Attached files
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2019
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file number: 000-55195
GI DYNAMICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 84-1621425 | |
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification Number) | |
| 320 Congress Street, 3rd Floor | ||
| Boston, Massachusetts | 02210 | |
| (Address of Principal Executive Offices) | (Zip Code) | |
(781) 357-3300
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Exchange Act: None
Securities registered pursuant to Section 12(g) of the Exchange Act: Common Stock, $0.01 par value per share
| Title of each class | Trading symbol(s) | Name
of each non-U.S. exchange on which registered | ||
| Common Stock, par value $0.01 | GID.ASX | Australia Securities Exchange |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ☐ No ☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days: Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files): Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Accelerated filer ☐ | |
| Large accelerated filer ☐ | Smaller reporting company ☒ |
| Non-accelerated filer ☒ | Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act): ☐ Yes ☒ No
The aggregate market value of the registrant’s common stock, in the form of CHESS Depositary Interests (“CDI” in singular, “CDIs” in plural, each CDI representing 1/50th of one share of common stock), held by non-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate), computed by reference to the price at which the CDIs were last sold on June 28 2019, the last business day of the registrant’s most recently completed second quarter, as reported on the Australian Securities Exchange, was $19,603,047 (A$27,952,442).
The registrant’s common stock is publicly traded on the ASX in the form of CDIs convertible at the option of the holders into shares of the registrant’s common stock on a 1-for-50 basis. The total number of shares of the registrant’s common stock outstanding on March 12, 2020, including shares of common stock underlying CDIs, was 36,598,291.
DOCUMENTS INCORPORATED BY REFERENCE
The registrant intends to file with the Securities and Exchange Commission a proxy statement pursuant to Regulation 14A or an amendment to this report filed under cover of Form 10-K/A containing the information required to be disclosed under Part III of Form 10-K within 120 days of the end of its fiscal year ended December 31, 2019. Portions of such proxy statement or Form 10-K/A are incorporated by reference into Part III of this Annual Report on Form 10-K.
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements as defined in Section 27A of the United States Securities Act of 1933, as amended, and the Securities Exchange Act of 1934, as amended. Throughout this Annual Report on Form 10-K, all references to the “Company” or “GI Dynamics,” unless where the context requires otherwise, refers to the consolidated entity of GI Dynamics, Inc. These forward-looking statements concern the Company’s business, operations, financial performance and condition as well as plans, objectives and expectations for the Company’s business, operations and financial performance and condition. Any statements contained in this Annual Report on Form 10-K that are not of historical facts may be deemed to be forward-looking statements. The forward-looking statements are contained principally in the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Forward-looking statements include, but are not limited to, statements about the Company’s:
| ● | expectations with respect to the Company’s intellectual property position; |
| ● | expectations with respect to clinical trials for EndoBarrier®; |
| ● | expectations with respect to regulatory submissions and receipt and maintenance of regulatory approvals; |
| ● | ability to commercialize products; |
| ● | ability to develop and commercialize new products; |
| ● | expectation with regard to product manufacture and inventory; and |
| ● | estimates regarding capital requirements and need for additional financing. |
In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “aims,” “assumes,” “goal,” “intends,” “objective,” “potential,” “positioned,” “target,” “continue,” “seek,” “vision,” or the negative thereof and similar expressions intended to identify forward-looking statements.
These forward-looking statements are based on current expectations, estimates, forecasts and projections about the Company’s business and the industry in which it operates and management’s beliefs and assumptions. These forward-looking statements are not guarantees of future performance or development and involve known and unknown risks, uncertainties and other factors that are in some cases beyond the Company’s control. As a result, any or all forward-looking statements in this Annual Report on Form 10-K may later become inaccurate. The Company may not actually achieve the plans, intentions or expectations disclosed in any forward-looking statements, and actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements made by the Company. The Company has included important factors in the cautionary statements included in this Annual Report on Form 10-K, particularly in the “Risk Factors” section, that could cause actual results or events to differ materially from the forward-looking statements made.
You are urged to consider these factors carefully in evaluating the forward-looking statements and are cautioned not to place undue reliance on the forward-looking statements. You should read this Annual Report on Form 10-K and the documents that the Company has filed as exhibits to the GI Dynamics Form 10-K completely and with the understanding that actual future results may be materially different from what the Company expects. These forward-looking statements speak only as at the date of this Annual Report on Form 10-K. Unless required by law, the Company does not intend to publicly update or revise any forward-looking statements to reflect new information or future events or otherwise. You should, however, review the factors and risks described in the reports the Company will file from time to time with the SEC after the date of this Annual Report on Form 10-K.
i
GI DYNAMICS, INC.
ANNUAL REPORT ON FORM 10-K
FOR THE YEAR ENDED DECEMBER 31, 2019
TABLE OF CONTENTS
ii
Overview
GI Dynamics, Inc. (“GI Dynamics” or “the Company”) is a clinical stage medical device company focused on the development and commercialization of EndoBarrier, a medical device system intended for treatment of patients with type 2 diabetes and reduction of obesity. EndoBarrier is a medical implant designated for the treatment of type 2 diabetes and the reduction of obesity with nearly 4,000 implants since inception and is the subject of an approved FDA pivotal trial in the United States under which the Company initiated patient implant procedures in January 2020. The Company believes that EndoBarrier represents a paradigm-breaking approach to traditional management of type 2 diabetes, which has focused historically solely on lifestyle intervention and diabetes medications. According to the article “Challenges in Diabetes Management: Glycemic Control, Medication Adherence, and Healthcare Costs,” published in the American Journal of Managed Care in 2017, these historical treatments for type 2 diabetes are limited in efficacy, with approximately 40% of patients achieving glycemic control.
EndoBarrier represents the first material departure from this historical approach. The Company believes that EndoBarrier offers an adjunct to non-insulin diabetes pharmacotherapy, while providing patients a chance to significantly reduce or eliminate insulin and increase the likelihood of avoiding invasive and permanent bariatric or metabolic surgery. EndoBarrier is designed to mimic the mechanism of action of the duodenal-jejunal exclusion currently created by gastric bypass surgery (which is referred to as Roux-en-Y Gastric Bypass, or RYGB), as explained in the article “EndoBarrier: A Safe and Effective Novel Treatment for Obesity and Type 2 Diabetes?”, published in Obesity Surgery in 2018 by Nisha Patel. After implanting EndoBarrier, the patient’s food that exits the stomach will flow through the inside of the EndoBarrier Liner, and therefore will not contact the wall of the upper intestine (duodenum and jejunum). The food will also not mix with fluids from the pancreas and bile duct until these fluids and the food reach the far end of the EndoBarrier Liner. It is believed that, in a similar fashion to RYGB, shunting the food to a more distant location in the upper intestines triggers hormonal responses that regulate key factors that help control hunger, satiety, and insulin sensitivity, which is also explained in the article by Nisha Patel.
EndoBarrier has been shown in multiple independent clinical studies to lower blood sugar levels (as measured by hemoglobin A1c or HbA1c), reduce excess body weight, increase insulin sensitivity, and positively affect other health metrics and comorbidities. Because it has been utilized in approximately 4,000 procedures, EndoBarrier has a thoroughly characterized benefit-risk profile. A recent independent meta-analysis published in 2018 by Pichamol Jirapinyo in American Diabetes Association (ADA) Diabetes Care, “Effects of the Duodenal-Jejunal Bypass Liner on Glycemic Control in Patients with Type 2 Diabetes with Obesity: A Meta-Analysis with Secondary Analysis on Weight Loss and Hormonal Changes,” provides the most comprehensive review and meta-analysis of EndoBarrier clinical data to date. In addition, two ongoing EndoBarrier registries in the UK and Germany have captured and reported clinical results on more than 800 patients between the two databases. These registries as well as other investigator-initiated trials continue to release clinical data on an annual basis, largely centered around the annual meetings hosted by DDW (Digestive Disease Week) in May, ADA (American Diabetes Association) in June, EASD (European Association for the Study of Diabetes) in October, and Obesity Week in November.
Business Strategy
The Company’s goal is to become the leading provider of alternative options for treating type 2 diabetes and concurrently reducing obesity. The Company’s corporate priorities include:
| 1) | US Clinical Operations: Conduct Stage 1 of the STEP-1 EndoBarrier pivotal trial in the United States. |
The Company is focused on the successful execution of the STEP-1 pivotal trial in the United States. In August 2018, GI Dynamics received notification from the FDA that it had approved the Investigational Device Exemption (“IDE”) application for EndoBarrier, pending Institutional Review Board (IRB) approval, which was received in February 2019. The approved EndoBarrier IDE represents the pivotal clinical trial for EndoBarrier.
The STEP-1 clinical trial is a randomized, controlled, double blinded trial with 3:1 randomization between patients who receive the EndoBarrier implant (3) and the control group made up of patients who will receive a sham procedure and not receive the EndoBarrier implant (1). Both arms will maintain pharmacotherapy within ADA guidelines and will receive lifestyle and nutritional counseling consistent with ADA guidelines. The primary endpoint of the trial is reduction of HbA1c from baseline to removal of the EndoBarrier at twelve months as compared to the control group. The study includes numerous secondary endpoints, including changes in weight, cardiovascular risk metrics, as well as health metrics related to NAFLD (non-alcoholic fatty liver disease) and NASH (non-alcoholic steatohepatitis), and CKD (chronic kidney disease).
1
The STEP-1 clinical trial consists of two stages. The first stage consists of 50 EndoBarrier patients and approximately 17 control patients. At the end of stage 1, the Company will submit four DMC (Data Monitoring Committee) reports to the FDA for review. Upon a successful completion of the stage 1 review, the Company will submit a request to the FDA to complete the study by conducting stage 2, which the Company anticipates will be comprised of 130 EndoBarrier patients and approximately 43 control patients for a study total of 240 patients (180 EndoBarrier and 60 control patients). There is no guarantee that this will be the final composition of the study, as the number of remaining stages or remaining patients may vary based on the stage 1 clinical data and the outcome of the stage 1 review with FDA. In addition to the multiple staged study, the FDA has mandated certain stopping rules which, if triggered, could result in the STEP-1 study having enrollment or treatment delayed or stopped.
On January 27, 2020, the first patient was randomized into the STEP-1Trial Protocol. The Company is focused on continuous recruitment and patient management of patients within the STEP-1 trial.
If the Company does not have adequate funds to ensure the complete treatment course of all patients to be enrolled in the STEP-1 clinical trial, the Company reserves funds at the time of implant, on a per implanted patient basis, to ensure the Company has adequate cash available to ensure patient safety through the completion of the patient care and patient safety monitoring course as detailed in the protocol. On the completion of sufficient financing to ensure funds will be available to ensure completion of the STEP-1 trial protocol for any patients implanted as part of the Step-1 study, these individual patient reserves will be returned to general operating funds. The Company expects to complete the enrollment of Stage 1 by the end of 2020.
| 2) | India Partnership: Conduct the I-STEP EndoBarrier pivotal trial in India as part of the Apollo Sugar partnership. |
GI Dynamics is focused on the successful execution of the EndoBarrier trial in India as part of its partnership with Apollo Sugar, which was announced on November 27, 2018.
The I-STEP study is also a randomized, controlled, double blinded trial with 3:1 randomization between patients who receive the EndoBarrier implant (3) and the control group made up of patients who will not receive the EndoBarrier implant (1). The primary endpoint of the trial is reduction of HbA1c from baseline to removal of the EndoBarrier at twelve months as compared to the control group. The study includes numerous secondary endpoints, including changes in weight and reduction in cardiovascular risk metrics. The study includes 100 patients in total, which consists of 75 patients who will receive the EndoBarrier implant and 25 control patients. Up to five clinical sites in India will participate in the study.
Apollo Sugar is a collaboration between Apollo Health & Lifestyle Limited and Sanofi. Apollo Sugar is a division of Apollo Hospitals Group (Apollo) focused on the treatment of metabolic disorders and operates an integrated network of centers of excellence for diabetes, obesity and endocrinology. Apollo is the largest private hospital system in India and has emerged as Asia’s foremost integrated healthcare services provider, maintaining a robust presence of hospitals, pharmacies, primary care and diagnostic clinics across the healthcare ecosystem. Since its inception, Apollo has treated over 65 million patients from 141 countries.
Management anticipates that the Company and Apollo Sugar will file a pre-trial application with the Central Drugs Standards Control Organization (CDSCO) in India to obtain approval to initiate the I-STEP trial. The Company expects to receive approval and initiate the trial prior to the end of the third quarter of 2020. The partners anticipate enrollment will take less than 12 months.
GI Dynamics and Apollo Sugar leadership are working to finalize the terms of the GI Dynamics-Apollo Sugar partnership that will focus on the marketing, distribution and clinical support of EndoBarrier to appropriate patients in India, Southeast Asia and the Middle East. Final terms of the proposed collaboration are subject to negotiation and will be disclosed upon completion.
| 3) | Gain CE Mark: Work with new Notified Body to gain EndoBarrier CE mark. |
CE Marking of a product is a manufacturer’s declaration that a product meets the applicable health, safety, and environmental requirements outlined in the appropriate European product legislation and has undergone the relevant conformity assessment procedure. A CE Mark is required before the Company can market EndoBarrier in the EU and certain Middle Eastern countries.
2
GI Dynamics previously announced that it was working with Intertek, a Notified Body headquartered in London, UK. GI Dynamics has ended this relationship and is working with a different Notified Body. A notified body evaluates the conformity of products and the associated quality systems for manufacturers that seek to sell products in Europe. The Company’s new Notified Body has initiated the process of working through EndoBarrier clinical data and the Company’s Quality Management System. The Company will release the name of the new Notified Body when the final agreement between GI Dynamics and the new Notified Body has been completed. Management believes that the attainment of CE Mark is achievable by the end of 2020.
| 4) | Ongoing clinical data: Support continued release of clinical data from registries and investigator-initiated clinical trials. |
GI Dynamics continues to support the ongoing efforts of multiple clinicians as they develop new EndoBarrier clinical data. Multiple investigator-initiated clinical trials released clinical data in 2019. It is expected that these clinical trials will continue to release data into 2020 and beyond.
GI Dynamics also supports two primary registries that continue to release clinical data. The Association of British Clinical Diabetologists (ABCD) Worldwide EndoBarrier Registry contains over 500 patient data points with the goal of analyzing and releasing clinical data for more than 1,000 patients. The German EndoBarrier Registry contains over 300 patient data points. The patients within these two registries are non-overlapping. Both registries are expected to continue releasing clinical data highlighting the EndoBarrier treatment effect on an annual basis.
The release of clinical data has historically focused and is expected to continue focusing on the annual clinical data releases at the following scientific meetings: Digestive Disease Week (DDW) in May, American Diabetes Association (ADA) in June, European Association for the Study of Diabetes (EASD) in October, and Obesity Week in November.
| 5) | Development of Intellectual Property: Continue developing the Company’s intellectual property and protecting its patents. |
GI Dynamics will continue to invest in expanding its intellectual property portfolio. The Company’s current patent portfolio is composed of 84 issued and pending U.S. and non-U.S. patents. The Company has 33 granted U.S. patents and 15 pending U.S. patent applications. The Company has also sought intellectual property protection outside the U.S. and has 8 issued patents in Germany and the United Kingdom and 28 pending patent applications across China, the European Patent Convention region, Hong Kong, Israel and India.
The Company’s current issued patents expire between 2023 and 2035. GI Dynamics also actively monitors its intellectual property by regularly reviewing new developments to identify extensions to the patent portfolio. The Company employs external patent attorneys to assist in managing the Company’s intellectual property portfolio.
Summary
In summary, GI Dynamics believes that EndoBarrier represents the most effective new treatment option in a market dominated by pharmaceutical companies generating annual revenue in excess $40 billion. The Company believes that EndoBarrier is poised to have a transformative and disruptive effect on the type 2 diabetes market. EndoBarrier is one of the few treatment options that treats type 2 diabetes, concurrently reduces obesity, and continues to show lasting treatment effects post treatment in many patients. As the duration of the EndoBarrier treatment effect continues to increase in published literature and because, to date, there has been minimal observed clinical risk after EndoBarrier removal, the EndoBarrier risk-benefit balance continues to evolve in an increasingly positive manner.
Background of the Disease
Diabetes mellitus type 2 (also known as type 2 diabetes) is a long-term progressive metabolic disorder characterized by high blood sugar, insulin resistance, and reduced insulin production. People with type 2 diabetes represent 90-95% of the worldwide diabetes population; only 5-10% of this population is diagnosed with type 1 diabetes (a form of diabetes mellitus wherein little to no insulin is produced).
Being overweight is a condition where the patient’s body mass index (BMI) is greater than 25 (kg/m2); obesity is a condition where the patient’s BMI is greater than 30, with some countries applying a lower metric to both definitions. Obesity and its comorbidities contribute to the progression of type 2 diabetes. Many experts believe obesity contributes to higher levels of insulin resistance, which creates a feedback loop that increases the severity of type 2 diabetes for many patients.
3
When considering treatment for type 2 diabetes, it is optimal to address obesity concurrently with diabetes. According to the article “Mechanisms of Insulin Resistance in Obesity,” published in Frontiers of Medicine in 2013 by Jianping Ye, absent the concurrent reduction of obesity with the treatment of type 2 diabetes, obesity will likely continue to contribute to the progressive nature of type 2 diabetes. It is the Company’s belief that the inability of the current pharmacologic options to fully treat type 2 diabetes is due to the fact that diabetes medications generally treat blood sugar levels only and do not contribute substantially to weight loss.
Current Treatment Options
According to the American Diabetes Association 2018 Standards of Medical Care in Diabetes, the current treatment paradigm for type 2 diabetes is lifestyle therapy combined with pharmacological treatment, whereby treating clinicians prescribe a treatment regimen of one to four concurrent medications that could include insulin. Insulin usage carries a significant risk of increased mortality and may contribute to weight gain, which in turn may lead to higher levels of insulin resistance and increased levels of blood sugar, as explained in the article “Insulin-associated weight gain in diabetes--causes, effects and coping strategies,” by Russel-Jones, D. Only 40% of patients treated pharmacologically for type 2 diabetes are adequately managed, meaning that medication does not lower blood sugar adequately and does not halt the progressive nature of diabetes for these patients, as explained in the article “Adherence to Therapies in Patients with Type 2 Diabetes,” by Luis-Emilio Garcia-Perez.
The current pharmacological treatment algorithms for type 2 diabetes fall short of ideal, creating a large and unfilled efficacy gap. GI Dynamics believes that EndoBarrier, which is designed to mimic the mechanism of action of duodenal-jejunal exclusion currently created by RYGB gastric bypass surgery and operates in a relatively similar manner by reducing both weight and long-term blood sugar levels, can fill this gap by treating type 2 diabetes and reducing obesity in a unique minimally invasive and reversible manner.
Market Opportunity
Unmet Clinical Needs in the Treatment of Type 2 Diabetes and Obesity.
In 2019, the International Diabetes Federation estimated there were approximately 463 million people with type 2 diabetes worldwide. Diabetes is the leading cause of cardiovascular disease, kidney failure, blindness, and lower-limb amputation in almost all countries.
Three years after initial diagnosis, over half of patients with type 2 diabetes require multiple drug therapies. Studies have shown that only 40% of the type 2 diabetes population is adequately managed pharmacologically, as explained in the article “Challenges in Diabetes Management: Glycemic Control, Medication Adherence, and Healthcare Costs,” published in the American Journal of Managed Care in 2017. At ten years post diagnosis, most patients, despite insulin use in many, struggle to reach their hemoglobin A1c (HbA1c) treatment goals. HbA1c is a glycosylated hemoglobin molecule found in the bloodstream that is formed when red blood cells are exposed to blood glucose. HbA1c has become the generally accepted gold standard biomarker for measuring levels of diabetes control in clinical practice and in human trials.
Many patients and health care systems struggle to meet the financial burden imposed by the numerous concurrent medications required to attempt to control the progressive nature of type 2 diabetes.
According to the World Health Organization, in 2016 more than 1.9 billion adults 18 and older were overweight. Of these, 650 million people worldwide are diagnosed with obesity (BMI ³ > 30 kg/m 2), a condition often leading to serious health consequences such as cardiovascular disease, diabetes, musculoskeletal disorders, and some cancers.
Those suffering from both type 2 diabetes and obesity, also referred to as diabesity, total more than 169 million worldwide, represent a significant public health problem not only in the United States (US) but also globally, as explained in the article “Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications,” published in the Circulation Research in 2016 by Shilpa Bhupathiraju. GI Dynamics believes that the unchecked worldwide rate of growth of the type 2 diabetes and obesity patient population represents one of the greatest unmanaged health risks in all of health care.
4
GI Dynamics believes EndoBarrier can treat patients with type 2 diabetes and reduce obesity in a safe, effective, nonpharmacological, and nonpermanent manner.
The Efficacy Gap
The Company’s intent in developing and seeking regulatory approval for EndoBarrier is to help clinicians deliver a unique treatment option to those patients suffering from type 2 diabetes, a disease state that sorely lacks innovative and broadly effective new treatment options.
GI Dynamics and its scientific advisors feel there must be a change in how the medical establishment currently treats obese patients suffering from type 2 diabetes because current treatment options are not effective. According to the International Diabetes Federation, the number of obese patients progressing to later stages of type 2 diabetes continues to grow at an alarming rate. Yet less than half of all type 2 diabetes patients are adequately managed by pharmacotherapy, and insulin carries serious risks and may in many cases contribute to the further progression of obesity. At the extreme end of the treatment spectrum, the treatment options are limited to different types of bariatric or metabolic surgery, which are highly invasive and irreversible procedures. Less than 1% of patients who are eligible for bariatric or metabolic surgery opt to undergo the procedure, as explained in the article “Recent advances in clinical practice challenges and opportunities in the management of obesity,” published by Andres Acosta in 2015 in the Gut .
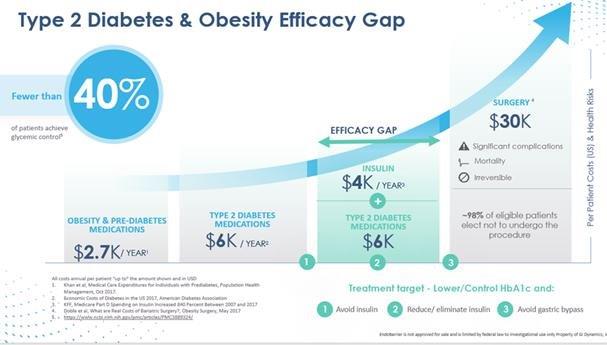
The graphic above illustrates the multiple treatment options during the course of progression of type 2 diabetes:
| ● | Risk associated with treatment increases from left to right. |
| ● | Progression of diabetes and obesity health risk and cost increase vertically. |
| ● | Lifestyle therapy (lifestyle counseling including nutrition and exercise) is the first line of defense against the progression of type 2 diabetes and obesity. |
| ● | Pharmacotherapy |
| o | Oral monotherapy follows lifestyle therapy, often with metformin as the first line of treatment. |
| o | Multiple combinations may then be administered, as recommended by the American Diabetes Association and other diabetes associations around the world. |
| o | Ultimately, as disease progression continues, injected insulin may be prescribed. |
| ● | Bariatric or metabolic gastric bypass surgery may represent a final option. |
5
A significant and rapidly growing patient population falls into the efficacy gap, wherein the patient is inadequately managed by medication yet unwilling to undergo gastric bypass surgery. For patients who elect to undergo bariatric or metabolic surgery, the clinical gains may not be permanent, while the risk associated with the procedure is permanent. These risks include, but are not limited to, excessive bleeding, blood clots, infection and in some cases death. GI Dynamics believes that because EndoBarrier is minimally invasive, can mimic the effects of gastric bypass, and has minimal side-effects, EndoBarrier can uniquely fill this efficacy gap, as explained in the article “EndoBarrier: A Safe and Effective Novel Treatment for Obesity and Type 2 Diabetes?” published in Obesity Surgery in 2018 by Nisha Patel.
The EndoBarrier Solution
EndoBarrier is intended for the treatment of type 2 diabetes and the reduction of obesity in a minimally invasive and reversible manner. EndoBarrier is designed to mimic the mechanism of action of duodenal-jejunal exclusion created by gastric bypass surgery. The EndoBarrier System is provided sterile and consists of an EndoBarrier Liner preloaded, packaged and sterilized within the EndoBarrier Delivery System. The EndoBarrier Delivery System is utilized to deliver the EndoBarrier Liner to the proximal small intestine. The EndoBarrier Liner is removed using the EndoBarrier Retrieval System.
The EndoBarrier System consists of three primary components:
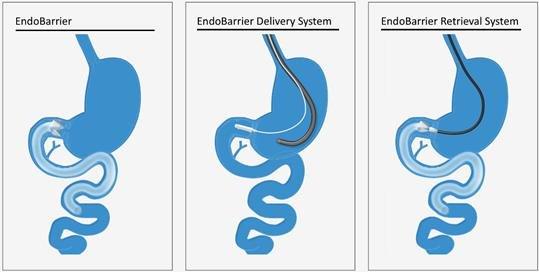
| ● | EndoBarrier — The EndoBarrier or EndoBarrier Liner is a 60-cm-long implant consisting of a thin, flexible, impermeable fluoropolymer liner coupled to a proprietary nitinol Anchor Mechanism. A gastroenterologist implants the EndoBarrier Liner into the patient’s duodenum in a minimally invasive manner using the EndoBarrier Delivery System and standard gastroscope. The EndoBarrier Liner is placed endoscopically (through the mouth and esophagus and into the stomach without cutting tissue). Once the EndoBarrier Liner is properly positioned in the patient’s duodenal bulb, the area within the duodenum just below the stomach, the EndoBarrier Delivery System is removed and the EndoBarrier Liner remains, held in place by a proprietary Anchor Mechanism. The EndoBarrier Liner remains in the body for a maximum intended duration of twelve months until removal, again via a minimally invasive endoscopic procedure using the EndoBarrier Retrieval System. |
6
| ● | EndoBarrier Delivery System — The EndoBarrier Liner is delivered using the Company’s proprietary sterile, single-use EndoBarrier Delivery System. This includes a custom-made Outer Catheter that is sufficiently flexible to allow for insertion through the patient’s mouth, through the stomach, and into the intestine without kinking. The EndoBarrier Liner is pre-assembled during manufacturing into a collapsed position inside the Capsule, which is located at the end of the Outer Catheter. The clinician uses the EndoBarrier Delivery System to advance the Capsule into the duodenum and deploy the EndoBarrier Liner from the Capsule. The delivery procedure is typically an outpatient procedure, during which the patient is under anesthesia or semi-sedation. |
| ● | EndoBarrier Removal System — The EndoBarrier Liner is removed at the end of the treatment period via a minimally invasive endoscopic procedure using the Company’s proprietary Retrieval System which is passed by the clinician through a standard gastroscope. The Hook of the EndoBarrier Removal System is used to pull either of two drawstrings located in the Anchor Mechanism. As the drawstring is pulled, the Anchor Mechanism is collapsed inward and disengages from the wall of the duodenum. The Hood, pre-placed on the end of a gastroscope, allows the EndoBarrier anchor assembly to be pulled and collapsed into the Hood with the Hood positioned to cover portions of the anchor. The EndoBarrier Liner is then safely removed through the patient’s stomach, esophagus, and mouth. The retrieval procedure is typically an outpatient procedure, during which the patient is under anesthesia or semi-sedation. |
EndoBarrier has been shown in multiple company-sponsored and independent clinical studies to lower blood sugar levels (hemoglobin A1c or HbA1c), reduce excess body weight, and positively affect other health metrics and comorbidities.
EndoBarrier is not currently approved or commercially available in any jurisdiction. In August 2018, GI Dynamics received notification from the FDA that it had approved its IDE application, pending IRB approval which was received in February 2019. To gain regulatory approval to commercialize EndoBarrier in the United States, the Company must submit a pre-market authorization (PMA) application for review and approval by the FDA. In January 2020, GI Dynamics initiated treatment of patients in the STEP-1 pivotal clinical trial, which is the registration trial for the PMA application.
The EndoBarrier Effect
Obesity has been shown to exacerbate insulin resistance and contribute to the progression of type 2 diabetes. In situations where lifestyle modification and pharmacotherapy have failed and surgery is not an option or is considered a therapy of last resort, EndoBarrier is intended to reduce blood sugar and weight. In clinical trials in both the United States and outside of the United States, EndoBarrier has been shown to:
| ● | significantly lower glucose levels; |
| ● | significantly lower body weight; |
| ● | lower cardiovascular-related risk factors. |
EndoBarrier utilization accomplishes this in many patients by affecting key hormones involved in insulin sensitivity, glucose metabolism, satiety, and food intake. (See the section titled “How EndoBarrier Works: EndoBarrier Mechanism of Action.”)
The Company’s intent in positioning EndoBarrier to fill the type 2 diabetes efficacy gap with concurrent reduction of obesity is to help patients and clinicians avoid the initiation of insulin therapy by helping many patients maintain lower HbA1c levels and slow or halt the progression of type 2 diabetes. Furthermore, for those patients whose type 2 diabetes has progressed to the point where insulin therapy is necessary, EndoBarrier has been shown in many cases to lower HbA1c levels to the point where insulin dosage can be reduced, or insulin is no longer needed. Finally, if the progression of type 2 diabetes is severe enough to warrant gastric bypass surgery, the Company expects that EndoBarrier either may serve as an opportunity to control type 2 diabetes so that surgery may not be needed, or at least better prepare the patient for bariatric surgery by lowering weight and helping control other comorbidities prior to surgery.
How EndoBarrier Works: EndoBarrier Mechanism of Action
The EndoBarrier mechanism of action is thought to be based on its functional similarities in many ways to RYGB. Once the EndoBarrier is implanted into the duodenum and proximal jejunum, ingested food passing through the EndoBarrier during the normal digestive process is prevented from interacting with the epithelium, microbiota, mucosal layer, or biliopancreatic secretions within the duodenum and proximal jejunum. In addition, EndoBarrier acts as a physical barrier that prevents the interaction of food with pancreatic enzymes and bile until reaching the end of the EndoBarrier Liner. Pancreatic enzymes and bile pass outside EndoBarrier and mix with the food at the distal end of the liner, where absorption ultimately takes place in the intestine. Thus, EndoBarrier creates a functional and reversible bypass of the upper intestine. Unlike RYGB surgery, EndoBarrier does not require an invasive and permanent surgical procedure or permanent physical modification of the stomach and exclusion of the distal stomach from the alimentary flow.
7
The Company’s scientific team and advisors have postulated the following EndoBarrier mechanisms of action, based on scientific evidence from such articles as “Effects of the Duodenal-Jejunal Bypass Liner on Glycemic Control in Patients with Type 2 Diabetes with Obesity: A Meta-Analysis with Secondary Analysis on Weight Loss and Hormonal Changes,” published in Diabetes Care in 2018 by Pichamol Jirapinyo and “The EndoBarrier: Duodenal-Jejunal Bypass Liner for Diabetes and Weight Loss,” published in Hindawi in 2018 by Aruchuna Ruban.:
Exclusion of the duodenum — This may offset an abnormality of gastrointestinal physiology responsible for insulin resistance and type 2 diabetes.
| ● | Increased nutrient delivery to the distal small bowel — Additional findings suggest that the exclusion of the proximal intestine (foregut theory) and increase in nutrient delivery to the distal small bowel (hindgut theory) created by EndoBarrier likely induce neuro-hormonal changes and nutrient sensing that affect energy balance and glucose homeostasis. |
| ● | Secretion of GLP-1 — Partially digested nutrients reach the distal ileum, which stimulates the secretion of GLP-1 by L-cells located in this area. GLP-1 is known to regulate insulin secretion and action. |
| ● | Increase in gut hormones — This contributes to the restoration of energy and glucose homeostasis. |
| ● | Elevated GLP-1 and PYY levels — Both levels are elevated as quickly as one-week post-implantation. Both hormones may play a role in satiety and body weight control. |
There is no known evidence of occurrence of clinically significant caloric malabsorption with EndoBarrier. EndoBarrier covers only 60 cm of duodenal and proximal jejunal mucosa, which most likely represents less than 10% of the length of the small intestine and leaves almost the entire jejunum and ileum for digestion and absorption.
Operations and Financing
GI Dynamics began selling EndoBarrier in Europe and South America in 2010 and in Australia in 2011. Since inception, the Company has distributed almost 4,000 units of EndoBarrier and generated a total of $7.8 million in revenue. The Company has incurred net losses in each year since its inception.
The Company has five subsidiaries: GI Dynamics Securities Corporation, a Massachusetts-incorporated nontrading entity; GID Europe Holding B.V., a Netherlands-incorporated nontrading holding company; GID Europe B.V., a Netherlands-incorporated company that conducts certain European business operations; GID Germany GmbH, a German-incorporated company that conducts certain European business operations; and GI Dynamics Australia Pty Ltd, an Australia-incorporated company that conducts Australian business operations.
GI Dynamics has raised net proceeds of approximately $273 million through sales of the Company’s equity and placement of debt, of which $9.3 million was received in 2019 as a result of the Company’s 2019 financings. Historically, the Company generated $75.7 million in proceeds, net of expenses, through the sale of convertible preferred stock to a number of US venture capital firms, two global medical device manufacturers, and qualified individual investors prior to going public. In June 2011, the Company issued convertible term promissory notes to several of its stockholders totaling $6 million. In September 2011, the Company conducted an Initial Public Offering (“IPO”) in Australia and simultaneously conducted a private placement of CDIs to accredited investors in the United States. Net proceeds from these financings approximated $72.5 million, net of expenses and repayment of the June 2011 notes. In connection with the IPO, all existing shares of preferred stock were converted into common stock.
In July and August 2013, GI Dynamics raised approximately $52.5 million, net of expenses, in an offering of the Company’s CDIs to qualified investors in Australia, the United States, and certain other jurisdictions. In May 2014, the Company raised approximately $30.8 million, net of expenses, in an offering of the Company’s CDIs to qualified investors in Australia, Hong Kong, the United Kingdom, and certain other jurisdictions.
8
In December 2016, GI Dynamics raised approximately $1 million, net of expenses, in an offering of the Company’s CDIs to qualified investors in Australia and certain other jurisdictions.
In January 2017, GI Dynamics raised approximately $0.2 million, net of expenses, in an offering of the Company’s CDIs under a Security Purchase Plan (“SPP”) available to security holders having a registered address in Australia or New Zealand.
In June 2017, GI Dynamics placed a Convertible Secured Term Promissory Note (the “2017 Note”) financing with Crystal Amber Fund Limited (“Crystal Amber”, the Company’s largest stockholder and a Related Party for ASX purposes) for a gross amount of $5 million. The 2017 Note accrues interest at 5% per annum, compounded annually. In December 2018, the maturity date of the 2017 Note was extended from December 31, 2018 to March 31, 2019 in exchange for payment of the total accrued interest on the 2017 Note at December 31, 2018, which approximated $394 thousand. The maturity date of the 2017 Note was extended four additional times in 2019, with the most recent being the August 21 extension of the maturity date from September 1, 2019 to March 31, 2020.
In July 2017, GI Dynamics signed a manufacturing agreement with its contract manufacturing partner Proven Process Medical Devices (“PPMD”).
In February and March 2018, GI Dynamics raised approximately $1.6 million in an offering of the Company’s CDIs to qualified investors, including certain existing investors, in Australia, the United States and the United Kingdom.
In May 2018, GI Dynamics placed a Convertible Term Promissory Note and Warrant (the “2018 Note and Warrant”) with Crystal Amber for a note principal amount of $1.75 million. The 2018 Note accrues interest at 10% per annum, compounded annually, and could be converted to CDIs at $0.018 per CDI until maturity on May 30, 2023. The warrants conferred the right to purchase 97,222,200 CDIs for $0.018 per CDI with anti-dilution protections.
In September and November 2018, GI Dynamics raised approximately $5 million in an offering of the Company’s CDIs to qualified investors, including certain existing investors, in Australia, the United States and the United Kingdom.
In October 2018, GI Dynamics announced that it signed an agreement with its new notified body Intertek to pursue CE marking of EndoBarrier in Europe. The Company has since suspended its relationship with Intertek and is working with a different notified body whose identity will be announced when the service agreement is finalized.
In November 2018, the Company signed a clinical trial agreement and a memorandum of understanding with its clinical and potential commercial partner Apollo Sugar, a division of Apollo Hospitals Group located in India.
In December 2018, GI Dynamics announced the appointment of Charles Carter, a consultant, as the Company’s Chief Financial Officer and Secretary. Mr. Carter became an employee of the Company on September 1, 2019.
In March 2019, the Company completed a Note Purchase Agreement (“March 2019 NPA”) detailing a convertible term promissory note (the “March 2019 Note”) and warrant (the “March 2019 Warrant”) financing with Crystal Amber for a gross amount of $1 million. The March 2019 Note compounded interest annually at 10% and, subject to Stockholder Approval required under ASX Listing Rules, Crystal Amber was conferred the right to optionally convert all unpaid principal and interest to CDIs at $0.0127 per CDI before the Note Maturity date in March 2024. Per the March 2019 NPA, the Company agreed to issue a warrant (the “March 2019 Warrant”) to Crystal Amber, pending stockholder approval, to purchase 78,984,823 CDIs (representing 1,579,696 shares of common stock) at an initial exercise price of $0.0127 per CDI. The issue of the March 2019 Warrant required the approval of stockholders and was not exercisable until its issue was approved on June 30, 2019. (See Note 9 of the Consolidated Financial Statements for a more complete description of the terms and conditions of the financing).
9
In March 2019, the maturity date of the 2017 Note was extended to May 1, 2019. In April 2019, the maturity date of the 2017 Note was extended to July 1, 2019.
In May 2019, the Company completed a convertible term promissory note (the “May 2019 Note”) and warrant (the “May 2019 Warrant”) financing with Crystal Amber for a gross amount of $3 million. The May 2019 Note compounded interest annually at 10% and, subject to Stockholder Approval required under ASX Listing Rules, Crystal Amber was conferred the right to optionally convert all unpaid principal and interest to CDIs at $0.0127 per CDI before the Note Maturity date in May 2024. Per the May 2019 NPA, the Company contingently issued a warrant (the “May 2019 Warrant”) to Crystal Amber, pending stockholder approval, to purchase 236,220,472 CDIs (representing 4,724,409 shares of common stock) at an initial exercise price of $0.0127 per CDI. The issue of the May 2019 Warrant required the approval of stockholders and was not exercisable until its issue was approved on June 30, 2019. (See Note 9 of the Consolidated Financial Statements for a more complete description of the terms and conditions of the financing).
In June 2019, the maturity date of the 2017 Note was extended to October 1, 2019.
On June 30, 2019, Crystal Amber elected to convert the 2018 Note, the March 2019 Note and the May 2019 Note into CDIs. Under the terms of the respective notes, an aggregate of 453,609,963 CDIs (representing approximately 9,072,197 shares of common stock) were subscribed but unissued on conversion and concurrent cancellation of the 2018 Note, the March 2019 Note and the May 2019 Note. The CDIs were issued on July 3, 2019.
On August 21, 2019, the Company and Crystal Amber entered into a securities purchase agreement for a total funding of up to approximately $10 million (the “August 2019 SPA”). The initial $5.4 million was comprised of existing warrant exercises scheduled between August 25, 2019 and November 15, 2019. Exercises included the 2018 Warrant, the March 2019 Warrant, and the May 2019 Warrant issued to Crystal Amber, as further detailed above. The remaining amount of the August 2019 funding was represented by a Convertible Term Promissory Note (“August 2019 Note”) of up to approximately $4.6 million and a related Warrant (“August 2019 Warrant”) in substantially the same form as the March 2019 and May 2019 convertible term promissory note. Under the terms of the August 2019 SPA and 2019 Note, the Company, at its sole discretion, elected to request the entire $4.6 million to be funded before December 6, 2019, but then amended the election to allow funding in tranches at Crystal Amber’s election before January 15, 2020. The conversion feature allows the conversion of the August 2019 Note unpaid principal and interest at $0.02 per CDI and the August 2019 Warrant, upon its issue, allowed the purchase for $0.02 per CDI of that number of CDIs represented by the August 2019 Note principal divided by $0.02 per CDI. (See Note 9 of the Consolidated Financial Statements for a more complete description of the terms and conditions of the financing).
On August 21, 2019, the maturity date of the 2017 Note was extended to March 31, 2020.
On August 25, 2019, Crystal Amber exercised the 2018 Warrant and a portion of the March 2019 Warrant in accordance with the terms of the August 2019 SPA. For an aggregate cash payment of $2 million, 97,222,200 CDIs (representing approximately 1,944,444 shares of common stock) were issued at $0.0144 per CDI under the 2018 Warrant and 47,244,119 CDIs (representing approximately 944,882 shares of common stock) were issued at $0.0127 per CDI under the March 2019 Warrant.
On September 30, 2019, Crystal Amber exercised the remaining portion of the March 2019 Warrant and a portion of the May 2019 Warrant in accordance with the August 2019 SPA. For an aggregate cash payment of $2 million, 31,740,704 CDIs (representing approximately 634,814 shares of common stock) were issued at $0.0127 per CDI under the March 2019 Warrant and 125,739,610 CDIs (representing approximately 2,514,792 shares of common stock) were issued on October 4, 2019 at $0.0127 per CDI under the May 2019 Warrant. As of September 30, 2019, this was recorded as a subscription receivable from a related party and the cash was received on October 1, 2019.
On October 31, 2019, Crystal Amber exercised another portion of the May 2019 Warrant in accordance with the August 2019 SPA. For an aggregate cash payment of $1 million, 78,740,157 CDIs (representing approximately 1,574,803 shares of common stock) were issued on November 4, 2019 at $0.0127 per CDI under the May 2019 Warrant. Cash was received on October 31, 2019.
10
On November 15, 2019, Crystal Amber exercised the final portion of the May 2019 Warrant in accordance with the August 2019 SPA. For an aggregate cash payment of approximately $0.4 million, 31,740,748 CDIs (representing approximately 634,814 shares of common stock) were issued on November 18, 2019 at $0.0127 per CDI under the May 2019 Warrant. Cash was received on November 15, 2019.
On December 2, 2019, GI Dynamics provided notice to Crystal Amber that the Company elected to place the August 2019 Note at the full amount, on or before December 6, 2019. In December, the Company and Crystal Amber agreed to confer the right to tranche the funding of the August 2019 Note in amounts and per timing chosen solely by Crystal Amber, provided the August 2019 Note total was funded on or before January 15, 2020.
On December 16, 2019, GI Dynamics stockholders approved the August 2019 Note conversion feature and the issuance of the August 2019 Warrant, pending funding of the August 2019 Note by Crystal Amber.
On January 13, 2020, GI Dynamics received approximately $4.6 million in cash representing the full funding of the August 2019 Note. As stockholder approval had been obtained in December 2019, the August 2019 Note became convertible at Crystal Amber’s sole discretion until maturity in August 2024. Additionally, the August 2019 Warrant was issued to Crystal Amber, providing for the purchase of up to 229,844,650 CDIs (representing 4,596,893 shares of common stock) at $0.02 per CDI.
The rights of the Company’s stockholders are governed by Delaware general corporation law.
GI Dynamics is headquartered in Boston, Massachusetts, where the majority of the Company’s employees work. The Company has subsidiaries in the Netherlands, Germany, and Australia, with employees in Germany and Australia.
2019 in Review
2019 was a year primarily focused on 4 operational priorities:
| 1. | STEP-1 US Pivotal Trial |
| 2. | I-STEP India Pivotal Trial with Apollo Sugar |
| 3. | CE Mark |
| 4. | Raising capital to finance operations |
The Company’s lead priority was the STEP-1 (Single Therapy Euglycemic Procedure) pivotal trial. The year was spent focusing on final training of clinical sites, study committee selection and running the STEP-1 Investigator Meeting. Additional focus was on selection, design, testing and deployment of multiple clinical trial management systems. Finally, the focus of efforts shifted to site initiations and initiation of patient recruitment efforts. The Company announced that the first patient was randomized in the STEP-1 trial in January 2020. Management expects that enrollment of Stage 1 (~67 patients; 50 EndoBarrier, ~17 control) will be enrolled by end of year 2020.
The Company, in concert with Apollo Sugar, has focused on preparation for initiation of the I-STEP (India Single Therapy Euglycemic Procedure) pivotal trial. The year was spent working with Apollo and CDSCO (Central Drugs Standard Control) and DCGI (Drugs Controller General of India) to receive approval to commence enrollment in the I-STEP study. In addition, the Company focused on conducting clinical site qualifications, software selection and design and integration to the Apollo Sugar patient management app. Management expects to begin enrollment in Q3 2020 and to be completely enrolled within 9 months, currently projected to be completely enrolled by the end of Q1 2021.
The Company is working with its new notified body towards receiving CE Mark designation. The Company previously announced that it was working with Intertek as its notified body. The Company terminated that relationship and has been working with a different notified body which will be announced in 2020 when the relationship is formalized under contract. Management is seeking CE Mark approval by the end of 2020; however, there is no assurance that CE Mark approval will be granted in the time frame anticipated by management or granted at any time in the future.
Finally, the Company focused on raising capital to adequately finance operations. Through a series of financings, management has been able to focus on reducing cash burn while decreasing risk, continuing to develop the Company’s intellectual property, and continuing to support the release of statistically significant clinical data from independent investigator-initiated trials and two registries.
GI Dynamics hired key staff and moved to a new office location in Boston, Massachusetts in 2019.
11
Strategic Focus in 2020: The Path Forward
The Company’s goal is to become the leading provider of alternative options for treating type 2 diabetes and concurrently reducing obesity. GI Dynamics intends to do this by conducting the following activities:
| 1) | U.S. clinical operations and pivotal trial: | Complete enrollment in Stage 1 of the STEP-1 pivotal trial in the US. | |
| 2) | India partnership and pivotal trial: | Initiate enrollment in the I-STEP pivotal trial in India. | |
| 3) | Gain CE Mark: | Work with the Company’s notified body to gain the EndoBarrier CE mark. | |
| 4) | Ongoing clinical data: | Support continued release of clinical data from registries and investigator- initiated clinical trials. | |
| 5) | Continued development of Intellectual Property: | Continue developing the Company’s intellectual property and protecting its patents. |
The Company will need to raise additional capital in 2020.
As of December 31, 2019, the Company’s primary source of liquidity is its cash, cash equivalents and restricted cash balances. GI Dynamics is currently focused primarily on its clinical trials, which will support future regulatory submissions and potential commercialization activities. Until GI Dynamics is successful in gaining regulatory approvals, the Company is unable to sell its product in any market at this time. Without revenues, GI Dynamics is reliant on funding obtained from investment in the Company to maintain business operations until the Company can generate positive cash flows from operations. The Company cannot predict the extent of its future operating losses and accumulated deficit, and it may never generate sufficient revenues to achieve or sustain profitability.
The Company has incurred operating losses since inception and at December 31, 2019, had an accumulated deficit of approximately $284 million and a working capital deficit of $3.4 million. The Company expects to incur significant operating losses for the next several years. At December 31, 2019, the Company had approximately $2.5 million in cash, cash equivalents and restricted cash.
The Company will need to restructure the terms of the 2017 Note before March 31, 2020 and raise additional capital before May 1, 2020 in order to continue to pursue its current business objectives as planned and to continue to fund its operations. The Company and Crystal Amber are currently in final discussions regarding the 2017 Note maturity extension. The Company is looking to raise additional funds through any combination of additional equity and debt financings or from other sources. However, the Company has no guarantee that the 2017 Note will not mature on March 31, 2020 and has no guaranteed source of capital that will accommodate repayment of the 2017 Note and sustain operations past March 31, 2020. If the 2017 Note terms are restructured, the Company anticipates operating cash being exhausted by May 1, 2020 and therefore requires additional financing. There can be no assurance that any such potential financing opportunities will be available on acceptable terms, if at all. If the Company is unable to raise sufficient capital on the Company’s required timelines and on acceptable terms to stockholders and the Board of Directors, it could be forced to cease operations, including activities essential to support regulatory applications to commercialize EndoBarrier. If access to capital is not achieved in the near term, it will materially harm the Company’s business, financial condition and results of operations to the extent that the Company may be required to cease operations altogether, file for bankruptcy, or undertake any combination of the foregoing. These factors raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that these consolidated financial statements are issued.
In addition, if the Company does not meet its payment obligations to third parties as they become due, the Company may be subject to litigation claims and its credit worthiness would be adversely affected. Even if the Company is successful in defending these claims, litigation could result in substantial costs and would be a distraction to management and may have other unfavorable results that could further adversely impact the Company’s financial condition.
As a result of the factors described above, the Company’s consolidated financial statements include a going-concern disclosure. See “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources” for further information regarding the Company’s funding requirements.
Implications of Being a Smaller Reporting Company
Section Rule 12b-2 of the Securities Exchange Act of 1934, as amended, (“the Exchange Act”), establishes a class of company called a “smaller reporting company,” which effective September 10, 2018, was amended to include companies with a public float of less than $250 million as of the last business day of its most recently completed fiscal quarter or, if such public float is less than $700 million, had annual revenues of less than $100 million during the most recently completed fiscal year for which audited financial statements are available. For the year ended December 31, 2019, the Company qualifies as a smaller reporting company.
12
As a smaller reporting company, the Company may take advantage of specified reduced disclosure and other requirements that are otherwise generally applicable to public companies that do not qualify for this classification. The provisions include:
| ● | Any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and financial statements, commonly known as an “auditor discussion and analysis”; | |
| ● | Allowance to report two years, instead of three, of audited financial statements; | |
| ● | Reduced disclosure about executive compensation arrangements; | |
| ● | Exemption from the auditor attestation requirement in the assessment of the Company’s internal control over financial reporting. |
For as long as GI Dynamics continues to be a smaller reporting company, the Company expects that it will take advantage of the reduced disclosure obligations available to it as a result of such classification. The Company will remain a smaller reporting company until it has a public float of $250 million or more as of the last business day of its most recently completed fiscal quarter, and the Company could retain its smaller reporting company status indefinitely depending on the size of its public float.
Employees
As of March 12, 2020, GI Dynamics has 15 full-time employees, 12 of whom are in the United States. None of the Company employees are represented by labor unions or covered by collective bargaining agreements.
Available Information
Financial and other information about GI Dynamics is available on the Company website, whose address is www.gidynamics.com. The Company makes available on its website, free of charge, copies of its Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after the Company electronically files such material with, or furnishes it to, the U.S. Securities and Exchange Commission (SEC) and the Australian Securities Exchange (ASX). The information contained in the Company website is not intended to be part of this filing.
The Company’s business faces many risks. The Company believes the risks described below are the material risks that it faces. However, the risks described below may not be the only risks that it faces. Additional unknown risks or risks that are currently considered immaterial, may also impair the Company’s business operations. If any of the events or circumstances described below actually occur, the Company’s business, financial condition or results of operations could suffer, and the trading price of its CDIs could decline significantly. You should consider the specific risk factors discussed below together with the cautionary statements under the caption “Forward-Looking Statements” and the other information and documents that the Company files from time to time with the Securities and Exchange Commission (SEC) and the Australian Securities Exchange (ASX).
Risks Related to the Company’s Business
GI Dynamics will need substantial additional funding and may be unable to raise capital when needed, which could force the Company to delay, reduce, or eliminate planned activities or result in its inability to operate as a going concern.
As of December 31, 2019, the Company’s primary source of liquidity is its cash, cash equivalents and restricted balances. GI Dynamics is currently focused primarily on its clinical trials, which will support future regulatory submissions and potential commercialization activities. Until the Company is successful in gaining regulatory approvals, it is unable to sell the Company’s product in any market at this time. Without revenues, GI Dynamics is reliant on funding obtained from investment in the Company to maintain business operations until the Company can generate positive cash flows from operations. The Company cannot predict the extent of future operating losses and accumulated deficit, and it may never generate sufficient revenues to achieve or sustain profitability.
The Company has incurred operating losses since inception and at December 31, 2019, had an accumulated deficit of approximately $284 million and a working capital deficit of $3.4 million. The Company expects to incur significant operating losses for the next several years. At December 31, 2019, the Company had approximately $2.5 million in cash, cash equivalents and restricted cash.
13
The Company will need to restructure the terms of the 2017 Note before March 31, 2020 and raise additional capital before May 1, 2020 in order to continue to pursue its current business objectives as planned and to continue to fund its operations. The Company and Crystal Amber are currently in final discussions regarding the 2017 Note maturity extension. The Company is looking to raise additional funds through any combination of additional equity and debt financings or from other sources. However, the Company has no guarantee that the 2017 Note will not mature on March 31, 2020 and has no guaranteed source of capital that will accommodate repayment of the 2017 Note and sustain operations past March 31, 2020. If the 2017 Note terms are restructured, the Company anticipates operating cash being exhausted by May 1, 2020 and therefore requires additional financing. There can be no assurance that any such potential financing opportunities will be available on acceptable terms, if at all. If the Company is unable to raise sufficient capital on the Company’s required timelines and on acceptable terms to stockholders and the Board of Directors, it could be forced to cease operations, including activities essential to support regulatory applications to commercialize EndoBarrier. If access to capital is not achieved in the near term, it will materially harm the Company’s business, financial condition and results of operations to the extent that the Company may be required to cease operations altogether, file for bankruptcy, or undertake any combination of the foregoing. These factors raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that these consolidated financial statements are issued.
In addition, if the Company does not meet its payment obligations to third parties as they become due, the Company may be subject to litigation claims and its credit worthiness would be adversely affected. Even if the Company is successful in defending these claims, litigation could result in substantial costs and would be a distraction to management and may have other unfavorable results that could further adversely impact the Company’s financial condition.
As a result of the factors described above, the Company’s consolidated financial statements include a going-concern disclosure.
The terms of the Company’s indebtedness, in particular the terms of the 2017 Note, may result in the liquidation or winding down of the business, which would have a negative impact on holders of the Company CDIs and common stock.
In June 2017, GI Dynamics completed its 2017 Note secured financing with Crystal Amber for a gross amount of $5.0 million that accrues annually compounded interest of 5% per annum. In December 2018, the maturity date of the 2017 Note was extended from December 31, 2018 to March 31, 2019 in exchange for payment of approximately $394 thousand, which was the total accrued interest on the 2017 Note at December 31, 2018. In 2019, the maturity of the 2017 Note was extended multiple times, most recently to March 31, 2020.
As of March 12, 2020, the Company had outstanding debt obligations of $9.9 million, including accrued interest. Approximately $5.3 million of this amount is due and payable on or before March 31, 2020 under the terms of the 2017 Note, unless (i) the 2017 Note is converted to equity prior to March 31, 2020 or (ii) the 2017 Note is restructured to extend the maturity date, as was last done in August 2019. The Company’s debt obligations are secured by all of the Company’s assets such that upon an event of default, the Company may be forced to sell some or all of its assets in order to make payment against the debt obligation when due. The proceeds from the sale of all of the Company’s assets may be insufficient to satisfy its debt obligations in full. As a result, it is unlikely that any proceeds would be available for distribution to holders of the Company’s CDIs or common stock on a sale of the Company’s assets or on a liquidation or winding down of the business.
Failure to achieve a positive outcome in the U.S. clinical trial of EndoBarrier could negatively impact the Company’s ability to raise additional capital and obtain regulatory approval in other countries.
GI Dynamics is conducting the U.S. pivotal trial of EndoBarrier under the FDA’s IDE. The Company refers to this trial as the GI Dynamics STEP-1 clinical trial in its statements and filings. Failure to achieve a positive outcome in this clinical trial could result in the failure of EndoBarrier to gain regulatory approval in the U. S. This outcome could negatively impact the Company’s ability to raise additional capital and obtain regulatory approval in other countries.
In order to complete the STEP-1 clinical trial, GI Dynamics will need to enroll patients that ultimately complete the trial protocol. If the Company is unable to enroll patients, if the enrollment pace is slower than anticipated, or if a number of enrolled patients do not complete the study protocol, it could have a negative effect on the Company’s ability to complete the clinical trial, which could adversely affect the Company’s business, operating results and prospects.
Conducting successful clinical studies will require the enrollment of patients, and suitable patients may be difficult to identify and recruit. Patient enrollment in clinical trials and completion of patient participation and follow-up depends on many factors, including the size of the patient population, the nature of the trial protocol, the desirability of, or the discomforts and risks associated with, the treatments received by enrolled subjects, the availability of appropriate clinical trial investigators and support staff, the proximity of patients to clinical sites, the ability of patients to comply with the eligibility and exclusion criteria for participation in the clinical trial and patient compliance. For example, patients may be discouraged from enrolling in the Company’s clinical trials if the trial protocol requires them to undergo extensive post-treatment procedures or follow-up to assess the safety and effectiveness of the Company’s product or if they determine that the treatments received under the trial protocols are not desirable or involve unacceptable risk or discomfort.
Development of sufficient and appropriate clinical protocols to demonstrate safety and efficacy are required and GI Dynamics may not adequately develop such protocols to support clearance or approval. Further, the FDA may require the Company to submit data on a greater number of patients than originally anticipated and/or for a longer follow-up period or change the data collection requirements or data analysis applicable to the Company’s clinical trials. Delays in patient enrollment or failure of patients to continue to participate in a clinical trial may cause an increase in costs and delays in the approval or clearance and attempted commercialization of the Company’s product or result in the failure of the clinical trial. In addition, despite considerable time and expense invested in clinical trials, the FDA may not consider the Company’s data adequate to demonstrate safety and efficacy. Such increased costs and delays or failures could adversely affect the Company’s business, operating results and prospects.
14
GI Dynamics or third parties upon whom it depends may be adversely affected by natural disasters and/or health epidemics, and the Company’s business, financial condition and results of operations could be adversely affected.
Natural disasters and/or health epidemics could severely disrupt the Company’s operations and have a material adverse effect on the Company’s business operations. If a natural disaster, health epidemic, or other event beyond the Company’s control occurred that prevented the Company or third parties on which GI Dynamics relies from using appropriate office, manufacturing, or warehouse space, that damaged critical infrastructure, negatively impacted the manufacturing capabilities of the Company’s third-party contract manufacturers, disabled broadband systems such that communication and access to records was impaired, or that otherwise disrupted operations, it may be difficult for GI Dynamics to continue its business for a substantial period of time. In December 2019, a novel strain of coronavirus was reported to have surfaced in Wuhan, China. In January 2020, this coronavirus spread to other parts of the world, including the United States and Europe, and efforts to contain the spread of this coronavirus intensified. In March 2020, the World Health Organization declared the Coronavirus outbreak a “pandemic.” The extent to which the coronavirus impacts the Company’s results will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of the coronavirus and the actions to contain the coronavirus or treat its impact, among others.
Should the coronavirus continue to spread in the United States and Europe, GI Dynamics business operations could be delayed or interrupted. For instance, the Company’s clinical trials may be impacted by containment policies enacted by the clinical practice, the respective state or the Federal government. This could result in lower than anticipated patient registration or enrollment and GI Dynamics may be forced to temporarily delay or pause ongoing trials. Further, if the spread of the coronavirus continues and the Company’s operations are impacted, GI Dynamics risks a delay, default and/or nonperformance under existing agreements arising from force majeure. If any of the foregoing were to occur, it could materially adversely affect the Company’s financial condition.
The FDA has mandated certain stopping rules in the STEP-1 clinical trial. If the stopping rules are triggered or other unanticipated adverse issues occur during the STEP-1 clinical trial, the FDA may not permit the trial to continue. If that were to happen, the Company’s business, operating results and prospects would be materially and adversely affected.
Clinical trials involve the administration of the biological product candidate to patients under the supervision of qualified investigators, generally physicians not employed by or under the trial sponsor’s control. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria, and the parameters to be used to monitor subject safety, including stopping rules that assure a clinical trial will be stopped if certain adverse events should occur. In the context of the GI Dynamics STEP-1 clinical trial, hepatic bleeding is an example of an adverse event that would be investigated and could trigger such stopping rules.
If, during the STEP-1 clinical trial, the stopping rules are triggered or other unanticipated adverse issues occur, the FDA may prevent the Company from enrolling additional patients in the clinical trial or may not permit the clinical trial to be completed. If that were to happen, the Company’s business, operating results and prospects would be materially and adversely affected.
In order to commercialize the Company’s product in the U.S. and certain other countries, GI Dynamics will need to obtain regulatory and other approvals. The Company’s inability to achieve, or a delay in achieving, such approvals could lead to the denial of marketing approval for EndoBarrier® or any of its other products.
At present, GI Dynamics has no regulatory approvals for the marketing and sale of EndoBarrier. In November 2017, the Company received notification from SGS, the Company’s now former notified body in Europe, that they were withdrawing the Certificate of Conformity for EndoBarrier. The Certificate of Conformity is required for the sale of any product under CE marking. As a result, GI Dynamics is not permitted to supply the EndoBarrier, EndoBarrier Delivery System and EndoBarrier Retrieval System in Europe.
There is no guarantee that GI Dynamics will obtain additional approvals from regulatory bodies in the future, including the FDA in the U.S. In the U.S., the Company stopped its pivotal trial of EndoBarrier in 2015. The Company will not be able to obtain FDA approval to commercialize EndoBarrier in the U.S. until the STEP-1 clinical trial is successfully completed and regulatory approval is obtained from the FDA through the PMA review process. The STEP-1 clinical trial will be conducted in two stages. At the end of the first stage, the FDA will review data collected to date from the STEP-1 clinical trial and must approve the continuation of the STEP-1 clinical trial to the second stage. There can be no assurance that the FDA will approve continuation of the trial at the end of the first stage.
Regulatory authorities in other countries may also require additional clinical trials. Any such clinical trials may be delayed, suspended or prematurely terminated because costs are greater than anticipated or for a variety of other reasons, including: delay or failure in reaching agreement with the foreign regulatory authority(ies) on a trial design that the Company is able to execute; delay or failure in obtaining authorization to commence a trial or inability to comply with conditions imposed by a regulatory authority regarding the scope or design of a clinical trial; or regulators may require that the Company suspend or terminate its clinical trials for various reasons, including noncompliance with regulatory requirements, unforeseen safety issues or adverse side effects, or a finding that the participants are being exposed to unacceptable health risks. Any of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of marketing approval for EndoBarrier or any of the Company’s other products. Necessary regulatory approvals could also be delayed, which could significantly impact the Company’s ability to commercialize the Company’s technology in the U.S. and other countries.
15
GI Dynamics has a history of net losses and may never achieve or maintain profitability.
GI Dynamics is a medical device company with a limited history of operations and has limited commercial experience with its product. As of December 31, 2019, the Company’s primary source of liquidity is its cash, cash equivalents and restricted cash balances. The Company continues to evaluate which markets are appropriate to continue pursuing reimbursement, market awareness and general market development and selling efforts, and continues to restructure its business and costs, establish new priorities, and evaluate strategic options. The Company expects to continue to incur significant operating losses for the foreseeable future as it incurs costs, including those associated with commercializing the Company’s products, conducting clinical trials to test its products, attempting to secure regulatory approvals for the Company’s products (in the U.S. and other countries) and increased costs associated with being a public company in the U.S. As a result, if the Company remains in business, it expects to incur significant operating losses for the next several years.
The Company has incurred operating losses since inception and at December 31, 2019, had an accumulated deficit of approximately $284 million and a working capital deficit of $3.4 million. The Company expects to incur significant operating losses for the next several years. At December 31, 2019, the Company had approximately $2.5 million in cash, cash equivalents and restricted cash.
GI Dynamics cannot predict the extent of future operating losses and accumulated deficit, and it may never generate sufficient revenues to achieve or sustain profitability.
GI Dynamics depends heavily on the success of the Company’s product, EndoBarrier. The Company cannot give any assurance that EndoBarrier will receive regulatory approval, which is necessary before it can be commercialized.
The Company’s ability to generate product revenues will depend heavily on the successful commercialization of its product, EndoBarrier, if approved by regulatory agencies in the U.S. or abroad. GI Dynamics cannot be certain that EndoBarrier will be successful in clinical trials or receive regulatory approval. Further, EndoBarrier may not receive regulatory approval even if it is successful in clinical trials. If the Company does not receive regulatory approvals for EndoBarrier, it may not be able to continue operations.
If GI Dynamics can obtain the required regulatory approvals in the U.S. and certain other countries, it expects to derive substantially all of its revenue from sales of EndoBarrier. Accordingly, the Company’s ability to generate revenues in the future relies on its ability to market and sell this product.
The degree of market acceptance for EndoBarrier will depend on a number of factors, including:
| ● | the efficacy, ease of use and perceived advantages and disadvantages of EndoBarrier over other available treatments and technologies for managing type 2 diabetes; |
| ● | the prevalence and severity of any adverse events or side effects of EndoBarrier; |
| ● | the extent to which physicians adopt EndoBarrier (which may be influenced by the Company’s ability to provide additional clinical data regarding the potential long-term benefits provided by EndoBarrier and the strength of the Company’s sales and marketing initiatives); |
| ● | the price of EndoBarrier and the third-party coverage and reimbursement for procedures using EndoBarrier; |
| ● | the extent to which reimbursement may be secured for each country in which EndoBarrier is commercialized; and |
| ● | the Company’s ability to attract and retain professional sales personnel to drive EndoBarrier revenue. |
16
GI Dynamics cannot predict the outcome and timing of its current and future human clinical trials of EndoBarrier products. If the trials do not produce positive results, the commercial prospects for EndoBarrier will be impaired.
The results of current and future human clinical trials, whether investigator initiated, or Company sponsored, cannot be predicted. If EndoBarrier or new products that the Company develops and tests in the future cause serious adverse events in future human clinical trials, these trials may need to be delayed or stopped. For example, GI Dynamics stopped its original U.S. pivotal trial, the ENDO trial, in 2015 because of adverse events associated with EndoBarrier in that trial. In addition, these clinical trials may not produce positive safety or efficacy results or may produce results that are not as favorable as those seen in previous clinical trials.
Negative safety or efficacy results of any future human clinical trials could require that the Company attempt to modify the EndoBarrier device or the treatment guidelines to address these issues and there is no guarantee that any potential modifications would be successfully developed.
If future human clinical trials of EndoBarrier products do not meet the required clinical specifications or cause serious adverse or unexpected events, such as those experienced in the ENDO trial, then these results could affect regulatory approvals and adoption and materially impact potential product sales and reimbursement. If the Company is not able to adequately address any adverse or unexpected events through training, education, changes in product design or product claims, this may significantly impair the commercial prospects for EndoBarrier.
Doctors may not accept EndoBarrier as a treatment option, which would harm the Company’s business and future revenues, if any.
The commercial success of EndoBarrier will require acceptance by physicians, who may be slow to adopt the Company’s product for the following reasons (among others):
| ● | lack of long-term clinical data supporting patient benefits or cost savings over existing alternative treatments; |
| ● | lack of experience with EndoBarrier and training time required before it can be used, which may drive preferences for other products or procedures; |
| ● | lack of adequate payment to the physician for implanting the device or caring for the patient (driven by availability of adequate coverage and reimbursement for hospitals and implanting physicians); |
| ● | perceived strength of products, procedures or pharmacotherapies as alternatives to EndoBarrier; and |
| ● | perceived liability risks associated generally with the use of new products and procedures. |
Although the Company has developed relationships with physicians who are key opinion leaders in certain countries, GI Dynamics cannot assure that these existing relationships and arrangements can be maintained or that new relationships will be established in support of its products. If physicians do not consider the Company’s products to be adequate for the treatment of type 2 diabetes or if a sufficient number of physicians recommend and use competing products or pharmacotherapies, it could harm the Company’s business and future revenues, if any.
The Company has limited sales, marketing and distribution experience; therefore, it may be unable to successfully commercialize its products.
There can be no guarantee that the Company will be able to effectively commercialize its products. Developing direct sales, distribution and marketing capabilities will require the devotion of significant resources and require GI Dynamics to ensure compliance with all legal and regulatory requirements for sales, marketing and distribution. Failure to develop these capabilities and meet these requirements could jeopardize the Company’s ability to market its products or could subject it to substantial liability. In addition, for those countries where the Company commercializes its products through distributors or other third parties, GI Dynamics will rely heavily on the ability of its partners to effectively market and sell its products to physicians and other end users in those countries. The Company cannot guarantee that distributors or other third parties will be effective in commercializing GI Dynamics products.
17
GI Dynamics may compete against companies that have longer operating histories, more established or approved products, and greater resources, which may prevent the Company from achieving market penetration with its products.
Competition in the medical device industry is intense and EndoBarrier will likely compete in part against more established procedures and products for the treatment of type 2 diabetes and obesity. Bariatric surgery, including gastric bypass surgery and the gastric band, have been used for many years with extensive publication histories on clinical effectiveness. Large multinational medical device companies sell supplies for these procedures and are formidable competitors to new entrants. In addition, certain drugs have been approved, and are used, for the treatment of type 2 diabetes and obesity. Pharmaceutical companies with significantly greater resources than the Company market these drugs, and GI Dynamics may be unable to compete effectively against these companies.
Many of the Company’s competitors have significantly greater sales, marketing, financial and manufacturing capabilities than it has and have established reputations and/or significantly greater name recognition. Accordingly, there is no assurance that GI Dynamics will be able to win market share from these competitors or that these competitors will not succeed in developing products that are more effective or economic.
Additionally, GI Dynamics is likely to compete with companies offering new technologies in the future. The Company may also face competition from other medical therapies, which may focus on the Company’s target market as well as competition from manufacturers of pharmaceutical and other devices that have not yet been developed. Competition from these companies could adversely affect the Company’s business.
GI Dynamics does not have data regarding the long-term benefits of EndoBarrier, which may affect market acceptance. Furthermore, if the long-term data, once obtained, does not indicate that EndoBarrier is as safe or effective as other treatment options, the Company’s business would be harmed.
An important factor that may be relevant to market acceptance of EndoBarrier is whether it improves or maintains glycemic control and maintains weight loss over extended periods of time after removal of the device. While the Company has tested and evaluated its technology in several clinical trials with hundreds of patients which, in the aggregate, have shown that EndoBarrier is an effective treatment for type 2 diabetes and also reduces obesity, GI Dynamics does not yet have sufficient data to demonstrate and claim any longer-term benefits of its product in the treatment of type 2 diabetes and the reduction of obesity following removal of the device from the patient.
GI Dynamics is continuing to monitor some patients who participated in the Company’s clinical trials after device removal to determine the ongoing effects and longevity of results, however, the Company does not currently have long-term data that supports the safety and efficacy of EndoBarrier. Accordingly, the Company cannot provide assurance that the long-term data, once obtained, will prove lower HbA1c levels compared to alternative treatment options for type 2 diabetes. If the results obtained from the Company clinical trials indicate that EndoBarrier is not as safe or effective as other treatment options or as effective as the Company’s current short-term data would suggest, EndoBarrier may not be approved, or its adoption may suffer, and the Company’s business would be harmed.
If GI Dynamics fails to obtain and maintain adequate levels of reimbursement for its products by health insurers and other third-party payers, there may be no commercially viable markets for its products, or the markets may be much smaller than expected.
If GI Dynamics products are approved for sale, health care providers, including hospitals and physicians that may purchase the Company’s products, generally rely on third-party payers, particularly government-sponsored health care and private health insurance providers, to pay for all or a portion of the costs of the procedures, including the cost of the products used in such procedures. Reimbursement and health care payment systems vary significantly by country. Third-party payers may attempt to limit coverage and the level of reimbursement of new therapeutic products.
If GI Dynamics fails to obtain and maintain adequate levels of reimbursement for its products by health insurers and other third-party payers, there may be few commercially viable markets for its products, or the markets may be much smaller than expected. Third-party payers may demand additional clinical data requiring new clinical trials or economic models showing the cost savings of using the Company’s product, each of which would consume resources and may delay the decision on reimbursement. If the results of such studies are not satisfactory to third-party payers, then reimbursement may not be received in an acceptable amount or at all. In addition, the efficacy, safety, performance and cost-effectiveness of the Company’s products in comparison to any competing products or therapies may determine the availability and level of reimbursement.
18
The Company believes that future reimbursement may be subject to increased restrictions both in the U.S. and in international markets. Future legislation, regulation or reimbursement policies of health insurers or third-party payers may adversely affect the demand for the Company’s current and future products or limit the Company’s ability to sell these products on a profitable basis.
The Company’s products may be subject to extensive, dynamic and ongoing regulation in countries where it plans to sell EndoBarrier, which may impede or hinder the approval or sale of its products and, in some cases, may ultimately result in the inability to obtain approval of certain products or may result in the recall or seizure of previously approved products.
The medical device industry is regulated extensively by governmental authorities, principally the FDA and corresponding state and foreign regulatory agencies and authorities, such as the E.U., legislative bodies and the European Economic Area, or EEA, Member State Competent Authorities. Additionally, the exit of Great Britain from the E.U. may create a separate and distinct regulatory agency with different requirements for regulatory approval in Great Britain.
Before GI Dynamics can market its products in the E.U., and in many other parts of the world, it must obtain and maintain CE Mark certification, which indicates that a product meets the essential requirements of applicable E.U. Directives and has been subject to the appropriate conformity assessment route. This conformity assessment procedure is often done through a self-certification, but depending on the type of product, may also require verification by an independent certification body, called a “notified body.” Notified bodies will also periodically conduct audits to ensure continued compliance with the applicable requirements. The CE Mark allows free movement of products in the E.U., the EEA and Switzerland although any of the member countries may require medical devices to be registered and impose requirements relating to the language of the device information. Many non-European countries also recognize and accept the CE Mark. Failure to support product performance claims and demonstrate continued compliance with the applicable E.U. requirements, can result in the loss of the right to affix the CE Mark to the product, which prevents the product from being sold within the territory and in other countries that recognize the CE Mark.
Before GI Dynamics can market its products in other parts of the world, which are not regulated via CE mark or FDA approvals, it must obtain and maintain regulatory approvals in those countries. These approvals are dependent on clinical data and may require specific clinical trial data to be collected before applications for approval can be made. The Company does not currently have any assurance in any jurisdiction that such regulatory approvals will be granted or that more extensive clinical trials will not be required to secure such approvals. In India, GI Dynamics is partnering with Apollo Sugar to initiate and conduct clinical trials to generate data required by the Indian regulatory authorities, but as with any market, the Company does not have any assurance that these trials will generate the complete data set required to support a marketing approval.
In addition, even if the Company receives regulatory approval of its products in existing markets, the Company will be subject to ongoing regulatory requirements relating to its existing products in those markets. These include the requirement to timely file various reports with regulatory authorities in the countries in which the Company markets products, including reports of adverse events such as those experienced in the ENDO trial, events that may have caused or contributed to a death or serious injury and malfunctions that would likely cause or contribute to a death or serious injury if the malfunction were to recur. If these reports are not timely filed, regulators may impose sanctions, including temporarily suspending existing market authorizations, and sales of EndoBarrier may suffer. In that case, the Company may be subject to product liability or regulatory enforcement actions, all of which could harm the Company’s business. The Company’s failure to comply with EEA or other foreign regulations applicable in the countries where it operates could lead to the issuance of warning letters or untitled letters, the imposition of injunctions, suspensions or loss of regulatory clearance or approvals, product recalls, termination of distribution, product seizures or civil penalties. In the most extreme cases, criminal sanctions or closure of the Company’s manufacturing facilities are possible.
In addition, numerous new regulatory changes in the E.U. came into effect in 2016. The Company may not be able to comply with the new regulations and standards, despite compliance with prior regulations and standards.
19
In some countries, GI Dynamics may rely on its foreign distributors and agents to assist in complying with foreign regulatory requirements, and the Company cannot be sure that they will always do so. If the Company or any of its suppliers, third-party manufacturers, distributors, agents or customers fail to comply with applicable requirements, the Company may face:
| ● | adverse publicity; | |
| ● | investigations by governmental authorities; | |
| ● | fines and prosecutions; | |
| ● | inability to raise capital; | |
| ● | inability to attract and retain sales professionals; | |
| ● | inability to attract and agree to terms with business partners; | |
| ● | increased difficulty in obtaining required approvals; | |
| ● | loss of approvals already granted; | |
| ● | delays in purchasing decisions by customers or cancellation of existing orders; and | |
| ● | the inability to sell or import the Company’s products into such countries. |
Regulatory requirements affecting the development, manufacture and sale of medical devices are evolving and subject to future change. GI Dynamics cannot predict what impact, if any, those changes might have on its business. Failure to comply with regulatory requirements could have a material adverse effect on the Company’s business, financial condition and results of operations. Later discovery of previously unknown problems with a product or manufacturer could result in fines, delays or suspensions of regulatory approvals. The failure to receive product approval on a timely basis, or the withdrawal of product approval by regulatory agencies, could have a material adverse effect on the Company’s business, financial condition or results of operations.
GI Dynamics has had historical challenges with regulatory compliance, which led to a 2014 shipping hold in the E.U., multiple observations by the Therapeutic Goods Administration (“TGA”) in Australia regarding the Company’s failure to comply with Essential Principals of the TGA, and compliance issues that led to the TGA’s cancellation of the EndoBarrier listing on the Australian Register of Therapeutic Goods (“ARTG”) in 2016. In addition, in May 2017, GI Dynamics received notification from its notified body, SGS, that the CE Mark for EndoBarrier had been suspended pending closure of non-conformances related to the Company’s quality management system required under International Organization for Standardization (“ISO”) regulations. On November 10, 2017, the Company received notification from SGS that SGS was withdrawing the Certificate of Conformance for EndoBarrier, ending the CE Marking of EndoBarrier in Europe and select Middle East countries.
Given the past challenges the Company has had in meeting all the requirements of a comprehensive global quality system, a risk exists that the Company will have compliance issues in the future.
Claims that the Company’s current or future products infringe or misappropriate the proprietary rights of others could adversely affect the Company’s ability to sell those products and cause it to incur additional costs.
If any third-party intellectual property claim against the Company is successful, the Company could be prevented from commercializing EndoBarrier or other Company products.
There are numerous issued patents and pending patent applications in the U.S. and internationally that are owned by third parties and that contain patent claims in areas that are the focus of the Company’s product development efforts. GI Dynamics is aware of patents owned by third parties, to which it does not have licenses, that relate to, among other things, liner materials and anchoring. The Company has also employed individuals who were previously employed at other medical device companies, including competitors or potential competitors, which may result in claims from third parties that the Company has inadvertently or otherwise used or disclosed alleged trade secrets or other proprietary information of former employers of its employees.
In addition, because patent applications can take many years to issue, there may be currently pending applications, unknown to the Company, which may later result in issued patents that pose a material risk to the Company.
20
GI Dynamics expects that it could be subject to third-party infringement claims if the number of competitors grows and the functionality of products and technology in different industry segments overlap. Third parties may currently have, or may eventually be issued, patents on which its current or future technologies may infringe.
If GI Dynamics is unable to obtain, maintain and enforce intellectual property protection covering its products, others may be able to make, use, or sell products substantially the same as the Company’s, which could adversely affect the Company’s ability to compete in the market.
The Company’s commercial success is dependent in part on obtaining, maintaining and enforcing intellectual property rights, including patents, covering EndoBarrier and the Company’s future products. If GI Dynamics is unable to obtain, maintain and enforce intellectual property protection covering its products, others may be able to make, use or sell products that are substantially the same as the Company’s without incurring the sizeable development costs that the Company has incurred, which would adversely affect the Company’s ability to compete in the market. Certain patents covering EndoBarrier will begin to expire in 2023.
Even if GI Dynamics patents are determined by a court to be valid and enforceable, they may not be sufficiently broad or enforceable to prevent others from marketing products similar to the Company’s or designing around the Company’s patents, despite its patent rights. GI Dynamics products may also not have freedom to operate unimpeded by the patent rights of others.
In addition to patented technology, the Company relies on a combination of non-patented proprietary technology, trade secrets, processes, procedures, technical knowledge and know-how accumulated or acquired since inception. Any of the Company’s intellectual property rights could be challenged, invalidated, circumvented or misappropriated. In order to preserve and enforce the Company’s patent and other intellectual property rights, GI Dynamics may need to make claims or file lawsuits against third parties. This can entail significant costs and divert management’s attention from developing and commercializing EndoBarrier.
GI Dynamics relies on suppliers for certain key components in the manufacture of the EndoBarrier system. If these suppliers were to become unavailable to the Company, there could be delays in the commercialization of the Company’s products.
GI Dynamics relies on suppliers for several key components of EndoBarrier, in particular the material used to manufacture the sleeve used in EndoBarrier. Except as discussed below, the Company or its manufacturing partner, PPMD, have supply agreements in place with suppliers of all components and services required to manufacture the EndoBarrier System. While GI Dynamics currently has adequate material required to make the sleeve to support the Company well into commercialization efforts, the Company does not presently have an active supply agreement with a manufacturer for additional material. The reliance on third-party suppliers subjects the Company to risks that could harm its business, particularly with respect to the supply of key components or processes. Although the Company believes that alternative suppliers are available, the process of identifying and qualifying new suppliers who can produce the components to the Company’s specifications could cause delays in the commercialization of the Company products.
The use, misuse or off-label use of the Company’s products by physicians may harm the Company’s image in the marketplace or result in injuries that lead to product liability.
GI Dynamics cannot prevent a physician from using EndoBarrier for purposes outside of its eventual approved and intended use, which is known as off-label use. If physicians attempt to use the Company’s products off-label, there may be an increased risk of adverse events. Further, the use of the Company’s products for uses other than those uses for which the products have been approved may not effectively treat such conditions, which could harm the Company’s reputation in the marketplace among physicians and patients.
Physicians may also misuse the Company’s products or use improper techniques if they are not adequately trained, potentially leading to injury and an increased risk of product liability for the Company. Physicians may also treat patients from other countries where the product is not approved, and the physician is then unable to properly monitor the patient’s progress. If GI Dynamics is deemed to have engaged in the promotion of any of its products for off-label use, the Company could be subject to action by regulatory authorities and the imposition of sanctions, which could also affect the Company’s reputation and position within the industry.
21
Patients may not follow instructions from their physicians. Patients who ignore training and ignore their clinician’s request to comply with dietary instructions or pharmacotherapy label instructions, or to remove EndoBarrier at the end of the 12-month indication for treatment may be exposed to greater physical and clinical risk.
Product liability claims could damage the Company’s reputation or adversely affect its business or financial position.
GI Dynamics may be exposed to the risk of product liability claims, which are inherent in the design, manufacturing, marketing and use of medical devices and, in particular, implantable medical devices. The Company holds product liability insurance; however, adequate product liability insurance may not continue to be available on commercially acceptable terms. Product liability claims may damage the Company’s reputation and, if the Company’s insurance coverage proves inadequate, may harm or destroy the Company’s business. Defending a suit, regardless of its merits, could be costly and could divert Company management’s attention from the Company’s core business activities.
GI Dynamics will rely on a third-party manufacturer for its products and this manufacturer is required to comply with regulatory requirements. Failure of the Company’s manufacturer or suppliers to comply with regulatory requirements could disrupt the manufacturing process, which would, in turn, have a material adverse effect on the Company’s business and results of operations.
Future clinical trials and commercialization of GI Dynamics products requires access to manufacturing facilities that meet applicable regulatory standards to manufacture a sufficient supply of the Company’s products. GI Dynamics will rely on a third-party manufacturer to meet the applicable regulatory requirements. Suppliers of components and products used to manufacture the Company’s products and the Company’s third-party manufacturer must also comply with applicable regulatory requirements. These often require significant time, resources, record-keeping and quality assurance efforts and subject the Company, its suppliers and third-party manufacturer to potential regulatory inspections and stoppages. Compliance with applicable regulatory requirements is subject to continual review and is rigorously monitored by regulatory authorities. Failure by GI Dynamics, its third-party manufacturer or suppliers to comply with regulatory requirements or failure to take satisfactory corrective action in response to an adverse inspection could result in enforcement actions that could have a material adverse effect on the Company’s business and results of operations.
In order to manufacture EndoBarrier in the quantities that GI Dynamics anticipates will be required to meet its clinical trial needs and potential future market demand, the Company will need to increase production capacity and efficiency over current levels, and the Company’s third-party manufacturer must therefore be able to provide the Company with sufficient quantities of its product in compliance with regulatory requirements, in accordance with agreed upon specifications, at acceptable cost and on a timely basis. The Company’s potential future growth could strain the ability of its third-party manufacturer to deliver the Company’s product and obtain materials and components in sufficient quantities. Third-party manufacturers often experience difficulties in scaling up production, including problems with production yields and quality control and assurance. If the Company is unable to obtain a sufficient or consistent supply of EndoBarrier or any other product it is developing, or if it cannot do so efficiently, the Company’s revenue, business and financial prospects would be adversely affected.
The Company announced on July 26, 2017 that it executed an agreement with PPMD to manufacture the Company’s product. GI Dynamics does not have extensive experience with this manufacturer, and the Company cannot guarantee that PPMD will manufacture its product in a validated manner or to acceptable levels of quality. The Company is seeking other suppliers who will be able to manufacture the Company’s product should the relationship with PPMD not prove successful.
GI Dynamics has been focused on efficiently running the Company’s operations in order to conserve resources which may impact its ability to retain its remaining employees and therefore the Company may not be able to sustain its business.
As of March 12, 2020, GI Dynamics has 15 full time employees. Since the Company’s initial restructuring efforts in 2015, the Company has been heavily dependent upon its ability to retain its remaining employees. The loss of service of any of the Company’s remaining employees, particularly members of the management team, may have an adverse effect on the Company’s business. Given the long period of reduced staff, the morale of the Company’s remaining employees may be lower, employees may be distracted and any one of the remaining employees could terminate his or her employment with the Company at any time. A departing employee’s expertise would be difficult to replace and the failure to do so on a timely basis could have a material adverse effect on the Company’s ability to achieve its business goals. There can be no assurance that the Company will have the financial resources to, or otherwise be successful in, retaining the Company’s remaining personnel and failure to do so could have a material adverse effect on the Company’s business, financial condition and results of operations.
22
If GI Dynamics is unable to retain or hire key personnel, it may not be able to sustain or grow its business.
The Company’s ability to operate successfully and manage potential future growth depends significantly upon the ability to attract, retain and motivate highly skilled and qualified research, technical, clinical, regulatory, sales, marketing, managerial and financial personnel. The competition for qualified employees in the medical device industry is intense and there are a limited number of persons with the necessary skills and experience.
The Company’s performance is substantially dependent on senior management and key technical staff to continue to develop and manage the Company operations. The loss or the inability to recruit and retain high-caliber staff could have a material adverse effect on the Company. GI Dynamics also relies on the technical and management abilities of certain key directors, key members of the executive team and employees, consultants and scientific advisers. The loss of any of these directors, members of the executive team, employees, consultants or scientific advisers could have an adverse effect on the Company’s business. On April 13, 2018 the Company terminated the employment of its Chief Compliance Officer and Vice President, Finance and Accounting for cost-management purposes. The Company hired a Chief Financial Officer in December 2018 and a Vice President of Clinical and Regulatory Affairs in January 2019.
GI Dynamics may be unable to effectively manage its anticipated growth.
To manage the Company’s anticipated growth and to commercialize its products, GI Dynamics will need to expand operations (research and development, product development, quality, regulatory, manufacturing, sales, marketing and administrative). This expansion will place a significant strain on management, infrastructure and operational and financial resources, particularly in light of the restructuring undertaken in the last few years. Specifically, the Company will need to manage relationships with various persons and entities participating in the Company’s clinical trials and quality systems, manufacturers, suppliers and other organizations, including various regulatory bodies in the U.S. and other jurisdictions. GI Dynamics may not be able to implement the required improvements in an efficient and timely manner and may discover deficiencies in existing systems and controls. The failure to accomplish any of these tasks could materially harm the Company’s business and its ability to commercialize EndoBarrier. As a result, the Company’s revenue, business and financial prospects would be adversely affected.
While GI Dynamics remains listed on the ASX, it will incur increased costs because the Company is a public company in the U.S. whose equity securities are listed on the ASX.
As of December 31, 2013, GI Dynamics became subject to the periodic reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Although the existing listing of the Company’s CDIs on the ASX requires the Company to file financial information and make certain other filings with the ASX, the Company’s status as a reporting company under the Exchange Act causes the Company to incur additional legal, accounting and other expenses, including costs related to compliance with the requirements of the Sarbanes-Oxley Act of 2002, insurance costs related to being an Exchange Act reporting company and other administrative costs. GI Dynamics also expects such rules and regulations may make it more difficult and more expensive for the Company to obtain director and officer liability insurance and the Company may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for the Company to attract and retain qualified individuals to serve on its Board of Directors or as executive officers. GI Dynamics cannot predict or estimate the amount of additional costs it may incur or the timing of such costs.
The Company’s shares of its common stock are publicly traded on the ASX in the form of CDIs. As a result, the Company must comply with the ASX Listing Rules. GI Dynamics has policies and procedures that the Company believes are designed to provide reasonable assurance of compliance with the ASX Listing Rules. If, however, the Company does not follow those procedures and policies, or they are not sufficient to prevent non-compliance, GI Dynamics could be subject to liability, fines and lawsuits. These laws, regulations and standards are subject to varying interpretations and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. The Company intends to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from revenue-generating activities to compliance activities. If, notwithstanding the Company’s efforts to comply with new laws, regulations and standards, it fails to comply, regulatory authorities may initiate legal proceedings against the Company and its business may be harmed.
23
If the Company fails to maintain proper and effective internal controls over financial reporting, its ability to produce accurate and timely financial statements could be impaired, which could harm the Company’s operating results, and investors could lose confidence in the Company’s financial reports, which could adversely affect the value of the Company’s CDIs and common stock.
As a public company in the U.S., GI Dynamics is required, pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, to furnish a report by management on, among other things, the effectiveness of its internal control over financial reporting as of the end of the fiscal year. The controls and other procedures are designed to ensure that information required to be disclosed by the Company in the reports that the Company files with the SEC is disclosed accurately and is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms. The Company’s first report on compliance with Section 404 was furnished in connection with the GI Dynamics financial statements for the year ended December 31, 2014. As a former emerging growth company and a current smaller reporting company, the Company has been and currently is exempted from the requirement to include an external auditor opinion on the Company’s internal controls in its filings. GI Dynamics continues to update, document and evaluate its internal control over financial reporting to the requirements of Section 404.
In Item 9A of this report, the Company discloses that with respect to the standards of Section 404, the Company concluded that its internal control over financial reporting was effective as of December 31, 2019. For additional information on this item, please see Item 9A. Controls and Procedures.
Although we concluded that our internal control over financial reporting was effective as of December 31, 2019, the Company has identified and reported material weaknesses in prior periods, and the Company cannot be certain that its internal control practices will ensure that the Company will have or maintain adequate internal control over financial reporting in future periods. Any failure to have or maintain such internal control could harm the Company’s operating results, and investors could lose confidence in the Company’s financial reports, which could adversely affect the value of the Company’s CDIs and common stock.
Fluctuations in foreign currency exchange rates could adversely affect the Company’s financial results.
In the past, when GI Dynamics sold EndoBarrier outside of the U.S., the Company’s activities produced revenues and GI Dynamics incurred expenses in a variety of different currencies, which exposed the Company to exchange rate risk. If GI Dynamics is successful in once again obtaining CE Mark designation or other foreign regulatory approvals that will allow it to sell EndoBarrier outside of the U.S., the Company will again be exposed to exchange rate risk. Exchange rate risk may affect the Company’s financial performance and position. Furthermore, some of the Company’s funds may be held in euros, Australian dollars or other currencies, while the Company’s functional currency is U.S. dollars. GI Dynamics is not currently hedging against exchange rate fluctuations, and consequently will be at the risk of any adverse movement in the U.S. dollar exchange rate if the Company exchanges funds held in one currency into another currency.
The Company’s shares of common stock, in the form of CDIs, are listed on the ASX and priced in Australian dollars. However, the Company’s reporting currency is U.S. dollars. As a result, movements in foreign exchange rates may cause the price of the Company’s securities to fluctuate for reasons unrelated to the Company’s financial condition or performance and may result in a discrepancy between the actual results of operations and investors’ expectations of returns on the Company’s securities expressed in Australian dollars.
24
Risks Related to the Medical Device Industry
The medical device industry is subject to rapid technology change, which may result in obsolescence of the Company’s products.
The medical device industry is subject to rapid technology change. For the Company to remain competitive and to retain and build market share, it must continually develop new products as well as improve its existing ones. Accordingly, The Company must devote substantial resources to research, development and commercialization activities.
There can be no assurance that GI Dynamics will be successful in developing competitive new products and/or improving existing products so that such products remain competitive and avoid obsolescence. There can also be no assurance that any or all Company research and development projects for new products will demonstrate safety and efficacy and result in commercial products, or that if such products are successfully designed and launched, they will be profitable.
Health care reform legislation could adversely affect the Company’s future revenue and financial condition.
In recent years, there have been numerous initiatives by governments throughout the world for comprehensive reforms affecting the delivery of and payment for health care. GI Dynamics cannot predict the changes that will be made and the effect such changes will have on the use of EndoBarrier. Decisions to increase or decrease reimbursement or coverage for treatments for type 2 diabetes and/or obesity could have a material impact on the Company’s business and results of operations.
CE Mark designation requires ongoing compliance, reporting and testing requirements established by regulatory agencies. While the Company has adopted comprehensive compliance programs to attempt to comply with these regulations, it is possible that some of its business activities could be subject to challenge. If the Company’s past or present operations, or those of its former independent sales agents and distributors, are found to be in violation of any of such applicable governmental regulations, GI Dynamics may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from health care reimbursement, product recall, or retraction of approval to market the Company’s product.
New legislation in the U.S. has also been enacted that imposes additional reporting requirements and penalties on the medical device industry. While GI Dynamics has adopted comprehensive compliance programs to attempt to comply with these regulations, it is possible that some of the Company’s business activities could be subject to challenge under one or more of such laws. If the Company’s past or present operations, or those of its former independent sales agents and distributors, are found to be in violation of any of such laws or any other applicable governmental regulations, GI Dynamics may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from health care reimbursement, product recall, or retraction of approval to market the Company’s product.
25
Subsequent to future regulatory approvals for EndoBarrier, pricing pressures in the health care industry could lead to demands for price concessions, which could have an adverse effect on the Company’s business, financial condition or results of operations.
Due to the significant rise in health care costs over the past decade, numerous initiatives and reforms initiated by governments and third-party payers to curb these costs have resulted in difficulties in maintaining or raising the number and price of procedures. The increase in pricing pressure is driven by the competitive environment in the medical device industry as many larger companies cut prices as they struggle to retain market share.
The type 2 diabetes and obesity market has seen increasing resistance from payers regarding local and national reimbursement coverage. GI Dynamics expects that market demand, government regulation, third-party reimbursement policies and societal pressures could exert downward pressure on the prices of the Company’s products in the future after regulatory approval is granted and may adversely impact the Company’s business, financial condition or results of operations on a country by country basis.
Manufacturing facilities for medical devices must comply with applicable regulatory requirements.
Completion of the Company’s current and future clinical trials and commercialization of its products requires access to manufacturing facilities that meet applicable regulatory standards to manufacture an adequate supply of the Company’s products. The third-party manufacturer and suppliers of components and parts that the Company intends to use to manufacture its products must also comply with applicable regulatory requirements, which often require significant time, money, resources and record-keeping and quality assurance efforts and subject the Company, its third-party manufacturer and its suppliers to potential regulatory inspections and stoppages.
Compliance with applicable regulatory requirements is subject to continual review and is rigorously monitored through periodic inspections by regulatory authorities. Failure by the Company, its third-party manufacturer or its suppliers to comply with regulatory requirements or failure to take satisfactory corrective action in response to an adverse inspection could result in enforcement actions, including a public warning letter, a shutdown of, or restrictions on, the Company’s ability to obtain sufficient quantities of its products, delays in approving or clearing a product, refusal to permit the import or export of its products or other enforcement action.
Critical elements of the manufacturing process may be found to be hazardous and alternative technologies may cost more and/or may be less effective.
Numerous materials are used in the process of manufacturing a patient-ready device. As chemical testing and empirical data collection continues, certain chemicals or processes may be declared hazardous and while suitable alternatives will be sought, there may be a period of short or no availability before a replacement chemical or process is adopted. For example, a widespread process used in medical device manufacture is the use of ethylene oxide (“EtO”) for low temperature gas sterilization. In early 2019, a large EtO sterilization plant was closed by state-level authorities over health concerns. The medical device industry, the contract manufacturing industry, state authorities, federal authorities, and scientists are seeking a suitable alternative. Until such a suitable replacement is found, there is a risk that there will be a shortage of sterilization options, which may require design changes, process changes or which may cause a delay in device production.
Inadequate funding for the FDA, the SEC and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent EndoBarrier from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of the Company’s business rely, which could negatively impact the Company’s business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of the SEC and other government agencies on which the Company’s operations may rely, including those that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
26
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs and medical devices to be reviewed and/or approved by necessary government agencies, which would adversely affect the Company’s business. For example, over the last several years, including beginning on December 22, 2018, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC and other government employees and stop critical activities. If a prolonged government shutdown occurs, it could significantly affect the ability of the FDA to timely review and process the Company’s regulatory submissions, which could have a material adverse effect on the Company’s business. Further, in the Company’s operations as a public company, future government shutdowns could impact the ability to access the public markets and obtain necessary capital in order to properly capitalize and continue operations.
Risks Related to GI Dynamics CDIs and common stock
There is no current trading market for GI Dynamics common stock in the U.S. and no such market may develop.
Although the Company’s CDIs are currently listed on the ASX in Australia, there is not any current trading market for the Company’s CDIs or the underlying shares of common stock in the U.S. Should at some stage in the future, GI Dynamics submits a listing application with Nasdaq (or any other exchange) to list its common stock on the Nasdaq Capital Market (or any other exchange), Nasdaq may not approve the listing application. Even if Nasdaq (or such other exchange) approves the Company’s listing application, an active trading market for the Company’s common stock may never develop in the U.S. (or other relevant country) and stockholders may not be able to transfer or resell their common stock at its fair value, or at all.
GI Dynamics is eligible to be treated as an “Smaller Reporting Company” as defined in the amended regulation S-K, section 210, and the Company cannot be certain if the reduced disclosure requirements applicable to Smaller Reporting Companies will make its common stock and CDIs less attractive to investors.
The Securities and Exchange Commission amended regulation S-K, section 210 in September 2018 to reduce the scale of reporting for Smaller Reporting Companies, keeping many of the scaled reporting requirements from the Emerging Growth Company requirements under the JOBS act. Smaller Reporting Companies are defined based on public capital and revenue limits and GI Dynamics qualifies as a Smaller Reporting Company for the period ended December 31, 2019. As a result, the Company’s reporting is compliant with the Smaller Reporting Company requirements. GI Dynamics cannot predict if investors will find its common stock or CDIs less attractive because it relies on these reporting exemptions. If some investors find the Company’s common stock or CDIs less attractive as a result, there may be a less active trading market for the Company’s common stock or CDIs and the Company’s stock price may be more volatile.
Changes in economic conditions may adversely affect the Company’s business.
Changes in the general economic climate in which the Company operates may adversely affect its financial performance and the value of its assets. Factors that contribute to that general economic climate include:
| ● | contractions in the world economy or increases in the rate of inflation; | |
| ● | international currency fluctuations; | |
| ● | changes in interest rates; | |
| ● | new or increased government taxes or duties or changes in taxation laws; or | |
| ● | changes in government regulatory policy. |
27
Stock market fluctuations may adversely affect the price of the Company’s CDIs and common stock.
There are a number of risks associated with any stock market investment. GI Dynamics CDIs have been traded on the ASX since September 7, 2011. The price of GI Dynamics CDIs has been, and is likely to continue to be, volatile, which means that the value could decline substantially within a short period of time. The value of the CDIs will be determined by the stock market and will be subject to a range of factors beyond the Company’s control. These factors include movements in local and international stock exchanges, local interest rates and exchange rates, domestic and international economic and political conditions, government taxation, market supply, competition and demand and other legal, regulatory or policy changes.
The trading volume of GI Dynamics CDIs is low, which may result in reduced liquidity for GI Dynamics stockholders.
GI Dynamics CDIs are only listed on the ASX and are not listed for trading on any other securities exchanges in Australia, the U.S. or elsewhere. As such, there can be no guarantee that an active market in the CDIs will develop or continue, or that the market price of the CDIs will increase. If a market does not develop or is not sustained, it may be difficult for investors to sell their CDIs. Furthermore, the market price for the CDIs may fall or be made more volatile because of the relatively low volume of trading in GI Dynamics securities. When trading volume is low, significant price movement can be caused by trading in a relatively small number of shares.
Sales of a substantial number of CDIs, or the perception that these sales may occur, could cause the market price of GI Dynamics CDIs to decline. Sales by current stockholders of a substantial number of CDIs, or the expectation that such sales may occur, could significantly reduce the market price of GI Dynamics CDIs. The Company may also offer additional CDIs in subsequent offerings, which may adversely affect the market price for the CDIs.
Some current stockholders can exert control over the Company and may not make decisions that are in the best interests of all stockholders.
As of December 31, 2019, two stockholders and their affiliated entities owned approximately 84% of the Company’s outstanding shares of common stock, or CDIs representing common stock, in the aggregate. One stockholder would be able to exert a significant degree of influence over the Company’s management of its affairs and over matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. The second largest stockholder, on matters which the largest stockholder were ineligible to vote, would be able to exert a significant degree of influence over the Company’s management of its affairs and over matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This concentration of ownership may harm the market price of the CDIs and the Company’s common stock by delaying or preventing a change in control, even if a change is in the best interests of other stockholders. This concentration of ownership may also delay, deter or prevent acts that may be favored by other stockholders, as the interests of these stockholders may not always coincide with the interests of other stockholders. In addition, this concentration of share ownership may adversely affect the trading price of the Company’s shares because it may limit the trading volume and purchase demand for outstanding shares, could adversely affect the Company’s stock price should any of these stockholders elect to sell some or all of their shares, and investors may perceive disadvantages in owning shares in a company with significant stockholders.
Provisions of the Company’s certificate of incorporation, the Company’s bylaws and Delaware law could make an acquisition of the Company more difficult.
Certain provisions of the Company’s certificate of incorporation and bylaws could discourage, delay or prevent a merger, acquisition or other change of control that the Company’s stockholders may consider favorable, including transactions in which stockholders might otherwise receive a premium for their CDIs or common stock. These provisions could also limit the price that investors might be willing to pay in the future for the CDIs or common stock, thereby depressing the market price of these securities. Stockholders who wish to participate in these transactions may not have the opportunity to do so. In addition, GI Dynamics is incorporated in the State of Delaware and, as such, is governed by the provisions of Section 203 of the Delaware General Corporation Law, which may, unless certain criteria are met, prohibit large stockholders, in particular those owning 15% or more of the voting rights on GI Dynamics common stock, from merging or combining with the Company for a prescribed period of time.
28
GI Dynamics does not intend to pay cash dividends on its common stock in the foreseeable future.
GI Dynamics has never declared or paid any cash dividends on its shares of common stock, and it currently does not anticipate paying any cash dividends in the foreseeable future. The Company intends to retain any earnings to finance the development and expansion of the Company’s products and business. Accordingly, the Company’s stockholders will not realize a return on their investment unless the trading price of GI Dynamics CDIs or common stock appreciates.
The Company’s ability to use its net operating loss carryforwards and certain other tax attributes may be limited.
The Company’s ability to utilize its federal net operating losses and federal tax credits may be limited under Sections 382 and 383 of the Internal Revenue Code. The limitations apply if an ownership change, as defined by Section 382, occurs. Generally, an ownership change occurs when certain stockholders increase their aggregated ownership by more than 50 percentage points over their lowest ownership percentage in a testing period (typically three years). The Company may already be subject to Section 382 limitations due to previous ownership changes. In addition, future changes in stock ownership may also trigger an ownership change and, consequently, a Section 382 limitation. Due to the significant complexity and cost associated with a change in control study, and the expectation of continuing to incur losses whereby the net operating losses and federal tax credits are not anticipated to be used in the foreseeable future, the Company has not assessed whether there have been changes in control since its formation. If GI Dynamics has experienced changes in control at any time since its formation, utilization of its net operating losses and research and development credit carryforwards would be subject to annual limitations under Sections 382 and 383, respectively. Any limitation may result in expiration of a portion of the net operating loss or research and development credit carryforwards before utilization, which would reduce the Company’s gross deferred tax assets and corresponding valuation allowance. As a result, if GI Dynamics earns net taxable income, its ability to use the pre-change net operating loss carryforwards to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to the Company.
Item 1B. UNRESOLVED STAFF COMMENTS
None.
In June 2016, GI Dynamics entered into a non-cancelable agreement to lease approximately 4,200 square feet of office and laboratory space in Boston, Massachusetts. The lease commenced in June 2016 and expired in April 2018.
In December 2018, GI Dynamics entered into a membership agreement with WeWork for 985 square feet of office space located in Boston, Massachusetts. The committed lease term expired in May 2019.
On April 22, 2019, GI Dynamics entered into a lease for 3,520 square feet of office space in Boston, Massachusetts. The lease period commenced on May 1, 2019 and expires in May 2022.
GI Dynamics is currently not involved in any litigation that it believes could have a materially adverse effect on its financial condition or results of operations. There is no action, suit, proceeding, inquiry or investigation before or by any court, public board, government agency, self-regulatory organization or body pending or, to the knowledge of the executive officers of the Company or any of its subsidiaries, threatened against or affecting the Company, its common stock, any of its subsidiaries or of the Company’s or its subsidiaries’ officers or directors in their capacities as such, in which an adverse decision could have a material adverse effect. From time to time, the Company may become involved legal proceedings, lawsuits, claims and regulations in the ordinary course of its business.
ITEM 4. MINE SAFETY DISCLOSURES
Not Applicable.
29
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
GI Dynamics CDIs, each representing one-fiftieth of one share of GI Dynamics common stock, have been listed on the ASX under the trading symbol “GID” since September 7, 2011. Prior to such time there was no public market for the Company’s securities.
On December 31, 2019, the last reported sale price of GI Dynamics CDIs was A$0.017 per CDI, or $0.60 per share of common stock.
As of December 31, 2019, 3,331,154 of GI Dynamics shares of common stock were subject to outstanding options, restricted stock units and warrants to purchase shares of common stock.
Holders
At March 12, 2020, the Company had 36,598,291 shares of common stock issued and outstanding with 19 holders of record. The holders included CHESS Depositary Nominees Pty Limited, or CDN, which held 36,524,665 shares of GI Dynamics common stock on behalf of the CDI holders; there were approximately 824 registered owners of GI Dynamics CDIs at March 12, 2020.
The Company’s transfer agent and registrar for its common stock is American Stock Transfer & Trust Company, LLC, 6201 15th Avenue, Brooklyn, New York 11219. The Company’s registrar for its CDIs is Link Market Services Limited, Level 12, 680 George Street, Sydney NSW 2000.
Equity Compensation Plan Information
The information required to be disclosed by Item 201(d) of Regulation S-K, “Securities Authorized for Issuance Under Equity Compensation Plans” is referenced under Item 12 of Part III of this Annual Report on Form 10-K.
ITEM 6. SELECTED CONSOLIDATED FINANCIAL DATA
Not applicable.
30
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of the Company’s financial condition and results of operations should be read in conjunction with the Company’s consolidated financial statements and related notes to those financial statements appearing elsewhere in this Annual Report on Form 10-K. This discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. You should review the “Risk Factors” section of this Annual Report on Form 10-K for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements described in the following discussion and analysis.
Overview
GI Dynamics is a clinical stage medical device company located in Boston, Massachusetts. The Company has developed EndoBarrier, a medical device intended to treat patients with type 2 diabetes and to reduce obesity. GI Dynamics is taking the steps necessary to obtain the regulatory approvals required to market this product. In order to market EndoBarrier in the U.S., GI Dynamics must obtain approval from the FDA. In order to market EndoBarrier outside of the U.S., GI Dynamics is required to comply with various regulations imposed by the countries in which it seeks to sell the product.
In 2010, EndoBarrier received CE Marking for sale in the European Union and in 2011, EndoBarrier was listed on the Australian Register of Therapeutic Goods. As a result, during 2013 and 2014, the Company received approximately $2.8 million and $2.3 million, respectively, in revenue from the sale of EndoBarrier in Europe, South America and the Asia Pacific region. In the U.S. in 2013, the Company began enrollment of patients in the initial pivotal trial of EndoBarrier, which is referred to as the ENDO trial.
In the third quarter of 2015, the Company announced its decision to discontinue the ENDO trial because patients were experiencing a higher than previously observed level of hepatic (liver) abscesses. In the fourth quarter of 2016, GI Dynamics received formal notification from the TGA of the Australian government of the cancellation of EndoBarrier’s inclusion on the ARTG. In the fourth quarter of 2017 the Company received formal notification of CE mark withdrawal from its notified body in Europe, preventing the sale of EndoBarrier in Europe and select Middle Eastern countries. The Company undertook comprehensive cost-cutting measures throughout 2015 and 2016, including significantly reducing the number of its employees.
Following the decision to discontinue the ENDO trial, GI Dynamics undertook significant investigational and scientific analyses with the goal of reducing the incidence rate and severity of hepatic abscess concurrent with the EndoBarrier treatment. This investigational work focused on understanding the root cause of hepatic abscess and how to reduce the rate of occurrence. This included: DNA analysis of normal EndoBarrier removals as well as hepatic abscess EndoBarrier removals, numerous meta-analyses and responder cohort analyses, investigation into the contributing factors represented by proton pump inhibitors (PPIs), leaky gut syndrome and microbiome analyses, among other research. This allowed the Company to modify the medications utilized with EndoBarrier, most notably discontinuation of chronic double-dose PPI usage during EndoBarrier implant.
As a result of the efforts described above, in August 2018, GI Dynamics received an IDE from the FDA to begin enrollment in a pivotal trial evaluating the safety and efficacy of EndoBarrier in the United States pending IRB approval, which was received on February 13, 2019. In this report, the Company refers to this pivotal trial as the GI Dynamics STEP-1 clinical trial. On January 27, 2020, the first patient was randomized into the STEP-1 Trial Protocol.
For financial reporting purposes, the Company have one reportable segment, which designs, manufactures and plans to market EndoBarrier.
To date, GI Dynamics has devoted substantially all of its efforts to research and development, business planning, clinical research, clinical study management, reimbursement development, product commercialization, acquiring operating assets and raising capital. GI Dynamics has incurred significant operating losses since its inception in 2003. As of December 31, 2019, the Company had an accumulated deficit of approximately $284 million. The Company expects to incur net losses for the next several years while it continues to evaluate which markets are appropriate to continue pursuing reimbursement, market awareness and general market development efforts, and continues to restructure its business and costs, establish new priorities, continue limited research, and evaluate strategic options.
31
GI Dynamics has raised net proceeds of approximately $273 million through sales of the Company’s equity and placement of debt, of which $9.3 million was raised through the Company’s 2019 financings.
In February and March 2018, GI Dynamics raised approximately $1.6 million in an offering of the Company’s CDIs to qualified investors, including certain existing investors, in Australia, the United States and the United Kingdom.
In May 2018, GI Dynamics placed a Convertible Term Promissory Note and Warrant (the “2018 Note and Warrant”) with Crystal Amber for a note principal amount of $1.75 million. The 2018 Note accrued interest at 10% per annum, compounded annually, and could be converted to CDIs at $0.018 per CDI until maturity on May 30, 2023. The warrants conferred the right to purchase 97,222,200 CDIs for $0.018 per CDI with anti-dilution protections.
In August 2018, GI Dynamics received notification from the FDA that it had approved the IDE application for EndoBarrier, pending IRB approval, which was received in February 2019.
In September and November 2018, GI Dynamics raised approximately $5 million in an offering of the Company’s CDIs to qualified investors, including certain existing investors, in Australia, the United States and the United Kingdom.
In October 2018, GI Dynamics announced that it signed an agreement with its new notified body Intertek to pursue CE marking of EndoBarrier in Europe. The Company has since suspended its relationship with Intertek and is working with a different notified body whose identity will be announced when the service agreement is finalized.
In November 2018, the Company signed a clinical trial agreement and a memorandum of understanding with its clinical and potential commercial partner Apollo Sugar, a division of Apollo Hospitals Group located in India.
In December 2018, GI Dynamics announced the appointment of Charles Carter, a consultant, as the Company’s Chief Financial Officer and Secretary. Mr. Carter became an employee of the Company on September 1, 2019.
In March 2019, the Company completed a convertible term promissory note (the “March 2019 Note”) and warrant (the “March 2019 Warrant”) financing with Crystal Amber for a gross amount of $1 million. The March 2019 Note compounded interest annually at 10% and, subject to Stockholder Approval required under ASX Listing Rules, Crystal Amber was conferred the right to optionally convert all unpaid principal and interest to CDIs at $0.0127 per CDI before the Note Maturity date in March 2024. Per the March 2019 NPA, the Company agreed to issue a warrant (the “March 2019 Warrant”) to Crystal Amber, pending Stockholder Approval, to purchase 78,984,823 CDIs (representing 1,579,696 shares of common stock) at an initial exercise price of $0.0127 per CDI. The issuance of the March 2019 Warrant required the approval of stockholders and was not exercisable until it was approved on June 30, 2019. (See Note 9 of the Consolidated Financial Statements for a more complete description of the terms and conditions of the financing).
In March 2019, the maturity date of the 2017 Note was extended to May 1, 2019. In April 2019, the maturity date of the 2017 Note was extended to July 1, 2019. Further extensions of the maturity date of the 2017 Note are described below.
In May 2019, the Company completed a convertible term promissory note (the “May 2019 Note”) and warrant (the “May 2019 Warrant”) financing with Crystal Amber for a gross amount of $3 million. The May 2019 Note compounded interest annually at 10% and, subject to Stockholder Approval required under ASX Listing Rules, Crystal Amber was conferred the right to optionally convert all unpaid principal and interest to CDIs at $0.0127 per CDI before the Note Maturity date in May 2024. Per the May 2019 NPA, the Company agreed to issue a warrant (the “May 2019 Warrant”) to Crystal Amber, pending Stockholder Approval, to purchase 236,220,472 CDIs (representing 4,724,409 shares of common stock) at an initial exercise price of $0.0127 per CDI. The issuance of the May 2019 Warrant required the approval of stockholders and was not exercisable until it was approved on June 30, 2019. (See Note 9 of the Consolidated Financial Statements for a more complete description of the terms and conditions of the financing).
In June 2019, the maturity date of the 2017 Note was extended to October 1, 2019.
On June 30, 2019, Crystal Amber elected to convert the 2018 Note, the March 2019 Note and the May 2019 Note into CDIs. Under the terms of the respective notes, an aggregate of 453,609,963 CDIs (representing approximately 9,072,197 shares of common stock) were subscribed but unissued on conversion and concurrent cancellation of the 2018 Note, the March 2019 Note and the May 2019 Note. The CDIs were issued on July 3, 2019.
32
On August 21, 2019, the Company and Crystal Amber entered into a securities purchase agreement for a total funding of up to approximately $10 million (the “August 2019 SPA”). The initial $5.4 million was comprised of existing warrant exercises scheduled between August 25, 2019 and November 15, 2019. Exercises included the 2018 Warrant, the March 2019 Warrant, and the May 2019 Warrant issued to Crystal Amber, as further detailed above. The remaining amount of the August 2019 funding was represented by a Convertible Term Promissory Note (“August 2019 Note”) of up to approximately $4.6 million and a related Warrant (“August 2019 Warrant”) in substantially the same form as the March 2019 and May 2019 convertible term promissory note. Under the terms of the August 2019 SPA and August 2019 Note, the Company, at its sole discretion, could elect to request any of the $4.6 million to be funded at any date on or before December 6, 2019. The conversion feature allows the conversion of the August 2019 Note’s unpaid principal and interest at $0.02 per CDI and the August 2019 Warrant, upon its issue, allowed the purchase for $0.02 per CDI a number of CDIs represented by the August 2019 Note principal divided by $0.02 per CDI. (See Note 9 of the Consolidated Financial Statements for a more complete description of the terms and conditions of the financing).
On August 21, 2019, the maturity date of the 2017 Note was extended to March 31, 2020.
On August 25, 2019, Crystal Amber exercised the 2018 Warrant and a portion of the March 2019 Warrant in accordance with the terms of the August 2019 SPA. For an aggregate cash payment of $2 million, 97,222,200 CDIs (representing approximately 1,944,444 shares of common stock) were issued at $0.0144 per CDI under the 2018 Warrant and 47,244,119 CDIs (representing approximately 944,882 shares of common stock) were issued at $0.0127 per CDI under the March 2019 Warrant.
On September 30, 2019, Crystal Amber exercised the remaining portion of the March 2019 Warrant and a portion of the May 2019 Warrant in accordance with the August 2019 SPA. For an aggregate cash payment of $2 million, 31,740,704 CDIs (representing approximately 634,814 shares of common stock) were issued at $0.0127 per CDI under the March 2019 Warrant and 125,739,610 CDIs (representing approximately 2,514,792 shares of common stock) were issued on October 4, 2019 at $0.0127 per CDI under the May 2019 Warrant. As of September 30, 2019, this was recorded as a subscription receivable from a related party and the cash was received on October 1, 2019.
On October 31, 2019, Crystal Amber exercised another portion of the May 2019 Warrant in accordance with the August 2019 SPA. For an aggregate cash payment of $1 million, 78,740,157 CDIs (representing approximately 1,574,803 shares of common stock) were issued on November 4, 2019 at $0.0127 per CDI under the May 2019 Warrant. Cash was received on October 31, 2019.
On November 15, 2019, Crystal Amber exercised the final portion of the May 2019 Warrant in accordance with the August 2019 SPA. For an aggregate cash payment of approximately $0.4 million, 31,740,748 CDIs (representing approximately 634,814 shares of common stock) were issued on November 18, 2019 at $0.0127 per CDI under the May 2019 Warrant. Cash was received on November 15, 2019.
On December 2, 2019, GI Dynamics provided notice to Crystal Amber that the Company elected to place the August 2019 Note at the full amount, on or before December 6, 2019. In December, the Company and Crystal Amber agreed to confer the right to tranche the funding of the August 2019 Note in amounts and per timing chosen solely by Crystal Amber, provided the August 2019 Note total was funded on or before January 15, 2020.
On December 16, 2019, GI Dynamics’ stockholders approved the August 2019 Note conversion feature and the issuance of the August 2019 Warrant, pending funding of the August 2019 Note by Crystal Amber.
On January 13, 2020, GI Dynamics received approximately $4.6 million in cash representing the full funding of the August 2019 Note. As stockholder approval had been obtained in December 2019, the August 2019 Note became convertible at Crystal Amber’s sole discretion until maturity in August 2024. Additionally, the August 2019 Warrant was issued to Crystal Amber, providing for the purchase of up to 229,844,650 CDIs (representing 4,596,893 shares of common stock) at $0.02 per CDI.
The Company’s costs include employee salaries and benefits, compensation paid to consultants, materials and supplies for research, costs associated with development activities including materials, sub-contractors, travel and administration, legal expenses, sales and marketing costs, general and administrative expenses, and other costs associated with an early stage, publicly-traded medical technology company. As of March 12, 2020, GI Dynamics has 15 full-time employees. The number of employees required to support the Company’s activities as it moves the EndoBarrier through the GI Dynamics STEP-1 clinical trial, as well as in the areas of research and development, sales and marketing, and general and administrative functions, may increase. GI Dynamics expects to continue to incur consulting expenses related to technology development that will increase as it enters into the recruitment phase of the STEP-1 and India clinical trials, and the Company expects to continue to incur expenses to protect its intellectual property.
33
The amount that GI Dynamics spends for any specific purpose may vary significantly from quarter to quarter or year to year, and could depend on a number of factors including, but not limited to, the pace of progress of the GI Dynamics STEP-1 and India clinical trials, commercialization and development efforts and actual needs with respect to development and research.
Research, development, and commercial acceptance of new medical technologies are, by their nature, unpredictable. Although the Company will undertake development and commercialization efforts with reasonable diligence, there can be no assurance that the net proceeds from the Company’s securities offerings will be sufficient to enable the Company to develop the Company’s technology to the extent needed to create future sales to sustain operations. If the net proceeds from these offerings are insufficient for this purpose, GI Dynamics will consider other options to continue its path to commercialization, including, but not limited to, additional financing through follow-on equity offerings, debt financing, co-development agreements, sale or licensing of developed intellectual or other property, or other alternatives.
GI Dynamics cannot assure that its technology will be accepted, that it will ever earn revenues sufficient to support its operations, or that it will ever be profitable. Furthermore, the Company has no committed source of financing and cannot assure that it will be able to raise money as and when it needs it to continue operations. If the Company cannot raise funds as and when it needs them, it may be required to scale back development plans by reducing expenditures for employees, consultants, business development and marketing efforts or to otherwise severely curtail, or even to cease, business operations.
The Company’s corporate headquarters are located in Boston, Massachusetts. The Company leases 3,520 square feet of office space with a lease expiration in May 2022. This space is adequate for current operations.
Critical Accounting Policies and Estimates
The Company’s discussion and analysis of its financial condition and results of operations is based upon the Company’s consolidated financial statements prepared in accordance with generally accepted accounting principles in the United States. GI Dynamics believes that its application of the following accounting policies, each of which require significant judgments and estimates on the part of management, are the most critical to aid in fully understanding and evaluating the Company’s reported financial results. The Company’s significant accounting policies are more fully described in Note 2, “Summary of Significant Accounting Policies and Basis of Presentation”, to the Company’s consolidated financial statements appearing elsewhere in this Annual Report on Form 10-K.
Use of Estimates
The preparation of consolidated financial statements in accordance with generally accepted accounting principles in the U.S. requires the Company’s management to make estimates and judgments that may affect the reported amounts of assets, liabilities, revenues and expenses, and the related disclosure of contingent assets and liabilities. On an ongoing basis, the Company’s management evaluates its estimates, including those related to impairment of long-lived assets, income taxes including the valuation allowance for deferred tax assets, research and development, contingencies, valuation of warrant liabilities and other derivative liabilities, estimates used to assess its ability to continue as a going concern and stock-based compensation. The Company bases its estimates on historical experience and on various other assumptions that are believed to be reasonable, the results of which form the basis for making judgments about the carrying values of assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions. Changes in estimates are reflected in reported results in the period in which they become known.
34
Going Concern Evaluation and Presentation
GI Dynamics analyzes its ability to continue as a going concern in each reporting period, assessing the likelihood that the company will be able to fund operations for the twelve months after the filing date of the financial statements. For further information regarding the Company’s funding requirements, see Note 1, “Nature of Business – Going Concern Evaluation”, to the Company’s consolidated financial statements. The Company has concluded and disclosed that there is substantial doubt in its ability to continue a going concern for a twelve-month period after the filing of this Annual Report on Form 10-K.
While there remains substantial doubt of the Company continuing as a going concern, the Company is seeking alternative financing sources and evaluating contingency plans that, while not guaranteed to yield the ability to fund operations at current levels, provide a reasonable probability that the Company will be able to maintain at least a subset of business operations. Therefore, the accompanying consolidated financial statements have been prepared assuming GI Dynamics will continue as a going concern, which contemplates the realization of assets and liabilities and commitments in the normal course of business. The consolidated financial statements for the year ended December 31, 2019 do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from uncertainty related to the Company’s ability to continue as a going concern within one year after the date that the consolidated financial statements are issued.
Inventory
GI Dynamics fully reserved its inventory as of December 31, 2017 and subsequently wrote off all inventory and reserves in 2018 as the materials on hand were not expected to be usable for future sales. At December 31, 2018 and 2019, there is no inventory or reserves against inventory on the balance sheet.
When the Company resumes recording inventory, it will state inventory at the lower of first-in, first-out cost or net realizable value. The Company will record a provision for excess, expired, and obsolete inventory based primarily on estimates of forecasted revenues. A significant change in the timing or level of demand for products as compared to forecasted amounts may result in the recording of additional provisions for excess, expired, and obsolete inventory. When capitalizing inventory, the Company will consider factors such as status of regulatory approval, alternative use of inventory, and anticipated commercial use of the product.
Fair Value of Derivative Instruments
GI Dynamics examines all financial instruments to determine if the financial instrument or any component feature that is a derivative requires liability treatment, predominantly due to a cash settlement feature. Such derivative liabilities are initially recorded at fair value with subsequent changes in value recorded in other income (expense) in the statements of operations. If the derivative instruments subsequently meet the requirements for classification as equity, the Company reclassifies the then fair value of the instrument to equity. The Company values its outstanding warrants and any other derivative liabilities identified using the Black-Scholes option pricing model. If multiple outcomes are probable, management assigns probability adjustments to determine the most likely probability adjusted fair value.
Research and Development Costs
Research and development costs are expensed when incurred. Research and development costs include costs of all basic research activities as well as other research, engineering, and technical effort required to develop a new product or service or make significant improvement to an existing product or manufacturing process. Research and development costs also include preapproval regulatory and clinical trial expenses.
Stock-Based Compensation
GI Dynamics accounts for stock-based compensation in accordance with the Financial Accounting Standards Board, (“FASB”) Accounting Standards Codification (“ASC”) 718, Stock Compensation (“ASC 718”), which requires that stock-based compensation be measured and recognized as an expense in the consolidated financial statements and that such expense be measured at the grant date fair value.
For awards that vest based on service conditions, the Company uses the straight-line method to allocate compensation expense to reporting periods. The grant date fair value of options granted is calculated using the Black-Scholes option pricing model, which requires the use of subjective assumptions including volatility, expected term and the fair value of the underlying common stock, among others.
35
The assumptions used in determining the fair value of stock-based awards represent management’s best estimates, but these estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change, and the Company uses different assumptions, its stock-based compensation could be materially different in the future. The risk-free interest rate used for each grant is based on a zero-coupon U.S. Treasury instrument with a remaining term similar to the expected term of the stock-based award. Because the Company does not have a sufficient stable history to estimate the expected term, the simplified method for estimating the expected term is used. The simplified method is based on the average of the vesting tranches and the contractual life of each grant. The Company uses historical price volatility data to estimate expected future stock volatility. GI Dynamics has not paid and does not anticipate paying cash dividends on shares of common stock; therefore, the expected dividend yield is assumed to be zero. The Company accounts for forfeitures as they occur, rather than applying an estimated forfeiture rate to the value of the grant on the grant date.
GI Dynamics periodically issues performance-based awards. For these awards, vesting will occur upon the achievement of certain milestones. When achievement of the milestone is deemed probable, the Company expenses the compensation of the respective stock award over the implicit service period.
Stock awards to non-employees were accounted for in accordance with ASC 505-50, Equity-Based Payments to Non-Employees (“ASC 505-50”). The measurement date for non-employee awards was generally the date performance of services required from the non-employee was complete. For non-employee awards that vest based on service conditions, the Company expensed the value of the awards over the related service period, provided the service condition was expected to be met. The Company recorded the expense of services rendered by non- employees based on the estimated fair value of the stock option using the Black-Scholes option pricing model over the contractual term of the non-employee award. At each reporting period, the fair value of unvested non-employee awards was remeasured and expensed on a straight-line basis over the vesting term of the underlying award. In June 2018, the FASB issued ASU No. 2018-07, Compensation – Stock Compensation (Topic 718: Improvements to nonemployee share-based payment accounting), or ASU 2018-07, which provides measurement provisions and clarifications for the accounting for Non-employee Share-Based Payments (“NESBP”). As a result, most of the guidance within ASC 718 associated with employee share-based payments, including most of its requirements related to classification and measurement, applies to NESBP. The Company adopted ASU 2018-07 as of January 1, 2019 and there was no impact to retained earnings or equity as no uncompleted NESBPs were outstanding when adopted. At December 31, 2019, the Company had options to purchase 120,000 shares of common stock granted to non-employees.
Impairment of Long-Lived Assets
GI Dynamics regularly reviews the carrying amount of its long-lived assets to determine whether indicators of impairment may exist that warrant adjustments to carrying values or estimated useful lives. If indications of impairment exist, projected future undiscounted cash flows associated with the asset are compared to the carrying amount to determine whether the asset’s value is recoverable. If the carrying value of the asset exceeds such projected undiscounted cash flows, the asset will be written down to its estimated fair value.
Smaller Reporting Company Status
The Securities and Exchange Commission amended regulation S-K, section 210 in September 2018 to reduce the scale of Smaller Reporting Companies. Smaller Reporting Companies are defined based on public capital and revenue limits and GI Dynamics qualifies as a Smaller Reporting Company for the year ended December 31, 2019. As a result, the Company’s reporting is compliant with the Smaller Reporting Company requirements.
36
Results of Operations
The following is a description of significant components of the Company’s operations, including significant trends and uncertainties that are believed to be important to an understanding of the Company’s business and results of operations.
Year Ended December 31, 2019 Compared to Year Ended December 31, 2018
GI Dynamics did not record any revenue in the years ended December 31, 2019 or 2018.
Operating Expenses
| Years Ended | ||||||||||||||||
| December 31, | Change | |||||||||||||||
| 2019 | 2018 | $ | % | |||||||||||||
| (dollars in thousands) | ||||||||||||||||
| Research and development expense | $ | 4,225 | $ | 1,947 | $ | 2,278 | 117.0 | % | ||||||||
| Sales and marketing expense | 22 | 866 | (844 | ) | (97.5 | )% | ||||||||||
| General and administrative expense | 5,295 | 4,809 | 486 | 10.1 | % | |||||||||||
| Total operating expenses | $ | 9,542 | $ | 7,622 | $ | 1,920 | 25.2 | % | ||||||||
Research and Development Expense. The increase in research and development expense of approximately $2.3 million for the year ended December 31, 2019 compared to the year ended December 31, 2018 was primarily due to an increase in spending on internal and external resources as the Company prepared to initiate the STEP-1 clinical trial in the United States, the I-STEP trial in India (under joint venture with Apollo Sugar) and prepare for audits related to filing for its CE Mark designation. In the same period in 2018, the Company had just received the IDE from the FDA and had not moved from the planning stages on all three strategic priorities.
Sales and Marketing Expense. The decrease in sales and marketing expense of approximately $844 thousand for the year ended December 31, 2019 compared to the year ended December 31, 2018 was primarily due to an overall decrease in sales and marketing activities and a redirection of existing overseas employee efforts to research and development.
General and Administrative Expense. The increase in general and administrative expense of approximately $486 thousand for the year ended December 31, 2019 compared to the year ended December 31, 2018 was primarily related to increased business insurance costs, increased support costs for higher employee counts and additional legal and insurance costs related to transacting multiple financing events as a public company that is subject to reporting in two jurisdictions.
GI Dynamics continues to look for ways to streamline the Company’s general and administrative expenses. In so doing, the Company recognizes that some costs, particularly those associated with being a public company with multiple reporting jurisdictions are unavoidable. GI Dynamics will continue to provide general and administrative support for the scope and size of the entire company.
Other Income (Expense), Net
| Years Ended | ||||||||||||||||
| December 31, | Change | |||||||||||||||
| 2019 | 2018 | $ | % | |||||||||||||
| (dollars in thousands) | ||||||||||||||||
| Other income (expense): | ||||||||||||||||
| Interest income | $ | 3 | $ | 29 | $ | (26 | ) | (89.7 | )% | |||||||
| Interest expense | (6,178 | ) | (1,151 | ) | (5,027 | ) | 436.8 | % | ||||||||
| Foreign exchange gain | 11 | 13 | (2 | ) | (15.4 | )% | ||||||||||
| Income from diverted funds and insurance claim, net | — | 224 | (224 | ) | (100.0 | )% | ||||||||||
| Re-measurement of derivative liabilities | (1,683 | ) | 482 | (2,165 | ) | (449.2 | )% | |||||||||
| Gain on write-off of accounts payable | 101 | — | 101 | (— | )% | |||||||||||
| Total other income (expense), net | $ | (7,746 | ) | $ | (403 | ) | $ | (7,343 | ) | 1,822.1 | % | |||||
37
Interest income. The decrease in interest income of approximately $26 thousand for the year ended December 31, 2019 compared to the year ended December 31, 2018 was due to lower average cash, cash equivalents and restricted cash balances in 2019.
Interest expense. Interest expense increased by approximately $5 million for the year ended December 31, 2019 compared to the year ended December 31, 2018 primarily due to non-cash interest expenses related to the conversion of the 2018, March 2019 and May 2019 Notes payable to Crystal Amber.
Income from diverted funds and insurance claim, net. In July 2018, after a third-party investigation isolated the activity, an insurance claim was filed for $271 thousand for fraudulent diversion of cash from the Company’s account into a personal account that occurred during the years 2016 through 2018. Income in the year ended December 31, 2018 represents net insurance proceeds on the claim less $22 thousand of research and development expense. The Company has since implemented internal controls to correct the identified control deficiencies associated with the fraud.
Foreign exchange gain. The change in the foreign exchange gain is minimal as the balances and transactions are comparable year over year
Re-measurement of derivative liabilities. The change in the re-measurement of derivative liabilities of approximately $2.2 million for the year ended December 31, 2019 compared to the year ended December 31, 2018 was primarily due to the revaluation of the March and May 2019 warrants prior to stockholder approval. During the intervening period between Note issuance and derivative remeasurement, the market price per CDI increased significantly which resulted in a higher value driven by the widened spread between the exercise price and the market price and less so, in the increased volatility resulting from the change in the price of the CDIs.
Liquidity and Capital Resources
As of December 31, 2019, the Company’s primary source of liquidity is its cash, cash equivalents and restricted cash balances. GI Dynamics is currently focused primarily on its clinical trials, which will support future regulatory submissions and potential commercialization activities. Until the Company is successful in gaining regulatory approvals, it is unable to sell the Company’s product in any market at this time. Without revenues, GI Dynamics is reliant on funding obtained from investment in the Company to maintain business operations until the Company can generate positive cash flows from operations. The Company cannot predict the extent of future operating losses and accumulated deficit, and it may never generate sufficient revenues to achieve or sustain profitability.
GI Dynamics has incurred operating losses since inception and at December 31, 2019, had an accumulated deficit of approximately $284 million and a working capital deficit of $3.4 million. The Company expects to incur significant operating losses for the next several years. At December 31, 2019, the Company had approximately $2.5 million in cash, cash equivalents and restricted cash.
The Company will need to restructure the terms of the 2017 Note before March 31, 2020 and raise additional capital before May 1, 2020 in order to continue to pursue its current business objectives as planned and to continue to fund its operations. The Company and Crystal Amber are currently in final discussions regarding the 2017 Note maturity extension. The Company is looking to raise additional funds through any combination of additional equity and debt financings or from other sources. However, the Company has no guarantee that the 2017 Note will not mature on March 31, 2020 and has no guaranteed source of capital that will accommodate repayment of the 2017 Note and sustain operations past March 31, 2020. If the 2017 Note terms are restructured, the Company anticipates operating cash being exhausted by May 1, 2020 and therefore requires additional financing. There can be no assurance that any such potential financing opportunities will be available on acceptable terms, if at all. If the Company is unable to raise sufficient capital on the Company’s required timelines and on acceptable terms to stockholders and the Board of Directors, it could be forced to cease operations, including activities essential to support regulatory applications to commercialize EndoBarrier. If access to capital is not achieved in the near term, it will materially harm the Company’s business, financial condition and results of operations to the extent that the Company may be required to cease operations altogether, file for bankruptcy, or undertake any combination of the foregoing. These factors raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that these consolidated financial statements are issued.
In addition, if the Company does not meet its payment obligations to third parties as they become due, the Company may be subject to litigation claims and its credit worthiness would be adversely affected. Even if the Company is successful in defending these claims, litigation could result in substantial costs and would be a distraction to management and may have other unfavorable results that could further adversely impact the Company’s financial condition.
38
As a result of the factors described above, the Company’s consolidated financial statements include a going-concern disclosure.
During the year ended December 31, 2019, the Company’s cash, cash equivalents and restricted cash balance decreased by approximately $1.3 million primarily due to cash payments related to, among other things, research and development and general and administrative expenses as GI Dynamics continued to focus on clinical and regulatory strategies that exceeded its various financings.
The following table sets forth the major sources and uses of cash for each of the periods set forth below:
| Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| (in thousands) | ||||||||
| Net cash (used in) provided by: | ||||||||
| Operating activities | $ | (10,612 | ) | $ | (7,324 | ) | ||
| Investing activities | (11 | ) | (3 | ) | ||||
| Financing activities | 9,316 | 8,099 | ||||||
| Net increase (decrease) in cash, cash equivalents and restricted cash | $ | (1,307 | ) | $ | 772 | |||
Cash Flows used in Operating Activities
Net cash used in operating activities totaled approximately $10.6 million for the year ended December 31, 2019. The primary uses of cash were:
| ● | to fund the Company’s net loss of approximately $17.3 million; |
| ● | a net negative adjustment to cash flow from changes in working capital of approximately $1.6 million resulting primarily from increases in prepaid expenses and decreases in accounts payable and accrued expenses; and |
| ● | a net positive adjustment to cash flow from non-cash items of approximately $8.3 million, primarily from non-cash interest expense, change in derivative valuation and stock-based compensation. |
Net cash used in operating activities totaled approximately $7.3 million for the year ended December 31, 2018. The primary uses of cash were:
| ● | to fund the Company’s net loss of approximately $8 million; |
| ● | a net negative adjustment to cash flow from changes in working capital of approximately $112 thousand resulting primarily from increases in accrued expenses and accounts receivable, partially offset by a decrease in accounts payable and an increase in prepaid expenses; and |
| ● | a net positive adjustment to cash flow from non-cash items of approximately $800 thousand, primarily from non-cash interest expense, change in derivative valuation, debt accretion and stock-based compensation. |
Cash Flows used in Investing Activities
Cash used in investing activities for the year ended December 31, 2019 totaled approximately $11 thousand from the purchase of office equipment.
Cash used in investing activities for the year ended December 31, 2018 totaled approximately $3 thousand from the purchase of office equipment.
Cash Flows provided by Financing Activities
Cash provided by financing activities for the year ended December 31, 2019 totaled approximately $9.3 million and primarily resulted from the exercise of related party warrants and proceeds from short and long term debt from a related party.
Cash provided by financing activities for the year ended December 31, 2018 totaled approximately $8.1 million and primarily resulted from the net proceeds from the Company’s private placement of CDIs.
39
Funding Requirements
As of December 31, 2019, the Company’s primary source of liquidity is its cash, cash equivalents and restricted cash balances. GI Dynamics is currently focused primarily on its clinical trials, which will support future regulatory submissions and potential commercialization activities. Until the Company is successful in gaining regulatory approvals, it is unable to sell the Company’s product in any market at this time. Without revenues, GI Dynamics is reliant on funding obtained from investment in the Company to maintain business operations until the Company can generate positive cash flows from operations. The Company cannot predict the extent of future operating losses and accumulated deficit, and it may never generate sufficient revenues to achieve or sustain profitability.
GI Dynamics has incurred operating losses since inception and at December 31, 2019, had an accumulated deficit of approximately $284 million and a working capital deficit of $3.4 million. The Company expects to incur significant operating losses for the next several years. At December 31, 2019, the Company had approximately $2.5 million in cash, cash equivalents and restricted cash.
Due to the numerous risks and uncertainties associated with securing regulatory approval for EndoBarrier, at this time GI Dynamics is unable to estimate precisely the amounts of capital outlays and operating expenditures necessary to complete the task of obtaining regulatory approval for EndoBarrier. Funding requirements will depend on many factors, including, but not limited to, the following:
| ● | the timing, cost, and resulting success of research and development efforts; | |
| ● | the rate of progress and cost of commercialization activities after regulatory approval; | |
| ● | the expenses the Company may incur in marketing and selling EndoBarrier subject to future regulatory approvals; | |
| ● | the timing and decisions of payer organizations related to reimbursement; | |
| ● | the revenue generated by sales of EndoBarrier; | |
| ● | the safety and efficacy of the Company’s product in treating diabetes and reducing obesity; | |
| ● | the success of the Company’s investment in its manufacturing and supply chain infrastructure; | |
| ● | the time and costs involved in obtaining regulatory approvals for EndoBarrier in new markets; | |
| ● | the costs associated with any additional clinical trial(s) required in the U.S. and other countries on a case by case basis; | |
| ● | the ability to ship CE marked products; | |
| ● | the emergence of competing or complementary developments; and | |
| ● | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights. |
GI Dynamics will continue to manage its capital structure and to consider all financing opportunities, whenever they may occur, that could strengthen its long-term liquidity profile. Any such capital transactions may or may not be similar to transactions in which the Company has engaged in the past and the ownership interests of existing stockholders may be materially diluted.
Off–Balance Sheet Arrangements
GI Dynamics does not have any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities, that would have been established for the purpose of facilitating off-balance sheet arrangements (as that term is defined in Item 303(a)(4)(ii) of Regulation S-K) or other contractually narrow or limited purposes. As such, the Company is not exposed to any financing, liquidity, market or credit risk that could arise if it had engaged in those types of relationships. GI Dynamics enters into guarantees in the ordinary course of business related to the guarantee of its own performance and the performance of its subsidiaries.
Contractual Obligations and Commitments
The Company’s commitments for operating leases relate to the lease of office space in Boston, Massachusetts which expires in May 2022.
Recent Accounting Pronouncements
For a discussion of recent accounting pronouncements please refer to Note 2, “Summary of Significant Accounting Policies and Basis of Presentation”, to the Company’s consolidated financial statements included in this Annual Report on Form 10-K.
40
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
GI Dynamics is focused on developing clinical data and regulatory approvals for EndoBarrier and the Company earnings and cash flows are exposed to market risk from changes in currency exchange rates and interest rates.
Interest Rate Sensitivity
The Company’s cash, cash equivalents and restricted cash of $2.5 million at December 31, 2019 consisted of cash and money market funds, all of which will be used for working capital purposes. The Company does not enter into investments for trading or speculative purposes. The goals of the Company’s investment policy are preservation of capital, fulfillment of liquidity needs and fiduciary control of cash and investments. The Company also seeks to maximize income from investments without assuming significant risk. The Company’s primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of interest rates in the U.S. and Australia. Because of the short-term nature of the Company’s cash, cash equivalents and restricted cash, GI Dynamics does not believe that it has any material exposure to changes in their fair values as a result of changes in interest rates.
Foreign Currency Risk
GI Dynamics conducts business in foreign countries. For U.S. reporting purposes, all assets and liabilities of non-U.S. entities are remeasured at the period-end exchange rate and income and expenses at the average exchange rates in effect during the periods. The net effect of these remeasurement adjustments is shown in the accompanying consolidated financial statements as a component of net loss. Continued fluctuation of these exchange rates could result in financial results that are not comparable from quarter to quarter. The Company does not currently utilize foreign currency contracts to mitigate the gains and losses generated by the remeasurement of non-functional currency assets and liabilities and do not currently hold material cash reserves in currencies in which certain expenditures are denominated.
Effects of Inflation
GI Dynamics does not believe that inflation and changing prices over the years ended December 31, 2019 and 2018 had a significant impact on its results of operations.
ITEM 8. CONSOLIDATED FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The Company’s consolidated financial statements, together with the independent registered public accounting firm report thereon, appear on pages F-1 through F-30 of this Annual Report on Form 10-K.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
On December 5, 2019, the Company dismissed Moody, Famiglietti & Andronico, LLP (“MFA”) as its independent registered public accounting firm. The decision to dismiss MFA was approved by the Company’s Board of Directors with the recommendation of its Audit Committee. The audit reports of MFA on the consolidated financial statements of the Company for each of the two most recent fiscal years ended December 31, 2018 and December 31, 2017 did not contain an adverse opinion or a disclaimer of opinion and were not qualified or modified as to uncertainty, audit scope or accounting principles. The audit reports for the years ended December 31, 2018 and December 31, 2017 contained an explanatory paragraph disclosing the uncertainty regarding the Company’s ability to continue as a going concern. During the Company’s two most recent fiscal years ended December 31, 2018 and December 31, 2017 and during the subsequent interim period from January 1, 2019 through December 5, 2019, (i) there were no disagreements with MFA on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedure that, if not resolved to MFA’s satisfaction, would have caused MFA to make reference to the subject matter of the disagreement in connection with its reports and (ii) there were no “reportable events” as defined in Item 304(a)(1)(v) of Regulation S-K.
On December 5, 2019, the Company engaged Wolf & Company, P.C. as its independent registered public accounting firm for the fiscal year ending December 31, 2019. The decision to engage Wolf & Company, P.C. was approved by the Company’s Board of Directors with the recommendation of its Audit Committee. During the two most recent fiscal years ended December 31, 2018 and December 31, 2017 and during the subsequent interim period from January 1, 2019 through December 5, 2019, neither the Company nor anyone acting on its behalf consulted with Wolf & Company, P.C. on (i) any matters regarding the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered with respect to the Company’s consolidated financial statements, and no written report or oral advice was provided to the Company that Wolf & Company, P.C. concluded was an important factor considered by the Company in reaching a decision as to any accounting, auditing or financial reporting issue, or (ii) any matter that was the subject of any disagreement (as described in Item 304(a)(1)(iv) of Regulation S-K and the related instructions thereto) or a reportable event (as defined in Item 304(a)(1)(v) of Regulation S-K).
41
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
In accordance with Exchange Act Rules 13a-15 and 15d-15(f), GI Dynamics carried out an evaluation, under the supervision and with the participation of management, including the Chief Executive Officer and Chief Financial Officer (the “Certifying Officers”), of the effectiveness of the Company’s disclosure controls and procedures as of the end of the period covered by this report. Based on that evaluation, the Company’s Certifying Officers concluded that the Company’s disclosure controls and procedures were effective as of December 31, 2019 in enabling GI Dynamics to record, process, summarize and report information required to be included in its periodic SEC filings within the required time period.
Management’s Annual Report on Internal Control over Financial Reporting
As a public company in the U.S., GI Dynamics is required, pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, to furnish a report by management on, among other things, the effectiveness of its internal control over financial reporting as of the end of the fiscal year. The controls and other procedures are designed to ensure that information required to be disclosed by the Company in the reports that the Company files with the SEC is disclosed accurately and is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms. The Company’s first report on compliance with Section 404 was furnished in connection with the GI Dynamics financial statements for the year ended December 31, 2014. As a former emerging growth company and a current smaller reporting company, the Company has been and currently is exempted from the requirement to include an external auditor opinion on the Company’s internal controls in its filings. GI Dynamics continues to update, document and evaluate its internal control over financial reporting to the requirements of Section 404.
The Company’s Certifying Officers are responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act.
Internal control over financial reporting is a process designed by, or under the supervision of, the Certifying Officers and effected by the Company’s Board of Directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| ● | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the Company’s assets; |
| ● | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that the Company’s receipts and expenditures are being made only in accordance with authorizations of the Company’s management and directors; and |
| ● | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition or disposition of the Company’s assets that could have a material effect on the financial statements. |
Internal control over financial reporting cannot provide absolute assurance of achieving financial reporting objectives because of its inherent limitations. It is a process that involves human diligence and compliance and is subject to lapses in judgment or breakdowns resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper management override. While process safeguards can reduce risks, because of inherent limitations, there is a risk that material misstatements may not be prevented or detected on a timely basis. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Company, under the supervision and with the participation of the Certifying Officers, has evaluated the effectiveness of the Company’s internal control over financial reporting as of December 31, 2019 based upon the framework in Internal Control Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Based on such evaluations, the Certifying Officers have concluded that the Company’s internal control over financial reporting was effective as of December 31, 2019.
Changes in Internal Control Over Financial Reporting
As required by Rule 13a-15(d) of the Exchange Act, the Certifying Officers conducted an evaluation of the internal control over financial reporting to determine whether any changes occurred during the fourth fiscal quarter that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting. The evaluation performed by the Certifying Officers resulted in a conclusion that no such changes occurred.
None.
42
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Certain information required by this Item 10 relating to the Company’s directors and executive officers is incorporated by reference herein to the Company’s Proxy Statement to be filed with the SEC in connection with its 2020 Annual Meeting of Stockholders or an amendment to this report filed under cover of Form 10-K/A not later than 120 days after the end of the fiscal year ended December 31, 2019.
Code of Business Conduct and Ethics
GI Dynamics has adopted a code of business conduct and ethics applicable to directors, executive officers and all other employees. A copy of that code is available on the Company’s corporate website at http://www.gidynamics.com. Any amendments to the code of business conduct and ethics, and any waivers thereto involving the Company executive officers, also will be available on the corporate website. A printed copy of these documents will be made available upon request. The content on the Company website is not incorporated by reference into this Annual Report on Form 10-K.
Directors of the Registrant
The following table sets forth the name, age and position of each of the Company’s directors as of March 2, 2020:
| Name | Age | Position | ||
| Daniel J. Moore (3) | 58 | Non-executive Chairman of the Board | ||
| Timothy J. Barberich (1)(2) | 72 | Non-executive Director | ||
| Juliet Thompson (1) | 53 | Non-executive Director | ||
| Oern R. Stuge, M.D. (2)(3) | 65 | Non-executive Director | ||
| Praveen Tyle, Ph.D. | 60 | Non-executive Director |
| 1. | Member of the audit committee. |
| 2. | Member of compensation committee. |
| 3. | Member of nominating and corporate governance committee. |
Daniel J. Moore has served as a director of GI Dynamics since 2014, as vice-chairman from March 2015 to April 2016 and chairman since May 2016. Mr. Moore’s extensive experience in domestic and international sales, operations and executive management in global medical device manufacturers and years of service on other boards makes him qualified to serve on the Company’s board of directors.
Mr. Moore has served as president, chief executive officer and director of Cyberonics, Inc., a medical technology company with core expertise in neuromodulation, from 2007 to October 2015. From 1989 to 2007, Mr. Moore held positions in sales, marketing, and senior management in the U.S. and in Europe at Boston Scientific Corporation, a diverse maker of minimally invasive medical products. His last position at Boston Scientific was President, International Distributor Management. Prior to that role, he held the position of President, Inter-Continental, the fourth largest business unit of Boston Scientific, with more than 1,000 global employees and revenues exceeding $700 million. Mr. Moore previously held senior management positions at several Boston Scientific U.S. and international divisions.
Mr. Moore currently serves as the chairman of LivaNova PLC (the company resulting from the merger of Sorin S.p.A. and Cyberonics, Inc.), chairman of ViewRay, a member of the board of directors for the Epilepsy Foundation of America, and as a member of the boards or advisory boards for Brainscope, BioHouston, Inc. and the Weldon School of Biomedical Engineering at Purdue University. Past board positions include Smiling Kids, Inc., the Epilepsy Foundation of Texas (past-Chair), the Epilepsy Foundation of Texas — Houston (past-President), the Medical Device Manufacturers Association (past-Chair), Cyberonics, Inc., Topera, Inc. (acquired by Abbott) and TriVascular Technologies, Inc. (acquired by Endologix).
43
Mr. Moore holds a B.A. from Harvard University and earned an MBA from Boston University.
Timothy J. Barberich has been a director of GI Dynamics since June 2011. Mr. Barberich has nearly 40 years’ experience in pharmaceutical and medical device companies, in technical, sales, marketing and management positions, including as chief executive officer and chairman of the board. Mr. Barberich is the founder and former president, chief executive officer and chairman of Sepracor, Inc., a NASDAQ-listed-pharmaceutical company based in Massachusetts, which was acquired by Dainippon Sumitomo Pharma Co., Ltd. in 2009. Mr. Barberich founded Sepracor in 1984 and served as its chief executive officer from 1984 to 2007 and chairman of the board from 1990 to 2007. From 2007 to 2008, Mr. Barberich served as executive chairman of Sepracor and then chairman of the board from 2008 to 2009. Mr. Barberich led Sepracor through its early-stage research and development, product approvals, commercialization, private financings and initial public offering, partnerships with major companies, several successful spin-outs and achievement of revenues in excess of $1 billion. Prior to founding Sepracor, Mr. Barberich spent 10 years as a senior executive at Millipore Corporation, a company that provides separations products to the life science research, pharmaceutical, biotechnology and electronic markets. Mr. Barberich brings to the Company’s board the knowledge and experience of leading a company in the health care industry through every stage of its life cycle. GI Dynamics believes this experience and familiarity with the types of risks that the Company may face, together with his broad medical device and pharmaceutical industry experience, makes Mr. Barberich uniquely suited to serve on the Company’s board.
Mr. Barberich is currently chairman and CEO of BioNevia Pharmaceuticals, Inc., a director of Verastem Oncology, a NASDAQ-listed biotechnology company, TScan and Frequency Therapeutics, both privately funded biotechnology companies. Mr. Barberich was formerly a director of Inotek Pharmaceuticals, a NASDAQ-listed biotechnology company, Neurovance, Inc., a privately held biotechnology company, HeartWare International, Inc., a NASDAQ-listed medical device company, Tokai Pharmaceuticals, Inc., a NASDAQ-listed biopharmaceutical company, MirImmune Inc., which was acquired in 2016, BioSphere Medical, Inc., a NASDAQ-listed biotechnology company, Gemin X Biotechnologies, Inc. and Resolvyx Pharmaceuticals, which were acquired in 2011 and 2010, respectively, and Virucon, a publicly traded biotechnology company.
Mr. Barberich holds a Bachelor of Science degree in Chemistry from Kings College in Pennsylvania and has taken graduate courses from the School of Chemistry at Rutgers University.
Juliet Thompson has been a director of GI Dynamics since August 22, 2017. Ms. Thompson also assumed the role of chair of the Company’s audit committee. Ms. Thompson has spent approximately 20 years working as an investment banker and strategic advisor to healthcare companies in Europe. Ms. Thompson’s extensive experience in investment banking, including capital raising and strategic initiatives, makes her qualified to serve on the Company’s board of directors.
Since March 2015, Ms. Thompson has served on the board of Nexstim Limited, a medical technology company listed on Nasdaq First North Finland and Sweden. Prior to that, Ms. Thompson led the European healthcare practice at Stifel Financial Corp., a diversified financial services holding company, serving as a partner from October 2013 to April 2015. In 2003, Ms. Thompson co-founded Code Securities, a healthcare investment banking firm that was purchased by Nomura and renamed Nomura Code Securities Limited (“Nomura Code”) in 2005 and served as Head of Corporate Finance and as a member of the board of Nomura Code until 2013. She is also currently a non-executive director of Vectura PLC, a company listed on the London Stock Exchange plc, and Novacyt S.A., a French-based company whose shares are admitted to trade on AIM, Ms. Thompson is a member of the Institute of Chartered Accountants in England and Wales (ACA) and holds a BSc degree in Economics from the University of Bristol. Her experience also includes roles at WestLB Panmure, ICI PLC, Deloitte and Touche and HM Treasury.
Oern R. Stuge, M.D. has served as a director of GI Dynamics since his appointment in January 2017. Dr. Stuge’s extensive experience in domestic and international sales, management and operations in a global medical device manufacturer makes him qualified to serve on the Company’s board of directors.
Dr. Stuge has served as an executive in various medical device, health care and life sciences companies over the last thirty years. Since January 2011, Dr. Stuge has been Chairman of Orsco Lifesciences AG, a management firm that specializes in medical technology through which he supports several companies. Prior to that, Dr. Stuge served in various positions, including as Senior Vice-President, at Medtronic Inc., from May 1998 to December 2009. Dr. Stuge is currently Chairman of Mainstay Medical Limited, a Euronext Paris-listed and Irish Stock Exchange-listed medical devices company and Luminas Limited, formerly a NASDAQ-listed medical company. Dr. Stuge is chairman of the board of OrthoD Ltd. and serves on the board of several private companies including Balt Extrusion SAS, Pulmonx International SA, and Phagenesis Limited. Furthermore, until December 2016, Dr. Stuge served on the board of Bonesupport AB, a private medical technology company.
44
Dr. Stuge received an M.D. from the University of Oslo, Norway, an M.B.A. from IMD and an INSEAD Certification in Corporate Governance.
Praveen Tyle, Ph.D. has served as a director since February 2020. Dr. Tyle’s executive research and development leadership experience, including within diabetes and obesity, and significant mergers and acquisitions and business development and licensing experience makes him qualified to serve on the Company’s board of directors.
Since May 2016, Dr. Tyle has served as Executive Vice President of Research and Development of Lexicon Pharmaceuticals. Dr. Tyle was previously a member of the executive management team at Osmotica Pharmaceutical Corp., serving as President and Chief Executive Officer from January 2013 through April 2016 and as Executive Vice President and Chief Scientific Officer from August 2012 to December 2012. He is also a member of the board of Orient EuroPharma Co., Ltd. of Taiwan. Dr. Tyle has nearly 30 years of experience in the pharmaceutical industry with the majority of his tenure in senior executive leadership positions in areas of research and development, manufacturing, quality, business development and operations. Prior to joining Osmotica Pharmaceutical Corp., Dr. Tyle served as Executive Vice President (from January 2012 to August 2012) and Chief Scientific Officer (from October 2011 to August 2012) for the United States Pharmacopeia, or USP. Prior to joining USP, Dr. Tyle from 2008 to 2011, served as the Senior Vice President and Global Head of Business Development and Licensing at Novartis Consumer Health from March 2009 to September 2011. At Novartis Consumer Health, Dr. Tyle also served as Senior Vice President & Global Head of Research and Development from March 2009 to February 2010. Dr. Tyle holds a doctorate in pharmaceutics and pharmaceutical chemistry from the Ohio State University and a BS in Pharmacy (honors) from the Institute of Technology, Banaras Hindu University in India.
Australian Disclosure Requirements
Because GI Dynamics is listed on the ASX, it is required to comply with various disclosure requirements as set out in the ASX Listing Rules. The following information is provided to comply with the ASX Listing Rules and is not intended to fulfill SEC information required by Part III of this Annual Report on Form 10-K.
Overview
GI Dynamics securities are listed for quotation in the form of CDIs on the ASX and trade under the symbol “GID.AX” Each share of GI Dynamics common stock is equivalent to fifty CDIs. The stockholder information below was applicable as at March 12, 2020.
GI Dynamics share capital was as follows:
| Type of Security | Number of Securities | Equivalent in CDIs | ||||||
| Common Stock | 73,631 | 3,681,550 | ||||||
| CDIs | 36,524,665 | 1,826,233,250 | ||||||
| Total | 1,829,914,800 | |||||||
| Options1 | 3,186,154 | 159,307,700 | ||||||
| Restricted stock units1 | 250,000 | 12,500,000 | ||||||
| Warrants | 4,625,425 | 231,271,250 | ||||||
| Total | 2,232,993,750 | |||||||
| 1. | As of March 12, 2020, an additional 574,866 shares of common stock (equivalent to 28,743,300 CDIs) were available for grant under the Company’s 2011 Employee, Director and Consultant Equity Incentive Plan. |
45
Substantial Holders
The number of CDIs held by the Company’s substantial stockholders (being stockholders who, together with their associates, have a relevant interest in at least 5% of the Company’s voting shares) assuming the conversion of common stock held by those stockholders into CDIs and based on the information in the substantial holder notices the Company received as of February 21, 2020, was as follows:
| Name of Holder | Number
of CDIs Held | %
of Total CDIs | ||||||
| Crystal Amber Fund Limited | 1,337,659,335 | 73.10 | % | |||||
| Richard Cashin | 199,364,713 | 10.89 | % | |||||
Distribution of Equity Security Holders
On March 12, 2020, a total of 36,598,291 shares of common stock were on issue, 36,524,665 of which were held as CDIs (being 1,826,233,250 CDIs in total). The table below presents the number of shares of common stock and the number of CDIs on issue, as well as the number of options, restricted stock units and warrants on issue by size of holding as of March 12, 2020:
| Common Stock (unlisted) | CDIs | Options (unlisted) | Restricted
Stock Units (unlisted) | Warrants (unlisted) | ||||||||||||||||||||||||||||||||||||
| Number | Number | Number | Number | Number | Number | Number | Number | Number | Number | |||||||||||||||||||||||||||||||
| of | of | of | of | of | of | of | of | of | of | |||||||||||||||||||||||||||||||
| Holders | Shares | Holders | Shares | Holders | Shares | Holders | Shares | Holders | Shares | |||||||||||||||||||||||||||||||
| 1 – 1,000 | — | — | 127 | 43,522 | 2 | 616 | — | — | — | — | ||||||||||||||||||||||||||||||
| 1,001 – 5,000 | 1 | 28 | 205 | 566,427 | 1 | 5,000 | — | — | — | — | ||||||||||||||||||||||||||||||
| 5,001 – 10,000 | 1 | 105 | 109 | 925,189 | 3 | 30,000 | — | — | — | — | ||||||||||||||||||||||||||||||
| 10,001 – 100,000 | 9 | 8,526 | 242 | 9,681,727 | 18 | 715,167 | — | — | 1 | 28,532 | ||||||||||||||||||||||||||||||
| 100,001 – and over | 7 | 64,972 | 142 | 1,815,016,385 | 3 | 2,435,371 | 1 | 250,000 | 1 | 4,596,893 | ||||||||||||||||||||||||||||||
| Total | 18 | 73,631 | 825 | 1,826,233,250 | 27 | 3,186,154 | 1 | 250,000 | 2 | 4,625,425 | ||||||||||||||||||||||||||||||
46
Unmarketable Parcels
As of March 12, 2020, the number of stockholders holding less than a marketable parcel (for the purposes of the ASX Listing Rules) was 664, based on the closing market price at March 12, 2020.
Top 20 Holders
Holders of CDIs only
The table below provides a list of the top 20 holders of GI Dynamics CDIs. Related but separate legal entities are not aggregated.
| No. | Name of Holder | Number of CDIs Held | % of Total CDIs | ||||||||
| 1 | Crystal Amber Fund | 1,337,659,335 | 48.93 | % | |||||||
| 2 | Mr Richard Cashin | 199,364,713 | 20.68 | % | |||||||
| 3 | Mr Paul Cozzi | 40,358,565 | 4.19 | % | |||||||
| 4 | MS Pace LP | 39,115,442 | 4.06 | % | |||||||
| 5 | Advanced Technology Ventures | 31,719,318 | 3.29 | % | |||||||
| 6 | Johnson & Johnson Development Corp | 28,278,460 | 2.93 | % | |||||||
| 7 | Polaris Venture Partners | 17,222,719 | 1.79 | % | |||||||
| 8 | Mr David Brock | 10,001,249 | 1.04 | % | |||||||
| 9 | Bank Leumi Le Israel BM a/c Main | 8,504,551 | 0.88 | % | |||||||
| 10 | Mr & Mrs Ian Moore | 7,000,060 | 0.73 | % | |||||||
| 11 | Fidelity Pacific Basin Fund | 5,561,290 | 0.58 | % | |||||||
| 12 | Invus Public Equity Advisors | 5,185,659 | 0.54 | % | |||||||
| 13 | Ms Rena C Merchant | 4,685,000 | 0.49 | % | |||||||
| 14 | Transact | 3,790,771 | 0.39 | % | |||||||
| 15 | MK Pension Plan | 3,437,363 | 0.36 | % | |||||||
| 16 | UBS Zurich | 3,419,165 | 0.35 | % | |||||||
| 17 | Mr Colin P Smith & Ms Mary Christoforou | 3,166,470 | 0.33 | % | |||||||
| 18 | Mr Timothy J Barberich | 3,103,705 | 0.32 | % | |||||||
| 19 | TD Ameritrade Clearing | 3,038,153 | 0.32 | % | |||||||
| 20 | E Trade Financial Corporation | 2,901,588 | 0.30 | % | |||||||
| Total CDIs held by top 20 CDI holders | 1,757,513,576 | 92.49 | % | ||||||||
| Total CDIs held by all other CDI holders | 68,719,674 | 7.51 | % | ||||||||
| TOTAL | 1,826,233,250 | ||||||||||
Holders of CDIs and common stock combined
The table below provides a list of the top 20 holders of GI Dynamics securities taking into account securities held in the form of both common stock and CDIs. Information presented below is prepared on the assumption that all shares of common stock on issue are held as CDIs. Related but separate legal entities are not aggregated.
47
Details of Stockholders if all shares of common stock on issue are held as CDIs
| No. | Name of Holder | Number of CDIs Held | % of Total CDIs | ||||||||
| 1 | Crystal Amber Fund | 1,337,659,335 | 73.10 | % | |||||||
| 2 | Mr Richard Cashin | 199,364,713 | 10.89 | % | |||||||
| 3 | Mr Paul Cozzi | 40,358,565 | 2.21 | % | |||||||
| 4 | MS Pace LP | 39,115,442 | 2.14 | % | |||||||
| 5 | Advanced Technology Ventures | 31,719,318 | 1.73 | % | |||||||
| 6 | Johnson & Johnson Development Corp | 28,278,460 | 1.55 | % | |||||||
| 7 | Polaris Venture Partners | 17,222,719 | 0.94 | % | |||||||
| 8 | Mr David Brock | 10,001,249 | 0.55 | % | |||||||
| 9 | Bank Leumi Le Israel BM a/c Main | 8,504,551 | 0.46 | % | |||||||
| 10 | Mr & Mrs Ian Moore | 7,000,060 | 0.38 | % | |||||||
| 11 | Fidelity Pacific Basin Fund | 5,561,290 | 0.30 | % | |||||||
| 12 | Invus Public Equity Advisors | 5,185,659 | 0.28 | % | |||||||
| 13 | Ms Rena C Merchant | 4,685,000 | 0.26 | % | |||||||
| 14 | Transact | 3,790,771 | 0.21 | % | |||||||
| 15 | MK Pension Plan | 3,437,363 | 0.19 | % | |||||||
| 16 | UBS Zurich | 3,419,165 | 0.19 | % | |||||||
| 17 | Mr Colin P Smith & Ms Mary Christoforou | 3,166,470 | 0.17 | % | |||||||
| 18 | Mr Timothy J Barberich | 3,103,705 | 0.17 | % | |||||||
| 19 | TD Ameritrade Clearing | 3,038,153 | 0.17 | % | |||||||
| 20 | E Trade Financial Corporation | 2,901,588 | 0.16 | % | |||||||
| Total securities held by top 20 securities holders | 1,757,513,576 | 96.04 | % | ||||||||
| Total securities held by all other securities holders | 72,401,224 | 3.96 | % | ||||||||
| 1,829,914,800 | |||||||||||
Options (not listed on ASX)
As of March 12, 2020, there are 3,186,154 options on issue to purchase shares of common stock under the 2011 Employee, Director and Consultant Equity Incentive Plan and the 2003 Omnibus Stock Plan with varying exercise prices. These options are held by 27 individuals.
Restricted Stock Units (not listed on ASX)
As of March 12, 2020, there are 250,000 performance related restricted stock units on issue for 250,000 shares of common stock under the 2011 Employee, Director and Consultant Equity Incentive Plan. These restricted stock units are held by 1 individual.
Warrants (not listed on ASX)
There are two warrants on issue as of March 12, 2020. One to subscribe for in aggregate 28,532 shares of common stock at an exercise price of $0.64 per share and another to subscribe for in aggregate 4,596,893 shares of common stock at an exercise price of $1.00.
Restricted Securities
There were 866,037,501 CDIs held by Crystal Amber on account with Link Market Services which are restricted as to sale or transfer until June 30, 2020 or later. These CDIs were issued with a one-year sale restriction as a result of the July 3, 2019 note conversions and the warrant exercises that were executed between August 23, 2019 and November 15, 2019.
Voting Rights
The Company’s bylaws provide that each stockholder has one vote for every share of common stock entitled to vote held of record by such stockholder and a proportionate vote for each fractional share of common stock entitled to vote so held, unless otherwise provided by Delaware General Corporation Law or in the certificate of incorporation.
Holders of CDIs have one vote for every fifty CDIs held of record by such stockholder. If holders of CDIs wish to attend the Company’s general meetings, they will be able to do so. Under the ASX Listing Rules, the Company, as an issuer of CDIs, must allow CDI holders to attend any meeting of the holders of the underlying securities unless relevant U.S. law at the time of the meeting prevents CDI holders from attending those meetings.
48
In order to vote at such meetings, CDI holders have the following options:
| a) | Instructing CHESS Depositary Nominees Pty Ltd (CDN), as the legal owner, to vote the shares of common stock underlying their CDIs in a particular manner. The instruction form must be completed and returned to the Company’s share registry prior to the meeting; |
| b) | Informing the Company that they wish to nominate themselves or another person to be appointed as CDN’s proxy for the purposes of attending and voting at the general meeting; and |
| c) | Converting their CDIs into a holding of shares of common stock and voting these at the meeting (however, if thereafter the former CDI holder wishes to sell their investment on the ASX, it would be necessary to convert the shares of common stock back to CDIs). This must be done prior to the record date for the meeting. |
As holders of CDIs will not appear on the Company’s share register as the legal holders of shares of common stock, they will not be entitled to vote at GI Dynamics stockholder meetings unless one of the above steps is undertaken.
Proxy forms and details of these alternatives will be included in each notice of meeting the Company sends to CDI holders.
Holders of restricted stock units, issued but unexercised options and warrants are not entitled to vote.
Required Statements
GI Dynamics, Inc. makes the following disclosures:
| a) | There is no current on-market buy-back of GI Dynamics securities. |
| b) | GI Dynamics, Inc. is incorporated in the state of Delaware in the United States of America. |
| c) | GI Dynamics, Inc. is not subject to Chapters 6, 6A, 6B or 6C of the Corporations Act 2001 (Cth), or Corporations Act, dealing with the acquisitions of shares (including substantial shareholdings and takeovers). |
| d) | Under the Delaware General Corporation Law, shares are generally freely transferable subject to restrictions imposed by U.S. federal or state securities laws, by the Company’s certificate of incorporation or bylaws, or by an agreement signed with the holders of the shares at issue. The Company’s amended and restated certificate of incorporation and bylaws do not impose any specific restrictions on transfer. Section 203 of the Delaware General Corporation Law prohibits a publicly-held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years following the time the person became an interested stockholder, unless the business combination or acquisition of shares that resulted in a stockholder becoming an interested stockholder is approved in a prescribed manner. A “business combination” can include a merger, asset or share sale, or other transaction resulting in a financial benefit to an interested stockholder. Generally, an interested stockholder is a person who, together with its affiliates and associates, owns (or within three years prior to the determination of interested stockholder status did own) 15% or more of a corporation’s voting shares. The existence of this provision would be expected to have an anti-takeover effect with respect to transactions not approved in advance by the board of directors, including discouraging attempts that might result in a premium over the market price for the shares of common stock held by stockholders. As a general matter, Section 203 applies solely to corporations with a class of voting stock listed on a national securities exchange in the U.S. or held of record by 2,000 or more stockholders, neither of which currently apply to the Company, but may at any time in the future. |
| e) | GI Dynamics has used the cash (and assets in a form readily convertible to cash) that it had at the time of admission to the ASX in a manner consistent with the Company’s stated business objectives (as described in the Australian prospectus lodged with the Australian Securities and Investments Commission with respect to the GI Dynamics IPO) from the time of the Company’s admission to the ASX through to December 31, 2019. |
| f) | The securities of GI Dynamics, Inc. are not quoted on any exchange other than the ASX. |
| g) | The name of the Company’s Corporate Secretary is Charles R. Carter. |
| h) | The address and telephone number of the Company’s principal registered office in Australia is: |
KPMG
Tower Three
International Towers Sydney
300 Barangaroo Avenue
Sydney NSW 2000 Australia
Telephone: +61 2 9335 8054
49
| i) | Registers of securities are held as follows: |
| 1. | for CDIs in Australia at: | |
| Link
Market Services Limited |
| 2. | for common stock in the United States at: | |
| American
Stock Transfer & Trust Company, LLC |
Australian Corporate Governance Statement
The Company’s board of directors, or Board, and employees are committed to developing, promoting and maintaining a strong culture of good corporate governance and ethical conduct.
The Board confirms that the Company’s corporate governance framework is generally consistent with the ASX Corporate Governance Council’s “Corporate Governance Principles and Recommendations 3rd Edition” (“ASX Governance Recommendations”), other than as set out below. To this end, the Company provides below a review of its corporate governance framework using the same numbering as adopted for the principles as set out in the ASX Governance Recommendations.
This corporate governance statement relates to the financial year ended December 31, 2019 and has been approved by the Board.
Copies of the Company’s codes and policies may be downloaded from the corporate governance section of the Company’s website at www.gidynamics.com.
Principle 1 – Lay solid foundations for management and oversight
Recommendation 1.1 – A listed entity should disclose:
| a) | the respective roles and responsibilities of its board and management; and |
| b) | those matters expressly reserved to the board and those delegated to management. |
The Board’s responsibilities are recognized and documented by the charter of the Board (“Board Charter”), a copy of which is available on the Company’s website at www.gidynamics.com, and there is a clear delineation between the Board’s responsibility for the Company’s strategy and activities, and the day-to-day management of operations conferred upon the Company’s officers.
The Board Charter provides that the role of the Board, as the body ultimately responsible for the corporate governance of the Company, includes the following major functions:
| ● | providing input into and final approval of management’s development of corporate strategy and performance objectives; |
| ● | reviewing, ratifying and monitoring systems of risk management and internal control, codes of conduct, and legal compliance; |
| ● | ensuring appropriate resources are available to senior executives; |
| ● | approving and monitoring the progress of major capital expenditure, capital management and acquisitions and divestments; |
50
| ● | approving and monitoring financial and other reporting; |
| ● | evaluating the overall effectiveness of the Board and its committees; and |
| ● | evaluating, selecting and recommending an appropriate slate of candidates for election as directors. |
Management is responsible for implementing the strategic objectives set by the Board, carrying out the day-to-day operations of the Company, and making accurate, timely, and clear reports to the Board.
Recommendation 1.2 – A listed entity should:
| a) | undertake the appropriate checks before appointing a person, or putting forward to security holders a candidate for election, as a director; and |
| b) | provide security holders with all material information in its possession relevant to a decision on whether or not to elect or re-elect a director. |
The nominating and corporate governance committee of GI Dynamics is responsible for reviewing, with the Board from time to time, the appropriate skills and characteristics required of Board members in the context of the current make-up of the Board and the Company’s business needs. When considering Board appointments, the committee ensures that appropriate checks are undertaken on the candidate’s character, education, qualifications, criminal record and bankruptcy history and that sufficient information is provided to security holders when a candidate is standing for election or re-election as a director to enable them to make an informed decision on whether or not to elect or re-elect the candidate. Information regarding the director who was re-appointed at the Company’s 2019 annual general meeting was provided in the notice of meeting disclosed to the ASX and stockholders on June 21, 2019.
Recommendation 1.3 – A listed entity should have a written agreement with each director and senior executive setting out the terms of their appointment.
The terms of Board membership are set forth in the GI Dynamics Board Charter and the remuneration paid to Board members is provided in accordance with stockholder approval (where required) following the compensation committee’s recommendation. While the Company does not have a separate written agreement with each of its Board members, it believes the terms set out in the Board Charter are adequate to provide a clear understanding of the roles and responsibilities of Board members. In the case of senior executives, the Company has provided a letter or contract of employment to each executive detailing the terms of employment and has developed job descriptions setting forth the position, duties, and reporting structure. Where there are any agreed entitlements upon termination, such agreed items are set forth in the employment letters or contracts.
Recommendation 1.4 - The company secretary of a listed entity should be accountable directly to the board, through the chair, on all matters to do with the proper functioning of the board:
The role and responsibilities of the Company’s secretary are set forth in the Company’s bylaws. The Board is responsible for electing or appointing the secretary and for prescribing the duties and powers of the secretary. Each director is able to communicate freely and directly with the secretary and vice versa. The secretary is accountable to the Board, through the chairman of the Board, for all matters to do with the proper functioning of the Board, including:
| ● | monitoring the Company’s compliance in respect of all corporate governance matters; |
| ● | drafting and circulating the minutes of meetings of the Board and all committees for approval at the next meeting; and |
| ● | monitoring the Company’s compliance with all disclosure obligations and regularly reviewing Company policies and procedures relating to compliance with such disclosure obligations. |
Recommendation 1.5 – A listed entity should:
| a) | have a diversity policy which includes requirements for the board or a relevant committee of the board to set measurable objectives for achieving gender diversity and to assess annually both the objectives and the entity’s progress in achieving them; |
| b) | disclose that policy or a summary of it; and |
51
| c) | disclose as at the end of each reporting period the measurable objectives for achieving gender diversity set by the board or a relevant committee of the board in accordance with the entity’s diversity policy and its progress towards achieving them, and either: |
| i. | the respective proportions of men and women on the board, in senior executive positions and across the whole organization (including how the entity has defined ‘senior executive’ for these purposes); or |
| ii. | if the entity is a “relevant employer” under the Workplace Gender Equality Act, the entity’s most recent “Gender Equality Indicators”, as defined in and published under that Act. |
The Company has adopted a Diversity Policy, a copy of which is available on the corporate governance section of the Company’s website. The Company’s Diversity Policy includes requirements for the Board to establish measurable objectives to assist the Company in achieving diversity.
The Board continued to evaluate the gender diversity of the Company’s employees, its senior executives, and the Board during 2019. During 2019, the Board, in considering measurable objectives to set for achieving gender diversity, concluded that, because of the current stage of the Company’s development and the ongoing restructuring efforts, the Company should continue to recruit employees from a diverse pool of talented candidates without regard to gender while continuing to focus on the necessary skills and experience required to achieve the Company’s performance objectives. As a result, the Company did not set measurable objectives for achieving gender diversity in 2019 but used 2018 data as a baseline to measure gender diversity among its employees, senior executives and Board for 2019.
At December 31, 2019, the proportion of women in the Company as a percentage of its total employees increased from 44% (4 out of 9 in 2018) to 50% (7 out of 14) based on data maintained by the Company’s human resources organization. In senior executive positions (vice president and above), the proportion of women remained the same at 0% (none out of 1 in 2018 and none of 3 in 2019). The proportion of women on the Board remained the same at 25% (1 out of 4 in 2018 and 2019).
Recommendation 1.6 – A listed entity should:
| a) | have and disclose a process for periodically evaluating the performance of the board, its committees and individual directors; and |
| b) | disclose, in relation to each reporting period, whether a performance evaluation was undertaken in the reporting period in accordance with that process. |
In accordance with the Company’s agreed evaluation process, during the reporting period ended December 31, 2019, the Board and each committee performed a self-evaluation. Each director provided their assessments of the effectiveness of the Board and the committees on which they serve to the nominating and corporate governance committee. The individual assessments were summarized by the nominating and corporate governance committee and reported for discussion to the full Board and the committees. The nominating and corporate governance committee completed its assessment of the Board’s compliance with the principles set forth in the Board Charter and did not identify any areas in which the Board or committees needed to improve performance and has reviewed and approved disclosures relating to any departures from the ASX Governance Recommendations. During the reporting period ended December 31, 2019, the nominating and corporate governance committee also evaluated individual directors in accordance with the criteria set by the nominating and corporate governance committee and the Board from time to time. Based on such assessments, the nominating and corporate governance committee has determined that the Board, its committees and each director were effective.
Recommendation 1.7 – A listed entity should:
| a) | have and disclose a process for periodically evaluating the performance of its senior executives; and |
| b) | disclose, in relation to each reporting period, whether a performance evaluation was undertaken in the reporting period in accordance with that process. |
52
Under the Board Charter, the directors of GI Dynamics are ultimately responsible for monitoring the performance of the senior management team and the compensation committee, in accordance with its charter, reviews and approves corporate and personal performance goals and objectives relevant to the compensation of all executive officers. At the end of each calendar year, the chief executive officer presents to the compensation committee his assessment of the performance during the year of each executive officer (other than himself) against pre-established performance objectives. The compensation committee considers this assessment and determines each executive officer’s (including the chief executive officer’s) compensation, including but not limited to salary, bonus, incentive compensation and equity awards based on such an evaluation. In addition, the compensation committee is responsible for regularly reviewing the Company’s compensation, recruitment, retention and termination policies for senior executives.
In 2020, a performance evaluation of the Company’s executive officers for the year ended December 31, 2019 was undertaken before March 15, 2020. Following each performance evaluation, the Company’s compensation committee reviewed and approved changes to the compensation of the Company’s executive officers based on the individual levels of achievement against pre-established performance objectives.
Further information regarding executive compensation for the year ended December 31, 2019, as required by Item 11 of this Annual Report on Form 10-K, is incorporated by reference to the applicable information to be included in the Company’s Proxy Statement to be filed with the SEC in connection with its 2020 Annual Meeting of Stockholders or an amendment to this report filed under cover of Form 10-K/A not later than 120 days after the end of the fiscal year ended December 31, 2019.
Principle 2 – Structure the board to add value
Recommendation 2.1 – The board of a listed entity should:
| a) | have a nomination committee which: |
| i. | has at least three members, a majority of whom are independent directors; and |
| ii. | is chaired by an independent director; |
and disclose:
| iii. | the charter of the committee; |
| iv. | the members of the committee; and |
| v. | as at the end of each reporting period the number of times the committee met throughout the period and the individual attendances of the members at those meetings. |
As of December 31, 2019, the members of the nominating and corporate governance committee were Daniel Moore and Dr. Oern Stuge (Chair). Mr. Moore and Dr. Stuge are considered independent directors for ASX, NASDAQ and SEC purposes and a copy of the Nominating and Corporate Governance Committee Charter is available on the corporate governance section of the Company’s website. The nominating and corporate governance committee met once during 2019 and both members were present at the meeting.
At December 31, 2019, there were only two directors appointed as members of the nominating and corporate governance committee (instead of the recommended three members), which was a decision made by the Board initially at the end of 2017 (and reported in the corporate governance statement for December 31, 2017) in order to properly utilize the resources of the four members of the Board across all of the various committees. As a result of only having two members, the Company was not fully compliant with recommendation 2.1 for the 2019 reporting period. The Board of Directors will continue to periodically assess the effectiveness of this committee, including the size and the experience of the members appointed, with a view to ensuring that the committee’s performance accords with the best possible practice in the context of the overall Board size and structure.
Recommendation 2.2 – A listed entity should have and disclose a board skills matrix setting out the mix of skills and diversity that the board currently has or is looking to achieve in its membership.
The nominating and corporate governance committee is responsible for reviewing with the Board from time to time the appropriate skills and characteristics required of Board members in the context of the current make-up of the Board and the Company’s business needs. This assessment includes, among other things, an individual’s business experience and skills (including skills in core areas such as operations, management, technology, medical device industry knowledge, accounting and finance, marketing, leadership, strategic planning and international markets), independence, judgment, integrity and ability to commit sufficient time and attention to the activities of the Board, as well as the absence of any potential conflicts with the Company’s interests. The nominating and corporate governance committee considers these criteria in the context of an assessment of the perceived needs of the Board as a whole and seeks to achieve diversity of occupational and personal backgrounds on the Board.
53
Information regarding the skills, experience and expertise relevant to each director is set out in the section titled “Directors of the Registrant” in this Item 10.
While the Board did not disclose the Board skills matrix for the reporting period the Board believes that its members possess the mix of skills and diversity that the Company needs at this stage of its development. The Board continues to evolve a board skills matrix setting out the mix of skills and diversity that the Board currently has or is looking to achieve in its membership.
Recommendation 2.3 – A listed entity should disclose:
| a) | the names of the directors considered by the board to be independent directors; |
| b) | if a director has an interest, position, association or relationship of the type described in this recommendation but the board is of the opinion that it does not compromise the independence of the director, the nature of the interest, position, association or relationship in question and an explanation of why the board is of that opinion; and |
| c) | the length of service of each director. |
The Company considers that a director is an independent director where that director is free from any interest and any business or other relationship which could, or could reasonably be perceived to, materially interfere with the director’s decisions relating to the Company or with the director’s ability to act in the best interests of the Company. The Company also assesses the independence of its directors with regard to requirements for independence set out under ASX Governance Recommendation 2.3.
The composition and tenure of the Board as of December 31, 2019, as well as each member’s independence status during 2019, was as follows:
| Committees | ||||||||||||||||||||||
| Nominating | ||||||||||||||||||||||
| and | ||||||||||||||||||||||
| Corporate | ||||||||||||||||||||||
| Director | Director Position | Tenure1 | Independent | Audit | Compensation | Governance | ||||||||||||||||
| Daniel J. Moore | Non-executive Chairman | 5.3 years | Yes | X | ||||||||||||||||||
| Timothy J. Barberich | Non-executive Director | 8.6 years | Yes | X | Chair | |||||||||||||||||
| Dr. Oern Stuge | Non-executive Director | 3.0 years | Yes | X | Chair | |||||||||||||||||
| Juliet Thompson | Non-executive Director | 2.3 years | Yes | Chair | ||||||||||||||||||
| 1. | Calculated as of December 31, 2019 |
The number of directors’ meetings (including meetings of committees) and number of meetings attended by each of the directors during the reporting period are as follows:
| Committee Meetings | ||||||||||||||||||||||||||||||||
| Nominating and | Compensation | |||||||||||||||||||||||||||||||
| Directors’ Meetings | Audit Committee | Corporate Governance | Committee | |||||||||||||||||||||||||||||
| Director | A | B | A | B | A | B | A | B | ||||||||||||||||||||||||
| Daniel J. Moore | 11 | 11 | — | — | 1 | 1 | — | — | ||||||||||||||||||||||||
| Timothy J. Barberich | 11 | 11 | 4 | 4 | — | — | 2 | 2 | ||||||||||||||||||||||||
| Dr. Oern Stuge | 11 | 11 | — | — | 1 | 1 | 2 | 2 | ||||||||||||||||||||||||
| Juliet Thompson | 11 | 11 | 4 | 4 | — | — | — | — | ||||||||||||||||||||||||
1 – Calculated as of December 31, 2019
A – Number of meetings attended.
B – Number of meetings held during the time the director held office during the reporting period.
54
Independent advice
At the Company’s expense, each member of the Board and each member of a committee of the Board is entitled to seek advice from independent external advisers in relation to any matter that is considered necessary to fulfil their relevant duties and responsibilities.
Recommendation 2.4 – A majority of the board of a listed entity should be independent directors.
The Board for the reporting period comprised a majority of independent directors.
Recommendation 2.5 – The chair of the board of a listed entity should be an independent director and, in particular, should not be the same person as the CEO of the entity.
In compliance with the ASX Governance Recommendations, the chairman of the Board is an independent director and the roles of the chairman and the chief executive officer of the Company are not currently exercised by the same individual. However, the Company’s Board Charter does not specifically address whether or not the offices of chairman and chief executive officer should be vested in the same person or two different people, or whether the chairman should be an employee of the Company or should be elected from among the non-executive directors. The needs of the Company and the individuals available to serve in these roles may dictate different outcomes at different times, and the Board believes that retaining flexibility in these decisions is in the best interest of the Company and its stockholders.
Recommendation 2.6 – A listed entity should have a program for inducting new directors and provide appropriate professional development opportunities for directors to develop and maintain the skills and knowledge needed to perform their roles as directors effectively.
The nominating and corporate governance committee of the Board continually assesses the needs of the Company and the skills and knowledge required of its Board members. On appointment, new directors are provided with induction information that generally includes historical information about the Company and its operations, details of the Company’s directors’ and officers’ insurance, the Company’s corporate governance guidelines, and other Company governance policies. The induction process also involves one-on-one discussions with the Chairman and other directors and briefings from senior management to help new directors participate actively in Board decision making at the earliest opportunity. When it is necessary, resources are provided for the Board as a whole, and for individual Board members as needed, to supplement their skills and knowledge and fill any identified gaps.
Principle 3 – Act ethically and responsibly
Recommendation 3.1 – A listed entity should:
| a) | have a code of conduct for its directors, senior executives and employees; and |
| b) | disclose that code or a summary of it. |
The Company has adopted a Code of Business Conduct and Ethics and an Insider Trading Policy, copies of which are available on the corporate governance section of the Company’s website.
Principle 4 – Safeguard integrity in corporate reporting
Recommendation 4.1 – The board of a listed entity should:
| a) | have an audit committee which: |
| i. | has at least three members, all of whom are non-executive directors and a majority of whom are independent directors; and |
| ii. | is chaired by an independent director, who is not the chair of the board; |
and disclose:
| iii. | the charter of the committee; |
| iv. | the relevant qualifications and experience of the members of the committee; and |
| v. | in relation to each reporting period, the number of times the committee met throughout the period and the individual attendances of the members at those meetings. |
55
The Board has established an audit committee to oversee the management of the Company’s financial and internal risks and reporting. As of December 31, 2019, the members of the audit committee were Juliet Thompson and Timothy Barberich, both independent, non-executive directors. The audit committee is chaired by Juliet Thompson who is an independent director and not chair of the Board.
The audit committee met four times during 2019 with Ms. Thompson and Mr. Barberich attending on all four occasions.
At December 31, 2019, there were only two directors appointed as members of the audit committee (instead of the recommended three members), which was a decision made by the Board initially at the end of 2017 (and reported in the corporate governance statement for December 31, 2017) in order to properly utilize the resources of the four members of the Board across all of the various committees. As a result of only having two members, the Company was not fully compliant with recommendation 4.1 for the 2019 reporting period. The Board of Directors will continue to periodically assess the effectiveness of this committee, including the size and the experience of the members appointed, with a view to ensuring that the committee’s performance accords with the best possible practice in the context of the overall Board size and structure.
The members of the audit committee must be financially literate and have familiarity with financial and accounting matters, with at least one member a qualified accountant or other financial professional with appropriate expertise in financial and accounting matters. The qualifications of those appointed to the audit committee are set out in the section titled “Directors of the Registrant” in this Item 10.
The audit committee is governed by the Audit Committee Charter, a copy of which is available on the corporate governance section of the Company’s website.
In its Audit Committee Charter, the Company has disclosed its policy for the selection and appointment of the Company’s independent auditor and for the rotation of the lead audit partner having primary responsibility for the audit and the audit partner responsible for reviewing the audit every five years. The audit committee will regularly report to the Board about committee activities, issues and related recommendations.
Recommendation 4.2 – The board of a listed entity should, before it approves the entity’s financial statements for a financial period, receive from its CEO and CFO a declaration that, in their opinion, the financial records of the entity have been properly maintained and that the financial statements comply with the appropriate accounting standards and give a true and fair view of the financial position and performance of the entity and that the opinion has been formed on the basis of a sound system of risk management and internal controls which is operating effectively.
As the Company prepares and files its consolidated financial statements under United States accounting practices and laws, management is required to provide representations to the Board on a wide range of issues, including the effectiveness of the Company’s disclosure controls and procedures as well as the design and operation of internal control over financial reporting. However, as the Company is incorporated in the State of Delaware, United States, it is not required to provide a declaration under section 295A of the Corporations Act. To this end, stockholders’ attention is drawn to Item 9A of this Annual Report on Form 10-K and the certifications provided by the principal executive officer and the principal financial officer at the end of the Annual Report on Form 10-K. As stated above, Item 9A discloses information regarding the Company’s controls and procedures and management’s evaluation of the effectiveness of its internal control over financial reporting. As required by Rule 13a-15(d) of the Exchange Act, management, including the principal executive officer and the principal financial and accounting officer, conducted an evaluation of the internal control over financial reporting to determine whether any changes occurred during the year ended December 31, 2019 that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting. Based on that evaluation, the principal executive officer and principal financial and accounting officer have concluded that, based on and as of the time of such evaluation, the Company’s disclosure controls and procedures were effective at the reasonable assurance level and believes the consolidated financial statements included in this Annual Report present, in all material respects, the Company’s financial position, results of operations and cash flows for the periods presented in conformity with U.S. generally accepted accounting principles.
56
Recommendation 4.3 – A listed entity that has an annual general meeting should ensure that its external auditor attends its AGM and is available to answer questions from security holders relevant to the audit.
The Company’s policy is to ensure its external auditor attends the annual general meeting of stockholders, in person, to have an opportunity to make a statement, if desired, and to respond to appropriate questions from security holders regarding the audit. The Company’s auditor for the year ended December 31, 2018 was Moody, Famiglietti & Andronico, LLP (“MFA”). MFA attended the 2019 annual general meeting with respect to the financial year ended December 31, 2018. The Company’s auditor for the year ended December 31, 2019 is Wolf and Company P.C. (“Wolf”) and Wolf intends to be present at the 2020 Annual Meeting of Stockholders.
Principle 5 – Make timely and balanced disclosure
Recommendation 5.1 – A listed entity should:
| a) | have a written policy for complying with its continuous disclosure obligations under the Listing Rules; and |
| b) | disclose that policy or a summary of it. |
The Company is committed to providing timely and balanced disclosure to the market in accordance with its continuous disclosure obligations. In accordance with its commitment to fully comply with its continuous disclosure obligations and to ensure accountability at a senior management level for that compliance, the Company has adopted a Continuous Disclosure Policy, together with other internal mechanisms and reporting requirements. A copy of the Company’s Continuous Disclosure Policy is available on the corporate governance section of the Company’s website. In addition, a copy of all of the Company’s ASX announcements, financial reports and related public information is also available on the Company’s website.
Principle 6 – Respect the rights of security holders
Recommendation 6.1 – A listed entity should provide information about itself and its governance to investors via its website.
The Company aims to provide stockholders with comprehensive and timely access to Company documents and releases through its website, including:
| ● | details of the Company’s certificate of incorporation and bylaws, Board and committee charters and key corporate governance policies; |
| ● | copies of all material information lodged with ASX and any other applicable securities regulators and securities exchanges; |
| ● | copies of material announcements, financial reports, briefings and speeches made to the market or media; |
| ● | a means for the stockholders to submit enquiries directly to the Company; |
| ● | the full text of notices of stockholder meetings and explanatory material; and |
| ● | advance notice of all open briefings to institutional investors and analysts, including copies of presentation materials. |
Other information may be provided to stockholders via periodic mail-outs. In addition, the Company allows stockholders to elect to receive email communications where appropriate.
Recommendation 6.2 – A listed entity should design and implement an investor relations program to facilitate effective two-way communications with investors.
The Company has adopted a Stockholder Communications Policy which supports effective two-way communication with its stockholders, a copy of which is available on the corporate governance section of the Company’s website. The Company seeks to utilize numerous modes of communication, including electronic communication, to ensure that its communication with stockholders is frequent, clear, and accessible. Stockholders are entitled to and encouraged to participate in briefing calls and/or contact the Company directly with questions or concerns. Contact information is provided in each communication with stockholders, as well as on the Company’s website.
57
Recommendation 6.3 – A listed entity should disclose the policies and processes it has in place to facilitate and encourage participation at meetings of security holders.
All stockholders are invited to attend the Company’s annual general meeting either in person or by proxy. The Board regards the annual general meeting as an excellent forum in which to discuss issues relevant to the Company and accordingly encourages full participation by stockholders. To facilitate attendance, the Company arranges the annual general meeting to be held in an easily accessed location and announces the date and location of the meeting in advance of the meeting. Stockholders have an opportunity to submit questions to the Board and the Company’s auditor. The meeting may also be audio cast and/or webcast to provide access to those stockholders who are unable to attend the annual general meeting in person.
Recommendation 6.4 – A listed entity should give security holders the option to receive communications from, and send communications to, the entity and its security registry electronically.
The Company provides its stockholders with the option to receive communications from, and send communications to, the Company and the Company’s share registry electronically.
Principle 7 – Recognize and manage risk
Recommendation 7.1 – The board of a listed entity should:
| a) | have a committee or committees to oversee risk, each of which: |
| i. | has at least three members, a majority of whom are independent directors; and |
| ii. | is chaired by an independent director; |
and disclose:
| iii. | the charter of the committee; |
| iv. | the members of the committee; and |
| v. | as at the end of each reporting period, the number of times the committee met throughout the period and the individual attendances of the members at those meetings. |
The risks that the Company faces are continually changing in line with the development of the Company. In simple terms, risk is inherent in all activities undertaken by the Company. Many of these risks are beyond the control of the Company and, as such, it is important that risk be mitigated on a continuous basis, particularly if the Company is to preserve stockholder value.
To ensure appropriate oversight and management of material business risks, the Company has adopted a Risk Management Policy that sets forth the process to identify, assess, and manage risk in the Company’s business operations. A copy of the policy is available on the corporate governance section of the Company’s website.
The day-to-day oversight and management of the Company’s risk management program has been conferred upon the audit committee. The audit committee is responsible for ensuring that the Company maintains effective risk management and internal control systems and processes and provides regular reports to the Board on the effectiveness of the risk management program in identifying and addressing material business risks. Details of the audit committee are set out above in response to ASX Governance Recommendation 4.1.
In addition, the Board is responsible for reviewing and ratifying the risk management structure, processes and guidelines which are developed and maintained by senior management. The audit committee or management may also refer particular risk management issues to the Board for final consideration and direction.
Recommendation 7.2 – The board or a committee of the board should:
| a) | review the entity’s risk management framework at least annually to satisfy itself that it continues to be sound; and |
| b) | disclose, in relation to each reporting period, whether such a review has taken place. |
58
While the Board does not currently conduct a formal annual review of the material risks to the Company and the methods used to identify and communicate those risks, the Board continually assesses these matters. The Board holds regular meetings by teleconference as well as at the Company’s facility in Boston, Massachusetts, for the purposes of discussing and reviewing operational developments and reviewing the effectiveness of the implementation of the Company’s risk management systems.
The Company’s Risk Management Policy (a copy of which is available on the corporate governance section of the Company’s website) also requires that management report on an on-going basis to the Board, primarily through the audit committee which has the responsibility for day-to-day oversight of the Company’s risk management program, on the status and effectiveness of the risk management program.
Recommendation 7.3 – A listed entity should disclose:
| a) | if it has an internal audit function, how the function is structured and what role it performs; or |
| b) | if it does not have an internal audit function, that fact and the processes it employs for evaluating and continually improving the effectiveness of its risk management and internal control processes. |
The Company does not currently have an internal audit function. Rather, the Company has implemented the following processes to evaluate and continually improve the effectiveness of its risk management and internal control processes:
| ● | the Board has conferred responsibility on senior management to develop and maintain a risk management program in light of the day-to-day needs of the Company; |
| ● | the Board has established three standing committees to provide focused support in key areas; namely the nominating and corporate governance committee, audit committee and compensation committee; |
| ● | management provides the Board with frequent updates on the state of the Company’s business, including the risks that the Company faces from time-to-time allowing the Board to assess the Company’s management of its material business risks. These updates include up-to-date financial information, operational activity, clinical status and competitor updates. These updates are founded on internal communications that are fostered internally through weekly management meetings and other internal communications; and |
| ● | these processes operate in addition to the Company’s system of internal controls over financial reporting, its quality system, complaint handling processes, employee policies and standard operating procedures, which are all designed to address various forms of risk. |
Recommendation 7.4 – A listed entity should disclose whether it has any material exposure to economic, environmental and social sustainability risks and, if it does, how it manages or intends to manage those risks.
The economic risks that the Company is subject to and must manage are set out in the “Risk Factors” section of this Annual Report on Form 10-K. In general, the Board considers that the Company is not susceptible to material environmental or social sustainability risks in operating its business.
Principle 8 – Remunerate fairly and responsibly
Recommendation 8.1 – The board of a listed entity should:
| a) | have a remuneration committee which: |
| i. | has at least three members, a majority of whom are independent directors; and |
| ii. | is chaired by an independent director; |
and disclose:
| iii. | that charter of the committee; |
| iv. | the members of the committee; and |
| v. | as at the end of each reporting period, the number of times the committee met throughout the period and the individual attendances of the members at those meetings. |
59
The Board has established a compensation committee to review and assess executive and director compensation. The compensation committee is governed by the Compensation Committee Charter, a copy of which is available on the corporate governance section of the Company’s website.
As of December 31, 2019, the members of the compensation committee were Timothy J. Barberich (Chair) and Dr. Oern Stuge both independent non-executive directors. The compensation committee met twice during 2019 and both members were present. At December 31, 2019, the committee has two directors appointed (instead of the recommended three members), which was a decision made by the Board initially during 2017 (and reported in the corporate governance statement for December 31, 2017) in order to properly utilize the resources of the four members of the Board across all of the various committees. As a result of only having two members, the Company was not fully compliant with recommendation 8.1 for the 2019 reporting period. The Board of Directors will periodically assess the effectiveness of this committee, including the size and the experience of the members appointed, with a view to ensuring that the committee’s performance accords with the best possible practice in the context of the overall Board size and structure.
While the compensation committee reviews and reports compensation items to the Board for both non-executive directors and executive management, including each individual’s skills, knowledge, and contributions to the Company, the compensation committee does not provide a separate report of compensation by gender.
Recommendation 8.2 – A listed entity should separately disclose its policies and practices regarding the remuneration of non-executive directors and the remuneration of executive directors and other senior executives.
In accordance with the Compensation Committee Charter, the compensation committee is responsible for ensuring that the structure of non-executive and executive directors’ and other senior executives’ compensation is clearly distinguished.
The Company has adopted a non-executive director compensation policy pursuant to which non-executive directors are compensated for their services to the Board which includes annual cash fees for serving as a member or the chair of the Board and for serving as a member or the chair of the Board committees. In addition, the policy provides that non-executive directors may receive grants of a fixed number of options upon their joining the Board and annual grants (which commenced in 2014) of a fixed number of options and restricted stock units, in each case subject to the terms of the non-executive director compensation policy as well as the approval of stockholders. Juliet Thompson was granted 30,000 non-qualified stock options on August 22, 2017, and approval was obtained at the Company’s May 2018 annual general meeting. Following receipt of stockholder approval in December 2019, each of the four current Non-Executive Directors were granted Non-qualified Stock Options conferring the right to purchase up to 30,000 common shares of the Company for $1.34 per share any time within the period starting October 29, 2020 and ending December 16, 2029.
The Company has adopted a separate executive compensation program that consists of base salary, equity-based incentives, performance-based cash bonuses, severance benefits, and other customary benefits such as health insurance on the same basis as provided to all other employees. None of the Company’s non-executive directors are entitled to any retirement benefits. The Company currently has no executive directors.
Further information regarding the compensation committee, as required by Item 10 of this Annual Report on Form 10-K, is incorporated by reference to the applicable information in the proxy statement for the GI Dynamics 2020 Annual Meeting of Stockholders to be filed with the SEC and ASX.
Recommendation 8.3 – A listed entity which has an equity-based remuneration scheme should:
| a) | have a policy on whether participants are permitted to enter into transactions (whether through the use of derivatives or otherwise) which limit the economic risk of participating in the scheme; and |
| b) | disclose that policy or a summary of it. |
The Company provides compensation in the form of equity-based awards to non-executive directors (upon approval by stockholders), senior executives, and employees of the Company. Awards are made under the Company’s 2011 Employee, Director and Consultant Equity Incentive Plan, as amended, which has been approved by stockholders. The Company’s Insider Trading Policy, a copy of which is available on the corporate governance section of the Company’s website, provides a summary of the Company’s policy on prohibiting entering into transactions in associated products which limit the economic risk of participating in unvested entitlements under any equity-based remuneration schemes. This policy operates to help limit the economic risk to the Company’s securities.
This report is made in accordance with a resolution of the Board.
60
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item 11 is incorporated by reference herein to the Company’s Proxy Statement to be filed with the SEC in connection with its 2020 Annual Meeting of Stockholders or an amendment to this report filed under cover of Form 10-K/A not later than 120 days after the end of the fiscal year ended December 31, 2019.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information relating to security ownership of certain beneficial owners of the Company’s common stock and information relating to the security ownership of management required by this Item 12 is incorporated by reference herein to the Company’s Proxy Statement to be filed with the SEC in connection with its 2020 Annual Meeting of Stockholders or an amendment to this report filed under cover of Form 10-K/A not later than 120 days after the end of the fiscal year ended December 31, 2019.
The table below sets forth information with regard to shares authorized for issuance under the Company’s equity compensation plans as of December 31, 2019. As of December 31, 2019, GI Dynamics had two active equity compensation plans, each of which was approved by GI Dynamics stockholders:
| ● | The GI Dynamics 2003 Omnibus Stock Plan; and |
| ● | The GI Dynamics 2011 Employee, Director and Consultant Equity Incentive Plan. |
| Plan Category | Number of shares to be issued upon exercise of outstanding options or vesting of restricted stock units | Weighted- average exercise price of outstanding options | Number of shares remaining available for future issuance under equity compensation plans1 | |||||||||
| Equity compensation plans approved by security holders | 3,346,154 | $ | 1.36 | 134,866 | ||||||||
| Equity compensation plans not approved by security holders | — | — | ||||||||||
| Total | 3,346,154 | $ | 1.36 | 134,866 | ||||||||
| 1) | The GI Dynamics 2011 Employee, Director and Consultant Equity Incentive Plan allows for an annual increase in the number of shares available for issue commencing on the first day of each fiscal year during the period beginning in fiscal year 2012 and ending in fiscal year 2020. The annual increase in the number of shares shall be equal to the lowest of: (i) 500,000 shares; (ii) 4% of the number of common shares outstanding as of such date; and (iii) an amount determined by the board of directors or the compensation committee. |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this Item 13 is incorporated by reference herein to the Company’s Proxy Statement to be filed with the SEC in connection with its 2020 Annual Meeting of Stockholders or an amendment to this report filed under cover of Form 10-K/A not later than 120 days after the end of the fiscal year ended December 31, 2019.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by this Item 13 is incorporated by reference herein to the Company’s Proxy Statement to be filed with the SEC in connection with its 2020 Annual Meeting of Stockholders or an amendment to this report filed under cover of Form 10-K/A not later than 120 days after the end of the fiscal year ended December 31, 2019.
61
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
| (a) | List of documents filed as part of this Annual Report on Form 10-K |
| (1) | Consolidated Financial Statements listed under Part II, Item 8 and included herein by reference. |
| (2) | Consolidated Financial Statement Schedules |
No schedules are submitted because they are not applicable, not required or because the information is included in the Consolidated Financial Statements or Notes to Consolidated Financial Statements.
| (3) | Exhibits |
The exhibits listed in the accompanying Exhibit Index are filed or incorporated by reference as part of this Annual Report on Form 10-K.
Not applicable.
62
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, hereunto duly authorized.
| GI Dynamics, Inc. | ||
| Date: March 26, 2020 | By: | /s/ SCOTT W. SCHORER |
| Name: | Scott W. Schorer | |
| Title: | President, Chief Executive Officer | |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ SCOTT W. SCHORER | President, Chief Executive Officer | March 26, 2020 | ||
| Scott W. Schorer | (Principal Executive Officer) | |||
| /s/ CHARLES R. CARTER | Chief Financial Officer, Treasurer, Secretary | March 26, 2020 | ||
| Charles R. Carter | (Principal Financial and Accounting Officer) | |||
| /s/ DANIEL J. MOORE | Chairman and Director | March 26, 2020 | ||
| Daniel J. Moore | ||||
| /s/ TIMOTHY J. BARBERICH | Director | March 26, 2020 | ||
| Timothy J. Barberich | ||||
| /s/ OERN STUGE, MD | Director | March 26, 2020 | ||
| Oern Stuge, MD | ||||
| /s/ JULIET THOMPSON | Director | March 26, 2020 | ||
| Juliet Thompson |
63
64
65
| * | Filed herewith. |
| ‡ | Furnished herewith. |
| † | Management contract or compensatory plan or arrangement. |
66
GI Dynamics, Inc. and Subsidiaries
Index to Consolidated Financial Statements
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
GI Dynamics, Inc.
Boston, Massachusetts
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of GI Dynamics, Inc. and Subsidiaries (the Company) as of December 31, 2019, the related consolidated statements of operations, stockholders’ deficit, and cash flows for the year ended December 31, 2019, and the related notes (collectively the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019, and the results of its operations and its cash flows for the year then ended in conformity with accounting principles generally accepted in the United States of America.
Emphasis of Matter Regarding Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has suffered losses from operations since inception and has an accumulated deficit and working capital deficiency that raises substantial doubt about the Company’s ability to continue as a going concern. Management’s evaluation of the events and conditions and management’s plans regarding these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our opinion is not modified with respect to this matter.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audit provides a reasonable basis for our opinion.
| /s/ Wolf and Company, P.C. | |
| We have served as the Company’s auditor since 2019. | |
| Boston, Massachusetts | |
| March 26, 2020 |
F-2
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
GI Dynamics, Inc.
Boston, Massachusetts
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of GI Dynamics, Inc. and Subsidiaries (the Company) as of December 31, 2018, and the related consolidated statements of operations, stockholders’ equity (deficit), and cash flows for each of the year ended December 31, 2018, and the related notes (collectively referred to as the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2018, and the results of their operations and their cash flows for each of the years in the two-year period then ended, in conformity with accounting principles generally accepted in the United States of America.
Going Concern Uncertainty
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has incurred operating losses since inception and at December 31, 2018, has an accumulated deficit and working capital deficiency. These matters raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
| /s/ Moody, Famiglietti & Andronico, LLP | |
| We have served as the Company’s auditor since 2016. | |
| Tewksbury, Massachusetts | |
| March 12, 2019 |
F-3
GI Dynamics, Inc. and Subsidiaries
Consolidated Balance Sheets
(In thousands, except share and per share amounts)
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 2,499 | $ | 3,806 | ||||
| Restricted cash | 30 | 30 | ||||||
| Prepaid expenses and other current assets | 1,230 | 530 | ||||||
| Total current assets | 3,759 | 4,366 | ||||||
| Property and equipment, net | 42 | 63 | ||||||
| Right-of-use operating lease, net of amortization | 386 | — | ||||||
| Total assets | $ | 4,187 | $ | 4,429 | ||||
| Liabilities and stockholders’ deficit | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 636 | $ | 1,050 | ||||
| Accrued expenses | 1,353 | 1,645 | ||||||
| Short term debt to related party, net of discount | 5,000 | 4,960 | ||||||
| Derivative liabilities | 10 | 51 | ||||||
| Short term lease liabilities | 169 | — | ||||||
| Total current liabilities | 7,168 | 7,706 | ||||||
| Long term debt to related party, net of discount | — | 168 | ||||||
| Long term lease liabilities | 217 | — | ||||||
| Total liabilities | 7,385 | 7,874 | ||||||
| Commitments and contingencies (Note 10) | ||||||||
| Stockholders’ deficit: | ||||||||
| Common stock, $0.01 par value – 75,000,000 and 50,000,000 shares authorized at December 31, 2019 and 2018, respectively; 36,598,291 and 19,277,545 shares issued and outstanding at December 31, 2019 and 2018, respectively | 366 | 193 | ||||||
| Additional paid-in capital | 280,928 | 263,521 | ||||||
| Accumulated deficit | (284,492 | ) | (267,159 | ) | ||||
| Total stockholders’ deficit | (3,198 | ) | (3,445 | ) | ||||
| Total liabilities and stockholders’ deficit | $ | 4,187 | $ | 4,429 | ||||
See reports of independent registered public accounting firms and the accompanying notes to these consolidated financial statements.
F-4
GI Dynamics, Inc. and Subsidiaries
Consolidated Statements of Operations
(In thousands, except share and per share amounts)
| Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Operating expenses: | ||||||||
| Research and development | $ | 4,225 | $ | 1,947 | ||||
| Sales and marketing | 22 | 866 | ||||||
| General and administrative | 5,295 | 4,809 | ||||||
| Total operating expenses | 9,542 | 7,622 | ||||||
| Loss from operations | (9,542 | ) | (7,622 | ) | ||||
| Other income (expense): | ||||||||
| Interest income | 3 | 29 | ||||||
| Interest expense | (6,178 | ) | (1,151 | ) | ||||
| Foreign exchange gain | 11 | 13 | ||||||
| Income (expense) from diverted funds and insurance claim, net | — | 224 | ||||||
| Re-measurement of derivative liabilities | (1,683 | ) | 482 | |||||
| Gain on write-off of accounts payable | 101 | — | ||||||
| Other expense, net | (7,746 | ) | (403 | ) | ||||
| Loss before income tax expense | (17,288 | ) | (8,025 | ) | ||||
| Income tax expense | 45 | 13 | ||||||
| Net loss | $ | (17,333 | ) | $ | (8,038 | ) | ||
| Basic and diluted net loss per common share | $ | (0.67 | ) | $ | (0.59 | ) | ||
| Weighted-average number of common shares used in basic and diluted net loss per common share | 25,886,615 | 13,699,585 | ||||||
See reports of independent registered public accounting firms and the accompanying notes to these consolidated financial statements.
F-5
GI Dynamics, Inc. and Subsidiaries
Consolidated Statements of Stockholders’ Deficit
(In thousands, except share amounts)
| Common Stock | Additional Paid-in | Accumulated | Total Stockholders’ | |||||||||||||||||
| Shares | Par Value | Capital | Deficit | Deficit | ||||||||||||||||
| Balance at December 31, 2017 | 11,157,489 | $ | 112 | $ | 255,294 | $ | (259,121 | ) | $ | (3,715 | ) | |||||||||
| Issuance of shares upon private placement, net of issuance costs | 8,120,056 | 81 | 6,353 | — | 6,434 | |||||||||||||||
| Beneficial conversion feature discounts associated with 2018 Note | — | — | 1,007 | — | 1,007 | |||||||||||||||
| Relative fair value of warrant issued with 2018 Note | — | — | 743 | — | 743 | |||||||||||||||
| Stock-based compensation expense | — | — | 124 | — | 124 | |||||||||||||||
| Net loss | — | — | — | (8,038 | ) | (8,038 | ) | |||||||||||||
| Balance at December 31, 2018 | 19,277,545 | 193 | 263,521 | (267,159 | ) | (3,445 | ) | |||||||||||||
| Reclassification of derivative liabilities to additional paid-in capital upon stockholder approval | — | — | 5,785 | — | 5,785 | |||||||||||||||
| Issuance of common stock upon conversion of notes payable to related party | 9,072,197 | 91 | 5,913 | — | 6,004 | |||||||||||||||
| Issuance of common stock upon exercise of related party warrants | 8,248,549 | 82 | 5,321 | — | 5,403 | |||||||||||||||
| Stock-based compensation expense | — | — | 388 | — | 388 | |||||||||||||||
| Net loss | — | — | — | (17,333 | ) | (17,333 | ) | |||||||||||||
| Balance at December 31, 2019 | 36,598,291 | $ | 366 | $ | 280,928 | $ | (284,492 | ) | $ | (3,198 | ) | |||||||||
See reports of independent registered public accounting firms and the accompanying notes to these consolidated financial statements.
F-6
GI Dynamics, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
(In thousands)
| Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Operating activities: | ||||||||
| Net loss | $ | (17,333 | ) | $ | (8,038 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 32 | 35 | ||||||
| Loss on disposal of leasehold improvements | — | 2 | ||||||
| Stock-based compensation expense | 388 | 124 | ||||||
| Non-cash interest expense | 6,173 | 683 | ||||||
| Re-measurement of derivative liabilities | 1,683 | (18 | ) | |||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | — | 40 | ||||||
| Prepaid expenses and other current assets | (700 | ) | (265 | ) | ||||
| Accounts payable | (414 | ) | (141 | ) | ||||
| Accrued expenses | (441 | ) | 265 | |||||
| Deferred revenue | — | (11 | ) | |||||
| Net cash used in operating activities | (10,612 | ) | (7,324 | ) | ||||
| Investing activities | ||||||||
| Purchases of property and equipment | (11 | ) | (3 | ) | ||||
| Net cash used in investing activities | (11 | ) | (3 | ) | ||||
| Financing activities | ||||||||
| Proceeds from issuance of common stock, net of issuance costs | — | 6,434 | ||||||
| Debt issuance costs | (87 | ) | (85 | ) | ||||
| Proceeds from exercise of related party warrants | 5,403 | |||||||
| Proceeds from short term and long term debt, related party | 4,000 | 1,750 | ||||||
| Net cash provided by financing activities | 9,316 | 8,099 | ||||||
| Net increase (decrease) in cash and cash equivalents | (1,307 | ) | 772 | |||||
| Cash, cash equivalents and restricted cash at beginning of year | 3,836 | 3,064 | ||||||
| Cash, cash equivalents and restricted cash at end of year | $ | 2,529 | $ | 3,836 | ||||
| Supplemental cash flow disclosures | ||||||||
| Income taxes paid | 45 | 12 | ||||||
| Beneficial conversion feature discount associated with 2018 Note | — | 1,007 | ||||||
| Relative fair value of warrant issued with 2018 Note | — | 743 | ||||||
| Beneficial conversion feature discount associated with 2017 Note modification | — | 40 | ||||||
| Interest paid | 394 | — | ||||||
| Right-of-use asset obtained in exchange for lease liability | 463 | — | ||||||
| Conversion of notes payable to related party into common stock | 6,004 | — | ||||||
| Reclassification of warrant from derivative liabilities to additional paid-in capital | 5,785 | — | ||||||
| Fair value of warrants issued with Notes to Related Party | 4,061 | — | ||||||
See reports of independent registered public accounting firms and the accompanying notes to these consolidated financial statements.
F-7
GI Dynamics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
1. Nature of Business
GI Dynamics® is a clinical stage medical device company focused on the development and commercialization of EndoBarrier, a medical device intended to treat patients with type 2 diabetes and to reduce obesity.
Diabetes mellitus type 2 (also known as type 2 diabetes) is a long-term progressive metabolic disorder characterized by high blood sugar, insulin resistance, and reduced insulin production. People with type 2 diabetes represent 90-95% of the worldwide diabetes population; only 5-10% of this population is diagnosed with type 1 diabetes (a form of diabetes mellitus wherein little to no insulin is produced).
Being overweight is a condition where the patient’s BMI is greater than 25 (kg/m2); obesity is a condition where the patient’s BMI is greater than 30. Obesity and its comorbidities contribute to the progression of type 2 diabetes. Many experts believe obesity contributes to higher levels of insulin resistance, which creates a feedback loop that increases the severity of type 2 diabetes.
When considering treatment for type 2 diabetes, it is optimal to address obesity concurrently with diabetes.
EndoBarrier® is intended for the treatment of type 2 diabetes and to reduce obesity in a minimally invasive and reversible manner.
The current treatment paradigm for type 2 diabetes is lifestyle therapy combined with pharmacological treatment, whereby treating clinicians prescribe a treatment regimen of one to four concurrent medications that could include insulin for patients with higher levels of blood sugar. Insulin carries a significant risk of increased mortality and may contribute to weight gain, which in turn may lead to higher levels of insulin resistance and increased levels of blood sugar. Fewer than 50% of patients treated pharmacologically for type 2 diabetes are adequately managed, meaning that medication does not lower blood sugar adequately and does not halt the progressive nature of diabetes of these patients.
The current pharmacological treatment algorithms for type 2 diabetes fall short of ideal, creating a large and unfilled efficacy gap.
The GI Dynamics vision is to make EndoBarrier the essential nonpharmacological and non-anatomy-altering treatment for patients with type 2 diabetes. The Company intends to achieve this vision by providing a safe and effective device, focusing on optimal patient care, supporting treating clinicians, adding to the extensive body of clinical evidence around EndoBarrier, gaining appropriate regulatory approvals, continuing to improve its products and systems, operating the Company in a lean fashion, and maximizing stockholder value.
EndoBarrier® is intended for the treatment of type 2 diabetes and to reduce obesity in a minimally invasive and reversible manner and is designed to mimic the mechanism of action of duodenal-jejunal exclusion created by gastric bypass surgery.
Since incorporation, the Company has devoted substantially all of its efforts to product commercialization, research and development, business planning, recruiting management and technical staff, acquiring operating assets, and raising capital. The Company currently operates in one reportable business segment.
Going Concern Evaluation
As of December 31, 2019, the Company’s primary source of liquidity is its cash, cash equivalents and restricted cash balances. GI Dynamics is currently focused primarily on its clinical trials, which will support future regulatory submissions and potential commercialization activities. Until the Company is successful in gaining regulatory approvals, it is unable to sell the Company’s product in any market at this time. Without revenues, GI Dynamics is reliant on funding obtained from investment in the Company to maintain business operations until the Company can generate positive cash flows from operations. The Company cannot predict the extent of future operating losses and accumulated deficit, and it may never generate sufficient revenues to achieve or sustain profitability.
F-8
GI Dynamics has incurred operating losses since inception and at December 31, 2019, had an accumulated deficit of approximately $284 million and a working capital deficit of $3.4 million. The Company expects to incur significant operating losses for the next several years. At December 31, 2019, the Company had approximately $2.5 million in cash, cash equivalents and restricted cash.
The Company will need to restructure the terms of the 2017 Note before March 31, 2020 and raise additional capital before May 1, 2020 in order to continue to pursue its current business objectives as planned and to continue to fund its operations. The Company and Crystal Amber Fund Limited (“Crystal Amber”, a Related Party for ASX purposes) are currently in final discussions regarding the 2017 Note maturity extension. The Company is looking to raise additional funds through any combination of additional equity and debt financings or from other sources. However, the Company has no guarantee that the 2017 Note will not mature on March 31, 2020 and has no guaranteed source of capital that will accommodate repayment of the 2017 Note and sustain operations past March 31, 2020. If the 2017 Note terms are restructured, the Company anticipates operating cash being exhausted by May 1, 2020 and therefore requires additional financing. There can be no assurance that any such potential financing opportunities will be available on acceptable terms, if at all. If the Company is unable to raise sufficient capital on the Company’s required timelines and on acceptable terms to stockholders and the Board of Directors, it could be forced to cease operations, including activities essential to support regulatory applications to commercialize EndoBarrier. If access to capital is not achieved in the near term, it will materially harm the Company’s business, financial condition and results of operations to the extent that the Company may be required to cease operations altogether, file for bankruptcy, or undertake any combination of the foregoing.
In addition, if the Company does not meet its payment obligations to third parties as they become due, the Company may be subject to litigation claims and its credit worthiness would be adversely affected. Even if the Company is successful in defending these claims, litigation could result in substantial costs and would be a distraction to management and may have other unfavorable results that could further adversely impact the Company’s financial condition.
These factors raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that these consolidated financial statements are issued.
The accompanying consolidated financial statements have been prepared assuming GI Dynamics will continue as a going concern, which contemplates the realization of assets and liabilities and commitments in the normal course of business. The consolidated financial statements for the year ended December 31, 2019 do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from uncertainty related to the Company’s ability to continue as a going concern within one year after the date that these consolidated financial statements are issued.
2. Summary of Significant Accounting Policies and Basis of Presentation
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of GI Dynamics, Inc. and its wholly owned subsidiaries. All intercompany transactions and balances are eliminated in consolidation.
The functional currency of GID Europe Holding B.V., GID Europe B.V., GID Germany GmbH and GI Dynamics Australia Pty Ltd is the U.S. dollar. Consolidated balance sheet accounts of the Company’s subsidiaries are remeasured into U.S. dollars using the exchange rate in effect at the consolidated balance sheet date while expenses are remeasured using the average exchange rate in effect during the period. Gains and losses arising from remeasurement of the wholly owned subsidiaries’ financial statements are included in the determination of net loss.
Segment Reporting
The Company has one reportable segment which designs, develops, manufactures and markets medical devices for non-surgical approaches to treating type 2 diabetes.
GI Dynamics does not report geographic segments as there were no product sales in 2019 or 2018 and at December 31, 2019 and 2018, all long-lived assets comprised of property and equipment were held in the U.S.
F-9
Use of Estimates
The preparation of consolidated financial statements in accordance with generally accepted accounting principles in the U.S. requires the Company’s management to make estimates and judgments that may affect the reported amounts of assets, liabilities, revenues and expenses, and the related disclosure of contingent assets and liabilities. On an ongoing basis, management evaluates its estimates, including those related to revenue recognition, inventory valuation including reserves for excess and obsolete inventory, impairment of long-lived assets, income taxes including the valuation allowance for deferred tax assets, research and development, contingencies, valuation of warrant and other derivative liabilities, estimates used to assess its ability to continue as a going concern and stock-based compensation. GI Dynamics bases its estimates on historical experience and on various other assumptions that are believed to be reasonable, the results of which form the basis for making judgments about the carrying values of assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions. Changes in estimates are reflected in reported results in the period in which they become known.
Cash, Cash Equivalents and Restricted Cash
GI Dynamics considers all highly liquid investment instruments with an original maturity when purchased of three months or less to be cash equivalents. Investments qualifying as cash equivalents primarily consist of money market funds and have a carrying amount that approximates fair value. The amount of cash equivalents included in cash and cash equivalents was approximately $0 and $1.1 million at December 31, 2019 and 2018, respectively.
At December 31, 2019 and 2018, GI Dynamics had approximately $2 and $0.01 million, respectively, of cash and cash equivalents denominated in Australian dollars or Euros that is subject to foreign currency gain and loss.
GI Dynamics has $30 thousand in restricted cash used to secure a corporate credit card account.
Property and Equipment
Property and equipment, are recorded at cost and are depreciated when placed in service using the straight-line method based on their estimated useful lives as follows:
| Asset Description | Estimated Useful Life (In Years) | |
| Laboratory and manufacturing equipment | 5 | |
| Computer equipment and software | 3 | |
| Office furniture and equipment | 5 |
F-10
Included in property and equipment are certain costs of software obtained for internal use. Costs incurred during the preliminary project stage are expensed as incurred, while costs incurred during the application development stage are capitalized and amortized over the estimated useful life of the software. GI Dynamics also capitalizes costs related to specific upgrades and enhancements when it is probable the expenditures will result in additional functionality. Maintenance and training costs related to software obtained for internal use are expensed as incurred.
Leasehold improvements are amortized over the shorter of the estimated useful life of the asset or the remaining lease term. Costs for capital assets not yet placed into service have been capitalized as construction in progress and will be depreciated in accordance with the above guidelines once placed into service. Maintenance and repair costs are expensed as incurred.
Derivative Liabilities
GI Dynamics examines all financial instruments to determine if the financial instrument or any component feature is a derivative under ASC 815, Derivatives and Hedging (“ASC 815”) and therefore requires liability classification. Certain warrants to purchase common stock did not meet the requirements for equity classification and are considered derivative instruments due to their cash settlement features. The derivative warrants are initially recorded at fair value with subsequent changes in fair value recorded in other income (expense) in the statements of operations. The Company estimates fair value using the Black-Scholes option pricing model. See Note 5 for inputs and assumptions used in the determination of the fair value. If the derivative instruments subsequently meet the requirements for classification as equity, the Company reclassifies the then fair value of the instrument to equity. If multiple outcomes are probable, management assigns probability adjustments to determine the most likely probability adjusted fair value.
Research and Development Costs
Research and development costs are expensed when incurred. Research and development costs include costs of all basic research activities as well as other research, engineering, and technical effort required to develop a new product or service or make significant improvement to an existing product or manufacturing process. Research and development costs also include preapproval regulatory and clinical trial expenses.
Patent Costs
GI Dynamics expenses as incurred all costs, including legal expenses, associated with obtaining patents until the patented technology becomes feasible. All costs incurred after the patented technology is feasible will be capitalized as an intangible asset. As of December 31, 2019, no such costs had been capitalized since inception of the Company. GI Dynamics has expensed approximately $200 thousand of patent costs within general and administrative expenses in the consolidated statements of operations for each of the years ended December 31, 2019 and 2018.
Stock-Based Compensation
GI Dynamics accounts for stock-based compensation in accordance with the Financial Accounting Standards Board, (“FASB”) Accounting Standards Codification (“ASC”) 718, Stock Compensation, or ASC 718, which requires that stock-based compensation be measured and recognized as an expense in the financial statements and that such expense be measured at the grant date fair value.
For awards that vest based on service conditions, GI Dynamics uses the straight-line method to allocate compensation expense to reporting periods. The grant date fair value of options granted is calculated using the Black-Scholes option pricing model, which requires the use of subjective assumptions including volatility, expected term and the fair value of the underlying common stock, among others.
The assumptions used in determining the fair value of stock-based awards represent management’s best estimates, but these estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change, and management uses different assumptions, the Company’s stock-based compensation could be materially different in the future. The risk-free interest rate used for each grant is based on a zero-coupon U.S. Treasury instrument with a remaining term similar to the expected term of the stock-based award. Because GI Dynamics does not have a sufficient stable history to estimate the expected term, it uses the simplified method for estimating the expected term. The simplified method is based on the average of the vesting tranches and the contractual life of each grant. Because there was no public market for GI Dynamics common stock prior to its IPO, GI Dynamics lacked company-specific historical and implied volatility information. The Company does have approximately 6 years of historical price volatility since its IPO in September 2011. Therefore, management estimates the expected stock volatility based on the to-date historical price volatility. GI Dynamics has not paid and does not anticipate paying cash dividends on its shares of common stock; therefore, the expected dividend yield is assumed to be zero.
F-11
GI Dynamics recognizes the impact of share-based award forfeitures only as they occur rather than by applying an estimated forfeiture rate.
GI Dynamics periodically issues performance-based awards. For these awards, vesting will occur upon the achievement of certain milestones. When achievement of the milestone is deemed probable, the Company records as compensation expense, the value of the respective stock award over the implicit remaining service period.
Stock awards to non-employees were accounted for in accordance with ASC 505-50, Equity-Based Payments to Non-Employees (“ASC 505-50”). The measurement date for non-employee awards was generally the date performance of services required from the non-employee was complete. For non-employee awards that vest based on service conditions, the Company expensed the value of the awards over the related service period, provided the service condition was expected to be met. The Company recorded the expense of services rendered by non- employees based on the estimated fair value of the stock option using the Black-Scholes option pricing model over the contractual term of the non-employee award. At each reporting period, the fair value of unvested non-employee awards was remeasured and expensed on a straight-line basis over the vesting term of the underlying award. In June 2018, the FASB issued ASU No. 2018-07, Compensation – Stock Compensation (Topic 718: Improvements to nonemployee share-based payment accounting), or ASU 2018-07, which provides measurement provisions and clarifications for the accounting for Non-employee Share-Based Payments (“NESBP”). As a result, most of the guidance within ASC 718 associated with employee share-based payments, including most of its requirements related to classification and measurement, applies to NESBP. The Company elects to use the contractual term of each award as the expected term for NESBP awards. The Company adopted ASU 2018-07 as of January 1, 2019 and there was no impact to retained earnings or equity as no uncompleted NESBPs were outstanding when adopted. At December 31, 2019, the Company had options to purchase 120,000 shares of common stock granted to non-employees.
Impairment of Long-Lived Assets
GI Dynamics regularly reviews the carrying amount of its long-lived assets to determine whether indicators of impairment may exist that merit adjustments to carrying values or estimated useful lives. If indications of impairment exist, projected future undiscounted cash flows associated with the asset are compared to the carrying amount to determine whether the asset’s value is recoverable. If the carrying value of the asset exceeds such projected undiscounted cash flows, the asset will be written down to its estimated fair value. No such impairments were recorded in 2019 or 2018.
Loss Contingencies
In accordance with ASC 450, Contingencies, GI Dynamics accrues anticipated costs of settlement, damages, and losses for loss contingencies based on historical experience or to the extent specific losses are probable and estimable. Otherwise, the Company expenses these costs as incurred. If the estimate of a probable loss is a range, and no amount within the range is more likely, GI Dynamics accrues the minimum amount of the range. No loss contingencies were recorded on the balance sheet as of December 31, 2019 or 2018.
Fraudulent Diversion of Funds and Related Insurance Proceeds
In July 2018, after a third-party investigation isolated the activity, an insurance claim was filed for $271 thousand for fraudulent diversion of cash from the Company’s account into a personal account that occurred during the years 2016 through 2018. Income in the year ended December 31, 2018 represents net insurance proceeds on the claim less $22 thousand of research and development expense. GI Dynamics has since implemented internal controls to correct the identified control deficiencies associated with the fraud and the employee responsible for the diverted funds is no longer with the Company.
Income Taxes
GI Dynamics uses the asset and liability method of accounting for income taxes. The Company records deferred tax assets and liabilities for the expected future tax consequences of temporary differences between its financial reporting and the tax bases of assets and liabilities measured using the enacted tax rates in effect in the years in which the differences are expected to reverse. The Company regularly assesses the need for a valuation allowance against its deferred tax assets. Future realization of the Company’s deferred tax assets ultimately depends on the existence of sufficient taxable income within the available carryback or carryforward periods. Sources of taxable income include taxable income in prior carryback years, future reversals of existing taxable temporary differences, tax planning strategies, and future taxable income. The Company records a valuation allowance to reduce its deferred tax assets to an amount it believes is more-likely-than-not to be realized. Deferred tax assets are reduced by a valuation allowance to reflect the uncertainty associated with their ultimate realization.
F-12
The Company assesses its income tax positions and records tax benefits based upon management’s evaluation of the facts, circumstances, and information available at the reporting date. For those tax positions where it is more-likely-than-not that a tax benefit will be sustained, the Company records the largest amount of tax benefit with a greater than 50 percent likelihood of being realized upon ultimate settlement with a taxing authority having full knowledge of all relevant information. For those income tax positions where it is not more-likely-than-not that a tax benefit will be sustained, no tax benefit is recognized in the financial statements. The Company classifies interest and penalties on uncertain tax positions as income tax expense.
Guarantees
GI Dynamics has identified the guarantees described below as disclosable, in accordance with ASC 460, Guarantees.
As permitted under Delaware law, GI Dynamics indemnifies its officers and directors for certain events or occurrences while the officer or director is, or was, serving at the Company’s request in such capacity. The maximum potential amount of future payments the Company could be required to make is unlimited; however, the Company maintains directors’ and officers’ insurance coverage that should limit its exposure and enable it to recover a portion of any future amounts paid.
GI Dynamics is a party to a number of agreements entered into in the ordinary course of business that contain typical provisions that obligate it to indemnify the other parties to such agreements upon the occurrence of certain events. Such indemnification obligations are usually in effect from the date of execution of the applicable agreement for a period equal to the applicable statute of limitations. The aggregate maximum potential future liability of the Company under such indemnification provisions is uncertain.
As of December 31, 2019, and 2018, GI Dynamics had not experienced any material losses related to these indemnification obligations, and no material claims with respect thereto were outstanding. The Company does not expect significant claims related to these indemnification obligations and, consequently, concluded that the fair value of these obligations is negligible. As a result, no related reserves have been established.
Recently Adopted Accounting Pronouncements
In February 2016, the FASB issued Accounting Standards Update, or ASU No. 2016-02, Leases (Topic 842), or ASU 2016-02. ASU 2016-02 requires that most operating leases be recorded on the balance sheet unless the practical expedient is elected for short-term operating leases. The lessee will record a lease liability, which is a lessee’s obligation to make lease payments arising from a lease, measured on a discounted basis, and a right-of-use asset, which is an asset representing the lessee’s right to use the underlying asset for the lease term. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years, with early adoption permitted. Lessees must apply a modified retrospective transition approach for leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements. The modified retrospective approach would not require any transition accounting for leases that expired before the earliest comparative period presented. Lessees may not apply a full retrospective transition approach. The Company elected to apply the practical expedient as it relates to short-term leases. The Company adopted ASU 2016-02 on January 1, 2019. There was no impact to retained earnings upon adoption as there were no long-term leases in effect at adoption (see Note 10 of the Consolidated Financial Statements for a more complete description of the lease accounting and impacts of adoption).
In June 2018, the FASB issued ASU No. 2018-07, Compensation – Stock Compensation (Topic 718: Improvements to nonemployee share-based payment accounting), or ASU 2018-07, which provides measurement provisions and clarifications for the accounting for Non-employee Share-Based Payments (“NESBP”). As a result, most of the guidance within ASC 718 associated with employee share-based payments, including most of its requirements related to classification and measurement, applies to NESBP. ASU 2018-07 is effective for public business entities for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years, with early adoption permitted but not in advance of adoption of Topic 606. Upon transition, the entity is required to measure and record these nonemployee awards at fair value as of the adoption date without subsequent remeasurement. The Company adopted ASU 2018-07 as of January 1, 2019 and there was no impact to retained earnings or equity as no uncompleted NESBPs were outstanding when adopted. At December 31, 2019, the Company had options to purchase 120,000 shares of common stock granted to non-employees.
F-13
Recently Issued Accounting Pronouncements
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurement (Topic 820), Disclosure Framework—Changes to the Disclosure Requirements for Fair Value Measurement, or ASU 2018-13, which provides guidance focused on the disclosure requirements for disclosing fair value estimates, assumptions, and methodology. Requirements removed include the requirement to disclose details around amount and reasoning for level 1 to level 2 transfers, timing policies for transfer between levels and the valuation processes for level 3 fair value measurements. Modified requirements include details regarding net asset redemption restrictions and timing related to uncertainty disclosures. Requirements added include disclosures of changes in unrealized gains and losses for recurring level 3 measurements held as of the reporting date and disclosures around the range and weighted average of significant inputs used to develop level 3 fair value measurements. These amendments are effective for all entities for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019. The amendments on changes in unrealized gains and losses, the range and weighted average of significant unobservable inputs used to develop Level 3 fair value measurements and the narrative description of measurement uncertainty should be applied prospectively for only the most recent interim or annual period presented in the initial fiscal year of adoption. All other amendments should be applied retrospectively to all periods presented upon their effective date. Early adoption is permitted upon issuance of this update, however the Company has declined early adoption. The Company will adopt this ASU in 2020 and expects no impact to its consolidated financial statements on adoption.
3. Net Loss per Common Share
Basic net loss per common share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period. Potential common stock equivalents are determined using the treasury stock method. For diluted net loss per share purposes, stock options and other stock-based awards are excluded, including shares issued as a result of option exercises but which are subject to repurchase by the Company, whose effect would be anti-dilutive from the calculation. For diluted net loss per share purposes, warrants conferring the right to purchase common shares and because CDIs and common shares are interchangeable at the election of the stockholder, warrants conferring the right to purchase CDIs are excluded from diluted net loss per share calculations as inclusion would have an anti-dilutive effect. During each of the years ended December 31, 2019 and 2018, common stock equivalents were excluded from the calculation of diluted net loss per common share, as their effect was anti-dilutive due to the net loss incurred. Therefore, basic and diluted net loss per share was the same in all periods presented.
The following potentially dilutive securities have been excluded from the computation of diluted weighted- average shares outstanding as of December 31, 2019 and 2018, as they would be anti-dilutive:
| Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Warrants to purchase common stock | 28,532 | 1,972,976 | ||||||
| Options to purchase common stock and other stock-based awards | 3,331,154 | 1,545,719 | ||||||
| Total | 3,359,686 | 3,518,695 | ||||||
4. Warrants to Purchase Common Stock or CDIs
The following series of warrants were outstanding and exercisable at December 31, 2019 and 2018 (warrants to purchase CDIs presented as shares at a 50 CDI per share ratio):
| Number of underlying | Exercise price | December 31, | ||||||||||||||||
| Warrant Series | Issue Date | shares | per share | 2019 | 2018 | |||||||||||||
| Consultant warrant | May 4, 2016 | 28,532 | $ | 0.64 | 28,532 | 28,532 | ||||||||||||
| 2018 Warrant (1) | May 24, 2018 | 1,944,444 | $ | 0.72 | - | 1,944,444 | ||||||||||||
| Total Outstanding and Exercisable | 28,532 | 1,972,976 | ||||||||||||||||
| Weighted average exercise price | $ | 0.64 | $ | 0.72 | ||||||||||||||
| (1) | Exercise price was initially $0.90 per share, but was adjusted to $0.72 in November 2018 |
F-14
The following table rolls forward the warrant activity during the year ended December 31, 2019:
| Shares underlying warrants | Weighted average exercise price per share | |||||||
| Outstanding and Exercisable at December 31, 2018 | 1,972,976 | $ | 0.72 | |||||
| Issuance of March 2019 Warrant | 1,579,696 | $ | 0.64 | |||||
| Issuance of May 2019 Warrant | 4,724,409 | $ | 0.64 | |||||
| Exercise of 2018 Warrant | (1,944,444 | ) | $ | 0.72 | ||||
| Exercise of March 2019 Warrant | (1,579,696 | ) | $ | 0.64 | ||||
| Exercise of May 2019 Warrant | (4,724,409 | ) | $ | 0.64 | ||||
| Outstanding and Exercisable at December 31, 2019 | 28,532 | $ | 0.64 | |||||
On August 21, 2019, GI Dynamics and Crystal Amber entered into a securities purchase agreement (“SPA”) for a total funding of up to approximately $10 million (the “August 2019 SPA”) comprised of the scheduled exercise of the 2018 Warrant, the March 2019 Warrant, and the May 2019 Warrant as detailed above and the sale of an Unsecured Convertible Note for up to approximately $4.6 million (the “August 2019 Note”), which included an agreement to issue a warrant (the “August 2019 Warrant”) to purchase up to 229,844,650 CDIs (representing 4,596,893 shares of common stock) for an exercise price of $0.02 per CDI. On December 16, 2019, stockholder approval for the warrant to be issued was obtained and it was issued on January 13, 2020.
5. Fair Value Measurements
The tables below present information about the Company’s assets and liabilities that are measured at fair value on a recurring basis as of December 31, 2019 and 2018 and indicates the fair value hierarchy of the valuation techniques the Company used to determine such fair value. In general, fair values determined by Level 1 inputs utilize observable inputs such as quoted prices in active markets for identical assets or liabilities. Fair values determined by Level 2 inputs utilize data points that are either directly or indirectly observable, such as quoted prices, interest rates and yield curves. Fair values determined by Level 3 inputs utilize unobservable data points in which there is little or no market data, requiring the Company to develop its own assumptions for the asset or liability.
The following tables present the assets and liabilities the Company has measured at fair value on a recurring basis (in thousands):
| Fair Value Measurements at | ||||||||||||||||
| Reporting Date Using | ||||||||||||||||
| Quoted Prices | Significant | |||||||||||||||
| in
Active Markets for | Other Observable | Significant Unobservable | ||||||||||||||
| December 31, | Identical Assets | Inputs | Inputs | |||||||||||||
| Description | 2019 | (Level 1) | (Level 2) | (Level 3) | ||||||||||||
| Liabilities | ||||||||||||||||
| Warrant liability | $ | 10 | $ | — | $ | — | $ | 10 | ||||||||
| Total liabilities | $ | 10 | $ | — | $ | — | $ | 10 | ||||||||
F-15
| Fair Value Measurements at | ||||||||||||||||
| Reporting Date Using | ||||||||||||||||
| Quoted Prices | Significant | |||||||||||||||
| in Active Markets for | Other Observable | Significant Unobservable | ||||||||||||||
| December 31, | Identical Assets | Inputs | Inputs | |||||||||||||
| Description | 2018 | (Level 1) | (Level 2) | (Level 3) | ||||||||||||
| Assets | ||||||||||||||||
| Money market funds (included in cash and cash equivalents) | $ | 1,097 | $ | 1,097 | $ | — | $ | — | ||||||||
| Total assets | $ | 1,097 | $ | 1,097 | $ | — | $ | — | ||||||||
| Liabilities | ||||||||||||||||
| Warrant liability | $ | 51 | $ | — | $ | — | $ | 51 | ||||||||
| Total liabilities | $ | 51 | $ | — | $ | — | $ | 51 | ||||||||
The assumptions used in the Black-Scholes option pricing model to determine the fair value of the Derivative Warrant Liabilities at December 31, 2019 and 2018 were as follows:
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Expected volatility | 130.0 | % | 134.0 | % | ||||
| Expected term (in years) | 1.35 | 2.35 | ||||||
| Risk-free interest rate | 1.59 | % | 2.48 | % | ||||
| Expected dividend yield | —% | —% | ||||||
The assumptions used in the Black-Scholes option pricing model to determine the fair value of the Derivative Warrant Liabilities related to the 2019 derivative warrants at each measurement period:
| March 2019 Warrants | May 2019 Warrants | |||||||||||||||||||
| March 15, | March 31, | June 28, | May 8, | June 28, | ||||||||||||||||
| 2019 | 2019 | 2019 | 2019 | 2019 | ||||||||||||||||
| Expected volatility | 130.7 | % | 131.2 | % | 134.0 | % | 134.0 | % | 137.3 | % | ||||||||||
| Expected term (in years) | 5.00 | 4.97 | 2.35 | 5.00 | 4.87 | |||||||||||||||
| Risk-free interest rate | 2.44 | % | 2.34 | % | 1.74 | % | 2.28 | % | 1.74 | % | ||||||||||
| Expected dividend yield | —% | —% | —% | —% | —% | |||||||||||||||
The following table rolls forward the fair value of the derivative liabilities, where fair value is determined by Level 3 inputs (in thousands):
| Balance at December 31, 2017 | $ | 493 | ||
| Decrease in fair value of 2017 Note-related conversion feature upon re-measurement | (464 | ) | ||
| Decrease in fair value of warrants upon re-measurement | (18 | ) | ||
| Fair value of beneficial conversion feature discount associated with 2017 Note modification | 40 | |||
| Balance at December 31, 2018 | $ | 51 | ||
| Increase in fair value of warrants upon re-measurement | 1,683 | |||
| Fair value of warrants issued with notes to related party | 4,061 | |||
| Reclassification of warrants to additional paid-in capital | (5,785 | ) | ||
| Balance at December 31, 2019 | $ | 10 |
Cash, cash equivalents, restricted cash, accounts receivable, prepaid expenses and other current assets, accounts payable, accrued expenses, short-term debt to related party and other current liabilities at December 31, 2019 and 2018 are carried at amounts that approximate fair value due to their short-term maturities and highly liquid nature of these instruments.
F-16
6. Concentrations of Credit Risk, Accounts Receivable and Related Valuation Account
Financial instruments that subject GI Dynamics to credit risk primarily consist of cash and cash equivalents, restricted cash and accounts receivable. Cash and cash equivalent balances are all maintained with high quality financial institutions, and consequently, the Company believes that such funds are subject to minimal credit risk. The Company’s short-term investments potentially subject the Company to concentrations of credit risk. GI Dynamics has adopted an investment policy that limits the amounts it may invest in any one type of investment and requires all held investments to hold at least an A rating from a recognized credit rating agency, thereby reducing credit risk concentration.
GI Dynamics reversed a $40 thousand allowance for doubtful accounts balance in 2018, resulting in no accounts receivable and allowance of doubtful accounts balances at December 31, 2019 or 2018. As such, there is no credit risk from receivables.
7. Property and Equipment
Property and equipment consisted of the following (in thousands):
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Laboratory and manufacturing equipment | $ | 591 | $ | 591 | ||||
| Computer equipment and software | 1,193 | 1,182 | ||||||
| Office furniture and equipment | 183 | 183 | ||||||
| 1,967 | 1,956 | |||||||
| Less accumulated depreciation and amortization | (1,925 | ) | (1,893 | ) | ||||
| Total | $ | 42 | $ | 63 | ||||
The Company recorded a loss on disposal of $2 thousand in connection with the expiration of its office lease and the related leasehold improvements on April 13, 2018.
At December 31, 2019 and 2018, the Company had no property and equipment assets financed in any capital lease arrangement.
Depreciation and amortization expense of property and equipment totaled approximately $32 thousand and $35 thousand for the years ended December 31, 2019 and 2018.
8. Accrued Expenses
Accrued expenses consisted of the following (in thousands):
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Payroll and related liabilities | $ | 531 | $ | 386 | ||||
| Professional fees | 335 | 573 | ||||||
| Credit refunds | 164 | 186 | ||||||
| Interest payable | 250 | 495 | ||||||
| Other | 73 | 5 | ||||||
| Total | $ | 1,353 | $ | 1,645 | ||||
In 2017, following the notification by MHRA, the Company notified its customers to return their inventory on hand. The Company calculated an estimate for returns, reversed its revenue and recorded an accrued expense estimate of $202 thousand of product return related costs in addition to $77 thousand of credit memos granted to customers. No related activity in 2018 and 2019 resulted in the amounts being unchanged and the Company still anticipates claims against these accruals in the future.
In 2017, the Company recorded $136 thousand of interest payable on the 2017 Note. In 2018, the Company recorded $359 thousand of interest payable on the 2017 and 2018 Notes. In December 2018, the maturity of the 2017 Note was extended to March 31, 2019 in exchange for payment of $394 thousand which was the total accrued interest on the 2017 Note at December 31, 2018.
F-17
9. Notes Payable
2017 Convertible Note Financing
On June 15, 2017, the Company entered into a Note Purchase Agreement (“2017 NPA”) by and between the Company and Crystal Amber, a Related Party. Pursuant to the 2017 NPA, the Company issued and sold to Crystal Amber, a Senior Secured Convertible Promissory Note in an aggregate original principal amount of $5.0 million (the “2017 Note”).
The 2017 Note accrues interest at an annually compounded rate of 5% per annum, other than during the continuance of an event of default, when the 2017 Note accrues interest at a rate of 8% per annum. The entire outstanding principal balance and all unpaid accrued interest thereon was initially due on the original maturity date of December 31, 2018, but the maturity date was extended multiple times as described below.
The 2017 Note is secured by a first priority security interest in substantially all tangible and intangible assets of the Company, including intellectual property (the “Collateral”). In the event of an uncured default, Crystal Amber, is authorized to sell, transfer, assign or otherwise deal in or with the Collateral or the proceeds thereof or any related goods securing the Collateral, as fully and effectually as if Crystal Amber were the absolute owner thereof.
The ASX provided the Company with a waiver to allow all asset liens (the “Security”) to be granted to Crystal Amber, without the customary requirement of having to obtain stockholder approval for the grant of a security to a Related Party of the Company. As a result of the waiver, the Security contains a provision that provides that if an event of default occurs and Crystal Amber exercises its rights under the Security, neither Crystal Amber nor any of its associates can acquire any legal or beneficial interest in an asset of the Company or its subsidiaries in full or partial satisfaction of the Company’s obligations under the Security, or otherwise deal with the assets of the Company or its subsidiaries, without the Company first having complied with any applicable ASX Listing Rules, including ASX Listing Rule 10.1, other than as required by law or through a receiver, manager, or analogous person appointed by Crystal Amber exercising its power of sale under the Security and selling the assets to an unrelated third party on arm’s length commercial terms and conditions and distributing the cash proceeds to Crystal Amber or any of its associates in accordance with their legal entitlements.
The 2017 Note was issued without stockholder approval of certain specific conversion features and as a consequence, Crystal Amber had no right to exercise any note conversion rights until stockholders approved the 2017 Note conversion terms on May 24, 2018. Subsequently, the entire outstanding principal balance under the 2017 Note and all unpaid accrued interest thereon is convertible into CDIs (i) prior to the maturity date, at the option of Crystal Amber at a conversion price calculated based on the five-day volume weighted average price of the Company’s CDIs traded on the ASX (“Optional Conversion Price”), or (ii) automatically upon the occurrence of an equity financing in which the Company raises at least $10 million (a “Qualified Financing”) at the price per CDI of the CDIs issued and sold in such financing.
In the event that the Company issues additional CDIs in a subsequent equity financing at a price per CDI that is less than the then-effective Optional Conversion Price, Crystal Amber has a 30-day option to convert at an adjusted conversion price reflecting, on a weighted average basis, the lower price per CDI. The number of CDIs that Crystal Amber may acquire upon conversion of the 2017 Note at this adjusted conversion price is limited to the number that maintains Crystal Amber’s fully-diluted ownership percentage of the Company at the same level as existed immediately preceding the applicable subsequent equity financing.
In addition, upon a change of control of the Company (other than a change of control resulting from a Qualified Financing) in which the Company’s stockholders receive cash consideration, the Company is obligated under the 2017 Note to pay all accrued and unpaid interest then due plus 110% of the remaining outstanding unconverted principal balance. If the change of control results in non-cash consideration, Crystal Amber may convert the entire outstanding principal balance under the 2017 Note and all unpaid accrued interest then due into CDIs at the abovementioned Optional Conversion Price. Other than as described above, the Company may not prepay the 2017 Note without the consent of Crystal Amber. The Company considers the change in control premium as a cash settleable feature, thereby requiring derivative liability classification. On applying a probability adjusted present value of the premium, the fair value was considered immaterial upon issuance and at all subsequent reporting period ends.
The 2017 NPA contains customary events of default including a failure to perform obligations under the 2017 NPA, bankruptcy, a decision by the board of directors of the Company to wind up the Company, or if the Company otherwise ceases to carry on its ongoing business operations. If a default occurs and is not cured within the applicable cure period or is not waived, any outstanding obligations under the 2017 Note may be accelerated. The 2017 NPA and related 2017 Note documents also contain additional representations and warranties, covenants and conditions, in each case customary for transactions of this type.
F-18
The Company recorded the $5 million 2017 Note, net of debt issuance costs of $115 thousand and amortized the debt issuance costs over the life of the 2017 Note.
In December 2018, the maturity date of the 2017 Note was extended to March 31, 2019 in exchange for payment of $394 thousand, which was the total interest accrued on the 2017 Note at December 31, 2018. Payment of this amount was made in January 2019.
In March 2019, the maturity date of the 2017 Note was extended to May 1, 2019. In April 2019, the maturity date of the 2017 Note was further extended to July 1, 2019. In June 2019, the maturity date of the 2017 Note was further extended to October 1, 2019. On August 21, 2019, the maturity date of the 2017 Note was further extended to March 31, 2020. These extensions of maturity were evaluated under ASC 470 to determine whether debt extinguishment or debt modification accounting applies. The Company concluded that the maturity extensions did not meet the characteristics of debt extinguishments under ASC 470 and no accounting recognition was required. For the years ended December 31, 2019 and 2018, the Company recognized interest expense of $248 and $257 thousand and amortization of debt issuance costs of $43 and $0 thousand, respectively, related to the 2017 Note.
2018 Convertible Note and Warrant Financing
On May 30, 2018, the Company entered into a Note Purchase Agreement (“2018 NPA”) by and between the Company and Crystal Amber. Pursuant to the 2018 NPA, the Company issued and sold to Crystal Amber a Senior Unsecured Convertible Promissory Note in an aggregate original principal amount of approximately $1.8 million (the “2018 Note”) with a maturity date of May 30, 2023. Interest accrued at an annually compounded rate of 10% per annum.
On May 30, 2018, having secured stockholder pre-approval of the conversion features on May 24, 2018 per ASX Listing Rule 10.11, the entire outstanding principal balance under the 2018 Note and all unpaid accrued interest thereon was immediately convertible into CDIs, at the option of Crystal Amber at an initial conversion price of $0.018 per CDI. Subsequently, the Company issued additional CDIs in an equity financing in September 2018 at a price per CDI of $0.0144, resulting in an adjustment of the conversion price to $0.0144 per CDI. Notice to convert the 2018 Note was provided by Crystal Amber, a Related Party on June 30, 2019. The 2018 Note converted to 134,852,549 CDIs (representing 2,697,050 shares of common stock). The principal of approximately $1.8 million converted to 121,527,778 CDIs (representing 2,430,555 shares of common stock) and the accrued interest of $192 thousand converted to 13,324,772 CDIs (representing 266,495 shares of common stock). As of June 30, 2019, the conversion of the CDIs had been executed, but not yet settled with the CDIs issued and available. The CDIs were issued on July 3, 2019 and have a one-year restriction on trading on the ASX.
On May 30, 2018, having secured stockholder pre-approval of the conversion features on May 24, 2018 per ASX Listing Rule 10.11, GI Dynamics issued to Crystal Amber a warrant (the “2018 Warrant”) to purchase 97,222,200 CDIs (representing 1,944,444 shares of common stock) at an initial exercise price of $0.018 per CDI. As per the 2018 Note conversion price, the warrant exercise price was subsequently adjusted to $0.0144 per CDI. The 2018 Warrant was exercised in full on August 25, 2019 for $1.4 million.
The Company has evaluated the guidance ASC 480-10 Distinguishing Liabilities from Equity, ASC 815-40 Contracts in an Entity’s Own Equity and ASC 470-20 Debt with Conversion and Other Options to determine the appropriate classification of the 2018 Note and 2018 Warrant. On issuance, having already obtained the required stockholder approval to reserve the CDIs underlying the conversion feature and the warrant, the 2018 Warrant was determined to be a freestanding instrument meeting the requirements for equity classification. Accordingly, the fair value estimated for the 2018 Warrant, totaling approximately $743 thousand, has been recorded as a discount to the debt with the offset to additional paid-in capital. The 2018 Note was also evaluated for a BCF subsequent to the allocation of proceeds among the 2018 Note and 2018 Warrant. Based upon the effective conversion price of the 2018 Note after considering the stock price at the date of issuance and the allocation of estimated fair value to the 2018 Warrant, it was determined that the 2018 Note contained a BCF. The value of the BCF was computed to be approximately $1.2 million but was capped at approximately $1 million so as to not exceed the total proceeds from the 2018 Note after deducting the value allocated to the 2018 Note and 2018 Warrant. The effective interest rate on the note after the discounts is 26.4%.
F-19
The Company recorded the 2018 Note at issuance, net of the total debt discount of $1.8 million and amortized the debt discount over the life of the 2018 Note. For the years ended December 31, 2019 and 2018, the Company recorded accrued interest expense of $91 and $102 thousand, debt discount amortization to interest expense of $146 and $168 thousand and interest expense derived from issuance costs of nil and $85 thousand, respectively.
March 2019 Convertible Note and Warrant Financing
On March 15, 2019, the Company entered into a Note Purchase Agreement (“March 2019 NPA”) by and between the Company and Crystal Amber. Pursuant to the March 2019 NPA, the Company issued and sold to Crystal Amber a Senior Unsecured Convertible Promissory Note in an aggregate original principal amount of $1 million (the “March 2019 Note”) with a maturity date of March 15, 2024. Interest accrued at an annually compounded rate of 10% per annum and issuance costs related to the March 2019 NPA were $50 thousand.
After the Company obtained stockholder approval to enable Crystal Amber’s conversion right under the March 2019 Note on June 30, 2019, the entire outstanding principal balance under the March 2019 Note and all unpaid accrued interest thereon was convertible into CDIs, at the option of Crystal Amber at a conversion price of $0.0127 per CDI. On June 30, 2019, Crystal Amber elected to convert the March 2019 Note into 81,070,003 CDIs (representing 1,621,400 shares of common stock) with the principal of $1 million converting to 78,740,157 CDIs (representing 1,574,803 shares of common stock) and the accrued interest of approximately $30 thousand converting to 2,329,846 CDIs (representing 46,597 shares of common stock). As of June 30, 2019, notice of the conversion had been provided by Crystal Amber and the CDIs were issued on July 3, 2019 and have a one-year restriction on trading on the ASX.
Per the March 2019 NPA, the Company agreed to issue a warrant (the “March 2019 Warrant”) to Crystal Amber, pending stockholder approval, to purchase 78,984,823 CDIs (representing 1,579,696 shares of common stock) at an initial exercise price of $0.0127 per CDI. The issue of the March 2019 Warrant required the approval of stockholders and was not exercisable until its issue was approved on June 30, 2019. On August 25, 2019, a portion of the March 2019 Warrant totaling 47,244,119 CDIs (equivalent to approximately 944,882 shares of common stock) was exercised for $600 thousand. The remaining portion of the March 2019 Warrant, totaling 31,740,704 CDIs (equivalent to 634,814 shares of common stock) was exercised for approximately $400 thousand on September 30, 2019.
The Company evaluated the guidance ASC 480-10, Distinguishing Liabilities from Equity, ASC 815-40 Contracts in an Entity’s Own Equity and ASC 470-20 Debt with Conversion and Other Options to determine the appropriate classification of the March 2019 Note and March 2019 Warrant. On the date of the issuance of the March 2019 Note, the March 2019 Warrant was determined to be a freestanding instrument meeting the requirements for liability classification due to a cash settlement recourse available on the condition of not obtaining stockholder approval. Accordingly, the fair value estimated for the March 2019 Warrant, totaling approximately $850 thousand, was recorded as a discount to the March 2019 Note with the offset to derivative liabilities. The Company then evaluated the March 2019 Note for a BCF. Based upon the effective conversion price of the March 2019 Note after considering the stock price at the commitment date and the allocation of estimated fair value to the March 2019 Warrant, it was determined that the March 2019 Note contained a contingent BCF. The value of the BCF was computed to be approximately $623 thousand but was capped at approximately $129 thousand so as to not exceed the total proceeds from the March 2019 Note after deducting the value allocated to the March 2019 Warrant.
In addition, upon a change of control of the Company (other than a change of control resulting from a Qualified Financing) in which the Company’s stockholders receive cash consideration, the Company is obligated under the March 2019 Note to pay all accrued and unpaid interest then due plus 110% of the remaining outstanding unconverted principal balance. If the change of control results in non-cash consideration, Crystal Amber may convert the entire outstanding principal balance under the March 2019 Note and all unpaid accrued interest then due into CDIs at the abovementioned Optional Conversion Price. Other than as described above, the Company may not prepay the March 2019 Note without the consent of Crystal Amber. The Company considers the change in control premium as a cash settleable feature, thereby requiring derivative liability classification. On applying a probability adjusted present value of the premium, the fair value was considered immaterial upon issuance and at all subsequent reporting period ends.
Upon approval of the conversion features of the March 2019 Note and issuance of the March 2019 Warrants on June 30, 2019, the Company remeasured the warrant liability and recorded a $576 thousand remeasurement loss to the consolidated statement of operations and then reclassified $1.4 million of fair value of March 2019 Warrant from derivative liability to equity as the warrant became immediately exercisable. The total debt discount on the March 2019 Note upon stockholder approval of its conversion feature was $1 million. The effective interest rate on the Note after the discounts was 29.4%. Upon conversion of the 2019 March Note on June 30, 2019, the Company recorded the contingent beneficial conversion feature of $129 thousand as non-cash interest expense and additional paid-in capital.
F-20
For the year ended December 31, 2019, the Company accrued interest expense of $30 thousand and recorded debt discount amortization to interest expense of $1 million.
May 2019 Convertible Note and Warrant Financing
On May 8, 2019, the Company entered into a Note Purchase Agreement (“May 2019 NPA”) by and between the Company and Crystal Amber. Pursuant to the May 2019 NPA, the Company issued and sold to Crystal Amber a Senior Unsecured Convertible Promissory Note in an aggregate original principal amount of $3.0 million (the “May 2019 Note”) with a maturity date of May 8, 2024. Interest accrued at an annually compounded rate of 10% per annum and issuance costs related to the May 2019 NPA were $34 thousand.
After the Company obtained stockholder approval to enable Crystal Amber’s, a Related Party, conversion rights under the May 2019 Note on June 30, 2019, the entire outstanding principal balance under the May 2019 Note and all unpaid accrued interest thereon was convertible into CDIs, at the option of Crystal Amber at a conversion price of $0.0127 per CDI. On June 30, 2019, Crystal Amber elected to convert the May 2019 Note into 237,687,411 CDIs (representing 4,753,748 shares of common stock). The principal of $3.0 million converted to 236,220,472 CDIs (representing 4,724,409 shares of common stock) and the accrued interest of approximately $19 thousand converted to 1,466,939 CDIs (representing 29,339 shares of common stock). As of June 30, 2019, notice of the conversion had been provided by Crystal Amber and the CDIs were issued on July 3, 2019 and have a one-year restriction on trading on the ASX.
Per the May 2019 NPA, the Company agreed to issue a warrant (the “May 2019 Warrant”) to Crystal Amber, pending stockholder approval, to purchase 236,220,472 CDIs (representing 4,724,409 shares of common stock) at an initial exercise price of $0.0127 per CDI. The issue of the May 2019 Warrant required the approval of stockholders and was not exercisable until its issue was approved on June 30, 2019. A portion of the May 2019 Warrant totaling 125,739,610 CDIs (equivalent to 2,514,792 shares of common stock) was exercised by Crystal Amber for approximately $1.6 million on September 30, 2019. Another portion of the May 2019 Warrant totaling 78,740,157 CDIs (equivalent to 1,574,803 shares of common stock) was exercised by Crystal Amber for approximately $1 million on October 31, 2019.
The Company evaluated the guidance ASC 480-10, Distinguishing Liabilities from Equity, ASC 815-40 Contracts in an Entity’s Own Equity and ASC 470-20 Debt with Conversion and Other Options to determine the appropriate classification of the May 2019 Note and May 2019 Warrant. On the date of the issuance of the May 2019 Note, the May 2019 Warrant was determined to be a freestanding instrument meeting the requirements for liability classification due to a cash settlement recourse available on the condition of not obtaining stockholder approval. Accordingly, the fair value estimated for the May 2019 Warrant, totaling approximately $3.2 million, was recorded to derivative liabilities with offsets of $3 million to a discount on the May 2019 Note and $200 thousand to derivative loss on the consolidated statements of operations. The Company then evaluated the May 2019 Note for a BCF. Based upon the effective conversion price of the May 2019 Note after considering the stock price at commitment date and the allocation of estimated fair value to the May 2019 Warrant, it was determined that the May 2019 Note contained a BCF. The value of the BCF was computed to be approximately $2 million but was not recorded as doing so would exceed the total proceeds from the May 2019 Note after recording the fair value of the May 2019 Warrant.
In addition, upon a change of control of the Company (other than a change of control resulting from a Qualified Financing) in which the Company’s stockholders receive cash consideration, the Company is obligated under the May 2019 Note to pay all accrued and unpaid interest then due plus 110% of the remaining outstanding unconverted principal balance. If the change of control results in non-cash consideration, Crystal Amber may convert the entire outstanding principal balance under the May 2019 Note and all unpaid accrued interest then due into CDIs at the abovementioned Optional Conversion Price. Other than as described above, the Company may not prepay the May 2019 Note without the consent of Crystal Amber. The Company considers the change in control premium as a cash settleable feature, thereby requiring derivative liability classification. On applying a probability adjusted present value of the premium, the fair value was considered immaterial upon issuance and at all subsequent reporting period ends.
Upon approval of the conversion features of the May 2019 Note and issuance of the May 2019 Warrant on June 30, 2019, the Company revalued the warrant liability and recorded a $1.1 million remeasurement loss to the consolidated statements of operations and then reclassified approximately $4.3 million of fair value of May 2019 Warrant from derivative liabilities to equity as the May 2019 Warrant became immediately exercisable. The total debt discount on the May 2019 Note upon stockholder approval was $3 million. The effective interest rate on the May 2019 Note after the discounts is 29.4%.
F-21
For the year ended December 31, 2019, the Company accrued interest expense of $19 thousand and recorded debt discount amortization to interest expense of $3 million.
August 2019 Share Purchase Agreement (“SPA”)
On August 21, 2019, the Company entered into the August 2019 SPA by and between the Company and Crystal Amber. The August 2019 SPA detailed a timeline wherein Crystal Amber would exercise the 2018 Warrant, the March 2019 Warrant, and the May 2019 Warrant. Additionally, pursuant to the August 2019 SPA, the Company issued and sold to Crystal Amber a senior unsecured convertible promissory note in an aggregate principal amount of approximately $4.6 million, or such lesser amount as may be set forth in a notice delivered by the Company to Crystal Amber (the “August 2019 Note”), to be funded on December 6, 2019, or such earlier or later date as may be requested by the Company (the “Funding Date”). In conjunction with the August 2019 Note, the Company agreed to issue to Crystal Amber a Warrant (the “August 2019 Warrant”) to purchase CDIs, subject to the receipt of required stockholder approval approving the issuance of the August 2019 Warrant and the funding of the August 2019 Note (For a detailed description of the exercises and the terms of the exiting warrants and for a description of the August 2019 Warrant, see Note 4 Warrants to purchase common stock or CDIs)
The August 2019 Note accrues interest at a rate equal to 10% per annum from the 2019 Note Funding Date, compounded annually, other than during the continuance of an event of default, when the August 2019 Note accrues interest at a rate of 16% per annum. The entire outstanding principal balance and all unpaid accrued interest thereon is due on the fifth anniversary of the Funding Date. Subject to the receipt of stockholder approval as required by Rule 10.11 of the ASX Listing Rules, the entire outstanding principal balance under the August 2019 Note and all unpaid accrued interest thereon is convertible into CDIs at the option of Crystal Amber at a conversion price equal to US$0.02 per CDI. In the event that the Company issues additional CDIs to a stockholder other than Crystal Amber in a subsequent equity financing at a price per CDI that is less than the conversion price under the August 2019 Note, the conversion price shall be reduced to the lowest such price per CDI. In addition, upon a change of control of the Company resulting in cash proceeds, Crystal Amber may, at its option, demand that the Company prepay all accrued and unpaid interest plus 110% of the remaining outstanding unconverted principal balance. The Company may not prepay the August 2019 Note without the consent of Crystal Amber, a Related Party. If the stockholder approvals required to issue the August 2019 Warrant or to approve the conversion rights under the August 2019 Note are not obtained, the Company is obligated to prepay all accrued and unpaid interest plus 110% of the remaining outstanding unconverted principal balance on the earlier of the Funding Date or the date that is six months following the date of the stockholder meeting at which the requisite approvals were not obtained. The Company considers the change in control premium and the stockholder approval premium to each represent a cash settleable feature, thereby requiring derivative liability classification. On applying a probability adjusted present value of the premiums, the fair value was considered immaterial upon issuance at all subsequent reporting period ends.
The August 2019 SPA contains customary events of default. If a default occurs and is not cured within the applicable cure period or is not waived, any outstanding obligations under the August 2019 Note may be accelerated. The August 2019 SPA and related August 2019 Note and August 2019 Warrant documents also contain additional representations and warranties, covenants and conditions, in each case customary for transactions of this type.
Prior to December 6, 2019, GI Dynamics notified Crystal Amber that it had elected to receive the full amount of approximately $4.6 million under the August 2019 Note, but agreed to timing extensions.
On December 16, 2019, stockholder approval was obtained pursuant to ASX Listing Rule 10.11, for the August 2019 Note conversion feature and the issuance of the August 2019 Warrant, contingent on receipt of the August 2019 Note proceeds.
On January 13, 2020, the full amount of approximately $4.6 million was received as proceeds from the August 2019 Note. On receipt of funds, the August 2019 Note conversion feature was immediately available to Crystal Amber. On January 13, 2020, GI Dynamics issued to Crystal Amber the August 2019 Warrant to purchase 229,844,650 CDIs (representing 4,596,893 shares of common stock) for an exercise price of $0.02 per CDI (see Note 4).
F-22
10. Commitments and Contingencies
Lease Commitments
In June 2016, the Company entered into a non-cancelable agreement to lease approximately 4,200 square feet of office space in Boston, Massachusetts. The lease commenced in June 2016 and expired in April 2018. Rent during the term was approximately $12 thousand per month.
In December 2018, the Company entered into a 6-month membership agreement with WeWork for 985 square feet of office space located in Boston, Massachusetts. The committed lease term expired in May 2019 and contained a two-month cancellation provision. The WeWork agreement contained no explicit or guaranteed extension provisions. At December 31, 2018, there remained approximately $91 thousand of lease obligation, assuming the company did not cancel early.
On April 22, 2019, the Company entered into a right-of-use lease for 3,520 square feet of office space in Boston, Massachusetts. The lease period contractually commenced June 1, 2019 and expires on May 31, 2022, but the space was available for occupancy on May 1, resulting in an effective period of May 2019 through May 2022, with no rent payment assessed in May 2019. The lease has defined escalating rent payments and contains no extension or expansion rights. On lease execution, the Company recorded the approximately $463 thousand present value of the lease liability in short-term and long-term liabilities and recorded a related right-of-use asset. The right-of-use asset will be amortized to lease expense and the liability will be reduced by the rent payments over the term of the lease.
The Company’s leases generally do not provide an implicit interest rate and therefore the Company uses 10% as an estimate of its incremental borrowing rate as the discount rate when measuring operating lease liabilities. The incremental borrowing rate represents an estimate of the interest rate the Company would incur at lease commencement to borrow an amount equal to the lease payments on a collateralized basis over the term of a lease in a similar economic environment. The Company had no leases currently classified as finance leases or previously classified as capital leases in either reporting period.
The Company’s operating lease is reflected in the balance sheets. The $102 thousand of lease expense included $7 thousand of common area rent. An additional $3 thousand of tax expense was accrued for property taxes associated with the leased facility. Other information related to leases was as follows:
| December 31, | ||||
| 2019 | ||||
| (in thousands) | ||||
| Operating cash flows from operating leases in lease liability measurement | $ | 148 | ||
| Operating cash flows from short term leases | 103 | |||
| Remaining long-term lease term in years | 2.4 | |||
| Discount rate | 10 | % | ||
The maturity of the Company’s operating lease liability as of December 31, 2019 is as follows:
| December 31, | ||||
| 2019 | ||||
| (in thousands) | ||||
| 2020 | 178 | |||
| 2021 | 182 | |||
| 2022 | 76 | |||
| Total future minimum lease payments | 436 | |||
| Less: imputed interest | (50 | ) | ||
| Total liabilities | $ | 386 | ||
Rent expense on non-cancelable operating leases was approximately $200 and $100 thousand for the years ended December 31, 2019 and 2018, respectively.
F-23
11. Stockholders’ Deficit
On December 19, 2019, GI Dynamics stockholders approved an increase of its authorized shares of common stock from 50 million to 75 million.
As of December 31, 2019, the authorized capital stock of the Company consists of 75.5 million shares, of which 75 million shares are designated as common stock and 500 thousand shares are designated as preferred stock.
In 2018, the Company received commitments for two private placements to sophisticated and professional investors in Australia, the United States and the United Kingdom, consisting of U.S. and non-U.S. persons (as defined in Regulation S (“Regulation S”) of the Securities Act of 1933 (the “Securities Act”) to raise up to approximately $6.6 million (the “2018 Placements”). The first placement (“First Quarter 2018 Placement”) consisted of a total of 58,780,619 fully paid CDIs of the Company (representing 1,175,612 shares of common stock) at an issue price of A$0.035 per CDI. The issue of CDIs under the First Quarter 2018 Placement occurred in two tranches. The first tranche closed on January 22, 2018 (US EST), pursuant to which the Company issued 28,467,063 CDIs (representing 569,341 shares of common stock) resulting in gross proceeds of approximately $781 thousand and related issuance costs of $63 thousand. The closing of the second tranche of the First Quarter 2018 Placement resulted in the raising of $824 thousand and related issuance costs of $40 thousand by the issue of 30,313,556 CDIs (representing 606,271 shares) following stockholder approval granted on February 27, 2018. There were two participants in the First Quarter 2018 Placement second tranche; Crystal Amber Fund, a related party for ASX purposes, purchased 27,391,756 CDIs. A Board member of the Company purchased 2,921,800 CDIs.
The second placement (“Autumn 2018 Placement”) consisted of a total of 347,222,250 fully paid CDIs of the Company (representing 6,944,445 shares of common stock) at an issue price of A$0.020 per CDI. The investors in the Autumn 2018 Placement included certain existing investors. The issue of these CDIs occurred in two tranches. The first tranche closed on September 20, 2018 (US EST), pursuant to which the Company issued 150,000,000 CDIs (representing 3,000,000 shares of common stock) resulting in gross proceeds of approximately $2.2 million and related issuance costs of $56 thousand. The closing of the second tranche resulted in the raising of $2.8 million and related issuance costs of $12 thousand by the issue of 197,222,250 CDIs (representing 3,944,445 shares of common stock) following stockholder approval at the adjourned Special Meeting of stockholders on October 29, 2018. There were three participants in the second tranche; Crystal Amber Fund, a related party for ASX purposes, purchased 168,194,450 CDIs. Existing investors in the United States and Australia also purchased 23,819,450 and 5,208,350 CDIs, respectively. All second tranche CDIs were allotted to investors in November 2018.
On June 30, 2019, Crystal Amber converted the 2018 Note to 134,852,549 CDIs (representing 2,697,050 shares of common stock).
On June 30, 2019, Crystal Amber converted the March 2019 Note to 81,070,003 CDIs (representing 1,621,400 shares of common stock).
On June 30, 2019, Crystal Amber converted the May 2019 Note to 237,687,411 CDIs (representing 4,753,748 shares of common stock).
On August 25, 2019, Crystal Amber exercised the 2018 Warrant totaling 97,222,200 CDIs (representing 1,944,444 shares of common stock) and a portion of the March 2019 Warrant totaling 47,244,119 CDIs (equivalent to approximately 944,882 shares of common stock) for an aggregate cash payment of $2 million.
On September 30, 2019, Crystal Amber exercised the remaining March 2019 Warrant totaling 31,740,704 CDIs (equivalent to 634,814 shares of common stock) and a portion of the May 2019 Warrant totaling 125,739,610 CDIs (equivalent to 2,514,792 shares of common stock) for an aggregate cash payment of $1.6 million. As of September 30, 2019, this was recorded as common stock – subscribed but unissued and the cash was received on October 1, 2019.
On October 31, 2019, Crystal Amber exercised another portion of May 2019 Warrant totaling 78,740,157 CDIs (equivalent to approximately 1,574,803 shares of common stock) for an aggregate cash payment of $1 million. Cash was received on October 31, 2019.
On November 15, 2019, Crystal Amber exercised the final portion of May 2019 Warrant totaling 31,740,704 CDIs (equivalent to approximately 634,814 shares of common stock) for an aggregate cash payment of approximately $403 thousand. Cash was received on November 15, 2019.
F-24
12. Share-Based Compensation
The Company has two stock-based compensation plans. The Board of Directors adopted the 2003 Omnibus Stock Plan (the “2003 Plan”), which provides for the grant of qualified incentive stock options and nonqualified stock options or other awards to the Company’s employees, officers, directors, advisors, and outside consultants to purchase up to an aggregate of 922,086 shares of the Company’s common stock.
In August 2011, the Board of Directors adopted the 2011 Employee, Director and Consultant Equity Incentive Plan (the “2011 Plan”, together with the 2003 Plan, the “Plans”) as the successor to the 2003 Plan. Under the 2011 Plan, the Company may grant incentive stock options, nonqualified stock options, restricted and unrestricted stock awards and other stock-based awards. The Company had initially reserved 450 thousand shares of its common stock for issue under the 2011 Plan. Awards that are returned to the Company’s 2003 Plan as a result of their forfeiture, expiration or cancellation without delivery of common stock shares or that result in the forfeiture of shares back to the Company on or after August 1, 2011, the date the 2011 Plan became effective, are automatically made available for issuance under the 2011 Plan. At August 1, 2011, 80,235 shares available for grant under the 2003 Plan were transferred to the 2011 Plan. As of December 31, 2019, an additional 1,173,917 shares of common stock were made available for grant under the Company’s 2011 Plan.
In addition, the 2011 Plan allows for an annual increase in the number of shares available for issue under the 2011 Plan commencing on the first day of each fiscal year during the period beginning in fiscal year 2012 and ending in fiscal year 2020. The annual increase in the number of shares shall be equal to the lowest of:
| · | 500 thousand shares; |
| · | 4% of the number of common shares outstanding as of such date; and |
| · | an amount determined by the Board of Directors or the Company’s compensation committee. Accordingly, during year ended December 31, 2019, 500 thousand shares were added to the 2011 Plan. During the year ended December 31, 2018, the board of directors elected to not add shares to the 2011 Plan, thereby reserving shares for other purposes. |
Stock-Based Compensation
Stock-based compensation is reflected in the consolidated statements of operations as follows for the years ended December 31, 2019 and 2018 (in thousands):
| Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Research and development | $ | 77 | $ | 6 | ||||
| Sales and marketing | — | 23 | ||||||
| General and administrative | 311 | 95 | ||||||
| $ | 388 | $ | 124 | |||||
The stock options granted under the Plans generally vest over a four-year period and expire ten years from the date of grant.
The weighted-average assumptions used to estimate the fair value of employee stock options using the Black-Scholes option-pricing model were as follows for the years ended December 31, 2019 and 2018:
| Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Expected volatility | 271.0 | % | 120.7 | % | ||||
| Expected term (in years) | 6.05 | 6.05 | ||||||
| Risk-free interest rate | 1.8 | % | 2.6 | % | ||||
| Expected dividend yield | 0 | % | 0 | % | ||||
F-25
Expected Volatility
Volatility measures the amount that a stock price has fluctuated or is expected to fluctuate during a period. As the Company was not publicly traded prior to September 2011 and therefore had no trading history, stock price volatility was estimated based on an analysis of historical and implied volatility of comparable public companies. The Company’s quoted publicly traded CDI price is used as a basis for calculating estimated volatility when there exists adequate history.
Expected Term
The Company has limited historical information to develop reasonable expectations about future exercise patterns and post-vesting employment termination behavior for its stock option grants. As a result, for stock option grants made during the years ended December 31, 2019 and 2018, the expected term was estimated using the “simplified method.” The simplified method is based on the average of the contractual term of the option and the weighted-average vesting period of the option. For options granted to non-employees, the Company used the remaining contractual life of the award to estimate the expected term of non-employee awards for the years ended December 31, 2019 and 2018.
Risk-Free Interest Rate
The risk-free interest rate used for each grant is based on the US Treasury published rate for a zero-coupon U.S. Treasury instrument with a remaining term similar to the expected term of the stock-based award.
Expected Dividend Yield
The Company has not paid and does not anticipate paying cash dividends on its shares of common stock in the foreseeable future; therefore, the expected dividend yield is assumed to be zero.
Stock Options
The following table summarizes share-based activity under the Company’s stock option plans:
| Shares of Common Stock Attributable to Options | Weighted- Average Exercise Price | Weighted- Average Fair Value per Share at Grant date | Weighted- Average Contractual Life | Aggregate Intrinsic Value | ||||||||||||||||
| (in years) | (in years) | (in thousands) | ||||||||||||||||||
| Outstanding at December 31, 2018 | 1,142,883 | $ | 2.24 | $ | 2.20 | 8.48 | $ | — | ||||||||||||
| Granted | 1,994,771 | $ | 1.08 | $ | 0.23 | |||||||||||||||
| Exercised | — | — | — | |||||||||||||||||
| Cancelled | (41,500 | ) | $ | 0.91 | $ | 0.91 | ||||||||||||||
| Outstanding at December 31, 2019 | 3,096,154 | $ | 1.47 | $ | 1.50 | 8.30 | — | |||||||||||||
| Vested or expected to vest at December 31, 2019 | 3,096,154 | — | ||||||||||||||||||
| Exercisable at December 31, 2019 | 685,015 | $ | 2.86 | $ | 0.04 | 7.10 | $ | — | ||||||||||||
The majority of the Company’s option grants vest 25% on the first anniversary of the grant date, and in a quarterly straight-line rate thereafter until fully vested on the fourth anniversary of the grant date. The weighted average grant date fair value for options granted in 2019 and 2018, was $1.04 and $0.97, respectively. The unrecognized stock compensation expense at December 31, 2019 was $2.4 million and was expected to be recognized over a weighted average period of 3.26 years from December 31, 2019.
F-26
Performance Stock Units
Each performance stock unit (“PSU”) represents a contingent right to receive one share of the Company’s common stock. There is no consideration payable on the vesting of PSUs issued under the Plans. Upon vesting, the PSUs are exercised automatically and settled in shares of the Company’s common stock. During the years ended December 31, 2019 and 2018, the Company awarded no PSUs to employees and directors of the Company. Due to the resignation of an employee, one grant of 142,659 PSUs was cancelled in 2018.
The following table summarizes information related to PSU activity during the year ended December 31, 2019:
| Number of Units | Weighted- Average Contractual Life | Aggregate Intrinsic Value | ||||||||||
| (in years) | (in thousands) | |||||||||||
| Outstanding at December 31, 2018 | 250,000 | 7.23 | $ | 141 | ||||||||
| Granted | — | |||||||||||
| Exercised | — | |||||||||||
| Cancelled | — | |||||||||||
| Outstanding at December 31, 2019 | 250,000 | 6.23 | $ | 149 | ||||||||
The aggregate intrinsic value at December 31, 2019 noted in the table above represents the closing price of the Company’s common stock multiplied by the number of PSUs outstanding. The fair value of each PSU award equals the closing price of the Company’s common stock on the date of grant.
At December 31, 2019, 250,000 of the PSUs outstanding vest on the achievement of certain milestones. When achievement of the milestone is deemed probable, the Company will expense the compensation of the respective stock award over the implicit service period.
During the years ended December 31, 2019 and 2018, the Company recognized no stock-based compensation related to performance-based vesting of PSUs.
As of December 31, 2019, there was approximately $200 thousand of unrecognized stock-based compensation expense related to non-vested PSU awards that have performance-based vesting.
Non-employee awards
The Company has recorded non-employee stock-based compensation expense of approximately $26 thousand and $20 thousand during the years ended December 31, 2019 and 2018, respectively, which is included in the total stock-based compensation expense. The unrecognized compensation expense associated with outstanding non-employee grants was $0 and $180 thousand at December 31, 2019 and 2018, respectively.
On October 29, 2019, the Company’s Board of Directors approved, subject to stockholder approval per ASX Listing Rule 10.14, the grant of stock options (“NED Options”) conferring the right to purchase up to 30,000 shares of the Company’s common stock to each of the Company’s four non-executive directors pursuant to the Company’s 2011 Plan. The exercise price is $1.34 per share of common stock and the NED Options will vest in full on October 29, 2020 if the individual remains a director of the Company through that date. The NED Options will vest immediately on a change in control event. The NED Options are immediately exercisable, subject to repurchase rights if purchased prior to vesting. The NED Options will be cancelled immediately upon termination of service as a director, unless such termination is the result of a defined change in control event, in which case the NED Options will be cancelled 12 months after such termination. On December 16, 2019, stockholders approved the NED Options.
F-27
13. Retirement Plans
The Company has a 401(k) retirement and savings plan (“401(k) Plan”) covering all qualified U.S. employees. The 401(k) Plan is a defined contribution plan and allows each participant to contribute up to 100% of the participant’s base wages up to an amount not to exceed an annual statutory maximum. The Company has made discretionary contributions to the 401(k) Plan and recorded expenses of approximately $48 and $24 thousand for the years ended December 31, 2019 and 2018, respectively.
The Company maintains a defined contribution plan for certain international employees. The Company contributes 100% of the cost of the defined contribution. The Company recorded expenses of approximately $9 and $12 thousand for the years ended December 31, 2019 and 2018, respectively, under this plan.
14. Income Taxes
The components of loss before provision for income taxes consisted of the following (in thousands):
| Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Domestic | $ | (17,376 | ) | $ | (8,054 | ) | ||
| Foreign | 88 | 29 | ||||||
| Total | $ | (17,288 | ) | $ | (8,025 | ) | ||
The components of the provision for income taxes in the accompanying consolidated statements of operations consisted of the following (in thousands):
| Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Current Provision: | ||||||||
| Federal | $ | — | $ | — | ||||
| State | 1 | 1 | ||||||
| Foreign | 42 | 13 | ||||||
| Total current | 43 | 14 | ||||||
| Deferred (Benefit) Provision: | ||||||||
| Federal | — | — | ||||||
| State | — | — | ||||||
| Foreign | 2 | (1 | ) | |||||
| Total deferred | 2 | (1 | ) | |||||
| Total provision | $ | 45 | $ | 13 | ||||
A reconciliation of the provision for income taxes from operations computed using the U.S. federal statutory rate of 21% to that reflected in operations follows (in thousands):
| Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Income tax benefit using U.S. federal statutory rate | $ | (3,624 | ) | $ | (1,675 | ) | ||
| State rate, net of federal benefit | (631 | ) | (438 | ) | ||||
| Permanent differences | ||||||||
| Nondeductible interest | 1,052 | 36 | ||||||
| Warrants | 353 | — | ||||||
| GILTI | 8 | — | ||||||
| Meals and entertainment | 6 | 3 | ||||||
| Other | 2 | 1 | ||||||
| Tax credits generated | (147 | ) | (45 | ) | ||||
| Change in valuation allowance | 2,888 | 2,041 | ||||||
| Foreign rate differential | 4 | 3 | ||||||
| Other items | 96 | 63 | ||||||
| Stock compensation | 38 | 24 | ||||||
| Total provision | $ | 45 | $ | 13 | ||||
F-28
Significant components of the Company’s deferred tax assets and liabilities are as follows (in thousands):
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carryforwards | $ | 66,859 | $ | 63,881 | ||||
| Research and development credit carryforwards | 4,083 | 4,026 | ||||||
| Capitalized research and development costs | — | 123 | ||||||
| Capitalized start-up expenses | 2,133 | 2,490 | ||||||
| Depreciation and other | 635 | 305 | ||||||
| Total deferred tax assets | 73,710 | 70,825 | ||||||
| Valuation allowance | (73,703 | ) | (70,815 | ) | ||||
| Net deferred tax asset | $ | 7 | $ | 10 | ||||
Management of the Company has evaluated the positive and negative evidence bearing upon the realizability of the Company’s deferred tax assets and determined that it is more likely than not that the Company will not recognize the benefits of the deferred tax assets related to the U.S. As a result, a valuation allowance of approximately $73.7 million and $70.8 million was established at December 31, 2019 and 2018, respectively. The valuation allowance increased by approximately $2.9 million during the year ended December 31, 2019, primarily due to the benefit of the current year tax loss. During the year ended December 31, 2019, the Company utilized its remaining carryforward net operating losses in the Netherlands. No valuation allowance is recorded against the net deferred tax assets in Australia related to future tax benefits that will offset future taxable income.
At December 31, 2019, the Company had U.S. federal and state net operating loss carryforwards of approximately $248.8 million and $231.1 million, respectively. These operating loss carryforwards will expire at various times beginning in 2024 through 2038 for federal purposes and begin to expire in 2030 and will continue to expire through 2039 for state purposes. Note that the $25.8 million net operating losses incurred in 2018 and 2019 can be carried forward indefinitely for federal purposes.
In addition, at December 31, 2019, the Company also has U.S. federal and state research and development tax credit carryforwards (excluding ASC 740, Income Taxes (“ASC 740”), reserve) of approximately $3.8 million and $2.0 million, respectively, available to offset future income taxes. These tax credit carryforwards will expire at various times beginning in 2023 through 2038 for federal purposes and will expire at various times beginning in 2019 through 2034 for state purposes.
The Company has not yet completed a study of its research and development credit carryforwards. Once completed, this study may result in an adjustment to the Company’s research and development credit carryforwards. A full valuation allowance has been provided against the Company’s research and development credits, and if an adjustment is required at the time the study is completed, this adjustment would be offset by an adjustment to the deferred tax asset established for the research and development credit carryforward and the valuation allowance.
Utilization of net operating loss carryforwards and research and development credit carryforwards may be subject to a substantial annual limitation due to ownership change limitations that have occurred previously or that could occur in the future in accordance with Sections 382 and Section 383 of the Internal Revenue Code of 1986, as well as similar state provisions. These ownership changes may limit the amount of net operating loss carryforwards and research and development credit carryforwards that can be utilized annually to offset future taxable income and taxes, respectively. In general, an ownership change, as defined by IRC Section 382, results from transactions increasing the ownership of certain stockholders or public groups in the stock of a corporation by more than 50 percentage points over a three-year period. The Company has completed several financings since its inception, which may have resulted in a change in control as defined by IRC Section 382 or could result in a change in control in the future. As of December 31, 2019, the Company has not, as yet, conducted an IRC Section 382 study, which could impact its ability to utilize net operating loss and tax credit carryforwards annually in the future to offset the Company’s taxable income, if any.
F-29
The Company applies ASC 740-10, which provides guidance on the accounting for uncertainty in income taxes recognized in financial statements and requires the impact of a tax position to be recognized in the financial statements if that position is more likely than not of being sustained by the taxing authority. When uncertain tax positions exist, the Company recognizes the tax benefit of tax positions to the extent that the benefit will more likely than not be realized. The determination as to whether the tax benefit will more likely than not be realized is based upon the technical merits of the tax position as well as consideration of the available facts and circumstances. At December 31, 2019 and 2018, the Company had unrecognized tax liabilities of approximately $1.5 million and $1.5 million, respectively.
The following is a roll forward of the Company’s unrecognized tax benefits (in thousands):
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Unrecognized tax benefit – as of the beginning of the year | $ | 1,462 | $ | 1,481 | ||||
| Gross decreases – expiration of underlying tax asset | (21 | ) | (35 | ) | ||||
| Gross increases – current period tax positions | 39 | 16 | ||||||
| Unrecognized tax benefits – as of the end of the year | $ | 1,480 | $ | 1,462 | ||||
The Company will recognize interest and penalties related to uncertain tax positions, should they be assessed, in income tax expense. As of December 31, 2019, and 2018, the Company had no accrued interest or penalties related to uncertain tax positions, and no amounts have been recognized in the Company’s consolidated statements of operations.
The statute of limitations for assessment by the Internal Revenue Service (“IRS”) and state tax authorities is open for tax years ended December 31, 2016 through December 31, 2019, although carryforward attributes that were generated prior to tax year 2016 may still be adjusted upon examination by the IRS or state tax authorities if they either have been or will be used in a future period. The statute of limitations for assessment by foreign tax authorities is open for tax years ended December 31, 2015 through December 31, 2019. There are currently no foreign, federal or state audits in progress.
15. Subsequent Events
On January 13, 2020, GI Dynamics received approximately $4.6 million pursuant to the August 2019 Note. On receipt of funds, the August 2019 Note became convertible per the August 2019 Note terms (see Note 9), and GI Dynamics issued the August 2019 Warrant (see Notes 4 and 9).
On March 11, 2020, the World Health Organization declared the outbreak of a coronavirus (COVID-19) a pandemic. As a result, economic uncertainties have arisen which are likely to negatively impact GI Dynamics short term-operations, for example in delays in timing of regulatory audits and in facing enrollment pauses in clinical trials. If the impacts are limited in duration, the Company anticipates an ability to overcome delays and return to original timing estimates, but there can be no guarantee the impacts will be limited to the degree required. Additionally, market-level impacts may affect the timing or the negotiation of terms in the Company’s financing efforts. Other financial impacts could occur though such potential impact is unknown at this time.
F-30
