Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a11-15509_28k.htm |
| EX-99.2 - EX-99.2 - VIVUS INC | a11-15509_2ex99d2.htm |
| EX-99.1 - EX-99.1 - VIVUS INC | a11-15509_2ex99d1.htm |
Exhibit 99.3
Below is a reproduction of the contents of the poster entitled, “Long-term Weight Loss and Improvement in Glycemic Parameters with Low-Dose, Controlled-Release Phentermine/Topiramate (PHEN/TPM CR)”:

Below is a reproduction of the contents of the poster entitled, “Long-term Weight Loss and Improvement in Glycemic Parameters with Low-Dose, Controlled-Release Phentermine/Topiramate (PHEN/TPM CR)”:
Authors: Robert F. Kushner, MD(a); W. Timothy Garvey, MD(b); Barbara Troupin, MD, MBA(c); Wesley W. Day, PhD(c)
(a)Northwestern University Feinberg School of Medicine, Chicago, IL; (b)University of Alabama at Birmingham; Birmingham VA Medical Center, Birmingham, AL; (c)Vivus, Inc., Mountain View, CA
Contact details: R. F. Kushner, MD; Northwestern University Feinberg School of Medicine, 750 North Lake Shore Drive, Rubloff 9-976, Chicago, IL 60611; Phone: (312) 503-6817; Fax: (312) 503-6743; E-mail: rkushner@northwestern.edu
· Introduction
· Obesity, a disease that has been associated with type 2 diabetes mellitus (T2DM), has reached epidemic proportions.(1)-(3)
· In patients with T2DM, modest weight loss of >5% has been associated with improvements in glycemic parameters and may potentially reduce the need for concomitant antidiabetic medications.(4)-(6)
· Phentermine/topiramate controlled-release (PHEN/TPM CR) is an investigational combination therapy that, in conjunction with lifestyle modifications, was developed to assist with weight loss and reduce weight-related comorbidities. Previously, weight loss induced by PHEN/TPM CR was associated with improvements in glycemic parameters through 56 weeks in obese patients with >2 weight-related comorbidities enrolled in the CONQUER study.(7)
· The SEQUEL study was a placebo-controlled 52-week extension of the CONQUER study.
· Objectives
· To assess the effects of lifestyle modification + PHEN/TPM CR on weight loss and glycemic parameters over 108 weeks in overweight/obese subjects with or without T2DM.
· Methods
· Overweight/obese adult subjects with comorbidities were studied over 108 weeks in 2 consecutive trials:
· CONQUER was a double-blind, placebo-controlled Phase 3 trial in which subjects (N=2487) were randomized to lifestyle + placebo, lifestyle + PHEN 7.5 mg/TPM CR 46 mg (lifestyle + 7.5/46), or lifestyle + PHEN 15 mg/TPM CR 92 mg (lifestyle + 15/92) for 56 weeks.(7)
· The double-blind extension study (SEQUEL) consisted of a 52-week treatment period in which eligible subjects from selected sites who completed CONQUER were maintained on their original blinded treatment.
· All subjects were encouraged to adhere to a dietary and lifestyle management program (the LEARN program). Subjects with diabetes were managed to American Diabetes Association standards of care.(5),(8)
· Assessments
Subject Criteria
· Subjects who completed CONQUER were eligible for participation in SEQUEL if they had a body mass index (BMI) >22 kg/m2 and did not have any conditions that might affect protocol compliance or contraindicate treatment with the study drug.
· Diabetic status was defined at the start of the CONQUER study based on medical history at study entry. Participants could be treated with diet and exercise or could be receiving metformin monotherapy for their T2DM. Other diabetes medications were excluded.
Efficacy Evaluations
· Primary efficacy endpoints included the percent weight loss from baseline (CONQUER Week 0) to Week 108, performed for the intent-to-treat (ITT) population, with last observation carried forward (LOCF).
· Additional endpoints included percent weight loss at 108 Weeks; change in fasting glucose, hemoglobin A1c (HbA1c), and fasting insulin from baseline (CONQUER Week 0) to Week 108 in patients with or without T2DM (ITT-LOCF); and change in concomitant antidiabetic medications.
Statistical Assessment
· Analysis of covariance (ANCOVA) was used to evaluate changes in primary and additional endpoints, with treatment, gender, and diabetic status as fixed effect factors and baseline weight as a covariate.
· Results
Baseline Demographics
· Baseline demographics for the overall population are shown in Table 1.
· Of the 675 subjects participating in SEQUEL, 145 (21.5%) had T2DM at baseline.
Table 1. Baseline Demographics: Overall Population (ITT).
|
|
|
Lifestyle + Placebo |
|
Lifestyle + 7.5/46 |
|
Lifestyle + 15/92 |
|
|
Mean age, years |
|
52.7 |
|
52.2 |
|
51.2 |
|
|
Gender, female, % |
|
64.8 |
|
69.3 |
|
66.1 |
|
|
Race, Caucasian, % |
|
87.2 |
|
87.6 |
|
82.7 |
|
|
Mean weight, kg |
|
101.1 |
|
102.2 |
|
101.9 |
|
|
Mean BMI, kg/m2 |
|
36.0 |
|
36.1 |
|
36.2 |
|
|
Presence of T2DM, % |
|
24.2 |
|
17.0 |
|
21.7 |
|
Weight Loss
· At Week 108, treatment with lifestyle + PHEN/TPM CR was associated with significantly greater least-squares (LS) mean percent weight loss compared with lifestyle + placebo in the overall ITT-LOCF population (P<.0001): -1.8%, -9.3%, and -10.5% for lifestyle + placebo, lifestyle + 7.5/46, and lifestyle + 15/92, respectively.
· Weight loss trends were similar in subjects with and without T2DM at baseline (Figure 1).

Glycemic Parameters: Non-T2DM and T2DM Subgroups
· At Week 108, LS mean change from baseline in fasting glucose significantly improved with lifestyle + PHEN/TPM CR vs lifestyle + placebo in subjects without T2DM; and comparably in both lifestyle + placebo and lifestyle + PHEN/TPM CR in subjects with T2DM (Figure 2).
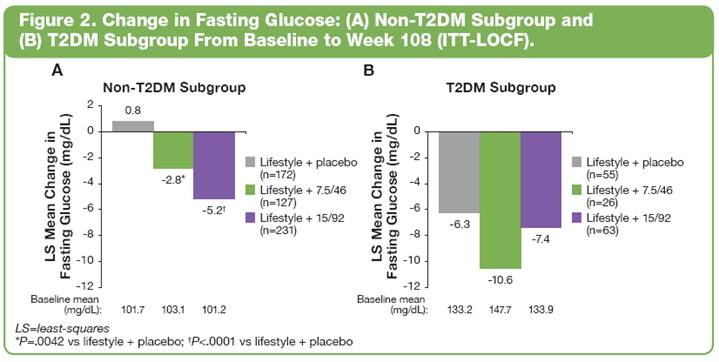
· In addition, LS mean change from baseline to Week 108 in HbA1c (Figure 3) and fasting insulin (Figure 4) were also improved with lifestyle + PHEN/TPM CR.


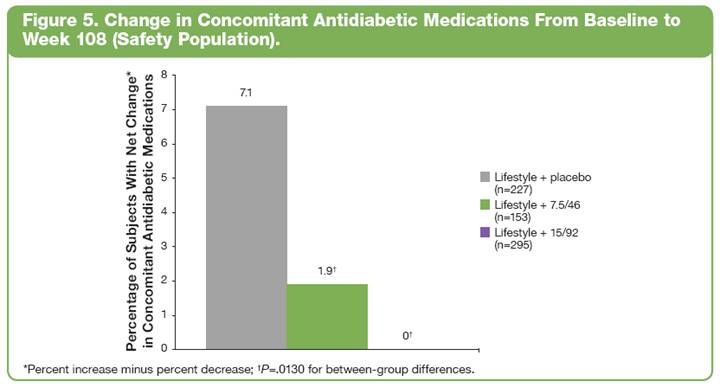
Change in Concomitant Antidiabetic Medications
· In the overall safety population, those subjects assigned to lifestyle + placebo required a greater increase in their concomitant antidiabetic medications than those receiving lifestyle + PHEN/TPM CR (Figure 5; P=.0130 for between-group differences).
Safety Summary: Overall Safety Population
· Out of 866 subjects eligible to enroll in SEQUEL, more subjects in the CONQUER lifestyle + placebo group elected not to enroll in the extension than subjects treated with lifestyle + PHEN/TPM CR (30.6%, 20.6%, and 14.5% for lifestyle + placebo, lifestyle + 7.5/46, and lifestyle + 15/92, respectively).
· Completion rates on study drug for subjects in SEQUEL were similar among treatment groups (lifestyle + placebo: 86.3%; lifestyle + 7.5/46: 82.5%; and lifestyle + 15/92: 83.1%).
· The most common treatment-emergent adverse events (TEAEs) are presented in Table 2.
· Discontinuations due to AEs during the extension study were 3.1%, 4.6%, and 4.4% for lifestyle + placebo, lifestyle + 7.5/46, and lifestyle + 15/92 groups, respectively.
· No new safety signals were seen between 56 and 108 weeks.
Table 2. Most Common TEAEs Between Baseline and Week 108
(Safety Population*).
|
TEAE, % |
|
Lifestyle + Placebo |
|
Lifestyle + 7.5/46 |
|
Lifestyle + 15/92 |
|
|
Upper respiratory tract infection |
|
32.6 |
|
27.5 |
|
28.5 |
|
|
Constipation |
|
9.7 |
|
22.2 |
|
22.7 |
|
|
Paresthesia |
|
2.6 |
|
14.4 |
|
22.4 |
|
|
Sinusitis |
|
13.7 |
|
15.7 |
|
21.0 |
|
|
Dry mouth |
|
2.6 |
|
14.4 |
|
20.7 |
|
|
Nasopharyngitis |
|
22.0 |
|
19.0 |
|
17.3 |
|
|
Dysgeusia |
|
1.8 |
|
11.8 |
|
13.6 |
|
|
Headache |
|
11.5 |
|
7.2 |
|
12.9 |
|
|
Insomnia |
|
9.7 |
|
12.4 |
|
11.5 |
|
|
Back pain |
|
11.5 |
|
11.1 |
|
11.5 |
|
*The safety population was defined as all subjects who received at least 1 dose of study drug.
· Overall, 7.0% of subjects had a serious AE (SAE) between baseline (CONQUER Week 0) and Week 108:
· 6.2% of subjects in the lifestyle + placebo group, 5.9% in the lifestyle + 7.5/46 group, and 8.1% in the lifestyle + 15/92 group; there were no drug-related SAEs.
· Conclusions
· Lifestyle + PHEN/TPM CR produced significant weight loss through 108 weeks in the overall patient population as well as specifically in those patients with T2DM.
· In addition to weight loss, subjects in the T2DM subgroup who were randomized to lifestyle + PHEN/TPM CR demonstrated improvements in glycemic parameters.
· Although not significant, subjects with T2DM had greater magnitudes of improvement in fasting glucose, HbA1c, and fasting insulin than subjects without T2DM.
· In addition, there was a greater reduction in use of concomitant antidiabetic medications in lifestyle + PHEN/TPM CR—treated subjects than in subjects treated with lifestyle + placebo, blunting the difference between treatment groups.
· Lifestyle + PHEN/TPM CR was generally well tolerated based on rates of joining the extension study, study completion and discontinuation rates, and overall AEs.
· These findings from the SEQUEL study demonstrate the sustained effectiveness and consistent tolerability profile of lifestyle + PHEN/TPM CR in the long-term management of obesity, as well as improvements in glycemic parameters with lifestyle + PHEN/TPM CR treatment in patients with and without T2DM.
This trial is registered at ClinicalTrials.gov, number NCT00796367.
References: (1) Flegal KM, et al. JAMA 2007;298(17):2028-2037. (2) Tzotzas T, et al. Obes Rev. 2011;12(5):e282-e289. (3) Reaven GM. Ann Intern Med 2003;138(5):420-423. (4) Hainer V, et al. Diabetes Care 2008;31(suppl 2):S269-S277. (5) American Diabetes Association. Diabetes Care 2010;33(suppl 1):S11-S61. (6) UKPDS Group. Metabolism 1990;39(9):905-912. (7) Gadde KM, et al. Lancet 2011;377(9774):1341-1352. (8) Brownell KD. The LEARN Program for Weight Management 2004.
Acknowledgements: We would like to acknowledge and thank the SEQUEL investigators and study coordinators, the Medpace team (study CRO), The Lockwood Group (for poster development assistance), and VIVUS internal contributors.
