Attached files
| file | filename |
|---|---|
| 8-K - 8-K - REGENERON PHARMACEUTICALS, INC. | a18-2403_18k.htm |
| EX-99.1 - EX-99.1 - REGENERON PHARMACEUTICALS, INC. | a18-2403_1ex99d1.htm |
2018 Financial overview Robert Landry Senior Vice President of Finance - Chief Financial Officer January 10th, 2018
NOTE REGARDING FORWARD-LOOKING STATEMENTS AND NON-GAAP FINANCIAL MEASURES 2 This presentation includes forward-looking statements that involve risks and uncertainties relating to future events and the future performance of Regeneron Pharmaceuticals, Inc. (“Regeneron” or the “Company”), and actual events or results may differ materially from these forward-looking statements. Words such as “anticipate,” “expect,” “intend,” “plan,” “believe,” “seek,” “estimate,” variations of such words, and similar expressions are intended to identify such forward-looking statements, although not all forward-looking statements contain these identifying words. These statements concern, and these risks and uncertainties include, among others, the nature, timing, and possible success and therapeutic applications of Regeneron’s products, product candidates, and research and clinical programs now underway or planned, including without limitation EYLEA® (aflibercept) Injection, Praluent® (alirocumab) Injection, Dupixent® (dupilumab) Injection, Kevzara® (sarilumab) Injection, cemiplimab, fasinumab, Regeneron’s earlier-stage product candidates, and the use of human genetics in Regeneron’s research programs; the extent to which the results from Regeneron’s research programs or preclinical testing may lead to advancement of product candidates to clinical trials or therapeutic applications; unforeseen safety issues resulting from the administration of products and product candidates in patients, including serious complications or side effects in connection with the use of Regeneron’s product candidates in clinical trials; the likelihood and timing of possible regulatory approval and commercial launch of Regeneron’s late-stage product candidates and new indications for marketed products, including without limitation EYLEA, Praluent, Dupixent, Kevzara, cemiplimab, and fasinumab; risks associated with intellectual property of other parties and pending or future litigation relating thereto, including without limitation the patent litigation proceedings relating to Praluent, the ultimate outcome of any such litigation proceeding, and the impact any of the foregoing may have on Regeneron’s business, prospects, operating results, and financial condition; the likelihood and timing of achieving any of the anticipated milestones described in this presentation; ongoing regulatory obligations and oversight impacting Regeneron’s marketed products (such as EYLEA, Praluent, Dupixent, and Kevzara), research and clinical programs, and business, including those relating to patient privacy; determinations by regulatory and administrative governmental authorities which may delay or restrict Regeneron’s ability to continue to develop or commercialize Regeneron’s products and product candidates; competing drugs and product candidates that may be superior to Regeneron’s products and product candidates; uncertainty of market acceptance and commercial success of Regeneron’s products and product candidates; the ability of Regeneron to manufacture and manage supply chains for multiple products and product candidates; the ability of Regeneron’s collaborators, suppliers, or other third parties to perform filling, finishing, packaging, labelling, distribution, and other steps related to Regeneron’s products and product candidates; coverage and reimbursement determinations by third-party payers, including Medicare and Medicaid; unanticipated expenses; the costs of developing, producing, and selling products; the ability of Regeneron to meet any of its sales or other financial projections or guidance and changes to the assumptions underlying those projections or guidance; and the potential for any license or collaboration agreement, including Regeneron’s agreements with Sanofi, Bayer, and Teva Pharmaceutical Industries Ltd. (or their respective affiliated companies, as applicable), to be cancelled or terminated without any further product success. A more complete description of these and other material risks can be found in Regeneron’s filings with the U.S. Securities and Exchange Commission, including its Form 10-K for the fiscal year ended December 31, 2016 and its Form 10-Q for the quarterly period ended September 30, 2017, including in each case in the section thereof captioned “Item 1A. Risk Factors.” Any forward-looking statements are made based on management’s current beliefs and judgment, and the reader is cautioned not to rely on any forward-looking statements made by Regeneron. Regeneron does not undertake any obligation to update publicly any forward-looking statement, including without limitation any financial projection or guidance, whether as a result of new information, future events, or otherwise. This presentation uses non-GAAP unreimbursed R&D and non-GAAP SG&A, which are financial measures that are not calculated in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”). These non-GAAP financial measures are computed by excluding certain non-cash and other items from the related GAAP financial measure. Non-GAAP adjustments also include the income tax effect of reconciling items. The Company makes such adjustments for items the Company does not view as useful in evaluating its operating performance. For example, adjustments may be made for items that fluctuate from period to period based on factors that are not within the Company’s control, such as the Company’s stock price on the dates share-based grants are issued. Management uses these and other non-GAAP measures for planning, budgeting, forecasting, assessing historical performance, and making financial and operational decisions, and also provides forecasts to investors on this basis. Additionally, such non-GAAP measures provide investors with an enhanced understanding of the financial performance of the Company’s core business operations. However, there are limitations in the use of these and other non-GAAP financial measures as they exclude certain expenses that are recurring in nature. Furthermore, the Company’s non-GAAP financial measures may not be comparable with non-GAAP information provided by other companies. Any non-GAAP financial measure presented by Regeneron should be considered supplemental to, and not a substitute for, measures of financial performance prepared in accordance with GAAP.

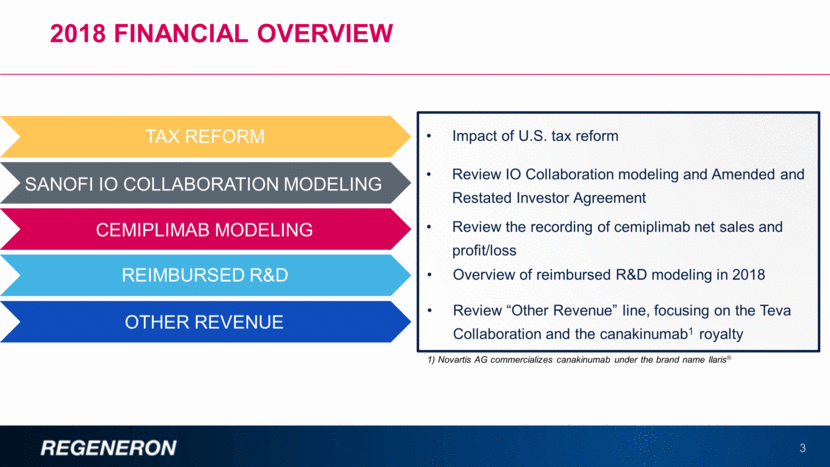
2018 FINANCIAL Overview 3 TAX REFORM SANOFI IO COLLABORATION MODELING CEMIPLIMAB MODELING REIMBURSED R&D OTHER REVENUE Impact of U.S. tax reform Review IO Collaboration modeling and Amended and Restated Investor Agreement Review the recording of cemiplimab net sales and profit/loss Overview of reimbursed R&D modeling in 2018 Review “Other Revenue” line, focusing on the Teva Collaboration and the canakinumab1 royalty 1) Novartis AG commercializes canakinumab under the brand name Ilaris®
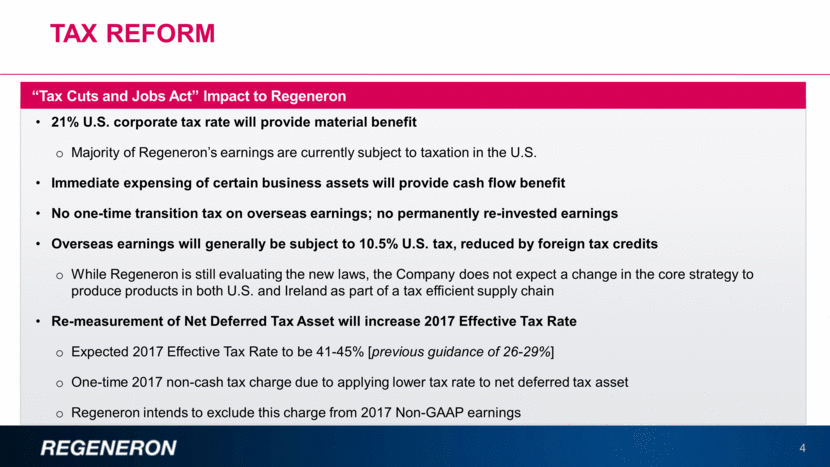
Tax reform 4 21% U.S. corporate tax rate will provide material benefit Majority of Regeneron’s earnings are currently subject to taxation in the U.S. Immediate expensing of certain business assets will provide cash flow benefit No one-time transition tax on overseas earnings; no permanently re-invested earnings Overseas earnings will generally be subject to 10.5% U.S. tax, reduced by foreign tax credits While Regeneron is still evaluating the new laws, the Company does not expect a change in the core strategy to produce products in both U.S. and Ireland as part of a tax efficient supply chain Re-measurement of Net Deferred Tax Asset will increase 2017 Effective Tax Rate Expected 2017 Effective Tax Rate to be 41-45% [previous guidance of 26-29%] One-time 2017 non-cash tax charge due to applying lower tax rate to net deferred tax asset Regeneron intends to exclude this charge from 2017 Non-GAAP earnings “Tax Cuts and Jobs Act” Impact to Regeneron
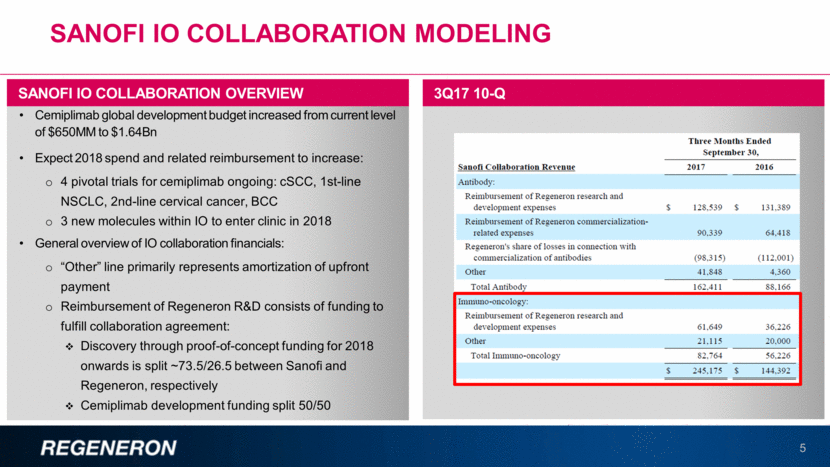
SANOFI IO COLLABORATION MODELING 5 Cemiplimab global development budget increased from current level of $650MM to $1.64Bn Expect 2018 spend and related reimbursement to increase: 4 pivotal trials for cemiplimab ongoing: cSCC, 1st-line NSCLC, 2nd-line cervical cancer, BCC 3 new molecules within IO to enter clinic in 2018 General overview of IO collaboration financials: “Other” line primarily represents amortization of upfront payment Reimbursement of Regeneron R&D consists of funding to fulfill collaboration agreement: Discovery through proof-of-concept funding for 2018 onwards is split ~73.5/26.5 between Sanofi and Regeneron, respectively Cemiplimab development funding split 50/50 SANOFI IO COLLABORATION OVERVIEW 3Q17 10-Q
Acceleration and Expanded investment of cemiplimab and dupilumab development programs 6 Cemiplimab global development budget increased from current level of $650MM to $1.64Bn Significant incremental investment in development plans for dupilumab and REGN3500 Changes to Amended and Restated Investor Agreement Allow Sanofi to sell in private transactions to Regeneron up to an aggregate of 1.4MM shares of Regeneron common stock through the end of 2020 Represents ~6% of the 23.9MM shares Sanofi currently owns As of October 20, 2017, there were 107.4 million shares of Regeneron capital stock outstanding For shares not purchased by Regeneron, Sanofi is capped in the number of shares they may sell in the open market Daily: Cannot exceed 10% of the average daily trading volume for 20 previous trading days Calendar Quarter: Cannot exceed 300,000 shares sold in any given calendar quarter
Cemiplimab modeling 7 U.S. cemiplimab net sales and U.S. cost of goods sold (COGS) will be recorded on Regeneron’s Income Statement (IS) Regeneron’s payment of Sanofi’s share of gross profit on U.S. sales of cemiplimab will be recorded in the COGS line on the Regeneron IS SG&A for U.S. cemiplimab will be recorded on Regeneron’s IS and will consist of Regeneron-incurred U.S. commercialization expenses, Regeneron’s reimbursement of 50% of Sanofi-incurred U.S. commercialization expenses, and will be offset by Sanofi’s reimbursement of 50% of Regeneron-incurred U.S. commercialization expenses Ex-U.S. cemiplimab net sales and ex-U.S. COGS will be recorded by our collaborator Sanofi Regeneron will recognize its share of ex-U.S. profits or losses within the Sanofi Collaboration Revenue line on the Regeneron IS Since cemiplimab development expenses are shared on a 50/50 basis, its development does not contribute to the IO development balance How to Record Net Sales, Cost of Goods Sold, and Share of Profit/Loss
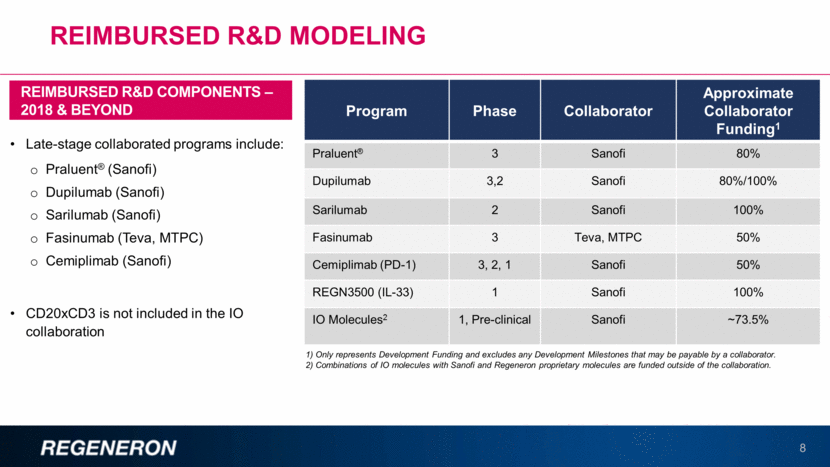
REIMBURSED R&D MODELING 8 Late-stage collaborated programs include: Praluent® (Sanofi) Dupilumab (Sanofi) Sarilumab (Sanofi) Fasinumab (Teva, MTPC) Cemiplimab (Sanofi) CD20xCD3 is not included in the IO collaboration REIMBURSED R&D COMPONENTS – 2018 & BEYOND Program Phase Collaborator Approximate Collaborator Funding1 Praluent® 3 Sanofi 80% Dupilumab 3,2 Sanofi 80%/100% Sarilumab 2 Sanofi 100% Fasinumab 3 Teva, MTPC 50% Cemiplimab (PD-1) 3, 2, 1 Sanofi 50% REGN3500 (IL-33) 1 Sanofi 100% IO Molecules2 1, Pre-clinical Sanofi ~73.5% 1) Only represents Development Funding and excludes any Development Milestones that may be payable by a collaborator. 2) Combinations of IO molecules with Sanofi and Regeneron proprietary molecules are funded outside of the collaboration.
R&D MODELING 9 Review of R&D expenses in the Quarterly and Annual filings, located in the Management’s Discussion and Analysis section, provides expense details for late-stage programs, as well as earlier candidates in development as a whole R&D FORECASTING * Certain prior year amounts have been reclassified to conform to the current year's presentation Source: 3Q17 10-Q
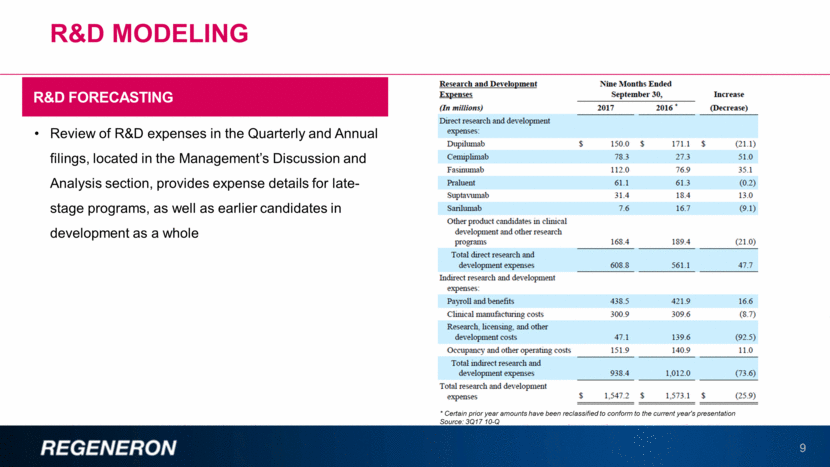
Other Revenue 10 Regeneron’s Quarterly and Annual filings include a table, located in the Management’s Discussion and Analysis section, that summarizes “Other Revenue” OTHER REVENUE COMPONENTS 3Q17 10-Q
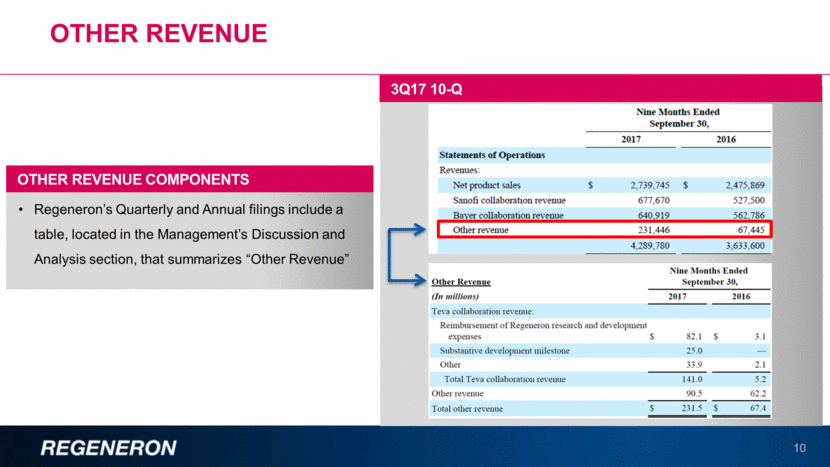
Other Revenue: TEVA collaboration & Other 11 Under the terms of the agreement, Regeneron and Teva split ongoing R&D costs 50/50, up to ~$1.0Bn Regeneron is entitled to receive up to $460MM in development milestones, of which $25MM was recorded in 2Q17 and $35MM in 4Q17 TEVA COLLABORATION REVENUE 3Q17 10-Q OTHER REVENUE EXAMPLES Under a 2009 agreement with Novartis, Regeneron receives a royalty on worldwide net sales of canakinumab The royalty rate starts at 4% and reaches 15% when canakinumab annual sales exceed $1.5Bn The royalty applies to currently approved indications and any potential sales for future indications Mitsubishi Tanabe Pharma collaboration revenue including development milestones Research and Development funding from BARDA (Biomedical Advanced Research and Development Authority) RGC (Regeneron Genetics Center) genetics consortium funding starting in 2018
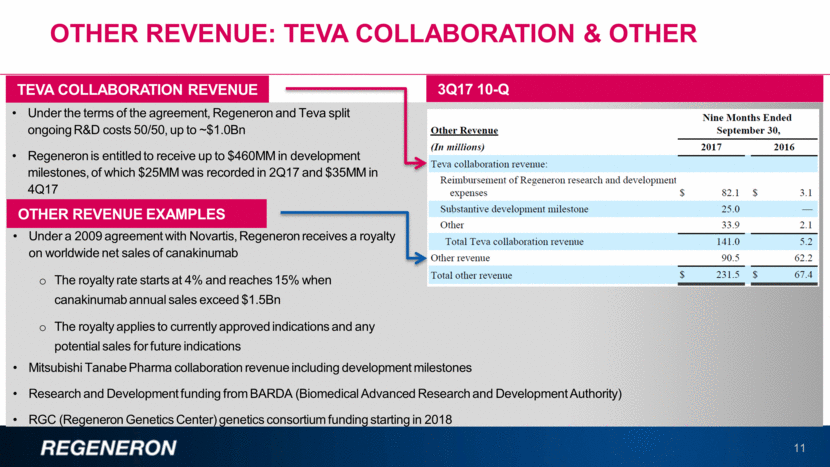
2018 FINANCIAL GUIDANCE1 12 $1,230MM - $1,330MM Non-GAAP Unreimbursed R&D: $1,350MM - $1,450MM Non-GAAP SG&A: $450MM - $500MM Sanofi Collaboration Revenue; Reimbursement of Regeneron Commercialization-Related Expenses: 15% - 19% Effective Tax Rate: $420MM - $500MM Capital Expenditures: As of January 8, 2018. The guidance does not assume the completion of any significant business development transaction that had not been completed as of the date of the guidance. Regeneron does not undertake any obligation to update publicly any financial projection or guidance, whether as a result of new information, future events, or otherwise.
13 Q&A

14 APPENDIX

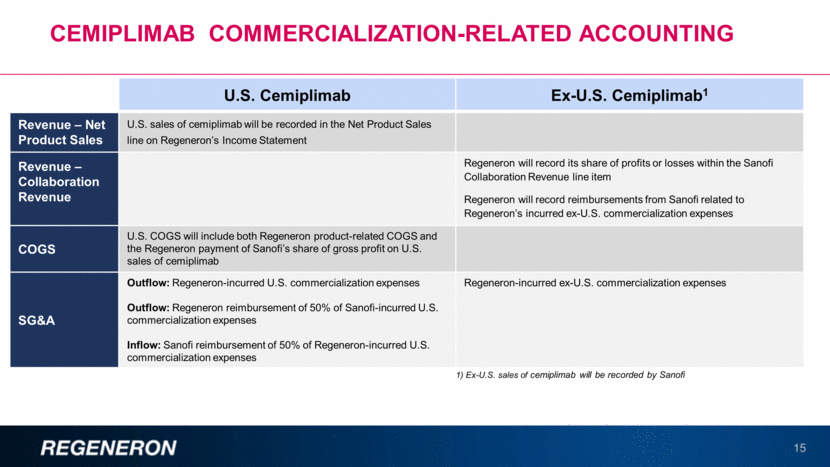
Cemiplimab commercialization-Related accounting 15 U.S. Cemiplimab Ex-U.S. Cemiplimab1 Revenue – Net Product Sales U.S. sales of cemiplimab will be recorded in the Net Product Sales line on Regeneron’s Income Statement Revenue – Collaboration Revenue Regeneron will record its share of profits or losses within the Sanofi Collaboration Revenue line item Regeneron will record reimbursements from Sanofi related to Regeneron’s incurred ex-U.S. commercialization expenses COGS U.S. COGS will include both Regeneron product-related COGS and the Regeneron payment of Sanofi’s share of gross profit on U.S. sales of cemiplimab SG&A Outflow: Regeneron-incurred U.S. commercialization expenses Outflow: Regeneron reimbursement of 50% of Sanofi-incurred U.S. commercialization expenses Inflow: Sanofi reimbursement of 50% of Regeneron-incurred U.S. commercialization expenses Regeneron-incurred ex-U.S. commercialization expenses 1) Ex-U.S. sales of cemiplimab will be recorded by Sanofi