Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - ALBIREO PHARMA, INC. | tm2030337d4_ex99-1.htm |
| 8-K - FORM 8-K - ALBIREO PHARMA, INC. | tm2030337-4_8k.htm |
Exhibit 99.2
PROSPECTUS SUPPLEMENT SUMMARY
Overview
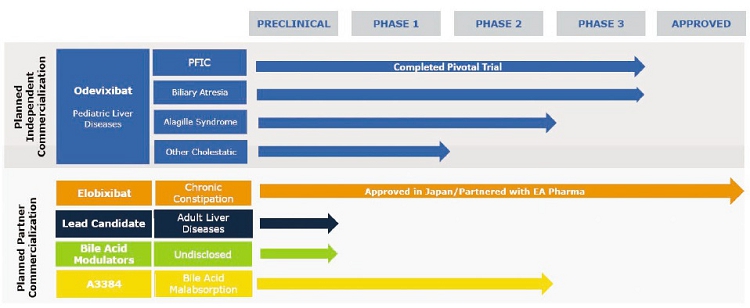
We are a biopharmaceutical company focused on the development and commercialization of novel bile acid modulators to treat orphan pediatric liver diseases and other liver or gastrointestinal diseases (GI) and disorders. We are pursuing the development of our lead product candidate, odevixibat (formerly known as A4250), for patients with progressive familial intrahepatic cholestasis, or PFIC, a rare, life-threatening genetic disorder affecting young children for which there is currently no approved drug treatment. In September 2020, we announced topline results from our Phase 3 trial in PFIC, and we intend to complete regulatory submissions in Europe and the United States no later than early 2021, in anticipation of potential regulatory approval, issuance of a rare pediatric disease priority review voucher and commercial launch in the second half of 2021, if approved. We are also pursuing the development of odevixibat in biliary atresia and in Alagille syndrome, or ALGS, each of which is a rare, life threatening disease that affects the liver and for which there is no approved pharmacologic treatment option. We initiated a pivotal clinical trial of odevixibat in biliary atresia, the BOLD trial, in the first half of 2020, and have enrolled the first patients in the trial. We plan to initiate a pivotal trial in ALGS by the end of 2020. Our most advanced product candidate in addition to odevixibat is elobixibat, which is approved in Japan for the treatment of chronic constipation. In August 2020, we announced topline results from our Phase 2 clinical trial as a treatment for nonalcoholic fatty liver disease, or NAFLD, and nonalcoholic steatohepatitis, or NASH, and based on the results of the trial, we decided not to pursue further development of elobixibat in NAFLD or NASH. We are exploring additional clinical development of our product candidate A3384 based on an evaluation of its patent coverage and our overall portfolio. We also have a preclinical program in adult liver disease, and expect to complete investigational new drug enabling studies in a lead preclinical candidate this year.
Odevixibat—Our Lead Product Candidate in PFIC. In September 2020, we announced topline results from PEDFIC 1, our Phase 3 clinical trial for odevixibat, given once per day as an oral capsule or sprinkled over food, in patients ages 6 months to 18 years with PFIC types 1 and 2, which was conducted at 45 global sites. PEDFIC 1 tested two doses of odevixibat, 40 µg/kg/day and 120 µg/kg/day, along with placebo, over a treatment period of 24 weeks. PEDFIC 1 met its two primary endpoints, demonstrating that odevixibat reduced serum bile acid responses, or sBAs, (p=0.003) and improved pruritus assessments (p=0.004) with a single digit diarrhea rate. In the primary analysis, PEDFIC 1 met the U.S. regulatory primary endpoint with the proportion of positive pruritus assessments being 53.5% in the odevixibat arms compared to 28.7% in the placebo arm (p=0.004). As a secondary endpoint, 42.9% of patients in the odevixibat arms had a clinically meaningful improvement in the pruritus score, defined as a drop from baseline of 1.0 point or more on the 0-4 point scale, at week 24 compared to 10.5% in the placebo arm (p=0.018). PEDFIC 1 also met the EU regulatory primary endpoint with 33.3% of subjects in the odevixibat arms experiencing either a 70% reduction in sBAs or reaching a level of 70 μmol/L compared to no patients in the placebo arm (p=0.003). As an EU regulatory secondary endpoint, mean reduction of bile acids was 114.3 µmol/L in the odevixibat arms compared to an increase of 13.1 µmol/L in the placebo arm (p=0.002). Both doses of odevixibat were statistically significant for each of the U.S. and EU primary endpoints. Odevixibat was well tolerated, with an overall adverse event incidence similar to placebo. There were no drug-related serious adverse events, or SAEs, reported during the study. Diarrhea/frequent bowel movements were the most common treatment-related gastrointestinal adverse events, which occurred in 9.5% of odevixibat treated patients vs. 5.0% of placebo patients. In June 2018, the FDA granted a rare pediatric disease designation to odevixibat for the treatment of PFIC, which affirms our eligibility to apply for a rare pediatric disease priority review voucher upon submission of a new drug application for odevixibat. In September 2018, the FDA granted fast track designation to odevixibat for the treatment of pruritus associated with PFIC. In the first quarter of 2019, we revised our statistical analysis methodology for PEDFIC 1, in line with guidance from the U.S. Food and Drug Administration, or FDA, which resulted in an improvement in the power of the study. We also submitted a protocol amendment for PEDFIC 2, our long term, open label extension study, which includes an additional cohort of PFIC patients who are not eligible for PEDFIC 1. The first sites have been activated and first patients enrolled in the expanded PEDFIC 2 cohort. In July 2020, we initiated an Expanded Access Program (EAP) for odevixibat in the United States, Canada, Australia and Europe.
| 1 |
We hold global rights to odevixibat unencumbered. Our current plan is to commercialize odevixibat ourselves in the United States and Europe, and we have begun the process of identifying potential partners for other regions. There are currently no drugs approved for the treatment of PFIC. First-line treatment for PFIC is typically off-label ursodeoxycholic acid, or UDCA, which is approved in the United States and elsewhere for the treatment of primary biliary cholangitis, or PBC. However, many PFIC patients do not respond well to UDCA, undergo partial external bile diversion, or PEBD, surgery and often require liver transplantation. PEBD surgery is a life-altering and undesirable procedure in which bile is drained outside the body to a stoma bag that must be worn by the patient 24 hours a day.
Other Indications Under Development for Odevixibat. We are also pursuing the development of odevixibat in patients with biliary atresia, another rare, life-threatening disease that affects the liver and for which there is no approved pharmacologic treatment option. In December 2018, the European Commission granted orphan designation to odevixibat for the treatment of biliary atresia, and in January 2019, the FDA granted orphan drug designation to odevixibat for the treatment of biliary atresia. We initiated the BOLD clinical trial, a global pivotal trial and the largest prospective intervention trial ever conducted in biliary atresia, in the first half of 2020. The first patients have been enrolled in the trial, and we plan for full site activation in the first half of 2021, subject to any potential impacts of COVID-19 on the enrollment. We believe biliary atresia is one of the most common rare pediatric liver diseases, and is the leading cause of liver transplants in children. Our double-blind, placebo controlled pivotal trial in biliary atresia is designed to enroll approximately 200 patients at 70 sites globally. Patients will receive either placebo or high-dose (120µg/kg) odevixibat once daily. The primary endpoint is survival with native liver after two years of treatment.
Biliary atresia is a partial or total blocking or absence of large bile ducts that causes cholestasis and resulting accumulation of bile that damages the liver. There are currently no drugs approved for the treatment of biliary atresia. The current standard of care is a surgery known as the Kasai procedure, or hepatoportoenterostomy, in which the obstructed bile ducts are removed and a section of the small intestine is connected to the liver directly. However, only an estimated 25% of those initially undergoing the Kasai procedure will survive to their twenties without need for liver transplantation.
| 2 |
In addition, we have agreed with the FDA and European Commission on a single pivotal study design for odevixibat in ALGS, and we plan to initiate the trial by the end of 2020. We expect topline data to be available before the announcement of the topline results from the BOLD clinical trial. ALGS is a genetic condition associated with liver, heart, eye, kidney and skeletal abnormalities. In particular, ALGS patients have fewer than normal bile ducts inside the liver, which leads to cholestasis and the accumulation of bile and causes scarring in the liver. There are currently no drugs approved for the treatment of ALGS. Current treatment for ALGS is generally in line with current treatments for PFIC as described above. In August 2012, the European Commission granted orphan designation to odevixibat for the treatment of ALGS. In October 2018, the FDA granted orphan drug designation to odevixibat for the treatment of ALGS.
We continue to evaluate potential clinical development in other indications, including primary sclerosing cholangitis, which refers to swelling (inflammation), scarring, and destruction of bile ducts inside and outside of the liver. The first symptoms are typically fatigue, itching and jaundice, and many patients with sclerosing cholangitis also suffer from inflammatory bowel disease. There are currently no drugs approved for the treatment of sclerosing cholangitis. First-line treatment is typically off-label UDCA, although UDCA has not been established to be safe and effective in patients with sclerosing cholangitis in well controlled clinical trials.
Our Pipeline
The following table summarizes our most advanced product candidates and programs:
Our Strategy
Our goal is to be a leader in the development and commercialization of novel therapeutics for orphan pediatric cholestatic liver diseases and disorders where there is high unmet medical need, while also leveraging our expertise in bile acid modulation to treat other liver and GI diseases and disorders. To achieve our goal, we intend to pursue the following strategies.
| • | Rapidly develop odevixibat to regulatory approval as a treatment for patients with PFIC. We recently announced topline results from our Phase 3 clinical trial in patients with PFIC, which we refer to as PEDFIC 1. It is our objective that PEDFIC 1, together with available data from PEDFIC 2, our long-term, open label extension study, forms the primary support for applications for marketing approval of odevixibat in both the United States and Europe. |
| 3 |
| • | Maximize the benefit and commercial potential of odevixibat by expanding development to additional orphan pediatric cholestatic indications. Although we have chosen PFIC as our lead program for odevixibat, we also believe odevixibat can benefit children suffering from other cholestatic diseases and disorders. We initiated a pivotal clinical trial with odevixibat for the treatment of biliary atresia, the BOLD trial, in the first half of 2020, and the first patients have been enrolled in the trial. We estimate biliary atresia impacts an aggregate of 15,000 to 20,000 patients in the United States and Europe, and is the cause of over 50% of liver transplants in children. We also plan to initiate a pivotal trial for odevixibat as a treatment for ALGS by the end of 2020. |
| • | Develop the capability to commercialize odevixibat to treat orphan pediatric liver diseases, if approved, through a targeted sales force in the United States and Europe and collaborate selectively to commercialize odevixibat outside of these regions. If we receive marketing approval in the United States or Europe for odevixibat as a treatment for patients with PFIC or any other pediatric cholestatic liver disease or disorder, we plan to build the capabilities to effectively commercialize odevixibat in the approved indication(s) in the applicable region. We believe that the required commercial organization would be modest in size and targeted to the relatively small number of specialists in the United States and Europe who treat children with cholestatic liver disease. If we receive marketing approval outside of the United States and Europe for odevixibat as a treatment for patients with PFIC or any other pediatric cholestatic liver disease or disorder, we plan to selectively utilize collaboration, distribution and other marketing arrangements with third parties to commercialize odevixibat in the approved indication(s) in the regions or markets where we receive approval. |
| • | Collaborate selectively to develop and commercialize product candidates targeting nonorphan indications, potentially including A3384 or any future product candidate to treat adult liver diseases. We intend to selectively seek alliances and collaborations to assist us in furthering the development or commercialization of product candidates targeting large primary care markets that must be served by large sales and marketing organizations. These product candidates may include A3384 and any potential future product candidate that arises from our preclinical program in adult liver diseases. |
| 4 |
