Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT ON FORM 8-K - Simulations Plus, Inc. | simulations_8k-120111.htm |
Exhibit 99.1

Simulations Plus, Inc. (NASDAQ:SLP) First Quarter of Fiscal Year 2012 (1QFY12) Conference Call and Webinar January 18, 2012
| 1 |

With the exception of historical information, the matters discussed in this presentation are forward - looking statements that involve a number of risks and uncertainties. The actual results of the Company could differ significantly from those statements. Factors that could cause or contribute to such differences include, but are not limited to: continuing demand for the Company’s products, competitive factors, the Company’s ability to finance future growth, the Company’s ability to produce and market new products in a timely fashion, the Company’s ability to continue to attract and retain skilled personnel, and the Company’s ability to sustain or improve current levels of productivity. Further information on the Company’s risk factors is contained in the Company’s quarterly and annual reports and filed with the Securities and Exchange Commission. Safe Harbor Statement
| 2 |

With the sale of our former Words+ subsidiary on November 30, 2011, we will now be reporting financials and comparing reporting periods for only the continuing operations, composed almost entirely of the pharmaceutical software and services business (Abbreviate!™ and FutureLab ™ contribute very little). Unless otherwise noted, all financials and comparisons are on a pro forma basis – in other words, as if the discontinued operations did not exist in the previous fiscal year. Because Words+ was operated through the end of the first quarter, we will be including the effect of its discontinued operations as appropriate for each quarter of this fiscal year and for the entire year at year’s end. When they are included, it will be so noted. Continuing and Discontinued Operations
| 3 |
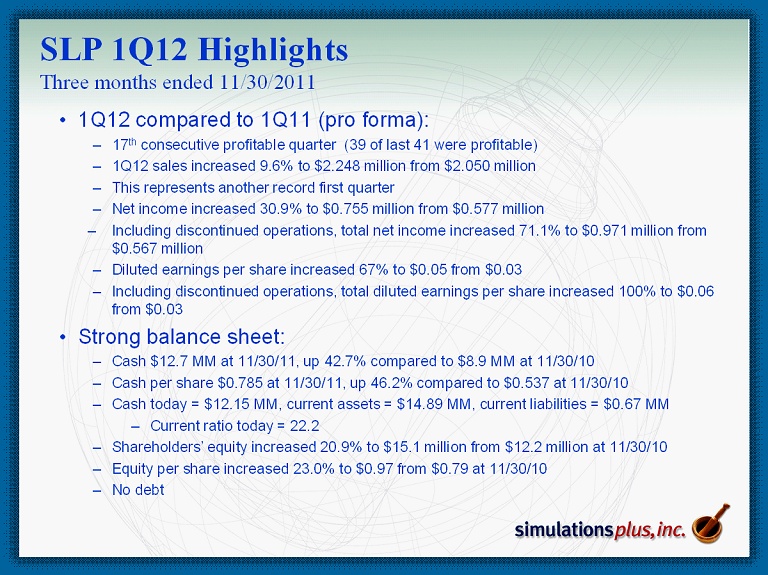
SLP 1Q12 Highlights Three months ended 11/30/2011 • 1Q12 compared to 1Q11 (pro forma): – 17 th consecutive profitable quarter (39 of last 41 were profitable) – 1Q12 sales increased 9.6% to $2.248 million from $2.050 million – This represents another record first quarter – Net income increased 30.9% to $0.755 million from $0.577 million – Including discontinued operations, total net income increased 71.1% to $0.971 million from $0.567 million – Diluted earnings per share increased 67% to $0.05 from $0.03 – Including discontinued operations, total diluted earnings per share increased 100% to $0.06 from $0.03 • Strong balance sheet: – Cash $12.7 MM at 11/30/11, up 42.7% compared to $8.9 MM at 11/30/10 – Cash per share $0.785 at 11/30/11, up 46.2% compared to $0.537 at 11/30/10 – Cash today = $12.15 MM, current assets = $14.89 MM, current liabilities = $0.67 MM – Current ratio today = 22.2 – Shareholders’ equity increased 20.9% to $15.1 million from $12.2 million at 11/30/10 – Equity per share increased 23.0% to $0.97 from $0.79 at 11/30/10 – No debt
| 4 |

Income Statement 1QFY12 vs 1QFY11 ($ millions) 1Q12 1Q11 (pro forma) Net sales 2.248 2.050 Gross profit 1.896 1.697 Gross profit margin 84.3% 82.8% SG&A 0.700 0.686 R&D 0.252 0.201 Total operating expenses 0.952 0.887 Income from operations 0.944 0.810 Other income 0.120 0.024 Income before income taxes 1.064 0.834 Results of discontinued operations, net of tax 0.216 (0.009) Net income 0.971 0.567 Earnings per share (diluted) 0.06 0.03
| 5 |

Pro Forma Income Statement Year Ended 8/31/2011 ($ millions) unaudited SLP Consolidated as reported Words+, Inc. as reported in consolidated Pro Forma adjustment (a) SLP Pro Forma as adjusted Net sales 11.720 2.981 (2.981) 8.739 Gross profit 8.841 1.600 (1.600) 7.241 Gross profit margin 75.4% 53.7% 53.7% 82.6% SG&A 4.305 1.467 (1.383)* 2.922 R&D 0.935 0.063 (0.063) 872 Total operating expenses 5.240 1.530 (1.446)* 3.794 Income from operations 3.601 0.070 (0.154) 3.447 Other income 0.169 0.002 (0.002) 0.167 Income before income taxes 3.770 0.072 (0.156)* 3.614 Net income from continuing operations 2.715 0.072 (0.156)* 2.559 Earnings per share (diluted) 0.17 -- (0.01) 0.16 (a) Reflects the elimination of Words+ operations; reflects intra - company cost sharing adjustments. * A significant adjustment was made for office lease cost sharing, which we treated as if a portion was subleased to Words+.
| 6 |
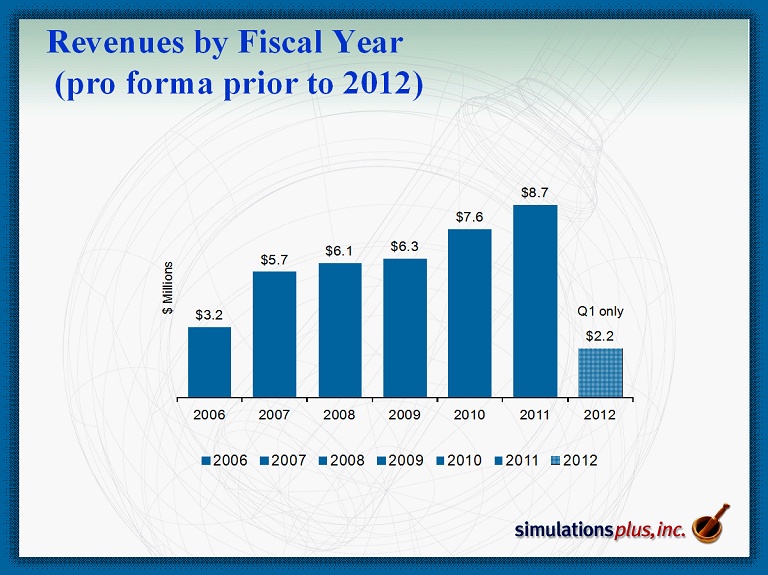
$3.2 $5.7 $6.1 $6.3 $7.6 $8.7 $2.2 2006 2007 2008 2009 2010 2011 2012 $ Millions 2006 2007 2008 2009 2010 2011 2012 Q1 only Revenues by Fiscal Year (pro forma prior to 2012)
| 7 |
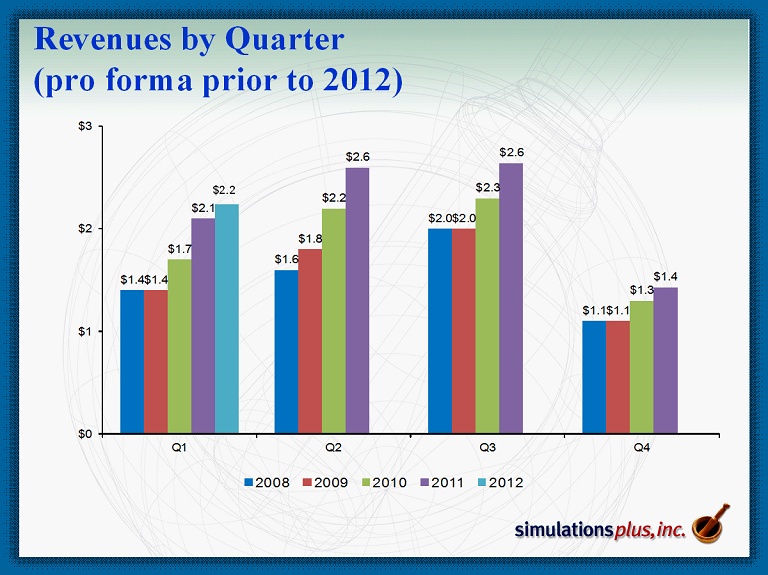
Revenues by Quarter (pro forma prior to 2012) $1.4 $1.6 $2.0 $1.1 $1.4 $1.8 $2.0 $1.1 $1.7 $2.2 $2.3 $1.3 $2.1 $2.6 $2.6 $1.4 $0 $1 $2 $3 Q1 Q2 Q3 Q4 2008 2009 2010 2011 2012 $2.2
| 8 |
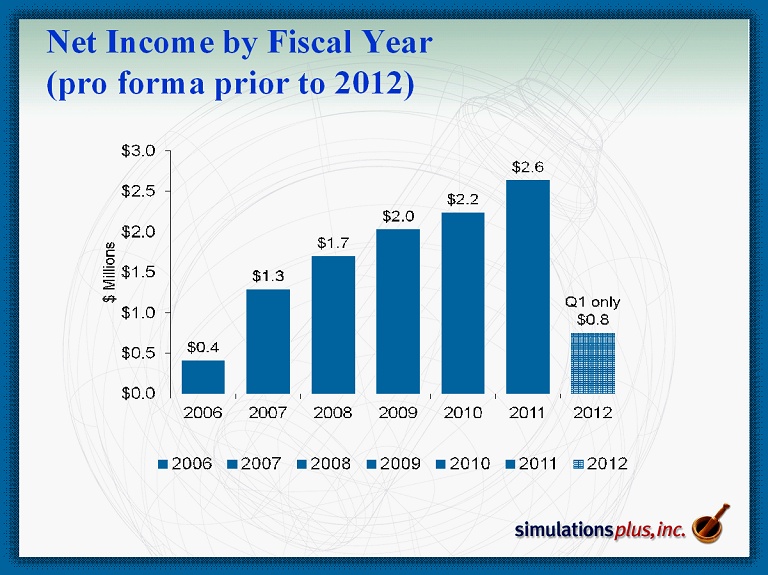
Net Income by Fiscal Year (pro forma prior to 2012)
| 9 |
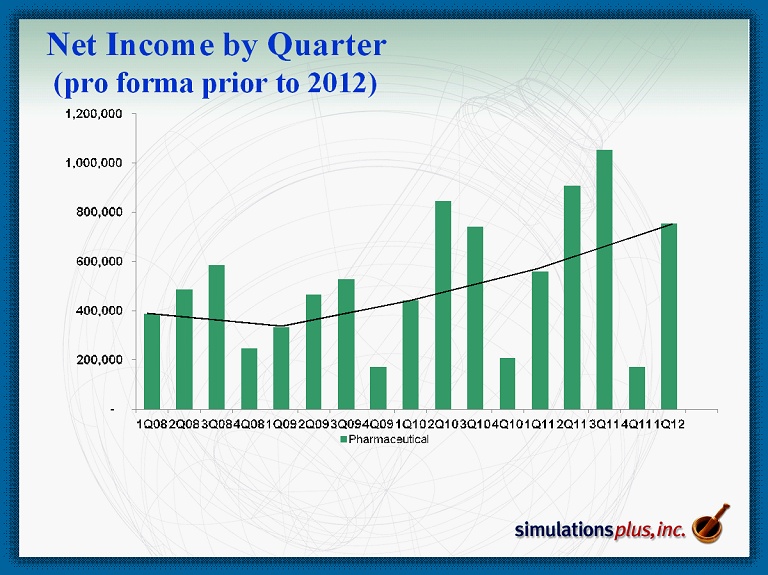
Net Income by Quarter (pro forma prior to 2012)
| 10 |
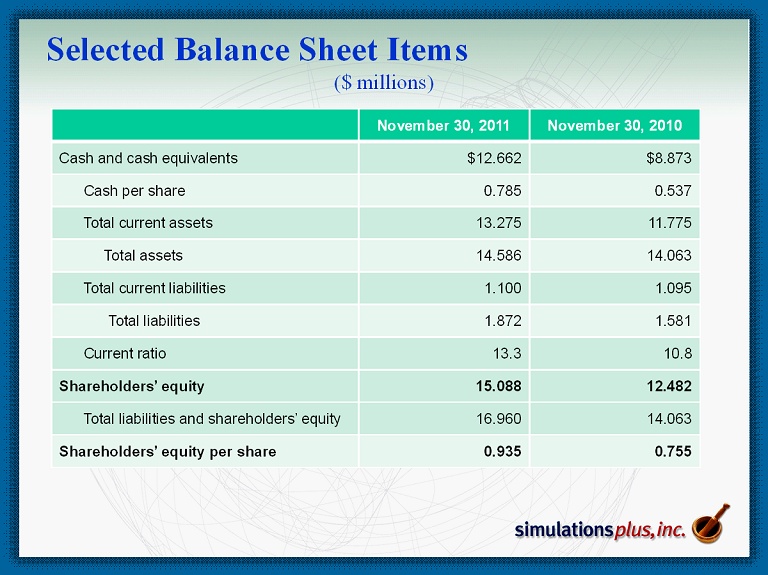
Selected Balance Sheet Items ($ millions) November 30, 2011 November 30 , 2010 Cash and cash equivalents $12.662 $8.873 Cash per share 0.785 0.537 Total current assets 13.275 11.775 Total assets 14.586 14.063 Total current liabilities 1.100 1.095 Total liabilities 1.872 1.581 Current ratio 13.3 10.8 Shareholders’ equity 15.088 12.482 Total liabilities and shareholders’ equity 16.960 14.063 Shareholders’ equity per share 0.935 0.755
| 11 |

Marketing and Sales Program • Conferences/Scientific Meetings – primary source of leads – we do 50 - 60/year, adding shows in China • Posters, Presentations, and Peer - Reviewed Publications • Training Workshops – now adding Basic GastroPlus training to ongoing Advanced GastroPlus training courses • Strategic Digital Marketing Initiatives • Collaborations/Consulting/Grants – Progressing on our 5 - year collaboration with the FDA Center for Food Safety and Applied Nutrition to build many toxicity models with ADMET Predictor/Modeler™ for food additives and contaminants – Consulting studies continue – provides exposure of software to new groups – Negotiating a new funded collaboration with a top - 5 pharmaceutical company to further enhance our GastroPlus software product – Negotiating a new collaboration with an important U.S. government agency for modifications to GastroPlus to better fit their needs for analysis • Fundamental industry shift continues – Software tools are constantly gaining wider acceptance and applications are growing – 20 new customers during 1QFY12 (includes new companies and new departments within existing large customers)
| 12 |
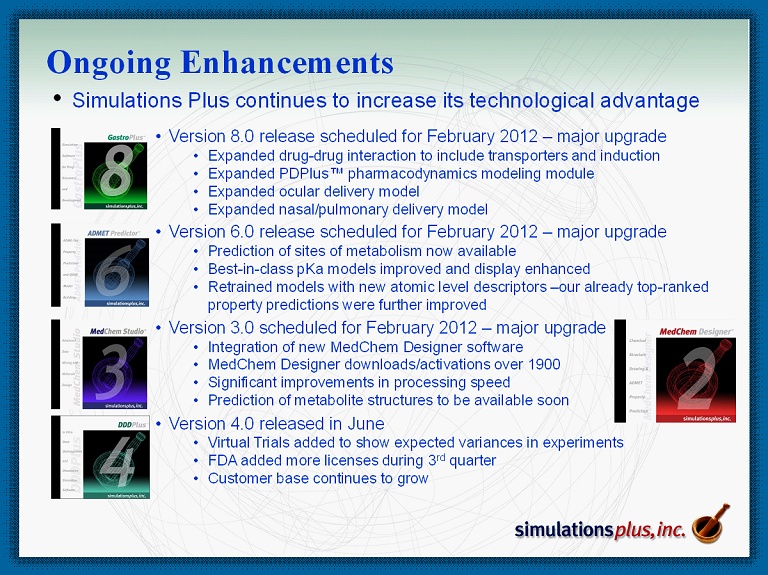
• Simulations Plus continues to increase its technological advantage Ongoing Enhancements • Version 8.0 release scheduled for February 2012 – major upgrade • Expanded drug - drug interaction to include transporters and induction • Expanded PDPlus™ pharmacodynamics modeling module • Expanded ocular delivery model • Expanded nasal/pulmonary delivery model • Version 6.0 release scheduled for February 2012 – major upgrade • Prediction of sites of metabolism now available • Best - in - class pKa models improved and display enhanced • Retrained models with new atomic level descriptors – our already top - ranked property predictions were further improved • Version 3.0 scheduled for February 2012 – major upgrade • Integration of new MedChem Designer software • MedChem Designer downloads/activations over 1900 • Significant improvements in processing speed • Prediction of metabolite structures to be available soon • Version 4.0 released in June • Virtual Trials added to show expected variances in experiments • FDA added more licenses during 3 rd quarter • Customer base continues to grow
| 13 |

FDA Food Safety Research Collaboration • We entered into a 5 - year renewable Research Collaboration with the Center for Food Safety and Applied Nutrition (CFSAN) to provide model - building capabilities for a large number (>10,000) of substances that can be in foods as additives or contaminants. • The FDA has data on a wide variety of toxicities for small subsets (typically hundreds to a few thousands) of the total molecular data set. • The purpose of the collaboration is to use the data from those molecules that have been tested for a particular toxicity to build predictive models for that toxicity using our ADMET Predictor/Modeler software. • Those models will then be used to predict the likely toxicity of the molecules that were not tested for that toxicity to see which ones appear to be toxic and should be tested. • As we began this effort, the FDA scientists wanted to train models using methods that were not yet a part of ADMET Predictor/Modeler. We have modified the code to include these new capabilities, and they are part of Version 6.0 to be released in February. • We have been using the modified code to build a few initial predictive models for some of the FDA data as part of our validation of the new methods. We expect to release a rodent carcinogenicity (tumor) model with Version 6.0.
| 14 |

NCE (New Chemical Entity) Project • When drug companies invent (“discover”) a new molecule, it is called a new chemical entity (NCE) • The process of getting to an approvable drug molecule usually involves making and test a large number of molecules to find good “lead candidates”. Lead candidates are molecules that show activity against the target and have some (but not all) acceptable properties. • Chemists then modify the lead candidates to try to make all properties acceptable. This often leads to circular activities because fixing one property can adversely affect others, and the chemist must iterate through the cycle until all measured properties are acceptable, and then those molecules are taken forward into preclinical development. • Using MedChem Studio/MedChem Designer/ADMET Predictor, we designed lead candidates to inhibit the malaria parasite in a small fraction of the time and cost normally required. These molecules were qualified for a large number of acceptable properties using ADMET Predictor predictions – no testing was required. The selected molecules are now being synthesized by a contractor. • If the molecules show significant promise as lead compounds, we will have completed an exciting proof - of - concept for our software tools. We may also be able to secure funding for additional testing and design studies, although our purpose was not so much to cure malaria, but to show the power of our molecule design tools.
| 15 |

Summary • For 1QFY11: - All financial performance measure are up nicely and strong growth trend for revenues and income continues - Sale of Words+ has simplified and focused the business – margins jump about 10%, reporting and auditing much simpler going forward • Continuing to Expand our Life Sciences team: - To promote faster development of new products and services - Also strengthens our marketing and sales efforts • Expanding Sales Team and Activities - Seeking chemist with sales experience for molecule design suite - Developing training courses for chemistry tools • Simulations Plus is globally recognized as a leader - Outstanding reputation for scientific expertise and innovation - Strong customer support • Strong cash position and no debt - Board of directors evaluating share repurchase, ongoing dividend, or combination of the two
| 16 |

Q&A
| 17 |
