapeutics directly from veterinarians. Pet owners increasingly could purchase pet therapeutics from sources other than veterinarians, such as Internet-based retailers, “big-box” retail stores or other over-the-counter distribution channels. This trend has been demonstrated by the significant shift away from the veterinarian distribution channel in the sale of parasiticides and vaccines in recent years. Pet owners also could decrease their reliance on, and visits to, veterinarians as they rely more on Internet-based animal health information. Because we expect to market our pet prescription therapeutics through the veterinarian distribution channel, any decrease in visits to veterinarians by pet owners could reduce our market share for such therapeutics and materially adversely affect our operating results and financial condition. In addition, pet owners may substitute human health products for pet therapeutics if human health products are deemed to be lower-cost alternatives.
Legislation has also been proposed in the United States, and may be proposed in the United States or abroad in the future, that could impact the distribution channels for our pet therapeutics. For example, such legislation may require veterinarians to provide pet owners with written prescriptions and disclosure that the pet owner may fill prescriptions through a third party, which may further reduce the number of pet owners who purchase their pet therapeutics directly from veterinarians. Such requirements may lead to increased use of generic alternatives to our therapeutics or the increased substitution of our therapeutics with other pet therapeutics or human health products if such other products are deemed to be lower-cost alternatives. Many states already have regulations requiring veterinarians to provide prescriptions to pet owners upon request and the American Veterinary Medical Association has long-standing policies in place to encourage this practice.
Over time, these and other competitive conditions may increase our reliance on Internet-based retailers, “big-box” retail stores or other over-the-counter distribution channels to sell our pet therapeutics. Any of these events could materially adversely affect our operating results and financial condition.
35
Consolidation of our customers could negatively affect the pricing of our therapeutics.
Veterinarians are our primary customers. In recent years, there has been a trend towards the concentration of veterinarians in large clinics and hospitals. For example, it was announced in January 2017 that Mars, Inc. (“Mars”) and VCA Inc. (“VCA”), a leading provider of pet health care services with nearly 800 small animal veterinary hospitals in the United States and Canada, had entered into an agreement under which Mars would acquire VCA and VCA would join Mars Petcare, one of the world’s leading pet care providers. If this trend towards consolidation continues, these customers could attempt to improve their profitability by leveraging their buying power to obtain favorable pricing. The resulting decrease in our prices could have a material adverse effect on our operating results and financial condition.
Our ability to use our net operating loss carryforwards to offset future taxable income may be subject to certain limitations.
As of December 31, 2017, we had net operating loss carryforwards, or NOLs, for federal and state income tax purposes of $94 million and $91 million, respectively, which may be available to offset our future taxable income, if any. Our federal NOLs begin to expire in 2031, and our state NOLs begin to expire in 2020. In general, under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, a corporation that undergoes an “ownership change” is subject to limitations on its ability to use its pre-change net operating loss carryforwards to offset future taxable income. If the Internal Revenue Service challenges our analysis that our existing NOLs will not expire before utilization due to previous ownership changes, or if we undergo an ownership change in the future, our ability to use our NOLs could be limited by Section 382 of the Code. Future changes in our stock ownership, some of which are outside of our control, could result in an ownership change under Section 382 of the Code. Furthermore, our ability to use NOLs of companies that we may acquire in the future may be subject to limitations. For these reasons, we may not be able to use a material portion of the NOLs reflected on our consolidated balance sheet, even if we attain profitability. We have not completed a study to assess whether an ownership change has occurred, or whether there have been multiple ownership changes since its formation, due to significant complexity and related costs associated with such a study.
New legislation on tax reform could have a material impact on the Company's financial position and/or results of operations.
Our business is subject to numerous international, federal, state, and other governmental laws, rules, and regulations that may adversely affect our operating results, including, taxation and tax policy changes, tax rate changes, new tax laws, or revised tax law interpretations, which individually or in combination may cause our effective tax rate to increase. For example, on December 22, 2017, the President of the United States signed into law the Tax Cuts and Jobs Act of 2017 (“TCJA”), which significantly changed existing United States tax laws, effective January 1, 2018, by a reduction of the corporate tax rate from a maximum of 35% to 21%, the implementation of a new system of taxation of non-United States earnings, and by expansion of the limitations on the deductibility of executive compensation and interest expense. The TCJA also provides that net operating losses generated in years ending after December 31, 2017, will be carried forward indefinitely and can no longer be carried back, and that net operating losses generated in years beginning after December 31, 2017, can only reduce taxable income by 80% when utilized in a future period. The exact ramifications of the legislation is subject to interpretation and could have a material impact on our financial position and/or results of operations. The TCJA is complex and far-reaching and we cannot predict with certainty the impact its enactment will have on us. Moreover, that effect, whether adverse or favorable, may not become evident for some period of time. We continue to analyze the full impact of enacted legislation and additional guidance as provided.
Generic products may be viewed as more cost-effective than our therapeutics.
We may face competition from products produced by other companies, including generic (or non-patented) alternatives to any of our therapeutics. We will depend on patents to provide us with exclusive marketing rights for some of our therapeutics. As of December 31, 2017, we had licensed an extensive portfolio of issued patents or pending patent applications relating to our AT-001, AT-002 and AT-003 compounds, including for GALLIPRANT, ENTYCE and NOCITA therapeutics, covering various composition of matter claims as well as methods of treatment and methods of manufacturing our therapeutics. In addition, as part of our Vet Therapeutics acquisition, we acquired a patent family related to the speciesization of antibodies that covers all Vet Therapeutics therapeutics. We also acquired a patent family related to antibody constant domain regions and uses thereof, which also covers all Vet Therapeutics therapeutics. We also acquired pending patent applications that cover specific canine monoclonal antibodies directed to various targets, including an allowed United States patent directed to the canine CD-52 development antibody. Further, as part of our acquisition of Okapi Sciences, we acquired two patent applications that cover formulations of AT-006 and commercially-viable methods of making the active ingredient of AT-006. Finally, we have a license to certain patent rights that covers composition and methods of use of AT-008 outside of North America. These patent rights will expire between 2024 and 2027.
The protection afforded to our patents, which varies from country to country, is limited by the scope and applicable terms of our patents and the availability of legal remedies in the applicable country. As a result, we may face competition from lower-priced generic alternatives to many of our therapeutics. Generic competitors are becoming more aggressive in terms of pricing, and generic products are an increasing percentage of overall animal health sales in certain regions. In addition, private label products may compete with our therapeutics. If pet therapeutics customers increase their use of new or existing generic or private label products, our operating results and financial condition could be materially adversely affected.
36
Our pet therapeutics are subject to unanticipated safety or efficacy concerns, which may harm our reputation.
Unanticipated safety or efficacy concerns can arise with respect to pet therapeutics, whether or not scientifically or clinically supported, leading to product recalls, withdrawals or suspended or declining sales, as well as product liability, and other claims. For example, although BLONTRESS and TACTRESS both received a full license from the USDA, we impaired the value of these assets and discontinued the products as of the fourth quarter of 2017, as studies indicated that these products were not as specific to the targets as expected and have uncertain clinical benefits. In addition, we depend on positive perceptions of the safety and quality of our therapeutics, and pet therapeutics generally, by our customers, veterinarians and end-users, and such concerns may harm our reputation. These concerns and the related harm to our reputation could materially adversely affect our operating results and financial condition, regardless of whether such reports are accurate.
Our business and operations would suffer in the event of system failures or security breaches.
Our internal computer systems and those of our current and future contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Any system failure or security breach by employees or others may pose a risk that sensitive data, including data from our target animal studies, intellectual property, trade secrets or personal information belonging to us may be exposed to unauthorized persons or to the public. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations. For example, the loss of data from completed or future studies could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we rely on third parties to manufacture our therapeutics and therapeutic candidates, and similar events relating to their computer systems could also have a material adverse effect on our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability, the further development our therapeutic candidates and commercialization of our therapeutics could be delayed, and the trading price of our common stock could be adversely affected.
Our Collaboration Agreement and Co-Promotion Agreement with Elanco are important to our business. If we or Elanco fail to adequately perform under the Collaboration Agreement and/or the Co-Promotion Agreement, or if we or Elanco terminate the Collaboration Agreement and/or the Co-Promotion Agreement, the development of grapiprant therapeutic candidates and commercialization of Grapiprant Products would be delayed or terminated and our business would be adversely affected.
The Collaboration Agreement and Co-Promotion Agreement are important to our business, and our ability to develop grapiprant therapeutic candidates and commercialize Grapiprant Products is dependent upon these agreements.
The Collaboration Agreement may be terminated by Elanco at any time upon 90 days’ written notice to us. The Collaboration Agreement may also be terminated by either party:
|
|
• |
|
for the other party’s material breach, where such breach is not cured within the timeframe specified by the agreement; |
|
|
• |
|
upon the bankruptcy, insolvency or dissolution of the other party; or; |
|
|
• |
|
for certain activities involving the challenge of certain patents licensed by us to Elanco. |
Upon Elanco’s voluntary termination or termination for Elanco’s breach, among other things, all licenses and rights granted to Elanco will terminate and revert to us, and Elanco has agreed to assign to us all registrations and trademarks obtained in connection with the products covered by the agreement. Upon termination for our breach, among other things, Elanco may elect to retain its rights to the licenses granted by us under the Collaboration Agreement subject to specified payment obligations.
Elanco may terminate the Co-Promotion Agreement in the event Elanco substantially stops marketing the products covered by the Collaboration Agreement, and either party may terminate the Co-Promotion Agreement upon the other party’s material breach, where such breach is not cured within the timeframe specified by the Co-Promotion Agreement. In addition, the Co-Promotion Agreement provides that it will automatically terminate if the Collaboration Agreement is terminated early.
Termination of the Collaboration Agreement and/or the Co-Promotion Agreement could cause significant delays in our product development and commercialization efforts that could prevent us from commercializing our Grapiprant Products without first expanding our internal capabilities, securing additional financing or entering into another agreement with a third party. Any alternative collaboration or license could also be on less favorable terms to us.
Under the Collaboration Agreement, Elanco has agreed to provide funding for certain clinical development activities. If the Collaboration Agreement were terminated, we may need to seek additional financing to support the research and development of any terminated products or discontinue any terminated products, which could adversely affect our business. In addition, under the Collaboration Agreement, Elanco is solely responsible for commercializing products outside the United States. We cannot directly control Elanco’s commercialization activities or the resources it allocates to our therapeutics. Our interests and Elanco’s interests may differ or conflict from time to time, or we may disagree with Elanco’s level of effort or resource allocation. Elanco may internally prioritize our therapeutics differently than we do or it may not allocate sufficient resources to effectively or optimally commercialize them or may change strategic direction in its business. For example, Eli Lilly & Co. announced in 2017 that it was performing a
37
strategic review of Elanco’s business, including a potential spin-off and initial public offering of Elanco in 2018. If these events were to occur, our business could be adversely affected.
Risks Related to Intellectual Property
We currently own a total of six issued patents and several patent applications, as well as foreign equivalent patents and applications. We also have licenses to issued patents covering our small molecule therapeutics and therapeutic candidates and have certain rights to prosecute and enforce those licensed patents. While we try to obtain patent coverage for our therapeutics where feasible and commercially reasonable, we cannot assure you that any patents based on any of our pending patent applications will ever be granted.
We currently own an issued United States patent related to the speciesization of antibodies that covers all Vet Therapeutics therapeutics, an issued United States patent related to antibody constant domain regions and uses thereof, which also covers all Vet Therapeutics therapeutics, an issued United States patent related to canine monoclonal antibodies directed to canine CD-52 and a filed patent application that specifically covers our canine monoclonal antibodies directed to canine CD-20, which was recently allowed in the United States, as well as pending related applications and foreign counterpart patents and applications.
We own a granted United States patent and related foreign patents and applications on a crystalline form of the active ingredient for GALLIPRANT. We also own a patent family relating to our AT-002 compounds, including ENTYCE, covering a method of treating inappetence using AT-002. We have a granted patent from this family in Australia and further applications pending in Australia, Argentina, Canada, China, European Patent Office, Japan and Taiwan. We cannot assure you that a patent based on any of these patent applications will ever be issued. We do not own any other patents or patent applications relating to AT-001 or any patents or patent applications relating to AT-003. We have exclusive license agreements in the field of animal health with RaQualia, pursuant to which we license key intellectual property relating to AT-001 and AT-002, including GALLIPRANT and ENTYCE, and with Pacira pursuant to which we license key intellectual property relating to AT-003, including NOCITA. Under each of the license agreements, RaQualia and Pacira retain ownership over the licensed patents and patent applications and retain control over the maintenance and prosecution of the licensed patents and patent applications. In the case of AT-003, we have no control over the manner in which Pacira chooses to maintain or prosecute its patent and patent applications and have no right to continue to prosecute any patents or patent applications that Pacira elects to abandon. We do not have the right to enforce patents licensed from Pacira against any third-party infringement, although we have certain limited rights to request our licensor to enforce such patents against infringement.
If we cannot obtain ownership of or adequate license rights to issued patents covering our therapeutic candidates or we cannot prosecute or enforce licensed patents, our business, results of operations, financial condition and prospects would be adversely affected.
If we fail to comply with our obligations under our intellectual property licenses with third parties, we could lose license rights that are essential to our business.
We are party to license agreements for our therapeutics and therapeutic candidates that are essential to our business, including the Collaboration Agreement with Elanco. These license agreements impose various payment and performance obligations on us. If we fail to comply with these obligations, RaQualia, Pacira or Elanco, as applicable, may have the right to terminate the relevant license agreement, in which event we would not be able to develop or commercialize those licensed compounds including GALLIPRANT, ENTYCE and/or NOCITA, as the case may be.
If we lose such license rights, our business, results of operations, financial condition and prospects would be adversely affected. We may enter into additional licenses in the future and if we fail to comply with obligations under those agreements, we could suffer adverse consequences.
We may not own any intellectual property rights we develop with respect to AT-003 or be able to share our licensed patent rights to AT-003 with future collaborators.
Our license agreement with Pacira contains certain obligations and restrictions on our ability to develop and commercialize AT-003, including NOCITA. All of the intellectual property rights that we develop with respect to AT-003 will be owned by Pacira upon termination of this license agreement. If we wish to enter into any collaboration agreements relating to AT-003, Pacira has the right to approve all of our sublicenses. Furthermore, Pacira has a right of first negotiation for shared commercialization rights to AT-003 in the United States. These restrictions may impair or delay our ability to engage third parties to commercialize AT-003, including NOCITA.
We may become subject to third parties’ claims alleging infringement of patents and proprietary rights or seeking to invalidate our patents or proprietary rights, which would be costly, time-consuming and, if successfully asserted against us, delay or prevent the development of our current or future therapeutic candidates or commercialization of our therapeutics.
There has been substantial litigation and other proceedings regarding patent and other intellectual property rights in the field of pet therapeutics, as well as patent challenge proceedings, including interference and administrative law proceedings before the United States PTO and oppositions and other comparable proceedings in foreign jurisdictions. Recently, under United States patent reform laws, new procedures including inter party review and post grant review have been implemented. As stated below, the novel implementation of such reform laws presents uncertainty regarding the outcome of challenges to our patents in the future. We cannot
38
assure you that any of our therapeutics or current or future therapeutic candidates will not infringe existing or future patents. Because we have not conducted a formal freedom to operate analysis for patents related to our therapeutics, we may not be aware of patents that have already been issued that a third party might assert are infringed by one of our therapeutics or current or future therapeutic candidates. Because patent applications can take many years to issue and may be confidential for eighteen months or more after filing, there also may be applications now pending of which we are unaware and which may later result in issued patents that we may infringe by commercializing any of our therapeutics or current or future therapeutic candidates.
We may be subject to third-party claims in the future against us or our collaborators that would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages, including treble damages and attorney’s fees if we are found to be willfully infringing a third party’s patents. If a patent infringement suit were brought against us or our collaborators, we or they could be forced to stop or delay research, development, manufacturing or sales of the therapeutic or therapeutic candidate that is the subject of the suit. As a result of patent infringement claims, or in order to avoid potential claims, we or our collaborators may choose to seek, or be required to seek, a license from the third party and would most likely be required to pay license fees, milestone payments, royalties or other payments. These licenses may not be available on acceptable terms, or at all. Even if we or our collaborators were able to obtain a license, the rights may be nonexclusive, which would give our competitors access to the same intellectual property. Ultimately, we could be prevented from commercializing a therapeutic, or forced to redesign it, or to cease some aspect of our business operations if, as a result of actual or threatened patent infringement claims, we or our collaborators are unable to enter into licenses on acceptable terms. Even if we are successful in defending such claims, infringement and other intellectual property litigation can be expensive and time-consuming to litigate and divert management’s attention from our core business. Any of these events could harm our business significantly.
In addition to infringement claims against us, if third parties have prepared and filed patent applications in the United States that also claim technology to which we have rights, we may have to participate in interference proceedings in the United States PTO to determine the priority of invention. Third parties may also attempt to initiate reexamination, post grant review or inter party review of our patents in the United States PTO. We may also become involved in similar opposition proceedings in the European Patent Office or similar offices in other jurisdictions regarding our intellectual property rights with respect to our therapeutics and technology. Moreover, we may face claims from non-practicing entities, which have no relevant product revenue and against whom our own patent portfolio may thus have no deterrent effect.
If our efforts to protect the proprietary nature of the intellectual property related to any of our therapeutics and current or future therapeutic candidates are not adequate, we may not be able to compete effectively in our market.
We rely upon a combination of patents, trade secret protection, confidentiality and license agreements to protect the intellectual property related to our therapeutics and current therapeutic candidates and our development programs.
Composition-of-matter patents on the active pharmaceutical ingredient are generally considered to be the strongest form of intellectual property protection for pharmaceutical products, including pet therapeutics, as such patents provide protection without regard to any particular method of use or manufacture. Method-of-use patents protect the use of a product for the specified method. This type of patent does not prevent a competitor from making and marketing a product that is identical to our therapeutic for an indication that is outside the scope of the patented method. Moreover, even if competitors do not actively promote their product for our targeted indications, veterinarians may recommend that pet owners use these products off label, or pet owners may do so themselves. Although off-label use may infringe or contribute to the infringement of method-of-use patents, the practice is common and such infringement is difficult to prevent or prosecute. Method of manufacturing patents protect a specific way to make a product and do not prevent a third party from making the product by a different method and then using the product for our uses. We cannot be certain that the claims in our patent applications will be considered patentable by the United States PTO and courts in the United States, or by the patent offices and courts in foreign countries.
The strength of patents in the field of pet therapeutics involves complex legal and scientific questions and can be uncertain. The patent applications that we own or license may fail to result in issued patents in the United States or in other foreign countries. Even if the patents do successfully issue, third parties may challenge the validity, enforceability or scope thereof, which may result in such patents being narrowed, invalidated or held unenforceable. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our therapeutics or our intellectual property or prevent others from designing around our claims. If the breadth or strength of protection provided by the patents and patent applications we own, in-license or pursue with respect to any of our therapeutics or current or future therapeutic candidates is threatened, it could threaten our ability to commercialize any of our therapeutics or current or future therapeutic candidates. Further, if we encounter delays in our development efforts, the period of time during which we could market any of our current or future therapeutic candidates under patent protection would be reduced. Since patent applications in the United States and most other countries are confidential for a period of time after filing, we cannot be certain that we were the first to file any patent application related to our therapeutics or therapeutic candidates. Furthermore, for patent applications in which claims are entitled to a priority date before March 16, 2013, an interference proceeding can be provoked by a third party or instituted by the United States PTO to determine who was the first to invent any of the subject matter covered by the patent claims of our applications. For patent applications containing a claim not entitled to a priority date before March 16, 2013, there is a greater level of uncertainty in the patent law due to the passage of the America Invents Act, which brings into effect significant changes to the United States patent laws that have yet to be well defined, and which introduces new procedures for challenging
39
pending patent applications and issued patents. A primary change under this reform is creating a “first to file” system in the United States, which requires us to minimize the time from invention to filing of a patent application.
We also rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable, processes for which patents are difficult to enforce and any other elements of our product development processes that involve proprietary know-how, information or technology that is not covered by patents. We cannot be certain that we have executed such agreements with all parties, including our collaborators and contract manufacturers, who may have helped to develop our intellectual property or had access to our proprietary information, nor that our agreements will not be breached. We cannot guarantee that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. If we are unable to prevent material disclosure of the intellectual property related to our technologies to third parties, we will not be able to establish or maintain a competitive advantage in our market, which could materially adversely affect our business, results of operations and financial condition.
Any disclosure to or misappropriation by third parties of our confidential proprietary information could enable competitors to quickly duplicate or surpass our technological achievements, thus eroding our competitive position in our market.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents, or patents that may be issued to us in the future, or the patents of our licensors that are licensed to us. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In addition, if we or one of our future collaborators were to initiate legal proceedings against a third party to enforce a patent covering our therapeutics or current therapeutic candidates, or one of our future therapeutics, the defendant could counterclaim that our patent is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the United States PTO, or made a materially misleading statement, during prosecution. Third parties may also raise similar claims before the United States PTO, even outside the context of litigation. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our therapeutics or current or future therapeutic candidates. Such a loss of patent protection could have a material adverse impact on our business.
Litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be unsuccessful, it could have an adverse effect on the price of our common stock.
Changes in United States patent law could diminish the value of patents in general, thereby impairing our ability to protect our therapeutics.
As is the case with other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves both technological and legal complexity. Therefore, obtaining and enforcing biopharmaceutical patents is costly, time-consuming and inherently uncertain. In addition, the United States has recently enacted and is currently implementing wide-ranging patent reform legislation. The Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the United States Congress, the federal courts, and the United States PTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing owned or licensed patents and patents that we might obtain in the future.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The United States PTO, the European Patent Office and various other foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case, which would have an adverse effect on our business.
40
We have pending trademark applications for our company name in the United States and certain other countries, and we have pending trademark applications in the United States for certain therapeutic candidates, and we have pending trademark applications for these therapeutic candidates in certain other countries; however, registration is not yet complete for certain of these filings, and failure to finally secure these registrations could adversely affect our business.
We have obtained six trademark registrations in the United States for our company name and design marks, and we have four pending foreign trademark applications for our company name and design marks and we have obtained eighteen foreign registrations for these marks, although we cannot make assurances that the trademark applications will become registered. We have two pending trademark applications in the United States for commercial trade names for our current therapeutic candidates, and we have obtained sixteen United States registrations for these candidates, and we have twenty-two pending foreign applications and we have obtained forty foreign registrations for these candidates, although we cannot make assurances that the trademark applications will become registered. During trademark registration proceedings, we have in the past and may in the future receive rejections. Although we are given an opportunity to respond to those rejections, we may be unable to overcome such rejections. In addition, in the USPTO and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and/or to seek to cancel registered trademarks. Opposition or cancellation proceedings have in the past and may in the future be filed against our trademark applications and/or registrations, and our trademark applications and/or registrations may not survive such proceedings. Additionally, we may need to enforce our trademark rights against third parties and expend significant additional resources to enforce such rights against infringements. Moreover, any name we propose to use with our therapeutic candidates in the United States must be approved by the CVM, the USDA and for use in Europe, by the EMA, regardless of whether we have registered it, or applied to register it, as a trademark. The CVM typically conducts a review of proposed product names, including an evaluation of potential for confusion with other product names. If the CVM, the USDA or the EMA object to any of our proposed proprietary product names (which they have done in the past done and may do in the future), we may be required to expend significant additional resources in an effort to identify a suitable substitute name that would qualify under applicable trademark laws, not infringe the existing rights of third parties and be acceptable to the CVM, the USDA or the EMA.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on therapeutics or therapeutic candidates throughout the world would be prohibitively expensive. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection, but where enforcement is not as strong as that in the United States. These products may compete with our therapeutics in jurisdictions where we do not have any issued or licensed patents and our patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions, including in Europe where our Aratana Therapeutics NV facilities are located. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties.
We have received confidential and proprietary information from third parties. In addition, we employ individuals who were previously employed at other biotechnology, pharmaceutical or animal health companies. We may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise improperly used or disclosed confidential information of these third parties or our employees’ former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial cost and be a distraction to our management and employees.
Risks Related to Government Regulation
The regulatory approval process is uncertain, requires us to utilize significant resources, and may prevent us from obtaining approvals for the commercialization of some or all of our therapeutic candidates.
The research, testing, manufacturing, labeling, approval, selling, import, export, marketing and distribution of pet therapeutics are subject to extensive regulation by the CVM, the USDA or the EMA and other regulatory authorities in the United States and other countries, which regulations differ from country to country. While it is unclear whether the recent political and regulatory uncertainty in the United States would have any impact on animal health industry in particular, because we make active in-licensing effort focused on identifying human therapeutics for development and commercialization as pet therapeutics, we may face similar regulatory risks that human pharmaceutical companies face in this current regulatory environment.
41
We are not permitted to market any of our current or future therapeutic candidates in the United States until we receive approval of an NADA from the CVM or a product license from the USDA. Obtaining approval of an NADA from CVM or a product license from the USDA can be an uncertain process that requires us to utilize significant resources. The CVM, the USDA or any foreign regulatory bodies can delay, limit or deny approval of any of our therapeutic candidates for many reasons, including:
|
|
• |
|
we are unable to demonstrate to the satisfaction of the CVM, the USDA, the EMA or the applicable foreign regulatory body that the therapeutic candidate is safe and effective for the requested indication; |
|
|
• |
|
the CVM, the USDA or the applicable foreign regulatory body may disagree with our interpretation of data from our target animal studies and other development efforts; |
|
|
• |
|
we may be unable to demonstrate that the therapeutic candidate’s benefits outweigh any safety or other actual or perceived risks; |
|
|
• |
|
the CVM, the USDA or the applicable foreign regulatory body may require additional studies; |
|
|
• |
|
the CVM, the USDA or the applicable foreign regulatory body may not approve of the formulation, labeling and/or the specifications of our current and future therapeutic candidates; |
|
|
• |
|
the CVM, the USDA or the applicable foreign regulatory body may fail to approve our manufacturing processes or facilities, or the manufacturing processes or facilities of third-party manufacturers with which we contract; and |
|
|
• |
|
the approval policies or regulations of the CVM, USDA or the applicable foreign regulatory body may significantly change in a manner rendering the data from our studies insufficient for approval. |
Failure to comply with CVM and other applicable United States and foreign regulatory requirements may subject us to administrative or judicially imposed sanctions, including: warning letters, civil and criminal penalties, injunctions, withdrawal of approved products from the market, product seizure or detention, product recalls, total or partial suspension of production, and refusal to approve pending NADAs or product licenses or supplements to approved NADAs or product licenses.
Regulatory approval of an NADA or supplement NADA, or of a product license, is not guaranteed, and the approval process requires us to utilize significant resources, may take several years, and is subject to the substantial discretion of the CVM, the USDA or the EMA. Despite the time and expense exerted, failure can occur at any stage, and we could encounter problems that cause us to abandon or repeat studies, or perform additional studies. If any of our current or future product candidates fails to demonstrate safety and efficacy in our studies, or for any other reason does not gain regulatory approval, our business and results of operations will be materially and adversely harmed.
Our therapeutics will be subject to ongoing CVM, USDA or EMA obligations and continued regulatory review even after the initial approval for commercialization, which may result in significant additional expense. Additionally, our therapeutics will be subject to labeling and manufacturing requirements and could be subject to other restrictions. Failure to comply with these regulatory requirements or the occurrence of unanticipated problems with our products could result in significant penalties.
Our therapeutics may be subject to conditions of approval or limitations on the approved indicated uses for which the product may be marketed, or may contain requirements for potentially costly surveillance to monitor the safety and efficacy of the therapeutics. In addition, if the CVM, the USDA or the EMA approves any of our current or future therapeutic candidates, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion and recordkeeping for the therapeutic will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with cGMP, GLP and cGCP, for any studies that we conduct post-approval. For example, in February 2017, we received a response from the CVM in connection with our PAS to transfer the manufacturing of ENTYCE to a new vendor in order to produce ENTYCE at commercial scale. The CVM requested additional information regarding the proposed transfer in order to complete the supplemental application, which delayed our commercialization of ENTYCE. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
|
• |
restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls; |
||||||
|
|
• |
|
fines, warning letters or holds on target animal studies; |
||||
|
|
|||||||
|
|
• |
|
refusal by the CVM, the USDA or the EMA to approve pending applications or supplements to approved applications filed by us or our strategic collaborators, or suspension or revocation of product license approvals; |
||||
|
|
|||||||
|
|
• |
|
product seizure or detention, or refusal to permit the import or export of products; and |
||||
|
|
|||||||
|
|
• |
|
injunctions or the imposition of civil or criminal penalties. |
||||
42
The CVM’s, USDA’s or the EMA’s policies may change and additional government regulations may be enacted that could prevent, limit or delay commercialization of our therapeutics or regulatory approval of our therapeutic candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability, which would adversely affect our business.
Failure to obtain regulatory approvals in foreign jurisdictions for our therapeutic candidates would prevent us from marketing our therapeutics internationally.
In order to market any product outside of the United States, including in the EEA (which is comprised of the 28 member states of the European Union plus Norway, Iceland and Liechtenstein) and many other foreign jurisdictions, separate regulatory approvals are required. More concretely, in the EEA, pet therapeutics can only be commercialized after obtaining a Marketing Authorization (“MA”). Before granting the MA, the EMA or the competent national authorities of the member states of the EEA make an assessment of the risk-benefit balance of the product on the basis of scientific criteria concerning its quality, safety and efficacy.
The approval procedures vary among countries and can involve additional studies and testing, and the time required to obtain approval may differ from that required to obtain CVM or USDA approval. Animal studies conducted in one country may not be accepted by regulatory authorities in other countries. Approval by the CVM or USDA does not ensure approval by regulatory authorities in other countries, and approval by one or more foreign regulatory authorities does not ensure approval by regulatory authorities in other foreign countries or by the CVM or the USDA. However, a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in others. The foreign regulatory approval process may include all of the risks associated with obtaining CVM or USDA approval. We may not be able to file for regulatory approvals or to do so on a timely basis and, even if we do file them, we may not receive necessary approvals to commercialize our therapeutics in any market.
If approved, any of our current or future therapeutics may cause or contribute to adverse medical events that we are required to report to the CVM, USDA and regulatory authorities in other countries and, if we fail to do so, we could be subject to sanctions that would materially harm our business.
If we are successful in commercializing any of our current or future therapeutics, regulations of the CVM, the USDA and of the regulatory authorities in other countries require that we report certain information about adverse medical events if those products may have caused or contributed to those adverse events. The timing of our obligation to report would be triggered by the date we become aware of the adverse event as well as the nature of the event. We may fail to report adverse events we become aware of within the prescribed timeframe. We may also fail to appreciate that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of our therapeutics. If we fail to comply with our reporting obligations, the CVM, USDA and regulatory authorities in other countries could take action including criminal prosecution, the imposition of civil monetary penalties, seizure of our therapeutics, or delay in approval or clearance of future therapeutics.
Legislative or regulatory reforms with respect to pet therapeutics may make it more difficult and costly for us to obtain regulatory clearance or approval of any of our current or future therapeutic candidates and to produce, market, and distribute our therapeutics after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in the United States Congress that could significantly change the statutory provisions governing the testing, regulatory clearance or approval, manufacture, and marketing of regulated products. In addition, CVM and USDA regulations and guidance are often revised or reinterpreted by the CVM and USDA in ways that may significantly affect our business and our therapeutics. Similar changes in laws or regulations can occur in other countries. Any new regulations or revisions or reinterpretations of existing regulations in the United States or in other countries may impose additional costs or lengthen review times of any of our current or future therapeutic candidates. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require:
|
|
• |
|
changes to manufacturing methods; |
|
|
• |
|
recall, replacement, or discontinuance of certain products; and |
|
|
• |
|
additional record keeping. |
Each of these would likely entail substantial time and cost and could materially harm our financial results. In addition, delays in receipt of or failure to receive regulatory clearances or approvals for any future products would harm our business, financial condition, and results of operations.
Our research and development relies on evaluations in animals, which may become subject to bans or additional regulations.
As a biopharmaceutical company with a focus on pet therapeutics, the evaluation of our existing and new products in animals is required to register our therapeutics. Animal testing in certain industries has been the subject of controversy and adverse publicity. Some organizations and individuals have attempted to ban animal testing or encourage the adoption of additional regulations
43
applicable to animal testing. To the extent that the activities of such organizations and individuals are successful, our research and development, and by extension our operating results and financial condition, could be materially adversely affected. In addition, negative publicity about us or our industry could harm our reputation.
Risks Related to Our Common Stock
Our stock price may be volatile and you may not be able to resell shares of our common stock at or above the price you paid.
The trading price of our common stock is volatile with trading prices ranging from $2.56 per share to $29.32 per share since our initial public offering in June 2013. The price of our common stock has been and could continue to be subject to wide fluctuations, including due to results from, and any delays in, our current and future target animal studies or delays in obtaining regulatory approval. For example, on September 25, 2015, following our announcement that we did not believe that BLONTRESS or TACTRESS would capture the desired lymphoma market opportunity, the price of our common stock fell from $17.49 on September 24, 2015 to $10.67 on September 25, 2015, a 39% reduction. And on February 6, 2017, following our announcement that we anticipated ENTYCE would be commercially available by late-2017 because of ongoing interactions with the FDA on our PAS to transfer manufacturing to a new vendor for commercial scale-up, the price of our common stock fell from $8.03 on February 3, 2017 to $6.59 on February 6, 2017, an 18% reduction. The price of our common stock could be volatile in the future in response to various factors, some of which are beyond our control. These factors include those discussed in this “Risk Factors” section and others, such as:
|
|
• |
|
delays in the commercialization of our therapeutics or current or future therapeutic candidates; |
|
|
• |
|
manufacturing and supply issues related to our therapeutics or current or future therapeutic candidates for our development programs and commercialization; |
|
|
• |
|
the termination of any of our existing license agreements; |
|
|
• |
|
announcements relating to future licensing or development agreements; |
|
|
• |
|
announcements of regulatory approval or disapproval of any of our current or future therapeutic candidates; |
|
|
• |
|
acquisitions and sales of new therapeutics, therapeutic candidates, technologies or businesses; |
|
|
• |
|
failure or discontinuation of any of our research programs; |
|
|
• |
|
quarterly variations in our results of operations or those of our future competitors; |
|
|
• |
|
changes in earnings estimates or recommendations by securities analysts; |
|
|
• |
|
announcements by us or our competitors of new therapeutics or therapeutic candidates, significant contracts, commercial relationships, acquisitions or capital commitments; |
|
|
• |
|
developments with respect to intellectual property rights; |
|
|
• |
|
our commencement of, or involvement in, litigation; |
|
|
• |
|
any major changes in our Board of Directors or management; |
|
|
• |
|
new legislation in the United States or other countries relating to the sale or pricing of pet therapeutics; |
|
|
• |
|
CVM or USDA or other United States or foreign regulatory actions affecting us or our industry; |
|
|
• |
|
product liability claims, other litigation or public concern about the safety of our therapeutics or therapeutic candidates or future therapeutics; |
|
|
• |
|
market conditions in the animal health sector and in the pet therapeutics market; |
|
|
• |
|
low daily trading volumes in our stock; and |
|
|
• |
|
general economic conditions in the United States and abroad. |
In addition, the stock market in general, or the market for stocks in our industry or industries related to our industry, may experience extreme volatility unrelated to the operating performance of the issuer. These broad market fluctuations may adversely affect the trading price or liquidity of our common stock. When the market price of a stock has been volatile, holders of that stock have sometimes instituted securities class action or other litigation against the issuer. If any of our stockholders were to bring such a lawsuit against us, such as the purported class action lawsuits filed as described under Item 3. “Legal Proceedings,” we could incur substantial costs defending the lawsuit and the attention of our management would be diverted from the operation of our business.
44
We are an “emerging growth company,” as defined in the JOBS Act, and as a result of the reduced disclosure and governance requirements applicable to emerging growth companies, our common stock may be less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive because we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an emerging growth company. We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) of 2018, (b) in which we have total annual gross revenue of at least $1.07 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior June 30, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
If we sell shares of our common stock in future financings, stockholders may experience immediate dilution and, as a result, our stock price may decline.
We may from time to time issue additional shares of common stock at the market price or at a discount from the current trading price of our common stock. As a result, our stockholders would experience immediate dilution upon the sale of any shares of our common stock. In addition, as opportunities present themselves, we may enter into financing or similar arrangements in the future, including the issuance of debt securities, preferred stock or common stock. If we issue common stock or securities convertible into common stock, our common stockholders would experience additional dilution and, as a result, our stock price may decline.
Our principal stockholders and management own a significant percentage of our stock and will be able to influence matters subject to stockholder approval.
As of March 9, 2018, our executive officers, directors, holders of 5% or more of our common stock and their respective affiliates beneficially owned approximately 32% of our voting stock. These stockholders will have the ability to influence us through this ownership position. For example, these stockholders may be able to influence elections of directors, amendments of our organizational documents, or approvals of any merger, sale of assets or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our common stock that stockholders may believe to be in their best interest.
Provisions in our charter documents and under Delaware law could discourage a takeover that stockholders may consider favorable and may lead to entrenchment of management.
Our restated certificate of incorporation and amended and restated bylaws contain provisions that could delay or prevent changes in control or changes in our management without the consent of our Board of Directors. These provisions include the following:
|
|
• |
|
a classified Board of Directors with three-year staggered terms, which may delay the ability of stockholders to change the membership of a majority of our Board of Directors; |
|
|
• |
|
no cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates; |
|
|
• |
|
the exclusive right of our Board of Directors to elect a director to fill a vacancy created by the expansion of the Board of Directors or the resignation, death or removal of a director, which prevents stockholders from being able to fill vacancies on our Board of Directors; |
|
|
• |
|
the ability of our Board of Directors to authorize the issuance of shares of preferred stock and to determine the terms of those shares, including preferences and voting rights, without stockholder approval, which could be used to significantly dilute the ownership of a hostile acquirer; |
|
|
• |
|
the ability of our Board of Directors to alter our bylaws without obtaining stockholder approval; |
|
|
• |
|
the required approval of the holders of at least two-thirds of the shares entitled to vote at an election of directors to adopt, amend or repeal our bylaws or repeal the provisions of our restated certificate of incorporation regarding the election and removal of directors; |
|
|
• |
|
a prohibition on stockholder action by written consent, which forces stockholder action to be taken at an annual or special meeting of our stockholders; |
|
|
• |
|
the requirement that a special meeting of stockholders may be called only by the chairman of the Board of Directors, the chief executive officer, the president or the Board of Directors, which may delay the ability of our stockholders to force consideration of a proposal or to take action, including the removal of directors; and |
45
|
|
• |
|
advance notice procedures that stockholders must comply with in order to nominate candidates to our Board of Directors or to propose matters to be acted upon at a stockholders’ meeting, which may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of us. |
In addition, these provisions would apply even if we were to receive an offer that some stockholders may consider beneficial.
We are also subject to the anti-takeover provisions contained in Section 203 of the Delaware General Corporation Law. Under Section 203, a corporation may not, in general, engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held the stock for three years or, among other exceptions, the Board of Directors has approved the transaction.
We do not currently intend to pay dividends on our common stock, and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common stock.
We do not currently intend to pay any cash dividends on our common stock for the foreseeable future. We currently intend to invest our future earnings, if any, to fund our growth. Additionally, the terms of our Loan and Security Agreement restrict our ability to pay dividends. Therefore, you are not likely to receive any dividends on your common stock for the foreseeable future. Since we do not intend to pay dividends, your ability to receive a return on your investment will depend on any future appreciation in the market value of our common stock. There is no guarantee that our common stock will appreciate or even maintain the price at which our holders have purchased it.
Item 1B. Unresolved Staff Comments
Not applicable.
Our corporate headquarters is located in Leawood, Kansas, where we lease and occupy approximately 17,600 square feet of office space pursuant to a lease that expires on February 28, 2021. Additionally, we lease approximately 400 square feet of office space in Leuven, Belgium, that expires on October 30, 2018.
We believe that our current facilities are adequate to support our existing operations. We also believe that we will be able to obtain suitable additional facilities on commercially reasonable terms on an “as needed basis.”
From time to time, we may become involved in legal proceedings arising in the ordinary course of our business. Except as described below, we are not presently a party to any litigation that we believe to be material and we are not aware of any pending or threatened litigation against us that we believe could have a material adverse effect on our business, operating results, financial condition or cash flows.
In February 2017, two purported class action lawsuits were filed in the United States District Court for the Southern District of New York against the Company and two of its current officers. Those cases have been consolidated into one purported class action lawsuit under the caption, In re Aratana Therapeutics, Inc. Securities Litigation, Case No. 1:17-cv-00880. The consolidated lawsuit, which was amended in August 2017, asserts claims under Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended, and is premised on allegedly false and/or misleading statements, and alleged non-disclosure of material facts, regarding the Company’s business, operations, prospects and performance during the proposed class period of March 16, 2015 to March 13, 2017. The Company is vigorously defending all claims asserted, including by filing a motion to dismiss. Given the early stage of the litigation, at this time a loss is not probable or reasonably estimable.
Item 4. Mine Safety Disclosures
Not applicable.
46
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our common stock has been publicly traded on the Nasdaq Global Select Market under the symbol “PETX” since our initial public offering on June 26, 2013. The following table sets forth, for the periods indicated, the high and low intraday sale prices of our common stock as reported by the Nasdaq Global Select Market.
|
|
|||||
|
|
High |
Low |
|||
|
2017 |
|||||
|
Fourth quarter |
$ |
7.28 |
$ |
4.85 | |
|
Third quarter |
$ |
7.67 |
$ |
5.18 | |
|
Second quarter |
$ |
7.45 |
$ |
5.02 | |
|
First quarter |
$ |
8.63 |
$ |
4.97 | |
|
|
|||||
|
|
High |
Low |
|||
|
2016 |
|||||
|
Fourth quarter |
$ |
10.73 |
$ |
6.64 | |
|
Third quarter |
$ |
9.90 |
$ |
6.29 | |
|
Second quarter |
$ |
7.75 |
$ |
5.16 | |
|
First quarter |
$ |
6.17 |
$ |
2.56 | |
As of March 9, 2018, there were approximately 75 holders of record and 45,854,403 shares of our common stock outstanding.
Dividend Policy
We have never declared or paid any cash dividends on our common stock. We intend to retain future earnings, if any, to finance the operation and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future. In addition, unless waived, the terms of our loan agreement with Pacific Western Bank and Oxford Finance LLC limit our ability to pay cash dividends. Any future determination related to dividend policy will be made at the discretion of our Board of Directors after considering our financial condition, results of operations, capital requirements, business prospects and other factors the Board of Directors deems relevant, and subject to the restrictions contained in our current or future financing instruments.
Unregistered Sales of Equity Securities
None.
Repurchases of Common Stock
The repurchase activity for the three months ended December 31, 2017, was as follows:
|
|
||||||||||
|
|
Total number of |
Maximum number of |
||||||||
|
|
Total number |
Average |
shares purchased |
shares that may yet be |
||||||
|
|
of shares |
price paid |
as part of publicly |
purchased under the |
||||||
|
|
purchased |
per share |
announced plan or program |
plan or program |
||||||
|
October 1 - October 31 |
— |
$ |
— |
— |
N/A |
|||||
|
November 1 - November 30 |
1,348 |
(1) |
5.86 |
— |
N/A |
|||||
|
December 1 - December 31 |
— |
— |
— |
N/A |
||||||
|
|
1,348 |
$ |
5.86 |
— |
N/A |
|||||
__________________
(1) For the three months ended December 31, 2017, 1,348 shares of restricted stock were withheld to satisfy employee tax withholding obligations arising in conjunction with the vesting of restricted stock pursuant to our 2013 Incentive Award Plan.
47
Stock Performance Graph
This performance graph shall not be deemed “soliciting material” or to be “filed” with the United States Securities and Exchange Commission (“SEC”) for purposes of Section 18 of the Securities Exchange Act of 1934, as amended the (“Exchange Act”), or otherwise subject to the liabilities under that Section, and shall not be deemed to be incorporated by reference into any of our filings under the Securities Act of 1933, as amended, or the Exchange Act.
The following graph shows a comparison from June 27, 2013 (the date our common stock commenced trading on the Nasdaq Global Select Market) through December 31, 2017 of the cumulative total return for our common stock, the Nasdaq Composite Index (the “Nasdaq Composite”), the Standard & Poor’s 500 Stock Index (the “S&P 500”), and the Nasdaq Biotechnology Index (the “NBI”). The graph assumes that $100 was invested at the market close on June 27, 2013 in the common stock of Aratana Therapeutics, Inc., the Nasdaq Composite, the S&P 500 and the NBI and data for the Nasdaq Composite, the S&P 500, and the NBI assumes reinvestments of dividends. The stock price performance of the following graph is not necessarily indicative of future stock price performance.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 27, 2013 |
|
|
2013 |
|
|
2014 |
|
|
2015 |
|
|
2016 |
|
|
2017 |
|
Aratana Therapeutics, Inc. |
$ |
100.00 |
|
$ |
231.23 |
|
$ |
215.74 |
|
$ |
67.55 |
|
$ |
86.92 |
|
$ |
63.68 | ||
|
Nasdaq Composite |
$ |
100.00 |
|
$ |
123.58 |
|
$ |
141.80 |
|
$ |
151.67 |
|
$ |
165.12 |
|
$ |
214.06 | ||
|
S&P 500 |
$ |
100.00 |
|
$ |
115.81 |
|
$ |
131.66 |
|
$ |
133.49 |
|
$ |
149.45 |
|
$ |
182.08 | ||
|
NBI |
$ |
100.00 |
|
$ |
130.35 |
|
$ |
175.20 |
|
$ |
195.81 |
|
$ |
154.01 |
|
$ |
187.33 | ||
48
Item 6. Selected Financial Data
The following tables set forth selected consolidated financial data of our company as of and for each of the years in the five-year period ended December 31, 2017, and should be read in conjunction with Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and notes thereto included elsewhere in this Annual Report on Form 10-K for the year ended December 31, 2017 (“2017 Annual Report”) and previously filed Annual Reports on Form 10-K.
We have derived the consolidated statements of operations for the years ended December 31, 2017, 2016 and 2015 and the consolidated balance sheet data as of December 31, 2017 and December 31, 2016 from our audited consolidated financial statements included in this 2017 Annual Report in Item 8. “Financial Statements and Supplementary Data.” The selected historical consolidated balance sheet data as of December 31, 2015, December 31, 2014 and December 31, 2013, presented below has been derived from our audited consolidated financial statements not included in this 2017 Annual Report. The revenues data for the years ended December 31, 2014 and 2013 is derived from our audited combined financial statements not included in this 2017 Annual Report.
For a discussion of certain factors that materially affect the comparability of the selected consolidated financial data or cause the data reflected herein not to be indicative of our future results of operations or financial condition, see Item 1A. “Risk Factors,” Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and notes to our consolidated financial statements included elsewhere in this report.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|||||||||||||
|
|
|
2017 |
|
2016 |
|
2015 |
|
2014 |
|
2013 |
|||||
|
|
|
(Dollars in thousands) |
|||||||||||||
|
Revenues |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Licensing and collaboration revenue (1) |
|
$ |
5,913 |
|
$ |
38,233 |
|
$ |
— |
|
$ |
500 |
|
$ |
15 |
|
Product sales (2) |
|
|
19,660 |
|
|
318 |
|
|
678 |
|
|
267 |
|
|
108 |
|
Total revenues |
|
|
25,573 |
|
|
38,551 |
|
|
678 |
|
|
767 |
|
|
123 |
|
Costs and expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of product sales |
|
|
16,387 |
|
|
3,139 |
|
|
365 |
|
|
333 |
|
|
108 |
|
Royalty expense |
|
|
1,821 |
|
|
106 |
|
|
84 |
|
|
72 |
|
|
1 |
|
Research and development |
|
|
15,126 |
|
|
30,462 |
|
|
24,964 |
|
|
19,985 |
|
|
10,925 |
|
Selling, general and administrative |
|
|
28,897 |
|
|
27,342 |
|
|
19,819 |
|
|
17,938 |
|
|
8,572 |
|
In-process research and development |
|
|
— |
|
|
— |
|
|
— |
|
|
2,157 |
|
|
— |
|
Amortization of intangible assets |
|
|
350 |
|
|
379 |
|
|
1,544 |
|
|
1,891 |
|
|
298 |
|
Impairment of intangible assets |
|
|
7,448 |
|
|
7,942 |
|
|
43,398 |
|
|
— |
|
|
— |
|
Total costs and expenses |
|
|
70,029 |
|
|
69,370 |
|
|
90,174 |
|
|
42,376 |
|
|
19,904 |
|
Loss from operations |
|
|
(44,456) |
|
|
(30,819) |
|
|
(89,496) |
|
|
(41,609) |
|
|
(19,781) |
|
Other income (expense) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income |
|
|
449 |
|
|
385 |
|
|
189 |
|
|
123 |
|
|
75 |
|
Interest expense |
|
|
(3,481) |
|
|
(3,396) |
|
|
(1,585) |
|
|
(1,060) |
|
|
(432) |
|
Other income (expense), net |
|
|
(22) |
|
|
255 |
|
|
5,140 |
|
|
2,287 |
|
|
478 |
|
Total other income (expense) |
|
|
(3,054) |
|
|
(2,756) |
|
|
3,744 |
|
|
1,350 |
|
|
121 |
|
Loss before income taxes |
|
|
(47,510) |
|
|
(33,575) |
|
|
(85,752) |
|
|
(40,259) |
|
|
(19,660) |
|
Income tax benefit |
|
|
— |
|
|
— |
|
|
1,698 |
|
|
1,443 |
|
|
12,722 |
|
Net loss |
|
$ |
(47,510) |
|
$ |
(33,575) |
|
$ |
(84,054) |
|
$ |
(38,816) |
|
$ |
(6,938) |
|
Net loss per share, basic and diluted |
|
$ |
(1.17) |
|
$ |
(0.95) |
|
$ |
(2.45) |
|
$ |
(1.30) |
|
$ |
(0.63) |
|
Weighted average shares outstanding, basic and diluted |
|
|
40,494,301 |
|
|
35,273,228 |
|
|
34,355,525 |
|
|
29,767,429 |
|
|
11,059,382 |
__________________
|
(1) |
Licensing and collaboration revenue reflects the impact of the following: |
- revenues recognized related to withdrawal of BLONTRESS from the market in the fourth quarter of 2017 ($480);
- revenues recognized related to the assumption of manufacturing responsibility for GALLIPRANT by Elanco in the third quarter of 2017 ($1,000);
- product launch of GALLIPRANT which commercial sales began in the first quarter of 2017;
- revenues recognized related to the upfront payment from the Collaboration Agreement for GALLIPRANT in the second quarter of 2016 ($38,000).
|
(2) |
Product sales reflect the impact of the following product launches: |
- commercial sales of ENTYCE, which began in the fourth quarter of 2017;
- commercial sales of GALLIPRANT, which began in the first quarter of 2017 and ended in the fourth quarter of 2017 upon the assumption of manufacturing responsibility by Elanco ($15,526);
- commercial sales of NOCITA, which began in the third quarter of 2016.
49
|
|
|||||||||||||||
|
|
As of December 31, |
||||||||||||||
|
|
2017 |
2016 |
2015 |
2014 |
2013 |
||||||||||
|
|
(Dollars in thousands) |
||||||||||||||
|
Consolidated Balance Sheet Data: |
|||||||||||||||
|
Cash, cash equivalents and short-term investments |
$ |
67,615 |
$ |
88,303 |
$ |
86,202 |
$ |
98,072 |
$ |
45,754 | |||||
|
Working capital (1) |
49,743 | 66,854 | 83,335 | 90,441 | 31,307 | ||||||||||
|
Total assets |
135,192 | 151,406 | 147,066 | 207,903 | 112,343 | ||||||||||
|
Total long-term debt, net of current portion |
19,492 | 25,775 | 39,710 | 14,963 | 9,310 | ||||||||||
|
Total stockholders’ equity |
$ |
80,134 |
$ |
90,403 |
$ |
101,550 |
$ |
181,832 |
$ |
83,390 | |||||
__________________
|
(1) |
We define working capital as current assets less current liabilities. |
50
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes and other financial information included elsewhere in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this annual report, including information with respect to our plans and strategy for our business, and expectations regarding product development and licensing, includes forward-looking statements that involve risks and uncertainties. You should review the “Risk Factors” section of this annual report for a discussion of important factors that could cause our actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis or elsewhere in this annual report.
Overview
We are a pet therapeutics company focused on licensing, developing and commercializing innovative therapeutics for dogs and cats. We operate in one business segment: pet therapeutics. Our current product portfolio includes multiple therapeutics and therapeutic candidates in development consisting of small molecule pharmaceuticals and molecule biologics that target large opportunities in unmet or underserved medical conditions in dogs and cats.
We have three United States Food and Drug Administration (“FDA”) approved therapeutics including, GALLIPRANT® (grapiprant tablets) for the control of pain and inflammation associated with osteoarthritis in dogs; ENTYCE® (capromorelin oral solution) for appetite stimulation in dogs; and NOCITA® (bupivacaine liposome injectable suspension) as a local post-operative analgesia for cranial cruciate ligament surgery in dogs. Additionally, we have our Canine Osteosarcoma Vaccine, Live Listeria Vector (AT-014) which is conditionally licensed by the United States Department of Agriculture ("USDA"). Our pipeline has multiple therapeutic candidates in development for the potential treatment of pain, viral diseases, allergy and cancer for dogs and cats.
We have incurred significant net losses since our inception. We incurred net losses of $47.5 million, $33.6 million and $84.1 million for the years ended December 31, 2017, 2016, and 2015, respectively. These losses have resulted principally from costs incurred in connection with in-licensing our therapeutic candidates, research and development activities, and selling, general and administrative costs associated with our operations. As of December 31, 2017, we had a deficit accumulated since inception of $233.3 million, and cash, cash equivalents, restricted cash and short-term investments of $68.0 million.
We expect to continue to incur operating losses for the foreseeable future as we work to develop and commercialize our therapeutics and therapeutic candidates. If we cannot generate sufficient cash from operations in the future, we may seek to fund our operations through collaborations and licensing arrangements, as well as public or private equity offerings or further debt (re)financings. If we are not able to raise additional capital on terms acceptable to us, or at all, as and when needed, we would be forced to delay, reduce, or eliminate certain research and development programs, reduce or eliminate discretionary operating expenses or grant rights to develop and market therapeutics or therapeutic candidates that we would otherwise prefer to develop and market ourselves, which could otherwise adversely affect our business prospects. As disclosed in Note 10 to our consolidated financial statements, we have a term loan and a revolving credit facility with an aggregate principal balance of $36.5 million as of December 31, 2017. The terms of the loan agreement require us to maintain certain minimum liquidity at all times (the greater of cash equal to fifty percent (50%) of outstanding balance or remaining months’ liquidity, which is calculated on an average trailing three (3) month basis, equal to six (6) months or greater), which as of December 31, 2017, was approximately $18.3 million. If the minimum liquidity is not met, we may be required to repay the loans prior to their scheduled maturity dates. At December 31, 2017, we were in compliance with all financial covenants. As of the date of the filing of this 2017 Annual Report, we believe that our existing cash, cash equivalents and short-term investments of $67.6 million on hand at December 31, 2017, together with the proceeds of the January 2018 at-the-market offering, will allow us to fund our operations and our debt obligations for at least one year from the issuance of our consolidated financial statements.
For more information regarding our business and the animal health industry, see Item 1. “Business.”
Recent Developments
For more information regarding research and development, manufacturing and sales and marketing refer to applicable sections in Item 1. “Business.”
Financial Overview
Revenues
Licensing and collaboration revenue consists primarily of revenues recognized related to our GALLIPRANT collaboration, license, development and commercialization agreement (the “Collaboration Agreement”) and co-promotion agreement (the “Co-Promotion Agreement,” and together with the Collaboration Agreement, the “Elanco Agreements”) with Elanco Animal Health, Inc. (“Elanco”), a division of Eli Lilly & Co.
51
Product sales consist primarily of net sales of our therapeutics NOCITA and ENTYCE, and sales of GALLIPRANT finished goods to Elanco under the supply terms of the Collaboration Agreement prior to the assumption of manufacturing responsibility by Elanco.
Costs and expenses
Cost of product sales consists primarily of the cost of direct materials, direct labor and overhead costs associated with the manufacturing of our products. Cost of product sales also included an inventory valuation loss as a result of GALLIPRANT inventories that were written down to net realizable value.
Royalty expense consists of royalty expenses associated with third-party intellectual property. Royalty expense primarily includes third-party royalties for licensed technologies pertaining to GALLIPRANT, NOCITA and ENTYCE. Royalties are either a minimum amount per year or a percentage of net product sales.
Research and development (“R&D”) expenses consist primarily of costs associated with our product development efforts (new product R&D and product lifecycle development), overhead costs associated with R&D and expenses related to regulatory approval of our products. Product development costs consist primarily of contracted development costs, manufacturing costs, wages, stock-based compensation, employee benefits for all employees engaged in scientific research and development functions and milestone payments made under our licensing agreements. Overhead costs associated with R&D consists of other operational costs related to our research and development activities, including facility-related expenses, regulatory, professional and consulting fees, travel costs, and allocated corporate costs.
We have been developing our lead programs in parallel and typically use our employee and infrastructure resources across multiple development programs. We track contracted development costs by development compound but do not allocate personnel or other internal costs related to development to specific programs or development compounds. These expenses are included in personnel costs and other internal costs, respectively.
Selling, general and administrative expenses consist primarily of personnel costs, including salaries, related benefits and stock-based compensation for employees in commercial, administration, finance, information technology, human resources, legal, and business development. Selling, general and administrative expenses also include allocated rent and other facilities costs; conference and sponsorship activities, information technology services, professional and consulting fees for general and commercial business purposes, for accounting and tax services, business development activities, general legal services; and travel and other costs.
Amortization of intangible assets consists primarily of the amortization expense for intangible assets capitalized in conjunction with approval/post-approval milestone payments made under our license agreements. These assets consist of intellectual property rights for currently marketed products.
Impairment of intangible assets consists solely of impairment charges for intangible assets that have been acquired through business combinations whose carrying amounts exceeded their fair value.
Other income (expense)
Interest income consists of interest earned on our cash, cash equivalents and short-term investments.
Interest expense consists of interest incurred on our borrowings.
A more detailed description of our Loan Agreement (as defined below) is available under the caption “Liquidity and Capital Resources.”
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of our consolidated financial statements and related disclosures requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, revenues, costs and expenses and related disclosures during the reporting periods. On an ongoing basis, we evaluate our estimates and judgments, including those described below. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 2 to our consolidated financial statements appearing elsewhere in this filing, we believe that the estimates and assumptions involved in the following accounting policies may have the greatest potential impact on our consolidated financial statements.
Revenue Recognition
We recognize revenue when all of the following conditions are met:
|
· |
persuasive evidence of an arrangement exists; |
|
· |
delivery has occurred or services have been rendered; |
52
|
· |
the seller’s price to the buyer is fixed or determinable; and |
|
· |
collectibility is reasonably assured. |
Our principal revenue streams and their respective accounting treatments are discussed below:
(i) Product sales - Revenue for the sale of products is recognized when delivery has occurred and substantially all the risks and rewards of ownership have been transferred to the customer. Revenue for the sale of products is recorded net of sales returns, allowances and discounts.
(ii) Royalty revenue - Royalty revenue relating to our out-licensed technology is recognized when reasonably estimable. The revenues are recorded based on the licensee’s sales that occurred during the relevant period. Differences between actual and estimated royalty revenues are adjusted in the period in which they become known, typically in the following quarter. If we are unable to reasonably estimate royalty revenue or do not have access to the information, then we record royalty revenue when the information needed for a reliable estimate becomes available. Royalty revenue is included in licensing and collaboration revenue in the consolidated statements of operations.
(iii) Licensing and collaboration revenues - Revenues derived from product out-licensing arrangements typically consist of an initial upfront payment at inception of the license and subsequent milestone payments contingent on the achievement of certain regulatory, development and commercial milestones.
Product out-licensing arrangements with multiple elements are divided into separate units of accounting if certain criteria are met. The upfront payment received is allocated among the separate units of accounting based on their fair values, and the applicable revenue recognition criteria are applied to each of the separate units of accounting. The application of the multiple element guidance requires subjective determinations, and requires us to make judgments about the individual deliverables and whether such deliverables are separable from the other aspects of the contractual relationship. Deliverables are considered separate units of accounting provided that:
(1) the delivered item(s) has value to the customer on a stand-alone basis and
(2) if the arrangement includes a general right of return relative to the delivered item(s), delivery or performance of the undelivered item(s) is considered probable and substantially in our control.
In determining the units of accounting, we evaluate certain criteria, including whether the deliverables have stand-alone value, based on the consideration of the relevant facts and circumstances for each arrangement. In addition, we consider whether the buyer can use the other deliverable(s) for their intended purpose without the receipt of the remaining element(s), whether the value of the deliverable is dependent on the undelivered item(s), and whether there are other vendors that can provide the undelivered element(s).
Arrangement consideration that is fixed or determinable is allocated among the separate units of accounting using the relative selling price method, and the applicable revenue recognition criteria. We determine the estimated selling price for deliverables within each agreement using vendor-specific objective evidence (“VSOE”) of selling price, if available, third-party evidence (“TPE”) of selling price if VSOE is not available, or management's best estimate of the selling price (“BESP”) if neither VSOE nor TPE is available. Determining the BESP for a unit of accounting requires significant judgment. In developing the BESP for a unit of accounting, we consider applicable market conditions and relevant entity-specific factors, including factors that were contemplated in negotiating the agreement with the customer and estimated costs.
If there are deliverables in an arrangement that are not separable from other aspects of the contractual relationship, they are treated as a combined unit of accounting, with the allocated revenue for the combined unit recognized in a manner consistent with the revenue recognition applicable to the final deliverable in the combined unit.
Amounts received prior to satisfying all relevant revenue recognition criteria are recorded as deferred revenue in the consolidated balance sheets and recognized as revenue when the related revenue recognition criteria are met. Amounts not expected to be recognized as revenue within the next twelve months of the consolidated balance sheet date are classified as long-term deferred revenue.
We recognize revenue contingent upon the achievement of a milestone in its entirety in the period in which the milestone is achieved only if the milestone meets all the criteria to be considered substantive. At the inception of each arrangement that includes milestone payments, we evaluate each contingent payment on an individual basis to determine whether they are considered substantive milestones, specifically reviewing factors such as the degree of certainty in achieving the milestone, the research and development risk and other risks that must be overcome to achieve the milestone, as well as the level of effort and investment required and whether the milestone consideration is reasonable relative to all deliverables and payment terms in the arrangement. This evaluation includes an assessment of whether (a) the consideration is commensurate with either (1) the entity’s performance to achieve the milestone, or (2) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the entity’s performance to achieve the milestone, (b) the consideration relates solely to past performance and (c) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement.
Milestone payments which are non-refundable, non-creditable and contingent on achieving certain development, regulatory, or commercial milestones are typically recognized as revenues either on achievement of such milestones or over the period we have continuing substantive performance obligations. We recognize revenue associated with the non-substantive milestones upon
53
achievement of the milestone if there are no undelivered elements and we have no remaining performance obligations. Revenues from commercial milestone payments are recorded as revenue upon achievement of the milestone, assuming all other revenue recognition criteria are met.
In the event that an agreement is terminated and we have no further performance obligations, we recognize as revenue any amounts that had not previously been recorded as revenue but were classified as deferred revenue at the date of such termination.
Cash consideration (including a sales incentive) given by us to a licensee/collaborator/customer is presumed to be a reduction of the selling prices of our products or services and is recognized as a reduction of revenue unless both of the following conditions are met:
a. We receive, or will receive, an identifiable benefit (goods or services) in exchange for the consideration. In order to meet this condition, the identified benefit must be sufficiently separable from the recipient’s purchase of our products such that we could have entered into an exchange transaction with a party other than a purchaser of its products or services in order to receive that benefit.
b. We can reasonably estimate the fair value of the benefit identified under the preceding condition. If the amount of consideration paid by us exceeds the estimated fair value of the benefit received, that excess amount shall be characterized as a reduction of revenue when recognized in our statements of operations.
If both conditions are met, the cash consideration is recognized as a cost incurred.
Research and Development
As part of the process of preparing our consolidated financial statements, we are required to estimate accrued research and development expenses. Examples of estimated accrued expenses include fees paid to clinical research organizations (“CROs”), in connection with target animal studies, to investigative sites in connection with target animal studies, to contract manufacturers in connection with the production of active pharmaceutical ingredient, and formulated drug, and to other parties for outsourced chemistry services.
We review new and open contracts and communicate with applicable internal and vendor personnel to identify services that have been performed on our behalf and estimate the level of service performed and the associated costs incurred for the service when we have not yet been invoiced or otherwise notified of the actual cost for accrued expenses. The majority of our service providers invoice us monthly in arrears for services performed or as milestones are achieved in relation to our contract manufacturers. We make estimates of our accrued expenses as of each consolidated balance sheet date.
We base our accrued expenses related to target animal studies on our estimates of the services received and efforts expended pursuant to contracts with CROs that conduct and manage target animal studies on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors such as the successful enrollment of animals and the completion of development milestones. We estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust the related expense accrual accordingly on a prospective basis. If we do not identify costs that have been incurred or if we underestimate or overestimate the level of services performed or the costs of these services, our actual expenses could differ from our estimates. To date, we have not made any material adjustments to our estimates of accrued research and development expenses or the level of services performed in any reporting period presented in this document.
Impairment of Intangible Assets and Goodwill
Indefinite-lived in-process research and development (“IPR&D”) intangible assets are assessed for impairment at least annually. In addition, all intangible assets are reviewed for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. Factors that we consider in deciding when to perform an impairment review include significant underperformance of the business in relation to expectations, significant negative industry or economic trends, and significant changes or planned changes in the use of the assets. If an impairment review is performed to evaluate a long-lived asset for recoverability, we compare forecasts of undiscounted cash flows for definite-lived intangible assets and discounted cash flows for indefinite-lived IPR&D intangible assets expected to result from the use and eventual disposition of the long-lived asset to its carrying value. An impairment loss would be recognized when estimated undiscounted (definite-lived) or discounted (indefinite-lived) future cash flows expected to result from the use of an asset are less than its carrying amount. The impairment loss would be based on the excess of the carrying value of the impaired asset over its fair value, determined based on discounted cash flows. In the years ended December 31, 2017 and 2016 and to date, we recorded $7.4 million and $7.9 million and $58.8 million, respectively, of impairment losses on intangible assets (see Note 8 to our consolidated financial statements).
We completed our annual indefinite-lived IPR&D intangible assets impairment testing during the fourth quarter of 2017. We elected to bypass the qualitative assessment. For purposes of impairment testing, the fair value of the indefinite-lived IPR&D intangible assets was determined by using the framework of ASC 820, Fair Value Measurement. When determining the fair value of the indefinite-lived IPR&D intangible assets, we revisited all assumptions used in measuring the indefinite-lived IPR&D intangible assets at the time of acquisition, and evaluated and considered new and updated data and information available. During the fourth quarter of 2017, we determined that events and changes in circumstances indicated that the IPR&D intangible assets might be impaired. During our
54
development program prioritization review, we considered a number of factors, including our inability to raise additional capital in November 2017, and we decided to further delay the development of AT-006 and AT-008. Due to this delay we revisited all assumptions used in measuring the fair values of AT-006 and AT-008. This interim review resulted in fair values of these intangible assets being less than their carrying values which resulted in an impairment charge of $7.4 million, which was recorded during the fourth quarter of 2017, reducing the carrying values of both AT-006 and AT-008 to $0.0 million.
We completed our annual goodwill impairment testing during the third quarter of 2017. We elected to bypass the qualitative assessment. We determined as of the testing date that we consisted of one operating segment, which is comprised of one reporting unit. In performing step one of the assessment, we determined that our fair value, determined to be our market capitalization, was greater than our carrying value, determined to be stockholders’ equity. Based on this result, we determined there was no impairment of goodwill during the third quarter of 2017.
Stock-Based Compensation
We measure stock-based awards granted to employees and directors at fair value on the date of grant and recognize the corresponding compensation expense of those awards over the requisite service period, which is generally the vesting period of the respective award. Stock-based compensation related to restricted stock awards is based on the market value of our common stock on the date of grant and is recognized as expense ratably over the requisite service period. Generally, we issue stock-based awards with only service-based vesting conditions and record compensation expense for these awards using the straight-line method. We grant stock-based awards with exercise prices equivalent to the fair value of our common share as of the date of grant.
Upon adoption of ASU 2016-09 (Compensation – Stock Compensation) on January 1, 2017, we elected to change our accounting policy to account for forfeitures as they occur. The change was applied on a modified retrospective basis with a cumulative-effect adjustment to accumulated deficit of $0.2 million (which increased the accumulated deficit) as of January 1, 2017. Prior to adoption of this guidance we estimated forfeitures.
We account for all stock-based awards issued to non-employees based on the fair value of the award on each measurement date. Stock-based awards granted to non-employees are subject to revaluation at each reporting date over their vesting terms. As a result, the charge to operations for non-employee awards with vesting conditions is affected each reporting period by changes in the fair value of our common stock.
The fair value of each stock-based award is estimated using the Black-Scholes option-pricing model. The risk-free interest rate is determined by reference to the United States Treasury yield curve in effect at the time of grant of the award for time periods approximately equal to the expected term of the award. The expected term of our awards has been determined utilizing the “simplified” method as we do not have sufficient historical experience for option grants overall, rendering existing historical experience irrelevant to expectations for current grants. Expected volatility for our awards is based on the historical volatility of our common stock. Expected dividend yield is based on the fact that we have never paid cash dividends and do not expect to pay any cash dividends in the foreseeable future.
The assumptions we used to determine the fair value of stock-based compensation attributable to stock options granted in each period were as follows, presented on a weighted average basis:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
||||||||||
|
|
|
2017 |
|
2016 |
|
2015 |
||||||
|
Risk-free interest rate |
|
|
1.99 |
% |
|
|
1.52 |
% |
|
|
1.38 |
% |
|
Expected term (in years) |
|
|
6.0 |
|
|
|
6.2 |
|
|
|
6.1 |
|
|
Expected volatility |
|
|
75 |
% |
|
|
77 |
% |
|
|
70 |
% |
|
Expected dividend yield |
|
|
— |
% |
|
|
— |
% |
|
|
— |
% |
These assumptions represent our best estimates, but the estimates involve inherent uncertainties and the application of our judgment. As a result, if factors change and we use significantly different assumptions or estimates, our stock-based compensation expense could be materially different. We had an aggregate of $4.50 million and $2.40 million of unrecognized stock-based compensation expense for options outstanding and restricted stock awards, respectively, as of December 31, 2017, which is expected to be recognized over a weighted-average period of 2.29 years for stock options and 1.77 years for restricted stock.
55
Income Taxes
We account for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in our consolidated financial statements or in our tax returns. Deferred taxes are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect in the years in which the differences are expected to reverse. Changes in deferred tax assets and liabilities, other than those arising from business combinations, are recorded in the provision for income taxes. We assess the likelihood that our deferred tax assets will be recovered from future sources of taxable income and, to the extent we believe, based upon the weight of available evidence, that it is more likely than not that all or a portion of deferred tax assets will not be realized, a valuation allowance is established through a charge to income tax expense. Potential for recovery of deferred tax assets is evaluated by estimating the future taxable profits expected and considering prudent and feasible tax planning strategies.
We account for uncertainty in income taxes recognized in our consolidated financial statements by applying a two-step process to determine the amount of tax benefit to be recognized. First, the tax position must be evaluated to determine the likelihood that it will be sustained upon external examination by the taxing authorities. If the tax position is deemed more-likely-than-not to be sustained, the tax position is then assessed to determine the amount of benefit to recognize in our consolidated financial statements. The amount of the benefit that may be recognized is the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement. The provision for income taxes includes the effects of any resulting tax reserves, or unrecognized tax benefits, that are considered appropriate as well as the related net interest and penalties.
JOBS Act
On April 5, 2012, the Jumpstart Our Business Startups Act (“JOBS Act”), was signed into law. The JOBS Act contains provisions that, among other things, reduce certain reporting requirements for an “emerging growth company.” As an “emerging growth company” we are electing not to take advantage of the extended transition period afforded by the JOBS Act for the implementation of new or revised accounting standards, and as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision not to take advantage of the extended transition period is irrevocable.
In addition, we continue to evaluate the benefits of relying on the other exemptions and reduced reporting requirements provided by the JOBS Act. Subject to certain conditions set forth in the JOBS Act, if as an “emerging growth company” we choose to rely on such exemptions, we may not be required to, among other things, (i) provide an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act, and (ii) comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the consolidated financial statements (auditor discussion and analysis). These exemptions will apply for a period of five years following the completion of our initial public offering, which such fifth anniversary will occur in 2018, or until we no longer meet the requirements of being an “emerging growth company,” whichever is earlier.
56
Results of Operations
Comparison of the Years Ended December 31, 2017 and 2016
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended |
|
|
|
||||
|
|
|
December 31, |
|
|
|
||||
|
|
|
2017 |
|
2016 |
|
% Change |
|||
|
|
|
(Dollars in thousands) |
|
|
|
||||
|
Revenues |
|
|
|
|
|
|
|
|
|
|
Licensing and collaboration revenue |
|
$ |
5,913 |
|
$ |
38,233 |
|
(84.5) |
% |
|
Product sales |
|
|
19,660 |
|
|
318 |
|
>100.0 |
% |
|
Total revenues |
|
|
25,573 |
|
|
38,551 |
|
(33.7) |
% |
|
Costs and expenses: |
|
|
|
|
|
|
|
|
|
|
Cost of product sales |
|
|
16,387 |
|
|
3,139 |
|
>100.0 |
% |
|
Royalty expense |
|
|
1,821 |
|
|
106 |
|
>100.0 |
% |
|
Research and development |
|
|
15,126 |
|
|
30,462 |
|
(50.3) |
% |
|
Selling, general and administrative |
|
|
28,897 |
|
|
27,342 |
|
5.7 |
% |
|
Amortization of intangible assets |
|
|
350 |
|
|
379 |
|
(7.7) |
% |
|
Impairment of intangible assets |
|
|
7,448 |
|
|
7,942 |
|
(6.2) |
% |
|
Total costs and expenses |
|
|
70,029 |
|
|
69,370 |
|
0.9 |
% |
|
Loss from operations |
|
|
(44,456) |
|
|
(30,819) |
|
44.2 |
% |
|
Other income (expense) |
|
|
|
|
|
|
|
|
|
|
Interest income |
|
|
449 |
|
|
385 |
|
16.6 |
% |
|
Interest expense |
|
|
(3,481) |
|
|
(3,396) |
|
2.5 |
% |
|
Other income (expense), net |
|
|
(22) |
|
|
255 |
|
<(100.0) |
% |
|
Total other expense |
|
|
(3,054) |
|
|
(2,756) |
|
10.8 |
% |
|
Net loss |
|
$ |
(47,510) |
|
$ |
(33,575) |
|
41.5 |
% |
Revenues
During the year ended December 31, 2017, total revenues decreased by $13.0 million as compared to 2016. The decrease was primarily due to a decrease of $32.8 million of licensing and collaboration revenue from the Elanco Agreements, partially offset by an increase of $19.3 million in net product sales primarily due to net sales of GALLIPRANT, NOCITA and ENTYCE and an increase of $0.5 million in licensing and collaboration revenue due to the derecognition of the remaining balance of a liability related to BLONTRESS. Total revenues for the year ended December 31, 2016, included $38.0 million of licensing and collaboration revenue recognized from the Elanco Agreements. During the year ended December 31, 2017, product sales consisted of net sales of GALLIPRANT, NOCITA, ENTYCE, BLONTRESS and TACTRESS. GALLIPRANT product sales during the year ended December 31, 2017, consisted of $15.5 million of product sales of finished goods prior to the assumption of manufacturing responsibility by Elanco under the supply terms of the Collaboration Agreement as compared to $0.0 million in 2016. During the years ended December 31, 2017 and 2016, NOCITA net sales were $2.8 million and $0.1 million, and ENTYCE net sales were $1.3 million and $0.0 million, respectively.
We believe that product sales in 2018 will be a combination of sales of ENTYCE, which began in late-2017, and NOCITA. Any licensing and collaboration revenue in 2018 will be substantially dependent on Elanco’s ability to successfully commercialize GALLIPRANT in accordance with the Elanco Agreements and the amount of research and development expenditures we incur towards the $7.0 million licensing and collaboration commitment in accordance with the Elanco Collaboration Agreement.
Cost of product sales
During the year ended December 31, 2017, cost of product sales increased by $13.2 million as compared to 2016, primarily as a result of cost of product sales of GALLIPRANT, NOCITA, BLONTRESS, and TACTRESS. During the year ended December 31, 2017, we recognized in cost of product sales an inventory valuation loss of $0.4 million as a result of GALLIPRANT inventories that were written down to net realizable value. During the year ended December 31, 2016, we recognized in cost of product sales an inventory valuation loss in the amount of $2.5 million from the write-off of BLONTRESS and TACTRESS inventories and pre-launch GALLIPRANT inventories written down to market value due to terms agreed upon in the Collaboration Agreement.
During the year ended December 31, 2017, cost of product sales as a percentage of product sales was largely impacted by the sale of GALLIPRANT inventories to Elanco which had lower margins as compared to ENTYCE and NOCITA margins. However, cost of product sales as a percentage of product sales improved in the fourth quarter of 2017 as a result of the sale of ENTYCE process validation batches, which were previously written down. We anticipate cost of product sales as a percentage of product sales will improve in 2018 as compared to 2017. This improvement is expected to be largely due to the fact that GALLIPRANT manufacturing responsibilities have been assumed by Elanco. However, this margin improvement for GALLIPRANT is expected to be offset by lower margins on ENTYCE after we sell process validation batches throughout the course of 2018 and begin to sell product that was not previously expensed.
57
Royalty expense
During the year ended December 31, 2017, royalty expense increased by $1.7 million as compared to 2016, primarily as a result of the sales of GALLIPRANT, NOCITA and ENTYCE. We believe any future royalty expense in 2018 will be substantially dependent on Elanco’s ability to successfully commercialize GALLIPRANT in accordance with the Elanco Agreements, and our continuing efforts to commercialize NOCITA and ENTYCE.
Research and development expense
|
|
|||||||||
|
|
Year Ended |
||||||||
|
|
December 31, |
||||||||
|
|
2017 |
2016 |
% Change |
||||||
|
|
(Dollars in thousands) |
||||||||
|
|
|||||||||
|
Contracted development costs |
$ |
11,086 |
$ |
18,349 | (39.6) |
% |
|||
|
Milestones |
250 | 6,950 | (96.4) |
% |
|||||
|
Personnel costs |
3,259 | 4,456 | (26.9) |
% |
|||||
|
Other costs |
531 | 707 | (24.9) |
% |
|||||
|
Total research and development |
$ |
15,126 |
$ |
30,462 | (50.3) |
% |
|||
During the year ended December 31, 2017, research and development expense decreased by $15.3 million as compared to 2016. This decrease was primarily due to a decrease of $6.7 million in milestone payments relating to GALLIPRANT, ENTYCE, NOCITA, and AT-016, and a decrease of $7.3 million in contracted development costs due to the prioritization of spending for ongoing programs, a $1.2 million decrease in personnel costs primarily due to a lower R&D headcount in 2017, and a $0.2 million decrease in other costs. During the year ended December 31, 2017, research and development expenses also included an inventory valuation loss of $0.4 million from the application of lower of cost and net realizable value related to raw materials purchased for future validation batches of GALLIPRANT that have been assumed by Elanco.
Since we have completed several of our pivotal studies and achieved several development milestones for GALLIPRANT, ENTYCE and NOCITA, we expect in 2018 our research and development expenses to be lower than in 2017. Research and development expenses in 2018 are expected to be primarily related to expanding the label of our approved therapeutics for additional indications and/or species and advancing our development portfolio.
Selling, general and administrative expense
During the year ended December 31, 2017, selling, general and administrative expense increased by $1.6 million as compared to 2016. The increase was primarily due to an increase of $2.8 million in personnel expenses primarily as a result of higher sales and marketing headcount, partially offset by a decrease of $1.2 million in other expenses due to the substantial completion of the implementation of our commercial infrastructure including systems and market preparation materials.
We expect selling, general and administrative expense in 2018 to remain relatively consistent with 2017 as we have substantially completed the build out of our sales organization and corporate infrastructure in support of the commercialization of NOCITA and ENTYCE, and our co-promotion of GALLIPRANT, with a slight increase to support further adoption and awareness for our marketed brands.
Impairment of intangible assets
During the year ended December 31, 2017, impairment of intangible assets expense decreased by $0.5 million as compared to 2016. The impairment of intangible assets in 2017 was related to impairment charges for AT-006 and AT-008, and in 2016 it was related to impairment charges for TACTRESS ($0.5 million), BLONTRESS ($5.2 million) and AT-007 ($2.2 million). The impairment charges related to AT-006 and AT-008 resulted from our decision to further delay the development of AT-006 and AT-008 due to our development program prioritization review, which included our consideration of a number of factors, including our inability to raise additional capital in November 2017, reducing the carrying values of both AT-006 and AT-008 to $0.0 million. The impairment charge related to TACTRESS resulted from updated sales expectations and resulted in a carrying value of $0.0 million for TACTRESS. The impairment charge related to AT-007 was the result of our decision to discontinue the development of AT-007 due to the return of global rights of AT-006 and ensuing development program portfolio prioritization, including consideration of our focus on commercial launch activities to support our recently approved products, and resulted in a carrying value of $0.0 million for AT-007. The impairment charge related to BLONTRESS resulted from updated sales expectations as result of the Mini B-CHOMP final study results. Unfavorable estimates of our therapeutics’ market opportunities, expected future cash flows and estimated useful lives could result in impairment charges in future periods. For more information regarding the impairment charges see Note 8 to our consolidated financial statements.
58
Comparison of the Years Ended December 31, 2016 and 2015
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended |
|
|
|
||||
|
|
|
December 31, |
|
|
|
||||
|
|
|
2016 |
|
2015 |
|
% Change |
|||
|
|
|
(Dollars in thousands) |
|
|
|
||||
|
Revenues |
|
|
|
|
|
|
|
|
|
|
Licensing and collaboration revenue |
|
$ |
38,233 |
|
$ |
— |
|
NA |
|
|
Product sales |
|
|
318 |
|
|
678 |
|
(53) |
% |
|
Total revenues |
|
|
38,551 |
|
|
678 |
|
>100.0 |
% |
|
Costs and expenses |
|
|
|
|
|
|
|
|
|
|
Cost of product sales |
|
|
3,139 |
|
|
365 |
|
>100.0 |
% |
|
Royalty expense |
|
|
106 |
|
|
84 |
|
26 |
% |
|
Research and development |
|
|
30,462 |
|
|
24,964 |
|
22 |
% |
|
Selling, general and administrative |
|
|
27,342 |
|
|
19,819 |
|
38 |
% |
|
Amortization of intangible assets |
|
|
379 |
|
|
1,544 |
|
(75) |
% |
|
Impairment of intangible assets |
|
|
7,942 |
|
|
43,398 |
|
(82) |
% |
|
Total costs and expenses |
|
|
69,370 |
|
|
90,174 |
|
(23) |
% |
|
Loss from operations |
|
|
(30,819) |
|
|
(89,496) |
|
(66) |
% |
|
Other income (expense) |
|
|
|
|
|
|
|
|
|
|
Interest income |
|
|
385 |
|
|
189 |
|
>100.0 |
% |
|
Interest expense |
|
|
(3,396) |
|
|
(1,585) |
|
>100.0 |
% |
|
Other income, net |
|
|
255 |
|
|
5,140 |
|
(95) |
% |
|
Total other income (expense) |
|
|
(2,756) |
|
|
3,744 |
|
<(100.0) |
% |
|
Loss before income taxes |
|
$ |
(33,575) |
|
$ |
(85,752) |
|
(61) |
% |
|
Income tax benefit |
|
|
— |
|
|
1,698 |
|
(100) |
% |
|
Net loss |
|
$ |
(33,575) |
|
$ |
(84,054) |
|
(60) |
% |
Revenues
During the year ended December 31, 2016, total revenues increased by $37.9 million as compared to 2015. The increase was primarily due to $38.0 million of licensing and collaboration revenue related to the Collaboration Agreement with Elanco entered into in April 2016, partially offset by a decrease of $0.4 million in product sales, which consisted of sales of NOCITA, BLONTRESS and TACTRESS.
Cost of product sales
During the year ended December 31, 2016, cost of product sales increased by $2.8 million as compared to 2015, primarily as a result of an inventory valuation loss in the amount of $2.5 million in 2016 from the write-off of BLONTRESS and TACTRESS inventories and pre-launch GALLIPRANT inventories written down to market value due to terms agreed upon in the Collaboration Agreement.
Royalty expense
During the year ended December 31, 2016, royalty expense increased by $22,000 as compared to 2015, primarily as a result of the sales of NOCITA.
Research and development expense
|
|
|||||||||
|
|
Year Ended |
||||||||
|
|
December 31, |
||||||||
|
|
2016 |
2015 |
% Change |
||||||
|
|
(Dollars in thousands) |
||||||||
|
|
|||||||||
|
Contracted development costs |
|
$ |
18,349 |
|
$ |
16,889 |
|
8.6 |
% |
|
Milestones |
|
|
6,950 |
|
|
700 |
|
>100.0 |
% |
|
Personnel costs |
|
|
4,456 |
|
|
5,726 |
|
(22.2) |
% |
|
Other costs |
|
|
707 |
|
|
1,649 |
|
(57.1) |
% |
|
Total research and development |
|
$ |
30,462 |
|
$ |
24,964 |
|
22.0 |
% |
During the year ended December 31, 2016, research and development expense increased by $5.5 million as compared to 2015. This increase was primarily due to an increase of $6.3 million in milestone payments relating to GALLIPRANT, ENTYCE, NOCITA, and AT-016, and an increase of $4.6 million in contracted development costs as a result of transfer and scale-up of manufacturing of ENTYCE, offset by a decrease of $3.1 million in contracted development costs due to the completion of several clinical studies for
59
our lead programs, a $1.3 million decrease in personnel costs primarily due to a lower headcount, and a $0.9 million decrease in other costs.
Selling, general and administrative expense
During the year ended December 31, 2016, selling, general and administrative expense increased by $7.5 million as compared to 2015. The increase was primarily due to an increase of $3.3 million in personnel expenses primarily as a result of higher sales and marketing headcount and an increase of $3.0 million incurred in preparation for the commercialization of product candidates for which we received regulatory approval. The increase in selling, general and administrative expenses was also due to a credit of $1.2 million recorded in the first quarter of 2015 to reduce the fair value of the contingent consideration to zero, which had originally been due under the Vet Therapeutics, Inc. merger agreement.
Amortization of intangible assets
During the year ended December 31, 2016, amortization of intangible assets decreased by $1.2 million as compared to 2015. The decrease reflects the impact of the impairment of BLONTRESS and TACTRESS in 2015 and 2016. The lower carrying value due to the impairment charges resulted in the lower expense being recognized.
Impairment of intangible assets
During the year ended December 31, 2016, impairment of intangible assets expense decreased by $35.5 million as compared to 2015. The impairment of intangible assets in 2016 was related to impairment charges for TACTRESS ($0.5 million), BLONTRESS ($5.2 million) and AT-007 ($2.2 million). The impairment charge related to TACTRESS resulted from updated sales expectations and resulted in a carrying value of $0.0 million for TACTRESS. The impairment charge related to AT-007 was the result of our decision to discontinue the development of AT-007 due to the return of global rights of AT-006 and ensuing development program portfolio prioritization, including consideration of our focus on commercial launch activities to support our recently approved products, and resulted in a carrying value of $0.0 million for AT-007. The impairment charge related to BLONTRESS resulted from updated sales expectations as result of the Mini B-CHOMP final study results. The impairment of intangible assets in 2015 was related to the impairment of BLONTRESS ($20.2 million), TACTRESS ($8.6 million), AT-007 ($8.7 million), and AT-011 ($5.9 million).
Interest income
During the year ended December 31, 2016, interest income increased by $0.2 million as compared to 2015. The increase was primarily related to interest earned at higher interest rates on higher deposits held at Square 1 Bank N.A., a division of Pacific Western Bank, and higher interest rates on certificates of deposit.
Interest expense
During the year ended December 31, 2016, interest expense increased by $1.8 million as compared to 2015. This increase was due to interest expense related to our term loan and our revolving credit facility, as discussed below in “Financial Condition, Liquidity and Capital Resources – Indebtedness,” which was entered into during October 2015. Accretion of the debt discount and deferred financing costs totaled $0.5 million, which is non-cash interest included in our interest expense above.
Other income, net
During the year ended December 31, 2016, other income, net decreased by $4.9 million as compared to 2015. The decrease was primarily related to the following non-recurring transactions in 2015: $3.5 million gain on the sale of Advaxis stock, $1.3 million gain related to the increase in fair value of the Advaxis warrant and a $0.3 million gain on the sale of shares received from the exercise of the Advaxis warrant. The decrease was partially offset by $0.3 million gain from deconsolidation of a variable interest entity.
Income tax benefit
During the year ended December 31, 2016, income tax benefit decreased by $1.7 million as compared to 2015. The income tax benefit recognized during 2015 was due to losses incurred in Aratana Therapeutics NV. The income tax benefit in 2015 was recognized for losses incurred that would reduce the amount of deferred tax liability related to intangible assets. There was no deferred tax benefit recognized for losses incurred in 2016 due to a full valuation allowance recognized against our deferred tax assets.
60
Financial Condition, Liquidity and Capital Resources
Our financial condition is summarized as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
December 31, 2017 |
|
December 31, 2016 |
|
Change % |
|||
|
|
|
(Dollars in thousands) |
|
|
|
||||
|
Financial assets: |
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
66,868 |
|
$ |
87,307 |
|
(23.4) |
% |
|
Marketable securities - short-term |
|
|
747 |
|
|
996 |
|
(25.0) |
% |
|
Total cash, cash equivalents and marketable securities |
|
$ |
67,615 |
|
$ |
88,303 |
|
(23.4) |
% |
|
Borrowings: |
|
|
|
|
|
|
|
|
|
|
Loans payable, net |
|
$ |
36,825 |
|
$ |
40,188 |
|
(8.4) |
% |
|
Working capital: |
|
|
|
|
|
|
|
|
|
|
Current assets |
|
$ |
85,239 |
|
$ |
101,542 |
|
(16.1) |
% |
|
Current liabilities |
|
|
35,496 |
|
|
34,688 |
|
2.3 |
% |
|
Total working capital |
|
$ |
49,743 |
|
$ |
66,854 |
|
(25.6) |
% |
We have incurred significant net losses since our inception. We incurred net losses of $47.5 million, $33.6 million and $84.1 million for the years ended December 31, 2017, 2016, and 2015, respectively. These losses have resulted principally from costs incurred in connection with in-licensing our product candidates, research and development activities and selling, general and administrative costs associated with our operations. As of December 31, 2017, we had an accumulated deficit of $233.3 million and cash, cash equivalents and short-term investments of $67.6 million.
We expect to continue to incur operating losses for the foreseeable future as we work to develop and commercialize our therapeutics and therapeutic candidates. If we cannot generate sufficient cash from operations in the future, we may seek to fund our operations through corporate collaborations and licensing arrangements, or other sources such as public or private equity and further debt (re)financings. If we are not able to raise additional capital on terms acceptable to us, or at all, as and when needed, we would be forced to delay, reduce, or eliminate certain research and development programs, reduce or eliminate discretionary operating expenses or grant rights to develop and market therapeutics or therapeutic candidates that we would otherwise prefer to develop and market ourselves, which could otherwise adversely affect our business prospects. Our failure to raise capital, as and when needed, would have a negative impact on our financial condition and our ability to pursue our business strategies as this capital is necessary for us to perform the research and development and commercial activities required to generate future revenue streams. As disclosed in Note 10 to our consolidated financial statements, we have a term loan and a revolving credit facility with an aggregate principal balance of $36.5 million as of December 31, 2017. The terms of the loan agreement require us to maintain certain minimum liquidity at all times, which as of December 31, 2017, was approximately $18.3 million. If the minimum liquidity is not met, we may be required to repay the loans prior to scheduled maturity dates. At December 31, 2017, we were in compliance with all financial covenants. As of the date of the filing of the 2017 Annual Report, we believe that our existing cash, cash equivalents and short-term investments of $67.6 million on hand at December 31, 2017, together with the proceeds of the January 2018 at-the-market offering, will be sufficient to fund our operations and debt obligations through at least through March 31, 2019.
Cash, Cash Equivalents and Investments
Until required for another use in our business, we typically invest our cash reserves in bank deposits, certificates of deposit, and other interest bearing debt instruments in accordance with our investment policy. It is our policy to mitigate credit risk in our cash reserves and investments by maintaining a well-diversified portfolio that limits the amount of exposure as to institution, maturity, and investment type. The value of our investments, however, may be adversely affected by increases in interest rates, instability in the global financial markets that reduces the liquidity of securities included in our portfolio, and by other factors which may result in declines in the value of the investments. Each of these events may cause us to record charges to reduce the carrying value of our investment portfolio if the declines are other-than-temporary or we sell investments for less than our acquisition cost, which could adversely impact our financial position and our overall liquidity.
Registered Direct Offering
On May 3, 2017, we entered into a Placement Agency Agreement (“PAA”) with Barclays Capital, Inc. (“Barclays”), pursuant to which Barclays agreed to serve as placement agent for an offering of shares of common stock. In conjunction with the PAA, on May 3, 2017, we also entered into a Securities Purchase Agreement with certain investors for the sale by us of 5,000,000 shares of common stock at a purchase price of $5.25 per share (the “Offering”). The shares of common stock were offered and sold pursuant to our previously filed and then effective registration statement on Form S-3 (File No. 333-197414) and a related prospectus supplement. We agreed to pay Barclays an aggregate fee equal to 6.0% of the gross proceeds received by us from the Offering. The Offering closed on May 9, 2017. We received aggregate net proceeds from the Offering of approximately $24.4 million, after deducting placement agent fees of $1.6 million and offering expenses of $0.3 million.
61
At-the-Market Offerings
Cowen and Company, LLC
On December 18, 2017, we entered into a Sales Agreement (“Cowen Sales Agreement”) with Cowen and Company, LLC (“Cowen”) pursuant to which we may sell from time to time, at our option, up to an aggregate of $50.0 million of shares of our common stock through Cowen, as sales agent. Sales of the shares of common stock will be made under our effective Registration Statement on Form S-3 (Reg. No. 333-219681), by means of ordinary brokers’ transactions on the Nasdaq Global Market or otherwise. Additionally, under the terms of the Cowen Sales Agreement, the shares of common stock may be sold at market prices, at negotiated prices or at prices related to the prevailing market price. We have agreed to pay Cowen a commission of 3% of the gross proceeds from the sale of such shares of common stock.
In January 2018, we sold 2,392,297 shares of common stock for aggregate net proceeds of $11.7 million. As of the date of this filing, approximately $38.0 million of shares of common stock remained available for sale under the Cowen Sales Agreement.
Barclays Capital, Inc.
On April 28, 2017, we terminated our prior at-the-market offering pursuant to a sales agreement with Barclays. As of that date, we had sold an aggregate of approximately $18.0 million of the $52.0 million available to be sold under the Barclays sales agreement, including 546,926 shares for aggregate net proceeds of $2.8 million in 2017.
Shelf Registration Statement
On August 4, 2017, we filed a new shelf registration statement on Form S-3 (Reg. No. 333-219681) (the “Shelf Registration Statement”) with the SEC. The Shelf Registration Statement was declared effective by the SEC on August 16, 2017.
The Shelf Registration Statement allows us to offer and sell, from time to time, up to $100.0 million of common stock, preferred stock, debt securities, warrants, units or any combination of the foregoing in one or more future public offerings. The terms of any future offering would be determined at the time of the offering and would be subject to market conditions and approval by our Board of Directors. Any offering of securities covered by the Shelf Registration Statement will be made only by means of a written prospectus and prospectus supplement authorized and filed by us.
Indebtedness
On October 16, 2015, we and Vet Therapeutics (together the “Borrowers”) entered into a Loan and Security Agreement, as amended on February 24, 2017 (the “Loan Agreement”), with Pacific Western Bank (“Pacific Western”) as collateral agent (“Collateral Agent”) and a lender and Oxford Finance LLC as a lender (“Oxford” and together with Pacific Western, the “Lenders”), pursuant to which the Lenders agreed to make available to the Borrowers, a term loan in an aggregate principal amount up to $35.0 million (the “Term Loan”), and a revolving credit facility in an aggregate principal amount up to $5.0 million (the “Revolving Line”), subject to certain conditions to funding. The Borrowers were required to make interest-only payments on the Term Loan for 18 months, and beginning on May 1, 2017, began to make payments of principal and accrued interest on the Term Loan in equal monthly installments over a term of 30 months. The Term Loan and the Revolving Line bear interest per annum at the greater of (i) 6.91% or (ii) 3.66% plus the prime rate, which is customarily defined. Under the Loan Agreement, all principal and accrued interest on the Term Loan is due on October 16, 2019 (the “Term Loan Maturity Date”), and all principal and accrued interest on the Revolving Line was due on October 16, 2017 (the “Prior Revolving Maturity Date”).
As security for their obligations under the Loan Agreement, the Borrowers granted a security interest in substantially all of their existing and after-acquired assets except for their intellectual property and certain other customary exclusions. Subject to customary exceptions, the Borrowers are not permitted to encumber their intellectual property.
Upon execution of the Loan Agreement, the Borrowers were obligated to pay a facility fee to the Lenders of $0.2 million and an agency fee to the Collateral Agent of $0.1 million. In addition, the Borrowers are or will be obligated to pay a final payment fee equal to 3.30% of such Term Loan being prepaid or repaid with respect to the Term Loan upon the earliest to occur of the Term Loan Maturity Date, the acceleration of any Term Loan or the prepayment of a Term Loan. The Borrowers were obligated to pay a termination fee equal to 3.30% of the highest outstanding amount of the Revolving Line with respect to the Revolving Line upon the earliest to occur of the Prior Revolving Maturity Date, the acceleration of the Revolving Line or the termination of the Revolving Line. The Borrowers will also be obligated to pay an unused-line fee equal to 0.25% per annum of the average unused portion of the Revolving Line.
Effective as of July 31, 2017, we amended the Loan Agreement (the “Second Amendment”). The terms of the Second Amendment, among other things, extend the maturity of the Revolving Line to October 16, 2019 (the “Revolving Line Maturity Date”), with amortized equal repayments of the principal outstanding under the Revolving Line beginning November 1, 2018, and provide a six (6) month interest only period for the Term Loan, starting on the date of the Second Amendment.
62
We are not subject to any new financial covenants as a result of the Second Amendment. At the closing of the Second Amendment, we paid the Lenders an amendment fee of $0.2 million and a facility fee of $0.1 million. We are obligated to pay a new termination fee equal to $0.2 million upon the earliest to occur of the Revolving Line Maturity Date, the acceleration of the Revolving Line or the termination of the Revolving Line. The existing termination fee of $0.2 million was due on the Prior Revolving Maturity Date, and was paid on October 17, 2017.
The Loan Agreement contains customary representations and warranties and customary affirmative and negative covenants, including, among others, limits or restrictions on the Borrowers’ ability to incur liens, incur indebtedness, make certain restricted payments, make certain investments, merge, consolidate, make an acquisition, enter into certain licensing arrangements and dispose of certain assets. In addition, the Loan Agreement contains customary events of default that entitle the Lenders to cause the Borrowers’ indebtedness under the Loan Agreement to become immediately due and payable. The events of default, some of which are subject to cure periods, include, among others, a non-payment default, a covenant default, the occurrence of a material adverse change in our business, the occurrence of an insolvency, a material judgment default, defaults regarding other indebtedness and certain actions by governmental authorities. Upon the occurrence and for the duration of an event of default, an additional default interest rate equal to 4% per annum will apply to all obligations owed under the Loan Agreement.
The Loan Agreement requires that we maintain certain minimum liquidity at all times, which as of December 31, 2017, was approximately $18.3 million. If the minimum liquidity requirement is not met, the Borrowers may be required to repay the loans prior to scheduled maturity dates. At December 31, 2017, the Borrowers were in compliance with all financial covenants, including the minimum liquidity covenant.
Working Capital
We define working capital as current assets less current liabilities. The decrease in working capital from December 31, 2016, reflects a decrease in total current assets of $16.3 million and an increase in current liabilities of $0.8 million. The decrease in total current assets was primarily driven by a decrease in cash and cash equivalents due to payments for our research and development activities related to our programs, payments for inventories, milestones and selling, general and administrative expenses. The increase in total current liabilities was primarily a result of a decrease in accrued expenses of $2.1 million primarily due to our payment of the accrued purchase commitment for ENTYCE inventories of $2.0 million, partially offset by an increase of $2.9 million in current portion – loans payables based on the payment terms of the Loan Agreement.
Cash Flows
The following table shows a summary of our cash flows for the periods set forth below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended |
|||||||
|
|
|
December 31, |
|||||||
|
|
|
2017 |
|
2016 |
|
2015 |
|||
|
|
|
(Dollars in thousands) |
|||||||
|
Net cash used in operating activities |
|
$ |
(38,185) |
|
$ |
(11,323) |
|
$ |
(38,495) |
|
Net cash provided by (used in) investing activities |
|
$ |
(5,752) |
|
$ |
57,285 |
|
$ |
34,013 |
|
Net cash provided by financing activities |
|
$ |
23,471 |
|
$ |
14,632 |
|
$ |
21,895 |
Net cash used in operating activities
During the year ended December 31, 2017, net cash used in operating activities was $38.2 million. We had a net loss of $47.5 million which included an adjustment of a non-cash expense for stock-based compensation of $7.1 million, a non-cash depreciation and amortization expense of $1.2 million, a non-cash impairment of intangible assets of $7.4 million, a non-cash interest expense of $0.5 million, and market value adjustments to inventories of $0.7 million. Our net loss was primarily attributed to our research and development activities related to our programs and our selling, general and administrative expenses, partially offset by product sales revenues and licensing and collaboration revenues from the Collaboration Agreement. Net cash used in operating assets and liabilities was primarily due to an increase in inventories of $3.2 million, a decrease in accrued expenses and other liabilities of $2.6 million and an increase in accounts receivable of $2.3 million, partially offset by a decrease in prepaid expenses and other current assets of $0.3 million, a decrease in other assets of $0.1 million, and an increase in accounts payable of $0.1 million. The increase in accounts receivable was primarily due to GALLIPRANT and ENTYCE receivables, the increase in inventories was primarily due to ENTYCE inventories and the decrease in accrued expenses was primarily due to $2.0 million payment of the accrued purchase commitment for ENTYCE inventories.
During the year ended December 31, 2016, net cash used in operating activities was $11.3 million. We had a net loss of $33.6 million which includes an adjustment of a non-cash expense for stock-based compensation of $8.5 million, a non-cash depreciation and amortization expense of $1.0 million, a non-cash impairment of intangible assets of $7.9 million, a non-cash gain on deconsolidation of a variable interest entity of $0.3 million, a non-cash interest expense of $0.5 million, and market value adjustments to inventories of $5.2 million. Our net loss was primarily attributed to our research and development activities related to our programs and our selling,
63
general and administrative expenses, partially offset by licensing and collaboration revenues of $38.0 million from the Collaboration Agreement. Net cash used in operating assets and liabilities consisted primarily of an increase in inventories of $15.0 million and an increase in prepaid expenses and other current assets of $0.8 million, partially offset by an increase in accounts payable of $6.2 million, an increase in accrued expenses and other liabilities of $2.1 million and an increase of $7.0 million in licensing and collaboration commitment under the Collaboration Agreement. The increase in inventories was primarily related to GALLIPRANT and NOCITA inventories, partially offset by a decrease in BLONTRESS and TACTRESS inventories. The increase in accounts payable was primarily related to GALLIPRANT inventories and trade payables.
During the year ended December 31, 2015, net cash used in operating activities was $38.5 million. We had a net loss of $84.1 million which includes a gain on sale of marketable securities of $3.9 million, an adjustment of a non-cash expense for stock-based compensation of $8.6 million, a non-cash depreciation and amortization expense of $1.8 million, and an impairment of intangible assets loss of $43.4 million, partially offset by a non-cash change in fair value of contingent consideration of $1.2 million, a non-cash change in fair value of derivative instruments of $1.3 million, and a non-cash deferred income tax benefit of $1.7 million. Our net losses were primarily attributed to the impairment of intangible assets, research and development activities related to our programs and our selling, general and administrative expenses. Net cash used in operating assets and liabilities consisted primarily of a decrease in accounts payable of $0.1 million, an increase in inventories of $0.9 million, an increase in prepaid expenses of $0.6 million, partially offset by an increase in accrued expenses and other liabilities of $1.2 million and a decrease in accounts receivable of $0.3 million.
Net cash provided by (used in) investing activities
During the year ended December 31, 2017, net cash used in investing activities was $5.8 million, which primarily consisted of $6.0 million in milestone payments for intangible assets for currently marketed products and the purchases of investments of $3.7 million, partially offset by proceeds from the maturities and sales of investments of $4.0 million.
During the year ended December 31, 2016, net cash provided by investing activities was $57.3 million, which primarily consisted of the proceeds from maturities and sales of investments of $288.3 million, partially offset by purchases of investments $229.8 million, net purchases of property and equipment of $0.1 million, cash contributed as investment in a noncontrolled entity of $0.1 million, and $1.0 million in milestone payments for intangible assets for currently marketed products.
During the year ended December 31, 2015, net cash provided by investing activities was $34.0 million, which related to $2,079 million from the proceeds of maturities of investments and $7.4 million from the sales of marketable securities, partially offset by $2,051 million for the purchase of investments and $2.2 million for purchases of property and equipment.
Net cash provided by financing activities
During the year ended December 31, 2017, net cash provided by financing activities was $23.5 million, which primarily consisted of the net proceeds from issuance of common stock of $27.5 million and proceeds from stock option exercises of $0.2 million, offset by $3.5 million in payments on loans payable, $0.3 million in payments for common stock issuance costs, $0.2 million in payments for debt issuance costs and a $0.2 million payment for the revolving credit facility termination fee.
During the year ended December 31, 2016, net cash provided by financing activities was $14.6 million, which primarily consisted of the net proceeds from issuance of common stock of $14.6 million, offset by $0.1 million in payments for stock issuance costs, and the proceeds of stock option exercises of $0.1 million.
During the year ended December 31, 2015, net cash provided by financing activities was $21.9 million. Net cash provided by financing activities primarily resulted from net cash received from our Loan Agreement of $24.8 million, partially offset by cash paid for contingent consideration of $3.0 million.
Future Funding Requirements
We anticipate that we will continue to incur net losses for the foreseeable future due to expenses for commercialization of our therapeutics and our development programs, including continuing studies in both cats and dogs for our programs in the United States and Europe and the in-licensing or acquisition of additional compounds for development as pet therapeutics.
As of the date of the filing of the 2017 Annual Report, we believe that our cash, cash equivalents and short-term investments on hand at December 31, 2017, together with the proceeds of the January 2018 at-the-market offering, will fund our operations and our debt obligations at least through March 31, 2019. However, our operating plan may change as a result of many factors currently unknown to us, and we may seek additional funds sooner than planned, through public or private equity or further debt (re)financings or other sources, such as strategic collaborations. Such (re)financings may result in dilution to stockholders, imposition of debt covenants and repayment obligations, or other restrictions that may affect our business. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement and involves risks and uncertainties, and actual results could vary as a result of a number of factors, including the factors discussed in the section of this filing entitled “Risk Factors.”
64
Our future capital requirements depend on many factors, including, but not limited to:
|
· |
the results of our target animal studies for our current and future therapeutic candidates; |
|
· |
the amount and timing of any milestone payments or royalties we must pay pursuant to our current or future license agreements or collaboration agreements; |
|
· |
the timing of, and the costs involved in, obtaining regulatory approvals for any of our current or future therapeutic candidates; |
|
· |
the upfront and other payments, and associated costs, related to our identifying, acquiring and in-licensing new therapeutic candidates; |
|
· |
the number and characteristics of the therapeutic candidates we pursue; |
|
· |
the scope, progress, results and costs of researching and developing any of our current or future therapeutic candidates and conducting target animal studies; |
|
· |
whether we acquire any other companies, assets, intellectual property or technologies in the future; |
|
· |
our ability to collaborate with companies with an established commercial presence in Europe to provide our products in that market; |
|
· |
the cost of commercialization activities, if any of our current or future therapeutic candidates are approved for sale, including marketing, sales and distribution costs; |
|
· |
cost of manufacturing our current and future therapeutic candidates and any therapeutics we successfully commercialize; |
|
· |
our ability to establish and maintain strategic collaborations, licensing or other arrangements and the financial terms of such agreements; |
|
· |
whether we are required to repay amounts that we received from government programs or other incentive programs; |
|
· |
whether we are able to service our debt and satisfy debt covenants; |
|
· |
the expenses needed to attract and retain skilled personnel; |
|
· |
the costs associated with being a public company; |
|
· |
the costs associated with any securities class action lawsuits and other litigation; and |
|
· |
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including litigation costs and the outcome of such litigation. |
Contractual Obligations and Commitments
Contractual Obligations
Our contractual obligations primarily consist of our obligations under our loans payable, contract manufacturer commitments, non-cancellable operating leases, minimum royalties and other purchase obligations, excluding amounts related to other funding commitments, contingent development, regulatory and commercial milestone payments, and off-balance sheet arrangements as described below.
65
The following table summarizes our contractual obligations as of December 31, 2017, and the effect such obligations are expected to have on our liquidity and cash flows in future periods:
|
|
|||||||||||||||
|
|
Payments Due by Fiscal Year |
||||||||||||||
|
|
Less Than |
More Than |
|||||||||||||
|
|
Total |
1 Year |
1-3 Years |
3-5 Years |
5 Years |
||||||||||
|
|
(Dollars in thousands) |
||||||||||||||
|
Loans payable (1) |
$ |
41,016 |
$ |
19,775 |
$ |
21,241 |
$ |
— |
$ |
— |
|||||
|
Manufacturing and supply chain (2) |
7,132 | 7,132 |
— |
— |
— |
||||||||||
|
Operating leases (3) |
1,410 | 444 | 966 |
— |
— |
||||||||||
|
Total (4), (5), (6), (7) |
$ |
49,558 |
$ |
27,351 |
$ |
22,207 |
$ |
— |
$ |
— |
|||||
______________
|
(1) |
Represents the contractually required principal and interest payments and termination fees on our term loan and our revolving credit facility in accordance with the required payment schedule. Amounts associated with future interest payments to be made were calculated using the interest rate in effect as of December 31, 2017, which was 8.16%. |
|
(2) |
The table includes minimum order commitments based upon an agreed upon demand forecast established each year and binding manufacturing commitments. |
|
(3) |
The table above includes payments for office equipment and rent for the lease of the corporate headquarters in Leawood, Kansas, through February 2021, and for office space in Leuven, Belgium, through October 2018. |
|
(4) |
The table above excludes flat rate royalty payments and/or milestone payments of up to $101.4 million that could become due in connection with various agreements. The milestones payments will become due as we achieve development, regulatory and commercial milestones and the royalty payments will be paid upon product sales. For more information regarding our milestone payments, see “Contingent Development, Regulatory and Commercial Milestone Payments” below. |
|
(5) |
The table above excludes potential licensing and collaboration commitment payments of up to $7.0 million that could become due in connection with the Collaboration Agreement. The licensing and collaboration payments will become due as related expenses are incurred by Elanco. |
|
(6) |
The table above excludes potential repayments of various government and other incentive programs of up to $0.8 million that could become due if certain criteria are not met or certain actions are taken by the Company. |
|
(7) |
The table above excludes the minimum royalty payment due each year based upon a Commercial License Agreement with Janssen Vaccines and Prevention B.V. (formerly, Crucell Holland B.V. (“Crucell”)) under which we received a commercial license to prepare recombinant antibodies. We are required to pay single digit royalties on net product sales by us allocable to Crucell’s producer cells and/or producer cell know-how, if any. We are required to pay Crucell a perpetual minimum royalty of $20,000 per year that is subject to a yearly inflation index adjustment. |
Contingent Development, Regulatory and Commercial Milestone Payments
Based on our development plans as of December 31, 2017, we have committed to make potential future milestone payments to third parties of up to approximately $101.4 million, of which $73.9 million are for commercial milestones, as part of our various collaborations, including licensing and development programs. Approximately $68.9 million of the commercial milestones relate to the achievement of various sales thresholds. Payments under these agreements generally become due and payable only upon achievement of certain development, regulatory or commercial milestones. Because the achievement of these milestones had not occurred or was not considered probable as of December 31, 2017, such contingencies have not been recorded in our consolidated financial statements.
As of December 31, 2017, we anticipate that we may pay approximately $2.0 million during the next 12 months, provided that various commercial milestones are achieved. Amounts related to contingent milestone payments are not considered contractual obligations as they are contingent on the successful achievement of certain development, regulatory approval and commercial milestones that may not be achieved.
Other Funding Commitments
As of December 31, 2017, we have several on-going development programs in various stages in the regulatory process. Our most significant expenditures are payments to clinical research and contract manufacturing organizations. The contracts are generally cancellable, with notice, at our option.
Off-Balance Sheet Arrangements
We have not engaged in the use of any off-balance sheet arrangements, such as structured finance entities or special purpose entities.
New Accounting Standards
For discussion of our new accounting standards, see notes to our consolidated financial statements Note 2. “Summary of Significant Accounting Policies-New Accounting Standards.”
66
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Interest Rate Fluctuation Risk
Our cash, cash equivalents and short-term investments as of December 31, 2017, consisted primarily of cash and certificates of deposit. Our primary exposure to market risk for our cash, cash equivalents and short-term investments is interest income sensitivity, which is affected by changes in the general level of United States interest rates. However, because of the short-term nature of the instruments in our portfolio, a sudden change in market interest rates would not be expected to have a material impact on our financial condition or results of operations.
We have borrowed $40.0 million under our Loan Agreement, consisting of a $35.0 million term loan and a $5.0 million revolving credit facility. The aggregate outstanding principal balance on the term loan and the revolving credit facility was $36.5 million as of December 31, 2017. We are obligated to make interest-only payments on the term loan and the revolving credit facility through February 1, 2018, and November 1, 2018, respectively, followed by monthly installments of outstanding principal and interest through October 1, 2019. The term loan and the revolving credit facility bear interest per annum at the greater of (i) 6.91% or (ii) 3.66% plus the prime rate. Given the amounts outstanding and available under the Loan Agreement, and the interest rate paid to date, we do not believe a 1.0% increase in the interest rate would have a material effect on our financial condition or results of operations.
Foreign Exchange Risk
We are exposed to market risk associated with foreign currency exchange rate fluctuations, and this market risk was further enhanced as a result of our acquisition of Okapi Sciences during January 2014. We face exposure to movements in foreign currency exchange rates whenever we enter into transactions with third parties that are denominated in currencies other than our functional currency. Intercompany transactions between entities that use different functional currencies also expose us to foreign currency risk.
Item 8. Financial Statements and Supplementary Data
The financial statements and supplementary data are listed under Item 15(a) and have been filed as part of this 2017 Annual Report on the pages indicated.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Limitations on Effectiveness of Controls and Procedures
In designing and evaluating our disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply judgment in evaluating the benefits of possible controls and procedures relative to their costs.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated, as of the end of the period covered by this 2017 Annual Report, the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended). Based on that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level as of December 31, 2017.
Management’s Annual Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) under the Securities Exchange Act of 1934, as amended.
Our management conducted an assessment of the effectiveness of our internal control over financial reporting as of December 31, 2017, based on the criteria set forth in “Internal Control-Integrated Framework (2013 Framework)” issued by the Committee of Sponsoring Organizations of the Treadway Commission.
Based on this assessment, management concluded that as of December 31, 2017, our internal control over financial reporting was effective.
As we are an emerging growth company, our independent registered public accounting firm is not required to attest to the effectiveness of our internal control over financial reporting.
67
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended) identified in connection with the evaluation of our internal control performed during the fiscal quarter ended December 31, 2017, that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
None.
68
Item 10. Directors, Executive Officers and Corporate Governance
The information concerning the Company’s executive officers and directors is contained in Part I of this Annual Report on Form 10-K. The rest of the information required to be disclosed by this item will be contained under the headings “Section 16(a) Beneficial Ownership Reporting Compliance,” “Corporate Governance – Code of Ethics,” and “Committees of the Board” in the Proxy Statement for the Company’s 2018 Annual Meeting of Stockholders and is incorporated herein by reference.
Item 11. Executive Compensation
The information required to be disclosed by this item will be contained under the headings “Executive and Director Compensation” and “Compensation Committee Interlocks and Insider Participation” in the Proxy Statement for the Company’s 2018 Annual Meeting of Stockholders and is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required to be disclosed by this item will be contained under the headings “Executive and Director Compensation – Equity Compensation Plan Information” and “Security Ownership of Certain Beneficial Owners and Management” in the Proxy Statement for the Company’s 2018 Annual Meeting of Stockholders and is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required to be disclosed by this item will be contained under the headings “Certain Relationships” and “Corporate Governance – Director Independence” in the Proxy Statement for the Company’s 2018 Annual Meeting of Stockholders and is incorporated herein by reference.
Item 14. Principal Accounting Fees and Services
The information required to be disclosed by this item will be contained under the heading “Independent Registered Public Accounting Firm Fees and Other Matters” in the Proxy Statement for the Company’s 2018 Annual Meeting of Stockholders and is incorporated herein by reference.
69
Item 15. Exhibits, Financial Statement Schedules
(a)(1), (a)(2) and (c). The response to this portion of Item 15 is submitted as a separate section of this report commencing on page F-1.
(a)(3) and (b). Exhibits (numbered in accordance with Item 601 of Regulation S-K).
|
|
|
|
|
|
|
|
|
|
|
Incorporated by Reference |
|
|||
|
Exhibit Number |
Exhibit Description |
Form |
File No. |
Exhibit |
Filing Date |
Filed/ Furnished Herewith |
|
2.1† |
8-K |
001-35952 |
10.1 |
10/16/2013 |
|
|
|
2.2 |
8-K |
001-35952 |
10.1 |
1/7/2014 |
|
|
|
3.1 |
8-K |
001-35952 |
3.1 |
7/3/2013 |
|
|
|
3.2 |
8-K |
001-35952 |
3.2 |
7/3/2013 |
|
|
|
4.1 |
Specimen stock certificate evidencing the shares of common stock |
S-1/A |
333-187372 |
4.1 |
6/6/2013 |
|
|
4.2 |
S-1/A |
333-187372 |
10.1 |
5/23/2013 |
|
|
|
10.1†† |
Form of Indemnification Agreement for Directors and Officers |
S-1 |
333-187372 |
10.3 |
3/20/2013 |
|
|
10.2†† |
S-1/A |
333-187372 |
10.4 |
5/23/2013 |
|
|
|
10.3†† |
S-1/A |
333-187372 |
10.8 |
5/23/2013 |
|
|
|
10.4†† |
8-K |
001-35952 |
10.1 |
11/14/2013 |
|
|
|
10.5(a)†† |
S-1/A |
333-193324 |
10.8(a) |
1/28/2014 |
|
|
|
10.5(b)†† |
S-1 |
333-187372 |
10.9(b) |
3/20/2013 |
|
|
|
10.5(c)†† |
S-1 |
333-187372 |
10.9(c) |
3/20/2013 |
|
|
|
10.5(d)†† |
Form of Stock Option Grant Notice and Stock Option Agreement under 2010 Equity Incentive Plan |
S-1 |
333-187372 |
10.9(d) |
3/20/2013 |
|
|
10.6(a)†† |
S-8 |
333-187372 |
99.1 |
1/21/2014 |
|
|
|
10.6(b)†† |
Form of Stock Option Grant Notice and Stock Option Agreement under 2013 Incentive Award Plan |
S-1/A |
333-187372 |
10.10(b) |
4/30/2013 |
|
70
|
|
|
|
|
|
|
|
|
|
|
Incorporated by Reference |
|
|||
|
Exhibit Number |
Exhibit Description |
Form |
File No. |
Exhibit |
Filing Date |
Filed/ Furnished Herewith |
|
10.6(c)†† |
Form of Restricted Stock Grant Notice and Restricted Stock Agreement under 2013 Incentive Award Plan |
S-1/A |
333-187372 |
10.10(c) |
4/30/2013 |
|
|
10.7†† |
10-Q |
001-35952 |
10.2 |
8/5/2016 |
|
|
|
10.8 |
8-K |
001-35952 |
10.1 |
10/13/15 |
|
|
|
10.9 |
10-K |
001-35952 |
10.11 |
3/15/2016 |
|
|
|
10.10(a) |
8-K |
001-35952 |
10.2 |
10/16/2015 |
|
|
|
10.10(b) |
10-K |
001-35952 |
10.11(b) |
3/14/2017 |
|
|
|
10.10(c) |
10-Q |
001-35952 |
10.4 |
8/4/2017 |
|
|
|
10.11(a)† |
S-1/A |
333-187372 |
10.18 |
6/6/2013 |
|
|
|
10.11(b) |
S-1/A |
333-187372 |
10.19 |
4/11/2013 |
|
|
|
10.11(c) |
10-K |
001-35952 |
10.13(c) |
3/14/2017 |
|
|
|
10.12(a)† |
S-1/A |
333-187372 |
10.20 |
6/6/2013 |
|
|
|
10.12(b) |
S-1/A |
333-187372 |
10.21 |
4/11/2013 |
|
|
|
10.12(c) |
10-K |
001-35952 |
10.14(c) |
3/14/2017 |
|
|
|
10.13† |
S-1/A |
333-187372 |
10.24 |
4/11/2013 |
|
|
|
10.14† |
S-1/A |
333-187372 |
10.25 |
4/11/2013 |
|
|
71
|
|
|
Incorporated by Reference |
|
|||
|
Exhibit Number |
Exhibit Description |
Form |
File No. |
Exhibit |
Filing Date |
Filed/ Furnished Herewith |
|
10.15†† |
8-K |
001-35952 |
10.1 |
6/30/2016 |
|
|
|
10.16(a)† |
10-Q |
001-35952 |
10.3 |
8/5/2016 |
|
|
|
10.16(b)† |
10-Q |
001-35952 |
10.3 |
8/4/2017 |
|
|
|
10.17† |
10-Q |
001-35952 |
10.4 |
8/5/2016 |
|
|
|
10.18†† |
8-K |
001-35952 |
10.1 |
9/1/2016 |
|
|
|
10.19 |
8-K |
001-35952 |
10.1 |
5/4/2017 |
|
|
|
10.20 |
8-K |
001-35952 |
10.2 |
5/4/2017 |
|
|
|
10.21 |
8-K |
001-35952 |
10.1 |
12/18/2017 |
|
|
|
21.1 |
|
|
|
|
* |
|
|
23.1 |
Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm |
|
|
|
|
* |
|
31.1 |
Rule 13a-14(a) / 15d-14(a) Certification of Chief Executive Officer |
|
|
|
|
* |
|
31.2 |
Rule 13a-14(a) / 15d-14(a) Certification of Chief Financial Officer |
|
|
|
|
* |
|
32.1 |
|
|
|
|
** |
|
|
32.2 |
|
|
|
|
** |
|
|
101.INS |
XBRL Instance Document |
|
|
|
|
* |
|
101.SCH |
XBRL Taxonomy Extension Schema Document |
|
|
|
|
* |
|
101.CAL |
XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
* |
|
101.DEF |
XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
|
* |
|
101.LAB |
XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
|
* |
|
101.PRE |
XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
|
* |
†Portions of this exhibit (indicated by asterisks) have been omitted pursuant to a confidential treatment order granted by the Securities and Exchange Commission pursuant to Rule 24b-2 under the Securities Exchange Act of 1934.
††Management contract or compensatory plan or arrangement.
* Filed herewith.
** Furnished herewith.
None.
72
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
ARATANA THERAPEUTICS, INC. |
||
|
BY: |
/s/ Steven St. Peter |
|
|
Steven St. Peter, M.D. President and Chief Executive Officer |
||
Date: March 14, 2018
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
|
|
|
|
|
|
SIGNATURE |
|
TITLE |
|
DATE |
|
|
|
|
||
|
/s/ Steven St. Peter Steven St. Peter, M.D. |
|
President, Chief Executive Officer and Director (principal executive officer) |
|
March 14, 2018 |
|
|
|
|
||
|
/s/ Craig Tooman Craig Tooman |
|
Chief Financial Officer and Treasurer (principal financial and accounting officer) |
|
March 14, 2018 |
|
|
|
|
||
|
/s/ Wendy L. Yarno Wendy L. Yarno |
|
Chairperson of the Board of Directors |
|
March 14, 2018 |
|
|
|
|
|
|
|
/s/ Laura A. Brege Laura A. Brege |
|
Director |
|
March 14, 2018 |
|
|
|
|
|
|
|
/s/ David L. Brinkley David L. Brinkley |
|
Director |
|
March 14, 2018 |
|
|
|
|
|
|
|
/s/ Robert Gerber Robert “Rip” Gerber |
|
Director |
|
March 14, 2018 |
|
|
|
|
|
|
|
/s/ Irvine O. Hockaday Irvine “Irv” O. Hockaday, Esq. |
|
Director |
|
March 14, 2018 |
|
|
|
|
|
|
|
/s/ Merilee Raines Merilee Raines |
|
Director |
|
March 14, 2018 |
|
|
|
|
|
|
|
/s/ Robert P. Roche Robert P. Roche |
|
Director |
|
March 14, 2018 |
|
|
|
|
|
|
|
/s/ John Vander Vort John Vander Vort, Esq. |
|
Director |
|
March 14, 2018 |
|
|
|
|
||
73
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Aratana Therapeutics, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Aratana Therapeutics, Inc. and its subsidiaries as of December 31, 2017 and 2016, and the related consolidated statements of operations, of comprehensive loss, of changes in stockholders’ equity and of cash flows for each of the three years in the period ended December 31, 2017, including the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2017 in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Emphasis of Matter
As discussed in Note 1 to the consolidated financial statements, the Company will require additional financing to fund future operations and debt payments which may be triggered if the minimum liquidity covenant requirement is not met. Management’s plans in regard to this matter are described in Note 1.
/s/ PricewaterhouseCoopers LLP
Boston, Massachusetts
March 14, 2018
We have served as the Company's auditor since 2012.
F-2
Consolidated Balance Sheets
(Amounts in thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2017 |
|
December 31, 2016 |
||
|
Assets |
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
66,868 |
|
$ |
87,307 |
|
Short-term investments |
|
|
747 |
|
|
996 |
|
Accounts receivable, net |
|
|
2,406 |
|
|
87 |
|
Inventories |
|
|
13,576 |
|
|
11,130 |
|
Prepaid expenses and other current assets |
|
|
1,642 |
|
|
2,022 |
|
Total current assets |
|
|
85,239 |
|
|
101,542 |
|
Property and equipment, net |
|
|
1,166 |
|
|
1,948 |
|
Goodwill |
|
|
41,295 |
|
|
39,382 |
|
Intangible assets, net |
|
|
6,616 |
|
|
7,639 |
|
Restricted cash |
|
|
350 |
|
|
350 |
|
Other long-term assets |
|
|
526 |
|
|
545 |
|
Total assets |
|
$ |
135,192 |
|
$ |
151,406 |
|
Liabilities and Stockholders’ Equity |
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
Accounts payable |
|
$ |
7,451 |
|
$ |
7,436 |
|
Accrued expenses |
|
|
3,712 |
|
|
5,827 |
|
Licensing and collaboration commitment |
|
|
7,000 |
|
|
7,000 |
|
Current portion – loans payable |
|
|
17,333 |
|
|
14,413 |
|
Other current liabilities |
|
|
— |
|
|
12 |
|
Total current liabilities |
|
|
35,496 |
|
|
34,688 |
|
Loans payable, net |
|
|
19,492 |
|
|
25,775 |
|
Other long-term liabilities |
|
|
70 |
|
|
540 |
|
Total liabilities |
|
|
55,058 |
|
|
61,003 |
|
Commitments and contingencies (Notes 5 and 16) |
|
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
|
Common stock, $0.001 par value; 100,000,000 shares authorized at December 31, 2017 and December 31, 2016, 42,532,725 and 36,607,922 shares issued and outstanding at December 31, 2017 and December 31, 2016, respectively |
|
|
43 |
|
|
37 |
|
Treasury stock, at cost; 80,916 and 78,226 shares at December 31, 2017 and December 31, 2016, respectively |
|
|
(1,107) |
|
|
(1,088) |
|
Additional paid-in capital |
|
|
321,599 |
|
|
286,909 |
|
Accumulated deficit |
|
|
(233,316) |
|
|
(185,593) |
|
Accumulated other comprehensive loss |
|
|
(7,085) |
|
|
(9,862) |
|
Total stockholders’ equity |
|
|
80,134 |
|
|
90,403 |
|
Total liabilities and stockholders’ equity |
|
$ |
135,192 |
|
$ |
151,406 |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Consolidated Statements of Operations
(Amounts in thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
Year Ended December 31, |
|||||||
|
|
2017 |
|
2016 |
|
2015 |
|||
|
Revenues |
|
|
|
|
|
|
|
|
|
Licensing and collaboration revenue |
$ |
5,913 |
|
$ |
38,233 |
|
$ |
— |
|
Product sales |
|
19,660 |
|
|
318 |
|
|
678 |
|
Total revenues |
|
25,573 |
|
|
38,551 |
|
|
678 |
|
Costs and expenses |
|
|
|
|
|
|
|
|
|
Cost of product sales |
|
16,387 |
|
|
3,139 |
|
|
365 |
|
Royalty expense |
|
1,821 |
|
|
106 |
|
|
84 |
|
Research and development |
|
15,126 |
|
|
30,462 |
|
|
24,964 |
|
Selling, general and administrative |
|
28,897 |
|
|
27,342 |
|
|
19,819 |
|
Amortization of intangible assets |
|
350 |
|
|
379 |
|
|
1,544 |
|
Impairment of intangible assets |
|
7,448 |
|
|
7,942 |
|
|
43,398 |
|
Total costs and expenses |
|
70,029 |
|
|
69,370 |
|
|
90,174 |
|
Loss from operations |
|
(44,456) |
|
|
(30,819) |
|
|
(89,496) |
|
Other income (expense) |
|
|
|
|
|
|
|
|
|
Interest income |
|
449 |
|
|
385 |
|
|
189 |
|
Interest expense |
|
(3,481) |
|
|
(3,396) |
|
|
(1,585) |
|
Other income (expense), net |
|
(22) |
|
|
255 |
|
|
5,140 |
|
Total other income (expense) |
|
(3,054) |
|
|
(2,756) |
|
|
3,744 |
|
Loss before income taxes |
$ |
(47,510) |
|
$ |
(33,575) |
|
$ |
(85,752) |
|
Income tax benefit |
|
— |
|
|
— |
|
|
1,698 |
|
Net loss |
$ |
(47,510) |
|
$ |
(33,575) |
|
$ |
(84,054) |
|
Net loss per share, basic and diluted |
$ |
(1.17) |
|
$ |
(0.95) |
|
$ |
(2.45) |
|
Weighted average shares outstanding, basic and diluted |
|
40,494,301 |
|
|
35,273,228 |
|
|
34,355,525 |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Consolidated Statements of Comprehensive Loss
(Amounts in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year December 31, |
|||||||
|
|
|
2017 |
|
2016 |
|
2015 |
|||
|
Net loss |
|
$ |
(47,510) |
|
$ |
(33,575) |
|
$ |
(84,054) |
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income (loss): |
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustment |
|
|
2,777 |
|
|
(542) |
|
|
(3,918) |
|
Unrealized gain on available-for-sale securities |
|
|
— |
|
|
— |
|
|
2,622 |
|
Net gain reclassified into income on sale of available-for-sale securities |
|
|
— |
|
|
— |
|
|
(3,874) |
|
Other comprehensive income (loss) |
|
|
2,777 |
|
|
(542) |
|
|
(5,170) |
|
Comprehensive loss |
|
$ |
(44,733) |
|
$ |
(34,117) |
|
$ |
(89,224) |
The accompanying notes are an integral part of these consolidated financial statements
F-5
Consolidated Statements of Changes in Stockholders’ Equity
(Amounts in thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
Treasury |
|
|
|||
|
|
|
|
|
|
|
|
Additional |
|
|
|
Other |
|
Stock |
|
Total |
|||||
|
|
|
Common Stock |
|
Paid-In |
|
Accumulated |
|
Comprehensive |
|
at |
|
Stockholders' |
||||||||
|
|
|
Shares |
|
Par Value |
|
Capital |
|
Deficit |
|
Income (Loss) |
|
Cost |
|
Equity |
||||||
|
Balance at December 31, 2014 |
|
34,147,861 |
|
$ |
34 |
|
$ |
254,993 |
|
$ |
(67,964) |
|
$ |
(4,150) |
|
$ |
(1,081) |
|
$ |
181,832 |
|
Compensation expense related to stock options and restricted awards |
|
— |
|
|
— |
|
|
8,592 |
|
|
— |
|
|
— |
|
|
— |
|
|
8,592 |
|
Vesting of restricted stock awards |
|
205,387 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Repurchase of common stock |
|
(859) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(7) |
|
|
(7) |
|
Vesting of stock awards early exercised |
|
121,014 |
|
|
1 |
|
|
44 |
|
|
— |
|
|
— |
|
|
— |
|
|
45 |
|
Issuance of common stock related to option exercises |
|
90,413 |
|
|
— |
|
|
312 |
|
|
— |
|
|
— |
|
|
— |
|
|
312 |
|
Other comprehensive loss |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(5,170) |
|
|
— |
|
|
(5,170) |
|
Net loss |
|
— |
|
|
— |
|
|
— |
|
|
(84,054) |
|
|
— |
|
|
— |
|
|
(84,054) |
|
Balance at December 31, 2015 |
|
34,563,816 |
|
$ |
35 |
|
$ |
263,941 |
|
$ |
(152,018) |
|
$ |
(9,320) |
|
$ |
(1,088) |
|
$ |
101,550 |
|
At-the-Market issuance of common stock, net of $262 of issuance costs |
|
1,629,408 |
|
|
2 |
|
|
14,323 |
|
|
— |
|
|
— |
|
|
— |
|
|
14,325 |
|
Compensation expense related to stock options and restricted awards |
|
— |
|
|
— |
|
|
8,476 |
|
|
— |
|
|
— |
|
|
— |
|
|
8,476 |
|
Vesting of restricted stock awards |
|
301,559 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Vesting of stock awards early exercised |
|
71,021 |
|
|
— |
|
|
31 |
|
|
— |
|
|
— |
|
|
— |
|
|
31 |
|
Issuance of common stock related to option exercises |
|
42,118 |
|
|
— |
|
|
138 |
|
|
— |
|
|
— |
|
|
— |
|
|
138 |
|
Other comprehensive loss |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(542) |
|
|
— |
|
|
(542) |
|
Net loss |
|
— |
|
|
— |
|
|
— |
|
|
(33,575) |
|
|
— |
|
|
— |
|
|
(33,575) |
|
Balance at December 31, 2016 |
|
36,607,922 |
|
$ |
37 |
|
$ |
286,909 |
|
$ |
(185,593) |
|
$ |
(9,862) |
|
$ |
(1,088) |
|
$ |
90,403 |
|
At-the-Market issuance of common stock, net of $73 of issuance costs |
|
546,926 |
|
|
1 |
|
|
2,714 |
|
|
— |
|
|
— |
|
|
— |
|
|
2,715 |
|
Registered direct offering of common stock, net of $273 of issuance costs |
|
5,000,000 |
|
|
5 |
|
|
24,398 |
|
|
— |
|
|
— |
|
|
— |
|
|
24,403 |
|
Compensation expense related to stock options and restricted awards |
|
— |
|
|
— |
|
|
7,331 |
|
|
(213) |
|
|
— |
|
|
— |
|
|
7,118 |
|
Vesting of restricted stock awards |
|
293,978 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Repurchase of common stock |
|
(2,690) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(19) |
|
|
(19) |
|
Vesting of stock awards early exercised |
|
438 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Issuance of common stock related to option exercises |
|
86,151 |
|
|
— |
|
|
247 |
|
|
— |
|
|
— |
|
|
— |
|
|
247 |
|
Other comprehensive income |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
2,777 |
|
|
— |
|
|
2,777 |
|
Net loss |
|
— |
|
|
— |
|
|
— |
|
|
(47,510) |
|
|
— |
|
|
— |
|
|
(47,510) |
|
Balance at December 31, 2017 |
|
42,532,725 |
|
$ |
43 |
|
$ |
321,599 |
|
$ |
(233,316) |
|
$ |
(7,085) |
|
$ |
(1,107) |
|
$ |
80,134 |
The accompanying notes are an integral part of these consolidated financial statements
F-6
Consolidated Statements of Cash Flows
(Amounts in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|||||||
|
|
2017 |
|
2016 |
|
2015 |
|||
|
Cash flows from operating activities |
|
|
|
|
|
|
|
|
|
Net loss |
$ |
(47,510) |
|
$ |
(33,575) |
|
$ |
(84,054) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
Stock-based compensation expense |
|
7,118 |
|
|
8,476 |
|
|
8,592 |
|
Depreciation and amortization expense |
|
1,162 |
|
|
991 |
|
|
1,840 |
|
Impairment of intangible assets |
|
7,448 |
|
|
7,942 |
|
|
43,398 |
|
Gain on sale of marketable securities |
|
— |
|
|
— |
|
|
(3,874) |
|
Gain on deconsolidation of a variable interest entity |
|
— |
|
|
(276) |
|
|
— |
|
Non-cash interest expense |
|
512 |
|
|
478 |
|
|
129 |
|
Creditor fees |
|
— |
|
|
— |
|
|
(150) |
|
Market value adjustments to inventories |
|
741 |
|
|
5,186 |
|
|
— |
|
Change in fair value of contingent consideration |
|
— |
|
|
— |
|
|
(1,248) |
|
Change in fair value of derivative instruments |
|
— |
|
|
— |
|
|
(1,274) |
|
(Gain) loss on disposition of property and equipment |
|
(30) |
|
|
2 |
|
|
— |
|
Deferred tax benefit |
|
— |
|
|
— |
|
|
(1,698) |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
Accounts receivable, net |
|
(2,319) |
|
|
(27) |
|
|
281 |
|
Inventories |
|
(3,187) |
|
|
(15,010) |
|
|
(879) |
|
Prepaid expenses and other current assets |
|
335 |
|
|
(771) |
|
|
(595) |
|
Other assets |
|
88 |
|
|
(5) |
|
|
(31) |
|
Accounts payable |
|
62 |
|
|
6,182 |
|
|
(117) |
|
Accrued expenses and other liabilities |
|
(2,605) |
|
|
2,084 |
|
|
1,185 |
|
Licensing and collaboration commitment |
|
— |
|
|
7,000 |
|
|
— |
|
Net cash used in operating activities |
|
(38,185) |
|
|
(11,323) |
|
|
(38,495) |
|
Cash flows from investing activities |
|
|
|
|
|
|
|
|
|
Milestone payments for intangible assets |
|
(6,000) |
|
|
(1,000) |
|
|
— |
|
Purchases of property and equipment, net |
|
(1) |
|
|
(72) |
|
|
(2,245) |
|
Proceeds from sales of marketable securities |
|
— |
|
|
— |
|
|
7,456 |
|
Purchases of investments |
|
(3,731) |
|
|
(229,836) |
|
|
(2,050,594) |
|
Proceeds from maturities of investments |
|
3,980 |
|
|
288,287 |
|
|
2,079,396 |
|
Cash contributed as investment in a noncontrolled entity |
|
— |
|
|
(94) |
|
|
— |
|
Net cash provided by (used in) investing activities |
|
(5,752) |
|
|
57,285 |
|
|
34,013 |
|
Cash flows from financing activities |
|
|
|
|
|
|
|
|
|
Proceeds from issuance of loans payable, net of discount |
|
— |
|
|
— |
|
|
24,779 |
|
Payments on loans payable |
|
(3,500) |
|
|
— |
|
|
— |
|
Taxes paid for awards vested under equity incentive plans |
|
(19) |
|
|
— |
|
|
(7) |
|
Proceeds from stock option exercises |
|
247 |
|
|
138 |
|
|
312 |
|
Proceeds from issuance of common stock, net of commission |
|
27,463 |
|
|
14,587 |
|
|
— |
|
Payments for common stock issuance costs |
|
(345) |
|
|
(93) |
|
|
(189) |
|
Payments for debt issuance costs |
|
(210) |
|
|
— |
|
|
— |
|
Payment for revolving credit facility termination fee |
|
(165) |
|
|
— |
|
|
— |
|
Cash paid for contingent consideration |
|
— |
|
|
— |
|
|
(3,000) |
|
Net cash provided by financing activities |
|
23,471 |
|
|
14,632 |
|
|
21,895 |
|
Effect of exchange rate on cash |
|
27 |
|
|
(42) |
|
|
(131) |
|
Net increase (decrease) in cash, cash equivalents and restricted cash |
|
(20,439) |
|
|
60,552 |
|
|
17,282 |
|
Cash, cash equivalents and restricted cash, beginning of period |
|
87,657 |
|
|
27,105 |
|
|
9,823 |
|
Cash, cash equivalents and restricted cash, end of period |
$ |
67,218 |
|
$ |
87,657 |
|
$ |
27,105 |
|
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
|
|
|
Cash paid for interest, net of amounts capitalized |
$ |
2,966 |
|
$ |
2,911 |
|
$ |
1,057 |
|
Supplemental disclosure of noncash investing and financing activities: |
|
|
|
|
|
|
|
|
|
Stock issuance costs included in accounts payable |
$ |
48 |
|
$ |
— |
|
$ |
— |
|
Non-cash exercise of warrant |
$ |
— |
|
$ |
— |
|
$ |
750 |
The accompanying notes are an integral part of these consolidated financial statements.
F-7
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
1. The Company and Basis of Presentation
The Company
Aratana Therapeutics, Inc., including its subsidiaries (the “Company,” or “Aratana”) was incorporated on December 1, 2010 under the laws of the State of Delaware. The Company is a pet therapeutics company focused on licensing, developing and commercializing of innovative therapeutics for dogs and cats. The Company has one operating segment: pet therapeutics.
Since its inception, the Company has devoted substantially all of its efforts to research and development, recruiting management and technical staff, building a commercial infrastructure, acquiring operating assets and raising capital.
The Company is subject to risks common to companies in the biotechnology and pharmaceutical industries. There can be no assurance that the Company’s licensing efforts will identify viable therapeutic candidates, that the Company’s research and development will be successfully completed, that adequate protection for the Company’s technology will be obtained, that any therapeutics developed will obtain necessary government regulatory approval or that any approved therapeutics will be commercially viable. The Company operates in an environment of substantial competition from other animal health companies. In addition, the Company is dependent upon the services of its employees and consultants, as well as third-party contract research organizations and manufacturers and collaborators.
Basis of Presentation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”) and on a basis which assumes that the Company will continue as a going concern and which contemplates the realization of assets and satisfaction of liabilities and commitments in the normal course of business.
The Company has incurred recurring losses and negative cash flows from operations and has an accumulated deficit of $233,316 as of December 31, 2017. The Company expects to continue to generate operating losses for the foreseeable future. The Company believes that its cash, cash equivalents and short-term investments on hand at December 31, 2017, together with the proceeds of the January 2018 at-the-market offering (Note 13), will be sufficient to fund operations and debt obligations at least through March 31, 2019. As disclosed in Note 10 to the consolidated financial statements, the Company has a term loan and a revolving credit facility with an aggregate principal balance of $36,500 as of December 31, 2017. The loan agreement requires that the Company maintain certain minimum liquidity at all times, which as of December 31, 2017, was approximately $18,250. If the minimum liquidity covenant is not met, the Company may be required to repay the loans prior to scheduled maturity dates.
The Company expects an increase in its investment related to commercial activities, including procuring of inventories needed to supply the marketplace, commercial investment to further support adoption and awareness of the Company’ marketed products and milestones related to approval and commencement of commercial sales. This will impact the minimum liquidity that needs to be maintained under the loan agreement. As a result, the Company will need additional capital to fund its operations and debt obligations beyond March 31, 2019, which the Company may obtain from corporate collaborations and licensing arrangements, or other sources, such as public or private equity and further debt (re)financings. The future viability of the Company beyond March 31, 2019, is dependent on its ability to raise additional capital to finance its operations, to fund on-going research and development costs, commercialization of its therapeutics and therapeutic candidates and to satisfy debt covenants. If the Company is not able to raise additional capital on terms acceptable to it, or at all, as and when needed, the Company would be forced to delay, reduce, or eliminate certain research and development programs, reduce or eliminate discretionary operating expenses or grant rights to develop and market therapeutics or therapeutic candidates that it would otherwise prefer to develop and market itself, which could otherwise adversely affect its business prospects. The Company’s failure to raise capital, as and when needed, would have a negative impact on its financial condition and its ability to pursue its business strategies as this capital is necessary for it to perform the research and development and commercial activities required to generate future revenue streams.
2. Summary of Significant Accounting Policies
Consolidation
The Company’s consolidated financial statements include its financial statements, and those of its wholly-owned subsidiaries and in prior periods, a consolidated variable interest entity through the deconsolidation date in December 2016 discussed further below. Intercompany balances and transactions are eliminated in consolidation.
To determine if the Company holds a controlling financial interest in an entity, the Company first evaluates if it is required to apply the variable interest entity (“VIE”) model to the entity. Where the Company holds current or potential rights that give it the power to direct the activities of a VIE that most significantly impact the VIE’s economic performance combined with a variable interest that gives it the right to receive potentially significant benefits or the obligation to absorb potentially significant losses, the Company is the primary beneficiary of that VIE. When changes occur to the design of an entity, the Company reconsiders whether it is subject to the VIE model. The Company continuously evaluates whether it is the primary beneficiary of a consolidated VIE and upon determination
F-8
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
that the Company no longer remains the primary beneficiary, the Company deconsolidates the entity and a gain or loss is recognized upon deconsolidation.
In December 2016, the Company concluded that it was no longer the primary beneficiary of a previously consolidated VIE and no longer consolidates the entity. The Company recognized a gain of $276 on deconsolidation of the VIE in other income (expense) in the quarter ended December 31, 2016. The Company’s remaining non-controlling investment in the VIE is not material and is accounted for using the cost method subsequent to deconsolidation as the Company’s remaining ownership interest is less than 20% and the Company has no board seat or other means to exert significant influence on the VIE.
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates, judgments and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Estimates are periodically reviewed in light of changes in circumstances, facts and experience. Actual results could differ from those estimates.
Cash and Cash Equivalents
The Company classifies all highly liquid investments with stated maturities of three months or less from the date of purchase as cash equivalents. Cash equivalents consisted of certificates of deposit (“CDs”) at December 31, 2017 and 2016.
Restricted Cash
Pursuant to the terms of the Loan and Security Agreement, the Company has posted collateral to Square 1 Bank N.A., a division of Pacific Western Bank, to collateralize corporate credit card services. The Company classifies the collateral as restricted cash.
Short-term Investments
The Company classifies reverse repurchase agreements other than overnight reverse repurchase agreements as short-term investments and as available-for-sale. Short-term investments in 2017 included CDs with original maturities greater than three months but less than 12 months. Short-term investments in 2016 included reverse repurchase agreements.
Marketable Securities
The Company classifies all highly liquid investments with stated maturities of greater than three months from the date of purchase as marketable securities. The Company determines the appropriate classification of investments in marketable securities at the time of purchase and re-evaluates such designation at each consolidated balance sheet date. The Company classifies and accounts for marketable securities as available-for-sale. The Company did not hold securities with stated maturities greater than 12 months as of December 31, 2017 or 2016. The Company reports available-for-sale investments at fair value as of each consolidated balance sheet date and records any unrealized gains and losses as a component of stockholders’ equity. The cost of securities sold is determined on a specific identification basis, and realized gains and losses are included in other income (expense) in the consolidated statements of operations. If any adjustment to fair value reflects a decline in the value of the investment, the Company considers available evidence to evaluate the extent to which the decline is “other than temporary” and recognizes the impairment by releasing other comprehensive income to the consolidated statement of operations. There were no such adjustments necessary during the years ended December 31, 2017 and 2016.
Accounts Receivable, net
Accounts receivable are uncollateralized customer obligations due under normal trade terms generally requiring payment within 30 days of the invoice date.
The Company provides an allowance for doubtful accounts equal to the estimated losses that will be incurred in collection of accounts receivable. This estimate is based on the current review of existing receivables and historical experience in the industry. The allowance and associated accounts receivable are reduced when the receivables are determined to be uncollectible. To date, the Company’s historical reserves and write-offs have not been significant. The Company also provides an allowance for estimated returns which is established based on the Company’s analysis of industry standards and its own history of actual returns.
Inventories
The Company states inventories at the lower of cost and net realizable value and consist of raw materials, work-in-process and finished goods. Cost is determined by the average cost method for raw materials and standard cost for work-in-process and finished goods, which approximates actual cost.
F-9
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
Pre-Launch Inventories
The Company may scale-up and make commercial quantities of certain of its product candidates prior to the date it anticipates that such products will receive final United States Food and Drug Administration (“FDA”)/United States Department of Agriculture (“USDA”) approval. The scale-up and commercial production of pre-launch inventories involves the risk that such products may not be approved for marketing by the FDA/USDA on a timely basis, or ever. Inventory costs associated with product candidates that have not yet received regulatory approval are capitalized if the Company believes there is probable future commercial use and future economic benefit. If the probability of future commercial use and future economic benefit cannot be reasonably determined, then pre-launch inventory costs associated with such product candidates are expensed as research and development expense during the period the costs are incurred. Specifically, the Company has determined that for FDA-regulated product candidates there is a probable future commercial use and future economic benefit upon the receipt of the three major technical section complete letters from the FDA’s Center for Veterinary Medicine (“CVM”). For USDA product candidates, the Company has determined there is a probable future commercial use and future economic benefit upon the receipt of a conditional license from the USDA’s Center for Veterinary Biologics. The Company makes at least quarterly reassessments of the probability of regulatory approval and useful life of the pre-launch inventory, and determines whether such inventory continues to have a probable future economic benefit.
Property and Equipment, net
The Company records property and equipment at historical cost less accumulated depreciation and amortization. Depreciation and amortization expense is recognized using the straight-line method over the following estimated useful lives:
|
|
||
|
Laboratory and office equipment |
3–10 years |
|
|
Computer software and equipment |
3–5 years |
|
|
Furniture |
3–7 years |
|
|
Vehicles |
3–5 years |
|
|
Leasehold improvements |
3–10 years |
Leasehold improvements are amortized over the shorter of the life of the related asset or the term of the lease.
Expenditures for repairs and maintenance of assets are charged to expense as incurred. Costs of major additions and betterments are capitalized and depreciated on a straight-line basis over their useful lives. When property and equipment are disposed of, the cost and respective accumulated depreciation and amortization are removed from the accounts. Any gain or loss on disposal is recorded in the consolidated statements of operations in other income (expense). Depreciation expense and gains or losses on disposal of property and equipment are classified within the corresponding operating expense categories in the consolidated statements of operations.
Goodwill
Goodwill relates to amounts that arose in connection with the Company’s business combinations and represents the difference between the purchase price and the estimated fair value of the identifiable tangible and intangible net assets when accounted for using the acquisition method of accounting. Goodwill is not amortized, but is subject to periodic review for impairment.
The Company tests goodwill at the reporting unit level for impairment on an annual basis and between annual tests, if events and circumstances indicate impairment may exist. Events that would indicate impairment and trigger an interim impairment assessment include, but are not limited to, current economic and market conditions, including a decline in market capitalization, a significant adverse change in legal factors, business climate or operational performance of the business and an adverse action or assessment by a regulator.
Intangible Assets, net
The Company’s intangible assets consist of intellectual property rights acquired for currently marketed products (amortized intangibles) and intellectual property rights acquired for in-process research and development (“IPR&D”) (unamortized intangibles). All of the Company’s IPR&D intangible assets were recorded in connection with the Company’s business combinations. All of the Company’s amortized intangibles were recorded in connection with the Company’s business combinations or approval/post-approval milestone payments made under the Company’s license agreements. The Company’s intangible assets are recorded at fair value at the time of their acquisition. The Company amortizes intangible assets over their estimated useful lives once the acquired technology is developed into a commercially viable product. The estimated useful lives of the individual categories of intangible assets are based on the nature of the applicable intangible asset and the expected future cash flows to be derived from the intangible asset. Amortization of intangible assets with finite lives is recognized over the time the intangible assets are estimated to contribute to future cash flows. The Company amortizes finite-lived intangible assets using the straight-line method as revenues cannot be reasonably estimated.
F-10
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
Indefinite-lived IPR&D intangible assets are assessed for impairment at least annually. In addition, all intangible assets are reviewed for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. Factors the Company considers in deciding when to perform an impairment review include significant underperformance of the business in relation to expectations, significant negative industry or economic trends, and significant changes or planned changes in the use of the assets. If an impairment review is performed to evaluate a long-lived asset for recoverability, the Company compares forecasts of undiscounted cash flows for definite-lived intangible assets and discounted cash flows for indefinite-lived IPR&D intangible assets expected to result from the use and eventual disposition of the long-lived asset to its carrying value. An impairment loss would be recognized when estimated undiscounted (definite-lived) or discounted (indefinite-lived) future cash flows expected to result from the use of an asset are less than its carrying amount. The impairment loss would be based on the excess of the carrying value of the impaired asset over its fair value, determined based on discounted cash flows.
Derivative Financial Instruments
The Company accounts for its derivative instruments as either assets or liabilities and carries them at fair value. The Company’s sole derivative (Note 9) was a warrant to purchase common stock and was adjusted to fair value through current income as it was not designated as a hedging instrument. In 2015, the Company exercised the warrant and subsequently sold the shares of common stock received upon exercise.
Foreign Currency
With the acquisition of Okapi Sciences in 2014, the Company is exposed to effects of foreign currency from translation. Transactions in foreign currencies are translated into the relevant functional currency at the rate of exchange at the date of the transaction. Transaction gains and losses are recognized in other income (expense) in the consolidated statements of operations. The results of operations for subsidiaries, whose functional currency is not the United States Dollar, are translated into the United States Dollar at the average rates of exchange during the period, with the subsidiaries’ balance sheets translated at the rates accumulated at the balance sheet date. The cumulative effect of these exchange rate adjustments is included in a separate component of other comprehensive income (loss) in the consolidated balance sheets. Gains and losses arising from intercompany foreign currency transactions are included in loss from operations unless the gains and losses arise from long-term investments in subsidiaries. Gains and losses from long-term investments in subsidiaries are included in a separate component of other comprehensive income (loss).
Deferred Public Offering and At-the-Market Offering Costs
The Company capitalizes certain legal, accounting and other third-party fees that are directly associated with in-process equity financings as other assets until such financings are consummated. After consummation of the equity financing, these costs are recorded in stockholders’ equity as a reduction of additional paid-in capital generated as a result of the offering. Should it no longer be considered probable that the equity financing will be consummated, the deferred offering costs would be expensed immediately as a charge to operating expenses in the consolidated statements of operations. The Company recorded $76 and $20 of deferred equity offering costs as of December 31, 2017 and 2016, respectively.
Income Taxes
The Company accounts for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the consolidated financial statements or in the Company’s tax returns. Deferred taxes are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect in the years in which the differences are expected to reverse. Changes in deferred tax assets and liabilities are recorded in the provision for income taxes. The Company assesses the likelihood that its deferred tax assets will be recovered from future taxable income and, to the extent it believes, based upon the weight of available evidence, that it is more likely than not that all or a portion of deferred tax assets will not be realized, a valuation allowance is established through a charge to income tax expense. Potential for recovery of deferred tax assets is evaluated by estimating the future taxable profits expected and considering prudent and feasible tax planning strategies.
The Company accounts for uncertainty in income taxes recognized in the consolidated financial statements by applying a two-step process to determine the amount of tax benefit to be recognized. First, the tax position must be evaluated to determine the likelihood that it will be sustained upon external examination by the taxing authorities. If the tax position is deemed more-likely-than-not to be sustained, the tax position is then assessed to determine the amount of benefit to recognize in the consolidated financial statements. The amount of the benefit that may be recognized is the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement. The provision for income taxes includes the effects of any resulting tax reserves, or unrecognized tax benefits, that are considered appropriate as well as the related net interest and penalties.
Debt Issuance Costs, net
Debt issuance costs, net represent legal and other direct costs related to the Company’s Loan and Security Agreement (Note 10). These costs are recorded as an offset to the carrying value of loans payable in the consolidated balance sheet at the time they are incurred and are amortized to interest expense through the scheduled final principal payment date. During the year ended
F-11
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
December 31, 2017 and 2015, the Company capitalized $210 and $360, respectively, of debt issuance costs in conjunction with the refinancings of debt. As of December 31, 2017 and 2016, deferred debt issuance costs totaled $330 and $259, respectively.
Revenue Recognition
The Company recognizes revenue when all of the following conditions are met:
|
· |
persuasive evidence of an arrangement exists; |
|
· |
delivery has occurred or services have been rendered; |
|
· |
the seller’s price to the buyer is fixed or determinable; and |
|
· |
collectibility is reasonably assured. |
The Company’s principal revenue streams and their respective accounting treatments are discussed below:
(i) Product sales - Revenue for the sale of products is recognized when delivery has occurred and substantially all the risks and rewards of ownership have been transferred to the customer. Revenue for the sale of products is recorded net of sales returns, allowances and discounts.
(ii) Royalty revenue - Royalty revenue relating to the Company’s out-licensed technology is recognized when reasonably estimable. The revenues are recorded based on the licensee’s sales that occurred during the relevant period. Differences between actual and estimated royalty revenues are adjusted in the period in which they become known, typically in the following quarter. If the Company is unable to reasonably estimate royalty revenue or does not have access to the information, then the Company records royalty revenue when the information needed for a reliable estimate becomes available. Royalty revenue is included in licensing and collaboration revenue in the consolidated statements of operations.
(iii) Licensing and collaboration revenues - Revenues derived from product out-licensing arrangements typically consist of an initial up-front payment at inception of the license and subsequent milestone payments contingent on the achievement of certain regulatory, development and commercial milestones.
Product out-licensing arrangements with multiple elements are divided into separate units of accounting if certain criteria are met. The up-front payment received is allocated among the separate units of accounting based on their respective fair values, and the applicable revenue recognition criteria are applied to each of the separate units of accounting. The application of the multiple element guidance requires subjective determinations, and requires the Company to make judgments about the individual deliverables and whether such deliverables are separable from the other aspects of the contractual relationship. Deliverables are considered separate units of accounting provided that:
(1) the delivered item(s) has value to the customer on a stand-alone basis and
(2) if the arrangement includes a general right of return relative to the delivered item(s), delivery or performance of the undelivered item(s) is considered probable and substantially in the Company's control.
In determining the units of accounting, the Company evaluates certain criteria, including whether the deliverables have stand-alone value, based on the consideration of the relevant facts and circumstances for each arrangement. In addition, the Company considers whether the buyer can use the other deliverable(s) for their intended purpose without the receipt of the remaining element(s), whether the value of the deliverable is dependent on the undelivered item(s), and whether there are other vendors that can provide the undelivered element(s).
Arrangement consideration that is fixed or determinable is allocated among the separate units of accounting using the relative selling price method, and the applicable revenue recognition criteria. The Company determines the estimated selling price for deliverables within each agreement using vendor-specific objective evidence (“VSOE”) of selling price, if available, third-party evidence (“TPE”) of selling price if VSOE is not available, or management's best estimate of the selling price (“BESP”) if neither VSOE nor TPE is available. Determining the BESP for a unit of accounting requires significant judgment. In developing the BESP for a unit of accounting, the Company considers applicable market conditions and relevant entity-specific factors, including factors that were contemplated in negotiating the agreement with the customer and estimated costs.
If there are deliverables in an arrangement that are not separable from other aspects of the contractual relationship, they are treated as a combined unit of accounting, with the allocated revenue for the combined unit recognized in a manner consistent with the revenue recognition applicable to the final deliverable in the combined unit.
Amounts received prior to satisfying all relevant revenue recognition criteria are recorded as deferred revenue in the consolidated balance sheets and recognized as revenue when the related revenue recognition criteria are met. Amounts not expected to be recognized as revenue within the next twelve months of the consolidated balance sheet date are classified as long-term deferred revenue.
F-12
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
The Company recognizes revenue contingent upon the achievement of a milestone in its entirety in the period in which the milestone is achieved only if the milestone meets all the criteria to be considered substantive. At the inception of each arrangement that includes milestone payments, the Company evaluates each contingent payment on an individual basis to determine whether they are considered substantive milestones, specifically reviewing factors such as the degree of certainty in achieving the milestone, the research and development risk and other risks that must be overcome to achieve the milestone, as well as the level of effort and investment required and whether the milestone consideration is reasonable relative to all deliverables and payment terms in the arrangement. This evaluation includes an assessment of whether (a) the consideration is commensurate with either (1) the entity’s performance to achieve the milestone, or (2) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the entity’s performance to achieve the milestone, (b) the consideration relates solely to past performance and (c) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement.
Milestone payments which are non-refundable and deemed substantive, non-creditable and contingent on achieving certain development, regulatory, or commercial milestones are typically recognized as revenues either on achievement of such milestones or over the period the Company has continuing substantive performance obligations. The Company recognizes revenue associated with the non-substantive milestones upon achievement of the milestone if there are no undelivered elements and the Company has no remaining performance obligations. Revenues from commercial milestone payments are recorded as revenue upon achievement of the milestone, assuming all other revenue recognition criteria are met.
In the event that an agreement is terminated and the Company then has no further performance obligations, the Company recognizes as revenue any amounts that had not previously been recorded as revenue but were classified as deferred revenue at the date of such termination.
Cash consideration (including a sales incentive) given by the Company to a licensee/collaborator/customer is presumed to be a reduction of the selling prices of the Company’s products or services and is recognized as a reduction of revenue unless both of the following conditions are met:
a. The Company receives, or will receive, an identifiable benefit (goods or services) in exchange for the consideration. In order to meet this condition, the identified benefit must be sufficiently separable from the recipient’s purchase of the Company’s products such that the Company could have entered into an exchange transaction with a party other than a purchaser of its products or services in order to receive that benefit.
b. The Company can reasonably estimate the fair value of the benefit identified under the preceding condition. If the amount of consideration paid by the Company exceeds the estimated fair value of the benefit received, that excess amount shall be characterized as a reduction of revenue when recognized in the Company’s statements of operations.
If both conditions are met, the cash consideration is recognized as a cost incurred.
Research and Development Costs
Research and development costs are expensed as incurred. Included in research and development costs are wages, stock-based compensation and employee benefits, and other operational costs related to the Company’s research and development activities, including facility-related expenses, external costs of outside contractors engaged to conduct both preclinical and clinical studies and allocation of corporate costs. If IPR&D is acquired in an asset purchase, then the acquired IPR&D is expensed on its acquisition date. Future costs to develop these assets are recorded to research and development expense as they are incurred.
Patent Costs
All patent-related costs incurred in connection with filing and prosecuting patent applications are recorded as selling, general and administrative expenses as incurred, as recoverability of such expenditures is uncertain.
Shipping
Shipping costs are included in cost of product sales.
Sales Tax
The Company collects and remits taxes assessed by various governmental authorities. These taxes may include sales, use and value added taxes. These taxes are recorded on a net basis and are excluded from sales.
Accounting for Stock-Based Compensation
The Company’s stock-based compensation program grants awards that may consist of stock options and restricted stock awards. The fair values of stock option grants are determined as of the date of grant using the Black-Scholes option pricing method. This method incorporates the fair value of the Company’s common stock at the date of each grant and various assumptions such as the risk-free interest rate, expected volatility based on the volatility of the Company’s common stock price, expected dividend yield, and expected term of the options. The fair values of restricted stock awards are determined based on the fair value of the Company’s common stock.
F-13
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
The fair values of the stock-based awards are then expensed over the requisite service period, which is generally the award’s vesting period. The Company classifies stock-based compensation expense in the consolidated statements of operations in the same manner in which the respective award recipient’s payroll costs are classified.
For stock-based awards granted to consultants and nonemployees, compensation expense is recognized over the period during which services are rendered by such consultants and nonemployees until completed. At the end of each financial reporting period prior to completion of the service, the value of these awards is re-measured using the then-current fair value of the Company’s common stock and updated assumption inputs in the Black-Scholes option pricing model.
Comprehensive Loss
In addition to the Company’s net loss, comprehensive loss during the years ended December 31, 2017, 2016 and 2015, includes foreign currency translation adjustments related to the translation of foreign subsidiaries’ balance sheets and unrealized holding gains and losses on available-for-sale securities.
Net Loss Per Share
The Company follows the two-class method when computing net loss per share, as the Company has issued shares that meet the definition of participating securities. The two-class method determines net loss per share for each class of common and participating securities according to dividends declared or accumulated and participation rights in undistributed earnings. The two-class method requires income available to common stockholders for the period to be allocated between common and participating securities based upon their respective rights to receive dividends as if all income for the period had been distributed.
Restricted stock awards granted by the Company entitle the holder of such awards to dividends declared or paid by the Board of Directors, regardless of whether such awards are unvested, as if such shares were outstanding common shares at the time of the dividend. However, the unvested restricted stock awards are not entitled to share in the residual net assets (deficit) of the Company. Accordingly, in periods in which the Company reports a net loss or a net loss attributable to common stockholders resulting from preferred stock dividends, accretion or modifications, net losses are not allocated to participating securities. The Company reported a net loss in each of the years ended December 31, 2017, 2016 and 2015.
Basic net loss per share is computed by dividing the net loss by the weighted average number of shares of common stock outstanding for the period. Diluted net loss is computed by adjusting net loss to reallocate undistributed earnings based on the potential impact of dilutive securities, including outstanding stock options. Diluted net loss per share is computed by dividing the diluted net loss by the weighted average number of shares of common stock, including potential dilutive shares of common stock assuming the dilutive effect of potentially dilutive securities. For periods in which the Company has reported net losses, diluted net loss per share is the same as basic net loss per share, since their impact would be anti-dilutive to the calculation of net loss per share. Diluted net loss per share is the same as basic net loss per share for each of the years ended December 31, 2017, 2016 and 2015.
Concentration of Credit Risk and of Significant Suppliers and Customers
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash, cash equivalents, short-term investments and accounts receivable. At December 31, 2017 and 2016, all of the Company’s fixed income marketable securities were invested in CDs insured by the Federal Deposit Insurance Corporation. The Company also generally maintains balances in various operating accounts in excess of federally insured limits at two accredited financial institutions. The Company does not believe that it is subject to unusual credit risk beyond the normal credit risk associated with commercial banking relationships.
Concentrations of credit risk with respect to accounts receivable, which are typically unsecured, are somewhat mitigated due to the wide variety of customers (large animal health companies, distributors, and veterinarians) purchasing the Company’s products. All of the Company’s accounts receivable arise from product sales sold by the Company in the United States and have standard payment terms which generally require payment within 30 days and licensing and collaboration revenue which require payment within 60 days. The Company monitors the financial health performance and credit worthiness of its customers so it can properly assess and respond to changes in their credit profile. The Company continues to monitor these conditions and assess their possible impact on it business. As of December 31, 2017, accounts receivable from two customers, Eli Lilly and Company, acting on behalf of its Elanco Animal Health Division (“Elanco”) and Henry Schein Animal Health, Inc. each accounted more than 10% of the Company’s accounts receivable, net, and on a combined basis accounted for approximately 79% of accounts receivable, net.
During the year ended December 31, 2017, the Company’s product sales to one customer, Elanco, accounted for approximately 79% of the Company’s total net product sales and 84% of total revenues.
The Company is dependent on a small number of third-party manufacturers to supply active pharmaceutical ingredients (“API”) and formulated drugs for research and development activities in its programs and commercial supply, which would be adversely affected by a significant interruption in supply.
The Company is also dependent on a combination of national and regional distributors for its product sales of ENTYCE.
F-14
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
Fair Value Measurements
Certain assets and liabilities are carried at fair value under GAAP. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. A fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last is considered unobservable, is used to measure fair value:
|
· |
Level 1—Quoted prices in active markets for identical assets or liabilities. |
|
· |
Level 2—Observable inputs (other than Level 1 quoted prices) such as quoted prices in active markets for similar assets or liabilities, quoted prices in markets that are not active for identical or similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data. |
|
· |
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to determining the fair value of the assets or liabilities, including pricing models, discounted cash flow methodologies and similar techniques. |
Segment and Geographic Information
Segment Assets
The Company manages its operations as a single segment for the purposes of assessing performance and making operating decisions. The Company is a pet therapeutics company developing compounds to address unmet and under-served medical needs in companion animals. All assets were held in the United States and Belgium as of December 31, 2017 and 2016. Total assets were $135,192 and $151,406 at December 31, 2017 and 2016, respectively.
Revenues by Geographic Region
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|||||||
|
|
|
2017 |
|
2016 |
|
2015 |
|||
|
|
|
(Dollars in thousands) |
|||||||
|
Revenues |
|
|
|
|
|
|
|
|
|
|
United States |
|
$ |
25,573 |
|
$ |
38,318 |
|
$ |
678 |
|
Belgium |
|
|
— |
|
|
233 |
|
|
— |
|
Total revenues |
|
$ |
25,573 |
|
$ |
38,551 |
|
$ |
678 |
Long-Lived Assets, Net by Geographic Region
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|||||||
|
|
|
2017 |
|
2016 |
|
2015 |
|||
|
|
|
(Dollars in thousands) |
|||||||
|
Long-lived assets, net |
|
|
|
|
|
|
|
|
|
|
United States |
|
$ |
1,166 |
|
$ |
1,947 |
|
$ |
2,460 |
|
Belgium |
|
|
— |
|
|
1 |
|
|
95 |
|
Total long-lived assets, net |
|
$ |
1,166 |
|
$ |
1,948 |
|
$ |
2,555 |
New Accounting Standards
Revenue from Contracts with Customers
In May 2014, the Financial Accounting Standards Board (“FASB”) issued guidance on recognizing revenue in contracts with customers. The guidance affects any entity that either enters into contracts with customers to transfer goods or services or enters into contracts for the transfer of nonfinancial assets unless those contracts are within the scope of other standards (e.g., insurance contracts or lease contracts). This guidance will supersede the revenue recognition requirements in topic, Revenue Recognition, and most industry-specific guidance. The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services.
F-15
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
These changes became effective for the Company on January 1, 2018, and the Company adopted the modified retrospective adoption method. The Company has identified variable consideration within its licensing and collaboration agreements that will result in the timing of revenue recognition to be different under the new guidance, specifically related to the timing of recognition of the $7,000 Elanco licensing and collaboration commitment (Note 12). Application of the new guidance to its licensing and collaboration agreements may result in a decrease in accumulated deficit, and may have a material impact to the consolidated balance sheet at initial adoption. The evaluation of the Company’s licensing and collaboration contracts subject to this guidance is ongoing, specifically the Company’s estimate of the amount of variable consideration that will be recognized as of the adoption date is being finalized during the first quarter of 2018. The Company also assessed product revenues under the revised guidance and no material differences have been identified with respect to product revenues. The Company continues to assess the new disclosures required by the new guidance to determine what additional information will need to be disclosed.
Inventory
In July 2015, the FASB issued guidance which requires entities to measure most inventory “at lower of cost and net realizable value” thereby simplifying the current guidance under which an entity must measure inventory at the lower of cost or market. This guidance is effective for financial statements issued for fiscal years beginning after December 15, 2016, and interim periods within those fiscal years. Early adoption is permitted and is to be applied on a prospective basis. The Company adopted this guidance on January 1, 2017, and the adoption did not have a material impact on its consolidated financial statements.
Leases
In February 2016, the FASB issued guidance which requires, for operating leases, a lessee to recognize a right-of-use asset and a lease liability, initially measured at the present value of the lease payments, in its balance sheet. The standard also requires a lessee to recognize a single lease cost, calculated so that the cost of the lease is allocated over the lease term, on a generally straight-line basis. This guidance is effective for financial statements issued for fiscal years beginning after December 15, 2018, and interim periods within those fiscal years. Early adoption is permitted and is to be applied on a modified retrospective transition. The Company is currently assessing the effect that adoption of this guidance will have on its consolidated financial statements.
Compensation – Stock Compensation
In March 2016, the FASB issued guidance that simplifies several aspects of the accounting for employee share-based payment transactions including accounting for income taxes, forfeitures and statutory tax withholding requirements, as well as classification in the statement of cash flows. This guidance is effective for financial statements issued for fiscal years beginning after December 15, 2016, and interim periods within those fiscal years. Early adoption is permitted. The Company adopted this guidance on January 1, 2017, and the adoption did not have a material impact on its consolidated financial statements.
Statement of Cash Flows
In August 2016, the FASB issued guidance on how certain cash receipts and cash payments are presented and classified in the statement of cash flows. This guidance addresses eight specific cash flow issues with the objective of reducing the existing diversity in practice. This guidance is effective for financial statements issued for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. Early adoption is permitted, provided that all of the amendments are adopted in the same period. The guidance requires application using a retrospective transition method. The Company adopted this guidance on January 1, 2017, and the adoption did not have a material impact on its consolidated financial statements.
Intangibles—Goodwill and Other
In January 2017, the FASB issued guidance on simplifying the subsequent measurement of goodwill by eliminating Step 2 (measuring a goodwill impairment loss by comparing the implied fair value of a reporting unit’s goodwill with the carrying amount of that goodwill) from the goodwill impairment test. Under the amendments in this guidance, an entity should perform its annual, or interim, goodwill impairment test by comparing the fair value of a reporting unit with its carrying amount. An entity should recognize an impairment charge for the amount by which the carrying amount exceeds the reporting unit’s fair value; however, the loss recognized should not exceed the total amount of goodwill allocated to that reporting unit. Additionally, an entity should consider income tax effects from any tax deductible goodwill on the carrying amount of the reporting unit when measuring the goodwill impairment loss, if applicable. This guidance is effective for annual or interim goodwill impairment tests in fiscal years beginning after December 15, 2019. Early adoption is permitted for interim or annual goodwill impairment tests performed on testing dates after January 1, 2017. The guidance requires application using a prospective method. The Company adopted this guidance on January 1, 2017, and the adoption did not have a material impact on its consolidated financial statements.
F-16
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
Compensation – Stock Compensation
In May 2017, the FASB issued guidance on determining which changes to the terms or conditions of share-based payment awards require an entity to apply modification accounting. This guidance is effective for financial statements issued for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. Early adoption is permitted, and is applied prospectively to changes in terms or conditions of awards occurring on or after the adoption date. The Company is currently assessing the effect that adoption of this guidance will have on its consolidated financial statements.
3. Fair Value of Financial Assets and Liabilities
Financial Assets and Liabilities Measured at Fair Value on a Recurring Basis
The carrying values and estimated fair values of the Company’s financial assets which are measured at fair value on a recurring basis was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value Measurements as of |
||||||||||
|
|
|
Carrying |
|
December 31, 2017 Using: |
|||||||||||
|
|
|
Value |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
|||||
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Certificates of deposit |
|
$ |
8,964 |
|
$ |
— |
|
$ |
8,964 |
|
$ |
— |
|
$ |
8,964 |
|
Short-term investments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short-term marketable securities - certificates of deposit |
|
|
747 |
|
|
— |
|
|
747 |
|
|
— |
|
|
747 |
|
|
|
$ |
9,711 |
|
$ |
— |
|
$ |
9,711 |
|
$ |
— |
|
$ |
9,711 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value Measurements as of |
||||||||||
|
|
|
Carrying |
|
December 31, 2016 Using: |
|||||||||||
|
|
|
Value |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
|||||
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Certificates of deposit |
|
$ |
7,719 |
|
$ |
— |
|
$ |
7,719 |
|
$ |
— |
|
$ |
7,719 |
|
Short-term investments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short-term marketable securities - certificates of deposit |
|
|
996 |
|
|
— |
|
|
996 |
|
|
— |
|
|
996 |
|
|
|
$ |
8,715 |
|
$ |
— |
|
$ |
8,715 |
|
$ |
— |
|
$ |
8,715 |
The financial assets above are measured at fair value using quoted prices in active markets for identical assets (Level 1); significant other observable inputs (Level 2); and significant unobservable inputs (Level 3). Certain estimates and judgments are required to develop the fair value amounts shown above. The fair value amounts shown above are not necessarily indicative of the amounts that the Company would realize upon disposition, nor do they indicate the Company’s intent or ability to dispose of the financial instrument.
The following methods and assumptions were used to estimate the fair value of each material class of financial instrument:
|
· |
Cash equivalents – the fair value of the cash equivalents has been determined to be amortized cost given the short duration of the securities. |
|
· |
Marketable securities (short-term) – the fair value of marketable securities has been determined to be amortized cost given the short duration of the securities. |
The Company had no financial liabilities measured at fair value on a recurring basis as of December 31, 2017 and 2016.
F-17
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
Financial Assets and Liabilities that are not Measured at Fair Value on a Recurring Basis
The carrying values and estimated fair values of the Company’s financial liabilities which are not measured at fair value on a recurring basis was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2017 |
||||
|
|
|
Carrying Value |
|
Fair Value |
||
|
Liabilities: |
|
|
|
|
|
|
|
Loans payable (Level 2) |
|
$ |
36,825 |
|
$ |
36,973 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2016 |
||||
|
|
|
Carrying Value |
|
Fair Value |
||
|
Liabilities: |
|
|
|
|
|
|
|
Loans payable (Level 2) |
|
$ |
40,188 |
|
$ |
40,709 |
The financial liabilities above are measured at fair value using quoted prices in active markets for identical assets (Level 1); significant other observable inputs (Level 2); and significant unobservable inputs (Level 3). Certain estimates and judgments were required to develop the fair value amounts. The fair value amount shown above is not necessarily indicative of the amounts that the Company would realize upon disposition, nor does it indicate the Company’s intent or ability to dispose of the financial instrument.
The fair value of loans payable was estimated using discounted cash flow analysis discounted at current rates.
The Company had no financial assets not measured at fair value on a recurring basis as of December 31, 2017 and 2016.
Fair value information about the intangible assets that were fully impaired during the years ended December 31, 2017 and 2016 (Note 8) was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value |
||||||||||
|
|
|
Carrying |
|
December 31, 2017 Using: |
|||||||||||
|
|
|
Value |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Impairment |
|||||
|
Intellectual property rights acquired for in-process research and development |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
7,448 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value |
||||||||||
|
|
|
Carrying |
|
December 31, 2016 Using: |
|||||||||||
|
|
|
Value |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Impairment |
|||||
|
Intellectual property rights for currently marketed products |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
5,711 |
|
Intellectual property rights acquired for in-process research and development |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,231 |
|
|
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
7,942 |
The fair value amount is presented as of the date of impairment, as these assets are not measured at fair value on a recurring basis. (Note 8). The fair value reflects intangible assets written down to fair value during the years ended December 31, 2017 and 2016. Fair value was determined using the income approach, specifically, the multi-period excess earnings method, a form of a discounted cash flow method. The Company started with a forecast of all the expected net cash flows associated with the asset and then it applied an asset-specific discount rate to arrive at a net present value amount. Some of the more significant estimates and assumptions inherent in this approach include: the amount and timing of the projected net cash flows, which includes the expected impact of competitive legal and/or regulatory forces on the product and the impact of technological risk associated with IPR&D intangible assets; the discount rate, which seeks to reflect the various risks inherent in the projected cash flows; and the tax rate, which seeks to incorporate the geographic diversity of the projected cash flows.
F-18
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
4. Investments
Marketable Securities
Marketable securities consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2017 |
||||||||||
|
|
|
|
|
|
Gross |
|
Gross |
|
|
|
||
|
|
|
Amortized |
|
Unrealized |
|
Unrealized |
|
Fair |
||||
|
|
|
Cost |
|
Losses |
|
Losses |
|
Value |
||||
|
Short-term marketable securities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Certificates of deposit |
|
$ |
747 |
|
$ |
— |
|
$ |
— |
|
$ |
747 |
|
Total |
|
$ |
747 |
|
$ |
— |
|
$ |
— |
|
$ |
747 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2016 |
||||||||||
|
|
|
|
|
|
Gross |
|
Gross |
|
|
|
||
|
|
|
Amortized |
|
Unrealized |
|
Unrealized |
|
Fair |
||||
|
|
|
Cost |
|
Losses |
|
Losses |
|
Value |
||||
|
Short-term marketable securities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Certificates of deposit |
|
$ |
996 |
|
$ |
— |
|
$ |
— |
|
$ |
996 |
|
Total |
|
$ |
996 |
|
$ |
— |
|
$ |
— |
|
$ |
996 |
At December 31, 2017 and 2016, short-term marketable securities consisted of investments that mature within one year. Short-term marketable securities are recorded as short-term investments in the consolidated balance sheets.
5. Inventories
Inventories are stated at the lower of cost and net realizable value and consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2017 |
|
December 31, 2016 |
||
|
Raw materials |
|
$ |
1,132 |
|
$ |
1,441 |
|
Work-in-process |
|
|
12,322 |
|
|
8,153 |
|
Finished goods |
|
|
122 |
|
|
1,536 |
|
|
|
$ |
13,576 |
|
$ |
11,130 |
As of December 31, 2017 and 2016, the Company had non-cancellable open orders for the purchase of inventories of approximately $7,132, which is expected to be paid in the next 12 months, and $17,800, respectively.
As of December 31, 2017, raw materials included $777 of GALLIPRANT® inventories. As part of the manufacturing transfer of GALLIPRANT (Note 12), the Company transferred these raw materials to Elanco. Elanco has agreed to reimburse the Company for these raw materials. During the year ended December 31, 2017, the Company recognized an inventory valuation loss related to these raw materials in the amount of $347 from the application of lower of cost and net realizable value in the research and development expenses.
During the year ended December 31, 2017, the Company recognized an inventory valuation loss in the amount of $394, from application of lower of cost and net realizable value in cost of product sales. The loss related to GALLIPRANT inventories that were written off.
Finished goods and work-in-process inventories at December 31, 2016, included $9,172 of pre-launch product costs of GALLIPRANT® (grapiprant tablets). GALLIPRANT was approved by the CVM for the control of pain and inflammation associated with osteoarthritis in dogs in the first quarter of 2016.
During the year ended December 31, 2016, the Company recognized an inventory valuation loss in the amount of $2,532 from application of lower of cost or market in cost of product sales. The loss related to BLONTRESS and TACTRESS inventories that were written off and pre-launch GALLIPRANT inventories written down to market value due to terms agreed upon in the Elanco collaboration agreement (Note 12).
F-19
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
During the fourth quarter of 2016, the Company expensed $2,639 of previously capitalized process validation batches of ENTYCE as research and development expenses due to the Company concluding that the future commercial use and future economic benefit could no longer be reasonably determined for process validation batches that were intended to be used as commercial launch inventories. As a result of the approval of the Company’s PAS from CVM in the fourth quarter of 2017, the Company is now selling the previously expensed process validation batches. In addition, the Company expensed $1,983 of costs incurred related to manufacturing of ENTYCE under a firm purchase commitment as research and development expenses due to the Company concluding that the future commercial use and future economic benefit could no longer be reasonably determined. At December 31, 2016, $1,983 was accrued as a loss on a firm purchase commitment in the consolidated balance sheets, and during the third quarter of 2017, the accrual was paid.
6. Property and Equipment, Net
Property and equipment, net consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2017 |
|
December 31, 2016 |
||
|
Laboratory and office equipment |
|
$ |
173 |
|
$ |
666 |
|
Computer equipment and software |
|
|
2,046 |
|
|
2,014 |
|
Furniture |
|
|
135 |
|
|
135 |
|
Construction in process |
|
|
— |
|
|
53 |
|
Total property and equipment |
|
|
2,354 |
|
|
2,868 |
|
Less: Accumulated depreciation and amortization |
|
|
(1,188) |
|
|
(920) |
|
Property and equipment, net |
|
$ |
1,166 |
|
$ |
1,948 |
Depreciation and amortization expense was $812, $609 and $296 for the years ended December 31, 2017, 2016 and 2015, respectively. During the year ended December 31, 2017, the Company recognized impairment charges of $317 related to equipment previously used in its former San Diego, California, property in cost of products sales. No significant gains/losses on disposal of property and equipment were recognized during the years ended December 31, 2017, 2016 and 2015.
7. Goodwill
Goodwill is recorded as an indefinite-lived asset and is not amortized for financial reporting purposes but is tested for impairment on an annual basis or when indications of impairment exist. No goodwill impairment losses have been recognized to date. Goodwill is not expected to be deductible for income tax purposes.
The Company completed its annual goodwill impairment testing during the third quarter of 2017. The Company elected to bypass the qualitative assessment. The Company determined as of the testing date that it consisted of one operating segment which is comprised of one reporting unit. In performing step one of the assessment, the Company determined that its fair value, determined to be its market capitalization, was greater than its carrying value, determined to be stockholders’ equity. Based on this result, the Company determined there was no impairment of goodwill as of the annual testing date.
Goodwill as of December 31, 2017, was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross |
|
Impairment |
|
Net |
|||
|
|
|
Carrying Value |
|
Losses |
|
Carrying Value |
|||
|
Goodwill |
|
$ |
41,295 |
|
$ |
— |
|
$ |
41,295 |
The change in the net book value of goodwill for the years ended December 31, 2017 and 2016, was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2017 |
|
2016 |
||
|
As of January 1, |
|
$ |
39,382 |
|
$ |
39,781 |
|
Effect of foreign currency exchange |
|
|
1,913 |
|
|
(399) |
|
As of the end of the period, |
|
$ |
41,295 |
|
$ |
39,382 |
F-20
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
8. Intangible Assets, Net
The change in the net book value of intangible assets for the years ended December 31, 2017 and 2016, was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2017 |
|
2016 |
||
|
As of January 1, |
|
$ |
7,639 |
|
$ |
15,067 |
|
Additions (Note 12) |
|
|
6,000 |
|
|
1,000 |
|
Amortization expense |
|
|
(350) |
|
|
(379) |
|
Effect of foreign currency exchange |
|
|
775 |
|
|
(107) |
|
Impairment |
|
|
(7,448) |
|
|
(7,942) |
|
As of the end of the period, |
|
$ |
6,616 |
|
$ |
7,639 |
The Company recognized amortization expense of $350, $379 and $1,544 for the years ended December 31, 2017, 2016 and 2015, respectively.
Amortization expense of intangible assets for each of the five succeeding years as of December 31, 2017, was as follows:
|
|
|
|
|
|
Year Ending December 31, |
|
|
|
|
2018 |
|
$ |
516 |
|
2019 |
|
|
516 |
|
2020 |
|
|
516 |
|
2021 |
|
|
516 |
|
2022 |
|
$ |
516 |
Unamortized Intangible Assets
The Company completed its annual indefinite-lived IPR&D intangible assets impairment testing during the fourth quarter of 2017. The Company elected to bypass the qualitative assessment. For purposes of impairment testing, the fair value of the indefinite-lived IPR&D intangible assets was determined by using the framework of ASC 820, Fair Value Measurement. When determining the fair value of the indefinite-lived IPR&D intangible assets, the Company revisited all assumptions used in measuring the indefinite-lived IPR&D intangible assets at the time of acquisition, and evaluated and considered new and updated data and information available.
Unamortized intangible assets as of December 31, 2017 and 2016, were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net |
||||
|
|
|
Carrying |
||||
|
|
|
Value |
||||
|
|
|
As of December 31, |
||||
|
|
|
2017 |
|
2016 |
||
|
Intellectual property rights acquired for in-process research and development |
|
$ |
— |
|
$ |
6,674 |
The net carrying value above includes asset impairment charges to date of $24,213.
Impairment of Unamortized Intangible Assets
AT-006 (eprociclovir) and AT-008 (rabacfosadine)
During the fourth quarter of 2017, the Company determined that events and changes in circumstances indicated that the IPR&D intangible assets might be impaired. During the Company’s development program prioritization review, which included the consideration of a number of factors, including the Company’s inability to raise additional capital in November 2017, the Company decided to further delay the development of AT-006 and AT-008. Due to this delay the Company revisited all assumptions used in measuring the fair values of AT-006 and AT-008. This interim review resulted in fair values of these intangibles being less than their carrying values which resulted in an impairment charge of $7,448, which was recorded during the fourth quarter of 2017, reducing the carrying values of both AT-006 and AT-008 to $0.
AT-007 (Feline immunodeficiency virus)
The Company had been considering out-licensing or internally advancing the AT-007 program for feline immunodeficiency virus since an impairment expense of $8,717 was recorded in 2015. Due to the return of the AT-006 global rights from Elanco in May 2016 (Note 12) and ensuing development program portfolio prioritization, including consideration of the Company’s focus on commercial
F-21
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
launch activities to support its recently approved products, the Company decided to discontinue the development of AT-007 during the second quarter of 2016. This resulted in an impairment charge of $2,229, which was recorded during the second quarter of 2016, reducing the carrying value of AT-007 to $0.
AT-011 (Parvovirus)
For AT-011, the Company had been conducting early pre-development studies, including lead selection and proof of concept on several molecules. In the third quarter of 2015, the Company completed its evaluation of AT-011. Based on this evaluation, the Company determined that none of the molecules being evaluated were suitable for advancement in development. As such, the Company decided to abandon the development of AT-011, resulting in an impairment charge of $5,819, the full carrying value of AT-011.
Amortized Intangible Assets
Amortized intangible assets as of December 31, 2017, were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross |
|
|
|
|
Net |
|
Weighted |
|||
|
|
|
Carrying |
|
Accumulated |
|
Carrying |
|
Average |
||||
|
|
|
Value |
|
Amortization |
|
Value |
|
Useful Life |
||||
|
Intellectual property rights for currently marketed products |
|
$ |
7,000 |
|
$ |
384 |
|
$ |
6,616 |
|
14.1 |
Years |
|
Intellectual property rights for formerly marketed products |
|
|
38,652 |
|
|
38,652 |
|
|
— |
|
N/A |
N/A |
|
|
|
$ |
45,652 |
|
$ |
39,036 |
|
$ |
6,616 |
|
|
|
Accumulated amortization for formerly marketed products includes both amortization expense and asset impairment charges. Asset impairment charges to date are $25,390 and $9,185 for BLONTRESS and TACTRESS, respectively.
Amortized intangible assets as of December 31, 2016, were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross |
|
|
|
|
Net |
|
Weighted |
|||
|
|
|
Carrying |
|
Accumulated |
|
Carrying |
|
Average |
||||
|
|
|
Value |
|
Amortization |
|
Value |
|
Useful Life |
||||
|
Intellectual property rights for currently marketed products |
|
$ |
39,652 |
|
$ |
38,687 |
|
$ |
965 |
|
12 |
Years |
Accumulated amortization includes both amortization expense and asset impairment charges. Asset impairment charges to date are $25,390 and $9,185 for BLONTRESS and TACTRESS, respectively.
Intellectual Property Rights for Currently Marketed Products
As of December 31, 2017, intellectual property rights for currently marketed products relate to intangible assets capitalized for NOCITA, GALLIPRANT and ENTYCE in conjunction with approval/post-approval milestone payments made under the Company's licensing agreements.
As of December 31, 2016, intellectual property rights for currently marketed products relate to intangible assets capitalized for NOCITA in conjunction with approval/post-approval milestone payment made under the Company's licensing agreement and BLONTRESS and TACTRESS that had been acquired through business combinations.
Impairment of Amortized Intangible Assets
Since the acquisition of Vet Therapeutics, Inc. (October 2013), the Company performed various scientific and clinical activities to gain further knowledge around the science and efficacy of BLONTRESS and TACTRESS.
BLONTRESS
In the third quarter of 2015, the Company noted that scientific studies suggested that BLONTRESS was not as specific to the target as previously expected. The Company’s market research and interactions with veterinary oncologists indicated that high specificity, including binding and depletion, will likely be necessary to drive wide adoption of monoclonal antibody therapy given that canine B-cell is generally chemotherapy sensitive. Furthermore, the Company was aware of other emerging therapies that would compete in the B-cell lymphoma market, and believed that products with break-through benefit will dominate the market. Given those scientific results and competitive assessment, the Company recorded an impairment expense of $20,228 in 2015. In the fourth quarter of 2016, the Company received final data from the Mini B-CHOMP study, which evaluated an abbreviated chemotherapy (CHOP) protocol in dogs with B-cell lymphoma. The results confirmed that BLONTRESS did not seem to be adding significant progression-free survival in canine B-cell lymphoma.
F-22
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
While BLONTRESS remained commercially available, the Company deemed the results of Mini B-CHOMP study and the updated commercial expectations as a result of the Mini B-CHOMP study results, as indicators of potential impairment of its finite-lived intangible asset BLONTRESS during the fourth quarter of 2016. The Company performed impairment testing for the intangible asset BLONTRESS as of December 31, 2016, and recorded an impairment expense of $5,162 during the fourth quarter of 2016, resulting in a net carrying value of $0 for BLONTRESS.
TACTRESS
In the third quarter of 2015, the Company’s interim analysis of the clinical results indicated that TACTRESS did not seem to be adding significant progression free survival in canine T-cell lymphoma; those results were confirmed in the final study results in July 2016. In addition, scientific studies suggested that TACTRESS was not as specific to the target as expected. Given those clinical and scientific results, the Company no longer believed that TACTRESS would capture the desired T-cell lymphoma market opportunity and recorded an impairment expense of $8,634 in 2015.
While TACTRESS remained commercially available, the use by oncologists had been more limited than the Company anticipated, resulting in sales during the second quarter of 2016, being significantly lower than forecasted. The Company deemed the events and market projections described above to be indicators of potential impairment of its finite-lived intangible asset TACTRESS during the second quarter of 2016. The Company performed impairment testing for the intangible asset TACTRESS as of June 30, 2016, and recorded an impairment expense of $551 during the second quarter of 2016, resulting in a net carrying value of $0 for TACTRESS.
Unfavorable estimates of the Company’s therapeutics’ market opportunities, expected future cash flows and estimated useful lives could result in impairment charges in future periods.
9. Derivative Financial Instruments
The Company records all derivatives in the consolidated balance sheets at fair value in other long-term assets. In 2015, the Company’s derivative financial instrument, the Advaxis warrant, was not designated as a hedging instrument and was adjusted to fair value through earnings in other income (expense). During the year ended December 31, 2015, the Company exercised the Advaxis warrant and subsequently sold the shares of common stock received upon exercise.
The gain recognized in other income (expense) for the years ended:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gain Recognized in |
|||||||
|
|
|
Other Income (expense) |
|||||||
|
|
|
Year Ended December 31, |
|||||||
|
|
|
2017 |
|
2016 |
|
2015 |
|||
|
Derivative assets: |
|
|
|
|
|
|
|
|
|
|
Warrant |
|
$ |
— |
|
$ |
— |
|
$ |
1,274 |
As the Company exercised the warrant and subsequently sold the shares of common stock received upon exercise during the second quarter of 2015, no gain was recorded during the years ended December 31, 2017 and 2016.
10. Debt
Loan and Security Agreements
Effective as of October 16, 2015, the Company and Vet Therapeutics, Inc., (the “Borrowers”), entered into a Loan and Security Agreement (“Loan Agreement”), with Pacific Western Bank, or Pacific Western, as a collateral agent and Oxford Finance, LLC, (the “Lenders”), pursuant to which the Lenders agreed to make available to the Company term loan in an aggregate principal amount up to $35,000 and a revolving credit facility in an aggregate principal amount up to $5,000 subject to certain conditions to funding. The term loan and the revolving credit facility are secured by all of the Borrowers’ personal property other than intellectual property and certain other customary exclusions. Subject to customary exceptions, the Company is not permitted to encumber its intellectual property. The outstanding principal under the Loan Agreement was $31,500 under the term loan and $5,000 under the revolving credit facility at December 31, 2017. Under the Loan Agreement, the Company was required to make interest-only payments on the term loan for 18 months, and beginning on May 1, 2017, began to make payments of principal and accrued interest on the term loan in equal monthly installments over a term of 30 months. The Company was required to make interest-only payments on the revolving credit facility until October 16, 2017, when all principal and accrued interest were due. The term loan and the revolving credit facility bear interest per annum at the greater of (i) 6.91% or (ii) 3.66% plus the prime rate, which is customarily defined. As of December 31, 2017, interest rate for the term loan and the revolving credit facility was 8.16%. During the years ended December 31, 2017 and 2016, the Company recognized interest expense of $3,481 and $3,396, respectively.
F-23
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
Upon execution of the Loan Agreement, the Company was obligated to pay a facility fee to the Lenders of $150, and an agency fee to the collateral agent of $100. In addition, the Company is or will be obligated to pay a final payment fee equal to 3.30% of such term loan being prepaid or repaid with respect to the term loan upon the earliest to occur: October 16, 2019, the acceleration of any term loan or the prepayment of a term loan. The Company was obligated to pay a termination fee equal to 3.30% of the highest outstanding amount of the revolving credit facility upon the earliest to occur of October 16, 2017, the acceleration of the revolving credit facility or the termination of the revolving credit facility. The Company will also be obligated to pay an unused-line fee equal to 0.25% per annum of the average unused portion of the revolving credit facility.
Effective as of July 31, 2017, the Borrowers and Lenders entered into a second amendment to the Loan Agreement (the “Second Amendment”). The terms of the Second Amendment, among other things, extend the maturity date of the existing revolving credit facility to October 16, 2019 (the “Revolving Line Maturity Date”), with amortized equal repayments of the principal outstanding under the revolving credit facility beginning November 1, 2018, and provide a six-month interest only period for the term loans, starting on the date of the Second Amendment. The Company is not subject to any new financial covenants as a result of the Second Amendment. At the closing of the Second Amendment, the Company paid the Lenders an amendment fee of $150 and a facility fee of $60. The Company is also obligated to pay a new termination fee equal to $165 upon the earliest to occur of the Revolving Line Maturity Date, the acceleration of the revolving credit facility or the termination of the revolving credit facility. The existing termination fee of $165 was due upon the original revolving maturity date, October 16, 2017, and was paid on October 17, 2017.
The Loan Agreement contains customary representations and warranties and customary affirmative and negative covenants, including, among others, limits or restrictions on the Borrowers’ ability to incur liens, incur indebtedness, make certain restricted payments, make certain investments, merge, consolidate, make an acquisition, enter into certain licensing arrangements and dispose of certain assets. In addition, the Loan Agreement contains customary events of default that entitle the Lenders to cause the Borrowers’ indebtedness under the Loan Agreement to become immediately due and payable. The events of default, some of which are subject to cure periods, include, among others, a non-payment default, a covenant default, the occurrence of a material adverse change in the Company’s business, the occurrence of an insolvency, a material judgment default, defaults regarding other indebtedness and certain actions by governmental authorities. Upon the occurrence and for the duration of an event of default, an additional default interest rate equal to 4% per annum will apply to all obligations owed under the Loan Agreement.
The Loan Agreement requires that the Company maintain certain minimum liquidity at all times (the greater of cash equal to fifty percent (50%) of outstanding balance or remaining months’ liquidity, which is calculated on an average trailing three (3) month basis, equal to six (6) months or greater), which as of December 31, 2017, was approximately $18,250. If the minimum liquidity covenant is not met, the Company may be required to repay the term loan and the revolving credit facility prior to their scheduled maturity dates. At December 31, 2017, the Company was in compliance with all financial covenants.
On the issuance date of the term loan and the revolving credit facility, the Company accounted for a portion of the transaction as a debt modification of the prior debt dated March 4, 2013 with Pacific Western Bank and a portion as a new financing for the term loan and the revolving credit facility from Oxford. In conjunction with the refinancing in 2015, the Company incurred $556 in lender and legal fees, of which $360 were recorded in the consolidated balance sheet as a reduction in loans payable and $196 were expensed as interest expense. The Company accounted for the Second Amendment as debt modification and capitalized incurred Lender fees of $210. The Lender fees and previously incurred debt issuance costs are amortized over the life of the term loan and the revolving credit facility using the straight-line method which materially approximates effective interest rate method. Final payment and termination fees related to the term loan and the revolving credit facility are being accreted to loans payable over the life of the term loan and the revolving credit facility using the straight-line method which materially approximates effective interest rate method. Amortization of debt issuance costs was $139, $108 and $129 for the years ended December 31, 2017, 2016 and 2015, respectively.
The Company’s loans payable balance as of December 31, 2017, as follows:
|
|
|
|
|
|
Principal amounts |
|
|
|
|
Term loan, 8.16%, principal payments from February 1, 2018 through October 1, 2019 |
|
$ |
31,500 |
|
Revolving credit facility, 8.16%, principal payments from November 1, 2018 through October 1, 2019 |
|
|
5,000 |
|
Add: accretion of final payment and termination fees |
|
|
655 |
|
Less: unamortized debt issuance costs |
|
|
(330) |
|
As of the end of the period |
|
$ |
36,825 |
As of December 31, 2017, $16,500 and $833 related to the term loan and the revolving credit facility, respectively, were classified as current portion – loans payable.
F-24
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
Estimated future principal payments under the Loan Agreement are as follows:
|
|
|
|
|
|
|
|
|
|
|
Year Ending December 31, |
|
|
|
|
2018 |
|
$ |
17,333 |
|
2019 |
|
|
19,167 |
|
2020 |
|
|
— |
|
2021 |
|
|
— |
|
2022 |
|
|
— |
|
Thereafter |
|
|
— |
|
Total |
|
$ |
36,500 |
11. Accrued Expenses
Accrued expenses consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2017 |
|
December 31, 2016 |
||
|
Accrued expenses: |
|
|
|
|
|
|
|
Payroll and related expenses |
|
$ |
2,314 |
|
$ |
2,321 |
|
Professional fees |
|
|
208 |
|
|
219 |
|
Royalty expense |
|
|
718 |
|
|
71 |
|
Interest expense |
|
|
249 |
|
|
247 |
|
Research and development costs |
|
|
5 |
|
|
364 |
|
Unbilled inventories |
|
|
— |
|
|
465 |
|
Accrued loss on a firm purchase commitment |
|
|
— |
|
|
1,983 |
|
Milestone |
|
|
— |
|
|
17 |
|
Other |
|
|
218 |
|
|
140 |
|
Total |
|
$ |
3,712 |
|
$ |
5,827 |
12. Agreements
RaQualia Pharma Inc. (“RaQualia”)
On December 27, 2010, the Company entered into two Exclusive License Agreements with RaQualia (as amended, the “RaQualia Agreements”) that granted the Company global rights, subject to certain exceptions for injectables in Japan, Korea, China and Taiwan for development and commercialization of licensed animal health products for compounds RQ-00000005 (ENTYCE®, also known as AT-002) and RQ-00000007 (GALLIPRANT®, also known as AT-001). The Company will be required to pay RaQualia remaining milestone payments associated with GALLIPRANT and ENTYCE of up to $4,000 and $3,000, respectively, upon the Company’s achievement of certain development, regulatory and commercial milestones, as well as mid-single digit royalties on the Company’s or the Company’s sublicensee’s product sales.
The Company achieved milestones totaling $6,000, $5,500 and $0 during the years ended December 31, 2017, 2016, and 2015, respectively. Milestones achieved in 2017 were capitalized as intangible assets and milestones achieved in 2016 were expensed within research and development expenses. As of December 31, 2017, the Company had paid $11,500 in milestone payments since execution of the RaQualia Agreements, and no milestone payments were accrued. It is possible that a milestone related to the RaQualia Agreements is achieved within the next twelve months totaling $2,000.
Pacira Pharmaceuticals, Inc. (“Pacira”)
On December 5, 2012, the Company entered into an Exclusive License, Development, and Commercialization Agreement with Pacira (the “Pacira Agreement”) that granted the Company global rights for development and commercialization of licensed animal health products for NOCITA® (also known as AT-003). The Company will be required to pay Pacira remaining milestone payments associated with NOCITA of up to $40,000 upon the Company’s achievement of certain commercial milestones, as well as tiered royalties on the Company’s product sales. The commercial milestones owed to Pacira under the Pacira Agreement begin to be triggered once NOCITA annual net sales reach $100,000 with the final tier being owed to Pacira once NOCITA annual net sales reach $500,000.
F-25
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
The Company achieved milestones totaling $0, $2,000 and $0 during the years ended December 31, 2017, 2016 and 2015, respectively. Of the $2,000 in achieved milestones in 2016, $1,000 was capitalized as intangible assets and the other $1,000 was expensed within research and development expenses. As of December 31, 2017, the Company had paid $2,500 in milestone payments since execution of the Pacira Agreement, and no milestone payments were accrued. The Company does not expect to achieve any milestones related to the Pacira Agreement in the next twelve months.
Elanco
BLONTRESS
On December 6, 2012, Vet Therapeutics entered into an Exclusive Commercial License Agreement with Elanco (formerly Novartis Animal Health, Inc.) (the “Elanco BLONTRESS Agreement”) under which Vet Therapeutics granted a commercial license to Elanco for BLONTRESS for the United States and Canada.
On January 2, 2015, the Company was granted a full product license from the USDA for BLONTRESS. The approval resulted in a $3,000 milestone payment being earned and due to the Company per the terms of the Elanco BLONTRESS Agreement. During the first quarter of 2015, the Company recognized $3,000 of licensing revenue related to the milestone payment.
On February 24, 2015, the Company and Elanco agreed to terminate the Elanco BLONTRESS Agreement. In consideration for the return of the commercial license granted to Elanco, the Company paid Elanco $2,500 in March 2015, and was to be required to pay an additional $500 upon the first commercial sale by the Company. At that time the Company determined that it was probable that the $500 payment will be paid, and recorded the $500 as a current liability in the first quarter of 2015. The first commercial sale occurred in March 2016. The Company recorded the $3,000 owed to Elanco as a reduction in revenues received from Elanco as the payment was to re-acquire rights that the Company had previously licensed to Elanco.
On February 25, 2016, the Company and Elanco agreed to amend the terms related to the $500 payment due upon the first commercial sale by the Company. Under the amended terms, upon the first commercial sale in March 2016, the Company was required to pay quarterly a royalty per vial sold until $500 in royalties were paid or the end of two years. After two years, the Company would have been required to pay Elanco $500 plus 10% interest, compounded annually against any unpaid balance, less any royalties paid during the two years. If during the two years following the first commercial sale the Company withdrew BLONTRESS from the market and ceased all commercialization, the remaining royalty and related interest would no longer be payable.
On November 13, 2017, the Company withdrew BLONTRESS from the market and ceased all commercialization making the remaining royalty and interest no longer payable. During the year ended December 31, 2017, the Company recognized $480 in licensing and collaboration revenue due to the derecognition of the remaining balance of the liability.
GALLIPRANT
On April 22, 2016, the Company entered into a Collaboration, License, Development and Commercialization Agreement (as amended, the “Collaboration Agreement”) with Elanco pursuant to which the Company granted Elanco rights to develop, manufacture, market and commercialize the Company’s products based on licensed grapiprant rights and technology, including GALLIPRANT (collectively, “Grapiprant Products”). Pursuant to the Collaboration Agreement, Elanco will have exclusive rights globally outside the United States and co-promotion rights with the Company in the United States during the term of the Collaboration Agreement.
Under the terms of the Collaboration Agreement, the Company received a non-refundable, non-creditable upfront payment of $45,000. The Company is entitled to a $4,000 milestone payment upon European approval of a Grapiprant Product for the treatment of pain and inflammation, another $4,000 payment upon achievement of a development milestone related to the manufacturing of a Grapiprant Product from an alternate supply source, and payments up to $75,000 upon the achievement of certain sales milestones. The sales milestone payments are subject to a one-third reduction for each year the occurrence of the milestone is not achieved beyond December 31, 2021, with any non-occurrence beyond December 31, 2023, cancelling out the applicable milestone payment obligation entirely.
The Collaboration Agreement also provides that Elanco will pay the Company royalty payments on a percentage of net sales in the mid-single to low double digits. The Company is responsible for all development activities required to obtain the first registration or regulatory approval for a Grapiprant Product for use in dogs in each of the European Union (“the EU Product Registration”) and the United States, and Elanco is responsible for all other development activities. First registration for a Grapiprant Product in the United States was achieved before the completion of the Collaboration Agreement. In addition, the Company and Elanco have agreed to pay 25% and 75%, respectively, of all third-party development fees and expenses through December 31, 2018, in connection with preclinical and clinical trials necessary for any additional registration or regulatory approval of Grapiprant Products, provided that the Company’s contribution to such development fees and expenses is capped at $7,000 (“R&D Cap”).
Commencing on the effective date of the Collaboration Agreement, the Company was responsible for the manufacture and supply of all of Elanco’s reasonable requirements of active pharmaceutical ingredient (“API”) and/or Grapiprant Products under the supply terms agreed upon pursuant to the Collaboration Agreement. However, Elanco retained the ability to assume all or a portion of the
F-26
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
manufacturing responsibility during the term of the Collaboration Agreement. On April 28, 2017, the Company and Elanco entered into an amendment (the “Amendment”) to the Collaboration Agreement. Under the Amendment, Elanco agreed to submit binding purchase orders to the Company, within 15 days of the effective date of the Amendment, for certain finished Grapiprant Products to be produced from certain batches of API the Company had agreed to purchase from its third-party manufacturer (the “API Batches”). In addition, Elanco agreed to pay the Company for the API Batches within 30 days after the Company provides Elanco with proof of payment to the manufacturer for such API Batches. The Amendment provides that, in the event Elanco provided notice of its intent to assume responsibility for manufacturing, Elanco would assume all responsibilities of the Company with respect to any undelivered API, including paying the third-party manufacturer for such undelivered API. In July 2017, pursuant to Sections 8.2.2 and 10.1(c) of the Collaboration Agreement, as amended, Elanco provided the Company notice of its intent to assume responsibility for manufacturing of the Grapiprant Products and its intent to assume the applicable regulatory approvals.
In September 2017, the Company and Elanco finalized the transfer of the applicable regulatory approvals in the United States and the responsibility for manufacturing of Grapiprant Products to Elanco. In connection with this assumption of manufacturing responsibility, Elanco compensated the Company $10,832 for certain Grapiprant Product inventories and manufacturing considerations. During the year ended December 31, 2017, the Company recognized $1,000 of licensing and collaboration revenues and $6,099 of product sales, as well as $3,733 in cash received for reimbursement of previously purchased inventories, related to the assumption of manufacturing responsibility by Elanco. In addition, the Company believes all residual matters related to the assumption of manufacturing responsibility by Elanco were concluded in the fourth quarter of 2017.
On April 22, 2016, in connection with the Collaboration Agreement, the Company entered into a Co-Promotion Agreement (the “Co-Promotion Agreement”) with Elanco to co-promote Grapiprant Products in the United States.
Under the terms of the Co-Promotion Agreement, Elanco has agreed to pay the Company, as a fee for promotional services performed and expenses incurred by the Company under the Co-Promotion Agreement, (i) 25% of the gross margin on net sales of Grapiprant Product sold in the United States under the Collaboration Agreement prior to December 31, 2018 (unless extended by mutual agreement), and (ii) a mid-single digit percentage of net sales of Grapiprant Product in the United States after December 31, 2018 through 2028 (unless extended by mutual agreement).
The Company concluded that the Collaboration Agreement and Co-Promotion Agreement represent a multiple-element arrangement, and evaluated if deliverables in the arrangement represent separate units of accounting. The Company identified the following deliverables under the agreement: (i) a royalty-bearing, sub-licensable, development, manufacturing and commercialization license; (ii) manufacturing and supply services; (iii) participation in a joint manufacturing subcommittee; and (iv) services associated with obtaining the EU Product Registration. The Company performed an assessment and concluded that the license had stand-alone value from the other undelivered elements in the arrangement. The Company’s best estimate of the selling price for the manufacturing subcommittee and the EU Product Registration services were immaterial and, therefore, no consideration was allocated to these deliverables. Under the manufacturing and supply services terms, Elanco will be obligated to pay for any future orders at a price per unit representative of market value, and, therefore, no upfront consideration was allocated to this deliverable. The Company allocated $38,000 of the $45,000 upfront payment to the license, and recognized $38,000 of licensing and collaboration revenue during the quarter ended June 30, 2016. The Company allocated $7,000 of upfront consideration to the R&D Cap, which was recorded as licensing and collaboration commitment liability in the consolidated balance sheet as a current liability at December 31, 2017. The licensing and collaboration commitment liability will be reduced in future periods as the related expenses are incurred by Elanco and paid for by the Company. Any remaining balance not paid to Elanco will be recognized as licensing and collaboration revenue on December 31, 2018, when the Company’s obligation to fund 25% of Elanco’s development efforts expires.
The Company evaluated if the sales and other milestones in the Collaboration Agreement are substantive. The Company determined that the milestones are non-substantive, and, therefore, these milestones will be allocated amongst the delivered, and any undelivered elements at the time the milestones are earned. If there are no undelivered elements, the milestone payments will be recognized as revenue in their entirety upon achievement of each milestone. For the year ended December 31, 2017, no milestones were achieved, and accordingly, no revenues were recognized from the milestones.
AT-006
On May 11, 2016, the Company and Elanco agreed to terminate the Elanco AT-006 Agreement that granted Elanco global rights for development and commercialization of licensed animal health products for an anti-viral for the treatment of feline herpes virus-induced ophthalmic conditions. In consideration for the return of the Elanco AT-006 Agreement global rights, the Company is required to pay Elanco a low single digit royalty on product sales, if any, up to an amount in the low-single digit millions.
Advaxis Inc. (“Advaxis”)
On March 19, 2014, the Company entered into an Exclusive License Agreement with Advaxis (the “Advaxis Agreement”) that granted the Company global rights for development and commercialization of licensed animal health products for Advaxis’ ADXS-cHER2 for the treatment of osteosarcoma in dogs (“AT-014”) and three additional cancer immunotherapy products for the treatment
F-27
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
of three other types of cancer. Under the terms of the Advaxis Agreement, the Company paid $2,500 in exchange for the license, 306,122 shares of common stock, and a warrant to purchase 153,061 shares of common stock. The consideration was allocated to the common stock and warrant based on their fair values on the date of issuance of $1,200 and $643, respectively. The remaining consideration of $657 was allocated to the licensed technology. On the date of acquisition, the licensed technology had not reached technological feasibility in animal health indications and had no alternative future use in the field of animal health. Accordingly, in-process research and development of $657 was expensed upon acquisition. The Company will be required to pay Advaxis remaining milestone payments of up to an additional $6,000 in clinical and regulatory milestones for each of the four products, assuming approvals in both cats and dogs, in both the United States and the European Union. In addition, the Company agreed to pay up to $28,500 in commercial milestones, as well as tiered royalties ranging from mid-single digit to 10% on the Company’s product sales, if any. As of December 31, 2017, the Company had not accrued or paid any milestone or royalty payments since execution of the Advaxis Agreement. The Company does not expect to achieve any milestones related to the Advaxis Agreement in the next twelve months.
Under the terms of the subscription agreement, the Company acquired 306,122 shares of common stock and a warrant to purchase another 153,061 shares of common stock for $1,843. The warrant was exercisable through March 19, 2024, at an exercise price of $4.90 per share of common stock and could have been settled through physical share issuance or net share settlement where the total number of issued shares is based on the amount the market price of common stock exceeds the exercise price of $4.90 on date of exercise. Neither the common stock nor warrant had registration rights. The Company allocated the consideration of $1,843 to Advaxis common stock ($1,200) and the Advaxis warrant ($643) based on their respective fair values and recorded the purchase in marketable securities and other long-term assets, respectively. In January 2015, Aratana sold 124,971 shares of Advaxis common stock for proceeds of $1,500 and recognized a gain of $1,010 in other income (expense). Further in April 2015, the Company sold the remaining 181,151 shares of Advaxis common stock for proceeds of $3,233, recognizing a gain of $2,523 in other income (expense) during the second quarter of 2015.
In May 2015, the Company, through net share settlement, exercised the Advaxis warrant for a total exercise price equivalent to $750 and received 116,411 net shares of Advaxis common stock. Subsequently, the Company sold this Advaxis common stock for proceeds of $2,724, a gain of $341, recorded in other income (expense) during the second quarter of 2015.
VetStem BioPharma, Inc. (“VetStem”)
On June 12, 2014, the Company entered into an Exclusive License Agreement with VetStem (as amended, the “VetStem Agreement”) that granted the Company the exclusive United States rights for commercialization and development of VetStem’s allogeneic stem cells being developed for the treatment of pain and inflammation of canine osteoarthritis (“AT-016”). VetStem was responsible for the development and obtaining regulatory approval of AT-016 and the Company was responsible for the commercialization of licensed products. Under the terms of the VetStem Agreement, the Company paid an initial license fee of $500. On the date of acquisition, the licensed technology had not reached technological feasibility in animal health indications and had no alternative future use in the field of animal health. Accordingly, in-process research and development of $500 was expensed upon acquisition. The Company was to be required to pay VetStem remaining milestone payments of up to $3,750 upon VetStem’s achievement of certain development and regulatory milestones, as well as tiered royalties in the low double digit percentages on the Company’s product sales, if any.
The Company achieved milestones totaling $250, $450 and $300 during the years ended December 31, 2017, 2016 and 2015, respectively, which were expensed within research and development expenses. As of December 31, 2017, the Company had paid $1,000 in milestone payments and no royalty payments since execution of the VetStem Agreement and no milestone payments or royalties were accrued.
In January 2018, the Company exercised its right to terminate the license agreement with VetStem effective as of mid-April 2018. As of December 31, 2017, the Company had reimbursed VetStem for all contractually obligated development expenses and had no further development funding obligations for any future development expenses. As a result of the termination of the VetStem Agreement, the Company does not anticipate having to reimburse any further development expenses or make milestone payments to VetStem.
Atopix Therapeutics Ltd. (“Atopix”)
On October 10, 2014, the Company entered into an Exclusive License Agreement with Atopix (the “Atopix Agreement”) that granted the Company an exclusive global license for development and commercialization of animal health products containing the active pharmaceutical ingredient included in Atopix’s CRTH2 antagonist product for the treatment of atopic dermatitis (“AT-018”). Under the terms of the Atopix Agreement, the Company paid an initial license fee of $1,000. On the date of acquisition, the licensed technology had not reached technological feasibility in animal health indications and had no alternative future use in the field of animal health. Accordingly, in-process research and development of $1,000 was expensed upon acquisition. The Company will be required to pay Atopix remaining milestone payments of up to an additional $4,000 in clinical and regulatory milestones, assuming approvals in both cats and dogs, in both the United States and the European Union, as well as tiered royalties in the mid-single digits on the Company’s product sales, if any.
F-28
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
The Company achieved milestones totaling $0, $0 and $500 during the years ended December 31, 2017, 2016 and 2015, respectively, which were expensed within research and development expenses. As of December 31, 2017, the Company had paid $500 in milestone payments and no royalty payments since execution of the Atopix Agreement and no milestone payments or royalties were accrued. The Company does not expect to achieve any milestones related to the Atopix Agreement in the next twelve months.
AskAt Inc. (“AskAt”)
AT-019
On February 28, 2018, the Company entered into an Exclusive License Agreement with AskAt (the “AskAt Agreement”) that granted the Company an exclusive global license for development and commercialization of compound AT-019 in the field of animal health. Under the terms of the AskAt Agreement, the Company will be required to pay an initial upfront license fee of $500. The Company will be required to pay AskAt remaining milestone payments associated with AT-019 of up to $15,500 upon the Company’s achievement of certain development/regulatory ($3,000) and commercial ($12,500) milestones, as well as tiered single digit royalties on the Company’s product sales, if any. The commercial milestones owed to AskAt under the AskAt Agreement begin to be triggered upon the first commercial sale with the final tier being owed to AskAt once annual net sales reach $100,000. Milestones, at the discretion of the Company, can be paid 50% in cash and 50% in a number of the Company’s shares as determined per the terms of the AskAt Agreement.
Collaboration and Option Agreement
On February 28, 2018, in connection with the AskAt Agreement, the Company entered into Collaboration and Option Agreement (the “COA”) with AskAt for animal health research, including an option agreement for multiple therapeutic candidates with potential in pain, allergy and cancer. Under the terms of the COA, the Company will be required to pay an initial upfront option fee of $500.
Government and Other Incentive Programs
The Company has received payments from various government and other incentive programs. Generally, under these programs the Company could be obligated to repay any payments received if certain criteria are not met or certain actions are taken by the Company. The Company could be required to repay up to $760 under these incentive programs as of December 31, 2017. The Company has determined these contingencies to be within its control and will only account for repayment(s) if it becomes probable that the Company will be obligated to repay as result of its actions.
13. Common Stock
Authorized Common Stock
As of December 31, 2017, the authorized number shares of common stock was 100,000,000, par value $0.001 per share.
Common Stock Outstanding
As of December 31, 2017 and 2016, there were 42,532,725 and 36,607,922 shares of the Company’s common stock outstanding respectively, net of 491,861 and 461,901 shares of unvested restricted common stock, respectively.
Treasury Stock
As of December 31, 2017 and 2016, there were 80,916 and 78,226 shares of the Company’s common stock held as treasury stock at a cost of $1,107 and $1,088, respectively. During the years ended December 31, 2017, 2016 and 2015, 2,690, 0 and 859 shares of restricted stock at a cost of $19, $0 and $7, respectively, were withheld to satisfy employee tax withholding obligations arising in conjunction with the vesting of restricted stock pursuant to the Company’s 2013 Incentive Award Plan.
Voting Rights
Each share of common stock entitles the holder to one vote on all matters submitted to a vote of the Company’s stockholders. Common stockholders are entitled to receive dividends, as may be declared by the Board of Directors, if any. As of December 31, 2017 and 2016, the Board of Directors had not declared any dividends in any period.
Stock-Based Awards
During the years ended December 31, 2017 and 2016, the Company issued common stock pursuant to the 2013 Incentive Award Plan (Note 14). During the years ended December 31, 2017 and 2016, the Company did not reacquire any unvested shares of common stock from its terminated employees that had been issued upon the exercise of a stock option prior to its vesting.
Registered Direct Offering
On May 3, 2017, the Company entered into a Placement Agency Agreement (“PAA”) with Barclays Capital Inc. (“Barclays”), pursuant to which Barclays agreed to serve as placement agent for an offering of shares of common stock. In conjunction with the
F-29
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
PAA, on May 3, 2017, the Company also entered into a Securities Purchase Agreement with certain investors for the sale by the Company of 5,000,000 shares of common stock at a purchase price of $5.25 per share (the “Offering”). The shares of common stock were offered and sold pursuant to the Company’s previously filed and then effective registration statement on Form S-3 (File No. 333-197414) and a related prospectus supplement. The Company agreed to pay Barclays an aggregate fee equal to 6.0% of the gross proceeds received by the Company from the Offering. The Offering closed on May 9, 2017, and the Company received aggregate net proceeds from the Offering of approximately $24,400, after deducting placement agent fees of $1,575 and offering expenses of $273.
At-the-Market Offerings
Cowen and Company, LLC
On December 18, 2017, the Company entered into a Sales Agreement (“Cowen Sales Agreement”) with Cowen and Company, LLC (“Cowen”) pursuant to which the Company may sell from time to time, at its option, up to an aggregate of $50,000 of shares of its common stock through Cowen, as sales agent. Sales of the shares of common stock will be made under the Company’s effective Registration Statement on Form S-3 (Reg. No. 333-219681), by means of ordinary brokers’ transactions on the Nasdaq Global Market or otherwise. Additionally, under the terms of the Cowen Sales Agreement, the shares of common stock may be sold at market prices, at negotiated prices or at prices related to the prevailing market price. The Company has agreed to pay Cowen a commission of 3% of the gross proceeds from the sale of such shares of common stock.
In January 2018, the Company sold 2,392,297 shares of common stock for aggregate net proceeds of $11,672. As of the date of this filing, approximately $38,000 of shares of common stock remained available for sale under the Cowen Sales Agreement.
Barclays Capital Inc.
On October 16, 2015, the Company entered into a sales agreement with Barclays pursuant to which the Company could sell from time to time, at its option, up to an aggregate of $52,000 of shares of its common stock through Barclays, as sales agent. Sales of the shares of common stock were made under the Company’s then effective registration statement on Form S-3 (Reg. No. 333-197414), by means of ordinary brokers’ transactions on the Nasdaq Global Market or otherwise. Additionally, under the terms of the Barclays sales agreement, the shares of common stock could be sold at market prices, at negotiated prices or at prices related to the prevailing market price. The Company paid Barclays a commission of 2.75% of the gross proceeds from the sale of the shares of common stock.
On April 28, 2017, the Company terminated its Barclays sales agreement. As of that date, the Company sold an aggregate of approximately $18,000 of the $52,000 available to be sold under the Barclays sales agreement, including 546,926 shares of common stock for aggregate net proceeds of $2,788.
Shelf Registration Statement
On August 4, 2017, the Company filed a shelf registration statement on Form S-3 (Reg. No. 333-219681) (the “Shelf Registration Statement”) with the SEC. The Shelf Registration Statement was declared effective by the SEC on August 16, 2017.
The Shelf Registration Statement allows the Company to offer and sell, from time to time, up to $100,000 of common stock, preferred stock, debt securities, warrants, units or any combination of the foregoing in one or more future public offerings. The terms of any future offering would be determined at the time of the offering and would be subject to market conditions and approval by the Company’s Board of Directors. Any offering of securities covered by the Shelf Registration Statement will be made only by means of a written prospectus and prospectus supplement authorized and filed by the Company.
14. Stock-Based Awards
2010 Equity Incentive Plan
In 2010, the Company’s Board of Directors adopted the 2010 Equity Incentive Plan (the “2010 Plan”). The 2010 Plan provided for the Company to sell or issue common stock or restricted common stock and to grant incentive stock options or nonqualified stock options for the purchase of common stock with a maximum term of ten years to employees, members of the Board of Directors and consultants of the Company. With the adoption and approval of the 2013 Incentive Award Plan (the “2013 Plan”), no further awards will be granted from the 2010 plan.
F-30
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
Stock Options
Activity related to stock options for the year ended December 31, 2017, was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
|
Shares |
|
Weighted |
|
Average |
|
|
||
|
|
|
Issuable |
|
Average |
|
Remaining |
|
Aggregate |
||
|
|
|
Under |
|
Exercise |
|
Contractual |
|
Intrinsic |
||
|
|
|
Options |
|
Price |
|
Term |
|
Value |
||
|
|
|
|
|
|
|
|
(In Years) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding as of December 31, 2016 |
|
65,931 |
|
$ |
3.73 |
|
6.09 |
|
$ |
228 |
|
Granted |
|
— |
|
|
— |
|
|
|
|
|
|
Exercised |
|
(8,537) |
|
|
0.40 |
|
|
|
|
|
|
Forfeited |
|
— |
|
|
— |
|
|
|
|
|
|
Expired |
|
— |
|
|
— |
|
|
|
|
|
|
Outstanding as of December 31, 2017 |
|
57,394 |
|
$ |
4.22 |
|
5.11 |
|
$ |
73 |
|
Options vested and expected to vest as of December 31, 2017 |
|
57,394 |
|
$ |
4.22 |
|
5.11 |
|
$ |
73 |
|
Options exercisable as of December 31, 2017 |
|
57,394 |
|
$ |
4.22 |
|
5.11 |
|
$ |
73 |
No stock options have been granted under the 2010 Plan since 2013. For the years ended December 31, 2017, 2016, and 2015, the total intrinsic value of options exercised was $53, $180 and $786, respectively. For the years ended December 31, 2017, 2016 and 2015, the total fair value of awards vested during the period was $8, $209 and $140, respectively. The Company received cash proceeds of $3, $9 and $25 from the exercise of stock options for the years ended December 31, 2017, 2016 and 2015, respectively, none of which were from the early exercise of stock options.
2013 Incentive Award Plan
In 2013, the Company’s Board of Directors adopted and stockholders approved the 2013 Plan which became effective upon the day prior to the effective date of the Company’s initial public offering. The 2013 Plan as of December 31, 2017 allows for the issuance of up to 5,629,036 shares of common stock, plus any additional shares represented by the 2010 Plan that are forfeited or lapse unexercised. The number of shares of common stock that may be issued under the plan is also subject to an annual increase on January 1st of each calendar year beginning in 2014 and ending in 2023, equal to the lesser of (i) 1,203,369 shares, (ii) 4% of the shares of common stock outstanding on the final day of the immediately preceding calendar year and (iii) an amount determined by the Board of Directors. As of December 31, 2017, there were 1,608,667 shares available for future grant under the 2013 Plan. On January 1, 2018, the annual increase was determined to be 1,203,369.
The 2013 Plan is administered by the Compensation Committee of the Board of Directors, which selects the individuals eligible to receive awards, determines or modifies the terms and condition of the awards granted, accelerates the vesting schedule of any award and generally administers and interprets the 2013 Plan. The 2013 Plan permits the granting of incentive and nonqualified stock options, with terms of up to ten years and the granting of restricted stock, restricted stock units, performance stock awards, dividend equivalent rights, stock payments (i.e. unrestricted stock), cash bonuses and stock appreciation rights to employees, consultants, and non-employee directors.
Stock Options
During the year ended December 31, 2017, the Company granted under the 2013 Plan stock options for the purchase of 540,900 shares of common stock to certain employees and non-employee directors. The vesting conditions for most of these awards are time-based, and the awards typically vest 25% after one year and monthly thereafter for the next 36 months. Awards typically expire after 10 years.
F-31
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
Activity related to stock options for the year ended December 31, 2017, was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighed |
|
|
|
|
|
|
Shares |
|
Weighted |
|
Average |
|
|
|
|
|
|
|
Issuable |
|
Average |
|
Remaining |
|
Aggregate |
||
|
|
|
Under |
|
Exercise |
|
Contractual |
|
Intrinsic |
||
|
|
|
Options |
|
Price |
|
Term |
|
Value |
||
|
|
|
|
|
|
|
|
(in years) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding as of December 31, 2016 |
|
2,251,518 |
|
$ |
12.43 |
|
7.78 |
|
$ |
2,261 |
|
Granted |
|
540,900 |
|
|
7.77 |
|
|
|
|
|
|
Exercised |
|
(77,614) |
|
|
3.14 |
|
|
|
|
|
|
Forfeited |
|
(36,295) |
|
|
10.77 |
|
|
|
|
|
|
Expired |
|
(121,366) |
|
|
18.75 |
|
|
|
|
|
|
Outstanding as of December 31, 2017 |
|
2,557,143 |
|
$ |
11.45 |
|
7.41 |
|
$ |
794 |
|
Options vested and expected to vest as of December 31, 2017 |
|
2,557,143 |
|
$ |
11.45 |
|
7.41 |
|
$ |
794 |
|
Options exercisable as of December 31, 2017 |
|
1,484,512 |
|
$ |
14.10 |
|
6.60 |
|
$ |
298 |
For the years ended December 31, 2017, 2016 and 2015, the weighted average grant date fair value of stock options granted was $5.13, $2.99 and $10.09, respectively. For the years ended December 31, 2017, 2016 and 2015, the total intrinsic value of options exercised was $185, $38 and $267, respectively. For the years ended December 31, 2017, 2016 and 2015, the total fair value of awards vested during the period was $4,424, $5,380 and $5,660, respectively. The Company received cash proceeds of $244, $129 and $287 from the exercise of stock options for the years ended December 31, 2017, 2016 and 2015, respectively.
Restricted Common Stock
The Company’s 2013 Plan provides for the award of restricted common stock. The Company has granted restricted common stock typically with time-based vesting conditions, having terms of between several months and three years. The awards granted in 2015 to executives and non-executives typically vest in three annual installments of 33.3% each year for three years. In 2016, the vesting conditions for executive awards changed so that the awards vest in 12 quarterly installments of 8.33% per quarter for three years. The awards granted in 2017 to executives and non-executives had the same vesting conditions as the awards granted in 2016, respectively for executives and non-executives. Awards granted to consultants typically vest in accordance with the expected term length of the consulting arrangement. Unvested shares of restricted common stock may not be sold or transferred by the holder. These restrictions lapse according to the time-based vesting.
Activity related to restricted stock for the year ended December 31, 2017, was as follows:
|
|
|||||
|
|
Weighted |
||||
|
|
Average Grant |
||||
|
|
Shares |
Date Fair Value |
|||
|
Unvested restricted common stock as of December 31, 2016 |
461,463 |
$ |
8.30 | ||
|
Issued |
339,700 | 7.85 | |||
|
Vested |
(293,978) | 9.01 | |||
|
Forfeited |
(15,324) | 7.63 | |||
|
Unvested restricted common stock as of December 31, 2017 |
491,861 |
$ |
7.59 | ||
For the years ended December 31, 2017, 2016 and 2015, the weighted average grant date fair value of restricted common stock granted was $7.85, $3.95 and $17.14, respectively. For the years ended December 31, 2017, 2016 and 2015, the total fair value of restricted common stock vested was $2,065, $1,559 and $1,893, respectively. The Company received no proceeds for any of the restricted common stock granted during the years ended December 31, 2017, 2016 and 2015.
Stock-Based Compensation
The fair value of each stock option award is estimated using the Black-Scholes option-pricing model. The expected volatility of the Company’s common stock is estimated based on historical volatility of the Company’s common stock. The expected term of the Company’s stock options has been determined utilizing the “simplified” method as the Company has insufficient historical experience for option grants overall, rendering existing historical experience irrelevant to expectations for current grants. The risk-free interest rate is determined by reference to the United States Treasury yield curve in effect at the time of grant of the award for time periods
F-32
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
approximately equal to the expected term of the award. Expected dividend yield is based on the fact that the Company has never paid cash dividends and does not expect to pay any cash dividends in the foreseeable future.
The relevant data used to determine the value of the stock option grants, presented on a weighted average basis, was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
||||||||||
|
|
|
2017 |
|
2016 |
|
2015 |
||||||
|
Risk-free interest rate |
|
|
1.99 |
% |
|
|
1.52 |
% |
|
|
1.38 |
% |
|
Expected term (in years) |
|
|
6.0 |
|
|
|
6.2 |
|
|
|
6.1 |
|
|
Expected volatility |
|
|
75 |
% |
|
|
77 |
% |
|
|
70 |
% |
|
Expected dividend yield |
|
|
— |
% |
|
|
— |
% |
|
|
— |
% |
Compensation expense related to restricted stock granted to employees and non-employee directors is equal to the fair value of the Company’s common stock on date of grant, multiplied by the number of shares of restricted common stock issued. Compensation expense related to restricted stock granted to non-employees is equal to the excess, if any, of the fair value of the Company’s common stock on date of vesting over the original purchase price per share, multiplied by the number of shares of restricted common stock vesting.
Upon adoption of ASU 2016-09 (Compensation – Stock Compensation) on January 1, 2017, the Company elected to change its accounting policy to account for forfeitures as they occur. The change was applied on a modified retrospective basis with a cumulative-effect adjustment to accumulated deficit of $213 (which increased the accumulated deficit) as of January 1, 2017. Prior to adoption of this guidance the Company estimated forfeitures.
The Company recorded stock-based compensation expense related to stock options and restricted stock as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|||||||
|
|
|
2017 |
|
2016 |
|
2015 |
|||
|
Research and development |
|
$ |
916 |
|
$ |
1,069 |
|
$ |
1,646 |
|
Cost of product sales and inventories |
|
|
153 |
|
|
116 |
|
|
118 |
|
Selling, general and administrative |
|
|
6,049 |
|
|
7,291 |
|
|
6,828 |
|
|
|
$ |
7,118 |
|
$ |
8,476 |
|
$ |
8,592 |
As of December 31, 2017, the Company had an aggregate of $4,546 and $2,375 of unrecognized stock-based compensation expense for options outstanding and restricted stock awards, respectively, which is expected to be recognized over 2.29 years and 1.77 years, respectively.
15. Net Loss Per Share
Basic and diluted net loss per share was calculated as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
Year Ended December 31, |
|||||||
|
|
|
2017 |
|
2016 |
|
2015 |
|||
|
Numerator: |
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(47,510) |
|
$ |
(33,575) |
|
$ |
(84,054) |
|
Denominator: |
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding, basic and diluted |
|
|
40,494,301 |
|
|
35,273,228 |
|
|
34,355,525 |
|
Net loss per share, basic and diluted |
|
$ |
(1.17) |
|
$ |
(0.95) |
|
$ |
(2.45) |
Stock options for the purchase of 2,614,537, 2,317,449 and 1,814,689 shares of common stock were excluded from the computation of diluted net loss per share for the years ended December 31, 2017, 2016 and 2015, respectively, because those options had an anti-dilutive impact due to the net loss incurred for the period.
F-33
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
16. Commitments and Contingencies
Operating Leases
Future minimum lease payments for operating leases as of December 31, 2017, were as follows:
|
|
|
|
|
|
|
|
|
|
|
Year Ending December 31, |
|
|
|
|
2018 |
|
$ |
444 |
|
2019 |
|
|
441 |
|
2020 |
|
|
450 |
|
2021 |
|
|
75 |
|
2022 |
|
|
— |
|
Thereafter |
|
|
— |
|
Total |
|
$ |
1,410 |
The Company leases facilities and certain operating equipment under operating leases expiring through 2021. The Company incurred rent expense of $725, $726 and $678 for the years ended December 31, 2017, 2016 and 2015, respectively.
Litigation
From time to time, the Company may become subject to legal proceedings, claims and litigation arising in the ordinary course of business, including those related to patents, product liability and government investigations. Except as described below, the Company is not presently a party to any litigation which it believes to be material, and is not aware of any pending or threatened litigation against the Company which it believes could have a material effect on its financial statements. The Company accrues contingent liabilities when it is probable that a future liability has been incurred and such liability can be reasonably estimated.
In February 2017, two purported class action lawsuits were filed in the United States District Court for the Southern District of New York against the Company and two of its current officers. Those cases have been consolidated into one purported class action lawsuit under the caption, In re Aratana Therapeutics, Inc. Securities Litigation, Case No. 1:17-cv-00880. The consolidated lawsuit, which was amended in August 2017, asserts claims under Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended, and is premised on allegedly false and/or misleading statements, and alleged non-disclosure of material facts, regarding the Company’s business, operations, prospects and performance during the proposed class period of March 16, 2015 to March 13, 2017. The Company is vigorously defending all claims asserted, including by filing a motion to dismiss. Given the early stage of the litigation, at this time a loss is not probable or reasonably estimable.
The Company currently is not a party to any threatened or pending litigation related to intellectual property. However, third parties might allege that the Company or its licensors are infringing their patent rights or that the Company is otherwise violating their intellectual property rights. Such third parties may resort to litigation against the Company or its licensors, which the Company has agreed to indemnify. With respect to some of these patents, the Company expects that it will be required to obtain licenses and could be required to pay license fees or royalties, or both. These licenses may not be available on acceptable terms, or at all. A costly license, or inability to obtain a necessary license, could have a material adverse effect on the Company’s financial condition, results of operations or cash flows.
Indemnification Agreements
In the ordinary course of business, the Company may provide indemnifications of varying scope and terms to customers, vendors, lessors, business partners, and other parties with respect to certain matters including, but not limited to, losses arising out of breach of such agreements, from services to be provided by the Company, or from intellectual property infringement claims made by third parties. In addition, the Company has entered into indemnification agreements with certain of its officers and members of its Board of Directors that will require the Company, among other things, to indemnify them against certain liabilities that may arise by reason of their status or service as directors or officers. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is, in many cases, not readily quantifiable. To date, the Company has not incurred any material costs as a result of such indemnifications. The Company does not believe that the outcome of any claims under indemnification arrangements will have a material effect on its financial position, results of operations or cash flows, and it has not accrued any liabilities related to such obligations in its consolidated financial statements as of December 31, 2017 or 2016.
F-34
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
17. Income Taxes
The components of loss from continuing operations before income taxes benefit were as follows:
|
|
|||||||||
|
|
Year Ended December 31, |
||||||||
|
|
2017 |
2016 |
2015 |
||||||
|
United States |
$ |
(38,920) |
$ |
(29,959) |
$ |
(65,481) | |||
|
Non-United States |
(8,590) | (3,616) | (20,271) | ||||||
|
Loss from continuing operations |
$ |
(47,510) |
$ |
(33,575) |
$ |
(85,752) | |||
The components of the income tax benefit were as follows:
|
|
|||||||||
|
|
Year Ended December 31, |
||||||||
|
|
2017 |
2016 |
2015 |
||||||
|
Current: |
|||||||||
|
Federal |
$ |
— |
$ |
— |
$ |
— |
|||
|
State |
— |
— |
— |
||||||
|
Deferred: |
|||||||||
|
Federal |
— |
— |
— |
||||||
|
State |
— |
— |
— |
||||||
|
Foreign |
— |
— |
1,698 | ||||||
|
Total |
$ |
— |
$ |
— |
$ |
1,698 | |||
A reconciliation of the United States federal statutory income tax rate to the Company’s effective income tax rate was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|||||||
|
|
|
|
2017 |
|
2016 |
|
2015 |
|||
|
Federal statutory income tax rate |
|
|
34.0 |
% |
|
34.0 |
% |
|
34.0 |
% |
|
State income taxes, net of federal tax benefit |
|
|
6.9 |
|
|
3.2 |
|
|
2.5 |
|
|
Non-deductible expenses |
|
|
(1.9) |
|
|
(1.3) |
|
|
(1.1) |
|
|
Research credits |
|
|
1.3 |
|
|
5.0 |
|
|
0.4 |
|
|
The United States Tax Cuts and Jobs Act of 2017 (“TCJA”) |
|
|
(42.8) |
|
|
— |
|
|
— |
|
|
Other |
|
|
(5.0) |
|
|
— |
|
|
— |
|
|
Change in valuation allowance |
|
|
7.5 |
|
|
(40.9) |
|
|
(33.8) |
|
|
Total |
|
|
— |
% |
|
— |
% |
|
2.0 |
% |
Net deferred tax assets consisted of the following:
|
|
|||||||||
|
|
Year Ended December 31, |
||||||||
|
|
2017 |
2016 |
2015 |
||||||
|
Net operating loss carry forwards |
$ |
31,061 |
$ |
27,244 |
$ |
26,670 | |||
|
Capitalized start-up costs |
5,052 | 5,990 | 6,645 | ||||||
|
Tax credit carry forwards |
3,737 | 2,996 | 1,308 | ||||||
|
Intangibles, net |
2,915 | 2,072 |
— |
||||||
|
Capitalized research and development, net |
6,083 | 10,005 | 11,911 | ||||||
|
Other temporary differences |
5,922 | 7,940 | 3,451 | ||||||
|
Total deferred tax assets |
54,770 | 56,247 | 49,985 | ||||||
|
Valuation allowance |
(54,636) | (56,116) | (46,885) | ||||||
|
Net deferred tax assets |
134 | 131 | 3,100 | ||||||
|
Intangibles, net |
— |
— |
(3,041) | ||||||
|
Depreciation |
(134) | (131) | (59) | ||||||
|
Total deferred tax liabilities |
(134) | (131) | (3,100) | ||||||
|
Net deferred tax liability |
$ |
— |
$ |
— |
$ |
— |
|||
F-35
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
As of December 31, 2017, the Company had net operating loss carryforwards for federal and state income tax purposes of $94,042 and $90,680, respectively, which begin to expire in fiscal year 2031 and 2020, respectively.
As of December 31, 2017, the Company had federal and state research and development tax credit carryforwards of $2,901 and $1,058, respectively, which begin to expire in fiscal year 2031 and until utilized, respectively. The Company has approximately $26,589 of foreign net operating loss carryforwards, which may be carried forward indefinitely.
Management of the Company has evaluated the positive and negative evidence bearing upon the realizability of its deferred tax assets, which are comprised principally of net operating loss carryforwards and research and development credits. Under the applicable accounting standards, management has considered the Company’s history of losses and concluded that it is more likely than not that the Company will not recognize the benefits of its deferred tax assets. Accordingly, a full valuation allowance of the net deferred tax asset had been established at December 31, 2017 and 2016.
Utilization of the net operating loss and research and development credit carryforwards may be subject to a substantial annual limitation under Section 382 of the Internal Revenue Code of 1986 due to ownership change limitations that have occurred previously or that could occur in the future. These ownership changes may limit the amount of net operating loss and research and development credit carryforwards that can be utilized annually to offset future taxable income and tax, respectively.
Changes in the valuation allowance for deferred tax assets during the years ended December 31, 2017, 2016 and 2015, were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|||||||
|
|
|
2017 |
|
2016 |
|
2015 |
|||
|
Valuation allowance as of beginning of year |
|
$ |
56,116 |
|
$ |
46,885 |
|
$ |
14,747 |
|
Changes due to operations, TCJA and other tax rates |
|
|
(1,480) |
|
|
9,231 |
|
|
32,138 |
|
Valuation allowance as of end of year |
|
$ |
54,636 |
|
$ |
56,116 |
|
$ |
46,885 |
The Company has not recorded any amounts for unrecognized tax benefits as of December 31, 2017 and 2016. The Company files tax returns as prescribed by the tax laws of the jurisdictions in which it operates. The Company’s major taxing jurisdictions include the United States (federal and states) and Belgium. In the normal course of business, the Company is subject to examination by federal, state and foreign jurisdictions, where applicable. The Company’s tax years are still open under statute from 2014 to the present. The Company’s policy is to record interest and penalties related to income taxes as part of its income tax expense in the consolidated statements of operations.
The TCJA was enacted on December 22, 2017, a tax reform bill which, amount other items, reduces the current corporate federal tax rate to 21% from 35%. The rate reduction is effective January 1, 2018. ASC Topic 740, Accounting for Income Taxes (“ASC 740”), requires companies to recognize the effect of tax law changes in the period of enactment even though the effective date for most provisions is for tax years beginning after December 31, 2017, or in the case of certain other provisions of the law, January 1, 2018. Accordingly, the Company remeasured its United States deferred tax assets and liabilities as of December 31, 2017, to reflect the reduced rate that is expected to apply in future periods when these deferred taxes will reverse, resulting in an estimated reduction of the Company’s net deferred tax assets by approximately $20.3 million, which was offset by a corresponding change in the valuation allowance. The TCJA includes numerous provisions, such as limitation of deduction for executive compensation, that could impact the Company’s United States deferred tax assets, which are subject to a full valuation allowance.
The SEC issued Staff Accounting Bulletin No. 118 (“SAB 118”) on December 22, 2017. SAB 118 measurement period from a registrant’s reporting period that includes the TCJA’s enactment date to allow the registrant sufficient time to obtain, prepare and analyze information to complete the accounting required under ASC 740. Although the Company made a reasonable estimate of the gross amounts of the deferred tax assets disclosed, a final determination of the TCJA’s impact on the deferred tax assets and related valuation allowance requirements remains incomplete pending a full analysis of the provisions of the TCJA and their interpretations. The ultimate impact of the TCJA on the Company’s reported results in 2018 and beyond may differ from the estimates provided therein, possibly materially, due to, among other things, changes in interpretations and assumptions the Company has made, guidance that may be issues, and other actions the Company may take as a result of the TCJA, different from what presently contemplated.
F-36
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
18. Related Party Transactions
MPM Asset Management, LLC
The Company subleased office space (Heartland House in Kansas City, Kansas) and received office related services from MPM Asset Management, LLC, formerly an affiliate of two of the Company’s principal stockholders. This sublease ended on December 31, 2015. Rent paid in the year ended December 31, 2015, was $50.
MPM Heartland House, LLC
The Company leased its former corporate headquarters office space in Kansas City, Kansas from MPM Heartland House, LLC, a company in which the current Chief Executive Officer and President of the Company, also a director of the Company, is the principal owner. The most recent lease period was from May 1, 2013 to December 31, 2015. Rent paid in the year ended December 31, 2015, was $131. The Company believes the terms of the lease agreement with MPM Heartland House were no less favorable than those that the Company could have obtained from an unaffiliated third party. Also, the Company had a services agreement with MPM Heartland House, LLC which included the lease of the furniture, janitorial and other services to care for the property. Service charges were $33 for each of the year ended December 31, 2015.
Indemnification Agreements
The Company has entered into indemnification agreements with each of its directors and executive officers. These agreements, among other things, require the Company or will require the Company to indemnify each director (and in certain cases their related venture capital funds) and executive officer to the fullest extent permitted by Delaware law, including indemnification of expenses such as attorneys’ fees, judgments, fines and settlement amounts incurred by the director or executive officer in any action or proceeding, including any action or proceeding by or in right of the Company, arising out of the person’s services as a director or executive officer.
19. Selected Quarterly Financial Data (unaudited)
Selected unaudited quarterly financial data for each of the quarters in the years ended December 31, 2017 and 2016 (in thousands, except share and per share data), was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2017 |
|
||||||||||
|
|
|
First |
|
Second |
|
Third |
|
Fourth |
|
||||
|
|
|
Quarter |
|
Quarter |
|
Quarter |
|
Quarter |
|
||||
|
Net revenues (1) |
|
$ |
3,795 |
|
$ |
5,158 |
|
$ |
6,163 |
(2) |
$ |
10,457 |
(3) |
|
Gross profit |
|
|
701 |
|
|
1,467 |
|
|
2,473 |
|
|
4,545 |
|
|
Net loss |
|
|
(12,612) |
|
|
(10,380) |
|
|
(8,920) |
|
|
(15,598) |
(4) |
|
Weighted average shares outstanding, basic and diluted |
|
|
36,711,601 |
|
|
40,206,042 |
|
|
42,445,553 |
|
|
42,493,514 |
|
|
Net loss per share, basic and diluted |
|
$ |
(0.34) |
|
$ |
(0.26) |
|
$ |
(0.21) |
|
$ |
(0.37) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
__________________
|
(1) |
Net revenues reflect the impact of the product launch of GALLIPRANT which commercial sales began in the first quarter of 2017 and which sales of finished goods in the amount of $15,526 to Elanco ended in the fourth quarter of 2017. |
|
(2) |
Net revenues in the third quarter or 2017 reflect the impact of revenues recognized in the amount of $1,000 of licensing and collaboration revenue related to the assumption of manufacturing responsibility by Elanco as further described in Note 12 to the consolidated financial statements included elsewhere in this 2017 Annual Report. |
|
(3) |
Net revenues in the fourth quarter of 2017 reflect the impact of revenues recognized related to the withdrawal of BLONTRESS from the market and the related derecognition of the remaining balance of the liability of $480 as further described in Note 12 to the consolidated financial statements included elsewhere in this 2017 Annual Report, and the impact of commercial sales of ENTYCE which began in the fourth quarter of 2017. |
|
(4) |
Net loss in the fourth quarter of 2017 reflects the impact of an intangible asset impairment charge of $7,448 related to AT-006 and AT-008 as further described in Note 8 to the consolidated financial statements included elsewhere in this 2017 Annual Report. |
|
|
F-37 |
ARATANA THERAPEUTICS, INC.
Notes to Consolidated Financial Statements
(Amounts in thousands, except share and per share data)
_
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2016 |
|
||||||||||
|
|
|
First |
|
Second |
|
Third |
|
Fourth |
|
||||
|
|
|
Quarter |
|
Quarter |
|
Quarter |
|
Quarter |
|
||||
|
Net revenues |
|
$ |
172 |
|
$ |
38,047 |
(1) |
$ |
40 |
|
$ |
292 |
(4) |
|
Gross profit |
|
|
153 |
|
|
36,306 |
|
|
(246) |
|
|
(801) |
|
|
Net income (loss) |
|
|
(18,067) |
|
|
21,196 |
(2) |
|
(13,371) |
(3) |
|
(23,333) |
(5) |
|
Net income attributable to participating securities |
|
|
— |
|
|
(20) |
|
|
— |
|
|
— |
|
|
Net income (loss) attributable to common stockholders (6) |
|
|
(18,067) |
|
|
21,176 |
|
|
(13,371) |
|
|
(23,333) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding, basic |
|
|
34,653,479 |
|
|
34,762,533 |
|
|
35,092,686 |
|
|
36,571,927 |
|
|
Weighted average shares outstanding, diluted |
|
|
34,653,479 |
|
|
34,938,455 |
|
|
35,092,686 |
|
|
36,571,927 |
|
|
Net income (loss) per share, basic and diluted (6) |
|
$ |
(0.52) |
|
$ |
0.61 |
|
$ |
(0.38) |
|
$ |
(0.64) |
|
_________________
|
(1) |
Net revenues in the second quarter of 2016 include revenues recognized related to the upfront payment from the collaboration agreement for GALLIPRANT as further described in Note 12 to the consolidated financial statements included elsewhere in this 2017 Annual Report. |
|
(2) |
Net income in the second quarter of 2016 reflects the impact of intangible asset impairment charges of $2,780 related to TACTRESS and AT-007 and the impact of inventory valuation losses of $1,553 related to GALLIPRANT and TACTRESS as further described in Notes 5 and 8 to the consolidated financial statements included elsewhere in this 2017 Annual Report. |
|
(3) |
Net loss in the third quarter of 2016 reflects the impact of inventory valuation losses of $2,750 related to ENTYCE and GALLIPRANT as further described in Note 5 to the consolidated financial statements included elsewhere in this 2017 Annual Report. |
|
(4) |
Net revenues in the fourth quarter of 2016 reflect the impact of the product launch of NOCITA which commercial sales began in the fourth quarter of 2016. |
|
(5) |
Net loss in the fourth quarter of 2016 reflects the impact of intangible impairment charge of $5,162 related to BLONTRESS and inventory valuation losses of $2,851 related to ENTYCE and BLONTRESS as further described in Notes 5 and 8 to the consolidated financial statements included elsewhere in this 2017 Annual Report. |
|
(6) |
Net income (loss) attributable to common stockholders and basic and diluted net income (loss) per share are computed consistent with annual per share calculations described in Notes 2 and 15 to the consolidated financial statements included elsewhere in this 2017 Annual Report. |
F-38