Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a10-18069_18k.htm |
| EX-99.4 - EX-99.4 - Innoviva, Inc. | a10-18069_1ex99d4.htm |
| EX-99.1 - EX-99.1 - Innoviva, Inc. | a10-18069_1ex99d1.htm |
| EX-99.6 - EX-99.6 - Innoviva, Inc. | a10-18069_1ex99d6.htm |
| EX-99.5 - EX-99.5 - Innoviva, Inc. | a10-18069_1ex99d5.htm |
| EX-99.3 - EX-99.3 - Innoviva, Inc. | a10-18069_1ex99d3.htm |
Exhibit 99.2
|
POSTER P1166 |
|
Fluticasone furoate (FF), a once-daily inhaled
corticosteroid (ICS), demonstrates dose-response |
|
Bateman ED(1), Bleecker ER(2), Busse W(3), Lötvall J(4), Woodcock A(5), Forth R(6), Medley H(7), Jacques L(7), Haumann B(7)
(1)Department of Medicine, University of Cape Town, Cape Town, South Africa; (2)Translational Sciences, Wake Forest University Health Sciences Winston-Salem, USA (3)Department of Medicine, University of Wisconsin; Madison, USA; (4)Krefting Research Centre, Gothenburg, Sweden; (5)School of Translational Medicine, University Hospital of Manchester Manchester, UK; (6)Respiratory Medicine Development Center, GlaxoSmithKline, North Carolina, USA; (7)Respiratory Medicine Development Centre, GlaxoSmithKline, Uxbridge, UK
ABSTRACT
Introduction: FF (GW685698X) is an ICS still active at 24h, in development as a once-daily treatment in combination with the long-acting beta2 agonist (LABA), vilanterol trifenatate (VI; GW642444M) for asthma and chronic obstructive pulmonary disease (COPD).
Objectives: To compare the efficacy and safety of FF (dry powder) at four doses administered via a novel single-step activation inhaler in patients >12 years old with persistent uncontrolled asthma. Fluticasone propionate (FP) served as an active control.
Methods: In a randomised, double-blind, placebo-controlled, parallel group study, 598 patients received one of six treatments: FF (25, 50, 100 or 200mcg) once daily, FP 100mcg twice daily or placebo for 8 weeks. The primary endpoint was change from baseline in trough (pre-dose) forced expiratory volume in 1 second (FEV1) at Week 8.
Results: A dose response was observed for trough FEV1 between FF 25–200mcg once daily including and excluding placebo (linear trend analysis p<0.001 and p=0.03, respectively). At Week 8, relative to placebo FF 50–200mcg once daily had significantly greater increases in trough FEV1 from baseline (p<0.05) with FF 100mcg and 200mcg achieving a >200mL increase. Secondary endpoints peak expiratory flow (PEF), symptom-free and rescue-free 24h periods supported the efficacy of FF 50–200mcg once daily doses and FP. Overall, FF was well tolerated. The incidence of oral candidiasis was low (0–4%). 24h urinary cortisol excretion ratios (Week 8/baseline) were similar across treatments (FF 0.98–1.21 and placebo 1.04).
Conclusion: The data support the use of FF (100 and 200mcg) as a once-daily treatment in persistent uncontrolled asthma.
INTRODUCTION
· FF is an ICS with a pharmacological profile that demonstrates greater retention in the lung and a longer duration of action than FP.(1)
· FF is under development as a once-daily inhaled asthma therapy, administered in a novel dry powder inhaler (DPI) formulation in combination with VI, a new LABA with 24h activity.(2)
OBJECTIVE
· To compare the efficacy and safety of FF (dry powder) versus placebo at four doses administered via a novel single-step activation inhaler in patients >12 years old with persistent uncontrolled asthma. FP served as an active control.
PATIENTS AND METHODS
Study design
· A phase IIb, randomised, double-blind, double-dummy, parallel-group, placebo- and active-controlled study conducted at 142 centres in 14 countries.
· Eligible patients had: persistent asthma (defined by the National Institutes of Health criteria)(3) and were symptomatic on short-acting beta2 agonists (SABAs) or other non-steroid agents; FEV1 (% of predicted normal) 40–90% (evening; PM) or 40–85% (morning; AM); reversibility of FEV1 of >12% and >200mL with albuterol/salbutamol aerosol inhaler. All patients had to be able to replace their current SABAs with albuterol/salbutamol aerosol for use as needed during the study period.
· After 4-weeks run-in (on usual medications), patients entered the treatment period if they were symptomatic (combined daily asthma symptom score >1 or albuterol/ salbutamol use on >4 of the last 7 days of run-in) and their evening pre-dose FEV1 was 40–90% of predicted normal.
· Eligible patients were randomised to one of six treatment groups for 8 weeks using a double dummy design (Figure 1): one of four doses of FF (25–200mcg once daily PM using a novel single-step DPI; these patients also took placebo twice daily via DiskusTM/AccuhalerTM); FP (twice daily via DiskusTM/AccuhalerTM; these patients also took placebo once daily via the novel DPI); or placebo (every evening via the novel DPI and twice daily via DiskusTM/AccuhalerTM). Patients were assessed at 1, 2, 4, 6 and 8 weeks during treatment.
Endpoints
· Primary: the mean change from baseline in trough (pre-dose, pre-rescue bronchodilator) evening FEV1 at Week 8 in each treatment group. The main treatment comparison was to test for a linear dose response across the four FF doses.
Figure 1. Study design.
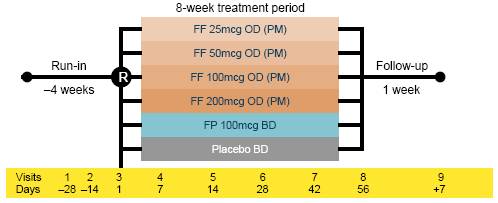
OD = once daily; BD = twice daily
· Secondary: mean change from baseline over the 8-week treatment period in daily trough (pre-dose, pre-rescue bronchodilator) PM PEF, daily AM PEF; % of symptom-free and rescue-free 24h periods; number of withdrawals due to lack of efficacy.
· Safety: incidence of adverse events (AEs) during treatment; evidence of oral candidiasis (visits 1, 3–8); haematology, clinical chemistry and urinalysis parameters before and end of study; 24h urinary cortisol excretion; vital signs (visits 1, 3–8).
RESULTS
· Of 601 patients randomised, 598 received at least one dose of study treatment (intent-to-treat [ITT] population). The demographics and baseline characteristics were similar across groups (Table 1): the patients’ mean age was 37–41 years; over half of them had a >10-year history of asthma, and around 20% had suffered an exacerbation during the last 6 months.
Table 1. Demographics and baseline characteristics (ITT population).
|
|
|
|
|
FF dose |
|
FP dose |
|
||||||
|
|
|
Placebo |
|
25mcg OD |
|
50mcg OD |
|
100mcg OD |
|
200mcg OD |
|
100mcg BD |
|
|
Mean age (years) |
|
39.2 |
|
37.7 |
|
38.3 |
|
36.8 |
|
40.7 |
|
39.9 |
|
|
Gender, n (%) |
|
47 (50) |
|
57 (59) |
|
59 (59) |
|
60 (55) |
|
60 (63) |
|
56 (55) |
|
|
Duration of asthma,
n (%) |
|
16 (17) |
|
19 (19) |
|
20 (20) |
|
26 (24) |
|
22 (23) |
|
19 (19) |
|
|
5 years to <10 years |
|
13 (14) |
|
20 (21) |
|
15 (15) |
|
19 (17) |
|
22 (23) |
|
16 (16) |
|
|
³10 years |
|
65 (69) |
|
58 (60) |
|
65 (65) |
|
65 (59) |
|
51 (54) |
|
67 (66) |
|
|
History of atopy, n (%) |
|
45 (48) |
|
38 (39) |
|
50 (50) |
|
47 (43) |
|
34 (36) |
|
39 (38) |
|
|
FEV1 at screening,
mean |
|
2.320 |
|
2.394 |
|
2.335 |
|
2.279 |
|
2.163 |
|
2.238 |
|
|
Pre-bronchodilator |
|
67.03 |
|
69.69 |
|
69.20 |
|
67.16 |
|
66.56 |
|
67.35 |
|
|
(% predicated) |
|
28.09 |
|
26.43 |
|
29.84 |
|
31.60 |
|
29.32 |
|
29.05 |
|
|
FEV1 at baseline, mean (L) |
|
2.373 |
|
2.456 |
|
2.427 |
|
2.419 |
|
2.210 |
|
2.343 |
|
|
DRC data at
baseline, mean |
|
355.4 |
|
370.8 |
|
364.5 |
|
372.9 |
|
337.3 |
|
347.2 |
|
|
AM PEF (L) |
|
342.7 |
|
359.0 |
|
356.3 |
|
362.2 |
|
326.0 |
|
337.2 |
|
|
Rescue-free 24h periods (%) |
|
10.5 |
|
9.5 |
|
13.1 |
|
15.4 |
|
9.0 |
|
9.8 |
|
|
Symptom-free 24h periods (%) |
|
13.5 |
|
11.0 |
|
9.5 |
|
14.2 |
|
8.3 |
|
9.2 |
|
Efficacy (ITT population)
· At Week 8, all active treatment groups showed a >200mL improvement in trough FEV1 from baseline; the FF 100mcg and 200mcg doses achieved a >200mL difference compared with placebo (p<0.001). FF 50mcg was also significantly better than placebo (129mL; p<0.05). A difference of 101mL was achieved with FF 25mcg, but this was not significantly better than placebo (p=0.095; Figure 2).
· There was a significant dose-response relationship for the baseline–Week 8 change in trough PM FEV1 across FF dose groups (25–200mcg), both with placebo (p<0.001) and without (p=0.03).
· Both AM and PM PEF values (Figure 3) were increased from baseline at Week 8 in all FF treatment groups. There were significantly greater improvements in PEF values versus placebo in all active treatment groups (p=0.041 to p<0.001).
Figure 2. Adjusted treatment differences of change from baseline in trough FEV1 (LOCF) at Week 8 (ITT population).

Note: analysis performed using analysis of covariance (ANCOVA) with covariates of baseline, country, sex, age and treatment
Figure 3. Change from baseline in PM and AM PEF over Weeks 1–8 (ITT population).

· The percentage of symptom-free 24h periods over Weeks 1–8 was significantly higher versus placebo for all treatment groups (p<0.005) except the FF 25mcg group (p=0.128); similarly, the percentage of rescue-free 24h periods over Weeks 1–8 was significantly higher versus placebo for all treatment groups (p<0.031) except the FF 25mcg group (p=0.110) (Figure 4).
· Withdrawal rates due to lack of efficacy were 15% and 11% for placebo and FP, respectively, and ranged from 3–9% for FF dose groups; there was a statistically significant difference for lower withdrawal rates due to lack of efficacy with the 50mcg (p=0.004) and 100mcg (p=0.032) FF dose groups versus placebo (Figure 5).
SAFETY
· FF therapy was generally well tolerated (Table 2). The most common AEs were headache, oropharyngeal pain and nasopharyngitis (Table 2). There was no evidence of a relationship between FF dose and the incidence of any of the most common AEs.
· Incidence of candidiasis was low (0–4%), but occurred at a higher incidence in the 50mcg (4%) and 100mcg (3%) FF once-daily PM dose groups.
Figure 4. Symptom-free and rescue-free 24h periods over Weeks 1–8.

Figure 5. Treatment discontinuation due to lack of efficacy (cumulative incidence).
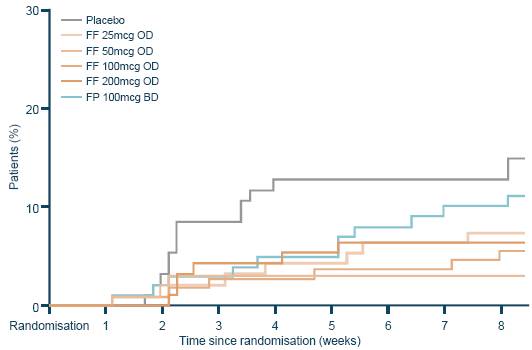
Note: patients are represented from their date of randomisation to their date of withdrawal due to lack of efficacy
· None of the six on-treatment serious AEs reported by four patients (snake bite [FF 25mcg], depression [FF 100mcg], gastritis, chest pain, hyperhidrosis, hypertension [all FP]) were considered to be study drug-related and there were no deaths or hospitalisations associated with AEs.
· There were no statistically significant differences in urinary cortisol excretion between placebo and any of the FF groups or FP (Figure 6) and no events attributable to systemic corticosteroid effects were reported. No clinically important changes in laboratory parameters or vital signs were recorded.
Table 2. Summary of AEs and most common on-treatment AEs (>3% incidence in any treatment group; ITT population).*
|
|
|
|
|
FF dose |
|
FP dose |
|
||||||
|
AE, n (%) |
|
Placebo |
|
25mcg OD |
|
50mcg OD |
|
100mcg OD |
|
200mcg OD |
|
100mcg BD |
|
|
Patients with any on-treatment AE, n (%) |
|
24 (26) |
|
19 (20) |
|
28 (28) |
|
35 (32) |
|
27 (28) |
|
35 (34) |
|
|
Patients with drug-related AE, n (%) |
|
2 (2) |
|
0 |
|
3 (3) |
|
7 (6) |
|
4 (4) |
|
6 (6) |
|
|
Patients with on-treatment SAEs, n (%) |
|
0 |
|
1 (1) |
|
0 |
|
1 (<1) |
|
0 |
|
2 (2) |
|
|
Patients with AEs leading to withdrawal, n (%) |
|
0 |
|
1 (1) |
|
1 (1) |
|
2 (2) |
|
1 (1) |
|
2 (2) |
|
|
Most common on-treatment AEs |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Headache |
|
10 (11) |
|
6 (6) |
|
6 (6) |
|
12 (11) |
|
5 (5) |
|
12 (12) |
|
|
Oropharyngeal pain |
|
1 (1) |
|
0 |
|
1 (1) |
|
4 (4) |
|
3 (3) |
|
2 (2) |
|
|
Nasopharyngitis |
|
1 (1) |
|
0 |
|
0 |
|
4 (4) |
|
3 (3) |
|
2 (2) |
|
|
Sinusitis |
|
1 (1) |
|
2 (2) |
|
0 |
|
0 |
|
2 (2) |
|
3 (3) |
|
|
Upper respiratory |
|
0 |
|
2 (2) |
|
1 (1) |
|
3 (3) |
|
0 |
|
1 (<1) |
|
|
tract infection Insomnia |
|
1 (1) |
|
0 |
|
1 (1) |
|
3 (3) |
|
0 |
|
1 (<1) |
|
|
Back pain |
|
0 |
|
0 |
|
3 (3) |
|
0 |
|
1 (1) |
|
1 (<1) |
|
*Oral candidiasis was diagnosed in 1 (1%), 1 (<1%) and 2 (2%) of patients in the FF 50, 100 and 200mcg OD arms, respectively when coded as ‘oropharyngeal candidiasis’, and in 1 (<1%) patients in the FP 100mcg BD arm. It was diagnosed in 1 (1%) and 2 (2%) of patients in the FF 50 and 100mcg OD arms, respectively when coded as ‘candidiasis’, and in 2 (2%) patients in the FF 50mcg OD arm when coded as ‘oral candidiasis’
Figure 6. Adjusted treatment ratios for 24h urinary cortisol excretion (urinary cortisol population).*

Note: analysis performed using ANCOVA with covariates of country, sex, age, treatment and the log of the baseline values; *The urinary cortisol population consisted of 425 (71%) patients in the ITT population who had urine samples with no factors that would confound the analysis of urinary cortisol
CONCLUSIONS
· A dose-response was observed for trough FEV1 between FF 25–200mcg once daily, including (p<0.001) and excluding (p=0.03) placebo; at Week 8 the 50–200mcg FF doses were all significantly better than placebo with the 100 and 200mcg FF doses resulting in a >200mL difference in FEV1 from baseline compared with placebo.
· Secondary endpoints, including PM and AM PEF and symptom-free and rescue-free 24h periods, further supported the efficacy of FF 50–200mg once-daily doses.
· The incidence of on-treatment AEs was low across the treatment groups and there was no evidence of FF dose impacting on incidence of AEs. 24h urinary cortisol excretion ratios (Week 8/baseline) were similar across treatment groups.
· FF 50–200mcg given once daily in the evening provided effective asthma control and was well tolerated in patients symptomatic on non-steroid asthma therapy. The data support the 100mcg FF once-daily dose as the optimal dose for further evaluation in phase III studies.
REFERENCES
(1) Salter M, et al. Am J Physiol Lung Cell Mol Physiol 2007;293:L660–67.
(2) Cazzola M, et al. Eur Respir J 2009;34:757–69.
(3) National Institutes of Health (NIH) 2007. NIH Publication No. 07-4051.
ACKNOWLEDGEMENTS
· This study was sponsored by GlaxoSmithKline (ClinicalTrials.gov: NCT00603382; protocol number FFA109687).
· Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Anna Ireland at Gardiner-Caldwell Communications and was funded by GlaxoSmithKline.
|
|
|
Presented at the Annual Conference of the European Respiratory Society (ERS), Barcelona, Spain, 18–22 September 2010 |

