Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - VIVUS INC | a10-19844_1ex99d2.htm |
| 8-K - 8-K - VIVUS INC | a10-19844_18k.htm |
Exhibit 99.1
|
|
Once-Daily, Controlled-Release Phentermine/Topiramate Results in Significant Weight Loss and Risk Factor Improvement in Overweight/Obese Subjects Over 56 Weeks Louis J. Aronne, MD New York-Presbyterian/Weill Cornell University, New York, NY Craig A. Peterson, MS Barbara Troupin, MD, MBA Wesley W. Day, PhD VIVUS, Inc. 1 |
|
|
Disclosures Dr. Aronne has the following consulting and financial relationships to disclose: Grant/Research Support: Amylin, Arena, Merck, Orexigen, Pfizer, Vivus Consultant: GlaxoSmithKline, Neurosearch, Pfizer Major Stock Shareholder: Cardiometabolic Support Network 2 |
|
|
Obesity: An Escalating Global Public Health Issue Obesity is associated with significantly increased morbidity, mortality related to cardiovascular disease, diabetes1,2 Prevalence is estimated at more than 1 billion overweight adults globally—at least 300 million are clinically obese2 Current pharmacologic weight-loss agents lead to limited weight loss at 1 year,6 potentially with safety/tolerability concerns7,8 NEW TOOLS TO ADDRESS THE EPIDEMIC ARE NEEDED 1. Reaven GM. Ann Intern Med 2003. 2. World Health Organization Web site. 3. Flegal KM JAMA 2007. 4. Flegal KM JAMA 2002. 5. NHLBI Obesity Education Initiative. Oct 2000. 6. Li Z, Ann Intern Med 2005. 7. Meridia PI Abbott July 2010. 8. Xenical PI Genentech, Inc; May 2010. 3 |
|
|
PHEN/TPM CR is an investigational, low-dose combination weight-loss therapy PHEN and TPM have different weight loss mechanisms PHEN: Used primarily for appetite suppression 1,2 TPM: Has demonstrated weight loss in obese individuals, and clinically meaningful improvements in glycemic control and blood pressure4-9 Greater response seen with combination than with either agent alone, allowing for lower doses of each agent1 Once-Daily, Low-Dose, Controlled-Release Phentermine/Topiramate (PHEN/TPM CR) 1. Vivus, Inc. Data on file. 2. Adipex-P PI Teva July 2005. 3. Topamax PI Ortho-McNeil-Janssen December 2009. 4. Bray GA. Obes Res 2003. 5. Wilding J. Int J Obes 2004. 6. Tonstad S. Am J Cardiol 2005. 7. Stenlof Kl. Diabetes Obes Metab 2007. 8. Toplak H. Int J Obes (Lond) 2007. 9. Rosenstock J. Diabetes Care 2007. 4 |
|
|
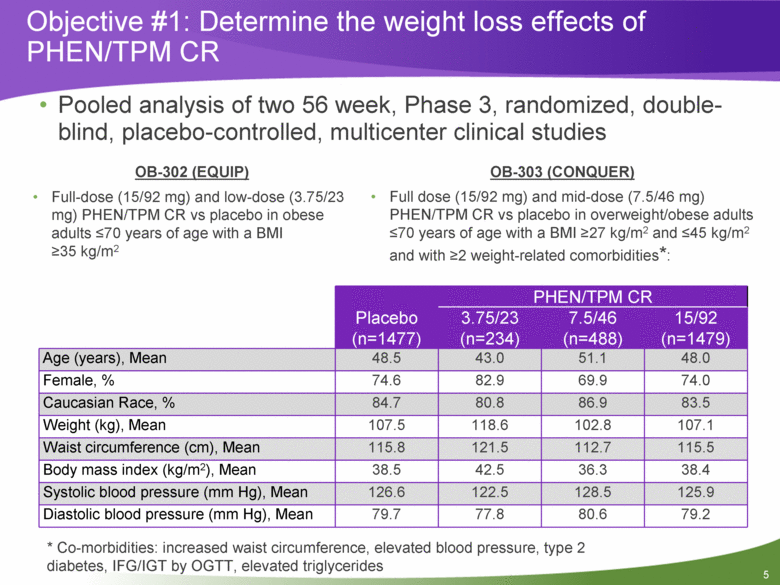
Objective #1: Determine the weight loss effects of PHEN/TPM CR Pooled analysis of two 56 week, Phase 3, randomized, double-blind, placebo-controlled, multicenter clinical studies OB-302 (EQUIP) Full-dose (15/92 mg) and low-dose (3.75/23 mg) PHEN/TPM CR vs placebo in obese adults <70 years of age with a BMI >35 kg/m2 OB-303 (CONQUER) Full dose (15/92 mg) and mid-dose (7.5/46 mg) PHEN/TPM CR vs placebo in overweight/obese adults <70 years of age with a BMI >27 kg/m2 and <45 kg/m2 and with >2 weight-related comorbidities*: Placebo (n=1477) PHEN/TPM CR 3.75/23 (n=234) 7.5/46 (n=488) 15/92 (n=1479) Age (years), Mean 48.5 43.0 51.1 48.0 Female, % 74.6 82.9 69.9 74.0 Caucasian Race, % 84.7 80.8 86.9 83.5 Weight (kg), Mean 107.5 118.6 102.8 107.1 Waist circumference (cm), Mean 115.8 121.5 112.7 115.5 Body mass index (kg/m2), Mean 38.5 42.5 36.3 38.4 Systolic blood pressure (mm Hg), Mean 126.6 122.5 128.5 125.9 Diastolic blood pressure (mm Hg), Mean 79.7 77.8 80.6 79.2 * Co-morbidities: increased waist circumference, elevated blood pressure, type 2 diabetes, IFG/IGT by OGTT, elevated triglycerides 5 |
|
|
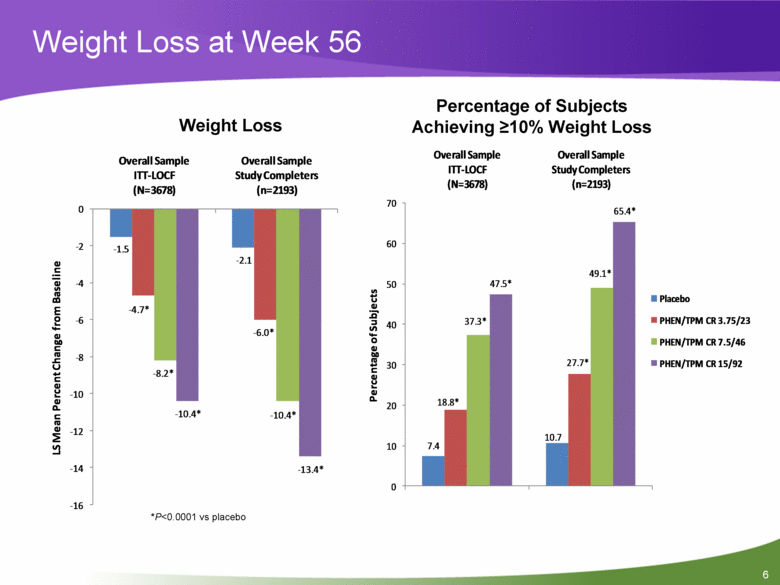
Weight Loss at Week 56 *P<0.0001 vs placebo Weight Loss Percentage of Subjects Achieving >10% Weight Loss 7.4 10.7 18.8* 27.7* 37.3* 49.1* 47.5* 65.4* 0 10 20 30 40 50 60 70 Overall Sample ITT-LOCF (N=3678) Overall Sample Study Completers (n=2193) Percentage of Subjects Placebo PHEN/TPM CR 3.75/23 PHEN/TPM CR 7.5/46 PHEN/TPM CR 15/92 - 1.5 - 2.1 - 4.7* - 6.0* - 8.2* - 10.4* - 10.4* - 13.4* - 16 - 14 - 12 - 10 - 8 - 6 - 4 - 2 0 Overall Sample ITT-LOCF (N=3678) Overall Sample Study Completers (n=2193) LS Mean Percent Change from Baseline 6 |
|
|
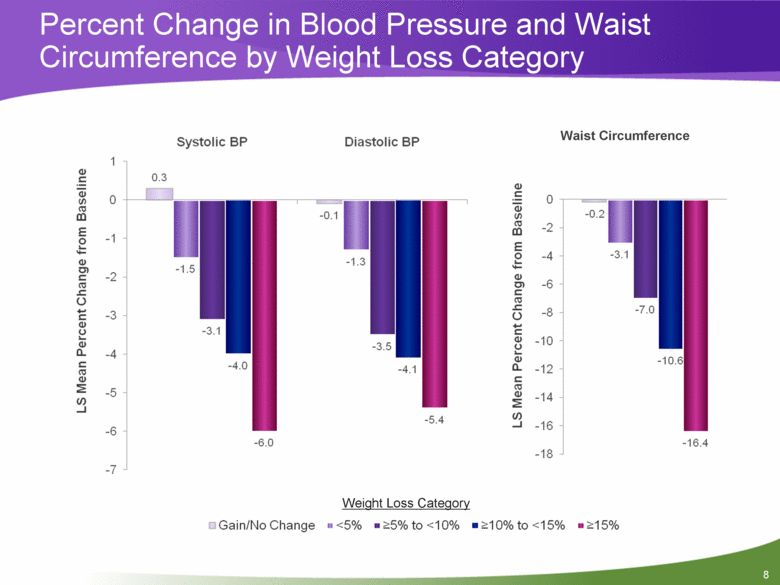
Objective #2: Relationship Between Weight Loss and Metabolic Improvements Overall, in a pooled patient population, weight loss was dose-dependent with higher doses of PHEN/TPM CR leading to greater weight loss A sub-analysis of weight loss by categories offers a more detailed look at the relationship between % weight loss and several cardiometabolic risk factors, irrespective of treatment group to assess whether a weight loss threshold exists for metabolic benefit Weight loss categories used in this analysis are: Gain/No Change <5% >5% to <10% >10% to <15% >15% 7 |
|
|
Percent Change in Blood Pressure and Waist Circumference by Weight Loss Category Weight Loss Category Waist Circumference 1 0.3 -0.1 0 -0.2 0 -1.5 -1.3 -2 -3.1 -1 -3.1 -3.5 -4 -7.0 -2 -4.0 -4.1 -6 -10.6 -3 -6.0 -5.4 -8 -16.4 -4 -10 -5 -12 -6 -14 -7 -16 -18 Systolic BP Diastolic BP 8 |
|
|
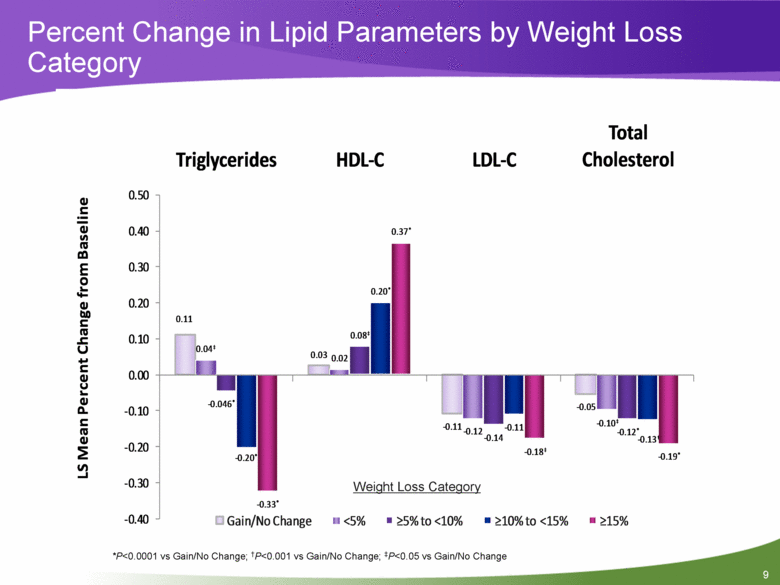
Percent Change in Lipid Parameters by Weight Loss Category *P<0.0001 vs Gain/No Change; †P<0.001 vs Gain/No Change; ‡P<0.05 vs Gain/No Change Weight Loss Category 0.11 0.03 - 0.11 - 0.05 0.04 ‡ 0.02 - 0.12 - 0.10 ‡ - 0.046 * 0.08 ‡ - 0.14 - 0.12 * - 0.20 * 0.20 * - 0.11 - 0.13 † - 0.33 * 0.37 * - 0.18 ‡ - 0.19 * - 0.40 - 0.30 - 0.20 - 0.10 0.00 0.10 0.20 0.30 0.40 0.50 Triglycerides HDL - C LDL - C Total Cholesterol LS Mean Percent Change from Baseline Gain/No Change <5% >5% to <10% >10% to <15% >15% 9 |
|
|
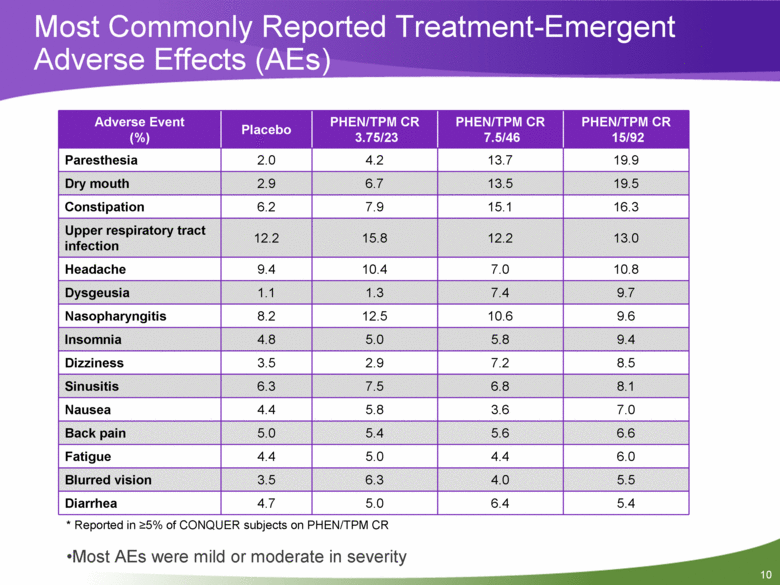
Most Commonly Reported Treatment-Emergent Adverse Effects (AEs) Adverse Event (%) Placebo PHEN/TPM CR 3.75/23 PHEN/TPM CR 7.5/46 PHEN/TPM CR 15/92 Paresthesia 2.0 4.2 13.7 19.9 Dry mouth 2.9 6.7 13.5 19.5 Constipation 6.2 7.9 15.1 16.3 Upper respiratory tract infection 12.2 15.8 12.2 13.0 Headache 9.4 10.4 7.0 10.8 Dysgeusia 1.1 1.3 7.4 9.7 Nasopharyngitis 8.2 12.5 10.6 9.6 Insomnia 4.8 5.0 5.8 9.4 Dizziness 3.5 2.9 7.2 8.5 Sinusitis 6.3 7.5 6.8 8.1 Nausea 4.4 5.8 3.6 7.0 Back pain 5.0 5.4 5.6 6.6 Fatigue 4.4 5.0 4.4 6.0 Blurred vision 3.5 6.3 4.0 5.5 Diarrhea 4.7 5.0 6.4 5.4 * Reported in >5% of CONQUER subjects on PHEN/TPM CR Most AEs were mild or moderate in severity 10 |
|
|
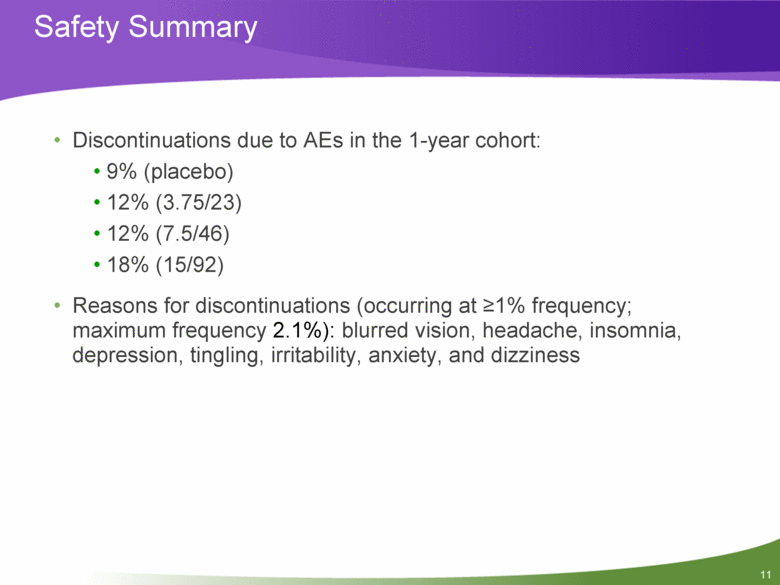
Safety Summary Discontinuations due to AEs in the 1-year cohort: 9% (placebo) 12% (3.75/23) 12% (7.5/46) 18% (15/92) Reasons for discontinuations (occurring at >1% frequency; maximum frequency 2.1%): blurred vision, headache, insomnia, depression, tingling, irritability, anxiety, and dizziness 11 |
|
|
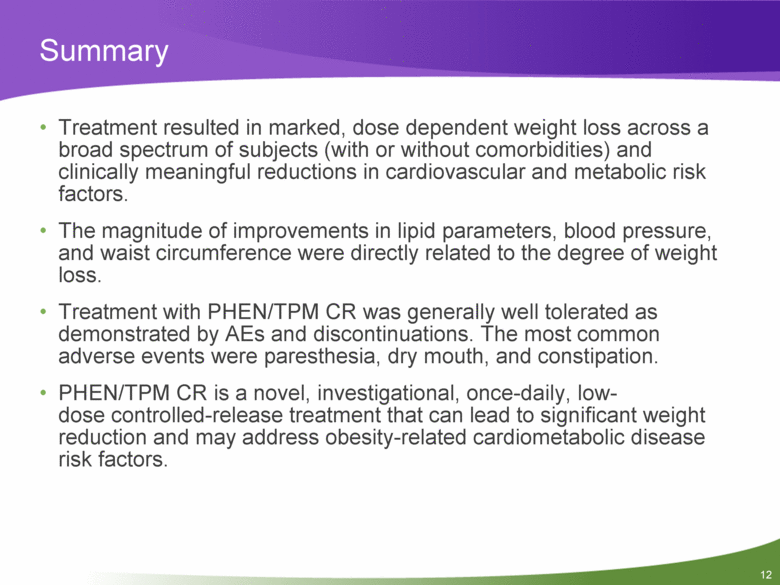
Summary Treatment resulted in marked, dose dependent weight loss across a broad spectrum of subjects (with or without comorbidities) and clinically meaningful reductions in cardiovascular and metabolic risk factors. The magnitude of improvements in lipid parameters, blood pressure, and waist circumference were directly related to the degree of weight loss. Treatment with PHEN/TPM CR was generally well tolerated as demonstrated by AEs and discontinuations. The most common adverse events were paresthesia, dry mouth, and constipation. PHEN/TPM CR is a novel, investigational, once-daily, low-dose controlled-release treatment that can lead to significant weight reduction and may address obesity-related cardiometabolic disease risk factors. 12 |