Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - REGENERON PHARMACEUTICALS, INC. | a19-1325_1ex99d2.htm |
| EX-99.1 - EX-99.1 - REGENERON PHARMACEUTICALS, INC. | a19-1325_1ex99d1.htm |
| 8-K - 8-K - REGENERON PHARMACEUTICALS, INC. | a19-1325_18k.htm |
2019 Financial Overview January 9th Executive Vice President of Finance – Chief Financial Officer Robert Landry

Note regarding FORWARD-LOOKING STATEMENTs and non-gaap financial measures 2 This presentation includes forward-looking statements that involve risks and uncertainties relating to future events and the future performance of Regeneron Pharmaceuticals, Inc. (“Regeneron” or the “Company”), and actual events or results may differ materially from these forward-looking statements. Words such as “anticipate,” “expect,” “intend,” “plan,” “believe,” “seek,” “estimate,” variations of such words, and similar expressions are intended to identify such forward-looking statements, although not all forward-looking statements contain these identifying words. These statements concern, and these risks and uncertainties include, among others, the nature, timing, and possible success and therapeutic applications of Regeneron’s products, product candidates, and research and clinical programs now underway or planned, including without limitation EYLEA® (aflibercept) Injection, Dupixent® (dupilumab) Injection, Praluent® (alirocumab) Injection, Kevzara® (sarilumab) Injection, Libtayo® (cemiplimab) Injection, fasinumab, evinacumab, Regeneron’s immuno-oncology programs (including its costimulatory bispecific portfolio), Regeneron’s earlier-stage product candidates, and the use of human genetics in Regeneron’s research programs; the extent to which the results from Regeneron’s research programs or preclinical testing may lead to advancement of product candidates to clinical trials or therapeutic applications; unforeseen safety issues resulting from the administration of products and product candidates in patients, including serious complications or side effects in connection with the use of Regeneron’s product candidates in clinical trials; the likelihood and timing of possible regulatory approval and commercial launch of Regeneron’s late-stage product candidates and new indications for marketed products, including without limitation EYLEA, Dupixent, Praluent, Kevzara, Libtayo, fasinumab, and evinacumab; the likelihood and timing of achieving any of the anticipated milestones described in this presentation; the extent to which the results from the research and development programs conducted by Regeneron or its collaborators may be replicated in other studies and lead to therapeutic applications; ongoing regulatory obligations and oversight impacting Regeneron’s marketed products (such as EYLEA, Dupixent, Praluent, Kevzara, and Libtayo), research and clinical programs, and business, including those relating to patient privacy; determinations by regulatory and administrative governmental authorities which may delay or restrict Regeneron’s ability to continue to develop or commercialize Regeneron’s products and product candidates; competing drugs and product candidates that may be superior to Regeneron’s products and product candidates; uncertainty of market acceptance and commercial success of Regeneron’s products and product candidates and the impact of studies (whether conducted by Regeneron or others and whether mandated or voluntary) on the commercial success of Regeneron's products and product candidates; the availability and extent of reimbursement of the Company’s products from third-party payers, including private payer healthcare and insurance programs, health maintenance organizations, pharmacy benefit management companies, and government programs such as Medicare and Medicaid; coverage and reimbursement determinations by such payers and new policies and procedures adopted by such payers; the ability of Regeneron to manufacture and manage supply chains for multiple products and product candidates; the ability of Regeneron’s collaborators, suppliers, or other third parties to perform filling, finishing, packaging, labeling, distribution, and other steps related to Regeneron’s products and product candidates; unanticipated expenses; the costs of developing, producing, and selling products; the ability of Regeneron to meet any of its sales or other financial projections or guidance and changes to the assumptions underlying those projections or guidance, including financial guidance relating to Sanofi collaboration revenue, non-GAAP unreimbursed R&D, non-GAAP SG&A, effective tax rate, and capital expenditures; risks associated with intellectual property of other parties and pending or future litigation relating thereto, including without limitation the patent litigation proceedings relating to EYLEA, Dupixent, and Praluent, the ultimate outcome of any such litigation proceeding, and the impact any of the foregoing may have on Regeneron’s business, prospects, operating results, and financial condition; and the potential for any license or collaboration agreement, including Regeneron’s agreements with Sanofi, Bayer, and Teva Pharmaceutical Industries Ltd. (or their respective affiliated companies, as applicable), to be cancelled or terminated without any further product success. A more complete description of these and other material risks can be found in Regeneron’s filings with the U.S. Securities and Exchange Commission, including its Form 10-K for the fiscal year ended December 31, 2017 and its Form 10-Q for the quarterly period ended September 30, 2018, including in each case in the section thereof captioned “Item 1A. Risk Factors.” Any forward-looking statements are made based on management’s current beliefs and judgment, and the reader is cautioned not to rely on any forward-looking statements made by Regeneron. Regeneron does not undertake any obligation to update publicly any forward-looking statement, including without limitation any financial projection or guidance, whether as a result of new information, future events, or otherwise. This presentation uses non-GAAP unreimbursed R&D and non-GAAP SG&A, which are financial measures that are not calculated in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”). These non-GAAP financial measures are computed by excluding certain non-cash and other items from the related GAAP financial measure. Non-GAAP adjustments also include the income tax effect of reconciling items. The Company makes such adjustments for items the Company does not view as useful in evaluating its operating performance. For example, adjustments may be made for items that fluctuate from period to period based on factors that are not within the Company’s control, such as the Company’s stock price on the dates share-based grants are issued. Management uses these and other non-GAAP measures for planning, budgeting, forecasting, assessing historical performance, and making financial and operational decisions, and also provides forecasts to investors on this basis. Additionally, such non-GAAP measures provide investors with an enhanced understanding of the financial performance of the Company’s core business operations. However, there are limitations in the use of these and other non-GAAP financial measures as they exclude certain expenses that are recurring in nature. Furthermore, the Company’s non-GAAP financial measures may not be comparable with non-GAAP information provided by other companies. Any non-GAAP financial measure presented by Regeneron should be considered supplemental to, and not a substitute for, measures of financial performance prepared in accordance with GAAP. A reconciliation of the Company's full year 2019 non-GAAP to GAAP financial guidance is provided at the end of this presentation.

2019 financial overview TAX OVERVIEW COGS & COCM LIBTAYO® ACCOUNTING REGENERON/SANOFI RESTRUCTURE IO COLLABORATION INTERACTIVE ANALYST CENTER 2018 tax review & 2019 and beyond guidance Review Cost of Goods Sold (COGS) & Cost of Collaboration and Contract Manufacturing (COCM) Review of accounting for net sales and profits/losses Overview of the changes to the IO Discovery and Development Agreement Introduction to the Interactive Analyst Center - a repository of financial information and operational data 3
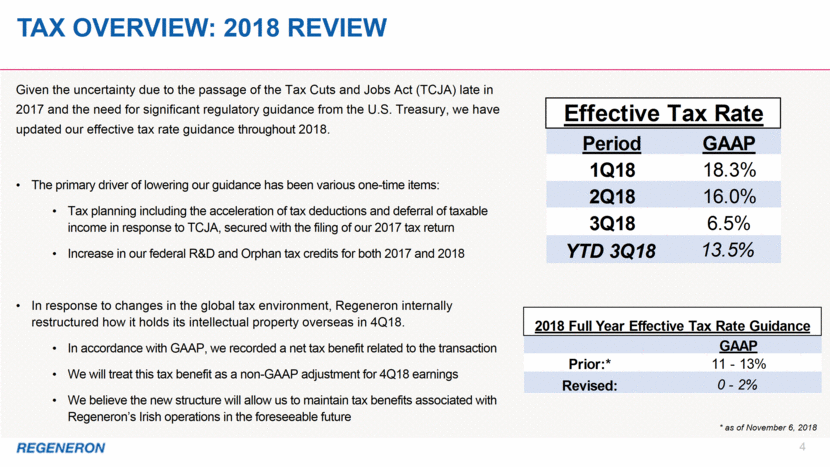
Tax overview: 2018 review 4 * as of November 6, 2018 Given the uncertainty due to the passage of the Tax Cuts and Jobs Act (TCJA) late in 2017 and the need for significant regulatory guidance from the U.S. Treasury, we have updated our effective tax rate guidance throughout 2018. The primary driver of lowering our guidance has been various one-time items: Tax planning including the acceleration of tax deductions and deferral of taxable income in response to TCJA, secured with the filing of our 2017 tax return Increase in our federal R&D and Orphan tax credits for both 2017 and 2018 In response to changes in the global tax environment, Regeneron internally restructured how it holds its intellectual property overseas in 4Q18. In accordance with GAAP, we recorded a net tax benefit related to the transaction We will treat this tax benefit as a non-GAAP adjustment for 4Q18 earnings We believe the new structure will allow us to maintain tax benefits associated with Regeneron’s Irish operations in the foreseeable future GAAP Prior:* 11 - 13% Revised: 0 - 2% 2018 Full Year Effective Tax Rate Guidance Period GAAP 1Q18 18.3% 2Q18 16.0% 3Q18 6.5% YTD 3Q18 13.5% Effective Tax Rate
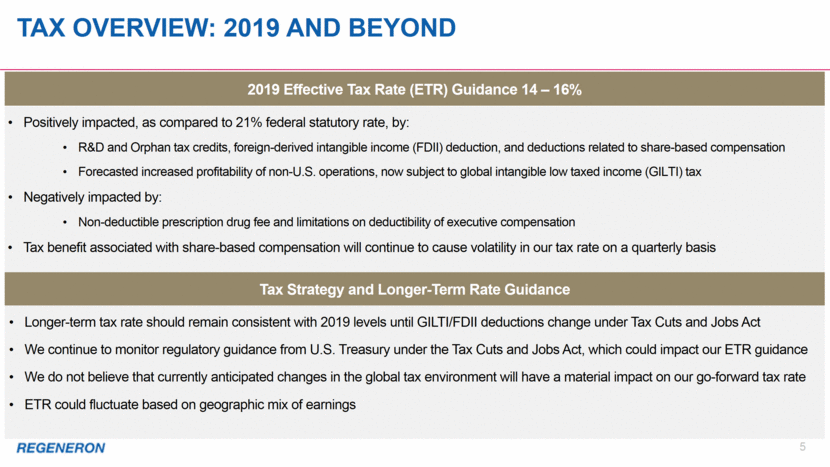
Tax Overview: 2019 and beyond 5 Tax Strategy and Longer-Term Rate Guidance 2019 Effective Tax Rate (ETR) Guidance 14 – 16% Positively impacted, as compared to 21% federal statutory rate, by: R&D and Orphan tax credits, foreign-derived intangible income (FDII) deduction, and deductions related to share-based compensation Forecasted increased profitability of non-U.S. operations, now subject to global intangible low taxed income (GILTI) tax Negatively impacted by: Non-deductible prescription drug fee and limitations on deductibility of executive compensation Tax benefit associated with share-based compensation will continue to cause volatility in our tax rate on a quarterly basis Longer-term tax rate should remain consistent with 2019 levels until GILTI/FDII deductions change under Tax Cuts and Jobs Act We continue to monitor regulatory guidance from U.S. Treasury under the Tax Cuts and Jobs Act, which could impact our ETR guidance We do not believe that currently anticipated changes in the global tax environment will have a material impact on our go-forward tax rate ETR could fluctuate based on geographic mix of earnings
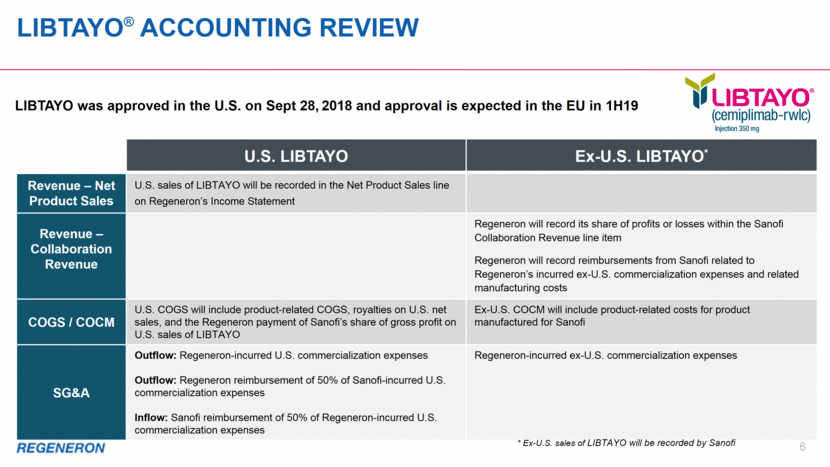
LIBTAYO was approved in the U.S. on Sept 28, 2018 and approval is expected in the EU in 1H19 LIBTAYO® ACCOUNTING REVIEW 6 Aflibercept is an investigational medicine for the indications listed here. The safety and efficacy of this drug candidate has not been fully evaluated by any regulatory authority for the disease categories indicated here. U.S. LIBTAYO Ex-U.S. LIBTAYO* Revenue – Net Product Sales U.S. sales of LIBTAYO will be recorded in the Net Product Sales line on Regeneron’s Income Statement Revenue – Collaboration Revenue Regeneron will record its share of profits or losses within the Sanofi Collaboration Revenue line item Regeneron will record reimbursements from Sanofi related to Regeneron’s incurred ex-U.S. commercialization expenses and related manufacturing costs COGS / COCM U.S. COGS will include product-related COGS, royalties on U.S. net sales, and the Regeneron payment of Sanofi’s share of gross profit on U.S. sales of LIBTAYO Ex-U.S. COCM will include product-related costs for product manufactured for Sanofi SG&A Outflow: Regeneron-incurred U.S. commercialization expenses Outflow: Regeneron reimbursement of 50% of Sanofi-incurred U.S. commercialization expenses Inflow: Sanofi reimbursement of 50% of Regeneron-incurred U.S. commercialization expenses Regeneron-incurred ex-U.S. commercialization expenses * Ex-U.S. sales of LIBTAYO will be recorded by Sanofi
Libtayo® accounting illustrative example 7 PLEASE NOTE ALL NUMBERS ARE ILLUSTRATIVE AND ARE NOT TO BE USED AS GUIDANCE Regeneron and Sanofi entered into a license agreement with Bristol-Myers Squibb Company, E. R. Squibb & Sons, L.L.C., and Ono Pharmaceutical Co., Ltd. and will pay royalties of 8.0% on worldwide sales of LIBTAYO through December 31, 2023, and royalties of 2.5% from January 1, 2024 through December 31, 2026. Regeneron will pay the royalties on U.S. net sales and Sanofi will pay the royalties on ex-U.S. net sales. LIBTAYO Alliance Product P&L U.S. Ex-U.S. Net Sales 500 A 250 Cost of Goods Sold (includes royalty) 100 B 75 F Gross Profit 400 C 175 SG&A REGN Expenses 200 D 25 G Sanofi Expenses 50 E 100 Total SG&A 250 125 Net Profit / (Loss) 150 50 REGN Share of Net Profit / (Loss) 75 25 H Regeneron Income Statement U.S. Ex-U.S. Net Product Sales 500 A Collaboration Revenue Profit Split 25 H Other Revenue 75 F Reimbursement of REGN SG&A 25 G Total Collaboration Revenue 125 Cost of Goods Sold (COGS) Product Supply Cost/Royalties 100 B Sanofi Share of U.S. Gross Profit 200 50% * C Total COGS 300 Cost of Collaboration and Contract Manufacturing (COCM) Product Supply Cost/Royalties 75 F SG&A REGN Incurred SG&A 200 D 25 G REGN Reimbursement of Sanofi SG&A 25 50% * E Sanofi Reimbursement of REGN SG&A -100 50% * D Total SG&A 125 REGN Share of Net Profit / (Loss) 75 25
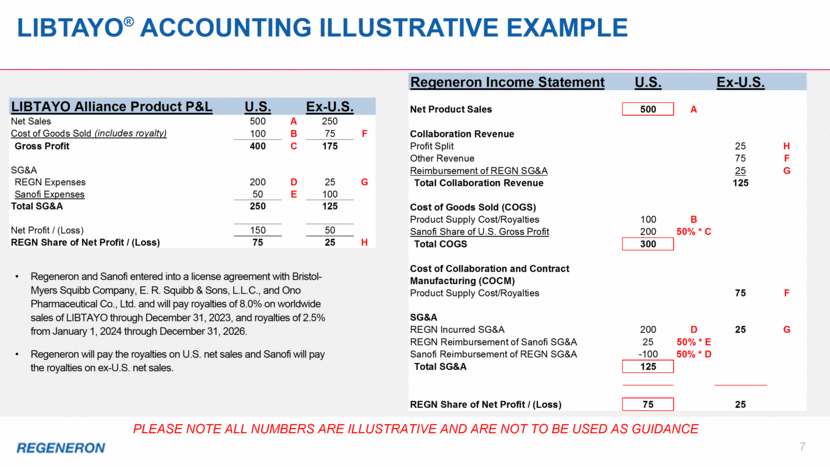
Cost of Goods sold (COGS) & Cost of Collaboration and Contract manufacturing (COCM) 8 Cost of Collaboration and Contract Manufacturing Cost of Goods Sold Costs related to products for which we record product sales (e.g., U.S. EYLEA®, ARCALYST®, U.S. LIBTAYO®): costs to manufacture commercial supplies royalties on products sold Starting in 4Q18, payment of Sanofi’s share of gross profit on U.S. sales of LIBTAYO Start-up costs and unabsorbed overhead costs in connection with our Limerick, Ireland manufacturing facility Costs related to product revenues recorded by our collaborators (e.g., Ex-U.S. EYLEA, DUPIXENT®, PRALUENT®, KEVZARA®, Ex-U.S. LIBTAYO): costs Regeneron incurs to manufacture Drug Product for products that are sold by our collaborators royalties Regeneron is contractually obligated to pay to third parties based on sales of product by our collaborators Costs associated with validation activities for collaborated products at our Limerick manufacturing facility Regeneron’s reimbursement of COCM by Sanofi and Bayer are recorded within the “Other Revenue” line item in the respective related collaboration revenue summary table in our MD&A
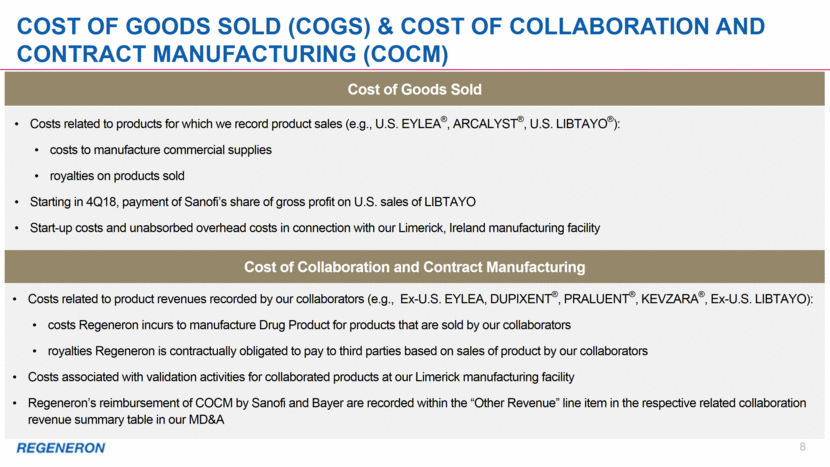
REGENERON/SANOFI RESTRUCTURE IO COLLABORATION 9 Key Terms of Restructured Agreement Sanofi will pay Regeneron $462 million – its funding obligation for the remainder of the term of the agreement. This payment includes Sanofi’s share of 4Q18 costs, up to $120 million in dedicated development funding for BCMAxCD3 and MUC16xCD3, and a termination payment. Regeneron will commit up to $70 million to further develop BCMAxCD3 and up to $50 million to further develop MUC16xCD3. Sanofi secures the right to opt-in to each program when the earlier of proof of concept is achieved or when the allocated funding is expended. BCMAxCD3 – Post opt-in, Sanofi will lead development and commercialization and fund 100% of development costs, with Regeneron reimbursing up to 50% out of its share of collaboration profits. Sanofi and Regeneron will share global profits equally. MUC16xCD3 – Post opt-in, Regeneron will lead development and lead commercialization in the U.S. The companies will share development costs and global profits equally. Sanofi will lead commercialization outside the U.S. The companies’ ongoing collaboration for the development and commercialization of LIBTAYO is unaffected by the amended IO Discovery and Development Agreement. Regeneron retains full rights to its other immuno-oncology programs that were previously in the immuno-oncology discovery program. Regeneron and Sanofi announce restructuring of the IO Collaboration for Discovery and Development Agreement Regeneron retains exclusive rights to its other immuno-oncology discovery and development programs The original 2015 IO Agreement was scheduled to end in approximately mid-2020 and this revision focuses ongoing development on two clinical-stage bispecific antibody programs (BCMAxCD3 and MUC16xCD3)
The Interactive Analyst Center is a repository of Regeneron’s historical reported financials and key operational data maintained by a third party This tool is meant to facilitate data gathering and to make researching Regeneron easier Access the Interactive Analyst Center via the Regeneron IR website: https://investor.regeneron.com/financial-information* Regeneron’s Interactive Analyst Center 10 Aflibercept is an investigational medicine for the indications listed here. The safety and efficacy of this drug candidate has not been fully evaluated by any regulatory authority for the disease categories indicated here. Available data includes GAAP financials, historic non-GAAP Measures, Reconciliation of non-GAAP Measures, Net Product Sales of Regeneron Discovered Products, Collaboration Revenues, and Other Revenues * The link and access to the Interactive Analyst Center are provided for convenience and should not be used as the sole basis of any analysis. Please refer to Regeneron’s reports filed with the U.S. Securities and Exchange Commission for further information.
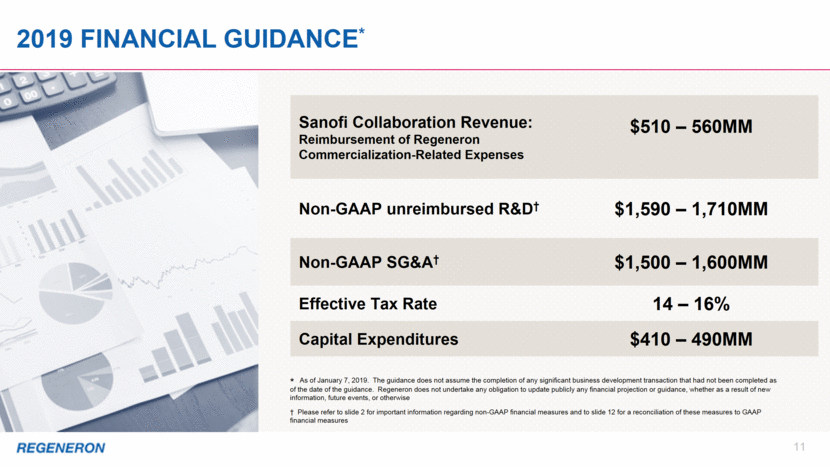
11 2019 financial Guidance* * As of January 7, 2019. The guidance does not assume the completion of any significant business development transaction that had not been completed as of the date of the guidance. Regeneron does not undertake any obligation to update publicly any financial projection or guidance, whether as a result of new information, future events, or otherwise Sanofi Collaboration Revenue: Reimbursement of Regeneron Commercialization-Related Expenses $510 – 560MM Non-GAAP unreimbursed R&D† $1,590 – 1,710MM Non-GAAP SG&A† $1,500 – 1,600MM Effective Tax Rate 14 – 16% Capital Expenditures $410 – 490MM † Please refer to slide 2 for important information regarding non-GAAP financial measures and to slide 12 for a reconciliation of these measures to GAAP financial measures
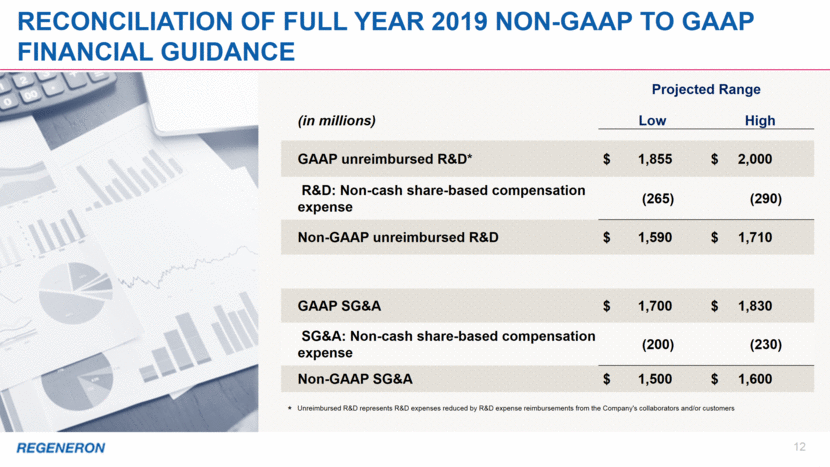
12 Reconciliation of full year 2019 Non-GAAP to GAAP Financial guidance * Unreimbursed R&D represents R&D expenses reduced by R&D expense reimbursements from the Company's collaborators and/or customers Projected Range (in millions) Low High GAAP unreimbursed R&D* $ 1,855 $ 2,000 R&D: Non-cash share-based compensation expense (265) (290) Non-GAAP unreimbursed R&D $ 1,590 $ 1,710 GAAP SG&A $ 1,700 $ 1,830 SG&A: Non-cash share-based compensation expense (200) (230) Non-GAAP SG&A $ 1,500 $ 1,600
13 Aflibercept is an investigational medicine for the indications listed here. The safety and efficacy of this drug candidate has not been fully evaluated by any regulatory authority for the disease categories indicated here. Q&A

