Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a13-12528_38k.htm |
| EX-99.1 - EX-99.1 - Innoviva, Inc. | a13-12528_3ex99d1.htm |
| EX-99.2 - EX-99.2 - Innoviva, Inc. | a13-12528_3ex99d2.htm |
| EX-99.3 - EX-99.3 - Innoviva, Inc. | a13-12528_3ex99d3.htm |
Exhibit 99.4
Poster No. 42583
Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25mcg in patients with COPD
Donohue, JF(1), Maleki-Yazdi, MR(2), Kilbride, S (3), Mehta, R(4), Kalberg, C(4), Church, A(4)
(1)University of North Carolina College of Medicine, Chapel Hill, NC, USA; (2)Women’s College Hospital, University of Toronto, Ontario, Canada; (3)GlaxoSmithKline, Stockley Park, Uxbridge, UK; (4)GlaxoSmithKline, Research Triangle Park, NC, USA
INTRODUCTION
· Current guidelines recommend treatment with one or more long-acting bronchodilators for patients with moderate-to-very severe chronic obstructive pulmonary disease (COPD).(1),(2)
· Umeclidinium (UMEC)/vilanterol (VI) is a novel long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA) combination bronchodilator in development for the maintenance treatment of COPD.
OBJECTIVES
· To evaluate the efficacy and safety of once-daily UMEC/VI 62.5/25 mcg compared with its components (UMEC and VI) and placebo in patients with COPD.
METHODS
Study design and treatments
· Multicenter, randomized, double-blind, placebo-controlled, parallel-group study (ClinicalTrials.gov: NCT01313650; protocol number: DB2113373).
· Key eligibility criteria: >40 years of age; clinically established history of COPD; current or former cigarette smokers with >10-pack-year smoking history; post-albuterol forced expiratory volume in 1 second (FEV1)/ forced vital capacity (FVC) <0.7 and predicted FEV1 <70%; and a mMRC dyspnea scale score >2.
· Following a 7–14-day run-in, patients were randomized 3:3:3:2 to 24 weeks treatment with UMEC/VI 62.5/25mcg, UMEC 62.5mcg, VI 25mcg or placebo once-daily via the ELLIPTA™ dry powder inhaler. Concurrent use of inhaled corticosteroids (ICS) and rescue albuterol was allowed.
· All patients were required to provide written informed consent prior to study participation. The study was conducted in accordance with the declaration of Helsinki, Good Clinical Practice guidelines, and IRB approval was obtained.
Endpoints
· Primary efficacy: trough FEV1 on Day 169, defined as the mean of the FEV1 values obtained 23 and 24 hours after dosing on Day 168.
· Additional efficacy included: 0–6h post-dose weighted mean (WM) FEV1; transition dyspnea index (TDI) focal score; St George’s Respiratory Questionnaire (SGRQ) score; rescue albuterol use; and time to first COPD exacerbation.
· Safety: adverse events (AEs); vital signs; 12-lead electrocardiography (ECG) and 24-h Holter ECG; and clinical chemistry and hematology.
· Plasma pharmacokinetics (PK) were analyzed using population PK methodology.
RESULTS
Patient demographics and baseline characteristics
· A total of 2210 patients were enrolled; 1532 were included in the intention-to-treat (ITT) population (i.e., randomized and received at least one dose of study medication).
· Patient demographics and baseline characteristics were similar across treatment groups (Table 1). ICS use was similar across active treatment groups (51–52%) and placebo (49%).
TABLE 1. PATIENT DEMOGRAPHICS AND BASELINE CHARACTERISTICS
|
|
|
|
|
UMEC |
|
VI |
|
UMEC/VI |
|
|
|
Placebo |
|
62.5 |
|
25 |
|
62.5/25 |
|
|
|
N=280 |
|
N=418 |
|
N=421 |
|
N=413 |
|
Age, years |
|
62.2 (9.04) |
|
64.0 (9.16) |
|
62.7 (8.52) |
|
63.1 (8.71) |
|
Sex, n (%) |
|
|
|
|
|
|
|
|
|
Male |
|
195 (70) |
|
298 (71) |
|
285 (68) |
|
305 (74) |
|
Race, n (%) |
|
|
|
|
|
|
|
|
|
White |
|
237 (85) |
|
354 (85) |
|
364 (86) |
|
348 (84) |
|
Patientswith cardiovascular risk factors, n (%)(a) |
|
174 (62) |
|
242 (58) |
|
268 (64) |
|
260 (63) |
|
Post-albuterol % predicted FEV1 |
|
46.7 (12.71) |
|
46.8 (13.39) |
|
48.2 (13.27) |
|
47.8 (13.19) |
|
Post-albuterol FEV1, L |
|
1.355 (0.4629) |
|
1.347 (0.4730) |
|
1.402 (0.5011) |
|
1.425 (0.5426) |
|
Post-albuterol FEV1/FVC |
|
47.082 (11.4695) |
|
46.775 (11.0696) |
|
47.372 (11.4928) |
|
48.011 (11.4189) |
|
Patients reversible to albuterol(b), n (%) |
|
91 (33) |
|
121 (29) |
|
155 (37) |
|
129 (31) |
Values are reported as mean (standard deviation) unless otherwise stated.
(a) Defined as current medical history of angina, myocardial infarction, stroke, diabetes, hypertension, or hyperlipidemia.
(b) Reversible was an increase in FEV1 of >12% and >200 mL following administration of 4 puffs of albuterol.
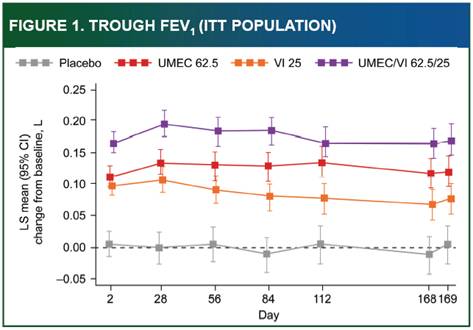
Efficacy: Trough FEV1
· Treatment with UMEC/VI 62.5/25 resulted in statistically significant improvements in trough FEV1 at Day 169 vs. VI, UMEC 62.5 and placebo (p<0.005, Table 2). Comparisons at all other visits were statistically significant, except for UMEC/VI 62.5/25 vs. UMEC 62.5 at Day 112 (Figure 1). All comparisons of UMEC 62.5 and VI vs. placebo were statistically significant (p<0.001).
Efficacy: additional endpoints
· Greater improvements in 0–6h post-dose WM FEV1 were shown for UMEC/VI 62.5/25 vs. VI, UMEC 62.5 and placebo (p<0.001 for all comparisons at all visits, Figure 2 and Table 2). Both UMEC 62.5 and VI consistently improved 0–6h post-dose WM FEV1 vs. placebo (p<0.001, Table 2).
· Greater improvements in TDI focal score, SGRQ score, and rescue albuterol use were shown with UMEC/VI 62.5/25 compared with placebo (Table 2).
· The incidence of COPD exacerbations was lower with UMEC/VI 62.5/25 (7%), UMEC 62.5 (8%), and VI (9%) compared with placebo (13%). Analysis of time to first exacerbation showed that patients on UMEC/VI 62.5/25 had a lower risk of exacerbation vs. placebo (hazard ratio: 0.5; 95% CI: 0.3, 0.8 [p=0.004]; corresponding to a risk reduction of 50%).
TABLE 2: EFFICACY ENDPOINT COMPARISONS
(ITT POPULATION)
|
|
|
UMEC |
|
VI |
|
UMEC/VI |
|
|
|
62.5 |
|
25 |
|
62.5/25 |
|
|
|
(N=418) |
|
(N=421) |
|
(N=413) |
|
Through FEV1 at Day 169, L(a) |
|
|
|
|
|
|
|
Difference vs. placebo |
|
0.115* |
|
0.072* |
|
0.167* |
|
(95% CI) |
|
(0.076, 0.155) |
|
(0.032, 0.112) |
|
(0.128, 0.207) |
|
UMEC/VI 62.5/25 vs. monotherapy |
|
0.052† |
|
0.095* |
|
|
|
(95% CI) |
|
(0.017, 0.087) |
|
(0.060, 0.130) |
|
|
|
0-6h WM FEV1 at Day 168, L(a) |
|
|
|
|
|
|
|
Difference vs. placebo |
|
0.150* |
|
0.122* |
|
0.242* |
|
(95% CI) |
|
(0.110, 0.190) |
|
(0.082, 0.162) |
|
(0.202, 0.282) |
|
UMEC/VI 62.5/25 vs. monotherapy |
|
0.092* |
|
0.120* |
|
|
|
(95% CI) |
|
(0.056, 0.127) |
|
(0.084, 0.155) |
|
|
|
TDI focal score at Day 168(a) |
|
|
|
|
|
|
|
Difference vs. placebo |
|
1.0* |
|
0.9* |
|
1.2* |
|
(95% CI) |
|
(0.5, 1.5) |
|
(0.4, 1.4) |
|
(0.7,1.7) |
|
OR vs. placebo (95% CI) |
|
1.6 (1.2, 2.3)† |
|
1.5 (1.1, 2.1)‡ |
|
2.0 (1.5, 2.8)* |
|
SGRQ score at Day 168 |
|
|
|
|
|
|
|
Difference vs. placebo |
|
-4.69* |
|
-5.19* |
|
-5.51* |
|
(95% CI) |
|
(-7.07, -2.31) |
|
(-7.58, -2.80) |
|
(-7.88, -3.13) |
|
OR vs. placebo (95% CI) |
|
1.6† (1.2, 2.3) |
|
1.9* (1.3, 2.6) |
|
2.0* (1.4, 2.8) |
|
Rescue albuterol use at weeks 1-24, puffs/day |
|
|
|
|
|
|
|
Difference vs. placebo (95% CI) |
|
-0.3 (-0.8, 0.2) |
|
-0.9* (-1.4, -0.4) |
|
-0.8* (-1.3,-0.3) |
(a) Values are differences in least squares mean (95% CI); OR, odds ratio (based on proportion of responders according to outcome measure).
* p<0.001 vs placebo,
† p<0.005 vs placebo,
‡ p<0.05 vs monotherapy. To account for multiplicity across treatment comparisons and endpoints, a step-down closed testing procedure was used.
Safety and pharmacokinetics
· Headache and nasopharyngitis were the most common AEs reported (Table 3). The incidence of dry mouth was low; reported for <1% of patients with UMEC 62.5, VI, and placebo and none treated with UMEC/VI 62.5/25.
· The incidence of serious AEs (SAEs) was similar across treatment groups (3–6%). The most common SAE was COPD.
· Nine deaths were reported (sudden death, COPD exacerbation, and COPD exacerbation/renal failure in VI group; COPD/acute respiratory failure, sudden death, cholecystitis/peritonitis in UMEC 62.5 group; COPD exacerbation/respiratory failure, myocardial infarction, and ‘death: undefined cause’ in UMEC/VI 62.5/25 group).
· No clinically meaningful treatment-related changes in vital signs, ECG, or clinical laboratory parameters were observed for active treatments compared with placebo.
· There were no differences in the systemic exposure of UMEC 62.5 or VI when administered in combination or as monotherapy. In addition, patient demographics did not influence PK parameters of either compound.
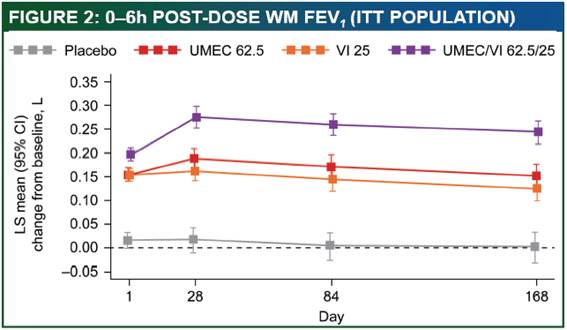
TABLE 3: OVERVIEW OF ADVERSE EVENTS
(ITT POPULATION)
|
|
|
|
|
UMEC |
|
VI |
|
UMEC/VI |
|
|
|
Placebo |
|
62.5 |
|
25 |
|
62.5/25 |
|
|
|
N=280 |
|
N=418 |
|
N=421 |
|
N=413 |
|
Any on-treatment AEs, n (%) |
|
130 (46) |
|
216 (52) |
|
204 (48) |
|
212 (51) |
|
AEs reported by >3% patients, n (%) |
|
|
|
|
|
|
|
|
|
Headache |
|
26 (9) |
|
32 (8) |
|
25 (6) |
|
35 (8) |
|
Nasopharyngitis |
|
16 (6) |
|
29 (7) |
|
26 (6) |
|
39 (9) |
|
Upper respiratory tract infection |
|
14 (5) |
|
21 (5) |
|
18 (4) |
|
13 (3) |
|
Cough |
|
7 (3) |
|
16 (4) |
|
15 (4) |
|
6 (1) |
|
Oropharyngeal pain |
|
4 (1) |
|
6 (1) |
|
14 (3) |
|
13 (3) |
|
Back pain |
|
7 (3) |
|
8 (2) |
|
7 (2) |
|
13 (3) |
|
Chronic obstructive pulmonary disease |
|
3 (1) |
|
12 (3) |
|
8 (2) |
|
7 (2) |
|
Arthralgia |
|
3 (1) |
|
12 (3) |
|
2 (<1) |
|
4 (<1) |
CONCLUSIONS
· Once-daily dosing with UMEC/VI 62.5/25 improved lung function compared with the UMEC and VI monotherapies and placebo in patients with COPD. Other efficacy assessments supported the efficacy of UMEC/VI 62.5/25.
· Safety and tolerability profiles of UMEC/VI 62.5/25 were similar to the monotherapies and placebo.
· This study supports the use of UMEC/VI 62.5/25 as a long-term maintenance treatment for COPD.
REFERENCES
(1) GOLD 2013. Available at: http://www.Goldcopd.org/ Last accessed March 2013.
(2) Celli BR, Macnee W. Eur Respir J 2004; 23:932–946.
ACKNOWLEDGEMENTS
· JFD and MRM-Y have been consultants for and received research grants from GlaxoSmithKline. SK, CK and AC are employees of GlaxoSmithKline and hold stocks/shares in GlaxoSmithKline.
· This study was sponsored by GlaxoSmithKline (ClinicalTrials.gov: NCT01313650; protocol number: DB2113373).
· Editorial support was provided by David Griffiths, PhD (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing), from Fishawack Scientific Communications Ltd, funded by GlaxoSmithKline.

Presented at the Annual Congress of the American Thoracic Society (ATS), Philadelphia, PA, USA, May 17–22, 2013
![]()
