Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a10-18267_18k.htm |
| EX-99.2 - EX-99.2 - Innoviva, Inc. | a10-18267_1ex99d2.htm |
Exhibit 99.1
|
|
Safety and efficacy of fluticasone furoate/vilanterol trifenatate (FF/VI) in COPD patients J Lötvall1, P Bakke2, L Bjermer3, S Steinshamn4, C Crim5, L Sanford6, C Scott-Wilson5, B Haumann6 1Krefting Research Centre, University of Gothenburg, Gothenberg, Sweden 2Department of Thoracic Medicine, University of Bergen and Haukeland University Hospital, Bergen, Norway 3Department of Respiratory Medicine and Allergology, Institute for Clinical Science, Lund, Sweden 4Lung Department, St. Olavs University Hospital of Trondheim, Trondheim, Norway 5Respiratory Medicine Development Centre, GlaxoSmithKline, RTP, USA 6Respiratory Medicine Development Centre, GlaxoSmithKline, London, UK |
|
|
CONFLICT OF INTEREST DISCLOSURE Jan Lötvall has the following, real or perceived conflicts of interest: Served as a consultant to, and received lecture fees from, or been sponsored to attend congresses by: AstraZeneca GlaxoSmithKline Merck Sharpe and Dohme Novartis Oriel Therapeutics UCB Pharma |
|
|
ACKNOWLEDGEMENTS Funded by GlaxoSmithKline Editorial support by Gardiner-Caldwell Communications, funded by GlaxoSmithKline Investigators and staff at the four study centres |
|
|
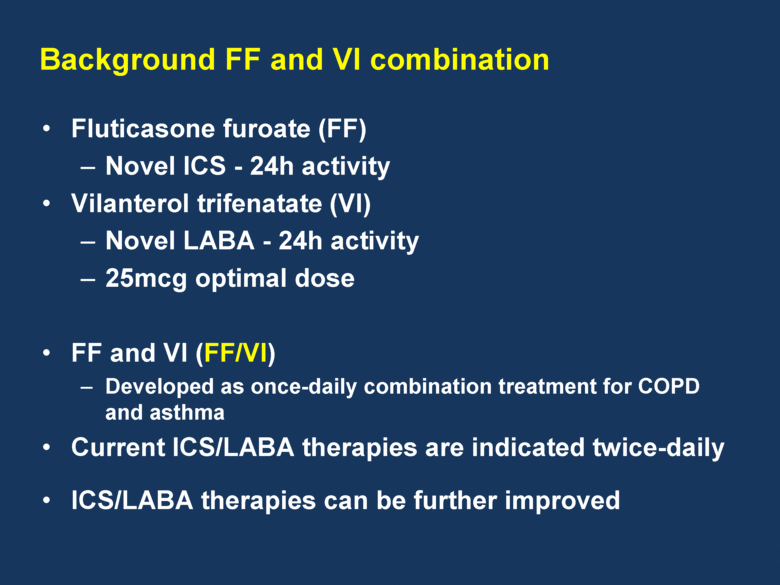
Fluticasone furoate (FF) Novel ICS - 24h activity Vilanterol trifenatate (VI) Novel LABA - 24h activity 25mcg optimal dose FF and VI (FF/VI) Developed as once-daily combination treatment for COPD and asthma Current ICS/LABA therapies are indicated twice-daily ICS/LABA therapies can be further improved Background FF and VI combination |
|
|
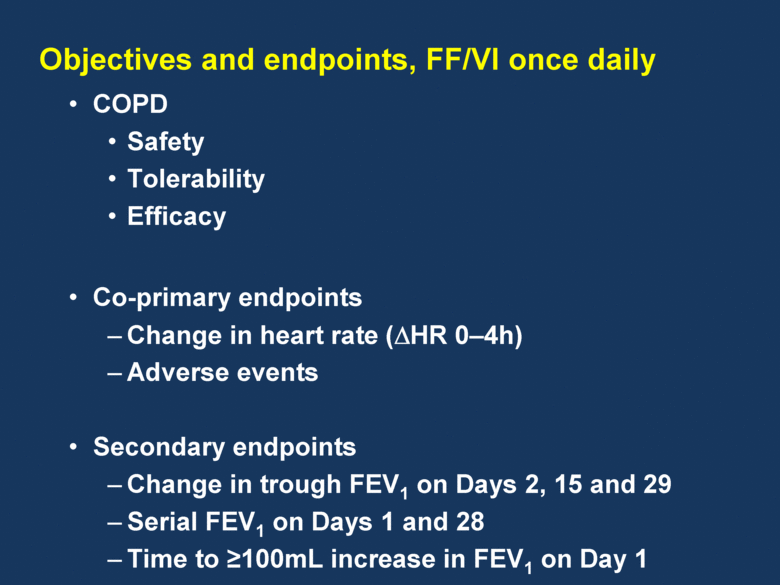
COPD Safety Tolerability Efficacy Co-primary endpoints Change in heart rate (HR 0–4h) Adverse events Secondary endpoints Change in trough FEV1 on Days 2, 15 and 29 Serial FEV1 on Days 1 and 28 Time to 100mL increase in FEV1 on Day 1 Objectives and endpoints, FF/VI once daily |
|
|
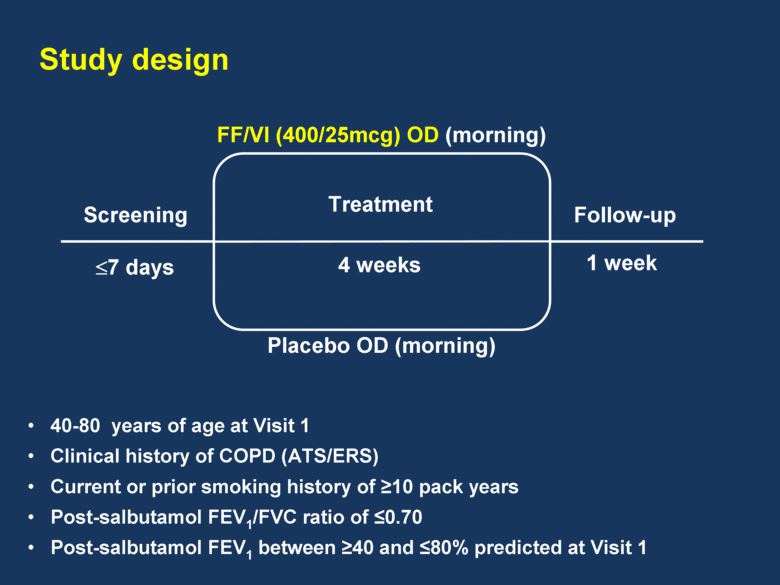
Screening 7 days FF/VI (400/25mcg) OD (morning) Placebo OD (morning) Follow-up 4 weeks 1 week Treatment Study design 40-80 years of age at Visit 1 Clinical history of COPD (ATS/ERS) Current or prior smoking history of 10 pack years Post-salbutamol FEV1/FVC ratio of 0.70 Post-salbutamol FEV1 between 40 and 80% predicted at Visit 1 |
|
|
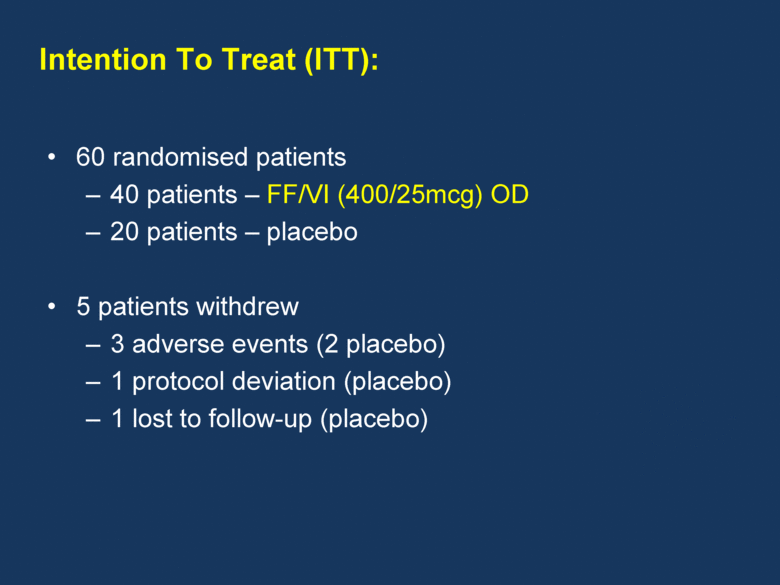
60 randomised patients 40 patients – FF/VI (400/25mcg) OD 20 patients – placebo 5 patients withdrew 3 adverse events (2 placebo) 1 protocol deviation (placebo) 1 lost to follow-up (placebo) Intention To Treat (ITT): |
|
|
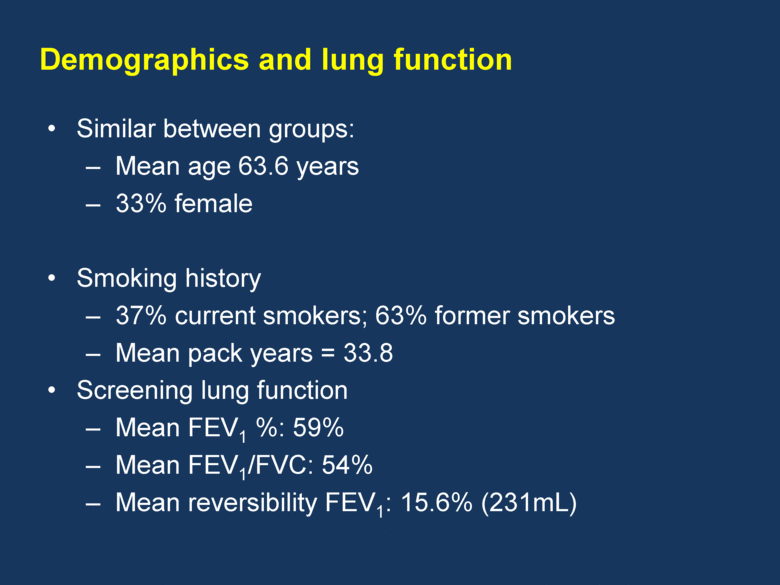
Similar between groups: Mean age 63.6 years 33% female Smoking history 37% current smokers; 63% former smokers Mean pack years = 33.8 Screening lung function Mean FEV1 %: 59% Mean FEV1/FVC: 54% Mean reversibility FEV1: 15.6% (231mL) Demographics and lung function |
|
|
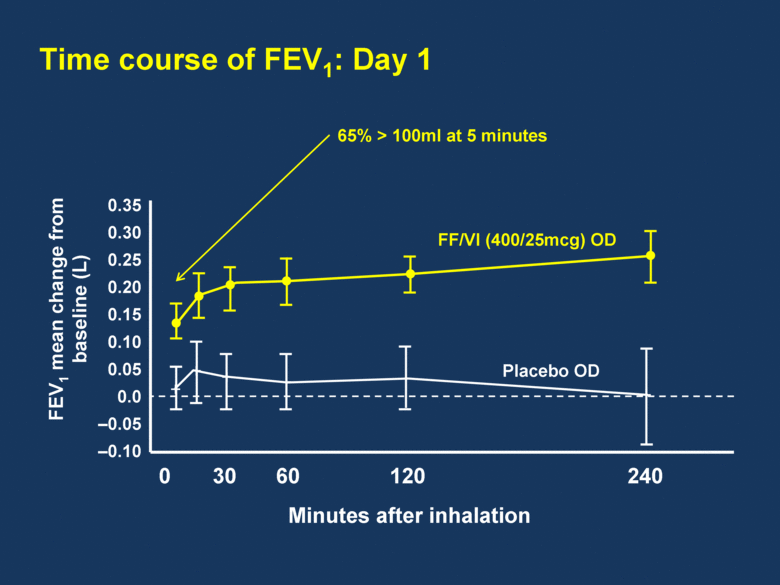
Time course of FEV1: Day 1 0 30 60 120 240 0.35 0.30 0.25 0.20 0.15 0.10 0.05 0.0 –0.05 –0.10 Minutes after inhalation FF/VI (400/25mcg) OD Placebo OD FEV1 mean change from baseline (L) 65% > 100ml at 5 minutes |
|
|
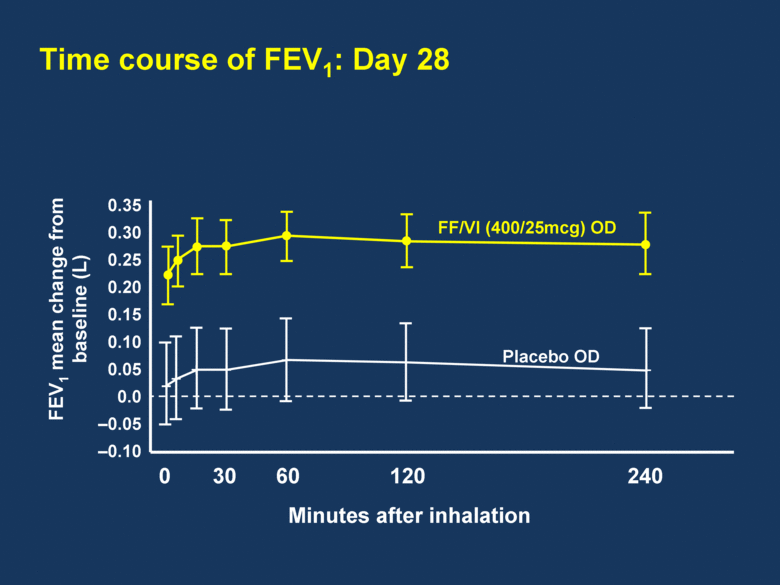
Time course of FEV1: Day 28 Minutes after inhalation 0.35 0.30 0.25 0.20 0.15 0.10 0.05 0.0 –0.05 –0.10 0 30 60 120 240 FF/VI (400/25mcg) OD Placebo OD FEV1 mean change from baseline (L) |
|
|
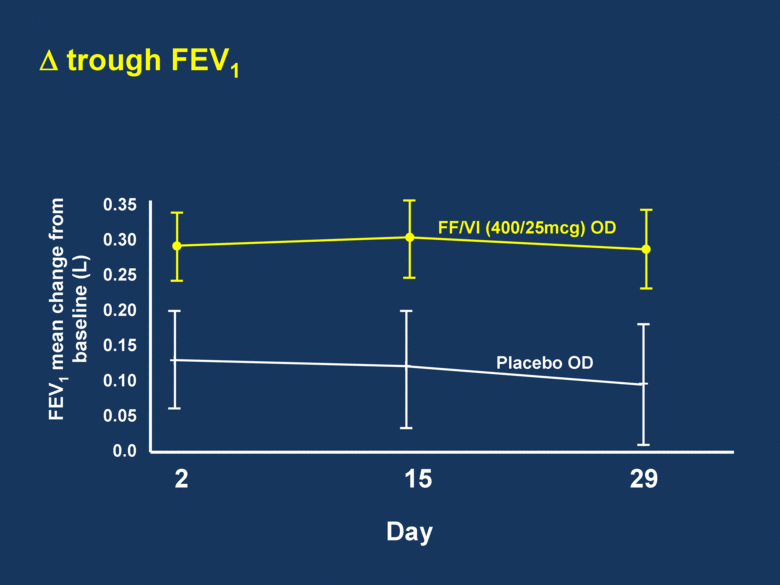
trough FEV1 0.35 0.30 0.25 0.20 0.15 0.10 0.05 0.0 2 15 29 Day FF/VI (400/25mcg) OD Placebo OD FEV1 mean change from baseline (L) |
|
|
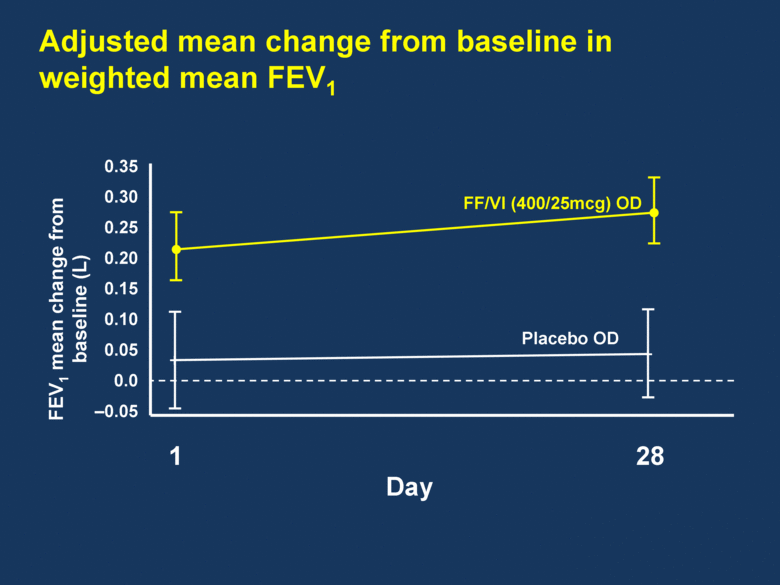
Adjusted mean change from baseline in weighted mean FEV1 1 28 0.35 0.30 0.25 0.20 0.15 0.10 0.05 0.0 –0.05 Day FF/VI (400/25mcg) OD Placebo OD FEV1 mean change from baseline (L) |
|
|
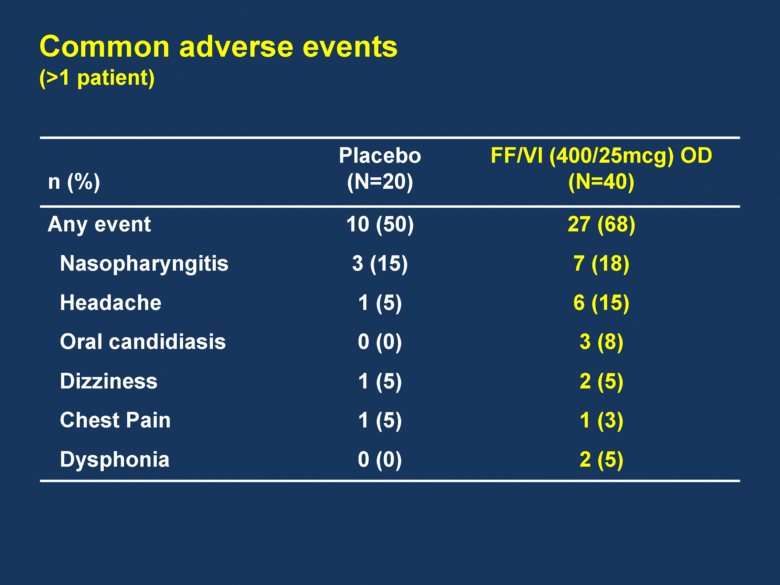
n (%) Placebo (N=20) FF/VI (400/25mcg) OD (N=40) Any event 10 (50) 27 (68) Nasopharyngitis 3 (15) 7 (18) Headache 1 (5) 6 (15) Oral candidiasis 0 (0) 3 (8) Dizziness 1 (5) 2 (5) Chest Pain 1 (5) 1 (3) Dysphonia 0 (0) 2 (5) Common adverse events (>1 patient) |
|
|
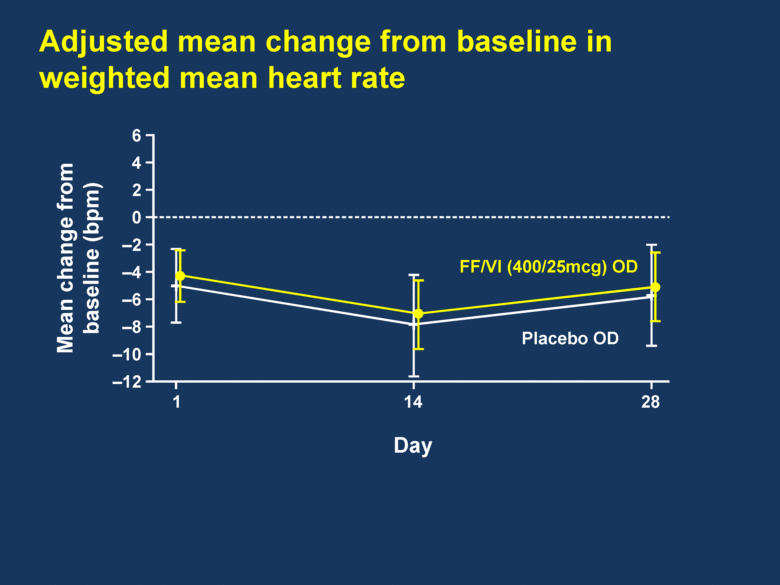
Adjusted mean change from baseline in weighted mean heart rate 6 4 2 0 –2 –4 –6 –8 –10 –12 Mean change from baseline (bpm) 1 14 28 FF/VI (400/25mcg) OD Placebo OD Day |
|
|
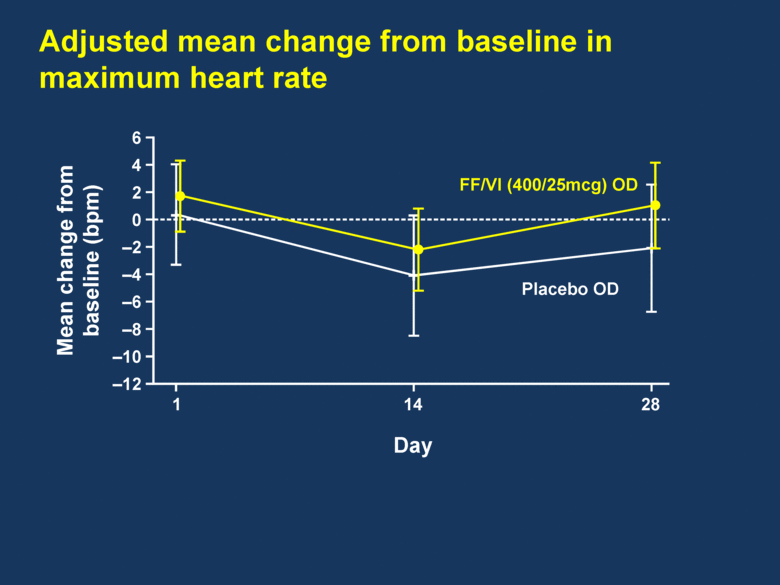
Adjusted mean change from baseline in maximum heart rate 6 4 2 0 –2 –4 –6 –8 –10 –12 Mean change from baseline (bpm) 1 14 28 FF/VI (400/25mcg) OD Placebo OD Day |
|
|
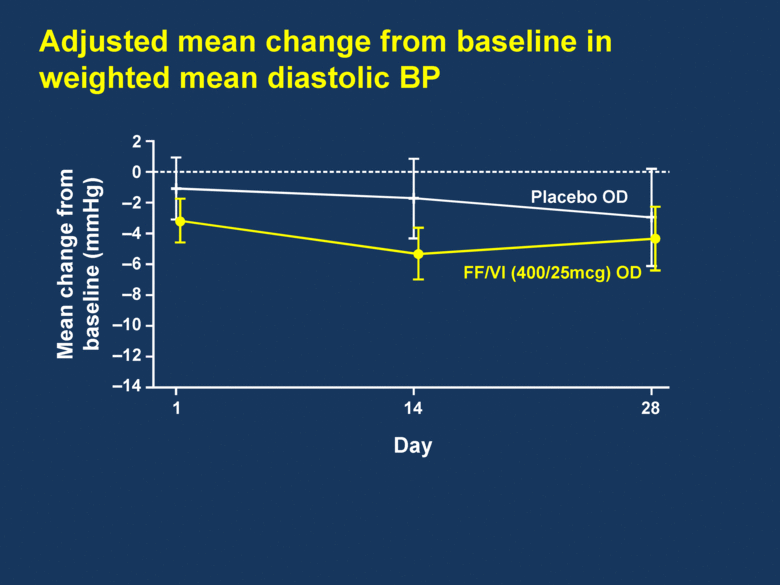
Adjusted mean change from baseline in weighted mean diastolic BP 2 0 –2 –4 –6 –8 –10 –12 –14 Mean change from baseline (mmHg) 1 14 28 FF/VI (400/25mcg) OD Placebo OD Day |
|
|
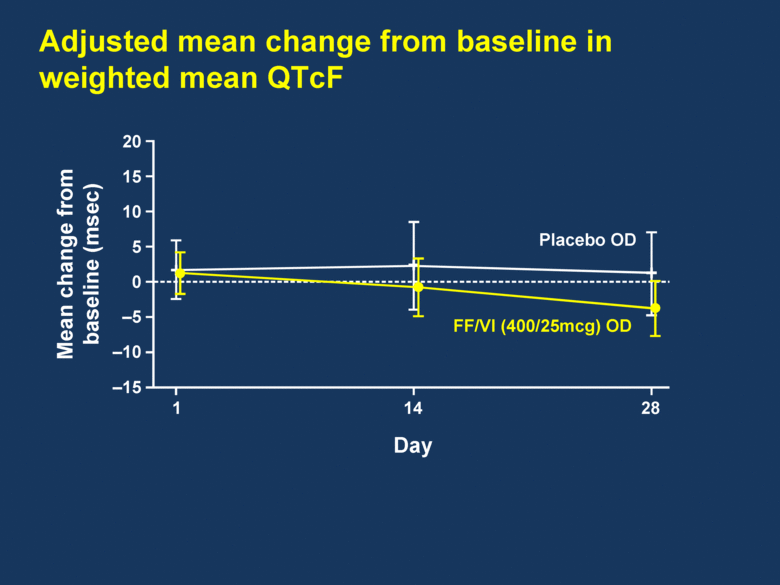
Adjusted mean change from baseline in weighted mean QTcF 20 15 10 5 0 –5 –10 –15 Mean change from baseline (msec) 1 14 28 FF/VI (400/25mcg) OD Placebo OD Day |
|
|
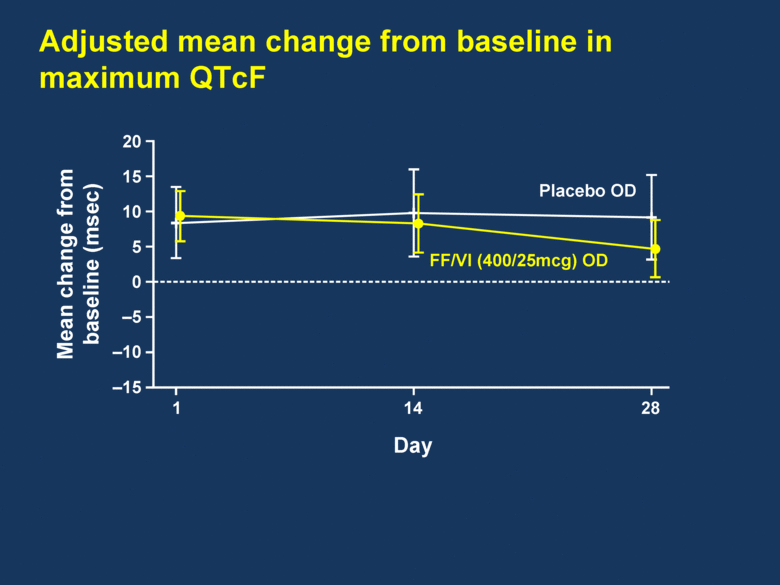
Adjusted mean change from baseline in maximum QTcF 20 15 10 5 0 –5 –10 –15 Mean change from baseline (msec) 1 14 28 FF/VI (400/25mcg) OD Placebo OD Day |
|
|
FF/VI demonstrated a clear improvement in 24h trough and serial FEV1 FF/VI was well tolerated over the 28-day treatment period FF/VI showed no adverse impact on HR or other vital signs FF/VI demonstrated no clinically relevant impact on QTc Summary |
|
|
FF/VI is well tolerated and induces improvements in lung function in patients with COPD FF/VI may function as a once-daily combination therapy in COPD Conclusions |