Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Viracta Therapeutics, Inc. | d15260dex991.htm |
| 8-K - 8-K - Viracta Therapeutics, Inc. | d15260d8k.htm |
| EX-99.3 - EX-99.3 - Viracta Therapeutics, Inc. | d15260dex993.htm |
| EX-10.4 - EX-10.4 - Viracta Therapeutics, Inc. | d15260dex104.htm |
| EX-10.3 - EX-10.3 - Viracta Therapeutics, Inc. | d15260dex103.htm |
| EX-10.2 - EX-10.2 - Viracta Therapeutics, Inc. | d15260dex102.htm |
| EX-10.1 - EX-10.1 - Viracta Therapeutics, Inc. | d15260dex101.htm |
| EX-3.1 - EX-3.1 - Viracta Therapeutics, Inc. | d15260dex31.htm |
| EX-2.1 - EX-2.1 - Viracta Therapeutics, Inc. | d15260dex21.htm |
Precision Oncology Company Targeting Virus-Associated Cancers November 30, 2020 Exhibit 99.2

This communication contains "forward-looking" statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, without limitation, statements regarding: expected timing, completion and effects of the proposed merger of Sunesis Pharmaceutics, Inc, (“Sunesis”) and Viracta Therapeutics, Inc. (“Viracta”) pursuant to which Viracta will combine with Sunesis in an all-stock transaction (the “Merger”), including listing on Nasdaq Global Market and estimated ownership percentages of the stockholders of each company; closing of Viracta’s financing; Viracta’s clinical development pipeline, including expected timing of the registration trial for EBV-associated lymphomas and the Phase 1b/2 trial in EBV-associated solid tumors; the combined company’s expected cash forecast and runway into 2024; the expected ability of Viracta to undertake certain activities and accomplish certain goals with respect to our clinical program in EBV+ lymphoma or other virus-associated malignancies, the projected timeline of clinical development activities related to our clinical program in EBV+ lymphoma or other virus-associated malignancies, and expectations regarding future therapeutic and commercial potential with respect to our clinical program in EBV+ lymphoma or other virus-associated malignancies; and other statements that are not historical facts. Sunesis’ expectations and beliefs regarding these matters may not materialize. Sunesis' actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation, risks relating to the ability of the parties to consummate the proposed Merger, satisfaction of closing conditions precedent to the consummation of the proposed Merger, potential delays in consummating the Merger, and the ability of Sunesis to timely and successfully achieve the anticipated benefits of the Merger. Risks and uncertainties related to Viracta that may cause actual results to differ materially from those expressed or implied in any forward-looking statement include, but are not limited to: risks relating to the ability of the parties to consummate the proposed Merger and the concurrent financing, satisfaction of closing conditions precedent to the consummation of the proposed Merger and the concurrent financing, potential delays in consummating the Merger and the concurrent financing, and the ability of Viracta to timely and successfully achieve the anticipated benefits of the Merger and the concurrent financing; Viracta’s ability to successfully enroll patients in and complete its ongoing and planned clinical trials; Viracta's plans to develop and commercialize its product candidates, including all oral combinations of nanatinostat and valganciclovir; the timing of initiation of Viracta's planned clinical trials; the timing of the availability of data from Viracta's clinical trials; previous preclinical and clinical results may not be predictive of future clinical results; the timing of any planned investigational new drug application or new drug application; Viracta's plans to research, develop and commercialize its current and future product candidates; the clinical utility, potential benefits and market acceptance of Viracta's product candidates; Viracta's ability to identify additional products or product candidates with significant commercial potential; developments and projections relating to Viracta's competitors and its industry; the impact of government laws and regulations; Viracta's ability to protect its intellectual property position; and Viracta's estimates regarding future expenses, capital requirements and need for additional financing following the proposed transaction. These risks and uncertainties may be amplified by the COVID-19 pandemic, which has caused significant economic uncertainty. If any of these risks materialize or underlying assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements. Additional risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included under the caption “Risk Factors” and elsewhere in Sunesis’ most recent filings with the SEC, including Sunesis’ Quarterly Report on Form 10-Q for the quarter ended September 30, 2020 and any subsequent reports on Form 10-K, Form 10-Q or Form 8-K filed with the SEC from time to time and available at www.sec.gov. These documents can be accessed on Sunesis’ Investor Relations page at https://ir.sunesis.com/shareholder-services/contact-ir by clicking on the link titled “SEC Filings.” The forward-looking statements included in this communication are made only as of the date hereof. Neither Sunesis nor Viracta assumes no obligation and does not intend to update these forward-looking statements, except as required by law or applicable regulation. Safe Harbor Statement

Additional Information on the Proposed Transaction Additional Information and Where to Find It Sunesis plans to file with the SEC, and the parties plan to furnish to the security holders of Viracta and Sunesis, a Registration Statement on Form S-4, which will constitute a prospectus of Sunesis and will include an information statement of Viracta, in connection with the proposed Merger, whereupon the separate corporate existence of Merger Sub shall cease and Viracta shall continue as the surviving corporation of the Merger as a wholly owned subsidiary of Sunesis. The prospectus/information statement described above will contain important information about Sunesis, Viracta, the proposed Merger and related matters. Investors and security holders are urged to read the prospectus/information statement carefully when it becomes available. Investors and security holders will be able to obtain free copies of these documents, and other documents filed with the SEC, by Sunesis through the website maintained by the SEC at www.sec.gov. In addition, investors and security holders will be able to obtain free copies of these documents from Sunesis by contacting Sunesis’ Investor Relations by telephone at 650-266-3784 or by going to Sunesis’ Investor Relations web page at https://ir.sunesis.com/shareholder-services/contact-ir and clicking on the link titled “SEC Filings.” Participants in the Solicitation The respective directors and executive officers of Sunesis and Viracta may be deemed to be participants in the solicitation of proxies and written consents from the security holders of Sunesis and Viracta, respectively, in connection with the proposed Merger. Information regarding the interests of these directors and executive officers in the transaction described herein will be included in the prospectus/information statement described above. Additional information regarding Sunesis’ directors and executive officers is included in Sunesis’ proxy statement for its Annual Meeting of Stockholders, which was filed with the SEC on April 17, 2020. This document is available from Sunesis free of charge as described in the preceding paragraph. No Offer or Solicitation This communication shall not constitute an offer to sell or the solicitation of an offer to buy any securities or a solicitation of any vote or approval, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended.

Viracta Therapeutics – Sunesis Pharmaceuticals Merger Viracta Therapeutics and Sunesis Pharmaceuticals agree to merge in an all-stock transaction and the combined company is expected to trade on Nasdaq under new symbol VIRX Viracta shareholders are expected to own approximately 86% of the combined company Sunesis shareholders are expected to own approximately 14% of the combined company Post-merger Board of Directors expected to include 6 board members from Viracta and 1 from Sunesis The transaction has been approved by the Board of Directors of both companies and is subject to approval of Sunesis stockholders and Viracta stockholders The transaction is expected to close in 1Q21 +
Viracta: Unique Convergence of Disciplines in Company to Treat Virus-Associated Malignancies Oncology Virology Immunology Epigenetics Viracta recently completed a $40 million Series E Preferred Stock equity financing, led by aMoon Concurrent with the execution of the merger agreement, Viracta entered to a $65 million private placement, led by BVF Funding of the private placement is expected to occur immediately prior to the closing of the merger The combined company is expected to have approximately $120 million in cash following the close of the merger Projected cash runway into 2024
Leadership Team Ivor Royston, MD President and Chief Executive Officer Hybritech Douglas Faller, MD, PhD Chief Scientific Officer Xiaohu Deng, PhD Senior VP, Product Development Lisa Rojkjaer, MD Chief Medical Officer Robert McRae VP, Operations & Strategic Alliances Daniel Chevallard, CPA Chief Financial Officer Mark McCamish, MD, PhD Strategic Advisor
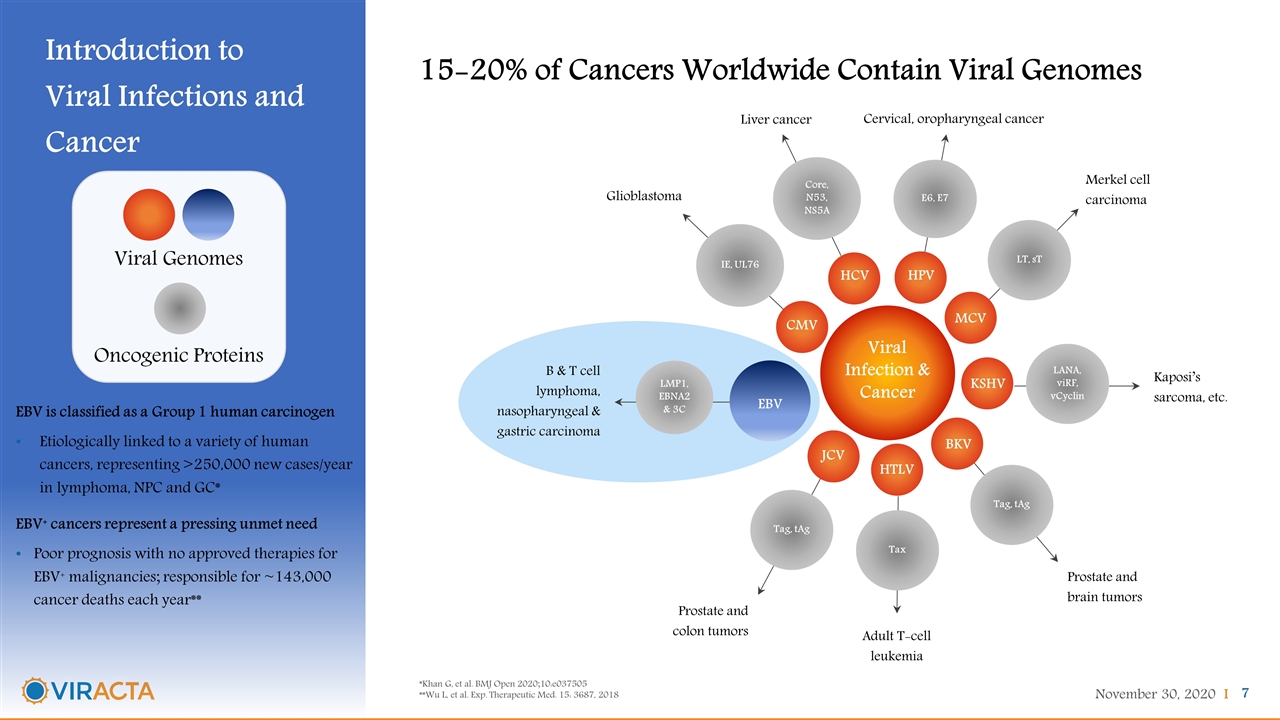
Introduction to Viral Infections and Cancer 15-20% of Cancers Worldwide Contain Viral Genomes Viral Genomes Oncogenic Proteins Viral Infection & Cancer HPV HCV Liver cancer Cervical, oropharyngeal cancer E6, E7 Core, N53, NS5A MCV Merkel cell carcinoma LT, sT KSHV Kaposi’s sarcoma, etc. LANA, viRF, vCyclin HTLV Adult T-cell leukemia Tax BKV Prostate and brain tumors Tag, tAg JCV Prostate and colon tumors Tag, tAg B & T cell lymphoma, nasopharyngeal & gastric carcinoma LMP1, EBNA2& 3C EBV CMV IE, UL76 Glioblastoma EBV is classified as a Group 1 human carcinogen Etiologically linked to a variety of human cancers, representing >250,000 new cases/year in lymphoma, NPC and GC* EBV+ cancers represent a pressing unmet need Poor prognosis with no approved therapies for EBV+ malignancies; responsible for ~143,000 cancer deaths each year** *Khan G, et al. BMJ Open 2020;10:e037505 **Wu L, et al. Exp. Therapeutic Med. 15: 3687, 2018
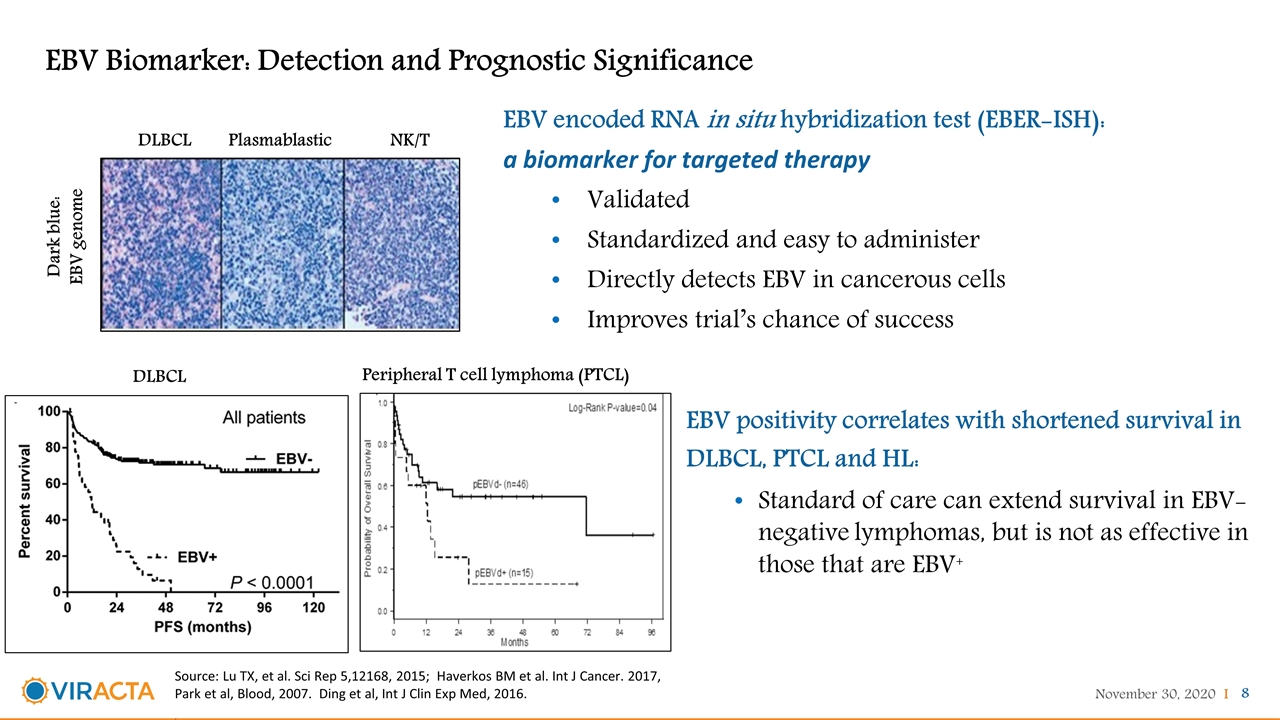
EBV Biomarker: Detection and Prognostic Significance Dark blue: EBV genome DLBCL Plasmablastic NK/T Source: Lu TX, et al. Sci Rep 5,12168, 2015; Haverkos BM et al. Int J Cancer. 2017, Park et al, Blood, 2007. Ding et al, Int J Clin Exp Med, 2016. . Peripheral T cell lymphoma (PTCL) DLBCL EBV positivity correlates with shortened survival in DLBCL, PTCL and HL: Standard of care can extend survival in EBV-negative lymphomas, but is not as effective in those that are EBV+ EBV encoded RNA in situ hybridization test (EBER-ISH): a biomarker for targeted therapy Validated Standardized and easy to administer Directly detects EBV in cancerous cells Improves trial’s chance of success
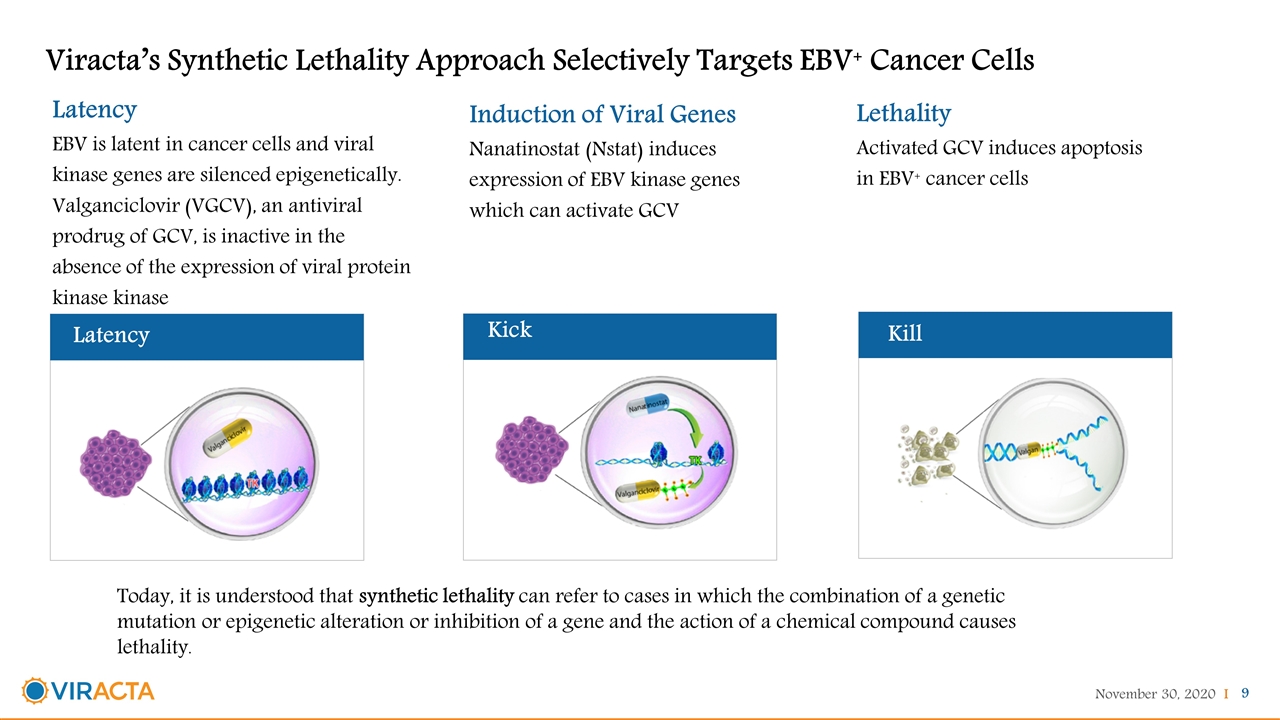
Viracta’s Synthetic Lethality Approach Selectively Targets EBV+ Cancer Cells Latency EBV is latent in cancer cells and viral kinase genes are silenced epigenetically. Valganciclovir (VGCV), an antiviral prodrug of GCV, is inactive in the absence of the expression of viral protein kinase kinase Latency Induction of Viral Genes Nanatinostat (Nstat) induces expression of EBV kinase genes which can activate GCV Kick Lethality Activated GCV induces apoptosis in EBV+ cancer cells Kill Today, it is understood that synthetic lethality can refer to cases in which the combination of a genetic mutation or epigenetic alteration or inhibition of a gene and the action of a chemical compound causes lethality.
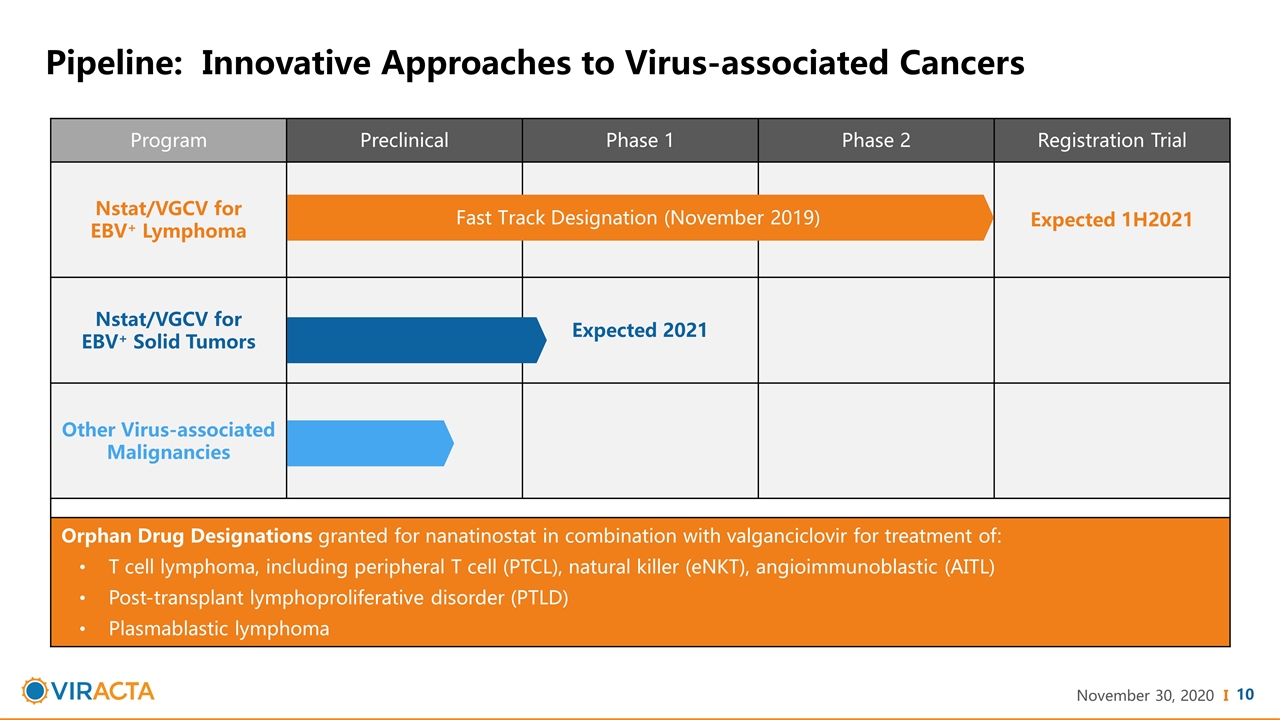
Program Preclinical Phase 1 Phase 2 Registration Trial Nstat/VGCV for EBV+ Lymphoma Expected 1H2021 Nstat/VGCV for EBV+ Solid Tumors Expected 2021 Other Virus-associated Malignancies Orphan Drug Designations granted for nanatinostat in combination with valganciclovir for treatment of: T cell lymphoma, including peripheral T cell (PTCL), natural killer (eNKT), angioimmunoblastic (AITL) Post-transplant lymphoproliferative disorder (PTLD) Plasmablastic lymphoma Pipeline: Innovative Approaches to Virus-associated Cancers Fast Track Designation (November 2019)

Viracta: Well-positioned to advance its novel oral therapy for EBV+ tumors EBV+ cancers: Orphan diseases with high unmet medical need, poor prognosis and no approved therapies Viracta’s approach: Novel biomarker-directed therapy with oral delivery - targets EBV+ tumor cells by “Synthetic Lethality” Lead program: Relapsed or refractory (R/R) EBV+ lymphoma Compelling Phase 1b data presented in oral session at ASH Meeting 2019 Updated Ph1b/2 data included in ASH 2020 abstract; accepted for poster presentation on December 5, 2020 Fast Track designation and multiple orphan drug designations granted for EBV+ lymphoma program Recent End of Phase 2 Type B FDA Meeting in November 2020; alignment with FDA on path to registration Registration trial in EBV+ lymphomas anticipated to start in 1H’2021 Pipeline expansion: EBV+ solid tumor Phase 1b trial planned for 2021, including nasopharyngeal (NPC) and gastric cancer (GC) IP estate: Issued patents and filed patent applications offering IP protection to 2040+ Transaction with Sunesis: Nasdaq-listed company expected to have approximately $120M cash at closing of the merger *Porcu P, et al. Blood 2019; 134 (Suppl 1): 465
