Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - IMMUNE PHARMACEUTICALS INC | tv485659_8k.htm |
Exhibit 99.1

www.immunepharma.com NASDAQ: IMNP BIO CEO and Investor Conference Elliot Maza – Chief Executive Officer February 12, 2018

Forward Looking Statements This presentation and oral statements made by representatives of the Company may contain projections or other forward - looking statements regarding future events or the future financial performance of the Company. Actual events or results may differ materially from those in the projections or other forward - looking statements. There can be no assurance that the Company will ever successfully complete its anticipated corporate restructuring, including the proposed spin - off of Cytovia Inc., or that the Company will be able to reduce expenses, capitalize on strategic alternatives, develop its assets, and generate value for shareholders. The Company may, at any time and for any reason until the proposed spin - off is complete, abandon the spin - off or modify its terms and conditions, or consider competing, alternate or complimentary transactions or offers by third parties at the discretion of Immune’s board of directors. Please see Immune’s filings with the Securities and Exchange Commission for a discussion of important risk factors that could cause actual events or results to differ materially from those in the projections or other forward - looking statements. 2

Overview • Two ongoing phase 2 studies - bullous pemphigoid and ulcerative colitis - Excellent safety and tolerability profile in over 100 subjects exposed - Multiple additional indications include atopic dermatitis, asthma and others • Potential to enter pivotal registration study in 2019 3 Developing Novel Therapeutics for Immunologic and Inflammatory Diseases Bertilimumab (anti - eotaxin mAb ) NanoCyclo • Topical formulation of cyclosporine, in late - stage preclinical development - Proprietary nanoencapsulation technology enhances penetration into the skin - Expected to enter clinic in 2018 New management team and corporate restructuring position Company for tremendous upside

Robust Pipeline Addresses Significant Unmet Needs 4 Program Indication Preclinical Phase 1 Phase 2 Phase 3 Bertilimumab Bullous Pemphigoid (BP) Ulcerative Colitis (UC) Allergic Rhinitis (AR) Allergic Conjunctivitis (AC) Atopic Dermatitis (AD) Other Inflammatory Conditions NanoCyclo Atopic Dermatitis (AD) Psoriasis Ongoing Ongoing Ongoing Ongoing Completed Completed Phase 2 Ready Phase 2 Ready
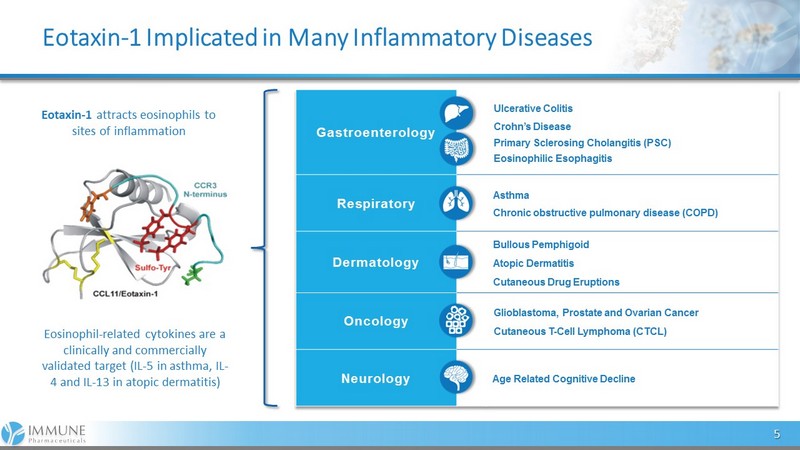
Eotaxin - 1 Implicated in Many Inflammatory Diseases 5 Gastroenterology Ulcerative Colitis Crohn’s Disease Primary Sclerosing Cholangitis (PSC) Eosinophilic Esophagitis Re s pira tory Asthma Chronic obstructive pulmonary disease (COPD) De rmatology Bullous Pemphigoid Atopic Dermatitis Cutaneous Drug Eruptions Oncology Glioblastoma, Prostate and Ovarian Cancer Cutaneous T - Cell Lymphoma (CTCL) Ne urol ogy Age Related Cognitive Decline Eotaxin - 1 attracts eosinophils to sites of inflammation Eosinophil - related cytokines are a clinically and commercially validated target (IL - 5 in asthma, IL - 4 and IL - 13 in atopic dermatitis)

Bertilimumab Blocks Eotaxin - 1 • Bertilimumab is a human antibody with picomolar affinity and high specificity for human eotaxin - 1 • Prevents eotaxin - 1 - induced chemotaxis and shape change of human eosinophils • Pharmacokinetic profile consistent with biweekly dosing • Clean safety profile in over 100 subjects treated to date - >60 received IV - 46 received ocular - 8 received intranasal - Well - tolerated by all routes of administration - Only one drug - related SAE, an infusion reaction that was self - limited 6 0.0 0.1 1.0 10.0 100.0 1000.0 0 100 200 300 400 500 600 700 800 900 Blood Bertilimumab Concentration (µg/mL) Hours Bertilimumab Concentration vs. Time 0.1 mg/kg 1 mg/kg 5 mg/kg 10 mg/kg
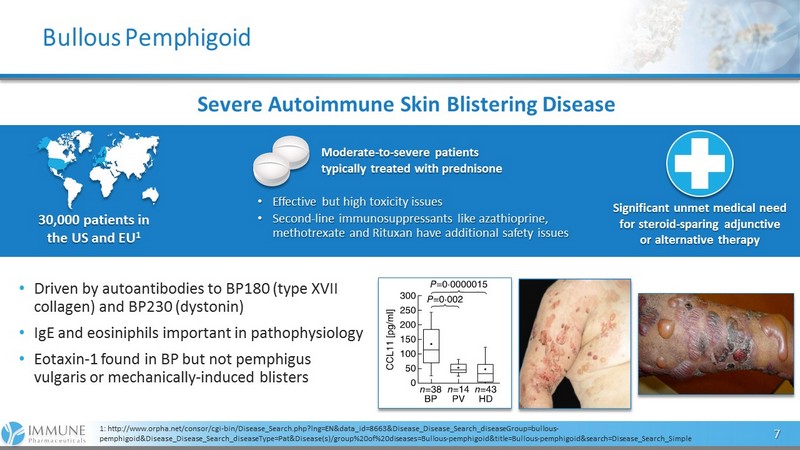
Bullous Pemphigoid 7 1: http://www.orpha.net/consor/cgi - bin/Disease_Search.php?lng=EN&data_id=8663&Disease_Disease_Search_diseaseGroup=bullous - pemphigoid&Disease_Disease_Search_diseaseType=Pat&Disease(s)/group%20of%20diseases=Bullous - pemphigoid&title=Bullous - pemphigoid&s earch=Disease_Search_Simple Severe Autoimmune Skin Blistering Disease 30,000 patients in the US and EU 1 Moderate - to - severe patients typically treated with prednisone Significant unmet medical need for steroid - sparing adjunctive or alternative therapy • Effective but high toxicity issues • Second - line immunosuppressants like azathioprine, methotrexate and Rituxan have additional safety issues • Driven by autoantibodies to BP180 (type XVII collagen) and BP230 ( dystonin ) • IgE and eosiniphils important in pathophysiology • Eotaxin - 1 found in BP but not pemphigus vulgaris or mechanically - induced blisters

Phase 2 Study in Bullous Pemphigoid - Positive Interim Data from First 6 Subjects 8 Expect to Complete Enrollment and Report Additional BP Data in Q2 2018 Single - arm, open - label phase 2 study ongoing 10 - 15 subjects with moderate - to - extensive disease 3 IV doses Every 2 weeks 84 day follow - up Additional Efficacy Endpoints: • BP Disease Area Index (BPDAI) • Pruritic Visual Analogue Scale (VAS) • % Responders • Quality of Life (QOL) Primary Endpoint: • Safety Enrolling at 6 sites in the US and 2 in Israel

Phase 2 Interim Results Demonstrated Rapid and Sustained Improvement in BPDAI Scores 9 0 10 20 30 40 50 60 70 0 14 28 42 56 70 84 BPDAI Total Activity Score Study Day BPDAI Total Activity Score First six subjects of a targeted 10 - 15; mean age 76 years 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0 14 28 42 56 70 84 Prednisone dose (mg/kg) Study Day Prednisone dose Standard Prednisone Done Mean starting dose of 26 mg Expected prednisone dose (based on Joly et al , 2002) Subjects received on average 2,555 mg less prednisone Tapered to 9 mg by study end

Positive Interim Phase 2 Could Lead to Pivotal Trial • 85% reduction in BPDAI Total Activity Index (p=0.0096) - All subjects achieved a >50% improvement - 4/6 with >90% improvement • Mean initial prednisone dose of 26 mg (0.34 mg/kg) tapered to 9 mg (0.13 mg/kg) (p=0.0145) - Standard regimen would have begun at ~60 mg and been at ~30 mg by day 84 - Subjects spared over 2,500 mg prednisone over 84 days - These patients should have fared poorly on the protocol - mandated prednisone dose • Excellent safety and tolerability of 3 bertilimumab doses • Potential to move directly into a pivotal phase 2/3 trial - Design protocol for controlled trial versus high - dose prednisone - Will explore steroid reduction endpoint - Get FDA feedback from an end - of - phase 2 meeting in mid - 2018 - Expect to launch pivotal study in 2019 10 Results to be Presented as Late - Breaker at AAD Meeting in February

Ulcerative Colitis 11 1: https://www.crohnsandcolitis.com/ulcerative - colitis Chronic, Inflammatory Bowel Disease Moderate - to - severe disease managed with TNF - blockers or other immunosuppressants • Many patients do not have adequate disease control or experience toxicities Control mAb Anti - eotaxin - 1 Eotaxin - 1 blockade effective in animal models of inflammatory bowel disease Eotaxin - 1 strongly implicated as a target in IBD • Tissue eotaxin - 1 levels correlated with Mayo Clinic DAI, mucosal injury and histologic severity • Greater eotaxin - 1 levels in areas of active vs. inactive disease ~ 700,000 patients in the US 1

Bertilimumab Proof of Concept Trial in Ulcerative Colitis 12 Randomized, double - blind, placebo - controlled trial 42 Subjects 2:1 randomization Patients selected based on Mayo UC Score and tissue eotaxin - 1 levels Every 2 weeks 90 day follow - up 3 IV doses Additional Efficacy Endpoints: • Mucosal injury • Fecal calprotectin (validated inflammatory marker) • Tissue eotaxin - 1 and eosinophil levels • Clinical remission Primary Endpoint: • Clinical response (UC Mayo Clinic Index) at Day 56 Expect to Complete Enrollment in Q3 2018 Enrolling at 5 sites in Israel and 4 in Russia
Additional Bertilimumab Development Plans • Several opportunities being vetted for next clinical indication - Asthma – lesson learned from IL - 5 inhibitors, which are only active in asthma patients with high eosinophil counts - Severe atopic dermatitis – developing subcutaneous formulation - Several orphan eosinophilic diseases with unmet medical needs • Manufacturing - New CHO cell line and new process more efficient and highly scalable - Will transfer process to a GMP facility for scale - up and validation starting Q2/18 - Extensive comparability testing already completed – may not need human bridging PK • Intellectual Property/Market Exclusivity - Current IP portfolio has several patents expiring in 2021 - 2022 that will be eligible for Patent Term Restoration of up to 5 years - Pursuing Orphan exclusivity for BP - 12 years of biologics exclusivity in the US and 10 years in the EU - Pursuing substantial new IP around new process bertilimumab 13

NanoCyclo - Topical Cyclosporine • Alternatives to topical corticosteroids remain in demand • Topical Calcineurin Inhibitors (TCIs) addressed this gap - Topical formulations of systemic immunosuppressants - Protopic® and Elidel® peaked at over $500 mm in WW sales - However, black - box for cancer risk impaired US marketing - Now both are off - patent, leaving a marketing void • Cyclosporine has poor skin penetration • NanoCyclo technology enhances penetration of cyclosporine by formulating it in polymer - coated nanoparticles • Extensive IP portfolio licensed from Yissum and BNS 14

NanoCyclo - Topical Cyclosporine • Down - regulates pro - inflammatory cytokines in the skin • Efficacy comparable to topical corticosteroids and TCIs in a human skin model of atopic dermatitis • Produces clinical improvement in mouse models • Intend to pursue 505(b)(2) pathway for atopic dermatitis and psoriasis • We believe black box may be avoided if there is no systemic absorption • Completing in vivo and in vitro studies in Q1 2018 to select formulation • GMP manufacturing expected to commence Q1 2018 • Expect to complete clinic - enabling toxicity study Q2 2018 and move into proof - of - concept clinic trial in H2 2018 15

Financial Snapshot – NASDAQ: IMNP 16 ~30M ~$17M 1.25M 1: As of February 7, 2018; closing price of $.55 per share Common Shares Outstanding Avg. Volume (3 month) 1 Market Cap. 1 Recent Financing of $ 18 Million Eliminates Debt and Strengthens Balance Sheet

Management Team 17 Elliot Maza, JD, CPA - President and CEO Extensive and successful experience in managing micro - cap biotech and drug manufacturing companies Tony Fiorino, MD, PhD - CMO/COO Broad experience in leading clinical - stage biotech companies and a former fund manager well - versed in finance John Zhang, MD, PhD - VP, R&D 20 years’ experience in preclinical/early clinical drug development in pharmaceutical/biotech industry
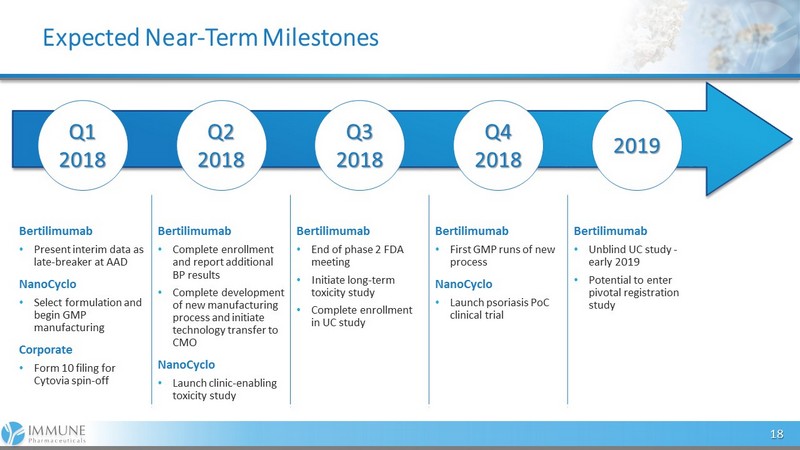
Bertilimumab • Present interim data as late - breaker at AAD NanoCyclo • Select formulation and begin GMP manufacturing Corporate • Form 10 filing for Cytovia spin - off Bertilimumab • Complete enrollment and report additional BP results • Complete development of new manufacturing process and initiate technology transfer to CMO NanoCyclo • Launch clinic - enabling toxicity study Bertilimumab • End of phase 2 FDA meeting • Initiate long - term toxicity study • Complete enrollment in UC study Bertilimumab • First GMP runs of new process NanoCyclo • Launch psoriasis PoC clinical trial Bertilimumab • Unblind UC study - early 2019 • Potential to enter pivotal registration study Expected Near - Term Milestones 18 Q2 2018 Q1 2018 Q3 2018 Q4 2018 2019

Investment Highlights • Lead program, bertilimumab, in two ongoing phase 2 studies with data expected in 2018 - Potential to enter pivotal registration study in 2019 • Potential to expand bertilimumab into multiple high value indications, including atopic dermatitis and asthma • NanoCyclo, polymer - coated nanoparticle formulation of cyclosporine, expected to enter a human PoC study in H2 2018 • Expect to spin - off non - core oncology assets to focus on execution of bertilimumab and NanoCyclo • New management team and corporate restructuring position Company for tremendous upside 19

www.immunepharma.com NASDAQ: IMNP Thank You!
