Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - Innoviva, Inc. | Financial_Report.xls |
| EX-32 - EX-32 - Innoviva, Inc. | a2223147zex-32.htm |
| EX-23.1 - EX-23.1 - Innoviva, Inc. | a2223147zex-23_1.htm |
| EX-21.1 - EX-21.1 - Innoviva, Inc. | a2223147zex-21_1.htm |
| EX-31.2 - EX-31.2 - Innoviva, Inc. | a2223147zex-31_2.htm |
| EX-10.63 - EX-10.63 - Innoviva, Inc. | a2223147zex-10_63.htm |
| EX-31.1 - EX-31.1 - Innoviva, Inc. | a2223147zex-31_1.htm |
Use these links to rapidly review the document
Table of Contents
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
ITEM 9B. OTHER INFORMATION
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| (Mark One) | ||
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the fiscal year ended December 31, 2014 |
||
Or |
||
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from to |
||
Commission File No. 000-30319
THERAVANCE, INC.
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
94-3265960 (I.R.S. Employer Identification No.) |
|
951 Gateway Boulevard, South San Francisco, California (Address of principal executive offices) |
94080 (Zip Code) |
Registrant's telephone number, including area code: 650-238-9600
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT:
| Title of Each Class | Name of Each Exchange On Which Registered | |
|---|---|---|
| Common Stock $0.01 Par Value | Nasdaq Global Market |
SECURITIES REGISTERED PURSUANT TO SECTION 12(g) OF THE ACT: NONE
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ý No o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether registrant is a large accelerated filer, an accelerated filer or a non-accelerated filer. See definition of "accelerated filer and large accelerated filer" in Rule 12b-2 of the Exchange Act (Check One):
| Large accelerated filer ý | Accelerated filer o | Non-accelerated filer o (Do not check if a smaller reporting company) |
Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant based upon the closing price of the registrant's Common Stock on The NASDAQ Global Market on June 30, 2014 was $1,411,186,319. Shares of Common Stock held by each executive officer and director and stockholders known by the registrant to own 10% or more of the outstanding stock based on public filings and other information known to the registrant have been excluded since such persons may be deemed affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
On February 12, 2015, there were 116,624,973 shares of the registrant's Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Specified portions of the registrant's definitive Proxy Statement to be issued in conjunction with the registrant's 2015 Annual Meeting of Stockholders, which is expected to be filed not later than 120 days after the registrant's fiscal year ended December 31, 2014, are incorporated by reference into Part III of this Annual Report. Except as expressly incorporated by reference, the registrant's Proxy Statement shall not be deemed to be a part of this Annual Report on Form 10-K.
THERAVANCE, INC.
2014 Form 10-K Annual Report
Table of Contents
2
Special Note Regarding Forward-Looking Statements
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Such forward-looking statements involve substantial risks, uncertainties and assumptions. All statements in this Annual Report on Form 10-K, other than statements of historical facts, including, without limitation, statements regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans, intentions, expectations, goals and objectives may be forward-looking statements. The words "anticipates," "believes," "could," "designed," "estimates," "expects," "goal," "intends," "may," "plans," "projects," "pursuing," "will," "would" and similar expressions (including the negatives thereof) are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions, expectations or objectives disclosed in our forward-looking statements and the assumptions underlying our forward-looking statements may prove incorrect. Therefore, you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions, expectations and objectives disclosed in the forward-looking statements that we make. All written and verbal forward-looking statements attributable to us or any person acting on our behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section.
Important factors that we believe could cause actual results or events to differ materially from our forward-looking statements include, but are not limited to, risks related to: the disruption of operations during the transition period following the Spin-Off, including the diversion of managements' and employees' attention, disruption of relationships with collaborators and increased employee turnover, lower than expected future royalty revenue from respiratory products partnered with GSK, delays or difficulties in commencing or completing clinical studies, the potential that results from clinical or non-clinical studies indicate product candidates are unsafe or ineffective, dependence on third parties to conduct its clinical studies, delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with third parties to discover, develop and commercialize products and risks discussed below in "Risk Factors" in Item 1A of Part I, "Management's Discussion and Analysis of Financial Condition and Results of Operations" in Item 7 of Part II and elsewhere in this Annual Report on Form 10-K. Our forward-looking statements in this Annual Report on Form 10-K are based on current expectations as of the date hereof and we do not assume any obligation to update any forward-looking statements on account of new information, future events or otherwise, except as required by law.
We encourage you to read Management's Discussion and Analysis of our Financial Condition and Results of Operations and our consolidated financial statements contained in this annual report on Form 10-K. We also encourage you to read Item 1A of Part I of this annual report on Form 10-K, entitled "Risk Factors," which contains a more complete discussion of the risks and uncertainties associated with our business. In addition to the risks described above and in Item 1A of this report, other unknown or unpredictable factors also could affect our results. Therefore, the information in this report should be read together with other reports and documents that we file with the Securities and Exchange Commission (SEC) from time to time, including on Form 10-Q and Form 8-K, which may supplement, modify, supersede or update those risk factors. As a result of these factors, we cannot assure you that the forward-looking statements in this report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all.
3
Overview
Theravance, Inc. ("Theravance", the "Company", the "Registrant" or "we" and other similar pronouns) is a royalty management company primarily focused on maximizing the potential value of the respiratory assets partnered with Glaxo Group Limited ("GSK"), including RELVAR®/BREO® ELLIPTA® (fluticasone furoate/ vilanterol, "FF/VI") and ANORO® ELLIPTA® (umeclidinium bromide/ vilanterol, "UMEC/VI"), with the intention of providing capital returns to stockholders. Under the Long-Acting Beta2 Agonist ("LABA") Collaboration Agreement and the Strategic Alliance Agreement with GSK (referred to herein collectively as the "GSK Agreements"), Theravance is eligible to receive the associated royalty revenues from RELVAR®/BREO® ELLIPTA® , ANORO® ELLIPTA® and if approved and commercialized, VI monotherapy. Theravance is also entitled to 15% of any future payments made by GSK under its agreements originally entered into with us, and since assigned to Theravance Respiratory Company, LLC ("TRC"), relating to the combination FF/UMEC/VI and the Bifunctional Muscarinic Antagonist-Beta2 Agonist ("MABA") program. We do not manufacture or sell any of the products commercialized under the GSK Agreements, as it is the exclusive responsibility of GSK.
Our headquarters are located at 951 Gateway Boulevard, South San Francisco, California 94080. Theravance was incorporated in Delaware in November 1996 under the name Advanced Medicine, Inc. and began operations in May 1997. The Company changed its name to Theravance, Inc. in April 2002.
On June 1, 2014, we separated our biopharmaceutical research and drug development operations from our late-stage partnered respiratory assets by transferring our research and drug development operations into our then wholly-owned subsidiary, Theravance Biopharma, Inc. ("Theravance Biopharma"). We contributed $393.0 million of cash, cash equivalents and marketable securities to Theravance Biopharma and all outstanding shares of Theravance Biopharma were then distributed to Theravance stockholders as a pro-rata dividend distribution on June 2, 2014 by issuing one ordinary share of Theravance Biopharma for every 3.5 shares held of our common stock to stockholders of record on May 15, 2014 (the "Spin-Off"). The Spin-Off resulted in Theravance Biopharma operating as an independent publicly-traded company. The results of operations for the former research and drug development operations conducted by us and by Theravance Biopharma until June 1, 2014 are included as part of this report as discontinued operations.
As a royalty management company, we have designed our company structure and organization to be focused on managing our respiratory assets with GSK, the commercial and developmental obligations associated with the GSK Agreements, intellectual property, licensing operations, and providing for certain essential reporting and management functions of a public company. As of December 31, 2014, we had ten employees. Our revenues consist of royalties and potential milestone payments, if any, from our respiratory partnership agreements with GSK.
Our Strategy
Our corporate strategy is focused on stockholder returns by:
- 1.
- Maximizing
the potential value of our respiratory assets partnered with GSK;
- 2.
- Providing
capital returns to our stockholders through dividends or share repurchases;
- 3.
- Reducing
our overall corporate cost of capital; and
- 4.
- Building a long term recurring revenue business.
4
Our Relationship with GSK
LABA Collaboration
In November 2002, we entered into our LABA Collaboration Agreement with GSK to develop and commercialize once-daily LABA products for the treatment of chronic obstructive pulmonary disease ("COPD") and asthma. For the treatment of COPD, the collaboration has developed two combination products: (1) RELVAR®/BREO® ELLIPTA® (FF/VI) (BREO® ELLIPTA® is the proprietary name in the U.S. and Canada and RELVAR® ELLIPTA® is the proprietary name outside the U.S. and Canada), a once-daily combination medicine consisting of a LABA, vilanterol (VI), and an inhaled corticosteroid (ICS), fluticasone furoate (FF) and (2) ANORO® ELLIPTA® (UMEC/VI), a once-daily medicine combining a long-acting muscarinic antagonist ("LAMA"), umeclidinium bromide (UMEC), with a LABA, VI. Under the collaboration agreements between the parties, GSK and Theravance are exploring various paths to create triple therapy medications. For the treatment of asthma, RELVAR® ELLIPTA® is approved in multiple regions outside of North America and the collaboration is further developing FF/VI for the U.S. The FF/VI program is aimed at developing a once-daily combination LABA/ICS to succeed GSK's Advair ® /Seretide™ (salmeterol and fluticasone as a combination) franchise, which had reported 2014 sales of approximately $7.0 billion, and to compete with Symbicort® (formoterol and budesonide as a combination), which had reported 2014 sales of approximately $3.8 billion. ANORO® ELLIPTA®, which is also a combination product, is targeted as an alternative treatment option to Spiriva® (tiotropium), a once-daily, single-mechanism bronchodilator, which had reported 2013 sales of approximately $4.7 billion.
As a result of the launch and approval of RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® in the U.S., Japan and Europe, we were obligated to pay milestone fees to GSK totaling $220.0 million, which we have paid in their entirety as of December 31, 2014. Although we have no further milestone payment obligations to GSK pursuant to the LABA Collaboration Agreement, we continue to have ongoing development and commercialization activities under the GSK Agreements that are expected to continue over the life of the agreements. The milestone fees paid to GSK were recognized as capitalized fees paid to a related party, which are being amortized over their estimated useful lives commencing upon the commercial launch of the product.
We are entitled to receive annual royalties from GSK on sales of RELVAR®/BREO® ELLIPTA® as follows: 15% on the first $3.0 billion of annual global net sales and 5% for all annual global net sales above $3.0 billion. Sales of single-agent LABA medicines and combination medicines would be combined for the purposes of this royalty calculation. For other products combined with a LABA from the LABA collaboration, such as ANORO™ ELLIPTA™, royalties are upward tiering and range from 6.5% to 10%.
2004 Strategic Alliance
In March 2004, we entered into the Strategic Alliance Agreement with GSK where GSK received an option to license exclusive development and commercialization rights to product candidates from certain of pre-Spin-Off our discovery programs on pre-determined terms and on an exclusive, worldwide basis. Upon GSK's decision to license a program, GSK is responsible for funding all future development, manufacturing and commercialization activities for product candidates in that program. In addition, GSK is obligated to use diligent efforts to develop and commercialize product candidates from any program that it licenses. If the program is successfully advanced through development by GSK, we are entitled to receive clinical, regulatory and commercial milestone payments and royalties on any sales of medicines developed from the program. If GSK chooses not to license a program, we retain all rights to the program and may continue the program alone or with a third party. GSK has no further option rights on any of our research or development programs under the strategic alliance.
5
In 2005, GSK licensed our MABA program for the treatment of COPD, and in October 2011, we and GSK expanded the MABA program by adding six additional Theravance-discovered preclinical MABA compounds (the "Additional MABAs"). GSK's development, commercialization, milestone and royalty obligations under the Strategic Alliance Agreement remain the same with respect to GSK961081 ('081), the lead compound in the MABA program. GSK is obligated to use diligent efforts to develop and commercialize at least one MABA within the MABA program, but may terminate progression of any or all Additional MABAs at any time and return them to us, at which point we may develop and commercialize such Additional MABAs alone or with a third party. Both GSK and we have agreed not to conduct any MABA clinical studies outside of the strategic alliance so long as GSK is in possession of the Additional MABAs. If a single-agent MABA medicine containing '081 is successfully developed and commercialized, GSK is required to pay royalties of between 10% and 20% of annual global net sales up to $3.5 billion, and 7.5% for all annual global net sales above $3.5 billion. If a MABA medicine containing '081 is commercialized as a combination product, such as a '081/FF, the royalty rate is 70% of the rate applicable to sales of the single-agent MABA medicine. For single-agent MABA medicines containing an Additional MABA, GSK is required to pay royalties of between 10% and 15% of annual global net sales up to $3.5 billion, and 10% for all annual global net sales above $3.5 billion. For combination products containing an Additional MABA, such as a MABA/ICS combination, the royalty rate is 50% of the rate applicable to sales of the single-agent MABA medicine. If a MABA medicine containing '081 is successfully developed and commercialized in multiple regions of the world, GSK could be required to pay total contingent payments of up to $125.0 million for a single-agent medicine and up to $250.0 million for both a single-agent and a combination medicine. If a MABA medicine containing an Additional MABA is successfully developed and commercialized in multiple regions of the world, GSK could be required to pay total contingent payments of up to $129.0 million. As a result of the transactions effected by the Spin-Off, we are only entitled to receive 15% of any contingent payments and royalties payable by GSK from sales of FF/UMEC/VI (and MABA, and MABA/FF) while Theravance Biopharma receives 85% of those same payments.
Agreements Entered into with GSK in Connection with the Spin-Off
On March 3, 2014, in contemplation of the Spin-Off of Theravance Biopharma, we, Theravance Biopharma and GSK entered into a series of agreements clarifying how the companies would implement the Spin-Off and operate following the Spin-Off. We, Theravance Biopharma and GSK entered into a three-way master agreement providing for GSK's consent to the Spin-Off provided certain conditions were met. In addition, we and GSK also entered into amendments to the GSK Agreements, and Theravance Biopharma and GSK entered into a governance agreement, a registration rights agreement and an extension agreement. The three-way master agreement was effective on June 1, 2014 when we transferred our research and drug development operations to Theravance Biopharma. Pursuant to a three-way master agreement entered into by and among us, Theravance Biopharma and GSK in connection with the Spin-Off, we agreed to sell a certain number of Theravance Biopharma shares withheld from a taxable dividend of Theravance Biopharma shares to GSK. After such Theravance Biopharma shares were sent to the transfer agent, we agreed to purchase the Theravance Biopharma shares from the transfer agent, rather than have them sold on the open market, in order to satisfy tax withholdings. GSK had a right to purchase these shares of Theravance Biopharma from us, but this right expired unexercised. Accordingly, at December 31, 2014, we owned 436,802 ordinary shares of Theravance Biopharma.
The amendments to the GSK Agreements do not change the economics or royalty rates under the GSK Agreements, though the assignment of the Strategic Alliance Agreement and portions of the LABA Collaboration Agreement to TRC do change how the economics are allocated between Theravance Biopharma and us. The amendments to the GSK Agreements do provide that GSK's diligent efforts obligations regarding commercialization matters under both agreements will change
6
upon regulatory approval in either the United States or the European Union (the "EU") of FF/UMEC/VI or a MABA in combination with FF. Upon such regulatory approval, GSK's diligent efforts obligations as to commercialization matters under the GSK Agreements will have the objective of focusing on the best interests of patients and maximizing the net value of the overall portfolio of products under the GSK Agreements. Since GSK's commercialization efforts following such regulatory approval will be guided by a portfolio approach across products in which we will retain our full interests upon the Spin-Off and also products in which we have retained only a portion of our interests following the Spin-Off, GSK's commercialization efforts may have the effect of reducing the overall value of our remaining interests in the GSK Agreements following the Spin-Off.
Purchases of Common Stock by GSK
Prior to 2014, affiliates of GSK purchased an aggregate of 29.9 million shares of our common stock. During 2014, GSK purchased 1.7 million shares of our common stock pursuant to its periodic "top-up" rights under our Amended and Restated Governance Agreement, dated as of June 4, 2004, as amended, among us, GSK and certain GSK affiliates, for an aggregate purchase price of $38.1 million. As of February 12, 2015, GSK beneficially owned approximately 27.1% of our outstanding capital stock.
Product Highlights
- 1.
- In
the fourth quarter 2014, sales for RELVAR®/BREO® ELLIPTA® by GSK were $62.2 million compared to
$25.6 million in the previous quarter, an increase of approximately 142%, resulting in total sales of $110.9 million in 2014.
- 2.
- In
the fourth quarter 2014, sales for ANORO® ELLIPTA® by GSK were $17.4 million compared to $1.8 million in the
previous quarter, a substantial increase resulting in total sales of $27.4 million in 2014.
- 3.
- GSK
announced that as of January 2015, U.S. Medicare Part D coverage has increased to 76 percent for BREO® ELLIPTA® and
to 65 percent for ANORO® ELLIPTA®. In addition, as of January 2015, 64 percent are insured through commercial plans for BREO® ELLIPTA® and
78 percent for ANORO® ELLIPTA®.
- 4.
- A
Phase 3 study evaluating the effectiveness of RELVAR®/BREO® ELLIPTA® compared to other COPD treatments, as
measured by the primary endpoint of the mean annual rate of moderate and severe exacerbations, one of the Salford Lung Studies being conducted, completed enrollment of 2,800 patients.
- 5.
- GSK secured reimbursement for ANORO® ELLIPTA® via the Australian Pharmaceutical Benefits Scheme (PBS) as a long-term once-daily, maintenance bronchodilator treatment to relieve symptoms in adult patients with COPD.
Manufacturing
Manufacturing of RELVAR®/BREO® ELLIPTA® (FF/VI) and ANORO™ ELLIPTA™ (UMEC/VI) and for the MABA program is handled by GSK.
Government Regulation
The development and commercialization of products and product candidates pursuant to the GSK Agreements are subject to extensive regulation by governmental authorities in the United States and other countries. Before marketing in the United States, any medicine must undergo rigorous preclinical studies and clinical studies and an extensive regulatory approval process implemented by the FDA under the Federal Food, Drug, and Cosmetic Act. Outside the United States, the ability to market a product depends upon receiving a marketing authorization from the appropriate regulatory authorities.
7
The requirements governing the conduct of clinical studies, marketing authorization, pricing and reimbursement vary widely from country to country. In any country, the commercialization of medicines is permitted only if the appropriate regulatory authority is satisfied that our collaborative partner has presented adequate evidence of the safety, quality and efficacy of such medicines.
Before commencing clinical studies in humans in the United States, our collaborative partner must submit to the FDA an Investigational New Drug application that includes, among other things, the results of preclinical studies. If the FDA accepts the Investigational New Drug submission, clinical studies are usually conducted in three phases and under FDA oversight. These phases generally include the following:
Phase 1. The product candidate is introduced into healthy human volunteers and is tested for safety, dose tolerance and pharmacokinetics.
Phase 2. The product candidate is introduced into a limited patient population to assess the efficacy of the drug in specific, targeted indications, assess dosage tolerance and optimal dosage, and identify possible adverse effects and safety risks.
Phase 3. If a compound is found to be potentially effective and to have an acceptable safety profile in Phase 2 evaluations, the clinical study will be expanded to further demonstrate clinical efficacy, optimal dosage and safety within an expanded patient population.
The results of product development, preclinical studies and clinical studies must be submitted to the FDA as part of a new drug application (NDA). The NDA also must contain extensive manufacturing information. NDAs for new chemical entities are subject to performance goals defined in the Prescription Drug User Fee Act (PDUFA) which suggests a goal for FDA action within six months of the 60-day filing date for applications that are granted priority review and ten months of the 60-day filing date for applications that receive standard review. For a product candidate no active ingredient of which has been previously approved by the FDA, the FDA must either refer the product candidate to an advisory committee for review or provide in the action letter on the application for the product candidate a summary of the reasons why the product candidate was not referred to an advisory committee prior to approval. In addition, under the 2009 Food and Drug Administration Amendments Act, the FDA has authority to require submission of a formal Risk Evaluation and Management Strategy (REMS) to ensure safe use of the product. At the end of the review period, the FDA communicates an approval of the NDA or issues a complete response listing the application's deficiencies.
Once approved, the FDA may withdraw the product approval if compliance with pre- and post-marketing regulatory standards is not maintained or if safety or quality issues are identified after the product reaches the marketplace. In addition, the FDA may require post-marketing studies, referred to as Phase 4 studies, to monitor the effect of approved products, and may limit further marketing of the product based on the results of these post-marketing studies. The FDA has broad post-market regulatory and enforcement powers, including the ability to suspend or delay issuance of approvals, seize products, withdraw approvals, enjoin violations, and institute criminal prosecution.
If regulatory approval for a medicine is obtained, the clearance to market the product will be limited to those diseases and conditions for which the medicine is effective, as demonstrated through clinical studies and included in the medicine's labeling. Even if this regulatory approval is obtained, a marketed medicine, its manufacturer and its manufacturing facilities are subject to continual review and periodic inspections by the FDA. The FDA ensures the quality of approved medicines by carefully monitoring manufacturers' compliance with its cGMP regulations. The cGMP regulations for drugs contain minimum requirements for the methods, facilities, and controls used in manufacturing, processing, and packaging of a medicine. The regulations are intended to make sure that a medicine is safe for use, and that it has the ingredients and strength it claims to have. Discovery of previously
8
unknown problems with a medicine, manufacturer or facility may result in restrictions on the medicine or manufacturer, including costly recalls or withdrawal of the medicine from the market.
We and our collaborative partner are also subject to various laws and regulations regarding laboratory practices, the experimental use of animals and the use and disposal of hazardous or potentially hazardous substances in connection with the development and commercialization of products and product candidates. In each of these areas, as above, the FDA and other regulatory authorities have broad regulatory and enforcement powers, including the ability to suspend or delay issuance of approvals, seize products, withdraw approvals, enjoin violations, and institute criminal prosecution, any one or more of which could have a material adverse effect upon our business, financial condition and results of operations.
Outside the United States our collaborative partner's ability to market partnered products will also depend on receiving marketing authorizations from the appropriate regulatory authorities. Risks similar to those associated with FDA approval described above exist with the regulatory approval processes in other countries.
Patents and Proprietary Rights
We and our collaborative partner will be able to protect our partnered technology from unauthorized use by third parties only to the extent that such technology is covered by valid and enforceable patents or is effectively maintained as trade secrets. Our success in the future will depend in part on us and our collaborative partner obtaining patent protection for our partnered products and product candidates. Accordingly, patents and other proprietary rights are essential elements of our business.
For proprietary know-how that is not patentable, processes for which patents are difficult to enforce and any other elements of our business that involve proprietary know-how and technology that is not covered by patent applications, we rely on trade secret protection and confidentiality agreements to protect our interests. We require all of our employees, consultants and advisors to enter into confidentiality agreements. Where it is necessary to share our proprietary information or data with outside parties, our policy is to make available only that information and data required to accomplish the desired purpose and only pursuant to a duty of confidentiality on the part of those parties.
As of December 31, 2014, we owned 37 issued United States patents and 192 granted foreign patents, as well as additional pending United States patent applications and foreign patent applications. The claims in these various patents and patent applications are directed to compositions of matter, including claims covering product candidates, lead compounds and key intermediates, pharmaceutical compositions, methods of use and processes for making our compounds.
United States issued patents and foreign patents generally expire 20 years after filing. Nevertheless, issued patents can be challenged, narrowed, invalidated or circumvented, which could limit our ability to stop competitors from marketing similar products and threaten our ability to commercialize our product candidates. Our patent position, similar to other companies in our industry, is generally uncertain and involves complex legal and factual questions. To maintain our proprietary position we will need to obtain effective claims and enforce these claims once granted. It is possible that, before any of our products can be commercialized, any related patent may expire or remain in force only for a short period following commercialization, thereby reducing any advantage of the patent. Also, we do not know whether any of our patent applications will result in any issued patents or, if issued, whether the scope of the issued claims will be sufficient to protect our proprietary position.
9
Competition
We anticipate that any approved product from our LABA collaboration with GSK, including RELVAR®/BREO® ELLIPTA® (FF/VI) and ANORO® ELLIPTA® (UMEC/VI), will compete with a number of approved bronchodilator drugs and drug candidates under development that are designed to treat asthma and COPD. These include but are not limited to:
- •
- Advair®/Seretide™ (salmeterol and fluticasone as a combination) marketed by GSK,
- •
- Foradil®/Oxis® (formoterol) marketed by a number of companies,
- •
- Symbicort® (formoterol and budesonide as a combination) marketed by AstraZeneca,
- •
- Dulera® (formoterol and mometasone as a combination) marketed by Merck,
- •
- Spiriva® (tiotropium) marketed by Boehringer Ingelheim and Pfizer,
- •
- Striverdi® Respimat® (olodaterol) marketed by Boehringer Ingelheim,
- •
- Onbrez®/Arcapta® (indacaterol) marketed by Novartis,
- •
- Tudorza ® (aclidinium) marketed by Forest/Actavis and Seebri® (glycopyrronium) were also launched in 2012
(Seebri, ex-U.S.),
- •
- Incruse® (Umec) and Arnuity® (FF), recently launched in January 2015 by GSK in the U.S. (we are not entitled
to any royalties from either product)
- •
- Indacaterol in combination with an ICS (mometasone), being developed by Novartis for markets outside the U.S.,
- •
- Indacaterol combined with a muscarinic antagonist glycopryyonium bromid (Ultibro®), developed by Novartis for the
treatment of COPD,
- •
- Ultibro®, approved and launched in European in 2013 and currently under regulatory review in the U.S.,
- •
- Tiotropium combined with the long acting beta agonist olodaterol, being developed by Boehringer Ingelheim for the treatment of COPD
and currently under regulatory review in the U.S.,
- •
- AirFluSal® (a branded generic containing salmeterol fluticasone), developed by the Sanoz division of Novartis and approved
in Denmark in late 2013 with further EU approval expected in coming months; and
- •
- Duaklir® Genuair® (aclidinium bromide/formoterol fumarate), developed by AstaZeneca and approved in November 2014 in the EU as a maintenance bronchodilator treatment for COPD.
In addition, several firms are reported to be developing new formulations of salmeterol fluticasone and formoterol budesonide which may be marketed as generics or branded generics relative to the existing products from GSK and AstraZeneca, respectively. All of these efforts represent potential competition for any of our partnered products.
Employees
After giving effect to the Spin-Off, as of December 31, 2014, we had ten employees. None of our employees are represented by a labor union. We consider our employee relations to be good.
10
Available Information
Our Internet address is www.thrxinc.com. Our investor relations website is located at http://investor.thrxinc.com. We make available free of charge on our investor relations website under "SEC Filings" our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, our directors' and officers' Section 16 Reports and any amendments to those reports as soon as reasonably practicable after filing or furnishing such materials to the U.S. Securities and Exchange Commission (SEC). The information found on our website is not part of this or any other report that we file with or furnish to the SEC. Theravance and the Theravance logo are registered trademarks of Theravance, Inc. Trademarks, tradenames or service marks of other companies appearing in this report are the property of their respective owners.
Risks Related to our Business
For the foreseeable future we will derive all of our royalty revenues from GSK and our future success depends on GSK's ability to successfully develop and commercialize the products in the respiratory programs partnered with GSK.
Pursuant to the GSK Agreements, GSK is responsible for the development and commercialization of products in the partnered respiratory programs. Through December 31, 2014, sales of both BREO® ELLIPTA® and ANORO® ELLIPTA® by GSK have been significantly below our expectations which resulted in a decline in our stock price. Although we may receive milestone payments from GSK if certain development milestones are achieved in our MABA program, we believe that royalty revenues from BREO® ELLIPTA® and ANORO® ELLIPTA® will represent the majority of our future revenues from GSK. The amount and timing of revenue from such royalties and milestones is unknown and highly uncertain. Our future success depends upon the performance by GSK of its commercial obligations under the GSK Agreements. We have no control over GSK's marketing and sales efforts, and GSK might not be successful, which would harm our business and cause the price of our securities to fall.
The amount of royalties and milestone payments, if any, we receive will depend on many factors, including the following:
- •
- the competitive landscape for approved products and developing therapies that compete with our partnered products, including other
products owned by GSK (such as Advair®) but which are not partnered with us and pricing pressure in the respiratory markets targeted by our partnered products;
- •
- the ability of patients to be able to afford our partnered products or obtain health care coverage that covers our partnered products;
- •
- acceptance of, and ongoing satisfaction with, our partnered products by the medical community, patients receiving therapy and third
party payors;
- •
- a satisfactory efficacy and safety profile as demonstrated in a broad patient population;
- •
- the size of the market for our partnered products;
- •
- the extent and effectiveness of the sales and marketing and distribution support GSK provides our partnered products;
- •
- safety concerns in the marketplace for respiratory therapies in general and with our partnered products in particular;
11
- •
- regulatory developments relating to the manufacture or continued use of our partnered products;
- •
- decisions as to the timing of product launches, pricing and discounts;
- •
- GSK's ability to expand the indications for which our partnered products can be marketed;
- •
- GSK's ability to successfully achieve development milestones with respect to our partnered MABA program;
- •
- GSK's ability to obtain regulatory approval of our partnered products in additional countries; or
- •
- the unfavorable outcome of any potential litigation relating to our partnered products.
Reductions on pricing and reimbursement from governments, payors, or other healthcare cost containment initiatives such as restrictions on use, may negatively impact royalties generated under the GSK Agreements.
The continuing efforts of governments, pharmaceutical benefit management organizations (PBMs), insurance companies, managed care organizations and other payors of health care costs to contain or reduce costs of health care has adversely affected the price, market access, and total revenues of BREO® ELLIPTA® and ANORO® ELLIPTA® and may continue to adversely affect them in the future.
The Patient Protection and Affordable Care Act and other potential legislative or regulatory action regarding healthcare and insurance matters, along with the trend toward managed healthcare in the U.S., could adversely influence the purchase of healthcare products and reduce demand and prices for our partnered products. This could harm GSK's ability to market our partnered products and significantly reduce future revenues. For example, when GSK launched BREO® ELLIPTA® for the treatment of COPD in the U.S. in October 2013, GSK experienced significant challenges gaining coverage at some of the largest PBMs, healthcare payors, and providers and lower overall prices that expected. Recent actions by U.S. PBMs in particular have increased discount levels for respiratory products resulting in lower net sales pricing realized for products in our collaboration. Further, if the ongoing Phase 3b studies with FF/VI do not show improved outcomes relative to the standard of care, obtaining payor coverage for RELVAR®/BREO® ELLIPTA® could become more difficult in the future. In addition, in certain foreign markets, the pricing of prescription drugs is subject to government control and reimbursement may in some cases be unavailable. We believe that pricing pressures will continue and may increase. This may make it difficult for GSK to sell our partnered products a price acceptable to us or GSK or to generate revenues in-line with our analysts' expectations, which may cause the price of our securities to fall.
If the commercialization of RELVAR®/BREO® ELLIPTA® or ANORO® ELLIPTA® in the countries in which they have received regulatory approval encounters any delays or adverse developments, or perceived delays or adverse developments, or if sales or payor coverage do not meet investor or our expectations, our business will be harmed, and the price of our securities could fall.
Under our agreements with our collaborative partner GSK, GSK has full responsibility for commercialization of RELVAR®/ BREO® ELLIPTA® and ANORO® ELLIPTA®. GSK has launched RELVAR®/ BREO® ELLIPTA® in a number of countries including the United States (U.S.), Canada, Japan, the United Kingdom, and Germany among others. The commercial launch of both products has been below our expectations primarily due to lower overall pricing levels in the U.S. and a longer timeframes to obtain payor coverage. For example, GSK recently stated that it has experienced more restrictive formulary access and lower net pricing in the U.S. respiratory market than it expected, which may indicate broader weakness in the respiratory markets targeted by RELVAR®/ BREO® ELLIPTA® and ANORO® ELLIPTA®. As a result, a number of analysts have adjusted their sales forecasts downward from previous projections. Any further delays or adverse developments or perceived
12
additional delays or adverse developments with respect to the commercialization of RELVAR®/ BREO® ELLIPTA® and ANORO® ELLIPTA® including if sales or payor coverage do not meet investor or our expectations, will significantly harm our business and the price of our securities could fall.
If the U.S. Food and Drug Administration ("FDA") does not approve the supplemental New Drug Application ("sNDA") for a fixed dose combination of FF/VI as a once-daily treatment for asthma in patients aged 12 years and older, or if the PDUFA date is extended, or if the approval contains restrictions or limitations on usage, our business will be significantly harmed, and the price of our securities could fall.
In June 2014, we and GSK announced the submission of a sNDA to the FDA for a fixed dose combination of FF/VI as a once-daily treatment for asthma in patients aged 12 years and older. The FDA determined the action target date under the Prescription Drug User Fee Act (PDUFA-V) to be April 30, 2015 and recently the FDA announced that on March 19, 2015, the FDA's Pulmonary-Allergy Drugs Advisory Committee and Drug Safety and Risk Management Advisory Committee will discuss the sNDA. Any adverse developments, results or delays or perceived adverse developments, results or delays with respect to the asthma sNDA, the Pulmonary-Allergy Drugs Advisory Committee and Drug Safety and Risk Management Advisory Committee meeting, or the FF/VI Phase 3 program will significantly harm our business and could cause the market price of our securities to decline. Examples of such adverse developments include, but are not limited to:
- •
- not every study, nor every dose in every study, in the Phase 3 asthma program for FF/VI achieved its primary endpoint and
regulatory authorities may determine that additional clinical studies are required;
- •
- safety, efficacy or other concerns arising from clinical or non-clinical studies in these programs, other studies of FF/VI, or
previous studies with other LABAs; and
- •
- any change in FDA policy or guidance regarding the use of LABAs to treat asthma.
On February 18, 2010, the FDA announced that LABAs should not be used alone in the treatment of asthma and it will require manufacturers to include this warning in the product labels of these drugs, along with taking other steps to reduce the overall use of these medicines. The FDA now requires that the product labels for LABA medicines reflect, among other things, that the use of LABAs is contraindicated without the use of an asthma controller medication such as an inhaled corticosteroid, that LABAs should only be used long-term in patients whose asthma cannot be adequately controlled on asthma controller medications, and that LABAs should be used for the shortest duration of time required to achieve control of asthma symptoms and discontinued, if possible, once asthma control is achieved. In addition, in March 2010, the FDA held an Advisory Committee to discuss the design of medical research studies (known as "clinical trial design") to evaluate serious asthma outcomes (such as hospitalizations, a procedure using a breathing tube known as intubation, or death) with the use of LABAs in the treatment of asthma in adults, adolescents, and children. Further, in April 2011, the FDA announced that to further evaluate the safety of LABAs, it is requiring the manufacturers of currently marketed LABAs to conduct additional randomized, double-blind, controlled clinical trials comparing the addition of LABAs to inhaled corticosteroids versus inhaled corticosteroids alone. Results from these post-marketing studies are expected in 2017. It is unknown at this time what, if any, effect these or future FDA actions will have on the prospects for FF/VI. The current uncertainty regarding the FDA's position on LABAs for the treatment of asthma and the lack of consensus expressed at the March 2010 Advisory Committee may result in the FDA requiring additional asthma clinical trials in the U.S. for FF/VI and increase the overall risk of FF/VI for the treatment of asthma in the U.S. We cannot predict the extent to which new FDA policy or guidance might significantly impede the discovery, development, production and marketing of FF/VI. Any adverse change in FDA
13
policy or guidance regarding the use of LABAs to treat asthma may significantly harm our business and the price of our securities could fall.
Any adverse developments to the regulatory status of either RELVAR®/BREO® ELLIPTA® or ANORO® ELLIPTA® in the countries in which they have received regulatory approval including labeling restrictions, safety findings, or any other limitation to usage, will harm our business and may cause the price of our securities to fall.
Although RELVAR®/BREO® ELLIPTA® or ANORO® ELLIPTA® are approved and marketed in a number of countries, it is possible that adverse changes to the regulatory status of these products could occur in the event new safety issues are identified, treatment guidelines are changed, or new studies fail to demonstrate product benefits. A number of notable pharmaceutical products have experienced adverse developments during commercialization that have resulted in the product being withdrawn, approved uses being limited, or new warnings being included. In the event that any adverse regulatory change were to occur to any of our products, our business will be harmed and the price of our securities will fall.
Any adverse developments or results or perceived adverse developments or results with respect to the ongoing Phase 3 programs for FF/VI in asthma or COPD, for UMEC/VI in COPD, or any future studies will significantly harm our business and the price of our securities could fall, and if regulatory authorities in those countries in which approval has not yet been granted determine that the Phase 3 programs for FF/VI in asthma or COPD or the Phase 3 programs for UMEC/VI for COPD do not demonstrate adequate safety and efficacy, the continued development of FF/VI or UMEC/VI or both may be significantly delayed, they may not be approved by these regulatory authorities, and even if approved it may be subject to restrictive labeling, any of which will harm our business, and the price of our securities could fall.
Although we have announced the completion of, and reported certain top-line data from, the Phase 3 registrational program for FF/VI in COPD and asthma, additional studies of FF/VI are underway. The Phase 3b program for FF/VI in COPD commenced in February 2011. Any adverse developments or perceived adverse developments with respect to the asthma sNDA, the COPD Phase 3b program or any future studies in these programs will significantly harm our business and the price of our securities could fall.
Although the FDA, the European Medicines Agency, the Japanese Ministry of Health, Labour and Welfare and Health Canada have approved ANORO® ELLIPTA®, it has not yet been approved in other countries. Any adverse developments or results or perceived adverse developments or results with respect to other pending or future regulatory submissions for the FF/VI program or the UMEC/VI program will significantly harm our business and the price of our securities could fall. Examples of such adverse developments include, but are not limited to:
- •
- not every study, nor every dose in every study, in the Phase 3 programs for FF/VI achieved its primary endpoint and regulatory
authorities may determine that additional clinical studies are required;
- •
- safety, efficacy or other concerns arising from clinical or non-clinical studies in these programs having to do with the LABA VI,
which is a component of FF/VI and UMEC/VI;
- •
- safety, efficacy or other concerns arising from clinical or non-clinical studies in these programs;
- •
- regulatory authorities determining that the Phase 3 programs in asthma or in COPD raise safety concerns or do not demonstrate
adequate efficacy; or
- •
- any change in FDA policy or guidance regarding the use of LABAs to treat asthma or the use of LABAs combined with a LAMA to treat COPD.
14
RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® face substantial competition for their intended uses in the targeted markets from products discovered, developed, launched and commercialized both by GSK and by other pharmaceutical companies, which could cause the royalties payable to us pursuant to the LABA Collaboration Agreement to be less than expected, which in turn would harm our business and the price of our securities could fall.
GSK has responsibility for obtaining regulatory approval, launching and commercializing RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® for their intended uses in the targeted markets around the world. While these products have received regulatory approval and been launched and commercialized in the U.S. and certain other targeted markets, the products face substantial competition from existing products previously developed and commercialized both by GSK and by other competing pharmaceutical companies and can expect to face additional competition from new products that are discovered, developed and commercialized by the same pharmaceutical companies and other competitors going forward. For example, sales of Advair®, GSK's approved medicine for both COPD and asthma, continue to be significantly greater than sales of RELVAR®/BREO® ELLIPTA®, and GSK has indicated publicly that it intends to continue commercializing Advair®.
Many of the pharmaceutical companies competing in respiratory markets are international in scope with substantial financial, technical and personnel resources that permit them to discover, develop, obtain regulatory approval and commercialize new products in a highly efficient and low cost manner at competitive prices to consumers. In addition, many of these competitors have substantial commercial infrastructures that facilitate commercializing their products in a highly efficient and low cost manner at competitive prices to consumers. The market for products developed for treatment of COPD and asthma continues to experience significant innovation and reduced cost in bringing products to market over time. There can be no assurance that RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® will not be replaced by new products that are deemed more effective at lower cost to consumers. The ability of RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® to succeed and achieve the anticipated level of sales depends on the commercial and development performance of GSK to achieve and maintain a competitive advantage over other products with the same intended use in the targeted markets.
If sales of RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® are less than anticipated because of existing or future competition in the markets in which they are commercialized, including competition from existing and new products that are perceived as lower cost or more effective, our royalty payments will be less than anticipated, which in turn would harm our business and the price of our securities could fall.
We and GSK are developing UMEC/VI/FF (LAMA/LABA/ICS) and MABA/FF as potential triple combination treatments for COPD and, potentially, asthma. As a result of the Spin-Off, most of our economic rights in these programs were assigned to Theravance Biopharma, Inc. If these programs are successful and GSK and the respiratory market in general views triple combination therapy as significantly more beneficial than existing therapies, including RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA®, our business could be harmed, and the price of our securities could fall.
Under our LABA Collaboration Agreement with GSK, we and GSK are exploring various paths to create triple therapy respiratory medications. The use of triple therapy is supported by the GOLD ("Global initiative for chronic Obstructive Lung Disease") guidelines in high-risk patients with severe COPD and a high risk of exacerbations. One potential triple therapy path is the combination of UMEC/VI (two separate bronchodilators) and FF (an inhaled corticosteroid), to be administered via the ELLIPTA® dry powder inhaler, referred to as UMEC/VI/FF or the "closed triple." Prior to the Spin-Off, we were entitled to receive 100% of any royalties payable under the GSK Agreements arising from sales of UMEC/VI/FF (as well as MABA and MABA/FF) if such products were successfully developed, approved and commercialized. In July 2014, we and GSK announced the initiation of a
15
large, global Phase 3 study for the closed triple in patients with COPD. If this Phase 3 study (or any other closed triple Phase 3 studies that may be initiated in the future) is successful, GSK and the respiratory market in general may view this triple combination therapy as significantly more beneficial than existing therapies, including RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA®. In such event the commercialization of RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® could be adversely affected, which in turn could result in lower royalties to us. Furthermore, if the closed triple (or MABA /FF) receives regulatory approval in either the U.S. or the EU, GSK's diligent efforts obligations regarding commercialization matters will have the objective of focusing on the best interests of patients and maximizing the net value of the overall portfolio of products under the GSK Agreements. Since GSK's commercialization efforts following such regulatory approval will be guided by a portfolio approach across products in which we have retained our full interest and also products in which we now have only a small portion of our former interest, GSK's commercialization efforts may have the effect of reducing the overall value of our remaining interests in the GSK Agreements in the future. As a result of the transactions effected by the Spin-Off, however, we are now only entitled to receive 15% of any contingent payments and royalties payable by GSK from sales of FF/UMEC/VI (and MABA, and MABA/FF) while Theravance Biopharma receives 85% of those same payments.
We have ongoing discussions with the SEC staff about the way we present and account for the different payments made and received under the LABA Collaboration Agreement in our current and historic financial statements. In the event the SEC staff disagrees with our accounting we may be required to restate prior financial statements which could, among other potential adverse effects, result in us incurring substantial costs, affect our ability to timely file our periodic reports, divert the attention of our management and employees and cause our stock price to decline.
Our consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States ("GAAP"). The preparation of these financial statements requires us to interpret accounting principles and guidance and make estimates and judgments that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported revenue generated and expenses incurred during the reporting periods. For instance, pursuant to GAAP, we are required to present and characterize the different payments made and received under the LABA Collaboration Agreement. Our most critical accounting estimates are described in "Management's Discussion and Analysis of Financial Condition and Results of Operations" in Part II, Item 7 of this Annual Report on Form 10-K. Our interpretations, estimates and judgments are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for the preparation of our financial statements. GAAP presentation is subject to interpretation by the United States Securities and Exchange Commission ("SEC"), the Financial Accounting Standards Board ("FASB") and various other bodies formed to interpret and create appropriate accounting principles and guidance. In the event that one of these bodies disagrees with our accounting recognition, measurement or disclosure or any of our accounting interpretations, estimates or assumptions, it may have a significant effect on our reported results and may retroactively affect previously reported results.
The SEC routinely reviews the periodic filings of public companies. For instance, we received in April 2014 a comment letter from the staff of the SEC in connection with a routine review of our Annual Report on Form 10-K for the year ended December 31, 2013 relating to our amortization of intangible assets and recognition of amortization charges associated with the LABA Collaboration Agreement. We responded to the comment letter in May 2014 and the SEC did not inquire further regarding the matters raised in the comment letter. More recently, the SEC inquired regarding our historical and current recognition of the up-front and milestone payments received from GSK pursuant to the LABA Collaboration Agreement between 2002 and 2006, and the milestone fees paid by us to GSK in 2013 and 2014. We are currently engaged in discussions with the SEC staff regarding our
16
recognition of the payments made and received under the LABA Collaboration Agreement. While we believe that our historical accounting related to the LABA Collaboration Agreement is appropriate and in accordance with U.S. generally accepted accounting principles, the SEC reserves the right to make further inquiries regarding our accounting treatment regarding the payments made and received under the LABA Collaboration Agreement. If the SEC disagrees with our accounting treatment, we may be required to restate our financial statements for prior periods. The need to restate our financial results could, among other potential adverse effects, result in us incurring substantial costs, affect our ability to timely file our periodic reports until such restatement is completed, divert the attention of our management and employees from managing our business, result in material changes to our historical and future financial results, result in investors losing confidence in our operating results, and cause our stock price to decline.
In addition, a restatement could also subject us to securities class action litigation. Defending against such potential litigation relating to a restatement of our financial statements would be expensive and would require significant attention and resources of our management. Moreover, our insurance to cover our obligations with respect to the ultimate resolution of any such litigation may be inadequate. As a result of these factors, any such potential litigation could have a material adverse effect on our financial results and cause our stock price to decline.
We were relying significantly upon Theravance Biopharma for a variety of services following the Spin-Off during which time we established our own separate administrative infrastructure, systems and controls to enable us to function as an independent public company and, if the new administrative infrastructure, systems and controls do not perform as expected, our business will be harmed and the price of our securities could fall.
Under the terms of a transition services agreement entered into between us and Theravance Biopharma, Theravance Biopharma has provided us with a variety of administrative services following the Spin-Off, including (i) record keeping support, (ii) finance, tax and accounting support to assist us in a secondary capacity to our own personnel, (iii) legal support, (iv) human resources support and (v) facilities support to the extent we continue to occupy separate space at our current South San Francisco, California facilities. We relied on Theravance Biopharma for execution of these administrative activities through a transition period extending into early 2015, which is a period when Theravance Biopharma personnel were highly focused on supporting its own new public company operations. We are in the process of establishing our own stand-alone capabilities, controls and systems including finance, tax, accounting, human resources, and IT systems, among others that are properly suited to our new post-spin business operations and to support our ongoing operations as an independent public company. If our new administrative infrastructure should cause us to be unable to comply with the accounting and legal standards required of publicly traded companies, our business and our reputation will be harmed, and the price of our securities could fall.
On June 2, 2014, we completed the separation of our businesses into two independent, publicly traded companies by separating our late-stage partnered respiratory assets from our biopharmaceutical operations; the lengthy, complicated process to separate the two businesses has diverted the attention of our management and employees, and has increased our professional services expenses in 2014 and will continue to do so in early 2015.
On April 25, 2013, we announced our intention to separate our businesses into two independent, publicly traded companies. On August 1, 2013, the company to be spun-off, Theravance Biopharma, filed a preliminary Form 10 with the SEC, and subsequent amendments throughout 2013 and the spring of 2014. The Spin-Off was completed on June 2, 2014. Theravance continues to be responsible for all development and commercial activities under the GSK Agreements. Theravance is eligible to receive the associated royalty revenues from FF/VI (RELVAR®/BREO® ELLIPTA®), UMEC/VI (ANORO®
17
ELLIPTA®) and potentially VI monotherapy and 15% of the aggregate potential royalty revenues payable to Theravance Respiratory Company, LLC from UMEC/VI/FF, MABA, and MABA/FF and other products that may be developed under the GSK Agreements. Theravance Biopharma is now a separate and independent publicly traded biopharmaceutical company focusing on the discovery, development and commercialization of small-molecule medicines in areas of significant unmet medical need.
In conjunction with the Spin-Off of Theravance Biopharma, on March 3, 2014, we, Theravance Biopharma and GSK entered into a series of agreements clarifying how the companies would implement the separation and operate following the Spin-Off. We, Theravance Biopharma and GSK entered into a three-way master agreement providing for GSK's consent to the Spin-Off provided certain conditions were met. We and GSK also entered into amendments of the GSK Agreements. The master agreement and the other agreements are all currently effective.
The amendments to the GSK Agreements do not change the royalty rates or other economic terms. The amendments do provide that GSK's diligent efforts obligations regarding commercialization matters under both agreements will change upon regulatory approval in either the U.S. or the EU of UMEC/VI/FF or a MABA combined with FF. Upon such regulatory approval, GSK's diligent efforts obligations as to commercialization matters under the GSK Agreements will have the objective of focusing on the best interests of patients and maximizing the net value of the overall portfolio of products under the GSK Agreements. Since GSK's commercialization efforts following such regulatory approval will be guided by a portfolio approach across products in which we have retained our full interest and also products in which we now have only a small portion of our former interest, GSK's commercialization efforts may have the effect of reducing the overall value of our remaining interests in the GSK Agreements in the future.
The process of planning for and effecting the business separation demanded a significant amount of time and effort from our management and certain employees, continued to do so in 2014 and will continue to do so until early 2015. The diversion of our management's and employees' attention to the business separation process and the post-separation transition has disrupted and may continue to disrupt our operations and may adversely impact our relationship with GSK and increase employee turnover.
We cannot assure you that we will not undertake additional restructuring activities, that the business separation will succeed in meeting our objectives and increasing stockholder value, or that the actual results will not differ materially from the results that we anticipate.
We have incurred and will continue to incur significant expenditures for professional services in connection with the business separation and our post-separation operations, including financial advisory, accounting and legal fees.
Under the terms of a separation and distribution agreement entered into between us and Theravance Biopharma, Theravance Biopharma will indemnify us from (i) all debts, liabilities and obligations transferred to Theravance Biopharma in connection with the Spin-Off (including its failure to pay, perform or otherwise promptly discharge any such debts, liabilities or obligations after the Spin-Off), (ii) any misstatement or omission of a material fact in its information statement filed with the SEC, resulting in a misleading statement and (iii) any breach by it of certain agreements entered into between the parties in connection with the Spin-Off. Theravance Biopharma's ability to satisfy these indemnities, if called upon to do so, will depend upon its future financial strength and if we are not able to collect on indemnification rights from Theravance Biopharma, our financial condition may be harmed.
18
We may not be able to utilize all of our net operating loss carryforwards.
We have net operating loss carryforwards and other significant U.S. tax attributes that we believe could offset otherwise taxable income in the U.S. As a part of the overall Spin-Off transaction, the transfer of certain assets by us to Theravance Biopharma and our distribution of Theravance Biopharma ordinary shares resulted in taxable transfers pursuant to applicable provisions of the Internal Revenue Code of 1986, as amended (the "Code") and Treasury Regulations. The taxable gain recognized by us attributable to the transfer of certain assets to Theravance Biopharma will generally equal the excess of the fair market value of each asset transferred over our adjusted tax basis in such asset. Although we will not recognize any gain with respect to the cash we transferred to Theravance Biopharma, we may recognize substantial gain based on the fair market value of the other assets (other than cash) transferred to Theravance Biopharma. The determination of the fair market value of these assets is subjective and could be subject to adjustments or future challenge by the Internal Revenue Service ("IRS"), which could result in an increase in the amount of gain realized by us as a result of the transfer. Our U.S. federal income tax resulting from any gain recognized upon the transfer of our assets to Theravance Biopharma (including any increased U.S. federal income tax that may result from a subsequent determination of higher fair market values for the transferred assets), may be reduced by our net operating loss carryforward. The net operating loss carryforwards available in any year to offset our net taxable income will be reduced following a more than 50% change in ownership during any period of 36 consecutive months (an "ownership change") as determined under the Internal Revenue Code of 1986 (the "Code"). As of December 31, 2014, we have conducted an analysis to determine whether an ownership change had occurred since inception through December 31, 2014, and concluded that we had undergone two ownership changes in prior years. We have approximately $1.4 billion of net operating loss carryforward available during 2014. We currently expect our net operating losses to be fully available to offset current year net taxable income after taking into account the taxable nature of the Spin-Off. With respect to our remaining net operating losses of approximately $1.2 billion as of December 31, 2014, there may be certain annual limitations for utilization based on the above-described ownership change provisions. In addition, we may not be able to have sufficient future taxable income prior to their expiration because net operating losses have carryforward periods. Future changes in federal and state tax laws pertaining to net operating loss carryforwards may also cause limitations or restrictions from us claiming such net operating losses. If the net operating loss carryforwards become unavailable to us or are fully utilized, our future taxable income will not be shielded from federal and state income taxation absent certain U.S. federal and state tax credits, and the funds otherwise available for general corporate purposes would be reduced.
Our stockholders who received ordinary shares of Theravance Biopharma in the Spin-Off and/or dividends we paid during 2014 could incur significant U.S. federal income tax liabilities as a result of the distributions.
The Theravance Biopharma ordinary shares received by our stockholders in the Spin-Off and dividends we paid during 2014, are expected to be taxable to stockholders. An amount equal to the fair market value of Theravance Biopharma ordinary shares received (including any fractional shares deemed to be received) on the distribution date, and/or the dividends we paid in 2014, will be treated as a taxable dividend to the extent of each Theravance stockholder's ratable share of any current and accumulated earnings and profits of Theravance, measured as of the end of 2014, with the excess treated as a non-taxable return of capital to the extent of such stockholder's tax basis in our common stock and any remaining excess treated as a capital gain. Accordingly, Theravance stockholders who received ordinary shares of Theravance Biopharma in the Spin-Off and/or dividends during 2014 could incur significant U.S. federal income tax liabilities as a result of the distribution. We periodically update Form 8937—Report of Organizational Actions Affecting Basis of Securities, which we post on our website, in order to provide investors with relevant information associated with the distributions.
19
The Spin-Off resulted in substantial changes in our Board, management, and employees. If we fail to hire and effectively integrate new executive officers into our organization, the future development and commercialization of our product candidates may suffer, harming future regulatory approvals, sales of our product candidates or our results of operations.
Since the Spin-Off, substantially all of our directors and senior management team has changed. Our current board and management team has only been working together for a relatively short period of time. In addition, Rick E Winningham resigned as our president and chief executive officer effective as of August 15, 2014 and as chairman of our Board and as a director effective as of October 30, 2014. We have appointed Michael W. Aguiar as our chief executive officer and as a member of our Board and appointed Eric d'Esparbes as our chief financial officer. We expect to continue to expand our management team in the future. Our future performance will depend significantly on our ability to successfully integrate our new directors into our Board and our new chief executive officer, chief financial officer and other recently and subsequently hired executive officers into our management team, and on those individuals' ability to develop and maintain an effective working relationship. Our failure to integrate recently and subsequently appointed directors and executive officers, including our new chief executive officer and chief financial officer, with other members of management could result in inefficiencies in the conduct of our business, which can adversely affect our results of operations.
If any product candidates in any respiratory program partnered with GSK are not approved by regulatory authorities or are determined to be unsafe or ineffective in humans, our business will be adversely affected and the price of our securities could fall.
The FDA must approve any new medicine before it can be marketed and sold in the U.S. Our partner GSK must provide the FDA and similar foreign regulatory authorities with data from preclinical and clinical studies that demonstrate that the product candidates are safe and effective for a defined indication before they can be approved for commercial distribution. GSK will not obtain this approval for a partnered product candidate unless and until the FDA approves a NDA. The processes by which regulatory approvals are obtained from the FDA to market and sell a new product are complex, require a number of years and involve the expenditure of substantial resources. In order to market medicines in foreign countries, separate regulatory approvals must be obtained in each country. The approval procedure varies among countries and can involve additional testing, and the time required to obtain approval may differ from that required to obtain FDA approval. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. Conversely, failure to obtain approval in one or more country may make approval in other countries more difficult.
Clinical studies involving product candidates partnered with GSK may reveal that those candidates are ineffective, inferior to existing approved medicines, unacceptably toxic, or that they have other unacceptable side effects. In addition, the results of preclinical studies do not necessarily predict clinical success, and larger and later-stage clinical studies may not produce the same results as earlier-stage clinical studies.
Frequently, product candidates that have shown promising results in early preclinical or clinical studies have subsequently suffered significant setbacks or failed in later clinical or non-clinical studies. In addition, clinical and non-clinical studies of potential products often reveal that it is not possible or practical to continue development efforts for these product candidates. If these studies are substantially delayed or fail to prove the safety and effectiveness of product candidates in development partnered with GSK, GSK may not receive regulatory approval for such product candidates and our business and financial condition will be materially harmed and the price of our securities may fall.
20
Several well-publicized Complete Response letters issued by the FDA and safety-related product withdrawals, suspensions, post-approval labeling revisions to include boxed warnings and changes in approved indications over the last several years, as well as growing public and governmental scrutiny of safety issues, have created a conservative regulatory environment. The implementation of new laws and regulations and revisions to FDA clinical trial design guidance have increased uncertainty regarding the approvability of a new drug. Further, there are additional requirements for approval of new drugs, including advisory committee meetings for new chemical entities, and formal risk evaluation and mitigation strategy at the FDA's discretion. These laws, regulations, additional requirements and changes in interpretation could cause non-approval or further delays in the FDA's review and approval of any product candidates in any respiratory program partnered with GSK.
Even if product candidates in any respiratory program partnered with GSK receive regulatory approval, as is the case with RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA®, commercialization of such products may be adversely affected by regulatory actions and oversight.
Even if GSK receives regulatory approval for product candidates in any respiratory program partnered with GSK, this approval may include limitations on the indicated uses for which GSK can market the medicines or the patient population that may utilize the medicines, which may limit the market for the medicines or put GSK at a competitive disadvantage relative to alternative therapies. These restrictions make it more difficult to market the approved products.
In addition, the manufacturing, labeling, packaging, adverse event reporting, advertising, promotion and recordkeeping for the approved product remain subject to extensive and ongoing regulatory requirements. If we or GSK become aware of previously unknown problems with an approved product in the U.S. or overseas or at contract manufacturers' facilities, a regulatory authority may impose restrictions on the product, the contract manufacturers or on GSK, including requiring it to reformulate the product, conduct additional clinical studies, change the labeling of the product, withdraw the product from the market or require the contract manufacturer to implement changes to its facilities. GSK is also subject to regulation by regional, national, state and local agencies, including the Department of Justice, the Federal Trade Commission, the Office of Inspector General of the U.S. Department of Health and Human Services and other regulatory bodies as well as governmental authorities in those foreign countries in which any of the product candidates in any respiratory program partnered with GSK are approved for commercialization. The Federal Food, Drug, and Cosmetic Act, the Public Health Service Act and other federal and state statutes and regulations govern to varying degrees the research, development, manufacturing and commercial activities relating to prescription pharmaceutical products, including non-clinical and clinical testing, approval, production, labeling, sale, distribution, import, export, post-market surveillance, advertising, dissemination of information and promotion. Any failure to maintain regulatory approval will limit GSK's ability to commercialize the product candidates in any respiratory program partnered with GSK, which would materially and adversely affect our business and financial condition and which may cause the price of our securities to fall.
We have incurred operating losses in each year since our inception and will continue to incur losses until royalties from the sales of RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® exceed total expenses, including interest expenses, and our revenues and operating results will likely fluctuate in future periods.
From mid-1997 until the Spin-Off, we were engaged in discovering and developing compounds and product candidates and we never generated sufficient revenue from the sale of medicines or royalties on sales by our partners to achieve sustained profitability. As of December 31, 2014, we had an accumulated deficit of approximately $1.7 billion. Although we expect to have a substantial reduction in our expenses in future periods as a result of the Spin-Off, we will continue to incur losses until royalties from the sales of RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® exceed total expenses, including interest expenses, and our revenues and operating results will likely fluctuate from
21
period to period. We are uncertain when or if we will be able to achieve or sustain profitability. Failure to become and remain profitable would adversely affect the price of our securities, our ability to return capital to stockholders and continue operations.
We may not be successful in our efforts to expand our portfolio of royalty generating products.
In the future, we may choose to acquire rights to one or more additional royalty generating products. However, we may be unable to license or acquire rights to suitable royalty generating products for a number of reasons. In particular, the licensing and acquisition of pharmaceutical product rights is a competitive area. Several more established companies are also pursuing strategies to license or acquire rights to royalty generating products. These established companies may have a competitive advantage over us. Other factors that may prevent us from licensing or otherwise acquiring rights to suitable royalty generating products include the following:
- •
- we may be unable to license or acquire the rights on terms that would allow us to make an appropriate return from the product;
- •
- companies that perceive us to be their competitors may be unwilling to assign or license their product rights to us; or
- •
- we may be unable to identify suitable royalty generating products.
If we are unable to acquire or license rights to suitable royalty generating product candidates, our business may suffer.
We have a significant amount of debt including Convertible Subordinated Notes and Fixed Rate Royalty notes that are senior in capital structure and cash flow, respectively, to our common stockholders. Satisfying the obligations relating to our debt could adversely affect the amount or timing of distributions to our stockholders.
As of December 31, 2014 we had approximately $725.6 million in total long-term liabilities outstanding, comprised primarily of $255.1 million in principal that remains outstanding under our 2.125% Convertible Subordinated Notes due 2023 (the "2023 Notes") and $470.5 million in principal that remains outstanding under our 9% Fixed Rate Royalty term notes due 2029 (the "2029 Notes" and with the 2023 Notes, the "Notes"). The 2023 Notes are unsecured debt and are not redeemable by us prior to the maturity date. Holders of the Notes may require us to purchase all or any portion of their Notes at 100% of their principal amount, plus any unpaid interest, upon a fundamental change. A fundamental change is generally defined to include a merger involving us, an acquisition of a majority of our outstanding common stock, and the change of a majority of our board without the approval of the board. In addition, to the extent we pursue and complete a monetization transaction, the structure of such transaction may qualify as a fundamental change under the Notes, which could trigger the put rights of the holders of the Notes, in which case we would be required to use a portion of the net proceeds from such transaction to repurchase any Notes put to us. Our 2029 Notes have rights to 40% of all royalty payments received from GSK related to RELVAR®/BREO® ELLIPTA®, ANORO® ELLIPTA®, and VI monotherapy until the notes are paid in full.
Satisfying the obligations of this debt could adversely affect the amount or timing of any distributions to our stockholders. We may choose to satisfy repurchase, or refinance this debt through public or private equity or debt financings if we deem such financings available on favorable terms. If any or all of the Convertible Subordinated Notes are not converted into shares of our common stock before the maturity date, we will have to pay the holders the full aggregate principal amount of the Notes then outstanding. If the Fixed Rate Royalty are not refinanced or paid in full, then they will receive 40% of all future economics associated with RELVAR®/BREO® ELLIPTA®, ANORO® ELLIPTA®, and VI monotherapy, until the notes are paid in full. Any of the above payments could have a material adverse effect on our cash position. If we fail to satisfy these obligations, it may result
22
in a default under the indenture, which could result in a default under certain of our other debt instruments, if any. Any such default would harm our business and the price of our securities could fall.
If we lose key management personnel, or if we fail to retain our key employees, our ability to manage our business will be impaired.
Following the Spin-Off, we have a much smaller management team and very few employees. We are highly dependent on principal members of our management team and a small group of key employees to operate our business. Our company is located in northern California, which is headquarters to many other biotechnology and biopharmaceutical companies and many academic and research institutions. As a result, competition for certain skilled personnel in our market remains intense. None of our employees have employment commitments for any fixed period of time and they all may leave our employment at will. If we fail to retain our qualified personnel or replace them when they leave, we may be unable to continue our business operations, which may cause the price of our securities to fall.
We rely and will continue to rely on outsourcing arrangements for many of our activities, including financial reporting and accounting and human resources.
We currently have only ten full-time employees and, as a result, we rely, and expect to continue to rely, on outsourcing arrangements for a significant portion of our activities, including financial reporting and accounting and human resources, as well as for certain functions as a public company through the end of the first quarter of 2015. We may have limited control over these third parties and we cannot guarantee that they will perform their obligations in an effective and timely manner.
As we continue to develop our business, our mix of assets and our sources of income may require that we register with the SEC as an "investment company" in accordance with the Investment Company Act of 1940.
We have not been and have no current intention to register as an "investment company" under the Investment Company Act of 1940, or the 40 Act, because we believe the nature of our assets and the sources of our income currently exclude us from the definition of an investment company pursuant to Sections (3)(a)(1)(A), (3)(a)(1)(C) under the 40 Act and Rule 270.3a-1 of Title 17 of the Code of Federal Regulations. Accordingly, we are not currently subject to the provisions of the 40 Act, such as compliance with the 40 Act's registration and reporting requirements, capital structure requirements, affiliate transaction restrictions, conflict of interest rules, requirements for disinterested directors, and other substantive provisions. Generally, to avoid being a company that is an "investment company" under the 40 Act, it must both: (a) not be or hold itself out as being engaged primarily in the business of investing, reinvesting or trading in securities, and (b) either (i) not be engaged or propose to engage in the business of investing in securities or own or propose to acquire investment securities having a value exceeding 40% of the value of its total assets (exclusive of U.S. government securities and cash items) on an unconsolidated basis or (ii) not have more than 45% of the value of its total assets (exclusive of Government securities and cash items) consist of or more than 45% of its net income after taxes (for the last four fiscal quarters combined) be derived from securities. In addition, we would not be an "investment company" if an exception, exemption, or safe harbor under the 40 Act applies.
We monitor our assets and income for compliance with the tests under the 40 Act and seek to conduct our business activities to ensure that we do not fall within its definitions of "investment company." If we were to become an "investment company" and be subject to the strictures of the 40 Act, the restrictions imposed by the 40 Act would likely require changes in the way we do business and add significant administrative burdens to our operations. In order to ensure that we do not fall within the 40 Act, we may need to take various actions which we might otherwise not pursue. These actions may include restructuring the Company and/or modifying our mixture of assets and income.
Specifically, our mixture of debt vs. royalty assets is important to our classification as an "investment company" or not. In this regard, while we currently believe that none of the definitions of
23
"investment company" apply to us, we may in the future rely on an exception under the 40 Act provided by Section 3(c)(5)(A). To qualify for Section 3(c)(5)(A), as interpreted by the staff of the SEC, we would be required to have at least 55% of our total assets in "notes, drafts, acceptances, open accounts receivable, and other obligations representing part or all of the sales price of merchandise, insurance, and services" (or Qualifying Assets). In a no-action letter issued to Royalty Pharma on August 13, 2010, the staff stated that royalty interests are Qualifying Assets under this exception. If the SEC or its staff in the future adopts a contrary interpretation or otherwise restricts the conclusions in the staff's no-action letter such that our royalty interests are no longer Qualifying Assets for purposes of Section 3(c)(5)(A), we could be required to register under the 40 Act.
The rules and interpretations of the SEC and the courts, relating to the definition of "investment company" are highly complex in numerous respects. While we currently intend to conduct our operations so that we will not be deemed an investment company, we can give no assurances that we will not determine it to be in the Company's and our stockholders' interest to register as an "investment company", not be deemed an "investment company" and not be required to register under the 40 Act.
Risks Related to our Alliance with GSK
Because all our current and projected revenues are derived from products under the GSK Agreements, disputes with GSK could harm our business and cause the price of our securities to fall.
All of our current and projected revenues are derived from products under the GSK Agreements. Any action or inaction by either GSK or us that results in a material dispute, allegation of breach, litigation, arbitration, or significant disagreement between the parties may be interpreted negatively by the market or by our investors, could harm our business and cause the price of our securities to fall. Examples of these kinds of issues include but are not limited to non-performance of contractual obligations and allegations of non-performance, disagreements over the relative marketing and sales efforts for our partnered products and other GSK respiratory products, disputes over public statements, and similar matters. In addition, while we obtained GSK's consent to the Spin-Off as structured, GSK could decide to challenge various aspects of our post-Spin-Off operation of Theravance Respiratory Company, LLC ("TRC"), the limited liability company jointly owned by us and Theravance Biopharma as violating or allowing it to terminate the GSK Agreements. Although we believe our operation of TRC fully complies with the GSK Agreements and applicable law, there can be no assurance that we would prevail against any such claims by GSK. Moreover, regardless of the merit of any claims by GSK, we may incur significant cost and diversion of resources in defending them. In addition, any market or investor uncertainty about the respiratory programs partnered with GSK or the enforceability of the GSK Agreements could result in significant reduction in the market price of our securities and other material harm to our business.
Because GSK is a strategic partner as well as a significant stockholder, it may take actions that in certain cases are materially harmful to both our business or to our other stockholders.
Although GSK beneficially owns approximately 27.1% of our outstanding capital stock as of February 12, 2015, it is also a strategic partner with rights and obligations under the GSK Agreements that cause its interests to differ from the interests of us and our other stockholders. In particular, GSK has a substantial respiratory product portfolio in addition to the partnered products that are covered by the GSK Agreements. GSK may make respiratory product portfolio decisions or statements about its portfolio which may be, or may be perceived to be, harmful to the respiratory products partnered with us. For example, GSK could promote its non-GSK/THRX respiratory products, delay or terminate the development or commercialization of the respiratory programs covered by the GSK Agreements, or take other actions, such as making public statements, that have a negative effect on our stock price. In this regard and by way of example, sales of Advair®, GSK's approved medicine for both COPD and asthma, continue to be significantly greater than sales of RELVAR®/BREO® ELLIPTA®, and GSK has
24
indicated publicly that it intends to continue commercializing Advair®. Also, given the potential future royalty payments GSK may be obligated to pay under the GSK Agreements, GSK may seek to acquire us to reduce those payment obligations. The timing of when GSK may seek to acquire us could potentially be when it possesses information regarding the status of drug programs covered by the GSK Agreements that has not been publicly disclosed and is not otherwise known to us. As a result of these differing interests, GSK may take actions that it believes are in its best interest but which might not be in the best interests of either us or our other stockholders. In addition, upon regulatory approval of UMEC/VI/FF or a MABA/ICS in either the U.S. or the EU, GSK's diligent efforts obligations as to commercialization matters under the GSK Agreements will have the objective of focusing on the best interests of patients and maximizing the net value of the overall portfolio of products under the GSK Agreements. Since GSK's commercialization efforts following such regulatory approval will be guided by a portfolio approach across products in which we have retained our full interest and also products in which we now have only a portion of our former interest, GSK's commercialization efforts may have the effect of reducing the overall value of our remaining interests in the products covered by the GSK Agreements in the future.
GSK has also indicated to us that it believes its consent may be required before we can engage in certain royalty monetization transactions with third parties, which may inhibit our ability to engage in these transactions.
In the course of our discussions with GSK concerning the Spin-Off of Theravance Biopharma, GSK indicated to us that it believes that its consent may be required before we can engage in certain transactions designed to monetize the future value of royalties that may be payable to us from GSK under the GSK Agreements. GSK has informed us that it believes that there may be certain covenants included in these types of transactions that might violate certain provisions of the GSK Agreements. Although we believe that we can structure royalty monetization transactions in a manner that fully complies with the requirements of the GSK Agreements without GSK's consent, a third party in a proposed monetization transaction may nonetheless insist that we obtain GSK's consent for the transaction or re-structure the transaction on less favorable terms. We have obtained GSK's agreement that (i) we may grant certain pre-agreed covenants in connection with monetization of our interests in RELVAR®/BREO® ELLIPTA®, ANORO® ELLIPTA® and vilanterol monotherapy and portions of our interests in TRC, and (ii) it will not unreasonably withhold its consent to our requests to grant other covenants, provided, among other conditions, that in each case, the covenants are not granted in favor of pharmaceutical or biotechnology company with a product either being developed or commercialized for the treatment of respiratory disease. If we seek GSK's consent to grant covenants other than pre-agreed covenants, we may not be able to obtain GSK's consent on reasonable terms, or at all. If we proceed with a royalty monetization transaction that is not otherwise covered by the GSK Agreement without GSK's consent, GSK could request that its consent be obtained or seek to enjoin or otherwise challenge the transaction as violating or allowing it to terminate the GSK Agreements. Regardless of the merit of any claims by GSK, we would incur significant cost and diversion of resources in defending against GSK's claims or asserting our own claims and GSK may seek concessions from us in order to provide its consent. Any uncertainty about whether or when we could engage in a royalty monetization transaction, the potential impact on the enforceability of the GSK Agreements or the loss of potential royalties from the respiratory programs partnered with GSK, could impair our ability to pursue a return of capital strategy for our stockholders ahead of our receipt of significant royalties from GSK, result in significant reduction in the market price of our securities and cause other material harm to our business.
25
GSK's ownership of a significant percentage of our stock and its ability to acquire additional shares of our stock may create conflicts of interest, and may inhibit our management's ability to continue to operate our business in the manner in which it is currently being operated.
As of February 12, 2015, GSK beneficially owned approximately 27.1% of our outstanding capital stock, and GSK has the right to acquire stock from us to maintain its percentage ownership of our capital stock in certain circumstances. GSK could have substantial influence in the election of our directors, delay or prevent a transaction in which stockholders might receive a premium over the prevailing market price for their shares and have significant control over certain changes in our business.
In addition, GSK may make an offer to our stockholders to acquire outstanding voting stock that would bring GSK's percentage ownership of our voting stock to no greater than 60%, provided that:
- •
- the offer includes no condition as to financing;
- •
- the offer is approved by a majority of our independent directors;
- •
- the offer includes a condition that the holders of a majority of the shares of the voting stock not owned by GSK accept the offer by
tendering their shares in the offer; and
- •
- the shares purchased will be subject to the same provisions of the governance agreement as are the shares of voting stock currently held by GSK.
If pursuant to the provision described above GSK's ownership of us is greater than 50.1%, then GSK is allowed to make an offer to our stockholders to acquire outstanding voting stock that would bring GSK's percentage ownership of our voting stock to 100%, provided that;
- •
- the offer includes no condition as to financing;
- •
- the offer is approved by a majority of our independent directors; and
- •
- the offer includes a condition that the holders of a majority of the shares of the voting stock not owned by GSK accept the offer by tendering their shares in the offer.
The procedures governing GSK offers to ours stockholders to acquire outstanding voting stock set forth in the preceding two paragraphs are applicable until the termination of the governance agreement on September 1, 2015 and thereafter the foregoing restrictions will not apply.
Further, pursuant to our Certificate of Incorporation, we renounce our interest in and waive any claim that a corporate or business opportunity taken by GSK constitutes a corporate opportunity of ours unless such corporate or business opportunity is expressly offered to one of our directors who is a director, officer or employee of GSK, primarily in his or her capacity as one of our directors.
GSK's significant ownership position and its rights under the governance agreement may deter or prevent efforts by other companies to acquire us, which could prevent our stockholders from realizing a control premium.
As of February 12, 2015, GSK beneficially owned approximately 27.1% of our outstanding capital stock. GSK may vote at its sole discretion on any proposal to effect a change of control of us or for us to issue equity securities to one or more parties that would result in that party or parties beneficially owning more than 20% of our outstanding capital stock. Our governance agreement with GSK requires us to exempt GSK from any stockholder rights plan we may adopt in the future, affords GSK certain rights to offer to acquire us in the event third parties seek to acquire our stock and contains other provisions that could deter or prevent another company from seeking to acquire us.
For example, GSK may offer to acquire 100% of our outstanding stock from stockholders in certain circumstances, such as if we are faced with a hostile acquisition offer or if our Board acts in a manner to facilitate a change in control of us with a party other than GSK. As a result of GSK's significant ownership and its rights under the governance agreement, other companies may be less
26
inclined to pursue an acquisition of us and therefore we may not have the opportunity to be acquired in a transaction that stockholders might otherwise deem favorable, including transactions in which our stockholders might realize a substantial premium for their shares.
GSK could sell or transfer a substantial number of shares of our common stock, which could depress the price of our securities or result in a change in control of our company.
Under our governance agreement with GSK, GSK could previously sell or transfer our common stock only pursuant to a public offering registered under the Securities Act or pursuant to Rule 144 of the Securities Act. GSK no longer has contractual restrictions on its ability to sell or transfer our common stock on the open market, in privately negotiated transactions or otherwise, and these sales or transfers could create substantial declines in the price of our securities or, if these sales or transfers were made to a single buyer or group of buyers, could contribute to a transfer of control of our company to a third party. Sales by GSK of a substantial number of shares, or the expectation of such sales, could cause a significant reduction in the market price of our common stock.
Risks Related to Legal and Regulatory Uncertainty
If the efforts of our partner, GSK, to protect the proprietary nature of the intellectual property related to products in any respiratory program partnered with GSK are not adequate, the future commercialization of any such product could be delayed, limited or prevented, which would materially harm our business and the price of our securities could fall.
To the extent the intellectual property protection of products in any respiratory program partnered with GSK are successfully challenged or encounter problems with the U.S. Patent and Trademark Office or other comparable agencies throughout the world, the commercialization of these products could be delayed, limited or prevented. Any challenge to the intellectual property protection of a late-stage development asset or approved product arising from any respiratory program partnered with GSK could harm our business and cause the price of our securities to fall.
Our commercial success depends in part on products in any respiratory program partnered with GSK not infringing the patents and proprietary rights of third parties. Third parties may assert that these products are using their proprietary rights without authorization. In addition, third parties may obtain patents in the future and claim that use of GSK's technologies infringes upon these patents. Furthermore, parties making claims against GSK may obtain injunctive or other equitable relief, which could effectively block GSK's ability to further develop or commercialize one or more of the product candidates or products in any respiratory program partnered with GSK.
In the event of a successful claim of infringement against GSK, it may have to pay substantial damages, obtain one or more licenses from third parties or pay royalties. In addition, even in the absence of litigation, GSK may need to obtain licenses from third parties to advance its research or allow commercialization of the products. GSK may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, GSK would be unable to further develop and commercialize one or more of the products, which could harm our business significantly. In addition, in the future GSK could be required to initiate litigation to enforce its proprietary rights against infringement by third parties. Prosecution of these claims to enforce its rights against others would involve substantial litigation expenses. If GSK fails to effectively enforce its proprietary rights related to our partnered respiratory programs against others, our business will be harmed, and the price of our securities could fall.
27
Risks Related to Ownership of our Common Stock
The price of our securities has been extremely volatile and may continue to be so, and purchasers of our securities could incur substantial losses.
The price of our securities has been extremely volatile and may continue to be so. Between January 1, 2014 and December 31, 2014, the high and low sales prices of our common stock as reported on The NASDAQ Global Market varied between $12.90 and $40.49 per share. The stock market in general and the market for biotechnology and biopharmaceutical companies in particular have experienced extreme volatility that has often been unrelated to the companies' operating performance, in particular during the last several years. The following factors, in addition to the other risk factors described in this section, may also have a significant impact on the market price of our securities:
- •
- any adverse developments or results or perceived adverse developments or results with respect to the commercialization of
RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® with GSK, including, without limitation, if payor coverage is lower than anticipated or if sales
of RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® are less than anticipated because of pricing pressure in the respiratory markets targeted by
our partnered products or existing or future competition in the markets in which they are commercialized, including competition from existing and new products that are perceived as lower cost or more
effective, and our royalty payments are less than anticipated;
- •
- any positive developments or results or perceived positive developments or results with respect to the development of UMEC/VI/FF with
GSK, including, without limitation if the new Phase 3 study (or any other closed triple Phase 3 studies that may be initiated in the future) is successful and GSK and the respiratory
market in general view this triple combination therapy as significantly more beneficial than existing therapies, including RELVAR®/BREO® ELLIPTA® and
ANORO® ELLIPTA®;
- •
- any adverse developments or results or perceived adverse developments or results with respect to the on-going development of FF/VI
with GSK, including, without limitation, any difficulties or delays encountered with regard to the regulatory path for FF/VI or any indication from clinical or non-clinical studies, including the
large Phase 3b program, that FF/VI is not safe or efficacious or does not sufficiently differentiate itself from alternative therapies;
- •
- any adverse developments or results or perceived adverse developments or results with respect to the on-going development of UMEC/VI
with GSK, including, without limitation, any difficulties or delays encountered with regard to the regulatory path for UMEC/VI, any indication from clinical or non-clinical studies that UMEC/VI is not
safe or efficacious;
- •
- any adverse developments or results or perceived adverse developments or results with respect to the sNDA submitted to the FDA for a
fixed dose combination of FF/VI as a once-daily treatment for asthma in patients aged 12 years and older;
- •
- any adverse developments or perceived adverse developments in the field of LABAs, including any change in FDA policy or guidance (such
as the pronouncement in February 2010 warning that LABAs should not be used alone in the treatment of asthma and related labeling requirements, the impact of the March 2010 FDA Advisory Committee
discussing LABA clinical trial design to evaluate serious asthma outcomes or the FDA's April 2011 announcement that manufacturers of currently marketed LABAs conduct additional clinical studies
comparing the addition of LABAs to inhaled corticosteroids versus inhaled corticosteroids alone);
- •
- GSK's decisions whether or not to purchase, on a quarterly basis, sufficient shares of our common stock to maintain its ownership percentage taking into account our preceding quarter's option exercise, equity vesting and debt conversion activity;
28
- •
- the occurrence of a fundamental change triggering a put right of the holders of the Notes or our inability, or perceived inability, to
satisfy the obligations under the Notes when they become due;
- •
- our incurrence of expenses in any particular quarter that are different than market expectations;
- •
- the extent to which GSK advances (or does not advance) FF/VI, UMEC/VI, UMEC/VI/FF, VI monotherapy and the MABA program through
development into commercialization in all indications in all major markets;
- •
- any adverse developments or perceived adverse developments with respect to our relationship with GSK, including, without limitation,
disagreements that may arise between us and GSK;
- •
- announcements regarding GSK generally;
- •
- announcements of patent issuances or denials, technological innovations or new commercial products by GSK;
- •
- publicity regarding actual or potential study results or the outcome of regulatory review relating to products under development by
GSK;
- •
- regulatory developments in the U.S. and foreign countries;
- •
- economic and other external factors beyond our control;
- •
- sales of stock by us or by our stockholders, including sales by certain of our employees and directors whether or not pursuant to
selling plans under Rule 10b5-1 of the Securities Exchange Act of 1934;
- •
- relative illiquidity in the public market for our common stock (our three largest stockholders other than GSK collectively owned
approximately 38.2% of our outstanding capital stock as of February 12, 2015 based on our review of publicly available filings);
- •
- any adverse developments or perceived adverse developments with respect to the business separation; and
- •
- potential sales or purchases of our capital stock by GSK.
We may be unable to or elect not to continue returning capital to our stockholders
We have a corporate goal of returning capital to stockholders and have paid quarterly dividends during the 3rd and 4th quarters of 2014. The payment of, or continuation of, capital returns to stockholders is at the discretion of our board of directors and is dependent upon our financial condition, results of operations, capital requirements, general business conditions, tax treatment of capital returns, potential future contractual restrictions contained in credit agreements and other agreements and other factors deemed relevant by our board of directors. Future capital returns may also be affected by, among other factors: our views on potential future capital requirements for investments in acquisitions and our working capital and debt maintenance requirements; legal risks; stock repurchase programs; changes in federal and state income tax laws or corporate laws; and changes to our business model. Our capital returns may change from time to time, and we cannot provide assurance that we will continue to provide any particular amounts. A reduction or suspension in our capital returns programs could have a negative effect on our stock price.
Concentration of ownership will limit your ability to influence corporate matters.
As of February 12, 2015, GSK beneficially owned approximately 27.1% of our outstanding capital stock and our directors, executive officers and investors affiliated with these individuals beneficially owned approximately 0.9% of our outstanding capital stock. Based on our review of publicly available filings as of February 12, 2015, our three largest stockholders other than GSK collectively owned approximately 38.2% of our outstanding capital stock. These stockholders could control the outcome of actions taken by us that require stockholder approval, including a transaction in which stockholders might receive a premium over the prevailing market price for their shares.
29
Anti-takeover provisions in our charter and bylaws, in our rights agreement and in Delaware law could prevent or delay a change in control of our company.
Provisions of our Certificate of Incorporation and Bylaws may discourage, delay or prevent a merger or acquisition that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions include:
- •
- requiring supermajority stockholder voting to effect certain amendments to our Certificate of Incorporation and Bylaws;
- •
- restricting the ability of stockholders to call special meetings of stockholders;
- •
- prohibiting stockholder action by written consent; and
- •
- establishing advance notice requirements for nominations for election to the Board or for proposing matters that can be acted on by stockholders at meetings.
In addition, our Board has adopted a rights agreement that may prevent or delay a change in control of us. Further, some provisions of Delaware law may also discourage, delay or prevent someone from acquiring us or merging with us.
ITEM 1B. UNRESOLVED STAFF COMMENTS
During the first quarter of 2015, the SEC staff has inquired regarding our historical and current recognition of the up-front and milestone payments received from GSK pursuant to the LABA Collaboration Agreement between 2002 and 2006, and the milestone fees paid by us to GSK in 2013 and 2014. We are currently engaged in discussions with the SEC staff regarding our recognition of the payments made and received under the LABA Collaboration Agreement. While we believe that our accounting related to the LABA Collaboration Agreement is appropriate and in accordance with U.S. generally accepted accounting principles, the SEC staff reserves the right to make further inquiries regarding our accounting treatment of the payments made and received under the LABA Collaboration Agreement. If the SEC staff disagrees with our accounting treatment, we may be required to restate our financial statements for prior periods.
Our former headquarters consisted of 150,000 square feet of office and laboratory space leased in two buildings located in South San Francisco, California. Pursuant to the Assignment and Assumption of Lease agreement between us and Theravance Biopharma, we assigned and Theravance Biopharma assumed all of the rights and obligations under the existing lease agreements for these two buildings. We also entered into a Sublease Agreement with Theravance Biopharma to sublease 4,847 square feet of space in Building 951, which expires in May 2020. Management believes that this facility is suitable and adequate to meet the company's anticipated near-term needs. We anticipate that following the expiration of the sublease, additional or alternative space will be available at commercially reasonable terms. We do not own or lease any other properties.
We are not a party to any material legal proceedings.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
30
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Price Range of Common Stock
Our common stock has been traded on The NASDAQ Global Market under the symbol "THRX" since October 5, 2004. The following table sets forth the high and low closing prices of our common stock on a per share basis for the periods indicated and as reported on The NASDAQ Global Market. On June 2, 2014, we completed the Spin-Off, in which each of our stockholders received one ordinary share of Theravance Biopharma for every 3.5 shares of our common stock. The closing price of Theravance Biopharma shares on the first day of regular trading was $23.51, which represents an adjustment of $6.72. The stock prices below have not been adjusted for the impact of the Spin-Off.
Calendar Quarter
|
High | Low | |||||
|---|---|---|---|---|---|---|---|
2014 |
|||||||
Fourth Quarter |
$ | 18.64 | $ | 12.90 | |||
Third Quarter |
$ | 30.40 | $ | 17.09 | |||
Second Quarter |
$ | 31.33 | $ | 23.10 | |||
First Quarter |
$ | 40.49 | $ | 30.17 | |||
2013 |
|||||||
Fourth Quarter |
$ | 41.53 | $ | 33.74 | |||
Third Quarter |
$ | 42.64 | $ | 35.82 | |||
Second Quarter |
$ | 41.87 | $ | 22.53 | |||
First Quarter |
$ | 24.84 | $ | 20.16 | |||
Holders
As of February 12, 2015, there were 141 stockholders of record of our common stock. As many of our shares of common stock are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of stockholders represented by these record holders.
Recent Sales of Unregistered Securities
In November 2014, we completed the sale of 832,456 shares of our common stock to an affiliate of GSK at a price of $15.36 per share, resulting in aggregate gross proceeds of approximately $12.8 million before deducting transaction expenses. Neither we nor the affiliate of GSK engaged any investment advisors with respect to the sale and no finders' fees were paid or will be paid to any party in connection with the sale. We issued and sold the shares in reliance upon an exemption from registration pursuant to Section 4(2) of the Securities Act of 1933, as amended.
Dividends
During the third and fourth quarters of 2014, we paid aggregate cash dividends of $57.0 million to our stockholders. On February 20, 2015, our board of directors declared a $0.25 per share dividend for the first quarter of 2015 for a total of approximately $29.2 million. The payment of, or continuation of, capital returns to stockholders is at the discretion of our board of directors and is dependent upon our financial condition, results of operations, capital requirements, general business conditions, tax treatment of capital returns, potential future contractual restrictions contained in credit agreements and other agreements and other factors deemed relevant by our board of directors.
31
On June 2, 2014, we completed the Spin-Off, in which each of our stockholders received one ordinary share of Theravance Biopharma for every 3.5 shares of our common stock.
Equity Compensation Plans
The following table provides certain information with respect to all of our equity compensation plans in effect as of December 31, 2014:
Plan Category
|
Number of securities to be issued upon exercise of outstanding options |
Weighted-average exercise price of outstanding options |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
(a) |
(b) |
(c) |
|||||||
Equity compensation plans approved by security holders |
6,097,875 | (1) | $ | 22.68 | (3) | 3,320,959 | (4) | |||
Equity compensation plans not approved by security holders |
99,500 | (2) | $ | 10.67 | (3) | — | ||||
| | | | | | | | | | | |
Total |
6,197,375 | (1)(2) | $ | 22.46 | (3) | 3,320,959 | (4) | |||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
- (1)
- Includes
5,322,719 shares issuable upon exercise of outstanding options and 775,156 shares issuable upon vesting of outstanding restricted stock units and
restricted stock awards.
- (2)
- Includes
99,500 shares issuable upon exercise of outstanding options and no outstanding restricted stock units.
- (3)
- Does
not take into account outstanding restricted stock units as these awards have no exercise price.
- (4)
- Includes 284,139 shares of common stock available under our Employee Stock Purchase Plan.
In May 2012, we adopted the 2012 Equity Incentive Plan ("2012 Plan"). The number of shares of our common stock originally reserved for issuance under the 2012 Plan is equal to 6,500,000 shares plus up to 12,667,411 additional shares that may be added to the 2012 Plan in connection with the forfeiture, repurchase, cash settlement or termination of awards outstanding under the 2004 Equity Incentive Plan ("2004 Plan"), the 2008 New Employee Equity Incentive Plan, the 1997 Stock Plan and the Long-Term Stock Option Plan (collectively, the "Prior Plans") as of December 31, 2011. In connection with the Spin-Off, outstanding stock options and other awards, along with the number of shares remaining available for future stock options and other awards, were adjusted pursuant to the anti-dilution provisions of the 2012 Plan and Prior Plans. An additional 1,373,201 shares were added to the 2012 Plan share reserve as a result of the anti-dilution adjustment of the outstanding stock options and other awards granted under the 2012 Plan and the shares remaining available for future grant under the 2012 Plan. The additional 993,130 shares added to the Prior Plans as a result of the anti-dilution provisions are included in the 12,667,411 additional shares that may be added to the 2012 Plan.
While a maximum of 12,667,411 shares could be added to the 2012 Plan from the Prior Plans, this assumes that all the awards outstanding on December 31, 2011 will be forfeited, repurchased, cash settled or terminated. Therefore, the actual number that may be added to the 2012 Plan share reserve will likely be lower. No additional awards were made after May 15, 2012 under the 2004 Plan. Stock options and stock appreciation rights ("SARs") will reduce the 2012 Plan reserve by one share for every share granted, and stock awards other than options and SARs granted will reduce the 2012 Plan share reserve by 1.45 shares for every share granted. The 2012 Plan share reserve was also reduced by
32
the number of stock awards granted under the 2004 Plan on or after January 1, 2012, using the same ratios described.
The 2012 Plan provides for the grant of incentive stock options, nonstatutory stock options, restricted stock awards, stock unit awards and SARs to our employees, non-employee directors and consultants. Stock options may be granted with an exercise price not less than the fair market value of the common stock on the grant date. Stock options granted to employees generally have a maximum term of 10 years and vest over a four year period from the date of grant; 25% vest at the end of one year, and 75% vest monthly over the remaining three years. We may grant options with different vesting terms from time to time. Unless an employee's termination of service is due to disability or death, upon termination of service, any unexercised vested options will be forfeited at the end of three months or the expiration of the option, whichever is earlier. Additional information regarding stock-based compensation is included in Note 1, "Description of Operations and Summary of Significant Accounting Policies," and Note 6, "Stock-Based Compensation," to the consolidated financial statements appearing in this Annual Report on Form 10-K.
Stock Performance Graph
The graph set forth below compares the cumulative total stockholder return on our common stock for the period commencing on December 31, 2009 and ending on December 31, 2014, with the cumulative total return of (i) the NASDAQ Composite Index, (ii) the NASDAQ Pharmaceutical Index and (iii) the NASDAQ Biotechnology Index over the same period. This graph assumes the investment of $100.00 on December 31, 2009 in each of (1) our common stock, (2) the Nasdaq Composite Index, (3) the NASDAQ Pharmaceutical Index and (4) the NASDAQ Biotechnology Index, and assumes the reinvestment of dividends.
The comparisons shown in the graph below are based upon historical data. We caution that the stock price performance shown in the graph below is not necessarily indicative of, nor is it intended to forecast, the potential future performance of our common stock. Information used in the graph was obtained from Research Data Group, Inc., a source believed to be reliable, but we are not responsible for any errors or omissions in such information.
Notwithstanding anything to the contrary set forth in any of our previous or future filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, that might incorporate this Annual Report on Form 10-K or future filings made by us under those statutes, this Stock Performance Graph section shall not be deemed filed with the SEC and shall not be deemed incorporated by reference into any of those prior filings or into any future filings made by us under those statutes.
33
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among
Theravance, Inc., the NASDAQ Composite Index, the NASDAQ Pharmaceutical Index,
and the NASDAQ Biotechnology Index
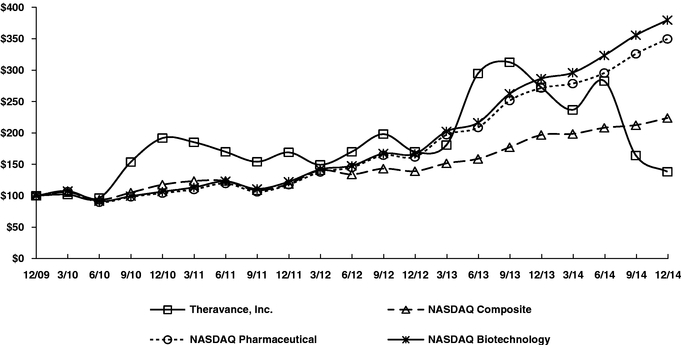
- *
- $100 invested on December 31, 2009 in stock or index, including reinvestment of dividends.
34
ITEM 6. SELECTED FINANCIAL DATA
The selected consolidated summary financial data below should be read in conjunction with Part II, Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations" and Part II, Item 8, "Financial Statements and Supplementary Data", in this Annual Report on Form 10-K. The historical results are not necessarily indicative of the results to be expected in any future period.
| |
Year ended December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2014 | 2013 | 2012 | 2011 | 2010 | |||||||||||
| |
(In thousands, except per share data) |
|||||||||||||||
CONSOLIDATED STATEMENTS OF OPERATIONS DATA |
||||||||||||||||
Net revenue |
$ | 8,433 | $ | 4,532 | $ | 5,613 | $ | 9,658 | $ | 9,827 | ||||||
Operating expenses: |
||||||||||||||||
Research and development |
7,498 | 9,038 | 8,153 | 8,560 | 7,959 | |||||||||||
General and administrative |
34,864 | 24,289 | 22,606 | 22,382 | 19,745 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total operating expenses(1) |
42,362 | 33,327 | 30,759 | 30,942 | 27,704 | |||||||||||
| | | | | | | | | | | | | | | | | |
Loss from operations |
(33,929 | ) | (28,795 | ) | (25,146 | ) | (21,284 | ) | (17,877 | ) | ||||||
Interest and other income, net |
(2,709 | ) | 7,510 | 460 | 415 | 505 | ||||||||||
Interest expense |
(36,892 | ) | (9,348 | ) | (6,003 | ) | (6,022 | ) | (6,044 | ) | ||||||
Loss from continuing operations |
(73,530 | ) | (30,633 | ) | (30,689 | ) | (26,891 | ) | (23,416 | ) | ||||||
Income (loss) from discontinued operations(1) |
(94,934 | ) | (140,068 | ) | 12,147 | (88,453 | ) | (60,446 | ) | |||||||
| | | | | | | | | | | | | | | | | |
Net loss |
$ | (168,464 | ) | $ | (170,701 | ) | $ | (18,542 | ) | $ | (115,344 | ) | $ | (83,862 | ) | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Basic and diluted net income (loss) per share: |
||||||||||||||||
Continuing operations, net of tax |
$ | (0.66 | ) | $ | (0.30 | ) | $ | (0.34 | ) | $ | (0.33 | ) | $ | (0.32 | ) | |
Discontinued operations |
(0.84 | ) | (1.37 | ) | 0.14 | (1.08 | ) | (0.84 | ) | |||||||
| | | | | | | | | | | | | | | | | |
Total |
$ | (1.50 | ) | $ | (1.67 | ) | $ | (0.20 | ) | $ | (1.41 | ) | $ | (1.16 | ) | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Shares used to compute basic and diluted net income (loss) per share |
112,059 | 102,425 | 90,909 | 82,051 | 72,070 | |||||||||||
Cash dividends declared per common share |
$ | 0.50 | $ | — | $ | — | $ | — | $ | — | ||||||
| |
As of December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2014 | 2013 | 2012 | 2011 | 2010 | |||||||||||
| |
(In thousands) |
|||||||||||||||
CONSOLIDATED BALANCE SHEETS DATA |
||||||||||||||||
Cash, cash equivalents and marketable securities |
$ | 283,354 | $ | 520,499 | $ | 343,683 | $ | 240,915 | $ | 309,634 | ||||||
Working capital |
238,426 | 398,794 | 231,167 | 199,267 | 276,300 | |||||||||||
Total assets |
521,654 | 681,255 | 368,582 | 258,782 | 331,202 | |||||||||||
Long-term liabilities(2) |
731,247 | 297,729 | 183,588 | 300,338 | 313,568 | |||||||||||
Accumulated (deficit) equity |
(1,673,667 | ) | (1,505,203 | ) | (1,334,502 | ) | (1,315,960 | ) | (1,200,616 | ) | ||||||
Total stockholders' (deficit) equity |
$ | (223,349 | ) | $ | 299,122 | $ | 155,028 | $ | (87,052 | ) | $ | (22,420 | ) | |||
- (1)
- Stock-based compensation expense included in total operating expenses is as follows:
| |
Year ended December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2014 | 2013 | 2012 | 2011 | 2010 | |||||||||||
| |
(In thousands) |
|||||||||||||||
Research and development |
$ | 2,781 | $ | 573 | $ | 475 | $ | 725 | $ | 229 | ||||||
General and administrative |
12,980 | 7,325 | 7,310 | 8,159 | 5,454 | |||||||||||
| | | | | | | | | | | | | | | | | |
Stock-based compensation from continuing operations |
15,761 | 7,898 | 7,785 | 8,884 | 5,683 | |||||||||||
Stock-based compensation from discontinued operations |
11,629 | 17,789 | 15,998 | 16,032 | 14,226 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total stock-based compensation |
$ | 27,390 | $ | 25,687 | $ | 23,783 | $ | 24,916 | $ | 19,909 | ||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- (2)
- Long-term liabilities include the long-term portion of deferred revenue as follows:
| |
Year ended December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2014 | 2013 | 2012 | 2011 | 2010 | |||||||||||
| |
(In thousands) |
|||||||||||||||
Deferred revenue |
$ | 3,788 | $ | 5,455 | $ | 6,014 | $ | 122,017 | $ | 137,425 | ||||||
35
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Management's Discussion and Analysis (MD&A) is intended to facilitate an understanding of our business and results of operations. This discussion and analysis should be read in conjunction with our consolidated financial statements and notes included in this Annual Report on Form 10-K. The information contained in this discussion and analysis or set forth elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business, our operating expenses, and future payments under our collaboration agreements, includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Such statements are based upon current expectations that involve risks and uncertainties. You should review the section entitled "Risk Factors" in Item 1A of Part I above for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis. See the section entitled "Special Note Regarding Forward Looking Statements" above for more information.
Management Overview
Theravance, Inc. is a royalty management company focused on maximizing the potential value of the respiratory assets partnered with Glaxo Group Limited ("GSK"), including RELVAR®/BREO® ELLIPTA® (fluticasone furoate/ vilanterol, "FF/VI") and ANORO® ELLIPTA® (umeclidinium bromide/ vilanterol, "UMEC/VI"), with the intention of providing capital returns to stockholders. Under the Long-Acting Beta2 Agonist ("LABA") Collaboration Agreement and the Strategic Alliance Agreement with GSK (referred to herein collectively as the "GSK Agreements"), Theravance is eligible to receive the associated royalty revenues from RELVAR®/BREO® ELLIPTA® , ANORO® ELLIPTA® and if approved and commercialized, VI monotherapy. Theravance is also entitled to 15% of any future payments made by GSK under its agreements originally entered into with us, and since assigned to Theravance Respiratory Company, LLC ("TRC"), relating to the combination FF/UMEC/VI and the Bifunctional Muscarinic Antagonist-Beta2 Agonist ("MABA") program, as monotherapy and in combination with other therapeutically active components, such as an inhaled corticosteroid, and any other product or combination of products that may be discovered and developed in the future under the LABA Collaboration Agreement, which has been assigned to TRC other than RELVAR®/BREO®ELLIPTA®, ANORO® ELLIPTA® and VI monotherapy.
On June 1, 2014, we separated our biopharmaceutical research and drug development operations from our late-stage partnered respiratory assets by transferring our research and drug development operations into our then wholly-owned subsidiary, Theravance Biopharma, Inc. ("Theravance Biopharma"). We contributed $393.0 million of cash, cash equivalents and marketable securities to Theravance Biopharma and all outstanding shares of Theravance Biopharma were then distributed to Theravance stockholders as a pro-rata dividend distribution on June 2, 2014 by issuing one ordinary share of Theravance Biopharma for every 3.5 shares held of our common stock to stockholders of record on May 15, 2014 (the "Spin-Off"). The Spin-Off resulted in Theravance Biopharma operating as an independent publicly-traded company. The results of operations for the former research and drug development operations conducted by us and by Theravance Biopharma until June 1, 2014 are included as part of this report as discontinued operations.
Pursuant to a three-way master agreement entered into by and among us, Theravance Biopharma and GSK in connection with the Spin-Off, we agreed to sell a certain number of Theravance Biopharma shares withheld from a taxable dividend of Theravance Biopharma shares to GSK. After such Theravance Biopharma shares were sent to the transfer agent, we agreed to purchase the Theravance Biopharma shares from the transfer agent, rather than have them sold on the open market, in order to satisfy tax withholdings. GSK had a right to purchase these shares of Theravance
36
Biopharma from us, but this right expired unexercised. Accordingly, at December 31, 2014, we owned 436,802 ordinary shares of Theravance Biopharma.
As a royalty management company, we have designed our company structure and organization to be focused on managing our respiratory assets with GSK, the commercial and developmental obligations associated with the GSK Agreements, intellectual property, licensing operations, and providing for certain essential reporting and management functions of a public company. As of December 31, 2014, we had ten employees. Our revenues consist of royalties and potential milestone payments, if any, from our respiratory partnership agreements with GSK.
Financial Highlights
In 2014, our net loss from our continuing operations was $73.5 million, an increase of $42.9 million from $30.6 million in 2013, primarily due to higher employee-related expenses, including stock-based compensation expense, and an increase in interest expense from our non-recourse notes payable due 2029 (the "2029 Notes"). Cash, cash equivalents, and marketable securities, totaled $283.4 million on December 31, 2014, a decrease of $237.1 million from December 31, 2013. The decrease was due primarily to the contribution of $393.0 million to Theravance Biopharma in connection with the Spin-Off, cash used in operations of $130.7 million, registrational and launch-related milestone payments to GSK of $135.0 million and payments of cash dividends of $57.0 million. These outflows were partially offset by net proceeds of $434.7 million from the issuance of our non-recourse notes due 2029, net proceeds of $48.9 million received from issuances of our common stock and $18.4 million from royalties earned from GSK.
Declaration and Payment of Cash Dividends
During each of the third and fourth quarters of 2014, our board of directors declared a quarterly dividend of $0.25 per share of common stock to stockholders resulting in aggregate cash dividends of $57.0 million paid to our stockholders in 2014. In connection with the payments of these cash dividends, the conversion rate with respect to our 2.125% Convertible Subordinated Notes due 2023 (the "2023 Notes") was adjusted.
Product Highlights
- 1.
- In
the fourth quarter 2014, sales for RELVAR®/BREO® ELLIPTA® by GSK were $62.2 million compared to
$25.6 million in the previous quarter, an increase of approximately 142%, resulting in total sales of $110.9 million in 2014.
- 2.
- In
the fourth quarter 2014, sales for ANORO® ELLIPTA® by GSK were $17.4 million compared to $1.8 million in the
previous quarter, a substantial increase resulting in total sales of $27.4 million in 2014.
- 3.
- GSK
announced that as of January 2015, U.S. Medicare Part D coverage has increased to 76 percent for BREO® ELLIPTA® and
to 65 percent for ANORO® ELLIPTA®. In addition, as of January 2015, 64 percent are insured through commercial plans for BREO® ELLIPTA® and
78 percent for ANORO® ELLIPTA®.
- 4.
- A
Phase 3 study evaluating the effectiveness of RELVAR®/BREO® ELLIPTA® compared to other COPD treatments, as
measured by the primary endpoint of the mean annual rate of moderate and severe exacerbations, one of the Salford Lung Studies being conducted, completed enrollment of 2,800 patients.
- 5.
- GSK secured reimbursement for ANORO® ELLIPTA® via the Australian Pharmaceutical Benefits Scheme (PBS) as a long-term once-daily, maintenance bronchodilator treatment to relieve symptoms in adult patients with COPD.
37
Collaborative Arrangements with GSK
LABA Collaboration
In November 2002, we entered into our LABA Collaboration Agreement with GSK to develop and commercialize once-daily LABA products for the treatment of chronic obstructive pulmonary disease ("COPD") and asthma. For the treatment of COPD, the collaboration has developed two combination products: (1) RELVAR®/BREO® ELLIPTA® (FF/VI) (BREO® ELLIPTA® is the proprietary name in the U.S. and Canada and RELVAR® ELLIPTA® is the proprietary name outside the U.S. and Canada), a once-daily combination medicine consisting of a LABA, vilanterol (VI), and an inhaled corticosteroid (ICS), fluticasone furoate (FF) and (2) ANORO® ELLIPTA® (UMEC/VI), a once-daily medicine combining a long-acting muscarinic antagonist ("LAMA"), umeclidinium bromide (UMEC), with a LABA, VI. Under the collaboration agreements between the parties, GSK and Theravance are exploring various paths to create triple therapy medications. For the treatment of asthma, RELVAR® ELLIPTA® is approved in multiple regions outside of North America and the collaboration is further developing FF/VI for the U.S. The FF/VI program is aimed at developing a once-daily combination LABA/ICS to succeed GSK's Advair ® /Seretide™ (salmeterol and fluticasone as a combination) franchise, which had reported 2014 sales of approximately $7.0 billion, and to compete with Symbicort® (formoterol and budesonide as a combination), which had reported 2014 sales of approximately $3.8 billion. ANORO® ELLIPTA®, which is also a combination product, is targeted as an alternative treatment option to Spiriva® (tiotropium), a once-daily, single-mechanism bronchodilator, which had reported 2013 sales of approximately $4.7 billion.
As a result of the launch and approval of RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® in the U.S., Japan and Europe, we were obligated to pay milestone fees to GSK totaling $220.0 million, which we have paid in their entirety as of December 31, 2014. Although we have no further milestone payment obligations to GSK pursuant to the LABA Collaboration Agreement, we continue to have ongoing development and commercialization activities under the GSK Agreements that are expected to continue over the life of the agreements. The milestone fees paid to GSK were recognized as capitalized fees paid to a related party, which are being amortized over their estimated useful lives commencing upon the commercial launch of the product.
We are entitled to receive annual royalties from GSK on sales of RELVAR®/BREO® ELLIPTA® as follows: 15% on the first $3.0 billion of annual global net sales and 5% for all annual global net sales above $3.0 billion. Sales of single-agent LABA medicines and combination medicines would be combined for the purposes of this royalty calculation. For other products combined with a LABA from the LABA collaboration, such as ANORO™ ELLIPTA™, royalties are upward tiering and range from 6.5% to 10%.
2004 Strategic Alliance
In March 2004, we entered into the Strategic Alliance Agreement with GSK where GSK received an option to license exclusive development and commercialization rights to product candidates from certain of our discovery programs on pre-determined terms and on an exclusive, worldwide basis. Upon GSK's decision to license a program, GSK is responsible for funding all future development, manufacturing and commercialization activities for product candidates in that program. In addition, GSK is obligated to use diligent efforts to develop and commercialize product candidates from any program that it licenses. If the program is successfully advanced through development by GSK, we are entitled to receive clinical, regulatory and commercial milestone payments and royalties on any sales of medicines developed from the program. If GSK chooses not to license a program, we retain all rights to the program and may continue the program alone or with a third party. GSK has no further option rights on any of our research or development programs under the strategic alliance.
38
In 2005, GSK licensed our MABA program for the treatment of COPD, and in October 2011, we and GSK expanded the MABA program by adding six additional Theravance-discovered preclinical MABA compounds (the "Additional MABAs"). GSK's development, commercialization, milestone and royalty obligations under the strategic alliance remain the same with respect to GSK961081 ('081), the lead compound in the MABA program. GSK is obligated to use diligent efforts to develop and commercialize at least one MABA within the MABA program, but may terminate progression of any or all Additional MABAs at any time and return them to us, at which point we may develop and commercialize such Additional MABAs alone or with a third party. Both GSK and we have agreed not to conduct any MABA clinical studies outside of the strategic alliance so long as GSK is in possession of the Additional MABAs. If a single-agent MABA medicine containing '081 is successfully developed and commercialized, GSK is required to pay royalties of between 10% and 20% of annual global net sales up to $3.5 billion, and 7.5% for all annual global net sales above $3.5 billion. If a MABA medicine containing '081 is commercialized as a combination product, such as a '081/FF, the royalty rate is 70% of the rate applicable to sales of the single-agent MABA medicine. For single-agent MABA medicines containing an Additional MABA, GSK is required to pay royalties of between 10% and 15% of annual global net sales up to $3.5 billion, and 10% for all annual global net sales above $3.5 billion. For combination products containing an Additional MABA, such as a MABA/ICS combination, the royalty rate is 50% of the rate applicable to sales of the single-agent MABA medicine. If a MABA medicine containing '081 is successfully developed and commercialized in multiple regions of the world, GSK could be required to pay total contingent payments of up to $125.0 million for a single-agent medicine and up to $250.0 million for both a single-agent and a combination medicine. If a MABA medicine containing an Additional MABA is successfully developed and commercialized in multiple regions of the world, GSK could be required to pay total contingent payments of up to $129.0 million. As a result of the transactions effected by the Spin-Off, we are only entitled to receive 15% of any contingent payments and royalties payable by GSK from sales of FF/UMEC/VI (and MABA, and MABA/FF) while Theravance Biopharma receives 85% of those same payments.
Agreements Entered into with GSK in Connection with the Spin-Off
On March 3, 2014, in contemplation of the Spin-Off of Theravance Biopharma, we, Theravance Biopharma and GSK entered into a series of agreements clarifying how the companies would implement the Spin-Off and operate following the Spin-Off. We, Theravance Biopharma and GSK entered into a three-way master agreement providing for GSK's consent to the Spin-Off provided certain conditions were met. In addition, we and GSK also entered into amendments to the GSK Agreements, and Theravance Biopharma and GSK entered into a governance agreement, a registration rights agreement and an extension agreement. The three-way master agreement was effective on June 1, 2014 when we transferred our research and drug development operations to Theravance Biopharma. Pursuant to a three-way master agreement entered into by and among us, Theravance Biopharma and GSK in connection with the Spin-Off, we agreed to sell a certain number of Theravance Biopharma shares withheld from a taxable dividend of Theravance Biopharma shares to GSK. After such Theravance Biopharma shares were sent to the transfer agent, we agreed to purchase the Theravance Biopharma shares from the transfer agent, rather than have them sold on the open market, in order to satisfy tax withholdings. GSK had a right to purchase these shares of Theravance Biopharma from us, but this right expired unexercised. Accordingly, at December 31, 2014, we owned 436,802 ordinary shares of Theravance Biopharma.
The amendments to the GSK Agreements do not change the economics or royalty rates under the GSK Agreements, though the assignment of the Strategic Alliance Agreement and portions of the LABA Collaboration Agreement to TRC do change how the economics are allocated between Theravance Biopharma and us. The amendments to the GSK Agreements do provide that GSK's diligent efforts obligations regarding commercialization matters under both agreements will change upon regulatory approval in either the United States or the European Union of FF/UMEC/VI or a
39
MABA in combination with FF. Upon such regulatory approval, GSK's diligent efforts obligations as to commercialization matters under the GSK Agreements will have the objective of focusing on the best interests of patients and maximizing the net value of the overall portfolio of products under the GSK Agreements. Since GSK's commercialization efforts following such regulatory approval will be guided by a portfolio approach across products in which we will retain our full interests upon the Spin-Off and also products in which we will have retained only a portion of our interests upon the planned Spin-Off transaction, GSK's commercialization efforts may have the effect of reducing the overall value of our remaining interests in the GSK Agreements after the Spin-Off.
Purchases of Common Stock by GSK
Prior to 2014, affiliates of GSK purchased an aggregate of 29.9 million shares of our common stock. During 2014, GSK purchased 1.7 million shares of our common stock pursuant to its periodic "top-up" rights under our Amended and Restated Governance Agreement, dated as of June 4, 2004, as amended, among us, GSK and certain GSK affiliates, for an aggregate purchase price of $38.1 million. As of February 12, 2015, GSK beneficially owned approximately 27.1% of our outstanding capital stock.
Critical Accounting Policies and Estimates
Our management's discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles ("GAAP"). The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported revenue generated and expenses incurred during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. We believe that the accounting policies discussed below are critical to understanding our historical and future performance, as these policies relate to the more significant areas involving management's judgments and estimates.
Revenue Recognition
Revenue is recognized when the four basic criteria of revenue recognition are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the fee is fixed or determinable; and (4) collectability is reasonably assured. Where the revenue recognition criteria are not met, we defer the recognition of revenue by recording deferred revenue until such time that all criteria are met.
Collaborative Arrangements and Multiple Element Arrangements
We generate revenue from collaboration and license agreements for the development and commercialization of product candidates. Collaboration and license agreements may include non-refundable upfront payments, partial or complete reimbursement of research and development costs, supply arrangement, contingent payments based on the occurrence of specified events under our collaborative arrangements, license fees and royalties on sales of product candidates if they are successfully approved and commercialized. Our performance obligations under the collaborations may include the transfer of intellectual property rights in the form of licenses, obligations to provide research and development services and related materials, supply of active pharmaceutical ingredient ("API") and/or drug product, and obligations to participate on certain development and/or commercialization committees with the collaborative partners. We make judgments that affect the periods over which we recognize revenue. We periodically review our estimated periods of performance
40
based on the progress under each arrangement and account for the impact of any changes in estimated periods of performance on a prospective basis.
On January 1, 2011, we adopted an accounting standards update that amends the guidance on accounting for new or materially modified multiple-element arrangements that we enter into subsequent to January 1, 2011. This guidance removed the requirement for objective and reliable evidence of fair value of the undelivered items in order to consider a deliverable a separate unit of accounting. It also changed the allocation method such that the relative-selling-price method must be used to allocate arrangement consideration to all the units of accounting in an arrangement. This guidance established the following hierarchy that must be used in estimating selling price under the relative-selling-price method: (1) vendor-specific objective evidence of fair value of the deliverable, if it exists, (2) third-party evidence of selling price, if vendor-specific objective evidence is not available or (3) vendor's best estimate of selling price ("BESP") if neither vendor-specific nor third-party evidence is available.
We may determine that the selling price for the deliverables within collaboration and license arrangements should be determined using BESP. The process for determining BESP involves significant judgment on our part and includes consideration of multiple factors such as estimated direct expenses and other costs, and available data. We have determined BESP for license units of accounting based on market conditions, similar arrangements entered into by third parties and entity-specific factors such as the terms of previous collaborative agreements, our pricing practices and pricing objectives, the likelihood that clinical trials will be successful, the likelihood that regulatory approval will be received and that the products will become commercialized. We have also determined BESP for services-related deliverables based on the nature of the services to be performed and estimates of the associated effort as well as estimated market rates for similar services.
For each unit of accounting identified within an arrangement, we determine the period over which the performance obligation occurs. Revenue is then recognized using either a proportional performance or straight-line method. We recognize revenue using the proportional performance method when the level of effort to complete our performance obligations under an arrangement can be reasonably estimated. Direct labor hours or full time equivalents are typically used as the measurement of performance. Any changes in the remaining estimated performance obligation periods under these collaborative arrangements will not have a significant impact on the results of operations, except for a change in estimated performance period resulting from the termination of a collaborative arrangement, which would result in immediate recognition of the related deferred revenue.
The GSK Agreements were entered into prior to January 1, 2011. The delivered items under these collaborative agreements did not meet the criteria required to be accounted for as separate accounting units for the purposes of revenue recognition. As a result, revenue from non-refundable, upfront fees and development contingent payments were recognized ratably over the expected term of our performance of research and development services under the agreements. These upfront or contingent payments received, pending recognition as revenue, were recorded as deferred revenue and recognized over the estimated performance periods.
Under the GSK Agreements, we recognized revenue of $8.4 million, $4.5 million and $5.6 million for the years ended December 31, 2014, 2013 and 2012. The remaining deferred revenue under the GSK Strategic Alliance Agreement is $4.9 million at December 31, 2014. Any change in the estimated performance period, which is predominantly based on GSK's development timeline, will not have a significant impact on the results of operations, except for a change in estimated performance period resulting from the termination of the MABA program that would result in immediate recognition of the deferred revenue.
On January 1, 2011, we also adopted an accounting standards update that provides guidance on revenue recognition using the milestone method. Payments that are contingent upon achievement of a
41
substantive milestone are recognized in their entirety in the period in which the milestone is achieved. Milestones are defined as events that can be achieved based only on our performance and as to which, at the inception of the arrangement, there is substantive uncertainty about whether the milestone will be achieved. Events that are contingent only on the passage of time or only on third-party performance are not considered milestones subject to this guidance. Further, the amounts received must relate solely to prior performance, be reasonable relative to all of the deliverables and payment terms in the agreement and commensurate with our performance to achieve the milestone after commencement of the agreement. Total contingent payments that may become payable to us under our collaborative agreements were up to $363.0 million at December 31, 2014 and are considered non-substantive.
Under the GSK Agreements, royalty revenue earned is reduced by amortization expense resulting from the fees paid to GSK, which were recognized as capitalized fees paid to a related party. When amortization expense exceeds amounts recognized for royalty revenues from GSK, negative revenue would be reported in our consolidated statements of operations.
Amounts related to research and development funding is recognized as the related services or activities are performed, in accordance with the contract terms. Payments may be made to us based on the number of full-time equivalent researchers assigned to the collaborative project and the related research and development expenses incurred. Accordingly, reimbursement of research and development expenses pursuant to the cost-sharing provisions of our agreements with certain collaborative partners are recognized as a reduction of research and development expenses.
Royalties
We recognize royalty revenue on licensee net sales of products with respect to which we have royalty rights in the period in which the royalties are earned and reported to us and collectability is reasonably assured. Royalties are recognized net of amortization of capitalized fees paid to a related party associated with any approval and launch milestone payments made to GSK.
Capitalized Fees paid to a Related Party
We capitalize fees paid to licensors related to agreements for approved products or commercialized products. We capitalize these fees as capitalized fees paid to a related party ("Capitalized Fees") and amortize these Capitalized Fees on a straight-line basis over their estimated useful lives upon the commercial launch of the product, which is expected to be shortly after regulatory approval of such product. The estimated useful lives of these Capitalized Fees are based on a country-by-country and product-by-product basis, as the later of the expiration or termination of the last patent right covering the compound in such product in such country and 15 years from first commercial sale of such product in such country, unless the agreement is terminated earlier. Consistent with our policy for classification of costs under the research and development collaborative arrangements, the amortization of these Capitalized Fees will be recognized as a reduction of royalty revenue.
We review our Capitalized Fees for impairment when events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. The recoverability of Capitalized Fees is measured by comparing the asset's carrying amount to the expected undiscounted future cash flows that the asset is expected to generate. The determination of recoverability typically requires various estimates and assumptions, including estimating the useful life over which cash flows will occur, their amount, and the asset's residual value, if any. We derive the required cash flow estimates from near-term forecasted product sales and long-term projected sales in the corresponding market.
Our gross Capitalized Fees of $220.0 million at December 31, 2014 consist of registrational and launch-related to milestone fees paid to GSK (see "Collaborative Arrangements with GSK" above for more information). These Capitalized Fees are amortized over their estimated useful lives using the straight-line method commencing upon commercial launch.
42
Fair Value of Stock-Based Compensation Awards
We use the Black-Scholes-Merton option pricing model to estimate the fair value of options at the date of grant. The Black-Scholes-Merton option valuation model requires the use of assumptions, including the expected term of the award and the expected stock price volatility. We use the "simplified" method as described in Staff Accounting Bulletin No. 107, "Share Based Payment," for the expected option term because the usage of our historical option exercise data is limited due to post-IPO exercise restrictions. Beginning April 1, 2011, we have used our historical volatility to estimate expected stock price volatility. Prior to April 1, 2011, we used our peer company price volatility to estimate expected stock price volatility due to our limited historical common stock price volatility since our initial public offering in 2004. The estimated fair value of the option is expensed on a straight-line basis over the expected term of the grant.
We estimated the fair value of restricted stock units ("RSUs") and restricted stock awards ("RSAs") based on the fair market values of the underlying stock on the dates of grant. The estimated fair value of time-based RSUs and RSAs is expensed on a straight-line basis over the expected term of the grant. The estimated fair value of performance-contingent RSUs and RSAs is expensed using an accelerated method over the requisite service period based on management's best estimate as to whether it is probable that the shares awarded are expected to vest. We assess the probability of the performance indicators being met on a continuous basis.
Stock-based compensation expense was calculated based on awards ultimately expected to vest and was reduced for estimated forfeitures at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differed from those estimates. The estimated annual forfeiture rates for stock options, RSUs and RSAs are based on our historical forfeiture experience.
We do not expect to recognize in the near future any tax benefit related to employee stock-based compensation expense as a result of the full valuation allowance on our deferred tax assets including deferred tax assets related to our net operating loss carry forwards.
For more information, refer to Note 6, "Stock-Based Compensation," to the consolidated financial statements appearing in this Annual Report on Form 10-K.
Amortization of Debt Issuance Costs from Non-recourse Notes Payable, due 2029
In April 2014, we entered into certain note purchase agreements relating to the private placement of $450.0 million aggregate principal amount of non-recourse 9% fixed rate term notes due 2029 (the "2029 Notes") issued by our wholly-owned subsidiary.
The 2029 Notes are secured exclusively by a security interest in a segregated bank account established to receive 40% of royalties due to us under the LABA Collaboration with GSK commencing on April 1, 2014 and ending upon the earlier of full repayment of principal or May 15, 2029. The funds in the segregated bank account can only be used to make principal and interest payments on the 2029 Notes.
The 2029 Notes bear an annual interest rate of 9%, with interest and principal paid quarterly beginning November 15, 2014. The 2029 Notes may be redeemed at any time prior to maturity, in whole or in part, at specified redemption premiums. Prior to May 15, 2016, in the event that the specified portion of royalties received in a quarter is less than the interest accrued for the quarter, the principal amount of the 2029 Notes will increase by the interest shortfall amount for that period.
As part of this sale, we incurred approximately $15.3 million in transaction costs, which will be amortized to interest expense over the estimated life of the 2029 Notes based on the effective interest method. Since the principal and interest payments on the 2029 Notes are based on royalties from product sales, which will vary from quarter to quarter, the 2029 Notes may be repaid prior to the final
43
maturity date in 2029. To the extent that the interest or principal payments are greater or less than our initial estimates or the timing of such payments is materially different than our original estimates, we will prospectively adjust the amortization of the debt issuance costs. There are a number of factors that could materially affect the amount and timing of the royalty payments due to us under the LABA Collaboration with GSK, most of which are not within our control. Such factors include, but are not limited to, the competitive landscape for approved products and developing therapies that compete with our partnered products, the ability of patients to be able to afford our partnered products, the size of the market for our partnered products, safety concerns in the marketplace for respiratory therapies in general and with our partnered products in particular, decisions as to the timing of product launches, pricing and discounts, and other events or circumstances that result in reduced royalty payments, all of which would result in an impact to the amount of debt issuance costs amortized.
Results of Operations
Net Revenue
Total net revenue from continuing operations, as compared to the prior years, was as follows:
| |
|
|
|
Change | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year Ended December 31, | 2014 | 2013 | |||||||||||||||||||
(In thousands)
|
2014 | 2013 | 2012 | $ | % | $ | % | |||||||||||||||
Royalties from a related party |
$ | 18,417 | $ | 1,945 | $ | — | $ | 16,472 | * | $ | 1,945 | * | ||||||||||
Less: amortization of capitalized fees paid to a related party |
(11,066 | ) | (743 | ) | — | (10,323 | ) | * | (743 | ) | * | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Royalty revenue |
7,351 | 1,202 | — | 6,149 | * | 1,202 | * | |||||||||||||||
LABA collaboration |
— | 1,815 | 3,629 | (1,815 | ) | (100 | )% | (1,814 | ) | (50 | )% | |||||||||||
Strategic alliance—MABA program license(1) |
1,082 | 1,515 | 1,984 | (433 | ) | (29 | ) | (469 | ) | (24 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Total net revenue from GSK |
$ | 8,433 | $ | 4,532 | $ | 5,613 | $ | 3,901 | 86 | % | $ | (1,081 | ) | (19 | ) | |||||||
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
- *
- Not
Meaningful
- (1)
- We revised the estimated performance period for the MABA program based on its progress as follows: (i) in the fourth quarter of 2012, resulting in an increase to net loss of $0.1 million for the year ended December 31, 2012 and (ii) in the fourth quarter of 2013, resulting in an increase to net loss of $0.1 million for the year ended December 31, 2013. We do not expect that these revisions will have a material impact on future revenue recognized under this program.
Total net revenue from continuing operations increased in 2014 compared to 2013 and decreased in 2013 compared to 2012.
Royalty revenue recognized in 2014 includes royalties from ANORO® ELLIPTA®, which was launched in 2014, and a full year of royalties from RELVAR®/ BREO® ELLIPTA®, which was launched in the fourth quarter of 2013. Royalty revenue recognized under the LABA Collaboration Agreement with GSK is reduced by amortization expense for Capitalized Fees, which commences upon commercial launch.
Revenue from collaborative arrangements includes deferred revenue under the LABA Collaboration Agreement with GSK, which was fully recognized by June 2013.
44
Research & Development
Research & Development (R&D) expenses from continuing operations, as compared to the prior years, were as follows:
| |
|
|
|
Change | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year Ended December 31, | 2014 | 2013 | |||||||||||||||||||
(In thousands)
|
2014 | 2013 | 2012 | $ | % | $ | % | |||||||||||||||
Research and development expenses |
$ | 7,498 | $ | 9,038 | $ | 8,153 | $ | (1,540 | ) | (17 | )% | $ | 885 | 11 | % | |||||||
R&D expenses from continuing operations decreased in 2014 compared to 2013 primarily due to fewer allocated costs as our ongoing operations are significantly smaller as a result of the Spin-Off.
R&D expenses from continuing operations increased in 2013 compared to 2012 primarily due to higher stock- based compensation costs as a result of the achievement of performance conditions under a special long-term retention and incentive equity award granted to certain employees in 2011 for which the expense commenced in 2013.
We expect R&D expenses in 2015 to decrease compared to 2014 due to the Spin-Off of our research and drug development operations. Currently, our research and development expenses are primarily due to expenses related to the late-stage partnered respiratory assets with GSK.
General & Administrative
General and administrative expenses from continuing operations, as compared to the prior years, were as follows:
| |
|
|
|
Change | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year Ended December 31, | 2014 | 2013 | |||||||||||||||||||
(In thousands)
|
2014 | 2013 | 2012 | $ | % | $ | % | |||||||||||||||
General and administrative expenses |
$ | 34,864 | $ | 24,289 | $ | 22,606 | $ | 10,575 | 44 | % | $ | 1,683 | 7 | % | ||||||||
General and administrative expenses from continuing operations increased in 2014 compared to 2013 primarily due to higher stock-based compensation expense and employee-related costs. Stock-based compensation expense and employee-related costs increased primarily due to the probable achievement of performance conditions under a special long-term retention and incentive equity and cash bonus awarded to certain employees in 2011.
General and administrative expenses from continuing operations increased in 2013 compared to 2012 primarily due to an increase in external legal and accounting fees in connection with our separation strategy and employee-related expenses.
We expect general and administrative expenses in 2015 to decrease due to the Spin-Off of our research and drug development operations and the significant reduction in our general and administrative cost structure. Since the Spin-Off, we significantly downsized our operations and as of December 31, 2014, had ten employees managing our respiratory assets with GSK, the commercial and developmental obligations associated with the GSK Agreements, intellectual property, licensing operations, and providing certain essential reporting and management functions of a public company.
45
Other Income (Expense), net and Interest Income
Other income (expense), net and interest income, as compared to the prior years, were as follows:
| |
|
|
|
Change | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year Ended December 31, | 2014 | 2013 | |||||||||||||||||||
(In thousands)
|
2014 | 2013 | 2012 | $ | % | $ | % | |||||||||||||||
Other income (expense), net |
$ | (3,272 | ) | $ | 6,732 | $ | — | $ | (10,004 | ) | (149 | )% | $ | 6,732 | * | |||||||
Interest income |
563 | 778 | 460 | (215 | ) | (28 | ) | 318 | 69 | % | ||||||||||||
- *
- Not Meaningful
Other income (expense), net in 2014 includes a charge of $3.8 million recognized for the unrealized loss as of December 31, 2014 on Theravance Biopharma, Inc. ordinary shares owned by us.
Other income (expense), net in 2013 includes net cash received from the termination of our royalty participation agreement with Elan Corporation, plc, partially offset by $1.4 million related to the change in fair value of the capped call instruments related to our convertible subordinated notes issued in 2013.
Interest income decreased in 2014 compared to 2013 primarily due to lower average cash balances resulting from the cash contribution to Theravance Biopharma in June 2014 and registrational and launch-related milestone payments to GSK during 2014.
Interest income increased in 2013 compared to 2012 primarily due to higher average cash balances resulting from the net proceeds received from the January 2013 issuance of 2.125% convertible subordinated notes due in 2023 ("2023 Notes") and from issuances of our common stock, partially offset by registrational and launch-related milestone payments to GSK
Interest Expense
Interest expense, as compared to the prior years, was as follows:
| |
|
|
|
Change | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year Ended December 31, | 2014 | 2013 | |||||||||||||||||||
(In thousands)
|
2014 | 2013 | 2012 | $ | % | $ | % | |||||||||||||||
Interest expense |
$ | 36,892 | $ | 9,348 | $ | 6,003 | $ | 27,544 | 295 | % | $ | 3,345 | 56 | % | ||||||||
Interest expense increased in 2014 compared to 2013 primarily due to the issuance of our 2029 Notes in April 2014. Interest expense increased in 2013 compared to 2012 primarily due to the issuance of our 2023 Notes in January 2013.
Income Taxes
At December 31, 2014, we had net operating loss carryforwards for federal income taxes of $1,158.3 million and federal research and development tax credit carryforwards of $45.2 million. We recorded a valuation allowance to offset in full the benefit related to our deferred tax assets because realization of these benefits is uncertain.
We had unrecognized tax benefits of $15.5 million as of December 31, 2014 and $57.4 million as of December 31, 2013. None of our currently unrecognized tax benefits would affect our effective income tax rate if recognized, due to the valuation allowance that currently offsets our deferred tax assets.
Utilization of net operating loss and tax credit carryforwards may be subject to a substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code and similar state provisions. We conducted an analysis through 2014 to determine whether an ownership change
46
had occurred since inception. The analysis indicated that two ownership changes occurred in prior years. However, notwithstanding the applicable annual limitations, we estimate that no portion of the net operating loss or credit carryforwards will expire before becoming available to reduce federal and state income tax liabilities. Annual limitations may result in expiration of net operating loss and tax credit carryforwards before some or all of such amounts have been utilized.
Discontinued Operations
On June 1, 2014, we separated our research and drug development businesses from our late-stage partnered respiratory assets. The significant components of the research and drug development operations, which are presented as discontinued operations on the consolidated statements of operations, were as follows:
| |
|
|
|
Change | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year Ended December 31, | 2014 | 2013 | |||||||||||||||||||
(In thousands)
|
2014 | 2013 | 2012 | $ | % | $ | % | |||||||||||||||
Net revenue |
$ | 3,129 | $ | 226 | $ | 130,145 | $ | 2,903 | * | $ | (129,919 | ) | * | |||||||||
Income (loss) from discontinued operations |
$ | (94,934 | ) | $ | (140,068 | ) | $ | 12,147 | $ | 45,134 | (32 | )% | $ | (152,215 | ) | * | ||||||
- *
- Not Meaningful
Net revenues primarily consist of revenue from collaborative arrangements and product sales.
Revenue from collaborative arrangements in 2014 includes deferred revenue recognized from our agreement with R-Pharm CJSC. Revenue from collaborative arrangements in 2012 includes $125.8 million of deferred revenue recognized from our global collaboration arrangement with Astellas Pharma, Inc. ("Astellas") for the development and commercialization of VIBATIV®, which was accelerated as a result of the termination of the Astellas agreement in January 2012, and the recognition of the upfront payment allocated to licensing of $4.4 million received under the collaborative arrangement with Merck, which was terminated in December 2013. Amounts from all three of these agreements were transferred to Theravance Biopharma as a part of the Spin-Off.
Products sales were generated from sales of VIBATIV® in the U.S. for which revenue recognition commenced in the first quarter of 2014.
Loss from discontinued operations decreased in 2014 compared to 2013 primarily as there was no impact of discontinued operations after the Spin-Off in June 2014. There was a loss from discontinued operations in 2013 compared to income from discontinued operations in 2012 primarily as result of net revenue. Included in the loss from discontinued operations for 2014 and 2013 are external legal and accounting fees in connection with our separation strategy and the additional stock-based compensation and cash bonus expense recognized due to the achievement of performance conditions under a special long-term retention and incentive equity and cash bonus awarded to certain employees in 2011 both of which we started to incur in 2013.
Liquidity and Capital Resources
Liquidity
Since our inception, we have financed our operations primarily through private placements and public offerings of equity and debt securities and payments received under collaborative arrangements. In 2014, we have also received royalty payments from GSK from the sale of a full year of royalties from RELVAR®/ BREO® ELLIPTA®, which was launched in the fourth quarter of 2013, and from
47
ANORO® ELLIPTA®, which was launched during 2014. At December 31, 2014, we had $283.4 million in cash, cash equivalents and marketable securities.
Our Board of Directors declared a $0.25 per share dividend for each of the third and fourth quarter of 2014 for all stockholders of record as of the close of business on specified dates resulting in a total of $57.0 million in cash dividends paid to our stockholders in 2014.
On June 1, 2014 we contributed $393.0 million of cash, cash equivalents and marketable securities to Theravance Biopharma as initial funds for their operations, based on anticipated operating plans and financial forecasts at the separation date. Although our cash on hand was reduced as a result of the Spin-Off, we expect that going forward our operating expenses will decrease significantly as our ongoing operations will be significantly smaller due to our focus on royalty management activities. As a result of the reduction in our operations, we believe that cash from future royalty revenues, net of operating expenses, debt service and cash on hand, will be sufficient to fund our operations for at least the next twelve months.
In April 2014, we entered into certain note purchase agreements relating to the private placement of $450.0 million aggregate principal amount of non-recourse 9% fixed rate term notes due 2029 ("2029 Notes"). The 2029 Notes are secured exclusively by a security interest in a segregated bank account established to receive 40% of the royalties from global net sales occurring on or after April 1, 2014 and ending upon the earlier of full repayment of principal or May 15, 2029 due to us under the LABA Collaboration Agreement with GSK. Prior to May 15, 2016, in the event that the specified portion of royalties received in a quarter is less than the interest accrued for the quarter, the principal amount of the 2029 Notes will increase by the interest shortfall amount for that period. As of December 31, 2014, interest expense of $20.5 million was added to the principal balance of the 2029 Notes. From the net proceeds of the offering of approximately $434.7 million, we established a milestone payment reserve account to fund 40% of any future milestone payments that could become payable under the LABA Collaboration Agreement with GSK. At December 31, 2014, the balance of the milestone reserve account and royalty collection account was not material. We incurred approximately $15.3 million in debt issuance costs, which are being amortized to interest expense over the estimated life of the 2029 Notes.
As a result of the launch and approval of RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® in the U.S., Japan and Europe, we were obligated to pay milestone fees to GSK totaling $220.0 million, which we have paid in their entirety as of December 31, 2014. These milestone fees paid to GSK were recognized as capitalized fees paid to a related party, which are being amortized over their estimated useful lives commencing upon commercial launch.
Adequacy of cash resources to meet future needs
We believe that our cash, cash equivalents and marketable securities will be sufficient to meet our anticipated operating needs for at least the next twelve months based upon current operating plans and financials forecasts. If our current operating plans and financial forecasts change, we may require additional funding sooner in the form of public or private equity offerings or debt financings. Furthermore, if in our view favorable financing opportunities arise, we may seek additional funding at any time. However, future financing may not be available in amounts or on terms acceptable to us, if at all. This could leave us without adequate financial resources to fund our operations as currently planned. In addition, we regularly explore debt restructuring and/or reduction alternatives, including through tender offers, redemptions, repurchases or otherwise, all consistent with the terms of our debt agreements.
48
Cash Flows
Cash flows, as compared to the prior years, were as follows:
| |
Year ended December 31, | Change | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
2014 | 2013 | 2012 | 2014 | 2013 | |||||||||||
Net cash used in operating activities |
$ | (130,723 | ) | $ | (129,602 | ) | $ | (127,513 | ) | $ | (1,121 | ) | $ | (2,089 | ) | |
Net cash used in investing activities |
(65,060 | ) | (219,580 | ) | (58,283 | ) | 154,520 | (161,297 | ) | |||||||
Net cash provided by financing activities |
149,073 | 397,843 | 235,867 | (248,770 | ) | 161,976 | ||||||||||
Cash Flows from Operating Activities
Cash used in operating activities is primarily driven by net loss, excluding the effect of non-cash charges or differences in the timing of cash flows and earnings recognition.
Net cash used in operating activities in 2014 of $130.7 million was primarily due to:
- •
- $100.5 million used in operating expenses, after adjusting for $68.2 million of non-cash related items, consisting
primarily of stock-based compensation expense of $27.4 million, interest expense added to the 2029 Notes of $20.5 million, depreciation and amortization expense of $12.2 million,
other than temporary impairment loss of $3.8 million from equity securities, amortization on premium of marketable securities of $1.7 million and amortization of debt issuance costs of
$2.4 million;
- •
- $15.9 million decrease in payable to Theravance Biopharma;
- •
- $4.8 million increase in interest payments on convertible subordinated notes payable;
- •
- $1.9 million used to increase inventories, all incurred prior to the Spin-Off;
- •
- $7.7 million decrease in accounts payable primarily due to the timing of payments and our ongoing operations being
significantly smaller due to the Spin-Off; and
- •
- $3.2 million from the decrease in deferred revenue.
Net cash used in operating activities in 2013 was $129.6 million, which was primarily due to:
- •
- $140.0 million used in operating expenses, after adjusting for non-cash related items of: $33.6 million consisting
primarily of stock- based compensation expense of $25.7 million and depreciation and amortization expenses of $8.2 million;
- •
- $8.0 million used for interest payments on convertible subordinated notes payable;
- •
- $3.1 million used to increase inventories;
- •
- $2.1 million used to increase receivable from collaborative arrangements related to royalty revenue and reimbursement of R&D
services;
- •
- $8.2 million increase for cash, net of third party expenses, for the termination of our royalty participation agreement;
- •
- $7.5 million increase in accrued liabilities due to $5.9 million increase in accrued personnel-related expenses, accrued
clinical and development expense, and other accrued liabilities, and $1.6 million increase in accounts payable primarily due to the timing of payments, and
- •
- $6.5 million received in upfront fees under our collaborative arrangements.
49
Net cash used in operating activities in 2012 was $127.5 million, which was primarily due to:
- •
- $118.4 million used in operating expenses, after adjusting for non-cash related items of $30.4 million consisting
primarily of stock- based compensation expense of $23.8 million, depreciation and amortization expenses of $7.3 million;
- •
- $5.2 million used for interest payments on convertible subordinated notes payable;
- •
- $4.8 million used to increase inventories;
- •
- $0.8 million used to increase receivable from collaborative arrangements related to reimbursement of R&D services
- •
- $3.3 million used to decrease accrued liabilities due to a $1.8 million decrease in accrued personnel-related expenses,
accrued clinical and development expense, and $1.5 million decrease in accounts payable primarily due to timing of payments; and
- •
- $6.0 million received in upfront fees under our collaborative arrangements.
Cash Flows from Investing Activities
Net cash used in investing activities in 2014 of $65.1 million was due to $135.0 million used for payments to GSK for registrational and launch-related milestone fees, partially offset by $69.7 million from the sale and maturities of marketable securities, net of purchases.
Net cash used in investing activities in 2013 was $219.6 million, which was primarily due to $131.9 million in cash balances being invested in available-for-sale securities and $85.0 million used for milestone payments to GSK.
Net cash used in investing activities in 2012 was $58.3 million, which was primarily due to $55.9 million in cash balances being invested in short- term investments and long-term marketable securities.
Cash Flows from Financing Activities
Net cash provided by financing activities in 2014 of $149.1 million was due to net proceeds of $434.7 million received from the private placement of our 2029 Notes and $48.9 million received from the issuance of our common stock. These increases were partially offset by $277.5 million of cash and cash equivalents contributed to Theravance Biopharma in connection with the Spin-Off and payments of cash dividends of $57.0 million to our stockholders.
Net cash provided by financing activities in 2013 of $397.8 million was primarily due to the net proceeds of $281.6 million received from the January 2013 issuance of 2.125% convertible subordinated notes due in 2023 and net proceeds from the issuances of our common stock of $153.0 million, which includes net proceeds of $126.0 million received from private placements of our common stock to an affiliate of GSK. These increases were partially offset by $36.8 million of payments on privately-negotiated capped call option transactions in connection with the issuance of the notes.
Net cash provided by financing activities in 2012 of $235.9 million was primarily due to net proceeds from the issuances of our common stock of $236.4 million, which includes net proceeds of $229.3 million received from private placements of our common stock to an affiliate of GSK.
Off-Balance Sheet Arrangements
Due to the Spin-Off, the leases for the facilities in South San Francisco, California, which formerly served as our headquarters, were assigned to Theravance Biopharma. We would be held liable by the landlord if Theravance Biopharma default under its lease obligations, and thus, we have in substance
50
guaranteed the payments under the lease agreements for the South San Francisco facilities. As of December 31, 2014, the total lease payments for the duration of the lease, which runs through May 2020, are approximately $33.4 million. We would be also responsible for lease related payments including utilities, property taxes, and common area maintenance, which may be as much as the actual lease payments. We recorded a long-term liability of $1.3 million on our consolidated balance sheet as of December 31, 2014 related to the estimated fair value of this guarantee.
Commitments and Contingencies
We indemnify our officers and directors for certain events or occurrences, subject to certain limits. We may be subject to contingencies that may arise from matters such as product liability claims, legal proceedings, shareholder suits and tax matters, as such, we are unable to estimate the potential exposure related to these indemnification agreements. We have not recognized any liabilities relating to these agreements as of December 31, 2014.
Special Long-Term Retention and Incentive Cash Awards Program
In 2011, we granted special long-term retention and incentive RSAs to members of senior management and special long-term retention and incentive cash bonus awards to certain employees. The awards have dual triggers of vesting based upon the achievement of certain performance conditions over a six-year timeframe from 2011 through December 31, 2016 and continued employment.
In connection with the Spin-Off, the Compensation Committee of our Board of Directors approved the modification of the remaining tranches related to these awards as the performance conditions associated with the remaining portions of these awards were unlikely to be consistent with the new strategies of each company following the separation. The modification acknowledged the Spin-Off and permitted recognition of achievement of certain of the original performance conditions that were met prior to the Spin-Off, triggering service-based vesting for a portion of the cash and equity awards. Stock-based compensation expense of $3.8 million associated with this portion of the equity awards after the modification is expected to be recognized by us during the twelve month period that commenced in June 2014. The remaining 63,000 RSAs for which service-based vesting was not triggered at the time of the Spin-Off remain subject to new performance conditions (as well as the original service conditions). In addition, the RSAs for which both the performance and service-based conditions were not achieved prior to the Spin-Off were entitled to the pro rata dividend distribution made by Theravance on June 2, 2014 of one ordinary share of Theravance Biopharma for every 3.5 shares of Theravance common stock subject to their awards. The Theravance Biopharma shares will be subject to the same new performance and service conditions as the original RSAs to which they relate. The amount payable by us under the modified cash bonus awards is $0.5 million and the remaining tranches of the cash bonus awards were forfeited.
Contractual Obligations and Commercial Commitments
As a result of the launch and approval of RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® in the U.S., Japan and Europe, we were obligated to pay milestone fees to GSK totaling $220.0 million, which we have paid in their entirety as of December 31, 2014. These milestone fees paid to GSK were recognized as capitalized fees paid to a related party, which are being amortized over their estimated useful lives commencing upon commercial launch. We have no further milestone payment obligations to GSK pursuant to the LABA Collaboration Agreement.
In April 2014, we entered into certain note purchase agreements relating to the private placement of $450.0 million aggregate principal amount of non-recourse 9% fixed rate term notes due 2029 issued by our wholly-owned subsidiary ("2029 Notes"). As of December 31, 2014, interest expense of $20.5 million was added to the principal balance of the 2029 Notes.
51
In the table below, we set forth our significant enforceable and legally binding obligations and future commitments.
| |
|
Years | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
Total | Within 1 | Over 1 to 3 | Over 3 to 5 | After 5 | |||||||||||
2023 Notes |
$ | 300,962 | $ | 5,421 | $ | 10,842 | $ | 10,842 | $ | 273,857 | ||||||
2029 Notes |
470,527 | * | * | * | * | |||||||||||
Facility leases** |
1,077 | 186 | 389 | 413 | 89 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total |
$ | 772,566 | $ | 5,607 | $ | 11,231 | $ | 11,255 | $ | 273,946 | ||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- *
- The
2029 Notes are secured by a security interest in a segregated bank account established to receive 40% of royalties due to us under the LABA Collaboration
with GSK commencing on April 1, 2014 and ending upon the earlier of full repayment of principal or May 15, 2029. The amounts in the segregated bank account can only be used to make
interest and principal payments on the 2029 Notes. In addition, prior to May 15, 2016, in the event that the specified portion of royalties received in a quarter is less than the interest
accrued for the quarter, the principal amount of the 2029 Notes will increase by interest shortfall amount for that period. Since the principal and interest payments on the 2029 Notes are based on
royalties from product sales recorded by GSK, which can vary from quarter to quarter and are unknown to us, these amounts are not included in the above table. See Note 7, "Long-Term Debt" of
the accompanying consolidated financial statements for further information.
- **
- Following the Spin-Off, we entered into a Sublease Agreement with Theravance Biopharma to sublease 4,847 square feet of office space in South San Francisco, California, which expires in May 2020. We do not own or lease any other properties.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Market Risk
We have invested primarily in money market funds, federal agency notes, corporate debt securities and U.S. treasury notes. To reduce the volatility relating to these exposures, we have put investment and risk management policies and procedures in place. The securities in our investment portfolio are not leveraged, are classified as available-for-sale and, due to their very short-term nature. We currently do not engage in hedging activities.
We performed a sensitivity analysis to determine the impact a change in interest rates would have on the value of our investment portfolio. Based on our investment positions as of December 31, 2014, a hypothetical 100 basis point increase in interest rates would result in a $1.3 million decline in the fair market value of the portfolio. Such losses would only be realized if we sold the investments prior to maturity. A hypothetical decrease in market interest rates by 10% would not have a material impact to our interest income from our investment portfolio.
Equity Market Risk
As of December 31, 2014, we held ordinary shares of Theravance Biopharma with a fair value of $6.5 million based on the closing sales price of $14.92 per share on December 31, 2014 as reported by The NASDAQ Global Market. These equity securities are restricted securities and could only be resold pursuant to a registration statement or an exemption from registration under the Securities Act of 1933, as amended (the "Securities Act"). We are able to sell these shares pursuant to Rule 144 promulgated under the Securities Act since the six-month holding period has been satisfied provided that Theravance Biopharma remains current with its SEC filing obligations. During the fourth quarter
52
of 2014 we took a charge in the consolidated statements of operations related to the loss in fair value of these equity securities. The fair value of the equity securities could further be adversely affected as ordinary shares are susceptible to stock market fluctuations and to volatile increases and decreases in value. As of December 31, 2014, a hypothetical 10% decrease in the fair value of this equity security would result in a loss in fair value of approximately $0.7 million.
Interest Rate Risk
As of December 31, 2014, the fair value of our convertible notes due in 2023 was estimated to be $197.1 million, based on available pricing information. The 2023 Notes bear interest at a fixed rate of 2.125%. The effective interest rate by year of expected maturity for our convertible notes or the earliest in which the note holders may put the debt to us is 2.38% each year.
As of December 31, 2014, the fair value of our non-recourse notes due 2029 was estimated to be $456.4 million, based on available pricing information. The 2029 Notes bear interest at a fixed rate of 9% per annum. The effective interest rate by year of expected maturity for our non-recourse notes due 2029 is 9.78% each year.
The obligations under these two facilities are subject to interest rate risk because the respective interest rates under the obligations may exceed current interest rates.
53
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
54
THERAVANCE, INC.
Consolidated Balance Sheets
(In thousands, except per share data)
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2014 | 2013 | |||||
Assets |
|||||||
Current assets: |
|||||||
Cash and cash equivalents |
$ | 96,800 | $ | 143,510 | |||
Short-term marketable securities |
143,698 | 321,615 | |||||
Accounts receivable, net of allowances of $89 at December 31, 2013 |
— | 199 | |||||
Receivables from collaborative arrangements (including amounts from a related party of $10,550 and $2,247 at December 31, 2014 and 2013) |
10,550 | 3,181 | |||||
Prepaid expenses and other current assets |
1,134 | 4,287 | |||||
Inventories |
— | 10,406 | |||||
| | | | | | | | |
Total current assets |
252,182 | 483,198 | |||||
Marketable securities |
42,856 | 55,374 | |||||
Restricted cash |
— | 833 | |||||
Property and equipment, net |
324 | 10,238 | |||||
Capitalized fees paid to a related party, net |
208,191 | 124,257 | |||||
Other assets |
18,101 | 7,355 | |||||
| | | | | | | | |
Total assets |
$ | 521,654 | $ | 681,255 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Liabilities and Stockholders' Equity (Deficit): |
|||||||
Current liabilities: |
|||||||
Accounts payable |
$ | — | $ | 7,583 | |||
Payable to a related party |
— | 40,000 | |||||
Payable to Theravance Biopharma, Inc. |
1,056 | — | |||||
Accrued personnel-related expenses |
1,959 | 10,881 | |||||
Accrued clinical and development expenses |
— | 9,714 | |||||
Accrued interest payable |
7,551 | 2,800 | |||||
Other accrued liabilities |
2,108 | 4,137 | |||||
Deferred revenue |
1,082 | 9,289 | |||||
| | | | | | | | |
Total current liabilities |
13,756 | 84,404 | |||||
Convertible subordinated notes |
255,109 | 287,500 | |||||
Non-recourse notes payable, due 2029 |
470,527 | — | |||||
Deferred rent |
105 | 4,774 | |||||
Other long-term liabilities |
1,718 | — | |||||
Deferred revenue |
3,788 | 5,455 | |||||
Commitments and contingencies (Notes 3, 6 and 8) |
|||||||
Stockholders' Equity (Deficit) : |
|||||||
Preferred stock, $0.01 par value: 230 shares authorized, no shares issued and outstanding at December 31, 2014 and 2013 |
— | — | |||||
Common stock, $0.01 par value; authorized: 200,000 shares; issued: 116,445 and 111,516 sahres at December 31, 2014 and 2013 |
1,164 | 1,115 | |||||
Treasury stock, 150 and 0 shares at December 31, 2014 and 2013 |
(3,263 | ) | — | ||||
Additional paid-in capital |
1,452,504 | 1,803,048 | |||||
Accumulated other comprehensive income (loss) |
(87 | ) | 162 | ||||
Accumulated deficit |
(1,673,667 | ) | (1,505,203 | ) | |||
| | | | | | | | |
Total stockholders' equity (deficit) |
(223,349 | ) | 299,122 | ||||
| | | | | | | | |
Total liabilities and stockholders' equity (deficit) |
$ | 521,654 | $ | 681,255 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
See accompanying notes to consolidated financial statements.
55
THERAVANCE, INC.
Consolidated Statements of Operations
(In thousands, except per share data)
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2014 | 2013 | 2012 | |||||||
Royalty revenue from a related party, net of amortization for capitalized fees paid to a related party of $11,066, $743 and $0 in 2014, 2013 and 2012 |
$ | 7,351 | $ | 1,202 | $ | — | ||||
Revenue from collaborative arrangements from a related party, net |
1,082 | 3,330 | 5,613 | |||||||
| | | | | | | | | | | |
Total net revenue |
8,433 | 4,532 | 5,613 | |||||||
Operating expenses: |
||||||||||
Research and development |
7,498 | 9,038 | 8,153 | |||||||
General and administrative |
34,864 | 24,289 | 22,606 | |||||||
| | | | | | | | | | | |
Total operating expenses |
42,362 | 33,327 | 30,759 | |||||||
| | | | | | | | | | | |
Loss from operations |
(33,929 | ) | (28,795 | ) | (25,146 | ) | ||||
Other income (expense), net |
(3,272 |
) |
6,732 |
— |
||||||
Interest income |
563 | 778 | 460 | |||||||
Interest expense |
(36,892 | ) | (9,348 | ) | (6,003 | ) | ||||
| | | | | | | | | | | |
Loss from continuing operations |
(73,530 | ) | (30,633 | ) | (30,689 | ) | ||||
Income (loss) from discontinued operations (Notes 10 and 11) |
(94,934 | ) | (140,068 | ) | 12,147 | |||||
| | | | | | | | | | | |
Net loss |
$ | (168,464 | ) | $ | (170,701 | ) | $ | (18,542 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Basic and diluted net loss per share: |
||||||||||
Continuing operations |
$ | (0.66 | ) | $ | (0.30 | ) | $ | (0.34 | ) | |
Discontinued operations |
(0.84 | ) | (1.37 | ) | 0.14 | |||||
| | | | | | | | | | | |
Basic and diluted net loss per share |
$ | (1.50 | ) | $ | (1.67 | ) | $ | (0.20 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Cash dividends declared per common share |
$ | 0.50 | $ | — | $ | — | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Shares used to compute basic and diluted net loss per share |
112,059 | 102,425 | 90,909 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
See accompanying notes to consolidated financial statements.
56
THERAVANCE, INC.
Consolidated Statements of Comprehensive Loss
(In thousands)
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2014 | 2013 | 2012 | |||||||
Net loss |
$ | (168,464 | ) | $ | (170,701 | ) | $ | (18,542 | ) | |
Other comprehensive income (loss): |
||||||||||
Unrealized gain (loss) on marketable securities |
(4,001 | ) | 63 | 83 | ||||||
Add: Reclassification adjustments for other-than temporary impairment loss included in net loss |
3,752 | — | — | |||||||
| | | | | | | | | | | |
Other comprehensive income (loss) |
(249 | ) | 63 | 83 | ||||||
| | | | | | | | | | | |
Comprehensive loss |
$ | (168,713 | ) | $ | (170,638 | ) | $ | (18,459 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
See accompanying notes to consolidated financial statements.
57
THERAVANCE, INC.
Consolidated Statements of Stockholders' Equity (Deficit)
(In thousands)
| |
Common Stock | |
Accumulated Other Comprehensive Income (Loss) |
|
Treasury Stock | Total Stockholders' Equity (Deficit) |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Additional Paid-In Capital |
Accumulated Deficit |
|||||||||||||||||||||||
(In thousands)
|
Shares | Amount | Shares | Amount | |||||||||||||||||||||
Balance at December 31, 2011 |
85,543 | $ | 855 | $ | 1,228,037 | $ | 16 | $ | (1,315,960 | ) | — | $ | — | $ | (87,052 | ) | |||||||||
Exercise of stock options, and issuance of common stock units, stock awards and purchase plan |
2,151 | 22 | 7,059 | — | — | — | — | 7,081 | |||||||||||||||||
Issuance of common stock in private placement to a related party, net of expenses of $0.4 million |
10,685 | 107 | 229,189 | — | — | — | — | 229,296 | |||||||||||||||||
Stock-based compensation |
— | — | 24,162 | — | — | — | — | 24,162 | |||||||||||||||||
Net loss |
— | — | — | — | (18,542 | ) | — | — | (18,542 | ) | |||||||||||||||
Other Comprehensive Income |
— | — | — | 83 | — | — | — | 83 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2012 |
98,379 | 984 | 1,488,447 | 99 | (1,334,502 | ) | — | — | 155,028 | ||||||||||||||||
Exercise of stock options, and issuance of common stock units, stock awards and purchase plan |
2,964 | 29 | 26,962 | — | — | — | — | 26,991 | |||||||||||||||||
Issuance of common stock in private placement to a related party |
3,505 | 35 | 125,995 | — | — | — | — | 126,030 | |||||||||||||||||
Stock-based compensation |
— | — | 25,858 | — | — | — | — | 25,858 | |||||||||||||||||
Conversion of convertible subordinated notes due 2015 |
6,668 | 67 | 171,164 | — | — | — | — | 171,231 | |||||||||||||||||
Capped call options associated with convertible subordinated notes due 2023 |
— | — | (35,378 | ) | — | — | — | — | (35,378 | ) | |||||||||||||||
Net loss |
— | — | — | — | (170,701 | ) | — | — | (170,701 | ) | |||||||||||||||
Other Comprehensive Income |
— | — | — | 63 | — | — | — | 63 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2013 |
111,516 | 1,115 | 1,803,048 | 162 | (1,505,203 | ) | — | — | 299,122 | ||||||||||||||||
Exercise of stock options, and issuance of common stock units and stock awards |
1,744 | 17 | 10,813 | — | — | — | — | 10,830 | |||||||||||||||||
Issuance of common stock in private placement to a related party |
1,665 | 17 | 38,078 | — | — | — | — | 38,095 | |||||||||||||||||
Stock-based compensation |
— | — | 27,485 | — | — | — | — | 27,485 | |||||||||||||||||
Conversion of convertible subordinated notes due 2023 |
1,520 | 15 | 31,756 | — | — | — | — | 31,771 | |||||||||||||||||
Repurchase of common stock |
— | — | 3,263 | — | — | (150 | ) | (3,263 | ) | — | |||||||||||||||
Guarantee issued in connection with distribution to Theravance Biopharma, Inc. related to lease agreements |
— | — | (1,300 | ) | — | — | — | — | (1,300 | ) | |||||||||||||||
Distribution to Theravance Biopharma, Inc. |
— | — | (402,787 | ) | — | — | — | — | (402,787 | ) | |||||||||||||||
Cash dividends declared, $0.50 per common share |
— | — | (57,852 | ) | — | — | — | — | (57,852 | ) | |||||||||||||||
Net loss |
— | — | — | — | (168,464 | ) | — | — | (168,464 | ) | |||||||||||||||
Other Comprehensive Loss |
— | — | — | (249 | ) | — | — | — | (249 | ) | |||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2014 |
116,445 | $ | 1,164 | $ | 1,452,504 | $ | (87 | ) | $ | (1,673,667 | ) | (150 | ) | $ | (3,263 | ) | $ | (223,349 | ) | ||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes to consolidated financial statements.
58
THERAVANCE, INC.
Consolidated Statements of Cash Flows
(In thousands)
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2014 | 2013 | 2012 | |||||||
Cash flows from operating activities |
||||||||||
Net loss |
$ | (168,464 | ) | $ | (170,701 | ) | $ | (18,542 | ) | |
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||
Depreciation and amortization |
12,175 | 3,458 | 3,251 | |||||||
Stock-based compensation |
27,390 | 25,687 | 23,783 | |||||||
Other-than-temporary impairment loss on marketable securities |
3,752 | — | — | |||||||
Amortization of premium on marketable securities |
1,742 | 3,794 | 3,248 | |||||||
Interest added to the principal balance of the non-recourse notes due 2029 |
20,527 | — | — | |||||||
Change in fair value of capped-call derivative assets |
— | 1,422 | — | |||||||
Amortization of debt issuance costs |
2,408 | 951 | 827 | |||||||
Other non-cash items |
(2 | ) | 17 | 187 | ||||||
Changes in operating assets and liabilities: |
||||||||||
Accounts receivable |
74 | 702 | — | |||||||
Receivables from collaborative arrangements |
(7,371 | ) | (2,117 | ) | (841 | ) | ||||
Prepaid expenses and other current assets |
(338 | ) | 36 | (441 | ) | |||||
Inventories |
(1,908 | ) | (3,100 | ) | (4,822 | ) | ||||
Other assets |
1,549 | (578 | ) | — | ||||||
Accounts payable |
(7,695 | ) | 1,613 | (1,480 | ) | |||||
Payable to Theravance Biopharma, Inc., net |
(15,916 | ) | — | — | ||||||
Accrued personnel-related expenses, accrued clinical and development expenses, and other accrued liabilities |
(491 | ) | 5,850 | (1,829 | ) | |||||
Accrued interest payable |
4,751 | 428 | — | |||||||
Deferred rent |
275 | (299 | ) | (747 | ) | |||||
Deferred revenue |
(3,181 | ) | 3,235 | (130,107 | ) | |||||
| | | | | | | | | | | |
Net cash used in operating activities |
(130,723 | ) | (129,602 | ) | (127,513 | ) | ||||
| | | | | | | | | | | |
Cash flows from investing activities |
||||||||||
Purchases of property and equipment |
(689 | ) | (2,734 | ) | (2,590 | ) | ||||
Purchases of marketable securities |
(276,914 | ) | (410,407 | ) | (330,484 | ) | ||||
Maturities of marketable securities |
339,359 | 255,861 | 224,902 | |||||||
Sales of marketable securities |
7,211 | 22,600 | 49,729 | |||||||
Payments for capitalized fees paid to a related party |
(135,000 | ) | (85,000 | ) | — | |||||
Change in restricted cash |
833 | — | 60 | |||||||
Issuances of notes receivable |
— | — | (140 | ) | ||||||
Payments received on notes receivable |
140 | 100 | 240 | |||||||
| | | | | | | | | | | |
Net cash used in investing activities |
(65,060 | ) | (219,580 | ) | (58,283 | ) | ||||
| | | | | | | | | | | |
Cash flows from financing activities |
||||||||||
Cash and cash equivalents contributed to Theravance Biopharma, Inc. |
(277,541 | ) | — | — | ||||||
Payments on note payable and capital leases |
— | — | (69 | ) | ||||||
Proceeds from issuances of common stock, net |
48,925 | 153,021 | 236,377 | |||||||
Payments of cash dividends to stockholders |
(56,988 | ) | — | — | ||||||
Purchase of capped-call options |
— | (36,800 | ) | — | ||||||
Proceeds from issuances of notes payable, net of debt issuance costs |
434,677 | 281,622 | (441 | ) | ||||||
| | | | | | | | | | | |
Net cash provided by financing activities |
149,073 | 397,843 | 235,867 | |||||||
| | | | | | | | | | | |
Net (decrease) increase in cash and cash equivalents |
(46,710 | ) | 48,661 | 50,071 | ||||||
Cash and cash equivalents at beginning of period |
143,510 | 94,849 | 44,778 | |||||||
| | | | | | | | | | | |
Cash and cash equivalents at end of period |
$ | 96,800 | $ | 143,510 | $ | 94,849 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Supplemental disclosure of cash flow information |
||||||||||
Cash paid for interest |
$ | 9,208 | $ | 7,970 | $ | 5,177 | ||||
Supplemental disclosure of noncash information |
||||||||||
Contribution of net assets, excluding cash and cash equivalents, to Theravance Biopharma, Inc. |
$ | 125,337 | $ | — | $ | — | ||||
Conversion of convertible subordinated notes into common stock |
$ | 32,391 | $ | 172,499 | $ | — | ||||
See accompanying notes to consolidated financial statements.
59
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Description of Operations and Summary of Significant Accounting Policies
Description of Operations
Theravance, Inc. ("Theravance", the "Company", or "we" and other similar pronouns) is a royalty management company focused on maximizing the potential value of the respiratory assets partnered with Glaxo Group Limited ("GSK"), including RELVAR®/BREO® ELLIPTA® (fluticasone furoate/ vilanterol, "FF/VI") and ANORO® ELLIPTA® (umeclidinium bromide/ vilanterol, "UMEC/VI"), with the intention of providing capital returns to stockholders. Under the Long-Acting Beta2 Agonist ("LABA") Collaboration Agreement and the Strategic Alliance Agreement with GSK (referred to herein as the "GSK Agreements"), Theravance is eligible to receive the associated royalty revenues from RELVAR®/BREO® ELLIPTA® , ANORO® ELLIPTA® and if approved and commercialized, VI monotherapy. Theravance is also entitled to 15% of any future payments made by GSK under its agreements originally entered into with us, and since assigned to Theravance Respiratory Company, LLC ("TRC"), relating to the combination FF/UMEC/VI and the Bifunctional Muscarinic Antagonist-Beta2 Agonist ("MABA") program, as monotherapy and in combination with other therapeutically active components, such as an inhaled corticosteroid, and any other product or combination of products that may be discovered and developed in the future under the LABA Collaboration Agreement ("LABA Collaboration"), which has been assigned to TRC other than RELVAR®/BREO®ELLIPTA®, ANORO® ELLIPTA® and VI monotherapy.
Business Separation
On June 1, 2014, we separated our biopharmaceutical research and drug development operations from our late-stage partnered respiratory assets by transferring our research and drug development operations into our then wholly-owned subsidiary, Theravance Biopharma, Inc. ("Theravance Biopharma"). We contributed $393.0 million of cash, cash equivalents and marketable securities to Theravance Biopharma and all outstanding shares of Theravance Biopharma were then distributed to Theravance stockholders as a pro-rata dividend distribution on June 2, 2014 by issuing one ordinary share of Theravance Biopharma for every 3.5 shares held of our common stock to stockholders of record on May 15, 2014 (the "Spin-Off"). The Spin-Off resulted in Theravance Biopharma operating as an independent, publicly traded company.
The results of operations for the former research and drug development operations conducted by us and by Theravance Biopharma until June 1, 2014 are included as part of this report as discontinued operations. Refer to Notes 10 and 11, "Spin-Off of Theravance Biopharma, Inc.," and "Discontinued Operations" for further information.
Pursuant to a three-way master agreement entered into by and among us, Theravance Biopharma and GSK in connection with the Spin-Off, we agreed to sell a certain number of Theravance Biopharma shares withheld from a taxable dividend of Theravance Biopharma shares to GSK. After such Theravance Biopharma shares were sent to the transfer agent, we agreed to purchase the Theravance Biopharma shares from the transfer agent, rather than have them sold on the open market, in order to satisfy tax withholdings. GSK had a right to purchase these shares of Theravance Biopharma from us, but this right expired unexercised. Accordingly, at December 31, 2014, we owned 436,802 ordinary shares of Theravance Biopharma, which are accounted for as marketable securities in the consolidated balance sheet. These equity securities are discussed further in Note 4, "Available-for-Sale Securities and Fair Value Measurements".
60
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
Principles of Consolidation
The consolidated financial statements include the accounts of Theravance and its wholly owned subsidiaries. All intercompany balances and transactions have been eliminated in consolidation.
Use of Management's Estimates
The preparation of consolidated financial statements in conformity with U.S. Generally Accepted Accounting Principles ("GAAP") requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Actual results could differ materially from those estimates. Management evaluates its significant accounting policies and estimates on an ongoing basis. We base our estimates on historical experience and other relevant assumptions that we believe to be reasonable under the circumstances. These estimates also form the basis for making judgments about the carrying values of assets and liabilities when these values are not readily apparent from other sources.
Segment Reporting
We operate in a single segment, which is to provide capital return to stockholders by maximizing the potential value of our respiratory assets partnered with GSK. Revenues are generated from our collaborative arrangements and royalty payment from GSK, located in Great Britain. Our facilities are located within the United States.
Variable Interest Entities
We evaluate our ownership, contractual and other interest in entities to determine if they are variable-interest entities ("VIE"), whether we have a variable interest in those entities and the nature and extent of those interests. Based on our evaluations, if we determine we are the primary beneficiary of such VIEs, we consolidate such entities into our financial statements. We consolidate the financial results of TRC, which we have determined to be a VIE, because we have the power to direct the economically significant activities of TRC and the obligation to absorb losses of, or the right to receive benefits from, TRC. The financial position and results of operations of TRC are not material as of and for the year ended December 31, 2014.
Cash and Cash Equivalents
We consider all highly liquid investments purchased with a maturity of three months or less on the date of purchase to be cash equivalents. Cash equivalents are carried at cost, which approximates fair value.
Prior to the Spin-Off, we pledged cash and cash equivalents as collateral under certain lease agreements and letters of credit. Restricted cash related to such agreements was $0 and $0.8 million as of December 31, 2014 and 2013.
Investments in Marketable Securities
We invest in short-term investments and marketable securities, primarily corporate notes, government, government agency, and municipal bonds. We limit the amount of credit exposure with any one issuer, industry or geographic area for investments other than instruments backed by the U.S.
61
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
federal government. We classify our marketable securities as available-for-sale securities and report them at fair value in cash equivalents, short-term investments or marketable securities on the consolidated balance sheets with related unrealized gains and losses included as a component of stockholders' equity (deficit). The amortized cost of debt securities is adjusted for amortization of premiums and accretion of discounts to maturity, which is included in interest income on the consolidated statements of operations. Realized gains and losses, if any, on available-for-sale securities are included in interest income. The cost of securities sold is based on the specific identification method. Interest and dividends on securities classified as available-for-sale are included in interest income.
We regularly review all of our investments for other-than-temporary declines in estimated fair value. Our review includes the consideration of the cause of the impairment, including the creditworthiness of the security issuers, the number of securities in an unrealized loss position, the severity and duration of the unrealized losses, whether we have the intent to sell the securities and whether it is more likely than not that we will be required to sell the securities before the recovery of their amortized cost basis. When we determine that the decline in estimated fair value of an investment is below the amortized cost basis and the decline is other-than-temporary, we reduce the carrying value of the security and record a loss for the amount of such decline to other income (expense), net.
Fair Value of Financial Instruments
We define fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date.
Our valuation techniques are based on observable and unobservable inputs. Observable inputs reflect readily obtainable data from independent sources, while unobservable inputs reflect our market assumptions. We classify these inputs into the following hierarchy:
Level 1—Quoted prices for identical instruments in active markets.
Level 2—Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations whose inputs are observable or whose significant value drivers are observable.
Level 3—Unobservable inputs and little, if any, market activity for the assets.
Financial instruments include cash equivalents, marketable securities, accounts receivable, receivables from collaborative arrangements, accounts payable, and accrued liabilities. Cash equivalents and marketable securities are carried at estimated fair value. The carrying value of accounts receivable, receivables from collaborative arrangements, accounts payable, and accrued liabilities approximate their estimated fair value due to the relatively short-term nature of these instruments.
Accounts Receivable
All trade accounts receivable were related to our former research and drug development operations and, thus, were contributed to Theravance Biopharma in connection with the Spin-Off. Accordingly, we have no trade accounts receivable as of December 31, 2014.
62
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
Prior to the Spin-Off, our trade accounts receivable were recorded net of allowances for wholesaler chargebacks related to government rebate programs, cash discounts for prompt payment and sales returns. Estimates for wholesaler chargebacks for government rebates, cash discounts and sales returns were based on contractual terms, historical trends and our expectations regarding the utilization rates for these programs. When appropriate, we recorded an allowance for doubtful accounts based upon our assessment of collectability. For the periods presented, we did not have any write-offs of accounts receivable.
Inventories
All inventories were related to our former research and drug development operations and, thus, were contributed to Theravance Biopharma in connection with the Spin-Off. Accordingly, we had no inventories as of December 31, 2014.
Prior to the Spin-Off, our inventories consisted of raw materials, work-in-process and finished goods. Inventories were stated at the lower of cost or market value. We determined the cost of inventory using the average-cost method for validation batches. We analyzed our inventory levels quarterly and wrote down any inventory that was expected to become obsolete, that had a cost basis in excess of its expected net realizable value or for inventory quantities in excess of expected requirements.
Inventories were as follows:
(In thousands)
|
December 31, 2013 | |||
|---|---|---|---|---|
Raw materials |
$ | 5,138 | ||
Work-in-process |
360 | |||
Finished goods |
4,908 | |||
| | | | | |
Total inventories |
$ | 10,406 | ||
| | | | | |
| | | | | |
| | | | | |
Property and Equipment
All property, equipment and leasehold improvements prior to the Spin-Off were related to our former research and drug development operations and thus, were contributed to Theravance Biopharma in connection with the Spin-Off.
63
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
Property and equipment consists of the following:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
(In thousands)
|
2014 | 2013 | |||||
Computer equipment |
$ | — | $ | 3,084 | |||
Software |
324 | 5,391 | |||||
Furniture and fixtures |
— | 3,890 | |||||
Laboratory equipment |
— | 31,910 | |||||
Leasehold improvements |
— | 17,769 | |||||
| | | | | | | | |
Subtotal |
324 | 62,044 | |||||
Less accumulated depreciation |
— | (51,806 | ) | ||||
| | | | | | | | |
Property and equipment, net |
$ | 324 | $ | 10,238 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Property, equipment and leasehold improvements are stated at cost and depreciated using the straight-line method as follows:
Leasehold improvements |
Shorter of remaining lease terms or useful life | |
Equipment, furniture and fixtures |
5 - 7 years | |
Software and computer equipment |
3 years |
Depreciation expense for the years ended December 31, 2014, 2013 and 2012 was $1.1 million, $2.7 million and $3.3 million. Depreciation expense for property and equipment used by our former research and drug development operations is classified within discontinued operations in the consolidated statements of operations. The change in accumulated depreciation is net of asset retirements.
Capitalized Software
We capitalize certain costs related to direct material and service costs for software obtained for internal use. Capitalized software costs are depreciated over 3 years.
Capitalized Fees paid to a Related Party
We capitalize fees paid to licensors related to agreements for approved products or commercialized products. We capitalize these fees as capitalized fees paid to a related party ("Capitalized Fees") and amortize these Capitalized Fees on a straight-line basis over their estimated useful lives upon the commercial launch of the product, which has been shortly after regulatory approval of such product. The estimated useful lives of these Capitalized Fees are based on a country-by-country and product-by-product basis, as the later of the expiration or termination of the last patent right covering the compound in such product in such country and 15 years from first commercial sale of such product in such country, unless the agreement is terminated earlier. Consistent with our policy for classification of costs under the research and development collaborative arrangements, the amortization of these Capitalized Fees are recognized as a reduction of royalty revenue. We review our Capitalized Fees for impairment when events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. The recoverability of Capitalized Fees is measured by comparing the asset's carrying amount to the expected undiscounted future cash flows that the asset is expected to generate.
64
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
The determination of recoverability typically requires various estimates and assumptions, including estimating the useful life over which cash flows will occur, their amount, and the asset's residual value, if any. We derive the required cash flow estimates from near-term forecasted product sales and long-term projected sales in the corresponding market.
Bonus Accruals
We have short-term bonus programs for eligible employees. Bonuses are determined based on various criteria, including the achievement of corporate, departmental and individual goals. Bonus accruals are estimated based on various factors, including target bonus percentages per level of employee and probability of achieving the goals upon which bonuses are based.
Deferred Rent
Deferred rent consists of the difference between cash payments and the recognition of rent expense on a straight-line basis under our lease agreements. Rent expense is recognized ratably over the life of the leases. Rent expenses associated with our operating leases for the years ended December 31, 2014, 2013 and 2012 were $3.0 million, $6.0 million, and $5.7 million, respectively.
The leases were assumed by Theravance Biopharma in connection with the Spin-Off. Prior to the Spin-Off, our deferred rent also included lease incentives of $2.6 million as of December 31, 2013, which were being recognized ratably over the life of the lease. The rent expense related to our former research and drug development operations is classified within discontinued operations in the consolidated statements of operations.
Revenue Recognition
Revenue is recognized when the four basic criteria of revenue recognition are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the fee is fixed or determinable; and (4) collectability is reasonably assured. Where the revenue recognition criteria are not met, we defer the recognition of revenue by recording deferred revenue until such time that all criteria are met.
Collaborative Arrangements and Multiple-Element Arrangements
Revenue from nonrefundable, up-front license or technology access payments under license and collaborative arrangements that are not dependent on any future performance by us is recognized when such amounts are earned. If we have continuing obligations to perform under the arrangement, such fees are recognized over the estimated period of continuing performance obligation.
We account for multiple element arrangements, such as license and development agreements in which a customer may purchase several deliverables, in accordance with Financial Accounting Standards Board ("FASB") Accounting Standards Codification ("ASC") Subtopic 605-25, "Multiple Element Arrangements." For new or materially amended multiple element arrangements, we identify the deliverables at the inception of the arrangement and each deliverable within a multiple deliverable revenue arrangement is accounted for as a separate unit of accounting if both of the following criteria are met: (1) the delivered item or items have value to the customer on a standalone basis and (2) for an arrangement that includes a general right of return relative to the delivered item(s), delivery or
65
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
performance of the undelivered item(s) is considered probable and substantially in our control. We allocate revenue to each non-contingent element based on the relative selling price of each element. When applying the relative selling price method, we determine the selling price for each deliverable using vendor-specific objective evidence ("VSOE") of selling price, if it exists, or third-party evidence ("TPE") of selling price, if it exists. If neither VSOE nor TPE of selling price exist for a deliverable, we use the best estimated selling price for that deliverable. Revenue allocated to each element is then recognized based on when the basic four revenue recognition criteria are met for each element.
For multiple-element arrangements entered into prior to January 1, 2011, we determined the delivered items under our collaborative arrangements did not meet the criteria to be considered separate accounting units for the purposes of revenue recognition. As a result, we recognized revenue from non-refundable, upfront fees and development contingent payments in the same manner as the final deliverable, which is ratably over the expected term of our performance of research and development services under the agreements. These upfront or contingent payments received, pending recognition as revenue, are recorded as deferred revenue and are classified as a short-term or long-term liability on the consolidated balance sheets and recognized over the estimated period of performance. We periodically review the estimated performance periods of our contracts based on the progress of our programs.
Where a portion of non-refundable upfront fees or other payments received are allocated to continuing performance obligations under the terms of a collaborative arrangement, they are recorded as deferred revenue and recognized as revenue, or as an accrued liability and recognized as a reduction of research and development expenses ratably over the term of our estimated performance period under the agreement. We determine the estimated performance periods, and they are periodically reviewed based on the progress of the related program. The effect of any change made to an estimated performance period and, therefore revenue recognized, would occur on a prospective basis in the period that the change was made.
Under certain collaborative arrangements, we have been reimbursed for a portion of our research and development expenses. These reimbursements have been reflected as a reduction of research and development expense in our consolidated statements of operations, as we do not consider performing research and development services to be a part of our ongoing and central operations. Therefore, the reimbursement of research and developmental services and any amounts allocated to our research and development services are recorded as a reduction of research and development expense.
Amounts deferred under a collaborative arrangement in which the performance obligations are terminated will result in an immediate recognition of any remaining deferred revenue and accrued liability in the period that termination occurred, provided that there are no remaining performance obligations.
We account for contingent payments in accordance with FASB Subtopic ASC 605-28 "Revenue Recognition—Milestone Method." We recognize revenue from milestone payments when (i) the milestone event is substantive and its achievability was not reasonably assured at the inception of the agreement and (ii) we do not have ongoing performance obligations related to the achievement of the milestone. Milestone payments are considered substantive if all of the following conditions are met: the milestone payment (a) is commensurate with either our performance to achieve the milestone or the enhancement of the value of the delivered item or items as a result of a specific outcome resulting from our performance to achieve the milestone, (b) relates solely to past performance, and (c) is
66
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
reasonable relative to all of the deliverables and payment terms (including other potential milestone consideration) within the arrangement.
Under our collaborative arrangements with GSK, royalty revenue earned is reduced by amortization expense resulting from the fees paid to GSK, which were recognized as capitalized fees paid to a related party. When amortization expense exceeds amounts recognized for royalty revenues from GSK, negative revenue would be reported in our consolidated statements of operations.
Royalties
We recognize royalty revenue on licensee net sales of products with respect to which we have contractual royalty rights in the period in which the royalties are earned and reported to us and collectability is reasonably assured. Royalties are recognized net of amortization of capitalized fees associated with any approval and launch milestone payments made to GSK.
Product Revenues
We currently have no product revenues following the Spin-Off.
Prior to the Spin-Off, we recognized revenues from product sales when there was persuasive evidence that an arrangement existed, title and risk of loss transferred, the price was fixed and determinable, and collectibility was reasonably assured. Product sales were recognized net of estimated allowances, discounts, sales returns, chargebacks and rebates. Such amounts are presented within discontinued operations in the consolidated statements of operations.
Allowance for Doubtful Accounts
We maintain a policy to record allowances for potentially doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. As of December 31, 2013, there were no allowances for doubtful accounts as we have not had any write-offs historically.
Research and Development Costs
Research and development costs are expensed in the period that services are rendered or goods are received. Research and development costs consist primarily of salaries and benefits. Prior to the Spin-Off, research and development costs also included laboratory supplies and facility costs, and fees paid to third parties that conduct certain research and development activities on behalf of us, net of certain external research and development costs reimbursed under collaborative arrangements, which are classified within discontinued operations in the consolidated statements of operations.
Preclinical Study and Clinical Study Expenses
All accrued clinical and development expenses were related to our former research and drug development operations and, thus, were contributed to Theravance Biopharma in connection with the Spin-Off. Accordingly, we have no accrued clinical and development expenses as of December 31, 2014.
67
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
Prior to the Spin-Off, a substantial portion of our preclinical studies and all of our clinical studies were performed by third-party contract research organizations (CROs). Some CROs billed monthly for services performed, while others billed based upon milestones achieved. We would review the activities performed under the significant contracts each quarter. For preclinical studies, the significant factors used in estimating accruals included the percentage of work completed to date and contract milestones achieved. For clinical study expenses, the significant factors used in estimating accruals included the number of patients enrolled and percentage of work completed to date. Vendor confirmations were obtained for contracts with longer duration when necessary to validate our estimate of expenses. Our estimates were highly dependent upon the timeliness and accuracy of the data provided by our CROs regarding the status of each program and total program spending and adjustments were made when deemed necessary.
Fair Value of Stock-Based Compensation Awards
We use the Black-Scholes-Merton option pricing model to estimate the fair value of options granted under our equity incentive plans and rights to acquire stock granted under our employee stock purchase plan (ESPP). The Black-Scholes-Merton option valuation model requires the use of assumptions, including the expected term of the award and the expected stock price volatility. We use the "simplified" method as described in Staff Accounting Bulletin No. 107, "Share-Based Payment," for the expected option term because the usage of its historical option exercise data is limited due to post-IPO exercise restrictions. Beginning April 1, 2011, we used our historical volatility to estimate expected stock price volatility. Prior to April 1, 2011, we used peer company price volatility to estimate expected stock price volatility due to our limited historical common stock price volatility since our initial public offering in 2004.
Restricted Stock Units ("RSUs") and Restricted Stock Awards ("RSAs") are measured based on the fair market values of the underlying stock on the dates of grant.
Stock-based compensation expense was calculated based on awards ultimately expected to vest and was reduced for estimated forfeitures at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differed from those estimates. Our estimated annual forfeiture rates for stock options, RSUs and RSAs are based on our historical forfeiture experience.
The estimated fair value of stock options, RSUs and RSAs is expensed on a straight-line basis over the expected term of the grant and the estimated fair value of performance-contingent RSUs and RSAs is expensed using an accelerated method over the term of the award once we have determined that it is probable that performance milestones will be achieved. Compensation expense for RSUs and RSAs that contain performance conditions is based on the grant date fair value of the award. Compensation expense is recorded over the requisite service period based on management's best estimate as to whether it is probable that the shares awarded are expected to vest. We assess the probability of the performance milestones being met on a continuous basis.
Compensation expense for purchases under the ESPP is recognized based on the fair value of the common stock on the date of offering, less the purchase discount percentage provided for in the plan.
We have not recognized, and do not expect to recognize in the near future, any income tax benefit related to employee stock-based compensation expense as a result of the full valuation allowance on our deferred tax assets including deferred tax assets related to our net operating loss carryforwards.
68
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
Amortization of Debt Issuance Costs from Non-recourse Notes Payable, due 2029
In April 2014, we entered into certain note purchase agreements relating to the private placement of $450.0 million aggregate principal amount of non-recourse 9% fixed rate term notes due 2029 (the "2029 Notes") issued by our wholly-owned subsidiary.
The 2029 Notes are secured by a security interest in a segregated bank account established to receive 40% of royalties due to us under the LABA Collaboration with GSK commencing on April 1, 2014 and ending upon the earlier of full repayment of principal or May 15, 2029. The funds in the segregated bank account can only be used to make principal and interest payments on the 2029 Notes.
The 2029 Notes bear an annual interest rate of 9%, with interest and principal paid quarterly beginning November 15, 2014. The 2029 Notes may be redeemed at any time prior to maturity, in whole or in part, at specified redemption premiums. Prior to May 15, 2016, in the event that the specified portion of royalties received in a quarter is less than the interest accrued for the quarter, the principal amount of the 2029 Notes will increase by the interest shortfall amount for that period.
As part of this sale, we incurred approximately $15.3 million in transaction costs, which will be amortized to interest expense over the estimated life of the 2029 Notes based on the effective interest method. Since the principal and interest payments on the 2029 Notes are based on royalties from product sales, which will vary from quarter to quarter, the 2029 Notes may be repaid prior to the final maturity date in 2029. To the extent that the interest or principal payments are greater or less than our initial estimates or the timing of such payments is materially different than our original estimates, we will prospectively adjust the amortization of the debt issuance costs. There are a number of factors that could materially affect the amount and timing of the royalty payments due to us under the LABA Collaboration with GSK, most of which are not within our control. Such factors include, but are not limited to, the competitive landscape for approved products and developing therapies that compete with our partnered products, the ability of patients to be able to afford our partnered products, the size of the market for our partnered products, safety concerns in the marketplace for respiratory therapies in general and with our partnered products in particular, decisions as to the timing of product launches, pricing and discounts, and other events or circumstances that result in reduced royalty payments, all of which would result in an impact to the amount of debt issuance costs amortized.
Other Income (Expense), net
In 2014, other income (expense), net includes the charge of $3.8 million recognized for the other-than-temporary loss on Theravance Biopharma ordinary shares owned by us.
In May 2013, we entered into a royalty participation agreement with Elan Corporation, plc ("Elan"). The closing of the transaction was subject to closing conditions, including the approval of the transaction by Elan's shareholders. Elan's shareholders did not approve the transaction at an Extraordinary General Meeting. Subsequently, we terminated the agreement and, as a result, Elan paid us a $10.0 million termination fee in June 2013, which is reflected in other income on the consolidated statements of operations. Other expense is comprised of third party expenses related to the aforementioned royalty participation agreement and the change in the estimated fair value of the capped-call instruments related to our convertible subordinated notes issued in January 2013, which is reflected in other expense.
69
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
Income Taxes
We utilize the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax basis of assets and liabilities and are measured using enacted tax rates and laws that will be in effect when the differences are expected to reverse. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized.
None of our currently unrecognized tax benefits would affect our effective income tax rate if recognized, due to the valuation allowance that currently offsets our deferred tax assets. We do not anticipate the total amount of unrecognized income tax benefits relating to uncertain tax positions existing at December 31, 2014 will significantly increase or decrease in the next 12 months.
We assess all material positions taken in any income tax return, including all significant uncertain positions, in all tax years that are still subject to assessment or challenge by relevant taxing authorities. Assessing an uncertain tax position begins with the initial determination of the position's sustainability and is measured at the largest amount of benefit that is greater than 50% likely to be realized upon ultimate settlement. As of each balance sheet date, unresolved uncertain tax positions must be reassessed, and we will determine whether: the factors underlying the sustainability assertion have changed and whether the amount of the recognized tax benefit is still appropriate.
The recognition and measurement of tax benefits requires significant judgment. Judgments concerning the recognition and measurement of a tax benefit might change as new information becomes available.
Comprehensive Loss
Comprehensive loss is comprised of net loss and other comprehensive income (loss). Other comprehensive income (loss) consists of changes in unrealized gains and losses on our marketable securities, net of tax.
Related Parties
Transactions with GSK are described in Note 3, "Collaborative Arrangements".
Prior to the Spin-Off, Robert V. Gunderson, Jr. was one of our directors. We have engaged Gunderson Dettmer Stough Villeneuve Franklin & Hachigian, LLP, of which Mr. Gunderson is a partner, as our primary legal counsel. Fees incurred in the ordinary course of business were $1.3 million in 2014, $3.2 million in 2013, $1.2 million in 2012.
Recently Issued Accounting Pronouncements Not Yet Adopted
In May 2014, the FASB issued Accounting Standards Update 2014-09, Revenue from Contracts with Customers ("ASU 2014-09"), which converges the FASB and the International Accounting Standards Board standards on revenue recognition. Areas of revenue recognition that will be affected include, but are not limited to, transfer of control, variable consideration, allocation of transfer pricing, licenses, time value of money, contract costs and disclosures. This guidance is effective for the fiscal years and interim reporting periods beginning after December 15, 2016, at which time we may adopt the new standard under the full retrospective method or the modified retrospective method. Early adoption is
70
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
not permitted. We are currently evaluating the impact of adopting ASU 2014-09 on our consolidated financial statements and related disclosures.
2. Net Loss per Share
Basic net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding, less RSAs subject to forfeiture. Diluted net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding, less RSAs subject to forfeiture, plus all additional common shares that would have been outstanding, assuming dilutive potential common shares had been issued for other dilutive securities.
For the years ended December 31, 2014, 2013 and 2012, diluted and basic net loss per common share was identical since potential common shares were excluded from the calculation, as their effect was anti-dilutive.
Anti-dilutive Securities
The following common equivalent shares were not included in the computation of diluted net loss per share because their effect was anti-dilutive:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
2014 | 2013 | 2012 | |||||||
Share issuances under equity incentive plan and ESPP |
6,239 | 4,095 | 5,367 | |||||||
Unvested RSAs |
1,772 | 2,364 | 2,501 | |||||||
Shares issuable upon conversion of convertible subordinated notes |
12,329 | 2,780 | 6,668 | |||||||
| | | | | | | | | | | |
|
20,340 | 9,239 | 14,536 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
3. Collaborative Arrangements
Net Revenue from Collaborative Arrangements
Net revenue from collaborative arrangements from continuing operations relates to our collaborative arrangement with GSK. Net revenue from other collaborative arrangements is reflected as discontinued operations in the consolidated statements of operations. Refer to Notes 1, 10 and 11, "Description of Operations and Summary of Significant Accounting Policies," "Spin-Off of Theravance Biopharma, Inc." and "Discontinued Operations" for further information.
71
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Collaborative Arrangements (Continued)
Net revenue recognized under our GSK Agreements was as follows:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
2014 | 2013 | 2012 | |||||||
Royalties from a related party |
$ | 18,417 | $ | 1,945 | $ | — | ||||
Less: amortization of capitalized fees paid to a related party |
(11,066 | ) | (743 | ) | — | |||||
| | | | | | | | | | | |
Royalty revenue |
7,351 | 1,202 | — | |||||||
LABA collaboration(1) |
— | 1,815 | 3,629 | |||||||
Strategic alliance—MABA program license(2) |
1,082 | 1,515 | 1,984 | |||||||
| | | | | | | | | | | |
Total net revenue from GSK |
$ | 8,433 | $ | 4,532 | $ | 5,613 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
- (1)
- Deferred
revenue under this agreement was fully recognized in 2013.
- (2)
- We revised the estimated performance period for the MABA program based on its progress as follows: (i) in the fourth quarter of 2012, resulting in an increase to net loss of $0.1 million for the year ended December 31, 2012 and (ii) in the fourth quarter of 2013, resulting in an increase to net loss of $0.1 million for the year ended December 31, 2013. We do not expect that these revisions will have a material impact on future revenue recognized under this program
LABA Collaboration
In November 2002, we entered into our LABA Collaboration Agreement with GSK to develop and commercialize once-daily LABA products for the treatment of chronic obstructive pulmonary disease ("COPD") and asthma. For the treatment of COPD, the collaboration has developed two combination products: (1) RELVAR®/BREO® ELLIPTA® (FF/VI), a once-daily combination medicine consisting of a LABA, vilanterol (VI), and an inhaled corticosteroid (ICS), fluticasone furoate (FF) and (2) ANORO® ELLIPTA® (UMEC/VI), a once-daily medicine combining a long-acting muscarinic antagonist ("LAMA"), umeclidinium bromide ("UMEC"), with a LABA, VI. For the treatment of asthma, RELVAR® ELLIPTA® is approved in multiple regions outside of North America and FF/VI is currently under regulatory review in the U.S.
As a result of the launch and approval of RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® in the U.S., Japan and Europe, we were obligated to pay milestone fees to GSK totaling $220.0 million, which have had paid in their entirety as of December 31, 2014. Although we have no further milestone payment obligations to GSK pursuant to the LABA Collaboration Agreement, we continue to have ongoing development and commercialization activities under the GSK Agreements that are expected to continue over the life of the agreements. The milestone fees paid to GSK were recognized as capitalized fees paid to a related party, which are being amortized over their estimated useful lives commencing upon the commercial launch of the product.
We are entitled to receive annual royalties from GSK on sales of RELVAR®/BREO® ELLIPTA® as follows: 15% on the first $3.0 billion of annual global net sales and 5% for all annual global net sales above $3.0 billion. Sales of single-agent LABA medicines and combination medicines would be combined for the purposes of this royalty calculation. For other products combined with a LABA from
72
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Collaborative Arrangements (Continued)
the LABA Collaboration, such as ANORO® ELLIPTA®, royalties are upward tiering and range from 6.5% to 10%.
Amortization expense resulting from the milestone fees paid to GSK, which are recognized as capitalized fees paid to a related party, is a reduction to royalty revenue. When amortization expense exceeds amounts recognized for royalty revenue, negative revenue would be reported in our consolidated statements of operations.
2004 Strategic Alliance
In March 2004, we entered into the Strategic Alliance Agreement with GSK where GSK received an option to license exclusive development and commercialization rights to product candidates from certain of our discovery programs on pre-determined terms and on an exclusive, worldwide basis. Upon GSK's decision to license a program, GSK is responsible for funding all future development, manufacturing and commercialization activities for product candidates in that program. In addition, GSK is obligated to use diligent efforts to develop and commercialize product candidates from any program that it licenses. If the program is successfully advanced through development by GSK, we are entitled to receive clinical, regulatory and commercial milestone payments and royalties on any sales of medicines developed from the program. If GSK chooses not to license a program, we retain all rights to the program and may continue the program alone or with a third party. GSK has no further option rights on any of our research or development programs under the strategic alliance.
In 2005, GSK licensed our MABA program for the treatment of COPD, and in October 2011, we and GSK expanded the MABA program by adding six additional Theravance-discovered preclinical MABA compounds (the "Additional MABAs"). GSK's development, commercialization, milestone and royalty obligations under the strategic alliance remain the same with respect to GSK961081 ('081), the lead compound in the MABA program. GSK is obligated to use diligent efforts to develop and commercialize at least one MABA within the MABA program, but may terminate progression of any or all Additional MABAs at any time and return them to us, at which point we may develop and commercialize such Additional MABAs alone or with a third party. Both GSK and we have agreed not to conduct any MABA clinical studies outside of the strategic alliance so long as GSK is in possession of the Additional MABAs. If a single-agent MABA medicine containing '081 is successfully developed and commercialized, GSK is required to pay royalties of between 10% and 20% of annual global net sales up to $3.5 billion, and 7.5% for all annual global net sales above $3.5 billion. If a MABA medicine containing '081 is commercialized as a combination product, such as '081/FF, the royalty rate is 70% of the rate applicable to sales of the single-agent MABA medicine. For single-agent MABA medicines containing an Additional MABA, GSK is required to pay royalties of between 10% and 15% of annual global net sales up to $3.5 billion, and 10% for all annual global net sales above $3.5 billion. For combination products containing an Additional MABA, such as a MABA/ICS combination, the royalty rate is 50% of the rate applicable to sales of the single-agent MABA medicine. If a MABA medicine containing '081 is successfully developed and commercialized in multiple regions of the world, GSK could be required to pay total contingent payments of up to $125.0 million for a single-agent medicine and up to $250.0 million for both a single-agent and a combination medicine. If a MABA medicine containing an Additional MABA is successfully developed and commercialized in multiple regions of the world, GSK could be required to pay contingent payments of up to $129.0 million. As a result of the transactions effected by the Spin-Off, however, we are now only entitled to receive 15% of
73
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Collaborative Arrangements (Continued)
any contingent payments and royalties payable by GSK from sales of FF/UMEC/VI (and MABA, and MABA/FF) while Theravance Biopharma receives 85% of those same payments.
Agreements Entered into with GSK in Connection with the Spin-Off
On March 3, 2014, in contemplation of the Spin-Off of Theravance Biopharma, we, Theravance Biopharma and GSK entered into a series of agreements clarifying how the companies would implement the Spin-Off and operate following the Spin-Off. We, Theravance Biopharma and GSK entered into a three-way master agreement providing for GSK's consent to the Spin-Off provided certain conditions were met. In addition, we and GSK also entered into amendments to the GSK Agreements, and Theravance Biopharma and GSK entered into a governance agreement, a registration rights agreement and an extension agreement. The three-way master agreement was effective on June 1, 2014 when we transferred our research and drug development operations to Theravance Biopharma. Pursuant to a three-way master agreement entered into by and among us, Theravance Biopharma and GSK in connection with the Spin-Off, we agreed to sell a certain number of Theravance Biopharma shares withheld from a taxable dividend of Theravance Biopharma shares to GSK. After such Theravance Biopharma shares were sent to the transfer agent, we agreed to purchase the Theravance Biopharma shares from the transfer agent, rather than have them sold on the open market, in order to satisfy tax withholdings. GSK had a right to purchase these shares of Theravance Biopharma from us, but this right expired unexercised. Accordingly, at December 31, 2014, we owned 436,802 ordinary shares of Theravance Biopharma, which are accounted for as marketable securities in the consolidated balance sheets.
The amendments to the GSK Agreements do not change the economics or royalty rates under the GSK Agreements, though the assignment of the Strategic Alliance Agreement and portions of the LABA Collaboration to TRC do change how the economics are allocated between Theravance Biopharma and us. The amendments to the GSK Agreements do provide that GSK's diligent efforts obligations regarding commercialization matters under both agreements will change upon regulatory approval in either the United States or the European Union of FF/UMEC/VI or a MABA in combination with FF. Upon such regulatory approval, GSK's diligent efforts obligations as to commercialization matters under the GSK Agreements will have the objective of focusing on the best interests of patients and maximizing the net value of the overall portfolio of products under the GSK Agreements. Since GSK's commercialization efforts following such regulatory approval will be guided by a portfolio approach across products in which we will retain our full interests upon the Spin-Off and also products in which we will have retained only a portion of our interests upon the planned Spin-Off transaction, GSK's commercialization efforts may have the effect of reducing the overall value of our remaining interests in the GSK Agreements after the Spin-Off.
Purchases of Common Stock under the Company's Governance Agreement and Common Stock Purchase Agreements with GSK
In 2014, GSK purchased approximately 1.7 million shares of our common stock pursuant to its periodic "top-up" rights under our Amended and Restated Governance Agreement, dated as of June 4, 2004, as amended, among us, GSK and certain GSK affiliates, for an aggregate purchase price of approximately $38.1 million.
74
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Collaborative Arrangements (Continued)
GSK Contingent Payments and Revenue
The potential future contingent payments receivable related to the MABA program of $363.0 million are not deemed substantive milestones due to the fact that the achievement of the event underlying the payment predominantly relates to GSK's performance of future development, manufacturing and commercialization activities for product candidates after licensing the program.
Reimbursement of Research and Development Costs
Reimbursement of research and development costs from continuing operations is solely related to the GSK Agreements. Under the GSK Agreements, we are entitled to reimbursement of certain research and development costs. For the years ended December 31, 2014, 2013 and 2012, research and development costs reimbursed from GSK was $0.1 million, $0.5 million and $0.2 million. Reimbursement of research and development costs from other collaborative arrangements has been reflected as discontinued operations in the consolidated statements of operations. Refer to Notes 1, 10 and 11, "Description of Operations and Summary of Significant Accounting Policies," "Spin-Off of Theravance Biopharma, Inc." and "Discontinued Operations" for further information.
4. Available-for-Sale Securities and Fair Value Measurements
Available-for Sale Securities
The classification of available-for-sale securities in the consolidated balance sheets is as follows:
(In thousands)
|
December 31, 2014 |
December 31, 2013 |
|||||
|---|---|---|---|---|---|---|---|
Cash and cash equivalents |
$ | 95,090 | $ | 125,009 | |||
Short-term marketable securities |
143,698 | 321,615 | |||||
Marketable securities |
42,856 | 55,374 | |||||
Restricted cash |
— | 833 | |||||
| | | | | | | | |
Total |
$ | 281,644 | $ | 502,831 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The estimated fair value of available-for-sale securities is based on quoted market prices for these or similar investments that were based on prices obtained from a commercial pricing service. Available-for-sale securities are summarized below:
| |
December 31, 2014 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
Amortized Cost | Gross Unrealized Gains |
Gross Unrealized Losses |
Other Than Temporary Impairment Loss |
Estimated Fair Value |
|||||||||||
U.S. government securities |
$ | 30,019 | $ | 24 | $ | — | $ | — | $ | 30,043 | ||||||
U.S. government agencies |
34,756 | 6 | (12 | ) | — | 34,750 | ||||||||||
U.S. corporate notes |
80,880 | 5 | (110 | ) | — | 80,775 | ||||||||||
U.S. commercial paper |
34,469 | — | — | — | 34,469 | |||||||||||
Ordinary shares of Theravance Biopharma |
10,269 | — | — | (3,752 | ) | 6,517 | ||||||||||
Money market funds |
95,090 | — | — | — | 95,090 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total |
$ | 285,483 | $ | 35 | $ | (122 | ) | $ | (3,752 | ) | $ | 281,644 | ||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
75
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Available-for-Sale Securities and Fair Value Measurements (Continued)
| |
December 31, 2013 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
Amortized Cost | Gross Unrealized Gains |
Gross Unrealized Losses |
Other Than Temporary Impairment Loss |
Estimated Fair Value |
|||||||||||
U.S. government securities |
$ | 42,104 | $ | 55 | $ | (1 | ) | $ | — | $ | 42,158 | |||||
U.S. government agencies |
141,278 | 61 | (8 | ) | — | 141,331 | ||||||||||
U.S. corporate notes |
94,923 | 54 | — | — | 94,977 | |||||||||||
U.S. commercial paper |
102,021 | 2 | (1 | ) | — | 102,022 | ||||||||||
Money market funds |
122,343 | — | — | — | 122,343 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total |
$ | 502,669 | $ | 172 | $ | (10 | ) | $ | — | $ | 502,831 | |||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
We determined that the unrealized loss on our Theravance Biopharma equity securities as of December 31, 2014 was other-than-temporary. Therefore, we recognized a loss of $3.8 million on these equity securities, which was charged to other income (expense), net on the consolidated statements of operations.
At December 31, 2014, all of the available-for-sale debt securities had contractual maturities within two years and the average duration of debt securities was approximately eight months. We do not intend to sell the investments in debt that are in an unrealized loss position, and it is unlikely that we will be required to sell the investments before recovery of their amortized cost basis, which may be maturity. We have determined that the gross unrealized losses on our available-for-sale debt securities at December 31, 2014 were temporary in nature. All available-for-sale debt securities with unrealized losses at December 31, 2014 have been in a loss position for less than twelve months.
During the years ended December 31, 2014, 2013 and 2012, we sold marketable securities totaling $7.2 million, $22.6 million and $49.7 million, and the related realized gains and losses were not significant in any of these periods.
76
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Available-for-Sale Securities and Fair Value Measurements (Continued)
Fair Value Measurements
Our available-for-sale securities are measured at fair value on a recurring basis and our debt is carried at the amortized cost basis. The estimated fair values were as follows:
| |
Estimated Fair Value Measurements at Reporting Date Using: | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Quoted Price in Active Markets for Identical Assets |
Significant Other Observable Inputs |
Significant Unobservable Inputs |
|
|||||||||
Types of Instruments (In thousands) |
Level 1 | Level 2 | Level 3 | Total | |||||||||
Assets at December 31, 2014 |
|||||||||||||
U.S. government securities |
$ | 30,043 | $ | — | $ | — | $ | 30,043 | |||||
U.S. government agencies |
— | 34,750 | — | 34,750 | |||||||||
U.S. corporate notes |
— | 80,775 | — | 80,775 | |||||||||
U.S. commercial paper |
— | 34,469 | — | 34,469 | |||||||||
Ordinary shares of Theravance Biopharma |
6,517 | — | — | 6,517 | |||||||||
Money market funds |
95,090 | — | — | 95,090 | |||||||||
| | | | | | | | | | | | | | |
Total assets measured at estimated fair value |
$ | 131,650 | $ | 149,994 | $ | — | $ | 281,644 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Liabilities at December 31, 2014 |
|||||||||||||
Convertible subordinated notes due 2023 |
$ | — | $ | 197,095 | $ | — | $ | 197,095 | |||||
Non-recourse notes due 2029 |
— | 456,411 | — | 456,411 | |||||||||
| | | | | | | | | | | | | | |
Total fair value of liabilities |
$ | — | $ | 653,506 | $ | — | $ | 653,506 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| |
Estimated Fair Value Measurements at Reporting Date Using: | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Quoted Price in Active Markets for Identical Assets |
Significant Other Observable Inputs |
Significant Unobservable Inputs |
|
|||||||||
Types of Instruments (In thousands) |
Level 1 | Level 2 | Level 3 | Total | |||||||||
Assets at December 31, 2013 |
|||||||||||||
U.S. government securities |
$ | 42,158 | $ | — | $ | — | $ | 42,158 | |||||
U.S. government agencies |
98,236 | 43,095 | — | 141,331 | |||||||||
U.S. corporate notes |
61,591 | 33,386 | — | 94,977 | |||||||||
U.S. commercial paper |
3,499 | 98,523 | — | 102,022 | |||||||||
Money market funds |
122,343 | — | — | 122,343 | |||||||||
| | | | | | | | | | | | | | |
Total assets measured at estimated fair value |
$ | 327,827 | $ | 175,004 | $ | — | $ | 502,831 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Liabilities at December 31, 2013 |
|||||||||||||
Convertible subordinated notes due 2023 |
$ | — | $ | 408,250 | $ | — | $ | 408,250 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
The fair value of our marketable securities classified within Level 2 is based upon observable inputs that may include benchmark yields, reported trades, broker/dealer quotes, issuer spreads, two-sided markets, benchmark securities, bids, offers and reference data including market research publications.
77
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Available-for-Sale Securities and Fair Value Measurements (Continued)
At December 31, 2014, securities with a total fair value of $8.0 million were measured using Level 2 inputs in comparison to December 31, 2013, at which time such securities had a fair value of $8.0 million and were measured using Level 1 inputs.
The fair value of our convertible subordinated notes due 2023 and non-recourse notes due 2029 is based on actual trading prices of the instruments, if applicable, or pricing models that utilize current observable market characteristics for similar types of instruments.
5. Capitalized Fees paid to a Related Party
Capitalized fees paid to a related party, which consist of registrational and launch-related milestone fees paid to GSK, were as follows:
| |
December 31, 2014 | December 31, 2013 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
Weighted Average Remaining Amortization Period (Years) |
Gross Carrying Value |
Accumulated Amortization |
Net Carrying Value |
Gross Carrying Value |
Accumulated Amortization |
Net Carrying Value |
|||||||||||||||
Approval and launch related milestone payments under the LABA Collaboration |
15.1 | $ | 220,000 | (11,809 | ) | $ | 208,191 | $ | 125,000 | (743 | ) | $ | 124,257 | |||||||||
These milestone fees are being amortized over their estimated useful lives commencing upon the commercial launch of the product in their respective regions with the amortization expense recorded as a reduction in revenue from collaborative arrangements. Additional information regarding these milestone fees is included in Note 3, "Collaborative Arrangements." Amortization expense for the years ended December 31, 2014, 2013 and 2012 were $11.1 million, $0.7 million and zero. The remaining estimated amortization expense is $13.8 million for each of the years from 2015 to 2019 and $139.2 million thereafter.
6. Stock-Based Compensation
Equity Incentive Plans
In May 2012, we adopted the 2012 Equity Incentive Plan (the "2012 Plan"). The number of shares of our common stock originally reserved for issuance under the 2012 Plan is equal to 6,500,000 shares plus up to 12,667,411 additional shares that may be added to the 2012 Plan in connection with the forfeiture, repurchase, cash settlement or termination of awards outstanding under the 2004 Equity Incentive Plan (the "2004 Plan"), the 2008 New Employee Equity Incentive Plan, the 1997 Stock Plan and the Long-Term Stock Option Plan (collectively, the "Prior Plans") as of December 31, 2011. In connection with the Spin-Off of Theravance Biopharma, Inc. on June 2, 2014, outstanding stock options and other awards, along with the number of shares remaining available for future stock options and other awards, were adjusted pursuant to the anti-dilution provisions of the 2012 Plan and Prior Plans. An additional 1,373,201 shares were added to the 2012 Plan share reserve as a result of the anti-dilution adjustment of the outstanding stock options and other awards granted under the 2012 Plan and the shares remaining available for future grant under the 2012 Plan. The additional 993,130 shares added to the Prior Plans as a result of the anti-dilution provisions are included in the 12,667,411
78
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
6. Stock-Based Compensation (Continued)
additional shares that may be added to the 2012 Plan. While a maximum of 12,667,411 shares could be added to the 2012 Plan from the Prior Plans, this assumes that all the awards outstanding on December 31, 2011 will be forfeited, repurchased, cash settled or terminated. Therefore, the actual number that may be added to the 2012 Plan share reserve will likely be lower. No additional awards were made after May 15, 2012 under the 2004 Plan. Stock options and stock appreciation rights (SARs) will reduce the 2012 Plan reserve by one share for every share granted, and stock awards other than options and SARs granted will reduce the 2012 Plan share reserve by 1.45 shares for every share granted. The 2012 Plan share reserve was also reduced by the number of stock awards granted under the 2004 Plan on or after January 1, 2012, using the same ratios described. As of December 31, 2014, total shares remaining available for issuance under the 2012 Plan were 3,036,820.
The 2012 Plan provides for the grant of incentive stock options, nonstatutory stock options, restricted stock awards, stock unit awards and SARs to employees, non-employee directors and consultants. Stock options may be granted with an exercise price not less than the fair market value of the common stock on the grant date. Stock options granted to employees generally have a maximum term of 10 years and vest over a four year period from the date of grant; 25% vest at the end of one year, and 75% vest monthly over the remaining three years. We may grant options with different vesting terms from time to time. Unless an employee's termination of service is due to disability or death, upon termination of service, any unexercised vested options will be forfeited at the end of three months or the expiration of the option, whichever is earlier.
Employee Stock Purchase Plan
Under the 2004 Employee Stock Purchase Plan (the "ESPP"), our non-officer employees may purchase common stock through payroll deductions at a price equal to 85 percent of the lower of the fair market value of the stock at the beginning of the offering period or at the end of each applicable purchase period. The ESPP provides for consecutive and overlapping offering periods of 24 months in duration, with each offering period composed of four consecutive six-month purchase periods. The purchase periods end on either May 15th or November 15th. ESPP contributions are limited to a maximum of 15% of an employee's eligible compensation.
Our ESPP plan also includes a feature that provides for a new offering period to begin when the fair market value of our common stock on any purchase date during an offering period falls below the fair market value of our common stock on the first day of such offering period. This feature is called a reset. We had resets for new twenty-four month offering periods on November 16, 2011, May 16, 2012 and November 16, 2012. We applied modification accounting to determine the incremental fair value associated with the ESPP resets and recognized the related incremental stock-based compensation expense.
As of December 31, 2013, a total of 2,025,000 shares of common stock were approved and authorized for issuance under the ESPP. Through December 31, 2013, we had issued 1,740,861 shares under the ESPP at an average price of $11.29 per share. As of December 31, 2013, total shares remaining available for issuance under the ESPP were 284,139. As a result of our announcement that our Board of Directors had approved plans to separate our businesses into two independent publicly traded companies, the ESPP was suspended after the November 15, 2013 purchase date and all monies remaining after the purchase were refunded to employees. Therefore, $0.8 million of compensation expense relating to purchase periods ending after November 15, 2013 was reversed in the fourth
79
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
6. Stock-Based Compensation (Continued)
quarter of 2013, and any remaining unamortized compensation expense relating to these purchase periods will not be recognized. The ESPP was resumed with the offering period commencing in November 2014.
Performance-Contingent RSAs
Over the past three years, the Compensation Committee of our Board of Directors (the "Compensation Committee") has approved grants of performance-contingent RSAs to senior management and a non-executive officer. Generally, these awards have dual triggers of vesting based upon the achievement of certain performance goals by a pre-specified date, as well as a requirement for continued employment. When the performance goals are probable of achievement for these types of awards, time-based vesting and, as a result, recognition of stock-based compensation expense commence. Included in these performance-contingent RSAs is the grant of 1,290,000 special long-term retention and incentive performance-contingent RSAs to senior management in 2011. The awards have dual triggers of vesting based upon the achievement of certain performance conditions over a six-year timeframe from 2011 through December 31, 2016 and require continued employment.
As of March 31, 2014, we determined that the achievement of the requisite performance conditions for vesting of the first tranche of these awards was probable and, as a result, $6.8 million of the total stock-based compensation expense was recognized in the first quarter of 2014. The total stock-based compensation expense of $7.0 million for the first tranche was recognized through May 2014.
In connection with the Spin-Off, our Compensation Committee approved the modification of the remaining tranches related to these awards as the performance conditions associated with the remaining portions of these awards were unlikely to be consistent with the new strategies of each company following the separation. The modification acknowledged the Spin-Off and permitted recognition of achievement of certain of the original performance conditions that were met prior to the Spin-Off, triggering service-based vesting for a portion of the equity awards, for which $3.8 million is expected to be recognized by us during the twelve-month period that commenced in June 2014. The remaining 63,000 RSAs for which service-based vesting was not triggered at the time of the Spin-Off remain subject to new performance conditions (as well as the original service conditions). In addition, the RSAs for which both the performance and service-based conditions were not achieved prior to the Spin-Off were entitled to the pro rata dividend distribution made by Theravance on June 2, 2014 of one ordinary share of Theravance Biopharma for every 3.5 shares of Theravance common stock subject to their awards, which will also be subject to the same new performance and service conditions as the original RSAs to which they relate. As of December 31, 2014, we determined that the achievement of the requisite performance conditions was not probable and, as a result, no compensation cost was recognized for the remaining equity awards.
Performance-Contingent RSUs
The Compensation Committee of the Company's Board of Directors has approved grants of performance-contingent RSUs to employees. These awards have dual triggers of vesting based upon the successful achievement of certain corporate operating milestones in specified timelines, as well as a requirement for continued employment. When the performance goals are deemed to be probable of achievement for these types of awards, time-based vesting and, as a result, recognition of stock-based compensation expense commences.
80
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
6. Stock-Based Compensation (Continued)
Director Compensation Program
Our non-employee directors receive compensation for services provided as a director. Each member of our Board of Directors who is not an employee receives an annual cash retainer for services as a director, member of a committee of the Board of Directors, lead independent director and chairman, as applicable. In addition, prior to the Spin-Off, each non-employee director was entitled to a cash fee for each board and committee meeting attended.
Each of our independent directors receives periodic automatic grants of equity awards under a program implemented under the 2012 Plan. These grants are non-discretionary. Only our independent directors or affiliates of such directors are eligible to receive automatic grants under the 2012 Plan. Under the program, as amended following the Spin-Off, each individual who first becomes a non-employee director will, on the date such individual joins the Board of Directors, automatically be granted a one-time grant of RSUs covering a number of shares of our common stock calculated as $250,000 divided by our common stock closing share price on the date of grant as reported on The NASDAQ Global Market, rounded down to the nearest whole share (the "Initial RSUs"), plus a one-time grant of RSUs covering a number of shares of our common stock calculated as $250,000 divided by our common stock closing share price on the date of grant as reported on The NASDAQ Global Market, which would be pro-rated for the number of whole months remaining until the anniversary of the prior year's stockholders' meeting, rounded down to the nearest whole share (the "Pro Rata RSUs"). The Initial RSUs vest in two equal annual installments, while Pro-Rata RSUs vest in a single installment at the sooner of the next annual stockholder meeting or the one-year grant anniversary, in each case subject to the non-employee director's continuous service through the applicable vesting date.
Annually, upon his or her re-election to the Board at the Annual Meeting of Stockholders, each non-employee director is automatically granted an RSU covering a number of shares of our common stock calculated as $250,000 divided by our common stock closing share price on the date of grant as reported on The NASDAQ Global Market, rounded down to the nearest whole share. Annual RSUs will vest at the sooner of the next annual stockholder meeting or the one-year anniversary of grant, subject to the non-employee director's continuous service through the applicable vesting date.
These RSUs will vest in full upon the director's death or the occurrence of a Change in Control before the director's service terminates. All director RSUs will be settled in shares of our common stock on the vesting date. Director RSUs will carry dividend equivalent rights to be credited with an amount equal to all cash dividends paid on the underlying shares of common stock while unvested. Dividend equivalents will be subject to the same terms and conditions, including vesting, as the RSUs to which they attach and will be paid in cash upon vesting.
Stock-Based Compensation Expense
In connection with the Spin-Off of Theravance Biopharma, all outstanding shares of Theravance Biopharma were distributed to our stockholders as a pro-rata dividend distribution on June 2, 2014 by issuing one ordinary share of Theravance Biopharma for every 3.5 shares held of Theravance common stock to stockholders of record on May 15, 2014. Outstanding stock options and RSUs that were not eligible for the dividend distribution were adjusted for the Spin-Off of Theravance Biopharma. The number of shares and exercise price for all outstanding stock options were adjusted and the number of shares for all outstanding RSUs was adjusted. All other terms of these grants remain the same;
81
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
6. Stock-Based Compensation (Continued)
provided, however, that the vesting and expiration of these grants are based on the holder's continuing employment or service with us or Theravance Biopharma, as applicable.
Although the anti-dilution adjustments were required pursuant to the terms of each stock plan, the anti-dilution adjustments were calculated using a volume-weighted average stock price, rather than the stock price as of the date of the dividend distribution, which resulted in incremental compensation expense. The accounting impact of the adjustment to the outstanding stock options and RSUs that occurred in connection with the Spin-Off of Theravance Biopharma was measured by comparing of the fair values of the modified stock options and RSUs to our employees and directors immediately before and after the adjustment. As a result, we recognized incremental stock-based compensation expense of $1.2 million in the second quarter of 2014, of which $0.9 million is included in discontinued operations. All remaining unrecognized stock-based compensation expense associated with this adjustment will be recognized by Theravance Biopharma as it pertains to stock options and RSUs held by individuals now employed by Theravance Biopharma or one if its affiliates.
Stock-based compensation expense is included in the consolidated statements of operations as follows:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
2014 | 2013 | 2012 | |||||||
Research and development |
$ | 2,781 | $ | 573 | $ | 475 | ||||
General and administrative |
12,980 | 7,325 | 7,310 | |||||||
| | | | | | | | | | | |
Stock-based compensation from continuing operations |
15,761 | 7,898 | 7,785 | |||||||
Stock-based compensation from discontinued operations |
11,629 | 17,789 | 15,998 | |||||||
| | | | | | | | | | | |
Total stock-based compensation expense |
$ | 27,390 | $ | 25,687 | $ | 23,783 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Stock-based compensation expense included in the consolidated statements of operations by award type is as follows:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
2014 | 2013 | 2012 | |||||||
Stock options |
$ | 4,658 | $ | 4,132 | $ | 3,417 | ||||
RSUs |
4,564 | 10,174 | 10,803 | |||||||
RSAs |
7,575 | 9,723 | 7,602 | |||||||
Performance RSUs |
3 | 61 | 743 | |||||||
Performance RSAs |
10,580 | 1,061 | 366 | |||||||
ESPP |
10 | 536 | 852 | |||||||
| | | | | | | | | | | |
Total stock-based compensation expense |
$ | 27,390 | $ | 25,687 | $ | 23,783 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Total stock-based compensation expense capitalized to inventory was not material for 2014, 2013 and 2012.
82
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
6. Stock-Based Compensation (Continued)
As of December 31, 2014, the unrecognized stock-based compensation cost, net of expected forfeitures for awards expected to vest, including performance-contingent RSAs for which the performance milestones were determined to be probable of achievement, and the estimated weighted-average amortization period, using the straight-line attribution method, was as follows:
(In thousands, except amortization period)
|
Unrecognized Compensation Cost |
Weighted- Average Amortization Period (Years) |
|||||
|---|---|---|---|---|---|---|---|
Stock options |
$ | 3,207 | 3.2 | ||||
RSUs |
1,814 | 1.4 | |||||
RSAs |
5,147 | 3.4 | |||||
Performance RSAs |
619 | 1.9 | |||||
| | | | | | | | |
Total stock-based compensation expense |
$ | 10,787 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Compensation Awards
The following table summarizes equity award activity under the 2012 Plan and Prior Plans and related information:
(In thousands, except per share data)
|
Number of Shares Subject to Outstanding Options |
Weighted- Average Exercise Price of Outstanding Options |
Number of Shares Subject to Outstanding RSUs |
Weighted- Average Fair Value per Share at Grant |
Number of Shares Outstanding Subject to Vesting or Performance Conditions with Vesting |
Weighted- Average Fair Value per Share at Grant |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Balance at December 31, 2013 |
4,824 | $ | 25.30 | 1,115 | $ | 21.53 | 2,365 | $ | 23.66 | ||||||||||
Granted |
885 | 30.42 | 157 | 23.08 | 684 | 23.36 | |||||||||||||
Exercised |
(1,177 | ) | 14.01 | — | — | — | — | ||||||||||||
Released RSUs/RSAs |
— | — | (538 | ) | 18.35 | (823 | ) | 23.20 | |||||||||||
Forfeited |
(393 | ) | 27.26 | (155 | ) | 22.00 | (454 | ) | 15.73 | ||||||||||
Anti-dilution adjustments |
1,283 | — | 196 | — | — | — | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2014 |
5,422 | 22.46 | 775 | 18.53 | 1,772 | 25.78 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
As of December 31, 2014, the aggregate intrinsic value of the options outstanding was $0.7 million and the aggregate intrinsic value of the options exercisable was $0.7 million.
The total intrinsic value of the options exercised was $17.5 million in 2014, $41.4 million in 2013 and $15.2 million in 2012. The total estimated fair value of options vested was $5.7 million in 2014, $3.7 million in 2013 and $4.1 million in 2012.
83
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
6. Stock-Based Compensation (Continued)
Valuation Assumptions
We based the range of weighted-average estimated values of employee stock option grants and rights granted under the ESPP, as well as the weighted-average assumptions used in calculating these values, on estimates at the date of grant, as follows:
| |
Year Ended December 31, | |||||
|---|---|---|---|---|---|---|
| |
2014 | 2013 | 2012 | |||
Employee stock options |
||||||
Risk-free interest rate |
1.6% - 2.1% | 0.8% - 2.0% | 0.7% - 1.2% | |||
Expected term (in years) |
5 - 6 | 5 - 6 | 5 - 6 | |||
Volatility |
52% - 60% | 58% - 60% | 55% - 60% | |||
Dividend yield |
3% - 4% | — | — | |||
Weighted-average estimated fair value of stock options granted |
$15.63 | $19.96 | $11.50 | |||
Employee stock purchase plan issuances |
||||||
Risk-free interest rate |
0.1% - 0.5% | 0.1% - 0.3% | 0.1% - 0.3% | |||
Expected term (in years) |
0.5 - 2 | 0.5 - 2 | 0.5 - 2 | |||
Volatility |
43% - 55% | 56% - 61% | 51% - 64% | |||
Dividend yield |
8% | — | — | |||
Weighted-average estimated fair value of stock options granted |
$4.49 | $16.44 | $8.07 | |||
7. Long-Term Debt
Our long-term debt consists of:
| |
As of December 31, | ||||||
|---|---|---|---|---|---|---|---|
(In thousands)
|
2014 | 2013 | |||||
Convertible Subordinated Notes due 2023 |
$ | 255,109 | $ | 287,500 | |||
Non-Recourse Notes Payable due 2029 |
470,527 | — | |||||
| | | | | | | | |
Total Long-Term Debt |
$ | 725,636 | $ | 287,500 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Convertible Subordinated Notes Due 2023
In January 2013, we completed an underwritten public offering of $287.5 million aggregate principal amount of unsecured convertible subordinated notes, which will mature on January 15, 2023 (the "2023 Notes"). The financing raised proceeds, net of issuance costs, of approximately $281.2 million, less $36.8 million to purchase two privately-negotiated capped call option transactions in connection with the issuance of the notes. The 2023 Notes bear interest at the rate of 2.125% per year that is payable semi-annually in arrears in cash on January 15 and July 15 of each year, beginning on July 15, 2013.
The 2023 Notes are convertible, at the option of the holder, into shares of our common stock at an initial conversion rate of 35.9903 shares per $1,000 principal amount of the 2023 Notes, subject to adjustment in certain circumstances, which represents an initial conversion price of approximately
84
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
7. Long-Term Debt (Continued)
$27.79 per share. Holders of the notes will be able to require us to repurchase some or all of their notes upon the occurrence of a fundamental change at 100% of the principal amount of the notes being repurchased plus accrued and unpaid interest. We may not redeem the notes prior to their stated maturity date.
In connection with the offering of the 2023 Notes, we entered into two privately-negotiated capped call option transactions with a single counterparty. The capped call option transaction is an integrated instrument consisting of a call option on our common stock purchased by us with a strike price equal to the conversion price of $27.79 per share for the underlying number of shares and a cap price of $38.00 per share. The cap component is economically equivalent to a call option sold by us for the underlying number of shares with a strike price of $38.00 per share. As an integrated instrument, the settlement of the capped call coincides with the due date of the convertible debt. At settlement, we would receive from our hedge counterparty a number of shares of our common shares that would range from zero, if the stock price was below $27.79 per share, to a maximum of 2,779,659 shares, if the stock price is above $38.00 per share. However, if the market price of our common stock, as measured under the terms of the capped call transactions, exceeds $38.00 per share, there is no incremental anti-dilutive benefit from the capped call. The aggregate cost of the capped call options was $36.8 million.
In accordance with the agreement for the 2023 Notes, the conversion rate was adjusted as a result of the completion of the Spin-Off of Theravance Biopharma. The conversion rate was adjusted based on the conversion rate immediately prior to the record date for the Spin-Off and the average of the stock dividend distributed to our common stockholders and our stock prices. This resulted in an adjusted conversion rate of 46.9087 shares per $1,000 principal amount of the 2023 Notes, which represents an adjusted conversion price of approximately $21.32 per share. As a result of the conversion rate adjustment, the capped call strike price and cap price were also adjusted accordingly as $21.32 and $29.16. On July 15, 2014, certain holders of the 2023 Notes converted their notes into 1,519,402 shares of our common stock at the adjusted conversion price of $21.32 per share. In connection with the partial conversion of the 2023 Notes, we received 149,645 shares of our common stock from our capped call option counterparty and the shares of common stock received were recorded as treasury stock.
In connection with the payments of the cash dividends during the year ended December 31, 2014, which is further discussed in Note 12, "Dividends Declared and Paid", the adjusted conversion rate with respect to our 2023 Notes was further adjusted in total from 46.9087 shares of our common stock per $1,000 principal amount of the 2023 Notes to 48.3294 shares of our common stock per $1,000 principal amount of the 2023 Notes, which represents an adjusted conversion price of approximately $20.6913 per share. As a result of the conversion rate adjustment, the capped call strike price and cap price were also adjusted accordingly to $20.69 and $28.30.
Private Placement of $450 Million of 9% Non-Recourse Notes
In April 2014, we entered into certain note purchase agreements relating to the private placement of $450.0 million aggregate principal amount of non-recourse 9% fixed rate term notes due 2029 (the "2029 Notes") issued by our wholly-owned subsidiary.
The 2029 Notes are secured by a security interest in a segregated bank account established to receive 40% of royalties due to us under the LABA Collaboration with GSK commencing on April 1,
85
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
7. Long-Term Debt (Continued)
2014 and ending upon the earlier of full repayment of principal or May 15, 2029. The amounts in the segregated bank account can only be used to make interest and principal payments on the 2029 Notes. At December 31, 2014, the balance of the segregated bank account was not material as the 40% of the royalties received under the LABA Collaboration during the three months ended December 31, 2014 were used to pay interest on the 2029 Notes prior to December 31, 2014.
The 2029 Notes bear an annual interest rate of 9%, with interest and principal paid quarterly beginning November 15, 2014. The 2029 Notes may be redeemed at any time prior to maturity, in whole or in part, at specified redemption premiums. Prior to May 15, 2016, in the event that the specified portion of royalties received in a quarter is less than the interest accrued for the quarter, the principal amount of the 2029 Notes will increase by the interest shortfall amount for that period. During the year ended December 31, 2014, $20.5 million of interest expense was added to the principal balance of the 2029 Notes. Since the principal and interest payments on the 2029 Notes are based on royalties from product sales recorded by GSK, which will vary from quarter to quarter and are unknown to us, the 2029 Notes may be repaid prior to the final maturity date in 2029.
From the net proceeds of the offering of approximately $434.7 million, we established a $32.0 million milestone payment reserve account to fund 40% of any future milestone payments that could become payable under the LABA Collaboration Agreement with GSK. This milestone reserve account was a segregated bank account and at December 31, 2014, the balance of this account was zero as we had fulfilled our obligation related to the milestone payments.
As part of this sale, we incurred approximately $15.3 million in transaction costs, which will be amortized to interest expense over the estimated life of the 2029 Notes.
8. Commitments and Contingencies
Operating Lease and Lease Guarantee
Due to the Spin-Off, the leases for the facilities in South San Francisco, California, which formerly served as our headquarters, were assigned to Theravance Biopharma. We would be held liable by the landlord if Theravance Biopharma defaults under its lease obligations, and thus, we have in substance guaranteed the payments under the lease agreements for these facilities. As of December 31, 2014, the total lease payments for the duration of the lease, which runs through May 2020, were $33.4 million. We would be responsible for lease-related payments including utilities, property taxes, and common area maintenance, which may be as much as the actual lease payments. We recorded a non-current liability of $1.3 million in our consolidated balance sheet as of December 31, 2014 related to the estimated fair value of this lease guarantee. We prepared a discounted, probability-weighted cash flow analysis to calculate the estimated fair value of the lease guarantee as of the Spin-Off. We were required to make assumptions regarding the probability of Theravance Biopharma's default on the lease payments, the likelihood of a sublease being executed, and the times at which these events could occur. The fair value of this lease guarantee was charged to additional paid in capital upon the Spin-Off, and any future adjustments to the carrying value of the obligation will be recorded in the consolidated statements of operations.
Following the Spin-Off, we entered into a Sublease Agreement with Theravance Biopharma to sublease 4,847 square feet of office space in South San Francisco, California, which expires in
86
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Commitments and Contingencies (Continued)
May 2020. We do not own or lease any other properties. Future minimum lease payments under this lease at December 31, 2014, were as follows:
(In thousands)
|
|
|||
|---|---|---|---|---|
Years Ending December 31: |
||||
2015 |
$ | 186 | ||
2016 |
192 | |||
2017 |
197 | |||
2018 |
203 | |||
2019 |
210 | |||
Thereafter |
89 | |||
| | | | | |
Total |
$ | 1,077 | ||
| | | | | |
| | | | | |
| | | | | |
Special Long-Term Retention and Incentive Cash Awards Program
In 2011, we granted special long-term retention and incentive cash bonus awards to certain employees. The awards have dual triggers of vesting based upon the achievement of certain performance conditions over a six-year timeframe from 2011 through December 31, 2016 and continued employment.
As of March 31, 2014, we determined that the achievement of the requisite performance conditions for the first tranche of these awards was probable and, as a result, $9.1 million of cash bonus expense was recognized in the first quarter of 2014, the majority of which is included in discontinued operations in the consolidated statements of operations. In May 2014, the total cash bonus of $9.5 million for the first tranche was paid.
In connection with the Spin-Off, the Compensation Committee approved the modification of the remaining tranches related to these awards as the performance conditions associated with the remaining portions of these awards were unlikely to be consistent with the new strategies of each company following the separation. The modification acknowledged the Spin-Off and permitted recognition of achievement of certain of the original performance conditions that were met prior to the Spin-Off, triggering service-based vesting for a portion of the cash awards. The amount payable by us under these modified cash bonus awards is $0.5 million. The remaining tranches of the cash awards were forfeited.
Guarantees and Indemnifications
We indemnify our officers and directors for certain events or occurrences, subject to certain limits. We believe the fair value of these indemnification agreements is minimal. Accordingly, we have not recognized any liabilities relating to these agreements as of December 31, 2014.
9. Income Taxes
Due to ongoing operating losses and the inability to recognize any income tax benefit, there is no provision for income taxes for any periods presented.
87
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
9. Income Taxes (Continued)
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of our deferred tax assets are as follows:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
(In thousands)
|
2014 | 2013 | |||||
Deferred tax assets: |
|||||||
Net operating loss carryforwards |
$ | 386,000 | $ | 480,000 | |||
Deferred revenues |
2,000 | 5,000 | |||||
Capitalized research and development expenditures |
— | 5,000 | |||||
Research and development tax credit carryforwards |
53,000 | 44,000 | |||||
Other |
18,000 | 28,000 | |||||
| | | | | | | | |
Total deferred tax assets |
459,000 | 562,000 | |||||
Valuation allowance |
(459,000 | ) | (562,000 | ) | |||
| | | | | | | | |
Net deferred tax assets |
$ | — | $ | — | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The differences between the U.S. federal statutory income tax rate to our effective tax rate are as follows:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2014 | 2013 | 2012 | |||||||
U.S. federal statutory income tax rate |
35.00 | % | 34.00 | % | 34.00 | % | ||||
Federal and state research credits |
12.66 | 3.63 | (4.21 | ) | ||||||
Non-deductible executive compensation |
(0.16 | ) | (0.07 | ) | (13.24 | ) | ||||
Stock-based compensation |
(1.11 | ) | 0.28 | (1.36 | ) | |||||
Expiration of net operating loss |
— | — | (1.81 | ) | ||||||
Effect of Spin-Off Transaction |
(203.2 | ) | — | — | ||||||
Other |
(4.04 | ) | (2.51 | ) | (2.09 | ) | ||||
Change in valuation allowance |
160.85 | (35.33 | ) | (11.29 | ) | |||||
| | | | | | | | | | | |
Effective tax rate |
(0.00 | )% | (0.00 | )% | (0.00 | )% | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Realization of deferred tax assets is dependent on future taxable income, if any, the timing and the amount of which are uncertain. Accordingly, the deferred tax assets have been fully offset by a valuation allowance. The valuation allowance decreased by $103.8 million in 2014, increased by $70.1 million in 2013, and increased by $3.0 million in 2012.
The decrease in the valuation allowance in 2014 was primarily a result of the tax impacts of the Spin-Off transaction. In 2014, the Company recorded a permanent difference related to the tax gain that was recognized in connection with the Spin-Off, which allowed the Company to utilize approximately $253.2 million of its federal net operating losses. Accordingly, the associated valuation allowance, of $88.6 million, was released. Additionally as discussed in Note 10 in connection with the Spin-Off, approximately $9.2 million of deferred tax assets were transferred to Theravance Biopharma, Inc. Accordingly, the associated valuation allowance was also decreased.
88
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
9. Income Taxes (Continued)
As of December 31, 2014, we had federal net operating loss carryforwards of approximately $1,158.3 million, which will expire from 2018 through 2033, and federal research and development tax credit carryforwards of approximately $45.2 million, which will expire from 2018 through 2034. We also had state net operating loss carryforwards of approximately $1,008.6 million expiring in the years 2015 through 2034 and state research tax credits of approximately $32.1 million, which do not expire.
The net operating loss deferred tax asset balances as of December 31, 2014 and 2013 do not include excess tax benefits from stock option exercises. Stockholders' equity will be credited if and when such excess tax benefits are ultimately realized.
Utilization of net operating loss and tax credit carryforwards may be subject to a substantial annual limitation due to ownership change limitations provided by the Internal Revenue Code and similar state provisions. Annual limitations may result in expiration of net operating loss and tax credit carryforwards before some or all of such amounts have been utilized.
Our policy is to recognize interest and/or penalties related to income tax matters in income tax expense. As of December 31, 2014 and 2013, we had no accrued interest or penalties due to our net operating losses available to offset any tax adjustment.
We conducted an analysis through 2014 to determine whether an ownership change had occurred since inception. The analysis indicated that two ownership changes occurred in prior years. However, notwithstanding the applicable annual limitations, no portion of the net operating loss or credit carryforwards are expected to expire before becoming available to reduce federal and state income tax liabilities.
Uncertain Tax Positions
A reconciliation of the beginning and ending balances of the total amounts of unrecognized tax benefits are as follows (in thousands):
Unrecognized tax benefits as of December 31, 2011 |
$ | 46,900 | ||
Gross decrease for tax positions for prior years |
— | |||
Gross increase in tax positions for current year |
5,600 | |||
| | | | | |
Unrecognized tax benefits as of December 31, 2012 |
52,500 | |||
Gross decrease for tax positions for prior years |
(565 | ) | ||
Gross increase in tax positions for current year |
5,485 | |||
| | | | | |
Unrecognized tax benefits as of December 31, 2013 |
57,420 | |||
Gross decrease for tax positions for prior years |
(42,650 | ) | ||
Gross increase in tax positions for current year |
689 | |||
| | | | | |
Unrecognized tax benefits as of December 31, 2014 |
$ | 15,459 | ||
| | | | | |
| | | | | |
| | | | | |
In the event that we are able to recognize these uncertain positions, most of the $15.5 million of the unrecognized benefit would reduce our effective tax rate. We currently have a full valuation allowance against our deferred tax assets, which would impact the timing of the effective tax rate benefit, should any of these uncertain positions be favorably settled in the future. We do not believe it is reasonably possible that our unrecognized tax benefits will significantly change within the next twelve months.
89
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
9. Income Taxes (Continued)
We are subject to taxation in the U.S. and various state jurisdictions. The tax years 1998 and forward remain open to examination by the federal and most state tax authorities due to net operating loss and overall credit carryforward positions.
10. Spin-Off of Theravance Biopharma, Inc.
On June 1, 2014, we separated our late-stage partnered respiratory assets from our biopharmaceutical research and drug development operations. We contributed the assets and certain liabilities from the research and drug development operations and $393.0 million of cash, cash equivalents and marketable securities to Theravance Biopharma. All outstanding shares of Theravance Biopharma were then distributed to our stockholders of record on May 15, 2014 as a pro-rata dividend distribution of one ordinary share of Theravance Biopharma for every 3.5 shares held of our common stock.
On June 1, 2014, we entered into a Separation and Distribution Agreement with Theravance Biopharma that set forth the terms and conditions of the separation of Theravance Biopharma from us. The Separation and Distribution Agreement sets forth a framework for the relationship between us and Theravance Biopharma following the separation regarding principal transactions necessary to separate Theravance Biopharma from us. This agreement also sets forth other provisions that govern certain aspects of our relationship with Theravance Biopharma after the completion of the separation from us and provides for the allocation of assets, liabilities and obligations between Theravance Biopharma and us in connection with the Spin-Off.
In addition, we entered into other definitive agreements in connection with the Spin-Off, including (1) a Transition Services Agreement pursuant to which Theravance Biopharma and we will provide each other with a variety of administrative services, including financial, tax, accounting, information technology, legal and human resources services, for a period of time of up to 12 months following the Spin-Off, (2) a Tax Matters Agreement that generally governs the parties' respective rights, responsibilities and obligations after the separation with respect to taxes, (3) a Sublease Agreement that provides for the sublease from Theravance Biopharma to us for certain office space to be utilized in our operations and (4) an Employee Matters Agreement that allocates liabilities and responsibilities relating to employee compensation, benefit plans, programs and other related matters in connection with the separation, including the treatment of outstanding incentive awards and certain retirement and welfare benefit obligations. These arrangements contain the provisions related to the Spin-Off and the distribution of Theravance Biopharma's ordinary shares to our stockholders.
90
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
10. Spin-Off of Theravance Biopharma, Inc. (Continued)
The total amount of the Theravance Biopharma share dividend of $402.9 million was based on the net book value of the net assets that were contributed to Theravance Biopharma in connection with the Spin-Off, as follows:
(In thousands)
|
June 2, 2014 |
|||
|---|---|---|---|---|
Cash and cash equivalents |
$ | 277,541 | ||
Marketable investment securities |
115,129 | |||
Accounts receivable |
125 | |||
Reimbursement of certain liabilities |
16,983 | |||
Prepaid and other current assets |
3,172 | |||
Inventories |
14,328 | |||
Fixed assets, net |
9,580 | |||
Accrued liabilities |
(22,342 | ) | ||
Deferred revenue |
(6,694 | ) | ||
Other liabilities |
(4,944 | ) | ||
| | | | | |
Net book value of assets contributed |
$ | 402,878 | ||
| | | | | |
| | | | | |
| | | | | |
Due to the Spin-Off, the leases for the facilities in South San Francisco, California, which formerly served as our headquarters, were assigned to Theravance Biopharma. We would be held liable by the landlord if Theravance Biopharma defaults under its lease obligations, and thus, we have in substance guaranteed the payments under the lease agreements for these facilities. We recorded a non-current liability of $1.3 million in our consolidated balance sheet as of December 31, 2014 related to the estimated fair value of this lease guarantee. See Note 8, "Commitments and Contingencies" for further information on this lease guarantee.
Theravance Biopharma's historical results of operations have been presented as discontinued operations in our consolidated statement of operations for the years ended December 31, 2014 and 2013. See Note 11, "Discontinued Operations," for further information.
11. Discontinued Operations
On June 1, 2014, we separated our research and drug development businesses from our late-stage partnered respiratory assets. For further information on the Spin-Off, refer to Notes 1 and 10, "Description of Operations and Summary of Significant Accounting Policies" and "Spin-Off of Theravance Biopharma, Inc.". The significant components of the research and drug development operations, which are presented as discontinued operations on the consolidated statements of operations, were as follows:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
2014 | 2013 | 2012 | |||||||
Net revenues(1) |
$ | 3,129 | $ | 226 | $ | 130,145 | ||||
Income (loss) from discontinued operations(2) |
(94,934 | ) | (140,068 | ) | 12,147 | |||||
- (1)
- Net revenues primarily consist of revenue from collaborative arrangements and product sales. Revenue from collaborative arrangements in 2014 includes deferred revenue recognized from our
91
THERAVANCE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
11. Discontinued Operations (Continued)
agreement with R-Pharm CJSC. Revenue from collaborative arrangements in 2012 includes $125.8 million of deferred revenue recognized from our global collaboration arrangement with Astellas for the development and commercialization of VIBATIV, which was accelerated as a result of the termination of the Astellas agreement in January 2012, and the recognition of the upfront payment allocated to licensing of $4.4 million received under the collaborative arrangement with Merck, which was terminated in December 2013. Amounts from all three of these agreements were transferred to Theravance Biopharma as a part of the Spin-Off.
Product sales were generated from sales of VIBATIV in the U.S. through a limited number of distributors, and title and risk of loss transfer upon receipt by these distributors. Healthcare providers ordered VIBATIV through these distributors. Commencing in the first quarter of 2014, revenue on the sale of VIBATIV was recorded on a sell-through basis, once the distributors sold the product to healthcare providers. Product sales were recorded net of estimated government-mandated rebates and chargebacks, distribution fees, estimated product returns and other deductions.
- (2)
- Loss from discontinued operations decreased in 2014 compared to 2013 primarily as there was no impact of discontinued operations after the Spin-Off occurring in June 2014. We had a loss from discontinued operations in 2013 compared to income from discontinued operations in 2012 primarily as result of net revenue. Included in the loss from discontinued operations for 2014 and 2013 are external legal and accounting fees in connection with our separation strategy which we started to incur in 2013 and the additional stock-based compensation and cash bonus expense recognized due to the achievement of performance conditions under a special long-term retention and incentive equity and cash bonus awarded to certain employees in 2011, which we started to incur in 2014.
12. Dividends Declared and Paid
During the third and fourth quarters of 2014, our Board of Directors declared a quarterly dividend of $0.25 per share of common stock to stockholders of record as of the close of business on August 28, 2014 and November 25, 2014. In 2014, we paid a total of $57.0 million in dividends. Unvested RSAs as of the record date are also entitled to dividends, which will only be paid when the RSAs vest and are released. For further information on the impact of the payments of the cash dividends on the 2023 Notes, refer to Note 7, "Long-Term Debt".
13. Subsequent Events
On February 20, 2015, our Board of Directors declared a $0.25 per share dividend for the first quarter of 2015 for all stockholders of record as of the close of business on March 12, 2015 for a total of approximately $29.2 million. Unvested RSAs as of the record date are also entitled to dividends, which will only be paid when the RSAs vest and are released. The dividend will be paid to our stockholders on March 31, 2015.
92
SUPPLEMENTARY FINANCIAL DATA (UNAUDITED)
(In thousands, except per share amounts)
The following table presents certain unaudited consolidated quarterly financial information for the eight quarters in the period ended December 31, 2014. This information has been prepared on the same basis as the audited consolidated financial statements and includes all adjustments (consisting only of normal recurring adjustments) necessary to present fairly the unaudited quarterly results of operations set forth herein.
| |
For the Quarters Ended(1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
March 31 | June 30 | September 30 | December 31 | |||||||||
2014 |
|||||||||||||
Total revenue |
$ | (780 | ) | $ | 934 | $ | 999 | $ | 7,280 | ||||
Operating expenses |
(13,943 | ) | (10,728 | ) | (10,541 | ) | (7,150 | ) | |||||
Loss from continuing operations, net of tax |
(16,182 | ) | (20,151 | ) | (21,271 | ) | (15,926 | ) | |||||
Loss from discontinued operations |
(51,521 | ) | (43,413 | ) | — | — | |||||||
Net loss |
$ | (67,703 | ) | $ | (63,564 | ) | $ | (21,271 | ) | $ | (15,926 | ) | |
Basic and diluted net loss per common share: |
|||||||||||||
Continuing operations, net of tax |
$ | (0.15 | ) | $ | (0.18 | ) | $ | (0.19 | ) | $ | (0.14 | ) | |
Discontinued operations |
(0.47 | ) | (0.39 | ) | — | — | |||||||
| | | | | | | | | | | | | | |
Basic and diluted net loss per common share |
$ | (0.62 | ) | $ | (0.57 | ) | $ | (0.19 | ) | $ | (0.14 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Cash dividends declared per share |
$ | — | $ | — | $ | 0.25 | $ | 0.25 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
2013 |
|||||||||||||
Total revenue |
$ | 1,322 | $ | 1,322 | $ | 415 | $ | 1,473 | |||||
Operating expenses |
(8,095 | ) | (8,220 | ) | (8,122 | ) | (8,890 | ) | |||||
Loss from continuing operations, net of tax(2) |
(10,746 | ) | (1,541 | ) | (9,454 | ) | (8,894 | ) | |||||
Loss from discontinued operations |
(26,614 | ) | (34,888 | ) | (37,531 | ) | (41,035 | ) | |||||
Net loss |
$ | (37,360 | ) | $ | (36,429 | ) | $ | (46,985 | ) | $ | (49,929 | ) | |
Basic and diluted net loss per common share: |
|||||||||||||
Continuing operations, net of tax |
$ | (0.11 | ) | $ | (0.02 | ) | $ | (0.09 | ) | $ | (0.08 | ) | |
Discontinued operations |
(0.28 | ) | (0.35 | ) | (0.35 | ) | (0.38 | ) | |||||
| | | | | | | | | | | | | | |
Basic and diluted net loss per common share |
$ | (0.39 | ) | $ | (0.37 | ) | $ | (0.44 | ) | $ | (0.46 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
- (1)
- Amounts
were computed independently for each quarter, and the sum of the quarters may not total the annual amounts.
- (2)
- Loss from continuing operations, net of tax for the second quarter of 2013 includes net cash received from the termination of our royalty participation agreement with Elan Corporation, plc.
93
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Theravance, Inc.
We have audited the accompanying consolidated balance sheets of Theravance, Inc. as of December 31, 2014 and 2013, and the related consolidated statements of operations, comprehensive loss, stockholders' equity (deficit) and cash flows for each of the three years in the period ended December 31, 2014. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Theravance, Inc. at December 31, 2014 and 2013, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2014, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Theravance Inc.'s internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (1992 framework) and our report dated February 27, 2015 expressed an unqualified opinion thereon.
/s/ ERNST & YOUNG LLP
San Jose, California
February 27, 2015
94
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures.
We conducted an evaluation as of December 31, 2014, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures, which are defined under SEC rules as controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files under the Securities Exchange Act of 1934 (Exchange Act) is recorded, processed, summarized and reported within required time periods. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of such date, our disclosure controls and procedures were effective.
Management's Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) of the Exchange Act. Internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on criteria established in the Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (1992 framework). Management's assessment included evaluation of such elements as the design and operating effectiveness of key financial reporting controls, process documentation, accounting policies, and our overall control environment. Based on this evaluation, our management concluded that our internal control over financial reporting was effective as of December 31, 2014.
Our independent registered public accounting firm, Ernst & Young LLP, has audited our internal control over financial reporting as of December 31, 2014. Their attestation report on the audit of our internal control over financial reporting is included below.
Limitations on the Effectiveness of Controls
Our management, including our Chief Executive Officer and Chief Financial Officer, does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all error and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefit of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within Theravance have been detected. Also, projections of any evaluation of
95
effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Changes in Internal Control over Financial Reporting
On June 2, 2014, we completed the Spin-Off of Theravance Biopharma, Inc. Since the Spin-Off of Theravance Biopharma, Inc., we have significantly downsized our operations and, as of December 31, 2014, we had ten employees focused on managing our respiratory assets with GSK, the commercial and developmental obligations associated with the GSK Agreements, intellectual property, licensing operations and providing for certain essential reporting and management functions of a public company. Under a transition services agreement, Theravance Biopharma, Inc. continues to support the financial reporting function for Theravance, Inc. during a transition period. There was no change in our internal control over financial reporting (as defined in Rule 13a-15(f) of the Exchange Act) identified in connection with the evaluation required by paragraph (d) of Rule 13a-15 of the Exchange Act, which occurred during the fourth fiscal quarter of the year ended December 31, 2014 which has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
96
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Theravance, Inc.
We have audited Theravance, Inc.'s internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (1992 framework) (the COSO criteria). Theravance, Inc.'s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Theravance, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2014, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Theravance, Inc. as of December 31, 2014 and 2013, and the related consolidated statements of operations, comprehensive loss, stockholders' equity (deficit) and cash flows for each of the three years in the period ended December 31, 2014 of Theravance, Inc. and our report dated February 27, 2015, expressed an unqualified opinion thereon.
/s/ ERNST & YOUNG LLP
San Jose, California
February 27, 2015
97
None
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
For the information required by this Item, see "Questions and Answers About this Proxy Material and Voting", "Election of Directors", "Nominees", "Audit Committee", "Meetings of the Board of Directors", "Code of Business Conduct", "Executive Officers", and "Section 16(a) Beneficial Ownership Reporting Compliance" in the Proxy Statement to be filed with the SEC, which sections are incorporated herein by reference.
ITEM 11. EXECUTIVE COMPENSATION
For the information required by this Item, see "2014 Director Compensation", "Compensation of Named Executive Officers", "Compensation Committee Report" and "Compensation Committee Interlocks and Insider Participation" in the Proxy Statement to be filed with the SEC, which sections are incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
For the information required by this Item, see "Security Ownership of Certain Beneficial Owners and Management" and "Securities Authorized for Issuance Under Equity Compensation Plans" in the Proxy Statement to be filed with the SEC, which sections are incorporated herein by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
For the information required by this Item, see "Independence of the Board of Directors" and "Review, Approval or Ratification of Transactions with Related Persons" in the Proxy Statement to be filed with the SEC, which sections are incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
For the information required by this Item, see "Ratification of Selection of Independent Registered Public Accounting Firm" and "Pre-Approval Policies and Procedures" in the Proxy Statement to be filed with the SEC, which sections are incorporated herein by reference.
98
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
- (a)
- The
following documents are filed as part of this Annual Report on Form 10-K:
- 1.
- Financial Statements:
The following financial statements and schedules of the Registrant are contained in Part II, Item 8, "Financial Statements and Supplementary Data" of this Annual Report on Form 10-K:
- 2.
- Financial Statement Schedules:
All schedules have been omitted because of the absence of conditions under which they are required or because the required information, where material, is shown in the financial statements, financial notes or supplementary financial information.
- (b)
- Exhibits required by Item 601 of Regulation S-K
The information required by this Item is set forth on the exhibit index that follows the signature page of this report.
99
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| THERAVANCE, INC. | ||||
Date: February 27, 2015 |
By: |
/s/ MICHAEL W. AGUIAR Michael W. Aguiar Chief Executive Officer |
||
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Michael W. Aguiar and Eric d'Esparbes, each of whom may act without joinder of the other, as their true and lawful attorneys-in-fact and agents, each with full power of substitution and resubstitution, for such person and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to the annual report on Form 10-K, and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or their substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ MICHAEL W. AGUIAR Michael W. Aguiar |
Chief Executive Officer (Principal Executive Officer) | February 27, 2015 | ||
/s/ ERIC D'ESPARBES Eric d'Esparbes |
Senior Vice President, Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
February 27, 2015 |
||
/s/ WILLIAM WALTRIP William H. Waltrip |
Chairman of the Board |
February 27, 2015 |
||
/s/ CATHERINE J. FRIEDMAN Catherine J. Friedman |
Director |
February 27, 2015 |
100
Signature
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ TERRENCE KEARNEY Terrence Kearney |
Director | February 27, 2015 | ||
/s/ PAUL PEPE Paul Pepe |
Director |
February 27, 2015 |
||
/s/ JAMES L. TYREE James L. Tyree |
Director |
February 27, 2015 |
101
| |
|
Incorporated by Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit Number |
Description | Form | Exhibit | Filing Date/Period End Date |
||||||
| 3.1 | Amended and Restated Certificate of Incorporation | S-1 | 3.3 | 7/26/04 | ||||||
| 3.2 | Certificate of Amendment of Restated Certificate of Incorporation | 10-Q | 3.4 | 3/31/07 | ||||||
| 3.3 | Amended and Restated Bylaws | 10-Q | 3.5 | 9/30/08 | ||||||
| 4.1 | Specimen certificate representing the common stock of the registrant | 10-K | 4.1 | 12/31/06 | ||||||
| 4.4 | Indenture dated as of January 24, 2013 by and between Theravance, Inc. and The Bank of New York Mellon Trust Company, N.A., as trustee | 8-K | 4.4 | 1/25/13 | ||||||
| 4.5 | Form of 2.125% Convertible Subordinated Note Due 2023 (included in Exhibit 4.4) | |||||||||
| 4.6 | Indenture, dated April 17, 2014. | 8-K | 10.1 | 4/21/14 | ||||||
| 4.7 | Form of 9.0% Convertible Subordinated Note Due 2029 | 8-K | 10.2 | 4/21/14 | ||||||
| 10.1 | + | 1997 Stock Plan | S-1 | 10.1 | 6/10/04 | |||||
| 10.2 | + | Long-Term Stock Option Plan | S-1 | 10.2 | 6/10/04 | |||||
| 10.3 | + | 2004 Equity Incentive Plan, as amended by the board of directors February 10, 2010 and approved by stockholders April 27, 2010 and forms of equity award | 10-K | 10.3 | 12/31/11 | |||||
| 10.4 | Employee Stock Purchase Plan, as amended April 27, 2010 | 10-Q | 10.4 | 6/30/10 | ||||||
| 10.5 | + | Change in Control Severance Plan, as amended and restated on July 27, 2007 | 10-Q | 10.8 | 6/30/08 | |||||
| 10.6 | Amended and Restated Lease Agreement, 951 Gateway Boulevard, between the registrant and HMS Gateway Office L.P., dated January 1, 2001 | S-1 | 10.8 | 6/10/04 | ||||||
| 10.7 | Lease Agreement, 901 Gateway Boulevard, between the registrant and HMS Gateway Office L.P., dated January 1, 2001 | S-1 | 10.9 | 6/10/04 | ||||||
| 10.8 | Collaboration Agreement between the registrant and Glaxo Group Limited, dated as of November 14, 2002 | 10-Q | 10.1 | 6/30/14 | ||||||
| 10.9 | + | Form of Indemnification Agreement for directors and officers of the registrant | S-1 | 10.11 | 6/10/04 | |||||
| 10.11 | Amended and Restated Investors' Rights Agreement by and among the registrant and the parties listed therein, dated as of May 11, 2004 | S-1 | 10.13 | 6/10/04 | ||||||
| 10.12 | Amended and Restated Governance Agreement by and among the registrant, SmithKline Beecham Corporation and GlaxoSmithKline dated as of June 4, 2004 | S-1 | 10.14 | 7/26/04 | ||||||
| 10.13 | * | Strategic Alliance Agreement between the registrant and Glaxo Group Limited, dated as of March 30, 2004 | 10-K | 10.13 | 12/31/13 | |||||
| |
|
Incorporated by Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit Number |
Description | Form | Exhibit | Filing Date/Period End Date |
||||||
| 10.15 | + | Offer Letter with Rick E Winningham dated August 23, 2001 | S-1 | 10.17 | 6/10/04 | |||||
| 10.17 | + | Offer Letter with Michael W. Aguiar dated as of January 31, 2005 | 10-K | 10.29 | 12/31/04 | |||||
| 10.18 | + | Form of Notice of Grant and Stock Option Agreement under 2004 Equity Incentive Plan | 10-K | 10.30 | 12/31/04 | |||||
| 10.19 | + | Form of Notice of Restricted Stock Award and Restricted Stock Agreement under 2004 Equity Incentive Plan (form in effect through 2010) | 10-Q | 10.31 | 6/30/07 | |||||
| 10.20 | + | Description of Cash Bonus Program, as amended | 10-K | 10.22 | 12/31/09 | |||||
| 10.24 | + | Amended and Restated 2008 New Employee Equity Incentive Plan and forms of equity award | 10-K | 10.24 | 12/31/11 | |||||
| 10.26 | + | Amendment to Offer Letter between the registrant and Rick E Winningham dated December 23, 2008 | 10-K | 10.48 | 12/31/08 | |||||
| 10.27 | + | Amendment to Change in Control Severance Plan effective December 16, 2009 | 10-K | 10.47 | 12/31/09 | |||||
| 10.28 | + | 2009 Change in Control Severance Plan adopted December 16, 2009 | 10-K | 10.48 | 12/31/09 | |||||
| 10.29 | First Amendment to Lease for 901 Gateway Boulevard effective as of June 1, 2010 between ARE-901/951 Gateway Boulevard, LLC and the registrant | 10-Q | 10.51 | 6/30/10 | ||||||
| 10.30 | First Amendment to Lease for 951 Gateway Boulevard effective as of June 1, 2010 between ARE-901/951 Gateway Boulevard, LLC and the registrant | 10-Q | 10.51 | 6/30/10 | ||||||
| 10.32 | Second Amendment to Amended and Restated Governance Agreement among the registrant, Glaxo Group Limited, GlaxoSmithKline plc and GlaxoSmithKline LLC, dated as of November 29, 2010 | 8-K | 10.2 | 11/29/10 | ||||||
| 10.33 | + | Form of Amendment to Restricted Stock Unit Agreements between the registrant and each current member of the Board of Directors outstanding as of December 31, 2010 | 10-K | 10.45 | 12/31/10 | |||||
| 10.34 | * | Amendment to Strategic Alliance Agreement dated October 3, 2011 | 10-K | 10.34 | 12/31/11 | |||||
| 10.35 | Common Stock Purchase Agreement, dated April 2, 2012, by and among Theravance, Inc., Glaxo Group Limited and GlaxoSmithKline LLC | 8-K | 10.1 | 4/2/12 | ||||||
| 10.36 | + | Form of Notice of Performance-Contingent Restricted Stock Award and Restricted Stock Award Agreement under 2004 Equity Incentive Plan (executive officer form) | 10-Q | 10.36 | 3/30/12 | |||||
| 10.37 | + | Form of Notice of Performance-Contingent Restricted Stock Award and Restricted Stock Award Agreement under 2004 Equity Incentive Plan | 10-Q | 10.37 | 3/30/12 | |||||
| |
|
Incorporated by Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit Number |
Description | Form | Exhibit | Filing Date/Period End Date |
||||||
| 10.38 | + | 2012 Equity Incentive Plan, as approved by the board of directors February 8, 2012 and approved by stockholders May 16, 2012 and forms of equity award | 10-Q | 10.38 | 6/30/12 | |||||
| 10.40 | Base Capped Call Transaction dated January 17, 2013 | 8-K | 10.1 | 1/23/13 | ||||||
| 10.41 | Additional Capped Call Transaction dated January 18, 2013 | 8-K | 10.2 | 1/23/13 | ||||||
| 10.43 | Master Agreement by and among Theravance, Inc., Theravance Biopharma, Inc. and Glaxo Group Limited, dated March 3, 2014 | 8-K/A | 10.1 | 3/6/14 | ||||||
| 10.44 | * | Collaboration Agreement Amendment by and between Theravance, Inc. and Glaxo Group Limited dated March 3, 2014 | 8-K/A | 10.2 | 3/6/14 | |||||
| 10.45 | * | Strategic Alliance Agreement Amendment by and between Theravance, Inc. and Glaxo Group Limited dated March 3, 2014 | 8-K/A | 10.3 | 3/6/14 | |||||
| 10.46 | Form of Note Purchase Agreement, dated April 17, 2014. | 8-K | 1.1 | 4/21/14 | ||||||
| 10.47 | Sale and Contribution Agreement, dated April 17, 2014. | 8-K | 10.1 | 4/21/14 | ||||||
| 10.48 | Servicing Agreement, dated April 17, 2014. | 8-K | 10.2 | 4/21/14 | ||||||
| 10.49 | Account Control Agreement, dated April 17, 2014. | 8-K | 10.3 | 4/21/14 | ||||||
| 10.50 | Limited Liability Agreement of LABA Royalty Sub LLC, dated April 17, 2014. | 8-K | 10.4 | 4/21/14 | ||||||
| 10.51 | Annex A—Rules of Construction and Defined Terms, dated April 17, 2014. | 8-K | 10.5 | 4/21/14 | ||||||
| 10.52 | + | Letter Agreement with Rick E Winningham dated May 5, 2014 | 8-K | 10.1 | 5/8/14 | |||||
| 10.53 | Separation and Distribution Agreement between Theravance and Theravance Biopharma, dated June 1, 2014 | 8-K | 10.1 | 6/5/14 | ||||||
| 10.54 | Transition Services Agreement between Theravance and Theravance Biopharma, dated June 2, 2014. | 8-K | 10.2 | 6/5/14 | ||||||
| 10.55 | Tax Matters Agreement between Theravance and Theravance Biopharma, dated June 2, 2014. | 8-K | 10.3 | 6/5/14 | ||||||
| 10.56 | Employee Matters Agreement between Theravance and Theravance Biopharma, dated June 1, 2014. | 8-K | 10.4 | 6/5/14 | ||||||
| 10.57 | Theravance Respiratory Company, LLC Limited Liability Company Agreement between Theravance and Theravance Biopharma, dated May 31, 2014. | 8-K | 10.5 | 6/5/14 | ||||||
| 10.58 | + | Equity Award Amendments for Employees VP Level or above remaining at Theravance, Inc. | 10-Q | 10.2 | 6/30/14 | |||||
| 10.59 | + | Policy for Non-Employee Director Stock Options (effective June 2, 2014) | 10-Q | 10.3 | 6/30/14 | |||||
| 10.60 | + | Offer Letter with Ted Witek dated May 2, 2014 | 10-Q | 10.4 | 6/30/14 | |||||
| 10.61 | + | Offer Letter with George Abercrombie dated May 30, 2014 | 10-Q | 10.5 | 6/30/14 | |||||
| |
|
Incorporated by Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit Number |
Description | Form | Exhibit | Filing Date/Period End Date |
||||||
| 10.62 | + | Offer Letter with Michael W. Aguiar dated August 5, 2014 | 10-Q | 10.1 | 9/30/14 | |||||
| 10.63 | + | Offer Letter with Eric d'Esparbes dated September 8, 2014 | ||||||||
| 21.1 | List of Subsidiaries | |||||||||
| 23.1 | Consent of Independent Registered Public Accounting Firm | |||||||||
| 24.1 | Power of Attorney (see signature page to this Annual Report on Form 10-K) | |||||||||
| 31.1 | Certification of Chief Executive Officer Pursuant to Rule 13a-14 under the Securities Exchange Act of 1934 | |||||||||
| 31.2 | Certification of Chief Financial Officer Pursuant to Rule 13a-14 under the Securities Exchange Act of 1934 | |||||||||
| 32 | Certifications Pursuant to 18 U.S.C. Section 1350 | |||||||||
| 101 | The following materials from Registrant's Annual Report on Form 10-K for the year ended December 31, 2014, formatted in Extensible Business Reporting Language (XBRL) includes: (i) Consolidated Balance Sheets at December 31, 2014 and 2013, (ii) Consolidated Statements of Income for the years ended December 31, 2014, 2013 and 2012, (iii) Consolidated Statements of Comprehensive Loss for the years ended December 31, 2014, 2013 and 2012, (iv) Consolidated Statements of Stockholders' Equity for the years ended December 31, 2014, 2013 and 2012, (v) Consolidated Statements of Cash Flows for years ended December 31, 2014, 2013 and 2012, and (vi) Notes to Consolidated Financial Statements. | |||||||||
- +
- Management
contract or compensatory plan or arrangement required to be filed pursuant to Item 15(b) of Form 10-K.
- *
- Confidential treatment has been granted for certain portions which are omitted in the copy of the exhibit electronically filed with the Securities and Exchange Commission. The omitted information has been filed separately with the Securities and Exchange Commission pursuant to Theravance, Inc.'s application for confidential treatment.
