Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a10-18267_18k.htm |
| EX-99.1 - EX-99.1 - Innoviva, Inc. | a10-18267_1ex99d1.htm |
Exhibit 99.2
|
|
24h duration of the novel long-acting 2 agonist vilanterol trifenatate in uncontrolled asthma Lötvall J1, Bateman ED2, Bleecker ER3, Busse W4, Woodcock A5, Follows R6, Lim J6, Stone S6, Jacques L6, Haumann B6 1Krefting Research Centre, University of Gothenburg, Gothenburg, Sweden 2Department of Medicine, University of Cape Town, Cape Town, South Africa 3Translational Sciences, Wake Forest University Health Sciences, Winston-Salem, NC, USA 4Department of Medicine, University of Wisconsin, Madison, WI, USA 5School of Translational Medicine, University of Manchester, Manchester, UK 6Respiratory Medicine Development Centre, GlaxoSmithKline, London, UK |
|
|
Conflict of interest disclosure Jan Lötvall has the following, real or perceived conflicts of interest: Served as a consultant to, and received lecture fees from, or been sponsored to attend congresses by: AstraZeneca GlaxoSmithKline Merck Sharpe and Dohme Novartis Oriel Therapeutics UCB Pharma |
|
|
Acknowledgements Funded by GlaxoSmithKline Editorial support by Gardiner-Caldwell Communications, funded by GlaxoSmithKline Study management: Suus Baggen (GlaxoSmithKline) Investigators and staff 88 centres |
|
|
Background Adding a LABA to ICS improves asthma control Current ICS/LABA therapies are indicated twice-daily ICS/LABA therapies can be further improved Adherence may improve with once daily treatment Vilanterol trifenatate (VI) Novel, inhaled LABA Inherent 24h activity Clinical efficacy not previously presented In development as combination therapy Asthma COPD |
|
|
Study objectives To evaluate VI in asthma Dose response Clinical efficacy Safety Five doses of VI / Placebo Dosing in evening Persistent asthma Regular treatment ICS |
|
|
Study design Multicentre, randomised, double-blind, placebo-controlled, parallel-group, dose-ranging study Endpoints primary: trough FEV1 Day 28 secondary: Mean 24h FEV1 Symptom-free days Safety and tolerability |
|
|
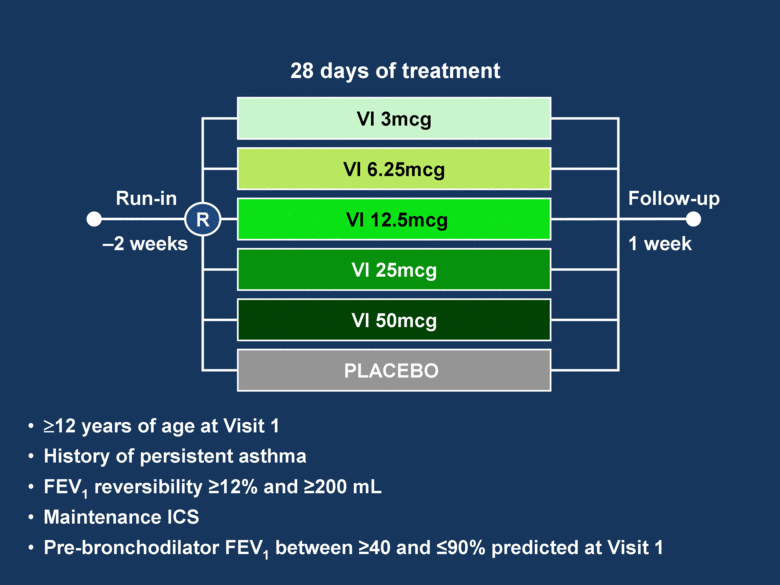
VI 12.5mcg VI 6.25mcg VI 3mcg VI 25mcg Follow-up PLACEBO VI 50mcg –2 weeks 1 week 28 days of treatment R Run-in 12 years of age at Visit 1 History of persistent asthma FEV1 reversibility 12% and 200 mL Maintenance ICS Pre-bronchodilator FEV1 between 40 and 90% predicted at Visit 1 |
|
|
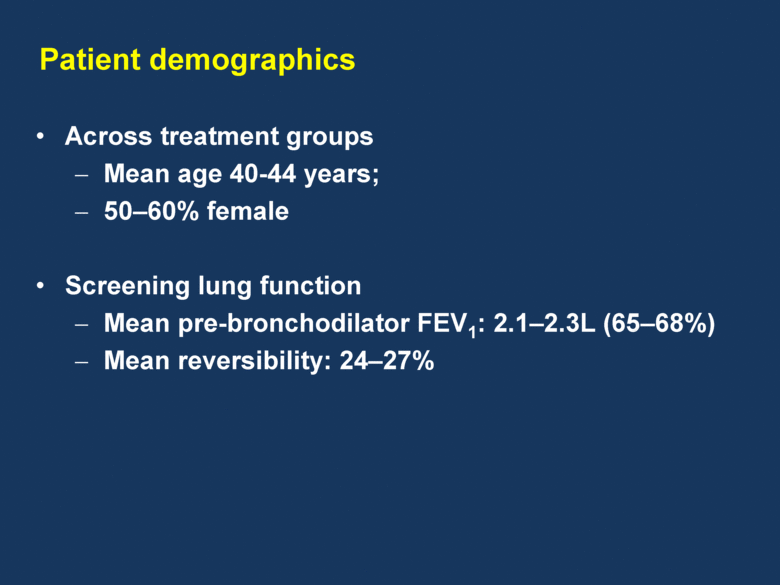
Patient demographics Across treatment groups Mean age 40-44 years; 50–60% female Screening lung function Mean pre-bronchodilator FEV1: 2.1–2.3L (65–68%) Mean reversibility: 24–27% |
|
|
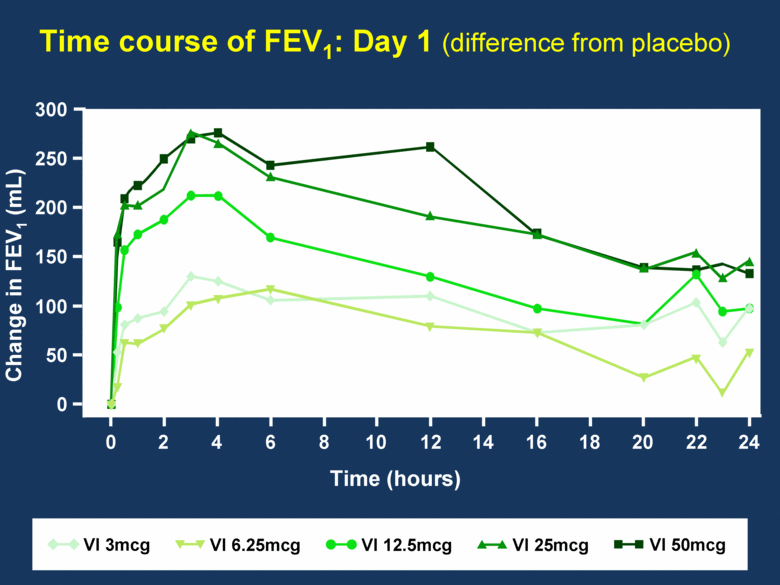
Time course of FEV1: Day 1 (difference from placebo) Change in FEV1 (mL) Time (hours) 300 250 200 150 100 50 0 VI 25mcg VI 6.25mcg VI 12.5mcg VI 50mcg VI 3mcg 0 2 4 6 8 10 12 14 16 18 20 22 24 |
|
|
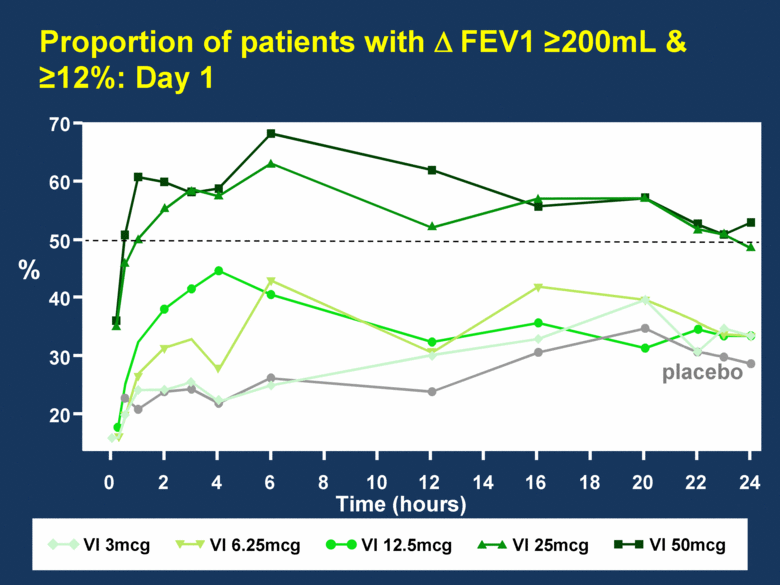
Proportion of patients with FEV1 200mL & 12%: Day 1 Time (hours) % 70 60 50 40 30 20 0 2 4 6 8 10 12 14 16 18 20 22 24 VI 25mcg VI 6.25mcg VI 12.5mcg VI 50mcg placebo VI 3mcg |
|
|
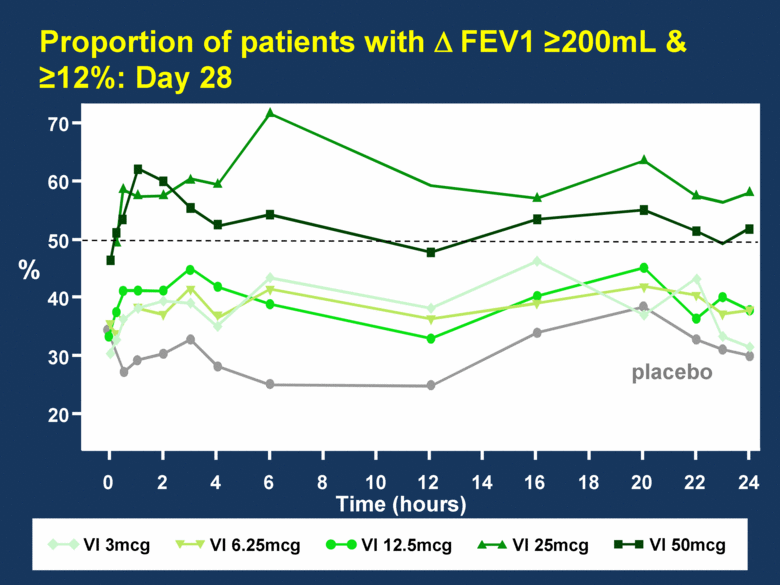
Proportion of patients with FEV1 200mL & 12%: Day 28 VI 25mcg VI 6.25mcg VI 12.5mcg VI 50mcg 70 60 50 40 30 20 0 2 4 6 8 10 12 14 16 18 20 22 24 % Time (hours) placebo VI 3mcg |
|
|
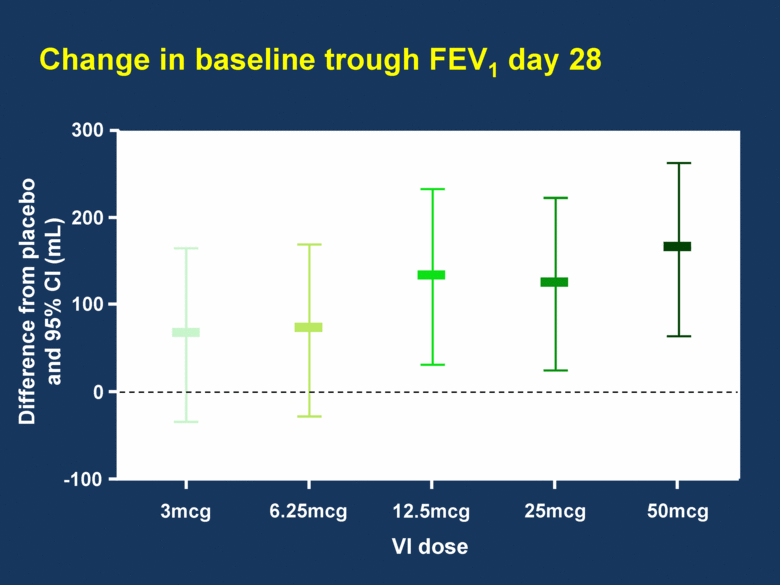
Change in baseline trough FEV1 day 28 300 3mcg 6.25mcg 12.5mcg 25mcg 50mcg 200 100 0 -100 VI dose Difference from placebo and 95% CI (mL) |
|
|
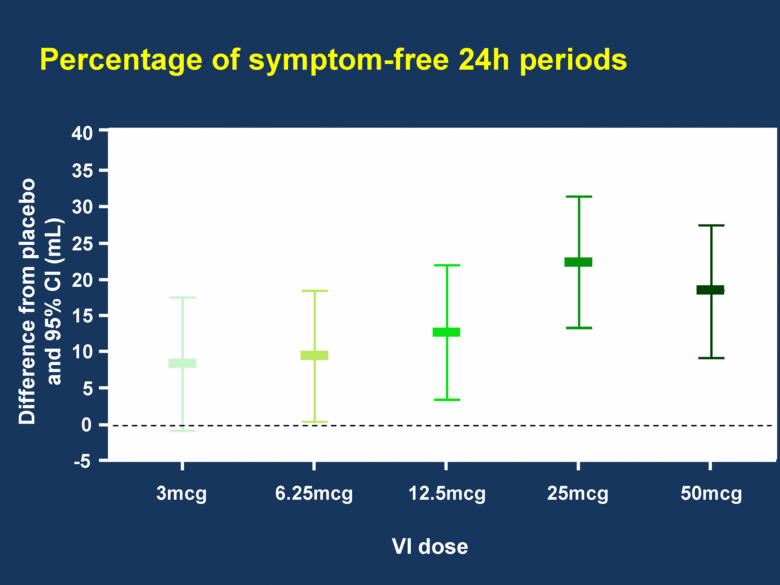
Percentage of symptom-free 24h periods 40 3mcg 6.25mcg 12.5mcg 25mcg 50mcg 35 30 25 20 15 10 5 0 -5 VI dose Difference from placebo and 95% CI (mL) |
|
|
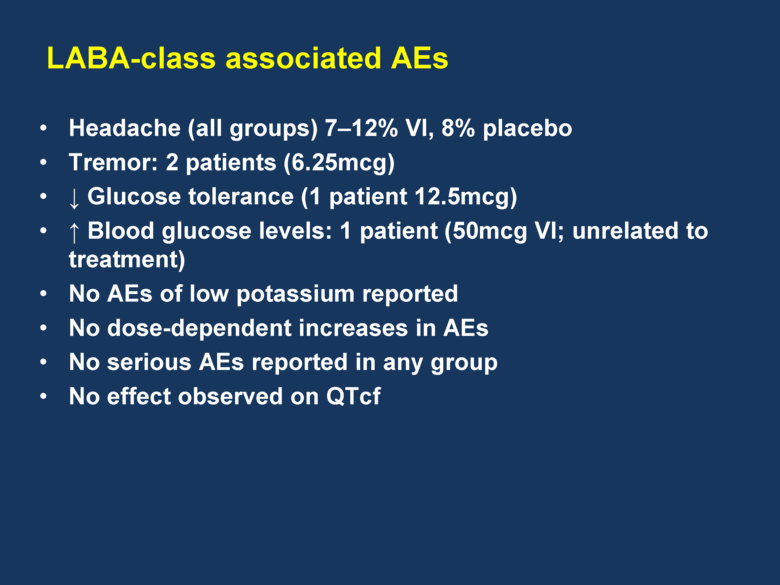
LABA-class associated AEs Headache (all groups) 7–12% VI, 8% placebo Tremor: 2 patients (6.25mcg) Glucose tolerance (1 patient 12.5mcg) Blood glucose levels: 1 patient (50mcg VI; unrelated to treatment) No AEs of low potassium reported No dose-dependent increases in AEs No serious AEs reported in any group No effect observed on QTcf |
|
|
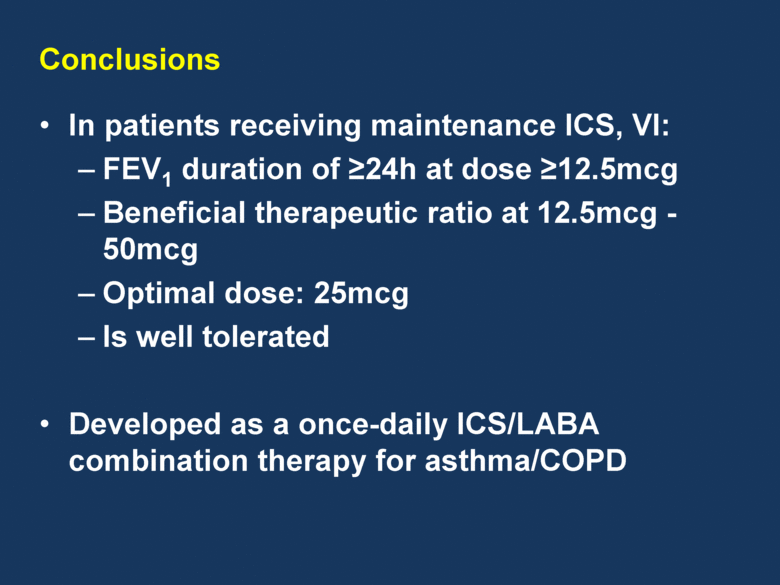
Conclusions In patients receiving maintenance ICS, VI: FEV1 duration of 24h at dose 12.5mcg Beneficial therapeutic ratio at 12.5mcg - 50mcg Optimal dose: 25mcg Is well tolerated Developed as a once-daily ICS/LABA combination therapy for asthma/COPD |