Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - PALISADE BIO, INC. | exh_322.htm |
| EX-32.1 - EXHIBIT 32.1 - PALISADE BIO, INC. | exh_321.htm |
| EX-31.2 - EXHIBIT 31.2 - PALISADE BIO, INC. | exh_312.htm |
| EX-31.1 - EXHIBIT 31.1 - PALISADE BIO, INC. | exh_311.htm |
| EX-23.01 - EXHIBIT 23.01 - PALISADE BIO, INC. | exh_2301.htm |
| EX-21.01 - EXHIBIT 21.01 - PALISADE BIO, INC. | exh_2101.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2020.
or
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to .
Commission File Number 001-33672
SENECA BIOPHARMA, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 52-2007292 | |
|
State or other jurisdiction of incorporation or organization |
(I.R.S. Employer Identification No.) | |
|
20271 Goldenrod Lane Germantown, Maryland |
20876 | |
| (Address of principal executive offices) | (Zip Code) |
(301) 366-4841
(Registrant’s telephone number, including area code)
|
|
Securities registered pursuant to Section 12(b) of the Act:
| Title of Class | Trading Symbol | Name of Each Exchange on Which Registered |
| Common Stock, $0.01 par value | SNCA | Nasdaq Capital Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☐ Yes ☒ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. ☐ Yes ☒ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒ Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ☒ Yes ☐ No
| 1 |
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ | |
| Non-accelerated filer ☒ | Smaller reporting company ☒ |
Emerging Growth Company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). ☐ Yes ☒ No
The aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the Company’s common equity was last sold as of the last business day of the registrant’s most recently completed second fiscal quarter based upon the closing price of the common stock as reported by NASDAQ on such date, was $12,444,149.
The number of shares outstanding of Registrant’s common stock, $0.01 par value at February 28, 2021 was 17,295,703.
DOCUMENTS INCORPORATED BY REFERENCE
None.
| 2 |
SENECA BIOPHARMA, INC
ANNUAL REPORT ON FORM 10-K
FOR THE YEAR ENDED DECEMBER 31, 2020
INDEX
| 3 |
PART I
We urge you to read this entire Annual Report on Form 10-K, including the “Risk Factors” section, the consolidated financial statements and the related notes included therein. As used in this Annual Report, unless context otherwise requires, the words “we,” “us,” “our,” “the Company,” “Neuralstem,” “Seneca” and “Registrant” refer to Seneca Biopharma, Inc. and its subsidiary. Also, any reference to “common share” or “common stock,” refers to our $.01 par value common stock. Additionally, any reference to our “Series A Preferred Stock” refers to our Series A 4.5% Convertible Preferred Stock.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Statements in this annual report that are not strictly historical are forward-looking statements made pursuant to the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995 and include statements about products in development, results and analyses of pre-clinical studies, clinical trials and studies, future research and development expenses, anticipated cash expenditures, regulatory applications and approvals, and third-party relationships, among other matters. You can identify these forward-looking statements because they involve our expectations, intentions, beliefs, plans, projections, anticipations, or other characterizations of future events or circumstances and may often be identified by words such as “expect,” “anticipate,” “intend,” “plan,” “believe,” “seek” or “will.” These forward-looking statements are not guarantees of future performance and are subject to substantial risks and uncertainties that may cause actual results to differ materially from those described in the forward-looking statements These Forward-looking statements by their nature address matters that are uncertain. Specific risks and uncertainties that could cause our actual results to differ materially from those expressed in our forward-looking statements include risks inherent in our ability to consummate the proposed merger with Leading BioSciences, Inc, identify and in-license compounds and/or assets, conduct and obtain successful results from our clinical trials, retain management and operate our business, commercialize our technology, obtain regulatory approval for our product candidates, contract with third parties to adequately test and manufacture our proposed products, protect our intellectual property rights and obtain additional financing to continue our development efforts and execute on our business plans. These forward-looking statements are based on current expectations and assumptions that are subject to risks and uncertainties, which could cause our actual results to differ materially from those reflected in the forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to those discussed in this Annual Report, and in particular, the risks discussed under the caption “Risk Factors” in Item 1A and those discussed in other documents we file with the Securities and Exchange Commission (SEC). We undertake no obligation to revise or publicly release the results of any revision to these forward-looking statements, except as required by law. Given these risks and uncertainties, readers are cautioned not to place undue reliance on such forward-looking statements.
The information contained herein is current as of the date of this Annual Report (December 31, 2020), unless another date is specified.
| ITEM 1. | BUSINESS |
Overview
Historically, we have been primarily focused on the research and development of nervous system therapies based on our proprietary human neural stem cells and our small molecule compounds with the ultimate goal of gaining approval from the “FDA”, and its international counterparts, to market and commercialize such therapies. In early 2019, we commenced a strategic assessment of our clinical programs to determinate how to maximize shareholder value. As a result, Seneca subsequently initiated an:
| · | in-licensing and acquisition strategy in which it is evaluating novel therapeutics that could benefit from our development experience with the goal of developing such technologies for commercialization; and |
| · | out-licensing strategy to find partners to acquire or license NSI-566 and NSI-189. |
In-licensing and Acquisition Strategy
In early 2019, we engaged Hibiscus Bioventures (“Hibiscus”) and initiated an in-licensing and/or acquisition strategy to expand our product pipeline. Our in-licensing strategy consists of evaluating novel therapeutics that could be synergistic to us with the goal of developing such candidates for commercialization. We believe that this element of our corporate strategy could provide new opportunities for product development and diversify risks inherent in focusing on a limited product portfolio and therapeutic areas, thus potentially increasing its probability of commercial success. In December 2019, we further expanded this initiative and engaged Solebury Capital LLC (“Solebury”) to help explore available strategic alternatives, including possible mergers and business combinations, a sale of part or all of our assets, and collaboration and licensing arrangements.
| 4 |
Out-Licensing and Sales Strategy
Based on our review of existing clinical programs, including required capital and time to market, we have initiated an out-licensing and sales strategy to find partners or interested parties to acquire or license NSI-566 (neural stem cell) and NSI-189 (small molecule) and their respective clinical and pre-clinical programs and development. As part of this strategy, we begun winding down our ongoing development efforts, pre-clinical and clinical stage studies.
In December of 2020, we licensed certain patents and technologies, including a sublicense, related to the NSI- 189 small molecule program for $100,000 up front for a three (3) year period, plus, upon the occurrence of certain events, the licensee has the right to purchase the NSI-189 small molecule program for $5,000,000 at any time before the three (3) year period expires.
Our Present Focus
After conducting a strategic alternatives evaluation, with a goal of maximizing stockholder value, we substantially reduced our workforce and have wound down and suspended our research and development activities. We continue to: (a) provide support for patients who remain in clinical trials, (b) conduct our day-to-day business operations including the limited remaining activities required to wrap up our trial (c) support our intellectual property portfolio with a goal of maximizing stockholder value and (d) undertake our out-licensing and in-licensing acquisition initiatives.
Following our assessment, we commenced a process of evaluating strategic alternatives to maximize stockholder value with the assistance of Hibiscus and Solebury. After conducting a diligent and extensive process of evaluating strategic alternatives and identifying and reviewing potential candidates for a strategic acquisition or other transaction, which included the receipt of 15 non-binding indications of interest from interested parties and careful evaluation and consideration of those proposals, and following extensive negotiation with a number of possible candidates, on December 17, 2020, Seneca and Leading BioSciences, Inc. (“LBS”) announced the signing of a merger agreement (“Merger Agreement”). Upon the terms and subject to the satisfaction of the conditions described in the Merger Agreement, including approval of the transaction by our stockholders, a wholly-owned subsidiary of Seneca will be merged with and into LBS, with LBS being the surviving entity and a wholly-owned subsidiary of Seneca (“Merger”).
Although we have entered into the Merger Agreement and intend to consummate the transaction, there is no assurance that we will be able to successfully consummate the proposed merger on a timely basis, or at all. If, for any reason, the merger is not completed, we will reconsider our strategic alternatives and could pursue one or more of the following courses of action:
| · | Dissolve and liquidate our assets. If, for any reason, the merger is not consummated and we are unable to identify and complete an alternative strategic transaction like a merger or potential collaborative, partnering or other strategic arrangements for our assets, or continue to operate our business due to the inability to raise additional funding, we may be required to dissolve and liquidate our assets. In such case, there can be no assurances as to the amount or timing of available cash left to distribute to our stockholders, if any, after paying our debts and other obligations and setting aside funds for reserves. |
| · | Pursue potential collaborative, partnering or other strategic arrangements for our assets, including a sale or other divestiture. |
| · | Continue to operate our business. Although presently not anticipated, we could elect to continue to operate our business and pursue licensing or partnering transactions or utilize our intellectual property and research and discovery platform. Based on our prior assessment, this would require a significant amount of time, financial resources, human capital and Seneca would be subject to all the risk and uncertainties involved in the development of product candidates. In such instance, there is no assurance that we could raise sufficient capital to support these efforts, that our development efforts would be successful or that we could successfully obtain the regulatory approvals required to market any product candidate we pursued. |
| · | Pursue another strategic transaction like the proposed merger. |
Seneca’s Proprietary Technology Platform
Our patented technology platform has three core components:
1. Over 300 lines of human, regionally specific neural stem cells, some of which have the potential to be used to treat serious or life-threatening diseases through direct transplantation into the central nervous system;
2. Proprietary screening capability – Seneca’s ability to generate human neural stem cell lines provides a platform for chemical screening and discovery of novel compounds against nervous system disorders; and
| 5 |
3. Small molecules that resulted from Seneca’s neurogenesis screening platform that may have the potential to treat a wide variety of nervous system conditions.
To date, our technology platform has produced two lead assets: our NSI-566 stem cell therapy program and our NSI-189 small molecule program. A component of our strategy is seeking an asset sale, out-license, or global development partnerships to further development of NSI-566 and NSI-189. We have recently initiated a formal initiative aimed at securing partners to advance the clinical development of these two programs.
We believe our technology, in partnership with an established biopharmaceutical company with the appropriate development expertise and financial resources, could facilitate the development and commercialization of products for use in the treatment of a wide array of nervous system disorders including neurodegenerative conditions and regenerative repair of acute and chronic disease.
Clinical Programs
Historically, we have devoted our efforts and financial resources primarily to the pre-clinical and clinical development of our small molecule compounds and our stem cell therapeutics.
Based on our cash position, we have refocused our efforts primarily on maintaining the cell lines, patents, clinical material and data, and relevant licenses associated with these clinical programs as we seek partners for further development.
Below is a description of our clinical programs, their intended indication and current stage of development:
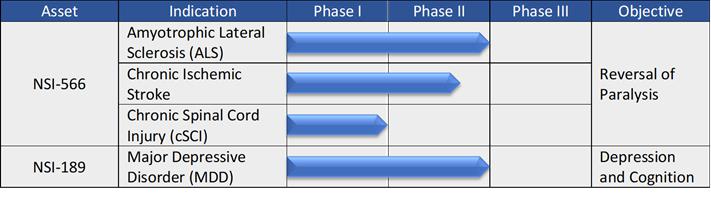
NSI - 566 (Stem Cells)
The human central nervous system (CNS) has limited capacity for regeneration following injury or the onset of disease. Traditional therapies have mainly focused on minimizing the progression or symptoms of CNS disease or injury but have not been effective at repairing the underlying cause of such disease. The goal of our cell therapy initiatives has been the regeneration of neural function which has been lost to disease or injury. We believe that neuroprotection, neuroregeneration, and/or bridging of damaged neural circuitry may be accomplished by implantation of NSI-566 at the injury site.
Clinical Experience with NSI-566
Chronic Spinal Cord Injury
In 2013, we received authorization from the FDA to commence a Phase 1 clinical trial to treat chronic spinal cord injury. The trial, which took place at The University of California, San Diego or UCSD, commenced in 2014 and the first subject was treated in October 2014. The study enrolled four AIS A classification thoracic spinal cord injury subjects (motor and sensory complete), one to two years’ post-injury at the time of stem cell treatment. In January of 2016 we reported six-month follow-up data on all four subjects. The stem cell treatment was found to be safe and well-tolerated by the subjects enrolled and there were no serious adverse events. In April of 2018 we enrolled the first subject in the second cohort of the trial, which included patients with AIS-A complete, quadriplegic, cervical injuries involving C5-C7 of their spinal cord. The final patient of this cohort was enrolled in March 2019.
In June 2018, the study investigators published the results of the first cohort in the journal Cell Stem Cell. The results support the potential of transplanted NSI-566 to benefit patients with cSCI. At 18 months to 27 months after surgery, the analysis of motor and sensory function and electrophysiology showed changes in three of the four patients after NSI-566 transplantation. There was no evidence of serious adverse events, suggesting the procedure is well-tolerated.
| 6 |
In January 2021, we announced preliminary, top-line results of the Company's placebo controlled Phase 2 stroke study (non-GCP) that was conducted in Beijing, China. The trial was designed to evaluate the relative safety of our human neural stem cell therapy, NSI-566, in patients with stable deficits in motor function resulting from ischemic stroke. Patients were eligible for the trial if they had documented history of ischemic stroke at least four months, but no more than 24 months, before surgery.
The study enrolled 23 patients who were randomly assigned to treatment or placebo arms. Patients in the treatment arm received intracerebral injection of 72 million stem cells, whereas those in the placebo group underwent a sham surgery procedure. Secondary objectives to evaluate efficacy were performed by qualified assessors who were blinded to treatment assignment, and included the Fugl-Meyer Motor Score (FMMS), an assessment of upper and lower motor function that comprises a 100-point scale and is widely used following stroke.
Patients enrolled in the treatment and placebo arms had similar baseline FMMS scores before surgery (mean ± SD: 36.80 ± 8.59 and 35.80 ± 4.66, respectively). While most participants showed some improvement in FMMS from pre-surgery scores, the mean improvement after one year was greater in those participants receiving NSI-566 (n=10, mean ± SD: 12.20 ± 14.15) compared to placebo (n=10, 6.30 ± 5.14), though the difference between groups did not reach statistical significance using the approximate Student's t-test (MMRM) (p=0.231). Two participants in the treatment arm showed clinically important improvements of 32 and 44 points on the FMMS following treatment with NSI-566, whereas the largest improvement observed in the placebo group was 17 points. Participants in the treatment arm experienced a total of three serious adverse events (SAE) that were considered by the investigator to be probably or possibly related to treatment, whereas no patients in the placebo arm experienced SAEs. Treatment-related SAEs were resolved with standard medical care and were limited to impaired healing at the incision site and wound dehiscence in one patient, and impaired hepatic function in another.
NSI-189 (Small Molecule Pharmaceutical Compound)
NSI-189 represents a new chemical entity that works through what appears to be a novel mechanism of action to stimulate neurogenesis of stem cells in the hippocampus, as well as generation of new synapses. Because impaired hippocampal neurogenesis has been linked with depression, we conducted clinical trials to evaluate the safety and effectiveness of NSI-189 in patients suffering from Major Depressive Disorder or MDD.
Out-license of NSI-189
In December 2020, we licensed certain patents and technologies, including a sublicense, related to the NSI- 189 small molecule program for $100,000 up front for a three (3) year period, plus, upon the occurrence of certain events, the licensee has the right to purchase the NSI-189 small molecule program for $5,000,000 at any time before the three (3) year period expires.
Seneca’s Technologies
Stem Cells
From a therapeutic perspective, our stem cell-based technology enables the isolation and large-scale expansion of regionally specific, human neural stem cells from all areas of the developing human brain and spinal cord thus enabling the generation of physiologically relevant human neurons of different types. We believe that our stem cell technology will enable the replacement or supplementation of malfunctioning or dead cells thereby creating a neurotrophic environment that offers protection to neural tissue as a way to treat disease and injury. Many significant and currently untreatable human diseases arise from the loss or malfunction of specific cell types in the body. Our focus is the development of effective methods to generate replacement cells from neural stem cells. We believe that creating a neurotrophic environment by replacing damaged, malfunctioning or dead neural cells with fully functional ones may be a useful therapeutic strategy in treating many diseases and conditions of the central nervous system.
Intellectual Property
We have developed and maintain a portfolio of patents and patent applications that form the proprietary base for our research and development efforts. We own or exclusively license 17 United States issued and pending patents and over 77 foreign issued and pending patents in the field of regenerative medicine, related to our stem cell technologies as well as our small molecule compounds. Our issued patents have expiration dates ranging from 2023 through 2038.
When appropriate, we seek patent protection for inventions in our core technologies and in ancillary technologies that support our core technologies or which we otherwise believe will provide us with a competitive advantage. We accomplish this by filing patent applications for discoveries we make, either alone or in collaboration with scientific collaborators and strategic partners. Typically, although not always, we file patent applications both in the United States and in select international markets. In addition, we plan to obtain licenses or options to acquire licenses to patent filings from other individuals and organizations that we anticipate could be useful in advancing our research, development and commercialization initiatives and our strategic business interests.
| 7 |
In addition to patenting our technologies, we also rely on confidential and proprietary information and take active measures to control access to that information, including the use of confidentiality agreements with our employees, consultants and certain of our contractors.
Our policy is to require our employees, consultants and significant scientific collaborators and sponsored researchers to execute confidentiality and assignment of invention agreements upon the commencement of an employment or consulting relationship with us. These agreements generally provide that all confidential information developed or made known to the individual by us during the course of the individual’s or entity’s relationship with us, is to be kept confidential and not disclosed to third parties except in specific circumstances. In the case of employees and consultants, the agreements generally provide that all inventions conceived by the individual or entity in the course of rendering services to us shall be our exclusive property.
Competition
The pharmaceutical and biotechnology industries are characterized by rapidly evolving technology and intense competition. Our competitors include major multinational pharmaceutical companies, specialty biotechnology companies and chemical and medical products companies. Many of these companies are well-established and possess greater resources for technical, research, development, financial, sales and marketing initiatives than we do. Other, less well-established companies have formed or may form strategic collaborations, partnerships and other types of joint ventures with larger, well established industry competitors that may provide research and development and commercialization advantages to these competitors. Academic institutions, governmental agencies and other public and private research organizations are also conducting and financing research activities which may produce products directly competitive to those we are developing. Moreover, many of these competitors may be able obtain patent protection, or FDA and other regulatory approvals that may impede on our freedom to develop and commercialize our proposed products.
The diseases and medical conditions we are targeting have a demographic in which there are large numbers of patients who do not respond to current therapies or have limited therapies available. Nevertheless, we expect that our technologies and product candidates, if or when approved, will compete with a variety of therapeutic products and procedures offered by other pharmaceutical and biotechnology companies. Many pharmaceutical and biotechnology companies are investigating new drugs and therapeutic approaches for the same or similar indications. These companies’ efforts may achieve new efficacy profiles, extend the therapeutic window for such products, alter the prognosis of these diseases, or prevent their onset. We believe that our products, if or when approved, will attempt to compete with these products principally on the basis of improved and extended efficacy and safety and their overall economic benefit to the health care system. Competition to our products may be in the form of existing and new drugs, other forms of cell transplantation, surgical procedures, gene therapy or other proprietary technology and expertise. We expect that all of these products will compete with our product candidates, if or when approved, based on efficacy, safety, cost and intellectual property positions. We cannot be certain that other entities have not filed patents that block our freedom to commercialize our programs and we may be required to seek licenses from these entities in order to commercialize certain of our proposed products, and such licenses may not be granted or be extremely expensive to obtain.
Government Regulation
Regulation by governmental authorities in the United States and other countries is a significant factor in our research and development and will be a significant factor in the manufacture and marketing of our proposed products. The nature and extent to which such regulation applies to our products will vary depending on the nature of any products we may develop. Governmental authorities, including the FDA and comparable regulatory authorities in other countries, regulate the design, development, testing, manufacturing, safety, efficacy, labeling, storage, record-keeping, advertising, promotion and marketing of pharmaceutical products, including drugs and biologics, under the Federal Food, Drug, and Cosmetic Act, or FDCA, and its implementing regulations, and, for biologics, under the Public Health Service Act, or PHSA, and its implementing regulations. Non-compliance with applicable requirements can result in fines and other judicially imposed sanctions, including product seizures, import restrictions, injunctive actions and criminal prosecutions of both companies and individuals. In addition, administrative remedies can involve requests to recall violative products; the refusal of the government to enter into supply contracts; or the refusal to approve pending product approval applications until manufacturing or other alleged deficiencies are brought into compliance. The FDA also has the authority to cause the withdrawal of approval of a marketed product or to impose labeling restrictions. The process of obtaining approvals and the subsequent compliance with appropriate statutes and regulations require the expenditure of substantial time and money, and there can be no guarantee that approvals will be granted.
During all phases of clinical development, regulatory agencies require extensive monitoring and auditing of all clinical activities, clinical data, and clinical trial investigators. Annual progress reports detailing the results of the clinical trials must be submitted to the FDA. Clinical trials may not be completed successfully within any specified period, if at all. The FDA or the sponsor or its data safety monitoring board may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research patients are being exposed to an unacceptable health risk, including risks inferred from other unrelated similar trials. Similarly, an institutional review board, or IRB, can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the product has been associated with unexpected serious harm to patients.
| 8 |
Human cell-based therapies in the field of regenerative medicine are relatively novel. Because this is a relatively new and expanding area of novel therapeutic interventions, there can be no assurance as to the length of the trial period, the number of patients the FDA will require to be enrolled in the trials in order to establish the safety, efficacy, purity and potency of such products, or that the data generated in these trials will be acceptable to the FDA to support marketing approval.
United States Review and Approval Process
After the completion of clinical trials of a product candidate, FDA approval of a BLA or NDA must be obtained before commercial marketing of the product. The BLA or NDA must include results of product development, laboratory and animal studies, human trials, information on the manufacture and composition of the product, proposed labeling and other relevant information as well as a significant user fee. The FDA may grant deferrals for submission of data, or full or partial waivers. The testing and approval processes require substantial time and effort and there can be no assurance that the FDA will accept the BLA or NDA for filing and, even if filed, that any approval will be granted on a timely basis, if at all.
The FDA may refuse to file any BLA or NDA that it deems incomplete or not properly reviewable at the time of submission, and may request additional information. Once the submission is accepted for filing, the FDA reviews the BLA or NDA to determine, among other things, whether the proposed product is safe, potent, and/or effective for its intended use, and has an acceptable purity profile, and whether the product is safe and effective for its intended use, and in each case, whether the product is being manufactured in accordance with cGMP or GTP, if applicable. During the product approval process, the FDA also will determine whether a Risk Evaluation and Mitigation Strategy, or REMS, is necessary to assure the safe use of the product. If the FDA concludes a REMS is needed, the sponsor of the BLA or NDA must submit a proposed REMS. The FDA will not approve a BLA or NDA without a REMS, if required.
Notwithstanding the submission of relevant data and information, the FDA may ultimately decide that the BLA or NDA does not satisfy its regulatory criteria for approval and deny approval via a letter detailing such deficiencies. Data obtained from clinical trials are not always conclusive and the FDA may interpret data differently than we interpret the same data. If the FDA denies an application, the applicant may either resubmit the BLA or NDA, addressing all of the deficiencies identified by the FDA, or withdraw the application.
United States Post-Approval Requirements
Any products for which we receive FDA approvals are subject to continuing regulation by the FDA, including, among other things, record-keeping requirements, reporting of adverse experiences with the product, providing the FDA with updated safety and efficacy information, product sampling and distribution requirements, and complying with FDA promotion and advertising requirements, which include, among others, standards for direct-to-consumer advertising, restrictions on promoting products for uses or in patient populations that are not described in the product’s approved uses, known as off-label use, limitations on industry-sponsored scientific and educational activities and requirements for promotional activities involving the internet.
In addition, quality control and manufacturing procedures must continue to conform to applicable manufacturing requirements after approval to ensure the long-term stability of the product. We rely, and expect to continue to rely, on third parties for the production of some, or all, clinical and commercial quantities of our products in accordance with cGMP and GTP regulations, as applicable. Manufacturers and other entities involved in the manufacture and distribution of approved products are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP, GTP and other laws.
The FDA also may require post-marketing testing, known as Phase 4 testing, and surveillance to monitor the effects of an approved product. Discovery of previously unknown problems with a product or the failure to comply with applicable FDA requirements can have negative consequences, including adverse publicity, judicial or administrative enforcement, warning letters from the FDA, mandated corrective advertising or communications with doctors, and civil or criminal penalties, among others. Also, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our product candidates under development.
European, China and Other Regulatory Review and Approval
Whether or not FDA approval has been obtained, approval of a product by comparable regulatory authorities in Europe, China and other countries will be necessary prior to commencement of marketing the product in such countries. The regulatory authorities in each country may impose their own requirements and may refuse to grant an approval, or may require additional data before granting it, even though the relevant product has been approved by the FDA or another authority. As with the FDA, the regulatory authorities in the European Union, China and other developed countries have lengthy approval processes for biological and pharmaceutical products. The process for gaining approval in particular countries varies, but generally follows a similar sequence to that described for FDA approval.
| 9 |
Other Health Care Laws
In the event any of our proposed products are ever approved for marketing, we may also be subject to healthcare regulation and enforcement by the federal government and the states and foreign governments where we may market our product candidates, if approved. These laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, physician sunshine and privacy and security laws and regulations.
Other Regulations
We are also subject to various U.S. federal, state, local and international laws, regulations and recommendations relating to safe working conditions, laboratory and manufacturing practices and the use and disposal of hazardous or potentially hazardous substances, including radioactive compounds and infectious disease agents, used in connection with our business. We cannot accurately predict the extent of government regulation which might result from future legislation or administrative action.
Employees
As of December 31, 2020, we had seven (7) full-time employees. We anticipate that upon the consummation of the merger with Leading BioSciences, Inc., we will terminate our current employees and consultants. We also use the services of several outside consultants in business and scientific matters. Historically, we have not implemented measures or objectives to address the development, attraction and retention of personnel and have instead hired employees and utilized the services of outside consultants as needed to run our operations.
Facilities
We currently operate one facility located in the United States and one facility located in China. Our corporate offices and primary research facilities are located in Germantown, Maryland, where we lease approximately 1,500 square feet. This lease provides for monthly payments of approximately $5,600 per month and expires on December 31, 2021.
We also lease approximately 11,300 square feet of research facility in the People’s Republic of China. This lease commenced in September 2019, provides for minimum lease payments of approximately $4,400 per month, expires in September 2024 and provides us with a future first right of refusal for extending the lease beyond its expiration.
Our Corporate Information
We were incorporated in Delaware in 2001 under the name Neuralstem, Inc. On October 28, 2019, we changed our name from Neuralstem, Inc. to Seneca Biopharma, Inc. Our principal executive offices are located at 20271 Goldenrod Lane, Germantown, Maryland 20876, and our telephone number is (301) 366-4841. Our website is located at www.senecabio.com.
We have not incorporated by reference into this report the information in, or that can be accessed through, our website and you should not consider it to be a part of this report.
Where to Find More Information
We make our public filings with the SEC, including our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and all exhibits and amendments to these reports. Also, our executive officers, directors and holders of more than 10% of our common stock, file reports with the SEC on Forms 3, 4 and 5 regarding their ownership of our securities. These materials are available on the SEC’s web site, http://www.sec.gov. You may also read or copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Alternatively, you may obtain copies of these filings, including exhibits, by writing or telephoning us at:
SENECA BIOPHARMA, INC
20271 Goldenrod Lane
Germantown, Maryland 20876
Attn: Investor Relations
Tel: (301) 366-4841
| 10 |
| ITEM 1A. | RISK FACTORS |
Investing in our common stock involves a high degree of risk. We have described below a number of uncertainties and risks which, in addition to uncertainties and risks presented elsewhere in this Quarterly Report, may adversely affect our business, operating results and financial condition. The uncertainties and risks enumerated below as well as those presented elsewhere in this Quarterly Report should be considered carefully when evaluating our company, business and the value of our securities.
Risks Related to the Merger
The Exchange Ratio is adjustable based on our net cash at closing and LBS’s Pre-Merger Financing, so the consideration at the closing of the Merger may have a greater or lesser value than at the time the Merger Agreement was signed.
The relative proportion of the combined company that our stockholders will own when the Merger closes will be based on the valuations of Seneca and LBS as negotiated by the parties and as specified in the Merger Agreement. Assuming a $22.5 million investment in LBS prior to the consummation of the Merger (“Pre-Merger Financing”), the outstanding equity of Seneca, as calculated on an adjusted fully diluted treasury stock method basis and after giving effect to such financing, is expected to be held as follows: equity holders of former LBS capital stock (prior to the Pre-Merger Financing) will hold approximately 25.2%; the investor in the Pre-Merger Financing will hold approximately 16.2%; pre-Merger Seneca equity holders will hold approximately 26.2%; and approximately 32.4% of the shares will be held in escrow to be distributed to the investor in the Pre-Merger Financing, or to be distributed to LBS Pre-Merger Financing equity holders. These estimates are based on the anticipated Exchange Ratio and are subject to adjustment as provided in the Merger Agreement. Prior to the consummation of the Merger, the Exchange Ratio at the closing of the Merger may be subject to either an upward or downward adjustment based on: (i) Seneca’s net cash, or (ii) the proceeds of the Pre-Merger Financing.
Failure to complete the Merger may result in us paying a termination fee to LBS and could harm our common stock price and our future business and operations.
If the Merger is not completed, each of Seneca and LBS is subject to the following risks:
| · | upon termination of the Merger Agreement, LBS may be required to pay Seneca a termination fee of $1.5 million, under certain circumstances, and/or up to $250,000 in expense reimbursements; |
| · | upon termination of the Merger Agreement, Seneca may be required to pay LBS a termination fee of $1.5 million, under certain circumstances, and/or up to $250,000 in expense reimbursements; |
| · | the parties will have incurred significant expenses related to the Merger, such as legal and accounting fees, which must be paid even if the Merger is not completed; and |
| · | Seneca may be forced to cease its operations, dissolve and liquidate its assets. |
In addition, if the Merger Agreement is terminated and our board of directors determines to seek another business combination, there can be no assurance that we will be able to find a partner willing to provide equivalent or more attractive consideration than the consideration to be provided in the Merger.
If the conditions to the closing of the Merger are not met, the Merger may not occur.
Even if the change of control and related share issuance are approved by our stockholders, specified conditions must be satisfied or waived to complete the Merger. We cannot assure you that all of the conditions will be satisfied or waived. If the conditions are not satisfied or waived, the Merger may not occur or will be delayed, we may lose some or all the intended benefits of the Merger.
The Merger may be completed even though material adverse changes may result from the announcement of the Merger, industry-wide changes and/or other causes.
In general, either Seneca or LBS can refuse to complete the Merger if there is a material adverse change affecting the other party between the date of the Merger Agreement, and the closing of the Merger. However, certain types of changes do not permit either party to refuse to complete the Merger, even if such change could be said to have a material adverse effect on Seneca or LBS, including:
| · | general business or economic conditions generally affecting the industry in which LBS or Seneca operate; |
| 11 |
| · | the taking of any action, or the failure to take any action, by the either party that is required to comply with the terms of Merger Agreement; |
| · | any natural disaster or epidemics, pandemics (including COVID-19 or other outbreaks of diseases or quarantine restrictions), or other force majeure events, or any act or threat of terrorism or war, any armed hostilities or terrorist activities; or |
| · | any change in, or any compliance with or action taken for the purpose of complying with, any law or GAAP (or interpretations of any law or GAAP). |
If adverse changes occur and Seneca and LBS still complete the Merger, the stock price of the combined company following the closing of the Merger may suffer. This in turn may reduce the value of the Merger to the stockholders of Seneca, LBS or both.
Some executive officers and directors of Seneca and LBS have interests in the Merger that are different from the respective stockholders of Seneca and LBS and that may influence them to support or approve the Merger without regard to the interests of the respective stockholders of Seneca and LBS.
Some officers and directors of Seneca and LBS are parties to arrangements that provide them with interests in the Merger that are different from the respective stockholders of Seneca and LBS, including, among others, service as an officer or director of the combined company following the closing of the Merger, severance benefits, the acceleration of equity award vesting, and continued indemnification.
Based on the terms of their respective agreements, Seneca’s recently terminated executive officers may be entitled to receive a total value of $3,425,613 (collectively, not individually) in connection with the consummation of the Merger, the associated termination of their employment from Seneca and the cancelation of their stock options. Additionally, Seneca’s recently terminated senior vice president of research and development will be entitled to receive a total value of $865,438. In addition, in connection with the Merger, the executive officers of LBS entered into new employment agreements and are entitled to receive cash bonuses, certain executive officers are entitled to receive equity grants, and members of the LBS Board are entitled to receive cash bonuses.
The market price of Seneca Common Stock following the Merger may decline as a result of the Merger.
The market price of Seneca Common Stock may decline as a result of the Merger for a number of reasons, including if:
| · | investors react negatively to the prospects of the combined company’s business and prospects following the closing of the Merger; |
| · | the effect of the Merger on the combined company’s business and prospects following the closing of the Merger is not consistent with the expectations of financial or industry analysts; or |
| · | the combined company does not achieve the perceived benefits of the Merger as rapidly or to the extent anticipated by stockholders or financial or industry analysts. |
| 12 |
LBS and Seneca securityholders will have a reduced ownership and voting interest in, and will exercise less influence over the management of, the combined company following the closing of the Merger as compared to their current ownership and voting interest in the respective companies.
After the completion of the Merger, the current securityholders of LBS and Seneca will own a smaller percentage of the combined company than their ownership in their respective companies prior to the Merger. Immediately after the Merger, it is currently estimated that the former LBS equity holders immediately before the Merger (including the investor in the Pre-Merger Financing) are expected to hold approximately 73.8% of the capital stock of Seneca outstanding immediately following the Merger and the equity holders of Seneca immediately before the Merger are expected to hold approximately 26.2% of the Seneca capital stock outstanding immediately following the Merger, in each case, as calculated on an adjusted fully diluted treasury stock method basis and after giving effect to the Pre-Merger Financing, but including 50% of the shares subject to the Equity Warrants. These estimates are based on the anticipated Exchange Ratio and are subject to adjustment as provided in the Merger Agreement.
During the pendency of the Merger, Seneca and LBS may not be able to enter into a business combination with another party at a favorable price because of restrictions in the Merger Agreement, which could adversely affect their respective businesses.
Covenants in the Merger Agreement impede the ability of Seneca and LBS to make acquisitions, subject to specified exceptions relating to fiduciary duties, or complete other mergers, sales of assets (other than the sale of the Seneca Legacy Technology) or other business combinations that are not in the ordinary course of business pending completion of the Merger. As a result, if the Merger is not completed, the parties may be at a disadvantage to their competitors during that period. In addition, while the Merger Agreement is in effect, each party is generally prohibited from soliciting, initiating, encouraging or entering into specified extraordinary transactions, such as a merger, sale of assets or other business combination, with any third party, subject to specified exceptions, even if any such transaction could be favorable to such party’s stockholders.
Certain provisions of the Merger Agreement may discourage third parties from submitting competing proposals, including proposals that may be superior to the arrangements contemplated by the Merger Agreement.
The terms of the Merger Agreement prohibit each of Seneca and LBS from soliciting competing proposals or cooperating with persons making unsolicited takeover proposals, except in limited circumstances when such party’s board of directors determines in good faith, after consultation with its independent financial advisor, if any, and outside counsel, that an unsolicited competing proposal constitutes, or would reasonably be expected to result in, a superior competing proposal and that failure to take such action would result in a breach of the fiduciary duties of the board of directors. In addition, if Seneca or LBS terminate the Merger Agreement under specified circumstances, including terminating because of a decision of a board of directors to recommend a superior competing proposal, LBS may be required to pay Seneca a termination fee of $1.5 million and/or $250,000 in expense reimbursements or Seneca may be required to pay LBS a termination fee of $1.5 million, or up to $250,000 in expense reimbursements.
Because the lack of a public market for LBS’s capital stock makes it difficult to evaluate the fairness of the Merger, the shareholders of LBS may receive consideration in the Merger that is less than the fair market value of LBS’s capital stock and/or Seneca may pay more than the fair market value of LBS’s capital stock.
The outstanding capital stock of LBS is privately held and is not traded in any public market. The lack of a public market makes it extremely difficult to determine the fair market value of LBS’s capital stock. Because the percentage of Seneca equity to be issued to LBS shareholders was determined based on negotiations between the parties, it is possible that the value of the Seneca Common Stock to be received by LBS shareholders will be less than the fair market value of LBS’s capital stock, or Seneca may pay more than the aggregate fair market value for LBS’s capital stock. The combined organization will incur significant transaction costs as a result of the Merger, including investment banking, legal and accounting fees. In addition, the combined organization will incur significant operating expenses which cannot be accurately estimated at this time. Actual transaction costs may substantially exceed the Party’s estimates and may have an adverse effect on the combined organization’s financial condition and operating results.
If Nasdaq does not approve our listing application for the combined company and we continue with the Merger, we may be subject to delisting.
Seneca has filed an initial listing application with Nasdaq pursuant to Nasdaq’s “reverse merger” rules. In the event our application is not accepted by the Nasdaq and the parties proceed with the merger, the combined company will be subject to delisting proceedings and could be delisted. If Seneca’s shares lose their status on the Nasdaq Capital Market, Seneca believes that its shares would likely be eligible to be quoted on the inter-dealer electronic quotation and trading system operated by Pink OTC Markets Inc., commonly referred to as the Pink Sheets and now known as the OTCQB market. These markets are generally considered not to be as efficient as, and not as broad as, the Nasdaq Capital Market. If Seneca’s common stock is delisted, this would, among other things, substantially impair its ability to raise additional funds and could result in a loss of institutional investor interest and fewer development opportunities for Seneca. Additionally, investors would find it more difficult to buy and sell shares of Seneca Common Stock.
| 13 |
Risks Related to Seneca’s Capital Requirements, Finances and Operations in the event the Merger is Not Completed
There is no assurance that the proposed Merger will be completed in a timely manner or at all. If the Merger is not consummated, our business could suffer materially, and its stock price could decline.
The consummation of the Merger is subject to a number of closing conditions, including approval by Seneca’s and LBS’s respective stockholders and other customary closing conditions. The parties are targeting a closing of the transaction in the first half of 2021, however, there can be no assurance that the merger will be consummated within this desired timeframe, or at all.
If the Merger is not consummated, we may be subject to a number of material risks, and our business and stock price could be adversely affected, as follows:
| · | We have incurred and expect to continue to incur significant expenses related to the Merger, even if the Merger is not consummated; |
| · | We could be obligated to pay a $1.5 million termination fee and expense reimbursements up to $250,000 in connection with the termination of the Merger Agreement, depending on the reason for the termination; |
| · | The market price of our Common Stock may decline to the extent that the current market price reflects a market assumption that the Merger will be completed; and |
| · | Nasdaq could determine to delist our Common Stock, which could have an adverse effect on the value of our Common Stock and any future ability to raise capital. |
If the Merger is not completed, we may be unsuccessful in completing an alternative transaction on terms that are as favorable as the terms of the proposed transaction, or at all, and we may be unable to reestablish a viable operating business.
We have generated limited revenue to date from royalties under a settlement agreement and have not generated revenue from any product sales. Our assets currently consist primarily of cash, cash equivalents and short-term investments, our intellectual property portfolio, a settlement agreement pursuant to which it has received royalties, its remaining assets and its listing on The Nasdaq Stock Market. While we have entered into the Merger Agreement, the consummation of the Merger may be delayed or may not occur at all. If the Merger is not completed, our board of directors may elect to pursue an alternative strategic transaction which is similar to the proposed Merger. Attempting to complete an alternative transaction will be costly and time consuming. If the Merger is not completed and our board of directors determines to pursue an alternative transaction, the terms of any such alternative transaction may not be as favorable to Seneca and its stockholders as the terms of the Merger. We can make no assurances that such an alternative transaction would occur at all. Further, if the Merger is not completed, given the level of investment and time that would be required to redesign its products or pursue the development of products and services pursuant to its collaboration agreements, it is unlikely that we would be able to obtain the funding required to recommence its product development activities on terms favorable to its stockholders, or at all.
If the Merger is not completed, our board of directors may decide to pursue a dissolution and liquidation of our business. In such an event, the amount of cash available for distribution to our stockholders will depend heavily on the timing of such liquidation as well as the amount of cash that will need to be reserved for commitments and contingent liabilities.
There can be no assurance that the Merger will be completed. If the Merger is not completed, our board of directors may decide to pursue a dissolution and liquidation of our assets. In such an event, the amount of cash available for distribution to our stockholders will depend heavily on the timing of such decision, as with the passage of time the amount of cash available for distribution will be reduced as we continue to fund our operations. In addition, if our board of directors were to approve and recommend, and our stockholders were to approve, a dissolution and liquidation of Seneca, we would be required under Delaware corporate law to pay our outstanding obligations, as well as to make reasonable provision for contingent and unknown obligations, prior to making any distributions in liquidation to its stockholders. As a result of this requirement, our remaining cash may need to be reserved pending the resolution of such obligations. In addition, we may be subject to litigation or other claims related to a dissolution and liquidation of our business. If a dissolution and liquidation were pursued, our board of directors, would need to evaluate these matters and make a determination about a reasonable amount to reserve. Accordingly, holders of our Common Stock could lose all or a significant portion of their investment in the event of a liquidation, dissolution or winding up.
| 14 |
If we were to continue to advance our research and development activities and pursue development of any of our pipeline products, it would require substantial additional funding. Raising additional capital would cause dilution to our existing stockholders and may restrict our operations or require us to relinquish rights to our technologies or to a product candidate.
We currently do not have any committed source of funds and do not expect to generate any commercial revenue in the foreseeable future. We believe in the event the Merger is not consummated that our existing cash, cash equivalents and marketable securities and interest thereon will be sufficient to fund our projected operating requirements under our current operating plan through at least March 2022. We have based our estimates on assumptions that may prove to be wrong, and it may use its available capital resources sooner than it currently expects if its operating plans change. If the Merger is not completed and Seneca decides to pursue further research and development activities, it will require substantial additional funding to operate, and would expect to finance these cash needs through a combination of equity offerings, debt financings, government or other third-party funding and licensing or collaboration arrangements.
To the extent that we raise additional capital through the sale of equity or convertible debt, the ownership interests of our stockholders will be diluted. In addition, the terms of any equity or convertible debt that we agree to issue may include liquidation or other preferences that adversely affect the rights of our stockholders. Convertible debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, and declaring dividends, and may impose limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business.
Additional funds may not be available to us when we need them on terms that are acceptable to us, or at all. Furthermore, the novel coronavirus (“COVID-19”) pandemic continues to rapidly evolve and has already resulted in a significant disruption of global financial markets. If the disruption persists and deepens, we could experience an inability to access additional capital, when and if needed. If adequate funds are not available to us on a timely basis, we may be required to curtail or cease its operations.
If the Merger is not completed, raising additional funding through debt or equity financing could be difficult or not successful at all, would be dilutive and may cause the market price of our Common Stock to further decline.
If the Merger is not completed, raising additional funding through debt or equity financing could be difficult or unavailable altogether given the turbulent financial markets. To the extent that Seneca raises additional capital through the sale of equity or convertible debt securities, the issuance of those securities would result in substantial dilution to our current stockholders and the terms may include liquidation or other preferences that adversely affect the rights of our current stockholders. Furthermore, the issuance of additional securities, whether equity or debt, or the possibility of such issuance, may cause the market price of its common stock to decline further and existing stockholders may not agree with its financing plans or the terms of such financings.
Risks Related to Seneca
We have a history of losses.
Since inception in 1996 through December 31, 2020, we have accumulated losses totaling approximately $238 million. As of December 31, 2020, we had a working capital surplus of approximately $10 million and stockholders’ equity of approximately $10 million. Our net losses for the two most recent fiscal years have been approximately $16 million and $8 million for 2020 and 2019, respectively.
To date, we have not generated any revenue from the commercial sale of our proposed products. No assurances can be given as to exactly when, if at all, we will be able to fully develop, commercialize, market, sell and/or derive any, let alone material, revenues from our proposed products.
We will need to raise additional capital to continue operations.
Since our inception, we have funded our operations through the sale of our securities, credit facilities, the exercise of options and warrants, and to a lesser degree, from grants and research contracts and other revenue generating activities such as licensing. As of December 31, 2020, we had cash, cash equivalents and short-term investments on hand of approximately $10.5 million. We anticipate that in the event the Merger is not consummated, and based on our cash position at December 31, 2020, we will be able to fund our operations beyond 12 months from this filing. We cannot assure you that we will be able to secure additional capital through financing transactions, including issuance of debt, licensing agreements or grants. Our inability to license our intellectual property, obtain grants or secure additional financing will materially impact our ability to fund our current and planned operations.
| 15 |
We are substantially dependent on our remaining employees and consultants to facilitate the consummation of the Merger.
As of March 17, 2021, as a result of entering into separation agreements with four employees, including our executive chairman, chief operating officer, chief financial officer, and Senior VP of R&D, we had only three full-time employees. Such remaining employees’ employment will be terminated upon the closing of the Merger. While we were able to secure consulting agreements with certain recently separated employees, our ability to successfully complete the Merger depends in large part on our ability to retain certain of our remaining personnel. Despite our efforts to retain these employees and consultants, one or more may terminate their employment or consulting agreements on short notice. The loss of the services of any of these employees or consultants could potentially harm our ability to consummate the Merger, to run our day-to-day business operations, as well as to fulfill our reporting obligations as a public company.
Management transition creates uncertainties and could harm Seneca’s business.
We have in the past, and expect to in the future, experience significant changes in executive leadership. Changes to company strategy, which can often times occur with the appointment of new executives, can create uncertainty, may negatively impact our ability to execute quickly and effectively, and may ultimately be unsuccessful. In addition, executive leadership transition periods are often difficult as the new executives gain detailed knowledge of Seneca’s operations, and friction can result from changes in strategy and management style. Management transition inherently causes some loss of institutional knowledge, which can negatively affect strategy and execution. Until we integrate new personnel, and unless they are able to succeed in their positions, we may be unable to successfully manage and grow our business, and our results of operations and financial condition could suffer as a result. In any event, changes in our organization as a result of executive management transition may have a disruptive impact on our ability to implement its strategy and could have a material adverse effect on our business, financial condition and results of operations.
The pendency of the Merger could have an adverse effect on the trading price of Seneca’s Common Stock and Seneca’s business, financial condition and prospects.
While there have been no significant adverse effects to date, the pendency of the Merger could disrupt Seneca’s business in many ways, including:
| · | the attention of our remaining management and employees may be directed toward the completion of the Merger and related matters and may be diverted from our day-to-day business operations; and |
| · | third parties may seek to terminate or renegotiate their relationships with us as a result of the Merger, whether pursuant to the terms of their existing agreements or otherwise. |
Should they occur, any of these matters could adversely affect the trading price of our Common Stock or harm our business, financial condition and prospects.
We may not be able to continue as a going concern if we do not obtain additional financing.
We have incurred losses since inception and have not demonstrated an ability to generate revenues from the sales of our proposed products. Our ability to continue as a going concern is dependent on raising capital from the sale of its common stock and/or obtaining debt financing. Our cash, cash equivalents and short-term investment balance at December 31, 2020 was approximately $10.5 million. Based on our current expected level of operating expenditures, and assuming the Merger is not consummated, we expect to be able to fund our operations beyond 12 months from this filing. Our ability to remain a going concern is wholly dependent upon our ability to continue to obtain sufficient capital to fund our operations. Despite our ability to secure capital in the past, there can be no assurance that additional equity or debt financing will be available to us when needed or that we may be able to secure funding from any other sources. In the event that we are not able to secure funding, we may be forced to curtail operations, cease operations altogether or file for bankruptcy.
Our auditors have expressed substantial doubt about our ability to continue as a going concern.
Our auditors’ report on our December 31, 2020 consolidated financial statements included an explanatory paragraph that expressed substantial doubt about its ability to continue as a going concern. Our current cash level raises substantial doubt about our ability to continue as a going concern at least through March 2022. If we do not obtain additional capital, we may no longer be able to continue as a going concern and may cease operation or seek bankruptcy protection.
| 16 |
We are involved in litigation in connection with the Merger and insurance coverage may not be sufficient to cover all related costs and damages.
Stockholder litigation frequently follows the announcement of certain significant business transactions, such as a business combination transaction. As of March 16, 2021, there were nine complaints filed by purported Seneca stockholders, Sheridan v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00166 (the “Sheridan Complaint”); Pirjamaat v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00172 (the “Pirjamaat Complaint”); Johnson v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00310 (the “Johnson Complaint”); Mathews v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00242 (the “Mathews Complaint”); Pechal v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00585 (the “Pechal Complaint”), Curtis v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00292 (the “Curtis Complaint”); Valdez v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00980 (the “Valdez Complaint”); Anderson v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00326 (the “Anderson Complaint”); and McIntire v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-01869 (the “Anderson Complaint” and, together with the Sheridan Complaint, the Pirjamaat Complaint, the Johnson Complaint, the Matthews Complaint, the Curtis Complaint, the Valdez Complaint, and the Anderson Complaint, the “Stockholder Complaints”). The Stockholder Complaints assert claims against us, the members of our board of directors as defendants under Section 14(a) of the Exchange Act and Rule 14a-9 promulgated thereunder for allegedly false and misleading statements in in the registration statement filed on Form S-4 in February 2021 and Section 20(a) of the Exchange Act for alleged “control person” liability with respect to such allegedly false and misleading statements. The Stockholder Complaints assert claims against Seneca, the members of the Seneca Board, and LBS as defendants under Section 14(a) of the Exchange Act and Rule 14a-9 promulgated thereunder for allegedly false and misleading statements in this proxy statement/prospectus/information statement and Section 20(a) of the Exchange Act for alleged “control person” liability with respect to such allegedly false and misleading statements. The Johnson Complaint also asserts that the members of the Seneca Board breached their fiduciary duties of candor/disclosure in connection with the Merger by purportedly failing to disclose material information about the Merger.
Each of the Stockholder Complaints seek, among other relief, injunctive relief, including enjoining the Merger unless and until the defendants disclose the allegedly omitted material information, as well as an award of attorneys’ and experts’ fees. The Mathews Complaint also seeks to enjoin any vote on the Merger; the Sheridan Complaint, the Johnson Complaint, and the McIntire Complaint seek damages; the Sheridan Complaint, the Pirjamaat Complaint, the Mathews Complaint, the Curtis Complaint, the Valdez Complaint, and the Anderson Complaint, seek, in the event the defendants consummate the merger, rescission of the Merger or an award of rescissory damages; the Pirjamaat Complaint, the Curtis Complaint, and the Valdez Complaint seek an order directing the Seneca Board to disseminate a revised registration statement in compliance with Sections 14(a) and/or 20(a) of the Exchange Act and Rule 14a-9; and the Pirjamaat Complaint, the Mathews Complaint, the Curtis Complaint, the Valdez Complaint, and the Anderson Complaint seek a declaration that defendants violated Sections 14(a) and/or 20(a) of the Exchange Act and Rule 14a-9.
We believe the allegations in the Stockholder Complaints are without merit.
Other stockholders may file additional lawsuits challenging the Merger, which may name us as well as members of our boards of directors and/or others as defendants. No assurance can be made as to the outcome of such lawsuits or the Stockholder Complaints, including the amount of costs associated with defending, or any other liabilities that may be incurred in connection with the litigation of, such claims. Litigation often is expensive and diverts management’s attention and resources, which could adversely affect our business. At present, we are unable to estimate potential losses, if any, related to the lawsuit.
Risks Relating to Seneca’s Business
Seneca’s business is dependent on the successful development of product candidates that it has yet to acquire or license.
Our business is significantly dependent on the successful development of product candidates that we have yet to acquire or license. If we are successful in-licensing or acquiring product candidates, the process to approve of such product candidates is time-consuming, involves substantial expenditures of resources, and depends upon a number of factors, including the availability of alternative treatments, and the risks and benefits demonstrated in its clinical trials. Our success will depend on our ability to achieve scientific and technological advances and to translate such advances into FDA-approvable, commercially competitive products on a timely basis. Failure can occur at any stage of the process. If we are not successful in our in-licensing and acquisition strategy, we will have invested substantial amounts of time and money without developing revenue-producing products.
Any product candidate we are able to license or acquire will likely not be commercially available for at least several years, if at all. Development schedules for future product candidates may be affected by a variety of factors, including difficulties in identifying and in-licensing or acquiring such future products candidates, technological difficulties, clinical trial delays or failures, regulatory hurdles, competitive products, intellectual property challenges and/or changes in governmental regulation, many of which will not be within our control. In light of the long-term nature of these types of projects, the technology potentially involved, and the other factors there can be no assurance that we will be able to successfully complete the development or marketing of any product candidates.
The technologies we intend to out-license may not be able to be commercially developed.
We have allocated most of our resources to the development of our stem cell and small molecule technologies. These are emerging technologies which may be deemed to have limited human application. If potential licensees or acquirors believe that these technologies have limited human applications, we may not be able to out-license, on acceptable terms or at all, our technologies. Failure to out-license or sell our stem cell or small molecule technologies may materially impact the value of its business.
We are unable to predict when or if we will be able to earn significant revenues.
Given that we have yet to in-license or acquire new technologies, it cannot predict when, or if ever, we will be able to realize revenues related to our future products. Even if in-licensed or acquired, these products are not likely to be commercially available for at least several or more years, if ever. Accordingly, we do not foresee generating any significant revenue during such time. As a result, we will be primarily dependent on its ability to raise capital through the sale of its securities to fund its operations for the foreseeable future.
| 17 |
We may be subject to litigation that will be costly to defend or pursue and uncertain in its outcome.
Our business may bring us into conflict with licensees, licensors, or others with whom we have contractual or other business relationships or with our competitors or others whose interests differ from ours. If we are unable to resolve these conflicts on terms that are satisfactory to all parties, we may become involved in litigation brought by or against such parties. Any litigation is likely to be expensive and may require a significant amount of management’s time and attention, at the expense of other aspects of our business. The outcome of litigation is always uncertain, and in some cases, could include judgments against us which could have a materially adverse effect on our business.
We depend on a limited number of employees and consultants for our continued operations and future success.
We are highly dependent on a limited number of employees and outside consultants. The loss of any of our employees or consultants could adversely affect our opportunities and materially harm our future prospects. In the event the Merger is not completed, and our board of directors elects to continue our business, we will need additional management personnel as well as the development of additional expertise by existing management personnel. There can be no assurance that we will be able to attract and retain the qualified personnel necessary for the development of our business.
We have entered into employment contracts with members of our senior management team that contain significant anti-termination provisions.
We have entered into employment agreements with members of its senior management team. These agreements require the payment of severance in the event one of these employees ceases to be employed. These provisions make the replacement of these employees very costly and could cause difficulty in effecting any required changes in management or a change in control. In the event the Merger is consummated, we will be obligated to pay members of our management team an aggregate of $4,291,051. Please see the Section of the Annual Report Entitled “Executive Compensation.”
Business or economic disruptions, or global health concerns could seriously harm our development efforts and increase our costs and expenses.
Broad-based business or economic disruptions could adversely affect our planned research and development activities as well as the execution of our acquisition and/or in-licensing strategy. For example, in December 2019 an outbreak of a novel strain of coronavirus originated in Wuhan, China, and has since spread around the world, including to the United States. To date, this outbreak has already resulted in extended shutdowns of many businesses around the world, including in the United States. At this time, the impact on our business has been that employees who previously worked in our corporate office and who traveled are now limited to home office work and virtual meetings. Global health concerns, such as coronavirus, could also result in social, economic, and labor instability in the countries in which we or the third parties with whom we engage operate. We cannot presently predict the scope, severity and longevity of any potential business shutdowns or disruptions, but if we or any of the third parties with whom it engages or plans to engage, including the suppliers, clinical trial sites, regulators and other third parties with whom we conduct business or plan to conduct business, were to experience shutdowns or other business disruptions, our ability to conduct our business in the manner and on the timelines presently planned could be materially and negatively impacted. It is also possible that global health concerns such as this one could disproportionately impact the hospitals and clinical sites in which we may conduct any of its clinical trials, which could have a material adverse effect on our business and results of operation and financial condition.
The increasing use of social media platforms presents new risks and challenges.
Social media is increasingly being used to communicate information about our products and the diseases that our therapies are designed to treat. Social media practices in our industry continue to evolve and regulations related to such use are not always clear. This evolution creates uncertainty and risk of noncompliance with regulations applicable to our business. For example, patients and others may use social media channels to comment on the effectiveness of a product or to report an alleged adverse event. When such disclosures occur, we may fail to monitor and comply with applicable adverse event reporting obligations or we may not be able to defend against political and market pressures generated by social media due to restrictions on what we may say about our products. There is also a risk of inappropriate disclosure of sensitive information or negative or inaccurate comments about us on any social networking website. If any of these events were to occur or Seneca otherwise fails to comply with applicable regulations, it could incur liability, face overly restrictive regulatory actions or incur other harm to its business.
| 18 |
Risks Relating to Seneca’s Intellectual Property
We may not be able to withstand challenges to our intellectual property rights.
We rely on our intellectual property, including issued and applied-for patents, as the foundation of our business. Our intellectual property rights may come under challenge. No assurances can be given that our current and potential future patents will survive such challenges. These cases are complex, lengthy, expensive, and could potentially be adjudicated adversely to our interests, removing the protection afforded by an issued patent. The viability of our business would suffer if such patent protection were limited or eliminated. Moreover, the costs associated with defending or settling intellectual property claims would likely have a material adverse effect on our business and future prospects.
We may not be able to adequately protect against the piracy of the intellectual property in foreign jurisdictions.
We have conducted research in countries outside of the U.S., including through our subsidiary in the People’s Republic of China. Several of our competitors are located in these countries and may be able to access our technology or test results. The laws protecting intellectual property in some of these countries may not adequately protect our trade secrets and intellectual property. The misappropriation of our intellectual property may materially impact our position in the market and any competitive advantages, if any, that it may have.
We may infringe on the intellectual property rights of others and may not be able to obtain necessary licenses to third-party patents and other rights.
A number of companies, universities and research institutions have filed patent applications or have received patents relating to technologies in our field. We cannot predict which, if any, of these applications will issue as patents or how many of these issued patents will be found valid and enforceable. There may also be existing issued patents on which we would infringe by the commercialization of our product candidates. If so, we may be prevented from commercializing these products unless the third party is willing to grant us a license. We may be unable to obtain licenses to the relevant patents at a reasonable cost, if at all, and may also be unable to develop or obtain alternative non-infringing technology. If we are unable to obtain such licenses or develop non-infringing technology at a reasonable cost, our business could be materially harmed. Any infringement lawsuits commenced against us may result in significant costs, divert its management’s attention and result in an award against it for substantial damages, or potentially prevent it from continuing certain operations.
Risks Related to Ownership of Our Common Stock
The market price for our common shares is particularly volatile.
The market for our common shares is characterized by significant price volatility when compared to seasoned issuers, and we expect that our share price will continue to be more volatile than those of a seasoned issuer. The volatility in our share price is attributable to a number of factors. Mainly however, we are a speculative or “risky” investment due to our limited operating history, lack of significant revenues to date and the uncertainty of FDA approval. By way of example, in July of 2019, we completed a firm commitment underwritten public offering of our securities. During the marketing of the offering and post-closing, the market price or our common stock decreased substantially. As a consequence of this enhanced risk, more risk-adverse investors may, under the fear of losing all or most of their investment in the event of negative news or lack of progress, be more inclined to sell their shares on the market more quickly and at greater discounts than would be the case with the stock of a seasoned issuer. Additionally, in the past, plaintiffs have often initiated securities class action litigation against a company following periods of volatility in the market price of its securities. Securities litigation could result in substantial costs and liabilities and could divert management’s attention and resources.
The following factors may add to the volatility in the price of our common shares: actual or anticipated variations in our quarterly or annual operating results; the results of clinical trials for our product candidates; FDA’s determination with respect to filings for new clinical studies, new drug applications and new indications; government regulations; announcements of significant acquisitions, strategic partnerships or joint ventures; our capital commitments; offerings of our securities and additions or departures of key personnel. Many of these factors are beyond our control and may decrease the market price of our common shares, regardless of our operating performance. We cannot make any predictions or projections as to what the prevailing market price for our common shares will be at any time, including as to whether our common shares will sustain their current market prices, or as to what effect the sale of shares or the availability of common shares for sale at any time will have on the prevailing market price.
If Seneca’s common stock were delisted from Nasdaq, Seneca would be subject to the risks relating to penny stocks.
If Seneca’s common stock were to be delisted from trading on the Nasdaq Capital Market and the trading price of its common stock were below $5.00 per share on the date its common stock is delisted, trading in Seneca’s common stock would also be subject to the requirements of certain rules promulgated under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). These rules require additional disclosure by broker-dealers in connection with any trades involving a stock defined as a “penny stock” and impose various sales practice requirements on broker-dealers who sell penny stocks to persons other than established customers and accredited investors, generally institutions. These additional requirements may discourage broker-dealers from effecting transactions in securities that are classified as penny stocks, which could severely limit the market price and liquidity of such securities and the ability of purchasers to sell such securities in the secondary market. A penny stock is defined generally as any non-exchange listed equity security that has a market price of less than $5.00 per share, subject to certain exceptions.
Future sales of our common stock could cause our stock price to fall.
In January 2020, we completed an inducement offering pursuant to which we reduced the exercise price of outstanding warrants in exchange for the holder exercising such warrants for cash. As a result, we issued 5,555,554 shares of common stock, or approximately 61% of our issued and outstanding common stock. Transactions, such as the inducement offering, that result in a large amount of newly issued shares that are readily tradable, or other events that cause current stockholders to sell shares, could place downward pressure on the trading price of our common stock. In addition, the lack of a robust trading market may require a stockholder who desires to sell a large number of shares of common stock to sell the shares in increments over time to mitigate any adverse impact of the sales on the market price of Seneca’s stock. If our stockholders sell, or the market perceives that our stockholders intend to sell for various reasons, substantial amounts of our common stock in the public market, including shares issued upon the exercise of outstanding options or warrants, the market price of our common stock could fall. Sales of a substantial number of shares of our common stock may make it more difficult for us to sell equity or equity-related securities on terms that we deem reasonable or appropriate.
| 19 |
Certain of our outstanding common stock purchase warrants contain price protection provisions (anti-dilution protection) in the event that we sell securities at prices lower than the current exercise price of such warrants.
As of December 31, 2020, we had 149,149 common stock purchase warrants outstanding that were issued in our May 2016 registered offering, May 2016 private placement and August 2017 registered offering. All of such warrants contain price protection provisions in the event that we sell securities at a price per share below their respective exercise prices (collectively “Price Protection Warrants”). Pursuant to our May 2020 common stock offering, the Price Protection Warrants all had their exercise prices adjusted to $0.90 per share. In the event that Seneca sells securities at a price per share lower than the current exercise price of the Price Protection Warrants, their exercise prices will be further reduced. Any future adjustments to the exercise prices of the Price Protection Warrants may have a negative impact on the trading price of Seneca’s common stock. Additionally, raising additional capital with new investors may be difficult as a result of the adjustment feature.
Certain of our outstanding common stock purchase options contain provisions (anti-dilution protection) in the event that we issue additional securities, which may have a negative impact on its capital structure and may result in significant dilution to our shareholders or impair our ability to raise capital.
As of December 31, 2020, we had 1,686,466 outstanding common stock purchase options held by certain members of its senior management team. These options contain provisions which have resulted in the adjustment of the shares underlying such options in order that the holder maintains his proportionate ownership. Any future adjustments to the number of shares may have a negative impact on our capital structure and dilute our other shareholders. Additionally, raising additional capital with new investors may be difficult as a result of the adjustment feature. As of the date hereof, each of the option holders have agreed, subject to entering into definitive agreements, to cancel their respective outstanding options in exchange for certain cash payments.
Our anti-takeover provisions may delay or prevent a change of control, which could adversely affect the price of its common stock.
Our amended and restated certificate of incorporation and amended and restated bylaws contain provisions that may make it difficult to remove its board of directors and management and may discourage or delay “change of control” transactions, which could adversely affect the price of its common stock. These provisions include, among others:
| · | Our board of directors are divided into three classes, with each class serving for a staggered three-year term, which prevents stockholders from electing an entirely new board of directors at an annual meeting; |
| · | Advance notice procedures that stockholders must comply with in order to nominate candidates to our board of directors and propose matters to be brought before an annual meeting of our stockholders may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of Seneca; and |
| · | Our board of directors may, without stockholder approval, issue series of preferred stock, or rights to acquire preferred stock, that could dilute the interest of, or impair the voting power of, holders of our common stock or could also be used as a method of discouraging, delaying or preventing a change of control. |
If securities or industry analysts do not publish research reports, or publish unfavorable research about our business, the price and trading volume of our common stock could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us and our business. We currently have limited research coverage by securities and industry analysts. In the event an analyst downgrades our securities, the price of our securities would likely decline. If analysts cease to cover us or fail to publish regular reports, interest in our securities could decrease, which could cause the price of our common stock and other securities and their trading volume to decline.
| 20 |
Our board of directors has broad discretion to issue additional securities, which might dilute the net tangible book value per share of our common stock for existing stockholders.
We are entitled under our certificate of incorporation to issue up to 300,000,000 shares of common stock and 7,000,000 “blank check” shares of preferred stock. Shares of our blank check preferred stock provide our board of directors with broad authority to determine voting, dividend, conversion, and other rights. As of December 31, 2020, we had issued and outstanding 17,295,703 shares of common stock and 6,750,287 shares of common stock reserved for future grants under its equity compensation plans and for issuances upon the exercise or conversion of currently outstanding options, warrants and convertible securities. As of December 31, 2020, we had 200,000 shares of preferred stock issued and outstanding which are convertible into 38,873 shares of common stock. Accordingly, as of December 31, 2020, we are entitled to issue up to 275,954,010 additional shares of common stock and 6,800,000 additional shares of “blank check” preferred stock. Our board of directors may generally issue those common and preferred shares, or convertible securities to purchase those shares, without further approval by our shareholders. Any preferred shares we may issue will have such rights, preferences, privileges and restrictions as may be designated from time-to-time by our board, including preferential dividend rights, voting rights, conversion rights, redemption rights and liquidation provisions. It is likely that we will be required to issue a large amount of additional securities to raise capital in order to further its development and marketing plans. It is also likely that we will be required to issue a large amount of additional securities to directors, officers, employees and consultants as compensatory grants in connection with their services, both in the form of stand-alone grants or under our various stock plans. The issuance of additional securities may cause substantial dilution to our existing shareholders.
Unstable market and economic conditions may have serious adverse consequences on Seneca’s business, financial condition and stock price.
From time to time, including recently as a result of the COVID-19 pandemic, global credit and financial markets have experienced extreme volatility and disruptions, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability. Seneca’s general business strategy may be adversely affected by any such economic downturn, volatile business environment and continued unpredictable and unstable market conditions. If the equity and credit markets deteriorate it may make any necessary debt or equity financing more difficult to complete, more costly, and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on Seneca’s growth strategy, financial performance and stock price and could require Seneca to delay or abandon clinical development plans. In addition, there is a risk that one or more of Seneca’s current service providers, manufacturers and other partners may not survive an economic down-turn, which could directly affect Seneca’s ability to attain Seneca’s operating goals on schedule and on budget.
Risks Related to Government Regulation and Approval of Therapeutic Product Candidates.
The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time consuming and inherently unpredictable, and our products may not receive regulatory approval.
The time required to obtain approval by the FDA and comparable foreign authorities is inherently unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a drug candidate’s clinical development and may vary among jurisdictions and countries.
If we are successful in in-licensing or acquiring therapeutic drug candidates, we could fail to receive regulatory approval for many reasons, including the following:
| • | the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials; |
| • | we may be unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that a product candidate is safe and effective for its proposed indication; |
| • | the results of clinical trials may not meet the level of statistical significance required by the FDA or comparable foreign regulatory authorities for approval; |
| • | we may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| • | the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; |
| • | the data collected from clinical trials of our product candidates may not be sufficient to support the submission of a BLA, NDA or other submission or to obtain regulatory approval in the United States or elsewhere; |
| 21 |
| • | the FDA or comparable foreign regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; or |
| • | the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. |
We cannot assure you that we will successfully in-license or acquire any technologies or complete any clinical trials in connection with such technologies. Further, we cannot predict when we might first submit any product license application (NDA or BLA) for FDA approval or whether any such product license application will be granted on a timely basis, if at all. Any delay in obtaining, or failure to obtain, such approvals could have a material adverse effect on the marketing of our products and our ability to generate product revenue.
Development of therapeutics product candidates is subject to extensive government regulation.
The process of obtaining FDA and other necessary regulatory approvals is lengthy, expensive and uncertain. FDA and other legal and regulatory requirements applicable to our proposed products, both in the U.S. and in foreign countries could substantially change. We may fail to obtain the necessary approvals to commence clinical testing or to manufacture or market our potential products in reasonable time frames, if at all. In addition, the U.S. Congress and other legislative bodies may enact regulatory reforms or restrictions on the development of new therapies that could adversely affect the regulatory environment in which we operate or the development of any products we may develop.
Noncompliance with applicable regulatory requirements can subject us, our third party suppliers and manufacturers and our other collaborators to administrative and judicial sanctions, such as, among other things, warning letters, fines and other monetary payments, recall or seizure of products, criminal proceedings, suspension or withdrawal of regulatory approvals, interruption or cessation of clinical trials, total or partial suspension of production or distribution, injunctions, limitations on or the elimination of claims we can make for our products, refusal of the government to enter into supply contracts or fund research, or government delay in approving or refusal to approve new drug applications.
We cannot predict if or when we will be able to commercialize our products due to regulatory constraints.
Federal, state and local governments and agencies in the U.S. (including the FDA) and governments in other countries have significant regulations in place that govern many of our activities. We are, or may become, subject to various federal, state and local laws, regulations and recommendations relating to safe working conditions, laboratory and manufacturing practices, the experimental use of animals and the use and disposal of hazardous or potentially hazardous substances used in connection with its research and development work. The preclinical testing and clinical trials of our proposed products are subject to extensive government regulation that may prevent us from creating commercially viable products. In addition, our sale of any commercially viable product will be subject to government regulation from several standpoints, including manufacturing, advertising, marketing, promoting, selling, labeling and distributing. If, and to the extent that, we are unable to comply with these regulations, our ability to earn revenues, if any, will be materially and negatively impacted.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None
| ITEM 2. | PROPERTIES |
We currently operate one facility located in the United States and one facility located in China. Our corporate offices and primary research facilities are located in Germantown, Maryland, where we lease approximately 1,500 square feet. This lease provides for monthly payments of approximately $5,600 per month and expires on December 31, 2021.
We also lease approximately 11,300 square feet of research facility in the People’s Republic of China. This lease commenced in September 2019, provides for minimum lease payments of approximately $4,400 per month, expires in September 2024 and provides us with a future first right of refusal for extending the lease beyond its expiration.
| 22 |
| ITEM 3. | LEGAL PROCEEDINGS |
As of the date of this Annual Report, except as described below, there are no material pending legal or governmental proceedings relating to our company or properties to which we are a party, and to our knowledge there are no material proceedings to which any of our directors, executive officers or affiliates are a party adverse to us or which have a material interest adverse to us.
Nine complaints have been filed by purported Seneca stockholders, each of which seeks to enjoin the Merger and other relief.
On January 8, 2021, Joseph Sheridan, a purported Seneca stockholder, filed a complaint in the United States District Court for the Southern District of New York against Seneca, the members of its board of directors, and LBS, captioned Sheridan v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00166 (the “Sheridan Complaint”).
Also, on January 8, 2021, Hesam Pirjamaat, a purported Seneca stockholder, filed a complaint in the United States District Court for the Southern District of New York against Seneca, the members of its board of directors, Townsgate Acquisition Sub 1, Inc., and LBS, captioned Pirjamaat v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00172 (the “Pirjamaat Complaint”).
On January 13, 2021, Brian Johnson, a purported Seneca stockholder, filed a complaint in the United States District Court for the Southern District of New York against Seneca and the members of its board of directors, captioned Johnson v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00310 (the “Johnson Complaint”).
On January 15, 2021, Vipin Mathews, a purported Seneca stockholder, filed a complaint in the United States District Court for the Eastern District of New York against Seneca and the members of its board of directors, captioned Mathews v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00242 (the “Mathews Complaint”).
On January 22, 2021, Emily Pechal, a purported Seneca stockholder, filed a complaint in the United States District Court for the Southern District of New York against Seneca and the members of its board of directors, captioned Pechal v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00585 (the “Pechal Complaint”).
On February 25, 2021, Marcie Curtis, a purported Seneca stockholder, filed a complaint in the United States District Court for the District of Delaware against Seneca and the members of its board of directors, captioned Curtis v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00292 (the “Curtis Complaint”).
On March 1, 2021, Juanesha Valdez, a purported Seneca stockholder, filed a complaint in the United States District Court for the Eastern District of Pennsylvania against Seneca, the members of its board of directors, Townsgate Acquisition Sub 1, Inc., and LBS, captioned Valdez v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00980 (the “Valdez Complaint”).
On March 2, 2021, Bryan Anderson, a purported Seneca stockholder, filed a complaint in the United States District Court for the District of Delaware against Seneca and the members of its board of directors, captioned Anderson v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00326 (the “Anderson Complaint”).
On March 3, 2021, Jack McIntire, a purported Seneca stockholder, filed a complaint in the United States District Court for the Southern District of New York against Seneca and the members of its board of directors, captioned McIntire v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-01869 (the “McIntire Complaint,” and, together with the Sheridan Complaint, the Pirjamaat Complaint, the Johnson Complaint, the Mathews Complaint, the Pechal Complaint, the Curtis Complaint, the Valdez Complaint, the Anderson Complaint, the “Stockholder Complaints”).
On February 26, 2021, the United States District Court for the Southern District of New York entered an order consolidating the Sheridan Complaint, the Pirjamaat Complaint, the Johnson Complaint, and the Pechal Complaint under Case No. 21-cv-0166.
The Stockholder Complaints assert claims against Seneca, the members of the Seneca Board, and LBS as defendants under Section 14(a) of the Exchange Act and Rule 14a-9 promulgated thereunder for allegedly false and misleading statements in this proxy statement/prospectus/information statement and Section 20(a) of the Exchange Act for alleged “control person” liability with respect to such allegedly false and misleading statements. The Johnson Complaint also asserts that the members of the Seneca Board breached their fiduciary duties of candor/disclosure in connection with the Merger by purportedly failing to disclose material information about the Merger.
Each of the Stockholder Complaints seek, among other relief, injunctive relief, including enjoining the Merger unless and until the defendants disclose the allegedly omitted material information, as well as an award of attorneys’ and experts’ fees. The Mathews Complaint also seeks to enjoin any vote on the Merger; the Sheridan Complaint, the Johnson Complaint, and the McIntire Complaint seek damages; the Sheridan Complaint, the Pirjamaat Complaint, the Mathews Complaint, the Curtis Complaint, the Valdez Complaint, and the Anderson Complaint, seek, in the event the defendants consummate the merger, rescission of the Merger or an award of rescissory damages; the Pirjamaat Complaint, the Curtis Complaint, and the Valdez Complaint seek an order directing the Seneca Board to disseminate a revised registration statement in compliance with Sections 14(a) and/or 20(a) of the Exchange Act and Rule 14a-9; and the Pirjamaat Complaint, the Mathews Complaint, the Curtis Complaint, the Valdez Complaint, and the Anderson Complaint seek a declaration that defendants violated Sections 14(a) and/or 20(a) of the Exchange Act and Rule 14a-9.
Seneca believe the allegations in the Complaints are without merit.
| ITEM 4. | MINE SAFETY DISCLOSURE |
Not Applicable
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock is traded on The Nasdaq Capital Market under the symbol "SNCA."
| 23 |
Holders
As of February 28, 2021, our common stock was held by approximately 195 record holders. Because many of our shares of common stock are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of stockholders represented by these holders.
Dividends
We have not paid any cash dividends to date and have no plans to do so in the immediate future. Additionally, we are prohibited from paying any cash dividends under the terms of certain agreements to which we are a party.
Equity Compensation Plan Information
See information contained in Part III, Item 12 of this Annual Report filed on Form 10-K.
Equity Compensation Plans Not Approved by Security Holders
See information contained in Part III, Item 12 of this this Annual Report filed on Form 10-K.
Recent Sales or Issuances of Unregistered Securities
The following information is given with regard to unregistered securities sold during the period covered by this report. The following securities were issued in private offerings pursuant to the exemption from registration contained in the Securities Act and the rules promulgated thereunder in reliance on Section 4(2) thereof, relating to offers of securities by an issuer not involving any public offering:
| • | On January 17, 2020, we issued an aggregate 2,777,777 Series P warrants and 2,777,777 Series Q warrants. The warrants were issued as an inducement for holders to exercise the Company’s Series M and N warrants for cash. As a result of the inducement, we received gross proceeds of approximately $7,555,553, not including closing costs and placement agent fees. The Series P Warrants have substantially the same terms as the Series M Warrants (except for provisions customary for an unregistered warrant, including a restricted legend) (i) a term of two (2) years from the date of issuance, and (ii) an exercise price per share of $1.23. The Series Q Warrants have substantially the same terms as the Series N Warrants (except for provisions customary for an unregistered warrant, including a restricted legend) (i) have a term of five (5) years from the date of issuance, and (iv) an exercise price per share of $1.23. |
In connection with the transactions we issued H.C. Wainwright & Co., LLC a common stock purchase warrant to purchase 44,444 shares of common stock. The warrants are substantially similar to the Series Q warrants but have an exercise price of $1.70 per share.
| • | In April 2020, in connection with Dane Saglio’s employment as Chief Financial Officer, we granted an inducement option from the Company’s Inducement Award Stock Option Plan to purchase 70,710 shares of common stock. The Inducement Option has an exercise price of $0.6199 per share, a term of ten (10) years, and vests as follows: (a) one quarter (1/4) of the options vest on the Effective Date, and (ii) the remaining three-quarters (3/4) of the options will vest on a monthly basis over the thirty-six (36) month period following the Effective Date. For a period of nine (9) months, subject to adjustment upon the Company’s issuance of common stock including by virtue of exercise, conversion or exchange of common stock equivalents, the shares underlying the options are subject to adjustment to maintain the percentage ownership that the option grant reflects on the date of grant. This resulted in the grant being increased to 129,745 shares of common stock through December 31, 2020. |
| • | In April 2020, in connection with Matthew Kalnik, PhD’s employment as President and Chief Operating Officer, we granted an inducement option from the Company’s Inducement Award Stock Option Plan to purchase 282,840 shares of common stock. The Inducement Option has an exercise price of $0.6199 per share, a term of ten (10) years, and vests as follows: (it) one quarter (1/4) of the options vest on the Effective Date, and (ii) the remaining three-quarters (3/4) of the options will vest on a monthly basis over the thirty-six (36) month period following the Effective Date. For a period of nine (9) months, subject to adjustment upon the Company’s issuance of common stock including by virtue of exercise, conversion or exchange of common stock equivalents, the shares underlying the options are subject to adjustment to maintain the percentage ownership that the option grant reflects on the date of grant. This resulted in the grant being increased to 518,979 shares of common stock through December 31, 2020. |
| 24 |
| • | Effective April 2020, Dr. Carter, our Executive Chairman, received a conditional option grant to purchase 471,400 shares of common stock, subject to the receipt of shareholder approval as well as the forfeiture of all of his previously issued vested and unvested grants. The option grant has a term of ten (10) years, and an exercise price of $0.6199. The option vests (i) one quarter (1/4) on the effective date and (ii) three quarters (3/4) on a monthly basis over the thirty-six (36) month period following the effective date, provided Dr. Carter remains a service provider to the Company over such period. For a period of nine (9) months, subject to adjustment upon the Company’s issuance of common stock including by virtue of exercise, conversion or exchange of common stock equivalents, the shares underlying the options are subject to adjustment to maintain the percentage ownership that the option grant reflects on the date of grant. This resulted in the grant being increased to 864,785 shares of common stock through December 31, 2020. This grant was approved by shareholders on September 9, 2020. |
| • | Effective April 2020, our Senior Vice President of Research and Development received a conditional option grant to purchase 94,280 shares of common stock, subject to the receipt of shareholder approval as well as the forfeiture of all of his previously issued vested and unvested grants. The option grant has a term of ten (10) years, and an exercise price of $0.6199. The option vests (i) one quarter (1/4) on the effective date and (ii) three quarters (3/4) on a monthly basis over the thirty-six (36) month period following the effective date, provided that such individual remains a service provider to the Company over such period. For a period of nine (9) months, subject to adjustment upon the Company’s issuance of common stock including by virtue of exercise, conversion or exchange of common stock equivalents, the shares underlying the options are subject to adjustment to maintain the percentage ownership that the option grant reflects on the date of grant. This resulted in the grant being increased to 172,957 shares of common stock through December 31, 2020. This grant was approved by shareholders on September 9, 2020. |
| • | On April 3, 2020, we issued an aggregate of 24,000 restricted stock units (6,000 to each of our four current directors) as partial compensation for their service on the board of directors. |
| • | In May 2020, in connection with the registered offering of our common stock, we issued our placement agent, H.C. Wainwright & Co., LLC a common stock purchase warrant to purchase 400,000 shares of common stock. The warrants have an exercise price of $1.25 per share and a term of five (5) years from issuance. |
| ITEM 6. | SELECTED FINANCIAL DATA |
Not Applicable.
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Our Management’s Discussion and Analysis of Financial Condition and Results of Operations or MD&A, is provided in addition to the accompanying consolidated financial statements and notes to assist readers in understanding our results of operations, financial condition and cash flows. Our MD&A is organized as follows:
| · | Executive Overview — Overview discussion of our business in order to provide context for the remainder of MD&A. |
| · | Trends & Outlook — Discussion of what we view as the overall trends affecting our business and the strategy for 2021. |
| · | Critical Accounting Policies— Accounting policies that we believe are important to understanding the assumptions and judgments incorporated in our reported financial results and forecasts. |
| · | Results of Operations— Analysis of our financial results comparing the: (i) year ended December 31, 2020 to the year ended December 31, 2019. |
| · | Liquidity and Capital Resources—Analysis of cash flows and discussion of our financial condition and future liquidity needs. |
Executive Overview
Historically, we have been primarily focused on the research and development of nervous system therapies based on our proprietary human neural stem cells and our small molecule compounds with the ultimate goal of gaining approval from the United States Food and Drug Administration (“FDA”), and its international counterparts, to market and commercialize such therapies. In early 2019, we also began an in-licensing and acquisition strategy by which we are evaluating novel therapeutics with the potential to be complimentary to our current technologies or that could benefit from our development experience with the goal of developing such technologies for commercialization.
| 25 |
Our patented technology platform has three core components:
| 1. | Over 300 lines of human, regionally specific neural stem cells, some of which have the potential to be used to treat serious or life-threatening diseases through direct transplantation into the central nervous system; |
| 2. | Proprietary screening capability – our ability to generate human neural stem cell lines provides a platform for chemical screening and discovery of novel compounds against nervous system disorders; and |
| 3. | Small molecules that resulted from Seneca’s neurogenesis screening platform that may have the potential to treat wide variety of nervous system conditions. |
To date, our technology platform has produced two lead assets in clinical development: our NSI-566 stem cell therapy program and our NSI-189 small molecule program. A component of our current strategy is out-licensing and we have recently initiated a formal out- licensing initiative aimed at securing partners to advance the clinical development of these two programs.
We believe this technology, in partnership with an established biopharmaceutical company with the appropriate development expertise and financial resources, could facilitate the development and commercialization of products for use in the treatment of a wide array of nervous system disorders including neurodegenerative conditions and regenerative repair of acute and chronic disease. We intend to maintain these programs with the goal of finding suitable development partners.
We are also seeking to in-license and acquire other novel therapeutics. On October 31, 2019, the Company announced it had entered into a non-binding term sheet with Jiangsu QYuns Therapeutics Co., Ltd., (“QYuns”) for an exclusive license agreement for certain of QYuns Therapeutics’ assets, a pipeline of cytokine-targeted monoclonal antibodies for the treatment of a range of auto-immune disease. Subsequently, on January 10, 2020, the Company filed a form 8-K disclosing that it was not able to reach an agreement on the exclusive license agreement and no longer expected to complete this transaction. However, we continue to seek other products to in-license or acquire.
Trends & Outlook
Revenue
We generated no revenues from the sale of our proposed therapies for any of the periods presented.
We have historically generated minimal revenue from the licensing of our intellectual property to third parties as well as payments under a settlement agreement.
On a long-term basis, we anticipate that our revenue will be derived primarily from licensing fees and sales of our products. Because we are at such an early stage in the clinical trials process, we are not yet able to accurately predict when we will have a product ready for commercialization, if ever.
Research and Development Expenses
Our research and development expenses consist primarily of clinical trial expenses, including payments to clinical trial sites that perform our clinical trials and clinical research organizations (CROs) that help us manage our clinical trials, manufacturing of small molecule drugs and stem cells for both human clinical trials and for pre-clinical studies and research, personnel costs for research and clinical personnel, and other costs including research supplies and facilities. Our research and development expenses reflect the costs of the technical evaluation of our internal programs as well as the evaluation of certain potential assets we considered for acquisition.
We focus on the development of therapies with potential uses in multiple indications and use employee and infrastructure resources across several projects. Accordingly, many of our costs are not attributable to a specifically identified product and we do not account for internal research and development costs on a project-by-project basis.
We expect that research and development expenses, which include expenses related to our ongoing ischemic stroke clinical trial, will decrease in the future as we seek partners to further the clinical development of our therapeutic programs. This could change if we are successful in our in-licensing and acquisition strategy in which we are evaluating novel therapeutics, our research and development expenditures will be primarily devoted to advancing the acquired programs towards or through later stage clinical trials.
We have a wholly owned subsidiary in the People’s Republic of China that primarily oversees our current clinical trial to treat motor deficits due to ischemic stroke.
| 26 |
In August 2017, we were awarded a Small Business Innovation Research (“SBIR”) grant by the National Institutes of Health (“NIH”) to evaluate in preclinical studies the potential of NSI-189, a novel small molecule compound, for the prevention and treatment of diabetic neuropathy. The award of approximately $1 million will be paid over a two-year period, if certain conditions are met as mid-term. The award performance period was extended through July 31, 2020 to complete the data collection and report writing. In June 2018, we were awarded a Department of Defense grant related to our efforts involving stem cell therapy for severe traumatic brain injury. The award of approximately $150,000 was received in 2019. The proceeds from the awards are recorded as a reduction of our gross research and development expenses, based on the terms and conditions of the grants.
Proposed Merger.
On December 17, 2020, we announced the signing of the Merger Agreement with LBS. Upon the terms and subject to the satisfaction of the conditions described in the Merger Agreement, including approval of the transaction by our stockholders, a wholly-owned subsidiary of Seneca will consummate the Merger. Upon the closing of the Merger, we will adopt the business and operating plan of LBS. In the event the Merger is not consummated, our Board will be required to develop a new business plan. At this time, we cannot ascertain such plan or the financial impact on Seneca.
General and Administrative Expenses
General and administrative expenses are primarily comprised of salaries, benefits and other costs associated with our operations including, finance, human resources, information technology, public relations and costs associated with maintaining a public company listing, legal, audit and compliance fees, facilities and other external general and administrative services.
Critical Accounting Policies
Our consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The preparation of these financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses. Note 2 of the Notes to Consolidated Financial Statements included elsewhere herein describes the significant accounting policies used in the preparation of the financial statements. Certain of these significant accounting policies are considered to be critical accounting policies, as defined below.
A critical accounting policy is defined as one that is both material to the presentation of our financial statements and requires management to make difficult, subjective or complex judgments that could have a material effect on our financial condition and results of operations. Specifically, critical accounting estimates have the following attributes: (1) we are required to make assumptions about matters that are highly uncertain at the time of the estimate; and (2) different estimates we could reasonably have used, or changes in the estimate that are reasonably likely to occur, would have a material effect on our financial condition or results of operations.
Estimates and assumptions about future events and their effects cannot be determined with certainty. We base our estimates on historical experience and on various other assumptions believed to be applicable and reasonable under the circumstances. These estimates may change as new events occur, as additional information is obtained and as our operating environment changes. These changes have historically been minor and have been included in the financial statements as soon as they became known. Based on a critical assessment of our accounting policies and the underlying judgments and uncertainties affecting the application of those policies, management believes that our financial statements are fairly stated in accordance with U.S. GAAP, and present a meaningful presentation of our financial condition and results of operations. We believe the following critical accounting policies reflect our more significant estimates and assumptions used in the preparation of our consolidated financial statements:
Use of Estimates - Our financial statements prepared in accordance with U.S. GAAP require us to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Specifically, we have estimated the expected economic life and value of our patent technology, our net operating loss carryforward and related valuation allowance for tax purposes the fair value of our liability classified warrants and our share-based compensation expenses related to employees, directors, consultants and investment banks. Actual results could differ from those estimates.
Long Lived Intangible Assets - Our long-lived intangible assets consist of our intellectual property patents including primarily legal fees associated with the filings and in defense of our patents. The assets are amortized on a straight-line basis over the expected useful life which we define as ending on the expiration of the patent group. These assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. We assess this recoverability by comparing the carrying amount of the asset to the estimated undiscounted future cash flows to be generated by the asset. If an asset is deemed to be impaired, we estimate the impairment loss by determining the excess of the asset’s carrying amount over the estimated fair value. These determinations use assumptions that are highly subjective and include a high degree of uncertainty. During the years ended December 31, 2020 and 2019, no significant impairment losses were recognized.
| 27 |
Fair Value Measurements - The fair value of our short-term financial instruments, which primarily include cash and cash equivalents, other short-term investments, accounts payable and accrued expenses, approximate their carrying values due to their short maturities. The fair value of our long-term indebtedness was estimated based on the quoted prices for the same or similar issues or on the current rates offered to the Company for debt of the same remaining maturities which approximates the carrying value. The fair values of our liability classified warrants are estimated using Level 3 unobservable inputs.
Share-Based Compensation - We account for share-based compensation at fair value; accordingly, we expense the estimated fair value of share-based awards over the requisite service period. Share-based compensation cost for stock options and warrants issued to employees, board members and non-employee consultants is generally determined at the grant date using an option pricing model. Option pricing models require us to make assumptions, including expected volatility and expected term of the options. If any of the assumptions we use in the model were to significantly change, share-based compensation expense may be materially different. Share-based compensation cost for restricted stock and restricted stock units issued to employees and board members is determined at the grant date based on the closing price of our common stock on that date. The value of the award that is ultimately expected to vest is recognized as expense on a straight-line basis over the requisite service period.
Comparison of Our Results of Operations for the Years Ended December 31, 2020 and 2019
Revenue
During each of the years ended December 31, 2020 and 2019, we recognized revenue of $10,000 related to ongoing fees pursuant to certain licenses of our intellectual property to third parties. In addition, during the years ended December 31, 2020 and 2019, we recognized $3,500 and $5,400 of royalty revenue related to a settlement of a prior patent infringement case.
Operating Expenses
5Operating expenses for 2020 and 2019 were as follows:
| Year Ended December 31, | Increase (Decrease) | |||||||||||||
| 2020 | 2019 | $ | % | |||||||||||
| Operating Expenses | ||||||||||||||
| Research & development costs | $ | 2,018,454 | $ | 4,061,450 | $ | (2,042,996 | ) | (50)% | ||||||
| General & administrative expenses | 8,670,612 | 4,585,638 | 4,084,974 | 89% | ||||||||||
| Total operating expense | $ | 10,689,066 | $ | 8,647,088 | $ | 2,041,978 | 24% | |||||||
Research and Development Expenses
The decrease of approximately $2,043,000 or 50% in research and development expenses was primarily attributable to the continued wind down of clinical activities for our stem cell and small molecule programs in 2020. In 2019, we incurred expenses related to external consulting services engaged in the technical evaluation of our internal programs as well as the evaluation of certain potential assets we considered for acquisition. If we are successful in the Merger, we will adopt the business and operating plan of LBS.
General and Administrative Expenses
G&A expenses increased approximately $4,085,000 or 89%. As noted above, we have shifted the Company’s strategy and focus from the development of the stem cell assets and initiated an out-licensing effort to partner these programs while seeking to in license or acquire novel therapeutics with the potential to be complimentary to our current technologies or that could benefit from our development experience with the goal of developing such technologies for commercialization. Associated with this shift in strategic focus our G&A expenses in the 2020 period reflect an enhanced internal management structure including individual consultants in key roles as well as the engagement of two executive officers in the second quarter of 2020.
Other income (expense)
Other expense, net in 2020 consisted primarily of a non-cash warrant inducement charge of approximately $5,620,000 partially offset by $20,000 of net interest income and $9,000 of non-cash gains related to the fair value adjustment of our liability classified warrants.
Other income, net in 2019 consisted of approximately $499,000 of non-cash gains related to the change in the fair value of our liability classified stock purchase warrants, $86,000 of sublease income and $68,000 of interest income partially offset by a $368,000 loss related to the write-off of a related party receivable.
| 28 |
Liquidity and Capital Resources
Since our inception, we have financed our operations through the sales of our securities, issuance of long-term debt, the exercise of investor warrants, and to a lesser degree from grants and research contracts as well as the licensing of our intellectual property to third parties.
We had cash and cash equivalents of approximately $10.5 million at December 31, 2021. In January 2020, we raised approximately $6.7 million of net proceeds from the exercise of certain common stock purchase warrants pursuant to an inducement offer and in May 2020, we raised approximately $4.4 million of net proceeds through the sale of our common stock as well as approximately $3.5 million from the exercise of warrants issued in the January inducement offer.
Based on our expected operating cash requirements, and assuming the Merger is not consummated, we anticipate our current cash and investments on hand will be sufficient to fund our operations, at least 12 months after this filing. However, we will require additional capital to execute our acquisition and/or in-licensing strategy as well as out-licensing initiatives and to fund our operations. Despite our ability to secure capital in the past, there can be no assurance that additional equity or debt financing will be available to us when needed or that we may be able to secure funding from any other sources. Consequently, as explained in Note 1 to our condensed consolidated financial statements, management has determined that there is substantial doubt about our ability to continue as a going concern.
We will require additional capital to pursue our acquisition and in-licensing strategy and continue our pre-clinical and clinical development plans. To continue to fund our operations and the development of our product candidates we anticipate raising additional cash through the private and public sales of equity or debt securities, collaborative arrangements, licensing agreements, asset sales or a combination thereof. Although management believes that such funding sources will be available, there can be no assurance that any such collaborative arrangement will be entered into or that financing will be available to us when needed in order to allow us to continue our operations, or if available, on terms acceptable to us. If we do not raise sufficient funds in a timely manner, we may be forced to curtail operations, delay or stop our ongoing clinical trials, cease operations altogether, or file for bankruptcy. We currently do not have commitments for future funding from any source. We cannot assure you that we will be able to secure additional capital or that the expected income will materialize. Several factors will affect our ability to raise additional funding, including, but not limited to market conditions, interest rates and, more specifically, our progress in our exploratory, preclinical and future clinical development programs.
Cash Flows – 2020 compared to 2019
| Year Ended December 31, | Increase (Decrease) | |||||||||||||
| 2020 | 2019 | $ | % | |||||||||||
| Net cash used in operating activities | $ | (9,025,129 | ) | $ | (7,255,680 | ) | $ | (1,769,449 | ) | 24% | ||||
| Net cash provided by investing | $ | - | $ | - | $ | - | - | |||||||
| Net cash provided by financing activities | $ | 14,465,916 | $ | 6,589,505 | $ | 7,876,411 | 120% | |||||||
Net Cash Used in Operating Activities
Cash used in operating activities for the year ended December 31, 2020, reflects our $16,267,000 loss for the period adjusted for certain non-cash items including: (a) $5,620,000 of expense related to our warrant inducement transaction, (ii) $954,000 of net cash inflows, including those resulting from increases in our prepaid expenses and accrued severance, related to changes in operating assets and liabilities, and (iii) $585,000 of share-based compensation.
Cash used in operating activities for the year ended December 31, 2019, of approximately $7,256,000 reflects our $8,352,000 loss for the period adjusted for certain non-cash items including: (i) $881,000 of share-based compensation, (ii) a ($499,000) gain related to the change in fair value of our liability classified warrants, (iii) $362,000 of write-off of related party receivable, (iv) $208,000 of net cash inflows related to changes in operating assets and liabilities and (v) $144,000 adjustment for amortization and depreciation.
Net Cash Used in Investing Activities
There were no investing activities in either of the years ended December 31, 2020 or 2019.
Net Cash Provided by Financing Activities
For the year ended December 31, 2020, cash provided by financing activities consisted of $11.2 million of net proceeds generated from the sale of our common stock and $3.5 million of net proceeds from the exercise of warrants partially offset by payments under our short-term debt used to finance insurance premiums.
For the year ended December 31, 2019, cash provided by financing activities consisted primarily of $6.6 million of net proceeds generated from the sale of our common stock and warrants coupled with borrowings and payments under our short-term debt used to finance insurance premiums.
Future Liquidity and Needs
We have incurred significant operating losses and negative cash flows since inception. We have not been able to generate significant revenues nor achieved profitability and may not be able to do so in the future. We do not expect to be profitable in the next several years, but rather expect to incur additional operating losses. We have limited liquidity and capital resources and must obtain significant additional capital resources in order to sustain our product development efforts, for acquisition of technologies and intellectual property rights, for preclinical and clinical testing of our anticipated products, pursuit of regulatory approvals, acquisition of capital equipment, laboratory and office facilities, establishment of production capabilities, for general and administrative expenses and other working capital requirements. We have relied on cash balances and the proceeds from the offering of our securities, exercise of outstanding warrants and grants to fund our operations.
| 29 |
We intend to pursue opportunities to obtain additional funds through the out-license or sale of our existing clinical programs in addition to financing in the future through the sale of our securities and additional research grants. On September 23, 2020, our shelf registration statement (Registration No. 333-248848), which replaced our prior expiring shelf registration statement, was declared effective by the SEC. Under such replacement shelf registration statement, we can offer and sell up to $100 million of our securities. Through September 30, 2020 we have not sold any securities under this registration statement. Based on our current market capitalization, we are limited to the use of our shelf registration statement by Item I.B.6 of Form S-3.
In July 2019, we completed a firm commitment underwritten public offering of our securities. The offering resulted in net proceeds of approximately $6.6 million, after deducting underwriting discounts and commissions and offering expenses. The securities in this offering were sold pursuant to a registration statement on Form S-1 (file no. 333- 232273).
In January 2020, pursuant to the terms of an inducement offer, certain holders of 5,555,554 of our common stock purchase warrants exercised their warrants at an exercise price of $1.36 per share generating approximately $6.7 million of net proceeds.
In May 2020, we completed an offering 5,000,000 shares of our common stock. The offering resulted in net proceeds of approximately $4.4 million, after deducting placement agent discounts and commissions and offering expenses. The common stock was offered and sold pursuant to our shelf registration statement on Form S-3 (file no. 333-218608).
In May 2020, we received approximately $3.5 million from the exercise of 2,871,296 outstanding common stock warrants at an exercise price of $1.23 per share.
As explained in the notes to our condensed consolidated financial statements, there continues to be substantial doubt as to our ability to continue as a going concern. The source, timing and availability of any future financing will depend principally upon market conditions, interest rates and, more specifically, current and future progress in our exploratory, preclinical and clinical development programs. Funding may not be available when needed, at all, or on terms acceptable to us. Lack of necessary funds may require us, among other things, to delay, scale back or eliminate some or all of our research and product development programs, planned clinical trials, and/or our capital expenditures or to license our potential products or technologies to third parties.
Off-balance Sheet Arrangements
None.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK |
Not Applicable.
| 30 |
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| 31 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders of Seneca Biopharma, Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Seneca Biopharma, Inc. (the “Company”) as of December 31, 2020 and 2019, and the related consolidated statements of operations and comprehensive loss, changes in stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2020, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2020, in conformity with U.S. generally accepted accounting principles.
Substantial Doubt about the Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has suffered recurring losses from operations and has accumulated deficit that raises substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1 to the consolidated financial statements. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter(s) below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which it relates.
| 32 |
Accounting for Warrant Inducement
As discussed in Note 4 to the consolidated financial statements, the Company issued replacement warrants as an inducement for warrant exercises. In January 2020, pursuant to the terms of an inducement offer, certain holders of 5,555,554 of the Company’s common stock purchase warrants exercised such warrants at a reduced exercise price of $1.36 per share generating approximately $7.6 million of gross proceeds. The warrants were evaluated for proper classification on the balance sheet and it was determined that the replacement warrants issued in the inducement offer should be classified within stockholders’ equity. The Company incurred expense in the consolidated statement of operations and comprehensive loss for the year ended December 31, 2020 of approximately $5.6 million representing the fair value of the inducement offer. The fair value is comprised of the fair value of the modification of the original warrants (the reduction in exercise price) and the fair value of the replacement warrants. The fair values were calculated using the Black-Scholes option pricing model.
We identified the accounting for the warrant inducement transaction as a critical audit matter. The principal considerations for our determination included the significant auditor judgments required to evaluate the accounting treatment of the replacement warrants, including the modification treatment and classification of the warrants.
The primary procedures we performed to address this critical audit matter included:
| · | We evaluated the appropriateness of the Company’s methodology to assess the accounting treatment associated with the warrant inducement. |
| · | We read the agreements related to the replacement warrants issued and evaluated the completeness and accuracy of management’s technical accounting analyses and application of the relevant account guidance. |
| · | We utilized subject matter experts in debt and equity accounting to assist in the evaluation of the appropriateness of management’s interpretation and application of relevant accounting guidance. |
/s/ Dixon Hughes Goodman LLP
We have served as the Company’s auditor since 2016.
Raleigh, North Carolina
March 22, 2021
| 33 |
Seneca Biopharma, Inc.
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash and cash equivalents | $ | 10,529,244 | $ | 5,114,917 | ||||
| Trade and other receivables | 116,279 | 21,064 | ||||||
| Prepaid expenses | 1,399,790 | 510,900 | ||||||
| Assets held for sale | 835,483 | - | ||||||
| Total current assets | 12,880,796 | 5,646,881 | ||||||
| Property and equipment, net | 10,776 | 41,036 | ||||||
| Patents, net | 147,133 | 668,936 | ||||||
| ROU and other assets | 10,439 | 227,036 | ||||||
| Total assets | $ | 13,049,144 | $ | 6,583,889 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable and accrued expenses | $ | 649,345 | $ | 824,406 | ||||
| Accrued severance and bonuses | 2,322,241 | 135,686 | ||||||
| Short-term notes and other current liabilities | - | 264,665 | ||||||
| Liabilities associated with assets held for sale | 234,344 | - | ||||||
| Total current liabilities | 3,205,930 | 1,224,757 | ||||||
| Warrant liabilities, at fair value | 75,298 | 84,596 | ||||||
| Lease liability, net of current portion | - | 148,543 | ||||||
| Total liabilities | 3,281,228 | 1,457,896 | ||||||
| Commitments and contingencies (Note 8) | ||||||||
| STOCKHOLDERS' EQUITY | ||||||||
| Preferred stock, 7,000,000 shares authorized, $0.01 par value; 200,000 shares issued and outstanding in 2020 and 2019 | 2,000 | 2,000 | ||||||
| Common stock, $0.01 par value; 300 million shares authorized, 17,295,703 and 3,866,457 shares issued and outstanding in 2020 and 2019, respectively | 172,957 | 38,665 | ||||||
| Additional paid-in capital | 247,836,057 | 227,067,058 | ||||||
| Accumulated other comprehensive loss | (734 | ) | (6,186 | ) | ||||
| Accumulated deficit | (238,242,364 | ) | (221,975,544 | ) | ||||
| Total stockholders' equity | 9,767,916 | 5,125,993 | ||||||
| Total liabilities and stockholders' equity | $ | 13,049,144 | $ | 6,583,889 | ||||
See accompanying notes to consolidated financial statements.
| 34 |
Seneca Biopharma, Inc.
Consolidated Statements of Operations and Comprehensive Loss
| Year Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Revenues | $ | 13,520 | $ | 15,394 | ||||
| Operating expenses: | ||||||||
| Research and development costs | 2,018,454 | 4,061,450 | ||||||
| General and administrative expenses | 8,670,612 | 4,585,638 | ||||||
| Total operating expenses | 10,689,066 | 8,647,088 | ||||||
| Operating loss | (10,675,546 | ) | (8,631,694 | ) | ||||
| Other income (expense): | ||||||||
| Interest income | 33,532 | 67,731 | ||||||
| Interest expense | (14,015 | ) | (8,920 | ) | ||||
| Gain from change in fair value of liability classified warrants | 9,298 | 499,138 | ||||||
| Warrant inducement and other expense | (5,620,089 | ) | (277,906 | ) | ||||
| Total other income (expense) | (5,591,274 | ) | 280,043 | |||||
| Net loss | $ | (16,266,820 | ) | $ | (8,351,651 | ) | ||
| Net loss per common share - basic and diluted | $ | (1.17 | ) | $ | (3.80 | ) | ||
| Weighted average common shares outstanding - basic and diluted | 13,869,272 | 2,197,434 | ||||||
| Comprehensive loss: | ||||||||
| Net loss | $ | (16,266,820 | ) | $ | (8,351,651 | ) | ||
| Foreign currency translation adjustment | 5,452 | (5,773 | ) | |||||
| Comprehensive loss | $ | (16,261,368 | ) | $ | (8,357,424 | ) | ||
See accompanying notes to consolidated financial statements.
| 35 |
Seneca Biopharma, Inc.
Consolidated Statements of Changes In Stockholders' Equity
| Preferred Stock Shares | Preferred Stock Amount | Common Stock Shares | Common Stock Amount | Additional Paid-In Capital | Accumulated Other Comprehensive Income (Loss) | Accumulated Deficit | Total Stockholders' Equity | |||||||||||||||||||||||||
| Balance at January 1, 2019 | 1,000,000 | $ | 10,000 | 910,253 | $ | 9,103 | $ | 219,654,753 | $ | (413 | ) | $ | (213,623,893 | ) | $ | 6,049,550 | ||||||||||||||||
| Share rounding adjustment related to 1:20 reverse stock split | - | - | 6,117 | 61 | (61 | ) | - | - | - | |||||||||||||||||||||||
| Share-based payments | - | - | - | - | 880,789 | - | - | 880,789 | ||||||||||||||||||||||||
| Issuance of common stock and warrants from capital raises, net | - | - | 416,315 | 4,163 | 6,548,679 | - | - | 6,552,842 | ||||||||||||||||||||||||
| Issuance of common stock for conversion of Series A Preferred Stock | (800,000 | ) | (8,000 | ) | 155,496 | 1,555 | 6,445 | - | - | - | ||||||||||||||||||||||
| Issuance of restricted stock awards | - | - | 15,688 | 157 | (157 | ) | - | - | - | |||||||||||||||||||||||
| Issuance of common stock for warrant exercises | - | - | 2,361,462 | 23,615 | (23,379 | ) | - | - | 236 | |||||||||||||||||||||||
| Issuance of common stock for RSU exercises | - | - | 1,126 | 11 | (11 | ) | - | - | - | |||||||||||||||||||||||
| Foreign currency translation adjustments | - | - | - | - | - | (5,773 | ) | - | (5,773 | ) | ||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | (8,351,651 | ) | (8,351,651 | ) | ||||||||||||||||||||||
| Balance at December 31, 2019 | 200,000 | 2,000 | 3,866,457 | 38,665 | 227,067,058 | (6,186 | ) | (221,975,544 | ) | 5,125,993 | ||||||||||||||||||||||
| Share-based payments | - | - | - | - | 584,991 | - | - | 584,991 | ||||||||||||||||||||||||
| Issuance of commn stock and inducement warrants for warrant exercises | - | - | 5,561,554 | 55,615 | 12,296,637 | - | - | 12,352,252 | ||||||||||||||||||||||||
| Issaunce of common stock and warrants from capital raises, net | - | - | 5,000,000 | 50,000 | 4,384,354 | - | - | 4,434,354 | ||||||||||||||||||||||||
| Issuance of common stock for warrant exercises | - | - | 2,871,296 | 28,713 | 3,502,981 | - | - | 3,531,694 | ||||||||||||||||||||||||
| Issuance of common stock for RSU exercises | - | - | 563 | 6 | (6 | ) | - | - | - | |||||||||||||||||||||||
| Forfeiure of restricted stock awards | - | - | (4,167 | ) | (42 | ) | 42 | - | - | - | ||||||||||||||||||||||
| Foreign currency translation adjustments | - | - | - | - | - | 5,452 | - | 5,452 | ||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | (16,266,820 | ) | (16,266,820 | ) | ||||||||||||||||||||||
| Balance at December 31, 2020 | 200,000 | $ | 2,000 | 17,295,703 | $ | 172,957 | $ | 247,836,057 | $ | (734 | ) | $ | (238,242,364 | ) | $ | 9,767,916 | ||||||||||||||||
See accompanying notes to consolidated financial statements.
| 36 |
Seneca Biopharma, Inc.
Consolidated Statements of Cash Flows
| Year Ended December 31, | ||||||||
| 2019 | 2019 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (16,266,820 | ) | $ | (8,351,651 | ) | ||
| Adjustments to reconcile net loss to cash used in operating activities: | ||||||||
| Depreciation and amortization | 92,272 | 143,859 | ||||||
| Share-based compensation expenses | 584,991 | 880,789 | ||||||
| Change in fair value of liability classified warrants | (9,298 | ) | (499,138 | ) | ||||
| Allowance for bad debt | - | 362,176 | ||||||
| Warrant inducement expense | 5,620,089 | - | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Trade and other receivables | (95,215 | ) | 272,993 | |||||
| Prepaid expenses | (1,024,494 | ) | (142,310 | ) | ||||
| ROU and other assets | 24,537 | 39,643 | ||||||
| Accounts payable and accrued expenses | (105,043 | ) | (15,741 | ) | ||||
| Accrued severance and bonuses | 2,186,555 | 135,686 | ||||||
| Other current liabilities | 5,508 | (47,414 | ) | |||||
| Lease and other long term liabilities | (38,211 | ) | (34,572 | ) | ||||
| Net cash used in operating activities | (9,025,129 | ) | (7,255,680 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Net cash provided by investing activities | - | - | ||||||
| Cash flows from financing activities: | ||||||||
| Proceeds from the sale of common stock and warrants, net | 11,150,318 | 6,552,842 | ||||||
| Proceeds from warrant exercises | 3,547,894 | 236 | ||||||
| Proceeds from short-term notes payable | - | 414,320 | ||||||
| Payments of short-term notes payable | (232,296 | ) | (377,893 | ) | ||||
| Net cash provided by financing activities | 14,465,916 | 6,589,505 | ||||||
| Effects of exchange rates on cash | (1,185 | ) | (6,018 | ) | ||||
| Net decrease in cash and cash equivalents | 5,439,602 | (672,193 | ) | |||||
| Cash and cash equivalents, beginning of year | 5,114,917 | 5,787,110 | ||||||
| Cash and cash equivalents, end of year | $ | 10,554,519 | $ | 5,114,917 | ||||
| Supplemental cash flow information: | ||||||||
| Cash paid for interest | 14,015 | 8,920 | ||||||
See accompanying notes to consolidated financial statements.
| 37 |
SENECA BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1. Organization and Business and Financial Condition
Nature of Business
In October 2019, we changed our name from Neuralstem, Inc. to Seneca Biopharma, Inc. Seneca Biopharma, Inc. and its subsidiary are referred to as “Seneca,” the “Company,” “us,” or “we” throughout this report. The operations of our wholly-owned and controlled subsidiary located in the People’s Republic of China are consolidated in our condensed consolidated financial statements and all intercompany activity has been eliminated. The Company operates in one business segment.
The Company was founded in 1997 and currently has laboratory and office space in Germantown, Maryland and laboratory facilities in the People’s Republic of China. Our operations to date have primarily focused on developing business strategies, raising capital, research and development activities, and conducting pre-clinical testing and human clinical trials of our product candidates.
Seneca Biopharma, Inc., is a clinical-stage biopharmaceutical company developing novel treatments for diseases of high unmet medical need. The Company had been in the process of transforming the organization through the acquisition and/or in-licensing of new science and technologies with the goal of developing and providing meaningful therapies for patients.
In December 2020, the Company entered into an Agreement and Plan of Merger (the “Merger Transaction”) with Leading BioSciences, Inc. (“LBS”) in an all-stock transaction. Upon completion of the Merger Transaction, the combined company will focus on advancing LBS’ lead asset. The closing of the Merger Transaction is subject to approval by the Company’s and LBS stockholders and such closing is expected to be in the first half of 2021.
On July 17, 2019, we effected a 1-for-20 reverse stock split of our common stock. Stockholders’ equity and all references to share and per share amounts in the accompanying unaudited consolidated financial statements have been retroactively adjusted to reflect the 1-for-20 reverse stock split for all periods presented.
Liquidity and Going Concern
The Company has incurred losses since its inception and has not demonstrated an ability to generate significant revenues from the sales of its therapies or services and has not yet achieved profitable operations. There can be no assurance that profitable operations will ever be achieved, or if achieved, could be sustained on a continuing basis. In addition, development activities, clinical and pre-clinical testing, and commercialization of our products will require significant additional financing. These factors create substantial doubt about the Company’s ability to continue as a going concern beyond one year after the date that the audited consolidated financial statements are issued. The audited consolidated financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern. Accordingly, the audited consolidated financial statements have been prepared on a basis that assumes the Company will continue as a going concern and which contemplates the realization of assets and satisfaction of liabilities and commitments in the ordinary course of business.
In making this assessment we performed a comprehensive analysis of our current circumstances including: our financial position at December 31, 2020, our cash flow and cash usage forecasts for the period covering one-year from the issuance date of this Annual Report filed on Form 10-K and our current capital structure including outstanding warrants and other equity-based instruments and our obligations and debts.
Assuming the Merger is not consummated, we expect that our existing cash and cash equivalents as of December 31, 2020 will be sufficient to enable us to fund our anticipated level of operations based on our current operating plans at least 12 months after this filing. However, we will require additional capital to execute our acquisition and/or in-licensing strategy as well as out-licensing initiatives and to fund our operations. We anticipate raising additional capital through the private and public sales of our equity or debt securities, collaborative arrangements, licensing agreements or a combination thereof. Although management believes that such capital sources will be available, there can be no assurance that any such collaborative or licensing arrangements will be entered into or that financing will be available to us when needed in order to allow us to continue our operations, or if available, on terms acceptable to us. If we do not raise sufficient capital in a timely manner, among other things, we may be forced to license our potential products or technologies to third parties on unfavorable terms or materially curtail our operations. We currently do not have any commitments for future funding from any source.
Based upon our out-licensing strategy, we have greatly reduced our spending on the research, development, pre-clinical and clinical testing of our small molecule and stem cell product candidates and have increased our spending on the evaluation of new assets and technologies with the goal of acquisition and/or entry into a strategic transaction. No assurance can be given that we will be successful in our out-licensing strategy and/or entry into a strategic transaction.
| 38 |
Note 2. Significant Accounting Policies and Basis of Presentation
Basis of Presentation
Our consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The consolidated financial statements include the accounts of the Company and our wholly owned subsidiary. All significant intercompany transactions and balances have been eliminated.
Use of Estimates
The preparation of consolidated financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. The consolidated financial statements include significant estimates for the expected economic life and value of our licensed technology and related patents, our net operating loss and related valuation allowance for tax purposes, the fair value of our liability classified warrants and our share-based compensation related to employees and directors, consultants and advisors, among other things. Because of the use of estimates inherent in the financial reporting process, actual results could differ significantly from those estimates.
Fair Value Measurements
The carrying amounts of our short-term financial instruments, which primarily include cash and cash equivalents, accounts payable and accrued expenses, approximate their fair values due to their short maturities. The fair value of our long-term indebtedness was estimated based on the quoted prices for the same or similar issues or on the current rates offered to the Company for debt of the same remaining maturities and approximates the carrying value. The fair values of our liability classified warrants were estimated using Level 3 unobservable inputs. See Note 3 for further details.
Foreign Currency Translation
The functional currency of our wholly owned foreign subsidiary is its local currency. Assets and liabilities of our foreign subsidiary are translated into United States dollars based on exchange rates at the end of the reporting period; income and expense items are translated at the weighted average exchange rates prevailing during the reporting period. Translation adjustments for subsidiary are accumulated in other comprehensive income or loss, a component of stockholders' equity. Transaction gains or losses are included in the determination of net loss.
Cash, Cash Equivalents and Credit Risk
Cash equivalents consist of investments in low risk, highly liquid money market accounts and certificates of deposit with original maturities of 90 days or less. Cash deposited with banks and other financial institutions may exceed the amount of insurance provided on such deposits. If the amount of a deposit at any time exceeds the federally insured amount at a bank, the uninsured portion of the deposit could be lost, in whole or in part, if the bank were to fail.
Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash equivalents. Our investment policy, approved by our Board of Directors, limits the amount we may invest in any one type of investment issuer, thereby reducing credit risk concentrations. We attempt to limit our credit and liquidity risks through our investment policy and through regular reviews of our portfolio against our policy. To date, we have not experienced any loss or lack of access to cash in our operating accounts or to our cash equivalents.
Cash and cash equivalents at December 31, 2020 consist of approximately $10,529,200 of cash held and used and $25,300 of cash included in disposal group assets held for sale.
Revenue
The Company analyzes contracts to determine the appropriate revenue recognition using the following steps: (i) identification of contracts with customers; (ii) identification of distinct performance obligations in the contract; (iii) determination of contract transaction price; (iv) allocation of contract transaction price to the performance obligations; and (v) determination of revenue recognition based on timing of satisfaction of the performance obligation. The Company recognizes revenues upon the satisfaction of its performance obligation (upon transfer of control of promised goods or services to customers) in an amount that reflects the consideration to which it expects to be entitled to in exchange for those goods or services. Deferred revenue results from cash receipts from or amounts billed to customers in advance of the transfer of control of the promised services to the customer and is recognized as performance obligations are satisfied. When sales commissions or other costs to obtain contracts with customers are considered incremental and recoverable, those costs are deferred and then amortized as selling and marketing expenses on a straight-line basis over an estimated period of benefit.
Research and Development
Research and development costs are expensed as they are incurred. Research and development expenses consist primarily of costs associated with the pre-clinical development and clinical trials of our product candidates. For the years ended December 31, 2020 and 2019, we recorded approximately $60,000 and $459,000, respectively of cost reimbursements from our grants as an offset to research and development expenses. The Company evaluated the grants and concluded that, based on the specific terms, they represent a cost reimbursement activity as opposed to a revenue generating activity, and are best reflected as an offset to the underlying research and development expense.
| 39 |
Income (Loss) per Common Share
Basic income (loss) per common share is computed by dividing total net income (loss) available to common stockholders by the weighted average number of common shares outstanding during the period.
For periods of net income when the effects are dilutive, diluted earnings per share is computed by dividing net income available to common stockholders by the weighted average number of shares outstanding and the dilutive impact of all dilutive potential common shares. Dilutive potential common shares consist primarily of convertible preferred stock, stock options, restricted stock units and common stock purchase warrants. The dilutive impact of potential common shares resulting from common stock equivalents is determined by applying the treasury stock method. Our unvested restricted shares contain non-forfeitable rights to dividends, and therefore are considered to be participating securities; the calculation of basic and diluted income per share excludes net income attributable to the unvested restricted shares from the numerator and excludes the impact of the shares from the denominator.
For all periods of net loss, diluted loss per share is calculated similarly to basic loss per share because the impact of all dilutive potential common shares is anti-dilutive due to the net losses; accordingly, diluted loss per share is the same as basic loss per share for the years ended December 31, 2020 and 2019. A total of approximately 6.4 and 7.3 million potential dilutive shares have been excluded in the calculation of diluted net income per share for the years ended December 31, 2020 and 2019, respectively as their inclusion would be anti-dilutive.
Share-Based Compensation
We account for share-based compensation at fair value. Share-based compensation cost for stock options and stock purchase warrants granted to employees, board members and non-employee consultants is generally determined at the grant date using an option pricing model that uses Level 3 unobservable inputs; share-based compensation cost for restricted stock and restricted stock units is determined at the grant date based on the closing price of our common stock on that date. The value of the award is recognized as expense on a straight-line basis over the requisite service period.
Intangible and Long-Lived Assets
We assess impairment of our long-lived assets using a "primary asset" approach to determine the cash flow estimation period for a group of assets and liabilities that represents the unit of accounting for a long-lived asset to be held and used. Long-lived assets to be held and used are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. The carrying amount of a long-lived asset is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposition of the asset. No impairment losses were recognized during the years ended December 31, 2020 or 2019.
Income Taxes
We account for income taxes using the asset and liability approach, which requires the recognition of future tax benefits or liabilities on the temporary differences between the financial reporting and tax bases of our assets and liabilities. A valuation allowance is established when necessary to reduce deferred tax assets to the amounts expected to be realized. We also recognize a tax benefit from uncertain tax positions only if it is “more likely than not” that the position is sustainable based on its technical merits. Our policy is to recognize interest and penalties on uncertain tax positions as a component of income tax expense.
Significant New Accounting Pronouncements
Recently Adopted Guidance
In August 2018, the FASB issued ASU 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework—Changes to the Disclosure Requirements for Fair Value Measurement. This ASU addresses the disclosure requirements for fair value measurements. The guidance intends to improve the effectiveness of the disclosures relating to recurring and nonrecurring fair value measurements. The guidance is effective for fiscal years beginning after December 15, 2019. Portions of the guidance are to be adopted prospectively while other portions are to be adopted retroactively. We adopted this guidance effective January 1, 2020. The adoption did not have a material impact to our consolidated financial statements.
Unadopted Guidance
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments – Credit Losses. This ASU relates to measuring credit losses on financial instruments, including trade receivables. The guidance eliminates the probable initial recognition threshold that was previously required prior to recognizing a credit loss on financial instruments. The credit loss estimate can now reflect an entity's current estimate of all future expected credit losses. Under the previous guidance, an entity only considered past events and current conditions. The guidance is effective for smaller reporting companies as defined by the SEC for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years and early adoption is permitted. The adoption of certain amendments of this guidance must be applied on a modified retrospective basis and the adoption of the remaining amendments must be applied on a prospective basis. We currently expect that the adoption of this guidance will likely change the way we assess the collectability of our receivables and recoverability of other financial instruments. We have not yet begun to evaluate the specific impacts of this guidance nor have we determined the manner in which we will adopt this guidance.
| 40 |
In August 2020, the FASB issued ASU No. 2020-06, Debt – Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging – Contracts in Entity’s Own Equity (Subtopic 815-40). This ASC addresses (i) accounting for convertible instruments, (ii) accounting for contracts in an entity’s own equity as derivatives and (iii) earnings per share calculations. The guidance attempts to simplify the accounting for convertible instruments by eliminating the requirement to separate embedded conversion options in certain circumstances. The guidance also provides for updated disclosure requirements for convertible instruments. The guidance further updates the criteria for determining whether a contract in an entity’s own equity can be classified as equity. Lastly, the guidance specifically addresses how to account for the effect of convertible instruments and potential cash settled instruments in calculating diluted earnings per share. The guidance is effective for smaller reporting companies as defined by the SEC for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years and early adoption is permitted. The adoption of this guidance may be applied on a modified retrospective basis or a full retrospective basis. We have not yet begun to evaluate the specific impacts of this guidance nor have we determined the manner in which we will adopt this guidance.
We have reviewed other recent accounting pronouncements and concluded that they are either not applicable to our business, or that no material effect is expected on our consolidated financial statements as a result of future adoption.
Note 3. Fair Value Measurements
Fair value is the price that would be received from the sale of an asset or paid to transfer a liability assuming an orderly transaction in the most advantageous market at the measurement date. U.S. GAAP establishes a hierarchical disclosure framework which prioritizes and ranks the level of observability of inputs used in measuring fair value. These levels are:
| • | Level 1 – inputs are based upon unadjusted quoted prices for identical instruments traded in active markets. |
| • | Level 2 – inputs are based upon quoted prices for similar instruments in active markets, quoted prices for identical or similar instruments in markets that are not active, and model-based valuation techniques (e.g. the Black-Scholes model) for which all significant inputs are observable in the market or can be corroborated by observable market data for substantially the full term of the assets or liabilities. Where applicable, these models project future cash flows and discount the future amounts to a present value using market-based observable inputs including interest rate curves, foreign exchange rates, and forward and spot prices for currencies and commodities. |
| • | Level 3 – inputs are generally unobservable and typically reflect management's estimates of assumptions that market participants would use in pricing the asset or liability. The fair values are therefore determined using model-based techniques, including option pricing models and discounted cash flow models. |
Financial Assets and Liabilities Measured at Fair Value on a Recurring Basis
We have segregated our financial assets and liabilities that are measured at fair value on a recurring into the most appropriate level within the fair value hierarchy based on the inputs used to determine the fair value at the measurement date.
At December 31, 2020 and 2019, we had certain common stock purchase warrants that were originally issued in connection with our May 2016 and August 2017 capital raises (See Note 4) that are accounted for as liabilities whose fair value was determined using Level 3 inputs. The following table identifies the carrying amounts of such liabilities:
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Liabilities | ||||||||||||||||
| Liability classified stock purchase warrants | $ | - | $ | - | $ | 84,596 | $ | 84,596 | ||||||||
| Balance at December 31, 2019 | $ | - | $ | - | $ | 84,596 | $ | 84,596 | ||||||||
| Liability classified stock purchase warrants | $ | - | $ | - | $ | 75,298 | $ | 75,298 | ||||||||
| Balance at December 31, 2020 | $ | - | $ | - | $ | 75,298 | $ | 75,298 | ||||||||
| 41 |
The following table presents the activity for those items measured at fair value on a recurring basis using Level 3 inputs for the year ended December 31, 2020:
| Mark-to-market liabilities - stock purchase warrants | ||||
| Balance at December 31, 2019 | $ | 84,596 | ||
| Change in fair value - gain | (9,298 | ) | ||
| Balance at December 31, 2020 | $ | 75,298 | ||
The following table presents the activity for those items measured at fair value on a recurring basis using Level 3 inputs for the year ended December 31, 2019:
| Mark-to-market liabilities - stock purchase warrants | ||||
| Balance at December 31, 2018 | $ | 583,734 | ||
| Change in fair value - gain | (499,138 | ) | ||
| Balance at December 31, 2019 | $ | 84,596 | ||
The gains resulting from the changes in the fair value of the liability classified warrants are classified as other income or expense in the accompanying consolidated statements of operations and comprehensive loss. The fair value of the common stock purchase warrants is determined based on the Black-Scholes option pricing model or other option pricing models as appropriate and includes the use of unobservable inputs such as the expected term, anticipated volatility and expected dividends. Changes in any of the assumptions related to the unobservable inputs identified above may change the embedded conversion options’ fair value; increases in expected term, anticipated volatility and expected dividends generally result in increases in fair value, while decreases in these unobservable inputs generally result in decreases in fair value.
Note 4. Stockholders’ Equity
We have granted share-based compensation awards to employees, board members and service providers. In addition, we have issued warrants to purchase common stock in conjunction with debt and equity offerings. Awards may consist of common stock, restricted common stock, restricted common stock units, common stock purchase warrants, or common stock purchase options. Our common stock purchase options and stock purchase warrants have lives of up to ten years from the grant date. Awards vest either upon the grant date or over varying periods of time. The stock options provide for exercise prices equal to or greater than the fair value of the common stock at the date of the grant. Restricted stock units grant the holder the right to receive fully paid common shares with various restrictions on the holder’s ability to transfer the shares. As of December 31, 2020, we have approximately 6.7 million shares of common stock reserved for issuance upon the exercise of share-based awards.
We record share-based compensation expense on a straight-line basis over the requisite service period. Share-based compensation expense included in the statements of operations and comprehensive loss was as follows:
| Year Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Research and development costs | $ | 30,883 | $ | 200,337 | ||||
| General and administrative expenses | 554,108 | 680,452 | ||||||
| Total | $ | 584,991 | $ | 880,789 | ||||
| 42 |
Stock Options
A summary of stock option activity and related information for the year ended December 31, 2020 follows:
| Number of Options | Weighted-Average Exercise Price | Weighted-Average Remaining Contractual Life (in years) | Aggregate Intrinsic Value | |||||||||||||
| Outstanding at January 1, 2020 | 271,660 | $ | 61.83 | 7.8 | $ | - | ||||||||||
| Granted | 1,686,466 | $ | 0.62 | $ | - | |||||||||||
| Exercised | - | - | ||||||||||||||
| Forfeited/Expired | (160,750 | ) | $ | 23.82 | ||||||||||||
| Outstanding at December 31, 2020 | 1,797,376 | $ | 7.80 | 9.0 | $ | 438,650 | ||||||||||
| Exercisable at December 31, 2020 | 812,742 | $ | 16.42 | 8.7 | $ | 182,771 | ||||||||||
| Range of Exercise Prices | Number of Options Outstanding | Weighted-Average Exercise Price | Weighted-Average Remaining Contractual Life (in years) | Aggregate Intrinsic Value | ||||||||||||
| $0.62 | 1,686,466 | $ | 0.62 | 9.3 | $ | 438,650 | ||||||||||
| $5.90 - $8.80 | 51,758 | $ | 6.32 | 8.5 | - | |||||||||||
| $22.20 - $80.60 | 23,326 | $ | 29.21 | 4.5 | - | |||||||||||
| $107.40 - $1,102.40 | 35,826 | $ | 333.81 | 1.8 | - | |||||||||||
| 1,797,376 | $ | 7.80 | 9.0 | $ | 438,650 | |||||||||||
The Company uses the Black-Scholes option pricing model for “plain vanilla” options and other pricing models as appropriate to calculate the fair value of options. Significant assumptions used in these models include:
| Year Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Annual dividend | - | - | ||||||
| Expected life (in years) | 4.0 | - | 5.2 | 4.8 | - | 5.5 | ||
| Risk free interest rate | 0.2% | - | 0.4% | 1.8% | - | 2.5% | ||
| Expected volatility | 110% | - | 111% | 97% | - | 115% | ||
Options granted in the years ended December 31, 2020 and 2019 had weighted average grant date fair values of $0.52 and $3.45, respectively. The total fair value of the options vested during the years ended December 31, 2020 and 2019 was approximately $500,000 and $671,100, respectively.
Unrecognized compensation cost for unvested stock option awards outstanding at December 31, 2020 was approximately $530,000 to be recognized over approximately 2.2 years.
In 2019, the Company modified certain awards in conjunction with an employee’s termination. The modification provided for the accelerated vesting of all unvested awards and the extension of the post-employment exercise period. The modifications resulted in approximately $102,000 of additional research and development expenses in 2019.
RSUs
We have granted restricted stock units (RSU’s) that entitle the holders to receive shares of our common stock upon vesting and subject to certain restrictions regarding the exercise of the RSU’s and the holders’ ability to transfer the shares received upon exercise. The fair value of RSU’s granted is based upon the market price of the underlying common stock as if they were vested and issued on the date of grant.
| 43 |
A summary of our RSU activity for the year ended December 31, 2020 follows:
| Number of RSU's | Weighted-Average Grant Date Fair Value | |||||||
| Outstanding at January 1, 2020 | 5,467 | $ | 29.62 | |||||
| Granted | 24,000 | $ | 0.70 | |||||
| Exercised and converted to common shares | (563 | ) | $ | 236.20 | ||||
| Forfeited | - | $ | - | |||||
| Outstanding at December 31, 2020 | 28,904 | $ | 1.58 | |||||
| Exercisable at December 31, 2020 | 22,904 | $ | 1.81 | |||||
The total intrinsic value of the outstanding RSU’s at December 31, 2020 was approximately $25,400. The total fair value of RSU’s vested during the years ended December 31, 2020 and 2019, was approximately $27,600 and $13,900, respectively. The total value of all RSU’s that were converted in the years ended December 31, 2020 and 2019 was approximately $300 and $10,400, respectively.
Unrecognized compensation cost for unvested RSU’s outstanding at December 31, 2020 was approximately $4,000 to be recognized over approximately 0.3 years.
Restricted Stock
We have granted restricted stock to certain board members.
A summary of our restricted stock activity for the year ended December 31, 2020 is as follows:
| Shares of Restricted Stock | Weighted-Average Grant Date Fair Value | |||||||
| Outstanding at January 1, 2020 | 7,981 | $ | 5.95 | |||||
| Granted | - | $ | - | |||||
| Vested | (3,814 | ) | $ | 5.90 | ||||
| Forfeited | (4,167 | ) | $ | 6.00 | ||||
| Outstanding at December 31, 2020 | - | $ | - | |||||
Restricted stock vesting in the year ended December 31, 2020 and 2019, had a total intrinsic value of approximately $2,600 and $14,500, respectively.
Stock Purchase Warrants
We have issued warrants to purchase common stock to certain officers, directors, stockholders and service providers as well as in conjunction with debt and equity offerings and at various times replacement warrants were issued as an inducement for warrant exercises.
In May 2016 and August 2017, we issued a total of 87,309 and 112,500 common stock purchase warrants, respectively in conjunction with the offering of our securities. Such warrants are classified as liabilities due to the existence of certain net cash settlement provisions contained in the warrants. At December 31, 2020, after giving effect to exercises, 149,136 of these common stock purchase warrants remain outstanding and are recorded at fair value as mark-to-market liabilities (see Note 3).
In January 2020, pursuant to the terms of an inducement offer, certain holders of 5,555,554 of our common stock purchase warrants exercised such warrants at an exercise price of $1.36 per share generating approximately $7.6 million of gross proceeds. As an inducement to exercise, we reduced the exercise price on the existing warrants from $2.70 to $1.36 and issued 5,555,554 replacement warrants with an exercise price of $1.23 per share. Of the replacement warrants, 2,777,777 have a two-year term and 2,777,777 have a five-year term. In conjunction with the transaction, we issued to the placement agent 444,445 common stock purchase warrants with an exercise price of $1.70 and a five-year term.
| 44 |
We recognized an expense in the accompanying consolidated statement of operations and comprehensive loss for the year ended December 31, 2020 of approximately $5.6 million representing the fair value of the inducement offer. The fair value is comprised of the fair value of the modification of the original warrants (the reduction in exercise price) and the fair value of the replacement warrants. The fair values were calculated using the Black-Scholes option pricing model.
In conjunction with our May 2020 Offering, we issued to the placement agent 400,000 common stock purchase warrants with an exercise price of $1.25 and a five-year term.
A summary of outstanding warrants at December 31, 2020 follows:
| Range of Exercise Prices | Number of Warrants Outstanding | Range of Expiration Dates | ||||
| $0.90 - $1.25 | 3,233,407 | May 2021 - May 2025 | ||||
| $1.70 - $3.38 | 1,080,333 | July 2024 - January 2025 | ||||
| $6.00 - $782.60 | 195,489 | July 2021 - April 2024 | ||||
| 4,509,229 | ||||||
Preferred and Common Stock
We have outstanding 200,000 shares of Series A 4.5% Convertible Preferred Stock issued in December 2016. Shares of the Series A 4.5% Convertible Preferred Stock are convertible into 38,873 shares of the Company’s common. In April and July 2019, 800,000 Series A 4.5% Convertible Preferred Stock shares were converted into 155,496 shares of common stock in accordance with their terms.
In May 2020, we completed a direct offering of 5,000,000 shares of common stock at a price of $1.00 per each share resulting in gross proceeds of $5.0 million. After deducting placement agent and other expenses related to the offering, we received approximately $4.4 million. The securities were sold pursuant to a registration statement on Form S-3 (file no. 333- 218608). In connection with the offering, we issued to the placement agent warrants to purchase 400,000 shares of our common stock at an exercise price of $1.25 per share. The warrants are exercisable immediately and expire 5 years from issuance.
Note 5. Property and Equipment
The major classes of property and equipment consist of the following at December 31:
| 2020 | 2019 | |||||||
| Furniture and fixtures | $ | 32,272 | $ | 35,407 | ||||
| Computers and office equipment | 138,897 | 138,897 | ||||||
| Lab equipment | 744,787 | 817,149 | ||||||
| 915,956 | 991,453 | |||||||
| Less accumulated depreciation | (905,180 | ) | (950,417 | ) | ||||
| Property and equipment, net | $ | 10,776 | $ | 41,036 | ||||
In addition to the above, we have approximately $1,000 of equipment, net located at our research facility in China which is classified as assets held for sale at December 31, 2020. Property and equipment are recorded at cost and are depreciated using the straight-line method over the estimated useful lives of the respective assets. Depreciation expense for the years ended December 31, 2020 and 2019, was approximately $29,000 and 49,000, respectively.
Note 6. Patents
The Company holds patents related to its stem cell and small molecule technologies. Patent costs are capitalized and are being amortized over the life of the patents. The weighted average remaining unamortized life of issued patents was approximately 7.3 years at December 31, 2020. Long-lived assets to be held and used are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. The carrying amount of a long-lived asset is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposition of the asset. Long-lived assets to be disposed of are reported at the lower of carrying amount or fair value less cost to sell. During the years ended December 31, 2020 and 2019, no impairment losses were recognized. The Company’s intangible assets and accumulated amortization consisted of the following at December 31:
| 45 |
| 2020 | 2019 | |||||||
| Patent asset | $ | 338,463 | $ | 2,006,443 | ||||
| Accumulated amortization | (191,330 | ) | (1,337,507 | ) | ||||
| Net intangibles | $ | 147,133 | $ | 668,936 | ||||
In addition to the above we have approximately $459,000 of intangible assets, net related to the Company’s neural stem cell program classified as assets held for sale at December 31, 2020.
Amortization expense for the years ended December 31, 2020 and 2019 was approximately $63,000 and $95,000, respectively. The expected average future annual amortization expense over the next five years is approximately $18,000 based on current balances of our intangible assets.
Note 7. Income Taxes
Our provision for income taxes for the years ended December 31, 2020 and 2019 consists of the following:
| 2020 | 2019 | |||||||
| Current provision: | ||||||||
| Federal | $ | - | $ | - | ||||
| State | - | - | ||||||
| Foreign | - | - | ||||||
| Total current provision | - | - | ||||||
| Deferred provision (benefit): | ||||||||
| Federal | (192,953 | ) | (545,792 | ) | ||||
| State | (1,893,740 | ) | 1,001,786 | |||||
| Foreign | - | - | ||||||
| Total deferred provision (benefit) | (2,086,693 | ) | 455,994 | |||||
| Valuation allowance | 2,086,693 | (455,994 | ) | |||||
| Consolidated income tax provision | $ | - | $ | - | ||||
We provide a full valuation allowance on our net deferred tax assets because management has determined that it is more likely than not that we will not earn income sufficient to realize the deferred tax assets during the asset reversal periods.
The difference between income taxes computed by applying the statutory federal income tax rate to consolidated losses before income taxes and the consolidated provision for income taxes is attributable to the following:
| 2020 | 2019 | |||||||
| Federal statutory rate | (21.0 | %) | (21.0 | %) | ||||
| State income taxes, net of Federal benefits | (5.0 | %) | (5.3 | %) | ||||
| Rate changes | 3.8 | % | (6.4 | %) | ||||
| Change in fair value of liability classified warrants | 0.0 | % | (1.6 | %) | ||||
| Warrant inducement expense | 9.0 | % | 0.0 | % | ||||
| Other, including non-deductible expenses | 26.0 | % | 28.8 | % | ||||
| Valuation allowance | (12.8 | %) | 5.5 | % | ||||
| Total | 0.0 | % | 0.0 | % | ||||
| 46 |
The tax effects of significant temporary differences representing deferred tax assets as of December 31 are:
| 2020 | 2019 | |||||||
| Net operating loss carryforwards | $ | 40,897,678 | $ | 43,190,604 | ||||
| Stock based compensation expense | 2,323,607 | 2,605,277 | ||||||
| Tax credit carryforwards and other | 1,377,275 | 889,372 | ||||||
| Gross deferred tax assets | 44,598,560 | 46,685,253 | ||||||
| Valuation allowance | (44,598,560 | ) | (46,685,253 | ) | ||||
| Net deferred tax assets | $ | - | $ | - | ||||
The Company had Federal net operating loss (“NOL”) carryforwards of approximately $158 million at December 31, 2020 of which $146 million was created prior to 2018 and began expiring in 2019. The Company also has certain Federal tax credit carryforwards that began expiring in 2020. The timing and manner in which these net operating loss carryforwards and credits may be used in any year will be limited to the Company’s ability to generate future earnings and also may be limited by certain provisions in the U.S. tax code. The Company has not identified any uncertain tax positions and did not recognize any adjustments for unrecognized tax benefits. The Company remains subject to examination for income tax returns dating back to 2017.
Note 8. Commitments and Contingencies
Leases
We currently operate one facility located in the United States and one facility located in China under leases which are both classified as operating leases.
Our corporate offices and primary research facilities are located in Germantown, Maryland, where we lease approximately 1,500 square feet. This lease provides for monthly payments of approximately $5,600 per month. This lease has an initial term of 12 months and expires on December 31, 2021. We did not establish a right of use (“ROU”) asset or lease liability for this short-term lease.
We also lease approximately 11,300 square feet of research facility in the People’s Republic of China. This lease commenced in September 2019, provides for minimum lease payments of approximately $4,400 per month, expires in September 2024 and provides us with a future first right of refusal for extending the lease beyond its expiration. This lease currently represents our lone long-term operating lease. This new lease obligation resulted in us obtaining an ROU asset of approximately $205,000.
Our long-term operating lease and related sublease for our San Diego facility both terminated in August 2019. We recognized other income of approximately $86,100 from this sublease for the year ended December 31, 2019.
We recognized total rent expense of approximately $113,100 and $194,200, in the years ended December 31, 2020 and 2019, respectively. Included in the expense is approximately $67,700 in each of the years ended December 31, 2020 and 2019 relating to our short-term leases. Lease costs, net of sublease income, for the years ended December 31 consisted of the following:
| 2020 | 2019 | |||||||
| Operating lease cost | $ | 113,100 | $ | 171,000 | ||||
| Variable lease cost | - | 23,200 | ||||||
| Sublease income | - | (86,100 | ) | |||||
| Total net lease cost | $ | 113,100 | $ | 108,100 | ||||
In the year ended December 31, 2019, we established approximately $204,300 of ROU assets as the result of entering into new lease arrangements.
At December 31, 2020, we have approximately $190,000 of ROU assets included in Disposal Group Assets Held for Sale and approximately $159,000 of lease liability included in Disposal Group Liabilities Associated with Assets Held for Sale in our consolidated balance sheets.
| 47 |
Future payments under our lone long-term operating lease as of December 31, 2020 are as follows:
Future undiscounted cash flows:
| 2021 | $ | 58,400 | ||
| 2022 | 60,200 | |||
| 2023 | 62,100 | |||
| 2024 | 14,600 | |||
| Total | 195,300 | |||
| Discount factor | (36,300 | ) | ||
| Lease liability | 159,000 | |||
| Less current liability | (40,500 | ) | ||
| Non-current lease liability | $ | 118,500 |
Accrued Severance
In connection with the Company’s reorganization resulting from the proposed Merger Transaction, the Company accrued approximately $2.3 million of severance payable to executives in accordance with their employment contracts. Such payment is not contingent on the closing of the Merger. The executives were terminated in March 2021 and the corresponding severance amounts will be paid in accordance with the terms of the employment contracts.
Other
From time to time, we are parties to legal proceedings that we believe to be ordinary, routine litigation incidental to the business. We are currently not a party to any litigation or legal proceeding. As a result of the Merger, we are currently involved in litigation related thereto as noted below.
On January 8, 2021, Joseph Sheridan, a purported Seneca stockholder, filed a complaint in the United States District Court for the Southern District of New York against Seneca, the members of its board of directors, and LBS, captioned Sheridan v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00166 (the “Sheridan Complaint”).
Also, on January 8, 2021, Hesam Pirjamaat, a purported Seneca stockholder, filed a complaint in the United States District Court for the Southern District of New York against Seneca, the members of its board of directors, Townsgate Acquisition Sub 1, Inc., and LBS, captioned Pirjamaat v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00172 (the “Pirjamaat Complaint”).
On January 13, 2021, Brian Johnson, a purported Seneca stockholder, filed a complaint in the United States District Court for the Southern District of New York against Seneca and the members of its board of directors, captioned Johnson v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00310 (the “Johnson Complaint”).
On January 15, 2021, Vipin Mathews, a purported Seneca stockholder, filed a complaint in the United States District Court for the Eastern District of New York against Seneca and the members of its board of directors, captioned Mathews v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00242 (the “Mathews Complaint”).
On January 22, 2021, Emily Pechal, a purported Seneca stockholder, filed a complaint in the United States District Court for the Southern District of New York against Seneca and the members of its board of directors, captioned Pechal v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00585 (the “Pechal Complaint”).
On February 25, 2021, Marcie Curtis, a purported Seneca stockholder, filed a complaint in the United States District Court for the District of Delaware against Seneca and the members of its board of directors, captioned Curtis v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00292 (the “Curtis Complaint”).
On March 1, 2021, Juanesha Valdez, a purported Seneca stockholder, filed a complaint in the United States District Court for the Eastern District of Pennsylvania against Seneca, the members of its board of directors, Townsgate Acquisition Sub 1, Inc., and LBS, captioned Valdez v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00980 (the “Valdez Complaint”).
| 48 |
On March 2, 2021, Bryan Anderson, a purported Seneca stockholder, filed a complaint in the United States District Court for the District of Delaware against Seneca and the members of its board of directors, captioned Anderson v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-00326 (the “Anderson Complaint”).
On March 3, 2021, Jack McIntire, a purported Seneca stockholder, filed a complaint in the United States District Court for the Southern District of New York against Seneca and the members of its board of directors, captioned McIntire v. Seneca Biopharma, Inc., et al., Case No. 1:21-cv-01869 (the “McIntire Complaint,” and, together with the Sheridan Complaint, the Pirjamaat Complaint, the Johnson Complaint, the Mathews Complaint, the Pechal Complaint, the Curtis Complaint, the Valdez Complaint, the Anderson Complaint, the “Stockholder Complaints”).
On February 26, 2021, the United States District Court for the Southern District of New York entered an order consolidating the Sheridan Complaint, the Pirjamaat Complaint, the Johnson Complaint, and the Pechal Complaint under Case No. 21-cv-0166.
We believe the allegations in the Stockholder Complaints are without merit.
Other stockholders may file additional lawsuits challenging the Merger, which may name us as well as members of our boards of directors and/or others as defendants. No assurance can be made as to the outcome of such lawsuits or the Stockholder Complaints, including the amount of costs associated with defending, or any other liabilities that may be incurred in connection with the litigation of, such claims. Litigation often is expensive and diverts management’s attention and resources, which could adversely affect our business. At present, we are unable to estimate potential losses, if any, related to the lawsuit.
Note 9. Related Party Receivable
On August 10, 2016, we entered into a reimbursement agreement with a former executive officer. Pursuant to the reimbursement agreement, the former officer agreed to repay the Company, over a six-year period, approximately $658,000 in expenses that the Company determined to have been improperly paid under the Company's prior expense reimbursement policies.
The $658,000 non-interest-bearing receivable was recorded net of a $199,000 discount to reflect the net present value of the future cash payments.
In March 2019, in conjunction with the former executive officer’s termination, we entered into a consulting agreement and release of claims agreement with the former executive officer. As partial consideration for the release, we modified the reimbursement agreement to change the payment terms, extend the maturity and forgive approximately 50% or $229,000 of the outstanding receivable. At December 31, 2020, $229,000 remains outstanding and is due in installments through July 2025. The Company has concluded that this outstanding balance is not recoverable and recorded an allowance against the entire remaining balance in 2019.
Note 10. Disposal Group Assets Held for Sale
In late 2020, the Company engaged in negotiations with an interested third party for the sale of all of its assets and liabilities related to its neural stem cell program (NSI-566). Those negotiations have subsequently ended. The Company is continuing the process to identify a purchaser for the assets and liabilities. The Company has concluded that it is probable that a sale will be completed within one year and that the assets and liabilities should be classified as a disposal group held for sale in its balance sheet at December 31, 2020. Assets and liabilities classified as held for sale will no longer be depreciated or amortized. Although the Company believes a sale will be consummated, no binding agreements have been entered into and there can be no assurance that a sale will ultimately be consummated or on what terms and conditions.
Based on current negotiations, the Company concluded the net proceeds from the sale are expected to exceed the net carrying value of the assets and liabilities and accordingly, no impairment charge has been recognized as of December 31, 2020.
| 49 |
The assets and liabilities classified as a disposal group held for sale at December 31, 2020 are comprised of the following:
| Amount | ||||
| Cash | $ | 25,275 | ||
| Prepaid expesnes | 146,051 | |||
| Property and equipment, net | 1,128 | |||
| Patents, net | 458,738 | |||
| ROU and other assets | 204,291 | |||
| Disposal group assets held for sale | $ | 835,483 | ||
| Accounts payable and accrued expenses | $ | 75,306 | ||
| Lease liabilities | 159,038 | |||
| Disposal group liabilities associated with assets held for sale | $ | 234,344 | ||
Note 11. Subsequent Events
On March 17, 2021, we terminated: (i) Kenneth Carter, PhD, Seneca’s executive chairman, (ii) Dane Saglio, Seneca’s chief financial officer, (iii) Matthew Kalnik, PhD, Seneca’s chief operating officer and (iv) Seneca’s Senior Vice President of R&D (collectively, the “Employees”) without cause. In connection with the Employees’ terminations, the Company entered into separation agreements (“Separation Agreement(s)”). The Separation Agreements contain mutual general releases of claims and acknowledge the amounts due to each Employee as a result of their terminations without cause as provided for in each of their respective employment agreements.
Such amounts are as follows:
| Name | Severance and Bonus | |||
| Kenneth Carter, PhD | $ | 816,995 | ||
| Dane Saglio | $ | 452,572 | ||
| Matthew Kalnik, PhD | $ | 599,868 | ||
| Senior VP of R&D | $ | 384,702 | ||
| Total: | $ | 2,254,137 |
Additionally, in the event that the Company consummates the Merger (as defined below), each employment agreement provides for the following additional severance and benefits:
Severance in Connection with a Change in Control
| Name | CIC Severance and Bonus1 | |||
| Kenneth Carter, PhD | $ | 277,248 | ||
| Dane Saglio | $ | 100,857 | ||
| Matthew Kalnik, PhD | $ | 150,225 | ||
| Senior VP of R&D | $ | 85,567 | ||
| Total: | $ | 613,897 |
_____________
| 1. | Represents additional severance benefits in connection with a termination without cause in connection with a change in control. |
Repurchase of Employee Stock Options
Immediately prior to the closing of the Merger, each respective Employee’s outstanding common stock options will be purchased by the Company for the following consideration:
| Name | Option Repurchase | |||
| Kenneth Carter, PhD | $ | 188,787 | ||
| Dane Saglio | $ | 362,391 | ||
| Matthew Kalnik, PhD | $ | 476,662 | ||
| Senior VP of R&D | $ | 395,166 | ||
| Total: | $ | 1,423,006 |
As a result of the Separation Agreements, the employment of the Employees was terminated on March 17, 2021. Dr. Carter will remain chairman of the Board.
Appointment of Mr. Saglio as Principal Executive Officer
On March 17, 2021, Mr. Saglio entered into a consulting agreement whereby he will perform the duties of principal executive and accounting officer of Seneca until such time as the Merger is consummated. Mr. Saglio will be paid on an hourly basis to perform such services at a rate of $250 per hour.
| 50 |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None
| ITEM 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Securities Exchange Act of 1934, as amended (the "Exchange Act"), is recorded, processed, summarized, and reported within the time periods specified in the Securities and Exchange Commission's rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed under the Exchange Act is accumulated and communicated to management, including our principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Based on an evaluation under the supervision and with the participation of the Company’s management, the Company’s principal executive officer and principal financial officer, concluded that the Company’s disclosure controls and procedures as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) were effective as of December 31, 2020 to provide reasonable assurance that information required to be disclosed by the Company in reports that it files or submits under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission rules and forms and (ii) accumulated and communicated to the Company’s management, including its principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Inherent Limitations Over Internal Controls
The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”). The Company’s internal control over financial reporting includes those policies and procedures that:
(i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the Company’s assets;
(ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with U.S. GAAP, and that the Company’s receipts and expenditures are being made only in accordance with authorizations of the Company’s management and directors; and
(iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the financial statements.
Management, including the Company’s principal executive officer and principal financial officer, does not expect that the Company’s internal controls will prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of internal controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. Also, any evaluation of the effectiveness of controls in future periods are subject to the risk that those internal controls may become inadequate because of changes in business conditions, or that the degree of compliance with the policies or procedures may deteriorate.
| 51 |
Management’s Annual Report on Internal Control Over Financial Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act). Management conducted an assessment of the effectiveness of the Company’s internal control over financial reporting based on the criteria set forth in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on the Company’s assessment, management has concluded that its internal control over financial reporting was effective as of December 31, 2020 to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with U.S. GAAP.
Changes in Internal Control Over Financial Reporting
There were no changes in the Company’s internal control over financial reporting during the fourth quarter of 2020, which were identified in connection with management’s evaluation required by paragraph (d) of rules 13a-15 and 15d-15 under the Exchange Act, that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
| ITEM 9B. | OTHER INFORMATION |
None
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The names of our directors and executive officers and their ages, positions, and biographies as of March 1, 2021 are set forth below. Our executive officers are appointed by and serve at the discretion of the Board. There are no family relationships among any of our directors or executive officers.
| Name | Position | Age | Position Since | |||||
| Named Executive Officers | ||||||||
| Kenneth Carter (1) | Executive Chairman | 61 | 2019 | |||||
| Dane Saglio (1) (3) | Chief Financial Officer | 63 | 2020 | |||||
| Matthew Kalnik, PhD (1) | Chief Operating Officer, President | 58 | 2020 | |||||
| Independent Directors | ||||||||
| Cristina Csimma, PharmD., MHP (2) | Director | 62 | 2017 | |||||
| David J. Mazzo, PhD | Director | 64 | 2019 | |||||
| Binxian Wei (2) | Director (Series A Preferred) | 51 | 2019 | |||||
| Mary Ann Gray, PhD (2) | Director | 68 | 2019 | |||||
| (1) | On March 17, 2021, Drs. Carter and Kalnik and Mr. Saglio entered into separation agreements where they were terminated by the Company “without cause” as defined in their respective employment agreements. |
| (2) | Pursuant to the Company’s anticipated merger transaction with Leading BioSciences, Inc., upon the effectiveness of the merger, Drs. Csimma and Gray, and Mr. Wei will continue to serve as directors of the combined company. |
| (3) | Pursuant to a consulting agreement entered into between Mr. Saglio and the Company, Mr. Saglio will act as principal executive and accounting officer until the consummation of the anticipated merger or until such agreement is terminated. |
Kenneth Carter PhD, has served as our executive chairman since January 2019. Dr. Carter has over 20 years of experience working in positions of substantial responsibility in the development and operations of early-stage biotechnology companies. Since 2010 when he co-founded the company, Dr. Carter has served as chairman of the board of directors of Noble Life Sciences, a private biotechnology company in Maryland. From 2011 through 2017, Dr. Carter served as president and chief executive officer of NexImmune, Inc., a private biopharmaceutical company in Maryland. He continues to serve as senior advisor of NexImmune. Prior to that, from 1999 through 2009, Dr. Carter served as president and chief executive officer of Avalon Pharmaceuticals, Inc. (NASDAQ: AVRX) until the company merged with Clinical Data, Inc. Dr. Carter also currently serves on the following boards of directors (i) since 2016, Antidote Therapeutics, Inc., a private biopharmaceutical company in Maryland, (ii) since 2011, BetaCat Pharmaceuticals, a private pharmaceutical company in Texas, and Maryland BioHealth Innovation, a biotechnology intermediary company in Maryland, and (iii) since 2007, Maryland Health Care Product Development Corporation, a biotechnology investment firm in Maryland. Dr. Carter additionally serves as a lecturer and Adjunct Faculty member of Johns Hopkins University in Maryland. Dr. Carter holds a BS in Biology and Chemistry from Abilene Christian University, a Ph.D. in Human Genetics and Cell Biology from the University of Texas Medical Branch, and a Postdoctoral degree in Cell and Molecular Biology from University of Massachusetts Medical School. In evaluating Dr. Carter’s specific experience, qualifications, attributes and skills in connection with his appointment to our board, we took into account his prior work with both public and private organizations, including his experience in building biopharmaceutical organizations, his strong business development background and his past experience and relationships in the biopharma and biotech fields.
| 52 |
Matthew Kalnik, PhD, has served as our President and Chief Operating Officer in April 2020. Dr. Kalnik has over 25 years of experience in senior R&D and business development roles leading multi-disciplinary teams in drug discovery and drug development. From 2013 through present, Dr. Kalnik has served as the Chairman and Chief Executive Officer of Antidote Therapeutics, a private biotechnology company. From 1997 through present, Dr. Kalnik has consulted for biotechnology / pharmaceutical companies related to portfolio analysis, licensing and M&A transactions. Prior to that, from 2009 through 2012, Dr. Kalnik served as Senior Vice President and Officer, Strategic Planning & Business Operations of Nabi Biopharmaceuticals, Inc. (NASDAQ: BOTA) a publicly traded biopharmaceutical company. Dr. Kalnik has also held leadership roles at Daiichi Medical Research (now Daiichi-Sankyo)c, Genaissance Pharmaceuticals, Inc. (now Allergan), Pfizer, Inc.and Biosym Technologies, Inc. (now Dassault Systèmes). He holds a Ph.D. in Biochemistry & Molecular Biophysics from Columbia University and conducted his post-doctoral fellowship at the Department of Molecular Biology at The Scripps Research Institute, La Jolla, CA.
Dane Saglio, has served as our Chief Financial Officer since April 2020. From July 2017 through July 2019, Mr. Saglio served as Executive Vice President and CFO of Celios Corporation, a private company focused on research, development, and commercialization of advanced air technologies. Prior to that, from November 2014 through June 2017, Mr. Saglio served as the CFO for Helomics Corporation (acquired in 2019 by Precision Therapeutics). Mr. Saglio has over 20 years of experience in financial positions with pharmaceutical and biotechnology companies. Mr. Saglio earned his BS in business administration from the University of Maryland and is a licensed CPA (inactive).
Cristina Csimma PharmD, MHP, has served on our board of directors since September 2017. She also serves on the Board of Directors of Idera Pharmaceuticals (NASDAQ: IDRA), a clinical stage biopharmaceutical company, Caraway Therapeutics, a preclinical stage biopharmaceutical company, and T1D Exchange, a nonprofit research organization for type 1 diabetes. She also serves on various advisory boards, including: the Muscular Dystrophy Association Venture Philanthropy Scientific Advisory Committee; the Executive Oversight Board to the National Institutes of Health (NIH) NeuroNext Network; the Harvard and Brigham and Women’s Hospital MRCT Center External Advisory Board, and the TREAT-NMD Advisory Committee for Therapeutics (TACT) She was previously the Executive Chair of the Board of Directors of Exonics Therapeutics, a Director of Juniper Pharmaceuticals (acquired in August 2018 by Catalent), Vtesse (acquired in March 2017 by Sucampo Pharmaceuticals) and Cydan, where she was also President and founding CEO, the Vice President of Drug Development at Virdante Pharmaceuticals Inc (acquired by Momenta), Principal at Clarus Ventures LLC, and held roles in Clinical Development and Translational Research at Wyeth (now Pfizer), Genetics Institute and Dana Farber Cancer Institute. Dr. Csimma holds both a Doctor of Pharmacy and a Bachelor of Science in Pharmacy from the Massachusetts College of Pharmacy and Allied Health Sciences, as well as a Master of Health Professions from Northeastern University. In selecting Dr. Csimma, the board took into account her vast experience in the pharmaceutical industry, including her successes in developing drugs for various diseases throughout her career.
Binxian Wei, has served on our board of directors since February 2019. He has been the V.P. of Darsheng Trade & Tech. Development Co, Ltd. (a subsidiary to Tianjin Tiayo Pharmaceutical Co., Ltd.) since 2015. He is responsible for API and finished dosage marketing for Chinese pharmaceutical companies. From 2008 through 2010, he worked as a business development manager for Sakai Trading. He holds a Master’s Degree in Mathematical & Computer Sciences from Colorado School of Mines, a Master’s Degree and Bachelor’s Degree in Chemical Engineering from Tianjin University in China. Bin-Xian Wei was appointed as the director representative of the Series A 4.5% Convertible Preferred Stock by Tianjin Pharmaceuticals Group International Holdings Co., LTD, the sole holder of the outstanding Series A 4.5% Convertible Preferred Stock.
David J. Mazzo, PhD, has served on our board of directors since June 2019. Dr. Mazzo brings over 35 years of experience in the pharmaceutical industry. Dr. Mazzo currently serves as President and Chief Executive Officer and a Director of Caladrius Biosciences (NASDAQ: CLBS), a late-stage therapeutics development biopharmaceutical company developing autologous cell therapies for select cardiovascular and autoimmune diseases. Dr. Mazzo also serves as the chairman of the Board of Directors of Visioneering Technology, Inc. (ASX: VTI), a medical device company with a focus on products for treating and preventing the progression pediatric myopia and presbyopia. Previously, Dr. Mazzo served from August 2008 to October 2014 as Chief Executive Officer and as a member of the Board of Directors of Regado Biosciences, Inc., (NASDAQ: RGDO) a pharmaceutical company focused on the development of novel antithrombotic drug systems for acute and sub-acute cardiovascular indications. Prior to his leading Regado, from March 2007 to April 2008, Dr. Mazzo was President, Chief Executive Officer and a Director of Æterna Zentaris, Inc., (NASDAQ: AEZS), an international biopharmaceutical company. From 2003 until 2007, Dr. Mazzo served as President, Chief Executive Officer and a director of Chugai Pharma USA, LLC, a biopharmaceutical company which was the U.S. subsidiary of Chugai Pharmaceutical Co., Ltd. of Japan and a member of the Roche Group (Switzerland). Prior to joining Chugai, Dr. Mazzo held executive positions at several large international pharmaceutical companies, including: Schering-Plough Corporation, a publicly held pharmaceutical company that was subsequently acquired by Merck & Co., Inc. where he was also a Director of the Essex Chimie European subsidiary; Hoechst Marion Roussel, Inc., the US subsidiary of Hoechst AG, which was subsequently acquired by Sanofi, a multinational pharmaceuticals company; and Rhone-Poulenc Rorer, Inc., a subsidiary of Rhone-Poulenc SA, a French pharmaceuticals company, which was subsequently acquired by Hoechst AG. From October 2005 through January 2015, he also served on the board of directors of Avanir Pharmaceuticals, a biopharmaceutical company which was sold to Otsuka Holdings in 2015. From August 2005 to June 2005, he served as a Director of EyePoint Pharmaceuticals (formerly known as pSivida, Inc. (NASDAQ: EYPT). Dr. Mazzo earned a B.A. in the Honors Program (Interdisciplinary Humanities) and a B.S. in Chemistry from Villanova University. In addition, Dr. Mazzo received his M.S. in chemistry and his Ph.D. degree in analytical chemistry from the University of Massachusetts, Amherst. He was also a research fellow at the Ecole Polytechnique Federale de Lausanne, Switzerland. In selecting Dr. Mazzo, the board took into account his vast experience in the pharmaceutical industry, as well as his service on other boards of directors in the biopharmaceutical industry.
| 53 |
Mary Ann Gray, PhD, has served on our board of directors since July 2019. From 2018 to current, Dr. Gray has served on the board of directors of Sarepta Therapeutics, Inc. From 2010 to 2018, Dr. Gray served as a member of the Board of Senomyx Inc., a biotechnology company working toward developing additives to amplify certain flavors and smells in foods. She served as a member of the compensation committee of Senomyx from May 2011 to November 2018, as the Chair of the Board and a member of the audit committee from May 2016 to November 2018, and as Lead Director from May 2017 to November 2018. Dr. Gray also served as a member of the Board and audit committee Chair of Juniper Pharmaceuticals, a women’s health company, from April 2016 to August 2018. From November 2014 to December 2016, she served as a Board member of TetraLogic, a publicly-held clinical-stage biopharmaceutical company focused on oncology and infectious diseases. She served as the Chair of the audit committee of Tetralogic from March 2015 to December 2016. Dr. Gray also served as a Board member of Acadia Pharmaceuticals, focused on commercialization of CNS therapies, from 2005 to 2016, and served as a member of the audit committee from 2005 to 2016 and as a member of the compensation committee from 2010 to 2016. She served as a Board member of Dyax Corp., a rare disease company acquired by Shire in 2016, from 2001 to 2016, serving as a Lead Director from 2008 to 2016, a member of the audit committee from 2004 to 2012, a member of the nominating and corporate governance committee from 2001 to 2016, and Chair of the compensation committee from 2012 to 2016. Dr. Gray is the President of Gray Strategic Advisors, LLC, a biotechnology strategic planning and advisory firm. Dr. Gray has a distinguished scientific background, completing pharmacology research in tumor biology, including the impact of therapeutics on cardiac membranes and beginning her career in biotechnology as a scientist focused on new drug development. She subsequently worked in equities research before becoming a senior analyst and portfolio manager. Dr. Gray earned a B.S. from University of South Carolina, a Ph.D. in pharmacology from the University of Vermont, and completed her post-doctoral work at Northwestern University Medical School and at the Yale University School of Medicine. Our nominating and corporate governance committee believes that Dr. Gray’s extensive experience in the biotechnology and biopharmaceutical industry qualifies her for service as a member of our Board.
SECTION 16(A) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Section 16(a) of the Exchange Act requires our officers, directors, and stockholders owning more than ten percent of our common stock, to file reports of ownership and changes in ownership with the SEC and to furnish us with copies of such reports. Based solely on our review of Form 3, 4 and 5’s, the following table provides information regarding any of the reports which were filed late during the fiscal year ended December 31, 2020:
| Name of Reporting Person | Type of Report and Number Filed Late | No. of Transactions Reported Late | ||
| Matthew Kalnik | Form 4 | 1 (1) | ||
| Dane Saglio | Form 4 | 1 (1) |
| (1) | Transaction not reported as of the date hereof |
Corporate Governance Guidelines and Code of Ethics
We have adopted Corporate Governance Guidelines that are intended to ensure that our Board has the necessary authority and practices in place to review and evaluate our business operations and to make decisions that are independent of management. The Corporate Governance Guidelines are intended to align the interests of directors and management with those of our shareholders and establish practices for the Board with regard to its oversight of the Company. Under our guidelines, the Board conducts a self-evaluation to assess adherence to the Corporate Governance Guidelines and identify opportunities to improve Board performance. A copy of our codes can be viewed on our website at www.senecabio.com under “Governance Documents” in the “Corporate Governance” section under the “Investors” tab.
In addition to our Corporate Governance Guidelines, we have adopted several guidelines intended to promote the honest and ethical conduct of our officers, directors, employees and consultants. They include, our "Code of Ethics” that applies to our officer, directors and employees and our “Finance Code of Professional Conduct” that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions, and any persons who participate in our financial reporting process. A copy of our codes can be viewed on our website at www.senecabio.com under “Governance Documents” in the “Corporate Governance” section under the “Investors” tab.
| 54 |
The codes incorporate our guidelines designed to deter wrongdoing and to promote honest and ethical conduct and compliance with applicable laws and regulations. The codes also incorporate our expectations of our officers, directors and employees that enable us to provide accurate and timely disclosure in our filings with the SEC and other public communications. In addition, the codes incorporate guidelines pertaining to topics such as complying with applicable laws, rules, and regulations; reporting violations; and maintaining accountability for adherence to the codes.
We intend to disclose future amendments to certain provisions of our codes, or waivers of such provisions on our web site within four business days following the date of such amendment or waiver.
Board of Directors
Our Board consists of five (5) members. Our business, property and affairs are managed under the direction of the Board. Members of the Board are kept informed of our business through discussions with the Executive Chairman and other members of management, by reviewing materials provided to them and by participating in meetings of the Board and its committees.
Our Board is responsible for establishing broad corporate policies and for overseeing our overall management. In addition to considering various matters which require its approval, the Board provides advice and counsel to, and ultimately monitors the performance of, our senior management.
Classification of Board
Pursuant to our bylaws, we have a classified Board which is divided into three classes with staggered three-year terms. Only one class may be elected each year, while the directors in the other classes continue to hold office for the remainder of their three-year terms. The Board may, on its own, determine the size of the exact number of directors on the Board and may fill vacancies on the Board. Notwithstanding, the holder of our Series A 4.5% Convertible Preferred Stock has the right to appoint one board member. Binxian Wei has been appointed and currently serves as such director since February 5, 2019. The procedure for electing and removing directors on a classified board of directors generally makes it more difficult for stockholders to change management control by replacing a majority of the board at any one time, and the classified board structure may discourage a third party tender offer or other attempt to gain control of the Company and may maintain the incumbency of directors. In addition, under our bylaws, directors may only be removed from office by a vote of the majority of the shares then outstanding and eligible to vote.
Independent Directors
Our common stock is listed on the Nasdaq Capital Market. As such, we are subject to the NASDAQ Stock Market LLC (“NASDAQ”) director independence standards. In accordance with these standards, in determining independence the Board affirmatively determines whether a director has a "material relationship" with Seneca Biopharma that would compromise his or her independence from management or would cause him or her to fail to meet the NASDAQ’s specific independence criteria. When assessing the "materiality" of a director's relationship with Seneca Biopharma the Board considers all relevant facts and circumstances, not merely from the director's standpoint, but from that of the persons or organizations with which the director has an affiliation, and, where applicable, the frequency and regularity of the services, and whether the services are being carried out at arm's length in the ordinary course of business. Material relationships can include commercial, consulting, charitable, familial and other relationships. A relationship is not material if, in the Board's judgment, it is not inconsistent with the NASDAQ’S director independence standards and it does not compromise a director's independence from management.
Applying the NASDAQ’s standards, the Board has determined that Mr. Wei and Drs. Mazzo, Gray, and Csimma are each “independent” as that term is defined by the NASDAQ’s standards.
Communications with Directors
We have adopted a formal process for shareholder communications with our independent directors. The policy, is available on our website, www.senecabio.com in the “Governance Documents” section in the “Corporate Governance” section under the “Investors” tab. The Document is named “Board Contact.” Individuals wanting to communicate with our directors are invited to communicate with the non-management members of the Board by sending correspondence to the non-management members of the Board of Directors, c/o Corporate Secretary, Seneca Biopharma, Inc., 20271 Goldenrod Lane, Suite 2024, Germantown, MD 20876.
The Corporate Secretary will review all such correspondence and forward to the non-management members of the Board a summary of all such correspondence received during the prior month and copies of all such correspondence that deals with the functions of the Board or committees thereof or that otherwise is determined to require attention of the non-management directors. Non-management directors may at any time review the log of all correspondence received by us that are addressed to the non-management members of the Board and request copies of any such correspondence. Concerns relating to accounting, internal controls or auditing matters will immediately be brought to the attention of the Chairman of the Audit Committee.
| 55 |
Stock Ownership Guidelines
On November 10, 2016, we adopted stock ownership guidelines for our Chief Executive Officer, Chief Scientific Officer and named executive officers. Under the guidelines, our CEO and CSO are expected to own shares of our common stock that have a value equal to 2x their respective annual salaries. All other named executive officers or Section 16 filing employees are expected to own shares of our common stock that have a value equal to 1x their respective annual salaries. Shares may be owned directly by the individual or owned jointly with or separately by the individual’s spouse, or held in trust for the benefit of the individual, the individual’s spouse or children. Share ownership requirements must be met within five years after first becoming subject to the guidelines.
Committees
We have established three (3) corporate governance committees comprised of the: (i) Audit Committee; (ii) Compensation Committee; and (iii) Governance and Nominating Committee. The committee membership and the function of each of the committees are described below. Each committee is governed by written committee charters. We periodically review such charters and may amend or update the process and procedures contained therein. In the event of such amendment or update, we will promptly post our revised charter on our website. In addition to our established committee, we may from time to time establish special committees as the Board deems necessary. A copy of each respective committee’s charter can be viewed on our website at www.senecabio.com under “Corporate Governance” under the “Investors” tab.
The table below identifies the Board’s standing committees and committee membership as of February 28, 2021:
| Director | Independent | Audit Committee | Governance and Nominating Committee | Compensation Committee | ||||
| David J. Mazzo, PhD | Yes | Member | --- | Chair | ||||
| Cristina Csimma, PharmD, MHP | Yes | Member | Chair | Member | ||||
| Mary Ann Gray, PhD | Yes | Chair | Member | --- |
Each member of the Audit Committee, the Compensation Committee and the Governing and Nominating Committee is considered independent under Nasdaq listing criteria.
Audit Committee
We have a designated audit committee in accordance with section 3(a)(58)(A) of the Exchange Act. Currently, we have three members of the Audit Committee, Drs. Gray, Csimma, and Mazzo. The main function of our Audit Committee is to oversee our accounting and financial reporting processes. The Audit Committee assists the Board in fulfilling its oversight and monitoring responsibility of reviewing the financial information provided to shareholders and others, appoints Seneca Biopharma’s independent registered public accounting firm, reviews the services performed by the independent registered public accounting firm and Seneca Biopharma’s finance department, evaluates Seneca Biopharma’s accounting policies and the system of internal controls established by management and the Board, reviews significant financial transactions, and oversees enterprise risk management.
The Board has determined that Dr. Gray is an “audit committee financial expert” within the meaning of SEC rules. An audit committee financial expert is a person who can demonstrate the following attributes: (1) an understanding of generally accepted accounting principles and financial statements; (2) the ability to assess the general application of such principles in connection with the accounting for estimates, accruals and reserves; (3) experience preparing, auditing, analyzing or evaluating financial statements that present a breadth and level of complexity of accounting issues that are generally comparable to the breadth and complexity of issues that can reasonably be expected to be raised by the Company’s financial statements, or experience actively supervising one or more persons engaged in such activities; (4) an understanding of internal controls and procedures for financial reporting; and (5) an understanding of audit committee functions.
Governance and Nominating Committee
Our Governance and Nominating Committee’s purpose is to assist our board of directors in identifying individuals qualified to become members of our Board consistent with criteria set by our Board, to oversee the evaluation of the board of directors and management, and to develop and update our corporate governance principles. Drs. Csimma and Gray are the members of the Governance and Nominating Committee.
| 56 |
The Governance and Nominating Committee evaluates candidates for the Board. Candidates may come to the attention of the Governance and Nominating Committee through current Board members, professional search firms, stockholders or other persons. The Governance and Nominating Committee will consider nominees recommended by our stockholders.
Compensation Committee
The Compensation Committee reviews and approves the compensation arrangements for Seneca Biopharma’s executive officers, including the Executive Chairman, administers our equity compensation plans, and reviews the Board’s compensation. Drs. Mazzo and Csimma are members of the Compensation Committee.
Leadership Structure
The Board does not have a policy regarding the separation of the roles of Chief Executive Officer and Chairman of the Board as the Board believes it is in the best interests of the Company to make that determination based on the position and direction of the Company and the membership of the Board. At present, the positions of Chairman and Chief Executive Officer are held by the same individual. Based on our Executive Chairman’s knowledge of the Company, its business and its industry, the Board believes this structure is currently in the best interest of the Company and its shareholders.
Risk Oversight
The Company has a risk management program overseen by our Principal Executive Officer. Material risks are identified and prioritized by management, and each prioritized risk is referred to a Board Committee or the full Board for oversight. For example, strategic risks are referred to the full Board while financial risks are referred to the Audit Committees. The Board regularly reviews information regarding the Company's liquidity and operations, as well as the risks associated with each, and annually reviews the Company's risks as a whole. Also, the Compensation Committee periodically reviews the most important risks to the Company to ensure that compensation programs do not encourage excessive risk-taking. The Company currently does not have a lead independent director as it has currently determined one to not be necessary given the Company’s size. The Company’s lead independent director has the following responsibilities:
| · | Advising the executive chairman of the Board as to the quality, quantity, and timeliness of the flow of information from management that is necessary for the independent directors to perform their duties effectively and responsibly. |
| · | Confirming the agenda with the Chief Executive Officer for meetings of the Board. |
| · | Coordinating and moderating executive sessions of the Board’s independent directors. |
| · | Acting as the principal liaison between the independent directors and the executive chairman of the Board on sensitive issues. |
| · | Performing such other duties as the Board may from time to time delegate in order to assist the Board in the fulfillment of its responsibilities. |
| ITEM 11. | EXECUTIVE COMPENSATION |
Our non-executive director and executive compensation programs impact all of our employees by establishing a general framework for compensation and creating a work environment focused on expectations, goals, and rewards. Because the performance of every employee is important to the overall success of the Company, our Board is mindful of the impact that our compensation programs have on all of our employees. In considering our compensation policies and practices, our Board balances the needs to conserve cash and minimize stockholder dilution against the requirements to attract, retain, and motivate our non-executive directors, executives and other employees while fostering an innovative and entrepreneurial corporate culture. Our Board strives to act in the long-term best interests of the Company and its stockholders, as well as ensure that the components of compensation do not, individually or in the aggregate, encourage excessive risk-taking.
Compensation-Setting Process
Role of the Board, Compensation Committee and Management
| 57 |
The Compensation Committee is responsible for overseeing, determining, recommending and approving the compensation of our non-executive directors, CEO and other executives, including the other Named Executive Officers. From time to time during the year, the Compensation Committee will review the compensation of our non-executive directors, CEO and other executives, determine whether to make any adjustments to their respective compensation. With regard to our executive officers, the Compensation Committee reviews base salaries, determine whether an annual incentive award was earned for the last completed fiscal year based on its assessment of the Company and individual performance for that period and, if so, the amount of any such bonuses, and determine whether to make equity awards based on Company and individual performance.
As described below, the Compensation Committee gives considerable weight to our CEO’s performance evaluation of the other executives because of his direct knowledge of each executive’s performance and contributions. The Compensation Committee conducts an annual review of our executives’ compensation and considers adjustments in executive compensation levels to ensure alignment with our compensation strategy and competitive market practices. During this process, the Compensation Committee is also mindful of the results of the shareholder’s Advisory Vote on Executive Compensation during the most recent vote and although not binding, is considered in the compensation setting process.
Role of Senior Management
The Compensation Committee typically seeks the input of our CEO when discussing the performance of and compensation for our other executives, including the other Named Executive Officers. In this regard, at the request of the Compensation Committee our CEO reviews the performance of the other executives, including the other Named Executive Officers, annually and presents to the Compensation Committee his conclusions and recommendations as to their compensation, including base salary adjustments, annual incentive awards, and long-term equity incentive awards. The Compensation Committee then uses these recommendations as one factor in its deliberations to determine the compensation of our executives.
Role of Compensation Consultant
The Compensation Committee is authorized to retain the services of one or more executive compensation advisors, as it sees fit, in connection with the oversight of our non-executive director and executive compensation program and related policies and practices. For compensation related to the year ended December 31, 2020, the Compensation Committee consulted with Nancy Arnosti and Associates (“Arnosti”), a compensation consulting firm with regard to our executive compensation program. Arnosti was engaged to provide the Compensation Committee with information, recommendations, and other advice relating to these compensation programs on an ongoing basis. Arnosti was directly engaged and serves at the discretion of the Compensation Committee and provides no other services to the Company.
Competitive Positioning
In making compensation decisions, the Compensation Committee reviews independent survey data, as well as publicly available data from companies with which we compete for executive talent. The companies chosen for comparison may differ from one executive to the next depending on the scope and nature of the business for which the particular executive is responsible.
Although the compensation data from comparable companies is useful comparative information, the Compensation Committee does not require that the compensation components of the non-executive directors or individual executives bear any particular relationship to the compensation of non-executive director or executives of similar positions of those comparable companies. In development-focused companies within the biopharmaceutical industry, many traditional measures of corporate performance, such as earnings-per-share or sales growth, may not readily apply in reviewing the performance of executives. Because of the Company’s current stage of development, the Compensation Committee evaluates other indications of performance, including progress towards the Company’s research and development programs and corporate development activities, as well as the Company’s success in securing capital sufficient to enable the Company to continue research and development activities, in its decision-making process.
Say-on-Pay
At our 2020 Annual Meeting of Stockholders held on August 7, 2020 and adjourned until September 4, 2020, we submitted two proposals to its stockholders regarding its executive compensation practices.
The first was an advisory vote on the 2019 executive compensation awarded to our named executive officers (commonly known as a “say-on-pay” vote). At our 2020 annual meeting, excluding broker non-votes, approximately 5,721,142 shares cast votes with regard to the say-on-pay proposal. Of those, 2,960,782, or approximately 51.8%, of the shares approved the compensation of named executive officers. We believe that the outcome of its say-on-pay vote signals its stockholders’ support of our compensation approach, specifically its efforts to retain and motivate its named executive officers. In light of this stockholder support, the Compensation Committee determined not to change its approach to compensation. However, even though in 2020 stockholders demonstrated support for its compensation approach during 2019, the Compensation Committee annually reevaluates Seneca’s compensation practices to determine how they might be improved. The Compensation Committee will continue to consider the outcome of say-on-pay votes when making future compensation decisions for Seneca’s named executive officers.
| 58 |
The second proposal was a vote on the frequency of future stockholder advisory votes regarding compensation awarded to named executive officers (commonly known as a “say-when-on-pay” vote). The frequency of every one (1) year received the highest number of votes cast. Notwithstanding these results, our Board determined that Seneca would hold our next say-on-pay vote in 2021.
Summary Compensation Table
The following table sets forth information regarding the compensation paid to, or earned by, our named executive officers for the years ended December 31, 2020 and 2019:
| Name and Principal Position | Year | Salary | Bonus | Stock Awards | Option Awards | Nonequity Incentive Plan Compensation | Non-qualified Deferred Compensation Earnings | All Other Compensation | Total | |||||||||||||||||||||||||
| (a) | (b) | ($) (c) | ($) (d) | ($) (e) | ($) (f) (2) | ($) (g) | ($) (h) | ($) (i) (1) | ($) (j) | |||||||||||||||||||||||||
| Kenneth Carter, | 2020 | $ | 492,500 | - | - | 409,864 | (3) | - | - | - | $ | 902,364 | ||||||||||||||||||||||
| Executive Chairman (9) | 2019 | $ | 395,000 | 20,000 | - | 425,409 | (4) | - | - | - | $ | 840,409 | ||||||||||||||||||||||
| Matthew Kalnik, PhD | 2020 | $ | 311,250 | - | - | 340,242 | (5) | - | - | 171,140 | (6) | $ | 822,632 | |||||||||||||||||||||
| Chief Operating Officer and President (9) | 2019 | $ | - | - | - | - | - | - | 358,750 | (6) | $ | 358,750 | ||||||||||||||||||||||
| Dane Saglio | 2020 | $ | 281,250 | - | - | 85,061 | (7) | - | - | 154,505 | (8) | $ | 520,816 | |||||||||||||||||||||
| Chief Financial Officer (9) | 2019 | $ | - | - | - | - | - | - | 84,750 | (8) | $ | 84,750 | ||||||||||||||||||||||
_________________________________
| (1) | Includes automobile allowance, relocation allowance, perquisites and other personal benefits. |
| (2) | For additional information regarding the valuation of Option Awards, refer to Note 4 of our financial statements contained in this report. |
| (3) | Represents a stock option granted conditionally issued on April 1, 2020 that
was approved by Seneca’s shareholders on September 4, 2020. The option grant was initially to purchase up to 471,000 shares of common stock at an exercise price of $0.6199. The option contains anti-dilution protection to maintain percentage ownership and as of December 31, 2020, pursuant to certain issuances, the option was increased to an aggregate of 864,785 shares. The option (including any true-up issuances) vests (i) one quarter (1/4) on the original issuance date and (ii) three quarters (3/4) on a monthly basis over the thirty-six (36) month period following the original issuance date. Dr. Carter agreed in principle, subject to entering into a definitive agreement, to cancel the option grant immediately prior to closing in exchange for $188,789. If the Merger does not close, Dr. Carter will retain his option grant. |
| (4) | Includes an Inducement Award of 40,000 options issued initially and an anti-dilution true-up issuance of an additional 116,253 options per Dr. Carter’s employment agreement. The options had an exercise price of $8.50 and vest over time and based on milestones. Pursuant to Dr. Carter’s employment agreement, as amended, as a result of the approval of Dr. Carter’s option award by Seneca’s shareholders on September 4, 2020, as described in Footnote 3 above, the Inducement Option award was cancelled. |
| (5) | Represents an inducement stock option granted on April 1, 2020. The option grant was initially to purchase up to 282,840 shares of common stock at an exercise price of $0.6199. The option contains anti-dilution protection to maintain percentage ownership and as of December 31, 2020, pursuant to certain issuances, the option was increased to an aggregate of 518,979 shares. The option (including any true-up issuances) vests (i) one quarter (1/4) on the original issuance date and (ii) three quarters (3/4) on a monthly basis over the thirty-six (36) month period following the original issuance date. Dr. Kalnik agreed in principle, subject to entering into a definitive agreement, to cancel the option grant immediately prior to closing in exchange for $476,663. If the Merger does not close, Dr. Kalnik will retain his option grant. |
| (6) | Represents cash compensation for professional consulting services prior to Dr. Kalnik being appointed as Chief Operating Officer and President effective April 1, 2020 along with reimbursements for certain expenses in 2020. |
| (7) | Represents an inducement stock option granted on April 1, 2020. The option grant was
initially to purchase up to 70,710 shares of common stock at an exercise price of $0.6199. The option contains anti-dilution protection to maintain percentage ownership and as of December 31, 2020, pursuant to certain issuances, the option was increased to an aggregate of 129,745 shares. The option (including any true-up issuances) vests (i) one quarter (1/4) on the original issuance date and (ii) three quarters (3/4) on a monthly basis over the thirty-six (36) month period following the original issuance date. Mr. Saglio agreed in principle, subject to entering into a definitive agreement, to cancel the option grant immediately prior to closing in exchange for $362,392. If the Merger does not close, Mr. Saglio will retain his option grant. |
| (8) | Represents cash compensation for professional consulting services prior to Mr. Saglio being appointed as Chief Financial Officer effective April 1, 2020 along for reimbursement of certain expenses in 2020. |
| (9) | Employees were terminated without cause on March 17, 2021. |
| 59 |
Original Employment Agreement with Kenneth Carter
On December 18, 2018, Dr. Kenneth Carter was appointed the executive chairman of Seneca to be effective January 1, 2019. In connection with Dr. Carter’s employment, Seneca entered into an at-will employment agreement. Pursuant to the terms of his employment agreement, he received a signing bonus of $20,000 and receives a base salary of $395,000 per year and is eligible to receive an annual cash bonus based on achievement of certain performance milestones with a target of 50% of his base salary.
Dr. Carter was also issued an inducement option to purchase 40,000 shares of common stock on December 12, 2018. The inducement option has an exercise price of $8.50 per share, a term of ten (10) years, and vests as follows: (i) 10,000 options on the effective date, (ii) 5,000 options on the six (6) month anniversary of the effective date, (iii) 5,000 options vest on the two (2) year anniversary of the effective date, and (iv) the remaining 20,000 vest upon the achievement of performance-based milestones. As of December 31, 2019, 27,000 shares have vested, 4,000 have been forfeited for failure to meet the milestone vesting requirements, and 9,000 are currently unvested, subject to meeting vesting conditions.
For a twelve (12) month period following the effective date, Dr. Carter’s employment agreement further calls for the adjustment in the number of shares underlying the inducement option in the event of a capital raising transaction such that Dr. Carter’s ownership percentage would remain the same prior and subsequent to such transaction. Pursuant to Seneca’s registered direct offering on July 30, 2019, Seneca issued Dr. Carter an additional 116,213 options as an adjustment. Of this issuance, 78,444 shares have vested, 11,621 have been forfeited for failure to meet the vesting requirements, and 26,148 remain unvested as of December 31, 2019. This option was cancelled pursuant to Seneca's shareholders approving the Option Grant described in Dr. Carter's Amendment to Employment Agreement described above.
Dr. Carter’s employment agreement also provides for severance in the event Seneca terminates his employment without “cause” or he resigns with “good reason,” or as a result of his death or disability as each term is defined in the employment agreement or upon termination due to death or disability, Dr. carter will be entitled to (i) payment of his accrued base salary, unreimbursed expenses, unpaid but earned bonuses, and accrued and unused vacation time; (ii) the accelerated vesting of 100% of Dr. Carter’s then outstanding unvested equity awards, (iii) the continued payment of his base salary for (a) eighteen (18) months following the termination if such termination occurs within six (6) months of the effective date or if termination occurs within the eighteen (18) month period following a “sale event” or “change of control” and (b) twelve (12) months following the termination date if termination occurs after the initial six (6) month period following the effective date and (iv) payment of a pro rata portion of his target annual bonus for the year in which termination occurs. Dr. Carter will not be entitled to any continued payment of salary after the twenty-four (24) month anniversary of the effective date.
Amendment to Employment Agreement with Kenneth Carter
On March 26, 2020, Seneca and Dr. Kenneth Carter, its Executive Chairman, entered into an amendment (the “Amendment”) to Dr. Carter’s employment agreement with an effective date of April 1, 2020. The material terms of the Amendment that control and supersede the prior employment agreement are described herein.
Dr. Carter is to be employed as Executive Chairman of Seneca and will spend substantially all of his duties, attention, skill, and efforts working for the Company. He will not receive any signing / retention bonus. Dr. Carter was reimbursed $5,000 in legal, accounting and other expenses related to the negotiation and drafting of the amendment.
Pursuant to the terms of the Amendment, Dr. Carter will continue to serve as the Executive Chairman of Seneca and will receive an annual base salary of $525,000. Additionally, on the effective date, Dr. Carter received a conditional option to purchase 471,400 shares of common stock (“Option Grant”) of Seneca, subject to the receipt of shareholder approval as well as the forfeiture of all of his previously issued vested and unvested grants. The Option Grant has a term of ten (10) years from issuance, and an exercise price equal to the closing trading price of Seneca’s common stock on the effective date. The Option Grant vests (i) one quarter (1/4) on the effective date and (ii) three quarters (3/4) on a monthly basis over the thirty-six (36) month period following the effective date, provided Dr. Carter remains a service provider to Seneca over such period. For a period of nine (9) months from the effective date (or until the closing of a transaction related to issuing securities that was approved during such nine (9) month period) (the “Measurement Period”), the Option Grant will be subject to adjustment to maintain the percentage ownership the Option Grant reflects on the date of grant in the event that (i) Seneca issues any common stock (including, without limitation, by virtue of exercise, conversion or exchange of any common stock equivalents that are issued and outstanding prior to the end of the Measurement Period) during the Measurement Period, or (ii) there is any exercise, conversion, or exchange of common stock equivalents that are issued and outstanding prior to the end of the Measurement Period.
Upon termination by reason of death or disability (as such terms are defined in the Amendment), Dr. Carter will be entitled to receive the “Accrued Obligations”.
| 60 |
Upon termination by Seneca for “Cause” or by Dr. Carter without “Good Reason,” as such terms are described in the Amendment, Dr. Carter will only be entitled to receive the Accrued Obligations.
Upon termination by Seneca without “Cause” or by Dr. Carter with “Good Reason,” Dr. Carter will be entitled to (i) the Accrued Obligations, (ii) the continued payment of his base salary for (a) twelve (12) months if termination occurs after the nine (9) month anniversary of the effective date or (b) seven (7) months if termination occurs prior to the nine (9) month anniversary of the effective date (each as applicable, the “Severance Term”) (iii) payment of his bonus pro-rata for the time employed during the year of termination, (iv) COBRA payments for the applicable Severance Term, and (v) the continued vesting of all outstanding equity grants for the earlier of (y) the term of the equity awards or (z) the applicable Severance Term. Dr. Carter will be considered a service provider under the applicable plan in which such grants were issued until the last day of the Severance Term.
Upon a termination by Seneca without “Cause” or by Dr. Carter with “Good Reason” three (3) months prior to or twelve (12) months subsequent to a Change of Control (as such term is defined in the Amendment), Dr. Carter will be entitled to (i) the Accrued Obligations, (ii) the continued payment of his base salary for (a) eighteen (18) months if termination occurs after the nine (9) month anniversary of the effective date, or (b) nine (9) months if termination occurs prior to the nine (9) month anniversary of the effective date (each as applicable, “Change of Control Severance Term”), (iii) payment of 100% of target cash bonus for year of termination, (iv) COBRA payments for the applicable Change of Control Severance Term, and (v) the full vesting of all outstanding equity grants on the date of termination. Dr. Carter will be considered a service provider under the applicable plan in which such grants were issued until the last day of the applicable Change of Control Severance Term.
Termination of Dr. Carter’s Employment
Effective March 17, 2021, Dr. Carter entered into a separation agreement with the Company whereby his employment was terminated. For a description of the compensation payable to Dr. Carter, please refer to the section in this Item 11 of Part III of this Annual Report on Form 10-K entitled “Merger Related Compensation Arrangements.”
Employment Agreement with Dane Saglio
Effective April 1, 2020, Dane Saglio was appointed chief financial officer of Seneca. In connection with Mr. Saglio’s employment, Seneca entered into an at-will employment agreement with Mr. Saglio. Pursuant to the terms of the employment agreement, Mr. Saglio will receive a base salary of $375,000 per year and will be eligible to receive an annual target cash bonus of 40% of his base salary, based upon the achievement of certain performance goals and at the discretion of Seneca’s Compensation Committee. Mr. Saglio will also be eligible to receive an annual market-based equity grant to be issued from one of Seneca’s equity compensation plans at the discretion of the Board. In addition, as an inducement to Mr. Saglio’s employment, Seneca granted him a non-qualified inducement option to purchase up to 70,710 shares of Common Stock. The option has an exercise price of $0.6199 per share, a term of ten (10) years, and vests as follows: (i) one quarter (1/4) of the options vest on the effective date, and (ii) the remaining three-quarters (3/4) of the options will vest on a monthly basis over the thirty-six (36) month period following the effective date. The option was issued from Seneca’s Inducement Plan.
For a period of nine (9) months from the effective date (or until the closing of a transaction related to the issuance of securities that was approved during such nine (9) month period) (the “Saglio Measurement Period”), the inducement option will be subject to adjustment to maintain the percentage ownership represented by the inducement option on the date of grant, in the event that (i) Seneca issues any Common Stock (including, without limitation, by virtue of exercise, conversion or exchange of any Common Stock equivalents that are issued and outstanding prior to the end of the Saglio Measurement Period) during the Saglio Measurement Period, or (ii) there is any exercise, conversion, or exchange of Common Stock equivalents that are issued and outstanding prior to the end of the Saglio Measurement Period.
| 61 |
Upon termination by reason of death or disability (as such terms are defined in the Employment Agreement), Mr. Saglio will be entitled to receive the “Accrued Obligations”.
Upon termination by Seneca for “Cause” or by Mr. Saglio without “Good Reason,” as such terms are described in the employment agreement, Mr. Saglio will only be entitled to receive the Accrued Obligations.
Upon termination by Seneca without “Cause” or by Mr. Saglio with “Good Reason,” (as those terms are defined in the employment agreement) Mr. Saglio will be entitled to receive (i) the Accrued Obligations, (ii) the continued payment of his base salary for (a) nine (9) months if termination occurs after the nine (9) month anniversary of the effective date or (b) five (5) months if termination occurs prior to the nine (9) month anniversary of the effective date (each as applicable, the “Saglio Severance Term”) (iii) payment of his bonus pro-rata for the time employed during the year of termination, (iv) COBRA payments for the applicable Saglio Severance Term, and (v) the continued vesting of all outstanding equity grants for the earlier of (y) the term of the equity awards or (z) the applicable Saglio Severance Term. Mr. Saglio will be considered a service provider under the Inducement Plan or any other applicable equity compensation plan of Seneca until the last day of the Saglio Severance Term.
Upon termination by Seneca without “Cause” or by Mr. Saglio with “Good Reason” during the period commencing three (3) months prior to and terminating twelve (12) months subsequent to a Change of Control (as such term is defined in the employment agreement), Mr. Saglio will be entitled to (i) the Accrued Obligations, (ii) the continued payment of his base salary for (a) twelve (12) months if termination occurs after the nine (9) month anniversary of the effective date, or (b) six (6) months if termination occurs prior to the nine (9) month anniversary of the effective date (each as applicable, “Saglio Change of Control Severance Term”), (iii) payment of 100% of target cash bonus for the entire year of termination, (iv) COBRA payments for the applicable Saglio Change of Control Severance Term, and (v) the full vesting of all outstanding equity grants on the date of termination. Mr. Saglio will be considered a service provider under the Inducement Plan or any other applicable equity compensation plan of Seneca until the last day of the applicable Saglio Change of Control Severance Term.
In addition, Mr. Saglio has also entered into (i) Seneca’s standard confidential information and invention assignment agreement governing the ownership of any inventions and confidential information and (ii) Seneca’s standard indemnification agreement which is entered into by Seneca’s officers and directors.
Termination of Mr. Saglio’s Employment
Effective March 17, 2021, Mr. Saglio entered into a separation agreement with the Company whereby his employment was terminated. For a description of the compensation payable to Mr. Saglio, please refer to the section in this Item 11 of Part III of this Annual Report on Form 10-K entitled “Merger Related Compensation Arrangements.”
Employment with Matthew Kalnik
Effective April 1, 2020, Matthew Kalnik was appointed President and Chief Operating Officer of Seneca. In connection with Dr. Kalnik’s employment, Seneca entered into an at-will employment agreement with Dr. Kalnik. Pursuant to the terms of the employment agreement, Dr. Kalnik will receive a base salary of $415,000 per year and will be eligible to receive an annual target cash bonus of 45% of his base salary, based upon the achievement of certain performance goals and at the discretion of the Compensation Committee. Dr. Kalnik will also be eligible to receive an annual market-based equity grant to be issued from one of Seneca’s equity compensation plans at the discretion of the Board. In addition, as an inducement to Dr. Kalnik’s employment, Seneca granted him a non-qualified inducement option to purchase up to 282,840 shares of Common Stock on the effective date. The option has an exercise price of $0.6199 per share, a term of ten (10) years, and vests as follows: (i) one quarter (1/4) of the options vest on the effective date, and (ii) the remaining three-quarters (3/4) of the options will vest on a monthly basis over the thirty-six (36) month period following the effective date. The option was issued from the Inducement Plan. Dr. Kalnik agreed in principle, subject to entering into a definitive agreement, to cancel the option grant immediately prior to closing in exchange for $476,663. If the merger with Leading BioSciences does not close, Dr. Kalnik will retain his option grant.
| 62 |
For a period of nine (9) months from the effective date (or until the closing of a transaction related to issuing securities that was approved during such nine (9) month period) (the “Kalnik Measurement Period”), the inducement option will be subject to adjustment to maintain the percentage ownership represented by the inducement option on the date of grant in the event that (i) Seneca issues any Common Stock (including, without limitation, by virtue of exercise, conversion or exchange of any Common Stock equivalents that are issued and outstanding prior to the end of the Kalnik Measurement Period) during the Kalnik Measurement Period, or (ii) there is any exercise, conversion, or exchange of Common Stock equivalents that are issued and outstanding prior to the end of the Kalnik Measurement Period.
Additionally, pursuant to the employment agreement, Seneca agreed to reimburse Dr. Kalnik up to $5,000 for legal and accounting expenses incurred in connection with the drafting and negotiation of his employment related agreements.
Upon termination by reason of death or disability (as such terms are defined in the employment agreement), Dr. Kalnik will be entitled to receive the Accrued Obligations.
Upon termination by Seneca for “Cause” or by Dr. Kalnik without “Good Reason,” as such terms are described in the employment agreement, Dr. Kalnik will only be entitled to receive the Accrued Obligations.
Upon termination by Seneca without “Cause” or by Dr. Kalnik with “Good Reason,” (as those terms are defined in the employment agreement) Dr. Kalnik will be entitled to receive (i) the Accrued Obligations, (ii) the continued payment of his base salary for (a) eleven (11) months if termination occurs after the nine (9) month anniversary of the effective date or (b) six (6) months if termination occurs prior to the nine (9) month anniversary of the effective date (each as applicable, the “Kalnik Severance Term”) (iii) payment of his bonus pro-rata for the time employed during the year of termination, (iv) COBRA payments for the applicable Kalnik Severance Term, and (v) the continued vesting of all outstanding equity grants for the earlier of (y) the term of the equity awards or (z) the applicable Kalnik Severance Term. Dr. Kalnik will be considered a service provider under the Inducement Plan or any other applicable equity compensation plan of Seneca until the last day of the Kalnik Severance Term.
Upon termination by Seneca without “Cause” or by Dr. Kalnik with “Good Reason” during the period commencing three (3) months prior to and terminating twelve (12) months subsequent to a Change of Control (as such term is defined in the employment agreement), Dr. Kalnik will be entitled to (i) the Accrued Obligations, (ii) the continued payment of his base salary for (a) fifteen (15) months if termination occurs after the nine (9) month anniversary of the effective date, or (b) eight (8) months if termination occurs prior to the nine (9) month anniversary of the effective date (each as applicable, “Kalnik Change of Control Severance Term”), (iii) payment of 100% of target cash bonus for the entire year of termination, (iv) COBRA payments for the applicable Kalnik Change of Control Severance Term, and (v) the full vesting of all outstanding equity grants on the date of termination. Dr. Kalnik will be considered a service provider under the Inducement Plan or any other applicable equity compensation plan of Seneca until the last day of the applicable Kalnik Change of Control Severance Term.
Termination of Dr. Kalnik’s Employment
Effective March 17, 2021, Dr. Kalnik entered into a separation agreement with the Company whereby his employment was terminated. For a description of the compensation payable to Dr. Kalnik, please refer to the section in this Item 11 of Part III of this Annual Report on Form 10-K entitled “Merger Related Compensation Arrangements.”
| 63 |
Merger Related Executive Compensation Arrangements
The following table and related footnotes present information about the compensation payable to Seneca’s named executive officers (who are the only executive officers of Seneca) in connection with the consummation of the Merger, and their associated termination without cause from Seneca that occurred on March 17, 2021. The compensation shown in the table below is intended to comply with Item 402(t) of Regulation S-K, which requires disclosure of information about compensation for each named executive officer that is based on or otherwise relates to the proposed Merger.
The values in the table below show amounts due and payable to executives as a result of their termination without cause on March 17, 2021, and amounts due upon consummation of the Merger, if and when it occurs.
| Name (a) | Cash
($) (b) | Equity
($) (c) | Pension/NQDC ($) (d) | Perquisites/Benefits
($) (e) | Tax
Reimbursement ($) (f) | Other
($) (g) | Total
($) (h) | |||||||||||||||||||||
| Kenneth Carter, PhD | 1,050,000 | 188,787 | (1) | - | 44,243 | - | - | 1,283,030 | ||||||||||||||||||||
| Mathew Kalnik, PhD | 705,500 | 476,662 | (1) | - | 44,593 | - | - | 1,226,755 | ||||||||||||||||||||
| Dane Saglio | 525,000 | 362,391 | (1) | - | 28,429 | - | - | 915,820 | ||||||||||||||||||||
| (1) | Represents anticipated payment for the cancellation of outstanding stock options. |
Equity Compensation Plans
Seneca currently has the following equity compensation plans outstanding as of the date hereof: (i) 2010 Equity Compensation Plan, (ii) 2019 Equity Incentive Plan, (iii) 2020 Equity Incentive Plan and (iv) the Inducement Award Stock Option Plan.
For information related to Seneca’s equity compensation plans from which Seneca’s officers and directors are issued securities, please see the sections below entitled “2010 Equity Compensation Plan,” “2019 Equity Incentive Plan,” “Equity Incentive Plan,” and “Inducement Award Stock Option Plan.”
2010 Equity Compensation Plan
Seneca’s 2010 Equity Compensation Plan, as amended (“2010 Plan”) was approved by our stockholders on June 22, 2017 and is administered by Seneca’s board or its compensation committee. The 2010 Plan provides for the grant of incentive stock options, nonstatutory stock options, restricted stock, performance units, performance shares, restricted stock units, and other stock-based awards to its employees, directors, and consultants. The purpose of the 2010 Plan is to attract and retain the best available personnel for positions of substantial responsibility, to provide additional incentive to Seneca’s employees, directors and consultants, and to promote the success of Seneca’s business. Under the terms of the 2010 Plan, Seneca currently has authorized 88,846 shares of Seneca Common Stock for the foregoing awards.
2019 Equity Incentive Plan
Seneca’s 2019 Equity Incentive Plan (“2019 Plan”) was approved by Seneca’s stockholders on June 12, 2019 and is administered by Seneca’s Board or its compensation committee. The 2019 Plan provides for the grant of incentive stock options, nonstatutory stock options, restricted stock, performance units, performance shares, restricted stock units, and other stock-based awards to our employees, directors, and consultants. The purpose of the 2019 Plan is to attract and retain the best available personnel for positions of substantial responsibility, to provide additional incentive to our employees, directors and consultants, and to promote the success of Seneca’s business. Under the terms of the 2019 Plan, Seneca initially reserved 200,000 shares of Seneca Common Stock, subject to an automatic increase on the first day of each calendar year by 4% of the total shares of Seneca Common Stock issued and outstanding on such date. The 2019 Plan further authorized the administrator to amend the exercise price and terms of certain awards thereunder.
Equity Incentive Plan
Our 2020 Equity Incentive Plan (“2020 Plan”) was approved our stockholders and is administered by our Board or our Compensation Committee. The 2020 Plan provides for the grant of incentive stock options, nonstatutory stock options, restricted stock, performance units, performance shares, restricted stock units, and other stock-based awards to our employees, directors, and consultants. The purpose of the 2020 Plan is to attract and retain the best available personnel for positions of substantial responsibility, to provide additional incentive to our employees, directors and consultants, and to promote the success of our business. Under the terms of the 2020 Plan, Seneca initially reserved 600,000 shares of common stock. The 2020 Plan provides that the shares under the plan, as well as the shares underlying grants, is subject to automatic increase upon the occurrence of certain dilutive events. The 2020 Plan further authorized the administrator to amend the exercise price and terms of certain awards thereunder.
| 64 |
Inducement Award Stock Option Plan
Seneca’s Inducement Award Stock Option Plan (“Inducement Plan”) is administered by Seneca’s Board or its compensation committee. The Inducement Plan is intended to be used in connection with the recruiting and inducement of senior management and employees. The issuance of awards under the Inducement Plan is at the discretion of the administrator which has the authority to determine the persons to whom any awards shall be granted and the terms, conditions and restrictions applicable to any award. Pursuant to the Inducement Plan, as amended and currently in effect, Seneca may grant stock options for up to a total of 215,000 shares of Seneca Common Stock to new employees of Seneca. As of December 31, 2019, 140,592 grants have been made pursuant to the Inducement Plan. On March 23, 2020 Seneca’s Board approved an amendment to the Inducement Plan increasing the numbers of shares authorized under the Inducement Plan to 715,000. The Inducement Plan is intended to qualify as an inducement plan under Nasdaq Listing Rule 5635(c)(4) and accordingly, Seneca did not seek stockholders’ approval.
Outstanding Equity Awards Value at Fiscal Year-End
The following table includes information with respect to the value of all outstanding equity awards previously awarded to our named executive officers as of December 31, 2020. All references to common stock, share, and per share amounts have been retroactively restated to reflect the 1:20 reverse stock split that became effective on July 17, 2019.
| Number of securities underlying unexercised options - exercisable | Number of securities underlying unexercised options - unexercisable | Equity incentive plan awards: Number of securities underlying unexercised unearned options | Option exercise price | Option expiration date | Number of shares or units of stock that have not vested | Market value of shares of units of stock that have not vested | Equity incentive plan award: Number of unearned shares, units or other rights that have not vested | Equity incentive plan awards: Market or payout value of unearned shares, units or other rights that have not vested | ||||||||||||||||||||||||||
| Name | (#) | (#) | (#) | ($) | (#) | ($) | (#) | (#) | ||||||||||||||||||||||||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | |||||||||||||||||||||||||
| Kenneth Carter (1) | 360,327 | 504,458 | - | $ | 0.6199 | 4/1/2030 | - | - | - | - | ||||||||||||||||||||||||
| Matthew Kalnik, PhD (2) | 216,241 | 302,738 | - | $ | 0.6199 | 4/1/2030 | - | - | - | - | ||||||||||||||||||||||||
| Dane Saglio (3) | 54,060 | 75,685 | - | $ | 0.6199 | 4/1/2030 | - | - | - | - | ||||||||||||||||||||||||
_____________
| (1) | Represents a stock option granted conditionally issued on April 1, 2020 that was approved by Seneca’s shareholders on September 4, 2020. The option grant was initially to purchase up to 471,000 shares of common stock at an exercise price of $0.6199. The option contains anti-dilution protection to maintain percentage ownership and as of December 31, 2020, pursuant to certain issuances, the option was increased to an aggregate of 864,785 shares. The option (including any true-up issuances) vests (i) one quarter (1/4) on the original issuance date and (ii) three quarters (3/4) on a monthly basis over the thirty-six (36) month period following the original issuance date. The options were all issued from our 2020 Equity Incentive Plan. Dr. Carter agreed in principle, subject to entering into a definitive agreement, to cancel the option grant immediately prior to closing in exchange for $188,789. If the Merger does not close, Dr. Carter will retain his option grant. |
| (2) | Represents an inducement stock option granted on April 1, 2020. The option grant was initially to purchase up to 282,840 shares of common stock at an exercise price of $0.6199. The option contains anti-dilution protection to maintain percentage ownership and as of December 31, 2020, pursuant to certain issuances, the option was increased to an aggregate of 518,979 shares. The option (including any true-up issuances) vests (i) one quarter (1/4) on the original issuance date and (ii) three quarters (3/4) on a monthly basis over the thirty-six (36) month period following the original issuance date. The options were all issued from our Inducement Stock Option Plan. Dr. Kalnik agreed in principle, subject to entering into a definitive agreement, to cancel the option grant immediately prior to closing in exchange for $476,663. If the Merger does not close, Dr. Kalnik will retain his option grant. |
| (3) | Represents an inducement stock option granted on April 1, 2020. The option grant was initially to purchase up to 70,710 shares of common stock at an exercise price of $0.6199. The option contains anti-dilution protection to maintain percentage ownership and as of December 31, 2020, pursuant to certain issuances, the option was increased to an aggregate of 129,745 shares. The option (including any true-up issuances) vests (i) one quarter (1/4) on the original issuance date and (ii) three quarters (3/4) on a monthly basis over the thirty-six (36) month period following the original issuance date. The options were all issued from our 2020 Equity Incentive Plan. Mr. Saglio agreed in principle, subject to entering into a definitive agreement, to cancel the option grant immediately prior to closing in exchange for $362,392. If the Merger does not close, Mr. Saglio will retain his option grant. |
DIRECTOR COMPENSATION
Board Compensation Arrangements
Our non-executive director compensation program is overseen and approved by our Compensation Committee and is designed to enable us to continue to attract and retain highly qualified directors by ensuring that director compensation is in line with peer companies competing for director talent, and is designed to address the time, effort, expertise, and accountability required of active board membership. In general, we believe that annual compensation for non-employee directors should be cash and equity based and designed to compensate members for their service on the Board and its committees, align the interests of directors and stockholders and, by vesting over time, to create an incentive for continued service on the Board. Our Compensation Committee annually reviews and approves compensation programs related to our non-employee members of the Board of Directors.
| 65 |
The following are the terms of our Director Compensation Plans pursuant to which non-employee directors are compensated:
Current Non-Employee Board Member Compensation Policy
Effective April 1, 2020, each non-employee Board member will receive the following compensation commencing April 1 and ending on March 31 (“Board Year”):
| · | A grant of 6,000 restricted stock units (“RSUs”) issued from one of Seneca’s equity compensation plans. The RSU’s will be granted on April 3, 2020 and then on April 1, of each subsequent year and will vest quarterly over the grant year on June 30, September 30, December 31 and March 31. |
| · | An annual cash fee of $40,000. |
In addition, non-employee Board members serving on committees will receive the following additional consideration:
| · | The lead independent director will receive an additional annual fee of $25,000; |
| · | Each member of the Audit Committee will receive an additional annual fee of $10,000; |
| · | Each member of the Compensation Committee will receive an additional annual fee of $7,500; and |
| · | Each member of the Governance and Nominating Committee will receive an additional annual fee of $5,000. |
In addition to any other consideration received, non-employee Board members serving as a Chairperson will receive the following additional consideration:
| · | The Audit Committee Chair will receive an additional annual fee of $10,000 (for chairing the committee in addition to the committee membership fee); |
| · | The Compensation Committee Chair will receive an additional annual fee of $7,500 (for chairing the committee in addition to the committee membership fee); and |
| · | The Governance and Nominating Committee Chair will receive an additional annual fee of $5,000 (for chairing the committee in addition to the committee membership fee). |
In addition, each non-employee Board member may elect to receive their respective shares of Seneca Common Stock upon vesting of the RSUs on a net basis to allow for tax withholdings by Seneca. Moreover, all cash compensation paid to non-employee Board members will be paid in arrears and on a quarterly basis over the Board Year.
Legacy Director Compensation Plan (no longer in effect)
Prior to April 1, 2020, each non-employee director received a $100,000 annual board fee. The annual board fee was payable as follows: (i) up to $50,000 in cash and (ii) the balance in equity grants consisting of common stock purchase options, restricted stock units or restricted stock, at the election of each non-employee director. Directors electing to receive a portion of their annual fee in cash received four equal quarterly payments during the year. Applicable equity grants were made as of July 1 of each year and vested quarterly over the grant year. Fees for new directors appointed or elected during the year were pro-rated and made on the fifth (5th) day following such approval and acceptance on the Board.
Each non-employee director continuing service was required to make an election to receive the board fee in either cash, restricted stock, restricted stock units, or common stock options or a combination thereof by June 19th of each year. All grants of restricted stock and restricted stock units were valued using the adjusted closing bid price of Seneca Common Stock on the applicable grant date. All option grants were valued using the Black-Scholes option pricing model and are subject to customary assumptions used in the preparation of the financial statements.
Board Compensation for 2020 Board Year
The following table summarizes compensation paid/to be paid to non-employee directors during the year ended December 31, 2020.
| 66 |
| Name | Fees Earned or Paid in Cash | Stock Awards | Option Awards | Nonequity Incentive Plan Compensation | Non-qualified Deferred Compensation Earnings | All Other Compensation | Total | |||||||||||||||||||||
| (a) | ($) (b) | ($) (c) | ($) (d) | ($) (e) | ($) (f) | ($) (g) | ($) (h) | |||||||||||||||||||||
| Scott Ogilvie | $ | - | ||||||||||||||||||||||||||
| Independent Director (1) (4) | $ | 12,500 | $ | - | $ | 25,000 | $ | - | $ | - | $ | 22,499 | $ | 59,999 | ||||||||||||||
| David J. Mazzon, PhD | $ | - | ||||||||||||||||||||||||||
| Independent Director (2) | $ | 61,500 | $ | 3,150 | $ | 25,000 | $ | - | $ | - | $ | - | $ | 89,650 | ||||||||||||||
| Cristina Csimma, PharmD, MHP | $ | - | ||||||||||||||||||||||||||
| Independent Director (2) | $ | 77,875 | $ | 3,150 | $ | 25,000 | $ | - | $ | - | $ | - | $ | 106,025 | ||||||||||||||
| Binxian Wei | $ | - | ||||||||||||||||||||||||||
| Independent Director (3) | $ | 30,000 | $ | 3,150 | $ | 50,000 | $ | - | $ | - | $ | - | $ | 83,150 | ||||||||||||||
| Sandford Smith | $ | - | ||||||||||||||||||||||||||
| Independent Director (4) | $ | 12,500 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | 12,500 | ||||||||||||||
| Mary Ann Gray, PhD | $ | - | ||||||||||||||||||||||||||
| Independent Director (5) | $ | 48,750 | $ | 10,586 | $ | 12,504 | $ | - | $ | - | $ | - | $ | 71,840 | ||||||||||||||
____________________________
| (1) | The Director’s compensation includes $24,999 in consulting fees earned after his board service ended. The compensation also includes vesting of stock options to purchase 5,054 shares of common stock at $6.00 per share. |
| (2) | The Director’s compensation includes vesting of stock options to purchase 5,054 shares of common stock at $6.00 per share and 5,400 restricted stock units. |
| (3) | The Director’s compensation includes vesting of stock options to purchase 10,106 shares of common stock at $6.00 per share and 5,400 restricted stock units. |
| (4) | The Directors resigned from the Board on March 26, 2020. |
| (5) | The Director’s compensation includes vesting of stock options to purchase 2,568 shares of common stock at $5.90 per share, 7,042 restricted stock units and 3,814 shares of restricted stock. |
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND |
RELATED STOCKHOLDER MATTERS.
Equity Compensation Plan Information
The following table sets forth information with respect to our equity compensation plans as of December 31, 2020.
| 67 |
| Number of Securities to be Issued upon Exercise of Outstanding Options and Rights | Weighted-Average Exercise Price for Outstanding Options and Rights | Number of Securities Remaining Available for Future Issuance under Equity compensation Plans (Excluding Securities Reflected in Column (a)) | ||||||||||
| Plan Category | (a) | (b) | (c) | |||||||||
| Equity compensation plans approved by security holders | ||||||||||||
| 2007 Stock Plan | 1,688 | $ | 173.10 | * | ||||||||
| 2010 Equity Compensation Plan | 57,464 | $ | 214.88 | 19,037 | ||||||||
| 2019 Equity Incentive Plan (1) | 80,662 | $ | 4.06 | 946,723 | ||||||||
| 2020 Equity Compensation Plan | 1,037,742 | $ | 0.62 | 34,320 | ||||||||
| Equity compensation plans not approved by security holders | ||||||||||||
| Inducement Plan | 648,724 | $ | 0.62 | 66,276 | ||||||||
| Total | 1,826,280 | $ | 7.67 | 1,066,356 | ||||||||
| * Our 2007 Stock Plan terminated. Accordingly, although certain outstanding awards under the plan can still be exercised, no additional grants may be made pursuant to such plan. |
| (1) On January 1 of each calendar year, the number of shares of common stock authroized under the 2019 Equity Incentive Plan increases by 4% of the total shares of common stock issued and outstanding on such date |
2019 Equity Incentive Plan
Our 2019 Equity Incentive Plan (“2019 Plan”) was approved by our stockholders on June 12, 2019 and is administered by our board or our compensation committee. The 2019 Plan provides for the grant of incentive stock options, nonstatutory stock options, restricted stock, performance units, performance shares, restricted tock units, and other stock-based awards to our employees, directors, and consultants. The purpose of the 2019 Plan is to attract and retain the best available personnel for positions of substantial responsibility, to provide additional incentive to our employees, directors and consultants, and to promote the success of our business. Under the terms of the 2019 Plan, we initially reserved 200,000 shares of common stock, subject to an automatic increase on the first day of each calendar year by 4% of the total shares of common stock issued and outstanding on such date. The 2019 Plan further authorized the administrator to amend the exercise price and terms of certain awards thereunder.
Equity Compensation Plans Not Approved by Security Holders
Inducement Plan
Our Inducement Award Stock Option Plan (“Inducement Plan”) is administered by our board or our compensation committee. The Inducement Plan is intended to be used in connection with the recruiting and inducement of senior management and employees. The issuance of awards under the Inducement Plan is at the discretion of the administrator which has the authority to determine the persons to whom any awards shall be granted and the terms, conditions and restrictions applicable to any award. Pursuant to the Inducement Plan, as amended and currently in effect, the Company may grant stock options for up to a total of 175,000 shares of common stock to new employees of the Company. As of December 31, 2019, 140,592 grants have been made pursuant to the Inducement Plan. The Inducement Plan is intended to qualify as an inducement plan under NASDAQ Listing Rule 5635(c)(4) and accordingly, the Company did not seek stockholders’ approval.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth, as of February 28, 2021, information regarding beneficial ownership of our capital stock by:
| · | each person, or group of affiliated persons, known by us to be the beneficial owner of 5% or more of any class of our voting securities; |
| · | each of our current directors and nominees; |
| · | each of our current named executive officers; and |
| · | all current directors and named executive officers as a group. |
| 68 |
Beneficial ownership is determined according to the rules of the SEC. Beneficial ownership means that a person has or shares voting or investment power of a security and includes any securities that person or group has the right to acquire within 60 days after the measurement date. This table is based on information supplied by officers, directors and principal stockholders. Except as otherwise indicated, we believe that each of the beneficial owners of the common stock listed below, based on the information such beneficial owner has given to us, has sole investment and voting power with respect to such beneficial owner’s shares, except where community property laws may apply.
| Common Stock | ||||||||||||||
| Name and Address of Beneficial Owner (1) | Shares | Shares Underlying Convertible Securities | Total | Percent of Class (2) | ||||||||||
| Directors and named executive officers | ||||||||||||||
| Kenneth Carter (5) | - | 396,360 | 396,360 | 2.24% | ||||||||||
| Cristina Csimma, Pharm.D, MHP | 7,430 | 13,089 | 20,519 | * | ||||||||||
| Binxian Wei (3) | 6,000 | 26,137 | 32,137 | * | ||||||||||
| David Mazzo | 6,000 | 10,561 | 16,561 | * | ||||||||||
| Mary Ann Gray, Ph.D | 18,259 | 4,954 | 23,213 | * | ||||||||||
| Matthew Kalnik, PhD (4) (5) | - | 237,865 | 237,865 | 1.36% | ||||||||||
| Dane Saglio (4) (5) | - | 59,466 | 59,466 | * | ||||||||||
| All directors and named executive officers as a group (7 individuals) | 786,121 | 4.41% | ||||||||||||
| 5% owners as reported on form SC 13G | ||||||||||||||
| None | ||||||||||||||
| All directors, named executive officers, and 5% owners as a group (7 entities) | 786,121 | 4.41% | ||||||||||||
________________________________
| * | Represents less than one percent |
| (1) | Except as otherwise indicated, the persons named in this table have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them, subject to community property laws where applicable and to the information contained in the footnotes to this table. Unless otherwise indicated, the address of the beneficial owner is c/o Seneca Biopharma, Inc. 20271 Goldenrod Lane, Germantown, MD 20876. |
| (2) | Pursuant to Rules 13d-3 and 13d-5 of the Exchange Act, beneficial ownership includes any shares as to which a shareholder has sole or shared voting power or investment power, and also any shares which the shareholder has the right to acquire within 60 days, including upon exercise of common share purchase options or warrants. There are 17,295,703 shares of common stock issued and outstanding as of November 15, 2020. |
| (3) | Mr. Wei is appointed by the Series A 4.5% Convertible Preferred Stock owners. |
| (4) | Dr. Kalnik was appointed as our Chief Operating Officer and President and Mr. Saglio was appointed as Seneca’s Chief Financial Officer effective April 1, 2020. |
| (5) | Holder has agreed in principle to the cancellation of all stock options prior to completion of Merger in exchange for cash consideration. In the event that the Merger does not close, holder will retain such options. |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
RELATED PARTY TRANSACTIONS
Related Party Transactions Procedures
We review all known relationships and transactions in which Seneca Biopharma and our directors, executive officers, and significant stockholders or their immediate family members are participants to determine whether such persons have a direct or indirect interest. Our management, in consultation with our outside legal consultants, determines based on specific fact and circumstances whether Seneca Biopharma or a related party has a direct or indirect interest in these transactions. In addition, our directors and executive officers are required to notify us of any potential related party transactions and provide us with the information regarding such transactions.
If it is determined that a transaction is a related party transaction, the Audit Committee must review the transaction and either approve or disapprove it. In determining whether to approve or ratify a transaction with a related party, the Audit Committee will take into account all of the relevant facts and circumstances available to it, including, among any other factors it deems appropriate:
| 69 |
| · | the benefits to us of the transaction; |
| · | the nature of the related party’s interest in the transaction; |
| · | whether the transaction would impair the judgment of a director or executive officer to act in the best interests of Seneca Biopharma and our stockholders; |
| · | the potential impact of the transaction on a director’s independence; and |
| · | whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances. |
Any member of the Audit Committee who is a related party with respect to a transaction under review may not participate in the deliberations or vote on the approval of the transaction.
Related Party Transactions
Summarized below are certain transactions and business relationships between Seneca Biopharma and persons who are or were an executive officer, director or holder of more than five percent of any class of our securities since January 1, 2020.
Information regarding disclosure of an employment relationship or transaction involving an executive officer and any related compensation solely resulting from that employment relationship or transaction is included in the Section of this Annual Report entitled “Director Compensation” and “Executive Compensation.”
Information regarding disclosure of compensation to a director is included in the Section of this Annual Report entitled “Director Compensation.”
Information regarding the identification of each independent director is included in the Section of this Annual Report entitled “Directors, Executive Officers and Corporate Governance.”
All of our officers and directors enter into our standard indemnification agreement.
| · | During the Board fiscal year of January 1, 2020 through December 31, 2020, we paid the following compensation to our non-employee board members: |
1. An aggregate of $243,925 in cash.
2. An aggregate of 24,000 restricted stock units valued at $16,800.
| · | On March 17, 2021, the Company terminated (i) Kenneth Carter, PhD, Seneca’s executive chairman, (ii) Dane Saglio, Seneca’s chief financial officer, (iii) Matthew Kalnik, PhD, Senecas’s chief operating officer and (iv) Seneca’s Senior Vice President of R&D (collectively, the “Employees”) without cause. In connection with the Employees’ terminations, the Company entered into separation agreements. The separation agreements contain mutual general releases of claims and acknowledge the amounts due to each Employee as a result of their terminations without cause as provided for in each of their respective employment agreements. As a result of their termination, the Company will repurchase their outstanding common stock purchase options for an aggregate of $1,423,012. For a further description of the payments to be made to each terminated employee, please see the section in Item 11 of Part III of this Annual Report on Form 10-K entitled “Merger Related Compensation Arrangements.” |
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
The following table summarizes the approximate aggregate fees billed to us or expected to be billed to us by our independent auditors, Dixon Hughes Goodman LLP for our 2020 and 2019 fiscal years, respectively:
| Type of Fees | 2020 | 2019 | ||||||
| Audit Fees | $ | 120,700 | $ | 119,350 | ||||
| Audit Related Fees | 11,280 | 30,000 | ||||||
| Tax Fees | 12,400 | 12,000 | ||||||
| All other Fees (1) | 15,500 | - | ||||||
| Total Fees | $ | 159,880 | $ | 161,350 | ||||
| (1) | Fees associated with registration statements and issuance of comfort letters |
Pre-Approval of Independent Auditor Services and Fees
Our audit committee reviewed and pre-approved all audit and non-audit fees for services provided by Dixon Hughes Goodman LLP and has determined that the provision of such services to us during fiscal 2020 and in connection with the audit of our 2020 consolidated financial statements is compatible with and did not impair independence. It is the practice of the audit committee to consider and approve in advance all auditing and non-auditing services provided to us by our independent auditors in accordance with the applicable requirements of the SEC. Dixon Hughes Goodman LLP did not provide us with any services, other than those listed above.
| 70 |
PART IV
| ITEM 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
2. Exhibits:
EXHIBIT INDEX
Exhibit No. |
Description | Filed/ Furnished Herewith | Form |
Exhibit No. |
File No. | Filing Date | ||||||
| 2.01# | Agreement and Plan of Merger, dated December 16, 2020, by and among Seneca Biopharma, Inc., Townsgate Acquisition Sub 1, Inc. and Leading BioSciences, Inc. | 8-K | 2.1 | 001-33672 | 12/21/20 | |||||||
| 2.02 | Form of Support Agreement, by and between Seneca Biopharma, Inc. and certain officers and directors of Seneca Biopharma, Inc. | 8-K | 10.1 | 001-33672 | 12/21/20 | |||||||
| 2.03 | Form of Support Agreement, by and between Seneca Biopharma, Inc. and certain officers, directors and stockholders of Leading BioSciences, Inc. | 8-K | 10.2 | 001-33672 | 12/21/20 | |||||||
| 2.04 | Form of Company Lock-Up Agreement | 8-K | 10.3 | 001-33672 | 12/21/20 | |||||||
| 2.05 | Form of LBS Lock-Up Agreement | 8-K | 10.4 | 001-33672 | 12/21/20 | |||||||
| 2.06 | Form of CVR Agreement. | S-4/A | 2.06 | 333-251659 | 2/9/2021 | |||||||
| 3.01(i) | Amended and Restated Certificate of Incorporation of Neuralstem, Inc. filed on 1/5/2017 | S-1/A | 3.01(i) | 001-33672 | 1/6/17 | |||||||
| 3.01(ii) | Amended and Restated Certificate of Incorporation of Neuralstem, Inc. effective on 7/17/2019 | 8-K | 3.01(i) | 001-33672 | 7/18/19 | |||||||
| 3.01(iii) | Amendment to Amended and Restated Certificate of Incorporation of Neuralstem, Inc. effective 10/28/19 | 8-K | 3.01 | 001-33672 | 10/30/19 | |||||||
| 3.01(iv) | Certificate of Validation of Certificate to the Amended and Restated Certificate of Incorporation of Seneca Biopharma, Inc. | S-4 | 3.01(iv) | 333-251659 | 12/23/20 | |||||||
| 3.02(i) | Certificate of Designation of Series A 4.5% Convertible Preferred Stock | 8-K | 3.01 | 001-33672 | 12/12/16 | |||||||
| 3.03(ii) | Amended and Restated Bylaws of Neuralstem, Inc. adopted on 11/10/2015 | 8-K | 3.01 | 001-33672 | 11/16/15 | |||||||
| 4.01** | Amended and Restated 2005 Stock Plan adopted on 6/28/07 | 10-QSB | 4.2(i) | 333-132923 | 8/14/07 | |||||||
| 4.02** | Neuralstem, Inc. 2007 Stock Plan | 10-QSB | 4.21 | 333-132923 | 8/14/07 | |||||||
| 4.03 | Form of Common Stock Purchase Warrant Issued to Karl Johe on 6/5/07 | 10-KSB | 4.22 | 333-132923 | 3/27/08 |
| 71 |
| 4.04 | Form of employee and consultant option grant pursuant to our 2007 Stock Plan and 2010 Equity Compensation Plan | 10-K | 4.23 | 001-33672 | 3/31/10 | |||||||
| 4.05** | Amended Neuralstem 2010 Equity Compensation Plan adopted on June 22, 2017 | DEF 14A | Appendix I | 001-33672 | 5/1/17 | |||||||
| 4.06** | Form of Restricted Stock Award Agreement pursuant to our 2007 Stock Plan and 2010 Equity Compensation Plan | S-8 | 4.06 | 333-172563 | 3/1/11 | |||||||
| 4.07** | Form of Restricted Stock Unit Agreement | S-8 | 4.08 | 333-172563 | 3/1/11 | |||||||
| 4.08 | Form of Consulting Warrant issued January 2011 and March 2012 | S-3 | 4.01 | 333-188859 | 5/24/13 | |||||||
| 4.09** | Inducement Stock Option Plan adopted 2/15/2016 and as amended on 12/12/2018, 9/13/2019, and 3/23/20 | 10-K | 4.31 | 001-33672 | 3/27/20 | |||||||
| 4.10** | Form of Inducement Award Non-Qualified Stock Option Grant pursuant to Inducement Stock Option Plan | 8-K | 4.02 | 001-33672 | 2/19/16 | |||||||
| 4.11 | Form of Common Stock Purchase Warrant from May 2016 Public Offering dated May 6, 2016 | 8-K | 4.01 | 001-33672 | 5/4/16 | |||||||
| 4.12 | Form of Common Stock Purchase Warrant from May 2016 Private Offering Dated May 12, 2016 | 8-K | 4.01 | 001-33672 | 5/13/16 | |||||||
| 4.13 | Form of Series A Preferred Stock Certificate | 8-K | 4.01 | 001-33672 | 9/12/16 | |||||||
| 4.14 | Form of Inducement Warrant issued March 20, 2017 and March 31, 2017 | 8-K | 4.01 | 001-33672 | 3/20/17 | |||||||
| 4.15 | Form of Common Stock Purchase Warrant from August 2017 Public Offering Dated August 1, 2017 | 8-K | 4.01 | 001-33672 | 7/28/17 | |||||||
| 4.16 | Form of Common Stock Purchase Warrant from October 2018 Offering | 8-K | 4.01 | 001-33672 | 10/29/18 | |||||||
| 4.17 | Form of Placement Agent Common Stock Purchase Warrant from October 2018 Offering | 8-K | 4.02 | 001-33672 | 10/29/18 | |||||||
| 4.18 | Consultant Warrant for Hibiscus BioVentures, LLC issued January 2019 | 10-Q | 4.40 | 001-33672 | 5/14/19 | |||||||
| 4.19** | Seneca Biopharma 2019 Equity Incentive Plan | DEF 14A | Appendix I | 001-33672 | 4/29/19 | |||||||
| 4.20** | Form of Restricted Stock Unit from 2019 Equity Incentive Plan | S-1 | 4.42 | 333-232273 | 6/21/19 | |||||||
| 4.21** | Form of Restricted Option Grant from 2019 Equity Incentive Plan | S-1 | 4.43 | 333-232273 | 6/21/19 | |||||||
| 4.22** | Form of Restricted Stock Grant from 2019 Equity Incentive Plan | S-1 | 4.44 | 333-232273 | 6/21/19 | |||||||
| 4.23 | Form of Series M and Series N warrant from July 2019 Offering | S-1/A | 4.45 | 333-232273 | 7/24/19 | |||||||
| 4.24 | Form of Series O Pre-Funded Warrant from July 2019 Offering | S-1/A | 4.46 | 333-232273 | 7/24/19 | |||||||
| 4.25 | Form of Series P Replacement Warrant issued in January 2020 Offering | 8-K | 4.01 | 001-33672 | 1/22/20 | |||||||
| 72 |
| 4.26 | Form of Series Q Replacement Warrant issued in January 2020 Offering | 8-K | 4.02 | 001-33672 | 1/22/20 | |||||||
| 4.27 | Form of Placement Agent Warrant issued in January 2020 Offering | 8-K | 4.03 | 001-33672 | 1/22/20 | |||||||
| 4.28 | Form of Placement Agent Warrant issued in May 2020 Offering | 8-K | 4.01 | 001-33672 | 5/27/20 | |||||||
| 4.29** | Seneca Biopharma 2020 Equity Incentive Plan | DEF 14A | Appendix C | 001-33672 | 6/24/20 | |||||||
| 4.31 | Registration Rights Agreement, by and between Seneca Biopharma, Inc. and the investor party thereto, dated December 16, 2020 | 8-K | 4.3 | 001-33672 | 12/21/20 | |||||||
| 10.01** | Employment Agreement with Kenneth Carter dated December 12, 2018 | 8-K | 10.01 | 001-33672 | 12/18/18 | |||||||
| 10.02 | Form of Securities Purchase Agreement from May 2016 Private Offering | 8-K | 10.01 | 001-33672 | 5/13/16 | |||||||
| 10.03 | Form of Securities Purchase Agreement between Neuralstem and Tianjin Pharmaceuticals Holdings, Ltd. | 8-K | 10.01 | 001-33672 | 9/12/16 | |||||||
| 10.04 | Form of Letter Agreement for Warrant Exercises on March 20, 2017 and March 30, 2017 | 8-K | 10.01 | 001-33672 | 3/20/17 | |||||||
| 10.05 | Form of Securities Purchase Agreement with Investors from October 2018 Offering | 8-K | 10.01 | 001-33672 | 10/29/18 | |||||||
| 10.06 | Form of Engagement Agreement with H.C. Wainwright & Co. Dated October 25, 2018 | 8-K | 10.02 | 001-33672 | 10/29/18 | |||||||
| 10.07** | Sample Confidential Information and Invention Assignment Agreement | 8-K | 10.02 | 001-33672 | 12/12/18 | |||||||
| 10.08** | Form of Indemnification Agreement for Directors and Officers | 8-K | 10.03 | 001-33672 | 12/12/18 | |||||||
| 10.09 | Letter Agreement from January 2020 Offering | 8-K | 10.01 | 001-33672 | 1/22/20 | |||||||
| 10.10 | Form of Placement Agent Agreement from January 2020 Offering | 8-K | 10.02 | 001-33672 | 1/22/20 | |||||||
| 10.11** | Amendment to Employment Agreement with Kenneth Carter effective April 1, 2020 | 10-K | 10.25 | 001-33672 | 3/27/20 | |||||||
| 10.12** | Employment Agreement with Dane Saglio | 8-K | 10.01 | 001-33672 | 4/2/20 | |||||||
| 10.13** | Employment Agreement with Matthew Kalnik, PhD | 8-K | 10.02 | 001-33672 | 4/2/20 | |||||||
| 10.14 | Form of Securities Purchase Agreement with Investors from May 2020 Offering | 8-K | 10.01 | 001-33672 | 5/27/20 | |||||||
| 10.15 | Securities Purchase Agreement, by and between Leading BioSciences, Inc. and the investor party thereto, dated December 16, 2020 | 8-K | 10.5 | 001-33672 | 12/21/20 | |||||||
| 10.16 | Securities Purchase Agreement, by and among Seneca Biopharma, Inc., Leading BioSciences, Inc. and the investor party thereto, dated December 16, 2020 | 8-K | 10.6 | 001-33672 | 12/21/20 | |||||||
| 73 |
| 10.17 | Form of Leak-Out Agreement, by and between Seneca Biopharma, Inc. and the investor party thereto | 8-K | 10.7 | 001-33672 | 12/21/20 | |||||||
| 10.18 | Form of Separation Agreement with Seneca Executives | 8-K | 10.01 | 001-33672 |
3/18/21 | |||||||
| 14.01 | Code of Ethics and Conduct | 10-K | 14.01 | 001-33672 | 4/2/18 | |||||||
| 14.02 | Financial Code of Professional Conduct | 10-K | 14.02 | 001-33672 | 3/27/20 | |||||||
| 21.01 | Subsidiaries of Registrant | * | ||||||||||
| 23.01 | Consent of Dixon Hughes Goodman LLP, independent registered public accounting firm to Seneca Biopharma, Inc. | * | ||||||||||
| 31.1 / 31.2 |
Certification of the Principal Executive Officer and Principal Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
* | ||||||||||
| 32.1 / 32.2 | Certification of Principal Executive Officer and Principal Financial Officer Pursuant to 18 U.S.C. § 1350 | * | ||||||||||
| 101.INS | XBRL Instance Document | * | ||||||||||
| 101.SCH | XBRL Taxonomy Extension Schema Document | * | ||||||||||
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | * | ||||||||||
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | * | ||||||||||
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document | * | ||||||||||
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | * |
| ITEM 16. | FORM 10-K SUMMARY |
None
| 74 |
SIGNATURES
In accordance with Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| SENECA BIOPHARMA, INC | ||||
| Dated: March 22, 2021 | By: | /S/ Dane Saglio | ||
| Dane Saglio | ||||
| Principal Executive and Financial Officer | ||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the following capacities and on the dates indicated.
| Name | Title | Date | ||
|
/s/Kenneth Carter Kenneth Carter |
Director (Chairman) | March 22, 2021 | ||
|
/s/Dane Saglio Dane Saglio |
Principal Executive and Principal Financial Officer | March 22, 2021 | ||
|
/s/ Cristina Csimma Cristina Csimma |
Director | March 22, 2021 | ||
|
/s/ Mary Ann Gray Mary Ann Gray |
Director | March 22, 2021 | ||
|
/s/ David Mazzo David Mazzo |
Director | March 22, 2021 | ||
|
/s/ Binxian Wei Binxian Wei |
Director | March 22, 2021 | ||
75
