Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a13-12528_38k.htm |
| EX-99.4 - EX-99.4 - Innoviva, Inc. | a13-12528_3ex99d4.htm |
| EX-99.2 - EX-99.2 - Innoviva, Inc. | a13-12528_3ex99d2.htm |
| EX-99.3 - EX-99.3 - Innoviva, Inc. | a13-12528_3ex99d3.htm |
Exhibit 99.1
POSTER NO. G16
Effect of fluticasone furoate (FF)/vilanterol (VI) compared with VI on COPD exacerbations: a pre-specified subgroup analysis
Dransfield MT(1), Calverley PMA(2), Bourbeau J(3), Jones P(4), Hanania NA(5), Mahler DA(6), Vestbo J(7), Wachtel A(8), Martinez F(9), Barnhart F(10), Midwinter DA(11), Lettis S(11), Crim C(10)
(1)University of Alabama at Birmingham, Birmingham, AL, USA; (2)University of Liverpool, Liverpool, UK; (3)McGill University, Montreal, Canada; (4)St George’s University of London, London, UK; (5)Baylor College of Medicine, Houston, TX, USA; (6)Dartmouth Medical School, Hanover, NH, USA; (7)Manchester Academic Health Sciences Centre, Manchester, UK; (8)UCLA School of Medicine, Los Angeles, CA, USA; (9)University of Michigan, Ann Arbor, MI, USA; (10)Respiratory Medicine Development Center, GlaxoSmithKline, RTP, NC, USA; (11)Quantitative Sciences Division, GlaxoSmithKline, Uxbridge, UK
INTRODUCTION
· FF/VI is a novel, once-daily ICS/LABA combination therapy for treatment of patients with COPD, which significantly reduces the annual rate of moderate/severe COPD exacerbations compared with VI alone in COPD patients.(1)
OBJECTIVE
· This analysis compares the effect of FF/VI vs. VI on the annual rate of moderate/severe exacerbations of COPD in seven pre-specified subgroups.
METHODS
· Data from two phase III, multi-center, randomized, double-blind, parallel-group studies identical in design, conduct and analysis, were pooled.
· Patients: post-bronchodilator FEV1 <70%; FEV1/FVC ratio <70%; smoking history >10 pack-years; >1 documented COPD exacerbation (requiring corticosteroids, antibiotics, or hospitalization) in the year prior to screening.
· Patients were randomized to receive one of the following once-daily treatments via ELLIPTATM dry powder inhaler for 52 weeks: FF/VI 50/25mcg, FF/VI 100/25mcg, FF/VI 200/25mcg or VI 25mcg.
· The primary endpoint was the annual rate of moderate/severe COPD exacerbations for FF/VI vs. VI
· moderate exacerbation: worsening symptoms of COPD requiring treatment with oral corticosteroids and/or antibiotics
· severe exacerbation: worsening symptoms of COPD requiring treatment with inpatient hospitalization.
· Pooled study data for FF/VI vs. VI were evaluated for the following subgroups:
· gender
· age
· reversibility (>12% and 200mL FEV1 post-albuterol/salbutamol)
· smoking status
· cardiovascular (CV) history/risk (current or past medical history of >1 of arrhythmia, cerebrovascular accident, congestive heart failure, coronary artery disease, diabetes mellitus, hypercholesterolemia, hypertension, myocardial infarction)
· geographic region
· race.
Table 1. Pooled patient demographics and screening characteristics (ITT population)
|
|
|
FF/VI |
|
FF/VI |
|
FF/VI |
|
VI |
|
|
|
|
50/25mcg |
|
100/25mcg |
|
200/25mcg |
|
25mcg |
|
|
|
|
(N=820) |
|
(N=806) |
|
(N=811) |
|
(N=818) |
|
|
Age, years |
|
63.6 (9.31) |
|
63.8 (9.17) |
|
63.6 (9.07) |
|
63.6 (9.36) |
|
|
Female sex, n (%) |
|
344 (42) |
|
353 (44) |
|
344 (42) |
|
344 (42) |
|
|
Current smoker, n (%) |
|
364 (44) |
|
359 (45) |
|
352 (43) |
|
364 (44) |
|
|
Smoking history, pack-years |
|
46.2 (26.7) |
|
46.6 (27.5) |
|
46.3 (29.5) |
|
45.7 (27.2) |
|
|
Post-bronchodilator FEV1, L |
|
1.29 (0.48) |
|
1.30 (0.48) |
|
1.27 (0.45) |
|
1.28 (0.46) |
|
|
% predicted post-bronchodilator FEV1 |
|
45.4 (13.6) |
|
46.0 (13.4) |
|
45.2 (13.4) |
|
45.2 (13.0) |
|
|
>1 exacerbation requiring oral/systemic corticosteroids and/or antibiotics, not requiring hospitalization, n (%) |
|
764 (93.2) |
|
744 (92.3) |
|
742 (91.5) |
|
755 (92.3) |
|
|
>1 exacerbation requiring hospitalization, n (%) |
|
173 (21.1) |
|
169 (21.0) |
|
174 (21.5) |
|
146 (17.8) |
|
Values are mean (SD) unless otherwise stated
RESULTS
Study population and demographics
· 3255 patients were randomized and received at least one dose of study medication (ITT population); 2406 patients completed the studies.
· Demographics and screening characteristics were well matched between the different treatment arms (Table 1).
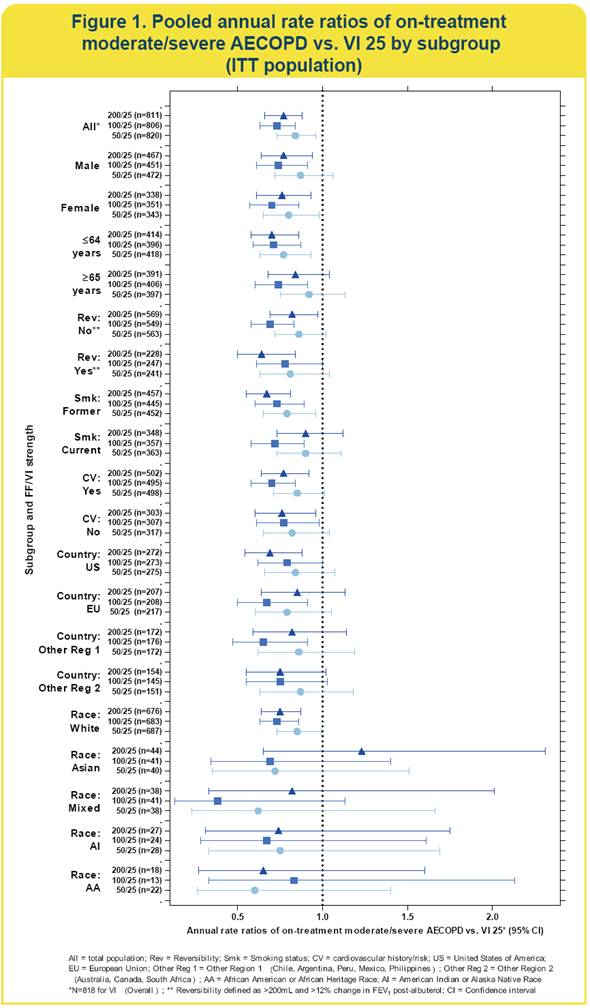
Efficacy: subgroup analysis (Figure 1)
· All three strengths of FF/VI reduced moderate/severe acute exacerbation of COPD (AECOPD) rates vs. VI in all subgroups, with the exception of Asians receiving 200/25mcg (n=44)
· because of the small sample in the non-White race subgroups, the confidence intervals for these treatment comparisons were very wide.
· In several clinically relevant subgroups (gender, smoking status, reversibility and CV history/risk), 100/25 and 200/25 strengths of FF/VI provided significant reduction in moderate/severe AECOPD vs. VI alone, except for current smokers (200/25) and reversible patients (100/25).
· A similar trend was observed for the remaining subgroups, although small populations within some of these subgroups limit interpretation of data.
Safety: subgroup analysis
· On-treatment AEs deemed to be drug related were reported in 21%, 17%, 17% and 14% of 50/25, 100/25, 200/25 and VI patients, respectively
· the AE profile did not markedly differ between the overall population and any specific subgroup (data not shown).
· Pneumonia rates were 27/818 (3%) in patients in the VI group, and 48/820 (6%), 51/806 (6%) and 55/811 (7%), respectively, in the FF/VI 50/25, 100/25 and 200/25 groups.(1)
· All subgroups, with the exception of EU patients, displayed a greater frequency of pneumonia in FF/VI treatment groups compared with VI alone.
· The greatest difference in pneumonia rates between VI and FF/VI patients were observed in the no CV history/risk subgroup: 7/315 (2%) in the VI group; 23/319 (7%), 22/307 (7%) and 23/305 (8%) in the FF/VI 50/25, 100/25 and 200/25 groups, respectively.
· The greatest frequency of pneumonia was observed in the Asian subgroup: 3/42 (7%) in the VI group; 5/40 (13%), 6/42 (14%) and 8/44 (18%) in the 50/25, 100/25 and 200/25 groups, respectively. The majority of patients in the Asian subgroup were of Japanese/East Asian origin.
CONCLUSIONS
· FF/VI once daily reduces the annual rate of moderate/severe exacerbations compared with VI alone in all seven pre-defined subgroups.
· Consistent with previous reports of an association of ICS use with an increased risk of pneumonia,(2) FF/VI treatment was associated with a higher incidence of pneumonia compared with VI alone, a trend that was more pronounced in certain subgroups.
REFERENCES
(1) Dransfield MT, et al. Lancet Respir Med 2013; 1:210–223.
(2) Crim C, et al. Eur Respir J 2009;34:641–7.
ACKNOWLEDGMENTS
· The presenting author, Mark Dransfield, declares the following real or perceived conflicts of interest during the last 3 years in relation to this presentation: has served as a consultant for Boehringer Ingelheim (BI), GlaxoSmithKline (GSK) and Ikaria. He has received grant funding from the NHLBI for COPD-related research and contracted research funding from Aeris, AstraZeneca, BI, Boston Scientific, Centocor, Forrest, GSK, Ikaria, MedImmune, Otsuka and Pfizer.
· These studies were funded by GlaxoSmithKline (GSK study codes HZC102871 & HZC102970, Clinicaltrials.gov NCT01009463 & NCT01017952).
· Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Vikas Sharma, PhD at Gardiner-Caldwell Communications (Macclesfield, UK) and was funded by GlaxoSmithKline.
ELLIPTA™ is a trade mark of GlaxoSmithKline

Presented at the American Thoracic Society Annual Congress, Philadelphia, PA, USA, 17—22 May 2013

