Attached files
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Year Ended December 31, 2009
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number 000-51531
SUNESIS PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
94-3295878 (I.R.S. Employer Identification Number) |
395 Oyster Point Boulevard, Suite 400
South San Francisco, California 94080
(Address of principal executive offices, including zip code)
(650) 266-3500
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class: |
Name of Each Exchange on Which Registered: | |
| Common Stock, par value $0.0001 per share | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark whether the registrant is a large accelerated filer, accelerated filer, a non-accelerated filer or a smaller reporting company. See definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ¨ | Accelerated filer ¨ | Non-accelerated filer ¨ (Do not check if a smaller reporting |
Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Exchange Act Rule 12b-2.) Yes ¨ No x
The aggregate market value of common stock held by non-affiliates of the registrant, based on the closing sales price for such stock on June 30, 2009, as reported by The Nasdaq Stock Market, was $11,301,666. The calculation of the aggregate market value of voting and non-voting stock excludes 6,854,138 shares of the registrant’s common stock held by current executive officers, directors and stockholders that the registrant has concluded are affiliates of the registrant. Exclusion of such shares should not be construed to indicate that any such person possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the registrant or that such person is controlled by or under common control with the registrant.
The total number of shares outstanding of the registrant’s common stock, $0.0001 par value per share, as of March 31, 2010, was 57,981,195.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Definitive Proxy Statement, to be filed with the Securities and Exchange Commission pursuant to Regulation 14A in connection with the 2010 Annual Meeting of Stockholders of Sunesis Pharmaceuticals, Inc. (hereinafter referred to as “Proxy Statement”) are incorporated by reference in Part III of this report. Such Proxy Statement will be filed with the Securities and Exchange Commission not later than 120 days after the conclusion of the registrant’s fiscal year ended December 31, 2009.
Table of Contents
| Page No. | ||||
| PART I | ||||
| ITEM 1. |
3 | |||
| ITEM 1A. |
17 | |||
| ITEM 1B. |
34 | |||
| ITEM 2. |
34 | |||
| ITEM 3. |
35 | |||
| ITEM 4. |
35 | |||
| PART II | ||||
| ITEM 5. |
35 | |||
| ITEM 6. |
37 | |||
| ITEM 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
39 | ||
| ITEM 7A. |
51 | |||
| ITEM 8. |
51 | |||
| ITEM 9. |
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
77 | ||
| ITEM 9A(T). |
77 | |||
| ITEM 9B. |
78 | |||
| PART III | ||||
| ITEM 10. |
79 | |||
| ITEM 11. |
79 | |||
| ITEM 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
79 | ||
| ITEM 13. |
Certain Relationships and Related Transactions, and Director Independence |
80 | ||
| ITEM 14. |
80 | |||
| PART IV | ||||
| ITEM 15. |
81 | |||
| 82 | ||||
| 83 | ||||
Table of Contents
PART I
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This report, including the information we incorporate by reference, contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that involve risks, uncertainties and assumptions. All statements, other than statements of historical facts, are “forward-looking statements” for purposes of these provisions, including without limitation any statements relating to the completion of any financing transaction or the satisfaction of closing conditions relating to any financing, any projections of revenue, expenses or other financial items, any statement of the plans and objectives of management for future operations, any statements concerning proposed clinical trials, regulatory activities or licensing or collaborative arrangements, any statements regarding future economic conditions or performance, and any statement of assumptions underlying any of the foregoing. In some cases, forward-looking statements can be identified by the use of terminology such as “anticipates,” “believe,” “continue,” “estimates,” “expects,” “intend,” “look forward,” “may,” “could,” “seeks,” “plans,” “potential,” or “will” or the negative thereof or other comparable terminology. Although we believe that the expectations reflected in the forward-looking statements contained herein are reasonable, there can be no assurance that such expectations or any of the forward-looking statements will prove to be correct, and actual results could differ materially from those projected or assumed in the forward-looking statements. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors, including but not limited to those set forth under “Risk Factors,” and elsewhere in this report. We urge you not to place undue reliance on these forward-looking statements, which speak only as of the date of this report. All forward-looking statements included in this report are based on information available to us on the date of this report, and we assume no obligation to update any forward-looking statements contained in this report.
In this report, “Sunesis,” the “Company,” “we,” “us,” and “our” refer to Sunesis Pharmaceuticals, Inc. and its wholly owned subsidiary, Sunesis Europe Limited, except where it is made clear that the term refers only to the parent company.
| ITEM 1. | BUSINESS |
General
We are a biopharmaceutical company focused on the development and commercialization of new oncology therapeutics for the treatment of hematologic and solid tumor cancers. Our efforts are currently focused primarily on the development of voreloxin for the treatment of acute myeloid leukemia, or AML. We have built a highly experienced cancer drug development organization committed to advancing our lead product candidate, voreloxin, in multiple indications to improve the lives of people with cancer.
We own worldwide development and commercialization rights to voreloxin and are currently preparing for anticipated Phase 3 development of the compound. Voreloxin is a first-in-class anti-cancer quinolone derivative, or AQD—a class of compounds that has not been used previously for the treatment of cancer. Quinolone derivatives have been shown to mediate anti-tumor activity by targeting mammalian topoisomerase II, an enzyme critical for cell replication, and have demonstrated promising preclinical anti-tumor activity.
We are currently completing three clinical trials of voreloxin: (i) a Phase 2 clinical trial (known as the REVEAL-1 trial) in previously untreated elderly patients with AML, for which enrollment was completed in October 2009, with a total of 113 patients dosed in one of three dosing schedules, (ii) a Phase 1b/2 clinical trial of voreloxin in combination with cytarabine for the treatment of patients with relapsed/refractory AML, for which enrollment was completed in January 2010, with a total of 108 patients dosed, and (iii) a Phase 2 single agent clinical trial in platinum-resistant ovarian cancer patients, for which enrollment was completed in
3
Table of Contents
December 2008, with a total of 137 patients dosed across one of three dosing schedules. In November 2009, we announced that the U.S. Food and Drug Administration, or FDA, had granted voreloxin orphan drug designation for the treatment of AML. In February 2010, we announced that we received formal guidance from the FDA from End-of-Phase 2 meetings regarding further development of voreloxin for AML. Based on this guidance, we will look to conduct a randomized, double-blind, placebo-controlled, pivotal trial evaluating the effect on overall survival of voreloxin in combination with cytarabine, a widely used chemotherapy in AML, compared to placebo with cytarabine, in patients with relapsed or refractory AML. We anticipate initiating this multi-national Phase 3 trial in the second half of 2010. Management is currently in the process of evaluating alternatives for funding the voreloxin development program.
The most recent data from our two Phase 2 trials of voreloxin in AML were presented at the 51st Annual Meeting of the American Society of Hematology (ASH) in December 2009. The most recent data from the Phase 2 trial of voreloxin in platinum-resistant ovarian cancer were presented at the American Society of Clinical Oncology (ASCO) 2009 Annual Meeting in June 2009. We believe the data from these three ongoing clinical trials demonstrate that voreloxin shows promising safety and efficacy in AML and in platinum-resistant ovarian cancer.
From our incorporation in 1998 through 2001, our operations consisted primarily of developing and refining our proprietary methods of discovering drugs in pieces, or fragments. From 2002 through June 2008, we focused on the discovery, in-licensing and development of novel small molecule drugs. In June 2008, we announced a corporate realignment to focus on the development of voreloxin. In conjunction with this strategic restructuring, or the 2008 Restructuring, we expanded our late-stage development team, announced the winding down of our internal discovery research activities, ceasing development of an enhanced fragment-based discovery platform, and reduced our workforce by approximately 60%.
We have also taken a number of other important steps to focus our resources and efforts on the advancement of voreloxin:
| • | We discontinued development of our product candidate, SNS-032, a selective inhibitor of cyclin-dependent kinases, or CDKs, 2, 7 and 9, which we had in-licensed from Bristol-Myers Squibb Company, or BMS. In March 2009, the license agreement was terminated and SNS-032 was returned to BMS. |
| • | In the first quarter of 2009, we completed a Phase 1 trial of SNS-314, a potent and selective pan-Aurora kinase inhibitor discovered internally at Sunesis, in patients with advanced solid tumors. As a maximum tolerated dose was not established in the trial and no responses were observed, further development of SNS-314 was suspended. |
| • | In March 2009, we announced the sale of our interest in all of our lymphocyte function-associated antigen-1, or LFA-1, patents and related know-how to SARcode Corporation, or SARcode, for total cash consideration of $2.0 million, which was recorded as revenue in the second quarter of 2009. In connection with the sale, the license agreement was terminated. SARcode had been the exclusive licensee of those assets since March 2006. |
| • | In February 2010, we granted Carmot Therapeutics, Inc. an exclusive license to our proprietary fragment-based lead discovery technology. We retain full rights to the technology for use in our future internal discovery efforts. |
In July 2009, we received a milestone of $1.5 million pursuant to a collaboration entered into with Biogen Idec in 2002 for Biogen Idec’s selection of a Raf kinase inhibitor development candidate for the treatment of cancer, which was earned and recorded as revenue in the second quarter. Biogen Idec is currently conducting IND-enabling preclinical work with the Raf kinase development candidate.
4
Table of Contents
On March 31, 2009, we entered into a securities purchase agreement with accredited investors, including certain members of management, providing for a private placement of up to $43.5 million, or the Private Placement. We completed the initial closing of $10.0 million of the Private Placement on April 3, 2009, and the second closing of $5.0 million on October 30, 2009. In the initial closing, $10.0 million of units were sold, resulting in net proceeds of $8.8 million, and in the second closing, $5.0 million of units were sold, resulting in net proceeds of $4.7 million. The units consist of Series A convertible preferred stock and warrants to purchase common stock. The Private Placement also contemplates the sale of up to the remaining $28.5 million in common stock at $0.275 per share to the same group of investors, subject to certain terms and conditions described in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” below.
On January 20, 2010, we entered into a controlled equity offering sales agreement with Cantor Fitzgerald & Co., or Cantor, pursuant to which we could issue and sell shares of our common stock having an aggregate offering price of up to $15.0 million from time to time through Cantor acting as agent and/or principal. Sales of our common stock through Cantor, if any, would be made on the NASDAQ Capital Market by means of ordinary brokers’ transactions at market prices, in block transactions or as otherwise agreed by Cantor and us. Cantor agreed to use its best efforts to sell our common stock from time to time, based upon our instructions (including any price, time or size limits or other customary parameters or conditions we might impose). We agreed to pay Cantor a commission rate ranging between 3.0% and 5.0% of the gross sales price per share of any common stock sold through Cantor as agent under the sales agreement. We also agreed to reimburse Cantor for certain expenses incurred in connection with entering into the sales agreement and provided Cantor with customary indemnification rights. Under the terms of the sales agreement, we may also sell shares of our common stock to Cantor, as principal for its own account, at a price negotiated at the time of sale. If we sell shares to Cantor in this manner, we will enter into a separate agreement setting forth the terms of any such transactions. As of March 31, 2010, the full $15.0 million available under the facility had been sold, for net proceeds of $14.2 million after commissions and expenses.
We have incurred significant losses in each year since our inception. As of December 31, 2009, we had an accumulated deficit of $356.4 million. We expect to continue to incur significant net losses for the foreseeable future, as we continue the development of, and seek regulatory approvals for, voreloxin. We believe that currently available cash, cash equivalents and marketable securities, including the net proceeds of $14.2 million from sales of common stock through March 31, 2010 under the agreement with Cantor, are sufficient to fund our operations through at least September 30, 2010. We will need to raise substantial additional funding in the near term in order to sustain operations beyond that date and before undertaking any additional clinical trials of voreloxin. Our significant negative cash flows and lack of financial resources raise substantial doubt as to our ability to continue as a going concern. Management is currently in the process of evaluating alternative funding sources. If we are unable to raise additional funding to meet our working capital needs, we will be forced to delay or reduce the scope of our voreloxin development program and/or limit or cease our operations.
On August 3, 2009, upon NASDAQ’s approval, the listing of our common stock was transferred from The NASDAQ Global Market to The NASDAQ Capital Market.
Voreloxin
Voreloxin is a first-in-class AQD—a class of compounds that has not been used previously for the treatment of cancer. Quinolone derivatives have been shown to mediate anti-tumor activity by targeting mammalian topoisomerase II, an enzyme critical for cell replication, and have demonstrated promising preclinical anti-tumor activity. Voreloxin acts by DNA intercalation and inhibition of topoisomerase II in replicating cancer cells. The resulting site-selective DNA damage rapidly causes the cancer cells to stop dividing and die. In preclinical studies, voreloxin demonstrates broad anti-tumor activity and appears to exhibit additive or synergistic activity when combined with several therapeutic agents currently used in the treatment of cancer. Clinical activity is observed in both solid and hematologic malignancies. We licensed worldwide development and commercialization rights to voreloxin from Dainippon Sumitomo Pharma Co., Ltd. in 2003.
5
Table of Contents
The following chart summarizes the status of the clinical trials that have been conducted or that we are currently conducting with voreloxin:
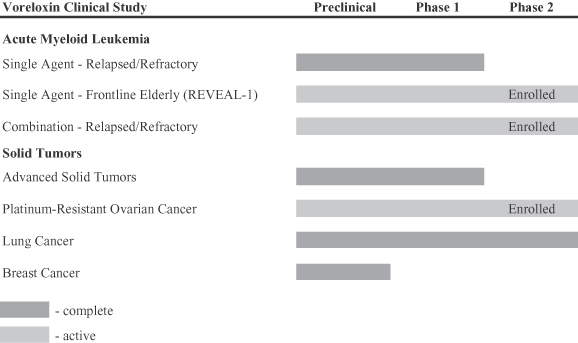
Since 2004, we have initiated eight clinical trials with voreloxin. Two Phase 1 clinical trials were conducted to evaluate doses and schedules of administration of voreloxin in patients with advanced solid tumors. We also conducted Phase 2 studies in non-small cell lung cancer and small cell lung cancer. Partial responses were observed in both lung cancer studies, but it was determined that voreloxin could be dosed with greater intensity given the low incidence of grade 3/4 neutropenia (15% or less). Thus, the studies were halted and we may consider future voreloxin studies in lung cancer either as a single agent at higher doses or in combination with other anti-cancer agents.
In January 2010, we completed enrollment of a Phase 1b/2 clinical trial of voreloxin in combination with cytarabine for the treatment of patients with relapsed/refractory AML The trial is designed to evaluate the safety, pharmacokinetics and anti-leukemic activity of escalating doses of voreloxin when administered in combination with cytarabine given either as continuous infusion or as a two hour IV infusion. A total of 66 patients have been treated in the expansion Phase 2 populations of the trial, which includes primary refractory and first relapse AML patients. Of these, 64 patients were evaluable for efficacy outcomes. Among evaluable first relapse (n=36) and primary refractory patients (n=28), preliminary median overall survival is 7.8 months and the remission rate is 31% (complete remission, or CR, is 27%, complete remission without full platelet recovery, or CRp, is 2%, and complete remission with incomplete recovery, or CRi, is 2%). Voreloxin in combination with either bolus or continuous infusion cytarabine was generally well-tolerated. Infection-related toxicities were the most common Grade 3 or higher non-hematologic adverse events. In addition, Grade 3 or higher oral mucositis was observed. All-cause mortality among these patients was 2% at 30 days and 8% at 60 days.
In October 2009, we completed enrollment in a Phase 2 single agent clinical trial of voreloxin in previously untreated elderly AML patients. The trial includes three dosing schedules: Schedule A, once weekly for three weeks (n=29); Schedule B, once weekly for two weeks (n=35); and Schedule C, on days one and four at either 72 mg/m2 (n=29) or 90 mg/m2 (n=20). Median survival was 8.7 months in Schedule A, 5.8 months in Schedule B, and 7.3 months (preliminary) in Schedule C (72 mg/m2 on days one and four). Median duration of remission was 10.7 months and one year survival was 38% for Schedule A. For the other schedules, median
6
Table of Contents
duration of remission has not been reached and one year survival is too early to evaluate. Patients age 75 or older (n=49) with at least 1 additional risk factor at diagnosis, a population identified by the National Comprehensive Cancer Network (2010) AML Guidelines as having poor outcome to standard treatment, experienced a CR rate of 30% and a 30-day all-cause mortality of 5%. Survival in these patients was too early to evaluate. Based on trial results, Schedule C has been determined to be the recommended pivotal dose regimen. For Schedule C, response rates (CR and CRp) are 38%; 30- and 60-day all-cause mortality are 7% and 17%, with improved tolerability over Schedule A.
In December 2008, we completed enrollment in a Phase 2 single agent trial of voreloxin in platinum-resistant ovarian cancer. Three dose cohorts of voreloxin were studied: 48 mg/m2 given every three weeks (n=65), 60 mg/m2 given every four weeks (n=37) and 75 mg/m2 given every four weeks (n=35). Data from this trial show encouraging durable anti-tumor activity across all three dose cohorts. The overall response rate, or ORR, was 11% for each of the three dosing cohorts. A total of 74 patients (52%) experienced disease control, defined as an objective response or stable disease for 12 weeks or more. The median progression free survival, or PFS, for cohort A was 82 days. The preliminary median PFS for cohorts B and C is 84 days and 109 days, respectively. Overall PFS was longer in the 60 and 75 mg/m2 cohorts vs. 48 mg/m2, suggesting a benefit to higher voreloxin doses. Four partial responses were achieved in the 44 women who were Doxil® failures for an ORR of 9% and 28, or 64%, achieved disease control. The preliminary median PFS in these Doxil® failure patients is 90 days. PFS was not statistically different from those who had not failed Doxil®. Overall, the adverse event profile was similar across cohorts and voreloxin was generally well-tolerated. Grade 3 or higher adverse events occurring in more than 10% of patients include neutropenia febrile neutropenia, and anemia.
Licensing Agreements
Dainippon Sumitomo Pharma Co., Ltd.
In October 2003, we entered into an agreement with Dainippon Sumitomo Pharma Co., Ltd., or Dainippon, to acquire exclusive worldwide development and marketing rights for our lead anti-cancer product candidate, voreloxin. We may in the future make a series of milestone payments of up to $7.5 million to Dainippon for starting Phase 3 clinical testing, for filing new drug applications, or NDAs, and for receiving regulatory approval in the United States, Europe and Japan for cancer treatment. If voreloxin is approved for a non-cancer indication, additional milestone payments become payable to Dainippon.
The agreement also provides for royalty payments to Dainippon at rates that are based on total annual net sales. Under the agreement, we may reduce our royalty payments to Dainippon if a third party markets a competitive product and we must pay royalties for third-party intellectual property rights necessary to commercialize voreloxin. Royalty obligations under the agreement continue on a country-by-country and product-by-product basis until the later of the date on which no valid patent claims relating to a product exist or 10 years from the date of the first sale of the product.
If we discontinue seeking regulatory approval and/or the sale of the product in a region, we are required to return to Dainippon its rights to the product in that region. The agreement may be terminated by either party for the other party’s uncured breach or bankruptcy.
Strategic Collaborations
Overview
Over the past three years, we generated revenue primarily through payments received in connection with our collaborations with Biogen Idec, Inc., Johnson & Johnson Pharmaceutical Research & Development LLC, or J&JPRD, and Merck & Co., Inc., or Merck, consisting principally of research funding and milestones paid by our collaborators, substantially offsetting our related research and development expenses. As of March 31, 2010, our
7
Table of Contents
only remaining ongoing collaboration is with Biogen Idec. Our collaboration with Merck will terminate effective as of June 8, 2010 and our collaboration with J&JPRD terminated on January 13, 2010.
Biogen Idec
In August 2004, we entered into a collaboration agreement with Biogen Idec, Inc., or Biogen Idec, to discover, develop and commercialize small molecule inhibitors of Raf kinase and up to five additional targets that play a role in oncology and immunology indications or in the regulation of the human immune system. Concurrent with the signing of the agreement, Biogen Idec paid a $7.0 million upfront technology access fee and made a $14.0 million equity investment in the company through the purchase of our Series C-2 preferred stock, which converted into common stock upon our initial public offering in September 2005. Biogen Idec’s equity ownership was 8.1% of our common shares outstanding as of December 31, 2009.
Pursuant to the terms of the collaboration agreement, we applied our proprietary fragment-based drug discovery technology, Tethering, to generate small molecule leads during the research term, for which we received research funding, which was paid in advance to support some of our scientific personnel. In connection with our 2008 Restructuring, the parties agreed to terminate the research term and related funding as of June 30, 2008. A total of $20.0 million of research funding was received through this date. We have received a total of $3.0 million in milestone payments for meeting certain preclinical milestones through December 31, 2009, including a $1.5 million milestone received in cash in July 2009 for Biogen Idec’s selection of a Raf kinase inhibitor development candidate for the treatment of cancer, which was recorded as revenue in June 2009, and a $0.5 million milestone received in cash and recorded as revenue in June 2008.
We may in the future receive pre-commercialization milestone payments of up to $60.5 million per target, as well as royalty payments depending on product sales. Royalty payments may be increased if we exercise our option to co-develop and co-promote product candidates for up to two targets worldwide (excluding Japan) and may be reduced if Biogen Idec is required to in-license additional intellectual property related to certain technology jointly developed under the collaboration agreement in order to commercialize a collaboration product. However, we do not expect to generate any royalty revenue from this collaboration in the foreseeable future, if at all.
Manufacturing
We do not have internal manufacturing capabilities and outsource the manufacture of the voreloxin active pharmaceutical ingredient, or API, and the finished drug product incorporating the API, or FDP, to third-party contract manufacturers. The voreloxin API is currently manufactured by one of two suppliers with whom we have an established relationship, through a multi-step convergent synthesis in which two intermediates are manufactured in a parallel process and then combined and de-protected in the final two steps. The API is then formulated and vials are filled and finished by one of two FDP suppliers with whom we have an established relationship. The API is classified as a toxic substance, and the number of suppliers qualified to manufacture it or the FDP is limited.
To date, voreloxin has been manufactured in sufficient quantities for our preclinical studies and clinical trials. New lots of FDP will need to be manufactured and released as required to support our current and planned clinical activities. Prior to being approved for commercial sale, we may need to manufacture API and FDP in larger quantities. Scale-up of manufacturing will be accompanied by validation studies, which will be reviewed by the FDA prior to approval. If we are unable to successfully increase the manufacturing capacity for voreloxin, the regulatory approval or commercial launch may be delayed or there may be a shortage in commercial supply.
Competition
We face significant competition from many pharmaceutical, biopharmaceutical and biotechnology companies that are researching, developing and marketing products designed to address the treatment of cancer,
8
Table of Contents
including AML and ovarian cancer. Many of our competitors have significantly greater financial, manufacturing, marketing and drug-development resources than we do. Large pharmaceutical companies in particular have extensive experience in the clinical testing of, obtaining regulatory approvals for, and marketing drugs.
Voreloxin is currently being clinically tested as a treatment for AML and platinum-resistant ovarian cancer. Some of the current key competitors to voreloxin in AML include Genzyme Corporation’s clofarabine, Eisai Corporation’s decitabine, Celgene Corporation’s azacitidine and Vion Pharmaceuticals, Inc.’s laromustine, any of which could change the treatment paradigm for acute leukemia. Each of these compounds is further along in clinical development than voreloxin. Liposomal doxorubicin and topotecan are current standards of care in platinum-resistant ovarian cancer patients, and we are aware that several of our competitors have initiated Phase 3 clinical trials for this indication.
We believe that our ability to successfully compete in the marketplace with voreloxin and any future product candidates, if any, will depend on, among other things:
| • | our ability to develop novel compounds with attractive pharmaceutical properties and to secure, protect and maintain intellectual property rights based on our innovations; |
| • | the efficacy, safety and reliability of our product candidates; |
| • | the speed at which we develop our product candidates; |
| • | our ability to design and successfully execute appropriate clinical trials; |
| • | our ability to maintain a good relationship with regulatory authorities; |
| • | our ability to obtain, and the timing and scope of, regulatory approvals; |
| • | our ability to manufacture and sell commercial quantities of future products to the market; and |
| • | acceptance of future products by physicians and other healthcare providers. |
Intellectual Property
We believe that patent protection is crucial to our business and that our future success depends in part on our ability to obtain patents protecting voreloxin or future drug candidates, if any. We have an exclusive license to 44 issued composition-of-matter patents that cover the voreloxin drug substance. The U.S. composition-of-matter patent is due to expire in October 2015 and most of its foreign counterparts are due to expire in June 2015. As of December 31, 2009, approximately 64 U.S. and foreign applications pertaining to voreloxin life cycle development were pending. When appropriate, we intend to seek patent term restoration, orphan drug status and/or data exclusivity in the United States and their equivalents in other relevant jurisdictions, to the maximum extent that the respective laws will permit at such time. In November 2009, we announced that the FDA had granted voreloxin orphan drug designation for the treatment of AML.
Historically we have patented a wide range of technology, inventions and improvements related to our business, but which we are no longer actively developing. As of December 31, 2009, we owned, co-owned or licensed rights to approximately 40 issued U.S. and foreign patents and approximately 104 pending U.S. and foreign patent applications relating to such intellectual property.
Our ability to build and maintain our proprietary position for voreloxin and any future drug candidates, if any, will depend on our success in obtaining effective claims and enforcing those claims if granted. The patent positions of biopharmaceutical companies like ours are generally uncertain and involve complex legal and factual
9
Table of Contents
questions for which some important legal principles remain unresolved. No consistent policy regarding the breadth of patent claims has emerged to date in the United States. The patent situation outside the United States is even more uncertain. We do not know whether any of our patent applications or those patent applications that we license will result in the issuance of any patents. Even if patents are issued, they may not be sufficient to protect voreloxin or future drug candidates, if any. The patents we own or license and those that may issue in the future may be challenged, invalidated or circumvented, and the rights granted under any issued patents may not provide us with proprietary protection or competitive advantages.
Patent applications filed before November 29, 2000 in the United States are maintained in secrecy until patents issue. Later filed U.S. applications and patent applications in most foreign countries generally are not published until at least 18 months after they are filed. Scientific and patent publication often occurs long after the date of the scientific discoveries disclosed in those publications. Accordingly, we cannot be certain that we were the first to invent the subject matter covered by any patent application or that we were the first to file a patent application for any inventions.
Our commercial success depends on our ability to operate without infringing patents and proprietary rights of third parties. We cannot determine with certainty whether patents or patent applications of other parties may materially affect our ability to conduct our business. The existence of third party patent applications and patents could significantly reduce the coverage of patents owned by or licensed to us and limit our ability to obtain meaningful patent protection. If patents containing competitive or conflicting claims are issued to third parties and these claims are ultimately determined to be valid, we may be enjoined from pursuing research, development or commercialization of voreloxin or future drug candidates, if any, or be required to obtain licenses to these patents or to develop or obtain alternative technology.
We may need to commence or defend litigation to enforce or to determine the scope and validity of any patents issued to us or to determine the scope and validity of third party proprietary rights. Litigation would result in substantial costs, even if the eventual outcome is favorable to us. An adverse outcome in litigation affecting proprietary rights we own or have licensed could present significant risk of competition for voreloxin or future drug candidates, if any, we market or seek to develop. Any adverse outcome in litigation affecting third party proprietary rights could subject us to significant liabilities to third parties and could require us to seek licenses of the disputed rights from third parties or to cease using the technology if such licenses are unavailable.
We also rely on trade secrets to protect our technology, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to maintain and do not protect technology against independent developments made by third parties.
We seek to protect our proprietary information by requiring our employees, consultants, contractors and other advisers to execute nondisclosure and assignment of invention agreements upon commencement of their employment or engagement. Agreements with our employees also prevent them from bringing the proprietary rights of third parties to us. We also require confidentiality or material transfer agreements from third parties that receive our confidential data or materials. There can be no assurance that these agreements will provide meaningful protection, that these agreements will not be breached, that we will have an adequate remedy for any such breach, or that our trade secrets will not otherwise become known or independently developed by a third party.
We seek to protect our company name and the names of our products and technologies by obtaining trademark registrations, as well as common law rights in trademarks and service marks, in the United States and in other countries. There can be no assurance that the trademarks or service marks we use or register will protect our company name or any products or technologies that we develop and commercialize, that our trademarks, service marks, or trademark registrations will be enforceable against third parties, or that our trademarks and service marks will not interfere with or infringe trademark rights of third parties.
10
Table of Contents
We may need to commence litigation to enforce our trademarks and service marks or to determine the scope and validity of our or a third party’s trademark rights. Litigation would result in substantial costs, even if the eventual outcome is favorable to us. An adverse outcome in litigation could subject us to significant liabilities to third parties and require us to seek licenses of the disputed rights from third parties or to cease using the trademarks or service marks if such licenses are unavailable.
Government Regulation
The FDA and comparable regulatory agencies in state and local jurisdictions and in foreign countries impose substantial requirements upon the clinical development, manufacture, marketing and distribution of drugs. These agencies and other federal, state and local entities regulate research and development activities and the testing, manufacture, quality control, safety, efficacy, labeling, storage, recordkeeping, approval, advertising and promotion of voreloxin and any future drug candidates we may develop. The application of these regulatory frameworks to the development, approval and commercialization of voreloxin or our future drug candidates, if any, will take a number of years to accomplish, if at all, and involve the expenditure of substantial resources.
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, as amended, and implementing regulations. The process required by the FDA before voreloxin and any future drug candidates may be marketed in the United States generally involves the following:
| • | completion of extensive preclinical laboratory tests, in vivo preclinical studies and formulation studies; |
| • | submission to the FDA of an Investigational New Drug, or IND, application which must become effective before clinical trials begin; |
| • | performance of adequate and well-controlled clinical trials to establish the safety and efficacy of the product candidate for each proposed indication; |
| • | submission of an NDA, to the FDA; |
| • | satisfactory completion of an FDA pre-approval inspection of the manufacturing facilities at which the product candidate is produced to assess compliance with current Good Manufacturing Practice, or cGMP, regulations; and |
| • | FDA review and approval of the NDA, including proposed labeling (package insert information) and promotional materials, prior to any commercial marketing, sale or shipment of the drug. |
The testing and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for voreloxin or our future drug candidates, if any, will be granted on a timely basis, if at all.
Preclinical Testing and INDs
Preclinical tests include laboratory evaluation of product chemistry, formulation and stability, as well as studies to evaluate toxicity in animals. Laboratories that comply with the FDA Good Laboratory Practice regulations must conduct preclinical safety tests. The results of preclinical tests, together with manufacturing information and analytical data, are submitted as part of an IND application to the FDA. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises concerns or questions about the conduct of the clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. Our submission of an IND, or those of our collaboration
11
Table of Contents
partners, may not result in FDA authorization to commence a clinical trial. A protocol amendment for an existing IND must be made for each successive clinical trial conducted during product development.
Clinical Trials
Clinical trials involve the administration of the investigational new drug to healthy volunteers or to patients under the supervision of a qualified principal investigator. Clinical trials are conducted in accordance with the FDA’s Protection of Human Subjects regulations and Good Clinical Practices, or GCP, under protocols that detail the objectives of the study, the parameters to be used to monitor safety, and the efficacy criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND.
In addition, each clinical study must be conducted under the auspices of an independent institutional review board, or IRB, at each institution where the study will be conducted. Each IRB will consider, among other things, ethical factors, the safety of human subjects and the possible liability of the institution. The FDA, an IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive GCP requirements and regulations for informed consent.
Clinical trials are typically conducted in the three sequential phases, which may overlap, sometimes followed by a fourth phase:
| • | Phase 1 clinical trials are initially conducted in a limited population to test the drug candidate for safety (adverse effects), dose tolerance, absorption, metabolism, distribution and excretion in healthy humans or, on occasion, in patients, such as cancer patients. In some cases, particularly in cancer trials, a sponsor may decide to conduct what is referred to as a “Phase 1b” evaluation, which is a second safety-focused Phase 1 clinical trial typically designed to evaluate the impact of the drug candidate in combination with currently approved drugs. |
| • | Phase 2 clinical trials are generally conducted in a limited patient population to identify possible adverse effects and safety risks, to determine the efficacy of the drug candidate for specific targeted indications and to determine dose tolerance and optimal dosage. Multiple Phase 2 clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more expensive Phase 3 clinical trials. In some cases, a sponsor may decide to conduct what is referred to as a “Phase 2b” evaluation, which is a second, confirmatory Phase 2 clinical trial that could, if positive and accepted by the FDA, serve as a pivotal clinical trial in the approval of a drug candidate. |
| • | Phase 3 clinical trials are commonly referred to as pivotal trials. When Phase 2 clinical trials demonstrate that a drug candidate has potential activity in a disease or condition and has an acceptable safety profile, Phase 3 clinical trials are undertaken to further evaluate clinical efficacy and to further test for safety in an expanded patient population at multiple, geographically dispersed clinical trial sites. |
| • | Phase 4 (post-marketing) clinical trials may be required by the FDA in some cases. The FDA may condition approval of an NDA for a drug candidate on a sponsor’s agreement to conduct additional clinical trials to further assess the drug’s safety and efficacy after NDA approval. Such post-approval trials are typically referred to as Phase 4 clinical trials. |
New Drug Applications
The testing and approval processes are likely to require substantial cost, time and effort, and there can be no assurance that any approval will be granted on a timely basis, if at all. The FDA may withdraw product
12
Table of Contents
approvals if compliance with regulatory standards is not maintained or if problems occur following initial marketing.
The results of development, preclinical testing and clinical trials, together with extensive manufacturing information, are submitted to the FDA as part of an NDA for approval of the marketing and commercial distribution of the drug. For priority reviews, once the NDA submission has been accepted for filing, the FDA has the goal of reviewing and acting on such NDA filing within 180 days of its receipt. The review process is often significantly extended by FDA requests for additional information or clarification. The FDA may refer the NDA to an advisory committee for review, evaluation and recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. The FDA may deny approval of an NDA if the applicable regulatory criteria are not satisfied, or it may require additional clinical testing. Even if data from such testing are obtained and submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Data from clinical trials are not always conclusive and the FDA may interpret data differently than we or our collaboration partners interpret data. If regulatory approval is granted, such approval may entail limitations on the indicated uses for which the product may be marketed.
Once issued, the FDA may withdraw drug approval if ongoing regulatory requirements are not met or if safety problems occur after the drug reaches the market. In addition, the FDA may require testing, including Phase 4 clinical trials, and surveillance programs to monitor the effect of approved products that have been commercialized, and the FDA has the power to prevent or limit further marketing of a drug based on the results of these post-marketing programs. Drugs may be marketed only for approved indications and in accordance with the provisions of the approved label. Further, if there are any modifications to the drug, including changes in indications, labeling, or manufacturing processes or facilities, we may be required to submit and obtain FDA approval of a new NDA or NDA supplement, which may require us to develop additional data or conduct additional preclinical studies and clinical trials.
Fast Track Designation
FDA’s fast track program is intended to facilitate the development, and to expedite the review, of drugs that are intended for the treatment of a serious or life-threatening condition for which there is no effective treatment and demonstrate the potential to address unmet medical needs for the condition. Under the fast track program, the sponsor of a new drug candidate must request that the FDA designate the drug candidate for a specific indication as a fast track drug concurrent with or after the filing of the IND for the drug candidate. The FDA must within 60 days of receipt of the sponsor’s request determine if the drug candidate qualifies for fast track designation.
If fast track designation is obtained, the FDA may initiate review of sections of an NDA before the application is complete. This rolling review is available if the applicant provides and the FDA approves a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, the time period specified in the Prescription Drug User Fees Act, which governs the time period goals the FDA has committed to reviewing an application, does not begin until the complete application is submitted. Additionally, the fast track designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
In some cases, a fast track designated drug candidate may also qualify for one or more of the following programs:
| • | Priority Review. Under FDA policies, a drug candidate is eligible for priority review, or review within six-months from the time a complete NDA is accepted for filing, if the drug candidate provides a significant improvement compared to marketed drugs in the treatment, diagnosis or prevention of a disease. A fast track designated drug candidate would ordinarily meet the FDA’s criteria for priority review. |
13
Table of Contents
| • | Accelerated Approval. Under the FDA’s accelerated approval regulations, the FDA is authorized to approve drug candidates that have been studied for their safety and efficacy in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit to patients over existing treatments based upon either a surrogate endpoint that is reasonably likely to predict clinical benefit or on the basis of an effect on a clinical endpoint other than patient survival. In clinical trials, surrogate endpoints are alternative measurements of the symptoms of a disease or condition that are substituted for measurements of observable clinical symptoms. A drug candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of Phase 4 clinical trials to validate the surrogate endpoint or confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or to validate a surrogate endpoint or confirm a clinical benefit during post-marketing studies, will allow the FDA to withdraw the drug from the market on an expedited basis. All promotional materials for drug candidates approved under accelerated regulations are subject to prior review by the FDA. |
When appropriate, we or our collaboration partners may seek fast track designation, accelerated approval or priority review for voreloxin or our future drug candidates, if any. We do not know whether voreloxin or our future drug candidates, if any, will receive a priority review designation or, if a priority designation is received, whether that review or approval will be faster than conventional FDA procedures. We also cannot predict whether voreloxin or our future drug candidates, if any, will obtain a fast track or accelerated approval designation, or the ultimate impact, if any, of the fast track or the accelerated approval process on the timing or likelihood of FDA approval of voreloxin or our future drug candidates, if any.
Satisfaction of FDA regulations and approval requirements or similar requirements of foreign regulatory agencies typically takes several years, and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease. Typically, if a drug candidate is intended to treat a chronic disease, as is the case with voreloxin, safety and efficacy data must be gathered over an extended period of time. Government regulation may delay or prevent marketing of drug candidates for a considerable period of time and impose costly procedures upon our activities. The FDA or any other regulatory agency may not grant approvals for new indications for our drug candidates on a timely basis, or at all. Even if a drug candidate receives regulatory approval, the approval may be significantly limited to specific disease states, patient populations and dosages. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a drug may result in restrictions on the drug or even complete withdrawal of the drug from the market. Delays in obtaining, or failures to obtain, regulatory approvals for any of our drug candidates would harm our business. In addition, we cannot predict what adverse governmental regulations may arise from future United States or foreign governmental action.
Other Regulatory Requirements
Any drugs manufactured or distributed by us or our collaboration partners pursuant to FDA approvals are subject to continuing regulation by the FDA, including recordkeeping requirements and reporting of adverse experiences associated with the drug. Drug manufacturers and their subcontractors are required to register with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMPs, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. Failure to comply with the statutory and regulatory requirements can subject a manufacturer to possible legal or regulatory action, such as warning letters, suspension of manufacturing, seizure of product, injunctive action or possible civil penalties.
The FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the Internet. A company can make only those claims relating to safety and efficacy that are approved by the FDA. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties. Physicians
14
Table of Contents
may prescribe legally available drugs for uses that are not described in the drug’s labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties, including cancer therapy. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, impose stringent restrictions on manufacturers’ communications regarding off-label use.
Foreign Regulation
In addition to regulations in the United States, we are subject to foreign regulations governing clinical trials and commercial sales and distribution of voreloxin or our future drug candidates, if any. We are currently conducting clinical trials in Canada and may in the future initiate clinical trials in countries in Europe, South America, or elsewhere. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
Under the Canadian regulatory system, Health Canada is the regulatory body that governs the sale of drugs for the purposes of use in clinical trials. Accordingly, any company that wishes to conduct a clinical trial in Canada must submit a clinical trial application to Health Canada. Health Canada reviews the application and notifies the company within 30 days if the application is found to be deficient. If the application is deemed acceptable, Health Canada will issue a no objection letter to the company within the 30-day review period which means the company may proceed with its clinical trial(s).
Under European Union regulatory systems, permission to conduct clinical research is granted by the Competent Authority of each European Member State, or MS, and the applicable Ethics Committees, or EC, through the submission of a Clinical Trial Application. An EC in the European Union serves the same function as an IRB in the United States. The review times vary by MS but may not exceed 60 days. The EC has a maximum of 60 days to give its opinion on the acceptability of the Clinical Trial Application to both the governing MS and the sponsor applicant. If the application is deemed acceptable, the MS informs the applicant (or does not within the 60 day window inform the applicant of non-acceptance) and the company may proceed with the clinical trial.
Under the European Union regulatory systems, marketing authorizations may be submitted either under a centralized or mutual recognition procedure. The centralized procedure provides for the grant of a single marketing authorization that is valid for all European Union member states. The mutual recognition procedure provides for mutual recognition of national approval decisions. Under this procedure, the holder of a national marketing authorization may submit an application to the remaining member states. Within 90 days of receiving the application and assessment report, each member state must decide whether to recognize approval.
In addition to regulations in the United States, Canada and the European Union, we will be subject to a variety of other foreign regulations governing clinical trials and commercial distribution of our current and possible future product candidates. Our ability to sell drugs will also depend on the availability of reimbursement from government and private practice insurance companies.
Research and Development Expenses
We incurred $13.2 million, $26.3 million and $36.1 million of research and development expenses in 2009, 2008 and 2007, respectively. As a result of our 2008 Restructuring and the resulting wind down of our research activities and focus on voreloxin development in the near term, we do not anticipate incurring any significant additional research expenses related to the discovery of additional product candidates, the development or application of our proprietary fragment-based drug discovery methods, the development of in-house research capabilities, or on the clinical development of product candidates other than voreloxin. In
15
Table of Contents
addition, we are no longer conducting any research activities in connection with any of our collaborations. However, we have incurred and expect to continue to incur substantial research and development expenses to conduct further clinical and related development of voreloxin.
Environment
We have made, and will continue to make, expenditures for environmental compliance and protection. In 2008, we incurred approximately $0.3 million in expenses related to the closure of our laboratory space at 341 Oyster Point Boulevard in South San Francisco, California, in accordance with environmental laws and regulations. We do not expect that expenditures for compliance with environmental laws will have a material effect on our capital expenditures or results of operations in the future.
Employees
As of December 31, 2009, our workforce consisted of 28 full-time employees. Of our total workforce, 18 are engaged in research and development and 10 are engaged in general and administrative functions. We have no collective bargaining agreements with our employees, and we have not experienced any work stoppages.
Corporate Background
We were incorporated in Delaware in February 1998 as Mosaic Pharmaceuticals, Inc., and subsequently changed our name to Sunesis Pharmaceuticals, Inc. Our offices are headquartered at 395 Oyster Point Boulevard, Suite 400, South San Francisco, California 94080, and our telephone number is (650) 266-3500. Our website address is www.sunesis.com. Information contained in, or accessible through, our website is not incorporated by reference into and does not form a part of this report.
16
Table of Contents
| ITEM 1A. | RISK FACTORS |
Investing in our common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below and all information contained in this report in weighing a decision to purchase our common stock. If any of the possible adverse events described below actually occurs, we may be unable to conduct our business as currently planned and our financial condition and operating results could be adversely affected. Additional risks not presently known to us or that we currently believe are immaterial may also significantly impair our business operations. In addition, the trading price of our common stock could decline due to the occurrence of any of these risks, and you may lose all or part of your investment. Please see “Special Note Regarding Forward-Looking Statements.”
Risks Related to Our Business
If we are unable to raise additional capital in the near term, we will not be able to continue to operate as a going concern.
We will need to raise substantial additional capital to continue the development and commercialization of voreloxin, and our business in general. We will need to raise substantial additional capital in the near term to:
| • | fund clinical trials and seek regulatory approvals; |
| • | continue and expand our development activities; |
| • | hire additional development personnel; |
| • | maintain, defend and expand the scope of our intellectual property portfolio; |
| • | implement additional internal systems and infrastructure; and |
| • | build or access manufacturing and commercialization capabilities. |
Our future funding requirements will depend on many factors, including but not limited to:
| • | the rate of progress and cost of our clinical trials, need for additional clinical trials, and other development activities; |
| • | the economic and other terms and timing of any licensing or other partnering arrangement into which we may enter; |
| • | the costs associated with building or accessing manufacturing and commercialization capabilities; |
| • | the costs of acquiring or investing in businesses, product candidates and technologies, if any; |
| • | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
| • | the costs and timing of seeking and obtaining FDA and other regulatory approvals; and |
| • | the effect of competing technological and market developments. |
On April 3, 2009, we closed the initial $10.0 million of the Private Placement of up to $43.5 million of our securities. On October 30, 2009, we completed the second closing of $5.0 million of the Private Placement. In the initial closing, $10.0 million of units were sold, resulting in net proceeds of $8.8 million, and in the second
17
Table of Contents
closing, $5.0 million of units were sold, resulting in net proceeds of $4.7 million. The units consist of Series A convertible preferred stock and warrants to purchase common stock, and were sold to accredited investors, including certain members of management. An additional $28.5 million of common stock may be sold at $0.275 per share in a common equity closing upon the election of the holders of a majority of the Series A convertible preferred stock issued in the Private Placement prior to the earlier of June 30, 2010, or a date determined with reference to our cash and investments balance dropping below $2.5 million. The common equity closing may also be completed upon our election prior to the earlier of June 30, 2010 or a qualifying alternative common stock financing, subject to the approval of the purchasers holding a majority of the Series A convertible preferred stock issued in the Private Placement and subject to us selling at least $28.5 million of common stock in the common equity closing. The common equity closing is entirely at the discretion of the investors in the Private Placement, and it is possible that they will not elect to complete that closing for reasons related to our business or other factors.
We believe that currently available cash, cash equivalents and marketable securities, including the net proceeds of $14.2 million from sales of common stock through March 31, 2010 under the agreement with Cantor, are sufficient to fund our operations through at least September 30, 2010. We will need to raise substantial additional funding in the near term in order to sustain operations beyond that date and before undertaking any additional clinical trials of voreloxin. If we are unable to raise substantial additional funding, we will be forced to delay or reduce the scope of our voreloxin development program and/or limit or cease our operations.
Until we can generate a sufficient amount of collaboration or product revenue to finance our cash requirements, which we may never do, we expect to finance future cash needs primarily through equity issuances (including the possible common equity closing of the Private Placement and subject to the satisfaction of the conditions described above), debt arrangements and/or a possible partnership or license of development and/or commercialization rights to voreloxin. We do not know whether additional funding will be available on acceptable terms, or at all.
We are currently conducting clinical trials of voreloxin in acute myeloid leukemia, or AML, and ovarian cancer. If we are not able to secure additional funding when needed, we may have to delay, reduce the scope of or eliminate one or more of our clinical trials, scale back our development program, conduct additional workforce or other expense reductions, or cease operations. Any one of the foregoing would have a material adverse effect on our business, financial condition and results of operations.
Our independent registered public accounting firm has indicated that our recurring operating losses raise substantial doubt as to our ability to continue as a going concern.
Our audited financial statements for the fiscal year ended December 31, 2009 were prepared on a basis that our business would continue as a going concern in accordance with United States generally accepted accounting principles. This basis of presentation assumes that we will continue in operation for the foreseeable future and will be able to realize our assets and discharge our liabilities and commitments in the normal course of business. However, our independent registered public accounting firm has indicated in their audit report on our 2009 consolidated financial statements that our recurring operating losses raise substantial doubt as to our ability to continue as a going concern. We will be forced to delay or reduce the scope of our voreloxin development program and/or limit or cease our operations if we are unable to raise substantial additional funding to meet our working capital needs. However, we cannot guarantee that we will be able to obtain sufficient additional funding when needed or that such funding, if available, will be obtainable on terms satisfactory to us. In the event that these plans cannot be effectively realized, there can be no assurance that we will be able to continue as a going concern.
Economic conditions may make it more difficult and costly to raise additional capital.
Recently, there has been turmoil in the U.S. economy, which has led to reduced credit availability. Banks have tightened their lending standards and investors have been unwilling to buy certain corporate stock and
18
Table of Contents
bonds. If economic conditions continue to affect the capital markets, our ability to raise capital may be adversely affected.
We have incurred losses since inception and anticipate that we will continue to incur losses for the foreseeable future. We may not ever achieve or sustain profitability.
We are not profitable and have incurred losses in each year since our inception in 1998. Our net losses for the years ended December 31, 2009, 2008 and 2007 were $40.2 million, $37.2 million and $38.8 million, respectively. As of December 31, 2009, we had an accumulated deficit of $356.4 million. We do not currently have any products that have been approved for marketing, and we continue to incur substantial development and general and administrative expenses related to our operations. We expect to continue to incur losses for the foreseeable future, and we expect these losses to increase significantly, especially upon commencing pivotal and Phase 3 clinical trials for voreloxin, as we conduct development of, and seek regulatory approvals for, voreloxin, and as we commercialize any approved drugs. Our losses, among other things, have caused and will continue to cause our stockholders’ equity and working capital to decrease.
Our business model had been based in part upon entering into strategic collaborations for the discovery and/or the development of some of our product candidates. To date, we have derived substantially all of our revenue from research collaboration agreements with Biogen Idec, Inc. Merck & Co., and Johnson & Johnson Pharmaceutical Research & Development LLC. As of March 31, 2010, our only remaining ongoing collaboration is with Biogen Idec; however, the research phase for this collaboration is completed. We do not expect to enter into any new collaboration agreement that will result in research revenue for us. We also do not anticipate that we will generate revenue from the sale of products for the foreseeable future. In the absence of additional sources of capital, which may not be available to us on acceptable terms, or at all, the development of voreloxin or future product candidates, if any, may be reduced in scope, delayed or terminated. If our product candidates or those of our collaborators fail in clinical trials or do not gain regulatory approval, or if our future products do not achieve market acceptance, we may never become profitable. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods.
The development of voreloxin could be halted or significantly delayed for various reasons; our clinical trials for voreloxin may not demonstrate safety or efficacy or lead to regulatory approval.
Voreloxin is vulnerable to the risks of failure inherent in the drug development process. We need to conduct significant additional preclinical studies and clinical trials before we can attempt to demonstrate that voreloxin is safe and effective to the satisfaction of the FDA and other regulatory authorities. Failure can occur at any stage of the development process, and successful preclinical studies and early clinical trials do not ensure that later clinical trials will be successful. A number of companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical trials, even after obtaining promising results in earlier trials.
For example, we terminated two Phase 2 trials of voreloxin in small cell and non-small cell lung cancer. We ceased development of SNS-032 and terminated our related license agreement with BMS after completion of a Phase 1 trial as no responses demonstrating efficacy were observed in that trial. In addition, in our Phase 1 trial of SNS-314, a maximum tolerated dose was not established and no responses were observed. As a result, we have suspended further development of SNS-314. If our clinical trials result in unacceptable toxicity or lack of efficacy, we may have to terminate them. If clinical trials are halted, or if they do not show that voreloxin is safe and effective in the indications for which we are seeking regulatory approval, our future growth will be limited and we may not have any other product candidates to develop.
We do not know whether our ongoing clinical trials or any other future clinical trials with voreloxin or any of our product candidates will be completed on schedule, or at all, or whether our ongoing or planned clinical trials will begin or progress on the time schedule we anticipate. The commencement of our planned clinical trials could be substantially delayed or prevented by several factors, including:
| • | delays or failures to raise additional funding; |
19
Table of Contents
| • | results of meetings with the FDA and/or other regulatory bodies; |
| • | a limited number of, and competition for, suitable patients with particular types of cancer for enrollment in our clinical trials; |
| • | delays or failures in obtaining regulatory approval to commence a clinical trial; |
| • | delays or failures in obtaining sufficient clinical materials; |
| • | delays or failures in obtaining approval from independent institutional review boards to conduct a clinical trial at prospective sites; or |
| • | delays or failures in reaching acceptable clinical trial agreement terms or clinical trial protocols with prospective sites. |
The completion of our clinical trials could also be substantially delayed or prevented by several factors, including:
| • | delays or failures to raise additional funding; |
| • | slower than expected rates of patient recruitment and enrollment; |
| • | failure of patients to complete the clinical trial; |
| • | unforeseen safety issues; |
| • | lack of efficacy during clinical trials; |
| • | inability or unwillingness of patients or clinical investigators to follow our clinical trial protocols; and |
| • | inability to monitor patients adequately during or after treatment. |
Additionally, our clinical trials may be suspended or terminated at any time by the FDA, other regulatory authorities, ourselves or, in some cases, our collaboration partners. Any failure to complete or significant delay in completing clinical trials for our product candidates could harm our financial results and the commercial prospects for our product candidates.
In March 2008, we informed the FDA of a stability observation in our voreloxin finished drug product, or FDP. Specifically, visible particles were observed during stability studies of one of our voreloxin FDP lots. We have since identified a process impurity in the voreloxin active pharmaceutical ingredient, or API, that, when formulated into the packaged vial of the voreloxin FDP, can result in the formation of particles over time. As a response to these findings, we implemented a revised manufacturing process to attempt to control the impurity and thereby prevent particle formation. Two lots of voreloxin API manufactured using the revised manufacturing process have been formulated into FDP lots that have completed up to 12 months of stability testing at room temperature without formation of particles. These FDP lots are currently being used in our clinical trials. It will take time to evaluate whether or not this revised manufacturing process for voreloxin API will be successful in stopping the formation of particles in these FDP lots over the longer term, and to evaluate whether or not such control of particle formation would also be reliably and consistently achieved in subsequent lots over the shorter or longer term. We provided updates on the results from our process optimization activities to the FDA in December 2008, and again most recently in October 2009 within the briefing materials that were discussed at the January 2010 CMC-focused End-of-Phase 2 meeting with the FDA. If the change in manufacturing process does
20
Table of Contents
not adequately control the formation of visible particles, we will need to discuss other possibilities with the FDA, which could include temporary clinical hold until the issue has been resolved to their satisfaction.
The failure to enroll patients for clinical trials may cause delays in developing voreloxin.
We may encounter delays if we are unable to enroll enough patients to complete clinical trials of voreloxin. Patient enrollment depends on many factors, including the size of the patient population, the nature of the protocol, the proximity of patients to clinical sites and the eligibility criteria for the trial. Moreover, when one product candidate is evaluated in multiple clinical trials simultaneously, patient enrollment in ongoing trials can be adversely effected by negative results from completed trials. Voreloxin is being tested in patients with AML and ovarian cancer, which can be difficult patient populations to recruit.
The results of preclinical studies and clinical trials may not satisfy the requirements of the FDA or other regulatory agencies.
Prior to receiving approval to commercialize voreloxin or future product candidates, if any, in the United States or abroad, we and our collaboration partners must demonstrate with substantial evidence from well-controlled clinical trials, to the satisfaction of the FDA and other regulatory authorities, that such product candidates are safe and effective for their intended uses. The results from preclinical studies and clinical trials can be interpreted in different ways. Even if we and our collaboration partners believe the preclinical or clinical data are promising, such data may not be sufficient to support approval by the FDA and other regulatory authorities.
We rely on third parties to manufacture our voreloxin drug product and its active pharmaceutical ingredient, and depend on one of two suppliers for production of the drug product and for production of the active pharmaceutical ingredient. There are a limited number of manufacturers that are capable of manufacturing voreloxin.
We do not currently own or operate manufacturing facilities and lack the capability to manufacture voreloxin on a clinical or commercial scale. As a result, we rely on third parties to manufacture both the voreloxin API and FDP. The API is classified as a toxic substance, limiting the available manufacturers. We believe that there are at least five contract manufacturers in North America with suitable capabilities for API manufacture, and at least four that can manufacture FDP. We currently have established relationships with only two manufacturers for API and two manufacturers for FDP. If either of our third-party API or FDP manufacturers is unable or unwilling to produce voreloxin, we may need to establish a contract with another supplier. However, establishing a relationship with an alternative supplier would likely delay our ability to produce API or FDP for six to nine months, during which time we would rely on current inventory to supply our drug product manufacturing activities. We expect to continue to depend on third-party contract manufacturers for all our API and FDP needs in the foreseeable future.
Voreloxin requires precise, high quality manufacturing. A contract manufacturer is subject to ongoing periodic unannounced inspection by the FDA and corresponding state agencies to ensure strict compliance with current Good Manufacturing Practice, or cGMP, and other applicable government regulations and corresponding foreign standards. Our contract manufacturer’s failure to achieve and maintain high manufacturing standards in compliance with cGMP regulations could result in manufacturing errors resulting in patient injury or death, product recalls or withdrawals, delays or interruptions of production or failures in product testing or delivery, delay or prevention of filing or approval of marketing applications for voreloxin, cost overruns or other problems that could seriously harm our business.
To date, voreloxin has been manufactured in small quantities for preclinical studies and clinical trials. Prior to being approved for commercial sale, we will need to manufacture finished drug product in larger quantities. Significant scale-up of manufacturing will be accompanied by significant validation studies, which
21
Table of Contents
will be reviewed by the FDA prior to approval. If we are unable to successfully increase the manufacturing capacity for voreloxin, the regulatory approval or commercial launch may be delayed or there may be a shortage in commercial supply.
Any performance failure on the part of a contract manufacturer could delay clinical development or regulatory approval of our product candidates or commercialization of our future products, depriving us of potential product revenue and resulting in additional losses. For example, because we rely on only two suppliers for voreloxin API and for FDP, the failure of such suppliers to have sufficient quantities of voreloxin or to supply it on a timely basis, or at all, would negatively affect us. In addition, our dependence on a third party for manufacturing may adversely affect our future profit margins. Our ability to replace an existing manufacturer may be difficult because the number of potential manufacturers is limited and the FDA must approve any replacement manufacturer before it can be an approved commercial supplier. Such approval would require new testing and compliance inspections. It may be difficult or impossible for us to identify and engage a replacement manufacturer on acceptable terms in a timely manner, or at all.
We expect to expand our clinical development capabilities, and any difficulties hiring or retaining key personnel or managing this growth could disrupt our operations.
We are highly dependent on the principal members of our development staff. We expect to expand our clinical development capabilities by increasing expenditures in these areas, hiring additional employees and expanding the scope of our current operations. Future growth will require us to continue to implement and improve our managerial, operational and financial systems and continue to retain, recruit and train additional qualified personnel, which may impose a strain on our administrative and operational infrastructure. The competition for qualified personnel in the biopharmaceutical field is intense. We are highly dependent on our continued ability to attract, retain and motivate highly qualified management and specialized personnel required for clinical development. Due to our limited resources, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. If we are unable to retain key personnel or manage our growth effectively, we may not be able to implement our business plan.
If we are sued for infringing intellectual property rights of third parties, litigation will be costly and time consuming and could prevent us from developing or commercializing voreloxin.
Our commercial success depends on not infringing the patents and other proprietary rights of third parties and not breaching any collaboration or other agreements we have entered into with regard to our technologies and product candidates. If a third party asserts that we are using technology or compounds claimed in issued and unexpired patents owned or controlled by the third party, we may need to obtain a license, enter into litigation to challenge the validity of the patents or incur the risk of litigation in the event that a third party asserts that we infringe its patents.
If a third party asserts that we infringe its patents or other proprietary rights, we could face a number of challenges that could seriously harm our competitive position, including:
| • | infringement and other intellectual property claims, which would be costly and time consuming to litigate, whether or not the claims have merit, and which could delay the regulatory approval process and divert management’s attention from our business; |
| • | substantial damages for past infringement, which we may have to pay if a court determines that voreloxin or any future product candidates infringe a third party’s patent or other proprietary rights; |
| • | a court order prohibiting us from selling or licensing voreloxin or any future product candidates unless a third party licenses relevant patent or other proprietary rights to us, which it is not required to do; and |
22
Table of Contents
| • | if a license is available from a third party, we may have to pay substantial royalties or grant cross-licenses to our patents or other proprietary rights. |
If our competitors develop and market products that are more effective, safer or less expensive than voreloxin, our commercial opportunities will be negatively impacted.
The life sciences industry is highly competitive, and we face significant competition from many pharmaceutical, biopharmaceutical and biotechnology companies that are researching, developing and marketing products designed to address the treatment of cancer, including AML and ovarian cancer. Voreloxin is a small molecule therapeutic that will compete with other drugs and therapies that currently exist or are being developed. Many of our competitors have significantly greater financial, manufacturing, marketing and drug development resources than we do. Large pharmaceutical companies in particular have extensive experience in clinical testing and in obtaining regulatory approvals for, and marketing, drugs.
We believe that our ability to successfully compete in the marketplace with voreloxin and any future product candidates, if any, will depend on, among other things:
| • | our ability to develop novel compounds with attractive pharmaceutical properties and to secure, protect and maintain intellectual property rights based on our innovations; |
| • | the efficacy, safety and reliability of our product candidates; |
| • | the speed at which we develop our product candidates; |
| • | our ability to design and successfully execute appropriate clinical trials; |
| • | our ability to maintain a good relationship with regulatory authorities; |
| • | our ability to obtain, and the timing and scope of, regulatory approvals; |
| • | our ability to manufacture and sell commercial quantities of future products to the market; and |
| • | acceptance of future products by physicians and other healthcare providers. |
Some of the current key competitors of voreloxin in AML include Genzyme Corporation’s clofarabine, Eisai Corporation’s decitabine, Celgene Corporation’s azacitidine and Vion Pharmaceuticals, Inc.’s laromustine, any or all of which could change the treatment paradigm of acute leukemia. Each of these compounds is further along in clinical development than is voreloxin. Liposomal doxorubicin and topotecan are current standards of care in platinum-resistant ovarian cancer patients, and we are aware that several of our competitors have initiated Phase 3 clinical trials for this indication.
We expect competition for voreloxin to increase as additional products are developed and approved to treat AML and ovarian cancer in various patient populations. If our competitors market products that are more effective, safer or less expensive than voreloxin or our other future products, if any, or that reach the market sooner we may not achieve commercial success or substantial market penetration. In addition, the biopharmaceutical industry is characterized by rapid change. Products developed by our competitors may render voreloxin or any future product candidates obsolete.
We rely on third parties to conduct our clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may be unable to obtain regulatory approval for or commercialize voreloxin.
We rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories, to conduct our planned and existing clinical trials for voreloxin. If the
23
Table of Contents
third parties conducting our clinical trials do not perform their contractual duties or obligations, do not meet expected deadlines or need to be replaced, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical trial protocols or for any other reason, we may need to enter into new arrangements with alternative third parties and our clinical trials may be extended, delayed or terminated or may need to be repeated, and we may not be able to obtain regulatory approval for or commercialize the product candidate being tested in such trials.
Our proprietary rights may not adequately protect voreloxin or future product candidates, if any.
Our commercial success will depend on our ability to obtain patents and maintain adequate protection for voreloxin and any future product candidates in the United States and other countries. We own, co-own or have rights to a significant number of issued U.S. and foreign patents and pending U.S. and foreign patent applications. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary technologies and future products are covered by valid and enforceable patents or are effectively maintained as trade secrets.
We apply for patents covering both our technologies and product candidates, as we deem appropriate. However, we may fail to apply for patents on important technologies or product candidates in a timely fashion, or at all. Our existing patents and any future patents we obtain may not be sufficiently broad to prevent others from practicing our technologies or from developing competing products and technologies. In addition, we generally do not exclusively control the patent prosecution of subject matter that we license to or from others. Accordingly, in such cases we are unable to exercise the same degree of control over this intellectual property as we would over our own. Moreover, the patent positions of biopharmaceutical companies are highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. As a result, the validity and enforceability of patents cannot be predicted with certainty. In addition, we do not know whether:
| • | we, our licensors or our collaboration partners were the first to make the inventions covered by each of our issued patents and pending patent applications; |
| • | we, our licensors or our collaboration partners were the first to file patent applications for these inventions; |
| • | others will independently develop similar or alternative technologies or duplicate any of our technologies; |
| • | any of our or our licensors’ pending patent applications will result in issued patents; |
| • | any of our, our licensors’ or our collaboration partners’ patents will be valid or enforceable; |
| • | any patents issued to us, our licensors or our collaboration partners will provide us with any competitive advantages, or will be challenged by third parties; |
| • | we will develop additional proprietary technologies that are patentable; or |
| • | the patents of others will have an adverse effect on our business. |
We also rely on trade secrets to protect some of our technology, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to maintain. While we use reasonable efforts to protect our trade secrets, our or our collaboration partners’ employees, consultants, contractors or scientific and other advisors, or those of our licensors, may unintentionally or willfully disclose our proprietary information to competitors. Enforcement of claims that a third party has illegally obtained and is using trade secrets is expensive, time consuming and uncertain. In addition, foreign courts are sometimes less willing than
24
Table of Contents
U.S. courts to protect trade secrets. If our competitors independently develop equivalent knowledge, methods and know-how, we would not be able to assert our trade secrets against them and our business could be harmed.
The composition of matter patents covering voreloxin are due to expire in 2015. Even if voreloxin is approved by the FDA, we may not be able to recover our development costs prior to the expiration of these patents.
The voreloxin API composition of matter is covered by U.S. patent 5,817,669 and its counterpart patents and patent applications in 43 foreign jurisdictions. U.S. patent 5,817,669 is due to expire in October 2015, and most of its foreign counterparts are due to expire in June 2015. We do not know whether patent term extensions and data exclusivity periods will be available in the future. Voreloxin must undergo extensive clinical trials before it can be approved by the FDA. We do not know when, if ever, voreloxin will be approved by the FDA. Even if voreloxin is approved by the FDA in the future, we may not have sufficient time to commercialize our voreloxin product to enable us to recover our development costs prior to the expiration of the U.S. and foreign patents covering voreloxin. Our obligation to pay royalties to Dainippon, the company from which we licensed voreloxin, may extend beyond the patent expiration, which would further erode the profitability of this product.
Any future workforce and expense reductions may have an adverse impact on our internal programs, our ability to hire and retain key personnel and may be distracting to management.
We have, in the past, implemented a number of workforce reductions. In light of our continued need for funding and expense control, we may be required to implement further workforce and expense reductions in the future. Further workforce and expense reductions could result in reduced progress on our internal programs. In addition, employees, whether or not directly affected by a reduction, may seek future employment with our business partners or competitors. Although our employees are required to sign a confidentiality agreement at the time of hire, the confidential nature of certain proprietary information may not be maintained in the course of any such future employment. Further, we believe that our future success will depend in large part upon our ability to attract and retain highly skilled personnel. We may have difficulty retaining and attracting such personnel as a result of a perceived risk of future workforce and expense reductions. In addition, the implementation of expense reduction programs may result in the diversion of efforts of our executive management team and other key employees, which could adversely affect our business.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of our employees’ former employers.
Many of our employees were previously employed at biotechnology or pharmaceutical companies, including our competitors or potential competitors. We may be subject to claims that we or our employees have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. A loss of key personnel or the work product of current or former personnel could hamper or prevent our ability to commercialize voreloxin, which could severely harm our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
We currently have limited marketing staff and no sales or distribution organization. If we are unable to develop a sales and marketing and distribution capability on our own or through collaborations with marketing partners, we will not be successful in commercializing voreloxin.
We currently have no sales or distribution capabilities and limited marketing staff. We intend to establish our own sales and marketing organization with technical expertise and supporting distribution capabilities to commercialize voreloxin in North America, which will be expensive and time consuming. Any failure or delay in the development of our internal sales, marketing and distribution capabilities would adversely impact the
25
Table of Contents
commercialization of these products. We plan to collaborate with third parties that have direct sales forces and established distribution systems to commercialize voreloxin. To the extent that we enter into co-promotion or other licensing arrangements, our product revenue is likely to be lower than if we marketed or sold voreloxin directly. In addition, any revenue we receive will depend upon the efforts of third parties, which may not be successful and are only partially within our control. If we are unable to enter into such arrangements on acceptable terms or at all, we may not be able to successfully commercialize voreloxin. If we are not successful in commercializing voreloxin or our future product candidates, if any, either on our own or through collaborations with one or more third parties, our future product revenue will suffer and we may incur significant additional losses.
We depend on various consultants and advisors for the success and continuation of development efforts.
We work extensively with various consultants and advisors, who provide advice and or services in various business and development functions, including clinical development, operations and strategy, regulatory matters, accounting and finance. The potential success of our drug development programs depends, in part, on continued collaborations with certain of these consultants and advisors. Our consultants and advisors are not our employees and may have commitments and obligations to other entities that may limit their availability to us. We do not know if we will be able to maintain such relationships or that such consultants and advisors will not enter into other arrangements with competitors, any of which could have a detrimental impact on our development objectives and our business.
If conflicts of interest arise between our collaboration partners and us, any of them may act in their self interest, which may be adverse to our interests.
If a conflict of interest arises between us and one or more of our collaboration partners, they may act in their own self interest or otherwise in a way that is not in the interest of our company or our stockholders. Our collaboration partners are conducting multiple product development efforts within the disease area that is the subject of collaboration with our company. In some of our collaborations, we have agreed not to conduct, independently or with any third party, any research that is competitive with the research conducted under our collaborations. Our collaboration partners, however, may develop, either alone or with others, products in related fields that are competitive with the product candidates that are the subject of these collaborations. Competing products, either developed by our collaboration partners or to which our collaboration partners have rights, may result in their withdrawal of support for a product candidate covered by the collaboration agreement.
If one or more of our collaboration partners were to breach or terminate their collaboration agreements with us or otherwise fail to perform their obligations thereunder in a timely manner, the preclinical or clinical development or commercialization of the affected product candidates could be delayed or terminated. We do not know whether our collaboration partners will pursue alternative technologies or develop alternative product candidates, either on their own or in collaboration with others, including our competitors, as a means for developing treatments for the diseases targeted by collaboration agreements with our company.
Our facilities are located near known earthquake fault zones, and the occurrence of an earthquake or other catastrophic disaster could cause damage to our facilities and equipment, which could require us to cease or curtail operations.
Our facilities are located in the San Francisco Bay Area near known earthquake fault zones and are vulnerable to significant damage from earthquakes. We are also vulnerable to damage from other types of disasters, including fires, floods, power loss, communications failures and similar events. If any disaster were to occur, our ability to operate our business at our facilities may be seriously or completely impaired and our data could be lost or destroyed.
26
Table of Contents
Compliance with changing regulation of corporate governance and public disclosure may result in additional expenses.
Changing laws, regulations and standards relating to corporate governance and public disclosure may create uncertainty regarding compliance matters. New or changed laws, regulations and standards are subject to varying interpretations in many cases. As a result, their application in practice may evolve over time. We are committed to maintaining high standards of corporate governance and public disclosure. Complying with evolving interpretations of new or changed legal requirements may cause us to incur higher costs as we revise current practices, policies and procedures, and may divert management time and attention from potential revenue-generating activities to compliance matters. If our efforts to comply with new or changed laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, our reputation may also be harmed. Further, our board members, chief executive officer and chief financial officer could face an increased risk of personal liability in connection with the performance of their duties. As a result, we may have difficulty attracting and retaining qualified board members and executive officers, which could harm our business.
Risks Related to Our Industry
The regulatory approval process is expensive, time consuming and uncertain and may prevent us from obtaining approval for the commercialization of voreloxin.
The research, testing, manufacturing, selling and marketing of product candidates are subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries, which regulations differ from country to country. Neither we nor our collaboration partners are permitted to market our product candidates in the United States until we receive approval of a new drug application or NDA, from the FDA, or in any other country without the equivalent marketing approval from such country. We have not received marketing approval for voreloxin. None of our collaboration partners have had a product resulting from our collaboration enter clinical trials. In addition, failure to comply with FDA and other applicable U.S. and foreign regulatory requirements may subject us to administrative or judicially imposed sanctions, including warning letters, civil and criminal penalties, injunctions, product seizure or detention, product recalls, total or partial suspension of production, and refusal to approve pending NDAs, supplements to approved NDAs or their foreign equivalents.
Regulatory approval of an NDA or NDA supplement or a foreign equivalent is not guaranteed, and the approval process is expensive and may take several years. Furthermore, the development process for oncology products may take longer than in other therapeutic areas. Regulatory authorities have substantial discretion in the drug approval process. Despite the time and expense exerted, failure can occur at any stage, and we could encounter problems that cause us to abandon clinical trials or to repeat or perform additional preclinical studies and clinical trials. The number of preclinical studies and clinical trials that will be required for marketing approval varies depending on the drug candidate, the disease or condition that the drug candidate is designed to address, and the regulations applicable to any particular drug candidate. While we plan to commence a pivotal clinical trial of voreloxin for the treatment of AML in 2010, we may not be able to reach agreement with the FDA on a development plan that would support potential regulatory approval based on the results of the clinical trials that we anticipate.
The FDA or a foreign regulatory authority can delay, limit or deny approval of a drug candidate for many reasons, including:
| • | the drug candidate may not be deemed safe or effective; |
| • | regulatory officials may not find the data from preclinical studies and clinical trials sufficient; |
| • | the FDA or foreign regulatory authority might not approve our or our third-party manufacturer’s processes or facilities; or |
| • | the FDA or foreign regulatory authority may change its approval policies or adopt new regulations. |
27
Table of Contents
We may be subject to costly claims related to our clinical trials and may not be able to obtain adequate insurance.
Because we conduct clinical trials in humans, we face the risk that the use of voreloxin or future product candidates, if any, will result in adverse side effects. We cannot predict the possible harms or side effects that may result from our clinical trials. Although we have clinical trial liability insurance for up to $10.0 million in aggregate, our insurance may be insufficient to cover any such events. We do not know whether we will be able to continue to obtain clinical trial coverage on acceptable terms, or at all. We may not have sufficient resources to pay for any liabilities resulting from a claim excluded from, or beyond the limit of, our insurance coverage. There is also a risk that third parties that we have agreed to indemnify could incur liability. Any litigation arising from our clinical trials, even if we were ultimately successful, would consume substantial amounts of our financial and managerial resources and may create adverse publicity.
Even if we receive regulatory approval to sell voreloxin, the market may not be receptive to voreloxin.
Even if voreloxin obtains regulatory approval, it may not gain market acceptance among physicians, patients, healthcare payors and/or the medical community. We believe that the degree of market acceptance will depend on a number of factors, including:
| • | timing of market introduction of competitive products; |
| • | efficacy of our product; |
| • | prevalence and severity of any side effects; |
| • | potential advantages or disadvantages over alternative treatments; |
| • | strength of marketing and distribution support; |
| • | price of voreloxin, both in absolute terms and relative to alternative treatments; and |
| • | availability of reimbursement from health maintenance organizations and other third-party payors. |
For example, the potential toxicity of single and repeated doses of voreloxin has been explored in a number of animal studies that suggest the dose-limiting toxicities in humans receiving voreloxin may be similar to some of those observed with approved cytotoxic agents, including reversible toxicity to bone marrow cells, the gastrointestinal system and other systems with rapidly dividing cells. In our Phase 1 and Phase 2 clinical trials of voreloxin, we have witnessed the following side effects, irrespective of causality, ranging from mild to more severe: lowered white blood cell count that may lead to a serious or possibly life-threatening infection, hair loss, mouth sores, fatigue, nausea with or without vomiting, lowered platelet count, which may lead to an increase in bruising or bleeding, lowered red blood cell count (anemia), weakness, tiredness, shortness of breath, diarrhea and intestinal blockage.
If voreloxin fails to achieve market acceptance, due to unacceptable side effects or any other reasons, we may not be able to generate significant revenue or to achieve or sustain profitability.
Even if we receive regulatory approval for voreloxin, we will be subject to ongoing FDA and other regulatory obligations and continued regulatory review, which may result in significant additional expense and limit our ability to commercialize voreloxin.
Any regulatory approvals that we or our collaboration partners receive for voreloxin or our future product candidates, if any, may also be subject to limitations on the indicated uses for which the product may be marketed or contain requirements for potentially costly post-marketing studies. In addition, even if approved, the
28
Table of Contents
labeling, packaging, adverse event reporting, storage, advertising, promotion and recordkeeping for any product will be subject to extensive and ongoing regulatory requirements. The subsequent discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, may result in restrictions on the marketing of the product, and could include withdrawal of the product from the market.
Regulatory policies may change and additional government regulations may be enacted that could prevent or delay regulatory approval of our product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are not able to maintain regulatory compliance, we might not be permitted to market voreloxin or our future products and we may not achieve or sustain profitability.
The coverage and reimbursement status of newly approved drugs is uncertain, and failure to obtain adequate coverage and reimbursement could limit our ability to market voreloxin and decrease our ability to generate revenue.
There is significant uncertainty related to the third party coverage and reimbursement of newly approved drugs both nationally and internationally. The commercial success of voreloxin and our future products, if any, in both domestic and international markets depends on whether third-party coverage and reimbursement is available for the ordering of our future products by the medical profession for use by their patients. Medicare, Medicaid, health maintenance organizations and other third-party payors are increasingly attempting to manage healthcare costs by limiting both coverage and the level of reimbursement of new drugs and, as a result, they may not cover or provide adequate payment for our future products. These payors may not view our future products as cost-effective, and reimbursement may not be available to consumers or may not be sufficient to allow our future products to be marketed on a competitive basis. Likewise, legislative or regulatory efforts to control or reduce healthcare costs or reform government healthcare programs could result in lower prices or rejection of our future products. Changes in coverage and reimbursement policies or healthcare cost containment initiatives that limit or restrict reimbursement for our future products may reduce any future product revenue.
Failure to obtain regulatory approval in foreign jurisdictions will prevent us from marketing voreloxin abroad.
We intend to market voreloxin in international markets. In order to market voreloxin in Canada, the European Union and many other foreign jurisdictions, we must obtain separate regulatory approvals. We have had limited interactions with foreign regulatory authorities, and the approval procedures vary among countries and can involve additional testing at significant cost. The time required to obtain approval may differ from that required to obtain FDA approval. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. The foreign regulatory approval processes may include all of the risks associated with obtaining FDA approval. We may not obtain foreign regulatory approvals on a timely basis, if at all. We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize voreloxin or any other future products in any market.
Foreign governments often impose strict price controls, which may adversely affect our future profitability.
We intend to seek approval to market voreloxin in both the United States and foreign jurisdictions. If we obtain approval in one or more foreign jurisdictions, we will be subject to rules and regulations in those jurisdictions relating to voreloxin. In some foreign countries, particularly in the European Union, prescription drug pricing is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a drug candidate. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of voreloxin to other available therapies. If reimbursement of voreloxin is
29
Table of Contents
unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability.
We may incur significant costs complying with environmental laws and regulations, and failure to comply with these laws and regulations could expose us to significant liabilities.
We use hazardous chemicals and radioactive and biological materials in our business and are subject to a variety of federal, state, regional and local laws and regulations governing the use, generation, manufacture, storage, handling and disposal of these materials. Although we believe our safety procedures for handling and disposing of these materials and waste products comply with these laws and regulations, we cannot eliminate the risk of accidental injury or contamination from the use, storage, handling or disposal of hazardous materials. In the event of contamination or injury, we could be held liable for any resulting damages, and any liability could significantly exceed our insurance coverage, which is limited to $50,000 for pollution cleanup, and we are uninsured for third-party contamination injury.
Risks Related to Our Common Stock
If we fail to maintain compliance with the continued listing requirements of The NASDAQ Capital Market, our common stock may be delisted and the price of our common stock and our ability to access the capital markets could be negatively impacted.
On August 3, 2009, we transferred the listing of our common stock from The NASDAQ Global Market to The NASDAQ Capital Market. To maintain a listing on The NASDAQ Capital Market, we are required to meet certain requirements, including, but not limited to, a minimum closing bid price of $1.00 per share, or the Bid Price Requirement, a market value of publicly held shares (excluding shares held by our executive officers, directors and 10% or more stockholders) of at least $1.0 million, and either a market value of listed securities of at least $35.0 million, or the Market Value Standard, or stockholders’ equity of at least $2.5 million, or the Equity Standard.
We announced on September 18, 2009 that we had received a letter, dated September 16, 2009, from the NASDAQ Listing Qualifications Staff, or the Staff, notifying us that, for the last 30 consecutive business days, the bid price for our common stock had closed below the Bid Price Requirement. In accordance with NASDAQ Listing Rules, we were given 180 calendar days, or until March 15, 2010, to regain compliance. To regain compliance, the bid price of our common stock needed to close at or above $1.00 for at least 10 consecutive business days at any time prior to March 15, 2010, which it did in the 10 consecutive business days ending on December 23, 2009. On December 24, 2009, we received notification from NASDAQ that we had regained compliance with the Bid Price Requirement. As of March 30, 2010, the bid price for our common stock had closed below the Bid Price Requirement for 30 consecutive business days. As a result, we expect to receive a letter shortly from the Staff, notifying us that we do not satisfy the Bid Price Requirement, and, in accordance with NASDAQ’s Listing Rules, that we will be afforded 180 calendar days to regain compliance. There can be no assurance that we will be able to regain compliance.
As of December 31, 2009, we believe we complied with the Market Value Standard, which is an element of one of the alternative tests for continued listing on The NASDAQ Capital Market. However, there is no assurance that our market value of listed securities will remain above this level in the future. If it does not, and we fail to meet an alternative test for continued listing on The NASDAQ Capital Market (for example, the test for which the Equity Standard is an element), we expect to receive a further letter from NASDAQ notifying us that we do not comply with the requirements for continued listing. If we fail to meet the continued listing requirements of The NASDAQ Capital Market in the future, our common stock could be subject to delisting.
30
Table of Contents
If we are delisted, we would expect our common stock to be traded in the over-the-counter market, which could adversely affect the liquidity of our common stock. Additionally, we could face significant material adverse consequences, including:
| • | a limited availability of market quotations for our common stock; |
| • | a reduced amount of news and analyst coverage for us; |
| • | a decreased ability to issue additional securities or obtain additional financing in the future; |
| • | reduced liquidity for our stockholders; |
| • | potential loss of confidence by collaboration partners and employees; and |
| • | loss of institutional investor interest and fewer business development opportunities. |
The closing of the Private Placement has resulted and could result in further substantial dilution to our stockholders. If we sell shares of our common stock in future financings or other arrangements, stockholders may experience additional dilution.
The closing of the Private Placement has resulted and could result in further substantial dilution to our stockholders. Immediately following the second closing for $5.0 million of units in the Private Placement, the holders of our common stock immediately prior to the initial closing of the Private Placement held approximately 44.2% of our outstanding common stock (assuming conversion of the Series A convertible preferred stock at the current conversion price), and would hold approximately 28.3% if the warrants issued at the initial and second closings are exercised in full. If the common equity closing had occurred on March 17, 2010, the holders of our common stock prior to the Private Placement would have held approximately 17.4% of our outstanding common stock (assuming conversion of the Series A convertible preferred stock at the current conversion price), and 14.7% if the remaining warrants outstanding that were issued at the initial and second closings had been exercised in full as of that date.
We need to raise substantial additional funding to continue our operations, fund additional clinical trials of voreloxin and potentially commercialize voreloxin. We plan to continue to finance our operations with a combination of equity issuances (including the possible common equity closing in the Private Placement and subject to the satisfaction of the conditions described above), debt arrangements and a possible partnership or license of development and/or commercialization rights to voreloxin. Any issuance of convertible debt securities, preferred stock or common stock may be at a discount from the then-current trading price of our common stock. If we issue additional common or preferred stock or securities convertible into common stock, our stockholders will experience additional dilution, which may be significant.
We may not have sufficient funding to distribute capital to our common stockholders or continue our business upon a change of control event.
If a change of control (as that term is defined in the certificate of designation related to the convertible Series A convertible preferred stock), which includes a sale or merger of Sunesis or a significant partnering transaction, occurs, the holders of the Series A convertible preferred stock would be entitled to receive, before any proceeds are distributed to common stockholders, three times the amount that the investors in the Private Placement paid for the units (i.e. three times the total of $15.0 million invested in the initial and second closings, or $45.0 million). We would not have any capital to distribute to our common stockholders if the consideration received in a transaction that triggers a change of control event under the certificate of designation is less than this liquidation preference amount. Further, if the investors elect to treat a partnering transaction as a change of
31
Table of Contents
control, entitling the holders of the convertible preferred to the liquidation preference described above, the holders of the Series A convertible preferred stock would be entitled to the full amount of any payments made by a corporate partner by surrendering the Series A convertible preferred stock, up to the liquidation preference amount, which may leave us with insufficient resources to continue our business. This right of the holders of the Series A convertible preferred stock may also impair our ability to enter into a significant partnering transaction since a partner would be willing to enter into a partnering agreement with us only if we have or had access to sufficient capital to satisfy our obligations under the partnering agreement. Whether or not we would have sufficient resources would depend on the terms of the partnering agreement and other cash resources available to us at that time.
We cannot take fundamental actions related to Sunesis without the consent of a majority of the holders of the convertible preferred stock issued in the Private Placement.
For as long as our convertible Series A convertible preferred stock is outstanding, the holders of the Series A convertible preferred stock will have a number of rights, including the right to approve any sale of the company, any significant partnering transaction, any issuance of debt or convertible preferred, and any issuance of common stock other than the common equity closing contemplated by the Private Placement. It is possible that the interests of the holders of the Series A convertible preferred stock and the holders of common stock may be inconsistent, resulting in the inability to obtain the consent of the holders of Series A convertible preferred stock to matters that may be in the best interests of the common stockholders.
The price of our common stock may continue to be volatile, and the value of an investment in our common stock may decline.
In 2009, our common stock traded as low as $0.05 and as high as $2.43. Factors that could cause continued volatility in the market price of our common stock include, but are not limited to:
| • | failure to raise additional capital to carry through with our clinical development plans and current and future operations; |
| • | results from, and any delays in or discontinuance of, ongoing and planned clinical trials for voreloxin; |
| • | announcements of FDA non-approval of voreloxin, delays in filing regulatory documents with the FDA or other regulatory agencies, or delays in the review process by the FDA or other foreign regulatory agencies; |
| • | announcements relating to our collaboration with Biogen Idec; |
| • | announcements relating to restructuring and other operational changes; |
| • | delays in the commercialization of voreloxin or our future products, if any; |
| • | market conditions in the pharmaceutical, biopharmaceutical and biotechnology sectors; |
| • | issuance of new or changed securities analysts’ reports or recommendations; |
| • | actual and anticipated fluctuations in our quarterly operating results; |
| • | developments or disputes concerning our intellectual property or other proprietary rights; |
| • | introduction of new products by our competitors; |
32
Table of Contents
| • | issues in manufacturing voreloxin drug substance or drug product, or future products, if any; |
| • | market acceptance of voreloxin or our future products, if any; |
| • | deviations in our operating results from the estimates of analysts; |
| • | third-party healthcare reimbursement policies; |
| • | FDA or other U.S. or foreign regulatory actions affecting us or our industry; |
| • | litigation or public concern about the safety of voreloxin or future products, if any; |
| • | failure to develop or sustain an active and liquid trading market for our common stock; |
| • | sales of our common stock by our officers, directors or significant stockholders; and |
| • | additions or departures of key personnel. |
In addition, the stock markets in general, and the markets for pharmaceutical, biopharmaceutical and biotechnology stocks in particular, have experienced extreme volatility that has often been unrelated to the operating performance of the issuer. These broad market fluctuations may adversely affect the trading price or liquidity of our common stock. In the past, when the market price of a stock has been volatile, holders of that stock have sometimes instituted securities class action litigation against the issuer. If any of our stockholders were to bring such a lawsuit against us, we could incur substantial costs defending the lawsuit and the attention of our management would be diverted from the operation of our business.
Provisions of our charter documents or Delaware law could delay or prevent an acquisition of our company, even if the acquisition would be beneficial to our stockholders, and could make it more difficult to change management.
Provisions of our amended and restated certificate of incorporation and amended and restated bylaws may discourage, delay or prevent a merger, acquisition or other change in control that stockholders might otherwise consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. In addition, these provisions may frustrate or prevent any attempt by our stockholders to replace or remove our current management by making it more difficult to replace or remove our board of directors. These provisions include:
| • | a classified board of directors so that not all directors are elected at one time; |
| • | a prohibition on stockholder action through written consent; |
| • | limitations on our stockholders’ ability to call special meetings of stockholders; |
| • | an advance notice requirement for stockholder proposals and nominations; and |
| • | the authority of our board of directors to issue preferred stock with such terms as our board of directors may determine. |
In addition, Delaware law prohibits a publicly held Delaware corporation from engaging in a business combination with an interested stockholder, generally a person who, together with its affiliates, owns or within the last three years has owned 15% of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a
33
Table of Contents
prescribed manner. Accordingly, Delaware law may discourage, delay or prevent a change in control of our company.
Provisions in our charter documents and provisions of Delaware law could limit the price that investors are willing to pay in the future for shares of our common stock.
The ownership of our capital stock is highly concentrated, and your interests may conflict with the interests of our existing stockholders.
Our executive officers and directors and their affiliates beneficially owned approximately 41.8% of our outstanding capital stock as of December 31, 2009, assuming the conversion of the Series A convertible preferred stock and the exercise in full of the warrants to purchase common stock held by these stockholders as of such date. Accordingly, these stockholders, acting as a group, could have significant influence over the outcome of corporate actions requiring stockholder approval, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets or any other significant corporate transaction. The significant concentration of stock ownership may adversely affect the trading price of our common stock due to investors’ perception that conflicts of interest may exist or arise.
We have never paid dividends on our capital stock and we do not anticipate paying any cash dividends in the foreseeable future.
We have never declared or paid cash dividends on our capital stock. We do not anticipate paying any cash dividends on our capital stock in the foreseeable future. We currently intend to retain all available funds and any future earnings to fund the development and growth of our business. As a result, capital appreciation, if any, of our common stock will be our stockholders’ sole source of gain for the foreseeable future.
We are at risk of securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology companies have experienced greater than average stock price volatility in recent years. If we faced such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
| ITEM 2. | PROPERTIES |
Prior to January 15, 2009, we leased approximately 54,000 square feet of office and laboratory space at 341 Oyster Point Boulevard in South San Francisco, California. As a result of the 2008 Restructuring, we vacated this building and consolidated our remaining employees to 395 Oyster Point Boulevard and 349 Allerton Avenue, as described below. In January 2009, we signed an agreement for the termination of the lease at 341 Oyster Point Boulevard and voluntarily surrendered the premises to our landlord.
In December 2006, we leased approximately 15,000 square feet of office space at 395 Oyster Point Boulevard in South San Francisco, California, which is currently our corporate headquarters. This lease expires in April 2013, subject to our option to extend the lease through February 2014. In October 2008, we leased approximately 5,500 square feet of laboratory space at 349 Allerton Avenue, South San Francisco, California. This lease expires in October 2010, with an option to extend the lease through October 2012. We believe that our current facilities will be sufficient to meet our needs through 2010.
34
Table of Contents
| ITEM 3. | LEGAL PROCEEDINGS |
From time to time, we may be involved in routine legal proceedings, as well as demands, claims and threatened litigation, which arise in the normal course of our business. The ultimate outcome of any litigation is uncertain and unfavorable outcomes could have a negative impact on our results of operations and financial condition. Regardless of outcome, litigation can have an adverse impact on us because of the defense costs, diversion of management resources and other factors.
We believe there is no litigation pending that could, individually or in the aggregate, have a material adverse effect on our results of operations or financial condition.
| ITEM 4. | (REMOVED AND RESERVED) |
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Our common stock is listed on the NASDAQ Capital Market under the symbol “SNSS”. From our initial public offering on September 27, 2005 until August 3, 2009 our common stock was listed on the NASDAQ Global Market under the same symbol. The following table sets forth the range of the high and low sales prices by quarter, as reported by NASDAQ.
| Year-Ended December 31, 2008 |
High | Low | ||||
| First Quarter |
$ | 2.01 | $ | 1.01 | ||
| Second Quarter |
$ | 2.10 | $ | 1.00 | ||
| Third Quarter |
$ | 1.85 | $ | 0.86 | ||
| Fourth Quarter |
$ | 1.31 | $ | 0.18 | ||
| Year-Ended December 31, 2009 |
High | Low | ||||
| First Quarter |
$ | 0.51 | $ | 0.16 | ||
| Second Quarter |
$ | 0.90 | $ | 0.05 | ||
| Third Quarter |
$ | 0.56 | $ | 0.26 | ||
| Fourth Quarter |
$ | 2.43 | $ | 0.27 | ||
As of March 17, 2010, there were approximately 163 holders of record of our common stock. In addition, we believe that a significant number of beneficial owners of our common stock hold their shares in nominee or in “street name” accounts through brokers. On March 17, 2010, the last sale price reported on the NASDAQ Capital Market for our common stock was $0.79 per share.
Dividend Policy
We have never paid cash dividends on our common stock. Any payment of dividends must be approved by the holders of at least a majority of the outstanding Series A Preferred Stock. We do not anticipate paying any cash dividends on our capital stock in the foreseeable future. While subject to periodic review, the current policy of our Board of Directors is to retain cash and investments primarily to provide funds for our future growth.
Unregistered Sales of Equity Securities
On April 3, 2009, we sold $10.0 million of units consisting of shares of our Series A convertible preferred stock and warrants to purchase our common stock in an initial closing of the Private Placement. On October 30, 2009, we sold $5.0 million of units in the second closing. The sales were to accredited investors, including
35
Table of Contents
certain members of management, and were exempt from the registration requirements of the Securities Act of 1933, as amended, pursuant to Rule 506 of Regulation D promulgated thereunder. We have used, and expect to use, the aggregate net proceeds of $13.4 million for working capital and other general corporate purposes.
In connection with the initial closing, we issued to the investors 2,898,544 shares of Series A convertible preferred stock, which are initially convertible into 28,985,440 shares of common stock, and warrants to purchase 28,985,440 shares of common stock. In connection with the second closing, we issued 1,449,268 shares of Series A convertible preferred stock, which are initially convertible into 14,492,680 shares of common stock, and warrants to purchase 14,492,680 shares of common stock. Each share of Series A convertible preferred stock is initially convertible into 10 shares of common stock, subject to adjustment for any stock dividends, combinations, stock splits, recapitalizations and the like. All outstanding shares of Series A convertible preferred stock are automatically converted into shares of common stock at the then-current conversion rate upon the earlier to occur of: (i) the affirmative election of the holders of at least a majority of the outstanding shares of the Series A convertible preferred stock; (ii) following the closing of a qualifying alternative common stock financing, on which the closing bid price has been equal to or at least $0.66 per share for a period of 30 trading days with an average trading volume during such period of at least 200,000 shares, or (iii) the common equity closing. Each holder of Series A convertible preferred stock also has the right to convert its Series A convertible preferred stock into common stock at the then-current conversion ratio at any time after the earlier of (i) the closing of a qualifying alternative common stock financing or (ii) January 24, 2011. In the event an investor fails to purchase its pro rata portion in the common equity closing, a pro rata portion (based on the extent of such investor’s failure to participate) of the shares of Series A convertible preferred stock then held by such investor (or all shares of Series A convertible preferred stock then held by the investor if the investor fails to participate at all) would automatically convert into common stock at a 1-to-1 conversion rate.
The warrants to purchase common stock may be exercised at the election of the holder at any time during their term of seven years from the date of issuance.
36
Table of Contents
| ITEM 6. | SELECTED FINANCIAL DATA |
The following selected financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and notes to those statements included elsewhere in this report.
| Year Ended December 31, | ||||||||||||||||||||
| Consolidated Statement of Operations: |
2009 | 2008 | 2007 | 2006 | 2005 | |||||||||||||||
| (In thousands, except per share amounts) | ||||||||||||||||||||
| Revenue: |
||||||||||||||||||||
| Collaboration revenue—related party |
$ | 1,500 | $ | 4,310 | $ | 7,587 | $ | 7,318 | $ | 9,018 | ||||||||||
| Collaboration revenue—other |
50 | 607 | 1,576 | 6,353 | 7,395 | |||||||||||||||
| License and other revenue |
2,212 | 500 | 500 | 38 | 109 | |||||||||||||||
| Total revenues |
3,762 | 5,417 | 9,663 | 13,709 | 16,522 | |||||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Research and development |
13,247 | 26,285 | 36,060 | 35,615 | 36,166 | |||||||||||||||
| General and administrative |
7,748 | 11,524 | 13,570 | 12,255 | 8,283 | |||||||||||||||
| Restructuring charges |
1,916 | 5,783 | 1,563 | — | — | |||||||||||||||
| Total operating expenses |
22,911 | 43,592 | 51,193 | 47,870 | 44,449 | |||||||||||||||
| Loss from operations |
(19,149 | ) | (38,175 | ) | (41,530 | ) | (34,161 | ) | (27,927 | ) | ||||||||||
| Interest income |
22 | 929 | 2,972 | 3,395 | 1,092 | |||||||||||||||
| Interest expense |
(1 | ) | (172 | ) | (210 | ) | (478 | ) | (674 | ) | ||||||||||
| Other income (expense), net(1) |
(21,098 | ) | 232 | 7 | 7 | 10 | ||||||||||||||
| Net loss |
(40,226 | ) | (37,186 | ) | (38,761 | ) | (31,237 | ) | (27,499 | ) | ||||||||||
| Deemed distribution to preferred stockholders(2) |
(27,563 | ) | — | — | — | (88,092 | ) | |||||||||||||
| Loss attributable to common stockholders |
$ | (67,789 | ) | $ | (37,186 | ) | $ | (38,761 | ) | $ | (31,237 | ) | $ | (115,591 | ) | |||||
| Basic and diluted loss attributable to common stockholders per common share |
$ | (1.97 | ) | $ | (1.08 | ) | $ | (1.20 | ) | $ | (1.13 | ) | $ | (17.41 | ) | |||||
| Shares used in computing basic and diluted loss attributable to common stockholders per common share |
34,480,716 | 34,387,177 | 32,340,203 | 27,758,348 | 6,637,935 | |||||||||||||||
| (1) | During 2009, we recorded non-cash charges of $21.0 million related to the accounting for the fair values of securities issued as part of a private placement, which provided for the sale of up to $15.0 million of units consisting of Series A convertible preferred stock and warrants to purchase common stock, and up to $28.5 million of common stock, in three closings. |
| (2) | During 2009, we recorded deemed distributions to preferred stockholders totaling $27.6 million, related to the accounting for the private placement of our securities. Of this amount, $26.4 million was due to the revaluation of certain securities upon an amendment of the private placement agreements in June 2009, and $1.2 million was due to the write-off of a discount for a beneficial conversion feature on the convertible preferred stock issued as part of the second closing of the private placement in October 2009. |
37
Table of Contents
| As of December 31, | ||||||||||||||||||||
| Consolidated Balance Sheet Data: |
2009 | 2008 | 2007 | 2006 | 2005 | |||||||||||||||
| (In thousands) | ||||||||||||||||||||
| Cash, cash equivalents and marketable securities |
$ | 4,259 | $ | 10,619 | $ | 47,684 | $ | 63,105 | $ | 48,333 | ||||||||||
| Working capital |
1,807 | 5,371 | 39,707 | 55,279 | 40,156 | |||||||||||||||
| Total assets |
5,169 | 12,784 | 53,246 | 69,276 | 54,708 | |||||||||||||||
| Long-term portion of equipment leases |
— | — | 1,353 | 956 | 1,306 | |||||||||||||||
| Convertible preferred stock |
60,005 | — | — | — | — | |||||||||||||||
| Common stock and additional paid-in capital |
298,473 | 322,675 | 320,583 | 298,077 | 249,692 | |||||||||||||||
| Accumulated deficit |
(356,418 | ) | (316,192 | ) | (279,006 | ) | (240,245 | ) | (209,008 | ) | ||||||||||
| Total stockholders’ equity |
2,060 | 6,491 | 41,394 | 56,804 | 38,466 | |||||||||||||||
38
Table of Contents
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion and analysis of our financial condition as of December 31, 2009 and results of operations for the year ended December 31, 2009 should be read together with our consolidated financial statements and related notes included elsewhere in this report. This discussion and analysis contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that involve risks, uncertainties and assumptions. All statements, other than statements of historical facts, are “forward-looking statements” for purposes of these provisions, including without limitation any statements relating to the completion of any financing transaction or the satisfaction of closing conditions relating to any financing, any projections of revenue, expenses or other financial items, any statement of the plans and objectives of management for future operations, any statements concerning proposed new clinical trials or licensing or collaborative arrangements, any statements regarding future economic conditions or performance, and any statement of assumptions underlying any of the foregoing. In some cases, forward-looking statements can be identified by the use of terminology such as “anticipates,” “believe,” “continue,” “estimates,” “expects,” “intend,” “look forward,” “may,” “could,” “seeks,” “plans,” “potential,” or “will” or the negative thereof or other comparable terminology. Although we believe that the expectations reflected in the forward-looking statements contained herein are reasonable, there can be no assurance that such expectations or any of the forward-looking statements will prove to be correct, and actual results could differ materially from those projected or assumed in the forward-looking statements. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors, including but not limited to those set forth under “Risk Factors,” and elsewhere in this report. We urge you not to place undue reliance on these forward-looking statements, which speak only as of the date of this report. All forward-looking statements included in this report are based on information available to us on the date of this report, and we assume no obligation to update any forward-looking statements contained in this report.
Overview
We are a biopharmaceutical company focused on the development and commercialization of new oncology therapeutics for the treatment of hematologic and solid tumor cancers. Our efforts are currently focused primarily on the development of voreloxin for the treatment of acute myeloid leukemia, or AML. We have built a highly experienced cancer drug development organization committed to advancing our lead product candidate, voreloxin, in multiple indications to improve the lives of people with cancer.
We own worldwide development and commercialization rights to voreloxin and are currently preparing for anticipated Phase 3 development of the compound. Voreloxin is a first-in-class anti-cancer quinolone derivative, or AQD—a class of compounds that has not been used previously for the treatment of cancer. Quinolone derivatives have been shown to mediate anti-tumor activity by targeting mammalian topoisomerase II, an enzyme critical for cell replication, and have demonstrated promising preclinical anti-tumor activity.
We are currently completing three clinical trials of voreloxin: (i) a Phase 2 clinical trial (known as the REVEAL-1 trial) in previously untreated elderly patients with AML, for which enrollment was completed in October 2009, with a total of 113 patients dosed in one of three dosing schedules, (ii) a Phase 1b/2 clinical trial of voreloxin in combination with cytarabine for the treatment of patients with relapsed/refractory AML, for which enrollment was completed in January 2010, with a total of 108 patients dosed, and (iii) a Phase 2 single agent clinical trial in platinum-resistant ovarian cancer patients, for which enrollment was completed in December 2008, with a total of 137 patients dosed across one of three dosing schedules. In November 2009, we announced that the U.S. Food and Drug Administration, or FDA, had granted voreloxin orphan drug designation for the treatment of AML. In February 2010, we announced that we received formal guidance from the FDA from End-of-Phase 2 meetings regarding further development of voreloxin for AML. Based on this guidance, we will look to conduct a randomized, double-blind, placebo-controlled, pivotal trial evaluating the effect on overall survival of voreloxin in combination with cytarabine, a widely used chemotherapy in AML, compared to placebo
39
Table of Contents
with cytarabine, in patients with relapsed or refractory AML. We anticipate initiating this multi-national Phase 3 trial in the second half of 2010. Management is currently in the process of evaluating alternatives for funding the voreloxin development program.
The most recent data from our two Phase 2 trials of voreloxin in AML were presented at the 51st Annual Meeting of the American Society of Hematology (ASH) in December 2009. The most recent data from the Phase 2 trial of voreloxin in platinum-resistant ovarian cancer were presented at the American Society of Clinical Oncology (ASCO) 2009 Annual Meeting in June 2009. We believe the data from these three ongoing clinical trials demonstrate that voreloxin shows promising safety and efficacy in AML and in platinum-resistant ovarian cancer.
During the year, we have taken a number of important steps to focus our resources and efforts on the advancement of voreloxin:
| • | We discontinued development of our product candidate, SNS-032, a selective inhibitor of cyclin-dependent kinases, or CDKs, 2, 7 and 9, which we had in-licensed from Bristol-Myers Squibb Company, or BMS. In March 2009, the license agreement was terminated and SNS-032 was returned to BMS. |
| • | In the first quarter of 2009, we completed a Phase 1 trial of SNS-314, a potent and selective pan-Aurora kinase inhibitor discovered internally at Sunesis, in patients with advanced solid tumors. As a maximum tolerated dose was not established in the trial and no responses were observed, further development of SNS-314 was suspended. |
| • | In March 2009, we announced the sale of our interest in all of our lymphocyte function-associated antigen-1, or LFA-1, patents and related know-how to SARcode Corporation, or SARcode, for total cash consideration of $2.0 million, which was recorded as revenue in the second quarter. In connection with the sale, the license agreement was terminated. SARcode had been the exclusive licensee of those assets since March 2006. |
| • | In February 2010, we granted Carmot Therapeutics, Inc. an exclusive license to our proprietary fragment-based lead discovery technology. We retain full rights to the technology for use in our future internal discovery efforts. |
In July 2009, we received a milestone of $1.5 million pursuant to a collaboration entered into with Biogen Idec in 2002 for Biogen Idec’s selection of a Raf kinase inhibitor development candidate for the treatment of cancer, which was earned and recorded as revenue in the second quarter. Biogen Idec is currently conducting IND-enabling preclinical work with the Raf kinase development candidate.
On March 31, 2009, we entered into a securities purchase agreement with accredited investors, including certain members of management, providing for a private placement of up to $43.5 million, or the Private Placement. We completed the initial closing of $10.0 million of the Private Placement on April 3, 2009, and the second closing of $5.0 million on October 30, 2009. In the initial closing, $10.0 million of units were sold, resulting in net proceeds of $8.8 million, and in the second closing, $5.0 million of units were sold, resulting in net proceeds of $4.7 million. The units consist of Series A convertible preferred stock and warrants to purchase common stock. The Private Placement also contemplates the sale of up to the remaining $28.5 million in common stock at $0.275 per share to the same group of investors, subject to terms and conditions described in ‘Sources of Liquidity’ below.
On January 20, 2010, we entered into a controlled equity offering sales agreement with Cantor Fitzgerald & Co., or Cantor, pursuant to which we could issue and sell shares of our common stock having an aggregate offering price of up to $15.0 million from time to time through Cantor acting as agent and/or principal. Sales of our common stock through Cantor, if any, would be made on the NASDAQ Capital Market by means of
40
Table of Contents
ordinary brokers’ transactions at market prices, in block transactions or as otherwise agreed by Cantor and us. Cantor agreed to use its best efforts to sell our common stock from time to time, based upon our instructions (including any price, time or size limits or other customary parameters or conditions we might impose). We agreed to pay Cantor a commission rate ranging between 3.0% and 5.0% of the gross sales price per share of any common stock sold through Cantor as agent under the sales agreement. We also agreed to reimburse Cantor for certain expenses incurred in connection with entering into the sales agreement and provided Cantor with customary indemnification rights. Under the terms of the sales agreement, we may also sell shares of our common stock to Cantor, as principal for its own account, at a price negotiated at the time of sale. If we sell shares to Cantor in this manner, we will enter into a separate agreement setting forth the terms of any such transactions. As of March 31, 2010, pursuant to the sales agreement with Cantor, we had sold an aggregate of 15,870,050 shares of common stock at an average price of approximately $0.95 per share for gross proceeds of the full $15.0 million available under the facility. Net proceeds were $14.2 million after deducting Cantor’s commission and costs to set up the facility.
We have incurred significant losses in each year since our inception. As of December 31, 2009, we had an accumulated deficit of $356.4 million. We expect to continue to incur significant net losses for the foreseeable future, as we continue the development of, and seek regulatory approvals for voreloxin. We believe that currently available cash, cash equivalents and marketable securities, including the net proceeds of $14.2 million from sales of common stock through March 31, 2010 under the agreement with Cantor, are sufficient to fund our operations through at least September 30, 2010. We will need to raise substantial additional funding in the near term in order to sustain operations beyond that date and before undertaking any additional clinical trials of voreloxin. Our significant negative cash flows and lack of financial resources raise substantial doubt as to our ability to continue as a going concern. Management is currently in the process of evaluating alternative funding sources. If we are unable to raise additional funding to meet our working capital needs, we will be forced to delay or reduce the scope of our voreloxin development program and/or limit or cease our operations.
On August 3, 2009, upon NASDAQ’s approval, the listing of our common stock was transferred from The NASDAQ Global Market to The NASDAQ Capital Market. To maintain a listing on The NASDAQ Capital Market, we are required to meet certain requirements, including a minimum closing bid price of $1.00 per share, a market value of publicly held shares of at least $1.0 million, and either a market value of listed securities of at least $35.0 million or stockholders’ equity of at least $2.5 million.
On September 16, 2009, we received a letter from the NASDAQ Listing Qualifications Staff, or the Staff, notifying us that we do not comply with the minimum $1.00 per share requirement for a continued listing, or the Bid Price Requirement. In accordance with NASDAQ Listing Rules, we were given 180 calendar days, or until March 15, 2010, to regain compliance. To regain compliance, the bid price of our common stock needed to close at or above $1.00 for at least 10 consecutive business days at any time prior to March 15, 2010, which it did in the 10 consecutive business days ending on December 23, 2009. On December 24, 2009, we received notification from NASDAQ that we had regained compliance with the minimum $1.00 per share Bid Price Requirement. As of March 30, 2010, the bid price for our common stock had closed below the required $1.00 per share minimum for 30 consecutive business days. As a result, we expect to receive a letter shortly from the Staff, notifying us that we do not satisfy the minimum $1.00 per share Bid Price Requirement, and, in accordance with NASDAQ’s Listing Rules, that we will be afforded 180 calendar days to regain compliance.
Critical Accounting Policies and the Use of Estimates
The accompanying discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements and the related disclosures, which have been prepared in accordance with U.S. generally accepted accounting principles, or GAAP. The preparation of these consolidated financial statements requires our management to make estimates, assumptions and judgments that affect the amounts reported in our financial statements and accompanying notes, including reported amounts of assets, liabilities and expenses and the disclosure of contingent assets and liabilities at the date of the consolidated financial
41
Table of Contents
statements, as well as revenue and expenses during the reporting periods. We evaluate our estimates, assumptions and judgments on an ongoing basis. We base our estimates on historical experience and on various other assumptions we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Management has discussed the development, selection and disclosure of these estimates with the Audit Committee of our Board of Directors. Actual results could differ materially from these estimates under different assumptions or conditions.
Our significant accounting policies are more fully described in Note 1 to our consolidated financial statements included elsewhere in this report. We believe the following critical accounting policies reflect our more significant estimates and assumptions used in the preparation of our consolidated financial statements.
Revenue Recognition
Revenue arrangements with multiple deliverables are accounted for in accordance with Financial Accounting Standards Board, or FASB, Accounting Standards Codification, or ASC, Subtopic 605-25, Multiple-Element Arrangements, or ASC 605-25. Under ASC 605-25, revenue arrangements with multiple deliverables are divided into separate units of accounting based on whether certain criteria are met, including whether the delivered item has stand-alone value to the customer and whether there is objective and reliable evidence of the fair value of the undelivered items. Consideration is allocated among the separate units of accounting based on their respective fair value, and the applicable revenue recognition is applied to each of the separate units.
Non-refundable fees where we have no continuing performance obligations are recognized as revenues when collection is reasonably assured. In situations where continuing performance obligations exist, nonrefundable fees are deferred and recognized ratably over the projected performance period.
Research funding from collaborations is recognized as revenue as the related research services are performed. This funding is generally based on a specified amount per full-time equivalent employee per year.
Milestone payments which are substantive and at risk at the time of the execution of the collaboration agreement, are recognized upon completion of the applicable milestone event. Any future royalty revenue will be recognized based on reported product sales by third-party licensees.
Clinical Trial Accounting
We record accruals for estimated clinical trial costs, which include payments for work performed by contract research organizations, or CROs, and participating clinical trial sites. These costs are generally a significant component of research and development expenses. Non-refundable costs of setting up clinical trial sites for participation in trials are expensed immediately, with any refundable advances related to enrollment of the first patient recorded as prepayments and assessed for recoverability on a quarterly basis. Costs related to patient enrollment are accrued as patients progress through the clinical trial, including amortization of any first-patient prepayments. This amortization generally matches when the related services are rendered, however, these cost estimates may or may not match the actual costs incurred by the CROs or clinical trial sites, and if we have incomplete or inaccurate information, our clinical trial accruals may not be accurate. The difference between accrued expenses based on our estimates and actual expenses have not been material to date.
Stock-Based Compensation
We grant options to purchase common stock to our employees, directors and consultants under our stock option plans. Under our Employee Stock Purchase Plan, eligible employees can also purchase shares of common stock at 85% of the lower of the fair market value of our common stock at the beginning of a 12-month offering period or at the end of one of the two related 6-month purchase periods.
42
Table of Contents
We value these share-based awards using the Black-Scholes model. The determination of fair value of share-based payment awards on the date of grant using the Black-Scholes model is affected by our stock price as well as assumptions regarding a number of highly complex and subjective variables. These variables include, but are not limited to, the expected stock price volatility over the term of the awards and actual and projected employee stock option exercise behaviors. Changes in these input variables would affect stock-based compensation expense.
Private Placement Accounting
The accounting for the initial and second closing of the sale of $10.0 million and $5.0 million of units, respectively, under our Private Placement, and subsequent revaluations of the related financial instruments, required fair values to be established at different dates, either individually or in aggregate, for the four primary components of the Private Placement: (a) the Series A convertible preferred stock, (b) the warrants to purchase common stock, (c) the option for the investors to participate in the second closing, or the Second Closing Option, and (d) the option for the investors to participate in the common equity closing, or the Common Equity Closing Option. The Option-Pricing Method, which utilizes the Black-Scholes model, was selected to determine these fair values, which were calculated as a series of call options on the potential enterprise value of the company at different valuation points at which the claims of the different stakeholder groups on the enterprise value would change. The results of the Black-Scholes model are affected by the company’s stock price, as well as assumptions regarding a number of highly subjective variables. These variables include the expected term of the financial instruments and our expected stock price volatility, risk-free interest rate and dividend rate over the expected term.
Alternative models could have been selected to calculate these fair values, which may have produced significantly different results. If we adopt a different valuation model in the future, this may result in a lack of consistency between periods and materially affect our fair value estimates. It may also result in a lack of comparability with other companies that use different models, methods and assumptions. Additionally, because the estimated fair values are affected by our stock price, fluctuations in our stock price, which has historically been volatile, may significantly affect our financial results.
Recent Accounting Pronouncements
The impact of recent accounting pronouncements that we have adopted is detailed in Note 1 to our consolidated financial statements.
Overview of Revenues
We have not generated any revenue from sales of commercial products and do not expect to generate any product revenue in the foreseeable future.
Collaboration Revenue
Over the past three years, we generated revenue primarily through payments received in connection with our collaborations with Biogen Idec, Inc., Johnson & Johnson Pharmaceutical Research & Development LLC, or J&JPRD, and Merck & Co., Inc., or Merck, consisting principally of revenue recognized from the amortization of upfront fees, and research funding and milestones paid by our collaborators, which substantially offset our related research and development expenses. From January 1, 2007 to December 31, 2009, we recorded an aggregate of $15.6 million in revenues from our collaboration partners. In 2007, 2008 and 2009, we received $7.6 million, $4.3 million and $1.5 million, respectively, from Biogen Idec, which represented 79%, 80% and 40% of our total revenue for these periods. Likewise, during this same three-year period, we received $1.6 million, $0.1 million and $50,000, respectively, from Merck, which represented 16%, 2% and 1% of our total revenues for these periods.
43
Table of Contents
As of March 31, 2010, our only remaining ongoing collaboration is with Biogen Idec. Our collaboration with Merck will terminate effective as of June 8, 2010 and our collaboration with J&JPRD terminated on January 13, 2010.
We are entitled to receive milestone payments under our collaboration with Biogen Idec upon the achievement of certain milestones by them. Additionally, we are entitled to receive royalty payments based on future sales of products, if any, resulting from this collaboration, although we do not expect to generate any royalty revenue from this collaboration in the foreseeable future, if at all. We expect to have substantially lower collaboration revenue in 2010 and in future years from our existing collaboration with Biogen Idec unless any products that may result from it advance to a level where significant milestones will be payable to us.
License and other revenue
In March 2009, SARcode acquired our interest in all of its LFA-1 patents and related know-how for a total cash consideration of $2.0 million, which was recorded as revenue in April 2009. In connection with the sale, the license agreement was terminated and we will not receive any future license fees, milestones or royalties under that license. We still hold three secured convertible promissory notes issued under the original license agreement, with a total principal value of $1.0 million, which are due in 2012 and are convertible into the preferred stock of SARcode at our option. We have yet to record the amount represented by these notes as revenue, due to uncertainty of their collectibility.
Overview of Operating Expenses
Research and Development Expense. Most of our operating expenses to date have been related to research and development activities, and include costs incurred:
| • | in the discovery and development of novel small molecule therapeutics and the advancement of product candidates towards clinical trials; |
| • | in the execution of clinical trials, including those for voreloxin; |
| • | in the development of novel fragment-based drug discovery methods; |
| • | in the development of in-house research, preclinical study and development capabilities; |
| • | in connection with in-licensing activities; and |
| • | in the conduct of activities we are required to perform in connection with our strategic collaborations. |
We expense all research and development costs as they are incurred.
The table below sets forth our research and development expense by program for each period presented:
| Year Ended December 31, | |||||||||
| 2009 | 2008 | 2007 | |||||||
| (In thousands) | |||||||||
| Voreloxin |
$ | 12,802 | $ | 16,544 | $ | 13,699 | |||
| SNS-032 |
236 | 3,480 | 3,723 | ||||||
| SNS-314 |
209 | 2,004 | 4,563 | ||||||
| Discovery programs and new technologies |
— | 2,233 | 4,128 | ||||||
| Other kinase inhibitors |
— | 2,024 | 9,947 | ||||||
| Total |
$ | 13,247 | $ | 26,285 | $ | 36,060 | |||
44
Table of Contents
As a result of the corporate realignment and reduction in force completed in June 2008, or the 2008 Restructuring, and the resulting wind-down of our research activities, we do not anticipate incurring any significant additional research expenses related to the discovery of additional product candidates, the development or application of our proprietary fragment-based drug discovery methods, or the development of in-house research capabilities. In addition, we are no longer conducting any research activities in connection with our collaborations.
As of December 31, 2009, we had incurred $63.7 million of expenses in the development of voreloxin since it was licensed from Dainippon Sumitomo Pharma Co., Ltd. in October 2003, and we expect to continue to incur significant expenses related to the development of voreloxin in 2010 and future years, including for the completion of the current Phase 2 clinical trials and in preparation for and conduct of anticipated pivotal trials. As a result, we expect research and development expense to increase significantly in 2010 as compared to 2009. However, due to the risks inherent in the clinical trial process, we are unable to estimate the additional substantial costs we will incur in the voreloxin development program.
We are currently focused on clinical trials of voreloxin in targeted indications and patient populations. Based on results of translational research, clinical results, regulatory and competitive concerns and our overall financial resources, we anticipate that we will make determinations as to which indications to pursue and patient populations to treat and how much funding to direct to each indication on an ongoing basis. This will affect our research and development expense going forward.
We are currently anticipating that development of voreloxin will be our highest priority. If we engage a development or commercialization partner for our voreloxin program, or if, in the future, we acquire additional product candidates, our research and development expenses could be significantly affected. We cannot predict whether future collaborative or licensing arrangements will be secured, if at all, and to what degree such arrangements would affect our development plans and capital requirements.
Under our Biogen Idec agreement, we have the right to participate in the co-development and co-promotion of product candidates for up to two targets including, at our option, the Raf kinase target, on a worldwide basis (excluding Japan). If we were to exercise our option on one or more product candidates, our research and development expense would increase significantly.
General and Administrative Expense. Our general and administrative expense consists primarily of salaries and other related costs for personnel in finance, legal, marketing, information technology, administration and general management, as well as non-cash stock-based compensation. Other significant costs include those related to facilities and fees paid to outside legal advisors and our independent registered public accounting firm. In 2010, we expect general and administrative expense to be generally comparable to 2009.
Results of Operations
Years Ended December 31, 2009 and 2008
Revenue. Total revenue decreased to $3.8 million in 2009 from $5.4 million in 2008. Collaboration revenue decreased to $1.6 million in 2009 from $4.9 million in 2008, primarily due to the completion of research funding and technology access fee amortization under the Biogen Idec collaboration in June 2008, partially offset by an increase in milestone revenue in 2009 as a result of a $1.5 million milestone earned from Biogen Idec’s selection of a Raf kinase inhibitor development candidate for the treatment of cancer. License and other revenue increased to $2.2 million in 2009 from $0.5 million in 2008, primarily due to the sale to SARcode in March 2009 of our interest in all patents and related know-how that had previously been the subject of a license agreement with them. The cash consideration of $2.0 million was recorded as revenue in April 2009, once all related materials had been transferred.
45
Table of Contents
Research and development expense. Research and development expense decreased to $13.2 million in 2009 from $26.3 million in 2008. The decrease was primarily due to the 2008 Restructuring, which resulted in decreases in headcount-related expenses of $4.2 million, allocated facility costs of $3.3 million, clinical expenses of $2.6 million and professional service costs of $1.8 million.
General and administrative expense. General and administrative expense decreased to $7.7 million in 2009 from $11.5 million in 2008. The decrease was primarily due to the 2008 Restructuring and a restructuring plan implemented in April 2009, or the 2009 Restructuring, which together resulted in decreases in headcount-related expenses of $2.1 million and facility costs of $0.9 million and also a reduction in professional service costs of $0.5 million.
Restructuring charges. Restructuring charges were $1.9 million in 2009 as compared to $5.8 million in 2008. The charges for 2009 included $1.3 million for lease termination activities related to the 2008 Restructuring and $0.6 million for employee severance and related benefit costs related to the 2009 Restructuring. The net charge for lease termination activities included $2.2 million for early lease termination fees paid to the landlord and $0.4 million for third party commission, partially offset by the reversal of $1.4 million in non-cash deferred rent on the facility. The 2008 charges were primarily comprised of $5.9 million related to the 2008 Restructuring.
Interest Income. Interest income decreased to $22,000 in 2009 from $0.9 million in 2008, primarily due to lower average balances of cash, cash equivalents and marketable securities and lower average interest rates during 2009.
Interest Expense. Interest expense decreased to $1,000 in 2009 from $0.2 million in 2008, due to full payment of the outstanding balance under our equipment financing agreement with General Electric Capital Corporation in November 2008.
Other Income (Expense), Net. Other expense, net was $21.1 million in 2009 as compared to other income, net of $0.2 million in 2008. The expense in 2009 was primarily due to non-cash charges of $21.0 million related to the accounting for the Private Placement, which consisted of $7.5 million recorded upon the initial closing in April 2009 and $13.5 million upon the revaluation in June 2009 of the options to participate in the second closing and common equity closing.
Years Ended December 31, 2008 and 2007
Revenue. Total revenue decreased to $5.4 million in 2008 from $9.7 million in 2007. Collaboration revenue decreased to $4.9 million in 2008 from $9.2 million in 2007, primarily due to a $3.3 million decrease in revenue from Biogen Idec resulting from the June 2008 termination of the research phase of our collaboration and a $1.5 million decrease in research revenue from our BACE program with Merck. Partially offsetting these decreases was a milestone payment from J&JPRD for the selection of a compound targeting the Cathepsin S enzyme.
Research and development expense. Research and development expense decreased to $26.3 million in 2008 from $36.1 million in 2007. The decrease was primarily due the 2008 Restructuring, which resulted in a $7.9 million decrease in expenses under our kinase inhibitors programs, a $2.8 million decrease in clinical trial activity related to SNS-314 and SNS-032, and a $1.9 million decrease in expenses for discovery and new technology programs. These decreases were partially offset by a $2.8 million increase in voreloxin expenses due to increased clinical development activities.
General and administrative expense. General and administrative expense decreased to $11.5 million in 2008 from $13.6 million in 2007. The decrease was primarily due the 2008 Restructuring, which resulted in decreases of $2.1 million in employee-related expenses, $0.3 million in office-related expenses and $0.1 million
46
Table of Contents
in professional services. These decreases were partially offset by a $0.4 million increase in facilities and related expenses.
Restructuring charges. Restructuring charges were $5.8 million in 2008 as compared to $1.6 million in 2007. The 2008 charges were primarily comprised of $5.9 million related to the 2008 Restructuring. Charges for the 2008 Restructuring consisted of $3.6 million for employee severance and related benefit costs, including non-cash stock-based compensation of $0.4 million, and $2.3 million related to asset impairment and facility exit costs. The 2007 charges were primarily comprised of severance costs and charges relating to leased facilities.
Interest income. Interest income decreased to $0.9 million in 2008 from $3.0 million in 2007, primarily due to lower average balances of cash, cash equivalents and marketable securities and lower average interest rates during 2008.
Interest expense. Interest expense was comparable for both 2008 and 2007, due to higher interest rates on lower outstanding debt obligation in 2008, compared to lower interest rates on higher outstanding debt obligations in 2007.
Income Taxes
Deferred tax assets or liabilities may arise from differences between the tax basis of assets or liabilities and their basis for financial reporting. Deferred tax assets or liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which temporary differences are expected to be recovered or settled. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized. Our policy is to recognize interest charges and penalties as other expense.
Since inception, we have incurred operating losses and, accordingly, have not recorded a provision for income taxes for any of the periods presented. As of December 31, 2009, we had net operating loss carry-forwards for federal and state income tax purposes of $233.2 million and $133.8 million, respectively. We also had federal and state research and development tax credit carry-forwards of $5.3 million and $5.2 million, respectively. If not utilized, the federal net operating loss and tax credit carry-forwards will expire at various dates beginning in 2018, and the state net operating loss will expire beginning in 2012. The state research and development tax credit carry-forwards do not expire. Utilization of these net operating loss and tax credits carry-forwards may be subject to a substantial annual limitation due to ownership change rules under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code. The limitations are applicable if an “ownership change,” as defined in the Code, is deemed to have occurred or occurs in the future. The annual limitation may result in the expiration of net operating loss and credit carry-forwards before they can be utilized.
Liquidity and Capital Resources
Sources of Liquidity
Since our inception, we have funded our operations primarily through the issuance of common and preferred stock, research funding, technology access fees and milestone payments from our collaboration partners, research grants, loans from Biogen Idec and other debt financings.
Our cash, cash equivalents and marketable securities totaled $4.3 million as of December 31, 2009, as compared to $10.6 million as of December 31, 2008. The decrease of $6.3 million was primarily due to $20.2 million of net cash used in operating activities, partially offset by net proceeds of $13.4 million received from the first and second closings of the Private Placement, as described below. No debt was outstanding at either balance sheet date.
On April 3, 2009, we completed the initial closing of $10.0 million of the Private Placement, and on October 30, 2009, we completed the second closing of $5.0 million. In the initial closing, $10.0 million of units
47
Table of Contents
were sold, resulting in net proceeds of $8.8 million, and in the second closing, $5.0 million of units were sold, resulting in net proceeds of $4.7 million. The units consist of Series A convertible preferred stock and warrants to purchase common stock, and were sold to accredited investors, including certain members of management.
Under the Private Placement, an additional $28.5 million of common stock may be sold at $0.275 per share in a common equity closing upon the election of the holders of a majority of the Series A convertible preferred stock issued in the Private Placement prior to the earlier of June 30, 2010, or a date determined with reference to our cash and investments balance dropping below $2.5 million. The common equity closing may also be completed upon our election prior to the earlier of June 30, 2010 and a qualifying alternative common stock financing, subject to the approval of the purchasers holding a majority of the Series A convertible preferred stock issued in the Private Placement and subject to us selling at least $28.5 million of common stock in the common equity closing.
On January 20, 2010, we entered into a controlled equity offering sales agreement with Cantor Fitzgerald & Co., or Cantor, pursuant to which we could issue and sell shares of our common stock having an aggregate offering price of up to $15.0 million from time to time through Cantor acting as agent and/or principal. We agreed to pay Cantor fees of between 3% and 5% of the gross proceeds from each sale. As of March 31, 2010, the full $15.0 million available under the facility had been sold for net proceeds of $14.2 million after commissions and expenses.
Cash Flows
Net cash used in operating activities was $20.2 million in 2009, compared to $35.5 million used in 2008 and $34.5 million used in 2007. Net cash used in 2009 resulted primarily from the net loss of $40.2 million, and changes in operating assets and liabilities of $1.3 million, partially offset by net adjustments for non-cash items of $21.4 million (including $21.0 million of charges related to the Private Placement and $1.3 million of stock-based compensation, partially offset by a $1.4 million credit for deferred rent related to the 2008 Restructuring). Net cash used in 2008 resulted primarily from the net loss of $37.2 million, and changes in operating assets and liabilities of $3.0 million (including decreases of $1.2 million in deferred revenue and $1.7 million in accrued compensation, partially offset by adjustments for non-cash items of $4.8 million (including $1.9 million of restructuring charges, $1.9 million of stock-based compensation and $1.1 million of depreciation and amortization). Net cash used in 2007 resulted primarily from the net loss of $38.8 million and changes in operating assets and liabilities of $1.0 million, partially offset by an adjustment for non-cash items of $5.3 million (including $3.2 million of stock-based compensation, $1.7 million of depreciation and amortization and $0.4 million of restructuring charges).
Net cash provided by investing activities was $4.7 million in 2009, compared to $32.3 million in 2008 and $19.7 million in 2007. Net cash provided in 2009 consisted of net proceeds from marketable securities transactions of $4.3 million and proceeds from the sale of property and equipment of $0.4 million. Net cash provided in 2008 consisted of net proceeds from marketable securities transactions of $31.6 million and $0.9 million from the sale of assets previously held-for sale, partially offset by capital expenditures of $0.2 million. Net cash provided in 2007 resulted primarily from net proceeds from marketable securities transactions of $21.2 million, partially offset by capital expenditures of $1.5 million.
Net cash provided by financing activities was $13.4 million in 2009, compared to net cash used of $2.2 million in 2008 and net cash provided of $20.5 million in 2007. Net cash provided in 2009 consisted primarily of net proceeds from the initial and second closings of the Private Placement. Net cash used in 2008 consisted primarily of equipment loan repayments of $2.3 million. Net cash provided in 2007 resulted primarily from net proceeds of $19.5 million from a common stock offering and $0.5 million from equipment loans.
Operating Cash Requirements
We expect to continue to incur substantial operating losses in the future. We will not receive any product revenue until a product candidate has been approved by the FDA or similar regulatory agencies in other
48
Table of Contents
countries, and has been successfully commercialized. We need to raise substantial additional funding to complete the development and commercialization of voreloxin. Additionally, we may evaluate in-licensing and acquisition opportunities to gain access to new drugs or drug targets that would fit with our strategy. Any such transaction would likely increase our funding needs in the future.
Our future funding requirements will depend on many factors, including but not limited to:
| • | the rate of progress and cost of our clinical trials, need for additional clinical trials, and other development activities; |
| • | the economic and other terms and timing of any licensing or partnering arrangement into which we may enter; |
| • | the costs associated with building or accessing manufacturing and commercialization capabilities; |
| • | the costs of acquiring or investing in businesses, product candidates and technologies, if any; |
| • | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
| • | the costs and timing of seeking and obtaining FDA and other regulatory approvals; and |
| • | the effect of competing technological and market developments. |
We believe that currently available cash, cash equivalents and marketable securities, including the net proceeds of $14.2 million from sales of common stock through March 31, 2010 under the agreement with Cantor, are sufficient to fund our operations through at least September 30, 2010. We will need to raise substantial additional funding in the near term in order to sustain operations beyond that date and before undertaking any additional clinical trials of voreloxin. Our significant negative cash flows and lack of financial resources raise substantial doubt as to our ability to continue as a going concern. Management is in the process of evaluating alternative funding sources, which may include raising proceeds from the third closing of the Private Placement, debt arrangements, a potential transaction related to rights associated with voreloxin, or otherwise.
Until we can generate a sufficient amount of collaboration or product revenue to finance our cash requirements, which we may never do, we expect to finance future cash needs primarily through equity issuances (including the possible third closing of the Private Placement and subject to the satisfaction of the conditions described above), debt arrangements and/or a possible partnership or license of development and/or commercialization rights to voreloxin. We do not know whether additional funding will be available on acceptable terms, or at all.
Our failure to raise significant additional capital in the near term would require us to delay or reduce the scope of our voreloxin development program and limit our ability to continue operations. Any one of the foregoing would have a material adverse effect on our business, financial condition and results of operations.
Contractual Obligations
Our operating lease obligations as of December 31, 2009 relate to the leases for two facilities in South San Francisco, California. In December 2006, we leased approximately 15,000 square feet of office space in a building at 395 Oyster Point Boulevard. This lease expires in April 2013, subject to our option to extend the lease through February 2014. In October 2008, we leased approximately 5,500 square feet of laboratory space at 349 Allerton Avenue. This lease expires in October 2010 with our option to extend the lease through October 2012.
49
Table of Contents
The lease for the facility located at 341 Oyster Point Boulevard, which formerly served as our headquarters and research and development facility, was terminated in the first quarter of 2009.
We also have agreements with clinical sites and contract research organizations for the conduct of our clinical trials. We generally make payments to these sites and organizations based upon the procedures to be performed in the particular clinical trial, the number of patients enrolled and the period of follow-up required for patients in the trial.
Off-Balance Sheet Arrangements
Since our inception, we have not had any off-balance sheet arrangements or relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or variable interest entities, which are typically established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes.
50
Table of Contents
| ITEM 7A: | QUALITATIVE AND QUANTITATIVE DISCLOSURES ABOUT MARKET RISK |
This item is not applicable to us as a smaller reporting company.
| ITEM 8: | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
Index to Consolidated Financial Statements
| Page | ||
| 52 | ||
| 53 | ||
| 54 | ||
| 55 | ||
| 56 | ||
| 57 |
51
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Sunesis Pharmaceuticals, Inc.
We have audited the accompanying consolidated balance sheets of Sunesis Pharmaceuticals, Inc. as of December 31, 2009 and 2008, and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2009. These financial statements are the responsibility of Sunesis Pharmaceuticals, Inc.’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Sunesis Pharmaceuticals, Inc. at December 31, 2009 and 2008, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2009, in conformity with U.S. generally accepted accounting principles.
As discussed in Note 1 to the financial statements, the Company’s recurring losses from operations and its cash and cash equivalents balance at December 31, 2009 raise substantial doubt about its ability to continue as a going concern. Management’s plans as to these matters are described in Note 1. The 2009 financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ ERNST & YOUNG, LLP
Palo Alto, California
March 31, 2010
52
Table of Contents
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| ASSETS |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 4,258,715 | $ | 6,296,942 | ||||
| Marketable securities |
— | 4,321,844 | ||||||
| Prepaids and other current assets |
583,030 | 934,429 | ||||||
| Total current assets |
4,841,745 | 11,553,215 | ||||||
| Property and equipment, net |
263,111 | 612,241 | ||||||
| Assets held-for-sale |
— | 470,547 | ||||||
| Deposits and other assets |
64,425 | 147,826 | ||||||
| Total assets |
$ | 5,169,281 | $ | 12,783,829 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 360,300 | $ | 790,546 | ||||
| Accrued clinical expense |
1,129,226 | 1,865,773 | ||||||
| Accrued compensation |
728,744 | 537,215 | ||||||
| Accrued restructuring charges |
11,982 | 191,170 | ||||||
| Other accrued liabilities |
749,494 | 1,360,434 | ||||||
| Current portion of deferred rent |
27,943 | 1,409,513 | ||||||
| Deferred revenue |
27,083 | 27,083 | ||||||
| Total current liabilities |
3,034,772 | 6,181,734 | ||||||
| Non-current portion of deferred rent |
74,105 | 110,919 | ||||||
| Commitments (Note 9) |
||||||||
| Stockholders’ equity: |
||||||||
| Convertible preferred stock, $0.0001 par value; (i) 10,000,000 and 5,000,000 shares authorized, (ii) 4,347,812 and 0 shares issued and outstanding, and (iii) aggregate liquidation preference of $44,999,854 and $0, as of December 31, 2009 and 2008, respectively |
60,004,986 | — | ||||||
| Common stock, $0.0001 par value; 400,000,000 and 100,000,000 shares authorized as of December 31, 2009 and 2008, respectively; 35,902,603 and 34,409,768 shares issued and outstanding as of December 31, 2009 and 2008, respectively |
3,590 | 3,441 | ||||||
| Additional paid-in capital |
298,469,584 | 322,671,604 | ||||||
| Accumulated other comprehensive income |
— | 7,841 | ||||||
| Accumulated deficit |
(356,417,756 | ) | (316,191,710 | ) | ||||
| Total stockholders’ equity |
2,060,404 | 6,491,176 | ||||||
| Total liabilities and stockholders’ equity |
$ | 5,169,281 | $ | 12,783,829 | ||||
See accompanying notes to consolidated financial statements.
53
Table of Contents
CONSOLIDATED STATEMENTS OF OPERATIONS
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Revenue: |
||||||||||||
| Collaboration revenue—related party |
$ | 1,500,000 | $ | 4,310,551 | $ | 7,586,903 | ||||||
| Collaboration revenue—other |
50,000 | 606,789 | 1,576,610 | |||||||||
| License and other revenue |
2,211,547 | 500,000 | 500,000 | |||||||||
| Total revenues |
3,761,547 | 5,417,340 | 9,663,513 | |||||||||
| Operating expenses: |
||||||||||||
| Research and development |
13,246,859 | 26,285,294 | 36,060,470 | |||||||||
| General and administrative |
7,748,243 | 11,524,198 | 13,569,578 | |||||||||
| Restructuring charges |
1,915,316 | 5,782,903 | 1,563,274 | |||||||||
| Total operating expenses |
22,910,418 | 43,592,395 | 51,193,322 | |||||||||
| Loss from operations |
(19,148,871 | ) | (38,175,055 | ) | (41,529,809 | ) | ||||||
| Interest income |
21,630 | 929,114 | 2,971,666 | |||||||||
| Interest expense |
(1,188 | ) | (171,308 | ) | (209,885 | ) | ||||||
| Other income (expense), net |
(21,097,617 | ) | 231,622 | 7,108 | ||||||||
| Net loss |
(40,226,046 | ) | (37,185,627 | ) | (38,760,920 | ) | ||||||
| Deemed distribution to preferred stockholders |
(27,563,400 | ) | — | — | ||||||||
| Loss attributable to common stockholders |
$ | (67,789,446 | ) | $ | (37,185,627 | ) | $ | (38,760,920 | ) | |||
| Basic and diluted loss attributable to common stockholders per common share |
$ | (1.97 | ) | $ | (1.08 | ) | $ | (1.20 | ) | |||
| Shares used in computing basic and diluted loss attributable to common stockholders per common share |
34,480,716 | 34,387,177 | 32,340,203 | |||||||||
See accompanying notes to consolidated financial statements.
54
Table of Contents
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
| Convertible Preferred Stock |
Common Stock | Additional Paid-In Capital |
Deferred Stock Compensation |
Accumulated Other Comprehensive Income (Loss) |
Accumulated Deficit |
Total Stockholders’ Equity |
||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||
| Balance as of December 31, 2006 |
— | $ | — | 29,443,079 | $ | 2,944 | $ | 298,073,896 | $ | (1,006,604 | ) | $ | (21,376 | ) | $ | (240,245,163 | ) | $ | 56,803,697 | |||||||||||
| Issuance of common stock pursuant to stock option exercises |
— | — | 68,913 | 8 | 161,008 | — | — | — | 161,016 | |||||||||||||||||||||
| Reversal of deferred stock-based compensation |
— | — | — | — | (76,980 | ) | 76,980 | — | — | — | ||||||||||||||||||||
| Amortization deferred stock-based compensation |
— | — | — | — | — | 633,023 | — | — | 633,023 | |||||||||||||||||||||
| Stock-based compensation expense—non-employees |
— | — | — | — | 2,394 | — | — | — | 2,394 | |||||||||||||||||||||
| Stock-based compensation expense—employees |
— | — | — | — | 2,468,898 | — | — | — | 2,468,898 | |||||||||||||||||||||
| Stock-based compensation expense—restructuring |
— | — | — | — | 126,456 | 45,000 | — | — | 171,456 | |||||||||||||||||||||
| Issuance of common stock under employee stock purchase plan |
— | — | 102,904 | 10 | 301,055 | — | — | 301,065 | ||||||||||||||||||||||
| Issuance of common stock pursuant to public offering, net of issuance costs of $1,519,513 |
— | — | 4,750,000 | 475 | 19,522,513 | — | — | — | 19,522,988 | |||||||||||||||||||||
| Components of comprehensive loss: |
||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (38,760,920 | ) | (38,760,920 | ) | ||||||||||||||||||||
| Unrealized gain on available-for-sale securities |
— | — | — | — | — | — | 90,638 | — | 90,638 | |||||||||||||||||||||
| Comprehensive loss |
(38,670,282 | ) | ||||||||||||||||||||||||||||
| Balance as of December 31, 2007 |
— | — | 34,364,896 | 3,437 | 320,579,240 | (251,601 | ) | 69,262 | (279,006,083 | ) | 41,394,255 | |||||||||||||||||||
| Issuance of stock to employees |
— | — | 70 | — | — | — | — | — | — | |||||||||||||||||||||
| Reversal of deferred stock-based compensation |
— | — | — | — | (28,500 | ) | 28,500 | — | — | — | ||||||||||||||||||||
| Amortization deferred stock-based compensation |
— | — | — | — | — | 223,101 | — | — | 223,101 | |||||||||||||||||||||
| Stock-based compensation expense—non-employees |
— | — | — | — | 828 | — | — | — | 828 | |||||||||||||||||||||
| Stock-based compensation expense—employees |
— | — | — | — | 1,686,827 | — | — | — | 1,686,827 | |||||||||||||||||||||
| Stock-based compensation expense— restructuring |
— | — | — | — | 366,637 | — | — | — | 366,637 | |||||||||||||||||||||
| Issuance of common stock under employee stock purchase plan |
— | — | 44,802 | 4 | 66,572 | — | — | — | 66,576 | |||||||||||||||||||||
| Components of comprehensive loss: |
||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | (37,185,627 | ) | (37,185,627 | ) | |||||||||||||||||||
| Unrealized loss on available-for-sale securities |
— | — | — | — | — | — | (61,421 | ) | — | (61,421 | ) | |||||||||||||||||||
| Comprehensive loss |
(37,247,048 | ) | ||||||||||||||||||||||||||||
| Balance as of December 31, 2008 |
— | — | 34,409,768 | 3,441 | 322,671,604 | — | 7,841 | (316,191,710 | ) | 6,491,176 | ||||||||||||||||||||
| Issuance of common stock pursuant to stock option exercises |
— | — | 4,557 | — | 6,562 | — | — | — | 6,562 | |||||||||||||||||||||
| Issuance of stock to employees |
— | — | 12 | — | — | — | — | — | — | |||||||||||||||||||||
| Issuance of $10,000,000 of units consisting of preferred stock and warrants in initial closing of Private Placement, recorded in liabilities |
2,898,544 | — | — | — | — | — | — | — | — | |||||||||||||||||||||
| Reclassification of preferred stock from liabilities to equity |
— | — | — | — | 20,126,000 | — | — | — | 20,126,000 | |||||||||||||||||||||
| Reclassification of second closing option of Private Placement from liabilities to equity and issuance of amended preferred stock instrument, net of issuance costs of $1,245,757 |
— | 56,146,243 | — | — | (46,501,000 | ) | — | — | — | 9,645,243 | ||||||||||||||||||||
| Issuance of $5,000,000 of units consisting of preferred stock and warrants in second closing of Private Placement, net of issuance costs of $321,185 |
1,449,268 | 2,670,343 | — | — | 2,008,472 | — | — | — | 4,678,815 | |||||||||||||||||||||
| Write-off of discount for beneficial conversion feature on second closing of Private Placement |
— | 1,188,400 | — | — | (1,188,400 | ) | — | — | — | — | ||||||||||||||||||||
| Issuance of common stock pursuant to warrant exercises |
— | — | 1,469,450 | 147 | (147 | ) | — | — | — | — | ||||||||||||||||||||
| Stock-based compensation expenses—non-employees |
— | — | — | — | 29,408 | — | — | — | 29,408 | |||||||||||||||||||||
| Stock-based compensation expenses—employees |
— | — | — | — | 1,310,945 | — | — | — | 1,310,945 | |||||||||||||||||||||
| Issuance of common stock under employee stock purchase plan |
— | — | 18,816 | 2 | 6,140 | — | — | — | 6,142 | |||||||||||||||||||||
| Components of comprehensive loss: |
||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | (40,226,046 | ) | (40,226,046 | ) | |||||||||||||||||||
| Unrealized loss on available-for-sale securities |
— | — | — | — | — | — | (7,841 | ) | — | (7,841 | ) | |||||||||||||||||||
| Comprehensive loss |
(40,233,887 | ) | ||||||||||||||||||||||||||||
| Balance as of December 31, 2009 |
4,347,812 | $ | 60,004,986 | 35,902,603 | $ | 3,590 | $ | 298,469,584 | $ | — | $ | — | $ | (356,417,756 | ) | $ | 2,060,404 | |||||||||||||
See accompanying notes to consolidated financial statements.
55
Table of Contents
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Cash flows from operating activities |
||||||||||||
| Net loss |
$ | (40,226,046 | ) | $ | (37,185,627 | ) | $ | (38,760,920 | ) | |||
| Adjustments to reconcile loss to net cash used in operating activities: |
||||||||||||
| Stock-based compensation expense |
1,340,353 | 1,910,755 | 3,189,048 | |||||||||
| Depreciation and amortization |
341,576 | 1,103,848 | 1,728,714 | |||||||||
| Non-cash expense related to private placement |
21,016,997 | — | — | |||||||||
| Non-cash restructuring charges (reversals), net |
(1,372,634 | ) | 1,937,821 | 359,865 | ||||||||
| Loss (gain) on sale or disposal of property and equipment |
56,188 | (189,111 | ) | (5,949 | ) | |||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Prepaids and other current assets |
351,399 | 11,154 | 138,064 | |||||||||
| Deposits and other assets |
83,401 | 229,972 | (17,824 | ) | ||||||||
| Accounts payable |
(430,246 | ) | (672,171 | ) | (1,014,939 | ) | ||||||
| Accrued clinical expense |
(736,547 | ) | 840,448 | 419,944 | ||||||||
| Accrued compensation |
191,529 | (1,688,653 | ) | (97,874 | ) | |||||||
| Accrued restructuring charges |
(179,188 | ) | (125,063 | ) | 316,233 | |||||||
| Other accrued liabilities |
(610,940 | ) | (378,082 | ) | 1,354,766 | |||||||
| Deferred rent |
(8,871 | ) | (56,302 | ) | 111,832 | |||||||
| Deferred revenue |
— | (1,199,948 | ) | (2,176,606 | ) | |||||||
| Net cash used in operating activities |
(20,183,029 | ) | (35,460,959 | ) | (34,455,646 | ) | ||||||
| Cash flows from investing activities |
||||||||||||
| Purchases of property and equipment, net |
(6,140 | ) | (179,148 | ) | (1,511,425 | ) | ||||||
| Proceeds from sale of property and equipment |
391,174 | 876,303 | 5,119 | |||||||||
| Purchases of marketable securities |
(503,107 | ) | (25,902,749 | ) | (92,679,521 | ) | ||||||
| Proceeds from maturities of marketable securities |
4,817,110 | 57,477,417 | 113,841,425 | |||||||||
| Net cash provided by investing activities |
4,699,037 | 32,271,823 | 19,655,598 | |||||||||
| Cash flows from financing activities |
||||||||||||
| Proceeds from borrowings under equipment financing |
— | — | 1,481,611 | |||||||||
| Payments on borrowing under equipment financing |
— | (2,306,624 | ) | (1,015,955 | ) | |||||||
| Proceeds from issuance of convertible preferred stock and warrants under private placement, net of issuance costs |
13,433,061 | — | — | |||||||||
| Proceeds from issuance of common stock and exercise of stock options |
12,704 | 66,576 | 19,985,069 | |||||||||
| Net cash provided by (used in) financing activities |
13,445,765 | (2,240,048 | ) | 20,450,725 | ||||||||
| Net increase (decrease) in cash and cash equivalents |
(2,038,227 | ) | (5,429,184 | ) | 5,650,677 | |||||||
| Cash and cash equivalents at beginning of period |
6,296,942 | 11,726,126 | 6,075,449 | |||||||||
| Cash and cash equivalents at end of period |
$ | 4,258,715 | $ | 6,296,942 | $ | 11,726,126 | ||||||
| Supplemental disclosure of cash flow information |
||||||||||||
| Interest paid |
$ | 1,187 | $ | 187,946 | $ | 193,247 | ||||||
| Supplemental disclosure of non-cash activities |
||||||||||||
| Deemed distributions to preferred stockholders |
$ | 27,563,400 | $ | — | $ | — | ||||||
| Beneficial conversion feature on preferred stock |
$ | 1,188,400 | $ | — | $ | — | ||||||
| Cashless exercise of warrants |
$ | 439,780 | $ | — | $ | — | ||||||
| Deferred stock-based compensation expense (reversal) |
$ | — | $ | (28,500 | ) | $ | (76,980 | ) | ||||
See accompanying notes to consolidated financial statements.
56
Table of Contents
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Summary of Significant Accounting Policies
Overview
Sunesis Pharmaceuticals, Inc. (the “Company” or “Sunesis”) was incorporated in the state of Delaware on February 10, 1998, and its facilities are located in South San Francisco, California. Sunesis is a biopharmaceutical company focused on the development and commercialization of new oncology therapeutics for the treatment of hematologic and solid tumor cancers. The Company’s primary activities since incorporation have been conducting research and development internally and through corporate collaborators, in-licensing and out-licensing pharmaceutical compounds and technology, conducting clinical trials and raising capital.
On March 31, 2009, the Company entered into agreements with accredited investors, including certain members of management, providing for the private placement of up to $15.0 million of units consisting of Series A convertible preferred stock and warrants to purchase common stock, and up to $28.5 million in common stock, in three closings, collectively referred to as the Private Placement. On April 3, 2009, the Company completed the initial closing of $10.0 million of units, and on October 30, 2009, completed the second closing of $5.0 million of units.
Significant Risks and Uncertainties
The Company has incurred significant losses and negative cash flows from operations since its inception, and as of December 31, 2009, had cash and cash equivalents totaling $4.3 million and an accumulated deficit of $356.4 million.
The Company believes that currently available cash and cash equivalents, including the net proceeds of $14.2 million from sales of common stock through March 31, 2010 under the sales agreement with Cantor Fitzgerald & Co., or Cantor (see Note 14), are sufficient to fund its operations through at least September 30, 2010. The Company will need to raise substantial additional funding in the near term in order to sustain operations beyond that date and before undertaking any additional clinical trials of voreloxin. The significant negative cash flows and lack of financial resources of the Company raise substantial doubt as to the Company’s ability to continue as a going concern. Management is in the process of evaluating alternative funding sources, which may include raising proceeds from the third closing of the Private Placement, debt arrangements, a potential transaction related to rights associated with voreloxin, or otherwise.
If the Company is unable to raise additional funding to meet its working capital needs, it will be forced to delay or reduce the scope of its voreloxin development program and/or limit or cease its operations. The accompanying consolidated financial statements do not reflect any adjustments relating to the recoverability and reclassification of assets and liabilities as that might be necessary if the Company is unable to continue as a going concern.
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles, or GAAP, and assume that the Company will continue as a going concern. The financial statements include a wholly owned subsidiary, Sunesis Europe Limited, a United Kingdom corporation. Management has determined that the Company operates as a single reportable segment. The financial statements include all adjustments (consisting only of normal recurring adjustments) that management believes are necessary for a fair presentation of the periods presented. Prior period revenues in the
57
Table of Contents
statements of operations and certain liabilities in the balance sheets and statements of cash flows have been reclassified to conform to the current year presentation.
Significant Estimates and Judgments
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the Company’s consolidated financial statements and accompanying notes. Actual results could differ materially from these estimates. Significant estimates, assumptions and judgments made by management include those related to revenue recognition, clinical trial accounting, stock-based compensation and the valuation of equity instruments.
Cash Equivalents and Marketable Securities
The Company considers all highly liquid securities with original maturities of three months or less from the date of purchase to be cash equivalents, which have generally consisted of money market funds and corporate debt securities. Marketable securities consist of securities with original maturities of greater than three months, which generally consist of U.S. government obligations and corporate debt securities.
Management determines the appropriate classification of securities at the time of purchase. The Company generally classifies its entire investment portfolio as available-for-sale. The Company views its available-for-sale portfolio as available for use in current operations. Accordingly, the Company classifies all investments as short-term, even though the stated maturity may be more than one year from the current balance sheet date. Available-for-sale securities are carried at fair value, with unrealized gains and losses reported in accumulated other comprehensive income (loss), which is a separate component of stockholders’ equity. Estimated fair values are determined by the Company using available market information.
The amortized cost of securities is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization is included in interest income. Realized gains and losses and declines in value judged to be other-than-temporary on available-for-sale securities, if any, are recorded in other income (expense), net. The cost of securities sold is based on the specific-identification method.
Concentrations of Credit Risk and Financial Instruments
The Company invests cash that is not currently being used for operational purposes in accordance with its investment policy. The policy allows for the purchase of low risk debt securities issued by U.S. government agencies and very highly rated banks and corporations, subject to certain concentration limits. The policy limits maturities of securities purchased to no longer than 18 months. Management believes these guidelines ensure both the safety and liquidity of any investment portfolio the Company may hold.
Financial instruments that potentially subject the Company to concentrations of credit risk generally consist of cash, cash equivalents and marketable securities. The carrying amounts of cash equivalents and marketable securities generally approximate fair value due to their short-term nature. The Company is exposed to credit risk in the event of default by the institutions holding its cash, cash equivalents and any marketable securities to the extent of the amounts recorded in the balance sheets.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation and amortization. Depreciation is determined using the straight-line method over the estimated useful lives of the respective assets, generally three to five years. Leasehold improvements are amortized on a straight-line basis over the shorter of their estimated useful lives or the term of the lease.
58
Table of Contents
Long-Lived Assets
The Company periodically assesses long-lived assets for potential impairment. An impairment review is performed whenever events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable, such as a significant industry or economic downturn, significant changes in the manner of use of the acquired assets, or changes in the Company’s business strategy.
If indicators of impairment exist, recoverability is assessed by comparing the estimated undiscounted cash flows resulting from the use and eventual disposition of the asset to its carrying value. If aggregate undiscounted cash flows are less than the carrying value, an impairment charge is recorded based on the excess of the carrying value of the asset over its fair value, with fair value determined based on estimated discounted future cash flows, or another appropriate measure.
Private Placement Accounting
The accounting for the initial and second closing of the sale of $10.0 million and $5.0 million of units, respectively, under the Private Placement, and subsequent revaluations of the related financial instruments, required fair values to be established at different dates, either individually or in aggregate, for the four primary components of the Private Placement: (a) the Series A convertible preferred stock, (b) the warrants to purchase common stock, (c) the option for the investors to participate in the second closing, or the Second Closing Option, and (d) the option for the investors to participate in the common equity closing, or the Common Equity Closing Option. The Option-Pricing Method, which utilizes the Black-Scholes model, was selected to determine these fair values, which were calculated as a series of call options on the potential enterprise value of the Company at different valuation points at which the claims of the different stakeholder groups on the enterprise value would change. The results of the Black-Scholes model are affected by the Company’s stock price, as well as assumptions regarding a number of highly subjective variables. These variables include the expected term of the financial instruments and the Company’s expected stock price volatility, risk-free interest rate and dividend rate over the expected term.
Revenue Recognition
Revenue arrangements with multiple deliverables are accounted for in accordance with the Financial Accounting Standards Board, or FASB, Accounting Standards Codification, or ASC, Subtopic 605-25, Multiple-Element Arrangements, or ASC 605-25. Under ASC 605-25, revenue arrangements with multiple deliverables are divided into separate units of accounting based on whether certain criteria are met, including whether the delivered item has stand-alone value to the customer and whether there is objective and reliable evidence of the fair value of the undelivered items. Consideration is allocated among the separate units of accounting based on their respective fair value, and the applicable revenue recognition is applied to each of the separate units.
Non-refundable fees where the Company has no continuing performance obligations are recognized as revenues when collection is reasonably assured. In situations where continuing performance obligations exist, nonrefundable fees are deferred and recognized ratably over the projected performance period.
Research funding from collaborations is recognized as revenue as the related research services are performed. This funding is generally based on a specified amount per full-time equivalent employee per year.
Milestone payments which are substantive and at risk at the time of the execution of the collaboration agreement are recognized upon completion of the applicable milestone event. Any future royalty revenue will be recognized based on reported product sales by third-party licensees.
Research and Development
All research and development costs, including those funded by third parties, are expensed as incurred. Research and development expenses consist primarily of costs related to employee salaries and benefits, clinical
59
Table of Contents
trials (including amounts paid to contract research organizations, or CROs, and participating clinical trial sites), consultants, other outside services, labs and other facilities.
Clinical Trial Accounting
The Company records accruals for estimated clinical trial costs, which include payments for work performed by contract research organizations, or CROs, and participating clinical trial sites. These costs are generally a significant component of research and development expenses. Non-refundable costs of setting up clinical trial sites for participation in trials are expensed immediately, with any refundable advances related to enrollment of the first patient recorded as prepayments and assessed for recoverability on a quarterly basis. Costs related to patient enrollment are accrued as patients progress through the clinical trial, including amortization of any first-patient prepayments. This amortization generally matches when the related services are rendered, however, these cost estimates may or may not match the actual costs incurred by the CROs or clinical trial sites, and if the Company has incomplete or inaccurate information, the clinical trial accruals may not be accurate. The difference between accrued expenses based on the Company’s estimates and actual expenses have not been material to date.
Stock-Based Compensation
The Company grants options to purchase common stock to its employees, directors and consultants under its stock option plans. Under the Company’s Employee Stock Purchase Plan, eligible employees can also purchase shares of common stock at 85% of the lower of the fair market value of the Company’s common stock at the beginning of a 12-month offering period or at the end of one of the two related six-month purchase periods.
The Company values these share-based awards using the Black-Scholes model. The determination of fair value of share-based payment awards on the date of grant using the Black-Scholes model is affected by the Company’s stock price as well as assumptions regarding a number of highly complex and subjective variables. These variables include, but are not limited to, the expected stock price volatility over the term of the awards and actual and projected employee stock option exercise behaviors.
Income Taxes
The Company accounts for income taxes under the liability method. Under this method, deferred tax assets and liabilities are determined based on the differences between the tax basis of assets and liabilities and their basis for financial reporting. Deferred tax assets or liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which temporary differences are expected to be recovered or settled. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized. The Company’s policy is to recognize interest charges and penalties as other expense.
Comprehensive Loss
The Company displays comprehensive loss and its components within the statements of stockholders’ equity, net of related tax effects. Comprehensive loss is comprised of net loss and unrealized gains or losses on available-for-sale securities.
Recent Accounting Pronouncements
In June 2009, the FASB established the FASB Accounting Standards Codification, or FASB ASC, as the source of authoritative accounting principles recognized by the FASB. The FASB will issue new standards in the form of Accounting Standards Updates, or FASB ASUs. The Company adopted the FASB ASC in the third quarter of 2009. The issuance of the FASB ASC does not change GAAP and therefore its adoption only affects the specific references to GAAP literature in these notes to the Company’s consolidated financial statements.
60
Table of Contents
In August 2009, the Company adopted the provisions of ASU 2009-04, which amends ASC Topic 480, Distinguishing Liabilities from Equity, or ASC 480. ASU 2009-04 clarifies the guidance under ASC 480, which was originally based on the pre-ASC guidance of Emerging Issues Task Force, or EITF, Topic D-98. The adoption of the amended provisions had no material effect on the Company’s financial condition or results of operations.
In October 2009, the FASB issued ASU 2009-13, Revenue Recognition (Topic 605): Multiple Deliverable Revenue Arrangements—A Consensus of the FASB Emerging Issues Task Force. This update provides application guidance on whether multiple deliverables exist, how the deliverables should be separated and how the consideration should be allocated to one or more units of accounting. This update establishes a selling price hierarchy for determining the selling price of a deliverable. The selling price used for each deliverable will be based on vendor-specific objective evidence, if available, third-party evidence if vendor-specific objective evidence is not available, or estimated selling price if neither vendor-specific or third-party evidence is available. The Company expects to adopt this guidance prospectively from January 1, 2011. The Company is assessing the impact of this guidance on its financial condition and results of operations.
2. Loss per Common Share
Basic loss attributable to common stockholders per common share is calculated by dividing loss attributable to common stockholders by the weighted-average number of common shares outstanding for the period. Diluted loss attributable to common stockholders per common share is computed by dividing loss attributable to common stockholders by the weighted-average number of common shares outstanding for the period plus dilutive potential common shares as determined using the as-if converted method for convertible preferred stock and the treasury stock method for options and warrants to purchase common stock. Convertible preferred stock, options and warrants to purchase common stock have been excluded from the calculation of diluted loss attributable to common stockholders per common share as their effect is anti-dilutive.
The following tables set forth the computation of basic and diluted loss attributable to common stockholders per common share and the excluded potential common shares for outstanding securities as of the related period end dates:
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Historical numerator: |
||||||||||||
| Net loss |
$ | (40,226,046 | ) | $ | (37,185,627 | ) | $ | (38,760,920 | ) | |||
| Deemed distribution to preferred stockholders |
(27,563,400 | ) | — | — | ||||||||
| Loss attributable to common stockholders |
$ | (67,789,446 | ) | $ | (37,185,627 | ) | $ | (38,760,920 | ) | |||
| Denominator: |
||||||||||||
| Weighted-average common shares outstanding |
34,480,716 | 34,387,177 | 32,340,203 | |||||||||
| Basic and diluted loss attributable to common stockholders per common share |
$ | (1.97 | ) | $ | (1.08 | ) | $ | (1.20 | ) | |||
| As of December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Outstanding securities not included in calculations: |
||||||||||||
| Convertible preferred stock, as-if converted |
43,478,120 | — | — | |||||||||
| Warrants to purchase common stock |
44,119,165 | 2,660,845 | 2,693,237 | |||||||||
| Options to purchase common stock |
6,407,309 | 4,650,955 | 5,099,847 | |||||||||
| 94,004,594 | 7,311,800 | 7,793,084 | ||||||||||
61
Table of Contents
3. License Agreements
SARcode Corporation
In March 2006, the Company entered into a license agreement with SARcode Corporation, or SARcode, a privately held biopharmaceutical company, granting SARcode an exclusive, worldwide license to all of the Company’s lymphocyte function-associated antigen-1, or LFA-1, patents and related know-how. Pursuant to the license agreement, the Company received a total of $1.0 million in license fees—$0.5 million in 2007 and $0.5 million in September 2008—which were recorded as revenue upon receipt. In addition, the Company received three secured convertible promissory notes, with a total principal amount of $1.0 million, which are due in 2012 and are convertible into the preferred stock of SARcode at the Company’s option. The Company has yet to record any amounts represented by these notes receivable as revenue, due to the uncertainty of their collectibility.
In March 2009, SARcode acquired the Company’s interest in all of its LFA-1 patents and related know-how for a total cash consideration of $2.0 million, which was recorded as revenue in April 2009, once all related materials had been transferred. This represented 53% of total revenue for the year ended December 31, 2009. In connection with the sale, the license agreement was terminated, but the promissory notes remain outstanding.
4. Strategic Collaborations
The table below summarizes collaboration revenues for the periods presented:
| Year Ended December 31, | |||||||||
| 2009 | 2008 | 2007 | |||||||
| Biogen Idec |
$ | 1,500,000 | $ | 4,310,551 | $ | 7,586,903 | |||
| Other |
50,000 | 606,789 | 1,576,610 | ||||||
| Total collaboration revenue |
$ | 1,550,000 | $ | 4,917,340 | $ | 9,163,513 | |||
In August 2004, the Company entered into a collaboration agreement with Biogen Idec, Inc., or Biogen Idec, to discover, develop and commercialize small molecule inhibitors of Raf kinase and up to five additional targets that play a role in oncology and immunology indications or in the regulation of the human immune system. Concurrent with the signing of the agreement, Biogen Idec paid a $7.0 million upfront technology access fee and made a $14.0 million equity investment in the Company through the purchase of the Company’s Series C-2 preferred stock, which converted into common stock upon the Company’s initial public offering in September 2005. Biogen Idec’s equity ownership was 8.1% of the Company’s common shares outstanding as of December 31, 2009.
Pursuant to the terms of the collaboration agreement, the Company applied its proprietary Tethering technology to generate small molecule leads during the research term, for which it received research funding, which was paid in advance to support some of the Company’s scientific personnel. In connection with the Company’s June 2008 restructuring, the parties agreed to terminate the research term and related funding as of June 30, 2008. A total of $20.0 million of research funding was received through this date. The Company had received a total of $3.0 million in milestone payments for meeting certain preclinical milestones through December 31, 2009, including a $1.5 million milestone for Biogen Idec’s selection of a Raf kinase inhibitor development candidate for the treatment of cancer, which was earned and recorded as revenue in June 2009, and the cash received in July 2009. Additionally, as part of this $3.0 million total, a $0.5 million milestone was received and recognized in June 2008.
The Company may in the future receive pre-commercialization milestone payments of up to $60.5 million per target, as well as royalty payments depending on product sales. Potential total royalty payments may be
62
Table of Contents
increased if the Company exercises its option to co-develop and co-promote product candidates for up to two targets worldwide (excluding Japan) and may be reduced if Biogen Idec is required to in-license additional intellectual property related to certain technology jointly developed under the collaboration agreement in order to commercialize a collaboration product.
Revenue from Biogen Idec represented 40%, 80% and 79% of total revenue for the years ended December 31, 2009, 2008 and 2007, respectively. Revenue from Merck & Co., Inc. was $1.6 million, or 16% of total revenue, for the year ended December 31, 2007. On March 10, 2010, Merck provided written notice that it was terminating the collaborations related to BACE inhibitors and small compounds derived from the Company’s Tethering technology, which it entered into with the Company in February 2003 and July 2004, respectively. In accordance with the terms of the collaboration agreements, the terminations will be effective on June 8, 2010. As a result, the Company does not expect to receive any additional funding from Merck relating to these agreements.
5. Financial Instruments
In accordance with applicable GAAP, the fair value of the Company’s financial instruments reflect the amounts that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date (i.e. the exit price). A fair value hierarchy is also utilized to prioritize valuation inputs, as follows:
| Level 1 - | quoted prices in active markets for identical assets and liabilities |
| Level 2 - | significant observable inputs other than Level 1 inputs, such as quoted prices in active markets for similar assets or liabilities; quoted prices for identical or similar assets or liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the asset or liability |
| Level 3 - | unobservable inputs |
The Company’s Level 2 valuations are generally based upon quoted prices in active markets for similar securities, with prices adjusted for yield and number of days to maturity.
As of December 31, 2009, the Company held no financial assets that were measured on a recurring basis other than money market funds. The following table summarizes the fair value of the Company’s financial assets measured on a recurring basis as of December 31, 2008, which is comprised solely of available-for-sale securities:
| December 31, 2008 |
Input Level | Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Estimated Fair Value | ||||||||||
| Money market funds |
Level 1 | $ | 5,417,903 | $ | — | $ | — | $ | 5,417,903 | ||||||
| U.S. treasury obligations |
Level 1 | 768,039 | — | (76 | ) | 767,963 | |||||||||
| U.S. government agency obligations |
Level 2 | 3,068,968 | 2,906 | — | 3,071,874 | ||||||||||
| Corporate debt obligations |
Level 2 | 996,102 | 3,898 | — | 1,000,000 | ||||||||||
| Commercial paper |
Level 2 | 248,857 | 1,113 | — | 249,970 | ||||||||||
| Total available-for-sale securities |
10,499,869 | 7,917 | (76 | ) | 10,507,710 | ||||||||||
| Less: amounts classified as cash equivalents |
6,185,942 | — | (76 | ) | 6,185,866 | ||||||||||
| Amounts classified as marketable securities |
$ | 4,313,927 | $ | 7,917 | $ | — | $ | 4,321,844 | |||||||
63
Table of Contents
There were no realized gains or losses on the sale of available-for-sale securities in the years ended December 31, 2009, 2008 and 2007.
6. Property and Equipment
Property and equipment is recorded at cost and consisted of the following as of December 31 of the periods presented:
| 2009 | 2008 | |||||||
| Computer equipment and software |
$ | 1,054,449 | $ | 1,353,231 | ||||
| Furniture and office equipment |
437,912 | 981,989 | ||||||
| Laboratory equipment |
855,678 | 901,694 | ||||||
| Leasehold improvements |
376,388 | 5,789,944 | ||||||
| 2,724,427 | 9,026,858 | |||||||
| Less accumulated depreciation and amortization |
(2,461,316 | ) | (8,414,617 | ) | ||||
| Net property and equipment |
$ | 263,111 | $ | 612,241 | ||||
7. Restructuring
2009 Restructuring
On March 30, 2009, the Compensation Committee of the Company’s board of directors, in conjunction with the anticipated closing of the initial closing of the Private Placement (see Note 10), committed to a restructuring plan, or the 2009 Restructuring, for an immediate reduction in force affecting six employees, including two executives. Employees were notified on March 31, 2009. All terminated employees were awarded severance payments and continuation of benefits based on length of service at the Company.
As a result of the 2009 Restructuring, the Company recorded a restructuring charge of $0.6 million in the first quarter of 2009 for employee severance and related benefit costs, which is included under “Restructuring charges” in the Company’s statement of operations. The severance payments were made in the second quarter of 2009, and other personnel-related expenses such as employee benefits were substantially paid over the remainder of 2009. No further charges are expected related to this restructuring.
The following table summarizes the changes in the 2009 Restructuring liabilities, for which balances are included under “Accrued restructuring charges” in the Company’s balance sheets:
| Employee Severance and Related Benefits |
||||
| Balance as of December 31, 2008 |
$ | — | ||
| Charges in period |
602,102 | |||
| Cash payments in period |
(571,668 | ) | ||
| Adjustments in period |
(18,452 | ) | ||
| Balance as of December 31, 2009 |
$ | 11,982 | ||
2008 Restructuring
In June 2008, the Company implemented a corporate realignment to focus on the development of voreloxin. In conjunction with this restructuring, or the 2008 Restructuring, the Company expanded its late-stage development leadership team, ceased its internal discovery research activities and reduced its workforce by
64
Table of Contents
approximately 60%. All terminated employees were awarded severance payments, continuation of benefits based on length of service at the Company and career transition assistance. The Company also consolidated its remaining employees into the leased office premises at 395 Oyster Point Boulevard and a small leased laboratory facility at 349 Allerton Avenue, both in South San Francisco.
In January 2009, the Company entered into an Agreement for Termination of Lease and Voluntary Surrender of Premises with ARE-Technology Center, SSF, LLC, or Alexandria, with respect to a leased facility located at 341 Oyster Point Boulevard, South San Francisco, which formerly served as the Company’s headquarters and research and development facility. Pursuant to the terms of the Termination Agreement, the Company was required to vacate the premises by February 28, 2009, and agreed to pay an aggregate fee of $2.2 million in consideration of early termination of the Lease Agreement. Under the original Lease Agreement, the Company was required to pay Alexandria base rents and operating expenses of $15.7 million between 2009 and 2013. The $2.2 million termination fee was paid in January 2009. In addition, the Company paid a commission of $0.4 million to a third party in January 2009 for negotiation of the lease termination.
In the first quarter of 2009, the Company recorded net restructuring charges of $1.3 million for these lease termination activities related to the 2008 Restructuring, which are included under “Restructuring charges” in the Company’s statement of operations. The net charge included the $2.2 million early termination fee and $0.4 million third-party commission, partially offset by the reversal of $1.4 million in non-cash deferred rent on this facility, which is recorded as a non-cash restructuring credit in the Company’s statement of cash flows.
The following table summarizes the changes in 2008 Restructuring liabilities, for which balances are included under “Accrued restructuring charges” in the Company’s balance sheets:
| Employee Severance and Related Benefits |
Facilities Related and Other Costs |
Total | ||||||||||
| Balance as of December 31, 2008 |
$ | 62,420 | $ | 128,750 | $ | 191,170 | ||||||
| Charges in period |
— | 2,654,360 | 2,654,360 | |||||||||
| Cash payments in period |
(62,420 | ) | (2,783,110 | ) | (2,845,530 | ) | ||||||
| Balance as of December 31, 2009 |
$ | — | $ | — | $ | — | ||||||
As of December 31, 2009, a total of $3.5 million of expenses had been incurred for the employee severance and related benefit costs, consisting of $3.2 million of severance and other benefit costs and $0.3 million of stock-based compensation. A total of $3.7 million had been incurred through December 31, 2009 related to facility exit costs, consisting of $2.2 million of lease termination fees, $1.6 million of asset impairments, $0.4 million of third-party commission and $0.9 million of other facility closure expenses, partially offset by the reversal of $1.4 million of deferred rent. No further charges are expected related to the 2008 Restructuring.
As part of the 2008 Restructuring, the Company implemented a corporate realignment to focus on the development of voreloxin. Due to the resulting termination of research activities, it was determined that laboratory equipment with a net book value of $1.2 million would be sold, and, accordingly, this equipment was recorded as held-for-sale. Held-for-sale equipment with a net book value of $0.5 million was sold in the year ended December 31, 2009, for net proceeds of $0.4 million. The loss on sale has been included in “Other income (expense), net” in the Company’s statement of operations.
2007 Restructuring
In August 2007, the Company implemented a restructuring, or the 2007 Restructuring, which included a 25% reduction in the Company’s workforce. As a result, the Company recorded total restructuring charges of
65
Table of Contents
$1.6 million in 2007 for employee severance and related benefit costs, including a non-cash portion related to stock-based compensation of $0.1 million, and $0.6 million of facilities exit costs, of which $0.3 million was related to the impairment of leasehold improvements and $0.3 million was related to the lease obligation on the property at 395 Oyster Point Boulevard which had been vacated in the 2007 consolidation.
8. Other Accrued Liabilities
Other accrued liabilities as of December 31 were as follows:
| 2009 | 2008 | |||||
| Accrued outside services |
$ | 390,418 | $ | 1,021,685 | ||
| Accrued professional services |
359,076 | 322,945 | ||||
| Sales taxes payable |
— | 15,804 | ||||
| Total other accrued liabilities |
$ | 749,494 | $ | 1,360,434 | ||
9. Commitments and Contingencies
Commitments
The Company’s operating lease obligations as of December 31, 2009 relate to the leases for two facilities in South San Francisco, California. In December 2006, the Company leased approximately 15,000 square feet of office space at 395 Oyster Point Boulevard, which is currently the Company’s headquarters. This lease expires in April 2013, subject to the Company’s option to extend the lease through February 2014. In October 2008, the Company leased approximately 5,500 square feet of laboratory space at 349 Allerton Avenue. This lease expires in October 2010 with the Company’s option to extend the lease through October 2012.
Aggregate non-cancelable future minimum rental payments under operating leases for each period presented are as follows:
| Payments | |||
| Year ended December 31, |
|||
| 2010 |
$ | 570,439 | |
| 2011 |
395,215 | ||
| 2012 |
404,441 | ||
| 2013 |
135,326 | ||
| 2014 and thereafter |
— | ||
| $ | 1,505,421 | ||
The operating lease agreements provide for increasing monthly rent payment over the lease term. The Company recognizes rent expense on a straight-line basis. The Company recorded rent expense of $0.8 million, $3.0 million and $3.1 million for the years ended December 31, 2009, 2008 and 2007, respectively. The deferred rent balances of $0.1 million and $1.5 million as of December 31, 2009 and 2008, respectively, represent the difference between actual rent payments and the straight-line rent expense.
Contingencies
From time to time, the Company may be involved in legal proceedings, as well as demands, claims and threatened litigation, which arise in the normal course of its business or otherwise. The ultimate outcome of any litigation is uncertain and unfavorable outcomes could have a negative impact on the Company’s results of operations and financial condition. Regardless of outcome, litigation can have an adverse impact on the Company
66
Table of Contents
because of the defense costs, diversion of management resources and other factors. The Company is not currently involved in any material legal proceedings.
10. Stockholders’ Equity
Preferred Stock
The Company has 10,000,000 shares of authorized preferred stock issuable in one or more series. Upon issuance, the Company can determine the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting any series or the designation of such series, any or all of which may be greater than the rights of common stock. There were 4,347,812 and 0 shares of preferred stock outstanding as of December 31, 2009 and 2008, respectively.
Common Stock
Holders of common stock are entitled to one vote per share on all matters to be voted upon by the stockholders of the Company. Subject to the preferences that may be applicable to any outstanding shares of preferred stock, the holders of common stock are entitled to receive ratably such dividends, if any, as may be declared by the Board of Directors. In accordance with the Private Placement agreements, any payment of dividends must be approved by the holders of at least a majority of the outstanding Series A Preferred Stock. No dividends have been declared to date.
Private Placement
Initial and Second Closings
The initial closing of $10.0 million of units of the Private Placement was completed on April 3, 2009, and the second closing of $5.0 million of units was completed on October 30, 2009. The per unit purchase price, which included a share of Series A convertible preferred stock and a warrant to purchase 10 shares of common stock, was $3.45 for both closings. The warrants have an exercise price of $0.22 per share and a term of seven years from the date of issuance. The net proceeds from the initial closing were $8.8 million, and net proceeds from the second closing were $4.7 million.
In the initial closing, the Company issued approximately 2.9 million shares of Series A convertible preferred stock, which are initially convertible into approximately 29.0 million shares of common stock and warrants to purchase an aggregate of approximately 29.0 million shares of common stock. In the second closing, the Company issued approximately 1.45 million shares of Series A preferred stock, which are initially convertible into 14.5 million shares of common stock, and warrants to purchase 14.5 million shares of common stock.
Common Equity Closing
Pursuant to the Private Placement, an additional $28.5 million of common stock may be sold in a common equity closing, (i) upon the election of the holders of a majority of the Series A convertible preferred stock issued in the Private Placement prior to a date determined with reference to the Company’s cash and investment balance dropping below $2.5 million, or (ii) at the Company’s election, subject to the approval of a majority of Series A convertible preferred stockholders and subject to a condition that the Company sells at least $28.5 million of common stock in the common equity closing. In the common equity closing, if completed, the Company would issue approximately 103.6 million shares of common stock at a purchase price of $0.275 per share. If a Series A convertible preferred stock investor declines to participate in the common equity closing, their shares of preferred stock will automatically be converted into shares of the Company’s common stock on a basis of one common share for each preferred share outstanding on the date of conversion.
67
Table of Contents
Other Investor Rights
In conjunction with the initial closing of the Private Placement, the investors received a number of additional rights as a result of their convertible preferred stock ownership, including the right to approve any sale of the Company, any issuance of debt or preferred stock and, unless certain conditions are met, any issuance of common stock, other than the second closing and the common equity closing described above. Upon any liquidation, dissolution or winding up of the Company, whether voluntary or involuntary, the holders of the Series A convertible preferred stock have a right to receive proceeds equal to $10.35 (i.e. three times the purchase price of each unit) for each share of Series A convertible preferred stock held, plus all declared and unpaid dividends, in preference to the holders of common stock. A change in control, as defined in the securities purchase agreement, shall be deemed a liquidation, and includes a consolidation or merger with or into any other corporation, or the sale, exclusive license or exclusive partnering of the majority of the Company’s assets.
On June 29, 2009, the Private Placement agreements were amended to: (a) clarify that in the event that an investor wishes to sell or transfer their shares of Series A convertible preferred stock, they must transfer all associated rights and obligations with such shares, including the option to participate in the second closing, or the Second Closing Option, and the option to participate in the common equity closing, or the Common Equity Closing Option, and (b) require approval by the Company’s board of directors of any transaction that constitutes a “change of control” event that does not already require approval of the board of directors by statute.
As of the initial closing, certain of the investors had the right to designate three of eight members of the Company’s board of directors. On October 27, 2009, the Private Placement agreements were amended such that the investors’ right to designate five of nine members of the Company’s board of directors, which would have otherwise been effective upon the second closing, was deferred until at least January 1, 2010. On March 29, 2010, the Private Placement agreements were amended once more, such that the investors’ right to designate additional members of the Company’s board of directors was deferred until at least May 1, 2010 (see Note 14). As a result, on May 1, 2010, the Series A convertible preferred stock will become potentially redeemable upon certain events that are outside of the control of the Company, and all Series A convertible preferred stock issued in the Private Placement that is outstanding at that time will be reclassified to mezzanine equity.
Accounting Treatment
On April 3, 2009, the fair values of the four primary components of the Private Placement: (a) the Series A convertible preferred stock, (b) the warrants to purchase common stock, (c) the Second Closing Option, and (d) the Common Equity Closing Option, were computed using the Black-Scholes model. The values were calculated as a series of call options on the potential enterprise value of the Company at different valuation points at which the claims of the different stakeholder groups on the enterprise value would change. On April 3, 2009, the Company determined that the Second Closing Option and Common Equity Closing Option were freestanding instruments, and as a result, their fair values of $7.3 million and $10.2 million were initially recorded as liabilities in the Company’s balance sheet. As the total fair value of these two components exceeded the gross proceeds of the initial closing of the Private Placement of $10.0 million, no value was attributed to the Series A convertible preferred stock or warrant components as of this date, and the $7.5 million excess of the fair value of the two closing options over the gross proceeds was recorded as a loss within other income (expense) in the Company’s statement of operations.
On June 18, 2009, the Company’s stockholders approved an increase in the authorized number of shares of common stock from 100 million to 400 million, subject to an administrative filing with the State of Delaware, which occurred on July 2, 2009. As a result, the Common Equity Closing Option liability was revalued to its fair value of $20.1 million as of June 18, 2009, resulting in a charge for the increase in fair value of $9.9 million, which was recorded within other income (expense) in the Company’s statement of operations. The Common Equity Closing Option was then reclassified from a liability to additional paid-in-capital in the Company’s balance sheet.
68
Table of Contents
On June 29, 2009, as a result of amendments to the Private Placement agreements, the convertible preferred stock, the Second Closing Option, and the Common Equity Closing Option were extinguished by the issuance of the amended convertible preferred stock instrument. The Second Closing Option liability was revalued upon extinguishment to its fair value of $10.9 million, resulting in a charge to other income (expense) for the increase in fair value of $3.6 million. The convertible preferred stock and Common Equity Closing Option were also revalued upon extinguishment to their fair values of $22.9 million and $23.6 million, respectively, resulting in an aggregate deemed distribution to preferred stockholders of $26.4 million. On June 29, 2009, the amended convertible preferred stock instrument was recorded as convertible preferred stock within stockholders equity, at its fair value of $57.4 million, less transaction costs of $1.2 million.
On October 30, 2009, the $5.0 million of gross proceeds from the second closing was allocated between the convertible preferred stock and warrants issued in the closing on a relative fair value basis, using the Black-Scholes model. The $4.2 million of gross proceeds allocated to the convertible preferred stock was discounted by $1.2 million to account for a beneficial conversion feature, which arose as the effective conversion price of the preferred stock was less than the fair value of the common stock on the date of closing, or commitment date. The discount was written off immediately and recorded as a deemed dividend, which increased the loss applicable to common stockholders in the calculation of basic and diluted net loss per share. The $0.8 million of gross proceeds allocated to the warrants was recorded to additional paid-in capital.
Stock Option Plans
The Company grants options primarily to: (i) new employees, 25% of which becomes exercisable on the first anniversary of the vesting commencement date, and 1/48th becomes exercisable each month over the remainder of the four-year vesting period, (ii) existing employees, 1/48 th of which becomes exercisable each month following the date of grant over a period of four years, (iii) new non-employee members of the board of directors, 50% of which becomes exercisable on each of the first and second anniversary of the vesting commencement date, and (iv) continuing non-employee members of the board of directors, 1/12th of which becomes exercisable each month following the date of grant over a period of one year.
2005 Equity Incentive Award Plan
In February 2005, the Board of Directors adopted and, in September 2005, the stockholders approved, the 2005 Equity Incentive Award Plan, or the 2005 Plan. The 2005 Plan is intended to serve as the successor equity incentive program to the 1998 Stock Plan and 2001 Stock Plan. The Company initially reserved a total of 1,779,396 shares of common stock for issuance under the 2005 Plan plus shares underlying any options granted under the Company’s 1998 Stock Plan or 2001 Stock Plan that expire unexercised or are repurchased by the Company pursuant to the terms of such options.
The number of shares of common stock reserved under the 2005 Plan automatically increases on the first trading day of each year by an amount equal to the lesser of: (i) 4% of the Company’s outstanding shares of common stock on such date, (ii) 1,082,352 shares, or (iii) an amount determined by the Board of Directors. The maximum aggregate number of shares which may be issued or transferred over the term of the 2005 Plan is 11,294,112 shares. In addition, no participant in the 2005 Plan may be issued or transferred more than 235,294 shares of common stock per calendar year.
On January 1, 2009, the number of shares of common stock reserved for future issuance under the 2005 Plan was increased by 1,082,352 shares pursuant to the evergreen provision detailed above. Options to purchase 4,105,000 shares of the Company’s common stock were granted under the 2005 Plan during the year ended December 31, 2009. As of December 31, 2009, options and awards for an aggregate of 8,586,748 shares of the Company’s common stock had been granted and 1,143,531 shares were available for future grants under the 2005 Plan.
69
Table of Contents
2006 Employment Commencement Incentive Plan
In November 2005, the Board of Directors adopted the 2006 Employment Commencement Incentive Plan, or the 2006 Plan, which became effective on January 1, 2006. Awards granted pursuant to the 2006 Plan are intended to be inducement awards pursuant to Nasdaq Marketplace Rule 4350(i)(1)(A)(iv). The 2006 Plan was not subject to the approval of the Company’s stockholders. Eligibility to participate in the 2006 Plan is limited to employees who have not previously been employees or directors of the Company, or following a bona fide period of non-employment by the Company. Additionally, grants awarded to such employees under the 2006 Plan must be made in connection with commencement of employment and must be an inducement material to the person entering into employment with the Company.
Effective January 1, 2009, the Board of Directors approved an amendment to increase the number of shares of common stock reserved for issuance under the 2006 Plan by 100,000 shares. No options were granted under the 2006 Plan during the year ended December 31, 2009. As of December 31, 2009, options to purchase an aggregate of 553,000 shares of the Company’s common stock had been granted and 467,000 shares were available for future grants under the 2006 Plan.
Employee Stock Purchase Plan
In February 2005, the Board of Directors adopted and, in September 2005, the stockholders approved the Company’s Employee Stock Purchase Plan, or ESPP. The ESPP permits eligible employees to purchase common stock at a discount through payroll deductions during defined offering periods. Eligible employees can purchase shares of the Company’s common stock at 85% of the lower of the fair market value of the common stock at the beginning of a 12-month offering period or at the end of one of the two related 6-month purchase periods. The Company initially reserved a total of 202,941 shares of common stock for issuance under the ESPP.
The number of shares of common stock reserved under the ESPP automatically increases on the first trading day each year, by an amount equal to the lesser of: (i) 0.5% of the Company’s outstanding shares of common stock on such date, (ii) 135,294 shares, or (iii) a lesser amount determined by the Board of Directors. The maximum aggregate number of shares which may be issued over the term of the ESPP is 1,352,941 shares. In addition, no participant in the ESPP may be issued or transferred shares of common stock valued at more than $25,000 per calendar year and no participant may purchase more than 1,176 shares during any purchase period.
A total of 18,816 shares were issued under the ESPP during the year ended December 31, 2009. As of December 31, 2009, 312,154 shares of the Company’s common stock had been issued and 233,637 shares were available for future issuance under the ESPP.
Warrants
The Company has the following warrants to purchase common stock outstanding as of December 31, 2009:
| Shares | Exercise Price | Expiration | |||||
| 41,176 | $ | 17.00 | May 2010 | ||||
| 256,740 | $ | 9.10 | July 2010 | ||||
| 1,046 | $ | 9.10 | September 2015 | ||||
| 164,830 | $ | 9.10 | August 2015 | ||||
| 1,582 | $ | 9.10 | June 2013 | ||||
| 757 | $ | 9.10 | June 2014 | ||||
| 2,173,914 | $ | 6.21 | March 2013 | ||||
| 26,986,440 | $ | 0.22 | April 2016 | ||||
| 14,492,680 | $ | 0.22 | October 2016 | ||||
| Total warrants outstanding |
44,119,165 | ||||||
70
Table of Contents
Reserved Shares
As of December 31, 2009, the Company’s shares of common stock reserved for future issuance were as follows:
| Shares Available for Future Grant |
Outstanding Securities |
Total Shares Reserved | ||||
| Convertible preferred stock, as-if converted |
— | 43,478,120 | 43,478,120 | |||
| Warrants |
— | 44,119,165 | 44,119,165 | |||
| Stock option plans |
1,610,531 | 6,407,309 | 8,017,840 | |||
| Employee stock purchase plan |
233,637 | — | 233,637 | |||
| Total reserved shares of common stock |
1,844,168 | 94,004,594 | 95,848,762 | |||
11. Stock-Based Compensation
Overview
Employee stock-based compensation expense is calculated based on the grant-date fair value of awards ultimately expected to vest, reduced for estimated forfeitures, and is recorded on a straight-line basis over the vesting period of the awards. Forfeitures are estimated at the time of grant, based on historical option cancellation information, and revised in subsequent periods if actual forfeitures differ from those estimates. Employee stock-based compensation expense related to the Company’s stock-based awards was as follows for the periods presented:
| Year ended December 31, | |||||||||
| 2009 | 2008 | 2007 | |||||||
| Research and development |
$ | 226,568 | $ | 644,549 | $ | 1,322,656 | |||
| General and administrative |
1,084,377 | 1,265,379 | 1,863,999 | ||||||
| Restructuring charges |
— | 366,637 | 126,456 | ||||||
| Total employee stock-based compensation expense |
$ | 1,310,945 | $ | 2,276,565 | $ | 3,313,111 | |||
71
Table of Contents
Fair Value of Awards
The Company determines the fair value of stock-based awards on the grant date using the Black-Scholes model, which is impacted by the Company’s stock price, as well as assumptions regarding a number of highly subjective variables. The following table summarizes the weighted-average assumptions used as inputs to the Black-Scholes model, and resulting weighted-average and total estimated grant date fair values of employee stock options granted during the periods presented:
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Stock Option Plans | ||||||||||||
| Assumptions: |
||||||||||||
| Expected term (years) |
4.5 | 5.0 | 5.0 | |||||||||
| Expected volatility |
86.7 | % | 72.4 | % | 68.5 | % | ||||||
| Risk-free interest rate |
1.9 | % | 3.3 | % | 4.3 | % | ||||||
| Dividend yield |
0.0 | % | 0.0 | % | 0.0 | % | ||||||
| Fair value: |
||||||||||||
| Weighted-average grant date fair value per share |
$ | 0.31 | $ | 0.89 | $ | 1.76 | ||||||
| Options granted to employees |
4,050,000 | 849,225 | 1,571,000 | |||||||||
| Total estimated grant date fair value |
$ | 1.3 million | $ | 0.8 million | $ | 2.8 million | ||||||
The estimated fair value of stock options that vested in the years ended December 31, 2009, 2008 and 2007, was $1.2 million, $2.2 million and $2.5 million, respectively.
Purchase rights for 18,816, 44,802 and 102,904 shares were granted under the ESPP during the years ended December 31, 2009, 2008 and 2007, respectively. The weighted-average estimated fair value of purchase rights granted under the ESPP for the years ended December 31, 2009, 2008 and 2007 was $0.32, $1.09 and $1.65 per share, respectively, using the Black-Scholes model with the following weighted-average assumptions:
| Year Ended December 31, | |||||||||
| 2009 | 2008 | 2007 | |||||||
| Employee Stock Purchase Plan | |||||||||
| Expected term (years) |
0.5 – 1.0 | 0.5 – 1.0 | 0.5 – 1.0 | ||||||
| Expected volatility |
157.0 | % | 93.4 | % | 68.5 | % | |||
| Risk-free interest rate |
1.1 | % | 0.4% – 5.1 | % | 3.2% – 5.1 | % | |||
| Dividend yield |
0.0 | % | 0.0 | % | 0.0 | % | |||
For employee stock options, the Company based its assumptions for the expected term on historical cancellation and exercise data, and the contractual term and vesting terms of the awards. Expected volatility is based on historical volatility of the Company’s common stock, as well as that for a mature peer group of companies in the same industry. For employee purchase rights under the ESPP, the expected term is equal to the purchase period. The risk-free interest rate assumptions are based upon observed interest rates appropriate for the expected life of the Company’s employee stock options and employee purchase rights. The Company does not anticipate paying any cash dividends in the foreseeable future, and therefore uses an expected dividend yield of zero.
72
Table of Contents
Option Plan Activity
The following table summarizes stock option activity for the Company’s stock option plans in the periods presented:
| Number of Shares |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Term (Years) |
Aggregate Intrinsic Value | ||||||||
| Outstanding as of December 31, 2006 |
3,942,435 | $ | 4.30 | ||||||||
| Options granted |
1,636,750 | $ | 3.04 | ||||||||
| Options exercised |
(68,813 | ) | $ | 2.34 | |||||||
| Options canceled/forfeited/expired |
(410,525 | ) | $ | 4.78 | |||||||
| Outstanding as of December 31, 2007 |
5,099,847 | $ | 3.83 | ||||||||
| Options granted |
874,225 | $ | 1.75 | ||||||||
| Options canceled/forfeited/expired |
(1,323,117 | ) | $ | 3.64 | |||||||
| Outstanding as of December 31, 2008 |
4,650,955 | $ | 3.44 | ||||||||
| Options granted |
4,105,000 | $ | 0.47 | ||||||||
| Options exercised |
(4,557 | ) | $ | 1.44 | |||||||
| Options canceled/forfeited/expired |
(2,344,089 | ) | $ | 3.18 | |||||||
| Outstanding as of December 31, 2009 |
6,407,309 | $ | 1.64 | 8.23 | $ | 2,358,200 | |||||
| Vested and expected to vest as of December 31, 2009 |
5,895,963 | $ | 1.73 | 8.11 | $ | 2,077,443 | |||||
| Exercisable as of December 31, 2009 |
2,454,203 | $ | 3.12 | 6.28 | $ | 315,483 | |||||
The aggregate intrinsic value in the table above represents the total pre-tax intrinsic value (i.e., the difference between the Company’s closing stock price on the last trading day of the period and the exercise price, multiplied by the number of in-the-money options) that would have been received by option holders if they had exercised all their options on December 31, 2009.
The intrinsic value of options exercised during the years ended December 31, 2009, 2008 and 2007 was $1,000, $0 and $0.1 million, respectively. As the Company believes it is more likely than not that no stock option related tax benefits will be realized, the Company does not record any net tax benefits related to exercised options.
Total estimated unrecognized stock-based compensation cost related to unvested stock options was $3.0 million as of December 31, 2009, which is expected to be recognized over the respective vesting terms of each award. The weighted average term of the unrecognized stock-based compensation expense is 2.9 years.
73
Table of Contents
12. Income Taxes
No provision for U.S. income taxes exists in the periods presented due to tax losses incurred in each period. The income tax provision differs from the amount computed by applying the statutory income tax rate of 34% to pre-tax loss as follows:
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Tax at statutory rate |
$ | (13,676,582 | ) | $ | (12,642,344 | ) | $ | (13,178,440 | ) | |||
| Current year net operating losses and temporary differences for which no tax benefit is recognized |
6,340,457 | 12,223,875 | 12,415,146 | |||||||||
| Non-cash expense related to Private Placement |
7,145,779 | — | — | |||||||||
| Other permanent differences |
190,346 | 418,469 | 763,294 | |||||||||
| Provision for income taxes |
$ | — | $ | — | $ | — | ||||||
Deferred income taxes reflect the net tax effects of loss and credit carry-forwards and temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets for federal and state income taxes are as follows:
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| Deferred tax assets: |
||||||||
| Net operating loss carry-forwards |
$ | 87,303,000 | $ | 74,711,000 | ||||
| Federal and state research credit carry-forwards |
8,852,000 | 8,328,000 | ||||||
| Capitalized research costs |
5,181,000 | 8,649,000 | ||||||
| Property and equipment |
181,000 | 1,966,000 | ||||||
| Accrued liabilities |
1,857,000 | 2,026,000 | ||||||
| Gross deferred tax assets |
103,374,000 | 95,680,000 | ||||||
| Valuation allowance |
(103,374,000 | ) | (95,680,000 | ) | ||||
| Net deferred tax assets |
$ | — | $ | — | ||||
Realization of the deferred tax assets is dependent upon future taxable income, if any, the amount and timing of which are uncertain. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance. The net valuation allowance increased by approximately $7.7 million, $15.0 million and $16.2 million during the years ended December 31, 2009, 2008 and 2007, respectively.
As of December 31, 2009, the Company had federal net operating loss carry-forwards of $233.2 million and federal research and development tax credit carry-forwards of $5.3 million. If not utilized, the federal net operating loss and tax credit carry-forwards will expire at various dates beginning in 2018. As of December 31, 2009, the Company had state net operating loss carry-forwards of $133.8 million, which expire beginning in 2012, and state research and development tax credit carry-forwards of $5.2 million, which do not expire.
Utilization of these net operating loss and tax credits carry-forwards may be subject to a substantial annual limitation due to the ownership change rules under Section 382 of the Internal Revenue Code of 1986, as amended, or The Code. The limitations are applicable if an “ownership change,” as defined in The Code, is deemed to have occurred or occurs in the future. The annual limitation may result in the expiration of net operating loss and credit carry-forwards before they can be utilized.
74
Table of Contents
The Company adopted the provisions of ASC Topic 740, previously referred to as FASB Interpretation No. 48, Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement No. 109, on January 1, 2007. ASC Topic 740 provides guidance on the recognition, measurement, classification and interest and penalties related to uncertain tax positions. In accordance with ASC Topic 740, the Company determines whether it is “more likely than not” that a tax position will be sustained upon examination by the appropriate taxing authorities before any tax benefit is recognized in the financial statements. As of December 31, 2009 and 2008, the Company had no unrecognized tax benefits.
The Company files U.S. federal and California tax returns. The Company’s wholly owned subsidiary files tax returns in the United Kingdom. To date, neither the Company nor its wholly owned subsidiary has been audited by the Internal Revenue Service, any state income tax authority or tax authority in the United Kingdom. Due to net operating loss carry-forwards, substantially all of the Company’s tax years remain open to federal tax examination. The tax return for California is subject to a four year statute of limitations.
13. Guarantees and Indemnification
As permitted under Delaware law and in accordance with the Company’s Bylaws, the Company indemnifies its officers and directors for certain events or occurrences, subject to certain limits, while the officer or director is or was serving at the Company’s request in such capacity. The indemnification agreements with the Company’s officers and directors terminate upon termination of their employment, but the termination does not affect claims for indemnification relating to events occurring prior to the effective date of termination. The maximum amount of potential future indemnification is unlimited; however, the Company’s officer and director insurance policy reduces the Company’s exposure and may enable the Company to recover a portion of any future amounts paid. The Company believes that the fair value of these indemnification agreements is minimal. In addition, in the ordinary course of business the Company enters into agreements, such as licensing agreements, clinical trial agreements and certain services agreements, containing standard indemnifications provisions. The Company believes that the likelihood of an adverse judgment related to such indemnification provisions is remote. Accordingly, the Company has not recorded any liabilities for any of these agreements as of December 31, 2009.
14. Subsequent Events
On January 20, 2010, the Company entered into a controlled equity offering sales agreement with Cantor Fitzgerald & Co., or Cantor, pursuant to which the Company could issue and sell shares of its common stock having an aggregate offering price of up to $15.0 million from time to time through Cantor acting as agent and/or principal. The Company agreed to pay Cantor a commission of between 3% and 5% of the gross proceeds from each sale. As of March 31, 2010, the Company had sold an aggregate of 15,870,050 shares of common stock at an average price of approximately $0.95 per share for gross proceeds of the full 15.0 million available under the facility. Net proceeds were $14.2 million after deducting Cantor’s commission and costs to set up the facility.
On January 1, 2010, due to amendments to the Private Placement agreements effected on October 27, 2009, the investors in the Private Placement received the right to designate five of nine members of the Company’s board of directors. As a result, on January 1, 2010, the Series A convertible preferred stock became potentially redeemable upon certain events that are outside of the control of the Company, and all Series A convertible preferred stock issued in the Private Placement that was outstanding at that time was reclassified to mezzanine equity, outside of stockholders’ equity. On March 29, 2010, the Private Placement agreements were amended once more, such that the investors’ right to designate additional members of the Company’s board of directors was deferred until at least May 1, 2010. As a result, on March 29, 2010, the Series A convertible preferred stock was reclassified back into stockholders’ equity.
75
Table of Contents
15. Selected Quarterly Financial Data (unaudited)
| Three Months Ended | ||||||||||||||||||||||||||||||||
| Mar. 31, 2009 |
June 30, 2009 |
Sep. 30, 2009 |
Dec. 31, 2009 |
Mar. 31, 2008 |
June 30, 2008 |
Sep. 30, 2008 |
Dec. 31, 2008 |
|||||||||||||||||||||||||
| Revenue |
$ | 224,047 | $ | 3,512,500 | $ | 12,500 | $ | 12,500 | $ | 2,303,183 | $ | 2,591,240 | $ | 510,417 | $ | 12,500 | ||||||||||||||||
| Net loss |
$ | (8,363,436 | ) | $ | (22,878,464 | ) | $ | (4,949,074 | ) | $ | (4,035,072 | ) | $ | (9,624,905 | ) | $ | (13,568,418 | ) | $ | (7,065,172 | ) | $ | (6,927,132 | ) | ||||||||
| Deemed distribution to preferred stockholders |
$ | — | $ | (26,375,000 | ) | $ | — | $ | (1,188,400 | ) | $ | — | $ | — | $ | — | $ | — | ||||||||||||||
| Loss attributable to common stockholders |
$ | (8,363,436 | ) | $ | (49,253,464 | ) | $ | (4,949,074 | ) | $ | (5,223,472 | ) | $ | (9,624,905 | ) | $ | (13,568,418 | ) | $ | (7,065,172 | ) | $ | (6,927,132 | ) | ||||||||
| Basic and diluted loss attributable to common stockholders per common share |
$ | (0.24 | ) | $ | (1.43 | ) | $ | (0.14 | ) | $ | (0.15 | ) | $ | (0.28 | ) | $ | (0.39 | ) | $ | (0.21 | ) | $ | (0.20 | ) | ||||||||
| Shares used in computing basic and diluted loss attributable to common stockholders per common share |
34,409,768 | 34,412,870 | 34,419,185 | 34,678,757 | 34,364,896 | 34,377,367 | 34,401,519 | 34,404,578 | ||||||||||||||||||||||||
76
Table of Contents
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
Not applicable.
| ITEM 9A(T). | CONTROLS AND PROCEDURES |
Disclosure Controls and Procedures
Based on their evaluation as of December 31, 2008, our Chief Executive Officer and Chief Financial Officer, with the participation of management, have concluded that, subject to the limitations described below, our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act) were effective at the reasonable assurance level.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2009. Management based its assessment on the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control – Integrated Framework. Based on this evaluation, our management concluded that as of December 31, 2009, our internal control over financial reporting was effective.
The Company’s internal control over financial reporting was not subject to attestation by the Company’s registered public accounting firm pursuant to temporary rules of the Securities and Exchange Commission that permit the Company to provide only management’s report in this annual report.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting during the quarter ended December 31, 2009 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations on the Effectiveness of Controls
Our disclosure controls and procedures provide our Chief Executive Officer and Chief Financial Officer with only reasonable assurances that our disclosure controls and procedures will achieve their objectives. However, our management, including our Chief Executive Officer and Chief Financial Officer, does not expect that our disclosure controls and procedures or our internal control over financial reporting can or will prevent all human error. A control system, no matter how well designed and implemented, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Furthermore, the design of a control system must reflect the fact that there are internal resource constraints, and the benefit of controls must be weighed relative to their corresponding costs. Because of the limitations in all control systems, no evaluation of controls can provide complete assurance that all control issues and instances of error, if any, within our company are detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and
77
Table of Contents
that breakdowns can occur due to human error or mistake. Additionally, controls, no matter how well designed, could be circumvented by the individual acts of specific persons within the organization. The design of any system of controls is also based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated objectives under all potential future conditions.
| ITEM 9B. | OTHER INFORMATION |
None.
78
Table of Contents
PART III
Certain information required by Part III is omitted from this report because we will file with the SEC a definitive proxy statement pursuant to Regulation 14A (the “Proxy Statement”) not later than 120 days after the year ended December 31, 2009, and certain information included therein is incorporated herein by reference.
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
Identification of Directors
Information responsive to this item is incorporated herein by reference to our definitive Proxy Statement.
Identification of Executive Officers
Information responsive to this item is incorporated herein by reference to our definitive Proxy Statement.
Identification of Audit Committee and Financial Expert
Information responsive to this item is incorporated herein by reference to our definitive Proxy Statement.
Material Changes to Procedures for Recommending Directors
Information responsive to this item is incorporated herein by reference to our definitive Proxy Statement.
Compliance with Section 16(a) of the Exchange Act
Information responsive to this item is incorporated herein by reference to our definitive Proxy Statement.
Code of Business Conduct & Ethics
We have adopted a Code of Business Conduct & Ethics which applies to all of our directors, officers and employees. A copy of our Code of Business Conduct & Ethics can be found on our website, www.sunesis.com, in the section titled “Investors and Media” under the subsection titled “Corporate Governance.” Information found on our website is not incorporated by reference into this report. In addition, we intend to promptly disclose (1) the nature of any amendment to our Code of Business Conduct & Ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or persons performing similar functions and (2) the nature of any waiver, including an implicit waiver, from a provision of our Code of Business Conduct & Ethics that is granted to one of these specified officers, the name of such person who is granted the waiver and the date of the waiver on our website in the future.
All additional information required by this Item 10 will be set forth in our definitive Proxy Statement and is incorporated in this report by reference.
| ITEM 11. | EXECUTIVE COMPENSATION |
Information responsive to this item is incorporated herein by reference to our definitive Proxy Statement.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Ownership of Sunesis Securities
Information responsive to this item is incorporated herein by reference to our definitive Proxy Statement.
79
Table of Contents
Equity Compensation Plan Information
The following table provides certain information with respect to our equity compensation plans in effect as of December 31, 2009:
| (A) | (B) | (C) | |||||||
| Plan Category |
Number of Securities to be Issued upon Exercise of Outstanding Options |
Weighted Average Exercise Price of Outstanding Options |
Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column A) |
||||||
| Equity Compensation Plans Approved by Stockholders(1) |
6,249,309 | (2) | $ | 1.64 | 1,377,168 | (3) | |||
| Equity Compensation Plans Not Approved by Stockholders(4) |
158,000 | $ | 1.54 | 467,000 | |||||
| Total |
6,407,309 | $ | 1.64 | 1,844,168 | |||||
| (1) | Includes securities issuable under our 2005 Equity Incentive Award Plan, or 2005 Plan, and Employee Stock Purchase Plan, or ESPP. |
| (2) | Excludes purchase rights currently accruing under the ESPP. Offering periods under the ESPP are 12-month periods, which are comprised of two six-month purchase periods. Eligible employees may purchase shares of common stock at a price equal to 85% of the lower of the fair market value of the common stock at the beginning of each offering period or the end of each semi-annual purchase period. Participation is limited to 20% of an employee’s eligible compensation, subject to limitations under the Internal Revenue Code. |
| (3) | Includes (i) 1,143,531 shares of common stock available for issuance under our 2005 Plan and (ii) 233,637 shares of common stock available for issuance under our ESPP. Beginning in 2006, the number of shares of common stock reserved under the 2005 Plan automatically increases on the first trading day each year by an amount equal to the lesser of: (i) 4% of the Company’s outstanding shares of common stock outstanding on such date, (ii) 1,082,352 shares, or (iii) an amount determined by the Board of Directors. The number of shares of common stock reserved under our ESPP automatically increases on the first trading day each year by an amount equal to the least of: (i) 0.5% of our outstanding shares of common stock outstanding on such date, (ii) 135,294 shares or (iii) a lesser amount determined by our Board of Directors. |
| (4) | Represents our 2006 Employment Commencement Incentive Plan, or 2006 Plan. |
The additional information required by this Item 12 concerning our non-stockholder approved equity compensation plans is discussed in the notes to our consolidated financial statements contained in Part II, Item 8 of this report and is incorporated herein by reference. Any other information required by this Item 12 will be set forth in our definitive Proxy Statement and is incorporated in this report by reference.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
Information responsive to this item is incorporated herein by reference to our definitive Proxy Statement.
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
Information responsive to this item is incorporated herein by reference to our definitive Proxy Statement.
80
Table of Contents
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
Exhibits and Financial Statement Schedules:
| (a)(1) | Financial Statements |
| Page | ||
| 52 | ||
| 53 | ||
| 54 | ||
| 55 | ||
| 56 | ||
| 57 |
| (a)(2) | Financial Statement Schedules |
All financial statement schedules are omitted because they are not applicable, or the information is included in the financial statements or notes thereto.
| (a)(3) | Exhibits |
A list of exhibits filed with this report or incorporated herein by reference is found in the Exhibit Index immediately following the signature page of this report.
81
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, Sunesis Pharmaceuticals, Inc. has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized on March 31, 2010.
| SUNESIS PHARMACEUTICALS, INC. | ||
| By: | /S/ ERIC H. BJERKHOLT | |
| Eric H. Bjerkholt | ||
| Senior Vice President, Corporate Development | ||
| and Finance, Chief Financial Officer | ||
KNOW ALL PERSONS BY THESE PRESENTS , that each person whose signature appears below constitutes and appoints Daniel N. Swisher, Jr. and Eric H. Bjerkholt, and each of them, as his true and lawful attorneys-in-fact and agents, with full power of substitution for him, and in his name in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, and any of them or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities on the dates indicated.
| Signature |
Title |
Date | ||
| /S/ JAMES W. YOUNG, PH.D. James W. Young, Ph.D. |
Chairman of the Board | March 31, 2010 | ||
| /S/ DANIEL N. SWISHER, JR. Daniel N. Swisher, Jr. |
President, Chief Executive Officer and Director (Principal Executive Officer) | March 31, 2010 | ||
| /S/ ERIC H. BJERKHOLT Eric H. Bjerkholt |
Senior Vice President, Corporate Development and Finance, Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
March 31, 2010 | ||
| /S/ MATTHEW K. FUST Matthew K. Fust |
Director | March 31, 2010 | ||
| /S/ EDWARD HURWITZ Edward Hurwitz |
Director | March 31, 2010 | ||
| /S/ HELEN S. KIM Helen S. Kim |
Director | March 31, 2010 | ||
| /S/ DAYTON MISFELDT Dayton Misfeldt |
Director | March 31, 2010 | ||
| /S/ HOMER L. PEARCE PH.D. Homer L. Pearce Ph.D. |
Director | March 31, 2010 | ||
| /S/ DAVID C. STUMP, M.D. David C. Stump, M.D. |
Director | March 31, 2010 | ||
82
Table of Contents
| Incorporated By Reference | ||||||||||||
| Exhibit |
Exhibit Description |
Form | File No. | Exhibit | Filing Date |
Filed Herewith | ||||||
| 3.1 | Amended and Restated Certificate of Incorporation of the Registrant | 10-K/A | 000-51531 | 3.1 | 5/23/07 | |||||||
| 3.2 | Amended and Restated Bylaws of the Registrant | 8-K | 000-51531 | 3.2 | 12/11/07 | |||||||
| 3.3 | Certificate of Designation of the Series A Preferred Stock of the Registrant | 8-K | 000-51531 | 3.3 | 4/3/09 | |||||||
| 3.4 | Certificate of Amendment to the Certificate of Designation of the Series A Preferred Stock of the Registrant | 8-K | 000-51531 | 3.4 | 11/2/09 | |||||||
| 3.5 | Certificate of Amendment to the Certificate of Designation of the Series A Preferred stock of the Registrant | 8-K | 000-51531 | 3.5 | 1/21/10 | |||||||
| 4.1 | Specimen Common Stock certificate of the Registrant | S-1 | 333-121646 | 4.1 | 12/23/04 | |||||||
| 4.2 | Investor Rights Agreement, dated April 3, 2009, by and among the Registrant and the purchasers identified on the signature pages thereto | 8-K | 000-51531 | 4.1 | 4/3/09 | |||||||
| 10.1* | 1998 Stock Plan and Form of Stock Option Agreement | S-1/A | 333-121646 | 10.1 | 1/27/05 | |||||||
| 10.2* | 2001 Stock Plan and Form of Stock Option Agreement | S-1 | 333-121646 | 10.2 | 12/23/04 | |||||||
| 10.3* | 2005 Equity Incentive Award Plan, as amended, and Form of Stock Option Agreement | 10-K/A | 000-51531 | 10.3 | 4/30/09 | |||||||
| 10.4* | Employee Stock Purchase Plan and Enrollment Form | 10-Q | 000-51531 | 10.4 | 11/9/06 | |||||||
| 10.5* | Form of Indemnification Agreement for directors and executive officers | S-1 | 333-121646 | 10.5 | 12/23/04 | |||||||
| 10.6 | Reserved | |||||||||||
| 10.7* | Warrant, dated April 9, 1998, issued to James A. Wells | S-1 | 333-121646 | 10.18 | 12/23/04 | |||||||
| 10.8 | Warrant, dated December 1, 1999, issued to Three Crowns Capital (Bermuda) Limited | S-1 | 333-121646 | 10.19 | 12/23/04 | |||||||
| 10.9 | Warrant, dated July 7, 2000, issued to Broadview Ltd. Limited and Amendment No. 1 thereto | S-1 | 333-121646 | 10.20 | 12/23/04 | |||||||
| 10.10 | Warrant, dated June 11, 2003, issued to General Electric Capital Corporation | S-1 | 333-121646 | 10.21 | 12/23/04 | |||||||
| 10.11 | Warrant, dated June 21, 2004, issued to General Electric Capital Corporation and Amendment No. 1 thereto, dated December 16, 2004 | S-1/A | 333-121646 | 10.22 | 4/29/05 | |||||||
| 10.12 | Agreement for Termination of Lease and Voluntary Surrender of Premises, dated as of January 15, 2009, by and between the Registrant and ARE-Technology Center, SSF, LLC | 10-K | 000-51531 | 10.13 | 4/3/09 | |||||||
| 10.13† | Collaboration Agreement, dated December 18, 2002, by and between the Registrant and Biogen Idec MA Inc. (successor to Biogen Inc.) | S-1/A | 333-121646 | 10.26 | 1/27/05 | |||||||
83
Table of Contents
| Incorporated By Reference | ||||||||||||
| Exhibit |
Exhibit Description |
Form | File No. | Exhibit | Filing Date |
Filed Herewith | ||||||
| 10.14† | Amendment No. 1 to Collaboration Agreement, dated June 17, 2003, between the Registrant and Biogen Idec MA Inc. | S-1/A | 333-121646 | 10.27 | 1/27/05 | |||||||
| 10.15† | Amendment No. 2 to Collaboration Agreement, dated September 17, 2003, between the Registrant and Biogen Idec MA Inc. | S-1/A | 333-121646 | 10.28 | 1/27/05 | |||||||
| 10.16† | Collaboration Agreement, dated August 25, 2004, between the Registrant and Biogen Idec, Inc. | S-1/A | 333-121646 | 10.29 | 4/29/05 | |||||||
| 10.17† | License Agreement, dated October 14, 2003, by and between the Registrant and Dainippon Sumitomo Pharma Co., Ltd. (formerly known as Dainippon Pharmaceutical Co., Ltd.) | S-1/A | 333-121646 | 10.36 | 4/29/05 | |||||||
| 10.18 | Warrant, dated August 25, 2005, issued to Horizon Technology Funding Company II LLC | S-1/A | 333-121646 | 10.40 | 9/1/05 | |||||||
| 10.19 | Warrant, dated August 25, 2005, issued to Horizon Technology Funding Company III LLC | S-1/A | 333-121646 | 10.41 | 9/1/05 | |||||||
| 10.20 | Warrant, dated August 25, 2005, issued to Oxford Finance Corporation | S-1/A | 333-121646 | 10.42 | 9/1/05 | |||||||
| 10.21* | Amended and Restated 2006 Employment Commencement Incentive Plan | 10-K/A | 000-51531 | 10.32 | 4/30/09 | |||||||
| 10.22 | Common Stock and Warrant Purchase Agreement, dated as of March 17, 2006, among the Registrant and the investors listed on the signature pages thereto | 8-K | 000-51531 | 10.44 | 3/22/06 | |||||||
| 10.23 | Registration Rights Agreement, dated as of March 17, 2006, among the Registrant and the investors listed on the signature pages thereto | 8-K | 000-51531 | 10.45 | 3/22/06 | |||||||
| 10.24 | Form of Warrant | 8-K | 000-51531 | 10.46 | 3/22/06 | |||||||
| 10.25† | Sublease, dated December 22, 2006, by and between the Registrant and Oncology Therapeutics Network Joint Venture, L.P., for office space located at 395 Oyster Point Boulevard, South San Francisco, California | 10-K | 000-51531 | 10.47 | 3/17/08 | |||||||
| 10.26* | Consulting Agreement, dated August 17, 2006, by and between the Registrant and Homer L. Pearce, Ph. D. | 10-Q | 000-51531 | 10.49 | 5/9/07 | |||||||
| 10.27* | Consulting Agreement, dated September 2, 2006, by and between the Registrant and David C. Stump, M. D. | 10-Q | 000-51531 | 10.50 | 5/9/07 | |||||||
| 10.28* | Forms of Stock Option Grant Notice and Stock Option Agreement under the 2005 Equity Incentive Award Plan | 8-K | 000-51531 | 10.52 | 9/19/07 | |||||||
| 10.29* | Amended and Restated Executive Severance Benefits Agreement, dated December 23, 2008, by and between the Registrant and Steven B. Ketchum, Ph.D. | 10-K | 000-51531 | 10.43 | 4/3/09 | |||||||
84
Table of Contents
| Incorporated By Reference | ||||||||||||
| Exhibit |
Exhibit Description |
Form | File No. | Exhibit | Filing Date |
Filed Herewith | ||||||
| 10.30* | Second Amended and Restated Executive Severance Benefits Agreement, dated December 24, 2008, by and between Registrant and Daniel N. Swisher, Jr. | 10-K | 000-51531 | 10.44 | 4/3/09 | |||||||
| 10.31* | Second Amended and Restated Executive Severance Benefits Agreement, dated December 24, 2008, by and between Registrant and Eric H. Bjerkholt | 10-K | 000-51531 | 10.45 | 4/3/09 | |||||||
| 10.32* | Second Amended and Restated Executive Severance Benefits Agreement, dated December 23, 2008, by and between Registrant and James W. Young, Ph.D. | 10-K | 000-51531 | 10.46 | 4/3/09 | |||||||
| 10.33* | Second Amended and Restated Executive Severance Benefits Agreement, dated December 24, 2008, by and between Registrant and Valerie L. Pierce | 10-K | 000-51531 | 10.47 | 4/3/09 | |||||||
| 10.34* | Forms of Stock Option Grant Notice and Stock Option Agreement for Automatic Grants to Outside Directors under the 2005 Equity Incentive Award Plan | 10-Q | 000-51531 | 10.69 | 11/7/08 | |||||||
| 10.35 | Reserved |
|||||||||||
| 10.36 | Forms of Stock Option Grant Notice and Stock Option Agreement under the Amended and Restated 2006 Employment Commencement Incentive Plan | 8-K | 000-51531 | 10.71 | 12/23/08 | |||||||
| 10.37 | Intellectual Property Assignment and License Termination Agreement by and between the Registrant and SARcode Corporation, dated March 6, 2009 | 8-K | 000-51531 | 10.72 | 3/10/09 | |||||||
| 10.38 | Form of Amended and Restated Convertible Secured Promissory Notes issued by SARcode Corporation to the Registrant, dated March 6, 2009 | 8-K | 000-51531 | 10.73 | 3/10/09 | |||||||
| 10.39 | Summary of Non-Employee Director Cash Compensation Arrangements | 10-K | 000-51531 | 10.59 | 4/3/09 | |||||||
| 10.40 | Intellectual Property Assignment and License Agreement, dated March 6, 2009, by and between the Company and SARcode Corporation, and related Exhibit 3.2 | 8-K | 000-51531 | 10.72, 10.73 |
3/10/09 | |||||||
| 10.41 | Securities Purchase Agreement, dated March 31, 2009, by and among the Registrant and the purchasers identified on the signature pages thereto | 8-K | 000-51531 | 10.1 | 4/3/09 | |||||||
| 10.42 | Form of Warrant to purchase shares of Common Stock | 8-K | 000-51531 | 10.2 | 4/3/09 | |||||||
| 10.43* | Sunesis Pharmaceuticals, Inc. Change of Control Payment Plan | 10-Q | 000-51531 | 10.62 | 7/28/09 | |||||||
| 10.44* | Sunesis Pharmaceuticals, Inc. 2009 Bonus Program | 10-Q | 000-51531 | 10.63 | 7/28/09 | |||||||
85
Table of Contents
| Incorporated By Reference | ||||||||||||
| Exhibit |
Exhibit Description |
Form | File No. | Exhibit | Filing Date |
Filed Herewith | ||||||
| 10.45 | Agreement Regarding Private Placement of Securities of Sunesis Pharmaceuticals, Inc., dated as of June 29, 2009, by and among the Registrant and the investors identified on the signature pages thereto | 8-K | 000-51531 | 10.1 | 7/2/09 | |||||||
| 10.46* | Medical benefits arrangement with James W. Young, Ph.D. | 10-Q | 000-51531 | 10.65 | 7/28/09 | |||||||
| 10.47 | Second Agreement Regarding Private Placement of Securities of Sunesis Pharmaceuticals, Inc., dated as of October 27, 2009, by and among the Registrant and the investors identified on the signature pages thereto | 8-K | 000-51531 | 10.66 | 11/2/09 | |||||||
| 10.48 | Third Agreement Regarding Private Placement of Securities of Sunesis Pharmaceuticals, Inc., dated as of January 19, 2010, by and among the Registrant and the investors identified on the signature pages thereto | 8-K | 000-57531 | 10.67 | 1/21/10 | |||||||
| 21.1 | Subsidiaries of the Registrant | 10-K | 000-51531 | 21.1 | 3/17/08 | |||||||
| 23.1 | Consent of Independent Registered Public Accounting Firm | X | ||||||||||
| 31.1 | Certification of Chief Executive Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Exchange Act | X | ||||||||||
| 31.2 | Certification of Chief Financial Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Exchange Act | X | ||||||||||
| 32.1# | Certification of Chief Executive Officer and Chief Financial Officer pursuant to 13a-14(b) or 15d-14(b) of the Exchange Act | X | ||||||||||
| * | Management contract, compensatory plan or arrangement. |
| † | Portions of the exhibit have been omitted pursuant to a request for confidential treatment. The omitted information has been filed separately with the Securities and Exchange Commission. |
| # | In accordance with Item 601(b)(32)(ii) of Regulation S-K and SEC Release Nos. 33-8238 and 34-47986, Final Rule; Management’s Reports on Internal Control over Financial Reporting and Certification of Disclosure in Exchange Act Periodic Reports, the Certification furnished in Exhibit 32.1 hereto is deemed to accompany this Form 10-K and will not be filed for purposes of Section 18 of the Exchange Act. Such certification will not be deemed incorporated by reference into any filing under the Securities Act or the Exchange Act, except to the extent that the registrant specifically incorporates it by reference. |
86
