Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Impax Laboratories, LLC | exhibit991.htm |
| 8-K - 8-K - Impax Laboratories, LLC | ipxl-3x01x2018x8k.htm |

1
Fourth Quarter and Full Year
2017 Results
March 1, 2018

2
Impax Cautionary Statement Regarding Forward
Looking Statements
"Safe Harbor" statement under the Private Securities Litigation Reform Act of 1995:
To the extent any statements made in this news release contain information that is not historical, these statements are forward-looking in nature and express the beliefs and expectations of
management. Such statements are based on current expectations and involve a number of known and unknown risks and uncertainties that could cause the Company’s future results,
performance, or achievements to differ significantly from the results, performance, or achievements expressed or implied by such forward-looking statements. Such risks and uncertainties
include, but are not limited to, fluctuations in the Company’s operating results and financial condition, the volatility of the market price of the Company’s common stock, the Company’s ability to
successfully develop and commercialize pharmaceutical products in a timely manner, the impact of competition, the effect of any manufacturing or quality control problems, the Company’s
ability to manage its growth, risks related to acquisitions of or investments in technologies, products or businesses, risks relating to goodwill and intangibles, the reduction or loss of business
with any significant customer, the substantial portion of the Company’s total revenues derived from sales of a limited number of products, the impact of continuing consolidation of the
Company’s customer base, the Company’s ability to sustain profitability and positive cash flows, the impact of any valuation allowance on the Company’s deferred tax assets, the restrictions
imposed by the Company’s credit facility and indenture, the Company’s level of indebtedness and liabilities and the potential impact on cash flow available for operations, the availability of
additional funds in the future, any delays or unanticipated expenses in connection with the operation of the Company’s manufacturing facilities or at its third party suppliers, the effect of foreign
economic, political, legal and other risks on the Company’s operations abroad, the uncertainty of patent litigation and other legal proceedings, the increased government scrutiny on the
Company’s agreements to settle patent litigations, product development risks and the difficulty of predicting FDA filings and approvals, consumer acceptance and demand for new
pharmaceutical products, the impact of market perceptions of the Company and the safety and quality of its products, the Company’s determinations to discontinue the manufacture and
distribution of certain products, the Company’s ability to achieve returns on its investments in research and development activities, changes to FDA approval requirements, the Company’s
ability to successfully conduct clinical trials, the Company’s reliance on third parties to conduct clinical trials and testing, the Company’s lack of a license partner for commercialization of
Numient® (IPX066) outside of the United States and Taiwan, the impact of illegal distribution and sale by third parties of counterfeits or stolen products, the availability of raw materials and
impact of interruptions in the Company’s supply chain, the Company’s policies regarding returns, rebates, allowances and chargebacks, the use of controlled substances in the Company’s
products, the effect of global economic conditions on the Company’s industry, business, results of operations and financial condition, disruptions or failures in the Company’s information
technology systems and network infrastructure caused by cyber-attacks or other third party breaches or other events, the Company’s reliance on alliance and collaboration agreements, the
Company’s reliance on licenses to proprietary technologies, the Company’s dependence on certain employees, the Company’s ability to comply with legal and regulatory requirements
governing the healthcare industry, the regulatory environment, the effect of certain provisions in the Company’s government contracts, the Company’s ability to protect its intellectual property,
exposure to product liability claims, changes in tax regulations, uncertainties involved in the preparation of the Company’s financial statements, the Company’s ability to maintain an effective
system of internal control over financial reporting, the effect of terrorist attacks on the Company’s business, the location of the Company’s manufacturing and research and development
facilities near earthquake fault lines, expansion of social media platforms, risks related to the Company’s proposed business combination with Amneal Pharmaceuticals, Inc. (“Amneal”),
including whether the transactions (the “Combination”) contemplated by the Business Combination Agreement dated as of October 17, 2017 by and among us, Amneal, Atlas Holdings, Inc.,
and K2 Merger Sub Corporation as amended by Amendment No. 1, dated November 21, 2017 and Amendment No. 2 dated December 16, 2017 (the “Business Combination Agreement”) will
be completed on the terms or timeline contemplated, if at all, the risk that governmental entities could take actions under antitrust laws to enjoin the completion of the Combination, business
uncertainties and contractual restrictions while the Combination is pending, challenges related to the Company’s integration with Amneal after the closing, the fact that ownership interests will
not be adjusted if there is a change in value of the Company or Amneal, provisions in the Business Combination Agreement that may discourage other companies from acquiring the Company,
transaction related costs related to the Combination and integration, the lower ownership and voting interests that the Company’s stockholders will have in New Amneal after the closing, the
pending litigation related to the Combination and other risks described in the Company’s periodic reports filed with the Securities and Exchange Commission. Forward-looking statements
speak only as to the date on which they are made, and the Company undertakes no obligation to update publicly or revise any forward-looking statement, regardless of whether new
information becomes available, future developments occur or otherwise.
Trademarks referenced herein are the property of their respective owners.
©2018 Impax Laboratories, Inc. All Rights Reserved.

3
Important Information for Investors and
Shareholders
Additional Information and Where to Find It
This communication does not constitute an offer to sell or the solicitation of an offer to buy any securities or a solicitation of any vote or approval. This communication may be deemed to be
solicitation material in respect of the proposed transaction between Impax Laboratories, Inc. (“Impax”) and Amneal Pharmaceuticals LLC (“Amneal”) pursuant to the Business Combination
Agreement dated as of October 17, 2017 by and among Impax, Amneal, Atlas Holdings, Inc. (“Holdco”), and K2 Merger Sub Corporation, as amended by Amendment No. 1, dated November
21, 2017, and Amendment No. 2, dated December 16, 2017. In connection with the proposed transaction, Holdco filed a registration statement on Form S- 4, containing a proxy
statement/prospectus, with the Securities and Exchange Commission (“SEC”) on November 21, 2017, Amendment No. 1 to the registration statement filed on December 29, 2017, Amendment
No. 2 to the registration statement filed on January 23, 2018, Amendment No. 3 to the registration statement filed on February 1, 2018 and Amendment No. 4 to the registration statement filed
on February 6, 2018, which was declared effective by the SEC on February 9, 2018. Impax has filed a definitive proxy statement on Schedule 14A with the SEC on February 12, 2018, and the
definitive proxy statement and a form of proxy have been mailed to the shareholders of Impax on or about February 13, 2018. This communication is not a substitute for the registration
statement, definitive proxy statement/prospectus or any other documents that Impax or Holdco may file or have filed with the SEC, or will send or have sent to stockholders in connection with
the proposed business combination. INVESTORS AND SECURITY HOLDERS OF IMPAX ARE URGED TO READ ALL RELEVANT DOCUMENTS FILED WITH THE SEC, INCLUDING THE
PROXY STATEMENT/PROSPECTUS, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION.
Investors and security holders will be able to obtain copies of the registration statement, including the proxy statement/prospectus and other documents filed with the SEC (when available) free
of charge at the SEC’s website, http://www.sec.gov. Copies of the documents filed with the SEC by Impax or Holdco will be available free of charge on Impax’s internet website at
http://www.impaxlabs.com or by contacting Mark Donohue, Investor Relations and Corporate Communications at (215) 558-4526.
Participants in Solicitation
Impax, Amneal, Holdco and their respective directors and executive officers may be deemed to be participants in the solicitation of proxies from Impax’s stockholders in respect of the proposed
transaction. Information about the directors and executive officers of Impax is set forth in its proxy statement for its 2017 annual meeting of stockholders, which was filed with the SEC on April
5, 2017, and in its Annual Report on Form 10-K for the year ended December 31, 2016. Other information regarding the participants in the proxy solicitations and a description of their direct
and indirect interests, by security holdings or otherwise, are contained in the proxy statement/prospectus regarding the proposed transaction and other relevant materials that have been or will
be filed with the SEC when they become available. You may obtain free copies of these documents as described in the preceding paragraph. This communication is not intended to and shall
not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities or a solicitation of any vote of approval, nor shall there be any sale of
securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of
securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended.
3
Important Information for Investors and
Shareholders

4
Paul Bisaro
President & CEO

5
4Q and Full Year 2017 Financial Summary
$ millions, except EPS 4Q 2017 4Q 2016 FY 2017 FY 2016
Total Revenues, net $183 $198 $776 $824
Generic, net $113 $139 $549 $606
Specialty, net $70 $59 $227 $218
Adjusted Gross Margin 50% 47% 49% 50%
Adjusted EBITDA $33 $37 $150 $200
Adjusted EPS $0.11 $0.16 $0.63 $1.16
Refer to the GAAP to non-GAAP reconciliation tables in the appendix for a reconciliation of non-GAAP results.

6
2017 Initiatives Original Timing Updated Timing
Consolidation of all generic R&D to Hayward, CA Completed mid-2017 Completed on-time
Closure of Middlesex packaging site Completion by end of 1Q18 Completed end of 2017
Strategic alternatives for Taiwan manufacturing site As late as end of 2019 Completed sale Feb. 2018
Rationalizing generic portfolio to eliminate low-value products Completion by 1Q18 Completed 1Q18
Reorganizing certain functions including quality, engineering and supply chain operations As late as end of 2019 Completed 1Q18
Impax Consolidation and Improvement Plan
~$85M Run-Rate Savings Achieved
2017
~$10 million
Q2 2018
Full run-rate savings of ~$85M
Completed more than one year ahead of schedule

7
Strategic Combination for Long-Term Growth
(1) Per Last Twelve Months IMS Gross Revenues as of June 2017.
(2) Includes expected Year 1 run-rate synergies.
(3) In addition to the previously announced Impax standalone cost savings initiatives.
Expanded Portfolio to Drive Growth
Creates 5th largest U.S. generics company(1)
Increases scale and diversification across
currently marketed product families and R&D
pipeline
High-margin specialty franchise is expected to
provide stable cash flow and a long-term growth
platform
Significant Financial Benefits
Annual double-digit revenue, adjusted EBITDA
and adjusted EPS growth over next 3 years
driven by already filed new product launches
Significant projected cash flow generation
enables de-leveraging and future investment in
high-growth specialty and other adjacencies
Accretive to Impax’s adjusted EPS in the first 12
months after close(2)
$200 million in expected annual synergies within
3 years(3)
Announced October 17, 2017

8
Transaction Timeline
Pre-close integration planning well underway
Enhanced leadership team of combined company
Regulatory review process progressing as expected
Impax shareholder meeting March 27, 2018 to approve combination
Developing debt structure to maximize flexibility and maintain low cost
Combined company 2018 financial guidance after close
Currently on target for close 2Q 2018

9
35 new products launched
36 ANDAs approved; 9 tentatively approved
48 ANDAs filed
Launched traimcinolone injection (first generic)
Launched thiotepa 15mg and 100mg injection (only
100mg product available)
Launched gAggrenox capsules and mometasone
nasal spray
Filgrastim (NeupogenTM) biosimilar filing accepted by
the FDA
Amneal Impax
9 new products launched
7 ANDAs approved; 2 tentatively approved
5 ANDAs filed
Positive phase IIb study for IPX203
Favorable district court decision on Zomig® Nasal
Spray patent challenge
Settled Opana® ER litigation with Endo
Announced sale of Taiwan facility for $18.5MM
Accelerated $85MM cost improvement program
Product Data as of December 31, 2017.
2017 Achievements

10
6 generic products approved
5 generic products launched including:
gConcerta® 18 mg, 27 mg, 36 mg and 54 mg
gAccutane® 10 mg, 20 mg, 30 mg and 40 mg
gEntocort® EL 3 mg
gNamenda XR® 7 mg, 14 mg, 21 mg and 28 mg
gTamiflu oral suspension 6 mg/ml
Amneal Impax
Launched authorized generic Solodyn® 65 mg
and 115 mg
Two player generic market for six months
Partner Perrigo received approval for generic
Estrace® cream
Impax expected to launch in second quarter 2018
New Rytary® patent issued – expires late 2028
Product Data as of March 1, 2018.
Solid Start to 2018

11
Bryan Reasons
Chief Financial Officer
12
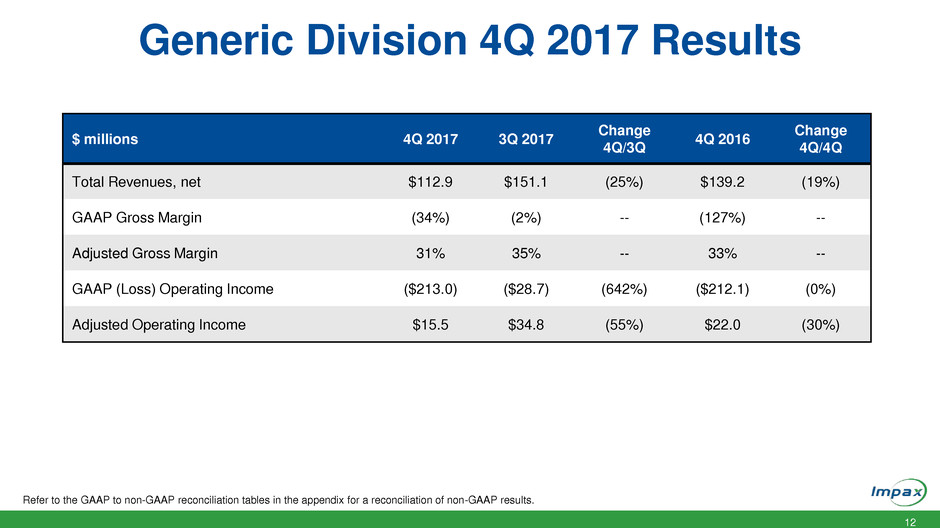
Generic Division 4Q 2017 Results
Refer to the GAAP to non-GAAP reconciliation tables in the appendix for a reconciliation of non-GAAP results.
$ millions 4Q 2017 3Q 2017
Change
4Q/3Q
4Q 2016
Change
4Q/4Q
Total Revenues, net $112.9 $151.1 (25%) $139.2 (19%)
GAAP Gross Margin (34%) (2%) -- (127%) --
Adjusted Gross Margin 31% 35% -- 33% --
GAAP (Loss) Operating Income ($213.0) ($28.7) (642%) ($212.1) (0%)
Adjusted Operating Income $15.5 $34.8 (55%) $22.0 (30%)

13
Specialty Pharma Division 4Q 2017 Results
Refer to the GAAP to non-GAAP reconciliation tables in the appendix for a reconciliation of non-GAAP results.
$ millions 4Q 2017 3Q 2017
Change
4Q/3Q
4Q 2016
Change
4Q/4Q
Total Revenues, net $70.0 $55.3 27% $59.2 18%
GAAP Gross Margin 72% 68% -- 26% --
Adjusted Gross Margin 80% 85% -- 79% --
GAAP Operating Income ($46.7) $16.4 (385%) ($17.4) (168%)
Adjusted Operating Income $36.1 $25.6 41% $26.3 37%

14
Selected 4Q 2017 Non-GAAP Adjustments
$ millions 4Q 2017 Related to:
Intangible Asset Impairment Charges $231
In-process R&D (gConcerta® and QVAR®) and
two marketed products acquired in 2016
Change in Fair Value of Contingent Consideration ($38)
Reduction in milestone liability related to
delayed launch of gConcerta
Fixed Asset Impairment Charges $80
Sale of Taiwan subsidiary/facility and
Middlesex, NJ facility
Restructuring and Severance Charges $13
Middlesex, NJ manufacturing, packaging and
R&D closure and Specialty Pharma division
reorg.
Refer to the GAAP to non-GAAP reconciliation tables in the appendix for a reconciliation of non-GAAP results.

15
Consolidated 4Q 2017 Results
Refer to the GAAP to non-GAAP reconciliation tables in the appendix for a reconciliation of non-GAAP results.
$ millions, except per share amounts 4Q 2017 3Q 2017
Change
4Q/3Q
4Q 2016
Change
4Q/4Q
EBITDA ($274.8) ($15.4) (1,684%) ($234.0) (17%)
Adjusted EBITDA $33.1 $45.6 (27%) $37.3 (11%)
GAAP Loss Per Share ($4.18) ($0.69) (506%) ($3.91) (7%)
Adjusted Diluted EPS $0.11 $0.23 (52%) $0.16 (31%)
GAAP Tax Rate 3% 6% -- (3%) --
Adjusted Tax Rate 49% 35% -- 23% --

16
Paul Bisaro
President & CEO

17
Position New
Amneal For
Sustainable
Long-Term
Growth
• Generics: Continuing internal R&D investment focused on all seven dosage forms
• Specialty: Continuing focus on Movement Disorders pipeline and opportunistically
in-license external opportunities
INVEST IN
ORGANIC
GROWTH
• Maintain high level of Quality and Compliance
• Continue to provide superior service levels
• Deliver differentiated products to our customers
MAINTAIN
CUSTOMER
FOCUS
• Strengthen Generic and Specialty franchises
and other adjacencies
PURSUE
CREATIVE
BUSINESS
DEVELOPMENT
Path Forward

18
Q&A

19
GAAP to Adjusted Results Reconciliation
The following table reconciles total Company reported cost of revenues to adjusted cost of revenues, adjusted gross profit, adjusted gross margin, adjusted
research and development expenses, and adjusted selling, general and administrative expenses.
Refer to the Fourth Quarter and Full Year 2017 Earnings Release for an explanation of adjusted items. The sum of the individual amounts may not equal due to rounding.
(Unaudited, In thousands) Three Months Ended Year Ended
Dec 31, 2017 Sept 30, 2017 Dec 31, 2016 2017 2016
Cost of revenues $126,480 $158,736 $129,047 $535,123 $486,899
Cost of revenues impairment charges $43,961 $13,623 $230,625 $96,865 $488,632
Adjusted to deduct:
Amortization $16,909 $17,015 $16,886 $68,375 $56,490
Intangible asset impairment charges $43,961 $13,623 $230,625 $96,865 $488,632
Business development - $55 - $112 -
Restructuring and severance charges $11,778 $13,836 $6,414 $44,136 $18,761
Inventory related charges $6,224 $20,478 - $26,702 -
Adjusted cost of revenues $91,569 $107,352 $105,747 $395,798 $411,648
Adjusted gross profit (a) $91,341 $99,040 $92,675 $379,989 $412,781
Adjusted gross margin (a) 49.9% 48.0% 46.7% 49.0% 50.1%
Research and development expenses $15,689 $15,821 $20,530 $80,847 $80,466
In-process research and development impairment charges $186,731 - $23,248 $192,809 $52,965
Adjusted to deduct:
Intangible asset impairment charges $186,731 - $23,248 $192,809 $52,965
Restructuring and severance charges $98 $356 - $3,378 -
Other - $60 $600 $2,534 $1,522
Adjusted research and development expenses $15,591 $15,405 $19,930 $74,935 $78,944
Selling, general and administrative expenses $64,016 $53,585 $57,586 $216,270 $201,830
Adjusted to deduct:
Business development expenses $8,061 $2,833 $251 $10,985 $4,540
Turing legal expenses $642 $214 $2,111 $451 $7,554
Restructuring and severance charges $3,676 $511 $5,291 $4,458 $5,455
Adjusted selling, general and administrative expenses $51,637 $50,027 $49,933 $200,376 $184,281

20
GAAP to Adjusted Net Income Reconciliation
The following table reconciles reported net loss to adjusted net income.
Refer to the Fourth Quarter and Full Year 2017 Earnings Release for an explanation of adjusted items. The sum of the individual amounts may not equal due to rounding.
Three Months Ended Year Ended
(Unaudited, In thousands, except per share and per share data) Dec 31, 2017 Sept 30, 2017 Dec 31, 2016 2017 2016
Net loss ($301,070) ($49,369) ($279,585) ($469,287) ($472,031)
Adjusted to add (deduct):
Amortization $16,909 $17,015 $16,886 $68,375 $56,490
Non-cash interest expense $6,660 $6,547 $6,241 $25,950 $22,846
Business development expenses $8,061 $2,888 $251 $11,097 $4,540
Intangible assets impairment charges $230,692 $13,623 $253,873 $289,674 $541,597
Fixed asset impairment charges $79,705 828 - $82,508 -
Reserve for Turing receivable $1,328 - ($7,731) $3,999 $40,312
Turing legal expenses $642 $214 $2,111 $451 $7,554
Restructuring and severance charges $13,483 $14,443 $11,705 $49,563 $24,040
Gain on sale of assets ($656) (4,379) - ($17,236) -
Loss on extinguishment of debt - - - $1,215 -
Inventory related charges $6,224 $20,478 - $26,702 -
Change in fair value of contingent consideration ($38,123) $6,333 - ($31,048) -
Legal settlements - - - $7,900 -
Other - $60 $2,762 $2,534 $3,684
Income tax effect ($16,213) ($11,998) $5,136 ($7,205) ($145,368)
Adjusted net income $7,642 $16,683 $11,649 $45,192 $83,664
Adjusted net income per diluted share $0.11 $0.23 $0.16 $0.63 $1.16
Net loss per diluted share ($4.18) ($0.69) ($3.91) ($6.53) ($6.63)
Diluted weighted-average common shares outstanding $72,635 $72,172 $71,489 $71,857 $71,830

21
GAAP to Adjusted EBITDA Reconciliation
Refer to the Fourth Quarter and Full Year 2017 Earnings Release for an explanation of adjusted items. The sum of the individual amounts may not equal due to rounding.
The following table reconciles reported net loss to adjusted EBITDA.
Three Months Ended Year Ended
(Unaudited, In thousands) Dec 31, 2017 Sept 30, 2017 Dec 31, 2016 2017 2016
Net loss ($301,070) ($49,369) ($279,585) ($469,287) ($472,031)
Adjusted to add (deduct):
Interest expense, net $13,672 $13,636 $13,440 $53,412 $40,419
Interest Income - ($336) - - -
Income taxes ($9,010) ($3,045) $8,572 $18,326 ($104,294)
Depreciation and amortization $21,570 $23,708 $23,573 $93,731 $82,879
EBITDA ($274,838) ($15,406) ($234,000) ($303,818) ($453,027)
Adjusted to add (deduct):
Share-based compensation expense $6,586 $6,490 $8,334 $26,258 $31,709
Business development expenses $8,061 $2,888 $251 $11,097 $4,540
Intangible asset impairment charges $230,692 $13,623 $253,873 $289,674 $541,597
Fixed assets impairment charges $79,705 828 - $82,508 -
Reserve for Turing receivable $1,328 - ($7,731) $3,999 $40,312
Turing legal expenses $642 $214 $2,111 $451 $7,554
Restructuring and severance charges $13,483 $14,443 $11,705 $49,563 $24,040
Gain on sale of assets ($656) ($4,379) - ($17,236) -
Loss on extinguishment of debt - - - $1,215 -
Inventory related charges $6,224 $20,478 - $26,702 -
Change in fair value of contingent consideration ($38,123) $6,333 - ($31,048) -
Legal settlements - - - $7,900 -
Other - $60 $2,762 $2,534 $3,684
Adjusted EBITDA $33,104 $45,572 $37,305 $149,799 $200,409

22
Generic Division GAAP to Adjusted Results
Reconciliation
The following tables reconcile the Impax Generics Division reported cost of revenues and loss from operations to adjusted cost of revenues, adjusted gross profit,
adjusted gross margin and adjusted operating income.
Refer to the Fourth Quarter and Full Year 2017 Earnings Release for an explanation of adjusted items. The sum of the individual amounts may not equal due to rounding.
(a) Adjusted gross profit is calculated as total revenues less adjusted cost of revenues. Adjusted gross margin is calculated as adjusted gross profit divided by total revenues.
Three Months Ended
(Unaudited, In thousands) Dec 31, 2017 Sept 30, 2017 Dec 31, 2016
Cost of revenues $106,801 $141,133 $109,380
Cost of revenues impairment charges $43,961 $13,623 $206,312
Adjusted to deduct:
Amortization $13,075 $13,181 $9,470
Intangible asset impairment charges $43,961 $13,623 $206,312
Restructuring and severance charges $9,960 $8,579 $6,414
Inventory related charges $6,224 $20,478 -
Adjusted cost of revenues $77,542 $98,895 $93,496
Adjusted gross profit (a) $35,401 $52,203 $45,730
Adjusted gross margin (a) 31.3% 34.5% 32.8%
GAAP Loss from operations ($212,951) ($28,658) ($212,088)
Adjusted to add (deduct):
Amortization $13,075 $13,181 $9,470
Intangible asset impairment charges $230,692 $13,623 $217,587
Restructuring and severance $10,058 $8,935 $6,414
Inventory related charges $6,224 $20,478 -
Fixed asset impairment charges $6,486 828 -
Change in fair value of contingent consideration ($38,123) 6,333 -
Other - 60 $600
Adjusted Income from operations $15,461 $34,780 $21,983

23
Specialty Pharma Division GAAP to Adjusted
Results Reconciliation
The following tables reconcile the Impax Specialty Pharma Division reported cost of revenues and (loss) income from operations to adjusted cost of revenues,
adjusted gross profit, adjusted gross margin and adjusted income from operations.
Refer to the Fourth Quarter and Full Year 2017 Earnings Release for an explanation of adjusted items. The sum of the individual amounts may not equal due to rounding.
(a) Adjusted gross profit is calculated as total revenues less adjusted cost of revenues. Adjusted gross margin is calculated as adjusted gross profit divided by total revenues.
Three Months Ended
(Unaudited, In thousands) Dec 31, 2017 Sept 30, 2017 Dec 31, 2016
Cost of revenues $19,679 $17,603 $19,667
Cost of revenues impairment charges - - $24,313
Adjusted to deduct:
Amortization $3,834 $3,834 $7,416
Restructuring and severance charges $1,818 $5,257 -
Intangible asset impairment charges - - $24,313
Adjusted cost of revenues $14,027 $8,512 $12,251
Adjusted gross profit (a) $55,940 $46,782 $46,945
Adjusted gross margin (a) 80.0% 84.6% 79.3%
GAAP (Loss) income from operations ($46,698) $16,364 ($17,437)
Adjusted to add:
Amortization $3,834 $3,834 $7,416
Intangible asset impairment charges - - $36,286
Restructuring and severance $4,825 $5,367 -
Fixed asset impairment charges $74,128 - -
Adjusted Income from operations $36,089 $25,565 $26,265
