Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - PALISADE BIO, INC. | f8k_012418.htm |
Exhibit 99.01

neuralstem.com Treatment of ALS with Neural Stem Cells Cell & Gene Therapy World 1 Karl Johe, Ph.D., CSO & Founder Neuralstem, Inc. January 24, 2018

neuralstem.com Safe Harbor Statement 2 Safe Harbor statements under the Private Securities Litigation Reform Act of 1995 : This presentation contains forward - looking statements as defined in Section 27 A of the Securities Act of 1933 as amended, and section 21 E of the Securities Exchange Act of 1934 , as amended . Such forward - looking statements are based upon Neuralstem, Inc . ’s management’s current expectations, estimates, beliefs, assumptions, and projections about Neuralstem’s business and industry . Words such as “anticipates,” “expects,” “intends,” “plans,” “predicts,” “believes,” “seeks,” “estimates,” “may,” “will,” “should,” “would,” “potential,” “continue,” and variations of these words (or negatives of these words) or similar expressions, are intended to identify forward - looking statements . In addition, any statements that refer to expectations, projections, or other characterizations of future events or circumstances, including any underlying assumptions, are forward - looking statements . These forward - looking statements are not guarantees of future performance and are subject to certain risks, uncertainties, and assumptions that are difficult to predict . Therefore, our actual results could differ materially and adversely from those expressed in any forward - looking statements as a result of various risk factors . These risks and uncertainties include the risks associated with the effect of changing economic conditions, trends in the products markets, variations in Neuralstem’s cash flow, market acceptance risks, technical development risks and other risk factors detailed in Neuralstem’s Securities and Exchange Commission filings . For links to SEC documents please visit the company’s Web site : neuralstem . com . Although we believe that we have a reasonable basis for each forward - looking statement contained in this presentation, we caution you that forward - looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward - looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10 - K for the year ended December 31 , 201 6 filed with the Securities and Exchange Commission on March 14 , 2017 , Form 10 - Q for the period ended September 30 , 2017 , an in other reports filed with the SEC . In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward - looking statements contained in this presentation, they may not be predictive of results or developments in future periods . Any forward - looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law . 2

neuralstem.com 6 Manufacturing at commercially viable scale while maintaining cell properties cGMP , 3 - tiered cell banks, fully tested & validated: • MCB (~250 vials, p6), WCB (~350 vials, p9), CCB (~400 vials, p12) — 2x10 7 cells/vial • Normal karyotype – 44 + XY 1. Large - scale, reproducible manufacturing of hNSC 3

neuralstem.com 2. MOA: Neuroprotection of host motor neurons 1. Xu L, et al. Neurosci Lett. 2011; 494(3): 222 - 6. 2. Xu L, et al. J Comp Neurol. 2009; 514(4):297 - 309. 3. Yan J, et al. PLoS Med. 2007 4(2): e39. 4. Xu L, et al. Transplantation. 2006; 82(7):865 - 75. 5. Yan J, et al. Stem Cells. 2006; 24(8):1976 - 85. 6. Hefferan MP, et al. PLoS One. 2012;7(8):e42614. 7. Hefferan MP, et al. Cell Transplant. 2011;20(8):1153 - 61. • Koliatsos et al., Johns Hopkins • Marsala et al., UCSD 4

neuralstem.com 3. Development of Cell Injection Device and Cannula: Precise, reproducible, accurate injections 5 28d DCX 7 wks 1. Gutierrez J, et al. Neurosurgery. 2015 Oct;77(4):604 - 12; discussion 612. 2. Federici T, et al. J Vis Exp. 2012 Dec 7;(70):e4371. 3. Riley JP, et al. Neurosurgery. 2011 Dec;69(2 Suppl Operative):ons147 - 54; discussion ons155. 4. Raore B, et al. Spine ( Phila Pa 1976). 2011;36(3):E164 - 71. 5. Dolezalova D, et al. J Comp Neurol. 2014; 522(12):2784 - 801. 6. Usvald D, et al. Cell Transplant. 2010;19(9):1103 - 22. • Boulis et al, Emory • Marsala et al., UCSD

neuralstem.com Cell Viability (%) Time after preparation ( hrs ) 0 24 48 72 Viability of Drug Product Temp ( o C ) Time after shipping ( hrs ) 0 4 6 8 10 12 14 16 18 2 Shipping Temperature of Drug Product 2 - 8 o C 4. Commercially Viable Formulation and Distribution of Drug Product: Live cell suspension, ready - to - inject, 48 - hr stability 6

neuralstem.com 5. NSI - 566: ALS Phase I : Team work! 7 A Phase I, Open - label, First - in - human, Feasibility and Safety Study of Human Spinal Cord derived Neural Stem Cell Transplantation for the Treatment of ALS Eva L. Feldman 1 , Jonathan D. Glass 2 , Nicholas M. Boulis 2 , Thais Federici 2 , Meraida Polak 2 , C. Kelly 2 and Karl Johe 3 1 University of Michigan, Ann Arbor, MI 2 Emory University, 3 Neuralstem, Inc

neuralstem.com 6. Phase I Clinical Design: Progressive Risks and Benefits (n=18) 8 A1: ½ million cells A2: 1 million cells L2 L3 L4 C3 C4 C5 L2 L3 L4 1 million cells ½ million cells ½ million cells 1.5 million cells B C D E C3 C4 C5 L2 L3 L4 L2 L3 L4 Compliments of Jon Glass MD A1/A2
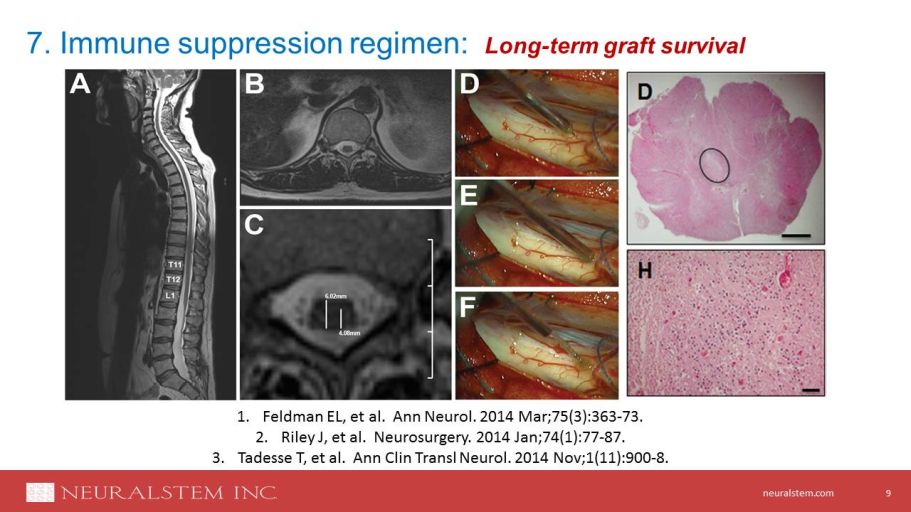
neuralstem.com 9 1. Feldman EL, et al. Ann Neurol. 2014 Mar;75(3):363 - 73. 2. Riley J, et al. Neurosurgery. 2014 Jan;74(1):77 - 87. 3. Tadesse T, et al. Ann Clin Transl Neurol. 2014 Nov;1(11):900 - 8. 7. Immune suppression regimen: Long - term graft survival

neuralstem.com NSI - 566: Long - term graft survival with transient immunosuppression 10 Tadesse T, et al. Ann Clin Transl Neurol. 2014 Nov;1(11):900 - 8. Patient number Gender # of days on FK506 # of days on MMF # of Days IM Meds Discontinued before death Survival Days % Donor DNA 1 M 177 165 216 394 0.06 – 5.40 2 M 107 503 67 572 0.18 – 0.93 3 M 259 259 0 259 0.03 – 2.39 4 M 189 192 133 325 0.07 – 4.20 5 M 94 283 638 921 0.14 – 0.67 6 F 139 134 57 196 0.06 - 0.96 FK506 = tacrolimus, MMF=mycophenolate mofetil, IM=immunomodulatory

neuralstem.com 11 NSI - 566: Long - term graft survival with transient immunosuppression

neuralstem.com NSI - 566 Phase I 12 Feldman EL, et al. Ann Neurol. 2014 Mar;75(3):363 - 73. Clear treatment - emergent improvement of function

neuralstem.com NSI - 566: ALS Phase II 13 A Phase II, Open - label, Dose Escalation and Safety Study of Human Spinal Cord derived Neural Stem Cell Transplantation for the Treatment of Amyotrophic Lateral Sclerosis Study Sites: University of Michigan, Emory University, Mass General Hospital • N=15 of variable disease profiles • Up to 16 million cells injected, intraspinal , C3 - C5/L2 - L5
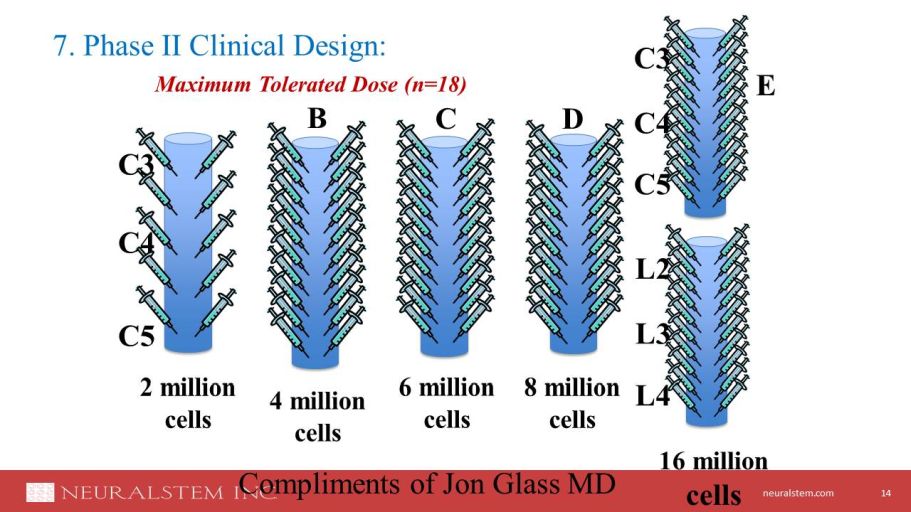
neuralstem.com 7. Phase II Clinical Design: Maximum Tolerated Dose (n=18) 14 2 million cells 4 million cells B 6 million cells C 8 million cells D C3 C4 C5 C3 C4 C5 16 million cells E L2 L3 L4 Compliments of Jon Glass MD
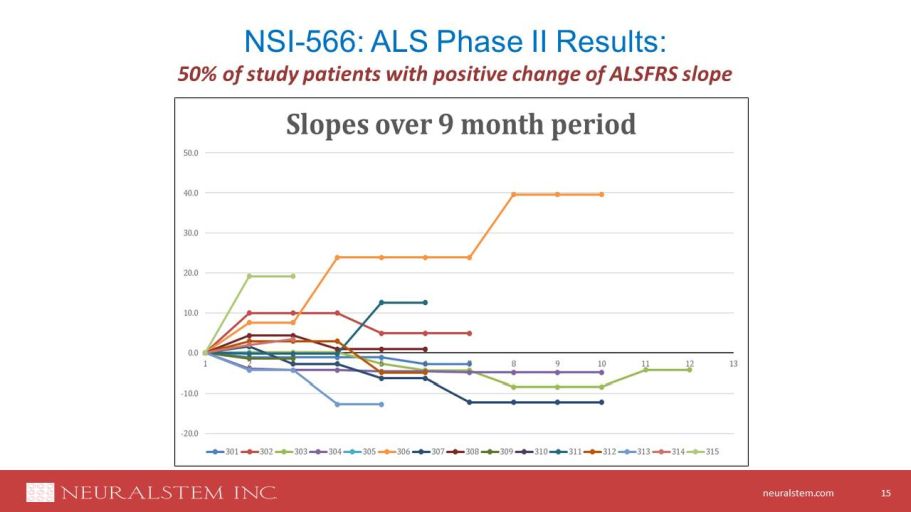
neuralstem.com NSI - 566: ALS Phase II Results: 15 50% of study patients with positive change of ALSFRS slope
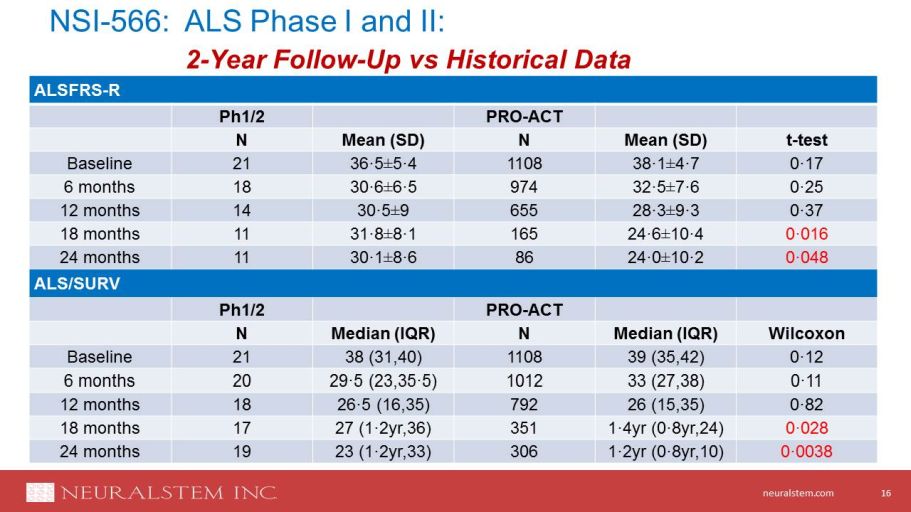
neuralstem.com NSI - 566 : ALS Ph ase I and II: 2 - Year Follow - Up vs Historical Data 10 16 ALSFRS - R Ph1/2 PRO - ACT N Mean (SD) N Mean (SD) t - test Baseline 21 36·5 ± 5·4 1108 38·1 ± 4·7 0·17 6 months 18 30·6 ± 6·5 974 32·5 ± 7·6 0·25 12 months 14 30·5 ± 9 655 28·3 ± 9·3 0·37 18 months 11 31·8 ± 8·1 165 24·6 ± 10·4 0·016 24 months 11 30·1 ± 8·6 86 24·0 ± 10·2 0·048 ALS/SURV Ph1/2 PRO - ACT N Median (IQR) N Median (IQR) Wilcoxon Baseline 21 38 (31,40) 1108 39 (35,42) 0·12 6 months 20 29·5 (23,35·5) 1012 33 (27,38) 0·11 12 months 18 26·5 (16,35) 792 26 (15,35) 0·82 18 months 17 27 (1·2yr,36) 351 1·4yr (0·8yr,24) 0·028 24 months 19 23 (1·2yr,33) 306 1·2yr (0·8yr,10) 0·0038

neuralstem.com 8. NSI - 566 Target Product Profile (TPP) for ALS 17 Mode of Action ▪ Human neural stem cells, as an allogeneic transplant, rescue motor neurons by: ▪ Expressing excitatory amino acid transporters to restore glutamate re - uptake ▪ Releasing neurotrophic growth factors that slow motor neuron degeneration Target Indication ▪ Sporadic or familial ALS with possible, laboratory supported probable, probable, or definite ALS by El Escorial Criteria Revised Dosage Form and Administration ▪ 2 spinal surgeries (laminectomy or laminoplasty) of 2 to 3 vertebral segments per procedure, one lumbar & one cervical ▪ Live cell suspension by intraspinal injections ▪ Repeat treatments at 2 - 5 years Clinical Benefits Primary Efficacy ▪ One year after treatment, slow disease progression by 5 points ALSFRS - R (most common rating scale used in ALS) in early stage patients compared to controls receiving SOC ▪ Increase life span by 5+ years Secondary Benefit ▪ Increased functionality (grip strength, Vital Capacity) and quality of life (patient reported outcomes) for 2 - 5 years Side Effects / Adverse Events ▪ Side effects associated with the procedure (e.g., incisional pain) or with the immunosuppressive therapy that is given for 1 - 3 months post surgery Contraindicated ▪ End stage patients who cannot be on anesthesia NSI - 566 Target Product Profile
